Abstract
Asthma is characterized by airway hyperreactivity and inflammation. In the lungs, parasympathetic and sensory nerves control airway tone and induce bronchoconstriction. Dysregulation of these nerves results in airway hyperreactivity. Humans with eosinophilic asthma have significantly increased sensory nerve density in airway epithelium, suggesting that type 2 cytokines and inflammatory cells promote nerve growth. Similarly, mice with congenital airway eosinophilia also have airway hyperreactivity and increased airway sensory nerve density. Here, we tested whether this occurs during development. We show that transgenic mice that overexpress IL-5, a cytokine required for eosinophil hematopoiesis, give birth to wild-type offspring that have significantly increased airway epithelial nerve density and airway hyperreactivity that persists into adulthood. These effects are caused by in utero exposure to maternal IL-5 and resulting fetal eosinophilia. Allergen exposure of these adult wild-type offspring results in severe airway hyperreactivity, leading to fatal reflex bronchoconstriction. Our results demonstrate that fetal exposure to IL-5 is a developmental origin of airway hyperreactivity, mediated by hyperinnervation of airway epithelium.
Keywords: asthma, airway hyperreactivity, eosinophil, sensory nerves, pregnancy
Asthma is a chronic airway disease that often begins during childhood and is characterized by airway hyperreactivity and inflammation. Airway hyperreactivity can be detected at birth and predicts future development of asthma in humans (1), suggesting that prenatal programming influences airway reactivity that, in combination with ex utero exposures, shapes lifelong lung health. Parental asthma is a particularly important risk factor for development of asthma in children (2–4). However, maternal asthma confers more risk than paternal asthma (5), and better asthma control during pregnancy decreases a child’s risk of developing asthma (6) and wheezy illnesses (7). Therefore, in utero exposure to maternal inflammation may be a unique risk factor for childhood asthma.
A majority of individuals with asthma have eosinophil-predominant inflammation mediated by increased expression of type 2 cytokines, such as IL-5, which stimulates eosinophil hematopoiesis, recruitment, and survival (8). During pregnancy, an increased ratio of maternal type 2 versus type 1 cytokines is associated with childhood asthma (9), suggesting that in utero cytokine exposures influence offspring airway reactivity. Similarly, treating allergen-exposed pregnant mice with IFN-γ (10) or IL-4 antagonists (11) reduces airway hyperreactivity in their offspring.
Type 2 cytokines may cause airway hyperreactivity in offspring by affecting airway nerves, given that nerves control airway tone, bronchoconstriction, and airway reactivity. Airway sensory nerves, originating in the nodose and jugular ganglia, innervate airway epithelium and respond to noxious inhaled stimuli. Activation of sensory nerves initiates a central neuronal reflex that stimulates efferent airway parasympathetic nerves, causing bronchoconstriction via release of acetylcholine onto smooth muscle M3 muscarinic receptors. Reflex bronchoconstriction is blocked by vagotomy, which simultaneously eliminates afferent and efferent nerve signaling.
In asthma, eosinophils increase bronchoconstriction by inducing parasympathetic and sensory nerve dysfunction. For example, adults with eosinophilic asthma have increased airway sensory innervation (12), which correlates with worse airflow obstruction. Similarly, eosinophils cause sensory nerve growth in airways of adult mice with chronic eosinophilia (12) and in skin of mice and humans with eosinophilic dermatitis (13). Eosinophils also increase acetylcholine release from parasympathetic nerves. Under normal conditions, acetylcholine activates prejunctional inhibitory M2 muscarinic receptors on parasympathetic nerves, which limits further acetylcholine release (14). In asthma, eosinophil major basic protein blocks M2 receptors (15) and their critical role inhibiting acetylcholine release is lost (16). Eosinophils are actively recruited to nerves via neuronal release of eotaxin (17), and physically adhere to nerves by binding ICAM (18). In a study of adults who died of fatal asthma, eosinophils were found to be increased specifically around airway nerves (19). Similarly, nerve-associated eosinophils were found to be increased in the airways of animals after antigen challenge (19). Nerve dysfunction is clearly relevant in humans with asthma because selective M3 muscarinic antagonists that block nerve reflex–induced bronchoconstriction, such as tiotropium, improve lung function in adults (20, 21) and children with asthma (22). Thus, interactions between eosinophils and airway nerves have profound consequences for the neuronal regulation of bronchoconstriction.
In this study, we tested whether airway hyperinnervation seen both in humans with type 2 high asthma and in mice with elevated IL-5 occurs during development and is influenced by maternal eosinophilic inflammation. Female IL-5 transgenic (IL5tg) mice were bred with wild-type (WT) male mice and the resulting WT offspring were tested for airway reactivity, inflammation, and airway innervation at baseline and after ex utero exposure to house dust mite (HDM). Some data from these studies have been previously reported as an abstract (23).
Methods
Mice
WT, eosinophil-deficient, and IL5tg male and female mice (all on a C57Bl/6J background) were used at ≥8 weeks of age for experiments. Eosinophil-deficient mice express diphtheria toxin under the eosinophil peroxidase promoter (24), causing congenital eosinophil deficiency. IL5tg mice have hemizygous expression of IL-5 under a CC10 promoter (25). The breeding strategy and genotyping methods used are described in the data supplement. Transgenic mice were a gift from James Lee (Mayo Clinic in Arizona). WT mice were purchased from The Jackson Laboratory. Mice were housed in the vivarium at Oregon Health and Science University with ad libitum access to food and water on a 12-hour light/dark cycle. Oregon Health and Science University’s Institutional Animal Care and Use Committee approved all experimental protocols. All data are available from the corresponding author upon reasonable request.
Allergen Sensitization and Challenge
Adult offspring mice were sensitized and challenged with HDM or vehicle as detailed in the data supplement. Control animals that were not exposed to isoflurane were also analyzed as detailed in the data supplement.
Airway Physiology
Bronchoconstriction in response to inhaled serotonin (26) was measured as detailed in the data supplement.
Adult and Fetal Tissue Collection
Details regarding the collection of airways, blood, and amniotic fluid are provided in the data supplement. IL-5, IL-13, GDNF (glia-derived neurotrophic factor), BDNF (brain-derived neurotrophic factor), eotaxin, and substance P (all from R&D Systems); NGF (nerve growth factor; Sigma); and NT4 (neurotrophin-4; LSBio) were measured in lavage fluid by ELISA.
Tissue Optical Clearing and Epithelial Nerve Modeling
Epithelial nerve density was quantified using confocal microscopy and three-dimensional nerve models as described by Scott and colleagues (27) and detailed in the data supplement.
Nodose/Jugular Ganglia Retrograde Tracing
Neurons were retrograde labeled as detailed in the data supplement.
Statistical Analysis
Data were graphed as the mean ± SEM and analyzed using GraphPad Prism 7. Each experimental group contained 5–10 individual animals that were derived from at least three separate litters. Littermates were limited to two offspring per treatment group to prevent overrepresentation of a single pregnancy. Serotonin dose–response curves were analyzed with a repeated-measures two-way ANOVA. Serotonin dose–response curves were tested in one or two animals from multiple treatment groups at a given time alongside tested control animals. Thus, Holm-Sidak’s post test was used to correct for multiple comparisons and control groups are shown for comparison in more than one graph, as indicated in the figure legend. The reported P values represent the post-test analysis. For experiments in which mice died of severe bronchoconstriction during the serotonin-dose response, data were graphed and analyzed only when all animals were still living (denoted in graphs with a truncated dose–response curve). Fetal blood eosinophils, amniotic fluid IL-5, PGP9.5 nerve length, and nodose/jugular ganglia neuron data were analyzed with an unpaired, two-tailed t test. All other data were analyzed with a two-way ANOVA (maternal genotype × offspring treatment) with Sidak’s post test and the reported P values represent either the main group effect (maternal treatment) or the post-test statistical value when significant.
Results
In Utero Exposure to IL-5 Increases Reflex Bronchoconstriction but Not Airway Inflammation
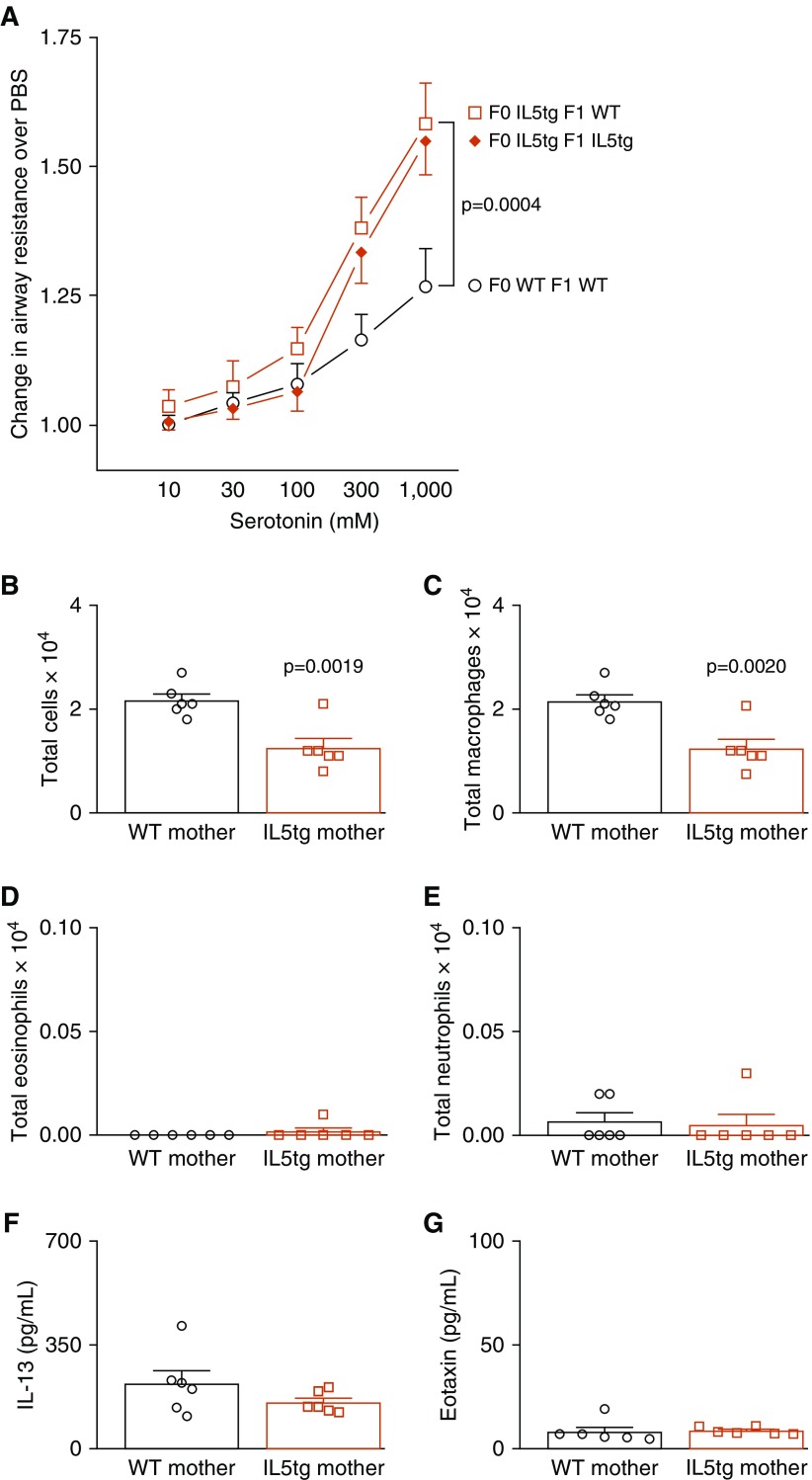
Baseline airway resistance before serotonin administration was the same among all offspring irrespective of maternal genotype. Bronchoconstriction caused by inhaled serotonin was significantly greater in adult WT offspring of IL5tg mice than in WT offspring of WT mice (Figure 1A). This appears to be fixed in utero, as airway reactivity was not further potentiated by continued postnatal exposure to IL-5 (see Figure 1A comparing WT mice exposed to maternal IL-5 with IL5tg mice that were also exposed to maternal IL-5).
Figure 1.
In utero exposure to IL-5 increased airway reactivity. (A) Exposure to maternal IL-5 increased reflex bronchoconstriction in adult wild-type (WT) offspring (open square, n = 6) compared with WT offspring of WT mice (open circle, n = 6). WT offspring exposed to maternal IL-5 in utero had the same airway hyperreactivity as IL-5 transgenic (IL5tg) offspring that inherited the IL-5 transgene (solid diamond, n = 8). (B–E) Airway inflammation was decreased in adult WT offspring born to IL5tg mice compared with WT offspring born to WT mice (B, P = 0.0019), with a decrease in macrophages (C, P = 0.0020) and no change in eosinophils (D) or neutrophils (E). (F and G) Lavage fluid IL-13 (F) and eotaxin (G) were the same in all offspring irrespective of maternal genotype. Data are presented as mean ± SEM.
Increased airway responsiveness to serotonin was unrelated to inflammation in the lung. Adult offspring of IL5tg mice had fewer total inflammatory cells than offspring of WT mice (Figure 1B) owing to a reduction in airway macrophages (Figure 1C). No differences in eosinophils (Figure 1D), neutrophils (Figure 1E), or lymphocytes (none detected) were observed among the offspring. There were also no differences in lavage IL-13 (Figure 1F), eotaxin (Figure 1G), or IL-5 (data for IL-5 are not shown, because it was below the limit of detection in lavage from all mice, irrespective of maternal genotype).
In Utero Exposure to IL-5 Potentiates Airway Hyperreactivity and Inflammation after HDM Exposure
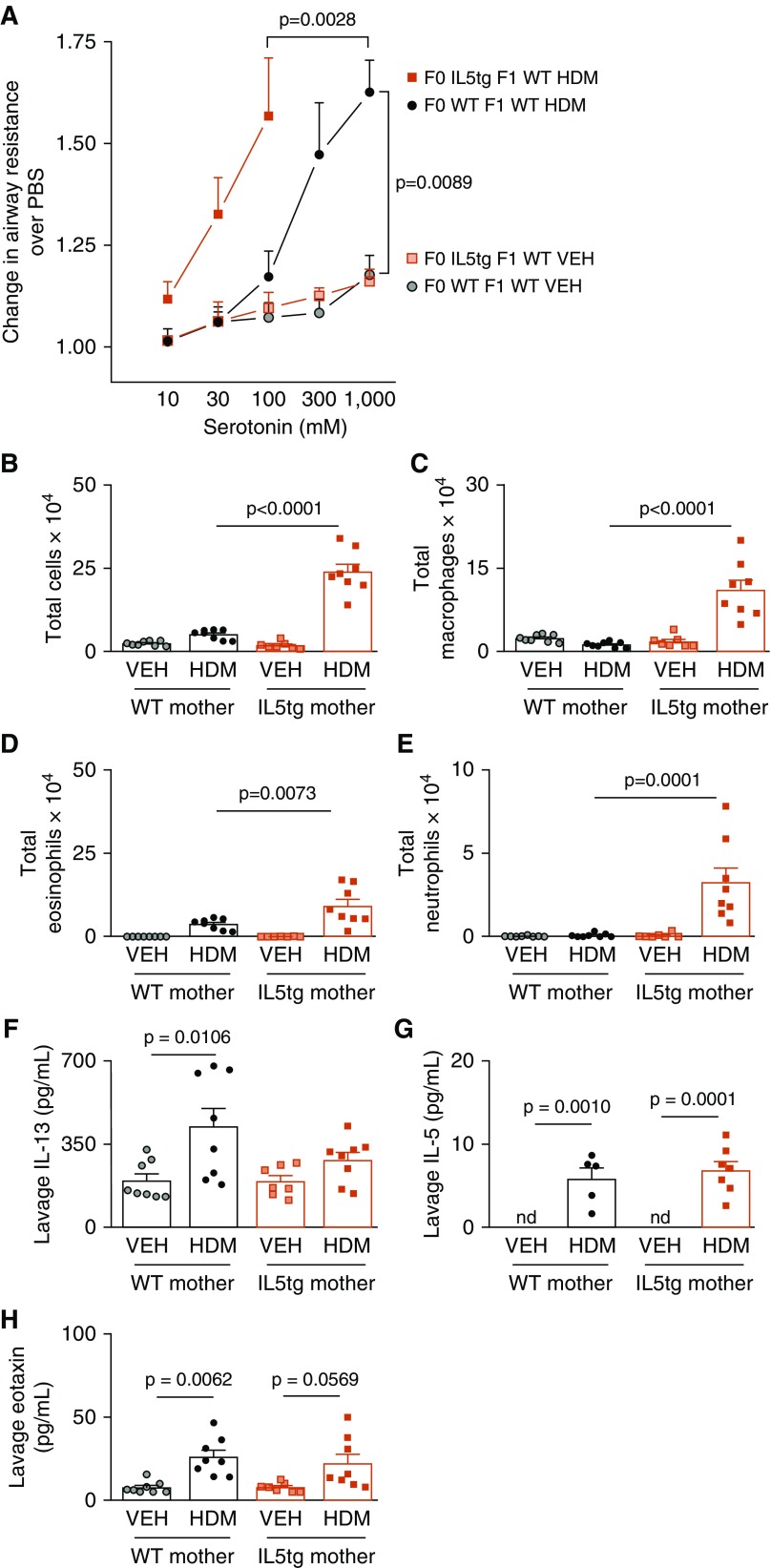
Sensitization and challenge with HDM caused significant airway hyperreactivity in all offspring (Figure 2A). However, HDM-induced airway hyperreactivity was significantly greater in offspring born to IL5tg mice than in offspring born to WT mice (Figure 2A). The dose–response curve for HDM-exposed WT offspring of IL5tg mice is truncated because bronchoconstriction was so severe in this group that only one of eight offspring survived the full dose–response curve (three died before administration of 300 mM serotonin and four died before administration of 1,000 mM serotonin).
Figure 2.
Maternal IL-5 increased offspring house dust mite (HDM)-induced airway hyperreactivity. (A) Airway reactivity was the same between vehicle (VEH)-exposed WT offspring born to IL5tg mice and WT mice (red shaded square, VEH-exposed WT offspring of IL5tg mice, n = 7; gray shaded circle, VEH-exposed WT offspring of WT mice, n = 8). Exposure to maternal IL-5 caused lethal HDM-induced airway hyperreactivity in WT offspring of IL5tg mice (red solid square, n = 8) compared with WT offspring of WT mice (black solid circle, n = 8). (B–E) Exposure to maternal IL-5 potentiated HDM-induced airway inflammation in WT offspring, including total cells (B, P < 0.0001), macrophages (C, P < 0.0001), eosinophils (D, P = 0.0073), and neutrophils (E, P = 0.0001), and as compared with HDM-exposed WT offspring of WT mice. (F–H) HDM significantly increased all inflammatory cells in WT offspring of IL5tg mice compared with VEH controls; however, P values were omitted for clarity where maternal genotype had effect. Exposure to maternal IL-5 in utero did not affect offspring lavage IL-13 (F), IL-5 (G), or eotaxin (H). Three HDM-exposed offspring of WT mice and one HDM-exposed offspring of IL-5tg mice had insufficient lavage fluid to measure IL-5 in replicate; thus, n = 5 and n = 7, respectively. Data are presented as mean ± SEM. nd = not detected.
HDM exposure increased airway inflammatory cells in all offspring, but offspring exposed to IL-5 in utero had significantly more macrophages, eosinophils, and neutrophils in their airways than the HDM-challenged offspring of WT mice (Figures 2B–2E). HDM exposure increased IL-13, IL-5, and eotaxin in WT offspring of WT mice, whereas it only significantly increased IL-5 in WT offspring of IL5tg mice (Figures 2F–2H).
In Utero Exposure to IL-5 Increases Fetal Eosinophils, which Are Required for Airway Hyperreactivity
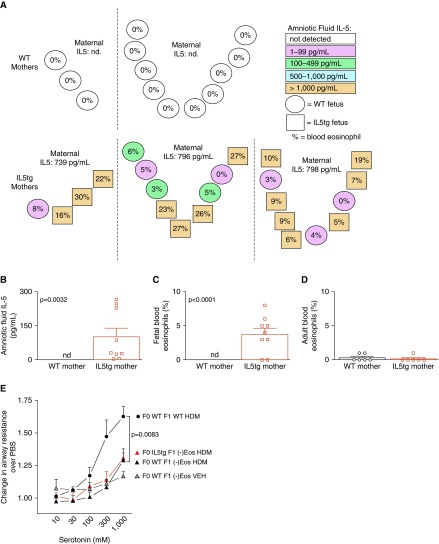
To test whether maternal IL-5 crossed the placenta and caused fetal eosinophilia, we collected fetal blood and amniotic fluid from IL5tg and WT mice between Gestation Days 18 and 20. In WT mice, IL-5 was below the limit of detection in amniotic fluid and there were no blood eosinophils in these fetuses (Figure 3A). In contrast, WT fetuses from IL5tg mice had elevated IL-5 in both the amniotic fluid and blood eosinophilia (Figures 3A–3C). Figure 3A illustrates the location of individual fetuses in utero, amniotic fluid IL-5 (color), and peripheral blood eosinophil percentages (%). Note that WT offspring developing between two IL5tg offspring do not have more IL-5 than WT offspring developing next to other WT offspring, suggesting that amniotic fluid IL-5 in WT offspring is likely derived solely from the mother and not from neighboring IL5tg offspring. Increased levels of fetal IL-5 and eosinophilia were not sustained into adulthood in WT offspring of IL5tg mice. At 8 weeks of age, IL-5 levels were undetectable and peripheral blood eosinophils were not increased compared with WT offspring of WT mice (Figure 3D).
Figure 3.
Eosinophils were required for offspring airway hyperreactivity. (A) Amniotic fluid IL-5 and peripheral eosinophils were elevated in WT fetuses of IL5tg mice. Each circle or square represents one fetus, with circles denoting WT fetuses and squares denoting IL5tg fetuses. The spatial orientation of circles and squares denotes the fetal position in utero (n = 2 WT pregnancies and n = 3 IL5tg pregnancies). Circle or square shading indicates the amniotic IL-5 level, and the percentage indicates the fetal blood eosinophil count. The maternal serum IL-5 level is denoted above each cluster of fetuses. (B and C) Graphical representation of (B) amniotic fluid IL-5 levels and (C) fetal blood eosinophil percentages presented in A. (D) Adult WT offspring born to IL5tg mice did not have peripheral eosinophilia. (E) Congenital eosinophil deficiency ([−]Eos) suppressed airway hyperreactivity in HDM-exposed offspring of both WT (solid black triangle, n = 7) and IL5tg mice (solid red triangle, n = 5, VEH: solid gray triangle, n = 8) (WT HDM vs. [−]Eos HDM, P = 0.0083). Data are presented as mean ± SEM. Note that HDM-exposed WT offspring of WT mice (solid black circle) were the same group shown in Figure 2A.
To test whether airway hyperresponsiveness caused by in utero exposure to IL-5 requires eosinophils in the developing fetus, we crossed IL5tg female mice with eosinophil-deficient male mice, resulting in eosinophil-deficient offspring that were exposed to maternal IL-5 in utero. Airway reactivity in response to serotonin was similar between eosinophil-deficient offspring born to IL5tg mice and those born to WT mice (Figure 3E), demonstrating that fetal eosinophils were required for development of airway hyperreactivity in offspring.
In Utero Exposure to IL-5 Increases Airway Innervation in Offspring
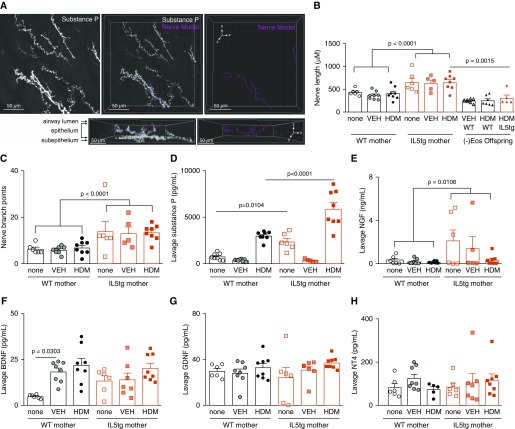
Airway epithelial nerve density was increased in WT offspring of IL5tg mice compared with WT offspring of WT mice (total length of PGP9.5+ nerves in airway epithelium: 905.1 ± 70.0 μM in offspring of IL5tg mice vs. 669.8 ± 59.9 μM in offspring of WT mice; P = 0.0229). Increased nerve lengths were also observed in the subpopulation of nerves expressing substance P (Figures 4A–4C), which corresponded with increased lavage substance P in these animals (Figure 4D). Congenital eosinophil deficiency prevented hyperinnervation caused by exposure to maternal IL-5 in utero (Figure 4B).
Figure 4.
Maternal IL-5 increased airway sensory innervation in offspring. Whole tracheas were stained with substance P and PGP9.5 antibodies, optically cleared, and imaged with a laser scanning microscope (63×, 1.4 numerical aperture). Three 63× images per animal were taken in the posterior midline at the level of the main bronchi bifurcation, with the z-stack depth extending from the serosal surface through the airway lumen. (A) Maximum intensity projection of a z-stack confocal image with substance P+ epithelial and subepithelial nerves (white), with an adjacent three-dimensional model of epithelial nerves (pink). The nerve models are full z-stack images oriented with the line of sight in the z-plane. The same image rotated around the x-axis demonstrates the epithelial location of the nerve models. (B–D) Maternal IL-5 increased airway substance P+ nerve length (B, P < 0.0001), branching (C, P < 0.0001), and lavage substance P (D) in offspring (circles represent WT offspring from WT mothers, squares represent WT offspring from IL5tg mothers, triangles represent eosinophil deficient offspring from WT mothers, diamonds represent eosinophil deficient offspring from IL5tg mothers). Eosinophil deficiency suppressed airway sensory hyperinnervation in offspring exposed to maternal IL-5 (B, P = 0.0015). (E–H) Maternal IL-5 increased WT offspring lavage NGF (nerve growth factor) (E, P = 0.0106) compared with offspring of WT mice, but did not change offspring lavage BDNF (brain-derived neurotrophic factor) (F), GDNF (glia-derived neurotrophic factor) (G), or NT4 (neurotrophin-4) (H). Data are presented as mean ± SEM. n = 8 VEH-exposed and n = 8 HDM-exposed offspring of WT mice, and n = 7 VEH-exposed and n = 8 HDM-exposed offspring of IL5tg mice for all measurements, with the following exceptions: lavage NGF n = 5 for VEH-exposed offspring of IL5tg mice, lavage NT4 n = 5 for HDM-exposed offspring of WT mice, and nerve length and branching n = 5 for VEH-exposed offspring of IL5tg mice.
Pulmonary-specific sensory nerves were labeled with the retrograde fluorescent tracer wheat germ agglutinin. There was no difference in the number of vagal nodose/jugular neurons innervating the lungs among offspring of mice with different genotypes (Figure E2). Thus, increased nerve density is due to increased structural complexity of sensory nerve axons rather than an increased number of nerves in the ganglia.
Among the four neurotrophins measured, only NGF was significantly increased in lavage from WT offspring of IL5tg mice compared with WT offspring of WT mice (Figure 4E). There was no significant difference in BDNF (Figure 4F), GDNF (Figure 4G), or NT4 (Figure 4H) in BAL fluid. HDM exposure did not increase any of the neurotrophins measured (Figures 4E–4H).
Maternal IL-5 Changes the Mechanism of Airway Hyperreactivity
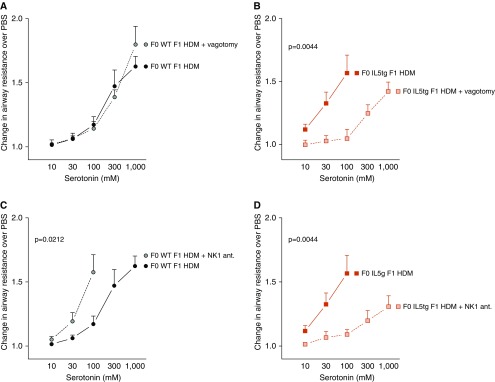
Airway hyperreactivity was similar in HDM-exposed WT offspring of WT mice before and after vagotomy (Figure 5A), indicating that vagal reflexes do not mediate serotonin-induced bronchoconstriction in WT offspring born to WT mice after acute HDM exposure. In contrast, vagotomy significantly reduced airway hyperreactivity in HDM-exposed WT offspring of IL5tg mice (Figure 5B). All vagotomized mice survived the dose–response curve, whereas seven of eight mice with intact vagus nerves died of lethal bronchoconstriction. Thus, IL-5 exposure in utero causes airway hyperreactivity in offspring by potentiating vagal reflex–mediated bronchoconstriction.
Figure 5.
Nerve-mediated airway hyperreactivity in offspring exposed to maternal IL-5. (A) Cutting the vagus nerves (solid gray circle, n = 8) did not inhibit serotonin-induced bronchoconstriction in HDM-exposed WT offspring of WT mice (vagus nerve intact, HDM-exposed mice, solid black circle, n = 8). (B) Cutting the vagus nerves (shaded red square, n = 6) suppressed hyperreactivity in HDM-exposed WT offspring of IL5tg mice (solid red square, HDM-exposed offspring of IL5tg mice intact vagus nerves, n = 8, P = 0.0128). (C) Blocking neurokinin-1 (NK1) receptors (antagonist [ant.], solid gray circle, n = 6) potentiated hyperreactivity in HDM-exposed WT offspring of WT mice (solid black circle, n = 8). (D) Blocking NK1 receptors (shaded red square, n = 7) suppressed hyperreactivity in HDM-exposed WT offspring of IL5tg mice (solid red square = HDM-exposed offspring of IL5tg mice, n = 8). Data are presented as mean ± SEM. Note that HDM-exposed WT offspring of WT mice (solid black circle) were the same group shown in Figures 2A and 3E.
In vagotomized animals, airway reactivity to inhaled methacholine was similar among all offspring regardless of maternal genotype, indicating that airway hyperreactivity in the offspring of IL5tg mice was not due to enhanced smooth muscle contractility (Figure E3).
We next tested whether sensory tachykinins contributed to airway hyperreactivity in offspring of IL5tg mice by pharmacologically blocking substance P receptors with the NK1 (neurokinin-1) antagonist CP99994. Surprisingly, pretreatment with the NK1 antagonist markedly worsened airway hyperreactivity in WT offspring of WT mice (Figure 5C). In the presence of the NK1 antagonist (1 μg i.p.), bronchoconstriction was so severe that only two of six survived the full serotonin dose–response curve (two died before administration of 300 mM serotonin and two died before administration of 1,000 mM serotonin). In contrast, the NK1 antagonist suppressed HDM-induced airway hyperreactivity in WT offspring born to IL5tg mice (Figure 5D). These data demonstrate that maternal IL-5 changes the role of substance P in offspring. Substance P is protective against airway hyperreactivity in WT offspring of WT mice, whereas airway hyperreactivity is mediated by substance P and NK1 receptors in WT offspring of IL5tg mice.
Discussion
We have shown that maternal IL-5 crosses the placenta and causes eosinophilia in the fetus, which increases airway sensory innervation and reflex bronchoconstriction. After birth, because there is no longer a source of IL-5 in the WT offspring, the eosinophilia disappears. However, the sensory hyperinnervation persists into adulthood, causing reflex-mediated airway hyperresponsiveness. Fetal eosinophilia, and not a direct effect of IL-5, is responsible for the hyperinnervation and hyperresponsiveness, as the effect was not seen when the fetus was eosinophil deficient.
Airway hyperinnervation has also been found in humans with eosinophilic asthma (12), and its presence may explain why therapies that deplete airway eosinophils, such as anti–IL-5 monoclonal antibodies, do not reduce airway hyperreactivity in adults with asthma (28) even though they reduce exacerbations (29). Whether blocking IL-5 in pregnant women with type 2 high asthma reduces the risk of asthma in their infants has not been tested. However, this approach is promising given that previous studies found that tighter control of asthma in pregnant women reduced the risk of asthma in their children (6).
The importance of persistent hyperinnervation in our studies is underscored by the effects of subsequent inflammation. HDM sensitization and challenge of the adult offspring of IL5tg mice further increased airway responsiveness, with reflex bronchoconstriction so severe that the mice died of bronchoconstriction at a dose of serotonin that caused little or no bronchoconstriction in normal controls (Figure 2A). Vagotomy substantially decreased bronchoconstriction in these animals, so that all WT offspring of IL5tg mice survived the full serotonin dose–response curve, clearly establishing that IL-5–induced airway hyperreactivity was mediated by a vagal reflex (Figure 5B). Likewise, treatment of these severely hyperresponsive mice with an NK1 antagonist markedly decreased responsiveness and prevented lethal bronchoconstriction (Figure 5D). These findings highlight an important difference between offspring of IL5tg mice and offspring of WT mice. In WT offspring of WT mice (which did not have increased nerve density), HDM-induced airway hyperreactivity was unaffected by vagotomy, indicating that vagal reflexes had little role in airway hyperreactivity after acute HDM exposure in these offspring.
In our study, offspring of IL5tg mice had more airway inflammation after HDM exposure than HDM-exposed offspring of WT mice (Figure 2B). Similarly, in a previous study, maternal HDM exposure during pregnancy potentiated airway inflammation and type 2 cytokines after HDM challenge in offspring (30). Neurons secrete chemoattractant factors (17) to recruit immune cells (19, 31, 32), including eotaxin, which attracts eosinophils specifically. In addition, neurotransmitters such as substance P activate immune cells (33) and potentiate immune responses (34). We postulate that increased baseline airway innervation in the offspring of IL5tg mice mediated the increased inflammatory cell recruitment after HDM sensitization and challenge, which in turn contributed to severe airway hyperreactivity. In humans, heightened airway responses in early infancy predict later development of asthma (35). Thus, it may be possible to prevent allergen-induced airway hyperreactivity in children if airway hyperinnervation is prevented in utero or decreased after birth.
IL-5–induced fetal eosinophilia may promote nerve growth by increasing expression of the neurotrophin NGF. Previous studies found that several neurotrophins, including NGF, increase sensory innervation in airways (36–39) and in the skin (13). NGF is also elevated in patients with asthma (40). NGF increases substance P expression (41) and controls the development of substance P–expressing sensory nerves (42). Innervation targets secrete NGF to promote both the development and maintenance of substance P–expressing nerve populations (43). Thus, the increased density of substance P–expressing epithelial nerves in the offspring of IL5tg mice may be caused by increased NGF expression by airway epithelium. In contrast to NGF, we observed no change in other neurotrophins, including BDNF, NT4, and GDNF. However, others have shown that NT4 increases airway innervation after allergen exposure in the immediate postnatal period (38, 39), possibly indicating that the timing of an inflammatory insult during development may determine neurotrophin expression and subsequent nerve growth.
We have shown a novel mechanism for in utero programming of airway hyperreactivity that is mediated by maternal IL-5 and fetal eosinophils. Exposure to maternal IL-5 causes profound changes in airway nerve structure in offspring, increasing nerve density and nerve-mediated airway hyperreactivity that persists into adulthood. Our results provide a mechanistic basis for human studies showing that tight control of asthma during pregnancy decreases the incidence of childhood asthma (6), and also suggest that selective targeting of type 2 inflammation during pregnancy may reduce the risk of childhood asthma. Furthermore, individuals with asthma and increased airway innervation may represent a distinct phenotype of asthma that would benefit the most from nerve-targeted therapies, such as anticholinergics and NK1 antagonists. The persistence of increased epithelial neurotrophins in offspring in our study may represent an additional therapeutic target. Understanding the mechanisms of maternal–fetal transmission of asthma may lead to novel therapeutic strategies for both mothers and children.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank James Lee for contributing reagents and animals. They also thank Z. Nie, G. D. Scott, S. Kaech Petri, and A. Snyder for technical help.
Footnotes
Supported by National Institutes of Health grants F30HL132414, R01HL124165, K08HL121254, R01HL113023, R01HL131525, HL-83808, and R01HL144008.
Author Contributions: K.M.L. and D.B.J. designed the study. K.M.L., M.G.D., A.D.F., and D.B.J. contributed discussion to guide experiments. K.M.L. and L.B.H.-B. performed experiments and analyzed data. All authors wrote the manuscript.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2019-0214OC on December 10, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Håland G, Carlsen KC, Sandvik L, Devulapalli CS, Munthe-Kaas MC, Pettersen M, et al. ORAACLE. Reduced lung function at birth and the risk of asthma at 10 years of age. N Engl J Med. 2006;355:1682–1689. doi: 10.1056/NEJMoa052885. [DOI] [PubMed] [Google Scholar]
- 2.Kelly YJ, Brabin BJ, Milligan P, Heaf DP, Reid J, Pearson MG. Maternal asthma, premature birth, and the risk of respiratory morbidity in schoolchildren in Merseyside. Thorax. 1995;50:525–530. doi: 10.1136/thx.50.5.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paaso EM, Jaakkola MS, Rantala AK, Hugg TT, Jaakkola JJ. Allergic diseases and asthma in the family predict the persistence and onset-age of asthma: a prospective cohort study. Respir Res. 2014;15:152. doi: 10.1186/s12931-014-0152-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Young S, Le Souëf PN, Geelhoed GC, Stick SM, Turner KJ, Landau LI. The influence of a family history of asthma and parental smoking on airway responsiveness in early infancy. N Engl J Med. 1991;324:1168–1173. doi: 10.1056/NEJM199104253241704. [DOI] [PubMed] [Google Scholar]
- 5.Lim RH, Kobzik L, Dahl M. Risk for asthma in offspring of asthmatic mothers versus fathers: a meta-analysis. PLoS One. 2010;5:e10134. doi: 10.1371/journal.pone.0010134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morten M, Collison A, Murphy VE, Barker D, Oldmeadow C, Attia J, et al. Managing Asthma in Pregnancy (MAP) trial: FENO levels and childhood asthma. J Allergy Clin Immunol. 2018;142:1765–1772.e4. doi: 10.1016/j.jaci.2018.02.039. [DOI] [PubMed] [Google Scholar]
- 7.Mattes J, Murphy VE, Powell H, Gibson PG. Prenatal origins of bronchiolitis: protective effect of optimised asthma management during pregnancy. Thorax. 2014;69:383–384. doi: 10.1136/thoraxjnl-2013-203388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levy BD, Noel PJ, Freemer MM, Cloutier MM, Georas SN, Jarjour NN, et al. Future research directions in asthma: an NHLBI working group report. Am J Respir Crit Care Med. 2015;192:1366–1372. doi: 10.1164/rccm.201505-0963WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rothers J, Stern DA, Lohman IC, Spangenberg A, Wright AL, DeVries A, et al. Maternal cytokine profiles during pregnancy predict asthma in children of mothers without asthma. Am J Respir Cell Mol Biol. 2018;59:592–600. doi: 10.1165/rcmb.2017-0410OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lima C, Souza VM, Faquim-Mauro EL, Hoshida MS, Bevilacqua E, Macedo MS, et al. Modulation of the induction of lung and airway allergy in the offspring of IFN-gamma-treated mother mice. J Immunol. 2005;175:3554–3559. doi: 10.4049/jimmunol.175.6.3554. [DOI] [PubMed] [Google Scholar]
- 11.Hamada K, Suzaki Y, Goldman A, Ning YY, Goldsmith C, Palecanda A, et al. Allergen-independent maternal transmission of asthma susceptibility. J Immunol. 2003;170:1683–1689. doi: 10.4049/jimmunol.170.4.1683. [DOI] [PubMed] [Google Scholar]
- 12.Drake MG, Scott GD, Blum ED, Lebold KM, Nie Z, Lee JJ, et al. Eosinophils increase airway sensory nerve density in mice and in human asthma. Sci Transl Med. 2018;10:eaar8477. doi: 10.1126/scitranslmed.aar8477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foster EL, Simpson EL, Fredrikson LJ, Lee JJ, Lee NA, Fryer AD, et al. Eosinophils increase neuron branching in human and murine skin and in vitro. PLoS One. 2011;6:e22029. doi: 10.1371/journal.pone.0022029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fryer AD, Maclagan J. Muscarinic inhibitory receptors in pulmonary parasympathetic nerves in the guinea-pig. Br J Pharmacol. 1984;83:973–978. doi: 10.1111/j.1476-5381.1984.tb16539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evans CM, Fryer AD, Jacoby DB, Gleich GJ, Costello RW. Pretreatment with antibody to eosinophil major basic protein prevents hyperresponsiveness by protecting neuronal M2 muscarinic receptors in antigen-challenged guinea pigs. J Clin Invest. 1997;100:2254–2262. doi: 10.1172/JCI119763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Minette PA, Lammers JW, Dixon CM, McCusker MT, Barnes PJ. A muscarinic agonist inhibits reflex bronchoconstriction in normal but not in asthmatic subjects. J Appl Physiol (1985) 1989;67:2461–2465. doi: 10.1152/jappl.1989.67.6.2461. [DOI] [PubMed] [Google Scholar]
- 17.Fryer AD, Stein LH, Nie Z, Curtis DE, Evans CM, Hodgson ST, et al. Neuronal eotaxin and the effects of CCR3 antagonist on airway hyperreactivity and M2 receptor dysfunction. J Clin Invest. 2006;116:228–236. doi: 10.1172/JCI25423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sawatzky DA, Kingham PJ, Court E, Kumaravel B, Fryer AD, Jacoby DB, et al. Eosinophil adhesion to cholinergic nerves via ICAM-1 and VCAM-1 and associated eosinophil degranulation. Am J Physiol Lung Cell Mol Physiol. 2002;282:L1279–L1288. doi: 10.1152/ajplung.00279.2001. [DOI] [PubMed] [Google Scholar]
- 19.Costello RW, Schofield BH, Kephart GM, Gleich GJ, Jacoby DB, Fryer AD. Localization of eosinophils to airway nerves and effect on neuronal M2 muscarinic receptor function. Am J Physiol. 1997;273:L93–L103. doi: 10.1152/ajplung.1997.273.1.L93. [DOI] [PubMed] [Google Scholar]
- 20.Peters SP, Kunselman SJ, Icitovic N, Moore WC, Pascual R, Ameredes BT, et al. National Heart, Lung, and Blood Institute Asthma Clinical Research Network. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. N Engl J Med. 2010;363:1715–1726. doi: 10.1056/NEJMoa1008770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang L, Zhou R, Xie X. Tiotropium added to low- to medium-dose inhaled corticosteroids (ICS) versus low- to medium-dose ICS alone for adults with mild to moderate uncontrolled persistent asthma: a systematic review and meta-analysis. J Asthma. 2019;56:69–78. doi: 10.1080/02770903.2018.1424192. [DOI] [PubMed] [Google Scholar]
- 22.Szefler SJ, Murphy K, Harper T, III, Boner A, Laki I, Engel M, et al. A phase III randomized controlled trial of tiotropium add-on therapy in children with severe symptomatic asthma. J Allergy Clin Immunol. 2017;140:1277–1287. doi: 10.1016/j.jaci.2017.01.014. [DOI] [PubMed] [Google Scholar]
- 23.Lebold KM, Drake MG, Fryer AD, Lee NA, Lee JJ, Jacoby DB. Maternal interleukin-5 and/or eosinophils affect airway physiology of offspring. Eur Respir J. 2017;50:PA2089. [Google Scholar]
- 24.Lee JJ, Dimina D, Macias MP, Ochkur SI, McGarry MP, O’Neill KR, et al. Defining a link with asthma in mice congenitally deficient in eosinophils. Science. 2004;305:1773–1776. doi: 10.1126/science.1099472. [DOI] [PubMed] [Google Scholar]
- 25.Lee JJ, McGarry MP, Farmer SC, Denzler KL, Larson KA, Carrigan PE, et al. Interleukin-5 expression in the lung epithelium of transgenic mice leads to pulmonary changes pathognomonic of asthma. J Exp Med. 1997;185:2143–2156. doi: 10.1084/jem.185.12.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin TR, Gerard NP, Galli SJ, Drazen JM. Pulmonary responses to bronchoconstrictor agonists in the mouse. J Appl Physiol (1985) 1988;64:2318–2323. doi: 10.1152/jappl.1988.64.6.2318. [DOI] [PubMed] [Google Scholar]
- 27.Scott GD, Blum ED, Fryer AD, Jacoby DB. Tissue optical clearing, three-dimensional imaging, and computer morphometry in whole mouse lungs and human airways. Am J Respir Cell Mol Biol. 2014;51:43–55. doi: 10.1165/rcmb.2013-0284OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haldar P, Brightling CE, Hargadon B, Gupta S, Monteiro W, Sousa A, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009;360:973–984. doi: 10.1056/NEJMoa0808991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ortega HG, Liu MC, Pavord ID, Brusselle GG, FitzGerald JM, Chetta A, et al. MENSA Investigators. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371:1198–1207. doi: 10.1056/NEJMoa1403290. [DOI] [PubMed] [Google Scholar]
- 30.Richgels PK, Yamani A, Chougnet CA, Lewkowich IP. Maternal house dust mite exposure during pregnancy enhances severity of house dust mite-induced asthma in murine offspring. J Allergy Clin Immunol. 2017;140:1404–1415.e9. doi: 10.1016/j.jaci.2016.12.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moriyama S, Brestoff JR, Flamar AL, Moeller JB, Klose CSN, Rankin LC, et al. β2-adrenergic receptor-mediated negative regulation of group 2 innate lymphoid cell responses. Science. 2018;359:1056–1061. doi: 10.1126/science.aan4829. [DOI] [PubMed] [Google Scholar]
- 32.Veres TZ, Rochlitzer S, Shevchenko M, Fuchs B, Prenzler F, Nassenstein C, et al. Spatial interactions between dendritic cells and sensory nerves in allergic airway inflammation. Am J Respir Cell Mol Biol. 2007;37:553–561. doi: 10.1165/rcmb.2007-0087OC. [DOI] [PubMed] [Google Scholar]
- 33.Evans CM, Belmonte KE, Costello RW, Jacoby DB, Gleich GJ, Fryer AD. Substance P-induced airway hyperreactivity is mediated by neuronal M(2) receptor dysfunction. Am J Physiol Lung Cell Mol Physiol. 2000;279:L477–L486. doi: 10.1152/ajplung.2000.279.3.L477. [DOI] [PubMed] [Google Scholar]
- 34.Wallrapp A, Riesenfeld SJ, Burkett PR, Abdulnour RE, Nyman J, Dionne D, et al. The neuropeptide NMU amplifies ILC2-driven allergic lung inflammation. Nature. 2017;549:351–356. doi: 10.1038/nature24029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palmer LJ, Rye PJ, Gibson NA, Burton PR, Landau LI, Lesouëf PN. Airway responsiveness in early infancy predicts asthma, lung function, and respiratory symptoms by school age. Am J Respir Crit Care Med. 2001;163:37–42. doi: 10.1164/ajrccm.163.1.2005013. [DOI] [PubMed] [Google Scholar]
- 36.Hoyle GW, Graham RM, Finkelstein JB, Nguyen KP, Gozal D, Friedman M. Hyperinnervation of the airways in transgenic mice overexpressing nerve growth factor. Am J Respir Cell Mol Biol. 1998;18:149–157. doi: 10.1165/ajrcmb.18.2.2803m. [DOI] [PubMed] [Google Scholar]
- 37.Tollet J, Everett AW, Sparrow MP. Development of neural tissue and airway smooth muscle in fetal mouse lung explants: a role for glial-derived neurotrophic factor in lung innervation. Am J Respir Cell Mol Biol. 2002;26:420–429. doi: 10.1165/ajrcmb.26.4.4713. [DOI] [PubMed] [Google Scholar]
- 38.Aven L, Paez-Cortez J, Achey R, Krishnan R, Ram-Mohan S, Cruikshank WW, et al. An NT4/TrkB-dependent increase in innervation links early-life allergen exposure to persistent airway hyperreactivity. FASEB J. 2014;28:897–907. doi: 10.1096/fj.13-238212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patel KR, Aven L, Shao F, Krishnamoorthy N, Duvall MG, Levy BD, et al. Mast cell-derived neurotrophin 4 mediates allergen-induced airway hyperinnervation in early life. Mucosal Immunol. 2016;9:1466–1476. doi: 10.1038/mi.2016.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Noga O, Hanf G, Schäper C, O’Connor A, Kunkel G. The influence of inhalative corticosteroids on circulating nerve growth factor, brain-derived neurotrophic factor and neurotrophin-3 in allergic asthmatics. Clin Exp Allergy. 2001;31:1906–1912. doi: 10.1046/j.1365-2222.2001.01249.x. [DOI] [PubMed] [Google Scholar]
- 41.Lindsay RM, Harmar AJ. Nerve growth factor regulates expression of neuropeptide genes in adult sensory neurons. Nature. 1989;337:362–364. doi: 10.1038/337362a0. [DOI] [PubMed] [Google Scholar]
- 42.Otten U, Goedert M, Mayer N, Lembeck F. Requirement of nerve growth factor for development of substance P-containing sensory neurones. Nature. 1980;287:158–159. doi: 10.1038/287158a0. [DOI] [PubMed] [Google Scholar]
- 43.McMahon SB, Gibson S. Peptide expression is altered when afferent nerves reinnervate inappropriate tissue. Neurosci Lett. 1987;73:9–15. doi: 10.1016/0304-3940(87)90022-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.