Abstract
Bovine viral diarrhea virus (BVDV) is a pestivirus member of the Flaviviridae family, closely related to, and used as a surrogate model for the hepatitis C virus. Its envelope contains the E1 and E2 glycoproteins, disulfide linked into homo- and heterodimers. In this study, we investigate the role of disulfide bond formation in the folding, assembly, and stability of BVDV glycoproteins. We provide molecular evidence that intact disulfide bonds are critical for the acquirement of a stable conformation of E2 monomers. Forcing the E2 glycoproteins to adopt a reduced conformation either co- or post-translationally before assembly into dimers, determines their misfolding and degradation by proteasome. In contrast, dimerization of E2 glycoproteins results in a conformation resistant to reducing agents and degradation. Furthermore, inhibition of the ER-α-mannosidase activity leads to impairment of misfolded E2 degradation, demonstrating the involvement of this enzyme in targeting viral proteins towards proteasomal degradation.
Keywords: BVDV, Disulfide bonds, DTT, Folding, Proteasome, Degradation
Bovine viral diarrhea virus (BVDV) is a pestivirus member of the Flaviviridae family [1]. Due to its resemblance to the hepatitis C virus (HCV) [2], [3], a hepacivirus member of the same family, BVDV is enjoying increasing attention. In the absence of a reliable cell culture system able to replicate HCV, BVDV has been adopted as a surrogate model for HCV, especially in studies which focus on infectivity [4], [5].
BVDV is an enveloped, positive single-stranded RNA virus. Its genome encodes a single polyprotein precursor that is co- and post-translationally processed by host and viral proteases to produce mature structural and nonstructural proteins of the virus [6]. The structural proteins comprise the nucleocapsid protein C and the three envelope glycoproteins Erns, E1, and E2. Unlike E1 and E2, Erns lacks a transmembrane domain and is secreted from infected cells [7].
Although the detailed structure of BVDV is not known, it is assumed that the viral nucleocapsid is surrounded by a lipid envelope containing the E1 and E2 glycoproteins. These proteins interact to form a heterodimeric complex, which has been proposed as a functional subunit of mature virions. An interesting difference between BVDV and HCV may reside in the nature of the linkage between E1 and E2 proteins within the dimers. While for BVDV the complex was clearly shown to be disulfide bonded both in cell lysates and mature virions [5], [8], purified HCV glycoprotein complexes expressed in recombinant systems were reported to be associated mainly in a noncovalent fashion [9]. Only a fraction of E1 and E2 present in the lysates of cells infected with vaccinia virus–HCV recombinants has been shown to be associated via disulfide linkages which was suggested to be part of a nonproductive pathway leading to misfolding and aggregation [10].
In a previous study, we followed the folding of BVDV E1 and E2 glycoproteins and showed that most of the E2 intramolecular disulfide bonds form co-translationally on the nascent polypeptide chain, while the intermolecular disulfide bonds form later, after the release of E1 from interactions with the ER-chaperone, calnexin [11]. However, very little is known about the role of the disulfide bonds in the folding of the BVDV envelope glycoproteins and the formation of a stable, native E1–E2 complex.
In this paper, we address the contribution of disulfide bonds to the folding and assembly of BVDV envelope glycoproteins, following the effect of a reducing agent, dithiothreitol (DTT), on the biosynthesis, stability and degradation of the E2 glycoprotein and its uncleaved precursor, E2-p7 [12]. Our studies show that preventing the formation of intramolecular disulfide bonds as well as reducing these bonds after they have formed determine misfolding of the E2/E2-p7 monomers and their subsequent degradation. Once the proteins have assembled into dimers, their conformation is such that they become resistant to DTT.
Using proteasome inhibitors we show that reduced viral proteins are mainly degraded by proteasome. The process was greatly impaired by inhibitors of the ER-mannosidase activity, suggesting a role for mannose trimming in targeting misfolded BVDV envelope glycoproteins to proteasome.
Methods
Cell culture and virus. Noncytopatic BVDV-free MDBK cells (European Collection of Animal Cell Cultures, Porton Down, UK) were grown in RPMI 1640 medium (Gibco/BRL) supplemented with 10% (v/v) BVDV-free fetal calf serum (PAA Laboratories, Teddington, UK), 50 U/ml penicillin, and 50 mg/ml streptomycin (Life Technologies). The cytopathic (cp) BVDV (NADL strain) was obtained from the American Type Culture Collection, Manassas, VA.
Reagents and antibodies. Dithiothreitol, l-methionine, protein G–Sepharose, and the protease inhibitor cocktail were from Sigma. [35S]Methionine/cysteine (Tran35S-label 1100 Ci/mmol) and the methionine- and cysteine-free RPMI 1640 (starvation) medium were from ICN flow (Thame, Oxfordshire, UK). The endoplasmic reticulum (ER) mannosidase inhibitor 1-deoxymannojirimicyn (DMJ), the proteaseome inhibitors N-acetyl-l-leucyl-l-leucyl-l-norleucinal (N-ALL-N), and lactacystin were obtained from Sigma. Monoclonal antibodies (MAbs) 214 against BVDV E2 glycoprotein were purchased from the Veterinary Laboratories Agency (Weybridge, UK). The polyclonal anti-actin antibodies were from Sigma. The polyclonal anti-calnexin antibodies were purchased from Bioquote (York, UK). The anti-mouse and anti-rabbit horseradish peroxidase-conjugated secondary antibodies were from Amersham.
Infection of MDBK cells with BVDV. MDBK cells were grown to subconfluent monolayers in six-well plates and infected with cp BVDV at a multiplicity of infection (MOI) of 1 plaque forming units (PFU)/cell, for 1 h at 37 °C, as previously described [13]. After the inoculum was removed, the cells were washed with phosphate-buffered saline (PBS). The incubation continued for 9 h postinfection (pi) in RPMI 1640 medium. The cells were then treated or not with different inhibitors, as detailed in the figure legend, harvested after the times indicated and lysed in a buffer containing 0.5% Triton X-100, 50 mM Tris–Cl (pH 7.5), 150 mM NaCl, and 2 mM EDTA (Triton–TSE buffer).
Western blotting. Proteins separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) were transferred to nitrocellulose membranes using a semidry electroblotter (Millipore) and detected with anti-BVDV E2 antibodies (MAb 214, diluted 1/1000 in PBS containing 1% dry milk), followed by anti-mouse antibodies (dilution 1/2000) conjugated to horseradish peroxidase. In experiments in which detection of actin and calnexin was used as a loading control, polyclonal anti-actin, and anti-calnexin antibodies were used (dilution 1/4000). The proteins were detected using an enhanced chemiluminescence detection system (Amersham) according to manufacturer's instructions.
Pulse-labeling and chase. Subconfluent MDBK cell monolayers were infected with BVDV at MOI 1. After 1 h of incubation at 37 °C, the viral inoculum was removed, the cells were washed once with PBS and incubated in medium containing 10% fetal calf serum. Eighteen hours pi the cells were washed with PBS and incubated in starvation buffer. When the effect of either proteasome or the ER-mannosidase inhibitors was analyzed, the corresponding drugs were included in starvation buffer, at concentrations indicated in the figure legend. After 1 h, the cells were pulse-labeled at 37 °C with 100 μCi of Tran35S-label per ml, in the presence or absence of 5 mM DTT, for the times indicated in the figures. Following labeling, the isotope-supplemented medium was removed, the cells were washed once with PBS and chased for various times in RPMI 1640 medium containing 10 mM unlabeled methionine. At the time points indicated in the figures, 5 mM DTT was added to the chase medium and the cells were further incubated in the presence of the reducing agent for various times. After discarding the chase medium the radioactively labeled cells were harvested and lysed in Triton–TSE buffer, and a mixture of protease inhibitors, for 1 h on ice. In experiments in which the association of the BVDV glycoproteins into disulfide-linked dimers was monitored, 20 mM iodoacetamide was included in the lysis buffer to prevent nonspecific formation of disulfide bonds and aggregation.
Immunoprecipitation and SDS–PAGE. Labeled cell lysates were clarified by centrifugation at 12,000g for 15 min, and precleared with 20 μl of protein G–Sepharose, for 1 h at 4 °C. The lysates were briefly centrifuged and the supernatants incubated with anti-BVDV E2 MAb 214 antibodies (diluted 1:50) overnight at 4 °C. Protein G–Sepharose (30 μl) was then added to the supernatants and the incubation continued for 1 h at 4 °C. The slurry was washed six times with 0.2% Triton X-100 in TSE buffer and the immunoprecipitated complexes were eluted by boiling the samples for 10 min in SDS–PAGE sample buffer, in the presence (reducing conditions) or absence (nonreducing conditions) of 5% 2-mercaptoethanol and followed by SDS–PAGE. The gels were then treated with Amplifier (Amersham), dried and exposed at −70 °C to Hyperfilm-MP (Amersham). The intensity of the bands on the resulting autoradiograms was measured by scan densitometry.
Results
Synthesis and stability of BVDV envelope glycoproteins in the presence of DTT
An important event in the assembly of pestiviruses is thought to be the formation of native E1–E2 heterodimers, the major components of two pestiviruses: the classical swine fever virus (CSFV) and BVDV [5], [8], [14]. These complexes begin their association through intermolecular disulfide bonds in the ER, after the monomers have acquired intramolecular disulfide bonds and have been released from calnexin [11]. To explore the role of co- and post-translationally formed disulfide bonds on the steady-state level of the BVDV glycoproteins expressed in MDBK cells, we employed a method described by several laboratories, in which the membrane permeable reducing agent DTT is used to produce proteins lacking disulfide bonds [15], [16].
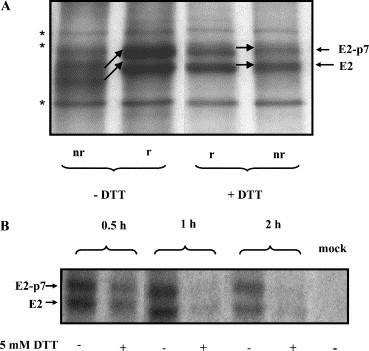
MDBK cells grown in monolayers were infected with BVDV. Five millimolar DTT was added to the cells 9 h pi, and synthesis of viral proteins was allowed to proceed in either presence or absence of the reducing agent for up to 2 h. The expression of E2 glycoproteins was analyzed by SDS–PAGE under reducing condition, followed by Western blotting. The MAb 214 antibodies, which recognize both the nonreduced and reduced forms of BVDV E2 glycoproteins [11], were used to detect the viral proteins. As expected, in untreated samples the E2/E2-p7 glycoproteins accumulated inside the cells due to continuous transcription and translation of viral RNA. Interestingly, the signal corresponding to E2/E2-p7 proteins in the DTT-treated samples decreased dramatically after only 0.5 h treatment and was almost completely lost at the end of the 2 h incubation period (Fig. 1A , upper panel). This suggests that either the biosynthesis of the E2/E2-p7 glycoproteins is impaired or the newly synthesized viral proteins are degraded in the presence of DTT, or that both events may contribute to the same effect. In the DTT-treated samples, the amount of E2/E2-p7 neither increases nor remains constant, which suggests that the viral proteins present in the cell prior to DTT incubation are also, to a certain extent, affected by the DTT treatment. As a sample loading control, detection of a soluble, cytoplasm-resident protein (actin), and a transmembrane, ER-localized protein (calnexin) was performed on the same Western blot (Fig. 1A, lower panel).
Fig. 1.
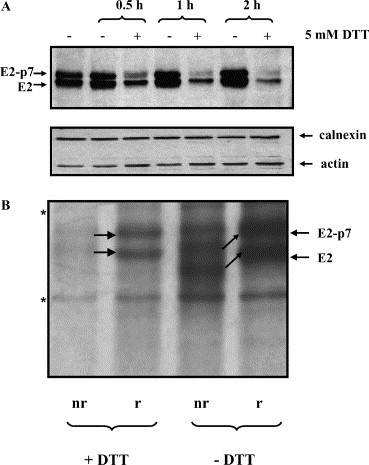
Biosynthesis of BVDV envelope glycoproteins in the presence of DTT. (A) Steady-state level of E2/E2-p7 glycoproteins in the presence of DTT. MDBK cells grown at 90% confluency were infected with BVDV at MOI 1. Nine hours pi cells were treated (+) or not (−) with 5 mM DTT for 0.5, 1, and 2 h before they were harvested and lysed. Equivalent amounts of whole-cell lysate (50 μg) were separated by SDS–10% PAGE under reducing conditions, transferred to an Immobilon membrane, and probed with anti-E2 MAb 214 (upper panel) or anti-actin and anti-calnexin antibodies (lower panel). (B) Effect of co-translational DTT treatment on the stability of E2/E2-p7 glycoproteins. MDBK cells were infected with BVDV as described above. Eighteen hours pi cells were pulse-labeled with [35S]methionine/cysteine for 15 min, in the presence (+) or absence (−) of 5 mM DTT. Cell lysates were immunoprecipitated with anti-E2 MAb 214 and bound proteins were analyzed by SDS–8% PAGE under nonreducing (nr) and reducing (r) conditions. The black arrows show the shift of the bands corresponding to the E2/E2-p7 glycoproteins run under nonreducing and reducing conditions. The bands marked with an asterisk represent unspecific contaminating proteins.
To investigate whether the observed reduction in the amount of BVDV proteins in the DTT-treated sample could be accounted for by a general decrease in total protein synthesis, a 0.5-h labeling of MDBK infected cells was performed in the presence and absence of the reducing agent. The rate of labeled amino acid incorporation into the cellular proteins was affected only to a minor extent in the DTT-treated samples (data not shown).
We have recently shown that the BVDV E2 glycoproteins acquire their intramolecular disulfide bonds co-translationally or immediately after the translation has been completed [11]. To determine if exogenously added DTT interferes with the formation of these rapidly acquired disulfide bonds, BVDV-infected MDBK cells were pulse-labeled for 15 min in the absence or continuous presence of DTT. The radioactively labeled viral proteins were then immunoprecipitated using MAb 214 and migrated on SDS–PAGE under nonreducing (nr) and reducing (r) conditions (Fig. 1B). In the untreated (−DTT) sample oxidation of the E2/E2-p7 glycoproteins occurs normally, as shown by the difference in the migration pattern between nonreduced and reduced samples, whereas the proteins pulsed in the presence of DTT showed an identical migration both under nonreducing and reducing conditions. Strikingly, more than 50% of the viral protein is lost in the presence of DTT, as revealed by scan densitometry analysis, suggesting that preventing the formation of disulfide bonds results in the synthesis of an unstable protein that is rapidly targeted for degradation. The difference in the intensities of the bands corresponding to the E2/E2-p7 glycoproteins run under nonreducing and reducing conditions is due to a more efficient elution of the bound proteins from protein G–Sepharose beads under reducing conditions.
The E2/E2-p7 monomers are susceptible to DTT-induced degradation
Having established that prevention of disulfide bond formation on the nascent viral polypeptides results in the biosynthesis of unstable, rapidly degraded proteins, we proceeded to determine whether the disulfides of the E2/E2-p7 monomers that have already folded would be accessible to DTT. BVDV-infected MDBK cells were pulse-labeled for 15 min and chased for 15 min in the absence of the reducing agent to allow normal folding and formation of the disulfide bonds to occur. After this initial chase, 5 mM DTT was added to the cells and the chase continued for additional 15 min. The cells were lysed, immunoprecipitated with MAb 214 and bound proteins were analyzed by SDS–PAGE under nonreducing and reducing conditions (Fig. 2A ). We have previously shown that within the first 30 min post-translation, the E2/E2-p7 glycoproteins exist mainly in the form of monomers, which will only later begin to associate into E2–E2 homodimers and E1–E2 heterodimers [11]. Therefore, within the 15 min of post-translational DTT treatment only the monomers will be exposed. As shown in Fig. 2A, the E2/E2-p7 monomers are oxidized normally (compare lanes nr and r, −DTT) in the untreated sample, while there is no difference in the migration pattern of the E2/E2-p7 proteins between the nonreduced and the reduced sample in DTT-treated samples. This suggests that the disulfide bonds of the E2/E2-p7 monomers were accessible to and reduced by DTT. Moreover, the intensity of the bands corresponding to the BVDV glycoproteins was reduced by approximately 25% in the presence of DTT, as compared to untreated sample, suggesting that reduced monomers are in an unstable conformation and therefore targeted to degradation. To investigate further the kinetics of this degradation we extended the chase for 0.5, 1, and 2 h. As shown in Fig. 2B, the amount of viral proteins immunoprecipitated with MAb 214 decreased dramatically throughout the chase in the DTT-treated samples, suggesting that the reduced monomers were unable to oligomerize and accumulate in the ER, and were targeted for degradation instead.
Fig. 2.
Stability of the E2/E2-p7 monomers in the presence of DTT. MDBK cells were infected with BVDV at MOI 1. Eighteen hours pi cells were pulse-labeled with [35S]methionine/cysteine for 15 min and chased for 15 min in the absence of DTT. After this initial chase, 5 mM DTT was added (+) or not (−) to the cells and the incubation continued for additional 15 min (A) or for the times indicated (B). After the chase, the cells were harvested, lysed, and immunoprecipitated with anti-E2 MAb 214. Proteins bound were separated by SDS–8% PAGE under both nonreducing (nr) and reducing (r) conditions (A), or reducing conditions only (B). The black arrows show the shift of the bands corresponding to the E2/E2-p7 glycoproteins run under nonreducing and reducing conditions. The bands marked with an asterisk represent unspecific contaminating proteins.
Degradation of reduced BVDV envelope glycoproteins is mediated by proteasome and depends on mannose trimming in the ER
An efficient “quality control system” operates in the ER to ensure that incorrectly folded proteins are selectively targeted to, and degraded by the proteasome [17]. To investigate whether the BVDV E2 glycoproteins synthesized in the presence of DTT were targeted to the proteasome, we followed the effect of two potent proteasome inhibitors, lactacystin and N-ALL-N, on the DTT-induced degradation of E2/E2-p7 glycoproteins. Western blot analysis showed that both proteasome inhibitors markedly blocked viral protein degradation, with lactacystin having the greatest effect (Fig. 3A ).
Fig. 3.
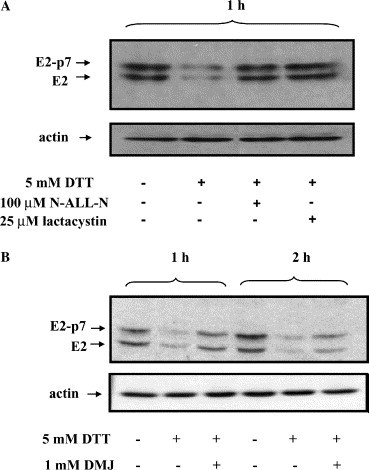
Steady-state level of reduced E2/E2-p7 glycoproteins in the presence of proteasome and ER-mannosidase inhibitors. MDBK cells grown at 90% confluency were infected with BVDV at MOI 1. Nine hours pi cells were incubated with 5 mM DTT for 1 h in the presence (+) or absence (−) of the proteasome inhibitors N-ALL-N (100 μM) and lactacystin (25 μM) (A) and for 1 and 2 h in the presence (+) or absence (−) of the ER-mannosidase inhibitor DMJ (1 mM) (B). After the incubation time indicated, cells were lysed separated by SDS–8% PAGE under reducing conditions, transferred to an Immobilon membrane, and probed with anti-E2 MAb 214 (upper panels A and B) or anti-actin antibodies (lower panels A and B).
It is thought that under physiological conditions the degradation of misfolded proteins takes place after a certain delay from synthesis, during which time mannose trimming occurs in the ER and persistently unfolded proteins are marked for degradation [17], [18]. However, the degradation of reduced BVDV E2/E2-p7 glycoproteins follows a rather unprecedented kinetic (Fig. 2B). We therefore attempted to determine whether glycan processing by the ER-mannosidases is also involved in this fast degradation. An experiment similar to that described in Fig 3A was performed and DMJ was used to inhibit the ER-mannosidase activity [19]. As shown in Fig. 3B, the inhibitor greatly suppressed the proteasome-associated degradation of reduced E2/E2-p7 proteins confirming that removal of mannose from asparagine-linked oligosaccharides precedes the viral protein proteolysis.
Dimerization of E2/E2-p7 glycoproteins determines protection against DTT action and degradation
DTT when added either co-translationally or immediately after the E2/E2-p7 monomers have formed, has a great effect on viral protein stability and promotes degradation. We further wanted to determine whether dimerization of the envelope proteins would have any effect in protecting them from reduction of the disulfide bonds by DTT.
A pulse-chase experiment was performed on BVDV-infected MDBK cells. Cells were labeled for 15 min and chased for 1.5 h in the absence of DTT, during which time most of the E1 and E2 glycoproteins associate into homo- and heterodimers [11]. Then 5-mM DTT was added to the cells and the chase continued for 1 h in presence of the reducing agent. The cells were lysed, immunoprecipitated with MAb 214 and the bound proteins were analyzed by SDS–PAGE under nonreducing and reducing conditions (Fig. 4 ). Interestingly, the intensity of the bands corresponding to E2/E2-p7 monomers was reduced by approximately 50% in the DTT-treated sample, as compared to only 10% reduction of the bands corresponding to the E1/E2 heterodimers (see nr lanes, ∓DTT). To better estimate the degree of the overall degradation of the E2/E2-p7 glycoproteins, we compared the intensities of the corresponding bands under reducing conditions (see r lanes, ∓DTT). The scan densitometry analysis showed that only a minor proportion of the E2/E2-p7 proteins was degraded (approximately 20%) after 1 h DTT treatment post-dimer formation. In contrast, 1 h DTT treatment of the E2/E2-p7 monomers resulted in a significant degradation (see Fig. 2B). Therefore, once the envelope proteins have assembled into dimers, the conformation of the complex is such that the disulfide bonds become resistant to disruption by DTT.
Fig. 4.
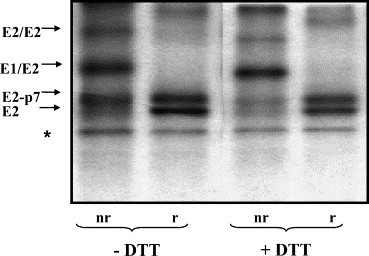
Effect of DTT treatment on the stability of BVDV envelope proteins, after their assembly into dimers. MDBK cells were infected with BVDV at MOI 1. Eighteen hours pi the cells were pulse-labeled with [35S]methionine/cysteine for 15 min and chased for 1 h in the absence of DTT. After this initial chase, 5 mM DTT was added (+) or not (−) to the cells and the incubation continued for 1 h. The cells were lysed and immunoprecipitated with anti-E2 MAb 214. Proteins bound were separated by SDS–10% PAGE under nonreducing (nr) and reducing (r) conditions. The bands marked with an asterisk represent nonspecific contaminating proteins.
Discussion
The assembly process of viral glycoproteins into functional oligomeric structures is part of a complex pathway that starts with the folding of the monomeric polypeptide components in the ER of the host cell, under the stringent “quality control” performed in this organelle [20], [21].
The BVDV E1–E2 heterodimer, which represents the major and probably the functional component of mature virions is covalently linked via disulfide bonds [5]. The rate limiting step in the assembly of the heterodimer is the folding of E1 which occurs slowly, while E2 undergoes very rapid and presumably co-translational intramolecular oxidation in the ER. Only when E1 has been released from the interaction with calnexin, the two glycoproteins can interact to form a disulfide bond-linked heterodimer [11].
In this paper, we have investigated the contribution of co- and post-translationally formed disulfide bonds to the folding and maturation of BVDV envelope glycoproteins. The reducing agent DTT was chosen as the tool to interfere with disulfide bonding at different steps of protein folding and BVDV maturation [15].
After synthesis in the presence of DTT, several viral proteins such as influenza haemaglutinin (HA) and the coronavirus S glycoprotein are able to acquire conformation-sensitive epitopes and are transport competent [15], [22]. In our system, DTT treatment during translation led to a massive reduction in the amount of viral protein accumulated during the pulse, suggesting that formation of co-translational disulfide bonds is crucial for achieving a stable conformation of the E2/E2-p7 glycoproteins. Failure to form these bonds could lead to abnormally folded molecules, which are rapidly degraded. To determine the fate of the E2/E2-p7 monomers when their assembly and oligomerization is prevented we performed a post-pulse DTT treatment experiment. This approach allowed proper oxidation of the monomers but prevented any further disulfide bond formation. It has been shown that several viral as well as cellular proteins acquire DTT resistance after some folding has taken place [15], [23]. Interestingly, reduction of the disulfide bonds in the BVDV E2/E2-p7 monomers and prevention of the oligomerization process resulted in their degradation. This result suggests that at this stage of the envelope protein biosynthesis, their folding is not complete. Therefore, the disruption of the disulfide bonds before final folding has achieved results in a disordered structure similar to that found when formation of the bonds is inhibited co-translationally. This confirms the earlier result that co-translationally formed disulfide bonds are crucial for the subsequent folding of BVDV E2/E2-p7 glycoproteins into their native forms.
This idea is supported by our data using proteasome inhibitors. N-ALL-N and lactacystin strongly inhibited the degradation of reduced E2/E2-p7, suggesting that DTT-induced modifications of the E2/E2-p7 proteins lead to them being recognized as misfolded by the ER “quality control” and targeted to proteasome, like other misfolded proteins [24], [17]. This hypothesis was further confirmed using an ER-mannosidase inhibitor that prevents formation of Man8GlcNAc2 glycoproteins, a prerequisite for targeting misfolded glycoproteins to proteasome-mediated degradation. Indeed, our data show that inhibition of ER mannosidases by DMJ greatly impaired degradation of E2/E2-p7 directly involving this activity in regulating the disposal of unfolded BVDV envelope glycoproteins.
An interesting finding emerging from this work is that DTT has very little effect on the E2/E2-p7 glycoproteins after they have assembled into dimers. In other words, dimerization results in a conformation that renders the disulfides inaccessible to DTT.
In conclusion, this study provides molecular evidence that disulfide bonds play a critical role in the folding and stability of the BVDV E2/E2-p7 glycoproteins. Forcing the viral proteins to adopt a reduced conformation at different stages of virus maturation process resulted in failure to acquire a stable conformation and led to proteasome-mediated degradation. It is interesting to note that the antiviral iminosugar N-butyl-deoxynojirimycin, an ER-α-glucosidase inhibitor, induces misfolding of BVDV E2/E2-p7 glycoproteins associated with at least one disulfide-bonded epitope [11]. Although in this case the viral proteins are not roughly misfolded and degraded in the ER, the assembly of the E1–E2 heterodimers is impaired, suggesting again that proper disulfide bonding is important for achieving a native conformation of the E2/E2-p7 monomers that would enable their subsequent assembly into functional heterodimers.
Acknowledgements
This work was supported by a Wellcome Trust International Research Development Award granted to Norica Branza-Nichita. Nicole Zitzmann is a Royal Society Dorothy Hodgkin Fellow and a Research Fellow of Wolfson College, Oxford.
References
- 1.Rice C.M. Flaviviridae: the viruses and their replication. In: Knipe D.M., Howley P.M., editors. Fields Virology. third ed. Lippincot-Raven Publishers; Philadelphia, PA: 1996. pp. 931–959. [Google Scholar]
- 2.Matsuura Y., Miyamura T. The molecular biology of hepatitis C virus. Sem. Virol. 1993;4:297–304. [Google Scholar]
- 3.Grakoui A., Lin Wychowski C., Feinstone S.M., Rice C.M. Expression and identification of hepatitis C virus polyprotein cleavage products. J. Virol. 1993;67:1385–1395. doi: 10.1128/jvi.67.3.1385-1395.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baginski S.G., Pevear D.C., Seipel M., Sun S.C., Benetatos C.A., Chunduru S.K., Rice C.M., Collett M.S. Mechanism of action of a pestivirus antiviral compound. Proc. Natl. Acad. Sci. USA. 2000;97:7981–7986. doi: 10.1073/pnas.140220397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Durantel D., Branza-Nichita N., Carrouee-Durantel S., Butters T.D., Dwek R.A., Zitzmann N. Study of the mechanism of antiviral action of iminosugar derivatives against bovine viral diarrhea virus. J. Virol. 2001;75:8987–8998. doi: 10.1128/JVI.75.19.8987-8998.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collett M.S., Wiskerchen M.A., Welniak E., Belzer S.K. Bovine viral diarrhea virus genomic organization. Arch. Virol. Suppl. 1991;3:19–27. doi: 10.1007/978-3-7091-9153-8_3. [DOI] [PubMed] [Google Scholar]
- 7.Rumenapf T., Unger G., Strauss J.H., Thiel H.-J. Processing of the envelope glycoproteins of pestiviruses. J. Virol. 1993;67:3288–3294. doi: 10.1128/jvi.67.6.3288-3294.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weiland E., Stark R., Haas B., Rumenapf T., Meyers G., Thiel H.-J. Pestivirus glycoprotein which induces neutralizing antibodies forms part of a disulfide-linked heterodimer. J. Virol. 1990;64:3563–3569. doi: 10.1128/jvi.64.8.3563-3569.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ralston R., Thudium K., Berger K., Kuo C., Gervase B., Hall J., Selby M., Kuo G., Houghton M., Choo Q.L. Characterization of hepatitis C virus envelope glycoprotein complexes expressed by recombinant vaccinia viruses. J. Virol. 1993;67:6753–6761. doi: 10.1128/jvi.67.11.6753-6761.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dubuisson J., Rice C.M. Hepatitis C virus glycoprotein folding: disulfide bond formation and association with calnexin. J. Virol. 1996;70:778–786. doi: 10.1128/jvi.70.2.778-786.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Branza-Nichita N., Durantel D., Carrouee-Durantel S., Dwek R.A., Zitzmann N. Antiviral effect of N-butyldeoxynojirimycin against bovine viral diarrhea virus correlates with misfolding of E2 envelope proteins and impairment of their association into E1–E2 heterodimers. J. Virol. 2001;75:3527–3536. doi: 10.1128/JVI.75.8.3527-3536.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harada T., Tautz N., Thiel H.J. E2-p7 region of the bovine viral diarrhea virus polyprotein: processing and functional studies. J. Virol. 2000;74:9498–9506. doi: 10.1128/jvi.74.20.9498-9506.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zitzmann N., Mehta A., Carrouee S., Butters T.D., Platt F.M., McCauley J., Blumberg B.S., Dwek R.A., Block T.M. Iminosugars inhibit the formation and secretion of bovine viral diarrhea virus, a pestivirus model of hepatitis C virus: implications for the development of broad spectrum anti-hepatitis virus agents. Proc. Natl. Acad. Sci. USA. 1999;96:11878–11882. doi: 10.1073/pnas.96.21.11878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Konig M., Lengsfeld T., Pauly T., Stark R., Thiel H.J. Classical swine fever virus: independent induction of protective immunity by two structural glycoproteins. J. Virol. 1995;69:6479–6486. doi: 10.1128/jvi.69.10.6479-6486.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Braakman I., Helenius J., Helenius A. Manipulating disulfide bond formation and protein folding in the endoplasmic reticulum. EMBO J. 1992;11:1717–1722. doi: 10.1002/j.1460-2075.1992.tb05223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tatu U., Braakman I., Helenius A. Membrane glycoprotein folding, oligomerization, and intracellular transport: effects of dithiothreitol in living cells. EMBO J. 1993;12:2151–2157. doi: 10.1002/j.1460-2075.1993.tb05863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ellgaard L., Helenius A. ER quality control: towards an understanding at the molecular level. Curr. Opin. Cell Biol. 2001;13:431–437. doi: 10.1016/s0955-0674(00)00233-7. [DOI] [PubMed] [Google Scholar]
- 18.Helenius A., Aebi M. Intracellular functions of N-linked glycans. Science. 2001;291:2364–2369. doi: 10.1126/science.291.5512.2364. [DOI] [PubMed] [Google Scholar]
- 19.Elbein A.D. Glycosidase inhibitors: inhibitors of N-linked oligosaccharide processing. FASEB J. 1991;5:3055–3063. doi: 10.1096/fasebj.5.15.1743438. [DOI] [PubMed] [Google Scholar]
- 20.Doms R.W., Lamb R.A., Rose J.R., Helenius A. Folding and assembly of viral membrane proteins. Virology. 1993;193:545–562. doi: 10.1006/viro.1993.1164. [DOI] [PubMed] [Google Scholar]
- 21.Bergeron J.J.M., Brenner M.B., Thomas D.Y., Williams D.B. Calnexin: a membrane-bound chaperone of the endoplasmic reticulum. Trend. Biochem. Sci. 1994;19:124–129. doi: 10.1016/0968-0004(94)90205-4. [DOI] [PubMed] [Google Scholar]
- 22.Opstelten D.E., DeGroote P., Horzinek M.C., Vennema H., Rottier P.J.M. Disulfide bonds in folding and transport of mouse hepatitis corobavirus glycoproteins. J. Virol. 1993;67:7394–7401. doi: 10.1128/jvi.67.12.7394-7401.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Negroiu G., Dwek R.A., Petrescu S.M. Folding and maturation of tyrosinase-related protein-1 are regulated by the post-translational formation of disulfide bonds and by N-glycan processing. J. Biol. Chem. 2000;275:32200–32207. doi: 10.1074/jbc.M005186200. [DOI] [PubMed] [Google Scholar]
- 24.Klausner R.D., Sitia R. Protein degradation in the endoplasmic reticulum. Cell. 1990;62:611–614. doi: 10.1016/0092-8674(90)90104-m. [DOI] [PubMed] [Google Scholar]


