Abstract
Abnormal activation of lung fibroblasts contributes to the initiation and progression of idiopathic pulmonary fibrosis (IPF). The objective of the present study was to investigate the role of fetal-lethal noncoding developmental regulatory RNA (FENDRR) in the activation of lung fibroblasts. Dysregulated long noncoding RNAs in IPF lungs were identified by next-generation sequencing analysis from the two online datasets. FENDRR expression in lung tissues from patients with IPF and mice with bleomycin-induced pulmonary fibrosis was determined by quantitative real-time PCR. IRP1 (iron-responsive element–binding protein 1), a protein partner of FENDRR, was identified by RNA pulldown-coupled mass spectrometric analysis and confirmed by RNA immunoprecipitation. The interaction region between FENDRR and IRP1 was determined by cross-linking immunoprecipitation. The in vivo role of FENDRR in pulmonary fibrosis was studied using adenovirus-mediated gene transfer in mice. The expression of FENDRR was downregulated in fibrotic human and mouse lungs as well as in primary lung fibroblasts isolated from bleomycin-treated mice. TGF-β1 (transforming growth factor-β1)–SMAD3 signaling inhibited FENDRR expression in lung fibroblasts. FENDRR was preferentially localized in the cytoplasm of adult lung fibroblasts and bound IRP1, suggesting its role in iron metabolism. FENDRR reduced pulmonary fibrosis by inhibiting fibroblast activation by reducing iron concentration and acting as a competing endogenous RNA of the profibrotic microRNA-214. Adenovirus-mediated FENDRR gene transfer in the mouse lung attenuated bleomycin-induced lung fibrosis and improved lung function. Our data suggest that FENDRR is an antifibrotic long noncoding RNA and a potential therapeutic target for pulmonary fibrosis.
Keywords: iron-responsive element–binding protein 1, iron, microRNA-214
Clinical Relevance
The abnormal activation of lung-resident fibroblasts is the major factor driving fibrotic progression in idiopathic pulmonary fibrosis. The regulation of this process by long noncoding RNAs is not completely understood. The present study identified fetal-lethal noncoding developmental regulatory RNA as an antifibrotic long noncoding RNA by inhibiting lung fibroblast activation and provided a potential therapeutic target for treating idiopathic pulmonary fibrosis.
Idiopathic pulmonary fibrosis (IPF) is a chronic and lethal fibrotic lung disease characterized by scarring of lung tissues and worsening lung function. Historically, lung tissues from patients with IPF show characteristics similar to those of usual interstitial pneumonia (1). The disease primarily occurs in individuals between the ages of 50 and 70 years and more frequently occurs in men (2). The course of IPF is difficult to predict, with a median survival time of 3–5 years after diagnosis; there is currently no effective therapy (3).
The human genome generates more noncoding RNA (ncRNA) than protein-coding RNA sequences. Most ncRNAs, including long noncoding RNAs (lncRNAs), are synthesized by polymerase II (4). lncRNAs are mRNA-like transcripts but are non–protein-encoding RNA molecules that are >200 bp long (5). lncRNAs are processed by capping, splicing, and polyadenylation, which is similar to the process of protein-coding genes (6). Only a small number of lncRNAs have been annotated functionally. lncRNAs are located in the nucleus, cytoplasm, and mitochondria (7), where they participate in various molecular functions, such as chromatin remodeling, transcriptional regulation, RNA splicing, RNA stability, and translational control (8–11). lncRNAs are important in controlling critical physiological functions, including gene imprinting; cell proliferation, differentiation, migration, and apoptosis; autophagy; immune responses; and chromosome structure (11–13). Aberrant expression of lncRNAs has been associated with a broad range of human diseases, including cardiovascular, neurodegenerative, metabolic, and lung diseases, as well as tumors and infections (14–19).
Fetal-lethal noncoding developmental regulatory RNA (FENDRR) is an lncRNA that is transcribed bidirectionally with FOXF1 (forkhead box protein F1) on its opposite strand. FENDRR binds to PRC2 (polycomb repressive complex 2) and/or TrxG (trithorax group)–MLL (mixed-lineage leukemia) complexes to epigenetically regulate the expression of its target genes (20). Murine Fendrr is essential for normal development of the lungs (21), heart, and body wall (22). LacZ reporter profiling has shown that Fendrr is highly expressed in embryonic and adult lung tissue. Fendrr homozygotes are embryonic lethal because of defective structural maturation of the lungs (21). Genomic deletion within the FENDRR gene was found in a human fatal lung development disorder, alveolar capillary dysplasia with misalignment of pulmonary veins (23). FENDRR has been linked to other human diseases, such as gastric cancer (24). However, it is unknown whether FENDRR is involved in the pathogenesis of IPF.
In the present study, we identified FENDRR as a downregulated lncRNA in the lungs of patients with IPF. We further determined the functional roles and underlying mechanisms of FENDRR in pulmonary fibrosis. Our results showed that FENDRR inhibited the activation of lung fibroblasts by binding IRP1 (iron-responsive element–binding protein 1) to control iron concentrations and by competing with the profibrotic microRNA-214 (miR-214). We also show that FENDRR functions as an antifibrotic lncRNA in vivo.
Methods
See the data supplement for details on the methods.
RNA-Sequencing Analyses
Two next-generation RNA-sequencing (RNA-seq) datasets from the lung tissues of patients with IPF are publicly available from the National Center for Biotechnology Information Sequence Read Archive (SRA accession number SRA048904) and Gene Expression Omnibus (GEO Series accession number GSE52463). We reanalyzed these datasets to identify altered lncRNAs in IPF lungs.
Cell Culture
Fibroblasts were grown and maintained with the following media supplemented with 10% FBS: HFL1 and LL29 cells cultured in F12K medium (Kaighn’s modification of Ham’s F-12 medium), CCD-8Lu cells cultured in Eagle’s minimum essential medium, and human pulmonary fibroblast (HPF) cells cultured in fibroblast medium with its supplements (PromoCell).
Isolation of Mouse Lung Fibroblasts
Primary lung fibroblasts were isolated from the lungs of saline- or bleomycin-treated mice according to a previously described protocol (25). Alveolar epithelial type II cells (AEC II) were also isolated from these mice and differentiated into alveolar epithelial type I cells (AEC I), as previously described (26).
Real-Time PCR
The mRNA and lncRNA expression levels were determined by SYBR Green I–based real-time PCR. The microRNA (miRNA) expression levels were determined by real-time PCR as previously described (27). The primers used are listed in Table E1 in the data supplement.
Droplet Digital PCR
The expression of FENDRR variants were determined by using the QX200 AutoDG droplet digital PCR system (Bio-Rad Laboratories) according to the manufacturer’s instructions. The expression of FENDRR variants was relative to the expression of ACTB (β-actin). The primers used are listed in Table E1.
Construction of Plasmids
FENDRR and miR-214 expression vectors
FENDRR was amplified by PCR using specific primers (Table E2) from human lung tissue cDNA. Mature miR-214 plus ∼200-bp flanking sequences at each end was amplified by PCR using specific primers (Table E2) from human genomic DNA. The fragments were inserted into adenoviral and lentiviral vectors as previously described (28, 29).
shRNA vectors
shRNAs were inserted into the lentiviral pSIH-H1 vector (System Biosciences). The shRNA sequences are listed in Table E2.
FENDRR promoter vector
The 5′-flanking region of FENDRR (−1653 to +90) was amplified by PCR using specific primers (Table E2) from human genomic DNA. The fragments were inserted into the luciferase reporter vector pGL3-Basic (catalog no. E1751; Promega).
miR-214 sensor vector
DNA containing four copies of the miR-214 binding site was inserted downstream of the firefly luciferase gene using the pmirGLO Dual-Luciferase miRNA Target Expression Vector (Promega). The sensor sequences are listed in Table E2.
Generation of Fibroblasts Stably Expressing FENDRR
We generated stable cell lines expressing FENDRR using a lentiviral FENDRR vector coupled with puromycin selection. The stable cell lines were used at passages 4 and 5.
RNA Pulldown and Mass Spectrometric Analysis
RNA pulldown was performed by using a Pierce Magnetic RNA-Protein Pull-Down Kit (catalog nos. 20163 and 20164; Thermo Scientific). The primers used are listed in Table E3. The RNA pulldown protein samples were analyzed by mass spectrometry (LTQ Orbitrap XL).
RNA Immunoprecipitation Assay
RNA immunoprecipitation assay was performed as described previously (30).
Cross-Linking Immunoprecipitation and qPCR Analysis
Cross-linking immunoprecipitation qPCR was performed as previously described (31). The sequences of the primers used for real-time PCR are shown in Table E4.
Iron Concentrations and Aconitase Activity Assay
Iron concentrations in the fibroblasts and lung tissues were determined by using an Iron Assay Kit purchased from Sigma-Aldrich (catalog no. MAK025) and expressed as nmol/mg protein. Aconitase activity was measured by using an Aconitase Enzyme Activity Microplate Assay Kit purchased from Abcam (catalog no. ab109712).
Gel Contraction Activity
Gel contraction activities were measured as previously described (32).
A Mouse Model of Bleomycin-induced Pulmonary Fibrosis
The animal procedures were approved by the institutional animal care and use committee at Oklahoma State University. C57BL/6 male mice (8–10 wk old) were used in this study. Adenovirus (5 × 109 infectious units per mouse) and bleomycin (1 U/kg body weight) (catalog no. B8416-15UN; Sigma-Aldrich) were delivered into the lung intranasally. The degree of fibrosis in the mouse lung was quantitated using an Ashcroft score in a blinded manner following the published method (33).
Results
FENDRR Is Downregulated in Fibrotic Lungs and Fibroblasts
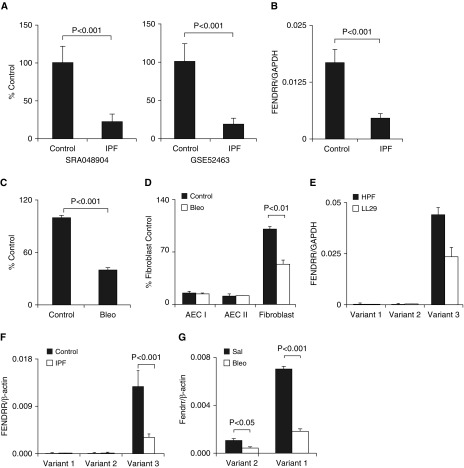
There are two publicly available RNA-seq datasets from IPF patient lungs: SRA048904, three IPF cases and three controls (34); and GSE52463, eight IPFs and seven controls (35). To identify dysregulated lncRNAs in the lungs of patients with IPF, we reanalyzed the datasets for lncRNA expression. We identified 174 (80 upregulated and 94 downregulated) and 56 (6 upregulated and 50 downregulated) dysregulated lncRNAs in IPF lungs from the SRA048904 and GSE52463 datasets, respectively. Of these, seven downregulated and zero upregulated lncRNAs were shared between the two datasets (Figure 1A and Table E5). We selected FENDRR for further studies for the following reasons: 1) FENDRR, but not other lncRNAs (except LINC00961), is conserved between humans and mice; 2) FENDRR had the fifth and third highest expression among all of the lncRNAs in normal human lungs and HPFs, respectively (data not shown); and 3) FENDRR is known to be essential for lung development (21, 23).
Figure 1.
FENDRR was downregulated in fibrotic lungs and fibroblasts. (A) Next-generation RNA-seq analysis showing the downregulation of FENDRR in IPF lungs in two published datasets. (B) Real-time PCR showing FENDRR downregulation in Lung Tissue Research Consortium IPF lungs (n = 7 for control; n = 27 for IPF). The primers detecting human FENDRR transcript variants 2 and 3 were used. (C) Real-time PCR showing FENDRR downregulation in fibrotic mouse lungs (n = 3). (D) Real-time PCR showing FENDRR expression in primary fibroblasts and AEC I and AEC II isolated from saline (Sal)- and bleomycin (Bleo)-treated mice (n = 3). The expression levels were normalized to GAPDH. Data are expressed as the percentage of the fibroblast control group. The primers detecting mouse Fendrr transcript variants 1 and 2 were used for C and D. (E) The copy number of human FENDRR transcript variants in the fibroblasts, as determined by absolute real-time qPCR (n = 3). (F) The copy number of human FENDRR variants in Lung Tissue Research Consortium IPF lungs, as determined by droplet digital PCR (n = 7 for control; n = 27 for IPF). (G) The copy number of murine Fendrr variants in the lungs of Sal- and Bleo-treated mice, as determined by droplet digital PCR (n = 3). The results are presented as the mean ± SEM. Student’s t test for A–C and ANOVA followed by Tukey’s honestly significant difference (HSD) test for D, F, and G. AEC = alveolar epithelial type cells; FENDRR = fetal-lethal noncoding developmental regulatory; HPF = human pulmonary fibroblast; IPF = idiopathic pulmonary fibrosis.
The downregulation of FENDRR in the lungs was confirmed using real-time PCR in an independent cohort of 27 patients with IPF from the Lung Tissue Research Consortium and 6 human adult normal lung tissues from the BioChain Institute (0.017 ± 0.003 in normal tissue vs. 0.0045 ± 0.001 in IPF tissue) (Figure 1B). Consistent with our findings using human lung tissues, the Fendrr concentration in mouse lung tissues with bleomycin-induced pulmonary fibrosis was 40.3 ± 2.4% of that in the control mouse lungs (Figure 1C). Fendrr downregulation was also observed in the fibroblasts, but not in the AEC I and AEC II isolated from the bleomycin-treated mice (Figure 1D).
Human FENDRR has two annotated splicing variants, transcript variant 1 (NR_036444) and transcript variant 2 (NR_033925), in the University of California Santa Cruz Genomics Institute Genome Browser (http://genome.ucsc.edu). When we attempted to clone transcript variant 2 using human lung tissue cDNA, we found that the cloned FENDRR was 531 bp longer than the transcript variant 2, which is 2,693 bp long (Figure E1). We named this FENDRR transcript “variant 3.” The cDNA sequence of variant 3 was submitted to GenBank and assigned the accession number MK522493.1. Using absolute real-time PCR, we found that the FENDRR transcript variant 3 was the major transcript in LL29 fibroblasts and HPFs (Figure 1E). Using droplet digit PCR, which provides highly sensitive and absolute quantitation of gene expression, we found that the FENDRR transcript variant 3 was also the major transcript in normal and IPF human lungs and was reduced in IPF lungs compared with normal lungs (Figure 1F). Furthermore, variants 1 and 2 were also essentially undetectable in normal and IPF lungs, even using the highly sensitive droplet digital PCR technique (Figure 1F).
There are two mouse Fendrr variants, which are highly homologous (Figure E1). The variant 1 was expressed at a much higher concentration than variant 2 in the mouse lungs. Both transcripts were decreased by bleomycin treatment (Figure 1G). Human FENDRR transcript variant 3 is positionally conserved to mouse Fendrr transcript variant 1 (Figure E1) and was used for in vivo mouse studies.
Unless otherwise noted, a primer pair common to human transcript variants 2 and 3 (Figure E1 and Table E1) was used for detecting human FENDRR expression in this study. This primer pair was designed and used before we discovered the human FENDRR transcript 3. Because the human transcript variant 2 is essentially undetectable in human lungs and human fibroblasts (Figures 1E and 1F), this primer pair mainly detects the human transcript variant 3.
TGF-β1 Inhibits FENDRR Expression via Smad3 in Pulmonary Fibroblasts
To assess whether TGF-β (transforming growth factor-β) regulates FENDRR expression, we treated HPFs with TGF-β1 and determined FENDRR concentrations. TGF-β1 reduced FENDRR expression by 42% as measured using the common primer pair to transcript variants 2 and 3 (Figure 2A). Similar results were observed using the primer pair specific to variant 3 and droplet digital PCR (Figure 2B). Promoter reporter luciferase assays indicated that TGF-β1 decreased the FENDRR promoter activity in primary HPFs and HFL1 fibroblasts (Figure 2C). We next determined whether the TGF-β1–mediated inhibition of FENDRR expression occurred through Smad transcription factors using RNA interference. The lentiviral shRNA treatment resulted in 79% and 98% decreases in the protein expression levels of Smad2 and Smad3, respectively, in LL29 fibroblasts (Figure 2D). The silencing of Smad3 but not Smad2 partially reversed the TGF-β1–mediated inhibition of FENDRR expression (Figure 2E). These results suggest that TGF-β1–SMAD3 signaling contributes to the downregulation of FENDRR in fibrotic lungs.
Figure 2.
TGF-β1–SMAD3 signaling inhibits FENDRR expression in lung fibroblasts. (A) Real-time PCR showing FENDRR downregulation in HPFs treated with TGF-β1 (5 ng/ml) for 48 hours (n = 3). (B) The copy number of FENDRR variants in control cells and TGF-β1–treated HPFs, as determined by droplet digital PCR (n = 4). (C) Dual luciferase reporter assay showing the inhibition of human FENDRR promoter activity by TGF-β1 in HPFs and HFL1 cells. Firefly luciferase activity was normalized to Renilla luciferase activity. Fold change was calculated relative to control (n = 3). (D) Western blot analysis showing knockdown of SMAD2/3 expression in LL29 cells by shRNA. (E) Real-time PCR showing that FENDRR expression in LL29 cells was inhibited by TGF-β1 and rescued by SMAD3 knockdown (n = 3). The results are presented as the mean ± SEM. Student’s t test for A and ANOVA followed by Tukey’s HSD test for B, C, and E. HFL1 = human fetal lung fibroblasts; TGF = transforming growth factor.
FENDRR Inhibits Fibroblast Activation
We overexpressed FENDRR in IPF LL29 fibroblasts using a lentiviral FENDRR (transcript variant 3) vector coupled with puromycin selection to generate stable cell lines and evaluated the effects of FENDRR overexpression on fibroblast activation. We observed a 13-fold increase in FENDRR concentrations in the FENDRR-overexpressing cells compared with the virus control cells (Figure 3A). FENDRR overexpression reduced TGF-β1–induced mRNA and protein expression of ACTA2, COL1A1 (collagen, α1[I] chain), and COL3A1 (collagen, α1[III] chain) (Figures 3B and 3C). Furthermore, FENDRR overexpression reduced TGF-β1–induced contractile activity, as determined by the collagen gel assay and stress fiber formation (Figures 3D and 3E). In contrast, knockdown of FENDRR using shRNA increased the mRNA and protein concentrations of ACTA2 and the collagens in HPF normal lung fibroblasts (Figures 3F and 3G). These results suggested that FENDRR inhibits TGF-β1–induced fibroblast activation.
Figure 3.
Overexpression of FENDRR inhibits TGF-β1–induced fibroblast activation. LL29 cells stably expressing FENDRR transcript variant 3 or a control (VC) were treated with 5 ng/ml of TGF-β1 for 48 hours. (A) FENDRR expression (n = 3). (B) FENDRR overexpression suppressed TGF-β1–induced mRNA expression of ACTA2 (α-SMA), COL1A1, and COL3A1. FENDRR and mRNA expression were determined by real-time PCR and normalized to GAPDH (n = 3). (C) Western blot analysis showing the suppression of TGF-β1–induced α-SMA, COL1A1, and COL3A1 protein expression levels by FENDRR overexpression. (D) FENDRR reduced TGF-β1–induced collagen gel contraction (n = 4). (E) Immunostaining showing the inhibition of fiber formation by FENDRR using anti–α-SMA antibodies and Alexa Fluor 546–conjugated secondary antibodies. Scale bar: 50 μm. (F and G) HPFs were infected with a lentiviral FENDRR shRNA or its control (shCON) (multiplicity of infection [MOI], 50) for 2 days, and the cells were collected for analysis. Real-time PCR and Western blotting results show that the knockdown of FENDRR enhanced mRNA expression of α-SMA, COL1A1, and COL3A1 and protein expression of α-SMA and COL1A1. The results are expressed as fold changes relative to shCON (n = 3). The results are presented as the mean ± SEM. Student’s t test for A and F. ANOVA followed by Tukey’s HSD test for B, and ANOVA followed by uncorrected Fisher’s least significant difference (LSD) test for D.
FENDRR Interacts with IRP1
As a first step in exploring the mechanisms of FENDRR activity, we determined the subcellular localization of FENDRR by measuring the FENDRR concentrations in the cytoplasmic and nuclear fractions of primary HPFs and LL29 fibroblasts extracted using a Cytoplasmic and Nuclear RNA Purification Kit (Norgen Biotek Corp.). FENDRR in both cells had a low ratio of nuclear to cytoplasmic concentration (∼0.2), similar to those of cytoplasmic GAPDH and ACTB but in contrast to that of nuclear RNA U2 (>2.5) (Figure 4A), suggesting that FENDRR is primarily located in the cytoplasm of fibroblasts.
Figure 4.
IRP1 is an interacting partner of FENDRR. (A) FENDRR is preferentially localized in the cytoplasm in fibroblasts. The RNA concentrations in cytoplasmic and nuclear fractions of LL29 fibroblasts and HPFs were determined by real-time PCR and calculated with the equation 2−Ct. GAPDH/ACTB and U2 small nuclear RNA were used as controls for cytoplasmic and nuclear RNA, respectively (n = 3). (B) RNA immunoprecipitation assay showing the interaction of FENDRR and IRP1 in LL29 fibroblasts (n = 3). (C) Mapping of the binding region of FENDRR with IRP1 by cross-linking immunoprecipitation qPCR analysis (n = 3). Data are presented as the mean ± SEM. Student’s t test for B. IRP1 = iron-responsive element-binding protein 1.
We hypothesized that FENDRR, as a cytoplasmic lncRNA, may perform its functions by interacting with cytoplasmic proteins. To identify such protein partners, we performed RNA pulldown-coupled mass spectrometric analysis. Twenty-nine proteins were enriched in the FENDRR pulldown group with a fold change >2 and a false discovery rate value <0.05 compared with the control RNA group (Table E6). These proteins included IRP1 (also named ACO1 [aconitase 1]), which controls iron homeostasis by binding to the iron-responsive element (IRE) of mRNAs related to iron transport and storage (36). Because iron overload is associated with fibrosis (37), we selected IRP1 for further study. We first validated the interaction of IRP1 and FENDRR in lung fibroblasts. RNA immunoprecipitation analysis showed the robust enrichment of FENDRR in an IRP1-interacting RNA fraction compared with that of an IgG control (Figure 4B). Moreover, we performed cross-linking immunoprecipitation qPCR analysis to determine the interaction region between FENDRR and IRP1. The results showed that the primary binding region between FENDRR and IRP1 was located at the 1,419–1,549-bp region (Figure 4C). RNA secondary structures of FENDRR, predicted using IPknot software (http://rtips.dna.bio.keio.ac.jp/ipknot/), are shown in Figure E2. The region of FENDRR that interacts with IRP1 involves the integration and formation of two independent RNA structures.
FENDRR Controls Iron Concentration by Suppressing IRP1
IRP1 is a dual-function protein with iron-regulatory ability and aconitase activity (36, 38). When cellular iron concentrations are low, IRP1 binds an IRE in either the 3′- or 5′-untranslated region of an mRNA to regulate transport and storage of iron. However, when cellular iron concentrations are high, IRP1 functions as the cytoplasmic isoform of aconitase to catalyze the interconversion of citrate into isocitrate through cis-aconitate. FENDRR overexpression reduced the iron concentration in LL29 fibroblasts, as determined using an Iron Assay Kit (Figure 5A), and the aconitase activity, as measured using an Aconitase Enzyme Activity Microplate Assay (Figure 5B). IRP1 controls iron homeostasis by binding the IRE of mRNAs related to iron transport and storage, including TFRC (transferrin receptor protein 1). FENDRR overexpression reduced TFRC mRNA concentrations (Figure 5C). The iron concentrations in fibroblasts isolated from bleomycin-treated mice were higher than those isolated from control mice (Figure 5D).
Figure 5.
FENDRR controls iron metabolism by interacting with IRP1. (A) FENDRR overexpression decreased iron concentrations in LL29 fibroblasts, as determined with an Iron Assay Kit (n = 4). (B) FENDRR overexpression inhibited aconitase activity in LL29 fibroblasts, as measured using an Aconitase Enzyme Activity Microplate Assay Kit (n = 3). (C) Real-time PCR showing the suppression of the expression of transferrin receptor (TFRC) mRNA with FENDRR overexpression (n = 4). (D) Iron concentrations were increased in primary fibroblasts isolated from the lungs of Bleo-treated mice compared with those isolated from Sal-treated mice (n = 3). (E) LL29 cells stably expressing FENDRR or VC were infected with three pooled lentiviral IRP1 shRNAs or shCON (MOI, 50) for 2 days, and the cells were then cultured in the serum-free RPMI 1640 medium (iron-free medium) or the complete RPMI 1640 medium containing 10 μM ferric ammonium citrate (iron-supplemented medium) for another 2 days. Western blotting shows the knockdown of IRP1 expression. IRP1 knockdown rescued the FENDRR-mediated decrease in cellular iron concentrations in iron-free medium (n = 6). Fold changes were calculated on the basis of VC and shCON in the iron-free medium. (F–H) LL29 lung fibroblasts were treated with 5 μM deferoxamine (DFO) and with or without TGF-β1 (5 ng/ml) for 48 hours. The mRNA expression levels of α-SMA, COL1A1, and COL3A1 were determined by real-time PCR (n = 3). Data are presented as the mean ± SEM. Student’s t test for (A–D), ANOVA followed by uncorrected Fisher’s LSD test for (E), and ANOVA followed by Tukey’s HSD for (F–H).
To determine whether FENDRR still regulates cellular iron concentrations when IRP1 is absent, we knocked down IRP1 using lentiviral shRNAs and examined the effects of IRP1 knockdown on cellular iron concentrations in the FENDRR-overexpressing fibroblasts. IRP1 protein expression was effectively reduced by a pool of three IRP1 shRNAs (Figure 5E). We then measured cellular iron concentrations in FENDRR-overexpressed and IRP1–knocked-down fibroblasts in the iron-free medium where IRP1 binds with iron metabolism mRNAs and the iron-supplemented medium where IRP1 exists in a free form in cytoplasm. In the iron-free medium, FENDRR reduced cellular iron concentration by 64%, and the knockdown of IRP1 rescued FENDRR-mediated reduction in cellular iron concentration (Figure 5E). However, in the iron-supplemented medium, FENDRR reduced only 33% of cellular iron concentration, and there were no differences in cellular iron concentrations between control shRNA and IRP1-knockdown conditions. These results suggest that FENDRR likely competes with the binding of IRP1 to iron metabolism genes under a low cellular iron concentration.
Iron Is Required for Fibroblast Activation
Iron overload has been shown to be associated with lung, liver, and renal fibrosis (37, 39). We evaluated the effects of iron on lung fibroblast activation. Iron depletion by treatment with the iron chelator deferoxamine suppressed the TGF-β1–induced mRNA expression of ACTA2, COL1A1, and COL3A1 in human lung LL29 fibroblasts (Figures 5F–5H). These results indicated that iron is required for TGF-β–induced fibroblast activation.
FENDRR Competes with Profibrotic miR-214
One of the mechanisms for lncRNA function involves sponging of miRNAs. To determine whether FENDRR could act as a competing endogenous RNA (ceRNA) or molecular sponge, we used the RNAhybrid tool to predict potential miRNA binding sites, and we found six binding sites for miR-214-3p on FENDRR (1028–1055, 1661–1676, 1876–1914, 2132–2147, 2698–2743, and 3010–3037) (Figure E3). To validate the prediction experimentally, we constructed an miR-214 sensor consisting of the firefly luciferase gene and four copies of miR-214 binding sites using the pmirGlo reporter vector. The miR-214 sensor activity was inhibited by endogenous miR-214 in the LL29 fibroblasts, and FENDRR overexpression increased the activity of the miR-214 sensor (Figure 6A). We then determined whether FENDRR directly competes with miR-214. Transfection of HEK293T cells with a FENDRR expression vector increased the miR-214 sensor activity. However, cotransfection with an miR-214 expression vector reduced the sensor activity in both control and FENDRR overexpression groups in an miR-214 dose-dependent manner (Figure 6B). These results suggest that FENDRR competes with miR-214.
Figure 6.
FENDRR sponges microRNA-214 (miR-214). (A) Luciferase assay showing that FENDRR overexpression increased the activity of miR-214 sensor in LL29 cells (n = 4). (B) Luciferase assay showing that FENDRR increased miR-214 sensor activity by competing with miR-214 in HEK293T cells. The effects of FENDRR on an miR-214 sensor were reversed by increasing the miR-214 concentration. HEK293T cells were cotransfected with 5 ng of miR-214 sensor and 20, 40, or 60 ng of miR-214/control and 150 ng of FENDRR-overexpressing vector/control. Luciferase activities were determined 48 hours after transfection (n = 4). (C and D) Real-time PCR and Western blot analysis showing increases in TGF-β1–induced COL1A1 mRNA and protein concentrations by miR-214 overexpression. LL29 cells were treated with lentiviral miR-214 or VC at an MOI of 50 for 48 hours. Then, the cells were stimulated with 5 ng/ml of TGF-β1 for 48 hours (n = 4). (E) Real-time PCR showing the expression of primary miR-214 (pri-miR214) and mature miR-214 in LL29 cells treated with DFO (n = 3). Data are presented as the mean ± SEM. ANOVA followed by uncorrected Fisher’s LSD test for A, ANOVA followed by Tukey’s HSD test for B and C, and Student’s t test for E.
miR-214 is upregulated in the fibrotic lung tissues of patients with IPF (40) and in other fibrotic tissues (41–43). miR-214 functions as a profibrotic agent in the kidneys, liver, and heart (41–43). We overexpressed miR-214 in LL29 fibroblasts with a lentiviral vector and examined the activation of fibroblasts. miR-214 increased TGF-β1–induced COL1A1 mRNA and protein expression (Figures 6C and 6D).
We next determined the relationship between iron concentrations and miR-214 expression. Iron depletion reduced primary and mature miR-214 expression in LL29 cells (Figure 6E), suggesting that iron overload increases miR-214 expression.
FENDRR Attenuates Bleomycin-induced Pulmonary Fibrosis in Mice
Because FENDRR inhibits lung fibroblast activation in vitro, we further examined the effects of FENDRR overexpression in mouse lungs on bleomycin-induced pulmonary fibrosis in vivo. We used adenovirus-mediated gene transfer to overexpress human FENDRR transcript variant 3, which is positionally conserved to mouse Fendrr major transcript variant 1. We observed 1.7-fold and 3.5-fold increases in FENDRR expression in the lungs of saline control– and bleomycin-treated mice, respectively (Figure 7A). Histopathological analysis showed reduced fibrosis in the FENDRR-treated group (Figure 7B). Quantitation of lung fibrosis in a blinded manner revealed that increased FENDRR expression significantly decreased the Ashcroft score (Figure 7C). Furthermore, increased FENDRR expression inhibited lung collagen concentrations, as measured by hydroxyproline assay (Figure 7D), and reduced bleomycin-induced COL1A1 and COL3A1 mRNA expression (Figures 7E and 7F) and protein expression of COL1A1 (Figures 7G and 7H). FENDRR also decreased elastance, indicating an improvement in lung function (Figure 7I).
Figure 7.
FENDRR overexpression attenuates Bleo-induced mouse lung fibrosis. On Day 0, mice were infected with adenovirus-mediated FENNDRR (AdFENDRR) or AdCON (5 × 109 infectious units) through nasal instillation. On Day 1, Sal or Bleo (1 U/kg body weight) was delivered into the mouse lungs through nasal instillation. At Day 14, the mice were subjected to an analysis of respiratory mechanics by using the flexiVent device (SCIREQ Scientific Respiratory Equipment Inc.) and then killed. The left lungs were collected for RNA and protein analysis, and the right lungs were fixed for histological analysis. (A) Real-time PCR showing FENDRR overexpression in mouse lungs. Primers detecting human FENDRR transcript variants 2 and 3 and murine Fendrr transcript variants 1 and 2 were used. Sal + AdCON (n = 5), Sal + AdFENDRR (n = 7), Bleo + AdCON (n = 6), Bleo + AdFENDRR (n = 8). (B) Hematoxylin and eosin staining showing fibrotic changes in mouse lungs induced by Bleo. FENDRR attenuated the fibrotic changes in Bleo-treated mouse lungs. Scale bars: 100 μm. (C) Ashcroft score showing that FENDRR lung transfer attenuated Bleo-induced mouse pulmonary fibrosis. Sal + AdCON (n = 15), Sal + AdFENDRR (n = 20), Bleo + AdCON (n = 21), Bleo + AdFENDRR (n = 24). (D) Hydroxyproline assay. Sal + AdCON (n = 5), Sal + AdFENDRR (n = 7), Bleo + AdCON (n = 6), Bleo + AdFENDRR (n = 7). (E and F) Real-time PCR analysis showing that FENDRR inhibited Bleo-induced Col1a1 and Col3a1 mRNA expression levels in mouse lungs. Sal + AdCON (n = 7), Sal + AdFENDRR (n = 12), Bleo + AdCON (n = 14), Bleo + AdFENDRR (n = 16). (G and H) Western blotting showing that FENDRR inhibited Bleo-induced COL1A1 protein expression in mouse lungs. Sal + AdCON (n = 6), Sal + AdFENDRR (n = 6), Bleo + AdCON (n = 6), Bleo + AdFENDRR (n = 6). (I) FENDRR improved respiratory function in Bleo-treated mice by flexiVent analysis. Elastance (Ers) was measured in a single-compartment model. Sal + AdCON (n = 6), Sal + AdFENDRR (n = 7), Bleo + AdCON (n = 6), Bleo + AdFENDRR (n = 7). The results are presented as the mean ± SEM. ANOVA followed by Tukey’s HSD test for A, C, E, F, and H. ANOVA followed by uncorrected Fisher’s LSD test for D and I.
Discussion
Currently, IPF remains a serious human disease. The lack of clarity surrounding the pathogenesis of IPF has resulted in a lack of effective treatments. In the present study, we discovered that FENDRR was downregulated in the fibrotic lungs of patients with IPF and bleomycin-treated mice. FENDRR expression was regulated by TGF-β/Smad3 signaling. Functionally, FENDRR reduced pulmonary fibrosis by inhibiting fibroblast activation through decreasing cellular iron concentration via interactions with IRP1 and acting as a ceRNA for profibrotic miR-214 (Figure E4).
Fendrr is highly expressed in the adult lung compared with other tissues, and it is confined to the mesenchyme in the developing lungs at Embryonic Day 14.5 (E14.5) and E18.5 (44). However, at E9.5, Fendrr is restricted to the caudal end of the lateral plate mesoderm (22). How FENDRR is regulated remains unknown. In the present study, we found that FENDRR is downregulated in fibrotic fibroblasts via TGF-β1–SMAD3 signaling. Downregulation of FENDRR has also been observed in gastric cancer (24). TGF-β–SMAD2/3 signaling has been shown to contribute to the pathogenesis of pulmonary fibrosis (45–47). Our present study demonstrated that TGF-β1 inhibited FENDRR promoter activity. Furthermore, knockdown of Smad3, but not Smad2, reversed TGF-β1–mediated reduction of FENDRR expression, supporting the contribution of TGF-β1–SMAD3 signaling to the FENDRR downregulation by TGF-β1.
Among ncRNAs, miRNAs have been studied extensively in IPF. However, little is known regarding the roles of lncRNAs in IPF. In this study, we found that FENDRR has antifibrotic functions. In in vitro studies, we revealed that FENDRR inhibited TGF-β–induced fibroblast activation, as demonstrated by the inhibition of collagen synthesis; reduced ACTA2 mRNA and protein expression; and decreased contractile activity and stress fiber formation. It is noted that FENDRR overexpression had little effects on these parameters under the basal conditions (without TGF-β). The possible explanation is that FENDRR may regulate the factors involved in the TGF-β signal pathway.
In in vivo studies, we further revealed that adenovirus-mediated FENDRR overexpression reduced collagen content and fibrosis in the lungs in response to bleomycin and improved pulmonary function. Pulmonary fibroblasts express low concentrations of CAR (coxsackievirus and adenovirus receptor). This raises a question whether adenovirus can deliver a gene to fibroblasts. It has been reported that adenoviral vector can be used to deliver ET1 (endothelin 1) or Fas to mouse or human lung fibroblasts in vitro, respectively (48, 49). Thus, it is possible that this may occur in vivo. However, we do not exclude the possibility that FENDRR is also delivered to the respiratory epithelial cells, which in turn would affect pulmonary fibroblasts.
We discovered that FENDRR was preferentially localized in the cytoplasm of adult lung fibroblasts. FENDRR has previously been shown to localize predominantly in the nucleus during murine lung development (22). However, cellular localization of lncRNAs can be changed under different physiological conditions. For example, lncRNA GAS5 (growth arrest–specific 5) translocates from the cytoplasm into the nucleus with the glucocorticoid receptor in response to dexamethasone treatment (50). Thus, shifting of FENDRR between the nucleus and cytoplasm might occur during development.
In a previous study, FENDRR was shown to function in development via epigenetic control mechanisms (20). FENDRR increased the PRC2 occupancy by forming dsDNA–RNA triplexes at target regulatory elements (20). Here, we demonstrated a novel mechanism of FENDRR activity in inhibiting fibroblast activation (i.e., regulating cellular iron concentrations). Iron is a trace element indispensable for nearly all living organisms because it participates in a variety of biological processes, including electron transport (51, 52), oxygen transport (53), and DNA synthesis (54). However, excessive iron can result in tissue damage due to the formation of free radicals (55, 56). Abnormal iron homeostasis causes a broad spectrum of human diseases. A number of studies have suggested that iron is associated with pulmonary fibrosis. For example, iron deficiency reduces the severity of bleomycin-induced pulmonary fibrosis in hamsters, possibly due to reduced iron-catalyzed oxygen radical formation, and lipid peroxidation (57). An accumulation of iron after silica instillation causes fibrotic lung injury in rats (58). Mobilization of iron from asbestos with a high percentage of iron can enhance collagen production in rat lung fibroblasts (59). Case studies have shown that interstitial lung disease might be linked to exposure to metal dust (60, 61). Two negative results have also been reported in which the treatment of rats or mice with the iron chelator deferoxamine did not inhibit bleomycin-induced lung fibrosis (62, 63). This lack of effects might have been due to the short period of treatment (62) or, in the long-term study (60 d) with the single dose of bleomycin treatment (63), to the fact that single-dose bleomycin-induced fibrosis normally resolves spontaneously within 4 weeks of treatment.
We propose that FENDRR inhibits fibroblast activation by reducing iron concentrations via interacting with IRP1, which is supported by the following observations: 1) IRP1 was identified as the target protein of FENDRR by RNA pulldown-coupled mass spectrometric analysis; 2) FENDRR overexpression inhibited ACO1 activity, reduced cellular iron concentrations, and decreased TFRC mRNA expression; and 3) iron was required for TGF-β–induced fibroblast activation.
How iron regulates lung fibroblast activation is still unclear and needs further studies. A few studies show that iron activates TGF-β signaling. One study shows that iron activates TGF-β signaling by increasing TGF-β receptor II and the phosphorylation of Smad2 in murine hepatic stellate cells (64). Another study reports that iron chelators inhibit the TGF-β/Smad pathway in prostate and colon cancer cells via the reduction in Smad2 expression and Smad3 phosphorylation due to an increase in NDRG1 (N-myc downstream-regulated gene 1) expression (65). Thus, it is possible that FENDRR reduces iron concentration, which in turn inhibits TGF-β signaling.
We also demonstrated another new mechanism for FENDRR activity regarding its antifibrotic effects (i.e., suppressing profibrotic miR-214 activity by acting as its ceRNA). A growing body of evidence suggests that lncRNAs can act as ceRNAs to sponge miRNAs (66). Our previous publication also indicated that lncRNAs function in pulmonary fibrosis by interacting with miRNAs to control fibroblast proliferation and activation (67). Here, we propose that FENDRR inhibits fibroblast activation by sponging miR-214. This is supported by the following observations: 1) miR-214 enhanced fibroblast activation; 2) FENDRR contains six binding sites for miR-214; 3) FENDRR overexpression increased the activity of an miR-214 sensor; and 4) FENDRR directly competed with miR-214 to affect miR-214 sensor activity. However, a rescue experiment is needed to address if miR-214 can reverse the antifibrotic activity of FENDRR. Furthermore, the relative contributions of iron-IRP1 and miR-214 to FENDRR activities remain to be determined. However, iron depletion reduces miR-214 expression, suggesting that these two pathways may cross-talk. In summary, we demonstrated that the lncRNA FENDRR is an antifibrotic lncRNA in the lung and that regulating cellular iron concentrations and competing with miR-214 are two novel mechanisms underlying FENDRR activity in pulmonary fibroblasts.
Supplementary Material
Acknowledgments
Acknowledgment
This study used biological specimens and data provided by the Lung Tissue Research Consortium supported by the National Heart, Lung, and Blood Institute.
Footnotes
Supported by the National Institutes of Health (grants R01HL135152, R01HL116876, and P20GM103648) (L.L.) and by the Oklahoma Center for Adult Stem Cell Research and the Lundberg-Kienlen Endowment fund (L.L.). Next-generation sequencing analysis in this project was performed at the OSU High Performance Computing Center at Oklahoma State University, which is supported in part through National Science Foundation grant OAC-1126330.
Author Contributions: C.H. and L.L. developed the study concept and design. C.H. performed and analyzed most of the experiments. Y.L. and L.K.S. helped to perform in vivo experiments. Y.L. performed fibrosis score analysis. X.Z., X.Y., and D.X. performed western blotting. X.G. performed flexiVent analysis. R.S. performed the gel contraction assay. P.W. critically reviewed the manuscript. C.H. and L.L. wrote the manuscript.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2018-0293OC on November 7, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Vourlekis JS, Brown KK, Schwarz MI. Acute interstitial pneumonitis: current understanding regarding diagnosis, pathogenesis, and natural history. Semin Respir Crit Care Med. 2001;22:399–408. doi: 10.1055/s-2001-17383. [DOI] [PubMed] [Google Scholar]
- 2.Raghu G, Rochwerg B, Zhang Y, Garcia CA, Azuma A, Behr J, et al. American Thoracic Society; European Respiratory Society; Japanese Respiratory Society; Latin American Thoracic Association. An official ATS/ERS/JRS/ALAT clinical practice guideline: treatment of idiopathic pulmonary fibrosis. An update of the 2011 clinical practice guideline. Am J Respir Crit Care Med. 2015;192:e3–e19. doi: 10.1164/rccm.201506-1063ST. [Published erratum appears in Am J Respir Crit Care Med 192:644.] [DOI] [PubMed] [Google Scholar]
- 3.Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G. Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2006;174:810–816. doi: 10.1164/rccm.200602-163OC. [DOI] [PubMed] [Google Scholar]
- 4.Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12:861–874. doi: 10.1038/nrg3074. [DOI] [PubMed] [Google Scholar]
- 5.Pelechano V, Steinmetz LM. Gene regulation by antisense transcription. Nat Rev Genet. 2013;14:880–893. doi: 10.1038/nrg3594. [DOI] [PubMed] [Google Scholar]
- 6.Geisler S, Lojek L, Khalil AM, Baker KE, Coller J. Decapping of long noncoding RNAs regulates inducible genes. Mol Cell. 2012;45:279–291. doi: 10.1016/j.molcel.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rinn JL, Chang HY. Genome regulation by long noncoding RNAs. Annu Rev Biochem. 2012;81:145–166. doi: 10.1146/annurev-biochem-051410-092902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Böhmdorfer G, Wierzbicki AT. Control of chromatin structure by long noncoding RNA. Trends Cell Biol. 2015;25:623–632. doi: 10.1016/j.tcb.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vance KW, Ponting CP. Transcriptional regulatory functions of nuclear long noncoding RNAs. Trends Genet. 2014;30:348–355. doi: 10.1016/j.tig.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonzalez I, Munita R, Agirre E, Dittmer TA, Gysling K, Misteli T, et al. A lncRNA regulates alternative splicing via establishment of a splicing-specific chromatin signature. Nat Struct Mol Biol. 2015;22:370–376. doi: 10.1038/nsmb.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fatica A, Bozzoni I. Long non-coding RNAs: new players in cell differentiation and development. Nat Rev Genet. 2014;15:7–21. doi: 10.1038/nrg3606. [DOI] [PubMed] [Google Scholar]
- 12.Xu D, Yang F, Yuan JH, Zhang L, Bi HS, Zhou CC, et al. Long noncoding RNAs associated with liver regeneration 1 accelerates hepatocyte proliferation during liver regeneration by activating Wnt/β-catenin signaling. Hepatology. 2013;58:739–751. doi: 10.1002/hep.26361. [DOI] [PubMed] [Google Scholar]
- 13.Monnier P, Martinet C, Pontis J, Stancheva I, Ait-Si-Ali S, Dandolo L. H19 lncRNA controls gene expression of the imprinted gene network by recruiting MBD1. Proc Natl Acad Sci USA. 2013;110:20693–20698. doi: 10.1073/pnas.1310201110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu JY, Yao J, Li XM, Song YC, Wang XQ, Li YJ, et al. Pathogenic role of lncRNA-MALAT1 in endothelial cell dysfunction in diabetes mellitus. Cell Death Dis. 2014;5:e1506. doi: 10.1038/cddis.2014.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009;136:629–641. doi: 10.1016/j.cell.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 16.Sakurai K, Reon BJ, Anaya J, Dutta A. The lncRNA DRAIC/PCAT29 locus constitutes a tumor-suppressive nexus. Mol Cancer Res. 2015;13:828–838. doi: 10.1158/1541-7786.MCR-15-0016-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Russell MR, Penikis A, Oldridge DA, Alvarez-Dominguez JR, McDaniel L, Diamond M, et al. CASC15-S is a tumor suppressor lncRNA at the 6p22 neuroblastoma susceptibility locus. Cancer Res. 2015;75:3155–3166. doi: 10.1158/0008-5472.CAN-14-3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang H, Cao Q, Ge J, Liu C, Ma Y, Meng Y, et al. LncRNA-regulated infection and inflammation pathways associated with pregnancy loss: genome wide differential expression of lncRNAs in early spontaneous abortion. Am J Reprod Immunol. 2014;72:359–375. doi: 10.1111/aji.12275. [DOI] [PubMed] [Google Scholar]
- 19.Wang K, Liu CY, Zhou LY, Wang JX, Wang M, Zhao B, et al. APF lncRNA regulates autophagy and myocardial infarction by targeting miR-188-3p. Nat Commun. 2015;6:6779. doi: 10.1038/ncomms7779. [DOI] [PubMed] [Google Scholar]
- 20.Grote P, Herrmann BG. The long non-coding RNA Fendrr links epigenetic control mechanisms to gene regulatory networks in mammalian embryogenesis. RNA Biol. 2013;10:1579–1585. doi: 10.4161/rna.26165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lai KM, Gong G, Atanasio A, Rojas J, Quispe J, Posca J, et al. Diverse phenotypes and specific transcription patterns in twenty mouse lines with ablated LincRNAs. PLoS One. 2015;10:e0125522. doi: 10.1371/journal.pone.0125522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grote P, Wittler L, Hendrix D, Koch F, Währisch S, Beisaw A, et al. The tissue-specific lncRNA Fendrr is an essential regulator of heart and body wall development in the mouse. Dev Cell. 2013;24:206–214. doi: 10.1016/j.devcel.2012.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Szafranski P, Dharmadhikari AV, Brosens E, Gurha P, Kolodziejska KE, Zhishuo O, et al. Small noncoding differentially methylated copy-number variants, including lncRNA genes, cause a lethal lung developmental disorder. Genome Res. 2013;23:23–33. doi: 10.1101/gr.141887.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu TP, Huang MD, Xia R, Liu XX, Sun M, Yin L, et al. Decreased expression of the long non-coding RNA FENDRR is associated with poor prognosis in gastric cancer and FENDRR regulates gastric cancer cell metastasis by affecting fibronectin1 expression. J Hematol Oncol. 2014;7:63. doi: 10.1186/s13045-014-0063-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bruce MC, Honaker CE, Cross RJ. Lung fibroblasts undergo apoptosis following alveolarization. Am J Respir Cell Mol Biol. 1999;20:228–236. doi: 10.1165/ajrcmb.20.2.3150. [DOI] [PubMed] [Google Scholar]
- 26.Mishra A, Guo Y, Zhang L, More S, Weng T, Chintagari NR, et al. A critical role for P2X7 receptor-induced VCAM-1 shedding and neutrophil infiltration during acute lung injury. J Immunol. 2016;197:2828–2837. doi: 10.4049/jimmunol.1501041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang C, Xiao X, Chintagari NR, Breshears M, Wang Y, Liu L. MicroRNA and mRNA expression profiling in rat acute respiratory distress syndrome. BMC Med Genomics. 2014;7:46. doi: 10.1186/1755-8794-7-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhaskaran M, Wang Y, Zhang H, Weng T, Baviskar P, Guo Y, et al. MicroRNA-127 modulates fetal lung development. Physiol Genomics. 2009;37:268–278. doi: 10.1152/physiolgenomics.90268.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao C, Huang C, Weng T, Xiao X, Ma H, Liu L. Computational prediction of microRNAs targeting GABA receptors and experimental verification of miR-181, miR-216 and miR-203 targets in GABA-A receptor. BMC Res Notes. 2012;5:91. doi: 10.1186/1756-0500-5-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khalil AM, Guttman M, Huarte M, Garber M, Raj A, Rivea Morales D, et al. Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proc Natl Acad Sci USA. 2009;106:11667–11672. doi: 10.1073/pnas.0904715106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoon JH, Gorospe M. Cross-linking immunoprecipitation and qPCR (CLIP-qPCR) analysis to map interactions between long noncoding RNAs and RNA-binding proteins. Methods Mol Biol. 2016;1402:11–17. doi: 10.1007/978-1-4939-3378-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiao X, Huang C, Zhao C, Gou X, Senavirathna LK, Hinsdale M, et al. Regulation of myofibroblast differentiation by miR-424 during epithelial-to-mesenchymal transition. Arch Biochem Biophys. 2015;566:49–57. doi: 10.1016/j.abb.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ashcroft T, Simpson JM, Timbrell V. Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J Clin Pathol. 1988;41:467–470. doi: 10.1136/jcp.41.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deng N, Sanchez CG, Lasky JA, Zhu D. Detecting splicing variants in idiopathic pulmonary fibrosis from non-differentially expressed genes. PLoS One. 2013;8:e68352. doi: 10.1371/journal.pone.0068352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nance T, Smith KS, Anaya V, Richardson R, Ho L, Pala M, et al. Transcriptome analysis reveals differential splicing events in IPF lung tissue. PLoS One. 2014;9:e92111. doi: 10.1371/journal.pone.0092111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaptain S, Downey WE, Tang C, Philpott C, Haile D, Orloff DG, et al. A regulated RNA binding protein also possesses aconitase activity. Proc Natl Acad Sci USA. 1991;88:10109–10113. doi: 10.1073/pnas.88.22.10109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arthur MJ. Iron overload and liver fibrosis. J Gastroenterol Hepatol. 1996;11:1124–1129. doi: 10.1111/j.1440-1746.1996.tb01840.x. [DOI] [PubMed] [Google Scholar]
- 38.Philpott CC, Klausner RD, Rouault TA. The bifunctional iron-responsive element binding protein/cytosolic aconitase: the role of active-site residues in ligand binding and regulation. Proc Natl Acad Sci USA. 1994;91:7321–7325. doi: 10.1073/pnas.91.15.7321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ikeda Y, Ozono I, Tajima S, Imao M, Horinouchi Y, Izawa-Ishizawa Y, et al. Iron chelation by deferoxamine prevents renal interstitial fibrosis in mice with unilateral ureteral obstruction. PLoS One. 2014;9:e89355. doi: 10.1371/journal.pone.0089355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pandit KV, Corcoran D, Yousef H, Yarlagadda M, Tzouvelekis A, Gibson KF, et al. Inhibition and role of let-7d in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2010;182:220–229. doi: 10.1164/rccm.200911-1698OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Denby L, Ramdas V, Lu R, Conway BR, Grant JS, Dickinson B, et al. MicroRNA-214 antagonism protects against renal fibrosis. J Am Soc Nephrol. 2014;25:65–80. doi: 10.1681/ASN.2013010072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iizuka M, Ogawa T, Enomoto M, Motoyama H, Yoshizato K, Ikeda K, et al. Induction of microRNA-214-5p in human and rodent liver fibrosis. Fibrogenesis Tissue Repair. 2012;5:12. doi: 10.1186/1755-1536-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun M, Yu H, Zhang Y, Li Z, Gao W. MicroRNA-214 mediates isoproterenol-induced proliferation and collagen synthesis in cardiac fibroblasts. Sci Rep. 2015;5:18351. doi: 10.1038/srep18351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sauvageau M, Goff LA, Lodato S, Bonev B, Groff AF, Gerhardinger C, et al. Multiple knockout mouse models reveal lincRNAs are required for life and brain development. Elife. 2013;2:e01749. doi: 10.7554/eLife.01749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Branton MH, Kopp JB. TGF-β and fibrosis. Microbes Infect. 1999;1:1349–1365. doi: 10.1016/s1286-4579(99)00250-6. [DOI] [PubMed] [Google Scholar]
- 46.Warburton D, Shi W, Xu B. TGF-β-Smad3 signaling in emphysema and pulmonary fibrosis: an epigenetic aberration of normal development? Am J Physiol Lung Cell Mol Physiol. 2013;304:L83–L85. doi: 10.1152/ajplung.00258.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roach KM, Wulff H, Feghali-Bostwick C, Amrani Y, Bradding P. Increased constitutive αSMA and Smad2/3 expression in idiopathic pulmonary fibrosis myofibroblasts is KCa3.1-dependent. Respir Res. 2014;15:155. doi: 10.1186/s12931-014-0155-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lagares D, Busnadiego O, García-Fernández RA, Lamas S, Rodríguez-Pascual F. Adenoviral gene transfer of endothelin-1 in the lung induces pulmonary fibrosis through the activation of focal adhesion kinase. Am J Respir Cell Mol Biol. 2012;47:834–842. doi: 10.1165/rcmb.2011-0446OC. [DOI] [PubMed] [Google Scholar]
- 49.Wynes MW, Edelman BL, Kostyk AG, Edwards MG, Coldren C, Groshong SD, et al. Increased cell surface Fas expression is necessary and sufficient to sensitize lung fibroblasts to Fas ligation-induced apoptosis: implications for fibroblast accumulation in idiopathic pulmonary fibrosis. J Immunol. 2011;187:527–537. doi: 10.4049/jimmunol.1100447. [DOI] [PubMed] [Google Scholar]
- 50.Kino T, Hurt DE, Ichijo T, Nader N, Chrousos GP. Noncoding RNA gas5 is a growth arrest- and starvation-associated repressor of the glucocorticoid receptor. Sci Signal. 2010;3:ra8. doi: 10.1126/scisignal.2000568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Semin BK, Seibert M. Iron bound to the high-affinity Mn-binding site of the oxygen-evolving complex shifts the pK of a component controlling electron transport via Y(Z) Biochemistry. 2004;43:6772–6782. doi: 10.1021/bi036047p. [DOI] [PubMed] [Google Scholar]
- 52.Gong XM, Agalarov R, Brettel K, Carmeli C. Control of electron transport in photosystem I by the iron-sulfur cluster FX in response to intra- and intersubunit interactions. J Biol Chem. 2003;278:19141–19150. doi: 10.1074/jbc.M301808200. [DOI] [PubMed] [Google Scholar]
- 53.Sanders-Loehr J. Involvement of oxo-bridged binuclear iron centers in oxygen transport, oxygen reduction, and oxygenation. Prog Clin Biol Res. 1988;274:193–209. [PubMed] [Google Scholar]
- 54.Liu Y, Templeton DM. Iron-loaded cardiac myocytes stimulate cardiac myofibroblast DNA synthesis. Mol Cell Biochem. 2006;281:77–85. doi: 10.1007/s11010-006-0388-9. [DOI] [PubMed] [Google Scholar]
- 55.Sledzinski M, Borkowska A, Sielicka-Dudzin A, Halon M, Wozniak M, Spodnik JH, et al. Cerulein-induced acute pancreatitis is associated with c-Jun NH(2)-terminal kinase 1-dependent ferritin degradation and iron-dependent free radicals formation. Pancreas. 2013;42:1070–1077. doi: 10.1097/MPA.0b013e318287d097. [DOI] [PubMed] [Google Scholar]
- 56.Nitenberg A, Ledoux S, Valensi P, Sachs R, Antony I. Coronary microvascular adaptation to myocardial metabolic demand can be restored by inhibition of iron-catalyzed formation of oxygen free radicals in type 2 diabetic patients. Diabetes. 2002;51:813–818. doi: 10.2337/diabetes.51.3.813. [DOI] [PubMed] [Google Scholar]
- 57.Chandler DB, Barton JC, Briggs DD, III, Butler TW, Kennedy JI, Grizzle WE, et al. Effect of iron deficiency on bleomycin-induced lung fibrosis in the hamster. Am Rev Respir Dis. 1988;137:85–89. doi: 10.1164/ajrccm/137.1.85. [DOI] [PubMed] [Google Scholar]
- 58.Ghio AJ, Jaskot RH, Hatch GE. Lung injury after silica instillation is associated with an accumulation of iron in rats. Am J Physiol. 1994;267:L686–L692. doi: 10.1152/ajplung.1994.267.6.L686. [DOI] [PubMed] [Google Scholar]
- 59.Gardi C, Calzoni P, Ferrali M, Comporti M. Iron mobilization from crocidolite as enhancer of collagen content in rat lung fibroblasts. Biochem Pharmacol. 1997;53:1659–1665. doi: 10.1016/s0006-2952(97)00050-6. [DOI] [PubMed] [Google Scholar]
- 60.Chu HQ, Liu JM, Gui T, Zhao L, Sun DY, Zhang JB, et al. Case of interstitial lung disease possibly induced by exposure to iron dust. Heart Lung. 2012;41:196–199. doi: 10.1016/j.hrtlng.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 61.Zakynthinos E, Vassilakopoulos T, Kaltsas P, Malagari E, Daniil Z, Roussos C, et al. Pulmonary hypertension, interstitial lung fibrosis, and lung iron deposition in thalassaemia major. Thorax. 2001;56:737–739. doi: 10.1136/thorax.56.9.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ward HE, Hicks M, Nicholson A, Berend N. Deferoxamine infusion does not inhibit bleomycin-induced lung damage in the rat. Am Rev Respir Dis. 1988;137:1356–1359. doi: 10.1164/ajrccm/137.6.1356. [DOI] [PubMed] [Google Scholar]
- 63.Teixeira KC, Soares FS, Rocha LG, Silveira PC, Silva LA, Valença SS, et al. Attenuation of bleomycin-induced lung injury and oxidative stress by N-acetylcysteine plus deferoxamine. Pulm Pharmacol Ther. 2008;21:309–316. doi: 10.1016/j.pupt.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 64.Mehta KJ, Coombes JD, Briones-Orta M, Manka PP, Williams R, Patel VB, et al. Iron enhances hepatic fibrogenesis and activates transforming growth factor-β signaling in murine hepatic stellate cells. Am J Med Sci. 2018;355:183–190. doi: 10.1016/j.amjms.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 65.Chen Z, Zhang D, Yue F, Zheng M, Kovacevic Z, Richardson DR. The iron chelators Dp44mT and DFO inhibit TGF-β-induced epithelial-mesenchymal transition via up-regulation of N-Myc downstream-regulated gene 1 (NDRG1) J Biol Chem. 2012;287:17016–17028. doi: 10.1074/jbc.M112.350470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Furió-Tarí P, Tarazona S, Gabaldón T, Enright AJ, Conesa A. spongeScan: a web for detecting microRNA binding elements in lncRNA sequences. Nucleic Acids Res. 2016;44:W176–W180. doi: 10.1093/nar/gkw443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huang C, Yang Y, Liu L. Interaction of long noncoding RNAs and microRNAs in the pathogenesis of idiopathic pulmonary fibrosis. Physiol Genomics. 2015;47:463–469. doi: 10.1152/physiolgenomics.00064.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.