Short abstract
Objectives
Des-gamma-carboxyprothrombin (DCP) is an important serum biomarker for the clinical screening of hepatocellular carcinoma (HCC). This study aimed to evaluate the value of DCP for the early diagnosis of alpha-fetoprotein (AFP)-negative hepatitis B virus (HBV)-related HCC.
Methods
We retrospectively enrolled patients with AFP-negative HBV-related HCC and benign liver disease. Serum DCP levels in all participants were measured by chemiluminescent enzyme immunoassay. The value of DCP for the early diagnosis of AFP-negative HBV-related HCC was evaluated by receiver operating characteristic curve (ROC) and area under the curve (AUC) analyses.
Results
A total of 210 patients, including 87 cases with AFP-negative HBV-related HCC and 123 control cases with chronic HBV infection (CHB) or liver cirrhosis (LC), were included. Serum DCP levels were significantly increased in patients with AFP-negative HBV-related HCC compared with those with CHB or LC. The AUC for DCP for distinguishing between the two groups was 0.731 (95% confidence interval (CI), 0.657 to 0.805) and that for early diagnosis was 0.685 (95%CI, 0.596 to 0.774).
Conclusions
DCP may be a favorable biomarker to improve the early diagnosis rate of AFP-negative HBV-related HCC.
Keywords: Des-gamma-carboxyprothrombin, hepatocellular carcinoma, alpha-fetoprotein, hepatitis B virus, serum level, early diagnosis
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors in adults in Asia and the third leading cause of cancer-related death.1,2 There are about 470,000 new cases of HCC in China every year, accounting for 55% of the worldwide incidence.3,4 The occurrence of HCC in China is mainly related to hepatitis virus infection, hepatic fibrosis, and biliary cirrhosis,5,6 and about 1% to 5% of cases of liver cirrhosis per year develop into HCC.7 Treatments for HCC include surgical resection, interventional embolization, radiofrequency ablation, liver transplantation, and chemotherapy.8 However, the postoperative recurrence and metastasis rates remain high and the long-term survival rate for more than 5 years is low.9
Chronic hepatitis B virus (HBV) infection (CHB) is the major risk factor for HCC in China.1 Although technologies such as ultrasound imaging, contrast-enhanced computed tomography, magnetic resonance imaging, and percutaneous transhepatic biopsy have greatly improved the rate of HCC diagnosis, these methods have some disadvantages, such as high cost, invasiveness, and a low efficiency for small lesions. Testing for serum tumor markers is thus an important complement for HCC diagnosis. Alpha-fetoprotein (AFP) is the most frequent biomarker used in the clinic; however, 30% to 40% of HCCs may present as AFP-negative.10,11 Furthermore, increased AFP levels may also be detected in patients with chronic active hepatitis.12 It thus remains difficult to improve the efficiency of the early diagnosis of AFP-negative HCC, especially HBV-related HCC.
Des-gamma-carboxyprothrombin (DCP; also known as protein induced by vitamin K deficiency or antagonist-II (PIVKA II)) is a prothrombin precursor synthesized by the liver. Liebman et al.13 first reported serum DCP in patients with liver cancer in 1984, and >90% of patients with HCC have since been shown to have strong positive expression of DCP.14 Sterling et al.15 found that more than half of patients with AFP-negative liver cancers had elevated DCP, while liver cancer could be ruled out in patients with normal DCP levels, despite positive nodules on ultrasound results. DCP is therefore considered to be a diagnostic marker for AFP-negative HCC and an independent prognostic indicator.16 This retrospective study aimed to evaluate the early diagnostic value of serum DCP for distinguishing AFP-negative HBV-related HCC.
Materials and methods
Study population and DCP measurement
This study retrospectively enrolled patients treated at Beijing YouAn Hospital between March 2010 and July 2018. The participants were divided into four subgroups: CHB group, liver cirrhosis (LC) group, early-stage HCC group, and advanced-stage HCC group. The inclusion criteria for the HCC groups in this study were: (1) diagnosis confirmed by histopathological examination, (2) chronic HBV infection, (3) age 20 to 80 years, (4) serum collected preoperatively, and (5) serum AFP level ≤20 ng/mL. The exclusion were: (1) intrahepatic cholangiocarcinoma or mixed HCC, (2) hepatitis C virus, alcoholic liver disease, or biliary cirrhosis, and (3) other cancers. Early-stage HCC was defined as a single lesion or up to three lesions ≤3 cm. Advanced-stage HCC was defined as lesions >3 cm or accompanied by metastasis. CHB and LC were diagnosed according to the Guideline of Prevention and Treatment for Chronic Hepatitis B (2015 update).17 This study was approved by the research ethics committee of Beijing YouAn Hospital. Written informed consent was obtained from all participants. Serum AFP and DCP levels were measured using an automated chemiluminescent enzyme immunoassay system (LUMIPULSE® G1200; Fujirebio Inc., Tokyo, Japan), according to the manufacturer’s instructions.
Statistical analysis
The data were analyzed using SPSS version 22.0 (IBM, Armonk, NY, USA) and GraphPad Software version 7 (Graph Pad Software, San Diego, CA, USA). Differences within subgroups were compared using the Mann–Whitney test (for non-normally distributed data) and Student’s t-test (for normally distributed data). The clinicopathological characteristics were compared with Pearson’s χ2 test. The diagnostic value of DCP was evaluated by analyzing the receiver-operating characteristic curves (ROC) and areas under the curves (AUC). A value of P < 0.05 was considered significant.
Results
Patient characteristics
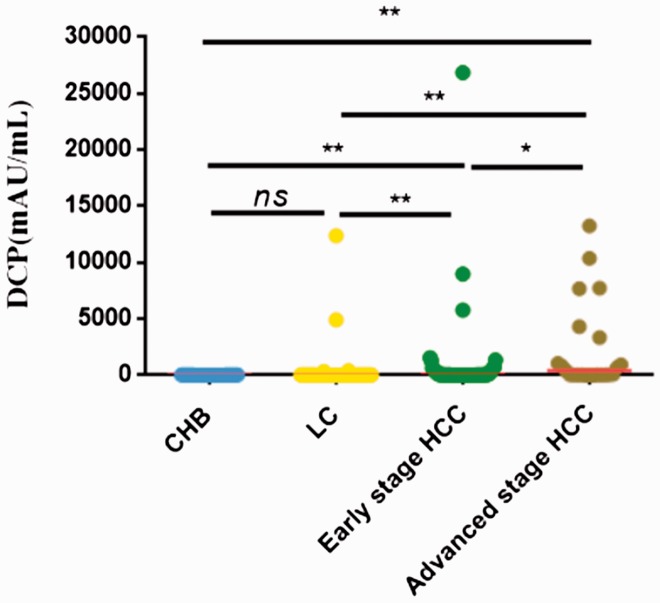
The baseline characteristics of the study population are presented in Table 1. A total of 210 patients were enrolled, including 87 patients with AFP-negative HBV-related HCC and 123 with CHB or LC. There was a male predominance in both groups (P = 0.008) (Table 1). The Child–Pugh grade was well-matched between the groups. There were significant differences among the HCC, LC, and CHB groups in terms of alanine aminotransferase, aspartate aminotransferase, total bilirubin, and total protein (all P < 0.05) (Table 1). Serum DCP levels were significantly higher in patients with early- or advanced-stage HCC compared with the CHB and LC groups (P = 0.032) (Figure 1).
Table 1.
Clinical features of the study population.
| Feature | CHB | LC | Early-stage HCC | Advanced-stage HCC | P value |
|---|---|---|---|---|---|
| Number | 51 | 72 | 62 | 25 | |
| Sex | |||||
| Male | 30 | 47 | 52 | 21 | 0.008 |
| Female | 21 | 25 | 10 | 4 | |
| Age (year) | |||||
| ≤55 | 45 | 44 | 39 | 10 | <0.001 |
| >55 | 6 | 28 | 23 | 15 | |
| Child–Pugh | |||||
| A | 51 | 51 | 52 | 21 | 0.183 |
| B | 0 | 17 | 10 | 3 | |
| C | 0 | 4 | 0 | 1 | |
| ALT, U/L | |||||
| ≤50 | 48 | 67 | 47 | 18 | 0.02 |
| >50 | 3 | 5 | 15 | 7 | |
| AST, U/L | |||||
| ≤40 | 46 | 54 | 47 | 13 | 0.03 |
| >40 | 5 | 18 | 15 | 12 | |
| TBil, µmol/L | |||||
| ≤21 | 41 | 37 | 44 | 19 | 0.04 |
| >21 | 10 | 35 | 18 | 6 | |
| TP, g/L | |||||
| ≤65 | 3 | 27 | 18 | 9 | 0.01 |
| >65 | 48 | 45 | 44 | 16 | |
| Cirrhosis | |||||
| Yes | 0 | 72 | 43 | 15 | <0.001 |
| No | 51 | 0 | 19 | 10 | |
| DCP(mAU/mL, median, range) | 24 (13, 49) | 24.5 (7, 12,391) | 34 (11, 26,858) | 406 (12, 75,000) | <0.001 |
HCC, hepatocellular carcinoma; LC, liver cirrhosis; CHB, chronic hepatitis B virus infection; TBil, total bilirubin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; TP, total protein; DCP, des-γ-carboxy prothrombin.
Figure 1.
Serum DCP levels in patients with AFP-negative HBV-related HCC, LC, and CHB. **P<0.001; *P<0.01. ns, not significant; HCC, hepatocellular carcinoma; HBV, hepatitis B virus; DCP, des-γ-carboxy prothrombin; AFP; alpha-fetoprotein. Note: Extreme values were removed.
Diagnostic value of DCP
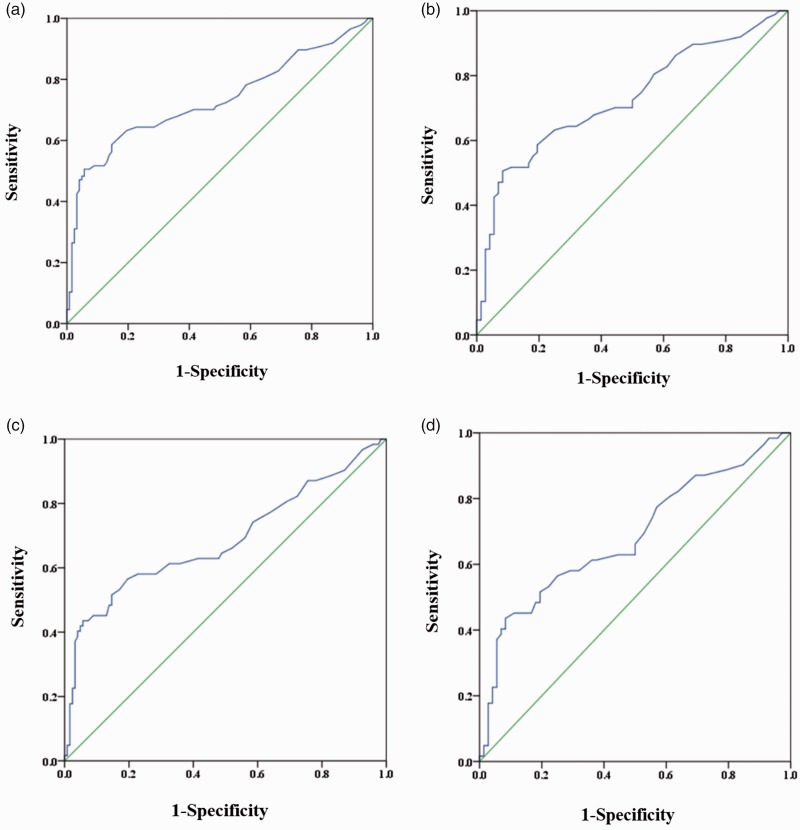
The diagnostic value of DCP for AFP-negative HBV-related HCC was evaluated by ROC curves. Based on the AUCs, DCP was able to distinguish between all HCCs and CHB or LC, all HCCs and LC, early-stage HCC and CHB or LC, early-stage HCC and LC (Table 2, Figure 2).
Table 2.
Diagnostic values of DCP for AFP-negative HBV-related HCC.
| Groups compared |
DCP |
|||||
|---|---|---|---|---|---|---|
| Cutoff (mAU/mL) | AUC (95%CI) | Sen (%) | Spe (%) | PPV (%) | NPV (%) | |
| All HCC vs LC or CHB | 45 | 0.731 (0.657–0.805) | 50.6 | 94.3 | 86.3 | 73.0 |
| All HCC vs LC | 41 | 0.728 (0.649–0.806) | 50.6 | 91.7 | 88.0 | 60.6 |
| Early-stage HCC vs LC, CHB | 45 | 0.685 (0.596–0.774) | 43.5 | 94.3 | 79.4 | 76.8 |
| Early-stage HCC vs LC | 41 | 0.683 (0.591–0.775) | 43.5 | 91.7 | 81.8 | 65.3 |
HCC, hepatocellular carcinoma; LC, liver cirrhosis; CHB, chronic hepatitis B virus infection; DCP, des-γ-carboxy prothrombin; AUC, area under curve; Sen, sensitivity; Spe, specificity; PPV, positive predictive value; NPV, negative predictive value.
Figure 2.
Values of DCP for early diagnosis of AFP-negative HBV-related HCC. (a) All HCC vs CHB and LC; (b) all HCC vs LC; (c) early-stage HCC vs CHB and LC; (d) early-stage HCC vs LC. HCC, hepatocellular carcinoma; LC, liver cirrhosis; CHB, chronic hepatitis B virus infection.
Discussion
The present study evaluated the performance of serum DCP for diagnosing AFP-negative HBV-related HCC in 87 patients with AFP-negative HBV-related HCC and 123 with CHB or LC. Serum DCP levels were significantly higher in the patients with HCC compared with the CHB and LC groups, indicating that DCP might be an excellent diagnostic biomarker for AFP-negative HBV-related HCC. The results of ROC curve analysis showed that DCP could effectively distinguish between all AFP-negative HCCs and CHB and LC (AUC 0.731) and between early-stage AFP-negative HBV-related HCC and CHB or LC (AUC 0.685). These results confirmed the value of DCP as a promising marker for diagnosing AFP-negative HBV-related HCC.
Most previous research suggested that the diagnostic efficiency of DCP for HCC was inferior to that of AFP.18–20 For instance, Choi et al.21 conducted a longitudinal assessment of the diagnostic value of DCP for detecting HCC and reported an AUC for DCP of 0.71 for diagnosing HCC. However, regarding AFP-negative HCC, a large-scale multicenter study by Ji et al.22 showed that DCP was superior to AFP for the surveillance of early HCC, and that DCP had an AUC of 0.856 for differentiating between patients with AFP-negative HCC and control subjects, including healthy individuals and patients with liver cirrhosis, liver hemangiomas, and chronic hepatitis B virus infection. The AUC for DCP in their study was higher than that in the current study, possibly because the former study included more patients with late-stage HCC and healthy controls. Another study reported by Wang et al.23 showed an AUC for DCP of 0.731 among patients with AFP-negative HBV-related HCC, which was consistent with the current results.
The ratio of fucosylated to total serum paraoxonase 1 was previously reported to show superior potential compared with AFP-L3 and glypican-3 for distinguishing between AFP-negative HCC and LC.24 In terms of other important markers, AFP-L3, as a specific type of AFP, was considered to be more specific than total AFP for diagnosing HCC, and might thus also improve the diagnosis of HCC in patients with low AFP levels.25 Although we did not compare the diagnostic values of DCP and AFP-L3 for differentiating HCC, Zhang et al.26 revealed that the maximum AUC of AFP-L3 for diagnosing AFP-negative HCC was 0.609 (95% confidence interval (CI) 0.599 to 0.799), which was much lower than that for DCP in the present study. Furthermore, Mao et al.27 showed that the AUCs of peripheral plasma Dickkopf-1 and Tie2-expressing monocytes for differentiating between AFP-negative HCC and LC and CHB were 0.649 (95%CI 0.542 to 0.755) and 0.708 (95%CI 0.605 to 0.812), respectively. However, the AUC for DCP in the current study was higher than these previous results, indicating that DCP was more promising than other potential biomarkers for diagnosing AFP-negative HBV-related HCC.
Different from previous studies, we also evaluated the value of DCP for the early diagnosis of AFP-negative HBV-related HCC, and showed that serum DCP might be a suitable biomarker to aid the early diagnosis of AFP-negative HCC.
This study had some limitations. First, the total number of included participants was relatively small. Second, this was a retrospective study from single center and not a prospective study. Third, we only focused on patients with HBV-related HCC.
In conclusion, DCP shows relative high efficiency for differentiating between AFP-negative HBV-related HCC and CHB or LC. DCP may thus be a suitable biomarker to improve the rate of early diagnosis of AFP-negative HBV-related HCC.
Availability of data and materials
The datasets supporting the conclusion of this study are included in the article.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD
References
- 1.Yuen MF, Hou JL, Chutaputti Aet al. Hepatocellular carcinoma in the Asia pacific region. J Gastroenterol Hepatol 2009; 24: 346–353. [DOI] [PubMed] [Google Scholar]
- 2.Moore MA, Ariyaratne Y, Badar Fet al. Cancer epidemiology in South Asia - past, present and future. Asian Pac J Cancer Prev 2010; 11: 49–66. [PubMed] [Google Scholar]
- 3.Wang FS, Fan JG, Zhang Zet al. The global burden of liver disease: the major impact of China. Hepatology 2014; 60: 2099–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018; 68: 7–30. [DOI] [PubMed] [Google Scholar]
- 5.Pungpapong S, Kim WR, Poterucha JJ. Natural history of hepatitis B virus infection: an update for clinicians. Mayo Clin Proc 2007; 82: 967–975. [DOI] [PubMed] [Google Scholar]
- 6.Nagaratnam N, Nagaratnam K, Cheuk G. Hepatocellular Carcinoma. Geriatric Diseases pp.1–4.
- 7.Ganem D, Prince AM. Hepatitis B virus infection–natural history and clinical consequences. N Engl J Med 2004; 350: 1118–1129. [DOI] [PubMed] [Google Scholar]
- 8.Marquardt JU, Galle PR, Teufel A. Molecular diagnosis and therapy of hepatocellular carcinoma (HCC): an emerging field for advanced technologies. J Hepatol 2012; 56: 267–275. [DOI] [PubMed] [Google Scholar]
- 9.Ju YC, Jun DW, Choi Jet al. Long term outcome of antiviral therapy in patients with hepatitis B associated decompensated cirrhosis. World J Gastroenterol 2018; 24: 4606–4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen S, Chen H, Gao Set al. Differential expression of plasma miR-125b in hepatitis B virus related liver diseases and diagnostic potential for hepatitis B virus induced hepatocellular carcinoma. Hepatol Res 2017; 47: 312–320. [DOI] [PubMed] [Google Scholar]
- 11.Han LL, Lv Y, Guo Het al. Implications of biomarkers in human hepatocellular carcinoma pathogenesis and therapy. World J Gastroenterol 2014; 20: 10249–10261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Males S, Gad RR, Esmat Get al. Serum alpha-foetoprotein level predicts treatment outcome in chronic hepatitis C. Antivir Ther 2007; 12: 797–803. [PubMed] [Google Scholar]
- 13.Liebman HA, Furie BC, Tong MJet al. Des-gamma-carboxy (abnormal) prothrombin as a serum marker of primary hepatocellular carcinoma. N Engl J Med 1984; 310: 1427–1431. [DOI] [PubMed] [Google Scholar]
- 14.Gao J, Feng X, Inagaki Yet al. Des-γcarboxy prothrombin and c-Met were concurrently and extensively expressed in hepatocellular carcinoma and associated with tumor recurrence. Biosci Trends 2012; 6: 153–159. [DOI] [PubMed] [Google Scholar]
- 15.Sterling RK, Jeffers L, Gordon Fet al. Utility of Lens culinaris agglutinin-reactive fraction of alpha-fetoprotein and des-gamma-carboxy prothrombin, alone or in combination, as biomarkers for hepatocellular carcinoma. Clin Gastroenterol Hepatol 2009; 7: 104–113. [DOI] [PubMed] [Google Scholar]
- 16.Song P, Feng X, Zhang Ket al. Perspectives on using des-gamma-carboxyprothrombin (DCP) as a serum biomarker: facilitating early detection of hepatocellular carcinoma in China. Hepatobiliary Surg Nutr 2013; 2: 227–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hou J, Wang G, Wang Fet al. Guideline of prevention and treatment for chronic hepatitis B (2015 update). J Clin Transl Hepatol 2017; 5: 297–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park SJ, Jang JY, Jeong SWet al. Usefulness of AFP, AFP-L3, and PIVKA-II, and their combinations in diagnosing hepatocellular carcinoma. Medicine (Baltimore) 2017; 96: e5811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao Q, Liu YY, Lei Xet al. PIVKA-II as a screening marker for hepatocellular carcinoma in patients with hepatitis B virus infection. World Chinese Journal of Digestology 2017; 9: 803. [Google Scholar]
- 20.Yoon YJ, Han KH, Kim DY. Role of serum prothrombin induced by vitamin K absence or antagonist-II in the early detection of hepatocellular carcinoma in patients with chronic hepatitis B virus infection. Scand J Gastroenterol 2009; 44: 861–866. [DOI] [PubMed] [Google Scholar]
- 21.Choi J, Kim GA, Han Set al. Longitudinal assessment of three serum biomarkers to detect very early-stage hepatocellular carcinoma. Hepatology 2019; 69: 1983–1994. [DOI] [PubMed] [Google Scholar]
- 22.Ji J, Wang H, Li Yet al. Diagnostic evaluation of des-gamma-carboxy prothrombin versus α-fetoprotein for hepatitis B virus-related hepatocellular carcinoma in China: a large-scale, multicentre study. PLoS One 2016; 11: e0153227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X, Zhang W, Liu Yet al. Diagnostic value of prothrombin induced by the absence of vitamin K or antagonist-II (PIVKA-II) for early stage HBV related hepatocellular carcinoma. Infect Agent Cancer 2017; 12: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shu H, Li W, Shang Set al. Diagnosis of AFP-negative early-stage hepatocellular carcinoma using Fuc-PON1. Discov Med 2017; 23: 163–168. [PubMed] [Google Scholar]
- 25.Luo P, Wu S, Yu Yet al. Current status and perspective biomarkers in AFP negative HCC: towards screening for and diagnosing hepatocellular carcinoma at an earlier stage. Pathol Oncol Res 2019. 10.1007/s12253-019-00585-5. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Z, Zhang Y, Wang Yet al. Alpha-fetoprotein-L3 and Golgi protein 73 may serve as candidate biomarkers for diagnosing alpha-fetoprotein-negative hepatocellular carcinoma. Onco Targets Ther 2015; 9: 123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mao L, Wang Y, Wang Det al. TEMs but not DKK1 could serve as complementary biomarkers for AFP in diagnosing AFP-negative hepatocellular carcinoma. PLoS One 2017; 12: e0183880. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets supporting the conclusion of this study are included in the article.