Abstract
Microbial infections affect humans worldwide. Many quaternary ammonium compounds have been synthesized that are not only antibacterial, but also possess antifungal, antiviral and anti-matrix metalloproteinase capabilities. Incorporation of quaternary ammonium moieties into polymers represents one of the most promising strategies for preparation of antimicrobial biomaterials. Various polymerization techniques have been employed to prepare antimicrobial surfaces with quaternary ammonium functionalities; in particular, syntheses involving controlled radical polymerization techniques enable precise control over macromolecular structure, order and functionality. Although recent publications report exciting advances in the biomedical field, some of these technological developments have also been accompanied by potential toxicological and antimicrobial resistance challenges. Recent evidenced-based data on the biomedical applications of antimicrobial quaternary ammonium-containing biomaterials that are based on randomized human clinical trials, the golden standard in contemporary medicinal science, are included in the present review. This should help increase visibility, stimulate debates and spur conversations within a wider scientific community on the implications and plausibility for future developments of quaternary ammonium-based antimicrobial biomaterials.
Keywords: Quaternary ammonium compounds, Antimicrobial surfaces, Toxicological aspects, Antimicrobial resistance
Nomenclature
- AAm
Acrylamide
- AME
Antimicrobial enzyme
- AMP
Antimicrobial peptide
- ATRP
Atom transfer radical polymerization
- BPEA
2-(2-Bromopropionate)-ethyl acrylate
- CCR5
C—C chemokine receptor type 5
- CRP
Controlled radical polymerization
- CTA
Chain transfer agent
- CuAAC
Cu-catalyzed azide–alkyne cycloaddition
- DADMAC
Diallyl-dimethylammonium chloride
- DBCO
Dibenzocyclooctynes
- DCAMA
Dipicolyl aminoethyl methacrylate
- DDMAI
2-Dimethyl-2-dodecyl-1-methacryl- oxyethyl ammonium iodine
- DEPN
N-tert-butyl-N-[1-diethylphosphono- (2,2-dimethylpropyl)]
- DMADDM
Dimethylaminododecyl methacrylate
- DMAEDM
Dimethylammoniumethyl dimethacrylate
- DMAEMA
2-Dimethylamino ethyl methacrylate
- DMAE-CB
Methacryloxylethyl cetyl ammonium chloride
- DMAHDM
Dimethylaminohexadecyl methacrylate
- DODAB
Dioctadecyldimethyl-ammonium bromide
- EA
Ethyl acrylate
- EDAX
Energy dispersive X-ray
- EPSiQA
Epoxy silicone quaternary ammonium salt
- EPS
Exopolysaccharides
- FAc
Fluorinated acrylate-heptadecafluorodecyl acrylate
- FE-SEM
Field emission scanning electron microscopy
- FRP
Free radical polymerization
- GIC
Glass ionomer cement
- gtf
Glucosyltransferase
- GTAC
Glycidyltrialkylammoniumchloride
- GTMAC
Glycidyltrimethylammonium chloride
- GTEACl
Glycidyltriehtylammonium chloride
- HACC
Hydroxypropyltrimethyl ammonium chloride chitosan
- HDPC
Human dental pulp cell
- HEMA
2-Hydroxyethyl methacrylate
- HGF
Human gingival fibroblast
- HSV
Herpes simplex virus
- IC50
Half maximal inhibitory concentration
- IDMA
Ionic dimethacrylate
- IMQ
N,N-bis[2-(3-(Methacry-loyloxy) propanamido)-ethyl]-N-methylhexa- decyl ammonium bromide
- LCST
Lower critical solution temperature
- LC50
Lethal concentration that kills 50% of a sample
- MAE-DB
2-Methacryloxylethyl dodecyl methyl ammonium bromide
- MBC
Minimum bactericidal concentration
- MDPB
12-Methacryloyloxy dodecyl pyridinium bromide
- MFC
Minimum fungicidal concentrations
- MIC
Minimum inhibitory concentration
- MMA
Methyl methacrylate
- MMP
Matrix metalloproteinase
- Monomer II
N-Benzyl-11-(methacryloyloxy)-N,N- dimethylundecan-1-aminium fluoride
- MRSA
Methicillin-resistant Staphylococcus aureus
- MRSE
Methicillin-resistant Staphylococcus epidermidis
- MTT
3-(4,5-Dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide
- MUPB
Methacryloyloxyundecylpyridinium bromide
- NACP
Nanoparticles of amorphous calcium phosphate
- NIPAAm
N-Isopropylacrylamide
- NM
Not mentioned
- NMR
Nuclear magnetic resonance
- NMP
Nitroxide-mediated polymerization
- NO
Nitric oxide
- NP
Nanoparticle
- Nrf2
Nuclear factor E2-related factor 2
- P(AA-co-Ada)
Poly(acrylic acid-co-1-adamantan- 1-ylmethyl acrylate)
- PAMAM
Poly amidoamine
- PAH
Poly(allylamine hydrochloride)
- PB
Poly(1-butene)
- PCL-PJDMA
Poly caprolactone-poly quaternary ammonium salt
- pDADMAC-PU
Poly diallyl-dimethylammonium chloride-polyurethane
- PDMS
Poly(dimethylsiloxane)
- PEG
Polyethylene glycol
- PMMA
Poly(methyl methacrylate)
- PTEA
2-Phenyl-2-(2,2,6,6-tetramethyl- piperidin-1-yloxy)ethyl acrylate
- PU
Polyurethane
- QA
Quaternary ammonium
- QAB
Quaternary ammonium bromide
- QAC
Quaternary ammonium compound
- QADM
Quaternary ammonium dimethacrylate
- QADMA
Quaternary amine dimethacrylate
- QADMAI- 12
N,N-bis[2-(3-(Methacryloyloxy)- propanamido)ethyl]-N-methyldodecyl ammonium iodide
- QADMAI-16
N,N-bis[2-(3-(Methacryloyloxy)- propanamido)ethyl]-N-methylhexadecyl-ammonium iodide
- QADMAI-18
N,N-bis[2-(3-(methacryloyloxy)- propanamido)ethyl]-N-methyloctadectyl ammonium iodide
- QAES
Quaternary ammonium epoxy silicate
- QAMA
Quaternized ethylene glycol dimethacrylate piperazine octyl ammonium iodide
- QASM
Quaternary ammonium salt containing monomers
- QCS
Quaternary ammonium chitosan derivative
- QPEI
Quaternary ammonium polyethylenimine
- QPGMA
Quaternized poly glycidyl methacrylate
- RAFT
Reversible addition fragmentation chain transfer
- ROS
Reactive oxygen species
- RCT
Randomized controlled trial
- ROP
Ring opening polymerization
- SEM
Scanning electron microscopy
- SIP
Surface-initiated polymerization
- SNP
Single nucleotide polymorphism
- SPAAC
Strain-promoted cycloaddition of alkynes and azide
- SPH
5,5-Dimethyl-3-(3′-triethoxysilyl- propyl)hydantoin
- SPMA
3-sulfopropylmethacrylate
- SPODA
3-(Trimethoxysilylpropyl) octadecyl dimethyl ammonium chloride
- TBAEMA
2-tert-Butylamino ethyl methacrylate
- TEM
Transmission electron microscopy
- Ti
Titanium
- TGA
Thermogravimetric analysis
- TMSPMA
Poly(3-(trimethoxysilyl)propyl methacrylate)
- TPGDA
Tripropylene glycol diacrylate
- XPS
X-ray photoelectron spectroscopy
- XRD
X-ray diffraction
- Zn
Zinc
- 2AE
[2-(Acryloyloxy)ethyl]trimethyl- ammonium chloride
- 4VP
4-Vinylpyridine
- 8QA
8-Quinolinyl acrylate
1. Introduction
Microbial infection is a major challenge to human health worldwide. Pathogenic microorganisms, including bacteria, viruses and fungi, are of special concern in hospitals and other health care settings, and adversely affect the optimal functioning of medical devices, drugs, surgical equipment, dental restorations and bone cements [1]. According to a systematic analysis, infectious diseases result in 9.2 million deaths in 2013 alone (about 17% of all deaths), most of which are associated with biofilm formation [2], [3]. The discovery that microorganisms cause infectious diseases resulted in development of antibiotics, disinfectants and antiseptics against those microbial pathogens [4], [5]. However, the widespread and injudicious use of antibiotics and disinfectants has resulted in the emergence of antibiotic-resistant bacterial strains that are becoming a serious threat to human health [6]. There has been a constant race between researchers developing new antimicrobial agents and bacteria acquiring resistance to those agents. According to the U.S. Center for Disease Control and Prevention, more than two million people are infected with antibiotic-resistant bacteria and at least 23,000 patients die annually from those infections [7]. The World Health Organization 2014 report on global surveillance of antimicrobial resistance surmised that the world is heading towards a post-antibiotic era in which what used to be common, treatable infections are rapidly becoming life-threatening diseases. The organization has appealed to researchers worldwide to prioritize their efforts toward concerted efforts in combating the spread of antimicrobial-resistant microorganisms [8]. Over the past decade, new strains of infectious pathogens, such as severe acute respiratory syndrome, antibiotic-resistant tuberculosis, avian influenza A (e.g. H5N1, H7N9), the Ebola and Zika viruses have emerged. These new life-threatening pathogens have the tendency to spread globally instead of being confined to their niches of origin. Pragmatically, there is increasing need for exploring more efficient, broad-spectrum and long-lasting antimicrobial agents for biomedical applications.
To circumvent the uneven release kinetics and reservoir exhaustion issues of release-based antimicrobial biomaterials, contact-killing surfaces incorporating immobilized bactericides have been developed in which monomeric antimicrobial agents are covalently attached to the polymer backbone [9]. Compared with release-based biomaterials, the contact-killing approach have distinct advantages, in that they: (1) improve and prolong antimicrobial activities; (2) possess non-toxic and non-irritant properties without affecting the interaction with host tissues or modifying the host immune responses; (3); exert no adverse effects on the physical and mechanical properties of the loading materials; (4) are different from antibiotics in that their mode of action involves physically puncturing and destroying bacterial cell walls and membranes as well as viral envelopes [10]; and (5) are unlikely to develop antibiotic resistance [11]. The most commonly used antimicrobial compounds include quaternary ammonium compounds (QACs), chitosan, silver nanoparticles (AgNPs), antimicrobial peptides (AMPs) and antimicrobial enzymes (AMEs) (Table 1 ).
Table 1.
Major antibacterial materials and their mechanisms of action.
| Material type | Representative compounds | Mechanisms of action | Refs. |
|---|---|---|---|
| Antibiotics | Aminoglycosides (e.g. gentamicin, tobramycin) | Bind to the bacterial 30S ribosomal subunit and inhibit protein synthesis | [12] |
| Glycopeptides (e.g. vancomycin) | Bind to amino acids and disrupt cell wall peptidoglycan synthesis | ||
| Penicillins (e.g. ampicillin) | Inhibit related enzymes and disrupt cell wall peptidoglycan synthesis | ||
| Quinolones (e.g. ciproflaxin, norfloxacin) | Inhibit DNA replication and transcription, targeting DNA topoisomerases II and IV | ||
| Rifamycins (e.g. rifampin) | Bind to RNA polymerase and inhibit transcription | ||
| Tetracyclines (e.g. minocycline, tetracycline) | Inhibit protein synthesis | ||
| Antimicrobial enzymes (AMEs) | Lysozyme | Catalyze glycosidic bond hydrolysis in bacterial cell wall peptidoglycans | [13] |
| Acylase | Quorum-quenching | ||
| Antimicrobial peptides (AMPs) | Natural AMPs (e.g. human β-defensin 1–3, magainin and nisin) | Transmembrane pore formation, intracellular targeting and metabolic inhibition mechanisms (inhibition of microbial functional proteins, DNA and RNA synthesis) | [14], [15] |
| Synthetic AMPs (e.g. β-17, human neutrophil peptides 1 and 2, histatins 5 and 8) | |||
| Cationic compounds | Chitosan | Interaction between positively charged chitosan molecules and negatively charged bacterial cell membranes leads to disruption of cell membrane | [16] |
| Chlorhexidine | Bind to negatively charged bacterial walls and disrupt cell walls | ||
| Poly(ε-lysine) | Electrostatic adsorption onto bacterial cell membranes and stripping of the outer membrane lead to cell death | ||
| Quaternary ammonium compounds (QACs) | Disruption of bacterial enzymes and cell membranes by positively charged polymers | ||
| Metal and metal oxides | AgNPs | Induce oxidative stresses, deactivate bacterial enzymes by binding to thiol groups and affect the function and permeability of the cell membranes | [17], [18] |
| CuNPs | Contribute to ROS formation and induce lipid peroxidation in bacterial membranes | ||
| TiO2NPs | Photocatalytically activate the production of ROS and interfere with phosphorylation, thereby causing oxidative cell death | ||
| ZnONPs | Generate ROS and binds to lipids and proteins, thus changing the osmotic balance and increasing membrane permeability | ||
| Other non-cationic compounds | Nitric oxide (NO) donors | Induce cellular nitrosative and oxidative stresses and act as a bacterial signaling disruptor | [19], [20] |
| Triclosan | Deactivate bacterial fatty acid biosynthesis through inhibition of the enoylacyl carrier protein reductase enzyme | [21], [22] |
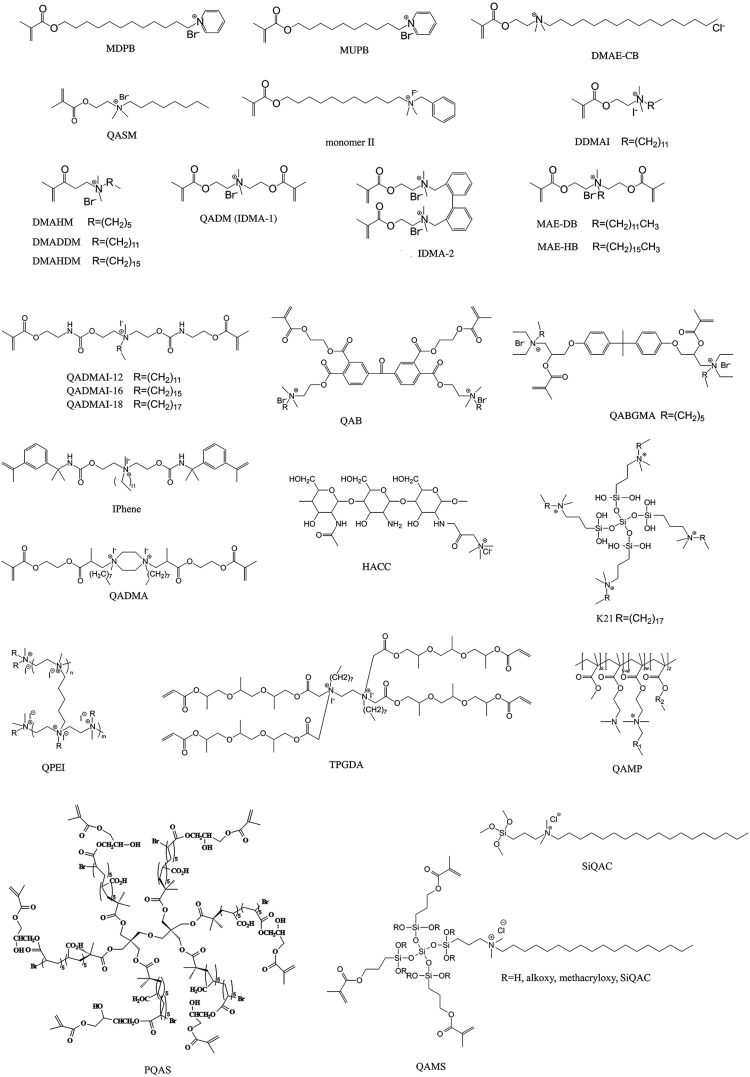
The use of QACs as antiseptics and disinfectants dated back to the 1930s [23]. The QACs employed in that era provided the first line of defense against pathogenic bacteria [24]. Structurally, QACs are composed of nitrogen (N+)-containing compounds in which the N atom is attached to four different groups by covalent bonds. The representative formula is N+R1R2R3R4X−, where R may be a hydrogen atom, a plain alkyl group or an alkyl group substituted with other functionalities, and X represents an anion, which is most often a halide anion. Most QAC salts are composed predominantly of chloride or bromide salts, while iodide salts tend to exhibit decreased solubility. Synthetic polymers with quaternary ammonium (QA) functionalities are produced using two general approaches: (1) quaternization of reactive precursor polymers (post-polymerization). This method generally generates products with variable degrees of cationization as a result of the unpredictable impact of steric hindrance from neighboring groups, and (2) direct copolymerization of monomers containing QA functional groups, coined as QA monomers, within the polymer network. These methods generate polymers with 100% functionality but with difficult molecular characterization (Fig. 1 ).
Fig. 1.
Schematic illustration of the use of quaternary ammonium compounds in antimicrobial biomedical materials.
There are already excellent reviews that summarize the synthesis of antimicrobial polymers and their chemical structures [16], [25], [26], [27], [28]. Hence, the present review is targeted toward providing an overview of the state-of-the-art of QACs and their antimicrobial applications in the biomedical field (Fig. 2 ), and describing the currently-accepted mechanisms on how antibacterial, antifungal, antiviral and the recently discovered anti-matrix metalloproteinase (MMP) activities of QACs are achieved. The use of controlled radical polymerization (CRP) techniques, in particular, has provided a major thrust in the synthesis of novel QA-based antimicrobial surfaces. Because of increasing concerns over the biocompatibility of QACs, a critique on the toxicological aspects of QACs is also included. Similar to antimicrobial peptides, QACs are potential candidates for combating antibiotic resistance. Thus, the potential impact of QACs on the emergence of antibiotic resistance is also discussed. Apart from reviewing bench-top work and animal studies, recent evidenced-based data on the biomedical applications of antimicrobial QA-containing copolymers based on randomized human clinical trials will also be highlighted in the present review.
Fig. 2.
Chemical structures of representative quaternary ammonium compounds in antimicrobial biomedical materials.
2. Bioactive functions of QACs
2.1. Antibacterial activities
Quaternary ammonium compounds are cationic surfactants and antimicrobials with a broad spectrum of activities [29] (Table 2 ). Although QACs are lethal to a wide variety of organisms, including vegetative cells of gram-positive and gram-negative bacteria, fungi, parasites (e.g. Leishmania major, Plasmodia falciparum), and lipophilic (enveloped) viruses [30], [31], [32], they are generally not considered sporicidal (e.g. Bacillus subtilis, Clostridium sporogenes), tuberculocidal (e.g. Mycobacterium tuberculosis, Mycobacterium bovis) or virucidal against hydrophilic (non-enveloped) viruses (e.g. Coxsackievirus, Rhinovirus) [27]. Although the exact antimicrobial mechanism of QACs has not been fully elucidated, it is generally believed that the predominant mode of action is disruption of the cell membrane [33]. The antimicrobial effect of QACs has been attributed to a multitude of factors [16]. The molecular weight of QACs has a profound impact on the efficacy of many QAC-based antimicrobial systems [34], [35]. The length of the N-alkyl chain affects the antimicrobial activities of QACs [36], [37], [38]. For different bacteria and fungi, the optimum chain length of QACs is different (14 carbons for gram-positive bacteria, 16 carbons for gram-negative bacteria and 12 carbons for yeast and filamentous fungi) [39], [40]. Counter anion has a profound effect on the efficiency and selectivity toward different microbes due to discrepancies in polymer morphology, binding affinity toward quaternary compounds and the solubility of polycations in water, resulting in variable degrees of antimicrobial performance [41], [42], [43], [44]. Some researchers found that the antimicrobial activity of QA dendrimers is dependent on the counter anion and biocides with bromide anions are more potent than those with chloride anions [35]. Conversely, other studies reported that counter anion species have no effect on antibacterial activities [45], [46]. In addition, molecular charge density also affects the antimicrobial actions of QACs [47], [48], [49]. For example, a threshold of immobilized surface QA groups is required to cause significant reduction in viable bacteria [49].
Table 2.
Antimicrobial activities of representative QACs against various pathogenic microorganisms and their cytocompatibility.
| Microorganism | QACs | Antimicrobial activity | Related niche | Cytotoxicity |
|---|---|---|---|---|
| A. israelii | MDPB | MIC 12.5 μg/mL [50] MBC 25.0 μg/mL [50] |
A/C/E | 0–40 μg/mL for 48 h. No cytotoxicity to HDPCs [51] 0–50 μg/mL for 3d. No cytotoxicity to mouse odontoblast-like MDPC-23 cells [52] IC50 62.5–75 μM to mouse osteoblast-like MC3T3-E1 cells [53] |
| A. gerencseriae | MDPB | MIC 3.13 μg/mL [50] MBC 6.25 μg/mL [50] |
A/E | |
| A. naeslundii | MDPB | MIC 3.13–25.0 μg/mL [50] MBC 6.25–50.0 μg/mL [50] |
A/C/E | |
| QAMP | MIC 20 μg/mL [54], [55] MBC 20 μg/mL [54], [55] |
Not assessed | ||
| A. odontolyticus | MDPB | MIC 6.25 μg/mL [50] MBC 12.5 μg/mL [50] |
A/C/E | |
| A. viscosus | DMADDM | MIC 4.9 μg/mL [56] MBC 9.8 μg/mL [56] |
A/C/E | LC50 20–40 μg/mL to HGFs [56] |
| DMAEDM | MIC 20,000 μg/mL [56] MBC 40,000 μg/mL [56] |
LC50 20–40 μg/mL to HGFs [56] | ||
| DMAE-CB | MIC 12.2 μg/mL [57] MBC 24.4 μg/mL [57] MIC 4.8 μg/mL[58] MBC 9.6 μg/mL [58] |
0–2 μg/mL for 24 h. No cytotoxicity to L929 mouse fibroblasts [59] 0–10 μM for 24 h. No cytotoxicity to HDPCs [60] IC50 25–35 μM to L929 mouse fibroblasts [61] |
||
| MAE-DB | MIC 6.1 μg/mL [57] MBC 12.2 μg/mL [57] |
LC50 10000–20000 μg/mL to HGFs [57] | ||
| MAE-HB | MIC 24.4 μg/mL [57] MBC 48.8 μg/mL [57] |
LC50 10000–20000 μg/mL to HGFs [57] | ||
| B. bifidum | MDPB | MIC 31.3 μg/mL [62] MBC 62.5 μg/mL [62] |
A/E | |
| B. subtilis | EPSiQA | MIC 2.5 μg/mL [63] | B/W | Not assessed |
| C. albicans | MDPB | MIC 3.13–12.5 μg/mL [50] MBC 3.13–12.5 μg/mL [50] |
A/B/E/W | |
| MUPB | MIC 630 μg/mL [64] MBC 5830 μg/mL [64] |
IC50 50 μg/mL to L929 mouse fibroblasts [64] | ||
| C. dubliniensis | MUPB | MIC 830 μg/mL [64] MBC 6670 μg/mL [64] |
A/E | |
| C. glabrata | MUPB | MIC 1040 μg/mL [64] MBC 5210 μg/mL [64] |
A/E | |
| Clinical isolute S. aureus | HACC | MIC <2.5 mg/mL [65] | B/W | 2500 μg/mL 6% or 18% substitution HACC for 48 h. No cytotoxicity to L929 mouse fibroblasts [65] |
| Clinical isolute S. epidermidis | HACC | MIC <2.5 mg/mL [65] | B/W | |
| E. alactolyticum | MDPB | MIC 31.3 μg/mL [62] MBC 125.0 μg/mL [62] |
A/E | |
| E. coli | EPSiQA | MIC 5.0 μg/mL [63] | A/B/C/E/W | Not assessed |
| E. faecalis | DMADDM | MIC 9.8 μg/mL [56] MBC 19.5 μg/mL [56] |
A/E | |
| DMAEDM | MIC 80,000 μg/mL [56] MBC 160,000 μg/mL [56] |
|||
| DMAE-CB | MIC 12.2 μg/mL [57] MBC 24.4 μg/mL [57] |
|||
| MAE-DB | MIC 12.2 μg/mL [57] MBC 24.4 μg/mL [57] |
|||
| MAE-HB | MIC 24.4 μg/mL [57] MBC 48.8 μg/mL [57] |
|||
| L. acidophilus | DMADDM | MIC 9.8 μg/mL [56] MBC 19.5 μg/mL [56] |
A/C/E | |
| DMAEDM | MIC 40,000 μg/mL [56] MBC 80,000 μg/mL [56] |
|||
| DMAE-CB | MIC 3.1 μg/mL [57] MBC 6.2 μg/mL [57] |
|||
| MAE-DB | MIC 6.1 μg/mL [57] MBC 12.2 μg/mL [57] |
|||
| MAE-HB | MIC 3.1 μg/mL [57] MBC 6.2 μg/mL [57] |
|||
| MDPB | MIC 15.6 μg/mL [62] MBC 62.5 μg/mL [62] |
|||
| L. brevis | MDPB | MIC 15.6 μg/mL [62] MBC 31.3 μg/mL [62] |
A/C/E | |
| L. casei | DMAE-CB | MIC 2.4 μg/mL [58] MBC 9.6 μg/mL [58] |
A/C/E | |
| MUPB | MIC 630 μg/mL [64] MBC 4580 μg/mL [64] |
|||
| QAMP | MIC 10 μg/mL [54], [55] MBC 20 μg/mL [54], [55] |
Not assessed | ||
| L. fermenti | MDPB | MIC 15.6 μg/mL [62] MBC 15.6 μg/mL [62] |
A/C/E | |
| L. paracasei | MDPB | MIC 15.6 μg/mL [62] MBC 62.5 μg/mL [62] |
A/C/E | |
| L. plantarum | MDPB | MIC 7.8 μg/mL [62] MBC 15.6 μg/mL [62] |
A/C/E | |
| Lactobacillus salivarius ssp. salivarius | MDPB | MIC 7.8 μg/mL [62] MBC 62.5 μg/mL [62] |
A/C/E | |
| Lactobacillus salivalius ssp. salicinius | MDPB | MIC 15.6 μg/mL [62] MBC 125 μg/mL [62] |
A/C/E | |
| Lactobacillus ssp . | MDPB | MIC 3.13-6.25 μg/mL [50] MBC 3.13-6.25 μg/mL [50] |
A/C/E | |
| methicillin-resistant S. aureus | HACC | MIC <2.5 mg/mL [65] | B/W | |
| P. acnes | MDPB | MIC 3.9 μg/mL [62] MBC 62.5 μg/mL [62] |
A/B/E/W | |
| P. asaccharolyticus | MDPB | MIC 31.3 μg/mL [62] MBC 31.3 μg/mL [62] |
A/E | |
| P. gingivalis | DMADDM | MIC 2.4 μg/mL [56] MBC 4.9 μg/mL [56] |
A/E | |
| DMAEDM | MIC 20,000 μg/mL [56] MBC 40,000 μg/mL [56] |
|||
| DMAE-CB | MIC 3.1 μg/mL [57] MBC 6.2 μg/mL [57] |
|||
| MAE-DB | MIC 6.1 μg/mL [57] MBC 12.2 μg/mL [57] |
|||
| MAE-HB | MIC 3.1 μg/mL [57] MBC 6.2 μg/mL [57] |
|||
| P. melaninogenica | DMADDM | MIC 2.4 μg/mL [56] MBC 4.9 μg/mL [56] |
A/E | |
| DMAEDM | MIC 20,000 μg/mL [56] MBC 40,000 μg/mL [56] |
|||
| DMAE-CB | MIC 6.1 μg/mL [57] MBC 12.2 μg/mL [57] |
|||
| MAE-DB | MIC 6.1 μg/mL [57] MBC 12.2 μg/mL [57] |
|||
| MAE-HB | MIC 6.1 μg/mL [57] MBC 12.2 μg/mL [57] |
|||
| S. aureus | DMADDM | MIC 4.9 μg/mL [56] MBC 9.8 μg/mL [56] |
B/W | |
| DMAEDM | MIC 40,000 μg/mL [56] MBC 80,000 μg/mL [56] |
|||
| DMAE-CB | MIC 12.2 μg/mL [57] MBC 24.4 μg/mL [57] MIC 1.2 μg/mL [58] MBC 2.4 μg/mL [58] |
|||
| EPSiQA | MIC 2.5 μg/mL [63] | Not assessed | ||
| MAE-DB | MIC 12.2 μg/mL [57] MBC 24.4 μg/mL [57] |
|||
| MAE-HB | MIC 24.4 μg/mL [57] MBC 48.8 μg/mL [57] |
|||
| MUPB | MIC 1750 μg/mL [64] MBC 5830 μg/mL [64] |
|||
| S. gordonii | MDPB | MIC 16.7 μg/mL [66] MBC 31.3 μg/mL [51] |
A/C/E | |
| S. mitis | MDPB | MIC 25.0 μg/mL [66] MBC 31.3 μg/mL [51] |
A/C/E | |
| S. mutans | DMADDM | MIC 4.9 μg/mL [56] MBC 9.8 μg/mL [56] MIC 6.0 μg/mL [67] MBC 12.0 μg/mL [67] MIC 4.9 μg/mL [68] MBC 9.7 μg/mL [68] |
A/C/E | |
| DMAEDM | MIC 20,000 μg/mL [56] MBC 40,000 μg/mL [56] |
|||
| DMAE-CB | MIC 3.1 μg/mL [57] MBC 6.2 μg/mL [57] MIC 2.4 μg/mL [58] MBC 4.8 μg/mL [58] |
|||
| DMAHDM | MIC 0.6 μg/mL [68] MBC 1.2 μg/mL [68] |
Not assessed | ||
| DDMAI | MIC 6.25 μg/mL [69] | Not assessed | ||
| MAE-DB | MIC 6.1 μg/mL [57] MBC 12.2 μg/mL [57] |
|||
| MAE-HB | MIC 6.1 μg/mL [57] MBC 12.2 μg/mL [57] |
|||
| MDPB | MIC 12.5 μg/mL [50] MBC 50.0 μg/mL [50] MIC 12.5 μg/mL [66] MBC 62.5 μg/mL [51] |
|||
| MUPB | MIC 1040 μg/mL [64] MBC 4170 μg/mL [64] |
|||
| PQAS | MIC 1.563 μg/mL [44] | Not assessed | ||
| QAB | MIC 25 μg/mL [70] | Not assessed | ||
| QADM | MIC 13,000 μg/mL [67] MBC 25,000 μg/mL [67] |
Not assessed | ||
| QAMP | MIC 20 μg/mL [54], [55] MBC 20 μg/mL [54], [55] |
Not assessed | ||
| S. oralis | MDPB | MIC 12.5 μg/mL [50] MBC 25.0 μg/mL [50] MIC 16.7 μg/mL [66] MBC 31.3 μg/mL [51] |
A/C/E | |
| S. salivarius | MDPB | MIC 6.25–12.5 μg/mL [50] MBC 12.5–50.0 μg/mL [50] MIC 15.6 μg/mL [66] MBC 31.3 μg/mL [51] |
C | |
| S. sanguis | DMADDM | MIC 1.2 μg/mL [56] MBC 2.4 μg/mL [56] |
C | |
| DMAEDM | MIC 20,000 μg/mL [56] MBC 40,000 μg/mL [56] |
|||
| DMAE-CB | MIC 6.1 μg/mL [57] MBC 12.2 μg/mL [57] |
|||
| MAE-DB | MIC 12.2 μg/mL [57] MBC 24.4 μg/mL [57] |
|||
| MAE-HB | MIC 6.1 μg/mL [57] MBC 12.2 μg/mL [57] |
|||
| MDPB | MIC 16.7 μg/mL [66] MBC 31.3 μg/mL [51] |
|||
| S. sobrinus | MDPB | MIC 7.8 μg/mL [66] MBC 62.5 μg/mL [51] |
A/C/E |
Related niche. A: apical periodontitis; B: bone infections; C: caries; E: endodontic infections; W: wound infections.
2.2. Antifungal activities
Fungi have a single membrane surrounded by a thick cell wall composed of glucan and chitin, similar to the cell envelope of gram-positive bacteria [71]. Previous studies attributed the antifungal mechanism of QACs to electrostatic interaction with the fungi cell membrane that results in cell lysis [72], [73], [74]. More recent work on QACs reported a different antifungal mechanism. In-situ hybridization with fluorescent oligonucleotide probes shows that fungi express hyphae on untreated prostheses but not on QAC-coated prostheses. This observation suggests that the antifungal activities of QACs may involve impediment of hyphae formation [75]. The micelle-forming cationic detergent cetyltrimethylammonium bromide (CTAB) does not cause disruption of the fungal cell membrane [76]. Instead, its antifungal activity may be associated with the reversal of cell surface charges from negative to positive, as determined from measurements of the cell electrophoretic mobility. The antifungal activities of QACs are largely dependent on molecular structures [77]. To cause fungus death, the cationic QA moiety has to be adsorbed onto the cell, alter the inherent charge of the cell wall and penetrate the latter to reach the fungal cell membrane. The antifungal activity of the bilayer-forming, cationic synthetic lipid dioctadecyldimethyl-ammonium bromide (DODAB) has been reported to be not as strong as CTAB. This phenomenon may be attributed to cell aggregation as a function of cell concentration [78]. Fungal cells that are inside cell aggregates cannot be reached by DODAB and those cells remain viable. Conversely, the aggregated cells are apparently defenseless in the presence of CTAB. These results suggest that CTAB molecules penetrate Candida albicans aggregates more effectively to exert their antimicrobial activity. In addition, the rigid gel state of the DODAB molecule may hamper its penetration into the fungal cell wall and cytoplasmic membrane [79].
The mode of action of gemini QACs appears to involve lysis of the cell membrane and organelles without fungal cell wall destruction or protein leakage. Gemini QACs containing two pyridinium residues [3,3′-(2,7-dioxaoctane)bis(1-decylpyridinium bromide)] per molecule. These residues possess fungicidal activity against Saccharomyces cerevisiae by causing respiration inhibition and cytoplasmic leakage of adenosine triphosphate, magnesium, and potassium ions [80]. Gemini QACs exhibit more effective antifungal activity in comparison with mono-QACs N-cetylpyridinium chloride [81]. In addition, the activity of the gemini surfactant against S. cerevisiae and C. albicans results in elevated levels of reactive oxygen species (ROS) under aerobic conditions. The potent antifungal activity of d-glucosamine QA derivatives against Coriolus versicolor and Poria placenta may be explained by their capacity to recognizedifferent kinds of enzymes released during fungal growth [82]. Apart from their effects on enzymes, d-glucosamine QA derivatives may also form complexes with vital metallic elements in the fungi to block or decrease fungal growth.
2.3. Antiviral activities
The virucidal capacity of Zephiran (alkyl-dimethylbenzylammonium chloride) against various types of viruses was summarized by Klein and Deforet as early as 1983 [27]. Zephiran effectively inactivates lipid-containing (enveloped) viruses such as vaccinia virus, and some non-lipid-containing (non-enveloped) viruses such as reovirus, and bacteriophages. However, it is ineffective against smaller non-lipid-containing viruses such as picornaviruses. In some studies, QACs were found to possess antiviral properties against enveloped Herpes simplex virus (HSV)-1 [83], [84]. The virucidal mechanism of QACs for lipophilic enveloped viruses appears to involve disruption or detachment of the viral envelope with subsequent release of the nucleocapsid. Disruption of the viral envelope may be attributed to the higher affinity of enveloped viruses for QAC through hydrophobic interactions. However, further disruption of the released nucleocapsids by QACs was not observed. The differential virucidal activities of QACs on enveloped or non-enveloped viruses were also investigated in another study [10]; the QACs tested were virucidal against enveloped Influenza A (H1N1) virus, but were ineffective against non-enveloped Poliovirus Sabin1. Several QA camphor derivatives have been evaluated against a broad range of influenza viruses [85], [86], [87], [88]. These compounds interfered with the viral fusion process and effectively inhibited influenza virus replication in cell cultures. Shiraishi et al. screened the Takeda chemical library for novel anti-HIV-1 agents and identified an anilide derivative with a QA moiety as a potent and selective small-molecule (C—C chemokine receptor type 5) CCR5 antagonist for inhibition of macrophage-tropic HIV-1 replication [89]. Although the exact mechanism has not been fully elucidated, the QA moiety was found to enhance CCR5 antagonistic activity.
The antiviral activities of QACs against enveloped viruses have gained widespread recognition. However, whether QACs are effective against non-enveloped viruses remains controversial. QACs have repeatedly been reported as effective against some specific non-enveloped viruses. In studies evaluating the virucidal activity of disinfectants against various viruses, QACs are virucidal against feline viral rhinotracheitis virus and feline herpesvirus, but ineffective against feline calicivirus, feline panleukopenia virus (a parvovirus), feline parvovirus, canine parvovirus, and foot-and-mouth disease virus [90], [91], [92], [93], [94]. The lethal activity of QACs on enveloped viruses is linked to detachment of the viral envelope. In non-enveloped viruses, QACs induce formation of nonstructural substances such as micelles but are not lethal to the viruses [95]. A meta-analysis on virus inactivation by chemical disinfectants indicates that QACs are relatively ineffective against human Norovirus [96]. Recently, a topical QA silane prepared by sol-gel reaction of an antimicrobial trialkoxysilane with an anchoring tetra-alkoxysilane (codenamed “K-21”) has been reported to inhibit the replication of enveloped and non-enveloped DNA and RNA viruses at non-toxic concentrations, including HSV-1, Human Herpesvirus-6 and Human Herpesvirus-7. These viruses have the capability to establish life-long latency in humans and can be reactivated later in life [97]. Because reactivation of the immunosuppressive and neurotrophic Human Herpesvirus-6 in human brain tissues can cause cognitive dysfunction, permanent disability or death, and may play a role in a subset of patients with chronic neurological conditions such as multiple sclerosis, mesial temporal lobe epilepsy, status epilepticus and chronic fatigue syndrome, there is an urgent need for more studies on the capability for the K-21 agent to inhibit the replication of Human Herpesvirus-6 in vivo.
2.4. Anti-MMP activities
Host-derived MMPs and cysteine cathepsins play a major role, among other factors, in compromising the durability of resin-dentin bonds in tooth-colored filling materials via enzymatic hydrolysis of the collagen matrix [98], [99]. During dentin formation, MMPs, a group of zinc- and calcium-dependent host-derived proteases, are highly active in enzymatic hydrolysis of the collagen matrix [100]. Inactive MMPs can be activated by mild acids during the dentin caries process [101]. Single nucleotide polymorphisms of MMP2 and MMP3 gene are involved in dental caries susceptibility [102], [103]. Dental adhesives containing 5 wt% 12-methacryloyloxy dodecyl pyridinium bromide (MDPB) exhibited 89% inhibition of soluble recombinant human MMP-9 and 90% inhibition of matrix-bound endogenous MMPs [104]. The inhibitory effect of MDPB on MMPs may contribute to the improved durability achieved by MDPB-containing adhesives. Since MDPB can be copolymerized with methacrylate resin comonomers in the adhesive-tooth interface, such MMP-inhibitors may keep inhibiting MMPs over time. The inhibitory effect of QA methacrylates on endogenous dentin MMPs has been attributed to prevention of the release of collagen degradation products, including cross-linked carboxyterminal telopeptide of type I collagen (ICTP) by MMPs and C-terminal crosslinked telopeptide of type I collagen fragments (CTX) by cathepsin K [105]. Although the exact mechanisms of the anti-MMP effects of QACs have not been elucidated, it has been hypothesized that cationic QACs can electrostatically act on the catalytic site of MMPs, thereby inhibiting MMP-catalyzed hydrolysis of the collagen matrix [106], [107].
Matrix metelloproteinases also play important roles in pulp tissue and periapical inflammation [108]. Bacterial lipopolysaccharide (LPS)-induced release of pro-inflammatory mediators elevate levels of MMP-1, -2 and -3 in acute pulpitis [109], [110]. Release of MMP-3 can activate other MMP members, mediate collagen degradation in the extracellular matrix, induce inflammation and promote angiogenesis [111]. MMP-13 is the major collagenase in dental pulp tissues and is effective in cleaving type II collagen. In periodontitis, MMP family members have been confirmed to be associated with severe tissue disorganization [112], [113], [114]. A recent clinical investigation identified higher levels of MMP-1, -2 and -9 in the periapical interstitial fluid of patients with apical periodontitis [115]. Hence, in addition to their antimicrobial properties, incorporation of QACs into sealers for root canal treatment has the potential to inhibit MMPs directly for controlling the severity of periapical inflammation.
3. QA-based antimicrobial surfaces
3.1. “Grafting onto” vs “grafting from” approaches
Tethering of functional polymers with QA functionalities is an effective means to impart antimicrobial properties to biomedical materials. Such antimicrobial surfaces are generally prepared via two major immobilization methods, the “grafting onto” and “grafting from” approaches [116] (Fig. 3 ).
Fig. 3.
Immobilization methods to produce antimicrobial surfaces by covalent attachment. [116], Copyright 2009.
Reproduced with permission from American Chemical Society.
In the “grafting onto” approach, polymer molecules from solution are directly immobilized on suitable surfaces. Although experimentally simple, such an approach suffers from the drawbacks of relatively low grafting density as a result of steric hindrance from the already-adsorbed/attached polymer chains. These polymers form mushroom-like structures and render potential reactive sites inaccessible, further limiting the grafting density [117]. Thus, only a small amount of the polymers can be immobilized onto the surface using the “grafting onto” approach. Furthermore, the reaction between the polymer end-groups and reactive groups on the substrate surfaces becomes less efficient as the molecular weight of the polymer in solution increases [118].
The “grafting from” approach, also known as “surface-initiated polymerization”, has attracted increasing interest in recent years due to its ability to produce grafted polymers with better control of polymer features including functionality, density and thickness of the grafted polymers. The surface is first modified with suitable initiators. This surface-bound initiator monolayer allows surface-initiated polymerization of monomers to produce functional polymers with optimal thickness and higher density. Steric hindrance is greatly reduced because of the addition of monomers to the end of the growing chains or to primary radicals. Matyjaszewski’s group investigated the differences in bactericidal efficacy between grafted-onto and grafted-from surfaces. Compared with the “grafting onto” approach, the “grafting from” approach produced surfaces with a higher charge density (1016 vs 6 × 1014 charges/cm2) and a higher biocidal efficacy [119] (Fig. 4 ). Based on the “grafting from” approach, many polymerization techniques including conventional free radical polymerization (FRP) and controlled radical polymerization (CRP) have found widespread use for the synthesis of antimicrobial surfaces.
Fig. 4.
Difference in biocidal activity of surfaces prepared by the “grafting onto” and the “grafting from” approaches. QA: quaternary amine group. [119], Copyright 2008.
Reproduced with permission from American Chemical Society.
3.2. Controlled radical polymerization
Conventional FRP is a type of instantaneous chain growth polymerization involving chain initiation, propagation and termination [120], [121]. The biggest drawback to this technique is the large dispersity and poor control over molecular mass. Free radical polymerization techniques usually produce final products with a broader distribution of polymer chain length and/or equivalent degree of polymerization and functional group density due to unavoidable, fast radical–radical termination reactions [122]. Nevertheless, the compliant nature of FRP, including its versatility in monomer selection, relatively mild polymerization conditions and tolerance to many different solvents (such as water) and impurity, makes this polymerization technique one of the most widely used processes for preparing QA-based polymers from the perspective of industrial production and applications.
Over the last two decades, the advent of CRP techniques enables precise control over macromolecular structure, order and functionality, which are important considerations for emerging biomedical designs. In the following sections, the major surface-initiated controlled radical polymerization (SI-CRP) techniques will be discussed. Table 3 summarizes the typical antimicrobial surfaces immobilized with QA moieties that have been prepared using SI-CRP.
Table 3.
Typical antimicrobial surfaces immobilized with QA moieties prepared via surface-initiated controlled radical polymerization (SI-CRP).
| Substrate | Grafted monomer | SI-CRP technique | Microorganisms tested | Refs. |
|---|---|---|---|---|
| Glass, filter paper | DMAEMA | SI-ATRP | E. coli, B. subtilis | [123] |
| Glass, silicon wafer | DMAEMA | SI-ATRP | E. coli | [124] |
| Glass | DMAEMA, TMSPMA | SI-ATRP | E. coli | [119] |
| Glass | DMAEMA | SI-ATRP | A. niger | [125] |
| Glass, PDMS, silicon wafer | DMAEMA, NIPAAm | SI-RAFT | E. coli, S. aureus | [126], [127] |
| Cellulose paper | DMAEMA | SI-RAFT | E. coli | [128] |
| Stainless steel | DMAEMA | SI-NMP | E. coli, S. aureus | [129] |
| Stainless steel | EA, PTEA, 8QA | SI-NMP | E. coli, S. aureus | [130] |
| Stainless steel | 4VP | SI-ATRP | D. desulfuricans | [131] |
| Stainless steel | DMAEMA | SI-ATRP | D. desulfuricans | [132] |
| Stainless steel | BPEA, FAc | SI-ATRP | S. aureus | [133] |
| Stainless steel | DMAEMA | SI-ATRP | D. desulfuricans | [134] |
| Fe3O4 magnetite nanoparticle | DMAEMA | SI-ATRP | E. coli | [135] |
| Fe(acac)3 magnetite nanoparticle | 4VP | SI-ATRP | NM | [136] |
| Titanium | HEMA | SI-ATRP | S. aureus | [137] |
| Gold, Si/SiO2 surface | SPMA | SI-ATRP | P. aeruginosa | [138] |
| Silicon wafer | DMAEMA | SI-ATRP | P. sp | [139] |
| Silicon wafer, gold-coated silicon wafer, glass, cellulose acetate, silicon nanowire array, PU, PDMS, stainless steel | P(AA-co-Ada), PAH | SI-RAFT | E. coli, S. aureus | [140] |
| Silicon rubber | AAm | SI-ATRP | S. aureus, E. coli | [141] |
| Silicon rubber | AAm | SI-ATRP | S. aureus, S. salivarius, C. albicans | [142] |
| Silicon nanowire array | DMAEMA | SI-ATRP | E. coli | [143] |
| Silicon catheter | DMAEMA | SI-ATRP | S. aureus | [144] |
| PVDF membrane | DMAEMA | SI-RAFT | E. coli | [145] |
| PVDF membrane | DMAEMA | SI-ATRP | E. coli | [146] |
| Polymer microsphere | DMAEMA | SI-ATRP | E. coli, S. aureus | [147] |
| Polyolefin | TBAEMA | SI-ATRP | E. coli | [148] |
| Polyolefin | PB | SI-NMP | NM | [149] |
| Polypropylene | DMAEMA | SI-ATRP | E. coli | [150] |
| Polypropylene | TBAEMA | SI-ATRP | E. coli | [151] |
| Polypropylene | DMAEMA | SI-ATRP | E. coli, S. aureus | [152] |
| Microfiber | DMAEMA | SI-ATRP | E. coli, S. aureus | [153] |
| Laponite clay platelet | DEPN | SI-NMP | NM | [154] |
Among the different CRP techniques that are available, atom transfer radical polymerization (ATRP) has been the most extensively studied [124], [155], [156] and reviewed for the preparation of a wide variety of polymeric materials [157], [158], [159], [160]. The first antimicrobial surface with QA moieties prepared via surface initiated-ATRP (SI-ATRP) was reported by Matyjaszewski’s group [123]. In that study, 2-(dimethylamino)ethyl methacrylate (DMAEMA) was polymerized directly onto filter paper via SI-ATRP. Subsequent quaternization of the amino groups of p(DMAEMA) generated a high concentration of QA groups on the polymer-modified surface (Fig. 5 ). These modified surfaces exhibited substantial antimicrobial activities against Escherichia coli and Bacillus subtilis. The authors found that biocidal activity increased with the density of available QA groups on the modified surface. Different functional monomers have subsequently been polymerized via SI-ATRP followed by quaternization to immobilize QA groups onto the polymer surfaces. Techniques involving SI-ATRP are excellent for preparing antimicrobial surfaces with QA groups because of their: (1) chemical versatility and compatibility with functional groups and monomers, (2) tolerance against a relatively high degree of impurities, and (3) relatively easy synthesis of surface-immobilizable initiators. Nevertheless, it is challenging to achieve controlled polymerization of pyridine-containing monomers, since the latter can complex or react with the metal catalysts. In addition, residual traces of catalysts are difficult to remove, which may result in undesirable toxicity in biomedical materials.
Fig. 5.
Representative quaternary ammonium-based antimicrobial coatings prepared by controlled radical polymerization (CRP). ATRP: atom transfer radical polymerization; CTA: chain transfer agent; DMAEMA: 2-(dimethylamino)ethyl methacrylate; NMP: nitroxide-medited polymerization; RAFT: reversible addition fragmentation chain transfer; SINMP: surface initiated nitroxide-medited polymerization. [123], Copyright 2004.
Reproduced with permission from American Chemical Society. [128], Copyright 2008, Reproduced with permission from American Chemical Society. [129], Copyright 2004, Reproduced with permission from American Chemical Society.
Reversible addition fragmentation chain transfer (RAFT) polymerization was introduced by Moad’s group [161], [162]. This technique possesses several advantages, the most important being the ability to synthesize well-defined polymers with various polar and nonpolar monomers under mild polymerization conditions. In addition, implementation of RAFT polymerization is relative simple and versatile by adding an appropriate chain transfer agent (CTA) into a conventional FRP system. Usually, the same monomers, initiators, solution and temperatures may be used. Hence, RAFT polymerization has great potential for preparation of antimicrobial coatings [163]. The most critical issue for successful RAFT polymerization is the selection of a suitable CTA. Commonly-used CTAs include dithioesters, xanthates, dithiocarbamates and trithiocarbonates [164]. Antimicrobial surfaces with QA moieties have been successfully prepared via surface initiated-RAFT (SI-RAFT) polymerization. For example, cellulose filter paper was polymerized with DMAEMA via SI-RAFT and further quaternized [128] (Fig. 5). When the modified materials were exposed to E. coli, cellulose fibers with the highest degree of quaternization or quaternized with the shortest alkyl chains exhibited more potent antimicrobial activities. Recently, Wang et al. reported the synthesis of a temperature-triggered, recycable bactericidal and antifouling surface [126], [127]. At a temperature above the lower critical solution temperature (LCST), the biomaterial surface is able to capture and effectively kill bacteria due to the presence of quaternized p(DMAEMA). Remarkably, the surface is capable of releasing the adhered bacteria corpses when the temperature is reduced to below the LCST. Moreover, the surface maintains its self-cleaning and bactericidal properties by simply washing with cold water and has good biocompatibility. This functionalized coating shows potential for multiple medical applications, including drug delivery, surface modification and tissue engineering. However, a major drawback of RAFT polymerization is that different monomers require specific CTAs, which are usually not commercially available. For example, methyl methacrylate (MMA) requires the use of a dithiobenzoate, whereas vinyl acetate can only be polymerized in the presence of a xanthate [165], [166].
Nitroxide-mediated polymerization (NMP), the simplest CRP technique, is accomplished by introducing a free nitroxide to the conventional FRP process. This type of polymerization is based on a reversible termination mechanism between the propagating radical and the nitroxide. The nitroxide acts as a control agent to yield alkoxyamine as the predominant species [167]. Owing to the living nature of NMP, this method can be applied to surface-initiated polymerization (SI-NMP) for preparing antimicrobial surfaces. In a typical SI-NMP process, binding of the initiator depends on the chemical nature of the surface, which may be achieved via covalent bonding, electrostatic interaction or hydrogen bonding. Ignatova et al. reported a two-step “grafting from” method for preparing antimicrobial stainless steel surfaces by sequential cathodic electrografting of an alkoxyamine-containing acrylate with SI-NMP of styrene and DMAEMA, followed by quaternization of the grafted polymers [129] (Fig. 5). The modified surface possesses significant antibacterial activities against both Staphylococcus aureus and E. coli. The thickness and hydrophilicity of the immobilized polymers may also be tailored by SI-NMP. Because no additional catalysts are required, this avoids the need for additional purification and reduces the chance to introduce impurities. Konn et al. used a similar alkoxyamine anchoring strategy with synthetic laponite clay platelets [154]. In this process, a quaternary ammonium alkoxyamine initiator is first intercalated into the clay galleries by cation exchange. Then, NMP of styrene was initiated from the surface of the functionalized clay platelets to obtain well-defined, ionically-bonded polystyrene chains with narrowly-distributed molecular weight. However, relatively high polymerization temperatures may cause problems when thermally-sensitive monomers are employed. In addition, the selection and synthesis of suitable nitroxides increase the workload in preparing antimicrobial coatings. Nitroxide-mediated polymerization may also be accomplished using the “grafting onto” approach. However, this synthesis pathway is not as popular due to steric hindrance of the grafted polymers.
3.3. Ring-opening polymerization
The history of ring-opening polymerization (ROP) dates back to the 1900s and presently, many polymers of industrial importance are produced using ROP [168]. In the ROP process, the terminal end of a polymer chain acts as a reactive center, which may be radical, anionic or cationic. Cyclic monomers can polymerize by opening the ring system using metal catalysts [169]. Depending on the nature of the initiator and catalyst, ROP may proceed via radical, cationic or anionic reaction mechanisms [170]. Examples of antimicrobial surfaces with QA moieties prepared by ROP have also been reported in the literature (Table 4 ). Wynne’s group prepared a series of quaternary/polyethylene glycol (PEG) copolyoxetanes via cationic ROP; these copolyoxetanes possessed biocidal activities against E. coli, S. aureus and Pseudomonas aeruginosa [171], [172]. The authors found that both linear charge density and quaternary alkyl chain length affected antimicrobial efficacy, hemolytic activity and cytotoxicity. Other workers concentrated on the synthesis of QA-functionalized surfaces/materials with dual antimicrobial actions [173], [174]. Nitric oxide (NO)-releasing, QA-functionalized silica nanoparticles are synthesized by first tethering glycidyltrialkylammoniumchloride (GTAC) via ROP. This is followed by functionalization of the secondary amine with N-diazeniumdiolate, the NO donor (Fig. 6 ).
Table 4.
Typical biomaterials grafted with QA-based polymers via ring-opening polymerization (ROP).
| Products | Microorganisms tested | Refs. |
|---|---|---|
| PEG/quaternary copolyoxetanes | S. aureus, E. coli, P. aeruginosa | [171], [172] |
| Polycarbonate hydrogels | S. aureus, E. coli, P. aeruginosa, C. albicans | [176] |
| QPGMA polymers | E. coli, S. aureus. | [177] |
| PAMAM dendrimers | S. aureus, P. aeruginosa | [173] |
| Silica nanoparticles | S. aureus, P. aeruginosa. | [174] |
Fig. 6.
Representative biomaterials grafted with QA-based polymers via ring-opening polymerization (ROP). A. Synthesis of nitric oxide (NO)-releasing QA-functionalized silica nanoparticles. B. Schematic illustration of nitric oxide (NO)-releasing QA-functionalized silica nanoparticles. [174], Copyright 2012.
Reproduced with permission from American Chemical Society.
Compared with nanoparticles with NO release or QA functionalities only, combining NO release with QA functionalities on the same nanoparticle resulted in augmented bactericidal efficacy against S. aureus. Nitric oxide is an endogenously synthesized small molecule involved in vital cellular functions and exhibits antimicrobial effects against a wide range of organisms. Hence, exogenous NO donors are promising bactericidal agents [19]. The development of novel monomers and catalysts enables more precise control over the molecular weight, structure and configuration of the polymers. In some cases, ROP and CRP techniques may be implemented together. For example, Leng et al. reported the synthesis of copolymer micelles of poly(caprolactone)-poly(quaternary ammonium salt) by a combination of ROP and ATRP. These copolymer micelles do not only possess antibacterial ability but may also be employed as carriers for antibacterial drugs [175].
3.4. Click chemistry
Click chemistry has recently emerged to become one of the most powerful tools in syntheses involving biomedical and chemical applications, including organic synthesis, medicinal chemistry, surface and polymer chemistry, drug discovery and chemical biology. The term “click chemistry” was coined by Sharpless and coworkers in 2001, and refers to a method for attaching a substrate of choice to specific biomolecules (site-specific bioconjugation) [178]. Click reactions are more broadly defined contemporarily as those that meet the criteria of ready availability of starting materials and reagents, high efficiency under mild conditions, regio- and stereo-selectivity, high yield, minimal by-products and limited side reactions. The aforementioned characteristics may be achieved by reactions possessing a high thermodynamic driving force (usually greater than 20 kcal mol−1). The most common methods for incorporating clickable groups into polymer chains include: (1) the use of functional initiators or transfer agents, (2) direct polymerization of functional monomers yielding pendant functionality and (3) post-polymerization transformation of end groups, each of which yielding terminal functionality [179]. Recently, the application of click techniques has found widespread use in the synthesis chemistry and there are a number of comprehensive reviews highlighting these exciting approaches [180], [181], [182], [183], [184]. Some of the most commonly used click reactions for creating antimicrobial surfaces with QA moieties, including Cu-catalyzed azide–alkyne cycloaddition (CuAAC), Cu-free click cycloaddition and thiol-ene reaction are illustrated in Fig. 7 .
Fig. 7.
Schematic illustration of reactions that best meet the criteria for a click reaction. A. Cu(I)-catalyzed azide–alkyne cycloaddition (CuAAC); B. Cu-free click cycloaddition; C. thiol–ene reaction. [183], Copyright 2011.
Reproduced with permission from Elsevier Science Ltd.
The quintessential example of click chemistry, CuACC, has generated the highest interest for producing QA antimicrobial surfaces [185], [186]. In a CuAAC reaction, [3 + 2] cycloaddition occurs between an organic azide and a terminal alkyne to produce Cu(I)-catalyzed 1,4-disubstituted 1,2,3-triazole [187]. In general, three key processes are involved in a CuAAC reaction: (1) initial formation of a 5-triazolyl copper intermediate, (2) coordination of the intermediate to organic azide and reaction between the nucleophilic carbon on the Cu(I) acetylide and the electrophilic terminal nitrogen on the azide, (3) ring contraction of the metallocycle and subsequent dissociation of the product to regenerate the catalyst. The reactions can accommodate a wide variety of functional groups and can proceed in many solvents (e.g. water), a wide range of pH values and over a broad temperature range. The reaction rate of CuAAC is more than 107 times faster than conventional reactions, which means that CuAAC may proceed efficiently at ambient temperature. It does not take long for chemists to realize the practicality and reliability of CuAAC in creating QA-based antimicrobial surfaces. For example, Ganewatta et al. recently produced QA-modified antimicrobial and biofilm-disrupting surfaces via CuAAC between surface-immobilized azide groups and the alkyne moiety on QACs [188] (Fig. 8 ). Other antimicrobial surfaces are produced by post-polymerization modification, via quaternization of tertiary amines to QA groups [189], [190], [191]. These reactions often involve production of cations of p(DMAEMA), p(2-vinylpyridine) (2-VP) and p(4-VP).
Fig. 8.
Representative quaternary ammonium-based antimicrobial surfaces prepared by Cu-catalyzed azide–alkyne cycloaddition (CuAAC). A. Synthesis of antimicrobial surfaces. B. Stained (live/dead stain) surfaces after 24 h of incubation with Staphylococcus aureus and Escherichia coli. S1, pristine surface; S3, quaternary ammonium-based surfaces. Cells with green fluorescence indicate live bacteria colonizing the surface, while dead cells exhibit red fluorescence. C. Schematic representation of the antimicrobial activity of the QA surfaces. [188], Copyright 2015. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Reproduced with permission from American Chemical Society.
An inherent limitation of CuAAC is the potential toxicity of catalyst Cu(I). To address this issue, Cu-free click chemistry has been developed [192]. Bertozzi et al. used strained alkynes (cyclooctynes) to facilitate strain-promoted cycloaddition of alkynes and azide (SPAAC) without a toxic catalyst [193], the reaction rate of which may be tuned by substituents on the cyclooctyne group. Copper-free click chemistry has attracted increasing interest in the fast-growing field of polymer and materials science because of its non-toxicity and biocompatibility. Applications of this strategy to QA surface modification have also been reported [194]. In that study, quantum dots were first modified with dibenzocyclooctynes (DBCO) to obtain DBCO-modified quantum dots. This was followed by SPAAC of the DBCO-modified quantum dots to azide-modified quaternized p(DMAEMA). Quaternary ammonium functionalized quantum dots possessed good antibacterial activity against E. coli and S. aureus, and exhibited no cytotoxicity on mammalian cells (Fig. 9 ).
Fig. 9.
Representative quaternary ammonium-based antimicrobial surfaces prepared by Cu-free click cycloaddition. A. Schematic of the synthesis of quaternary ammonium-functionalized quantum dots (QA-QDs). B. Representative scanning electron microscopy images of Escherichia coli incubated without (A–C) and with QPA-QDs for 20 min (D–F). C. Optical phase contrast images of cells double-stained with acridine orange and propidium iodide (AO/PI), after culturing with HepG2, A549, and HUVEC–C cells for 20 min with QPA-QDs (400× magnification). Cells with green fluorescence indicate live cells, while dead cells displayed red fluorescence. [194], Copyright 2016. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Reproduced with permission from The Royal Society of Chemistry.
Thiol-ene reaction has also been used for surface functionalization due to its efficiency, simplicity and high degree of conversion under mild conditions. Attachment of a molecule with a sterically accessible alkene or thiol group to a surface of interest enables surface functionalization via thiol-ene reaction. Tian et al. synthesized QA-functionalized antimicrobial wool fabric through thiol-ene reaction. The wool fabric was first treated with tris(2-carboxyethyl)phosphine to produce thiol groups for subsequent reaction with the C C groups of a QA salt [195]. The antimicrobial efficiency of the modified fabric against E. coli and S. aureus was 86.3% and 90.1%, respectively. In radical initiated polymerization, three distinct processes including initiation, polymerization and termination are involved in thiol-ene reaction [196]. In an ideal thiol-ene reaction, the combined propagation and chain transfer reaction proceed at equivalent rates and one thiol reacts with one-ene to produce an addition product. This inherent nature of the reaction results in homogenous and uniform end products. Photochemistry may also be used to initiate the thiol-ene reaction. For example, Wen et al. synthesized a series of QA-functionalized hyperbranched polyglycerols via ultraviolet light-initiated thiol-ene click chemistry [197] (Fig. 10 ). Compared with other methodologies such as thermal and redox initiation, the use of photoinitiation is particularly attractive because it allows both spatial and temporal control over the progress of the reaction.
Fig. 10.
Synthesis of sulfabetaine/quaternary ammonium modified, hyperbranched polyglycerols (HPGs) via ultraviolet light-initiated thiol-ene “click” chemistry. [197], Copyright 2016.
Reproduced with permission from The Royal Society of Chemistry.
4. Orthopedics-related materials
Despite the adoption of stringent sterilization and aseptic procedures, biomedical material-associated infections remain a critical challenge in orthopedics. The incidence of peri-prosthetic infection is 0.5–5%, depending on the surgical site and the procedure undertaken [198]. Among the diverse microorganisms present, gram-positive pathogens are predominant in deep infections, predisposing individuals to pain, high treatment cost and reduced treatment success. Hence, biomedical materials with anti-infective properties are highly desirable in orthopedics to prevent potential infections, and to serve as local delivery systems for antimicrobial agents to treat deep bone infections (osteomyelitis). Controlled release of antibiotics such as gentamicin from bone cements and beads represent the current gold standard for local antibiotic delivery, for preventing infections after total joint replacement and for treating osteomyelitis [199], [200], [201]. Non-degradable poly(methyl methacrylate) (PMMA) bone cements and spacer beads loaded with antibiotics can release drugs from the cement over time with a high initial release rate followed by sustained release. Nevertheless, their use is also associated with drawbacks such as unpredictable long-term efficacy, potential antibiotic resistance and local tissue toxicity. These issues may compromise cell and bone regeneration and implant osseointegration [202], [203]. The effectiveness of such devices is strongly dependent on the release rate of the loaded drugs [204]. In addition, long-term low-level release below the minimum inhibitory concentration of antibiotics following the “initial burst release” may contribute to the development of antibiotic-resistant bacteria strains.
Several publications support that the use of QACs in providing stable and permanent antimicrobial properties to bone cements (Table 5 ). Punyani et al. developed QA-based PMMA bone cements using a free radical bulk polymerization technique [205]. The bone cements exhibit a broad spectrum of contact-killing antimicrobial properties against E. coli and S. aureus. The modified cements and their eluants elicited negligible immune response and chronic inflammation in the host tissues [206]. These materials effectively kill bacterial cells attached to the cement surface. Incorporation of less than 15 wt% of these QACs to the bone cements results in no alteration of mechanical properties; the methacryloxy functionality renders these QACs polymerizable with methacrylate bone cements. Compared with linear polymers, dendrimers have many advantages as antimicrobial agents. The controlled synthetic process produces final molecules with well-defined architectures. The specific spherical-shaped structure provides high surface functional group concentration [295]. Hyperbranched QAC-functionalized tripropylene glycol diacrylate (TPGDA) dendrimers have been incorporated into bone cements due to their large number of QA functionalities [208]. The modified cement can kill up to 108 CFU/mL of common hospital-acquired bacteria such as E. coli, S. aureus and P. aeruginosa by contact-killing, with sustained bactericidal activity over a 30-day period. These antimicrobial properties render the modified bone cement promising for combating infections related with prostheses and surgeries.
Table 5.
Quaternary ammonium-based biomedical materials, QA-based nanoparticles, and the combination of QACs and nanomaterials.
| Applications | QACs | Microorganisms tested | Antibacterial methodologies | Details | Refs. | |
|---|---|---|---|---|---|---|
| Orthopedics-related materials | Bone cements | QADMA | E. coli | Agar diffusion test, bacteria viability (colony counts), SEM | QADMA-modified bone cement showed contact killing antimicrobial properties without releasing any bioactive agents. | [207] |
| QAMA | E. coli, S. aureus | Agar diffusion test, bacteria viability (colony counts), SEM | Inclusion of QAMA produced bone cements with antibacterial properties, lowered polymerization exotherm and comparable mechanical properties without evoking any cytotoxic response. | [205], [206] | ||
| TPGDA | E. coli, S. aureus, P. aeruginosa | Bacteria viability (colony counts), agar diffusion test | The novel dendritic structures showed promise for clinical antimicrobial activity while retaining the mechanical properties of bone cements. | [208] | ||
| HACC | Clinical isolate S. aureus, clinical isolate S. epidermidis, S. epidermidis, methicillin-resistant S. aureus, methicillin-resistant S. epidermidis | Biofilm inhibition test, bacteria viability (colony counts) fluores, SEM, Real-time PCR, rabbit model, bacteria viability (colony counts) | PMMA bone cements loaded with HACC exhibited improved surface roughness and wettability, increased porosity and better attachment and spreading of human-marrow-derived mesenchymal stem cells on the cement surface. These cements also prevented bacterial biofilm formation and exhibited improved physical properties and better osteogenic activity than gentamicin-loaded PMMA. In a rabbit model, HACC-loaded PMMA inhibits in vivo bone infections induced by methicillin-resistant S. epidermidis, with no bone lysis, periosteal reactions, cyst formation and sequestral bone formation. | [65], [209], [210], [211], [212] | ||
| QCS NPs | S. aureus, S. epidermidis | Bacteria viability (colony counts), biofilm inhibition test | Incorporation of the NPs in bone cements resulted in effective antibacterial action, and enhanced the antibacterial efficacy of gentamicin-loaded bone cements without compromising mechanical properties or causing cytotoxicity. | [213] | ||
| QPEI NPs | S. aureus, E. faecalis | Direct contact test, agar diffusion test | Incorporation of QPEI nanoparticles in bone cements had a long-lasting antibacterial effect without compromising the cement’s biocompatibility and physical properties. | [214] | ||
| Titanium implant coatings | HACC | S. epidermidis | Bacterial viability (colony counts), rat model, SEM, CLSM | The antibacterial implant coating decreased infection rates associated with orthopedic implantation and promotes implant osseointegration. | [215] | |
| Wound dressing materials | Sutures | 2AE | E. coli | Bacterial viability (colony counts), agar diffusion test | The modified keratin substrate exhibited a multifunctional effect including antibacterial and antistatic properties, improved liquid moisture management property, improved dyeing ability and a non-leaching characteristic of the treated substrate. | [216] |
| K21 | P. gingivalis, E. faecalis | Agar diffusion test | K21-coated surgical sutures exhibited antimicrobial activity for bacterial species of direct relevance to postoperative infection and bacteremia. | [217] | ||
| IDMA | E. coli, S. aureus | Agar diffusion test | The modified wool fabric showed good antibacterial and antistatic properties. Its mechanical properties were improved by the chemical bonds of the modification. | [195] | ||
| Cotton fibers | SPH, SPODA | S. aureus, E. coli | Agar diffusion test | The cotton fabric coated with both SPH and SPODA exhibited antibacterial activity. | [218] | |
| GTAC, GTAC + AgNPs | E. coli, methicillin-resistant S. aureus, P. aeruginosa, S. typhimurium | Bacterial viability (colony counts), agar diffusion test | Cotton fibers treated chemically with GTAC and coated with AgNPs exhibited increased antibacterial efficacy, enhanced hydrophilicity but lower heat stability. | [219] | ||
| Wound dressings and hydrogels | pDADMAC | A. baumannii, P. aeruginosa, S. aureus | Bacterial viability (colony counts), CLSM, SEM, bacterial migration test | The modified dressing inhibited growth and migration of bacteria. | [220] | |
| GTEACl | S. aureus, E. coli | Agar diffusion test, shaking flask test | The polyurethane membranes with appropriate loading of GTEACl showed biocompatibility, antibacterial activity, and possessed appropriate hydrophilicity and water vapor transmission rate. | [221] | ||
| EPSiQA | A. niger, B. subtilis, E. coli, S. aureus | MIC determination | EPSiQA-gelatin had excellent antibacterial property. | [63] | ||
| Monomer 1 | S. aureus, E. coli, P. aeruginosa, C. albicans | Bacterial viability (colony counts), FE-SEM | The quaternized hydrogels showed fast degradation at room temperature, with broad-spectrum antimicrobial activity. These properties made the modified hydrogels ideal candidates for wound healing and implantable biomaterials. | [176] | ||
| GTMAC | E. coli, S. epidermidis | Shaking flask test, bacterial viability (colony counts), rat wound model | The efficiency in wound healing of the gel rendered it a promising material for treatment of full-thickness open wound. | [222], [223] | ||
| Dental materials | Resin composites | MDPB | S. mutans, S. sobrinus, S. oralis, S. mitis, S. sanguis, S. gordonii, S. salicarius | Agar diffusion test, bacteria viability (colony counts) | Resin composites with MDPB after curing possessed antibacterial activity on contact against bacteria, with no adverse effects on mechanical properties and curing performance. | [66], [224], [225], [226], [227], [228] |
| QPEI NPs | E. coli, E. faecalis, P. aeruginosa, S. epidermidis, S. mutans, S. aureus, human saliva | Direct contact test, agar diffusion test, in vivo model, SEM, bacteria viability (colony counts), CLSM | QPEI nanoparticles immobilized resin composite exhibited strong and long-lasting in vitro and in vivo antibacterial activity against a broad spectrum of bacteria, without leaching of the nanoparticles or compromising mechanical properties. | [229], [230], [231] | ||
| QADM/DMADDM/DMAHDM + NACP | Human saliva, rat saliva | Dental plaque microcosm model, MIC and MBC determination, biofilm accumulation (MTT), bacteria viability (colony counts) fluores, biofilm inhibition test, rat tooth cavity model | The nanocomposite exhibited antibacterial and remineralization potential in vitro without compromising load-bearing properties. Milder pulpal inflammation and more extensive tertiary dentin formation in vivo, indicating its potential to combat bulk tooth fracture, secondary caries, and facilitate the healing of the dentin–pulp complex. | [232], [233], [234], [235], [236], [237] | ||
| QADM + AgNPs + NACP | S. mutans, Human saliva | Biofilm accumulation (MTT), bacteria viability (colony counts) | The QADM + AgNPs + NACP composite possessed the double benefits of remineralization and antibacterial capabilities. The composite was found to be potentially useful for inhibiting dental caries with no adverse effects on mechanical properties. The anti-biofilm activity was maintained after 12 months of water-aging. | [238], [239] | ||
| QADM | S. mutans | CLSM | Incorporation of 10 wt% QADM reduced bacterial colonization, and increased the viscosity, degree of conversion and surface charge density. | [240] | ||
| DDMAI | S. mutans | MIC determination, bacteria viability (colony counts), SEM | Incorporation of 5% DDMAI had no adverse effect on the conversion and flexural strength of the resin composite and provided radio-opaque and antibacterial effects. | [69], [241], [242] | ||
| QADMAIs | S. mutans | Agar diffusion test, biofilm inhibition test | QADMAIs had no adverse impact on the degree of conversion and flexural strength. However, antibacterial activity, flexural strength, flexural modulus and radio-opacity were affected by the alky chain length of QAC. | [243] | ||
| MAE-DB | S. mutans | Bacteria viability (colony counts), biofilm inhibition, RT-PCR | MAE-DB-containing resin blends exhibited long-term antibacterial effects after polymerization by attenuating gtfB expression and impairing membrane integrity. | [57], [244] | ||
| IPhene | S. mutans | Biofilm inhibition test | Incorporation of 30 wt% IPhene endowed dental resins with both antibacterial and radio-opacity, with lower flexural strength and modulus, lower volumetric shrinkage and higher fracture energy. | [245] | ||
| QAB | S. mutans | MIC determination, bacteria viability (colony counts) | QAB-modified composites showed significant antibacterial activity. However, mechanical strength was reduced. | [70] | ||
| Monomer II | S. mutans | Biofilm inhibition test, FE-SEM | Resin composite containing 3% of monomer II significantly reduced against S. mutans biofilm formation without major adverse effects on its physical and mechanical properties. | [246] | ||
| QASM | S. aureus, E. coli, S. mutans | Agar diffusion test, direct contact test | Thiol–ene rich resins produced low shrinkage, homogeneous networks with adequate water uptake. Bactericidal activity was present on the matrix surface without sacrificing the physico-mechanical properties of the cured resin. | [247] | ||
| IMQ-16 | S. mutans | Biofilm Inhibition test, agar diffusion test, direct contact test | Incorporation of IMQ-16 into resin provided significant antibacterial activity and equivalent physicochemical properties. | [248], [249] | ||
| Dental adhesives | MDPB |
P. acnes, E. alactolyticum, B. bifidum, P. asaccharolyticus, L. acidophilus, L. aracasei ssp, L. plantarum, L. salivarius ssp, L. brevis, L. fermentum, L. casei, S. mutans, dog saliva, human saliva |
Agar diffusion test, bacterial viability (colony counts), MIC and MBC determination, biofilm accumulation, direct contact test, microcosm biofilm model, dog model, in situ | Antibacterial adhesive or primer containing MDPB before curing acted as effective cavity disinfectants to directly kill bacteria. After polymerization, the adhesive or primer exhibited long-lasting contact inhibition effects on bacteria contacting the cured surface, without diffusion of soluble components, or significant decline in bond strength or curing performance. | [62], [224], [250], [251], [252], [253], [254], [255], [256], [257], [258] | |
| DMAE-CB | S. mutans, A. viscosus, S. aureus, L. casei | Bacteria viability (colony counts), biofilm accumulation, RT-PCR, MIC and MBC determination, SEM | The modified dental adhesive had strong and long-lasting contact antibacterial activity after polymerization without negatively influencing bonding ability. | [58], [59], [259], [260], [261] | ||
| DMAHDM, DMADDM |
S. mutans | Bacteria viability (colony counts), biofilm inhibition test, SEM, EPS Staining, pH Measurement, RT-PCR | Increasing the alkyl chain length and quaternary amine charge density of dentin bonding agent resin greatly enhanced antibacterial and anti-biofilm activity without compromising dentin bond strength. | [68], [262], [263], [264], [265] | ||
| QAMP | S. mutans, L. casei, A. naeslundii | MIC and MBC determination, bacteria viability (colony counts), agar diffusion test | The use of QAMP in an adhesive system demonstrated effective bond strength, acceptable degree of conversion, and long-lasting antibacterial effects. | [54], [55] | ||
| MDPB + AgNPs | Human saliva | Dental plaque microcosm model, bacteria viability (colony counts), agar diffusion test | Dual agents (MDPB + AgNPs) method yielded potent antibacterial properties without reducing dentin bond strength. | [266], [267] | ||
| QADM + AgNPs | S. mutans, human saliva | Bacteria viability (colony counts) fluores, biofilm inhibition test, RT-PCR, dental plaque microcosm model, agar diffusion test and inhibitory effects, TEM | Adhesive containing QADM and AgNPs had long-distance killing capability and inhibited bacteria on contact and away from its surface, without adversely affecting bond strength. Potential to inhibit secondary caries. | [268], [269], [270], [271] | ||
| DMADDM + NACP | Human saliva | Dental plaque microcosm model, bacteria viability (colony counts), biofilm inhibition test | Bonding agent containing DMADDM and NACP inhibited biofilms and dramatically increased the Ca and P ion release at cariogenic pH 4, without affecting dentin bond strength. | [272] | ||
| GICs and resin modified GICs | PQAS | S. mutans, human saliva | MIC determination, bacteria viability (colony counts) | The cement exhibited long-lasting antibacterial activity and high mechanical strength, indicating its clinical potential. | [44], [273], [274], [275] | |
| DMADDM | Human saliva, S. mutans | In situ dental biofilm model, biofilm inhibition test, fluoride releasing, atomic force microscope, live/dead bacteria and EPS staining, lactic acid measurement, RT-PCR | The cement strongly inhibited in situ S. mutans biofilms and had the potential to improve the management of secondary caries. | [276], [277] | ||
| QPEI NPs | S. mutans, L. casei | Direct contact test, agar diffusion test | QPEI nanoparticles when incorporated in GICs at a low concentration (1%) exhibited strong antibacterial effect that lasted for at least one month. | [214] | ||
| Root canal sealers | MDPB | E. faecalis, F. nucleatum, P. nigrescens | Root canal infection model, bacteria viability (colony counts), bacterial adherence, MIC and MBC determination, agar diffusion test | The root canal filling system had the ability to effectively disinfect infected root canals and achieved good sealing. | [50], [278], [279] | |
| QAES NPs | A. naeslundii, C. albicans, E. faecalis, S. mutans | Direct contact test, biofilm inhibition test, bacteria viability (colony counts) | The root canal sealer possessed long-lasting antibacterial activity for both planktonic bacteria and biofilms. | [280], [281] | ||
| QPEI NPs | E. faecalis, clinical isolate E. faecalis | Direct contact test, membrane-restricted experiments, biofilm inhibition test, agar diffusion test, SEM | Addition of QPEI NPs improved the long-term antibacterial activity of root canal sealers without relevant changes in physicochemical and mechanical properties. | [282], [283], [284] | ||
| Pit-and-fissure sealants | DMAE-CB | S. mutans | Bacteria viability (colony counts) | Incorporation of DMAE-CB provided the sealant with contact-liking antibacterial activity without negatively influencing physicochemical and mechanical properties. | [285] | |
| MAE-DB | S. mutans | Bacteria viability (colony counts), CLSM | Incorporation of MAE-DB rendered the sealant with contact antibacterial activity after polymerization by influencing the growth, adherence and membrane integrity of S. mutans. | [286] | ||
| Pulp capping materials | MAE-DB | S. mutans | Bacteria viability (colony counts), biofilm accumulation | Pulp capping materials with strong and long-lasting contact-killing antibacterial activity in vitro. | [287] | |
| Acrylic resin | QAMS | S. mutans, A. naeslundii, C. albicans | Bacterial viability (colony counts), CLSM, RCT | QAMS-containing acrylic resins exhibited long-lasting antimicrobial activities, decreased water wettability and improved toughness, without adversely affecting the flexural strength and modulus, water sorption and solubility. The acrylic resin had the potential for preventing bacteria- and fungus-induced stomatitis. | [288], [289], [290], [291] | |
| MUPB | C. albicans, C. dubliniensis, C. glabrata, L. casei, S. aureus, S. mutans | MIC and MBC determination, bacterial viability (colony counts), bacterial adherence | Antimicrobial activity of MUPB after incorporation in a denture base acrylic resin was not dependent on elution of the antimicrobial monomer. Incorporation of MUPB slightly reduced the mechanical properties of denture base acrylic resin. | [64], [292] | ||
| QCS |
S. mutans, C. albicans |
Bacterial viability (colony counts) | Chitosan quaternary ammonium salt-modified resin denture base materials showed antimicrobial properties, without significant reduction in their tensile strength and cytotoxicity. | [293] | ||
| Dental implants | DCAMA | S. aureus | Bacterial adherence, CLSM | The coatings demonstrated antibacterial activity against bacteria adherent to the surface but exhibited poor cyto-compatibility. | [294] | |
Quaternary ammonium polyethylenimine (QPEI) nanoparticles and quaternized chitosan derivatives are two examples of QA-functionalized fillers incorporated into PMMA bone cements. The QPEI nanoparticles have been used in various dental materials (see Section 6) because of their excellent antibacterial activities, mechanical properties and biosafety. Bone cements loaded with the nanoparticles retain effective antibacterial activity after a 4-week aging period, without altering the biocompatibility or mechanical properties of PMMA [209]. From a clinical perspective, PMMA bone cements loaded with 26 wt% hydroxypropyltrimethyl ammonium chloride chitosan (HACC), a quaternized chitosan derivative, prevent bacterial biofilm formation of antibiotic-resistant bacteria strains (methicillin-resistant S. aureus standard strains and clinically-isolated methicillin-resistant Staphylococcus epidermidis) and down-regulate virulence-associated gene expressions on the cement surface [209]. The HACC-loaded PMMA was found to induce better stem cell proliferation, osteogenic differentiation and osteogenesis-associated genes expression when compared with gentamicin-loaded PMMA because local release of antibiotics in bone cements suppresses osseointegration [210] (Fig. 11 ).
Fig. 11.
A. Cytoskeletal morphology of human mesenchymal stem cells (hMSCs) exposed to different polymethyl methacrylate (PMMA)-based bone cements. B. Relative alkaline phosphatase activity (indicative of bone forming potential) of hMSCs after 6, 10, and 14 days of culture. For each time period, columns connected with a horizontal bar are not significantly different (P > 0.05). C. Representative images showing hMSCs with positive alkaline phosphatase staining on the four PMMA-based bone cements at day 14. PMMA-C: chitosan-loaded PMMA; PMMA-G: gentamicin-loaded PMMA; PMMA-H: hydroxypropyltrimethyl ammonium chloride chitosan (HACC)-loaded PMMA. [210], Copyright 2012.
Reproduced with permission from Elsevier Science Ltd.
The favorable results achieved by HACC-loaded PMMA may be attributed to improved surface roughness and wettability, increased porosity and better attachment and spreading of human-marrow-derived mesenchymal stem cells on the cement surface. In a rabbit model, HACC-loaded PMMA inhibits in vivo bone infections induced by methicillin-resistant S. epidermidis, with no bone lysis, periosteal reactions, cyst formation and sequestration of non-vital bone [211], [212]. The quaternized chitosan derivative may also be immobilized on titanium implant surfaces via silanization with aminopropyltriethoxysilane [215]. Incorporation of silyl groups enables covalent attachment of the quaternized chitosan derivative on the metal or hydroxylated surfaces, while the QA group exerts long-term antibacterial activity. Titanium surfaces modified with HACC prevent bacteria attachment in vitro and reduce infection in vivo. The coated titanium implants are well-integrated with the surrounding bone without bacterial growth. Nevertheless, clinical trials and FDA approval are required before clinical translation and commercialization of this technology can be successfully achieved.
Incorporation of both contact-based biocides such as QACs, and release-based biocides such as AgNPs [296], [297] or silver bromide (AgBr) NPs [298] produces surfaces with dual-functional antibacterial activities. This strategy is of particular interest in orthopedic operations because of its unique antibacterial characteristics. Dual-functional coatings exhibit potent initial antibacterial properties due to the release of bioactive biocides and maintain long-term antibacterial activities via the immobilized QA groups. The first 6 h after surgical implantation is highly susceptible to bacterial invasion and surface colonization, and has been coined the “decisive period” [299]. A high initial burst release offers protection against the initial elevated risk of infection, while sustained contact-killing provides surveillance against subsequent latent infections.
5. Sutures and wound dressings
Wound management is a critical healthcare component that has attracted much global concerns. Sutures are used to approximate tissues to facilitate closure and healing of wounds [300]. Bacteria attachment and proliferation on suture materials are significant risk factors for wound infections [301]. Wound dressings generally consist of bilayer structures, including an upper dense “skin” layer and a lower spongy layer. Although dressings are designed to protect the wound against microorganisms [302], the wounded site in the vicinity of the dressing provides a favorable environment for bacterial growth. Intensive research has been conducted on antibacterial modification of wound dressings to prevent infections and provide an optimal milieu for tissue regeneration [303], [304]. These antibacterial materials are produced predominantly by electrospinning [305]. Sustained broad-spectrum antimicrobial activity is desirable for optimal wound healing because complex tissue interactions between cells, extracellular matrix molecules and soluble mediators are involved in the regeneration of damaged skin or other tissues. Previous studies reported addition of small molecule QACs such as benzalkonium chloride, N,N,n,n,-didecyl-N,N-dimethylammonium chloride to polymer solutions for electrospinning of antibacterial nanofibers [306], [307]. Such methods suffer from drawbacks such as reduction in fiber diameter and a burst release of QACs. Compared with the attachment of QACs to a wound dressing through ionic absorption, immobilization of QA moieties on the dressing surface via covalent bonding provides long-term antibacterial protection and eradication of bacteria by contact-killing (Table 5). Various polymerization techniques including layer-by-layer assembly, click chemistry, FRP and CRP have been employed [308], [309], [310]. For example, Dan et al. reported the preparation of antimicrobial keratin fibers for medical applications through thiol-ene click chemistry [216]. The presence of QA moieties on keratin structures was confirmed with Raman and infrared spectroscopy. The modified sutures exhibit excellent antimicrobial activity, antistatic property and significantly improved moisture management capability. Sutures and dental floss that were coated with the QA compound “K21” demonstrated dose-dependent antibacterial activities against the periodontal pathogen P. gingivalis and the endodontic pathogen Enterococcus faecalis [217]. Liu et al. found that compared with traditional pad-dry-cure process, QA-functionalized cotton fabric prepared using a pad-dry process showed a relatively higher antibacterial efficacy [218]. The phenomenon may be attributed to increased hydrophobicity of the coated samples under high coating temperature in the pad-dry-cure process. Recently, Kang et al. reported inproved antibacterial efficacy in antimicrobial cotton fibers that are chemically treated with glycidyltrimethylammonium chloride and AgNPs [219] (Fig. 12 ). In addition, the coated fibers exhibit increased moisture regain as a result of the enhanced hydrophilicity.
Fig. 12.
Representative scanning electron microscopy images of A. Untreated cotton; B. Cotton treated with silver nanoparticles (AgNPs). C. Cotton treated cotton with quaternary ammonium compound. D. Cotton treated with quaternary ammonium compound and AgNPs. [219], Copyright 2016.
Reproduced with permission from Elsevier Science Ltd.
Attempts have also been made to incorporate QA groups into wound dressing materials. Attachment of QA groups to a polyurethane foam wound dressing [220] resulted in inhibition of biofilm development of three major wound pathogens (S. aureus, P. aeruginosa and Acinetobacter baumannii) both within the wound and in the wound dressing. Wound healing requires a moist and warm environment, and dressing materials with good air permeability and moisture absorption are desirable. Novel QA-containing polyurethane membranes used as wound dressings exhibit antibacterial activity against S. aureus and E. coli [221]. The modified antibacterial dressings exhibit good biocompatibility for fibroblasts and epidermal keratinocytes. More importantly, the modified dressings possess excellent water vapor transmission to prevent accumulation of exudates and decrease surface inflammation within the wounded area. Hydrogels based on synthetic or natural polymers may be used as wound dressings to locally deliver a wide range of bioactive agents in a controlled and sustained manner to regulate stem cells that are encapsulated within the three-dimensional polymer network [311]. Because of their biocompatibility, low immunogenicity and rapid hydrolysis under physiologic conditions, some hydrogels have been commercialized for clinical use [312]. Recently, a gelatin hydrogel grafted with an epoxy silicone QA salt exhibits excellent antibacterial property against Gram-positive and Gram-negative bacteria, including S. aureus, E. coli and Bacillus subtilis [63]. The quaternized polycarbonate hydrogels have been successfullysynthesized via ROP [176]. In addition to their broad-spectrum antimicrobial activity, the gels showed fast degradation at room temperature (4−6 days). This makes them promising for incorporation into wound healing and implantable biomaterials. Pilakasiri et al. found that QA-modified chitin gels are promising candidates for treatment of full-thickness open wounds, with better wound healing than a clinically available dressing [223].
6. Dental materials
Dental caries is one of the most prevalent bacteria-related human infectious diseases [313]. During the development of dental caries, acidogenic and aciduric bacteria such as Streptococcus mutans accumulate on teeth and/or dental fillings and cause demineralization of enamel and dentin via acid generation [314]. Following demineralization, salivary proteases or endogenous peptidases such as MMPs and cysteine cathepsins induce degradation of the demineralized collagen matrix [315]. To address the growing need for antibacterial dental materials, a useful approach is to permanently copolymerize QACs with resin materials by forming covalent bonds with the polymer network. Such stable non-leaching antibacterial materials can provide long-term protection via contact of the microorganisms with the biocidal surface, without sacrificing the mechanical properties and polymerization behavior of the original non-antibacterial resin formulations [70], [226]. In particular, QA-based resin materials exhibit good biocompatibility that is evidenced by minimal toxicity, allergenicity and tissue irritability [51]. Based on the aforementioned advantages, QACs are considered highly desirable for managing and combating dental caries.
The first synthetic QAC to be incorporated in antibacterial dental materials is 12-methacryloyloxy dodecyl pyridinium bromide (MDPB) [66]. Polymerized dental adhesives containing MDPB significantly exhibit contact-killing effects on S. mutans when these bacteria come into contact with the specimens’ surface [253]. Composites incorporated with MDPB exhibit long-term antibacterial activity without eluting antibacterial components. Dental materials containing methacryloxylethyl cetyl ammonium chloride (DMAE-CB) exhibit strong, fast and long-term killing effects [58], [261] (Fig. 13 ). Adhesives incorporating DMAE-CB suppress the expression of glucosyltransferase genes which contribute to plaque biofilm accumulation [259], [260]. The application of QACs in dental materials has been published in several reviews [316], [317]. These discoveries led to the development of various kinds of non-releasing antibacterial dental materials including resin composites, adhesives, glass ionomer cements and resin-modified glass ionomer cements, root canal sealers, pit-and-fissure sealants, pulp capping materials, acrylic resin dentures, orthodontic retainers, denture lining materials and dental implants (Table 5).
Fig. 13.
Antibacterial activity of polymerized dental adhesives containing MDPB or DMAE-CB. A-C. Representative confocal laser scanning microscopy images of Streptococcus mutans adhered on the control adhesive (A), adhesive containing MDPB (B) and adhesive containing DMAE-CB (C) after 24-h incubation. Bacteria with integral membranes were stained with green fluorescence and those with compromised membranes were stained with red fluorescence. D. Fluorescence intensity values of the two channels for adhesives derived from the three groups. E. Schematic representation of polymerized adhesive containing DMAE-CB. F-H. Representative scanning electron microscopy images of Streptococcus mutans biofilms on the control adhesive (F), adhesive containing MDPB (G) and adhesive containing DMAE-CB (H) after 4-h incubation. I-K. Representative scanning electron microscopy images of Streptococcus mutans biofilms on the control adhesive (I), adhesive containing MDPB (J) and adhesive containing DMAE-CB (K) after 24-h incubation. [260], Copyright 2009. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Reproduced with permission from Elsevier Science Ltd. [259], Copyright 2009, Reproduced with permission from International & American Associations for Dental Research.
Over the last decade, researches on QACs have benefited from ground breaking advancements in organic and inorganic chemistry as well as materials science. These improvements have contributed to the introduction of strategies to develop innovative QACs with improved properties. Theoretically, antibacterial materials incorporated with more QACs provide better antimicrobial activities. However, the capability of a resin polymer network to copolymerize with QACs is limited (e.g. only 5 wt% MDPB and 3 wt% DMAE-CB are employed in adhesives). Incorporating too high concentrations of these mono-methacrylates (i.e. QACs with one methacrylate group) beyond the polymerizable capability of the resin polymer network may adversely affect its structural and mechanical properties. To address these problems, QACs with two methacrylate groups have been developed for incorporation in antibacterial dental materials [57], [240], [244], [287], [318].
In the investigation of the structure-property relationship of QACs [67], [68], those with a chain length of 12 or 16 were found to possess higher antibacterial potency. Due to the high electron density of iodine, substitution of the halogen anions in QACs with iodine as the counter ion results in radiopaque antibacterial monomers [69], [241], [242], [243], [245].
Most of the reported QACs have only antibacterial effects. Apart from bacteria, fungi play an important role in oral cavity infections such as denture-related stomatitis. Co-colonization of S. mutans and Candida albicans in the form of mixed dental plaque biofilms can occur on the surface of removable orthodontic appliances or removable dentures [319], [320]. Recently, quaternary ammonium methacryloxy silicate (QAMS) has been developed with both antibacterial and anti-fungal properties for incorporation into dental removable appliances fabricated from acrylic resin (i.e. PMMA) [288], [289], [290]. In those in vitro studies, acrylic resin containing 5 wt% QAMS exhibits contact-killing microbiocidal activities on S. mutans, Actinomyces naeslundii and C. albicans single species biofilms. Thus, QAMS has the potential for fabrication of antibacterial/anti-fungal partial/full denture bases, as well as for preparation of removable orthodontic appliances and retainers.
Polymerization shrinkage stresses and strains are generally considered the most pertinent issues associated with the clinical use of resin-based dental filling materials. These stresses and resulting strains may contribute to crack propagation in the filling material, leakage along the tooth-filling interface, bacterial invasion and secondary caries [321], [322]. Burujeny and colleagues fabricated thiol-ene-methacrylate containing quaternary amine moieties with reduced polymerization shrinkage stress and strain and improved antibacterial effectiveness [247]. Reduction in polymerization shrinkage has been attributed to the nature of thiol-ene polymerization; delayed gelation produces more homogeneous structure with narrow glass transition temperature ranges and reduced brittleness in comparison with classic methacrylate resin systems [323], [324]. In addition, the presence of flexible thiol-ether linkages provide a better opportunity for exposure of reactive quaternary amine groups on the surface of the polymerized resin matrix, which, in turn, provides better bactericidal function.
Biomedical materials have evolved with the advent of nanotechnology, making it possible to develop antibacterial dental materials with QAC-functionalized nano-fillers. Quaternary ammonium polyethylenimine (QPEI) nanoparticles are effective against a variety of Gram-positive and Gram-negative bacteria, including clinical isolates of pathogenic bacteria and bacteria in contaminated water [325], [326]. The antimicrobial potency of these nanoparticles is attributed to the abundance of QA groups along its backbone. The QPEI is a synthetic, non-biodegradable, cationic polymer with primary, secondary and tertiary amine functionalities, which may be subjected to a wide range of chemical modifications to produce desirable physicochemical properties [16]. Because of the small size and large surface area of the nanoparticles, as well as their capability to release high concentrations of ions with low filler content, only a small amount is required for incorporation into dental materials. Incorporation of low concentration of these nanoparticles (1–2 wt%) into dental materials [229], [230], [282], [284], [327] provides potent and broad-spectrum antimicrobial effects. Silane-quaternary amine-based antimicrobials, generally consisting of an organic shell with antimicrobial functionality and a silica core with high colloidal stability, have been shown to be effective antimicrobial fillers for more than 40 years [328]. Reactive silane functionalized antimicrobial agents are resistant to microbial growth and biofilm formation. These antimicrobial agents also possess other benefits, including tunable surface functionality, low cytotoxicity and easily controllable size. Hence, they are widely used in the field of antimicrobials [329], [330], [331]. Silica-quaternary ammonium QPEI nanoparticles, prepared by surface modification of silica particles with QA groups, distribute more homogenously in the polymeric coatings, with less nanoparticle aggregation when compared with QPEI nanoparticles [332]. For example, quaternary ammonium epoxy silicate nanoparticles applied as QA compounds conjugated to organosilanes and silica nanoparticles, and also to epoxy silicates, were synthesized by a silane-based sol-gel reaction and incorporated into an epoxy resin-based root canal sealer. The sealer exhibited antimicrobial activities against E. faecalis biofilms [281]. Resins incorporating functionalized silica nanoparticles possess sufficient antimicrobial activity as well as high strength and modulus of elasticity values even at low filler concentrations [333].
Dental materials with both QACs and nano-sized antibacterial agents incorporated into one system operate with dual functions [334]. For example, incorporation of both QACs and AgNPs can effectively kill bacterial both on (contact-based killing) and away from the surface of the materials (release-based killing) [268], [269]. The AgNPs are small enough (mean diameter 40 nm) to infiltrate dentinal tubules to kill residual intratubular bacteria [270]. Incorporation of nanoparticles of amorphous calcium phosphate (NACPs) into QAC-based dental materials bestows those materials with remineralization capabilities for reversing the tooth decay process [335]. The QAC-based nanocomposites and adhesives containing NACPs possess “smart” properties by increasing Ca and P ion release at low cariogenic pH values, when these ions are most needed for inhibiting caries, neutralizing the demineralization of enamel and dentin by lactic acid, and preventing caries formation [232]. However, one should be careful in balancing the antibacterial effects of those nano-fillers with their cytotoxicity due to latent nanoparticles-related toxicity.
Although in vitro models have been adopted by researchers for initial screening of new materials and techniques for developing QACs and to justify their potential for clinical applications, there are other in vivo factors that cannot be duplicated in in vitro studies. To date, the majority of the work performed on the antimicrobial properties of QACs is in vitro nature. Although the recent use of multi-species oral biofilm models has contributed to the understanding of intraoral microbial adhesion and biofilm formation, these models are unlikely to simulate the variability and in vivo dynamics of dental plaque biofilms. For example, the antibacterial and immunoregulatory effects of antimicrobial peptides such as α- and β-defensins, and the cathelicidin LL-37 in the oral cavity, as well as histatins derived from the saliva cannot be neglected [336], [337]. Other factors include the role of saliva in slowing down tooth decay by neutralizing plaque acids [338]. In addition, saliva-derived protein films can be adsorbed on dental restoration surfaces and provide anchoring sites for bacteria [339], block functional antibacterial groups on the material surfaces, thereby reducing the antibacterial activity of QA-based materials. Indeed, several publications show that the presence of a saliva-derived protein film (i.e. pellicle) on the surface of QA-based materials reduce their bactericidal effects [224], [340]. Because pre-coating of salivary pellicles on resin surfaces significantly decreases the antibacterial effects of polymerized antibacterial resins, in vivo and in-situ models are highly desirable for evaluating treatment outcomes, which, unfortunately, are desperately lacking. Animal models have been developed for comparing the functional efficacy of QA-based dental materials. Generally, those animal models were designed to examine: a) the biocompatibility of the materials (pulp response and reparative dentin formation) [341]; b) the presence of bacteria within the intraradicular dentin [254]; c) bonding ability using QA-based dental adhesives [256] and restorative materials. Using a rat tooth cavity model, QA-based NACP-containing nanocomposites and adhesives were found to combat oral pathogens and biofilms effectively and facilitate healing of the dentin-pulp complex [234] (Fig. 14 ).
Fig. 14.
Evaluation of antibacterial and remineralizing nanocomposite and adhesive containing nanoparticles of amorphous calcium phosphate (NACP) and dimethylaminododecyl methacrylate (DMADDM) in a rat tooth cavity model. A. Schematic representation of rat tooth cavity model. B. Rat right and left molar teeth were used. C. Occlusal cavity was prepared. D. Cavity was restored with adhesive and nanocomposite and light-cured. E-H. Histological analyses of rat tooth cavities at 8 days in the control group (E and F) and the DMADDM + NACP group (G and H). The same four groups at a higher magnification. The control group exhibited disruption of the odontoblast layer associated with a medium inflammatory response in the pulp. Blood vessels are observed and a thin layer of tertiary dentin can be identified. The DMADDM + NACP group has normal pulp tissues, with much thicker tertiary dentin (a type of reparative dentin) than the control. Stars indicate areas with inflammatory cell infiltration. I and J. Representative hematoxylin and eosin-stained images at 30 days in control group (I) and the DMADDM + NACP group (J). Stars indicate areas with inflammatory cells. Blood vessels are indicated by arrows. The control group exhibits slight inflammatory responses. The DMADDM + NACP group shows normal pulp tissue without inflammatory response, and greater tertiary dentin thickness. [234], Copyright 2014.
Reproduced with permission from Elsevier Science Ltd.
It must be pointed out that data derived from animal studies are somewhat different from the clinical effects experienced by humans. Apart from differences in structural characteristics between in vitro and in vivo biofilms, the presence of host defenses such as antimicrobial peptides derived from saliva is seldom taken into account in in vitro multi-species biofilm models [342]. Plaque biofilm profiles vary among individuals because they are modulated by different environmental factors as well as quorum sensing signals derived from adjacent microorganisms [343], [344], [345]. These confounding factors may temper the efficacy of antimicrobial polymers in vivo. In the contemporary world of evidence-based medicine and dentistry which demands the conscientious, explicit and judicious use of current best evidence in making decisions about the care of individual patients [346], it is crucial to ensure that QACs that have been tested in vitro have equivalent antimicrobial potential in in vivo settings before they are marketed commercially as “antimicrobial dental materials”. Moreover, clinically relevant in-situ models are required to validate the effects of novel antibacterial dental materials. Double-blind randomized control clinical trials remain the gold standard in evaluating treatment outcomes [347], [348]. In a case-controlled observational mini-study (ClinicalTrials.gov: registration number NCT00299598), ten volunteers wore removable acrylic appliances with two control resin composite specimens and two resin composite specimens incorporating 1 wt% QPEI nanoparticles for 4 h to develop intraoral biofilms [230]. The vitality of biofilms on QPEI-containing specimens was reduced by more than 50%, while those grown on the control resin composite discs were predominantly viable. No inflammation or allergic reaction developed after wearing of the appliances containing the experimental specimens. Recently, a double-blind, randomized clinical trial (RCT) was conducted to examine the in vivo antimicrobial efficacy of QAMS-containing orthodontic acrylic (ClinicalTrials.gov: registration number NCT02525458) [291]. The trial involved intraoral wearing of custom-made removable retainers by thirty-two human subjects to create 48-h multi-species plaque biofilms, using a split-mouth study design. Using confocal laser scanning microscopy, it was found that incorporation of 5 wt% QAMS in a PMMA-based orthodontic acrylic resin enables killing of microorganisms in the plaque biofilms without harm to the oral mucosa or systemic health (Fig. 15 ).
Fig. 15.
Randomized clinical trial on the in vivo antimicrobial efficacy of quaternary ammonium methacryloxy siloxane (QAMS)-containing orthodontic acrylic. A. Schematic illustrating the relationship between a test disk and its fitting well within the retainer appliance. The disk surface to be examined was turned toward the palate but not in direct contact with the palate. Each well was protected from the oral cavity by orthodontic acrylic to prevent disturbance of the biofilm by the tongue. B. Each appliance contained 4 wells to house two 6-mm diameter retrievable control disks and 2 similar diameter retrievable experimental PMMA disks (containing 5 wt% QAMS). C. BacLight-stained confocal laser scanning microscopy merged image showing the presence of live (green) bacteria and fungal hyphae (open arrowhead) within the biovolume of a biofilm taken from a control disk of one subject. D. The corresponding BacLight-stained merged image showing the presence of dead (red) bacteria and fungal hyphae killed by the QAMS-containing acrylic from an experimental disk of the same subject. [291], Copyright 2016. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Reproduced with permission from Scientific Reports, Nature Publishing Group.
It was previously perceived that polymerized “contact killing” antibacterial resin polymers exhibit antimicrobial activities only via direct contact of bacteria, without antibacterial effects against planktonic (free-floating) bacteria [349]. However, recent studies of QA-containing materials conducted with confocal laser scanning microscopy and three-dimensional reconstruction of biofilms stained with live-dead fluorescent stains [350], [351] indicate that those materials possess a reducing spectrum of biocidal activities from the substrate surface to the top of the biofilms [230], [288], [289], [352], [353]. That is, the presence of dead microorganisms is not limited to the bottom of the biofilms that is in contact with the antibacterial surface. This phenomenon may be explained by the ability of bacteria to undergo quorum-sensing signaling-induced programmed cell death in responses to environmental stresses [354], [355]. Activation of altruistic cell death mechanisms stimulates the release of pheromones such as the toxin component of toxin-antitoxin systems or bacteriocins (bacteriolytic molecules) [356], [357]. This coordinated sacrificial lifestyle in the biofilm community enables elimination of non-competent members of the community to sustain survival of the remaining members or for re-colonization. For S. mutans biofilms, a quorum-sensing pathway is present for inducing bacteriocin production in response to environmental stresses. The pathway responds to the intracellular accumulation of competence-stimulating peptide pheromone [358]. The occurrence of apoptosis-like programmed cell death in bacteria provides a rational explanation for the observation of three-dimensional killing by QACs within in vivo plaque biofilms that mimic the process of diffusional-killing by leached antimicrobial agents incorporated into polymers (Video 1 in Supplementary material; https://youtu.be/Iw5StFwE_FU).
7. Toxicological aspects of QACs
Although QAC-based biomedical materials have seen exciting advances, there are public concerns and even frustrations regarding their potential harm to the ecosystem and human health due to recent toxicological reports on QACs. Detection of QACs and their degradation products have been reported in diverse environments such as surface water, sediment and sludge-amended soil. The toxicity of QACs has received considerable attention because in addition to microorganisms, QACs are toxic to aquatic organisms, soil organisms, animals and humans [359]. Toxicity data from laboratory studies are essential for evaluation of the possible biological risks of QACs. Sandbacka et al. reported that QACs exhibited acute toxicity on fish cells, daphnia magna and even white fish [360]. Other studies reported that QACs exhibited higher toxicity in algae than fish and crustaceans [359], [361], [362], [363], due to their high affinity for negatively-charged algal cell walls. Sludge containing QACs also exhibit toxicity towards soil organisms such as earthworms [364]. Accumulation of QACs, together with other contaminants in soil over time, may change the soil environment and lead to potential bioaccumulation in soil organisms, resulting in potential biological risks [365]. Hence, the toxicological effects of QACs after their long-term exposure to the environment warrant further investigation [366].
Special attention should be paid to the toxicity of QACs towards animals and humans. The common QAC surfactant benzalkonium chloride causes toxicity and genotoxicity in Daphnia magna and Ceriodaphnia dubia models [367]. Using a eukaryotic cell model, genotoxicity has been observed for two QACs, benzalkonium chloride and dimethyldioctadecyl-ammonium bromide, at concentrations commonly found in waste water [368] and in commercially available nasal preparations [369]. These data suggest that direct contact with QA-containing detergents and pharmaceuticals that contain substantially higher QAC concentrations may cause potential DNA-damaging effects in humans. Different cell types have dissimilar susceptibility to the same QAC. An in vitro study conducted by Sokolova and coworkers reported that QACs exhibit selective toxic action among different cell lines [85]. Such a phenomenon may be explained by the different cell culture conditions and selective toxicity of QACs against lymphoblastoid cells compared with cells of epithelial origin. Melin et al. reported that QACs significantly reduced reproductive function in both male and female mice [370], [371]. Larsen et al. also found that inhalation of QAC aerosols results in acute airway irritation and inflammation in mice [372]. Theoretically, the QACs cannot be released from QA-based biomedical materials. Thus, a better understanding of the mechanisms of QAC-related toxicity should promote the innocuous use of these technologies.
The antimicrobial activity of QACs has generally been attributed to their ability to destroy cell membrane structure. To investigate the mechanisms responsible for the toxicity of QACs, Vieira’s group compared the toxicity of QACs to cells cultured in vitro with their ability to kill pathogens [373]. The authors also evaluated the cytotoxicity of QACs toward several mammalian cell lines [374]. Unexpectedly, they found that cytotoxicity is intracellular in origin at low QAC concentrations; abnormality or damage of intracellular biochemical processes are involved in lieu of membrane disintegration or cell lysis. It has been shown that QACs at low concentrations induce oxidative stresses, trigger apoptotic signals and destroy cells by apoptosis [375], [376]. Similarly, DMAE-CB monomers cause overproduction of reactive oxygen species, cell cycle arrest, reduction in mitochondrial membrane potential and enhanced caspase-3 activity [61]. Mitochondria are believed to be a core component of the cell death machinery. Mitochondria act as sensors and amplifiers in determining the execution of cell death or apoptosis through regulation of cellular energy metabolism (mitochondrial respiration and oxidative phosphorylation) [377]. Mitochondrial dysfunction and perturbation of cellular energetic mechanisms are involved in QAC-induced toxicity [367], [378], [379], [380]. At very low concentrations, QACs inhibit mitochondrial respiration and oxidative phosphorylation. Apoptosis occurs when the cellular energy charge is reduced by more than 50%. An in vitro study showed that QACs alter DNA structure and compromise vital cellular processes [381]. Recently, DMAE-CB monomers were found to induce intracellular oxidative stress, oxidative DNA damage and induction of intrinsic mitochondrial apoptosis [382]. Taking together, oxidative stress and oxidative damage are involved in QAC-induced cytotoxicity.
Based on the finding that oxidative stresses are involved in the cytotoxic effects of QACs, antioxidants such as N-acetyl cysteine (NAC) have been used for scavenging ROS and antioxidants to reduce the cytotoxicity of QACs [53], [60]. Modification of the molecular structures of QACs (e.g. preparation of pegylated-polymers) is an alternative strategy for combating their toxicity. It has been demonstrated that incorporation of poly(ethylene glycol) in QACs completely suppresses hemolysis of human red blood cells [383], [384]. Well-defined pegylated-polymers have been synthesized via RAFT polymerization [385]. By modifying the content of hydrophobic groups and molecular weights, the antimicrobial and hemolytic activities of amphiphilic polymethacrylate derivatives can be tailored. Compared with pegylated-polymers consisting of longer alkyl spacers, polymers with a shorter alkyl or hydroxyl group exhibit minimal hemolysis of red blood cells while retaining potent antimicrobial activities.
Quaternary ammonium-based nanomaterials may cause latent nanoparticle-related toxicity. Nano-sized particles have unique bio-distribution due to their highly different pharmacokinetic properties. Although existing drugs or materials are safe in the micrometer dimensions, unpredictable adverse and toxicological effects may emerge when they are formulated at the nanoscale. Nanoparticles are small enough to penetrate blood-brain barrier in the body, causing neurotoxicity [386]. Several approaches have been suggested and identified to achieve safe design of nanomaterials, based on the understanding of the bio-nanointerface [387] (Fig. 16 ). These approaches include: (1) changing the surface characteristics (such as size, surface charge, and dispersibility, particularly the effect of hydrophobicity) and the ability of nanoparticles to aggregate, (2) coating nanoparticles with protective shells, and (3) designing nanoparticles with different oxidative states.
Fig. 16.
Schematic representation of the interface between a nanoparticle and the lipid bilayer of a cell membrane. The nano-bio interface comprises the surface of the nanoparticle; the solid-liquid interface and the effects of the surrounding medium; and the interface’s contact zone with biological substrates.
8. QACs and antimicrobial resistance
Antimicrobial resistance of pathogens is rapidly becoming the most serious concern in contemporary infection control [388]. A new post-antibiotic era has instigated since the discovery of untreatable strains of Enterobacteriaceae that are resistant to the carbapenem class of antibiotics, considered the “drug of last resort” for such infections [389]. Until recently, QACs were thought to be invulnerable to the development of antimicrobial resistance [390]. However, recently acquired data has shown this to be far from the truth. Therefore, comprehending the contributory conditions and underlying mechanisms that result in antimicrobial resistance of QACs are still required.
At concentrations above the minimum inhibitory concentration, it is well accepted that QACs interact with cell membranes of microorganisms, disrupt their membrane integrity and cause leakage of cellular contents [47]. However, QACs are biodegradable under aerobic conditions. As a result, microorganisms are dynamically exposed to QACs over a wide range of concentrations (i.e., non-inhibitory, sub-inhibitory, over-inhibitory concentrations). In general, environmental concentrations of QACs, including those that are found in sewage, biological wastewater treatment units, surface water and sediments, are well below minimum inhibitory concentration values. Thus, it is possible to prolong the contact between QACs and microorganisms at sub-inhibitory concentrations. Such environments with QACs at low chemical reactivity become selective and favorable for the survival of clones with lower susceptibility, ultimately leading to evolution and selection of QAC-resistant bacteria [391]. Many excellent reviews indicate that QAC-related antimicrobial resistance occurs at sub-inhibitory concentrations through both intrinsic and acquired resistance mechanisms [392], [393] (Fig. 17 ). Bacteria compensate for the oxidative stresses created by exposure to QACs at sub-inhibitory concentrations via SOS-response and induction of stress-response sigma factors rpoS inducing modification of cell membrane structure and composition, enhanced biofilm formation, hyper-expression of efflux pumps and acquisition of efflux pump genes through mobile recombination elements [394]. Mutations in QAC-resistant bacterial strains cause modifications in cell membrane, resulting in reduction in cell permeability and a more anionic and hydrophobic cell surface. Such modifications help reduce or restrict QAC-induced contact killing [395]. Exposure to QACs at sub-inhibitory concentrations may enhance biofilm formation [396], [397] through augmentation of certain gene expression, such as stress response sigma factor HrcA. The latter encodes a transcriptional regulator of the class I heat-shock response [398], and DnaK which encodes a class I heat-shock response chaperone protein [399]. The reduced expression of porins, which results in the changes of membrane protein composition, is also attributed to QAC resistance [400], [401]. Efflux pumps, which are generally chromosomally-encoded, help remove antimicrobial agents from the inside of bacterial cell [402]. Efflux-mediated QAC resistance may involve two mechanisms. First, QAC resistance is achieved by hyper-expression of efflux pumps or increase in their extrusion efficiency, including regulatory modifications or mutations of efflux determinants [403]. For instance, a study by Guo et al. showed that over-expression of efflux-related genes in a variant of Salmonella typhimurium increases their tolerance to benzalkonium chloride [404]. Another study by Buffet-Bataillon et al. demonstrated that clinical E. coli strains, variants of which are pathogenic, exhibit higher minimum inhibitory concentrations of QACs and ciprofloxacin. Their antibiotic resistance depends on the AcrAB-TolC efflux pump and its regulatory genes [405]. The second mechanism of QAC resistance is through acquisition of genes for specialized QAC efflux pumps through mobile recombination elements, such as plasmids, transposon or integrons. Acquisition of genetic elements, which can be collaterally transferred between bacteria of the same or different genera via quorum sensing, may result in co-resistance (two or more resistance mechanisms in one organism) or cross-resistance (one resistance mechanism that counteracts two or more antibacterial agents). The emergence of “small multidrug resistance” includes expressions of both multidrug efflux pumps [406] and QAC-specific efflux determinants [407]. Accordingly, antibiotic efflux mechanism is likely to be a potential therapeutic target to counteract antimicrobial resistance. There is limited in vitro evidence showing that efflux inhibitors such as verapamil and reserpine can block efflux pumps [408]. Further research is required to enhance the pharmacokinetics of these drugs and improve their biocompatibility to target QAC resistance by bacteria.
Fig. 17.
Pathways and mechanisms of antimicrobial resistance in quaternary ammonium compounds (QACs). Bacteria compensate for exposure to QACs at sub-minimal inhibitory conditions via SOS-response and stress-response sigma factors rpoS-induction. These factors, in turn, induce modifications in bacteria cell physiology that result in the development QAC resistance. These modifications include induction of QAC biodegradation, enhanced biofilm formation, modifications in cell membrane via reduced expression of porins, acquisition of efflux genes such as plasmids, transposon or integrons, and over-expression of efflux pumps which helps remove antimicrobial agents from the inside of a bacterial cell with high extrusion efficiency. These modifications help reduce or restrict QAC-induced contact-killing. [394], Copyright 2009.
Reproduced with permission from Elsevier Science Ltd.
Regarding QAC-based biomedical materials, there is an urgent need to evaluate whether the long-term use of such technologies results in reduced microbial susceptibility to the QAC and the emergence of QAC-resistant strains. A recent study investigated whether S. mutans and E. faecalis could develop resistance to the cationic biocides [409]. There were no changes in the minimum inhibitory concentration of MDPB to S. mutans and E. faecalis even after ten passages, suggesting that no antimicrobial resistance has been developed. Although experimental and clinical evidence on the causal link between QAC-based biomedical materials and antimicrobial resistance are rare, it is important to bear in mind that the use of QACs at sub-inhibitory concentrations helps develop QAC-resistant strains. Caution would be required in the use of QAC-based biomedical materials.
9. Concluding remarks and future perspectives
In addition to their broad-spectrum antimicrobial activity, biomaterials with QA functionalities possess potent and long-term biocidal efficacy with non-leachable active species, better biocompatibility and no adverse effects on the mechanical properties of the carrier materials. The advent of new technologies and biomaterials design, especially the emergence of nanotechnology, brings unique opportunities and hope to the formulation of novel biomedical materials that contain QA-functionalized fillers/nano-fillers, to address the difficulties and drawbacks of incorporating antibacterial agents that exist in their conventional dimensions into those materials. Nevertheless, there is an ever-growing need to develop antimicrobial materials aiming at multi-species pathogenic microbes, since many microorganisms including bacteria, fungi, protozoa, prions and viruses are involved in microbial infections. Over the past two decades, the advent of various polymerization techniques and post-modification strategies has made it possible to produce QA-based antimicrobial surfaces with an unprecedented level of control over composition, structure, and properties. Despite phenomenal advances in the variety of polymerization techniques, the ongoing progress in each of these arenas indicates that there is still plenty of room for future development.
There are also growing concerns regarding the biocompatibility QACs. Considerable evidence points to their potential toxicological side-effects which may hamper their safe and population-wide applications. Along with the efforts to enhance their antimicrobial properties, lower cytotoxicity also needs to be achieved to expand their clinical use. Currently, many research studies are engaged in investigating structure-bioactivity relationship of QA-based biomedical materials to balance their antimicrobial activity and cytotoxicity. Although the exact and detailed mechanism is still largely unknown, prior reports suggest that oxidative stress plays an important role in their cytotoxicity. Experimental evidence suggests that the use of antioxidants helps reduce their cytotoxicity [53], [60], [410]. Some QACs may induce differential changes in expression of antioxidant enzymes such as superoxide dismutase, glutathione peroxidase, and catalase [382]. The expression of these antioxidant enzymes is under the control of nuclear factor E2-related factor 2 (Nrf2), a transcription factor that plays a key role in the intracellular anti-oxidative defense system to maintain redox homeostasis [411]. The aforementioned pathway also plays a protective role in nanomaterial-induced oxidative DNA damage and cell death [412], [413]. Accordingly, modulation of the Nrf2-mediated cellular defense response may help control QAC-induced cytotoxicity, an important issue that requires further investigation.
Sub-inhibitory concentrations of QACs may be involved in the evolution and selection of QAC-resistant bacteria through intrinsic or acquired resistance mechanisms. To date, there are no relevant studies that examine whether QA-based biomedical materials cause antimicrobial resistance. Nevertheless, one has to be careful with the use of QA-based biomedical materials. The biological environment within the human body is complex, and different fields of application require antimicrobial biomaterials with additional specific properties (e.g. remineralization or protein-repellent properties). Some research groups have incorporated additional components such as AgNPs [268], [269], [270], NO-releasing agents [173], [174], fluoride [414], NACPs [232], [233], [234], [235], [236], [237], [335] and 2-methacryloyloxyethyl phosphorylcholine [415], [416], [417] into QA-based biomedical materials to achieve augmented antimicrobial or multifunctional applications.
Biofilms are made up of a diverse community of interacting microorganisms with complex three-dimensional structures, including channels, micro-colonies and mushroom-like protrusions [418]. The properties of biofilms are distinct from those exhibited by planktonic species. Biofilms are more tolerant of adverse growth conditions such as antimicrobial agents, stress and host defense peptides. Thus, a better understanding of every aspect of the biofilm, especially with fluorescence microscopy coupled with functionalized nano-probes which allow in-situ investigations of the three-dimensional structure of biofilms, may help manage and eradicate biofilms. Recently, luminescent silica nanoparticles with QA functionality have been designed and synthesized [419]. Using confocal laser scanning microscopy, narrow diffusion paths of these luminescent nanoparticles were identified in P. aeruginosa biofilms. In addition, pH-sensitive luminescent silica nanoparticles have also been used to investigate pH microenvironment of biofilms of E. coli [420]. Future investigations with these functionalized nano-probes may render it possible to create a luminescent adhesive or resin composite with self-diagnostic capability using this technology. To expand the clinical use and commercialization of more QA-based biomedical materials, appropriately designed and well-structured multicenter clinical trials are critically needed to obtain reliable comparative data. Acquisition of these important information should help identify the most effective antibacterial solutions and the optimal circumstances for the use of these materials.
Acknowledgements
This work was supported by grant 81470773, grant 81130078 and grant 81400555 from National Nature Science Foundation of China, grant 2015AA020942 from National High Technology Research and Development Program of China and Program No. IRT13051 for Changjiang Scholars and Innovative Research Team in University.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.progpolymsci.2017.03.001.
Contributor Information
Franklin R. Tay, Email: ftay@augusta.edu.
Ji-hua Chen, Email: jhchen@fmmu.edu.cn.
Appendix A. Supplementary data
The following is Supplementary data to this article:
Representative three-dimensional confocal laser scanning microscopy (CLSM) video of biofilm grown on the surface of quaternary ammonium methacryloxy silane-containing acrylic resin (green channel: live microorganisms; red channel: dead microorganisms). https://youtu.be/Iw5StFwE_FU.
References
- 1.Avila M., Said N., Ojcius D.M. The book reopened on infectious diseases. Microbes Infect. 2008;10:942–947. doi: 10.1016/j.micinf.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD 2013 Mortality and Causes of Death Collaborators Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the global burden of disease study 2013. Lancet. 2015;385:117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salwiczek M., Qu Y., Gardiner J., Strugnell R.A., Lithgow T., McLean K.M., et al. Emerging rules for effective antimicrobial coatings. Trends Biotechnol. 2014;32:82–90. doi: 10.1016/j.tibtech.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 4.Oyston P.C., Fox M.A., Richards S.J., Clark G.C. Novel peptide therapeutics for treatment of infections. J Med Microbiol. 2009;58:977–987. doi: 10.1099/jmm.0.011122-0. [DOI] [PubMed] [Google Scholar]
- 5.Lakshmaiah Narayana J., Chen J.Y. Antimicrobial peptides: possible anti-infective agents. Peptides. 2015;72:88–94. doi: 10.1016/j.peptides.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 6.Campoccia D., Montanaro L., Speziale P., Arciola C.R. Antibiotic-loaded biomaterials and the risks for the spread of antibiotic resistance following their prophylactic and therapeutic clinical use. Biomaterials. 2010;31:6363–6377. doi: 10.1016/j.biomaterials.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 7.Talbot G.H., Bradley J., Edwards J.E., Jr., Gilbert D., Scheld M., Bartlett J.G. Antimicrobial availability task force of the Infectious Diseases Society of America: bad bugs need drugs: an update on the development pipeline from the antimicrobial availability task force of the Infectious Diseases Society of America. Clin Infect Dis. 2006;42:657–668. doi: 10.1086/499819. [DOI] [PubMed] [Google Scholar]
- 8.Organization WH Antimicrobial resistance: global report on surveillance. Australas Med J. 2014;7:695–704. [Google Scholar]
- 9.Isquith A., Abbott E., Walters P. Surface-bonded antimicrobial activity of an organosilicon quaternary ammonium chloride. Appl Microbiol. 1972;24:859–863. doi: 10.1128/am.24.6.859-863.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tuladhar E., de Koning M.C., Fundeanu I., Beumer R., Duizer E. Different virucidal activities of hyperbranched quaternary ammonium coatings on poliovirus and influenza virus. Appl Environ Microbiol. 2012;78:2456–2458. doi: 10.1128/AEM.07738-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deka S.R., Sharma A.K., Kumar P. Cationic polymers and their self-assembly for antibacterial applications. Curr Top Med Chem. 2015;15:1179–1195. doi: 10.2174/1568026615666150330110602. [DOI] [PubMed] [Google Scholar]
- 12.Campoccia D., Montanaro L., Arciola C.R. A review of the biomaterials technologies for infection-resistant surfaces. Biomaterials. 2013;34:8533–8554. doi: 10.1016/j.biomaterials.2013.07.089. [DOI] [PubMed] [Google Scholar]
- 13.Cloutier M., Mantovani D., Rosei F. Antibacterial coatings: challenges, perspectives, and opportunities. Trends Biotechnol. 2015;33:637–652. doi: 10.1016/j.tibtech.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Mai S., Mauger M.T., Niu L.N., Barnes J.B., Kao S., Bergeron B.E., et al. Potential applications of antimicrobial peptides and their mimics in combating caries and pulpal infections. Acta Biomater. 2016;49:16–35. doi: 10.1016/j.actbio.2016.11.026. [DOI] [PubMed] [Google Scholar]
- 15.Lima S.M., de Pádua G.M., Sousa M.G., Freire de M.S., Franco O.L., Rezende T.M. Antimicrobial peptide-based treatment for endodontic infections-biotechnological innovation in endodontics. Biotechnol Adv. 2015;33:203–213. doi: 10.1016/j.biotechadv.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 16.Jain A., Duvvuri L.S., Farah S., Beyth N., Domb A.J., Khan W. Antimicrobial polymers. Adv Healthc Mater. 2014;3:1969–1985. doi: 10.1002/adhm.201400418. [DOI] [PubMed] [Google Scholar]
- 17.Padovani G.C., Feitosa V.P., Sauro S., Tay F.R., Durán G., Paula A.J., et al. Advances in dental materials through nanotechnology: facts, perspectives and toxicological aspects. Trends Biotechnol. 2015;33:621–636. doi: 10.1016/j.tibtech.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 18.Paladini F., Pollini M., Sannino A., Ambrosio L. Metal-based antibacterial substrates for biomedical applications. Biomacromolecules. 2015;16:1873–1885. doi: 10.1021/acs.biomac.5b00773. [DOI] [PubMed] [Google Scholar]
- 19.Seabra A.B., Justo G.Z., Haddad P.S. State of the art challenges and perspectives in the design of nitric oxide-releasing polymeric nanomaterials for biomedical applications. Biotechnol Adv. 2015;33:1370–1379. doi: 10.1016/j.biotechadv.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 20.Michl T.D., Coad B.R., Doran M., Osiecki M., Kafshgari M.H., Voelcker N.H., et al. Nitric oxide releasing plasma polymer coating with bacteriostatic properties and no cytotoxic side effects. Chem Commun. 2015;51:7058–7060. doi: 10.1039/c5cc01722j. [DOI] [PubMed] [Google Scholar]
- 21.Weber D.J., Rutala W.A. Self-disinfecting surfaces: review of current methodologies and future prospects. Am J Infect Control. 2013;41:S31–5. doi: 10.1016/j.ajic.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 22.Yueh M.F., Tukey R.H. Triclosan: a widespread environmental toxicant with many biological effects. Annu Rev Pharmacol Toxicol. 2016;56:251–272. doi: 10.1146/annurev-pharmtox-010715-103417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Domagk G. A new class of disinfectants. Dtsch Med Wochenscher. 1935;61:829–832. [Google Scholar]
- 24.Jennings M.C., Buttaro B.A., Minbiole K.P.C., Wuest W.M. Bioorganic investigation of multicationic antimicrobials to combat QAC-resistant Staphylococcus aureus. ACS Infect Dis. 2015;1:304–309. doi: 10.1021/acsinfecdis.5b00032. [DOI] [PubMed] [Google Scholar]
- 25.Xue Y., Xiao H., Zhang Y. Antimicrobial polymeric materials with quaternary ammonium and phosphonium salts. Int J Mol Sci. 2015;16:3626–3655. doi: 10.3390/ijms16023626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carmona-Ribeiro A.M., de Melo Carrasco L.D. Cationic antimicrobial polymers and their assemblies. Int J Mol Sci. 2013;14:9906–9946. doi: 10.3390/ijms14059906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muñoz-Bonilla A., Fernández-García M. Polymeric materials with antimicrobial activity. Prog Polym Sci. 2012;37:281–339. [Google Scholar]
- 28.Jaeger W., Bohrisch J., Laschewsky A. Synthetic polymers with quaternary nitrogen atoms-synthesis and structure of the most used type of cationic polyelectrolytes. Prog Polym Sci. 2010;35:511–577. [Google Scholar]
- 29.Marcotte L., Barbeau J., Lafleur M. Permeability and thermodynamics study of quaternary ammonium surfactants-phosphocholine vesicle system. J Colloid Interface Sci. 2005;292:219–227. doi: 10.1016/j.jcis.2005.05.060. [DOI] [PubMed] [Google Scholar]
- 30.Haldar J., An D., de Cienfuegos L.Á., Chen J., Klibanov A.M. Polymeric coatings that inactivate both influenza virus and pathogenic bacteria. Proc Natl Acad Sci USA. 2006;103:17667–17671. doi: 10.1073/pnas.0608803103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kenawy el R., Worley S.D., Broughton R. The chemistry and applications of antimicrobialpolymers: a state-of-the-art review. Biomacromolecules. 2007;8:1359–1384. doi: 10.1021/bm061150q. [DOI] [PubMed] [Google Scholar]
- 32.Oblak E., Piecuch A., Krasowska A., Luczynski J. Antifungal activity of gemini quaternary ammonium salts. Microbiol Res. 2013;168:630–638. doi: 10.1016/j.micres.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 33.Wessels S., Ingmer H. Modes of action of three disinfectant active substances: a review. Regul Toxicol Pharmacol. 2013;67:456–467. doi: 10.1016/j.yrtph.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 34.Ikeda T., Hirayama H., Yamaguchi H., Tazuke S., Watanabe M. Polycationic biocides with pendant active groups: molecular weight dependence of antibacterial activity. Antimicrob Agents Chemother. 1986;30:132–136. doi: 10.1128/aac.30.1.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen C.Z., Beck-Tan N.C., Dhurjati P., van Dyk T.K., LaRossa R.A., Cooper S.L. Quaternary ammonium functionalized poly (propylene imine) dendrimers as effective antimicrobials: structure-activity studies. Biomacromolecules. 2000;1:473–480. doi: 10.1021/bm0055495. [DOI] [PubMed] [Google Scholar]
- 36.Gilbert P., Al-Taae A. Antimicrobial activity of some alkyltrimethylammonium bromides. Lett App Microbiol. 1985;1:101–104. [Google Scholar]
- 37.Caillier L., de Givenchy E.T., Levy R., Vandenberghe Y., Geribaldi S., Guittard F. Synthesis and antimicrobial properties of polymerizable quaternary ammoniums. Eur J Med Chem. 2009;44:3201–3208. doi: 10.1016/j.ejmech.2009.03.031. [DOI] [PubMed] [Google Scholar]
- 38.He J., Soderling E., Vallittu P.K., Lassila L.V. Investigation of double bond conversion, mechanical properties, and antibacterial activity of dental resins with different alkyl chain length quaternary ammonium methacrylate monomers (QAM) J Biomater Sci Polym Ed. 2013;24:565–573. doi: 10.1080/09205063.2012.699709. [DOI] [PubMed] [Google Scholar]
- 39.Kong M., Chen X.G., Xing K., Park H.J. Antimicrobial properties of chitosan and mode of action: a state of the art review. Int J Food Microbiol. 2010;144:51–63. doi: 10.1016/j.ijfoodmicro.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 40.Gilbert P., Moore L.E. Cationic antiseptics: diversity of action under a common epithet. J Appl Microbiol. 2005;99:703–715. doi: 10.1111/j.1365-2672.2005.02664.x. [DOI] [PubMed] [Google Scholar]
- 41.Garg G., Chauhan G.S., Gupta R., Ahn J.H. Anion effects on anti-microbial activity of poly[1-vinyl-3-(2-sulfoethyl imidazolium betaine)] J Colloid Interface Sci. 2010;344:90–96. doi: 10.1016/j.jcis.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 42.Ingalsbe M.L., Denis J.D., McGahan M.E., Steiner W.W., Priefer R. Development of a novel expression, ZI MAX/K ZI, for determination of the counter-anion effect on the antimicrobial activity of tetrabutylammonium salts. Bioorg Med Chem Lett. 2009;19:4984–4987. doi: 10.1016/j.bmcl.2009.07.066. [DOI] [PubMed] [Google Scholar]
- 43.Sharma S.K., Chauhan G.S., Gupta R., Ahn J.H. Tuning anti-microbial activity of poly(4-vinyl 2-hydroxyethyl pyridinium) chloride by anion exchange reactions. J Mater Sci Mater Med. 2010;21:717–724. doi: 10.1007/s10856-009-3932-9. [DOI] [PubMed] [Google Scholar]
- 44.Xie D., Weng Y., Guo X., Zhao J., Gregory R.L., Zheng C. Preparation and evaluation of a novel glass-ionomer cement with antibacterial functions. Dent Mater. 2011;27:487–496. doi: 10.1016/j.dental.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 45.Panarin E.F., Solovskii M.V., Zaikina N.A., Afinogenov G.E. Biological activity of cationic polyelectrolytes. Makromol Chem. 1985;9:25–33. [Google Scholar]
- 46.Mizerska U., Fortuniak W., Chojnowski J., Halasa R., Konopacka A., Werel W. Polysiloxane cationic biocides with imidazolium salt (ImS) groups, synthesis and antibacterial properties. Eur Polym J. 2009;45:779–787. [Google Scholar]
- 47.Tischer M., Pradel G., Ohlsen K., Holzgrabe U. Quaternary ammonium salts and their antimicrobial potential: targets or nonspecific interactions. ChemMedChem. 2012;7:22–31. doi: 10.1002/cmdc.201100404. [DOI] [PubMed] [Google Scholar]
- 48.Gottenbos B., Grijpma D.W., van der Mei H.C., Feijen J., Busscher H.J. Antimicrobial effects of positively charged surfaces on adhering Gram-positive and Gram-negative bacteria. J Antimicrob Chemother. 2001;48:7–13. doi: 10.1093/jac/48.1.7. [DOI] [PubMed] [Google Scholar]
- 49.Cavallaro A., Mierczynska A., Barton M., Majewski P., Vasilev K. Influence of immobilized quaternary ammonium group surface density on antimicrobial efficacy and cytotoxicity. Biofouling. 2016;32:13–24. doi: 10.1080/08927014.2015.1115977. [DOI] [PubMed] [Google Scholar]
- 50.Yoshikawa K., Clark D.T., Brailsford S.R., Beighton D., Watson T.F., Imazato S., et al. The effect of antibacterial monomer MDPB on the growth of organisms associated with root caries. Dent Mater J. 2007;26:388–392. doi: 10.4012/dmj.26.388. [DOI] [PubMed] [Google Scholar]
- 51.Imazato S., Ebi N., Tarumi H., Russell R.R., Kaneko T., Ebisu S. Bactericidal activity and cytotoxicity of antibacterial monomer MDPB. Biomaterials. 1999;20:899–903. doi: 10.1016/s0142-9612(98)00247-6. [DOI] [PubMed] [Google Scholar]
- 52.Nishida M., Imazato S., Takahashi Y., Ebisu S., Ishimoto T., Nakano T., et al. The influence of the antibacterial monomer 12-methacryloyloxydodecylpyridinium bromide on the proliferation, differentiation and mineralization of odontoblast-like cells. Biomaterials. 2010;31:1518–1532. doi: 10.1016/j.biomaterials.2009.11.023. [DOI] [PubMed] [Google Scholar]
- 53.Ma S., Imazato S., Takahashi Y., Kiba W., Takeda K., Izutani N., et al. Mechanism of detoxification of the cationic antibacterial monomer12-methacryloyloxydodecylpyridiniumbromide (MDPB) by N-acetyl cysteine. Dent Mater. 2013;29:1219–1227. doi: 10.1016/j.dental.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 54.Pupo Y.M., Farago P.V., Nadal J.M., Simao L.C., Esmerino L.A., Gomes O.M., et al. Effect of a novel quaternary ammonium methacrylate polymer (QAMP) on adhesion and antibacterial properties of dental adhesives. Int J Mol Sci. 2014;15:8998–9015. doi: 10.3390/ijms15058998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pupo Y.M., Farago P.V., Nadal J.M., Esmerino L.A., Maluf D.F., Zawadzki S.F., et al. An innovative quaternary ammonium methacrylate polymer can provide improved antimicrobial properties for a dental adhesive system. J Biomater Sci Polym Ed. 2013;24:1443–1458. doi: 10.1080/09205063.2013.766784. [DOI] [PubMed] [Google Scholar]
- 56.Li F., Weir M.D., Fouad A.F., Xu H.H. Time-kill behaviour against eight bacterial species and cytotoxicity of antibacterial monomers. J Dent. 2013;41:881–891. doi: 10.1016/j.jdent.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang L., Xiao Y.H., Xing X.D., Li F., Ma S., Qi L.L., et al. Antibacterial activity and cytotoxicity of two novel cross-linking antibacterial monomers on oral pathogens. Arch Oral Biol. 2011;56:367–373. doi: 10.1016/j.archoralbio.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 58.Xiao Y.H., Chen J.H., Fang M., Xing X.D., Wang H., Wang Y.J., et al. Antibacterial effects of three experimental quaternary ammonium salt (QAS) monomers on bacteria associated with oral infections. J Oral Sci. 2008;50:323–327. doi: 10.2334/josnusd.50.323. [DOI] [PubMed] [Google Scholar]
- 59.Chai Z., Li F., Fang M., Wang Y., Ma S., Xiao Y., et al. The bonding property and cytotoxicity of a dental adhesive incorporating a new antibacterial monomer. J Oral Rehabil. 2011;38:849–856. doi: 10.1111/j.1365-2842.2011.02212.x. [DOI] [PubMed] [Google Scholar]
- 60.Jiao Y., Ma S., Li J., Shan L., Wang Y., Tian M., et al. N-acetylcysteine (NAC)-directed detoxification of methacryloxylethyl cetyl ammonium chloride (DMAE-CB) PLoS One. 2015;10 doi: 10.1371/journal.pone.0135815. e0135815/1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ma S., Shan L.Q., Xiao Y.H., Li F., Huang L., Shen L., et al. The cytotoxicity ofmethacryloxylethyl cetyl ammonium chloride, a cationic antibacterial monomer, is related to oxidative stress and the intrinsic mitochondrial apoptotic pathway. Braz J Med Biol Res. 2011;44:1125–1133. doi: 10.1590/s0100-879x2011007500130. [DOI] [PubMed] [Google Scholar]
- 62.Imazato S., Torii Y., Takatsuka T., Inoue K., Ebi N., Ebisu S. Bactericidal effect of dentinprimer containing antibacterial monomer methacryloyloxydodecylpyridinium bromide(MDPB) against bacteria in human carious dentin. J Oral Rehabil. 2001;28:314–319. doi: 10.1046/j.1365-2842.2001.00659.x. [DOI] [PubMed] [Google Scholar]
- 63.Li J., Sha Z., Zhang W., Tao F., Yang P. Preparation and antibacterial properties of gelatin grafted with an epoxy silicone quaternary ammonium salt. J Biomater Sci Polym Ed. 2016;27:1017–1028. doi: 10.1080/09205063.2016.1175784. [DOI] [PubMed] [Google Scholar]
- 64.Regis R.R., Vecchia M.P.D., Pizzolitto A.C., Compagnoni M.A., Souza P.P.C., Souza R.F.D. Antimicrobial properties and cytotoxicity of an antimicrobial monomer for application in prosthodontics. J Prosthodont. 2012;21:283–290. doi: 10.1111/j.1532-849X.2011.00815.x. [DOI] [PubMed] [Google Scholar]
- 65.Peng Z.X., Wang L., Du L., Guo S.R., Wang X.Q., Tang T.T. Adjustment of the antibacterial activity and biocompatibility of hydroxypropyltrimethyl ammonium chloride chitosan by varying the degree of substitution of quaternary ammonium. Carbohydr Polym. 2010;81:275–283. [Google Scholar]
- 66.Imazato S., Torii M., Tsuchitani Y., McCabe J.F., Russell R.R. Incorporation of bacterial inhibitor into resin composite. J Dent Res. 1994;73:1437–1443. doi: 10.1177/00220345940730080701. [DOI] [PubMed] [Google Scholar]
- 67.Cheng L., Weir M.D., Zhang K., Arola D.D., Zhou X., Xu H.H. Dental primer and adhesive containing a new antibacterial quaternary ammonium monomer dimethylaminododecyl methacrylate. J Dent. 2013;41:345–355. doi: 10.1016/j.jdent.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li F., Weir M.D., Xu H.H. Effects of quaternary ammonium chain length on antibacterialbonding agents. J Dent Res. 2013;92:932–938. doi: 10.1177/0022034513502053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.He J., Söderling E., Österblad M., Vallittu P.K., Lassila L.V. Synthesis of methacrylate monomers with antibacterial effects against S. mutans. Molecules. 2011;16:9755–9763. doi: 10.3390/molecules16119755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weng Y., Guo X., Chong V.J., Howard L., Gregory R.L., Xie D. Synthesis and evaluation of a novel antibacterial dental resin composite with quaternary ammonium salts. J Biomed Sci Eng. 2011;4:147–157. [Google Scholar]
- 71.Lewis K. In search of natural substrates and inhibitors of MDR pumps. J Mol Microbiol Biotechnol. 2001;3:247–254. [PubMed] [Google Scholar]
- 72.Leclercq L., Lubart Q., Dewilde A., Aubry J.M., Nardello-Rataj V. Supramolecular effects on the antifungal activity of cyclodextrin/di-n-decyldimethylammonium chloride mixtures. Eur J Pharm Sci. 2012;46:336–345. doi: 10.1016/j.ejps.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 73.Nurdin N., Helary G., Sauvet G. Biocidal polymers active by contact: III. Ageing of biocidal polyurethane coatings in water. J Appl Polym Sci. 1993;50:671–678. [Google Scholar]
- 74.Obłak E., Lachowicz T.M., Luczyński J., Witek S. Comparative studies of the biological activities of lysosomotropic aminoesters and quaternary ammonium salts on the yeast Saccharomyces cerevisiae. Cell Mol Biol Lett. 2001;6:871–880. [PubMed] [Google Scholar]
- 75.Oosterhof J.J., Buijssen K.J., Busscher H.J., van der Laan B.F., van der Mei H.C. Effects of quaternary ammonium silane coatings on mixed fungal and bacterial biofilms on tracheoesophageal shunt prostheses. Appl Environ Microbiol. 2006;72:3673–3677. doi: 10.1128/AEM.72.5.3673-3677.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vieira D.B., Carmona-Ribeiro A.M. Cationic lipids and surfactants as antifungal agents:mode of action. J Antimicrob Chemother. 2006;58:760–767. doi: 10.1093/jac/dkl312. [DOI] [PubMed] [Google Scholar]
- 77.Carmona-Ribeiro A.M., Vieira D.B., Lincopan N. Cationic surfactants and lipids as anti-infective agents. Anti-Infe Agents Med Chem. 2006;5:33–51. [Google Scholar]
- 78.Reboiras M.D., Miller M.J., Jones M.N. Liposome adsorption to Candida albicans. Colloids Surf B. 1997;9:101–107. [Google Scholar]
- 79.Campanhã M., Mamizuka E., Carmona-Ribeiro A. Interactions between cationic vesicles and Candida albicans. J Phys Chem B. 2001;105:8230–8236. [Google Scholar]
- 80.Shirai A., Sumitomo T., Kurimoto M., Maseda H., Kourai H. The mode of the antifungal activity of gemini-pyridinium salt against yeast. Biocontrol Sci. 2009;14:13–20. doi: 10.4265/bio.14.13. [DOI] [PubMed] [Google Scholar]
- 81.Shirai A., Ueta S., Maseda H., Kourai H., Omasa T. Action of reactive oxygen species in the antifungal mechanism of gemini-pyridinium salts against yeast. Biocontrol Sci. 2012;17:77–82. doi: 10.4265/bio.17.77. [DOI] [PubMed] [Google Scholar]
- 82.Muhizi T., Coma V., Grelier S. Synthesis of D glucosamine quaternary ammonium derivatives and evaluation of their antifungal activity together with aminodeoxyglucose derivatives against two wood fungi Coriolus versicolor and Poria placenta: structure-activity relationships. Pest Manag Sci. 2011;67:287–293. doi: 10.1002/ps.2063. [DOI] [PubMed] [Google Scholar]
- 83.Tsao I.F., Wang H.Y., Shipman C., Jr. Interaction of infectious viral particles with a quaternary ammonium chlorid (QAC) surface. Biotechnol Bioeng. 1989;34:639–646. doi: 10.1002/bit.260340508. [DOI] [PubMed] [Google Scholar]
- 84.Walters P.A., Abbott E.A., Isquith A.J. Algicidal activity of a surface-bonded organosilicon quaternary ammonium chloride. Appl Microbiol. 1973;25:253–256. doi: 10.1128/am.25.2.253-256.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sokolova A.S., Yarovaya C.O., Shernyukov C.A., Pokrovsky C.E., Pokrovsky C.A., Lavrinenko V.A., et al. New quaternary ammonium camphor derivatives and their antiviral activity, genotoxic effects and cytotoxicity. Bioorgan Med Chem. 2013;21:6690–6698. doi: 10.1016/j.bmc.2013.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sokolova A.S., Yarovaya O.I., Shernyukov A.V., Gatilov Y.V., Razumova Y.V., Zarubaev V.V., et al. Discovery of a new class of antiviral compounds: camphor imine derivatives. Eur J Med Chem. 2015;105:263–273. doi: 10.1016/j.ejmech.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 87.Sokolova A.S., Yarovaya O.C., Korchagina D.V., Zarubaev V.V., Tretiak T.S., Anfimov P.M., et al. Camphor-based symmetric diimines as inhibitors of influenza virus reproduction. Bioorgan Med Chem. 2014;22:2141–2148. doi: 10.1016/j.bmc.2014.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zarubaev V.V., Garshinina A.V., Tretiak T.S., Fedorova V.A., Shtro A.A., Sokolova A.S., et al. Broad range of inhibiting action of novel camphor-based compound with anti-hemagglutinin activity against influenza viruses in vitro and in vivo. Antiviral Res. 2015;120:126–133. doi: 10.1016/j.antiviral.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 89.Shiraishi M., Aramaki Y., Seto M., Imoto H., Nishikawa Y., Kanzaki N., et al. Discovery of novel, potent, and selective small-molecule CCR5 antagonists as anti-HIV-1 agents: synthesis and biological evaluation of anilide derivatives with a quaternary ammonium moiety. J Med Chem. 2000;43:2049–2063. doi: 10.1021/jm9906264. [DOI] [PubMed] [Google Scholar]
- 90.Scott F.W. Virucidal disinfectants and feline viruses. Am J Vet Res. 1980;41:410–414. [PubMed] [Google Scholar]
- 91.Kennedy M.A., Mellon V.S., Caldwell G., Potgieter L. Virucidal efficacy of the newerquaternary ammonium compounds. J Am Anim Hosp Assoc. 1994;31:254–258. doi: 10.5326/15473317-31-3-254. [DOI] [PubMed] [Google Scholar]
- 92.Eleraky N.Z., Potgieter L.N., Kennedy M.A. Virucidal efficacy of four new disinfectants. J Am Anim Hosp Assoc. 2002;38:231–234. doi: 10.5326/0380231. [DOI] [PubMed] [Google Scholar]
- 93.Harada Y., Lekcharoensuk P., Furuta T., Taniguchi T. Inactivation of foot-and-mouth disease virus by commercially available disinfectants and cleaners. Biocontrol Sci. 2015;20:205–208. doi: 10.4265/bio.20.205. [DOI] [PubMed] [Google Scholar]
- 94.Tung G., Macinga D., Arbogast J., Jaykus L.A. Efficacy of commonly used disinfectants for inactivation of human noroviruses and their surrogates. J Food Prot. 2013;76:1210–1217. doi: 10.4315/0362-028X.JFP-12-532. [DOI] [PubMed] [Google Scholar]
- 95.Shirai J., Kanno T., Tsuchiya Y., Mitsubayashi S., Seki R. Effects of chlorine, iodine, and quaternary ammonium compound disinfectants on several exotic disease viruses. J Vet Med Sci. 2000;62:85–92. doi: 10.1292/jvms.62.85. [DOI] [PubMed] [Google Scholar]
- 96.Hoelzer K., Fanaselle W., Pouillot R., Van Doren J.M., Dennis S. Virus inactivation on hard surfaces or in suspension by chemical disinfectants: systematic review and meta-analysis of norovirus surrogates. J Food Prot. 2013;76:1006–1016. doi: 10.4315/0362-028X.JFP-12-438. [DOI] [PubMed] [Google Scholar]
- 97.Gulve N., Kimmerling K., Johnston A.D., Krueger G.R., Ablashi D.V., Prusty B.K. Anti-herpesviral effects of a novel broad range anti-microbial quaternary ammonium silane, K21. Antiviral Res. 2016;131:166–173. doi: 10.1016/j.antiviral.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 98.Mazzoni A., Tjäderhane L., Checchi V., Di Lenarda R., Salo T., Tay F.R., et al. Role of dentin MMPs in caries progression and bond stability. J Dent Res. 2015;94:241–251. doi: 10.1177/0022034514562833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chaussain-Miller C., Fioretti F., Goldberg M., Menashi S. The role of matrix metalloproteinases (MMPs) in human caries. J Dent Res. 2006;85:22–32. doi: 10.1177/154405910608500104. [DOI] [PubMed] [Google Scholar]
- 100.Palosaari H., Pennington C.J., Larmas M., Edwards D.R., Tjäderhane L., Salo T. Expressionprofile of matrix metalloproteinases (MMPs) and tissue inhibitors of MMPs in mature human odontoblasts and pulp tissue. Eur J Oral Sci. 2003;111:117–127. doi: 10.1034/j.1600-0722.2003.00026.x. [DOI] [PubMed] [Google Scholar]
- 101.Sulkala M., Wahlgren J., Larmas M., Sorsa T., Teronen O., Salo T., et al. The effects of MMP inhibitors on human salivary MMP activity and caries progression in rats. J Dent Res. 2001;80:1545–1549. doi: 10.1177/00220345010800061301. [DOI] [PubMed] [Google Scholar]
- 102.Menezes-Silva R., Khaliq S., Deeley K., Letra A., Vieira A.R. Genetic susceptibility to periapical disease: conditional contribution of MMP2 and MMP3 genes to the development of periapical lesions and healing response. J Endod. 2012;38:604–607. doi: 10.1016/j.joen.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Karayasheva D., Glushkova M., Boteva E., Mitev V., Kadiyska T. Association study for the role of matrix metalloproteinases 2 and 3 gene polymorphisms in dental caries susceptibility. Arch Oral Biol. 2016;68:9–12. doi: 10.1016/j.archoralbio.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 104.Tezvergil-Mutluay A., Agee K.A., Uchiyama T., Imazato S., Mutluay M.M., Cadenaro M., et al. The inhibitory effects of quaternary ammonium methacrylates on soluble and matrix-bound MMPs. J Dent Res. 2011;90:535–540. doi: 10.1177/0022034510389472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tezvergil-Mutluay A., Agee K.A., Mazzoni A., Carvalho R.M., Carrilho M., Tersariol I.L., et al. Can quaternary ammonium methacrylates inhibit matrix MMPs and cathepsins. Dent Mater. 2015;31:e25–32. doi: 10.1016/j.dental.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liu Y., Tjäderhane L., Breschi L., Mazzoni A., Li N., Mao J., et al. Limitations in bonding to dentin and experimental strategies to prevent bond degradation. J Dent Res. 2011;90:953–968. doi: 10.1177/0022034510391799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Carrilho M.R. Can exogenous protease inhibitors control dentin matrix degradation. J Dent Res. 2012;91:1099–1102. doi: 10.1177/0022034512462399. [DOI] [PubMed] [Google Scholar]
- 108.Jain A., Bahuguna R. Role of matrix metalloproteinases in dental caries, pulp and periapical inflammation: an overview. J Oral Biol Craniofac Res. 2015;5:212–218. doi: 10.1016/j.jobcr.2015.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chang Y.C., Yang S.F., Hsieh Y.S. Regulation of matrix metalloproteinase-2 production by cytokines and pharmacological agents in human pulp cell cultures. J Endod. 2001;27:679–682. doi: 10.1097/00004770-200111000-00007. [DOI] [PubMed] [Google Scholar]
- 110.Lin S.K., Wang C.C., Huang S., Lee J.J., Chiang C.P., Lan W.H., et al. Induction of dental pulp fibroblast matrix metalloproteinase-1 and tissue inhibitor of metalloproteinase-1 gene expression by interleukin-1alpha and tumor necrosis factor-alpha through a prostaglandin-dependent pathway. J Endod. 2001;27:185–189. doi: 10.1097/00004770-200103000-00012. [DOI] [PubMed] [Google Scholar]
- 111.Goda S., Kato Y., Domae E., Hayashi H., Tani-Ishii N., Iida J., et al. Effects of JNK1/2 on the inflammation cytokine TNF-alpha-enhanced production of MMP-3 in human dental pulp fibroblast-like cells. Int Endod J. 2015;48:1122–1128. doi: 10.1111/iej.12411. [DOI] [PubMed] [Google Scholar]
- 112.de Paula-Silva F.W., D'Silva N.J., da Silva L.A., Kapila Y.L. High matrix metalloproteinaseactivity is a hallmark of periapical granulomas. J Endod. 2009;35:1234–1242. doi: 10.1016/j.joen.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Leonardi R., Caltabiano R., Loreto C. Collagenase-3 (MMP-13) is expressed in periapical lesions: an immunohistochemical study. Int Endod J. 2005;38:297–301. doi: 10.1111/j.1365-2591.2005.00943.x. [DOI] [PubMed] [Google Scholar]
- 114.Wahlgren J., Salo T., Teronen O., Luoto H., Sorsa T., Tjäderhane L. Matrix metalloproteinase-8 (MMP-8) in pulpal and periapical inflammation and periapical root-canal exudates. Int Endod J. 2002;35:897–904. doi: 10.1046/j.1365-2591.2002.00587.x. [DOI] [PubMed] [Google Scholar]
- 115.Martinho F.C., Teixeira F.F., Cardoso F.G., Ferreira N.S., Nascimento G.G., Carvalho C.A., et al. Clinical investigation of matrix metalloproteinases, tissue inhibitors of matrix metalloproteinases, and matrix metalloproteinase/tissue inhibitors of matrix metalloproteinase complexes and their networks in apical periodontitis. J Endod. 2016;42:1082–1088. doi: 10.1016/j.joen.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 116.Zhao B., Brittain W.J. Polymer brushes: surface-immobilized macromolecules. Prog Polym Sci. 2000;25:677–710. [Google Scholar]
- 117.Zhou T., Zhu Y., Li X., Liu X., Yeung K.W.K., Wu S., et al. Surface functionalization of biomaterials by radical polymerization. Prog Mater Sci. 2016;83:191–235. [Google Scholar]
- 118.Barbey R., Lavanant L., Paripovic D., Schüwer N., Sugnaux C., Tugulu S., et al. Polymer brushes via surface-initiated controlled radical polymerization: synthesis, characterization, properties, and applications. Chem Rev. 2009;109:5437–5527. doi: 10.1021/cr900045a. [DOI] [PubMed] [Google Scholar]
- 119.Huang J., Koepsel R.R., Murata H., Wu W., Lee S.B., Kowalewski T., et al. Nonleaching antibacterial glass surfaces via grafting onto: the effect of the number of quaternary ammonium groups on biocidal activity. Langmuir. 2008;24:6785–6795. doi: 10.1021/la8003933. [DOI] [PubMed] [Google Scholar]
- 120.Davies M.L., Hamilton C.J., Murphy S.M., Tighe B.J. Polymer membranes in clinical sensor applications: I. An overview of membrane function. Biomaterials. 1992;13:971–978. doi: 10.1016/0142-9612(92)90147-g. [DOI] [PubMed] [Google Scholar]
- 121.Qiu J., Charleux B., Matyjaszewski K. Controlled/living radical polymerization inaqueous media: homogeneous and heterogeneous systems. Prog Polym Sci. 2001;26:2083–2134. [Google Scholar]
- 122.Mastan E., Li X., Zhu S. Modeling and theoretical development in controlled radical polymerization. Prog Polym Sci. 2015;45:71–101. [Google Scholar]
- 123.Lee S.B., Koepsel R.R., Morley S.W., Matyjaszewski K., Sun Y., Russell A.J. Permanent, nonleaching antibacterial surfaces: 1. Synthesis by atom transfer radical polymerization. Biomacromolecules. 2004;5:877–882. doi: 10.1021/bm034352k. [DOI] [PubMed] [Google Scholar]
- 124.Murata H., Koepsel R.R., Matyjaszewski K., Russell A.J. Permanent, non-leaching antibacterial surfaces. 2: How high density cationic surfaces kill bacterial cells. Biomaterials. 2007;28:4870–4879. doi: 10.1016/j.biomaterials.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 125.Ravikumar T., Murata H., Koepsel R.R., Russell A.J. Surface-active antifungal polyquaternary amine. Biomacromolecules. 2006;7:2762–2769. doi: 10.1021/bm060476w. [DOI] [PubMed] [Google Scholar]
- 126.Wang B., Xu Q., Ye Z., Liu H., Lin Q., Nan K., et al. Copolymer brushes with temperature-triggered, reversibly switchable bactericidal and antifouling properties for biomaterial surfaces. ACS Appl Mater Interfaces. 2016;8:27207–27217. doi: 10.1021/acsami.6b08893. [DOI] [PubMed] [Google Scholar]
- 127.Wang B., Ye Z., Xu Q., Liu H., Lin Q., Chen H., et al. Construction of a temperature-responsive terpolymer coating with recyclable bactericidal and self-cleaning antimicrobial properties. Biomater Sci. 2016;4:1731–1741. doi: 10.1039/c6bm00587j. [DOI] [PubMed] [Google Scholar]
- 128.Roy D., Knapp J.S., Guthrie J.T., Perrier S. Antibacterial cellulose fiber via RAFT surface graft polymerization. Biomacromolecules. 2008;9:91–99. doi: 10.1021/bm700849j. [DOI] [PubMed] [Google Scholar]
- 129.Ignatova M., Voccia S., Gilbert B., Markova N., Mercuri P.S., Galleni M., et al. Synthesis of copolymer brushes endowed with adhesion to stainless steel surfaces and antibacterial properties by controlled nitroxide-mediated radical polymerization. Langmuir. 2004;20:10718–10726. doi: 10.1021/la048347t. [DOI] [PubMed] [Google Scholar]
- 130.Voccia S., Ignatova M., Jérôme R., Jérôme C. Design of antibacterial surfaces by a combination of electrochemistry and controlled radical polymerization. Langmuir. 2006;22:8607–8613. doi: 10.1021/la0606087. [DOI] [PubMed] [Google Scholar]
- 131.Yuan S.J., Pehkonen S.O., Ting Y.P., Neoh K.G., Kang E.T. Inorganic-organic hybrid coatings on stainless steel by layer-by-layer deposition and surface-initiated atom-transfer-radical polymerization for combating biocorrosion. ACS Appl Mater Interfaces. 2009;1:640–652. doi: 10.1021/am800182d. [DOI] [PubMed] [Google Scholar]
- 132.Yuan S.J., Pehkonen S.O., Ting Y.P., Neoh K.G., Kang E.T. Antibacterial inorganic-organic hybrid coatings on stainless steel via consecutive surface-initiated atom transfer radical polymerization for biocorrosion prevention. Langmuir. 2010;26:6728–6736. doi: 10.1021/la904083r. [DOI] [PubMed] [Google Scholar]
- 133.Ignatova M., Voccia S., Gabriel S., Gilbert B., Cossement D., Jerome R., et al. Stainless steel grafting of hyperbranched polymer brushes with an antibacterial activity: synthesis, characterization, and properties. Langmuir. 2009;25:891–902. doi: 10.1021/la802472e. [DOI] [PubMed] [Google Scholar]
- 134.Yuan S.J., Xu F.J., Pehkonen S.O., Ting Y.P., Neoh K.G., Kang E.T. Grafting of antibacterial polymers on stainless steel via surface-initiated atom transfer radical polymerization for inhibiting biocorrosion by Desulfovibrio desulfuricans. Biotechnol Bioeng. 2009;103:268–281. doi: 10.1002/bit.22252. [DOI] [PubMed] [Google Scholar]
- 135.Dong H., Huang J., Koepsel R.R., Ye P., Russell A.J., Matyjaszewski K. Recyclable antibacterial magnetic nanoparticles grafted with quaternized poly(2-(dimethylamino)ethyl methacrylate) brushes. Biomacromolecules. 2011;12:1305–1311. doi: 10.1021/bm200031v. [DOI] [PubMed] [Google Scholar]
- 136.Rutnakornpituk B., Wichai U., Vilaivan T., Rutnakornpituk M. Surface-initiated atom transfer radical polymerization of poly(4-vinylpyridine) from magnetite nanoparticle. J Nanopart Res. 2011;13:6847–6857. [Google Scholar]
- 137.Zhang F., Shi Z.L., Chua P.H., Kang E.T., Neoh K.G. Functionalization of titanium surfacesvia controlled living radical polymerization: from antibacterial surface to surface for osteoblast adhesion. Ind Eng Chem Res. 2007;46:9077–9086. [Google Scholar]
- 138.Ramstedt M., Cheng N., Azzaroni O., Mossialos D., Mathieu H.J., Huck W.T. Synthesis and characterization of poly(3-sulfopropylmethacrylate) brushes for potential antibacterial applications. Langmuir. 2007;23:3314–3321. doi: 10.1021/la062670+. [DOI] [PubMed] [Google Scholar]
- 139.Xu F.J., Yuan S.J., Pehkonen S.O., Kang E.T., Neoh K.G. Antimicrobial surfaces of viologen-quaternized poly((2-dimethyl amino)ethyl methacryIate)-Si(100) hybrids from surface-initiated atom transfer radical polymerization. Nanobiotechnology. 2006;2:123–134. [Google Scholar]
- 140.Wei T., Zhan W., Cao L., Hu C., Qu Y., Yu Q., et al. Multifunctional and regenerable antibacterial surfaces fabricated by a universal strategy. ACS Appl Mater Interfaces. 2016;8:30048–30057. doi: 10.1021/acsami.6b11187. [DOI] [PubMed] [Google Scholar]
- 141.Fundeanu I., Klee D., Schouten A.J., Busscher H.J., van der Mei H.C. Solvent-free functionalization of silicone rubber and efficacy of PAAm brushes grafted from an amino-PPX layer against bacterial adhesion. Acta Biomater. 2010;6:4271–4276. doi: 10.1016/j.actbio.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 142.Fundeanu I., van der Mei H.C., Schouten A.J., Busscher H.J. Polyacrylamide brush coatings preventing microbial adhesion to silicone rubber. Colloids Surf B. 2008;64:297–301. doi: 10.1016/j.colsurfb.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 143.Wang H., Wang L., Zhang P., Yuan L., Yu Q., Chen H. High antibacterial efficiency of pDMAEMA modified silicon nanowire arrays. Colloids Surf B. 2011;83:355–359. doi: 10.1016/j.colsurfb.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 144.Vaterrodt A., Thallinger B., Daumann K., Koch D., Guebitz G.M., Ulbricht M. Antifouling and antibacterial multi-functional polyzwitterion/enzyme coating on silicone catheter material prepared by electrostatic layer-by-layer assembly. Langmuir. 2016;32:1347–1359. doi: 10.1021/acs.langmuir.5b04303. [DOI] [PubMed] [Google Scholar]
- 145.Peng Q., Lu S., Chen D., Wu X., Fan P., Zhong R., et al. Poly(vinylidenefluoride)-graft-poly(N-vinyl-2-pyrrolidone) copolymers prepared via a RAFT-mediated process and their use in antifouling and antibacterial membranes. Macromol Biosci. 2007;7:1149–1159. doi: 10.1002/mabi.200700068. [DOI] [PubMed] [Google Scholar]
- 146.Zhai G., Shi Z.L., Kang E.T., Neoh K.G. Surface-initiated atom transfer radical polymerization on poly(vinylidene fluoride) membrane for antibacterial ability. Macromol Biosci. 2005;5:974–982. doi: 10.1002/mabi.200500079. [DOI] [PubMed] [Google Scholar]
- 147.Cheng Z., Zhu X., Shi Z.L., Neoh K.G., Kang E.T. Polymer microspheres with permanentantibacterial surface from surface-initiated atom transfer radical polymerization. Ind Eng Chem Res. 2005;44:7098–7104. [Google Scholar]
- 148.Lenoir S., Pagnoulle C., Galleni M., Compere P., Jerome R., Detrembleur C. Polyolefin matrixes with permanent antibacterial activity: preparation, antibacterial activity, and action mode of the active species. Biomacromolecules. 2006;7:2291–2296. doi: 10.1021/bm050850c. [DOI] [PubMed] [Google Scholar]
- 149.Jo T.S., Yang M.L., Brownell L.V., Bae C. Synthesis of quaternary ammonium ion-grafted polyolefins via activation of inert C–H bonds and nitroxide mediated radical polymerization. J Polym Sci Part A Polym Chem. 2009;47:4519–4531. [Google Scholar]
- 150.Huang J., Murata H., Koepsel R.R., Russell A.J., Matyjaszewski K. Antibacterial polypropylene via surface-initiated atom transfer radical polymerization. Biomacromolecules. 2007;8:1396–1399. doi: 10.1021/bm061236j. [DOI] [PubMed] [Google Scholar]
- 151.Thomassin J.M., Lenoir S., Riga J., Jerome R., Detrembleur C. Grafting of poly[2-(tert-butylamino)ethyl methacrylate] onto polypropylene by reactive blending and antibacterial activity of the copolymer. Biomacromolecules. 2007;8:1171–1177. doi: 10.1021/bm0611228. [DOI] [PubMed] [Google Scholar]
- 152.Fang Y., Fu G.D., Zhao J., Kang E.T., Neoh K.G. Antibacterial effect of surface-functionalized polypropylene hollow fiber membrane from surface-initiated atom transfer radical polymerization. J Membrane Sci. 2008;319:149–157. [Google Scholar]
- 153.Fu G.D., Yao F., Li Z.G., Li X.S. Solvent-resistant antibacterial microfibers of self-quaternized block copolymers from atom transfer radical polymerization and electrospinning. J Mater Chem. 2008;18:859–867. [Google Scholar]
- 154.Konn C., Morel F., Beyou E., Chaumont P., Bourgeat-Lami E. Nitroxide-mediated polymerization of styrene initiated from the surface of laponite clay platelets. Macromolecules. 2007;40:7464–7472. [Google Scholar]
- 155.Wang J.S., Matyjaszewski K. Controlled/living radical polymerization: atom transfer radical polymerization in the presence of transition-metal complexes. J Am Chem Soc. 1995;117:5614–5615. [Google Scholar]
- 156.Kato M., Kamigaito M., Sawamoto M., Higashimura T. Polymerization of methyl methacrylate with the carbon tetrachloride/dichlorotris- (triphenylphosphine)ruthenium(II)/methylaluminum bis(2,6-di-tert-butylphenoxide) initiating system: possibility of living radical polymerization. Macromolecules. 1995;28:1721–1723. [Google Scholar]
- 157.Matyjaszewski K., Tsarevsky N.V. Macromolecular engineering by atom transfer radical polymerization. J Am Chem Soc. 2014;136:6513–6533. doi: 10.1021/ja408069v. [DOI] [PubMed] [Google Scholar]
- 158.Matyjaszewski K. Atom transfer radical polymerization (ATRP): Current status and future perspectives. Macromolecules. 2012;45:4015–4039. [Google Scholar]
- 159.Ran J., Wu L., Zhang Z., Xu T. Atom transfer radical polymerization (ATRP): a versatileand forceful tool for functional membranes. Prog Polym Sci. 2013;39:124–144. [Google Scholar]
- 160.Siegwart D.J., Oh J.K., Matyjaszewski K. ATRP in the design of functional materials for biomedical applications. Prog Polym Sci. 2012;37:18–37. doi: 10.1016/j.progpolymsci.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Mayadunne R.T., Rizzardo E., Chiefari J., Chong Y.K., Moad G., Thang S.H. Living radical polymerization with reversible addition-fragmentation chain transfer (RAFT polymerization) using dithiocarbamates as chain transfer agents. Macromolecules. 1999;32:6977–6980. [Google Scholar]
- 162.Chong Y., Le T.P., Moad G., Rizzardo E., Thang S.H. A more versatile route to block copolymers and other polymers of complex architecture by living radical polymerization: the RAFT process. Macromolecules. 1999;32:2071–2074. [Google Scholar]
- 163.Barner Kowollik C., Perrier S. The future of reversible addition fragmentation chain transfer polymerization. J Polym Sci Part A Polym Chem. 2008;46:5715–5723. [Google Scholar]
- 164.Zhang H. Controlled/living radical precipitation polymerization: a versatile polymerization technique for advanced functional polymers. Eur Polym J. 2013;49:579–600. [Google Scholar]
- 165.Gregory A., Stenzel M.H. Complex polymer architectures via RAFT polymerization: from fundamental process to extending the scope using click chemistry and nature's building blocks. Prog Polym Sci. 2012;37:38–105. [Google Scholar]
- 166.Benaglia M., Chiefari J., Chong Y.K., Moad G., Rizzardo E., Thang S.H. Universal (switchable) RAFT agents. J Am Chem Soc. 2009;131:6914–6915. doi: 10.1021/ja901955n. [DOI] [PubMed] [Google Scholar]
- 167.Nicolas J., Guillaneuf Y., Lefay C., Bertin D., Gigmes D., Charleux B. Nitroxide-mediated polymerization. Prog Polym Sci. 2013;38:63–235. [Google Scholar]
- 168.Zhao L., Li N., Wang K., Shi C., Zhang L., Luan Y. A review of polypeptide-based polymersomes. Biomaterials. 2014;35:1284–1301. doi: 10.1016/j.biomaterials.2013.10.063. [DOI] [PubMed] [Google Scholar]
- 169.Nuyken O., Pask S.D. Ring-opening polymerization – an introductory review. Polymers. 2013;5:361–403. [Google Scholar]
- 170.Penczek S., Cypryk M., Duda A., Kubisa P., Slomkowski S. Living ring-opening polymerizations of heterocyclic monomers. Prog Polym Sci. 2007;32:247–282. [Google Scholar]
- 171.King A., Chakrabarty S., Zhang W., Zeng X., Ohman D.E., Wood L.F., et al. High antimicrobial effectiveness with low hemolytic and cytotoxic activity for PEG/quaternary copolyoxetanes. Biomacromolecules. 2014;15:456–467. doi: 10.1021/bm401794p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Chakrabarty S., King A., Kurt P., Zhang W., Ohman D.E., Wood L.F., et al. Highly effective, water-soluble, hemocompatible 1,3-propylene oxide-based antimicrobials: poly[(3,3-quaternary/PEG)-copolyoxetanes] Biomacromolecules. 2011;12:757–769. doi: 10.1021/bm101381y. [DOI] [PubMed] [Google Scholar]
- 173.Worley B.V., Slomberg D.L., Schoenfisch M.H. Nitric oxide-releasing quaternary ammonium-modified poly(amidoamine) dendrimers as dual action antibacterial agents. Bioconjug Chem. 2014;25:918–927. doi: 10.1021/bc5000719. [DOI] [PubMed] [Google Scholar]
- 174.Carpenter A.W., Worley B.V., Slomberg D.L., Schoenfisch M.H. Dual action antimicrobials: nitric oxide release from quaternary ammonium-functionalized silica nanoparticles. Biomacromolecules. 2012;13:3334–3342. doi: 10.1021/bm301108x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Leng M., Hu S., Lu A., Cai M., Luo X. The anti-bacterial poly(caprolactone)-poly(quaternary ammonium salt) as drug delivery carriers. Appl Microbiol Biotechnol. 2015;100:3049–3059. doi: 10.1007/s00253-015-7126-8. [DOI] [PubMed] [Google Scholar]
- 176.Pascual A., Tan J.P., Yuen A., Chan J.M., Coady D.J., Mecerreyes D., et al. Broad-spectrum antimicrobial polycarbonate hydrogels with fast degradability. Biomacromolecules. 2015;16:1169–1178. doi: 10.1021/bm501836z. [DOI] [PubMed] [Google Scholar]
- 177.Liang Z., Zhu M., Yang Y.W., Gao H. Antimicrobial activities of polymeric quaternary ammonium salts from poly(glycidyl methacrylate)s. Polym Adv Technol. 2014;25:117–122. [Google Scholar]
- 178.Kolb H.C., Finn M.G., Sharpless K.B. Click chemistry: diverse chemical function from a few good reactions. Angew Chem Int Ed. 2001;40:2004–2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 179.Golas P.L., Matyjaszewski K. Marrying click chemistry with polymerization: expanding the scope of polymeric materials. Chem Soc Rev. 2010;41:1338–1354. doi: 10.1039/b901978m. [DOI] [PubMed] [Google Scholar]
- 180.Galvin C.J., Genzer J. Applications of surface-grafted macromolecules derived from post-polymerization modification reactions. Prog Polym Sci. 2012;37:871–906. [Google Scholar]
- 181.Moses J.E., Moorhouse A.D. The growing applications of click chemistry. Chem Soc Rev. 2007;36:1249–1262. doi: 10.1039/b613014n. [DOI] [PubMed] [Google Scholar]
- 182.Sheiko S.S., Sumerlin B.S., Matyjaszewski K. Cylindrical molecular brushes: synthesis, characterization, and properties. Prog Polym Sci. 2008;33:759–785. [Google Scholar]
- 183.Such G.K., Johnston A.P.R., Liang K., Caruso F. Synthesis and functionalization of nanoengineered materials using click chemistry. Prog Polym Sci. 2012;37:985–1003. [Google Scholar]
- 184.Tasdelen M.A., Kiskan B., Yagci Y. Externally stimulated click reactions for macromolecular syntheses. Prog Polym Sci. 2015;52:19–78. [Google Scholar]
- 185.Rostovtsev V.V., Green L.G., Fokin V.V., Sharpless K.B. A stepwise huisgen cycloaddition process: copper(I)-catalyzed regioselective ligation of azides and terminal alkynes. Angew Chem Int Ed. 2002;41:2596–2599. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 186.Tornoe C.W., Christensen C., Meldal M. Peptidotriazoles on solid phase: [1,2,3]-triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J Org Chem. 2002;67:3057–3064. doi: 10.1021/jo011148j. [DOI] [PubMed] [Google Scholar]
- 187.Bock V.D., Hiemstra H., Van Maarseveen J.H. CuI-catalyzed alkyne-azide click cycloadditions from a mechanistic and synthetic perspective. Eur J Org Chem. 2006;37:51–68. [Google Scholar]
- 188.Ganewatta M.S., Miller K.P., Singleton S.P., Mehrpouya-Bahrami P., Chen Y.P., Yan Y., et al. Antibacterial and biofilm-disrupting coatings from resin acid-derived materials. Biomacromolecules. 2015;16:3336–3344. doi: 10.1021/acs.biomac.5b01005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Qin T., Chang T., Ma T., Long P., Wang J. Click synthesis of quaternized poly(dimethylaminoethyl methacrylate) functionalized graphene oxide with improved antibacterial and antifouling ability. Colloids Surf B. 2016;141:196–205. doi: 10.1016/j.colsurfb.2016.01.046. [DOI] [PubMed] [Google Scholar]
- 190.Riva R., Lussis P., Lenoir S., Jérôme C., Jérôme R., Lecomte P. Contribution of click chemistry to the synthesis of antimicrobial aliphatic copolyester. Polymer. 2008;49:2023–2028. [Google Scholar]
- 191.Yao K., Chen Y., Zhang J., Clay B., Tang C. Cationic salt-responsive bottle-brush polymers. Macromol Rapid Comm. 2013;34:645–651. doi: 10.1002/marc.201300088. [DOI] [PubMed] [Google Scholar]
- 192.Jewett J.C., Bertozzi C.R. Cu-free click cycloaddition reactions in chemical biology. Chem Soc Rev. 2010;39:1272–1279. doi: 10.1039/b901970g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Jewett J.C., Sletten E.M., Bertozzi C.R. Rapid Cu-free click chemistry with readily synthesized biarylazacyclooctynones. J Am Chem Soc. 2010;132:3688–3690. doi: 10.1021/ja100014q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Tu Q., Ma C., Tian C., Yuan M., Han X., Wang D.E., et al. Quantum dots modified with quaternized poly(dimethylaminoethyl methacrylate) for selective recognition and killing of bacteria over mammalian cells. Analyst. 2016;141:3328–3336. doi: 10.1039/c6an00725b. [DOI] [PubMed] [Google Scholar]
- 195.Tian W., Hu Y., Wang W., Yu D. Synthesis of a gemini quaternary ammonium salt and its reaction with wool fabric using click chemistry. RSC Adv. 2015;5:91932–91936. [Google Scholar]
- 196.Hoyle C.E., Lowe A.B., Bowman C.N. Thiol-click chemistry: a multifaceted toolbox for small molecule and polymer synthesis. Chem Soc Rev. 2010;39:1355–1387. doi: 10.1039/b901979k. [DOI] [PubMed] [Google Scholar]
- 197.Wen J., Weinhart M., Lai B., Kizhakkedathu J., Brooks D.E. Reversible hemostatic properties of sulfabetaine/quaternary ammonium modified hyperbranched polyglycerol. Biomaterials. 2016;86:42–55. doi: 10.1016/j.biomaterials.2016.01.067. [DOI] [PubMed] [Google Scholar]
- 198.Zilberman M., Elsner J.J. Antibiotic-eluting medical devices for various applications. J Control Release. 2008;130:202–215. doi: 10.1016/j.jconrel.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 199.Buchholz H., Engelbrecht H. Über die Depotwirkung einiger Antibiotika beiVermischung mit dem kunstharz Palacos. Chirurg. 1970;41:511–515. [PubMed] [Google Scholar]
- 200.Vecsei V., Barquet A. Treatment of chronic osteomyelitis by necrectomy and gentamicin-PMMA beads. Clin Orthop Relat Res. 1981;159:201–207. [PubMed] [Google Scholar]
- 201.Hendriks J.G., van Horn J.R., van der Mei H.C., Busscher H.J. Backgrounds of antibiotic-loaded bone cement and prosthesis-related infection. Biomaterials. 2004;25:545–556. doi: 10.1016/s0142-9612(03)00554-4. [DOI] [PubMed] [Google Scholar]
- 202.van de Belt H., Neut D., van Horn J.R., van der Mei H.C., Schenk W., Busscher H.J. Antibiotic resistance—to treat or not to treat. Nat Med. 1999;5:358–359. doi: 10.1038/7330. [DOI] [PubMed] [Google Scholar]
- 203.Bunetel L., Segui A., Cormier M., Percheron E., Langlais F. Release of gentamicin from acrylic bone cement. Clin Pharmacokinet. 1989;17:291–297. doi: 10.2165/00003088-198917040-00006. [DOI] [PubMed] [Google Scholar]
- 204.Wu P., Grainger D.W. Drug/device combinations for local drug therapies and infectionprophylaxis. Biomaterials. 2006;27:2450–2467. doi: 10.1016/j.biomaterials.2005.11.031. [DOI] [PubMed] [Google Scholar]
- 205.Punyani S., Singh H. Preparation of iodine containing quaternary amine methacrylate copolymers and their contact killing antimicrobial properties. J Appl Polym Sci. 2006;102:1038–1044. [Google Scholar]
- 206.Deb S., Doiron R., Disilvio L., Punyani S., Singh H. PMMA bone cement containing a quaternary amine comonomer with potential antibacterial properties. J Biomed Mater Res B. 2008;85:130–139. doi: 10.1002/jbm.b.30925. [DOI] [PubMed] [Google Scholar]
- 207.Punyani S., Deb S., Singh H. Contact killing antimicrobial acrylic bone cements: preparation and characterization. J Biomater Sci Polym Ed. 2007;18:131–145. doi: 10.1163/156856207779116748. [DOI] [PubMed] [Google Scholar]
- 208.Abid C.K., Jain S., Jackeray R., Chattopadhyay S., Singh H. Formulation and characterization of antimicrobial quaternary ammonium dendrimer in poly(methyl methacrylate) bone cement. J Biomed Mater Res B. 2015:10. doi: 10.1002/jbm.b.33553. [DOI] [PubMed] [Google Scholar]
- 209.Tan H., Peng Z., Li Q., Xu X., Guo S., Tang T. The use of quaternised chitosan-loadedPMMA to inhibit biofilm formation and downregulate the virulence-associated gene expression of antibiotic-resistant staphylococcus. Biomaterials. 2012;33:365–377. doi: 10.1016/j.biomaterials.2011.09.084. [DOI] [PubMed] [Google Scholar]
- 210.Tan H., Guo S., Yang S., Xu X., Tang T. Physical characterization and osteogenic activity of the quaternized chitosan-loaded PMMA bone cement. Acta Biomater. 2012;8:2166–2174. doi: 10.1016/j.actbio.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 211.Tan H., Ao H., Ma R., Tang T. Quaternised chitosan-loaded polymethylmethacrylate bone cement: biomechanical and histological evaluations. J Orthop Trans. 2013;1:57–66. [Google Scholar]
- 212.Tan H.L., Ao H.Y., Ma R., Lin W.T., Tang T.T., Tan H.L. In vivo effect of quaternized chitosan-loaded PMMA bone cement on methicillin-resistant staphylococcus infection of the tibial metaphysis in a rabbit model. Antimicrob Agents Chemother. 2014;58:6016–6023. doi: 10.1128/AAC.03489-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Shi Z., Neoh K.G., Kang E.T., Wang W. Antibacterial and mechanical properties of bone cement impregnated with chitosan nanoparticles. Biomaterials. 2006;27:2440–2449. doi: 10.1016/j.biomaterials.2005.11.036. [DOI] [PubMed] [Google Scholar]
- 214.Beyth S., Polak D., Milgrom C., Weiss E.I., Matanis S., Beyth N. Antibacterial activity of bone cement containing quaternary ammonium polyethyleneimine nanoparticles. J Antimicrob Chemother. 2014;69:854–855. doi: 10.1093/jac/dkt441. [DOI] [PubMed] [Google Scholar]
- 215.Peng Z.X., Ao H.Y., Wang L., Guo S.R., Tang T.T. Quaternised chitosan coating on titanium provides a self-protective surface that prevents bacterial colonisation and implant-associated infections. RSC Adv. 2015;5:54304–54311. [Google Scholar]
- 216.Dan Y., Cai J.Y., Xin L., Church J.S., Wang L. Novel immobilization of a quaternary ammonium moiety on keratin fibers for medical applications. Int J Biol Macromol. 2014;70:236–240. doi: 10.1016/j.ijbiomac.2014.06.025. [DOI] [PubMed] [Google Scholar]
- 217.Meghil M.M., Rueggeberg F., El-Awady A., Miles B., Tay F., Pashley D., et al. Novel coating of surgical suture confers antimicrobial activity against porphyromonas gingivalis and Enterococcus faecalis. J Periodontol. 2015;86:788–794. doi: 10.1902/jop.2015.140528. [DOI] [PubMed] [Google Scholar]
- 218.Liu Y., Ma K., Li R., Ren X., Huang T.S. Antibacterial cotton treated with N-halamine and quaternary ammonium salt. Cellulose. 2013;20:3123–3130. [Google Scholar]
- 219.Kang C.K., Kim S.S., Kim S., Lee J., Lee J.H., Roh C., et al. Antibacterial cotton fibers treated with silver nanoparticles and quaternary ammonium salts. Carbohyd Polym. 2016;151:1012–1018. doi: 10.1016/j.carbpol.2016.06.043. [DOI] [PubMed] [Google Scholar]
- 220.Tran P.L., Hamood A.N., de Souza A., Schultz G., Liesenfeld B., Mehta D., et al. A study on the ability of quaternary ammonium groups attached to a polyurethane foam wound dressing to inhibit bacterial attachment and biofilm formation. Wound Repair Regen. 2015;23:74–81. doi: 10.1111/wrr.12244. [DOI] [PubMed] [Google Scholar]
- 221.Yari A., Yeganeh H., Bakhshi H. Synthesis and evaluation of novel absorptive and antibacterial polyurethane membranes as wound dressing. J Mater Sci Mater Med. 2012;23:2187–2202. doi: 10.1007/s10856-012-4683-6. [DOI] [PubMed] [Google Scholar]
- 222.Uppanan P., Channasanon S., Veeranondh S., Tanodekaew S. Synthesis of GTMAC modified chitin-PAA gel and evaluation of its biological properties. J Biomed Mater Res A. 2011;98:185–191. doi: 10.1002/jbm.a.33104. [DOI] [PubMed] [Google Scholar]
- 223.Pilakasiri K., Molee P., Sringernyuang D., Sangjun N., Channasanon S., Tanodekaew S. Efficacy of chitin-PAA-GTMAC gel in promoting wound healing: animal study. J Mater Sci Mater Med. 2011;22:2497–2504. doi: 10.1007/s10856-011-4420-6. [DOI] [PubMed] [Google Scholar]
- 224.Imazato S., Ebi N., Takahashi Y., Kaneko T., Ebisu S., Russell R.R. Antibacterial activity of bactericide-immobilized filler for resin-based restoratives. Biomaterials. 2003;24:3605–3609. doi: 10.1016/s0142-9612(03)00217-5. [DOI] [PubMed] [Google Scholar]
- 225.Imazato S., Imai T., Russell R.R., Torii M., Ebisu S. Antibacterial activity of cured dental resin incorporating the antibacterial monomer MDPB and an adhesion-promoting monomer. J Biomed Mater Res. 1998;39:511–515. doi: 10.1002/(sici)1097-4636(19980315)39:4<511::aid-jbm1>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 226.Imazato S., McCabe J.F. Influence of incorporation of antibacterial monomer on curing behavior of a dental composite. J Dent Res. 1994;73:1641–1645. doi: 10.1177/00220345940730100901. [DOI] [PubMed] [Google Scholar]
- 227.Imazato S., Russell R.R., McCabe J.F. Antibacterial activity of MDPB polymer incorporated in dental resin. J Dent. 1995;23:177–181. doi: 10.1016/0300-5712(95)93576-n. [DOI] [PubMed] [Google Scholar]
- 228.Imazato S., Tarumi H., Kato S., Ebisu S. Water sorption and colour stability of composites containing the antibacterial monomer MDPB. J Dent. 1999;27:279–283. doi: 10.1016/s0300-5712(98)00006-2. [DOI] [PubMed] [Google Scholar]
- 229.Beyth N., Yudovin-Farber I., Bahir R., Domb A.J., Weiss E.I. Antibacterial activity of dental composites containing quaternary ammonium polyethylenimine nanoparticles against Streptococcus mutans. Biomaterials. 2006;27:3995–4002. doi: 10.1016/j.biomaterials.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 230.Beyth N., Yudovin-Farber I., Perez-Davidi M., Domb A.J., Weiss E.I. Polyethyleneimine nanoparticles incorporated into resin composite cause cell death and trigger biofilm stress in vivo. Proc Natl Acad Sci USA. 2010;107:22038–22043. doi: 10.1073/pnas.1010341107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Yudovin-Farber I., Beyth N., Nyska A., Weiss E.I., Golenser J., Domb A.J. Surface characterization and biocompatibility of restorative resin containing nanoparticles. Biomacromolecules. 2008;9:3044–3050. doi: 10.1021/bm8004897. [DOI] [PubMed] [Google Scholar]
- 232.Cheng L., Weir M.D., Zhang K., Wu E.J., Xu S.M., Zhou X., et al. Dental plaquemicrocosm biofilm behavior on calcium phosphate nanocomposite with quaternary ammonium. Dent Mater. 2012;28:853–862. doi: 10.1016/j.dental.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Cheng L., Weir M.D., Zhang K., Xu S.M., Chen Q., Zhou X., et al. Antibacterial nanocomposite with calcium phosphate and quaternary ammonium. J Dent Res. 2012;91:460–466. doi: 10.1177/0022034512440579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Li F., Wang P., Weir M.D., Fouad A.F., Xu H.H. Evaluation of antibacterial and remineralizing nanocomposite and adhesive in rat tooth cavity model. Acta Biomater. 2014;10:2804–2813. doi: 10.1016/j.actbio.2014.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Wu J., Weir M.D., Melo M.A., Xu H.H. Development of novel self-healing and antibacterial dental composite containing calcium phosphate nanoparticles. J Dent. 2015;43:317–326. doi: 10.1016/j.jdent.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Wu J., Zhou H., Weir M.D., Melo M.A., Levine E.D., Xu H.H. Effect of dimethylaminohexadecyl methacrylate mass fraction on fracture toughness and antibacterial properties of CaP nanocomposite. J Dent. 2015;43:1539–1546. doi: 10.1016/j.jdent.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Zhou C., Weir M.D., Zhang K., Deng D., Cheng L., Xu H.H. Synthesis of new antibacterialquaternary ammonium monomer for incorporation into CaP nanocomposite. Dent Mater. 2013;29:859–870. doi: 10.1016/j.dental.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Cheng L., Weir M.D., Xu H.H., Antonucci J.M., Kraigsley A.M., Lin N.J., et al. Antibacterial amorphous calcium phosphate nanocomposites with a quaternary ammonium dimethacrylate and silver nanoparticles. Dent Mater. 2012;28:561–572. doi: 10.1016/j.dental.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Cheng L., Zhang K., Zhou C.C., Weir M.D., Zhou X.D., Xu H.H.K. One-year water-ageing of calcium phosphate composite containing nano-silver and quaternary ammonium to inhibit biofilms. Int J Oral Sci. 2016;8:172–181. doi: 10.1038/ijos.2016.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Antonucci J.M., Zeiger D.N., Tang K., Lin-Gibson S., Fowler B.O., Lin N.J. Synthesis and characterization of dimethacrylates containing quaternary ammonium functionalities for dental applications. Dent Mater. 2012;28:219–228. doi: 10.1016/j.dental.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.He J., Soderling E., Lassila L.V., Vallittu P.K. Incorporation of an antibacterial and radiopaque monomer in to dental resin system. Dent Mater. 2012;28:e110–e117. doi: 10.1016/j.dental.2012.04.026. [DOI] [PubMed] [Google Scholar]
- 242.He J., Soderling E., Vallittu P.K., Lassila L.V. Preparation and evaluation of dental resinwith antibacterial and radio-opaque functions. Int J Mol Sci. 2013;14:5445–5460. doi: 10.3390/ijms14035445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.He J., Söderling E., Lassila L.V., Vallittu P.K. Synthesis of antibacterial and radio-opaquedimethacrylate monomers and their potential application in dental resin. Dent Mater. 2014;30:968–976. doi: 10.1016/j.dental.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 244.Huang L., Sun X., Xiao Y.H., Dong Y., Tong Z.C., Xing X.D., et al. Antibacterial effect of a resin incorporating a novel polymerizable quaternary ammonium salt MAE-DB against Streptococcus mutans. J Biomed Mater Res B. 2012;100:1353–1358. doi: 10.1002/jbm.b.32703. [DOI] [PubMed] [Google Scholar]
- 245.He J., Soderling E., Lassila L.V., Vallittu P.K. Preparation of antibacterial and radio-opaque dental resin with new polymerizable quaternary ammonium monomer. Dent Mater. 2015;31:575–582. doi: 10.1016/j.dental.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 246.Xu X., Wang Y., Liao S., Wen Z.T., Fan Y. Synthesis and characterization of antibacterial dental monomers and composites. J Biomed Mater Res B. 2012;100:1151–1162. doi: 10.1002/jbm.b.32683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Burujeny S.B., Atai M., Yeganeh H. Assessments of antibacterial and physico-mechanical properties for dental materials with chemically anchored quaternary ammonium moieties: thiol-ene-methacrylate vs. conventional methacrylate system. Dent Mater. 2015;31:244–261. doi: 10.1016/j.dental.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 248.Liang X., Soderling E., Liu F., He J., Lassila L.V., Vallittu P.K. Optimizing the concentration of quaternary ammonium dimethacrylate monomer in bis-GMA/TEGDMA dental resin system for antibacterial activity and mechanical properties. J Mater Sci Mater Med. 2014;25:1387–1393. doi: 10.1007/s10856-014-5156-x. [DOI] [PubMed] [Google Scholar]
- 249.Huang Q.T., He J.W., Lin Z.M., Liu F., Lassila L.V., Vallittu P.K. Physical and chemical properties of an antimicrobial Bis-GMA free dental resin with quaternary ammonium dimethacrylate monomer. J Mech Behav Biomed Mater. 2016;56:68–76. doi: 10.1016/j.jmbbm.2015.10.028. [DOI] [PubMed] [Google Scholar]
- 250.Banzi E.C., Costa A.R., Puppin-Rontani R.M., Babu J., Garcia-Godoy F. Inhibitory effects of a cured antibacterial bonding system on viability and metabolic activity of oral bacteria. Dent Mater. 2014;30:e238–44. doi: 10.1016/j.dental.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 251.da Silva B.M., Franca F.M., Florio F.M., Basting R.T. In situ anticariogenic effect ofadhesive systems containing fluoride and MDPB. Am J Dent. 2010;23:75–80. [PubMed] [Google Scholar]
- 252.Gondim J.O., Duque C., Hebling J., Giro E.M. Influence of human dentine on the antibacterial activity of self-etching adhesive systems against cariogenic bacteria. J Dent. 2008;36:241–248. doi: 10.1016/j.jdent.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 253.Imazato S., Ehara A., Torii M., Ebisu S. Antibacterial activity of dentine primer containing MDPB after curing. J Dent. 1998;26:267–271. doi: 10.1016/s0300-5712(97)00013-4. [DOI] [PubMed] [Google Scholar]
- 254.Imazato S., Kaneko T., Takahashi Y., Noiri Y., Ebisu S. In vivo antibacterial effects of dentin primer incorporating MDPB. Oper Dent. 2004;29:369–375. [PubMed] [Google Scholar]
- 255.Imazato S., Kinomoto Y., Tarumi H., Torii M., Russell R., McCabe J. Incorporation of antibacterial monomer MDPB into dentin primer. J Dent Res. 1997;76:768–772. doi: 10.1177/00220345970760030901. [DOI] [PubMed] [Google Scholar]
- 256.Imazato S., Tay F.R., Kaneshiro A.V., Takahashi Y., Ebisu S. An in vivo evaluation of bonding ability of comprehensive antibacterial adhesive system incorporating MDPB. Dent Mater. 2007;23:170–176. doi: 10.1016/j.dental.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 257.Turkun M., Turkun L., Ergucu Z., Ates M. Is an antibacterial adhesive system more effective than cavity disinfectants. Am J Dent. 2006;19:166–170. [PubMed] [Google Scholar]
- 258.Uysal T., Amasyali M., Ozcan S., Koyuturk A.E., Sagdic D. Effect of antibacterial monomer-containing adhesive on enamel demineralization around orthodontic brackets: an in-vivo study. Am J Orthod Dentofacial Orthop. 2011;139:650–656. doi: 10.1016/j.ajodo.2009.06.038. [DOI] [PubMed] [Google Scholar]
- 259.Li F., Chai Z.G., Sun M.N., Wang F., Ma S., Zhang L., et al. Anti-biofilm effect of dental adhesive with cationic monomer. J Dent Res. 2009;88:372–376. doi: 10.1177/0022034509334499. [DOI] [PubMed] [Google Scholar]
- 260.Li F., Chen J., Chai Z., Zhang L., Xiao Y., Fang M., et al. Effects of a dental adhesive incorporating antibacterial monomer on the growth, adherence and membrane integrity of Streptococcus mutans. J Dent. 2009;37:289–296. doi: 10.1016/j.jdent.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 261.Xiao Y.H., Ma S., Chen J.H., Chai Z.G., Li F., Wang Y.J. Antibacterial activity and bondingability of an adhesive incorporating an antibacterial monomer DMAE-CB. J Biomed Mater Res B. 2009;90:813–817. doi: 10.1002/jbm.b.31350. [DOI] [PubMed] [Google Scholar]
- 262.Li F., Weir M.D., Chen J., Xu H.H. Effect of charge density of bonding agent containing a new quaternary ammonium methacrylate on antibacterial and bonding properties. Dent Mater. 2014;30:433–441. doi: 10.1016/j.dental.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Wang S., Zhang K., Zhou X., Xu N., Xu H.H., Weir M.D., et al. Antibacterial effect of dental adhesive containing dimethylaminododecyl methacrylate on the development of Streptococcus mutans biofilm. Int J Mol Sci. 2014;15:12791–12806. doi: 10.3390/ijms150712791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Zhang K., Wang S., Zhou X., Xu H.H., Weir M.D., Ge Y., et al. Effect of antibacterial dental adhesive on multispecies biofilms formation. J Dent Res. 2015;94:622–629. doi: 10.1177/0022034515571416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Zhou H., Li F., Weir M.D., Xu H.H. Dental plaque microcosm response to bonding agents containing quaternary ammonium methacrylates with different chain lengths and charge densities. J Dent. 2013;41:1122–1131. doi: 10.1016/j.jdent.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Zhang K., Cheng L., Imazato S., Antonucci J.M., Lin N.J., Lin-Gibson S., et al. Effects of dual antibacterial agents MDPB and nano-silver in primer on microcosm biofilm, cytotoxicity and dentine bond properties. J Dent. 2013;41:464–474. doi: 10.1016/j.jdent.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Zhang K., Li F., Imazato S., Cheng L., Liu H., Arola D.D., et al. Dual antibacterial agents of nano-silver and 12-methacryloyloxydodecylpyridinium bromide in dental adhesive to inhibit caries. J Biomed Mater Res B. 2013;101:929–938. doi: 10.1002/jbm.b.32898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Cheng L., Zhang K., Weir M.D., Liu H., Zhou X., Xu H.H. Effects of antibacterial primers with quaternary ammonium and nano-silver on Streptococcus mutans impregnated in human dentin blocks. Dent Mater. 2013;29:462–472. doi: 10.1016/j.dental.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Zhang K., Melo M.A., Cheng L., Weir M.D., Bai Y., Xu H.H. Effect of quaternary ammonium and silver nanoparticle-containing adhesives on dentin bond strength and dental plaque microcosm biofilms. Dent Mater. 2012;28:842–852. doi: 10.1016/j.dental.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Cheng L., Zhang K., Melo M.A., Weir M.D., Zhou X., Xu H.H. Anti-biofilm dentin primer with quaternary ammonium and silver nanoparticles. J Dent Res. 2012;91:598–604. doi: 10.1177/0022034512444128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Li F., Weir M.D., Chen J., Xu H.H. Comparison of quaternary ammonium-containing with nano-silver-containing adhesive in antibacterial properties and cytotoxicity. Dent Mater. 2013;29:450–461. doi: 10.1016/j.dental.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Chen C., Weir M.D., Cheng L., Lin N.J., Lin-Gibson S., Chow L.C., et al. Antibacterial activity and ion release of bonding agent containing amorphous calcium phosphate nanoparticles. Dent Mater. 2014;30:891–901. doi: 10.1016/j.dental.2014.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Weng Y., Guo X., Gregory R., Xie D. A novel antibacterial dental glass-ionomer cement. Eur J Oral Sci. 2010;118:531–534. doi: 10.1111/j.1600-0722.2010.00770.x. [DOI] [PubMed] [Google Scholar]
- 274.Weng Y., Howard L., Chong V.J., Sun J., Gregory R.L., Xie D. A novel furanone-modified antibacterial dental glass ionomer cement. Acta Biomater. 2012;8:3153–3160. doi: 10.1016/j.actbio.2012.04.038. [DOI] [PubMed] [Google Scholar]
- 275.Weng Y., Guo X., Gregory R.L., Xie D. Preparation and evaluation of an antibacterial dental cement containing quaternary ammonium salts. J Appl Polym Sci. 2011;122:2542–2551. [Google Scholar]
- 276.Feng J., Cheng L., Zhou X., Xu H.H., Weir M.D., Meyer M., et al. In situ antibiofilm effect of glass-ionomer cement containing dimethylaminododecyl methacrylate. Dent Mater. 2015;31:992–1002. doi: 10.1016/j.dental.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 277.Wang S.P., Ge Y., Zhou X.D., Xu H.H., Weir M.D., Zhang K.K., et al. Effect of anti-biofilm glass-ionomer cement onStreptococcus mutans biofilms. Int J Oral Sci. 2016;8:76–83. doi: 10.1038/ijos.2015.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Izutani N., Imazato S., Noiri Y., Ebisu S. Antibacterial effects of MDPB against anaerobes associated with endodontic infections. Int Endod J. 2010;43:637–645. doi: 10.1111/j.1365-2591.2010.01716.x. [DOI] [PubMed] [Google Scholar]
- 279.Kitagawa R., Kitagawa H., Izutani N., Hirose N., Hayashi M., Imazato S. Development of an antibacterial root canal filling system containing MDPB. J Dent Res. 2014;93:1277–1282. doi: 10.1177/0022034514549808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Gong S.Q., Epasinghe D.J., Zhang W., Zhou B., Niu L.N., Ryou H., et al. Synthesis of antimicrobial silsesquioxane-silica hybrids by hydrolytic co-condensation of alkoxysilanes. Polym Chem. 2014;5:454–462. [Google Scholar]
- 281.Gong S.Q., Huang Z.B., Shi W., Ma B., Tay F.R., Zhou B. In vitro evaluation of antibacterial effect of AH Plus incorporated with quaternary ammonium epoxy silicate against Enterococcus faecalis. J Endod. 2014;40:1611–1615. doi: 10.1016/j.joen.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 282.Barros J., Silva M.G., Rocas I.N., Goncalves L.S., Alves F.F., Lopes M.A., et al. Antibiofilm effects of endodontic sealers containing quaternary ammonium polyethylenimine nanoparticles. J Endod. 2014;40:1167–1171. doi: 10.1016/j.joen.2013.12.021. [DOI] [PubMed] [Google Scholar]
- 283.Barros J., Silva M.G., Rodrigues M.A., Alves F.R., Lopes M.A., Pina-Vaz I., et al. Antibacterial, physicochemical and mechanical properties of endodontic sealers containing quaternary ammonium polyethylenimine nanoparticles. Int Endod J. 2014;47:725–734. doi: 10.1111/iej.12207. [DOI] [PubMed] [Google Scholar]
- 284.Kesler Shvero D., Abramovitz I., Zaltsman N., Perez Davidi M., Weiss E., Beyth N. Towards antibacterial endodontic sealers using quaternary ammonium nanoparticles. Int Endod J. 2013;46:747–754. doi: 10.1111/iej.12054. [DOI] [PubMed] [Google Scholar]
- 285.Li F., Li F., Wu D., Ma S., Gao J., Li Y., et al. The effect of an antibacterial monomer on the antibacterial activity and mechanical properties of a pit-and-fissure sealant. J Am Dent Assoc. 2011;142:184–193. doi: 10.14219/jada.archive.2011.0062. [DOI] [PubMed] [Google Scholar]
- 286.Yu F., Yu H., Lin P., Dong Y., Zhang L., Sun X., et al. Effect of an antibacterial monomer on the antibacterial activity of a pit-and-fissure sealant. PLoS One. 2016;11 doi: 10.1371/journal.pone.0162281. e0162281/1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Yang Y., Huang L., Dong Y., Zhang H., Zhou W., Ban J., et al. In vitro antibacterial activity of a novel resin-based pulp capping material containing the quaternary ammonium salt MAE-DB and Portland cement. PLoS One. 2014;9 doi: 10.1371/journal.pone.0112549. e112549/1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Gong S.Q., Epasinghe D.J., Zhou B., Niu L.N., Kimmerling K.A., Rueggeberg F.A., et al. Effect of water-aging on the antimicrobial activities of an ORMOSIL-containing orthodontic acrylic resin. Acta Biomater. 2013;9:6964–6973. doi: 10.1016/j.actbio.2013.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Gong S.Q., Epasinghe J., Rueggeberg F.A., Niu L.N., Mettenberg D., Yiu C.K., et al. An ORMOSIL-containing orthodontic acrylic resin with concomitant improvements in antimicrobial and fracture toughness properties. PLoS One. 2012;7 doi: 10.1371/journal.pone.0042355. e42355/1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Gong S.Q., Niu L.N., Kemp L.K., Yiu C.K., Ryou H., Qi Y.P., et al. Quaternary ammonium silane-functionalized, methacrylate resin composition with antimicrobial activities and self-repair potential. Acta Biomater. 2012;8:3270–3282. doi: 10.1016/j.actbio.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Liu S.Y., Tonggu L., Niu L.N., Gong S.Q., Fan B., Wang L., et al. Antimicrobial activity of a quaternary ammonium methacryloxy silicate-containing acrylic resin: a randomised clinical trial. Sci Rep. 2016;6:21882/1–21882/14. doi: 10.1038/srep21882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Regis R.R., Zanini A.P., Della Vecchia M.P., Silva-Lovato C.H., Oliveira Paranhos H.F., deSouza R.F. Physical properties of an acrylic resin after incorporation of an antimicrobial monomer. J Prosthodont. 2011;20:372–379. doi: 10.1111/j.1532-849X.2011.00719.x. [DOI] [PubMed] [Google Scholar]
- 293.Song R., Zhong Z., Lin L. Evaluation of chitosan quaternary ammonium salt-modified resin denture base material. Int J Biol Macromol. 2016;85:102–110. doi: 10.1016/j.ijbiomac.2015.12.052. [DOI] [PubMed] [Google Scholar]
- 294.Wassmann M., Winkel A., Haak K., Dempwolf W., Stiesch M., Menzel H. Influence of quaternization of ammonium on antibacterial activity and cytocompatibility of thin copolymer layers on titanium. J Biomater Sci Polym Ed. 2016;27:1507–1519. doi: 10.1080/09205063.2016.1214001. [DOI] [PubMed] [Google Scholar]
- 295.Kalomiraki M., Thermos K., Chaniotakis N.A. Dendrimers as tunable vectors of drug delivery systems and biomedical and ocular applications. Int J Nanomed. 2015;11:1–12. doi: 10.2147/IJN.S93069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Li Z., Lee D., Sheng X., Cohen R.E., Rubner M.F. Two-level antibacterial coating with both release-killing and contact-killing capabilities. Langmuir. 2006;22:9820–9823. doi: 10.1021/la0622166. [DOI] [PubMed] [Google Scholar]
- 297.Eby D.M., Luckarift H.R., Johnson G.R. Hybrid antimicrobial enzyme and silver nanoparticle coatings for medical instruments. ACS Appl Mater Interfaces. 2009;1:1553–1560. doi: 10.1021/am9002155. [DOI] [PubMed] [Google Scholar]
- 298.Varun S., Macbride M.M., Peterson B.R., Ayusman S. Silver bromide nanoparticle/polymer composites: dual action tunable antimicrobial materials. J Am Chem Soc. 2006;128:9798–9808. doi: 10.1021/ja061442z. [DOI] [PubMed] [Google Scholar]
- 299.Hetrick E.M., Schoenfisch M.H. Reducing implant-related infections: active release strategies. Chem Soc Rev. 2006;35:780–789. doi: 10.1039/b515219b. [DOI] [PubMed] [Google Scholar]
- 300.Mackenzie D. The history of sutures. Med Hist. 1973;17:158–168. doi: 10.1017/s0025727300018469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Masini B.D., Stinner D.J., Waterman S.M., Wenke J.C. Bacterial adherence to suture materials. J Surg Educ. 2011;68:101–104. doi: 10.1016/j.jsurg.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 302.Mogosanu G.D., Grumezescu A.M. Natural and synthetic polymers for wounds and burns dressing. Int J Pharm. 2014;463:127–136. doi: 10.1016/j.ijpharm.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 303.Windler L., Height M., Nowack B. Comparative evaluation of antimicrobials for textile applications. Environ Int. 2013;53:62–73. doi: 10.1016/j.envint.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 304.Dastjerdi R., Montazer M. A review on the application of inorganic nano-structured materials in the modification of textiles: focus on anti-microbial properties. Colloids Surf B. 2010;79:5–18. doi: 10.1016/j.colsurfb.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 305.Supaphol P., Suwantong O., Sangsanoh P., Srinivasan S., Jayakumar R., Nair S.V. Electrospinning of biocompatible polymers and their potentials in biomedical applications. Adv Polym Sci. 2011;246:213–239. [Google Scholar]
- 306.Gliścińska E., Gutarowska B., Brycki B., Krucińska I. Electrospun polyacrylonitrile nanofibers modified by quaternary ammonium salts. J Appl Polym Sci. 2013;128:767–775. [Google Scholar]
- 307.Uykun N., Ergal I., Kurt H., Gokceoren A.T., Gocek I., Kayaoglu B.K., et al. Electrospun antibacterial nanofibrous polyvinylpyrrolidone/cetyltrimethylammonium bromide membranes for biomedical applications. J Bioact Compat Polym. 2014;29:382–397. [Google Scholar]
- 308.Cerkez I., Kocer H.B., Worley S.D., Broughton R.M., Huang T.S. N-halamine biocidal coatings via a layer-by-layer assembly technique. Langmuir. 2011;27:4091–4097. doi: 10.1021/la104923x. [DOI] [PubMed] [Google Scholar]
- 309.Liu Y., Liu Y., Ren X., Huang T.S. Antimicrobial cotton containing N-halamine andquaternary ammonium groups by grafting copolymerization. Appl Surf Sci. 2014;296:231–236. [Google Scholar]
- 310.Duque Sanchez L., Brack N., Postma A., Pigram P.J., Meagher L. Surface modification of electrospun fibres for biomedical applications: a focus on radical polymerization methods. Biomaterials. 2016;106:24–45. doi: 10.1016/j.biomaterials.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 311.DeForest C.A., Anseth K.S. Advances in bioactive hydrogels to probe and direct cell fate. Annu Rev Chem Biomol Eng. 2012;3:421–444. doi: 10.1146/annurev-chembioeng-062011-080945. [DOI] [PubMed] [Google Scholar]
- 312.Malda J., Visser J., Melchels F.P., Jüngst T., Hennink W.E., Dhert W.J., et al. 25th anniversary article: engineering hydrogels for biofabrication. Adv Mater. 2013;25:5011–5028. doi: 10.1002/adma.201302042. [DOI] [PubMed] [Google Scholar]
- 313.Bagramian R.A., Garcia-Godoy F., Volpe A.R. The global increase in dental caries: apending public health crisis. Am J Dent. 2009;22:3–8. [PubMed] [Google Scholar]
- 314.Totiam P., Gonzalez-Cabezas C., Fontana M.R., Zero D.T. A new in vitro model to study the relationship of gap size and secondary caries. Caries Res. 2007;41:467–473. doi: 10.1159/000107934. [DOI] [PubMed] [Google Scholar]
- 315.Tjäderhane L., Buzalaf M.A.R., Carrilho M., Chaussain C. Matrix metalloproteinases and other matrix proteinases in relation to cariology: the era of dentin degradomics. Caries Res. 2015;49:193–208. doi: 10.1159/000363582. [DOI] [PubMed] [Google Scholar]
- 316.Imazato S., Ma S., Chen J.H., Xu H.H. Therapeutic polymers for dental adhesives: loading resins with bio-active components. Dent Mater. 2014;30:97–104. doi: 10.1016/j.dental.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Ge Y., Wang S., Zhou X., Wang H., Xu H.H., Cheng L. The use of quaternary ammonium to combat dental caries. Materials. 2015;8:3532–3549. doi: 10.3390/ma8063532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Zhou W., Niu L.N., Huang L., Fang M., Chang G., Shen L.J., et al. Improved secondary caries resistance via augmented pressure displacement of antibacterial adhesive. Sci Rep. 2016;6:22269/1–22269/10. doi: 10.1038/srep22269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Batoni G., Pardini M., Giannotti A., Ota F., Giuca M.R., Gabriele M., et al. Effect of removable orthodontic appliances on oral colonisation by mutans streptococci in children. Eur J Oral Sci. 2001;109:388–392. doi: 10.1034/j.1600-0722.2001.00089.x. [DOI] [PubMed] [Google Scholar]
- 320.Arendorf T., Addy M. Candidal carriage and plaque distribution before, during and afterremovable orthodontic appliance therapy. J Clin Periodontol. 1985;12:360–368. doi: 10.1111/j.1600-051x.1985.tb00926.x. [DOI] [PubMed] [Google Scholar]
- 321.Ferracane J.L., Hilton T.J. Polymerization stress-is it clinically meaningful. Dent Mater. 2016;32:1–10. doi: 10.1016/j.dental.2015.06.020. [DOI] [PubMed] [Google Scholar]
- 322.Kleverlaan C.J., Feilzer A.J. Polymerization shrinkage and contraction stress of dental resin composites. Dent Mater. 2005;21:1150–1157. doi: 10.1016/j.dental.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 323.Lee T.Y., Carioscia J., Smith Z., Bowman C.N. Thiol-allyl ether-methacrylate ternary systems: evolution mechanism of polymerization-induced shrinkage stress and mechanical properties. Macromolecules. 2007;40:1473–1479. [Google Scholar]
- 324.Boulden J.E., Cramer N.B., Schreck K.M., Couch C.L., Bracho-Troconis C., Stansbury J.W., et al. Thiol-ene-methacrylate composites as dental restorative materials. Dent Mater. 2011;27:267–272. doi: 10.1016/j.dental.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Ortega A., Farah S., Tranque P., Ocana A.V., Nam-Cha S.H., Beyth N., et al. Antimicrobial evaluation of quaternary ammonium polyethyleneimine nanoparticles against clinical isolates of pathogenic bacteria. IET Nanobiotechnol. 2015;9:342–348. doi: 10.1049/iet-nbt.2014.0078. [DOI] [PubMed] [Google Scholar]
- 326.Farah S., Aviv O., Laout N., Ratner S., Beyth N., Domb A.J. Quaternary ammonium polyethylenimine nanoparticles for treating bacterial contaminated water. Colloids Surf B. 2015;128:614–619. doi: 10.1016/j.colsurfb.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 327.Beyth N., Houri-Haddad Y., Baraness-Hadar L., Yudovin-Farber I., Domb A.J., Weiss E.I. Surface antimicrobial activity and biocompatibility of incorporated polyethylenimine nanoparticles. Biomaterials. 2008;29:4157–4163. doi: 10.1016/j.biomaterials.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 328.Speier J. Development of an organosilicone antimicrobial agent for the treatment of surfaces. J Ind Text. 1982;12:38–45. [Google Scholar]
- 329.Jang J., Kim Y. Fabrication of monodisperse silica-polymer core-shell nanoparticles with excellent antimicrobial efficacy. Chem Commun. 2008:4016–4018. doi: 10.1039/b809137d. [DOI] [PubMed] [Google Scholar]
- 330.Song J., Kong H., Jang J. Enhanced antibacterial performance of cationic polymer modified silica nanoparticles. Chem Commun. 2009;541:5418–5420. doi: 10.1039/b908060k. [DOI] [PubMed] [Google Scholar]
- 331.Song J., Kong H., Jang J. Bacterial adhesion inhibition of the quaternary ammonium functionalized silica nanoparticles. Colloids Surf B. 2011;82:651–656. doi: 10.1016/j.colsurfb.2010.10.027. [DOI] [PubMed] [Google Scholar]
- 332.Farah S., Aviv O., Laout N., Ratner S., Beyth N., Domb A.J. Antimicrobial silica particles loaded with quaternary ammonium polyethyleneimine network. Polym Adv Technol. 2014;25:689–692. [Google Scholar]
- 333.Makvandi P., Ghaemy M., Ghadiri A.A., Mohseni M. Photocurable antimicrobial quaternary ammonium-modified nanosilica. J Dent Res. 2015;94:1401–1407. doi: 10.1177/0022034515599973. [DOI] [PubMed] [Google Scholar]
- 334.Yu Q., Wu Z., Chen H. Dual-function antibacterial surfaces for biomedical applications. Acta Biomater. 2015;16:1–13. doi: 10.1016/j.actbio.2015.01.018. [DOI] [PubMed] [Google Scholar]
- 335.Zhang K., Cheng L., Weir M.D., Bai Y.X., Xu H.H. Effects of quaternary ammonium chain length on the antibacterial and remineralizing effects of a calcium phosphate nanocomposite. Int J Oral Sci. 2016;8:45–53. doi: 10.1038/ijos.2015.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Putsep K., Carlsson G., Boman H.G., Andersson M. Deficiency of antibacterial peptides in patients with morbus Kostmann: an observation study. Lancet. 2002;360:1144–1149. doi: 10.1016/S0140-6736(02)11201-3. [DOI] [PubMed] [Google Scholar]
- 337.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 338.Hay D.I. Salivary factors in caries models. Adv Dent Res. 1995;9:239–243. doi: 10.1177/08959374950090030801. [DOI] [PubMed] [Google Scholar]
- 339.Lendenmann U., Grogan J., Oppenheim F.G. Saliva and dental pellicle – a review. Adv Dent Res. 2000;14:22–28. doi: 10.1177/08959374000140010301. [DOI] [PubMed] [Google Scholar]
- 340.Li F., Weir M.D., Fouad A.F., Xu H.H. Effect of salivary pellicle on antibacterial activity of novel antibacterial dental adhesives using a dental plaque microcosm biofilm model. Dent Mater. 2014;30:182–191. doi: 10.1016/j.dental.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Tziafas D., Koliniotou-Koumpia E., Tziafa C., Papadimitriou S. Effects of a new antibacterial adhesive on the repair capacity of the pulp-dentine complex in infected teeth. Int Endod J. 2007;40:58–66. doi: 10.1111/j.1365-2591.2006.01183.x. [DOI] [PubMed] [Google Scholar]
- 342.Bjarnsholt T., Alhede M., Alhede M., Eickhardt-Sorensen S.R., Moser C., Kühl M., et al. The in vivo biofilm. Trends Microbiol. 2013;21:466–474. doi: 10.1016/j.tim.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 343.Toyofuku M., Inaba T., Kiyokawa T., Obana N., Yawata Y., Nomura N. Environmental factors that shape biofilm formation. Biosci Biotechnol Biochem. 2015;80:7–12. doi: 10.1080/09168451.2015.1058701. [DOI] [PubMed] [Google Scholar]
- 344.Goo E., An J.H., Kang Y., Hwang I. Control of bacterial metabolism by quorum sensing. Trends Microbiol. 2015;23:567–576. doi: 10.1016/j.tim.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 345.Dixon E.F., Hall R.A. Noisy neighbourhoods: quorum sensing in fungal-polymicrobial infections. Cell Microbiol. 2015;17:1431–1441. doi: 10.1111/cmi.12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Metzdorff M.T. Evidence-based medicine: what it is, what it isn’t, and are we practicing it? J Trauma Acute Care Surg. 2013;75:927–935. doi: 10.1097/TA.0b013e3182932bac. [DOI] [PubMed] [Google Scholar]
- 347.Paik S., Sechrist C., Torabinejad M. Levels of evidence for the outcome of endodontic retreatment. J Endod. 2004;30:745–750. doi: 10.1097/01.don.0000137636.93933.51. [DOI] [PubMed] [Google Scholar]
- 348.Torabinejad M., Kutsenko D., Machnick T.K., Ismail A., Newton C.W. Levels of evidence for the outcome of nonsurgical endodontic treatment. J Endod. 2005;31:637–646. doi: 10.1097/01.don.0000153593.64951.14. [DOI] [PubMed] [Google Scholar]
- 349.Tiller J.C., Liao C.J., Lewis K. Klibanov AM.V Designing surfaces that kill bacteria on contact. Proc Natl Acad Sci USA. 2001;98:5981–5985. doi: 10.1073/pnas.111143098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Netuschil L., Reich E., Unteregger G., Sculean A., Brecx M. A pilot study of confocal laser scanning microscopy for the assessment of undisturbed dental plaque vitality and topography. Arch Oral Biol. 1998;43:277–285. doi: 10.1016/s0003-9969(97)00121-0. [DOI] [PubMed] [Google Scholar]
- 351.Auschill T.M., Arweiler N.B., Netuschil L., Brecx M., Reich E., Sculean A. Spatial distribution of vital and dead microorganisms in dental biofilms. Arch Oral Biol. 2001;46:471–476. doi: 10.1016/s0003-9969(00)00136-9. [DOI] [PubMed] [Google Scholar]
- 352.Zhou H., Weir M.D., Antonucci J.M., Schumacher G.E., Zhou X.D., Xu H.H. Evaluation of three-dimensional biofilms on antibacterial bonding agents containing novel quaternary ammonium methacrylates. Int J Oral Sci. 2014;6:77–86. doi: 10.1038/ijos.2014.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Zhou H., Liu H., Weir M.D., Reynolds M.A., Zhang K., Xu H.H. Three-dimensional biofilm properties on dental bonding agent with varying quaternary ammonium charge densities. J Dent. 2016;26:30141–30145. doi: 10.1016/j.jdent.2016.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Bayles K.W. Bacterial programmed cell death: making sense of a paradox. Nat Rev Microbiol. 2014;12:63–69. doi: 10.1038/nrmicro3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Rice K.C., Bayles K.W. Molecular control of bacterial death and lysis. Microbiol Mol Biol Rev. 2008;72:85–109. doi: 10.1128/MMBR.00030-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Allocati N., Masulli M., Di Ilio C., De Laurenzi V. Die for the community: an overviewof programmed cell death in bacteria. Cell Death Dis. 2015;6 doi: 10.1038/cddis.2014.570. e1609/1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Gerdes K., Christensen S.K., Løbner-Olesen A. Prokaryotic toxin-antitoxin stress response loci. Nat Rev Microbiol. 2005;3:371–382. doi: 10.1038/nrmicro1147. [DOI] [PubMed] [Google Scholar]
- 358.Senadheera D.B., Cordova M., Ayala E.A., Chavez de Paz L.E., Singh K., Downey J.S., et al. Regulation of bacteriocin production and cell death by the VicRK signaling system in Streptococcus mutans. J Bacteriol. 2012;194:1307–1316. doi: 10.1128/JB.06071-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Zhang C., Cui F., Zeng G.M., Jiang M., Yang Z.Z., Yu Z.G., et al. Quaternary ammonium compounds (QACs): a review on occurrence, fate and toxicity in the environment. Sci Total Environ. 2015:518–519. doi: 10.1016/j.scitotenv.2015.03.007. 352-62. [DOI] [PubMed] [Google Scholar]
- 360.Sandbacka M., Christianson I., Isomaa B. The acute toxicity of surfactants on fish cells, Daphnia magna and fish-a comparative study. Toxicol In Vitro. 2000;14:61–68. doi: 10.1016/s0887-2333(99)00083-1. [DOI] [PubMed] [Google Scholar]
- 361.van Wijk D., Gyimesi-van den Bos M., Garttener-Arends I., Geurts M., Kamstra J., Thomas P. Bioavailability and detoxification of cationics: I. Algal toxicity of alkyltrimethyl ammonium salts in the presence of suspended sediment and humic acid. Chemosphere. 2009;75:303–309. doi: 10.1016/j.chemosphere.2008.12.047. [DOI] [PubMed] [Google Scholar]
- 362.Guohua J., Zuoming Z., Jing Z. Quantitative structure-activity relationship (QSAR) study of toxicity of quaternary ammonium compounds on Chlorella pyrenoidosa and Scenedesmus quadricauda. Chemosphere. 2012;86:76–82. doi: 10.1016/j.chemosphere.2011.09.021. [DOI] [PubMed] [Google Scholar]
- 363.Liang Z., Ge F., Zeng H., Xu Y., Peng F., Wong M. Influence of cetyltrimethyl ammoniumbromide on nutrient uptake and cell responses of Chlorella vulgaris. Aquat Toxicol. 2013;138–139:81–87. doi: 10.1016/j.aquatox.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 364.Sarkar B., Megharaj M., Shanmuganathan D., Naidu R. Toxicity of organoclays to microbial processes and earthworm survival in soils. J Hazard Mater. 2013;261:793–800. doi: 10.1016/j.jhazmat.2012.11.061. [DOI] [PubMed] [Google Scholar]
- 365.Sarkar B., Megharaj M., Xi Y., Krishnamurti G.S., Naidu R. Sorption of quaternary ammonium compounds in soils: implications to the soil microbial activities. J Hazard Mater. 2010;184:448–456. doi: 10.1016/j.jhazmat.2010.08.055. [DOI] [PubMed] [Google Scholar]
- 366.Grabinska-Sota E. Genotoxicity and biodegradation of quaternary ammonium salts in aquatic environments. J Hazard Mater. 2011;195:182–187. doi: 10.1016/j.jhazmat.2011.08.026. [DOI] [PubMed] [Google Scholar]
- 367.Lavorgna M., Russo C., D’Abrosca B., Parrella A., Isidori M. Toxicity and genotoxicity of the quaternary ammonium compound benzalkonium chloride (BAC) using Daphnia magna and Ceriodaphnia dubia as model systems. Environ Pollut. 2016;210:34–39. doi: 10.1016/j.envpol.2015.11.042. [DOI] [PubMed] [Google Scholar]
- 368.Ferk F., Misik M., Hoelzl C., Uhl M., Fuerhacker M., Grillitsch B., et al. Benzalkonium chloride (BAC) and dimethyldioctadecyl-ammonium bromide (DDAB), two common quaternary ammonium compounds, cause genotoxic effects in mammalian and plant cells at environmentally relevant concentrations. Mutagenesis. 2007;22:363–370. doi: 10.1093/mutage/gem027. [DOI] [PubMed] [Google Scholar]
- 369.Deutschle T., Porkert U., Reiter R., Keck T., Riechelmann H. In vitro genotoxicity and cytotoxicity of benzalkonium chloride. Toxicol In Vitro. 2006;20:1472–1477. doi: 10.1016/j.tiv.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 370.Melin V.E., Potineni H., Hunt P., Griswold J., Siems B., Werre S.R., et al. Exposure to common quaternary ammonium disinfectants decreases fertility in mice. Reprod Toxicol. 2014;50:163–170. doi: 10.1016/j.reprotox.2014.07.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Melin V.E., Melin T.E., Dessify B.J., Nguyen C.T., Shea C.S., Hrubec T.C. Quaternary ammonium disinfectants cause subfertility in mice by targeting both male and female reproductive processes. Reprod Toxicol. 2016;59:159–166. doi: 10.1016/j.reprotox.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 372.Larsen S.T., Verder H., Nielsen G.D. Airway effects of inhaled quaternary ammonium compounds in mice. Basic Clin Pharmacol Toxicol. 2012;110:537–543. doi: 10.1111/j.1742-7843.2011.00851.x. [DOI] [PubMed] [Google Scholar]
- 373.Vieira O.V., Hartmann D.O., Cardoso C.M., Oberdoerfer D., Baptista M., Santos M.A., et al. Surfactants as microbicides and contraceptive agents: a systematic in vitro study. PLoS One. 2008;3 doi: 10.1371/journal.pone.0002913. e2913/1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Inácio Â.S., Mesquita K.A., Baptista M., Ramalho-Santos J., Vaz W.L., Vieira O.V. In vitro surfactant structure-toxicity relationships: implications for surfactant use in sexually transmitted infection prophylaxis and contraception. PLoS One. 2011;6:1851–1864. doi: 10.1371/journal.pone.0019850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Debbasch C., Brignole F., Pisella P.J., Warnet J.M., Rat P., Baudouin C. Quaternary ammoniums and other preservatives' contribution in oxidative stress and apoptosis on Chang conjunctival cells. Invest Ophthalmol Vis Sci. 2001;42:642–652. [PubMed] [Google Scholar]
- 376.Perani A., Gerardin C., Stacey G., Infante M.R., Vinardell P., Rodehuser L., et al. Interactions of surfactants with living cells: induction of apoptosis by detergents containing a beta-lactam moiety. Amino Acids. 2001;21:185–194. doi: 10.1007/s007260170025. [DOI] [PubMed] [Google Scholar]
- 377.Green D.R. Reed JC. mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 378.Yip K.W., Mao X., Au P.Y., Hedley D.W., Chow S., Dalili S., et al. Benzethonium chloride: a novel anticancer agent identified by using a cell-based small-molecule screen. Clin Cancer Res. 2006;12:5557–5569. doi: 10.1158/1078-0432.CCR-06-0536. [DOI] [PubMed] [Google Scholar]
- 379.Levine S.L., Han Z., Liu J., Farmer D.R., Papadopoulos V. Disrupting mitochondrial function with surfactants inhibits MA-10 Leydig cell steroidogenesis. Cell Biol Toxicol. 2007;23:385–400. doi: 10.1007/s10565-007-9001-6. [DOI] [PubMed] [Google Scholar]
- 380.Inacio A.S., Costa G.N., Domingues N.S., Santos M.S., Moreno A.J., Vaz W.L., et al. Mitochondrial dysfunction is the focus of quaternary ammonium surfactant toxicity to mammalian epithelial cells. Antimicrob Agents Chemother. 2013;57:2631–2639. doi: 10.1128/AAC.02437-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Gaweda S., Moran M.C., Pais A.A., Dias R.S., Schillen K., Lindman B., et al. Cationic agents for DNA compaction. J Colloid Interface Sci. 2008;323:75–83. doi: 10.1016/j.jcis.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 382.Jiao Y., Ma S., Wang Y., Li J., Shan L., Sun J., et al. Methacryloxylethyl cetyl ammonium chloride induces DNA damage and apoptosis in human dental pulp cells via generation of oxidative stress. Int J Biol Sci. 2016;12:580–593. doi: 10.7150/ijbs.14578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Allison B.C., Applegate B.M., Youngblood J.P. Hemocompatibility of hydrophilic antimicrobial copolymers of alkylated 4-vinylpyridine. Biomacromolecules. 2007;8:2995–2999. doi: 10.1021/bm7004627. [DOI] [PubMed] [Google Scholar]
- 384.Sellenet P.H., Allison B., Applegate B.M., Youngblood J.P. Synergistic activity of hydrophilic modification in antibiotic polymers. Biomacromolecules. 2007;8:19–23. doi: 10.1021/bm0605513. [DOI] [PubMed] [Google Scholar]
- 385.Venkataraman S., Zhang Y., Liu L., Yang Y.Y. Design, syntheses and evaluation of hemocompatible pegylated-antimicrobial polymers with well-controlled molecular structures. Biomaterials. 2010;31:1751–1756. doi: 10.1016/j.biomaterials.2009.11.030. [DOI] [PubMed] [Google Scholar]
- 386.Feng X., Chen A., Zhang Y., Wang J., Shao L., Wei L. Application of dental nanomaterials: potential toxicity to the central nervous system. Int J Nanomed. 2015;10:3547–3565. doi: 10.2147/IJN.S79892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Nel A.E., Madler L., Velegol D., Xia T., Hoek E.M., Somasundaran P., et al. Understanding biophysicochemical interactions at the nano-bio interface. Nat Mater. 2009;8:543–557. doi: 10.1038/nmat2442. [DOI] [PubMed] [Google Scholar]
- 388.Laxminarayan R., Duse A., Wattal C., Zaidi A.K., Wertheim H.F., Sumpradit N., et al. Antibiotic resistance-the need for global solutions. Lancet Infect Dis. 2013;13:1057–1098. doi: 10.1016/S1473-3099(13)70318-9. [DOI] [PubMed] [Google Scholar]
- 389.Centers for Disease Control and Prevention (CDC) Vital signs: carbapenem-resistant enterobacteriaceae. MMWR Morb Mortal Wkly Rep. 2013;62:165–170. [PMC free article] [PubMed] [Google Scholar]
- 390.Jennings M.C., Minbiole K.P.C., Wuest W.M. Quaternary ammonium compounds: an antimicrobial mainstay and platform for innovation to address bacterial resistance. ACS Infect Dis. 2015;1:288–303. doi: 10.1021/acsinfecdis.5b00047. [DOI] [PubMed] [Google Scholar]
- 391.McBain A.J., Rickard A.H., Gilbert P. Possible implications of biocide accumulation in the environment on the prevalence of bacterial antibiotic resistance. J Ind Microbiol Biotechnol. 2002;29:326–330. doi: 10.1038/sj.jim.7000324. [DOI] [PubMed] [Google Scholar]
- 392.Buffet-Bataillon S., Tattevin P., Bonnaure-Mallet M., Jolivet-Gougeon A. Emergence of resistance to antibacterial agents: the role of quaternary ammonium compounds? A critical review. Int J Antimicrob Agents. 2012;39:381–389. doi: 10.1016/j.ijantimicag.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 393.Hegstad K., Langsrud S., Lunestad B.T., Scheie A.A., Sunde M., Yazdankhah S.P. Does the wide use of quaternary ammonium compounds enhance the selection and spread of antimicrobial resistance and thus threaten our health. Microb Drug Resist. 2010;16:91–104. doi: 10.1089/mdr.2009.0120. [DOI] [PubMed] [Google Scholar]
- 394.Tezel U., Pavlostathis S.G. Quaternary ammonium disinfectants: microbial adaptation, degradation and ecology. Curr Opin Biotechnol. 2015;33:296–304. doi: 10.1016/j.copbio.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 395.Fox E.M., Leonard N., Jordan K. Physiological and transcriptional characterization of persistent and nonpersistent Listeria monocytogenes isolates. Appl Environ Microbiol. 2011;77:6559–6569. doi: 10.1128/AEM.05529-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Pagedar A., Singh J., Batish V.K. Adaptation to benzalkonium chloride and ciprofloxacin affects biofilm formation potential, efflux pump and haemolysin activity of Escherichia coli of dairy origin. J Dairy Res. 2012;79:383–389. doi: 10.1017/S0022029912000295. [DOI] [PubMed] [Google Scholar]
- 397.Nakamura H., Takakura K., Sone Y., Itano Y., Nishikawa Y. Biofilm formation andresistance to benzalkonium chloride in Listeria monocytogenes isolated from a fish processing plant. J Food Prot. 2013;76:1179–1186. doi: 10.4315/0362-028X.JFP-12-225. [DOI] [PubMed] [Google Scholar]
- 398.Stijn V.D.V., Tjakko A. Importance of SigB for Listeria monocytogenes static andcontinuous-flow biofilm formation and disinfectant resistance. Appl Environ Microbiol. 2010;76:7854–7860. doi: 10.1128/AEM.01519-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.van der Veen S., Abee T. HrcA and DnaK are important for static and continuous-flow biofilm formation and disinfectant resistance in Listeria monocytogenes. Microbiology. 2010;156:3782–3790. doi: 10.1099/mic.0.043000-0. [DOI] [PubMed] [Google Scholar]
- 400.Atsushi T., Hideaki N., Takuya M., Keiji M., Yoichiro M., Hiroki K. Correlation between resistance of Pseudomonas aeruginosa to quaternary ammonium compounds and expression of outer membrane protein OprR. Antimicrob Agents Chemother. 2008;47:2093–2099. doi: 10.1128/AAC.47.7.2093-2099.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Karatzas K.A., Randall L.P., Webber M., Piddock L.J., Humphrey T.J., Woodward M.J., et al. Phenotypic and proteomic characterization of multiply antibiotic-resistant variants of Salmonella enterica serovar Typhimurium selected following exposure to disinfectants. Appl Environ Microbiol. 2008;74:1508–1516. doi: 10.1128/AEM.01931-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Swartjes J.J., Sharma P.K., van Kooten T.G., van der Mei H.C., Mahmoudi M., Busscher H.J., et al. Current developments in antimicrobial surface coatings for biomedical applications. Curr Med Chem. 2015;22:2116–2129. doi: 10.2174/0929867321666140916121355. [DOI] [PubMed] [Google Scholar]
- 403.Grkovic S., Brown M.H., Skurray R.A. Regulation of bacterial drug export systems. Microbiol Mol Biol Rev. 2002;66:671–701. doi: 10.1128/MMBR.66.4.671-701.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Guo W., Cui S., Xu X., Wang H. Resistant mechanism study of benzalkonium chloride selected Salmonella Typhimurium mutants. Microb Drug Resist. 2014;20:11–16. doi: 10.1089/mdr.2012.0225. [DOI] [PubMed] [Google Scholar]
- 405.Buffet-Bataillon S., Le Jeune A., Le Gall-David S., Bonnaure-Mallet M., Jolivet-Gougeon A. Molecular mechanisms of higher MICs of antibiotics and quaternary ammonium compounds for Escherichia coli isolated from bacteraemia. J Antimicrob Chemother. 2012;67:2837–2842. doi: 10.1093/jac/dks321. [DOI] [PubMed] [Google Scholar]
- 406.He G.X., Zhang C., Crow R.R., Thorpe C., Chen H., Kumar S., et al. SugE, a new member of the SMR family of transporters, contributes to antimicrobial resistance in Enterobacter cloacae. Antimicrob Agents Chemother. 2011;55:3954–3957. doi: 10.1128/AAC.00094-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Braga T.M., Marujo P.E., Pomba C., Lopes M.F.S. Involvement, and dissemination, of the enterococcal small multidrug resistance transporter QacZ in resistance to quaternary ammonium compounds. J Antimicrob Chemother. 2011;66:283–286. doi: 10.1093/jac/dkq460. [DOI] [PubMed] [Google Scholar]
- 408.Turner R.J., Taylor D.E., Weiner J.H. Expression of Escherichia coli TehA gives resistance to antiseptics and disinfectants similar to that conferred by multidrug resistance efflux pumps. Antimicrob Agents Chemother. 1997;41:440–444. doi: 10.1128/aac.41.2.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Kitagawa H., Izutani N., Kitagawa R., Maezono H., Yamaguchi M., Imazato S. Evolution of resistance to cationic biocides in Streptococcus mutans and Enterococcus faecalis. J Dent. 2016;47:18–22. doi: 10.1016/j.jdent.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 410.Jiao Y., Ma S., Wang Y., Li J., Shan L., Chen J. Epigallocatechin-3-gallate reduces cytotoxic effects caused by dental monomers: a hypothesis. Medi Sci Monit. 2015;21:3197–3202. doi: 10.12659/MSM.895628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Suzuki T., Motohashi H., Yamamoto M. Toward clinical application of the Keap1-Nrf2 pathway. Trends Pharmacol Sci. 2013;34:340–346. doi: 10.1016/j.tips.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 412.Shi Z., Niu Y., Wang Q., Shi L., Guo H., Liu Y., et al. Reduction of DNA damage induced by titanium dioxide nanoparticles through Nrf2 in vitro and in vivo. J Hazard Mater. 2015;298:310–319. doi: 10.1016/j.jhazmat.2015.05.043. [DOI] [PubMed] [Google Scholar]
- 413.Kang S.J., Ryoo I.G., Lee Y.J., Kwak M.K. Role of the Nrf2-heme oxygenase-1 pathway in silver nanoparticle-mediated cytotoxicity. Toxicol Appl Pharmacol. 2012;258:89–98. doi: 10.1016/j.taap.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 414.de Carvalho F.G., Puppin-Rontani R.M., Soares L.E., Santo A.M., Martin A.A., Nociti-Junior F.H. Mineral distribution and CLSM analysis of secondary caries inhibition by fluoride/MDPB-containing adhesive system after cariogenic challenges. J Dent. 2009;37:307–314. doi: 10.1016/j.jdent.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 415.Zhang N., Melo M.A., Chen C., Liu J., Weir M.D., Bai Y., et al. Development of a multifunctional adhesive system for prevention of root caries and secondary caries. Dent Mater. 2015;31:1119–1131. doi: 10.1016/j.dental.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Zhang N., Weir M.D., Romberg E., Bai Y., Xu H.H. Development of novel dental adhesivewith double benefits of protein-repellent and antibacterial capabilities. Dent Mater. 2015;31:845–854. doi: 10.1016/j.dental.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 417.Zhang N., Ma J., Melo M.A., Weir M.D., Bai Y., Xu H.H. Protein-repellent and antibacterial dental composite to inhibit biofilms and caries. J Dent. 2015;43:225–234. doi: 10.1016/j.jdent.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Marsh P. Controlling the oral biofilm with antimicrobials. J Dent. 2010;38:S11–S15. doi: 10.1016/S0300-5712(10)70005-1. [DOI] [PubMed] [Google Scholar]
- 419.Mauline L., Gressier M., Roques C., Hammer P., Ribeiro S.J., Caiut J.M., et al. Bifunctional silica nanoparticles for the exploration of biofilms of Pseudomonas aeruginosa. Biofouling. 2013;29:775–788. doi: 10.1080/08927014.2013.798866. [DOI] [PubMed] [Google Scholar]
- 420.Hidalgo G., Burns A., Herz E., Hay A.G., Houston P.L., Wiesner U., et al. Functional tomographic fluorescence imaging of pH microenvironments in microbial biofilms by use of silica nanoparticle sensors. Appl Environ Microbiol. 2009;75:7426–7435. doi: 10.1128/AEM.01220-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Representative three-dimensional confocal laser scanning microscopy (CLSM) video of biofilm grown on the surface of quaternary ammonium methacryloxy silane-containing acrylic resin (green channel: live microorganisms; red channel: dead microorganisms). https://youtu.be/Iw5StFwE_FU.