Abstract
Pulmonary arterial hypertension (PAH) is an incurable disease characterized by disordered and dysfunctional angiogenesis leading to small-vessel loss and an obliterative vasculopathy. The pathogenesis of PAH is not fully understood, but multiple studies have demonstrated links between elevated angiostatic factors, disease severity, and adverse clinical outcomes. ES (endostatin), one such circulating angiostatic peptide, is the cleavage product of the proteoglycan COL18A1 (collagen α1[XVIII] chain). Elevated serum ES is associated with increased mortality and disease severity in PAH. A nonsynonymous variant of ES (aspartic acid–to-asparagine substitution at amino acid 104; p.D104N) is associated with differences in PAH survival. Although COL18A1/ES expression is markedly increased in remodeled pulmonary vessels in PAH, the impact of ES on pulmonary endothelial cell (PEC) biology and molecular contributions to PAH severity remain undetermined. In the present study, we characterized the effects of exogenous ES on human PEC biology and signaling. We demonstrated that ES inhibits PEC migration, proliferation, and cell survival, with significant differences between human variants, indicating that they are functional genetic variants. ES promotes proteasome-mediated degradation of the transcriptional repressor ID1, increasing expression and release of TSP-1 (thrombospondin 1). ES inhibits PEC migration via an ID1/TSP-1/CD36-dependent pathway, in contrast to proliferation and apoptosis, which require both CD36 and CD47. Collectively, the data implicate ES as a novel negative regulator of ID1 and an upstream propagator of an angiostatic signal cascade converging on CD36 and CD47, providing insight into the cellular and molecular effects of a functional genetic variant linked to altered outcomes in PAH.
Keywords: apoptosis, proliferation, migration, endostatin, pulmonary arterial hypertension
Clinical Relevance
The molecular and genetic determinants of disease heterogeneity in pulmonary arterial hypertension are incompletely defined. This study provides additional evidence that endostatin is a novel negative regulator of ID1 and an upstream propagator of an angiostatic signal cascade converging on CD36 and CD47. In addition, it provides insight into the cellular and molecular effects of a functional genetic variant linked to altered outcomes in pulmonary arterial hypertension.
Pulmonary arterial hypertension (PAH) is a disease of the pulmonary vasculature with diverse etiologies. Although the pathogenesis of PAH is complex and multifactorial, it is clear that pulmonary endothelial cell (PEC) dysfunction is a central feature of the disease (1). The mechanism(s) responsible for initiating and propagating PEC dysfunction in PAH have not been fully elucidated. A leading hypothesis in the field is that PEC dysfunction and apoptosis are sentinel events in PAH-associated vascular remodeling. A number of the stimuli linked to the development of disease, including shear stress, hypoxia, infection, autoimmunity, and genetic susceptibility, promote PEC injury leading to activation and apoptosis (2). Robust preclinical models of PAH are characterized by widespread PEC apoptosis, which is necessary for subsequent disease development (3, 4). Inactivating mutations in the bone morphogenetic protein (BMP) receptor type II (BMPR2) found in familial PAH promote PEC apoptosis, and, consistent with this hypothesis, BMP–BMPR2 signaling promotes PEC survival (5, 6). It is postulated that PEC apoptosis and dysfunction destabilize the vascular intima and thus promote smooth muscle cell proliferation, perivascular inflammation, and endothelial–mesenchymal transdifferentiation (2). Collectively, these events would give rise to the obliterative plexiform lesions pathognomonic of advanced PAH and contribute to the pathologic signaling milieu characterized by excess ET-1 (endothelin 1) and insufficient nitric oxide and prostacyclin (1, 7, 8).
ES (endostatin) is a potent circulating angiostatic factor derived from the cleavage of the matrix molecule COL18A1 (collagen α1[XVIII] chain) localized within the perivascular space (9). COL18A1/ES expression is significantly increased in the intima and media of remodeled vessels in PAH lungs (10). Elevated circulating ES concentrations are observed in PAH and correlate with adverse pulmonary hemodynamics. In PAH, ES is strongly associated with increased mortality, making it a novel prognostic biomarker (11). Genetic studies in PAH have identified a nonsynonymous variant of COL18A1 associated with survival differences in PAH, implicating COL18A1/ES as a genetic modifier of clinical phenotype and outcomes (11). ES has dramatic effects on endothelial cell (EC) gene and protein expression as well as the capacity to inhibit EC migration, proliferation, and survival (12–16). However, the effects of ES on PECs and the cellular and molecular impacts of identified nonsynonymous variants remain unknown.
To further understanding of the role of ES in disease, we defined the effects of ES on primary human PECs and assessed the impact of the genetic polymorphisms. We hypothesized that COL18A1/ES contributes to PAH severity via its effects on PEC biology and that this is modified by the observed human polymorphism. Collectively, our findings indicate that ES acts as a novel negative regulator of ID1, an established BMP/BMPR2 target, and propagates angiostatic signaling through release of TSP-1 (thrombospondin 1). Clinically relevant genetic polymorphisms in COL18A1/ES impact the peptide’s angiostatic properties in PECs, specifically impacting the antimigratory and proapoptotic properties of ES. Some of the results of these studies were previously reported in the form of abstracts (17, 18).
Methods
Reagents and Cell Lines
Primary human PECs and human lung microvascular endothelial cells (HLMVECs) derived from three individual donors (CC-2530 and CC-2527; Lonza) were maintained according to the manufacturer’s recommendations in endothelial cell growth media 2 (EGM-2) supplemented with the EGM-2 BulletKit (Lonza). Primary human pulmonary artery vascular smooth muscle cells (HPASMCs) (CC-2581; Lonza) were maintained according to the manufacturer’s recommendations provided with the SmGM 2 Smooth Muscle Cell Growth Medium-2 BulletKit (CC-3182; Lonza).
Cells were analyzed between passages 5 and 9 and cultured, unless otherwise indicated, in complete media. Experiments were conducted in complete media. For each individual experiment, cells were matched for donor and passage number. Reagents used included recombinant human endostatin (rES) and recombinant human TSP-1 (R&D Systems) and the proteasome inhibitor MG132 (Cell Signaling Technology). Peptides were synthesized as previously described [(ESD104 99–111 [RIFSFDGKDVLRH] and ESN104 99–111 [RIFSFNGKDVLRH])] (19). The reference protein sequence of full-length COL18A1 containing variant rs12483377 was ENST00000359759.8:c.5023G>A (NP_085059.2:p.Asp1437Asn; www.ncbi.nlm.nih.gov/clinvar/47079898/).
Duplex RNAs encoding nontargeting negative control siRNA (ON-TARGETplus, Non-Targeting Pool) were manufactured by Dharmacon Inc. The transfection of duplex RNA was performed using Geneporter B reagent (Genlantis) according to the manufacturer’s recommendations, as previously described (20).
ID1 Adenovirus Overexpression
PECs were infected with ID1 adenovirus or a null adenovirus (Adeno CMV Null adenovirus; Applied Biological Materials Inc.) at a multiplicity of infection of 10. Twenty-four hours after viral infection, PECs were treated with the indicated dose of ES.
Western Blotting
Specific proteins were detected with the following antibodies: anti-ID1 (Calbioreagents), anti–TSP-1 (Cell Signaling Technology), anti-CD36 (STEMCELL Technologies), anti-CD47 (Santa Cruz Biotechnology), and antiactin (horseradish peroxidase conjugated) (Cell Signaling Technology).
qRT-PCR
The following primers were used for qRT-PCR: ID1/ID1 (Qiagen), THDS1/TSP-1 (Qiagen), and ACTB/Actin, β (Qiagen).
Proliferation Assay
Proliferation was assessed after 48 hours of rES exposure. Cells were treated with fixation/permeabilization concentrate (Thermo Fisher Scientific), and permeabilized cells were stained with anti-Ki67. Flow cytometry was performed with a FACSAria cell sorter (BD Biosciences) and analyzed using FlowJo software (FlowJo, LLC).
Migration Assay
Migration was assessed after 4 hours of rES exposure via Transwell assay using Corning Transwell filters (Sigma-Aldrich), and cells were scraped, fixed, and stained before counting using Hema 3 Stat Pack (Thermo Fisher Scientific).
Apoptosis
Morphology
Apoptotic cells were identified on the basis of altered nuclear morphology after staining with Hoechst dye 33342 (Invitrogen) as previously described (21). A minimum of 300 cells per sample were evaluated using fluorescence microscopy. Sample identification was blinded during both image acquisition and analysis to minimize the potential for bias.
Annexin 5 assay and caspase 3 activity flow cytometry
Apoptotic cells were evaluated using an annexin V–FITC apoptosis kit (Thermo Fisher Scientific) with live cells or intracellular staining with cleaved caspase 3 Alexa Fluor 488 antibody (R&D Systems).
Statistics
All data are expressed as mean ± SEM. Comparison of means between two groups was made using an unpaired Student’s t test or two-way ANOVA when appropriate. Differences were considered significant at P ≤ 0.05. Additional methods are described in the data supplement.
Results
ES Exposure Inhibits Human PEC Proliferation and Migration and Promotes PEC Apoptosis
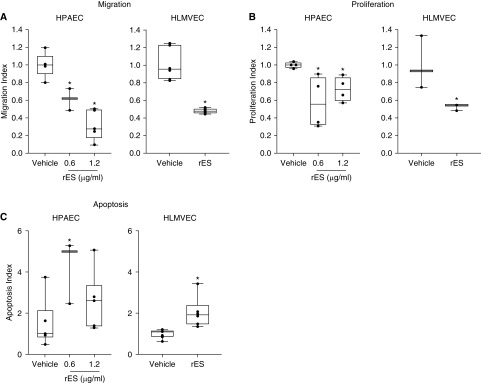
To determine the cellular and molecular impact of ES on the pulmonary circulation, we characterized the effects of rES on primary human macro- and microvascular PECs (human pulmonary artery endothelial cells [HPAECs] and human lung micro-vascular endothelial cells [HLMVECs], respectively) and HPASMCs in vitro. Migration was quantified using Transwell migration assays. Cells were exposed to rES for 4 hours. Migration in rES-exposed cells was normalized to migration in vehicle-treated cells. Cells exposed to rES demonstrated a significant dose-dependent decrease in migration (72 ± 7% reduction achieved at 1.2 μg/ml) compared with vehicle-treated HPAECs (Figure 1A). This dose similarly reduced migration in HLMVECs by 52 ± 2% (Figure 1A).
Figure 1.
Endostatin exposure inhibits pulmonary endothelial cell (PEC) proliferation and migration and promotes PEC cell death. Human macrovascular pulmonary endothelial cells (HPAECs) (left) and human microvascular pulmonary endothelial cells (HLMVECs) (right). (A) PECs were treated with vehicle or recombinant endostatin (rES) (0.6 μg/ml or 1.2 μg/ml) for 4 hours, after which migration was assessed with a modified Boyden Transwell assay. Migration Index = normalized number of PECs relative to vehicle-treated arm. (B) PECs treated with vehicle or rES (0.6 μg/ml or 1.2 μg/ml) for 48 hours, after which proliferative antigen Ki67 was quantified using flow cytometry. Proliferative Index = percentage of Ki67-positive cells normalized to vehicle control. (C) PECs were exposed to rES for 18 hours, and the percentage of cells staining for active caspase 3 were assessed with flow cytometry. Apoptosis Index = percentage of caspase 3–positive cells normalized to vehicle control. n = 4 per treatment arm. *P ≤ 0.05 compared with vehicle. Data expressed as mean ± SEM.
Proliferation was quantified using the proliferative antigen Ki67 and flow cytometry. PECs were exposed to rES for 48 hours. Treatment with rES resulted in a significant decrease in proliferation in both HPAECs and HLMVECs (Figure 1B).
Apoptosis was evaluated using three complementary assays. First, apoptosis was quantified by nuclear morphology using microscopy. PECs were exposed to increasing concentrations of rES (0.156 μg/ml to 2.5 μg/ml) or vehicle for 24 hours. Apoptotic cells were identified after staining with the nuclear dye Hoechst 33342. Treatment of PECs with rES resulted in a dose-dependent increase in cell death within 24 hours. The apoptosis dose–response curve plateaued at 0.6 μg/ml, resulting in a 2.4-fold increase in apoptosis (17 ± 7% rES vs. 7 ± 1% vehicle) (Figure E1A in the data supplement). Subsequent experiments used a dose of 0.6 μg/ml or 1.2 μg/ml as indicated.
Apoptosis was confirmed with two additional assays. Active caspase 3– and/or annexin V–positive cells were identified with flow cytometry when indicated. PECs were exposed to rES or vehicle for 18 hours. PEC cultures treated with rES had a 1.7–2.5-fold increase in active caspase 3–positive cells compared with vehicle-treated cells (Figure 1C). Similarly, PECs treated with rES had higher rates of annexin V positivity than vehicle-treated cells (16–17% compared with 10%, respectively), indicating increased rates of apoptosis with rES exposure (Figure E1B).
ES Antagonizes ID1 to Modify PEC Biology
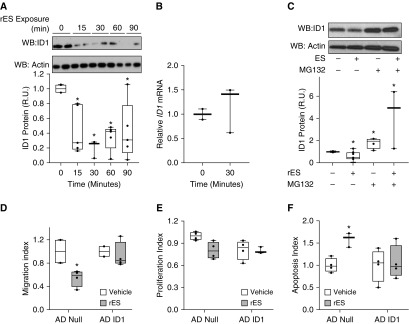
Having established that rES alters PEC phenotype, we evaluated potential molecular mechanisms. Published transcriptional profiling of human umbilical vein endothelial cells treated with ES suggests that ES can alter mRNA expression of the ID1 transcript (12). Because ID proteins have been widely implicated in EC growth and differentiation, and because the expression of ID1 is altered in PAH, we hypothesized that ID1 may be a critical effector of ES in PECs. PECs were treated with rES or vehicle, and both mRNA and protein were assessed. Recombinant ES promptly reduced ID1 protein expression in PECs, with greater than 75% reduction of total cellular ID1 observed within 30 minutes of exposure (Figure 2A). In contrast to the effects reported in human umbilical vein endothelial cells, rES did not decrease ID1 mRNA expression (Figure 2B), implicating an alternative post-transcriptional mechanism. We speculated that rES was acting at the level of protein turnover, and thus we evaluated the role of the proteasome in rES-dependent ID1 suppression. Cells were treated with the proteasome inhibitor MG132 or its carrier (PBS). Each arm was then challenged with vehicle or rES. ID1 expression was stabilized in the presence of MG132, and rES treatment no longer reduced ID1 expression (Figure 2C). This suggested that proteasome-mediated degradation was required for rES-induced reductions in ID1 expression. Together, these observations indicate that rES altered ID1 expression via a post-transcriptional, proteasome-dependent mechanism.
Figure 2.
Endostatin suppresses ID1 protein expression through a post-transcriptional proteasome-dependent mechanism, and ID1 is necessary for rES-induced angiostasis. (A) PECs were exposed to rES for an increasing length of time (15–90 min), and total cellular ID1 protein expression was evaluated by Western blot analysis (WB). WB is representative of several independent experiments. The total number per treatment arm included in the analysis was between four and nine samples. (B) PECs were exposed to rES (1.2 μg/ml) or vehicle for 30 minutes. RNA was extracted, and ID1 mRNA expression was assessed relative to ACTB/actin expression with qRT-PCR. (C) PECs were exposed to rES and the proteasome inhibitor MG132 (1 μM) or vehicle for 30 minutes. ID1 protein expression was evaluated with WB analysis. For WB, ID1 expression was quantified by protein densitometry, normalized to actin, and displayed as the proportion of ID1 expression in vehicle-treated cells. (D–F) PECs were transduced with adenovirus encoding ID1 (AD ID1) or null adenovirus (AD Null). (D) Cells were exposed to rES for 4 hours, and migration was assessed with a Transwell assay. (E) Cells were exposed to rES for 48 hours, and proliferation was assessed via flow cytometry. (F) Cells were exposed to rES for 18 hours, and caspase 3 activity was assessed with flow cytometry. For all experiments, data are displayed relative to vehicle treatment arm for each respective adenovirus treatment arm. n = 4–9 per treatment arm. *P ≤ 0.05 compared with vehicle. Data expressed as mean ± SEM. R.U. = relative units.
We then evaluated the ability of forced overexpression of ID1 protein to block rES-mediated effects on PECs. Adenoviral gene transduction achieved high levels of ID1 protein expression in PECs (Figure E2A). PECs transduced with a null adenovirus or ID1-expressing adenovirus were challenged with rES or vehicle. Migration and proliferation were quantified using Transwell assays and flow cytometry, respectively. Apoptosis was quantified by flow for active caspase 3. Basal rates of apoptosis assessed by activated caspase 3 were decreased by ID1 forced overexpression (Figure E2B). Forced overexpression of ID1 completely inhibited rES-mediated effects on migration, proliferation, and apoptosis (Figures 2D–2F). Thus, suppression of ID1 is required for rES-mediated effects on PEC biology.
TSP-1 Is an Effector of ES Function
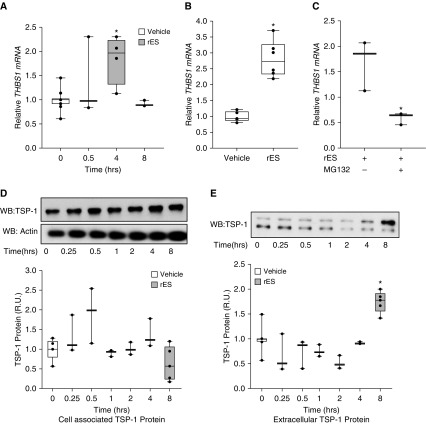
Having established that ID1 is an effector of ES in PECs, we focused on potential transcriptional targets of ID1. Expression of THBS1 (thrombospondin 1), the gene encoding TSP-1, is an angiostatic ID1 target previously implicated both in preclinical models of pulmonary hypertension and in human PAH (22, 23). Thus, we hypothesized that rES may increase expression of THBS1/TSP-1. We evaluated the effects of rES on the THBS1 gene and protein expression. HPAECs were exposed to rES or vehicle for 30 minutes to 8 hours, and total RNA was extracted to analyze THBS1 mRNA expression by qPCR (Figure 3A). Recombinant ES increased THBS1 mRNA 1.8-fold, with a peak change at 4 hours. A similar increase in THBS1 mRNA expression was observed in HLMVECs (Figure 3B) after 4 hours of exposure (4-h time point selected on the basis of peak effect observed in HPAEC time course). Having established that MG132 prevents rES-mediated suppression of ID1, we evaluated its impact on rES-induced changes in THBS1 mRNA. PECs were treated in the presence of MG132 or its carrier and challenged with rES. Total RNA was extracted, and THBS1 mRNA was quantified by qRT-PCR and expressed relative to baseline concentrations. In the presence of MG132, rES treatment failed to increase THBS1 mRNA expression (Figure 3C), suggesting that suppressing ID1 is necessary for rES-mediated alterations to THBS1 expression.
Figure 3.
Endostatin exposure results in increased THBS1/TSP-1 expression. (A) HPAECs were exposed to rES or vehicle for increasing durations (0.5–8 h). RNA was extracted, and THBS1 mRNA expression was assessed relative to ACTB/actin expression with qRT-PCR. (B) HLMVECs were exposed to rES for 4 hours, mRNA was extracted, and THBS1 mRNA expression was assessed relative to ACTB/actin via qRT-PCR. (C) PECs were treated with the proteasome inhibitor MG132 (1 μM) or its carrier and then challenged with rES or vehicle for 4 hours. RNA was extracted, and THBS1 mRNA expression was assessed relative to ACTB/actin expression with qRT-PCR. Data are displayed as the proportion of THBS1 mRNA expression in vehicle-treated cells. (D and E) PECs were exposed to rES for increasing durations (15 min to 24 h). Cellular supernatants (extracellular) and lysates (cell associated) were collected, and protein expression was evaluated via WB analysis. (D) WB of cell-associated protein expression of TSP-1 and quantified by protein densitometry. Cell-associated TSP-1 normalized to actin and displayed as the proportion of TSP-1 protein expression in vehicle-treated cells. (E) WB of extracellular protein expression of TSP-1 quantified by densitometry. Extracellular TSP-1 expression displayed as the proportion of TSP-1 expression in vehicle-treated cells. n = 3–6 per treatment arm. *P ≤ 0.05 compared with vehicle. Data are expressed as mean ± SEM. TSP-1 = thrombospondin 1.
At the level of protein expression, total cell-associated TSP-1 expression did not significantly change in response to rES (Figure 3D). In contrast, treatment of PECs with rES resulted in a 1.7-fold increase in TSP-1 released from PECs in vitro (extracellular TSP-1) (Figure 3E). Notably, with reducing SDS-PAGE, we detected two motilities for TSP-1 in the extracellular pool and a single band in cell lysates. The additional faster-mobility band was unique to the extracellular pool. The ability of extracellular proteases to liberate such smaller bioactive forms of TSP-1 has previously been described (24).
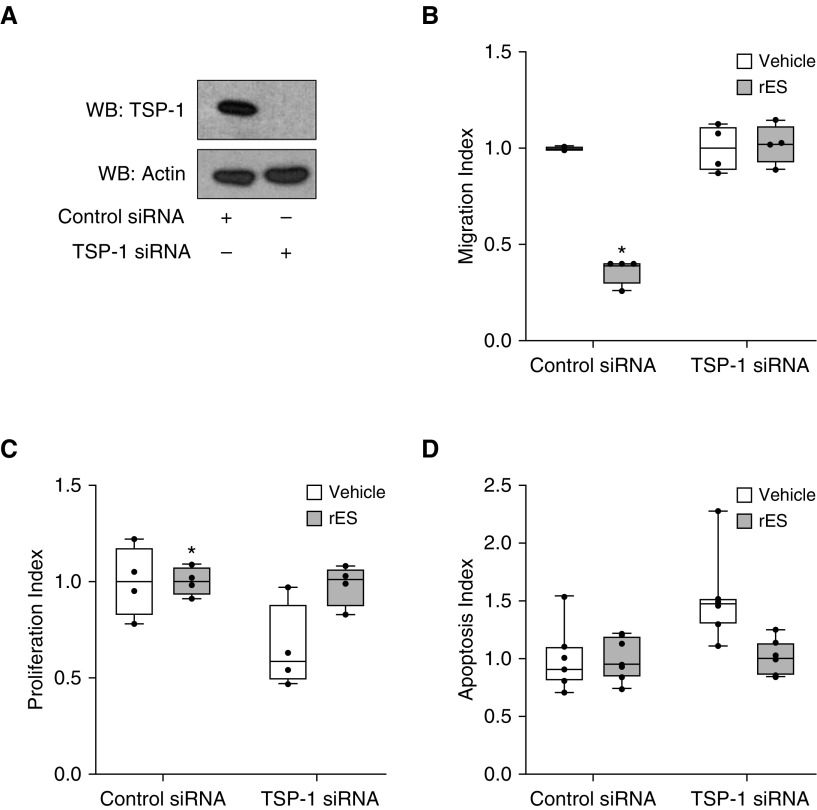
The induction and release of TSP-1 from PECs in response to rES suggest that TSP-1 may function to propagate ES-mediated effects in an autocrine or paracrine manner. To test this, we first confirmed that primary PECs were sensitive to exogenous TSP-1 in vitro. PECs were treated with recombinant TSP-1 (rTSP-1) at increasing doses and assayed for changes in migration, proliferation, and survival (data not shown). To determine if endogenous TSP-1 was an obligatory downstream effector of rES, we suppressed TSP-1 using RNA interference. PECs were transfected with TSP-1 or control siRNA and challenged with rES or vehicle. TSP-1 siRNA efficiently decreased TSP-1 protein (Figure 4A). TSP-1 deficiency had no demonstrable effects on basal rates of cell death, migration, or proliferation (Figure E3). Migratory, proliferative, and apoptotic responses of PECs to ES in the setting of TSP-1 knockdown were analyzed and were displayed indexed to individual siRNA control arms to emphasize the role of ES specifically (Figure 4). Data were also analyzed to demonstrate control versus TSP-1 siRNA effect by normalizing only to control siRNA vehicle-treated cells (Figure E4). Importantly, TSP-1–deficient (TSP-1 siRNA) cells were insensitive to rES-mediated effects on migration, proliferation, and apoptosis (Figures 4B–4D). TSP-1–deficient PECs were still sensitive to the proapoptotic and antimigratory effects of exogenous rTSP-1 (data not shown), providing additional evidence of specificity.
Figure 4.
TSP-1 is necessary for rES-induced alterations of angiostatic functions. (A) PECs were transfected with control siRNA (−) or TSP-1 siRNA (+), and TSP-1 protein expression relative to actin was evaluated in cell lysates after 24 hours. rES was administered at 1.2 μg/ml. (B) Four hours after rES treatment, migration was assessed via Transwell assay. (C) Forty-eight hours after rES treatment, proliferation was assessed via flow cytometry. (D) Twenty-four hours after rES treatment, Hoechst dye was applied, and cell death was assessed by apoptotic nuclear morphologic changes. A minimum of 350 cells were counted per condition. Data are expressed relative to vehicle treatment in the respective (control or TSP-1) siRNA treatment arm. n = 4–7 per treatment arm. *P ≤ 0.05. Data are expressed as mean ± SEM.
Receptor Requirements for ES-mediated Effects on PECs
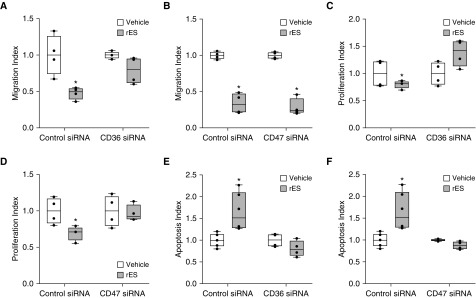
Given that endogenous TSP-1 is a downstream effector of rES, we assessed the role of established TSP-1 cell surface receptors in the signaling pathways. Although there are multiple binding partners for TSP-1, two receptors, CD36 and CD47, have been linked with TSP-1–mediated angiostatic activities and implicated in PAH pathobiology (25). We evaluated the role of CD36 and CD47 in PECs individually using RNA interference (Figure E5). Migratory, proliferative, and apoptotic responses of PECs to ES in the setting of receptor siRNA knockdown were analyzed and were displayed indexed to individual siRNA control arms to emphasize the role of ES specifically (Figure 5). Data were also analyzed to demonstrate the control versus target siRNA effect by normalizing to only control siRNA vehicle-treated cells (Figure E6). To assess the role of these receptors in rES-dependent signaling in PECs, control and receptor-specific siRNA-transfected PECs were then challenged with vehicle or rES in vitro. CD36 expression contributes to rES effects on PEC migration, because CD36-deficient PECs are resistant to the antimigratory effects of rES (Figure 5A). Similar results were observed in rTSP-1–treated cells (Figure E7A, left). However, CD47-deficient PECs remained sensitive to rES (Figure 5B). Thus, CD47 is not required for rES to inhibit PEC migration.
Figure 5.
Role of CD36 and CD47 in rES-induced alterations to angiostatic functions. Cells were transfected with control siRNA or CD36 or CD47 siRNA. After transfection, cells were exposed to rES or vehicle. (A and B) After 4 hours of rES treatment, migration was assessed via Transwell assay. (C and D) After 48 hours of rES treatment, proliferation was assessed via flow cytometry. (E and F) After 18 hours of rES treatment, caspase 3 activity was assessed via flow cytometry. Data are expressed relative to vehicle treatment in the respective (control or CD36 or CD47) siRNA treatment arm. n = 4 per treatment arm, *P ≤ 0.05. Data are expressed as mean ± SEM.
Recombinant ES-dependent effects on proliferation were lost in CD36-deficient cells (Figure 5C). This indicates that CD36 is required for rES signaling PECs. With CD47 deficiency, treatment with rTSP-1 failed to inhibit proliferation (Figure E7B, right), suggesting that CD47 is necessary for the effects of TSP-1 on PEC proliferation. Similarly, rES’s ability to inhibit proliferation was lost in the absence of CD47 expression (Figure 5D). Thus, CD47 expression appears to be necessary for rES-mediated effects on PEC proliferation, presumably via a TSP-1–dependent mechanism.
rES’s ability to induce apoptosis was similarly lost in CD36- and CD47-deficient PECs (Figures 5E and 5F, respectively). Similarly, the capacity of rTSP-1 to trigger PEC death was also lost in CD36- or CD47-deficient PECs (Figure E7C). Thus, both CD36 and CD47 are required for rES- and rTSP-1–induced apoptosis of human PECs.
Collectively, the loss-of-function studies indicate that expression of CD36 and CD47 is required for rES-induced apoptosis and proliferation, whereas only expression of CD36 is obligatory for an rES-dependent effect on migration. The receptor requirements overlap those of rTSP-1, suggesting that ES acts on these receptors through TSP-1. Our observations support a model in which rES works via ID1–TSP-1 to alter PEC phenotype via autocrine/paracrine signaling mediated by CD36 and CD47.
Natural Variants in ES Impact Function
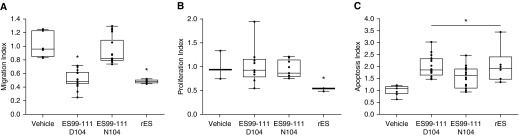
To assess the impact of human genetic variants of the COL18A1 gene on ES function, we generated ES peptides overlapping the amino acid of interest. Prior work based on the crystal structure of ES has identified smaller ES-derived peptides that maintain biological activity (19). One such peptide spans amino acids 99–111 (ES99–111) and thus overlaps with the identified natural variant linked to differences in clinical outcomes in PAH (residue p.D104N) (11). We synthesized peptides corresponding to human ES residues 99–111 with either aspartic acid (D) to asparagine (N) at residue 104 (ES99–111D104 and ES99–111N104, respectively). PECs were exposed to equal concentrations of full-length rES, ES99–111D104 or ES99–111N104 peptides, or vehicle and analyzed for migration, proliferation, and cell death. Both rES and ES99–111D104 treatment resulted in significant decreases in PEC migration (∼50% reduction) compared with vehicle (Figure 6A). In contrast, ES99–111N104 peptides did not significantly modify PEC migration. This suggests that residues 99–111 of ES are sufficient to mediate the antimigratory effects and that the aspartic acid–to-asparagine substitution disrupts that function. Neither the ES99–111D104 peptide nor the ES99–111N104 peptide altered PEC proliferation as assessed by Ki67 and flow cytometry. This is in contrast to full-length rES, which reduced proliferation by half (Figure 6B). This suggests either that the residue(s) required for antagonism of proliferation are outside of this region or that a much higher concentration of peptide is required. ES99–111D104 was as potent as full-length rES in triggering apoptosis, whereas ES99–111N104 had an intermediate effect on cell death (Figure 6C).
Figure 6.
Role of natural ES variants in migration, proliferation, and apoptosis. PECs were exposed to ES99–111D104 or ES99–111N104 peptides, rES, or vehicle. (A) Four hours after treatment, migration was assessed via Transwell assay. (B) Forty-eight hours after treatment, proliferation was assessed via flow cytometry. (C) Eighteen hours after treatment, caspase 3 activity was assessed via flow cytometry. n = 4 per treatment arm. Data are expressed as mean ± SEM. *P < 0.05.
Discussion
There is a growing body of literature linking angiostatic factors, including ES, TSP-1, and angiostatin, to adverse hemodynamics and decreased survival in PAH (11, 22, 26). Defining the cellular and molecular impacts of these factors on the pulmonary circulation is critical to understanding their contribution to disease severity and their potential as exploitable therapeutic targets.
In the present study, we established that rES promotes apoptosis and inhibits proliferation and migration of human PECs derived from both the conduit and microvascular pulmonary beds (i.e., macrovascular and microvascular pulmonary ECs). The impact of rES on PECs is linked to increased expression and secretion of TSP-1, a second angiostatic protein implicated in disease pathobiology. The data supported a model in which TSP-1 acts as a downstream effector of rES in PECs. The complete loss of rES-mediated effects in TSP-1–deficient cells and the ability of rTSP-1 to trigger PEC apoptosis and inhibit migration and proliferation similar to rES were consistent with such a model.
ES’s induction of TSP-1 occurred via an ID1-dependent mechanism. In contrast to results reported in ECs derived from other vascular beds (12), rES did not significantly alter ID1 mRNA in PECs. Recombinant ES triggered a rapid decrease in ID1 protein expression, implicating a distinct post-transcriptional mechanism. TGF-β (transforming growth factor-β), VEGF (vascular endothelial growth factor), and BMPs are established positive regulators of ID1 expression, acting at the transcriptional level (27, 28), whereas post-transcriptional regulation of ID1 occurs via ubiquitin-mediated proteasome degradation (29, 30). Treatment with the proteasome inhibitor MG132 antagonized rES-mediated ID1 degradation and prevented rES-mediated THBS1 mRNA expression. Loss of basal ID1 expression in PECs was sufficient to increase THBS1 mRNA, implicating basal ID1 as a transcriptional suppressor of THBS1 in PECs. Importantly, the capacity of rES to further modify THBS1 expression was lost in ID1-deficient cells (data not shown). In contrast, forced overexpression of ID1 antagonized the effects of rES on PEC biology. Collectively, the data indicated that rES destabilizes ID1 via a proteasome-dependent mechanism and that degradation was required for THBS1 expression and angiostatic functions on PECs.
ES binds cell surface heparin sulfate proteoglycans and integrins (31). The peptide’s antiproliferative and antimigratory properties have been linked to activation of the tyrosine kinase Src (19) and inhibition of other kinases, specifically focal adhesion kinase and Jnk (c-Jun terminal kinase) (32, 33). Less is known about the signaling required for ES-mediated apoptosis. Although ES exposure alters expression of both pro- and antiapoptotic genes, specifically Bcl2 and Bax, the upstream signaling mediators are unknown (15). In the present study, we provide evidence that rES promoted PEC apoptosis via liberation of a second angiostatic and apoptogenic protein, TSP-1. The region within the peptide containing amino acids 99–111 of ES was sufficient to promote both apoptosis and inhibit cell migration but had no discernible impact on PEC proliferation. The nonsynonymous variant, ES99–111N104, induced less apoptosis, suggesting that the variant impacts ES-mediated cell death. In contrast, the ancestral ES99–111D104 was as potent as full-length rES in terms of apoptosis, indicating that this region was sufficient to induce cell death. ES99–111D104, but not ES99–111N104, was a potent inhibitor of PEC migration in vitro. Neither truncated ES peptide retained antiproliferative functions. We emphasize that this is an observation we made in HPAECs and that this may not be generalizable to ECs from other vascular beds, which will have distinct phenotypes and molecular signaling control. Both cell types used in the present study, micro- and macrovascular PECs, are widely used in the study of PAH pathobiology, likely because of multiple observations linking PEC dysfunction to disease (1). Collectively, the data suggest that different regions within the peptide are responsible for its divergent effects on PEC function. Furthermore, it provides evidence that the nonsynonymous p.D104N variant in ES linked to improved survival in PAH (11) is functional, impacting effects of ES on PECs, although based on our published work, we do believe this to be not a causal variant but a modifier of disease risk.
Like ES, TSP-1 has multiple cell surface receptors, including CD36 and CD47, both of which have been implicated in apoptosis and inhibition of migration and proliferation (34–37). CD36 is a multifunctional glycoprotein and member of the class B scavenger receptor family, which plays an important role in fatty acid and glucose metabolism. In addition to TSP-1, it acts as a receptor for a broad range of other ligands, including fibronectin, collagen, phospholipids, and oxidized lipoprotein. It is dependent on coreceptor signaling for ligand specificity (25). CD36 binds to TSP-1, dimerizes, and becomes actively involved in signal transduction–promoting apoptosis (35) as well as inhibiting migration (36). CD47 is a membrane protein that interacts with TSP-1 in the COOH-terminal domain to impact cell adhesion, spreading, and proliferation. In addition, CD47 has been implicated in TSP-1–mediated regulation of nitric oxide and ET-1 signaling, two major pathways currently targeted with PAH-specific therapies (38, 39). ES-mediated apoptosis and inhibition of PEC proliferation required both CD36 and CD47, whereas antimigratory signaling only required CD36.
Disordered and dysfunctional angiogenic function is present in vascular smooth muscle cells (VSMCs) in addition to PECs in PAH (40). VSMCs express TSP-1, CD36, and CD47 (25, 41, 42), so, although prior studies have indicated that the cell biologic properties of ES were restricted to ECs (16), we briefly explored the PASMC response to rES. Studies in HPASMCs confirmed that these cells were directly insensitive to the rES-induced apoptosis, and furthermore, rES did not alter expression of ID1 or TSP-1 protein in human PASMCs (Figure E8). Although ES may not directly impact other vascular cell types, its capacity to trigger PEC release of TSP-1 raises the possibility of its contributing to remodeling via paracrine signaling. TSP-1 alters VSMC function as a mitogen and chemoattractant (43). It may also influence vascular tone and remodeling via its effects on ET-1 and endothelial nitric oxide synthase and activation of latent TGF-β (23, 38, 39). Thus, in contrast to the observed cell-restricted direct effects of rES in vitro, the effects of ES in vivo may be mediated secondarily through TSP-1. Our data indicate that ES acts via ID1 and THBS1/TSP-1, TSP-1 release, and autocrine/paracrine signaling through CD36 and/or CD47 to modify PEC phenotype.
Although rES-dependent effects require ID1 and its target THBS1, it is reasonable to suspect that ES may also alter expression of other ID1 targets. The ID family of proteins has a dominant-negative effect on the expression of many genes regulating angiogenesis and EC phenotype (44). ID proteins, including ID1, are known transcriptional targets of BMP and TGF-β (28, 45–47). In patients with PAH, ID1 expression is lower in PECs and VSMCs relative to control cells (46, 48). Thus, PAH is characterized by depressed ID1 expression. Intriguingly, pharmacologic enhancement of ID1 expression protects PECs from apoptosis in vitro and reverses pulmonary hypertension in vivo (49, 50). Loss-of-function mutations in BMPR2 and other TGF-β receptors are associated with decreased ID1 expression (5, 51); however, mutations in these receptors are not observed in most cases of sporadic PAH. The most recent PAH guidelines estimate that, on the basis of current genetic and genomic studies published to date, 70–75% of cases of IPAH occur without an identified receptor mutation (52), suggesting that other events are responsible for ID1 suppression in the majority of patients with PAH. The capacity of ES to inhibit ID1 may provide another mechanism accounting for ID1 suppression in human PAH, highlighting its potential role as a therapeutic target.
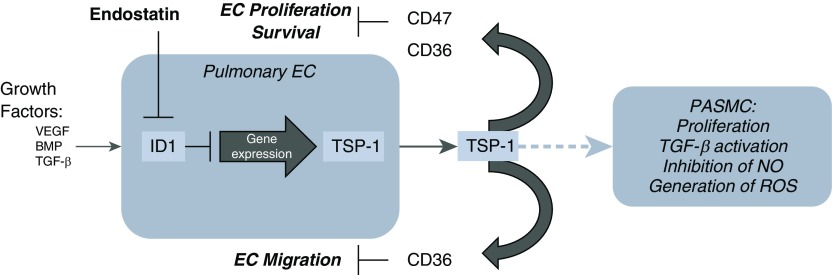
In summary, ES alters PEC biology via an ID1/TSP-1–dependent pathway (Figure 7). These properties are altered in human genetic variants linked to differences in PAH outcomes. We have identified ES as a novel regulator of TSP-1 expression and provide evidence that ES acts via proteasome-mediated degradation to suppress ID1 to alter PEC gene expression. Further studies, informed by this work, will define the contribution of this ES/ID1/TSP-1 pathway in PAH sensitivity and severity.
Figure 7.
Proposed rES/ID1/TSP-1 signaling pathway. In the absence of rES, ID1 regulates growth factor (BMP and TGF-β) signaling by acting as a transcriptional repressor via dimerization with other basic helix–loop–helix transcription factors. This results in maintenance of vascular homeostasis via regulation of PEC proliferation, migration, and activation; via alterations in endothelial progenitor cell migration and proliferation; and via modification of the vascular smooth muscle cell (SMC) phenotype. In our proposed pathway, in the presence of rES, ID1 is degraded via the proteasome, alleviating transcriptional repression of ID1 target genes, including THBS1. Increased TSP-1 protein expression promotes PEC apoptosis, inhibits migration and proliferation, and conversely promotes SMC proliferation. TSP-1 has also been implicated in TGF-β activation, regulation of nitric oxide (NO) signaling, and promoting reactive oxygen species (ROS), all of which are pathways implicated in the molecular pathobiology of pulmonary arterial hypertension. BMP = bone morphogenetic protein; EC = endothelial cell; PASMC = pulmonary artery vascular smooth muscle cells; TGF-β = transforming growth factor-β; VEGF = vascular endothelial growth factor.
Supplementary Material
Footnotes
Supported by American Heart Association grant 16POST30980016 (A.M.G.), and National Institutes of Health grants HL132153/R01 (P.M.H. and R.L.D.), HL114910/R01 (P.M.H. and R.L.D.), and T32 HL007534-31A1 (A.M.G. and G.L.P.).
Author Contributions: Study conception and design: A.M.G. and R.L.D. Data acquisition, analysis, and interpretation: A.M.G., A.M., M.M., L.C.V., L.W., L.F.J., M.M.A., G.L.P., B.S.K., M.D., K.S., T.S., T.M.K., P.M.H., and R.L.D. Manuscript preparation: A.M.G., M.M., P.M.H., and R.L.D.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2019-0056OC on January 10, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Budhiraja R, Tuder RM, Hassoun PM. Endothelial dysfunction in pulmonary hypertension. Circulation. 2004;109:159–165. doi: 10.1161/01.CIR.0000102381.57477.50. [DOI] [PubMed] [Google Scholar]
- 2.Sakao S, Tatsumi K, Voelkel NF. Endothelial cells and pulmonary arterial hypertension: apoptosis, proliferation, interaction and transdifferentiation. Respir Res. 2009;10:95. doi: 10.1186/1465-9921-10-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taraseviciene-Stewart L, Kasahara Y, Alger L, Hirth P, Mc Mahon G, Waltenberger J, et al. Inhibition of the VEGF receptor 2 combined with chronic hypoxia causes cell death-dependent pulmonary endothelial cell proliferation and severe pulmonary hypertension. FASEB J. 2001;15:427–438. doi: 10.1096/fj.00-0343com. [DOI] [PubMed] [Google Scholar]
- 4.Jurasz P, Courtman D, Babaie S, Stewart DJ. Role of apoptosis in pulmonary hypertension: from experimental models to clinical trials. Pharmacol Ther. 2010;126:1–8. doi: 10.1016/j.pharmthera.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 5.Lane KB, Machado RD, Pauciulo MW, Thomson JR, Phillips JA, III, Loyd JE, et al. International PPH Consortium. Heterozygous germline mutations in BMPR2, encoding a TGF-β receptor, cause familial primary pulmonary hypertension. Nat Genet. 2000;26:81–84. doi: 10.1038/79226. [DOI] [PubMed] [Google Scholar]
- 6.Majka S, Hagen M, Blackwell T, Harral J, Johnson JA, Gendron R, et al. Physiologic and molecular consequences of endothelial Bmpr2 mutation. Respir Res. 2011;12:84. doi: 10.1186/1465-9921-12-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Masri FA, Xu W, Comhair SA, Asosingh K, Koo M, Vasanji A, et al. Hyperproliferative apoptosis-resistant endothelial cells in idiopathic pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol. 2007;293:L548–L554. doi: 10.1152/ajplung.00428.2006. [DOI] [PubMed] [Google Scholar]
- 8.Abe K, Toba M, Alzoubi A, Ito M, Fagan KA, Cool CD, et al. Formation of plexiform lesions in experimental severe pulmonary arterial hypertension. Circulation. 2010;121:2747–2754. doi: 10.1161/CIRCULATIONAHA.109.927681. [DOI] [PubMed] [Google Scholar]
- 9.Miosge N, Simniok T, Sprysch P, Herken R. The collagen type XVIII endostatin domain is co-localized with perlecan in basement membranes in vivo. J Histochem Cytochem. 2003;51:285–296. doi: 10.1177/002215540305100303. [DOI] [PubMed] [Google Scholar]
- 10.Hoffmann J, Marsh LM, Pieper M, Stacher E, Ghanim B, Kovacs G, et al. Compartment-specific expression of collagens and their processing enzymes in intrapulmonary arteries of IPAH patients. Am J Physiol Lung Cell Mol Physiol. 2015;308:L1002–L1013. doi: 10.1152/ajplung.00383.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Damico R, Kolb TM, Valera L, Wang L, Housten T, Tedford RJ, et al. Serum endostatin is a genetically determined predictor of survival in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2015;191:208–218. doi: 10.1164/rccm.201409-1742OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdollahi A, Hahnfeldt P, Maercker C, Gröne HJ, Debus J, Ansorge W, et al. Endostatin’s antiangiogenic signaling network. Mol Cell. 2004;13:649–663. doi: 10.1016/s1097-2765(04)00102-9. [DOI] [PubMed] [Google Scholar]
- 13.Kim YM, Jang JW, Lee OH, Yeon J, Choi EY, Kim KW, et al. Endostatin inhibits endothelial and tumor cellular invasion by blocking the activation and catalytic activity of matrix metalloproteinase. Cancer Res. 2000;60:5410–5413. [PubMed] [Google Scholar]
- 14.Taddei L, Chiarugi P, Brogelli L, Cirri P, Magnelli L, Raugei G, et al. Inhibitory effect of full-length human endostatin on in vitro angiogenesis. Biochem Biophys Res Commun. 1999;263:340–345. doi: 10.1006/bbrc.1999.1342. [DOI] [PubMed] [Google Scholar]
- 15.Dhanabal M, Ramchandran R, Waterman MJ, Lu H, Knebelmann B, Segal M, et al. Endostatin induces endothelial cell apoptosis. J Biol Chem. 1999;274:11721–11726. doi: 10.1074/jbc.274.17.11721. [DOI] [PubMed] [Google Scholar]
- 16.O’Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, et al. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 17.Goyanes A, Wang L, Varela L, Johnston L, Aladdin M, Kolb TM, et al. Endostatin suppresses the inhibitors of DNA binding/differentiation transcription factors (ID1) and promotes pulmonary artery endothelial cell death [abstract] Am J Respir Crit Care Med. 2017;195:A2244. [Google Scholar]
- 18.Goyanes AM, Wang L, Varela L, Johnston L, Peloquin G, Berger A, et al. Endostatin-ID1-thrombospondin-1 signaling alters endothelial cell survival and is associated with poor clinical outcomes in pulmonary arterial hypertension [abstract] Am J Respir Crit Care Med. 2018;197:A2885. [Google Scholar]
- 19.Wickström SA, Alitalo K, Keski-Oja J. Endostatin associates with integrin α5β1 and caveolin-1, and activates Src via a tyrosyl phosphatase-dependent pathway in human endothelial cells. Cancer Res. 2002;62:5580–5589. [PubMed] [Google Scholar]
- 20.Damico RL, Chesley A, Johnston L, Bind EP, Amaro E, Nijmeh J, et al. Macrophage migration inhibitory factor governs endothelial cell sensitivity to LPS-induced apoptosis. Am J Respir Cell Mol Biol. 2008;39:77–85. doi: 10.1165/rcmb.2007-0248OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Damico R, Simms T, Kim BS, Tekeste Z, Amankwan H, Damarla M, et al. p53 mediates cigarette smoke-induced apoptosis of pulmonary endothelial cells: inhibitory effects of macrophage migration inhibitor factor. Am J Respir Cell Mol Biol. 2011;44:323–332. doi: 10.1165/rcmb.2009-0379OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaiser R, Frantz C, Bals R, Wilkens H. The role of circulating thrombospondin-1 in patients with precapillary pulmonary hypertension. Respir Res. 2016;17:96. doi: 10.1186/s12931-016-0412-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar R, Mickael C, Kassa B, Gebreab L, Robinson JC, Koyanagi DE, et al. TGF-β activation by bone marrow-derived thrombospondin-1 causes Schistosoma- and hypoxia-induced pulmonary hypertension. Nat Commun. 2017;8:15494. doi: 10.1038/ncomms15494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee NV, Sato M, Annis DS, Loo JA, Wu L, Mosher DF, et al. ADAMTS1 mediates the release of antiangiogenic polypeptides from TSP1 and 2. EMBO J. 2006;25:5270–5283. doi: 10.1038/sj.emboj.7601400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lopez-Dee Z, Pidcock K, Gutierrez LS. Thrombospondin-1: multiple paths to inflammation. Mediators Inflamm. 2011;2011:296069. doi: 10.1155/2011/296069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jurasz P, Ng D, Granton JT, Courtman DW, Stewart DJ. Elevated platelet angiostatin and circulating endothelial microfragments in idiopathic pulmonary arterial hypertension: a preliminary study. Thromb Res. 2010;125:53–60. doi: 10.1016/j.thromres.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 27.Yang J, Li X, Morrell NW. Id proteins in the vasculature: from molecular biology to cardiopulmonary medicine. Cardiovasc Res. 2014;104:388–398. doi: 10.1093/cvr/cvu215. [DOI] [PubMed] [Google Scholar]
- 28.Liang YY, Brunicardi FC, Lin X. Smad3 mediates immediate early induction of Id1 by TGF-β. Cell Res. 2009;19:140–148. doi: 10.1038/cr.2008.321. [DOI] [PubMed] [Google Scholar]
- 29.Bounpheng MA, Dimas JJ, Dodds SG, Christy BA. Degradation of Id proteins by the ubiquitin-proteasome pathway. FASEB J. 1999;13:2257–2264. [PubMed] [Google Scholar]
- 30.Trausch-Azar JS, Lingbeck J, Ciechanover A, Schwartz AL. Ubiquitin-proteasome-mediated degradation of Id1 is modulated by MyoD. J Biol Chem. 2004;279:32614–32619. doi: 10.1074/jbc.M403794200. [DOI] [PubMed] [Google Scholar]
- 31.Faye C, Moreau C, Chautard E, Jetne R, Fukai N, Ruggiero F, et al. Molecular interplay between endostatin, integrins, and heparan sulfate. J Biol Chem. 2009;284:22029–22040. doi: 10.1074/jbc.M109.002840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yin G, Liu W, An P, Li P, Ding I, Planelles V, et al. Endostatin gene transfer inhibits joint angiogenesis and pannus formation in inflammatory arthritis. Mol Ther. 2002;5:547–554. doi: 10.1006/mthe.2002.0590. [DOI] [PubMed] [Google Scholar]
- 33.Dixelius J, Cross M, Matsumoto T, Sasaki T, Timpl R, Claesson-Welsh L. Endostatin regulates endothelial cell adhesion and cytoskeletal organization. Cancer Res. 2002;62:1944–1947. [PubMed] [Google Scholar]
- 34.Isenberg JS, Ridnour LA, Perruccio EM, Espey MG, Wink DA, Roberts DD. Thrombospondin-1 inhibits endothelial cell responses to nitric oxide in a cGMP-dependent manner. Proc Natl Acad Sci USA. 2005;102:13141–13146. doi: 10.1073/pnas.0502977102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiménez B, Volpert OV, Crawford SE, Febbraio M, Silverstein RL, Bouck N. Signals leading to apoptosis-dependent inhibition of neovascularization by thrombospondin-1. Nat Med. 2000;6:41–48. doi: 10.1038/71517. [DOI] [PubMed] [Google Scholar]
- 36.Dawson DW, Pearce SF, Zhong R, Silverstein RL, Frazier WA, Bouck NP. CD36 mediates the in vitro inhibitory effects of thrombospondin-1 on endothelial cells. J Cell Biol. 1997;138:707–717. doi: 10.1083/jcb.138.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Graf R, Freyberg M, Kaiser D, Friedl P. Mechanosensitive induction of apoptosis in fibroblasts is regulated by thrombospondin-1 and integrin associated protein (CD47) Apoptosis. 2002;7:493–498. doi: 10.1023/a:1020634924760. [DOI] [PubMed] [Google Scholar]
- 38.Rogers NM, Sharifi-Sanjani M, Yao M, Ghimire K, Bienes-Martinez R, Mutchler SM, et al. TSP1-CD47 signaling is upregulated in clinical pulmonary hypertension and contributes to pulmonary arterial vasculopathy and dysfunction. Cardiovasc Res. 2017;113:15–29. doi: 10.1093/cvr/cvw218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bauer PM, Bauer EM, Rogers NM, Yao M, Feijoo-Cuaresma M, Pilewski JM, et al. Activated CD47 promotes pulmonary arterial hypertension through targeting caveolin-1. Cardiovasc Res. 2012;93:682–693. doi: 10.1093/cvr/cvr356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rabinovitch M. Molecular pathogenesis of pulmonary arterial hypertension. J Clin Invest. 2012;122:4306–4313. doi: 10.1172/JCI60658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ricciarelli R, Zingg JM, Azzi A. Vitamin E reduces the uptake of oxidized LDL by inhibiting CD36 scavenger receptor expression in cultured aortic smooth muscle cells. Circulation. 2000;102:82–87. doi: 10.1161/01.cir.102.1.82. [DOI] [PubMed] [Google Scholar]
- 42.Kaur S, Kuznetsova SA, Pendrak ML, Sipes JM, Romeo MJ, Li Z, et al. Heparan sulfate modification of the transmembrane receptor CD47 is necessary for inhibition of T cell receptor signaling by thrombospondin-1. J Biol Chem. 2011;286:14991–15002. doi: 10.1074/jbc.M110.179663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patel MK, Lymn JS, Clunn GF, Hughes AD. Thrombospondin-1 is a potent mitogen and chemoattractant for human vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 1997;17:2107–2114. doi: 10.1161/01.atv.17.10.2107. [DOI] [PubMed] [Google Scholar]
- 44.Sikder HA, Devlin MK, Dunlap S, Ryu B, Alani RM. Id proteins in cell growth and tumorigenesis. Cancer Cell. 2003;3:525–530. doi: 10.1016/s1535-6108(03)00141-7. [DOI] [PubMed] [Google Scholar]
- 45.Valdimarsdottir G, Goumans MJ, Rosendahl A, Brugman M, Itoh S, Lebrin F, et al. Stimulation of Id1 expression by bone morphogenetic protein is sufficient and necessary for bone morphogenetic protein-induced activation of endothelial cells. Circulation. 2002;106:2263–2270. doi: 10.1161/01.cir.0000033830.36431.46. [DOI] [PubMed] [Google Scholar]
- 46.Yang J, Davies RJ, Southwood M, Long L, Yang X, Sobolewski A, et al. Mutations in bone morphogenetic protein type II receptor cause dysregulation of Id gene expression in pulmonary artery smooth muscle cells: implications for familial pulmonary arterial hypertension. Circ Res. 2008;102:1212–1221. doi: 10.1161/CIRCRESAHA.108.173567. [DOI] [PubMed] [Google Scholar]
- 47.Goumans MJ, Valdimarsdottir G, Itoh S, Lebrin F, Larsson J, Mummery C, et al. Activin receptor-like kinase (ALK)1 is an antagonistic mediator of lateral TGFβ/ALK5 signaling. Mol Cell. 2003;12:817–828. doi: 10.1016/s1097-2765(03)00386-1. [DOI] [PubMed] [Google Scholar]
- 48.Sa S, Gu M, Chappell J, Shao NY, Ameen M, Elliott KA, et al. Induced pluripotent stem cell model of pulmonary arterial hypertension reveals novel gene expression and patient specificity. Am J Respir Crit Care Med. 2017;195:930–941. doi: 10.1164/rccm.201606-1200OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Long L, Ormiston ML, Yang X, Southwood M, Gräf S, Machado RD, et al. Selective enhancement of endothelial BMPR-II with BMP9 reverses pulmonary arterial hypertension. Nat Med. 2015;21:777–785. doi: 10.1038/nm.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spiekerkoetter E, Tian X, Cai J, Hopper RK, Sudheendra D, Li CG, et al. FK506 activates BMPR2, rescues endothelial dysfunction, and reverses pulmonary hypertension. J Clin Invest. 2013;123:3600–3613. doi: 10.1172/JCI65592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pousada G, Baloira A, Vilariño C, Cifrian JM, Valverde D. Novel mutations in BMPR2, ACVRL1 and KCNA5 genes and hemodynamic parameters in patients with pulmonary arterial hypertension. PLoS One. 2014;9:e100261. doi: 10.1371/journal.pone.0100261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morrell NW, Aldred MA, Chung WK, Elliott CG, Nichols WC, Soubrier F, et al. Genetics and genomics of pulmonary arterial hypertension. Eur Respir J. 2019;53:1801899. doi: 10.1183/13993003.01899-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.