Short abstract
Objectives
The purpose of this paper was to synthesize 4-amino-1,2,4-triazole Schiff base derivative and to evaluate its antitumor activity.
Methods
2,4,6-Tris (4-formylphenoxy)-1,3,5-triazine was synthesized by nucleophilic substitution with reaction of p-hydroxybenzaldehyde with cyanuric chloride. Then, the intermediate was reacted with 1,2,4-triazole to form 4-amino-1,2,4-triazole Schiff base derivative by reduction and ammonification of aldehydes. The 4-amino-1,2,4-triazole Schiff base derivative was characterized by infrared spectroscopy, nuclear magnetic resonance, and elemental analyses. Finally, the anticancer effect of 4-amino-1,2,4-triazole Schiff base derivative was evaluated by MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay on a lung adenocarcinoma cell line (A549) and a human hepatoma cell line (Bel7402).
Results
The product synthesized in this study was confirmed to be 4-amino-1,2,4-triazole Schiff base derivative, and it significantly suppressed the proliferation of cancer cell lines in a dose-dependent manner.
Conclusions
We successfully prepared 4-amino-1,2,4-triazole Schiff base derivative and verified its antitumor activity.
Keywords: Nucleophilic substitution; reduction ammonification reaction; 4-amino-1,2,4-triazole Schiff base derivative; product characterization; anticancer; MTT assay
Introduction
Triazole Schiff base derivatives have many important applications in industry, agriculture, and medicine.1,2 They can be used as fungicides, anticancer drugs, pharmaceutical intermediates, antioxidants of polymers, and ultraviolet absorbers.3,4 Triazole Schiff base derivatives, as five-membered heterocyclic compounds, contain the basic structural skeleton of a Schiff base in their molecular structure, so they can also be used as ligands to chelate some trace metal ions in organisms, thus having a wide range of biological activities and playing an important role in pharmacodynamics.5,6 These compounds have good bioactivities and are widely used in medicine, materials, and other fields. They can also be used as antibacterial agents, insecticides, and plant-growth regulators in medicine and agriculture.7,8
In recent years, research on triazole Schiff base derivatives has attracted increasing attention. Patents for synthesis of new triazole Schiff base derivatives and novel applications have been reported.9,10 Therefore, the preparation and characterization of 4-amino-1,2,4-triazole Schiff base derivative is of great importance. In this study, we first synthesized and characterized a 4-amino-1,2,4-triazole Schiff base derivative. Subsequently, we demonstrated that the 4-amino-1,2,4-triazole Schiff base derivative inhibited tumor cell proliferation.
Materials and methods
Ethical approval
Ethical approval was not required because our study used cell lines, not samples from humans or animals.
Equipment and reagents
An AdvanceIII HD-400 NMR spectrometer (Bruker, Fällanden, Switzerland), Tensor27 IR spectrometer (Bruker), Vario ELIII elemental analyzer (Elementar Analysensysteme GmbH, Langenselbold, Germany), and SGW X-4 micro-melting-point apparatus (Shanghai Shen Guang Instrument Co. Ltd., Shanghai, China) were used in all procedures. Para-hydroxybenzaldehyde (analytical purity), cyanuric chloride (analytical purity), 1,4-dioxane (analytical purity), triethylamine, 10% anhydrous Na2CO3 (analytical purity), ethyl acetate (analytical purity), glacial acetic acid (analytical purity), 4-amino-1,2,4-triazole, trichloromethane (analytical purity), ethanol (analytical purity), sodium carboxymethyl cellulose, and silica gel GF254 (analytical purity) were purchased from SinoPharm (Shanghai, China). The human lung adenocarcinoma cell line A549 and human hepatoma cell line Bel7402 were purchased from the cell bank of the Chinese Academy of Sciences (Shanghai, China). RPMI-1640 culture medium, trypsinase, and fetal calf serum were obtained from Gibco (Carlsbad, CA, USA). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was purchased from Invitrogen (Carlsbad, CA, USA).
Preparation and characterization of 4-amino-1,2,4-triazole Schiff base derivative
According to the published reference11 and preliminary experiments, the synthetic route of 4-amino-1,2,4-triazole Schiff base derivative is shown in Figure 1. To prepare 4-amino-1,2,4-triazole Schiff base derivative, 0.22 g of 2,4,6-tris (4-formylphenoxy)-1,3,5-triazine and 0.47 g of 4-amino-1,2,4-triazole were dissolved with 10 mL of glacial acetic acid in a four-mouthed flask. Then, the reaction was heated to 118°C and refluxed for 4 hours. After the reaction, the solution (a light yellow color) was filtered, precipitated on ice, and dried in a vacuum. A white powder solid was obtained as crude product. The crude product was washed with ethanol and then chloroform and purified by silica gel column chromatography. The white powder was dried into a pure form with a melting point of 289°C. Subsequently, a small amount of purified and refined sample was dried at 90°C for 30 minutes, and then a portion of the sample and potassium bromide were mixed at a ratio of 1:20 and ground uniformly.
Figure 1.
Synthetic route of 4-amino-1,2,4-triazole Schiff base derivative.
After pressing the mixture, the product was characterized by infrared spectroscopy, 1H nuclear magnetic resonance (NMR), and 13C NMR. 1H NMR and 13C NMR were performed using deuterium instead of dimethyl sulfoxide (DMSO) as solvent. The Vario EL III elemental analyzer was used for elemental analysis.
Cell culture
The A549 and Bel7402 cell lines were grown in RPMI-1640 culture medium with 100 mL/L fetal calf serum, 1 × 105 U/L penicillin, and 100 mg/L streptomycin, and then incubated (Thermo Fisher Scientific, Waltham, MA, USA) at 37°C with 5% CO2.
MTT assay
The A549 and Bel7402 cells (6 × 103 cells/well) were seeded in 96-well plates (Corning Inc., Corning, NY, USA) for 24 hours. The culture medium was then discarded and replaced with RPMI-1640 culture medium containing various concentrations of 4-amino-1, 2, 4-triazole Schiff base derivative (0, 50, 100, 150, 200, 300, and 400 µg/mL) and cultured for another 24 hours. Then, 20 µL of MTT (5 mg/mL) was added to each well, incubated for 4 hours and discarded; 100 µL of DMSO was added to fully dissolve the formazan precipitate. The absorbance (optical density, OD) was measured at 570 nm. Relative cell viability was calculated by comparing with vehicle control. Three independent experiments were performed.
Lactate dehydrogenase assay of cell toxicity
The A549 and Bel7402 cells were seeded in 96-well plates for 24 hours, and then treated with different concentrations of 4-amino-1,2,4-triazole Schiff base derivative (0, 100, 200, and 300 µg/mL) for another 24 hours. The cells were collected in a 1.5-mL Eppendorf tube for the lactate dehydrogenase (LDH) assay (BioVision Inc., Milpitas, CA, USA). The LDH concentration of cell lysates was detected in accordance with the instructions of the LDH kit. The absorbance of the resultant product was measured at 450 nm, using an ELISA microplate reader (Thermo Labsystems, Waltham, MA, USA). The LDH concentration in lysate was then calculated using the following formula: Viability (U/L) = (ODtreated − ODcontrol)/(ODstandard − ODblank) × concentration of standards (U/L). Three independent experiments were performed.
Results
Infrared absorption spectrum
Figure 2 shows the characteristic absorption peaks of the benzene rings at 1605, 1495, and 1466 cm−1, as well as characteristic peaks of the triazine rings at 1532, 1376, and 798 cm−1. The absorption peaks at 2983 and 2942 cm−1 indicate the C–H stretching vibration, whereas those at 1460, 1250, and 678 cm−1 indicate the C–O–C absorption peaks, and the absorption peak at 1260 cm−1 was the stretching vibration peak of the triazole ring (N–N=C). We noted a lack of typical aldehyde carbonyl absorption peaks near 1705 to 1725 cm−1, indicating that C=O in the raw material took part in the chemical reaction. We concluded that the infrared spectra of the synthesized product conformed to the molecular conformation of 4-amino-1,2, 4-triazole-n-isopropyl butylamine.
Figure 2.
Infrared absorption spectra of product. Black arrows indicate the characteristic absorption peaks of benzene rings, purple arrows indicate the characteristic absorption peaks of the triazine rings, blue arrows indicate the C–H stretching vibration peaks, red arrows indicate the characteristic absorption peaks of C–O–C, and the yellow arrow indicates the N–N=C stretching vibration peaks of the triazole rings.
1H NMR spectrum
Figure 3 shows the 21 hydrogen atoms in the final product, which had good structural symmetry. Only four types of hydrogen were found: two on the benzene rings, one on –C=N, and another on the triazole rings. At 7.454–7.949 ppm, the signal peaks of hydrogen represent the benzene rings. At 1.863–2.490 ppm, the signal peaks of hydrogen represent the triazole rings. At 3.274–3.324 ppm, the signal peaks of hydrogen represent –C=N. At the same time, the resonance absorption of some hydrogen nucleus on –C=N and on the triazole rings shifted to 9.095–9.126 ppm due to the deshielding effect of the benzene and azole rings and the electronegativity of nitrogen. We concluded that the hydrogen spectra of the synthesized product conformed to the hydrogen atom conformation of 4-amino-1,2,4-triazole Schiff base derivative.
Figure 3.
1H Nuclear magnetic resonance spectrum of the final product.
13C NMR spectrum
Figure 4 shows the seven types of carbon in the final product: one on the triazine rings, four on the benzene rings, one on –C=N, and one on the triazole rings. In Figure 4, 172.779 ppm was the signal peak of carbon on the triazine rings, 153.987–157.050 ppm indicated the carbon on –C=N, 38.909–40.156 ppm indicated the carbon on the benzene rings, and 122.310–138.875 ppm indicated the carbon on the triazole rings, which was in accord with the basic law of chemical shift of aromatic ring carbon. In conclusion, the carbon spectra of the synthesized product conformed to the carbon atom conformation of 4-amino-1,2,4-triazole Schiff base derivative.
Figure 4.
13C Nuclear magnetic resonance spectrum of the final product.
Elemental analysis
Elemental analysis showed that the constituent elements in the synthesized product were as follows: C, 56.34%; H, 3.29%; O, 7.51%; and N, 32.86%. These results were in accord with the content of each element in 4-amino-1,2,4-triazole Schiff base derivative (C30H21O3N15).
Complete name of the prepared compound
Combining the results of infrared absorption spectrum, 1H NMR spectrum, 13C NMR spectrum, and elemental analysis, the product name was 4-amino-1,2, 4-triazole-n-isopropyl butylamine, also known as 4-amino-1,2,4-triazole Schiff base derivative (C30H21O3N15).
Effects of C30H21O3N15 on proliferation of A549 and Bel7402 cells
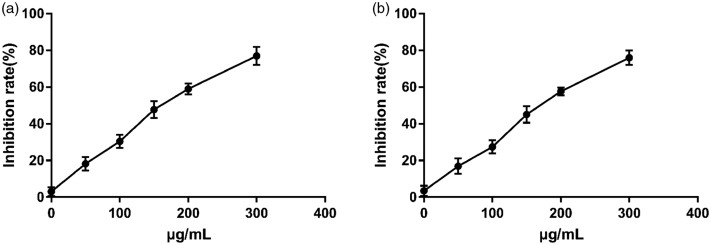
The normal morphology of A549 and Bel7402 cells is shown in Figure 5. To explore the effects of 4-amino-1,2,4-triazole Schiff base derivative on growth and proliferation of cancer cells, A549 and Bel7402 cells were treated with different concentrations of the chemical compound for 24 hours and viability was tested by MTT assay. The cell viability results indicated that 4-amino-1,2,4-triazole Schiff base derivative dose-dependently inhibited cancer cell growth (Figure 6a, b). In A549 and Bel7402 cells, the half-maximal inhibitory concentration (IC50) of 4-amino-1,2,4-triazole Schiff base derivative was 144.1± 4.68 µg/mL and 195.6 ± 1.05 µg/mL, respectively. These results indicated that 4-amino-1,2,4-triazole Schiff base derivative had antitumor activity.
Figure 5.
The normal morphology of (a) A549 cells (lung adenocarcinoma) and (b) Bel7402 (human hepatoma) cells.
Figure 6.
4-Amino-1,2,4-triazole Schiff base derivative inhibited cell viability in cancer cell lines. (a) Human lung adenocarcinoma cell line A549 and (b) human hepatoma cell line Bel7402 were treated with various concentrations (0 to 400 µg/mL) of 4-amino-1,2,4-triazole Schiff base derivative for 24 hours, and cell viability was measured by MTT assay. Error bars indicate standard deviations.
Cytotoxicity evaluation
LDH exists in the cytoplasm of all tissues and cells and it is not released from healthy cells. However, upon cellular damage, LDH is released into the medium, which produces a brownish red color in alkaline solution. Thus, the LDH assay can be used to ascertain the toxicity of drugs. At a concentration of 200 µg/mL or below, the crude extract (C30H21O3N15) did not result in obvious cytotoxicity (Figure 7a, b) in the two cell lines, confirming that 4-amino-1,2,4-triazole Schiff base derivative was not toxic at this concentration. Thus, in future experiments, concentrations below 200 µg/mL could be used for antitumor experiments.
Figure 7.
Effects of C30H21O3N15 (4-amino-1,2,4-triazole Schiff base derivative) on LDH contents of two tumor cell lines. LDH was released in a dose-dependent manner in (a) A549 and (b) Bel7402 cell lines following treatment with crude extract. **P < 0.01 vs. the control group. Error bars indicate standard deviations. LDH, lactate dehydrogenase.
Discussion
The biological activities of triazole compounds show that the azole ring is the necessary structural group.12 The four nitrogen atoms of triazole coordinate strongly with the iron atoms of heme to inhibit the activity of cytochrome P-450, and this inhibition can exert biological activity.13,14 When the heme iron atom is replaced by other groups, this activity is lost. Research shows that the polystereochemistry of azole compounds is very strict, and the stereoisomers are related to biological activity.15
Amination with triazole ring and aldehydes are raw materials for the synthesis of triazole Schiff bases. Generally, triazole Schiff bases are synthesized using anhydrous organic reagents. In addition, anhydrous ethanol, dimethyl formamide (DMF), or acetic acid is often used as the reaction solvent, with glacial acetic acid as the catalyst16 and refluxing is done for several hours. Then, the reaction solution is concentrated after cooling to room temperature. A large amount of precipitate (triazole Schiff base) is obtained, which is filtered, washed, and dried. When anhydrous ethanol or DMF is used as solvent, the reaction time is longer, the post-treatment is more difficult, and the yield is lower. We used acetic acid as solvent, which greatly reduced the reaction time. Our method for the synthesis of triazole Schiff base was short and simple and the yield was high.
Triazole Schiff base derivatives are currently a focus in heterocyclic research. The derivatives are widely used for their anticancer and antifungal activities.17 Xuan et al.18 proposed that novel 1,2,4-triazole Schiff base derivatives had a bactericidal effect, and Li et al.19 found that the 1,2,4-triazole-5-thione bis-Schiff base significantly inhibited the activities of HeLa and Bel7402 cells. Therefore, preparation of a triazole Schiff base derivative with high biological activity could have important social and economic significance. In our experiment, glacial acetic acid was chosen as the reaction solvent, which could fully dissolve the reactant. Moreover, glacial acetic acid can be used as a catalyst to synthesize the target product triazole Schiff base, accelerating the reaction and inducing it to fully proceed, which is more conducive to formation of the specific product.
Although we report here that 4-amino-1,2,4-triazole Schiff base derivative has an antitumor effect, the current study lacks experimental data. This study confirmed that 4-amino-1,2,4-triazole Schiff base derivative inhibited the proliferation of A549 and Bel7402 cells and thus could play an important biological role in medicine. Currently, there are no suitable Schiff base derivatives in clinical use as positive control drugs. Further studies are needed to fully explore the antitumor effect of 4-amino-1,2,4-triazole Schiff base derivative in vitro and in vivo. We also need to determine the biological target of 4-amino-1,2,4-triazole Schiff base derivative. In later experiments, isomers will need to be prepared and their activities assessed.
Conclusions
We first successfully synthesized 4-amino-1,2,4-triazole Schiff base derivative and then characterized it by infrared spectroscopy, nuclear magnetic resonance, and elemental analyses. These methods confirmed that the product synthesized by this method was 4-amino-1,2,4-triazole Schiff base derivative. The synthesized Schiff base derivative was found to significantly suppress the proliferation of cancer cell lines in a dose-dependent manner.
Authors’ contributions
Guo-wei Jiang and Qing-qing Yi designed the study; Yong-jun Meng and Dong-yu Liang collected and analyzed the data; Yu-ting Zhang participated in some experiments; Qing Chang reviewed the article; and Qing-qing Yi submitted the article.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This study was supported by institutional funding from the National Natural Science Foundation of China (grant number: 81670968).
ORCID iD
Qing-qing Yi https://orcid.org/0000-0003-4955-5851
References
- 1.Süleymanoğlu N, Ustabaş R, Direkel Şet al. 1,2,4-Triazole derivative with Schiff base; thiol-thione tautomerism, DFT study and antileishmanial activity. J Mol Struct 2017; 1150: 82–87. [Google Scholar]
- 2.Karrouchi K, Chemlal L, Taoufik Jet al. Synthesis, antioxidant and analgesic activities of Schiff bases of 4-amino-1,2,4-triazole derivatives containing a pyrazole moiety. Ann Pharm Fr 2016; 74: 431–438. [DOI] [PubMed] [Google Scholar]
- 3.Liu XL, Zhao ZG, Shi ZCet al. Microwave-prompted synthesis and bioactivity of novel substituted bis-triazole Schiff bases containing pyridine rings. Journal of Southwest Minzu University (Natural Science Edition) 2017; 43: 469–473. [Google Scholar]
- 4.Wang BL, Zhang LY, Zhan YZet al. Synthesis and biological activities of novel 1,2,4-triazole thiones and bis(1,2,4-triazole thiones)containing phenylpyrazole and piperazine moieties. J Fluor Chem 2016; 184: 36–44. [Google Scholar]
- 5.Zhang SH, Wang JM, Zhang HYet al. Highly efficient electrochemiluminescence based on 4-amino-1,2,4-triazole Schiff base two-dimensional Zn/Cd coordination polymers. Dalton Trans 2017; 46: 410–419. [DOI] [PubMed] [Google Scholar]
- 6.Hermanowicz KW, Pieniążczak D, Wróbel Ret al. A study on the condensation reaction of aryl substituted 4-amine-1,2,4-triazole with benzaldehydes: structures and spectroscopic properties of schiff bases and stable hemiaminals. J Mol Struct 2016; 1114: 108–122. [Google Scholar]
- 7.Sun Q, Zheng F, Sun Xet al. Construction of a dinuclear silver (I) coordination complex with a Schiff base containing 4-amino-1,2,4-triazole ligands. Acta Crystallogr Sect E Struct Rep Online 2009; 65: m283–m284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jin RY, Zeng CY, Liang XHet al. Design, synthesis, biological activities and DFT calculation of novel 1,2,4-triazole Schiff base derivatives. Bioorg Chem 2018; 80, 253–260. [DOI] [PubMed] [Google Scholar]
- 9.Alphonse R, Varghese A, George Let al. Estimation of ground state and excited state dipole moments of a novel Schiff base derivative containing 1,2,4-triazole nucleus by solvatochromic method. J Mol Liq 2016; 215: 387–395. [Google Scholar]
- 10.Duan HD, Wang LZ, Qin DWet al. Synthesis and characterization of some new star-shaped polydentate ligands from 2,4,6-trichloro-1,3,5-triazine. Synth Commun 2011; 41: 380–384. [Google Scholar]
- 11.Zhang LX, Zhu HY. Recent research progress on Schiff Base chemistry. Chem Eng 2015; 236: 64–66. [Google Scholar]
- 12.Menendez C, Saffon N, Lherbet Cet al. ChemInform abstract: synthesis and biological activities of triazole derivatives as inhibitors of InhA and antituberculosis agents. Eur J Med Chem 2011; 46: 5524–5531. [DOI] [PubMed] [Google Scholar]
- 13.Zhang N. Synthesis of ionic metal complexes of ferrocenylmethyltriazole (imidazole) and their catalysis in combustion process. Shaanxi Normal University, 2018. [Google Scholar]
- 14.Liu MM. Synthesis and catalytic properties of Dinuclear ferrocenyltriazole ionic metal complexes. Shaanxi Normal University, 2018. [Google Scholar]
- 15.Paqhaleh DMS, Hashemi L, Amani Vet al. Synthesis of two new nano-structured mercury (II) complexes with 4-methyl-4H-1,2,4-triazole-3-thiol ligand by sonochemical method. Inorganica Chim Acta 2013; 407: 1–6. [Google Scholar]
- 16.Ishak NNM, Jamsari J, Ismail AZet al. Synthesis, characterisation and biological studies of mixed-ligand nickel (II) complexes containing imidazole derivatives and thiosemicarbazide Schiff bases. J Mol Struct 2019. DOI: 10.1016/j.molstruc.2019.126888. [Google Scholar]
- 17.Gouranourimi A, Ghassemzadeh M, Bahemmat Set al. New palladium (II) complexes containing 3-mercapto-1,2,4-triazole ligands: synthesis, characterization, crystal structure, and density functional theory calculations. Monatsh Chem 2015; 146: 57–67. [Google Scholar]
- 18.Xuan F, Xu J, Zhang CL. Synthesis and antibacterial activities of novel 1, 2, 4-triazole schiff-base derivatives. Chemical Industry Times 2017; 31 16–18. [Google Scholar]
- 19.Li M, Zhang YN, Tian YS. Synthesis and antitumor activity evaluation of the derivatives of 1, 2, 4-triazole-5-thione bis-schiff base. J Pharm Res 2016; 35: 376–378. [Google Scholar]