Abstract
Bronchiectasis is an increasingly recognised respiratory condition with limited therapeutic options and a complex spectrum of clinical manifestations that invariably includes chronic cough. As the primary presentation of bronchiectasis in most cases, chronic cough and its mechanistic underpinnings are of central importance but remain poorly understood in this setting. Bronchiectasis is also increasingly identified as an underlying cause of chronic cough highlighting the interrelationship between the two conditions that share overlapping clinical features. Several therapeutic approaches have illustrated positive effects on bronchiectasis-associated cough, however, more focused treatment of heterogeneous cough subtypes may yield better outcomes for patients. A current challenge is the identification of bronchiectasis and cough endophenotypes that may allow improved patient stratification and more targeted therapeutic matching of the right treatment to the right patient. Here we discuss the complex disease phenotypes of bronchiectasis and their interrelationship with cough while considering current and emerging treatment options. We discuss some key cough promoters in bronchiectasis including infection, allergy and immune dysfunction.
1. The bronchiectasis ‘challenge’
Bronchiectasis is an increasingly recognised respiratory syndrome defined by permanent and irreversible dilatation of the bronchi [1]. Cole's vicious cycle remains the central model of disease pathogenesis whereby a self-perpetuating cycle of infection and inflammation precipitates damage to the bronchial wall leading to impaired mucociliary clearance and predisposition to recurrent infection. The primary clinical presentation of bronchiectasis includes chronic cough and recurrent respiratory infections responsible for the increased inflammation, airway damage and shortness of breath with eventual lung function decline, respiratory failure and death [1]. An increased awareness of disease heterogeneity, including its presence in overlap syndromes has brought renewed focus upon potential underlying molecular endophenotypes that may better define specific disease traits and subtypes amenable to treatment [[2], [3], [4], [5]]. Endotypes represent disease presentations characterised by distinct functional or pathobiological mechanisms. Critically, a clinical phenotype can demonstrate multiple endotypes while a single endotype may be present in more than one clinical phenotype. This inherent disease heterogeneity and its geographic variability are recognised as a major barriers to success in clinical trials and questions remain over how to better stratify patients for targeted therapy to improve clinical outcomes [1,6]. A recent example is the failure to reproduce findings between two replicate clinical trials that recruited from geographically different regions. RESPIRE1: recruited patients from Europe, North and South America, Australia and Japan while RESPIRE2 focused on Asian and Eastern Europeans [[6], [7], [8]]. In addition, only modest benefit from directed pathogen-drug treatment is observed, which appears in contrast to that expected from Cole's vicious cycle hypothesis. This likely reflects the complexity of this disease of which infection is only one of many other pulmonary, extra-pulmonary, aetiological and environmental factors influencing disease [3,9,10]. Newer emerging models such as the ‘vicious vortex’ proposed by Flume and colleagues perhaps offers a more complete picture of disease pathogenesis [9]. As such, improved patient stratification and identifying disease endophenotypes that respond optimally to therapy has become a key focus of current bronchiectasis research [3]. While work has been published on the pathophysiology of cough phenotypes, the driving factors in bronchiectasis remain to be well defined [[11], [12], [13], [14]]. Although cough is important for lung homeostasis, irritants and microbes causing inflammation can aberrantly prime neuro-immune pathways leading to excessive cough and tissue damage, a process requiring detailed study in bronchiectasis. The identification of specific cough phenotypes in bronchiectasis would provide scope for potential cough-directed interventions and further our understanding of this complex disease without a currently licensed therapy and where up to half of all cases remain idiopathic.
2. Cough in bronchiectasis
While a small but significant proportion of chronic cough (2–4%) is attributable to bronchiectasis, almost all patients with bronchiectasis (>90%) present with persistent cough [[15], [16], [17]]. Bronchiectasis is therefore an important contributor to the diagnostic spectrum of chronic cough: a comparatively heterogeneous pathology associated with over 100 disorders [18]. In parallel, chronic productive cough is an important clinical manifestation of bronchiectasis and the first recognised symptom in many cases antecedent to a confirmatory diagnosis [1,16,19]. Given its central importance in the diagnosis and pathology of bronchiectasis, a clearer understanding of its mechanistic underpinnings is desirable. The importance of cough in bronchiectasis is illustrated by implementation of the Leicester Cough Questionnaire (LCQ); a measure of cough symptoms that has been validated in bronchiectasis and correlates with disease severity reflecting the underlying association between cough and disease progression [20]. More recent evidence corroborates this finding, highlighting objectively monitored cough frequency as an important predictor of sputum production and exacerbations (though not lung function) in bronchiectasis [21]. Indeed, such is the importance of cough, specifically cough hypersensitivity, that it has been advanced as a ‘treatable trait’ of bronchiectasis and proposed as a potential target of individualised therapies to alleviate cough in particularly symptomatic individuals [3,10]. Therapies such as use of antitussives, inhaled corticosteroids (ICS) or chest physiotherapy may therefore alleviate symptoms in individuals with problematic cough if appropriately targeted [10,22]. While bronchiectasis mandates multifaceted management with consideration of heterogeneous clinical features, co-morbidities, microbiology, inflammation and therapeutic responses, cough remains an important common phenotypic trait but also a key symptom in the definition of a bronchiectasis exacerbation – a major endpoint applied in almost all clinical trials and an important prognostic indicator of disease progression [23,24].
3. The inter-relationship between cough and bronchiectasis
Both chronic cough and bronchiectasis exhibit comparable aetiological profiles and illustrate high prevalence rates of idiopathic aetiology (Table 1 ) [1,13]. This further underscores the shortcomings in our understanding of both conditions and the need for greater diagnostic resolution [3,18]. In a large study of chronic cough among the general adult population, bronchiectasis was identified as the highest ranked risk factor at the individual level (according to age-adjusted odds ratio), followed by asthma, occupational exposure to dust or fumes, airflow limitation and gastro-oesophageal reflux disease (GORD) [15]. These latter conditions also co-exist in bronchiectasis where asthma, GORD and airflow limitation (i.e. COPD) are themselves recognised as important co-morbidities highlighting the potentially overlapping underlying pathology between cough and bronchiectasis (Table 1) [25]. While associated risks of occupational exposure to dust or fumes remains to be clearly demonstrated in bronchiectasis, a case cross-over study has linked exacerbations in bronchiectasis to fluctuations in outdoor air pollution adding to the body of literature on air quality and respiratory illness further serving to highlight aetiological overlap between these conditions [26]. The central importance of microbes and microbial infection in both conditions is another common feature reflected in epidemiological work. The most commonly cited cause of chronic cough is respiratory infection while up to 50% of bronchiectasis is classified as ‘post-infective’ (Table 1) [27,28]. These observations reflect the fundamental role of cough in the clearance of potentially harmful microbes and maintaining lung homeostasis while highlighting its clearly aberrant status in bronchiectasis. Though infectious causes of cough generally remain unresolved, bacterial pathogens including Bordetella pertussis, Bordetella parapertussis, Mycoplasma pneumonia and Chlamydophila (Chlamydia) pneumoniae are most frequently identified [29]. Fungi are also implicated, triggering cough hypersensitivity via activation of allergic responses, while viral causes of neural dysfunction following respiratory infection have been linked to aberrant cough sensitisation that persists beyond initial infection [30,31]. The primary bacterial species identified in bronchiectasis include Haemophilus influenzae and Pseudomonas aeruginosa and their detection has a major bearing on antimicrobial treatment choices [32]. The importance of microbes in bronchiectasis is further substantiated through culture-independent analysis of the lung microbiome illustrating the influence of macrolide therapy on microbiome composition and the association of microbial consortia with important clinical outcomes including exacerbation [33,34]. Work from our group and others has extensively characterised the fungal component of the bronchiectasis microbiome (the Mycobiome) and highlights a high frequency of fungal sensitisation associated with worsening disease severity, lung function and exacerbations [35]. In chronic cough, microbiome analysis has been applied to paediatric patients which has illustrated an increased abundance of commensal taxa in the lower airway and the potential association of specific microbiome signatures with particular patients including asthma, bacterial bronchitis or neurologically impaired orally fed subjects [36]. These observations highlight the potential for characterising cough endotypes based on microbiome analysis, however, the specific influence of the microbiome in bronchiectasis-associated cough remains to be defined. Asthma is an important bronchiectasis comorbidity complicating its diagnosis and treatment particularly in cases of asthma-bronchiectasis overlap syndrome (ABOS). Asthma itself however exhibits strong associations with chronic cough most notably in cough-variant asthma where cough is the predominant symptom [13,25]. While not a strong predictor of bronchiectasis, cigarette smoking is a significant risk factor for chronic cough. Smoking does predispose however to chronic obstructive pulmonary disease (COPD) and bronchiectasis-COPD overlap syndrome (BCOS) [37]. Smoking may therefore importantly underlie some subtypes of cough (COPD-associated cough) which on occasion relate to bronchiectasis (e.g. BCOS) [18,37]. Some aetiologies are distinct to bronchiectasis and include immune dysfunction; primary ciliary dyskinesia, allergic bronchopulmonary aspergillosis and cystic fibrosis while bronchiectasis itself represents an important cause of chronic cough (Table 1). Rhinosinusitis is of relevance to both disease states; reported in up to 93% of chronic cough patients and in 84% of patients with idiopathic bronchiectasis [38,39]. Nasal sections associated with rhinosinusitis triggers excessive coughing perpetuating a chronic disease state of relevance to bronchiectasis where co-existing rhinosinusitis associates with increased exacerbations and high rates of allergy [40,41]. Given these observations, the association of particular cough phenotypes such as those driven by rhinosinusitis could represent one of several distinct cough subtypes that occur in bronchiectasis. Sinobronchial disease, associated with both cough and bronchiectasis, and of high prevalence in Japan, is an example reflecting geographic or environmental influences on underlying aetiology (Table 1). While known aetiologies of bronchiectasis intersect with those of chronic cough, their influence on cough in bronchiectasis remains unclear given the lack of dedicated study directly assessing such associations (Fig. 1 ).
Table 1.
Major aetiologies of chronic cough and bronchiectasis.
| Chronic cough | Bronchiectasis |
|---|---|
| Idiopathic [12–42%] [89] | Idiopathic [7–74%] [28] |
| Acute/Chronic infection [13–27%] [27] | Post‐infective [10–50%] [28] |
| Cough variant asthma [10–59%] [13] | Asthma [1%] [90] |
| Cigarette smoking [22–48%] [15,91] | Immune dysfunction [5%] [90] |
| Occupational exposure to dust/fumes [7–15%] [15] | Primary Cilliary dyskinesia [3%] [90] |
| Allergic bronchopulmonary aspergillosis [3%] [90] | |
| GORD [5–73%] [13] | GORD [1%] [90] |
| COPD [19–26%] [91,92] | COPD [4%] [90] |
| Bronchiectasis [2–4%] [15,17] | Cystic fibrosis [1%] [90] |
| Rhinosinusitis [6–93%] [13] | Rhinosinusitis [27–70%] [93,94] |
| Sinobronchial disease [17% - Japan] [95] | Sinobronchial disease [25% - Japan] [96] |
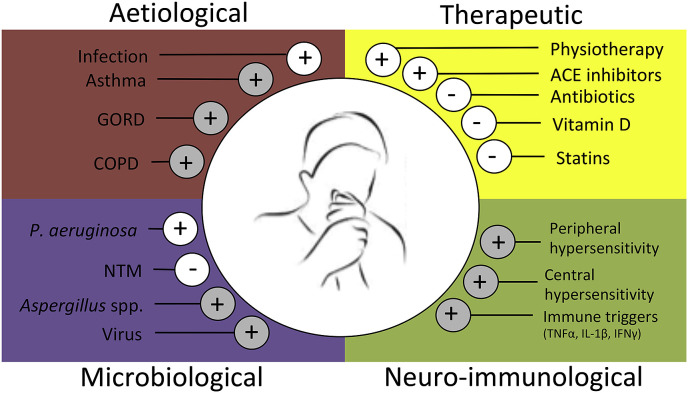
Fig. 1.
Factors influencing cough in bronchiectasis. Aetiological, therapeutic microbiological and neuro-immunological influencers of cough in bronchiectasis are indicated. Plus and minus symbols illustrate the nature of the effect; (+) = an increase in cough symptoms, (−) = a decrease in cough symptoms. White circles indicate influences supported by studies where cough was specifically measured. Grey circles indicate presumed influences based on other associated disease severity measures or observations confirmed in chronic cough that may be applicable in bronchiectasis but require dedicated investigation.
4. Treating cough in bronchiectasis: non-pharmacological approaches
The most widely accepted treatment for bronchiectasis-associated cough is physiotherapy emphasising pulmonary hygiene and airway clearance [16,42]. The rationale for this is enhancement of the mucociliary escalator with consequent removal of purulent airway secretions which subsequently reduces the burden of inciting microbial pathogens and inflammation [1]. Initial work by Murray et al. demonstrated improvements in Leicester Cough Questionnaire (LCQ) scores following chest physiotherapy using an oscillatory positive expiratory pressure device [43]. This generated important evidence as appraised in a Cochrane review on the subject concluding overall positive effects albeit with the recommendation that further confirmatory trials be conducted to support the evidence base [42,43]. Subsequent studies support the use of positive expiratory pressure in bronchiectasis with improvement of cough [44,45]. From a mechanistic perspective, the use of positive expiratory pressure devices improves mucus transport by coughing and contributes to the reduction in total number of inflammatory cells seen in respiratory secretions in bronchiectasis [46,47]. Recent work has shown the positive impact of high-intensity inspiratory muscle training on cough as seen in declining (better) LCQ scores post therapy [48]. This suggests improvement to respiratory muscle strength which enhances respiratory secretion transport properties and a resulting beneficial impact on cough.
5. Treating cough in bronchiectasis: pharmacological approaches
5.1. Antimicrobial therapy
Despite weak empirical evidence and the lack of success of several large clinical trials in bronchiectasis, antibiotics remain a therapeutic option for the treatment of bronchiectasis associated infection in selected patients [32,49]. Disappointing outcomes seen in clinical trials investigating inhaled antibiotic formulations raise concerns over trial design itself as well as our appreciation of disease complexity and heterogeneity. Concern surrounds the use of exacerbations as a clinical trial endpoint that overlooks other important disease measures including cough frequency and severity [50]. To date, macrolides have shown greatest promise in the treatment of bronchiectasis demonstrating a reduction in exacerbations compared to placebo, however, with selection of increased antibiotic resistance [51,52]. While no effect of macrolides on cough was observed in these studies, macrolides have shown some efficacy in treating cough among patients without bronchiectasis suggesting their potential suitability, if appropriately targeted to specific bronchiectasis-cough subtypes [53,54]. As such, cough in bronchiectasis may benefit from a more directed, focused and personalised therapeutic approach. However current guidelines, and most clinicians do not support targeted use of macrolides for cough given the absence of larger trials investigating their use for this specific purpose in bronchiectasis. Given the observed role of fungi in both bronchiectasis and chronic cough, antifungal therapy represents an additional therapeutic avenue for investigation. While data remains limited, Ogawa et al. have demonstrated a positive effect of low-dose itraconazole therapy [55]. Effects were most pronounced in specific patient subgroups – such as those colonised by basidiomycetous fungi – reinforcing the importance of microbiology and the targeted, personalised approach likely required. The potential application of antifungal therapeutics in bronchiectasis should however be targeted to specific disease subtypes, such as those driven by fungal allergy and, here, the identity of the inciting fungal species may be critically important [4,5,10,35,56].
5.2. Vitamin D supplementation
Vitamin D is a central modulator of both innate and adaptive immune responses [57]. Potential immunological functions of Vitamin D include direct down-regulation of pro-inflammatory cytokines IL-8, IL-6 and TNF-α, direct up-regulation of regulatory cytokines IL-10 and inhibition of MAPK and NF-κB innate and inflammatory pathways with major implication for respiratory disease [58]. Deficiency in Vitamin D leads to increased autoimmunity and susceptibility to infection and has been reported in 50% of bronchiectasis patients in the UK where it is associated with higher frequencies of exacerbation and infection, however, it remains unclear if Vitamin D deficiency in this setting is associated with or is a consequence of inactivity in bronchiectasis [59]. Early bronchiectasis studies have illustrated favourable outcomes with supplementation including improvements in LCQ suggesting a direct benefit to cough in this setting [60]. Vitamin D supplementation therefore represents an interesting area for future investigation in terms of its therapeutic potential in bronchiectasis. While less is known about the role of Vitamin D in chronic cough, lower serum levels are reported in children with cough and the associated protection from respiratory infection afforded by Vitamin D supplementation is supported [61,62]. Together, these data support further investigation in regard to Vitamin D supplementation and cough in bronchiectasis.
5.3. Statins
Statins are members of a pharmaceutical class with a primary indication of lowering serum cholesterol. Statins exert powerful immunological effects and have antimicrobial properties [63,64]. Given these pleiotropic effects, the potential therapeutic application of statins to other conditions including respiratory disease has been studied [63]. While mechanisms by which statins exert effects in bronchiectasis remains unclear, significant improvements are seen in LCQ in patients treated with atorvastatin versus placebo. These improvements occur in association with reductions in sputum neutrophils and inflammatory markers IL-8 and C-reactive protein [65]. Interestingly, improvements in cough were not observed in patients colonised with P. aeruginosa emphasising the potential impact of microbiology and microbiome composition on treatment outcome [66]. Currently however, use of statins is not recommended as a bronchiectasis treatment option given the small sample size of trials, potential high rates of adverse events in treatment groups and, inconsistencies in their reported findings [32]. Conversely, use of Angiotensin-converting enzyme (ACE)-inhibitors induce cough [67]. While the burden of ACE-inhibitor-induced cough remains to be determined, it has some contribution in bronchiectasis given the higher associated cardiovascular disease prevalence [68].
5.4. Antitussives
The evidence for antitussive use in bronchiectasis is lacking and potential benefits must be weighed carefully against the potential for sputum retention that could further promote infection. In contrast, antitussive agents have been extensively investigated in cough where their use represents a therapeutic mainstay for treatment of a heightened cough reflex [69]. Cough-suppressant drugs function by targeting either hypersensitisation of the central nervous system or the peripheral receptors that instigate the cough response; mainly the transient receptor potential (TRP) ion channels and nociceptive sensory neurons that orchestrate cough reflexes and the release of pro-inflammatory neuropeptides [70]. As such, cough therapies are divided into centrally acting agents such as opioids (targeting the neural sensitisation in the central nervous system) and receptor agonists (that target the peripheral receptors involved in stimulation of the cough response) [69,70]. The active area of cough-suppressant neuromodulator research may have relevance to bronchiectasis, as novel antitussive therapies to alleviate cough hypersensitivity may in fact prove efficacious in this setting although further work is required [71]. While central and peripheral neuro-immunological triggers of cough remain to be delineated in bronchiectasis, they are clearly perturbed given the hypersensitisation observed in bronchiectasis, and therefore represent potential targets for cough suppressant therapy (Fig. 1). Non-pharmacological cough suppression techniques for bronchiectasis should also be noted since volitional cough suppression, encouraged through physiotherapy, can achieve the same effect – reduction of cough symptoms. Whether this represents a viable therapeutic strategy in the context of bronchiectasis remains to be robustly validated but emerging evidence has shown promise for both pharmaceutical and non-pharmaceutical approaches for selected bronchiectasis patients [72].
6. Should cough itself be a clinically measured endpoint in bronchiectasis?
Cough in bronchiectasis exists as a prelude to formal diagnosis and as a correlate of worsening disease. Despite this, most clinical trials have thus far focused on exacerbation as a primary clinical endpoint while cough symptoms or cough frequency rarely measured may serve an alternative and powerful clinical indicators [50,73] Wearable technologies such as acoustic cough monitors can further enhance cough assessment by more robustly capturing symptoms and offering a more accurate objective and holistic alternative to self-reported cough scores with widespread application for clinical trials and longitudinal studies [21,74,75]. While exacerbations are a major focus of bronchiectasis research, cough is a burdensome symptom for patients with significant impact on quality of life [50]. Revisiting the assessment of cough as a clinical endpoint, perhaps with incorporation of enhanced monitoring via emerging wearable technologies, may allow assessment of therapeutic effects with greater precision compared to the status quo of exacerbation rates or self-reported cough questionnaires. While several clinical trials in bronchiectasis use questionnaires incorporating cough-related sections, they generally report aggregate symptom scores. We propose that re-analysis of such trial data, particularly those evaluating macrolides and inhaled antibiotics, may provide important information regarding the particular impact of these drugs on cough-specific domains. A better understanding of cough subtypes and mechanisms in bronchiectasis may ultimately provide new or improved clinical trial selection criteria and end-point measures that require careful assessment in bronchiectasis given the already mixed outcomes of several (largely unsuccessful) clinical trials [73].
7. Cough promoters in bronchiectasis
Bronchiectasis exhibits heightened cough sensitivities – a condition associated with several respiratory disease states – measured as response to the cough-stimulating agent capsaicin [[76], [77], [78]]. The cough response to capsaicin is mediated via slow-conducting, unmyelinated, jugular ganglia neurons (nociceptors) and may be elevated by aberrant inflammation associated with pulmonary disease that activates and sensitises cough neural pathways [11]. While observed associations between cough and disease severity may reflect the need to clear excess secretions caused by infection, an elevated cough sensitivity suggests a more fundamental change to cough physiology in bronchiectasis as opposed to a clearing response to recurrent infection or inflammatory cues. The cough response in bronchiectasis is primed and easily triggered leading to an enhanced cough reflex, which contributes to pathological progression. In this regard, the treatment of cough, or its direct causes alone may not solely alleviate symptoms but arrest further airway damage resulting from an aberrant cough response [14]. The heightened cough sensitivity seen in bronchiectasis associates with several notable clinical parameters including duration of bronchiectasis symptoms, worsening HRCT score, increased sputum volume, higher bronchiectasis severity index (BSI) and sputum purulence as well as sputum culture positivity for P. aeruginosa [78].
7.1. Microbial cough promoters
A prime example of the bacterial promotion of cough in bronchiectasis is the association between cough and P. aeruginosa, which in itself is interesting in the context of microbial contribution to distinct cough phenotypes. In contrast to P. aeruginosa-colonised patients, reduced cough symptomology is apparent in those who exhibit non-tuberculous Mycobacteria (NTM) culture positivity [79]. Though unreported, the cough sensitivity status of NTM-colonised bronchiectasis patients compared to those dominated by P. aeruginosa or other relevant microbes would be an interesting comparison for future investigation as differences in sensitivity may be linked to differing microbial profiles in bronchiectasis. In support of this, animal studies have demonstrated that sentinel cough-mediating nociceptor sensory neurons residing in the lung can orchestrate distinct host responses in the presence of specific bacteria [80]. This provides a plausible framework for the emergence of specific microbe-driven cough phenotypes in bronchiectasis. The role of fungi in bronchiectasis remains an active area of research by our group and others [35,[81], [82], [83]]. The presence of fungi, in particular those of the genus Aspergillus, is of clear clinical significance in bronchiectasis and associated with a spectrum of disease phenotypes that range from chronic pulmonary aspergillosis (CPA), asymptomatic colonisation, fungal sensitisation and allergic bronchopulmonary aspergillosis (ABPA) [81,83]. The presence of these immunological manifestations associate with disease severity, lung function decline and exacerbation even in apparently stable patients [35]. As the role of fungi on cough in bronchiectasis remains to be fully explicated, the impact of fungal-driven immune responses on neuro-immunological cough pathways are a potential area for future work. Viruses, also implicated in bronchiectasis, remains to be precisely defined in their role in disease and influencing cough. Among those reported are coronavirus, rhinovirus, and influenza A/B, which exhibit higher prevalence during exacerbation [28,84]. A conclusive association between viruses and cough remains to be confirmed in bronchiectasis however, the established relationship between viruses and hypersensitivity in chronic cough suggests that this area is important for future epidemiological and mechanistic studies in the setting of bronchiectasis [85].
7.2. Immunological cough promoters
Airway secretions in bronchiectasis are dominated by neutrophils and associated cytokines including, TNF-α, IL-β, IL-8 and neutrophil elastase among other neutrophil-associated products [86]. Precise pathways by which this immunological milieu drives cough in the setting of bronchiectasis remains to be established however known neuro-immunological pathways of cough are likely involved. Pathogen-incited inflammatory events likely impinge on stimulation of peripheral vagal sensory nerves responsible for the cough reflex that harbours many immune receptors at their neuron terminals [11,87]. The constant barrage of inflammatory signals in the bronchiectatic airway is likely to trigger these neuronal pathways that would otherwise remain silent, possibly explaining the heightened cough hypersensitivity observed in bronchiectasis and the potential establishment of central hypersensitisation via neuro-inflammatory triggers (Fig. 1). This likely begins by immunologic excitation of pulmonary sensory neurons by microbial airway triggers. Work by Baral et al. highlights the role played by nociceptors in suppression of neutrophil recruitment, an observation with important implications in respect to neutrophillic immunopathology seen in bronchiectasis [80]. The specific airway immune profile in bronchiectasis likely has direct consequences for cough hypersensitivity and cough phenotypes in bronchiectasis. Recently, our group has explored the role of sensitisation and allergy in bronchiectasis and identified high levels of atopic sensitisation, even in stable patients [5]. These observations have parallels with global atopic tendency in patients with chronic cough reflecting the central role of fungi and other allergens in allergic respiratory disease where cough is a predominant symptom [30] Importantly, our work also characterised the airway immune profile in bronchiectasis and revealed the existence of two distinct ‘immuno-allertypes’ defined by specific immune profiles. These distinct inflammatory profiles in the lungs of stable bronchiectasis patients provide insight into the potential inflammatory promoters of cough hypersensitisation. Notably, the more severe fungal-driven pro-inflammatory (FDPI) immuno-allertype is characterised by pro-inflammatory signals including tumour necrosis factor α (TNF-α), which itself has an established role in potentiating the effect of rat neuronal TRP ion channels to capsaicin contributing to airway hyperresponsiveness [88]. Further immunological characterisation of the bronchiectasis airway and the influence of specific cytokine patterns on hyperresponsiveness may in future shed light on neuro-inflammatory pathways that drive cough hypersensitivity in bronchiectasis.
8. Conclusions
Cough and bronchiectasis are remarkable in their heterogeneity of clinical phenotypes. As a strong risk factor for cough, bronchiectasis may be viewed as a cough subtype amenable to specific treatment. From the bronchiectasis standpoint, cough is a ubiquitous symptom and the question of whether specific cough phenotypes merit particular attention as treatable traits of disease is an important one. Neuronal pathways of cough driven by inflammatory mediators have been described in great detail but remain to be fully assessed in bronchiectasis. Emerging molecular endophenotyping in bronchiectasis is uncovering microbial and immunological disease signatures that may explain cough hypersensitivity responses in this setting. Confirmation of cough subtypes in bronchiectasis could in future provide scope for novel therapeutic approaches, such as the targeted application of novel neuro-immune therapies already in development for chronic cough.
Acknowledgments
This research is supported by the Singapore Ministry of Health’s National Medical Research Council under its Transition Award (NMRC/TA/0048/2016) (S.H.C), the Singapore Ministry of Health's National Medical Research Council under its Clinician-Scientist Individual Research Grant (MOH-000141) (S.H.C), the NTU Integrated Medical, Biological and Environmental Life Sciences (NIMBELS), Nanyang Technological University, Singapore [NIM/03/2018] and the Lee Kong Chian School of Medicine, Nanyang Technological University Start-Up Grant.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pupt.2019.101812.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Chalmers J.D., Chang A.B., Chotirmall S.H., Dhar R., McShane P.J. Bronchiectasis. Nat. Rev. Dis.Prim. 2018;4(1):45. doi: 10.1038/s41572-018-0042-3. [DOI] [PubMed] [Google Scholar]
- 2.Poh T.Y., Mac Aogain M., Chan A.K., Yii A.C., Yong V.F., Tiew P.Y. Understanding COPD-overlap syndromes. Expert Rev. Respir. Med. 2017;11(4):285–298. doi: 10.1080/17476348.2017.1305895. [DOI] [PubMed] [Google Scholar]
- 3.Chalmers J.D., Chotirmall S.H. Bronchiectasis: new therapies and new perspectives. Lancet Respir. Med. 2018;6(9):715–726. doi: 10.1016/S2213-2600(18)30053-5. [DOI] [PubMed] [Google Scholar]
- 4.McShane P.J. A new bronchiectasis endophenotype: immuno-allertypes. Am. J. Respir. Crit. Care Med. 2019 Apr 1;199(7):811–812. doi: 10.1164/rccm.201810-1949ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mac Aogain M., Tiew P.Y., Lim A.Y.H., Low T.B., Tan G.L., Hassan T. Distinct 'Immuno-Allertypes' of disease and high frequencies of sensitisation in non-cystic-fibrosis bronchiectasis. Am. J. Respir. Crit. Care Med. 2019 Apr 1;199(7):842–853. doi: 10.1164/rccm.201807-1355OC. [DOI] [PubMed] [Google Scholar]
- 6.Chotirmall S.H., Chalmers J.D. RESPIRE: breathing new life into bronchiectasis. Eur. Respir. J. 2018;51(1) doi: 10.1183/13993003.02444-2017. [DOI] [PubMed] [Google Scholar]
- 7.De Soyza A., Aksamit T., Bandel T.J., Criollo M., Elborn J.S., Operschall E. RESPIRE 1: a phase III placebo-controlled randomised trial of ciprofloxacin dry powder for inhalation in non-cystic fibrosis bronchiectasis. Eur. Respir. J. 2018;51(1) doi: 10.1183/13993003.02052-2017. [DOI] [PubMed] [Google Scholar]
- 8.Aksamit T., De Soyza A., Bandel T.J., Criollo M., Elborn J.S., Operschall E. RESPIRE 2: a phase III placebo-controlled randomised trial of ciprofloxacin dry powder for inhalation in non-cystic fibrosis bronchiectasis. Eur. Respir. J. 2018;51(1) doi: 10.1183/13993003.02053-2017. [DOI] [PubMed] [Google Scholar]
- 9.Flume P.A., Chalmers J.D., Olivier K.N. Advances in bronchiectasis: endotyping, genetics, microbiome, and disease heterogeneity. Lancet. 2018;392(10150):880–890. doi: 10.1016/S0140-6736(18)31767-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boaventura R., Sibila O., Agusti A., Chalmers J.D. Treatable traits in bronchiectasis. Eur. Respir. J. 2018;52(3) doi: 10.1183/13993003.01269-2018. [DOI] [PubMed] [Google Scholar]
- 11.McGovern A.E., Short K.R., Kywe Moe A.A., Mazzone S.B. Translational review: neuroimmune mechanisms in cough and emerging therapeutic targets. J. Allergy Clin. Immunol. 2018;142(5):1392–1402. doi: 10.1016/j.jaci.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 12.Mazzone S.B., Farrell M.J. Heterogeneity of cough neurobiology: clinical implications. Pulm. Pharmacol. Therapeut. 2019 Apr;55:62–66. doi: 10.1016/j.pupt.2019.02.002. Epub 2019 Feb 11. [DOI] [PubMed] [Google Scholar]
- 13.Chung K.F., Pavord I.D. Prevalence, pathogenesis, and causes of chronic cough. Lancet. 2008;371(9621):1364–1374. doi: 10.1016/S0140-6736(08)60595-4. [DOI] [PubMed] [Google Scholar]
- 14.Niimi A., Torrego A., Nicholson A.G., Cosio B.G., Oates T.B., Chung K.F. Nature of airway inflammation and remodeling in chronic cough. J. Allergy Clin. Immunol. 2005;116(3):565–570. doi: 10.1016/j.jaci.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 15.Colak Y., Nordestgaard B.G., Laursen L.C., Afzal S., Lange P., Dahl M. Risk factors for chronic cough among 14,669 individuals from the general population. Chest. 2017;152(3):563–573. doi: 10.1016/j.chest.2017.05.038. [DOI] [PubMed] [Google Scholar]
- 16.Pasteur M.C., Bilton D., Hill A.T. British thoracic society bronchiectasis non CFGG. British thoracic society guideline for non-CF bronchiectasis. Thorax. 2010;65(Suppl 1):i1–58. doi: 10.1136/thx.2010.136119. [DOI] [PubMed] [Google Scholar]
- 17.Rosen M.J. Chronic cough due to bronchiectasis: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(1 Suppl):122S–131S. doi: 10.1378/chest.129.1_suppl.122S. [DOI] [PubMed] [Google Scholar]
- 18.Mazzone S.B., Chung K.F., McGarvey L. The heterogeneity of chronic cough: a case for endotypes of cough hypersensitivity. Lancet Respir. Med. 2018;6(8):636–646. doi: 10.1016/S2213-2600(18)30150-4. [DOI] [PubMed] [Google Scholar]
- 19.Vendrell M., de Gracia J., Olveira C., Martínez M.Á., Girón R., Máiz L. Diagnosis and treatment of bronchiectasis. Arch. Bronconeumol. 2008;44(11):629–640. doi: 10.1157/13128330. [DOI] [PubMed] [Google Scholar]
- 20.Murray M.P., Turnbull K., MacQuarrie S., Pentland J.L., Hill A.T. Validation of the leicester cough questionnaire in non-cystic fibrosis bronchiectasis. Eur. Respir. J. 2009;34(1):125–131. doi: 10.1183/09031936.00160508. [DOI] [PubMed] [Google Scholar]
- 21.Spinou A., Lee K.K., Sinha A., Elston C., Loebinger M.R., Wilson R. The objective assessment of cough frequency in bronchiectasis. Lung. 2017;195(5):575–585. doi: 10.1007/s00408-017-0038-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bolser D.C. Cough suppressant and pharmacologic protussive therapy: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(1 Suppl):238S–249S. doi: 10.1378/chest.129.1_suppl.238S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hill A.T., Haworth C.S., Aliberti S., Barker A., Blasi F., Boersma W. Pulmonary exacerbation in adults with bronchiectasis: a consensus definition for clinical research. Eur. Respir. J. 2017;49(6) doi: 10.1183/13993003.00051-2017. [DOI] [PubMed] [Google Scholar]
- 24.Mac Aogáin M., Chandrasekaran R., Lim A.Y.H., Low T.B., Tan G.L., Hassan T. Immunological corollary of the pulmonary mycobiome in bronchiectasis: the CAMEB study. Eur. Respir. J. 2018;52(1):1800766. doi: 10.1183/13993003.00766-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McDonnell M.J., Aliberti S., Goeminne P.C., Restrepo M.I., Finch S., Pesci A. Comorbidities and the risk of mortality in patients with bronchiectasis: an international multicentre cohort study. Lancet Respir. Med. 2016;4(12):969–979. doi: 10.1016/S2213-2600(16)30320-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goeminne P.C., Cox B., Finch S., Loebinger M.R., Bedi P., Hill A.T. The impact of acute air pollution fluctuations on bronchiectasis pulmonary exacerbation: a case-crossover analysis. Eur. Respir. J. 2018;52(1) doi: 10.1183/13993003.02557-2017. [DOI] [PubMed] [Google Scholar]
- 27.Irwin R.S., French C.L., Chang A.B., Altman K.W., Panel* C.E.C. Classification of cough as a symptom in adults and management algorithms: CHEST guideline and expert panel report. Chest. 2018;153(1):196–209. doi: 10.1016/j.chest.2017.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chandrasekaran R., Mac Aogain M., Chalmers J.D., Elborn S.J., Chotirmall S.H. Geographic variation in the aetiology, epidemiology and microbiology of bronchiectasis. BMC Pulm. Med. 2018;18(1):83. doi: 10.1186/s12890-018-0638-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morice A.H., Kastelik J.A. Cough. 1: chronic cough in adults. Thorax. 2003;58(10):901–907. doi: 10.1136/thorax.58.10.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ogawa H., Fujimura M., Ohkura N., Makimura K. Atopic cough and fungal allergy. J. Thorac. Dis. 2014;6(Suppl 7):S689–S698. doi: 10.3978/j.issn.2072-1439.2014.09.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Undem B.J., Zaccone E., McGarvey L., Mazzone S.B. Neural dysfunction following respiratory viral infection as a cause of chronic cough hypersensitivity. Pulm. Pharmacol. Therapeut. 2015;33:52–56. doi: 10.1016/j.pupt.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Polverino E., Goeminne P.C., McDonnell M.J., Aliberti S., Marshall S.E., Loebinger M.R. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur. Respir. J. 2017;50(3) doi: 10.1183/13993003.00629-2017. [DOI] [PubMed] [Google Scholar]
- 33.Rogers G.B., Bruce K.D., Martin M.L., Burr L.D., Serisier D.J. The effect of long-term macrolide treatment on respiratory microbiota composition in non-cystic fibrosis bronchiectasis: an analysis from the randomised, double-blind, placebo-controlled BLESS trial. Lancet Respir. Med. 2014;2(12):988–996. doi: 10.1016/S2213-2600(14)70213-9. [DOI] [PubMed] [Google Scholar]
- 34.Rogers G.B., Zain N.M., Bruce K.D., Burr L.D., Chen A.C., Rivett D.W. A novel microbiota stratification system predicts future exacerbations in bronchiectasis. Ann. Am. Thorac. Soc. 2014;11(4):496–503. doi: 10.1513/AnnalsATS.201310-335OC. [DOI] [PubMed] [Google Scholar]
- 35.Mac Aogain M., Chandrasekaran R., Lim A.Y.H., Low T.B., Tan G.L., Hassan T. Immunological corollary of the pulmonary mycobiome in bronchiectasis: the CAMEB study. Eur. Respir. J. 2018;52(1) doi: 10.1183/13993003.00766-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kazachkov M., Kapoor B.C., Malecha P.W., Wu B.G., Li Y., Levine J. Aerodigestive dysbiosis in children with chronic cough. Pediatr. Pulmonol. 2018;53(9):1288–1298. doi: 10.1002/ppul.24115. [DOI] [PubMed] [Google Scholar]
- 37.Martinez-Garcia M.A., Miravitlles M. Bronchiectasis in COPD patients: more than a comorbidity? Int. J. Chronic Obstr. Pulm. Dis. 2017;12:1401–1411. doi: 10.2147/COPD.S132961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carney I.K., Gibson P.G., Murree-Allen K., Saltos N., Olson L.G., Hensley M.J. A systematic evaluation of mechanisms in chronic cough. Am. J. Respir. Crit. Care Med. 1997;156(1):211–216. doi: 10.1164/ajrccm.156.1.9605044. [DOI] [PubMed] [Google Scholar]
- 39.Shoemark A., Ozerovitch L., Wilson R. Aetiology in adult patients with bronchiectasis. Respir. Med. 2007;101(6):1163–1170. doi: 10.1016/j.rmed.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 40.Tatar M., Plevkova J., Brozmanova M., Pecova R., Kollarik M. Mechanisms of the cough associated with rhinosinusitis. Pulm. Pharmacol. Therapeut. 2009;22(2):121–126. doi: 10.1016/j.pupt.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 41.Shteinberg M., Nassrallah N., Jrbashyan J., Uri N., Stein N., Adir Y. Upper airway involvement in bronchiectasis is marked by early onset and allergic features. ERJ Open Res. 2018;4(1) doi: 10.1183/23120541.00115-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee A.L., Burge A.T., Holland A.E. Airway clearance techniques for bronchiectasis. Cochrane Database Syst. Rev. 2015;11 doi: 10.1002/14651858.CD008351.pub3. CD008351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murray M.P., Pentland J.L., Hill A.T. A randomised crossover trial of chest physiotherapy in non-cystic fibrosis bronchiectasis. Eur. Respir. J. 2009;34(5):1086–1092. doi: 10.1183/09031936.00055509. [DOI] [PubMed] [Google Scholar]
- 44.Lee A.L., Burge A.T., Holland A.E. Positive expiratory pressure therapy versus other airway clearance techniques for bronchiectasis. Cochrane Database Syst. Rev. 2017;9:CD011699. doi: 10.1002/14651858.CD011699.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uzmezoglu B., Altiay G., Ozdemir L., Tuna H., Sut N. The efficacy of flutter((R)) and active cycle of breathing techniques in patients with bronchiectasis: a prospective, randomized, comparative study. Turk. Thorac. J. 2018;19(3):103–109. doi: 10.5152/TurkThoracJ.2018.17050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tambascio J., de Souza H.C.D., Martinez R., Baddini-Martinez J.A., Barnes P.J., Gastaldi A.C. Effects of an airway clearance device on inflammation, bacteriology, and mucus transport in bronchiectasis. Respir. Care. 2017;62(8):1067–1074. doi: 10.4187/respcare.05214. [DOI] [PubMed] [Google Scholar]
- 47.Tambascio J., de Souza L.T., Lisboa R.M., Passarelli Rde C., de Souza H.C., Gastaldi A.C. The influence of Flutter(R)VRP1 components on mucus transport of patients with bronchiectasis. Respir. Med. 2011;105(9):1316–1321. doi: 10.1016/j.rmed.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 48.Ozalp O., Inal-Ince D., Cakmak A., Calik-Kutukcu E., Saglam M., Savci S. High-intensity inspiratory muscle training in bronchiectasis: a randomized controlled trial. Respirology. 2019 Mar;24(3):246–253. doi: 10.1111/resp.13397. Epub 2018 Sep 12. [DOI] [PubMed] [Google Scholar]
- 49.Kaehne A., Milan S.J., Felix L.M., Sheridan E., Marsden P.A., Spencer S. Head-to-head trials of antibiotics for bronchiectasis. Cochrane Database Syst. Rev. 2018;9 doi: 10.1002/14651858.CD012590.pub2. CD012590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Metersky M., Chalmers J. Bronchiectasis insanity: doing the same thing over and over again and expecting different results? F1000Research. 2019;8 doi: 10.12688/f1000research.17295.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wong C., Jayaram L., Karalus N., Eaton T., Tong C., Hockey H. Azithromycin for prevention of exacerbations in non-cystic fibrosis bronchiectasis (EMBRACE): a randomised, double-blind, placebo-controlled trial. Lancet. 2012;380(9842):660–667. doi: 10.1016/S0140-6736(12)60953-2. [DOI] [PubMed] [Google Scholar]
- 52.Serisier D.J., Martin M.L., McGuckin M.A., Lourie R., Chen A.C., Brain B. Effect of long-term, low-dose erythromycin on pulmonary exacerbations among patients with non-cystic fibrosis bronchiectasis: the BLESS randomized controlled trial. J. Am. Med. Assoc. 2013;309(12):1260–1267. doi: 10.1001/jama.2013.2290. [DOI] [PubMed] [Google Scholar]
- 53.Martin M.J., Lee H., Clayton C., Pointon K., Soomro I., Shaw D.E. Idiopathic chronic productive cough and response to open-label macrolide therapy: an observational study. Respirology. 2019 Jun;24(6):558–565. doi: 10.1111/resp.13483. (Epub 2019 Feb 5) [DOI] [PubMed] [Google Scholar]
- 54.Hodgson D., Anderson J., Reynolds C., Oborne J., Meakin G., Bailey H. The effects of azithromycin in treatment-resistant cough: a randomized, double-blind, placebo-controlled trial. Chest. 2016;149(4):1052–1060. doi: 10.1016/j.chest.2015.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ogawa H., Fujimura M., Takeuchi Y., Makimura K. Clinical experience with low-dose itraconazole in chronic idiopathic cough. Cough. 2013;9(1):1. doi: 10.1186/1745-9974-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jhun B.W., Jeon K., Eom J.S., Lee J.H., Suh G.Y., Kwon O.J. Clinical characteristics and treatment outcomes of chronic pulmonary aspergillosis. Med. Mycol. 2013;51(8):811–817. doi: 10.3109/13693786.2013.806826. [DOI] [PubMed] [Google Scholar]
- 57.Aranow C. Vitamin D and the immune system. J. Investig. Med. 2011;59(6):881–886. doi: 10.231/JIM.0b013e31821b8755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moustaki M., Loukou I., Priftis K.N., Douros K. Role of vitamin D in cystic fibrosis and non-cystic fibrosis bronchiectasis. World J. Clin. Pediatr. 2017;6(3):132–142. doi: 10.5409/wjcp.v6.i3.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chalmers J.D., McHugh B.J., Docherty C., Govan J.R., Hill A.T. Vitamin-D deficiency is associated with chronic bacterial colonisation and disease severity in bronchiectasis. Thorax. 2013;68(1):39–47. doi: 10.1136/thoraxjnl-2012-202125. [DOI] [PubMed] [Google Scholar]
- 60.Bartley J., Garrett J., Camargo C.A., Jr., Scragg R., Vandal A., Sisk R. Vitamin D3 supplementation in adults with bronchiectasis: a pilot study. Chronic Respir. Dis. 2018;15(4):384–392. doi: 10.1177/1479972318761646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Martineau A.R., Jolliffe D.A., Hooper R.L., Greenberg L., Aloia J.F., Bergman P. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356 doi: 10.1136/bmj.i6583. i6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ozdemir B., Koksal B.T., Karakas N.M., Tekindal M.A., Ozbek O.Y. Serum vitamin D levels in children with recurrent respiratory infections and chronic cough. Indian J. Pediatr. 2016;83(8):777–782. doi: 10.1007/s12098-015-2010-1. [DOI] [PubMed] [Google Scholar]
- 63.Hothersall E., McSharry C., Thomson N.C. Potential therapeutic role for statins in respiratory disease. Thorax. 2006;61(8):729–734. doi: 10.1136/thx.2005.057976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hennessy E., Adams C., Reen F.J., O'Gara F. Is there potential for repurposing statins as novel antimicrobials? Antimicrob. Agents Chemother. 2016;60(9):5111–5121. doi: 10.1128/AAC.00192-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mandal P., Chalmers J.D., Graham C., Harley C., Sidhu M.K., Doherty C. Atorvastatin as a stable treatment in bronchiectasis: a randomised controlled trial. Lancet Respir. Med. 2014;2(6):455–463. doi: 10.1016/S2213-2600(14)70050-5. [DOI] [PubMed] [Google Scholar]
- 66.Bedi P., Chalmers J.D., Graham C., Clarke A., Donaldson S., Doherty C. A randomized controlled trial of atorvastatin in patients with bronchiectasis infected with Pseudomonas aeruginosa: a proof of concept study. Chest. 2017;152(2):368–378. doi: 10.1016/j.chest.2017.05.017. [DOI] [PubMed] [Google Scholar]
- 67.Dicpinigaitis P.V. Angiotensin-converting enzyme inhibitor-induced cough: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(1 Suppl):169S–173S. doi: 10.1378/chest.129.1_suppl.169S. [DOI] [PubMed] [Google Scholar]
- 68.Navaratnam V., Millett E.R., Hurst J.R., Thomas S.L., Smeeth L., Hubbard R.B. Bronchiectasis and the risk of cardiovascular disease: a population-based study. Thorax. 2017;72(2):161–166. doi: 10.1136/thoraxjnl-2015-208188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pavord I.D., Chung K.F. Management of chronic cough. Lancet. 2008;371(9621):1375–1384. doi: 10.1016/S0140-6736(08)60596-6. [DOI] [PubMed] [Google Scholar]
- 70.Ryan N.M., Vertigan A.E., Birring S.S. An update and systematic review on drug therapies for the treatment of refractory chronic cough. Expert Opin. Pharmacother. 2018;19(7):687–711. doi: 10.1080/14656566.2018.1462795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chung K.F. Advances in mechanisms and management of chronic cough: the ninth london international cough symposium 2016. Pulm. Pharmacol. Therapeut. 2017;47:2–8. doi: 10.1016/j.pupt.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 72.McCallion P., De Soyza A. Cough and bronchiectasis. Pulm. Pharmacol. Therapeut. 2017;47:77–83. doi: 10.1016/j.pupt.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 73.Crichton M.L., Aliberti S., Chalmers J.D. A systematic review of pharmacotherapeutic clinical trial end-points for bronchiectasis in adults. Eur. Respir. Rev. 2019;28(151) doi: 10.1183/16000617.0108-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Amoh J., Odame K., editors. IEEE Biomedical Circuits and Systems Conference (BioCAS) vol. 2015. IEEE; 2015. DeepCough: a deep convolutional neural network in a wearable cough detection system. [Google Scholar]
- 75.Birring S.S., Fleming T., Matos S., Raj A.A., Evans D.H., Pavord I.D. The Leicester Cough Monitor: preliminary validation of an automated cough detection system in chronic cough. Eur. Respir. J. 2008;31(5):1013–1018. doi: 10.1183/09031936.00057407. [DOI] [PubMed] [Google Scholar]
- 76.Doherty M.J., Mister R., Pearson M.G., Calverley P.M. Capsaicin responsiveness and cough in asthma and chronic obstructive pulmonary disease. Thorax. 2000;55(8):643–649. doi: 10.1136/thorax.55.8.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Torrego A., Haque R.A., Nguyen L.T., Hew M., Carr D.H., Wilson R. Capsaicin cough sensitivity in bronchiectasis. Thorax. 2006;61(8):706–709. doi: 10.1136/thx.2005.049767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Guan W.J., Gao Y.H., Xu G., Lin Z.Y., Tang Y., Li H.M. Capsaicin cough sensitivity and the association with clinical parameters in bronchiectasis. PLoS One. 2014;9(11) doi: 10.1371/journal.pone.0113057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Aksamit T.R., O'Donnell A.E., Barker A., Olivier K.N., Winthrop K.L., Daniels M.L.A. Adult patients with bronchiectasis: a first look at the US bronchiectasis research registry. Chest. 2017;151(5):982–992. doi: 10.1016/j.chest.2016.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Baral P., Umans B.D., Li L., Wallrapp A., Bist M., Kirschbaum T. Nociceptor sensory neurons suppress neutrophil and gammadelta T cell responses in bacterial lung infections and lethal pneumonia. Nat. Med. 2018;24(4):417–426. doi: 10.1038/nm.4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chotirmall S.H., Martin-Gomez M.T. Aspergillus species in bronchiectasis: challenges in the cystic fibrosis and non-cystic fibrosis airways. Mycopathologia. 2018;183(1):45–59. doi: 10.1007/s11046-017-0143-7. [DOI] [PubMed] [Google Scholar]
- 82.Yii A.C., Koh M.S., Lapperre T.S., Tan G.L., Chotirmall S.H. The emergence of Aspergillus species in chronic respiratory disease. Front. Biosci. 2017;9:127–138. doi: 10.2741/s477. [DOI] [PubMed] [Google Scholar]
- 83.De Soyza A., Aliberti S. Bronchiectasis and Aspergillus: how are they linked? Med. Mycol. 2017;55(1):69–81. doi: 10.1093/mmy/myw109. [DOI] [PubMed] [Google Scholar]
- 84.Gao Y.H., Guan W.J., Xu G., Lin Z.Y., Tang Y., Lin Z.M. The role of viral infection in pulmonary exacerbations of bronchiectasis in adults: a prospective study. Chest. 2015;147(6):1635–1643. doi: 10.1378/chest.14-1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Abdullah H., Heaney L.G., Cosby S.L., McGarvey L.P. Rhinovirus upregulates transient receptor potential channels in a human neuronal cell line: implications for respiratory virus-induced cough reflex sensitivity. Thorax. 2014;69(1):46–54. doi: 10.1136/thoraxjnl-2013-203894. [DOI] [PubMed] [Google Scholar]
- 86.Chalmers J.D., Hill A.T. Mechanisms of immune dysfunction and bacterial persistence in non-cystic fibrosis bronchiectasis. Mol. Immunol. 2013;55(1):27–34. doi: 10.1016/j.molimm.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 87.Wang J., Kollarik M., Ru F., Sun H., McNeil B., Dong X. Distinct and common expression of receptors for inflammatory mediators in vagal nodose versus jugular capsaicin-sensitive/TRPV1-positive neurons detected by low input RNA sequencing. PLoS One. 2017;12(10) doi: 10.1371/journal.pone.0185985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hu Y., Gu Q., Lin R.L., Kryscio R., Lee L.Y. Calcium transient evoked by TRPV1 activators is enhanced by tumor necrosis factor-{alpha} in rat pulmonary sensory neurons. Am. J. Physiol. Lung Cell Mol. Physiol. 2010;299(4):L483–L492. doi: 10.1152/ajplung.00111.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.McGarvey L.P. Idiopathic chronic cough: a real disease or a failure of diagnosis? Cough. 2005;1:9. doi: 10.1186/1745-9974-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gao Y.H., Guan W.J., Liu S.X., Wang L., Cui J.J., Chen R.C. Aetiology of bronchiectasis in adults: a systematic literature review. Respirology. 2016;21(8):1376–1383. doi: 10.1111/resp.12832. [DOI] [PubMed] [Google Scholar]
- 91.Koo H.K., Jeong I., Lee S.W., Park J., Kim J.H., Park S.Y. Prevalence of chronic cough and possible causes in the general population based on the Korean National Health and Nutrition Examination Survey. Medicine (Baltim.) 2016;95(37) doi: 10.1097/MD.0000000000004595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.de Marco R., Accordini S., Cerveri I., Corsico A., Anto J.M., Kunzli N. Incidence of chronic obstructive pulmonary disease in a cohort of young adults according to the presence of chronic cough and phlegm. Am. J. Respir. Crit. Care Med. 2007;175(1):32–39. doi: 10.1164/rccm.200603-381OC. [DOI] [PubMed] [Google Scholar]
- 93.King P.T., Holdsworth S.R., Freezer N.J., Villanueva E., Holmes P.W. Characterisation of the onset and presenting clinical features of adult bronchiectasis. Respir. Med. 2006;100(12):2183–2189. doi: 10.1016/j.rmed.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 94.King P.T., Holdsworth S.R., Farmer M., Freezer N., Villanueva E., Holmes P.W. Phenotypes of adult bronchiectasis: onset of productive cough in childhood and adulthood. COPD. 2009;6(2):130–136. doi: 10.1080/15412550902766934. [DOI] [PubMed] [Google Scholar]
- 95.Fujimura M., Abo M., Ogawa H., Nishi K., Kibe Y., Hirose T. Importance of atopic cough, cough variant asthma and sinobronchial syndrome as causes of chronic cough in the Hokuriku area of Japan. Respirology. 2005;10(2):201–207. doi: 10.1111/j.1440-1843.2005.00686.x. [DOI] [PubMed] [Google Scholar]
- 96.Kadowaki T., Yano S., Wakabayashi K., Kobayashi K., Ishikawa S., Kimura M. An analysis of etiology, causal pathogens, imaging patterns, and treatment of Japanese patients with bronchiectasis. Respair. Invest. 2015;53(1):37–44. doi: 10.1016/j.resinv.2014.09.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.