Abstract
Background
Notoginsenoside R1 (NR) is a major dynamic constituent of Panax notoginseng found to possess anti-inflammatory activity against various inflammatory diseases. However, its protective effects against renal ischemia-reperfusion (I/R) injury have not been elucidated. In male Wistar rats, we induced I/R under general anesthesia by occluding the renal artery for 60 min, followed by reperfusion and right nephrectomy.
Material/Methods
Rats were randomized to 4 groups: a sham group, an I/R group, an NR-pretreated (50 mg/kg) before I/R induction group, and an NR control group. All animals were killed at 72 h after I/R induction. Blood and renal tissues were collected, and histological and basic renal function parameters were assessed. In addition, levels of various kidney markers and proinflammatory cytokines were measured using RT-PCR, ELISA, and immunohistochemistry analysis.
Results
After I/R induction, the onset of renal dysfunction was shown by the elevated levels of serum urea, creatinine levels, and histological evaluation, showing a 2-fold increase in the renal failure markers kim-1 and NGAL compared to control rats. Rats pretreated with NR before I/R induction had significantly better renal functions, with attenuated levels of oxidative markers, restored levels of inflammatory cytokines such as tumor necrosis factor-α (TNF-α), tumor growth factor-β1 (TGF-β1), INF-γ, and IL-6, and increased anti-inflammatory cytokine levels (IL-10) compared to I/R-induced rats.
Conclusions
NR suppressed I/R-induced inflammatory cytokines production by suppressing oxidative stress and kidney markers, suggesting that NR is a promising drug candidate for prevention, progression, and treatment of renal dysfunction.
MeSH Keywords: Acute Kidney Injury, Anti-Inflammatory Agents, Ginsenosides
Background
Renal ischemia can cause acute kidney injury during major cardiovascular surgery and renal transplantation, known as ischemia-reperfusion injury (I/R). This is characterized by a blood flow restriction (ischemia) to the kidneys and subsequently oxygenating it by restoring the blood flow (reperfusion). Both of these actions can contribute to renal injuries that occur during organ transplantation or after infarction, causing tissue damage. The damage can occur in the renal microvascular architecture due to energy shortage in the blood for ion transport, and the condition can spread and worsen due to the absence of a repair mechanism normally performed by angiogenesis [1]. When the blood supply is restored, the tissue repair mechanism is activated, with the dedifferentiated cells at the site of injury undergoing mitosis and differentiation to establish the functional integrity of the microvascular structure of the nephrons.
Tissue damage during renal injury activates an inflammatory response by producing reactive oxygen species, cytokines, and chemokines, as well as infiltration of leukocytes to the site of injury [2]. The combined action of all these factors further damage the site of injury by increasing the tissue damage until the blood supply is restored. During reperfusion, oxygen supply to the kidneys is restored and free-radical-mediated cellular damage occurs, which overwhelms the kidney detoxification ability [1]. The kidney damage is due to inflammatory responses and the generation of ROS, which can cause cell injury. The extent of tissue damage due to ROS can be measured by the extent of toxic products derived from the peroxidation of protein and lipids and DNA. These serve as markers for assessment of the extent of renal damage due to ROS in I/R. It is important to find ways to limit ROS generation and prevent oxidative damage and reperfusion injury to treat the post-ischemic renal failure.
Hence, we assessed the ability of notoginsenoside R1 (NR1) to perform essential anti-inflammatory and antioxidative functions. NR1 is a major phytoestrogen isolated from the traditional medicinal herb Panax notoginseng, which is used against inflammation and oxidative stress and also has anti-apoptotic properties [3–7]. Since the use of NR1 in treatment of I/R injury in the kidneys has not yet been thoroughly studied, we propose Notoginsenoside NR1 would perform essential anti-inflammatory and antioxidative functions. Therefore, in the present investigation, we induced I/R in rats and treated them with NR1 to evaluate the effects of NR1 on I/R. Our results suggest the value of NR1 in attenuating the effects of I/R by reducing the effects of inflammatory mediators and ROS after kidney transplantation.
Material and Methods
Chemicals
Notoginsenoside R1 was acquired from Sigma Aldrich (St. Louis, USA). We used a High-Capacity cDNA Reverse Transcription Kit (Thermo-Fisher, USA) and a Master Mix Rotor-Gene SYBR® Green PCR Kit (Qiagen, USA). Oxidative marker enzymes and antioxidant enzymes were obtained from Cayman Chemicals (USA). Primary antibodies for iNOS and COX-2, HRP conjugated secondary antibody were obtained from Santa Cruz Biotechnology, Inc. (USA). ELISA assay kits for Cystatin-C, NGAL, β2-microglobulin, NAG, IL-18, kim-1, TNF-α, IL-2, IL-17, IL-8, IL-6, and IL-1β were obtained from Fine Biotech, China. All other chemicals used were of reagent grade.
IR rat model
For the study, male Wistar rats weighing 160–180 g were used. The experimental protocol was approved by the Institutional Animal Ethical Committee of the university. All animal care and experiments were carried out strictly in accordance with the institutional guidelines with prior approval by the committee. All surgery procedures were performed under anesthesia, and all efforts were made to minimize animal suffering. Rat renal IR injury models were created as per previously published articles [6]. Briefly, rats were anesthetized by isoflurane inhalation induction followed by intraperitoneal ketamine (650 mg/kg) and xylazine (10 mg/kg) administration. The analgesic buprenorphine hydrochloride (0.06 mg/kg) was injected subcutaneously and eye lubricant was applied to the eyes to prevent corneal drying. Analgesics were administered pre-operatively to aid post-surgical recovery. As per protocol, right nephrectomy was performed after ligating the renal artery and vein. Then, ischemia was induced in the left kidney by applying a microaneurysm clamp onto the artery and vein, and ischemia was confirmed visually by a gradual uniform darkening of the kidney. The rats were kept on heat-controlled thermal pads to maintain body temperature throughout the process. After 45 min of warm ischemia, the renal clamp was removed and reperfusion was confirmed by the change in kidney color from pale to bright red.
Experimental plan
The investigation included 4 experimental groups: Group 1 was Sham-operated, Group 2 was I/R-induced, Group 3 was NR pretreatment before I/R induction, and Group 4 was NR pretreatment. All rats were killed 72 h after reperfusion, then urine, blood, and kidneys were collected for histology, biochemical, functional biomarker, and molecular marker analyses. Serum creatinine, blood urea nitrogen (BUN), oxidative marker enzymes, and antioxidant enzymes were analyzed using commercial kits.
Renal markers and cytokine analysis
The onset of acute injury was determined using the markers Cystatin-C, NGAl, β2-microglobulin (Urine), NAG (Urine), IL-18, and kim-1 by use of ELISA and commercial assay kits. The cytokines TNF-α, IL-2, IL-17, IL-8, IL-6, and IL-1β were also analyzed using commercial kits.
Reverse transcription-PCR
To assess the mRNA expression, total RNA was extracted from the kidney tissues using TRIzol® and chloroform method. Briefly, tissues were homogenized in TRIzol and incubated for 5 min at room temperature, then chloroform was added and the mixture was centrifuged at 12 000 g for 15 min at 4°C. The top aqueous phase was mixed with isopropanol and kept for 10 min at 25±1°C, followed by centrifugation at 12 000 g for 10 min at 4°C. The obtained RNA pellet was washed with 70% ethanol, and the total RNA was quantified using a Nanoquant spectrophotometer. A known amount of RNA sample was transcribed to cDNA using a High-Capacity cDNA Reverse Transcription Kit (Thermo-Fisher). Real-time RT-PCR was performed for specific genes using the Rotor-Gene SYBR® Green PCR Kit (Qiagen). The gene-specific primers are listed in Table 1. The data obtained were analyzed by comparison with house-keeping control genes. The PCR reaction was performed with the initial temperature conditions at 95°C for 5 min, 30 cycles at 95°C for 10 s and 60°C for 1 min, followed by 1 cycle at 95°C for 1 min, 55°C for 30 s, and 95°C for 30 s for the dissociation curve. The Ct values were calculated, and the gene expressions were determined by the comparative Ct method (ΔΔCT).
Table 1.
Oligonucleotides used in this study.
| Gene | Primer | Sequence | Annealing | Accession number |
|---|---|---|---|---|
| TLR-4 | F | GAGGACAATGCTCTGGGGAG | 58 | NM_019178 |
| R | ATGGGTTTTAGGCGCAGAGT | |||
| PLD2 | F | CCTTGAACCCCTACATGCCC | 57 | NM_033299.2 |
| R | TCCAAGAAGAAGCATGGCCT | |||
| PLA2 | F | TCAGAACTGGGTATGTTCCACG | 59 | NM_001106015.1 |
| R | TTGAAGAAAGCCACGCCCAT | |||
| IL-16 | F | CTGTACCAGGAGGGTCAGGGA | 59 | NM_001105749.1 |
| R | GTCATCACTGGTCTTGGCGTT | |||
| CXCL10 | F | TGCAAGTCTATCCTGTCCGC | 58 | NM_139089.1 |
| R | CTCTCTGCTGTCCATCGGTC | |||
| IL-10 | F | CCTCTGGATACAGCTGCGAC | 57 | NM_012854.2 |
| R | GTAGATGCCGGGTGGTTCAA | |||
| GAPDH | F | AGTGCCAGCCTCGTCTCATA | 58 | NM_017008.4 |
| R | GGTAACCAGGCGTCCGATAC |
Immunohistochemical analysis
Briefly, the paraffin sections were rehydrated using a series of ethanol, and the antigen was retrieved using citrate buffer (pH 6.0). The nonspecific binding was blocked using 3% BSA in PBS. The tissue sections were incubated with iNOS and COX-2 specific primary polyclonal antibody (1: 150) diluted in 1% skimmed milk powder in PBS for 2 h at room temperature, followed by washing and then incubating with corresponding HRP-labeled secondary antibodies for 1 h at room temperature. The antigen expression was visualized using peroxidase activity developed by DAB and counterstained with hematoxylin.
Statistical data
Statistical significance was evaluated with one-way analysis of variance (ANOVA) followed by a post hoc (Bonferroni) test for multiple group comparison. For two-group comparison, the t test was used. Differences with a p-value less than 0.05 were considered statistically significant.
Results
To evaluate the protective effect of notoginsenoside R1 (NR) against renal ischemia-reperfusion (I/R) injury, the experimental model of I/R was created in Wistar rats, and the results are presented here. The blood levels of creatinine, BUN, albumin, potassium, and lactate dehydrogenase are presented in Table 2. Rats with I/R injury demonstrated a significant increase in levels of BUN, creatinine, and other parameters compared to controls. Rats in the NR pretreatment group exhibited reduced levels of these blood analytes in I/R animals but not equal to levels in control animals (Table 2).
Table 2.
The blood levels of creatinine, BUN, albumin, potassium, and LDH of control and experimental rats.
| Parameters | Control | I/R-Induced | I/R + NR | NR |
|---|---|---|---|---|
| Creatinine (mmol/L) | 22±2.4 | 117±16*** | 43±6.5## | 23±4.1 |
| BUN (mmol/L) | 9±4.35 | 39±4.2*** | 15±3.7 | 10±2.5 |
| Albumin (g/dl) | 3±3.1 | 8±1.5** | 4±2.4# | 2±4.1 |
| Potassium (meq/L) | 5±2.1 | 7±1.88ns | 5±3.3ns | 4±1.6 |
| LDH (U/L) | 144±22 | 1517±26*** | 283±45### | 153±34 |
Values are expressed as mean±SE (n=6). Statistical significance is expressed as
p<0.01,
p<0.01 compared to sham-operated controls,
p<0.05,
p<0.01 NR compared to I/R rats;
denotes non-significant.
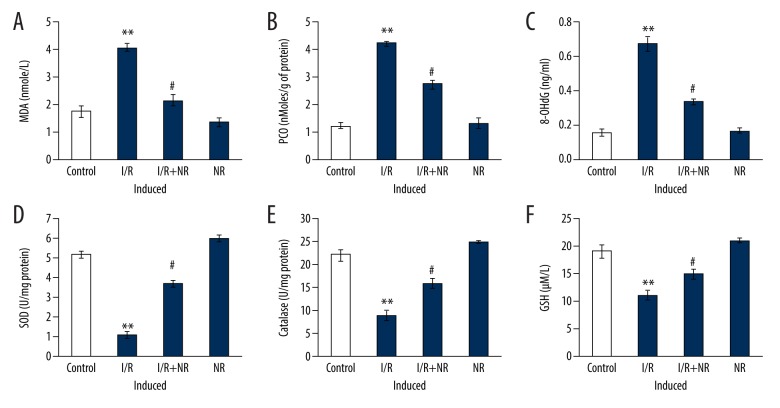
Further, to explore whether the renoprotection conferred by NR in I/R was associated with oxidative stress, the oxidative stress markers were examined, and the results are presented in Figure 1. The levels of oxidative stress markers MDA, PCO, and 8-hydroxydeoxyguanosine (8-OHdG) were increased and renal antioxidant enzymes such as SOD, catalase, and GSH level were decreased in I/R rats compared with the sham-operated group. Rats with NR pretreatment before I/R had levels near normal compared to I/R rats (Figure 1).
Figure 1.
(A–F) Show the levels of MDA, PCO, 8-hydroxydeoxyguanosine (8-OHdG), and renal antioxidant enzymes in the control and experimental groups of rats. The experimental details were mentioned in the methodology section. Values are expressed as mean±SE (n=6). Statistical significance is expressed as ** p<0.01 compared to sham-operated controls, # p<0.05 NR compared to I/R rats.
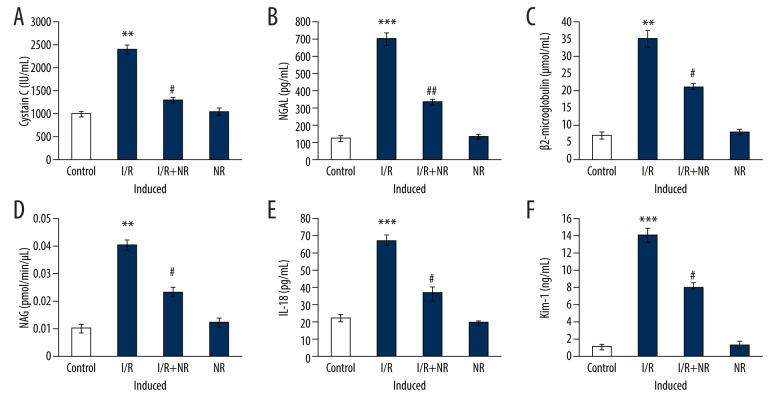
In addition, to evaluate the condition of acute kidney injury, markers of acute injury – Cystatin-C, NGAL, β2-microglobulin, NAG, IL-18and kim-1 levels – were assessed using ELISA. From the above serum biochemical results, the acute injury was observed in I/R animals. Conversely, the molecular markers demonstrated significant increases in the levels of Cystatin-C, NGAL, β2-microglobulin, NAG, IL-18, and kim-1 in I/R rats compared to control. Conversely, rats with NR exposure had decreased levels of these biomarkers, showing that NR exerts protection (Figure 2).
Figure 2.
(A–F) Show the levels of renal markers of an acute injury such as Cystatin-C, NGAl, β2-microglobulin, NAG, IL-18, kim-1 in the control and experimental groups of rats. Values are expressed as mean±SE (n=6). Statistical significance is expressed as ** p<0.01, *** p<0.001 compared to sham-operated controls, # p<0.05, ## p<0.01 NR compared to I/R rats.
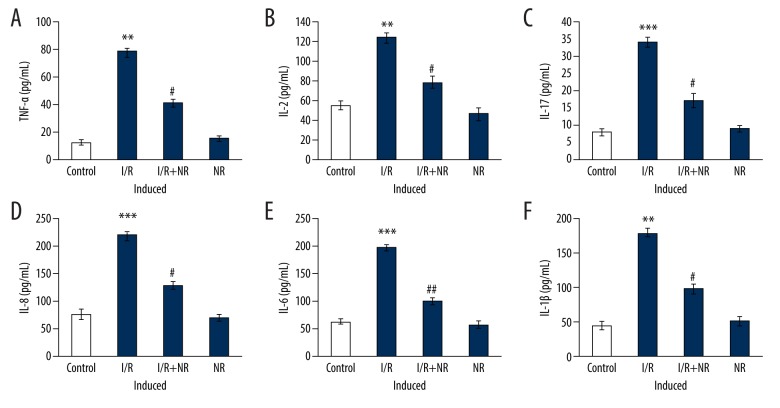
Further experiments to assess cytokine levels were carried out, and the results are presented in Figure 3. Rats with induced I/R had substantial increases in levels of cytokines TNF-α (p<0.01), IL-2 (p<0.05), IL-17A (p<0.01), IL-6 (p<0.001), IL-8 (p<0.01), and IL-1β (p<0.05) compared to control. These inflammatory cytokines were diminished in the NR pretreatment group, and I/R progression was reduced by NR, probably through reducing the inflammatory signaling (Figure 3).
Figure 3.
(A–F) Show cytokine expression of TNF-α, IL-2, IL-17, IL-8, IL-6, and IL-1β in the control and experimental groups of rats. Values are expressed as mean±SE (n=6). Statistical significance is expressed as ** p<0.01, *** p<0.001 compared to sham-operated controls, # p<0.05, ## p<0.01 NR compared to I/R rats.
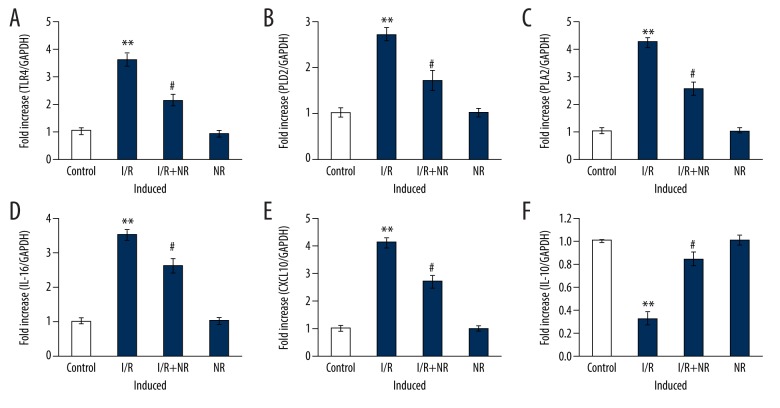
To determine the role of NR on the modulation of inflammatory signaling, the mRNA levels of cytokines were assessed by real-time quantitative PCR of the control gene, and the results are shown in Figure 4. The results show a profound increase (p<0.01) in the mRNA expression of TLR-4 (4-fold), PLD2 (5.2-fold), PLA2 (2-fold), IL-16 (3-fold), and CXCL10 (2-fold), with reduced IL-10 levels in I/R rats, compared to control. However, the increased levels of these chemokines’ genes were reduced in the NR pretreatment group, indicating that the drug restored normal functioning (Figure 4).
Figure 4.
(A–F) Show qRT-PCR mRNA expression of TLR-4, PLD2, PLA2, IL-16, CXCL10, and IL-10 in the control and experimental groups of rats. The experimental details of the PCR method are provided in the methodology section. Values are expressed as mean±SE (n=6). Statistical significance is expressed as ** p<0.01 compared to sham-operated controls, # p<0.05 NR compared to I/R rats.
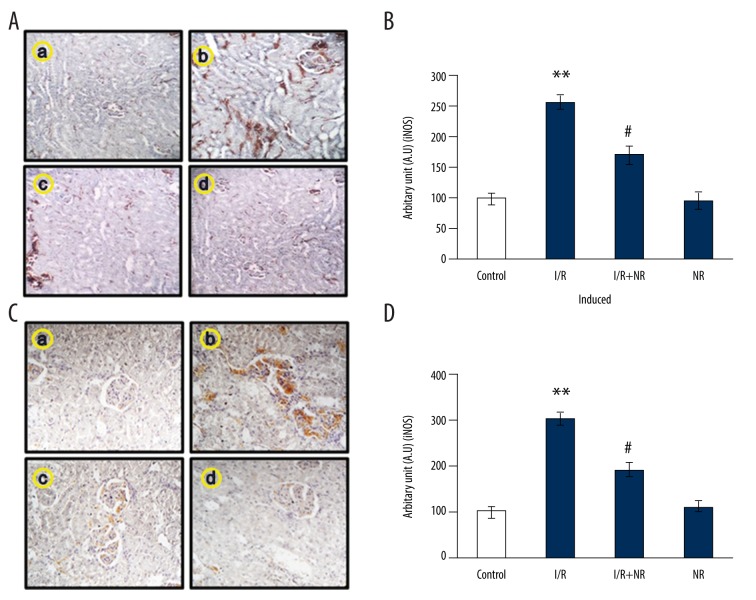
Figure 5 shows the immunohistochemical (IHC) analysis in the kidney tissues of control and NR rats. We assessed the tissue-level expression of inflammatory mediators and performed IHC analysis of iNOS and COX-2 expression. The results demonstrated an increase (p<0.05) in chemical reactivity for the iNOS and COX-2 proteins in I/R-induced rats, while the levels of these proteins were lower in NR-pretreated rats compared to induced rats. No significant changes in the expression of these proteins were detected in the kidney tissue of control rats (Figure 5).
Figure 5.
(A–D) Show results of immunohistochemical (IHC) analysis in the kidney tissues for iNOS and COX-2 expression in the control and experimental groups of rats. The experimental details of the IHC analysis is provided in the methodology section. Values are expressed as mean±SE (n=6). Statistical significance is expressed as ** p<0.01 compared to sham-operated controls, # p<0.05 NR compared to I/R rats.
Discussion
When the renal blood supply is cut off in conditions such as kidney transplantation, nephrectomy, and aortic bypass surgery, and then restored, it will result in renal I/R injury [1]. Such conditions may aggravate into decreased glomerular filtration and blood flow to the kidney, which result in natriuresis and dysregulated urine output. This condition is known as renal failure and is characterized by damage to glomerular tubules, glomerular injury, and necrosis, followed by blockage of renal tubules with cell debris [8–10]. Ischemia-reperfusion injuries are caused by damage to cellular components by ROS that are generated during oxidative stress and by the additional lipid peroxidation that they initiate [2]. They react with proteins, lipids, and nucleic acids to generate toxic peroxidation products that inhibit normal functioning of the kidney and DNA repair, resulting in death [11,12]. Perturbation of peroxidation of polyunsaturated fatty acids that may have methylene-interrupted double bonds can generate malondialdehyde (MDA), which is the most reliable lipoperoxidation marker (protein carbonyl concentration as a biomarker for development and mortality in sepsis-induced acute kidney injury) of oxidative stress under ischemic conditions [13–15]. When we conducted our investigation, we found that the untreated I/R rats had an upsurge in MDA and protein carbonyls (Figure 1A, 1B) as a result of ROS generation, and, due to the antioxidative capacity of the SOD, catalase enzymes were reduced [16] (Figure 1D, 1E).
This increase in MDA and PCO indicates the oxidative injury [17,18] caused by oxidative stress due to the successful onset of I/R on the renal tissues, leading to membrane cross-linking and ion permeability [19,20], which is an indicator of cell damage. The increase in renal damage was evident in the I/R groups, which had high MDA, but which was controlled in rats with NR1 pretreatment. The extent of renal damage is proportional to the extent of MDA increase [21] in cells. Renal tubules injury compromises the normal functioning of the kidney and hence increases the serum BUN and creatinine levels (Table 2) [1], which is indicative of decreased renal function. Compared to the controls, I/R-induced rats showed an increase in extracellular lactate dehydrogenase, which is an indicator of decreased cell viability and a biomarker for cell death [16]. Such increases were controlled in rats pretreated with NR and then induced with I/R, which protected the kidneys by preventing the protein and lipid peroxidation by improving the antioxidant activity of pretreated rats (Figure 1A, 1B).
Damage to cellular DNA takes place by the constant interaction of free oxygen radicals reacting with the DNA and by the formation of 8-hydroxy guanine (8-OHG), which is an indicator of the oxidative damage to cellular DNA [23]. We have observed that the renal cells in the I/R groups had higher levels of 8-OHG than in the groups pretreated with NR and in the sham group (Figure 1C). The reduced levels of 8-OHG is an indication of the healthy renal cells in the pretreated rats. Ischemic tissues generally show relatively lower expression of GSH [24,25]. The amount of oxidative stress can be assessed by measuring the activity of GSH, which is an antioxidant that is expressed to fight against the ROS. Since the ischemia followed by reperfusion has damaged the renal tissues, levels and the activity of GSH in acting against the ROS is compromised [21] and cannot process peroxides and free radicals into simpler non-toxic products, thus protecting the renal cells against oxidative damage. These conditions were seen in the I/R rats, but not in the NR-pretreated rats with I/R. The normalcy of antioxidants was restored in the rats with I/R that were pretreated (Figure 1F).
An increase in oxidative stress stimulates the antioxidant enzyme superoxide dismutase, which catalyzes the conversion of superoxide free radical (O2−.) into hydrogen peroxide (H2O2) and molecular oxygen (O2). The free radicals generated as a result of the ischemic process due to onset of oxidative stress were neutralized and helped protect the renal tissues from the toxicity of the free radicals and active oxygen species [26]. In the analysis of cytokine expression promoting inflammation [27,28] IL-2, IL-6, IL-8, and IL-17 were highly expressed in the IR-induced group (Figure 3). Among them, the proinflammatory cytokine IL-7, secreted by Th17 cells, and NK cells recruit neutrophils to the site of inflammation [29] and trigger the inflammatory cascade through NF-κB activation [30]. The antioxidant capacity of NR1 reduced the oxidative stress in pretreated rats and thereby blocked NF-κB activation [31], which regulated the expression of cytokines IL-1β, IL-6, and TNF-α [32]. Our ELISA results indicated that NR1 reduced the proinflammatory effect in pretreated rats by reducing IL-17 (Figure 3), which is the upstream activator of NF-κB [33]. Moreover, qPCR experimental results show an increase in the expression of TLR-4 mRNA in the renal cells of I/R-induced rats (Figure 4A). These results agree with our data showing the increased levels of proinflammatory cytokines as reported in induced ischemia [34]. Further, these inflammatory cytokines increased the expression of cyclooxygenase 2 (COX-2) (Figure 5D), and a there was a direct correlation between the expression of COX2 and renal ischemic injury in rats [35]. The retreated rats, but not the I/R-induced rats, showed a decrease in COX2 expression with the immunomodulatory action of NR1 [36,37]. Levels of Cxcl10 (chemokine [C-X-C motif] ligand 10) were reported to be overexpressed in I/R rats when multiple pathogenic events such as oxidative stress and inflammatory response caused reperfusion injury [38], and this was decreased in NR-pretreated rats in the present study (Figure 4E).
A strong increase in the mRNA expression of IL-16 (Figure 4D) indicates that the renal injury is complete and it has affected various segments of the nephron and proximal and distal tubules in the medulla, recruiting leukocytes and causing inflammation [39]. The effect was more prominent in I/R-induced rats, but NR1 modulated the inflammation by downregulating the expression of IL-16 and upregulating the anti-inflammatory cytokine IL-10 (Figure 4F) to inhibit the acute renal injury [40,41]. Hence, NR1 has an inhibitory effect [42,43] on such inflammatory cytokines in NR-pretreated rats with IR. In the aftermath of reperfusion in rat kidneys, there is an imbalance in the oxygen supply and a decrease in activity of mitochondria in scavenging the ROS. This results in increased ROS, and they attach to proteins and upregulate the superoxide anions in mitochondria, which, with the active participation of nitric oxide [1,44], results in reperfusion injury [44] in IR-induced rats without any pretreatment, as shown in our immunohistochemistry analysis showing that inducible nitric oxide synthase (iNOS) expression has increased (Figure 5A, 5B) and it played a key role in the onset of reperfusion injury in that group. Similar levels of iNOS expression were not observed in NR-pretreated rats [1], as iNOS inhibition did not result in renal failure.
To evaluate the severity of renal injury due to reperfusion, we assessed the acute kidney injury markers (Figure 2) neutrophil gelatinase-associated lipocalin (NGAL)-induced renal injury, N-acetyl-β-glucosaminidase (NAG)-tubular injury, Cystatin-C and β2-microglobulin-tubular dysfunction, IL-18-ischemic injury [41], and kidney injury molecule (kim-1) expressed in proximal tubules in post-ischemic kidneys in rats [41,45,46]. Treatment with NR1 decreased the levels of NGAL, as previously reported [47–50]. All these molecules found to be elevated in I/R-induced rats by ELISA were found to be reduced with NR1 pretreatment. The reduction in acute renal injury markers indicates that extended protection has been made possible by NR1 pretreatment in IR-induced rats.
Conclusions
We showed that rats pretreated with NR-1 had lower levels of inflammatory cytokines arising from I/R, and the iNOS mediating the oxidative damage and NF-KB activation are controlled, thus improving renal function after I/R. Our findings could be helpful as we have identified a potential drug candidate for use as a therapeutic strategy in treating I/R injury in patients hospitalized for renal transplantation. Our results need to be verified in animal models and in humans.
Footnotes
Conflict of interest
None.
Source of support: Departmental sources
References
- 1.Karaman A, Turkmen E, Gursul C, et al. Prevention of renal ischemia/reperfusion-induced injury in rats by leflunomide. Int J Urol. 2006;13(11):1434–41. doi: 10.1111/j.1442-2042.2006.01592.x. [DOI] [PubMed] [Google Scholar]
- 2.Glantzounis GK, Tselepis AD, Tambaki AP, et al. Laparoscopic surgery-induced changes in oxidative stress markers in human plasma. Surg Endosc. 2001;15(11):1315–19. doi: 10.1007/s00464-001-0034-2. [DOI] [PubMed] [Google Scholar]
- 3.Zhang WJ, Wojta J, Binder BR. Notoginsenoside R1 counteracts endotoxin-induced activation of endothelial cells in vitro and endotoxin-induced lethality in mice in vivo. Arterioscler Thromb Vasc Biol. 1997;17(3):465–74. doi: 10.1161/01.atv.17.3.465. [DOI] [PubMed] [Google Scholar]
- 4.Zhang HS, Wang SQ. Notoginsenoside R1 from Panax notoginseng inhibits TNF-alpha-induced PAI-1 production in human aortic smooth muscle cells. Vascul Pharmacol. 2006;44(4):224–30. doi: 10.1016/j.vph.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Sun K, Wang CS, Guo J, et al. Protective effects of ginsenoside Rb1, ginsenoside Rg1, and notoginsenoside R1 on lipopolysaccharide-induced microcirculatory disturbance in rat mesentery. Life Sci. 2007;81(6):509–18. doi: 10.1016/j.lfs.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 6.Liu WJ, Tang HT, Jia YT, et al. Notoginsenoside R1 attenuates renal ischemia-reperfusion injury in rats. Shock. 2010;34(3):314–20. doi: 10.1097/SHK.0b013e3181ceede4. [DOI] [PubMed] [Google Scholar]
- 7.Chen WX, Wang F, Liu YY, et al. Effect of notoginsenoside R1 on hepatic microcirculation disturbance induced by gut ischemia and reperfusion. World J Gastroenterol. 2008;14(1):29–37. doi: 10.3748/wjg.14.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barnes JL, Osgood RW, Reineck HJ, et al. Glomerular alterations in an ischemic model of acute renal failure. Lab Invest. 1981;45(4):378–86. [PubMed] [Google Scholar]
- 9.Finn WF. Nephron heterogeneity in polyuric acute renal failure. J Lab Clin Med. 1981;98(1):21–29. [PubMed] [Google Scholar]
- 10.Chatterjee PK, Cuzzocrea S, Thiemermann C. Inhibitors of poly (ADP-ribose) synthetase protect rat proximal tubular cells against oxidant stress. Kidney Int. 1999;56(3):973–84. doi: 10.1046/j.1523-1755.1999.00644.x. [DOI] [PubMed] [Google Scholar]
- 11.Chatterjee PK, Cuzzocrea S, Brown PA, et al. Tempol, a membrane-permeable radical scavenger, reduces oxidant stress-mediated renal dysfunction and injury in the rat. Kidney In. 2000;58(2):658–73. doi: 10.1046/j.1523-1755.2000.00212.x. [DOI] [PubMed] [Google Scholar]
- 12.Chatterjee PK, Zacharowski K, Cuzzocrea S, et al. Inhibitors of poly (ADP-ribose) synthetase reduce renal ischemia-reperfusion injury in the anesthetized rat in vivo. FASEB J. 2000;14(5):641–51. doi: 10.1096/fasebj.14.5.641. [DOI] [PubMed] [Google Scholar]
- 13.Del Rio D, Stewart AJ, Pellegrini N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr Metab Cardiovasc Dis. 2005;15(4):316–28. doi: 10.1016/j.numecd.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 14.Sammour T, Mittal A, Loveday BP, et al. Systematic review of oxidative stress associated with pneumoperitoneum. Br J Surg. 2009;96(8):836–50. doi: 10.1002/bjs.6651. [DOI] [PubMed] [Google Scholar]
- 15.Luo CF, Tsai YF, Chang CH, et al. Increased oxidative stress and gut ischemia caused by prolonged pneumoperitoneum in patients undergoing robot-assisted laparoscopic radical prostatectomy. Acta Anaesthesiol Taiwan. 2011;49(2):46–49. doi: 10.1016/j.aat.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 16.Yu Y, Sun G, Luo Y, et al. Cardioprotective effects of Notoginsenoside R1 against ischemia/reperfusion injuries by regulating oxidative stress- and endoplasmic reticulum stress-related signaling pathways. Sci Rep. 2016;6:21730. doi: 10.1038/srep21730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levine RL, Garland D, Oliver CN, et al. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990;186:464–78. doi: 10.1016/0076-6879(90)86141-h. [DOI] [PubMed] [Google Scholar]
- 18.Fadillioglu E, Oztas E, Erdogan H, et al. Protective effects of caffeic acid phenethyl ester on doxorubicin-induced cardiotoxicity in rats. J Appl Toxicol. 2004;24(1):47–52. doi: 10.1002/jat.945. [DOI] [PubMed] [Google Scholar]
- 19.Wilhelm J. Metabolic aspects of membrane lipid peroxidation. Acta Univ Carol Med Monogr. 1990;137:1–53. [PubMed] [Google Scholar]
- 20.Niki E, Yoshida Y, Saito Y, et al. Lipid peroxidation: Mechanisms, inhibition, and biological effects. Biochem Biophys Res Commun. 2005;338(1):668–76. doi: 10.1016/j.bbrc.2005.08.072. [DOI] [PubMed] [Google Scholar]
- 21.Celik O, Turkoz Y, Hascalik S, et al. The protective effect of caffeic acid phenethyl ester on ischemia-reperfusion injury in rat ovary. Eur J Obstet Gynecol Reprod Biol. 2004;117(2):183–88. doi: 10.1016/j.ejogrb.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 22.Basile DP, Bonventre JV, Mehta R, et al. Progression after AKI: Understanding maladaptive repair processes to predict and identify therapeutic treatments. J Am Soc Nephrol. 2016;27(3):687–97. doi: 10.1681/ASN.2015030309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grollman AP, Moriya M. Mutagenesis by 8-oxoguanine: an enemy within. Trends Genet. 1993;9(7):246–49. doi: 10.1016/0168-9525(93)90089-z. [DOI] [PubMed] [Google Scholar]
- 24.Cadirci E, Oral A, Odabasoglu F, et al. Atorvastatin reduces tissue damage in rat ovaries subjected to torsion and detorsion: Biochemical and histopathologic evaluation. Naunyn Schmiedebergs Arch Pharmacol. 2010;381(5):455–66. doi: 10.1007/s00210-010-0504-y. [DOI] [PubMed] [Google Scholar]
- 25.Ingec M, Isaoglu U, Yilmaz M, et al. Prevention of ischemia-reperfusion injury in rat ovarian tissue with the on-off method. J Physiol Pharmacol. 2011;62(5):575–82. [PubMed] [Google Scholar]
- 26.Arosio B, Gagliano N, Fusaro LM, et al. Aloe-Emodin quinone pretreatment reduces acute liver injury induced by carbon tetrachloride. Pharmacol Toxicol. 2000;87(5):229–33. doi: 10.1034/j.1600-0773.2000.d01-79.x. [DOI] [PubMed] [Google Scholar]
- 27.Zhang JM, An J. Cytokines, inflammation, and pain. Int Anesthesiol Clin. 2007;45(2):27–37. doi: 10.1097/AIA.0b013e318034194e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chae MS, Kim Y, Chung HS, et al. Predictive role of serum cytokine profiles in acute kidney injury after living donor liver transplantation. Mediators Inflamm. 2018;2018 doi: 10.1155/2018/8256193. 8256193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mehrotra P, Collett JA, McKinney SD, et al. IL-17 mediates neutrophil infiltration and renal fibrosis following recovery from ischemia reperfusion: Compensatory role of natural killer cells in athymic rats. Am J Physiol Renal Physiol. 2017;312(3):F385–97. doi: 10.1152/ajprenal.00462.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma J, Li YJ, Chen X, et al. Interleukin 17A promotes diabetic kidney injury. Sci Rep. 2019;9(1):2264. doi: 10.1038/s41598-019-38811-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu X, Wang JM. Iridoid glycosides fraction of Folium syringae leaves modulates NF-kappaB signal pathway and intestinal epithelial cells apoptosis in experimental colitis. PLoS One. 2011;6(9):e24740. doi: 10.1371/journal.pone.0024740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tak PP, Firestein GS. NF-kappaB: A key role in inflammatory diseases. J Clin Invest. 2001;107(1):7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sonder SU, Saret S, Tang W, et al. IL-17-induced NF-kappaB activation via CIKS/Act1: Physiologic significance and signaling mechanisms. J Biol Chem. 2011;286(15):12881–90. doi: 10.1074/jbc.M110.199547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu H, Chen G, Wyburn KR, et al. TLR4 activation mediates kidney ischemia/reperfusion injury. J Clin Invest. 2007;117(10):2847–59. doi: 10.1172/JCI31008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsuyama M, Yoshimura R, Hase T, et al. Study of cyclooxygenase-2 in renal ischemia-reperfusion injury. Transplant Proc. 2005;37(1):370–72. doi: 10.1016/j.transproceed.2004.12.246. [DOI] [PubMed] [Google Scholar]
- 36.Chen J, Si M, Wang Y, et al. Ginsenoside metabolite compound K exerts anti-inflammatory and analgesic effects via downregulating COX2. Inflammopharmacology. 2019;27(1):157–66. doi: 10.1007/s10787-018-0504-y. [DOI] [PubMed] [Google Scholar]
- 37.Lee SM. Anti-inflammatory effects of ginsenosides Rg5, Rz1, and Rk1: Inhibition of TNF-alpha-induced NF-kappaB, COX-2, and iNOS transcriptional expression. Phytother Res. 2014;28(12):1893–96. doi: 10.1002/ptr.5203. [DOI] [PubMed] [Google Scholar]
- 38.Yu L, Moriguchi T, Kaneko H, et al. Reducing inflammatory cytokine production from renal collecting duct cells by inhibiting GATA2 ameliorates acute kidney injury. Mol Cell Biol. 2017;37(22) doi: 10.1128/MCB.00211-17. pii: e00211-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang S, Diao H, Guan Q, et al. Decreased renal ischemia-reperfusion injury by IL-16 inactivation. Kidney Int. 2008;73(3):318–26. doi: 10.1038/sj.ki.5002692. [DOI] [PubMed] [Google Scholar]
- 40.Deng J, Kohda Y, Chiao H, et al. Interleukin-10 inhibits ischemic and cisplatin-induced acute renal injury. Kidney Int. 2001;60(6):2118–28. doi: 10.1046/j.1523-1755.2001.00043.x. [DOI] [PubMed] [Google Scholar]
- 41.Sakai K, Nozaki Y, Murao Y, et al. Protective effect and mechanism of IL-10 on renal ischemia-reperfusion injury. Lab Invest. 2019;99(5):671–83. doi: 10.1038/s41374-018-0162-0. [DOI] [PubMed] [Google Scholar]
- 42.Niu J, Wang K, Graham S, et al. MCP-1-induced protein attenuates endotoxin-induced myocardial dysfunction by suppressing cardiac NF-small ka, CyrillicB activation via inhibition of Ismall ka, CyrillicB kinase activation. J Mol Cell Cardiol. 2011;51(2):177–86. doi: 10.1016/j.yjmcc.2011.04.018. [DOI] [PubMed] [Google Scholar]
- 43.Sun Q, Meng QT, Jiang Y, et al. Protective effect of ginsenoside Rb1 against intestinal ischemia-reperfusion induced acute renal injury in mice. PLoS One. 2013;8(12):e80859. doi: 10.1371/journal.pone.0080859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ozyurt H, Irmak MK, Akyol O, et al. Caffeic acid phenethyl ester changes the indices of oxidative stress in serum of rats with renal ischaemia-reperfusion injury. Cell Biochem Funct. 2001;19(4):259–63. doi: 10.1002/cbf.923. [DOI] [PubMed] [Google Scholar]
- 45.Han WK, Bailly V, Abichandani R, et al. Kidney injury molecule-1 (KIM-1): A novel biomarker for human renal proximal tubule injury. Kidney Int. 2002;62(1):237–44. doi: 10.1046/j.1523-1755.2002.00433.x. [DOI] [PubMed] [Google Scholar]
- 46.Ferguson MA, Vaidya VS, Bonventre JV. Biomarkers of nephrotoxic acute kidney injury. Toxicology. 2008;245(3):182–93. doi: 10.1016/j.tox.2007.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haase M, Bellomo R, Devarajan P, et al. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: A systematic review and meta-analysis. Am J Kidney Dis. 2009;54(6):1012–24. doi: 10.1053/j.ajkd.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 48.Mori K, Nakao K. Neutrophil gelatinase-associated lipocalin as the real-time indicator of active kidney damage. Kidney Int. 2007;71(10):967–70. doi: 10.1038/sj.ki.5002165. [DOI] [PubMed] [Google Scholar]
- 49.Hjortrup PB, Haase N, Wetterslev M. Clinical review: Predictive value of neutrophil gelatinase-associated lipocalin for acute kidney injury in intensive care patients. Crit Care. 2013;17(2):211. doi: 10.1186/cc11855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun B, Xiao J, Sun XB. Notoginsenoside R1 attenuates cardiac dysfunction in endotoxemic mice: An insight into oestrogen receptor activation and PI3K/Akt signalling. Br J Pharmacol. 2013;168(7):1758–70. doi: 10.1111/bph.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]