Abstract
Early detection and identification of SARS-CoV-infected patients and actions to prevent transmission are absolutely critical to prevent another SARS outbreak. Antibodies that specifically recognize the SARS-CoV spike and nucleocapsid proteins may provide a rapid screening method to allow accurate identification and isolation of patients with the virus early in their infection. For this reason, we raised peptide-induced polyclonal antibodies against SARS-CoV spike protein and polyclonal antibodies against SARS-CoV nucleocapsid protein using 6× His nucleocapsid recombinant protein. Western blot analysis and immunofluorescent staining showed that these antibodies specifically recognized SARS-CoV.
Keywords: SARS, Spike, Nucleocapsid
Coronaviruses are enveloped viruses with positive-stranded, capped, and polyadenylated RNA genomes ranging in size between 28 and 32 kb [1]. Two-thirds of the viral genome starting from the 5′ end encodes replicase proteins, Rep1a and Rep1b, for amplication of coronavirus RNA. Structural proteins, including spike, envelope, membrane, nucleocapsid, and several other proteins with unknown functions, are also expressed in the coronaviruses [1]. In March 2003, a new member of coronavirus, the severe acute respiratory syndrome coronavirus (SARS-CoV), was discovered in association with cases of SARS [2], [3], [4], [5]. Ultimately, the virus infected more than 8000 people and killed more than 700, mostly in Asia.
The sequence of the complete SARS-CoV genome was determined and found to be 29,727 nucleotides in length, with 11 open reading frames. The organization of the genome is similar to those of other coronaviruses. Phylogenetic analyses and sequence comparisons show that SARS-CoV is not closely related to any of the previously characterized coronaviruses, with only an approximate 25–30% identity with other members of the coronavirus family [2], [3], [4]. Therefore, it was postulated that SARS-CoV is an animal virus that was only recently transmitted to humans. Examination of domestic and wild mammals in Guangdong Province, southern China, suggested that the Himalayan palm civet and raccoon dog might have been in the route of interspecies transmission [6]. In addition, ferrets and domestic cats are susceptible to infection by SARS-CoV and they may efficiently transmit the virus to previously uninfected animals that are housed with them [7], indicating that the natural reservoir of SARS-CoV involves a range of animal species.
Human coronavirus HcoV-229E belongs to group I coronavirus that causes common colds [8]. Human aminopeptidase N (CD13, hAPN) has been demonstrated to be a receptor for this virus [9]. Studies on the interaction between HcoV-229E spike protein and hAPN show that the domain of the spike protein between amino acids 417 and 547 is essential for the binding of HcoV-229E to its hAPN receptor. Furthermore, soluble hAPN neutralized the infectivity of HcoV-229E virions at 37 °C [10], [11]. The SARS-CoV spike gene encodes a glycoprotein of approximate 150 kDa containing 1255 amino acids. More recently, angiotensin-converting enzyme 2 (ACE2) has been identified to be a functional receptor for the SARS coronavirus [12]. The spike protein may mediate membrane fusion and induce neutralizing antibodies in the host, raising the possibility that antibodies against the SARS-CoV spike protein may be good tools for early detection and neutralization of SARS-CoV infection.
The SARS-CoV nucleocapsid gene encodes a 50 kDa protein harboring a putative nuclear localization signal (KKDKKKK, a.a. 370–376). The biological function of coronavirus nucleocapsid protein is thought to participate in the replication and transcription of viral RNA and interfere with cell cycle processes of host cells [13], [14]. In addition, the nucleocapsid protein in many coronaviruses is highly immunogenic and abundantly expressed during infection [15], [16], again suggesting that antibodies that specifically recognize this protein might be useful in early diagnosis of the infection. In this study, we describe the generation of polyclonal anti-SARS-CoV spike and nucleocapsid antibodies.
Materials and methods
Molecular cloning of spike and nucleocapsid cDNA. A total of six specimens from three probable SARS-CoV patients, according to the WHO case definition, were collected by nasopharyngeal swabs. The viruses were immediately inactivated by immersion in Trizol reagent (Gibco, Rockville, MD). Viral RNA was extracted according to the manufacturer’s instructions. To obtain spike and nucleocapsid cDNAs, specific primers designed for the spike and nucleocapsid genes were retrieved from the SARS-CoV TOR2 strain (GenBank Accession No. NC_004718), and RT-PCR was carried out using a Qiagen one step RT-PCR kit (Hilden, Germany). PCR products were cloned into pGEM-T easy vector (Promega, Madison, WI) and positive clones were analyzed with ABI Prism 377.
Expression and purification of 6× His-tagged spike and nucleocapsid protein. For the expression of 6× His-tagged SARS-CoV spike and nucleocapsid recombinant protein, the open reading frames of spike and nucleocapsid cDNA were cloned into the pQE30 vector (Qiagen). We designed two sets of primers for spike cDNA, specific for a.a. 10–647 and a.a. 648–1255, and thereby the spike protein was expressed in these two fragments. The pQE constructs were transformed into Escherichia coli strain M15 (pREP4) and expression of His-tagged fusion protein was induced with 0.1 mM isopropyl-1-thio-d-galactopyranoside (IPTG) at 30 °C for 5 h. Bacterial pellets were sonicated and solubilized in lysis buffer (1.5% N-lauroylsarcosine, 1% Triton X-100, 150 mM NaCl, and 10 mM Tris, pH 8.0). The purification of His-tagged fusion protein was carried out according to the manufacturer’s instructions (Qiagen).
Antibodies. Anti-spike polyclonal antiserum was raised in New Zealand rabbits using six different spike specific peptides, SP1: N-RCTTFDDVQAPNYTQHTSSMR-C, a.a. 18–38, SP2: N-DVSEKSGNFKHLREFVFKNKD-C, a.a. 171–191, SP3: N-KYDENGTITDAVDCSQNPLA-C, a.a. 265–284 SP4: N-NYNYKYRYLRHGKLRPFERD-C, a.a. 435–454, SP5: N-DVSDFTDSVRDPKTSEILDI-C, a.a. 555–574, and SP6: N-DVDLGDISGINASVVNIQKEIDR-C, a.a. 1145–1167. Anti-nucleocapsid antiserum was produced using 6× His nucleocapsid recombinant protein. Antibodies were further purified with an Affi-10 affinity column (Bio-Rad, Hercules, CA). Monoclonal anti-fibrillarin antibody (38F3) was purchased from Abcam (Cambridge, UK).
Western blot analysis. Human 293T cells were transfected with pcDNA3.1-nucleocapsid for 36 h using Superfect transfection reagent (Qiagen). Cell extracts were prepared by gentle pipetting and was lysed in lysis buffer (1% Triton, 150 mM NaCl, and 20 mM Tris–HCl, pH 7.5). Western blot analysis was carried out using affinity-purified antibodies and an ECL system (Amersham–Pharmacia Biotech, Piscataway, NJ).
Immunofluorescence. Peptide-induced anti-spike and affinity-purified anti-nucleocapsid antibodies were applied to BIOCHIPs pre-coated with SARS-CoV infected Vero E6 cells (Euroimmun AG, Lübeck, Germany) and incubated at 37 °C for 1 h. After rinsing with PBS, BIOCHIPs were incubated with anti-rabbit IgG antibodies conjugated with rhodamine (Jackson Immuno Research, West Grove, PA) at 37 °C for 30 min. Subsequently, DNA was labeled with 4,6-diamidino-2-phenylindole (Sigma, St. Louis, MO) and coverslips were mounted using Sigma mounting media. Immunostained images were visualized and recorded using a Zeiss Axioplan2 imaging microscope (Carl Zeiss, Göttingen, Germany).
Results
Detection of SARS-CoV spike protein using peptide specific antibodies
Using computer analysis to predict hydrophobic regions of SARS spike protein (http://searchlauncher.bcm.tmc.edu/seq-search/struc-predict.html), we designed six specific peptides of SARS-CoV spike protein to raise polyclonal antibodies, SP1–SP6 (Fig. 1A ). To evaluate the specificity of these peptide-induced antibodies, Western blot analysis was conducted. Immunoblotting showed that peptide SP1- and SP4-induced polyclonal antibodies recognized the 6× His-tagged N-terminal fragment (a.a. 10–647) of SARS-CoV spike protein, but not the C-terminal fragment (a.a. 648–1255) (Figs. 1B and C). By contrast, peptide SP6-induced polyclonal antibody recognized the 6× His-tagged C-terminal spike protein, but not the N-terminal end (Fig. 1D).
Fig. 1.
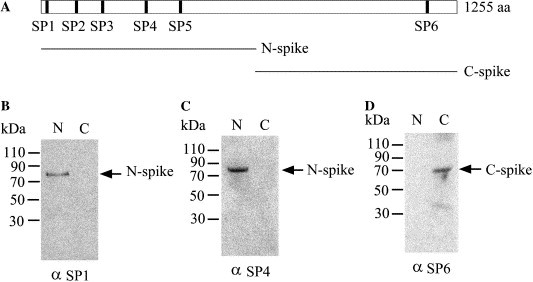
(A) Schematic position of specific peptides of SARS-CoV spike protein and N-spike (a.a. 10–647) and C-spike (a.a. 648–1255) of SARS-CoV spike protein. 6× His N-spike and C-spike proteins were expressed in E. coli JM109 and affinity-purified by Ni–NTA–agarose. Western blot analysis was performed using peptide-induced polyclonal antibodies against SP1 (B), SP4 (C), and SP6 (D).
Structural predictions suggest that SARS-CoV spike protein is a highly glycosylated protein. To investigate whether SP1-, SP4-, and SP6-induced antibodies recognize glycosylated spike protein during SARS-CoV infection, immunofluorescent assay was carried out. Peptide-induced SP1 and SP4 polyclonal antibodies preferentially detected the same signals as did positive control sera obtained from patients who had recovered from probable SARS infection (Fig. 2 ). In contrast, SP6-induced antiserum did not yield a positive IFA result (data not shown), suggesting that SP6-induced antiserum is useful in Western blot analysis, but not in the immunofluorescence test. To exclude the possibility of cross-reaction from peptide-induced polyclonal antibodies, SP1 and SP4 pre-immunized rabbit antisera were applied to BIOCHIPs and these sera did not recognize SARS-infected cells. Furthermore, SP1 and SP4 polyclonal antibodies did not detect any SARS-CoV spike protein after peptide competition (data not shown), demonstrating that SP1- and SP4-induced polyclonal antibodies specifically recognize SARS-CoV spike protein.
Fig. 2.
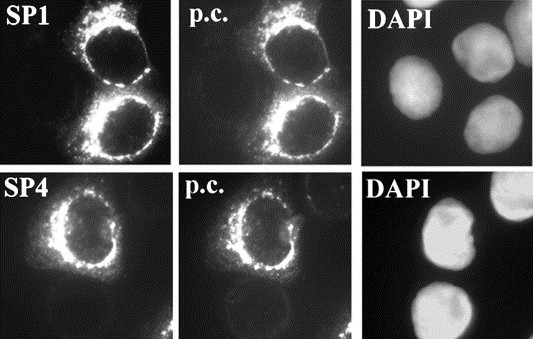
Antibody detection of SARS-CoV spike protein. BIOCHIPS coated with SARS-CoV infected Vero E6 cells were immunostained with SP1 and SP4 peptide-induced anti-spike polyclonal antibodies. The p.c. indicates positive control serum obtained from patients who had recovered from probable SARS infection. The DAPI staining represents the position of DNA.
Expression of SARS-CoV nucleocapsid protein in mammalian cells
Affinity purification of 6× His-tagged recombinant nucleocapsid protein was performed (Fig. 3A ). Anti-nucleocapsid polyclonal antibody was raised using 6× His-tagged nucleocapsid protein and the specificity of affinity-purified antibody was determined by Western blot analysis. No obvious signal was observed in mock-transfected 293T cell extracts (Fig. 3B, lane 1). By contrast, one major band with an approximate molecular mass of 50 kDa was detected in cell extracts of 293T cells transfected with pcDNA3.1-nucleocapsid construct (Fig. 3B, lane 2).
Fig. 3.
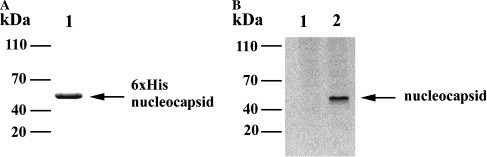
Expression of SARS-CoV nucleocapsid protein. (A) SARS-CoV nucleocapsid protein was expressed in E. coli JM109 and purified using Ni–NTA–agarose. (B) 293T cells were mock-transfected with pcDNA3.1 empty vector (lane 1) or transfected with cDNA3.1-nucleocapsid construct (lane 2). Thirty hours after transfection, cell extracts were prepared and Western blot analysis was performed using affinity-purified anti-nucleocapsid polyclonal antibody.
Subcellular localization of SARS-CoV nucleocapsid protein
To investigate the subcellular localization of SARS-CoV nucleocapsid protein, indirect immunofluorescence staining using BIOCHIPs pre-coated with SARS-CoV infected Vero E6 cells was carried out. Interestingly, we did not observe nuclear signals located in the nucleolus, which is a common feature of other coronavirus nucleocapsid members. By contrast, positive signals were largely distributed in the cytoplasm (Fig. 4A ), suggesting that SARS-CoV nucleocapsid protein does not behave like that in other coronaviruses. In control experiments, cells not infected with SARS-CoV did not show any staining signal (data not shown), demonstrating that polyclonal antibodies specifically recognize viral particles containing nucleocapsid protein.
Fig. 4.

The subcellular localization of nucleocapsid protein. (A) BIOCHIPS coated with SARS-CoV infected Vero E6 cells were immunostained with affinity-purified anti-nucleocapsid antibodies. The DAPI staining represents the position of DNA (A′). Human 293T (B) and green monkey Vero cells (C) were transfected with pcDNA3.1-nucleocapsid expression construct. Thirty hours post-transfection, cells were fixed in cold methanol and immunostained.
Moreover, to investigate the localization of nucleocapsid protein in mammalian cells, human 293T (Fig. 4C) and green monkey Vero (Fig. 4D) cells were transfected with pcDNA3.1-nucleocapsid, and immunofluorescent staining was performed. Positive anti-nucleocapsid signals were mostly visualized in the cytoplasm, very weakly in the nucleus and nucleolus, indicating that SARS-CoV nucleocapsid protein does not predominantly localize in the nucleolus. In addition, 293T cells transfected by HA-, FLAG-, and GFP-tagged nucleocapsid protein also showed the subcellular localization in the cytoplasm (data not shown), further supporting our observations.
Discussion
SARS-CoV spike protein is essential for viral infection and nucleocapsid protein is important for viral RNA transcription. Both proteins can serve as targets for early diagnosis of infection. In this study, we found two peptide-induced antibodies against spike protein and polyclonal antibodies against nucleocapsid protein could specifically recognize SARS-CoV infected cells by immunostaining. These data may provide a basis for diagnostic purpose and reveal a potential viral packaging pathway.
The spike protein of bovine coronavirus (BCV) encodes a 151 kDa protein. With its 19 potential glycosylation sites, the S protein has a size of 190 kDa when fully glycosylated [17], [18]. BCV spike protein consists of two subunits, N-terminal S1 (110 kDa) and C-terminal S2 (100 kDa). S1, which forms the bulb portion of the spike, contains domains responsible for viral attachment to the receptor of host cells, while S2, anchoring the spike into the envelope, possesses regions essential for fusion between the virus and cell membrane during infection [19]. Structural analysis predicts that peptides (ISGINASVVNIQKEIDRLNEVAKNLNESLIDLQEL) derived from the SARS-CoV S2 protein might inhibit virus-induced membrane fusion, thereby blocking SARS-CoV infection [20]. Coincidentally, our SP6 (DVDLGDISGINASVVNIQKEIDR, a.a. 1145–1167) partially covers the above peptide. Peptide SP6-induced polyclonal antibody was adequate for Western blot analysis but did not render a good result for immunofluorescence assay. Instead, polyclonal antibodies against peptide SP1 and SP4 (a.a. 18–38 and a.a. 435–454, respectively) detected the SARS-CoV fixed on the biochip. Interestingly, the region between a.a. 417 and 547 of the HCoV-229E spike protein was found to be essential for binding to the hAPN receptor for HcoV-229E [10]. Whether SP4 peptide binds to the unknown receptor for SARS-CoV spike protein and may thus neutralize the virus requires further investigation.
In 20 patients who recovered from SARS-CoV infection, IgG antibodies were detectable after week 3 and remained at high levels for at least three months. IgM titers peaked during the acute or early convalescent phase and then declined, disappearing by the end of week 12 [21]. Lin et al. successfully raised one peptide-induced polyclonal antibody against SARS-CoV nucleocapsid protein. IgG antibody against this N1 peptide was detectable in the sera of patients with SARS [22]. Our preliminary results showed that those high levels of IgG were largely antibodies against nucleocapsid protein (data not shown), suggesting an intriguing role for the nucleocapsid in the primary humoral immune response against SARS-CoV infection.
Three groups of coronaviruses have been identified to date, although sequence conservation of nucleocapsid proteins within the genus is low. For instance, the nucleocapsid proteins of coronaviruses IBV (group III) and porcine transmissible gastroenteritis virus (TGEV, group I) have only 29% identity with that of bovine coronavirus (BcoV, group II). Within the group II coronaviruses, the N proteins of MHV and BcoV have 70% identity [23]. The subcellular localizations of infectious avian bronchitis virus, porcine transmissible gastroenteritis virus, and mouse hepatitis virus (MHV) nucleocapsid protein in both cytoplasm and nucleolus have been described [24], [25]. The nucleolus is a structure within the nucleus that is only present during interphase. It is the site where rRNA is synthesized and processed. Biogenesis of pre-ribosomal subunits and polymerase III transcripts also takes place in the nucleolus [26]. One possible explanation for the entry of nucleocapsid protein into the nucleolus is that coronaviruses need to control host ribosomes to produce thousands of viral RNA copies. Over-expression of coronavirus nucleocapsid protein appeared to induce a cell cycle arrest, most likely in the G2/M phase [24], indicating that nucleocapsid protein may interact with unknown cytoplasmic and nucleolar proteins and delay the cell cycle progression. Interestingly, however, our study shows that SARS-CoV nucleocapsid protein distributed predominantly in the cytoplasm of SARS-CoV permissive Vero E6 cells, and nucleocapsid-transfected 293T and Vero cells, but not in the nucleolus. This suggests that SARS-CoV nucleocapsid protein may interact with cytoplasmic proteins rather than nucleolar proteins to localize at certain organelles in the cytoplasm. It is possible, then, that the biological function of SARS-CoV nucleocapsid protein is totally different from those in other coronaviruses. This unusual localization and behavior of SARS-CoV nucleocapsid protein may have contributed in some way to the severity of the recent outbreak of SARS.
Acknowledgements
This work was partly supported by grants from National Science Council to Y.T.L. (NSC 92-2751-B-195-001-Y), M.S.C. (NSC 92-2311-B-195-001, NSC 92-2751-B-195-002-Y), and Y.C.Y. (NSC 92-2134-B-195-029). We are grateful to Dr. Mary Jeanne Buttrey for critical comments on this article.
References
- 1.Lai M.M.C., Cavanagh D. The molecular biology of coronaviruses. Adv. Virus Res. 1997;48:1–100. doi: 10.1016/S0065-3527(08)60286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ksiazek A novel coronavirus associated with severe acute respiratory syndrome. New Engl. J. Med. 2003;348:1947–1959. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- 3.Rota Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science. 2003;300:1394–1399. doi: 10.1126/science.1085952. [DOI] [PubMed] [Google Scholar]
- 4.Marra The genome sequence of the SARS-associated coronavirus. Science. 2003;300:1399–1404. doi: 10.1126/science.1085953. [DOI] [PubMed] [Google Scholar]
- 5.Kuiken Newly discovered coronavirus as the primary cause of severe acute respiratory syndrome. Lancet. 2003;362:263–270. doi: 10.1016/S0140-6736(03)13967-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guan Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science. 2003;302:276–278. doi: 10.1126/science.1087139. [DOI] [PubMed] [Google Scholar]
- 7.Martina B.E.E., Haagmans B.L., Kuiken T., Fouchier R.A.M., Rimmelzwaan G.F., van Amerongen G., Peiris J.S.M., Lim W., Osterhaus A.D.M.E. SARS virus infection of cats and ferrets. Nature. 2003;425:915. doi: 10.1038/425915a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holmes K.V. Coronaviruses. In: Knipe D.M., Howley P.M., editors. fourth ed. vol. 1. Lippincott Williams & Wilkins; Philadelphia: 2001. pp. 1187–1203. (Fields Virology). [Google Scholar]
- 9.Yeager C.L., Ashmun R.A., Williams R.K., Cardellichio C.B., Shapiro L.H., Look A.T., Holmes K.V. Human aminopeptidase N is a receptor for human coronavirus 229E. Nature. 1992;357:420–422. doi: 10.1038/357420a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonavia A., Zelus B.D., Wentworth D.E., Talbot P.J., Holmes K. Identification of a receptor-binding domain of the spike glycoprotein of human coronavirus HcoV-229E. J. Viol. 2003;77:2530–2538. doi: 10.1128/JVI.77.4.2530-2538.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Breslin J.J., Mork I., Smith M.K., Vogel L.K., Hemmila E.M., Bonavia A., Talbot P.J., Sjostrom H., Noren O., Holmes K.V. Human coronavirus 229E: receptor binding domain and neutralization by soluble receptor at 37 °C. J. Virol. 2003;77:4435–4438. doi: 10.1128/JVI.77.7.4435-4438.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greenough T.C., Choe H., Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parker M.M., Masters P.S. Sequence comparison of the N genes of 5 strains of the coronavirus mouse hepatitis virus suggests a 3 domain-structure for the nucleocapsid protein. Virology. 1990;179:463–468. doi: 10.1016/0042-6822(90)90316-J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuo L., Masters P.S. Genetic evidence for a structural interaction between the carboxy termini of the membrane and nucleocapsid proteins of mouse hepatitis virus. J. Virol. 2002;76:4987–4999. doi: 10.1128/JVI.76.10.4987-4999.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Narayanan K., Chen C.-J., Maeda J., Makino S. Nucleocapsid-independent specific viral RNA packaging via viral envelope protein and viral RNA signal. J. Virol. 2003;77:2922–2927. doi: 10.1128/JVI.77.5.2922-2927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu DNA mediated immunization with encoding the nucleoprotein gene of porcine transmissible gastroenteritis virus. Virus Res. 2001;80:75–82. doi: 10.1016/s0168-1702(01)00333-1. [DOI] [PubMed] [Google Scholar]
- 17.St. Cyroats K., Storz J. Bovine coronavirus induced cytopathic expression and plaque formation: host cell and virus strain determine trypsin dependence. J. Vet. Med. 1988;35:48–56. doi: 10.1111/j.1439-0450.1988.tb00465.x. [DOI] [PubMed] [Google Scholar]
- 18.Cavanagh D. The coronavirus surface glycoprotein. In: Siddell S.G., editor. Coronaviridae. Plenum Press; New York: 1995. pp. 73–113. [Google Scholar]
- 19.Popova R., Zhang X. The spike but not the hemagglutinin/esterase protein of bovine coronavirus is necessary and sufficient for viral infection. Virology. 2002;294:222–236. doi: 10.1006/viro.2001.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kliger Y., Levanon E.Y. Cloaked similarity between HIV-1 and SARS-CoV suggests an anti-SARS strategy. BMC Microbiol. 2003;3:20. doi: 10.1186/1471-2180-3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li G., Chen X., Xu A. Profile of specific antibodies to the SARS-associated coronavirus. N. Engl. J. Med. 2003;349:508–509. doi: 10.1056/NEJM200307313490520. [DOI] [PubMed] [Google Scholar]
- 22.Lin Identification of an epitope of SARS-coronavirus nucleocapsid protein. Cell Res. 2003;13:141–145. doi: 10.1038/sj.cr.7290158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lapps W., Hogue B.G., Brian D.A. Sequence analysis of the bovine coronavirus nucleocapsid and matrix protein gene. Virology. 1987;157:47–57. doi: 10.1016/0042-6822(87)90312-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hiscox J.A., Wurm T., Wilson L., Britton P., Cavanagh D., Brooks G. The coronavirus infectious bronchitis virus nucleoprotein localizes to the nucleolus. J. Virol. 2001;75:506–512. doi: 10.1128/JVI.75.1.506-512.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wurm T., Chen H., Hodgson T., Britton P., Brooks G., Hiscox J.A. Localization to the nucleolus is a common feature of coronavirus nucleoproteins, and the protein may disrupt host cell division. J. Virol. 2001;75:9345–9356. doi: 10.1128/JVI.75.19.9345-9356.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shaw P.J., Jordan E.G. The nucleolus. Annu. Rev. Cell Dev. Biol. 1995;11:93–121. doi: 10.1146/annurev.cb.11.110195.000521. [DOI] [PubMed] [Google Scholar]


