Abstract
Atherosclerosis with associated cardiovascular diseases remains one of the main causes of disability and death worldwide, requiring development of new solutions for prevention and treatment. Macrophages are the key effectors of a series of events involved in atherogenesis, such as inflammation, plaque formation, and changes in lipid metabolism. Some of these events were shown to be associated with mitochondrial dysfunction and excessive mitochondrial DNA (mtDNA) damage. Moreover, macrophages represent a promising target for novel therapeutic approaches that are based on the expression of various receptors and nanoparticle uptake. Lipid‐based gene delivery to mitochondria is considered to be an interesting strategy for mtDNA damage correction. To date, several nanocarriers and their modifications have been developed that demonstrate high transfection efficiency and low cytotoxicity. This review discusses the possibilities of lipid‐based gene delivery to macrophage mitochondria for atherosclerosis therapy.
Keywords: atherosclerosis, gene delivery, liposomes, macrophages, mitochondrial dysfunction, mtDNA damage
Abbreviations
- ASCVD
atherosclerotic cardiovascular disease
- CRP
C‐reactive protein
- NA
nucleic acid
- NP
nanoparticle
- SAMS
statin‐associated muscle symptoms
1. INTRODUCTION
Atherosclerosis is a multifactorial disease, which is characterized by the formation of fatty plaques in the arterial wall and chronic inflammatory response. Atherosclerosis leads to narrowing of the lumen of the affected vessel and increases the risk of thrombosis, which can be followed by lethal events, such as ischemic stroke and sudden cardiac death. 1 , 2 It is well‐known that atherosclerosis development can be a result of acquired and inherited factors. To date, numerous genetic variations and mutations have been shown to predispose humans to atherogenesis. Among the risk factors of atherosclerosis are dyslipidemia, arterial hypertension, diabetes mellitus, and old age. 3 Monocytes and macrophages play leading roles at all stages of atherosclerosis development, contributing to the local inflammatory response, cholesterol accumulation, and plaque growth. 4
Modern advances of genetics and molecular biology have improved our understanding of atherosclerosis pathogenesis and opened new perspectives for developing diagnostic and therapeutic approaches. 5 It is evident that treatment of atherosclerosis requires implementation of complex approaches that employ a combination of physical, chemical, and biological methods, such as those provided by nanomedicine. 6
Mitochondrial dysfunction caused by mitochondrial DNA (mtDNA) damage is a well‐studied cause of cell dysfunction and death observed in atherosclerosis. 7 Correspondingly, dysfunctional mtDNA is regarded as a promising target for atherosclerosis treatment strategies. The latest tendencies of treatment of mitochondrial diseases include direct nucleic acid (NA) delivery into these organelles. Currently, specially developed nanocarriers for DNA delivery into the mitochondria demonstrate sufficient efficacy and low cytotoxicity. 9 Lipid‐based nanocarriers are currently well investigated and widely used in research and therapy. 10 Being constantly improved and upgraded, liposomes often demonstrate biocompatibility and transfection efficiency, which makes them an advantageous agent for immunostimulation, drug, and NA delivery not only to cells but also to mitochondria. 11 The aim of this review is to summarize current knowledge on the use of lipid‐based nanocarriers for targeted gene delivery to macrophage mitochondria and assess its role in future atherosclerosis therapy.
2. CURRENT CARDIOVASCULAR DISEASE MANAGEMENT STRATEGIES
Current approaches for treatment of atherosclerosis are mainly focused on lipid lowering with statins, reduction in risk of thrombosis with anticoagulants, and alleviation of inflammation by means of immunomodulation. 12 , 13 During the recent years, substantial progress was made in the improvement of atherosclerosis treatment. Currently, a number of therapeutic agents applicable for atherosclerotic cardiovascular disease (ASCVD) treatment are being used. Having proved efficacy in atherosclerosis therapy, some of these agents can be associated with high residual cardiovascular risk, low cost‐efficiency, various contraindications, and side effects. Among them, lipid lowering statin drugs, fibrates, PCSK‐9 inhibitors, and niacin can be highlighted as important therapeutic agents that deserve to be discussed in detail.
Statins (HMG‐CoA reductase inhibitors) are used for the first‐line treatment for ASCVD reduction. These drugs can be used in different groups of patients providing low‐density lipoprotein‐cholesterol (LDL‐C) lowering effect of desirable intensity (low, medium, or high) with up to more than 50% LDL‐C reduction. 14 While lipid lowering effect can be regarded as the primary effect of statins, they also have secondary effects (so‐called pleiotropic effects) that can contribute to ASCVD management. Importantly, anti‐inflammatory activity of statins is well‐known. It was shown that statins reduce the levels of proinflammatory cytokines and C‐reactive protein (CRP), which is a nonspecific, but highly sensitive biomarker of inflammation. 15 , 16 Moreover, clinical studies reported statins to stabilize the plaque and reduce vascular wall inflammation. 17
However, statins are not free from adverse effects, drug interactions, and intolerance in some patients. 18 That is why clinician‐patient risk discussion as well as risk‐benefit assessment are recommended before statin prescription. 19 Despite this fact, in some cases it is still necessary to stop statin therapy, mainly because of statin‐associated muscle symptoms (SAMS), which can vary from myalgia to rhabdomyolysis and associated disorders. 20 Therapeutic alternatives to statins have been considered, and several other drug classes have been developed, some of them being able to potentiate the ongoing statin therapy.
Fibrates (agonists of peroxisome proliferator‐activated receptor‐α (PPAR‐α)) are another group of medications that belong to nonstatin therapy, mainly aimed on triglyceride level control. 21 It has also been reported that fibrates can be used in combination with statins to correct dyslipidemia and residual cardiovascular risk. 22 The results of some studies have indicated anti‐inflammatory potential of fibrates. 23 Nevertheless, the results of studies assessing the effect of fibrates on human lipid profile are sometimes contradictory. 24 Moreover, presence of adverse effects is also notable, including nausea, myositis, and gallstones. 25 Obviously, more studies are needed to assess the efficacy of fibrates and associated drug combinations in different groups of patients.
Recently, a new group of drugs, PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitors, came to clinical practice. The mechanism of action of these drugs relates to the reduction in the plasma level of PCSK9, therefore reducing its binding to low‐density lipoprotein receptor (LDLR). Using of PCSK9 inhibitors opens new opportunities for high‐risk patients, and is recommended in cases when general LDL‐lowering therapy is not sufficient. 26 However, these drugs have their limitations. Firstly, the relatively high price of treatment with PCSK9 inhibitors makes them cost ineffective and hinders their widespread use. 27 Secondly, little is known to date about the long‐term tolerance of these drugs due to their recent approval. 28 Future studies will add more detail on the PCSK9 inhibitors safety and effectiveness, allowing for a better positioning of these drugs within the spectrum of antiatherosclerosis and antihyperlipidemia treatments.
Niacin was one of the first effective lipid‐lowering agents that have been discovered, which acts through LDL and HDL lowering in the plasma. 29 However, clinical trials did not confirm a beneficial effect on niacin on cardiovascular events incidence, while the drug's side effects, including skin, gastrointestinal, and musculoskeletal effects, have been observed. Thus, there are currently no medications with niacin approved in Europe, and no recommendations to use it in the US guidelines. 30 , 31
Modern guidelines for cardiovascular disease prevention and therapy suggest low‐lipid diet and active lifestyle as primary measures to reduce cardiovascular risk. Dietary supplements also have their place as risk‐reducing agents helping to normalize the blood lipid profile. Omega‐3 fatty acids, according to the results of several trials, were shown to reduce plasma triglycerides, and were associated with the reduction on fatal and nonfatal cardiovascular events. 32 , 33 The most stable eicosapentaenoic acid, icosapent ethyl, is currently indicated to reduce overall cardiovascular risk and is used as adjuvant to statin and nonstatin therapy. 34
Accumulating clinical evidence suggests that existing lipid‐lowering strategies alone may not be sufficient to eliminate the cardiovascular risk. These results encouraged multiple studies to investigate the best treatment combinations (statin + nonstatin approaches) in order to lower the cardiovascular risk in a more reliable way. 35 , 36 Generally, various dyslipidemias that can occur in different groups of patients are mentioned as the main cause of residual cardiovascular events. 37 At the same time, chronic inflammation, which plays a key role in atherosclerosis development, is likely to be a prominent risk factor of cardiovascular events. Despite the improvements achieved in clinical practice recently, atherosclerosis still remains a serious cause of disability and death. 38 Future studies are needed to identify the optimal therapeutic strategies that allow for a more profound and stable reduction in cardiovascular risk in different groups of patients. Moreover, novel solutions are needed to combat the development of atherosclerosis by effectively preventing the formation of new plaques and induce regression of the existing plaques.
3. NANOTECHNOLOGIES FOR ATHEROSCLEROSIS THERAPY
One of the most recent treatment strategies of atherosclerosis is nanomedicine, which comprises chemical, biological, and physical technological applications. 39 Several novel techniques, including nanoparticle (NP) target therapy, drug delivery, nanovisualization, have been introduced and are being tested. As atherosclerosis is a multifactorial disease, there are number of possible targets for nanomedical methods in the atherosclerotic plaque: extracellular matrix, endothelial cells, and macrophages. 40 Macrophages are considered to be a promising target mainly due to the possibility of effective NP targeting. 10 NP design can provide combinative approaches for targeted treatment of atherosclerosis by carrying therapeutic agents, such as lipid‐lowering and anticoagulant drugs, siRNA, and DNA plasmids. Delivery of such agents directly into the target cells showed antiatherogenic effects in vitro as well as in vivo. 41 , 42 In addition, NPs can be used for accurate visualization of vessels and plaques. 43
Novel drug delivery strategies include methods of selective targeting of mitochondria with lipid‐based carriers. This approach is aimed at reducing mitochondrial dysfunction and alleviating mtDNA damage, which contributes to the development of pathological conditions in atherosclerosis. 44
4. ROLE OF MACROPHAGES IN ATHEROSCLEROSIS
Several recent studies conducted in vitro on animal models (mostly ApoE−/− and LDLR−/− mice) and human rupture plaques have determined the critical role of macrophages in atherosclerosis pathogenesis. 45 In growing atherosclerotic plaques, macrophages actively participate in lipid accumulation giving rise to foam cells and expanding the plaque. Moreover, macrophages also contribute to the immune response by releasing cytokines and chemokines providing the inflammatory component of atherosclerosis. 46 In atherosclerosis, the heterogeneity of monocytes/macrophages is shifted toward the prevalence of proinflammatory activation. 47 Proinflammatory or M1 macrophages release tumor necrosis factor‐alpha (TNF‐α), interleukins, and chemokines, and also produce high levels of ROS and nitric oxide (NO). 48 At the same time, anti‐inflammatory M2 macrophage phenotype is characterized by IL‐10 and IL‐1 receptor agonist secretion and may induce plaque regression and tissue repair. 49
Macrophages are involved in lipid metabolism via cholesterol efflux and oxidized LDL (oxLDL) uptake. 50 OxLDL particles are internalized through interaction with macrophage scavenger receptors (SR) (CD36, SR‐A1, and lectin‐like oxLDL receptor‐1 (LOX)) and then processed in the lysosomes. Subsequently, free cholesterol is released from the macrophages via ABC (ATP‐binding cassette) transporters (ABCA1 and ABCG1) facilitating the formation of HDL and removing the excess cholesterol. However, when the cholesterol efflux system is dysregulated due to excess plasma lipid levels, foam cells develop, which is believed to be a hallmark of atherosclerosis. 51 In the arterial wall, macrophages participate in the immune response via a number of cell receptors, such as Toll‐like receptors (TLR), mannose‐receptor (MR), SRs, and Fc receptors. 52 Some of these molecules are currently considered as potential targets for atherosclerosis therapy.
5. TARGETING OF MACROPHAGES RECEPTORS
A number of known macrophage surface receptors have been considered as potential targets for molecular therapeutic approaches. 10 , 52 , 53 NPs containing ligands for these receptors demonstrate significantly higher selectivity and transfection efficiency. 54 , 55 , 56 The mannose receptor (MR), which is a C‐type lectin I transmembrane protein, is believed to be a promising target for nanocarriers. 57 The MR is expressed on dendritic cells, tissue macrophages, and liver sinusoidal endothelial cell. 58 The MR plays an important role in antigen presentation, inflammatory response, and endocytosis, and can recognize the residues of mannose, fucose, and N‐acetylglucosamine. Incorporation of this ligands into the liposomes was shown to demonstrate high transfection efficiency and low cytotoxicity. 59 In atherosclerotic plaque, high expression of MR is present in macrophages of the fibrous cap, while in macrophages of the lipid core, where the most of mtDNA alterations occur, expression is lower. 60 Together, with presence of MR in other cell types, this target needs further investigation.
Another class of surface proteins that can be used for targeted drug delivery is integrins that play a crucial role in cell adhesion. Integrins can recognize proteins, components of extracellular matrix, phospholipids, as well as various amino acid sequences. 61 Integrins are heterodimeric proteins, with each subunit containing ligand‐binding sites. Conjugation of certain peptides to nanocarriers demonstrated increased cellular uptake through integrin‐mediated pathway. 62 , 63
Pattern recognition receptors (PRRs) can be activated by damaged cell fragments or debris (damage‐associated molecular patterns, or DAMP) and bacterial LPS or DNA (pathogen‐ associated molecular patterns, or PAMP) (Schiltze and Schmidt, 2015). Toll‐like receptors (TLR) are one of the most studied family of receptors, which may be used for drug targeting and immunostimulating therapy. 64 , 65 However, the activation of PRRs is associated with inflammatory response and leads to T‐cell activation and cytokine release, which is obviously an undesirable event in atherosclerosis. 66
6. MITOCHONDRIAL DNA DAMAGE
Mitochondria play a crucial role in regulating cell death, generating ROS, and maintaining cell metabolism and growth. 67 The mitochondrial genome contains 2‐10 copies of mtDNA, each of them consisting of 16 569 base pairs and encoding 37 genes, including ETC (electron transport chain) components, tRNAs and rRNAs. 68 Unlike nuclear DNA, mtDNA is not protected by histones and lacks efficient repair mechanisms, being therefore more susceptible for damage and accumulation of mutations, which increases with age. It is worth mentioning that mitochondrial dysfunction usually occurs only if more than 80% is damaged. 69 One of the factors promoting mtDNA damage is constant ROS generation during the oxidative phosphorylation in the inner mitochondrial membrane.
Mitochondrial genome defects are well investigated and often associated with so‐called mitochondrial diseases. 70 , 71 However, mtDNA damage occurs in other diseases, including atherosclerosis, that can also be associated with mitochondrial dysfunction. 72 Both animal and human studies showed that damaged mtDNA is more common in the sites of atherosclerotic lesions. Moreover, mitochondria appear to be considerably affected in macrophages, thus contributing to a number of atherogenesis events. 73 , 74
7. MITOCHONDRIAL DNA DAMAGE IN MACROPHAGES
Although mtDNA damage, in general, believed to be caused by excessive ROS generation in the mitochondria, recent in vivo studies demonstrated occurrence of mtDNA defects in atherosclerosis independently from oxidative stress. These studies were conducted in apoE−/− mice with downregulated polymerase‐γ (polG) proofreading activity. These mice showed high rate of mtDNA damage without increase in ROS and oxidative phosphorylation intensity. In comparison to classical apoE−/− models, polG‐deficient mice had increased hyperlipidemia and atherosclerosis. Moreover, polG−/−/apoE−/− monocytes were characterized by increased inflammatory cytokine secretion. These findings confirm possible development of atherosclerotic plaques and vessel damage promoted by damaged mtDNA with no associated ROS increase. 75
A number of studies reported apoptosis of macrophages and vessel smooth muscle cells (VSMC) induced by mitochondrial dysfunction. 76 , 77 , 78 As mentioned above, mitochondrial dysfunction can often be a result of accumulated mtDNA damage, subsequently leading to ROS generation and membrane defects. These conditions can stimulate the release of cytochrome C, an important cell death regulator, and promote apoptosis. 79 Macrophage apoptosis in atherosclerotic plaques contributes to the necrotic core formation thus reducing the plaque stability and promoting thrombogenesis. 80
The inflammatory response associated with atherosclerosis can be stimulated by endogenous antigens such as damaged mtDNA. 81 According to the results of recent studies, a number of events can contribute to this process. 82 The activation of TLRs under mitochondrial oxidative stress induces the NF‐κB pathway, which facilitates further immune response. It was also shown that the NF‐κB pathway in the atherosclerotic lesions’ macrophages promoted monocytes infiltration and plaque development. 83 Moreover, oxidized mtDNA, which escaped degradation by autophagy, was reported to activate the NLRP3 inflammasome thus regulating the release of cytokines, such as IL‐1β and IL‐18. 84 , 85 In addition, mitochondrial dysfunction was also shown to affect the cholesterol efflux in macrophages. 86 As this process is maintained by ATP‐dependent ABCA1 and ABCG1 transporters, the impaired ATP synthesis associated with mitochondrial dysfunction can inhibit the cholesterol efflux, therefore, disturbing lipid metabolism. 87 Moreover, ABC transporters were also shown to mediate about 70% of the cholesterol efflux from the foam cells,therefore, their inhibition further facilitates foam cells formation. 88
8. LIPID CARRIERS FOR GENE DELIVERY TO MITOCHONDRIA
One of the latest nanomedical tendencies of targeted therapy of mitochondrial dysfunction is using nanocarriers for gene delivery directly to the mitochondrion. This strategy aims to correct the mtDNA damage. 89 Implementation of this strategy requires overcoming of several obstacles. First of them is the presence of two negatively charged mitochondrial membranes. While the outer membrane is quite similar to the cellular membrane by its composition, the inner membrane contains cardiolipin, which makes it impermeable for hydrophilic molecules. In order to pass this obstacle, the carrier must contain some hydrophobic and positively charged ligands. 90 , 91 Another challenge for targeted drug delivery to the mitochondria is endocytosis. To escape from the endosome, the carriers must be designed to contain ligands facilitating such transport. 92
As mentioned above, accumulation of mtDNA damage contributes greatly to mitochondrial dysfunction as well as in atherogenesis. As mitochondrial genome consists of only 37 genes, it becomes possible to identify the potential targets for gene therapy in atherosclerosis. According to studies on ruptured plaques, arterial intima, and blood samples, a number of coding and noncoding mitochondrial genes, if mutated or damaged, were shown to cause various cell impairments and to be associated with atherogenesis. Among them are ETC proteins (NADH dehydrogenase, ATP synthase, cytochrome b, and cytochrome c oxidase subunits) and tRNA genes. 93 , 94 , 95 Transfection of these genes may result in decrease in plaque progression and atherosclerotic lesion development.
Currently, a wide diversity of transport systems is known, including physical, chemical, biological, and combinatorial approaches. Several comparative analyses have been conducted to assess the toxicity, efficiency, and specificity of different methods of gene delivery into the mitochondria. Although all of them were far from implementation into the clinical practice, some of the methods demonstrate low cytotoxicity and high efficiency. 96 , 97 , 98 The most promising technology is probably the use of lipid‐based nanocarriers. Such lipid carriers can be extensively modified to lower cytotoxicity and increase selectivity of delivered NA. 99 , 100 As well as in classical concept, any liposome contains lipid bilayer and aqueous core, which allow the carrier to fuse with cell membrane and subsequently release its content. 101 However, this mechanism is obviously not enough for mitochondrial delivery. According to that, firstly endocytosis should be involved, followed by endosome formation and further endosomal escape. Only after being released from the endosome, the carrier will be able to go through both outer and inner mitochondrial membranes. 102 Thus, successful gene delivery to mitochondria must include: 1 adsorption of liposomes on the cell surface,2 carrier endocytosis with endosome formation; 3 endosomal escape and migration to mitochondrion; and 4 mitochondrial membrane fusion and therapeutic agent release (Figure 1). 103
Figure 1.
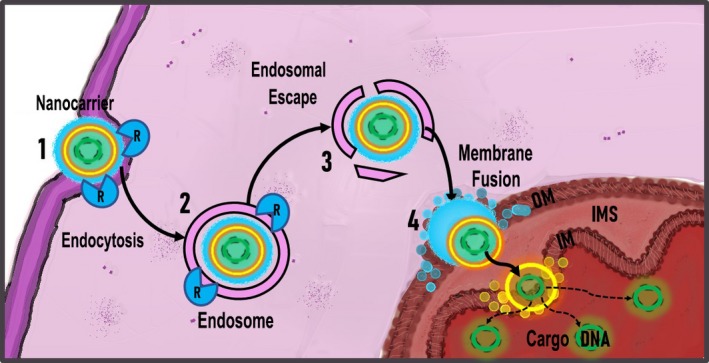
Lipid‐based mitochondria gene delivery mechanism. 1 – absorption of lipid carrier on macrophage surface; 2 – receptor‐mediated endocytosis of the carrier; 3 – endosome formation; 4 – endosomal escape and migration to mitochondrion; 5 – mitochondrial membrane fusion and cargo DNA release
Successful gene delivery to the mitochondria by lipid‐based nanocarriers depends on the proper design of the carriers, including the appropriate molar ratio of liposome components, adequate size, molecular weight, and N/P ratio (the ratio of cationic amine groups to anionic phosphates of NA). 104 These parameters were shown to influence both the transfection efficacy and cytotoxicity. While the molecular weight of a carrier can be increased through incorporation of ligands thus enhancing its specificity and serum resistance, large liposomes demonstrate low transfection rate and relatively high cytotoxicity. 105 , 106 High N/P ratio was reported to improve carrier‐cell interaction (due to high positive charge of the liposome), however, such lipids showed increased cytotoxicity. 107 , 108 To date, various types of lipid carriers have been described that are characterized by different lipid composition and parameters of transfection efficacy. The next step in improving the nanocarrier design will be the use of specific mitochondria‐targeting molecules. 89 , 109 , 110
9. CURRENT MITOCHONDRIAL GENE DELIVERY APPROACHES
Notably, there are currently other potential strategies for gene therapy being developed, such as viral vectors, CRISPR/Cas9, and stem cells–based therapy. In spite of significant efficacy and convenience for some purposes, these methods are not flawless and have obstacles to overcome before application to mitochondria. 111 Viral‐based methods, although demonstrate high transfection efficiency, are prone to initiate host immune response and have a limit in size of delivered genes. 112 CRISPR/Cas9, which is a well‐known method of nuclear genome editing, still remains uncertain for the mitochondrial genome. There is complication of guide RNA import to these organelles as well as unwanted total mtDNA reduction under action of cleaving enzymes. 113 Stem cells are frequently discussed in cardiovascular disease treatment and can be observed in many studies as an effective therapeutic approach. However, obviously more researches in this area are needed because of wide diversity of stem cell available with unknown signaling pathways and genetic instability. 114 Moreover, a lot of complications are related to high costs and difficulties in maintaining cell culture. 115 In comparison to abovementioned strategies, lipid‐based gene delivery has the main advantage of various design facilities, thus making it available to be customized and structured according to the purpose and also suitable for mitochondria targeting.
Early techniques of mitochondria‐specific lipid‐based NA carriers creation included the use of DQAsome (dequalimium chloride), early MITO‐porter, and STPP‐L (stearyl triphenylphosphonium‐modified liposomes). These approaches demonstrated relatively low specificity, substantial cytotoxicity, and, in some cases, low transfection efficacy, which considerably limited their use. 116 , 117 , 118 Further development of nanocarriers employed modifying their biocompatibility and incorporating certain mitochondria‐specific molecules. 119
In order to improve biocompatibility, new variations in liposomes were designed and tested in vitro and in vivo. TPP‐based carriers were incorporated with PGE‐PE (polyethylene glycol‐phosphatidyl ethanolamine) polymers which managed to decrease cytotoxicity and increase the lifetime of the carriers in blood serum. 120 MITO‐Porter was modified with S2 peptide (Dmt‐D‐Arg‐FK‐Dmt‐D‐Arg‐FK‐NH2) and showed improved cell viability rate. 121 At the same time, attempts were made to increase specificity of the carriers by supplementing them with mitochondria‐fusogenic lipids and targeting molecules. 103
Currently, lipid‐based carriers for gene delivery can provide for a wide range of therapeutic effects for mtDNA damage correction in different states, including atherosclerosis. Moreover, these particles are characterized by specific and accurate action in comparison to currently approved treatment methods, thus avoiding adverse effects. In relation to atherosclerosis, such targeted therapy may correct the mtDNA damage, which currently cannot be repaired with any other available tools, and thus alleviate the mitochondrial dysfunction.
Although lipid‐based gene delivery to macrophage mitochondria represents a novel technology in atherosclerosis prevention and treatment, there is still a serious lack of studies further characterizing this approach in vivo. It is also worth mentioning that liposome‐based atherosclerosis therapy had been discussed before. However, no clinical trial results are available to be observed at the moment because of problems needed to be solved first. Most notably, the problem of inequality between cell cultures, animal model and human, hence there is always a difference in structure, morphology, and biochemistry of the plaque. 122 , 123 This fact, on the one hand, can alter expected cytotoxicity, and, on the other hand, explains the importance of searching for efficient plaque macrophage targets. Another problem is distribution and plaque targeting due to interactions with plasma proteins and high hemodynamics in the arteries. Unlike NP‐based cancer therapy, the carriers targeting atherosclerotic plaques cannot be administered locally due to plaques presence in many vessels. That is why DNA vector and the carrier must remain intact from serum components to achieve proper bioavailability, reach mitochondria, and interact with mtDNA. In this case, a number of solutions in carrier design have been suggested, and some of them are currently being tested in models. Moreover, there is always a question of cost effectiveness related to nanomaterials and carrier preparation. 124 Today we can conclude that more preclinical studies are needed to reach the step of clinical approval of this promising therapeutic approach.
10. CONCLUSION
Converging evidence identifies macrophages as key players in atherosclerosis pathogenesis and, consequently, as potential therapeutic targets. Macrophage mitochondria, in particular, appear to be interesting from the point of view of future therapies development, as mtDNA damage is associated with the pathology development. Lipid‐based nanocarriers may provide a solution for targeted gene delivery into macrophage mitochondria to alleviate atherosclerosis‐associated mitochondrial dysfunction and oxidative stress. These agents are characterized by high transfection efficacy and low cytotoxicity. More studies are needed, however, to translate the results obtained in in vitro experiments to clinical practice.
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
AUTHORS’ CONTRIBUTION
FHZ wrote the manuscript text; all other authors contributed to the manuscript development and review.
Zakirov FH, Zhang D, Grechko AV, Wu W‐K, Poznyak AV, Orekhov AN. Lipid‐based gene delivery to macrophage mitochondria for atherosclerosis therapy. Pharmacol Res Perspect. 2020;8:e00584 10.1002/prp2.584
Funding information
This work was supported by the Russian Science Foundation (Grant #19‐15‐00010).
REFERENCES
- 1. Brown RA, Shantsila E, Varma C, Lip GYH. Current understanding of atherogenesis. Am J Med. 2017;130:268‐282. 10.1016/j.amjmed.2016.10.022. [DOI] [PubMed] [Google Scholar]
- 2. Orekhov AN, Ivanova EA. Introduction of the special issue "atherosclerosis and related diseases". Vessel Plus. 2017;1:163‐165. [Google Scholar]
- 3. Banerjee C, Turan TN. Large artery atherosclerosis: extracranial and intracranial. Semin Neurol. 2017;37:307‐315. 10.1055/s-0037-1603588. [DOI] [PubMed] [Google Scholar]
- 4. Moroni F, Ammirati E, Norata GD, Magnoni M, Camici PG. The Role of monocytes and macrophages in human atherosclerosis, plaque neoangiogenesis, and atherothrombosis. Mediators Inflamm. 2019;2019:7434376 10.1155/2019/7434376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gaurav C, Saurav B, Goutam R, Goyal A. Nano‐Systems for advanced therapeutics and diagnosis of atherosclerosis. CPD. 2015;21:4498‐4508. 10.2174/1381612821666150917094215. [DOI] [PubMed] [Google Scholar]
- 6. Vaidyanathan K, Gopalakrishnan S. Nanomedicine in the diagnosis and treatment of atherosclerosis‐a systematic review. Cardiovasc Hematol Disord Drug Targets. 2017;17:119‐131. 10.2174/1871529X17666170918142653. [DOI] [PubMed] [Google Scholar]
- 7. Sinyov VV, Sazonova MA, Ryzhkova AI, et al. Potential use of buccal epithelium for genetic diagnosis of atherosclerosis using mtDNA mutations. Vessel Plus. 2017;1:145‐150. [Google Scholar]
- 8. Sobenin IA. (2017) Mitochondrial DNA Damage in Atherosclerosis. Genetic Polymorphisms September 2017. doi:10.5772/intechopen.69622.
- 9. Wang Z, Guo W, Kuang X, Hou S, Liu H. Nanopreparations for mitochondria targeting drug delivery system: Current strategies and future prospective. Asian J Pharm Sci. 2017;12:498‐508. 10.1016/j.ajps.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kelly C, Jefferies C, Cryan S‐A. Targeted liposomal drug delivery to monocytes and macrophages. J Drug Deliv. 2011;2011:727241 10.1155/2011/727241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chernyak BV, Antonenko YN, Domnina LV, et al. Novel penetrating cations for targeting mitochondria. Curr Pharm Des. 2013;19:2795‐2806. [DOI] [PubMed] [Google Scholar]
- 12. Alipov VI, Sukhorukov VN, Karagodin VP, Grechko AV, Orekhov AN. Chemical composition of circulating native and desialylated low density lipoprotein: what is the difference? Vessel Plus. 2017;1:107‐115. [Google Scholar]
- 13. Weber C, Badimon L, Mach F, van der Vorst EPC. Therapeutic strategies for atherosclerosis and atherothrombosis: Past, present and future. Thromb Haemost. 2017;117:1258‐1264. 10.1160/TH16-10-0814. [DOI] [PubMed] [Google Scholar]
- 14. Soran H, Dent R, Durrington P. Evidence‐based goals in LDL‐C reduction. Clin Res Cardiol. 2017;106(4):237‐248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Antonopoulos AS, Margaritis M, Lee R, Channon K, Antoniades C. Statins as anti‐inflammatory agents in atherogenesis: molecular mechanisms and lessons from the recent clinical trials. Curr Pharm Des. 2012;18(11):1519‐1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Arévalo‐Lorido JC. Clinical relevance for lowering C‐reactive protein with statins. Ann Med. 2016;48(7):516‐524. [DOI] [PubMed] [Google Scholar]
- 17. Oesterle A, Laufs U, Liao JK. Pleiotropic effects of statins on the cardiovascular system. Circ Res. 2017;120(1):229‐243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Laufs U, Scharnagl H, März W. Statin intolerance. Curr Opin Lipidol. 2015;26(6):492‐501. [DOI] [PubMed] [Google Scholar]
- 19. Taylor BA, Thompson PD. Statin‐associated muscle disease: advances in diagnosis and management. Neurotherapeutics. 2018;15(4):1006‐1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Thompson PD, Panza G, Zaleski A, Taylor B. Statin‐associated side effects. J Am Coll Cardiol. 2016;67(20):2395‐2410. [DOI] [PubMed] [Google Scholar]
- 21. Saxon DR, Eckel RH. Statin intolerance: a literature review and management strategies. Prog Cardiovasc Dis. 2016;59(2):153‐164. [DOI] [PubMed] [Google Scholar]
- 22. Hegele RA, Gidding SS, Ginsberg HN, et al. ATVB council statement: non‐statin LDL‐lowering therapy and cardiovascular risk reduction. Arterioscler Thromb Vasc Biol. 2015;35(11):2269‐2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tziomalos K, Athyros VG, Karagiannis A, Mikhailidis DP. Anti‐inflammatory effects of fibrates: an overview. Curr Med Chem. 2009;16(6):676‐684. [DOI] [PubMed] [Google Scholar]
- 24. Athyros VG, Doumas M, Imprialos KP, et al. Diabetes and lipid metabolism. Hormones (Athens). 2018;17(1):61‐67. [DOI] [PubMed] [Google Scholar]
- 25. Remick J, Weintraub H, Setton R, Offenbacher J, Fisher E, Schwartzbard A. Fibrate therapy: an update. Cardiol Rev. 2008;16(3):129‐141. [DOI] [PubMed] [Google Scholar]
- 26. Wang Y, Liu Z‐P. PCSK9 Inhibitors: novel therapeutic strategies for lowering LDLCholesterol. Mini Rev Med Chem. 2019;19(2):165‐176. [DOI] [PubMed] [Google Scholar]
- 27. Hlatky MA, Kazi DS. PCSK9 Inhibitors: economics and policy. J Am Coll Cardiol. 2017;70(21):2677‐2687. [DOI] [PubMed] [Google Scholar]
- 28. Adhyaru BB, Jacobson TA. Safety and efficacy of statin therapy. Nat Rev Cardiol. 2018;15(12):757‐769. [DOI] [PubMed] [Google Scholar]
- 29. Schandelmaier S, Briel M, Saccilotto R, et al. Niacin for primary and secondary prevention of cardiovascular events. Cochrane Database Syst Rev. 2017;2017(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;74(10):e177‐e232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Atherosclerosis. 2019;290:140‐205. [DOI] [PubMed] [Google Scholar]
- 32. Bäck M. Omega‐3 fatty acids in atherosclerosis and coronary artery disease. Future Sci OA. 2017;3(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chang CL, Deckelbaum RJ. Omega‐3 fatty acids: mechanisms underlying “protective effects” in atherosclerosis. Curr Opin Lipidol. 2013;24(4):345‐350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bhatt DL, Steg PHG, Brinton EA, et al. Rationale and design of REDUCE‐IT: reduction of cardiovascular events with Icosapent ethyl‐intervention trial. Clin Cardiol. 2017;40(3):138‐148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nichols GA, Philip S, Reynolds K, Granowitz CB, Fazio S. Increased residual cardiovascular risk in patients with diabetes and high versus normal triglycerides despite statin‐controlled LDL cholesterol. Diabetes Obes Metab. 2019;21(2):366‐371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Reith C, Armitage J. Management of residual risk after statin therapy. Atherosclerosis. 2016;245:161‐170. [DOI] [PubMed] [Google Scholar]
- 37. Hernández‐Mijares A, Ascaso JF, Blasco M, et al. Residual cardiovascular risk of lipid origin. Components and pathophysiological aspects. Clínica e Investigación en Arteriosclerosis. (English Edition). 2019;31(2):75‐88. [DOI] [PubMed] [Google Scholar]
- 38. Giordano A, Peruzzi M, Marullo AG, et al. What we learned with recent network meta‐analyses on atherosclerosis prevention and treatment. Curr Atheroscler Rep. 2017;19:8 10.1007/s11883-017-0645-2. [DOI] [PubMed] [Google Scholar]
- 39. Martín Giménez VM, Ruiz‐Roso MB, Camargo AB, Kassuha D, Manucha W. Nanotechnology, a new paradigm in atherosclerosis treatment. Clin Investig Arterioscler. 2017;29:224‐230. 10.1016/j.arteri.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 40. Chan CKW, Zhang L, Cheng CK, et al. Recent advances in managing atherosclerosis via nanomedicine. Small. 2018;14: 10.1002/smll.201702793. [DOI] [PubMed] [Google Scholar]
- 41. Deshpande D, Janero DR, Segura‐Ibarra V, Blanco E, Amiji MM. Nucleic acid delivery for endothelial dysfunction in cardiovascular diseases. Methodist Debakey Cardiovasc J. 2016;12:134‐140. 10.14797/mdcj-12-3-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sato Y, Nakamura T, Yamada Y, Akita H, Harashima H. Multifunctional enveloped nanodevices (MENDs). Adv Genet. 2014;88:139‐204. 10.1016/B978-0-12-800148-6.00006-7. [DOI] [PubMed] [Google Scholar]
- 43. McAteer MA, Choudhury RP. Noninvasive molecular imaging of mouse atherosclerosis. Methods Mol Biol. 2015;1339:61‐83. 10.1007/978-1-4939-2929-0_4. [DOI] [PubMed] [Google Scholar]
- 44. Jang Y, Lim K. Recent advances in mitochondria‐targeted gene delivery. Molecules. 2018;23: 10.3390/molecules23092316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341‐355. 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Moore K, Sheedy F, Fisher E. Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol. 2013;13:709‐721. 10.1038/nri3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tabas I, Bornfeldt KE. Macrophage phenotype and function in different stages of atherosclerosis. Circ Res. 2016;118:653‐667. 10.1161/CIRCRESAHA.115.306256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bobryshev YV, Ivanova EA, Chistiakov DA, Nikiforov NG, Orekhov AN. Macrophages and their role in atherosclerosis: pathophysiology and transcriptome analysis. Biomed Res Int. 2016;2016: 10.1155/2016/9582430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chistiakov DA, Bobryshev YV, Orekhov AN. Macrophage‐mediated cholesterol handling in atherosclerosis. J Cell Mol Med. 2016;20:17‐28. 10.1111/jcmm.12689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zakiev ER, Sukhorukov VN, Melnichenko AA, Sobenin IA, Ivanova EA, Orekhov AN. Lipid composition of circulating multiple‐modified low density lipoprotein. Lipids Health Dis. 2016;15:134 10.1186/s12944-016-0308-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chistiakov DA, Melnichenko AA, Myasoedova VA, Grechko AV, Orekhov AN. Mechanisms of foam cell formation in atherosclerosis. J Mol Med. 2017;95:1153‐1165. 10.1007/s00109-017-1575-8. [DOI] [PubMed] [Google Scholar]
- 52. Quail DF, Joyce JA. Molecular pathways: deciphering mechanisms of resistance to macrophage‐targeted therapies. Clin Cancer Res. 2017;23:876‐884. 10.1158/1078-0432.CCR-16-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Singh A, Talekar M, Raikar A, Amiji M. Macrophage‐targeted delivery systems for nucleic acid therapy of inflammatory diseases. J Control Release. 2014;190:515‐530. 10.1016/j.jconrel.2014.04.021. [DOI] [PubMed] [Google Scholar]
- 54. Costa A, Sarmento B, Seabra V. Mannose‐functionalized solid lipid nanoparticles are effective in targeting alveolar macrophages. Eur J Pharm Sci. 2018;114:103‐113. 10.1016/j.ejps.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 55. Fukuda I, Mochizuki S, Sakurai K. Macrophage‐targeting gene delivery using a micelle composed of mannose‐modified lipid with triazole ring and Dioleoyl Trimethylammonium propane. Biomed Res Int. 2015;2015:350580 10.1155/2015/350580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Nakamura K, Kuramoto Y, Mukai H, Kawakami S, Higuchi Y, Hashida M. Enhanced gene transfection in macrophages by histidine‐conjugated mannosylated cationic liposomes. Biol Pharm Bull. 2009;32:1628‐1631. [DOI] [PubMed] [Google Scholar]
- 57. Kermanizadeh A, Villadsen K, Østrem RG, Jensen KJ, Møller P, Loft S. Integrin targeting and toxicological assessment of peptide‐conjugated liposome delivery systems to activated endothelial cells. Basic Clin Pharmacol Toxicol. 2017;120:380‐389. 10.1111/bcpt.12692. [DOI] [PubMed] [Google Scholar]
- 58. Vigerust DJ, Vick S, Shepherd VL. Stable expression and characterization of an optimized mannose receptor. J Clin Cell Immunol. 2015;6(3):330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kong F, Zhou F, Ge L, Liu X, Wang Y. Mannosylated liposomes for targeted gene delivery. Int J Nanomedicine. 2012;7:1079‐1089. 10.2147/IJN.S29183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chinetti‐Gbaguidi G, Baron M, Bouhlel MA, et al. Human atherosclerotic plaque alternative macrophages display low cholesterol handling but high phagocytosis because of distinct activities of the PPARγ and LXRα pathways. Circ Res. 2011;108(8):985‐995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Woodside DG, Tanifum EA, Ghaghada KB, et al. Magnetic resonance imaging of atherosclerotic plaque at clinically relevant field strengths (1T) by targeting the integrin α4β1. Sci Rep. 2018;8:3733 10.1038/s41598-018-21893-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hamano N, Negishi Y, Fujisawa A, et al. Modification of the C16Y peptide on nanoparticles is an effective approach to target endothelial and cancer cells via the integrin receptor. Int J Pharm. 2012;428:114‐117. 10.1016/j.ijpharm.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 63. Schultze JL, Schmidt SV. Molecular features of macrophage activation. Semin Immunol. 2015;27:416‐423. 10.1016/j.smim.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 64. Krishnan J, Lee G, Choi S. Drugs targeting toll‐like receptors. Arch Pharm Res. 2009;32:1485 10.1007/s12272-009-2100-6. [DOI] [PubMed] [Google Scholar]
- 65. Zhao X, Li X, Zhao Y, et al. Immune activities of polycationic vectors for gene delivery. Front Pharmacol. 2017;8:510 10.3389/fphar.2017.00510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Satoh T, Akira S. Toll‐Like receptor signaling and its inducible proteins. Microbiology Spectrum. 2016;4: 10.1128/microbiolspec.MCHD-0040-2016. [DOI] [PubMed] [Google Scholar]
- 67. Kauppila JHK, Stewart JB. Mitochondrial DNA: radically free of free‐radical driven mutations. Biochim Biophys Acta. 2015;1847:1354‐1361. 10.1016/j.bbabio.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 68. Kolesnikov AA. The mitochondrial genome. The Nucleoid. Biochemistry Mosc. 2016;81:1057‐1065. 10.1134/S0006297916100047. [DOI] [PubMed] [Google Scholar]
- 69. Young MJ, Copeland WC. Human mitochondrial DNA replication machinery and disease. Curr Opin Genet Dev. 2016;38:52‐62. 10.1016/j.gde.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Alston CL, Rocha MC, Lax NZ, Turnbull DM, Taylor RW. The genetics and pathology of mitochondrial disease. J Pathol. 2017;241:236‐250. 10.1002/path.4809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Stein A, Sia EA. Mitochondrial DNA repair and damage tolerance. Front Biosci (Landmark Ed). 2017;22:920‐943. [DOI] [PubMed] [Google Scholar]
- 72. Yu EPK, Bennett MR. The role of mitochondrial DNA damage in the development of atherosclerosis. Free Radic Biol Med. 2016;100:223‐230. 10.1016/j.freeradbiomed.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 73. Kattoor AJ, Pothineni NVK, Palagiri D, Mehta JL. Oxidative stress in atherosclerosis. Curr Atheroscler Rep. 2017;19:42 10.1007/s11883-017-0678-6. [DOI] [PubMed] [Google Scholar]
- 74. Lane RK, Hilsabeck T, Rea SL. The role of mitochondrial dysfunction in age‐related diseases. Biochim Biophys Acta. 2015;1847:1387‐1400. 10.1016/j.bbabio.2015.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Yu E, Calvert PA, Mercer JR, et al. Mitochondrial DNA damage can promote atherosclerosis independently of reactive oxygen species through effects on smooth muscle cells and monocytes and correlates with higher‐risk plaques in humans. Circulation. 2013;128:702‐712. 10.1161/CIRCULATIONAHA.113.002271. [DOI] [PubMed] [Google Scholar]
- 76. Hulsmans M, Van Dooren E, Holvoet P. Mitochondrial reactive oxygen species and risk of atherosclerosis. Curr Atheroscler Rep. 2012;14:264‐276. 10.1007/s11883-012-0237-0. [DOI] [PubMed] [Google Scholar]
- 77. Li H, Xiao Y, Tang L, et al. Adipocyte fatty acid‐binding protein promotes palmitate‐induced mitochondrial dysfunction and apoptosis in macrophages. Front Immunol. 2018;9:81 10.3389/fimmu.2018.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Yu EPK, Reinhold J, Yu H, et al. Mitochondrial respiration is reduced in atherosclerosis, promoting necrotic core formation and reducing relative fibrous cap thickness. Arterioscler Thromb Vasc Biol. 2017;37:2322 10.1161/ATVBAHA.117.310042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kulikov AV, Shilov ES, Mufazalov IA, Gogvadze V, Nedospasov SA, Zhivotovsky B. Cytochrome c: the Achilles’ heel in apoptosis. Cell Mol Life Sci. 2012;69:1787‐1797. 10.1007/s00018-011-0895-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Gonzalez L, Trigatti BL. Macrophage apoptosis and necrotic core development in atherosclerosis: a rapidly advancing field with clinical relevance to imaging and therapy. Can J Cardiol. 2017;33:303‐312. 10.1016/j.cjca.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 81. Ding Z, Liu S, Wang X, et al. LOX‐1, oxidant stress, mtDNA damage, autophagy, and immune response in atherosclerosis. Can J Physiol Pharmacol. 2014;92:524‐530. 10.1139/cjpp-2013-0420. [DOI] [PubMed] [Google Scholar]
- 82. West AP, Shadel GS. Mitochondrial DNA in innate immune responses and inflammatory pathology. Nat Rev Immunol. 2017;17:363‐375. 10.1038/nri.2017.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wang Y, Wang GZ, Rabinovitch PS, Tabas I. Macrophage mitochondrial oxidative stress promotes atherosclerosis and nuclear factor‐κB‐mediated inflammation in macrophages. Circ Res. 2014;114:421‐433. 10.1161/CIRCRESAHA.114.302153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Baldrighi M, Mallat Z, Li X. NLRP3 inflammasome pathways in atherosclerosis. Atherosclerosis. 2017;267:127‐138. 10.1016/j.atherosclerosis.2017.10.027. [DOI] [PubMed] [Google Scholar]
- 85. Ding Z, Liu S, Wang X, et al. LOX‐1, mtDNA damage, and NLRP3 inflammasome activation in macrophages: implications in atherogenesis. Cardiovasc Res. 2014;103:619‐628. 10.1093/cvr/cvu114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Litvinov YD, Savushkin VE, Garaeva AE, Dergunov DA. Cholesterol efflux and reverse cholesterol transport: experimental approaches. CMC. 2016;23:3883‐3908. 10.2174/0929867323666160809093009. [DOI] [PubMed] [Google Scholar]
- 87. Graham A, Allen A‐M. Mitochondrial function and regulation of macrophage sterol metabolism and inflammatory responses. World J Cardiol. 2015;7:277‐286. 10.4330/wjc.v7.i5.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Yvan‐Charvet L, Wang N, Tall AR. The role of HDL, ABCA1 and ABCG1 transporters in cholesterol efflux and immune responses. Arterioscler Thromb Vasc Biol. 2010;30:139‐143. 10.1161/ATVBAHA.108.179283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ishikawa T, Somiya K, Munechika R, Harashima H, Yamada Y. Mitochondrial transgene expression via an artificial mitochondrial DNA vector in cells from a patient with a mitochondrial disease. J Controlled Release. 2018;274:109‐117. 10.1016/j.jconrel.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 90. Bae Y, Jung MK, Song SJ, et al. Functional nanosome for enhanced mitochondria‐targeted gene delivery and expression. Mitochondrion. 2017;37:27‐40. 10.1016/j.mito.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 91. Pathak RK, Kolishetti N, Dhar S. Targeted nanoparticles in mitochondrial medicine. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2015;7:315‐329. 10.1002/wnan.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Wongrakpanich A, Geary SM, Joiner MA, Anderson ME, Salem AK. Mitochondria‐targeting particles. Nanomedicine (Lond). 2014;9:2531‐2543. 10.2217/nnm.14.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Forrester SJ, Griendling KK. Mitochondrial respiration and atherosclerosis. Arterioscler Thromb Vasc Biol. 2017;37(12):2229‐2230. 10.1161/ATVBAHA.117.310298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Sobenin IA, Orekhov AN. Mitochondrial genetic variation: an association with subclinical carotid atherosclerosis. J Am Coll Cardiol. 2014;63:A1517 10.1016/S0735-1097(14)61520-5. [DOI] [Google Scholar]
- 95. Volobueva A, Grechko A, Yet S‐F, Sobenin I, Orekhov A. Changes in mitochondrial genome associated with predisposition to atherosclerosis and related disease. Biomolecules. 2019;9(8):377 10.3390/biom9080377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Coutinho E, Batista C, Sousa F, Queiroz J, Costa D. Mitochondrial gene therapy: advances in mitochondrial gene cloning, plasmid production, and nanosystems targeted to mitochondria. Mol Pharm. 2017;14:626‐638. 10.1021/acs.molpharmaceut.6b00823. [DOI] [PubMed] [Google Scholar]
- 97. Ibrahim N, Handa H, Cosset A, et al. DNA delivery to mitochondria: sequence specificity and energy enhancement. Pharm Res. 2011;28:2871‐2882. 10.1007/s11095-011-0516-4. [DOI] [PubMed] [Google Scholar]
- 98. Yamada Y, Harashima H. Targeting the mitochondrial genome via a dual function MITO‐Porter: evaluation of mtDNA levels and mitochondrial function. Methods Mol Biol. 2015;1265:123‐133. 10.1007/978-1-4939-2288-8_10. [DOI] [PubMed] [Google Scholar]
- 99. Weissig V. DQAsomes as the prototype of mitochondria‐targeted pharmaceutical nanocarriers: preparation, characterization, and use. Methods Mol Biol. 2015;1265:1‐11. 10.1007/978-1-4939-2288-8_1. [DOI] [PubMed] [Google Scholar]
- 100. Yamada Y, Harashima H. MITO‐porter for mitochondrial delivery and mitochondrial functional analysis. Handb Exp Pharmacol. 2017;240:457‐472. 10.1007/164_2016_4. [DOI] [PubMed] [Google Scholar]
- 101. Sercombe L, Veerati T, Moheimani F, Wu SY, Sood AK, Hua S. Advances and challenges of liposome assisted drug delivery. Front Pharmacol. 2015;6: 10.3389/fphar.2015.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Weissig V, Boddapati SV, Cheng S‐M, D’souza GGM. Liposomes and liposome‐like vesicles for drug and DNA delivery to mitochondria. J Liposome Res. 2006;16(3):249‐264. 10.1080/08982100600851169. [DOI] [PubMed] [Google Scholar]
- 103. Yamada Y, Furukawa R, Yasuzaki Y, Harashima H. Dual function MITO‐porter, a nano carrier integrating both efficient cytoplasmic delivery and mitochondrial macromolecule delivery. Mol Ther. 2011;19:1449‐1456. 10.1038/mt.2011.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Ren H, He Y, Liang J, et al. Role of liposomes size, surface charge and PEGylation on rheumatoid arthritis targeting therapy. ACS Appl Mater Interfaces. 2019;11:20304‐20315. 10.1021/acsami.8b22693. [DOI] [PubMed] [Google Scholar]
- 105. Bozzuto G, Molinari A. Liposomes as nanomedical devices. Int J Nanomedicine. 2015;10:975‐999. 10.2147/IJN.S68861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Lee S‐H, Sato Y, Hyodo M, Harashima H. Size‐dependency of the surface ligand density of liposomes prepared by post‐insertion. Biol Pharm Bull. 2017;40:1002‐1009. 10.1248/bpb.b16-00990. [DOI] [PubMed] [Google Scholar]
- 107. Alameh M, Dejesus D, Jean M, et al. Low molecular weight chitosan nanoparticulate system at low N: P ratio for nontoxic polynucleotide delivery. IJN. 2012;7:1399‐1414. 10.2147/IJN.S26571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Lechanteur A, Sanna V, Duchemin A, Evrard B, Mottet D, Piel G. Cationic liposomes carrying siRNA: impact of lipid composition on physicochemical properties, Cytotoxicity and Endosomal Escape. Nanomaterials. 2018;8:270 10.3390/nano8050270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Selmin F, Magri G, Gennari CGM, Marchianò S, Ferri N, Pellegrino S. Development of poly(lactide‐co‐glycolide) nanoparticles functionalized with a mitochondria penetrating peptide. J Pept Sci. 2017;23:182‐188. 10.1002/psc.2952. [DOI] [PubMed] [Google Scholar]
- 110. Yamada Y, Furukawa R, Harashima H. A dual‐ligand liposomal system composed of a cell‐penetrating peptide and a mitochondrial RNA aptamer synergistically facilitates cellular uptake and mitochondrial targeting. J Pharm Sci. 2016;105:1705‐1713. 10.1016/j.xphs.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 111. Patananan AN, Wu T‐H, Chiou P‐Y, Teitell MA. Modifying the mitochondrial genome. Cell Metab. 2016;23(5):785‐796. 10.1016/j.cmet.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Gonçalves GAR, de Paiva R, MA,. Gene therapy: advances, challenges and perspectives. Einstein (Sao Paulo). 2017;15(3):369‐375. 10.1590/S1679-45082017RB4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Gammage PA, Moraes CT, Minczuk M. Mitochondrial genome engineering: the revolution may not be CRISPR‐Ized. Trends Genet. 2018;34(2):101‐110. 10.1016/j.tig.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Sun Q, Zhang Z, Sun Z. The potential and challenges of using stem cells for cardiovascular repair and regeneration. Genes Dis. 2014;1(1):113‐119. 10.1016/j.gendis.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Alenzi FQ, Lotfy M, Tamimi WG, Wyse RKH. Review: Stem cells and gene therapy. Lab Hematol. 2010;16(3):53‐73. 10.1532/LH96.10010. [DOI] [PubMed] [Google Scholar]
- 116. Boddapati SV, Tongcharoensirikul P, Hanson RN, D’Souza GGM, Torchilin VP, Weissig V. Mitochondriotropic liposomes. J Liposome Res. 2005;15:49‐58. 10.1081/LPR-64958. [DOI] [PubMed] [Google Scholar]
- 117. Weissig V, Lasch J, Erdos G, Meyer HW, Rowe TC, Hughes J. DQAsomes: a novel potential drug and gene delivery system made from Dequalinium. Pharm Res. 1998;15:334‐337. [DOI] [PubMed] [Google Scholar]
- 118. Yamada Y, Akita H, Kamiya H, et al. MITO‐Porter: a liposome‐based carrier system for delivery of macromolecules into mitochondria via membrane fusion. Biochim Biophys Acta. 2008;1778:423‐432. 10.1016/j.bbamem.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 119. Niazi AK, Mileshina D, Cosset A, Val R, Weber‐Lotfi F, Dietrich A. Targeting nucleic acids into mitochondria: progress and prospects. Mitochondrion. 2013;13:548‐558. 10.1016/j.mito.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 120. Biswas S, Dodwadkar NS, Deshpande PP, Torchilin VP. Liposomes loaded with paclitaxel and modified with novel Triphenylphosphonium‐PEG‐PE conjugate possess low toxicity, target mitochondria and demonstrate enhanced antitumor effects in vitro and in vivo. J Control Release. 2012;159:393‐402. 10.1016/j.jconrel.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Yamada Y, Fukuda Y, Harashima H. An analysis of membrane fusion between mitochondrial double membranes and MITO‐Porter, mitochondrial fusogenic vesicles. Mitochondrion. 2015;24:50‐55. 10.1016/j.mito.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 122. Alaarg A, Hamers AA, Versloot M, et al. Targeted liposomal drug delivery to inhibit atherosclerotic plaque inflammation. Atherosclerosis. 2015;241(1):e87 10.1016/j.atherosclerosis.2015.04.306. [DOI] [Google Scholar]
- 123. Lobatto ME, Calcagno C, Metselaar JM, et al. Imaging the efficacy of anti‐inflammatory liposomes in a rabbit model of atherosclerosis by non‐invasive imaging. Methods Enzymol. 2012;508:211‐228. 10.1016/B978-0-12-391860-4.00011-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Nakhlband A, Eskandani M, Omidi Y, et al. Combating atherosclerosis with targeted nanomedicines: recent advances and future prospective. Bioimpacts. 2018;8(1):59‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]


