Abstract
The serum concentration of haptoglobin (S-Hp) was measured in 833 group-housed dairy calves from nine herds in south-west Sweden once at 24–56 days of age to evaluate the potential of S-Hp as an indicator of clinical respiratory-tract disease (CRD). Presence of disease (treated and non-treated) was assessed clinically by farmers and by a project veterinarian visiting the farms every third week.
The median S-Hp of healthy calves was 0.06 g/L (80% central range: 0.04–0.23), of calves with diarrhoea within the 10 days before sampling 0.07 g/L (80% central range: 0.04–0.63), and of calves with CRD within the 14 days before sampling 0.09 g/L (80% central range: 0.04–0.69). Eight different cut-off values were used to define a positive S-Hp analysis result: >0.05, >0.06, >0.07, >0.08, >0.09, >0.10, >0.15 and >0.20 g/L. A rectal temperature >39.5 °C was denoted as fever. A positive result of five different diagnostic tests for CRD was defined as: (1) a positive S-Hp with fever absent, (2) a positive S-Hp with fever present, (3) either a positive S-Hp or fever, (4) both a positive S-Hp and fever, and (5) fever (regardless of S-Hp). The sensitivity (Se) and specificity (Sp) of each test were calculated from regression coefficients of generalized linear mixed models of the binary test results, applying a logit link. Apart from CRD status (within the 14 days before sampling; no or yes), the models included sex (bull or heifer), and for the test based on S-Hp alone, also rectal temperature (fever, no or yes). Confidence intervals (CI) of Se and Sp were estimated by simulation.
Based on Se, Sp, and areas under Receiver Operating Characteristics curves, test 3 was considered the best. At optimal performance, giving equal importance to type I and II errors, i.e. at a S-Hp cut-off of 0.15 g/L in heifer calves, Se was 0.64 (95% CI 0.50–0.77) and Sp 0.71 (95% CI 0.60–0.80), and at a S-Hp cut-off of 0.08 g/L in bulls, Se was 0.52 (95% CI 0.40–0.64) and Sp 0.80 (95% CI 0.74–0.85). The other tests were judged as unsatisfactory indicators of CRD. In heifers, the proportion of CRD-positive calves in the herd was strongly associated with the proportion of test positives (S-Hp or fever; S-HP and fever), suggesting potential as a herd-level indicator.
Keywords: Clinical respiratory-tract disease, Dairy calf, Diagnostic test, Haptoglobin, Rectal temperature
1. Introduction
Together with diarrhoea, clinical respiratory-tract disease (CRD) constitutes the main health problem in young dairy calves (Olsson et al., 1993, Sivula et al., 1996, Virtala et al., 1996, Wells et al., 1996, Svensson et al., 2003). Furthermore, CRD is the most common disease in older calves (Gardner et al., 1990, Svensson et al., 2006), and pneumonia the overall most common cause of mortality in dairy calves and replacement heifers (Agerholm et al., 1993, Svensson et al., in press). Svensson et al. (2003) and Svensson et al. (2006) reported an increased risk of CRD in calves housed in large group pens with automatic milk feeders, compared to individually housed calves. In Sweden, the use of group housing systems is increasing, especially the use of large group pens with rationalised milk feeding (Pettersson et al., 2001), and hence increased problems with CRD can be expected unless proper measures are taken. CRD was also found to be the most common indication for antibiotic drug use in Swedish calves (Ortman and Svensson, 2004).
CRD is considered to be a multi-factorial disease with causative agents, calf factors and environmental factors as components. The most common agents found in association with CRD are viruses such as bovine respiratory syncytial virus (BRS), parainfluenza-3 virus, adenovirus and bovine coronavirus. However, secondary bacterial infections, e.g. Pasteurella multocida, Mannheimia haemolytica and Haemophilus somnus are not uncommon (Radostits et al., 2000). Except for respiratory disease due to BRS, cases involving bacterial agents generally are considered to be associated with more severely affected general condition and prolonged convalescence.
Acute phase proteins (APP) are mostly glycoproteins produced in reduced (negative APP) or increased (positive APP) amounts in response to inflammatory processes. Previous studies have shown that haptoglobin is an important positive APP in cattle. Under normal conditions haptoglobin is absent or present in very low concentrations in serum ranging from 0.05 to 0.10 g/L, but the concentration may increase 50–100 times in response to different diseases including CRD (Conner et al., 1989, Skinner et al., 1991, Alsemgeest et al., 1994, Godson et al., 1996, Hirvonen et al., 1996, Heegard et al., 2000, Gånheim et al., 2003). Wittum et al. (1996) found the serum-haptoglobin concentration (S-Hp) to be associated with CRD in feedlot cattle, and suggested that analyses of this glycoprotein should be included in disease screening or used as an indication of microbial infection.
S-Hp was used to discriminate between acute and chronic inflammation (Alsemgeest et al., 1994, Horadagoda et al., 1999) and was suggested an indicator of the effect of treatment (Carter et al., 2002, Berry et al., 2004) and prognosis (Godson et al., 1996). Gray et al. (1996) reported it to be a useful tool for distinguishing healthy animals from those that may need further examination at slaughter, and Saini et al. (1998) also suggested it to be of value as an aid in improving food safety. Carter et al. (2002) concluded that S-Hp was a better tool for discriminating between calves that became ill and those that did not, compared to other APP.
The objective of the present study was to test the hypothesis that S-Hp at 3–8 weeks of age is a useful indicator of CRD in dairy calves under field conditions.
2. Material and methods
The data were from nine commercial dairy farms located in south-west Sweden and participating in an experiment on the effect of group size on health and growth rate of calves from September 2002 to December 2003 (Svensson and Liberg, 2006). Each farm had 80–120 dairy cows and housed their young calves in two group pens with automatic milk feeders, one holding 6–9 calves and the other 12–18 calves. The present study comprised 833 of the 892 calves from the above experiment, i.e. all calves which were successfully sampled for S-Hp at between 3 and 8 weeks of age. The number of studied calves per herd was 42–155 (median: 103). The calves were Swedish Reds (52%), Swedish Holsteins (40%), crossbreeds between milk breeds including Jersey (6%) or crossbreeds with a beef breed, such as Aberdeen Angus, Charolais, Hereford or Simmental (3%).
Except for two herds, in which the calves were transferred to individual pens immediately after birth, each calf was kept together with its dams in a calving pen for 0.5–3 days. The animal was then housed in a single calf pen, and at 2–35 (median: 12) days of age transferred to a group pen on the farm, randomly allocated to one of the two group-size treatments (one pen per treatment per farm). Except in one herd, where the calves were kept in cubicles bedded with sawdust with access to a concrete feeding alley, the group pens were straw-bedded or, on three farms, consisted of a straw-bedded lying area and a concrete or slatted-floor feeding alley. The total areas of the pens used for the small-sized group ranged from 11 to 40 (median: 18) m2, and the pens for the large-sized group measured from 19.5 to 42.2 (median: 27.0) m2. The lying area per calf measured 1.4 to 3.2 (median: 1.6) m2 in the small-sized groups and 1.4 to 2.4 (median: 1.6) m2 in the large-sized groups.
The calves received their first meal of colostrum through a nipple in six of the herds and by suckling the dam in the rest of the herds. They then received whole milk (4 herds), milk replacer (1 herd) or combinations of milk replacer and whole milk (4 herds) until weaning at 8–11 weeks of age. The calves had free access to roughage (hay, silage or a grain and silage mix) from 1 to 10 days of age and free access to concentrates in the form of pelleted calf feed or, in the case of one herd, crushed grain, starting from 1 to 14 days of age. The calves had free access to water from cups from 4 to 14 days of age. The same feeding and management regimes were applied to all calves within a herd.
2.1. Data collection
The calves were monitored from birth to 56 days of age. The farmers were asked to record on individual health forms, the breed and sex of each calf, the parity of the dam, and all cases of disease (treated and untreated) together with information about treatments and about the calves’ appetite, and general condition during the disease periods. Every third week the herds were visited by the same project veterinarian, who conducted a brief physical examination of all the calves present, auscultated their lungs and recorded diseases not detected by the farmers.
CRD was defined alternatively as coughing or sneezing for >2 days, as severely increased respiratory sounds at lung auscultation, or as moderately increased respiratory sounds together with coughing and/or nasal discharge. Diarrhoea was defined as a faecal consistency that remained softer than is regularly observed in clinically healthy calves for two days or more. Cases of other diseases of presumed infectious origin, including arthritis, cheek abscess, omphalophlebitis/umbilical abscess, weak-calf syndrome were categorised as “other infectious disease”. Arthritis (no case reported) was defined as swelling of one or more joints accompanied by lameness and fever. Cheek abscess was defined as a protrusion on the cheek accompanied by a moderately to severely affected general condition. Omphalophlebitis/umbilical abscess was defined as a warm swelling or abscess formation around the umbilical cord accompanied by a moderately to severely affected general condition. Weak-calf syndrome was diagnosed when an animal showed inappetence and dullness for at least two days, with or without increased rectal temperature, but lacked other symptoms of disease.
Blood samples were taken from the calves once during the study period from 24 to 56 (median: 41) days of age. The rectal temperature was measured on the same occasion (there were four missing values). The blood samples were sent promptly to the Department of Clinical Chemistry of the Swedish University of Agricultural Sciences in Uppsala, where they were centrifuged the next day and serum samples were stored at −18 °C until analysis. S-Hp was analysed using a commercial kit (PHASE Range Haptoglobin Assay Kit), with a detection level of 0.05 g/L. Samples below the detection level were given a value of 0.04 g/L. The assay was based on Hp-haemoglobin binding and preservation of the peroxidase activity of the bound haemoglobin. The peroxidase activity was measured by spectrophotometry using a Cobas Mira (Roche Basel). In cases of haemolysis, calves were subjected to new sampling.
2.2. Data editing and statistical analysis
Microsoft Office Excel 2003 (Microsoft Corp.) and JMP Statistical Discovery Software, Release 6 (SAS Systems) were used for data coding, checking and descriptive statistics.
Eight different cut-off values were used to define a positive S-Hp analysis result: >0.05, >0.06, >0.07, >0.08, >0.09, >0.10, >0.15 and >0.20 g/L. Five diagnostic tests were constructed using the results from S-Hp analyses (with each of the cut-off values) and rectal temperature measurements (fever when >39.5 °C) with a positive test defined as (1) S-Hp positive with fever absent (HP(T−) test), (2) S-Hp positive with fever present (HP(T+) test), (3) either S-Hp or fever positive (HP/TEMP test), (4) both S-Hp and fever positive (HP + TEMP test), and (5) fever (regardless of S-Hp; TEMP test), respectively. CRDtest was defined as CRD diagnosed within 14 days before blood sampling.
The analysis of the tests as indicators of CRD in individual calves was performed in two steps. Firstly, a generalized linear mixed model with test result (0 = test negative; 1 = test positive) as outcome was constructed for each test and each S-Hp cut-off value, using the GLIMMIX procedure of the SAS software package, Version 9 (SAS Systems) and a logit link. Considering all different tests and eight different S-Hp cut-off values, 25 models were thus constructed. Apart from the disease status (CRDtest: 0 = no; 1 = yes), variables representing parity of the dam, breed, sex, rectal temperature, month of year, and week of age were tested for inclusion as predictors. Only sex and rectal temperature were statistically significant (P ⩽ 0.05 in most cases). Sex was retained in all models, rectal temperature was retained in the model of the HP(T−) and HP(T+) tests, while the remaining predictors (P > 0.05) were omitted. To account for herd-level clustering, herd was included in all models as a random-intercept effect. The overall model fit was good (generalized Chi-square statistic divided by its degrees of freedom 0.97–1.03). Based on point estimates of the regression coefficients from the models, test sensitivity (Se) and specificity (Sp) were calculated separately for each model as described by Dohoo et al. (2003).
In the second step, simulation by the @RISK add-in for Microsoft Excel (Palisade Corp.) was used to produce 95% confidence intervals for Se and Sp estimates, assuming a Normal uncertainty distribution. The Se and Sp estimates were depicted graphically against S-Hp cut-off values, and Receiver Operating Characteristics (ROC) curves were constructed (Hanley and McNeil, 1982). The areas under ROC curves (AUC) were calculated non-parametrically by summing up trapezoid segments, as a measure of the discriminative ability of each test. AUC together with Se and Sp estimates were used to choose the test with the best performance. For each test the optimal S-Hp cut-off value was determined, given equal priorities to type I and type II errors, by selecting the point on the ROC curve closest to the upper left corner of the diagram, i.e., minimizing the distance d = ((1 − Se)2 + (1 − Sp)2)0.5. Predictive values for positive and negative tests were calculated from the Se and Sp estimates for a CRD prevalence between 10% and 50%.
The usefulness of each test for estimating the herd prevalence of CRD was assessed by modelling the risk of CRDtest in a calf as a function of the proportion of test-positive animals in the herds, assuming a binomial distribution and using the LOGISTIC procedure of the SAS software package. A separate logistic regression model was constructed for each diagnostic test and its combinations of sex and rectal temperature, all in all ten models. The change in the odds of CRDtest per percentage unit of test-positive calves in the herds and the Pearson’s coefficient of correlation r (on the logit scale) were used to quantify the association at the herd level.
3. Results
Weekly incidence rates of CRD, diarrhoea and other infectious disease (all recorded cases), and weekly proportions of S-Hp samples taken from 1 to 8 weeks of age are shown in Fig. 1 . Among all 833 calves, S-Hp ranged from 0.05 to 2.25 (median: 0.07) g/L. Calves with CRD within 14 days prior to sampling (n = 153) had a median of 0.09 (80% central range: 0.04–0.69) g/L, calves with diarrhoea within 10 days prior to sampling (n = 57) a median of 0.07 (80% central range: 0.04–0.63) g/L, calves with other infectious disease within 10 days prior to sampling (n = 12) a median of 0.09 (80% central range, 0.05–0.48) g/L, and calves without CRD within 14 days before sampling or diarrhoea or other infectious disease within 10 days before sampling (n = 630) a median of 0.06 (80% central range: 0.04–0.23).
Fig. 1.
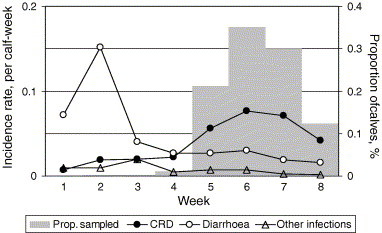
Weekly incidence rates of clinical respiratory-tract disease (CRD), diarrhoea and other infectious disease, and proportion of blood samples for analysis of serum concentration of haptoglobin taken from weeks 1 to 8 of age (833 dairy calves in nine Swedish herds 2002–2003; one sample per calf).
One hundred and fifty-three calves (18.4%) had CRD within 14 days before sampling, 57 (6.84%) had diarrhoea within 10 days before sampling, and 12 (1.44%) other infectious disease within 10 days before sampling. The herd-level incidence of CRD within 14 days before sampling ranged from 2.9% to 31% (median 17%), diarrhoea within 10 days before sampling from 0.97% to 12% (median: 6.7%) and other infectious disease within 10 days before sampling from 0% to 3.2% (median: 0.97%).
Heifer calves (n = 391) had a median S-Hp of 0.07 (80% central range: 0.04–0.39) g/L and 16% of them had CRDtest. In bull calves (n = 442), median S-Hp was 0.06 (80% central range: 0.04–0.26) g/L and 20% had CRDtest. Calves with a normal rectal temperature (⩽39.5 °C; n = 664) had a median S-Hp of 0.06 (80% central range: 0.04–0.13) g/L and 13% had CRDtest. In calves with fever (>39.5 °C; n = 165), median S-Hp was 0.12 (80% central range: 0.04–0.88) g/L and 42% had CRDtest.
Fig. 2 a–d shows the variation of Se and Sp with S-Hp cut-off value for four of the diagnostic tests. With the tests based on S-Hp alone, sensitivities were generally considerably higher and specificities lower in calves with fever (HP(T+); Fig. 2b) than in those with normal rectal temperature (HP(T−); Fig. 2a). Also, sensitivities were generally somewhat higher and specificities generally lower in bull calves than in heifers (not shown). Test optima were obtained at S-Hp cut-off values between 0.06 and 0.15 g/L for the HP test (Se: 0.54–0.69; Sp: 0.59–0.71; Fig. 2a and b), between 0.08 and 0.15 g/L for the HP/TEMP test (Se: 0.65–0.72; Sp: 0.69–0.73; Fig. 2c) and at 0.05 g/L for the HP+TEMP test (Se: 0.42–0.57; Sp: 0.84–0.91; Fig. 2d).
Fig. 2.
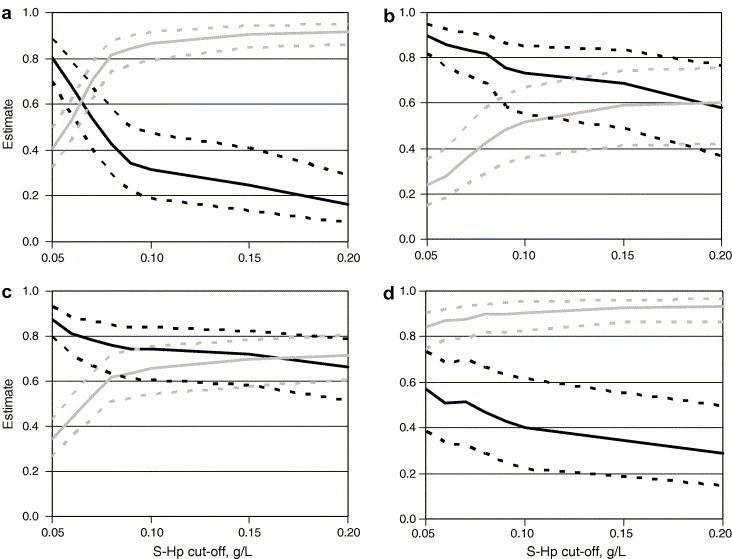
Sensitivity (black) and specificity (grey) estimates when testing for clinical respiratory-tract disease in heifer calves at 3–8 weeks of age using four different diagnostic tests: (a) an elevated serum concentration of haptoglobin (S-Hp) (cut-off from 0.05 to 20 g/L) at rectal temperature ⩽39.5 °C; (b) an elevated S-Hp at rectal temperature >39.5 °C; (c) either an elevated S-Hp or an elevated rectal temperature (>39.5 °C); (d) both an elevated S-Hp level and an elevated rectal temperature; point estimates (whole line) and 95% confidence limits (dotted lines) (391 calves in nine Swedish dairy herds 2002–2003).
For the TEMP test, Se was 0.49–0.60 and Sp 0.78–0.84. Fig. 3 shows an example of a ROC curve, based on the HP/TEMP test, which performed best with sensitivities >0.48 and specificities >0.35 throughout the studied S-Hp cut-off range. Table 1 summarises the discriminative ability of the different tests, showing the AUC and minimum distances from the ROC curve to the upper left corner of the diagram for different sexes and rectal temperatures. At the observed overall CRDtest prevalence of 18%, the predictive value (at the optimum cut-off) was 0.35 for a positive HP/TEMP test and 0.90 (bulls) to 0.92 (heifers) for a negative test. Assuming the CRDtest prevalence to change from 10% to 50%, the predictive value (at the optimum cut-off) increased by 49–50% units for a positive and dropped by 25–27% units for a negative test.
Fig. 3.
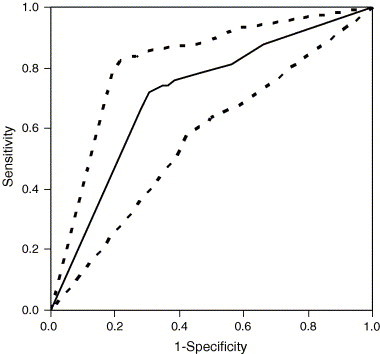
Example of Receiver Operating Characteristics curve for testing for clinical respiratory-tract disease at 3–8 weeks of age, using a diagnostic test based on either an elevated serum concentration of haptoglobin (cut-off from 0.05 to 0.20 g/L) or an elevated rectal temperature (>39.5 °C); point estimates (whole line) and 95% confidence limits (dotted lines) (391 heifer calves in nine Swedish dairy herds 2002–2003).
Table 1.
Areas under Receiver Operating Characteristics (ROC) curves (AUC), their 95% confidence limits (CL), and minimum distance (d) from the curve to the upper left corner of the ROC diagram for different diagnostic tests, sexes and rectal temperatures when testing for clinical respiratory-tract disease at 3–8 weeks of age (833 dairy calves in nine Swedish herds 2002–2003)
| Test | Sex | AUC | 95% CL | Min. d |
|---|---|---|---|---|
| HP(T−)a | Bull | 0.65 | 0.54–0.75 | 0.56 |
| Heifer | 0.66 | 0.50–0.79 | 0.55 | |
| HP(T+)b | Bull | 0.64 | 0.46–0.79 | 0.52 |
| Heifer | 0.63 | 0.35–0.80 | 0.51 | |
| HP/TEMPc | Bull | 0.71 | 0.60–0.80 | 0.44 |
| Heifer | 0.71 | 0.57–0.82 | 0.42 | |
| HP+TEMPd | Bull | 0.66 | 0.57–0.76 | 0.59 |
| Heifer | 0.71 | 0.56–0.83 | 0.46 | |
| TEMPe | Bull | – | – | 0.53 |
| Heifer | – | – | 0.46 |
Based on elevated serum concentration of haptoglobin (S-Hp; cut-off values from 0.05 to 0.20 g/L) alone in calves with normal rectal temperature (⩽39.5 °C).
Based on elevated S-Hp alone in calves with elevated rectal temperature (>39.5 °C).
Based on either elevated S-Hp or elevated rectal temperature.
Based on both elevated S-Hp and elevated rectal temperature.
Based on elevated rectal temperature alone.
In models of herd prevalence, the association between the proportion of CRDtest-positives and the proportion of test-positives was substantially stronger in heifers than in bull calves. The herd-level r was 0.40 and 0.68 for the HP test, 0.41 and 0.81 for the HP/TEMP test, 0.44 and 0.95 for the HP+TEMP test, and 0.33 and 0.77 for the TEMP test, for bulls and heifers respectively. Fig. 4 shows the relationship between the proportion of calves with CRDtest (on the logit scale) and the proportion of HP/TEMP test positive bull and heifer calves, respectively (cut-off at 0.08 g/L in bulls and 0.15 g/L in heifers). In this case, the model predicted a 1.3-fold increase in the odds of a bull calf having CRDtest and a 1.9-fold increase of a heifer for every increase by 10% units of test-positive calves in the herd.
Fig. 4.
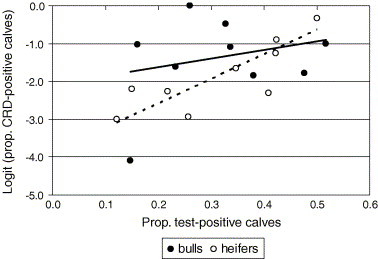
Relationship between proportions of calves with clinical respiratory-tract disease (CRD; on the logit scale) and test-positive bull and heifer calves, respectively, in the study herds using a diagnostic test based on either an elevated serum concentration of haptoglobin (cut-off at 0.08 g/L in bulls and 0.15 g/L in heifers) or an elevated rectal temperature (>39.5 °C) (442 bull and 391 heifer calves in nine Swedish dairy herds 2002–2003; one data point for bulls omitted due to zero prevalence).
4. Discussion
In the present study, most clinically healthy calves had S-Hp below 0.10 g/L, considered by several researchers to be the reference limit for S-Hp (Saini and Webert, 1991, Eckersall et al., 1988). Calves with diarrhoea had numerically higher S-Hp than healthy calves, in accordance with findings by Okamoto et al., 1998, Deignan et al., 2000, but the levels of S-Hp were even higher in calves with CRD. S-Hp varied considerably between calves, both in healthy and diseased individuals.
The discriminative ability of S-Hp for indicating CRD was overall poor, not better than that of rectal temperature, and significantly affected by sex and rectal temperature. When the two parameters were combined into the HP/TEMP test the best test performance was achieved in heifer calves at a S-Hp cut-off of 0.15 g/L, with Se 0.72 and Sp 0.59.
In the present study, a large proportion of the cases of CRD were diagnosed by the project veterinarian at the day of blood sampling. Although the time of diagnosis may not truly reflect the time of onset of the disease, it is likely nevertheless that most of the diseased animals had a short history of disease. Most of the cases of CRDtest therefore may have been of a quite acute type and probably to a large extent caused by viral infections. The majority of these cases went unrecognized by the calf carers, and were mild. It has generally been considered that viral infections give a weaker acute phase reaction than bacterial infections (Spooner and Miller, 1971, Godson et al., 1996, van Leeuwen and van Rijswiijk, 1994), although corresponding levels as induced by bacterial infections have been observed in for example BRS infections (Heegard et al., 2000).
Although S-Hp has been seen to increase rapidly in experimentally infected cattle, the increase in S-Hp was found to coincide with the onset of clinical signs (Höfner et al., 1994, Heegard et al., 2000, Gånheim et al., 2003), indicating that APP cannot be used to predict disease during the incubation period. Similarly, Alsemgeest et al. (1994) found that S-Hp did not increase in very acute inflammatory conditions such as peracute pneumonia. In fact, they found the largest concentrations in animals with serious, often chronic inflammatory diseases. However, Eckersall et al., 2001, Horadagoda et al., 1999 reported haptoglobin to be more affected by acute than chronic conditions. The design of the study and the detection of mild cases (unnoticed by the calf caretakers) may have contributed to the generally low sensitivities of the diagnostic tests evaluated in our study. The age distribution of disease and of S-Hp sampling, as well as the occurrence of other (infectious) diseases with an effect on S-Hp levels may alter the behaviour of the test.
Eckersall et al. (2001) found S-Hp (cut-off: 0.05 mg/mL) to have a Se of 0.82 and Sp of 0.94, in differentiating between healthy cows and those with mastitis detected by dairymen. However, compared to CRD, mastitis is relatively easy for dairymen to detect and blood samples for S-Hp analysis were collected within 2–4 h of the mastitis being detected. In their analysis Eckersall et al. (2001) did not account for other factors affecting the discriminative ability of the test.
Because of the difficulties for farmers to detect CRD and hence to get an overview of its occurrence in their herd a diagnostic tool would be very useful for on-farm monitoring of the disease. It could for instance be used as a key figure in herd health services, animal welfare programmes, or to follow up prophylactic measures to reduce CRD problems. The usefulness of individual S-Hp sampling for herd-level diagnosis will depend on the prevalence of disease and the number of calves sampled, as well as on the age of the calves at sampling in relation to the onset of the CRD. In the present study the age at sampling coincided well with the age when the calves developed the disease.
Alsemgeest (1994) reported that S-Hp in combination with other APP seemed to be a promising indicator of disease on a herd-level basis. Gånheim (2004) investigated the potential of APP (Hp, fibrinogen and serum amyloid A) as an objective tool to evaluate animal health and management in two groups of calves with different clinical health status and reported the largest difference in number of days per calf with supra-normal concentrations to occur for S-Hp, suggesting this to be the most useful APP to measure. Our results further support that S-Hp, when combined with rectal temperature may be a valuable parameter in herd-level diagnostics at least in heifers. Our data comprised only nine herds and further field studies are needed to confirm the usefulness of the test.
5. Conclusion
We conclude that S-Hp alone is not useful in diagnosing CRD in individual dairy calves 24–56 days of age. However, if combined with rectal temperature measurements, a reasonable discriminative ability in heifer calves may be obtained when the test is constructed as either S-Hp >0.15 g/L or rectal temperature > 39.5 C, and in bull calves as either S-Hp >0.08 g/L or temperature >39.5 °C. Furthermore, there is some support for such a test or one using S-Hp plus rectal temperature to be valuable in herd-level diagnostics in heifer calves.
Acknowledgements
The study was supported financially by the Swedish Farmers’ Foundation for Agricultural Research and by the Intervet research fund. The authors thank the participating farmers for their interest and support. We are also very grateful to Gunilla Jacobsson (of the Swedish University of Agricultural Sciences) for practical data processing.
References
- Agerholm J.S., Basse A., Krogh H.V., Christensen K., Rønsholt L. Abortion and calf mortality in Danish cattle herds. Acta Veterinaria Scandinavica. 1993;34:371–377. doi: 10.1186/BF03548180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsemgeest, S.P.M., 1994. Blood concentrations of acute-phase proteins in cattle as markers for disease. Doctoral thesis, Utrecht University.
- Alsemgeest S.P., Kalsbeek H.C., Wensing T., Koeman T., van Ederen A.M., Gruys E. Concentrations of serum amyloid-A (SAA) and haptoglobin (HP) as parameters of inflammatory disease in cattle. The Veterinary Quarterly. 1994;16:21–23. doi: 10.1080/01652176.1994.9694410. [DOI] [PubMed] [Google Scholar]
- Berry B.A., Confer A.W., Krehbiel C.R., Gill D.R., Smith R.A., Montelongo M. Effects of dietary energy and starch concentrations for newly received feedlot calves. II. Acute-phase protein response. Journal of Animal Science. 2004;82:845–850. doi: 10.2527/2004.823845x. [DOI] [PubMed] [Google Scholar]
- Carter J.N., Meredith G.L., Montelongo M., Donald R.G., Krehbiel C.R., Payton M.E., Confer A.W. The relationship of vitamin E supplementation and antimicrobial treatment with acute phase protein response in cattle affected by naturally acquired respiratory tract disease. American Journal of Veterinary Research. 2002;63:1111–1117. doi: 10.2460/ajvr.2002.63.1111. [DOI] [PubMed] [Google Scholar]
- Conner J.G., Eckersall P.D., Wiseman A., Bain R.K., Douglas T.A. Acute phase response in calves following infection with Pasteurella haemolytica, Ostertagia ostertagi and endotoxin administration. Research in Veterinary Science. 1989;47:203–207. [PubMed] [Google Scholar]
- Deignan T., Alwan A., Kelly J., McNair J., Warren T., O’Farelly C. Serum haptoglobin: an objective indicator of experimentally-induced Salmonella infection in calves. Research in Veterinary Science. 2000;69:153–158. doi: 10.1053/rvsc.2000.0403. [DOI] [PubMed] [Google Scholar]
- Dohoo I., Martin W., Stryhn H. AVC Inc.; Charlottetown, Prince Edward Island, Canada: 2003. Veterinary Epidemiologic Research. [Google Scholar]
- Eckersall P.D., Parton H., Conner J.G. Acute phase reactants in diseases of dog and cattle. In: Blackstone D.J., editor. Animal clinical biochemistry – the future. Cambridge University Press; Cambridge: 1988. pp. 225–228. [Google Scholar]
- Eckersall P.D., Young F.J., McComb C., Hogarth C.J., Safi S., Weber A., McDonald T., Nolan A.M., Fitzpatrick J.L. Acute phase proteins in serum and milk from dairy cows with clinical mastitis. The Veterinary Record. 2001;148:35–41. doi: 10.1136/vr.148.2.35. [DOI] [PubMed] [Google Scholar]
- Gardner I.A., Hird D.W., Utterback W.W., Danaye-Elmi C., Heron B.R., Christansen K.H., Sischo W.M. Mortality, morbidity, case-fatality, and culling rates for California dairy cattle as evaluated by the National Animal Health Monitoring System, 1986–1987. Preventive Veterinary Medicine. 1990;8:157–170. [Google Scholar]
- Godson D.L., Campos M., Attah-Poku S.K., Redmond M.J., Cordeiro D.M., Sethi M.S., Harland R.J., Babiuk L.A. Serum haptoglobin as an indicator of the acute phase response in bovine respiratory disease. Veterinary Immunology and Immunopathology. 1996;51:277–292. doi: 10.1016/0165-2427(95)05520-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray M.L., Young C., Stanker L.H., Bounous D.I. Measurement of serum haptoglobin in neonatal farm-raised and bob veal calves using two immunoassay methods. Veterinary Clinical Pathology. 1996;25:38–42. doi: 10.1111/j.1939-165x.1996.tb00965.x. [DOI] [PubMed] [Google Scholar]
- Gånheim, C. 2004. Studies on the acute phase reaction during respiratory infections in calves. Doctoral thesis. Swedish University of Agricultural Sciences, Uppsala.
- Gånheim C., Hultén C., Carlsson U., Kindahl H., Niskanen R., Persson Waller K. The acute phase response in calves experimentally infected with bovine viral diarrhoea virus and or Mannheimia haemolytica. Journal of Veterinary Medicine, B. 2003;50:183–190. doi: 10.1046/j.1439-0450.2003.00658.x. [DOI] [PubMed] [Google Scholar]
- Hanley J.A., McNeil B.J. The meaning and use of the area under the Receiver Operating Characteristic (ROC) curve. Radiology. 1982;143:29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- Heegard P.M.H., Godson D.L., Toussaint M.J.M., Tjørnehøj K., Larsen L.E., Viuff B., Rønsholt L. The acute phase response of haptoglobin and serum amyloid A (SAA) in cattle undergoing experimental infection with bovine respiratory syncytial virus. Veterinary Immunology and Immunopathology. 2000;77:151–159. doi: 10.1016/S0165-2427(00)00226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirvonen J., Pyärölä S., Jousimies–Somer H. Acute phase response in heifers with experimentally induced mastitis. Journal of Dairy Research. 1996;64:351–360. doi: 10.1017/s0022029900031873. [DOI] [PubMed] [Google Scholar]
- Höfner M.C., Fosbery M.W., Eckersall P.D., Donaldson A.I. Haptoglobin response of cattle infected with foot-and-mouth disease virus. Research in Veterinary Science. 1994;56:125–128. doi: 10.1016/0034-5288(94)90093-0. [DOI] [PubMed] [Google Scholar]
- Horadagoda N.U., Knox K.M.G., Gibbs H.A., Reid S.W.J., Horagoda A., Edwards S.E.R., Eckersall P.D. Acute phase proteins in cattle: discrimination between acute and chronic inflammation. The Veterinary Record. 1999;144:437–441. doi: 10.1136/vr.144.16.437. [DOI] [PubMed] [Google Scholar]
- van Leeuwen M.A., van Rijswiijk M.H. Acute phase proteins in the monitoring of inflammatory disorders. Baillière’s Clinical Rheumatology. 1994;8:531–552. doi: 10.1016/s0950-3579(05)80114-1. [DOI] [PubMed] [Google Scholar]
- Okamoto K., Harada M., Akuzawa M., Deguchi E. Peripheral blood lymphocyte blastogenesis and concentrations of serum haptoglobin and α1-acid glycoprotein in normal calves and calves on the first onset of diarrhea. Animal Science and Technology (Japan) 1998;69:683–689. [Google Scholar]
- Olsson S.-O., Viring S., Emanuelson U., Jacobsson S.-O. Calf diseases and mortality in Swedish dairy herds. The Veterinary Record. 1993;34:263–269. doi: 10.1186/BF03548190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortman K., Svensson C. Use of antimicrobial drugs in Swedish dairy calves and replacement heifers. The Veterinary Record. 2004;154:136–140. doi: 10.1136/vr.154.5.136. [DOI] [PubMed] [Google Scholar]
- Pettersson K., Svensson C., Liberg P. Housing, feeding and management of calves and replacement heifers in Swedish dairy herds. The Veterinary Record. 2001;42:465–478. doi: 10.1186/1751-0147-42-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radostits O.M., Gay C.C., Bloos D.C., Hinchcliff K.W. W.B. Saunders Company Ltd.; London: 2000. Veterinary Medicine. [Google Scholar]
- Saini P.K., Riaz M., Webert D.W., Eckersall P.D., Young C.R., Stanker L.H., Chakrabarti E., Judkins J.C. Development of a simple enzyme immunoassay for blood haptoglobin concentration in cattle and its application in improving food safety. American Journal of Veterinary Research. 1998;59:1101–1107. [PubMed] [Google Scholar]
- Saini P.K., Webert D.W. Application of acute phase reactants during antemortem and postmortem meat inspection. Journal of American Veterinary Medical Association. 1991;198:1898–1901. [PubMed] [Google Scholar]
- Sivula N.J., Ames T.R., Marsh W.E., Werdin R.E. Descriptive epidemiology of morbidity and mortality in Minnesota dairy heifer calves. Preventive Veterinary Medicine. 1996;27:155–171. [Google Scholar]
- Skinner J.G., Brown R.A., Roberts L. Bovine haptoglobin response in clinically defined field conditions. The Veterinary Record. 1991;128:147–149. doi: 10.1136/vr.128.7.147. [DOI] [PubMed] [Google Scholar]
- Spooner R.L., Miller J.K. The measurement of haemoglobin reactive proteins in ruminants as an aid to diagnosis of acute inflammation. The Veterinary Record. 1971;88:2–4. doi: 10.1136/vr.88.1.2. [DOI] [PubMed] [Google Scholar]
- Svensson C., Liberg P. The effect of group size on health and growth rate of Swedish dairy calves housed in pens with automatic milk-feeders. Preventive Veterinary Medicine. 2006;73:43–53. doi: 10.1016/j.prevetmed.2005.08.021. [DOI] [PubMed] [Google Scholar]
- Svensson C., Lundborg K., Emanuelson U., Olsson S.-O. Morbidity in Swedish dairy calves from birth to 90 days of age and individual calf-level risk factors for infectious diseases. Preventive Veterinary Medicine. 2003;58:179–197. doi: 10.1016/s0167-5877(03)00046-1. [DOI] [PubMed] [Google Scholar]
- Svensson C., Hultgren J., Oltenacu P.A. Morbidity in Swedish dairy calves from 3 to 7 months of age and risk factors for diarrhoea and respiratory disease. Preventive Veterinary Medicine. 2006;74:162–179. doi: 10.1016/j.prevetmed.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Svensson, C., Linder, A., Olsson, 2006. Mortality in Swedish dairy calves and replacement heifers. Journal of Dairy Science, in press. [DOI] [PubMed]
- Virtala A.-M., Mechor G.D., Gröhn Y.T., Erb H.E. Morbidity from nonrespiratory diseases and mortality in dairy heifers during the first three months of life. Journal of American Veterinary Medical Association. 1996;208:2043–2046. [PubMed] [Google Scholar]
- Wells S.J., Garber L.P., Hill G.W. Health status of preweaned dairy heifers in the United States. Preventive Veterinary Medicine. 1996;29:185–199. doi: 10.1016/s0167-5877(96)01078-1. [DOI] [PubMed] [Google Scholar]
- Wittum T.E., Young C.R., Stanker L.H., Griffin D.D., Perino L.J., Littledike E.T. Haptoglobin response to clinical respiratory tract disease in feedlot cattle. American Journal of Veterinary Research. 1996;57:646–649. [PubMed] [Google Scholar]


