Abstract
Context
Neonatal severe hyperparathyroidism (NSHPT) is rare and potentially lethal. It is usually from homozygous or heterozygous germline-inactivating CASR variant(s). NSHPT shows a puzzling range of serum calcium and parathyroid hormone (PTH) levels. Optimal therapy is unclear.
Evidence acquisition
We categorized genotype/phenotype pairings related to CASRs. For the 2 pairings in NSHPT, each of 57 cases of neonatal severe hyperparathyroidism required calcium, PTH, upper normal PTH, and dosage of a germline pathogenic CASR variant.
Evidence synthesis
Homozygous and heterozygous NSHPT are 2 among a spectrum of 9 genotype/phenotype pairings relating to CASRs and NSHPT. For the 2 NSHPT pairings, expressions differ in CASR allelic dosage, CASR variant severity, and sufficiency of maternofetal calcium fluxes. Homozygous dosage of CASR variants was generally more aggressive than heterozygous. Among heterozygotes, high-grade CASR variants in vitro were more pathogenic in vivo than low-grade variants. Fetal calcium insufficiency as from maternal hypoparathyroidism caused fetal secondary hyperparathyroidism, which persisted and was reversible in neonates. Among NSHPT pairings, calcium and PTH were higher in CASR homozygotes than in heterozygotes. Extreme hypercalcemia (above 4.5 mM; normal 2.2–2.6 mM) is a robust biomarker, occurring only in homozygotes (83% of that pairing). It could occur during the first week.
Conclusions
In NSHPT pairings, the homozygotes for pathogenic CASR variants show higher calcium and PTH levels than heterozygotes. Calcium levels above 4.5 mM among NSHPT are frequent and unique only to most homozygotes. This cutoff supports early and robust diagnosis of CASR dosage. Thereby, it promotes definitive total parathyroidectomy in most homozygotes.
Keywords: CASR, GNA11, AP2S1, gene dosage, gene variant, hyperparathyroid, parathyroidectomy, hypercalcemia, severe, hypocalciuric, neonate, homozygous
Neonatal severe hyperparathyroidism (NSHPT) (OMIM 239200) is a rare disorder with approximately 100 reported cases (1–5). Half were reported after 1993, the year of identification of the CASR gene (OMIM 601199) and the year of first showing CASR’s frequent and likely pathogenic variants in NSHPT (2, 3).Two NSHPT phenotypes with biallelic versus monoallelic variants of the CASR gene are part of a wide spectrum of 9 genotype/phenotype pairings related to inactivation of CASRs.
If untreated, NSHPT is often lethal or otherwise catastrophic during infancy (6,[meeting abstract] 7). However, very early therapy, using major interventions of medicine and/or parathyroidectomy (PTX), may support a promising long-term survival (4). Unfortunately, neuromotor retardation may persist after otherwise successful therapy. This retardation likely correlates with microcephaly and/or with the duration or amplitude of hypercalcemia.
Many patients with NSHPT have family members with the closely related genotype/phenotype pairing of familial hypocalciuric hypercalcemia (OMIM 145980) (FHH) (8, 9); FHH expresses lifelong autosomal dominant and usually benign hypercalcemia. Sixty percent of patients with FHH kindreds have a heterozygous inactivating and pathogenic variant in CASR; these kindreds are also termed FHH type 1 (FHH1) (OMIM 145980). The CASR gene encodes a plasma membrane calcium-sensing receptor (CaSR); it is a G-protein-coupled receptor most densely located in the parathyroid glands (2, 3). This location helps to explain why germline CASR variant(s) cause NSHPT, a disorder arising mainly in the parathyroid glands.
Most patients with NSHPT have homozygous or heterozygous germline-inactivating and pathogenic variant(s) of the CASR gene (2–5). In FHH1 there is 3% to 4% nonsense variant(s) of the CASR gene as compared with 30% to 40% in NSHPT(2, 10). Currently, the biochemical features of patients with NSHPT with homozygous versus heterozygous variants of CASR are not clearly distinguished from one another. For example, there is a relative hypocalciuria in FHH1 and in NSHPT, but these have not been compared or analyzed in NSHPT (11).
A diagnosis of NSHPT should be made at the earliest possible age; it should lead both to urgent efforts at stabilization of the neonate and also to early major interventions. The interventions have focused mainly upon total or subtotal parathyroidectomy (PTX) (1–5); subtotal PTX can include total PTX and autograft of fresh parathyroid tissue. Furthermore, some patients benefit before surgery or even without surgery, due only to having received major medical interventions (bisphosphonates, calcimimetics, electrolyte management, dialysis, and/or respiratory support) (4).
Among all of the reports of NSHPT in homozygotes or in heterozygotes for CASR variants, there has been a very large and even puzzling range of the core values of serum calcium and parathyroid hormone (PTH). Roles of dosage of the CASR variant and grade of pathogenicity of the variant in understanding this range have been suggested but not analyzed in detail (4, 12). Herein, we found that the maximal value of serum calcium was highly characteristic and distinct within the homozygote group.
Methods
Genotype and phenotype
We analyzed expressions of genotypes and phenotypes related to CASRs and to NSHPT in order to clarify 2 allelic dosages of CASRs, 5 grades of pathogenicity of CASR variants, and sufficiency of external calcium fluxes for the fetus. One large, composite display included a brief overview of the specific phenotype that accompanied each of the CASR allelic genotypes.
Case reports of NSHPT
Case reports of NSHPT were identified and retrieved from PubMed searches using the terms hyperparathyroid, hypercalcemia, severe, crisis, fetus, utero, newborn, neonatal, infancy, CASR, AP2S1, GNA11, calcium-sensing, calcimimetic, bisphosphonate, parathyroidectomy, and autograft. All available case reports of NSHPT, including abstracts and posters, through September 31, 2019, were included. If a case was reported in more than one publication, the findings were combined, and it was entered one time. Although neonatal generally implies the first 3 months of life, a case was included in the main analysis if the onset of symptoms or signs was at less than the age of 7 months (OMIM 145980). Furthermore, a case was tabulated in the data analysis only if it included each of the following: serum calcium, serum PTH, the normal upper limit of PTH (for a ratio calculation), and report of mono- versus biallelic dosage of germline and likely pathogenic variant(s) of CASR. Reports of CASR variants were accepted with or without demonstration about pathogenic properties of the variant via an in vitro assay of calcium responsivity (11).
Only severely affected neonates were included in the analysis of the 2 formats of NSHPT. Criteria for severe disease in NSHPT have not been established, nor even discussed. We used a broad but demanding definition. To be labeled as severe, the hypercalcemic neonate had to express any one among the following: (1) the term severe or its equivalent in the title or in the text of the case report; (2) a skeletal feature of severe hyperparathyroidism: bell-shaped thorax, narrowed thorax, multiple fractures of the ribs and/or long bones, disorganized metaphyses, osteopenia, osteoporosis, or subperiosteal resorption (13); (3) a biochemical feature of severe hyperparathyroidism: extreme hypercalcemia (defined as calcium above 4.5 mM) or very high PTH, defined as a ratio greater than 10 for [maximal PTH] divided by [upper normal limit of normal PTH]; (4) another severe systemic feature presumed to have resulted from primary or secondary hyperparathyroidism (HPT): hypotonia (or floppy baby), respiratory distress including advanced pneumonia, lethargy, failure to thrive, poor sucking, constipation, dehydration, or obtundation.
Laboratory values in NSHPT
Data were tabulated from NSHPT reports for the maximal serum calcium, maximal serum PTH, and upper limit of normal for PTH. The data also included codon identification, location in the CaSR protein for the variant codon, and allelic dosage of the pathogenic or likely pathogenic variant(s) of CASR. Serum calcium or PTH were entered as one value at the maximum for that case from the text, tables, and figures. PTH was analyzed as the ratio of [its maximal value] divided by [upper limit of normal in that immunoassay]. This ratio method for PTH allowed the comparisons of PTH measurements from immunoassays with widely differing normal ranges such as those based on different antisera and/or different assay standards (14).
Kindreds or neonates with NSHPT and CASR heterozygosity were classified as 2 genotype/phenotypes, specifically with low-grade or high-grade FHH1. This was based on the mean serum calcium per kindred or per neonatal case being below or above an inflection point. This inflection was at 2.9 mM in a visual fit to the assembled calcium data, averaged by kindred or by case. This inflection was derived from 3 series, each of which supported the derivation of this parameter among 15 or more families or cases (15–17). The breaks or inflections in data for average serum calcium were at 3.0, 2.8, and 3.0 mM for series (15), series (16), and series (17), respectively. The inflection in the data on familial calcium or neonatal calcium resulted in classifying approximately 50% of heterozygous families as low grade and 50% as high grade. Similarly, each heterozygous CASR variant codon accompanying FHH1 was also classified as expressing that same grade of severity in silico as in the heterozygous family in vivo. We classified phenotypes and allelic variants as grades 0 to 4 with grade 0 representing benign (or normal or wild type) and grade 4 as pathogenic, the highest grade encountered in reports or in theory. These 5 grades were similar to the 5 tiers for variants in the consensus classification of severity for variants, and usually replacing use of the terms mutation or polymorphism. (18)
Statistics
Data are analyzed by simple descriptive statistics or by frequencies and percentages. All data distributions on their raw and logarithmic transformed scales were assessed for assumptions; nonparametric tests were carried out as applicable to confirm consistency of conclusions. Wilcoxon rank-sum tests and t tests compared maximal serum calcium and maximal PTH data between the homozygote and heterozygote groups. Linear regression analysis tested the relation of the paired maximal PTH and maximal calcium and compared the 2 groups. Data were analyzed using SAS version 9.4 (SAS Institute, Inc, Cary, NC). Differences between groups were based on magnitude of differences and confidence intervals.
Results
Overview of NSHPT genotypes and phenotypes
Spectrum of 9 genotype/phenotype pairings.
Variant(s) of the CASR gene cause the vast majority of NSHPT kindreds and cases in a spectrum of related genotypes/phenotypes. The size of this majority may reflect a selection bias against reporting several thoroughly evaluated cases with no identified variant of an identified and causative gene. We explored the possibility of 2 distinct formats for NSHPT within this spectrum, representing biallelic versus monoallelic dosage for pathogenic variants of the CASR gene.
In fact, these would be just 2 important formats among a broader and partly speculative distribution of 9 genotype/phenotypes related closely to the dosage of CASR and to distinct phenotypes related to NSHPT. The extended display of 9 genotype/phenotype pairings related to CASR and to NSHPT helps to clarify complexities within this broad spectrum. (Fig. 1-A to Fig. 1-I) (19–43).
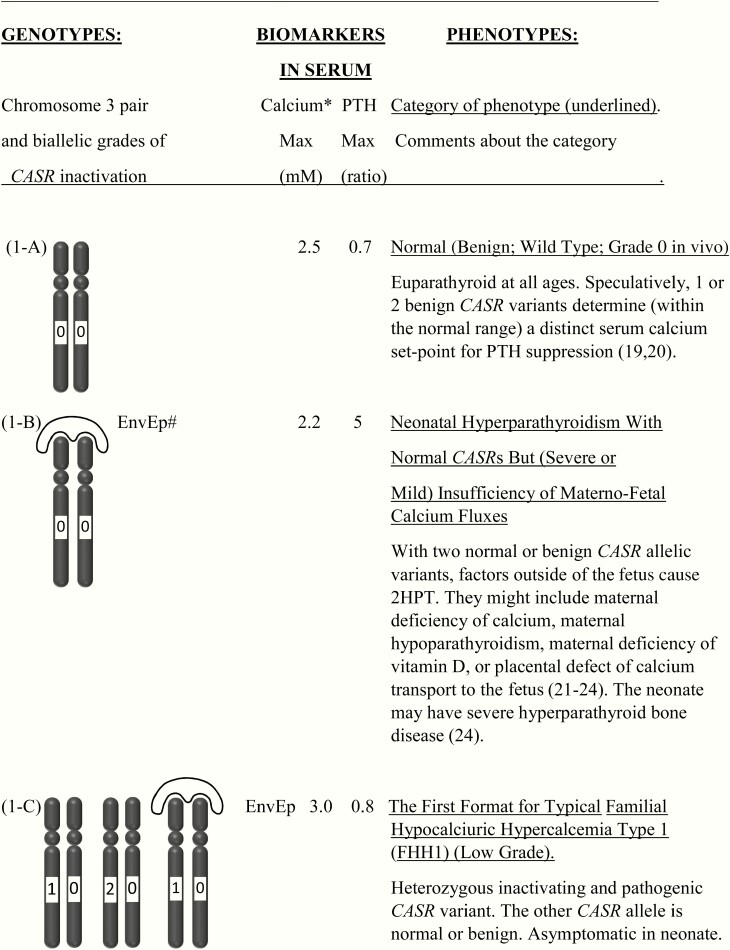
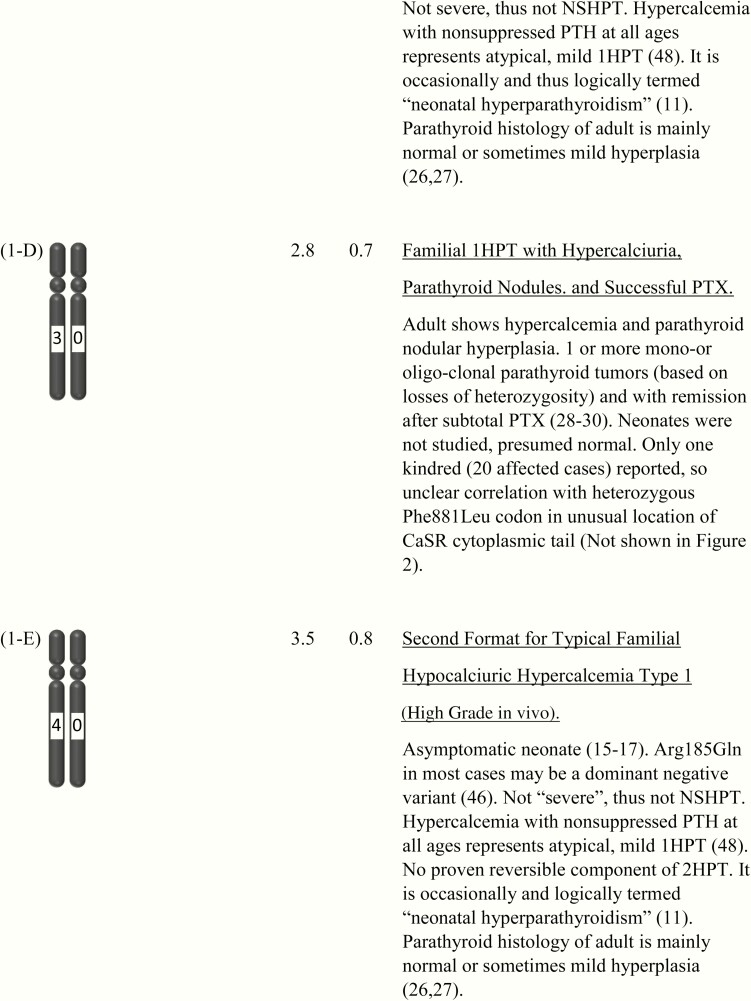
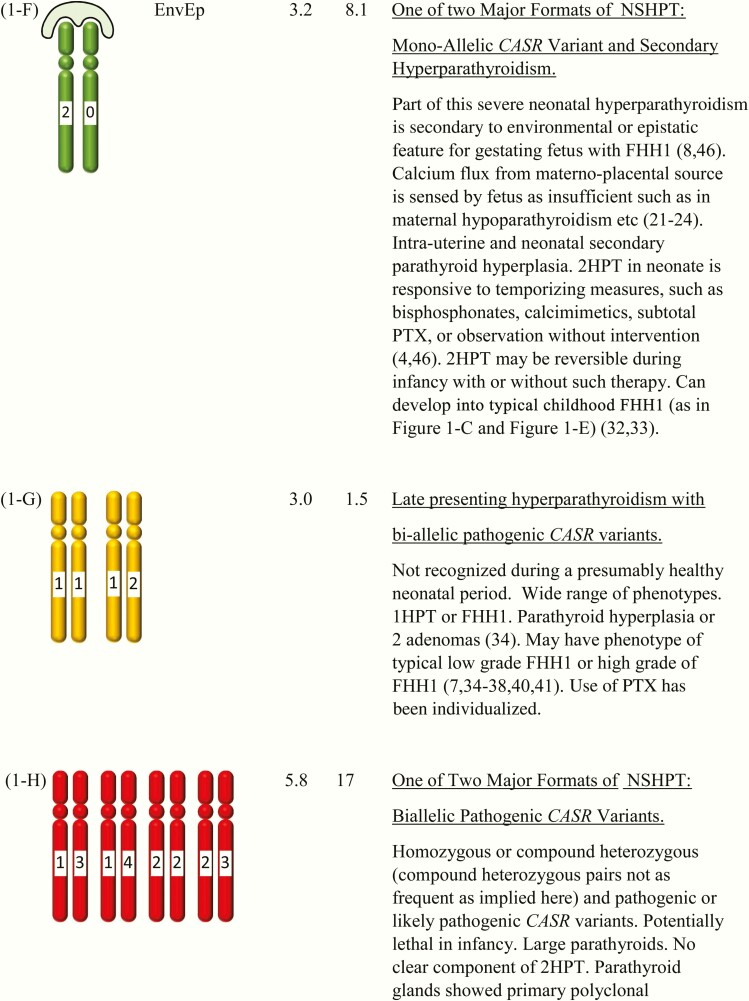
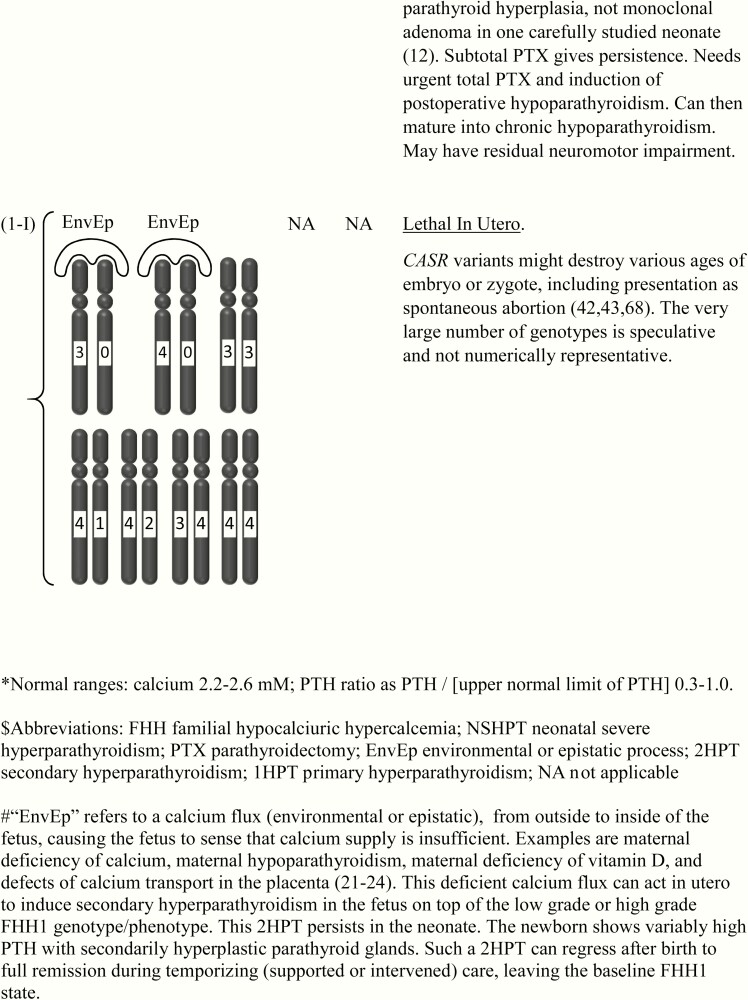
Figure 1.
This shows the category related to neonatal severe hyperparathyroidism (NSHPT), dependent on secondary hyperparathyroidism and/or upon the dosage and stages of inactivation of CASR alleles at chromosome 3q13.3-q21.1. This is a speculative synthesis of data from human and animal species, intended to give a logical background for a spectrum of phenotypes and genotypes. The number of chromosomal pairs in a subpanel is somewhat arbitrary and intended to accommodate overall most of the many possibilities considering 5 grades of severity for each allele. The CASR also causes monogenic phenotypes by activating variants such as in autosomal dominant hypoparathyroidism, autosomal resistant hypoparathyroidism, or type V Bartter syndrome (2, 3); these activating variants are not shown here. Degree of inactivation varies from 0 to 4 or from benign (no inactivation) to pathogenic (maximal inactivation). Degree of inactivation of the calcium-sensing receptor in vitro correlates with serum calcium in vivo (11). The GNA11 and AP2S1 genes can be incorporated into this speculative scheme in similar but more complicated ways (not shown). Red is NSHPT with biallelic-inactivating and pathogenic variant of the CASR (Fig. 1-H). Green is NSHPT with monoallelic-inactivating and pathogenic or likely pathogenic CASR variant and secondary hyperparathyroidism (Fig. 1-F). Yellow is late-onset biallelic-inactivating and pathogenic or likely pathogenic variant of the CASR (Fig. 1-G).
Germline CASR-inactivating and pathogenic variant(s) present as a monogenic disturbance with a strong penetrance in the form of several phenotypes related to NSHPT. The spectrum of phenotypes varies from the mildest expressions (speculatively with benign variant(s) of CASR as a determinant of the serum calcium set point for suppression of PTH secretion inside of the normal range) (19, 20) to the severest expressions (speculatively as unrecognized lethality from higher-grade pathogenic variants of CASR, expressed during early gestation) (42, 43). NSHPT and 3 other genotype/phenotype pairs most closely related to NSHPT are treated in more detail in the Discussion.
Differences in grades of variant pathogenicity.
The concept of 2 groups based on 2 categories of DNA dosage supports the possibility that the severely affected homozygous cases of NSHPT usually express 2 copies of a low grade of pathogenic CASR variants. This is often evidenced by high normal to minimally elevated serum calcium in one or both of their affected parents and in other heterozygous relatives (38, 44, 45).
Many but not all heterozygotes express in vivo one copy of a high grade of a CASR variant, especially the arginine to glutamine variant at amino acid codon 185 (Arg185Gln) (11, 15–17) (Fig. 2). Other heterozygotes express one copy of a lower grade CASR variant. Among heterozygous NSHPT, a hot spot for the heterozygous variant at Arg185Gln (see below) is the highest grade of CASR variant, which could be characterized from the available data in vitro and in vivo (11). This pathogenic variant (and perhaps several other similarly toxic ones) may be particularly high grade by exerting a dominant negative effect, as was suggested from a study in vitro (46). However, there has been no report confirming an in vivo dominant negative effect by featuring a true heterozygote with a presentation as if it were a severely affected homozygote ([meeting abstract] 47).
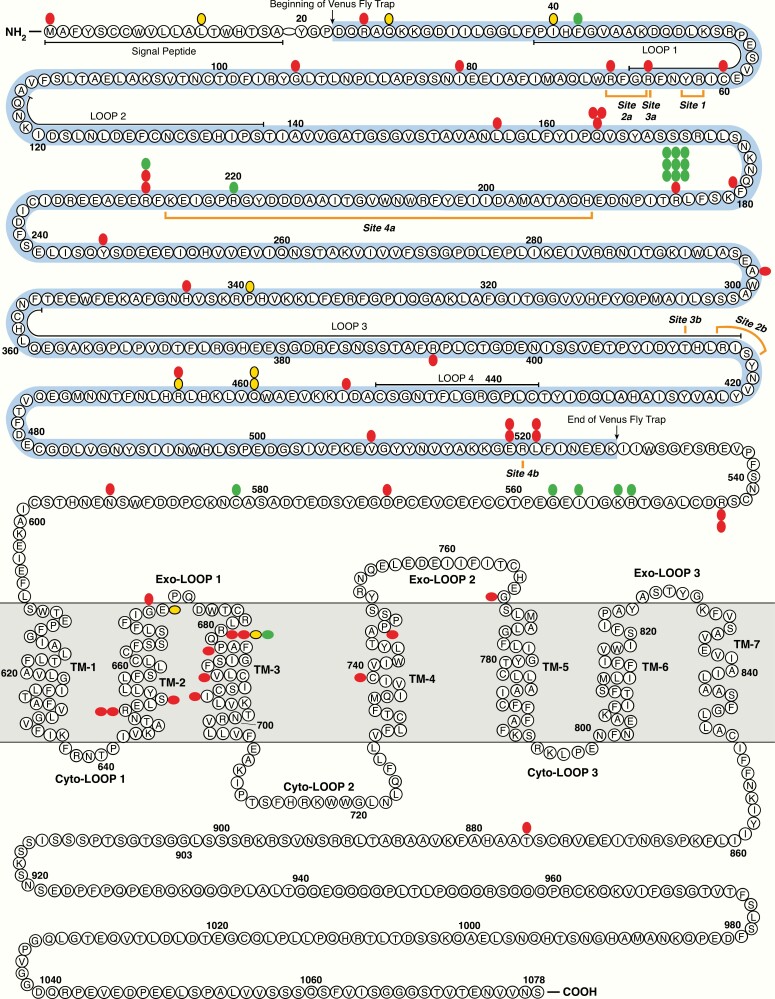
Figure 2.
Two-dimensional (linear) display of the domains of the calcium-sensing receptor (CaSR) and codons of pathogenic or likely pathogenic inactivating variants. Predicted domains of the CaSR are modified from (51). The Venus fly-trap domain is colored (light blue). Seven transmembrane domains are abbreviated as TM1-7. The 4 theoretical calcium-binding sites (outlined in orange) are as determined from crystallography of the extracellular domain of the CaSR (52); site 1 R62-Y63; site 2a R66-R69 site 2b R415-S417; site 3a R66 site 3b T412; site 4a H192-K225 and Site 4b R520. Another group also used crystallography but identified the calcium-binding sites in partly different locations (53). Homozygous severe neonatal-presenting variants (red); compound heterozygous NSHPT variants as 2 separate mutant codons (also red). Heterozygous severe neonatal variants (green), homozygous late-presenting variants (yellow). Three different homozygous splicing variants (in severely affected neonates) are not included.
Heterozygotes with secondary hyperparathyroidism caused by fetal calcium insufficiency.
In detail and particularly among heterozygotes with NSHPT, the phenotypes can differ with respect to exogenous (environmental and epistatic) disturbances related to the sufficiency of maternofetal calcium fluxes (Fig. 1-A to Fig. 1-I). The possible environmental or epistatic disturbances in calcium fluxes include maternal calcium deficiency, maternal hypoparathyroidism, maternal deficiency of vitamin D, and defects of calcium transport in the placenta (21–24). Each can cause secondary hyperparathyroidism (2HPT) in utero, and the 2HPT of each can persist into the neonate.
The genotype/phenotype disturbances also include several complex phenotypes such as NSHPT with a heterozygous pathogenic CASR variant (Fig. 1-F), which share combinations between primary hyperparathyroidism and 2HPT (8, 45, 46). These complex genotypes are highlighted by a logo drawn outside of the chromosomes with the abbreviation of EnvEp; this abbreviation implies an environmental or epistatic influence on calcium fluxes (Fig. 1).
Analysis of NSHPT mutations and biochemistry
Codon dosages and locations.
An inactivating germline variant of GNA11 (OMIM 19313) or AP2S1 (OMIM 602242) can occasionally cause FHH, termed FHH2 or FHH3 respectively (OMIM 145981 and 600740) (2). However, there were no reports of NSHPT with a homozygous or heterozygous variant of GNA11. There were 3 case reports of NSHPT with a heterozygous variant of AP2S1 and none with a homozygous AP2S1 variant (48,[meeting abstract] 49, 50). These 3 cases were excluded from the present analysis for simplification. Their inclusion would have had no important effect on any of the analyses.
There were 36 NSHPT probands with biallelic and pathogenic or likely pathogenic variants of CASR: homozygous (N = 31) or compound heterozygous (N = 5) (Table1). There were 21 cases of NSHPT with a monoallelic and pathogenic or likely pathogenic variant of CASR. The similar frequency of reports for either homozygotes or heterozygotes suggests that the 2 allelic dosages of CASR variants for NSHPT are about equally represented in the general population. This makes it all the more important to distinguish promptly the CASR allelic dosage in each case.
Table 1.
Descriptive statistics for serum calcium and serum PTH between heterozygotes versus homozygotes for CASR variant(s).a,b,c
| Heterozygotes (Monoallelic) n = 21 | Homozygotes (Biallelic) n = 36 | P Value | |
|---|---|---|---|
| Maximal calcium (mM) | 3.20 ± 0.26 (3.08–3.32) | 5.83 ± 1.54 (5.31–6.35) | < .001 |
| Maximal PTH (ratio) | 8.14 ± 8.98 (4.06–12.23) | 17.42 ± 11.87 (13.41–21.44) | .003 |
| Slope of maximal calcium (mM) versus maximal PTH (ratio) | -0.00238 ± 0.0300 (–0.01608 to +0.01132) | 0.01413 ± 0.1326 (–0.03077 to +0.05903) | 0.52 |
| Maximal calcium at PTH = 0 (mM) | 3.22 ± 0.36 (3.06–3.38) | 5.58 ± 2.78 (4.64–6.53) | < .001 |
Data are mean ± standard deviation (and 95% confidence interval).
aSerum calcium was scored as the maximal value for that case. Serum PTH was scored as the ratio of [maximal PTH] divided by [upper normal PTH].
bFive compound heterozygotes are included within the homozygote group.
cNormal ranges: calcium 2.2 to 2.6 mM; PTH ratio 0.3 to 1.0.
Abbreviation: PTH, parathyroid hormone.
Both the homozygous and the heterozygous germline variant codons for 3 phenotypes clustered in the predicted extracellular and transmembrane domains of the CaSR; thus they almost excluded the predicted long cytoplasmic tail (10, 51–53) (Fig. 2). There were several differences between the distributions of the homozygous versus heterozygous variants (Fig. 2). Codons 43 to 178 had no variants in heterozygotes but 10 pathogenic variants in homozygotes; similarly, the transmembrane domains 2 to 5 had only 1 codon with a pathogenic variant among heterozygotes but 12 codons with pathogenic variants in homozygotes. Crystallography has mapped these inactivating (and activating not shown) pathogenic variants mainly to the hinge region between the 2 major subdomains of the Venus fly trap domain and to the dimerization interface (52, 53).
Mutational hot spots.
The extracellular domain of the CaSR has arginine at 25 of 606 (4.1%) residues (Fig. 2). Among 52 codons in this domain with a pathogenic or likely pathogenic variant, 10 (19%) are arginine.
Several of the variants repeated in identical codon locations 2 to 3 times (mini hot spots) in apparently unrelated families. In particular, among 21 cases of heterozygous NSHPT, 10 cases from 9 apparently unrelated kindreds have the same Arg185Gln variant (Fig. 2); this was caused by a CGG to CAG transition (46). These 9 kindreds included at least 2 with a proved de novo variant of Arg185Gln; there were another 2 variants highly likely to be de novo based on normal serum calcium in both parents (46, 54–56). One NSHPT family with a compound heterozygous variant included a heterozygous arginine to termination variant at codon 185 (57). All of these findings favor an independent variant hot spot at codon 185 and weigh strongly against common ancestry for the variant among most or all of these 9 families. Arginine to glutamine is also a typical hot spot for variants in other genes, attributable in part to frequent mutation at CpG bases (58–62).
Among 17 different heterozygous CASR variants compared previously in vivo versus in vitro, neonatal cases with Arg185Gln expressed the highest average serum calcium of 3.2 mM and the greatest disturbance of calcium responsivity in vitro (11). Codon 185 is centered among the 4 predicted calcium-binding sites of the CaSR, but other lower grades of variant codons have a similar position (52). Thus, the frequency and pathogenicity of the codon 185 hot spot in vivo have not been fully explained.
Comparison of serum calcium and PTH between mono- and biallelic forms of NSHPT.
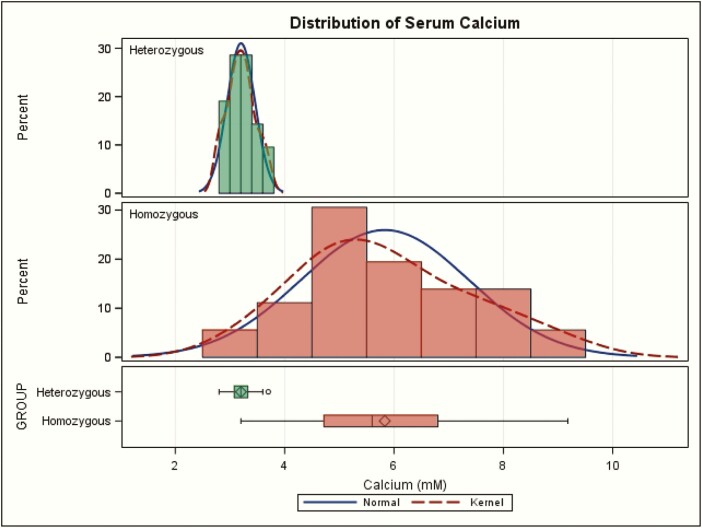
Maximal serum calcium values showed a slightly skewed distribution within the homozygote group or within the heterozygote group (Fig. 3). Logarithmic transformation did not normalize the distributions, and nonparametric comparison yielded the same conclusions (not shown). There was a highly significant difference of maximal serum calcium means [standard deviation (SD)] between the 2 groups: 5.83 [1.54] mM versus 3.20 [0.26] mM P < 0.001 (normal 2.2–2.6 mM) Table 1 and Fig. 3. Hypercalcemia values above 3.7 mM were seen only in the CASR homozygotes. Thus, extreme hypercalcemia (defined empirically as greater than 4.5 mM) is a cutoff that is highly conservative and approximately100% specific for homozygous NSHPT. It comfortably excludes all heterozygotes and 6 homozygotes, but it still encompasses 30 of 36 (83%) among homozygotes. It occurred during presentation at initial testing as early as at 1 to 6 days of life (63,[meeting abstract] 64, 65–67) but not always at this earliest age (68). A more aggressive empirical cutoff at 4.0 mM calcium would retain 100% specificity and encompass 34 of 36 (94%) of homozygotes.
Figure 3.
Maximal serum calcium values in homozygotes (red) versus heterozygotes (green) for CASR variant(s) in neonatal severe hyperparathyroidism. The kernel plot is a variation of the histogram that shows a smoother line. The sideway box at the bottom shows the following: length represents the interquartile range (distance between the lower (25th) and higher (75th) percentiles); symbol in the box is the group mean; line in the middle of the box is the group median; the whiskers extend to the minimum and maximum values.
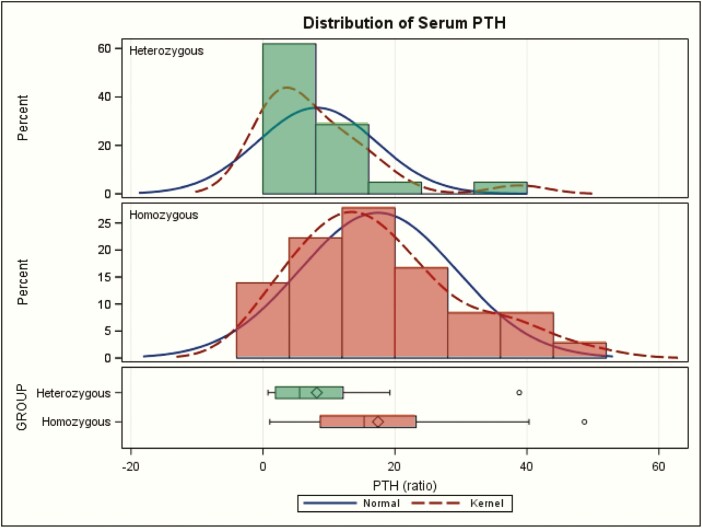
Like serum calcium, the maximum serum PTH values also showed a mildly skewed distribution within homozygotes or within heterozygotes (Fig. 4). Logarithmic transformation did not normalize the distributions, and nonparametric comparison yielded the same conclusions (not shown). There was a significant difference of maximal serum PTH between the 2 groups (ratio of 17 ± 12 versus 8 ± 9 P = 0.003; normal 0.3–1 (Table 1 and Fig. 4), but there was also substantial overlap in these 2 distributions (Fig. 4).
Figure 4.
Maximal serum parathyroid (PTH) values (as ratio of [maximal PTH] divided by [upper normal PTH]) in homozygotes (red) versus heterozygotes (green) for CASR variants with neonatal severe hyperparathyroidism. The kernel plot is a variation of the histogram that shows a smoother line. The sideway box at the bottom shows the following: length represents the interquartile range (distance between the lower (25th) and higher (75th) percentiles); symbol in the box is the group mean; line in the middle of the box is the group median; the whiskers extend to the minimum and maximum values.
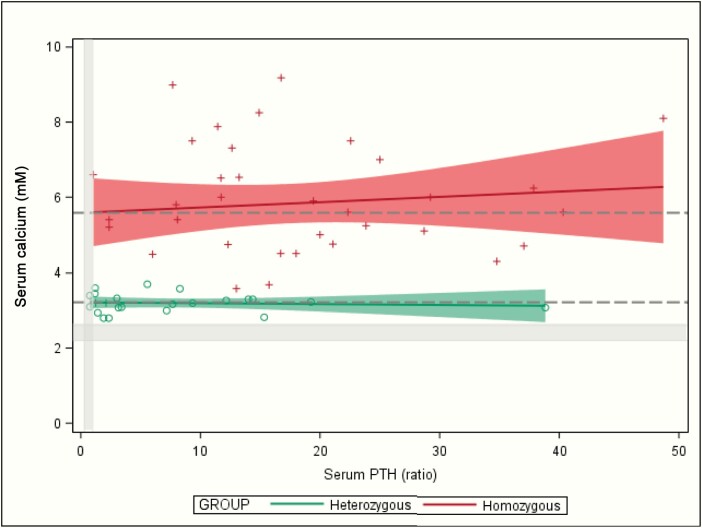
Paired maxima (PTH versus calcium) showed a linear distribution with a slightly positive slope in homozygotes but not in heterozygotes (Table 1 and Fig. 5). With extrapolation of PTH back to zero (ie, a model for postoperative total hypoparathyroidism), the PTH intercept of maximal calcium was 5.6 mM in homozygotes and 3.2 in heterozygotes; P < 0.001( Table 1 and Fig. 5; [normal calcium 2.2–2.6 mM] Fig. 5).
Figure 5.
Regression of maximal serum parathyroid hormone (PTH) versus maximal serum calcium in homozygotes (red) versus heterozygotes (green) for CASR variants with neonatal severe hyperparathyroidism. The red and green shaded zones are the 95% confidence ranges for the regressions. The 2 Y-intercepts (for calcium at PTH = zero) are drawn as 2 dashed horizontal lines across the whole figure. Normal ranges are narrow on these wide scales and are shaded gray: calcium 2.2 to 2.6 mM; PTH ratio 0.3 to 1.0.
Discussion
Genotype/phenotype pairings related to CASR and to NSHPT
This analysis has featured some 9 pairings of genotypes and phenotypes, each with variable inactivation of the CASR alleles and related to formats like NSHPT. Defining characteristics among the genotype/phenotype pairings were attributed to differences in allelic dosage of CASR, differences in grade of pathogenicity of variants of CASR, and differences in sufficiency of calcium fluxes between the maternoplacental unit and the fetus. A similar tripartite formulation can be informative for congenital overactivity of other genes and in other tissues.
The first characterized among 2 major formats of NSHPT: biallelic pathogenic variant(s) of CASR
The first case reports of clearcut NSHPT were in 1947 and 1948. Maximum serum calcium levels were 5.5 mM and 5.5 mM; those 2 patients died at ages 5 and 11 months, respectively (69, 70). Over 5 decades each of 7 (or more) reports centered upon a further step in understanding discrete findings about the same case of homozygous NSHPT (44, 70–75). The second of these 2 patients had consanguineous parents who gave birth to another severely affected sibling 15 years after the birth of their first child (71). Thus, both familial occurrence and recessive transmission already had been suggested for NSHPT cases in that family in 1962. Several other kindreds with presumed recessive cases of NSHPT have other family members with low grades of FHH, suggesting FHH as the trait occurring on a recessive background in those severely affected neonates (7, 44, 68). This was supported further by haplotype mapping in 4 such consanguineous kindreds; the neonate with NSHPT had inherited from each parent one copy of the same 3q haplotype (containing CASR at 13q13.3-q21.1)(75). Also, there has been proof of a CASR variant in both parents of a child with homozygous NSHPT (44, 45). In sum, NSHPT is proved (based on several lines of evidence) as often to be an expression of biallelic inactivating and pathogenic variants of CASR (Fig. 1-H).
The second major genotype/phenotype format of NSHPT: monoallelic pathogenic variants of CASR with reversible secondary hyperparathyroidism
Other findings suggest a different and usually milder form of NSHPT (Fig. 1-F), with only one variant CASR allele; thus, these neonates express states closely related to the 2 formats of heterozygous FHH1 (See Fig. 1-C and Fig. 1-E). Several families have had an autosomal dominant transmission pattern with either mild or severe clinical features around a similar degree of hypercalcemia in the unaffected neonates and in the affected heterozygous neonates to support this concept (8, 9). Furthermore, several cases of NSHPT were managed without PTX and eventually matured to express a similar grade of asymptomatic hypercalcemia with typical FHH (32, 33, 76). CASR gene testing proved that several families with such NSHPT heterozygotes showed an autosomal dominant (ie, monoallelic) transmission pattern (77, 78). These heterozygous CASR variants are pathogenic, causing inactivation of the CaSR according to in vitro tests of calcium responsivity (11, 79). The germline pathogenic variants of CASR in NSHPT cause differing degrees of inactivation of the CaSR, and these correlate in vitro and in vivo (11, 79). The CaSR functions as a homodimer and a heterodimer (3), suggesting that monoallelic variants would cause 25% of dimers to be homozygous benign; from these, they might express as a relatively mild phenotype.
Disproportionately, a large majority of such heterozygous cases of NSHPT were born to a normocalcemic mother. This resulted from paternal transmission of the CASR pathogenic variant to the case of NSHPT or from a de novo variant (perhaps as a mosaic) in the neonate (46, 63, 64). NSHPT could, therefore, reflect a CASR heterozygous fetus, having gestated with FHH1 and simultaneously having sensed the maternoplacental supply of calcium as insufficient (8, 24). The heterozygous fetus could, therefore, develop mild or severe 2HPT in utero in addition to its embryologic expressions of typical FHH1.
More specifically, 2HPT might arise in utero due to several exogenous states wherein maternoplacental calcium supply is sensed by the fetus as insufficient (8, 46). The environmental states that could cause fetal 2HPT include maternal euparathyroidism with unrecognized calcium deficiency, maternal calcium deficiency, maternal hypoparathyroidism, and maternal deficiency of vitamin D (21–23) (Fig. 1-F). Alternately, hyperparathyroidism (HPT) can arise secondarily in utero as caused by the variant of an epistatic gene such as maternal/placental TRPV6 (24). Epistasis is the interaction between genes that are not alleles of each other (31). Homozygous TRPV6 in the mother causes a placental defect in calcium transport. The fetal pairing, featuring a euparathyroid mother giving birth to a case of NSHPT, was not inevitable in kindreds with FHH1, indicating that other features must contribute (15, 26, 27). Indeed, a heterozygous NSHPT case has rarely inherited the CASR variant from its mother (47, 80).
Another genotype/phenotype format: late onset primary hyperparathyroidism with biallelic and likely pathogenic variant(s) of CASR.
A homozygous inactivating CASR variant per se is not an obligatory cause of NSHPT. Eleven cases of homozygous CASR variant in 9 families with 8 different biallelic and likely pathogenic variants expressed onset of hypercalcemia at later than 6 months of age and well into adulthood (mean onset age of 23 years [range 3–59 years]) (7, 35–41) (Fig. 1-G). Their status as neonates was not reported, but all or most are presumed to have seemed healthy. The degrees of primary hyperparathyroidsm were highly variable but generally mild. And only 1 of 8 families met one criterion for severe disorder, based on its level of hypercalcemia (maximal calcium 6.2 mM) (7). The very late onset age or late recognition age led us to separate each of these cases from the main analyses of neonates. The parathyroid histology was usually hyperplasia, with 2 adenomas in one case (34). We speculate that most of the underlying CASR variants were likely to be of low grade (grades 1 or 2) in the monoallelic format (Fig. 1-G).
Yet 2 other genotype/phenotype formats: neonatal cases of low-grade or high-grade FHH1, seen as a monoallelic pathogenic variant without secondary hyperparathyroidism
Neonatal 2HPT can occur secondary to an environmental or epistatic interaction, without any CASR variant (21–24) (Fig. 1-B); this genotype/phenotype pairing is not treated further here.
Asymptomatic neonatal cases with a CASR variant, low-grade or high-grade hypercalcemia, and typical FHH1 may be without either 2HPT or NSHPT (Fig. 1-C and Fig. 1-E). In fact, some adult (and perhaps also neonatal) carriers of a low-grade variant are intermittently euparathyroid (38, 43–45). These 2 low-grade and high-grade phenotypes in vivo are attributed to the normal neonatal expressions of FHH1 from a heterozygous inactivating variant of CASR (Fig. 1-C and Fig. 1-E). Some of these cases have logically been termed neonatal hyperparathyroidism. (11) They do not meet any of our physiologic criteria for NSHPT, but they might have arbitrarily been described under the term of NSHPT. Cases in these categories may receive aggressive interventions, but they usually require no intervention, and they continue to express typical low-grade or high-grade FHH1 as they mature (8, 32, 33, 81).
Mouse models of fetal parathyroid development relevant to NSHPT
Information from mouse models is relevant, although it is difficult to establish a uniform definition of the neonatal interval, applicable to both mouse and man. Engineered mice with homozygous germline-inactivating and pathogenic variant of CASR develop features including relative hypocalciuria analogous to features of NSHPT in man. Interestingly, these CASR knockout mice show hyperplasia of the parathyroids in utero (82); by fetal day 18.5, PTH is 20-fold of that in the normal fetal mouse (83). Thus, homozygous inactivating variant of CASR in the mouse causes not only a defect in parathyroid gland secretion but also a defect in fetal parathyroid gland growth (82, 84).
Tissue-selective biallelic knockout of both somatic GNAQ and GNA11 in the mouse parathyroid glands causes a mouse syndrome similar to NSHPT (without relative hypocalciuria); this suggested a central G protein (likely G alpha-11 or G alpha-q) in the CaSR pathway of the parathyroid gland (85). This was supported by identification of GNA11 mutation as the cause of FHH2 (2). GNA11 encodes G alpha-11.
Parathyroid hyperplasia in the CASR knockout mouse at postnatal day 10 is even greater when the parathyroid gland-specific knockout of CASR is combined with parathyroid gland-specific knockout of Klotho (86); this somatic combination provides a mouse model for one type of epistatic effect, contributing toward NSHPT.
Basic implications for NSHPT
The findings about NSHPT in man, supplemented by mouse models, have many basic implications.
(1) The fetal parathyroid glands are highly responsive even to mild shifts of ambient fetal calcium concentration, including at early stages in utero even though the placenta prevents wide swings in fetal serum calcium concentration (83).
(2) The fetus regulates calcium biology by effects on its parathyroid gland secretion of PTH and by effects on growth of its own parathyroid glands.
(3) The secondary effects on fetal parathyroid growth can persist as 2HPT after birth.
(4) The neonatal 2HPT is reversible after birth spontaneously or with therapies.
(5) The placenta does not protect the fetus from high fetal PTH levels. Very high circulating PTH in the fetus causes characteristic and highly deleterious rachitic effects upon the developing fetal skeleton.
(6) These rachitic skeletal effects are similar or identical whether in utero hyperparathyroidism is primary (as in a homozygote with NSHPT (Fig. 1-H)) or secondary (as with a defect in maternal placental transport of calcium etc (Fig. 1-B) (21–24)).
(7) If the newborn survives the traumas of birth, and if the neonate then receives appropriate support, skeletal normalization is eventually likely. The undermineralization from in utero primary or secondary hyperparathyroidism can usually be reversed during the first postnatal months. The time to reversal or healing likely depends on the degrees of severity of the skeletal defects and on the level of support (for example, amount of calcium intake).
(8) The long-term prognosis of the associated severe thoracic narrowing has been reported rarely; importantly, 2 cases of NSHPT with all the severe neonatal skeletal defects showed complete normalization of the skeleton when first retested extensively at age 6 months and at age 6 years (24, 87).
(9) While the placenta protects the fetus from severe hypercalcemia, this protection is rapidly lost within several days after birth. Extreme hypercalcemia may occur in CASR homozygotes as early as in the first week of life.
(10) The human neonate can express and tolerate serum calcium values that are far higher than those encountered in pathophysiology of the adult man. (11) Finally, the CASR may also regulate serum calcium in utero and/or after birth by effects outside of the parathyroid gland. For example, calcium transport effects of the CaSR on the renal tubule are well established and probably contribute to regulation of serum calcium levels (74, 88). Other tissues such as thyroidal C-cell (regulation of calcitonin secretion), osteoblast, bone marrow, or elsewhere may also participate in fetal or neonatal systemic regulation of mineral metabolism (89–93). This might be recognizable by analyses in cases with postoperative chronic hypoparathyroidism (74).
Hypercalcemia in NSHPT: clinical implications of a robust biomarker and its limitations
Our findings reveal for the first time that serum calcium measurements can serve as a biomarker for robust and very early genetic diagnosis among NSHPT cases. If any serum calcium value in NSHPT is above 4.5 mM, then a homozygous germline-inactivating and pathogenic or likely pathogenic variant of CASR is virtually certain (approximately 100% specificity). Such values have sometimes been recorded in the first week of life (45, 77–79). Therefore, in the future, robust detection of extreme hypercalcemia in most homozygotes could promote individualized and very early total PTX, the presumed definitive management for homozygous cases of NSHPT.
At the same time, calcium values at or below 4.5 mM should be interpreted with caution. Some 6 of 36 (17%) homozygotes had maximal serum calcium at 4.5 mM or lower. And prospective experience with extreme hypercalcemia may not be identical to retrospective experience. For example, past family history could promote extremely early intervention in a case with NSHPT (94). Such improved medical and surgical management could result in curtailed maximal values of both serum calcium and serum PTH. Furthermore, although not recognized to date, a concomitant major illness such as severe vitamin D deficiency, respiratory failure, or septicemia could blunt the maximal calcium in an ill homozygote (76). Lastly, if there were very few calcium measurements, then the estimate of the true maximum could be grossly too low.
There are limitations in our analyses of serum calcium and serum PTH, each as one maximal value. Other parameters such as the initial value of each, or a mean value could be informative. In the future, it will be particularly useful to define the full perinatal time course of serum calcium values, including the determinants of the typical time to reach its maximum (46, 68). Even with the above considerations, the tabulation of a maximal value has special value; it brings a focus onto both the capacity for the severest expression of the syndrome and also the single parameter that was most likely to be reported similarly in each case.
Pairing of PTH and calcium in NSHPT
When examined together as simple ellipsoids (not shown), there was no overlap of the paired calcium and PTH domains between the 2 NSHPT groups (Fig. 5). This nomogram shows an added diagnostic value of the 2 variables together.
Homozygotes showed a serum calcium intercept at PTH = 0 (ie, a model of postoperative total hypoparathyroidism) far higher than did the heterozygotes. This interpretation should be cautious since neither intercept is in the hypocalcemic range, typical of the occasional untreated hypoparathyroid status in these cases; furthermore, there is modest variability to the data, and lastly, the data points do not have any representation in the normal range, closer to the zero intercept.
Speculations about issues related to NSHPT
These findings establish biochemical (serum calcium and serum PTH) differences between the 2 deoxyribonucleic acid (DNA)-dosage-based groups of NSHPT. Other differences between the 2 groups are likely to be uncovered later, both in the clinical presentation and also in the responsivity to therapy.
(1) For example, the higher mean PTH postnatally in homozygotes may reflect that the homozygotes had typically suffered a higher grade of hyperparathyroid bone disease in utero.
(2) The amplitude and/or duration of extreme hypercalcemia in homozygotes might contribute to lifelong neuromotor retardation.
(3) Other electrolyte differences such as degree and duration of hypophosphatemia (contributing to hypotonia, respiratory distress, and other compromises) could distinguish the 2 groups.
(4) The 2 groups may differ in most appropriate surgery. Only homozygotes may require total PTX. Subtotal PTX may be a more successful temporizing measure in treating the 2HPT in heterozygotes than in homozygotes (that may not have an important component of 2HPT) (46, 81).
(5) Surprisingly, the extrapolation of serum calcium at PTH = zero in homozygotes suggests that maintenance of normocalcemia after total PTX might require lower doses of calciotropic support in homozygotes than in heterozygotes.
(6) The appropriate target level of serum calcium, particularly in homozygotes, has not been established (4). Is lifelong moderate hypercalcemia desirable in some homozygotes? For the same reason, is chronic hypocalcemia particularly harmful to homozygotes?
(7) Occasional pathogenic variants of other genes (for example, GNA11, AP2S1, TRPV6, or SPINK1) may come within the spectrum of NSHPT (32, 33, 46, 50, 53).
(8) Is variant codon type (missense, nonsense, frameshift, etc) selective for mono- or biallelic dosage of the CASR, or is it selective for grade of severity within a dosage group?
(9) Rapid and inexpensive DNA diagnosis in the future (via currently developing methods) might change the landscape for diagnosis and management of newborns with NSHPT and states resembling this (95). It might even eclipse the value of rapid and inexpensive measurement of serum calcium for diagnosis of gene dosage. This is already within reach of current methods. Assuming that access and/or cost will not be prohibitive, future diagnosis by DNA sequencing could quickly define many cases of a NSHPT-related disorder as a homozygote or a heterozygote for a variant of CASR. These methods could also clarify cases affected by a related gene in the calcemic pathway such as maternal TRPV6 or neonatal AP2S1.
(10) Most importantly, the robust, early feedback about calcium or DNA presents the likelihood of improved management and improved prognosis in the potentially devastating disorder that is NSHPT.
Conclusions
NSHPT is composed of 2 distinct pairings in a wide spectrum of 9 genotype/phenotype pairings. The 2 pairings with NSHPT represent homozygosity versus heterozygosity for pathogenic germline-inactivating CASR variant(s). This is also evidenced by major biochemical differences with the CASR homozygotes, showing higher serum calcium and serum PTH than the heterozygotes. In fact, extreme hypercalcemia with a serum calcium value above 4.5 mM (normal 2.2–2.6 mM) is a conservative cutoff; it is virtually diagnostic of a biallelic pathogenic variant of the CASR. When measured early, this robust cutoff should promote early and definitive total PTX in a large majority of homozygous cases with NSHPT.
Acknowledgments
Jancarlos Camacho, Mari Suzuki, Crystal Kamilaris, and Adela Leahu helped to assemble and proof the data. Faith Williams and Amy Parkhurst provided expert assistance with graphics. Constantine Stratakis gave support and helpful comments. A preliminary analysis was presented as a poster: S.J Marx, J. Camacho, M. Suzuki, C.D.C Kamilaris, A. Leahu, N. Sinaii, and C.A. Stratakis. Neonatal severe hyperparathyroidism (NSHPT): Striking biochemical differences between mono- and diallelic variants of the CASR. 16th International Multiple Endocrine Neoplasia Workshop, Houston, TX; March 27–29, 2019. Thurs-13 [Abstract and Poster].
Financial Support: This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health.
Glossary
Abbreviations
- 2HPT
secondary hyperparathyroidism
- Arg185Gln
arginine to glutamine variant at amino acid codon 185
- CaSR
calcium-sensing receptor
- DNA
deoxyribonucleic acid
- FHH
familial hypocalciuric hypercalcemia
- FHH1
FHH type 1
- HPT
hyperparathyroidism
- NSHPT
neonatal severe hyperparathyroidism
- PTH
parathyroid hormone
- PTX
parathyroidectomy
Additional Information
Disclosure Summary : The authors have no conflict around this work
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Ross AJ 3rd, Cooper A, Attie MF, Bishop HC. Primary hyperparathyroidism in infancy. J Pediatr Surg. 1986;21(6):493–499. [DOI] [PubMed] [Google Scholar]
- 2. Hannan FM, Babinsky VN, Thakker RV. Disorders of the calcium-sensing receptor and partner proteins: insights into the molecular basis of calcium homeostasis. J Mol Endocrinol. 2016;57(3):R127–R142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brown EM. Disorders of the calcium-sensing receptor: familial hypocalciuric hypercalcemia and autosomal dominant hypocalcemia. In: Rosen CJ, ed. UpToDate. UpToDate: Waltham, MA. (Accessed on September 13, 2019) 2019. www.uptodate.com [Google Scholar]
- 4. Mayr B, Schnabel D, Dörr HG, Schöfl C. Genetics in endocrinology: gain and loss of function mutations of the calcium-sensing receptor and associated proteins: current treatment concepts. Eur J Endocrinol. 2016;174(5):R189–R208. [DOI] [PubMed] [Google Scholar]
- 5. Marx SJ, Goltzman D. Evolution of our understanding of the hyperparathyroid syndromes: a historical perspective. J Bone Miner Res. 2019;34(1):22–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cole De, Forsythe CR, Dooley JM, Grantmyre EB, Salisbury SR. Primary neonatal hyperparathyroidism: a devastating neurodevelopmental disorder if left untreated. J Craniofac Genet Dev Biol. 1990;10(2):205–214. [PubMed] [Google Scholar]
- 7. Schnabel D, Letz S, Lankes E, Mayr B, Schofl C. Severe but not neonatally lethal. A homozygous inactivating CaSR mutation in a 3 year old child. Abstract P04 Exp Clin Endocr Diab. 2014;122(3):P041. [Google Scholar]
- 8. Marx SJ, Attie MF, Spiegel AM, Levine MA, Lasker RD, Fox M. An association between neonatal severe primary hyperparathyroidism and familial hypocalciuric hypercalcemia in three kindreds. N Engl J Med. 1982;306(5):257–264. [DOI] [PubMed] [Google Scholar]
- 9. Lillquist K, Illum N, Jacobsen BB, Lockwood K. Primary hyperparathyroidism in infancy associated with familial hypocalciuric hypercalcemia. Acta Paediatr Scand. 1983;72(4):625–629. [DOI] [PubMed] [Google Scholar]
- 10. Hannan FM, Nesbit MA, Zhang C, et al. Identification of 70 calcium-sensing receptor mutations in hyper- and hypo-calcaemic patients: evidence for clustering of extracellular domain mutations at calcium-binding sites. Hum Mol Genet. 2012;21(12):2768–2778. [DOI] [PubMed] [Google Scholar]
- 11. Glaudo M, Letz S, Quinkler M, et al. Heterozygous inactivating CaSR mutations causing neonatal hyperparathyroidism: function, inheritance and phenotype. Eur J Endocrinol. 2016;175(5):421–431. [DOI] [PubMed] [Google Scholar]
- 12. Corrado KR, Andrade SC, Bellizzi J, D’Souza-Li L, Arnold A. Polyclonality of parathyroid tumors in neonatal severe hyperparathyroidism. J Bone Miner Res. 2015;30(10):1797–1802. [DOI] [PubMed] [Google Scholar]
- 13. Bulloch B, Schubert CJ, Brophy PD, Johnson N, Reed MH, Shapiro RA. Cause and clinical characteristics of rib fractures in infants. Pediatrics. 2000;105(4):E48. [DOI] [PubMed] [Google Scholar]
- 14. Souberbielle JC, Brazier F, Piketty ML, Cormier C, Minisola S, Cavalier E. How the reference values for serum parathyroid hormone concentration are (or should be) established? J Endocrinol Invest. 2017;40(3):241–256. [DOI] [PubMed] [Google Scholar]
- 15. Marx SJ, Attie MF, Levine MA, Spiegel AM, Downs RW Jr, Lasker RD. The hypocalciuric or benign variant of familial hypercalcemia: clinical and biochemical features in fifteen kindreds. Medicine (Baltimore). 1981;60(6):397–412. [DOI] [PubMed] [Google Scholar]
- 16. Rajala MM, Heath H 3rd. Distribution of serum calcium values in patients with familial benign hypercalcemia (hypocalciuric hypercalcemia): evidence for a discrete genetic defect. J Clin Endocrinol Metab. 1987;65(5):1039–1041. [DOI] [PubMed] [Google Scholar]
- 17. Christensen SE, Nissen PH, Vestergaard P, Mosekilde L. Familial hypocalciuric hypercalcaemia: a review. Curr Opin Endocrinol Diabetes Obes. 2011;18(6):359–370. [DOI] [PubMed] [Google Scholar]
- 18. Richards S, Aziz N, Bale S, et al. ; ACMG Laboratory Quality Assurance Committee . Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cole DE, Peltekova VD, Rubin LA, et al. A986S polymorphism of the calcium-sensing receptor and circulating calcium concentrations. Lancet. 1999;353(9147):112–115. [DOI] [PubMed] [Google Scholar]
- 20. Scillitani A, Guarnieri V, De Geronimo S, et al. Blood ionized calcium is associated with clustered polymorphisms in the carboxyl-terminal tail of the calcium-sensing receptor. J Clin Endocrinol Metab. 2004;89(11):5634–5638. [DOI] [PubMed] [Google Scholar]
- 21. Khan AA, Clarke B, Rejnmark L, Brandi ML. Management of endocrine disease: hypoparathyroidism in pregnancy: review and evidence-based recommendations for management. Eur J Endocrinol. 2019;180(2):R37–R44. [DOI] [PubMed] [Google Scholar]
- 22. Innes AM, Seshia MM, Prasad C, et al. Congenital rickets caused by maternal vitamin D deficiency. Paediatr Child Health. 2002;7(7):455–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kovacs CS. Maternal vitamin D deficiency: fetal and neonatal implications. Semin Fetal Neonatal Med. 2013;18(3):129–135. [DOI] [PubMed] [Google Scholar]
- 24. Suzuki Y, Chitayat D, Sawada H, et al. TRPV6 variants interfere with maternal-fetal calcium transport through the placenta and cause transient neonatal hyperparathyroidism. Am J Hum Genet. 2018;102(6):1104–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Marx SJ. Familial hypocalciuric hypercalcemia as an atypical form of primary hyperparathyroidism. J Bone Miner Res. 2018;33(1):27–31. [DOI] [PubMed] [Google Scholar]
- 26. Thorgeirsson U, Costa J, Marx SJ. The parathyroid glands in familial hypocalciuric hypercalcemia. Hum Pathol. 1981;12(3):229–237. [DOI] [PubMed] [Google Scholar]
- 27. Law WM Jr, Carney JA, Heath H 3rd. Parathyroid glands in familial benign hypercalcemia (familial hypocalciuric hypercalcemia). Am J Med. 1984;76(6):1021–1026. [DOI] [PubMed] [Google Scholar]
- 28. Carling T, Szabo E, Bai M, et al. Familial hypercalcemia and hypercalciuria caused by a novel mutation in the cytoplasmic tail of the calcium receptor. J Clin Endocrinol Metab. 2000;85(5):2042–2047. [DOI] [PubMed] [Google Scholar]
- 29. Szabo E, Hellman P, Lundgren E, Carling T, Rastad J. Parathyroidectomy in familial hypercalcemia with clinical characteristics of primary hyperparathyroidism and familial hypocalciuric hypercalcemia. Surgery. 2002;131(3):257–263. [DOI] [PubMed] [Google Scholar]
- 30. Szabo E, Carling T, Hessman O, Rastad J. Loss of heterozygosity in parathyroid glands of familial hypercalcemia with hypercalciuria and point mutation in calcium receptor. J Clin Endocrinol Metab. 2002;87(8):3961–3965. [DOI] [PubMed] [Google Scholar]
- 31. van de Haar J, Canisius S, Yu MK, Voest EE, Wessels LFA, Ideker T. Identifying epistasis in cancer genomes: a delicate affair. Cell. 2019;177(6):1375–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Page LA, Haddow JE. Self-limited neonatal hyperparathyroidism in familial hypocalciuric hypercalcemia. J Pediatr. 1987;111(2):261–264. [DOI] [PubMed] [Google Scholar]
- 33. Harris SS, D’Ercole AJ. Neonatal hyperparathyroidism: the natural course in the absence of surgical intervention. Pediatrics. 1989;83(1):53–56. [PubMed] [Google Scholar]
- 34. Hannan FM, Nesbit MA, Christie PT, et al. A homozygous inactivating calcium-sensing receptor mutation, Pro339Thr, is associated with isolated primary hyperparathyroidism: correlation between location of mutations and severity of hypercalcaemia. Clin Endocrinol (Oxf). 2010;73(6):715–722. [DOI] [PubMed] [Google Scholar]
- 35. Miyashiro K, Kunii I, Manna TD, et al. Severe hypercalcemia in a 9-year-old Brazilian girl due to a novel inactivating mutation of the calcium-sensing receptor. J Clin Endocrinol Metab. 2004;89(12):5936–5941. [DOI] [PubMed] [Google Scholar]
- 36. Chikatsu N, Fukumoto S, Suzawa M, et al. An adult patient with severe hypercalcaemia and hypocalciuria due to a novel homozygous inactivating mutation of calcium-sensing receptor. Clin Endocrinol (Oxf). 1999;50(4):537–543. [DOI] [PubMed] [Google Scholar]
- 37. Aida K, Koishi S, Inoue M, Nakazato M, Tawata M, Onaya T. Familial hypocalciuric hypercalcemia associated with mutation in the human Ca(2+)-sensing receptor gene. J Clin Endocrinol Metab. 1995;80(9):2594–2598. [DOI] [PubMed] [Google Scholar]
- 38. Lietman SA, Tenenbaum-Rakover Y, Jap TS, et al. A novel loss-of-function mutation, Gln459Arg, of the calcium-sensing receptor gene associated with apparent autosomal recessive inheritance of familial hypocalciuric hypercalcemia. J Clin Endocrinol Metab. 2009;94(11):4372–4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Szczawinska D, Schnabel D, Letz S, Schöfl C. A homozygous CaSR mutation causing a FHH phenotype completely masked by vitamin D deficiency presenting as rickets. J Clin Endocrinol Metab. 2014;99(6):E1146–E1153. [DOI] [PubMed] [Google Scholar]
- 40. Maltese G, Izatt L, McGowan BM, Hafeez K, Hubbard JG, Carroll PV. Making (mis) sense of asymptomatic marked hypercalcemia in pregnancy. Clin Case Rep. 2017;5(10):1587–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Borsari S, Marcocci C, Cetani F. Familial hypocalciuric hypercalcemia type 1 due to a novel homozygous mutation of the calcium-sensing receptor gene. J Endocrinol Invest. 2017;40(11):1271–1272. [DOI] [PubMed] [Google Scholar]
- 42. Papaioannou VE, Behringer RR. Early embryonic lethality in genetically engineered mice: diagnosis and phenotypic analysis. Vet Pathol. 2012;49(1):64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Alazami AM, Awad SM, Coskun S, et al. TLE6 mutation causes the earliest known human embryonic lethality. Genome Biol. 2015;16(1):240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Marx SJ, Fraser D, Rapoport A. Familial hypocalciuric hypercalcemia. Mild expression of the gene in heterozygotes and severe expression in homozygotes. Am J Med. 1985;78(1):15–22. [DOI] [PubMed] [Google Scholar]
- 45. Cole DE, Janicic N, Salisbury SR, Hendy GN. Neonatal severe hyperparathyroidism, secondary hyperparathyroidism, and familial hypocalciuric hypercalcemia: multiple different phenotypes associated with an inactivating Alu insertion mutation of the calcium-sensing receptor gene. Am J Med Genet. 1997;71(2):202–210. [DOI] [PubMed] [Google Scholar]
- 46. Bai M, Pearce SH, Kifor O, et al. In vivo and in vitro characterization of neonatal hyperparathyroidism resulting from a de novo, heterozygous mutation in the Ca2+-sensing receptor gene: normal maternal calcium homeostasis as a cause of secondary hyperparathyroidism in familial benign hypocalciuric hypercalcemia. J Clin Invest. 1997;99(1):88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Arunchaiya S, Pollak MR, Seidman CA, et al. Marked hypercalcemia in a five month old male associated with heterozygous point mutation in the calcium-sensing receptor gene. Program and Abstracts of the 80th Annual Meeting of the Endocrinology Society; 1998, New Orleans, p. 493, (Abstract P3-535). [Google Scholar]
- 48. Fujisawa Y, Yamaguchi R, Satake E, et al. Identification of AP2S1 mutation and effects of low calcium formula in an infant with hypercalcemia and hypercalciuria. J Clin Endocrinol Metab. 2013;98(12):E2022–E2027. [DOI] [PubMed] [Google Scholar]
- 49. Goldsweig B, Brownstein C, Towne M, Agrawal P, Beggs A, Carpenter TO. Familial hypocalciuric hypercalcemia type 3 presenting as neonatal bone disease: a dual treatment approach. Program and Abstracts of the 98th Annual Meeting of the Endocrine Society; 2016, Boston, (Abstract SUN 383). [Google Scholar]
- 50. Scheers I, Sokal E, Limaye N, et al. Cinacalcet sustainedly prevents pancreatitis in a child with a compound heterozygous SPINK1/AP2S1 mutation. Pancreatology. 2019;19(6):801–804. [DOI] [PubMed] [Google Scholar]
- 51. Hu J, Spiegel AM. Structure and function of the human calcium-sensing receptor: insights from natural and engineered mutations and allosteric modulators. J Cell Mol Med. 2007;11(5):908–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Geng Y, Mosyak L, Kurinov I, et al. Structural mechanism of ligand activation in human calcium-sensing receptor. Elife. 2016;5:e13662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhang C, Zhang T, Zou J, et al. Structural basis for regulation of human calcium-sensing receptor by magnesium ions and an unexpected tryptophan derivative co-agonist. Sci Adv. 2016;2(5):e1600241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Reh CM, Hendy GN, Cole DE, Jeandron DD. Neonatal hyperparathyroidism with a heterozygous calcium-sensing receptor (CASR) R185Q mutation: clinical benefit from cinacalcet. J Clin Endocrinol Metab. 2011;96(4):E707–E712. [DOI] [PubMed] [Google Scholar]
- 55. Fisher MM, Cabrera SM, Imel EA. Successful treatment of neonatal severe hyperparathyroidism with cinacalcet in two patients. Endocrinol Diabetes Metab Case Rep. 2015;2015:150040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Forman TE, Niemi AK, Prahalad P, Shi RZ, Nally LM. Cinacalcet therapy in an infant with an R185Q calcium-sensing receptor mutation causing hyperparathyroidism: a case report and review of the literature. J Pediatr Endocrinol Metab. 2019;32(3):305–310. [DOI] [PubMed] [Google Scholar]
- 57. Kobayashi M, Tanaka H, Tsuzuki K, et al. Two novel missense mutations in calcium-sensing receptor gene associated with neonatal severe hyperparathyroidism. J Clin Endocrinol Metab. 1997;82(8):2716–2719. [DOI] [PubMed] [Google Scholar]
- 58. Rauh G, Schuster H, Fischer J, Keller C, Wolfram G, Zöllner N. Familial defective apolipoprotein B-100: haplotype analysis of the arginine(3500)––glutamine mutation. Atherosclerosis. 1991;88(2-3):219–226. [DOI] [PubMed] [Google Scholar]
- 59. Alexandrov LB, Nik-Zainal S, Wedge DC, et al. ; Australian Pancreatic Cancer Genome Initiative; ICGC Breast Cancer Consortium; ICGC MMML-Seq Consortium; ICGC PedBrain . Signatures of mutational processes in human cancer. Nature. 2013;500(7463):415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Baugh EH, Ke H, Levine AJ, Bonneau RA, Chan CS. Why are there hotspot mutations in the TP53 gene in human cancers? Cell Death Differ. 2018;25(1):154–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rani DS, Nallari P, Priyamvada S, Narasimhan C, Singh L, Thangaraj K. High prevalence of arginine to glutamine substitution at 98, 141 and 162 positions in troponin I (TNNI3) associated with hypertrophic cardiomyopathy among Indians. BMC Med Genet. 2012;13:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Buisson R, Langenbucher A, Bowen D, et al. Passenger hotspot mutations in cancer driven by APOBEC3A and mesoscale genomic features. Science. 2019;364(6447):eaaw2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Waller S, Kurzawinski T, Spitz L, et al. Neonatal severe hyperparathyroidism: genotype/phenotype correlation and the use of pamidronate as rescue therapy. Eur J Pediatr. 2004;163(10):589–594. [DOI] [PubMed] [Google Scholar]
- 64. Despert F, Lienhardt-Roussie A, Lardy H, et al. Severe neonatal hyperparathyroidism due to a large homozygous deletion of the calcium-sensing receptor gene requiring peritoneal diaysis before surgical care. Program and Abstracts of the 87th Annual Meeting of the Endocrine Society; 2005, San Diego, p. 510, (Abstract P2-607). [Google Scholar]
- 65. Dong Q, Cheng Z, Chang W, et al. Naturally-occurring mutation in the calcium-sensing receptor reveals the significance of extracellular domain loop III region for class C G-protein-coupled receptor function. J Clin Endocrinol Metab. 2010;95(10):E245–E252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kahvecioglu D, Atasay B, Berberoglu M, et al. A novel mutation in the calcium sensing receptor gene in a neonate with severe hyperparathyroidism. Genet Couns. 2014;25(3):331–335. [PubMed] [Google Scholar]
- 67. Murphy H, Patrick J, Báez-Irizarry E, et al. Neonatal severe hyperparathyroidism caused by homozygous mutation in CASR: a rare cause of life-threatening hypercalcemia. Eur J Med Genet. 2016;59(4):227–231. [DOI] [PubMed] [Google Scholar]
- 68. Steinmann B, Gnehm HE, Rao VH, Kind HP, Prader A. Neonatal severe primary hyperparathyroidism and alkaptonuria in a boy born to related parents with familial hypocalciuric hypercalcemia. Helv Paediatr Acta. 1984;39(2):171–186. [PubMed] [Google Scholar]
- 69. Pratt EL, Geren BB, Neuhauser EB. Hypercalcemia and idiopathic hyperplasia of the parathyroid glands in an infant. J Pediatr. 1947;30(4):388–399. [DOI] [PubMed] [Google Scholar]
- 70. Philips RN. Primary diffuse parathyroid hyperplasia in an infant of 4 months. Pediatrics. 1948;2(4):428–434. [PubMed] [Google Scholar]
- 71. Hillman DA, Scriver CR, Pedvis S, Shragovitch I. Neonatal familial primary hyperparathyroidism. N Engl J Med. 1964;270:483–490. [DOI] [PubMed] [Google Scholar]
- 72. Chou YH, Pollak MR, Brandi ML, et al. Mutations in the human Ca(2+)-sensing-receptor gene that cause familial hypocalciuric hypercalcemia. Am J Hum Genet. 1995;56(5):1075–1079. [PMC free article] [PubMed] [Google Scholar]
- 73. D’Souza-Li L, Canaff L, Janicic N, Cole DE, Hendy GN. An acceptor splice site mutation in the calcium-sensing receptor (CASR) gene in familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidism. Hum Mutat. 2001;18(5):411–421. [DOI] [PubMed] [Google Scholar]
- 74. Attie MF, Gill JR Jr, Stock JL, et al. Urinary calcium excretion in familial hypocalciuric hypercalcemia. Persistence of relative hypocalciuria after induction of hypoparathyroidism. J Clin Invest. 1983;72(2):667–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Pollak MR, Chou YH, Marx SJ, et al. Familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidism. Effects of mutant gene dosage on phenotype. J Clin Invest. 1994;93(3):1108–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Meeran K, Husain M, Puccini M, et al. Neonatal primary hyperparathyroidism masked by vitamin D deficiency. Clin Endocrinol (Oxf). 1994;41(4):531–534. [DOI] [PubMed] [Google Scholar]
- 77. Fox L, Sadowsky J, Pringle KP, et al. Neonatal hyperparathyroidism and pamidronate therapy in an extremely premature infant. Pediatrics. 2007;120(5):e1350–e1354. [DOI] [PubMed] [Google Scholar]
- 78. Zajickova K, Vrbikova J, Canaff L, Pawelek PD, Goltzman D, Hendy GN. Identification and functional characterization of a novel mutation in the calcium-sensing receptor gene in familial hypocalciuric hypercalcemia: modulation of clinical severity by vitamin D status. J Clin Endocrinol Metab. 2007;92(7):2616–2623. [DOI] [PubMed] [Google Scholar]
- 79. Pearce SH, Bai M, Quinn SJ, Kifor O, Brown EM, Thakker RV. Functional characterization of calcium-sensing receptor mutations expressed in human embryonic kidney cells. J Clin Invest. 1996;98(8):1860–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Schwarz P, Larsen NE, Lønborg Friis IM, Lillquist K, Brown EM, Gammeltoft S. Familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidism associated with mutations in the human Ca2+-sensing receptor gene in three Danish families. Scand J Clin Lab Invest. 2000;60(3):221–227. [DOI] [PubMed] [Google Scholar]
- 81. Panda AK, Nandi SK, Chakraborty A, Nagaraj RH, Biswas A. Differential role of arginine mutations on the structure and functions of α-crystallin. Biochim Biophys Acta. 2016;1860(1 Pt B):199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kovacs CS, Ho-Pao CL, Hunzelman JL, et al. Regulation of murine fetal-placental calcium metabolism by the calcium-sensing receptor. J Clin Invest. 1998;101(12):2812–2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ho C, Conner DA, Pollak MR, et al. A mouse model of human familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidism. Nat Genet. 1995;11(4):389–394. [DOI] [PubMed] [Google Scholar]
- 84. Marx SJ, Lourenço DM Jr. Familial hyperparathyroidism - disorders of growth and secretion in hormone-secretory tissue. Horm Metab Res. 2017;49(11):805–815. [DOI] [PubMed] [Google Scholar]
- 85. Wettschureck N, Lee E, Libutti SK, Offermanns S, Robey PG, Spiegel AM. Parathyroid-specific double knockout of Gq and G11 alpha-subunits leads to a phenotype resembling germline knockout of the extracellular Ca2+ -sensing receptor. Mol Endocrinol. 2007;21(1):274–280. [DOI] [PubMed] [Google Scholar]
- 86. Fan Y, Liu W, Bi R, et al. Interrelated role of klotho and calcium-sensing receptor in parathyroid hormone synthesis and parathyroid hyperplasia. Proc Natl Acad Sci U S A. 2018;115(16):E3749–E3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Tõke J, Czirják G, Patócs A, et al. Neonatal severe hyperparathyroidism associated with a novel de novo heterozygous R551K inactivating mutation and a heterozygous A986S polymorphism of the calcium-sensing receptor gene. Clin Endocrinol (Oxf). 2007;67(3):385–392. [DOI] [PubMed] [Google Scholar]
- 88. Toka HR, Al-Romaih K, Koshy JM, et al. Deficiency of the calcium-sensing receptor in the kidney causes parathyroid hormone-independent hypocalciuria. J Am Soc Nephrol. 2012;23(11):1879–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Garrett JE, Tamir H, Kifor O, et al. Calcitonin-secreting cells of the thyroid express an extracellular calcium receptor gene. Endocrinology. 1995;136(11):5202–5211. [DOI] [PubMed] [Google Scholar]
- 90. Kantham L, Quinn SJ, Egbuna OI, et al. The calcium-sensing receptor (CaSR) defends against hypercalcemia independently of its regulation of parathyroid hormone secretion. Am J Physiol Endocrinol Metab. 2009;297(4):E915–E923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Goltzman D, Hendy GN. The calcium-sensing receptor in bone–mechanistic and therapeutic insights. Nat Rev Endocrinol. 2015;11(5):298–307. [DOI] [PubMed] [Google Scholar]
- 92. Dvorak-Ewell MM, Chen TH, Liang N, et al. Osteoblast extracellular Ca2+ -sensing receptor regulates bone development, mineralization, and turnover. J Bone Miner Res. 2011;26(12):2935–2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Adams GB, Chabner KT, Alley IR, et al. Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature. 2006;439(7076):599–603. [DOI] [PubMed] [Google Scholar]
- 94. Goldbloom RB, Gillis DA, Prasad M. Hereditary parathyroid hyperplasia: a surgical emergency of early infancy. Pediatrics. 1972;49(4):514–523. [PubMed] [Google Scholar]
- 95. Farnaes L, Hildreth A, Sweeney NM, et al. Rapid whole-genome sequencing decreases infant morbidity and cost of hospitalization. NPJ Genom Med. 2018;3:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.