Abstract
Objective
We aimed to determine the prevalence of insomnia and insomnia symptoms and its association with metabolic parameters and glycemic control in people with type 2 diabetes (T2D) in a systematic review and meta-analysis.
Data Sources
A systematic literature search was conducted in PubMed/Embase until March 2018.
Study Selection
Included studies described prevalence of insomnia or insomnia symptoms and/or its association with metabolic parameters or glycemic control in adults with T2D.
Data Extraction
Data extraction was performed independently by 2 reviewers, on a standardized, prepiloted form. An adaptation of Quality Assessment Tool for Quantitative Studies was used to assess the methodological quality of the included studies.
Data Synthesis
When possible, results were meta-analyzed using random-effects analysis and rated using Grading of Recommendations Assessment, Development and Evaluation (GRADE).
Results
A total of 11 329 titles/abstracts were screened and 224 were read full text in duplicate, of which 78 studies were included. The pooled prevalence of insomnia (symptoms) in people with T2D was 39% (95% confidence interval, 34–44) with I2 statistic of 100% (P < 0.00001), with a very low GRADE of evidence. Sensitivity analyses identified no clear sources of heterogeneity. Meta-analyses showed that in people with T2D, insomnia (symptoms) were associated with higher hemoglobin A1c levels (mean difference, 0.23% [0.1–0.4]) and higher fasting glucose levels (mean difference, 0.40 mmol/L [0.2–0.7]), with a low GRADE of evidence. The relative low methodological quality and high heterogeneity of the studies included in this meta-analysis complicate the interpretation of our results.
Conclusions
The prevalence of insomnia (symptoms) is 39% (95% confidence interval, 34–44) in the T2D population and may be associated with deleterious glycemic control.
Keywords: type 2 diabetes, insomnia, HbA1c, glucose, prevalence
Insomnia is defined as chronic difficulty of falling asleep, staying asleep or waking up early, despite opportunity to sleep, at least 3 times a week during 1 month, resulting in daytime impairment (1). In the general population the prevalence of symptoms of insomnia ranges from 30% to 40% (1–3), whereas the prevalence of insomnia is about 6% to 20% (3–5). Insomnia and insomnia symptoms are thought to affect a wide range of body functions, including metabolic (decreased glucose tolerance, insulin resistance) (6) and endocrine regulation (elevated cortisol levels) (7). Cross-sectional studies have shown that in healthy people, insomnia (symptoms) are associated with an increased risk of obesity with odds ratios ranging between 1.07 (1.0–1.1) (8) and 1.66 (1.4–1.9) (9) as well as disturbances in glucose homeostasis and tolerance, with insomnia being associated with homeostatic model assessment of insulin sensitivity 0.03% (0.01–0.05) (10). In addition, in the general population insomnia (symptoms) are associated with an increased risk of type 2 diabetes (T2D), with odds ranging between 1.52 (1.05–2.20) and 2.98 (1.36–6.53), as well as with an increased risk of cardiovascular disease with a risk ratio of 1.05 (1.01–1.08) for hypertension incidence (11–15).
Although the association between insomnia (symptoms) and the increased risk of developing T2D is consistently shown (16), it is still unclear whether people with T2D also have an increased risk of having insomnia and insomnia symptoms. Despite plenty of studies examining this association, the prevalence rates of insomnia and insomnia symptoms in people with T2D are inconclusive, ranging from 6% (17) to 80% (18). This range could be due to different study populations, measurement instruments, and comorbidities; however, this was never studied. Also, the inconsistent definition of insomnia, using insomnia, insomnia symptoms, and low sleep quality interchangeably, might explain the range. In this review, we will therefore use the terms insomnia and insomnia symptoms.
A recent meta-analysis (19) did show that low sleep quality, assessed with the Pittsburgh Sleep Quality Index (PSQI) questionnaire, is associated with higher hemoglobin A1c (HbA1c) levels in adults with T2D. However, there is a lack of meta-analytic evidence on the other parameters of glycemic control and metabolic parameters such as: fasting glucose levels, high-density lipoprotein (HDL)/low-density lipoprotein (LDL) / total cholesterol levels, triglyceride levels, body mass index (BMI), waist circumference, and blood pressure (systolic and diastolic). Also, this meta-analysis did not include other measures of insomnia symptoms or insomnia, such as self-report and other questionnaires, and did not study the prevalence of insomnia. Therefore, the aim of our current study is to determine the prevalence of insomnia and insomnia symptoms and its association with metabolic parameters and glycemic control in people with T2D in a systematic review and meta-analysis.
Methods
Data sources and searches
A systematic search of the literature was conducted in MEDLINE (PubMed) and Embase until March 2018 by investigators A.D.M.K. and F.R., assisted by a librarian. Reference lists of included studies were searched manually for additional studies. In short, the search strategy focused on a combination of these terms and their synonyms: (Type 2 diabetes OR NIIDM OR T2DM OR diabetes) AND (Insomnia OR sleep quality OR disturbed sleep). The full search strategy is provided in File 1. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines were followed for experimental studies (20) and the Meta-analysis of Observational Studies in Epidemiology guidelines for observational studies (21). The protocol of this review was registered in the PROSPERO database under number CRD42018089917.
File 1. Search Strategy PubMed (MEDLINE) Database.
“Diabetes Mellitus, Type 2”[Mesh] OR diabetes*[tiab] OR diabetic*[tiab] OR dm2[tiab] OR niddm[tiab] OR dm 2[tiab] OR t2d*[tiab] OR dm type 2[tiab] OR DMII[tiab] OR dm type II[tiab]
AND
“Sleep initiation and maintenance disorders”[Mesh:NoExp] OR sleep quality[tiab] OR disturbed sleep[tiab] OR fragmented sleep[tiab] OR insomni*[tiab] OR dyssomni*[tiab] OR chronic insomnia[tiab] OR poor sleep[tiab] OR sleep*[tiab] OR poor sleep quality[tiab] OR sleep impairment[tiab] OR sleep disturbance*[tiab] OR sleep disorder*[tiab] OR sleep-disorder*[tiab] OR sleep complaint*[tiab] OR sleep problem*[tiab] OR sleep difficult*[tiab] OR sleep latency[tiab] OR sleep efficiency[tiab] OR nonrestorative sleep[tiab] OR non-restorative sleep[tiab] OR disorders of initiating and maintaining sleep[tiab] OR DIMS[tiab] OR early awakening[tiab] OR sleep initiating dysfunction*[tiab] OR sleep initiation [tiab] OR initiating sleep[tiab] OR sleep onset[tiab] OR maintaining sleep[tiab] OR sleep maintenance[tiab] OR wakeful*[tiab] OR sleepless*[tiab] OR difficulty in initiating and maintaining sleep[tiab] OR insomnia disorder*[tiab]
NOT
“Animals”[Mesh] NOT “Humans”[Mesh]
Abbreviations: [tiab] = title and/or abstract only.
Search strategy was adapted for other databases.
Selection of studies
Studies were included if: 1) the study population consisted of adults (≥18 years) with T2D; 2) article was available as full text, either via our library or upon request authors; 3) the article was written in English or Dutch; 4) prevalence of insomnia or insomnia symptoms was reported as a percentage or could be calculated from the results; and/or 5) the association between insomnia (symptoms) and glycemic control, defined as HbA1c levels and fasting plasma glucose levels, or metabolic parameters were studied, defined as BMI, waist circumference, levels of triglycerides, LDL/HDL/(total) cholesterol levels and/or systolic/diastolic blood pressure. Studies were excluded if they reported solely on patients with obstructive sleep apnea and/or sleep apnea. When results from a study were reported more than once, the most recent article was used.
All studies identified in the literature search were screened for eligibility on title and abstract by 2 reviewers (T.D. and F.R. or A.D.M.K.). The full text versions of potentially eligible studies were independently assessed for inclusion by 2 reviewers (T.D. and F.R. or A.D.M.K.). Discrepancies were resolved through discussion, consulting a third reviewer, F.R. or A.D.M.K.
Data extraction
Data extraction was performed independently by 2 reviewers, T.D. and F.R. or A.D.M.K. A standardized, prepiloted form was used to extract data from the included studies. Data extraction included: setting, country, years when data were collected, number of participants (% men), age, diabetes duration, diabetes diagnosis (eg, self-report, clinically diagnosed), diabetes treatment, insomnia or insomnia symptoms measure, and metabolic and glycemic control parameters. If studies reported multiple outcomes, all were extracted and reported separately. Discrepancies identified during the duplicate data extraction were resolved through discussion, consulting a third reviewer, J.W.B.
Methodological quality assessment
An adaptation of the Quality Assessment Tool for Quantitative Studies, as developed by the Effective Public Health Practice Project, was used to assess the methodological quality of the included studies (22). This 19-item tool, adapted by Mackenbach et al. 2014, is suitable for assessing the methodological quality of studies of observational and experimental design (23). It contains 8 domains of methodological quality on which studies were assessed: 1) study design; 2) blinding; 3) representativeness with regard to selection bias; 4) representativeness with regard to withdrawals/dropouts; 5) confounders; 6) data collection; 7) data analysis; and 8) reporting. The quality assessment was based on the outcome of interest, independent of the primary aim of the particular study.
The methodological quality was assessed separately for insomnia and insomnia symptoms prevalence and the association with metabolic parameters or glycemic control resulting in 2 ratings for methodological quality when a study examined both outcomes. For the assessment of prevalence studies, we scored “no rate” on the domains confounders and data analysis, because these domains were not applicable. Consequently, the prevalence outcome could only have 4 to 6 component ratings, whereas studies containing metabolic or glycemic control parameters could have 6 to 8 component ratings, resulting in 1 overall rating, ranging from low risk of bias (high methodological quality) to high risk of bias (low methodological quality). If 4 ratings were given, the overall rating was scored as follows: high methodological quality was attributed to those studies with no “weak” rating and at least 2 “strong” ratings; moderate methodological quality was attributed to those studies with 1 weak rating or fewer than 2 strong ratings; low methodological quality was attributed to those studies with 2 or more weak ratings. If 6 ratings were given, the overall rating was the same, except that there should be at least 3 strong ratings to be scored as a study with high methodological quality.
All included studies were independently assessed for methodological quality by 2 raters, A.D.M.K. and F.R./J.B. The ratings of each domain and the overall ratings were compared between the 2 raters to reach consensus.
The measurement of insomnia and insomnia symptoms
The primary aim was to determine the prevalence of insomnia and insomnia symptoms in adults with T2D. Insomnia or having insomnia symptoms was defined as having the following symptoms: chronic difficulty of falling asleep, staying asleep or waking up early at least 3 times per week during 1 month (1), although an unequivocal way for measuring insomnia (symptoms) does not exist. The PSQI questionnaire was most frequently used, with the questionnaire aiming to assess overall sleep quality, with a score of >5 points on the PSQI indicating poorer sleep quality, often referred to as insomnia (symptoms). But as depicted in Table 1 and Table 2, other methods also are used, such as self-report on having insomnia, the Insomnia Severity Index and the Medical Outcomes Study - Sleep Scale.
Table 1.
Characteristics of Included Studies in the Systematic Review (n = 78), Studies A-K
| Author (Y), Reference | - Setting - Country (Period of Analysis) | - N (% men) - Age ± SD/[IQR] | - Diabetes Duration - Diabetes Diagnosis - Diabetes Treatment | - Insomnia or Insomnia Symptoms Measure - Distribution/Cutoff | Metabolic Parameters and Glycemic Control | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| None | HbA1c | FPG | BMI | Waist | HDL | LDL | Chol | TG | SBP | DBP | |||||
| Abdelgadir (2009) (24) | - Outpatient clinic - Sudan (NR) | - 60 (67%) - 57 y | - 16 ± NR - Clinically diagnosed - NR | - MOS-SS - Insomnia yes/no | x | ||||||||||
| Al Tannir (2016) (25) | - General population - Saudi Arabia (2014-2015) | - 161 DM (42% in general population) - General population: 33 ± 12 y | - NR - Self-report - NR | - Unknown questionnaire - Sleep disturbance yes or maybe/no | x | ||||||||||
| Aribas (2015) (26) | - Outpatient clinic - Turkey (NR) | - 78 (39%) - 50 ± 9 y | - 6 [2–12] y - Clinically diagnosed - NR | - PSQI - PSQI >5 = insomnia symptoms | x | x | x | x | x | x | x | x | x | x | |
| Bani-Issa (2017) (27) | - Community health care setting - UAE (NR) | - 268 (38%) - 42 ± 13 y | - 75% = 0–10 y - Clinically diagnosed - NR | - PSQI - PSQI ≥5 = insomnia symptoms | x | ||||||||||
| Bedi (2011) (28) | - Outpatient clinic - India (NR) | - 201 (50%) - 40-60 y | - NR - Clinically diagnosed - 100% oral medication | - PSQI - PSQI >5 = insomnia symptoms | x | ||||||||||
| Bener (2010) (29) | - Health care center/ primary care - Qatar (2009) | - 847 (47%) - 59% = 40–59 y | - NR - Clinically diagnosed - 100% oral medication | - PSQI - PSQI >5 = insomnia symptoms | x | ||||||||||
| Bhaskar (2016) (30) | - Outpatient clinic - India (2015) | - 68 (NR) - 18-60 y | - NR - NR - NR | - AIS - Score >6 = insomnia | x | ||||||||||
| Bilge (2016) (18) | - Outpatient clinic - Turkey (2015) | - 40 (30%) - 48 ± 10 y | - NR - NR - NR | - PSQI - PSQI >5 = insomnia symptoms | x | ||||||||||
| Budhiraja (2011) (31) | - General population - USA (<2010) | - 207 (NR) - General population: 42 ± 13y | - NR - Self-report - NR | - DSM-IV insomnia criteria - yes/no | x | ||||||||||
| Celik (2012) (32) | - Tertiary care - Turkey (NR) | - 46 (52%) - 59 ± 12 y | - NR - Clinically diagnosed - NR | - PSQI - PSQI >5 = insomnia symptoms | x | ||||||||||
| Chang (2017) (33) | - Secondary care - Taiwan (2013–2014) | - 275 (0%) - 58 ± 8 y | - ≥3 mo - Clinically diagnosed - 78% OAD 14% OAD + insulin 4% diet + exercise | - PSQI - PSQI >6 = insomnia symptoms | x | ||||||||||
| Cheng (2019) (34) | - Secondary care - Taiwan (2014-2016) | - 201 (52%) - 70 ± 6.9 y | - NR - Self-report - NR | - Self-report - Sleep disturbance ≥1 night per week yes/no | x | ||||||||||
| Cho (2014) (35) | - Secondary care - South Korea (2011) | - 614 (62%) - 60 ± 11y | - 10 ± 8 y - Clinically diagnosed - NR | Insomnia variable 1: - Self-report - Insomnia = difficulty falling asleep, maintaining sleep, early morning waking and non- restorative sleep ≥3 times/wk Insomnia variable 2: - PSQI - PSQI ≥5 = insomnia symptoms | x | ||||||||||
| Colbay (2015) (36) | - Secondary care - Turkey (2011) | - 53 (42%) - 51 ± 8 y | - 8 y - Clinically diagnosed - 49% OAD 42% OAD + insulin 9% insulin | - PSQI - PSQI >5 = insomnia symptoms | x | ||||||||||
| Cuellar (2008) (37) | - Secondary care - USA (2004-2005) | - 35 (44%) - 61 ± 11 y | - NR - Clinically diagnosed - NR | - PSQI - PSQI >6 = insomnia symptoms | x | ||||||||||
| Cunha (2008) (38) | - Secondary care - Brazil (2005) | - 50 (24%) - Median: 62 (range 44–79) y | - 38% >10 y - Clinically diagnosed - NR | - PSQI - PSQI >5 = insomnia symptoms | x | ||||||||||
| El-Aghoury (2017) (39) | - NR - Egypt (NR) | - 46 (NR) - 48 ± 7 y | - NR - Clinically diagnosed - NR | - NHANES sleep questionnaire - Insomnia = difficulty initiating or maintaining sleep | x | ||||||||||
| Ford (2015) (40) | - General population - USA (2002, 2007, 2012) | DM: - 2002: 2179 (43%) - 2007: 2028 (44%) - 2012: 3526 (44%) - Mean age: 45 y in 2002; 47 y in 2012 | - NR - Self-report - NR | - Self-report - “During the past 12 months, have you regularly had insomnia or trouble sleeping?” | x | ||||||||||
| Fritschi 2017 (41) | - Veteran Hospital + flyers - USA (2012–2013) | - 80 (53%) - 58 ± 8 y | - 9 ± 7 y - Self-report - 70% metformin | - Actigraph: sleep efficiency, wake after sleep onset - Not applicable | x | ||||||||||
| Fukui (2012) (42) | - Outpatient clinic - Japan (NR) | - 296 (100%) - 64 ± 10 y | - 14 ± 11 - Clinically diagnosed - 63% OAD 25% insulin 12% diet | - PSQI - PSQI >5 = insomnia symptoms | x | ||||||||||
| Gozashti (2016) (43) | - Outpatient clinic - Iran (2014) | - 118 (76%) - 58 ± 11 y | - NR - Clinically diagnosed - 100% OAD | - PSQI - PSQI >5 = insomnia symptoms | x | x | x | x | x | x | |||||
| Grandner (2011) (44) | - General population - USA (2006) | - 18 888 DM (41%) - General population: ±53 y | - NR - Self-report - NR | - Self-report - Sleep complaints: reporting difficulty falling asleep, staying asleep or sleeping too much ≥6 days over 2 weeks | x | ||||||||||
| Han (2002) (45) | - Hospital - Korea (NR) | - 82 (61%) - 50 ± 9 y | - With insomnia: 26 ± 23 y, Without insomnia: 20 ± 19 y - Clinically diagnosed - NR | - Self-report - Reporting difficulty falling asleep, awakening during the night, or/ and early morning awakening for ≥2 months = insomnia | x | x | |||||||||
| Hayashino (2013) (46) | - Outpatient clinic - Japan (2009–2010) | - 1513 (51%) - 63 ± 13 y | - 17 ± 10 y - Clinically diagnosed - 100% insulin | - PSQI - PSQI >5 = insomnia symptoms | x | x | |||||||||
| Hood (2014) (47) | - Endocrinology clinic - USA (NR) | - 194 (30%) - 58 ± 13 y | - NR - Clinically diagnosed - NR | - PSQI - PSQI >5 = insomnia symptoms | x | ||||||||||
| Huang (2017) (48) | - Endocrinology department in hospital - China (2014–2015) | - 81 (56%) - 66 ± 10 y | - NR - Clinically diagnosed - Diet or OAD | - PSQI - PSQI >7 = insomnia symptoms | x | x | x | x | x | x | x | x | x | ||
| Hung (2013) (49) | - Prevention Health Center - Taiwan (2002–2006) | - 103 (66%) - 56 ± 9 y | - NA: newly diagnosed - Clinically diagnosed - NA: newly diagnosed | - PSQI - PSQI >5 = insomnia symptoms | x | ||||||||||
| Hyyppa (1989) (50) | - Diabetics born and living in a particular district - Finland (NR) | - 63 (NR) - 45–65 y | - 1-14 y - Clinically diagnosed - NR | - Questionnaire on sleep habits 1. Sleep latency >50 min 2. Habitual insomnia 3. Difficulty maintaining sleep | x | ||||||||||
| Jain (2012) (51) | - Diabetic clinic - USA (NR) | - 81 (28%) - ±50 y | - NR - Clinically diagnosed - 0% insulin | - History of insomnia - Yes/no | x | x | x | ||||||||
| Johnson (2017) (52) | - Diabetic clinic - USA (NR) | - 168 (54%) - 66 ± 10 y | - 14 ± 9 - Clinically diagnosed - NR | - Self-report - “Ever been told by a doctor or health professional that you have a sleep disorder?” | x | ||||||||||
| Kara (2015) (53) | - Outpatient clinic - Turkey (2013–2014) | - 180 (41%) - 55 ± 17 y | - 11 ± 9 y - Clinically diagnosed - NR | - PSQI - PSQI ≥5 = insomnia symptoms | x | ||||||||||
| Kasenova (2017) (54) | - NR - Kazakhstan (NR) | - 136 (34%) - 59 ± 6 y | - 10 ± 7 y - Clinically diagnosed - NR | Insomnia variable 1: - PSQI - PSQI >5 = insomnia symptoms | x | ||||||||||
| Katic (2015) (17) | - Websurvey - USA (2013) | - 405 DM (49%) - 56 ± 10 y | - 11 ± 9 y - Self-report - NR | Insomnia variable 1: - Self-report - At risk for insomnia: waking up unrefreshed, difficulty falling asleep, waking in the middle of the night, or waking tooearly at least a few nights/week and affectingdaily activities Insomnia variable 2: - Self-report - Diagnosed with insomnia yes/no | x | ||||||||||
| Keskin (2015) (55) | - Family medicine clinics - Turkey (2014) | - 575 (33%) - 57 [50–64] y | - 7 [3–12] y - Clinically diagnosed - 66% OAD 30% insulin | - PSQI - PSQI ≥5 = insomnia symptoms | x | x | x | x | |||||||
| Keskin (2016) (56) | - Outpatient clinic - Turkey (2014) | - 208 (29%) - Adult group: 53 ± 9 y; geriatric group: 71 ± 5 y | - NR - Clinically diagnosed - NR | - PSQI - PSQI ≥5 = insomnia symptoms | x | ||||||||||
| Khosravan (2015) (57) | - Diabetes clinic - Iran (2012) | - 1600 (NR) - 35–70 y | - NR - Clinically diagnosed - NR | - PSQI - PSQI >5 = insomnia symptoms | x | ||||||||||
| Knutson (2011) (58) | - General population - USA (2003–2006) | - 40 DM (30%) - 46 ± 4 y | - NR - Clinically diagnosed - NR | - PSQI + actigraph - Insomnia: not falling asleep <30 min >3 times/wk or waking up in the middle of the night >3 times/wk + sleep efficiency <80% | x | ||||||||||
| Knutson (2006) (6) | - Tertiary care - USA (NR) | - 161 (26%) - 57 ± 13 y | - 11 ± 9 y - Clinically diagnosed - 48% insulin or in combination with OAD | - PSQI - PSQI >5 = insomnia symptoms | x |
Abbreviations: AIS, Athens Insomnia Scale; CES-D, Center for Epidemiologic Studies Depression scale; DM, diabetes mellitus; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders-IV; GDS, Geriatric Depression Scale; GLP, glucagon-like peptide; MOS-SS, Medical Outcomes Study - Sleep Scale; NA, not applicable; NHANES, National Health and Nutrition Examination Survey; NR, not reported; OAD, oral antidiabetic drugs; PHQ9, Patient Health Questionnaire; PSQI, Pittsburgh Sleep Quality Index; UAE, United Arabic Emirates.
Table 2.
Characteristics of Included Studies in the Systematic Review (n = 78), Studies L-Z
| Author (Y), Reference | - Setting - Country (Period of Analysis) | - N (% men) - Age ± SD/[IQR] | - Diabetes Duration - Diabetes Diagnosis - Diabetes Treatment | - Insomnia or Insomnia Symptoms Measure - Distribution/Cutoff | Metabolic Parameters and Glycemic Control | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| None | HbA1c | FPG | BMI | Waist | HDL | LDL | Chol | TG | SBP | DBP | |||||
| Koyanagi (2014) (65) | - General population - Finland, Poland, Spain (2011–2012) China, Ghana, India, Mexico, Russia, South Africa (2007–2010) | - 3285 DM (NR) - Median: 60–65 y | - NR - Self-report - NR | - Self-report - Sleep problems: severe or extreme problems with falling asleep, waking up frequently during the night or waking up too early in the morning the last 30 days | x | ||||||||||
| Lecube (2016) (66) | - Outpatient clinic - Spain (2013–2014) | - 135 (44%) - 61 ± 13 y | - >5 y - Clinically diagnosed - NR | - PSQI - PSQI >5 = insomnia symptoms | x | ||||||||||
| Lopes (2005) (67) | - Outpatient clinic - Brazil (NR) | - 100 (27%) - 58 ± 12 y | - 10 ± 8 y - Clinically diagnosed - 59% OAD 32% insulin | - PSQI - PSQI ≥6 = insomnia symptoms | x | ||||||||||
| Lou (2012) (68) | - General population - China (2008) | - 954 DM (43%) - 49 ± 13 y | - NR - Clinically diagnosed - NR | - Self-report - Sleep quality during previous year = good, common or poor | x | ||||||||||
| Lou (2015) (69) | - Health centers - China (NR) | - 944 (39%) - 64 ± 10y | - 6 ± 5y - Clinically diagnosed - 12% insulin | - PSQI - PSQI ≥8 = insomnia symptoms | x | x | |||||||||
| Luyster (2011) (70) | - NR - USA (NR) | - 300 (43%) - 64 ± 10y | - 9 ± 7y - Clinically diagnosed - 12% insulin | - PSQI - PSQI >5 = insomnia symptoms | x | ||||||||||
| Manodpitipong (2017) (71) | - Hospital - Thailand (2014) | - 189 (40%) - Unemployed: 65 ± 8 Day work: 53 ± 9 | - Unemployed: 14 ± 10 Day work: 9 ± 8 - Clinically diagnosed - Unemployed: 42% Day work: 35% | - PSQI - NR | x | ||||||||||
| Mahmood (2013) (72) | - Diabetes clinic - Ireland (NR) | - 114 (64%) - Healthy: 64 ± 11 Insomnia: 66 ± 10 | - NR - Clinically diagnosed - Healthy/insomnia: 66%/65% OAD 23%/15% insulin 21%/15% diet | - PSQI - PSQI ≥6 = insomnia symptoms | x | x | x | x | x | x | x | x | x | ||
| Medeiros (2013) (73) | - Outpatient clinic - Brazil (NR) | - 110 (35%) - 58 ± 11 y | - NR - Clinically diagnosed - NR | - PSQI - PSQI >6 = insomnia symptoms | x | ||||||||||
| Meng (2015) (74) | - Hospital - China (2014-2015) | - 332 (57%) - Insomnia: 59 ± 9 y Healthy: 53 ± 14 y | - Insomnia:12 ± 7 y Healthy: 7 ± 7 y - Clinically diagnosed - NR | - PSQI - PSQI ≥7 = insomnia symptoms | x | x | x | x | x | x | x | x | |||
| Narisawa (2017) (75) | - Outpatient clinic - Japan (2014) | - 622 (76%) - 57 ± 10 y | - NR - Clinically diagnosed - 10% OAD 27% insulin | - PSQI - PSQI >5.5 = insomnia symptoms | x | ||||||||||
| Nefs (2015) (76) | - Websurvey - Netherlands (2011) | - 361 (54%) - 62 ± 9 | - 11 ± 8 y - Self-report - 44% OAD 49% insulin | - PSQI - PSQI >5 = insomnia symptoms | x | ||||||||||
| Osonoi (2015) (77) | - Outpatient clinic - Japan (2013–2014) | - 724 (63% ) - 58 ± 9 y | - 10 ± 7 y - Clinically diagnosed - 86% OAD/insulin/ both | - PSQI - PSQI ≥9 = insomnia symptoms | x | x | x | x | x | x | x | x | |||
| Rajendran (2012) (78) | - Tertiary care - India (2010–2011) | - 120 (54%) - 54 ± 9 y | - 7 ± 6 y - Clinically diagnosed - 100% OAD/insulin/ both | - PSQI - PSQI ≥5 = insomnia symptoms | x | ||||||||||
| Ramos (2015) (79) | - Registry black participants - USA (NR) | - 612 (NR) - 62 ± 14 y | - NR - Clinically diagnosed - NR | - Unspecified questionnaire - Insomnia symptoms yes/no | x | ||||||||||
| Ramtahal (2015) (80) | - Outpatient clinics - Trinidad and Tobago (2013) | - 291 (33%) - 59 ± 11 y | - 10 [6–19] y - Clinically diagnosed - 30% OAD 17% insulin 47% both | - NHANES sleep questionnaire - Insomnia = patients answered “often” or “almost always” to sleep-related questions | x | ||||||||||
| Sakamoto (2018) (81) | - Hospital - Japan (2014–2016) | - 3294 (61%) - 65 [55–72] y | - 11 [5–17] y - Clinically diagnosed - 60% OAD 29% insulin/GLP | - PSQI - PSQI >5 = insomnia symptoms | x | ||||||||||
| Seligowski (2013) (82) | - Primary care - USA (NR) | - 86 (97%) - 62 ± 8 y | - NR - Clinically diagnosed - 100% insulin or OAD | - PSQI - NR | x | ||||||||||
| Shamshirgaran (2017) (83) | - Diabetes clinic - Iran (2013-2014) | - 256 (29%) - 54 ± 9 y | - NR - Clinically diagnosed - 58% OAD 29% OAD + insulin | - PSQI - PSQI >5 = insomnia symptoms | x | x | x | ||||||||
| Shim (2011) (84) | - Outpatient clinic - Korea (2008) | - 784 (50%) - 54 ± 12 y | - 9 ± 7 y - Clinically diagnosed - 0% insulin | - PSQI - PSQI >5 = insomnia symptoms | x | ||||||||||
| Skomro (2001) (85) | - Outpatient clinic - Canada (NR) | - 58 (50%) - 57 ± 15 y | - 10 y - Clinically diagnosed - NR | - Interview - Difficulty with sleep onset or maintenance ≥3 times/wk | x | ||||||||||
| Sokwalla (2017) (86) | - Outpatient clinic - Kenya (2012) | - 228 (42%) - 57 ± 12 y | - 10 ± 8 y - Clinically diagnosed - 36% OAD 13% insulin 50% insulin + OAD | - PSQI - PSQI >5 = insomnia symptoms | x | x | |||||||||
| Song (2013) (87] | - Outpatient clinic - China (2012) | - 140 (59%) - 57 ± 14 y | - 20% = >10 y - Clinically diagnosed - 100% insulin | - PSQI - PSQI >5 = insomnia symptoms | x | ||||||||||
| Sridhar (1994) (88) | - Diabetes center - India (NR) | - 184 (82%) - 46 ± NR | - Normal sleep: 5 ± 6 y Abnormal sleep: 4 ± 5 y - Clinically diagnosed - NR | - Self-report - Variable 1: difficulty falling asleep ≥3 times/ wk for ≥2 wks - Variable 2: difficulty in maintaining sleep; interrupted sleep ≥2/night and problems going back to sleep | x | ||||||||||
| Sudore (2012) (89) | - Diabetes registry - USA (2005–2006) | - 13 171 (52%) - 60 ± 10 y | - 10 ± 8 y - Clinically diagnosed - 21% insulin | - PHQ9 - Sleep disturbance = almost every day difficulty initiating or maintaining sleep or excessive sleep | x | ||||||||||
| Tang (2014) (90) | - Hospital - China (2013–2014) | - 551 (55%) - 57 ± 10 y | - 9 ± 8 y - Clinically diagnosed - NR | - PSQI - PSQI >5 = insomnia symptoms | x | ||||||||||
| Tanjani (2015) (91) | - General population - Iran (2012) | - 297 DM (41%) - 60–76 y | - NR - Self-report - NR | - GDS - Insomnia: yes/no | x | ||||||||||
| Telford (2018) (92) | - Primary care clinic - USA (NR) | - 281 (52%) - 62 ± 9 y | - NR - Clinically diagnosed - 44% insulin | - PSQI - PSQI >5 = insomnia symptoms | x | x | x | x | x | ||||||
| Thongsai (2013) (93) | - Outpatient clinic - Thailand (2013) | - 209 (40%) - 40% > 60 y | - 62% = 3–5 y 38% >5 y - Clinically diagnosed - NR | - CES-D questionnaire - Difficulty sleeping (never, sometimes, quite often, always) | x | ||||||||||
| Torella (2015) (94) | - Diabetes clinic - Spain (2011–2013) | - 145 (51%) - 60 ± 10 y | - 14 ± 10 y - Clinically diagnosed - 73% insulin | - PSQI - PSQI >5 = insomnia symptoms | x | ||||||||||
| Trento (2008) (95) | - NR - Italy (NR) | - 47 (68%) - 61 ± 5 y | - 17 (8–31) y - Clinically diagnosed - 100% OAD | - Actigraphy: sleep efficiency + sleep latency - NA | x | ||||||||||
| Tsai (2012) (96) | - Outpatient clinic - Taiwan (2009) | - 46 (61%) - 60 ± 10 y | - >1 y - Clinically diagnosed - NR | - PSQI - PSQI ≥8 = insomnia symptoms | x | ||||||||||
| Tsujimura (2009) (97) | - NR - Japan (NR) | - 19 (58%) - 63 ± 10 y | - 7 (1–20) y - WHO 1981 - NR | - Actigraphy: sleep efficiency + wake after sleep onset - NA | x | x | |||||||||
| Vernon (2008) (98) | - Clinical centers - North America, Australia, Germany, Hungary, Poland, South Africa, United Kingdom | - 388 (±58) - ±59 (21–85) y | - ±11 y - Clinically diagnosed - NR | - MOS-SS questionnaire - MOS score >52.5 = sleep disturbance | x | ||||||||||
| Wei (2017) (99) | - Outpatient clinic - China (2015) | - 206 (50%) - 60 [56–63] y | - Newly diagnosed - WHO 1999 - 0% OAD | - PSQI - PSQI >5 = insomnia symptoms | x | x | x | x | x | x | x | x | |||
| Yagi (2011) (100) | - Outpatient clinic - Japan (baseline 1996–1998) | - 270 (55%) - 67 ± 10 y | - 18 ± 9 y - Clinically diagnosed - 41% insulin | - PSQI - PSQI >5.5 = insomnia symptoms | x | ||||||||||
| Zelman (2006) (101) | - Tertiary care - USA (2003) | - 255 (45%) - 61 ± 13 y | - 9 [4–18] y - Clinically diagnosed - NR | - MOS-SS - No, some or sleep problems | x | ||||||||||
| Zhang (2016) (63) | - T2D registry - China (2012) | - 944 (39%) - 64 ± 10 y | - 6 ± 5 y - Clinically diagnosed - 12% insulin | - PSQI - PSQI ≥7 = insomnia symptoms | x | ||||||||||
| Zhu (2014) (102) | - Hospital - China (2013–2014) | - 206 (66%) - 57 ± 11 y | - 10 ± 7 y - Clinically diagnosed - 60% insulin | - PSQI - PSQI ≥8 = insomnia symptoms | x | x | x | x | x | x | x | x | |||
| Zhu (2018) (103) | - Convenience sample - USA (2013–2014) | - 90 (48%) - 57 ± 8 y | - 9 ± 7 y - Self-report - NR | - PROMIS - Sleep disturbance = perceived difficulties in getting or staying asleep | x |
Abbreviations: AIS, Athens Insomnia Scale; BMI, body mass index; CES-D, Center for Epidemiologic Studies Depression scale; DBP, diastolic blood pressure; DM, diabetes mellitus; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders-IV; FPG, fasting plasma glucose; GDS, Geriatric Depression Scale; GLP, glucagon-like peptide; HDL, high-density lipoprotein; IQR, interquartile range; LDL, low-density lipoprotein; MOS-SS, Medical Outcomes Study - Sleep Scale; NA, not applicable; NHANES, National Health and Nutrition Examination Survey; NR, not reported; OAD, oral antidiabetic drugs; PHQ9, Patient Health Questionnaire; PSQI, Pittsburgh Sleep Quality Index; SBP, systolic blood pressure; SD, standard deviation; TG, triglyceride; UAE, United Arabic Emirates; WHO, World Health Organization.
Quantitative data synthesis and analysis
Meta-analyses were performed, using Review Manager 5.3 (Nordic Cochrane Center). Because the inclusion criteria allowed for a large range of studies (ie, all countries and all types of adults with T2D), random-effects meta-analyses were performed (59). Prevalence data were entered on a log scale to correct for studies with a low variance resulting from a high or low prevalence. The level of evidence was applied to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) criteria and reported. As is customary with GRADE, randomized controlled trials start as high quality and observational studies as low quality. While no trials were included, all studies start off with a low GRADE quality.
Statistical heterogeneity was assessed with the I2 statistic, with the I2 reflecting the percentage of the variability in effect estimates that is due to heterogeneity rather than sampling error (chance), with 0% reflecting no heterogeneity and 100% high heterogeneity (60). A P value <0.05 was considered statistically significant. Funnel plots were used to assess publication bias.
Prevalence of insomnia and insomnia symptoms.
Sensitivity analyses were performed to gain insight into how certain subgroups influenced the prevalence of insomnia and insomnia symptoms and to identify sources of heterogeneity. The sensitivity analysis for methodological quality of the studies (low/moderate/high) was prespecified. In addition, because of the high heterogeneity, sensitivity analyses were performed on age of participants (age ≥60 years), presence of comorbidities (eg, lower limb amputation, neuropathy), geographical location (Asia vs Europe/America), year of analysis (year ≥2010), sample size (N < 100, N = 100–199, N = 200–299, N = 300–999, N > 1000), and insomnia or insomnia symptoms measurement method (PSQI or not). Several studies have used different cutoff points for the PSQI; however, we could not assess the effect of these different cutoffs in a sensitivity analysis because the studies in this meta-analysis using a higher cutoff are mostly Asian studies and in Asia the validated PSQI cutoff is >8 instead of >5. After these primary sensitivity analyses, variables that explained most of the heterogeneity were selected and stratified for some of the remaining variables that could still make a large enough subgroup to assess if more heterogeneity could be explained.
Association between insomnia, insomnia symptoms and metabolic parameters, and glycemic control.
The quantitative association between insomnia, insomnia symptoms and metabolic parameters, or glycemic control was examined in 2 ways. First, we included studies that examined the association by means of adjusted regression analyses, resulting in a beta-coefficient or risk estimate with 95% confidence intervals (CI). Studies were pooled in case of 3 or more studies reported on the same metabolic or glycemic control parameter, with the same regression analyses (logistic or linear). Second, we included studies that compared insomnia (symptoms) versus no insomnia (symptoms) and estimated the mean difference or risk ratio and 95% CI. When median values instead of mean values were reported, the mean and standard deviation were estimated from the sample size, median, and interquartile range according to the method of Wan et al. 2014 (61). Studies were pooled in case of 3 or more studies reported on the same outcome.
Subgroup analyses were performed to examine possible sources of heterogeneity by excluding poor-quality studies and outliers. In addition, for the analyses on the mean differences, a sensitivity analysis was performed on studies with a reported mean versus a calculated mean.
Results
Description of included studies
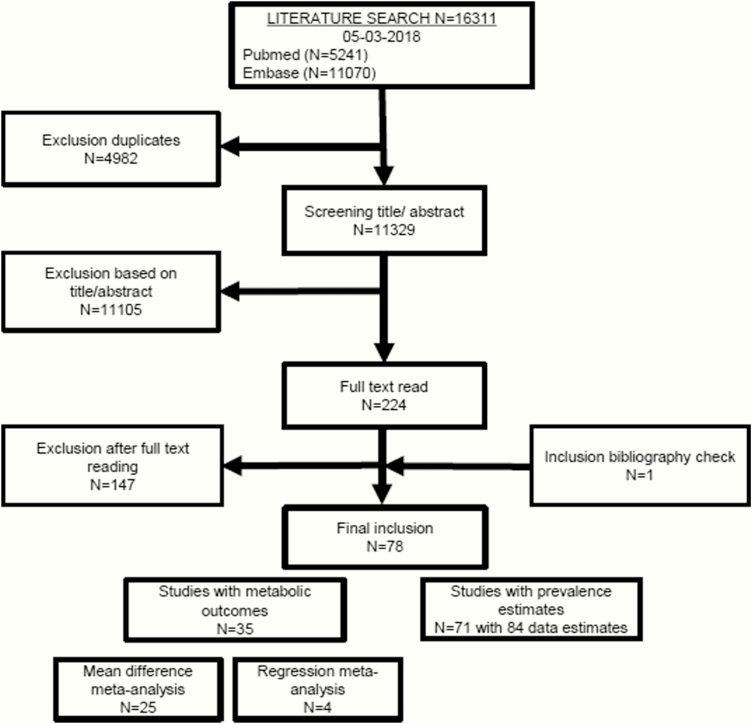
The systematic literature search identified 11 329 articles. After screening titles and abstracts, 224 potentially eligible articles were read full text. Because 3 studies reported the prevalence of insomnia and insomnia symptoms in the same population (62–64), only the most recent (63) was included. In total, 78 studies met the inclusion criteria and were therefore included in this systematic review (Fig. 1). Of these 78 studies, 71 reported on the insomnia and insomnia symptoms prevalence in T2D and 35 on the associations with metabolic parameters or glycemic control. An overview of the 147 excluded studies and reason for exclusion is provided in File 2.
Figure 1.
Flowchart of the search and selection process.
File 2. List of Excluded Full-text Studies (N = 147).
| Author, Y | Reason for Exclusion | Author, Y | Reason for Exclusion |
|---|---|---|---|
| A˚berg Löfvenborg, 2012 | A | Ebrahimi, 2017 | E |
| Abolfotouh, 2015 | B | Ekici, 2017 | E |
| Ajayi, 2016 | A | Engeda, 2012 | A |
| Alemohammed, 2017 | A | Engeda, 2013 | D |
| Alfawaz, 2016 | A | Eriksson, 2009 | A |
| Alfawaz, 2016 | A | Etzioni, 2012 | A |
| Altaf, 2017 | A | Fernandez, 2012 | F |
| Aminian, 2014 | A | Finkelstein, 2009 | A |
| Arora, 2014 | C | Finn, 2010 | A |
| Arora, 2016 | D | Foley, 1999 | G |
| Balci, 2017 | A | Foley, 2004 | B |
| Bedi, 2013 | C | Fiorentini, 2007 | B |
| Bener, 2011 | C | Fritschi, 2013 | A |
| Bermudez-Millan, 2015 | A | Fritschi, 2015 | E |
| Bermudez-Millan, 2016 | E | Fritschi, 2017 | A |
| Bolge, 2010 | A | Gangwisch, 2015 | A |
| Bonn, 2018 | A | Garcia, 2008 | A |
| Bradai, 2011 | A | Garcia, 2018 | A |
| Brady, 2016 | A | Garnwich, | A |
| Brod, 2013 | B | Gebel, 2011 | A |
| Carnethon, 2012 | A | Gebel, 2011 | A |
| Cassidy, 2016 | A | Givens, 2015 | E |
| Cespedes, 2016 | B | Gooneratne, 2011 | A |
| Chasens, 2007 | A | Grandner, 2012 | E |
| Chasens, 2009 | E | Gribble, 2009 | A |
| Chasens, 2013 | A | Groenewald, 2016 | A |
| Chasens, 2013 | A | Guo, 2015 | F |
| Chasens, 2013 | D | Hayashino, 2012 | A |
| Chasens, 2014 | D | Holingue, 2018 | A |
| Chasens, 2014 | C | Hughes, 2016 | A |
| Chasens, 2016 | A | Hung, 2014 | A |
| Chasens, 2016 | D | Hung, 2018 | B |
| Chasens, 2017 | A | Ip, 2007 | C |
| Chen, 2017 | A | Johnson, 2015 | C |
| Chiang, 2011 | A | Kessler, 2009 | A |
| Chiang, 2014 | A | Khandelwal, 2017 | A |
| Danielle, 2013 | E | Kim, 2013 | E |
| Dobrosielski, 2010 | A | Kim, 2016 | A |
| Donjacour, 2009 | A | Kim, 2016 | A |
| Klimek, 2015 | B | Ren, 2015 | F |
| Ko, 2011 | A | Ren, 2015 | F |
| Kondo, 2011 | A | Reutrakul, 2014 | A |
| Konrade, 2017 | A | Rodriguez, 2014 | A |
| Kubota, 2017 | A | Savas, 2013 | A |
| Lalau, 2013 | B | Sekine, 2011 | A |
| Lecube, 2005 | A | Seo, 2011 | A |
| Leger, 2010 | A | Shan, 2015 | A |
| Liao, 2011 | A | Singh, 2013 | B |
| Liao, 2012 | A | Singh, 2014 | A |
| Liao, 2014 | A | Sivertsen, 2009 | D |
| Liao, 2014 | A | Smitherman, 2010 | A |
| Libman, 2010 | A | Sokwalla, 2014 | A |
| Lou, 2015 | D | Sridhar, 2003 | G |
| Luo, 2011 | F | Sridhar, 2006 | E |
| Mahapatra, 2017 | A | St-Onge, 2012 | B |
| Mahmood, 2011 | A | Sturrock, 1995 | G |
| Maracy, 2011 | F | Suhaym, 2016 | A |
| Martínez, 2014 | A | Sun, 2016 | C |
| Martínez, 2016 | A | Szu-Hua, 2016 | A |
| Matsunaga, 2011 | A | Talwalkar, 2017 | A |
| Meng, 2015 | A | Tanaka, 2011 | A |
| Morris, 2016 | A | Urry, 2014 | A |
| Morris, 2017 | A | Vgontzas, 2009 | A |
| Naseri, 2017 | E | Vgontzas, 2009 | B |
| Nefs, 2015 | C | Voinescu, 2010 | A |
| Öztürk, 2013 | A | Wandell, 2000 | E |
| Ozturk, 2015 | E | Wang, 2005 | F |
| Perfect, 2010 | D | Weatherall, 2017 | A |
| Pisanti, 2012 | A | Yoda, 2015 | B |
| Plantinga, 2012 | B | Zaraspe, 2017 | A |
| Quinn, 2016 | A | Zhang, 2005 | F |
| Reader, 2015 | A | Zhang, 2016 | F |
| Ren, 2015 | A | Zhao, 2016 | C |
| Ren, 2015 | F |
Abbreviations: A, abstract, review, letter, design article; B, wrong outcome; C, duplicate study; D, wrong patient population; E, data cannot be extracted; F, language; G, no full text.
An overview of the characteristics of the included studies is shown in Tables 1 and 2. Sample sizes ranged from 35 (37) to 18 888 (44) participants, with only 6 studies reporting on >1000 participants. Both male and female participants were included except for 2 studies, which included only women (33) or men (42). Most studies were conducted in Asia (35 studies). Thirty-two studies were conducted in outpatient and diabetes clinics and 11 studies were conducted in the general population.
As shown in Tables 1 and 2, insomnia and insomnia (symptoms) were measured differently among the studies. Of the 78 studies, 50 used the PSQI questionnaire to determine insomnia symptoms. Among others, also self-reported diagnosis of insomnia by a physician, the Medical Outcomes Study-Sleep Scale, and the Athens Insomnia Scale were used and additionally only after a diagnostic interview was used to assess insomnia and insomnia symptoms.
Methodological quality rating
An overview of the methodological quality assessment of the studies is presented in Table 3. With regard to the prevalence outcome, the methodological quality of the studies was considered to be strong (low risk of bias) in 31 studies, moderate (moderate risk of bias) in 36 studies, and weak (high risk of bias) in 4 studies. With regard to metabolic parameters or glycemic control, the quality assessment resulted in 6 strong methodological quality studies, 9 moderate quality studies, and 20 weak studies. Only 2 studies adjusted for age, sex, and diabetes duration and were therefore rated as strong regarding the domain confounding in the methodological quality assessment. The other studies did not adjust for diabetes duration and were therefore rated as moderate, but these studies did adjust for other confounders such as BMI and insulin use.
Table 3.
Methodological Quality Rating per Domain per Study
| Author, Y (Reference) | Prevalence Outcome (P) | SD | BL | RSB | RWD | CF | DC | DA | RP | Overall |
|---|---|---|---|---|---|---|---|---|---|---|
| Metabolic/Glycemic (M) | ||||||||||
| Abdelgadir 2009 (24) | P | M | NR | W | NR | NR | S | NR | M | Moderate |
| Al Tannir 2016 (25) | P | M | NR | M | NR | NR | W | NR | S | Moderate |
| Aribas 2015 (26) | P | M | NR | W | NR | NR | S | NR | S | Moderate |
| Aribas 2015 | M | M | NR | W | NR | W | W | M | S | Weak |
| Bani-Issa 2017 (27) | P | M | NR | S | NR | NR | S | NR | S | Strong |
| Bani-Issa 2017 | M | M | NR | S | NR | W | W | M | S | Weak |
| Bedi 2011 (28) | P | M | NR | W | NR | NR | S | NR | S | Moderate |
| Bener 2010 (29) | P | M | NR | M | NR | NR | S | NR | S | Strong |
| Bhaskar 2016 (30) | P | M | NR | S | NR | NR | M | NR | S | Strong |
| Bilge 2016 (18) | P | M | NR | W | NR | NR | S | NR | S | Moderate |
| Budhiraja 2011 (31) | P | M | NR | S | NR | NR | S | NR | S | Strong |
| Celik 2012 (32) | P | M | NR | W | NR | NR | S | NR | S | Moderate |
| Chang 2017 (33) | P | M | NR | W | NR | NR | S | NR | S | Moderate |
| Cheng 2017 (34) | P | M | NR | W | NR | NR | W | NR | S | Weak |
| Cheng 2017 | M | M | NR | W | NR | W | W | M | S | Weak |
| Cho 2014 (35) | P | M | NR | M | NR | NR | S | NR | S | Strong |
| Colbay 2015 (36) | P | M | NR | M | NR | NR | M | NR | S | Moderate |
| Colbay 2015 | M | M | NR | M | NR | W | S | M | S | Moderate |
| Cuellar 2008 (37) | P | M | NR | W | NR | NR | S | NR | S | Moderate |
| Cunha 2008 (38) | P | M | NR | W | NR | NR | S | NR | S | Moderate |
| Cunha 2008 | M | M | NR | W | NR | W | W | W | S | Weak |
| El Aghoury 2017 (39) | P | M | NR | W | NR | NR | S | NR | S | Moderate |
| Ford 2015 (40) | P | M | NR | S | NR | NR | W | NR | S | Moderate |
| Fritschi 2017 (41) | M | M | NR | M | NR | W | W | M | M | Weak |
| Fukui (42) | P | M | NR | W | NR | NR | S | NR | M | Moderate |
| Gozashti 2016 (43) | P | M | NR | W | NR | NR | S | NR | S | Moderate |
| Gozashti 2016 | M | M | NR | W | NR | W | W | S | S | Weak |
| Grandner 2011 (44) | P | M | NR | S | NR | NR | W | NR | S | Moderate |
| Han 2002 (45) | P | M | NR | W | NR | NR | W | NR | S | Weak |
| Han 2002 | M | M | NR | W | NR | W | M | M | S | Weak |
| Hayashino 2013 (46) | P | M | NR | M | NR | NR | S | NR | S | Strong |
| Hayashino 2013 | M | M | NR | M | NR | W | W | M | S | Weak |
| Hood 2014 (47) | P | M | NR | M | NR | NR | S | NR | S | Strong |
| Huang 2017 (48) | P | M | NR | W | NR | NR | S | NR | S | Moderate |
| Huang 2017 | M | M | NR | W | NR | W | W | M | S | Weak |
| Hung 2013 (49) | P | M | NR | S | NR | NR | S | NR | S | Strong |
| Hyyppa 1989 (50) | P | M | NR | M | NR | NR | S | NR | S | Strong |
| Jain 2012 (51) | P | M | NR | M | NR | NR | W | NR | M | Moderate |
| Jain 2012 | M | M | NR | M | NR | W | W | M | M | Weak |
| Johnson 2017 | P | M | NR | S | NR | NR | W | NR | M | Moderate |
| Kara 2015 (53) | P | M | NR | M | NR | NR | S | NR | S | Strong |
| Kasenova 2017 (54) | P | M | NR | W | NR | NR | M | NR | S | Moderate |
| Katic 2015 (17) | P | M | NR | M | NR | NR | M | NR | S | Moderate |
| Keskin 2016 (56) | P | M | NR | S | NR | NR | S | NR | S | Strong |
| Keskin 2016 | M | M | NR | S | NR | M | M | S | S | Strong |
| Keskin 2015 (55) | P | M | NR | S | NR | NR | S | NR | S | Strong |
| Keskin 2015 | M | M | NR | S | NR | W | S | M | S | Moderate |
| Khosravan 2015 (57) | P | M | NR | M | NR | NR | S | NR | M | Moderate |
| Knutson 2006 (6) | P | M | NR | S | NR | NR | S | NR | S | Strong |
| Knutson 2006 | M | M | NR | S | NR | M | S | S | S | Strong |
| Knutson 2011 (58) | P | M | NR | M | NR | NR | S | NR | S | Strong |
| Knutson 2011 | M | M | NR | M | NR | M | S | S | S | Strong |
| Koyagani 2014 (65) | P | M | NR | S | NR | NR | W | NR | S | Moderate |
| Lecube 2016 (66) | P | M | NR | W | NR | NR | S | NR | S | Moderate |
| Lecube 2016 | M | M | NR | W | NR | W | W | W | S | Weak |
| Lopes 2005 (67) | P | M | NR | M | NR | NR | S | NR | S | Strong |
| Lopes 2005 | M | M | NR | M | NR | W | W | W | S | Weak |
| Lou 2012 (68) | P | M | NR | M | NR | NR | M | NR | S | Moderate |
| Lou 2015 (69) | P | M | NR | S | NR | NR | S | NR | S | Strong |
| Lou 2015 | M | M | NR | S | NR | W | S | M | S | Moderate |
| Luyster 2011 (70) | P | M | NR | W | NR | NR | S | NR | S | Moderate |
| Mahmood 2013 (72) | P | M | NR | S | NR | NR | S | NR | S | Strong |
| Mahmood 2013 | M | M | NR | S | NR | M | W | S | S | Moderate |
| Manodpitipong 2017 (71) | M | M | NR | M | NR | M | S | S | S | Strong |
| Medeiros 2013 (73) | P | M | NR | S | NR | NR | S | NR | S | Strong |
| Meng 2015 (74) | P | M | NR | W | NR | NR | S | NR | S | Moderate |
| Meng 2015 | M | M | NR | W | NR | W | M | M | S | Weak |
| Narisawa 2017 (75) | P | M | NR | S | NR | NR | S | NR | S | Strong |
| Nefs 2015 (76) | P | M | NR | M | NR | NR | S | NR | S | Strong |
| Nefs 2015 | M | M | NR | M | NR | W | W | M | S | Weak |
| Osonoi 2015 (77) | P | M | NR | M | NR | NR | S | NR | S | Strong |
| Osonoi 2015 | M | M | NR | M | NR | M | M | S | S | Moderate |
| Rajendran 2012 (78) | P | M | NR | W | NR | NR | S | NR | S | Moderate |
| Ramos 2015 (79) | P | M | NR | W | NR | NR | S | NR | S | Moderate |
| Ramtahal 2015 (80) | P | M | NR | S | NR | NR | S | NR | S | Strong |
| Sakamoto 2018 (81) | P | M | NR | S | NR | NR | S | NR | S | Strong |
| Seligowski 2013 (82) | M | M | NR | M | NR | W | W | M | M | Weak |
| Shamshirgaran 2017 (83) | P | M | NR | W | NR | NR | S | NR | S | Moderate |
| Shamshirgaran 2017 | M | M | NR | W | NR | W | W | M | S | Weak |
| Shim 2011 (84) | P | M | NR | M | NR | NR | S | NR | S | Strong |
| Skomro 2001 (85) | P | M | NR | W | NR | NR | M | NR | S | Moderate |
| Sokwalla 2017 (86) | P | M | NR | M | NR | NR | S | NR | S | Strong |
| Sokwalla 2017 | M | M | NR | M | NR | W | S | M | S | Moderate |
| Song 2013 (87) | P | M | NR | W | NR | NR | S | NR | S | Moderate |
| Sridhar 1994 (88) | P | M | NR | W | NR | NR | M | NR | S | Moderate |
| Sudore 2012 (89) | P | M | NR | S | NR | NR | M | NR | S | Strong |
| Tang 2014 (90) | P | M | NR | M | NR | NR | S | NR | S | Strong |
| Tang 2014 | M | M | NR | M | NR | S | M | S | S | Strong |
| Tanjani 2015 (91) | P | M | NR | W | NR | NR | W | NR | M | Weak |
| Telford 2018 (92) | P | M | NR | W | NR | NR | S | NR | S | Moderate |
| Telford 2018 | M | M | NR | W | NR | M | M | S | S | Moderate |
| Thongsai 2013 (93) | P | M | NR | W | NR | NR | W | NR | S | Weak |
| Torrella 2015 (94) | P | M | NR | W | NR | NR | S | NR | S | Moderate |
| Torrella 2015 | M | M | NR | W | NR | M | M | S | S | Moderate |
| Trento 2008 (95) | M | M | NR | W | NR | W | W | M | S | Weak |
| Tsai 2012 (96) | P | M | NR | M | NR | NR | S | NR | S | Strong |
| Tsai 2012 | M | M | NR | M | NR | M | M | S | S | Moderate |
| Tsujimura 2009 (97) | M | M | NR | W | NR | W | M | M | M | Weak |
| Vernon 2008 (98) | P | M | NR | W | NR | NR | S | NR | S | Moderate |
| Wei 2017 | M | M | NR | W | NR | W | S | M | S | Weak |
| Yagi 2011 (100) | P | M | NR | M | NR | NR | S | NR | S | Strong |
| Zelman 2006 (101) | P | M | NR | W | NR | NR | S | NR | M | Moderate |
| Zhang 2016 (63) | P | M | NR | M | NR | NR | S | NR | S | Strong |
| Zhu 2014 (102) | P | M | NR | M | NR | NR | S | NR | S | Strong |
| Zhu 2014 | M | M | NR | M | NR | S | S | S | S | Strong |
| Zhu 2018 (103) | M | M | NR | W | NR | W | W | M | M | Weak |
Abbreviations: BL, blinding; BMI, body mass index; CF, confounding; DA, data-analysis; DC, data-collection; M, moderate; NR, no rating; RP, reporting; RSB, representativeness with regard to selection bias; RWD, representativeness with regard to withdrawals/dropouts; S, strong; SD, study design; W, weak.
Prevalence of insomnia and insomnia symptoms in T2D
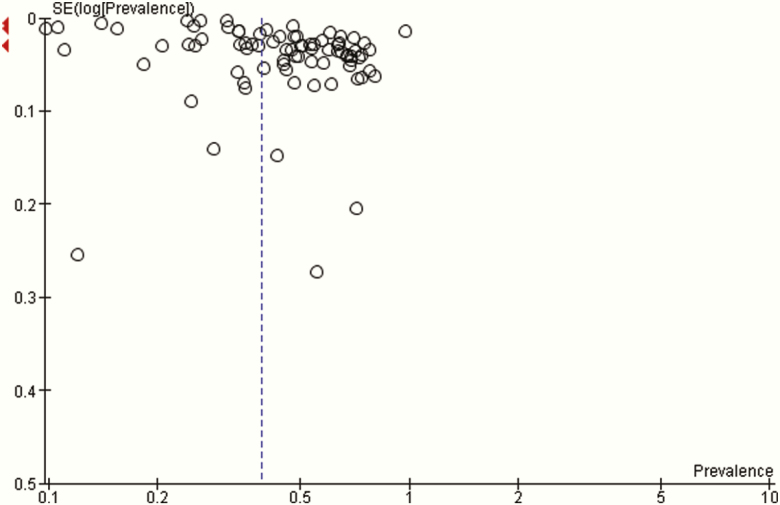
A random-effects meta-analysis of 71 studies with 84 prevalence estimates revealed an insomnia and insomnia symptoms prevalence of 39% with a 95% CI of 34% to 44%. Heterogeneity among the 71 studies was high with an I2 statistic of 100% (P < 0.00001) (Table 4). Visual examination of the funnel plot showed there was no publication bias (Fig. 2).
Table 4.
Prevalence, 95% Confidence Intervals (CI) and I2 Statistic Overall and for Several Sensitivity Analyses
| No. Prevalence Estimates | Prevalence (%) | 95% CI | I2 (P Value) | |
|---|---|---|---|---|
| Overall | 84 | 39 | 34–44 | 100% (P < 0.00001) |
| Sensitivity analyses | ||||
| Age <60 y | 56 | 37 | 31–44 | 100% (P < 0.00001) |
| Age ≥60 y | 28 | 44 | 36–55 | |
| Year of analysis <2010 |
31 53 |
35 42 |
31–40 32–54 |
100% (P < 0.00001) |
| Year of analysis ≥2010 | ||||
| PSQI | 48 | 46 | 35–62 | 100% (P < 0.00001) |
| Other than PSQI | 36 | 31 | 27–36 | |
| Strong quality | 34 | 39 | 33–47 | 100% (P < 0.00001) |
| Moderate quality | 46 | 39 | 32–48 | 100% (P < 0.00001) |
| Weak quality | 4 | 44 | 29–68 | 99% (P < 0.00001) |
| With comorbidities | 9 | 60 | 46–79 | 99% (P < 0.00001) |
| No comorbidities | 74 | 37 | 33–43 | 100% (P < 0.00001) |
| N < 100 | 22 | 40 | 25–64 | 100% (P < 0.00001) |
| N = 100–199 | 21 | 55 | 46–67 | 99% (P < 0.00001) |
| N = 200–299 | 14 | 46 | 38–55 | 99% (P < 0.00001) |
| N = 300–999 | 18 | 32 | 22–47 | 100% (P < 0.00001) |
| N > 1000 | 9 | 24 | 17–34 | 100% (P < 0.00001) |
| Studies with no comorbidities, with PSQI, of strong quality and with sample size >200 | 17 | 39 | 30–51 | 100% (P < 0.00001) |
Abbreviation: PSQI = Pittsburgh Sleep Quality Index.
Figure 2.
Funnel plot of the studies with prevalence estimates.
Sensitivity analyses showed that the prevalence of insomnia and insomnia symptoms was higher when age was ≥60 years (44%) and when the study was conducted in 2010 or later (42%). Additionally, the sensitivity analyses showed that when the PSQI questionnaire was used (46%), when the methodological quality of the studies was weak (44%), and when participants with comorbidities (lower limb amputation, neuropathy) were studied (60%) the prevalences were also higher (Table 4). Stratifying for geographical location showed the prevalence was 49% versus 40% in Asia versus Europe/America. Stratifying for sample size showed that in studies with >1000 participants, the prevalence was the lowest (24%); however, no clear pattern of larger studies reporting a lower prevalence of insomnia and insomnia symptoms was observed. Although in some subgroups (comorbidities and PSQI use), differences were observed, the I2 statistic remained 100% in almost all subgroups. Because of limited reporting in the original papers, we could not investigate type of population (population-based or hospital), type of diabetes medication, or glycemic control as possible sources of heterogeneity. Also as a result of the large unexplained heterogeneity, we did not test for statistical differences in prevalence between the subgroups.
Because the first set of sensitivity analyses showed that the presence of comorbidities was the largest source of heterogeneity, followed by using the PSQI or not, we performed an additional sensitivity analysis including only the studies without comorbidities, which used the PSQI, were of strong methodological quality, and had a sample size >200 participants to examine the prevalence in the most reliable subset of studies (n = 17). This sensitivity analysis resulted in a prevalence of insomnia and insomnia symptoms of 39% (95% CI, 30-51). However, heterogeneity remained very high with an I2 statistic of 100% (P < 0.00001). The level of evidence for the prevalence of insomnia and insomnia symptoms by GRADE was very low quality because of the findings being downgraded due to heterogeneity.
Insomnia and insomnia symptoms in T2D and metabolic parameters and glycemic control
Insomnia and insomnia symptoms and glycemic control unadjusted for confounders
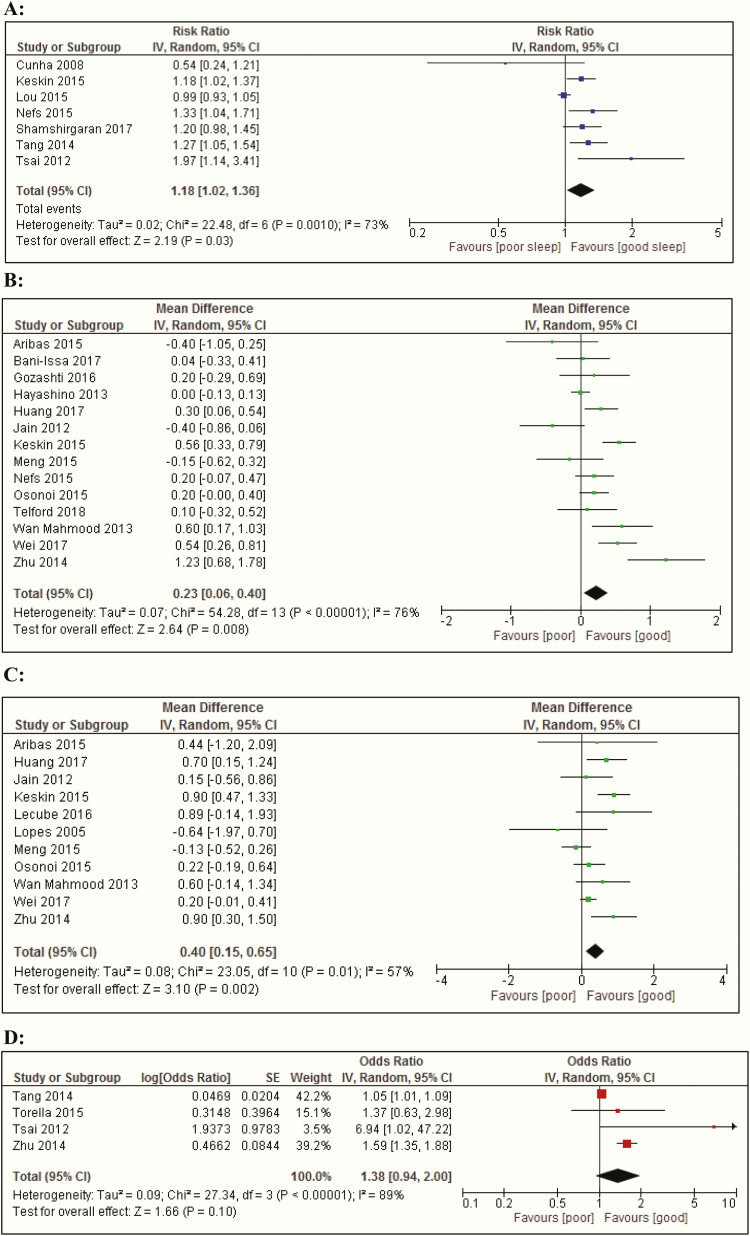
We investigated whether an elevated level of insomnia and insomnia symptoms were associated with glycemic control. For the meta-analysis of the HbA1c levels, the data are presented in 2 ways in Fig. 3: as a dichotomized and as a continuous variable. Data from 7 cross-sectional studies (38,55,69,76,83,90,96) showed that insomnia and insomnia symptoms were associated with an increased risk of an elevated HbA1c level (Fig. 3A), defined as HbA1c levels >6.5% (>48 mmol/mol) or ≥7% (>53 mmol/mol) (risk ratio, 1.18; 95% CI, 1.0-1.4; I2 = 73% [P = 0.001]). Similar results were observed when HbA1c levels from 14 studies that analyzed continuous variables (26, 27, 43, 46, 48, 51, 55, 72, 74, 76, 77, 92, 99, 102); compared to people with T2D without insomnia (symptoms), those with insomnia (symptoms) showed significantly higher HbA1c levels with a mean difference (MD) of: 0.23% (95% CI, 0.1-0.4); I2 = 76%, P < 0.00001 (Fig. 3B).
Figure 3.
Forest plots of meta-analyses of mean differences and regression analysis of metabolic and glycemic parameters between people with T2D with and without insomnia symptoms. (A) Poor glycemic control: HbA1c levels >6.5/7.0% (>48/53 mmol/mol). (B) HbA1c levels. (C) Fasting plasma glucose levels. (D) BMI. (E) Odds ratio for poor glycemic control: HbA1c levels >7/8.5% (>53/69 mmol/mol). Abbreviation: BMI, body mass index; CI, confidence interval; HbA1c, hemoglobin A1c; IV, inverse variance; random, random effects model.
The pooled data of 11 studies (26, 48, 51, 55, 66, 67, 72, 74, 77, 99, 102) on fasting glucose levels showed that fasting glucose levels were 0.40 mmol/L (95% CI, 0.2-0.7) higher in people with T2D and insomnia (symptoms), compared with people with T2D without insomnia (symptoms) (I2 = 57%, P = 0.01) (Fig. 3C).
Insomnia, insomnia symptoms, and BMI and waist circumference
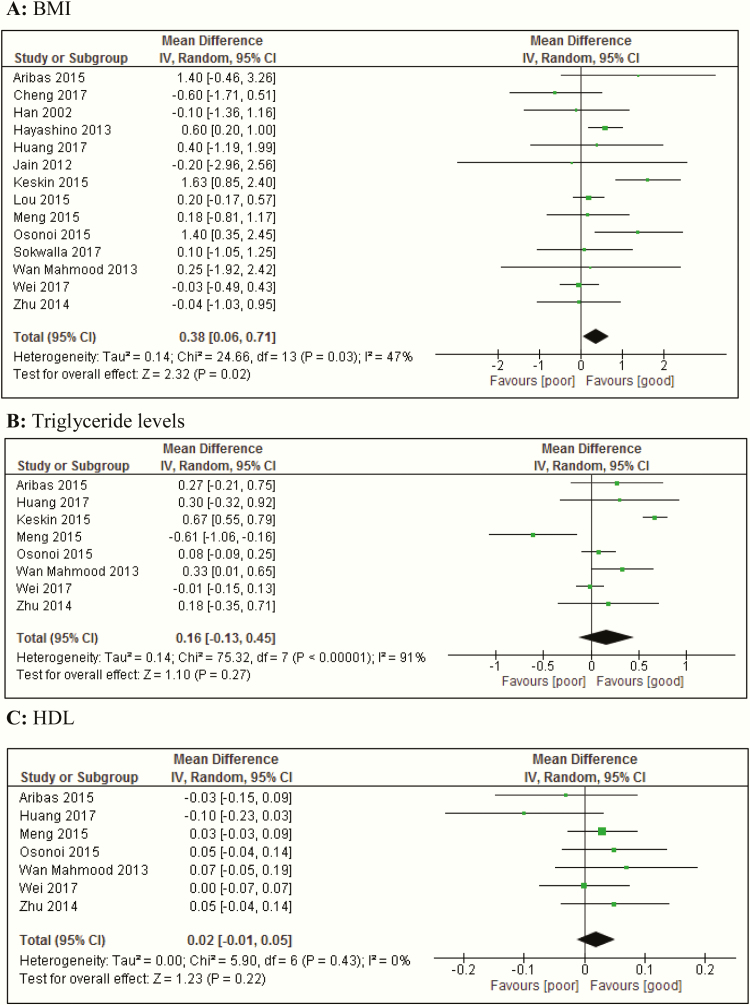
The pooled data of 14 studies (26, 34, 45, 46, 48, 51, 55, 69, 72, 74, 77, 86, 99, 102) on BMI showed that people with T2D and insomnia (symptoms) had a significantly higher BMI, compared with those without insomnia (symptoms) (MD, 0.38 kg/m2; 95% CI, 0.1-0.7; I2 = 47%, P = 0.03) (Fig. 4A).
Figure 4.
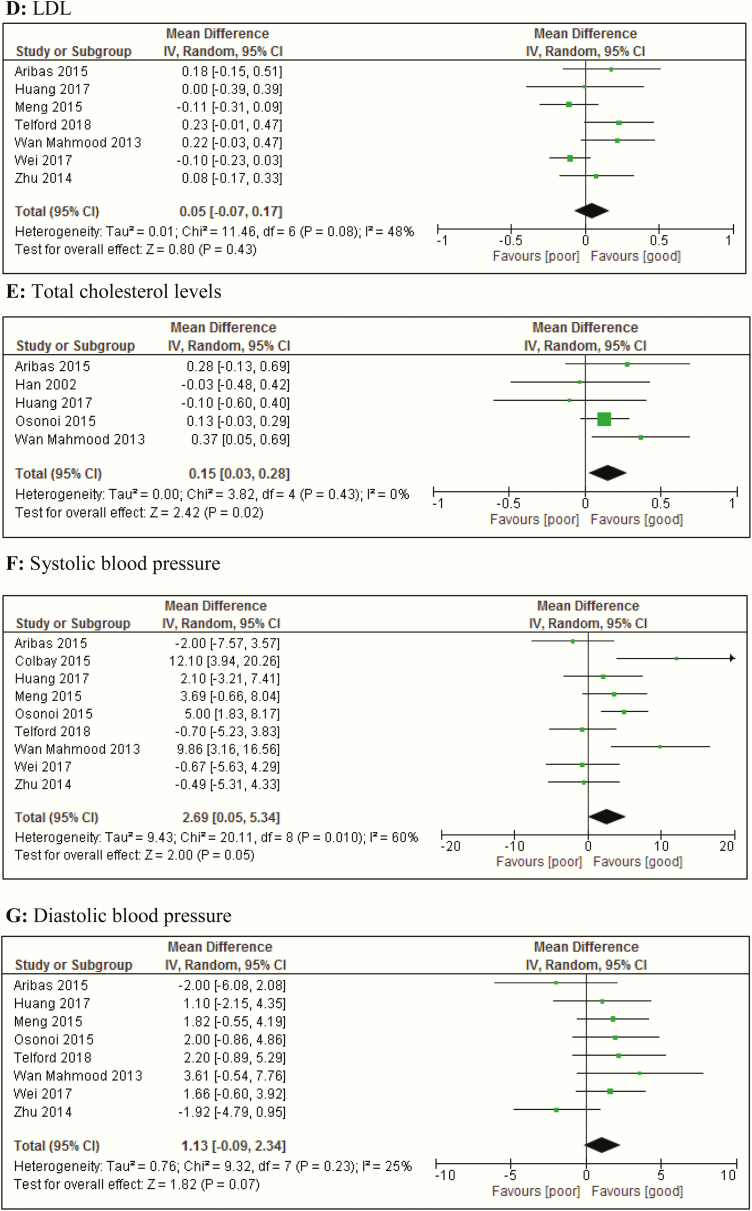
Forest plots of meta-analyses of mean differences and regression analysis of metabolic and glycemic parameters between people with T2D with and without insomnia symptoms. (A) BMI. (B) Triglyceride levels. (C) HDL levels. (D) LDL levels. (E) Total cholesterol levels. (F) Systolic blood pressure. (G) Diastolic blood pressure. Abbreviation: BMI, body mass index; CI, confidence interval; HDL, high-density lipoprotein; IV, inverse variance; LDL, low-density lipoprotein; random, random effects model; T2D, type 2 diabetes.
Studies on waist circumference could not be pooled in a meta-analysis because there were only 2 studies reporting on waist circumference (26, 86).
Insomnia, insomnia symptoms, and lipid levels
The pooled data of 8 studies (26, 48, 55, 72, 74, 77, 99, 102) on triglyceride levels showed that there were no differences in triglyceride levels between the groups (Fig. 4B). Similarly, for levels of HDL (26, 48, 72, 74, 77, 99, 102) and LDL (26, 48, 72, 74, 92, 99, 102), the meta-analysis showed no differences for people with T2D with and without insomnia (symptoms) (Fig. 4C and 4D).
The pooled data of 5 studies (26, 45, 48, 72, 77) on total cholesterol levels showed that people with T2D and insomnia (symptoms) had significantly higher total cholesterol levels compared with people with T2D without insomnia (symptoms) (MD, 0.15 mmol/L; 95% CI, 0.03-0.3; I2 = 0%, P = 0.43) (Fig. 4E).
Insomnia, insomnia symptoms, and blood pressure
The pooled data on blood pressure showed that the systolic (26, 36, 48, 72, 74, 77, 92, 99, 102) blood pressure was 2.69 mm Hg higher (95% CI, 0.1-5.3) in people with T2D and insomnia (symptoms), compared with those without insomnia (symptoms) (Fig. 4F). We observed a nonsignificant difference in diastolic blood pressure (26, 48, 72, 74, 77, 92, 99, 102) of 1.13 mm Hg (95% CI, -0.1 to 2.3) (Fig. 4G), higher in people with T2D and insomnia (symptoms), compared with those without insomnia (symptoms). I2 statistic was 60% (P = 0.01) and 25% (P = 0.23) for systolic and diastolic blood pressure, respectively.
Insomnia, insomnia symptoms, and glycemic control corrected for possible confounders
Of the 12 studies that performed regression analysis adjusted for confounders, 8 could not be included in the meta-analysis because fewer than 3 reported on the same parameter and/or with same type of regression analysis. Only for HbA1c levels could the data of the adjusted association be pooled. A random-effects meta-analysis of 4 studies (90, 94, 96, 102) showed that people with T2D and insomnia (symptoms), according to the PSQI, had a higher, albeit nonsignificant, odds (odds ratio [OR], 1.38; 95% CI, 0.9-2.0) for poor glycemic control, defined as HbA1c levels >7/8.5% (>53/69 mmol/mol) compared to people with T2D and insomnia (symptoms). Heterogeneity between studies was high with I2 statistic of 89% (P < 0.00001) (Fig. 3D). Excluding the study (94) that defined poor glycemic control as HbA1c >8.5% (>69 mmol/mol) and included only people with T2D with poor glycemic control did not affect the OR; 1.38 (95% CI, 0.9-2.1) with I2 statistic of 91%. Excluding the study (96) with a small sample size of only 46 people with T2D decreased the OR to 1.29 (95% CI, 0.9-1.9), with I2 = 92%.
Sensitivity analyses
Table 5 shows that, when excluding studies with low methodological quality, the mean difference between people with diabetes and with or without insomnia (symptoms) remained significant and increased for HbA1c levels, fasting glucose levels, and systolic blood pressure, compared to those with T2D and no insomnia (symptoms). The differences in triglycerides, HDL levels, and diastolic blood pressure were not significant and remained nonsignificant after removing the studies with low quality. The difference in BMI and total cholesterol attenuated to nonsignificant when excluding low methodological quality studies; for LDL levels, the opposite association was observed. However, in general, excluding low-quality studies resulted in a small number of studies left that could be pooled.
Table 5.
Sensitivity Analyses of the Mean Difference Analyses in Metabolic and Glycemic Parameters Between Type 2 Diabetes Patients With and Without Insomnia (Symptoms)
| Metabolic Parameter | Type of Analysis | No. Studies (Left) | MDa | 95% CI | I2 (P Value) |
|---|---|---|---|---|---|
| HbA1c levels (%) | Overall | 14 | 0.23 | 0.1–0.4 | 76% (P < 0.00001) |
| Excluding studies with calculated mean | 12 | 0.12 | -0.1–0.3 | 68% (P = 0.0006) | |
| Excluding studies with weak quality | 5 | 0.49 | 0.2–0.8 | 77% (P = 0.002) | |
| Poor glycemic control (risk ratio) | Overall | 7 | 1.18 | 1.0–1.4 | 73% (P = 0.001) |
| Excluding studies with weak quality | 4 | 1.22 | 1.1–1.4 | 30% (P = 0.23) | |
| Excluding outlier | 6 | 1.22 | 1.1–1.3 | 0% (P = 0.44) | |
| Fasting glucose levels (mmol/L) | Overall | 11 | 0.40 | 0.2–0.7 | 57% (P = 0.01) |
| Excluding studies with calculated mean | 8 | 0.33 | 0–0.7 | 50% (P = 0.05) | |
| Excluding studies with weak quality | 4 | 0.64 | 0.3–1.0 | 50% (P = 0.11) | |
| BMI (kg/m 2) | Overall | 14 | 0.38 | 0.1–0.7 | 47% (P = 0.03) |
| Excluding studies with calculated mean | 12 | 0.34 | 0.1–0.6 | 5% (P = 0.40) | |
| Excluding studies with weak quality | 4 | 0.63 | -0.01–1.3 | 67% (P = 0.01) | |
| Triglycerides (mmol/L) | Overall | 8 | 0.16 | -0.1–0.5 | 91% (P < 0.00001) |
| Excluding studies with calculated mean | 4 | 0.02 | -0.4–0.5 | 68% (P = 0.02) | |
| Excluding studies with weak quality | 4 | 0.33 | -0.04–0.7 | 91% (P < 0.00001) | |
| Excluding outlier | 7 | 0.26 | -0.02–0.5 | 90% (P < 0.00001) | |
| HDL levels (mmol/L) | Overall | 7 | 0.02 | -0.01–0.1 | 0% (P = 0.43) |
| Excluding studies with calculated mean | 6 | 0.02 | -0.01–0.1 | 10% (P = 0.35) | |
| Excluding studies with weak quality | 3 | 0.05 | 0–0.1 | 0% (P = 0.96) | |
| LDL levels (mmol/L) | Overall | 7 | 0.05 | -0.1–0.2 | 48% (P = 0.08) |
| Excluding studies with calculated mean | 6 | 0.09 | -0.03–0.2 | 28% (P = 0.23) | |
| Excluding studies with weak quality | 3 | 0.18 | 0.04–0.3 | 0% (P = 0.64) | |
| Total cholesterol levels (mmol/L) | Overall | 5 | 0.15 | 0.03–0.3 | 0% (P = 0.43) |
| Excluding studies with weak quality | 2 | 0.21 | -0.01–0.4 | 42% (P = 0.19) | |
| Systolic blood pressure (mm Hg) | Overall | 9 | 2.69 | 0.1–5.3 | 60% (P = 0.01) |
| Excluding studies with calculated mean | 8 | 3.14 | 0.3–6.0 | 62% (P = 0.01) | |
| Excluding studies with weak quality | 5 | 4.42 | 0.1–8.7 | 72% (P = 0.006) | |
| Diastolic blood pressure (mm Hg) | Overall | 8 | 1.13 | -0.1–2.3 | 25% (P = 0.23) |
| Excluding studies with calculated mean | 7 | 1.00 | -0.5–2.5 | 34% (P = 0.27) | |
| Excluding studies with weak quality | 4 | 1.28 | -1.1–3.6 | 54% (P = 0.09) |
Abbreviations: BMI, body mass index; CI, confidence interval; HDL, high-density lipoprotein; LDL, low-density lipoprotein; MD, mean difference.
aFor poor glycemic control, it is not the mean difference but the Risk ratio. Boldface type indicates P < 0.05.
Sensitivity analyses performed on studies with a reported mean versus a calculated mean had small effects on the mean differences, for example the mean HbA1c level difference changed from 0.23% (0.1-0.4) to 0.12% (-0.1 to 0.3). The overall level of evidence for the association of insomnia, insomnia symptoms, metabolic parameters, and glycemic control by GRADE was low quality because of the studies being observational, with no reasons to upgrade.
Discussion
This systematic review with meta-analysis aimed to determine the prevalence of insomnia and insomnia symptoms and the association with metabolic parameters and glycemic control in people with T2D. First, we identified 78 studies and our meta-analysis revealed a prevalence of 39% (95% CI, 34-44) of insomnia and insomnia symptoms with a very high degree of heterogeneity, with an I2 of 100%. Second, we showed that in unadjusted studies people with T2D and insomnia (symptoms) had a small, but significantly higher level of fasting glucose, HbA1c, and total cholesterol and a higher BMI and systolic blood pressure, compared to people with T2D without insomnia (symptoms). Overall, these results show a high prevalence of insomnia and insomnia symptoms in the T2D population, which may be associated with deleterious glycemic control. Because of the cross-sectional nature of the papers included in the review (as is standard with GRADE) and the large heterogeneity for the prevalence estimates, the level of evidence by GRADE was downgraded to (very) low, so the findings should be interpreted as (very) uncertain and likely to change after future research.
The first point of discussion is the high degree of heterogeneity of the insomnia and insomnia symptoms prevalence estimate, which could not be accounted for by a range of sensitivity analyses. One of the main sources of the high heterogeneity seems to be the presence of comorbidities, with people with comorbidities having a higher prevalence of insomnia (symptoms) as well as poorer metabolic parameters or poorer glycemic control (3). Although we explored several potential sources of heterogeneity, not all possible sources of heterogeneity could be assessed in this meta-analysis, such as whether people were treated in primary, secondary, or tertiary care, with the latter 2 groups representing those with many more comorbidities. Most of the studies included in the review did not provide information on the true source of people with T2D, which made it impossible to determine if they came from primary, secondary, or tertiary care. Second, information regarding important possible confounding factors such as (diabetes) medication or other sleep problems were not reported and could be a source of heterogeneity. For example, obstructive sleep apnea, a highly prevalent comorbid condition in T2D, has been associated with increased HbA1c levels (104, 105) and is associated with insomnia and insomnia symptoms. Participants could be reporting insomnia (symptoms), which could be due to sleep apnea. Finally, information on certain mediating factors has not been reported in most studies, such as the comorbidities restless leg syndrome, depression, or hypoglycemia (46), which are prevalent comorbidities in people with T2D (106) and can affect sleep as well as glycemic control (107).
A second point of discussion is that the method of insomnia and insomnia symptoms measurement seems to play an important role in the high heterogeneity. Most methods define insomnia and insomnia symptoms in liberal terms, such as the PSQI (108, 109), which solely focus on the presence of nocturnal sleep disturbances (eg, sleep initiation, maintenance difficulties), whereas the more conservative definitions (4, 110) require additional functional impairment (eg, sleep dissatisfaction). In addition, because insomnia (symptoms) is a combination of symptoms, the heterogeneity might be due to differences in presence of certain symptoms (ie, is a patient with high sleep latency but low daytime dysfunction the same as a patient with low sleep latency and high daytime dysfunction), whereas both are defined as having insomnia (symptoms). We chose not to define specific inclusion criteria for the insomnia and/or insomnia symptoms measure, resulting in included studies that used a wide range of measures to assess insomnia and insomnia symptoms. Although in our meta-analysis, most of the measures used liberal terms, this may suggest that our meta-analysis provided a pooled prevalence of 39% with a 95% CI of 34% to 44% for insomnia symptoms rather than insomnia. These estimates are slightly higher compared to the prevalence estimates in the general population (3–5). For future research, insomnia and insomnia symptoms should be operationalized more clearly with less variation in measures of insomnia or a clear definition of insomnia symptoms and insomnia, for example by using one instrument to measure them (ie, the Insomnia Severity Index) (111).
A final point of discussion is that the 4 studies that we could meta-analyze, which adjusted for confounders, showed no significant association among insomnia, insomnia symptoms, and poor glycemic control, although a trend toward an increased risk was visible. However, in addition to real confounders, corrections also included mediators, such as neuropathy, depression, and BMI. Data from strong methodological studies, correcting or stratifying for at least age, sex, and diabetes duration are therefore necessary to draw firm conclusions regarding the association among insomnia, insomnia symptoms, and metabolic parameters and glycemic control. Overall, this study adds to the previous meta-analysis of Lee et al. 2017 (19), whereas we studied the prevalence of insomnia, insomnia symptoms, as well as the association with metabolic parameters.
Possible mechanisms
The mechanisms underlying the association among insomnia, insomnia symptoms, and glycemic control are complex and bidirectional. In other words, T2D can cause sleep disorders, for example, via nightly hypoglycemia, neuropathic pain, and nocturia (112). On the other hand, insomnia and insomnia symptoms can disturb glycemic control via several physiological pathways (113), including decreased brain glucose utilization, which leads to hyperglycemia. Second, this can occur via activation of the hypothalamic-pituitary-adrenal axis, which in turn promotes insulin resistance and hyperglycemia (113). Third, symptoms can manifest via an alteration in appetite-regulating hormones, including ghrelin and leptin (113). Fourth, insomnia symptoms can occur via behavioral changes such as suboptimal self-care activities, with fatigue leading to impaired decision-making (eg, unhealthy food choice, sedentary behaviors) (114) and less medication adherence (115), which in turn may lead to poorer glycemic control. Finally, concomitant sleep disorders to insomnia and insomnia symptoms, such as sleep fragmentation and intermittent hypoxia, might trigger some of the changes in glycemic control. However, further research is required to explore whether improving insomnia and insomnia symptoms improves glycemic control as well as the underlying mechanisms.
Implications
Our results suggest that although insomnia and insomnia symptoms are common in T2D and associated with metabolic parameters and glycemic control, this may offer an opportunity for improvement of T2D treatment. Health care providers should be more aware of the magnitude and impact of insomnia and insomnia symptoms. Efforts toward educating people with T2D about the importance of sleep could be a first strategy to help patients improve their glycemic control. Furthermore, an improvement of insomnia and insomnia symptoms by improvement of sleep hygiene, cognitive behavioral therapy, or certain types of sleep medication will improve quality of life. In addition, improvement of insomnia symptoms could improve glycemic control, although not many studies have been conducted on this topic (116, 117). To assess which treatment is optimal to improve insomnia symptoms and if glycemic control is improved, we need intervention studies in people with T2D.
Strengths and limitations
This is the first systematic review and meta-analysis to quantify and assess, when weighted for risk of bias, the prevalence of insomnia, insomnia symptoms, and associated metabolic parameters and glycemic control (other than HbA1c levels) in adults with T2D. Strengths of this review include the methodological quality assessment of each study, the inclusion of 78 publications, and inclusion of studies from all continents. However, some limitations must be taken into account. First, not all gray literature was captured because unpublished reports and non-English or non-Dutch papers were not included. Second, most studies that investigated metabolic parameters and glycemic control were of low methodological quality because they scored weakly on the domains confounding and data-analysis. Third, there was little consensus regarding the choice of confounders, and lack of adjustment may have overestimated the observed association among insomnia, insomnia symptoms, and metabolic parameters and glycemic control. Fourth, there was a lack of a clear conceptualization of the term insomnia and insomnia symptoms and standardization of the measurement of these constructs, which results in a combined construct, possibly complicating the interpretation of our results. Finally, even after stratifying, the included prevalence studies were characterized by a high heterogeneity, with an I2 of 100%.
In conclusion, the prevalence of symptoms of insomnia and insomnia is 39% (95% CI, 34-44) in the T2D population and may be associated with deleterious glycemic control. Future prospective studies should aim to explain the heterogeneity and account for confounding factors. Overall, these findings point toward insomnia and insomnia symptoms screening in care for adults with T2D, where treatment could help minimize its negative metabolic impact.
Glossary
Abbreviations
- BMI
body mass index
- CI
confidence interval
- HbA1c
hemoglobin A1c
- GRADE
Grading of Recommendations Assessment, Development and Evaluation
- HDL
high-density lipoprotein
- LDL
low-density lipoprotein
- MD
mean difference
- OR
odds ratio
- PSQI
Pittsburgh Sleep Quality Index
- T2D
type 2 diabetes
Acknowledgments
Author Contributions: A.D.M.K. performed the literature search, study selection, data extraction, quality assessment and data synthesis, and drafted the manuscript, tables, and figures. T.D. performed study selection, data extraction, and data synthesis. J.W.B. performed the quality assessment, provided support in design and execution of the review and meta-analyses, and made major revisions to the manuscript. F.P., M.A.B, and A.v.S. provided support in design and execution of the review and meta-analyses, and made major revisions to the manuscript. F.R. performed study selection and quality assessment, made major revisions to the manuscript, is the guarantor of this work, and takes responsibility for the integrity of the work and analyses. This manuscript has not been submitted elsewhere and it is original.
Additional Information
Disclosure Summary: The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Data availability: Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References and Notes
- 1. Roth T. Insomnia: definition, prevalence, etiology, and consequences. J Clin Sleep Med. 2007;3(5 Suppl):S7–S10. [PMC free article] [PubMed] [Google Scholar]
- 2. Walsh JK, Coulouvrat C, Hajak G, et al.. Nighttime insomnia symptoms and perceived health in the America Insomnia Survey (AIS). Sleep. 2011;34(8):997–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6(2):97–111. [DOI] [PubMed] [Google Scholar]
- 4. Buysse DJ. Insomnia. JAMA. 2013;309(7):706–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Roth T, Coulouvrat C, Hajak G, et al. Prevalence and perceived health associated with insomnia based on DSM-IV-TR; International statistical classification of diseases and related health problems, tenth revision; and research diagnostic criteria/international classification of sleep disorders, second edition criteria: results from the America Insomnia Survey. Biol Psychiatry. 2011;69(6):592–600. [DOI] [PubMed] [Google Scholar]
- 6. Knutson KL, Ryden AM, Mander BA, Van Cauter E. Role of sleep duration and quality in the risk and severity of type 2 diabetes mellitus. Arch Intern Med. 2006;166(16):1768–1774. [DOI] [PubMed] [Google Scholar]
- 7. Schmid SM, Hallschmid M, Schultes B. The metabolic burden of sleep loss. Lancet Diabetes Endocrinol. 2015;3(1):52–62. [DOI] [PubMed] [Google Scholar]
- 8. Rahe C, Czira ME, Teismann H, Berger K. Associations between poor sleep quality and different measures of obesity. Sleep Med. 2015;16(10):1225–1228. [DOI] [PubMed] [Google Scholar]
- 9. Park SK, Jung JY, Oh CM, McIntyre RS, Lee JH. Association between sleep duration, quality and body mass index in the Korean population. J Clin Sleep Med. 2018;14(8):1353–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Byberg S, Hansen AL, Christensen DL, et al.. Sleep duration and sleep quality are associated differently with alterations of glucose homeostasis. Diabet Med. 2012;29(9):e354–e360. [DOI] [PubMed] [Google Scholar]
- 11. Meisinger C, Heier M, Loewel H; MONICA/KORA Augsburg Cohort Study Sleep disturbance as a predictor of type 2 diabetes mellitus in men and women from the general population. Diabetologia. 2005;48(2):235–241. [DOI] [PubMed] [Google Scholar]
- 12. Kawakami N, Takatsuka N, Shimizu H. Sleep disturbance and onset of type 2 diabetes. Diabetes Care. 2004;27(1):282–283. [DOI] [PubMed] [Google Scholar]
- 13. Nilsson PM, Rööst M, Engström G, Hedblad B, Berglund G. Incidence of diabetes in middle-aged men is related to sleep disturbances. Diabetes Care. 2004;27(10):2464–2469. [DOI] [PubMed] [Google Scholar]
- 14. Khan MS, Aouad R. The effects of insomnia and sleep loss on cardiovascular disease. Sleep Med Clin. 2017;12(2):167–177. [DOI] [PubMed] [Google Scholar]
- 15. Meng L, Zheng Y, Hui R. The relationship of sleep duration and insomnia to risk of hypertension incidence: a meta-analysis of prospective cohort studies. Hypertens Res. 2013;36(11):985–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2010;33(2):414–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Katic B, Heywood J, Turek F, et al.. New approach for analyzing self-reporting of insomnia symptoms reveals a high rate of comorbid insomnia across a wide spectrum of chronic diseases. Sleep Med. 2015;16(11):1332–1341. [DOI] [PubMed] [Google Scholar]
- 18. Bilge NSY, Guler S, Bilge U.. Comparison of the frequency of sleep disorders among patients with rheumatoid arthritis and type 2 diabetic patients. Konuralp Tip Dergisi. 2016;8(2):100–103. [Google Scholar]
- 19. Lee SWH, Ng KY, Chin WK. The impact of sleep amount and sleep quality on glycemic control in type 2 diabetes: a systematic review and meta-analysis. Sleep Med Rev. 2017;31:91–101. [DOI] [PubMed] [Google Scholar]
- 20. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–9, W64. [DOI] [PubMed] [Google Scholar]
- 21. Stroup DF, Berlin JA, Morton SC, et al.. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. [DOI] [PubMed] [Google Scholar]
- 22. Thomas BH, Ciliska D, Dobbins M, Micucci S. A process for systematically reviewing the literature: providing the research evidence for public health nursing interventions. Worldviews Evid Based Nurs. 2004;1(3):176–184. [DOI] [PubMed] [Google Scholar]
- 23. Mackenbach JD, Rutter H, Compernolle S, et al.. Obesogenic environments: a systematic review of the association between the physical environment and adult weight status, the SPOTLIGHT project. BMC Public Health. 2014;14:233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Abdelgadir M, Shebeika W, Eltom M, Berne C, Wikblad K. Health related quality of life and sense of coherence in Sudanese diabetic subjects with lower limb amputation. Tohoku J Exp Med. 2009;217(1):45–50. [DOI] [PubMed] [Google Scholar]
- 25. Al-Tannir M, Kobrosly SY, Al-Badr AH, Salloum NA, Altannir YM. Characterizing sleeping habits and disturbances among Saudi adults. Saudi Med J. 2016;37(12):1372–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Aribas A, Kayrak M, Tekinalp M, et al.. The relationship between serum asymmetric dimethylarginine levels and subjective sleep quality in normotensive patients with type 2 diabetes mellitus. Korean J Intern Med. 2015;30(3):316–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bani-Issa W, Al-Shujairi AM, Patrick L. Association between quality of sleep and health-related quality of life in persons with diabetes mellitus type 2. J Clin Nurs. 2018;27(7-8):1653–1661. [DOI] [PubMed] [Google Scholar]
- 28. Bedi U, Mittal G, Arora R. Daytime sleepiness and quality of sleep in Punjabi diabetic population. J Clin Diagn Res. 2011;5(5):1051–1055. [Google Scholar]
- 29. Bener A, Al-Hamaq AOAA. Sleep quality and excessive daytime sleepiness in a arab diabetic population. Biomed Res. 2010;21(4):333–340. [Google Scholar]
- 30. Bhaskar S, Hemavathy D, Prasad S. Prevalence of chronic insomnia in adult patients and its correlation with medical comorbidities. J Family Med Prim Care. 2016;5(4):780–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Budhiraja R, Roth T, Hudgel DW, Budhiraja P, Drake CL. Prevalence and polysomnographic correlates of insomnia comorbid with medical disorders. Sleep. 2011;34(7):859–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Celik G, Annagür BB, Yılmaz M, Kara F. Findings of multidimensional instruments for determining psychopathology in diabetic and non-diabetic hemodialysis patients. Int J Clin Exp Med. 2012;5(4):346–354. [PMC free article] [PubMed] [Google Scholar]
- 33. Chang CJ, Pei D, Wu CC, Palmer MH, Su CC, Kuo SF, Liao YM. Correlates of nocturia and relationships of nocturia with sleep quality and glycemic control in women with type 2 diabetes. J Nurs Scholarsh. 2017;49(4):400–410. [DOI] [PubMed] [Google Scholar]
- 34. Cheng HP, Chen CH, Lin MH, et al. Gender differences in the relationship between walking activity and sleep disturbance among community-dwelling older adult with diabetes in Taiwan. J Women Aging. 2019;31(2):108–116. [DOI] [PubMed] [Google Scholar]
- 35. Cho EH, Lee H, Ryu OH, Choi MG, Kim SW. Sleep disturbances and glucoregulation in patients with type 2 diabetes. J Korean Med Sci. 2014;29(2):243–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Colbay G, Cetin M, Colbay M, Berker D, Guler S. Type 2 diabetes affects sleep quality by disrupting the respiratory function. J Diabetes. 2015;7(5):664–671. [DOI] [PubMed] [Google Scholar]
- 37. Cuellar NG, Ratcliffe SJ. A comparison of glycemic control, sleep, fatigue, and depression in type 2 diabetes with and without restless legs syndrome. J Clin Sleep Med. 2008;4(1):50–56. [PMC free article] [PubMed] [Google Scholar]
- 38. Cunha MC, Zanetti ML, Hass VJ. Sleep quality in type 2 diabetics. Rev Lat Am Enfermagem. 2008;16(5):850–855. [DOI] [PubMed] [Google Scholar]
- 39. El-Aghoury AA, Elsherbiny TM, Lewis N, Salem MT, Osman N. Characterization of abnormal sleep patterns in patients with obesity, type 2 diabetes, or combined. Alexandria J Med. 20174;455–462. [Google Scholar]
- 40. Ford ES, Cunningham TJ, Giles WH, Croft JB. Trends in insomnia and excessive daytime sleepiness among U.S. adults from 2002 to 2012. Sleep Med. 2015;16(3):372–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fritschi C, Bronas UG, Park CG, Collins EG, Quinn L. Early declines in physical function among aging adults with type 2 diabetes. J Diabetes Complications. 2017;31(2):347–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fukui M, Tanaka M, Toda H, et al.. Andropausal symptoms in men with type 2 diabetes. Diabet Med. 2012;29(8):1036–1042. [DOI] [PubMed] [Google Scholar]
- 43. Gozashti MH, Eslami N, Radfar MH, Pakmanesh H. Sleep pattern, duration and quality in relation with glycemic control in people with type 2 diabetes mellitus. Iran J Med Sci. 2016;41(6):531–538. [PMC free article] [PubMed] [Google Scholar]
- 44. Grandner MA, Patel NP, Perlis ML, et al.. Obesity, diabetes, and exercise associated with sleep-related complaints in the American population. Z Gesundh Wiss. 2011;19(5):463–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Han SY, Yoon JW, Jo SK, et al. Insomnia in diabetic hemodialysis patients. Prevalence and risk factors by a multicenter study. Nephron. 2002;92(1):127–132. [DOI] [PubMed] [Google Scholar]
- 46. Hayashino Y, Tsujii S, Ishii H; Diabetes Distress and Care Registry at Tenri Study Group High frequency of non-nocturnal hypoglycemia was associated with poor sleep quality measure by Pittsburg sleep quality index in patients with diabetes receiving insulin therapy: diabetes distress and care registry at Tenri (DDCRT 4). Exp Clin Endocrinol Diabetes. 2013;121(10):628–634. [DOI] [PubMed] [Google Scholar]
- 47. Hood MM, Reutrakul S, Crowley SJ. Night eating in patients with type 2 diabetes. Associations with glycemic control, eating patterns, sleep, and mood. Appetite. 2014;79:91–96. [DOI] [PubMed] [Google Scholar]
- 48. Huang Y, Wang H, Li Y, Tao X, Sun J. Poor sleep quality is associated with Dawn phenomenon and impaired circadian clock gene expression in subjects with type 2 diabetes mellitus. Int J Endocrinol. 2017;2017:4578973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hung HC, Yang YC, Ou HY, Wu JS, Lu FH, Chang CJ. The relationship between impaired fasting glucose and self-reported sleep quality in a Chinese population. Clin Endocrinol (Oxf). 2013;78(4):518–524. [DOI] [PubMed] [Google Scholar]
- 50. Hyyppä MT, Kronholm E. Quality of sleep and chronic illnesses. J Clin Epidemiol. 1989;42(7):633–638. [DOI] [PubMed] [Google Scholar]
- 51. Jain SK, Kahlon G, Morehead L, Lieblong B, Stapleton T, Hoeldtke R, Bass PF 3rd, Levine SN. The effect of sleep apnea and insomnia on blood levels of leptin, insulin resistance, IP-10, and hydrogen sulfide in type 2 diabetic patients. Metab Syndr Relat Disord. 2012;10(5):331–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Johnson ST, Thiel D, Al Sayah F, et al.. Objectively measured sleep and health-related quality of life in older adults with type 2 diabetes: a cross-sectional study from the Alberta’s Caring for Diabetes Study. Sleep Health. 2017;3(2):102–106. [DOI] [PubMed] [Google Scholar]
- 53. Kara B, Kılıç Ö. Predictors of poor sleep quality and excessive daytime sleepiness in Turkish adults with type 2 diabetes. J Clin Nurs. 2015;24(9-10):1436–1439. [DOI] [PubMed] [Google Scholar]
- 54. Kasenova AS, Eszhanova LE, Tursbekova DD, Durmanova AK. The influence of sleep disorders on cognitive functions of a brain at patients with type 2 diabetes. Drug Invent Today. 2017;9(3):26–29. [Google Scholar]
- 55. Keskin A, Ünalacak M, Bilge U, et al.. Effects of sleep disorders on hemoglobin A1c levels in type 2 diabetic patients. Chin Med J (Engl). 2015;128(24):3292–3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Keskin A, Bilge U, Unalacak M, et al. Effects of sleep disorders and 25-OH vitamin D levels on HbA1c levels in geriatric type 2 diabetic patients. Biomed Res. 2016;27(2):537–542. [Google Scholar]
- 57. Khosravan S, Alami A, Golchin Rahni S. Effects of continuous care model based non-pharmacological intervention on sleep quality in patients with type 2 diabetes mellitus: a randomized controlled clinical trial. Int J Community Based Nurs Midwifery. 2015;3(2):96–104. [PMC free article] [PubMed] [Google Scholar]
- 58. Knutson KL, Van Cauter E, Zee P, Liu K, Lauderdale DS. Cross-sectional associations between measures of sleep and markers of glucose metabolism among subjects with and without diabetes: the Coronary Artery Risk Development in Young Adults (CARDIA) Sleep Study. Diabetes Care. 2011;34(5):1171–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta-analyses. Bmj. 2011;342:d549. [DOI] [PubMed] [Google Scholar]
- 60. Cuijpers P. Meta-analyses in mental health research. A practical guide. 2016. Available at: http://bit.do/meta-analysis
- 61. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sun N, Lou P, Shang Y, et al.. Prevalence and determinants of depressive and anxiety symptoms in adults with type 2 diabetes in China: a cross-sectional study. BMJ Open. 2016;6(8):e012540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zhang P, Lou P, Chang G, et al.. Combined effects of sleep quality and depression on quality of life in patients with type 2 diabetes. BMC Fam Pract. 2016;17:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zhao J, Li XL, Han K, Tao ZQ, Wu ZM. Biological interaction between sleep quality and depression in type 2 diabetes. Eur Rev Med Pharmacol Sci. 2016;20(14):3087–3091. [PubMed] [Google Scholar]
- 65. Koyanagi A, Garin N, Olaya B, et al.. Chronic conditions and sleep problems among adults aged 50 years or over in nine countries: a multi-country study. Plos One. 2014;9(12):e114742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lecube A, Sanchez E, Gomez-Peralta F, et al. Daytime sleepiness and quality of sleep in type 2 diabetes mellitus: the role of related questionnaires to evaluate its negative effect on sleep breathing. Diabetes. 2015;64(SUPPL. 1):A129. [Google Scholar]
- 67. Lopes LA, Lins Cde M, Adeodato VG, et al.. Restless legs syndrome and quality of sleep in type 2 diabetes. Diabetes Care. 2005;28(11):2633–2636. [DOI] [PubMed] [Google Scholar]
- 68. Lou P, et al. Relation of sleep quality and sleep duration to type 2 diabetes: a population-based cross-sectional survey. BMJ Open, 2012;2(4):e000956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lou P, Qin Y, Zhang P, et al.. Association of sleep quality and quality of life in type 2 diabetes mellitus: a cross-sectional study in China. Diabetes Res Clin Pract. 2015;107(1):69–76. [DOI] [PubMed] [Google Scholar]
- 70. Luyster FS, Dunbar-Jacob J. Sleep quality and quality of life in adults with type 2 diabetes. Diabetes Educ. 2011;37(3):347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Manodpitipong A, Saetung S, Nimitphong H, et al.. Night-shift work is associated with poorer glycaemic control in patients with type 2 diabetes. J Sleep Res. 2017;26(6):764–772. [DOI] [PubMed] [Google Scholar]
- 72. Wan Mahmood WA, Draman Yusoff MS, Behan LA, et al.. Association between sleep disruption and levels of lipids in Caucasians with type 2 diabetes. Int J Endocrinol. 2013;2013:341506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Medeiros C, Bruin V, Férrer D, et al.. Excessive daytime sleepiness in type 2 diabetes. Arq Bras Endocrinol Metabol. 2013;57(6):425–430. [DOI] [PubMed] [Google Scholar]
- 74. Meng LL, Tang YZ, Ni CL, et al.. Impact of inflammatory markers on the relationship between sleep quality and incident cardiovascular events in type 2 diabetes. J Diabetes Complications. 2015;29(7):882–886. [DOI] [PubMed] [Google Scholar]
- 75. Narisawa H, Komada Y, Miwa T, et al.. Prevalence, symptomatic features, and factors associated with sleep disturbance/insomnia in Japanese patients with type-2 diabetes. Neuropsychiatr Dis Treat. 2017;13:1873–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Nefs G, Donga E, van Someren E, Bot M, Speight J, Pouwer F. Subjective sleep impairment in adults with type 1 or type 2 diabetes: results from diabetes MILES–The Netherlands. Diabetes Res Clin Pract. 2015;109(3):466–475. [DOI] [PubMed] [Google Scholar]
- 77. Osonoi Y, Mita T, Osonoi T, et al.. Poor sleep quality is associated with increased arterial stiffness in Japanese patients with type 2 diabetes mellitus. BMC Endocr Disord. 2015;15:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Rajendran A, Parthsarathy S, Tamilselvan B, Seshadri KG, Shuaib M. Prevalence and correlates of disordered sleep in southeast asian indians with type 2 diabetes. Diabetes Metab J. 2012;36(1):70–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ramos AR, Wallace DM, Pandi-Perumal SR, et al.. Associations between sleep disturbances and diabetes mellitus among blacks with metabolic syndrome: results from the Metabolic Syndrome Outcome Study (MetSO). Ann Med. 2015;47(3):233–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ramtahal R, Khan C, Maharaj-Khan K, et al.. Prevalence of self-reported sleep duration and sleep habits in type 2 diabetes patients in South Trinidad. J Epidemiol Glob Health. 2015;5(4 Suppl 1):S35–S43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Sakamoto R, Yamakawa T, Takahashi K, et al.. Association of usual sleep quality and glycemic control in type 2 diabetes in Japanese: a cross sectional study. Sleep and Food Registry in Kanagawa (SOREKA). Plos One. 2018;13(1):e0191771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Seligowski AV, Pless Kaiser AP, Niles BL, Mori DL, King LA, King DW. Sleep quality as a potential mediator between psychological distress and diabetes quality of life in veterans with type 2 diabetes. J Clin Psychol. 2013;69(10):1121–1131. [DOI] [PubMed] [Google Scholar]
- 83. Shamshirgaran SM, Ataei J, Malek A, Iranparvar-Alamdari M, Aminisani N. Quality of sleep and its determinants among people with type 2 diabetes mellitus in Northwest of Iran. World J Diabetes. 2017;8(7):358–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Shim U, Lee H, Oh JY, Sung YA. Sleep disorder and cardiovascular risk factors among patients with type 2 diabetes mellitus. Korean J Intern Med. 2011;26(3):277–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Skomro RP, Ludwig S, Salamon E, Kryger MH. Sleep complaints and restless legs syndrome in adult type 2 diabetics. Sleep Med. 2001;2(5):417–422. [DOI] [PubMed] [Google Scholar]
- 86. Sokwalla SM, Joshi MD, Amayo EO, Acharya K, Mecha JO, Mutai KK. Quality of sleep and risk for obstructive sleep apnoea in ambulant individuals with type 2 diabetes mellitus at a tertiary referral hospital in Kenya: a cross-sectional, comparative study. BMC Endocr Disord. 2017;17(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Song Y, Ye X, Ye L, Li B, Wang L, Hua Y. Disturbed subjective sleep in Chinese females with type 2 diabetes on insulin therapy. Plos One. 2013;8(1):e54951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Sridhar GR, Madhu K. Prevalence of sleep disturbances in diabetes mellitus. Diabetes Res Clin Pract. 1994;23(3):183–186. [DOI] [PubMed] [Google Scholar]
- 89. Sudore RL, Karter AJ, Huang ES, et al.. Symptom burden of adults with type 2 diabetes across the disease course: diabetes & aging study. J Gen Intern Med. 2012;27(12):1674–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Tang Y, Meng L, Li D, et al.. Interaction of sleep quality and sleep duration on glycemic control in patients with type 2 diabetes mellitus. Chin Med J (Engl). 2014;127(20):3543–3547. [PubMed] [Google Scholar]
- 91. Taheri Tanjani P, Moradinazar M, Esmail Mottlagh M, Najafi F. The prevalence of diabetes mellitus (DM) type II among Iranian elderly population and its association with other age-related diseases, 2012. Arch Gerontol Geriatr. 2015;60(3):373–379. [DOI] [PubMed] [Google Scholar]
- 92. Telford O, Diamantidis CJ, Bosworth HB, et al. The relationship between Pittsburgh sleep quality index subscales and diabetes control. Chronic Illn. 2019;15(3):210–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Thongsai S, Watanabenjasopa S, Youjaiyen M. Depression in patients with type II diabetes: case study at diabetic outpatient clinic, in Samut Prakan. Glob J Health Sci. 2013;6(1):127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Torrella M, Castells I, Gimenez-Perez G, et al.. Intermittent hypoxia is an independent marker of poorer glycaemic control in patients with uncontrolled type 2 diabetes. Diabetes Metab. 2015;41(4):312–318. [DOI] [PubMed] [Google Scholar]
- 95. Trento M, Broglio F, Riganti F, et al.. Sleep abnormalities in type 2 diabetes may be associated with glycemic control. Acta Diabetol. 2008;45(4):225–229. [DOI] [PubMed] [Google Scholar]
- 96. Tsai YW, Kann NH, Tung TH, et al.. Impact of subjective sleep quality on glycemic control in type 2 diabetes mellitus. Fam Pract. 2012;29(1):30–35. [DOI] [PubMed] [Google Scholar]
- 97. Tsujimura T, Matsuo Y, Keyaki T, Sakurada K, Imanishi J. Correlations of sleep disturbance with the immune system in type 2 diabetes mellitus. Diabetes Res Clin Pract. 2009;85(3):286–292. [DOI] [PubMed] [Google Scholar]
- 98. Vernon MK, Brandenburg NA, Alvir JM, Griesing T, Revicki DA. Reliability, validity, and responsiveness of the daily sleep interference scale among diabetic peripheral neuropathy and postherpetic neuralgia patients. J Pain Symptom Manage. 2008;36(1):54–68. [DOI] [PubMed] [Google Scholar]
- 99. Wei H, Qu H, Wang H, Ji B, Deng H. Serum brain-derived neurotrophic factor levels and sleep disorders in Chinese healthy and newly diagnosed type 2 diabetic subjects. J Diabetes. 2017;9(2):180–189. [DOI] [PubMed] [Google Scholar]
- 100. Yagi A, Nishio Y, Ugi S, et al. The role of sleep disturbance and depression in patients with type 2 diabetes. Sleep and Biological Rhythms, 2011;9(4):320. [Google Scholar]
- 101. Zelman DC, Brandenburg NA, Gore M. Sleep impairment in patients with painful diabetic peripheral neuropathy. Clin J Pain. 2006;22(8):681–685. [DOI] [PubMed] [Google Scholar]
- 102. Zhu BQ, Li X-M, Wang D, Yu X-F. Sleep quality and its impact on glycaemic control in patients with type 2 diabetes mellitus. Int J Nurs Sci. 2014;1(3):260–265. [Google Scholar]
- 103. Zhu B, Quinn L, Fritschi C. Relationship and variation of diabetes related symptoms, sleep disturbance and sleep-related impairment in adults with type 2 diabetes. J Adv Nurs. 2018;74(3):689–697. [DOI] [PubMed] [Google Scholar]
- 104. Aronsohn RS, Whitmore H, Van Cauter E, Tasali E. Impact of untreated obstructive sleep apnea on glucose control in type 2 diabetes. Am J Respir Crit Care Med. 2010;181(5):507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Kent BD, Grote L, Ryan S, et al.. Diabetes mellitus prevalence and control in sleep-disordered breathing: the European Sleep Apnea Cohort (ESADA) study. Chest. 2014;146(4):982–990. [DOI] [PubMed] [Google Scholar]
- 106. Talbot F, Nouwen A. A review of the relationship between depression and diabetes in adults: is there a link? Diabetes Care. 2000;23(10):1556–1562. [DOI] [PubMed] [Google Scholar]
- 107. Pouwer F, Snoek FJ. Association between symptoms of depression and glycaemic control may be unstable across gender. Diabet Med. 2001;18(7):595–598. [DOI] [PubMed] [Google Scholar]
- 108. Klink ME, Quan SF, Kaltenborn WT, Lebowitz MD. Risk factors associated with complaints of insomnia in a general adult population. Influence of previous complaints of insomnia. Arch Intern Med. 1992;152(8):1634–1637. [PubMed] [Google Scholar]
- 109. Quera-Salva MA, Orluc A, Goldenberg F, Guilleminault C. Insomnia and use of hypnotics: study of a French population. Sleep. 1991;14(5):386–391. [DOI] [PubMed] [Google Scholar]
- 110. Ohayon MM, Caulet M, Guilleminault C. How a general population perceives its sleep and how this relates to the complaint of insomnia. Sleep. 1997;20(9):715–723. [DOI] [PubMed] [Google Scholar]
- 111. Morin CM, Belleville G, Bélanger L, Ivers H. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34(5): 601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Lamond N, Tiggemann M, Dawson D. Factors predicting sleep disruption in Type II diabetes. Sleep. 2000;23(3):415–416. [PubMed] [Google Scholar]
- 113. Reutrakul S, Van Cauter E. Interactions between sleep, circadian function, and glucose metabolism: implications for risk and severity of diabetes. Ann N Y Acad Sci. 2014;1311:151–173. [DOI] [PubMed] [Google Scholar]
- 114. Larcher S, Benhamou PY, Pépin JL, Borel AL. Sleep habits and diabetes. Diabetes Metab. 2015;41(4):263–271. [DOI] [PubMed] [Google Scholar]
- 115. Chasens ER, Korytkowski M, Sereika SM, Burke LE. Effect of poor sleep quality and excessive daytime sleepiness on factors associated with diabetes self-management. Diabetes Educ. 2013;39(1):74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Tannas CL. Type 2 diabetes and insomnia: impact on metabolic control. Department of Nursing: Wayne State University Dissertations, 2012. [Google Scholar]
- 117. Toi N, Inaba M, Kurajoh M, et al.. Improvement of glycemic control by treatment for insomnia with suvorexant in type 2 diabetes mellitus. J Clin Transl Endocrinol. 2019;15:37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]