Graphical abstract
Keywords: Norovirus, Antiviral drugs, Small animal models, Cell culture models, Antiviral targets
Abstract
The development of antiviral strategies to treat or prevent norovirus infections is a pressing matter. Noroviruses are the number 1 cause of acute gastroenteritis, of foodborne illness, of sporadic gastroenteritis in all age groups and of severe acute gastroenteritis in children less than 5 years old seeking medical assistance [USA/CDC]. In developing countries, noroviruses are linked to significant mortality (∼200 000 children <5 years old). Noroviruses are a major culprit for the closure of hospital wards, and associated with increased hospitalization and mortality among the elderly. Transplant patients have significant risk of acquiring persistent norovirus gastroenteritis. Control and prevention strategies are limited to the use of disinfectants and hand sanitizers, whose efficacy is frequently insufficient. Hence, there is an ample need for antiviral treatment and prophylaxis of norovirus infections.
The fact that only a handful of inhibitors of norovirus replication have been reported can largely be attributable to the hampering inability to cultivate human noroviruses in cell culture. The Norwalk replicon-bearing cells and the murine norovirus-infected cell lines are the available models to assess in vitro antiviral activity of compounds. Human noroviruses have been shown to replicate (to some extent) in mice, calves, gnotobiotic pigs, and chimpanzees. Infection of interferon-deficient mice with the murine norovirus results in virus-induced diarrhea. Here we review recent developments in understanding which norovirus proteins or host cell factors may serve as targets for inhibition of viral replication. Given the recent advances, significant progress in the search for antiviral strategies against norovirus infections is expected in the upcoming years.
1. Norovirus burden of disease, epidemiology and transmission
Noroviruses are the number 1 cause of foodborne illness around the world, producing extensive outbreaks of gastroenteritis that attract media attention and raise concern of healthcare professionals and consumers. About 58% of domestically-acquired foodborne illnesses, 26% of hospitalizations and 11% deaths are attributable to noroviruses [1]. The complete scope of foodborne diseases is hard to tackle, however building capacity to detect, control and prevent them is an urgent matter. Gastroenteritis remains an important health issue that affects all age groups but can be particularly severe for children, the elderly and immunocompromised. In developing countries, still today, one million children die annually before they reach the age of five due to diarrhea [2]. Of those, ∼200 000 deaths are estimated to be caused by noroviruses [3]. The introduction of vaccines against rotavirus had in recent years a major impact on reducing the total number of deaths due to diarrhea; however the set Millennium Development Goal 4 is still far from being attained [2]. Noroviruses are currently the most important pathogen causing severe childhood diarrhea in countries where vaccination against rotavirus is routinely implemented [4]. It is the reason for 1 million pediatric medical care visits annually in the United States [4]. Conversely, the elderly account for the majority of gastroenteritis-associated hospitalizations and deaths [5]. Indeed, norovirus gastroenteritis is linked with increased risk of hospitalization and mortality in the elderly population (in spite of remaining underestimated) and thus should not be regarded as a trivial illness of short duration [5], [6]. Long-term care facilities are the most common setting for norovirus outbreaks, followed by restaurants, schools, hospitals and cruise ships [7]. Noroviruses are a major culprit for the closure of hospital wards [8]. Nearly 4000 hospital outbreaks were reported in the UK in a 2-year period, affecting ∼13 000 patients and ∼3400 staff members and resulting in almost 9000 days of ward closure [9].
Symptomatic norovirus infection is generally acute and self-limited, with 24–48 h of incubation after which there is an acute onset of vomiting, nausea, abdominal cramps, myalgias and intense watery non-bloody diarrhea, that commonly resolves in 2–3 days [10]. However, prolonged and severe disease – including dehydrating diarrhea – occurs in vulnerable populations (children, elderly and immunocompromised). Post-infectious functional gastrointestinal disorders such as irritable bowel syndrome have been reported after norovirus gastroenteritis [11]. Other reported sequelae include necrotizing enterocolitis, convulsions and encephalopathy [12], [13], [14]. Detection of norovirus in the serum of patients has been scarcely reported, but the significance of such findings is not yet well understood. In immunocompromised individuals norovirus gastroenteritis can become chronic and persist for weeks to years, having an estimated prevalence of 17–18% [15]. Indeed, an increasing number of studies show that immunosuppressive therapy is a risk factor for norovirus infection [15]. Such patients often present dramatic weight loss due to prolonged norovirus-related diarrhea which together with malnutrition, dehydration, and altered intestinal mucosal barrier may contribute to increased morbidity and aggravate the outcome of underlying disease [16]. Transplant recipients with such chronic norovirus infections may require adjustment (reduction) of immunosuppressive therapy [15].
The transmission of norovirus occurs via the fecal-oral route through the consumption of contaminated food or water (at the source or by food handlers), through fomites, recreational waters or via aerosolized particles from vomitus or stool [10]. The main route of transmission in over 70% of outbreaks is person-to-person [7] thus favoring the occurrence of large outbreaks in semi-closed environments. The extensiveness of such outbreaks is explained by the low infectious dose of norovirus (18 virus particles are sufficient for infection), with shedding in stool for 20 to 40 days (also present in asymptomatic individuals) and by its long persistence in the environment (resistance to disinfectants, heating, freezing), up to two weeks on surfaces [17], [18], [19]. In addition, norovirus infections can occur repeatedly through life due to lack of complete cross-protection against the diverse norovirus strains and the lack of long-lasting immunity [10].
Currently treatment for norovirus infections is merely supportive in nature, with electrolyte replenishment for dehydrated individuals. Outbreak control and prevention strategies are limited to the use of disinfectants and hand sanitizers, whose efficacy against this enteric pathogen may be limited or insufficient. Hence, there is ample need for specific antiviral treatment and prophylaxis of norovirus infections.
2. Genetic diversity and genome organization
Noroviruses belong to the family Caliciviridae that is comprised of five genera: Norovirus, Sapovirus, Lagovirus, Vesivirus, and Nebovirus. Other tentative genera have been proposed following the characterization of the simian Tulane virus, the porcine St. Valérian virus and the avian Bayern virus. The two genera Norovirus and Sapovirus comprise human viruses that cause gastroenteritis, while the other genera represent animal viruses [10]. Noroviruses are divided into six genogroups based on the amino acid sequence diversity of the major structural protein VP1, of which genogroup I (GI) is further divided into 9 genotypes and GII into 22 genotypes. The (human) Norwalk virus is the prototype of the genus and is designated GI.1; while human pathogens are found in GI, GII and (rarely) GIV, GIII comprises bovine and ovine strains and GV murine noroviruses. In addition, swine strains are classified into GII and canine strains into GIV and GVI [20]. Due to the close genetic relatedness of humans and animal noroviruses, plus the detection of antibodies to animal strains in humans and to human strains in pigs, the possibility of a zoonotic transmission has been raised [21]. In humans, GII.4 noroviruses cause the vast majority of outbreaks; novel GII.4 variants have emerged and become globally predominant multiple times in the past decades [18], [22]. This cyclic emergence of novel strains and displacement of the previously predominant ones, causing worldwide epidemics suggests a pattern of epochal evolution resembling that of influenza [23].
Noroviruses are small non-enveloped viruses of 27–32 nm with a linear positive-sense single-stranded (ss) RNA genome of 7.4–7.7 kb, organized into three open reading frames (ORF1-3). While ORF1, in the 5′ end, encodes a polyprotein of six/seven nonstructural protein products, ORF2 and 3 encode the major and minor structural capsid proteins VP1 and VP2, respectively. Murine norovirus (MNV) genomes comprise an additional ORF4, which encodes virulence factor 1 (VF1) [24], [25]. The viral protein VPg (virion protein, genome-linked) is covalently linked to the 5′ end of the norovirus genome, which is polyadenlyated at the 3′ end [10]. The ORF1 of norovirus encodes the six/seven nonstructural proteins in the following order: the p48/N-terminal protein (or NS1-2), the NTPase (NS3), the p22 (NS4), the VPg (NS5), the viral protease (Pro, NS6), and the viral RNA-dependent RNA polymerase (RdRp, NS7). Virions contain 180 copies or 90 dimers of VP1, the major structural protein, that assemble into icosahedral particles. The VP1 protein is divided into a conserved internal shell domain (S) and a more variable protruding domain, the P domain, which forms the arch-like protrusions. Its P2 subdomain is located at the outmost surface of the viral capsid and comprises a hypervariable region, where resides the binding interface for histo-blood group antigen (HBGA) association with norovirus. The VP2 is a small basic structural protein which is present in one or two copies per virion and associates with the VP1 S domain at the interior surface of the capsid, being likely involved in the capsid assembly and genome encapsidation [26]. Norovirus virus-like particles (VLPs) formed by the independent expression and self-assembly of the VP1 are morphologically and antigenically indistinguishable from the native forms of viruses found in human stools, and retain the binding properties of native norovirus virions at least in terms of carbohydrate association.
3. Immune response and vaccine development
Susceptibility to norovirus infection is estimated to be present in over 80% of adults and to be largely dependent of the host genotype, particularly in the presence of specific human HBGA receptors in the gut of susceptible hosts [10], [22]. There is limited knowledge of the immune response against human norovirus, most of which derives from volunteer studies. The inability to measure serum or intestinal secretory neutralizing antibodies in tissue culture has made it hard to study protective immunity. Such studies, performed with multiple norovirus genotypes had variable outcomes, being likely affected by pre-exposure history of volunteers [27]. Short-term immunity was demonstrated and likely virus or serotype specific, while long-term immunity has been harder to put in evidence. The levels of intestinal secretory antibodies appeared to correlate with protection at a higher level than serum antibody titers, particularly in natural infections.
Norovirus VLPs or P particles (24 P domains of VP1) have been proposed as vaccine candidates [28], [29]. Clinical efficacy studies with Norwalk virus VLPs administered intranasally (two doses three weeks apart) to healthy adult volunteers showed homotypic protection [30]. The vaccine reduced the frequency of Norwalk virus gastroenteritis (69% of placebo recipients vs. 37% of vaccine recipients) and specific IgA antibodies were detected in high levels in 70% of vaccine recipients. The optimal antigen dose, route of administration, adjuvants and type of norovirus strains present in a norovirus vaccine are yet to be determined. Other important aspects to address are whether there is immunogenicity and protective efficacy in the elderly population and young children and primarily the duration of protective immunity of such vaccine. This could become a major challenge given the great antigenic and genetic diversity of noroviruses and the practical usefulness of the vaccine requiring a one-year minimum of protective immunity to be feasible. More recently, a GI.1/GII.4 bivalent VLP vaccine (two doses, intramuscular injection) has been carried out (NCT01168401, www.clinicaltrials.gov); however, results have not yet been officially disclosed. In any case, these trials constitute an important step to the feasibility of a norovirus vaccine. Conversely, the need for specific antiviral therapy remains in the case of chronically infected immunocompromised patients, elderly or children with severe on-going infections and prophylactic control of fast-growing outbreaks.
4. In vitro and in vivo model systems
4.1. Surrogate viruses
Animal viruses within the calicivirus family have been used as surrogates for the yet non-cultivable human norovirus. These include the porcine enteric calicivirus (PEC), a Sapovirus, the feline calicivirus (FCV), a Vesivirus and the Tulane Virus, tentatively a Recovirus (Table 1 ). While these viruses have been used in persistence and inactivation studies, the differences in terms of genome organization, route of infection and pathogenesis make them far from ideal for human norovirus antiviral drug discovery. The more closely related murine norovirus, a GV norovirus has been more widely used and is to date the only Norovirus known to replicate in a cell line and small animal model (Table 1) [31], [32]. This virus has allowed the study of multiple aspects of norovirus biology and is presently considered a central tool for antiviral studies of human norovirus [33]. The genome of MNV has the three ORFs characteristic of noroviruses; the ORF1 encodes the nonstructural proteins and ORFs 2 and 3 encode the structural proteins VP1 and VP2. It has however the additional ORF4 that overlaps with ORF2 in a different reading frame and encodes VF1, a protein involved in the host innate immune response [24], [25]. The similarities in genome organization, protein function and structure allow to assume that the fundamental aspects of MNV and human norovirus replication are highly conserved [33]. Furthermore, MNV replicates in mice, spreads through the fecal-oral route and is shed at high levels in the feces. It is thus an enteric virus, capable of inducing gastroenteritis, with similarities in pathogenesis to human norovirus [31], [32].
Table 1.
Human norovirus and its surrogates.
| Virus | Classification | Genome organization | Natural host | In vitro replication | Reverse genetic system | Causes diarrhea in natural host | Fecal-oral route/shedding in feces | Small animal model |
|---|---|---|---|---|---|---|---|---|
| Human norovirus | GI, GII, GIV Norovirus | 3 ORF | Humans | No. Norwalk replicon-bearing cell line | No | Yes | Yes | Yes. Rag-γc-deficient mice |
| Murine norovirus | GV Norovirus | 3 ORF + ORF4 (VF1) | Mice | Yes. Murine macrophages (RAW264.7) and dendritic cells; murine microglial BV-2 cell line | Yes | Yes | Yes | Yes. Wild-type, RAG/STAT1−/−; IFN receptor deficient− |
| Feline calicivirus | Vesivirus | 3 ORF | Cats | Yes. Feline kidney cell line (CFRK) | Yes | No | No | No |
| Porcine enteric calicivirus | Sapovirus | 2 ORF | Swine | Yes. Porcine kidney cell line (LLC-PK) | Yes | Yes | Yes | No |
| Tulane virus | Recovirus | 3 ORF | Macaques | Yes. Monkey kidney cell line (LLC-MK2) | Yes | Yes | Yes | No |
4.2. In vitro cell culture models for norovirus
Murine norovirus has been shown to replicate in mice in Kupffer cells in the liver and in splenic cells with macrophage-like morphology [32], [34]. Based on these observations research has been carried out to study the replication of norovirus in cell cultures from monocyte/macrophage origin. In vitro cell culture models for murine norovirus reveal that this virus replicates in murine bone marrow derived macrophages and dendritic cells and also in different macrophage cell lines (ex. RAW 264.7) [32]. These in vitro systems have been extremely important to study the murine norovirus life cycle.
Despite extensive efforts, no in vitro cell culture models are available for human norovirus. Duizer et al. explored ∼25 human and animal cell lines (mainly derived from the gastrointestinal system) but none of these supported significant replication of human norovirus [35]. Others investigated the propagation of human norovirus in three-dimensional cultures in intestinal epithelial cells. Also these efforts remained unsuccessful or did not yield reproducible results [36], [37]. Other laboratories have focused on human norovirus replication in cell cultures of hematopoietic origin. One group investigated whether macrophages and dendritic cells, differentiated in vitro from human peripheral blood mononuclear cells, can sustain human norovirus replication [38]. While this study revealed that a low number of dendritic cells can be infected with Norwalk virus in vitro (2–3 cells per 104 inoculated cells) it was insufficient to cause an increase in viral RNA. It should be mentioned that in this study only Norwalk virus and no other human norovirus strains were employed. Similar efforts to cultivate human norovirus in human and murine cell lines of hematopoietic origin remained also unsuccessful [39]. However, in the same study, infection and productive replication of human norovirus in an ex vivo culture using adult human duodenal tissues was observed and some limited replication was noted in a glandular epithelial cell line HIEC-6.
The lack of a cell culture model of human norovirus is perhaps the most important challenge for the discovery of novel antiviral strategies. Fortunately, an alternative approach to at least study norovirus RNA replication is available. A human norovirus replicon-bearing cell line was created by transfecting a plasmid containing nearly the complete Norwalk virus genome into mammalian cell lines [40]. While this system does not allow to study the entry or maturation of human norovirus, it can be used to investigate the activity and inhibition of all the replicative enzymes and nonstructural proteins. In this way it can be employed to find inhibitors of these viral proteins in a cellular context. In addition, the system is also able to study the activity and inhibition of some host factors involved in human norovirus replication.
4.3. Animal models
Small animal models for human norovirus infection are key tools not only to study viral pathogenesis but also to enable proof of concept studies of small molecule inhibitors of norovirus replication (Table 2 ). It was recently reported that Rag−/− γ−/− BALB/c mice support limited replication of human norovirus [41]. However, infection via the oral route was not sufficient to cause infection, mice had to be injected via the intraperitoneal (ip) route. Infected mice did not develop clinical symptoms and the virus was cleared by day 3 post infection (pi).
Table 2.
Norovirus in vivo infection models.
| Virus/strain | Host | Immune status of host | Route of infection | Duration of infection | Symptoms of infection | RNA detected in tissue | Viral antigens in organs | Histological changes in intestine | Shedding in stool | Antibodies in serum | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Human norovirus/GI, GII pool | Mouse (BALB/c) | Rag-γc-deficient | Intraperitoneal (ip); ip + oral | <3 Days | None | Intestinal tract, liver, spleen, heart, lung, mesenteric lymph nodes, kidney | Small intestine, spleen, liver (Kupffer cells) | Non-significant | Ip: no; Ip + oral: yes | Not reported | [41] |
| Human norovirus/Norwalk virus | Chimpanzee | Wild-type | Intravenous (iv) | 2–6 Weeks | None | Intestine, liver | Small intestine | Yes (2–6 weeks); max 106-7 RNA copies/g stool | Yes | [42] | |
| Human norovirus/GII.4 stool filtrate | Pig | Gnotobiotic | Oral | <1 Week | Diarrhea | Blood | Small intestine | Mild lesions | Yes (2–3 days) | Yes | [43] |
| Human norovirus/GII.4 stool filtrate | Calves | Gnotobiotic | Oral | <1 Week | Diarrhea | Small intestine | Mild lesions | Yes (3 days) | Yes | [44] | |
| Murine norovirus/MNV-1.CW1, MNV-1.CW3, MNV-3, MNV.CR6 | Mouse (129SvEv, CD1, C57BL/6) | Wild-type | Oral | CW1—<2 days CW3—< 1 week MNV-3—weeks CR6—weeks |
CW3—fecal inconsistency Otherwise non significant |
CW3, MNV-3, CR6—small intestine, spleen, liver, lung, and mesenteric lymph nodes (MLN); [CW1—only small intestine and spleen at 24 h pi] | CW3—small intestines at 24 h pi [CW1—not reported] | Mild inflammation [CW1–not reported] | Yes CW3—<1 week; MNV-3, CR6—weeks; [CW1—not reported] | Yes | [24], [47], [48], [51], [52], [96] |
| Murine norovirus/MNV-1.CW3, MNV-3 | Mouse (129SvEv, CD1, C57BL/6 | RAG/STAT1−/−; IFN receptor deficient− | Oral | Acute, ∼100% mortality 4–9 days after infection | CW3—diarrhea, gastric bloating, weight loss, mortality, MNV-3—weight loss, fecal inconsistency | Liver, spleen, intestine, MLN, lungs | Not reported | CW3—severe, acute necrosis, MNV-3—mild inflammation | Yes | Yes | [31], [48], [49], [51], [52], [96] |
| Murine norovirus/MNV.CR6 | Mouse (129SvEv, C57BL/6 | STAT1−/− | Oral | Persistent, weeks to months | CR6—no symptoms | Spleen; intestine, MLNs | Not reported | CR6—not reported? | Yes (>7 weeks) | Yes | [24], [52] |
| Porcine sapovirus/Cowden | Pig | Gnotobiotic | Oral/iv | < 1 Week | Diarrhea | Blood | Small intestine | Mild lesions | Yes (7 days) | Yes | [45] |
| Tulane virus | Rhesus macaque | Wild-type | Oral | <10 Days | diarrhea, fever | Not reported | Small intestine | Mild lesions | Yes (8–10 days) max.105 TV-RNA copies per gram of stools | Yes | [46], [97] |
A number of large animal models have been shown to host human norovirus replication, namely chimpanzees, gnotobiotic pigs and calves (Table 2). While no diarrhea was observed in chimpanzees, intravenous (iv) infection induced week-lasting shedding in stool and serum antibody response. Virus was detected in the intestinal and liver biopsies [42]. In gnotobiotic pigs and calves, however, diarrhea and mild lesions in the intestine were observed as a result of an oral infection with a stool filtrate containing GII.4 norovirus [43], [44]. Such large animal models can help shedding light into the (inhibition of) replication of this human pathogen, however large-scale studies will hardly be feasible.
Additional animal models using surrogate viruses are also available. Large-animal models for the porcine sapovirus (PEC/Cowden strain) replicating in swine, or the Tulane virus replicating in rhesus macaques, also provide an opportunity to study clinical features and pathogenesis of caliciviruses [45], [46]. All of the 30 known strains of murine norovirus replicate in mice. The majority of these cause a persistent asymptomatic infection in immunocompetent mice (Table 2) [24]. However, in mice lacking components of innate immunity such as signal transducer and activator of transcription 1 (STAT1) or alpha/beta (IFN-α/β) and gamma (IFN-γ) interferon receptors, MNV can cause either a persistent or an acute and lethal infection. When infected with the MNV-1 strain innate immunity deficient mice develop severe diarrhea and weight loss, with ∼100% mortality four to nine days after infection [31], [32]. Viral RNA is detected in these mice in high levels in the intestine, lung, liver, spleen, brain, blood, and feces. The clinical symptoms observed are severe pneumonia and destruction of splenic and liver tissues that cause fatal disease in most cases [31]. This mortality model displays features similar to the pathogenesis of human norovirus infection in humans namely, infection via the oral route, diarrhea, gastric bloating, shedding in stool in high levels [31], [47]. Two MNV-1 variants were selected from the initially isolated virus by plaque purification and passaging in cell culture, MNV-1.CW1 (P3) and MNV-1.CW3 (P3), which genetically differ in four amino acids [32], [47]. Only MNV-1.CW3 is equally virulent as the parental strain in immunocompromised mice. MNV-1.CW1 is attenuated and detected in vivo solely at 24 h pi [47]. In immunocompetent mice, however, MNV-1.CW3 infection causes modest intestinal pathology and fecal inconsistency, which is cleared in less than one week [48].
More recently, other MNV strains such as the MNV-3 and MNV.CR6 have been identified, and amino acid changes in the genome of such strains have been linked to persistence and tropism [49], [50]. The MNV-3 causes mild symptoms in STAT1-deficient mice, in comparison with the CW3 strain, with less weight loss and more moderate gastric bloating and fecal inconsistency, being cleared after one week. In wild-type mice MNV-3 infection is asymptomatic and can persist for weeks to months [49], [51]. Conversely, the CR6 strain persists asymptomatically in both wild-type and innate immune-deficient mice, presenting thus a different phenotype [52]. These (and potentially other) persistent MNV strains may be used as a model to study norovirus persistence. This might also become useful for antiviral studies given the increased recognition of prolonged norovirus infections in immunocompromised patients.
5. Targets for antiviral drug discovery
5.1. Viral entry
Norovirus virions recognize HBGAs which are key players in the initial viral attachment, acting most likely as cellular receptors or co-receptors of norovirus for cell entry (Table 3 ) [53]. The HBGA-binding interfaces are located in the P domain of the viral capsid protein. Here, a conformational pocket on the distal surface of the viral capsid interacts with individual oligosaccharide residues of the HBGA molecules. Structural studies that demonstrated this for human norovirus have been mainly performed using crystallization and X-ray diffraction [54] but recently an STD-NMR was successful used yielding similar results [55]. Insight in the molecular interaction of HBGA with the human norovirus capsid has been used as a starting point for computer-assisted drug discovery. This approach, in which ∼2 million chemical structures were screened in silico for their potential to compete with HBGA binding, resulted in the identification of several compounds that could block the HBGA-P domain interaction in vitro (Fig. 1 ) [56]. None of these molecules have yet been tested in cell culture or in in vivo models.
Table 3.
Molecular targets for inhibition of norovirus replication.
| Target | Function | Inhibitors | Tools |
|---|---|---|---|
| HBGA/capsid Interaction | Receptor binding before cell entry | Dimethyl cyclopenta-α-phenanthren analogues |
In silico struct. Biology[56] Crystallography/X-ray[54] Surface plasma resonance technology [56] Mass spectrometry [57] STD NMR [55] |
| Virus uptake (endocytosis) and uncoating | Cell entry and release of viral RNA | None | Pharmacological inhibitors, neutral red infectious center assay, dominant-negative constructs and siRNA [59], [60]. |
| NS1/2 | Intracellular membrane reorganization Determines viral persistence |
None | Expression, sub cellular localization and organelle morphology studies[66] Generation of recombinant MNV[50] |
| NS3 | Putative RNA helicase | None | Picornavirus 2C inhibitors [69], [70] |
| NS4 | Intracellular membrane reorganization, antagonizes secretory pathways | None | Picornavirus PI4KIIIb inhibitors [74] |
| NS5—VPg | Priming for genome polymerization viral protein translation | None | NMR spectroscopy [77] |
| NS6—protease | Maturation of viral proteins | Substrate-based aldehyde inhibitors [82] Michael-acceptor polypeptide inhibitor [81] |
Crystallography/X-ray Biochemical protease assay |
| NS7—RNA dependent RNA polymerase | Replication of virus genome | 2′-C-methylcytidine [98], [99] Favipiravir[100] β-d-N(4)-hydroxycytidine, 2′-F-2′-C-methylcytidine, Ribavirin [99] |
Cell culture models Animal models |
| 5-Nitrocytidine triphosphate [86] 2′-Amino-2′-deoxycytidine-5′-triphosphate (ACT) [87] |
Crystallography/X-Ray of RDRP with primer–template | ||
| Suramin, NF023 and NAF2 and PPNDS [88], [89] |
In silico struct. Biology Crystallography/X-Ray of RDRP in absence of primer–template Biochemical polymerase assay |
Fig. 1.
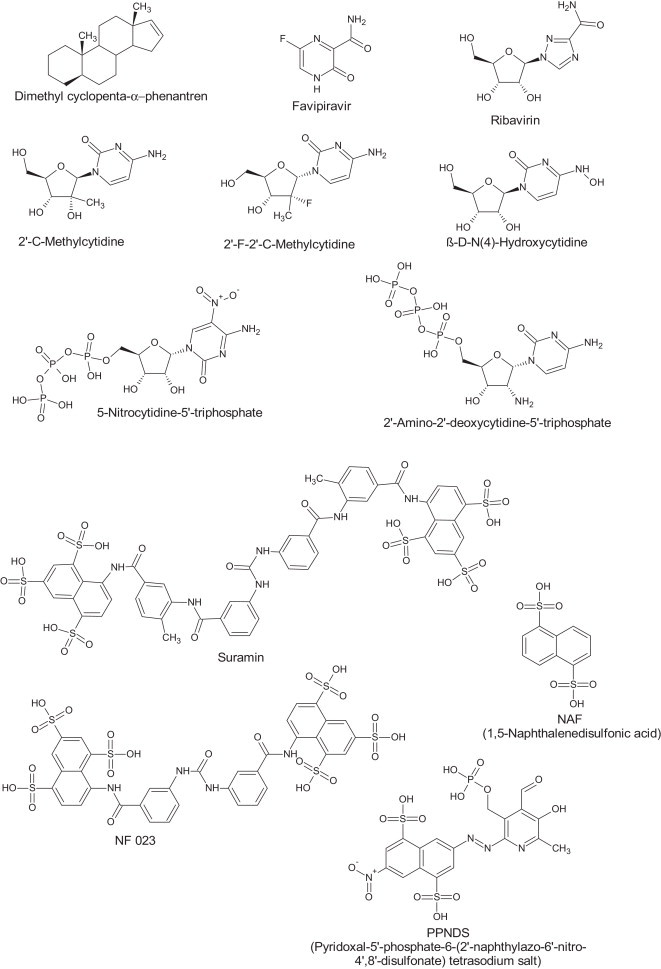
Structural formulae molecules inhibiting norovirus replication.
Recently new methodologies, using surface plasma resonance technology or mass spectrometry, have been developed to determine the binding of HBGAs to the viral capsid protein [57], [58]. These techniques proved also valuable for the characterization of the binding of other oligosaccharides, including human milk glycans, with the norovirus capsid making them potential anti-adhesive agents for norovirus prophylaxis [58].
Following receptor binding viruses take advantage of cellular processes to enter the cell. MNV enters the cell via a clathrin/caveolin-independent mechanism that is mediated by dynamin II and cholesterol (Table 3) [59], [60]. Insights in the mechanism of human norovirus entry are lacking due to the inability to grow the virus in cell culture. The observation that simvastatin (which lowers cholesterol levels) enhance the replication of a human norovirus replicon in vitro and increases the replication of human norovirus in vivo further highlights the not yet fully understood role of cholesterol in human norovirus entry [61], [62].
Uncoating of MNV is a pH-independent process. This is not a surprise as MNV is an enteric virus that must travel through the acidic pH of the stomach to reach its site of replication. In contrast to many other viruses, a low intracellular pH does not trigger the required conformational changes in the capsid [63]. This implicates that chemicals frequently used to prevent acidification of endosomes and as such inhibit virus uncoating will not be active on norovirus.
5.2. Targeting nonstructural proteins of norovirus
The genome of noroviruses encodes six/seven nonstructural proteins: the NS1/2 (N-terminal protein p48), the NS3 (NTPase), the NS4 (p22), the NS5 (VPg), the viral protease (Pro, NS6), and the viral RNA-dependent RNA polymerase (RdRp, NS7) [Table 3]. The nomenclature and functions of these ORF1-encoded proteins were predicted based on the sequence similarities with their picornavirus counterparts. The norovirus RdRp is therefore referred to as 3D-like, the protease as 3C-like, the p22 as 3A-like, the NTPase as 2C-like and the p48/N-term was linked with the 2B protein [10]. These similarities can be an important step-up for the search of antivirals against norovirus as some of the picornavirus counterparts are proven targets for inhibition of viral replication.
For MNV it has been shown that all nonstructural proteins play a role in norovirus replication and that they co-localize with the replication complex and the viral RNA intermediate dsRNA [64]. As for many other RNA viruses the replication complex of MNV is associated with the membranes of the endoplasmic reticulum (ER), the Golgi apparatus and endosomes. These intracellular membranes reorganize upon virus infection [32], [64].
5.3. NS1/2—N-terminal protein
The function of p48 or NS1/2 is not yet well defined. Based on sequence similarity it is thought to be comparable to the picornavirus 2B protein, which participates in intracellular membrane changes that follow infection. In the case of Norwalk virus infection p48 seems to interfere with disassembly of the Golgi complex and cellular protein trafficking [65]. Studies with MNV reveal that p48 is associated with the recruitment of ER membranes to the site of replication [66]. This recruitment of membranes is probably vital for the formation of replication complexes and the synthesis of new viral proteins [32], [64]. More recently the N-terminal domain of the p48 was shown to determine virus colonic tropism and persistence [50]. As the exact mechanism is still unknown, it is speculated that structural differences of a novel protein fold in the N-terminal domain of p48 influences cellular pathways during viral infection, resulting in evasion of immunity and consequently viral persistence [67]. Given its pleiotropic functions, the mechanisms controlled by p48 in the membrane rearrangements could be a good strategy to stop the norovirus life cycle.
5.4. NS3—Helicase/NTPase
The NS3 protein has been classified in the protein superfamily 3 of RNA helicases [68]. It shares sequence motifs with the picornavirus 2C protein and the flavivirus NS3 helicase/NTPase. Helicases are enzymes involved in the unwinding of nucleic acids which is catalyzed by the hydrolysis of NTP. However, for the norovirus NS3 protein (as is the case for the picornavirus 2C protein) only NTPase activity has been demonstrated. The norovirus helicase is, akin to the picornavirus helicase, a ring helicase (unlike for example the flavivirus helicase). Attempts to solve the structure of this protein have so far been unsuccessful.
Several molecules have been shown to directly or indirectly interfere with the picornavirus 2C protein function and as such block viral replication [69], [70]. The mechanism of action of these molecules is still not fully characterized. There has been recent progress towards solving the structure of the 2C protein [71]. If this could be achieved it might give insights in the mechanism of action of these inhibitors and generate opportunities for characterization of the norovirus NS3 protein. Recently, a novel class of picornavirus 2C targeting compounds was discovered [72]. These molecules showed potent and broad-spectrum entero/rhinovirus activity including excellent activity in animal models for enterovirus infection.
5.5. NS4
Functional studies with NS4 from human norovirus have shown that this protein affects the Golgi-phenotype and antagonizes the secretory pathways of mammalian cells [73]. Both activities correlate with the preservation of an endoplasmic reticulum export signal (MERES) motif. The MNV NS4 causes a similar shift in the Golgi-phenotype but has less effect on the secretory pathways.
The picornavirus 3A protein and the norovirus NS4 protein are considered orthologous as both proteins share the same location in the viral genome and both interfere with the host secretory pathway. The picornavirus 3A protein recruits phospatidylinositol-4-kinase IIIb (PI4KIIIb) to secretory organelle membranes. This promotes a phosphatidylinositol-4-phosphate (PI4P) lipid-rich environment which the 3D polymerase can bind leading to the formation of replication complexes [74]. Various molecules have been shown to inhibit PI4KIIIb and thereby also viral replication. Interestingly the effect of the picornavirus 3A protein is also linked with a reduction of the secretion of several cytokines and the down regulation of TNF-receptors and MHC class I molecules on the cell surface [75]. Inhibitors of PI4KIIIb have been tested on norovirus replication but no significant antiviral activity has been found (our unpublished observations).
5.6. NS5—VPg
VPg is a viral protein that is covalently linked to the 5′ end of RNA genome of picorna- and caliciviruses. These viruses have evolved a protein-primed mechanism of genome replication in which VPg is used as a protein primer for the viral RNA polymerase. In addition, VPg is thought to have many other functions that have recently been reviewed [76]. VPg probably protects the 5′ end of the RNA from being detected by the cytoplasmic sensors RIG-I and PKR. The protein also interacts directly with the cap-binding protein eIF4E and other proteins of the translation initiation complex. Current hypothesis hold that VPg and its interacting partners form a hub for the assembly of the translation and replication complex.
Recently, the first high-resolution structural analysis (NMR) of calicivirus and MNV VPg proteins was reported [77]. In contrast with the VPg proteins of picornaviruses that seem to have an intrinsically disordered structure, the VPg protein of MNV contains α-helical cores flanked by long unstructured termini. The essential Tyr residue that is nucleotidylated by the viral polymerase to form primers for RNA synthesis is located on the first α-helix and exposed to the solvent. When the MNV VPg was docked manually into the crystal structure of the MNV NS7 RdRp the Tyr residue could not be placed in sufficient proximity to the active site to allow for a nucleotidylation reaction. However when the first α-helix is detached from the VPg core, or when the thumb and fingers domains of the polymerase are moved apart then a productive complex could be formed. No inhibitors of VPg functions are known, but its multiple protein-protein interactions may provide suitable targets for antivirals. It would be important to solve the structure of the NS7-VPg complex and to explore whether this interaction can be annihilated by specific inhibitors. Recently a picornavirus VPg-uridylylation AlphaScreen has been developed for high-throughput screening [78]. A similar approach can be envisioned to find inhibitors of the norovirus VPg-guanylation reaction.
5.7. NS6—Protease
The atomic structure of the Norwalk protease was first resolved to high resolution in 2006 [79]. It is a cysteine protease that adopts a chymotrypsin-like fold comprised of two domains similar to other viral cysteine proteases, like those of picornavirus 3C proteases [79]. The catalytic triad in the active site consists of a cysteine as the nucleophile, a histidine residue as the general base catalyst and a glutamic acid to stabilize the imidazole ring of the histidine. Recently NMR also has been added as a tool to complement these structural studies of the Norovirus protease [80].
Detailed structural analysis of the norovirus protease with its natural substrate or with inhibitors in its active site have been performed [81], [82]. To prevent cleavage of the natural substrate during crystallization the active site cysteine was mutated to alanine. The structures obtained show that the protease induces an extended β-strand conformation in the substrate. In addition they explain why the cleavage specificity-determining interactions are conserved across viral serine-like proteases. Comparative analysis of the structures of caliciviruses, picornaviruses and coronavirus proteases reveal that the Glu or Gln in the P1 position make identical sets of interactions involving highly conserved His, Thr, and Tyr residues in the S1 pocket. The S2 pocket show great flexibility in association with substrate binding and these changes are correlated with alterations in the S4 pocket. The inhibitors tested were all irreversible inhibitors that covalently link to the active site cysteine residue (Fig. 1). Different reactive groups, such as bisulfite, esters, aldehyde, ketoamides and ketones have been used [81], [82], [83]. Functional assays for the detection of protease activity of calicivirus, using fluorogenic substrate peptides or bioluminescence technologies have been developed [79], [84]. While these show that the biochemical activity on the Norovirus protease inhibitors is limited (K i ∼ 1–0.1 μM) some of these molecules showed broad-spectrum antiviral activity in cell culture (EC50 ∼ 1 μM) on various members of the calici-, picorna- and coronavirus families [83].
5.8. NS7—RNA-dependent RNA polymerase
The calicivirus RdRps have all the sequence and structural motifs found in canonical viral RdRp. Different nucleoside analogues have been co-crystallized in the active site of MNV RdRp (Fig. 1). Surprisingly no primer-template RNA is needed. Also there was no need to use a phosphorylated form of the nucleoside [85]. This explains why the nucleosides tested [5-fluorouracil (5FU), 2-thiouridine (2TU) and ribavirin] bind with some flexibility and/or slightly shifted from the expected conformation. The relevance of these approaches to find inhibitors can be questioned. For example while 2TU and ribavirin show a similar binding mode in these crystal structures they differ significantly in their potency to block MNV replication in vitro [85].
Structures of the human norovirus RdRp bound to an RNA primer–template duplex and a nucleoside-triphosphate analogue have also been obtained and these are likely to be more relevant to understand how the norovirus polymerase can be targeted. In one study the binding mode of the natural substrate CTP and a potent competitive inhibitor 5-nitro-cytidine-5′-triphosphate were determined [86]. The precise positioning of these nucleotides exerts significant effects on the efficiency of the nucleotidyl transfer reaction and suggests that a series of hydrogen-bonding and van der Waals interactions with the ribose moiety may play an important role in nucleotide recognition and positioning within the active site. In a second study the binding of 2′-amino-2′-deoxycytidine-5′-triphosphate was investigated. The binding mode of this nucleotide in the active site forces the enzyme to remain in an open “inactive” conformation, which can be regarded as a novel mechanism of inhibition. These structural changes are attributable to the replacement of the 2′-hydroxyl group with an amino group that rearranges the polymerase active site and disrupts the coordination shells of the active-site metal ions. Such replacement of the 2′-hydroxyl group therefore suggests a general approach for the design of RdRp inhibitors [87].
Recently, co-crystals of the interaction of non-nucleotide inhibitors of human norovirus RdRp in the absence of an RNA primer–template duplex were solved [88]. Interestingly this study started from the crystal structure of the human norovirus RdRp bound to an RNA primer–template duplex and CTP and removed both the CTP and the short dsRNA [86]. Based on this structure the authors screened a small set of ∼1200 molecules for their potential to bind to the active site in silico and the two most interesting compounds showed clear inhibition of human norovirus polymerase in vitro. Crystallization and X-ray diffraction experiments together with mutational analysis subsequently demonstrated that, in absence of any substrate, these molecules bind to the active site of MNV and human norovirus polymerase. In addition a follow-up study using reverse fragment screening unraveled a novel binding site for a third inhibitor in the RdRp thumb domain [89].
6. Targeting host cell factors to stop norovirus replication
Viruses require and interact with the cellular machinery to facilitate their replication. Inhibition of host cell factors that are essential for viral replication is a potentially good antiviral strategy, particularly because these may be less prone to drug resistance.
Conserved secondary structures of the norovirus genome were found to be critical for the replication of MNV and for its infectivity [90]. These regions in the genomic and subgenomic RNA of norovirus interact with host factors [such as polypyrimidine tract-binding protein (PTB), protein La, and DDX3] but their precise function in the norovirus life cycle has yet to be elucidated [91]. Inhibitors of such host factors could potentially be good antiviral targets and help to unravel more details of the norovirus life cycle.
Cellular pathways for cholesterol and carbohydrate biosynthesis were found to be altered in Norwalk replicon-bearing cells by DNA microarray analysis [61]. While in the hepatitis C virus (HCV) replication cholesterol biosynthesis is upregulated and cholesterol-lowering statins act as antivirals through blockage of protein geranylgeranylation [92], the opposite was reported for norovirus. Statins such as simvastatin increased the replication of Norwalk virus which was correlated with an increased expression of low density lipoprotein receptor (LDLR). It was postulated that LDLR could play an important direct role in virus replication such as participating in viral replication complexes as an essential cofactor [61]. In addition, bile acids [for which cholesterol is a biosynthesis precursor] were found essential for the in vitro replication of the PEC/Cowden Sapovirus [93]. In gnotobiotic pigs, simvastatin increased the infectivity of human norovirus, which was linked to a suppressive effect on innate immune response via interferon-α [62], [94]. The use of statins has indeed been regarded as a risk factor for severe norovirus gastroenteritis [95]. The activity of acyl-CoA:cholesterol acyltransferase (ACAT) is also an important factor for cholesterol biosynthesis and unlike statins, treatment with ACAT inhibitors resulted in reduced levels of Norwalk replication [and of LDLR]. This indicates that ACAT could be a target for inhibition of norovirus replication [61].
7. Final remarks
Norovirus is currently an important pathogen, however no vaccines or antivirals are available to prevent or treat norovirus infection. Here we reviewed the progress made on the development of different tools to facilitate anti-norovirus research. Many of the human norovirus proteins have now been studied functionally and structurally, some in the presence of small-molecule inhibitors. The first small animal model for human norovirus was reported. Models for acute and persistence MNV infection have been reported. However the lack of a convenient in vitro cell culture system remains the main hurdle to study human norovirus replication and its inhibition. For other viruses this has proven critical for discovery and optimization of small molecule antivirals.
Acknowledgment
This project has received funding from the European Union's Seventh Framework Programme for research, technological development and demonstration under Grant Agreement no. 2260644-SILVER.
References
- 1.Scallan E., Hoekstra R.M., Angulo F.J., Tauxe R.V., Widdowson M.A., Roy S.L. Foodborne illness acquired in the United States—major pathogens. Emerg Infect Dis. 2011;17:7–15. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu L., Johnson H.L., Cousens S., Perin J., Scott S., Lawn J.E. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379:2151–2161. doi: 10.1016/S0140-6736(12)60560-1. [DOI] [PubMed] [Google Scholar]
- 3.Patel M.M., Widdowson M.A., Glass R.I., Akazawa K., Vinje J., Parashar U.D. Systematic literature review of role of noroviruses in sporadic gastroenteritis. Emerg Infect Dis. 2008;14:1224–1231. doi: 10.3201/eid1408.071114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Payne D.C., Vinje J., Szilagyi P.G., Edwards K.M., Staat M.A., Weinberg G.A. Norovirus and medically attended gastroenteritis in U.S. children. N Engl J Med. 2013;368:1121–1130. doi: 10.1056/NEJMsa1206589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hall A.J., Curns A.T., McDonald L.C., Parashar U.D., Lopman B.A. The roles of Clostridium difficile and norovirus among gastroenteritis-associated deaths in the United States, 1999–2007. Clin Infect Dis. 2012;55:216–223. doi: 10.1093/cid/cis386. [DOI] [PubMed] [Google Scholar]
- 6.van Asten L., Siebenga J., van den Wijngaard C., Verheij R., van Vliet H., Kretzschmar M. Unspecified gastroenteritis illness and deaths in the elderly associated with norovirus epidemics. Epidemiology. 2011;22:336–343. doi: 10.1097/EDE.0b013e31821179af. [DOI] [PubMed] [Google Scholar]
- 7.Vega E., Barclay L., Gregoricus N., Shirley S.H., Lee D., Vinje J. Genotypic and epidemiologic trends of norovirus outbreaks in the United States, 2009 to 2013. J Clin Microbiol. 2014;52:147–155. doi: 10.1128/JCM.02680-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hansen S., Stamm-Balderjahn S., Zuschneid I., Behnke M., Ruden H., Vonberg R.P. Closure of medical departments during nosocomial outbreaks: data from a systematic analysis of the literature. J Hosp Infect. 2007;65:348–353. doi: 10.1016/j.jhin.2006.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harris J.P., Adams N.L., Lopman B.A., Allen D.J., Adak G.K. The development of web-based surveillance provides new insights into the burden of norovirus outbreaks in hospitals in England. Epidemiol Infect. 2014;142:1590–1598. doi: 10.1017/S0950268813002896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Green K.Y. Caliciviridae: the noroviruses. In: Knipe D.M., Howley P.M., editors. Fields virology. sixth ed. Lippincott Williams & Wilkins; Philadelphia, PA: 2013. pp. 583–609. [Google Scholar]
- 11.Zanini B., Ricci C., Bandera F., Caselani F., Magni A., Laronga A.M. Incidence of post-infectious irritable bowel syndrome and functional intestinal disorders following a water-borne viral gastroenteritis outbreak. Am J Gastroenterol. 2012;107:891–899. doi: 10.1038/ajg.2012.102. [DOI] [PubMed] [Google Scholar]
- 12.Chen S.Y., Tsai C.N., Lai M.W., Chen C.Y., Lin K.L., Lin T.Y. Norovirus infection as a cause of diarrhea-associated benign infantile seizures. Clin Infect Dis. 2009;48:849–855. doi: 10.1086/597256. [DOI] [PubMed] [Google Scholar]
- 13.Ito S., Takeshita S., Nezu A., Aihara Y., Usuku S., Noguchi Y. Norovirus-associated encephalopathy. Pediatr Infect Dis J. 2006;25:651–652. doi: 10.1097/01.inf.0000225789.92512.6d. [DOI] [PubMed] [Google Scholar]
- 14.Stuart R.L., Tan K., Mahar J.E., Kirkwood C.D., Andrew Ramsden C., Andrianopoulos N. An outbreak of necrotizing enterocolitis associated with norovirus genotype GII.3. Pediatr Infect Dis J. 2010;29:644–647. doi: 10.1097/inf.0b013e3181d824e1. [DOI] [PubMed] [Google Scholar]
- 15.Bok K., Green K.Y. Norovirus gastroenteritis in immunocompromised patients. N Engl J Med. 2012;367:2126–2132. doi: 10.1056/NEJMra1207742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwartz S., Vergoulidou M., Schreier E., Loddenkemper C., Reinwald M., Schmidt-Hieber M. Norovirus gastroenteritis causes severe and lethal complications after chemotherapy and hematopoietic stem cell transplantation. Blood. 2011;117:5850–5856. doi: 10.1182/blood-2010-12-325886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lopman B., Gastanaduy P., Park G.W., Hall A.J., Parashar U.D., Vinje J. Environmental transmission of norovirus gastroenteritis. Curr Opin Virol. 2012;2:96–102. doi: 10.1016/j.coviro.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 18.Siebenga J.J., Beersma M.F., Vennema H., van Biezen P., Hartwig N.J., Koopmans M. High prevalence of prolonged norovirus shedding and illness among hospitalized patients: a model for in vivo molecular evolution. J Infect Dis. 2008;198:994–1001. doi: 10.1086/591627. [DOI] [PubMed] [Google Scholar]
- 19.Teunis P.F., Moe C.L., Liu P., Miller S.E., Lindesmith L., Baric R.S. Norwalk virus: how infectious is it. J Med Virol. 2008;80:1468–1476. doi: 10.1002/jmv.21237. [DOI] [PubMed] [Google Scholar]
- 20.Kroneman A., Vega E., Vennema H., Vinje J., White P.A., Hansman G. Proposal for a unified norovirus nomenclature and genotyping. Arch Virol. 2013;158:2059–2068. doi: 10.1007/s00705-013-1708-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bank-Wolf B.R., Konig M., Thiel H.J. Zoonotic aspects of infections with noroviruses and sapoviruses. Vet Microbiol. 2010;140:204–212. doi: 10.1016/j.vetmic.2009.08.021. [DOI] [PubMed] [Google Scholar]
- 22.Lindesmith L.C., Donaldson E.F., Lobue A.D., Cannon J.L., Zheng D.P., Vinje J. Mechanisms of GII.4 norovirus persistence in human populations. PLoS Med. 2008;5:e31. doi: 10.1371/journal.pmed.0050031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glass R.I., Parashar U.D., Estes M.K. Norovirus gastroenteritis. N Engl J Med. 2009;361:1776–1785. doi: 10.1056/NEJMra0804575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thackray L.B., Wobus C.E., Chachu K.A., Liu B., Alegre E.R., Henderson K.S. Murine noroviruses comprising a single genogroup exhibit biological diversity despite limited sequence divergence. J Virol. 2007;81:10460–10473. doi: 10.1128/JVI.00783-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McFadden N., Bailey D., Carrara G., Benson A., Chaudhry Y., Shortland A. Norovirus regulation of the innate immune response and apoptosis occurs via the product of the alternative open reading frame 4. PLoS Pathog. 2011;7:e1002413. doi: 10.1371/journal.ppat.1002413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vongpunsawad S., Venkataram Prasad B.V., Estes M.K. Norwalk virus minor capsid protein VP2 associates within the VP1 shell domain. J Virol. 2013;87:4818–4825. doi: 10.1128/JVI.03508-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Donaldson E.F., Lindesmith L.C., Lobue A.D., Baric R.S. Norovirus pathogenesis: mechanisms of persistence and immune evasion in human populations. Immunol Rev. 2008;225:190–211. doi: 10.1111/j.1600-065X.2008.00680.x. [DOI] [PubMed] [Google Scholar]
- 28.El-Kamary S.S., Pasetti M.F., Mendelman P.M., Frey S.E., Bernstein D.I., Treanor J.J. Adjuvanted intranasal Norwalk virus-like particle vaccine elicits antibodies and antibody-secreting cells that express homing receptors for mucosal and peripheral lymphoid tissues. J Infect Dis. 2010;202:1649–1658. doi: 10.1086/657087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tan M., Huang P., Xia M., Fang P.A., Zhong W., McNeal M. Norovirus P particle, a novel platform for vaccine development and antibody production. J Virol. 2011;85:753–764. doi: 10.1128/JVI.01835-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Atmar R.L., Bernstein D.I., Harro C.D., Al-Ibrahim M.S., Chen W.H., Ferreira J. Norovirus vaccine against experimental human Norwalk virus illness. N Engl J Med. 2011;365:2178–2187. doi: 10.1056/NEJMoa1101245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karst S.M., Wobus C.E., Lay M., Davidson J., Virgin H.W.T. STAT1-dependent innate immunity to a Norwalk-like virus. Science. 2003;299:1575–1578. doi: 10.1126/science.1077905. [DOI] [PubMed] [Google Scholar]
- 32.Wobus C.E., Karst S.M., Thackray L.B., Chang K.O., Sosnovtsev S.V., Belliot G. Replication of Norovirus in cell culture reveals a tropism for dendritic cells and macrophages. PLoS Biol. 2004;2:e432. doi: 10.1371/journal.pbio.0020432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wobus C.E., Thackray L.B., Virgin H.W. Murine norovirus: a model system to study norovirus biology and pathogenesis. J Virol. 2006;80:5104–5112. doi: 10.1128/JVI.02346-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ward J.M., Wobus C.E., Thackray L.B., Erexson C.R., Faucette L.J., Belliot G. Pathology of immunodeficient mice with naturally occurring murine norovirus infection. Toxicol Pathol. 2006;34:708–715. doi: 10.1080/01926230600918876. [DOI] [PubMed] [Google Scholar]
- 35.Duizer E., Schwab K.J., Neill F.H., Atmar R.L., Koopmans M.P., Estes M.K. Laboratory efforts to cultivate noroviruses. J Gen Virol. 2004;85:79–87. doi: 10.1099/vir.0.19478-0. [DOI] [PubMed] [Google Scholar]
- 36.Herbst-Kralovetz M.M., Radtke A.L., Lay M.K., Hjelm B.E., Bolick A.N., Sarker S.S. Lack of norovirus replication and histo-blood group antigen expression in 3-dimensional intestinal epithelial cells. Emerg Infect Dis. 2013;19:431–438. doi: 10.3201/eid1903.121029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takanashi S., Saif L.J., Hughes J.H., Meulia T., Jung K., Scheuer K.A. Failure of propagation of human norovirus in intestinal epithelial cells with microvilli grown in three-dimensional cultures. Arch Virol. 2014;159:257–266. doi: 10.1007/s00705-013-1806-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lay M.K., Atmar R.L., Guix S., Bharadwaj U., He H., Neill F.H. Norwalk virus does not replicate in human macrophages or dendritic cells derived from the peripheral blood of susceptible humans. Virology. 2010;406:1–11. doi: 10.1016/j.virol.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leung W.K., Chan P.K., Lee N.L., Sung J.J. Development of an in vitro cell culture model for human noroviruses and its clinical application. Hong Kong Med J = Xianggang yi xue za zhi/Hong Kong Acad Med. 2010;16:18–21. [PubMed] [Google Scholar]
- 40.Chang K.O., Sosnovtsev S.V., Belliot G., King A.D., Green K.Y. Stable expression of a Norwalk virus RNA replicon in a human hepatoma cell line. Virology. 2006;353:463–473. doi: 10.1016/j.virol.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 41.Taube S., Kolawole A.O., Hohne M., Wilkinson J.E., Handley S.A., Perry J.W. A mouse model for human norovirus. mBio. 2013;4:e00450–e513. doi: 10.1128/mBio.00450-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bok K., Parra G.I., Mitra T., Abente E., Shaver C.K., Boon D. Chimpanzees as an animal model for human norovirus infection and vaccine development. Proc Nat Acad Sci USA. 2011;108:325–330. doi: 10.1073/pnas.1014577107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheetham S., Souza M., Meulia T., Grimes S., Han M.G., Saif L.J. Pathogenesis of a genogroup II Human norovirus in gnotobiotic pigs. J Virol. 2006;80:10372–10381. doi: 10.1128/JVI.00809-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Souza M., Azevedo M.S., Jung K., Cheetham S., Saif L.J. Pathogenesis and immune responses in gnotobiotic calves after infection with the genogroup II.4-HS66 strain of human norovirus. J Virol. 2008;82:1777–1786. doi: 10.1128/JVI.01347-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guo M., Hayes J., Cho K.O., Parwani A.V., Lucas L.M., Saif L.J. Comparative pathogenesis of tissue culture-adapted and wild-type Cowden porcine enteric calicivirus (PEC) in gnotobiotic pigs and induction of diarrhea by intravenous inoculation of wild-type PEC. J Virol. 2001;75:9239–9251. doi: 10.1128/JVI.75.19.9239-9251.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sestak K., Feely S., Fey B., Dufour J., Hargitt E., Alvarez X. Experimental inoculation of juvenile rhesus macaques with primate enteric caliciviruses. PLoS One. 2012;7:e37973. doi: 10.1371/journal.pone.0037973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mumphrey S.M., Changotra H., Moore T.N., Heimann-Nichols E.R., Wobus C.E., Reilly M.J. Murine norovirus 1 infection is associated with histopathological changes in immunocompetent hosts, but clinical disease is prevented by STAT1-dependent interferon responses. J Virol. 2007;81:3251–3263. doi: 10.1128/JVI.02096-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu G., Kahan S.M., Jia Y., Karst S.M. Primary high-dose murine norovirus 1 infection fails to protect from secondary challenge with homologous virus. J Virol. 2009;83:6963–6968. doi: 10.1128/JVI.00284-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arias A., Bailey D., Chaudhry Y., Goodfellow I. Development of a reverse-genetics system for murine norovirus 3: long-term persistence occurs in the caecum and colon. J Gen Virol. 2012;93:1432–1441. doi: 10.1099/vir.0.042176-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nice T.J., Strong D.W., McCune B.T., Pohl C.S., Virgin H.W. A single-amino-acid change in murine norovirus NS1/2 is sufficient for colonic tropism and persistence. J Virol. 2013;87:327–334. doi: 10.1128/JVI.01864-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kahan S.M., Liu G., Reinhard M.K., Hsu C.C., Livingston R.S., Karst S.M. Comparative murine norovirus studies reveal a lack of correlation between intestinal virus titers and enteric pathology. Virology. 2011;421:202–210. doi: 10.1016/j.virol.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Strong D.W., Thackray L.B., Smith T.J., Virgin H.W. Protruding domain of capsid protein is necessary and sufficient to determine murine norovirus replication and pathogenesis in vivo. J Virol. 2012;86:2950–2958. doi: 10.1128/JVI.07038-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tan M., Jiang X. Norovirus gastroenteritis, carbohydrate receptors, and animal models. PLoS Pathog. 2010;6:e1000983. doi: 10.1371/journal.ppat.1000983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tan M., Jiang X. Virus-host interaction and cellular receptors of caliciviruses. In: Grant S., Hansman XJJaKYG, editors. Caliciviruses: molecular and cellular virology. Academic Press; Caister: 2010. pp. 111–130. [Google Scholar]
- 55.Hansman G.S., Shahzad-Ul-Hussan S., McLellan J.S., Chuang G.Y., Georgiev I., Shimoike T. Structural basis for norovirus inhibition and fucose mimicry by citrate. J Virol. 2012;86:284–292. doi: 10.1128/JVI.05909-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang X.F., Tan M., Chhabra M., Dai Y.C., Meller J., Jiang X. Inhibition of histo-blood group antigen binding as a novel strategy to block norovirus infections. PLoS One. 2013;8:e69379. doi: 10.1371/journal.pone.0069379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Han L., Kitova E.N., Tan M., Jiang X., Klassen J.S. Identifying carbohydrate ligands of a norovirus P particle using a catch and release electrospray ionization mass spectrometry assay. J Am Soc Mass Spectrom. 2014;25:111–119. doi: 10.1007/s13361-013-0752-4. [DOI] [PubMed] [Google Scholar]
- 58.Shang J., Piskarev V.E., Xia M., Huang P., Jiang X., Likhosherstov L.M. Identifying human milk glycans that inhibit norovirus binding using surface plasmon resonance. Glycobiology. 2013;23:1491–1498. doi: 10.1093/glycob/cwt077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gerondopoulos A., Jackson T., Monaghan P., Doyle N., Roberts L.O. Murine norovirus-1 cell entry is mediated through a non-clathrin-, non-caveolae-, dynamin- and cholesterol-dependent pathway. J Gen Virol. 2010;91:1428–1438. doi: 10.1099/vir.0.016717-0. [DOI] [PubMed] [Google Scholar]
- 60.Perry J.W., Wobus C.E. Endocytosis of murine norovirus 1 into murine macrophages is dependent on dynamin II and cholesterol. J Virol. 2010;84:6163–6176. doi: 10.1128/JVI.00331-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chang K.O. Role of cholesterol pathways in norovirus replication. J Virol. 2009;83:8587–8595. doi: 10.1128/JVI.00005-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jung K., Wang Q., Kim Y., Scheuer K., Zhang Z., Shen Q. The effects of simvastatin or interferon-alpha on infectivity of human norovirus using a gnotobiotic pig model for the study of antivirals. PLoS One. 2012;7:e41619. doi: 10.1371/journal.pone.0041619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Perry J.W., Taube S., Wobus C.E. Murine norovirus-1 entry into permissive macrophages and dendritic cells is pH-independent. Virus Res. 2009;143:125–129. doi: 10.1016/j.virusres.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hyde J.L., Sosnovtsev S.V., Green K.Y., Wobus C., Virgin H.W., Mackenzie J.M. Mouse norovirus replication is associated with virus-induced vesicle clusters originating from membranes derived from the secretory pathway. J Virol. 2009;83:9709–9719. doi: 10.1128/JVI.00600-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fernandez-Vega V., Sosnovtsev S.V., Belliot G., King A.D., Mitra T., Gorbalenya A. Norwalk virus N-terminal nonstructural protein is associated with disassembly of the Golgi complex in transfected cells. J Virol. 2004;78:4827–4837. doi: 10.1128/JVI.78.9.4827-4837.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hyde J.L., Mackenzie J.M. Subcellular localization of the MNV-1 ORF1 proteins and their potential roles in the formation of the MNV-1 replication complex. Virology. 2010;406:138–148. doi: 10.1016/j.virol.2010.06.047. [DOI] [PubMed] [Google Scholar]
- 67.Borin B.N., Tang W., Nice T.J., McCune B.T., Virgin H.W., Krezel A.M. Murine norovirus protein NS1/2 aspartate to glutamate mutation, sufficient for persistence, reorients side chain of surface exposed tryptophan within a novel structured domain. Proteins. 2014;82:1200–1209. doi: 10.1002/prot.24484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pfister T., Wimmer E. Polypeptide p41 of a Norwalk-like virus is a nucleic acid-independent nucleoside triphosphatase. J Virol. 2001;75:1611–1619. doi: 10.1128/JVI.75.4.1611-1619.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.De Palma A.M., Heggermont W., Lanke K., Coutard B., Bergmann M., Monforte A.M. The thiazolobenzimidazole TBZE-029 inhibits enterovirus replication by targeting a short region immediately downstream from motif C in the nonstructural protein 2C. J Virol. 2008;82:4720–4730. doi: 10.1128/JVI.01338-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Norder H., De Palma A.M., Selisko B., Costenaro L., Papageorgiou N., Arnan C. Picornavirus non-structural proteins as targets for new anti-virals with broad activity. Antiviral Res. 2011;89:204–218. doi: 10.1016/j.antiviral.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 71.Sara T., Konrat R., Skern T. Strategies for purifying variants of human rhinovirus 14 2C protein. Protein Expr Purif. 2014;95:28–37. doi: 10.1016/j.pep.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 72.Thibaut H.J., Malpani Y., Ahn S.H., Lee C.K., Tijsma A., Verbeken E. A novel class of highly potent small molecule inhibitors of entero/rhinovirus replication with an excellent safety and pharmacokinetic profile are highly effective against enterovirus infections in mice. 26th International Conference on Antiviral Research; San Francisco, CA, USA; 2013. p. O123. [Google Scholar]
- 73.Sharp T.M., Crawford S.E., Ajami N.J., Neill F.H., Atmar R.L., Katayama K. Secretory pathway antagonism by calicivirus homologues of Norwalk virus nonstructural protein p22 is restricted to noroviruses. Virol J. 2012;9:181. doi: 10.1186/1743-422X-9-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hsu N.Y., Ilnytska O., Belov G., Santiana M., Chen Y.H., Takvorian P.M. Viral reorganization of the secretory pathway generates distinct organelles for RNA replication. Cell. 2010;141:799–811. doi: 10.1016/j.cell.2010.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.De Palma A.M., Thibaut H.J., van der Linden L., Lanke K., Heggermont W., Ireland S. Mutations in the nonstructural protein 3A confer resistance to the novel enterovirus replication inhibitor TTP-8307. Antimicrob Agents Chemother. 2009;53:1850–1857. doi: 10.1128/AAC.00934-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Goodfellow I. The genome-linked protein VPg of vertebrate viruses—a multifaceted protein. Curr Opin Virol. 2011;1:355–362. doi: 10.1016/j.coviro.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Leen E.N., Kwok K.Y., Birtley J.R., Simpson P.J., Subba-Reddy C.V., Chaudhry Y. Structures of the compact helical core domains of feline calicivirus and murine norovirus VPg proteins. J Virol. 2013;87:5318–5330. doi: 10.1128/JVI.03151-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gingras R., Mekhssian K., Fenwick C., White P.W., Thibeault D. Human rhinovirus VPg uridylylation AlphaScreen for high-throughput screening. J Biomol Screen. 2014;19:259–269. doi: 10.1177/1087057113494805. [DOI] [PubMed] [Google Scholar]
- 79.Zeitler C.E., Estes M.K., Venkataram Prasad B.V. X-ray crystallographic structure of the Norwalk virus protease at 1.5-A resolution. J Virol. 2006;80:5050–5058. doi: 10.1128/JVI.80.10.5050-5058.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Takahashi D., Kim Y., Lovell S., Prakash O., Groutas W.C., Chang K.O. Structural and inhibitor studies of norovirus 3C-like proteases. Virus Res. 2013;178:437–444. doi: 10.1016/j.virusres.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hussey R.J., Coates L., Gill R.S., Erskine P.T., Coker S.F., Mitchell E. A structural study of norovirus 3C protease specificity: binding of a designed active site-directed peptide inhibitor. Biochemistry. 2011;50:240–249. doi: 10.1021/bi1008497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Muhaxhiri Z., Deng L., Shanker S., Sankaran B., Estes M.K., Palzkill T. Structural basis of substrate specificity and protease inhibition in Norwalk virus. J Virol. 2013;87:4281–4292. doi: 10.1128/JVI.02869-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim Y., Lovell S., Tiew K.C., Mandadapu S.R., Alliston K.R., Battaile K.P. Broad-spectrum antivirals against 3C or 3C-like proteases of picornaviruses, noroviruses, and coronaviruses. J Virol. 2012;86:11754–11762. doi: 10.1128/JVI.01348-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Oka T., Takagi H., Tohya Y., Murakami K., Takeda N., Wakita T. Bioluminescence technologies to detect calicivirus protease activity in cell-free system and in infected cells. Antiviral Res. 2011;90:9–16. doi: 10.1016/j.antiviral.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Alam I., Lee J.H., Cho K.J., Han K.R., Yang J.M., Chung M.S. Crystal structures of murine norovirus-1 RNA-dependent RNA polymerase in complex with 2-thiouridine or ribavirin. Virology. 2012;426:143–151. doi: 10.1016/j.virol.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 86.Zamyatkin D.F., Parra F., Alonso J.M., Harki D.A., Peterson B.R., Grochulski P. Structural insights into mechanisms of catalysis and inhibition in Norwalk virus polymerase. J Biol Chem. 2008;283:7705–7712. doi: 10.1074/jbc.M709563200. [DOI] [PubMed] [Google Scholar]
- 87.Zamyatkin D.F., Parra F., Machin A., Grochulski P., Ng K.K. Binding of 2′-amino-2′-deoxycytidine-5′-triphosphate to norovirus polymerase induces rearrangement of the active site. J Mol Biol. 2009;390:10–16. doi: 10.1016/j.jmb.2009.04.069. [DOI] [PubMed] [Google Scholar]
- 88.Mastrangelo E., Pezzullo M., Tarantino D., Petazzi R., Germani F., Kramer D. Structure-based inhibition of Norovirus RNA-dependent RNA polymerases. J Mol Biol. 2012;419:198–210. doi: 10.1016/j.jmb.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 89.Tarantino D., Pezzullo M., Mastrangelo E., Croci R., Rohayem J., Robel I. Naphthalene-sulfonate inhibitors of human norovirus RNA-dependent RNA-polymerase. Antiviral Res. 2014;102:23–28. doi: 10.1016/j.antiviral.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 90.Simmonds P., Karakasiliotis I., Bailey D., Chaudhry Y., Evans D.J., Goodfellow I.G. Bioinformatic and functional analysis of RNA secondary structure elements among different genera of human and animal caliciviruses. Nucleic Acids Res. 2008;36:2530–2546. doi: 10.1093/nar/gkn096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vashist S., Urena L., Chaudhry Y., Goodfellow I. Identification of RNA-protein interaction networks involved in the norovirus life cycle. J Virol. 2012;86:11977–11990. doi: 10.1128/JVI.00432-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ye J., Wang C., Sumpter R., Jr., Brown M.S., Goldstein J.L., Gale M., Jr. Disruption of hepatitis C virus RNA replication through inhibition of host protein geranylgeranylation. Proc Nat Acad Sci USA. 2003;100:15865–15870. doi: 10.1073/pnas.2237238100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chang K.O., Sosnovtsev S.V., Belliot G., Kim Y., Saif L.J., Green K.Y. Bile acids are essential for porcine enteric calicivirus replication in association with down-regulation of signal transducer and activator of transcription 1. Proc Nat Acad Sci USA. 2004;101:8733–8738. doi: 10.1073/pnas.0401126101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bui T., Kocher J., Li Y., Wen K., Li G., Liu F. Median infectious dose of human norovirus GII.4 in gnotobiotic pigs is decreased by simvastatin treatment and increased by age. J Gen Virol. 2013;94:2005–2016. doi: 10.1099/vir.0.054080-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rondy M., Koopmans M., Rotsaert C., Van Loon T., Beljaars B., Van Dijk G. Norovirus disease associated with excess mortality and use of statins: a retrospective cohort study of an outbreak following a pilgrimage to Lourdes. Epidemiol Infect. 2011;139:453–463. doi: 10.1017/S0950268810000993. [DOI] [PubMed] [Google Scholar]
- 96.Hsu C.C., Riley L.K., Wills H.M., Livingston R.S. Persistent infection with and serologic cross-reactivity of three novel murine noroviruses. Comp Med. 2006;56:247–251. [PubMed] [Google Scholar]
- 97.Farkas T., Cross R.W., Hargitt E., 3rd, Lerche N.W., Morrow A.L., Sestak K. Genetic diversity and histo-blood group antigen interactions of rhesus enteric caliciviruses. J Virol. 2010;84:8617–8625. doi: 10.1128/JVI.00630-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rocha-Pereira J., Jochmans D., Dallmeier K., Leyssen P., Cunha R., Costa I. Inhibition of norovirus replication by the nucleoside analogue 2′-C-methylcytidine. Biochem Biophys Res Commun. 2012;427:796–800. doi: 10.1016/j.bbrc.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 99.Costantini V.P., Whitaker T., Barclay L., Lee D., McBrayer T.R., Schinazi R.F. Antiviral activity of nucleoside analogues against norovirus. Antiviral Ther. 2012;17:981–991. doi: 10.3851/IMP2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rocha-Pereira J., Jochmans D., Dallmeier K., Leyssen P., Nascimento M.S., Neyts J. Favipiravir (T-705) inhibits in vitro norovirus replication. Biochem Biophys Res Commun. 2012;424:777–780. doi: 10.1016/j.bbrc.2012.07.034. [DOI] [PubMed] [Google Scholar]


