Abstract
Respiratory viral infections (RVI) are important in hematopoietic stem cell transplantations (HSCT) and knowledge regarding incidence, morbidity, mortality, and long-term pulmonary complications is limited. We report a study to evaluate incidence and outcomes, both short and long-term, of RVI in children receiving HSCT. Between January 2000 and December 2012, 844 patients underwent hematopoietic stem cell transplantation (HSCT) at the Hospital for Sick Children: 491 were allogeneic and 353 were autologous. When screening for causes of death in the first year after HSCT in the 844 patients, we found that RVI as a cause of death was only evident in the first 100 days after HSCT. Fifty-four (6.5%) patients were found to have an RVI within the first 100 days after HSCT (allogeneic = 32, autologous = 22). Upper and lower respiratory tract infections were documented in 31 (57%) and 23 (43%) patients, respectively. Viruses were parainfluenza (35%), respiratory syncytial virus (28%), influenza (22%), adenovirus (7%), human metapneumovirus (4%), coronavirus (2%), and rhinovirus (2%). Three patients relapsed with their primary disease before day 100 and were excluded. The overall mortality for the remaining 51 patients was 10% (allogeneic = 4, autologous = 1). All 5 deaths were directly attributable to RVI and all 5 deaths occurred in patients with a lower respiratory tract infection. The remaining patients were followed for a median of 4.3 years (range, 1.4 to 11.8) and no chronic pulmonary complications were observed. A clear seasonal pattern for contracting RVI was evident with 65% of total RVI occurring between October and March (35 of 427 versus 19 of 417, P = .03). Given the significant mortality from RVI and the challenges in preventing them, choosing the time to start HSCT, whenever possible, may help prevent RVI and improve outcomes.
Key Words: Respiratory virus infection, Hematopoietic stem cell transplantation, Children, Mortality
Highlights
-
•
We found significant mortality of respiratory virus infection in the first 100 days after hematopoietic stem cell transplantation
-
•
We attempted to identify risk factors for adverse outcome of respiratory virus infection after hematopoietic stem cell transplantation
-
•
We found a seasonal pattern of respiratory virus infection in children after hematopoietic stem cell transplantation in Canada
Introduction
Infections are 1 of the main complications after hematopoietic cell transplantation (HSCT) and respiratory viral infections (RVI) are being increasingly recognized as important complications after HSCT with variable degrees of morbidity and mortality 1, 2, 3, 4. In recent years, the growing number of HSCT utilizing alternative donors and cord progenitor stem cells, along with the common use of serotherapy, has contributed to a significant increase in viral infections after HSCT 5, 6. Some well-known viruses, such herpes simplex virus (HSV) and cytomegalovirus (CMV), were major causes of morbidity and mortality in HSCT 7, 8. However, since prophylactic or preemptive anti-HSV/CMV therapy became standard practice, morbidity and mortality from HSV or CMV disease are rare 9, 10. Nonetheless, respiratory viruses such as influenza, parainfluenza, and respiratory syncytial virus (RSV) continue to be a major cause of morbidity and mortality in patients after HSCT and there is increased awareness for the diagnosis of these infections. However, no prophylactic approach for such viruses exists in HSCT recipients. Hence, this study's aim was to investigate the incidence and risk factors of RVI in children after HSCT and describe the short- and long-term outcomes of these patients.
Patients and Methods
This study was approved by our institutional research ethics board. The medical records of children who received HSCT from January 2000 to December 2012 at the Hospital for Sick Children, Toronto were reviewed to identify those patients who had a positive test for respiratory virus obtained by nasopharyngeal aspirate (NPA), bronchoalveolar lavage (BAL), pleural tap, or lung biopsy specimen. Microbiological testing included the following methodologies: (1) virus isolation in cell culture (2000 to 2007 for NPA and 2000 to 2011 for BAL and biopsies) for influenza A and B, RSV, parainfluenza virus 1 through 3, and adenovirus; (2) direct fluorescent antibody testing (2000 to 2012) on all NPAs, bronchoscopic BALs for 8 viruses (influenza A and B, RSV, parainfluenza virus 1 through 3, adenovirus, and human metapneumovirus (hMPV); (3) PCR for the pandemic influenza strain (May to December 2009); (4) PCR for adenovirus (2002 to 2012); and (5) multiplex PCR (May 2011 to December 2012) for influenza A and B, RSV, parainfluenza virus 1 through 4, adenovirus, hMPV, enterovirus, rhinovirus, bocavirus, and coronaviruses. Data collected included patient demographics, indication for HSCT, type of transplantation (allogeneic or autologous), graft type, conditioning regimen, donor details for allogeneic patients, graft-versus-host disease (GVHD) prophylaxis, time from HSCT to positive respiratory virus result, clinical course, transplantation-related mortality (TRM) and cause, long-term pulmonary complications, and overall survival.
Definitions
The following definitions were used in the study. An upper respiratory tract infection (URTI) was defined as detection of an RVI from upper respiratory secretions together with symptoms from the upper respiratory tract (nose and throat). A lower respiratory tract infection (LRTI) was defined as either hypoxia or pulmonary infiltrates together with identification of an RVI in upper respiratory secretion, BAL, pleural tap, or lung biopsy. The day of engraftment was defined as the first of 3 consecutive days in which the peripheral absolute neutrophil count was > .5 × 109/L. TRM was defined as being either due to an RVI or other transplantation toxicities. Death due to RVI was defined as death from respiratory failure with no other cause of the pneumonia. Long-term pulmonary complication was defined as any limitation of pulmonary status, including pulmonary function tests. Relapse was defined by the finding of hematologic or cytogenetic recurrence or by the initiation of therapy for recurrence. Progressive disease was defined as tumor growth of more than 20% or spreading since undergoing transplantation [11].
Supportive Care
All patients were nursed in protective isolation in single rooms with high-efficiency particulate air filters. All patients had indwelling central venous catheters and the majority received nutritional support with nasogastric feeding or total parenteral nutrition. Infection prophylaxis included fluconazole for fungal prophylaxis, ganciclovir for CMV prophylaxis (2000 to 2005), and preemptive strategy from 2005 onwards. Growth factors were given as indicated, and laminar air flow rooms were used from day 0 until engraftment. Intravenous immunoglobulin at a dose of .5 g/kg was supplemented if the IgG level was less than 400 mg/dL. The IgG level was routinely monitored every week in all patients. Pneumocystis jiroveci prophylaxis was given before HSCT and after HSCT for at least 6 months. Pneumococcal prophylaxis with penicillin continued for at least 1 year or until the administration of pneumococcal vaccine. Blood products were transfused to maintain hemoglobin concentration > 70 mg/L and platelet counts of 20,000/mm3. Fever during the neutropenic phase was treated with broad-spectrum antibiotics and amphotericin or caspofungin, as necessary, and modified subsequently according to the results of blood or tissue cultures.
Clinical Follow-Up
Patients received their preparatory regimen, underwent transplantation, and recovered in the hospital, where they were examined by the medical team twice each day until they achieved engraftment. All patients with respiratory symptoms were investigated for the presence of respiratory viruses. Chest radiographs and computed tomography scans were obtained if clinically indicated. NPAs, throat swaps, or nasal swabs were used for obtaining upper respiratory specimens. BAL or pleural tap and/or lung biopsy were used for obtaining lower respiratory specimens.
Endpoints
The primary endpoint of the study was to review all RVI-positive patients after HSCT and describe their outcome in terms of TRM and long-term pulmonary complications.
Statistics
Univariate logistic regression models were constructed to analyze the impact of risk factors on TRM within the first 100 days, including transplantation type (allogeneic versus autologous), graft type (bone marrow versus peripheral blood stem cells versus cord blood), utilization of serotherapy (antithymocyte globulin, Alemtuzumab [MabCampath; Genzyme Canada Inc., Mississauga, Ontario], and no serotherapy), and underlying disorder (malignant versus benign). All reported P values were 2-sided, and a significance level of .05 was used. All statistical analyses were carried out using SPSS version 12.5.
Results
Eight hundred forty-four patients underwent HSCT at the Hospital for Sick Children, Toronto, Canada during the study period and comprise the study population. The transplant recipients consisted of 341 female and 503 male patients, with a median age of 7.5 years (range, 1 month to 17.8 years). The HSCT was allogeneic in 491 patients and autologous in 353 patients. The diseases for which HSCT was performed were acute lymphoblastic leukemia (n = 138), acute myeloid leukemia (n = 110), myelodysplastic syndrome (n = 21), severe aplastic anemia (n = 49), inherited marrow failure (n = 29), primary immune deficiency (n = 58), non-Hodgkin lymphoma (n = 44), Hodgkin disease (n = 28), chronic myelogenous leukemia (n = 16), metabolic diseases (n = 25), hemophagocytic lymphohistiocytosis (n = 17), brain tumors (n = 103), solid tumors (n = 179), thalassemia (n = 15), and others (n = 12).
Incidence of RVI
During the study period and among the 844 HSCT recipients, 96 patients with RVI were documented. Screening for causes of death in the 96 patients, we found that RVI was never the cause of death after day 100 after HSCT. In the first 100 days after HSCT, there were 54 patients with RVI. As these 54 patients were the focus of the study, their diagnosis and conditioning regimen including use of serotherapy are described in Table 1 . Only 1 patient with diagnosis of severe combined immune deficiency (SCID)/Omenn's syndrome received conditioning with busulfan and cyclophosphamide. The remaining 5 SCID patients did not receive any conditioning regimen. When analyzing utilization of serotherapy, we found 11 patients who received antithymocyte globulin and 1 who received alemtuzumab.
Table 1.
Characteristics of Patients with Identified Respiratory Virus Infections
| Characteristic | Value |
|---|---|
| Age, median (range) | 7.5 yr (1 mo-17.8 yr) |
| Diagnosis | |
| Malignant disorders | 38 |
| Neuroblastoma | 12 |
| Acute lymphoblastic leukemia | 6 |
| Acute myeloid leukemia | 6 |
| Medulloblastoma | 5 |
| Hemophagocytic lymphohistiocytosis | 3 |
| Non-Hodgkin lymphoma | 1 |
| Hodgkin lymphoma | 1 |
| MDS | 1 |
| Atypical rhabdoid/teratoid tumor | 1 |
| Pineoblastoma | 1 |
| Ependymoblastoma | 1 |
| Benign disorders | 16 |
| SCID | 6 |
| Severe aplastic anemia | 3 |
| Wiskott Aldrich syndrome | 3 |
| Thalassemia | 1 |
| Sickle cell disease | 1 |
| Hurler's syndrome | 1 |
| Fanconi's anemia | 1 |
| Type of transplant | |
| Allogeneic | 32 |
| HLA-identical sibling | 14 |
| Family mismatch | 1 |
| Unrelated donor | 17 |
| Autologous | 22 |
| Conditioning regimen | |
| TBI + chemotherapy | 6 |
| Chemotherapy alone | 43 |
| No conditioning regimen | 5 |
| Serotherapy | 12 |
| ATG | 11 |
| Alemtuzumab | 1 |
| None given | 42 |
| Graft type | |
| Bone marrow | 25 |
| Peripheral blood stem cells | 22 |
| Cord blood | 7 |
| Type of viral infection | |
| Parainfluenza | 19 |
| RSV | 15 |
| Influenza A | 8 |
| Influenza B | 4 |
| Adenovirus | 4 |
| hMPV | 2 |
| Rhinovirus | 1 |
| Coronavirus | 1 |
MDS indicates myelodysplastic syndrome; TBI, total body irradiation; ATG, antithymocyte globulin.
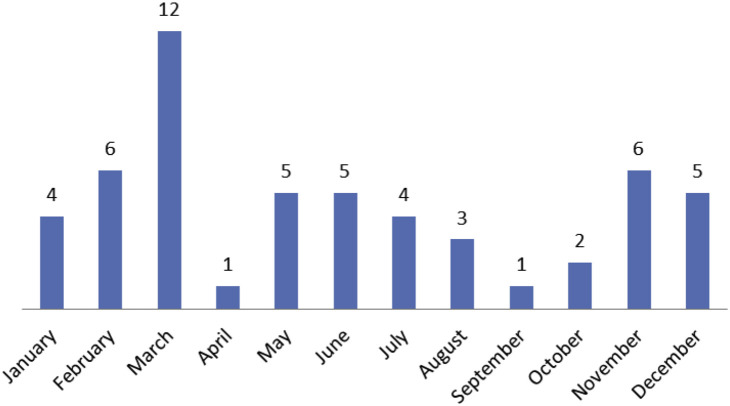
The overall frequency of documented RVI in the first 100 days after HSCT was 6.4%. The frequency was slightly higher in allogeneic (6.5%) than in autologous (6.2%) transplantations (P = .867). Twenty-three patients had LRTI: 17 occurring in allogeneic patients and 6 in autologous HSCT patients. The frequency of a LRTI was 3.5% in allogeneic and 1.7% in autologous HSCT patients. Nineteen patients had parainfluenza infections (11 allogeneic, 8 autologous). Six patients had LRTI and 13 patients had URTI. The median time to diagnosis of parainfluenza infection was 25 days (range, 1 to 81 days) after HSCT. One patient with LRTI was treated was with ribavirin. Fifteen patients had RSV infection (9 allogeneic, 6 autologous). Eight had LRTI and 7 had URTI. The median time to diagnosis of RSV infection was 14 days (range, 1 to 52 days) after HSCT. Six patients with LRTI were treated with ribavirin immediately after RSV was identified. Health Canada approved palivizumab (RSV Ig) in 2012 and only 1 patient received it. Twelve patients had influenza infection (7 allogeneic, 5 autologous). Eight patients had influenza A and 4 had influenza B. Four patients had LRTI and 8 patients had URTI. The median time to diagnosis of influenza infection was 31 days (range, 1 to 100 days) after HSCT. All 12 patients received oseltamivir. Four patients had adenovirus infections (3 allogeneic, 1 autologous) proven by adenovirus PCR positive from BAL or pleural fluid and/or lung biopsy. All of them had LRTI and subsequently developed respiratory distress. The median time to diagnosis of adenovirus infection was 35 days (range, 8 to 100 days) after HSCT. All patients with adenovirus received cidofovir therapy. The remaining 4 patients (2 allogeneic, 2 autologous) were documented to have hMPV (2 patients), coronavirus (1 patient) and rhinovirus infections (1 patient). Of the 32 patients who received allogeneic HSCT, 12 were receiving therapy for acute GVHD grade II to IV. The number of patients who contracted RVI and the type of viruses are summarized in Table 1. The seasonal pattern of RVI is shown in Figure 1 , with the number of patients who contracted RVI in each month displayed. Thirty-five of the 54 total RVI were contracted between October and March, which is the high flu season in Canada. with March being the worst month. Comparing positive RVI patients versus negative patients, there was a statistically significant difference for contracting RVI in the high season versus the low season (35 of 427 versus 19 of 417, P = .03).
Figure 1.
Seasonal pattern of 54 patients with RVI.
Outcome of RVI in the First 100 Days after HSCT
Eleven patients required mechanical ventilation (parainfluenza, 4; influenza, 3; RSV, 2; adenovirus, 2). Four allogeneic and 1 autologous HSCT recipients died within 100 days after transplantation because of the RVI and all of them were mechanically ventilated. Thus, the overall mortality was 10.0% (13.3% allogeneic and 4.8% autologous, P = .304). None of the 4 allogeneic transplant recipients who died had acute GVHD. Two of the 19 patients with parainfluenza infections died (1 patient was treated with ribavirin). Two additional patients with parainfluenza infection died; 1 from liver veno-occlusive disease and another from pulmonary hypertension. Thus, the mortality caused by parainfluenza was 10.5% and the total mortality with a diagnosis of parainfluenza was 21%. For those with adenovirus respiratory infection (n = 4), 2 patients died of adenovirus infections. The mortality caused by adenovirus infection was 50%. One of 15 patients died of RSV infection. The mortality caused by RSV infection was 6.7% and that patient was treated with ribavirin. None of 12 patients with influenza, hMPV, coronavirus, and rhinovirus infections died from respiratory complications. After day 100 after HSCT, 3 more patients died from TRM but death was not related to respiratory problems. The remaining 43 patients were followed for a median of 4.3 years (range, 1.4 to 11.8). No chronic pulmonary complication or allo-immune lung syndrome was observed.
Details of Patients Who Died from RVI (Table 2)
Table 2.
Details of Patients who Died from RVI after HSCT
| Diagnosis | Age at HSCT | Conditioning Regimen | Stem Cell | Respiratory Viruses | Interval, HSCT to Infection | Cause of Death |
|---|---|---|---|---|---|---|
| Severe aplastic anemia | 11 yr | Fludarabine/cyclophosphamide/ATG | Unrelated cord 5/6 | Adenovirus | 98 d | Pneumonitis, ARDS |
| Neuroblastoma, stage 4 | 18 mo | Carboplatin/etoposide/melphalan | Autologous | Adenovirus | 21 d | Pneumonitis, ARDS |
| SCID | 2 mo | Busulfan/cyclophosphamide | Matched sibling BM | RSV | 11 d | Pneumonitis, ARDS |
| Relapsed AML | 2 yr | Busulfan/cyclophosphamide | Matched sibling BM | Parainfluenza type 1 | 19 d | Pneumonitis, ARDS, pulmonary hypertension |
| Wiskott-Aldrich syndrome | 1 yr | Busulfan/cyclophosphamide/ATG | Unrelated cord 6/6 | Parainfluenza type 3 | 24 d | Pneumonitis, ARDS, pulmonary hypertension |
AML indicates acute myeloid leukemia; BM, bone marrow.
Parainfluenza
Two patients died from parainfluenza LRTI. A 1-year-old patient with Wiskott-Aldrich syndrome who underwent unrelated cord blood transplantation developed respiratory distress on day 24 after transplantation. NPA was positive for parainfluenza type 3 antigen. Ribavirin, nitric oxide, and high-frequency oscillator support were given. The second patient was a 2-year-old boy with diagnosis of relapse acute myeloid leukemia who underwent matched sibling bone marrow transplantation. Parainfluenza type 1 antigen was positive from NPA on day 19 after HSCT. This patient developed acute respiratory distress syndrome (ARDS) with radiographic findings consistent with pneumonitis.
RSV
A 2-month-old patient with diagnosis of SCID with Omenn's syndrome underwent matched sibling bone marrow transplantation. This patient developed ARDS from RSV pneumonitis on day 11 after HSCT.
Adenovirus
Two patients died from adenovirus pneumonitis and ARDS. An 18-month-old patient with diagnosis of neuroblastoma stage 4 underwent autologous HSCT after high-dose chemotherapy. This patient had delayed engraftment and developed ARDS, which was positive for adenovirus from BAL and pleural fluid. Another patient was an 11-year-old boy who underwent unrelated cord blood transplantation for severe aplastic anemia. This patient had primary graft failure, subsequently developed respiratory distress, and required high-frequency oscillation. Adenovirus was positive from BAL and lung biopsy.
Univariate Analysis
Type of transplantation, graft type, utilization of serotherapy, and underlying malignant disorder were analyzed using univariate analysis. None of these factors were significantly correlated with TRM (type of transplantation [P = .347], graft type [P = .213], serotherapy [P = .405], and underlying malignant disorder [P = .156]).
Discussion
In this large pediatric study, we found that 6.5% of patients receiving allogeneic or autologous HSCT had a positive RVI that led to 10% overall mortality in the first 100 days after HSCT. Mortality was higher for allogeneic recipients: 13.3% compared with 4.8% for autologous recipients. Furthermore, for a median follow-up of 4.3 years (range, 1.4 to 11.8) patients who tested positive for respiratory virus but did not die had no long-term pulmonary complications and patients who contracted the virus after 100 days after HSCT did not have an adverse event.
The total incidence of RVI in this study was consistent within the range of 5% to 11% by previous published data of pediatric HSCT recipients 12, 13. However, our study particularly reported the incidence of RVI within the first 100 days after transplantation, as it is a very distinct pattern in our population and different from aforementioned studies. Our data showed that RVI episodes were clustering within the first 100 days after transplantation. Only 1 patient developed influenza A URTI at 5 months after HSCT but no serious respiratory complication was observed. Similar to patients who were diagnosed with URTI and LRTI after 6 months after HSCT, none had prolonged admission or died from respiratory illnesses.
We supplemented our routine viral respiratory diagnostics with PCR testing (adenovirus PCR, pandemic influenza PCR, multiplex respiratory PCR), if requested by the treating physician. PCR has been proven to be a more specific, sensitive, and rapid method for detecting respiratory viruses compared with antigen testing and viral culture and was likely responsible for increasing our rates of detection, though changes in methodology over the study time frame mean that testing algorithms were applied differently as testing methodologies changed 14, 15, 16.
Even with a lower incidence of adenovirus compared with parainfluenza and RSV infections in this study, the respiratory-related mortality was higher for adenovirus, ie, 50%. All 4 patients were confirmed to have a positive adenovirus PCR from either BAL or pleural fluid and lung biopsy after diagnosis of LRTI. Moreover, 2 patients who died from severe pneumonitis and ARDS with a positive adenovirus PCR test did not have another apparent cause of respiratory illness. It was described that disseminated adenovirus infection can cause pulmonary consolidation, such as bacterial pneumonia 17, 18. Because of similar clinical and radiographic findings, adenovirus infection may be under-recognized as a cause of fatal pneumonia in immunocompromised patients with severe lymphopenia or lymphocyte dysfunction. Likewise, our 2 patients with adenovirus infection both had delayed engraftment.
Adenovirus infection is associated with high mortality, up to 100% in the pediatric HSCT 19, 20, 21. Routine monitoring for adenoviremia is recommended after HSCT because of asymptomatic reactivation in most cases [22]. Cidofovir is widely used for both preemptive and therapeutic treatment of adenovirus infection despite well-recognized toxicities 23, 24. Identification of patients at risk of adenovirus infection is very crucial, as immune reconstitution is a key factor for reactivation. Rapid withdrawal of immunosuppressive medications should also be considered whenever possible [25].
Respiratory-related mortality from parainfluenza virus in this study is lower, 10.5%, than in other studies, which showed approximately 30% to 70%. This could be explained by very early years when transplantations were completed and the mainly adult study population in previous reports 26, 27, 28, 29. A large retrospective cohort study from Fred Hutchinson Cancer Research Center described unrelated transplantation as the major risk factor for parainfluenza virus acquisition as well the use of high-dose corticosteroids [29]. For our 2 patients who died from parainfluenza virus pneumonia, 1 with diagnosis of Wiskott-Aldrich syndrome received methylprednisolone as GVHD prophylaxis for an unrelated cord blood transplantation. However, another patient with relapsed acute myeloid leukemia did not develop GVHD or receive any corticosteroids treatment. Aerosolized ribavirin was given to the first patient but it was given later during the course of pneumonia. The benefit of using aerosolized ribavirin in the treatment of parainfluenza pneumonia among HSCT recipients is controversial 26, 28. The early diagnosis of parainfluenza infection could expedite ribavirin treatment initiation and improve the outcome [25].
The incidence of RSV infection in this study was 28% and LRTI composed more than one half of all RSV infections. Interestingly, the mortality is only 6.7%, which is lower than 20% to 40% in other studies in HSCT recipients [1]. Treatment with aerosolized ribavirin in 6 of 7 patients with RSV LRTI may potentially be the reason for lowered mortality, although 1 fatal case was observed after the patient received ribavirin. HSCT recipients have high potential for progression from URTI to severe LRTI without well-defined and specific risk factors for death 4, 30, 31. A pooled analysis by Shah and Chemaly suggested that ribavirin treatment with/without RSV-specific immunoglobulin or palivizumab had lower rates of mortality by almost one-third in a review of 21 separate studies [32]. Another small retrospective analysis of palivizumab given for RSV URTI did not show protective effect of progression to LRTI 33, 34, 35.
No death associated with influenza, hMPV, rhinovirus, and coronavirus infections was observed in this study. High mortality between 15% and 70% has been reported in HSCT recipients with influenza infection, but recent data showed an improvement with the availability of neuraminidase inhibitors 36, 37. An inactivated vaccine is the only prophylactic approach for influenza infection, but it appears to be less effective in HSCT recipients, particularly in the first few months after transplantation, as well as in patients who required immunosuppressive agents for GVHD treatment 38, 39, 40, 41. However, the influenza vaccine is considered to be safe as no major local or general adverse effects were reported after use in transplant recipients [42]. hMPV infection has been reported with an incidence of 3% to 9% in adult HSCT recipients and patients with hematologic malignancies. hMPV may be associated with severe pneumonia and fatal lower -respiratory tract disease in HSCT recipients [43]. Even though hMPV infections are documented in asymptomatic cases [44], further analysis of a larger number of patients is necessary to identify its real impact in after transplantation population. For human rhinovirus and coronavirus, despite the high incidence of infections among HSCT recipients, morbidity and mortality are both relatively low. Asymptomatic detections are common, accounting for 13% and 41% of patients with rhinovirus and coronavirus, respectively [45].
Mortality is higher in allogeneic HSCT and recipients of cord blood transplants. However, no significant difference was observed by univariate analysis. Previous studies revealed GVHD and use of immunosuppressive agents as major risk factors associated with LRTI and mortality 46, 47, 48. Because of the small number of patients who developed GVHD in our study, logistic regression analysis could not be performed. Likewise, various conditioning regimens were used in our 5 patients who died from RVI. We are unable to establish the correlation between transplantation type and respiratory virus–associated death.
The strength of our study is in the large number of patients examined from a single center with a uniform isolation practice, contact precautions, and supportive care. The limitation is in the retrospective nature of the study design. Nonetheless, with an incidence of 6.5% among HSCT recipients, prospective studies could be a challenge and when using multiple centers, another challenge arises in terms of isolation practices, infection precautions, and supportive care heterogeneity among HSCT centers.
Finally, given the high mortality that is associated with viral infections in the first 100 days after HSCT in our study, it may be intuitive to avoid admission of patients in the high season for respiratory viruses, especially in a country such as Canada where there is a significant difference between summer and winter temperatures. In Canada, the high season for respiratory viral infections span between October and March and in our study, 35 patients (65%) contracted RVI during the high season, compared with 19 patients (35%) in the remaining months. This may not be feasible for children who need their HSCT urgently, for example those with aggressive leukemia, but may be appropriate for those with elective nonmalignant HSCT to minimize their risk of acquiring these infections early after HSCT.
Acknowledgments
Financial disclosure: The authors have nothing to disclose.
Conflict of interest statement: There are no conflicts to disclose.
Footnotes
Financial disclosure: See Acknowledgments on page 1806.
References
- 1.Kim Y.J., Boeckh M., Englund J.A. Community respiratory virus infections in immunocompromised patients: hematopoietic stem cell and solid organ transplant recipients, and individuals with human immunodeficiency virus infection. Semin Respir Crit Care Med. 2007;28:222–242. doi: 10.1055/s-2007-976494. [DOI] [PubMed] [Google Scholar]
- 2.Mikulska M., Del Bono V., Gandolfo N. Epidemiology of viral respiratory tract infections in an outpatient haematology facility. Ann Hematol. 2014;93:669–676. doi: 10.1007/s00277-013-1912-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ison M.G., Hayden F.G. Viral infections in immunocompromised patients: what's new with respiratory viruses? Curr Opin Infect Dis. 2002;15:355–367. doi: 10.1097/00001432-200208000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Ljungman P., Ward K.N., Crooks B.N. Respiratory virus infections after stem cell transplantation: a prospective study from the Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2001;28:479–484. doi: 10.1038/sj.bmt.1703139. [DOI] [PubMed] [Google Scholar]
- 5.Kontoyiannis D.P., Lewis R.E., Marr K. The burden of bacterial and viral infections in hematopoietic stem cell transplant. Biol Blood Marrow Transplant. 2009;15(1 Suppl):128–133. doi: 10.1016/j.bbmt.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Crocchiolo R., Bramanti S., Vai A. Infections after T-replete haploidentical transplantation and high-dose cyclophosphamide as graft-versus-host disease prophylaxis. Transpl Infect Dis. 2015;17:242–249. doi: 10.1111/tid.12365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Green M.L., Leisenring W.M., Xie H. CMV reactivation after allogeneic HCT and relapse risk: evidence for early protection in acute myeloid leukemia. Blood. 2013;122:1316–1324. doi: 10.1182/blood-2013-02-487074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zaucha-Prazmo A., Wojcik B., Drabko K. Cytomegalovirus (CMV) infections in children undergoing hematopoetic stem cell transplantation. Pediatr Hematol Oncol. 2005;22:271–276. doi: 10.1080/08880010590935158. [DOI] [PubMed] [Google Scholar]
- 9.Hazar V., Kansoy S., Kupesiz A. High-dose acyclovir and pre-emptive ganciclovir in prevention of cytomegalovirus disease in pediatric patients following peripheral blood stem cell transplantation. Bone Marrow Transplant. 2004;33:931–935. doi: 10.1038/sj.bmt.1704463. [DOI] [PubMed] [Google Scholar]
- 10.Hiwarkar P., Gaspar H.B., Gilmour K. Impact of viral reactivations in the era of pre-emptive antiviral drug therapy following allogeneic haematopoietic SCT in paediatric recipients. Bone Marrow Transplant. 2013;48:803–808. doi: 10.1038/bmt.2012.221. [DOI] [PubMed] [Google Scholar]
- 11.Therasse P., Arbuck S.G., Eisenhauer E.A. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 12.Lujan-Zilbermann J., Benaim E., Tong X. Respiratory virus infections in pediatric hematopoietic stem cell transplantation. Clin Infect Dis. 2001;33:962–968. doi: 10.1086/322628. [DOI] [PubMed] [Google Scholar]
- 13.Lee J.H., Jang J.H., Lee S.H. Respiratory viral infections during the first 28 days after transplantation in pediatric hematopoietic stem cell transplant recipients. Clin Transplant. 2012;26:736–740. doi: 10.1111/j.1399-0012.2012.01607.x. [DOI] [PubMed] [Google Scholar]
- 14.Osiowy C. Direct detection of respiratory syncytial virus, parainfluenza virus, and adenovirus in clinical respiratory specimens by a multiplex reverse transcription-PCR assay. J Clin Microbiol. 1998;36:3149–3154. doi: 10.1128/jcm.36.11.3149-3154.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Elden L.J., Nijhuis M., Schipper P. Simultaneous detection of influenza viruses A and B using real-time quantitative PCR. J Clin Microbiol. 2001;39:196–200. doi: 10.1128/JCM.39.1.196-200.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Elden L.J., van Kraaij M.G., Nijhuis M. Polymerase chain reaction is more sensitive than viral culture and antigen testing for the detection of respiratory viruses in adults with hematological cancer and pneumonia. Clin Infect Dis. 2002;34:177–183. doi: 10.1086/338238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee Y.J., Palomino-Guilen P., Babady N.E. Disseminated adenovirus infection in cancer patients presenting with focal pulmonary consolidation. J Clin Microbiol. 2014;52:350–353. doi: 10.1128/JCM.01893-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mochizuki K., Kondo Y., Hosokawa K. Adenovirus pneumonia presenting with nodular shadows on chest X-ray in two unrelated allogeneic bone marrow transplant recipients. Intern Med. 2014;53:499–503. doi: 10.2169/internalmedicine.53.1192. [DOI] [PubMed] [Google Scholar]
- 19.Mynarek M., Ganzenmueller T., Mueller-Heine A. Patient, virus, and treatment-related risk factors in pediatric adenovirus infection after stem cell transplantation: results of a routine monitoring program. Biol Blood Marrow Transplant. 2014;20:250–256. doi: 10.1016/j.bbmt.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 20.Robin M., Marque-Juillet S., Scieux C. Disseminated adenovirus infections after allogeneic hematopoietic stem cell transplantation: incidence, risk factors and outcome. Haematologica. 2007;92:1254–1257. doi: 10.3324/haematol.11279. [DOI] [PubMed] [Google Scholar]
- 21.Lion T., Kosulin K., Landlinger C. Monitoring of adenovirus load in stool by real-time PCR permits early detection of impending invasive infection in patients after allogeneic stem cell transplantation. Leukemia. 2010;24:706–714. doi: 10.1038/leu.2010.4. [DOI] [PubMed] [Google Scholar]
- 22.Lindemans C.A., Leen A.M., Boelens J.J. How I treat adenovirus in hematopoietic stem cell transplant recipients. Blood. 2010;116:5476–5485. doi: 10.1182/blood-2010-04-259291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yusuf U., Hale G.A., Carr J. Cidofovir for the treatment of adenoviral infection in pediatric hematopoietic stem cell transplant patients. Transplantation. 2006;81:1398–1404. doi: 10.1097/01.tp.0000209195.95115.8e. [DOI] [PubMed] [Google Scholar]
- 24.Bhadri V.A., Lee-Horn L., Shaw P.J. Safety and tolerability of cidofovir in high-risk pediatric patients. Transpl Infect Dis. 2009;11:373–379. doi: 10.1111/j.1399-3062.2009.00391.x. [DOI] [PubMed] [Google Scholar]
- 25.Chakrabarti S., Mautner V., Osman H. Adenovirus infections following allogeneic stem cell transplantation: incidence and outcome in relation to graft manipulation, immunosuppression, and immune recovery. Blood. 2002;100:1619–1627. doi: 10.1182/blood-2002-02-0377. [DOI] [PubMed] [Google Scholar]
- 26.Wendt C.H., Weisdorf D.J., Jordan M.C. Parainfluenza virus respiratory infection after bone marrow transplantation. N Engl J Med. 1992;326:921–926. doi: 10.1056/NEJM199204023261404. [DOI] [PubMed] [Google Scholar]
- 27.Whimbey E., Vartivarian S.E., Champlin R.E. Parainfluenza virus infection in adult bone marrow transplant recipients. Eur J Clin Microbiol Infect Dis. 1993;12:699–701. doi: 10.1007/BF02009383. [DOI] [PubMed] [Google Scholar]
- 28.Lewis V.A., Champlin R., Englund J. Respiratory disease due to parainfluenza virus in adult bone marrow transplant recipients. Clin Infect Dis. 1996;23:1033–1037. doi: 10.1093/clinids/23.5.1033. [DOI] [PubMed] [Google Scholar]
- 29.Nichols W.G., Corey L., Gooley T. Parainfluenza virus infections after hematopoietic stem cell transplantation: risk factors, response to antiviral therapy, and effect on transplant outcome. Blood. 2001;98:573–578. doi: 10.1182/blood.v98.3.573. [DOI] [PubMed] [Google Scholar]
- 30.Small T.N., Casson A., Malak S.F. Respiratory syncytial virus infection following hematopoietic stem cell transplantation. Bone Marrow Transplant. 2002;29:321–327. doi: 10.1038/sj.bmt.1703365. [DOI] [PubMed] [Google Scholar]
- 31.Martino R., Porras R.P., Rabella N. Prospective study of the incidence, clinical features, and outcome of symptomatic upper and lower respiratory tract infections by respiratory viruses in adult recipients of hematopoietic stem cell transplants for hematologic malignancies. Biol Blood Marrow Transplant. 2005;11:781–796. doi: 10.1016/j.bbmt.2005.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shah J.N., Chemaly R.F. Management of RSV infections in adult recipients of hematopoietic stem cell transplantation. Blood. 2011;117:2755–2763. doi: 10.1182/blood-2010-08-263400. [DOI] [PubMed] [Google Scholar]
- 33.de Fontbrune F.S., Robin M., Porcher R. Palivizumab treatment of respiratory syncytial virus infection after allogeneic hematopoietic stem cell transplantation. Clin Infect Dis. 2007;45:1019–1024. doi: 10.1086/521912. [DOI] [PubMed] [Google Scholar]
- 34.Kassis C., Champlin R.E., Hachem R.Y. Detection and control of a nosocomial respiratory syncytial virus outbreak in a stem cell transplantation unit: the role of palivizumab. Biol Blood Marrow Transplant. 2010;16:1265–1271. doi: 10.1016/j.bbmt.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 35.Khanna N., Widmer A.F., Decker M. Respiratory syncytial virus infection in patients with hematological diseases: single-center study and review of the literature. Clin Infect Dis. 2008;46:402–412. doi: 10.1086/525263. [DOI] [PubMed] [Google Scholar]
- 36.Nichols W.G., Guthrie K.A., Corey L., Boeckh M. Influenza infections after hematopoietic stem cell transplantation: risk factors, mortality, and the effect of antiviral therapy. Clin Infect Dis. 2004;39:1300–1306. doi: 10.1086/425004. [DOI] [PubMed] [Google Scholar]
- 37.Chemaly R.F., Ghosh S., Bodey G.P. Respiratory viral infections in adults with hematologic malignancies and human stem cell transplantation recipients: a retrospective study at a major cancer center. Medicine (Baltimore) 2006;85:278–287. doi: 10.1097/01.md.0000232560.22098.4e. [DOI] [PubMed] [Google Scholar]
- 38.Haining W.N., Evans J.W., Seth N.P. Measuring T cell immunity to influenza vaccination in children after haemopoietic stem cell transplantation. Br J Haematol. 2004;127:322–325. doi: 10.1111/j.1365-2141.2004.05204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Issa N.C., Marty F.M., Gagne L.S. Seroprotective titers against 2009 H1N1 influenza A virus after vaccination in allogeneic hematopoietic stem cell transplantation recipients. Biol Blood Marrow Transplant. 2011;17:434–438. doi: 10.1016/j.bbmt.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ljungman P., Avetisyan G. Influenza vaccination in hematopoietic SCT recipients. Bone Marrow Transplant. 2008;42:637–641. doi: 10.1038/bmt.2008.264. [DOI] [PubMed] [Google Scholar]
- 41.Machado C.M., Cardoso M.R., da Rocha I.F. The benefit of influenza vaccination after bone marrow transplantation. Bone Marrow Transplant. 2005;36:897–900. doi: 10.1038/sj.bmt.1705159. [DOI] [PubMed] [Google Scholar]
- 42.Dhedin N., Krivine A., Le Corre N. Comparable humoral response after two doses of adjuvanted influenza A/H1N1pdm2009 vaccine or natural infection in allogeneic stem cell transplant recipients. Vaccine. 2014;32:585–591. doi: 10.1016/j.vaccine.2013.11.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cane P.A., van den Hoogen B.G., Chakrabarti S. Human metapneumovirus in a haematopoietic stem cell transplant recipient with fatal lower respiratory tract disease. Bone Marrow Transplant. 2003;31:309–310. doi: 10.1038/sj.bmt.1703849. [DOI] [PubMed] [Google Scholar]
- 44.Debiaggi M., Canducci F., Sampaolo M. Persistent symptomless human metapneumovirus infection in hematopoietic stem cell transplant recipients. J Infect Dis. 2006;194:474–478. doi: 10.1086/505881. [DOI] [PubMed] [Google Scholar]
- 45.Milano F., Campbell A.P., Guthrie K.A. Human rhinovirus and coronavirus detection among allogeneic hematopoietic stem cell transplantation recipients. Blood. 2010;115:2088–2094. doi: 10.1182/blood-2009-09-244152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reid G., Huprikar S., Patel G. A multicenter evaluation of pandemic influenza A/H1N1 in hematopoietic stem cell transplant recipients. Transpl Infect Dis. 2013;15:487–492. doi: 10.1111/tid.12116. [DOI] [PubMed] [Google Scholar]
- 47.Ison M.G. Adenovirus infections in transplant recipients. Clin Infect Dis. 2006;43:331–339. doi: 10.1086/505498. [DOI] [PubMed] [Google Scholar]
- 48.Bruno B., Gooley T., Hackman R.C. Adenovirus infection in hematopoietic stem cell transplantation: effect of ganciclovir and impact on survival. Biol Blood Marrow Transplant. 2003;9:341–352. doi: 10.1016/s1083-8791(03)00102-2. [DOI] [PubMed] [Google Scholar]