Abstract
Objective
To synthesize available data related to the complex associations among viral infections, atopy, and asthma.
Data Sources
Key historical articles, articles highlighted in our recent review of most significant recent asthma advancements, and findings from several birth cohorts related to asthma and viral infections were reviewed. In addition, PubMed was searched for review articles and original research related to the associations between viral infection and asthma, using the search words asthma, viral infections, atopy, development of asthma, rhinovirus (RV), and respiratory syncytial virus (RSV).
Study Selections
Articles were selected based on novelty and relevance to our topic of interest, the role of asthma and viral infections, and possible mechanisms to explain the association.
Results
There is a large body of evidence demonstrating a link between early viral infections (especially RV and RSV) and asthma inception and exacerbations. RV-induced wheezing is an important risk factor for asthma only when atopy is present, with much evidence supporting the idea that sensitization is a risk factor for early RV-induced wheezing, which in turn is a risk factor for asthma. RSV, on the other hand, is a more important risk factor for nonatopic asthma, with severe infections conferring greater risk.
Conclusion
There are important differences in the development of atopic and nonatopic asthma, with several proposed mechanisms explaining the association between viral infections and the development of asthma and asthma exacerbations. Understanding these complex associations is important for developing asthma prevention strategies and targeted asthma therapies.
Key Messages.
-
•
Viral infections in infancy, particularly with rhinovirus (RV) or respiratory syncytial virus (RSV), are important risk factors for wheezing episodes, asthma, and asthma exacerbations.
-
•
RV infection is a more important risk factor for the development of atopic asthma, likely through a mechanism that relies on allergic sensitization.
-
•
RSV infection is a more important risk factor for the development of nonatopic asthma, likely through a different mechanism than RV.
-
•
Neutrophils and dendritic cell responses to viral infection appear to be important contributors to the TH2 response that defines atopic asthma.
-
•
Understanding the association among viral infections, atopy, and asthma can help guide appropriate treatment strategies for viral-induced or exacerbated asthma.
Introduction
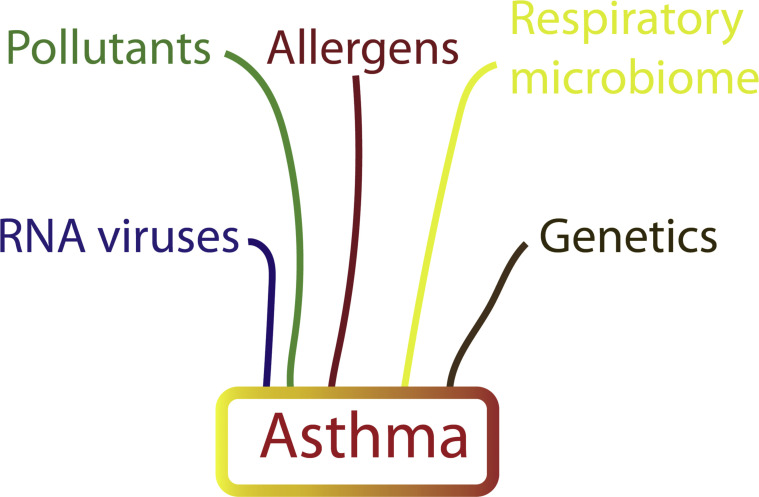
Asthma is the most common chronic medical condition in the pediatric population, affecting 5% to 10% of children and adolescents,1 and is characterized by chronic inflammation of the airways, excessive mucous production, bronchial hyperreactivity, and reversible airflow obstruction that leads to recurrent episodes of wheezing, dyspnea, and cough. This review discusses the strong link between asthma and viral infections. Viral infections trigger infant wheezing episodes, have been implicated in the inception of asthma, and are recognized as an important risk factor for asthma exacerbations (Fig 1 ).
Figure 1.
Factors involved in the development of asthma. Many factors combine to drive the development of asthma, of which respiratory viruses are only 1 component. These factors include RNA viruses, such as respiratory syncytial virus and rhinovirus, airway pollutants, allergens, genetics, and the components of the respiratory microbiome.
Acute Wheezing Episodes in Infancy
Many children wheeze in the first few years of life, with some studies citing up to 50% of children wheezing before school age.2 Not all these children develop asthma, and a subset of children develop asthma even without wheezing as an infant.1 Almost all wheezing episodes in the first few years of life occur with viral infections, most commonly rhinovirus (RV) and respiratory syncytial virus (RSV), but enterovirus, bocavirus, parainfluenza virus, coronavirus, metapneumovirus, influenza virus, and adenovirus have also been implicated.3
Bronchiolitis is a common condition characterized by inflammation of the small bronchioles and surrounding tissue in children up to 2 years of age and is caused by viral infection of the lower airways. Bronchiolitis can affect up to 20% to 30% infants in the first year of life and up to 10% to 20% of infants in the second year of life.3 Wheezing is often a characteristic finding of bronchiolitis but not mandatory. Other symptoms include dry cough, tachypnea, widespread crackles, and breathing difficulty.4
RSV is the most common cause of bronchiolitis in the first year of life, usually occurring in winter.5 RSV often affects infants during a critical time in lung development and consequently may affect long-term lung development.4 Although RSV may lead to severe infections, most infections are asymptomatic or present with a mild illness. Risk factors for RSV-induced wheezing include prematurity; heart, lung, and immune system abnormalities; and the age and season of birth (being 2-6 months of age during the winter RSV season).5
Whereas RSV is the most common cause of infant wheezing in the first year of life, RV is the most often detected virus with wheezing in older infants and toddlers6 and is the second most common cause of bronchiolitis in infants younger than 12 months.2 RV infections can present year-round, with a peak incidence in late autumn and early spring.4 RV infections are nonenveloped positive-strand RNA viruses with many distinct genotypes that are divided into 3 different species (RV-A, RV-B, RV-C) based on genetic properties. Because RV-C viruses do not grow in conventional cell cultures, their identification was delayed, and their detection continues to rely on polymerase chain reaction. RV-A and RV-C are more often associated with wheezing illnesses in infants than RV-B.2
Risk Factors for Development of Asthma
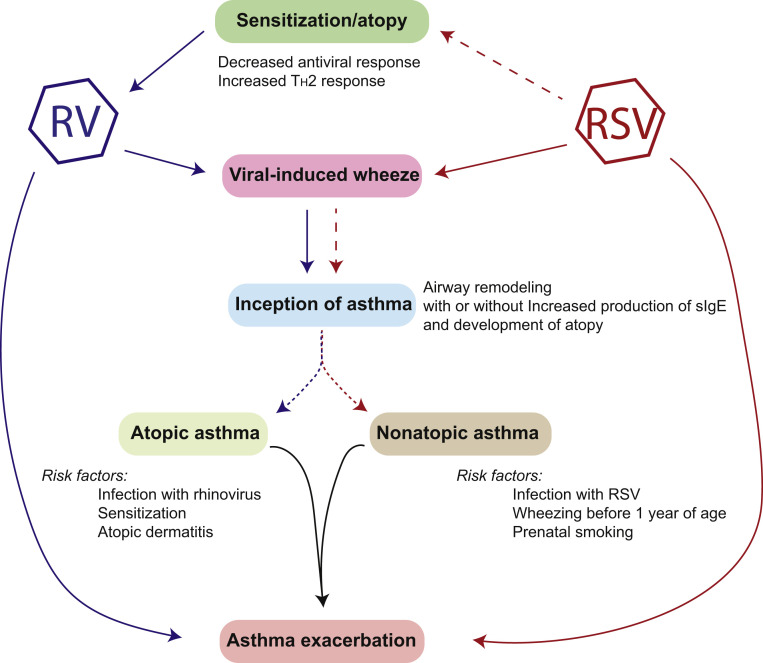
Understanding the risk factors for progression from wheezing as an infant to development of asthma is important for targeted asthma prevention strategies and for offering anticipatory guidance after an initial wheezing episode. Although many viruses have been associated with wheeze as an infant, most data suggest that the greatest association is between RV and RSV recurrent wheeze and development of asthma (Fig 2 ).2, 6, 7, 8, 9, 10
Figure 2.
Association among respiratory viruses, atopy, wheeze, and asthma. As discussed in the text, respiratory syncytial virus (RSV) has been strongly associated with the development of wheeze in infants who have had a severe infection but without any preexisting atopy. Less clear (dashed lines) are the association between RSV infection and development of atopy, as well as asthma by adolescence. Rhinovirus (RV), on the other hand, drives the development of wheeze and asthma but only in the presence of preexisting atopy. There is an unclear association with the factors that drive atopic and nonatopic asthma (dotted lines). Viral infections, including with RV or RSV, are also able to exacerbate existing asthma.
Risk factors can differ for allergic and nonallergic asthma. The modified Asthma Predictive Index (API), mainly based on atopic characteristics, is often used to predict risk of school-age asthma and in birth cohort studies has been predictive of atopic asthma but not nonatopic asthma.11 Lukkarinen et al10 considered the characteristics used in the API, in combination with viral origin, in infants hospitalized with first-time wheeze and found that RV-induced wheezing, alone or with sensitization and eczema, predicted atopic asthma at school age. On the other hand, parental smoking, RSV infection with first wheeze, and wheezing before 1 year of age were found to be risk factors for nonatopic asthma at school-age.10 These differences in risk factors highlight important mechanistic differences between RV-induced and RSV-induced disease, with RV-induced wheezing being a more important risk factor in atopic individuals (Fig 2).
Many studies have found that early RV-induced wheezing is an important asthma risk factor.9, 12, 13, 14, 15, 16, 17 Some studies estimate that 60% of children who wheeze with RV in the first 2 years of life will develop asthma.6 In the Childhood Origins of Asthma (COAST) study, a birth cohort study that prospectively followed up children who had at least 1 atopic parent, infants who wheezed with RV alone were more likely to develop asthma than those who wheezed with RSV alone or RSV and RV.18
Many studies have found a link between atopy and RV-induced wheezing/9, 13, 14, 16, 17, 19 The risk of asthma increases markedly in infants with RV-triggered wheeze who also have evidence of allergen sensitization. An Australian birth cohort demonstrated the risk for wheezing at 5 years of age was only associated with infant RV wheezing in children who had evidence of sensitization.7 Furthermore, in the COAST study, the greatest risk for asthma was in infants who had both RV wheezing and sensitization to at least one aeroallergen.18 Although it is unclear whether sensitization or viral infection came first, it seems most likely that allergic sensitization itself is a risk factor for RV-induced wheezing.13, 20 In the COAST study, early-life aeroallergen sensitization preceded RV infection, leading the authors to hypothesize that allergic sensitization may be what drives viral wheezing.13 Children sensitized to multiple aeroallergens early in life have the highest risk for persistent wheeze, severe asthma exacerbations requiring hospitalization, and impaired lung function.21, 22 There are many pathways between RV infections and allergic inflammation that can potentially explain the increased risk of asthma that occurs with RV wheezing and atopy, and these are briefly reviewed in the mechanism section below.
Others have challenged the finding that specific viral infections are significant, hypothesizing that the number of lower respiratory tract infections in the first year of life is a more significant risk factor that any specific viral origin.23 The number of early viral infections in the lower airways is certainly an important factor. The COAST study found that the number of RV wheezing episodes is most closely associated with asthma risk.24 However, most studies reflect the belief that RV-induced wheeze is a significant risk factor for the development of asthma, and likely frequent infant RV-wheezing episodes confers a higher risk. A meta-analysis examining 15 original articles found an association between RV-induced wheezing and development of childhood asthma.25
Although a link between RSV and asthma has long been suspected, the association is less clear. One-third of children with RSV develop recurrent wheezing episodes.11 RSV-induced bronchiolitis in birth cohort studies has been associated with asthma but not with allergic sensitization.26, 27 There have been conflicting results regarding the importance of RSV infection in the development of asthma. Sigurs et al28 were the first to conduct a prospective study that included controls matched for age, birthdate, sex, and residence and found that there was an increased rate of asthma in the infants who were hospitalized with RSV in infancy. In fact, they followed up the cohort until 18 years of age and reported an increase in asthma, allergic sensitization, and clinical allergy and a decrease in pulmonary function on spirometry in the RSV group.8 The Tucson respiratory study, a nonselected birth cohort study, linked RSV-induced bronchiolitis to asthma for up to 13 years.26 In this study, RSV-induced wheeze was a risk factor for recurrent wheeze, but the risk of wheeze decreased and was insignificant by 13 years of age, conflicting with the results from the study by Sigurs et al.28 A Finnish study found an association between RSV and self-reported asthma in children 15 to 18 years of age.29 Another study found that infants born 3 months before the RSV season had the highest risk of asthma between 4 and 5 years of age.29 Contrary to these studies, the COAST study and an Australian birth cohort, both high-risk birth cohorts, did not identify an association between RSV as an infant and the development of asthma in school age/30 The ambiguity in results among these studies is likely attributable to the differences in population among the studies because mechanisms differ between RV and RSV for causing virus-induced disease. Most notably, sensitization does not seem to be a risk factor for infants who develop RSV-induced wheezing26 but is an important risk factor in infants who have RV-triggered wheeze. Hence, RSV will be less significant in atopic populations, such as those present in the COAST study and the Australian study group.
The severity of RSV infection is likely the most significant risk factor for development of asthma after RSV-induced wheezing. This risk factor is likely attributable to different disease mechanisms involved in the antiviral response, including enhanced TH2 responses and genetic polymorphisms,31, 32 which are briefly discussed in the Mechanism section of this review.
Parainfluenza viruses have also been implicated in the development of asthma.33 A study in Sweden found that infections with RSV, influenza, or parainfluenza in children younger than 3 months lead to the development of a TH2 cytokine profile.34
Despite this increasing body of evidence showing a pivotal link between RV- and RSV-induced wheezing episodes and development of asthma, it remains unclear whether infection with RV and/or RSV in infancy contributes to the development of asthma or is simply a marker of asthma susceptibility.20, 35 There are many dynamics that come together to produce asthma, including host and environmental factors. Children with severe virus-inducted bronchiolitis were born with increased bronchial responsiveness compared with children without bronchiolitis,13 and patients with asthma have an altered epithelial immune response to RV.36 Many posit that infant RV-wheezing episodes occur in infants predisposed to develop asthma.4 The host factors that contribute to severe viral infection, predispose patients to atopy, and respond to viral wheezing with enhanced airway damage may actually be significant risk factors for the development of asthma. There is a likely a complex interplay among viral infections, sensitization, and environmental exposures in a susceptible host that promote asthma.
Asthma Exacerbations
Viral infections are often associated with asthma exacerbations, contributing to up to 90% of acute asthma exacerbations and serving as the main trigger of asthma in school-aged children and adults.37, 38 Viral infections are particularly important when children return to school after summer and spring breaks.39 Weeks in which respiratory viruses are circulating have been associated with greater asthma symptoms and more frequent loss of asthma control.40, 41 Children with chronic asthma and greater symptoms with a respiratory viral infection often have reduced airway function. In fact, children with asthma harbor viruses in their lower airways even when they are not wheezing.38 However, viruses tend to be found more often during asthma exacerbations than when asymptomatic.38
Many viruses have been associated with asthma exacerbations, including RSV, RV, and influenza and to a lesser extent coronavirus, parainfluenza, adenovirus, and metapneumovirus.42 However, RV is the most common virus associated with childhood asthma exacerbations, probably because RV is the most prevalent of the respiratory viruses. RV can cause a wide spectrum of disease, ranging from asymptomatic to severe infection requiring hospitalization. Most RV infections in children with asthma do not lead to an exacerbation, however. Several factors predispose children to an exacerbation, most notably of which is a history of atopy. Studies in the emergency department have found that detection of respiratory virus, allergen sensitization,43, 44 and eosinophil inflammation are risk factors for acute wheezing. Higher levels of aeroallergen specific IgE imparted the greatest risk, further supporting the synergistic association between RV infection and IgE, leading to increased risk of wheezing.43, 44 Similar to the greater association with wheezing infections in infants, RV-A and RV-C infections lead to more severe illness and are more often associated with asthma exacerbations than RV-B.2, 4 Furthermore, RV-C might be more strongly associated with severe asthma exacerbations and asthma hospitalizations because of a stronger inflammatory response being elicited by the virus.45
Viral infections can increase the presence of bacterial colonization and infection in the airways. Prospective studies have found that RV infections increase the frequency and quantity of Streptococcus pneumoniae, Moraxella catarrhalis, and Haemophilus influenzae detected in airway secretions.41 Detection of these bacterial pathogens was associated with asthma exacerbations in children with asthma and symptomatic illness in children without asthma.41 The role of bacteria in asthma development and exacerbation is beyond the scope of this review, but clearly there is a potential interaction between respiratory viruses and bacterial biome in the airways that could also drive disease development and/or exacerbation.
Prevention and Treatment
Understanding the role of viral infections in asthma development has been the basis for attempts to prevent disease development and to reduce the severity and frequency of asthma exacerbations. Palivizumab, a monoclonal antibody directed against RSV and thus able to reduce the rate of severe RSV infections, decreases early episodes of recurrent wheezing in nonatopic children (but not atopic children) when administered to high-risk preterm infants.21, 46, 47 The differences between the 2 populations is consistent with the observation that RSV appears to be more important in nonatopic asthma. Despite the reduction in early wheeze, there was no reduction in the rate of asthma.47, 48 This finding was not surprising given the fact that RSV is a risk factor for wheezing in infants but this risk of recurrent wheeze diminishes with age. Another RSV antibody, motavizumab, administered to a high-risk group in the first year of life reduced the risk of hospital admissions for wheezing by 87% but did not lead to a reduction in medically treated wheezing between 1 and 3 years of age.49 Taken together, these studies indicate that monoclonal antibody therapy directed against RSV in preterm infants can reduce bronchiolitis and recurrent wheezing. Whether similar results would be seen in full-term infants remains to be demonstrated.
Studies have found that oral steroid use in hospitalized infants with first wheeze can decrease the risk of recurrent wheeze and asthma in children affected by RV, eczema, or both.12, 15, 16, 19, 50 The mechanism(s) of this protection are not known but likely is mediated through decreased inflammatory response to the viral infection. In general, RV-induced wheezing is more responsive to oral steroids, whereas RSV-induced wheezing is less likely to respond to oral steroids.16, 51
Omalizumab, a monoclonal antibody that prevents IgE from binding to its receptor, has been effectively used in the treatment of moderate to severe asthma, reducing the number of asthma exacerbations and seasonal variation in exacerbations.52, 53 Because seasonal peaks in asthma exacerbations are thought to be mainly attributable to viral illnesses, the reduction in exacerbations likely means that anti-IgE has an effect on the immune response to the viral infection. Indeed, treatment with omalizumab, either year-round or seasonal pretreatment, led to a decrease in seasonal exacerbations and a decrease in RV in nasal secretions, especially among children with severe asthma.52, 53 The mechanism of this effect is not known. We found that humans make IgE against RV (and others have found that IgE can be made against RSV).54, 55 This finding suggests that anti-IgE might block asthma exacerbations in a similar manner to how it is able to inhibit airway allergen–induced asthma. An alternate explanation is based on the observation that reduction of IgE led to increased type I interferon production from plasmacytoid dendritic cells ex vivo.56 This finding suggest that anti-IgE might increase antiviral immunity. Consistent with both of these observations, treatment with omalizumab decreases the severity of asthma exacerbations associated with RV.57 As a side note, a randomized clinical trial of inhaled interferon beta (type I interferon) found no benefit with treatment in the primary end points of Asthma Control Questionnaire score and forced expiratory volume in 1 second, suggesting that decreased type I interferon is not the mechanism of asthma exacerbations. Some have argued that this study was not adequately powered to demonstrate power for the entire study population. In particular, the inclusion of patients with mild asthma who experienced upper respiratory tract infection–induced exacerbations may have skewed the results toward no benefit. However, a benefit was seen in a subpopulation with severe asthma in Asthma Control Questionnaire score and peak expiratory flow.58
Mechanisms
Decreased Type I Interferon Production and Increased Susceptibility to Viral Infections
As mentioned above, one potential mechanistic link between RV infections and asthma exacerbations could be the production of type I interferon. Patients with asthma have been reported to have a delayed and decreased antiviral response, likely thought to be attributable to decreased production of innate interferons.59, 60, 61 Impaired type I interferon expression likely contributes to asthma by allowing uncontrolled replication of viral infections and by leaving TH2 inflammation unchecked; however, this assertion has not been well proved. In fact, although some studies have supported the idea that there is decreased interferon expression in patients with asthma,35, 59, 60, 62, 63 others have disputed this finding.64, 65 It is believed, however, that there is at least a subgroup of patients with asthma who have impaired type I interferon responses. One birth cohort study found that the population of patients with asthma and impaired IFN response to RV at 11 years of age also had increased wheeze in infancy, asthma in adolescence, and more frequent asthma exacerbations and were more likely to have allergic sensitization in infancy.10 Type I interferon levels demonstrate an inverse association with changes in lung function, eosinophilia, and other markers of inflammation after RV infection.61 However, as mentioned above, administration of type I interferon did not have a significant effect in improving asthma symptoms or lung function in most patients with asthma.58
Neutrophils and Dendritic Cells
Neutrophils are believed to play an important role in the interplay between viral respiratory infections and asthma.66, 67, 68 Infants hospitalized with RSV and other viruses have robust neutrophil influx in the airway epithelium.69 We identified a potential pathway for the role of neutrophils in virus-induced disease in a mouse model, where, using Sendai virus (a rodent respiratory virus similar to RSV), we found that the virally infected mice developed postviral atopic disease via recruitment of a subset of neutrophils (CD49d expressing) through production of cysteinyl leukotrienes. These neutrophils then induced expression of the high-affinity IgE receptor (FcƐRI) on lung conventional dendritic cells. At the same time, the mice made IgE against Sendai virus, which then bound the FcƐRI expressing conventional dendritic cells and could be crosslinked by the virus. This process led to production of CCL28, a TH2 cell chemoattractant, which drove an influx of TH2 cells and interleukin (IL) 13 production, and the subsequent airway hyperreactivity and mucous cell hyperplasia.66, 70, 71
In the human, we have found evidence of a similar pathway. CD49d-expressing neutrophils can be found in the peripheral blood and nasal lavage fluid, and their presence was strongly associated with allergic disease or symptoms of a respiratory viral infection.67, 68 Like their murine counterparts, human CD49d-expressing neutrophils also express cysteinyl leukotriene receptor 1, a receptor for cysteinyl leukotriene.68 Crosslinking IgE on peripheral blood human conventional dendritic cells will also lead to the production of CCL28.72 Finally, others have found that during respiratory viral infections (most often RV), total serum IgE levels increase, as does expression of FcƐRI on peripheral blood conventional dendritic cells.73
Role of Sensitization
As described previously, there is link between RV infection and allergic inflammation in both the development of asthma and triggering of asthma exacerbations. Some studies have found sensitization preceding viral infection.13, 20 Allergic inflammation can increase airway responsiveness to RV infection74 and has been suggested to lead to greater viral replication.75, 76 As described previously, RSV infections have a greater association with the development of nonatopic asthma and RV infections with atopic asthma. However, RSV has been associated with the development of atopy in a subset of patients. In fact, viral infections, including RSV and paramyxoviral infections, have been associated with development of asthma and atopy.70 In a mouse model, we found that exposure to a nonviral antigen during the viral infection was sufficient to drive specific IgE production against the nonviral antigen, outlining a potential mechanism for postviral development of atopy.77 Furthermore, viral infections damage the barrier function of airway epithelium, which can allow for greater absorption of aeroallergens, leading to a potential increase in allergic sensitization.75, 76
Healthy airway epithelium is relatively resistant to RV infection. However, disrupted airway epithelium, such as that present when allergen sensitization is present, may promote viral replication by providing access to deeper cell layers, which are reported to favor RV replication, in part through expression of more intercellular adhesion molecule 1 receptors, which are one of the receptors through which RV infects cells. The disrupted airway epithelium that promotes RV replication also invites absorption of aeroallergens and infection by bacterial pathogens.4, 75
Viral infection, specifically RV infection, has been associated with production of IL-25 and IL-33, which promote type 2 airway inflammation and remodeling primarily through driving production of type 2 innate lymphoid cells.78 These cells are capable of producing large amounts of type 2 cytokines and driving the atopic response.78 A recent publication also found that IL-33 can dampen innate and adaptive TH1-like and cytotoxic responses in response to viral infection, which may be another factor in driving virus-induced asthma exacerbations.79
Genetic Polymorphisms
The genetic components that underlie or possibly drive the risk of asthma development after respiratory viral infection are still being explored. One locus associated with RV-induced disease is located at 17q21. The presence of a polymorphism at this locus influences the risk of RV-induced wheezing, as well as the risk of RV-wheezing infants progressing to develop asthma, in both the COAST and Copenhagen Prospective Studies on Asthma in Childhood birth cohorts.80 Another polymorphism associated with the risk of asthma is the cadherin-related family member 3, which was identified as an entry factor on airway epithelial cells for RV-C.81 Although there are likely genetic polymorphisms that may predispose infants to more severe RSV infections, more research is needed to better understand these. One potential genetic association has linked TLR4 and LPS polymorphisms to TH2 immune responses that favor the development of asthma after RSV infection.82
Conclusion
Viral infections are important triggers of acute wheezing episodes in infancy and the inception and exacerbation of asthma. The 2 most common respiratory viruses to be associated with development and/or exacerbation of asthma, RSV and RV, seem to have disparate mechanisms that lead to disease. RSV appears to drive asthma in infants without a preexisting history of atopy, whereas RV is more likely to lead to asthma in children who have already developed an allergic phenotype. Understanding the differences in these mechanisms will be important as we begin to explore potential primary prevention strategies and novel therapeutics to prevent viral-induced wheeze, asthma, and development of atopy.
Footnotes
Disclosures: Dr Grayson has served on advisory boards for AstraZeneca, Genentech, and Novartis. He is an associate editor for the Annals of Allergy, Asthma, and Immunology. No other disclosures were reported.
Funding Sources: This work was funded in part by grants HL087778 and AI120655 from the National Institutes of Health (Dr Grayson).
References
- 1.Lai C.K., Beasley R., Crane J., et al. Global variation in the prevalence and severity of asthma symptoms: phase three of the International Study of Asthma and Allergies in Childhood (ISAAC) Thorax. 2009;64:476–483. doi: 10.1136/thx.2008.106609. [DOI] [PubMed] [Google Scholar]
- 2.Jackson D.J., Gern J.E., Lemanske R.F., Jr. The contributions of allergic sensitization and respiratory pathogens to asthma inception. J Allergy Clin Immunol. 2016;137:659–665. doi: 10.1016/j.jaci.2016.01.002. quiz 666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meissner H.C. Viral Bronchiolitis in children. N Engl J Med. 2016;374:62–72. doi: 10.1056/NEJMra1413456. [DOI] [PubMed] [Google Scholar]
- 4.Jartti T., Gern J.E. Role of viral infections in the development and exacerbation of asthma in children. J Allergy Clin Immunol. 2017;140:895–906. doi: 10.1016/j.jaci.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geevarghese B., Simoes E.A. Antibodies for prevention and treatment of respiratory syncytial virus infections in children. Antivir Ther. 2012;17:201–211. doi: 10.3851/IMP2061. [DOI] [PubMed] [Google Scholar]
- 6.Kotaniemi-Syrjanen A., Vainionpaa R., Reijonen T.M., Waris M., Korhonen K., Korppi M. Rhinovirus-induced wheezing in infancy: the first sign of childhood asthma? J Allergy Clin Immunol. 2003;111:66–71. doi: 10.1067/mai.2003.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kusel M.M., de Klerk N.H., Kebadze T., et al. Early-life respiratory viral infections, atopic sensitization, and risk of subsequent development of persistent asthma. J Allergy Clin Immunol. 2007;119:1105–1110. doi: 10.1016/j.jaci.2006.12.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sigurs N., Aljassim F., Kjellman B., et al. Asthma and allergy patterns over 18 years after severe RSV bronchiolitis in the first year of life. Thorax. 2010;65:1045–1052. doi: 10.1136/thx.2009.121582. [DOI] [PubMed] [Google Scholar]
- 9.Midulla F., Pierangeli A., Cangiano G., et al. Rhinovirus bronchiolitis and recurrent wheezing: 1-year follow-up. Eur Respir J. 2012;39:396–402. doi: 10.1183/09031936.00188210. [DOI] [PubMed] [Google Scholar]
- 10.Lukkarinen M., Koistinen A., Turunen R., Lehtinen P., Vuorinen T., Jartti T. Rhinovirus-induced first wheezing episode predicts atopic but not nonatopic asthma at school age. J Allergy Clin Immunol. 2017;140:988–995. doi: 10.1016/j.jaci.2016.12.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castro-Rodriguez J.A., Holberg C.J., Wright A.L., Martinez F.D. A clinical index to define risk of asthma in young children with recurrent wheezing. Am J Respir Crit Care Med. 2000;162:1403–1406. doi: 10.1164/ajrccm.162.4.9912111. [DOI] [PubMed] [Google Scholar]
- 12.Lehtinen P., Ruohola A., Vanto T., Vuorinen T., Ruuskanen O., Jartti T. Prednisolone reduces recurrent wheezing after a first wheezing episode associated with rhinovirus infection or eczema. J Allergy Clin Immunol. 2007;119:570–575. doi: 10.1016/j.jaci.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jackson D.J., Evans M.D., Gangnon R.E., et al. Evidence for a causal relationship between allergic sensitization and rhinovirus wheezing in early life. Am J Respir Crit Care Med. 2012;185:281–285. doi: 10.1164/rccm.201104-0660OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kusel M.M., Kebadze T., Johnston S.L., Holt P.G., Sly P.D. Febrile respiratory illnesses in infancy and atopy are risk factors for persistent asthma and wheeze. Eur Respir J. 2012;39:876–882. doi: 10.1183/09031936.00193310. [DOI] [PubMed] [Google Scholar]
- 15.Lukkarinen M., Lukkarinen H., Lehtinen P., Vuorinen T., Ruuskanen O., Jartti T. Prednisolone reduces recurrent wheezing after first rhinovirus wheeze: a 7-year follow-up. Pediatr Allergy Immunol. 2013;24:237–243. doi: 10.1111/pai.12046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jartti T., Nieminen R., Vuorinen T., et al. Short- and long-term efficacy of prednisolone for first acute rhinovirus-induced wheezing episode. J Allergy Clin Immunol. 2015;135:691–698, e699. doi: 10.1016/j.jaci.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turunen R., Koistinen A., Vuorinen T., et al. The first wheezing episode: respiratory virus etiology, atopic characteristics, and illness severity. Pediatr Allergy Immunol. 2014;25:796–803. doi: 10.1111/pai.12318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jackson D.J., Gangnon R.E., Evans M.D., et al. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med. 2008;178:667–672. doi: 10.1164/rccm.200802-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lukkarinen M.M., Koistinen A.P., Turunen R.M., Jartti T.T. Toward primary prevention of asthma: role of corticosteroids for the first rhinovirus wheeze. Am J Respir Crit Care Med. 2015;192:1018–1019. doi: 10.1164/rccm.201506-1226LE. [DOI] [PubMed] [Google Scholar]
- 20.Holt P.G., Sly P.D. Viral infections and atopy in asthma pathogenesis: new rationales for asthma prevention and treatment. Nat Med. 2012;18:726–735. doi: 10.1038/nm.2768. [DOI] [PubMed] [Google Scholar]
- 21.Blanken M.O., Rovers M.M., Bont L., Dutch R.S.V.N.N. Respiratory syncytial virus and recurrent wheeze. N Engl J Med. 2013;369:782–783. doi: 10.1056/NEJMc1307429. [DOI] [PubMed] [Google Scholar]
- 22.Stoltz D.J., Jackson D.J., Evans M.D., et al. Specific patterns of allergic sensitization in early childhood and asthma & rhinitis risk. Clin Exp Allergy. 2013;43:233–241. doi: 10.1111/cea.12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonnelykke K., Vissing N.H., Sevelsted A., Johnston S.L., Bisgaard H. Association between respiratory infections in early life and later asthma is independent of virus type. J Allergy Clin Immunol. 2015;136:81–86, e84. doi: 10.1016/j.jaci.2015.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson H.M., Lemanske R.F., Jr., Evans M.D., et al. Assessment of wheezing frequency and viral etiology on childhood and adolescent asthma risk. J Allergy Clin Immunol. 2017;139:692–694. doi: 10.1016/j.jaci.2016.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu L., Pan Y., Zhu Y., et al. Association between rhinovirus wheezing illness and the development of childhood asthma: a meta-analysis. BMJ Open. 2017;7:e013034. doi: 10.1136/bmjopen-2016-013034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stein R.T., Sherrill D., Morgan W.J., et al. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet. 1999;354:541–545. doi: 10.1016/S0140-6736(98)10321-5. [DOI] [PubMed] [Google Scholar]
- 27.Henderson J., Hilliard T.N., Sherriff A., Stalker D., Al Shammari N., Thomas H.M. Hospitalization for RSV bronchiolitis before 12 months of age and subsequent asthma, atopy and wheeze: a longitudinal birth cohort study. Pediatr Allergy Immunol. 2005;16:386–392. doi: 10.1111/j.1399-3038.2005.00298.x. [DOI] [PubMed] [Google Scholar]
- 28.Sigurs N., Bjarnason R., Sigurbergsson F., Kjellman B., Bjorksten B. Asthma and immunoglobulin E antibodies after respiratory syncytial virus bronchiolitis: a prospective cohort study with matched controls. Pediatrics. 1995;95:500–505. [PubMed] [Google Scholar]
- 29.Ruotsalainen M., Hyvarinen M.K., Piippo-Savolainen E., Korppi M. Adolescent asthma after rhinovirus and respiratory syncytial virus bronchiolitis. Pediatr Pulmonol. 2013;48:633–639. doi: 10.1002/ppul.22692. [DOI] [PubMed] [Google Scholar]
- 30.Wu P., Dupont W.D., Griffin M.R., et al. Evidence of a causal role of winter virus infection during infancy in early childhood asthma. Am J Respir Crit Care Med. 2008;178:1123–1129. doi: 10.1164/rccm.200804-579OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rubner F.J., Jackson D.J., Evans M.D., et al. Early life rhinovirus wheezing, allergic sensitization, and asthma risk at adolescence. J Allergy Clin Immunol. 2017;139:501–507. doi: 10.1016/j.jaci.2016.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aberle J.H., Aberle S.W., Dworzak M.N., et al. Reduced interferon-gamma expression in peripheral blood mononuclear cells of infants with severe respiratory syncytial virus disease. Am J Respir Crit Care Med. 1999;160:1263–1268. doi: 10.1164/ajrccm.160.4.9812025. [DOI] [PubMed] [Google Scholar]
- 33.Caballero M.T., Serra M.E., Acosta P.L., et al. TLR4 genotype and environmental LPS mediate RSV bronchiolitis through Th2 polarization. J Clin Invest. 2015;125:571–582. doi: 10.1172/JCI75183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumar A., Grayson M.H. The role of viruses in the development and exacerbation of atopic disease. Ann Allergy Asthma Immunol. 2009;103:181–186. doi: 10.1016/S1081-1206(10)60178-0. quiz 186-187, 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kristjansson S., Bjarnarson S.P., Wennergren G., et al. Respiratory syncytial virus and other respiratory viruses during the first 3 months of life promote a local TH2-like response. J Allergy Clin Immunol. 2005;116:805–811. doi: 10.1016/j.jaci.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 36.Edwards M.R., Bartlett N.W., Hussell T., Openshaw P., Johnston S.L. The microbiology of asthma. Nat Rev Microbiol. 2012;10:459–471. doi: 10.1038/nrmicro2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Edwards M.R., Regamey N., Vareille M., et al. Impaired innate interferon induction in severe therapy resistant atopic asthmatic children. Mucosal Immunol. 2013;6:797–806. doi: 10.1038/mi.2012.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnston N.W., Johnston S.L., Duncan J.M., et al. The September epidemic of asthma exacerbations in children: a search for etiology. J Allergy Clin Immunol. 2005;115:132–138. doi: 10.1016/j.jaci.2004.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnston S.L., Pattemore P.K., Sanderson G., et al. Community study of role of viral infections in exacerbations of asthma in 9-11 year old children. BMJ. 1995;310:1225–1229. doi: 10.1136/bmj.310.6989.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnston N.W., Johnston S.L., Norman G.R., Dai J., Sears M.R. The September epidemic of asthma hospitalization: school children as disease vectors. J Allergy Clin Immunol. 2006;117:557–562. doi: 10.1016/j.jaci.2005.11.034. [DOI] [PubMed] [Google Scholar]
- 41.Olenec J.P., Kim W.K., Lee W.M., et al. Weekly monitoring of children with asthma for infections and illness during common cold seasons. J Allergy Clin Immunol. 2010;125:1001–1006, e1001. doi: 10.1016/j.jaci.2010.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kloepfer K.M., Lee W.M., Pappas T.E., et al. Detection of pathogenic bacteria during rhinovirus infection is associated with increased respiratory symptoms and asthma exacerbations. J Allergy Clin Immunol. 2014;133:1301–1307, e1301-e1303. doi: 10.1016/j.jaci.2014.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arden K.E., Chang A.B., Lambert S.B., Nissen M.D., Sloots T.P., Mackay I.M. Newly identified respiratory viruses in children with asthma exacerbation not requiring admission to hospital. J Med Virol. 2010;82:1458–1461. doi: 10.1002/jmv.21819. [DOI] [PubMed] [Google Scholar]
- 44.Heymann P.W., Carper H.T., Murphy D.D., et al. Viral infections in relation to age, atopy, and season of admission among children hospitalized for wheezing. J Allergy Clin Immunol. 2004;114:239–247. doi: 10.1016/j.jaci.2004.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Soto-Quiros M., Avila L., Platts-Mills T.A., et al. High titers of IgE antibody to dust mite allergen and risk for wheezing among asthmatic children infected with rhinovirus. J Allergy Clin Immunol. 2012;129:1499–1505, e1495. doi: 10.1016/j.jaci.2012.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nakagome K., Bochkov Y.A., Ashraf S., et al. Effects of rhinovirus species on viral replication and cytokine production. J Allergy Clin Immunol. 2014;134:332–341. doi: 10.1016/j.jaci.2014.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mochizuki H., Kusuda S., Okada K., et al. Palivizumab prophylaxis in preterm infants and subsequent recurrent wheezing. six-year follow-up study. Am J Respir Crit Care Med. 2017;196:29–38. doi: 10.1164/rccm.201609-1812OC. [DOI] [PubMed] [Google Scholar]
- 48.Yoshihara S., Kusuda S., Mochizuki H., et al. Effect of palivizumab prophylaxis on subsequent recurrent wheezing in preterm infants. Pediatrics. 2013;132:811–818. doi: 10.1542/peds.2013-0982. [DOI] [PubMed] [Google Scholar]
- 49.Carroll K.N., Gebretsadik T., Escobar G.J., et al. Respiratory syncytial virus immunoprophylaxis in high-risk infants and development of childhood asthma. J Allergy Clin Immunol. 2017;139:66–71, e63. doi: 10.1016/j.jaci.2016.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O'Brien K.L., Chandran A., Weatherholtz R., et al. Efficacy of motavizumab for the prevention of respiratory syncytial virus disease in healthy Native American infants: a phase 3 randomised double-blind placebo-controlled trial. Lancet Infect Dis. 2015;15:1398–1408. doi: 10.1016/S1473-3099(15)00247-9. [DOI] [PubMed] [Google Scholar]
- 51.Lukkarinen M., Vuorinen T., Lehtinen P., Ruuskanen O., Jartti T. Sensitization at the first wheezing episode increases risk for long-term asthma therapy. Pediatr Allergy Immunol. 2015;26:687–691. doi: 10.1111/pai.12439. [DOI] [PubMed] [Google Scholar]
- 52.Koistinen A., Lukkarinen M., Turunen R., et al. Prednisolone for the first rhinovirus-induced wheezing and 4-year asthma risk: a randomized trial. Pediatr Allergy Immunol. 2017;28:557–563. doi: 10.1111/pai.12749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Busse W.W., Morgan W.J., Gergen P.J., et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N Engl J Med. 2011;364:1005–1015. doi: 10.1056/NEJMoa1009705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Teach S.J., Gill M.A., Togias A., et al. Preseasonal treatment with either omalizumab or an inhaled corticosteroid boost to prevent fall asthma exacerbations. J Allergy Clin Immunol. 2015;136:1476–1485. doi: 10.1016/j.jaci.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tam J.S., Jackson W.T., Hunter D., Proud D., Grayson M.H. Rhinovirus specific IgE can be detected in human sera. J Allergy Clin Immunol. 2013;132:1241–1243. doi: 10.1016/j.jaci.2013.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Welliver R.C., Sun M., Rinaldo D., Ogra P.L. Predictive value of respiratory syncytial virus-specific IgE responses for recurrent wheezing following bronchiolitis. J Pediatr. 1986;109:776–780. doi: 10.1016/s0022-3476(86)80692-8. [DOI] [PubMed] [Google Scholar]
- 57.Gill M.A., Liu A.H., Calatroni A., et al. Enhanced plasmacytoid dendritic cell antiviral responses after omalizumab. J Allergy Clin Immunol. 2018;141:1735–1743, e1739. doi: 10.1016/j.jaci.2017.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kantor D.B., McDonald M.C., Stenquist N., et al. Omalizumab is associated with reduced acute severity of rhinovirus-triggered asthma exacerbation. Am J Respir Crit Care Med. 2016;194:1552–1555. doi: 10.1164/rccm.201606-1145LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Djukanovic R., Harrison T., Johnston S.L., et al. The effect of inhaled IFN-beta on worsening of asthma symptoms caused by viral infections: a randomized trial. Am J Respir Crit Care Med. 2014;190:145–154. doi: 10.1164/rccm.201312-2235OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bufe A., Gehlhar K., Grage-Griebenow E., Ernst M. Atopic phenotype in children is associated with decreased virus-induced interferon-alpha release. Int Arch Allergy Immunol. 2002;127:82–88. doi: 10.1159/000048173. [DOI] [PubMed] [Google Scholar]
- 61.Wark P.A., Johnston S.L., Bucchieri F., et al. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med. 2005;201:937–947. doi: 10.1084/jem.20041901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Contoli M., Message S.D., Laza-Stanca V., et al. Role of deficient type III interferon-lambda production in asthma exacerbations. Nat Med. 2006;12:1023–1026. doi: 10.1038/nm1462. [DOI] [PubMed] [Google Scholar]
- 63.Baraldo S., Contoli M., Bazzan E., et al. Deficient antiviral immune responses in childhood: distinct roles of atopy and asthma. J Allergy Clin Immunol. 2012;130:1307–1314. doi: 10.1016/j.jaci.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 64.Uller L., Leino M., Bedke N., et al. Double-stranded RNA induces disproportionate expression of thymic stromal lymphopoietin versus interferon-beta in bronchial epithelial cells from donors with asthma. Thorax. 2010;65:626–632. doi: 10.1136/thx.2009.125930. [DOI] [PubMed] [Google Scholar]
- 65.Patel D.A., You Y., Huang G., et al. Interferon response and respiratory virus control are preserved in bronchial epithelial cells in asthma. J Allergy Clin Immunol. 2014;134:1402–1412, e1407. doi: 10.1016/j.jaci.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cakebread J.A., Xu Y., Grainge C., et al. Exogenous IFN-beta has antiviral and anti-inflammatory properties in primary bronchial epithelial cells from asthmatic subjects exposed to rhinovirus. J Allergy Clin Immunol. 2011;127:1148–1154, e1149. doi: 10.1016/j.jaci.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 67.Holtzman M.J. Asthma as a chronic disease of the innate and adaptive immune systems responding to viruses and allergens. J Clin Invest. 2012;122:2741–2748. doi: 10.1172/JCI60325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sigua J.A., Buelow B., Cheung D.S., et al. CD49d-expressing neutrophils differentiate atopic from nonatopic individuals. J Allergy Clin Immunol. 2014;133:901–904, e905. doi: 10.1016/j.jaci.2013.09.035. [DOI] [PubMed] [Google Scholar]
- 69.Cheung D.S., Sigua J.A., Simpson P.M., et al. Cysteinyl leukotriene receptor 1 expression identifies a subset of neutrophils during the antiviral response that contributes to postviral atopic airway disease. J Allergy Clin Immunol. 2018;142:1206–1217, e1205. doi: 10.1016/j.jaci.2017.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pitrez P.M., Brennan S., Sly P.D. Inflammatory profile in nasal secretions of infants hospitalized with acute lower airway tract infections. Respirology. 2005;10:365–370. doi: 10.1111/j.1440-1843.2005.00721.x. [DOI] [PubMed] [Google Scholar]
- 71.Cheung D.S., Grayson M.H. Role of viruses in the development of atopic disease in pediatric patients. Curr Allergy Asthma Rep. 2012;12:613–620. doi: 10.1007/s11882-012-0295-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Grayson M.H., Cheung D., Rohlfing M.M., et al. Induction of high-affinity IgE receptor on lung dendritic cells during viral infection leads to mucous cell metaplasia. J Exp Med. 2007;204:2759–2769. doi: 10.1084/jem.20070360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Khan S.H., Grayson M.H. Cross-linking IgE augments human conventional dendritic cell production of CC chemokine ligand 28. J Allergy Clin Immunol. 2010;125:265–267. doi: 10.1016/j.jaci.2009.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Subrata L.S., Bizzintino J., Mamessier E., et al. Interactions between innate antiviral and atopic immunoinflammatory pathways precipitate and sustain asthma exacerbations in children. J Immunol. 2009;183:2793–2800. doi: 10.4049/jimmunol.0900695. [DOI] [PubMed] [Google Scholar]
- 75.Gern J.E., Calhoun W., Swenson C., Shen G., Busse W.W. Rhinovirus infection preferentially increases lower airway responsiveness in allergic subjects. Am J Respir Crit Care Med. 1997;155:1872–1876. doi: 10.1164/ajrccm.155.6.9196088. [DOI] [PubMed] [Google Scholar]
- 76.Jakiela B., Brockman-Schneider R., Amineva S., Lee W.M., Gern J.E. Basal cells of differentiated bronchial epithelium are more susceptible to rhinovirus infection. Am J Respir Cell Mol Biol. 2008;38:517–523. doi: 10.1165/rcmb.2007-0050OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lachowicz-Scroggins M.E., Boushey H.A., Finkbeiner W.E., Widdicombe J.H. Interleukin-13-induced mucous metaplasia increases susceptibility of human airway epithelium to rhinovirus infection. Am J Respir Cell Mol Biol. 2010;43:652–661. doi: 10.1165/rcmb.2009-0244OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cheung D.S., Ehlenbach S.J., Kitchens T., Riley D.A., Grayson M.H. Development of atopy by severe paramyxoviral infection in a mouse model. Ann Allergy Asthma Immunol. 2010;105:437–443, e431. doi: 10.1016/j.anai.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Beale J., Jayaraman A., Jackson D.J., et al. Rhinovirus-induced IL-25 in asthma exacerbation drives type 2 immunity and allergic pulmonary inflammation. Sci Transl Med. 2014;6:256ra134. doi: 10.1126/scitranslmed.3009124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ravanetti L., Dijkhuis A., Dekker T., et al. IL-33 drives influenza-induced asthma exacerbations by halting innate and adaptive antiviral immunity. J Allergy Clin Immunol. 2019;143:1355–1370, e1316. doi: 10.1016/j.jaci.2018.08.051. [DOI] [PubMed] [Google Scholar]
- 81.Caliskan M., Bochkov Y.A., Kreiner-Moller E., et al. Rhinovirus wheezing illness and genetic risk of childhood-onset asthma. N Engl J Med. 2013;368:1398–1407. doi: 10.1056/NEJMoa1211592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bochkov Y.A., Watters K., Ashraf S., et al. Cadherin-related family member 3, a childhood asthma susceptibility gene product, mediates rhinovirus C binding and replication. Proc Natl Acad Sci U S A. 2015;112:5485–5490. doi: 10.1073/pnas.1421178112. [DOI] [PMC free article] [PubMed] [Google Scholar]