Abstract
Platensimycin (PTM), originally isolated from soil bacteria Streptomyces platensis, is a potent FabF inhibitor against many gram-positive pathogens, such as methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant enterococci. However, the further clinical development of PTM is hampered by its poor bioavailability. In this study, twenty PTM derivatives were prepared by Suzuki-Miyaura cross-coupling reactions catalyzed by Pd (0)/C. Compared to PTM, 6-pyrenyl PTM (6t) showed improved antibacterial activity against MRSA in a mouse peritonitis model. Our results support the strategy to target the essential fatty acid synthases in major pathogens, in order to discover and develop new generations of antibiotics.
Keywords: platensimycin, antibiotics, cross-coupling reactions
Graphical Abstract
INTRODUCTION
Bacterial resistance to current antibiotic treatment is a major public health crisis worldwide.1 Type II fatty acid synthases (FASII) catalyze the biosynthesis of fatty acids, essential for the survival of many bacterial pathogens.2, 3 Therefore they are promising drug targets for antibiotic discovery and development. A milestone discovery of natural FASII inhibitors is the isolation of platensimycin (PTM) from soil-dwelling Streptomyces platensis, which targets the bacterial condensing enzyme FabF in FASII and represents a new molecular scaffold with potent antibacterial activities against a wide range of Gram-positive pathogens1, such as methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant enterococci (VRE).4 However, the poor pharmacokinetics of PTM prevents its further development into clinics.5–8
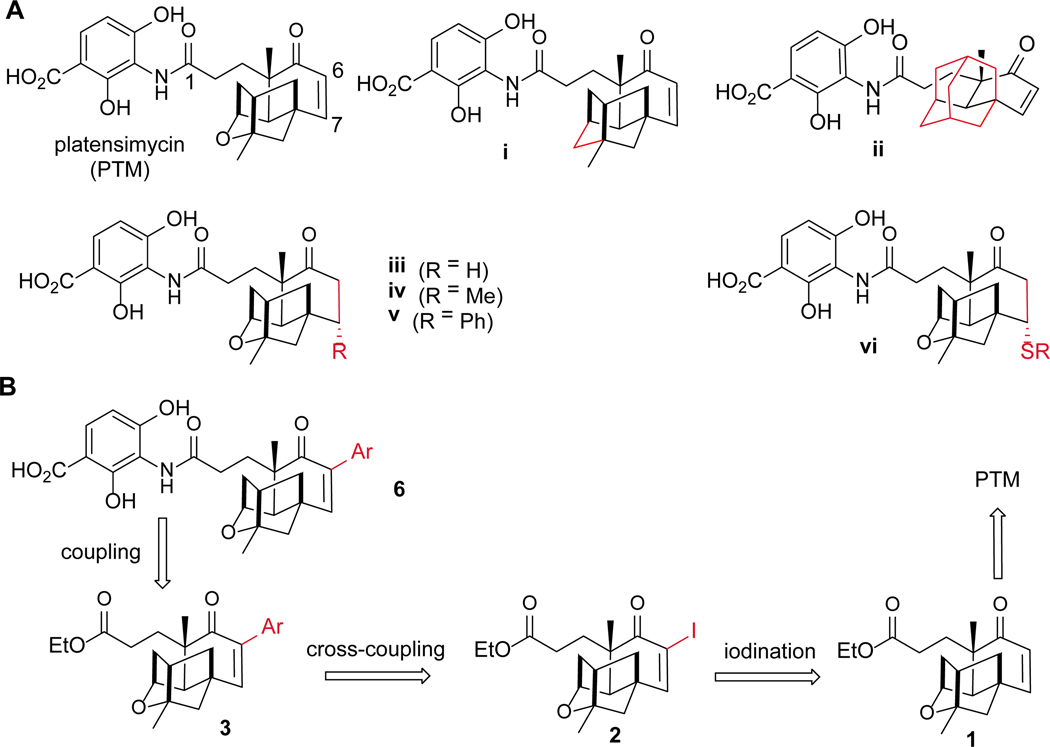
The previous structure-activity relationship study of PTM has revealed that its 3-amino-2, 4-dihydroxybenzoic acid (ADHBA) moiety is located in the deep hole of the catalytic pocket of FabF.4, 9 Even small modifications on the ADHBA usually result in a dramatic decrease in its antibacterial activity.10, 11 The ketolide moiety of PTM is solvent-exposed and has few interactions with the active site, proper modification of this moiety could thus retain the antibiotic activities (Figure 1A).11–16 For example, potent PTM analogue 7-phenylplatensimycin could be generated by the conjugation of a phenyl group on the β-position (C-7) of ketolide moiety of PTM.16 However, it took 15 synthetic steps to be prepared with a low overall yield. In the past decades, more than one hundred of PTM analogues were obtained from fermentation or through synthesis.8–18 However, most approaches yielded only a few active analogues with very limit quantity, which likely prevented further study of their antibacterial properties in vivo.
Figure 1.
The strategy to modify PTM terpene cage. (A) The structures of PTM, carboplatensimycin (i), adamantaplatensimycin (ii), dihydroplatensimycin (iii), 7-methyl-platensimycin (iv) and 7-phenylplatensimycin (v) and PTM sulfa-Michael adducts (vi); (B) The retrosynthetic analysis of 6-aryl conjugated PTM analogues 6 through Suzuki-Miyaura cross-coupling reactions.
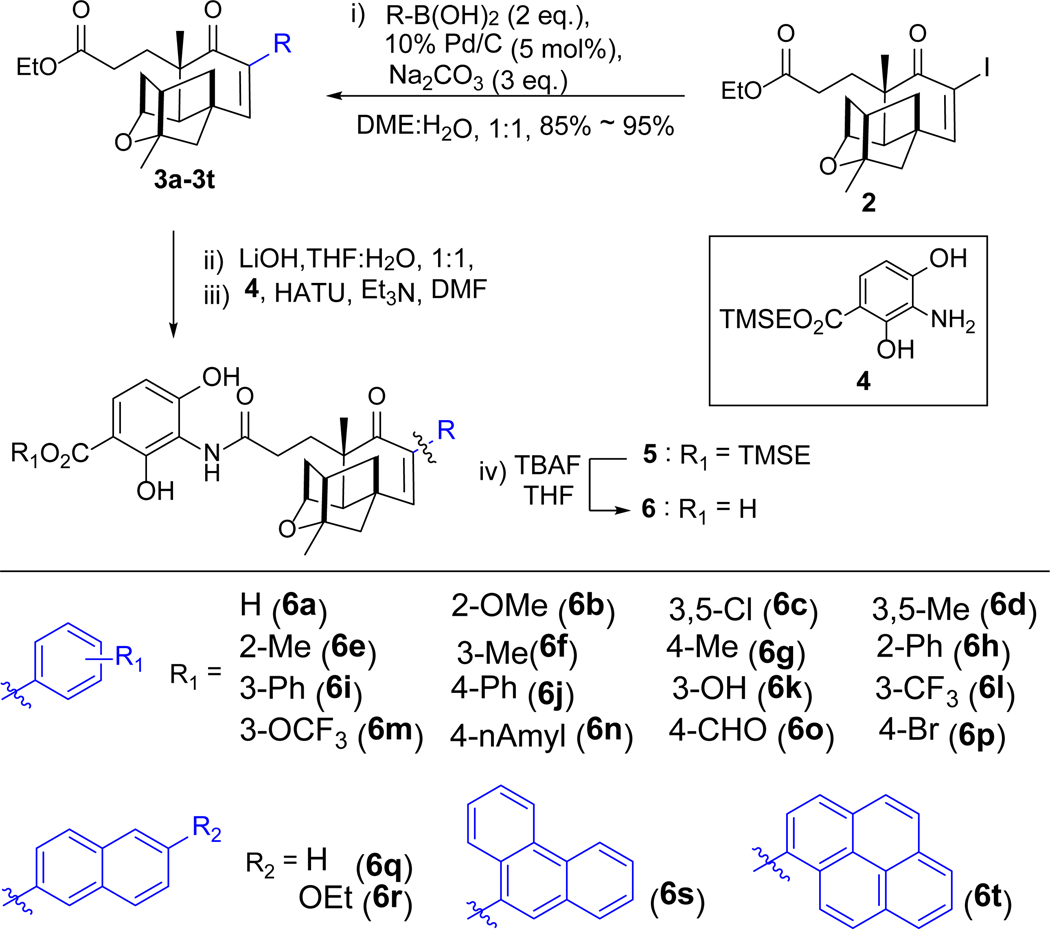
The generation of a PTM high-yield producing strain S. platensis SB12026 and the pilot scale production of PTM in decagram quantity provided us excellent opportunities to prepare more PTM analogues for structure-activity relationship studies through semisynthesis.19, 20 The Suzuki-Miyaura reaction plays critical roles in palladium-catalyzed C-C bond formation, applicable to a wide range of substrates and organoboronic acids. The recent developed heterogeneous palladium catalyst 10% Pd(0)/C is environmentally benign and the cross-coupling reactions are not sensitive to water and air, in contrast to the classical homogeneous conditions.21 In this study, we applied Suzuki-Miyaura reactions to synthesize PTM derivatives with a diverse set of aryl groups on C-6 of PTM with potent antibiotic activities in vitro and 6-pyrenyl PTM (6t) showed improved antibacterial activity in a mouse peritonitis model, in comparison to PTM (Figure 1B).
RESULTS AND DISCUSSION
Semisynthesis of PTM Derivatives 6a – 6t
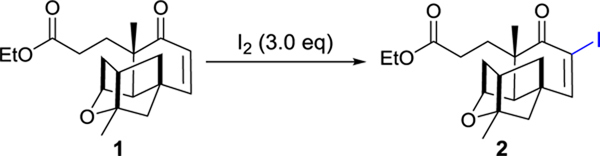
After the failed attempt to prepare 6-iodo-PTM as the substrate for Suzuki-Miyaura cross-coupling reaction by direct iodination of PTM with I2, we opted to synthesize the iodine intermediate 2 using platensic acid (PTMA) ethyl ester 1 as the starting material. In our previous study, compound 1 was obtained from purified PTM by hydrolysis with 90% yield.10 In order to develop a more efficient method to obtain PTM, the fermentation supernatant containing PTM was firstly adsorbed to porous hydrophobic resins, which were air-dried and contained about 33.0 mg of PTM per gram resin. The adsorbed PTM was released with ethanol and directly hydrolyzed by sulfuric acid to afford 1, which was subsequently purified through column chromatography (Figure S1). Over 10 g of 1 could be obtained from 500 g dried resins, which was sufficient for further structural modifications.
In order to synthesize iodo-PTMA ethyl ester 2, we applied the reported iodination conditions for cycloalkenones using iodine, by varying different solvents, catalyst loading and reaction temperature (Table 1).22, 23 Compound 2 was conveniently obtained with 95% yield, when DMAP was used as the catalyst in tetrachloromethane/pyridine under 90 oC.
Table 1.
Optimization of iodination reaction conditions
 | ||||
|---|---|---|---|---|
| entry | Cat. | solvent | T (°C) | yielda (%) |
| 1 | K2CO3 | THF/H2O (1/1) | 25 | <5 |
| 2 | K2CO3 | THF/H2O (1/1) | 50 | 35 |
| 3 | Et3N | THF/H2O (1/1) | 25 | <5 |
| 4 | DMAP | THF/Py (10/1) | 25 | <5 |
| 5 | DMAP | DCM/Py (3/1) | 25 | <5 |
| 6 | DMAP | DCM/Pip (3/1) | 25 | 27 |
| 7 | DMAP | CHCl3/Py (3/1) | 25 | <5 |
| 8 | DMAP | Et2O/Py (3/1) | 25 | <5 |
| 9 | DMAP | CCl4/Py (2/1) | 90 | >95 |
Yields were calculated according to HPLC analysis.
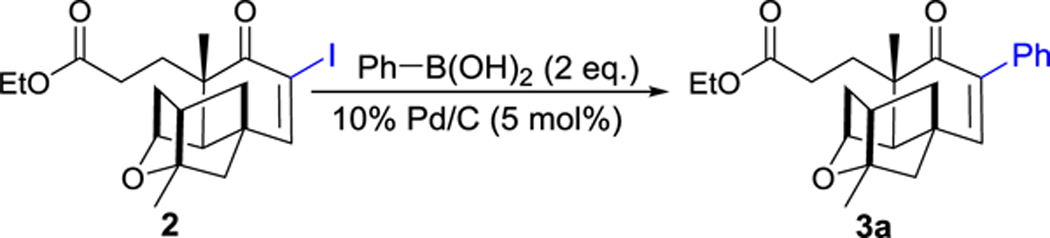
The borophenylic acid was next used as the model substrate to couple to 2, using the Suzuki-Miyaura cross-coupling reactions.21 In order to identify the proper condition for the cross-coupling reaction, we varied the bases, solvents, reaction temperature and reaction time (Table 2). The desired aryl-substituted PTMA ethyl ester 3a was obtained in 87% yield in a 1:1 mixture of ethanol and H2O in the presence of 2 eq. of borophenylic acid, 3 eq. of Na2CO3 and catalytic amount of 10% Pd(0)/C (Table 2, entry 1). Its structure was subsequently determined based on HRMS, 1H and 13C NMR spectra (Figure S2). The yield of compound 3a could be further improved to 95%, when a 1:1 mixture of glycol dimethyl ether (DME) and H2O was used as the solvents under room temperature. Compound 3a was partially hydrolyzed to the corresponding acid when higher temperature (80 oC) or K2CO3 was applied (Table 2, entries 2, 3 and 8–9). No products were observed when stronger bases, such as NaOH and KOH, and organic base trimethylamine were used (Table 2, entries 6–7, 10)
Table 2.
Optimization of coupling reaction conditions
 | |||||
|---|---|---|---|---|---|
| entry | base (3.0 eq.) | solvents | T (°C) | t (h) | yielda (%) |
| 1 | Na2CO3 | EtOH/H2O (1/1) | 25 | 12 | 87 |
| 2 | Na2CO3 | EtOH/H2O (1/1) | 80 | 6 | 69b |
| 3 | Na2CO3 | DME/H2O (95/5) | 80 | 6 | 85b |
| 4 | Na2CO3 | DME/H2O (1/1) | 25 | 12 | 95 |
| 5 | Na2CO3 | DME/H2O (1/1) | 80 | 6 | 73 b |
| 6 | NaOH | DME/H2O (1/1) | 25 | 12 | <5 b |
| 7 | KOH | DME/H2O (1/1) | 25 | 12 | <5 b |
| 8 | K2CO3 | DME/H2O (1/1) | 25 | 12 | 78 b |
| 9 | K2CO3 | DME/H2O (1/1) | 80 | 12 | <5 b |
| 10 | Et3N | DME/H2O (1/1) | 25 | 12 | <5 |
Yields are calculated according to HPLC.
Parts of the products were hydrolyzed in such conditions
The optimized cross-coupling reaction condition (Table 2, entry 4) was subsequently applied to other 19 different organoboronic acids, and most of them could efficiently react with 2 to generate 3b – 3t (Scheme 1). Interestingly, no obvious differences in yields were observed among small and big substituents bearing organoboronic acids, suggesting that the steric effects of PTMA were less important to this reaction. Compounds 3a – 3t were hydrolyzed with 2M LiOH in THF /water (1: 1) to generate the corresponding aryl-PTMAs in quantitative yields. The trimethylsilane (TMS) ester derivative of the ADHBA moiety 4 was prepared based on the previous procedure in gram-scale.11 Coupling of the newly prepared PTMA derivatives with compound 4, using HATU as the catalyst in dry DMF, afforded the corresponding trimethylsilylethyl (TMSE) protected PTM derivatives 5.10 Finally the TMSE protecting group of 5 was removed with catalytic amount of tetrabutylammonium fluoride (TBAF) in THF, affording 6a – 6t with yields from 70–85% (Figure S22 – S43).
Scheme 1.
Synthesis of PTM derivatives 6a – 6t. Compounds 3a – 3t and 5a – 5t have the same substituents with compounds 6a – 6t. DME: dimethoxyethane; Et3N: triethylamine; HATU: 2-(7-Azabenzotriazol-1-yl)-N,N,N’,N’-tetramethyluronium hexafluorophosphate. TBAF: tetrabutylammonium fluoride; TMSE: trimethylsilylethyl.
In Vitro Evaluation of the Antibacterial Activities of PTM Derivatives 6a – 6t
The antibacterial activities for the 20 PTM derivatives 6a – 6t were evaluated against S. aureus ATCC 29213, four MRSA strains and two methicillin-sensitive S. aureus (MSSA) strains, as well as the gram-negative pathogens Klebsiella pneumoniae and Escherichia coli, isolated from local hospitals.24 Similar to other reported PTM analogues, compounds 6a – 6t showed potent antibacterial activities against the gram-positive S. aureus strains, while no antibacterial activities against the tested K. pneumoniae and E. coli strains. The minimum inhibitory concentrations (MICs) against S. aureus strains for 6a – 6t were subsequently determined using agar dilution method (Table 1). Impressively, these analogues showed MICs ranging from 2 – 32 μg/mL against the tested S. aureus strains. Several PTM analogues, such as 6p, 6s and 6t, only showed about 2 – 4 fold less antibacterial activities (2 – 4 μg/mL), in comparison with PTM (0.5 – 1 μg/mL). These data were consistent with the previous design principle that the modification of the PTM terpene cage would result in active PTM analogues.11 PTM benzyl derivative 6a – 6p have various substituents on the benzene ring, with their MIC ranging from 2 – 32 μg/mL, which suggests that the size, polarity and position of the substituents on the benzyl group would likely affect their antibacterial activities in vitro.
Molecular Docking Studies
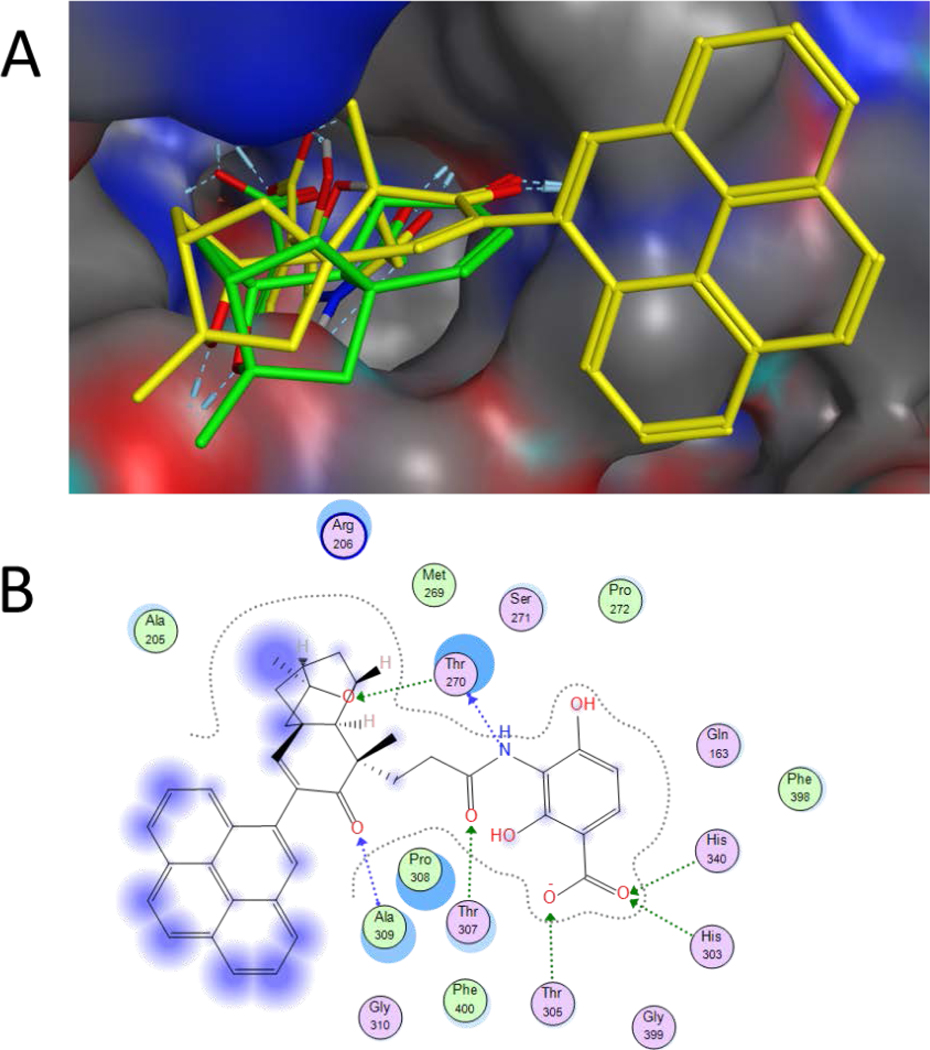
Molecular docking studies of 6a – 6t with FabF of E. coli FabF (C163Q) were performed to rationalize the obtained biological results and understand their interactions with FabF (Figures 2 and S44).25 All Docking experiments were performed using the MOE (Molecular Operating Environment) platform by using ecFabF (C163Q) co-crystallized with PTM (PDB ID: 2GFX) as a template. One hundred docking iterations followed with force field refinement had been taken for each analogue. Docking of compound 6t bearing rigid pyrenyl substituent to ecFabF (C163Q), revealed that the appended pyrenyl group on the PTM ketolide would form hydrophobic interactions with residues located at the edge of the FabF active site (Figure 2). In comparison to PTM, the ADHBA moiety of 6t also formed hydrogen bonds with His303 and His340 of ecFabF (C163Q), which correlated well with its strong antibacterial activities against S. aureus. Analysis of the docking results for compounds 6a – 6s also reveals the presence of the critical interactions of their ADHBA moiety with His303 and His340 in the ecFabF (C163Q) active site (Figure S44).
Figure 2.
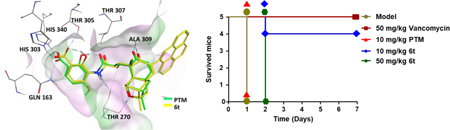
The predicted docking mode of analogue 6t in ecFabF (C163Q). (A) The docking mode of 6t (yellow) is similar to that of PTM (green). (B) The binding mode of 6t with ecFabF (C163Q) was shown in 2D model, with interactions with His303, His340, Thr270, Thr307 and Ala309 of the amino acid residues in the enzyme active site.
Further Evaluation of the Antibacterial Activities of 6s and 6t
The antimicrobial activity of the most potent PTM analogues 6s and 6t were evaluated using resazurin based microtiter dilution assay, with or without addition of 10% human serum (Figure S45). Consistent with the previous report, serum addition significantly reduced the antibiotic activities of PTM, with a MIC of 8 μg/mL against the tested MRSA strain.4 In contrast, compound 6t has a MIC of 4 μg/mL in the presence of 10% human serum, compared favorably to PTM.
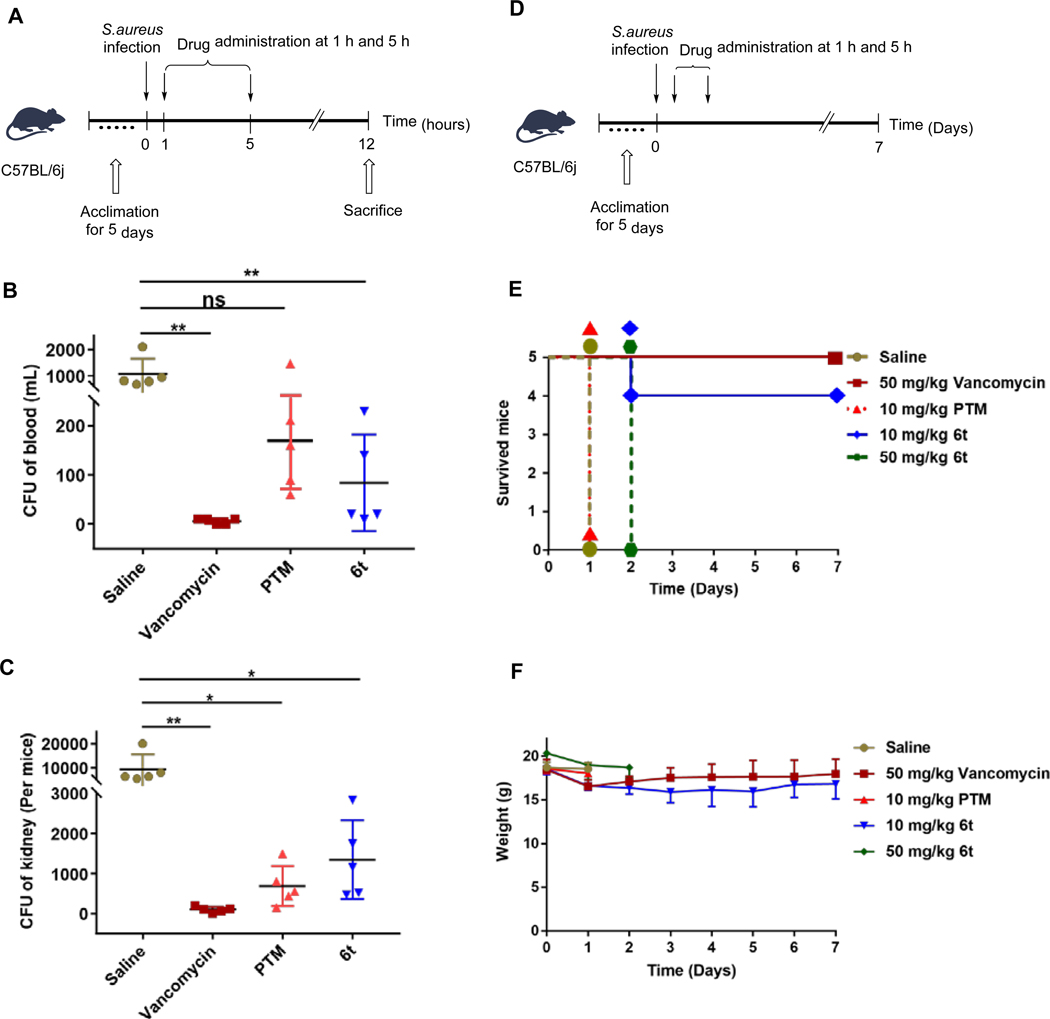
Therefore the in vivo antibacterial activity of 6t was evaluated in a mouse peritonitis model, using PTM and vancomycin as controls (Figures 3A–3C). Twenty C57BL/6J male mice were inoculated intraperitoneally with 2 × 107 colony forming units (CFUs) of the previous MRSA strain, in combination of 5% (w/v) hog gastric mucin.26, 27 The infected mice were intraperitoneally treated with 50 mg/kg of 6t, PTM, vancomycin or saline (n = 5) at 1 h and 5 h after bacterial injection. The bacterial load in blood and kidneys of these mice were examined at 12 h after MRSA infection. Administration of vancomycin, 6t and PTM significantly reduced the bacterial load in blood and kidneys in infected mice, compared to the saline-treated mice. Interestingly, 6t tended to clear more S. aureus in blood compared to PTM, while PTM-treated mice had slightly less S. aureus load in kidneys than 6t-treated mice.
Figure 3.
The antibacterial activities of compound 6t in vivo. (A) The timeline to evaluate the antibacterial activities of 6t in in a mouse peritonitis model at 12 h after MRSA infection. PTM and vancomycin (50 mg/kg) was used as the positive controls and physiological saline was used as the negative control. (B) The CFUs of S. aureus in the blood of infected mice with different treatments. (C) The CFUs of S. aureus in the kidney of infected mice with different treatments. Each point represents data from a single mouse. Mean values are presented, n = 5. Error bars indicate ± SEM. The CFUs were analyzed using unpaired two-tailed Student’s t-test. A probability value of p < 0.05 (∗) or p < 0.01 (∗∗) was considered significant, while p > 0.05 (ns) was considered insignificant. (D) The timeline to evaluate the antibacterial activities of 6t in in a mouse peritonitis model for 7 days after MRSA infection, in comparison to PTM (n = 5 mice per group). Vancomycin was used as the positive control and physiological saline was used as the negative control. The infected mice were inspected twice every day and their survival and body weights were tracked for 7 days. (E) The NO. of survived mice after treatment. (F) The weights of the survived mice in the period of 7 days.
Using the similar mouse peritonitis model, different dosage of 6t was further evaluated and the mice were inspected for 7 days (Figures 3D–3F). Four out of five 6t-treated mice (10 mg/kg) survived after 7 days, while none of the five mice in the PTM-treated group (10 mg/kg) survived beyond 24 h. In addition, the weights of survived mice in 6t-treated group (10 mg/kg) were comparable to those in vancomycin-treated group. However, the mice in 6t-treated group (50 mg/kg) died within 48 h, suggesting its toxicity when used in high dosage. These results suggested that 6t exhibited improved antibacterial activities against the tested MRSA strain than PTM in vivo.
CONCLUSION
One important approach to alleviate the current antibiotic crisis is to discover antibiotics against novel bacterial targets.1 Bacteria FASs have been considered as promising antibacterial targets, due to its structural difference with their human type I FASs.2, 3 Although isoniazid has been used as a prodrug to treat Mycobacteria tuberculosis infection over 60 years, by targeting enoyl-acyl carrier protein reductase to block the synthesis of essential mycolic acids, the discovery of novel bacterial FAS inhibitors remains difficult. For example, PTM was discovered as a potent inhibitor of bacterial FASs in 2006, but the poor bioavailability limits its further drug development in the past decade. 4, 8
In this study, we have discovered that 6-pyrenyl PTM 6t showed improved in vivo efficacy against MRSA infection in a mouse peritonitis model, compared to its parent compound PTM. The in vivo antibacterial activity of 6t might partly result from its enhanced retention in the mouse circulation system (Figure 3B). Although its toxicity might be some concern for the future drug development of 6t, our results have set the stage to discover more PTM derivatives with improved in vivo efficacy against drug-resistant pathogens. The facile Suzuki-Miyaura cross-coupling reactions on the PTM scaffold and hundreds of commercially available organoboron compounds should greatly speed up this process. Although fatty acid synthesis is not essential in certain bacteria in the presence of exogenous fatty acids, such as Streptococcus pneumoniae, type II FASs are validated antibacterial targets in most major pathogens, including Staphylococcus, Enterococcus and Clostridium difficile.3 The discovery and development of PTM and its derivatives should inspire the future efforts to discover and develop other inhibitors targeting the essential bacterial fatty acid biosynthetic pathway in many major pathogens.
EXPERIMENTAL SECTION
General Experimental Procedure
The commercial reagents were used as received. All 1H and 13C NMR spectra were recorded on a Brucker 500 MHz or 400 MHz spectrometer. Chemical shifts were reported in ppm relative to the internal standard tetramethylsilane (δ = 0 ppm) for 1H NMR and deuterio chloroform (δ = 77.00 ppm) for 13C NMR spectroscopy. The following abbreviations were used to designate chemical shift multiplicities: s = singlet, d =doublet, t = triplet, q = quartet, m = multiplet, br = broad. HRMS spectra were recorded on a LTQ-ORBITRAP-ETD instrument. Samples were analysed on a Waters e2695 HPLC system equipped with a PDA detector and a Waters Sunfire C18 column (150 × 4.6 mm). The mobile phase consisted of buffer A (ultrapure H2O containing 0.1 % HCOOH and 0.1 % CH3CN) and buffer B (chromatographic grade CH3CN containing 0.1 % HCOOH) was applied at a flow rate of 1 mL min−1. All compounds examined possessed a purity of at least 95%.
Chemistry
Synthesis of PTMA Ethyl Ester (1)
PTM-containing dried resins (500 g) were eluted with 8 L ethanol to release PTM from the resin. The eluent was concentrated and re-dissolved in 300 ml ethanol. Concentrated sulphuric acid (1.5 mL, 15.0 mmol) was added to this solution and stirred under reflux conditions for 48 hours. Then the reaction mixture was cooled to room temperature and 500 mL brine was added. The resulting mixture was extracted with EtOAc (3 × 100 mL), and the combined organic portions were washed with fresh water and dried over anhydrous sodium sulfate. Concentration followed by flash column chromatography (eluent: EtOAc/PE 1:30 to 1:5) afforded PTMA ethyl ester 1 10.9 g in 89% yield. 1H NMR (400 MHz, CDCl3) δ 6.43 (d, J = 10.1 Hz, 1H), 5.84 (d, J = 10.1 Hz, 1H), 4.35 (s, 1H), 4.10 – 4.05 (m, 2H), 2.37 (t, J = 6.5 Hz, 1H), 2.32 (s, 1H), 2.30 – 2.11 (m, 3H), 2.03 (dd, J = 6.3, 5.3 Hz, 1H), 1.99 – 1.94 (m, 2H), 1.81 (dd, J = 11.1, 3.6 Hz, 1H), 1.77 – 1.64 (m, 2H), 1.61 – 1.54 (m, 1H), 1.40 (s, 3H), 1.19 (s, 3H), 1.19 (dd, J = 6.3, 3.2 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 203.25, 173.31, 153.51, 127.22, 86.96, 76.44, 60.35, 54.90, 46.27, 45.94, 44.62, 43.13, 40.55, 30.70, 29.32, 24.50, 23.00, 14.18.
Synthesis of Iodo-PTMA Ethyl Ester (2)
To a solution of PTMA ethyl ester 1 (4.0 g, 12.6 mmol) in pyridine/CCl4 (20 mL/40 mL) was added DMAP (0.2 g, 1.2 mmol) and iodine (9.6 g, 37.8 mmol). The mixture was stirred under reflux conditions for 24 hours. Solvent was removed in vacuo after the reaction mixture was cooled to room temperature. Then the resulting dark gum was dissolved in EtOAc (100 mL) which was successively washed with a 10% aqueous solution of Na2S2O3 and 5% aqueous HCl and dried over Na2SO4. Last, the solvent was distilled under vacuo to give an amber residue, which was subjected to column chromatography with a gradient of EtOAc and petroleum ether (1:4) to give iodo-PTMA ethyl ester 2 as an oil (5.0 g, 90%). 1H NMR (500 MHz, CDCl3) δ 7.28 (s, 1H), 4.32 (s, 1H), 4.05 (tdd, J = 9.3, 6.3, 2.1 Hz, 2H), 2.41 – 2.33 (m, 2H), 2.32 – 2.21 (m, 2H), 2.20 – 2.09 (m, 1H), 2.07 – 1.97 (m, 2H), 1.94 (d, J = 11.6 Hz, 1H), 1.85 (dd, J = 11.2, 3.5 Hz, 1H), 1.79 – 1.74 (m, 1H), 1.73 – 1.67 (m, 1H), 1.59 (d, J = 11.0 Hz, 1H), 1.39 (s, 3H), 1.20 (s, 3H), 1.18 (dd, J = 7.3, 1.4 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 196.21, 172.94, 162.37, 102.29, 86.73, 76.25, 60.40, 54.55, 50.17, 47.04, 45.88, 44.27, 42.89, 40.39, 31.63, 29.21, 24.58, 22.91, 14.20. HRMS (ESI) m/z calcd for C19H25IO4, [M+H]+ 445.0798; Found: 445.0798.
General Procedure for Suzuki Cross-Coupling Reaction to Prepare Compounds 3
To a solution of iodo-PTMA ethyl ester 2 (0.3 mmol) in DME (5.0 mL) and H2O (5.0 mL) were added Na2CO3 (318 mg, 0.9 mmol), Ar-B(OH)2 (0.6 mmol) and 10% Pd/C (16.0 mg, 5 mol%). The resulting mixture was stirred 12 h at room temperature. Then Pd/C was filtered, and the filtrate was extracted with EtOAc (3 × 5.0 mL). The organic extracts were dried (Na2SO4) and distilled under vacuo to give an amber residue, which was subjected to column chromatography (eluent: EtOAc/PE 1:5 to 1:3) to give aryl-PTMA ethyl esters 3.
General Procedure for Compounds 6
Hydrolysis.
To a solution of aryl-PTMA ethylester 3 (0.24 mmol) in 10 mL THF was added LiOH-H2O (3.0 mL, 2 M). The mixture was stirred overnight at room temperature. The reaction was then quenched with 1 N HCl solution and extracted with EtOAc (3 × 10 mL), and the extract was washed with fresh water and dried over anhydrous Na2SO4, which further concentrated in vacuo to generate the corresponding aryl-PTMAs.
Conjugation.
To a solution of aryl-PTMAs (obtained in previous step) and aminobenzoates 4 (0.36 mmol) in DMF (6.0 mL) at room temperature were added Et3N (0.63 mmol) and HATU (114.2 mg, 0.30 mmol). The mixture was stirred at room for 12 h. Then brine (20 mL) was added and extracted with EtOAc (3 × 10.0 mL), and the extract was washed with fresh water and dried over anhydrous Na2SO4, which further concentrated in vacuo. Aryl-PTM TMSEs 5 were finally prepared through flash column chromatography (eluent: EtOAc/PE 1:5 to 1:3).
Deprotection.
TBAF (0.48 mmol) was added to a solution of aryl-PTM TMSEs 5 from the previous step in THF (6.0 mL). Then the mixture was stirred at room temperature for 12 h and concentrated in vacuo. The residue was dissolved with 30 mL Et2O/EtOAc (5/1 to 1/1) and washed with 0.5 N HCl. The organic layer was next concentrated and purified through flash chromatography (60:30:1 EtoAc/PE/AcOH) to provided aryl-PTMs 6.
The MIC Determination Using the Agar Dilution Method
In vitro bioassay for tested compounds were carried out using S. aureus ATCC 29213, MSSA and MRSA, E.coli and K. pneumoniae strains isolated from patients in local hospitals in central China.10, 24 All tested compounds were prepared with methanol and 150 μl of each compound with different concentration was used to mix with 15 ml melted LB agar in each petri dish, resulting a final compound concentrations of 0.5 – 64 μg/ml each plate. Linezolid and PTM were used as controls. The tested bacteria were first cultivated in LB broth and incubated for 16 h at 37 °C and 250 rpm. The bacteria suspensions were diluted till the OD600 reaches to 0.25±0.05, which were further diluted for 104 folds. The diluted bacteria (2 μl) were added to each plate and incubated for 16 h at 37 °C. The MICs were determined to be the lowest compound concentration with no visible growth of bacterial colonies on the plates.
Molecular Docking
The molecular modeling studies were carried out on an Intel core i5 2.5 GHz processor, 4 GB memory with Windows 10 operating system using Molecular Operating Environment (MOE 2010.06; Chemical Computing Group, Canada) as the computational platform. All the energy minimizations were performed with MOE until a RMSD gradient of 0.05 Kcal mol−1 Å−1 with MMFF94X force-field and the partial charges were automatically calculated. The X-ray crystallographic structure of ecFabF (C163Q) complexed with PTM (PDB ID: 2GFX) was obtained from the Protein Data Bank. The enzyme was prepared for docking studies based on our previous report.25
In Vitro Antibacterial Assay in 96 Well Plates with or without Human Serum
One representative MRSA strain was grown to exponential phase (OD600 = 0.5) and diluted to 106 CFU/ml in Mueller-Hinton broth. Different concentrations of 6s, 6t and PTM (100 μl) and 100 μl of bacterial solution with or without 10% serum per well were mixed and incubated for 18 h at 37 °C. After the incubation, 50 μl resazurin (0.66 mM) were added into each well and visualized.26
Bacteria Count in the Blood and Kidney after Treatment
Twenty six to eight-week-old female mice (18–21 g) were purchased (Hunan Silaikejingda Experimental Animal Company Limited) and maintained under specific pathogen-free conditions at 25 ± 2 oC and 50 ± 5% humidity, on a 12 h/12 h light/dark cycle in Department of Laboratory Animals in Central South University (Changsha, China). The mice were intraperitoneally injected with 2 × 107 CFU of the overnight MRSA inoculum in 0.2 ml physiological saline containing 5% hog gastric mucin.27, 28 The concentration of 6t and PTM was 5 mg/mL, which were formulated with 12.5% DMSO, 30% propanediol, 30% polyethylene glycol 400 and 27.5% physiological saline. Vancomycin was dissolved in physiological saline to a final concentration of 5 mg/mL. The mice were then divided into four groups, and injected with physiological saline, 6t, PTM and vancomycin in 50 mg/kg at 1 and 5 h post-infection. At 12 h of post-infection, the mice were sacrificed to obtain their blood and kidneys. The blood were diluted and plated on LB agar plates. The mouse kidneys were homogenized in 4.0 mL physiological saline. The kidney homogenate was diluted and plated on LB agar plates. After overnight incubation at 37 °C, the number of bacterial colonies for each mice (n = 5) was counted.
Mouse Peritonitis Model
Twenty-five six to Eight-week-old female C57BL/6j mice (18–21 g) were intraperitoneally injected with 2 × 107 CFU of the overnight MRSA inoculum in 0.5 ml physiological saline containing 5% (wt/v) mucin to the generate lethal peritonitis mice model. After 1 h and 5 h post-infection, the mice were divided into 5 groups (n = 5) and injected with 0.2 mL/mouse of 6t (10 or 50 mg/kg), PTM (10 mg/kg), vancomycin (10 mg/kg) and saline. Compound 6t (50 mg/kg) was formulated in 12.5% DMSO, 30% propanediol, 30% polyethylene glycol 400 and 27.5% physiological saline to 5 mg/mL for injection. PTM (10 mg/kg) and 6t (10 mg/kg) were formulated with 2.5% DMSO, 30% propanediol, 30% polyethylene glycol 400 and 37.5% physiological saline to 1 mg/mL for injection. Vancomycin was dissolved in physiological saline to a final concentration of 5 mg/mL. The ability of the tested compounds to rescue mice from lethal peritonitis was observed twice daily and mice survival was tracked for 7 days.
ASSOCIATED CONTENT
Animal protocols
All animal protocols were approved by the Animal Care and Use Committee of Central South University. All experimental procedures complied with the Guide for the Care and Use of Laboratory Animals (1996).
Supplementary Material
Table 3.
The MICs of PTM derivatives 6a – 6t against S. aureus ATCC 29213, and several clinical MSSA and MRSA strains using agar dilution method (data from at least two independent experiments).
 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Compounds | R group | S. aureus ATCC | MSSAs | MRSAs | ||||
| 29213 | 1 | 2 | 3 | 4 | 5 | 6 | ||
| PTM | - | 0.5 | 0.5 | 0.5 | 0.5 | 1 | 1 | 1 |
| Linezolid | - | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 6a | -H | 16 | 16 | 16 | 16 | 16 | 16 | 16 |
| 6b | 2-OMe | 8 | 8 | 16 | 16 | 16 | 16 | 16 |
| 6c | 3,5-Cl | 8 | 8 | 8 | 8 | 8 | 8 | 8 |
| 6d | 3,5-Me | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| 6e | 2-Me | 8 | 8 | 16 | 16 | 16 | 16 | 16 |
| 6f | 3-Me | 8 | 4 | 4 | 8 | 8 | 8 | 8 |
| 6g | 4-Me | 8 | 8 | 8 | 8 | 16 | 16 | 16 |
| 6h | 2-Ph | 4 | 8 | 8 | 8 | 8 | 8 | 8 |
| 6i | 3-Ph | 8 | 8 | 8 | 8 | 8 | 8 | 8 |
| 6j | 4-Ph | 4 | 8 | 8 | 8 | 8 | 8 | 8 |
| 6k | 3-OH | 32 | 32 | 32 | 32 | 32 | 32 | 32 |
| 6l | 3-CF3 | 4 | 4 | 8 | 8 | 8 | 8 | 8 |
| 6m | 3-OCF3 | 8 | 8 | 8 | 8 | 8 | 8 | 8 |
| 6n | 4-nAmyl | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| 6o | 4-CHO | 32 | 16 | 16 | 32 | 32 | 32 | 32 |
| 6p | 4-Br | 2 | 2 | 4 | 4 | 4 | 2 | 2 |
| 6q | -H | 8 | 8 | 8 | 8 | 8 | 8 | 8 |
| 6r | -OEt | 8 | 8 | 8 | 8 | 8 | 8 | 8 |
| 6s | -Phenanthryl | 2 | 2 | 2 | 4 | 2 | 4 | 2 |
| 6t | -Pyrenyl | 2 | 2 | 2 | 4 | 2 | 4 | 2 |
ACKNOWLEDGMENT
We are grateful to the Center for Advanced Research in CSU, including the High Resolution MS Facility and the NMR facility, for the HRMS and NMR experiments.
Funding Sources
This work was supported in part by NSFC grants 81473124 (to Y.H.), the Chinese Ministry of Education 111 Project B0803420 (to Yanwen Duan), U. S. National Institutes of Health Grants GM114353 (to B.S.), the Fundamental Research Funds for the Central Universities of Central South University (CSU) 1053320181889 (to Z.W.), 1053320181907 (to Youchao Deng) and 1053320181621 (to M.S.).
ABBREVIATIONS
- PTM
Platensimycin
- FASII
Type II fatty acid synthases
- MRSA
methicillin-resistant Staphylococcus aureus
- MSSA
methicillin-sensitive Staphylococcus aureus
- VRE
Vancomycin-resistant Enterococci
- ADHBA
3-Amino-2,4-dihydroxybenzoic acid
- PTMA
Platensic acid
- TMS
Trimethylsilane
- TMSE
Trimethylsilylethyl
- TBAF
Tetrabutylammonium fluoride
- MIC
minimum inhibitory concentration
- EtOAc
Ethyl acetate
- PE
Petroleum ether
- DME
Glycol dimethyl ether
- LiOH
Lithium hydrate
- THF
Tetrahydrofuran
- HCl
Hydrochloric acid
- DMF
Dimethylformamide
- HATU
2-(7-Azabenzotriazol-1-yl)-N,N,N’,N’-tetramethyluronium hexafluorophosphate
- Et3N
Triethylamine
Footnotes
The authors declare no competing financial interest.
Supporting Information.
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.jmedchem.
Characterization data of 3a – 3t and 6a – 6t, copies of NMR spectra and HRMS of 2, 3a – 3t and 6a – 6t, predicted binding modes of 6a – 6t with ecFabF(C163Q), 96-well plate assay of 6s, 6t and PTM
Molecular formula strings
REFERENCES
- 1.Walsh CT; Wencewicz TA Prospects for new antibiotics: a molecule-centered perspective. J. Antibiot. 2014, 67, 7–22. [DOI] [PubMed] [Google Scholar]
- 2.Wright HT; Reynolds KA Antibacterial targets in fatty acid biosynthesis. Curr. Opin. Microbiol. 2007, 10, 447–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yao J; Rock CO Bacterial fatty acid metabolism in modern antibiotic discovery. Biochim. Biophys. Acta. 2017, 1862, 1300–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang J; Soisson SM; Young K; Shoop W; Kodali S; Galgoci A; Painter R; Parthasarathy G; Tang YS; Cummings R; Ha S; Dorso K; Motyl M; Jayasuriya H; Ondeyka J; Herath K; Zhang CW; Hernandez L; Allocco J; Basilio A; Tormo JR; Genilloud O; Vicente F; Pelaez F; Colwell L; Lee SH; Michael B; Felcetto T; Gill C; Silver LL; Hermes JD; Bartizal K; Barrett J; Schmatz D; Becker JW; Cully D; Singh SB Platensimycin is a selective FabF inhibitor with potent antibiotic properties. Nature. 2006, 441, 358–361. [DOI] [PubMed] [Google Scholar]
- 5.Dong L-B; Rudolf JD; Lin L; Ruiz C; Cameron MD; Shen B In vivo instability of platensimycin and platencin: Synthesis and biological evaluation of urea-and carbamate-platensimycin. Bioorg. Med. Chem. 2017, 25, 1990–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martens E; Demain A Platensimycin and platencin: promising antibiotics for future application in human medicine. J. Antibiot. 2011, 43, 705–710. [DOI] [PubMed] [Google Scholar]
- 7.Singh SB; Young K; Miesel L Screening strategies for discovery of antibacterial natural products. Expert Rev. Anti. Infect. Ther. 2011, 9, 589–613. [DOI] [PubMed] [Google Scholar]
- 8.Rudolf JD; Dong L-B; Shen B Platensimycin and platencin: inspirations for chemistry, biology, enzymology, and medicine. Biochem. Pharmacol. 2017, 133, 139–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang J; Sintim HO Dialkylamino-2,4-dihydroxybenzoic acids as easily synthesized analogues of platensimycin and platencin with comparable antibacterial properties. Chem. Eur. J. 2011, 17, 3352–3357. [DOI] [PubMed] [Google Scholar]
- 10.Qiu L; Tian K; Pan J; Jiang L; Yang H; Zhu X; Shen B; Duan Y; Huang Y A facile semi-synthetic approach towards halogen-substituted aminobenzoic acid analogues of platensimycin. Tetrahedron. 2017, 73, 771–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nicolaou KC; Stepan AF; Lister T; Li A; Montero A; Tria GS; Turner CI; Tang Y; Wang J; Denton RM; Edmonds DJ Design, synthesis, and biological evaluation of platensimycin analogues with varying degrees of molecular complexity. J. Am. Chem. Soc. 2008, 130, 13110–13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nicolaou KC; Lister T; Denton RM; Montero A; Edmonds DJ Adamantaplatensimycin: a bioactive analogue of platensimycin. Angew. Chem. Int. Ed. 2007, 46, 4712–4714. [DOI] [PubMed] [Google Scholar]
- 13.Nicolaou KC; Tang Y; Wang J; Stepan AF; Li A; Montero A Total synthesis and antibacterial properties of carbaplatensimycin. J. Am. Chem. Soc. 2007, 129, 14850–14851. [DOI] [PubMed] [Google Scholar]
- 14.Qiu L; Tian K; Wen Z; Deng Y; Kang D; Liang H; Zhu X; Shen B; Duan Y; Huang Y Biomimetic stereoselective sulfa-Michael addition leads to platensimycin and platencin sulfur analogues against methicillin-resistant Staphylococcus aureus. J. Nat. Prod. 2018, 81, 316–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen HC; Ding F-X; Singh SB; Parthasarathy G; Soisson SM; Ha SN; Chen X; Kodali S; Wang J; Dorso K; Tata JR; Hammond ML; MacCoss M; Colletti SL Synthesis and biological evaluation of platensimycin analogs. Bioorg. Med. Chem. Lett. 2009, 19, 1623–1627. [DOI] [PubMed] [Google Scholar]
- 16.Jang KP; Kim CH; Na SW; Jang DS; Kim H; Kang H; Lee E 7-Phenylplatensimycin and 11-methyl-7-phenylplatensimycin: more potent analogs of platensimycin. Bioorg. Med. Chem. Lett. 2010, 20, 2156–2158. [DOI] [PubMed] [Google Scholar]
- 17.Dong L-B; Rudolf JD; Shen B A mutasynthetic library of platensimycin and platencin analogues. Org. Lett. 2016, 18, 4606–4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dong L-B; Rudolf JD; Shen B Antibacterial sulfur-containing platensimycin and platencin congeners from Streptomyces platensis SB12029. Bioorg. Med. Chem. 2016, 24, 6348–6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hindra T; Huang D; Yang JD; Rudolf P; Xie G; Xie Q; Teng JR; Lohman X; Zhu Y; Huang Y; Zhao LX; Jiang Y; Duan Y; Shen B Strain prioritization for natural product discovery by a high-throughput real-time PCR Method. J. Nat. Prod. 2014, 77, 2296–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi J; Pan J; Liu L; Yang D; Lu S; Zhu X; Shen B; Duan Y; Huang Y Titer improvement and pilot-scale production of platensimycin from Streptomyces platensis SB12026. J. Ind. Microbiol. Biotechnol. 2016, 43, 1027–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Felpin FX Practical and efficient Suzuki-Miyaura cross-coupling of 2-iodocycloenones with arylboronic acids catalyzed by recyclable Pd(0)/C. J. Org. Chem. 2005, 70, 8575–8578. [DOI] [PubMed] [Google Scholar]
- 22.Li K; Alexakis A Asymmetric conjugate addition to α‐halo enones: dramatic effect of styrene on the enantioselectivity. Angew. Chem. Int. Ed. 2006, 45, 7600–7603. [DOI] [PubMed] [Google Scholar]
- 23.Johnson CR; Adams JP; Braun MP; Senanayake CBW; Wovkulich PM; Uskoković MRJTL Direct α-iodination of cycloalkenones. Tetrahedron Lett. 1992, 33, 917–918. [Google Scholar]
- 24.Wiegand I; Hilpert K; Hancock RE Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc. 2008, 3, 163–175. [DOI] [PubMed] [Google Scholar]
- 25.Deng YC; Kang DD; Shi J; Zhou WQ; Sun AJ; Ju JH; Zhu XC; Shen B; Duan YW; Huang Y The semi-synthesis, biological evaluation and docking analysis of the oxime, hydrazine and hydrazide derivatives of platensimycin. Med. Chem. Comm. 2018, 9, 789–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haste NM; Hughes CC; Tran DN; Fenical W; Jensen PR; Nizet V; Hensler ME Pharmacological properties of the marine natural product marinopyrrole A against Methicillin-Resistant Staphylococcus aureus. 2011, 55, 3305–3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Horn KS; Burda WN; Fleeman R; Shaw LN; Manetsch R Antibacterial activity of a series of N2,N4-disubstituted quinazoline-2,4-diamines. J Med Chem. 2014, 57, 3075–3093. [DOI] [PubMed] [Google Scholar]
- 28.Knudsen JD; Fuursted K; Raber S; Espersen F; Frimodt-Moller N Pharmacodynamics of glycopeptides in the mouse peritonitis model of Streptococcus pneumoniae or Staphylococcus aureus infection. Antimicrob Agents Chemother. 2000, 44, 1247–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.