Summary
Early mortality syndrome (EMS) in cultivated shrimp is of complex aetiology. One of the causes is acute hepatopancreatic necrosis disease (AHPND) caused by unique Vibrio isolates that carry two Pirvp toxin genes, but other causes of EMS remain mostly unexplained. Here, we describe the discovery of a Shewanella isolate TH2012T from an EMS/AHPND outbreak pond and demonstrate its virulence for shrimp (the mean lethal concentration of 105 colony‐forming units per millilitre by immersion challenge) accompanied by distinctive histopathology, particularly of the ventral nerve cord and lymphoid organ but also including the digestive tract. On the basis of its complete genome sequence, multilocus phylogenetic trees, digital DNA–DNA hybridization analysis and differential phenotypic characteristics, we propose that Shewanella isolate TH2012T represents a novel species, separated sufficiently from the type strains S. litorisediminis and S. amazonensis to justify naming it Shewanella khirikhana sp. nov. Analysis of the TH2012T genome revealed no homologues of the Pirvp toxin genes but revealed a number of other potential virulence factors. It constitutes the first Shewanella isolate reported to be pathogenic to shrimp.
A new species, Shewanella khirikhana has been isolated from shrimp during the early mortality syndrome (EMS) in 2012 in Thailand. It causes distinctive histopathological lesions at the hepatopancreas, which is the same target with EMS/AHPND‐ inducing bacteria (VP‐AHPND) resulting in severe mortality of shrimp in the infected pond.
Introduction
The history and current status of early mortality syndrome (EMS) and its component acute hepatopancreatic necrosis disease (AHPND) have recently been reviewed (Thitamadee et al., 2016; Prachumwat et al., 2019). Briefly, outbreaks began in China around 2009 and spread to several countries in Asia (Thitamadee et al., 2016; Prachumwat et al., 2019) before reaching Mexico (Nunan et al., 2014) in 2013. More recently, outbreaks have been reported from the Philippines (De La Peña et al., 2015), Australia (World Organization for Animal Health, 2016) and the USA (Dhar et al., 2018). The AHPND component of EMS is the most severe bacterial disease so far reported for cultivated shrimp and has caused massive production losses since 2009. In 2013, the cause of AHPND was reported to be unique isolates of Vibrio parahaemolyticus (VPAHPND) that produced Pirvp toxins A and B from genes on a conjugative plasmid called pVA (Tran et al., 2013; Lee et al., 2015; Sirikharin et al., 2015). AHPND accounts for only a portion of the disease outbreaks that shrimp farmers collectively call early mortality syndrome (EMS) (Sanguanrut et al., 2018) and so cannot be equated with EMS that shrimp farmers use to refer to any early mortality whatever the cause (Thitamadee et al., 2016). Thus, all reports of putative AHPND outbreaks that arise by equating EMS with AHPND must be considered scientifically unreliable, unless they are accompanied by confirmation in the form of evidence for pathognomonic AHPND lesions, PCR detection of VPAHPND or immunological detection of Pirvp toxins A and B. At the same time, EMS outbreaks not caused by AHPND remain unexplained and possible causes could include other bacterial species.
At the same time, variation in the virulence of VPAHPND isolates has been reported (Joshi et al., 2014; Lai et al., 2015) but no basis for it has so far been clearly identified. It has been suggested that such variation may be due to other VPAHPND virulence factors that may or may not be carried by the pVA plasmid (Sirikharin et al., 2015; Tinwongger et al., 2016; Han et al., 2017). In addition, other species of Vibrio have been reported to carry pVA plasmids, produce Pirvp toxins A and B and cause pathognomonic AHPND lesions (Kondo et al., 2015; Liu et al., 2015; Han et al., 2017). Even more recently, archived isolates of Vibrio campbellii from Thailand in 2002 (named V. harveyii at the time of their isolation) have been reported to produce Pirvp toxins A and B and to cause AHPND (Wangman et al., 2018). However, before the discovery of VPAHPND, it was found that some bacterial genera not previously associated with shrimp disease occurred at a higher proportion in EMS ponds than in normal ponds (Prachumwat et al., 2012; FAO, 2013). Thus, after the discovery of VPAHPND, it was hypothesized that non‐AHPND isolates previously obtained from AHPND outbreak ponds might have the ability to potentiate the virulence of a VPAHPND isolate (FAO, 2013). Results of the study revealed that the cause of mortality in some EMS ponds was unknown (Sanguanrut et al., 2018), and reports on wide variation in the virulence of AHPND isolates lent some support to this 2013 hypothesis. Thus, in a continued effort to test the hypothesis, we have been screening other bacterial isolates that have been obtained from EMS/AHPND ponds. Recently, we reported the complete genome of one such Thai isolate TH2012T with a high similarity of 16S rRNA to those of species in the genus Shewanella (Wechprasit et al., 2019). Here, we demonstrate that TH2012T itself is lethal to juvenile Pacific whiteleg shrimp Penaeus (Litopenaeus) vannamei in laboratory challenges, and we reveal by full genome analysis that it is a new species of the genus Shewanella with a number of potential virulence factor genes.
Results
Organism information, genome, taxonomic classification and phenotypic features
Bacterial isolate TH2012T was obtained together with V. parahaemolyticus AHPND (VPAHPND) isolates 3HP and 5HP (Joshi et al., 2014) from the hepatopancreas of moribund shrimp specimens obtained from a shrimp pond experiencing an EMS/AHPND outbreak. TH2012T was initially identified tentatively based on the very high sequence identity of a 762‐nucleotide 16S rRNA PCR amplicon to matching sequences from species in the genus Shewanella. The complete genome sequence and genomic annotation of TH2012T have been previously described (see Supporting information) (Wechprasit et al., 2019). Details of its taxonomic classification and phenotypic features are described below.
Phylogenetic analysis
A neighbour‐joining tree constructed from the concatenated multiple sequence alignments of 16S rRNA, atpA, mreB and rpoA genes revealed that TH2012T resided in a clade (bootstrap re‐sampling value 100%) together with other two Shewanella species (three strains shown in Fig. 1). The tree placed TH2012T as a sister taxon of a subclade containing S. litorisediminis SMK1‐12T and TBRC 5001 and S. amazonensis SB2BT (bootstrap 100%). Similarly, the placement of TH2012T as a sister taxon with a subclade containing SMK1‐12T, TBRC 5001 and SB2BT was also recovered by all of the other three algorithms (the minimum evolution, maximum‐likelihood and maximum‐likelihood algorithms; Fig. 1). The same topology of TH2012T and the other three strains was also observed in individual trees of atpA, mreB and rpoA genes (Figs S3, S4 and S5). However, different topologies of these four strains were observed in trees of 16S rRNA genes (Figs S1 and S2). All trees of 16S rRNA sequences revealed that TH2012T was most closely linked to the type strain of S. amazonensis SB2BT and then clustered with the type strain of S. litorisediminis SMK1‐12T. Among these 16S rRNA trees, TBRC 5001 was clustered with TH2012T or S. amazonensis SB2BT (depending on algorithms) but with lower bootstrap values. Our phylogenetic tree analysis further suggested that TH2012T and other two isolates with partial 16S rRNA sequences (S. sp. AK55 and S. litorisediminis LV 5) might be representatives of a single species (Fig. S2). Nucleotide identities of TH2012T to homologous sequences in the other strains were 98.4–100% for 16S rRNA and 91–97% for atpA, mreB and rpoA genes (Table S2).
Figure 1.
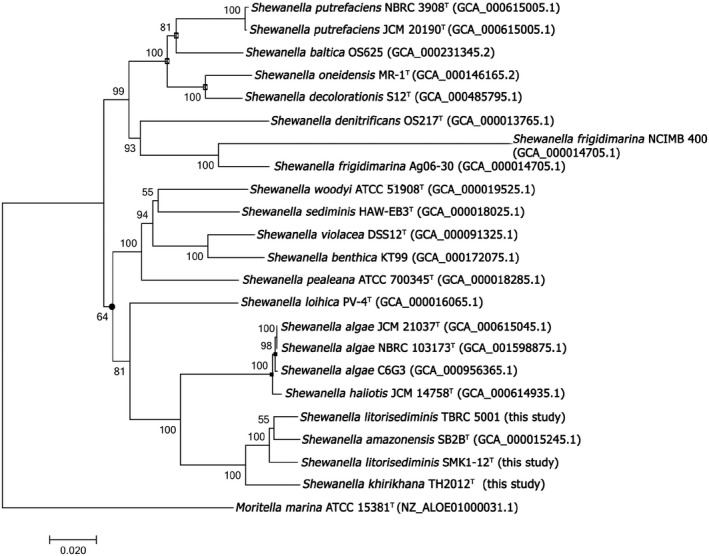
Phylogenetic positions of S. khirikhana TH2012T and other Shewanella species. The neighbour‐joining phylogenetic tree with Jukes–Cantor distance was based on 4290 aligned positions of concatenated 16S rDNA, atpA, mreB and rpoA gene sequences. Moritella marina ATCC 15381 was used as an outgroup. Bootstrap values (expressed as percentages of 1000 replications) are shown at branching points. Most of the nodes were also recovered in the trees generated with the minimum evolution (Jukes–Cantor distance), maximum‐likelihood (Jukes–Cantor distance) and maximum parsimonious algorithms based on the same set of sequences, except those with Filled circles, Opened squares or Filled squares that were not recovered in the trees of maximum parsimonious, maximum‐likelihood, or both maximum parsimonious and maximum‐likelihood algorithms respectively. The scale bar indicates estimated sequence divergence (substitutions per nucleotide position). Sequence accession numbers are shown in the parentheses after the species names.
DNA–DNA relatedness
Using complete genomes of TH2012T (Wechprasit et al., 2019) and S. amazonensis SB2BT to calculate dDDH similarities (see Experimental Procedures), it was found that the dDDH value was 25.2% (model 95% C.I. 22.9–27.7%). This value suggested that TH2012T and SB2BT were isolates of different species based on the recommendation that an dDDH > 70 % should be obtained for isolates belonging to the same species (Meier‐Kolthoff et al., 2013). The genomic G + C contents of TH2012T and SB2BT were 54.80% and 53.58% respectively. This difference in genomic G + C content of 1.22% also indicates that TH2012T and SB2BT are different species. Furthermore, the average nucleotide identity (ANI) scores between the complete genomes of TH2012T and SB2BT were 81.7%‐81.8%. These ANI scores supported the proposal that TH2012T and SB2BT are different species, according to a cut‐off at ANI > 95% identity for species boundary separation (Goris et al., 2007).
Morphological and physiological characteristics of TH2012T
TH2012T cells are free‐living, Gram‐negative, straight motile rods, and their colonies on TSA + 1.5% NaCl are yellowish in colour, translucent, circular, smooth and convex with entire edges, but with no evidence of spore formation (Figs S6 and S7). Cells from broth culture measured 0.58 ± 0.05 × 2.02 ± 0.38 µm (n = 50) in Gram‐stained smears (Fig. S6B). We tested various parameters (temperatures, pH and % NaCl) on TH2012T growth (see Supporting information) as it has been cultured in TSB supplemented by 1.5% (w/v) NaCl at 30°C in our laboratory. We found that TH2012T could grow at temperatures 25–37°C, with supplementation of 0.5–5% (w/v) NaCl (0% NaCl was not tested), and over a pH range of 6.5–9.5, but could not grow at 4 and 42°C, with 6–8% (w/v) NaCl or at pH of 6. Since TSB already contained 0.5% NaCl, TH2012T could grow in supplementation with 0–5% NaCl, resulted in medium finally containing 0.5–5.5% (w/v) NaCl. Its optimal growth occurred at 30°C, pH 7–7.5 and 1.5–2% (w/v) NaCl (i.e. TSB supplemented 1–1.5% NaCl), and this constituted our routine laboratory culture conditions (Table S5, Figs S8 and S9). It died when stored at 4°C, so refrigerated storage is not recommended. TH2012T produced H2S, showed oxidase activity and could reduce nitrate to nitrite but did not ferment glucose and citrate and did not show ornithine decarboxylase activity. In addition, it could hydrolyse Tween 20, gelatin and casein but not urea. S. amazonensis SB2BT (= ATCC 700329T) also had similar characteristics to TH2012T for the above investigated features, but lacked information on urease activity and gave conflicting reports on ability to ferment d‐glucose and citrate (Venkateswaran et al., 1998; Lee and Yoon, 2012). S. litorisediminis SMK1‐12T (= KCTC 23961T) had similar features as TH2012T and SB2BT but was reported as non‐motile with capability to ferment d‐glucose (Lee and Yoon, 2012). However, we found that KCTC 23961T (= SMK1‐12T) could not ferment d‐glucose and did not show ornithine decarboxylase activity. The full phenotypic characteristics of TH2012T in comparison with SMK1‐12T and SB2BT are given in Table 1. TH2012T showed exponential growth from the end of the first to the fourth hours of liquid culture with OD600 increasing from 0.1 to 2.5, respectively, and with viable cell counts of 107 to 109 CFU ml−1 at the end of the second and fourth hours respectively (Figs S10 and S11).
Table 1.
Differential phenotypic characteristics of Shewanella khirikhana TH2012T, S. litorisediminis SMK1‐12T (= KCTC 23961T) and S. amazonensis SB2BT (= ATCC 700329T).
| Characteristics | Shewanella | ||
|---|---|---|---|
| khirikhana TH2012T | litorisediminis SMK1‐12T a | amazonensis SB2BT b | |
| Colony pigment colour | Yellowish | Yellowish | Pinkish |
| Gram staining | − | − | − |
| Motility | + | − | + |
| Shape | Rod | Rod | Rod |
| Ornithine decarboxylase | − | nd/+c | − |
| H2S production | + | + | + |
| Oxidase test | + | + | + |
| Reduction nitrate to nitrite | + | + | + |
| Urea hydrolysis | − | − | nd |
| Fermentation of | |||
| D−glucose | − | +/−c | −/+d |
| Citrate | − | − | +/−d |
| Hydrolysis of Tween 20 | + | + | + |
| Enzyme activity | |||
| Caseinase | + | +/ndc | nd/+d |
| Gelatinase | + | + | + |
+, positive; −, negative; nd, no data.
Data based on both (Lee and Yoon, 2015) and this study.
The conflicting data between (Lee and Yoon, 2015)/this study (KCTC 23961T) or not determined in this study (KCTC 23961T).
The conflicting data between (Venkateswaran et al., 1998)/(Lee and Yoon, 2015) (ATCC 700329T) or not reported in (Venkateswaran et al., 1998); All three strains were negative for fermentation of L‐arabinose ((Lee and Yoon, 2015) and this study), but TH2012T was also negative for fermentation of d‐mannitol, inositol, d‐sorbitol, l‐rhamnose, d‐sucrose, d‐melibiose and amygdalin.
TH2012T satisfies Koch’s postulates as a potential shrimp pathogen
In shrimp immersion challenge tests with TH2012T at 104 to 106 CFU ml−1, mortality was first observed at 48 hpi and reached 100% by 72 hpi at all concentrations of TH2012T except 104 that gave 100% mortality at 84 hpi. There was no mortality within 96 hpi in the unchallenged, negative control group consisting of untreated shrimp. Thus, the mean lethal concentration (LC50) for 60 h exposure to TH2012T was estimated to be ~ 105 CFU ml−1 (Fig. S12). Histological analysis was carried out with the control and test shrimp to screen for any pathognomonic histopathology (see below).
To satisfy Koch’s postulates, stomach and HP tissues from the replicated challenge tests above were aseptically and individually removed from moribund shrimp, homogenized and used as an inoculum in selective TSB for re‐isolation of S. khirikhana TH2012T. From TSB, the turbid culture broth was streaked on selective TSA, and colonies with appropriate morphology were re‐streaked on fresh selective TSA. One such isolate, confirmed as S. khirikhana TH2012T by 16S rRNA PCR followed by amplicon sequencing analysis and chemical characteristics with API 20E biochemical strip tests (data not shown), was used to prepare inoculum for an additional immersion challenge test of 105 CFU ml−1 in the challenge group along with an untreated control group (see Experimental Procedures). From this second challenge series to confirm Koch’s postulates, the results showed that TH2012T caused 100% mortality within 141 hpi. The same unique histopathological lesions were observed for both this and the first series of bacterial challenge tests (see below).
Clinical signs of TH2012T challenged shrimp
Similar to AHPND diseased shrimp (Lightner et al., 2012; Naca, 2012; Tran et al., 2013), TH2012T‐infected shrimp showed lethargy and swimming in a slow movement or in a spiral direction. Unlike AHPND diseased shrimp, TH2012T‐infected shrimp had visibly white abdominal muscle but did not show other clinical signs of AHPND such as empty or interrupted gut contents, pale‐to‐white hepatopancreata (HP) and significant atrophy of HP.
TH2012T kills shrimp with unique histopathology but no pathognomonic AHPND lesions
Altogether, 40 shrimp were examined from three challenge experiments carried out independently with separate sources of shrimp. Shrimp examined in the first two challenge experiments (total 15 each) included three control shrimp and 12 moribund shrimp (four each from immersion challenges at 104 to 106 CFU ml−1), whereas in the third experiment (total 10 shrimp), three control shrimp and seven moribund shrimp (three each from immersion challenges 104 and 105 CFU ml−1 and only one from immersion challenge 106 since all others from that group had died before they could be retrieved for fixation while still alive).
From the three challenge experiments, none of the shrimp in the control (total nine examined) or in the immersion challenge groups (total 31 examined) showed the pathognomonic lesions of acute AHPND characterized by massive sloughing of the HP tubular epithelial cells (Lightner et al., 2012; Naca, 2012; Tran et al., 2013). The most consistent and prominent histopathological abnormality in the moribund shrimp challenged by immersion exposure to TH2012T were bacteria‐free lesions in the ventral nerve cord that were absent in all nine control shrimp examined. These lesions consisted of abnormal nerve cord nuclei distorted in the two‐dimensional sections into variable shapes somewhat resembling jigsaw puzzle pieces (‘jigsaw‐piece nuclei’ or JPN) (Fig. 2A–E) and/or highly vacuolated (‘foamy’) cytoplasm in the giant nerve cells (FGC) of associated nerve ganglia (Fig. 3). Out of the 31 moribund shrimp tissue sections examined, two did not include ventral nerve cord tissue, but all of the remaining 29 (100%) showed JPN and/or FGC. We propose this previously unreported feature of JPN to be used as a strong presumptive indicator pathognomonic for disease caused by S. khirikhana. Inspection of JPN with the oil emersion lens suggested that the misshapen nuclei were caused by pressure from cytoplasmic vacuoles (Fig. 2F). Whether or not any of these vacuoles are intra‐nuclear would need to be resolved by using transmission electron microscopy. The histological features described above could be clearly visualized using a light microscope with a 40x objective. They indicated a severe negative response to S. khirikhana challenge in the shrimp nervous system, and the absence of bacterial cells in the lesions suggested the possibility that this response involved a causal neurotoxin.
Figure 2.
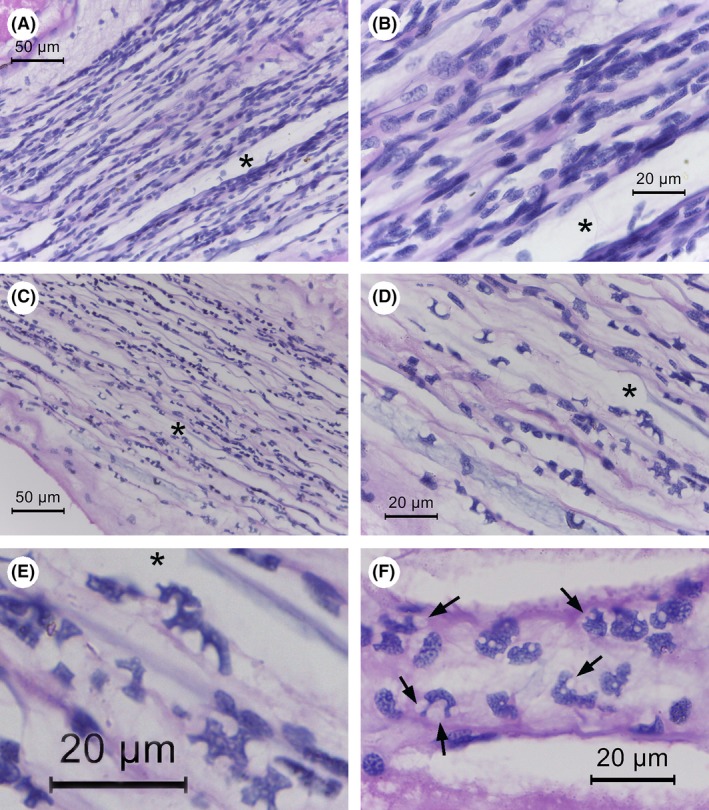
Photomicrographs of ventral nerve cord histology of normal and moribund shrimp from S. khirikhana TH2012T challenge tests. Asterisks (*) indicate the same location in photomicrographs at different magnifications.
A and B. A control shrimp showing normal nerve cord histology at low and high magnification respectively.
C, D and E. Moribund shrimp histopathology at progressively higher magnifications showing irregularly shaped nuclei reminiscent of scattered jigsaw puzzle pieces (JSN).
F. Example of JSN in a section where pressure from adjacent vacuoles appears to be the cause of their abnormal shapes (arrows).
Figure 3.
Photomicrographs of ventral nerve cord ganglia of normal and moribund shrimp from S. khirikhana TH2012T challenge tests. Asterisks (*) indicate the same location in photomicrographs at different magnifications.
A and B. A control shrimp showing normal nerve cord histology at low and high magnification, respectively.
C–F. Moribund shrimp histopathology at progressively higher magnifications showing highly vacuolated cytoplasm of giant nerve cells.
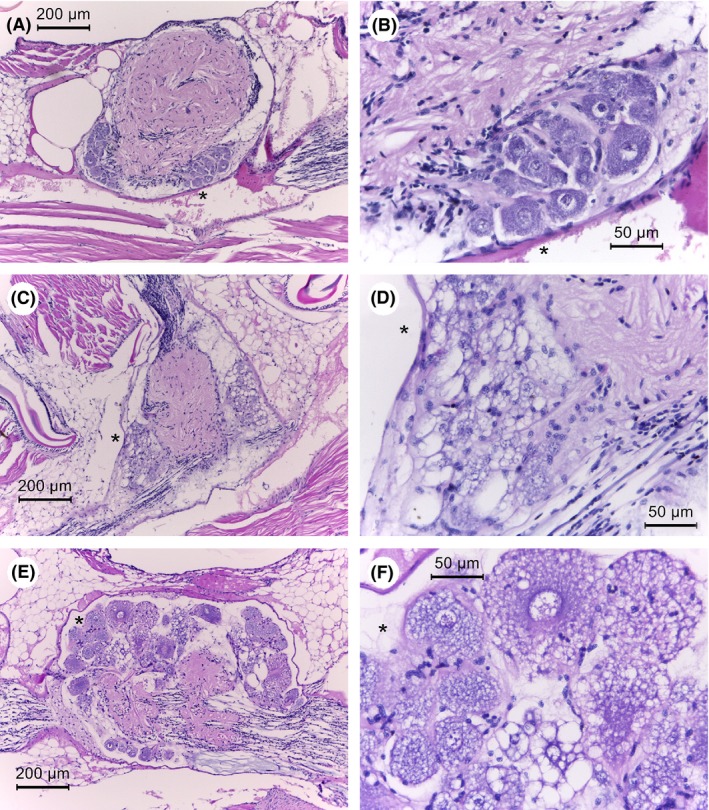
The next most consistent lesion in the 31 moribund shrimp (absent in the control shrimp and also clearly visualized using a light microscope with a 40x objective) was the abnormal presence of cytoplasmic vacuoles in the tubule matrix cells of the lymphoid organ (LO) with bacteria absent for 25/26 (96%) of the specimens with LO tissue in their sections (Fig. 4). This gave rise to a marked decrease in the normal nuclear density of the LO tubule matrices that was clearly visible using a light microscope with a 40x objective. Many of these vacuoles contained eosinophilic inclusions of variable shape but smaller than the adjacent nuclei. In most cases, the interstitial spaces between the tubules also contained abnormal vacuoles with eosinophilic inclusions. Also, in some specimens these features were accompanied by scattered pyknotic and karyorrhectic nuclei (8/27 specimens). All seven control shrimp sections that included LO (i.e. two sections without LO) showed normal LO histology (Fig. 4). For the two moribund shrimp specimens whose sections lacked nervous tissue (see above) both contained LO tissue that showed LO lesions. Thus, combined examination of both the nervous and LO tissues for lesions was sufficient to yield a positive presumptive diagnosis result for disease caused by S. khirikhana in all 31 of the moribund shrimp specimens examined. There was no indication of accompanying LO spheroid formation as is frequently reported during the shrimp response to viral and bacterial pathogens (Hasson et al., 1999; Anggraeni and Owens, 2000; Van De Braak et al., 2002; Owens, 2010). It is possible that the loss of LO function was prevented or too rapid to allow for spheroid formation.
Figure 4.
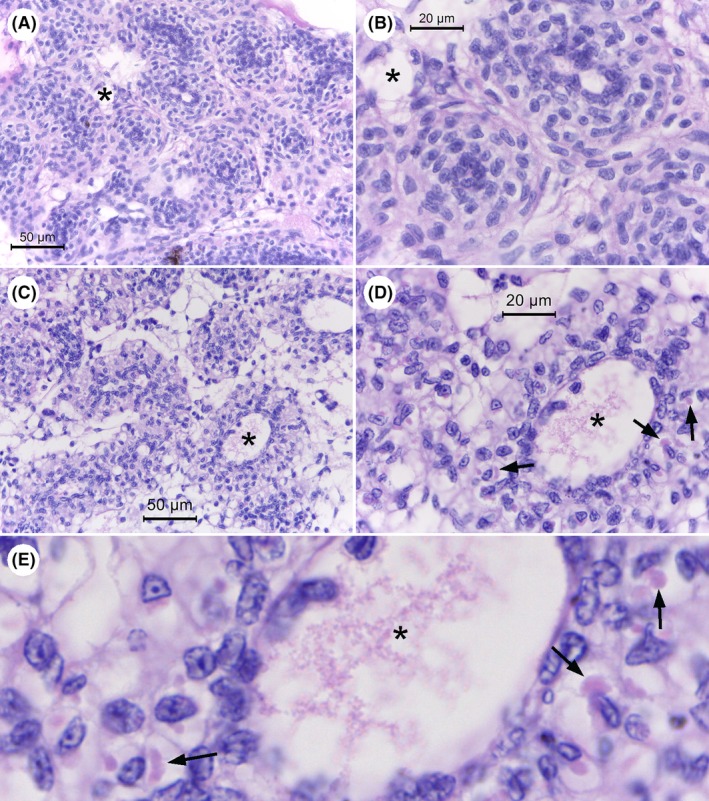
Photomicrographs of histology of the lymphoid organ (LO) of normal shrimp and moribund shrimp from immersion with S. khirikhana TH2012T. Asterisks (*) indicate the same location in photomicrographs at different magnifications.
A and B. Example of normal LO tissue from control shrimp at low and high magnification respectively.
C–E. Example of histopathology of LO tissue from test shrimp at progressively higher magnifications showing extensive cell vacuolization with some cells showing the presence of eosinophilic cytoplasmic inclusions (arrows).
Additional, but less ubiquitous distinctive histological abnormalities presented in the moribund shrimp but absent in the control shrimp are described in Supporting information. These included the digestive system with unusual vacuolization of (i) the anterior midgut caecum (AMC) (22/26 = 85% of specimens with AMC tissue in the section; Fig. S13), of (ii) nearby subcuticular epithelial cells of the stomach (24/26 = 92% of specimens with appropriate tissue in the section; Fig. S14) and of (iii) E‐cells of the tubule epithelium of the hepatopancreas (HP) (22/31 = 71% of specimens; Fig. S15). Also found were hematopoietic tissue (HT) lesions showing prominent eosinophilic, cytoplasmic inclusions (sometimes within vacuoles) (20/31 = 65% of moribund shrimp specimens examined; Fig. S16), and focal gill lesions showing pyknotic and karyorrhectic nuclei, eosinophilic cytoplasmic inclusions and loss of gill structure (25/31 = 81% of moribund shrimp; data not shown). None of these are reliable for presumptive diagnosis when compared to the nerve cell and LO lesions due to their low prevalence, excessive time needed for analysis or difficulty in detection (i.e. an 100x oil immersion lens is required for HT examination in contrast to the 40x lens sufficient for examination of nervous and LO tissues). Note that no bacterial cells were observed in the lesions described above, but that they were seen occasionally in the HP tubule lumens of moribund shrimp specimens from the bacterial challenge (11/31 = 35.5% moribund shrimp; Fig. S17).
Putative virulence factors
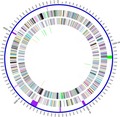
In the TH2012T genome, no Pirvp sequences of VPAHPND (Han et al., 2015; Lee et al., 2015; Xiao et al., 2017) or Pirvp‐like sequences of S. violacea and no significant nucleotide similarity of pSTH1 to the Pirvp toxin carrying plasmids were found by exhaustive searches (Supporting information) (Wechprasit et al., 2019). GIPSy (Soares et al., 2016) reported six PAIs and two RIs in the TH2012T genome (Fig. 5). Two PAIs gave strong prediction scores and one gave a normal score, and the others gave weak scores. The two with strong scores contained putative genes of the Type VI secretion system (T6SS) along with fimbrial and colonization factor genes, lysozyme, extracellular phospholipase, microbial serine proteinase and hypothetical proteins. There were two putative RIs, one each with strong and weak prediction score. The weak score RI was in the same region as one of the three weak score PAIs (Fig. 5). Genes in the RIs included putative multidrug efflux pumps MacA–MacB pumps, MarR family genes, several permease proteins, OmpF porin, and evgS and PhoP genes. In addition, the TH2012T genome contains a putative prophage (Fig. 5) (Wechprasit et al., 2019) and some virulence genes found in some Shewanella isolates (Paździor, 2016; Yousfi et al., 2017) such as putative haemolysins, chitinases and proteases (Wechprasit et al., 2019).
Figure 5.
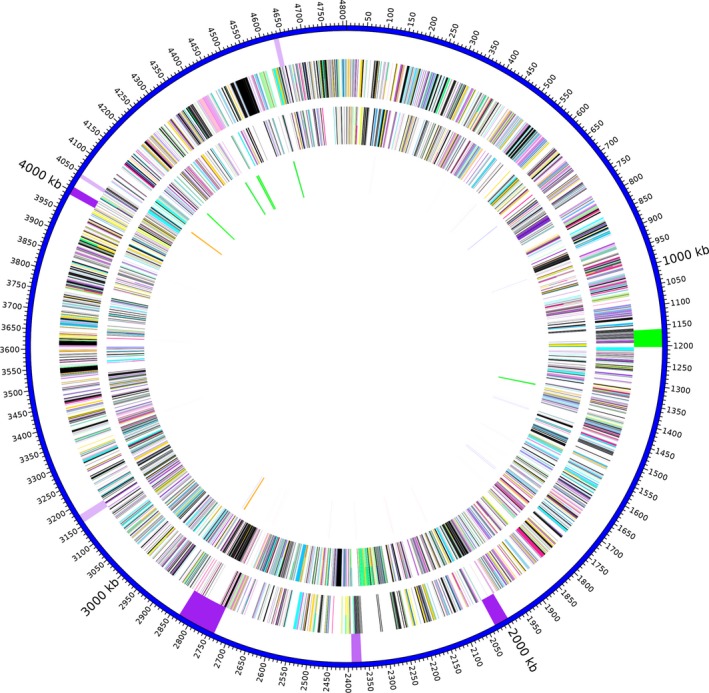
Graphical map of the chromosome of S. khirikhana TH2012T. From the outside to the centre: pathogenicity or resistance islands (darker purple indicates stronger prediction scores) and prophage (green), genes on the forward strand (coloured by COG categories), genes on the reverse strand (coloured by COG categories) and RNA genes (tRNAs forward strand blue, tRNAs on reverse strand pink, rRNAs on forward strand green, rRNAs on reverse strand orange and tmRNAs on forward strand tan). COG colour and functional designations are described in Table S5.
Discussion
The phylogenetic tree analysis (Fig. 1, Figs S2–S6) and DNA–DNA relatedness indicated that isolate TH2012T represents a new species of Shewanella, separated from other Shewanella species including type strains of S. amazonensis and S. litorisediminis. This was supported by differential morphological and physiological characteristics (Table 1 and Supporting information). Recently, an independent phylogenomic analysis of our published TH2012T genome sequence (Wechprasit et al., 2019) also supported this erection of a new species within the genus (Thorell et al., 2019). Thus, we propose the name Shewanella khirikhana to indicate that TH2012T originated from Prachuap Khirikhan Province, Thailand. The full description of the species is given below. Our phylogenetic tree analysis also raised the question as to whether TBRC 5001 and LV 5 can be included as representatives of S. litorisediminis based solely on analysis of partial 16S rRNA sequences (Fig. S3). Lacking full genome sequences for the type strain of S. litorisediminis SMK1‐12T, for the strains of TBRC 5001 and LV 5, and for the unidentified isolate S. sp. AK55, similar comparisons could not be carried out to establish clear placement of TBRC 5001, LV 5 and AK55.
By satisfying Koch’s postulates, we have demonstrated that S. khirikhana TH2012T is lethal to shrimp (60‐h LC50 ~ 105 CFU ml−1) and is the first from its genus to constitute an emerging shrimp pathogen. Its origin from moribund shrimp in an EMS/AHPND outbreak pond lends some support to the hypothesis that it could possibly cause some of the unexplained mortality in EMS ponds (Sanguanrut et al., 2018) or even in other non‐EMS ponds with unexplained mortality. In addition, it is possible that it might act together with AHPND bacteria in an additive or synergistic way to exacerbate shrimp mortality as earlier hypothesized (FAO, 2013). However, these speculations need to be confirmed or dismissed via subsequent comparison of results from single and co‐challenge tests in the laboratory. For such work, the molecular tools described herein will be useful.
So far, within the genus Shewanella, the species S. algae and S. putrefaciens have been reported as the two major species infecting humans (e.g. septicaemia, cellulitis, arthritis, otitis and pneumonia). Both are often recovered from mixed microbial flora of patients (Satomi, 2013; Yousfi et al., 2017). They have also been reported to be opportunistic pathogens in aquatic species. For example, S. algae caused abalone mortalities in China and Taiwan (Cai et al., 2006) and ulcer disease in marine channel bass in China ((Chen et al., 2003) in (Cai et al., 2006)). S. putrefaciens has been reported from several counties as a marine and freshwater fish pathogen for tilapia, European sea bass, rabbitfish, loach, siberian sturgeon, hybrid sturgeon, rainbow trout and carp. Recently, outbreaks have been reported in farmed freshwater fish in Poland (for review see (Paździor, 2016)). It also caused mortality in freshwater zebra mussels in North America (Gu and Mitchell, 2002). In China, S. marisflavi caused high mortality in sea cucumbers (Li et al., 2010), and S. aquimarina was associated with a lesion syndrome of sea urchin (Wang et al., 2013). In shrimp, Shewanella species, and especially S. putrefaciens and S. baltica, were associated with spoilage of P. vannamei stored at low temperature (Qian et al., 2013; Qian et al., 2015; Zhu et al., 2017), and Shewanella species were also found to be a normal part of the bacterial flora in the P. vannamei gut (Suo et al., 2017).
Histopathology caused by S. khirikhana TH2012T in infected shrimp was unique (Figs 2, 3, 4, Figs S13–S17) and did not show the pathognomonic lesions of acute AHPND (Lightner et al., 2012; Naca, 2012; Tran et al., 2013). Based on prevalence, unique character and ease of examination using a 40x objective with a light microscope, we recommend that histological analysis of shrimp suspected of exposure to S. khirikhana focus initially on the tissue of the ventral nerve cord and the LO in standard, mid‐longitudinal cephalothorax sections. This would allow for presumptive diagnosis based on the distinctive histopathology for the nerve cord (Figs 2 and 3) and the LO (Fig. 4). The other histopathological lesions described in Supporting information can be used as backups, if by chance the nerve cord and the LO are absent in a tissue section. However, this is unlikely since it is usually recommended to examine at least 10 shrimp specimens from a disease outbreak pond and it is usual by these standard procedures (Bell and Lightner, 1988) that most tissue sections would include both the ventral nerve cord and the LO, rarely only one or the other and very rarely neither.
We further recommend that any presumptive diagnosis should be confirmed by PCR testing for S. khirikhana followed by sequencing of any PCR amplicons. This would be facilitated since tests of EMS shrimp for AHPND bacteria involves an enrichment step that would probably also enrich for S. khirikhana such that the same DNA extract could be used to test for both pathogens. This would help in determining prevalence of S. khirikhana in EMS outbreak ponds and also indicate whether there is any positive association between occurrence of S. khirikhana and AHPND. We would welcome cooperation with anyone who is testing for AHPND bacteria using DNA extracts but does not or cannot test for S. khirikhana (see Supporting information for our free sample testing).
The nerve cord, LO and other distinctive tissue pathological lesions described here for S. khirikhana showed no visible bacterial cells, and since free, rod‐shaped bacterial cells were seen in hepatopancreatic tubule lumens only occasionally (11/31 = 35.5%; see Supporting information). Thus, the overall results for the S. khirikhana immersion challenges were similar to those for the acute phase of AHPND where bacterial cells are absent in the HP lesions at acute stage of disease. This suggests, like AHPND, that the cause of the unique histological changes in the moribund shrimp from our immersion challenge was one or more toxic substances produced by S. khirikhana located in the immersion water or in the shrimp stomach.
The fact that S. khirikhana often causes distinctive histopathological lesions (i.e. vacuolization in the E‐cells of the HP) indicates that it targets, at least in part, in the same organ that is the main target of the Pirvp toxins of AHPND‐causing bacteria. Because of this, it is possible that mixed infections of S. khirikhana with AHPND bacteria could interact in an additive or synergistic manner such that normally non‐lethal concentrations for each would become lethal when combined. The prospect of a synergistic increase in virulence in mixed infections is of particular concern and should be further investigated.
Absence of Pirvp (VPAHPND) and Pirvp–like (S. violacea) homologous sequences in the genome of TH2012T (Wechprasit et al., 2019) suggests that one or more of other putative toxins or virulence factors may be involved in its pathogenesis. Information on virulence factors in the genus Shewanella is still limited. In humans, a higher virulence for S. algae than for S. putrefaciens was found to be associated with the production of beta‐haemolysin and other extracellular enzymes (Khashe and Michael Janda, 1998; Yousfi et al., 2017). Genomic analysis of S. algae MARs 14 (a multidrug‐resistant pneumonia agent) revealed putative virulence factors, including haemolysin and flagellum system genes along with drug‐resistance genes and multidrug efflux pump genes (Cimmino et al., 2016).
Six putative PAIs and two putative RIs were detected in TH2012T (Fig. 5). T6SS is known to play an important role in pathogenesis and bacterial competition by translocating toxic effector proteins into hosts or other bacterial cells (e.g. Jani and Cotter, 2010; Russell et al., 2014)). EvgS is a member of the two‐component regulatory system EvgS/EvgA, and PhoP is a member of another two‐component system PhoPQ. These two systems are known to be involved in regulation of expression of the AcrAB‐TolC efflux system of E. coli (for review see (Li et al., 2015)). The MarR family genes are known to be involved in a regulatory cascade that decreases OmpF porin expression, resulting in decreased drug uptake, which in turn leads to drug resistance (see (Fernández and Hancock, 2012; Li et al., 2015)). Interestingly, many transposases (24 ORFs out of the total 78 ORFs) were found scattering in one PAI, suggesting that genes in this region might be strongly linked with mobile genetic elements. Along with a prophage, other virulence genes in some Shewanella isolates (Paździor, 2016; Yousfi et al., 2017) such as haemolysins, chitinases and proteases are also present in the TH2012T genome (Wechprasit et al., 2019).
In summary, we have described a new bacterial species Shewanella khirikhana sp. nov. TH2012T isolated from the hepatopancreas of a shrimp specimen taken from a cultivation pond experiencing an outbreak of EMS/AHPND. It is distinct from its currently known closest phylogenetic sister species S. amazonensis and S. litorisediminis. We have shown that S. khirikhana TH2012T is lethal to shrimp and causes distinctive histopathology that differs markedly from the pathognomonic lesions of AHPND. It can be used as a preliminary, presumptive diagnosis for infection with S. khirikhana. In addition, it harbours several virulence factors in putative pathogenicity and resistance islands. However, its impact on shrimp production in terms of a direct or potentiating cause of mortality remains to be determined. The complete genome sequence of TH2012T is a ready source for the development of a highly sensitive and specific detection method to study the prevalence of S. khirikhana in order to determine its full impact on shrimp culture and to control its spread.
Description of Shewanella khirikhana sp. nov
The specific epithet in the name Shewanella khirikhana (khi.ri.khan.a, adj. khirikhana) refers to its origin from a shrimp pond in Prachuap Khirikhan Province, Thailand, where the shrimp samples were collected for isolation of the type strain.
Cells are facultative anaerobic, free‐living, Gram‐negative, straight rods and motile. Colonies are yellowish, translucent, circular, smooth and convex with entire edges. Cultures grow at 25–37°C over the salinity range of 0.5–5.5% (w/v) NaCl and over the pH range 6.5–9 with optimal growth at 30 °C, pH 7–7.5 and 1.5–2% NaCl. Cultures die at 4°C and so should not be stocked in a refrigerator. However, they can be stored using standard methods at −80°C with cryoprotectant. TH2012T produces H2S, shows oxidase activity and can reduce nitrate to nitrite, hydrolyse Tween 20, gelatin and casein but not urea, and it does not ferment glucose and citrate, and does not show ornithine decarboxylase, beta‐galactosidase, arginine dihydrolase, lysine decarboxylase and tryptophan deaminase activities. d‐glucose, d‐mannitol, inositol, d‐sorbitol, l‐rhamnose, d‐melibiose, amygdalin, citrate, l‐arabinose and d‐sucrose are not utilized as carbon source. Production of indole and acetoin is absent.
The sequenced genome of the type strain TH2012T has a G + C content of 54.88%, is 4.85 megabase pairs (Mbp) in size and contains a single circular chromosome (4.80 Mbp) and a circular plasmid pSTH1 (0.05 Mbp). The sequences of 16S rRNA, atpA (ATP synthase F1 subunit alpha), mreB (rod shape‐determining protein) and rpoA (DNA directed RNA polymerase subunit alpha) genes of the type strain TH2012T are deposited under the GenBank/EMBL/DDBJ accession numbers MH719102, MH719105, MH705614, MH705617 and MH705620 respectively. The complete genome sequence of the type strain TH2012T has also been deposited at GenBank/EMBL/DDBJ under the accession number CP020373 and at DOE JGI Integrated Microbial Genomes & Microbiomes under GOLD ID Gp0206473. Strain TH2012T is the type strain of Shewanella khirikhana sp. nov. and has been deposited as TBRC 8956T and NBRC 113603T at Thailand Bioresource Research Center and Japan Biological Resource Center, NITE respectively.
Experimental procedures
Bacterial isolates and cultures
The bacterial isolate TH2012T was obtained during bacterial isolation from hepatopancreatic tissue of diseased shrimp from an EMS/AHPND outbreak in 2012 (Joshi et al., 2014) and was identified as a putative member of the genus Shewanella (Wechprasit et al., 2019). Two other Shewanella isolates, S. litorisediminis KCTC 23961T (= SMK1‐12T) and S. litorisediminis TBRC 5001, were obtained from the Korean Collection for Type Cultures (KCTC) and the Thailand Bioresource Research Center (TBRC) respectively. All isolates were revived from cryopreserved glycerol stocks by overnight culturing aerobically at 30°C in tryptic soy broth (TSB) supplemented by 1.5% (w/v) NaCl under shaking conditions before subsequent use. Note that all TSB and tryptic soy agar (TSA) used in this study were supplemented by 1.5% NaCl (resulting in a final NaCl content of 2.0%) unless otherwise indicated.
Morphological and physiological characteristic tests
The procedures to investigate cell morphology, biochemical characteristics with API®biomerieux API 20E stripe tests, enzyme activities (casein hydrolysis, lipase activity and gelatin hydrolysis), effects of temperature, salinity and pH on TH2012T growth and its growth curve are described in Supporting information.
Shrimp immersion bioassay and histopathology
Penaeus vannamei (3–5 g) purchased from a local hatchery were maintained in 50 litre (L) aerated tanks containing 20 L artificial seawater (Marinium) at 15 ppt salinity and 30 ºC. Shrimp (10) were acclimatized in each tank for 3 days. Then, they were challenged by immersion with TH2012T at the concentrations of 104, 105 and 106 colony‐forming units per millilitre (CFU ml−1) with two replicate tanks for each concentration. Stock inoculum of TH2012T for addition to shrimp immersion water was prepared by addition of as a starter culture from cryopreserved bacteria inoculated to achieve optical density at 600 nm (OD600) ~ 0.05 cultivation to obtain OD600 of 2.6 (~ 109 CFU ml−1 variable bacterial cell count; see Supporting information). Such stock inoculum was diluted in TSB such that 200 ml added to each shrimp culture tank would achieve the desired final bath‐challenge bacterial concentrations in 20 L artificial sea water. Negative control groups were two tanks without any treatment. For 102 h post immersion (hpi), moribund and dead shrimp were recorded to determine the 50% lethal concentration at 60 hpi. Whole moribund shrimp and normal control shrimp were fixed in Davidson’s fixative for histopathological analysis according to Bell and Lightner (Bell and Lightner, 1988) (see Supporting information). Similarly, the subsequent bacterial immersion challenge carried out to satisfy Koch’s postulates used TH2012T re‐isolated from a moribund shrimp at 105 CFU ml−1 with 1–2 g shrimp.
Phylogenetic analysis by multilocus sequence analysis and genome comparison
Sequences of 16S rRNA, atpA (ATP synthase F1 subunit alpha), mreB (rod shape‐determining protein) and rpoA (DNA directed RNA polymerase subunit alpha) genes of TH2012T were obtained from the TH2012T genome assembly (Wechprasit et al., 2019), and these genes were previously used for phylogenetic analysis of Shewanella (Dikow, 2011). The homologous sequences in S. litorisediminis SMK1‐12T and TBRC 5001 were obtained by sequencing PCR amplicons of genomic DNA template (see Supporting information). The homologous sequences in other Shewanella species were obtained from GenBank (ftp://ftp.ncbi.nlm.nih.gov/genomes/all/). Multiple sequence alignments were performed with MUSCLE (Edgar, 2004) using concatenated multilocus sequence alignment with genes in the order of 16S rRNA, atpA, mreB and rpoA genes, and phylogenetic trees were constructed with the neighbour‐joining, minimum evolution, maximum‐likelihood and maximum parsimonious algorithms (all with Jukes–Cantor distance) in MEGA 7 (Kumar et al., 2016). Complete genomes of TH2012T (Wechprasit et al., 2019) and S. amazonensis SB2BT (Accession: NC_008700) were used to calculate digital DNA:DNA hybridization (dDDH) similarities by the Genome‐to‐Genome Distance Calculator version 2.1 formula 2 (http://ggdc.dsmz.de (Meier‐Kolthoff et al., 2013)) as well as to calculate average nucleotide identity (ANI) scores in IMG/MER (Chen et al., 2017). GIPSy (Soares et al., 2016) was used to detect pathogenicity islands and resistance islands by using the genome sequence of TH2012T as a query against the whole genome sequence of S. amazonensis SB2BT (NC_008700) as a reference.
Conflict of interest
None declared.
Supporting information
Appendix S1. Supporting Information, Figs S1–S18 and Tables S1–S5.
Fig. S1. Phylogeny of 16S rDNA genes of Shewanella khirikhana TH2012T and other Shewanella species. The neighbour‐joining phylogenetic tree with Jukes‐Cantor distance was based on 1452 aligned positions of 16S rDNA gene sequences. Moritella marina ATCC 15381 was used as an outgroup. Bootstrap values (expressed as percentages of 1000 replications) are shown at branching points. Most of the nodes were also recovered in the trees generated with the minimum evolution (Jukes‐Cantor distance), maximum‐likelihood (Jukes‐Cantor distance) and maximum parsimonious algorithms based on the same set of sequences, except those with Filled circles, or Filled squares were not recovered in the trees of maximum parsimonious, or both maximum parsimonious and maximum‐likelihood algorithms, respectively. Scale bar indicates estimated sequence divergence (substitutions per nucleotide position). Sequence accession numbers are shown in the parentheses after the species names.
Fig. S2. Phylogeny 16S rDNA genes of Shewanella khirikhana TH2012T and other Shewanella species including strains LV 5 and AK55. The neighbour‐joining phylogenetic tree with Jukes‐Cantor distance was based on 1176 aligned positions of 16S rDNA gene sequences. Moritella marina ATCC 15381 was used as an outgroup. Bootstrap values (expressed as percentages of 1000 replications) are shown at branching points. Most of the nodes were also recovered in the trees generated with the minimum evolution (Jukes‐Cantor distance), maximum‐likelihood (Jukes‐Cantor distance) and maximum parsimonious algorithms based on the same set of sequences, except those with Filled circles, or Opened squares were not recovered in the trees of maximum parsimonious, or maximum‐likelihood algorithms, respectively. Scale bar indicates estimated sequence divergence (substitutions per nucleotide position). Sequence accession numbers are shown in the parentheses after the species names.
Fig. S3. Phylogeny of atpA genes of Shewanella khirikhana TH2012T and other Shewanella species. The neighbour‐joining phylogenetic tree with Jukes‐Cantor distance was based on 1262 aligned positions of atpA gene sequences. Moritella marina ATCC 15381 was used as an outgroup. Bootstrap values (expressed as percentages of 1000 replications) are shown at branching points. Most of the nodes were also recovered in the trees generated with the minimum evolution (Jukes‐Cantor distance), maximum‐likelihood (Jukes‐Cantor distance) and maximum parsimonious algorithms based on the same set of sequences, except those with Opened squares, or Filled squares were not recovered in the trees of maximum‐likelihood, or both maximum parsimonious and maximum‐likelihood algorithms, respectively. Scale bar indicates estimated sequence divergence (substitutions per nucleotide position). Sequence accession numbers are shown in the parentheses after the species names.
Fig. S4. Phylogeneny of mreB genes of Shewanella khirikhana TH2012T and other Shewanella species. The neighbour‐joining phylogenetic tree with Jukes‐Cantor distance was based on 785 aligned positions of mreB gene sequences. Moritella marina ATCC 15381 was used as an outgroup. Bootstrap values (expressed as percentages of 1000 replications) are shown at branching points. Most of the nodes were also recovered in the trees generated with the minimum evolution (Jukes‐Cantor distance), maximum‐likelihood (Jukes‐Cantor distance) and maximum parsimonious algorithms based on the same set of sequences, except those with Filled circles, or Filled squares were not recovered in the trees of maximum parsimonious, or both maximum parsimonious and maximum‐likelihood algorithms, respectively. Scale bar indicates estimated sequence divergence (substitutions per nucleotide position). Sequence accession numbers are shown in the parentheses after the species names.
Fig. S5. Phylogeny of rpoA genes of Shewanella khirikhana TH2012T and other Shewanella species. The neighbour‐joining phylogenetic tree with Jukes‐Cantor distance was based on 789 aligned positions of rpoA gene sequences. Moritella marina ATCC 15381 was used as an outgroup. Bootstrap values (expressed as percentages of 1000 replications) are shown at branching points. Most of the nodes were also recovered in the trees generated with the minimum evolution (Jukes‐Cantor distance), maximum‐likelihood (Jukes‐Cantor distance) and maximum parsimonious algorithms based on the same set of sequences, except those with Filled circles, Opened squares, or Filled squares were not recovered in the trees of maximum parsimonious, maximum‐likelihood, or both maximum parsimonious and maximum‐likelihood algorithms, respectively. Scale bar indicates estimated sequence divergence (substitutions per nucleotide position). Sequence accession numbers are shown in the parentheses after the species names.
Fig. S6. Photomicrographs of unstained cell morphology and Gram stain morphology of Shewanella khirikhana TH2012T.
Fig. S7. Colony characteristics of Shewanella khirikhana TH2012T from stereomicroscope (A) and light microscope (B).
Fig. S8. Graph showing time course OD600 of Shewanella khirikhana TH2012T cultured at various salinities (%NaCl).
Fig. S9. Graph showing time course OD600 of Shewanella khirikhana TH2012T cultured at various pH’s.
Fig. S10. Growth profiles of Shewanella khirikhana TH2012T measured by optical density at 600 nm (OD600).
Fig. S11. Graph showing Shewanella khirikhana TH2012T viable bacterial cell counts (CFU ml−1) vs. OD600 during hours 2–4 of the growth curve experiment (hours are indicated by colors) in Fig. S10.The grey area denotes the 95% confidence level interval for predictions from a linear model of log10 CFU ml−1 versus log10 OD600. R 2 = 0.7814, P = 4.902 x 10‐8.
Fig. S12. Graph showing the regression line for shrimp mortality versus bacterial concentration (log10 CFU ml−1) for Shewanella khirikhana TH2012T immersion bioassay up to 60 h post immersion. The grey area denotes the 95% confidence level interval for predictions from a linear model. R 2 = 0.6945, P = 0.03933.
Fig. S13. Photomicrographs of histology of the anterior midgut cecum (AMC) of normal shrimp and moribund shrimp from immersion with Shewanella khirikhana TH2012T. (A & B) Example of normal AMC tissue at low and high magnification, respectively. (C & D) Example of histopathology from a moribund test shrimp with abnormally vacuolated epithelial cells containing few eosinophilic inclusions. (E & F) Example histopathology showing vacuolated epithelial cells containing many eosinophilic inclusions.
Fig. S14. Example photomicrographs of histology of the sub‐cuticular epithelium of the stomach of normal shrimp and of moribund shrimp from immersion with Shewanella khirikhana TH2012T. (A & B) Normal sub‐cuticular epithelial cells at low and high magnification showing relatively non‐vacuolated cytoplasm. (C & D) Abnormally vacuolated sub‐cuticular epithelial cells from a moribund shrimp specimen at low and high magnification. Asterisks (*) indicate the same position in the low and high magnification photomicrographs.
Fig. S15. Photomicrographs of histology of the E‐cell region of the hepatopancreas of normal shrimp and moribund shrimp from immersion with Shewanella khirikhana TH2012T. (A & B) Example of normal E‐cells in tubular cross section at low and high magnification, respectively, showing dense non‐vacuolated cytoplasm. (C & D) Example of abnormally vacuolated E‐cells in cross‐section. (E & F) Similar to C and D except that the tubules are in longitudinal section. Asterisks (*) indicate the same position in the low and high magnification photomicrographs.
Fig. S16. Example photomicrographs of histology of hematopoietic tissue (HT) of normal shrimp and of moribund shrimp from immersion with Shewanella khirikhana TH2012T. (A, B & C) Normal HT at progressively higher magnification showing cells that lack eosinophilic cytoplasmic inclusions. In B and C, the arrows indicate the same cell nucleus at low and high magnification. (D, E & F) Abnormal HT at progressively higher magnification showing the presence of eosinophilic cytoplasmic inclusions. The asterisks indicate the same position at different magnifications D, E and F and the arrows mark the same eosinophilic inclusion in E & F. Note that the highest magnification (100x objective) is needed for easy identification of the unique inclusions.
Fig. S17. Photomicrographs of bacterial cells seen in some of the moribund, challenged shrimp. (A) Bacterial cells of mixed size and morphology together with sloughed epithelial cells in the lumen of a hepatopancreatic (HP) tubule. (B) Few bacterial cells of uniform morphology in the lumen of an HP tubule with an intact epithelial cell layer.
Fig. S18. Graphical map of the plasmid pSTH1. From outside to the center: Genes on forward strand (colored by COG categories), Genes on reverse strand (colored by COG categories). COG color and functional designations are described in Supporting Information Table S5.
Table S1. PCR primer sequences used in this study.
Table S2. Length (bp) of atpA, mreB, and rpoA sequences in strains TH2012T, TBRC 5001, SMK1‐12T, and SB2BT, and percentage of nucleotide identities of TH2012T to corresponding orthologous sequences in TBRC 5001, SMK1‐12T, and SB2BT.
Table S3. Shewanella khirikhana TH2012T viable bacterial cell counts (CFU ml−1) from incubation experiments at various temperature (ºC). UD, unable to determine due to either no colony formation or the number of colonies were not in the range of 10–300 colonies.
Table S4. Shewanella khirikhana TH2012T genome project information.
Table S5. Codes, number and percentage of genes, descriptions and colors associated with general COG functional categories.
Acknowledgements
We would like to thank N. Munkongwongsiri, R. Suebsing, P. Sanguanrut and W. Eamsaard for their help in laboratory, BIOTEC’s Biostatistics & Informatics Laboratory, and Aung T.R.H. and K. Anekthanakul for their programing and HPC support. This work was supported by the Agricultural Research Development Agency (ARDA) (grant number 8669), Mahidol University and the National Center for Genetic Engineering and Biotechnology (BIOTEC) of the National Science and Technology Development Agency (NSTDA). PW also would like to acknowledge the support from Thailand Graduate Institute of Science and Technology Scholarship (TGIST grant number SCA‐CO‐2560‐4497‐TH).
Microbial Biotechnology (2020) 13(3), 781–795
Funding Information
This work was supported by the Agricultural Research Development Agency (ARDA) (grant number 8669), Mahidol University and the National Center for Genetic Engineering and Biotechnology (BIOTEC) of the National Science and Technology Development Agency (NSTDA). PW also would like to acknowledge the support from Thailand Graduate Institute of Science and Technology Scholarship (TGIST grant number SCA‐CO‐2560‐4497‐TH).
References
- Anggraeni, M.S. , and Owens, L. (2000) The haemocytic origin of lymphoid organ spheroid cells in the penaeid prawn Penaeus monodon. Dis Aquat Organ 40: 85–92. [DOI] [PubMed] [Google Scholar]
- Bell, T. A. , and Lightner, D. V. (1988) A Handbook of Normal Penaeid Shrimp Histology. Baton Rouge, LO: World Aquaculture Society. [Google Scholar]
- Cai, J. , Chen, H. , Thompson, K.D. , and Li, C. (2006) Isolation and identification of Shewanella alga and its pathogenic effects on post‐larvae of abalone Haliotis diversicolor supertexta . J Fish Dis 29: 505–508. [DOI] [PubMed] [Google Scholar]
- Chen, C. , Hu, C.Q. , Chen, X.Y. , and Zhang, L.P. (2003) Identification and characterization of Shewanella alga as a novel pathogen of ulcer disease of fish Scinenops ocellata. Oceanologia et Limnologia Sinica 34: 2–8. [Google Scholar]
- Chen, I.‐M.A. , Markowitz, V.M. , Chu, K. , Palaniappan, K. , Szeto, E. , Pillay, M. , et al (2017) IMG/M: integrated genome and metagenome comparative data analysis system. Nucleic Acids Res 45: D507–D516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimmino, T. , Olaitan, A.O. , and Rolain, J.‐M. (2016) Whole genome sequence to decipher the resistome of Shewanella algae, a multidrug‐resistant bacterium responsible for pneumonia, Marseille, France. Expert Rev Anti Infect Ther 14: 269–275. [DOI] [PubMed] [Google Scholar]
- De La Peña, L.D. , Cabillon, N.A.R. , Catedral, D.D. , Amar, E.C. , Usero, R.C. , Monotilla, W.D. , et al (2015) Acute hepatopancreatic necrosis disease (AHPND) outbreaks in Penaeus vannamei and p. monodon cultured in the Philippines. Dis Aquat Organ 116: 251–254. [DOI] [PubMed] [Google Scholar]
- Dhar, A. K. , Piamsomboon, P. , Aranguren Caro, L. F. , and Kanrar, S. (2018). First report of the presence of acute hepatopancreatic necrosis disease (AHPND) in Texas, USA. URL https://www.was.org/meetings/ShowAbstract.aspx?Id=75594 [DOI] [PubMed]
- Dikow, R.B. (2011) Genome‐level homology and phylogeny of Shewanella (Gammaproteobacteria: lteromonadales: Shewanellaceae). BMC Genom 12: 237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar, R.C. (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32: 1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO (2013) FAO Report of the FAO/MARD technical workshop on early mortality syndrome (EMS) or acute hepatopancreatic necrosis syndrome (AHPNS) of cultured Shrimp (under TCP/VIE/3304), Hanoi, Viet Nam on 25–27 June 2013. Rome, p. 55. [Google Scholar]
- Fernández, L. , and Hancock, R.E.W. (2012) Adaptive and mutational resistance: role of porins and efflux pumps in drug resistance. Clin Microbiol Rev 25: 661–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goris, J. , Konstantinidis, K.T. , Klappenbach, J.A. , Coenye, T. , Vandamme, P. , and Tiedje, J.M. (2007) DNA‐DNA hybridization values and their relationship to whole‐genome sequence similarities. Int J Syst Evol Microbiol 57: 81–91. [DOI] [PubMed] [Google Scholar]
- Gu, J.‐D. , and Mitchell, R. (2002) Indigenous microflora and opportunistic pathogens of the freshwater zebra mussel, Dreissena polymorpha . Hydrobiologia 474: 81–90. [Google Scholar]
- Han, J.E. , Tang, K.F.J. , Tran, L.H. , and Lightner, D.V. (2015) Photorhabdus insect‐related (Pir) toxin‐like genes in a plasmid of Vibrio parahaemolyticus, the causative agent of acute hepatopancreatic necrosis disease (AHPND) of shrimp. Dis Aquat Organ 113: 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, J.E. , Tang, K.F.J. , Aranguren, L.F. , and Piamsomboon, P. (2017) Characterization and pathogenicity of acute hepatopancreatic necrosis disease natural mutants, pirABvp(‐) V. parahaemolyticus, and pirABvp (+) V. campbellii strains. Aquaculture 470: 84–90. [Google Scholar]
- Hasson, K.W. , Lightner, D.V. , Mohney, L.L. , Redman, R.M. , and White, B.M. (1999) Role of lymphoid organ spheroids in chronic Taura syndrome virus (TSV) infections in Penaeus vannamei . Dis Aquat Organ 38: 93–105. [Google Scholar]
- Jani, A. . , and Cotter, P.A. (2010) Type VI Secretion: not just for pathogenesis anymore. Cell Host Microbe 8: 2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi, J. , Srisala, J. , Truong, V.H. , Chen, I.‐T. , Nuangsaeng, B. , Suthienkul, O. , et al (2014) Variation in Vibrio parahaemolyticus isolates from a single Thai shrimp farm experiencing an outbreak of acute hepatopancreatic necrosis disease (AHPND). Aquaculture 428–429: 297–302. [Google Scholar]
- Khashe, S. , and Michael Janda, J. (1998) Biochemical and pathogenic properties of Shewanella alga and Shewanella putrefaciens . J Clin Microbiol 36: 783–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo, H. , Van, P.T. , Dang, L.T. , and Hirono, I. (2015) Draft genome sequence of non‐Vibrio parahaemolyticus acute hepatopancreatic necrosis disease strain KC13.17.5, isolated from diseased shrimp in Vietnam. Genome Announcements 3: e00978-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, S. , Stecher, G. , and Tamura, K. (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33: 1870–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai, H.‐C. , Ng, T.H. , Ando, M. , Lee, C.‐T. , Chen, I.‐T. , Chuang, J.‐C. , et al (2015) Pathogenesis of acute hepatopancreatic necrosis disease (AHPND) in shrimp. Fish Shellfish Immunol 47: 1006–1014. [DOI] [PubMed] [Google Scholar]
- Lee, M.H. , and Yoon, J.H. (2012) Shewanella litorisediminis sp. nov., a gammaproteobacterium isolated from a tidal flat sediment. Antonie Van Leeuwenhoek 102: 591–599. [DOI] [PubMed] [Google Scholar]
- Lee, C.‐T. , Chen, I.‐T. , Yang, Y.‐T. , Ko, T.‐P. , Huang, Y.‐T. , Huang, J.‐Y. , et al (2015) The opportunistic marine pathogen Vibrio parahaemolyticus becomes virulent by acquiring a plasmid that expresses a deadly toxin. Proc Natl Acad Sci USA 112: 10798–10803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, H. , Qiao, G. , Gu, J.‐Q. , Zhou, W. , Li, Q. , Woo, S.‐H. , et al (2010) Phenotypic and genetic characterization of bacteria isolated from diseased cultured sea cucumber Apostichopus japonicus in northeastern China. Dis Aquat Organ 91: 223–235. [DOI] [PubMed] [Google Scholar]
- Li, X.‐Z. , Plésiat, P. , and Nikaido, H. (2015) The challenge of efflux‐mediated antibiotic resistance in Gram‐negative bacteria. Clin Microbiol Rev 28: 337–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightner, D.V. , Redman, R. , Pantoja, C. , Noble, B. , and Tran, L. (2012) Early mortality syndrome affects shrimp in Asia. Glob Aquacult Advocate 15: 40. [Google Scholar]
- Liu, L. , Xiao, J. , Xia, X. , Pan, Y. , Yan, S. , and Wang, Y. (2015) Draft genome sequence of Vibrio owensii strain SH‐14, which causes shrimp acute hepatopancreatic necrosis disease. Genome Announcements 3: e01395‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier‐Kolthoff, J.P. , Auch, A.F. , Klenk, H.‐P. , and Göker, M. (2013) Genome sequence‐based species delimitation with confidence intervals and improved distance functions. BMC Bioinformatics 14: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naca (2012) Report of the Asia Pacific Emergency Regional Consultation on the Emerging Shrimp Disease: Early Mortality Syndrome (EMS)/acute Hepatopancreatic Necrosis Syndrome (AHPNS). Asia Pacific Emergency Regional Consultation on the Emerging Shrimp Disease: Early Mortality Syndrome (EMS)/Acute Hepatopancreatic Necrosis Syndrome (AHPNS).
- Nunan, L. , Lightner, D. , Pantoja, C. , and Gomez‐Jimenez, S. (2014) Detection of acute hepatopancreatic necrosis disease (AHPND) in Mexico. Dis Aquat Organ 111: 81–86. [DOI] [PubMed] [Google Scholar]
- Owens, L. (2010) Insight into the lymphoid organ of penaeid prawns: a review. Fish Shellfish Immunol 29: 367–377. [DOI] [PubMed] [Google Scholar]
- Paździor, E. (2016) Shewanella putrefaciens ‐ A new opportunistic pathogen of freshwater fish. J Vet Res (Poland) 60: 429–434. [Google Scholar]
- Prachumwat, A. , Thitamadee, S. , Sriurairatana, S. , Chuchird, N. , Limsuwan, C. , Jantratit, W. , et al (2012) Shotgun sequencing of bacteria from AHPNS, a new shrimp disease threat for Thailand. Poster. URL http://library.enaca.org/Health/DiseaseLibrary/ahpns-poster-nru-summit.pptx
- Prachumwat, A. , Taengchaiyaphum, S. , Mungkongwongsiri, N. , Aldama‐Cano, D.J. , Flegel, T.W. , and Sritunyalucksana, K. (2019) Update on early mortality syndrome/acute hepatopancreatic necrosis disease by April 2018. J World Aquaculture Soc 50: 5–17. [Google Scholar]
- Qian, Y.‐F. , Xie, J. , Yang, S.‐P. , and Xiong, Q. (2013) Study of the microbiota in Pacific white shrimp (Litopenaeus vannamei) under varying O2 modified atmosphere packaging using PCR‐DGGE. J Pure Appl Microbi 7: 87–93. [Google Scholar]
- Qian, Y. , Yang, S. , Xie, J. , Wu, W. , Xiong, Q. , and Gao, Z. (2015) Studies on the putrefaction potential of the specific spoilage organisms from modified atmosphere packaged Litopenaeus vannamei . J Chin Institute Food Sci Technol 15: 85–91. [Google Scholar]
- Russell, A.B. , Peterson, S.B. , and Mougous, J.D. (2014) Type VI secretion system effectors: poisons with a purpose. Nat Rev Microbiol 12: 137–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanguanrut, P. , Munkongwongsiri, N. , Kongkumnerd, J. , Thawonsuwan, J. , Thitamadee, S. , Boonyawiwat, V. , et al (2018) A cohort study of 196 Thai shrimp ponds reveals a complex etiology for early mortality syndrome (EMS). Aquaculture 493: 26–36. [Google Scholar]
- Satomi, M. (2013) The family shewanellaceae. The Prokaryotes: Gammaproteobacteria: 597–625.
- Sirikharin, R. , Taengchaiyaphum, S. , Sanguanrut, P. , Chi, T.D. , Mavichak, R. , Proespraiwong, P. , et al (2015) Characterization and PCR detection of binary, pir‐like toxins from Vibrio parahaemolyticus isolates that cause acute hepatopancreatic necrosis disease (AHPND) in shrimp. PLoS ONE 10: e0126987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares, S.C. , Geyik, H. , Ramos, R.T.J. , de Sá, P.H.C.G. , Barbosa, E.G.V. , Baumbach, J. , et al (2016) GIPSy: genomic island prediction software. J Biotechnol 232: 2–11. [DOI] [PubMed] [Google Scholar]
- Suo, Y. , Li, E. , Li, T. , Jia, Y. , Qin, J. G. , Gu, Z. , and Chen, L. (2017) Response of gut health and microbiota to sulfide exposure in Pacific white shrimp Litopenaeus vannamei . Fish Shellfish Immunol 63: 87–96. [DOI] [PubMed] [Google Scholar]
- Thitamadee, S. , Prachumwat, A. , Srisala, J. , Jaroenlak, P. , Salachan, P.V. , Sritunyalucksana, K. , et al (2016) Review of current disease threats for cultivated penaeid shrimp in Asia. Aquaculture 452: 69–87. [Google Scholar]
- Thorell, K. , Meier‐Kolthoff, J.P. , Sjöling, Å. , and Martín‐Rodríguez, A.J. (2019) Whole‐genome sequencing redefines Shewanella taxonomy. Front Microbiol 10: 1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinwongger, S. , Nochiri, Y. , Thawonsuwan, J. , Nozaki, R. , Kondo, H. , Awasthi, S.P. , et al (2016) Virulence of acute hepatopancreatic necrosis disease PirAB‐like relies on secreted proteins not on gene copy number. J Appl Microbiol 121: 1755–1765. [DOI] [PubMed] [Google Scholar]
- Tran, L. , Nunan, L. , Redman, R.M. , Mohney, L.L. , Pantoja, C.R. , Fitzsimmons, K. , and Lightner, D.V. (2013) Determination of the infectious nature of the agent of acute hepatopancreatic necrosis syndrome affecting penaeid shrimp. Dis Aquat Organ 105: 45–55. [DOI] [PubMed] [Google Scholar]
- Van De Braak, C.B. , Botterblom, M.H.A. , Taverne, N. , Van Muiswinkel, W.B. , Rombout, J.H.W.M. , and Van Der Knaap, W.P.W. (2002) The roles of haemocytes and the lymphoid organ in the clearance of injected Vibrio bacteria in Penaeus monodon shrimp. Fish Shellfish Immunol 13: 293–309. [DOI] [PubMed] [Google Scholar]
- Venkateswaran, K. , Dollhopf, M.E. , Aller, R. , Stackebrandt, E. , and Nealson, K.H. (1998) Shewanella amazonensis sp. nov., a novel metal‐reducing facultative anaerobe from Amazonian shelf muds. Int J Syst Bacteriol 48 (Pt 3): 965–972. [DOI] [PubMed] [Google Scholar]
- Wang, Y. , Feng, N. , Li, Q. , Ding, J. , Zhan, Y. , and Chang, Y. (2013) Isolation and characterization of bacteria associated with a syndrome disease of sea urchin Strongylocentrotus intermedius in North China. Aquac Res 44: 691–700. [Google Scholar]
- Wangman, P. , Longyant, S. , Taengchaiyaphum, S. , Senapin, S. , Sithigorngul, P. , and Chaivisuthangkura, P. (2018) PirA & B toxins discovered in archived shrimp pathogenic Vibrio campbellii isolated long before EMS/AHPND outbreaks. Aquaculture 497: 494–502. [Google Scholar]
- Wechprasit, P. , Panphloi, M. , Thitamadee, S. , Sritunyalucksana, K. , and Prachumwat, A. (2019) Complete genome sequence of Shewanella sp. strain TH2012, isolated from shrimp in a cultivation pond exhibiting early mortality syndrome. Microbiol Resour Announc 8: e01703–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Organization for Animal Health (2016) Event summary: Hepatopancreatitis in Prawns, Australia. World Organization for Animal Health; URL http://www.oie.int/wahis_2/public/wahid.php/Reviewreport/Review/viewsummary?fupser=%26dothis=%26reportxml:id=19665 [Google Scholar]
- Xiao, J. , Liu, L. , Ke, Y. , Li, X. , Liu, Y. , Pan, Y. , et al (2017) Shrimp AHPND‐causing plasmids encoding the PirAB toxins as mediated by pirAB‐Tn903 are prevalent in various Vibrio species. Sci Rep 7: 42177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousfi, K. , Bekal, S. , Usongo, V. , and Touati, A. (2017) Current trends of human infections and antibiotic resistance of the genus Shewanella. Eur J Clin Microbiol Infect Dis 36: 1353–1362. [DOI] [PubMed] [Google Scholar]
- Zhu, S. , Zhang, C. , Wu, H. , Jie, J. , Zeng, M. , Liu, Z. , et al (2017) Spoilage of refrigerated (4°C) Litopenaeus vannamei: cooperation between Shewanella species and contribution of cyclo‐(L‐Pro‐L‐Leu)‐dependent quorum sensing. Int J Food Sci Technol 52: 1517–1526. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information, Figs S1–S18 and Tables S1–S5.
Fig. S1. Phylogeny of 16S rDNA genes of Shewanella khirikhana TH2012T and other Shewanella species. The neighbour‐joining phylogenetic tree with Jukes‐Cantor distance was based on 1452 aligned positions of 16S rDNA gene sequences. Moritella marina ATCC 15381 was used as an outgroup. Bootstrap values (expressed as percentages of 1000 replications) are shown at branching points. Most of the nodes were also recovered in the trees generated with the minimum evolution (Jukes‐Cantor distance), maximum‐likelihood (Jukes‐Cantor distance) and maximum parsimonious algorithms based on the same set of sequences, except those with Filled circles, or Filled squares were not recovered in the trees of maximum parsimonious, or both maximum parsimonious and maximum‐likelihood algorithms, respectively. Scale bar indicates estimated sequence divergence (substitutions per nucleotide position). Sequence accession numbers are shown in the parentheses after the species names.
Fig. S2. Phylogeny 16S rDNA genes of Shewanella khirikhana TH2012T and other Shewanella species including strains LV 5 and AK55. The neighbour‐joining phylogenetic tree with Jukes‐Cantor distance was based on 1176 aligned positions of 16S rDNA gene sequences. Moritella marina ATCC 15381 was used as an outgroup. Bootstrap values (expressed as percentages of 1000 replications) are shown at branching points. Most of the nodes were also recovered in the trees generated with the minimum evolution (Jukes‐Cantor distance), maximum‐likelihood (Jukes‐Cantor distance) and maximum parsimonious algorithms based on the same set of sequences, except those with Filled circles, or Opened squares were not recovered in the trees of maximum parsimonious, or maximum‐likelihood algorithms, respectively. Scale bar indicates estimated sequence divergence (substitutions per nucleotide position). Sequence accession numbers are shown in the parentheses after the species names.
Fig. S3. Phylogeny of atpA genes of Shewanella khirikhana TH2012T and other Shewanella species. The neighbour‐joining phylogenetic tree with Jukes‐Cantor distance was based on 1262 aligned positions of atpA gene sequences. Moritella marina ATCC 15381 was used as an outgroup. Bootstrap values (expressed as percentages of 1000 replications) are shown at branching points. Most of the nodes were also recovered in the trees generated with the minimum evolution (Jukes‐Cantor distance), maximum‐likelihood (Jukes‐Cantor distance) and maximum parsimonious algorithms based on the same set of sequences, except those with Opened squares, or Filled squares were not recovered in the trees of maximum‐likelihood, or both maximum parsimonious and maximum‐likelihood algorithms, respectively. Scale bar indicates estimated sequence divergence (substitutions per nucleotide position). Sequence accession numbers are shown in the parentheses after the species names.
Fig. S4. Phylogeneny of mreB genes of Shewanella khirikhana TH2012T and other Shewanella species. The neighbour‐joining phylogenetic tree with Jukes‐Cantor distance was based on 785 aligned positions of mreB gene sequences. Moritella marina ATCC 15381 was used as an outgroup. Bootstrap values (expressed as percentages of 1000 replications) are shown at branching points. Most of the nodes were also recovered in the trees generated with the minimum evolution (Jukes‐Cantor distance), maximum‐likelihood (Jukes‐Cantor distance) and maximum parsimonious algorithms based on the same set of sequences, except those with Filled circles, or Filled squares were not recovered in the trees of maximum parsimonious, or both maximum parsimonious and maximum‐likelihood algorithms, respectively. Scale bar indicates estimated sequence divergence (substitutions per nucleotide position). Sequence accession numbers are shown in the parentheses after the species names.
Fig. S5. Phylogeny of rpoA genes of Shewanella khirikhana TH2012T and other Shewanella species. The neighbour‐joining phylogenetic tree with Jukes‐Cantor distance was based on 789 aligned positions of rpoA gene sequences. Moritella marina ATCC 15381 was used as an outgroup. Bootstrap values (expressed as percentages of 1000 replications) are shown at branching points. Most of the nodes were also recovered in the trees generated with the minimum evolution (Jukes‐Cantor distance), maximum‐likelihood (Jukes‐Cantor distance) and maximum parsimonious algorithms based on the same set of sequences, except those with Filled circles, Opened squares, or Filled squares were not recovered in the trees of maximum parsimonious, maximum‐likelihood, or both maximum parsimonious and maximum‐likelihood algorithms, respectively. Scale bar indicates estimated sequence divergence (substitutions per nucleotide position). Sequence accession numbers are shown in the parentheses after the species names.
Fig. S6. Photomicrographs of unstained cell morphology and Gram stain morphology of Shewanella khirikhana TH2012T.
Fig. S7. Colony characteristics of Shewanella khirikhana TH2012T from stereomicroscope (A) and light microscope (B).
Fig. S8. Graph showing time course OD600 of Shewanella khirikhana TH2012T cultured at various salinities (%NaCl).
Fig. S9. Graph showing time course OD600 of Shewanella khirikhana TH2012T cultured at various pH’s.
Fig. S10. Growth profiles of Shewanella khirikhana TH2012T measured by optical density at 600 nm (OD600).
Fig. S11. Graph showing Shewanella khirikhana TH2012T viable bacterial cell counts (CFU ml−1) vs. OD600 during hours 2–4 of the growth curve experiment (hours are indicated by colors) in Fig. S10.The grey area denotes the 95% confidence level interval for predictions from a linear model of log10 CFU ml−1 versus log10 OD600. R 2 = 0.7814, P = 4.902 x 10‐8.
Fig. S12. Graph showing the regression line for shrimp mortality versus bacterial concentration (log10 CFU ml−1) for Shewanella khirikhana TH2012T immersion bioassay up to 60 h post immersion. The grey area denotes the 95% confidence level interval for predictions from a linear model. R 2 = 0.6945, P = 0.03933.
Fig. S13. Photomicrographs of histology of the anterior midgut cecum (AMC) of normal shrimp and moribund shrimp from immersion with Shewanella khirikhana TH2012T. (A & B) Example of normal AMC tissue at low and high magnification, respectively. (C & D) Example of histopathology from a moribund test shrimp with abnormally vacuolated epithelial cells containing few eosinophilic inclusions. (E & F) Example histopathology showing vacuolated epithelial cells containing many eosinophilic inclusions.
Fig. S14. Example photomicrographs of histology of the sub‐cuticular epithelium of the stomach of normal shrimp and of moribund shrimp from immersion with Shewanella khirikhana TH2012T. (A & B) Normal sub‐cuticular epithelial cells at low and high magnification showing relatively non‐vacuolated cytoplasm. (C & D) Abnormally vacuolated sub‐cuticular epithelial cells from a moribund shrimp specimen at low and high magnification. Asterisks (*) indicate the same position in the low and high magnification photomicrographs.
Fig. S15. Photomicrographs of histology of the E‐cell region of the hepatopancreas of normal shrimp and moribund shrimp from immersion with Shewanella khirikhana TH2012T. (A & B) Example of normal E‐cells in tubular cross section at low and high magnification, respectively, showing dense non‐vacuolated cytoplasm. (C & D) Example of abnormally vacuolated E‐cells in cross‐section. (E & F) Similar to C and D except that the tubules are in longitudinal section. Asterisks (*) indicate the same position in the low and high magnification photomicrographs.
Fig. S16. Example photomicrographs of histology of hematopoietic tissue (HT) of normal shrimp and of moribund shrimp from immersion with Shewanella khirikhana TH2012T. (A, B & C) Normal HT at progressively higher magnification showing cells that lack eosinophilic cytoplasmic inclusions. In B and C, the arrows indicate the same cell nucleus at low and high magnification. (D, E & F) Abnormal HT at progressively higher magnification showing the presence of eosinophilic cytoplasmic inclusions. The asterisks indicate the same position at different magnifications D, E and F and the arrows mark the same eosinophilic inclusion in E & F. Note that the highest magnification (100x objective) is needed for easy identification of the unique inclusions.
Fig. S17. Photomicrographs of bacterial cells seen in some of the moribund, challenged shrimp. (A) Bacterial cells of mixed size and morphology together with sloughed epithelial cells in the lumen of a hepatopancreatic (HP) tubule. (B) Few bacterial cells of uniform morphology in the lumen of an HP tubule with an intact epithelial cell layer.
Fig. S18. Graphical map of the plasmid pSTH1. From outside to the center: Genes on forward strand (colored by COG categories), Genes on reverse strand (colored by COG categories). COG color and functional designations are described in Supporting Information Table S5.
Table S1. PCR primer sequences used in this study.
Table S2. Length (bp) of atpA, mreB, and rpoA sequences in strains TH2012T, TBRC 5001, SMK1‐12T, and SB2BT, and percentage of nucleotide identities of TH2012T to corresponding orthologous sequences in TBRC 5001, SMK1‐12T, and SB2BT.
Table S3. Shewanella khirikhana TH2012T viable bacterial cell counts (CFU ml−1) from incubation experiments at various temperature (ºC). UD, unable to determine due to either no colony formation or the number of colonies were not in the range of 10–300 colonies.
Table S4. Shewanella khirikhana TH2012T genome project information.
Table S5. Codes, number and percentage of genes, descriptions and colors associated with general COG functional categories.


