Highlights
-
•
A broad molecular- and biomarker-based microbiologic workup identified causative pathogens in 70% of HSCT recipients with acute respiratory failure admitted to the intensive care unit.
-
•
Fungi were the most frequently detected pathogens (42%), followed by viruses (40%) and bacteria (27%). Polymicrobial findings involving several pathogen groups occurred in 30% of patients.
-
•
Bronchoalveolar lavage may enhance pathogen detection and therapy guidance in settings of high prevalence of invasive mold infections including non-Aspergillus strains and emerging azole resistance.
Key Words: Acute respiratory failure, Intensive care unit, ICU, Hematopoietic stem cell transplantation, HSCT
Abstract
Allogeneic hematopoietic stem cell transplantation (HSCT) recipients frequently develop acute respiratory failure (ARF) with pulmonary infiltrates. Molecular- and biomarker-based assays enhance pathogen detection, but data on their yield in this population are scarce. This was a retrospective single-center study of 156 consecutive HSCT recipients admitted to the intensive care unit (ICU) between May 2013 and July 2017. Findings from a microbiologic diagnostic workup using currently available methods on bronchoalveolar lavage (BAL) and blood samples from 66 patients (age, 58 years [range, 45 to 64]; HSCT to ICU, 176 days [range, 85 to 407]) with ARF and pulmonary infiltrates were analyzed. In 47 patients (71%) a causative pathogen was identified (fungal, n = 28; viral, n = 26; bacterial, n = 18). Polymicrobial findings involving several pathogen groups occurred in 20 patients (30%). Culture (12/16, 75%), galactomannan (13/15, 87%), and Aspergillus-PCR (8/9, 89%) from BAL but not serum galactomannan (6/14, 43%) helped to diagnose invasive aspergillosis (n = 16, 24%). Aspergillus-PCR detected azole resistance in 2 cases. Mucorales was found in 7 patients (11%; BAL culture, n = 6; Mucorales-PCR, n = 1). Patients with identified pathogens had higher Simplified Acute Physiology Score II scores (P = .049) and inferior ICU survival (6% versus 37%, P < .01), which largely related to the presence of an invasive fungal infection. Eight patients (12%) had 1 or more viruses with uncertain lung pathogenicity as the sole microbiologic finding. A diagnostic microbiologic workup incorporating molecular- and biomarker-based assays identified pathogens in most HSCT recipients with ARF and pulmonary infiltrates admitted to the ICU. Implications of polymicrobial infection and pathogen patterns in these patients warrant further investigation.
Introduction
Pulmonary complications are a significant contributor to morbidity and nonrelapse mortality after allogeneic hematopoietic stem cell transplantation (HSCT). Acute respiratory failure (ARF) occurs in up to 16% of patients within the first year after HSCT [1] and is the main reason for intensive care unit (ICU) admission in this population [2]. Because of the broad range of possible causative pathogens or noninfectious processes, dedicated algorithms have been proposed to determine the cause of pulmonary infiltrates in immunocompromised hosts 3, 4.
In the course of recent decades diagnostic testing has become more sophisticated, and modern approaches now include molecular (eg, PCR) and other nonculture tests (eg, antigen assays) to detect fungal, bacterial, and/or viral pathogens in various types of specimens [5]. In addition to these technical advances, the list of respiratory and opportunistic viruses linked to documented cases of lower respiratory tract disease in immunocompromised hosts has been extended over the last years 6, 7, 8. These developments have led to an increase in the number of diagnosed infections in HSCT recipients with ARF that would have previously been diagnosed with a noninfectious post-HSCT lung injury syndrome. In fact, a study was able to identify pathogens in more than half of the cases previously diagnosed with idiopathic pneumonia syndrome using molecular and nonculture tests for a wide array of pathogens on bronchoalveolar lavage (BAL) samples [9]. Still, situations remain where noninfectious post-HSCT lung injury syndromes become the diagnosis of exclusion [10].
In the absence of comparable data in the literature, our study had 2 aims. The first was to analyze the findings of a modern infection detection panel performed on BAL and blood samples in a real-world cohort of critically ill HSCT recipients with ARF and pulmonary infiltrates. Second, we wanted to infer possible clinical and scientific implications.
Methods
This retrospective study included consecutive adult (ie, ≥18 years old) HSCT recipients treated at the Department of Bone Marrow Transplantation at the University Hospital Essen, Germany. The study was approved by the ethics committee of the University of Duisburg-Essen (EC 15-6446-BO) and conducted in accordance with Good Clinical Practice guidelines and the amended Declaration of Helsinki. Our center performs around 200 adult HSCTs per year in 2 inpatient units with a total capacity of 20 beds. Critically ill patients are treated in a third affiliated unit (ICU) providing 17 closed beds run by trained intensivists and hemato-oncologists.
Consecutive adult HSCT recipients admitted to the ICU between May 2013 (introduction of a PCR-based viral panel for respiratory specimen testing) and July 2017 were screened and included in the study cohort (1) if they had a BAL with available results performed during their ICU stay and (2) if the BAL was conducted as part of a workup of ARF (new requirement of supplemental oxygen or ventilatory support during hospitalization) with pulmonary infiltrates documented on x-ray or computed tomography (CT) of the lungs ±7 days from the procedure.
Data shown in Table 1, Table 2 were obtained by reviewing the patients' medical charts, radiographic images, and discharge letters. Disease risk was categorized according to Seo et al. [11]. The Simplified Acute Physiology Score II (SAPSII) was used to assess the severity of illness at ICU admission [12]. Absolute neutrophil counts and absolute lymphocyte counts were included if results were available ±2 days from BAL.
Table 1.
Cohort Characteristics
| All Patients (n = 66) |
Established Pathogen (n = 47) |
Without Established Pathogen (n = 19) |
P | |
|---|---|---|---|---|
| Sex | .29 | |||
| Male | 38 (58) | 25 (53) | 13 (68) | |
| Female | 28 (42) | 22 (47) | 6 (32) | |
| Age at ICU admission, yr | 58 (45-64) | 58 (47-64) | 49 (32-63) | .20 |
| Disease risk at transplantation | .78 | |||
| Standard | 43 (65) | 30 (64) | 13 (68) | |
| High | 23 (35) | 17 (36) | 6 (32) | |
| Cell source | .57 | |||
| Bone marrow | 4 (6) | 2 (4) | 2 (11) | |
| Peripheral blood stem cells | 62 (93) | 45 (96) | 17 (90) | |
| Donor type | .16 | |||
| Matched related | 16 (24) | 11 (23) | 5 (26) | |
| Mismatched related | 2 (3) | 0 | 2 (11) | |
| Matched unrelated | 36 (55) | 26 (55) | 10 (53) | |
| Mismatched unrelated | 12 (18) | 10 (21) | 2 (11) | |
| Conditioning regimen | .85 | |||
| MA including TBI ≥ 8 Gy | 29 (44) | 20 (43) | 9 (47) | |
| MA without TBI | 7 (11) | 6 (13) | 1 (5) | |
| Reduced intensity | 30 (45) | 21 (45) | 9 (47) | |
| GVHD prophylaxis | .22 | |||
| CNI + MTX | 52 (79) | 36 (77) | 16 (84) | |
| CNI + MMF | 10 (15) | 9 (19) | 1 (5) | |
| Others | 4 (6) | 2 (4) | 2 (11) | |
| ATG during conditioning | 47 (71) | 34 (72) | 13 (68) | .77 |
| HSCT to ICU admission, days | 176 (85-407) | 173 (76-288) | 201 (87-415) | .46 |
| ICU during hospitalization for HSCT | 14 (21) | 11 (23) | 3 (16) | .74 |
| SAPSII at ICU admission | 38 (27-51) | 45 (28-53) | 32 (26-39) | .05* |
| GVHD during ICU or at admission | ||||
| Acute GVHD | 16 (24) | 10 (21) | 6 (32) | .53 |
| Chronic GVHD | 18 (27) | 14 (30) | 4 (21) | .55 |
| Etiology of ARF according to discharge diagnosis | <.01 | |||
| Fungal pneumonia | 20 (30) | 20 (43) | 0 | |
| Viral pneumonia | 15 (23) | 11 (23) | 4 (21) | |
| Clinically documented pneumonia | 15 (23) | 3 (6) | 12 (63) | |
| Polymicrobial pneumonia | 7 (11) | 7 (15) | 0 | |
| Bacterial pneumonia | 6 (9) | 6 (13) | 0 | |
| Noninfectious etiology | 3 (5) | 0 | 3 (16) | |
| Life-supporting interventions | ||||
| Invasive mechanical ventilation | 63 (96) | 45 (96) | 18 (95) | 1.0 |
| P/F ratio at initiation of IMV | 167 (129-250) | 162 (127-249) | 177 (140-253) | .32 |
| Vasopressors | 61 (92) | 45 (96) | 16 (84) | .14 |
| Renal replacement therapy | 38 (58) | 31 (66) | 7 (37) | .05 |
| Extracorporeal life support | 12 (18) | 8 (17) | 4 (21) | .73 |
| ICU length of stay, days | 16 (6-27) | 14 (5-24) | 22 (11-38) | .09 |
| ICU survivors | 10 (15) | 3 (6) | 7 (37) | <.01 |
Values are absolute number (%) or median (IQR). P < .05 are shown in bold type. MA indicates myeloablative; TBI, total body irradiation; GVHD, graft-versus-host disease; CNI, calcineurin inhibitor; MMF, mycophenolate mofetil; MTX, methotrexate; ATG, antithymocyte globulin; P/F, PaO2/FiO2 ratio.
Exact P = .049.
Table 2.
Characteristics at the Time of BAL
| All Patients (n = 66) |
Established Pathogen (n = 47) |
Without Established Pathogen (n = 19) |
P | |
|---|---|---|---|---|
| ICU admission to BAL, days | 2 (1-6) | 2 (0-8) | 3 (1-6) | .76 |
| Infiltrates on chest x-ray* | .91 | |||
| Focal | 11 (17) | 7 (15) | 4 (22) | |
| Multifocal/diffuse | 45 (70) | 33 (72) | 12 (67) | |
| None | 8 (13) | 6 (13) | 2 (11) | |
| Pleural effusion | 15 (23) | 10 (22) | 5 (28) | .74 |
| Number of quadrants involved on x-ray | 3 (1-4) | 4 (1-4) | 3 (1-4) | .76 |
| Absolute neutrophil count, g/L† | 1.89 (.3-5.1) | 1.89 (.21-5.99) | 2.04 (.39-4.80) | .88 |
| Absolute lymphocyte count, g/L† | .17 (.06-0.54) | .14 (.02-0.51) | .40 (.13-0.56) | .05 |
| Immunosuppressive therapies‡ | ||||
| Prednisone > 1 mg/kg | 21 (34) | 17 (39) | 4 (24) | .37 |
| Calcineurin inhibitor | 13 (21) | 9 (21) | 4 (24) | 1.0 |
| Mycophenolate mofetil | 17 (28) | 13 (30) | 4 (24) | .76 |
| No. of immunosuppressants | 1 (1-2) | 2 (1-2) | 1 (1-2) | .79 |
| Antimicrobial therapy‡ | ||||
| Intravenous broad-spectrum antibiotic | 57 (93) | 41 (93) | 16 (94) | 1.0 |
| Trimethoprim-sulfamethoxazole | 15 (25) | 8 (19) | 7 (41) | .10 |
| Anti-mold coverage | 54 (89) | 39 (89) | 15 (88) | 1.0 |
| Antiviral therapy | 26 (43) | 18 (41) | 8 (47) | .78 |
Values are number (%) or median (interquartile range).
Data available for 64 patients; the remaining 2 patients had a chest CT around BAL showing infiltrates.
Data available for 59 patients.
Data available for 61 patients.
Standard diagnostic tests performed on BAL included conventional culture for bacteria and fungi; direct microscopy; galactomannan (Bio-Rad, Munich, Germany); PCR testing for the detection of 2 fungal, 3 bacterial, and 20 viral pathogens (Pneumocystis jirovecii [Sacace, Como, Italy], Aspergillus spp. [AsperGenius PN001, Maastricht, The Netherlands; available from March 2015], atypical bacteria [Legionella pneumophila, Mycoplasma pneumoniae, and Chlamydia pneumoniae; all sacace], cytomegalovirus [CMV], herpes simplex virus types 1 and 2, varicella zoster virus [all Qiagen, Hilden, Germany], human herpesvirus 6 [HHV-6; Altona, Hamburg, Germany]; and the respiratory panel (all from Qiagen) including influenza A (including H3N2 and H1N1) and B viruses, parainfluenza virus types 1 to 3, human metapneumovirus, human coronavirus (types OC43, NL63, and 229E), respiratory syncytial virus, adenoviruses (AdV), enteroviruses, human bocavirus, and human rhinoviruses (HRV). Because of limited sample material or technical reasons, not all tests were performed or provided results in all patients. Positive reads of CMV were only included if the viral load was >500 copies/mL as measured by quantitative PCR [13]. Molecular testing for azole-resistant strains of Aspergillus spp. (AsperGenius PN002 [14], Maastricht) and for the detection of Mucorales [15] were available from January 2017. Other BAL tests were performed as clinically indicated (Table 3 ).
Table 3.
Pathogen Findings in BAL
| Findings in BAL | No. of Tests | No. of Positive Tests (% Positive) | Percent of Patients with Positive Test |
|---|---|---|---|
| Fungal | |||
| Culture* | 66 | 18 (27) | 27 |
| Aspergillus spp. | 13 (20) | 20 | |
| Mucorales | 6 (9) | 9 | |
| Talaromyces | 1 (2) | 2 | |
| Galactomannan (OD ≥ .5) | 64 | 17 (27) | 26 |
| Aspergillus PCR | 40 | 8 (20) | 12 |
| Mucorales PCR | 3 | 1 (33) | 2 |
| Pneumocystis PCR† | 63 | 5 (8) | 8 |
| Viral | |||
| CMV | 61 | 19 (31) | 29 |
| Varicella zoster virus‡ | 57 | 5 (9) | 8 |
| Herpes simplex 1‡ | 59 | 5 (9) | 8 |
| Herpes simplex 2‡ | 59 | 0 | 0 |
| HHV-6‡ | 40 | 10 (25) | 15 |
| Influenza viruses | 62 | 3 (5) | 5 |
| Parainfluenza viruses | 59 | 1 (2) | 2 |
| Human metapneumovirus | 59 | 1 (2) | 2 |
| Human coronaviruses‡ | 59 | 5 (9) | 8 |
| Respiratory syncytial virus | 62 | 2 (3) | 3 |
| Adenoviruses‡ | 61 | 10 (16) | 15 |
| Enteroviruses‡ | 59 | 1 (2) | 2 |
| Human bocavirus‡ | 59 | 5 (9) | 8 |
| Human rhinoviruses‡ | 59 | 12 (20) | 18 |
| Bacterial | |||
| Culture | 66 | 17 (26) | 26 |
| Gram-negative | 16 (24) | 24 | |
| Gram-positive | 1 (2) | 2 | |
| Atypical bacteria PCR | 63 | 1 (2) | 2 |
| Toxoplasma PCR | 10 | 1 (10) | 2 |
| Mycobacteria culture | 4 | 0 | 0 |
Two patients with Aspergillus spp. + Mucorales.
One patient with additional positive direct microscopy.
Classified as virus without established pathogenicity in the lung (see Methods).
Results from blood cultures, serum CMV, and serum galactomannan testing were analyzed if they were obtained ±7 days from BAL. Results of galactomannan testing from BAL or blood are reported as positive using an optical density index ≥ .5 [16]. Viral pathogens were graded as “established” or “uncertain” lung pathogenicity in the setting of HSCT according to Seo et al. [9]. The recovery of Candida spp. or Enterococcus spp. from BAL was not reported in the absence of positive blood cultures. Documented diagnoses of probable invasive aspergillosis were in line with De Pauw et al. [17]. Standard antimicrobial prophylaxis consisted of ciprofloxacin and metronidazole from conditioning start until engraftment [18]. Patients with prolonged neutropenia or on systemic corticosteroids at a dose of >.5 mg/kg body weight prednisone or equivalent received anti-mold prophylaxis with voriconazole or posaconazole. Prophylaxis against P. jirovecii using trimethoprim-sulfamethoxazole or pentamidine as well as antiviral prophylaxis using acyclovir or valaciclovir followed national guideline recommendations [19].
Continuous data are presented as median and interquartile range (IQR; 25% to 75%) and dichotomous data as number and percentage. Group comparisons were made using Fisher's exact test and the Mann-Whitney U test as appropriate. Results were considered statistically significant when P < .05. Because of the exploratory nature of the study, no adjustments for multiple testing were made. Statistical tests were performed using the SPSS 23 software package (IBM, Armonk, NY).
Results
Patient Demographics and Characteristics
Of 156 HSCT recipients who were admitted to the ICU between May 2013 and July 2017, 66 patients (42%; 38 men and 28 women; age, 58 years [IQR, 45 to 64]) fulfilled the inclusion criteria for this study (Figure 1 ). Table 1 shows the patients' characteristics regarding HSCT, severity of illness and organ support measures, and ICU outcome.
Figure 1.
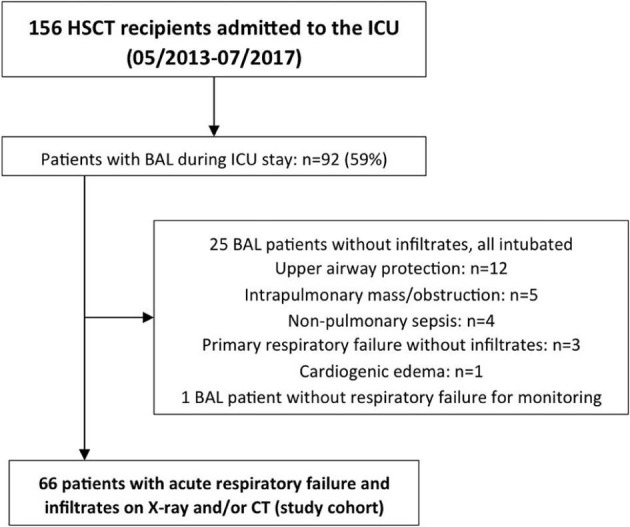
Patient flowchart.
ICU referral occurred 5 days (IQR, 0 to 30) after hospital admission, 176 days (IQR, 85 to 407) after HSCT, and during hospitalization for transplantation in 14 patients (21%). BAL was performed 2 days (IQR, 1 to 6) after ICU admission (Table 2), and 1194 tests were performed on the respective samples (Table 3). Most patients presented with multifocal/diffuse infiltrates on chest x-ray (n = 45, 70%), whereas 11 (17%) had a focal infiltrate (3 quadrants involved [IQR, 1 to 4]). A chest CT ±7 days from BAL was available in 41 patients (62%) and was the only method to detect infiltrates in 8 patients (12%) with a negative chest x-ray. At the time of BAL 57 patients (86%) received invasive mechanical ventilation (time from intubation to BAL, 0 days [IQR, 0 to 1]), 4 patients (6%) received noninvasive ventilation via face mask, 3 patients (5%) received high-flow nasal cannula oxygen, and 2 patients (3%) received conventional oxygen therapy. Most patients (n = 55, 90%) were at least on 1 immunosuppressive agent when BAL was performed (medication data available for 61 patients; Table 2). No transbronchial biopsy was performed in any patient.
Bacterial Pathogens
A bacterial pathogen was identified in BAL specimens of 18 patients (27%), mostly in the context of positive culture growth (Table 3). The nonfermenters Stenotrophomas maltophilia (n = 5) and Pseudomonas aeruginosa (n = 4) were the most abundant bacterial pathogens alongside other gram-negative bacilli. A full list of detected pathogen species is given in Figure 2 . There was nearly universal coverage with anti-pseudomonal beta-lactam antibiotics (n = 57, 93%) at the time of BAL. Concomitant blood cultures were available in all patients and yielded noncontaminant bacteria in 5 patients (8%), and only 2 patients with bacteria detected in BAL also had positive blood cultures. Older patient age (62 year [IQR, 59 to 68] versus 52 years [IQR, 42 to 63], P < .01) and time from HSCT to ICU admission (234 days [IQR, 155 to 1128] versus 143 days [IQR, 52 to 321], P = .03) were associated with the detection of a bacterial pathogen (Supplementary Tables 2 and 3).
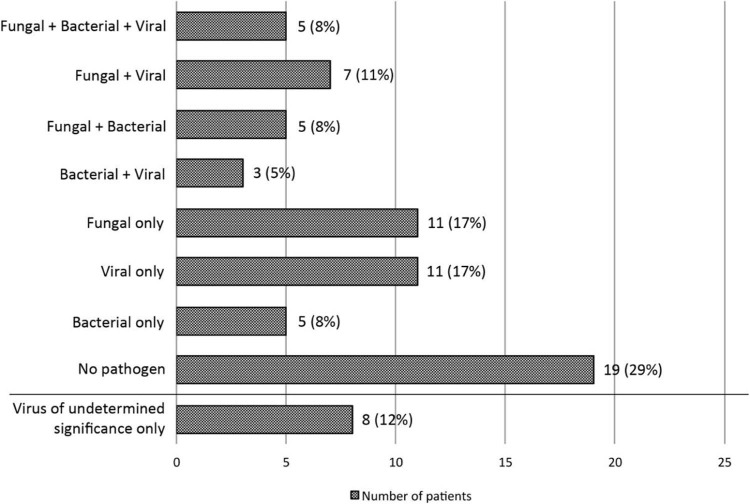
Figure 2.
Pathogen distribution among patients. Numbers of patients according to pathogen distribution are plotted against the horizontal axis. The following pathogens were observed in each group: Fungal + Bacterial + Viral: Talaromyces (n = 1), Aspergillus (n = 4), Stenotrophomonas (n = 3), Pseudomonas (n = 1), Escherichia coli (n = 1), CMV (n = 4), parainfluenza virus (n = 1); Fungal + Viral: Aspergillus (n = 4), Mucorales (n = 3), pneumocystis (n = 2), CMV (n = 5), influenza (n = 1), human metapneumovirus (n = 1); Fungal + Bacterial: Aspergillus (n = 4), Mucor (n = 1) pneumocystis (n = 1), Stenotrophomonas (n = 2), Klebsiella (n = 2), Pseudomonas (n = 1), Hemophilus influenzae (n = 1); Bacterial + Viral: CMV (n = 1), respiratory syncytial virus (n = 1), influenza (n = 1), Pseudomonas (n = 1), Enterobacter (n = 1), Legionella (n = 1); Fungal only: Aspergillus (n = 8), Mucorales (n = 3), pneumocystis, (n = 2); Viral only: CMV (n = 9), respiratory syncytial virus (n = 1), influenza (n = 1); Bacterial only: E. coli (n = 2), Staphylococcus aureus (n = 1), Klebsiella (n = 1), Pseudomonas (n = 1); Virus of uncertain lung pathogenicity: AdV (n = 4), HHV-6 (n = 3), HRV (n = 2), human bocavirus (n = 2), varicella zoster virus (n = 1), herpes simplex virus type 1 (n = 1). Only pathogen findings with established pathogenicity as outlined in Methods section were included in the first 7 groups.
Fungal Pathogens
Fungi represented the most prominent pathogen group and were identified in 28 patients (42%). Most belonged to mold species, which were detected by positive culture growth in 18 patients (27%; Aspergillus spp., n = 13; Mucorales, n = 6; Talaromyces, n = 1; Table 3). Forty-five patients (89%) received mold-active antifungal agents at the time of BAL. Considering culture results and nonculture tests from BAL (galactomannan and PCR) and blood (galactomannan), the diagnostic workup provided mycologic evidence of Aspergillus spp. in 20 patients (30%). Sixteen (80%) of these patients had a documented diagnosis of probable invasive aspergillosis.
Figure 3 shows the sensitivity and gives information about the overall test performance in these patients and in the overall cohort. BAL culture was the only positive test in 2 cases and BAL galactomannan in 1 case, respectively, with the remainder having had at least 2 different positive tests. Serum galactomannan was positive in 43% (6/14 tests) with a documented diagnosis of probable invasive aspergillosis. Of 3 Aspergillus DNA-positive samples tested, 2 were found with mutations in the cyp51A gene conferring azole resistance. Seven patients (11%) were diagnosed with invasive mucormycosis, 6 patients based on positive culture growth and 1 additional patient by positive PCR. We checked for established risk factors for invasive mucormycosis in our cohort and found an association with relapsed disease (4 [57%] versus 7 [12%], P = .01) and neutropenia (absolute neutrophil count, .12 g/L [IQR, 0 to 1.76] versus 2.25 g/L [IQR, .3 to 5.12]; P = .047), respectively (Supplementary Table 4). Pneumonia due to P. jirovecii was documented as the clinical diagnosis in 4 of 5 patients with a positive PCR, the other patient having been diagnosed with probable invasive aspergillosis. Severity of illness as measured by the SAPSII score (47 [IQR, 35 to 57] versus 34 [IQR, 26 to 46], P = .02), high-dose systemic corticosteroids (prednisone > 1 mg/kg; 13 [52%] versus 8 [22%]; P = .03), and low absolute lymphocyte counts (.09 g/L [IQR, .01 to .2] versus .35 g/L [IQR, .13 to .61], P < .01) were associated with the detection of a fungal pathogen (Supplementary Tables 2 and 3).
Figure 3.
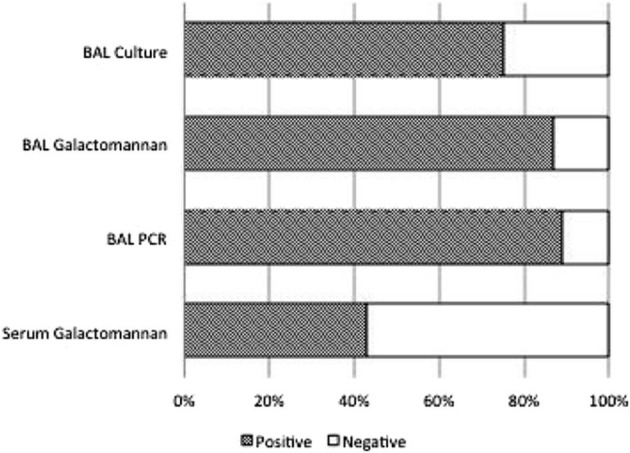
Sensitivity of diagnostic tests in 16 patients with a documented diagnosis of probable invasive aspergillosis. Number of positive tests: BAL culture, 12 of 16 (sensitivity 75%; specificity 98%, PPV 92%, NPV 92%); BAL galactomannan, 13 of 15 (sensitivity 87%, specificity 92%, PPV 76%, NPV 96%); BAL PCR, 8 of 9 (sensitivity 89%, specificity 100%, PPV 100%, NPV 97%); and serum galactomannan, 6 of 14 (sensitivity 43%, specificity 92%, PPV 86%, NPV 86%). NPV indicates negative predictive value; PPV, positive predictive value.
Viral Pathogens
A PCR-based panel identified at least 1 viral pathogen in BAL specimens of 26 patients (40%). In relation to the number of tests conducted, CMV (19/61, 31%) was the most abundant virus followed by several viruses with uncertain lung pathogenicity (HHV-6 [10/40, 25%], HRV [12/59, 20%], and AdV [10/61, 16%], etc.) (Table 3). The median CMV DNA load in BAL was 4.08 log10 copies/mL (IQR, 3.39 to 4.96). Fifteen patients (79%) with CMV detected in BAL also were tested positive for viral DNA in plasma at highest median loads of 4.15 log10 copies/mL (IQR, 3.53 to 5.12), whereas 12 of 31 patients (39%) were positive in blood but had CMV not detected or a viral load < 500 copies/mL in BAL.
Viruses with established lung pathogenicity other than CMV were found in 7 cases (influenza type A, n = 3; parainfluenza virus, n = 1; human metapneumovirus, n = 1; and respiratory syncytial virus, n = 2). Eight patients (12%) had 1 or more viruses with uncertain lung pathogenicity identified as the sole pathogens in BAL (AdV, n = 4; HHV-6, n = 3; HRV, n = 2; human bocavirus, n = 2; varicella zoster virus, n = 1; and herpes simplex virus 1, n = 1). Five of these patients had diffuse ground-glass opacities as their main CT finding, 2 patients had patchy alveolar consolidations, and 1 patient had no CT scan but had a focal infiltrate on chest x-ray. Twenty-six patients (43%) in the overall cohort received antiviral therapy at the time of BAL. Regarding patient and baseline characteristics, only the donor type showed an association with the identification of an established viral pathogen (P = .04; Supplementary Tables 2 and 3).
Pathogen Patterns, Polymicrobial Events, and Clinical Outcome
Counting only fungal and bacterial pathogens deemed likely to be causative of pneumonia and viruses with established lung pathogenicity in the setting of HSCT, 47 patients (71%) had at least 1 pathogen identified. In 20 cases (30%) significant pathogens from at least 2 different groups could be found (Figure 2). We checked for associations between pathogen groups and between individual pathogens but found no statistically significant evidence of such (Supplementary Table 1). Patients with an identified pathogen had a higher SAPSII score at ICU admission (45 [IQR, 28 to 53] versus 32 [IQR, 26 to 39], P = .049), a trend toward a more frequent need for renal replacement therapy during the ICU stay (31 [66%] versus 7 [37%], P = .053), and lower lymphocyte counts at the time of BAL (.14 g/L [IQR, .02 to .51] versus .40 g/L [IQR, .13 to .56], P = .054) and showed inferior ICU survival (3 [6%] versus 7 [37%], P < .01). In subgroup analysis these findings were largely attributable to the characteristics and outcomes observed in patients with and without a fungal pathogen (see above; ICU survival, 1 [4%] versus 9 [24%]; P = .04; Supplementary Table 2). ICU survival was 1 in 20 patients (5%) with polymicrobial infection as compared with 9 in 46 patients (20%) in the remainder (P = .26). Of the 8 patients with 1 or more viruses with uncertain lung pathogenicity as the sole microbiologic finding, 2 patients survived to ICU discharge without specific antiviral therapy (1 patient with HRV and 1 with AdV, respectively).
Discussion
Panel-based multiplex pathogen testing designed to achieve rapid turnaround times and to detect a large number of micro-organisms is increasingly used to assist in the diagnosis of respiratory tract infections, bloodstream infections, and others. Immunocompromised patients in particular could benefit from this approach, because a broad range of pathogens may cause their clinical presentation in this population, which often come with atypical signs and symptoms [5]. This is the first study to report comprehensive findings of a diagnostic workup including currently available panel-based methods in a real-life cohort of critically ill patients with ARF and pulmonary infiltrates after HSCT. We found that around 70% of patients had a pathogen known to cause pneumonia and that polymicrobial findings were frequent. An additional 12% had 1 or more viruses with uncertain lung pathogenicity as the sole microbiologic finding.
Fungi were the most commonly detected pathogens, with microbiologic evidence of such found in 42% of our patients. Their prevalence thus appeared somewhat higher than previously reported in noncritically ill HSCT recipients with pulmonary infiltrates (10% to 30%) 20, 21 or in the general population of critically ill immunocompromised patients (30%) [22]. Importantly, probable invasive aspergillosis occurred despite standard prophylaxis and almost universal anti-mold coverage at the time of BAL in several of our patients, and at least 2 of these cases were associated with genotypic azole resistance of the respective strain. Insufficient drug levels during azole therapy may also have led to breakthrough infections [23], but the respective data were not available for analysis. Regarding non-Aspergillus mold infections, a significant proportion of patients (11%) suffered from an invasive mucormycosis. Broad anti-mold antifungal is a known risk factor for this condition [24], although other risk factors, such as preceding graft-versus-host disease, older age, disease relapse, or profound neutropenia, have also been taken into account [25]. In our cohort we found an association with mucormycosis only for both of the latter, but statistical power of the analysis was limited. Emerging azole resistance and an increasing number of non-Aspergillus mold infections have previously been described in HSCT recipients 26, 27, and our findings probably outline this increasing complexity of fungal infection patterns in the HSCT population. Ultimately, their distribution may have clinical implications regarding regimens of antifungal prophylaxis and empirical therapy and the timing of BAL in nonintubated patients, because noninvasive tests (eg, galactomannan) fail to identify cases of mucormycosis or azole-resistant Aspergillus strains [3]. However, further studies in that respect are warranted. Importantly, even within this cohort with high baseline immunosuppression, evidence of impaired immune reconstitution as indicated by low absolute lymphocyte counts and high-dose corticosteroid therapy helped to identify individuals at the highest risk for invasive fungal disease.
As the second most common pathogen group, 1 or more viruses were detected in BAL samples of 40% of our patients after the implementation of a broad viral PCR panel. CMV was the most abundant virus and was detected at a viral load > 500 copies/mL in every third patient. Although this cut-off was recently established to support the diagnosis of CMV pneumonia in the context of HSCT [13], only very few of these patients had a respective clinical diagnosis documented. However, even in the absence of overt disease, subclinical CMV infection in the lungs has been shown to produce significant biologic effects such as inflammation and fibrosis [28]. Similar considerations apply for the 12% of our patients with the finding of 1 or more viruses of uncertain lung pathogenicity. Although several of these viruses such as HHV-6 [6], human coronavirus [7], or HRV [8] are increasingly recognized to cause lower respiratory tract disease after HSCT, evidence accumulates for a variety of subclinical pathogenic processes to be induced in the lungs upon infection with these viruses 29, 30. Although more research on these host responses is needed, studies including newer antiviral agents and focusing on preventive strategies in patients with asymptomatic shedding or upper respiratory tract disease are already ongoing [31]. Given the inferences from the high rate of polymicrobial findings in our patients (see below), these strategies may be more suited to improve outcomes than interventions in patients who already developed ARF, and we support further such studies.
Bacterial pathogens were detected in BAL samples of 27% of our patients with a clear predominance of gram-negative bacilli. Older patient age and time from HSCT to ICU admission were the only factors associated with the identification of bacterial pathogen, whereas time from hospital to ICU admission—a possible surrogate for the acquisition of hospital-acquired pneumonia—did not correlate with this finding (data not shown). Previous exposure to antibiotics as well as hospitalizations would have been other variables of interest in this context, but we could not obtain the respective data. Interestingly, time from HSCT to ICU admission did not correlate with the detection of the other pathogen groups [32].
The polymicrobial findings in almost a third of cases is another important observation from our study, although polymicrobial pneumonia was the clinical diagnosis in only 11% of patients. This is probably because clinicians attributed lung injury patterns to specific pathogens according to the patients' history, clinical assessment, and radiographic findings [4]. However, both clinical assessment and radiographic findings lack specificity in many instances 33, 34. On the other hand, as outlined above for CMV, microbiologic findings may still be significant even if they do not seem to account for the predominant pattern of lung injury 28, 29. Hence, the mutual interaction of pathogens or their inherent pathogenicity acting as competing conditions could be a significant promoter of lung injury in these patients. In both cases the observation of frequent polymicrobial findings would be important, because it reduced the chance of any single pathogen-directed intervention to be successful, either in clinical practice or in clinical trials.
Detection of a causative pathogen enhances diagnostic certainty and guides antimicrobial therapy. Furthermore, the inability to identify the cause of ARF in hematologic patients is an independent factor associated with mortality [35]. Although the analysis of outcome measures was not the scope of our study, the finding of increased mortality in patients with a detected pathogen was therefore surprising. However, a similar observation was made in the study of Seo et al. [9]. Three factors may have accounted for this observation. First, invasive fungal infections are associated with excess mortality of up to 90% in HSCT recipients 2, 22, and our detection rate was high. Indeed, only 1 patient with an invasive fungal infection in our cohort survived to ICU discharge. Second, even with respect to HSCT, patients in our cohort were highly immunosuppressed, most having been on multiagent immunosuppressive therapy including corticosteroids, and having shown very low absolute lymphocyte counts [36]. Detection of a pathogen may therefore have been deleterious in these patients with significantly impaired infection control capabilities, especially if regarded as a possible consequence of high pathogen burden. Third, patients with a detected pathogen were more severely ill than those without. However, this may have been regarded either as a consequence of the respective pathogen(s) or as a factor having influenced detection rates in our patients [37].
A major strength of our study was that it included a reasonable number of patients from a contemporary cohort of HSCT recipients admitted to the ICU, and findings may thus reflect current patterns observed in this population. On the other hand, microbiologic findings regarding pathogen occurrence and resistance always follow local conditions and have thereby been put into place. Because of the retrospective observational nature of our study, several technical aspects such as standardized conduction of BALs and uniform sample processing cannot be ensured. Other limitations related to the study design concern data completeness, quality, and interpretation. Finally, our study provides a mere description of microbiologic findings and does not allow inferences to relative contributions of single pathogens to ARF and outcome.
In conclusion, by using a microbiologic diagnostic workup incorporating molecular- and biomarker-based assays on BAL and blood samples, pathogens known to cause pneumonia were found in 70% of HSCT recipients with ARF admitted to the ICU. An additional 12% had a virus of undetermined lung pathogenicity as the sole microbiologic finding. BAL may enhance pathogen detection and therapy guidance in settings of high prevalence of invasive mold infections including non-Aspergillus strains and of emerging azole resistance. The polymicrobial findings in almost a third of our patients warrant consideration in clinical practice and in the design of future interventional studies.
Acknowledgments
Financial disclosure: The authors have nothing to disclose.
Conflict of interest statement: J.S. has received honoraria for lectures and advisory board meetings from Gilead Sciences and TEVA.
Authorship statement: P.W. and T.L. were responsible for the conception and design of the study; the acquisition, analysis, and interpretation of data; and for drafting the manuscript. A.T.T., J.S., M.F., N.K.S., and D.W.B. were involved in the analysis and interpretation of data and critically revised the manuscript for important intellectual content. All authors read and approved the final manuscript.
Footnotes
Financial disclosure: See Acknowledgments on page 1713.
Supplementary data related to this article can be found online at doi:10.1016/j.bbmt.2018.03.007.
Supplementary Data
The following is the supplementary data to this article:
Supplementary Tables 1–4.
References
- 1.Yadav H., Nolan M.E., Bohman J.K. Epidemiology of acute respiratory distress syndrome following hematopoietic stem cell transplantation. Crit Care Med. 2016;44:1082–1090. doi: 10.1097/CCM.0000000000001617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lengline E., Chevret S., Moreau A.S. Changes in intensive care for allogeneic hematopoietic stem cell transplant recipients. Bone Marrow Transplant. 2015;50:840–845. doi: 10.1038/bmt.2015.55. [DOI] [PubMed] [Google Scholar]
- 3.Azoulay E., Mokart D., Lambert J. Diagnostic strategy for hematology and oncology patients with acute respiratory failure: randomized controlled trial. Am J Respir Crit Care Med. 2010;182:1038–1046. doi: 10.1164/rccm.201001-0018OC. [DOI] [PubMed] [Google Scholar]
- 4.Schnell D., Mayaux J., Lambert J. Clinical assessment for identifying causes of acute respiratory failure in cancer patients. Eur Respir J. 2013;42:435–443. doi: 10.1183/09031936.00122512. [DOI] [PubMed] [Google Scholar]
- 5.Ramanan P., Bryson A.L., Binnicker M.J., Pritt B.S., Patel R. Syndromic panel-based testing in clinical microbiology. Clin Microbiol Rev. 2018;31 doi: 10.1128/CMR.00024-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mariotte E., Schnell D., Scieux C. Significance of herpesvirus 6 in BAL fluid of hematology patients with acute respiratory failure. Infection. 2011;39:225–230. doi: 10.1007/s15010-011-0114-8. [DOI] [PubMed] [Google Scholar]
- 7.Milano F., Campbell A.P., Guthrie K.A. Human rhinovirus and coronavirus detection among allogeneic hematopoietic stem cell transplantation recipients. Blood. 2010;115:2088–2094. doi: 10.1182/blood-2009-09-244152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seo S., Waghmare A., Scott E.M. Human rhinovirus detection in the lower respiratory tract of hematopoietic cell transplant recipients: association with mortality. Haematologica. 2017;102:1120–1130. doi: 10.3324/haematol.2016.153767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seo S., Renaud C., Kuypers J.M. Idiopathic pneumonia syndrome after hematopoietic cell transplantation: evidence of occult infectious etiologies. Blood. 2015;125:3789–3797. doi: 10.1182/blood-2014-12-617035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Panoskaltsis-Mortari A., Griese M., Madtes D.K. An official American Thoracic Society research statement: noninfectious lung injury after hematopoietic stem cell transplantation: idiopathic pneumonia syndrome. Am J Respir Crit Care Med. 2011;183:1262–1279. doi: 10.1164/rccm.2007-413ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seo S., Campbell A.P., Xie H. Outcome of respiratory syncytial virus lower respiratory tract disease in hematopoietic cell transplant recipients receiving aerosolized ribavirin: significance of stem cell source and oxygen requirement. Biol Blood Marrow Transplant. 2013;19:589–596. doi: 10.1016/j.bbmt.2012.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le Gall J.R., Lemeshow S., Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270:2957–2963. doi: 10.1001/jama.270.24.2957. [DOI] [PubMed] [Google Scholar]
- 13.Boeckh M., Stevens-Ayers T., Travi G. Cytomegalovirus (CMV) DNA quantitation in bronchoalveolar lavage fluid from hematopoietic stem cell transplant recipients with CMV pneumonia. J Infect Dis. 2017;215:1514–1522. doi: 10.1093/infdis/jix048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chong G.L., van de Sande W.W., Dingemans G.J. Validation of a new Aspergillus real-time PCR assay for direct detection of Aspergillus and azole resistance of Aspergillus fumigatus on bronchoalveolar lavage fluid. J Clin Microbiol. 2015;53:868–874. doi: 10.1128/JCM.03216-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Springer J., Lackner M., Ensinger C. Clinical evaluation of a Mucorales-specific real-time PCR assay in tissue and serum samples. J Med Microbiol. 2016;65:1414–1421. doi: 10.1099/jmm.0.000375. [DOI] [PubMed] [Google Scholar]
- 16.Fisher C.E., Stevens A.M., Leisenring W., Pergam S.A., Boeckh M., Hohl T.M. The serum galactomannan index predicts mortality in hematopoietic stem cell transplant recipients with invasive aspergillosis. Clin Infect Dis. 2013;57:1001–1004. doi: 10.1093/cid/cit393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Pauw B., Walsh T.J., Donnelly J.P. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46:1813–1821. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beelen D.W., Elmaagacli A., Muller K.D., Hirche H., Schaefer U.W. Influence of intestinal bacterial decontamination using metronidazole and ciprofloxacin or ciprofloxacin alone on the development of acute graft-versus-host disease after marrow transplantation in patients with hematologic malignancies: final results and long-term follow-up of an open-label prospective randomized trial. Blood. 1999;93:3267–3275. [PubMed] [Google Scholar]
- 19.Ullmann A.J., Schmidt-Hieber M., Bertz H. Infectious diseases in allogeneic haematopoietic stem cell transplantation: prevention and prophylaxis strategy guidelines 2016. Ann Hematol. 2016;95:1435–1455. doi: 10.1007/s00277-016-2711-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forslow U., Remberger M., Nordlander A., Mattsson J. The clinical importance of bronchoalveolar lavage in allogeneic SCT patients with pneumonia. Bone Marrow Transplant. 2010;45:945–950. doi: 10.1038/bmt.2009.268. [DOI] [PubMed] [Google Scholar]
- 21.Shannon V.R., Andersson B.S., Lei X., Champlin R.E., Kontoyiannis D.P. Utility of early versus late fiberoptic bronchoscopy in the evaluation of new pulmonary infiltrates following hematopoietic stem cell transplantation. Bone Marrow Transplant. 2010;45:647–655. doi: 10.1038/bmt.2009.203. [DOI] [PubMed] [Google Scholar]
- 22.Azoulay E., Lemiale V., Mokart D. Acute respiratory distress syndrome in patients with malignancies. Intensive Care Med. 2014;40:1106–1114. doi: 10.1007/s00134-014-3354-0. [DOI] [PubMed] [Google Scholar]
- 23.Park W.B., Kim N.H., Kim K.H. The effect of therapeutic drug monitoring on safety and efficacy of voriconazole in invasive fungal infections: a randomized controlled trial. Clin Infect Dis. 2012;55:1080–1087. doi: 10.1093/cid/cis599. [DOI] [PubMed] [Google Scholar]
- 24.Kontoyiannis D.P., Lewis R.E. How I treat mucormycosis. Blood. 2011;118:1216–1224. doi: 10.1182/blood-2011-03-316430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riches M.L., Trifilio S., Chen M. Risk factors and impact of non-Aspergillus mold infections following allogeneic HCT: a CIBMTR infection and immune reconstitution analysis. Bone Marrow Transplant. 2015;51:277. doi: 10.1038/bmt.2015.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steinmann J., Hamprecht A., Vehreschild M.J. Emergence of azole-resistant invasive aspergillosis in HSCT recipients in Germany. J Antimicrob Chemother. 2015;70:1522–1526. doi: 10.1093/jac/dku566. [DOI] [PubMed] [Google Scholar]
- 27.Wiederhold N.P., Patterson T.F. Emergence of azole resistance in Aspergillus. Semin Respir Crit Care Med. 2015;36:673–680. doi: 10.1055/s-0035-1562894. [DOI] [PubMed] [Google Scholar]
- 28.Cook C.H., Zhang Y., Sedmak D.D., Martin L.C., Jewell S., Ferguson R.M. Pulmonary cytomegalovirus reactivation causes pathology in immunocompetent mice. Crit Care Med. 2006;34:842–849. doi: 10.1097/01.ccm.0000201876.11059.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khalfaoui S., Eichhorn V., Karagiannidis C. Lung infection by human bocavirus induces the release of profibrotic mediator cytokines in vivo and in vitro. PLoS One. 2016;11 doi: 10.1371/journal.pone.0147010. e0147010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abbas S., Raybould J.E., Sastry S., de la Cruz O. Respiratory viruses in transplant recipients: more than just a cold. Clinical syndromes and infection prevention principles. Int J Infect Dis. 2017;62:86–93. doi: 10.1016/j.ijid.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 31.Waghmare A., Englund J.A., Boeckh M. How I treat respiratory viral infections in the setting of intensive chemotherapy or hematopoietic cell transplantation. Blood. 2016;127:2682–2692. doi: 10.1182/blood-2016-01-634873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tomblyn M., Chiller T., Einsele H. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant. 2009;15:1143–1238. doi: 10.1016/j.bbmt.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leung A.N., Gosselin M.V., Napper C.H. Pulmonary infections after bone marrow transplantation: clinical and radiographic findings. Radiology. 1999;210:699–710. doi: 10.1148/radiology.210.3.r99mr39699. [DOI] [PubMed] [Google Scholar]
- 34.Wingard J.R., Hiemenz J.W., Jantz M.A. How I manage pulmonary nodular lesions and nodular infiltrates in patients with hematologic malignancies or undergoing hematopoietic cell transplantation. Blood. 2012;120:1791–1800. doi: 10.1182/blood-2012-02-378976. [DOI] [PubMed] [Google Scholar]
- 35.Contejean A., Lemiale V., Resche-Rigon M. Increased mortality in hematological malignancy patients with acute respiratory failure from undetermined etiology: a Groupe de Recherche en Reanimation Respiratoire en Onco-Hematologique (Grrr-OH) study. Ann Intensive Care. 2016;6:102. doi: 10.1186/s13613-016-0202-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim H.T., Armand P., Frederick D. Absolute lymphocyte count recovery after allogeneic hematopoietic stem cell transplantation predicts clinical outcome. Biol Blood Marrow Transplant. 2015;21:873–880. doi: 10.1016/j.bbmt.2015.01.019. [DOI] [PubMed] [Google Scholar]
- 37.Walton A.H., Muenzer J.T., Rasche D. Reactivation of multiple viruses in patients with sepsis. PLoS One. 2014;9 doi: 10.1371/journal.pone.0098819. e98819. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Tables 1–4.