Abstract
Practical relevance Blood transfusions are a potentially life-saving procedure that are within the reach of most small animal practitioners. Only minimal equipment is required.
Patient group Any cat with clinical signs attributable to a reduced red blood cell mass that is affecting oxygen transport (as a result of reduced packed cell volume or acute blood loss) is a potential candidate for a transfusion.
Clinical challenges Although the principles of transfusion medicine are not complicated, there can be fatal consequences if certain steps are omitted.
Diagnostics Blood typing kits and blood filters are readily available from veterinary wholesalers, laboratories and blood banking services.
Evidence base Over the past three decades, a substantial body of clinical research and reports has built up covering feline blood types and transfusion medicine. This article draws on that research to provide clinical guidance aimed at all veterinarians in feline or small animal practice who either currently practise transfusion medicine or plan to do so.
Anaemia presents a challenge in terms of stabilising and supporting the patient. Appropriate administration of transfusion products allows the clinician more time to investigate and manage the underlying cause.
Introduction to feline transfusion medicine
Transfusions are uncommonly performed in general practice. Yet, with adequate planning, and due care and attention, they are readily achievable and, for many anaemic cats, stabilisation with blood or blood products is an essential step prior to definitive investigation.
Currently, feline blood is a precious resource. Given that the availability of feline blood products is limited, this article focuses on the transfusion of whole blood, collected acutely from a normal cat or the practice's blood donors. In time, the increasing availability of veterinary blood banks throughout the world may simplify feline transfusion medicine.
Indications for a transfusion
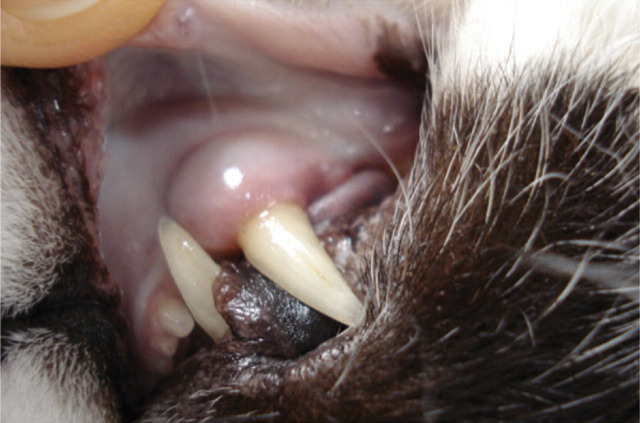
The main indication for transfusion of whole blood in cats is a decreased red cell mass. 1–4 Other, occasional indications include severe methaemoglobinaemia (as seen with paracetamol toxicity, Fig 1). Common to all patients requiring transfusion of whole blood is a lack of effective red blood cell mass, which results in reduced oxygen-carrying capacity and, in turn, tissue hypoxia. Those patients suffering an acute reduction in blood cell mass (eg, due to haemorrhage or rapid immune-mediated destruction) are often more severely affected than those with chronic non-regenerative anaemia, and usually become symptomatic at a higher packed cell volume (PCV). 2,5 Patients with hypovolaemia will benefit from aggressive initial stabilisation, aimed at maintaining intravascular volume and tissue oxygen delivery, prior to transfusion.
FIG 1.
Mucous membranes of a cat with methaemoglobinaemia
Cats have marked adaptive capabilities in the face of chronic anaemia. It is not uncommon for a cat to show only pallor despite an extremely severe anaemia (PCV <10%).
Common causes of anaemia in cats requiring transfusion are haemorrhage (as a result of peri- or postoperative bleeding, trauma, gastrointestinal bleeding, abdominal neoplasia, primary immune-mediated thrombocytopenia and coagulopathies), 6–8 primary immune-mediated haemolytic anaemia, 9 and ineffective, or absent, erythropoeisis. 2 Anaemia is also seen in young cats with a heavy flea burden, hepatic necrosis or neonatal isoerythrolysis.
In most situations, the anaemia is severe prior to transfusion, with pre-transfusion PCVs typically being 12–14%. 1–3 Multiple transfusions (three or more per hospitalisation period) are less commonly performed in cats than in dogs. This is partly due to the difficulty of sourcing large quantities of type-specific blood, but partly also because, in those cats requiring multiple transfusions, bone marrow failure is the most common cause of anaemia. 4
Anaemia is also reported in a number of infectious diseases — in particular, feline immuno-deficiency virus (FIV) and feline leukaemia virus (FeLV) infections, and feline infectious peritonitis. 10–12 However, transfusion is rarely performed in these patients. Haemoplasmas (Mycoplasma haemofelis, ‘Candidatus Mycoplasma haemominutum’ and ‘Candidatus Mycoplasma turicensis') have additionally been associated with anaemia caused by immune-mediated destruction of red blood cells. This may occur in the acute phase of the disease or during relapse of remission once in a carrier state. 13–15 Generally, ‘Candidatus M haemominutum’ and ‘Candidatus M turicensis’ are thought to be poorly pathogenic. 15,16 Due to the nature of this disease, anaemia is rarely reported in surveyed positive cats. 17,18 Moreover, haemoplasma species and retroviruses were uncommonly detected in anaemic cats in the UK. 19 There may be regional variation in this respect, however, as haemoplasmosis-associated anaemia is reported as one of the most frequent indications for transfusion in South Africa. 20 Other, rarely reported infectious organisms that have been associated with anaemia include Bartonellaspecies (which can cause transient anaemia), Ehrlichiaspecies, Neorickettsia risticii, Anaplasma phagocytophilum, Cytauxzoon felis and Rickettsia felis. 21,22
Transfusion ‘triggers'
Transfusion ‘triggers’ are physical examination findings and haematological parameters that have been used to define when a blood transfusion should be given. These triggers have long been debated in the human and veterinary literature. Current thinking in the human critical care field tends towards a ‘less is more’ approach, 23 in the light of findings such as a similar 30-day mortality in a critical care setting despite the use of lower transfusion triggers. 24 Another study reported increased mortality in human patients that received transfusions compared with those that refused blood transfusions. 25 There is increasing evidence in people and animals that transfusion of blood products is not always favourable and can in some cases be associated with a poorer prognosis, although the veterinary studies are hard to interpret due to confounding factors such as severity of illness. 2,3,26,27
Current veterinary recommendations are that a haemoglobin concentration ([Hb]) of 7 g/dl (equivalent to a PCV of approximately 21%), or in cases where the patient requires surgery a [Hb] of 10 g/dl, should be used as a guide for transfusion. 28,29 These recommendations are somewhat cautious as many normal adult cats have a [Hb] of 10 g/dl and clinical signs of anaemia will not be present at these levels in the absence of hypovolaemia. Given the variety of causes and potential chronicity of anaemia, it is inadvisable to use PCV or [Hb] alone as a trigger for transfusion. The best way of determining whether a blood transfusion is indicated is by assessing the patient for clinical signs referable to anaemia, through physical examination, in concert with clinicopathological data (see box on page 13).
Feline blood groups and types
The nomenclature used to describe different blood groups and types can be confusing, as these terms are used interchangeably. The nomenclature used in this article is the feline AB blood group system (comprising of types A, B and AB), and Mik group system (comprising of types Mik positive and Mik negative).
Mik group.
Mik is a newly described blood group in cats that has been identified as a cause of incompatibilities between donor and recipient blood that are not related to the AB blood group system. Mik antibodies (named after the alloantibody identified in the first blood donor cat, Mike) were discovered in three cats that had not previously received transfusions. 36 The clinical relevance of this is that haemolytic transfusion reactions can occur in previously naive, AB-typed cats. As it is not possible to examine for the presence of this blood group, cross-matching has been recommended by some authors. Reactions can be subclinical and result in more rapid destruction of donor blood cells. The importance of this blood group has not been investigated outside of the USA.
Is a transfusion required?
Clinical signs of anaemia include lethargy and altered mentation, increased respiratory effort, pale mucous membranes and tachycardia. Physiologically, the body undergoes a number of adaptive responses to maintain delivery of oxygen to the tissues. Adaptive mechanisms in chronic anaemia include an increase in stroke volume, through sodium and water retention, to increase the cardiac output. 30–32 Importantly, these feline patients may not be tachycardic, although they will have hyperdynamic femoral and peripheral arterial pulses. Tachycardia is a more reliable indicator of anaemia following acute haemorrhage, particularly once the patient has undergone adequate volume resuscitation.
Measurement of serum lactate can be useful to demonstrate anaerobic metabolism. Other supportive evidence of significant anaemia may be identified by examining arteriovenous oxygen extraction or electrocardiographic changes, although these have not been evaluated clinically. Normal tissue oxygen extraction (oxygen saturation of haemoglobin in arterial blood minus mixed venous oxygen saturation of haemoglobin) is approximately 10–20% and this is increased in anaemia. An extraction percentage of 50–60% in a patient with progressive anaemia has been suggested as a transfusion trigger. Electrocardiographic signs of myocardial ischaemia, similar to those identified in human patients with myocardial infarction, can occur with anaemia, 33 and have been identified in dogs with artificially produced acute haemorrhage 34 and dogs with babesiosis. 35 The most common changes included a low R amplitude, prolonged QRS duration, ST deviation (high, depressed or elevated) and high T amplitude.
As alluded to, cats have marked adaptive capabilities in the face of chronic anaemia. It is not uncommon for a cat to show only pallor despite an extremely severe anaemia (PCV <10%). PCVs as low as 5% have been reported anecdotally in living cats.
The best way of determining whether a transfusion is indicated is by assessing the patient for clinical signs referable to anaemia, through physical examination, in concert with clinicopathological data.
Guidelines for transfusion used by the authors
Research milestones
Feline blood groups have been studied since the early 20th century. Ingebrigtsen, in 1912, 37 used Epstein and Ottenberg's 38 technique to look for agglutination of blood cells and serum from different cats. He identified strong agglutination in one or two cases but concluded that they did not define any blood group. His experiments were repeated in 1915, 39 but again did not identify any group system, although this study did report the first feline transfusion reactions. It was not until 1950 that two blood types were defined. 40 Two years on, Holmes 41 postulated that cats might have three blood types; however, this was not confirmed until 1981. 42 The nomenclature of blood types A and B was first defined by Eyquem, Podliachouk and Millot in 1962. 43
Types A and B — determinants, genetics and relative prevalence
Blood types are defined by antigens expressed on the surface of the red blood cell. These vary both within and between species and, therefore, the blood types of dogs, cats and humans are not comparable. The molecular nature of the different antigens of the blood types (A and B) have been identified: type A cats express N-glycolylneuraminic acid and small amounts of N-acetylneuraminic acid, whereas type B cats express only N-acetylneuraminic acid. 44 N-acetylneuraminic acid undergoes enzymatic hydroxylation to N-glycolylneuraminic acid, which suggests that type B cats might lack this particular hydroxylase. 45
Approximately 20% of type A cats have anti-B antibodies, which are generally weak. All type B cats have strong anti-A antibodies, while type AB cats do not have alloantibodies.
Unlike dogs, cats have preformed ‘non-self’ antibodies, which can result in potentially fatal antibody-mediated reactions to non-self red blood cells. Approximately 20% of type A cats have anti-B antibodies, which are generally weak. All type B cats have strong anti-A antibodies, while type AB cats do not have alloantibodies. 46,47
Genetically, blood type A (A/A) is dominant over blood type B (homozygous b/b). A third allele (ab), recessive to a and co-dominant to b, leads to the expression of both A and B molecules. Type AB is not obtained from mating a type A cat with a type B cat, unless type A is heterozygous (A/ab).
Generally, type A cats are more common than type B. A recent UK study identified that 68% of non-pedigree cats were type A, 30% were type B and 2% were type AB. 48 It is, thus, important to have both type A and type B blood donors available in the practice, or a list of suitable donors and their blood type.
There are important implications for breeders, too, with respect to avoiding the risk of neonatal isoerythrolysis.
Blood typing
Due to the risk of life-threatening reactions, it is imperative to blood type cats prior to a transfusion. The high titres of naturally occurring anti-A antibodies in type B cats 75 result in rapid intravascular destruction of transfused type A red blood cells. This process is thought to be mediated by IgM and complement. By contrast, the destruction of type B blood cells transfused to a type A cat is mostly an extravascular process mediated by IgG and IgM without complement activation, and is typically less severe. 47
Feline blood groups worldwide.
The prevalence of blood groups throughout the world has been studied since Holmes’ first report of two distinct blood groups in cats in Manchester, UK. 40 Most studies have been in North America, 49–52 Europe 43,48,53–64 and Australia, 42,65 with a few studies in South America 66 and Japan. 67,68 Throughout the world type A is the most common blood type, particularly within domestic shorthairs, type B is less common and type AB is considered rare. Certain breeds, such as the Siamese, have been reported to be 100% type A; others, such as the Devon Rex, are more commonly type B. This underlines the need to type cats prior to any blood transfusion. Tables are available to highlight the risk of blood transfusions to unmatched donors and recipients of different breeds. 69–72
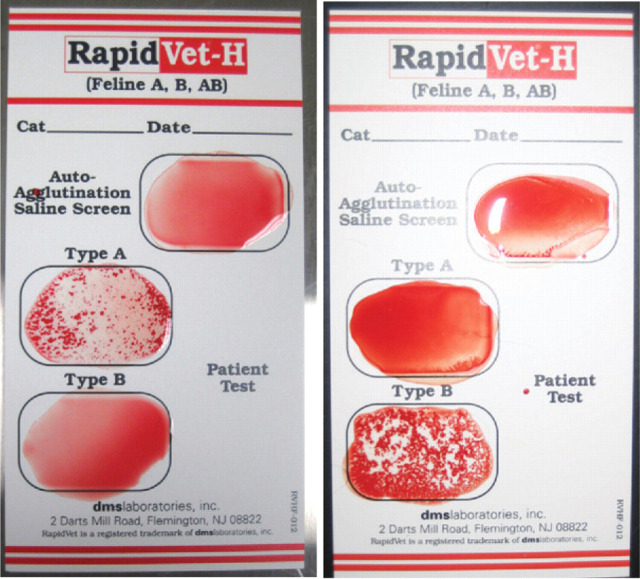
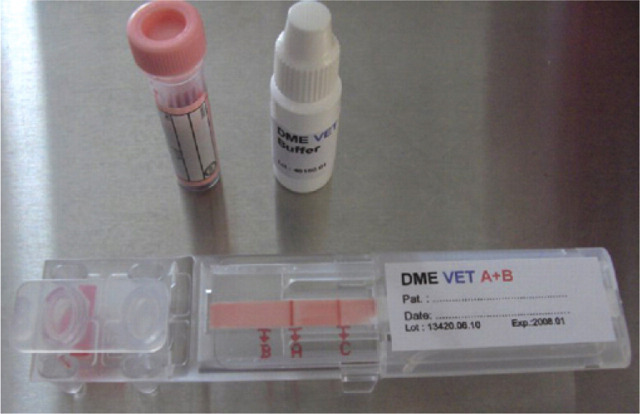
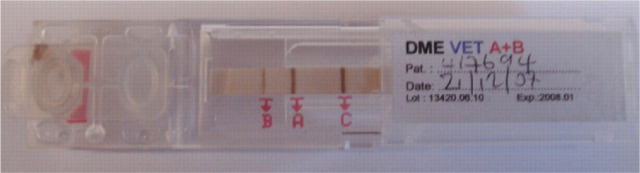
Blood typing can be performed at any commercial laboratory on an EDTA anticoagulated whole blood sample. Alternatively, several methods can be used in clinical practice including a card system (RapidVet-H Feline; DMS Laboratories, Fig 2) and a migration paper strip cartridge (DME VET A+B; Alvedia, Fig 3). The cards depend on an agglutination reaction using anti-A antiserum for detecting type A antigen and Triticum vulgaris lectin for detecting type B antigen. The migration paper strip cartridges use monoclonal antibodies to differentiate blood types and, therefore, have the added bonus of being able to differentiate the rare type AB (Fig 4), which has proved difficult with the card system as there are reports of false positive results due to a number of causes. 76
FIG 2.
RapidVet-H feline blood typing cards, revealing type A (left) and type B (right) blood
FIG 3.
DME blood typing kit, complete with reagent strip and buffer. EDTA blood is required for typing, which in this case reveals type A
FIG 4.
Blood type AB, determined using the DME blood typing system
Implications for breeders.
Type A or AB kittens born from a type B queen are at risk of neonatal isoerythrolysis. 73 The clinical signs shown by these so-called ‘fading’ kittens are variable depending on the concentration of anti-A antibodies in the queen's colostrum, the amount consumed and the amount absorbed.
Kittens at risk of neonatal isoerythrolysis should be removed from the queen in the first day of life so that they do not ingest and absorb maternal antibodies directed at their red blood cells. Absorption of antibodies drops significantly by 16 h, 74 indicating that preventing sucking for the first 24 h of life should be sufficient to prevent maternal antibody transfer. Treatment of this condition is technically challenging. The kitten should be prevented from sucking and a type A blood transfusion may be required, which can be administered using the intraosseous or intraperitoneal route if intravenous access is not possible.
Breeders should be aware of the devastating consequences of this condition and be urged to type the queen, and the tom if the queen is found to be type B. Selective breeding can be instituted to reduce the risk of producing type A or AB kittens from a type B queen. However, this method might decrease the genetic pool of available toms and queens, which in certain breeds is undesirable as there may already be a high incidence of inbreeding.
‘Fading’ kittens can show:
Weakness
Jaundice
Tail tip necrosis
Pigmenturia
Sudden death
Throughout the world type A is the most common, particularly within domestic shorthairs, type B is less common and type AB is considered rare. Certain breeds, such as the Siamese, have been reported to be 100% type A, while the Devon Rex is more commonly type B. This underlines the need to type cats prior to any blood transfusion.
The blood typing cards have been compared with commercially available methods, including the GEL test card (Diamed), the University of Pennsylvania slide and tube tests (considered the gold standard), the monoclonal antibody tube test (Shigeta) and alloantibody test (agglutination of cat's serum/plasma with known type A or B positive red blood cells), and have been shown to give inaccurate results when both A and B wells agglutinate. It is recommended that, should this occur, the result is confirmed at an external laboratory. 77 Although the migration paper strip cartridges (DME VET A+B; Alvedia) were not included in this study, they work in a similar way to the monoclonal antibody tube test (Shigeta), which has been shown to be reliable for typing the feline AB blood group system. Details on how to use the cards and kits can be found on the respective company websites, listed on page 21 under ‘Useful resources'.
Feline blood products
Currently, fresh whole blood is the most common product used in cats; however, stored whole blood, packed red blood cells and fresh frozen plasma (FFP) are also given as transfusions. 1,4 The open collection system most commonly used to obtain feline blood (see later) means that storage is not advisable for longer than 24 h. Feline blood products are commercially available in the USA and steps are being taken in the UK to develop similar ‘blood banks'. At time of writing, however, most donations are from cats known to the practice.
Storing feline blood products.
Whole blood and packed red cells need to be stored at 4°C, preferably in a refrigerator that can record temperature fluctuations. If this facility is not available, a refrigerator that is seldom used can be used to attempt to keep the temperature constant. Stored samples need to be identified with the blood type, donor name and collection date. This information should also be noted on the recipient's transfusion sheet and clinical record. Fresh frozen plasma needs to be kept frozen at −30°C until used and, again, should be identified with the donor blood type, name and collection date. If samples are thawed and not used they should not be refrozen but instead discarded or stored in a fridge and used within 12–24 h.
Alternatives to blood products.
Alternatives to blood products based on haemoglobin have been used in humans since the 1960s and have the benefits of being immediately available and not requiring compatibility testing. 28 Currently, the only commercially available alternative to red cell transfusion is an ultra-purified polymerised bovine haemoglobin solution (Oxyglobin; OPK Biotech). Although this is not licensed in cats, it has been used to support cats with clinical signs of anaemia, 78,79 and as a therapy for carbon monoxide poisoning. 80 Informed consent should be obtained from clients prior to this therapy being instituted. The main risk associated with administration is volume overload as it is a potent colloid (colloid osmotic pressure 43 mmHg). 81 Conservative administration rates are recommended (as low as 0.2–0.4 ml/kg/h and to a maximum of 1 ml/kg/h) in patients with normovolaemic anaemia. 82 Patients should be carefully monitored, paying particular attention to their heart and respiratory rate. The plasma half-life of Oxyglobin is dose-dependent; therefore, giving larger doses will provide a longer duration of effect. The product should be used within 24 h once removed from the protective foil packaging as it oxidises to methaemoglobin on exposure to light. Administration of this product changes the colour of the patient's serum and will, therefore, invalidate certain serum biochemical tests that use colorimetric methodology. Generally, it should not interfere with measurement of electrolytes. 83 Once Oxyglobin therapy has been started, monitoring the PCV is no longer a reflection of the patient's oxygen-carrying capability and measurement of [Hb] is recommended using a haemoglobinometer. Clinical signs should also improve following administration.
Oxyglobin can be used repeatedly with care, although it is often used in the initial management of a clinically affected anaemic patient when there is no immediate access to a blood donor, or if there has been a transfusion reaction.
The long term availability of the product is currently uncertain.
Donor cats and the blood donation process
Donor requirements and screening
Feline blood donors should be healthy, indoor-only cats with an agreeable temperament for easy handling and restraint. Owned pet cats should not donate more often than once every 2 months. While cats weighing more than 4 kg can be used, 84 larger donors should experience fewer side effects associated with donation, so a 5 kg minimum (lean body mass) is recommended by the authors. Donors should be of normal body condition.
It is preferable to leave at least a month after vaccination, routine surgery (such as neutering) or medical treatment prior to donation. Cats on long term medication of any sort (including non-steroidal anti-inflammatory agents) should not be used. They should be vaccinated as appropriate for the region. (For information relevant to Europe, see the guidelines of the European Advisory Board on Cat Diseases [ABCD] on the prevention and management of feline panleukopenia, 85 feline herpesvirus infection, 86 feline calicivirus infection, 87 feline leukaemia, 88 feline immuno-deficiency, 89 feline infectious peritonitis, 90 feline rabies, 91 Chlamydophila felis 92 and Bordetella bronchiseptica infection in cats. 93 The guidelines are also available at www.abcd-vets.org/guidelines.) Haematology and biochemistry should be checked annually in regularly used donors, and prior to transfusion in donors used less frequently.
Donors should be screened for infectious diseases applicable to their region to minimise the risk of transmission. Transmission of M haemofelis and ‘Candidatus M haemominutum’ to naive cats via administration of infected blood has been demonstrated after storage for 1 h and 1 week. 94 The American College of Veterinary Internal Medicine (ACVIM) consensus statement on canine and feline blood donor screening for infectious disease 95 recommends testing for certain vector-borne diseases in all areas (M haemofelis infection, ‘Candidatus M haemominutum’ infection and bartonellosis) and conditional testing depending on region for others (cytauxzoonosis, ehrlichiosis, anaplasmosis and neorickettsiosis). Testing for non-vector-borne diseases including FeLV and FIV infection is recommended; testing for feline coronavirus and toxoplasmosis is not recommended, however, due to the low risk of transmission.
While cats weighing more than 4 kg can be used as donors, larger cats should experience fewer side effects associated with donation, so a 5 kg minimum (lean body mass) is recommended by the authors.
Physical examination and blood sampling
Prior to donation the cat should undergo a thorough physical examination, paying particular attention to the cardiovascular and respiratory systems, as it will likely have to undergo deep sedation or anaesthesia for the donation process. Cats with significant abnormalities on physical examination, such as heart murmurs or gallop rhythms on auscultation, should not be used.
A blood sample should be taken to measure the PCV or [Hb]. It is preferable to use cats with a PCV of 30–35%; cats with low—normal PCVs should not be used. If indoor—outdoor cats are used for donation, FIV and FeLV status should be rechecked prior to each donation using a bench-side test (albeit results might be difficult to assess if the donor has been vaccinated against FIV).
Sedation/anaesthesia
Most cats require sedation or general anaesthesia to facilitate the donation process. A variety of protocols can be used including parenteral administration of combinations of ketamine hydrochloride, midazolam and butorphanol tartrate, or mask administration of sevoflurane. 96,97 Mild hypo tension is frequently observed following donation. The use of sevoflurane is associated with greater decreases in blood pressure, heart rate and PCV. 98
Open versus closed blood collection systems
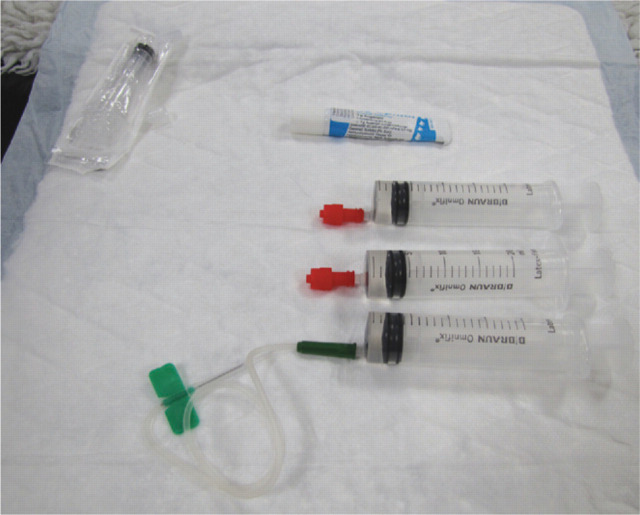
In the majority of cases, feline blood is collected into open systems (Fig 5), which are made up of individual components that are sterilised at the time of donation. Currently, closed systems — that is, those pre-prepared ‘all-in-one’ and sterilised as such — are not available for cats. Although blood collection kits for cats can be purchased, they are not pre-filled with anticoagulant and, therefore, not truly ‘closed’ systems.
FIG 5.
Equipment for an open blood collection system, including butterfly catheter and syringes pre-filled with anticoagulant
Blood donation protocol.
There are a few considerations that make the donation procedure easier to manage, such as getting everything ready in advance and making sure there is at least one assistant to hand; it is also very advisable to preplace an intravenous catheter in case of problems and to use more than one syringe for collecting the donated blood. The following protocol is used at the authors’ institution.
A 20–22 gauge intravenous catheter is preplaced in the cephalic vein and patency is confirmed by flushing with saline.
Sedation is administered. The authors prefer to use a 1:1 combination of ketamine 100 mg/ml and midazolam 5 mg/ml, which is made up in a small syringe and given intravenously up to a maximum dose of 5 mg/kg ketamine (0.1 ml/kg of combination). Sedation can be given subcutaneously or intramuscularly to facilitate intravenous catheterisation. Additionally inhalation agents (eg, sevoflurane) can be administered by mask to improve the sedation.
The area over the jugular vein is clipped and aseptically prepared.
Three 20 ml syringes are each pre-filled with 3 ml of acid citrate dextrose or citrate phosphate dextrose anticoagulant. Using three syringes makes collection of a full unit of blood (50–60 ml) easier. Although not recommended (as it might induce platelet aggregation and inhibit coagulation factors), in an emergency situation heparin can alternatively be used as an anticoagulant, at 5–10 units of heparin/ml of blood. 20,99,100
The patient is adequately restrained.
A 19–21 G butterfly needle is used to access the jugular vein and the blood is collected over a total of 10–15 mins. Each syringe is gently rotated during and after the donation to make sure the anticoagulant is adequately distributed throughout the collected blood. A maximum of 10–12 ml/kg blood can be donated at one time.
The cat is given isotonic crystalloid fluid therapy (eg, Hartmann's) post-donation at a rate of 60 ml/h for 3 h. This may also be started during the donation procedure.
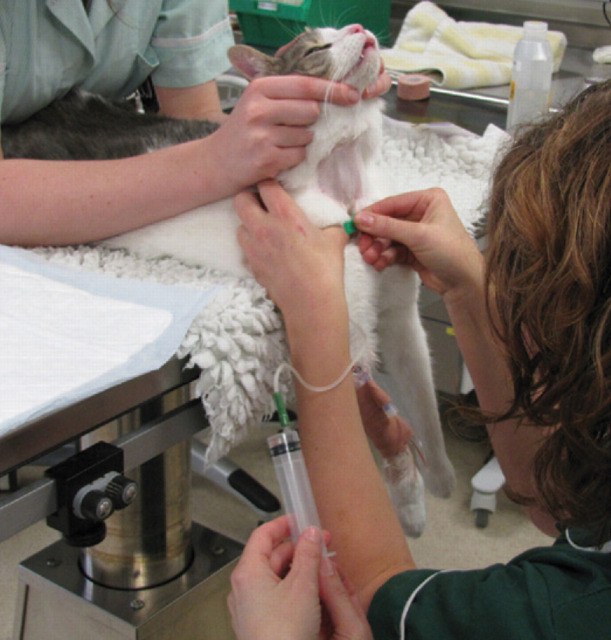
Donor restraint and positioning for blood collection
Blood collection using an open collection system
A maximum of 10-12 ml/kg blood can be donated at one time.
MULTIMEDIA.
A video clip showing blood collection from a donor cat is included in the online version of this article at doi:10.1016/j.jfms.2010.11.006
The distinction between the two types of collection system is important as the risk of bacterial contamination is higher in open systems, which is relevant when blood is to be stored. Blood banking services and certain small animal hospitals that use stored whole blood and packed red blood cells collected in as near to a closed system as possible do not report any bacterial contamination or complications associated with the use of stored products; nevertheless the authors would not recommend storing blood collected this way for more than 24 h in general small animal practice.
Transfusion of whole blood and other blood products to cats
The goal of therapy in most cases is to increase the patient's PCV sufficiently to reverse the signs of anaemia. A PCV of 20% should be the aim, although in practice the post-transfusion PCV is not always this high.
The volume of whole blood to be administered can be calculated from the following equation:
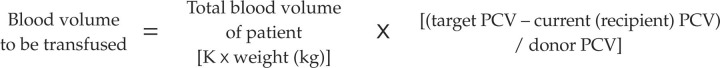 |
Clinically, a unit of blood is usually donated and administered to the recipient. This is usually sufficient to see an improvement in the transfused patient's clinical signs.
This formula estimates that 2 ml/kg of whole blood will increase the recipient's PCV by 1%, assuming a donor PCV of 30%. Although the formula is embedded in the literature it has not been validated. Other formulae have been described, but may not be clinically useful in cats. 5 Clinically, a unit is usually donated and administered to the recipient. This is usually sufficient to see an improvement in the transfused patient's clinical signs.
Administration of FFP can be used for the treatment of a single or multiple clotting factor deficiency, vitamin K deficiency or antagonism, surgical bleeding or where a massive transfusion is required. 4 In cats, hypoalbuminaemia and coagulopathies (mainly due to liver disease) are the main reported indications for FFP transfusions. 1 Unlike dogs, only type-specific plasma should be administered to cats as they have antibodies to non-self blood types within the plasma. Whole blood can be separated into FFP and packed red cells if it is taken aseptically using one of the commercially available systems. The blood should be spun at 3800 rpm at 10°C in a refrigerated centrifuge for 12 mins. The plasma is extracted using a plasma extractor and stored at −20°C. 102
An increase in clotting times (activated partial thromboplastin time, prothrombin time) by >30%, particularly if associated with active bleeding, is considered to be a ‘trigger’ for administering a FFP transfusion. A generic dose of 5–20 ml/kg is recommended and the effectiveness of administration is objectively measured by repeating the clotting times. Although hypoalbuminaemia has been an indication for FFP transfusion, no post-transfusion albumin concentrations have been recorded in the clinical or experimental literature. Extrapolating from canine patients that have received FFP for the treatment of hypo-albuminaemia, large doses (>10 ml/kg initial dose) are needed to significantly increase albumin levels. Such large doses of FFP are difficult to source in many countries, and the dose of plasma available in most cases is likely to be ineffective in significantly raising albumin levels. 103
Lyophilised feline serum albumin is not yet commercially available for therapeutic use, but human serum albumin has been used in cats. 104
Preparation and route of administration
Prior to administration, the gold standard approach would be that the donor and recipient are cross-matched (see below). This, however, is not routinely performed at the authors’ institution unless there is a transfusion reaction.
The main route of administration is intravascular, through a peripheral or centrally placed catheter. 5,106,107 The whole blood can either go through a designated port on a T-piece connector attached to the catheter, or through a separate line if the patient is also receiving intravenous fluids, providing the fluid does not contain calcium. Intraosseous catheters can also be used to administer all blood products, 108 which is useful in collapsed neonatal patients where vascular access is difficult. Although whole blood can be infused into the peritoneal cavity, 109 its slow rate of absorption (40% in 24 h) limits its potential therapeutic use and this route is, therefore, not recommended. 5
Unlike dogs, only type-specific plasma should be administered to cats as they have antibodies to non-self blood types within the plasma.
Cross-matching — the gold standard.
A major cross-match identifies incompatibilities between the donor's red blood cells and recipient's plasma. A minor cross-match identifies incompatibilities between the donor's plasma and recipient's red blood cells. An in-house cross-match kit is commercially available (RapidVet-H; DMS Laboratories) and is recommended to be used in an emergency or when external laboratory facilities are not available.
Some authors recommend that cross-matching is performed prior to any transfusion, as there are blood types that have not been described and it is not possible to type for Mik. This is likely to be a little overcautious; however, cross-matching should be performed if the recipient has already received a transfusion more than 4 days previously. 105
Major and minor cross-matches can be performed in-house with collected donor blood.
For a major cross-match:
Collect donor blood into EDTA anticoagulant
Centrifuge sample at 3000 rpm for 10 mins
Discard supernatant (plasma and buffy coat)
Add saline (0.9% NaCl) to precipitate (erythrocytes) and resuspend. This ‘wash’ can be repeated three times
Resuspend the erythrocytes in saline (0.9% NaCl) to make a 3–5% solution
Place 1–2 drops of this erythrocyte suspension on to a glass slide
Add 1–2 drops of recipient (heparinised) plasma
Check for agglutination or haemolysis
This process can be repeated with recipient erythrocytes and donor plasma for a minor cross-match
If whole blood has been refrigerated then it should be warmed to room temperature and gently agitated to resuspend the red blood cells prior to administration. Colder blood has a higher viscosity, which limits the rate of infusion. 110
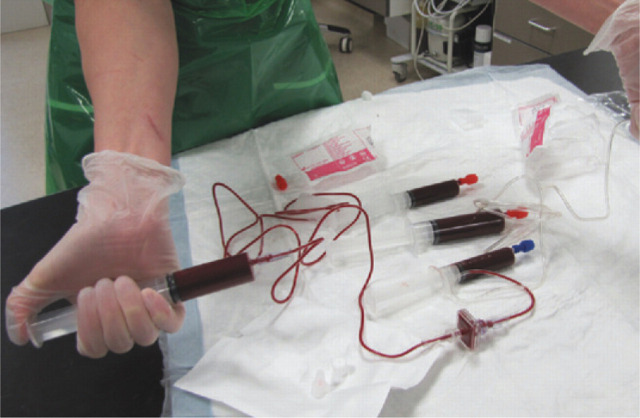
Whole blood should ideally be administered through a commercially available filter, which can be attached to a product bag (eg, transfusion/blood set with 200 μm filter). If the whole blood is administered through a syringe, a disposable 18 micron blood filter (eg, Hemo-Nate; Utah Medical Products — see page 21) can be attached in series (Fig 6). Sterility should be maintained when preparing giving sets/filters with blood products.
FIG 6.
Priming the blood through extension tubing and a disposable 18 micron filter, prior to transfusion
Rate of administration
The transfusion should be started slowly using a syringe driver, if possible, or by hand, at 0.25 ml/kg/h. The rate can be increased if no adverse affects are witnessed after the first 30–60 mins of administration. The rate of administration may vary depending on the urgency of the requirement for whole blood (eg, haemorrhagic shock) and any underlying concurrent disease (eg, risk of volume overload with cardiac disease). In cases of acute blood loss, for example, whole blood can be administered at a faster rate, but rates >20 ml/kg/h are not recommended. 107
The transfusion should be completed in 4–6 h once the whole blood is brought up to room temperature.
Monitoring the transfusion
Very low rates of transfusion reactions have been reported in cats receiving typed or cross-matched transfusions. Review of the available literature identified reports of 16 transfusion reactions from a total of 514 type-specific transfusions of whole blood or packed red blood cells. 1–4 No reactions have been reported following transfusions of FFP in cats. 1
Autotransfusion — an emergency measure.
Autotransfusion — the collection and re-transfusion of the cat's own blood — is a useful technique in an emergency situation. 111 It can be achieved when animals bleed into body cavities, but should not be performed if the blood is contaminated with urine, bacteria or bile. Blood is collected from the body cavity in a sterile manner and re-transfused into the patient through an appropriate filter (eg, 18 micron Hemo-Nate blood filter — see page 21). Anticoagulant (eg, acid citrate dextrose) should be included at a ratio of 1:7 to prevent clotting.
Transfusion reactions.
Type A cats should receive type A blood only
Type B cats should receive type B blood only
Type AB cats should receive type AB blood, if possible, or type A blood
It is, therefore, imperative to blood type all cats, without exception, prior to their first transfusion
If a transfusion reaction is suspected the transfusion should be stopped immediately and the recipient monitored continually for deterioration. Acute haemolytic transfusion reactions from naturally occurring alloantibodies are the most severe and have been well documented. 46,47,112 This type of reaction needs to be ruled out by spinning down the recipient's blood prior to recommencing the transfusion at a slower rate.
Clinical signs include restlessness, vocalisation, tachypnoea, bradycardia, tachycardia, hypotension and hypertension. 5,112 Other adverse reactions include transfusion-related pyrexia (increase in core body temperature >1°C during or within 4 h of a transfusion) and vomiting. Pyrexia is commonly due to reactions to donor leukocytes, platelets and plasma proteins; 5 vomiting can be reduced by starving cats 6 h prior to administration of blood. Urticaria is rare in cats.
There is also the potential for hypocalcaemia when administering large volumes of blood products due to binding by citrate. Therefore, calcium should be measured if the patient is showing clinical signs of hypocalcaemia (eg, muscle fasciculations, seizures, etc).
Review of the available literature identified reports of 16 transfusion reactions in cats from a total of 514 type-specific transfusions of whole blood or packed red blood cells. No reactions have been reported following transfusions of fresh frozen plasma.
Adequate care and attention, and close monitoring, is nonetheless required with any transfusion. Prior to the procedure the recipient should be carefully examined and its heart rate, respiratory rate, mucous membrane colour, capillary refill time and temperature recorded. The PCV and total plasma protein should also be record-ed. 113 A transfusion monitoring sheet, as shown in Fig 7, can assist in the collection of this data.
FIG 7.
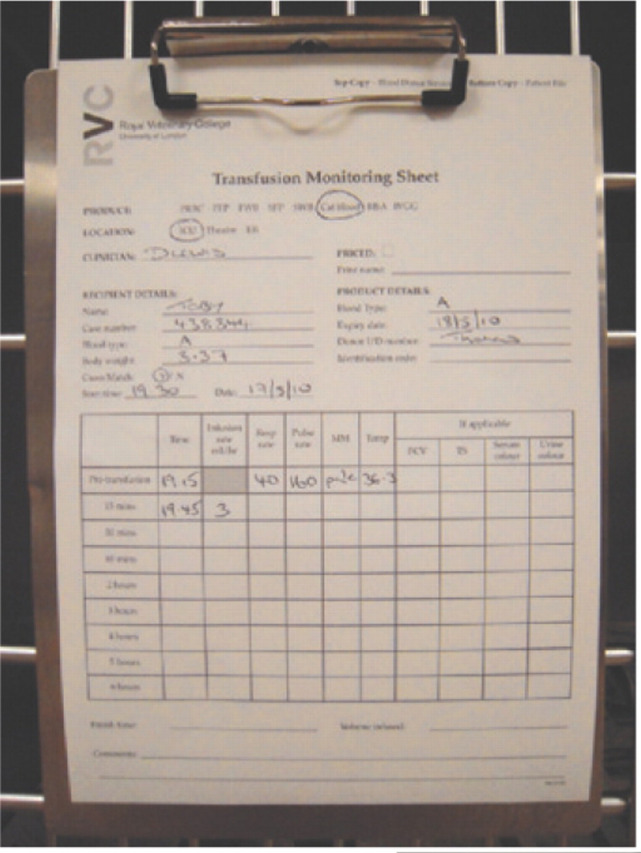
Transfusion monitoring sheet on a hospital cage. The first row of data comprises the pre-transfusion clinical parameters
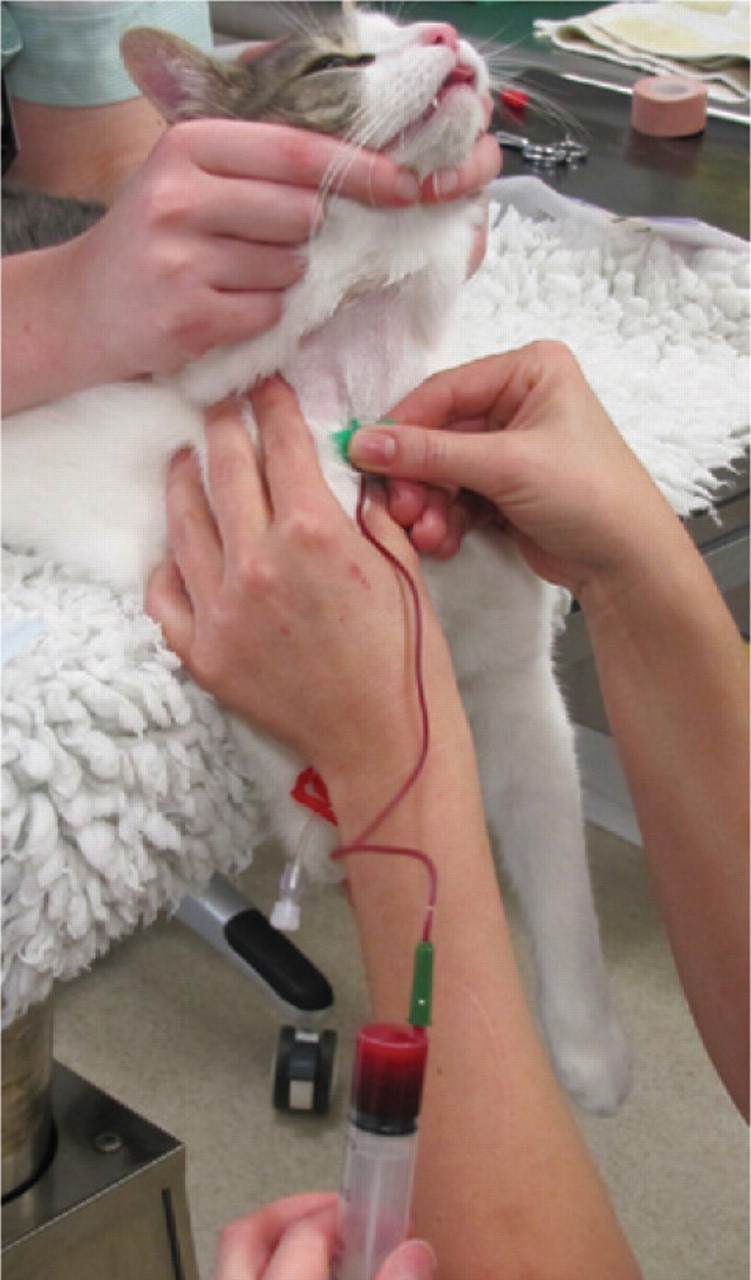
Once the transfusion has started (Fig 8), the patient's vital signs should be assessed every 15 mins of the first hour. The physical parameters should be repeated every hour for the remainder of the transfusion. Patients that are euvolaemic or volume loaded prior to transfusion (such as those with chronic non-regenerative anaemia) are at risk of volume overload and should be monitored especially closely. The jugular veins should be examined for distension or pulsation, and the respiratory rate measured regularly. Thoracic auscultation should also be performed regularly throughout the transfusion to check for signs of pulmonary oedema associated with over-perfusion. Patients at risk of volume overload may benefit from slower administration of the transfusion. Where the donation has been taken into three syringes this is readily achieved by keeping the unused syringes in the fridge until required and then bringing them up to room temperature prior to administering each syringe over a maximum of 4 h.
FIG 8.
Patient receiving a typed blood transfusion. Note the Hemo-Nate blood filter in series with the extension tubing
The underlying disease is key to the prognosis
Anaemia, no matter what the underlying cause, presents a challenge for the clinician in terms of stabilising and supporting the patient. The survival rate has been reported as 84% in the first 24 h, for all reasons for a transfusion, and 75% and 49.6% at 10 days, for blood loss anaemia and ineffective erythropoeisis, respectively. 2 Appropriate administration of whole blood allows the clinician more time to investigate and manage the cause of a patient's anaemia. As emphasised in this article, a good understanding of feline blood groups and blood typing will limit the risks of transfusion reactions. The difficulty in procuring feline blood products in some countries makes it important that veterinarians have knowledge of donation and transfusion medicine, and access to donor cats. If donor cats are not available then referral to a suitable facility needs to be considered.
Approximately 60% of cats requiring transfusion are ultimately discharged from hospital. 3,4 It should be recognised that the requirement for a transfusion is a negative prognostic indicator for hospitalised cats, 3 and that it is the underlying disease necessitating the blood transfusion that is the real prognostic indicator.
Future for feline blood products.
Blood banks selling feline blood products are already present within North America. However, the size of our feline patients, the inherent difficulties in obtaining blood donations, the commercial viability of blood banks and country-specific laws mean that it is likely to be some time before a blood bank facility that will sell feline products to local veterinarians is generally available in other countries. Until that time, most small animal and feline practices will manage donations and transfusions within the clinic, using staff-owned or client-owned cats. Potentially, other products that may become commercially available include feline serum albumin, feline platelets and plasma- or recombinant-derived clotting factor components. It is important to remember that any intravenous transfusion of a foreign protein may cause a reaction and monitoring should be conducted in the same way as for a blood transfusion.
KEY POINTS.
All donors and recipients should be blood typed.
Cats must receive appropriate AB type-specific blood.
Blood should be collected from donors and administered to recipients aseptically.
Donors should be monitored after donation and supported with intravenous fluids.
Recipients of transfusions should be closely monitored for reactions.
If a transfusion reaction is detected a haemolytic cause needs to be ruled out.
The clinician should be alert to the risk of volume overload, and monitor the recipient's heart and respiratory rate.
Acknowledgements
The authors would like to thank Robyn Taylor, the RVC's dedicated blood donation nurse, for all her assistance with this article. They also thank Brian Cox for his technical help with the multimedia clip.
Useful resources
Websites
-
‘Practice pointers’ on blood transfusions are available within the ‘toolbox’ section.
-
Includes information on blood types and the associated genetics, as well as information on many cat viruses.
Blood typing systems, other products and equipment
Rapid Vet-H Feline blood typing, DMS Laboratories, Flemmington, NJ, USA. www.rapidvet.com
DME VET A+B, Scientific Service Laboratory, Alvedia, Lyon, France. www.alvedia.com
Vet ID Card A+B GEL test, DiaMed AG, Cressier sur Morat, Switzerland
Transfusion laboratory, Section of Medical Genetics, School of Veterinary Medicine, University of Pennsylvania, Philadelphia, USA. http://research.vet.upenn.edu/penngen
Shigeta_s blood-typing kit, Shigeta Animal Pharmaceuticals, Komoridani, Oyabe City, Toyama Pref, Japan
Oxyglobin, OPK Biotech, Cambridge, MA, USA. www.oxyglobin.com
Transfusion/blood set. 200 μm filter. Cardinal Health, Rolle, Switzerland
Hemo-Nate, disposable 18 micron blood filter. Gesco. Utah Medical Products. www.utahmed.com
Rapid Vet-H Companion Animal Crossmatch; DMS Laboratories, Flemmington, NJ, USA. www.rapidvet.com
References
- 1. Castellanos I, Couto CG, Gray TL. Clinical use of blood products in cats: a retrospective study (1997–2000). J Vet Intern Med 2004; 18: 529–32. [DOI] [PubMed] [Google Scholar]
- 2. Weingart C, Giger U, Kohn B. Whole blood transfusions in 91 cats: a clinical evaluation. J Feline Med Surg 2004; 6: 139–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Klaser DA, Reine NJ, Hohenhaus AE. Red blood cell transfusions in cats: 126 cases (1999). J Am Vet Med Assoc 2005; 226: 920–23. [DOI] [PubMed] [Google Scholar]
- 4. Roux FA, Deschamps JY, Blais MC, Welsh DM, Delaforcade-Buress AM, Rozanski EA. Multiple red cell transfusions in 27 cats (2003–2006): indications, complications and outcomes. J Feline Med Surg 2008; 10: 213–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Griot-Wenk ME, Giger U. Feline transfusion medicine. Blood types and their clinical importance. Vet Clin North Am Small Anim Pract 1995; 25: 1305–22. [DOI] [PubMed] [Google Scholar]
- 6. Kohn B, Weingart C, Giger U. Haemorrhage in seven cats with suspected anticoagulant rodenticide intoxication. J Feline Med Surg 2003; 5: 295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wondratschek C, Weingart C, Kohn B. Primary immune-mediated thrombocytopenia in cats. J Am Anim Hosp Assoc 2010; 46: 12–19. [DOI] [PubMed] [Google Scholar]
- 8. Culp WTN, Weisse C, Kellogg ME, et al. Spontaneous hemoperitoneum in cats: 65 cases (1994–2006). J Am Vet Med Assoc 2010; 236: 978–82. [DOI] [PubMed] [Google Scholar]
- 9. Kohn B, Weingart C, Eckmann V, Ottenjann M, Leibold W. Primary immune-mediated hemolytic anemia in 19 cats: diagnosis, therapy, and outcome (1998–2004). J Vet Intern Med 2006; 20: 159–66. [DOI] [PubMed] [Google Scholar]
- 10. Shelton GH, Linenberger ML. Hematologic abnormalities associated with retroviral infections in the cat. Semin Vet Med Surg 1995; 10: 220–33. [PubMed] [Google Scholar]
- 11. Shelton GH, Linenberger ML, Persik MT, Abkowitz JL. Prospective hematologic and clinicopathological study of asymptomatic cats with naturally acquired feline immuno-deficiency virus-infection. J Vet Intern Med 1995; 9: 133–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Norris JM, Bosward KL, White JD, Baral RM, Catt MJ, Malik R. Clinicopathological findings associated with feline infectious peritonitis in Sydney, Australia: 42 cases (1990–2002). Aust Vet J 2005; 83: 666–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Flint JC, Roepke MH, Jensen R. Feline infectious anemia. I. Clinical aspects. Am J Vet Res 1958; 19: 164–68. [PubMed] [Google Scholar]
- 14. Foley JE, Harrus S, Poland A, Chomel B, Pedersen NC. Molecular, clinical, and pathologic comparison of two distinct strains of Haemobartonella felis in domestic cats. Am J Vet Res 1998; 59: 1581–88. [PubMed] [Google Scholar]
- 15. Reynolds CA, Lappin MR. ‘Candidatus Mycoplasma haemominutum’ infections in 21 client-owned cats. J Am Anim Hosp Assoc 2007; 43: 249–57. [DOI] [PubMed] [Google Scholar]
- 16. Foley JE, Pedersen NC. ‘Candidatus Mycoplasma haemominutum’, a low-virulence epierythrocytic parasite of cats. Int J Syst Evol Micr 2000; 51: 815–17. [DOI] [PubMed] [Google Scholar]
- 17. Juvet F, Lappin MR, Brennan S, Mooney CT. Prevalence of selected infectious agents in cats in Ireland. J Feline Med Surg 2010; 12: 476–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Macieira DB, de Menezes Rde C, Damico CB, et al. Prevalence and risk factors for hemoplasmas in domestic cats naturally infected with feline immunodeficiency virus and/or feline leukemia virus in Rio de Janeiro — Brazil. J Feline Med Surg 2008; 10: 120–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tasker S, Murray JK, Knowles TG, Day MJ. Coombs’, haemoplasma and retrovirus testing in feline anaemia. J Small Anim Pract 2010; 51: 192–99. [DOI] [PubMed] [Google Scholar]
- 20. Dippenaar T. Feline transfusion practice in South Africa: current status and practical solutions. J S Afr Vet Assoc 1999; 70: 135–37. [DOI] [PubMed] [Google Scholar]
- 21. Sykes JE. Ehrlichia, anaplasmosis, Rocky Mountain spotted fever, and neorickettsial infection. In: Ettinger SJ, Feldman BF, eds. Textbook of veterinary internal medicine. 7th edn. Saunders; Elsevier, 2010: 901–9. [Google Scholar]
- 22. Guptill LF. Feline bartonella. In: Ettinger SJ, Feldman EC, eds. Textbook of veterinary internal medicine. 7th edn: Oxford, Saunders; Elsevier, 2010: 896–900. [Google Scholar]
- 23. Corwin HL, Carson JL. Blood transfusion — when is more really less? New Engl J Med 2007; 356: 1667–69. [DOI] [PubMed] [Google Scholar]
- 24. Hebert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. New Engl J Med 1999; 340: 409–17. [DOI] [PubMed] [Google Scholar]
- 25. Carson JL, Noveck H, Berlin JA, Gould SA. Mortality and morbidity in patients with very low postoperative Hb levels who decline blood transfusion. Transfusion 2002; 42: 812–18. [DOI] [PubMed] [Google Scholar]
- 26. Marik PE, Corwin HL. Efficacy of red blood cell transfusion in the critically ill: a systematic review of the literature. Crit Care Med 2008; 36: 2667–74. [DOI] [PubMed] [Google Scholar]
- 27. Prittie JE. Controversies related to red blood cell transfusion in critically ill patients. J Vet Emerg Crit Care 2010; 20: 167–76. [DOI] [PubMed] [Google Scholar]
- 28. Wingfield WE. The transfusion trigger. In: Wingfield WE, Raffe MR, eds. The veterinary ICU. Jackson: Teton NewMedia, 2002: 989–1003. [Google Scholar]
- 29. Marino PL. Anemia and red blood cell transfusions in the ICU. The ICU book. 3rd edn. Lippincott Williams & Wilkins; 2007: 659–80. [Google Scholar]
- 30. Duke M, Abelmann WH. The hemodynamic response to chronic anemia. Circulation 1969; 39: 503–15. [DOI] [PubMed] [Google Scholar]
- 31. Varat MA, Adolph RJ, Fowler NO. Cardiovascular effects of anemia. Am Heart J 1972; 83: 415–17. [DOI] [PubMed] [Google Scholar]
- 32. Hebert PC, Van der Linden P, Biro G, Hu LQ. Physiologic aspects of anemia. Crit Care Clin 2004; 20: 187–89. [DOI] [PubMed] [Google Scholar]
- 33. Michael MA, El Masry H, Khan BR, Das MK. Electrocardiographic signs of remote myocardial infarction. Prog Cardiovasc Dis 2007; 50: 198–208. [DOI] [PubMed] [Google Scholar]
- 34. Della Torre PK, Zaki S, Govendir M, Church DB, Malik R. Effect of acute haemorrhage on QRS amplitude of the lead II canine electrocardiogram. Aust Vet J 1999; 77: 298–300. [DOI] [PubMed] [Google Scholar]
- 35. Dvir E, Lobetti RG, Jacobson LS, Pearson J, Becker PJ. Electrocardiographic changes and cardiac pathology in canine babesiosis. J Vet Cardiol 2004; 6: 15–23. [DOI] [PubMed] [Google Scholar]
- 36. Weinstein NM, Blais MC, Harris K, Oakley DA, Aronson LR, Giger U. A newly recognized blood group in domestic shorthair cats: the Mik red cell antigen. J Vet Intern Med 2007; 21: 287–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ingebrigtsen R. The influence of isoagglutinins on the final results of homoplastic transplantations of arteries. J Exp Med 1912; 16: 169–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Epstein AA, Ottenberg R. Simple method of performing serum reactions. Proceed New York Patholog Soc 1908; 8: 117–23. [Google Scholar]
- 39. Ottenberg R, Thalhimer W. Studies in experimental transfusion. J Med Res 1915; 33: 213–29. [PMC free article] [PubMed] [Google Scholar]
- 40. Holmes R. Blood groups in cats. J Physiol 1950; 111: 61. [PubMed] [Google Scholar]
- 41. Holmes R. The occurrence of blood groups in cats. J Exper Biol 1952; 30: 350–57. [Google Scholar]
- 42. Auer L, Bell K. The AB blood group system of cats. Anim Blood Groups Biochem Genet 1981; 12: 287–97. [DOI] [PubMed] [Google Scholar]
- 43. Eyquem A, Podliachouk L, Millot P. Blood groups in chimpanzees, horses, sheep, pigs, and other mammals. Ann N Y Acad Sci 1962; 97: 320–28. [DOI] [PubMed] [Google Scholar]
- 44. Andrews GA, Chavey PS, Smith JE, Rich L. N-glycolyl-neuraminic acid and N-acetylneuraminic acid define feline blood group A and B antigens. Blood 1992; 79: 2485–91. [PubMed] [Google Scholar]
- 45. Schauer R. Biosynthese der N-glykoloylneuraminsaure durch eine von ascorbinsaure bzw. NADPH abhangige N-acetyl-hydroxylierende N-acetylneuraminat: O2-reducktase in homogenaten der unterkieferespeicheldruse vom schwein. Hope-Seyler's Z Physiol Chem 1970; 351: 783–91. [PubMed] [Google Scholar]
- 46. Auer L, Bell K, Coates S. Blood transfusion reactions in the cat. J Am Vet Med Assoc 1982; 180: 729–30. [PubMed] [Google Scholar]
- 47. Giger U, Bucheler J. Transfusion of type-A and type-B blood to cats. J Am Vet Med Assoc 1991; 198: 411–18. [PubMed] [Google Scholar]
- 48. Forcada Y, Guitian J, Gibson G. Frequencies of feline blood types at a referral hospital in the south east of England. J Small Anim Pract 2007; 48: 570–73. [DOI] [PubMed] [Google Scholar]
- 49. Giger U, Griotwenk M, Bucheler J, et al. Geographical variation of the feline blood-type frequencies in the United-States. Feline Pract 1991; 19: 21–27. [Google Scholar]
- 50. Giger U, Kilrain CG, Filippich LJ, Bell K. Frequencies of feline blood-groups in the United-States. J Am Vet Med Assoc 1989; 195: 1230–32. [PubMed] [Google Scholar]
- 51. Giger U, Bucheler J, Patterson DF. Frequency and inheritance of A and B blood types in feline breeds of the United States. J Hered 1991; 82: 15–20. [DOI] [PubMed] [Google Scholar]
- 52. Bird MS, Cotter SM, Gibbons G, Harris S. Blood-groups in cats. Compan Anim Pract 1988; 2: 31–33. [Google Scholar]
- 53. Silvestre-Ferreira AC, Pastor J, Almeida O, Montoya A. Frequencies of feline blood types in northern Portugal. Vet Clin Path 2004; 33: 240–43. [DOI] [PubMed] [Google Scholar]
- 54. Knottenbelt CM, Addie DD, Day MJ, Mackin AJ. Determination of the prevalence of feline blood types in the UK. J Small Anim Pract 1999; 40: 115–18. [DOI] [PubMed] [Google Scholar]
- 55. Ruiz De Gopegui R, Velasquez M, Espada Y. Survey of feline blood types in the Barcelona area of Spain. Vet Rec 2004; 154: 794–99. [DOI] [PubMed] [Google Scholar]
- 56. Jensen AL, Olesen AB, Arnbjerg J. Distribution of feline blood types detected in the Copenhagen area of Denmark. Acta Vet Scand 1994; 35: 121–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mylonakis ME, Koutinas AF, Saridomichelakis M, Leontidis L, Papadogiannakis M, Plevraki K. Determination of the prevalence of blood types in the non-pedigree feline population in Greece. Vet Rec 2001; 149: 213–14. [DOI] [PubMed] [Google Scholar]
- 58. Bagdi N, Magdus M, Leidinger E, Leidinger J, Voros K. Frequencies of feline blood types in Hungary. Acta Vet Hung 2001; 49: 369–75. [DOI] [PubMed] [Google Scholar]
- 59. Arikan S, Gurkan M, Ozaytekin E, Dodurka T, Giger U. Frequencies of blood type A, B and AB in non-pedigree domestic cats in Turkey. J Small Anim Pract 2006; 47: 10–13. [DOI] [PubMed] [Google Scholar]
- 60. Haarer M, Grunbaum EG. [Blood group typing in the cat.] Tierarztl Prax 1993; 21: 339–43. [PubMed] [Google Scholar]
- 61. Hubler M, Arnold S, Casal M, Fairburn A, Nussbaumer M, Rusch P. [The blood group distribution in domestic cats in Switzerland.] Schweiz Arch Tierheilkd 1993; 135: 231–35. [PubMed] [Google Scholar]
- 62. Leidinger J, Leidinger E, Giger U. Distribution and importance of feline blood type-A and type-B in Austria. Wien Tierarztl Monat 1993; 80: 10–14. [Google Scholar]
- 63. Silvestre-Ferreira AC, Pastor J, Sousa AP, et al. Blood types in the non-pedigree cat population of Gran Canaria. Vet Rec 2004; 155: 778–79. [PubMed] [Google Scholar]
- 64. Weingart C, Arndt G, Kohn B. Prevalence of feline blood types A, B and AB in non-pedigree and purebred cats in Berlin and Brandenburg. Kleintierpraxis 2006; 51: 189–97. [Google Scholar]
- 65. Malik R, Griffin DL, White JD, et al. The prevalence of feline A/B blood types in the Sydney region. Aust Vet J 2005; 83: 38–44. [DOI] [PubMed] [Google Scholar]
- 66. Medeiros MAS, Soares AM, Alviano DS, Ejzemberg R, da Silva MH, Almosny NR. Frequencies of feline blood types in the Rio de Janeiro area of Brazil. Vet Clin Path 2008; 37: 272–76. [DOI] [PubMed] [Google Scholar]
- 67. Ikemoto S, Sakurai Y, Fukui M. Individual difference within the cat-blood group detected by isohemagglutinin. Jpn J Vet Sci 1981; 43: 433–35. [DOI] [PubMed] [Google Scholar]
- 68. Ejima H, Kurokawa K, Ikemoto S. Feline red-blood-cell groups detected by naturally-occurring isoantibody. Jpn J Vet Sci 1986; 48: 971–76. [DOI] [PubMed] [Google Scholar]
- 69. Gunn-Moore DA, Simpson KE, Day MJ. Blood types in Bengal cats in the UK. J Feline Med Surg 2009; 11: 826–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Knottenbelt CM, Day MJ, Cripps PJ, Mackin AJ. Measurement of titres of naturally occurring alloantibodies against feline blood group antigens in the UK. J Small Anim Pract 1999; 40: 365–70. [DOI] [PubMed] [Google Scholar]
- 71. Gurkan M, Arikan S, Ozaytekin E, Dodurka T. Titres of alloantibodies against A and B blood types in non-pedigree domestic cats in Turkey: assessing the transfusion reaction risk. J Feline Med Surg 2005; 7: 301–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Knottenbelt CM. The feline AB blood group system and its importance in transfusion medicine. J Feline Med Surg 2002; 4: 69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Griot-Wenk ME, Callan MB, Casal ML, et al. Blood type AB in the feline AB blood group system. Am J Vet Res 1996; 57: 1438–42. [PubMed] [Google Scholar]
- 74. Casal ML, Jezyk PF, Giger U. Transfer of colostral antibodies from queens to their kittens. Am J Vet Res 1996; 57: 1653–58. [PubMed] [Google Scholar]
- 75. Feldman BF. In-house canine and feline blood typing. J Am Anim Hosp Assoc 1999; 35: 455–56. [DOI] [PubMed] [Google Scholar]
- 76. Barrs VR, Giger U, Wilson B, et al. Erythrocytic pyruvate kinase deficiency and AB blood types in Australian Abyssinian and Somali cats. Aust Vet J 2009; 87: 39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Stieger K, Palos H, Giger U. Comparison of various blood-typing methods for the feline AB blood group system. Am J Vet Res 2005; 66: 1393–99. [DOI] [PubMed] [Google Scholar]
- 78. Gibson GR, Callan MB, Hoffman V, Giger U. Use of a hemoglobin-based oxygen-carrying solution in cats: 72 cases (1998–2000). J Am Vet Med Assoc 2002; 221: 96–102. [DOI] [PubMed] [Google Scholar]
- 79. Weingart C, Kohn B. Clinical use of haemoglobin-based oxygen carrying solution (Oxyglobin (R)) in cats: 48 cases (2002–2006). J Feline Med Surg 2008; 10: 431–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Berent AC, Todd J, Sergeeff J, Powell LL. Carbon monoxide toxicity: a case series. J Vet Emerg Crit Care 2005; 15: 128–35. [Google Scholar]
- 81. Chan DL, Freeman LM, Rozanski EA, Rush JE. Colloid osmotic pressure of parenteral nutrition components and intravenous fluids. J Vet Emerg Crit Care 2001; 11: 269–73. [Google Scholar]
- 82. Adamantos S, Boag A, Hughes D. Clinical use of a haemoglobin-based oxygen-carrying solution in dogs and cats. In Pract 2005; 27: 399–404. [Google Scholar]
- 83. Kerl ME, Langdon PF, Wiedmeyer CE, Branson KR. Evaluation of hematological, chemistry and blood gas values in dogs receiving hemoglobin glutamer-200. J Vet Emerg Crit Care 2007; 17: 37–44. [Google Scholar]
- 84. Knottenbelt G, Mackin A. Blood transfusions in the dog and cat — Part 1. Blood collection techniques. In Pract 1998; 20: 110–14. [Google Scholar]
- 85. Truyen U, Addie D, Belak S, et al. Feline panleukopenia. ABCD guidelines on prevention and management. J Feline Med Surg 2009; 11: 538–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Thiry E, Addie D, Belak S, et al. Feline herpesvirus infection. ABCD guidelines on prevention and management. J Feline Med Surg 2009; 11: 547–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Radford AD, Addie D, Belak S, et al. Feline calicivirus infection. ABCD guidelines on prevention and management. J Feline Med Surg 2009; 11: 556–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Lutz H, Addie D, Belak S, et al. Feline leukaemia. ABCD guidelines on prevention and management. J Feline Med Surg 2009; 11: 565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Hosie MJ, Addie D, Belak S, et al. Feline immunodeficiency. ABCD guidelines on prevention and management. J Feline Med Surg 2009; 11: 575–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Addie D, Belak S, Boucraut-Baralon C, et al. Feline infectious peritonitis. ABCD guidelines on prevention and management. J Feline Med Surg 2009; 11: 594–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Frymus T, Addie D, Belak S, et al. Feline rabies. ABCD guidelines on prevention and management. J Feline Med Surg 2009; 11: 585–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Gruffydd-Jones T, Addie D, Belak S, et al. Chlamydophila felis infection. ABCD guidelines on prevention and management. J Feline Med Surg 2009; 11: 605–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Egberink H, Addie D, Belak S, et al. Bordetella bronchiseptica infection in cats. ABCD guidelines on prevention and management. J Feline Med Surg 2009; 11: 610–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Gary AT, Richmond HL, Tasker S, Hackett TB, Lappin MR. Survival of Mycoplasma haemofelis and ‘Candidatus Mycoplasma haemominutum’ in blood of cats used for transfusions. J Feline Med Surg 2006; 8: 321–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Wardrop KJ, Reine N, Birkenheuer A, et al. Canine and feline blood donor screening for infectious disease. J Vet Intern Med 2005; 19: 135–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Killos MB, Graham LF, Lee J. Comparison of two anesthetic protocols for feline blood donation. Vet Anaesth Analg 2010; 37: 230–39. [DOI] [PubMed] [Google Scholar]
- 97. Tzannes S, Govendir M, Zaki S, Miyake Y, Packiarajah P, Malik R. The use of sevoflurane in a 2:1 mixture of nitrous oxide and oxygen for rapid mask induction of anaesthesia in the cat. J Feline Med Surg 2000; 2: 83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Iazbik MC, Gomez Ochoa P, Westendorf N, Charske J, Couto CG. Effects of blood collection for transfusion on arterial blood pressure, heart rate, and PCV in cats. J Vet Intern Med 2007; 21: 1181–84. [DOI] [PubMed] [Google Scholar]
- 99. Giger U. Feline transfusion medicine. Probl Vet Med 1992; 4: 600–11. [PubMed] [Google Scholar]
- 100. Wardrop KJ. Selection of anticoagulant-preservatives for canine and feline blood storage. Vet Clin North Am Small Anim Pract 1995; 25: 1263–76. [DOI] [PubMed] [Google Scholar]
- 101. Greene CE, ed. Practical considerations of blood transfusion therapy. AAHA 47th Annual Meetings Proceedings, 1980.
- 102. Lucas RL, Lentz KD, Hale AS. Collection and preparation of blood products. Clin Tech Small Anim Pract 2004; 19: 55–62. [DOI] [PubMed] [Google Scholar]
- 103. Logan JC, Callan MB, Drew K, et al. Clinical indications for use of fresh frozen plasma in dogs: 74 dogs (October through December 1999). J Am Vet Med Assoc 2000; 218: 1449–55. [DOI] [PubMed] [Google Scholar]
- 104. Vigano F, Perissinotto L, Bosco VR. Administration of 5% human serum albumin in critically ill small animal patients with hypoalbuminemia: 418 dogs and 170 cats (1994–2008). J Vet Emerg Crit Care (San Antonio) 2010; 20: 237–43. [DOI] [PubMed] [Google Scholar]
- 105. Tocci LJ, Ewing PJ. Increasing patient safety in veterinary transfusion medicine: an overview of pretransfusion testing. J Vet Emerg Crit Care 2009; 19: 66–73. [DOI] [PubMed] [Google Scholar]
- 106. Turnwald GH, Pichler ME. Blood-transfusion in dogs and cats 2. Administration, adverse-effects, and component therapy. Comp Contin Educ Vet 1985; 7: 115–17. [Google Scholar]
- 107. Knottenbelt C, Mackin A. Blood transfusions in the dog and cat — Part 2. Indications and safe administration. In Pract 1998; 20: 191–99. [Google Scholar]
- 108. Corley EA. Intramedullary transfusion in small animals. J Am Vet Med Assoc 1963; 142: 1005–6. [PubMed] [Google Scholar]
- 109. Clark CH, Woodley CH. The absorption of red blood cells after parenteral injection at various sites. Am J Vet Res 1959; 20: 1062–66. [Google Scholar]
- 110. Dula DJ, Muller HA, Donovan JW. Flow rate variance of commonly used IV infusion techniques. J Trauma 1981; 21: 480–82. [PubMed] [Google Scholar]
- 111. Rozanski E, de Laforcade AM. Transfusion medicine in veterinary emergency and critical care medicine. Clin Tech Small Anim Pract 2004; 19: 83–87. [DOI] [PubMed] [Google Scholar]
- 112. Auer L, Bell K. Transfusion reactions in cats due to AB blood group incompatibility. Res Vet Sci 1983; 35: 145–52. [PubMed] [Google Scholar]
- 113. Chiaramonte D. Blood-component therapy: selection, administration and monitoring. Clin Tech Small Anim Pract 2004; 19: 63–67. [DOI] [PubMed] [Google Scholar]
Further reading
- Giger U. Blood-typing and cross matching. In: Bonagura JD, Twedt DC, eds. Current veterinary therapy XIV. Oxford; Elsevier, 2009: 260–65. [Google Scholar]
- Hohenhaus AE. Blood transfusions, component therapy, and oxygen-carrying solutions. In: Ettinger SJ, Feldman EC, eds. Textbook of veterinary internal medicine. 7th edn. Oxford; Saunders, 2010: 537–45. [Google Scholar]
- Bighignoli B, Owens SD, Froenicke L, Lyons LA. Blood types of domestic cats. In: August JR, ed. Consultations in feline internal medicine 6. Saunders, 2010: 628–38. [Google Scholar]
- BSAVA Manual of haematology and transfusion medicine. Gloucester, British Small Animal Veterinary Association. [Google Scholar]