Graphical abstract
Chemical compounds studied in this article: 3-Deazaneplanocin A (PubChem CID: 73087), BCX4430 (PubChem CID: 69211190), BCX-1777 (PubChem CID: 11493192), Favipiravir (PubChem CID: 492405), FGI-103 (PubChem CID: 5477931), NSC62914 (PubChem CID: 66662), LJ 001 (PubChem CID: 49777349), dUY11 (PubChem CID: 24771429), Clomifene (PubChem CID: 1548953), Amiodarone (PubChem CID: 2157), Dronedarone (PubChem CID: 208898), Verapamil (PubChem CID: 2520), Chloroquine (PubChem CID: 2719)
Keywords: Ebola, VSV (vesicular stomatitis virus), BCX4430, 3-Deazaneplanocin A, Favipiravir, Filoviridae
Abstract
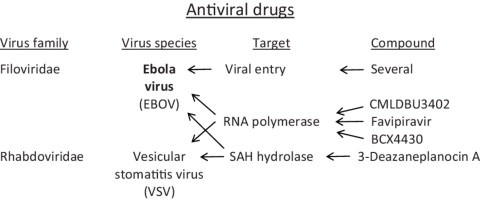
Within less than a year after its epidemic started (in December 2013) in Guinea, Ebola virus (EBOV), a member of the filoviridae, has spread over a number of West-African countries (Guinea, Sierra Leone and Liberia) and gained allures that have been unprecedented except by human immunodeficiency virus (HIV). Although EBOV is highly contagious and transmitted by direct contact with body fluids, it could be counteracted by the adequate chemoprophylactic and -therapeutic interventions: vaccines, antibodies, siRNAs (small interfering RNAs), interferons and chemical substances, i.e. neplanocin A derivatives (i.e. 3-deazaneplanocin A), BCX4430, favipiravir (T-705), endoplasmic reticulum (ER) α-glucosidase inhibitors and a variety of compounds that have been found to inhibit EBOV infection blocking viral entry or by a mode of action that still has to be resolved. Much has to be learned from the mechanism of action of the compounds active against VSV (vesicular stomatitis virus), a virus belonging to the rhabdoviridae, that in its mode of replication could be exemplary for the replication of filoviridae.
1. Introduction
On 23 March 2014, the World Health Organization (WHO) reported on a new outbreak of Ebola virus (EBOV) infection which began in December 2013 in the Republic of Guinea, initially in the Prefecture of Guéckédou [1], and which would shortly thereafter spread to other West African countries, viz. Sierra Leone and Liberia. The number of cases reported in Guinea, Liberia and Sierra Leone for the period of January–September 2014 (Fig. 1 ) [2] give little indication that the incidence of EBOV infection has begun to decline [3]. According to the WHO the EBOV epidemic is still growing and the doubling time was estimated 15.7 days in Guinea, 23.6 days in Liberia and 30.2 days in Sierra Leone [2]. EBOV infection is a severe hemorrhagic fever caused by the negative-stranded, non-segmented RNA virus belonging to the genus Ebolavirus (family Filoviridae, order Mononegavirales). The second genus in this family is Marburgvirus, causing a similar disease to EBOV infection; the third genus, Cuevavirus (prototype: Cueva del Lloviu) [4], is confined to bat hosts. Bats, and in particular the fruit bat, Myonycteris torquata, seem to be the leading suspect as the reservoir of Ebola virus infections, but the bats do not seem to get sick from the virus [5]. Humans, however, present with fever, headache, joint muscle and abdominal pain accompanied by diarrhea and vomiting after a highly variable incubation period of 1–25 days; in this stage, EBOV infection could be easily confused with other tropical fevers such as malaria or dengue, until the appearance of the hemorrhagic terminal phase presenting with the characteristic internal and subcutaneous bleedings [6]. To date treatment against EBOV infection is mostly asymptomatic and consists of rehydration, stabilization of blood pressure and control of fever and pain.
Fig. 1.
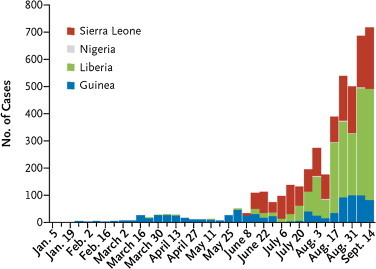
Numbers of confirmed and probable Ebola cases reported weekly from Guinea, Sierra Leone, and Liberia from January 5, 2014, to September 14, 2014 [2].
Reprinted with permission of the New England Journal of Medicine.
EBOV is subdivided into 5 species: Zaire (EBOV-Z), Sudan (EBOV-S), Reston (EBOV-R), Tai Forest (EBOV-TF), which was also known as Côte d’Ivoire Ebola virus until 2010, and Bundibugyo (EBOV-B) [7]. EBOV-Z and EBOV-S are the predominant EBOVs associated with known outbreaks, and are more pathogenic than EBOV-R, which has caused fatal infection only in non-human primates, and EBOV-TF, which has only caused a single non-fatal human infection [7]. EBOV-Z, EBOV-S and EBOV-B have often caused severe hemorrhagic disease with markedly high case fatality rates (40–90%) (Table 1 ) [8]. EBOV has been classified as a BSL4 (biosafety level 4) agent or Category A potential bioterrorism agent, by the Centers for Disease Control (CDC) and Prevention. It was first described in 1976 [9].
Table 1.
Ebola hemorrhagic fever cases in Africa (1976–2014).
| Year | Country | Town | Cases, n | Deaths, n | Species |
|---|---|---|---|---|---|
| 1976 | Democratic Republic of the Congo | Yambuku | 318 | 280 | EBOV |
| 1976 | South Sudan | Nzara | 284 | 151 | SUDV |
| 1977 | Democratic Republic of the Congo | Tandala | 1 | 1 | EBOV |
| 1979 | South Sudan | Nzara | 34 | 22 | SUDV |
| 1994 | Gabon | Mekouka | 52 | 31 | EBOV |
| 1994 | Ivory Coast | Tai Forest | 1 | 0 | TAFV |
| 1995 | Democratic Republic of the Congo | Kikwit | 315 | 250 | EBOV |
| 1996 | Gabon | Mayibout | 37 | 21 | EBOV |
| 1996 | Gabon | Booué | 60 | 45 | EBOV |
| 1996 | South Africa | Johannesburg | 2 | 1 | EBOV |
| 2000 | Uganda | Gulu | 425 | 224 | EBOV |
| 2001 | Gabon | Libreville | 65 | 53 | EBOV |
| 2001 | Republic of the Congo | Not specified | 57 | 43 | EBOV |
| 2002 | Republic of the Congo | Mbomo | 143 | 128 | EBOV |
| 2003 | Republic of the Congo | Mbomo | 35 | 29 | EBOV |
| 2004 | South Sudan | Yambio | 17 | 7 | EBOV |
| 2007 | Democratic Republic of the Congo | Luebo | 264 | 187 | EBOV |
| 2007 | Uganda | Bundibugyo | 149 | 37 | BDBV |
| 2008 | Democratic Republic of the Congo | Luebo | 32 | 15 | EBOV |
| 2011 | Uganda | Luwero District | 1 | 1 | SUDV |
| 2012 | Uganda | Kibaale District | 11a | 4a | SUDV |
| 2012 | Democratic Republic of the Congo | Isiro Health Zone | 36a | 13a | BDBV |
| 2012 | Uganda | Luwero District | 6a | 3a | SUDV |
| 2014 | Guinea, Sierra Leone, Liberia, Nigeria | Multiple | 1009a | 574a | EBOV |
BDBV, Bundibugyo virus; EBOV, Ebola virus; SUDV, Sudan virus; TAFV, Tai Forest Virus.
Laboratory-confirmed cases only.
According to Del Rio et al. [8].
The filoviridae (Ebola, Marburg), together with the paramyxoviridae, rhabdoviridae and bornaviridae, belong to the order of the Mononegavirales. Ebola virus has a uniform diameter of 80 nm and form filaments of 800–1100 nm long (Fig. 2 ) [10]. The classical virion contains a single genome copy, but polyploid virions have also been described that contain two or more copies of the genome [11]. The viral RNA genome encodes seven proteins: NP (nucleoprotein), VP35 (polymerase cofactor), VP40 (matrix protein), GP (glycoprotein), VP30 (transcription activator), VP24 (secondary matrix protein), and L (“Large”), RNA-dependent RNA polymerase [6]. Whereas NP, VP24 and GP may be involved in viral entry, the L polymerase may be an attractive target for viral RNA synthesis inhibitors.
Fig. 2.
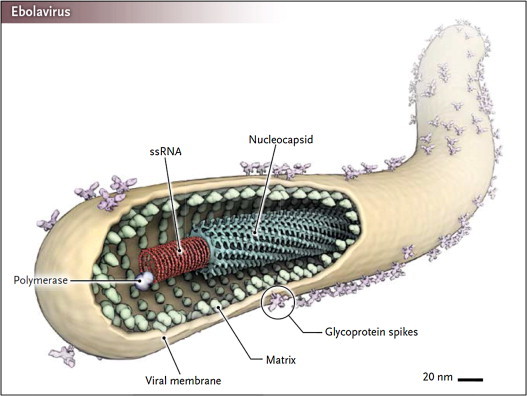
Structure of Ebola virus. An ebolavirus particle and its characteristic filamentous shape are shown. The negative-strand RNA genome is found in the center of particles in an encapsidated form as the nucleocapsid, together with the polymerase complex. Embedded in the virus membrane are trimeric glycoprotein spikes. Beneath the membrane is the matrix protein, which facilitates morphogenesis and budding of virus particles [10].
Reprinted with permission of the New England Journal of Medicine.
2. Post-exposure (non-antiviral) strategies
Therapeutic strategies against EBOV infection can be classified into different categories according to their target of action: (i) recombinant nematode anticoagulant protein c2 (rNAPc2) [12] and recombinant human activated protein C (rhAPC), which are aimed at treating clinical symptoms of coagulopathy and sepsis, respectively, which are observed in infected patients but not specific for EBOV infection; (ii) small, interfering RNAs (siRNAs) such as the positively charged phosphorodiamidate morpholino oligomers (PMO plus) and (iii) monoclonal antibodies (mAbs) to suppress viremia and virus spread [13]. PMO plus antisense therapies have been shown to protect > 60% of rhesus monkeys against EBOV-Z and 100% of cynomolgus monkeys against Marburg virus infection [14], and the safety and pharmacokinetic profiles of PMO plus (AVI-6002, AVI-6003) have been further documented [15]. The PMO AVI-6002 is composed of AVI-7537 and AVI-7539 and AVI-6003 is composed of AVI-7287 and AVI-7288. AVI-7537 targets the VP24 gene of EBOV and AVI-7288 targets the NP gene of Marburg virus. They are now progressing to the late stage of clinical development [16]. Meanwhile, the potential of siRNAs as a postexposure treatment strategy for people infected with EBV has been convincingly demonstrated [17]. Post-exposure antibody prophylaxis has been shown to protect nonhuman primates (NHPs) from filovirus (either Marburg or Ebola virus) infections, even when delayed for 48 hours [18]. The reversion of advanced EBOV infection in nonhuman primates with ZMapp (100% protection of rhesus macaques) [19] has had such an impact that ethical considerations have trespassed the demand for the material [20].
3. Vaccination
The time to deploy Ebola vaccines has now come [21]. Viable Ebola vaccine candidates are rVSV (recombinant vesicular stomatitis virus) + EBOV-Z-GP (glycoprotein), rRABV (recombinant rabies virus) + EBOV-Z-GP, rAd5 (recombinant adenovirus serotype 5) + EBOV-Z-GP, VLP (virus-like particles) + EBOV-Z-GP, rHPIV3 (recombinant human parainfluenza virus type 3) + EBOV-Z-GP, rCMV (recombinant cytomegalovirus) + EBOV-Z-NP (nucleoprotein) and rEBOV (recombinant Ebola virus) subunit vaccine + TLR (toll-like receptor) agonist [21]. That it would be feasible to develop a preventive vaccine against Ebola virus infection in primates, i.e. cynomolgus macaques, was already demonstrated in 2000 by Nabel and his co-workers [22]. VLPs have subsequently been shown to protect nonhuman primates against a lethal Ebola virus challenge [23]; VSV-based vaccines expressing the EBOV-Z glycoprotein completely protect cynomolgus macaques against an aerosol challenge of EBOV-Z [24]. Complete protection in cynomolgus macaques against Bundibugyo Ebola virus challenge was also achieved with a VSV-based vaccine [25]. A single intramuscular vaccination with Venezuelan equine encephalitis virus (VEEV) replicon particle (VRP) expressing EBOV-S-GP combined with VRP expressing EBOV GP provided complete protection against intramuscular challenge with either EBOV-S or EBOV-Z in cynomolgus macaques [26]. Antibodies play a critical role in rVSV- EBOV-Z-GP-mediated protection against a lethal EBOV-Z challenge in cynomolgus macaques [27]. A highly immunogenic fragment [MFL (aa 393–556)] has been derived from EBOV-Z-GP that elicits high levels of neutralizing antibody in mice [28]. And a VLP vaccine would hold great potential in the fight against wild ape extinction, as it could be used for vaccinating captive chimpanzees to protect wild chimpanzees [29]. While several phase I vaccination clinical trials are in progress or about to start, it is not expected to get any vaccine commercially available before the end of 2015.
4. Interferon
Although interferon was discovered at the end of the 1950s [30], its medical use has been limited, essentially because of its severe side effects (which are, in principle, similar to those that are experienced during an acute influenza virus infection). Yet, interferon has for the last decade, been part, together with ribavirin, of the standard of care (SOC) in the treatment of hepatitis C [31], [32], [33]. Whenever a new virus emerges (or re-emerges), however, so does the potential use of interferon. This was the case, in 2003, at the outbreak of the SARS coronavirus epidemic [34], and now is interferon envisaged again for the therapy of EBOV infections [35]. From a practical viewpoint, the potential use of (pegylated) interferon in the treatment of EBOV infections should be facilitated by its increased availability now that its usefulness in the treatment of hepatitis C will be overtaken by the direct-acting antivirals (DAAs). In addition, interferons could induce a number of IFITMs (interferon-induced transmembrane proteins), which exert antiviral activity against a broad range of viruses, including not only HIV-1, HCV, SARS coronavirus, but also VSV, EBOV, Marburg and West Nile virus and, possibly, other viruses which could considerably extend the scope for interferon-based therapy [36], [37].
5. Neplanocin A, 3-deazaneplanocin A
A surprising observation made in 2002 by Bray et al. [38] is that 3-deazaneplanocin A, an S-adenosyl-l-homocysteine (SAH) hydrolase inhibitor [40] could induce massively increased interferon-α production in EBOV-infected mice. Whether this massive interferon production was only epiphenomenal or causally related to the protective effect of 3-deazaneplanocin A against Ebola has never been resolved. Nor has been the reason for the induction of the massive interferon induction by 3-deazaneplanocin A. A possible hypothesis is that 3-deazaneplanocin, being a SAH hydrolase inhibitor, blocks the methylation of the (+)RNA transcribed from the (−)RNA filovirus genome, thus preventing the release of the mRNA from the (−)RNA·(+)RNA duplex and generating increased levels of double-stranded (ds)RNA molecules which then act as powerful inducers of interferon. SAH hydrolase inhibitors may specifically block the capping (ribose 2′-O-methylation) of viral mRNAs, as it may provide a molecular signature for the distinction of self from non-self mRNA dependent on the RNA sensor Mda5 [41]. In addition to the natural neplanocin A, B, C, D and F, the enantiomers of 1′,6′-isomer of neplanocin A have been synthesized (Fig. 3 ) [42], but their potential for in vivo therapy of EBOV infections remains to be assessed.
Fig. 3.
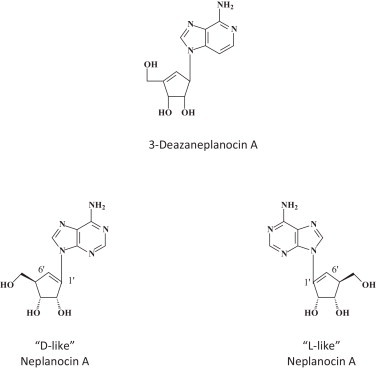
Structure of 3-deazaneplanocin A [38], [39] and neplanocin A (D-like and L-like) analogs [41].
6. BCX4430
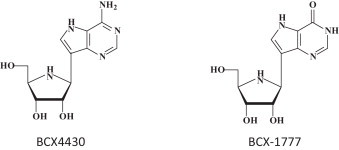
BCX4430 (Fig. 4 ) was described as an inhibitor of the RNA-dependent RNA polymerase hailed as a possible leap ahead in filovirus therapeutics [43]. BCX4430 was proposed to function as a non-obligate RNA chain terminator [44], and its role as a possible SAH hydrolase inhibitor was not even considered. Even more importantly, its potential activity against the rhabdovirus VSV was not even touched upon, although much has to be learned for filovirus therapeutics from their action against rhabdoviruses (such as VSV), especially with regard to their mode of action at the RNA polymerase level. BCX4430 can be considered as an adenosine analog with 2 structural modifications: (i) it is a C-nucleoside instead of the usual N-glycoside, and (ii) the 1,4-oxygen has been replaced by a 1,4-imino group. The original compound synthesized in this series was BCX-1777 (Fig. 4), the hypoxanthine derivative of BCX4430 [45]. BCX-1777 was reported as a purine nucleoside phosphorylase transition-state inhibitor. No antiviral activity was reported for BCX-1777. Being a hypoxanthine derivative, it probably has no antiviral effects.
Fig. 4.
7. Favipiravir (T-705)
I have amply discussed previously [46], [47] the potential of favipiravir for its broad-spectrum activity, that it shares with ribavirin, against a wide variety of both (−)RNA viruses [i.e. influenza (it has been approved in Japan for the treatment of influenza A virus infections), arena, bunya) and (+)RNA viruses (i.e. flavi, picorna, noro]. Hence, it is not surprising that it is also active against the filoviridae, in casu EBOV [48], [49]. Structurally, favipiravir is closely related to ribavirin (Fig. 5 ), with which it shares a carboxamide (C–(O)–NH2) moiety. Perhaps, favipiravir could be considered as a more specific antiviral version of ribavirin; they are both targeted at the viral RNA polymerase, although ribavirin is principally targeted at the IMP dehydrogenase [50]. To be converted to its active metabolite, acting at the viral RNA polymerase, favipiravir should first be converted to its phosphoribosyl derivative and subsequently to the triphosphate (Fig. 6 ) before it could interact as a RNA polymerase inhibitor, principally in direct competition with GTP. Again, it should be mentioned that VSV would serve as an adequate surrogate virus to judge the potential of favipiravir in the treatment of EBOV infections. An in vivo animal model for VSV infection in newborn mice has been described many years ago [51].
Fig. 5.
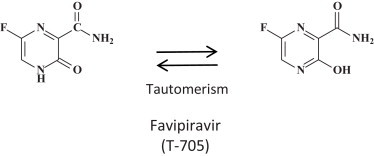
Structure of favipiravir (T-705) [47].
Fig. 6.
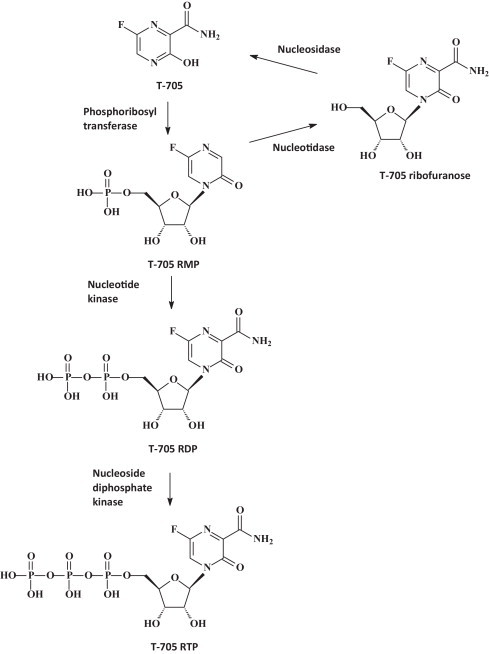
Metabolic pathways of T-705 (favipiravir) [47].
8. Lectins
Griffithsin is a red-alga derived lectin that binds to the terminal mannose residues of the asparagine(N)-linked Man 5–9 GlcNAc2 structures found on the envelopes of HIV-1, HIV-2, HCV, SARS coronavirus and EBOV. Griffithsin and similar lectins may have potential usefulness in the treatment of EBOV infections [52]. Numerous lectins, starting with concanavalin A, cyanovirin N and other mannose-specific plant lectins have been described as potential antiviral agents [53]. They have been proven particularly active against HIV-1 [54], [55].
9. Endoplasmic reticulum (ER) glucosidase inhibitors
Host cellular ER α-glucosidases I and II are essential for the maturation of viral glycosylated envelope proteins. Inhibition of these glycan processing enzymes leads to the misfolding and degradation of viral glycoproteins. The imino sugar 1-deoxynojirimycin and its derivatives are glucose mimics with a nitrogen atom replacing the oxygen and competitively inhibit ER α-glucosidases I and II [56]. One of these derivatives, CM-10-18, is efficacious against a lethal Dengue virus infection in mouse models [57]. Three derivatives of CM-10-18, namely IHVR11029, IHVR17028 and IHVR19029 (Fig. 7 ) suppressed the mortality of Marburg and Ebola virus infection, in mice [58].
Fig. 7.
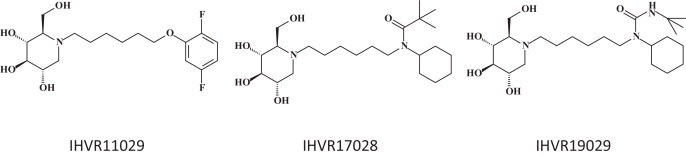
Endoplasmic reticulum (ER) glucosidase inhibitors: IHVR11029, IHVR17028 and IHVR19029 [58].
10. The FGI (Functional Genetics Inc.) compounds
From FGI (Gaithersburg, MD), three compounds (FGI-103, FGI-104 and FGI-106) were reported to exhibit in vivo efficacy against EBOV, the first one (FGI-103) also exhibiting activity against Marburg virus, the third one (FGI-106) being active against Rift Valley virus and Dengue Fever virus, as well as EBOV. The structures of FGI-103 and FGI-106 were revealed (Fig. 8 ); the structure of FGI-104 was not. The mode of action of FGI-103 [59], FGI-104 [60], or FGI-106 [61], can only be speculated upon. Fascinating is the perfectly symmetrical structure of FGI-106. This should tell us something about its mode of antiviral action, which, nevertheless, has remained enigmatic so far.
Fig. 8.
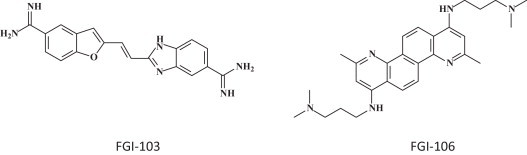
The FGI (Functional Genetics Inc.) compounds: FGI 103 [59] and FGI 106 [61].
11. Antioxidant NSC62914
NSC62914 was found to exhibit anti-filovirus activity in vitro and in vivo, in mice infected with EBOV or Marburg virus [62]. NSC62914 (Fig. 9 ) was found to act as a scavenger of reactive oxygen species. In vitro it was also inhibitory to Rift Valley fever virus, Lassa virus and Venezuelan equine encephalitis virus.
Fig. 9.
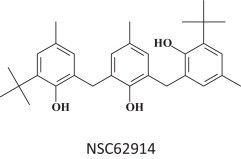
Antioxidant NSC62914 [62].
12. Benzylpiperazine adamantane diamides and benzodiazepine derivatives
Ebola virus entry into the host cells requires the cholesterol transporter Niemann-Pick C1 [63] and this viral entry can be blocked by benzylpiperazine adamantane diamides (Fig. 10 ) [64]. Various other hit compounds, among which the benzodiazepine compound 7 have also been identified as entry inhibitors for filoviruses (Fig. 10) [65].
Fig. 10.
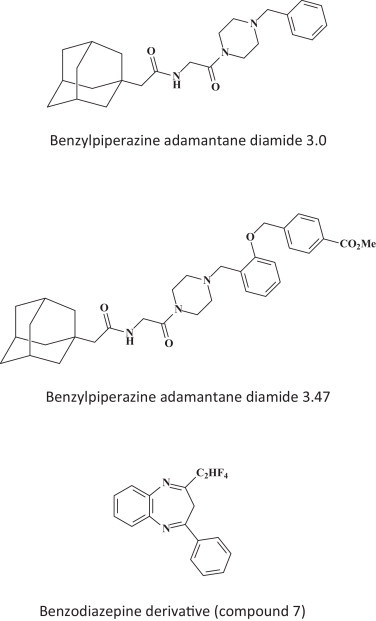
Viral entry inhibitors, benzylpiperazine adamantine diamides 3.0 and 3.47 [55] and benzodiazepine derivative (compound 7) [65].
13. LJ-001 and dUY11
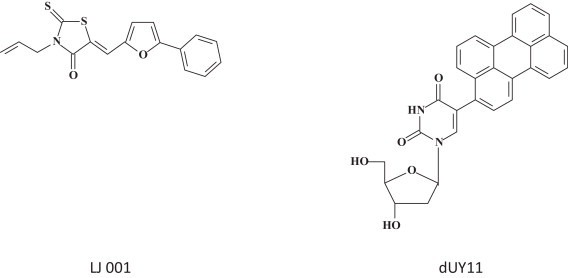
Two structurally unrelated compounds (Fig. 11 ), namely LJ-001, a rhodamine derivative [66], and dUY11, a rigid amphipathic fusion inhibitor (RAFI) [67] prevent the fusion of the viral and cellular membranes and are specifically active against enveloped viruses. That LJ-001 inhibits the entry of filoviruses including EBOV, and enveloped viruses such as influenza A, HIV, pox-, arena-, bunya-, paramyxo- and flaviviruses has been directly demonstrated [66]. For dUY11, it has only been surmised that it would inhibit the replication of filoviruses such as EBOV. As it has a relatively simple structure, and as it has also been shown effective in preventing virus-induced mortality from EBOV, LJ-001 should be considered a prime candidate to curtail the ongoing EBOV epidemics.
Fig. 11.
14. Selective estrogen receptor modulators (SERMS)
SERMS, previously approved by the FDA were, totally by chance, found to inhibit EBOV infection (Fig. 12 ) [68]. The compounds concerned are clomiphene and toremifene. They would be active against EBOV through an off-target effect where the compounds interfere with a late step of viral entry and likely affect the triggering of fusion [68]. The SERMS are an immediately actionable class of FDA-approved drugs that can be readily repurposided for the treatment of filovirus infections.
Fig. 12.
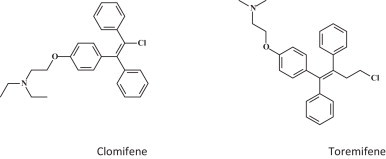
Selective estrogen receptor modulators (SERMS): clomiphene and toremifene [68].
15. Ion channel blockers
The ion channel blockers amiodarone, dronedarone and verapamil were found to inhibit the cell entry of filoviruses (i.e. EBOV) [69]. In particular, amiodarone, a multi-ion channel inhibitor used clinically as an anti-arrhythmic agent, inhibited filovirus entry within the range achieved in serum during anti-arrhythmic therapy in humans, i.e. 1.5–2.5 μg/ml [70]. Amiodarone also inhibited the New World arenavirus Guanarito, while the Old World arenavirus Lassa and the rhabdoviridae (vesicular stomatitis virus) and bunyaviridae (Hantaan) were not inhibited [69] (Fig. 13 ).
Fig. 13.
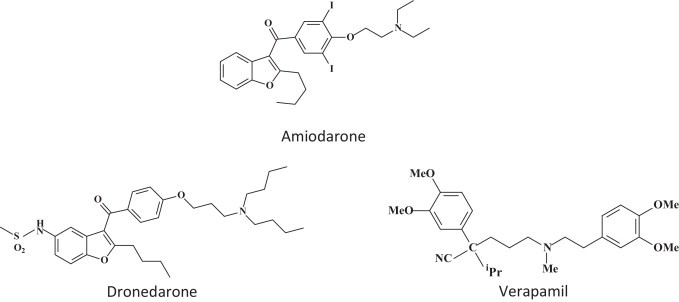
Ion channel blockers amiodarone, dronedarone and verapamil [69].
16. CMLDBU3402: EBOV RNA transcription inhibitor
CMLDBU3402 (Fig. 14 ) was found to inhibit the replication of the non-segmented negative-strand RNA viruses, EBOV and VSV (vesicular stomatitis virus) [71]. In earlier studies Connor et al. [72] and Smith et al. [73] had noted that inhibition of VSV (i.e. through inhibition of heat-shock protein 90) presaged inhibition of EBOV replication.
Fig. 14.
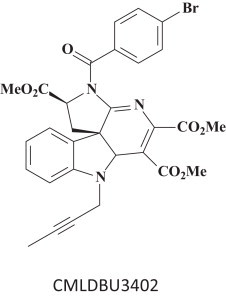
CMLDBU3402, an indoline alkaloid-type compound [71].
17. HSPA5: an essential host factor for EBOV infection
The endoplasmic reticulum (ER) chaperone HSPA5 (heat shock 70 kDa protein 5) has been identified as EBOV-associated host factor and other enveloped viruses such as VSV [74]. The small molecule (−)-epigallocatechin gallate (Fig. 15 ) binds to the ATP-binding site of HSPA5, and thereby disturbs its chaperone function required for EBOV infection. Besides (−)-epigallocatechin gallate, varying other molecules have been identified as HSPA5 inhibitors [75]. Whether they are also inhibitory to VSV and EBOV infection, remains to be determined.
Fig. 15.
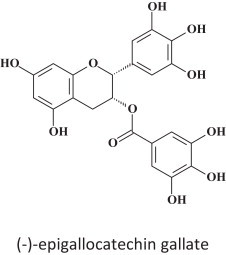
(−)-Epigallocatechin gallate.
18. Heme oxygenase-1 (HO-1)
HO-1 is an enzyme that catalyzes the first and rate-limiting step in the degradation of heme to carbon monoxide (CO), free iron (Fe++, which is subsequently oxidized to Fe+++ and stored as ferritin) and biliverdin (which is subsequently reduced to bilirubin). HO-1 is upregulated not only by its substrate, heme, but also by various nonheme inducers, such as heat shock, inflammatory cytokines, endotoxin, and oxidative stress. It would also suppress EBOV replication, not at the level of viral entry (or budding), but at the level of EBOV transcription/replication [76]. It would now also seem mandatory to examine whether HO-1 also suppresses VSV replication. It certainly represents a novel therapeutic strategy against EBOV infection.
19. Miscellaneous compounds preventing cathepsin L cleavage
A number of small molecules preventing cathepsin L cleavage of viral glycoproteins have been identified to inhibit the entry of SARS coronavirus, Hendra, Nipah and/or EBOV (Fig. 16 ) [77]. These compounds need to be further optimized and developed into antiviral drugs useful for the treatment of any of the target viruses.
Fig. 16.
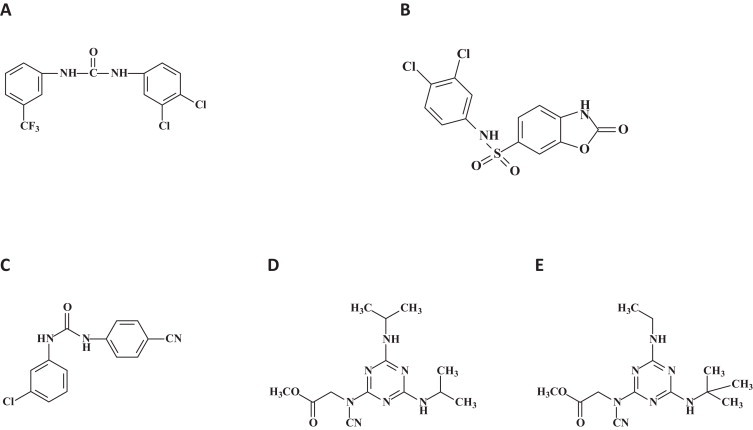
Miscellaneous compounds preventing cathepsin L cleavage of viral glycoproteins derived from SARS coronavirus, Hendra, Nipah or EBOV. Chemical structures of the small molecules identified by pseudovirus inhibition assay. Four small molecules showed inhibition of both EBOV and SARS-CoV pseudotyped virus entry. (A) Compound 5182554 {N-(3,4-dichlorophenyl)-N′-[3-(trifluoromethyl)phenyl]urea}; (B) compound 7910528 [N-(3,4-dichlorophenyl)-2-oxo-2,3-dihydro-1,3-benzoxazole-6-sulfonamide]; (C) compound 7914021 [N-(3-chlorophenyl)-N′-(4-cyanophenyl)urea]; (D and E) compound 5705213 {methyl-N-[4,6-bis(isopropylamino)-1,3,5-triazin-2-yl]-N-cyanoglycinate}. (D) and (E) its derivative 7402683 {methyl-N-[4-(tert-butylamino)-6-(ethylamino)-1,3,5-triazin-2-yl]-N-cyanoglycinate} [77].
20. Chloroquine
Chloroquine is a 9-aminoquinoline known since 1934. It was specifically synthesized as an antimalarial agent but gradually dismissed from antimalarial therapy and prophylaxis due to the continuous emergence of chloroquine-resistant Plasmodium falciparum strains. It has a pleiade of antiviral effects varying from the endocytosis to the exocytosis of viral particles, and, in addition, downregulates IFN-γ and TNF-α production and TNF-α receptors [78]. It was shown to have anti-HIV-1 activity [79] and to inhibit SARS coronavirus [80] and to inhibit human coronavirus OC43 infection in newborn mice [81]. Not surprisingly, it was also found to protect mice against EBOV infection in vivo [82] (Fig. 17 ).
Fig. 17.
Chloroquine.
21. Conclusion
Ebola virus (EBOV) was first identified as a hemorrhagic fever virus in 1976, that is 5 years before AIDS was recognized, and 7 years before HIV was discovered as its etiologic agent. EBOV has regularly led to the emergence of epidemics, particularly in Congo (Zaire), Sudan and Uganda, but it only recently stirred up worldwide concern with its breakthrough in West Africa. This started in December 2013, has spread over three countries, Guinea, Sierra Leone and Liberia, and with a mortality rate of up to 90%, it has reached a global death toll of about 5000 (and still rising). There is still no vaccine or treatment available, although EBOV, while highly contagious, is very sensitive to varying well-defined compounds. The majority of these compounds (Table 2 ) are targeted at either viral entry or virus replication/transcription. To work with EBOV, BSL 4 (Biosafety level 4, the highest level) is required, which makes that EBOV can only be handled in very few laboratories over the world. It should be pointed out, however, that the mechanism of replication of EBOV, which belongs to the filoviridae, follows a strategy that is similar to that of vesicular stomatitis virus (VSV), which belongs to the rhabdoviridae. In this sense, VSV could be considered as a surrogate virus for EBOV. This means that several compounds that were previously described as inhibitors of VSV should be revisited as therapeutic agents for EBOV, and, vice versa, potential anti-EBOV therapies could be pre-evaluated for their anti-VSV activity. This is most pertinent for compounds, such as neplanocin A derivatives, that are targeted at the S-adenosyl-l-homocysteine (SAH) hydrolase, or favipiravir, which is targeted at the viral RNA polymerase.
Table 2.
Chemical agents representing therapeutic strategies for EBOV infection.
| Compound | Viral target |
|---|---|
| Neplanocin A | SAH hydrolase |
| 3-Deazaneplanocin A | SAH hydrolase |
| BCX4430 | RNA polymerase |
| Favipiravir (T-705) | RNA polymerase |
| Lectins | Viral entry |
| Glucosidase inhibitors | Viral entry |
| FGI compounds | Unknown |
| Antioxidant NSC62914 | Reactive oxygen species (ROS) |
| Benzylpiperazine adamantane diamides | Viral entry |
| LJ-001 | Viral entry |
| dUY11 | Viral entry |
| SERMS (clomiphene, toremifene) | Viral entry |
| Ion channel blockers | Viral entry |
| CMLDBU3402 | RNA polymerase |
| HSPA5 inhibitors | Unknown |
| Heme oxygenase-1 (HO-1) | Unknown |
| Miscellaneous inhibitors of cathepsin L cleavage | Viral entry |
| Chloroquine | Unknown |
Acknowledgments
I thank Mrs. Christiane Callebaut and Mrs. Cathy De Meyer for their proficient editorial assistance.
References
- 1.Gatherer D. The 2014 Ebola virus disease outbreak in West Africa. J Gen Virol. 2014;95:1619–1624. doi: 10.1099/vir.0.067199-0. [DOI] [PubMed] [Google Scholar]
- 2.WHO Ebola Response Team Ebola virus disease in West Africa – the first 9 months of the epidemic and forward projections. N Engl J Med. 2014;371:1481–1495. doi: 10.1056/NEJMoa1411100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Briand S., Bertherat E., Cox P., Formenty P., Kieny M.P., Myhre J.K. The international ebola emergency. N Engl J Med. 2014;371:1180–1183. doi: 10.1056/NEJMp1409858. [DOI] [PubMed] [Google Scholar]
- 4.Negredo A., Palacios G., Vázquez-Morón S., González F., Dopazo H., Molero F. Discovery of an ebolavirus-like filovirus in Europe. PLoS Pathog. 2011;7:e1002304. doi: 10.1371/journal.ppat.1002304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vogel G. Infectious disease. Are bats spreading Ebola across sub-Saharan Africa? Science. 2014;344:140. doi: 10.1126/science.344.6180.140. [DOI] [PubMed] [Google Scholar]
- 6.Paessler S., Walker D.H. Pathogenesis of the viral hemorrhagic fevers. Annu Rev Pathol Mech Dis. 2013;8:411–440. doi: 10.1146/annurev-pathol-020712-164041. [DOI] [PubMed] [Google Scholar]
- 7.Li Y.H., Chen S.P. Evolutionary history of Ebola virus. Epidemiol Infect. 2014;142:1138–1145. doi: 10.1017/S0950268813002215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Del Rio C., Mehta A.K., Lyon G.M., III, Guarner J. Ebola hemorrhagic Fever in 2014: the tale of an evolving epidemic. Ann Intern Med. 2014;161:746–748. doi: 10.7326/M14-1880. [DOI] [PubMed] [Google Scholar]
- 9.Pattyn S., Jacob W., van der Groen G., Piot P., Courteille G. Isolation of Marburg-like virus from a case of haemorrhagic fever in Zaire. Lancet. 1977;309:573–574. doi: 10.1016/s0140-6736(77)92002-5. [DOI] [PubMed] [Google Scholar]
- 10.Feldmann H. Ebola – a growing threat? N Engl J Med. 2014;371:1375–1378. doi: 10.1056/NEJMp1405314. [DOI] [PubMed] [Google Scholar]
- 11.Booth T.F., Rabb M.J., Beniac D.R. How do filovirus filaments bend without breaking. Trends Microbiol. 2013;21:583–593. doi: 10.1016/j.tim.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 12.Geisbert T.W., Hensley L.E., Jahrling P.B., Larsen T., Geisbert J.B., Paragas J. Treatment of Ebola virus infection with a recombinant inhibitor of factor VIIa/tissue factor: a study in rhesus monkeys. Lancet. 2003;362:1953–1958. doi: 10.1016/S0140-6736(03)15012-X. [DOI] [PubMed] [Google Scholar]
- 13.Wong G., Qiu X., Oliger G.G., Kobinger G.P. Post-exposure therapy of filovirus infections. Trends Microbiol. 2014;22:456–463. doi: 10.1016/j.tim.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 14.Warren T.K., Warfield K.L., Wells J., Swenson D.L., Donner K.S., Van Tongeren S.A. Advanced antisense therapies for postexposure protection against lethal filovirus infections. Nat Med. 2010;16:991–994. doi: 10.1038/nm.2202. [DOI] [PubMed] [Google Scholar]
- 15.Heald A.E., Iversen P.L., Saoud J.B., Sazani P., Charleston J.S., Axtelle T. Safety and pharmacokinetic profiles of phosphorodiamidate morpholino oligomers with activity against Ebola virus and Marburg virus: results of two single-ascending-dose studies. Antimicrob Agents Chemother. 2014;58:6639–6647. doi: 10.1128/AAC.03442-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iversen P.L., Warren T.K., Wells J.B., Garza N.L., Mourich D.V., Welch L.S. Discovery and early development of AVI-7537 and AVI-7288 for the treatment of Ebola virus and Marburg virus infections. Viruses. 2012;4:2806–2830. doi: 10.3390/v4112806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geisbert T.W., Lee A.C., Robbins M., Geisbert J.B., Honko A.N., Sood V. Postexposure protection of non-human primates against a lethal Ebola virus challenge with RNA interference: a proof-of-concept study. Lancet. 2010;375:1896–1905. doi: 10.1016/S0140-6736(10)60357-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dye J.M., Herbert A.S., Kuehne A.I., Barth J.F., Muhammad M.A., Zak S.E. Postexposure antibody prophylaxis protects nonhuman primates from filovirus disease. Proc Natl Acad Sci USA. 2012;109:5034–5039. doi: 10.1073/pnas.1200409109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qiu X., Wong G., Audet J., Bello A., Fernando L., Alimonti J.B. Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature. 2014;514:47–53. doi: 10.1038/nature13777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rid A. Emanuel EJ. Ethical considerations of experimental interventions in the Ebola outbreak. Lancet. 2014;384:1896–1899. doi: 10.1016/S0140-6736(14)61315-5. [DOI] [PubMed] [Google Scholar]
- 21.Galvani A.P., Ndeffo-Mbah M.L., Wenzel N., Childs J.E. Ebola vaccination: if not now, when? Ann Intern Med. 2014;161:749–750. doi: 10.7326/M14-1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sullivan N.J., Sanchez A., Rollin P.E., Yang Z.Y., Nabel G.J. Development of a preventive vaccine for Ebola virus infection in primates. Nature. 2000;408:605–609. doi: 10.1038/35046108. [DOI] [PubMed] [Google Scholar]
- 23.Warfield K.L., Swenson D.L., Olinger G.G., Kalina W.V., Aman M.J., Bavari S. Ebola virus-like particle-based vaccine protects nonhuman primates against lethal Ebola virus challenge. J Infect Dis. 2007;196(Suppl. 2):S430–S437. doi: 10.1086/520583. [DOI] [PubMed] [Google Scholar]
- 24.Geisbert T.W., Daddario-Dicaprio K.M., Geisbert J.B., Reed D.S., Feldmann F., Grolla A. Vesicular stomatitis virus-based vaccines protect nonhuman primates against aerosol challenge with Ebola and Marburg viruses. Vaccine. 2008;26:6894–6900. doi: 10.1016/j.vaccine.2008.09.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mire C.E., Geisbert J.B., Marzi A., Agans K.N., Feldmann H., Geisbert T.W. Vesicular stomatitis virus-based vaccines protect nonhuman primates against Bundibugyo ebolavirus. PLoS Negl Trop Dis. 2013;7:e2600. doi: 10.1371/journal.pntd.0002600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herbert A.S., Kuehne A.I., Barth J.F., Ortiz R.A., Nichols D.K., Zak S.E. Venezuelan equine encephalitis virus replicon particle vaccine protects nonhuman primates from intramuscular and aerosol challenge with ebolavirus. J Virol. 2013;87:4952–4964. doi: 10.1128/JVI.03361-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marzi A., Engelmann F., Feldmann F., Haberthur K., Shupert W.L., Brining D. Antibodies are necessary for rVSV/ZEBOV-GP-mediated protection against lethal Ebola virus challenge in nonhuman primates. Proc Natl Acad Sci USA. 2013;110:1893–1898. doi: 10.1073/pnas.1209591110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y., Liu Z., Dai Q. A highly immunogenic fragment derived from Zaire Ebola virus glycoprotein elicits effective neutralizing antibody. Virus Res. 2014;189:254–261. doi: 10.1016/j.virusres.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 29.Warfield K.L., Goetzmann J.E., Biggins J.E., Kasda M.B., Unfer R.C., Vu H. Vaccinating captive chimpanzees to save wild chimpanzees. Proc Natl Acad Sci USA. 2014;111:8873–8876. doi: 10.1073/pnas.1316902111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Isaacs A., Lindenmann J. Virus interference. I. The interferon. Proc R Soc Lond B Biol Sci. 1957;147:258–267. [PubMed] [Google Scholar]
- 31.Manns M.P., McHutchison J.G., Gordon S.C., Rustgi V.K., Shiffman M., Reindollar R. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958–965. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 32.Fried M.W., Shiffman M.L., Reddy K.R., Smith C., Marinos G., Gonçales F.L., Jr. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 33.McHutchison J.G., Lawitz E.J., Shiffman M.L., Muir A.J., Galler G.W., McCone J. Peginterferon alfa-2b or alfa-2a with ribavirin for treatment of hepatitis C infection. N Engl J Med. 2009;361:580–593. doi: 10.1056/NEJMoa0808010. [DOI] [PubMed] [Google Scholar]
- 34.Haagmans B.L., Kuiken T., Martina B.E., Fouchier R.A., Rimmelzwaan G.F., van Amerongen G. Pegylated interferon-alpha protects type 1 pneumocytes against SARS coronavirus infection in macaques. Nat Med. 2004;10:290–293. doi: 10.1038/nm1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith L.M., Hensley L.E., Geisbert T.W., Johnson J., Stossel A., Honko A. Interferon-β therapy prolongs survival in rhesus macaque models of Ebola and Marburg hemorrhagic fever. J Infect Dis. 2013;208:310–318. doi: 10.1093/infdis/jis921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chutiwitoonchai N., Hiyoshi M., Hiyoshi-Yoshidomi Y., Hashimoto M., Tokunaga K., Suzu S. Characteristics of IFITM, the newly identified IFN-inducible anti-HIV-1 family proteins. Microbes Infect. 2013;15:280–290. doi: 10.1016/j.micinf.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perreira J.M., Chin C.R., Feeley E.M., Brass A.L. IFITMs restrict the replication of multiple pathogenic viruses. J Mol Biol. 2013;425:4937–4955. doi: 10.1016/j.jmb.2013.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bray M., Raymond J.L., Geisbert T., Baker R.O. 3-Deazaneplanocin A induces massively increased interferon-α production in Ebola virus-infected mice. Antiviral Res. 2002;55:151–159. doi: 10.1016/s0166-3542(02)00018-9. [DOI] [PubMed] [Google Scholar]
- 39.De Clercq E., Cools M., Balzarini J., Marquez V.E., Borcherding D.R., Borchardt R.T. Broad-spectrum antiviral activities of neplanocin A, 3-deazaneplanocin A, and their 5′-nor derivatives. Antimicrob Agents Chemother. 1989;33:1291–1297. doi: 10.1128/aac.33.8.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bray M., Driscoll J., Huggins J.W. Treatment of lethal Ebola virus infection in mice with a single dose of an S-adenosyl-l-homocysteine hydrolase inhibitor. Antiviral Res. 2000;45:135–147. doi: 10.1016/s0166-3542(00)00066-8. [DOI] [PubMed] [Google Scholar]
- 41.Züst R., Cervantes-Barragan L., Habjan M., Maier R., Neuman B.W., Ziebuhr J. Ribose 2′-O-methylation provides a molecular signature for the distinction of self and non-self mRNA dependent on the RNA sensor Mda5. Nat Immunol. 2011;12:137–143. doi: 10.1038/ni.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ye W., Schneller S.W. The enantiomers of the 1′,6′-isomer of neplanocin A: synthesis and antiviral properties. Bioorg Med Chem. 2014;22:5315–5319. doi: 10.1016/j.bmc.2014.07.051. [DOI] [PubMed] [Google Scholar]
- 43.Falzarano D., Feldmann H. Possible leap ahead in filovirus therapeutics. Cell Res. 2014;24:647–648. doi: 10.1038/cr.2014.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Warren T.K., Wells J., Panchal R.G., Stuthman K.S., Garza N.L., Van Tongeren S.A. Protection against filovirus diseases by a novel broad-spectrum nucleoside analogue BCX4430. Nature. 2014;508:402–405. doi: 10.1038/nature13027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kilpatrick J.M., Morris P.E., Serota D.G., Jr., Phillips D., Moore D.R., Bennett J.C. Intravenous and oral pharmacokinetic study of BCX-1777, a novel purine nucleoside phosphorylase transition-state inhibitor. In vivo effects on blood 2′-deoxyguanosine in primates. Int Immunopharmacol. 2003;3:541–548. doi: 10.1016/S1567-5769(03)00044-4. [DOI] [PubMed] [Google Scholar]
- 46.De Clercq E. A cutting-edge view on the current state of antiviral drug development. Med Res Rev. 2013;33:1249–1277. doi: 10.1002/med.21281. [DOI] [PubMed] [Google Scholar]
- 47.De Clercq E. Dancing with chemical formulae of antivirals: a panoramic view (Part 2) Biochem Pharmacol. 2013;86:1397–1410. doi: 10.1016/j.bcp.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 48.Smither S.J., Eastaugh L.S., Steward J.A., Nelson M., Lenk R.P., Lever M.S. Post-exposure efficacy of oral T-705 (favipiravir) against inhalational Ebola virus infection in a mouse model. Antiviral Res. 2014;104:153–155. doi: 10.1016/j.antiviral.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 49.Oestereich L., Lüdtke A., Wurr S., Rieger T., Munoz-Fontela C., Günther S. Successful treatment of advanced Ebola virus infection with T-705 (favipiravir) in a small animal model. Antiviral Res. 2014;105:17–21. doi: 10.1016/j.antiviral.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 50.Streeter D.G., Witkowski J.T., Khare G.P., Sidwell R.W., Bauer R.J., Robins R.K. Mechanism of action of 1-β-d-ribofuranosyl-1,2,4-triazole-3-carboxamide (Virazole), a new broad-spectrum antiviral agent. Proc Natl Acad Sci USA. 1973;70:1174–1178. doi: 10.1073/pnas.70.4.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.De Clercq E., De Somer P. Protective effect of interferon and polyacrylic acid in newborn mice infected with a lethal dose of vesicular stomatitis virus. Life Sci. 1968;7:925–933. doi: 10.1016/0024-3205(68)90098-2. [DOI] [PubMed] [Google Scholar]
- 52.Barton C., Kouokam J.C., Lasnik A.B., Foreman O., Cambon A., Brock G. Activity of and effect of subcutaneous treatment with the broad-spectrum antiviral lectin griffithsin in two laboratory rodent models. Antimicrob Agents Chemother. 2014;58:120–127. doi: 10.1128/AAC.01407-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Balzarini J., Hatse S., Vermeire K., Princen K., Aquaro S., Perno C.F. Mannose-specific plant lectins from the Amaryllidaceae family qualify as efficient microbicides for prevention of human immunodeficiency virus infection. Antimicrob Agents Chemother. 2004;48:3858–3870. doi: 10.1128/AAC.48.10.3858-3870.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Balzarini J., Van Laethem K., Hatse S., Vermeire K., De Clercq E., Peumans W. Profile of resistance of human immunodeficiency virus to mannose-specific plant lectins. J Virol. 2004;78:10617–10627. doi: 10.1128/JVI.78.19.10617-10627.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mori T., Boyd M.R., Cyanovirin N. a potent human immunodeficiency virus-inactivating protein, blocks both CD4-dependent and CD4-independent binding of soluble gp120 (sgp120) to target cells, inhibits sCD4-induced binding of sgp120 to cell-associated CXCR4, and dissociates bound sgp120 from target cells. Antimicrob Agents Chemother. 2001;45:664–672. doi: 10.1128/AAC.45.3.664-672.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dwek R.A., Butters T.D., Platt F.M., Zitzmann N. Targeting glycosylation as a therapeutic approach. Nat Rev Drug Discov. 2002;1:65–75. doi: 10.1038/nrd708. [DOI] [PubMed] [Google Scholar]
- 57.Chang J., Schul W., Yip A., Xu X., Guo J.T., Block T.M. Competitive inhibitor of cellular α-glucosidases protects mice from lethal dengue virus infection. Antiviral Res. 2011;92:369–371. doi: 10.1016/j.antiviral.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chang J., Warren T.K., Zhao X., Gill T., Guo F., Wang L. Small molecule inhibitors of ER α-glucosidases are active against multiple hemorrhagic fever viruses. Antiviral Res. 2013;98:432–440. doi: 10.1016/j.antiviral.2013.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Warren T.K., Warfield K.L., Wells J., Enterlein S., Smith M., Ruthel G. Antiviral activity of a small-molecule inhibitor of filovirus infection. Antimicrob Agents Chemother. 2010;54:2152–2159. doi: 10.1128/AAC.01315-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kinch M.S., Yunus A.S., Lear C., Mao H., Chen H., Fesseha Z. FGI-104: a broad-spectrum small molecule inhibitor of viral infection. Am J Transl Res. 2009;1:87–98. [PMC free article] [PubMed] [Google Scholar]
- 61.Aman M.J., Kinch M.S., Warfield K., Warren T., Yunus A., Enterlein S. Development of a broad-spectrum antiviral with activity against Ebola virus. Antiviral Res. 2009;83:245–251. doi: 10.1016/j.antiviral.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 62.Panchal R.G., Reid S.P., Tran J.P., Bergeron A.A., Wells J., Kota K.P. Identification of an antioxidant small-molecule with broad-spectrum antiviral activity. Antiviral Res. 2012;93:23–29. doi: 10.1016/j.antiviral.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 63.Carette J.E., Raaben M., Wong A.C., Herbert A.S., Obernosterer G., Mulherkar N. Ebola virus entry requires the cholesterol transporter Niemann-Pick C1. Nature. 2011;477:340–343. doi: 10.1038/nature10348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Côté M., Misasi J., Ren T., Bruchez A., Lee K., Filone C.M. Small molecule inhibitors reveal Niemann-Pick C1 is essential for Ebola virus infection. Nature. 2011;477:344–348. doi: 10.1038/nature10380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Basu A., Li B., Mills D.M., Panchal R.G., Cardinale S.C., Butler M.M. Identification of a small-molecule entry inhibitor for filoviruses. J Virol. 2011;85:3106–3119. doi: 10.1128/JVI.01456-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wolf M.C., Freiberg A.N., Zhang T., Akyol-Ataman Z., Grock A., Hong P.W. A broad-spectrum antiviral targeting entry of enveloped viruses. Proc Natl Acad Sci USA. 2010;107:3157–3162. doi: 10.1073/pnas.0909587107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.St Vincent M.R., Colpitts C.C., Ustinov A.V., Muqadas M., Joyce M.A., Barsby N.L. Rigid amphipathic fusion inhibitors, small molecule antiviral compounds against enveloped viruses. Proc Natl Acad Sci USA. 2010;107:17339–17344. doi: 10.1073/pnas.1010026107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Johansen L.M., Brannan J.M., Delos S.E., Shoemaker C.J., Stossel A., Lear C. FDA-approved selective estrogen receptor modulators inhibit Ebola virus infection. Sci Transl Med. 2013;5 doi: 10.1126/scitranslmed.3005471. 190ra79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gehring G., Rohrmann K., Atenchong N., Mittler E., Becker S., Dahlmann F. The clinically approved drugs amiodarone, dronedarone and verapamil inhibit filovirus cell entry. J Antimicrob Chemother. 2014;69:2123–2131. doi: 10.1093/jac/dku091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Goldschlager N., Epstein A.E., Naccarelli G., Olshansky B., Singh B. Practical guidelines for clinicians who treat patients with amiodarone. Practice Guidelines Subcommittee, North American Society of Pacing and Electrophysiology. Arch Intern Med. 2000;160:1741–1748. doi: 10.1001/archinte.160.12.1741. [DOI] [PubMed] [Google Scholar]
- 71.Filone C.M., Hodges E.N., Honeyman B., Bushkin G.G., Boyd K., Platt A. Identification of a broad-spectrum inhibitor of viral RNA synthesis: validation of a prototype virus-based approach. Chem Biol. 2013;20:424–433. doi: 10.1016/j.chembiol.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Connor J.H., McKenzie M.O., Parks G.D., Lyles D.S. Antiviral activity and RNA polymerase degradation following Hsp90 inhibition in a range of negative strand viruses. Virology. 2007;362:109–119. doi: 10.1016/j.virol.2006.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Smith D.R., McCarthy S., Chrovian A., Olinger G., Stossel A., Geisbert T.W. Inhibition of heat-shock protein 90 reduces Ebola virus replication. Antiviral Res. 2010;87:187–194. doi: 10.1016/j.antiviral.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Reid S.P., Shurtleff A.C., Costantino J.A., Tritsch S.R., Retterer C., Spurgers K.B. HSPA5 is an essential host factor for Ebola virus infection. Antiviral Res. 2014;109:171–174. doi: 10.1016/j.antiviral.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 75.Huang M., Li Z., Li D., Walker S., Greenan C., Kennedy R. Structure-based design of HSPA5 inhibitors: from peptide to small molecule inhibitors. Bioorg Med Chem Lett. 2013;23:3044–3050. doi: 10.1016/j.bmcl.2013.03.035. [DOI] [PubMed] [Google Scholar]
- 76.Hill-Batorski L., Halfmann P., Neumann G., Kawaoka Y. The cytoprotective enzyme heme oxygenase-1 suppresses Ebola virus replication. J Virol. 2013;87:13795–13802. doi: 10.1128/JVI.02422-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Elshabrawy H.A1, Fan J., Haddad C.S., Ratia K., Broder C.C., Caffrey M. Identification of a broad-spectrum antiviral small molecule against severe acute respiratory syndrome coronavirus and Ebola, Hendra, and Nipah viruses by using a novel high-throughput screening assay. J Virol. 2014;88:4353–4365. doi: 10.1128/JVI.03050-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Savarino A., Boelaert J.R., Cassone A., Majori G., Cauda R. Effects of chloroquine on viral infections: an old drug against today's diseases. Lancet Infect Dis. 2003;3:722–727. doi: 10.1016/S1473-3099(03)00806-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Savarino A., Gennero L., Sperber K., Boelaert J.R. The anti-HIV-1 activity of chloroquine. J Clin Virol. 2001;20:131–135. doi: 10.1016/s1386-6532(00)00139-6. [DOI] [PubMed] [Google Scholar]
- 80.Keyaerts E., Vijgen L., Maes P., Neyts J., Van Ranst M. In vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine. Biochem Biophys Res Commun. 2004;323:264–268. doi: 10.1016/j.bbrc.2004.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Keyaerts E., Li S., Vijgen L., Rysman E., Verbeeck J., Van Ranst M. Antiviral activity of chloroquine against human coronavirus OC43 infection in newborn mice. Antimicrob Agents Chemother. 2009;53:3416–3421. doi: 10.1128/AAC.01509-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Madrid P.B., Chopra S., Manger I.D., Gilfillan L., Keepers T.R., Shurtleff A.C. A systematic screen of FDA-approved drugs for inhibitors of biological threat agents. PLOS ONE. 2013;8:e60579. doi: 10.1371/journal.pone.0060579. [DOI] [PMC free article] [PubMed] [Google Scholar]


