Abstract
Prohibitins (PHBs) are scaffold proteins that modulate many signaling pathways controlling cell survival, metabolism, and inflammation. Several drugs that target PHBs have been identified and evaluated for various clinical applications. Preclinical and clinical studies indicate that these PHB ligands may be useful in oncology, cardiology, and neurology, as well as against obesity. This review covers the physiological role of PHBs in health and diseases and current developments concerning PHB ligands.
Graphical Abstract
Highlights
► Prohibitins regulate cell survival, metabolism, and inflammation ► Prohibitins act as a hub for many signaling pathways ► Prohibitin ligands are examined in cancer, Parkinson’s disease, and obesity
Prohibitins are scaffold proteins that modulate many signaling pathways, controlling cell survival, metabolism, and inflammation. Thuaud et al. review prohibitins in health and diseases, current developments of small molecules targeting prohibitins, and their therapeutic potential.
Main Text
Introduction
Prohibitin-1 (PHB1, formerly known as BAP32) and its homolog PHB2 (formerly BAP37, REA, or prohibitone) are pleiotropic proteins with multiple functions. PHB1 was initially identified in 1989 by McClung and collaborators by screening for potential tumor suppressors with antiproliferative activities that were highly expressed in normal resting, but not regenerating, rat liver (McClung et al., 1989; Nuell et al., 1991). PHBs have since been shown to act as a hub for many signaling pathways triggered by growth factors, the immune response, and steroid hormones regulating metabolism, mitochondrial biogenesis, cell migration, division, and survival.
PHBs are composed of an N-terminal transmembrane domain, an evolutionarily conserved PHB domain that is common to other scaffold proteins (including stomatin, flotillin, and HflK/C), and a C-terminal coiled-coil domain that is involved in the interaction between PHB1 and PHB2 (Figure S1 available online). Although the structure of PHBs has not been solved, Winter et al. (2007) were able to generate structural models of human PHBs based on the known structure of flotillin-2, which belongs to the same family of membrane proteins.
Mitochondrial Functions of PHBs
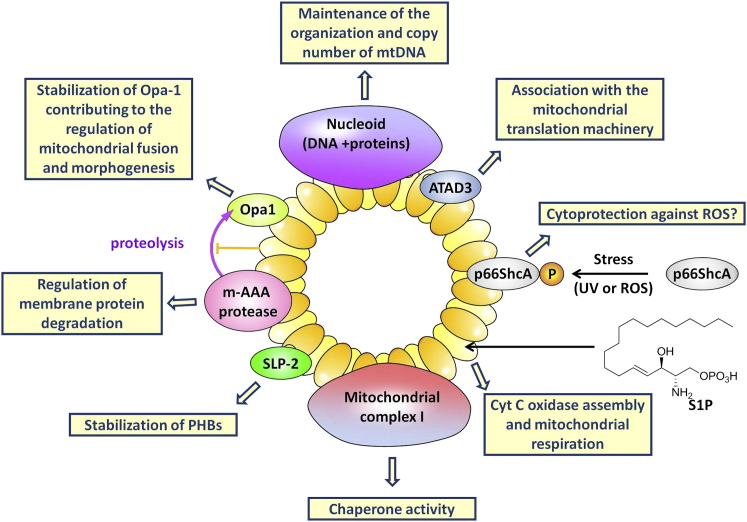
Located in the mitochondrial inner membrane, PHB1 and PHB2 interact with each other to form heterodimers that are organized in ring-like structures with a diameter of 20–25 nm (Figure 1 ; Back et al., 2002; Coates et al., 2001). These supercomplexes maintain the structure of mitochondria and regulate their functions (Tatsuta et al., 2005). PHBs protect newly imported proteins from degradation by the m-AAA (mitochondrial ATPases Associated with diverse cellular Activities) protease (Steglich et al., 1999), promote mitochondrial protein synthesis (He et al., 2012), maintain the organization and copy number of mitochondrial DNA (mtDNA; Kasashima et al., 2008), and act as a chaperones for newly synthesized proteins of the mitochondrial complex I (Nijtmans et al., 2000, 2002) and the GTPase optic atrophy 1 (Opa1) during mitochondrial fission and morphogenesis (Merkwirth et al., 2008). PHB1 also interacts with the adaptor protein p66Shc, a major mediator of stress-induced apoptosis (Madireddi, 2006). This adaptor protein is activated by UVC, H2O2, and reactive oxygen species (ROS), and induces the generation of ROS in mitochondria, leading to apoptosis (Gertz et al., 2008). It remains unclear whether PHB1 modulates this proapoptotic response; nevertheless, it is tempting to speculate that p66Shc is involved in the cytoprotective activities of PHBs and their ligands, particularly against oxidative stress (Bernard et al., 2011; Fahrig et al., 2005; Kathiria et al., 2012a; Liu et al., 2009b; Ribeiro et al., 2012a).
Figure 1.
Function of PHB1 and PHB2 in Mitochondria
In the mitochondrial inner membrane, PHB1 and PHB2 are organized in ring-like structures that maintain the structure and regulate the functions of mitochondria through interaction with OPA-1, m-AAA protease, SLP-2 (stomatin-like protein 2), ATAD3 (ATPase family AAA Domain-containing protein 3), and mtDNA.
PHB2 is also regulated by a second messenger, sphingosine-1-phosphate (S1P), which controls diverse physiological processes through several targets. S1P binds with high affinity to PHB2 and thereby maintains the integrity of the mitochondrial respiration machinery (Strub et al., 2011). This process is involved in the preconditioning protection of the heart against ischemia-reperfusion injury (Gomez et al., 2011).
Regulation and Physiological Role of PHBs
PHBs are not limited to mitochondria; they are also found in the nucleus, the endoplasmic reticulum, the plasma membrane, and macrophage phagosomes (Garin et al., 2001), where they modulate many aspects of cell physiology. Their roles are extremely complex, partly because PHBs are themselves regulated by several tyrosine and serine phosphorylations, O-GlcNAc modifications, palmitoylations, transamidations, and tyrosine nitrosylations (Mishra et al., 2010). PHB1 interacts physically with the second messenger PIP3, and PHB2 interacts with S1P (Ande and Mishra, 2009; Strub et al., 2011).
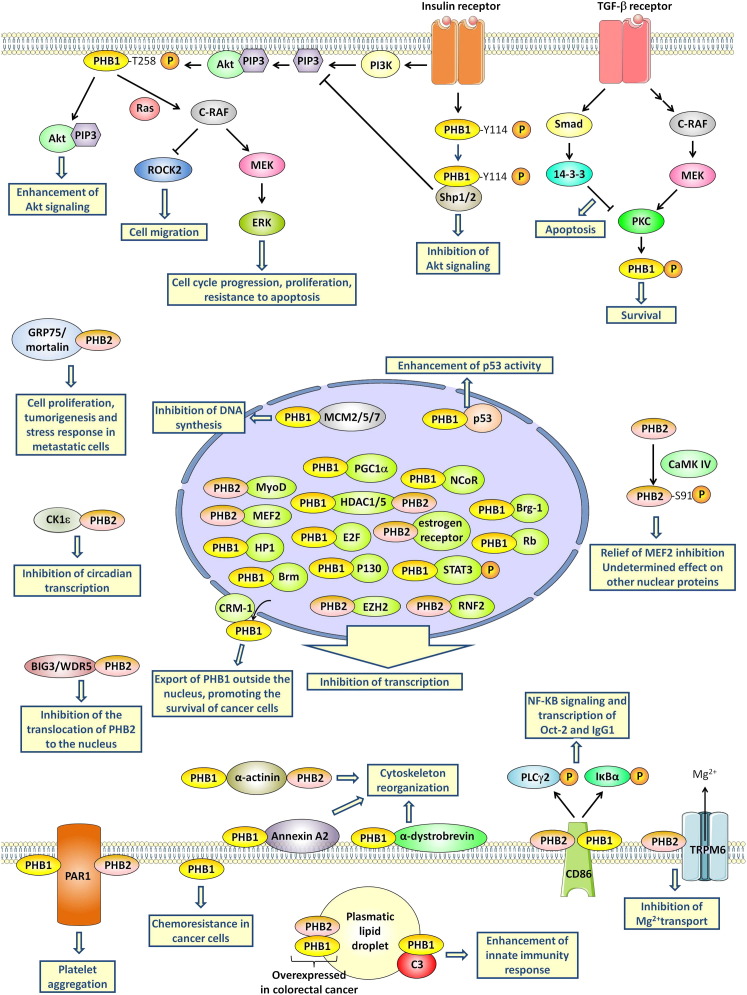
Each posttranslational modification of the PHBs has substantial effects on their activity (Figure 2 ). One of the best-documented examples is Akt phosphorylation of PHB1 at Thr258, which blocks its interaction with Shp1/2 and facilitates Akt signaling (Ande and Mishra, 2009). Shp1/2 is a phosphatase that facilitates Akt signaling and enhances insulin signaling. Additionally, the phosphorylation of PHB1 at Thr258 is necessary for the activation of C-Raf (Raf-1) by Ras (Chiu et al., 2013). Rajalingam et al. (2005) established that the activation of C-Raf by Ras requires the heterodimerization of phospho-PHB1(Thr258) with C-Raf. The phosphorylation of PHB1 at Thr258 in the plasma membrane of cancer cells activates PI3K/Akt and C-Raf/ERK pathways, which promote proliferation and metastasis (Chiu et al., 2012). By contrast, insulin-receptor-induced phosphorylation of PHB1 at Tyr114 promotes its heterodimerization with the phosphatase Shp1 and blocks Akt signaling (Ande et al., 2009a).
Figure 2.
PHB Signaling
PHBs integrate many signaling pathways (e.g., Akt, C-RAF-MEK-ERK, CaMK, and PKC) to orchestrate various aspects of cell physiology, including metabolism, transcription, apoptosis/survival, cytoskeleton reorganization, and differentiation.
It has been suggested that the regulation of PHB1 by transforming growth factor β (TGF-β) is responsible for the dual effect of this cytokine in prostate cancer (Zhu et al., 2010). In the early stage of tumorigenesis, TGF-β acts as a tumor suppressor, but subsequently it promotes metastatic spread during cancer progression. The binding of TGF-β to its receptor triggers the C-Raf/MEK/ERK pathway and also activates Smad 2/3 or Smad 1/5, thereby causing opposite effects (Figure 2). ERK activates protein kinase C δ (PKC-δ), which leads to the phosphorylation of PHB1 and consequently to cell survival and invasion. However, Smad signaling also upregulates 14-3-3 protein, which inhibits PKC-δ, leading to a hypophosphorylation of PHB1 that promotes apoptosis.
The activity of PHB2 is regulated by serine and threonine phosphorylation, in particular at Ser91 and Tyr248 (Ross et al., 2008; Sun et al., 2011). Calcium/calmodulin-dependent protein kinase IV (CaMKIV) phosphorylates PHB2 at Ser91, impeding its ability to repress the transcriptional activity of myocyte enhancer factor 2 (MEF2; Sun et al., 2011). The consequence of this phosphorylation of PHB2 on the activity of other transcription factors has not been reported. PHB2 also inhibits circadian transcription by interacting with casein kinase 1 ε (CK1ε; Kategaya et al., 2012).
PHBs have been detected at the surface of platelets, microglial cells (Wintachai et al., 2012), endothelial cells in adipose tissue (Kolonin et al., 2004), paclitaxel-resistant cancer cells (Patel et al., 2010), intestinal epithelial cells (Sharma and Qadri, 2004), and activated T cells (Yurugi et al., 2012). In platelets, PHB1 and PHB2 interact directly with protease-activated receptor 1 (PAR1) to promote platelet aggregation (Zhang et al., 2012). The physiological role of PHB1 at the surface of microglial cells and endothelial cells of adipose tissue remains elusive, but it has been demonstrated that PHB1 at the surface of cancer cells is involved in resistance to paclitaxel (Patel et al., 2010). A virulence antigen of Salmonella typhi, the causative agent of typhoid, interacts with PHB1 and PHB2 at the surface of intestinal epithelial cells, resulting in downregulation of the mitogen-activated protein kinase (MAPK) cascade and inhibition of early inflammatory responses (Patel et al., 2010). Similarly, considerable proportions of total PHB1 and PHB2 are located on the surface of activated T cells, where they contribute to the activation of ERK induced by T cell activation (Yurugi et al., 2012).
PHB1 is also present in human serum, embedded in lipid droplets where it interacts with the complement protein C3 to promote innate immunity (Mishra et al., 2007). Interestingly, serum PHB1 and PHB2 concentrations were found to be significantly elevated in patients with colorectal cancer, suggesting that they may serve as a biomarker for this type of cancer (Mengwasser et al., 2004).
A brief presentation of the interactions between PHBs and other proteins is provided in Table 1 (for a list of other putative partners of PHB1, see Table S1).
Table 1.
Proteins that Interact with PHBs
| Binding Partners | PHB | Biological Consequences | References |
|---|---|---|---|
| Mitochondrial Proteins | |||
| m-AAA protease | PHB1 | regulation of membrane protein degradation | Steglich et al., 1999 |
| ATAD3 | PHB1 | mitochondrial protein synthesis | He et al., 2012 |
| nucleoids (mitochondrial DNA associated to nucleoproteins) | PHB1/2 | maintenance of the organization and copy number of the mtDNA | Kasashima et al., 2008 |
| dynamin-like GTPase OPA-1 | PHB1 | stabilization of the structure of mitochondria during their fusion and morphogenesis | Merkwirth et al., 2008 |
| subunits of cytochrome c oxidase | PHB1 | function of the mitochondrial respiratory chain | Tsutsumi et al., 2009 |
| NADH-ubiquinone oxidoreductase 30 kDa subunit | PHB1 | stabilization of mitochondrial complex I | Park et al., 2010 |
| ND4 and ND5 | PHB1 | assembly of mitochondrial complex I | Bourges et al., 2004 |
| SLP-2 | PHB1/2 | stabilization of PHBs | Da et al., 2008 |
| TFAM | PHB1 | regulation of copy number of mitochondrial DNA | Kasashima et al., 2008 |
| ANT2 | PHB2 | unknown | Kasashima et al., 2006 |
| VDAC2 | PHB2 | unknown | Kasashima et al., 2006 |
| Hax-1 | PHB2 | stabilization of antiapoptotic Hax-1 | Kasashima et al., 2006 |
| nitric oxide-associated protein 1 (mNOA1) | PHB1 | regulation of mitochondrial protein translation and respiration | Heidler et al., 2011 |
| phosphorylated p66ShcA | PHB1 | putative protection against oxidative stress | Madireddi, 2006 |
| Transcription Factors | |||
| estrogen receptor | PHB1/2 | repression of estrogen-receptor activity | Delage-Mourroux et al., 2000; He et al., 2008; Montano et al., 1999 |
| E2Fs | PHB1 | repression of E2F activities | Gamble et al., 2007; Joshi et al., 2003, 2007; Schneider et al., 2010 |
| p53 | PHB1 | enhancement of p53-mediated transcriptional activity and chaperone activity of PHB1 for p53 | Chander et al., 2011; Fusaro et al., 2003; Joshi et al., 2007 |
| MyoD and MEF2 | PHB2 | repression of MyoD and MEF2 activities | Sun et al., 2004, 2011 |
| chicken ovalbumin upstream binding transcription factors I and II | PHB2 | transcriptional repression | Kurtev et al., 2004 |
| phosphorylated STAT3 | PHB1 | modulation of p53- and STAT3-mediated apoptosis | Kathiria et al., 2012b |
| Other Nuclear Proteins | |||
| HDAC1 and HDAC5 | PHB1/2 | transcriptional repression | Kurtev et al., 2004 |
| Rb and its family members p107 and p130 | PHB1 | repression of E2F-mediated transcription and inhibition of cell proliferation | Wang et al., 1999a, 1999b, 2002 |
| Brg-1 and Brm | PHB1 | repression of E2F-mediated transcription and inhibition of cell proliferation | Wang et al., 2002 |
| heterochromatin protein 1 (HP1) family proteins | PHB1 | repression of E2F-mediated transcription and induction of cellular senescence | Rastogi et al., 2006a |
| RING finger protein 2 (RNF2) | PHB1/2 | repression of the transcriptional activity of E2F1 and CP2c | Choi et al., 2008; Lee et al., 2008 |
| histone methyltransferase EZH2 | PHB2 | repression of estrogen-dependent transcription | Hwang et al., 2008 |
| PGC1α (PPARγ coactivator 1) | PHB2 | inhibition of the transcriptional activity of PPARγ | Endo, 2011 |
| minichromosomes maintenance complex of proteins MCM2, MCM5, and MCM 7 | PHB1 | inhibition of DNA replication | Rizwani et al., 2009 |
| nuclear receptor corepressor 1 (NCoR) | PHB1 | transcriptional repression of E2F family members | Wang et al., 2002 |
| Other Signaling Proteins | |||
| IgM receptor | PBH1 | phosphorylation of PHB1 | Terashima et al., 1994 |
| protease-activated receptor 1 | PHB1/2 | platelet aggregation | Zhang et al., 2012 |
| C-Raf | PBH1 | phosphorylation of C-Raf by Ras and consequently activation of the MEK-eIF4 pathway | Chiu et al., 2012; Chiu et al., 2013; Rajalingam et al., 2005 |
| Akt2 | PHB2 | cell-cycle exit and myogenic differentiation | Héron-Milhavet et al., 2008; Sun et al., 2004 |
| WD repeat domain 5 (WDR5, BIG3) | PHB2 | inhibition of the translocation of PHB2 to the nucleus | Kim et al., 2009 |
| Skp2B | PHB1/2 | attenuation of the transcriptional activity of p53, release of the repression of the estrogen receptor | Chander et al., 2010, 2011; Umanskaya et al., 2007 |
| MLK2 (mixed lineage kinase 2) | PHB1 | unknown | Rasmussen et al., 1998 |
| Shp1 (phosphatase) | phospho-Y114-PHB1 | modulation of insulin signaling | Ande et al., 2009a |
| PKC-δ | PHB1 | phosphorylation of PHB1 leading to a decrease in the inner mitochondrial membrane permeability and to cell survival | Zhu et al., 2010 |
| 14-3-3 protein | PHB1 | inhibition of PHB1 phosphorylation leading to an increase in the inner mitochondrial membrane permeability and to apoptosis | Zhu et al., 2010 |
| α-dystrobrevin | PHB1 | NB4 cell granulocytic differentiation | Borutinskaite et al., 2011 |
| transient Receptor Potential Melastatin 6 (TRPM6) | PHB2 | inhibition of TRPM6-mediated transepithelial Mg2+ transport | Cao et al., 2009 |
| HSP70 | PHB1 | resistance to stress | Liu et al., 2010 |
| glucose-regulated protein 75 (GRP75)/mortalin | PHB1 | cell proliferation, tumorigenesis and stress response in metastatic cells | Martín et al., 2008 |
| phospho- Ser291- Syk | PHB1 | B cell antigen-receptor signaling | Paris et al., 2010 |
| CRM-1 | PHB1 | export of PHB1 outside of the nucleus selectively in cancer cells | Rastogi et al., 2006b |
| Pex14p | PHB1 | regulation of the peroxisomal importomer | Oeljeklaus et al., 2012 |
| annexin A2 | PHB1/2 | interaction blocked by calcium, association with lipid rafts | Zhu et al., 2010 |
| alpha-actinin | PHB1/2 | interaction blocked by calcium, association with lipid rafts | Bacher et al., 2002 |
| EHD2 | Palmitoylated PHB1 | inhibition of pyruvate carboxylase leading to a decrease in glucose and fatty acid oxidation | Ande and Mishra, 2010; Vessal et al., 2006 |
| C3 | PHB1 | enhancement of innate immunity response | Mishra et al., 2007 |
| CK1ε | PHB2 | inhibition of circadian transcription | Kategaya et al., 2012 |
| Proteins of Pathogens | |||
| SV40Tag (viral oncoprotein) | PHB1 | interruption of the association between PHB1 and Brg-1/Brm | Wang et al., 2002 |
| HIV-1 glycoprotein | PHB1/2 | viral spread in nonpermissive cells | Emerson et al., 2010 |
| DENV-2 E protein | PHB2 | internalization of the dengue virus | Kuadkitkan et al., 2010 |
| Chikungunya virus E2 protein | PHB1 | internalization of the chikungunya virus | Wintachai et al., 2012 |
| SARS-CoV nonstructural protein nsp2 | PHB1/2 | supposed disruption of intracellular host signaling during SARS-CoV infection | Cornillez-Ty et al., 2009 |
| Vi polysaccharide of Salmonella typhi | PHB1/2 | inhibition of the inflammatory response upon infection with Vi- S. typhi. | Sharma and Qadri, 2004 |
| capsid protein VP1 of foot-and-mouth disease virus | PHB1 | dephosphorylation of PHB T258 | Chiu et al., 2012 |
| macrophage surface HSP70 | Leishmania donovani PHB1 | induction of a strong humoral response in leishmaniasis patients | Jain et al., 2010 |
See also Table S1.
PHBs and Cancer
Both PHB1 and PHB2 are involved in growth, resistance to chemotherapy, and metastasis through several mechanisms, including activation of the Ras-C-Raf-MEK-ERK pathway, modulation of TGF-β signaling, and transcriptional regulation. Indeed, PHB1 interacts with the transcription factor p53 in the nucleus of cancer cells to increase its transcriptional activity (Fusaro et al., 2003). p53 is a tumor suppressor that controls the cell cycle, apoptosis, genomic stability, and angiogenesis (Chander et al., 2010, 2011; Fusaro et al., 2003; Joshi et al., 2007). A component of ubiquitin ligase complexes, Skp2B, which is overexpressed in many breast cancers, interacts with both PHB1 and PHB2 to promote their degradation (Chander et al., 2010, 2011; Umanskaya et al., 2007). Because PHB1 acts as an activator (Fusaro et al., 2003) and chaperone (Chander et al., 2011) for p53, this depletion in PHB1 leads to an attenuated activity of p53 in cancer cells overexpressing Skp2B and consequently promotes their survival.
PHB1 is also an important regulator of the retinoblastoma tumor suppressor protein (Rb) and its family members P107 and p130 (Wang et al., 1999b). These proteins bind and inhibit E2F transcription factors, leading to cell-cycle arrest. PHB1 represses the activity of E2F transcription factors by interacting with several proteins, including Rb, histone deacetylases (HDACs), and the nucleosome-remodeling proteins Brg-1 and Brm (Wang et al., 1999a, 1999b, 2002).
A growing body of evidence indicates that the cellular localization of PHBs is a determinant of their function, especially in cancer cells. Patel et al. (2010) found that PHB1 is overexpressed on the surface of cancer cells that are resistant to taxoids, doxorubicin, and etoposide. They also demonstrated that the redirection of PHB1 from the cytoplasm to the cell surface is critical for drug resistance, and their work suggests that cell-surface PHB1 may serve as a biomarker of chemoresistance in cancer patients.
In normal epithelial breast cells, PHB1 is located primarily in the mitochondria, but in invasive and noninvasive breast cancer cells it is mainly confined to the nucleus, suggesting that PHB1 localization is associated with tumorigenesis (Chen et al., 2011). Confocal microscopy experiments demonstrated that PHBs colocalize with the proto-oncogenes c-myc, c-fos, p53, and Rb in neuroblastoma SK-N-SH cells (Li et al., 2011).
Treatment with the anticancer drug camptothecin leads to PHB1 and p53 being exported from the nucleus to the mitochondria of breast cancer cell lines (Fusaro et al., 2003). This camptothecin-induced translocation of PHB1, which is dependent on the protein transporter CRM-1, occurs preferentially in transformed cell lines and not in untransformed or primary cells. A peptide corresponding to the nuclear export signal of PHB1 prevented the export of PHB1, resulting in the protection of cells from apoptosis, indicating that the translocation of PHB1 from the nucleus to the mitochondria contributes to its tumor-suppressive effects (Rastogi et al., 2006b). One mechanism by which the intracellular location of PHBs is controlled involves the signaling protein BIG3/WDR5, which traps PHB2 in the cytoplasm and consequently stimulates the estrogen-signaling pathway in the hormone-related growth of breast cancer cells (Kim et al., 2009).
Beyond these activities of PHBs as proteins, the 3′-UTR of PHB1 messenger RNA (mRNA) was found to suppress both the proliferation of human breast cancer cells in vitro and the growth of breast tumors in xenografted mice in vivo (Manjeshwar et al., 2003). This was the first demonstration that an RNA molecule can function as a tumor suppressor in human breast cancer.
The tumor-suppressor activity of PHB1 and its mRNA 3′-UTR is further supported by the discovery that the oncogenic microRNA-27a (miR-27a), which is upregulated in many cancers (Chhabra et al., 2010), targets the 3′-UTR of PHB1 and downregulates PHB1 in human gastric adenocarcinoma (Liu et al., 2009a). Fletcher et al. (2012) demonstrated that miR-27a is upregulated by androgen receptor in prostate cancer, resulting in reduced PHB1 mRNA and protein levels and increased cancer cell growth. Interestingly, adding PHB1 to cells overexpressing miR-27a blocked miR-27a-induced growth, demonstrating that the oncogenic activity of miR-27a strongly involves PHB1 signaling in prostate cancer.
An important question that needs to be examined is why PHBs display both anti- and protumorigenic roles in cancers (summarized in Table 2 ). This dichotomy may reflect the heterogeneity of cancer cell types, which are manifested by an alteration of different signaling pathways. Consequently, the regulation of transcription, translation, and posttranslational modification of PHBs probably depends on the cancerous cell type involved. These posttranslational events (e.g., phosphorylation, SUMOylation, O-GlcNAc modification, palmitoylation, and transamidation) modulate both the subcellular localization of PHBs and their downstream effects on proliferation and survival of cells. On the cell surface PHB1 promotes chemoresistance, but on the inner face of the plasma membrane it can contribute to the oncogenic activation of C-Raf. However, it is tempting to speculate that in the nucleus PHB1 displays antitumorigenic activity due to its action on p53, Rb, p107, p130, and components of the mammalian replication machinery. Additional studies are required to decipher the roles played by signaling and intracellular localization of PHBs in oncogenesis.
Table 2.
Roles of PHB Signaling in Cancer
| Antitumorigenic Roles of PHBs | References |
|---|---|
| PHB1 activates the tumor suppressor p53 | Chander et al., 2011; Fusaro et al., 2003; Joshi et al., 2007 |
| PHB1 interacts with the tumors suppressors Rb, p107, and p130 to repress E2F-mediated transcription and inhibit cell proliferation | Wang et al., 1999a, 1999b, 2002 |
| PHB1 represses DNA replication by interacting with components of the mammalian replication machinery | Rizwani et al., 2009 |
| Protumorigenic Roles of PHBs | |
| PHB1 and PHB2 are necessary for the activation of C-RAF by Ras | Rajalingam et al., 2005 |
| phosphorylated PHB1 promotes the survival of prostate cancer cells | Zhu et al., 2010 |
| the oncomir miR-27a downregulates PHB1 | Liu et al., 2010; Fletcher et al., 2012 |
| localized on the cell surface, PHB1 mediates resistance to taxoids | Patel et al., 2010 |
PHBs and Cytoprotection
Many studies have indicated that PHBs protect cells from oxidative stress, which is due to an excessive production or an impaired elimination of ROS (Jones, 2008). It is closely associated with mitochondrial dysfunction and is an important component of the etiology of cancers, inflammatory, cardiovascular, and neurodegenerative diseases, and diabetes mellitus. In view of the central role of PHBs in maintaining the normal structure and function of mitochondria, it is unsurprising that PHBs can alleviate the deleterious effect of oxidative stress.
Many stresses, in particular oxidative stress, upregulate PHB1 and PHB2 to promote cell survival. Indeed, PHB1 expression is enhanced in neurons by various stresses, including electrical stimulation, hypoxia-ischemia, oxygen-glucose deprivation (Zhou et al., 2012), exercise-induced neuroplasticity (Ding et al., 2006), injection of the neurotoxin 6-hydroxydopamine (Park et al., 2010), and schizophrenia-induced oligodendrocyte dysfunction (Bernstein et al., 2012). PHB1 is also upregulated in the liver of steatohepatitis patients (Tsutsumi et al., 2009), in fetal rabbit lung after exposure to hyperoxic conditions (Henschke et al., 2006), in pancreatic β-cells after ethanol intoxication, (Lee et al., 2010b), and in cardiac cells after ischemic-hypoxic preconditioning (Kim et al., 2006; Muraguchi et al., 2010) or chronic restraint stress (Liu et al., 2004). This cytoprotectant response involves the translocation of PHB1 from the nucleus and the cytoplasm to mitochondria in pancreatic β-cells (Lee et al., 2010b), in the retina and retinal epithelium (Lee et al., 2010a), and in ovarian granulosa cells (Chowdhury et al., 2007; Figure S2). Sripathi et al. (2011) suggested that the localization and trafficking of PHBs are determined by the modulation of their binding to specific lipids. Indeed, strong binding of PHB1 to cardiolipin was shown to correlate with the localization of PHB1 within mitochondria in transformed epithelial cells after an oxidative stress. By contrast, under normal conditions in these cells, PHB1 binds strongly to PIP3 but not to cardiolipin.
Overexpression of PHB1 protects pancreatic β-cells, ovarian granulosa cells, and cardiomyocytes from apoptosis induced by ethanol, ceramide, staurosporine, serum withdrawal, and oxidative stress-induced injury, consistent with PHB1 having a cytoprotective role (Chowdhury et al., 2007, 2011; Lee et al., 2010b; Liu et al., 2009b).
Merkwirth et al. (2012) recently described a critical role of PHB2 in the survival of neurons. Neuron-specific deletion of PHB2 in the mouse forebrain impaired mitochondrial architecture, leading to tau hyperphosphorylation and filament formation. This phenotype was accompanied by severe behavioral and cognitive dysfunctions that were reminiscent of Alzheimer’s disease.
The details of the mechanisms of this cytoprotection remain poorly documented. The only other actors that have been identified as being involved are S1P and STAT3. S1P protects the heart from ischemia-reperfusion damage by directly activating PHB2, thereby regulating respiration and cytochrome oxidase subunit IV assembly and decreasing the heart’s susceptibility to opening of the permeability transition pore (PTP; Gomez et al., 2011).
Interleukin-6 (IL-6) increases PHB1 levels through phosphorylation of the transcription factor STAT3 to promote the survival of intestinal cells and cardiomyocytes (Gratia et al., 2012; Theiss et al., 2007b). In intestinal epithelial cells in vivo, PHB1 interacts with STAT3 to modulate STAT3-mediated apoptosis (Kathiria et al., 2012b). In cardiomyocytes, IL-6-induced upregulation of PHB1 is central to the cardioprotective effects of IL-6 against oxidative stress (Gratia et al., 2012). Importantly, phosphorylated STAT3 protects cardiomyocytes against oxidative stress by stimulating respiration and inhibiting the PTP within mitochondria, and not through a transcriptional effect (Boengler et al., 2010). It would be interesting to determine whether PHB1 modulates this mitochondrial action of STAT3.
PHBs and Inflammatory Diseases
Inflammatory bowel diseases (IBDs) are characterized by strong oxidative stresses associated with downregulated antioxidant enzymes in the intestinal mucosa (Lih-Brody et al., 1996). Several studies in vitro and in vivo have indicated that PHB1 alleviates intestinal inflammation and promotes the survival of cells exposed to oxidative stress. In intestinal epithelial cells, PHB1 binds to a Salmonella typhi antigen to inhibit the inflammatory response to S. typhi infection (Sharma and Qadri, 2004). PHB1 expression is abnormally low during ulcerative colitis and Crohn’s disease, the two most common forms of IBD (Hsieh et al., 2006; Theiss et al., 2007a; Yeo et al., 2006), due to an overstimulation of tumor necrosis factor α (TNF-α) signaling in the inflamed colon (Theiss et al., 2009a). This downregulation of PHB1 increases ROS signaling and TNF-α-induced autophagy, promoting inflammation in patients with IBD (Kathiria et al., 2012a). Interestingly, transgenic mice overexpressing PHB1 in intestinal epithelial cells exhibit reduced proinflammatory nuclear factor κB (NF-κB) signaling in the colonic mucosa after treatment with TNF-α, demonstrating that PHB1 plays a critical role in inflammation. A detailed examination of this mechanism of action revealed that PHB1 inhibits TNF-α-induced translocation of NF-κB by downregulating the expression of importin α3, a protein involved in NF-κB nuclear import (Theiss et al., 2009a). In an in vivo model of IBD, PHB1 transgenic mice exhibited lower oxidative stress and colitis than wild-type mice (Theiss et al., 2009b). This cytoprotective effect is due to the upregulation of nuclear factor erythroid 2-related factor 2 (Nrf2), a transcriptional activator of antioxidant responses. Further evidence that PHB1 is a putative therapeutic target for treating IBD comes from the observations that adenovirus-directed administration by enema and nanoparticle-based colonic delivery of PHB1 effectively enhanced the levels of PHB1 at the surface of colonic epithelial cells and alleviated colitis induced by dextran sodium sulfate (Theiss et al., 2011).
Picard et al. (2013) recently demonstrated that PHB1 accumulates in the nucleus of osteoarthritic chondrocytes, where it represses the expression of the transcription factor PITX1, suggesting an involvement of PHB1 in the etiology of osteoarthritis.
In addition to PHB1, PHB2 is also involved in inflammation. Indeed, heterozygous PHB2+/− were shown to be more sensitive to liver insults and inflammatory aggressions than wild-type animals, confirming a cytoprotective and anti-inflammatory role of PHB2 in liver (Sánchez-Quiles et al., 2012).
Lucas et al. (2013) recently discovered how PHB1 and PHB2 induce an immune response in B cells. The interaction of CD86 with PHBs was shown to induce the phosphorylation of IκBα, phospholipase Cγ2, and protein kinase Cα/β(II), leading to the nuclear translocation of NF-κB (p65) into the nucleus and the subsequent transcription of Oct-2 and IgG1.
PHBs and Metabolic Diseases
Ande and Mishra (2009) and Ande et al. (2009a) established that PHB1 is a key regulator of insulin signaling and adipocyte differentiation. Indeed, PHB1 is directly phosphorylated by the insulin receptor to promote insulin signaling and block Akt signaling, whereas phosphorylation of PHB1 by Akt has the opposite effect.
Interestingly, PHB1 is not only phosphorylated but is also conjugated to O-linked β-N-acetylglucosamine in myoblast cells in response to insulin and high glucose. This glucose-induced O-GlcNAc modification and phosphorylation of PHB1 may be associated with insulin resistance modification and tyrosine phosphorylation of PHB (Ande et al., 2009b; Gu et al., 2011).
PHB1 inhibits pyruvate carboxylase and decreases insulin-stimulated oxidation of glucose and fatty acid in adipocytes, implying that it has a role in promoting lipid accumulation (Vessal et al., 2006). Crosslinking experiments showed that PHB1 associates with pyruvate carboxylase and Eps 15 homology domain protein 2 (EHD2), suggesting that PHB1 shuttles between the extracellular space and the mitochondria by a mechanism involving lipid rafts and EHD2.
Recently, Ande et al. (2012) demonstrated that treatment of preadipocytes with insulin or a peroxisome proliferator-activated receptor γ (PPARγ) agonist upregulates PHB1, thereby promoting preadipocyte differentiation into adipocytes. Remarkably, overexpression of PHB1 was sufficient to induce adipogenesis. PHB1 on the cell surface is also a marker of adipose vasculature and may be a possible therapeutic target for obesity (see below).
PHBs and Pathogenic Agents
Several recent studies demonstrated that the internalization of some viruses involves PHBs. For instance, PHB1 interacts with chikungunya virus (Wintachai et al., 2012), PHB2 interacts with dengue virus (Kuadkitkan et al., 2010), and both PHB1 and PHB2 interact with severe acute respiratory syndrome coronavirus (SARS-CoV; Cornillez-Ty et al., 2009). PHB1 and PHB2 also interact with the HIV-1 glycoprotein to promote replicative spread in nonpermissive cells (Emerson et al., 2010). Interestingly, recombinant capsid protein VP1 (rVP1) of foot-and-mouth disease virus dephosphorylates Akt and phospho-PHB Ttr258 in lipid rafts to downregulate C-Raf/ERK signaling and inhibit metastasis of cancer cells, both in vitro and in vivo (Chiu et al., 2012). Moreover, using high-throughput mass spectrometry, Jang et al. (2012) discovered that lipoteichoic acid, a major virulence factor of Gram-positive bacteria, binds to PHB2, implicating PHB2 in host immune responses to infections.
Drug Development Outlook
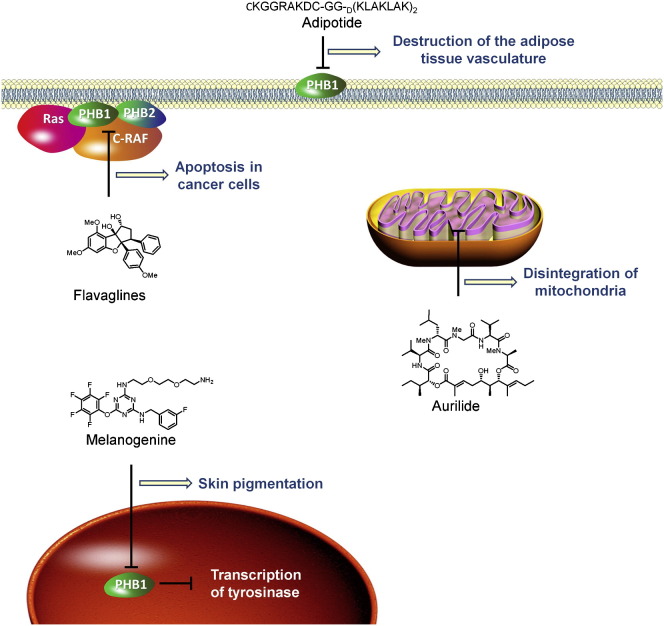
The activities of PHBs are affected by some natural products (e.g., flavaglines and aurilide), fully synthetic small molecules (e.g., melanogenin), and adipotide, a chimeric peptide that is currently in phase I clinical trial. These compounds (except for adipotide) were examined for a variety of activities before it became clear that PHBs were their molecular targets. The best-studied PHB ligands are flavaglines.
Flavaglines
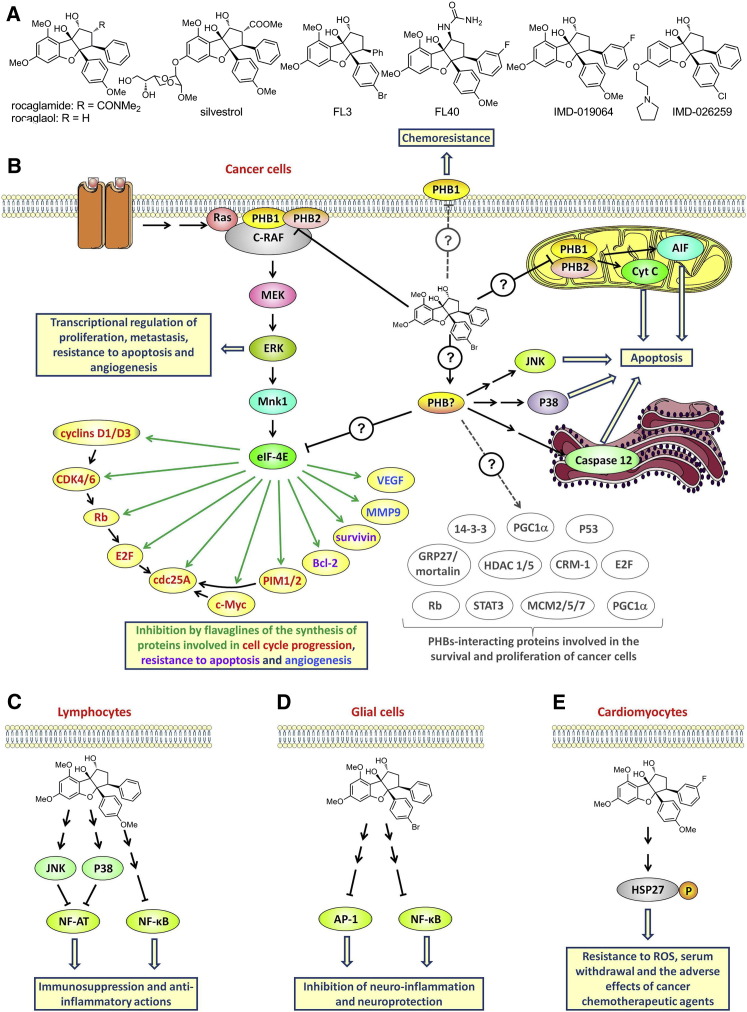
Flavaglines have a unique cyclopenta[b]benzofuran skeleton (Ebada et al., 2011; Ribeiro et al., 2012b). Rocaglamide, the first of these compounds to be described, was isolated more than 30 years ago by King et al. (1982) from medicinal plants of the genus aglaia (Meliaceae) in Southeast Asia. Since then, ∼100 other flavaglines, including rocaglaol and silvestrol, have been identified (Figure 3 A; Figure S3).
Figure 3.
Structure and Pharmacological Actions of Flavaglines
(A) Structure of selected natural (rocaglamide, rocaglaol, and silvestrol) and synthetic (FL3, FL40, and IMD-019064) flavaglines.
(B–E) Proposed models of the mode of action of flavaglines in cancer cells (B), lymphocytes (C), glial cells (D), and cardiomyocytes (E).
See also Figure S2, which illustrates the translocalization of PHB1 to mitochondria in noncancerous cells to promote survival.
Although flavaglines display a myriad of pharmacological effects due to their anticancer, anti-inflammatory, neuroprotective, and cardioprotective properties, it is the anticancer properties that have attracted the most attention from scientists (Ebada et al., 2011; Ribeiro et al., 2012b). At concentrations in the low-nanomolar range, flavaglines inhibit the proliferation of tumor cells, are not toxic to normal cells (Hausott et al., 2004; Ribeiro et al., 2012a; Su et al., 2006; Thuaud et al., 2009, 2011; Zhu et al., 2007), and show no sign of toxicity in mice (Bernard et al., 2011; Cencic et al., 2009; Lee et al., 1998; Thuaud et al., 2009; Zhu et al., 2009). King et al. (1982) were the first to demonstrate the antileukemic activity of rocaglamide in a mouse model. Subsequently, Ohse et al. (1996) established that flavaglines strongly inhibit protein synthesis, and Lee et al. (1998) demonstrated that these compounds delay the growth of human breast cancer cells in athymic mice. However, because the tumors were not eradicated, these investigations were interrupted. Since then, with the development of targeted therapies, approaches to the development of cytostatic compounds have changed radically (Gutierrez et al., 2009). As a consequence, there is now a renewed interest in flavaglines as potential anticancer agents.
Although flavaglines have shown significant anticancer effects in mouse models of cancer (Alinari et al., 2012; Cencic et al., 2009; Hwang et al., 2004; King et al., 1982; Lee et al., 1998; Lucas et al., 2009; Meurer-Grimes et al., 2002; Thuaud et al., 2011), their most promising potential is associated with their ability to enhance the in vivo efficacy of other anticancer drugs, and in particular to relieve the resistance of tumors to chemotherapies. Indeed, flavaglines were shown to enhance doxorubicin chemosensitivity in several mouse lymphoma models (Bordeleau et al., 2008; Cencic et al., 2009; Zhu et al., 2009). Importantly, flavaglines did not display any overt sign of toxicity in mice in these studies, consistent with previous observations.
Very recently, using affinity chromatography-coupled mass spectrometry, Polier et al. (2012) identified PHB1 and PHB2 as the direct targets of flavaglines. The binding of flavaglines to PHBs inhibits Ras-C-Raf-MEK-ERK signaling, and such signaling is essential for the survival of cancer cells (Figure 3B). C-Raf needs to interact with PHBs to be phosphorylated by Ras and thus participate in signaling (Rajalingam et al., 2005). The binding of flavaglines to PHBs prevents this interaction both in vitro and in vivo. Knockdown of either PHB1 or PHB2 with small interfering RNA (siRNA) mimicked the effects of flavaglines through inhibition of cap-dependent translation and an arrest of cell-cycle progression via a depletion of cyclin D3, CDK4, CDK6, and cdc25A. Moreover, rocaglamide strongly depleted PHB1 and phosphorylated C-Raf at the plasma membrane, suggesting that the relocation of PHBs to the membrane is involved in the mode of action of flavaglines. These various observations support the idea that flavaglines exert their anticancer effects through their action on PHBs.
These findings are of particular interest because we now have evidence that Ras-C-Raf-ERK constitutes an overactivated pathway in many human cancers, and that this pathway is a promising target for treatments in oncology (Matallanas et al., 2011; Maurer et al., 2011). Bleumink et al. (2011) showed that this inhibition of C-Raf-ERK signaling blocks cap-dependent protein synthesis. Indeed, ERK activates Mnk1, which phosphorylates eIF4E, allowing cap-dependent synthesis to occur. Few proteins require phosphorylated eIF4E for their synthesis, and most of them are involved in oncogenesis, angiogenesis, and chemoresistance. Thus, the in vivo anticancer effects of flavaglines seems to be a consequence of the inhibition of the synthesis of cyclins D1 and D3, CDK4, CDK6, cdc25A c-Myc, Bcl-2, survivin, Mcl-1, the PIM1/2 kinases, vascular endothelial growth factor (VEGF), and matrix metallopeptidase 9 (MMP9) (Bordeleau et al., 2008; Cencic et al., 2009; Nasr et al., 2013; Polier et al., 2012; Schatz et al., 2011).
Although inhibition of the Ras-C-Raf-ERK signaling pathway may be involved in the flavagline-induced suppression of cap-dependent synthesis in cells (Bleumink et al., 2011), it is more difficult to explain the observed inhibition of protein synthesis in vitro, where C-Raf, MEK, and ERK are absent. This observation suggests either that another molecular target is involved in the inhibition of translation by flavaglines or that PHBs interact with the translational machinery to control its activity.
Flavaglines induce apoptosis in cancer cells through a myriad of mechanisms (Ribeiro et al., 2012b) involving cytochrome c (Mi et al., 2006b; Zhu et al., 2007), apoptosis-inducing factor (Thuaud et al., 2009), caspase 12 (Thuaud et al., 2009), and the MAPKs JNK and p38 (Zhu et al., 2007, 2009). Whether these mechanisms involve PHB1 and PHB2 or another target remains to be determined. The effect of flavaglines on PHBs-interacting proteins other than C-Raf is also an issue that needs to be examined.
Silvestrol is the flavagline that has been the most studied in vivo for its anticancer properties (Alinari et al., 2012; Bordeleau et al., 2008; Cencic et al., 2009; Hwang et al., 2004; Kim et al., 2007; Mi et al., 2006a; Robert et al., 2009; Schatz et al., 2011). However, the development of this compound is severely limited by its sensitivity to P-glycoprotein-mediated multidrug resistance and its suboptimal absorption, distribution, metabolism, and excretion (ADME) characteristics (Gupta et al., 2011; Liu et al., 2012). Fortunately, structure-activity relationship (SAR) studies have established that the deletion of the methyl ester or the amide in position 2 results in compounds that escape this multidrug resistance without any loss of cytotoxicity (Figure S3; Ribeiro et al., 2012a; Thuaud et al., 2009, 2011).
In addition to their anticancer effects, flavaglines also display potent anti-inflammatory and neuroprotective activities. Indeed, flavaglines at nanomolar concentrations act as immunosuppressors by inhibiting the production of interferon-γ, TNF-α, IL-2, and IL-4 by T lymphocytes (Proksch et al., 2005). This immunosuppression is mediated by activation of the MAPKs JNK and p38, which selectively inactivate nuclear factor of activated T cells (NFAT; Figure 3C). It is still not known how flavaglines activate JNK and p38. At higher concentrations, flavaglines also inhibit NF-κB, another transcription factor that is involved in inflammation (Baumann et al., 2002). Interestingly, the synthetic flavagline IMD-019064 inhibits NF-κB signaling and displays potent in vitro anti-inflammatory effects that manifest as an inhibition of proinflammatory mediator release from astrocytes, microglia, and endothelial cells (Fahrig et al., 2005; Figure 3D). More importantly, IMD-019064 protects dopaminergic neurons both in vitro and in vivo in models of Parkinson’s disease (MPP+-induced neurotoxicity) and traumatic brain injury. Recent SAR studies led to the identification of FL40, which provides greater neuroprotection in vitro than IMD-019064 (Ribeiro et al., 2012a). The neuroprotective activity of flavaglines is currently being actively explored by the pharmaceutical company Intermed Discovery, which has been developing IMD-026259 (Figure 3A) for the treatment of Parkinson’s disease.
These studies have prompted evaluations of the cytoprotective potential of flavaglines in other tissues. The synthetic flavagline FL3 (Figure 3A), which displays a potent cytotoxicity in cancer cells (Thuaud et al., 2009), was shown to protect cardiomyocytes in vitro and in vivo against various stresses, including anthracycline cardiotoxicity (Bernard et al., 2011). The heart is extremely sensitive to the toxicity of anthracyclines, such as doxorubicin. These medicines are among the most effective anticancer drugs available and have antitumor activity against both hematopoietic and solid tumors; unfortunately, their clinical utility is markedly hampered by their cardiotoxicity, which can lead to dilated cardiomyopathy and congestive heart failure (Minotti et al., 2004).
FL3 protects cardiomyocytes from the apoptosis induced by both doxorubicin and serum starvation (Bernard et al., 2011). Interestingly, these stresses are of different natures: doxorubicin causes an oxidative stress, whereas serum starvation blocks the growth factor signaling that is necessary for cell survival, mimicking an important component of myocardial ischemia. Treatment with FL3 significantly reduces mortality and attenuates both apoptosis and fibrosis in the hearts of doxorubicin-treated mice. Thus, flavaglines may both enhance the anticancer efficacy of anthracyclines and alleviate their main adverse effect, cardiotoxicity. Anthracyclines are central to cancer chemotherapies, so the discovery of a new class of cardioprotective agents would be extremely valuable clinically, and further studies to examine this therapeutic application are therefore warranted.
The cardioprotective effects of flavaglines are mediated through the phosphorylation of the small heat shock protein Hsp27 (Figure 3E). Hsp27 is critical for protecting cells against many types of damage, including the cardiotoxicity of doxorubicin, and exerts its effects in several ways, including chaperone activity, control of redox homeostasis, and inhibition of apoptosis (Kostenko and Moens, 2009). Whether PHBs or another target is involved in this cardioprotective effect of flavaglines has not been examined.
Although the cytoprotective and anti-inflammatory effects of flavaglines have not yet been demonstrated to be mediated through a binding to PHBs, it is unlikely that another target is involved. Indeed, both their pharmacological effects and cytotoxicity in cancer cells occur at the same range of concentrations. As far as we know, no other natural product that possesses such a complex three-dimensional structure has been shown to bind to different classes of proteins with a comparably high affinity.
It would be useful to further evaluate this therapeutic application of flavaglines and elucidate the mechanisms involved. It may seem odd than an anticancer drug can also display various cardio- or neuroprotective effects; however, there are precedents. For example, rapamycin derivatives (Erlich et al., 2007; Khan et al., 2006; Malagelada et al., 2010) and HDAC inhibitors are both cardio- and neuroprotectants in addition to having anticancer effects (Bush and McKinsey, 2009; Mai et al., 2009). A first clue to understanding this paradox may be that PHB1 is localized mainly in the mitochondria of normal cells and in the nucleus in cancer cells (Chen et al., 2011). The translocation of PHB1 from the nucleus and cytosol to mitochondria promotes survival in normal cells but apoptosis in cancer cells (Fusaro et al., 2003). It is therefore tempting to suggest that the opposite behaviors of flavaglines in cancer and normal cells are the consequences of different intracellular localizations of PHBs.
Although many academic studies have demonstrated a therapeutic potential of flavaglines in preclinical models of cancers and inflammatory and cardiac diseases, these compounds have not yet been examined in a clinical trial. To our knowledge, only two pharmaceutical companies (Intermed Discovery and Infinity Pharmaceutical) are currently developing flavaglines for the treatment of Parkinson’s disease and cancers, respectively.
Aurilide
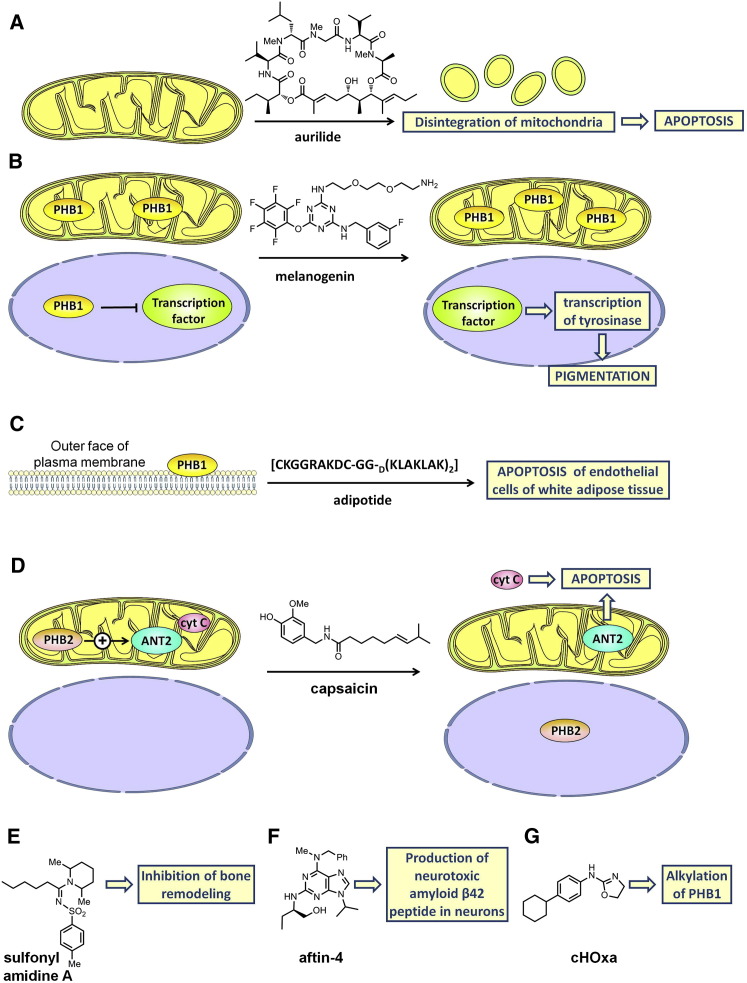
Isolated from the Japanese sea hare (Dolabella auricularia; Suenaga et al., 2004; Suenaga et al., 1996), aurilide is a cyclic depsipeptide that displays a strong cytotoxicity at nanomolar and subnanomolar concentrations on a panel of cancer cell lines (Figure 4 A; Figure S4). Work involving affinity chromatography identified PHB1 as the molecular target of aurilide (Sato et al., 2011). The binding of this toxin activates the proteolytic processing of the dynamin-like GTPase optic atrophy 1 (OPA1), which in turn triggers remodeling of the mitochondrial cristae, leading to apoptosis. This devastating effect on mitochondria probably explains the extreme toxicity of this compound, which impedes its study in animal models and precludes its further development as a medicament.
Figure 4.
Proposed Mechanism of Action of Various PHB Ligands
(A) Aurilide is a cytotoxic depsipeptide that binds to PHB1 and triggers remodeling of the mitochondrial cristae to lead to apoptosis.
(B) Melanogenin binds to PHB1 to induce the expression of tyrosinase, the rate-limiting enzyme in melanogenesis.
(C) Adipotide is a chimeric peptide that targets PHB1 at the surface of the vascular endothelial cells of white adipose tissue. Its proapoptotic sequence is responsible for the damage to this tissue.
(D) Binding of capsaicin to PHB2 induces its translocation from mitochondria to the nucleus, a noncompetitive inhibition of the ANT2 and the release of cytochrome c into the cytosol, leading to the apoptosis of myeloid leukemia cells.
(E) Sulfonyl amidine (A) inhibits the osteoclastogenesis involved in bone remodeling. This compound binds to PHB1 and three other proteins. Which of these proteins is the relevant target involved in this antiresorptive activity remains unknown.
(F) Aftin-4, which binds to PHB1, VDAC1, and mitofilin, promotes in neurons the production of the neurotoxic peptide Aβ42, which is involved in the etiology of Alzheimer’s disease.
(G) The dihydrooxazole cHOxa alkylates PHB1, suggesting that it could be used as a chemical probe to analyze the structure of PHB1.
Melanogenin
In a quest to identify compounds that modulate skin pigmentation, Snyder et al. (2005) screened a tagged-triazine library on unpigmented melanocytes (Figure 4B; Figure S5). They identified melanogenin as an inducer of pigmentation with an EC50 of 2.5 μM. This compound upregulates tyrosinase, the rate-limiting enzyme in the biosynthesis of melanin.
Snyder et al. (2005) conjugated melanogenin to an agarose support, which enabled them to identify PHB1 as the molecular target by affinity chromatography. Further biological investigations confirmed that PHB1 is responsible for the induction of pigmentation. Immunofluorescence microscopy studies showed that PHB1 was localized only in mitochondria, which led these authors to suggest that the binding of melanogenin to PHB1 may disrupt the interaction between PHB1 and a transcriptional factor, thereby causing its translocation to the nucleus and induction of the expression of tyrosinase, the rate-limiting enzyme in melanogenesis.
This study was the first to demonstrate and unravel the involvement of PHB1 in the regulation of mammalian pigmentation. Although these discoveries may provide a basis for the development of novel cosmetics and drugs for the treatment of pigment disorders, no further investigation in this direction has been reported.
Recently, Wu and Wu (2012) demonstrated that PHB1 is enriched in lipid rafts in HaCaT keratinocytes after UVB irradiation, and that this protects the cells against UVB-induced apoptosis. These findings are consistent with the notion that PHB1 contributes to protection of the skin against UVB.
Adipotide
Kolonin et al. (2004) and Staquicini et al. (2011) used phage display techniques to identify a peptide homologous to a region of annexin A2 that targets PHB1 at the surface of the vascular endothelial cells of white adipose tissue (Figure 4C). By fusion with a proapoptotic sequence, they obtained adipotide (CKGGRAKDC-GG-D(KLAKLAK)2), a chimeric peptide that damages the vasculature of the adipose tissue and consequently disrupts the blood supply to adipocytes. In obese mice, adipotide induced a substantial loss of weight and normalized metabolism, leading to a reversal of obesity without any adverse sign of toxicity. Surprisingly, food intake was reduced, suggesting that there is an unexpected relationship between the adipose tissue vasculature and the regulation of feeding behavior (Kim et al., 2010).
In obese rhesus monkeys, 4 weeks of adipotide treatment led to a 28% reduction in body fat and also reduced food intake and improved the animals’ metabolic status (i.e., reduced insulin resistance); these findings have implications for the development of treatment for type 2 diabetes (Barnhart et al., 2011). Adipotide is currently being developed by the biotech company Arrowhead Research Corporation, and entered a phase I trial involving obese patients with prostate cancer in July 2012. Because adipotide has a negative endocrine effect on tumor growth, it is expected to slow tumor growth in these patients.
Capsaicin
Capsaicin is a component of hot chili peppers that inhibits the proliferation of many cancer cell lines. Using affinity chromatography, Fletcher et al. (2012) demonstrated that capsaicin (0.1–1 mM) binds to PHB2, and that this binding induces a series of proapoptotic events in human myeloid leukemia cells, including (1) the translocation of PHB2 from mitochondria to the nucleus; (2) the dissociation of PHB2 from Adenine Nucleotide Translocator 2 (ANT2), leading to a noncompetitive inhibition of ANT activity; and (3) the release of cytochrome c into the cytosol (Figure 4D). The latter two effects could be caused by the depletion of PHB2 in mitochondria, which would compromise mitochondrial integrity and promote apoptosis.
Capsaicin was used in these experiments at concentrations that are much too high to be therapeutically relevant, but this work may constitute the basis for an optimization program to develop a clinically useful drug.
Sulfonyl Amidine Derivatives
Sulfonyl amidine A inhibits the osteoclastogenesis involved in bone remodeling with an IC50 of 1.8 μM (Lee et al., 2010c; Figure 4E). Chang et al. (2011) used affinity chromatography to identify the molecular target involved in this process and found that this compound binds to PHB1 and three other proteins. Which of these proteins is the relevant target involved in this antiresorptive activity remains to be determined.
Aftin-4
While investigating how the toxic amyloid-β42 (Aβ42) peptide is produced in Alzheimer’s disease, Bettayeb et al. (2012) observed that the adenine derivative Aftin-4 promotes Aβ42 production in neurons (Figure 4F). Aftin-4 was found to interact with three proteins related to neural degeneration: PHB1, voltage-dependent anion channel 1 (VDAC1), and mitofilin.
cHOxa
The dihydrooxazole cHOxa, which displays some cytotoxicity with a GI50 of 7.2 μM in B16F0 cells, was shown to alkylate the Asp40 of PHB1, suggesting that it could be used as a chemical probe to analyze the structure of PHB1 (Trzeciakiewicz et al., 2011; Figure 4G).
Conclusions
The regulation by PHBs of cell survival, apoptosis, metabolism, and inflammation makes these proteins promising therapeutic targets for novel treatments for cancer and neurodegenerative, metabolic, and inflammatory diseases. Their intracellular localization and their translocation in response to proapoptotic signals differ substantially between normal and cancer cells. This divergence of behavior, which is not fully understood, may be exploited to develop therapeutic agents. The phase I trial of adipotide for the treatment of obese patients with prostate cancer may be the first step on the road to developing PHB ligands of clinical value. The recent discovery that flavaglines target PHBs warrants further studies of this class of anticancer and cytoprotective agent. In addition to the ongoing exploration of the roles of PHBs in physiology and physiopathology, we anticipate that this field will stimulate research addressing other classes of PHB ligands with original pharmacological properties.
Over the last few years, PHBs have been found to interact with more than 60 other proteins to regulate a myriad of cellular events. There is no doubt that in the coming years, new partners and new cellular regulations will be uncovered. An important issue will be to determine when the alterations of PHB expression, subcellular localization, and signaling are involved in the etiology of not only cancers but also cardiac, neurological, inflammatory, and metabolic diseases. Translating this knowledge into clinical applications may lead to the development of new medicines and biomarkers for diagnosis and prognosis.
Acknowledgments
We thank Professor Roger A. Johnson for useful discussions. Financial support for this work was provided to L.D. by the Association pour la Recherche sur le Cancer (ARC, grants 3940 and SFI20111204054), Conectus, Labex Medalis, and the Fondation pour la Recherche Médicale. We also thank ARC (N.R.) and MNESR (F.T.) for fellowships.
Footnotes
Supplemental Information includes five figures and one table and can be found with this article online at http://dx.doi.org/10.1016/j.chembiol.2013.02.006.
Supplemental Information
References
- Alinari L., Prince C.J., Edwards R.B., Towns W.H., Mani R., Lehman A., Zhang X., Jarjoura D., Pan L., Kinghorn A.D. Dual targeting of the cyclin/Rb/E2F and mitochondrial pathways in mantle cell lymphoma with the translation inhibitor silvestrol. Clin. Cancer Res. 2012;18:4600–4611. doi: 10.1158/1078-0432.CCR-12-0839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ande S.R., Mishra S. Prohibitin interacts with phosphatidylinositol 3,4,5-triphosphate (PIP3) and modulates insulin signaling. Biochem. Biophys. Res. Commun. 2009;390:1023–1028. doi: 10.1016/j.bbrc.2009.10.101. [DOI] [PubMed] [Google Scholar]
- Ande S.R., Mishra S. Palmitoylation of prohibitin at cysteine 69 facilitates its membrane translocation and interaction with Eps 15 homology domain protein 2 (EHD2) Biochem. Cell Biol. 2010;88:553–558. doi: 10.1139/o09-177. [DOI] [PubMed] [Google Scholar]
- Ande S.R., Gu Y., Nyomba B.L.G., Mishra S. Insulin induced phosphorylation of prohibitin at tyrosine114 recruits Shp1. Biochim. Biophys. Acta. 2009;1793:1372–1378. doi: 10.1016/j.bbamcr.2009.05.008. [DOI] [PubMed] [Google Scholar]
- Ande S.R., Moulik S., Mishra S. Interaction between O-GlcNAc modification and tyrosine phosphorylation of prohibitin: implication for a novel binary switch. PLoS ONE. 2009;4:e4586. doi: 10.1371/journal.pone.0004586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ande S.R., Xu Z., Gu Y., Mishra S. Prohibitin has an important role in adipocyte differentiation. Int J Obes. 2012;36:1236–1244. doi: 10.1038/ijo.2011.227. [DOI] [PubMed] [Google Scholar]
- Bacher S., Achatz G., Schmitz M.L., Lamers M.C. Prohibitin and prohibitone are contained in high-molecular weight complexes and interact with α-actinin and annexin A2. Biochimie. 2002;84:1207–1220. doi: 10.1016/s0300-9084(02)00027-5. [DOI] [PubMed] [Google Scholar]
- Back J.W., Sanz M.A., De Jong L., De Koning L.J., Nijtmans L.G.J., De Koster C.G., Grivell L.A., Van Der Spek H., Muijsers A.O. A structure for the yeast prohibitin complex: Structure prediction and evidence from chemical crosslinking and mass spectrometry. Protein Sci. 2002;11:2471–2478. doi: 10.1110/ps.0212602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnhart K.F., Christianson D.R., Hanley P.W., Driessen W.H., Bernacky B.J., Baze W.B., Wen S., Tian M., Ma J., Kolonin M.G. A peptidomimetic targeting white fat causes weight loss and improved insulin resistance in obese monkeys. Sci. Transl. Med. 2011;3 doi: 10.1126/scitranslmed.3002621. 108ra112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann B., Bohnenstengel F., Siegmund D., Wajant H., Weber C., Herr I., Debatin K.M., Proksch P., Wirth T. Rocaglamide derivatives are potent inhibitors of NF-kappa B activation in T-cells. J. Biol. Chem. 2002;277:44791–44800. doi: 10.1074/jbc.M208003200. [DOI] [PubMed] [Google Scholar]
- Bernard Y., Ribeiro N., Thuaud F., Türkeri G., Dirr R., Boulberdaa M., Nebigil C.G., Désaubry L. Flavaglines alleviate doxorubicin cardiotoxicity: implication of Hsp27. PLoS ONE. 2011;6:e25302. doi: 10.1371/journal.pone.0025302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein H.-G., Smalla K.-H., Dürrschmidt D., Keilhoff G., Dobrowolny H., Steiner J., Schmitt A., Kreutz M.R., Bogerts B. Increased density of prohibitin-immunoreactive oligodendrocytes in the dorsolateral prefrontal white matter of subjects with schizophrenia suggests extraneuronal roles for the protein in the disease. Neuromolecular Med. 2012;14:270–280. doi: 10.1007/s12017-012-8185-y. [DOI] [PubMed] [Google Scholar]
- Bettayeb K., Oumata N., Zhang Y., Luo W., Bustos V., Galons H., Greengard P., Meijer L., Flajolet M. Small-molecule inducers of Aβ-42 peptide production share a common mechanism of action. FASEB J. 2012;26:5115–5123. doi: 10.1096/fj.12-212985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleumink M., Köhler R., Giaisi M., Proksch P., Krammer P.H., Li-Weber M. Rocaglamide breaks TRAIL resistance in HTLV-1-associated adult T-cell leukemia/lymphoma by translational suppression of c-FLIP expression. Cell Death Differ. 2011;18:362–370. doi: 10.1038/cdd.2010.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boengler K., Hilfiker-Kleiner D., Heusch G., Schulz R. Inhibition of permeability transition pore opening by mitochondrial STAT3 and its role in myocardial ischemia/reperfusion. Basic Res. Cardiol. 2010;105:771–785. doi: 10.1007/s00395-010-0124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordeleau M.E., Robert F., Gerard B., Lindqvist L., Chen S.M., Wendel H.G., Brem B., Greger H., Lowe S.W., Porco J.A., Jr., Pelletier J. Therapeutic suppression of translation initiation modulates chemosensitivity in a mouse lymphoma model. J. Clin. Invest. 2008;118:2651–2660. doi: 10.1172/JCI34753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borutinskaite V.V., Magnusson K.E., Navakauskiene R. α-Dystrobrevin distribution and association with other proteins in human promyelocytic NB4 cells treated for granulocytic differentiation. Mol. Biol. Rep. 2011;38:3001–3011. doi: 10.1007/s11033-010-9965-9. [DOI] [PubMed] [Google Scholar]
- Bourges I., Ramus C., Mousson de Camaret B., Beugnot R., Remacle C., Cardol P., Hofhaus G., Issartel J.P. Structural organization of mitochondrial human complex I: role of the ND4 and ND5 mitochondria-encoded subunits and interaction with prohibitin. Biochem. J. 2004;383:491–499. doi: 10.1042/BJ20040256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush E.W., McKinsey T.A. Targeting histone deacetylases for heart failure. Expert Opin. Ther. Targets. 2009;13:767–784. doi: 10.1517/14728220902939161. [DOI] [PubMed] [Google Scholar]
- Cao G., van der Wijst J., van der Kemp A., van Zeeland F., Bindels R.J., Hoenderop J.G. Regulation of the epithelial Mg2+ channel TRPM6 by estrogen and the associated repressor protein of estrogen receptor activity (REA) J. Biol. Chem. 2009;284:14788–14795. doi: 10.1074/jbc.M808752200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cencic R., Carrier M., Galicia-Vázquez G., Bordeleau M.E., Sukarieh R., Bourdeau A., Brem B., Teodoro J.G., Greger H., Tremblay M.L. Antitumor activity and mechanism of action of the cyclopenta[b]benzofuran, silvestrol. PLoS ONE. 2009;4:e5223. doi: 10.1371/journal.pone.0005223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chander H., Halpern M., Resnick-Silverman L., Manfredi J.J., Germain D. Skp2B attenuates p53 function by inhibiting prohibitin. EMBO Rep. 2010;11:220–225. doi: 10.1038/embor.2010.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chander H., Halpern M., Resnick-Silverman L., Manfredi J.J., Germain D. Skp2B overexpression alters a prohibitin-p53 axis and the transcription of PAPP-A, the protease of insulin-like growth factor binding protein 4. PLoS ONE. 2011;6:e22456. doi: 10.1371/journal.pone.0022456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S.Y., Bae S.J., Lee M.Y., Baek S.H., Chang S., Kim S.H. Chemical affinity matrix-based identification of prohibitin as a binding protein to anti-resorptive sulfonyl amidine compounds. Bioorg. Med. Chem. Lett. 2011;21:727–729. doi: 10.1016/j.bmcl.2010.11.123. [DOI] [PubMed] [Google Scholar]
- Chen Y.W., Chou H.C., Lyu P.C., Yin H.S., Huang F.L., Chang W.S., Fan C.Y., Tu I.F., Lai T.C., Lin S.T. Mitochondrial proteomics analysis of tumorigenic and metastatic breast cancer markers. Funct. Integr. Genomics. 2011;11:225–239. doi: 10.1007/s10142-011-0210-y. [DOI] [PubMed] [Google Scholar]
- Chhabra R., Dubey R., Saini N. Cooperative and individualistic functions of the microRNAs in the miR-23a∼27a∼24-2 cluster and its implication in human diseases. Mol. Cancer. 2010;9:232. doi: 10.1186/1476-4598-9-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi D., Lee S.J., Hong S., Kim I.H., Kang S. Prohibitin interacts with RNF2 and regulates E2F1 function via dual pathways. Oncogene. 2008;27:1716–1725. doi: 10.1038/sj.onc.1210806. [DOI] [PubMed] [Google Scholar]
- Chiu C.-F., Peng J.-M., Hung S.-W., Liang C.-M., Liang S.-M. Recombinant viral capsid protein VP1 suppresses migration and invasion of human cervical cancer by modulating phosphorylated prohibitin in lipid rafts. Cancer Lett. 2012;320:205–214. doi: 10.1016/j.canlet.2012.02.027. [DOI] [PubMed] [Google Scholar]
- Chiu C.F., Ho M.Y., Peng J.M., Hung S.W., Lee W.H., Liang C.M., Liang S.M. Raf activation by Ras and promotion of cellular metastasis require phosphorylation of prohibitin in the raft domain of the plasma membrane. Oncogene. 2013;32:777–787. doi: 10.1038/onc.2012.86. [DOI] [PubMed] [Google Scholar]
- Chowdhury I., Xu W., Stiles J.K., Zeleznik A., Yao X., Matthews R., Thomas K., Thompson W.E. Apoptosis of rat granulosa cells after staurosporine and serum withdrawal is suppressed by adenovirus-directed overexpression of prohibitin. Endocrinology. 2007;148:206–217. doi: 10.1210/en.2006-0187. [DOI] [PubMed] [Google Scholar]
- Chowdhury I., Branch A., Olatinwo M., Thomas K., Matthews R., Thompson W.E. Prohibitin (PHB) acts as a potent survival factor against ceramide induced apoptosis in rat granulosa cells. Life Sci. 2011;89:295–303. doi: 10.1016/j.lfs.2011.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates P.J., Nenutil R., McGregor A., Picksley S.M., Crouch D.H., Hall P.A., Wright E.G. Mammalian prohibitin proteins respond to mitochondrial stress and decrease during cellular senescence. Exp. Cell Res. 2001;265:262–273. doi: 10.1006/excr.2001.5166. [DOI] [PubMed] [Google Scholar]
- Cornillez-Ty C.T., Liao L., Yates J.R., 3rd, Kuhn P., Buchmeier M.J. Severe acute respiratory syndrome coronavirus nonstructural protein 2 interacts with a host protein complex involved in mitochondrial biogenesis and intracellular signaling. J. Virol. 2009;83:10314–10318. doi: 10.1128/JVI.00842-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da C.S., Parone P.A., Gonzalo P., Bienvenut W.V., Tondera D., Jourdain A., Quadroni M., Martinou J.-C. SLP-2 interacts with prohibitins in the mitochondrial inner membrane and contributes to their stability. Biochim. Biophys. Acta, Mol. 2008;1783:904–911. doi: 10.1016/j.bbamcr.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Delage-Mourroux R., Martini P.G., Choi I., Kraichely D.M., Hoeksema J., Katzenellenbogen B.S. Analysis of estrogen receptor interaction with a repressor of estrogen receptor activity (REA) and the regulation of estrogen receptor transcriptional activity by REA. J. Biol. Chem. 2000;275:35848–35856. doi: 10.1074/jbc.M001327200. [DOI] [PubMed] [Google Scholar]
- Ding Q., Vaynman S., Souda P., Whitelegge J.P., Gomez-Pinilla F. Exercise affects energy metabolism and neural plasticity-related proteins in the hippocampus as revealed by proteomic analysis. Eur. J. Neurosci. 2006;24:1265–1276. doi: 10.1111/j.1460-9568.2006.05026.x. [DOI] [PubMed] [Google Scholar]
- Ebada S.S., Lajkiewicz N., Porco J.A., Jr., Li-Weber M., Proksch P. Chemistry and biology of rocaglamides (= flavaglines) and related derivatives from aglaia species (meliaceae) Fortschr. Chem. Org. Naturst. 2011;94:1–58. doi: 10.1007/978-3-7091-0748-5_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson V., Holtkotte D., Pfeiffer T., Wang I.H., Schnölzer M., Kempf T., Bosch V. Identification of the cellular prohibitin 1/prohibitin 2 heterodimer as an interaction partner of the C-terminal cytoplasmic domain of the HIV-1 glycoprotein. J. Virol. 2010;84:1355–1365. doi: 10.1128/JVI.01641-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo H. PHB2-PGC1alpha complex, adipogenesis controllers containing substance which controls formation of the complex or fragment or mutant of PHB2, PGC1alpha controllers containing fragment or mutant of PHB2, and screening of adipogenesis controllers. Japanese Patent. 2011 2011116753A. [Google Scholar]
- Erlich S., Alexandrovich A., Shohami E., Pinkas-Kramarski R. Rapamycin is a neuroprotective treatment for traumatic brain injury. Neurobiol. Dis. 2007;26:86–93. doi: 10.1016/j.nbd.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Fahrig T., Gerlach I., Horváth E. A synthetic derivative of the natural product rocaglaol is a potent inhibitor of cytokine-mediated signaling and shows neuroprotective activity in vitro and in animal models of Parkinson’s disease and traumatic brain injury. Mol. Pharmacol. 2005;67:1544–1555. doi: 10.1124/mol.104.008177. [DOI] [PubMed] [Google Scholar]
- Fletcher C.E., Dart D.A., Sita-Lumsden A., Cheng H., Rennie P.S., Bevan C.L. Androgen-regulated processing of the oncomir miR-27a, which targets Prohibitin in prostate cancer. Hum. Mol. Genet. 2012;21:3112–3127. doi: 10.1093/hmg/dds139. [DOI] [PubMed] [Google Scholar]
- Fusaro G., Dasgupta P., Rastogi S., Joshi B., Chellappan S. Prohibitin induces the transcriptional activity of p53 and is exported from the nucleus upon apoptotic signaling. J. Biol. Chem. 2003;278:47853–47861. doi: 10.1074/jbc.M305171200. [DOI] [PubMed] [Google Scholar]
- Gamble S.C., Chotai D., Odontiadis M., Dart D.A., Brooke G.N., Powell S.M., Reebye V., Varela-Carver A., Kawano Y., Waxman J., Bevan C.L. Prohibitin, a protein downregulated by androgens, represses androgen receptor activity. Oncogene. 2007;26:1757–1768. doi: 10.1038/sj.onc.1209967. [DOI] [PubMed] [Google Scholar]
- Garin J., Diez R., Kieffer S., Dermine J.F., Duclos S., Gagnon E., Sadoul R., Rondeau C., Desjardins M. The phagosome proteome: insight into phagosome functions. J. Cell Biol. 2001;152:165–180. doi: 10.1083/jcb.152.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertz M., Fischer F., Wolters D., Steegborn C. Activation of the lifespan regulator p66Shc through reversible disulfide bond formation. Proc. Natl. Acad. Sci. USA. 2008;105:5705–5709. doi: 10.1073/pnas.0800691105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez L., Paillard M., Price M., Chen Q., Teixeira G., Spiegel S., Lesnefsky E.J. A novel role for mitochondrial sphingosine-1-phosphate produced by sphingosine kinase-2 in PTP-mediated cell survival during cardioprotection. Basic Res. Cardiol. 2011;106:1341–1353. doi: 10.1007/s00395-011-0223-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratia S., Kay L., Michelland S., Sève M., Schlattner U., Tokarska-Schlattner M. Cardiac phosphoproteome reveals cell signaling events involved in doxorubicin cardiotoxicity. J. Proteomics. 2012;75:4705–4716. doi: 10.1016/j.jprot.2012.02.004. [DOI] [PubMed] [Google Scholar]
- Gu Y., Ande S.R., Mishra S. Altered O-GlcNAc modification and phosphorylation of mitochondrial proteins in myoblast cells exposed to high glucose. Arch. Biochem. Biophys. 2011;505:98–104. doi: 10.1016/j.abb.2010.09.024. [DOI] [PubMed] [Google Scholar]
- Gupta S.V., Sass E.J., Davis M.E., Edwards R.B., Lozanski G., Heerema N.A., Lehman A., Zhang X., Jarjoura D., Byrd J.C. Resistance to the translation initiation inhibitor silvestrol is mediated by ABCB1/P-glycoprotein overexpression in acute lymphoblastic leukemia cells. AAPS J. 2011;13:357–364. doi: 10.1208/s12248-011-9276-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez M.E., Kummar S., Giaccone G. Next generation oncology drug development: opportunities and challenges. Nat. Rev. Clin. Oncol. 2009;6:259–265. doi: 10.1038/nrclinonc.2009.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausott B., Greger H., Marian B. Flavaglines: a group of efficient growth inhibitors block cell cycle progression and induce apoptosis in colorectal cancer cells. Int. J. Cancer. 2004;109:933–940. doi: 10.1002/ijc.20033. [DOI] [PubMed] [Google Scholar]
- He B., Feng Q., Mukherjee A., Lonard D.M., DeMayo F.J., Katzenellenbogen B.S., Lydon J.P., O’Malley B.W. A repressive role for prohibitin in estrogen signaling. Mol. Endocrinol. 2008;22:344–360. doi: 10.1210/me.2007-0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J., Cooper H.M., Reyes A., Di Re M., Sembongi H., Litwin T.R., Gao J., Neuman K.C., Fearnley I.M., Spinazzola A. Mitochondrial nucleoid interacting proteins support mitochondrial protein synthesis. Nucleic Acids Res. 2012;40:6109–6121. doi: 10.1093/nar/gks266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidler J., Al-Furoukh N., Kukat C., Salwig I., Ingelmann M.-E., Seibel P., Krüger M., Holtz J., Wittig I., Braun T., Szibor M. Nitric oxide-associated protein 1 (NOA1) is necessary for oxygen-dependent regulation of mitochondrial respiratory complexes. J. Biol. Chem. 2011;286:32086–32093. doi: 10.1074/jbc.M111.221986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henschke P., Vorum H., Honoré B., Rice G.E. Protein profiling the effects of in vitro hyperoxic exposure on fetal rabbit lung. Proteomics. 2006;6:1957–1962. doi: 10.1002/pmic.200500245. [DOI] [PubMed] [Google Scholar]
- Héron-Milhavet L., Mamaeva D., Rochat A., Lamb N.J.C., Fernandez A. Akt2 is implicated in skeletal muscle differentiation and specifically binds Prohibitin2/REA. J. Cell. Physiol. 2008;214:158–165. doi: 10.1002/jcp.21177. [DOI] [PubMed] [Google Scholar]
- Hsieh S.-Y., Shih T.-C., Yeh C.-Y., Lin C.-J., Chou Y.-Y., Lee Y.-S. Comparative proteomic studies on the pathogenesis of human ulcerative colitis. Proteomics. 2006;6:5322–5331. doi: 10.1002/pmic.200500541. [DOI] [PubMed] [Google Scholar]
- Hwang B.Y., Su B.N., Chai H., Mi Q., Kardono L.B., Afriastini J.J., Riswan S., Santarsiero B.D., Mesecar A.D., Wild R. Silvestrol and episilvestrol, potential anticancer rocaglate derivatives from Aglaia silvestris. J. Org. Chem. 2004;69:3350–3358. doi: 10.1021/jo040120f. [DOI] [PubMed] [Google Scholar]
- Hwang C., Giri V.N., Wilkinson J.C., Wright C.W., Wilkinson A.S., Cooney K.A., Duckett C.S. EZH2 regulates the transcription of estrogen-responsive genes through association with REA, an estrogen receptor corepressor. Breast Cancer Res. Treat. 2008;107:235–242. doi: 10.1007/s10549-007-9542-7. [DOI] [PubMed] [Google Scholar]
- Jain R., Ghoshal A., Mandal C., Shaha C. Leishmania cell surface prohibitin: role in host-parasite interaction. Cell. Microbiol. 2010;12:432–452. doi: 10.1111/j.1462-5822.2009.01406.x. [DOI] [PubMed] [Google Scholar]
- Jang K.-S., Baik J.E., Kang S.-S., Jeon J.H., Choi S., Yang Y.-H., Kim B.-G., Yun C.-H., Han S.H. Identification of staphylococcal lipoteichoic acid-binding proteins in human serum by high-resolution LTQ-Orbitrap mass spectrometry. Mol. Immunol. 2012;50:177–183. doi: 10.1016/j.molimm.2011.11.012. [DOI] [PubMed] [Google Scholar]
- Jones D.P. Radical-free biology of oxidative stress. Am. J. Physiol. Cell Physiol. 2008;295:C849–C868. doi: 10.1152/ajpcell.00283.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi B., Ko D., Ordonez-Ercan D., Chellappan S.P. A putative coiled-coil domain of prohibitin is sufficient to repress E2F1-mediated transcription and induce apoptosis. Biochem. Biophys. Res. Commun. 2003;312:459–466. doi: 10.1016/j.bbrc.2003.10.148. [DOI] [PubMed] [Google Scholar]
- Joshi B., Rastogi S., Morris M., Carastro L.M., DeCook C., Seto E., Chellappan S.P. Differential regulation of human YY1 and caspase 7 promoters by prohibitin through E2F1 and p53 binding sites. Biochem. J. 2007;401:155–166. doi: 10.1042/BJ20060364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasashima K., Ohta E., Kagawa Y., Endo H. Mitochondrial functions and estrogen receptor-dependent nuclear translocation of pleiotropic human prohibitin 2. J. Biol. Chem. 2006;281:36401–36410. doi: 10.1074/jbc.M605260200. [DOI] [PubMed] [Google Scholar]
- Kasashima K., Sumitani M., Satoh M., Endo H. Human prohibitin 1 maintains the organization and stability of the mitochondrial nucleoids. Exp. Cell Res. 2008;314:988–996. doi: 10.1016/j.yexcr.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Kategaya L.S., Hilliard A., Zhang L., Asara J.M., Ptáček L.J., Fu Y.-H. Casein kinase 1 proteomics reveal prohibitin 2 function in molecular clock. PLoS ONE. 2012;7:e31987. doi: 10.1371/journal.pone.0031987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathiria A.S., Butcher L.D., Feagins L.A., Souza R.F., Boland C.R., Theiss A.L. Prohibitin 1 modulates mitochondrial stress-related autophagy in human colonic epithelial cells. PLoS ONE. 2012;7:e31231. doi: 10.1371/journal.pone.0031231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathiria A.S., Neumann W.L., Rhees J., Hotchkiss E., Cheng Y., Genta R.M., Meltzer S.J., Souza R.F., Theiss A.L. Prohibitin attenuates colitis-associated tumorigenesis in mice by modulating p53 and STAT3 apoptotic responses. Cancer Res. 2012;72:5778–5789. doi: 10.1158/0008-5472.CAN-12-0603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S., Salloum F., Das A., Xi L., Vetrovec G.W., Kukreja R.C. Rapamycin confers preconditioning-like protection against ischemia-reperfusion injury in isolated mouse heart and cardiomyocytes. J. Mol. Cell. Cardiol. 2006;41:256–264. doi: 10.1016/j.yjmcc.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Kim N., Lee Y., Kim H., Joo H., Youm J.B., Park W.S., Warda M., Cuong D.V., Han J. Potential biomarkers for ischemic heart damage identified in mitochondrial proteins by comparative proteomics. Proteomics. 2006;6:1237–1249. doi: 10.1002/pmic.200500291. [DOI] [PubMed] [Google Scholar]
- Kim S., Hwang B.Y., Su B.N., Chai H., Mi Q., Kinghorn A.D., Wild R., Swanson S.M. Silvestrol, a potential anticancer rocaglate derivative from Aglaia foveolata, induces apoptosis in LNCaP cells through the mitochondrial/apoptosome pathway without activation of executioner caspase-3 or -7. Anticancer Res. 2007;27:2175–2183. [PMC free article] [PubMed] [Google Scholar]
- Kim J.-W., Akiyama M., Park J.-H., Lin M.-L., Shimo A., Ueki T., Daigo Y., Tsunoda T., Nishidate T., Nakamura Y., Katagiri T. Activation of an estrogen/estrogen receptor signaling by BIG3 through its inhibitory effect on nuclear transport of PHB2/REA in breast cancer. Cancer Sci. 2009;100:1468–1478. doi: 10.1111/j.1349-7006.2009.01209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D.H., Woods S.C., Seeley R.J. Peptide designed to elicit apoptosis in adipose tissue endothelium reduces food intake and body weight. Diabetes. 2010;59:907–915. doi: 10.2337/db09-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King M.L., Chiang C.C., Ling H.C., Fujita E., Ochiai M., McPhail A.T. X-Ray crystal structure of rocaglamide, a novel antileukemic 1H-cyclopenta[b]benzofuran from Aglaia elliptifolia. J. Chem. Soc., Chem. Commun. 1982;20:1150–1151. [Google Scholar]
- Kolonin M.G., Saha P.K., Chan L., Pasqualini R., Arap W. Reversal of obesity by targeted ablation of adipose tissue. Nat. Med. 2004;10:625–632. doi: 10.1038/nm1048. [DOI] [PubMed] [Google Scholar]
- Kostenko S., Moens U. Heat shock protein 27 phosphorylation: kinases, phosphatases, functions and pathology. Cell. Mol. Life Sci. 2009;66:3289–3307. doi: 10.1007/s00018-009-0086-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuadkitkan A., Wikan N., Fongsaran C., Smith D.R. Identification and characterization of prohibitin as a receptor protein mediating DENV-2 entry into insect cells. Virology. 2010;406:149–161. doi: 10.1016/j.virol.2010.07.015. [DOI] [PubMed] [Google Scholar]
- Kurtev V., Margueron R., Kroboth K., Ogris E., Cavailles V., Seiser C. Transcriptional regulation by the repressor of estrogen receptor activity via recruitment of histone deacetylases. J. Biol. Chem. 2004;279:24834–24843. doi: 10.1074/jbc.M312300200. [DOI] [PubMed] [Google Scholar]
- Lee S.K., Cui B., Mehta R.R., Kinghorn A.D., Pezzuto J.M. Cytostatic mechanism and antitumor potential of novel 1H-cyclopenta[b]benzofuran lignans isolated from Aglaia elliptica. Chem. Biol. Interact. 1998;115:215–228. doi: 10.1016/s0009-2797(98)00073-8. [DOI] [PubMed] [Google Scholar]
- Lee S.J., Choi D., Rhim H., Choo H.J., Ko Y.G., Kim C.G., Kang S. PHB2 interacts with RNF2 and represses CP2c-stimulated transcription. Mol. Cell. Biochem. 2008;319:69–77. doi: 10.1007/s11010-008-9878-2. [DOI] [PubMed] [Google Scholar]
- Lee H., Arnouk H., Sripathi S., Chen P., Zhang R., Bartoli M., Hunt R.C., Hrushesky W.J.M., Chung H., Lee S.H., Jahng W.J. Prohibitin as an oxidative stress biomarker in the eye. Int. J. Biol. Macromol. 2010;47:685–690. doi: 10.1016/j.ijbiomac.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.H., Nguyen K.H., Mishra S., Nyomba B.L.G. Prohibitin is expressed in pancreatic β-cells and protects against oxidative and proapoptotic effects of ethanol. FEBS J. 2010;277:488–500. doi: 10.1111/j.1742-4658.2009.07505.x. [DOI] [PubMed] [Google Scholar]
- Lee M.Y., Kim M.H., Kim J., Kim S.H., Kim B.T., Jeong I.H., Chang S., Kim S.H., Chang S.Y. Synthesis and SAR of sulfonyl- and phosphoryl amidine compounds as anti-resorptive agents. Bioorg. Med. Chem. Lett. 2010;20:541–545. doi: 10.1016/j.bmcl.2009.11.104. [DOI] [PubMed] [Google Scholar]
- Li Q.-F., Liang Y., Shi S.-L., Liu Q.-R., Xu D.-H., Jing G.-J., Wang S.-Y., Kong H.-Y. Localization of prohibitin in the nuclear matrix and alteration of its expression during differentiation of human neuroblastoma SK-N-SH cells induced by retinoic acid. Cell. Mol. Neurobiol. 2011;31:203–211. doi: 10.1007/s10571-010-9608-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lih-Brody L., Powell S.R., Collier K.P., Reddy G.M., Cerchia R., Kahn E., Weissman G.S., Katz S., Floyd R.A., McKinley M.J. Increased oxidative stress and decreased antioxidant defenses in mucosa of inflammatory bowel disease. Dig. Dis. Sci. 1996;41:2078–2086. doi: 10.1007/BF02093613. [DOI] [PubMed] [Google Scholar]
- Liu X.H., Qian L.J., Gong J.B., Shen J., Zhang X.M., Qian X.H. Proteomic analysis of mitochondrial proteins in cardiomyocytes from chronic stressed rat. Proteomics. 2004;4:3167–3176. doi: 10.1002/pmic.200300845. [DOI] [PubMed] [Google Scholar]
- Liu T., Tang H., Lang Y., Liu M., Li X. MicroRNA-27a functions as an oncogene in gastric adenocarcinoma by targeting prohibitin. Cancer Lett. 2009;273:233–242. doi: 10.1016/j.canlet.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Liu X., Ren Z., Zhan R., Wang X., Wang X., Zhang Z., Leng X., Yang Z., Qian L. Prohibitin protects against oxidative stress-induced cell injury in cultured neonatal cardiomyocyte. Cell Stress Chaperones. 2009;14:311–319. doi: 10.1007/s12192-008-0086-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X.H., Zhang Z.Q., Zhan R., Wang X.X., Wang L.Q., Leng X., Gao X.J., Qian L.J. Identification of interaction between HSP70 and prohibitin by co-immunoprecipitation. Jiefangjun Yufang Yixue Zazhi. 2010;28:316–319. [Google Scholar]
- Liu T., Nair S.J., Lescarbeau A., Belani J., Peluso S., Conley J., Tillotson B., O’Hearn P., Smith S., Slocum K. Synthetic silvestrol analogues as potent and selective protein synthesis inhibitors. J. Med. Chem. 2012;55:8859–8878. doi: 10.1021/jm3011542. [DOI] [PubMed] [Google Scholar]
- Lucas D.M., Edwards R.B., Lozanski G., West D.A., Shin J.D., Vargo M.A., Davis M.E., Rozewski D.M., Johnson A.J., Su B.N. The novel plant-derived agent silvestrol has B-cell selective activity in chronic lymphocytic leukemia and acute lymphoblastic leukemia in vitro and in vivo. Blood. 2009;113:4656–4666. doi: 10.1182/blood-2008-09-175430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas C.R., Cordero-Nieves H.M., Erbe R.S., McAlees J.W., Bhatia S., Hodes R.J., Campbell K.S., Sanders V.M. Prohibitins and the cytoplasmic domain of CD86 cooperate to mediate CD86 signaling in B lymphocytes. J. Immunol. 2013;190:723–736. doi: 10.4049/jimmunol.1201646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madireddi, M. (June 2006). Protein-protein interactions of p66.sup.ShcA. U.S. Patent 7,056,684.
- Mai A., Rotili D., Valente S., Kazantsev A.G. Histone deacetylase inhibitors and neurodegenerative disorders: holding the promise. Curr. Pharm. Des. 2009;15:3940–3957. doi: 10.2174/138161209789649349. [DOI] [PubMed] [Google Scholar]
- Malagelada C., Jin Z.H., Jackson-Lewis V., Przedborski S., Greene L.A. Rapamycin protects against neuron death in in vitro and in vivo models of Parkinson’s disease. J. Neurosci. 2010;30:1166–1175. doi: 10.1523/JNEUROSCI.3944-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjeshwar S., Branam D.E., Lerner M.R., Brackett D.J., Jupe E.R. Tumor suppression by the prohibitin gene 3’untranslated region RNA in human breast cancer. Cancer Res. 2003;63:5251–5256. [PubMed] [Google Scholar]
- Martín B., Sanz R., Aragüés R., Oliva B., Sierra A. Functional clustering of metastasis proteins describes plastic adaptation resources of breast-cancer cells to new microenvironments. J. Proteome Res. 2008;7:3242–3253. doi: 10.1021/pr800137w. [DOI] [PubMed] [Google Scholar]
- Matallanas D., Birtwistle M., Romano D., Zebisch A., Rauch J., von Kriegsheim A., Kolch W. Raf family kinases: old dogs have learned new tricks. Genes Cancer. 2011;2:232–260. doi: 10.1177/1947601911407323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer G., Tarkowski B., Baccarini M. Raf kinases in cancer-roles and therapeutic opportunities. Oncogene. 2011;30:3477–3488. doi: 10.1038/onc.2011.160. [DOI] [PubMed] [Google Scholar]
- McClung J.K., Danner D.B., Stewart D.A., Smith J.R., Schneider E.L., Lumpkin C.K., Dell’Orco R.T., Nuell M.J. Isolation of a cDNA that hybrid selects antiproliferative mRNA from rat liver. Biochem. Biophys. Res. Commun. 1989;164:1316–1322. doi: 10.1016/0006-291x(89)91813-5. [DOI] [PubMed] [Google Scholar]
- Mengwasser J., Piau A., Schlag P., Sleeman J.P. Differential immunization identifies PHB1/PHB2 as blood-borne tumor antigens. Oncogene. 2004;23:7430–7435. doi: 10.1038/sj.onc.1207987. [DOI] [PubMed] [Google Scholar]
- Merkwirth C., Dargazanli S., Tatsuta T., Geimer S., Löwer B., Wunderlich F.T., von Kleist-Retzow J.C., Waisman A., Westermann B., Langer T. Prohibitins control cell proliferation and apoptosis by regulating OPA1-dependent cristae morphogenesis in mitochondria. Genes Dev. 2008;22:476–488. doi: 10.1101/gad.460708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkwirth C., Martinelli P., Korwitz A., Morbin M., Brönneke H.S., Jordan S.D., Rugarli E.I., Langer T. Loss of prohibitin membrane scaffolds impairs mitochondrial architecture and leads to tau hyperphosphorylation and neurodegeneration. PLoS Genet. 2012;8:e1003021. doi: 10.1371/journal.pgen.1003021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meurer-Grimes B.M., Yu J., Vairo G.L. Therapeutic compounds and methods. Australian Patent AU2001268848. November 2002 [Google Scholar]
- Mi Q., Kim S., Hwang B.Y., Su B.N., Chai H., Arbieva Z.H., Kinghorn A.D., Swanson S.M. Silvestrol regulates G2/M checkpoint genes independent of p53 activity. Anticancer Res. 2006;26(5A):3349–3356. [PubMed] [Google Scholar]
- Mi Q., Su B.N., Chai H., Cordell G.A., Farnsworth N.R., Kinghorn A.D., Swanson S.M. Rocaglaol induces apoptosis and cell cycle arrest in LNCaP cells. Anticancer Res. 2006;26(2A):947–952. [PubMed] [Google Scholar]
- Minotti G., Menna P., Salvatorelli E., Cairo G., Gianni L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol. Rev. 2004;56:185–229. doi: 10.1124/pr.56.2.6. [DOI] [PubMed] [Google Scholar]
- Mishra S., Moulik S., Murphy L.J. Prohibitin binds to C3 and enhances complement activation. Mol. Immunol. 2007;44:1897–1902. doi: 10.1016/j.molimm.2006.09.025. [DOI] [PubMed] [Google Scholar]
- Mishra S., Ande S.R., Nyomba B.L.G. The role of prohibitin in cell signaling. FEBS J. 2010;277:3937–3946. doi: 10.1111/j.1742-4658.2010.07809.x. [DOI] [PubMed] [Google Scholar]
- Montano M.M., Ekena K., Delage-Mourroux R., Chang W., Martini P., Katzenellenbogen B.S. An estrogen receptor-selective coregulator that potentiates the effectiveness of antiestrogens and represses the activity of estrogens. Proc. Natl. Acad. Sci. USA. 1999;96:6947–6952. doi: 10.1073/pnas.96.12.6947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraguchi T., Kawawa A., Kubota S. Prohibitin protects against hypoxia-induced H9c2 cardiomyocyte cell death. Biomed. Res. 2010;31:113–122. doi: 10.2220/biomedres.31.113. [DOI] [PubMed] [Google Scholar]
- Nasr Z., Robert F., Porco J.A., Jr., Muller W.J., Pelletier J. eIF4F suppression in breast cancer affects maintenance and progression. Oncogene. 2013;32:861–871. doi: 10.1038/onc.2012.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijtmans L.G.J., de Jong L., Artal Sanz M., Coates P.J., Berden J.A., Back J.W., Muijsers A.O., van der Spek H., Grivell L.A. Prohibitins act as a membrane-bound chaperone for the stabilization of mitochondrial proteins. EMBO J. 2000;19:2444–2451. doi: 10.1093/emboj/19.11.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijtmans L.G.J., Artal S.M., Grivell L.A., Coates P.J. The mitochondrial PHB complex: roles in mitochondrial respiratory complex assembly, ageing and degenerative disease. Cell. Mol. Life Sci. 2002;59:143–155. doi: 10.1007/s00018-002-8411-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuell M.J., Stewart D.A., Walker L., Friedman V., Wood C.M., Owens G.A., Smith J.R., Schneider E.L., Dell’ Orco R., Lumpkin C.K. Prohibitin, an evolutionarily conserved intracellular protein that blocks DNA synthesis in normal fibroblasts and HeLa cells. Mol. Cell. Biol. 1991;11:1372–1381. doi: 10.1128/mcb.11.3.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeljeklaus S., Reinartz B.S., Wolf J., Wiese S., Tonillo J., Podwojski K., Kuhlmann K., Stephan C., Meyer H.E., Schliebs W. Identification of core components and transient interactors of the peroxisomal importomer by dual-track stable isotope labeling with amino acids in cell culture analysis. J. Proteome Res. 2012;11:2567–2580. doi: 10.1021/pr3000333. [DOI] [PubMed] [Google Scholar]
- Ohse T., Ohba S., Yamamoto T., Koyano T., Umezawa K. Cyclopentabenzofuran lignan protein synthesis inhibitors from Aglaia odorata. J. Nat. Prod. 1996;59:650–652. doi: 10.1021/np960346g. [DOI] [PubMed] [Google Scholar]
- Paris L.L., Hu J.-J., Galan J., Ong S.-S., Martin V.A., Ma H.-Y., Tao W.A., Harrison M.L., Geahlen R.L. Regulation of Syk by phosphorylation on serine in the linker insert. J. Biol. Chem. 2010;285:39844–39854. doi: 10.1074/jbc.M110.164509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park B., Yang J., Yun N., Choe K.-M., Jin B.K., Oh Y.J. Proteomic analysis of expression and protein interactions in a 6-hydroxydopamine-induced rat brain lesion model. Neurochem. Int. 2010;57:16–32. doi: 10.1016/j.neuint.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Patel N., Chatterjee S.K., Vrbanac V., Chung I., Mu C.J., Olsen R.R., Waghorne C., Zetter B.R. Rescue of paclitaxel sensitivity by repression of Prohibitin1 in drug-resistant cancer cells. Proc. Natl. Acad. Sci. USA. 2010;107:2503–2508. doi: 10.1073/pnas.0910649107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard C., Pellicelli M., Taheri M., Lavoie J.F., Doucet R., Wang D., Bernard L., Bouhanik S., Lavigne P., Moreau A. Nuclear accumulation of PHB1 in osteoarthritic chondrocytes downregulates PITX1 expression levels. Arthritis Rheum. 2013 doi: 10.1002/art.37837. in press. [DOI] [PubMed] [Google Scholar]
- Polier G., Neumann J., Thuaud F., Ribeiro N., Gelhaus C., Schmidt H., Giaisi M., Köhler R., Müller W.W., Proksch P. The natural anticancer compounds rocaglamides inhibit the Raf-MEK-ERK pathway by targeting prohibitin 1 and 2. Chem. Biol. 2012;19:1093–1104. doi: 10.1016/j.chembiol.2012.07.012. [DOI] [PubMed] [Google Scholar]
- Proksch P., Giaisi M., Treiber M.K., Palfi K., Merling A., Spring H., Krammer P.H., Li-Weber M. Rocaglamide derivatives are immunosuppressive phytochemicals that target NF-AT activity in T cells. J. Immunol. 2005;174:7075–7084. doi: 10.4049/jimmunol.174.11.7075. [DOI] [PubMed] [Google Scholar]
- Rajalingam K., Wunder C., Brinkmann V., Churin Y., Hekman M., Sievers C., Rapp U.R., Rudel T. Prohibitin is required for Ras-induced Raf-MEK-ERK activation and epithelial cell migration. Nat. Cell Biol. 2005;7:837–843. doi: 10.1038/ncb1283. [DOI] [PubMed] [Google Scholar]
- Rasmussen R.K., Ji H., Eddes J.S., Moritz R.L., Reid G.E., Simpson R.J., Dorow D.S. Two-dimensional electrophoretic analysis of mixed lineage kinase 2 N-terminal domain binding proteins. Electrophoresis. 1998;19:809–817. doi: 10.1002/elps.1150190535. [DOI] [PubMed] [Google Scholar]
- Rastogi S., Joshi B., Dasgupta P., Morris M., Wright K., Chellappan S. Prohibitin facilitates cellular senescence by recruiting specific corepressors to inhibit E2F target genes. Mol. Cell. Biol. 2006;26:4161–4171. doi: 10.1128/MCB.02142-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastogi S., Joshi B., Fusaro G., Chellappan S. Camptothecin induces nuclear export of prohibitin preferentially in transformed cells through a CRM-1-dependent mechanism. J. Biol. Chem. 2006;281:2951–2959. doi: 10.1074/jbc.M508669200. [DOI] [PubMed] [Google Scholar]
- Ribeiro N., Thuaud F., Bernard Y., Gaiddon C., Cresteil T., Hild A., Hirsch E.C., Michel P.P., Nebigil C.G., Désaubry L. Flavaglines as potent anticancer and cytoprotective agents. J. Med. Chem. 2012;55:10064–10073. doi: 10.1021/jm301201z. [DOI] [PubMed] [Google Scholar]
- Ribeiro N., Thuaud F., Nebigil C., Désaubry L. Recent advances in the biology and chemistry of the flavaglines. Bioorg. Med. Chem. 2012;20:1857–1864. doi: 10.1016/j.bmc.2011.10.048. [DOI] [PubMed] [Google Scholar]
- Rizwani W., Alexandrow M., Chellappan S. Prohibitin physically interacts with MCM proteins and inhibits mammalian DNA replication. Cell Cycle. 2009;8:1621–1629. doi: 10.4161/cc.8.10.8578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert F., Carrier M., Rawe S., Chen S., Lowe S., Pelletier J. Altering chemosensitivity by modulating translation elongation. PLoS ONE. 2009;4:e5428. doi: 10.1371/journal.pone.0005428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross J.A., Nagy Z.S., Kirken R.A. The PHB1/2 phosphocomplex is required for mitochondrial homeostasis and survival of human T cells. J. Biol. Chem. 2008;283:4699–4713. doi: 10.1074/jbc.M708232200. [DOI] [PubMed] [Google Scholar]
- Sánchez-Quiles V., Segura V., Bigaud E., He B., O’Malley B.W., Santamaría E., Prieto J., Corrales F.J. Prohibitin-1 deficiency promotes inflammation and increases sensitivity to liver injury. J. Proteomics. 2012;75:5783–5792. doi: 10.1016/j.jprot.2012.08.009. [DOI] [PubMed] [Google Scholar]
- Sato S.-I., Murata A., Orihara T., Shirakawa T., Suenaga K., Kigoshi H., Uesugi M. Marine natural product aurilide activates the OPA1-mediated apoptosis by binding to prohibitin. Chem. Biol. 2011;18:131–139. doi: 10.1016/j.chembiol.2010.10.017. [DOI] [PubMed] [Google Scholar]
- Schatz J.H., Oricchio E., Wolfe A.L., Jiang M., Linkov I., Maragulia J., Shi W., Zhang Z., Rajasekhar V.K., Pagano N.C. Targeting cap-dependent translation blocks converging survival signals by AKT and PIM kinases in lymphoma. J. Exp. Med. 2011;208:1799–1807. doi: 10.1084/jem.20110846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A., Qadri A. Vi polysaccharide of Salmonella typhi targets the prohibitin family of molecules in intestinal epithelial cells and suppresses early inflammatory responses. Proc. Natl. Acad. Sci. USA. 2004;101:17492–17497. doi: 10.1073/pnas.0407536101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M., Schambony A., Wedlich D. Prohibitin1 acts as a neural crest specifier in Xenopus development by repressing the transcription factor E2F1. Development. 2010;137:4073–4081. doi: 10.1242/dev.053405. [DOI] [PubMed] [Google Scholar]
- Snyder J.R., Hall A., Ni-Komatsu L., Khersonsky S.M., Chang Y.T., Orlow S.J. Dissection of melanogenesis with small molecules identifies prohibitin as a regulator. Chem. Biol. 2005;12:477–484. doi: 10.1016/j.chembiol.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Sripathi S.R., He W., Atkinson C.L., Smith J.J., Liu Z., Elledge B.M., Jahng W.J. Mitochondrial-nuclear communication by prohibitin shuttling under oxidative stress. Biochemistry. 2011;50:8342–8351. doi: 10.1021/bi2008933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staquicini F.I., Cardó-Vila M., Kolonin M.G., Trepel M., Edwards J.K., Nunes D.N., Sergeeva A., Efstathiou E., Sun J., Almeida N.F. Vascular ligand-receptor mapping by direct combinatorial selection in cancer patients. Proc. Natl. Acad. Sci. USA. 2011;108:18637–18642. doi: 10.1073/pnas.1114503108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steglich G., Neupert W., Langer T. Prohibitins regulate membrane protein degradation by the m-AAA protease in mitochondria. Mol. Cell. Biol. 1999;19:3435–3442. doi: 10.1128/mcb.19.5.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strub G.M., Paillard M., Liang J., Gomez L., Allegood J.C., Hait N.C., Maceyka M., Price M.M., Chen Q., Simpson D.C. Sphingosine-1-phosphate produced by sphingosine kinase 2 in mitochondria interacts with prohibitin 2 to regulate complex IV assembly and respiration. FASEB J. 2011;25:600–612. doi: 10.1096/fj.10-167502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su B.N., Chai H., Mi Q., Riswan S., Kardono L.B., Afriastini J.J., Santarsiero B.D., Mesecar A.D., Farnsworth N.R., Cordell G.A. Activity-guided isolation of cytotoxic constituents from the bark of Aglaia crassinervia collected in Indonesia. Bioorg. Med. Chem. 2006;14:960–972. doi: 10.1016/j.bmc.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Suenaga K., Mutou T., Shibata T., Itoh T., Kigoshi H., Yamada K. Isolation and stereostructure of aurilide, a novel cyclodepsipeptide from the Japanese sea hare Dolabella auricularia. Tetrahedron Lett. 1996;37:6771–6774. [Google Scholar]
- Suenaga K., Mutou T., Shibata T., Itoh T., Fujita T., Takada N., Hayamizu K., Takagi M., Irifune T., Kigoshi H., Yamada K. Aurilide, a cytotoxic depsipeptide from the sea hare Dolabella auricularia: Isolation, structure determination, synthesis, and biological activity. Tetrahedron. 2004;60:8509–8527. [Google Scholar]
- Sun L., Liu L., Yang X.-J., Wu Z. Akt binds prohibitin 2 and relieves its repression of MyoD and muscle differentiation. J. Cell Sci. 2004;117:3021–3029. doi: 10.1242/jcs.01142. [DOI] [PubMed] [Google Scholar]
- Sun L., Cao X., Liu B., Huang H., Wang X., Sui L., Yin W., Ma K. CaMK IV phosphorylates prohibitin 2 and regulates prohibitin 2-mediated repression of MEF2 transcription. Cell. Signal. 2011;23:1686–1690. doi: 10.1016/j.cellsig.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsuta T., Model K., Langer T. Formation of membrane-bound ring complexes by prohibitins in mitochondria. Mol. Biol. Cell. 2005;16:248–259. doi: 10.1091/mbc.E04-09-0807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terashima M., Kim K.-M., Adachi T., Nielsen P.J., Reth M., Köhler G., Lamers M.C. The IgM antigen receptor of B lymphocytes is associated with prohibitin and a prohibitin-related protein. EMBO J. 1994;13:3782–3792. doi: 10.1002/j.1460-2075.1994.tb06689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theiss A.L., Idell R.D., Srinivasan S., Klapproth J.M., Jones D.P., Merlin D., Sitaraman S.V. Prohibitin protects against oxidative stress in intestinal epithelial cells. FASEB J. 2007;21:197–206. doi: 10.1096/fj.06-6801com. [DOI] [PubMed] [Google Scholar]
- Theiss A.L., Obertone T.S., Merlin D., Sitaraman S.V. Interleukin-6 transcriptionally regulates prohibitin expression in intestinal epithelial cells. J. Biol. Chem. 2007;282:12804–12812. doi: 10.1074/jbc.M609031200. [DOI] [PubMed] [Google Scholar]
- Theiss A.L., Jenkins A.K., Okoro N.I., Klapproth J.M., Merlin D., Sitaraman S.V. Prohibitin inhibits tumor necrosis factor alpha-induced nuclear factor-kappa B nuclear translocation via the novel mechanism of decreasing importin alpha3 expression. Mol. Biol. Cell. 2009;20:4412–4423. doi: 10.1091/mbc.E09-05-0361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theiss A.L., Vijay-Kumar M., Obertone T.S., Jones D.P., Hansen J.M., Gewirtz A.T., Merlin D., Sitaraman S.V. Prohibitin is a novel regulator of antioxidant response that attenuates colonic inflammation in mice. Gastroenterology. 2009;137:199–208. doi: 10.1053/j.gastro.2009.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theiss A.L., Laroui H., Obertone T.S., Chowdhury I., Thompson W.E., Merlin D., Sitaraman S.V. Nanoparticle-based therapeutic delivery of prohibitin to the colonic epithelial cells ameliorates acute murine colitis. Inflamm. Bowel Dis. 2011;17:1163–1176. doi: 10.1002/ibd.21469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuaud F., Bernard Y., Türkeri G., Dirr R., Aubert G., Cresteil T., Baguet A., Tomasetto C., Svitkin Y., Sonenberg N. Synthetic analogue of rocaglaol displays a potent and selective cytotoxicity in cancer cells: involvement of apoptosis inducing factor and caspase-12. J. Med. Chem. 2009;52:5176–5187. doi: 10.1021/jm900365v. [DOI] [PubMed] [Google Scholar]
- Thuaud F., Ribeiro N., Gaiddon C., Cresteil T., Désaubry L. Novel flavaglines displaying improved cytotoxicity. J. Med. Chem. 2011;54:411–415. doi: 10.1021/jm101318b. [DOI] [PubMed] [Google Scholar]
- Trzeciakiewicz A., Fortin S., Moreau E., C-Gaudreault R., Lacroix J., Chambon C., Communal Y., Chezal J.M., Miot-Noirault E., Bouchon B., Degoul F. Intramolecular cyclization of N-phenyl N’(2-chloroethyl)ureas leads to active N-phenyl-4,5-dihydrooxazol-2-amines alkylating β-tubulin Glu198 and prohibitin Asp40. Biochem. Pharmacol. 2011;81:1116–1123. doi: 10.1016/j.bcp.2011.02.014. [DOI] [PubMed] [Google Scholar]
- Tsutsumi T., Matsuda M., Aizaki H., Moriya K., Miyoshi H., Fujie H., Shintani Y., Yotsuyanagi H., Miyamura T., Suzuki T., Koike K. Proteomics analysis of mitochondrial proteins reveals overexpression of a mitochondrial protein chaperon, prohibitin, in cells expressing hepatitis C virus core protein. Hepatology. 2009;50:378–386. doi: 10.1002/hep.22998. [DOI] [PubMed] [Google Scholar]
- Umanskaya K., Radke S., Chander H., Monardo R., Xu X., Pan Z.-Q., O’Connell M.J., Germain D. Skp2B stimulates mammary gland development by inhibiting REA, the repressor of the estrogen receptor. Mol. Cell. Biol. 2007;27:7615–7622. doi: 10.1128/MCB.01239-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vessal M., Mishra S., Moulik S., Murphy L.J. Prohibitin attenuates insulin-stimulated glucose and fatty acid oxidation in adipose tissue by inhibition of pyruvate carboxylase. FEBS J. 2006;273:568–576. doi: 10.1111/j.1742-4658.2005.05090.x. [DOI] [PubMed] [Google Scholar]
- Wang S., Nath N., Adlam M., Chellappan S. Prohibitin, a potential tumor suppressor, interacts with RB and regulates E2F function. Oncogene. 1999;18:3501–3510. doi: 10.1038/sj.onc.1202684. [DOI] [PubMed] [Google Scholar]
- Wang S., Nath N., Fusaro G., Chellappan S. Rb and prohibitin target distinct regions of E2F1 for repression and respond to different upstream signals. Mol. Cell. Biol. 1999;19:7447–7460. doi: 10.1128/mcb.19.11.7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Zhang B., Faller D.V. Prohibitin requires Brg-1 and Brm for the repression of E2F and cell growth. EMBO J. 2002;21:3019–3028. doi: 10.1093/emboj/cdf302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintachai P., Wikan N., Kuadkitkan A., Jaimipuk T., Ubol S., Pulmanausahakul R., Auewarakul P., Kasinrerk W., Weng W.Y., Panyasrivanit M. Identification of prohibitin as a Chikungunya virus receptor protein. J. Med. Virol. 2012;84:1757–1770. doi: 10.1002/jmv.23403. [DOI] [PubMed] [Google Scholar]
- Winter A., Kamarainen O., Hofmann A. Molecular modeling of prohibitin domains. Proteins. 2007;68:353–362. doi: 10.1002/prot.21355. [DOI] [PubMed] [Google Scholar]
- Wu Q., Wu S. Lipid rafts association and anti-apoptotic function of prohibitin in ultraviolet B light-irradiated HaCaT keratinocytes. Exp. Dermatol. 2012;21:640–642. doi: 10.1111/j.1600-0625.2012.01547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo M., Kim D.K., Park H.J., Oh T.Y., Kim J.H., Cho S.W., Paik Y.K., Hahm K.B. Loss of transgelin in repeated bouts of ulcerative colitis-induced colon carcinogenesis. Proteomics. 2006;6:1158–1165. doi: 10.1002/pmic.200500390. [DOI] [PubMed] [Google Scholar]
- Yurugi H., Tanida S., Ishida A., Akita K., Toda M., Inoue M., Nakada H. Expression of prohibitins on the surface of activated T cells. Biochem. Biophys. Res. Commun. 2012;420:275–280. doi: 10.1016/j.bbrc.2012.02.149. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Wang Y., Xiang Y., Lee W., Zhang Y. Prohibitins are involved in protease-activated receptor 1-mediated platelet aggregation. J. Thromb. Haemost. 2012;10:411–418. doi: 10.1111/j.1538-7836.2011.04607.x. [DOI] [PubMed] [Google Scholar]
- Zhou P., Qian L., D’Aurelio M., Cho S., Wang G., Manfredi G., Pickel V., Iadecola C. Prohibitin reduces mitochondrial free radical production and protects brain cells from different injury modalities. J. Neurosci. 2012;32:583–592. doi: 10.1523/JNEUROSCI.2849-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J.Y., Lavrik I.N., Mahlknecht U., Giaisi M., Proksch P., Krammer P.H., Li-Weber M. The traditional Chinese herbal compound rocaglamide preferentially induces apoptosis in leukemia cells by modulation of mitogen-activated protein kinase activities. Int. J. Cancer. 2007;121:1839–1846. doi: 10.1002/ijc.22883. [DOI] [PubMed] [Google Scholar]
- Zhu J.Y., Giaisi M., Köhler R., Müller W.W., Mühleisen A., Proksch P., Krammer P.H., Li-Weber M. Rocaglamide sensitizes leukemic T cells to activation-induced cell death by differential regulation of CD95L and c-FLIP expression. Cell Death Differ. 2009;16:1289–1299. doi: 10.1038/cdd.2009.42. [DOI] [PubMed] [Google Scholar]
- Zhu B., Zhai J., Zhu H., Kyprianou N. Prohibitin regulates TGF-β induced apoptosis as a downstream effector of Smad-dependent and -independent signaling. Prostate. 2010;70:17–26. doi: 10.1002/pros.21033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.