Abstract
The pro- and antiapoptotic proteins belonging to the B-cell lymphoma-2 (Bcl-2) family exert a critical control over cell-death processes by enabling or counteracting mitochondrial outer membrane permeabilization. Beyond this mitochondrial function, several Bcl-2 family members have emerged as critical modulators of intracellular Ca2+ homeostasis and dynamics, showing proapoptotic and antiapoptotic functions. Bcl-2 family proteins specifically target several intracellular Ca2+-transport systems, including organellar Ca2+ channels: inositol 1,4,5-trisphosphate receptors (IP3Rs) and ryanodine receptors (RyRs), Ca2+-release channels mediating Ca2+ flux from the endoplasmic reticulum, as well as voltage-dependent anion channels (VDACs), which mediate Ca2+ flux across the mitochondrial outer membrane into the mitochondria. Although the formation of protein complexes between Bcl-2 proteins and these channels has been extensively studied, a major advance during recent years has been elucidating the complex interaction of Bcl-2 proteins with IP3Rs. Distinct interaction sites for different Bcl-2 family members were identified in the primary structure of IP3Rs. The unique molecular profiles of these Bcl-2 proteins may account for their distinct functional outcomes when bound to IP3Rs. Furthermore, Bcl-2 inhibitors used in cancer therapy may affect IP3R function as part of their proapoptotic effect and/or as an adverse effect in healthy cells.
B-CELL LYMPHOMA-2 (Bcl-2) FAMILY OF PROTEINS
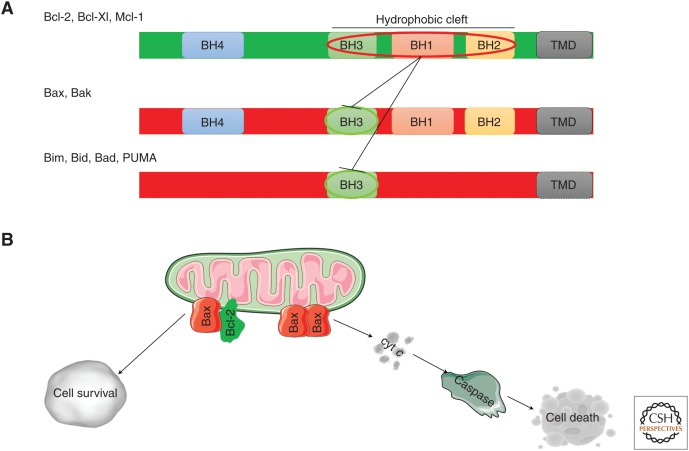
The Bcl-2 family of proteins consists of pro- and antiapoptotic members, which are characterized by the presence of at least one of the four highly conserved α-helical motifs, termed Bcl-2 homology (BH) domains (Adams and Cory 1998). The antiapoptotic family members, such as Bcl-2, Bcl-Xl, and Mcl-1, contain all four BH domains where the BH1, BH2, and BH3 domains form a hydrophobic cleft (Fig. 1A). The hydrophobic cleft is separated from the amino-terminal BH4 domain by an unstructured loop. The proapoptotic family members include the multidomain Bax, Bak, and Bok, which also contain all four BH domains (Westphal et al. 2011), and the BH3-only family members. On activation, Bax/Bak form oligomeric proteinaceous pores in the outer mitochondrial membrane (OMM), which function as channels. The Bax/Bak pores release apoptotic factors such as cytochrome c and SMAC/Diablo into the cytosol to activate apoptosis, and also enable mitochondrial DNA loss (Kalkavan and Green 2018; McArthur et al. 2018; Riley et al. 2018). BH3-only proteins, such as Bim and Bid (which becomes proaptotic following proteolytic cleavage), are termed activators, owing to their ability to directly activate Bax/Bak. Other BH3-only proteins, like Bad and Noxa, are termed sensitizers because they antagonize the action of the antiapoptotic members without binding and directly activating Bax/Bak (Chipuk et al. 2008). Most of the Bcl-2 family proteins contain a carboxy-terminal transmembrane domain (TMD), which targets the proteins to various intracellular membranes, including the mitochondrial and the endoplasmic reticulum (ER) membranes (Akao et al. 1994; García-Sáez 2012; Popgeorgiev et al. 2018).
Figure 1.
Bcl-2 family of proteins. (A) Representation of the linear structure of the Bcl-2 proteins. The antiapoptotic (green) and the proapoptotic (red) family members are shown. The BH1-4 domains, the transmembrane domain (TMD), and the hydrophobic cleft are indicated. The sequestration of the BH3 domain of the proapoptotic proteins by the hydrophobic cleft of the antiapoptotic members, which mediates apoptosis prevention, is also indicated. (B) Bcl-2 proteins at the mitochondria. The antiapoptotic Bcl-2 proteins bind to the proapoptotic Bax/Bak proteins and BH3-only proteins, thereby neutralizing their proapoptotic activity and facilitating cell survival. Oligomerization of the proapoptotic Bax/Bak proteins in response to activator BH3-only proteins (such as Bim and truncated Bid) results in outer mitochondrial membrane (OMM) permeabilization, enabling cytochrome c (cyt c) release into the cytosol with subsequent caspase 3/7 activation, eventually leading to cell death.
The pro- and antiapoptotic Bcl-2 family members interact with each other, forming a network of protein complexes that tightly control apoptosis. The interactions within the family revolve around the pro-death role of the BH3 domain. Indeed, the antiapoptotic members use their hydrophobic cleft to scaffold and neutralize the BH3 domain of the proapoptotic family members. The BH4 domains of Bcl-2 and Bcl-Xl were also implicated in counteracting Bax activation (Ding et al. 2010, 2014; Barclay et al. 2015). The interactions within the Bcl-2 family of proteins occur mainly at the mitochondria, where antiapoptotic Bcl-2 family members prevent Bax/Bak oligomerization, thereby suppressing apoptosis (Fig. 1B; Adams and Cory 1998; Chipuk et al. 2008, 2010; Shamas-Din et al. 2013).
Many of the Bcl-2 family proteins also localize at the ER (Popgeorgiev et al. 2018), where they may act as modulators of Ca2+ signals (Ferrari et al. 2002; Vervliet et al. 2016), which play a central role in the regulation of cell survival and death (Orrenius et al. 2003; Zecchini et al. 2007; Marchi et al. 2008; Zhivotovsky and Orrenius 2011). Here, we summarize the important role of Bcl-2 family members as direct modulators of the stability or function of organellar Ca2+ channels: the inositol 1,4,5-trisphosphate receptors (IP3Rs) and the ryanodine receptors (RyRs) at the ER, and the voltage-dependent anion channels (VDACs) at the OMM. Our main focus is the IP3R, for which the crystal structure was recently solved in both ligand-free (apo) (Fan et al. 2015, 2018) and ligand-bound states (Fan et al. 2018).
Emerging topics in this field and recent insights are that:
Different Bcl-2-family members, including Bok, Bcl-2, Bcl-Xl, Mcl-1, and Bcl-2L10/Nrh directly target IP3Rs;
Each Bcl-2-family member modulates the properties of the IP3R in a unique way, impacting IP3R stability (Bok) or function, resulting in IP3R inhibition (Bcl-2, Bcl-Xl at high concentrations, Bcl-2L10/Nrh) or IP3R sensitization (Bcl-Xl at low concentrations, Mcl-1);
The interaction between Bcl-2 proteins and IP3Rs often involves multiple binding sites, enabling modulatory Ca2+-signaling outputs;
The binding of certain Bcl-2 family members to IP3R impacts properties of the Bcl-2 protein; for instance, Bok is stabilized on binding to IP3Rs;
Bcl-2 family members exert part of their cell death and survival functions by forming complexes with IP3R: Bcl-2 prevents proapoptotic Ca2+ transients, whereas Bcl-Xl promotes prosurvival Ca2+ oscillations;
Targeting IP3R/Bcl-2-family protein complexes offers novel means to interfere with Bcl-2 protein function in malignancies that are dependent on these proteins, for instance, peptides targeting IP3R/Bcl-2 complexes can kill B-cell cancers, whereas peptides targeting IP3R/Bcl-2L10 complex can kill breast cancer cells; and
Other organellar Ca2+ channels such as RyRs and VDACs are targeted and modulated by Bcl-2 family members.
These aspects are discussed below in more detail.
STRUCTURE AND FUNCTION OF IP3Rs
IP3Rs function as tetrameric Ca2+-release channels with a monomeric molecular mass of ∼300 kDa. In vertebrates, IP3Rs exist as three isoforms (IP3R1, IP3R2, and IP3R3), which are encoded by three different genes (ITPR1, ITPR2, and ITPR3) (Foskett et al. 2007; Mikoshiba 2007; Parys and De Smedt 2012). All three isoforms show strong sequence homology (∼70% sequence identity), especially within their TMDs (∼90% sequence identity), yet they show significant functional differences that result in isoform-specific properties. The diversity of the IP3Rs is further expanded by alternative gene-splicing variants and by homo- and heterotetrameric assembly of isoforms and splice variants into functional channels (Monkawa et al. 1995; Patel et al. 1999; Onoue et al. 2000).
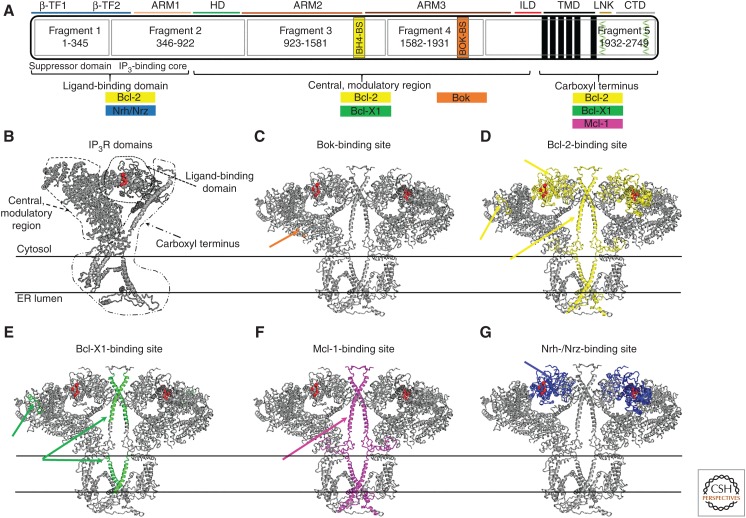
The IP3R monomer is organized into three functional regions. The amino-terminal region, consisting of ∼600 amino acids (aa), represents the ligand-binding domain (LBD), which contains the suppressor domain and the IP3-binding core (IBC). The LBD is followed by the central, modulatory region, which couples the ligand binding to the channel opening. Finally, the carboxyl terminus consists of six TMDs, whereby the channel pore is located between the fifth and the sixth TMD, and a cytosolic tail (Fig. 2A,B; Mignery et al. 1990; Südhof et al. 1991; Michikawa et al. 1994). Structural information on IP3R channels was previously limited to X-ray structures of IP3R1's soluble LBD (Lin et al. 2011; Seo et al. 2012), which represent only ∼15% of the protein sequence. Yet, the detailed mechanism by which the channel agonists activate the passage of Ca2+ through the channel remained unresolved. With major advances in the fields of cryo-electron microscopy (cryo-EM) (Kühlbrandt 2014) and the availability of biochemically optimized cryo-specimen (Murray et al. 2013), the structure of the full-length, tetrameric IP3R1 channel was determined to a resolution of 4.7 Å (Fan et al. 2015, 2018). The channel assembly shows a unique architecture, whereby each of the subunits is arranged around two four-helix bundles, forming a central core along the fourfold axis (Fan et al. 2015). The transmembrane bundle is formed by the sixth TMD helix from each subunit packed in a right-handed fashion and the cytosolic left-handed packed bundle is formed by the ∼80 Å long helix from the CTD of each subunit.
Figure 2.
Inositol 1,4,5-trisphosphate receptor (IP3R) structure with indication of the binding sites for the different Bcl-2-family members. (A) Representation of the linear structure of IP3R1. The structural domains according to Fan et al. (2015) are depicted as colored lines. The functional regions (i.e., ligand-binding domain with its suppressor domain and IP3-binding core; central, modulatory region, and carboxy-terminal region with the transmembrane domains [TMDs]) are indicated in black, and the five fragments resulting from trypsinization are indicated in blue. The Bcl-2 family members are indicated in different colors and positioned along the structure, according to the targeted region. Wherever possible, the exact binding site is indicated: the BH4-binding site (BH4-BS) for Bcl-2 in ARM2 in the fragment 3 is indicated as a yellow box; the binding site for Bok (Bok-BS) in ARM3 in the fragment 4 is indicated as an orange box; the helix 1 and 4 in the carboxyl terminus of IP3R, targeted by Bcl-Xl are indicated in green. (B) IP3R monomer viewed along the membrane plane. IP3R monomer is depicted in grey with its ligand-binding domain, central, modulatory region, and carboxyl terminus. The IP3-binding site is indicated in red. (C–G) IP3R dimers viewed along the membrane plane. The binding sites for Bok (C), Bcl-2 (D), Bcl-Xl (E), Mcl-1 (F), and Nrh/Nrz (G) are indicated in the same color as in A.
At the monomer level, the cytoplasmic part of the channel appears to be rich in α-helices, enabling flexibility and fast changes in the channel conformation on ligand binding. The apical portion of the structure contains the amino-terminal LBD, which is comprised of two β-trefoil domains (β-TF1 and β-TF2, aa 1–436, according to rat IP3R1 sequence) and a consecutive armadillo solenoid fold (ARM1, aa 436–714). ARM1 is followed by a helical domain, which together with two other α-helical domains of armadillo repeats (ARM2, aa 1030–1494 and ARM3, aa 1594–2192) form the central, modulatory region. The ARM3 expands into two antiparallel β-strands followed by a helix–turn–helix motif. This extension is called the intervening lateral domain (ILD, aa 2193–2272) and together with the ARM2 and ARM3 facilitates the communication between the LBD and the carboxyl terminus, where the ILD connects to the six α-helical TMDs. The sixth TMD helix contains the bulky hydrophobic residues (F2586 and I2590), which constitute a physical gate and can function to either hinder or permit ion translocation across the ER membrane. The sixth TMD helix extends beyond the lipid membrane boundaries into the cytosol where it is connected to a helical linker domain (LNK, aa 2609–2681) followed by the long cytosolic α-helix, designated as a carboxy-terminal domain (CTD, aa 2682–2750) (Fan et al. 2015).
A striking feature of IP3R1 monomer arrangement is the sandwich formed by the LNK domain surrounded by the β-strands and the helix–turn–helix regions of the ILD. With both domains directly connected to the TMD, the ILD/LNK unit is uniquely positioned to integrate all channel gating signals arising from the cytosolic domains to the gating machinery within the TMD. Further details regarding the molecular mechanism of channel gating revealed that the cytosolic carboxy-terminal helix of one subunit directly interacts with the amino-terminal LBD, in particular the β-TF2 domain, of the adjacent subunit, suggesting an allosteric type of regulation of the channel (Fan et al. 2015).
Recent structures of the IP3R1 in a ligand-bound (4.1 Å) and apo state (3.9 Å) solved by cryo-EM revealed considerable changes in both the pore and cytoplasmic domains on binding of activating ligands (Fan et al. 2018). Thereby, a network of intra- and interdomain interfaces responsible for conformational coupling between ligand binding and gating activation were identified. Adenophostin A, an IP3 analog and potent IP3R agonist of fungal origin (Takahashi et al. 1994), evoked structural changes in the LBD, where the β-TF2 and ARM1 undergo a 5 Å closure of the cleft between them. The changes in the LBD trigger pronounced rearrangements of the cytoplasmic domains, rotation of the CTD helix and lateral movements in the interfacial ILD/LNK region. The ILD/LNK region appears to be mechanically connected to pore opening, whereby changes in the ILD/LNK interface traverse to the TMDs. Ligand gating would require the pore-forming helical bundle comprised of four pairs of the inner sixth TMDs to dilate at the hydrophobic constrictions formed by the side-chains of F2586 and I2590. Mutations within the ILD (Hamada et al. 2017) and LNK (Uchida et al. 2003; Bhanumathy et al. 2012) domains support the mechanism of signal transduction through the ILD/LNK assembly formed at the membrane cytosol interface. Overall, the structures of IP3R1 provide the basis for understanding the allosteric regulation of channel gating.
Other IP3-bound IP3R structures were determined either by X-ray crystallography (in the case of isolated LBD [Lin et al. 2011; Seo et al. 2012] and large, monomeric cytoplasmic portion of IP3R1 [Hamada et al. 2017]) or by cryo-EM (IP3R3) (Paknejad and Hite 2018). These structures described conformational changes within some cytoplasmic domains upon ligand binding. However, it remains unclear whether these changes are representative of the ligand-evoked channel gating, because either the TMD was not present (Hamada et al. 2017) or no conformational changes within the TMD were described for the ligand-bound channel (Paknejad and Hite 2018). Structural studies of IP3R1 in multiple functional states guided by biophysical characterizations and in physiological conditions will be necessary to fully understand the allosteric mechanism of IP3R channel gating and regulation.
New insights in the mechanism of activation were gained by using concatenated IP3Rs. This study showed that proper IP3R activation and initiation of Ca2+ release occurs only when all four subunits are occupied by IP3 (Alzayady et al. 2016). In addition, previous work showed that the controlled trypsinization of each monomeric IP3R1 resulted in five fragments, as follows according to the mouse IP3R1 sequence: fragment 1 (aa 1–345), fragment 2 (aa 346–922), fragment 3 (aa 923–1581), fragment 4 (aa 1582–1931), and fragment 5 (aa 1932–2749) (Fig. 2A). Interestingly, when co-expressed, these five fragments were able to self-assemble into a functional IP3R1 channel that enabled Ca2+ release from the ER in response to IP3 (Yoshikawa et al. 1999; Alzayady et al. 2013).
The ability of IP3Rs to respond adequately to the cell's requirements and to tightly control versatile Ca2+-dependent processes is a result of a precise regulation. The basic regulators of IP3Rs are IP3, Ca2+, ATP, and various posttranslational modifications like phosphorylation, glycosylation, palmitoylation, thiol modification, and oxidation by reactive oxygen species, such as H2O2 (Bezprozvanny 2005; Foskett et al. 2007; Mikoshiba 2007; Vanderheyden et al. 2009; Booth et al. 2016; Joseph et al. 2018). An important part of the IP3R population resides at specialized domains at which the ER is in close apposition with other organelles, including the mitochondria (Decuypere et al. 2011; Raturi and Simmen 2013; Marchi et al. 2014) and the lysosomes (Kilpatrick et al. 2013; Atakpa et al. 2018). These domains are often referred to as membrane contact sites and represent bidirectional interactions between the ER and these organelles through IP3R and Ca2+ signaling (La Rovere et al. 2016; Roest et al. 2017). Furthermore, there is an increasing number of documented interactions with regulatory proteins, including several proteins directly involved in the regulation of cell-death and -survival processes such as apoptosis, metabolism, unfolded protein responses, and autophagy (Choe and Ehrlich 2006; Ivanova et al. 2014; Prole and Taylor 2016; Parys and Vervliet, in press).
REGULATION OF IP3R BY Bcl-2 FAMILY PROTEINS
Proapoptotic Bok as a Stabilizer of IP3R Proteins
The multidomain proapoptotic Bcl-2-family-member Bok, which failed to bind to any of the Bcl-2 family members (Echeverry et al. 2013; Llambi et al. 2016), emerged as a prominent binding partner of IP3Rs (Fig. 2; Table 1). The interaction, which occurred between the amino terminus of Bok and a small site in the central, modulatory region of IP3Rs and in particular within the fragment 4 (aa 1895–1903 of mouse IP3R1, part of the ARM3 domain), appeared to control the proteolytic degradation of IP3Rs (Schulman et al. 2013). Vice versa, it was shown that Bok is constitutively bound to IP3Rs, which promotes Bok stabilization. The unbound Bok is ubiquitinated by the AMFR/gp78 E3 ubiquitin ligase complex, targeted to the proteasome through VCP/p97 and degraded through the ER-associated degradation pathway (Schulman et al. 2016). Bok showed greater affinity for IP3R1 and IP3R2, while barely binding to IP3R3. It is well established that cells stimulated with IP3-generating agonists for prolonged time down-regulate their IP3Rs via a process that involves ubiquitination and proteasomal degradation (Oberdorf et al. 1999; Wojcikiewicz et al. 2009). Cells exposed to chronic IP3R activation showed a concomitant decline in both Bok and IP3R levels, whereas other Bcl-2-family members remained unaltered. Thus, it seems that IP3R/Bok complexes are degraded as a “combined unit” (Schulman et al. 2013). In in vitro cleavage experiments, IP3Rs from Bok−/− cells were more susceptible to cleavage by chymotrypsin. This correlated with in cellulo experiments demonstrating that caspase-3-mediated cleavage of IP3R1 on exposure to staurosporine was more readily observed in Bok−/− cells than in wild-type cells. Yet, wild-type mouse embryonic fibroblasts (MEFs) and Bok−/− MEF cells displayed very similar IP3R-mediated Ca2+ responses when intact cells were stimulated with lysophosphatidic acid, or if IP3Rs were directly activated by addition of IP3 to permeabilized cells. It should be noted that Bok−/− cells derived from Bok−/− mice displayed increased IP3R1 levels, but a marked decline in IP3R2 levels and a modest decline in IP3R3 levels (Schulman et al. 2013). Thus, it remains possible that changes in IP3R function caused by loss of Bok were masked by changes in IP3R expression. To perform a more direct assessment of the impact of Bok on IP3R function, Bok-deficient MEF cells have recently been generated by CRISPR/Cas9 (Schulman et al. 2019). In these models, the expression levels of the different IP3R isoforms were unaffected on deletion of Bok, indicating that IP3R stability in basal conditions is not affected by Bok. However, also these Bok−/− MEF cells displayed similar levels of IP3R-mediated Ca2+ release and furthermore of mitochondrial Ca2+ influx as the wild-type cells. Interestingly, the deletion of Bok led to mitochondrial fragmentation, rather than altered Ca2+ signaling. The mitochondrial fragmentation was attributed to a decreased mitochondrial fusion rate. This also increased the mitochondrial spare respiratory capacity in Bok−/− cells, potentially the result of an increased mitochondrial surface (Schulman et al. 2019). The exact mechanism of how Bok, a protein localized at the ER, has such tremendous effect on mitochondria requires further investigation. The impact of Bok on the mitochondria appeared to be independent of its sequestration by IP3R, which was elegantly shown by using a Bok mutant defective in IP3R binding. Therefore, these investigators speculated that IP3R-bound Bok is not only stabilized, but could also maintain the normal rate of mitochondrial fusion, thereby preventing excessive mitochondrial fusion promoted by unbound Bok (Schulman et al. 2019).
Table 1.
Bcl-2-family members as modulators of IP3R
| Protein | Impact on cell fate | Effect on [Ca2+] | Effect on IP3R | Binding site on IP3R | Binding site on Bcl-2 family members | References |
|---|---|---|---|---|---|---|
| Bok | Proapoptotic | No apparent effect on Ca2+-flux properties | Increased stability | Fragment 4 (ARM3 in the modulatory region) | Amino terminus | Schulman et al. 2013 |
| Bcl-2 | Antiapoptotic | Suppresses the Ca2+ release from the endoplasmic reticulum (ER), decreasing the ER–mitochondrial Ca2+ transfer | Inhibition | IP3-binding core (IBC); fragment 3 (ARM2 in the modulatory region) and carboxyl terminus | BH4 and terminal transmembrane domain (TMD) | Rong et al. 2009; Monaco et al. 2012; Ivanova et al. 2016; Ivanova et al. 2019 |
| Promotes prosurvival Ca2+ oscillations, increasing the Ca2+ flux in the mitochondria | Sensitization | Carboxyl terminus | ? | Zhong et al. 2006; Eckenrode et al. 2010 | ||
| Reduces the [Ca2+]ER, decreasing the ER–mitochondrial Ca2+ transfer | Pinton et al. 2001; Oakes et al. 2005 | |||||
| Bcl-XI | Antiapoptotic | Suppresses the Ca2+ release from the ER, decreasing the ER–mitochondrial Ca2+ transfer | Inhibition | Fragment 3 (ARM2 in the modulatory region) and helix 1 (carboxyl terminus) | Hydrophobic cleft | Yang et al. 2016 |
| Reduces the [Ca2+]ER, decreasing the ER–mitochondrial Ca2+ transfer | Sensitization | Helices 1 and 4 (carboxyl terminus) | Hydrophobic cleft | Li et al. 2007; Yang et al 2016 | ||
| Promotes prosurvival Ca2+ oscillations, increasing the Ca2+ flux in the mitochondria | White et al. 2005; Yang et al. 2016 | |||||
| Mcl-1 | Antiapoptotic | Reduces the [Ca2+]ER, decreasing the ER–mitochondrial Ca2+ transfer | Sensitization | Carboxyl terminus | ? | Eckenrode et al. 2010 |
| Nrh | Antiapoptotic | Suppresses the Ca2+ release from the ER, decreasing the ER–mitochondrial Ca2+ transfer | Inhibition | IBC (β-TF2 in the ligand-binding domain, LBD) | BH4 | Bonneau et al. 2016 |
| Nrz | Antiapoptotic | Suppresses the Ca2+ release from the ER, decreasing the ER–mitochondrial Ca2+ transfer | Inhibition | LBD | BH4, BH1, and BH3 | Bonneau et al. 2014 |
Summarizing the different Bcl-2 family members with their effect on apoptosis and intracellular Ca2+, the impact they exert on IP3R and the binding determinants underlying the protein–protein complexes.
The role of Bok as a Bcl-2-family member in apoptosis remains controversial. The results vary from reporting Bok as a proapoptotic protein that functions in a Bax/Bak-dependent (Echeverry et al. 2013) or independent manner (Llambi et al. 2016), through suggesting that Bok can exert antiapoptotic effects (D'Orsi et al. 2016) and finally to the recent findings indicating the Bok does not directly affect stimuli-induced apoptosis (Schulman et al. 2019). In any case, it is clear that Bok recruitment to IP3R with concomitant interference of the channel proteolytic cleavage could represent a novel and intriguing mechanism of IP3R abundance regulation. Furthermore, differences in Bok expression and/or in association with IP3Rs might account for the different susceptibilities reported for IP3R cleavage by caspase-3. These findings might also have implications for human cancers, because high-resolution analyses of somatic copy-number alterations from more than 3000 cancer specimens revealed the loss of Bok as a significant alteration (Beroukhim et al. 2010).
Antiapoptotic Bcl-2 Family Proteins as Modulators of IP3R Activity
Bcl-2
The ability of Bcl-2 to directly modulate IP3R activity was first observed in T-lymphocytes. Overexpression of Bcl-2 in an immature T-cell model (WEHI7.2 cells), which displays very low endogenous levels of Bcl-2, dampened T-cell receptor (TCR)-induced IP3R-mediated Ca2+ release (Chen et al. 2004). In the same study, an endogenous Bcl-2/IP3R-protein complex was identified in S49.A2 lymphocytes, and since then, the Bcl-2/IP3R interaction was further confirmed in various cell models from different origin (Xu et al. 2007; Hanson et al. 2008; Rong et al. 2008; Akl et al. 2013). Moreover, different cancer cell types, including lymphoma, leukemia, lung, and ovarian cancer cells have been described as dependent on the Bcl-2/IP3R interaction for their survival, because disruption of this complex led to proapoptotic Ca2+ signals and cell death (Zhong et al. 2011; Akl and Bultynck 2013; Akl et al. 2013, 2015; Greenberg et al. 2015; Lavik et al. 2015; Xie et al. 2018). The effect of Bcl-2 on Ca2+ signaling was extensively studied in T-lymphocytes, in which the TCR-mediated Ca2+ release plays a crucial role for cell-fate decisions (Feske 2007; Fracchia et al. 2013; Joseph et al. 2014). Bcl-2 displayed divergent impacts on proapoptotic versus prosurvival Ca2+ signals. Although it suppressed the proapoptotic cytosolic Ca2+ transients with high amplitude, triggered by strong TCR activation, Bcl-2 did not affect prosurvival Ca2+ oscillatory signals, triggered by weak TCR activation (Zhong et al. 2006).
Of note, Bcl-2 was also proposed as able to sensitize IP3R. This notion was based on Bcl-2's tendency to increase Ca2+ oscillation frequency, although not statistically significantly different (Zhong et al. 2006). This observation relates to another study, in which the open probability (Po) of single IP3R channels and the IgM-induced Ca2+ oscillations in DT40 cells were monitored. The analyses showed that Bcl-2 was capable of promoting IP3R activity and enhancing the frequency of Ca2+ oscillations (Eckenrode et al. 2010). The proposed Bcl-2-mediated increase of IP3R activity has also been linked to a lower steady-state ER Ca2+ content in cells overexpressing Bcl-2. Effectively, the increase of IP3R activity acts as constitutive ER Ca2+ leak (Oakes et al. 2005). The ability of Bcl-2 to lower steady-state ER Ca2+ levels was originally proposed to be the key component of its antiapoptotic effect (Pinton et al. 2001), although now it is clear that Bcl-2 can fulfill its antiapoptotic function in cells while not affecting the Ca2+ store content (Hanson et al. 2008; Monaco et al. 2012b; Ivanova et al. 2016). Here, the increased IP3R activity was explained by more binding of Bcl-2 to the IP3R and an enhanced protein kinase A–dependent phosphorylation state of the IP3R1, thereby sensitizing IP3R-mediated Ca2+ release to basal concentrations of IP3. In that context, it is important to mention that Bcl-2 can regulate the phosphorylation state of the IP3R by protein kinase A, via its scaffolding of DARPP-32 and calcineurin (Chang et al. 2014a). Thus, Bcl-2 interaction with IP3Rs appears to be modulated by additional factors and proteins.
Bcl-2-mediated decrease of ER Ca2+ stores has also been shown to be dependent on other proteins besides IP3Rs, including Bax Inhibitor-1 (BI-1) and CISD2. First, Bcl-2 overexpression failed to lower ER Ca2+ levels in cells lacking BI-1, the founding member of the transmembrane Bax inhibitor motif (TMBIM)–containing protein family (Xu et al. 2008). BI-1 and the other TMBIM-family members function as ancestral regulators of cell death (Rojas-Rivera and Hetz 2015; Carrara et al. 2017; Liu 2017). This function is tightly connected to Ca2+ dynamics, because BI-1 operates as an ER Ca2+-leak channel (Bultynck et al. 2012, 2014), a function conserved during evolution (Chang et al. 2014b; Guo et al. 2019). Second, CISD2, a protein involved in longevity (Chen et al. 2009; Wu et al. 2012), breast cancer (Sohn et al. 2013), and Wolfram syndrome type 2 (Rigoli and Di Bella 2012) has been identified to be present in IP3R/Bcl-2 multiprotein complexes, where it enables Bcl-2 to lower the ER Ca2+ content (Chang et al. 2010). Thus, also in cells lacking CISD2, Bcl-2 fails to reduce the ER Ca2+ levels.
As we discussed, the modulation of IP3R activity by Bcl-2 is complex, with alternative outcomes being proposed: inhibition and sensitization with reduced ER Ca2+ content. The molecular mechanism of interaction between Bcl-2 and IP3R has been best studied in the context of channel inhibition. The interaction between these proteins relies on complex multidomain-binding determinants (Fig. 2; Table 1; Parys 2014). IP3R inhibition (Fig. 3) was initially attributed to binding between Bcl-2's BH4 domain and the fragment 3 of the IP3R (aa 923–1581) (Rong et al. 2008, 2009a,b; Ivanova et al. 2017). This interaction appeared to be highly conserved during vertebrate evolution (Ivanova et al. 2017). Based on detailed molecular insights in the Bcl-2-binding determinants within the fragment 3, the precise location of the Bcl-2-binding site was identified (aa 1389–1408). This 20-aa region, located in the ARM2 domain in the central, modulatory region of IP3R1, is highly conserved among all three IP3R isoforms and during evolution (Monaco et al. 2012b). Consistent with this, Bcl-2 binding to the IP3R was observed for all three IP3R isoforms. A peptide corresponding to the Bcl-2-binding site on IP3R1 (IP3R-derived peptide [IDP]) was able to disrupt endogenous IP3R/Bcl-2 complexes, antagonizing the ability of Bcl-2 to inhibit IP3Rs in a variety of experimental models (Rong et al. 2008; Zhong et al. 2011; Akl et al. 2013). These findings have recently been corroborated by an electrophysiological analysis performed on giant unilamellar vesicles prepared from Bcl-2-overexpressing WEHI7.2 cells (Shapovalov et al. 2017). Addition of IDP-augmented IP3-induced channel activity caused by a dramatic increase in the Po of IP3Rs. A cell-permeable variant of the IDP peptide (TAT-IDP) enhanced Ca2+-dependent cell death in T-cell models (cell death was triggered by strong TCR activation) (Rong et al. 2008). A stabilized, proteolysis-resistant, form of TAT-IDP (Bcl-2/IP3 receptor disruptor-2, BIRD-2) provoked cell death by itself in a variety of cancer cell models, particularly in those that are less sensitive to venetoclax, a BH3-mimetic Bcl-2 inhibitor (Vervloessem et al. 2017b). In diffuse large B-cell lymphoma cells, BIRD-2-induced cell death depended on the combination of high IP3R2 expression levels (Akl et al. 2013) and of elevated chronic IP3 signaling, which occurs downstream from the B-cell receptor in these cells (Bittremieux et al. 2019). Furthermore, recent work indicated that both Ca2+ release from the ER and influx of extracellular Ca2+ contributed to BIRD-2-induced cell death (Bittremieux et al. 2018). A further in-depth discussion on the IP3R-mediated function of Bcl-2 in cancer (Akl et al. 2014) and its targeting by BIRD-2 has been provided in recent reviews (Distelhorst 2018; Kerkhofs et al. 2019a,b). The investigative path that resulted in IP3R-derived peptides as novel Bcl-2 inhibitors with anticancer potential and how this inspired the development of novel first-in-class small-molecule Bcl-2 inhibitors that provoke cell death in chronic lymphocytic leukemia through Ca2+ overload was reviewed elsewhere in this collection (Distelhorst and Bootman 2019).
Figure 3.
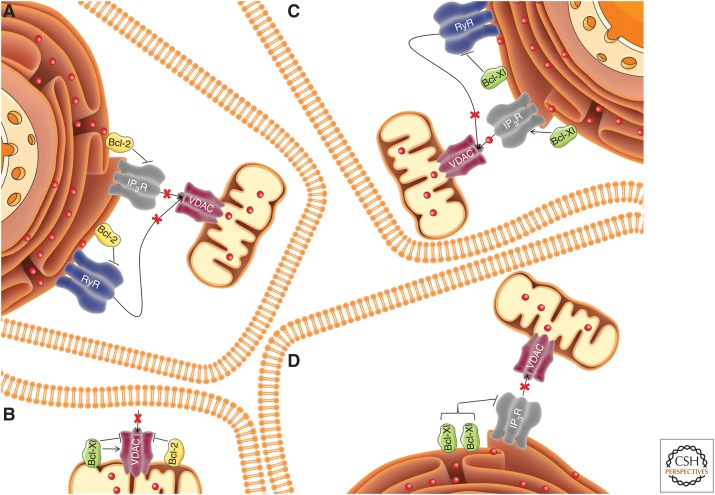
Overview of Bcl-2's and Bcl-Xl's effects on inositol 1,4,5-trisphosphate receptor (IP3R), ryanodine receptor (RyR), and voltage-dependent anion channel (VDAC) activity. The various regulatory mechanisms are illustrated in four cells with their endoplasmic reticulum and mitochondria. Bcl-2, depicted in yellow is able to inhibit all three of the discussed intracellular Ca2+ channels: (A) IP3R (gray), RyR (blue), and (B) VDAC (purple). Depending on its concentration, Bcl-Xl (green), could sensitize (C) or inhibit IP3R (D). Similarly to Bcl-2, Bcl-Xl also inhibits RyR (C). Finally, the effect of Bcl-Xl on VDAC is still under debate as both stimulation and inhibition were reported (B).
As stated above, the interaction between Bcl-2 and IP3R is multimodal, underlying the complex IP3R channel modulation by Bcl-2. Such interaction profiles enable complex signaling outputs. For instance, Bcl-2 fails to inhibit IP3R-mediated Ca2+ signaling provoked by supramaximal concentrations of (1) IP3 in 45Ca2+ fluxes using permeabilized cells and in single IP3R channel recordings using nuclear patch clamping, or (2) agonist in intact cells using Ca2+ measurements at the single-cell level (Hanson et al. 2008; Ivanova et al. 2019). The molecular basis for the ability of Bcl-2 to sensitize or inhibit IP3R activity depending on the level of IP3/agonist stimulation were not clear until very recently, when a novel interaction between the BH4 domain of Bcl-2 and the LBD of IP3R1, in particular the IBC, was identified (Fig. 2; Ivanova et al. 2019). Furthermore, the interaction was antagonized by physiological (IP3) and pharmacological (adenophostin A) IP3R agonists that target the LBD. Vice versa, the BH4 domain of Bcl-2 decreased the ligand binding to the LBD in in vitro Förster resonance energy transfer (FRET) measurements. This finding is consistent with a previous observation that Bcl-2 overexpression interfered with the ligand–receptor interaction (Hanson et al. 2008). As such, a mutual antagonism between Bcl-2 and IP3 for controlling IP3R channel activity seems to exist. In line with the existence of this second BH4 domain-binding region, Bcl-2 was able to inhibit a mutated IP3R1 channel that lacked the binding site in the fragment 3 (IP3RΔ1389-1408). Although, at first sight, this study challenges the existing model of IP3R inhibition by Bcl-2, whereby the BH4 domain and its binding site in fragment 3 are the driving force, notably compared with the wild-type channel, the inhibition of IP3RΔ1389–1408 was less prominent. Thus, instead of contradicting, these new findings might represent an additional layer of the complex mechanism of IP3R modulation by Bcl-2. This is also supported by the 3D cryo-EM structure of IP3R1 (Fan et al. 2015, 2018), where aa 1389–1408 from each subunit appeared to be located relatively close to the LBD from the neighboring IP3R1 subunit. However, the importance of both BH4 domain-binding sites, that is, LBD and aa 1389–1408, and their interplay with Bcl-2, require further investigation. A fascinating consequence of Bcl-2 binding to the LBD of IP3R is that in theory one Bcl-2 molecule per channel could be sufficient to inhibit IP3R activity, because all four subunits ought to be occupied by IP3 to open the channel (Alzayady et al. 2016; Taylor and Konieczny 2016). Yet, further work will be needed to determine the stoichiometry of IP3R/Bcl-2 complexes and the minimal ratio of Bcl-2 proteins per IP3R to impose IP3R inhibition.
As the BH4 domain of Bcl-2 is crucial for the modulation of IP3R, different studies were dedicated to elucidate its unique characteristics. It is clear that Bcl-2's BH4 domain is necessary and sufficient for IP3R inhibition (Rong et al. 2009b). As such, Bcl-2 lacking its BH4 domain did not bind to the ARM2 in the fragment 3 of IP3R. A number of critical surface-accessible residues (R6, S7, D10, R12, K17, H20, Y21, Q25, R26, Y28), which contribute to the inhibitory effect of the BH4 domain on IP3R, were identified (Rong et al. 2008, 2009a; Monaco et al. 2012b). Among them, the K17 is of a particular interest. This positively charged residue represents a critical difference with the BH4 domain of the other major antiapoptotic member, Bcl-Xl, where it corresponds to a negatively charged D11. In contrast to the BH4 domain of Bcl-2, the BH4 domain of Bcl-Xl displayed poor IP3R-binding properties and was much less effective in inhibiting IP3Rs. Interestingly, a mutated version of the BH4 domain of Bcl-2 (BH4-Bcl-2 K17D), which mimics the BH4 domain of Bcl-Xl, was severely compromised in binding and inhibiting IP3Rs. The K17 was important also at the full-length protein level, because the full-length Bcl-2 mutant K17D was impaired in inhibiting agonist-induced Ca2+ release within intact cells and preventing apoptosis, triggered by staurosporine (Monaco et al. 2012b). The α-helical properties of the BH4 domain of Bcl-2 have also been found to be essential for IP3R binding and inhibition. Indeed, replacing I14 and V15, two α-helical backbone residues, by two G residues reduced the propensity of the BH4 domain to form an α-helix. These mutations strongly impaired the interaction of the BH4 domain with fragment 3 of the IP3R and the ability of the BH4 domain to inhibit IP3R function and to protect against Ca2+-dependent apoptosis (Monaco et al. 2013). The I14G/V15G mutated version of full-length Bcl-2 also abrogated Bcl-2's ability to inhibit IP3R-mediated Ca2+ release. However, the underlying mechanism appeared complex. Indeed, the protein levels of ectopically expressed Bcl-2 I14G/V15G were consistently lower than those of ectopically expressed wild-type Bcl-2. Additional biophysical experiments indicated that I14G/V15G mutations impacted Bcl-2's conformation, caused a destabilization, and shortened half-life of the protein in cells (Monaco et al. 2018). This indicates that the BH4 domain is also important for the overall stability of the Bcl-2 proteins. Of importance, Bcl-2 K17D was not severely destabilized, indicating that the reduced ability of Bcl-2 K17D to inhibit IP3Rs was not implicitly the result of a decrease in Bcl-2 stability. Furthermore, these data also hold a warning on the interpretation of results obtained with BH4 domain mutants, as their ability to abrogate a particular function of Bcl-2 may not imply a specific prevention of an interaction or a novel function/target for the BH4 domain itself, but rather an effect on overall Bcl-2's structural organization and/or stability. Consequently, Bcl-2 I14G/V15G failed to bind Bax and to inhibit IP3R, both contributing to its reduced antiapoptotic properties (Monaco et al. 2018).
Besides the central, modulatory region and the LBD, the carboxyl terminus of IP3R has also been proposed as a Bcl-2-binding site. The sixth TMD appeared as a critical binding determinant, because Bcl-2 displayed efficient binding only to a GST protein, corresponding to the carboxy-terminal tail together with the sixth TMD of IP3R1 (aa 2512–2749 according to the mIP3R1 sequence), but not to the carboxy-terminal IP3R fragment lacking the sixth TMD (aa 2590–2749 according to the mIP3R1 sequence) (Rong et al. 2008; Eckenrode et al. 2010; Monaco et al. 2012a). Bcl-2 binding to the carboxyl terminus of IP3R was associated with sensitization of the channel (Eckenrode et al. 2010) and it was proposed that the hydrophobic cleft of Bcl-2 might be involved in the interaction (Yang et al. 2016). Using genetic and pharmacological approaches to neutralize the hydrophobic cleft of Bcl-2, this part of the protein appeared dispensable for the binding and the regulation of IP3R. Consistent with that, BH3-mimetic drugs that occupy Bcl-2's hydrophobic cleft and abolish the interaction with proapoptotic Bcl-2-family members did not cause Ca2+ dysregulation and did not alter agonist-induced Ca2+ increases in a variety of cell lines, cancer cells, and primary cells such as pancreatic acinar cells (Vervloessem et al. 2017a; Jakubowska et al. 2018). In contrast to the hydrophobic cleft, the TMD of Bcl-2 was identified as a binding partner of the carboxyl terminus of IP3R and this interaction was required for the efficient IP3R inhibition. It is anticipated that the binding between both IP3R and Bcl-2 carboxyl termini augments the local concentration of Bcl-2 and thus of its BH4 domain in the proximity of the IP3R, and in particular of the fragment 3 binding site, enabling in cellulo interaction and inhibition of Ca2+ release (Ivanova et al. 2016). It is important to note that the isolated BH4 domain displays a rather low-affinity inhibition of the IP3R (half-maximal inhibitory concentration [IC50] value of ∼30 µm) and a binding affinity in the low µm range for the isolated fragment 3 and IBC of IP3R1. Thus, inhibition of IP3Rs by Bcl-2's BH4 domain likely requires a high local concentration of Bcl-2 in a cellular context.
Bcl-Xl
Bcl-Xl has been shown to bind to IP3R1, IP3R2, and IP3R3 (Fig. 2; Table 1; White et al. 2005). The functional relevance of the Bcl-Xl–IP3R interaction was studied by patch clamp electrophysiological experiments, using Sf9 (White et al. 2005) and DT40 (Li et al. 2007) cells, where the Po of IP3Rs was measured. Purified recombinant Bcl-Xl, applied in the low µm range, enhanced the channel activity triggered by submaximal IP3 concentration in both models (White et al. 2005; Li et al. 2007), but did not display any significant effect when IP3R was activated by supramaximal (IP3) in Sf9 cells (White et al. 2005). Furthermore, Bcl-Xl overexpression in DT40 cells increased the frequency of spontaneous and IgM-induced Ca2+ oscillations and the number of oscillating cells (White et al. 2005). Similar results were obtained in a study that aimed to decipher the impact of Bcl-Xl on the various IP3R isoforms (Li et al. 2007). Recombinant Bcl-Xl was able to sensitize each IP3R isoform to low concentration of IP3 in patch clamp experiments, causing an increase in the Po of the IP3R. Consistent with this, the overexpression of Bcl-Xl in DT40 cells with a triple knockout of all three IP3R isoforms (DT40-TKO) with reconstituted IP3R1, IP3R2, or IP3R3 resulted in an increased frequency of the spontaneous Ca2+ oscillations. Interestingly, although Bcl-Xl appeared to decrease the ER Ca2+ content and the amplitude of IgM-induced Ca2+ signals in DT40-TKO cells expressing IP3R3, overexpression of Bcl-Xl resulted in suppressed IgM-induced apoptosis in DT40-TKO cells expressing any of the three IP3R isoforms (Li et al. 2007). This was in line with the observation that Bcl-Xl protected DT40 cells against IgM-induced apoptosis by promoting mitochondrial bioenergetics leading to cell survival (White et al. 2005). Moreover, it was shown that Bcl-Xl-mediated protection against cellular toxins was strongly dependent on the expression of IP3R, as Bcl-Xl overexpression particularly protected DT40-TKO cells when IP3Rs were reintroduced (Li et al. 2007).
More light was shed on the mechanism of IP3R regulation by Bcl-Xl by a study elucidating the differential effect of Bcl-Xl levels in patch clamp experiments. Low Bcl-Xl concentrations, in a range with a maximal potentiating effect at 1 µm, enhanced IP3R activity, whereas high concentrations (10 µm or more) inhibited the channels (Fig. 3; Yang et al. 2016). These divergent effects of Bcl-Xl were underscored by its binding to alternative IP3R regions with different affinities. In contrast to Bcl-2, Bcl-Xl displayed a much higher binding efficiency for the carboxy terminal tail fragment compared with the central fragment 3 (Monaco et al. 2012a; Yang et al. 2016). The carboxy-terminal region of IP3R contains two adjacent sites targeted by Bcl-Xl, helix 1, and helix 4, which both contain BH3-domain-like features. Bcl-Xl binding to both helixes was required for the activation of IP3R, whereas Bcl-Xl binding to helix 1 and to ARM2 domain in the fragment 3 resulted in inhibition of the channel, as measured by the decrease of the Po (Yang et al. 2016). Disturbance of the interactions of Bcl-Xl with the carboxyl terminus of IP3R affected cell survival. Indeed, DT40-TKO cells expressing IP3Rs that carry a mutation in the BH3-domain-like sequences of helix 1 (F2585A D2590A) and helix 4 (L2716A D2721A) were more sensitive to proapoptotic stimuli than DT40-TKO cells expressing wild-type IP3Rs, at least when these IP3Rs were expressed at low-to-medium levels. In addition, abrogating the high-affinity interaction of Bcl-Xl with IP3Rs using cell-permeable peptides representing the helices 1 and 4 of IP3R enhanced the apoptotic effect of staurosporine in DT40 cells. Finally, helix 1 and 4 as peptides were able to induce cell death by themselves in breast cancer cells and in Burkitt's lymphoma cells (Yang et al. 2016). However, it is not clear whether the cell death effects were solely caused by disruption of the IP3R/Bcl-Xl complexes or may be in part caused by the disturbance of Bcl-Xl complexes with proapoptotic Bcl-2-family members at the mitochondrial membranes.
As stated above, IP3R inhibition by Bcl-Xl requires the simultaneous binding of Bcl-Xl to fragment 3 and to helix 1 in the carboxyl terminus. Previously, it was shown that the BH4 domain of Bcl-Xl lacked sufficient affinity or selectivity to bind fragment 3 and inhibit IP3R (Monaco et al. 2012b). Yet, in a recent study, it was speculated that the inhibitory interaction might be executed by Bcl-Xl's BH4 domain, because a synthetic peptide, corresponding to the BH4 domain, could antagonize IP3R inhibition by high concentrations of Bcl-Xl (Yang et al. 2016). Of note, in a study elucidating the role of Bcl-Xl at ER–mitochondrial contact sites, the BH4 domain and the TMD of Bcl-Xl were proposed to be involved in the interaction with IP3R, because mutants lacking either of these domains displayed decreased binding to the receptor (Williams et al. 2016). Indeed, the lack of the TMD caused a ∼90% reduction in the binding, suggesting a crucial role for Bcl-Xl localization at the ER–mitochondria contact sites, where it would bind to and regulate IP3R activity. Nevertheless, deletions of the BH4 domains of Bcl-Xl/Bcl-2 might result in conformational changes and less stable proteins. The binding to the carboxy-terminal tail of the IP3R occurred via Bcl-Xl's hydrophobic cleft, because mutations that abrogate this functional domain resulted in failure of Bcl-Xl to form complexes with both IP3R helices (helix 1 and helix 4) identified as its binding partners (Yang et al. 2016). Furthermore, such mutated Bcl-Xl proteins were severely impaired in regulating IP3R function. The contribution of the hydrophobic cleft of Bcl-Xl was also emphasized by experiments using ABT-737, antimycin A, and a peptide derived from the BH3 domain of Bax, which all allegedly target the hydrophobic cleft. All these compounds prevented the effects of recombinant Bcl-Xl on IP3R activity (Yang et al. 2016). This is in line with a previous study reporting that recombinant Bax and Bid disrupt the interaction of Bcl-Xl and IP3R and overcome the sensitizing effect of Bcl-Xl on the channel activity (White et al. 2005).
Mcl-1
Similarly to Bcl-2 and Bcl-Xl, Mcl-1 also binds to and regulates IP3Rs (Fig. 2; Table 1). Mcl-1 interacts with all three isoforms of the IP3R, targeting their carboxyl terminus. Mcl-1 overexpression in DT40 cells resulted in a decreased Ca2+ level in the ER, suggesting that the sensitivity of the IP3R is enhanced by the presence of Mcl-1. Furthermore, Mcl-1 displayed a positive effect on spontaneous Ca2+ oscillations, as well as those induced by IgM, with increased peak amplitudes and total amount of released Ca2+. Finally, Mcl-1 overexpression in DT40 cells resulted in enhanced protection against staurosporine- or etoposide-induced apoptosis compared with DT40-TKO cells, suggesting that IP3R potentiates the antiapoptotic activity of Mcl-1 (Eckenrode et al. 2010). However, at this point, not much is known about the molecular determinants responsible for IP3R/Mcl-1 complex formation and more work is required to reveal them.
Bcl-2L10 and Its Orthologs
Nrz, a zebrafish ortholog of the antiapoptotic Bcl-2 homolog Bcl-2L10, has been shown to decrease IP3 binding to the IP3R, a process implicated in the regulation of epiboly in zebrafish embryos (Popgeorgiev et al. 2011; Bonneau et al. 2014). Via its BH4 domain, Nrz directly binds to the ligand-binding region of the IP3R (Fig. 2; Table 1), thereby antagonizing the interaction of IP3 to its receptor (Bonneau et al. 2014). Because Ca2+ release occurs only when the four IP3R monomers are occupied by IP3 (Alzayady et al. 2016), such interference with IP3 binding to the ligand-binding region might be exploited for achieving efficient inhibition of the IP3R channel activity, as is the case with Nrz. This prevents the phosphorylation of myosin light chain and the premature contraction of the actin–myosin ring, thereby ensuring epiboly (Popgeorgiev et al. 2011; Bonneau et al. 2014). The efficient IP3R inhibition by Nrz required the presence of the BH3 and BH1 domains in addition to the BH4 domain. Furthermore, only phosphorylated Nrz was able to bind and thereby inhibit IP3R (Bonneau et al. 2014), confirming the complex mechanism of IP3R modulation by Bcl-2 proteins.
The human ortholog of Bcl-2L10, Nrh, was also shown to bind through its BH4 domain to the LBD of IP3R and to decrease Ca2+ release (Fig. 2; Table 1; Bonneau et al. 2016). Nrh appeared to participate in a three-protein complex together with IP3R and IP3R-binding protein released with IP3 (IRBIT), resulting in inhibition of IP3R activity. The investigators speculated that one part of the BH4 helix is involved in the interaction with the LBD of IP3R, whereas the other part binds to IRBIT. Nrh and IRBIT colocalized at the membrane contact sites between ER and mitochondria. IRBIT appeared to promote the formation of ER–mitochondrial contact sites. Furthermore, IRBIT and Nrh cooperated to suppress the Ca2+ transfer between ER and mitochondria, thereby conferring apoptotic resistance. In cells undergoing apoptosis, IRBIT becomes dephosphorylated and promotes apoptosis. First, dephosphorylated IRBIT fails to bind IP3Rs and thus does not compete with IP3 anymore, which potentially increases IP3-induced Ca2+ release. Second, dephosphorylated IRBIT antagonized Nrh's ability to bind and inhibit IP3Rs, thereby counteracting its antiapoptotic effects on IP3R channels. The investigators proposed a model in which dephosphorylated IRBIT, which still binds to Nrh, but not to IP3R, displaces Nrh from IP3Rs. As a consequence, ER–mitochondrial Ca2+ transfer will increase and promote apoptotic cell death (Bonneau et al. 2016). In this context, a recent study suggested that targeting Nrh interaction with IP3R can be a promising strategy in breast cancer treatment (Nougarede et al. 2018).
OTHER INTRACELLULAR Ca2+ CHANNELS REGULATED BY Bcl-2 PROTEINS
Structure and Function of RyRs and Their Regulation by Bcl-2 Proteins
RyRs form another protein family of Ca2+ release channels, which are present at the ER or sarcoplasmic reticulum (SR) membranes of several, although not all, cell types (Lanner et al. 2010). RyRs are large tetrameric Ca2+-release channels (monomers >500 kDa). Similar to the IP3Rs, three isoforms of RyRs are known to exist in vertebrates. These isoforms have a distinct expression pattern with RyR1 predominantly expressed in skeletal muscle cells (Lai and Meissner 1989), RyR2 in the heart (Imagawa et al. 1989), whereas all three isoforms are expressed in different parts of the brain (Martin et al. 1998). RyRs are also expressed at lower levels in pancreatic acinar cells and β cells, T cells, smooth muscle cells, and liver cells (Lanner et al. 2010). A major activator of RyRs is Ca2+ itself (Meissner et al. 1997). As such, RyRs are often seen as amplifiers of smaller Ca2+ signals, which may originate at different sites of the cell. In the heart for instance, Ca2+ influx over the plasma membrane through voltage-gated L-type Ca2+ channels triggers RyR2 activation via Ca2+-induced Ca2+ release, which then provides the Ca2+ increase necessary for contraction. Given the restricted expression pattern of RyRs, these channels contribute to specialized cellular functions, including muscle contraction, memory formation, and secretion of insulin and digestive enzymes (Unni et al. 2004; Lanner et al. 2010; Lanner 2012; Rebbeck et al. 2014; Llanos et al. 2015).
Excessive RyR-mediated Ca2+ release is known to contribute to the progression of several diseases (Lanner 2012). Acute pancreatitis, for instance, is characterized by excessive intracellular Ca2+ release, which triggers the premature activation of digestive enzymes, resulting in necrosis of the pancreatic acinar cells (Gerasimenko et al. 2014). Inhibition of IP3R- (Huang et al. 2017) or RyR-mediated Ca2+ release (Husain et al. 2012) has been shown to inhibit necrosis, and as such may be beneficial for treating acute pancreatitis. The excessive Ca2+ release responsible for the onset of acute pancreatitis is usually triggered by the presence of toxic agents, such as alcohol metabolites, bile acids, and certain chemotherapy (Petersen and Sutton 2006; Peng et al. 2016), resulting in hyperactivation of the RyRs. However, in other diseases, like malignant hyperthermia and catecholaminergic polymorphic ventricular tachycardia, mutations in RyR1 and RyR2 are very well known to be the underlying cause for the diseases (Yano et al. 2006). Three mutational hotspots exist on RyR1 and RyR2 in which more than 100 point mutations have been identified. These mutations lead to aberrant RyR-mediated Ca2+ release, which is causal in these diseases. As such, compounds that stabilize/normalize RyR-mediated Ca2+ release under disease conditions may hold significant therapeutic value.
Bcl-2
Recently, it was shown that RyR activity can be modulated by antiapoptotic Bcl-2 proteins. Sequence alignment revealed high similarity between the 20 aa of the Bcl-2-binding site identified in the regulatory region of the IP3R, and a stretch of 22 aa in the central region of the RyR (Vervliet et al. 2014). Interestingly, this 22-residue (aa 2448–2469 for rabbit RyR1) region is located within one of the previously described mutational hotspots contributing to malignant hyperthermia and catecholaminergic polymorphic ventricular tachycardia (Yano et al. 2006). The region also contains a proposed FKBP12.6-binding site, a major regulator of RyR activity (Marx et al. 2000; Vervliet et al. 2015c), further highlighting the importance of this region for channel regulation.
In line with the high degree of similarity between the described amino acid stretches in the central regions of IP3R and RyR, it was shown that full-length Bcl-2 binds to RyR1 and RyR3 in human embryonic kidney (HEK) cells overexpressing these particular RyR isoforms. Furthermore, the interaction occurs between the BH4 domain of Bcl-2 and the central region of RyRs, as shown using GST-fused fragments corresponding to the central region of each RyR isoform. Consistently, Bcl-2 appeared to be a potent inhibitor of RyR-mediated Ca2+ release (Fig. 3) without altering the ER store content (Vervliet et al. 2014). In addition, a completely endogenous interaction between RyRs and Bcl-2 was observed in lysates from rat hippocampus. Introducing the BH4 domain of Bcl-2 into HEK cells overexpressing RyRs or in dissociated hippocampal neurons inhibited RyR activity. More details of the Bcl-2–RyR interaction at the molecular level were revealed and it appeared that the hydrophobic cleft of Bcl-2 does not contribute to the interaction with RyR (Vervliet et al. 2015b), which is another similarity to its binding to IP3R. Despite the analogy in the mechanisms of interaction of Bcl-2 with the two channels, a critical difference at the level of the BH4 domain was revealed. Strikingly, the above-described mutant Bcl-2 K17D, which was impaired in binding and inhibiting IP3R (Monaco et al. 2012b), retained its ability to interact with RyR and to inhibit its function (Vervliet et al. 2014).
Bcl-Xl
The other major regulator of IP3Rs from the Bcl-2 family of proteins, Bcl-Xl also modulates the activity of RyRs (Fig. 3). In contrast to the interaction with IP3Rs, in which the BH4 domain of Bcl-Xl appeared dispensable for the complex formation, here the BH4 domain binds to full-length RyRs. Moreover, it targets the same region in RyR where Bcl-2 binds (Vervliet et al. 2015a). In addition to the BH4 domain, K87, a residue located in the beginning of the BH3 domain of Bcl-Xl, was required for efficient binding and regulation of RyR (Vervliet et al. 2015a). While Bcl-Xl appeared to modulate IP3R activity, depending on its concentration (Yang et al. 2016), only an inhibitory effect was observed when studying the impact of full-length Bcl-Xl or of its BH4 domain on RyR activity (Vervliet et al. 2015b). Nevertheless, the relation between the level of Bcl-Xl and its effect on RyR was not addressed.
The above-discussed studies suggest that the RyR-inhibitory abilities of the BH4 domains of Bcl-2 and Bcl-Xl could be good starting points for developing peptidomimetic drugs for treating diseases with excessive RyR-mediated Ca2+ release. This idea was recently illustrated in the framework of acute pancreatitis. Using primary isolated pancreatic acinar cells from mice, it was shown that in the acinar cells the BH4 domains of Bcl-2 and Bcl-Xl inhibit physiological RyR-mediated Ca2+ release (Vervliet et al. 2018). In addition, both BH4 domains were similarly capable of inhibiting pathological bile acid–induced Ca2+ release and subsequent necrosis, two hallmarks of acute pancreatitis. These results show that the BH4 domains, and/or drugs derived from them, may be therapeutically relevant in diseases caused by excessive RyR activity.
Structure and Function of VDACs and Their Regulation by Bcl-2 Proteins
VDACs are members of the protein family of porins, which are mainly located on the OMM. Three isoforms of VDACs, with ∼70% of sequence similarity and a molecular weight of ∼30 kDa (Messina et al. 2012), have been identified. VDACs are best known for acting as large conductance channels that permeate metabolites, nucleotides, and ions, such as Ca2+, across the OMM (Magrì et al. 2018). Each isoform is widely expressed in most mammalian tissues (Messina et al. 2012). Exceptionally, VDAC2 and VDAC3 are highly expressed in testis, whereas mouse VDAC1 is poorly expressed in this tissue. Nevertheless, VDAC1 is the most abundant isoform, and is ubiquitously expressed across all other tissue types. This isoform contributes the most to mitochondrial Ca2+ fluxes in concert with the mitochondrial Ca2+ uniporter that in turn allows for Ca2+ transport across the inner mitochondrial membrane (De Stefani et al. 2012; Nemani et al. 2018). This Ca2+ transfer to the mitochondria is further facilitated by an IP3R–VDAC1 physical link provided by the chaperone glucose-regulated protein 75 (Szabadkai et al. 2006). Additionally, in cardiomyocytes, proper Ca2+ transfer from SR to the mitochondria is ensured by the interaction between RyR2 and VDAC2 (Min et al. 2012; Naghdi and Hajnóczky 2016).
Close regulation of mitochondrial Ca2+ uptake via VDACs is critical for cell life or death by maintaining mitochondrial energy production or driving mitochondrial Ca2+ overload, respectively (Shoshan-Barmatz et al. 2018). Studies using various cell models (e.g., HeLa, MEF, skeletal myotubes, endothelial cells) showed that ectopic overexpression of VDAC1 leads to increased levels of mitochondrial Ca2+ and apoptosis under both resting and stress conditions (Rapizzi et al. 2002; Yuan et al. 2008; Monaco et al. 2015; Shoshan-Barmatz et al. 2018). Conversely, VDAC1 genetic silencing attenuates mitochondrial Ca2+ uptake and cell apoptosis induced by several agents or stressors (e.g., H2O2, ceramide) (Yuan et al. 2008; De Stefani et al. 2012; Shoshan-Barmatz et al. 2018). Intriguingly, a variety of pathological conditions associated with aberrant intracellular Ca2+ signaling are characterized by increased VDAC1 expression levels in the affected tissues. Pathological overexpression of VDAC1 is, for instance, observed in cancer (Shoshan-Barmatz and Ben-Hail 2012; Shoshan-Barmatz and Golan 2012), cardiovasculopathies (Branco et al. 2011; Liao et al. 2015), Alzheimer disease (Manczak et al. 2006; Cuadrado-Tejedor et al. 2011), and type 2 diabetes (Ahmed et al. 2010; Zhang et al. 2019).
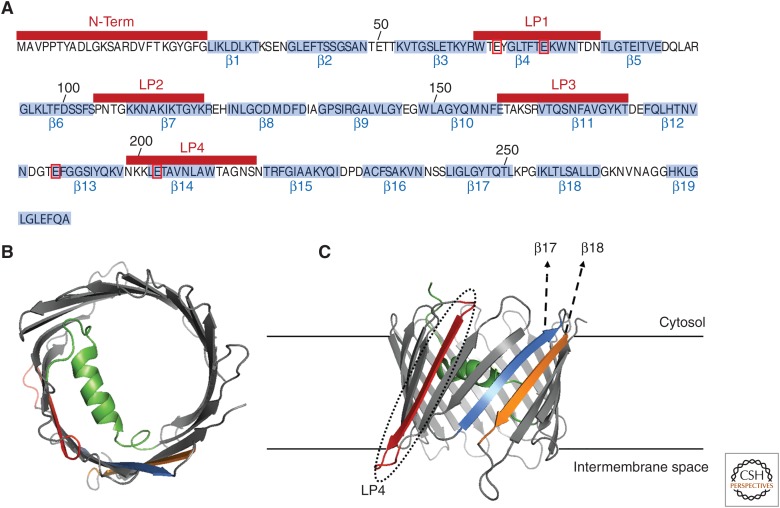
VDAC1 is composed of 19 antiparallel β-strands that form a transmembrane β-barrel pore (Fig. 4A; Hiller et al. 2008; Zeth and Zachariae 2018). The amino-terminal domain of this channel, encompassing the first 25 residues, is a short α-helical stretch that flips in and out of the pore lumen (Geula et al. 2012; Shuvo et al. 2016). A glycine-rich sequence (21GYGFG25) was proposed to provide the flexibility required for such properties. It remains under debate whether the amino terminus of VDAC1, and/or part of its pore wall, would serve as a voltage sensor (Song et al. 1998; Teijido et al. 2012). However, it is now generally accepted that the high flexibility of both the amino-terminal α-helix (Mertins et al. 2012; Shuvo et al. 2016) and the VDAC1 β-barrel (Grosse et al. 2014; Ge et al. 2016; Zeth and Zachariae 2018) underlies the ion permeation and gating mechanisms of VDAC1. A narrower, partially “closed state” of the VDAC1 pore, was described as favorable for Ca2+ and cations’ conductance while obstructing the passage of metabolites and anions (Tan and Colombini 2007; Shoshan-Barmatz et al. 2018). Further experiments, exploiting reagents known to specifically interact with several Ca2+-binding proteins (Gincel et al. 2002; Israelson et al. 2005), revealed that VDAC1 is equipped with two low-affinity Ca2+-binding sites shaped by the pore β-strands 4, 13, and 14 (residues E66, E73, E189, and E203) (Israelson et al. 2007, 2008). Pertinently, cellular events or agents (e.g., apoptosis-inducing agents) that promote an increase in cytosolic Ca2+ have been shown to increase VDAC1 permeability to Ca2+ (Bathori et al. 2006), decrease channel plasticity (Ge et al. 2016), and in many cases stimulate VDAC1 protein expression and its proapoptotic oligomerization (Weisthal et al. 2014; Shoshan-Barmatz et al. 2018). Finally, some of the VDAC1 regions previously assumed to be loop regions (namely, LP1, LP2, LP3, and LP4 and including β-strands 4, 7, 11, and 14, respectively), as well as the amino-terminal α-helix itself (Fig. 4B), might act as a docking site for several cytosolic-modulatory proteins, including anti- and proapoptotic Bcl-2 family proteins (Hiller et al. 2008; Geula et al. 2012).
Figure 4.
Voltage-dependent anion channel 1 (VDAC1) structure. (A) Linear sequence of hVDAC1 (UniProtKB, P21796). The amino acids encompassing for the 19 b-stands (b1–b19) are highlighted in light blue. The amino-terminal (N-term) and the loop peptide (LP) regions (LP1–4) are shown above in red bars. The four key residues allegedly encompassing the Ca2+-binding properties of VDAC1 (E66, E73, E189, and E203) are boxed in red. (B,C) Color-coded views of the structure model of hVDAC1 (PDB:2K4T) with the key regions involved in its interaction with Bcl-Xl highlighted in color: N-term (green), LP4 (red), β17 (light blue), and β18 (orange). A top view is presented in B and a side view in C. The latter structure is membrane-embedded to facilitate the visualization of the possible interaction interfaces.
Bcl-2, Bcl-Xl, and Mcl-1
Beyond their role as modulators of ER-resident Ca2+ channels, Bcl-2, Bcl-Xl, and Mcl-1 have the ability to interact with VDAC1. In experimental settings precluding the contribution of IP3Rs, some studies found that the antiapoptotic Bcl-2 members inhibit Ca2+ uptake into the mitochondria and protect cells from OMM permeabilization induced by Ca2+ overload (Tornero et al. 2011; Arbel et al. 2012; Huang et al. 2014; Monaco et al. 2015). Alternatively, other reports indicated that Bcl-Xl and Mcl-1 could promote VDAC1-mediated Ca2+ transfer into the mitochondria (Fig. 3; Huang et al. 2013, 2014). Indeed, Bcl-Xl or Mcl-1 overexpression enhanced mitochondrial Ca2+ uptake in line with observations in knockout cells for either of the proteins. Accordingly, VDAC1-deficient cells failed to show any Bcl-Xl or Mcl-1-mediated regulation of mitochondrial Ca2+ uptake (Huang et al. 2013, 2014). A more recent study (Morciano et al. 2016) revealed that the exogenous overexpression of the short proapoptotic Mcl-1 isoform was also accompanied by increased mitochondrial Ca2+ uptake, thereby increasing susceptibility to apoptotic stimuli. Although apparently conflicting and certainly puzzling, these evidences on VDAC1 modulation presumably underscore the activities of the channel as both driver of basal mitochondrial bioenergetics and gateway to cell death (Michels et al. 2013; Shoshan-Barmatz et al. 2017).
The molecular basis for Bcl-2, Bcl-X, and Mcl-1's modulatory effect on VDAC1 remains poorly characterized, especially with regard to its Ca2+-transporting activity. Bcl-2, Bcl-Xl, and Mcl-1 target the amino terminus of VDAC1, as shown by using VDAC1-amino-terminal derived (N-Ter) peptides, which also interfered with the antiapoptotic function of all three proteins when intracellularly delivered in multiple cell models (Abu-Hamad et al. 2009; Arbel and Shoshan-Barmatz 2010; Arbel et al. 2012; Huang et al. 2013, 2014; Morciano et al. 2016). The BH4 domain of Bcl-Xl, but not the one of Bcl-2, appears as the sole molecular determinant sufficient for binding and suppressing ATP-induced and VDAC1-mediated Ca2+ uptake. Consistently, N-Ter peptides would only counteract the channel inhibitory action of BH4-Bcl-Xl but not of BH4-Bcl-2 (Monaco et al. 2015). Among the VDAC1–cytosol accessible regions, the LP4 stands out as a conserved and validated binding site for Bcl-2, Bcl-Xl, and Mcl-1 (Abu-Hamad et al. 2009; Arbel and Shoshan-Barmatz 2010; Arbel et al. 2012; Huang et al. 2013, 2014). Importantly, stable and cell-permeable versions of VDAC1-amino-terminal and -LP4-derived peptides are under scrutiny as future cancer therapeutics because of their ability to induce cell death by, at least in part, modulating the Ca2+-transport activity of VDAC1 (Pittala et al. 2018; Shteinfer-Kuzmine et al. 2018). Finally, nuclear magnetic resonance (NMR) mapping experiments and detailed biochemical analyses revealed that, in a membrane-mimicking environment, the primary interacting interface is shaped by the carboxy-terminal portion of Bcl-Xl (encompassing BH1, BH2, and TMD) and by the β-strands 17 and 18 of VDAC1 (Losonczi et al. 2000; Malia and Wagner 2007; Hiller et al. 2008). The latter β-strands are relatively proximal to β-strand 14 (Fig. 4C), which contains the above-mentioned LP4 region. Therefore, in this “membrane-embedded” interaction model, the BH4 region would only partially contribute to the binding by reaching over the top of the VDAC pore (Malia and Wagner 2007; Hiller et al. 2008), in agreement with the data suggesting a putative pore contact between the mobile VDAC1-amino-terminal and BH4-Bcl-Xl (Monaco et al. 2015). Analogous analyses are still missing to conclusively determine whether or not the VDAC1–Bcl-2 and VDAC1–Mcl-1 interactions retain similar binding interfaces.
Proapoptotic Bcl-2 Proteins
Finally, the proapoptotic Bcl-2 relatives (i.e., Bak, Bax, tBid, and BNIP3) have been shown to interact with VDAC1 (Shoshan-Barmatz et al. 2018). However, there are (1) no direct and validated evidences for their regulation of VDAC1-mediated Ca2+ transport, and (2) insufficient information about the molecular determinants responsible for their complex formation with VDAC1.
CONCLUSIONS
Various Bcl-2 family members, including anti- and proapoptotic proteins, were identified as direct binding partners of intracellular Ca2+ channels. Despite the similar structure and function of antiapoptotic Bcl-2-family members as Bax/Bak inhibitors, they display divergent binding profiles for IP3Rs, RyRs, and VDACs, translating to distinct impacts on Ca2+ homeostasis and dynamics and subsequently cell death and survival. Gaining more insights in the molecular determinants underlying these interactions will be instrumental for the successful development of selective strategies and tools for modulating the function of these channels, including in pathological conditions associated with altered IP3R, RyR, or VDAC activity.
ACKNOWLEDGMENTS
Work performed in the authors’ laboratory was supported by grants from the Research Foundation-Flanders (FWO Grants G.0819.13 to G.B., G.0C91.14 to G.B. and J.B.P., G.0A34.16 to G.B., G.0901.18 to G.B. and D.I.Y.), by the Research Council of the KU Leuven (OT Grant 14/101, CELSA/18/040, and C14/19/099) and by the National Institutes of Health (NIH) (5R21NS106968-02 to I.I.S.). G.B., J.B.P., I.I.S., and D.I.Y. are partners of the FWO Scientific Research Network (CaSign W0.019.17N). H.I., T.V., and G.M. are recipients of postdoctoral fellowships of the FWO. H.I. was supported by a mobility grant from the FWO for a stay in the team of Dr. Yule (Rochester University, Rochester, NY).
Footnotes
Editors: Geert Bultynck, Martin D. Bootman, Michael J. Berridge, and Grace E. Stutzmann
Additional Perspectives on Calcium Signaling available at www.cshperspectives.org
REFERENCES
*Reference is also in this collection.
- Abu-Hamad S, Arbel N, Calo D, Arzoine L, Israelson A, Keinan N, Ben-Romano R, Friedman O, Shoshan-Barmatz V. 2009. The VDAC1 N-terminus is essential both for apoptosis and the protective effect of anti-apoptotic proteins. J Cell Sci 122: 1906–1916. 10.1242/jcs.040188 [DOI] [PubMed] [Google Scholar]
- Adams JM, Cory S. 1998. The Bcl-2 protein family: Arbiters of cell survival. Science 281: 1322–1326. 10.1126/science.281.5381.1322 [DOI] [PubMed] [Google Scholar]
- Ahmed M, Muhammed SJ, Kessler B, Salehi A. 2010. Mitochondrial proteome analysis reveals altered expression of voltage dependent anion channels in pancreatic β-cells exposed to high glucose. Islets 2: 283–292. 10.4161/isl.2.5.12639 [DOI] [PubMed] [Google Scholar]
- Akao Y, Otsuki Y, Kataoka S, Ito Y, Tsujimoto Y. 1994. Multiple subcellular localization of bcl-2: Detection in nuclear outer membrane, endoplasmic reticulum membrane, and mitochondrial membranes. Cancer Res 54: 2468–2471. [PubMed] [Google Scholar]
- Akl H, Bultynck G. 2013. Altered Ca2+ signaling in cancer cells: Proto-oncogenes and tumor suppressors targeting IP3 receptors. Biochim Biophys Acta 1835: 180–193. 10.1016/j.bbcan.2012.12.001 [DOI] [PubMed] [Google Scholar]
- Akl H, Monaco G, La Rovere R, Welkenhuyzen K, Kiviluoto S, Vervliet T, Molgo J, Distelhorst CW, Missiaen L, Mikoshiba K, et al. 2013. IP3R2 levels dictate the apoptotic sensitivity of diffuse large B-cell lymphoma cells to an IP3R-derived peptide targeting the BH4 domain of Bcl-2. Cell Death Dis 4: e632 10.1038/cddis.2013.140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akl H, Vervloessem T, Kiviluoto S, Bittremieux M, Parys JB, De Smedt H, Bultynck G. 2014. A dual role for the anti-apoptotic Bcl-2 protein in cancer: Mitochondria versus endoplasmic reticulum. Biochim Biophys Acta 1843: 2240–2252. 10.1016/j.bbamcr.2014.04.017 [DOI] [PubMed] [Google Scholar]
- Akl H, La Rovere RM, Janssens A, Vandenberghe P, Parys JB, Bultynck G. 2015. HA14-1 potentiates apoptosis in B-cell cancer cells sensitive to a peptide disrupting IP3 receptor/Bcl-2 complexes. Int J Dev Biol 59: 391–398. 10.1387/ijdb.150213gb [DOI] [PubMed] [Google Scholar]
- Alzayady KJ, Chandrasekhar R, Yule DI. 2013. Fragmented inositol 1,4,5-trisphosphate receptors retain tetrameric architecture and form functional Ca2+ release channels. J Biol Chem 288: 11122–11134. 10.1074/jbc.M113.453241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzayady KJ, Wang L, Chandrasekhar R, Wagner LE II, Van Petegem F, Yule DI. 2016. Defining the stoichiometry of inositol 1,4,5-trisphosphate binding required to initiate Ca2+ release. Sci Signal 9: ra35 10.1126/scisignal.aad6281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbel N, Shoshan-Barmatz V. 2010. Voltage-dependent anion channel 1-based peptides interact with Bcl-2 to prevent antiapoptotic activity. J Biol Chem 285: 6053–6062. 10.1074/jbc.M109.082990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbel N, Ben-Hail D, Shoshan-Barmatz V. 2012. Mediation of the antiapoptotic activity of Bcl-xL protein upon interaction with VDAC1 protein. J Biol Chem 287: 23152–23161. 10.1074/jbc.M112.345918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atakpa P, Thillaiappan NB, Mataragka S, Prole DL, Taylor CW. 2018. IP3 Receptors preferentially associate with ER–lysosome contact sites and selectively deliver Ca2+ to lysosomes. Cell Rep 25: 3180–3193.e7. 10.1016/j.celrep.2018.11.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay LA, Wales TE, Garner TP, Wachter F, Lee S, Guerra RM, Stewart ML, Braun CR, Bird GH, Gavathiotis E, et al. 2015. Inhibition of pro-apoptotic BAX by a noncanonical interaction mechanism. Mol Cell 57: 873–886. 10.1016/j.molcel.2015.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bathori G, Csordas G, Garcia-Perez C, Davies E, Hajnoczky G. 2006. Ca2+-dependent control of the permeability properties of the mitochondrial outer membrane and voltage-dependent anion-selective channel (VDAC). J Biol Chem 281: 17347–17358. 10.1074/jbc.M600906200 [DOI] [PubMed] [Google Scholar]
- Beroukhim R, Mermel CH, Porter D, Wei G, Raychaudhuri S, Donovan J, Barretina J, Boehm JS, Dobson J, Urashima M, et al. 2010. The landscape of somatic copy-number alteration across human cancers. Nature 463: 899–905. 10.1038/nature08822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezprozvanny I. 2005. The inositol 1,4,5-trisphosphate receptors. Cell Calcium 38: 261–272. 10.1016/j.ceca.2005.06.030 [DOI] [PubMed] [Google Scholar]
- Bhanumathy C, da Fonseca PC, Morris EP, Joseph SK. 2012. Identification of functionally critical residues in the channel domain of inositol trisphosphate receptors. J Biol Chem 287: 43674–43684. 10.1074/jbc.M112.415786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittremieux M, La Rovere RM, Schuermans M, Luyten T, Mikoshiba K, Vangheluwe P, Parys JB, Bultynck G. 2018. Extracellular and ER-stored Ca2+ contribute to BIRD-2-induced cell death in diffuse large B-cell lymphoma cells. Cell Death Discov 4: 101 10.1038/s41420-018-0118-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittremieux M, La Rovere RM, Akl H, Martines C, Welkenhuyzen K, Dubron K, Baes M, Janssens A, Vandenberghe P, Laurenti L, et al. 2019. Constitutive IP3 signaling underlies the sensitivity of B-cell cancers to the Bcl-2/IP3 receptor disruptor BIRD-2. Cell Death Differ 26: 531–547. 10.1038/s41418-018-0142-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonneau B, Nougarede A, Prudent J, Popgeorgiev N, Peyrieras N, Rimokh R, Gillet G. 2014. The Bcl-2 homolog Nrz inhibits binding of IP3 to its receptor to control calcium signaling during zebrafish epiboly. Sci Signal 7: ra14 10.1126/scisignal.2004480 [DOI] [PubMed] [Google Scholar]
- Bonneau B, Ando H, Kawaai K, Hirose M, Takahashi-Iwanaga H, Mikoshiba K. 2016. IRBIT controls apoptosis by interacting with the Bcl-2 homolog, Bcl2l10, and by promoting ER–mitochondria contact. eLife 5: e19896 10.7554/eLife.19896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth DM, Enyedi B, Geiszt M, Varnai P, Hajnoczky G. 2016. Redox nanodomains are induced by and control calcium signaling at the ER–mitochondrial interface. Mol Cell 63: 240–248. 10.1016/j.molcel.2016.05.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branco AF, Pereira SL, Moreira AC, Holy J, Sardão VA, Oliveira PJ. 2011. Isoproterenol cytotoxicity is dependent on the differentiation state of the cardiomyoblast H9c2 cell line. Cardiovasc Toxicol 11: 191–203. 10.1007/s12012-011-9111-5 [DOI] [PubMed] [Google Scholar]
- Bultynck G, Kiviluoto S, Henke N, Ivanova H, Schneider L, Rybalchenko V, Luyten T, Nuyts K, De Borggraeve W, Bezprozvanny I, et al. 2012. The C terminus of Bax inhibitor-1 forms a Ca2+-permeable channel pore. J Biol Chem 287: 2544–2557. 10.1074/jbc.M111.275354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bultynck G, Kiviluoto S, Methner A. 2014. Bax inhibitor-1 is likely a pH-sensitive calcium leak channel, not a H+/Ca2+ exchanger. Sci Signal 7: pe22 10.1126/scisignal.2005764 [DOI] [PubMed] [Google Scholar]
- Carrara G, Parsons M, Saraiva N, Smith GL. 2017. Golgi anti-apoptotic protein: A tale of camels, calcium, channels and cancer. Open Biol 7: 170045 10.1098/rsob.170045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang NC, Nguyen M, Germain M, Shore GC. 2010. Antagonism of Beclin 1-dependent autophagy by BCL-2 at the endoplasmic reticulum requires NAF-1. EMBO J 29: 606–618. 10.1038/emboj.2009.369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang MJ, Zhong F, Lavik AR, Parys JB, Berridge MJ, Distelhorst CW. 2014a. Feedback regulation mediated by Bcl-2 and DARPP-32 regulates inositol 1,4,5-trisphosphate receptor phosphorylation and promotes cell survival. Proc Natl Acad Sci 111: 1186–1191. 10.1073/pnas.1323098111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y, Bruni R, Kloss B, Assur Z, Kloppmann E, Rost B, Hendrickson WA, Liu Q. 2014b. Structural basis for a pH-sensitive calcium leak across membranes. Science 344: 1131–1135. 10.1126/science.1252043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Valencia I, Zhong F, McColl KS, Roderick HL, Bootman MD, Berridge MJ, Conway SJ, Holmes AB, Mignery GA, et al. 2004. Bcl-2 functionally interacts with inositol 1,4,5-trisphosphate receptors to regulate calcium release from the ER in response to inositol 1,4,5-trisphosphate. J Cell Biol 166: 193–203. 10.1083/jcb.200309146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YF, Kao CH, Chen YT, Wang CH, Wu CY, Tsai CY, Liu FC, Yang CW, Wei YH, Hsu MT, et al. 2009. Cisd2 deficiency drives premature aging and causes mitochondria-mediated defects in mice. Genes Dev 23: 1183–1194. 10.1101/gad.1779509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipuk JE, Fisher JC, Dillon CP, Kriwacki RW, Kuwana T, Green DR. 2008. Mechanism of apoptosis induction by inhibition of the anti-apoptotic BCL-2 proteins. Proc Natl Acad Sci 105: 20327–20332. 10.1073/pnas.0808036105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipuk JE, Moldoveanu T, Llambi F, Parsons MJ, Green DR. 2010. The BCL-2 family reunion. Mol Cell 37: 299–310. 10.1016/j.molcel.2010.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe CU, Ehrlich BE. 2006. The inositol 1,4,5-trisphosphate receptor (IP3R) and its regulators: Sometimes good and sometimes bad teamwork. Sci STKE 2006: re15 10.1126/stke.3632006re15 [DOI] [PubMed] [Google Scholar]
- Cuadrado-Tejedor M, Vilarino M, Cabodevilla F, Del Rio J, Frechilla D, Perez-Mediavilla A. 2011. Enhanced expression of the voltage-dependent anion channel 1 (VDAC1) in Alzheimer's disease transgenic mice: An insight into the pathogenic effects of amyloid-β. J Alzheimers Dis 23: 195–206. 10.3233/JAD-2010-100966 [DOI] [PubMed] [Google Scholar]
- Decuypere JP, Monaco G, Bultynck G, Missiaen L, De Smedt H, Parys JB. 2011. The IP3 receptor–mitochondria connection in apoptosis and autophagy. Biochim Biophys Acta 1813: 1003–1013. 10.1016/j.bbamcr.2010.11.023 [DOI] [PubMed] [Google Scholar]
- De Stefani D, Bononi A, Romagnoli A, Messina A, De Pinto V, Pinton P, Rizzuto R. 2012. VDAC1 selectively transfers apoptotic Ca2+ signals to mitochondria. Cell Death Differ 19: 267–273. 10.1038/cdd.2011.92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J, Zhang Z, Roberts GJ, Falcone M, Miao Y, Shao Y, Zhang XC, Andrews DW, Lin J. 2010. Bcl-2 and Bax interact via the BH1-3 groove-BH3 motif interface and a novel interface involving the BH4 motif. J Biol Chem 285: 28749–28763. 10.1074/jbc.M110.148361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J, Mooers BH, Zhang Z, Kale J, Falcone D, McNichol J, Huang B, Zhang XC, Xing C, Andrews DW, et al. 2014. After embedding in membranes antiapoptotic Bcl-XL protein binds both Bcl-2 homology region 3 and helix 1 of proapoptotic Bax protein to inhibit apoptotic mitochondrial permeabilization. J Biol Chem 289: 11873–11896. 10.1074/jbc.M114.552562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distelhorst CW. 2018. Targeting Bcl-2-IP3 receptor interaction to treat cancer: A novel approach inspired by nearly a century treating cancer with adrenal corticosteroid hormones. Biochim Biophys Acta Mol Cell Res 1865: 1795–1804. 10.1016/j.bbamcr.2018.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Distelhorst CW, Bootman MD. 2019. Creating a new cancer therapeutic agent by targeting the interaction between Bcl-2 and IP3 receptors. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a035196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Orsi B, Engel T, Pfeiffer S, Nandi S, Kaufmann T, Henshall DC, Prehn JH. 2016. Bok is not pro-apoptotic but suppresses poly ADP-ribose polymerase-dependent cell death pathways and protects against excitotoxic and seizure-induced neuronal injury. J Neurosci 36: 4564–4578. 10.1523/jneurosci.3780-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echeverry N, Bachmann D, Ke F, Strasser A, Simon HU, Kaufmann T. 2013. Intracellular localization of the BCL-2 family member BOK and functional implications. Cell Death Differ 20: 785–799. 10.1038/cdd.2013.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckenrode EF, Yang J, Velmurugan GV, Foskett JK, White C. 2010. Apoptosis protection by Mcl-1 and Bcl-2 modulation of inositol 1,4,5-trisphosphate receptor-dependent Ca2+ signaling. J Biol Chem 285: 13678–13684. 10.1074/jbc.M109.096040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan G, Baker ML, Wang Z, Baker MR, Sinyagovskiy PA, Chiu W, Ludtke SJ, Serysheva II. 2015. Gating machinery of InsP3R channels revealed by electron cryomicroscopy. Nature 527: 336–341. 10.1038/nature15249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan G, Baker MR, Wang Z, Seryshev AB, Ludtke SJ, Baker ML, Serysheva II. 2018. Cryo-EM reveals ligand induced allostery underlying InsP3R channel gating. Cell Res 28: 1158–1170. 10.1038/s41422-018-0108-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari D, Pinton P, Szabadkai G, Chami M, Campanella M, Pozzan T, Rizzuto R. 2002. Endoplasmic reticulum, Bcl-2 and Ca2+ handling in apoptosis. Cell Calcium 32: 413–420. 10.1016/S0143416002002014 [DOI] [PubMed] [Google Scholar]
- Feske S. 2007. Calcium signalling in lymphocyte activation and disease. Nat Rev Immunol 7: 690–702. 10.1038/nri2152 [DOI] [PubMed] [Google Scholar]
- Foskett JK, White C, Cheung KH, Mak DO. 2007. Inositol trisphosphate receptor Ca2+ release channels. Physiol Rev 87: 593–658. 10.1152/physrev.00035.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fracchia KM, Pai CY, Walsh CM. 2013. Modulation of T cell metabolism and function through calcium signaling. Front Immunol 4: 324 10.3389/fimmu.2013.00324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Sáez AJ. 2012. The secrets of the Bcl-2 family. Cell Death Differ 19: 1733–1740. 10.1038/cdd.2012.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge L, Villinger S, Mari SA, Giller K, Griesinger C, Becker S, Müller DJ, Zweckstetter M. 2016. Molecular plasticity of the human voltage-dependent anion channel embedded into a membrane. Structure 24: 585–594. 10.1016/j.str.2016.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerasimenko JV, Gerasimenko OV, Petersen OH. 2014. The role of Ca2+ in the pathophysiology of pancreatitis. J Physiol 592: 269–280. 10.1113/jphysiol.2013.261784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geula S, Ben-Hail D, Shoshan-Barmatz V. 2012. Structure-based analysis of VDAC1: N-terminus location, translocation, channel gating and association with anti-apoptotic proteins. Biochem J 444: 475–485. 10.1042/BJ20112079 [DOI] [PubMed] [Google Scholar]
- Gincel D, Vardi N, Shoshan-Barmatz V. 2002. Retinal voltage-dependent anion channel: Characterization and cellular localization. Invest Ophthalmol Vis Sci 43: 2097–2104. [PubMed] [Google Scholar]
- Greenberg EF, McColl KS, Zhong F, Wildey G, Dowlati A, Distelhorst CW. 2015. Synergistic killing of human small cell lung cancer cells by the Bcl-2-inositol 1,4,5-trisphosphate receptor disruptor BIRD-2 and the BH3-mimetic ABT-263. Cell Death Dis 6: e2034 10.1038/cddis.2015.355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosse W, Psakis G, Mertins B, Reiss P, Windisch D, Brademann F, Bürck J, Ulrich A, Koert U, Essen LO. 2014. Structure-based engineering of a minimal porin reveals loop-independent channel closure. Biochemistry 53: 4826–4838. 10.1021/bi500660q [DOI] [PubMed] [Google Scholar]
- Guo G, Xu M, Chang Y, Luyten T, Seitaj B, Liu W, Zhu P, Bultynck G, Shi L, Quick M, et al. 2019. Ion and pH sensitivity of a TMBIM Ca2+ channel. Structure 27: 1013–1021. 10.1016/j.str.2019.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada K, Miyatake H, Terauchi A, Mikoshiba K. 2017. IP3-mediated gating mechanism of the IP3 receptor revealed by mutagenesis and X-ray crystallography. Proc Natl Acad Sci 114: 4661–4666. 10.1073/pnas.1701420114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson CJ, Bootman MD, Distelhorst CW, Wojcikiewicz RJ, Roderick HL. 2008. Bcl-2 suppresses Ca2+ release through inositol 1,4,5-trisphosphate receptors and inhibits Ca2+ uptake by mitochondria without affecting ER calcium store content. Cell Calcium 44: 324–338. 10.1016/j.ceca.2008.01.003 [DOI] [PubMed] [Google Scholar]
- Hiller S, Garces RG, Malia TJ, Orekhov VY, Colombini M, Wagner G. 2008. Solution structure of the integral human membrane protein VDAC-1 in detergent micelles. Science 321: 1206–1210. 10.1126/science.1161302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Hu X, Eno CO, Zhao G, Li C, White C. 2013. An interaction between Bcl-xL and the voltage-dependent anion channel (VDAC) promotes mitochondrial Ca2+ uptake. J Biol Chem 288: 19870–19881. 10.1074/jbc.M112.448290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Shah K, Bradbury NA, Li C, White C. 2014. Mcl-1 promotes lung cancer cell migration by directly interacting with VDAC to increase mitochondrial Ca2+ uptake and reactive oxygen species generation. Cell Death Dis 5: e1482 10.1038/cddis.2014.419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Cane MC, Mukherjee R, Szatmary P, Zhang X, Elliott V, Ouyang Y, Chvanov M, Latawiec D, Wen L, et al. 2017. Caffeine protects against experimental acute pancreatitis by inhibition of inositol 1,4,5-trisphosphate receptor-mediated Ca2+ release. Gut 66: 301–313. 10.1136/gutjnl-2015-309363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain SZ, Orabi AI, Muili KA, Luo Y, Sarwar S, Mahmood SM, Wang D, Choo-Wing R, Singh VP, Parness J, et al. 2012. Ryanodine receptors contribute to bile acid-induced pathological calcium signaling and pancreatitis in mice. Am J Physiol Gastrointest Liver Physiol 302: G1423–G1433. 10.1152/ajpgi.00546.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imagawa T, Takasago T, Shigekawa M. 1989. Cardiac ryanodine receptor is absent in type I slow skeletal muscle fibers: Immunochemical and ryanodine binding studies. J Biochem 106: 342–348. 10.1093/oxfordjournals.jbchem.a122855 [DOI] [PubMed] [Google Scholar]
- Israelson A, Arzoine L, Abu-hamad S, Khodorkovsky V, Shoshan-Barmatz V. 2005. A photoactivable probe for calcium binding proteins. Chem Biol 12: 1169–1178. 10.1016/j.chembiol.2005.08.006 [DOI] [PubMed] [Google Scholar]
- Israelson A, Abu-Hamad S, Zaid H, Nahon E, Shoshan-Barmatz V. 2007. Localization of the voltage-dependent anion channel-1 Ca2+-binding sites. Cell Calcium 41: 235–244. 10.1016/j.ceca.2006.06.005 [DOI] [PubMed] [Google Scholar]
- Israelson A, Zaid H, Abu-Hamad S, Nahon E, Shoshan-Barmatz V. 2008. Mapping the ruthenium red-binding site of the voltage-dependent anion channel-1. Cell Calcium 43: 196–204. 10.1016/j.ceca.2007.05.006 [DOI] [PubMed] [Google Scholar]
- Ivanova H, Vervliet T, Missiaen L, Parys JB, De Smedt H, Bultynck G. 2014. Inositol 1,4,5-trisphosphate receptor-isoform diversity in cell death and survival. Biochim Biophys Acta 1843: 2164–2183. 10.1016/j.bbamcr.2014.03.007 [DOI] [PubMed] [Google Scholar]
- Ivanova H, Ritane A, Wagner L, Luyten T, Shapovalov G, Welkenhuyzen K, Seitaj B, Monaco G, De Smedt H, Prevarskaya N, et al. 2016. The trans-membrane domain of Bcl-2α, but not its hydrophobic cleft, is a critical determinant for efficient IP3 receptor inhibition. Oncotarget 7: 55704–55720. 10.18632/oncotarget.11005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova H, Luyten T, Decrock E, Vervliet T, Leybaert L, Parys JB, Bultynck G. 2017. The BH4 domain of Bcl-2 orthologues from different classes of vertebrates can act as an evolutionary conserved inhibitor of IP3 receptor channels. Cell Calcium 62: 41–46. 10.1016/j.ceca.2017.01.010 [DOI] [PubMed] [Google Scholar]
- Ivanova H, Wagner LE II, Tanimura A, Vandermarliere E, Luyten T, Welkenhuyzen K, Alzayady K, Wang L, Hamada K, Mikoshiba K, et al. 2019. Bcl-2 and IP3 compete for the ligand-binding domain of IP3Rs modulating Ca2+ signaling output. Cell Mol Life Sci 10.1007/s00018-019-03091-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubowska MA, Kerkhofs M, Martines C, Efremov DG, Gerasimenko JV, Gerasimenko OV, Petersen OH, Bultynck G, Vervliet T, Ferdek PE. 2018. ABT-199 (Venetoclax), a BH3-mimetic Bcl-2 inhibitor, does not cause Ca2+-signalling dysregulation or toxicity in pancreatic acinar cells. Br J Pharmacol 10.1111/bph.14505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph N, Reicher B, Barda-Saad M. 2014. The calcium feedback loop and T cell activation: How cytoskeleton networks control intracellular calcium flux. Biochim Biophys Acta 1838: 557–568. 10.1016/j.bbamem.2013.07.009 [DOI] [PubMed] [Google Scholar]
- Joseph SK, Young M, Alzayady K, Yule DI, Ali M, Booth DM, Hajnóczky G. 2018. Redox regulation of type-I inositol trisphosphate receptors in intact mammalian cells. J Biol Chem 293: 17464–17476. 10.1074/jbc.RA118.005624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkavan H, Green DR. 2018. MOMP, cell suicide as a BCL-2 family business. Cell Death Differ 25: 46–55. 10.1038/cdd.2017.179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerkhofs M, Bultynck G, Vervliet T, Monaco G. 2019a. Therapeutic implications of novel peptides targeting ER–mitochondria Ca2+-flux systems. Drug Discov Today 24: 1092–1103. 10.1016/j.drudis.2019.03.020 [DOI] [PubMed] [Google Scholar]
- Kerkhofs M, Vervloessem T, Bittremieux M, Bultynck G. 2019b. Recent advances in uncovering the mechanisms contributing to BIRD-2-induced cell death in B-cell cancer cells. Cell Death Dis 10: 42 10.1038/s41419-018-1297-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick BS, Eden ER, Schapira AH, Futter CE, Patel S. 2013. Direct mobilisation of lysosomal Ca2+ triggers complex Ca2+ signals. J Cell Sci 126: 60–66. 10.1242/jcs.118836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühlbrandt W. 2014. Cryo-EM enters a new era. eLife 3: e03678 10.7554/eLife.03678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rovere RM, Roest G, Bultynck G, Parys JB. 2016. Intracellular Ca2+ signaling and Ca2+ microdomains in the control of cell survival, apoptosis and autophagy. Cell Calcium 60: 74–87. 10.1016/j.ceca.2016.04.005 [DOI] [PubMed] [Google Scholar]
- Lai FA, Meissner G. 1989. The muscle ryanodine receptor and its intrinsic Ca2+ channel activity. J Bioenerg Biomembr 21: 227–246. 10.1007/BF00812070 [DOI] [PubMed] [Google Scholar]
- Lanner JT. 2012. Ryanodine receptor physiology and its role in disease. Adv Exp Med Biol 740: 217–234. 10.1007/978-94-007-2888-2_9 [DOI] [PubMed] [Google Scholar]
- Lanner JT, Georgiou DK, Joshi AD, Hamilton SL. 2010. Ryanodine receptors: Structure, expression, molecular details, and function in calcium release. Cold Spring Harb Perspect Biol 2: a003996 10.1101/cshperspect.a003996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavik AR, Zhong F, Chang MJ, Greenberg E, Choudhary Y, Smith MR, McColl KS, Pink J, Reu FJ, Matsuyama S, et al. 2015. A synthetic peptide targeting the BH4 domain of Bcl-2 induces apoptosis in multiple myeloma and follicular lymphoma cells alone or in combination with agents targeting the BH3-binding pocket of Bcl-2. Oncotarget 6: 27388–27402. 10.18632/oncotarget.4489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Wang X, Vais H, Thompson CB, Foskett JK, White C. 2007. Apoptosis regulation by Bcl-xL modulation of mammalian inositol 1,4,5-trisphosphate receptor channel isoform gating. Proc Natl Acad Sci 104: 12565–12570. 10.1073/pnas.0702489104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Z, Liu D, Tang L, Yin D, Yin S, Lai S, Yao J, He M. 2015. Long-term oral resveratrol intake provides nutritional preconditioning against myocardial ischemia/reperfusion injury: Involvement of VDAC1 downregulation. Mol Nutr Food Res 59: 454–464. 10.1002/mnfr.201400730 [DOI] [PubMed] [Google Scholar]
- Lin CC, Baek K, Lu Z. 2011. Apo and InsP3-bound crystal structures of the ligand-binding domain of an InsP3 receptor. Nat Struct Mol Biol 18: 1172–1174. 10.1038/nsmb.2112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q. 2017. TMBIM-mediated Ca2+ homeostasis and cell death. Biochim Biophys Acta Mol Cell Res 1864: 850–857. 10.1016/j.bbamcr.2016.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llambi F, Wang YM, Victor B, Yang M, Schneider DM, Gingras S, Parsons MJ, Zheng JH, Brown SA, Pelletier S, et al. 2016. BOK is a non-canonical BCL-2 family effector of apoptosis regulated by ER-associated degradation. Cell 165: 421–433. 10.1016/j.cell.2016.02.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llanos P, Contreras-Ferrat A, Barrientos G, Valencia M, Mears D, Hidalgo C. 2015. Glucose-dependent insulin secretion in pancreatic β-cell islets from male rats requires Ca2+ release via ROS-stimulated ryanodine receptors. PLoS ONE 10: e0129238 10.1371/journal.pone.0129238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losonczi JA, Olejniczak ET, Betz SF, Harlan JE, Mack J, Fesik SW. 2000. NMR studies of the anti-apoptotic protein Bcl-xL in micelles. Biochemistry 39: 11024–11033. 10.1021/bi000919v [DOI] [PubMed] [Google Scholar]
- Magrì A, Reina S, De Pinto V. 2018. VDAC1 as pharmacological target in cancer and neurodegeneration: Focus on its role in apoptosis. Front Chem 6: 108 10.3389/fchem.2018.00108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malia TJ, Wagner G. 2007. NMR structural investigation of the mitochondrial outer membrane protein VDAC and its interaction with antiapoptotic Bcl-xL. Biochemistry 46: 514–525. 10.1021/bi061577h [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manczak M, Anekonda TS, Henson E, Park BS, Quinn J, Reddy PH. 2006. Mitochondria are a direct site of Aβ accumulation in Alzheimer's disease neurons: Implications for free radical generation and oxidative damage in disease progression. Hum Mol Genet 15: 1437–1449. 10.1093/hmg/ddl066 [DOI] [PubMed] [Google Scholar]
- Marchi S, Rimessi A, Giorgi C, Baldini C, Ferroni L, Rizzuto R, Pinton P. 2008. Akt kinase reducing endoplasmic reticulum Ca2+ release protects cells from Ca2+-dependent apoptotic stimuli. Biochem Biophys Res Commun 375: 501–505. 10.1016/j.bbrc.2008.07.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchi S, Patergnani S, Pinton P. 2014. The endoplasmic reticulum-mitochondria connection: One touch, multiple functions. Biochim Biophys Acta 1837: 461–469. 10.1016/j.bbabio.2013.10.015 [DOI] [PubMed] [Google Scholar]
- Martin C, Chapman KE, Seckl JR, Ashley RH. 1998. Partial cloning and differential expression of ryanodine receptor/calcium-release channel genes in human tissues including the hippocampus and cerebellum. Neuroscience 85: 205–216. 10.1016/S0306-4522(97)00612-X [DOI] [PubMed] [Google Scholar]
- Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, Marks AR. 2000. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): Defective regulation in failing hearts. Cell 101: 365–376. 10.1016/S0092-8674(00)80847-8 [DOI] [PubMed] [Google Scholar]
- McArthur K, Whitehead LW, Heddleston JM, Li L, Padman BS, Oorschot V, Geoghegan ND, Chappaz S, Davidson S, San Chin H, et al. 2018. BAK/BAX macropores facilitate mitochondrial herniation and mtDNA efflux during apoptosis. Science 359: eaao6047 10.1126/science.aao6047 [DOI] [PubMed] [Google Scholar]
- Meissner G, Rios E, Tripathy A, Pasek DA. 1997. Regulation of skeletal muscle Ca2+ release channel (ryanodine receptor) by Ca2+ and monovalent cations and anions. J Biol Chem 272: 1628–1638. 10.1074/jbc.272.3.1628 [DOI] [PubMed] [Google Scholar]
- Mertins B, Psakis G, Grosse W, Back KC, Salisowski A, Reiss P, Koert U, Essen LO. 2012. Flexibility of the N-terminal mVDAC1 segment controls the channel's gating behavior. PLoS ONE 7: e47938 10.1371/journal.pone.0047938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messina A, Reina S, Guarino F, De Pinto V. 2012. VDAC isoforms in mammals. Biochim Biophys Acta 1818: 1466–1476. 10.1016/j.bbamem.2011.10.005 [DOI] [PubMed] [Google Scholar]
- Michels J, Kepp O, Senovilla L, Lissa D, Castedo M, Kroemer G, Galluzzi L. 2013. Functions of BCL-XL at the interface between cell death and metabolism. Int J Cell Biol 2013: 705294 10.1155/2013/705294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michikawa T, Hamanaka H, Otsu H, Yamamoto A, Miyawaki A, Furuichi T, Tashiro Y, Mikoshiba K. 1994. Transmembrane topology and sites of N-glycosylation of inositol 1,4,5-trisphosphate receptor. J Biol Chem 269: 9184–9189. 10.1016/s0921-8696(05)80732-7 [DOI] [PubMed] [Google Scholar]
- Mignery GA, Newton CL, Archer BT III, Sudhof TC. 1990. Structure and expression of the rat inositol 1,4,5-trisphosphate receptor. J Biol Chem 265: 12679–12685. [PubMed] [Google Scholar]
- Mikoshiba K. 2007. The IP3 receptor/Ca2+ channel and its cellular function. Biochem Soc Symp 74: 9–22. 10.1042/BSS2007c02 [DOI] [PubMed] [Google Scholar]
- Min CK, Yeom DR, Lee KE, Kwon HK, Kang M, Kim YS, Park ZY, Jeon H, Kim DH. 2012. Coupling of ryanodine receptor 2 and voltage-dependent anion channel 2 is essential for Ca2+ transfer from the sarcoplasmic reticulum to the mitochondria in the heart. Biochem J 447: 371–379. 10.1042/BJ20120705 [DOI] [PubMed] [Google Scholar]
- Monaco G, Beckers M, Ivanova H, Missiaen L, Parys JB, De Smedt H, Bultynck G. 2012a. Profiling of the Bcl-2/Bcl-XL-binding sites on type 1 IP3 receptor. Biochem Biophys Res Commun 428: 31–35. 10.1016/j.bbrc.2012.10.002 [DOI] [PubMed] [Google Scholar]
- Monaco G, Decrock E, Akl H, Ponsaerts R, Vervliet T, Luyten T, De Maeyer M, Missiaen L, Distelhorst CW, De Smedt H, et al. 2012b. Selective regulation of IP3-receptor-mediated Ca2+ signaling and apoptosis by the BH4 domain of Bcl-2 versus Bcl-Xl. Cell Death Differ 19: 295–309. 10.1038/cdd.2011.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaco G, Decrock E, Nuyts K, Wagner LE, Luyten T, Strelkov SV, Missiaen L, De Borggraeve WM, Leybaert L, Yule DI, et al. 2013. α-Helical destabilization of the Bcl-2-BH4-domain peptide abolishes its ability to inhibit the IP3 receptor. PLoS ONE 8: e73386 10.1371/journal.pone.0073386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaco G, Decrock E, Arbel N, van Vliet AR, La Rovere RM, De Smedt H, Parys JB, Agostinis P, Leybaert L, Shoshan-Barmatz V, et al. 2015. The BH4 domain of anti-apoptotic Bcl-XL, but not that of the related Bcl-2, limits the voltage-dependent anion channel 1 (VDAC1)-mediated transfer of pro-apoptotic Ca2+ signals to mitochondria. J Biol Chem 290: 9150–9161. 10.1074/jbc.M114.622514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaco G, La Rovere R, Karamanou S, Welkenhuyzen K, Ivanova H, Vandermarliere E, Di Martile M, Del Bufalo D, De Smedt H, Parys JB, et al. 2018. A double point mutation at residues Ile14 and Val15 of Bcl-2 uncovers a role for the BH4 domain in both protein stability and function. FEBS J 285: 127–145. 10.1111/febs.14324 [DOI] [PubMed] [Google Scholar]
- Monkawa T, Miyawaki A, Sugiyama T, Yoneshima H, Yamamoto-Hino M, Furuichi T, Saruta T, Hasegawa M, Mikoshiba K. 1995. Heterotetrameric complex formation of inositol 1,4,5-trisphosphate receptor subunits. J Biol Chem 270: 14700–14704. 10.1074/jbc.270.24.14700 [DOI] [PubMed] [Google Scholar]
- Morciano G, Giorgi C, Balestra D, Marchi S, Perrone D, Pinotti M, Pinton P. 2016. Mcl-1 involvement in mitochondrial dynamics is associated with apoptotic cell death. Mol Biol Cell 27: 20–34. 10.1091/mbc.E15-01-0028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray SC, Flanagan J, Popova OB, Chiu W, Ludtke SJ, Serysheva II. 2013. Validation of cryo-EM structure of IP3R1 channel. Structure 21: 900–909. 10.1016/j.str.2013.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naghdi S, Hajnóczky G. 2016. VDAC2-specific cellular functions and the underlying structure. Biochim Biophys Acta 1863: 2503–2514. 10.1016/j.bbamcr.2016.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemani N, Shanmughapriya S, Madesh M. 2018. Molecular regulation of MCU: Implications in physiology and disease. Cell Calcium 74: 86–93. 10.1016/j.ceca.2018.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nougarede A, Popgeorgiev N, Kassem L, Omarjee S, Borel S, Mikaelian I, Lopez J, Gadet R, Marcillat O, Treilleux I, et al. 2018. Breast cancer targeting through inhibition of the endoplasmic reticulum-based apoptosis regulator Nrh/BCL2L10. Cancer Res 78: 1404–1417. 10.1158/0008-5472.CAN-17-0846 [DOI] [PubMed] [Google Scholar]
- Oakes SA, Scorrano L, Opferman JT, Bassik MC, Nishino M, Pozzan T, Korsmeyer SJ. 2005. Proapoptotic BAX and BAK regulate the type 1 inositol trisphosphate receptor and calcium leak from the endoplasmic reticulum. Proc Natl Acad Sci 102: 105–110. 10.1073/pnas.0408352102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdorf J, Webster JM, Zhu CC, Luo SG, Wojcikiewicz RJ. 1999. Down-regulation of types I, II and III inositol 1,4,5-trisphosphate receptors is mediated by the ubiquitin/proteasome pathway. Biochem J 339: 453–461. 10.1042/bj3390453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onoue H, Tanaka H, Tanaka K, Doira N, Ito Y. 2000. Heterooligomer of type 1 and type 2 inositol 1, 4, 5-trisphosphate receptor expressed in rat liver membrane fraction exists as tetrameric complex. Biochem Biophys Res Commun 267: 928–933. 10.1006/bbrc.1999.2065 [DOI] [PubMed] [Google Scholar]
- Orrenius S, Zhivotovsky B, Nicotera P. 2003. Regulation of cell death: The calcium-apoptosis link. Nat Rev Mol Cell Biol 4: 552–565. 10.1038/nrm1150 [DOI] [PubMed] [Google Scholar]
- Paknejad N, Hite RK. 2018. Structural basis for the regulation of inositol trisphosphate receptors by Ca2+ and IP3. Nat Struct Mol Biol 25: 660–668. 10.1038/s41594-018-0089-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parys JB. 2014. The IP3 receptor as a hub for Bcl-2 family proteins in cell death control and beyond. Sci Signal 7: pe4 10.1126/scisignal.2005093 [DOI] [PubMed] [Google Scholar]
- Parys JB, De Smedt H. 2012. Inositol 1,4,5-trisphosphate and its receptors. Adv Exp Med Biol 740: 255–279. 10.1007/978-94-007-2888-2_11 [DOI] [PubMed] [Google Scholar]
- Parys JB, Vervliet T. 2019. New insights in the IP3 receptor and its regulation. Adv Exp Med Biol (in press). [DOI] [PubMed] [Google Scholar]
- Patel S, Joseph SK, Thomas AP. 1999. Molecular properties of inositol 1,4,5-trisphosphate receptors. Cell Calcium 25: 247–264. 10.1054/ceca.1999.0021 [DOI] [PubMed] [Google Scholar]
- Peng S, Gerasimenko JV, Tsugorka T, Gryshchenko O, Samarasinghe S, Petersen OH, Gerasimenko OV. 2016. Calcium and adenosine triphosphate control of cellular pathology: Asparaginase-induced pancreatitis elicited via protease-activated receptor 2. Philos Trans R Soc Lond B Biol Sci 371: 20150423 10.1098/rstb.2015.0423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen OH, Sutton R. 2006. Ca2+ signalling and pancreatitis: Effects of alcohol, bile and coffee. Trends Pharmacol Sci 27: 113–120. 10.1016/j.tips.2005.12.006 [DOI] [PubMed] [Google Scholar]
- Pinton P, Ferrari D, Rapizzi E, Di Virgilio F, Pozzan T, Rizzuto R. 2001. The Ca2+ concentration of the endoplasmic reticulum is a key determinant of ceramide-induced apoptosis: Significance for the molecular mechanism of Bcl-2 action. EMBO J 20: 2690–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittala S, Krelin Y, Shoshan-Barmatz V. 2018. Targeting liver cancer and associated pathologies in mice with a mitochondrial VDAC1-based peptide. Neoplasia 20: 594–609. 10.1016/j.neo.2018.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popgeorgiev N, Bonneau B, Ferri KF, Prudent J, Thibaut J, Gillet G. 2011. The apoptotic regulator Nrz controls cytoskeletal dynamics via the regulation of Ca2+ trafficking in the zebrafish blastula. Dev Cell 20: 663–676. 10.1016/j.devcel.2011.03.016 [DOI] [PubMed] [Google Scholar]
- Popgeorgiev N, Jabbour L, Gillet G. 2018. Subcellular localization and dynamics of the Bcl-2 family of proteins. Front Cell Dev Biol 6: 13 10.3389/fcell.2018.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prole DL, Taylor CW. 2016. Inositol 1,4,5-trisphosphate receptors and their protein partners as signalling hubs. J Physiol 594: 2849–2866. 10.1113/JP271139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapizzi E, Pinton P, Szabadkai G, Wieckowski MR, Vandecasteele G, Baird G, Tuft RA, Fogarty KE, Rizzuto R. 2002. Recombinant expression of the voltage-dependent anion channel enhances the transfer of Ca2+ microdomains to mitochondria. J Cell Biol 159: 613–624. 10.1083/jcb.200205091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raturi A, Simmen T. 2013. Where the endoplasmic reticulum and the mitochondrion tie the knot: The mitochondria-associated membrane (MAM). Biochim Biophys Acta 1833: 213–224. 10.1016/j.bbamcr.2012.04.013 [DOI] [PubMed] [Google Scholar]
- Rebbeck RT, Karunasekara Y, Board PG, Beard NA, Casarotto MG, Dulhunty AF. 2014. Skeletal muscle excitation–contraction coupling: Who are the dancing partners? Int J Biochem Cell Biol 48: 28–38. 10.1016/j.biocel.2013.12.001 [DOI] [PubMed] [Google Scholar]
- Rigoli L, Di Bella C. 2012. Wolfram syndrome 1 and Wolfram syndrome 2. Curr Opin Pediatr 24: 512–517. [DOI] [PubMed] [Google Scholar]
- Riley JS, Quarato G, Cloix C, Lopez J, O'Prey J, Pearson M, Chapman J, Sesaki H, Carlin LM, Passos JF, et al. 2018. Mitochondrial inner membrane permeabilisation enables mtDNA release during apoptosis. EMBO J 37: e99238 10.15252/embj.201899238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roest G, La Rovere RM, Bultynck G, Parys JB. 2017. IP3 receptor properties and function at membrane contact sites. Adv Exp Med Biol 981: 149–178. 10.1007/978-3-319-55858-5_7 [DOI] [PubMed] [Google Scholar]
- Rojas-Rivera D, Hetz C. 2015. TMBIM protein family: Ancestral regulators of cell death. Oncogene 34: 269–280. 10.1038/onc.2014.6 [DOI] [PubMed] [Google Scholar]
- Rong YP, Aromolaran AS, Bultynck G, Zhong F, Li X, McColl K, Matsuyama S, Herlitze S, Roderick HL, Bootman MD, et al. 2008. Targeting Bcl-2–IP3 receptor interaction to reverse Bcl-2's inhibition of apoptotic calcium signals. Mol Cell 31: 255–265. 10.1016/j.molcel.2008.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong YP, Barr P, Yee VC, Distelhorst CW. 2009a. Targeting Bcl-2 based on the interaction of its BH4 domain with the inositol 1,4,5-trisphosphate receptor. Biochim Biophys Acta 1793: 971–978. 10.1016/j.bbamcr.2008.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong YP, Bultynck G, Aromolaran AS, Zhong F, Parys JB, De Smedt H, Mignery GA, Roderick HL, Bootman MD, Distelhorst CW. 2009b. The BH4 domain of Bcl-2 inhibits ER calcium release and apoptosis by binding the regulatory and coupling domain of the IP3 receptor. Proc Natl Acad Sci 106: 14397–14402. 10.1073/pnas.0907555106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulman JJ, Wright FA, Kaufmann T, Wojcikiewicz RJ. 2013. The Bcl-2 protein family member Bok binds to the coupling domain of inositol 1,4,5-trisphosphate receptors and protects them from proteolytic cleavage. J Biol Chem 288: 25340–25349. 10.1074/jbc.M113.496570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulman JJ, Wright FA, Han X, Zluhan EJ, Szczesniak LM, Wojcikiewicz RJ. 2016. The stability and expression level of Bok are governed by binding to inositol 1,4,5-trisphosphate receptors. J Biol Chem 291: 11820–11828. 10.1074/jbc.M115.711242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulman JJ, Szczesniak LM, Bunker EN, Nelson HA, Roe MW, Wagner LE II, Yule DI, Wojcikiewicz RJH. 2019. Bok regulates mitochondrial fusion and morphology. Cell Death Differ doi: 10.1038/s41418-019-0327-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo MD, Velamakanni S, Ishiyama N, Stathopulos PB, Rossi AM, Khan SA, Dale P, Li C, Ames JB, Ikura M, et al. 2012. Structural and functional conservation of key domains in InsP3 and ryanodine receptors. Nature 483: 108–112. 10.1038/nature10751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamas-Din A, Kale J, Leber B, Andrews DW. 2013. Mechanisms of action of Bcl-2 family proteins. Cold Spring Harb Perspect Biol 5: a008714 10.1101/cshperspect.a008714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapovalov G, Ritaine A, Bidaux G, Slomianny C, Borowiec AS, Gordienko D, Bultynck G, Skryma R, Prevarskaya N. 2017. Organelle membrane derived patches: Reshaping classical methods for new targets. Sci Rep 7: 14082 10.1038/s41598-017-13968-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoshan-Barmatz V, Ben-Hail D. 2012. VDAC, a multi-functional mitochondrial protein as a pharmacological target. Mitochondrion 12: 24–34. 10.1016/j.mito.2011.04.001 [DOI] [PubMed] [Google Scholar]
- Shoshan-Barmatz V, Golan M. 2012. Mitochondrial VDAC1: Function in cell life and death and a target for cancer therapy. Curr Med Chem 19: 714–735. 10.2174/092986712798992110 [DOI] [PubMed] [Google Scholar]
- Shoshan-Barmatz V, Maldonado EN, Krelin Y. 2017. VDAC1 at the crossroads of cell metabolism, apoptosis and cell stress. Cell Stress 1: 11–36. 10.15698/cst2017.10.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoshan-Barmatz V, Krelin Y, Shteinfer-Kuzmine A. 2018. VDAC1 functions in Ca2+ homeostasis and cell life and death in health and disease. Cell Calcium 69: 81–100. 10.1016/j.ceca.2017.06.007 [DOI] [PubMed] [Google Scholar]
- Shteinfer-Kuzmine A, Amsalem Z, Arif T, Zooravlov A, Shoshan-Barmatz V. 2018. Selective induction of cancer cell death by VDAC1-based peptides and their potential use in cancer therapy. Mol Oncol 12: 1077–1103. 10.1002/1878-0261.12313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuvo SR, Ferens FG, Court DA. 2016. The N-terminus of VDAC: Structure, mutational analysis, and a potential role in regulating barrel shape. Biochim Biophys Acta 1858: 1350–1361. 10.1016/j.bbamem.2016.03.017 [DOI] [PubMed] [Google Scholar]
- Sohn YS, Tamir S, Song L, Michaeli D, Matouk I, Conlan AR, Harir Y, Holt SH, Shulaev V, Paddock ML, et al. 2013. NAF-1 and mitoNEET are central to human breast cancer proliferation by maintaining mitochondrial homeostasis and promoting tumor growth. Proc Natl Acad Sci 110: 14676–14681. 10.1073/pnas.1313198110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Midson C, Blachly-Dyson E, Forte M, Colombini M. 1998. The sensor regions of VDAC are translocated from within the membrane to the surface during the gating processes. Biophys J 74: 2926–2944. 10.1016/S0006-3495(98)78000-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof TC, Newton CL, Archer BT III, Ushkaryov YA, Mignery GA. 1991. Structure of a novel InsP3 receptor. EMBO J 10: 3199–3206. 10.1002/j.1460-2075.1991.tb04882.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabadkai G, Bianchi K, Várnai P, De Stefani D, Wieckowski MR, Cavagna D, Nagy AI, Balla T, Rizzuto R. 2006. Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. J Cell Biol 175: 901–911. 10.1083/jcb.200608073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S, Kinoshita T, Takahashi M. 1994. Adenophostins A and B: Potent agonists of inositol-1,4,5-trisphosphate receptor produced by Penicillium brevicompactum. Structure elucidation. J Antibiot (Tokyo) 47: 95–100. 10.7164/antibiotics.47.95 [DOI] [PubMed] [Google Scholar]
- Tan W, Colombini M. 2007. VDAC closure increases calcium ion flux. Biochim Biophys Acta 1768: 2510–2515. 10.1016/j.bbamem.2007.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CW, Konieczny V. 2016. IP3 receptors: Take four IP3 to open. Sci Signal 9: pe1 10.1126/scisignal.aaf6029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teijido O, Ujwal R, Hillerdal CO, Kullman L, Rostovtseva TK, Abramson J. 2012. Affixing N-terminal α-helix to the wall of the voltage-dependent anion channel does not prevent its voltage gating. J Biol Chem 287: 11437–11445. 10.1074/jbc.M111.314229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornero D, Posadas I, Cena V. 2011. Bcl-xL blocks a mitochondrial inner membrane channel and prevents Ca2+ overload-mediated cell death. PLoS ONE 6: e20423 10.1371/journal.pone.0020423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida K, Miyauchi H, Furuichi T, Michikawa T, Mikoshiba K. 2003. Critical regions for activation gating of the inositol 1,4,5-trisphosphate receptor. J Biol Chem 278: 16551–16560. 10.1074/jbc.M300646200 [DOI] [PubMed] [Google Scholar]
- Unni VK, Zakharenko SS, Zablow L, DeCostanzo AJ, Siegelbaum SA. 2004. Calcium release from presynaptic ryanodine-sensitive stores is required for long-term depression at hippocampal CA3-CA3 pyramidal neuron synapses. J Neurosci 24: 9612–9622. 10.1523/jneurosci.5583-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderheyden V, Devogelaere B, Missiaen L, De Smedt H, Bultynck G, Parys JB. 2009. Regulation of inositol 1,4,5-trisphosphate-induced Ca2+ release by reversible phosphorylation and dephosphorylation. Biochim Biophys Acta 1793: 959–970. 10.1016/j.bbamcr.2008.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vervliet T, Decrock E, Molgo J, Sorrentino V, Missiaen L, Leybaert L, De Smedt H, Kasri NN, Parys JB, Bultynck G. 2014. Bcl-2 binds to and inhibits ryanodine receptors. J Cell Sci 127: 2782–2792. 10.1242/jcs.150011 [DOI] [PubMed] [Google Scholar]
- Vervliet T, Lemmens I, Vandermarliere E, Decrock E, Ivanova H, Monaco G, Sorrentino V, Nadif Kasri N, Missiaen L, Martens L, et al. 2015a. Ryanodine receptors are targeted by anti-apoptotic Bcl-XL involving its BH4 domain and Lys87 from its BH3 domain. Sci Rep 5: 9641 10.1038/srep09641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vervliet T, Lemmens I, Welkenhuyzen K, Tavernier J, Parys JB, Bultynck G. 2015b. Regulation of the ryanodine receptor by anti-apoptotic Bcl-2 is independent of its BH3-domain-binding properties. Biochem Biophys Res Commun 463: 174–179. 10.1016/j.bbrc.2015.04.131 [DOI] [PubMed] [Google Scholar]
- Vervliet T, Parys JB, Bultynck G. 2015c. Bcl-2 and FKBP12 bind to IP3 and ryanodine receptors at overlapping sites: The complexity of protein–protein interactions for channel regulation. Biochem Soc Trans 43: 396–404. 10.1042/BST20140298 [DOI] [PubMed] [Google Scholar]
- Vervliet T, Parys JB, Bultynck G. 2016. Bcl-2 proteins and calcium signaling: Complexity beneath the surface. Oncogene 35: 5079–5092. 10.1038/onc.2016.31 [DOI] [PubMed] [Google Scholar]
- Vervliet T, Gerasimenko JV, Ferdek PE, Jakubowska MA, Petersen OH, Gerasimenko OV, Bultynck G. 2018. BH4 domain peptides derived from Bcl-2/Bcl-XL as novel tools against acute pancreatitis. Cell Death Discov 4: 58 10.1038/s41420-018-0054-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vervloessem T, Ivanova H, Luyten T, Parys JB, Bultynck G. 2017a. The selective Bcl-2 inhibitor venetoclax, a BH3 mimetic, does not dysregulate intracellular Ca2+ signaling. Biochim Biophys Acta 1864: 968–976. 10.1016/j.bbamcr.2016.11.024 [DOI] [PubMed] [Google Scholar]
- Vervloessem T, Akl H, Tousseyn T, De Smedt H, Parys JB, Bultynck G. 2017b. Reciprocal sensitivity of diffuse large B-cell lymphoma cells to Bcl-2 inhibitors BIRD-2 versus venetoclax. Oncotarget 8: 111656–111671. 10.18632/oncotarget.22898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisthal S, Keinan N, Ben-Hail D, Arif T, Shoshan-Barmatz V. 2014. Ca2+-mediated regulation of VDAC1 expression levels is associated with cell death induction. Biochim Biophys Acta 1843: 2270–2281. 10.1016/j.bbamcr.2014.03.021 [DOI] [PubMed] [Google Scholar]
- Westphal D, Dewson G, Czabotar PE, Kluck RM. 2011. Molecular biology of Bax and Bak activation and action. Biochim Biophys Acta 1813: 521–531. 10.1016/j.bbamcr.2010.12.019 [DOI] [PubMed] [Google Scholar]
- White C, Li C, Yang J, Petrenko NB, Madesh M, Thompson CB, Foskett JK. 2005. The endoplasmic reticulum gateway to apoptosis by Bcl-XL modulation of the InsP3R. Nat Cell Biol 7: 1021–1028. 10.1038/ncb1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A, Hayashi T, Wolozny D, Yin B, Su TC, Betenbaugh MJ, Su TP. 2016. The non-apoptotic action of Bcl-xL: Regulating Ca2+ signaling and bioenergetics at the ER–mitochondrion interface. J Bioenerg Biomembr 48: 211–225. 10.1007/s10863-016-9664-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcikiewicz RJ, Pearce MM, Sliter DA, Wang Y. 2009. When worlds collide: IP3 receptors and the ERAD pathway. Cell Calcium 46: 147–153. 10.1016/j.ceca.2009.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CY, Chen YF, Wang CH, Kao CH, Zhuang HW, Chen CC, Chen LK, Kirby R, Wei YH, Tsai SF, et al. 2012. A persistent level of Cisd2 extends healthy lifespan and delays aging in mice. Hum Mol Genet 21: 3956–3968. 10.1093/hmg/dds210 [DOI] [PubMed] [Google Scholar]
- Xie Q, Xu Y, Gao W, Zhang Y, Su J, Liu Y, Guo Y, Dou M, Hu K, Sun L. 2018. TAT-fused IP3R-derived peptide enhances cisplatin sensitivity of ovarian cancer cells by increasing ER Ca2+ release. Int J Mol Med 41: 809–817. 10.3892/ijmm.2017.3260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Kong D, Zhu L, Zhu W, Andrews DW, Kuo TH. 2007. Suppression of IP3-mediated calcium release and apoptosis by Bcl-2 involves the participation of protein phosphatase 1. Mol Cell Biochem 295: 153–165. 10.1007/s11010-006-9285-5 [DOI] [PubMed] [Google Scholar]
- Xu C, Xu W, Palmer AE, Reed JC. 2008. BI-1 regulates endoplasmic reticulum Ca2+ homeostasis downstream of Bcl-2 family proteins. J Biol Chem 283: 11477–11484. 10.1074/jbc.M708385200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Vais H, Gu W, Foskett JK. 2016. Biphasic regulation of InsP3 receptor gating by dual Ca2+ release channel BH3-like domains mediates Bcl-xL control of cell viability. Proc Natl Acad Sci 113: E1953–E1962. 10.1073/pnas.1517935113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano M, Yamamoto T, Ikeda Y, Matsuzaki M. 2006. Mechanisms of disease: Ryanodine receptor defects in heart failure and fatal arrhythmia. Nat Clin Pract Cardiovasc Med 3: 43–52. 10.1038/ncpcardio0419 [DOI] [PubMed] [Google Scholar]
- Yoshikawa F, Iwasaki H, Michikawa T, Furuichi T, Mikoshiba K. 1999. Trypsinized cerebellar inositol 1,4,5-trisphosphate receptor. Structural and functional coupling of cleaved ligand binding and channel domains. J Biol Chem 274: 316–327. 10.1074/jbc.274.1.316 [DOI] [PubMed] [Google Scholar]
- Yuan S, Fu Y, Wang X, Shi H, Huang Y, Song X, Li L, Song N, Luo Y. 2008. Voltage-dependent anion channel 1 is involved in endostatin-induced endothelial cell apoptosis. FASEB J 22: 2809–2820. 10.1096/fj.08-107417 [DOI] [PubMed] [Google Scholar]
- Zecchini E, Siviero R, Giorgi C, Rizzuto R, Pinton P. 2007. Mitochondrial calcium signalling: Message of life and death. Ital J Biochem 56: 235–242. [PubMed] [Google Scholar]
- Zeth K, Zachariae U. 2018. Ten Years of high resolution structural research on the voltage dependent anion channel (VDAC)—Recent developments and future directions. Front Physiol 9: 108 10.3389/fphys.2018.00108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang E, Mohammed Al-Amily I, Mohammed S, Luan C, Asplund O, Ahmed M, Ye Y, Ben-Hail D, Soni A, Vishnu N, et al. 2019. Preserving insulin secretion in diabetes by inhibiting VDAC1 overexpression and surface translocation in β cells. Cell Metab 29: 64–77.e6. 10.1016/j.cmet.2018.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhivotovsky B, Orrenius S. 2011. Calcium and cell death mechanisms: A perspective from the cell death community. Cell Calcium 50: 211–221. 10.1016/j.ceca.2011.03.003 [DOI] [PubMed] [Google Scholar]
- Zhong F, Davis MC, McColl KS, Distelhorst CW. 2006. Bcl-2 differentially regulates Ca2+ signals according to the strength of T cell receptor activation. J Cell Biol 172: 127–137. 10.1083/jcb.200506189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong F, Harr MW, Bultynck G, Monaco G, Parys JB, De Smedt H, Rong YP, Molitoris JK, Lam M, Ryder C, et al. 2011. Induction of Ca2+-driven apoptosis in chronic lymphocytic leukemia cells by peptide-mediated disruption of Bcl-2-IP3 receptor interaction. Blood 117: 2924–2934. 10.1182/blood-2010-09-307405 [DOI] [PMC free article] [PubMed] [Google Scholar]