Abstract
Ozone causes airway hyperresponsiveness, a defining feature of asthma. We have reported that the gut microbiome contributes to sex differences in ozone-induced airway hyperresponsiveness. Altering dietary fiber affects the gut microbiome. The purpose of this study was to determine the effects of dietary fiber on pulmonary responses to ozone and whether these effects differ by sex. We fed male and female mice fiber-free diets or diets enriched in one of two types of dietary fiber, cellulose and pectin, for 3 days before ozone exposure. Compared with control diets or pectin-enriched diets, cellulose-enriched diets attenuated ozone-induced airway hyperresponsiveness in male but not female mice. In contrast, fiber-free diets augmented responses to ozone in female but not male mice. Analysis of 16S rRNA sequencing of fecal DNA also indicated sex differences in the impact of dietary fiber on the gut microbiome and identified bacterial taxa that were associated with ozone-induced airway hyperresponsiveness. Our data suggest that microbiome-based therapies such as prebiotics may provide an alternative therapeutic strategy for air pollution–triggered asthma, but they indicate that such therapeutics may need to be tailored differently for males and females.
Keywords: airway responsiveness, microbiome, neutrophil, 16S rRNA sequencing
Clinical Relevance
Our data confirm that the gut microbiome impacts pulmonary responses to the air pollutant ozone. The data also suggest that microbiome-based therapies such as prebiotics may provide a therapeutic strategy for air pollution–triggered asthma, but they indicate that such therapeutics may need to be tailored differently for males and females.
Exposure to ozone (O3), a common air pollutant, is associated with respiratory problems, including asthma (1). O3 causes asthma symptoms, reduction in lung function, and airway hyperresponsiveness (AHR), a defining feature of asthma (2, 3). Hospitalizations and emergency room visits related to asthma are also increased after days of high ambient O3 concentrations (4). Greater understanding of the mechanistic basis for air pollution–triggered asthma is important for the development of effective treatments (1, 5).
There is increasing evidence for a role of the gut microbiome in human disease, including asthma (6). For example, in children, the relative abundance of several bacterial taxa in the gut is associated with development of asthma-related symptoms and risk of asthma (7, 8). Studies using antibiotics to deplete the microbiome and studies using germ-free mice also indicate a role for the microbiome in mouse models of allergic asthma (9–11). We have reported that the gut microbiome also contributes to pulmonary responses to O3, including O3-induced AHR (12–14).
Sex differences in the community structure of the gut microbiome exist, and they have important functional consequences (12, 15, 16). For example, in a mouse model of type 1 diabetes, the incidence of diabetic symptoms is much greater in female than in male mice (15). This sex difference is abolished under germ-free conditions. Moreover, transfer of male fecal material into female mice protects the females from development of diabetic symptoms. We also observed sex differences in the role of the microbiome in O3-induced AHR (12). The magnitude of O3-induced AHR is greater in male than in female mice (12, 17, 18). Treatment with a cocktail of antibiotics attenuates O3-induced AHR in male mice but augments O3-induced AHR in female mice, ablating the usual sex difference (12). These data suggest that the gut microbiome could be a therapeutic target for regulating O3-induced pulmonary responses, but they also suggest that the outcome of such therapeutic strategies may depend on sex.
Altering dietary fiber is a well-established means of manipulating the gut microbiome (19–23). Importantly, others have reported that manipulating dietary fiber may have beneficial effects for asthma. In mouse models of allergic airway disease, fiber-enriched diets attenuate, whereas fiber-reduced diets worsen, allergic airway responses in females (19, 22). Similarly, a single soluble fiber meal attenuates sputum markers of inflammation and improves lung function in patients with asthma (24). Sex differences were not explored in any of these studies, nor was the response to nonatopic asthma triggers evaluated.
To determine the impact of dietary fiber on pulmonary responses to O3, we fed mice either fiber-free diets or diets enriched in one of two types of dietary fiber: cellulose and pectin. To determine whether there were sex differences in the response to fiber, we examined both male and female mice. Our results indicate sex differences in the impact of dietary fiber on pulmonary responses to O3.
Methods
Animals
All protocols were approved by the Harvard Medical Area Standing Committee on Animals. To examine sex differences in the impact of dietary fiber on pulmonary responses to O3, 8-week-old female and male C57BL/6 mice were purchased from Taconic Biosciences and housed under specific-pathogen–free conditions with a 12-h/12-h light/dark cycle in the Harvard T.H. Chan School of Public Health vivarium.
Protocol
Three experimental protocols were performed. In each, C57BL/6 male and female mice were fed a control diet from Research Diets, Inc. (Table E1 in the data supplement) for 7 days after arriving from Taconic Biosciences. In the first protocol, mice either remained on the control diet or were switched to a cellulose- or a pectin-enriched diet for 3 days. On the fourth day, fecal pellets were harvested for 16S rRNA sequencing, and the mice were then exposed to room air or to O3 (2 ppm for 3 h). Twenty-four hours after cessation of O3, mice were anesthetized for the measurement of airway responsiveness to inhaled aerosolized methacholine, after which the mice were killed with an overdose of sodium pentobarbital. Blood was harvested by cardiac puncture, and BAL was performed. Effects of pectin versus cellulose on O3-induced AHR and neutrophil recruitment in the male mice were previously reported (13) but are included here for comparison with the female mice that were studied within the same time frame. In the second protocol, C57BL/6 male and female mice either remained on the control diet or were fed a fiber-free diet for 3 days. On the fourth day, mice were exposed to room air or to O3 and evaluated as described above. In the case of mice fed special diets, the experimental food was continued in the period between cessation of exposure and subsequent evaluation of the mice. In the third protocol, female mice were fed a fiber-free diet for 3 days as described above. In half of the mice, sodium propionate (200 mM) was added to the drinking water during the fiber-free diet feeding period (12, 13). In the other half, the mice received regular drinking water. Additional methodologic details are provided in the data supplement.
Statistical Analysis
Except for microbial community analysis (see data supplement), the significance of differences between groups was assessed using ANOVA or factorial analysis of variance combined with Fisher’s least significant difference post hoc analysis (Statistica Software) using sex, exposure, and diet as main effects. A P value less than 0.05 (two tailed) was considered significant. All values are expressed as mean ± SEM.
Results
Sex Differences in the Effect of Dietary Fiber on Pulmonary Responses to O3
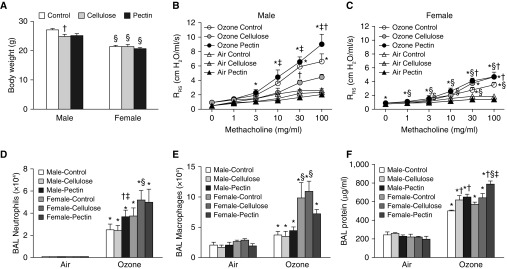
To evaluate the role of increasing dietary fiber on pulmonary responses to O3, we fed male and female mice control diets, cellulose-enriched diets, or pectin-enriched diets for 3 days before exposure. These diets were continued in the 24-hour period after exposure but before evaluation. Body weight measured just before exposure was not different among the three dietary groups in either males or females (Figure 1A), perhaps because of the short (3-d) period of feeding. In air-exposed mice, airway responsiveness was not different across diet groups in either males or females (Figures 1B and 1C). Compared with air, acute O3 exposure increased airway responsiveness in both male and female mice that were fed control diets (Figures 1B and 1C), though the magnitude of the increase was greater in the males, as we and others have reported (12, 17, 18). In male mice, O3-induced AHR was not different between mice fed control or pectin-enriched diets, except at the highest dose of methacholine, the response to which was significantly greater in the pectin-fed mice than in the control mice (Figure 1B). In contrast, O3-induced AHR was significantly lower in male mice fed cellulose-enriched diets than in those fed control diets or pectin-enriched diets (Figure 1B). In female mice, airway responsiveness was not different in O3-exposed mice fed pectin- versus cellulose-enriched diets, but in both groups, airway responsiveness was greater than in mice fed control diets (Figure 1C). The net effect of these changes was such that in mice fed cellulose-enriched diets, there was no longer any sex difference in the magnitude of O3-induced AHR. Thus, increasing dietary fiber affects O3-induced AHR in male and female mice, with the outcome depending on the nature of the fiber.
Figure 1.
Effect of fiber-enriched diets on pulmonary responses to O3. Male and female mice were fed either control, cellulose-enriched, or pectin-enriched diets for 3 days. Mice were then exposed to room air or to O3 (2 ppm for 3 h) and evaluated 24 hours later. Shown are (A) body weight measured before exposure, (B) airway responsiveness of male mice exposed to air or O3, (C) airway responsiveness of female mice exposed to air or O3, (D) BAL neutrophils, (E) BAL macrophages, and (F) BAL protein. Note that for airway responsiveness, the air-exposed cellulose–treated mice virtually overlap with the air-exposed pectin–treated mice. Results for ozone-exposed mice are mean ± SE of data from eight mice per group and were gathered over 7 and 4 experimental days, respectively, in male and female mice. On each day, control, pectin-fed, and cellulose-fed mice were studied. Results for air-exposed mice are mean ± SE of data from six mice per group and were gathered over 4 and 3 experimental days for male and female mice, respectively. *P < 0.05 compared with air, †P < 0.05 compared with control diet–fed mice, ‡P < 0.05 compared with cellulose-enriched diet–fed mice, and §P < 0.05 compared with male mice. Evaluation of the effects of diet and ozone was done within each sex. Evaluation of sex differences was performed by combining data from both sexes. Airway responsiveness and BAL neutrophil data for the male mice were previously reported (13) and are shown here for comparison with the female mice, which were studied within the same time frame. RRS = respiratory system resistance.
Compared with air, exposure to O3 increased BAL neutrophils (Figure 1D), macrophages (Figure 1E), and BAL protein (Figure 1F), a marker of O3-induced lung epithelial injury (25). In males but not females, BAL neutrophils were greater in mice fed the pectin diet than in either control diet– or cellulose diet–fed mice (Figure 1D). Dietary fiber did not affect BAL macrophages in either male or female mice (Figures 1D and 1E), though within certain diet groups, there were differences in both BAL neutrophils and macrophages between the males and the females. In both males and females, BAL protein was significantly higher in mice fed the pectin-enriched diet than in those fed the control diet. BAL protein was also higher in male but not female mice fed cellulose-enriched versus control diets (Figure 1F). Thus, effects of dietary fiber on O3-induced AHR and injury were dissociated: After O3 exposure, cellulose-fed male mice had greater BAL protein than control animals despite lower AHR, and cellulose diet–fed female mice had greater AHR than control animals despite no change in BAL protein. The data suggest that fiber-related changes in O3-induced lung injury are unlikely to account for fiber-related differences in O3-induced AHR.
We considered the possibility that changes in sex hormones might account for the impact of fiber-enriched diets on pulmonary responses to O3. Others have reported that fiber-enriched diets alter the gut microbiome (22, 23), and the gut microbiome has the capacity to alter sex hormones (15, 26–28). Importantly, both male and female sex hormones also impact responses to O3 (29, 30). Furthermore, pulmonary sensory afferents are stimulated by O3 and relay to the hypothalamus, resulting in reduction in anterior pituitary release of luteinizing hormone (LH), which leads to reduction in sex steroids (31). We observed both O3- and fiber-related changes in male and female sex hormones (Figure E1). However, these changes do not appear to account for effects of dietary fiber on responses to O3. For example, there were reductions in serum testosterone in O3- versus air-exposed male mice (Figure E1). However, serum testosterone was significantly lower in pectin diet–fed than in either control diet– or cellulose diet–fed mice exposed to O3 (Figure E1). Because androgens augment rather than attenuate responses to O3, the data do not support the hypothesis that fiber-related changes in testosterone (Figure E1) account for fiber-related differences in O3-induced AHR observed in male mice (Figure 1B). We also considered the possibility that changes in sex hormones might account for the impact of fiber-enriched diets on pulmonary responses to O3 in female mice. O3-induced AHR and O3-induced neutrophil release are greater when mice are in the follicular phase of the estrous cycle, when LH and estradiol are high, than in the luteal phase of the estrous cycle, when progesterone is high (30). As in the male mice, there were both O3- and fiber-related effects on serum LH and progesterone (Figure E1). LH was greater in pectin diet– than in cellulose diet–fed mice exposed to O3 (Figure E1), but there was no difference in airway responsiveness between these two groups of mice (Figure 1C). Thus, our data do not support the hypothesis that changes in sex hormones account for the effects of fiber-enriched diets. Note that we did not examine serum estradiol, because we have previously reported that O3 exposure reduces estradiol to undetectable concentrations (12).
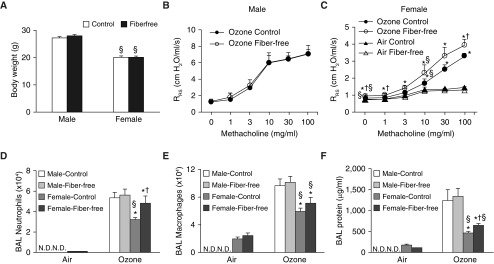
Because there were marked sex differences in the impact of increased dietary fiber on pulmonary responses to O3 (Figures 1B and 1C), we also evaluated the impact of fiber-free diets. Male and female mice were fed control diets or fiber-free diets for 3 days before exposure. Body weight was not different in mice fed control versus fiber-free diets, regardless of whether the mice were male or female (Figure 2A). Compared with the control diet, there was no effect of the fiber-free diet on the magnitude of O3-induced AHR in male mice (Figure 2B). Therefore, air-exposed mice were not evaluated. In female mice, there was no effect of the fiber-free diet on airway responsiveness in air-exposed mice, but the fiber-free diet increased airway responsiveness in O3-exposed mice (Figure 2C).
Figure 2.
Effect of fiber-free diets on pulmonary responses to O3. Male and female mice were fed either a control diet or a fiber-free diet for 3 days. Shown are (A) body weight, (B) airway responsiveness of male mice exposed to O3 (air-exposed male mice were not examined), (C) airway responsiveness of female mice exposed to room air or O3, (D) BAL neutrophils, (E) BAL macrophages, and (F) BAL protein. Results are mean ± SE of data from 4 (air) or 8–10 (ozone) mice per group. Data were gathered over 2 and 4 experimental days for the male and female mice, respectively. On each day, fiber-free diet–fed and control diet–fed mice were studied. *P < 0.05 compared with air, †P < 0.05 compared with control diet–fed mice, and §P < 0.05 compared with male mice. Evaluation of statistical significance was performed separately for male and female mice. N.D. = not done.
In male mice, the fiber-free diet did not affect BAL neutrophils, macrophages, or protein (Figures 2D–2F). However, in female mice, O3 caused greater increases in BAL neutrophils (Figure 2D) and protein (Figure 2F), but not in macrophages (Figure 2E), in mice fed fiber-free versus control diets. These data indicate that a lack of dietary fiber augments O3-induced AHR, neutrophilic airway inflammation, and airway injury in female but not male mice.
A variety of cytokines and chemokines are released in response to O3 and some, including IL-6, CXCL1, CXCL2, and IL-1α, have been reported to contribute to O3-induced AHR (32–34). To determine whether the effects of fiber-free diets on O3-induced AHR observed in the female mice were the result of effects on cytokine and chemokine release, we performed ELISAs and multiplex assays on BAL fluid from mice fed pectin- or cellulose-enriched diets. Of the various cytokines and chemokines assessed, BAL concentrations of IL-5, IL-6, CCL2, and CCL11 were each significantly higher in female mice fed fiber-free versus control diets (Table E2). In contrast, in males, BAL concentrations of these cytokines were not altered by fiber-free diets, but BAL IL-17A was significantly higher and BAL granulocyte colony-stimulating factor was significantly lower in male mice fed fiber-free versus control diets (Table E2). We also observed no change in BAL cytokines in male mice fed cellulose-enriched versus pectin-enriched diets, except for a decrease in BAL IL-1α in the pectin diet– versus cellulose diet–fed mice (Table E3).
As in the mice on fiber-enriched diets, we also considered the possibility that changes in sex hormones might account for the observed impact of fiber-free diets on pulmonary responses to O3 in female mice. However, neither serum LH nor serum progesterone was affected by fiber-free diet feeding in O3-exposed female mice (Figure E2). Similarly, we observed no difference in serum LH or serum testosterone in male mice fed control versus fiber-free diets (Figure E2).
Similarly to the increased response to O3 reported here in female mice fed fiber-free diets (Figure 2), others have reported increases in allergic airway disease in female mice fed fiber-free diets (19, 22). In these allergic models, loss of the beneficial effects of short-chain fatty acids (SCFAs), products of bacterial metabolism of dietary fiber (23), appear to account for the deleterious effects of the fiber-free diet because these deleterious effects can be corrected by adding propionate to the drinking water (22). To determine whether loss of bacterial production of SCFAs might also account for the augmented responses to O3 observed in fiber-free diet–fed female mice (Figure 2), we added the SCFA propionate to the drinking water of female mice fed fiber-free diets. Propionate was administered in concentrations that are effective in allergic airway disease models (22) and in male chow-fed mice exposed to O3 (12). Compared with control drinking water, addition of propionate to the drinking did not alter O3-induced AHR, nor did it affect O3-induced recruitment of neutrophils and macrophages to the lungs in female mice fed fiber-free diets (Figure E3). The data suggest that loss of SCFAs does not account for the augmented response to O3 observed in these fiber-deficient mice. Instead, SCFA-independent effects of dietary fibers likely account for these observations (23).
Sex Differences in the Effect of Dietary Fiber on the Gut Microbiome
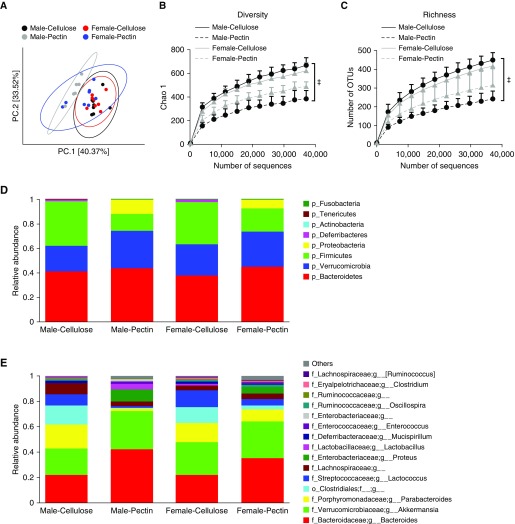
Others have reported effects of dietary fiber on the gut microbiome (23), and we have reported sex differences in the microbiome (12). To determine whether there were sex differences in the impact of dietary fiber on the gut microbiome that might account for observed sex differences in the impact of dietary fiber on O3-induced AHR (Figures 1 and 2), we performed 16S rRNA sequencing of fecal DNA. Fecal pellets were harvested before air or O3 exposure. Principal coordinate analysis (PCoA) of 16S rRNA sequencing data indicated differences in the community structure of the gut microbiome between cellulose-fed and pectin-fed male mice (Figure 3A). Diversity and richness were also significantly lower in pectin-fed than in cellulose-fed male mice (Figures 3B and 3C). Compared with males, differences in the community structure of the gut microbiome between cellulose-fed and pectin-fed mice were less pronounced in females (Figure 3A), and in females there was no significant difference in diversity or richness in cellulose- versus pectin-fed mice (Figures 3B and 3C).
Figure 3.
Differences in the gut microbiomes of male and female mice fed cellulose-enriched or pectin-enriched diets for 3 days as assessed by 16S rRNA sequencing of fecal DNA. Fecal pellets were harvested before exposure. (A) Principal coordinate (PC) analysis calculated by Bray-Curtis distances. (B) Rarefaction curves of diversity on Chao 1 index. (C) Rarefaction curve of richness. (D) Relative abundance of bacterial phyla. (E) Relative abundance of top 15 genus-level taxa. n = 8 mice per group. ‡P < 0.05 compared with cellulose-enriched diet–fed mice. OTUs = operational taxonomic units.
Examination of both phylum-level taxa (Figure 3D) and the 15 most abundant lower-order taxa (Figure 3E) indicated differences in gut microbiomes of cellulose- versus pectin-fed mice, regardless of whether the mice were male or female. For example, compared with cellulose-fed mice, there was a decrease in the relative abundance of Firmicutes and an increase in Proteobacteria and Verrucomicrobia in pectin-fed mice, regardless of whether the mice were male or female, though the magnitude of the change was greater in the males (Figure 3D). Similarly, the abundance of Proteus and Lactobacillus was increased and the abundance of Parabacteroides, Clostridiales, and Lachnospiraceae was decreased in pectin- versus cellulose-fed mice, but the magnitude of the diet-related changes was greater in the male than in the female mice (Figure 3E).
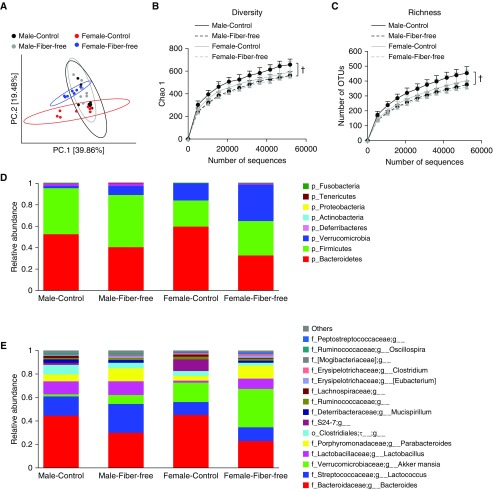
There were also sex differences in the impact of the fiber-free diet on the gut microbiome. In male mice, PCoA indicated little effect of the fiber-free diet on the community structure of the gut microbiome (Figure 4A), though both diversity and richness of the gut microbiome were lower in the fiber-free mice than in the control mice (Figures 4B and 4C). In contrast, in female mice, PCoA indicated a substantial effect of the fiber-free diet on the community structure of the gut microbiome (Figure 4A).
Figure 4.
Differences in gut microbiomes of male and female mice fed control or fiber-free diet for 3 days as assessed by 16S rRNA sequencing of fecal DNA. Fecal pellets were harvested before exposure. (A) Principal coordinate analysis calculated by Bray-Curtis distances. (B) Rarefaction curves of diversity on Chao 1 index. (C) Rarefaction curve of richness. (D) Relative abundance of bacterial phyla. (E) Relative abundance of top 15 genus-level taxa. n = 8 mice per group. †P < 0.05 compared with control diet–fed mice.
Examination of both phylum-level taxa (Figure 4D) and the 15 most abundant genus-level taxa (Figure 4E) indicated sex differences in gut microbiomes of male and female mice fed control diets, consistent with previous reports (12, 15, 16). Compared with the control diet, the fiber-free diet had a more profound effect on the gut microbiome of the female than on that of the male mice (Figures 4D and 4E). For example, the relative abundance of Bacteroidetes was decreased and the abundance of Lactobacillus and Parabacteroides was increased in fiber-free versus control diet–fed mice, regardless of whether the mice were male or female, but the magnitude of the changes was greater in the female mice (Figures 4D and 4E).
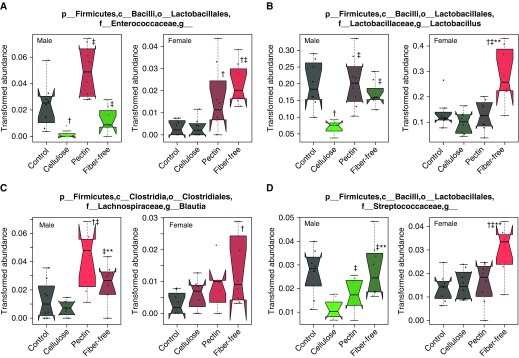
The data above indicated that the impact of fiber-enriched diets on the gut microbiome was greater in males than in females, whereas the effect of fiber-free diets on the gut microbiome was greater in females than in males. Our data indicated similar sex differences in the impact of dietary fiber manipulation on O3-induced AHR: a larger effect of pectin-enriched versus cellulose-enriched diets in males than in females, but a larger effect of fiber-free diets in females than in males (Figures 1 and 2). Because we have previously reported sex differences in the role of the microbiome in O3-induced AHR (12), we sought to identify bacterial taxa that associated with O3-induced AHR. To do so, we performed statistical analysis using Multivariate Association with Linear Models (MaAsLin) (35). In male mice and also in female mice, we combined the 16S rRNA from the mice fed the cellulose- versus pectin-enriched diets (Figure 3) and the mice fed the control versus fiber-free diets (Figure 4). In male mice, MaAsLin identified 81 genus-level taxa that were significantly different among those four groups of mice. From among these 81 taxa, we identified taxa for which the effect of the fiber-altered diet on taxon abundance paralleled the effect of the fiber-altered diet on O3-induced AHR (i.e., significantly different in pectin- vs. cellulose-fed mice [Figure 1B] but not different in control vs. fiber-free diet–fed mice [Figure 2B]). MaAsLin indicated 21 genus-level taxa that fulfilled these criteria (Table E4). In female mice, MaAsLin identified 56 genus-level taxa that were significantly different among the four groups of mice. From among these 56 taxa, we identified taxa for which the effect of the fiber-altered diet paralleled the effect of the fiber-altered diet on O3-induced AHR (i.e., not different in pectin- vs. cellulose-fed mice [Figure 1C] but different in control vs. fiber-free diet–fed mice [Figure 2C]). MaAsLin indicated 14 genus-level taxa that fulfilled these criteria (Table E5). Only four taxa were on both the male and female lists of taxa: Enterococcaceae, Lactobacillus, Blautia, and Streptococcaceae (Figure 5). Each of these taxa was significantly increased in pectin- versus cellulose-fed male but not female mice, increased in fiber-free versus control diet–fed female but not male mice, and thus associated with O3-induced AHR.
Figure 5.
Relative abundances of taxa that associated with O3-induced airway hyperresponsiveness. Analysis was performed using Multivariate Association with Linear Models (35). (A) Enterococcaceae. (B) Lactobacillus. (C) Blautia. (D) Streptococcaceae. The trapezoid boxes indicate the 25th and 75th percentiles. The whiskers indicate the minimum and maximum values, and each dot denotes one mouse. Tukey’s notches on either side of the median line indicate within-sample variance. Red means higher abundance than in mice fed a control diet, and green means lower relative abundance than in mice fed a control diet. n = 8 mice/group. †P < 0.05 and q < 0.25 compared with control diet–fed mice, ‡P < 0.05 and q < 0.25 compared with cellulose-enriched diet–fed mice, and **P < 0.05 and q < 0.25 compared with pectin-enriched diet–fed mice.
Discussion
Our data indicated sex differences in the impact of dietary fiber manipulation on pulmonary responses to O3. Compared with control diets, the pectin-enriched diet increased O3-induced AHR in both males and females, but cellulose-enriched diets reduced O3-induced AHR in males but augmented O3-induced AHR in females (Figures 1B and 1C). Indeed, the greater magnitude of O3-induced AHR typically observed in control diet–fed male versus female mice was abolished in mice fed cellulose-enriched diets (Figures 1B and 1C). Compared with control diets, fiber-free diets augmented O3-induced AHR, and they augmented O3-induced neutrophilic inflammation and injury in females but not in males. Sex differences in other physiological responses to dietary fiber have been reported. For example, in patients with hyperlipidemia, reductions in serum low-density lipoprotein in patients fed diets rich in soluble versus insoluble fiber are greater in males than in females (36). Similarly, increasing dietary fiber consumption reduces colorectal adenoma recurrence in men but not in women (37). In contrast, in the European Youth Heart Studies, dietary fiber intake was associated with improvements in insulin resistance in girls but not in boys (38).
We observed marked effects of dietary fiber manipulation on the gut microbiome (Figures 3 and 4), consistent with previous reports (19–23). The fiber-induced changes in the gut microbiome occurred rapidly, within 3 days. Others have also noted rapid changes in the gut microbiome with other types of dietary intervention (39). Importantly, there were also sex differences in the impact of dietary fiber on the microbiome (Figures 3 and 4): Differences in the gut microbiomes of mice fed cellulose- versus pectin-enriched diets were greater in male than in female mice (Figure 3), whereas differences in the gut microbiomes of mice fed control versus fiber-free diets were greater in female than in male mice (Figure 4). Sex differences in the gut microbiomes of mice fed control diets are well established (12, 15, 16). However, to our knowledge, this is the first report indicating sex differences in the impact of fiber-induced changes on the gut microbiome, though others have reported sex differences in the impact of other dietary factors on the gut microbiome (40). Notably, sex differences in the impact of dietary fiber on the gut microbiome paralleled sex differences in the effects of dietary fiber on pulmonary responses to O3, and we identified several bacterial taxa that associated with O3-induced AHR (Figure 5). Among these taxa, Lactobacillus was the most abundant. Probiotics containing bacteria of the Lactobacillus genus have been shown to attenuate allergen-induced AHR (41–43), but in our study, the abundance of Lactobacillus was associated with dietary fiber conditions that resulted in greater O3-induced AHR (Figure 5). We have also observed an association between greater Lactobacillus and greater O3-induced AHR in male wild-type and ST2-deficient mice (18). Studies examining the effects of probiotics containing Lactobacillus on O3-induced AHR will be required to resolve differences in the impact of Lactobacillus on O3- versus allergen-induced AHR.
Exactly how fiber-related changes in the gut microbiome lead to alterations in the pulmonary response to O3 remains to be established. Current concepts regarding the role of the gut microbiome in other health conditions posit that gut bacteria may communicate with other organs by stimulating intestinal afferents leading to reflex responses or by altering hormone release (44). For example, gut bacteria can affect both male and female hormones (15, 26–28). Importantly, both male and female sex steroids have been demonstrated to alter the response to O3 (29, 30). However, although there were differences in sex hormones in mice fed fiber-enriched diets (Figure E1), these changes could not explain the impact of these diets on O3-induced AHR, nor was there any impact of fiber-free diets on female hormones (Figure E2), even though these diets did alter responses to O3 in females (Figure 2).
Gut bacteria also produce numerous biochemicals that can cross the gut mucosa, enter the portal blood, circulate to distal organs and tissues, and exert effects in those locations (44). For example, gut bacteria ferment dietary fiber, resulting in the production of SCFAs (23), and low-fiber diets reduce SCFAs (22, 45). We considered the possibility that fiber-related changes in SCFAs might account for the changes in O3-induced AHR observed in fiber-altered diets. However, our data do not support this hypothesis. In male mice, pectin feeding, which increases serum SCFAs (13), resulted in greater O3-induced AHR than cellulose feeding (Figure 1B), but fiber-free diets, which reduce SCFAs (22, 45), had no effect (Figure 2B). In female mice, fiber-free diets augmented O3-induced AHR (Figure 1C), but supplementation of these fiber-free diet–fed mice with exogenous propionate had no effect (Figure E3), nor was there any difference in O3-induced AHR in pectin- versus cellulose-fed female mice (Figure 1). Instead, it is likely that SCFA-independent effects of dietary fibers account for these observations. For example, dietary fiber impacts microbial production of ferulic acid, which has both antioxidant and antiinflammatory properties (23) and could thus modify responses to O3. Given the marked differences in the composition of the gut microbiome in mice on fiber-altered diets (Figures 3–5), it is also possible that changes in other microbially derived metabolites might account for the impact of dietary fiber on pulmonary responses to O3. For example, exposure to O3 alters the serum metabolome, and we have reported marked differences in the impact of O3 on the serum metabolome in control and antibiotic-treated mice (46). O3-induced changes in serum concentration of polyamines, long-chain fatty acids, and bile acids were each significantly different in antibiotic-treated versus control mice (46). Notably, each of these metabolites has the capacity to alter airway responsiveness and/or affect neutrophil recruitment.
One important limitation to this study relates to the magnitude of the changes in dietary fiber we employed. The fiber-enriched diets we used contained 30% fiber by weight. Even relatively fiber-rich human diets do not contain this much fiber, although the amount of fiber in diets consumed by our ancestors, during the time when symbiosis between us and our gut microbiomes was being established, was higher than what is consumed now (23). Similarly, we used fiber-free rather than fiber-reduced diets, even though most human diets do not lack fiber altogether. Our goal was to use these diets as proof of concept that dietary fiber might be a therapeutic target for manipulating responses to O3. Follow-up studies will require use of intermediary amounts of fiber as well as different types of dietary fiber.
In conclusion, our data indicate substantial effects of manipulating dietary fiber on pulmonary responses to O3. Importantly, we also demonstrated marked sex differences in the impact of dietary fiber on responses to O3 and on the gut microbiome. The data suggest that therapeutic strategies which include altering the gut microbiome by dietary fiber manipulation will need to be tailored differently for male and female subjects.
Supplementary Material
Acknowledgments
Acknowledgment
The Massachusetts Host Microbiome Center, Brigham and Women’s Hospital, Boston, Massachusetts, performed the 16S rRNA sequencing.
Footnotes
Supported by National Institutes of Health grants ES-013307 (S.A.S.), ES-024032 (S.A.S.), HL007118, and ES-000002 and a gift from Paul and Mary Finnegan.
Author Contributions: H.T., D.I.K., R.S.O., T.B., Y.C., and S.A.S. conceived and designed the experiments. H.T. and A.C. performed the experiments and analyzed the data. H.T. and S.A.S. wrote the paper. H.T., D.I.K., R.S.O., T.B., Y.C., and S.A.S. reviewed, revised, and approved the final version of the manuscript.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2019-0124OC on January 8, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Brunekreef B, Holgate ST. Air pollution and health. Lancet. 2002;360:1233–1242. doi: 10.1016/S0140-6736(02)11274-8. [DOI] [PubMed] [Google Scholar]
- 2.Foster WM, Brown RH, Macri K, Mitchell CS. Bronchial reactivity of healthy subjects: 18-20 h postexposure to ozone. J Appl Physiol (1985) 2000;89:1804–1810. doi: 10.1152/jappl.2000.89.5.1804. [DOI] [PubMed] [Google Scholar]
- 3.Gent JF, Triche EW, Holford TR, Belanger K, Bracken MB, Beckett WS, et al. Association of low-level ozone and fine particles with respiratory symptoms in children with asthma. JAMA. 2003;290:1859–1867. doi: 10.1001/jama.290.14.1859. [DOI] [PubMed] [Google Scholar]
- 4.Ji M, Cohan DS, Bell ML. Meta-analysis of the association between short-term exposure to ambient ozone and respiratory hospital admissions. Environ Res Lett. 2011;6:024006. doi: 10.1088/1748-9326/6/2/024006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guarnieri M, Balmes JR. Outdoor air pollution and asthma. Lancet. 2014;383:1581–1592. doi: 10.1016/S0140-6736(14)60617-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang YJ, Boushey HA. The microbiome in asthma. J Allergy Clin Immunol. 2015;135:25–30. doi: 10.1016/j.jaci.2014.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stiemsma LT, Arrieta MC, Dimitriu PA, Cheng J, Thorson L, Lefebvre DL, et al. Canadian Healthy Infant Longitudinal Development (CHILD) Study Investigators. Shifts in Lachnospira and Clostridium sp. in the 3-month stool microbiome are associated with preschool age asthma. Clin Sci (Lond) 2016;130:2199–2207. doi: 10.1042/CS20160349. [DOI] [PubMed] [Google Scholar]
- 8.Arrieta MC, Arevalo A, Stiemsma L, Dimitriu P, Chico ME, Loor S, et al. Associations between infant fungal and bacterial dysbiosis and childhood atopic wheeze in a nonindustrialized setting. J Allergy Clin Immunol. 2018;142:424–434, e10. doi: 10.1016/j.jaci.2017.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cait A, Hughes MR, Antignano F, Cait J, Dimitriu PA, Maas KR, et al. Microbiome-driven allergic lung inflammation is ameliorated by short-chain fatty acids. Mucosal Immunol. 2018;11:785–795. doi: 10.1038/mi.2017.75. [DOI] [PubMed] [Google Scholar]
- 10.Olszak T, An D, Zeissig S, Vera MP, Richter J, Franke A, et al. Microbial exposure during early life has persistent effects on natural killer T cell function. Science. 2012;336:489–493. doi: 10.1126/science.1219328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arrieta MC, Sadarangani M, Brown EM, Russell SL, Nimmo M, Dean J, et al. A humanized microbiota mouse model of ovalbumin-induced lung inflammation. Gut Microbes. 2016;7:342–352. doi: 10.1080/19490976.2016.1182293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cho Y, Abu-Ali G, Tashiro H, Brown TA, Osgood RS, Kasahara DI, et al. Sex differences in pulmonary responses to ozone in mice: role of the microbiome. Am J Respir Cell Mol Biol. 2019;60:198–208. doi: 10.1165/rcmb.2018-0099OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho Y, Abu-Ali G, Tashiro H, Kasahara DI, Brown TA, Brand JD, et al. The microbiome regulates pulmonary responses to ozone in mice. Am J Respir Cell Mol Biol. 2018;59:346–354. doi: 10.1165/rcmb.2017-0404OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tashiro H, Cho Y, Kasahara DI, Brand JD, Bry L, Yeliseyev V, et al. Microbiota contribute to obesity-related increases in the pulmonary response to ozone. Am J Respir Cell Mol Biol. 2019;61:702–712. doi: 10.1165/rcmb.2019-0144OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Markle JG, Frank DN, Mortin-Toth S, Robertson CE, Feazel LM, Rolle-Kampczyk U, et al. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science. 2013;339:1084–1088. doi: 10.1126/science.1233521. [DOI] [PubMed] [Google Scholar]
- 16.Org E, Mehrabian M, Parks BW, Shipkova P, Liu X, Drake TA, et al. Sex differences and hormonal effects on gut microbiota composition in mice. Gut Microbes. 2016;7:313–322. doi: 10.1080/19490976.2016.1203502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Birukova A, Cyphert-Daly J, Cumming RI, Yu YR, Gowdy KM, Que LG, et al. Sex modifies acute ozone-mediated airway physiologic responses. Toxicol Sci. 2019;169:499–510. doi: 10.1093/toxsci/kfz056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kasahara DI, Wilkinson JE, Cho Y, Cardoso AP, Huttenhower C, Shore SA. The interleukin-33 receptor contributes to pulmonary responses to ozone in male mice: role of the microbiome. Respir Res. 2019;20:197. doi: 10.1186/s12931-019-1168-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Z, Shi L, Pang W, Liu W, Li J, Wang H, et al. Dietary fiber intake regulates intestinal microflora and inhibits ovalbumin-induced allergic airway inflammation in a mouse model. PLoS One. 2016;11:e0147778. doi: 10.1371/journal.pone.0147778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marchesi JR, Adams DH, Fava F, Hermes GD, Hirschfield GM, Hold G, et al. The gut microbiota and host health: a new clinical frontier. Gut. 2016;65:330–339. doi: 10.1136/gutjnl-2015-309990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Desai MS, Seekatz AM, Koropatkin NM, Kamada N, Hickey CA, Wolter M, et al. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell. 2016;167:1339–1353, e21. doi: 10.1016/j.cell.2016.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med. 2014;20:159–166. doi: 10.1038/nm.3444. [DOI] [PubMed] [Google Scholar]
- 23.Makki K, Deehan EC, Walter J, Bäckhed F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe. 2018;23:705–715. doi: 10.1016/j.chom.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 24.Halnes I, Baines KJ, Berthon BS, MacDonald-Wicks LK, Gibson PG, Wood LG. Soluble fibre meal challenge reduces airway inflammation and expression of GPR43 and GPR41 in asthma. Nutrients. 2017;9:57. doi: 10.3390/nu9010057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhalla DK. Ozone-induced lung inflammation and mucosal barrier disruption: toxicology, mechanisms, and implications. J Toxicol Environ Health B Crit Rev. 1999;2:31–86. doi: 10.1080/109374099281232. [DOI] [PubMed] [Google Scholar]
- 26.Kamimura I, Watarai A, Takamura T, Takeo A, Miura K, Morita H, et al. Gonadal steroid hormone secretion during the juvenile period depends on host-specific microbiota and contributes to the development of odor preference. Dev Psychobiol. 2019;61:670–678. doi: 10.1002/dev.21827. [DOI] [PubMed] [Google Scholar]
- 27.Kunc M, Gabrych A, Witkowski JM. Microbiome impact on metabolism and function of sex, thyroid, growth and parathyroid hormones. Acta Biochim Pol. 2016;63:189–201. doi: 10.18388/abp.2015_1093. [DOI] [PubMed] [Google Scholar]
- 28.Shin JH, Park YH, Sim M, Kim SA, Joung H, Shin DM. Serum level of sex steroid hormone is associated with diversity and profiles of human gut microbiome. Res Microbiol. 2019;170:192–201. doi: 10.1016/j.resmic.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 29.Osgood RS, Kasahara DI, Tashiro H, Cho Y, Shore SA. Androgens augment pulmonary responses to ozone in mice. Physiol Rep. 2019;7:e14214. doi: 10.14814/phy2.14214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fuentes N, Cabello N, Nicoleau M, Chroneos ZC, Silveyra P. Modulation of the lung inflammatory response to ozone by the estrous cycle. Physiol Rep. 2019;7:e14026. doi: 10.14814/phy2.14026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Henriquez AR, House JS, Snow SJ, Miller CN, Schladweiler MC, Fisher A, et al. Ozone-induced dysregulation of neuroendocrine axes requires adrenal-derived stress hormones. Toxicol Sci. 2019;172:38–50. doi: 10.1093/toxsci/kfz182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnston RA, Mizgerd JP, Shore SA. CXCR2 is essential for maximal neutrophil recruitment and methacholine responsiveness after ozone exposure. Am J Physiol Lung Cell Mol Physiol. 2005;288:L61–L67. doi: 10.1152/ajplung.00101.2004. [DOI] [PubMed] [Google Scholar]
- 33.Johnston RA, Schwartzman IN, Flynt L, Shore SA. Role of interleukin-6 in murine airway responses to ozone. Am J Physiol Lung Cell Mol Physiol. 2005;288:L390–L397. doi: 10.1152/ajplung.00007.2004. [DOI] [PubMed] [Google Scholar]
- 34.Michaudel C, Maillet I, Fauconnier L, Quesniaux V, Chung KF, Wiegman C, et al. Interleukin-1α mediates ozone-induced myeloid differentiation factor-88-dependent epithelial tissue injury and inflammation. Front Immunol. 2018;9:916. doi: 10.3389/fimmu.2018.00916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morgan XC, Tickle TL, Sokol H, Gevers D, Devaney KL, Ward DV, et al. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 2012;13:R79. doi: 10.1186/gb-2012-13-9-r79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jenkins DJ, Wolever TM, Rao AV, Hegele RA, Mitchell SJ, Ransom TP, et al. Effect on blood lipids of very high intakes of fiber in diets low in saturated fat and cholesterol. N Engl J Med. 1993;329:21–26. doi: 10.1056/NEJM199307013290104. [DOI] [PubMed] [Google Scholar]
- 37.Jacobs ET, Lanza E, Alberts DS, Hsu CH, Jiang R, Schatzkin A, et al. Fiber, sex, and colorectal adenoma: results of a pooled analysis. Am J Clin Nutr. 2006;83:343–349. doi: 10.1093/ajcn/83.2.343. [DOI] [PubMed] [Google Scholar]
- 38.Kynde I, Johnsen NF, Wedderkopp N, Bygbjerg IB, Helge JW, Heitmann BL. Intake of total dietary sugar and fibre is associated with insulin resistance among Danish 8-10- and 14-16-year-old girls but not boys. European Youth Heart Studies I and II. Public Health Nutr. 2010;13:1669–1674. doi: 10.1017/S1368980010000285. [DOI] [PubMed] [Google Scholar]
- 39.Carmody RN, Gerber GK, Luevano JM, Jr, Gatti DM, Somes L, Svenson KL, et al. Diet dominates host genotype in shaping the murine gut microbiota. Cell Host Microbe. 2015;17:72–84. doi: 10.1016/j.chom.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wankhade UD, Zhong Y, Lazarenko OP, Chintapalli SV, Piccolo BD, Chen JR, et al. Sex-specific changes in gut microbiome composition following blueberry consumption in C57BL/6J mice. Nutrients. 2019;11:313. doi: 10.3390/nu11020313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spacova I, Petrova MI, Fremau A, Pollaris L, Vanoirbeek J, Ceuppens JL, et al. Intranasal administration of probiotic Lactobacillus rhamnosus GG prevents birch pollen-induced allergic asthma in a murine model. Allergy. 2019;74:100–110. doi: 10.1111/all.13502. [DOI] [PubMed] [Google Scholar]
- 42.Karimi K, Inman MD, Bienenstock J, Forsythe P. Lactobacillus reuteri-induced regulatory T cells protect against an allergic airway response in mice. Am J Respir Crit Care Med. 2009;179:186–193. doi: 10.1164/rccm.200806-951OC. [DOI] [PubMed] [Google Scholar]
- 43.Hsieh MH, Jan RL, Wu LS, Chen PC, Kao HF, Kuo WS, et al. Lactobacillus gasseri attenuates allergic airway inflammation through PPARγ activation in dendritic cells. J Mol Med (Berl) 2018;96:39–51. doi: 10.1007/s00109-017-1598-1. [DOI] [PubMed] [Google Scholar]
- 44.Rastelli M, Knauf C, Cani PD. Gut microbes and health: a focus on the mechanisms linking microbes, obesity, and related disorders. Obesity (Silver Spring) 2018;26:792–800. doi: 10.1002/oby.22175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kovatcheva-Datchary P, Shoaie S, Lee S, Wahlstrom A, Nookaew I, Hallen A, et al. Simplified intestinal microbiota to study microbe-diet-host interactions in a mouse model. Cell Rep. 2019;26:3772–3783, e6. doi: 10.1016/j.celrep.2019.02.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cho Y, Osgood RS, Bell LN, Karoly ED, Shore SA. Ozone-induced changes in the serum metabolome: role of the microbiome. PLoS One. 2019;14:e0221633. doi: 10.1371/journal.pone.0221633. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.