TO THE EDITOR
The skin barrier defect underlying autosomal recessive congenital ichthyoses leads to excessive transepidermal water loss immediately after birth. Affected infants are often born as collodion babies reflecting a physical compensation for the defective permeability barrier. In the available knockout mouse models, however, extrauterine survival is limited to a few hours. These animals, therefore, do not allow evaluation of any postnatal therapy.
Mutations in 10 different genes have been reported to cause autosomal recessive congenital ichthyoses (Hotz et al., 2018). Seven of them encode proteins involved in the biosynthesis of omega-hydroxyceramides (ω-OH-Cer) and, thus, in the formation of the corneocyte lipid envelope. TGM1, the most frequently affected gene, codes for transglutaminase-1, an enzyme that cross-links cornified envelope (CE) proteins and might play a role in corneocyte lipid envelope formation (Crumrine et al., 2019). There is no cure for autosomal recessive congenital ichthyoses yet. Symptomatic treatment is based on emollients, complemented by keratolytic agents and, if needed, systemic retinoid therapy. To elucidate pathogenic processes, animal models that faithfully recapitulate the cardinal features of the disease are required. We developed traditional knockout mouse models for Alox12b (Epp et al., 2007) and Aloxe3 (Krieg et al., 2013) encoding 12R-LOX and eLOX-3, respectively. Constitutive inactivation of either gene resulted in a rapidly fatal water loss, confirming that the successive oxygenation of the linoleate moiety of omega-hydroxyacyl-sphingosines by 12R-LOX and eLOX-3 is a crucial step in the biosynthesis of ω-OH-Cer. Homozygous knockout mice did not develop a typical ichthyosiform phenotype because they died too early.
Here we report on an epidermis-specific conditional knockout of Alox12b that was established using the Cre-Lox system. Mice homozygous for a LoxP-flanked Alox12b allele (Alox12bfl/fl) were crossed with mice that express the tamoxifen-dependent Cre-ERT2 recombinase driven by the cytokeratin K14 promoter. The resultant Alox12bfl/fl/K14-CreJ offspring developed no skin phenotype in the absence of tamoxifen. It was backcrossed with hairless SKH1 mice to facilitate manipulation of the skin, topical treatment, and observation of the cutaneous response. All animal experiments were conducted in accordance with the legal requirements and were approved by the local government authorities.
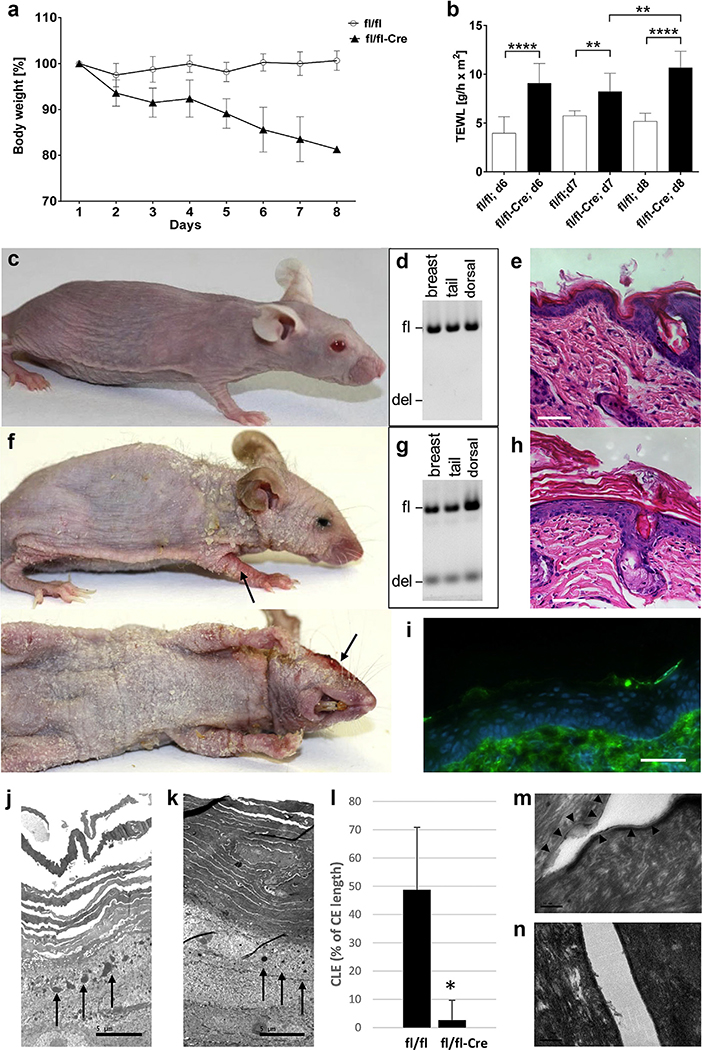
Alox12b inactivation in keratinocytes of 8-week old Alox12bfl/fl/K14-CreJ mice was induced by repeated intraperitoneal injection of tamoxifen. Alox12bfl/fl mice treated in the same manner served as controls. Tamoxifen-induced deletion of exon 8 of Alox12b occurred in all K14-expressing stratified epithelia, such as epidermis, tongue, esophagus, and forestomach, whereas in other tissues Alox12b alleles remained unaffected (Supplementary Figure S1). Upon tamoxifen treatment, Alox12bfl/fl/K14-CreJ mice, but not Alox12bfl/fl control mice, showed incessant weight loss (Figure 1a). Their transepidermal water loss doubled (Figure 1b). In contrast to Alox12bfl/fl control mice (Figure 1c–e), Alox12bfl/fl/K14-CreJ mice developed inflamed skin areas and excessive generalized scaling, predominantly on neck, paws, and ears (Figure 1f and Supplementary Figure S2). Histological examination revealed severe hyperkeratosis (Figure 1h), and frequent scratching was observed. Six days after initiation of tamoxifen treatment, a cachectic appearance with muscular and adipose tissue atrophy, kyphosis, and failing locomotor functions became evident, so that the animals had to be killed for animal welfare reasons by day 8 at the latest. Immunofluorescence staining indicated the absence of 12R-LOX protein (Figure 1i). Electron microscopy showed an increased number of cell layers in the stratum corneum (Figure 1j and k), reduced size and density of keratohyalin granules, and a decomposition of the corneocyte lipid envelope (Figure 1l–n) in tamoxifen-treated Alox12bfl/fl/K14-CreJ mice compared with Alox12bfl/fl control mice.
Figure 1. Effects of tamoxifen-induced Alox12b knockout in murine epidermis.
(a) Weight loss over time (percentage of initial body weight) of control mice (fl/fl; n = 9) and Alox12bfl/fl/K14-CreJ mice (fl/fl-Cre; n = 10). (b) TEWL of control and Alox12bfl/fl/K14-CreJ mice 6 days (n = 9), 7 days (n = 6), and 8 days (n = 3) after the first tamoxifen treatment. Photographs of representative (c) Alox12bfl/fl and (f) Alox12bfl/fl/K14-CreJ mice at day 8 after initiation of tamoxifen treatment (inflamed skin areas marked with arrows). (d, g) PCR-based genotype analyses of skin samples, and representative sections from (e) control and (h) Alox12bfl/fl/K14-CreJ skin stained with hematoxylin and eosin. Scale bar = 50 μm. (i) Lack of 12R-LOX immunofluorescence staining in Alox12bfl/fl/K14-CreJ skin. Scale bar = 50 μm. Electron microscopy of (j) control and (k) Alox12bfl/fl/K14-CreJ epidermis indicated hypogranulosis (arrows). Scale bar = 5 μm. (l) Quantification of the CLE (± SEM; *P = 3.6 × 10−6). Representative electron micrographs of (m) control and (n) Alox12bfl/fl/K14-CreJ epidermis; CLE indicated by arrowheads. Scale bar = 100 nm. CLE, corneocyte lipid envelope; fl/fl, control mice; fl/fl-Cre, Alox12bfl/fl/K14-CreJ mice; SEM, standard error of the mean; TEWL, transepidermal water loss; 12R-LOX, arachidonate 12R-lipoxygenase.
The corneocyte lipid envelope, a monolayer of ω-OH-Cer covalently attached to the outer surface of the CE, is an essential component of the epidermal barrier. Its formation depends on 12R-LOX activity, as the enzyme catalyzes the oxidation of free precursor esterified omega-hydroxyacyl sphingosines, resulting in ω-OH-Cer bound to CE proteins. We determined the content of free and bound ceramides in the epidermis of Alox12bfl/fl/K14-CreJ mice after tamoxifen treatment by liquid chromatography tandem mass spectrometry. This revealed an increase in free extractable ceramide species with long-chain fatty acids (C30–C36) in Alox12bfl/fl/K14-CreJ mice compared with controls (Supplementary Figure S3). The accumulation of acylceramides after 12R-LOX inactivation indicates that functional 12R-LOX is crucial for adequate processing of esterified omega-hydroxyacyl sphingosines in the skin. To clarify whether the ichthyosislike phenotype is linked to a lack of protein-bound ω-OH-Cer, we quantified omega-hydroxyacyl sphingosines in epidermal extracts. In contrast to the constitutive knockout (Rosenberger et al., 2014), conditional inactivation of 12R-LOX did not lead to a detectable reduction of omega-hydroxyacyl sphingosine levels in mouse epidermis harvested 6–8 days after the first tamoxifen injection (Supplementary Figure S3). At this time-point, the epidermal barrier is already disrupted because 12R-LOX-driven processing of esterified omega-hydroxyacyl sphingosines and production of protein-bound ω-OH-Cer are blocked. However, pre-existing omega-hydroxyacyl sphingosine molecules are not yet degraded and are still attached to the CE proteins. This may offer a time window for the evaluation of topical therapies, such as creams containing ω-OH-Cer.
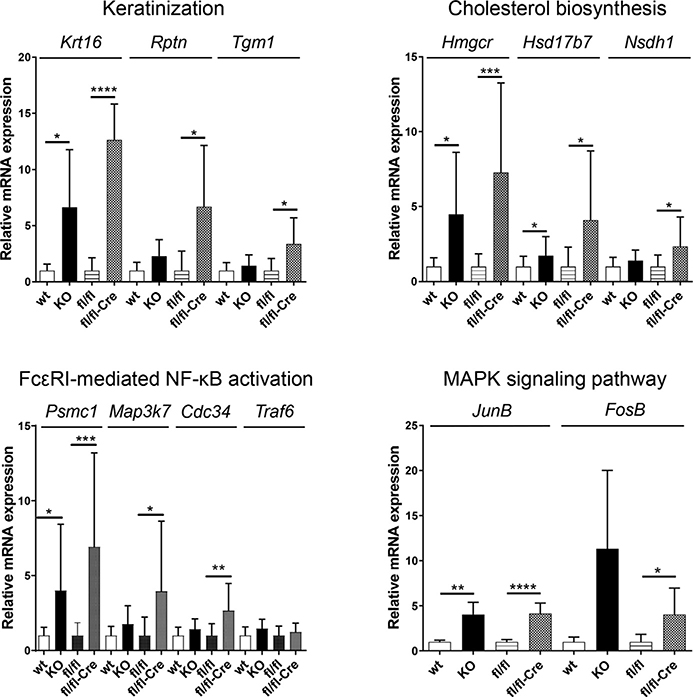
We also compared gene expression in five epidermal samples of Alox12bfl/fl/K14-CreJ mice 6 days after initiation of tamoxifen treatment with that of control animals, as well as epidermal gene expression in constitutive Alox12b knockout and newborn wild-type mice. Among the 20,604 genes analyzed, 3,677 showed significantly upregulated transcription. The 100 most abundantly upregulated gene products included 12 associated with keratinization, epidermal growth, and formation of the CE, such as keratins and small proline-rich proteins. Reactome database analyses provided further insight into the molecular effects of Alox12b inactivation. Among eight significantly upregulated pathways in Alox12bfl/fl/K14-CreJ mouse epidermis (false discovery rate <5%) were “keratinization,” “cholesterol biosynthesis,” and “Fc epsilon receptor-mediated NF-kB activation” (Supplementary Figures S4–S6). Increased keratinization and cholesterol biosynthesis is most likely associated with compensatory skin barrier repair, whereas Fc epsilon receptor-mediated NF-kB activation indicates a proinflammatory response. Representative genes involved in keratinization (Krt16, Rptn, Tgm1), cholesterol biosynthesis (Hmgcr1, Hsd17b7, Nsdh1), or Fc epsilon receptor-mediated NF-kB activation (Psmc1, Map3k7, Cdc34) showed higher expression than in Alox12bfl/fl control mice (Figure 2). We also analyzed transcripts of the same genes in the epidermis of constitutive Alox12b knockouts in comparison with wild-type pups and observed increased expression in newborn 12R-LOX-deficient mice. In addition, quantitative real-time reverse transcriptase–PCR data showed significant upregulation of JunB and FosB mRNA in the epidermis of both constitutive and conditional Alox12b knockouts, indicating activation of the mitogen-activated protein kinase signaling pathway. Changes in gene expression profiles, however, might be secondary effects of the development of ichthyosislike skin lesions, but not the primary effects of Alox12b inactivation.
Figure 2. Increased expression of individual genes associated with keratinization, cholesterol biosynthesis, FcεRI-mediated NF-kB activation, and the MAPK pathway in the epidermis of neonatal Alox12b knockout mice and in adult mice upon tamoxifen-induced Alox12b inactivation.
Data were obtained by QRT-PCR from independent epidermis samples (wild-type, n = 5; Alox12b-KO, n = 5; fl/fl control, n = 8; fl/fl-Cre, n = 9). Means ± SD of fold inductions relative to the expression in wild-type epidermis and fl/fl control epidermis, respectively, are shown. *P < 0.05, **P < 0.001, ***P < 0.0005, ****P < 0.0001. FcεRI, Fc epsilon receptor; fl/fl, control mice; fl/fl-Cre, Alox12bfl/fl/K14-CreJ mice; KO, knockout; MAPK, mitogen-activated protein kinase; QRT-PCR, quantitative real-time reverse transcriptase–PCR; SD, standard deviation.
In summary, mice with conditional Alox12b knockout are a viable rodent model resembling human autosomal recessive congenital ichthyoses and could be a useful tool for the development of pathogenesis-directed therapeutic strategies, such as the topical application of missing lipids.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants from the German Research Foundation (KR 905/7-2 and SCHN 569/4-2). We are grateful to Julia Panzer, Elisabeth Koppmann, and Philipp Stöhr for excellent technical assistance, and to Bodo Dobner and colleagues from Martin-Luther University Halle-Wittenberg for providing the Cer [EOS]-D3-br-D3 internal standard. We acknowledge the microarray unit of the DKFZ Genomics and Proteomics Core Facility for providing the Illumina Whole-Genome Expression Beadchips and related services.
Abbreviations
- CE
cornified envelope
- ω-OH-Cer
omega-hydroxyceramides
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
SUPPLEMENTARY MATERIAL
Supplementary material is linked to the online version of the paper at www.jidonline.org, and at https://doi.org/10.1016/j.jid.2019.06.134.
Data availability statement
All datasets related to this article will be made available free of charge to researchers upon reasonable request. The two gene array datasets can be found at [https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE127434 and https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE127435], hosted at NCBI GEO.
REFERENCES
- Crumrine D, Khnykin D, Krieg P, Man MQ, Celli A, Mauro TM, et al. Mutations in recessive congenital ichthyoses illuminate the origin and functions of the corneocyte lipid envelope. J Invest Dermatol 2019;139:760–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epp N, Fürstenberger G, Müller K, de Juanes S, Leitges M, Hausser I, et al. 12R-lipoxygenase deficiency disrupts epidermal barrier function. J Cell Biol 2007;177:173–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotz A, Bourrat E, Küsel J, Oji V, Alter S, Hake L, et al. Mutation update for CYP4F22 variants associated with autosomal recessive congenital ichthyosis. Hum Mutat 2018;39:1305–13. [DOI] [PubMed] [Google Scholar]
- Krieg P, Rosenberger S, de Juanes S, Latzko S, Hou J, Dick A, et al. Aloxe3 knockout mice reveal a function of epidermal lipoxygenase-3 as hepoxilin synthase and its pivotal role in barrier formation. J Invest Dermatol 2013;133:172–80. [DOI] [PubMed] [Google Scholar]
- Rosenberger S, Dick A, Latzko S, Hausser I, Stark HJ, Rauh M, et al. A mouse organotypic tissue culture model for autosomal recessive congenital ichthyosis. Br J Dermatol 2014;171:1347–57. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.