Short abstract
Objective
To examine whether lifestyle-related factors and resilience predict health-related quality of life (HRQoL) in a sample of early adolescents.
Methods
A total of 611 eighth grade pupils (response rate: 79%) participated in this cross-sectional study. The variables measured were physical activity (accelerometer), cardiorespiratory fitness (Andersen test) and a questionnaire assessing dietary habits, sleep disturbance, resilience (Resilience Scale for Adolescents) and HRQoL (KIDSCREEN-27).
Results
A total of 36% of the sample met the official recommendations of 60 minutes of daily physical activity. Univariate analysis identified physical activity, dietary habits, sleep disturbances, body mass index (BMI), cardiorespiratory fitness and resilience, but not sedentary time, as predictors of HRQoL. Multivariate regression analysis identified resilience as a positive predictor (β 0.18 to 0.27) of all HRQoL domains and sleep disturbance as a negative predictor (β −0.65 to −0.24) of four HRQoL domains. BMI (β = −0.27) and cardiorespiratory fitness (β = 0.021) were predictors of the HRQoL domain physical well-being. Adherence to dietary recommendations was both a positive and a negative predictor of HRQoL (β −0.45 to 0.59).
Conclusion
Resilience and sleep disturbances were the main predictors of HRQoL.
Keywords: Public health, adolescents, exercise, sleep, mental health, resilience, health promotion
Introduction
Adolescence is a key transitional stage in which lifestyle choices that determine long-term health, well-being and quality of life are made.1 It is also a life phase characterized by substantial physical, psychological, social and emotional changes that lead to greater vulnerability related to health and well-being.2 Some adolescent lifestyle changes are temporary, whereas others continue into adulthood. Physical activity level declines and sedentary time increases rapidly from childhood to adolescence.3 To some extent, physical activity and sedentary behaviour in adolescence predict such behaviour in adulthood.4,5 Currently, only approximately half of Norwegian adolescents adhere to the official recommendations of 60 minutes of daily physical activity6 Dietary habits also persist from adolescence to adulthood,7,8 and in Norwegian adolescents they are currently characterized by an irregular meal pattern with frequent snacking and intake of light meals.9 Alarmingly, few Norwegian adolescents adhere to the official dietary recommendations of (for example) an intake of >five portions of fruit/vegetables/berries (10% adherence) and a sugar limit of <10% of total daily energy intake (17% adherence).10,11 There is evidence that dietary adherence is associated with parental socioeconomic status (SES).10
Adequate sleep is fundamental for health and well-being.12 Sleeps affects both biological processes, such as energy expenditure, appetite regulation and hormonal regulation, and psychological processes such as attention and memory.13 Sleep is one of the most prominent lifestyle-related behaviour changes from childhood to adolescence; these changes include altered bedtimes, poorer sleep quality and more daytime sleepiness.14 Social changes during adolescence, such as less parental control, more social interaction with peers and increased use of electronic devices, may affect several aspects of sleep.15,16 Despite this, sleep has been neglected in studies of lifestyle and health among adolescents.14 To acknowledge the importance for health and well-being of lifestyle behaviour throughout the 24-hour period, the Canadian 24-hour Movement Guidelines were published in 2016.17 These guidelines provide recommendations for sleep hygiene (i.e. 8–10 hours of uninterrupted sleep per night for adolescents) in addition to recommendations for physical activity (i.e. 60 minutes per day) and sedentary time (i.e. 2 hours maximum of daily recreational screen-time, limited sitting for extended periods). Although adherence to sleep recommendations is better than adherence to physical activity and dietary recommendations, an Australian study found that as many as 44% of adolescents sleep for an insufficient number of hours each night.18 It is therefore important to include sleeping patterns and disturbances in any analysis of lifestyle factors that predict health and well-being.
Health-related quality of life (HRQoL) has been defined by the World Health Organization as a multidimensional and integrative construct comprising physical, psychological and social well-being and functioning.19 HRQoL is a core aspect of general health and well-being, and it is relatively stable in healthy adolescents.20,21 Associations between HRQoL and sleep,14 physical activity and sedentary behaviour,22 dietary habits23 and cardiorespiratory fitness24 have been found in adolescents. Unfortunately, most studies consider only a limited set of lifestyle-related factors as predictors of HRQoL. Muros et al.25 found that dietary habits, physical activity and body mass index (BMI) explained 20% of the variance in HRQoL in adolescents aged 11 to 14 years, but this study did not measure sleep and cardiorespiratory fitness. Although physical activity and cardiorespiratory fitness are related, they are different constructs. Physical activity is a behavioural component and is the most important contributor to physical fitness,26 whereas cardiorespiratory fitness is one of several components of physical fitness.26 Both physical activity and physical fitness are independently associated with various domains of HRQoL. A study using path analysis demonstrated that physical fitness mediates the relationship between physical activity and HRQoL in children.27 This indicates the importance of examining both these constructs as possible predictors of HRQoL.
Furthermore, most existing studies have not included important confounding factors such as resilience. Resilience is a protective factor that contributes to good outcomes despite the substantial risk of disease burden,28 and is associated with both HRQoL29 and lifestyle30 in adolescents. Resilience also mediates 60% of the relationship between physical activity and mental health.31 To our knowledge, this current study is the first to examine the relationship between resilience and a wide set of lifestyle-related factors as predictors of HRQoL. The study aim was to examine 1) lifestyle-related factors, resilience and HRQoL, and 2) the relative importance of lifestyle-related factors and resilience as predictors of HRQoL in a sample of early adolescents. The results have the potential to increase the understanding of the relative importance of various lifestyle-related factors, and hence provide evidence for future experimental studies to improve adolescent HRQoL.
Materials and methods
Sample and study design
The cross-sectional data presented in this paper are baseline data from the quasi-experimental study ‘Active and Healthy Kids in Telemark’, a study initiated and implemented by the Telemark County Council Department of Public Health, Telemark, Norway. Participants were recruited using a non-probability convenience sampling method. Inclusion criteria were enrolment as an eighth grade pupil in school year 2017/2018 at a public secondary school in one of the six municipalities that had agreed to participate in the Telemark study. Telemark County Council selected four rural municipalities and two urban municipalities for participation; these municipalities contained 15 secondary schools. Exclusion criteria for participation were language barriers, and any injuries or illnesses that affected the assessment of physical fitness and physical activity.
Data collection was conducted during one school day within 4 weeks after the start of the school semester; pupils absent from school that day were unable to participate. The research group provided oral and written information about the study to school principals and staff. Prior to data collection, the primary teachers for each included class distributed written information about the study to the pupils and their parents on behalf of the research group prior to data collection. This study was conducted in accordance with the Helsinki Declaration, was evaluated by the Regional Committee for Medical and Health Research Ethics (ID 2017/387), approved by the Norwegian Data Protection Services (ID number 54327) and registered in ClinicalTrials.gov (NCT03906851). Parents of the included participants gave written consent for participation. The data presented in this paper were collected during September 2017.
Assessment
SES
Parental education level is considered the most fundamental SES indicator.32 The parents of each pupil were thus asked to classify their completed educational level as one of the five following categories: ‘elementary school’, ‘vocational school’, ‘high school’, ‘higher education, undergraduate level’ or ‘higher education, graduate level’. If each parent of a child had different completed educational levels, the highest level was used for the analyses.
Physical activity
Physical activity was measured objectively using a triaxial Actigraph accelerometer (ActiGraph GT3X+, LLC, Pensacola, FL, USA). Each participant was fitted with an accelerometer in an elastic belt, fitted around the waist and placed on the right hip. The accelerometer was worn for 4 consecutive days (2 weekdays and 2 weekend days). Participants were instructed to wear their accelerometers for the whole day, except during water-based activities or while sleeping. Accelerometers were initialized to start recording at 06:00 on the day after they were distributed. Epoch length was set to 10-second intervals. The criterion for a valid day was set as a weartime of 480 minutes/day between 06:00 and 24:00. A total of ≥2 days (weekdays and/or weekend days) was the criteria for a valid measurement, following Kolle et al.33 All sequences of ≥20 minutes of consecutive zero counts from each subject’s recording were excluded and defined as non-weartime, as this indicates periods in which participants did not wear the accelerometer.34 We used ActiLife software (ActiGraph, LLC, Pensacola, FL, USA) to initialize the monitors and to download the accelerometer files. The outcomes for total physical activity were counts per minute (cpm) from the accelerometer’s vertical axis (cpm axis 1). Sedentary time was defined as all activities less than 100 cpm, a threshold that corresponds with sitting, reclining or lying down.35,36 Evenson’s criteria35 were used to define cutoffs for moderate-to-vigorous physical activity. We analysed all accelerometer data using ActiLife software (ActiGraph, LLC, Pensacola, FL, USA).
Cardiorespiratory fitness
The Andersen test, which has been shown to provide reliable and valid data on a group level,37 was used to assess cardiorespiratory fitness. The Andersen test is a 10-minute intermittent running test with periods of 15 seconds work and 15 seconds rest typically signalled by a test leader blowing a whistle.38 Participants run between two parallel lines 20 m apart. At each line, they must touch the floor behind the line before turning around to run to the other line. The aim of the test is to cover the longest distance possible during the 10-minute run; the test result is the distance covered in metres. Participants were divided into pairs for the testing; half the pupils completed the test while the other half assisted in counting by writing down the number of laps on a standardized form. Afterwards, they switched tasks. Each test was filmed by a test assistant. If a counter lost count, the test leader would recount the number of laps by watching the film. The film was immediately deleted after the test results were registered. Verbal encouragements were given during all tests to motivate participants to give their best performance. After each test, the test leader and test assistants evaluated subjective criteria for near-maximal performance. Participants who clearly did not give near-maximal efforts (assessed using physical and verbal behaviour during and immediately after the test, as well as running and breathing patterns) were excluded from the test results.
Anthropometric measurements
Height and body weight were measured wearing light clothing and without shoes. Height was measured to the nearest 0.1 cm using a wall-mounted measuring tape or stadiometer. Body weight was measured to the nearest 0.1 kg using electronic scales belonging to the school nurse’s office at each school. BMI was calculated as weight in kilograms divided by height in metres squared (kg/m2). The children were categorized as normal weight, overweight or obese according to the criteria by Cole and Lobstein.39
Dietary index
Dietary information was collected using a short food frequency questionnaire (FFQ), based on a previously validated FFQ used with Norwegian adolescents.40 The FFQ included questions about habitual intake of 12 types of foods/drinks with nine answer options ranging from seldom/never to several times/day. Responses were coded from 0 to 8. Based on current Norwegian general dietary recommendations,41 we chose four dietary indicators to form a dietary index: fruits/berries and vegetables, whole grain cereals, fish for dinner/on bread, and high intake of sugar products (sweets/bakery goods/sugary soda). Based on the recommendations, each indicator was given scores on a scale from 0 to 10; a score of 10 indicated that the respondent had a recommended/optimal intake of the respective indicator (Table 1).42 Respondents with other intake frequencies were given scores proportionally. For indicators that included more than one question (fruits/berries and vegetables, high sugar products), a mean score for each indicator was calculated. Finally, each participant was given a mean dietary score (0–10); higher scores on the index indicated a healthier diet. The variable was labelled ‘recommended diet’.
Table 1.
Dietary indicators and calculation of dietary index.
|
Cutoff points = criteria for max score ( = 10) |
Proportion fulfilling recommendations |
|||
|---|---|---|---|---|
| Dietary index indicators | Recommended frequency | Corresponding value (answer options 0–8) | Boys (%) | Girls (%) |
| Whole grain cereals | Daily | 7 | 17% | 18% |
| Fish (for dinner and on bread) | ≥3 times/week* | 3 | 24% | 25% |
| Sweets/bakery goods/sugary soda (mean of the 3 variables) | ≤1 time/week** | 1 | 32% | 33% |
| Fruits/berries, and vegetables (mean of the 2 variables) | Several times a day | 7.5 | 6% | 14% |
*≥2 dinners/week and on sandwiches **not on schooldays/weekdays, (1 time/week).
Sleep
Sleep quality, sleep latency and daytime sleepiness were assessed using six questions adapted from the Norwegian cross-national youth survey Ungdata.43 The sleep items were as follows: ‘During the past month, how many days have you … 1. had problems getting to sleep after you have switched the light off? 2. not felt properly rested after sleeping? 3. been so sleepy/tired that it has affected your schoolwork or leisure activities? 4. had difficulty waking up at the right time in the morning? 5. not managed to fall asleep before 2 a.m.? 6. woken up too early and been unable to get back to sleep?’ Questions are categorized into the following five response options: 0 to 5 times (1), 6 to 10 times (2), 11 to 15 times (3), 16 to 20 times (4), and 20 times or more (5). The questions used in this survey reflect the categories outlined in the BEARS screening tool44 and sleep concepts assessed in other widely used subjective sleep measures.45 To generate a sleep index, we recoded item 2 so that a higher score on all items indicated higher frequency of problems. The index was a sum score of the six items; total scores ranged from 6 to 30.
Resilience
To measure participants’ resilience we used the 28-item Resilience Scale for Adolescents (READ).28,46 The scale comprises five subscales labelled ‘personal competence’, ‘social competence’, ‘structured style’, ‘family cohesion’ and ‘social resources’. Responses are on a 5-point Likert scale ranging from strongly disagree (1) to strongly agree (5). All items are positively phrased, such that high scores indicate high resilience for all items. Initial work on the READ scale has shown the instrument to have adequate internal reliability and factorial validity.46 The alpha coefficient in this study was 0.95 for the mean total READ score and ranged from 0.69 to 0.86 for the subscales; these values were deemed acceptable.
HRQoL
KIDSCREEN is a multidimensional, widely used and validated instrument that assesses physical, psychological, social and behavioural components of well-being in children and adolescents aged 8 to 18 years.47,48 The KIDSCREEN-27 was developed to obtain a shorter version of the original KIDSCREEN-52, and it consists of the five domains: ‘physical well-being’, ‘psychological well-being’, ‘social support and peers’, ‘parent relations and autonomy’, and ‘school environment’.49 Cronbach’s alpha in this sample ranged from 0.79 to 0.82 for the five domains.
Statistical analysis
Missing data for the independent variables (physical activity (18%), sedentary activity (18%), BMI (17%) and physical condition (11%)) were imputed using mean values for all analysis. Excluding all cases with missing values did not significantly alter the results. The Pearson correlations between the independent variables ranged from −0.360 for BMI and physical condition and 0.339 for physical condition and physical activity. Sex differences were analysed using the independent t-test. Univariate linear regression was used to assess univariate associations between the independent variables and the five domains of the KIDSCREEN-27. Stepwise linear regression was used to assess associations between all the independent variables and the five KIDSCREEN-27 domains, adjusted for each other and SES, sex and place of birth, resulting in a model with only significant associated predictors. P < 0.05 was considered to indicate significance in all analyses. IBM SPSS version 25.0 (IBM Corp., Armonk, NY, USA) was used for all analyses.
Results
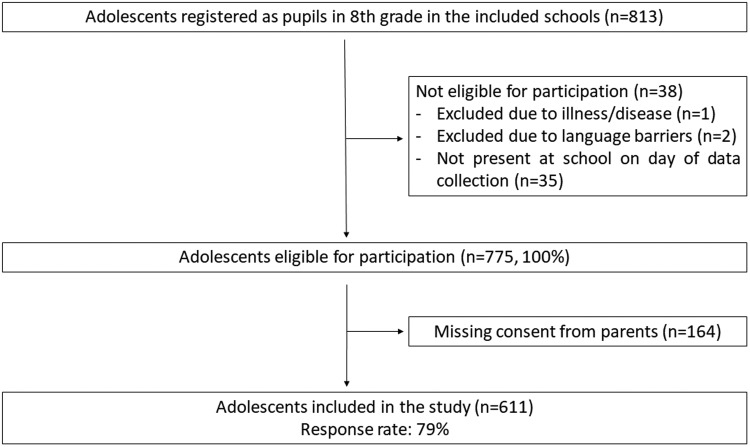
As shown in Figure 1, 611 pupils participated in this cross-sectional data collection (response rate: 79%). The mean respondent age was 13.2 years (Table 2). There was an equal distribution of female (49.1%) and male (50.9%) respondents; 9% of the respondents were immigrants (Table 2). As shown in Table 2, 69% of the respondents had high SES. A total of 36% of the sample met the recommendations of 60 minutes of daily physical activity; of these, more boys met the recommendations than girls. The READ scores showed higher personal competence (P < 0.05) and lower social competence (P < 0.05), social resources (P < 0.001) and family cohesion (P < 0.05) in boys compared with girls (Table 2). The sleep data showed that 23% of participants reported daytime sleepiness >1 time/week, 27% reported feeling rested after sleep <5 times/month, and 29% reported long sleep latency >1 time/week. Girls reported a higher frequency of feeling inadequately rested after sleep compared with boys (P < 0.05) (Table 2). Sex differences were found for two of the five HRQoL domains (parent relations and autonomy (P < 0.05) and social support and peers (P < 0.01)) (Table 2).
Figure 1.
Flow chart of study participation.
Table 2.
Descriptive data.
| Boys (n = 311) | Girls (n = 300) | Difference | Total (n = 611) | |
|---|---|---|---|---|
| Background | ||||
| Age, years, mean (SD) | 13.2 (0.3) | 13.2 (0.3) | t = 0.65 | 13.2 (0.3) |
| Immigrants, n (%) | 26 (9) | 25 (9) | χ2(1) = 0.01 | 51 (9) |
| Parental higher education, n (%) | 133 (69) | 132 (70) | χ2(1) = 0.72 | 265 (69) |
| Lifestyle-related variables | ||||
| BMI, kg/m2, mean (SD) | 19.6 (3.2) | 20.5 (3.6) | t = 2.84** | 20.1 (3.4) |
| Overweight/obesity, n (%) | 41 (17) | 54 (22) | χ2(1) = 2.13 | 95 (19) |
| Physical activity, CPM, mean (SD) | 513.0 (186.7) | 445.2 (155.6) | t = 4.36*** | 476.8 (174.0) |
| MVPA, minute/day, mean (SD) | 58.4 (24.8) | 50.3 (20.3) | t = 3.95*** | 54.1 (22.9) |
| Compliance with PA recommendations, n (%) | 94 (41) | 80 (31) | χ2(1) = 5.98 | 174 (36) |
| Sedentary time, hours/day, mean (SD) | 8.8 (1.3) | 9.1 (1.1) | t = 3.02** | 9.0 (1.2) |
| Andersen test, mean (SD) | 1018 (128) | 973 (111) | t = 4.31*** | 996.4 (122.2) |
| Recommended dietary intake, index, mean (SD) | 5.8 (1.7) | 6.4 (1.8) | t = 3.84*** | 6.2 (1.8) |
| Sleep item #1a mean (SD) | 1.50 (1.06) | 1.66 (1.16) | t = 1.69 | 1.58 (1.11) |
| Sleep item #2b mean (SD) | 2.82 (1.59) | 3.14 (1.50) | t = 2.48* | 2.98 (1.56) |
| Sleep item #3c mean (SD) | 1.42 (0.85) | 1.34 (0.80) | t = 1.09 | 1.38 (0.83) |
| Sleep item #4d mean (SD) | 2.25 (1.42) | 2.37 (1.46) | t = 1.00 | 2.31 (1.44) |
| Sleep item #5e mean (SD) | 1.26 (0.78) | 1.22 (0.69) | t = 0.70 | 1.24 (0.74) |
| Sleep item #6f mean (SD) | 1.43 (0.82) | 1.32 (0.76) | t = 1.65 | 1.37 (0.80) |
| Sleep index, mean (SD) | 10.68 (3.49) | 11.04 (3.54) | t = 1.26 | 10.86 (3.5) |
| Resilience | ||||
| READ, mean | 3.99 (0.65) | 4.96 (0.60) | t = 1.36 | 4.02 (0.63) |
| Personal competence | 3.97 (0.68) | 3.85 (0.60) | t = 2.00* | 3.91 (0.69) |
| Structured style | 3.63 (0.76) | 3.69 (0.75) | t = 1.09 | 3.66 (0.76) |
| Social competence | 3.79 (0.79) | 3.94 (0.75) | t = 2.38* | 3.87 (0.77) |
| Social resources | 4.28 (0.74) | 4.49 (0.61) | t = 3.77*** | 4.39 (0.69) |
| Family cohesion | 4.17 (0.74) | 4.30 (0.68) | t = 2.37* | 4.23 (0.71) |
| Health-related quality of life | ||||
| Physical well-being | 46.7 (9.4) | 46.1 (9.1) | t = 0.70 | 46.4 (9.2) |
| Psychological well-being | 51.0 (9.3) | 50.5 (9.6) | t = 0.68 | 50.7 (9.5) |
| Parent relations and autonomy | 52.4 (10.1) | 54.1 (9.6) | t = −2.07* | 53.3 (9.9) |
| Social support and peers | 50.3 (10.1) | 52.4 (9.4) | t = −2.61** | 51.4 (9.8) |
| School environment | 51.5 (9.7) | 52.6 (9.4) | t = −1.30 | 52.0 (9.6) |
*P < 0.05. **P < 0.01. ***P < 0.001. CPM: counts per minute; MVPA: moderate-to-vigorous physical activity; PA: physical activity; SD: standard deviation; BMI: body mass index; READ: Resilience Scale for Adolescents. aDuring the past month, how many days have you had problems getting to sleep after you have switched the light off? bDuring the past month, how many days have you not felt properly rested after sleeping? cDuring the past month, how many days have you been so sleepy/tired that it has affected your schoolwork or leisure activities? dDuring the past month, how many days have you had difficulty waking up at the right time in the morning? eDuring the past month, how many days have you not managed to fall asleep before 2 a.m.? fDuring the past month, how many days have you woken up too early and been unable to get back to sleep?
Predictors of HRQoL
Sedentary time did not predict scores on any of the HRQoL domains. Physical activity and cardiorespiratory fitness (β = 0.021) were positive predictors of physical well-being and psychological well-being, BMI was a negative predictor of physical well-being (β = −0.27), and sleep disturbance was a negative predictor of four of the five HRQoL domains (β −0.65 to −0.24) (all P < 0.05) (Table 3). Recommended dietary intake and resilience were positive predictors of physical well-being, psychological well-being, parent relations and autonomy, and school environment (all P < 0.05). The multivariate analyses identified resilience as a positive predictor for all five HRQoL domains (β 0.18 to 0.27; all P < 0.05). Sleep disturbance negatively predicted four out of five domains (all P < 0.05). Recommended dietary intake positively predicted physical well-being and school environment and negatively predicted social support and peers (β −0.45 to 0.59), whereas cardiorespiratory fitness positively predicted physical well-being (all P < 0.05) (Table 4). Physical activity level and sedentary time did not predict scores on any of the HRQoL domains in the multivariate analyses (Table 4).
Table 3.
Univariate linear regression of association between lifestyle-related factors, resilience and each of the five KIDSCREEN-27 (HRQoL) dimensions.
| Physical well-being | Psychological well-being | Parent relations and autonomy | Social support and peers | School environment | |
|---|---|---|---|---|---|
| Beta (95% CI) | Beta (95% CI) | Beta (95% CI) | Beta (95% CI) | Beta (95% CI) | |
| Physical activity | 0.010 (0.005, 0.014) | 0.0044 (0.0005, 0.0092) | −0.0003 (−0.0054, 0.0047) | 0.0030 (−0.0020, 0.0080) | 0.0007 (−0.0042, 0.0056) |
| Sedentary time | −0.6 (−1.2, 0.1) | −0.1 (−0.8, 0.6) | 0.2 (−0.5, 0.9) | −0.2 (−0.9, 0.5) | 0.6 (−0.1, 1.3) |
| Recommended dietary intake | 1.2 (0.8, 1.6) | 0.7 (0.3, 1.2) | 0.8 (0.4, 1.3) | 0.2 (−0.2, 0.7) | 1.1 (0.7, 1.6) |
| BMI | −0.57 (−0.81, −0.34) | −0.05 (−0.29, 0.19) | 0.03 (−0.22, 0.29) | −0.13 (−0.38, 0.13) | −0.02 (−0.27, 0.22) |
| Sleep disturbance | −0.50 (−0.71, −0.29) | −0.82 (−1.03, −0.61) | −0.49 (−0.72, −0.27) | −0.12 (−0.34, 0.11) | −0.63 (−0.84, −0.41) |
| Cardiorespiratory fitness | 0.028 (0.022, 0.034) | 0.007 (0.001, 0.013) | 0.001 (−0.006, 0.008) | 0.003 (−0.004, 0.010) | 0.002 (−0.004, 0.009) |
| Resilience | 0.21 (0.17, 0.25) | 0.28 (0.25, 0.32) | 0.26 (0.22, 0.30) | 0.26 (0.22, 0.30) | 0.29 (0.26, 0.33) |
Bold text: significance level P < 0.05. Beta values are unstandardized. SES: socioeconomic status; BMI: body mass index (kg/m2); CI: confidence interval.
Table 4.
Stepwise multivariate linear regression of association between lifestyle-related factors, resilience and each of the five KIDSCREEN-27 dimensions (HRQoL), adjusted for sex, SES and place of birth.
| Physical well-being | Psychological well-being | Parent relations and autonomy | Social support and peers | School environment | |
|---|---|---|---|---|---|
| Beta (95% CI) | Beta (95% CI) | Beta (95% CI) | Beta (95% CI) | Beta (95% CI) | |
| Physical activity | |||||
| Sedentary time | |||||
| Recommended dietary intake | 0.59 (0.22, 0.96) | −0.45 (−0.86, 0.04) | 0.40 (0.02, 0.78) | ||
| BMI | −0.27 (−0.48, -0.06) | ||||
| Sleep disturbance | −0.24 (−0.42, −0.06) | −0.65 (−0.83, −0.47) | −0.34 (−0.54, −0.14) | −0.42 (−0.60, −0.24) | |
| Cardiorespiratory fitness | 0.021 (0.016, 0.027) | ||||
| Resilience | 0.18 (0.15, 0.22) | 0.27 (0.23, 0.30) | 0.25 (0.22, 0.29) | 0.27 (0.23, 0.31) | 0.27 (0.24, 0.31) |
Bold text: significance level P < 0.05. Beta values are unstandardized. SES: socioeconomic status; BMI: body mass index; CI: confidence interval.
Discussion
The main findings of this study were that sleep and resilience predicted scores on most HRQoL domains in early adolescents, and that physical activity disappeared as a predictor of HRQoL in the multivariate analysis. In addition, an alarmingly low percentage of the sample met the recommended level of physical activity and the dietary recommendations (and the percentage was lower than reported in other national and international studies of adolescents).3,11,50 Together with the lower cardiorespiratory fitness and higher BMI than found in previous studies,33,51 this indicates a general poor physical health profile in the sample. Reported sleep was more in accordance with findings from other studies of the same age group,52 as were the mean READ total and subscale scores.28 Telemark County’s public health profile shows a lower SES, shorter life expectancy and higher prevalence of mental health challenges in adolescents and early adults compared with the national average for Norway;53 therefore, the low adherence to both physical activity and dietary recommendations was not surprising.
The total scores for the HRQoL subscale physical well-being were lower in this sample compared with norm data for a similar age group provided by the KIDSCREEN group,47 and Norwegian data on 10-year-old children.54 This was the only domain for which BMI and cardiorespiratory fitness remained as predictors in the multivariate analysis, indicating that the lower scores on these variables may explain the overall lower level of physical well-being in this sample. Interestingly, the total scores for parent relations and autonomy and school environment were higher in this sample compared with the norm data for this age group.47 Scores on school environment were comparable with other Norwegian data,54 suggesting some cultural differences within the school system. Scores on parent relations and autonomy are higher in high-SES groups; however, the scores in this sample are even higher than in the high-SES group identified by Ravens-Sieberer et al.49 Norway is a high-income country with relatively low rates of poverty, which means that scores on items related to personal/family economy may show less variance in Norwegian adolescent samples than in adolescent samples from other countries. We cannot exclude the possibility that there are culture-specific differences that affect parent–child relationships; these may contribute to our respondents perceiving more autonomy and parental support than adolescents in other countries.
The multivariate analysis showed that sleep was a more important lifestyle-related predictor of the five HRQoL domains than physical activity, sedentary time, dietary habits, BMI and cardiorespiratory fitness. Sleep index was a negative predictor, indicating that a higher level of sleep disturbance negatively affects various aspects of HRQoL. It is well documented that sleep disturbances are associated with impairments in psychological functioning and physical health in adolescents,55 and our finding is in line with previous research on adults.56 One possible explanation for this finding may be that sleep is more fundamental for daytime functioning than other lifestyle-related behaviours. In addition, sleep affects behaviour, and therefore has a major influence on the various HRQoL domains. For instance, sleep disturbances have been shown to affect appetite regulation in a way that influences dietary habits,12 and sleep disturbance can impair athletic performance.57 Although the data in this study are cross-sectional, other studies have found alterations in sleep from childhood to adolescence.14 It is therefore possible that sleep and sleep disturbances play a more central role in the HRQoL of adolescents than in other age groups.
Resilience was the most prominent positive predictor of scores on the various HRQoL domains. This prediction was strongest for the domains psychological well-being, social support and peers and school environment; all of which are great sources of stress in adolescence.29 Higher scores on resilience indicate greater capability to cope with stress, so these results were not surprising. Interestingly, resilience was a significant predictor, together with sleep and/or dietary habits, for several of the HRQoL domains. This is in accordance with previous study findings showing that the interaction between resilience and sleep comprises a protective factor against depression in children and adolescents,58 and that resilience influences the relationship between lifestyle and depression/anxiety.30 Previous studies have mostly focused on resilience and lifestyle-related behaviours from a risk factor and preventive perspective. Our study adds to that knowledge by showing that these factors also promote quality of life and well-being. Especially interesting is the finding that resilience, sleep and adherence to dietary recommendations are significant predictors of the HRQoL school environment domain. The data were obtained from eighth grade secondary school pupils within 4 weeks of the start of a new school semester. For all the respondents, this meant new schools, new classes and new teachers. The transition from elementary school to secondary school may be a time in which resilience and lifestyle factors such as sleep and diet are particularly important in promoting school-related HRQoL. We have not been able to identify any studies showing an interaction between resilience and lifestyle as predictors of HRQoL in this domain. Therefore, future studies should aim to replicate these data using longitudinal designs to examine if this interaction changes as adolescents become more adapted to secondary school.
Adherence to dietary recommendations (i.e. high score on the dietary index) appeared to be an independent positive predictor of physical well-being and school environment, and a negative predictor of social support and peers. This is partly consistent with findings from a recent Australian study.23 The negative predictive effect of healthy diet on social support and peers was a little surprising, but can perhaps be interpreted in light of previous findings on social norms for dietary behaviour among early adolescents.59
Strengths and limitations
The high response rate, use of well-known, acknowledged and validated assessment methods, and the generalizability of the results are the main strengths of this study. The cross-sectional design makes it impossible to determine causal relationships, and self-reported dietary data may be negatively affected by response bias or recall bias. However, the dietary questions have demonstrated good reliability and validity in comparison with other validated instruments.40 Self-assessment methods have several limitations when used to assess sleep habits.45 The questions used to assess sleep quality, latency and daytime sleepiness in the present study do not address possible underlying mechanisms causing the sleep disturbances. Nevertheless, sleep information self-reported by adolescents rather than by parents is more accurate, as parents tend to report more idealized sleeping patterns.60
Implications
The findings indicate a need to acknowledge the importance of sleep as a lifestyle-related factor that should be more prominent in health promotion strategies for early adolescents. Efforts are also needed to advise parents of ways to detect sleep patterns and disturbances among adolescents. Future work should explore whether interventions for adolescent sleep patterns improve well-being and HRQoL. Although physical activity level and sedentary time were not found to be independent predictors of HRQoL in this study, the low level of physical activity observed may have been a confounding factor. Research is therefore needed to examine if similar results are obtained from a more active population. Future studies should include objective tools (e.g. accelerometer and/or polysomnograph) to obtain a more detailed understanding of sleep patterns and disturbances and their association with HRQoL in early adolescents. Future intervention studies should also take into account the complex way in which adherence to dietary recommendations predicts HRQoL both positively and negatively in this age group.
To conclude, this sample of early adolescents showed a low adherence to physical activity and dietary recommendations, but resilience scores similar to those found in previous studies. HRQoL differed from the norm data on three out of five domains, with no sex difference. Resilience and sleep were the most prominent predictors of HRQoL, whereas adherence to dietary recommendations was both a positive and negative predictor for the different HRQoL domains.
Acknowledgements
We are grateful to Dr. Shea Allison Sundstøl, University of South-Eastern Norway, for providing valuable comments on the manuscript and correcting the English expression.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This study was partly funded by Sparebankstiftelsen Sparebanken Sør. The funders had no role in the design of the study, collection, analysis, and interpretation of data, or in writing the manuscript.
ORCID iD
Solfrid Bratland-Sanda https://orcid.org/0000-0002-4202-5439
References
- 1.Patton GC, Olsson CA, Skirbekk Vet al. Adolescence and the next generation. Nature 2018; 554: 458 DOI: 10.1038/nature25759 https://www.nature.com/articles/nature25759#supplementary-information. [DOI] [PubMed] [Google Scholar]
- 2.Frisen A. Measuring health-related quality of life in adolescence. Acta Paediatr 2007; 96: 963–968. DOI: 10.1111/j.1651-2227.2007.00333.x. [DOI] [PubMed] [Google Scholar]
- 3.Cooper AR, Goodman A, Page ASet al. Objectively measured physical activity and sedentary time in youth: the International children’s accelerometry database (ICAD). Int J Behav Nutr Phys Act 2015; 12: 113. DOI: 10.1186/s12966-015-0274-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Telama R, Yang X, Viikari Jet al. Physical activity from childhood to adulthood: a 21-year tracking study. Am J Prev Med 2005; 28: 267–273. DOI: 10.1016/j.amepre.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Cleland V, Dwyer T, Venn A. Which domains of childhood physical activity predict physical activity in adulthood? A 20-year prospective tracking study. Br J Sports Med 2012; 46: 595–602. DOI: 10.1136/bjsports-2011-090508. [DOI] [PubMed] [Google Scholar]
- 6.Dalene KE, Anderssen SA, Andersen LBet al. Secular and longitudinal physical activity changes in population-based samples of children and adolescents. Scand J Med Sci Sports 2018; 28: 161–171. DOI: 10.1111/sms.12876. [DOI] [PubMed] [Google Scholar]
- 7.Lien N, Lytle LA, Klepp KI. Stability in consumption of fruit, vegetables, and sugary foods in a cohort from age 14 to age 21. Prev Med 2001; 33: 217–226. DOI: 10.1006/pmed.2001.0874. [DOI] [PubMed] [Google Scholar]
- 8.Fismen AS, Smith OR, Torsheim Tet al. A school based study of time trends in food habits and their relation to socio-economic status among Norwegian adolescents, 2001-2009. Int J Behav Nutr Phys Act 2014; 11: 115. DOI: 10.1186/s12966-014-0115-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Samuelson G. Dietary habits and nutritional status in adolescents over Europe. An overview of current studies in the Nordic countries. Eur J Clin Nutr 2000; 54: S21–S28. [DOI] [PubMed] [Google Scholar]
- 10.Handeland K, Kjellevold M, Wik Markhus Met al. A diet score assessing Norwegian adolescents’ adherence to dietary recommendations-development and test-retest reproducibility of the score. Nutrients 2016; 8: pii: E467. DOI: 10.3390/nu8080467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hansen LB, Myshre JB, Johansen AMWet al. UNGKOST 3, Nationwide dietary survey among pupils in 4th and 8th grade in Norway, 2015. Oslo: Norwegian Directorate of Health, 2016. [Google Scholar]
- 12.Chattu VK, Manzar MD, Kumary Set al. The global problem of insufficient sleep and its serious public health implications. Healthcare (Basel) 2018; 7: pii: E1. DOI: 10.3390/healthcare7010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matricciani L, Bin YS, Lallukka Tet al. Past, present, and future: trends in sleep duration and implications for public health. Sleep Health 2017; 3: 317–323. DOI: 10.1016/j.sleh.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 14.Roeser K, Eichholz R, Schwerdtle Bet al. Relationship of sleep quality and health-related quality of life in adolescents according to self- and proxy ratings: a questionnaire survey. Front Psychiatry 2012; 3: 76–76. DOI: 10.3389/fpsyt.2012.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dahl RE, Lewin DS. Pathways to adolescent health sleep regulation and behavior. J Adolesc Health 2002; 31: 175–184. [DOI] [PubMed] [Google Scholar]
- 16.Hysing M, Pallesen S, Stormark KMet al. Sleep and use of electronic devices in adolescence: results from a large population-based study. BMJ Open 2015; 5: e006748. DOI: 10.1136/bmjopen-2014-006748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tremblay MS, Carson V, Chaput JPet al. Canadian 24-Hour Movement Guidelines for Children and Youth: An Integration of Physical Activity, Sedentary Behaviour, and Sleep. Appl Physiol Nutr Metab 2016; 41: S311–S327. DOI: 10.1139/apnm-2016-0151. [DOI] [PubMed] [Google Scholar]
- 18.Hardy LL, Mihrshahi S, Bellew Wet al. Children’s adherence to health behavior recommendations associated with reducing risk of non-communicable disease. Prev Med Rep 2017; 8: 279–285. DOI: 10.1016/j.pmedr.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Group TWS. Study protocol for the World Health Organization project to develop a Quality of Life assessment instrument (WHOQOL). Qual Life Res 1993; 2: 153–159. [PubMed] [Google Scholar]
- 20.Meade T, Dowswell E. Adolescents’ health-related quality of life (HRQoL) changes over time: a three year longitudinal study. Health Qual Life Outcomes 2016; 14: 14. DOI: 10.1186/s12955-016-0415-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gillison F, Skevington S, Standage M. Exploring response shift in the quality of life of healthy adolescents over 1 year. Qual Life Res 2008; 17: 997–1008. DOI: 10.1007/s11136-008-9373-y. [DOI] [PubMed] [Google Scholar]
- 22.Wu XY, Han LH, Zhang JHet al. The influence of physical activity, sedentary behavior on health-related quality of life among the general population of children and adolescents: a systematic review. PLoS One 2017; 12: e0187668. DOI: 10.1371/journal.pone.0187668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bolton KA, Jacka F, Allender Set al. The association between self-reported diet quality and health-related quality of life in rural and urban Australian adolescents. Aust J Rural Health 2016; 24: 317–325. DOI: 10.1111/ajr.12275. [DOI] [PubMed] [Google Scholar]
- 24.Evaristo S, Moreira C, Lopes Let al. Muscular fitness and cardiorespiratory fitness are associated with health-related quality of life: results from labmed physical activity study. J Exerc Sci Fit 2019; 17: 55–61. DOI: 10.1016/j.jesf.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muros JJ, Salvador Perez F, Zurita Ortega Fet al. The association between healthy lifestyle behaviors and health-related quality of life among adolescents. J Pediatr (Rio J) 2017; 93: 406–412. DOI: 10.1016/j.jped.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 26.Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep 1985; 100: 126–131. [PMC free article] [PubMed] [Google Scholar]
- 27.Gu X, Chang M, Solmon MA. Physical activity, physical fitness, and health-related quality of life in school-aged children. J Teach Phys Educ 2016; 35: 117–126. [Google Scholar]
- 28.Hjemdal O, Friborg O, Stiles TCet al. A new scale for adolescent resilience: grasping the central protective resources behind healthy development. Meas Eval Couns Dev 2006; 39: 84–96. [Google Scholar]
- 29.Simon-Saiz MJ, Fuentes-Chacon RM, Garrido-Abejar Met al. Influence of resilience on health-related quality of life in adolescents. Enferm Clin 2018; 28: 283–291. DOI: 10.1016/j.enfcli.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 30.Skrove M, Romundstad P, Indredavik MS. Resilience, lifestyle and symptoms of anxiety and depression in adolescence: the Young-HUNT study. Soc Psychiatry Psychiatr Epidemiol 2013; 48: 407–416. DOI: 10.1007/s00127-012-0561-2. [DOI] [PubMed] [Google Scholar]
- 31.Ho FK, Louie LH, Chow CBet al. Physical activity improves mental health through resilience in Hong Kong Chinese adolescents. BMC Pediatr 2015; 15: 48. DOI: 10.1186/s12887-015-0365-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adler NE, Newman K. Socioeconomic disparities in health: pathways and policies. Health Aff (Millwood) 2002; 21: 60–76. DOI: 10.1377/hlthaff.21.2.60. [DOI] [PubMed] [Google Scholar]
- 33.Kolle E, Steene-Johannessen J, Andersen LBet al. Objectively assessed physical activity and aerobic fitness in a population-based sample of Norwegian 9- and 15-year-olds. Scand J Med Sci Sports 2010; 20: e41–e47. DOI: 10.1111/j.1600-0838.2009.00892.x. [DOI] [PubMed] [Google Scholar]
- 34.Migueles JH, Cadenas-Sanchez C, Ekelund Uet al. Accelerometer data collection and processing criteria to assess physical activity and other outcomes: a systematic review and practical considerations. Sports Med 2017; 47: 1821–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Evenson KR, Catellier DJ, Gill Ket al. Calibration of two objective measures of physical activity for children. J Sports Sci 2008; 26: 1557–1565. [DOI] [PubMed] [Google Scholar]
- 36.Kim Y, Beets MW, Welk GJ. Everything you wanted to know about selecting the “right” Actigraph accelerometer cut-points for youth, but …: a systematic review. J Sci Med Sport 2012; 15: 311–321. [DOI] [PubMed] [Google Scholar]
- 37.Aadland E, Terum T, Mamen Aet al. The Andersen aerobic fitness test: reliability and validity in 10-year-old children. PLoS One 2014; 9: e110492. DOI: 10.1371/journal.pone.0110492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Andersen LB, Andersen TE, Andersen Eet al. An intermittent running test to estimate maximal oxygen uptake: the Andersen test. J Sports Med Phys Fitness 2008; 48: 434–437. [PubMed] [Google Scholar]
- 39.Cole TJ, Lobstein T. Extended international (IOTF) body mass index cut-offs for thinness, overweight and obesity. Pediatr Obes 2012; 7: 284–294. DOI: 10.1111/j.2047-6310.2012.00064.x. [DOI] [PubMed] [Google Scholar]
- 40.Andersen LF, Nes M, Lillegaard ITet al. Evaluation of a quantitative food frequency questionnaire used in a group of Norwegian adolescents. Eur J Clin Nutr 1995; 49: 543–554. [PubMed] [Google Scholar]
- 41.Norwegian Directorate of Health (Helsedirektoratet). Anbefalinger om kosthold, ernæring og fysisk aktivitet. Oslo: Helsedirektoratet, 2014. [Google Scholar]
- 42.Lioret S, McNaughton SA, Cameron AJet al. Three-year change in diet quality and associated changes in BMI among schoolchildren living in socio-economically disadvantaged neighbourhoods. Br J Nutr 2014; 112: 260–268. DOI: 10.1017/s0007114514000749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frøyland LR. Ungdata–Lokale ungdomsundersøkelser. Dokumentasjon av variablene i spørreskjemaet Hentet fra: http://www ungdata no/Forskning/Metode-ogdokumentasjon/Ungdata-dokumentasjonsrapport-2010–2019, 2017.
- 44.Owens JA, Dalzell V. Use of the ‘BEARS’ sleep screening tool in a pediatric residents’ continuity clinic: a pilot study. Sleep Med 2005; 6: 63–69. DOI: 10.1016/j.sleep.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 45.Ji X, Liu J. Subjective sleep measures for adolescents: a systematic review. Child Care Health Dev 2016; 42: 825–839. DOI: 10.1111/cch.12376. [DOI] [PubMed] [Google Scholar]
- 46.Soest T, Mossige S, Stefansen Ket al. A validation study of the Resilience Scale for Adolescents (READ). J Psychopathol Behav Assess 2009; 32: 215–225. DOI: 10.1007/s10862-009-9149-x. [Google Scholar]
- 47.Ravens-Sieberer U, Gosch A, Erhart Met al. The KIDSCREEN questionnaires: quality of life questionnaires for children and adolescents. Handbook. Lengerich, Germany: Pabst Science Publ, 2006. [Google Scholar]
- 48.Ravens-Sieberer U, Herdman M, Devine Jet al. The European KIDSCREEN approach to measure quality of life and well-being in children: development, current application, and future advances. Qual Life Res 2014; 23: 791–803. DOI: 10.1007/s11136-013-0428-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ravens-Sieberer U, Auquier P, Erhart Met al. The KIDSCREEN-27 quality of life measure for children and adolescents: psychometric results from a cross-cultural survey in 13 European countries. Qual Life Res 2007; 16: 1347–1356. DOI: 10.1007/s11136-007-9240-2. [DOI] [PubMed] [Google Scholar]
- 50.Corder K, Sharp SJ, Atkin AJet al. Age-related patterns of vigorous-intensity physical activity in youth: The International Children’s Accelerometry Database. Prev Med Rep 2016; 4: 17–22. DOI: 10.1016/j.pmedr.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gebremariam MK, Frost Andersen L, Bjelland Met al. Are weight-related attitudes and behaviours associated with the accuracy of BMI derived from self-reported weight and height among 13-year-olds? Scand J Public Health 2015; 43: 130–137. DOI: 10.1177/1403494814563370. [DOI] [PubMed] [Google Scholar]
- 52.Lang C, Kalak N, Brand Set al. The relationship between physical activity and sleep from mid adolescence to early adulthood. A systematic review of methodological approaches and meta-analysis. Sleep Med Rev 2016; 28: 32–45. DOI: 10.1016/j.smrv.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 53.Health NIoP. Public Health Profile for Telemark County 2019, https://khp.fhi.no/PDFVindu.aspx?Nr=08&sp=1&PDFAar=2019 (2019, accessed 03.07.2019 2019).
- 54.Andersen JR, Natvig GK, Haraldstad Ket al. Psychometric properties of the Norwegian version of the Kidscreen-27 questionnaire. Health Qual Life Outcomes 2016; 14: 58. DOI: 10.1186/s12955-016-0460-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brand S, Kirov R. Sleep and its importance in adolescence and in common adolescent somatic and psychiatric conditions. Int J Gen Med 2011; 4: 425–442. DOI: 10.2147/IJGM.S11557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ding D, Rogers K, Macniven Ret al. Revisiting lifestyle risk index assessment in a large Australian sample: should sedentary behavior and sleep be included as additional risk factors? Prev Med 2014; 60: 102–106. DOI: 10.1016/j.ypmed.2013.12.021. [DOI] [PubMed] [Google Scholar]
- 57.Chennaoui M, Arnal PJ, Sauvet Fet al. Sleep and exercise: a reciprocal issue? Sleep Med Rev 2015; 20: 59–72. DOI: 10.1016/j.smrv.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 58.Silk JS, Vanderbilt-Adriance E, Shaw DSet al. Resilience among children and adolescents at risk for depression: mediation and moderation across social and neurobiological contexts. Dev Psychopathol 2007; 19: 841–865. DOI: 10.1017/s0954579407000417. [DOI] [PubMed] [Google Scholar]
- 59.Draper CE, Grobler L, Micklesfield LKet al. Impact of social norms and social support on diet, physical activity and sedentary behaviour of adolescents: a scoping review. Child Care Health Dev 2015; 41: 654–667. DOI: 10.1111/cch.12241. [DOI] [PubMed] [Google Scholar]
- 60.Short MA, Gradisar M, Lack LCet al. Estimating adolescent sleep patterns: parent reports versus adolescent self-report surveys, sleep diaries, and actigraphy. Nat Sci Sleep 2013; 5: 23–26. DOI: 10.2147/nss.s38369. [DOI] [PMC free article] [PubMed] [Google Scholar]