Abstract
Background
The Middle East respiratory syndrome coronavirus (MERS-CoV) has been reported to have a high case-fatality rate. Currently, there is no specific therapy or vaccine with proven effectiveness for MERS-CoV infections.
Methods
A combination of ribavirin and interferon therapy was used for the treatment of five MERS-CoV-positive patients. We reviewed the therapeutic schedule and the outcome of these patients.
Results
All patients were critically ill with acute respiratory distress syndrome treated with adjunctive corticosteroids and were on mechanical ventilation at the time of initiation of therapy. The median time from admission to therapy with ribavirin and interferon was 19 (range 10–22) days. None of the patients responded to the supportive or therapeutic interventions and all died of their illness.
Conclusions
While ribavirin and interferon may be effective in some patients, our practical experience suggests that critically ill patients with multiple comorbidities who are diagnosed late in the course of their illness may not benefit from combination antiviral therapy as preclinical data suggest. There is clearly an urgent need for a novel effective antiviral therapy for this emerging global threat.
Keywords: MERS-CoV, Interferon, Ribavirin, Pegylated interferon
1. Introduction
Since the discovery of Middle East respiratory syndrome coronavirus (MERS-CoV), the virus has caused 163 cases of disease, with a fatality rate of 43–50%.1, 2 The disease was initially described in a patient from Saudi Arabia in June 2012.3 MERS-CoV has caused sporadic cases and clusters in families and healthcare settings.4, 5, 6, 7 The best treatment option for the virus is not known. In view of the high case-fatality rate and the potential global spread of the virus, there is an urgent need to develop effective therapies for this infection. In a recent review, based on therapies used for the related severe acute respiratory syndrome (SARS) coronavirus, the possible use of interferon and ribavirin was considered as a therapeutic option.8 The purpose of this study was to describe the outcome of the use of a combination of interferon-α2b and ribavirin in the management of five patients with MERS-CoV infections.
2. Materials and methods
This was a retrospective observational study of five MERS-CoV-positive patients who received a combination therapy of interferon-α2b and ribavirin. We describe the therapeutic schedule, the clinical course, clinical and laboratory parameters, and outcomes.
2.1. MERS-CoV testing
Either Dacron flocked nasopharyngeal swabs or tracheal aspirates were obtained from the patients, and these were submitted to the Ministry of Health MERS-CoV laboratory for MERS-CoV diagnosis. The clinical samples were screened at the Ministry of Health laboratory using a real-time reverse transcriptase PCR.9, 10 Amplification targeted both the upstream E protein (upE gene) and open reading frame 1a (ORF1a) for MERS-CoV testing, and a case was considered positive if both assays were positive, as described previously (Fig. 1 ).5
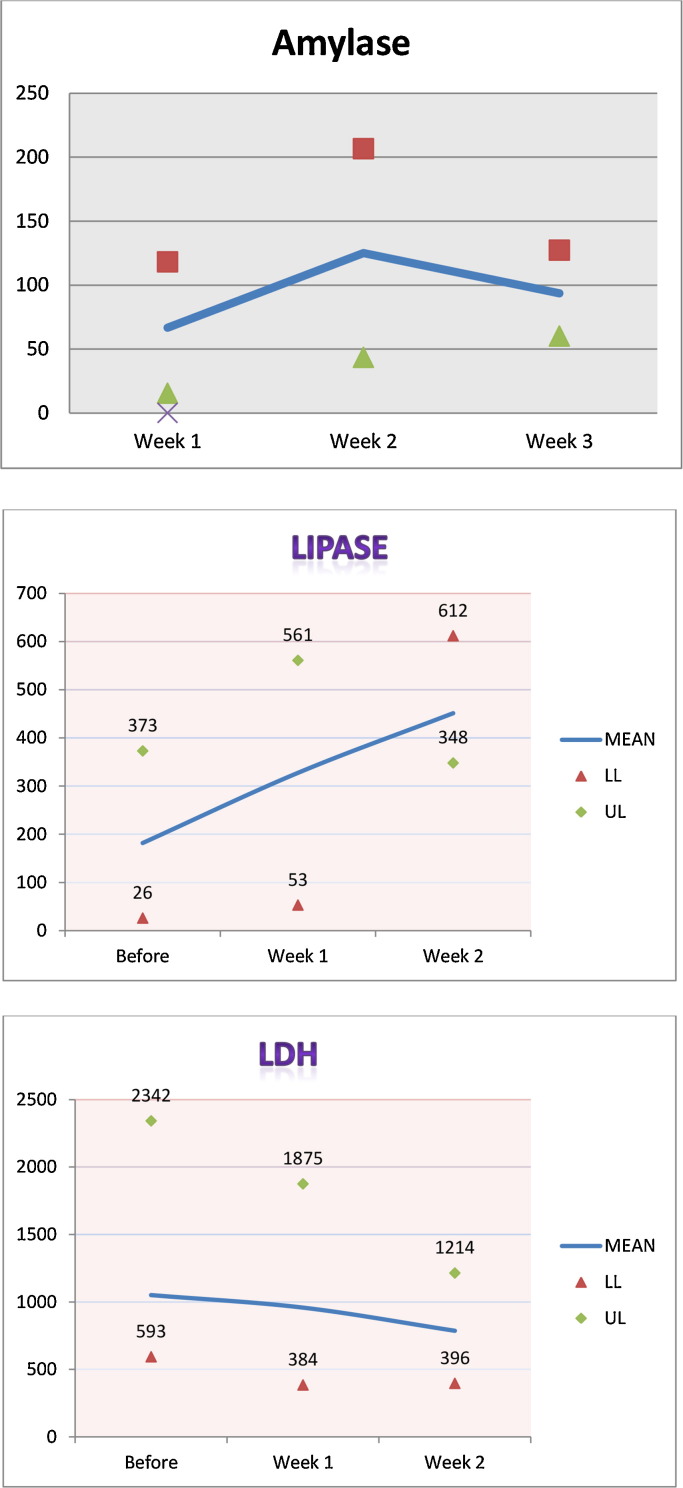
Figure 1.
Median, minimum, and maximum values for amylase, lipase, and lactate dehydrogenase (LDH).
3. Case reports
3.1. Case 1
A 62-year-old woman with diabetes, hypertension, and end-stage renal disease (ESRD) on hemodialysis was admitted with fever, cough, and respiratory failure of 3-day duration (table 1 ). She tested positive for MERS-CoV by PCR. Baseline laboratory data are shown in Table 2, Table 3 . On admission, the patient was started on oseltamivir 75 mg once daily for 5 days and levofloxacin 500 mg intravenous (IV) every 2 days for 15 days. Imipenem was added on day 5 for 3 days. On day 21 after admission, she was started on ribavirin for 5 days with a loading dose of 2000 mg via nasogastric tube, followed by 400 mg per os (PO) every 8 h, one dose of interferon-α2b 130 μg subcutaneously, and methylprednisolone 40 mg IV every 8 h for 1 day, then twice daily for 1 day, then daily for 2 days. She had a mild rise in amylase and lipase (from 61 to 126 U/l and from 274 to 348 U/l, respectively). The patient remained critically ill on mechanical ventilation requiring inotropic support. She died 34 days after admission from respiratory and multi-organ failure.
Table 1.
Patient demographics and underlying conditions
| Case No. | Age, years | Gender | Body weight, kg | Hypertension | Diabetes mellitus | Chronic renal disease | ClCr, ml/min | Dialysis | Other chronic conditions |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 62 | F | 78 | Yes | Yes | Yes | 8.5 | HD | No |
| 2 | 58 | M | 52 | Yes | Yes | Yes | 15.8 | HD | No |
| 3 | 63 | F | 96 | Yes | Yes | Yes | 15.7 | No | Asthma, obstructive sleep apnea, coronary artery disease |
| 4 | 81 | M | 71 | Yes | Yes | Yes | 40.7 | HD | Atrial fibrillation |
| 5 | 24 | M | 58 | No | No | Yes | 12.8 | HD | End-stage renal disease |
ClCr, creatinine clearance; F, female; M, male; HD, hemodialysis.
Table 2.
Laboratory results of patients at baseline and at 1 and 2 weeks after the initiation of interferon and ribavirin therapy
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | ||
|---|---|---|---|---|---|---|
| Serum creatinine, mg/dl | Baseline | 5.8 | 4.9 | 0.6 | 0.7 | 4.5 |
| Week 1 | 3.9 | 5.4 | 2.9 | 0.7 | 3.5 | |
| Week 2 | 5.7 | 3.8 | 1.7 | 7.3 | ||
| Week 3 | 0.7 | |||||
| Platelet count, × 109/l | Baseline | 290 | 408 | 272 | 74 | 194 |
| Week 1 | 166 | 239 | 408 | 163 | 160 | |
| Week 2 | 233 | 43 | 106 | 234 | ||
| Week 3 | 85 | |||||
| Hemoglobin, g/dl | Baseline | 11.3 | 9.3 | 10.5 | 11.7 | 8.4 |
| Week 1 | 9.3 | 10.2 | 10.7 | 10 | 8.1 | |
| Week 2 | 9.4 | 9.1 | 9.1 | 8.7 | ||
| Week 3 | 8.7 | |||||
| WBC count, × 109/l | Baseline | 12.9 | 5.5 | 6.3 | 9.5 | 32.1 |
| Week 1 | 7.2 | 4.3 | 20 | 10.7 | 15.5 | |
| Week 2 | 25.5 | 2.7 | 5.1 | 28.6 | ||
| Week 3 | 4.7 | |||||
| Lymphocytes (%) | Baseline | 13 | 17 | 8 | 8 | 4 |
| Week 1 | 17 | 18 | 2 | 2 | 19 | |
| Week 2 | 3 | 62 | 3 | 4 | ||
| Week 3 | 13 |
Table 3.
Hepatic and pancreatic enzymes of patients at baseline and at 1 and 2 weeks after the initiation of interferon and ribavirin therapy
| Liver function testa |
Pancreatic enzymesb |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Test | Baseline | Week 1 | Week 2 | Week 3 | Test | Baseline | Week 1 | Week 2 | |
| Case 1 | ALP | 123 | 62 | 119 | Amylase | 61 | 105 | 126 | |
| ALT | 15 | 24 | 22 | ||||||
| AST | 20 | 27 | 34 | Lipase | 274 | 380 | 348 | ||
| LDH | 677 | 543 | 936 | ||||||
| Total bilirubin | 1 | 0.7 | 0.8 | ||||||
| Case 2 | ALP | 93 | 75 | Amylase | <30 | 45 | |||
| ALT | 32 | 17 | |||||||
| AST | 27 | 10 | Lipase | 26 | 152 | ||||
| LDH | 593 | 543 | |||||||
| Total bilirubin | 0.7 | 1.5 | |||||||
| Case 3 | ALP | 41 | 74 | Amylase | 35 | 255 | |||
| ALT | 22 | 49 | |||||||
| AST | 25 | 126 | Lipase | 55 | 53 | ||||
| LDH | 1102 | 1389 | |||||||
| Total bilirubin | 0.6 | 0.4 | |||||||
| Case 4 | ALP | 342 | 293 | 269 | 548 | Amylase | 141 | 148 | 59 |
| ALT | 53 | 41 | 48 | 68 | |||||
| AST | 161 | 79 | 64 | 125 | Lipase | 373 | 492 | 394 | |
| LDH | 2342 | 1875 | 1214 | 995 | |||||
| Total bilirubin | 0.7 | 1.2 | 3.4 | 7.4 | |||||
| Case 5 | ALP | 230 | 136 | 101 | Amylase | 75 | 96 | ||
| ALT | 83 | 47 | 17 | ||||||
| AST | 65 | 14 | 15 | Lipase | 561 | 612 | |||
| LDH | 762 | 384 | 547 | ||||||
| Total bilirubin | 3.9 | 1.1 | 1.2 | ||||||
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; LDH, lactate dehydrogenase.
ALP, U/L; ALT, IU/L; AST, IU/L; LDH, IU/L; bilirubin, mg/dl.
Amylase, U/L; lipase, U/L
3.2. Case 2
A 58-year-old man with diabetes, hypertension, and ESRD on hemodialysis was admitted with cough, fever, and hypoxemic respiratory failure of 2-day duration. He tested positive for MERS-CoV by PCR. Baseline laboratory data are shown in Table 2, Table 3. On admission, the patient was started on oseltamivir 75 mg once daily for 5 days, levofloxacin 500 mg IV every 2 days for 6 days, and imipenem 250 mg IV twice daily for 7 days. On day 6, he was prescribed methylprednisolone 60 mg IV every 6 h for 6 days, then every 8 h for 1 day, then 40 mg every 12 h for 1 day, followed by a prednisolone tapered dose for another 10 days. On day 13 after admission, he was started on ribavirin for 5 days with a loading dose of 2000 mg PO, followed by 400 mg every 8 h and two doses of interferon-α2b 100 μg subcutaneously once per week. Two weeks after the initiation of the therapy, platelets dropped from 408 to 43 × 109/l (Table 2). The patient remained on the ventilator for more than 30 days. He died 35 days after admission from multi-organ failure.
3.3. Case 3
A 63-year-old woman with a history of severe bronchial asthma and obstructive sleep apnea (on continuous positive airway pressure (CPAP)), with coronary artery disease, diabetes, hypertension, and chronic kidney disease (estimated glomerular filtration rate 15.7 ml/min), was admitted with fever, cough, and dyspnea of 3-day duration and a diagnosis of pneumonia. She tested positive for MERS-CoV by PCR. Baseline laboratory data are shown in Table 2, Table 3. On admission, the patient was also started on oseltamivir 75 mg once daily for 5 days, levofloxacin 750 mg IV every 2 days for 7 days, and imipenem 500 mg IV every 6 h for 8 days. On day 11, she was started on ribavirin for 5 days, with a loading dose of 2000 mg via nasogastric tube followed by 600 mg PO every 8 h, one dose of interferon-α2b 144 μg subcutaneously, and prednisone 40 mg PO daily, tapered to 10 mg PO daily for 10 days. One week after initiation of the antiviral and steroid therapy, serum creatinine, aspartate aminotransferase (AST) Table 2, Table 3, and amylase increased (from 0.6 to 2.9 mg/dl, from 6.3 to 20 × 109/l, from 25 to 126 IU/l, and from 35 to 255 U/l, respectively). The patient was diagnosed with pancreatitis and thus further interferon and ribavirin was not given. The patient remained critically ill on mechanical ventilation. She died 45 days after admission from multi-organ failure.
3.4. Case 4
An 81-year-old man with a history of atrial fibrillation, diabetes mellitus, and hypertension was admitted with progressive respiratory distress and hypoxemia with fever for 2 days. He tested positive for MERS-CoV by PCR. Baseline laboratory data are shown in Table 2, Table 3. On the first day of hospitalization, the patient was started on oseltamivir 75 mg once daily for 6 days, levofloxacin 750 mg every 48 h for 5 days, and imipenem 250 mg IV every 6 h for 6 days. On day 20 after admission, he was started on ribavirin for 9 days, with a loading dose of 2000 mg via nasogastric tube followed by 800 mg via nasogastric tube every 8 h, two doses of interferon-α2b 100 μg subcutaneously once per week on hospital days 20 and 27, and methylprednisolone 40 mg IV every 8 h for 4 days, then 40 mg IV twice daily for 2 days, then 40 mg daily for 2 days (started on hospital day 22). Three weeks after the initiation of therapy, his hemoglobin dropped (from 11.7 to 8.7 g/dl) (Table 2), while bilirubin increased (from 0.7 to 7.4 mg/dl), suggestive of hemolysis. The patient died 52 days after admission from multi-organ failure.
3.5. Case 5
A 24-year-old man with a history of ESRD on hemodialysis was admitted with a febrile illness, a cough for 10 days, and respiratory failure requiring mechanical ventilation. He tested positive for MERS-CoV by PCR. Baseline laboratory data are shown in Table 2, Table 3. On the first day of hospitalization, the patient was started on oseltamivir 75 mg once daily for 2 days and imipenem 250 mg IV twice daily for 11 days. On day 19 of admission, he was started on ribavirin for 11 days, with a loading dose of 2000 mg via nasogastric tube, followed by 400 mg via nasogastric tube every 8 h, and two doses of interferon-α2b 100 μg subcutaneously once per week on hospital days 19 and 26. On the same day he started ribavirin, he was also started on methylprednisolone 40 mg IV every 8 h for 4 days, then 40 mg IV twice daily for 2 days, and then 40 mg daily for 2 days. Two weeks after the initiation of therapy, lipase increased (from 561 to 612 U/l). The patient died 32 days after admission from multi-organ failure.
4. Results
We reviewed the cases of five patients with MERS-CoV (Table 1). Their median age was 62 (range 24–81) years. The patients had chronic kidney disease and four were on maintenance hemodialysis. There were three men and two women. All patients tested negative for influenza. The median number of days from admission to therapy with ribavirin and interferon was 19 (range 10–22) (Table 4 ). All patients received adjunctive corticosteroid therapy for acute respiratory distress syndrome. Two patients developed increased lipase after the initiation of therapy, but both of them were given corticosteroids at the same time as antiviral therapy. One patient (case 2) developed thrombocytopenia, with a platelet count that dropped from 408 to 49 × 109/l (Table 2, Table 3) and one patient had possible hemolysis (case 3). All patients had severe disease with progressive respiratory failure, developed multi-organ failure, and died a mean 39.6 (standard deviation 8.5) days after admission. None of the patients had bacteremia or fungemia during their hospital stay. Figure 1 shows the median, minimum, and maximum values for amylase, lipase, and lactate dehydrogenase (LDH) for all the patients before initiation of therapy and at week1 and 2 after therapy initiation.
Table 4.
Days from admission to initiation of therapy or death
| Patient | Days to antiviral therapy | Days to steroid therapy | Days to death |
|---|---|---|---|
| 1 | 21 | 21 | 34 |
| 2 | 22 | 6 | 35 |
| 3 | 10 | 0 | 15 |
| 4 | 19 | 19 | 53 |
| 5 | 18 | 18 | 33 |
5. Discussion
Since the emergence of MERS-CoV in 2012, the virus has caused a total of 163 cases of disease, with a high case-fatality rate of 43%.2 There is an urgent need for effective therapeutic agents. To date, no data are available on the use of any agents for the therapy of MERS-CoV-positive patients. In this report we have described the therapy of five MERS-CoV patients who received interferon and ribavirin.
The patients were treated during the initial phase of the Al-Hasa outbreak in April and May 2013, a time at which the disease epidemiology and clinical characteristics were not known. Hence, there was an inevitable time lapse from admission to the initiation of therapy. All patients were already on mechanical ventilation by the time interferon and ribavirin therapy was instituted and all had a fatal outcome. The patients received the therapy late in the course of the disease, at a median of 19 (range 10–22) days after admission. Late therapy may have contributed to the poor outcome, in addition to the severity of the disease and the multiple comorbidities of the patients.
The timing of initiation of antiviral therapy is critical in the treatment of most viral infections. In the treatment of SARS-CoV, no effect of oseltamivir or ribavirin was observed when these agents were started 6–14 days after symptom onset.11, 12, 13 Early therapy with ribavirin, within 48 h of hospitalization or after the diagnosis of SARS, has been shown to be associated with better outcomes, although the numbers of patients enrolled in these studies has been small.7, 14, 15, 16, 17 In the treatment of influenza, oseltamivir therapy early in the disease resulted in reduced mortality when this was started not later than 8 days after the onset of symptoms.18 Early in vitro studies showed that ribavirin and interferon have anti-MERS-CoV activity.19 The in vitro activity of interferon was augmented by the concomitant use of ribavirin.20 In a rhesus macaque model of MERS-CoV infection, the combination of interferon-α2b and ribavirin therapy was effective in limiting the disease and resulted in very mild radiographic evidence of pneumonia.21 The treatment was given within 8 h after inoculation of the rhesus macaques. Treated animals had lower levels of systemic and local proinflammatory markers and fewer viral genome copies.21 Although these preclinical data are promising, our report illustrates some of the real world issues of dealing with a novel emerging viral infection. Our patients were not diagnosed until a week into their illnesses, by which time all five were on mechanical ventilation. They had multiple comorbidities, which most likely adversely affected their clinical outcomes. In addition, we had no serial virologic determination of MERS-CoV levels in airway secretions to shed light on the possibility of virological clearance, virological failure, or clinical failure of therapy.
In our small series, adverse effects from combination ribavirin and interferon therapy were observed in three cases. These side effects have been noted before.22 Two patients had pancreatic enzyme elevation and one had significant hemolysis. These findings were complicated by the presence of abnormal laboratory findings even before the initiation of combination therapy, so it is difficult to determine which side effects were due to disease progression and which were due to the therapeutic drugs.
There is an urgent need for large-scale clinical trials to determine the safest and most effective regime for the treatment of this novel highly fatal emerging infection. While ribavirin and interferon may show promise, their use needs to be prompt and adverse effects monitored closely. This therapeutic approach should be tested in careful clinical studies.
Acknowledgements
The authors (JAT, HM, and JD) wish to acknowledge the use of the Saudi Aramco Medical Services Organization (SAMSO) facilities for the data and study, which resulted in this paper. Opinions expressed in this article are those of the authors and not necessarily of SAMSO. The authors thank Dr Paul Anantharajah Tambyah of the National University of Singapore Department of Medicine for his critical review of the manuscript.
Financial support: All authors have no funding.
Conflict of interest: All authors have no conflict of interest to declare.
Corresponding Editor: Eskild Petersen, Aarhus, Denmark
References
- 1.Penttinen P.M., Kaasik-Aaslav K., Friaux A., Donachie A., Sudre B., Amato-Gauci A.J. Taking stock of the first 133 MERS coronavirus cases globally—is the epidemic changing? Euro Surveill. 2013;18 doi: 10.2807/1560-7917.es2013.18.39.20596. pii: 20596. [DOI] [PubMed] [Google Scholar]
- 2.World Health Orzination . WHO; Geneva: 2013. Middle East respiratory syndrome coronavirus (MERS-CoV)—update. Available at: http://www.who.int/csr/don/2013_12_02/en/index.html last (accessed December 3) [Google Scholar]
- 3.Zaki A.M., van Boheemen S., Bestebroer T.M., Osterhaus A.D., Fouchier R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 4.Omrani A.S., Matin M.A., Haddad Q., Al-Nakhli D., Memish Z.A., Albarrak A.M. A family cluster of Middle East respiratory syndrome coronavirus infections related to a likely unrecognized asymptomatic or mild case. Int J Infect Dis. 2013;17:e668–e672. doi: 10.1016/j.ijid.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Assiri A., Al-Tawfiq J.A., Al-Rabeeah A.A., Al-Rabiah F.A., Al-Hajjar S., Al-Barrak A. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. 2013;13:752–761. doi: 10.1016/S1473-3099(13)70204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Assiri A., McGeer A., Perl T.M., Price C.S., Al Rabeeah A.A., Cummings D.A., KSA MERS-CoV Investigation Team Hospital outbreak of Middle East respiratory syndrome coronavirus. N Engl J Med. 2013;369:407–416. doi: 10.1056/NEJMoa1306742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Memish Z.A., Zumla A.I., Al-Hakeem R.F., Al-Rabeeah A.A., Stephens G.M. Family cluster of Middle East respiratory syndrome coronavirus infections. N Engl J Med. 2013;368:2487–2494. doi: 10.1056/NEJMoa1303729. [DOI] [PubMed] [Google Scholar]
- 8.Momattin H., Mohammed K., Zumla A., Memish Z.A., Al-Tawfiq J.A. Therapeutic options for Middle East respiratory syndrome coronavirus (MERS-CoV)—possible lessons from a systematic review of SARS-CoV therapy. Int J Infect Dis. 2013;17:e792–e798. doi: 10.1016/j.ijid.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corman V.M., Eckerle I., Bleicker T., Zaki A., Landt O., Eschbach-Bludau M. Detection of a novel human coronavirus by real-time reverse-transcription polymerase chain reaction. Euro Surveill. 2012;17 doi: 10.2807/ese.17.39.20285-en. pii: 20285. [DOI] [PubMed] [Google Scholar]
- 10.Corman V.M., Müller M.A., Costabel U., Timm J., Binger T., Meyer B. Assays for laboratory confirmation of novel human coronavirus (hCoV–EMC) infections. Euro Surveill. 2012;17 doi: 10.2807/ese.17.49.20334-en. pii: 20334. [DOI] [PubMed] [Google Scholar]
- 11.Hsu L.Y., Lee C.C., Green J.A., Ang B., Paton N.I., Lee L. Severe acute respiratory syndrome (SARS) in Singapore: clinical features of index patient and initial contacts. Emerg Infect Dis. 2003;9:713–717. doi: 10.3201/eid0906.030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sung J.J., Wu A., Joynt G.M., Yuen K.Y., Lee N., Chan P.K. Severe acute respiratory syndrome: report of treatment and outcome after a major outbreak. Thorax. 2004;59:414–420. doi: 10.1136/thx.2003.014076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leong H.N., Ang B., Earnest A., Teoh C., Xu W., Leo Y.S. Investigational use of ribavirin in the treatment of severe acute respiratory syndrome, Singapore, 2003. Trop Med Int Health. 2004;9:923–927. doi: 10.1111/j.1365-3156.2004.01281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poutanen S.M., Low D.E., Henry B., Finkelstein S., Rose D., Green K. Identification of severe acute respiratory syndrome in Canada. N Engl J Med. 2003;348:1995–2005. doi: 10.1056/NEJMoa030634. [DOI] [PubMed] [Google Scholar]
- 15.Tsang K.W., Ho P.L., Ooi G.C., Yee W.K., Wang T., Chan-Yeung M. A cluster of cases of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348:1977–1985. doi: 10.1056/NEJMoa030666. [DOI] [PubMed] [Google Scholar]
- 16.Peiris J.S., Chu C.M., Cheng V.C., Chan K.S., Hung I.F., Poon L.L. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361:1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao Z., Zhang F., Xu M., Huang K., Zhong W., Cai W. Description and clinical treatment of an early outbreak of severe acute respiratory syndrome (SARS) in Guangzhou, PR China. J Med Microbiol. 2003;52:715–720. doi: 10.1099/jmm.0.05320-0. [DOI] [PubMed] [Google Scholar]
- 18.Adisasmito W., Chan P.K., Lee N., Oner A.F., Gasimov V., Aghayev F. Effectiveness of antiviral treatment in human influenza A(H5N1) infections: analysis of a global patient registry. J Infect Dis. 2010;202:1154–1160. doi: 10.1086/656316. [DOI] [PubMed] [Google Scholar]
- 19.Chan JF, Chan KH, Kao RY, To KK, Zheng BJ, Li CP, et al. Broad-spectrum antivirals for the emerging Middle East respiratory syndrome coronavirus. J Infect 2013 Oct 3. pii: S0163-4453(13)00298-3. [DOI] [PMC free article] [PubMed]
- 20.Falzarano D., de Wit E., Martellaro C., Callison J., Munster V.J., Feldmann H. Inhibition of novel β coronavirus replication by a combination of interferon-α2b and ribavirin. Sci Rep. 2013;3:1686. doi: 10.1038/srep01686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Falzarano D., de Wit E., Rasmussen A.L., Feldmann F., Okumura A., Scott D.P. Treatment with interferon-α2b and ribavirin improves outcome in MERS-CoV-infected rhesus macaques. Nat Med. 2013;19:1313–1317. doi: 10.1038/nm.3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schering Plough Corporation; Kenilworth, NJ: 2009. Product Information: REBETOL (R) oral capsules solution, ribavirin oral capsules solution. [Google Scholar]