Highlights
-
•
More than one-third of critically ill patients with Middle East Respiratory Syndrome Coronavirus (MERS-CoV) received empiric therapy with macrolides.
-
•
Macrolide therapy is not significantly associated with a reduction in 90-day mortality of critically ill patients with MERS-CoV.
-
•
Macrolide therapy is not significantly associated with improvement in MERS-CoV RNA clearance of critically ill patients with MERS-CoV.
Keywords: Macrolides, MERS-CoV, Critical care, Pneumonia, Influenza, Azithromycin
Abstract
Objectives
Macrolides have been reported to be associated with improved outcomes in patients with viral pneumonia related to influenza and other viruses, possibly because of their immune-modulatory effects. Macrolides have frequently been used in patients with Middle East Respiratory Syndrome (MERS). This study investigated the association of macrolides with 90-day mortality and MERS coronavirus (CoV) RNA clearance in critically ill patients with MERS.
Methods
This retrospective analysis of a multicenter cohort database included 14 tertiary-care hospitals in five cities in Saudi Arabia. Multivariate logistic-regression analysis was used to determine the association of macrolide therapy with 90-day mortality, and the Cox-proportional hazard model to determine the association of macrolide therapy with MERS-CoV RNA clearance.
Results
Of 349 critically ill MERS patients, 136 (39%) received macrolide therapy. Azithromycin was most commonly used (97/136; 71.3%). Macrolide therapy was commonly started before the patient arrived in the intensive care unit (ICU) (51/136; 37.5%), or on day1 in ICU (53/136; 39%). On admission to ICU, the baseline characteristics of patients who received and did not receive macrolides were similar, including demographic data and sequential organ failure assessment score. However, patients who received macrolides were more likely to be admitted with community-acquired MERS (P = 0.02). Macrolide therapy was not independently associated with a significant difference in 90-day mortality (adjusted odds ratio [OR]: 0.84; 95% confidence interval [CI] :0.47–1.51; P = 0.56) or MERS-CoV RNA clearance (adjusted HR: 0.88; 95% CI:0.47–1.64; P = 0.68).
Conclusions
These findings indicate that macrolide therapy is not associated with a reduction in 90-day mortality or improvement in MERS-CoV RNA clearance.
Introduction
Macrolides are bacteriostatic antibiotics with a broad spectrum of activity against many Gram-positive and atypical bacterial species commonly associated with respiratory tract infections (Min and Jang, 2012, NIH, 2017). As well as their antibacterial effects, macrolides have been shown to have immune-modulatory and anti-inflammatory effects (Amsden, 2005, Kanoh and Rubin, 2010, Nakamura et al., 1999, Wales and Woodhead, 1999, Zarogoulidis et al., 2012). Morbidity and mortality caused by respiratory viral infections is associated with the excessive elaboration of cytokines and immunopathologic host inflammatory responses (Min and Jang, 2012, Mosquera et al., 2014, Us, 2008, Wang et al., 2012). Pre-clinical and clinical studies have shown that macrolides downregulate the inflammatory response, attenuating extreme cytokine production and promote the induction of immunoglobulin antibodies (Bermejo-Martin et al., 2009), which may reduce the complications of respiratory viral infections (Lee et al., 2017, Lendermon et al., 2017, Min and Jang, 2012, Us, 2008, Wang et al., 2012). Given these properties, macrolides (e.g., azithromycin, clarithromycin, erythromycin, fidaxomicin, telithromycin) have been studied for their potential use as targeted therapy for a wide spectrum of viral respiratory infections including influenza (Bermejo-Martin et al., 2009, Min and Jang, 2012, Suzuki et al., 2002, Tahan et al., 2007, Zhang et al., 2010).
Clinical studies of macrolides in patients with viral respiratory infections have yielded contradictory results. In a retrospective analysis, use of clarithromycin with oseltamivir or zanamivir was associated with restoration of attenuated antiviral mucosal and systemic immunity and reduction in the re-infection rate in pediatric patients with influenza (Shinahara et al., 2013). In another study, pediatric patients with influenza-related symptoms were randomly assigned to groups receiving either cephalosporins or macrolides. In this study, macrolides were found to be more effective for alleviating fever and reducing the occurrence of pneumonia (Ninomiya et al., 2002). In a double-blind randomised controlled trial (RCT) involving infants with respiratory syncytial virus (RSV)-related bronchiolitis, administration of clarithromycin daily for three weeks was associated with reduction in hospital length of stay (LOS), supplemental oxygen, the need for β2-agonists, and hospital readmission. In addition, it was associated with reduction in plasma interleukin-4 (IL-4), IL-8 and eotaxin levels (Tahan et al., 2007). In an open-label, prospective RCT, clarithromycin was associated with fever reduction in adult patients with influenza A/H1N1pdm09 infection who were treated with neuraminidase inhibitors. However, there was no difference in the reduction of serum IL-6 levels between the study groups (Higashi et al., 2014). Similarly, in a multicenter, open-label RCT, a combination therapy of osteltamivir and azithromycin was associated with early resolution of some clinical symptoms but no difference in inflammatory cytokine levels (Kakeya et al., 2014). In a recent open-label RCT of hospitalized adults, early combination therapy with clarithromycin, naproxen and oseltamivir was associated with reduced 30-day and 90-day mortality and hospital LOS compared to oseltamivir monotherapy (Hung et al., 2017). Similar results were obtained from secondary analysis of a multicenter RCT, in which use of the macrolides azithromycin, clarithromycin, or erythromycin was associated with improved outcomes, including mortality, in patients with acute lung injury (Walkey and Wiener, 2012).
On the other hand, in a multicenter, double-blind, placebo-controlled equivalence RCT, use of azithromycin to treat infants and young children with RSV was not associated with improved clinical symptoms (Kneyber et al., 2008). Similarly, placebo-controlled RCTs in infants and children have shown that use of azithromycin for bronchiolitis does not reduce length of stay, oxygen requirement or readmission (McCallum et al., 2013, Pinto et al., 2012). In one multicenter, observational study, macrolides were not associated with improved survival in critically ill patients with A(H1N1)pdm09-associated primary viral pneumonia (Martin-Loeches et al., 2013).
In patients with Middle East Respiratory Syndrome (MERS), macrolides are often prescribed as part of the empiric treatment regimen for pneumonia, often before the detection of MERS coronavirus (MERS-CoV) However, the association of macrolides with MERS outcomes has not been investigated rigorously. Using a cohort of critically ill patients with MERS, the aim of this study was to determine whether there was an association between macrolide therapy and 90-day mortality, and between macrolide treatment and MERS-CoV RNA clearance from respiratory secretions.
Material and methods
Setting
This was a retrospective analysis of a multicenter cohort study, which included 14 tertiary care hospitals in five cities in Saudi Arabia. Details of the original cohort have been previously published (Arabi et al., 2017). The study was approved by the institutional review boards of all participating centers. Patient-level informed consent was not required due to its observational nature.
Patients
The study included all patients who were admitted to the participating hospitals between September 2012 and January 2018 and who were critically ill with laboratory-confirmed MERS. In this retrospective analysis, patients were divided into one of two treatment groups: a ‘macrolide therapy group’, in which patients received macrolide treatment (azithromycin, clarithromycin or erythromycin) within three days before admission to ICU, or at any time during their stay in ICU, up to 28 days and a ‘no macrolide therapy’ (control) group consisting of patients not given macrolides during the same timeframe. Macrolides were typically administered as part of empiric therapy for pneumonia, although some patients received erythromycin as a gastrointestinal prokinetic agent.
Data collection
A standardised case report form was used for data collection (ISARIC). Data extracted included patient demographic features; underlying comorbidities; and the durations between symptom onset and presentation to the Emergency Department, ICU admission and intubation. Severity of patients’ illness was assessed using the sequential organ failure assessment (SOFA) score, as well as laboratory and ventilator parameters on days 1, 3, 7, 14 and 28 of ICU admission (Vincent et al., 1998). The results of diagnostic tests were documented, including those for bacterial co-pathogens; ‘atypical’ pathogens including legionella, chlamydia and mycoplasma; and other viral infections in the first three days of ICU admission. Details of any other antibiotics given within the first three days of ICU admission were also documented. The primary outcome was 90-day all-cause mortality. We also examined the time to clearance of MERS-CoV rRT-PCR defined as the time from ICU admission until the test was negative on two occasions, without a positive test afterward. Clearance of MERS-CoV rRT-PCR was calculated on patients who had at least one follow-up rRT-PCR test in the ICU from the date of ICU admission, and censored by the date of last test or death whichever comes first.
Statistical analysis
We compared the baseline characteristics, co-interventions, and outcomes of patients who received macrolide therapy and those who did not. For categorical variables, the Chi-square test or Fisher’s exact test were used, and for continuous variables, Student’s t-test or the Mann–Whitney U test were used as appropriate.
To examine the association between macrolide therapy and 90-day mortality, we performed multivariable logistic regression analysis, with macrolide therapy being the independent variable. The model included baseline variables of clinical interest, decided a priori, and significant co-variables at the univariable level (P ≤ 0.2) that included: body mass index (BMI), SOFA score at admission to ICU, community-acquired (versus hospital-acquired) infection, healthcare worker-associated infection, non-invasive ventilation at ICU admission, malignancy, chronic pulmonary disease, and liver disease. Sensitivity analysis was conducted by excluding patients who received macrolides after the third day of ICU admission.
To examine the association between macrolide therapy and MERS-CoV RNA clearance, we used a Cox proportional hazard model, with macrolide therapy being the independent variable. We included in this model the same covariables mentioned above. Because of the competing nature of MERS-CoV RNA clearance and death, we performed further analysis restricting to survivors.
Results
Patient characteristics
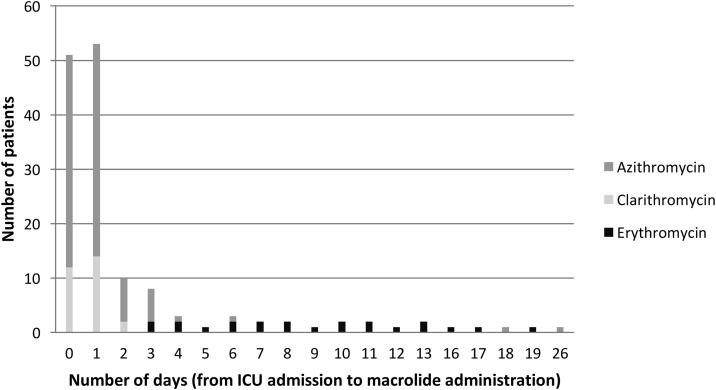
Out of 349 critically ill MERS patients, 136 (39%) received macrolide therapy. Of these, 97/136 (71.3%) received azithromycin, 28/136 (20.6%) received clarithromycin, and 22/136 (16.2%) received erythromycin with 11 patients received two macrolides at different times during the study period. Most patients who received macrolides had already been started on this therapy before arriving to ICU (51/136; 37.5%) or were started on the first day in ICU (53/136; 39%) (Figure 1 ).
Figure 1.
Time to initiation of macrolide therapy from ICU admission among critically ill patients with Middle East Respiratory Syndrome (MERS). Day 0 includes patients who were already on macrolides therapy before being admitted to ICU. There were 11 patients who recieved two macrolides at different times during the study period, and one patient with missing data regarding the date of macrolide therapy.
The demographic characteristics, physiological parameters, comorbidities and presenting symptoms of patients between the two study groups were very similar (Table 1 and Supplementary Table 1). However, patients in the macrolide therapy group were more likely to have community-acquired MERS (81/136; 59.6%) than those in the control group (104/213; 48.8%; P = 0.02), and had a slightly higher mean arterial pressure at ICU admission. Patients not receiving macrolides had more liver disease, had slightly higher international normalised ratio (INR) readings, and slightly higher bilirubin levels at ICU admission.
Table 1.
Baseline characteristics and physiological parameters of critically ill patients with Middle East Respiratory Syndrome (MERS) who received or did not receive macrolide therapy.
| Variables | Macrolides group (n = 136) |
No macrolides group (n = 213) |
P-value |
|---|---|---|---|
| Age (years), median (Q1, Q3) | 56 (42, 67) | 58 (47, 71) | 0.23^ |
| BMI (kg/m2), median (Q1, Q3) | 29 (25, 34) | 28 (23, 33) | 0.08^ |
| Male gender, n (%) | 97 (71.3) | 144 (67.6) | 0.46** |
| Occupation | |||
| Healthcare worker, n (%) | 16 (11.8) | 16 (7.5) | 0.02** |
| Community-acquired, n (%) | 81 (59.6) | 104 (48.8) | |
| Hospital-acquired, n (%) | 39 (28.7) | 93 (43.7) | |
| Duration of illness | |||
| Days from onset of symptoms to emergency room presentation, median (Q1, Q3) | 5 (3, 8) | 4 (3, 8) | 0.79^ |
| Days from onset of symptoms to ICU admission, median (Q1, Q3) | 7 (4, 10) | 7 (4, 12) | 0.23^ |
| Days from onset of symptoms to intubation, median (Q1, Q3), N = 214 | 8 (5, 11) | 8 (5, 13) | 0.11^ |
| Comorbidities | |||
| Any comorbidity, n (%) | 106 (77.9) | 175 (82.2) | 0.33** |
| Diabetes with chronic complications, n (%) | 72 (52.9) | 98 (46.0) | 0.21** |
| Asthma/chronic pulmonary disease, n (%) | 14 (10.3) | 32 (15.0) | 0.20** |
| Liver disease, n (%) | 4 (2.9) | 18 (8.5) | 0.04** |
| Renal disease, n (%) | 41 (30.1) | 68 (31.9) | 0.73** |
| Chronic cardiac disease, n (%) | 54 (39.7) | 83 (39.0) | 0.89** |
| Chronic neurological disease/hemiplegia or paraplegia or dementia, n (%) | 12 (8.8) | 26 (12.2) | 0.32** |
| Rheumatological disease, n (%) | 1 (0.7) | 6 (2.8) | 0.25^^ |
| Any malignancy (solid tumors, leukemia or lymphoma), n (%) | 9 (6.6) | 25 (11.7) | 0.12** |
| Immunosuppressant use prior to admission, n (%) | 8 (5.9) | 13 (6.1) | 0.93** |
| Physiological parameters on ICU day 1 | |||
| SOFA score, median (Q1, Q3) | 9 (5, 11.5) | 9 (6, 13) | 0.14^ |
| Glascow coma score, median (Q1, Q3) | 11 (3, 15) | 10 (3, 15) | 0.70^ |
| Tidal volume (ml), median (Q1, Q3) | 394 (350, 433) | 400 (350, 450) | 0.37^ |
| PEEP (cmH20), median (Q1, Q3) | 12 (10, 15) | 10 (8, 14) | 0.14^ |
| Plateau pressure (cmH20), median (Q1, Q3) | 28 (23, 30) | 28 (22, 32) | 0.77^ |
| PaO2/FiO2 ratio, median (Q1, Q3) | 97 (64, 163) | 113 (69, 156) | 0.75^ |
| Mean arterial pressure (mmHg), median (Q1, Q3) | 71 (60, 83) | 68 (58, 79) | 0.04^ |
| White blood cell count (×109/L), median (Q1, Q3) | 6 (4, 10) | 7.7 (5, 12) | 0.07^ |
| Lactate (mmol/L), median (Q1, Q3) | 1.6 (1.0, 2.4) | 1.9 (1.2, 3.1) | 0.06^ |
| INR, median (Q1, Q3) | 1.1 (1.0, 1.2) | 1.2 (1.0, 1.4) | 0.0004^ |
| Creatinine (μmol/L), median (Q1, Q3) | 133.5 (74, 312) | 125.5 (75, 252) | 0.71^ |
| Bilirubin level (μmol/L), median (Q1, Q3) | 10 (6.8, 18) | 13 (7.8, 24) | 0.02^ |
| Platelets (×109/L), median (Q1, Q3) | 182 (120, 253) | 170 (113, 252) | 0.59^ |
| ICU intervention at day 1 | |||
| Non-invasive positive pressure ventilation, n (%) | 26 (19.1) | 25 (11.7) | 0.06** |
| Invasive ventilation, n (%) | 87 (64.0) | 127 (59.6) | 0.42** |
| High-frequency oscillation ventilation, n (%) | 1 (0.7) | 2 (0.9) | >0.99^^ |
| ECMO, n (%) | 2 (1.5) | 4 (1.9) | >0.99^^ |
| Nitric oxide, n (%) | 3 (2.2) | 2 (0.9) | 0.38^^ |
| Prone positioning, n (%) | 4 (2.9) | 6 (2.8) | >0.99^^ |
| Vasopressors, n (%) | 57 (41.9) | 103 (48.4) | 0.24** |
| Intravenous immunoglobin, n (%) | 2 (1.5) | 1 (0.5) | 0.56^^ |
| Renal replacement therapy, n (%) | 25 (18.4) | 34 (16.0) | 0.56** |
SOFA: sequential organ failure assessment, FiO2: denotes the fraction of inspired oxygen, PaO2: partial pressure of oxygen in arterial blood, PEEP: positive end-expiratory pressure, WBC: white blood cells, INR: international normalised ratio. For continuous variables, the ^Mann–Whitney U test was used to calculate P values except for those labelled with *, which indicates the use of Student’s t-test. For categorical variables, the **Chi-square test was used to calculate P values except for those labelled with ^^, which indicates the use of Fisher’s exact test.
There was no significant difference between the groups in terms of the number of days from onset of symptoms to emergency room presentation, to ICU admission and to intubation (Table 1).
Co-pathogens and other antibiotics
As shown in Supplementary Table 2, co-infection with a respiratory virus was confirmed in 21 patients (10 patients in the macrolide therapy group and 11 in the control group). Atypical co-pathogens were diagnosed in a small number of patients (legionella, 1; chlamydia, 1; mycoplasma, 3). Other antibiotics received by patients are shown in Supplementary Table 3.
Co-interventions
Throughout their stay in ICU, patients in the macrolide therapy group received more high-frequency oscillation ventilation (19/136; 14%) than those in the control group (7/213; 3.3%; P = 0.0002), oseltamiver therapy (99/136; 72.8% versus 97/213; 45.5%; P = 0.0001), intravenous immunoglobin (15/136; 11% versus 9/213; 4.2%; P = 0.01), and more renal replacement therapy (79/136; 58.1% versus 95/213; 44.6%; P = 0.01) (Table 2 ).
Table 2.
ICU course and outcomes among critically ill patients with Middle East Respiratory Syndrome (MERS) who received or did not receive macrolide therapy.
| Variables | Macrolide group (n = 136) |
No macrolides group (n = 213) |
P-value |
|---|---|---|---|
| Non-invasive positive pressure ventilation, n (%) | 49 (36.0) | 57 (26.8) | 0.07** |
| Invasive ventilation, n (%) | 117 (86.0) | 180 (84.5) | 0.70** |
| Neuromuscular blockade, n (%) | 59 (43.4) | 74 (34.7) | 0.11** |
| High-frequency oscillation ventilation, n (%) | 19 (14.0) | 7 (3.3) | 0.0002** |
| ECMO, n (%) | 10 (7.4) | 12 (5.6) | 0.52** |
| Nitric oxide, n (%) | 23 (16.9) | 21 (9.9) | 0.05** |
| Prone positioning, n (%) | 18 (13.2) | 15 (7.0) | 0.05** |
| Vasopressors, n (%) | 107 (78.7) | 169 (79.3) | 0.88** |
| Antivirals, n (%) | 118 (86.8) | 169 (79.3) | 0.08** |
| Oseltamivir, n (%) | 99 (72.8) | 97 (45.5) | < 0.0001** |
| Corticosteroids, n (%) | 76 (55.9) | 102 (47.9) | 0.15** |
| Intravenous immunoglobin, n (%) | 15 (11.0) | 9 (4.2) | 0.01** |
| Renal replacement therapy, n (%) | 79 (58.1) | 95 (44.6) | 0.01** |
| ICU mortality, n (%) | 81 (59.6) | 146 (68.5) | 0.09** |
| Hospital mortality, n (%) | 86 (63.2) | 151 (70.9) | 0.14** |
| 90-day mortality, n (%) | 82 (60.3) | 150 (70.4) | 0.05** |
| MERS-CoV RNA clearancea, days, median (Q1, Q3) | 26 (19, 33) | 21 (17, 28) | 0.93 |
| ICU length of stay, days, median (Q1, Q3) | 11 (6, 21) | 8 (5, 17) | 0.09^ |
| Hospital length of stay, days, median (Q1,Q3) | 16 (8.5, 34) | 20 (11, 35) | 0.08^ |
| Invasive ventilation duration, days, median (Q1, Q3) | 11 (6, 18) | 8 (4, 15) | 0.04^ |
ECMO: extracorporeal membrane oxygenation, ICU: intensive care unit.
For continuous variables, the ^Mann–Whitney U test was used to calculate P values except for those labelled with *, which indicates the use of Student’s t-test. For categorical variables, the **Chi-square test was used to calculate P values except for those labelled with ^^, which indicates the use of Fisher’s exact test.
Clearance of MERS-CoV rRT-PCR was calculated on patients who had at least one follow-up rRT-PCR test in the ICU from the date of ICU admission, and censored by the date of last test or death whichever comes first.
Clinical outcomes
Mortality
As shown in Table 2, there were no statistically significant differences between crude ICU and hospital mortality, 90-day mortality and ICU, and hospital LOS between the ‘macrolide therapy’ group and ‘no macrolide therapy’ group. After adjusting baseline variables, macrolide therapy was not associated with a reduction in 90-day mortality compared with the no macrolide therapy group (adjusted OR: 0.84; 95% CI: 0.47–1.51; P = 0.56) (Table 3 ). Sensitivity analysis excluding those patients who received macrolides after day 3 showed similar results (adjusted OR: 0.70; 95% CI: 0.39–1.28; P = 0.25).
Table 3.
Association of macrolide therapy with 90-day mortality and with MERS-CoV RNA clearance in critically ill patients with Middle East Respiratory Syndrome (MERS).
| Day-90 mortality |
MERS-CoV RNA clearancea |
|||||
|---|---|---|---|---|---|---|
| Variables | Logistic regression |
Cox proportional hazard model |
||||
| n | OR (95% CI) |
P-value | n | HR (95% CI) |
P-value | |
| Macrolides vs no macrolides (ref) | 268 | 0.84 (0.47, 1.51) |
0.56 | 137 | 0.88 (0.47, 1.64) |
0.68 |
| Macrolides vs no macrolides (ref) among survivors | 55 | 0.75 (0.35, 1.62) |
0.46 | |||
Clearance of MERS-CoV rRT-PCR was calculated on patients who had at least one follow-up rRT-PCR test in the ICU from the date of ICU admission, and censored by the date of last test or death whichever comes first. For the cox proportional analysis: Event – cleared, Censored – not cleared – Discharged Alive, Palliative Discharge, Still In Hospital, Transferred To Other Facility.
MERS-CoV RNA clearance
Cox proportional hazards model revealed that macrolide therapy was not associated with difference in MERS-CoV RNA clearance (adjusted HR: 0.88; 95% CI: 0.47–1.64; P = 0.68). Similar results were revealed when this analysis was restricted to survivors (adjusted HR: 0.75; 95% CI: 0.35–1.62; P = 0.46) (Table 3).
Discussion
This cohort study found no association between macrolide therapy and 90-day mortality or MERS-CoV RNA clearance among critically ill patients with MERS.
Few studies have investigated the association between macrolide therapy and mortality in patients with influenza or respiratory viral infections (Hung et al., 2017, Martin-Loeches et al., 2013, Walkey and Wiener, 2012). However, these results cannot necessarily be generalised to MERS. This study showed when adjusted for possible confounders using logistic regression analysis, macrolide therapy was not associated with a significant change in 90-day mortality. This may suggest that immunomodulatory effects of macrolides alone may not be effective on improving mortality rate in patients with MERS in the absence of effective antiviral drugs against MERS-CoV.
Although macrolides are known to downregulate inflammatory responses and reduce the excessive cytokine secretions that are associated with respiratory viral infections (Amsden, 2005, Beigelman et al., 2010, Kanoh and Rubin, 2010, Lendermon et al., 2017, Zarogoulidis et al., 2012), their direct effects on viral clearance in patients with respiratory viral infection are uncertain. An in vitro study on human bronchial epithelial cells showed that azithromycin significantly reduced rhinovirus replication and release (Gielen et al., 2010, Schogler et al., 2015). However, another study reported that azithromycin did not directly alter viral replication or clearance of parainfluenza type 1, Sendai Virus in mice (Beigelman et al., 2010). Similarly, in an RCT, azithromycin therapy in infants with severe RSV bronchiolitis did not facilitate RSV clearance from the upper airway compared to placebo (Beigelman et al., 2015). In an RCT, adults hospitalized for laboratory confirmed influenza were randomized to receive oseltamivir and azithromycin or oseltamivir alone, both for 5 days (Lee et al., 2017). Pro-inflammatory cytokines declined faster in the oseltamivir-azithromycin group. However, viral RNA decline was not affected (Lee et al., 2017). Our study is the first to have investigated the association between macrolide therapy and MERS-CoV RNA clearance. Our results showed that macrolide therapy had no significant association.
Previous studies demonstrated improvement of symptoms of respiratory viruses including influenza patients who were treated with macrolides (Ninomiya et al., 2002, Tahan et al., 2007, Higashi et al., 2014, Kakeya et al., 2014). Due to the nature of the population in our study (critically ill patients), this study does not measure the difference in symptoms improvement between the group who received macrolides and the group who did not receive macrolides. Measuring the potential improvement of MERS-CoV symptoms after macrolides therapy may be considered in future studies.
Our results should be interpreted in light of its strengths and weaknesses. This study is the first to examine the use of macrolide therapy in patients with MERS-CoV. It is derived from a collaborative, multicenter, observational database of critically ill patients with MERS-CoV, enhancing generalizability of our findings and mitigating the possibility of centre-related effect. This study also used an international standardised data collection tool. We adjusted for possible confounders including community-acquired infection, because of the probable association of this variable with both the exposure (macrolide therapy) and outcomes. Since macrolide therapy was initiated early during the ICU course, the risk of immortal time bias (survivorship bias) is probably limited. The main limitation is the retrospective nature of this observational study, and the potential that unmeasured confounders may influence the relationships we sought to examine. Also we did not measure cytokine levels or immune measures that might have been affected by macrolide administration.
Conclusions
In our study, more than one-third of critically ill patients with MERS-CoV received empiric therapy with macrolides. However, we found that macrolide therapy is not significantly associated with a reduction in 90-day mortality or improvement in MERS-CoV RNA clearance.
Acknowledgements
We would like to acknowledge the International Severe Acute Respiratory and Emerging Infection Consortium (ISARIC) for their support in the database.
Acknowledgments
Funding
There was no specific funding to this project, and data have been generated as part of the routine work of the participating organizations.
Competing interests
The authors declare that they have no competing interests. Dr Arabi is the principal investigator on the MIRACLE trial: MERS-CoV Infection tReated With A Combination of Lopinavir/Ritonavir and Interferon Beta-1b (ClinicalTrials.gov Identifier: NCT02845843). He and FGH are also nonpaid consultants on antivirals active for MERS for Gilead Sciences, SAB Biotherpautics and Regeneron.
Ethical approval
The study was approved by the institutional review boards of all participating centers. Patient-level informed consent was not required due to its observational nature.
Corresponding Editor: Eskild Petersen, Aarhus, Denmark
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.ijid.2019.01.041.
Contributor Information
Yaseen M. Arabi, Email: arabi@ngha.med.sa.
Ahmad M. Deeb, Email: rn_a_deeb@hotmail.com.
Fahad Al-Hameed, Email: Hameedf@ngha.med.sa.
Yasser Mandourah, Email: Yasser.mandourah@me.com.
Ghaleb A. Almekhlafi, Email: gmekhlafi@yahoo.com.
Anees A. Sindi, Email: ansindi@gmail.com.
Awad Al-Omari, Email: dr_awad_ksa@yahoo.com.
Sarah Shalhoub, Email: sarah.shalhoub@googlemail.com.
Ahmed Mady, Email: afmady@hotmail.com.
Basem Alraddadi, Email: basemalraddadi@gmail.com.
Abdullah Almotairi, Email: aalmotairi@kfmc.med.sa.
Kasim Al Khatib, Email: kasimalkhatib@yahoo.com.
Ahmed Abdulmomen, Email: aturk@ksu.edu.sa.
Ismael Qushmaq, Email: iqushmaq@kfshrc.edu.sa.
Othman Solaiman, Email: omsmd@yahoo.com.
Abdulsalam M. Al-Aithan, Email: AithanA@ngha.med.sa.
Rajaa Al-Raddadi, Email: saudiresearcher@yahoo.com.
Ahmad Ragab, Email: ahmadragab63@hotmail.com.
Abdulrahman Al Harthy, Email: a_almshal@hotmail.com.
Ayman Kharaba, Email: a7yman@hotmail.com.
Jesna Jose, Email: joseje@ngha.med.sa.
Tarek Dabbagh, Email: DabbaghT@ngha.med.sa.
Robert A. Fowler, Email: rob.fowler@sunnybrook.ca.
Hanan H. Balkhy, Email: BalkhyH@ngha.med.sa.
Laura Merson, Email: laura.merson@ndm.ox.ac.uk.
Frederick G. Hayden, Email: fgh@virginia.edu.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Amsden G.W. Anti-inflammatory effects of macrolides—an underappreciated benefit in the treatment of community-acquired respiratory tract infections and chronic inflammatory pulmonary conditions? J Antimicrob Chemother. 2005;55(1):10–21. doi: 10.1093/jac/dkh519. [DOI] [PubMed] [Google Scholar]
- Arabi Y.M., Al-Omari A., Mandourah Y., Al-Hameed F., Sindi A.A., Alraddadi B. Critically ill patients with the middle east respiratory syndrome: a multicenter retrospective cohort study. Crit Care Med. 2017;45(10):1683–1695. doi: 10.1097/CCM.0000000000002621. [DOI] [PubMed] [Google Scholar]
- Beigelman A., Bacharier L.B., Baty J., Buller R., Mason S., Schechtman K.B. Does azithromycin modify viral load during severe respiratory syncytial virus bronchiolitis? J Allergy Clin Immunol. 2015;136(4):1129–1131. doi: 10.1016/j.jaci.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beigelman A., Mikols C.L., Gunsten S.P., Cannon C.L., Brody S.L., Walter M.J. Azithromycin attenuates airway inflammation in a mouse model of viral bronchiolitis. Respir Res. 2010;11:90. doi: 10.1186/1465-9921-11-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermejo-Martin J.F., Kelvin D.J., Eiros J.M., Castrodeza J., Ortiz de Lejarazu R. Macrolides for the treatment of severe respiratory illness caused by novel H1N1 swine influenza viral strains. J Infect Dev Ctries. 2009;3(3):159–161. doi: 10.3855/jidc.18. [DOI] [PubMed] [Google Scholar]
- Gielen V., Johnston S.L., Edwards M.R. Azithromycin induces anti-viral responses in bronchial epithelial cells. Eur Respir J. 2010;36(3):646–654. doi: 10.1183/09031936.00095809. [DOI] [PubMed] [Google Scholar]
- Higashi F., Kubo H., Yasuda H., Nukiwa T., Yamaya M. Additional treatment with clarithromycin reduces fever duration in patients with influenza. Respir Investig. 2014;52(5):302–309. doi: 10.1016/j.resinv.2014.05.001. [DOI] [PubMed] [Google Scholar]
- Hung I.F.N., To K.K.W., Chan J.F.W., Cheng V.C.C., Liu K.S.H., Tam A. Efficacy of clarithromycin-naproxen-oseltamivir combination in the treatment of patients hospitalized for influenza A(H3N2) infection: an open-label randomized, controlled, phase IIb/III trial. Chest. 2017;151(5):1069–1080. doi: 10.1016/j.chest.2016.11.012. [DOI] [PubMed] [Google Scholar]
- ISARIC. The international severe acute respiratory and emerging infection consortium case report forms. Available from: https://isaric.tghn.org/. [Accessed 1 November 2018].
- Kakeya H., Seki M., Izumikawa K., Kosai K., Morinaga Y., Kurihara S. Efficacy of combination therapy with oseltamivir phosphate and azithromycin for influenza: a multicenter, open-label, randomized study. PLoS One. 2014;9(3) doi: 10.1371/journal.pone.0091293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanoh S., Rubin B.K. Mechanisms of action and clinical application of macrolides as immunomodulatory medications. Clin Microbiol Rev. 2010;23(3):590–615. doi: 10.1128/CMR.00078-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneyber M.C., van Woensel J.B., Uijtendaal E., Uiterwaal C.S., Kimpen J.L. Azithromycin does not improve disease course in hospitalized infants with respiratory syncytial virus (RSV) lower respiratory tract disease: a randomized equivalence trial. Pediatr Pulmonol. 2008;43(2):142–149. doi: 10.1002/ppul.20748. [DOI] [PubMed] [Google Scholar]
- Lee N., Wong C.K., Chan M.C.W., Yeung E.S.L., Tam W.W.S., Tsang O.T.Y. Anti-inflammatory effects of adjunctive macrolide treatment in adults hospitalized with influenza: a randomized controlled trial. Antiviral Res. 2017;144:48–56. doi: 10.1016/j.antiviral.2017.05.008. [DOI] [PubMed] [Google Scholar]
- Lendermon E.A., Coon T.A., Bednash J.S., Weathington N.M., McDyer J.F., Mallampalli R.K. Azithromycin decreases NALP3 mRNA stability in monocytes to limit inflammasome-dependent inflammation. Respir Res. 2017;18(1):131. doi: 10.1186/s12931-017-0608-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Loeches I., Bermejo-Martin J.F., Valles J., Granada R., Vidaur L., Vergara-Serrano J.C. Macrolide-based regimens in absence of bacterial co-infection in critically ill H1N1 patients with primary viral pneumonia. Intensive Care Med. 2013;39(4):693–702. doi: 10.1007/s00134-013-2829-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallum G.B., Morris P.S., Chatfield M.D., Maclennan C., White A.V., Sloots T.P. A single dose of azithromycin does not improve clinical outcomes of children hospitalised with bronchiolitis: a randomised, placebo-controlled trial. PLoS One. 2013;8(9) doi: 10.1371/journal.pone.0074316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min J.Y., Jang Y.J. Macrolide therapy in respiratory viral infections. Mediators Inflamm. 2012;2012 doi: 10.1155/2012/649570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosquera R.A., Stark J.M., Atkins C.L., Colasurdo G.N., Chevalier J., Samuels C.L. Functional and immune response to respiratory syncytial virus infection in aged BALB/c mice: a search for genes determining disease severity. Exp Lung Res. 2014;40(1):40–49. doi: 10.3109/01902148.2013.859334. [DOI] [PubMed] [Google Scholar]
- Nakamura H., Fujishima S., Inoue T., Ohkubo Y., Soejima K., Waki Y. Clinical and immunoregulatory effects of roxithromycin therapy for chronic respiratory tract infection. Eur Respir J. 1999;13(6):1371–1379. doi: 10.1183/09031936.99.13613809. [DOI] [PubMed] [Google Scholar]
- NIH. Macrolide antibiotics; 2017. Available from: https://livertox.nih.gov/MacrolideAntibiotics.htm. [Accessed 1 November 2018].
- Ninomiya K., Fukui T., Imai T., Matsui M., Matsuoka K. Effect of maclorides on duration and resolution of symptoms and complication of pneumonia in children with influenza. J Nippon Med Sch = Nippon Ika Daigaku zasshi. 2002;69(1):53–57. doi: 10.1272/jnms.69.53. [DOI] [PubMed] [Google Scholar]
- Pinto L.A., Pitrez P.M., Luisi F., de Mello P.P., Gerhardt M., Ferlini R. Azithromycin therapy in hospitalized infants with acute bronchiolitis is not associated with better clinical outcomes: a randomized, double-blinded, and placebo-controlled clinical trial. J Pediatr. 2012;161(6):1104–1108. doi: 10.1016/j.jpeds.2012.05.053. [DOI] [PubMed] [Google Scholar]
- Schogler A., Kopf B.S., Edwards M.R., Johnston S.L., Casaulta C., Kieninger E. Novel antiviral properties of azithromycin in cystic fibrosis airway epithelial cells. Eur Respir J. 2015;45(2):428–439. doi: 10.1183/09031936.00102014. [DOI] [PubMed] [Google Scholar]
- Shinahara W., Takahashi E., Sawabuchi T., Arai M., Hirotsu N., Takasaki Y. Immunomodulator clarithromycin enhances mucosal and systemic immune responses and reduces re-infection rate in pediatric patients with influenza treated with antiviral neuraminidase inhibitors: a retrospective analysis. PLoS One. 2013;8(7) doi: 10.1371/journal.pone.0070060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T., Yamaya M., Sekizawa K., Hosoda M., Yamada N., Ishizuka S. Erythromycin inhibits rhinovirus infection in cultured human tracheal epithelial cells. Am J Respir Crit Care Med. 2002;165(8):1113–1118. doi: 10.1164/ajrccm.165.8.2103094. [DOI] [PubMed] [Google Scholar]
- Tahan F., Ozcan A., Koc N. Clarithromycin in the treatment of RSV bronchiolitis: a double-blind, randomised, placebo-controlled trial. Eur Respir J. 2007;29(1):91–97. doi: 10.1183/09031936.00029206. [DOI] [PubMed] [Google Scholar]
- Us D. Cytokine storm in avian influenza. Mikrobiyol Bul. 2008;42(2):365–380. [PubMed] [Google Scholar]
- Vincent J.L., de Mendonca A., Cantraine F., Moreno R., Takala J., Suter P.M. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit Care Med. 1998;26(11):1793–1800. doi: 10.1097/00003246-199811000-00016. [DOI] [PubMed] [Google Scholar]
- Wales D., Woodhead M. The anti-inflammatory effects of macrolides. Thorax. 1999;54(Suppl. 2):S58–62. doi: 10.1136/thx.54.2008.s58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walkey A.J., Wiener R.S. Macrolide antibiotics and survival in patients with acute lung injury. Chest. 2012;141(5):1153–1159. doi: 10.1378/chest.11-1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Nikrad M.P., Travanty E.A., Zhou B., Phang T., Gao B. Innate immune response of human alveolar macrophages during influenza A infection. PLoS One. 2012;7(3) doi: 10.1371/journal.pone.0029879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarogoulidis P., Papanas N., Kioumis I., Chatzaki E., Maltezos E., Zarogoulidis K. Macrolides: from in vitro anti-inflammatory and immunomodulatory properties to clinical practice in respiratory diseases. Eur J Clin Pharmacol. 2012;68(5):479–503. doi: 10.1007/s00228-011-1161-x. [DOI] [PubMed] [Google Scholar]
- Zhang C., Xu Y., Jia L., Yang Y., Wang Y., Sun Y. A new therapeutic strategy for lung tissue injury induced by influenza with CR2 targeting complement inhibitor. Virol J. 2010;7:30. doi: 10.1186/1743-422X-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.