Abstract
In the nervous system, calcium signals play a major role in the conversion of synaptic stimuli into transcriptional responses. Signal-regulated gene transcription is fundamental for a range of long-lasting adaptive brain functions that include learning and memory, structural plasticity of neurites and synapses, acquired neuroprotection, chronic pain, and addiction. In this review, we summarize the diverse mechanisms governing calcium-dependent transcriptional regulation associated with central nervous system plasticity. We focus on recent advances in the field of synapse-to-nucleus communication that include studies of the signal-regulated transcriptome in human neurons, identification of novel regulatory mechanisms such as activity-induced DNA double-strand breaks, and the identification of novel forms of activity- and transcription-dependent adaptations, in particular, metabolic plasticity. We summarize the reciprocal interactions between different kinds of neuroadaptations and highlight the emerging role of activity-regulated epigenetic modifiers in gating the inducibility of signal-regulated genes.
A remarkable feature of the brain is its ability to adapt its structure and function in response to sensory stimulation, learning, or pathological conditions. At the cellular level, structural and functional plasticity has mostly been studied in excitatory principal neurons, but it is now known to also occur in inhibitory interneurons (Debanne et al. 2019; Lamsa and Lau 2019), astrocytes (Shao and McCarthy 1994; Theodosis et al. 2008; Zhang et al. 2017), and oligodendroglia (Birey et al. 2017; Kaller et al. 2017). Brain plasticity is, however, a double-edged sword. On the one hand, it allows, for example, for the coding of sensory experiences into long-lasting memories or for the acquisition of novel motor skills (Dayan and Cohen 2011; Mansvelder et al. 2019). On the other hand, it enables maladaptive changes in brain circuitry that mediate the development of pathologies like chronic pain or addiction. In both cases, long-lasting adaptations of brain structure and function require changes in gene transcription. This, in turn, necessitates ways of communication between synapses and the nucleus. Such communication can be brought about by diffusible proteins, the stimulation of diverse signaling cascades, and also, most notably, activity-dependent calcium signals.
In this article, we will first summarize the molecular mechanisms through which calcium signaling mediates the translation of synaptic activity into a transcriptional response. We will then discuss a range of activity- and calcium-dependent physiological and pathophysiological neuroadaptations with a focus on metabolic plasticity.
MECHANISMS OF CALCIUM-DEPENDENT SYNAPSE-TO-NUCLEUS COMMUNICATION
To couple synaptic input to a transcriptional response, the nucleus needs to sense levels and timing of synaptic activity. This information can be conveyed via different signaling modes that act on diverse time scales. First, several proteins have been described that are located at pre- or postsynaptic sites and, upon synaptic stimulation, can travel to the nucleus over time periods on the order of minutes to hours. Progress in the field of synaptonuclear protein messengers and their role in activity-dependent gene transcription has recently been covered in elegant reviews (Herbst and Martin 2017; Marcello et al. 2018). Second, activation of G-protein-coupled receptors and receptor tyrosine kinases can result in the generation of second messengers such as cyclic AMP (cAMP), which, in turn, trigger further signaling cascades on the order of seconds. Third, calcium that enters the cytoplasm from intracellular or extracellular sources upon synaptic activity can trigger signaling cascades that reach the nucleus on the order of seconds. Fourth, calcium itself can enter the nucleus to activate calcium-sensitive transcriptional regulators on the order of milliseconds to seconds. The signaling mechanisms that underlie calcium-dependent synapse-to-nucleus communication and gene transcription have been covered in several comprehensive reviews (Flavell and Greenberg 2008; Hagenston and Bading 2011; Bengtson and Bading 2012; Bading 2013). In this section, we will briefly summarize these mechanisms with a focus on new findings from recent years.
Conversion of Calcium Signals into Transcriptional Responses
Calcium signals trigger transcriptional responses in neurons by numerous mechanisms, including (1) the posttranslational modification of transcription factors such as the cAMP response element-binding protein (CREB) and the ternary complex factor (TCF), Elk1, (2) the modulation of transcriptional regulators such as downstream regulatory element (DRE) antagonist modulator (DREAM) and calmodulin (CaM)-binding transcription activator 1 (CAMTA1), (3) the up-regulated expression of transcription factors like neuronal PAS domain protein 4 (NPAS4), (4) the modulated nucleocytoplasmic shuttling of transcription factors like Foxo3A, and (5) the altered expression and/or regulation of proteins that influence chromatin structure and DNA methylation state, including histone acetyl transferases (HATs), histone deacetylases (HDACs), DNA methyltransferases (DNMTs), and DNA-methyl-binding proteins (Carrion et al. 1999; Finkler et al. 2007; Dick and Bading 2010; Hagenston and Bading 2011; Bading 2013; Schlumm et al. 2013; Mellström et al. 2014; Bas-Orth et al. 2016; Oliveira et al. 2016; Sun and Lin 2016; Litke et al. 2018). Yet another intriguing mechanism that contributes to activity-regulated gene transcription is the activity-dependent formation of DNA double-strand breaks (DSBs). These breaks transiently occur during physiological activity in vivo (Suberbielle et al. 2013) and have been shown to be necessary and sufficient to induce the transcription of several neuronal immediate-early genes, including Fos, Zif268, and Npas4 (Madabhushi et al. 2015). Activity-dependent DSBs depend on N-methyl-d-aspartate receptor (NMDAR) signaling and are mediated by DNA topoisomerases SPO11 and TOP2B that probably promote genomic rearrangements to relieve spatial restraints on transcription. TOP2B has been shown to preferentially bind to promoter regions that contain H3K4 methylation marks (Tiwari et al. 2012), but given that TOP2B appears to control the expression of around 20–100 genes only (Tiwari et al. 2012; Madabhushi et al. 2015), how this enzyme is recruited to specific genes remains an open question. Likewise, how topoisomerases are activated by synaptic activity and whether this involves calcium signaling remains to be investigated.
Pathways of Calcium Entry and Signal Propagation
Synaptic activity leads to transient changes in the cytosolic calcium concentration via several mechanisms. First, glutamate that is released from presynaptic terminals binds to and activates postsynaptic ligand-gated ion channels like the α-amino-3-hydroxyl-5-methyl-4-isoxazolepropionate receptor (AMPAR) or the NMDAR that are permeable for sodium and calcium. Subsequent depolarization of the postsynaptic membrane leads to opening of L-type voltage-dependent calcium channels (VDCCs) and additional influx of calcium from the extracellular space. Second, binding of synaptically released glutamate and other neurotransmitters to G-protein-coupled metabotropic receptors results in the generation of the second messenger inositol 1,4,5-trisphosphate (IP3) that binds to its receptor on the endoplasmic reticulum and causes release of calcium from this intracellular store (Power and Sah 2002; Watanabe et al. 2006; Hagenston et al. 2008). Third, intracellular calcium transients can be amplified by binding of calcium to the ryanodine receptor on the endoplasmic reticulum, leading to calcium-induced calcium release. Although the endoplasmic reticulum is considered the predominant source for activity-dependent intracellular calcium release, other cellular compartments might contribute to calcium signaling as well. For example, recent work has revealed a physiological role for activity- and glutamate receptor–dependent release of calcium from the endolysosomal compartment (Hui et al. 2015; Morgan 2016; Shang et al. 2016; Padamsey et al. 2017). Whether calcium release from endolysosomes functions in local signaling only, or whether it also affects synapse-to-nucleus communication remains to be investigated.
Among the signaling cascades that can relay cytosolic calcium signals to the nucleus, the RAS/mitogen-activated protein kinase (RAS/MAPK) and calcium/CaM-dependent protein kinase (CaMK) pathways have been the best studied (Greer and Greenberg 2008; Hagenston and Bading 2011; Bading 2013). Stimulation of the classical RAS/MAPK signaling cascade initiates at the synapse via the calcium-dependent activation of RAS/GRF and the inhibition by calcium/CaM-dependent protein kinase II (CaMKII) of a RAS GTPase-activating protein. The spatial spread of subsequent signaling events downstream from RAS is limited, however, by diffusion and by the rapid inactivation of second messenger kinases. Accordingly, signaling to the nucleus by distal synapses via the RAS/MAPK cascade seems unlikely (Hagenston and Bading 2011; Wiegert and Bading 2011). L-type VDCCs, on the other hand, are distributed on somatic and proximal dendritic membranes and are open in response to neuronal depolarization such as occurs during an action potential. These channels, which may dominate depolarization-induced transcriptional responses (Ma et al. 2012), were recently linked to the RAS/MAPK signaling cascade and to depolarization-triggered gene transcription, albeit by a mechanism that is independent of their ability to conduct calcium ions (Servili et al. 2018). In particular, this study suggests that activation of H-RAS is initiated during membrane depolarization, and calcium binding in the pore-forming α11.2 subunit of the VDCC is conveyed by a conformational change from the α11.2 to the β2b subunit, and is mediated by the direct interaction of the β2b subunit with H-RAS (Servili et al. 2018).
Cytoplasmic rises in calcium, particularly those mediated by voltage-dependent calcium entry from the extracellular space, stimulate the activity of cytoplasmic calcium/CaM-dependent protein kinase kinase (CaMKK), which in turn phosphorylates and activates calcium/CaM-dependent protein kinase IV (CaMKIV). This kinase phosphorylates and activates a range of effector proteins in the nucleus including CREB (Soderling 1999; Means 2000; Hagenston and Bading 2011). Activation of transcription mediated by CREB, CREST, and other transcription factors requires the additional stimulation of the cofactor CREB-binding protein (CBP) by CaMKIV (Chawla et al. 1998), thus endowing this particular CaMK with a central role in synapse-to-nucleus communication (Bading 2013). Indeed, overexpression of dominant-negative CaMKIV effectively blunts activity-dependent gene transcription, transcription-dependent long-term potentiation (LTP) of synaptic strength, memory consolidation, and basal- and activity-induced dendrite complexity and spine density (Kang et al. 2001; Limback-Stokin et al. 2004; Zhang et al. 2009; Mauceri et al. 2011).
CaMKIV is predominantly expressed in the nucleus (Jensen 1991; Nakamura 1995; Bito 1996). Accordingly, stimulation of CaMKIV, the first step being its association with calcium-bound CaM (calcium/CaM), requires a nuclear elevation in the levels of calcium/CaM. This elevation is accomplished by direct entry of calcium into the nucleoplasm, via nuclear pores, where it binds to nuclear-localized CaM (Hardingham et al. 2001), although cytoplasm-to-nucleus translocation of calcium-bound CaM has also been suggested (Cohen et al. 2015). Another mechanism by which neuronal excitation and the stimulation of calcium entry via VDCCs could lead to the nuclear accumulation of calcium-bound CaM may involve shuttling of the γ isoform of CaMKII (Ma et al. 2014; Cohen et al. 2015). According to this model, γCaMKII binds calcium/CaM near the membrane in certain cell types, after which it is phosphorylated at T287 by α/βCaMKII, trapping the calcium/CaM cargo and dephosphorylated at S334 by calcineurin, resulting in the increased exposure of an adjacent nuclear localization signal that directs the calcium/CaM-bound kinase shuttle to the nucleus. Once in the nucleus, γCaMKII is dephosphorylated by the CaMKIV-associated protein phosphatase PP2A, releasing calcium/CaM that can then immediately activate CaMKIV (Ma et al. 2014; Cohen et al. 2015, 2018). Although this mode of signaling to the nucleus, which relies on the cytoplasm-to-nucleus transport of the calcium/CaM shuttle, is supported by other studies (Cohen et al. 2016; Herbst and Martin 2017; Wang et al. 2017; Zalcman et al. 2018), it remains to be seen how these ideas can be reconciled with conflicting observations that hippocampal neurons express high levels of CaM in the cell nucleus and that this nuclear accumulation of CaM is independent of synaptic activity (Hardingham et al. 2001). Also, immunostaining studies and biochemical subcellular fractionations of hippocampal neurons showed that the localization of γCaMKII in the cytoplasm and in the nucleus does not change upon synaptic activity (Buchthal et al. 2012). Finally, and most importantly, activity-dependent activation of CREB in hippocampal neurons occurs even when nuclear transport is completely blocked by microinjection into the cytoplasm of wheat germ agglutinin (WGA), a potent blocker of nuclear import (Hardingham et al. 2001).
Nuclear Calcium Signaling
In addition to activating signaling cascades in the cytosol, calcium can directly enter the nucleus via passive diffusion through nuclear pores (Eder and Bading 2007). Once in the nucleus, calcium can contribute to activity-dependent transcription via its association with CaM, but also by direct interaction with calcium-sensitive transcriptional regulators such as the transcriptional repressor, DREAM (Carrion et al. 1999; Osawa et al. 2001). DREAM achieves transcriptional control by high-affinity binding to DRE sequences in DNA, and its calcium-dependent unbinding results in the derepression of several genes, including Bdnf and prodynorphin, both of which contribute to pain chronicity (Costigan and Woolf 2002; Rivera-Arconada et al. 2010). Several calcium-dependent transcription factors that have established roles in synaptic plasticity and learning and memory are regulated by DREAM: NPAS4, NR4A1, MEF2C, JUNB, and cFOS (Mellström et al. 2014). Additionally, DREAM may interact with diverse nuclear and cytosolic proteins to influence neuronal functions, such as the transcription factors CREB and ATF6, NMDARs and potassium channels, presenilin-2, and others (Rivas et al. 2011; Burgoyne and Haynes 2012; Wang and Wang 2012). Studies investigating the functional consequences of nuclear calcium signaling, using the intranuclear injection of a nondiffusible dextran-coupled calcium chelator, the transgenic expression of a nuclearly localized calcium/CaM inhibitor, or the nuclear expression of the calcium-buffering protein parvalbumin, have demonstrated that nuclear calcium signaling is a potent regulator of activity-dependent gene transcription and is required for the maintenance of dendritic structure and synapse number, synaptic plasticity, memory consolidation, acquired neuroprotection, and pain chronicity (Hardingham et al. 1997; Chawla et al. 1998; Limback-Stokin et al. 2004; Zhang et al. 2009; Mauceri et al. 2011, 2015; Simonetti et al. 2013; Weislogel et al. 2013; Hemstedt et al. 2017; Chandrasekar et al. 2018). In addition, transgenic mice lacking DREAM or expressing a calcium-insensitive DREAM mutant exhibit decreased dendritic arborization and spine density, altered synaptic plasticity, impaired learning and memory, and diminished pain hypersensitivity (Costigan and Woolf 2002; Rivera-Arconada et al. 2010; Mellström et al. 2016; López-Hurtado et al. 2018). Taken together, these findings highlight the relevance of nuclear calcium signaling for synapse-to-nucleus communication and central nervous system neuroadaptations.
Calcium signaling in the neuronal nucleus appears particularly relevant for calcium entering the cell via somatodendritic L-type VDCCs. Indeed, synapse-independent “replay” of the membrane potential changes induced by bursts of action potentials was shown to generate nuclear calcium transients that had amplitudes nearly 70% of their original synaptic activity-generated counterparts, and that could be abolished by L-type VDCC blockers, indicating that this channel type represents the major source of nuclear calcium signals evoked during trains of action potentials triggered by synaptic activity (Bengtson et al. 2013). Other sources of nuclear calcium include calcium that enters via NMDARs and calcium that is released via ryanodine and IP3 receptors from the endoplasmic reticulum and perhaps the nuclear envelope (Hagenston and Bading 2011; Bengtson et al. 2013; Martins et al. 2016). In some cell types, including neurons, IP3 receptors may be localized to the inner nuclear envelope, raising the possibility that calcium may be released directly into the nucleus either subsequent to the activation of intranuclear G-protein-coupled receptors (Jong et al. 2005, 2007) or, following the synaptic mobilization of IP3, which as a result of its small size can pass freely through nuclear pores (Humbert et al. 1996; Echevarría et al. 2003; O'Malley et al. 2003; Jong et al. 2005, 2007, 2018; Marchenko et al. 2005; Marchenko and Thomas 2006; Kumar et al. 2008; Bootman et al. 2009; Rodrigues et al. 2009; Olivares-Florez et al. 2018). Consistent with their generation via the diffusion of calcium from the soma and through the nuclear pores, nuclear calcium transients triggered by bursts of synaptic activity closely follow their cytoplasmic counterparts with respect to both amplitude and duration (Eder and Bading 2007; Mauceri et al. 2015). In the fruit fly brain in vivo and in brain and spinal cord slices ex vivo, nuclear calcium transients are evoked by stimuli that trigger and/or mimic afferent synaptic activity, including those that are known to induce transcription-dependent plasticity and to trigger LTP, like 100 Hz and Θ-burst stimulation (Bengtson et al. 2010; Simonetti et al. 2013; Weislogel et al. 2013). The amplitude and duration of nuclear calcium transients evoked by these stimuli is closely correlated to the intensity and duration of synaptic activity. The transcriptional machinery, in turn, is rapidly activated upon increases in nuclear calcium and rapidly shuts off after the transient has ceased (Chawla and Bading 2001). In an effort to determine the minimum signal strength that is required to induce gene transcription, the authors of a recent study used the highly sensitive catFISH method to characterize activity-dependent transcription of the immediate early gene Arc (Yu et al. 2017). They found that a single burst of action potential that consisted of ∼25 action potentials and was associated with a single ∼10 sec nuclear calcium transient was sufficient to trigger Arc transcription. Moreover, consistent with the dependency of CREB, CBP, and serum response factor (SRF) activity on the duration of nuclear calcium elevations (Chawla and Bading 2001), increasing the duration of stimuli—and thereby the number of evoked action potential bursts—elevated both the number of Arc-positive cells and the total amount of Arc messenger RNA (mRNA) transcribed (Yu et al. 2017). Thus, nuclear calcium transients provide a quantitative and highly sensitive means of translating synaptic activity into a transcriptional response.
Activity-Dependent Gene Transcription in Human Neurons
Activity- and calcium-dependent gene transcription has mostly been studied in cultured rodent neurons and in the rodent brain. A long-standing question in the field, therefore, is whether the subset of activity-regulated genes and the corresponding signaling mechanisms are conserved in humans. Technical progress in the generation of human-induced pluripotent stem cells (hiPSCs) and their differentiation into neurons (hiPSC-derived neurons) has allowed for first insights into this question. Recent studies that compared activity-dependent gene transcription between primary and stem-cell-derived mouse neurons and primary and iPSC-derived human neurons revealed that activity-dependent gene transcription is largely conserved between rodent and human neurons (Ataman et al. 2016; Qiu et al. 2016; Pruunsild et al. 2017). However, specific differences were identified. For several genes, the kinetics of the transcriptional response to neuronal activity differed markedly between mouse and human neurons. In addition, several genes were found to be induced by neuronal activity in human but not mouse neurons. These changes are mediated by the acquisition of cis-regulatory elements, such as cAMP-response elements (CREs) and MEF2-responsive elements (MREs), in the promoter and enhancer regions of the respective genes. Intriguingly, genes that have acquired responsiveness to synaptic activity in the primate lineage include Osteocrin and Camta1. These genes are involved in cortical development (Ataman et al. 2016) and long-term memory (Huentelman et al. 2007; Bas-Orth et al. 2016), respectively, which suggests that evolutionary changes in activity-dependent gene transcription may underlie the development of cognitive abilities in the primate lineage (Hardingham et al. 2018).
FUNCTIONAL CONSEQUENCES OF CALCIUM-DEPENDENT GENE TRANSCRIPTION
Recent years have provided a growing list of neuroadaptations that are governed by calcium-dependent gene transcription. These adaptations span several levels of organization, from the structure and function of individual neurons to circuit functions and to behavior. They support physiological functions like development, memory, and neuroprotection, but play a role as well as in maladaptations like chronic pain and addiction. In this section, we provide a brief summary of some of these adaptations.
Long-Term Memory
Our current understanding of the molecular biology of learning and memory was born with the pioneering discovery in the 1960s that the formation of long-lasting memories depends on de novo gene transcription (Hernandez and Abel 2008). Subsequent studies in PC12 cells, in cultured neurons, and in the rodent brain revealed that activity-dependent gene transcription is triggered by the depolarization-induced influx of calcium into postsynaptic neurons (Greenberg et al. 1986; Morgan and Curran 1986, 1988; Bading et al. 1993). The importance of calcium signaling in memory formation was underscored by the discovery of a number of calcium-regulated transcription factors and transcriptional regulators that are required for the formation of long-lasting memories in sea snails, fruit flies, and mice. These transcriptional regulators include CREB, CBP, SRF/p62Elk1, NPAS4, NR4A1, and CAMTA1 (Dash et al. 1990; Bourtchuladze et al. 1994; Yin et al. 1994; Kida et al. 2002; Pittenger et al. 2002; Wood et al. 2005; Etkin et al. 2006; Ploski et al. 2011; Ramamoorthi et al. 2011; Coutellier et al. 2012; McNulty et al. 2012; Bas-Orth et al. 2016). Several of these proteins are regulated by calcium/CaM signaling within the cell nucleus. Accordingly, nuclear calcium/CaM signaling was found to be required for long-term memory in fruit flies and mice (Kang et al. 2001; Limback-Stokin et al. 2004; Weislogel et al. 2013).
How exactly activity- and calcium-dependent gene transcription contributes to the formation of long-lasting memories is still incompletely understood. It is thought, however, that the expression of genes targeted by activity-regulated transcription factors, such as Arc, Homer1, and Bdnf, is required to modify synaptic strength and neuronal connectivity. In addition to its effects at the synaptic level, calcium/CaM-dependent gene transcription has also been shown to modify the overall excitability of activated neurons. This global effect has been suggested to promote the recruitment of coactivated neurons into neuronal assemblies that are thought to represent the physical basis of memory traces (Buzsaki and Draguhn 2004; Holtmaat and Caroni 2016; Lisman et al. 2018). The activity-dependent expression of memory-related genes is further controlled by epigenetic regulators that in turn are controlled by calcium signaling. These include both regulators of DNA methylation patterns and regulators of histone modifications. For example, expression of the de novo DNA methyltransferase DNMT3A2 is induced by synaptic activity in a nuclear calcium/CaM-dependent manner (Oliveira et al. 2012). DNMT3A2 facilitates the expression of the immediate-early genes Arc and Bdnf, is required for the formation, consolidation, and extinction of long-term memories, and contributes to the establishment of nociceptive hypersensitivity in chronic inflammatory pain (Oliveira et al. 2012, 2016, 2019). The subcellular localization of class IIa histone deacetylases ([HDACs] is controlled by synaptic activity and nuclear calcium/CaM signaling (Chawla et al. 2003; Schlumm et al. 2013). HDACs generally act as transcriptional repressors either by interfering with DNA-binding proteins or by catalyzing the removal of acetyl groups from histones, resulting in denser chromatin with more limited accessibility for transcriptional machinery. The nucleocytoplasmic shuttling specifically of HDAC4 and HDAC5 is induced by synaptic activity and NMDAR activation both in vitro and in vivo and can be inhibited by the nuclear expression of either a calcium/CaM buffer or the calcium-binding protein parvalbumin (Chawla et al. 2003; Sando et al. 2012; Schlumm et al. 2013; Chen et al. 2014). Among the targets of HDAC4/5 are a number of genes essential for synaptic function and plasticity, including Npas4, CaMKIIα, and HomerI, but also regulators of dendritic structure (Mauceri et al. 2011; Sando et al. 2012; Taniguchi et al. 2017; Litke et al. 2018). Numerous lines of evidence implicate class IIa HDAC function in nonassociative learning, and a recent study demonstrated its involvement in spatial learning (Sando et al. 2012; Rahn et al. 2013; Uchida and Shumyatsky 2018). Because activity-dependent expression of epigenetic regulators and subsequent modification of histones and DNA takes time, these mechanisms seem not to play a major role in the acute transcriptional response to synaptic activity. Rather, by modifying the chromatin landscape, they have a gating function and control the permissiveness of the genome for transcriptional responsiveness to future synaptic stimuli (Oliveira et al. 2012; Baker-Andresen et al. 2013; Gulmez Karaca et al. 2018). Thus, in the control of long-term memory, calcium signaling acts on two time scales. First, via calcium-regulated transcription factors, it mediates the acute expression of proteins that are required for long-term memory formation. Second, via calcium-dependent epigenetic regulators, it maintains the responsiveness of the transcriptional machinery so that future stimuli can be efficiently translated into long-lasting memories.
Morphological Plasticity
Synaptic activity controls the development and maintenance of neuronal morphology, including the number and size of synapses and the size and complexity of the dendritic arbor (Marie et al. 2005; Flavell et al. 2006; Shalizi et al. 2006; Wayman et al. 2006; Saneyoshi et al. 2010; Mauceri et al. 2011). The development, maintenance, and plasticity of neuronal structure and interneuronal connectivity depend on calcium-regulated gene expression. For example, the nuclear calcium/CaM-dependent transcription factor NPAS4, an immediate early gene expressed in both excitatory and inhibitory neurons following synaptic activity in vitro and diverse stimuli in vivo (Lin et al. 2008; Zhang et al. 2009; Ramamoorthi et al. 2011; Taniguchi et al. 2017; Weng et al. 2018), plays a role in establishing and maintaining the balance of excitation and inhibition in neuronal networks involved in adaptive plastic processes (Sun and Lin 2016). In particular, the activity-driven expression of NPAS4, which is selectively induced by nuclear calcium (Zhang et al. 2009) and depends on DREAM-mediated transcriptional derepression (Lin et al. 2008; Mellström et al. 2014), was shown to trigger unique homeostatic transcriptional responses in excitatory and inhibitory neurons. These responses trigger an up-regulation of the numbers of inhibitory synapses formed onto excitatory neurons, and a concomitant up-regulation of the numbers of excitatory synapses formed onto inhibitory neurons, resulting in an overall restriction of excitatory neuronal output following robust synaptic activity. Loss of NPAS4, by contrast, was shown to result in an overall disinhibition of neuronal networks (Bloodgood et al. 2013; Spiegel et al. 2014). Interestingly, NPAS4 was recently also shown to modulate the synaptic connectivity between excitatory neurons. Specifically, while enhanced activation of dentate granule cells triggered the homeostatic down-regulation in the size and functional efficacy of synaptic contacts made between their mossy fibers and the thorny excrescences of CA3 pyramidal neurons, constitutive or conditional deletion of NPAS4 from CA3 had the opposite effect (Weng et al. 2018). Expression of NPAS4 was also demonstrated to be controlled by a class IIa HDAC, HDAC5 (Taniguchi et al. 2017), the nucleocytoplasmic shuttling of which is synaptic activity- and nuclear calcium/CaM-dependent (Schlumm et al. 2013; Simonetti et al. 2013). Nuclear calcium/CaM signaling through class IIa HDACs also controls the expression of two further genes that regulate neuronal structure and connectivity. In particular, by stimulating the nucleocytoplasmic shuttling of HDAC4, nuclear calcium/CaM signaling curbs the expression of the secreted complement C1Q subunit C (C1QC) and maintains physiological expression levels of vascular endothelial growth factor D (VEGFD), a secreted molecule that is required to maintain dendritic geometry in mature neurons (Mauceri et al. 2011; Schlumm et al. 2013; Simonetti et al. 2013; Litke et al. 2018). Accordingly, inhibition of nuclear calcium signaling causes a reduction in the number and size of dendritic spines and a loss of dendritic length and complexity in mature neurons, effects that are replicated by exogenous application of C1QC, which acts as a synapse pruning factor, or by short-hairpin RNA (shRNA)-mediated knockdown of Vegfd, respectively. Conversely, shRNA-mediated knockdown of C1QC can increase the spine number on dendrites of spinal neurons and overexpression or exogenous application of VEGFD supports maintenance of the structural integrity of dendritic arbors (Mauceri et al. 2011; Schlumm et al. 2013; Simonetti et al. 2013; Litke et al. 2018). In vivo, C1QC is required for physiological synapse refinement during development, but might also contribute to the pathogenesis of neurodegenerative diseases (Presumey et al. 2017). It therefore provides a putative link between dysregulated calcium signaling and synapse loss in aging and neurodegeneration. Loss of VEGFD in the mouse brain leads to reduced length and complexity of dendrites, causing impaired synapse-to-nucleus calcium signaling, impaired expression of plasticity-related genes, and impaired formation and extinction of long-term memories (Mauceri et al. 2011; Hemstedt et al. 2017). Together, these findings highlight the complex interplay between neuronal structure and function. They reveal a reciprocal regulatory loop in which calcium-dependent gene transcription maintains neuronal structure, which, in turn, is a prerequisite for efficient activity-dependent calcium signaling and calcium-dependent synapse-to-nucleus communication.
Acquired Neuroprotection
Synaptic stimulation of neurons leads to the buildup of a long-lasting protected state in which neurons are more resistant to harmful stimuli. This acquired neuroprotection can be observed after action potential bursting in cultured hippocampal neurons in vitro (Hardingham et al. 2002; Lee et al. 2005; Papadia et al. 2005), and after exposure of rodents to an enriched/novel environment in vivo (Hannan 2014). It includes increased resistance against proapoptotic stimuli such as growth factor withdrawal and staurosporine treatment, and against excitotoxic stimuli such as glutamate and kainic acid treatment. The buildup of acquired neuroprotection depends on nuclear calcium signaling and gene transcription. A series of transcriptome analyses revealed a core set of so-called activity-regulated inhibitor of death (AID) genes that mediate acquired neuroprotection (Zhang et al. 2007, 2009). Their protein products include the transcriptional regulators ATF3, GADD45β, GADD45γ, NPAS4, and NR4A1; the nuclear proteins BTG2 and interferon-activated gene 202B (IFI202B); and the secreted proteins inhibin β-A (INHBA)/Activin-A and SerpinB2. At least six of the nine AID genes have been observed to exhibit a significant up-regulation following exposure to an enriched/novel environment in vivo (Lacar et al. 2016; AM Hagenston and H Bading, unpubl.). The expression of AID genes is modulated by synaptic NMDAR activity and nuclear calcium, and they confer robust neuroprotection both in vitro and in vivo. Neuroprotection against ischemic brain damage in vivo has been achieved in mice by virus-mediated overexpression of individual AID genes and, in the case of Activin-A and SerpinB2, by postinjury protein delivery via a nasal spray (Zhang et al. 2011a; Lau et al. 2015; Buchthal et al. 2018). Overexpression of several individual AID genes has been shown to attenuate mitochondrial permeability transition during excitotoxicity. This and other observations lead to the notion that, mechanistically, acquired neuroprotection appears to work mainly via the protection of mitochondrial structure and function, either directly or indirectly (Lau and Bading 2009; Zhang et al. 2009; Leveille et al. 2010; Bas-Orth and Bading 2013; Bading 2017). An example of a direct link between AID gene expression and mitochondrial protection is the NPAS4-mediated transcriptional repression of the mitochondrial calcium uniporter (MCU), which protects mitochondria from calcium overload, oxidative damage, and permeability transition under conditions of glutamate excitotoxicity (Qiu et al. 2013; Depp et al. 2018). Protection of mitochondria is further achieved by the activity- and calcium-dependent expression of antioxidant defense genes (Papadia et al. 2008; Soriano et al. 2009; Baxter et al. 2015) and, potentially, by a transcription-dependent switch of neuronal energy metabolism toward aerobic glycolysis (see below).
Chronic Pain
Although acute physiological pain is essential for survival, chronic pathological pain is a debilitating disease that is associated with intense individual suffering as well as significant social and financial burden. Such chronic pain may result from an injury causing tissue inflammation or damage to a nerve or the spinal cord, cancer, viral infections, diabetes, or chemotherapy. Mechanistically, chronic pain syndromes are associated with a range of functional and structural plastic changes in neuronal networks at all levels of the pain axis extending from peripheral sensory neurons for both pain and touch, to the spinal cord circuits that receive and transmit painful sensory information, to the somatosensory and limbic circuits that assign meaning and emotional valence (Kuner 2010; Hagenston and Simonetti 2014). Two key features of pathological pain are (1) its persistence well after the initial injury or insult has healed, and (2) its occurrence in the absence of any obvious pathological trigger. Both in terms of its persistence and with relation to its cellular and molecular biological underpinnings, chronic pain shares many features in common with learning and memory (Rahn et al. 2013; Price and Inyang 2015). At the level of the spinal cord, for instance, pain chronicity involves both LTP at the synapses between peripheral afferents and central neurons and an increase in neuronal excitability, processes that depend on calcium signaling and calcium-dependent transcriptional responses (Ji et al. 2003; Sandkühler 2009; Hagenston and Simonetti 2014). Indeed, spinal central sensitization, for which LTP represents the synaptic correlate, is linked to the altered expression of genes controlled by CREB and DREAM, but also nuclear calcium/CaM, class II HDACs, and the de novo DNA methyltransferase, DNMT3A2 (Cheng et al. 2002; Bai et al. 2010; Kuner 2010; Rivera-Arconada et al. 2010; Long et al. 2011; Zhang et al. 2011b; Tochiki et al. 2012; Descalzi et al. 2015; Oliveira et al. 2019).
Drug Addiction
Drug addiction is a chronic, relapsing disorder that is characterized by compulsive drug seeking and taking, despite adverse consequences. It typically develops during repeated exposure to drugs of abuse that are known to cause structural and functional neuroadaptations in the mesolimbic reward circuit. Development of an addicted state can be considered a form of maladaptive brain plasticity or learning (Hyman et al. 2006; Kauer and Malenka 2007). Similar to physiological forms of learning, drug-induced neuroadaptations require calcium signaling through NMDARs and the MAPK and CaMK pathways (Konradi et al. 1996; Cahill et al. 2014; Hopf 2017; Morisot and Ron 2017; Takemoto-Kimura et al. 2017). These signaling cascades culminate in transcriptional responses and epigenetic modifications that are thought to mediate the persistent neuronal modifications that underlie addictive behavior. Examples of calcium-dependent transcriptional and epigenetic regulators that are involved in this process include CREB, CBP, ΔFOSB, HDAC5, and DNMT3A2 (Robison and Nestler 2011; Taniguchi et al. 2017; Cannella et al. 2018; Walker and Nestler 2018).
Activity-Dependent Control of Transcriptional Permissiveness
Most work in the field of activity-dependent gene transcription has focused on the acute mechanisms and targets of signal-regulated gene transcription to explain how synaptic activity promotes long-lasting neuronal adaptations. Whereas initial work focused on activity-regulated transcription factors, we now know that synaptic activity regulates the expression and activity of histone modifiers, DNA methyltransferases, DNA topoisomerases, and noncoding RNAs as well. According to the classical concept of signal-regulated gene transcription, these can be considered as additional parts of the machinery that translates synaptic signals into acute transcriptional responses. An emerging concept in activity-regulated gene transcription suggests, however, that these epigenetic modifiers do not primarily contribute to acute gene expression, but instead act on a longer time scale to control the transcriptional responsiveness of the genome, thereby gating the inducibility of signal-regulated genes. This concept was first proposed in the context of drug addiction, based on an analogy between the long-lasting epigenetic control of transcriptional states during development and activity-dependent chromatin modifications in mature neurons (Nestler 2001). In recent years, it has gained increasing experimental support and has been increasingly recognized in the fields of addiction, chronic pain, and memory (Robison and Nestler 2011; Oliveira et al. 2012, 2019; Baker-Andresen et al. 2013; Descalzi et al. 2015; Cannella et al. 2018; Walker and Nestler 2018). Thus, a general functional consequence of synaptic activity-dependent gene transcription appears to be an adaptation of the transcriptional environment itself. This form of transcriptional plasticity ensures the maintenance of a permissive chromatin landscape that allows for efficient activity-induced gene expression needed for a large range of long-term neuroadaptations.
ACTIVITY-DEPENDENT METABOLIC PLASTICITY
In recent studies, synaptic activity was shown to regulate the expression of several metabolic enzymes in astrocytes and neurons. These transcriptional changes are thought to underlie long-lasting adaptations in astrocytic glycogen handling and neuronal energy metabolism. In this section, we discuss the molecular mechanisms and potential functions of this metabolic plasticity in both astrocytes and neurons.
Acute Metabolic Responses and Delayed, Transcription-Dependent Metabolic Plasticity in Astrocytes
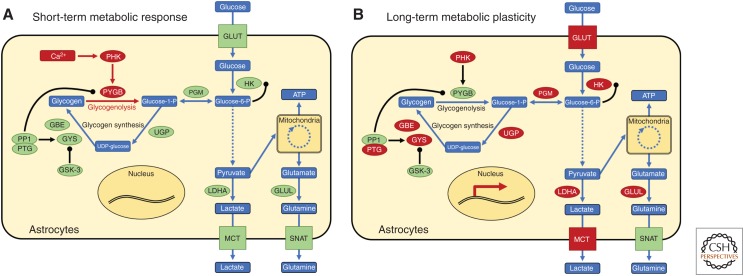
The functional contributions of astrocytes in neuroglial networks are diverse, from the regulation of ion homeostasis and neurotransmitter uptake to the stimulation of neighboring cells by so-called gliotransmitters to the modulation of synaptic structure and function and the availability of energetic substrates (Araque et al. 2014; Kim et al. 2017; Jha and Morrison 2018; Mederos et al. 2018). Accordingly, astrocyte-neuron coupling is increasingly implicated in a range of physiological and pathophysiological adaptive processes in the central nervous system, including learning and memory, drug addiction, and chronic pain, as described in detail in a number of excellent reviews (Hansen and Malcangio 2013; Ji et al. 2013; Lacagnina et al. 2017; Alberini et al. 2018; Santello et al. 2019). One critical process in astrocyte-neuron coupling is the stimulation of glycogen metabolism during and following periods of elevated neuronal activity (Hertz et al. 2015; Waitt et al. 2017; Hertz and Chen 2018). Glycogen breakdown, known as glycogenolysis, is triggered in astrocytes by intracellular calcium elevations that result from the neurotransmitter-dependent activation of G-protein-coupled receptors and the release of calcium from intracellular stores, and is promoted by elevated extracellular potassium levels (Hertz et al. 2013; Waitt et al. 2017). The rate-limiting enzyme in glycogenolysis in the central nervous system is the brain isoform of glycogen phosphorylase (PYGB), the activity of which hinges on its phosphorylation by the calcium-dependent kinase, phosphorylase kinase (PHK). Once broken down to glucose by PYGB, metabolized glycogen feeds into the glycolytic pathway resulting in the production of ATP, which is essential for maintaining the activity of sodium/potassium ATPases that normalize intra- and extracellular ion concentrations following neuronal activity. Metabolized glycogen can also be converted into glutamine, which is required as a glutamate and GABA precursor by excitatory and inhibitory neurons, respectively. Further, astrocytic glycogenolysis results in the production of lactate, which can function as a signaling molecule and possibly also an energetic substrate for neighboring neurons (Fig. 1A; Hertz et al. 2013; DiNuzzo 2016; Waitt et al. 2017; Jourdain et al. 2018; Magistretti and Allaman 2018; Margineanu et al. 2018, but see Yellen 2018; Dienel 2019). Thus, via the calcium-dependent stimulation of glycogenolysis, activation of astrocytes may alter their metabolism in the short term to meet local energy and metabolic demands under acute conditions of increased synaptic activity (Fig. 1A). This short-term astrocytic metabolic response is paralleled by the initiation of delayed transcription-dependent changes in the astrocytes’ metabolic capacity. Despite the existence, for at least 10 years, of methods that allow for cell-type-specific transcriptional analyses, and although these methods have been used to study activity-dependent gene transcription in diverse populations of neurons in response to physiological stimuli (Cahoy et al. 2008; Sanz et al. 2009; Wu et al. 2017; Sathyamurthy et al. 2018; Sloan and Barres 2018; Zeisel et al. 2018), surprisingly little is known about astrocytes’ transcriptional responses to stimuli that result in long-lasting nervous system adaptations.
Figure 1.
Short-term metabolic response and long-term metabolic plasticity in astrocytes. Neuronal activity triggers (A) a rapid, calcium-dependent metabolic response in astrocytes involving glycogenolysis and the release of energetic and signaling substrates and, in parallel, (B) a transcription-mediated up-regulation of astrocytes’ metabolic capacity (Hasel et al. 2017). In this way, astrocytes can meet local energy, homeostasis, and metabolic demands under acute conditions of increased synaptic activity, and, via changes in gene expression, provide a permissive environment for the sustained enhancement of synaptic transmission that accompanies functional adaptations. Metabolic intermediates and end points are depicted as blue rectangles, enzymes are depicted as green/red ellipses, and transporters as orange/red squares. Dashed lines indicate processes with multiple steps that are not shown here. Up-regulated activity (A) and expression (B) of enzymes and transporters are indicated with color changes to red. GBE, glycose branching enzyme; GLUL, glutamine synthetase; GLUT, glucose transporter (Slc2a family); GSK-3, glycogen synthase kinase 3; GYS, glycogen synthase; HK, hexokinase; LDHA, lactate dehydrogenase; MCT, monocarboxylate transporter (Slc16a family); PGM, phosphoglucomutase; PHK, phosphorylase kinase; PP1, protein phosphatase 1; PTG, protein targeting to glycogen; PYGB, glycogen phosphorylase brain form; SNAT, sodium-coupled neutral amino acid transporter; UGP, UDP-glucose pyrophosphorylase.
A few recent studies have begun to explore the activity-dependent astrocyte transcriptome. Notably, among the induced genes identified in these studies are a number that are implicated in astrocytic energy metabolism, including key players in glycogen synthesis and glycogenolysis (Fig. 1B; Hasel et al. 2017; Hrvatin et al. 2018; McGann and Mandel 2018). For instance, one gene showing up-regulated levels of expression following synaptic activity in vitro and neuronal activity, behavioral training, or visual stimulation in vivo was Ppp1r3c (Hasel et al. 2017; Hrvatin et al. 2018). The protein product of Ppp1r3c, protein targeting to glycogen (PTG), acts as a glycogen-targeting subunit for the protein phosphatase 1 (PP1) complex, which positively regulates glycogen synthesis by stimulating glycogen synthase (GYS), triggering glycogen production, and inhibiting glycogen phosphorylase (PYGB, limiting glycogen breakdown) (Fig. 1; Ruchti et al. 2016). Up-regulation of PTG is associated with glycogen accumulation, and its down-regulation with glycogen depletion in astrocytes (Ruchti et al. 2016). Thus, the synaptic activity-dependent up-regulation Ppp1r3c/PTG in vivo might be expected to result in a build-up of astrocytic glycogen stores. Among the other metabolism-related genes having elevated expression levels after neuronal activity were the muscle form of glycogen synthase (Gys1), which catalyzes the extension of glycogen chains by progressively adding glucose molecules and glycogen branching enzyme 1 (Gbe1), where the branches extended glycogen polymers. Also, dynamically regulated by activity was the gene coding for phosphorylase kinase β (PHKβ), a regulatory subunit of the calcium-dependent kinase that specifically activates glycogen phosphorylase (PYGB), the rate-limiting enzyme responsible for glycogenolysis, as well as several other genes that influence glucose uptake, glycolysis, and lactate export (Hasel et al. 2017). Viewed together, these data suggest that synaptic activity-evoked transcriptional changes lead to an enhancement of astrocytes’ capacity both to store glucose as glycogen, and to rapidly metabolize glycogen to meet local energy, homeostasis, and signaling demands. It will be exciting in the future to uncover the extent to which astrocytic metabolism and glycogen levels are altered following physiological and pathophysiological stimuli in vivo, and to learn what role this long-term metabolic plasticity plays in establishing a permissive environment for functional adaptations in both cerebral and spinal neuroglial networks.
In an effort to uncover the molecular mechanisms underlying the observed changes in metabolic gene expression, Hasel and colleagues analyzed the promoter regions of highly induced genes, and found that they were highly enriched for CREB-family binding sites. In parallel, the authors demonstrated that, in astrocytes, calcium rises, cAMP/PKA signaling, and CREB-mediated transcription were triggered by neuronal activity (Hasel et al. 2017; Pardo et al. 2017). Diverse stimuli and second messenger signaling cascades can induce CREB-mediated gene transcription in astrocytes, and the set of genes that is transcribed varies according to the stimulus given (Carriba et al. 2012; Karki et al. 2013; Pardo et al. 2017; Koppel et al. 2018). Thus, like neurons, it seems likely that activity-dependent astrocytic transcriptional responses may be tunable according to nature and relative strength of the stimuli these cells receive. As our collective interest in astrocytes grows, ongoing and future studies will surely reveal how physiological stimuli in vivo influence the astrocytic transcriptome, which second messenger signaling cascades underlie activity-dependent gene transcription in astrocytes, and how activity-triggered changes in the astrocytic transcriptome influence not only astrocyte cell biology, structure, and function, but also the neurons and neuronal networks with which they communicate.
Transcription-Dependent Plasticity of Neuronal Energy Metabolism
In this section, we will focus on synaptic activity- and gene transcription-dependent long-lasting changes in neuronal energy metabolism that manifest as increased glycolytic flux and decreased mitochondrial respiration. In particular, we will discuss the underlying molecular mechanisms, functional consequences, and potential physiological benefits of this form of neuronal metabolic plasticity.
Activity-Dependent Transcription of Metabolic Genes in Neurons
A series of recent publications has revealed that synaptic activity controls the neuronal expression of several genes that are involved in glycolysis and energy metabolism (Table 1; Zhang et al. 2007, 2009; Bas-Orth et al. 2017; Segarra-Mondejar et al. 2018). These genes include glucose and monocarboxylate transporters, rate-limiting enzymes of glycolysis such as hexokinase and pyruvate kinase, activators of glycolysis such as the enzyme 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 (Pfkfb3), and a potent regulator of mitochondrial energy metabolism, pyruvate dehydrogenase kinase 3 (Pdk3). Activity-dependent expression of metabolic genes was found in cultured neurons and in the mouse and rat hippocampus in vivo. Experiments in pure neuronal cultures and neuronal cultures with varying amounts of astrocytes, as well as FACS-sorted neurons from mixed neuron-glia cocultures confirmed that activity-dependent regulation of metabolic genes occurs in neurons. Analysis of transcriptome data from recent studies in human iPSC-derived neurons (Ataman et al. 2016; Qiu et al. 2016) further revealed that activity-regulated expression of key metabolic genes is evolutionarily conserved (Table 1). On a molecular level, activity-dependent expression of metabolic genes is triggered by a signaling cascade that includes synaptic NMDARs, nuclear calcium signaling, and the transcription factor CREB (Rajakumar et al. 2004; Bas-Orth et al. 2017; Segarra-Mondejar et al. 2018). Target genes of CREB include the glucose transporter Glut3 and the E3 ubiquitin ligase Siah2, which promotes stabilization of hypoxia-inducible factor (HIF), which in turn drives expression of the glycolytic genes hexokinase, pyruvate kinase, and Pfkfb3.
Table 1.
List of synaptic activity-regulated genes that promote glycolysis
| Symbol | Description/function | FC range | Species | Supported by in vivo data | Neuron-specific data available | References |
|---|---|---|---|---|---|---|
| Slc2a1 | Glucose transporter (GLUT1) | 1.2–3.4 | Hs, Mm, Rn | ✓ | Maher and Simpson 1994; Zhang et al. 2007, 2009; Tadi et al. 2015; Qiu et al. 2016; Bas-Orth et al. 2017 | |
| Slc2a3 | Glucose transporter (GLUT3) | 1.2–4.4 | Hs, Mm, Rn | ✓ | ✓ | Maher and Simpson 1994; Zhang et al. 2007, 2009; Tadi et al. 2015; Ataman et al. 2016; Qiu et al. 2016; Bas-Orth et al. 2017; Segarra-Mondejar et al. 2018 |
| Slc16a1 | Monocarboxylate transporter (MCT1) | 1.3–3.8 | Hs, Mm | ✓ | ✓ | Zhang et al. 2007, 2009; Tadi et al. 2015; Qiu et al. 2016; Bas-Orth et al. 2017 |
| Slc16a7 | Monocarboxylate transporter (MCT2) | 0.7–1.2 | Mm | ✓ | ✓ | Tadi et al. 2015; Bas-Orth et al. 2017 |
| Slc16a3 | Monocarboxylate transporter (MCT4) | 1.2–2.0 | Hs, Mm | ✓ | Tadi et al. 2015; Qiu et al. 2016; Bas-Orth et al. 2017 | |
| Hk2 | Hexokinase, converts glucose to glucose-6P | 1.3–5.9 | Hs, Mm | Qiu et al. 2016; Segarra-Mondejar et al. 2018 | ||
| Pkm | Pyruvate kinase, converts PEP to pyruvate | 1.2–1.5 | Hs, Mm | Qiu et al. 2016; Segarra-Mondejar et al. 2018 | ||
| Pdk1 | Pyruvate dehydrogenase kinase 1, inhibits PDH | 1.2–1.5 | Hs, Mm | ✓ | Tadi et al. 2015; Qiu et al. 2016 | |
| Pdk2 | Pyruvate dehydrogenase kinase 2, inhibits PDH | 0.9–1.2 | Hs, Mm | ✓ | Tadi et al. 2015; Qiu et al. 2016; Bas-Orth et al. 2017 | |
| Pdk3 | Pyruvate dehydrogenase kinase 3, inhibits PDH | 1.8–2.1 | Hs, Mm, Rn | ✓ | ✓ | Zhang et al. 2007; Tadi et al. 2015; Qiu et al. 2016; Bas-Orth et al. 2017 |
| Pdk4 | Pyruvate dehydrogenase kinase 4, inhibits PDH | 1.3–1.9 | Hs, Mm | ✓ | Tadi et al. 2015; Qiu et al. 2016 | |
| Pfkfb3 | Stimulates conversion of Fruc-6P to Fruc-1,6-BP | 1.9–2.3 | Hs, Mm | Zhang et al. 2007; Qiu et al. 2016; Bas-Orth et al. 2017; Segarra-Mondejar et al. 2018 | ||
| Atpaf1 | ATP synthase mitochondrial F1 complex assembly factor 1 | 0.6–0.9 | Hs, Mm | ✓ | ✓ | Zhang et al. 2007; Qiu et al. 2016; Bas-Orth et al. 2017 |
| Atpaf2 | ATP synthase mitochondrial F1 complex assembly factor 2 | 1.5 | Hs | Qiu et al. 2016 | ||
| Tfam | Mitochondrial transcription factor A | 0.6 | Mm | ✓ | ✓ | Zhang et al. 2007; Bas-Orth et al. 2017 |
| Tfb2m | Mitochondrial transcription factor B2 | 0.6 | Mm | ✓ | ✓ | Zhang et al. 2007; Bas-Orth et al. 2017 |
| Ucp1 | Uncoupling protein 1 | 8.2 | Mm | ✓ | Bas-Orth et al. 2017 |
FC range, range of fold changes that were observed in different studies; PDH, pyruvate dehydrogenase; PEP, phosphoenolpyruvate; Fruc-6P, fructose-6-phosphate; Fruc-1,6-BP, fructose-1,6-bisphosphate; Hs, Homo sapiens; Mm, Mus musculus; Rn, Rattus norvegicus.
Activity-Regulated Long-Lasting Changes in Neuronal Glucose Metabolism
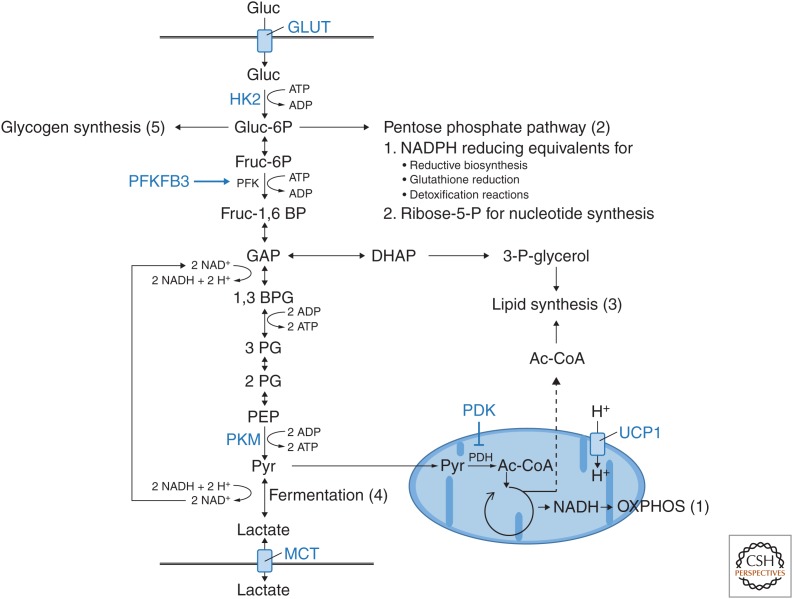
How do these changes in gene expression affect cellular energy metabolism? To address this question, we first need to revisit the different possible metabolic fates of glucose (Fig. 2). After being taken up via glucose transporters and being phosphorylated by hexokinase, glucose can be converted to pyruvate through glycolysis and used for oxidative ATP generation in mitochondria (oxidative phosphorylation [oxphos], reaction 1). A varying fraction of glucose does not enter glycolysis but instead is rerouted to the pentose phosphate pathway (PPP, reaction 2) to generate building blocks for nucleotide synthesis and NADPH for reductive biosynthesis, glutathione reduction, and detoxification reactions. Furthermore, dihydroxyacetone phosphate (DHAP) and pyruvate that are generated through glycolysis can be used for lipid synthesis instead of ATP production (reaction 3). In addition, glucose can be used for glycolytic ATP generation via lactic acid fermentation, which involves reduction of glycolysis-derived pyruvate to lactate (reaction 4). This step is required to prevent the accumulation of glycolysis-derived NADH that would otherwise inhibit glycolysis (Yellen 2018). Finally, glucose can be converted to glycogen for intracellular storage (reaction 5), a process thought to be of minor importance in neurons (Brown and Ransom 2007; Saez et al. 2014). Increased flux through reactions 2–5 results in a decreased oxygen-glucose index as more glucose is taken up by the cells than is oxidized in their mitochondria. Such nonoxidative use of glucose under normoxic conditions is commonly referred to as aerobic glycolysis. Note, however, that aerobic glycolysis can be defined in different ways. Especially in brain imaging studies, it is typically measured by comparing the rate of brain oxygen consumption (CMRO2) to the rate of brain glucose consumption (CMRgluc) (Goyal et al. 2014; Yellen 2018). In this case, any use of glucose that is not oxphos (i.e., reactions 2–5) contributes to aerobic glycolysis. In contrast, if the rate of oxygen consumption (CMRO2) is compared to the rate of lactate generation then aerobic glycolysis is defined by aerobic lactic acid fermentation also known as the Warburg effect.
Figure 2.
Synaptic activity drives the expression of key regulatory genes that control neuronal glucose metabolism. In general, glucose can be converted to pyruvate or lactate through glycolysis for oxidative (OXPHOS) or glycolytic (fermentation) ATP generation, respectively. In addition, intermediate metabolites from glycolysis can be used for redox homeostasis (2), biosynthesis of macromolecules (2, 3), and storage (5). Genes whose expression in neurons is increased upon synaptic activity are depicted in blue and include (1) transporters for glucose and lactate, (2) enzymes that increase glycolytic flux (HK2, PFKFB3, PKM), and (3) enzymes and transporters that reduce TCA cycle activity and oxidative phosphorylation. Functionally, expression of this activity-regulated gene program results in a shift of neuronal energy metabolism toward aerobic glycolysis. GLUT, Glucose transporter (Slc2a family); MCT, monocarboxylate transporter (Slc16a family); HK2, hexokinase 2; PFKFB3, 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3; PKM, pyruvate kinase M; PDK, pyruvate dehydrogenase kinase; UCP1, uncoupling protein 1.
Taking a closer look at the set of activity-regulated metabolic genes, we find that they are located at strategic positions in the metabolic network to change the balance between oxphos and lactic acid fermentation (Fig. 2). Increased expression of glucose transporters and glycolytic enzymes allows for increased uptake and turnover of glucose that can be used for storage, the PPP, lipid synthesis, and ATP production. Indeed, using stable carbon isotope tracing, a recent study revealed that neurons expressing the activity-regulated metabolic gene program take up more glucose and use its carbon atoms for lipid biosynthesis to promote neurite growth (Segarra-Mondejar et al. 2018). In addition, increased expression of PDK3 inhibits the activity of its target, pyruvate dehydrogenase (PDH), that catalyzes the conversion of pyruvate into acetyl coenzyme A. PDK3-mediated inhibition of PDH thus results in reduced rates of mitochondrial TCA cycle activity and respiration. As a result, neurons increase their rate of lactic acid fermentation to maintain ATP levels, which has been detected as increased release of lactate via monocarboxylate transporters upon activation of the metabolic gene program (Bas-Orth et al. 2017). Thus, via calcium-dependent gene transcription, synaptic activity promotes a long-lasting adaptive shift in the balance between oxphos and lactic acid fermentation, which has been described as the “neuronal Warburg effect” (Bading 2013; Bas-Orth et al. 2017). Of note, this long-lasting change is different from the acute response to neuronal activation that involves a transient increase in both respiration and glycolysis.
Potential Physiological Benefits of Activity-Dependent Aerobic Glycolysis
As discussed above, the activity-dependent expression of a metabolic gene program is conserved between mice and humans. From an evolutionary point of view, this implies that the activity-dependent shift in neuronal energy metabolism confers a specific advantage to these cells. What could this advantage be? Conceptually, the metabolic gene program can be divided into two parts. The first part, which includes the increased expression of glucose transporters and of enzymes that increase glycolytic flux, promotes a general increase in glucose turnover. The second part, which includes increased expression of PDK enzymes and lactate transporters, promotes a shift from oxphos toward lactic acid fermentation. One can easily imagine how the first part confers a benefit to cells by supporting cellular energy production, redox protection, and activity-dependent growth. In contrast, given that ATP generation via lactic acid fermentation is much less efficient than oxphos, the second part may seem disadvantageous in highly energy-demanding neurons.
Aerobic glycolysis reduces ROS generation
One likely benefit of the neuronal Warburg effect is a reduced generation of reactive oxygen species (ROS). Mitochondrial ATP generation via electron transfer and oxidative phosphorylation generates ROS as a toxic by-product (Barja 2004). Accordingly, the rate of mitochondrial respiration has been tightly linked to neuronal vulnerability. In this sense, the highly respiratory active, highly vulnerable dopaminergic neurons in the substantia nigra serve as a prime example (Pacelli et al. 2015). Of note, ROS production strongly depends on the mitochondrial membrane potential. Accordingly, mild mitochondrial uncoupling by uncoupling proteins (UCPs) in neurons was shown to reduce ROS generation and to increase neuronal resistance to oxidative stress and excitotoxic insults (Liu et al. 2006; Ramsden et al. 2012; Barnstable et al. 2016). Similarly, a reduction in mitochondrial respiration mediated by mitochondrial small conductance calcium-activated potassium channels was recently shown to reduce ROS production and to confer neuroprotection against glutamate toxicity in HT22 neuronal cells (Honrath et al. 2017), and overexpression of aerobic glycolysis-promoting enzymes PDK1 and LDHA in the B12 neuronal cell line conferred protection against amyloid β toxicity (Newington et al. 2012).
Aerobic glycolysis supports fast and local ATP supply
Although mitochondria can generate large amounts of ATP from glucose, due to their size they cannot reach small neuronal subcompartments such as dendritic spines or small presynaptic terminals. Also, while mitochondrial ATP generation is highly efficient, it is much slower than glycolytic ATP generation (Pfeiffer et al. 2001). This might explain why even in larger presynaptic terminals that contain mitochondria, glycolysis is required to maintain ATP levels during high-frequency neuronal activity (Rangaraju et al. 2014; Jang et al. 2016). Thus, ATP generation via aerobic glycolysis appears to be especially well suited as a fast-acting, local energy source.
Aerobic glycolysis increases bioenergetic robustness
Increased levels of PDK enzymes will increase the phosphorylation of PDH and will thus reduce the activity of PDH and the TCA cycle under basal conditions. This does not mean, however, that mitochondrial respiration is permanently disabled. Rather, the rise of mitochondrial calcium levels that is associated with acute neuronal activation will lead to rapid dephosphorylation and thus stimulation of PDH by calcium-dependent PDH phosphatases (Glancy and Balaban 2012). Thus, by tuning down basal mitochondrial respiration in a reversible manner, the neuronal Warburg effect might increase mitochondrial spare respiratory capacity, which would allow the cells to better respond to transient elevations of energy demand. ATP generation via aerobic glycolysis has the additional advantage of rendering neurons less dependent on mitochondrial function. Treatment of cultured neurons with the mitochondrial uncoupler CCCP results in a drastic loss of cellular ATP levels. This loss, however, is much attenuated if neurons have experienced an episode of action potential bursting before the onset of CCCP treatment (Segarra-Mondejar et al. 2018). Although neurons still lose considerable amounts of ATP in the latter case, these experiments suggest that they can better tolerate an impairment of mitochondrial function if they have previously undergone a switch to aerobic glycolysis. Thus, by providing neurons with increased mitochondrial spare respiratory capacity and an increased capacity for fermentation-based ATP generation, the adaptive metabolic shift increases the bioenergetic robustness of neurons.
Taken together, enhanced bioenergetic robustness, improved local ATP supply and protection from ROS might all contribute to enhanced neuronal resilience against stressful conditions. This view is supported by a series of studies in which a pharmacologically or genetically induced metabolic switch toward aerobic glycolysis provided neuroprotection in models of mitochondrial stress, glutamate excitotoxicity, amyloid β toxicity, and Leigh syndrome (Liu et al. 2006; Newington et al. 2012; Jain et al. 2016; Sun et al. 2016; Ferrari et al. 2017).
CONCLUDING REMARKS
Synaptic activity-dependent calcium rises trigger the activation of diverse signaling cascades and second messengers that converge in the nucleus to regulate gene transcription. Calcium-dependent transcriptional responses, in turn, regulate a wide range of processes in the central nervous system (CNS) and are intricately linked to both physiological and pathophysiological neuroadaptations. Astrocytic and neuronal metabolism are gaining recognition as plastic processes subject to control by activity-dependent gene transcription. We anticipate that ongoing and future studies will delineate the prevalence, cellular and subcellular localization, and precise involvement of metabolic plasticity in functional neuroadaptations ranging from learning and memory to chronic pain.
ACKNOWLEDGMENTS
This work was supported by the Deutsche Forschungsgemeinschaft (DFG) (SFB1158, A05; BA1007/7-1; FOR2289; BA1007/9-1; FOR2289; BA3679/4-2).
Footnotes
Editors: Geert Bultynck, Martin D. Bootman, Michael J. Berridge, and Grace E. Stutzmann
Additional Perspectives on Calcium Signaling available at www.cshperspectives.org
REFERENCES
- Alberini CM, Cruz E, Descalzi G, Bessières B, Gao V. 2018. Astrocyte glycogen and lactate: New insights into learning and memory mechanisms. Glia 66: 1244–1262. 10.1002/glia.23250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A, Carmignoto G, Haydon PG, Oliet SH, Robitaille R, Volterra A. 2014. Gliotransmitters travel in time and space. Neuron 81: 728–739. 10.1016/j.neuron.2014.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ataman B, Boulting GL, Harmin DA, Yang MG, Baker-Salisbury M, Yap EL, Malik AN, Mei K, Rubin AA, Spiegel I, et al. 2016. Evolution of Osteocrin as an activity-regulated factor in the primate brain. Nature 539: 242–247. 10.1038/nature20111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bading H. 2013. Nuclear calcium signalling in the regulation of brain function. Nat Rev Neurosci 14: 593–608. 10.1038/nrn3531 [DOI] [PubMed] [Google Scholar]
- Bading H. 2017. Therapeutic targeting of the pathological triad of extrasynaptic NMDA receptor signaling in neurodegenerations. J Exp Med 214: 569–578. 10.1084/jem.20161673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bading H, Ginty DD, Greenberg ME. 1993. Regulation of gene expression in hippocampal neurons by distinct calcium signaling pathways. Science 260: 181–186. 10.1126/science.8097060 [DOI] [PubMed] [Google Scholar]
- Bai G, Wei D, Zou S, Ren K, Dubner R. 2010. Inhibition of class II histone deacetylases in the spinal cord attenuates inflammatory hyperalgesia. Mol Pain 6: 51 10.1186/1744-8069-6-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker-Andresen D, Ratnu VS, Bredy TW. 2013. Dynamic DNA methylation: A prime candidate for genomic metaplasticity and behavioral adaptation. Trends Neurosci 36: 3–13. 10.1016/j.tins.2012.09.003 [DOI] [PubMed] [Google Scholar]
- Barja G. 2004. Free radicals and aging. Trends Neurosci 27: 595–600. 10.1016/j.tins.2004.07.005 [DOI] [PubMed] [Google Scholar]
- Barnstable CJ, Reddy R, Li H, Horvath TL. 2016. Mitochondrial uncoupling protein 2 (UCP2) regulates retinal ganglion cell number and survival. J Mol Neurosci 58: 461–469. 10.1007/s12031-016-0728-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bas-Orth C, Bading H. 2013. The divergence-convergence model of acquired neuroprotection. Mech Dev 130: 396–401. 10.1016/j.mod.2012.09.008 [DOI] [PubMed] [Google Scholar]
- Bas-Orth C, Tan YW, Oliveira AM, Bengtson CP, Bading H. 2016. The calmodulin-binding transcription activator CAMTA1 is required for long-term memory formation in mice. Learn Mem 23: 313–321. 10.1101/lm.041111.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bas-Orth C, Tan YW, Lau D, Bading H. 2017. Synaptic activity drives a genomic program that promotes a neuronal Warburg effect. J Biol Chem 292: 5183–5194. 10.1074/jbc.M116.761106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter PS, Bell KF, Hasel P, Kaindl AM, Fricker M, Thomson D, Cregan SP, Gillingwater TH, Hardingham GE. 2015. Synaptic NMDA receptor activity is coupled to the transcriptional control of the glutathione system. Nat Commun 6: 6761 10.1038/ncomms7761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtson CP, Bading H. 2012. Nuclear calcium signaling. Adv Exp Med Biol 970: 377–405. 10.1007/978-3-7091-0932-8_17 [DOI] [PubMed] [Google Scholar]
- Bengtson CP, Freitag HE, Weislogel JM, Bading H. 2010. Nuclear calcium sensors reveal that repetition of trains of synaptic stimuli boosts nuclear calcium signaling in CA1 pyramidal neurons. Biophys J 99: 4066–4077. 10.1016/j.bpj.2010.10.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtson CP, Kaiser M, Obermayer J, Bading H. 2013. Calcium responses to synaptically activated bursts of action potentials and their synapse-independent replay in cultured networks of hippocampal neurons. Biochim Biophys Acta 1833: 1672–1679. 10.1016/j.bbamcr.2013.01.022 [DOI] [PubMed] [Google Scholar]
- Birey F, Kokkosis AG, Aguirre A. 2017. Oligodendroglia-lineage cells in brain plasticity, homeostasis and psychiatric disorders. Curr Opin Neurobiol 47: 93–103. 10.1016/j.conb.2017.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bito H, Deisseroth K, Tsien RW. 1996. CREB phosphorylation and dephosphorylation: A Ca2+- and stimulus duration-dependent switch for hippocampal gene expression. Cell 87: 1203–1214. 10.1016/S0092-8674(00)81816-4 [DOI] [PubMed] [Google Scholar]
- Bloodgood BL, Sharma N, Browne HA, Trepman AZ, Greenberg ME. 2013. The activity-dependent transcription factor NPAS4 regulates domain-specific inhibition. Nature 503: 121–125. 10.1038/nature12743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bootman MD, Fearnley C, Smyrnias I, Macdonald F, Roderick HL. 2009. An update on nuclear calcium signalling. J Cell Sci 122: 2337–2350. 10.1242/jcs.028100 [DOI] [PubMed] [Google Scholar]
- Bourtchuladze R, Frenguelli B, Blendy J, Cioffi D, Schutz G, Silva AJ. 1994. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell 79: 59–68. 10.1016/0092-8674(94)90400-6 [DOI] [PubMed] [Google Scholar]
- Brown AM, Ransom BR. 2007. Astrocyte glycogen and brain energy metabolism. Glia 55: 1263–1271. 10.1002/glia.20557 [DOI] [PubMed] [Google Scholar]
- Buchthal B, Lau D, Weiss U, Weislogel JM, Bading H. 2012. Nuclear calcium signaling controls methyl-CpG-binding protein 2 (MeCP2) phosphorylation on serine 421 following synaptic activity. J Biol Chem 287: 30967–30974. 10.1074/jbc.M112.382507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchthal B, Weiss U, Bading H. 2018. Post-injury nose-to-brain delivery of activin A and SerpinB2 reduces brain damage in a mouse stroke model. Mol Ther 26: 2357–2365. 10.1016/j.ymthe.2018.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne RD, Haynes LP. 2012. Understanding the physiological roles of the neuronal calcium sensor proteins. Mol Brain 5: 2 10.1186/1756-6606-5-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G, Draguhn A. 2004. Neuronal oscillations in cortical networks. Science 304: 1926–1929. 10.1126/science.1099745 [DOI] [PubMed] [Google Scholar]
- Cahill E, Salery M, Vanhoutte P, Caboche J. 2014. Convergence of dopamine and glutamate signaling onto striatal ERK activation in response to drugs of abuse. Front Pharmacol 4: 172 10.3389/fphar.2013.00172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, et al. 2008. A transcriptome database for astrocytes, neurons, and oligodendrocytes: A new resource for understanding brain development and function. J Neurosci 28: 264–278. 10.1523/jneurosci.4178-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannella N, Oliveira AMM, Hemstedt T, Lissek T, Buechler E, Bading H, Spanagel R. 2018. Dnmt3a2 in the nucleus accumbens shell is required for reinstatement of cocaine seeking. J Neurosci 38: 7516–7528. 10.1523/jneurosci.0600-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carriba P, Pardo L, Parra-Damas A, Lichtenstein MP, Saura CA, Pujol A, Masgrau R, Galea E. 2012. ATP and noradrenaline activate CREB in astrocytes via noncanonical Ca2+ and cyclic AMP independent pathways. Glia 60: 1330–1344. 10.1002/glia.22352 [DOI] [PubMed] [Google Scholar]
- Carrion AM, Link WA, Ledo F, Mellstrom B, Naranjo JR. 1999. DREAM is a Ca2+-regulated transcriptional repressor. Nature 398: 80–84. 10.1038/18044 [DOI] [PubMed] [Google Scholar]
- Chandrasekar A, Olde Heuvel F, Tar L, Hagenston AM, Palmer A, Linkus B, Ludolph AC, Huber-Lang M, Boeckers T, Bading H, et al. 2018. Parvalbumin interneurons shape neuronal vulnerability in blunt TBI. Cereb Cortex 29: 2701–2715. 10.1093/cercor/bhy139 [DOI] [PubMed] [Google Scholar]
- Chawla S, Bading H. 2001. CREB/CBP and SRE-interacting transcriptional regulators are fast on-off switches: Duration of calcium transients specifies the magnitude of transcriptional responses. J Neurochem 79: 849–858. 10.1046/j.1471-4159.2001.00645.x [DOI] [PubMed] [Google Scholar]
- Chawla S, Hardingham GE, Quinn DR, Bading H. 1998. CBP: A signal-regulated transcriptional coactivator controlled by nuclear calcium and CaM kinase IV. Science 281: 1505–1509. 10.1126/science.281.5382.1505 [DOI] [PubMed] [Google Scholar]
- Chawla S, Vanhoutte P, Arnold FJ, Huang CL, Bading H. 2003. Neuronal activity-dependent nucleocytoplasmic shuttling of HDAC4 and HDAC5. J Neurochem 85: 151–159. 10.1046/j.1471-4159.2003.01648.x [DOI] [PubMed] [Google Scholar]
- Chen Y, Wang Y, Modrusan Z, Sheng M, Kaminker JS. 2014. Regulation of neuronal gene expression and survival by basal NMDA receptor activity: A role for histone deacetylase 4. J Neurosci 34: 15327–15339. 10.1523/jneurosci.0569-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng HY, Pitcher GM, Laviolette SR, Whishaw IQ, Tong KI, Kockeritz LK, Wada T, Joza NA, Crackower M, Goncalves J, et al. 2002. DREAM is a critical transcriptional repressor for pain modulation. Cell 108: 31–43. 10.1016/S0092-8674(01)00629-8 [DOI] [PubMed] [Google Scholar]
- Cohen SM, Li B, Tsien RW, Ma H. 2015. Evolutionary and functional perspectives on signaling from neuronal surface to nucleus. Biochem Biophys Res Commun 460: 88–99. 10.1016/j.bbrc.2015.02.146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SM, Ma H, Kuchibhotla KV, Watson BO, Buzsáki G, Froemke RC, Tsien RW. 2016. Excitation-transcription coupling in parvalbumin-positive interneurons employs a novel CaM kinase-dependent pathway distinct from excitatory neurons. Neuron 90: 292–307. 10.1016/j.neuron.2016.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SM, Suutari B, He X, Wang Y, Sanchez S, Tirko NN, Mandelberg NJ, Mullins C, Zhou G, Wang S, et al. 2018. Calmodulin shuttling mediates cytonuclear signaling to trigger experience-dependent transcription and memory. Nat Commun 9: 2451 10.1038/s41467-018-04705-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costigan M, Woolf CJ. 2002. No DREAM, no pain. Closing the spinal gate. Cell 108: 297–300. 10.1016/S0092-8674(02)00640-2 [DOI] [PubMed] [Google Scholar]
- Coutellier L, Beraki S, Ardestani PM, Saw NL, Shamloo M. 2012. Npas4: A neuronal transcription factor with a key role in social and cognitive functions relevant to developmental disorders. PLoS ONE 7: e46604 10.1371/journal.pone.0046604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash PK, Hochner B, Kandel ER. 1990. Injection of the cAMP-responsive element into the nucleus of Aplysia sensory neurons blocks long-term facilitation. Nature 345: 718–721. 10.1038/345718a0 [DOI] [PubMed] [Google Scholar]
- Dayan E, Cohen LG. 2011. Neuroplasticity subserving motor skill learning. Neuron 72: 443–454. 10.1016/j.neuron.2011.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debanne D, Inglebert Y, Russier M. 2019. Plasticity of intrinsic neuronal excitability. Curr Opin Neurobiol 54: 73–82. 10.1016/j.conb.2018.09.001 [DOI] [PubMed] [Google Scholar]
- Depp C, Bas-Orth C, Schroeder L, Hellwig A, Bading H. 2018. Synaptic activity protects neurons against calcium-mediated oxidation and contraction of mitochondria during excitotoxicity. Antioxid Redox Signal 29: 1109–1124. 10.1089/ars.2017.7092 [DOI] [PubMed] [Google Scholar]
- Descalzi G, Ikegami D, Ushijima T, Nestler EJ, Zachariou V, Narita M. 2015. Epigenetic mechanisms of chronic pain. Trends Neurosci 38: 237–246. 10.1016/j.tins.2015.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick O, Bading H. 2010. Synaptic activity and nuclear calcium signaling protect hippocampal neurons from death signal-associated nuclear translocation of FoxO3a induced by extrasynaptic N-methyl-d-aspartate receptors. J Biol Chem 285: 19354–19361. 10.1074/jbc.M110.127654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienel GA. 2019. Brain glucose metabolism: Integration of energetics with function. Physiol Rev 99: 949–1045. 10.1152/physrev.00062.2017 [DOI] [PubMed] [Google Scholar]
- DiNuzzo M. 2016. Astrocyte-neuron interactions during learning may occur by lactate signaling rather than metabolism. Front Integr Neurosci 10: 2 10.3389/fnint.2016.00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echevarría W, Leite MF, Guerra MT, Zipfel WR, Nathanson MH. 2003. Regulation of calcium signals in the nucleus by a nucleoplasmic reticulum. Nat Cell Biol 5: 440–446. 10.1038/ncb980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eder A, Bading H. 2007. Calcium signals can freely cross the nuclear envelope in hippocampal neurons: Somatic calcium increases generate nuclear calcium transients. BMC Neurosci 8: 57 10.1186/1471-2202-8-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Alarcón JM, Weisberg SP, Touzani K, Huang YY, Nordheim A, Kandel ER. 2006. A role in learning for SRF: Deletion in the adult forebrain disrupts LTD and the formation of an immediate memory of a novel context. Neuron 50: 127–143. 10.1016/j.neuron.2006.03.013 [DOI] [PubMed] [Google Scholar]
- Ferrari M, Jain IH, Goldberger O, Rezoagli E, Thoonen R, Cheng KH, Sosnovik DE, Scherrer-Crosbie M, Mootha VK, Zapol WM. 2017. Hypoxia treatment reverses neurodegenerative disease in a mouse model of Leigh syndrome. Proc Natl Acad Sci 114: E4241–E4250. 10.1073/pnas.1621511114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkler A, Ashery-Padan R, Fromm H. 2007. CAMTAs: Calmodulin-binding transcription activators from plants to human. FEBS Lett 581: 3893–3898. 10.1016/j.febslet.2007.07.051 [DOI] [PubMed] [Google Scholar]
- Flavell SW, Greenberg ME. 2008. Signaling mechanisms linking neuronal activity to gene expression and plasticity of the nervous system. Annu Rev Neurosci 31: 563–590. 10.1146/annurev.neuro.31.060407.125631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell SW, Cowan CW, Kim TK, Greer PL, Lin Y, Paradis S, Griffith EC, Hu LS, Chen C, Greenberg ME. 2006. Activity-dependent regulation of MEF2 transcription factors suppresses excitatory synapse number. Science 311: 1008–1012. 10.1126/science.1122511 [DOI] [PubMed] [Google Scholar]
- Glancy B, Balaban RS. 2012. Role of mitochondrial Ca2+ in the regulation of cellular energetics. Biochemistry 51: 2959–2973. 10.1021/bi2018909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal MS, Hawrylycz M, Miller JA, Snyder AZ, Raichle ME. 2014. Aerobic glycolysis in the human brain is associated with development and neotenous gene expression. Cell Metab 19: 49–57. 10.1016/j.cmet.2013.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg ME, Ziff EB, Greene LA. 1986. Stimulation of neuronal acetylcholine receptors induces rapid gene transcription. Science 234: 80–83. 10.1126/science.3749894 [DOI] [PubMed] [Google Scholar]
- Greer PL, Greenberg ME. 2008. From synapse to nucleus: Calcium-dependent gene transcription in the control of synapse development and function. Neuron 59: 846–860. 10.1016/j.neuron.2008.09.002 [DOI] [PubMed] [Google Scholar]
- Gulmez Karaca K, Brito DVC, Zeuch B, Oliveira AMM. 2018. Adult hippocampal MeCP2 preserves the genomic responsiveness to learning required for long-term memory formation. Neurobiol Learn Mem 149: 84–97. 10.1016/j.nlm.2018.02.010 [DOI] [PubMed] [Google Scholar]
- Hagenston AM, Bading H. 2011. Calcium signaling in synapse-to-nucleus communication. Cold Spring Harb Perspect Biol 3: a004564 10.1101/cshperspect.a004564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenston AM, Simonetti M. 2014. Neuronal calcium signaling in chronic pain. Cell Tissue Res 357: 407–426. 10.1007/s00441-014-1942-5 [DOI] [PubMed] [Google Scholar]
- Hagenston AM, Fitzpatrick JS, Yeckel MF. 2008. MGluR-mediated calcium waves that invade the soma regulate firing in layer V medial prefrontal cortical pyramidal neurons. Cereb Cortex 18: 407–423. 10.1093/cercor/bhm075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannan AJ. 2014. Environmental enrichment and brain repair: Harnessing the therapeutic effects of cognitive stimulation and physical activity to enhance experience-dependent plasticity. Neuropathol Appl Neurobiol 40: 13–25. 10.1111/nan.12102 [DOI] [PubMed] [Google Scholar]
- Hansen RR, Malcangio M. 2013. Astrocytes–multitaskers in chronic pain. Eur J Pharmacol 716: 120–128. 10.1016/j.ejphar.2013.03.023 [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Chawla S, Johnson CM, Bading H. 1997. Distinct functions of nuclear and cytoplasmic calcium in the control of gene expression. Nature 385: 260–265. 10.1038/385260a0 [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Arnold FJ, Bading H. 2001. Nuclear calcium signaling controls CREB-mediated gene expression triggered by synaptic activity. Nat Neurosci 4: 261–267. 10.1038/85109 [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Fukunaga Y, Bading H. 2002. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat Neurosci 5: 405–414. 10.1038/nn835 [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Pruunsild P, Greenberg ME, Bading H. 2018. Lineage divergence of activity-driven transcription and evolution of cognitive ability. Nat Rev Neurosci 19: 9–15. 10.1038/nrn.2017.138 [DOI] [PubMed] [Google Scholar]
- Hasel P, Dando O, Jiwaji Z, Baxter P, Todd AC, Heron S, Markus NM, McQueen J, Hampton DW, Torvell M, et al. 2017. Neurons and neuronal activity control gene expression in astrocytes to regulate their development and metabolism. Nat Commun 8: 15132 10.1038/ncomms15132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemstedt TJ, Bengtson CP, Ramírez O, Oliveira AMM, Bading H. 2017. Reciprocal interaction of dendrite geometry and nuclear calcium-VEGFD signaling gates memory consolidation and extinction. J Neurosci 37: 6946–6955. 10.1523/jneurosci.2345-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst WA, Martin KC. 2017. Regulated transport of signaling proteins from synapse to nucleus. Curr Opin Neurobiol 45: 78–84. 10.1016/j.conb.2017.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez PJ, Abel T. 2008. The role of protein synthesis in memory consolidation: Progress amid decades of debate. Neurobiol Learn Mem 89: 293–311. 10.1016/j.nlm.2007.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz L, Chen Y. 2018. Glycogenolysis, an astrocyte-specific reaction, is essential for both astrocytic and neuronal activities involved in learning. Neuroscience 370: 27–36. 10.1016/j.neuroscience.2017.06.025 [DOI] [PubMed] [Google Scholar]
- Hertz L, Xu J, Song D, Du T, Yan E, Peng L. 2013. Brain glycogenolysis, adrenoceptors, pyruvate carboxylase, Na+,K+-ATPase and Marie E. Gibbs’ pioneering learning studies. Front Integr Neurosci 7: 20 10.3389/fnint.2013.00020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz L, Xu J, Song D, Du T, Li B, Yan E, Peng L. 2015. Astrocytic glycogenolysis: Mechanisms and functions. Metab Brain Dis 30: 317–333. 10.1007/s11011-014-9536-1 [DOI] [PubMed] [Google Scholar]
- Holtmaat A, Caroni P. 2016. Functional and structural underpinnings of neuronal assembly formation in learning. Nat Neurosci 19: 1553–1562. 10.1038/nn.4418 [DOI] [PubMed] [Google Scholar]
- Honrath B, Matschke L, Meyer T, Magerhans L, Perocchi F, Ganjam GK, Zischka H, Krasel C, Gerding A, Bakker BM, et al. 2017. SK2 channels regulate mitochondrial respiration and mitochondrial Ca2+ uptake. Cell Death Differ 24: 761–773. 10.1038/cdd.2017.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf FW. 2017. Do specific NMDA receptor subunits act as gateways for addictive behaviors? Genes Brain Behav 16: 118–138. 10.1111/gbb.12348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrvatin S, Hochbaum DR, Nagy MA, Cicconet M, Robertson K, Cheadle L, Zilionis R, Ratner A, Borges-Monroy R, Klein AM, et al. 2018. Single-cell analysis of experience-dependent transcriptomic states in the mouse visual cortex. Nat Neurosci 21: 120–129. 10.1038/s41593-017-0029-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huentelman MJ, Papassotiropoulos A, Craig DW, Hoerndli FJ, Pearson JV, Huynh KD, Corneveaux J, Hanggi J, Mondadori CR, Buchmann A, et al. 2007. Calmodulin-binding transcription activator 1 (CAMTA1) alleles predispose human episodic memory performance. Hum Mol Genet 16: 1469–1477. 10.1093/hmg/ddm097 [DOI] [PubMed] [Google Scholar]
- Hui L, Geiger NH, Bloor-Young D, Churchill GC, Geiger JD, Chen X. 2015. Release of calcium from endolysosomes increases calcium influx through N-type calcium channels: Evidence for acidic store-operated calcium entry in neurons. Cell Calcium 58: 617–627. 10.1016/j.ceca.2015.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humbert JP, Matter N, Artault JC, Köppler P, Malviya AN. 1996. Inositol 1,4,5-trisphosphate receptor is located to the inner nuclear membrane vindicating regulation of nuclear calcium signaling by inositol 1,4,5-trisphosphate. Discrete distribution of inositol phosphate receptors to inner and outer nuclear membranes. J Biol Chem 271: 478–485. 10.1074/jbc.271.1.478 [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. 2006. Neural mechanisms of addiction: The role of reward-related learning and memory. Annu Rev Neurosci 29: 565–598. 10.1146/annurev.neuro.29.051605.113009 [DOI] [PubMed] [Google Scholar]
- Jain IH, Zazzeron L, Goli R, Alexa K, Schatzman-Bone S, Dhillon H, Goldberger O, Peng J, Shalem O, Sanjana NE, et al. 2016. Hypoxia as a therapy for mitochondrial disease. Science 352: 54–61. 10.1126/science.aad9642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S, Nelson JC, Bend EG, Rodriguez-Laureano L, Tueros FG, Cartagenova L, Underwood K, Jorgensen EM, Colon-Ramos DA. 2016. Glycolytic enzymes localize to synapses under energy stress to support synaptic function. Neuron 90: 278–291. 10.1016/j.neuron.2016.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha MK, Morrison BM. 2018. Glia-neuron energy metabolism in health and diseases: New insights into the role of nervous system metabolic transporters. Exp Neurol 309: 23–31. 10.1016/j.expneurol.2018.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen KF, Ohmstede CA, Fisher RS, Sahyoun N. 1991. Nuclear and axonal localization of Ca2+/calmodulin-dependent protein kinase type Gr in rat cerebellar cortex. Proc Natl Acad Sci 88: 2850–2853. 10.1073/pnas.88.7.2850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji RR, Kohno T, Moore KA, Woolf CJ. 2003. Central sensitization and LTP: Do pain and memory share similar mechanisms? Trends Neurosci 26: 696–705. 10.1016/j.tins.2003.09.017 [DOI] [PubMed] [Google Scholar]
- Ji R-R, Berta T, Nedergaard M. 2013. Glia and pain: Is chronic pain a gliopathy? Pain 154: S10–S28. 10.1016/j.pain.2013.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jong YJ, Kumar V, Kingston AE, Romano C, O'Malley KL. 2005. Functional metabotropic glutamate receptors on nuclei from brain and primary cultured striatal neurons. Role of transporters in delivering ligand. J Biol Chem 280: 30469–30480. 10.1074/jbc.M501775200 [DOI] [PubMed] [Google Scholar]
- Jong YJ, Schwetye KE, O'Malley KL. 2007. Nuclear localization of functional metabotropic glutamate receptor mGlu1 in HEK293 cells and cortical neurons: Role in nuclear calcium mobilization and development. J Neurochem 101: 458–469. 10.1111/j.1471-4159.2006.04382.x [DOI] [PubMed] [Google Scholar]
- Jong YI, Harmon SK, O'Malley KL. 2018. GPCR signalling from within the cell. Br J Pharmacol 175: 4026–4035. 10.1111/bph.14023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdain P, Rothenfusser K, Ben-Adiba C, Allaman I, Marquet P, Magistretti PJ. 2018. Dual action of L-lactate on the activity of NR2B-containing NMDA receptors: From potentiation to neuroprotection. Sci Rep 8: 13472 10.1038/s41598-018-31534-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaller MS, Lazari A, Blanco-Duque C, Sampaio-Baptista C, Johansen-Berg H. 2017. Myelin plasticity and behaviour-connecting the dots. Curr Opin Neurobiol 47: 86–92. 10.1016/j.conb.2017.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H, Sun LD, Atkins CM, Soderling TR, Wilson MA, Tonegawa S. 2001. An important role of neural activity-dependent CaMKIV signaling in the consolidation of long-term memory. Cell 106: 771–783. 10.1016/S0092-8674(01)00497-4 [DOI] [PubMed] [Google Scholar]
- Karki P, Webb A, Smith K, Lee K, Son DS, Aschner M, Lee E. 2013. cAMP response element-binding protein (CREB) and nuclear factor κB mediate the tamoxifen-induced up-regulation of glutamate transporter 1 (GLT-1) in rat astrocytes. J Biol Chem 288: 28975–28986. 10.1074/jbc.M113.483826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC. 2007. Synaptic plasticity and addiction. Nat Rev Neurosci 8: 844–858. 10.1038/nrn2234 [DOI] [PubMed] [Google Scholar]
- Kida S, Josselyn SA, Pena de Ortiz S, Kogan JH, Chevere I, Masushige S, Silva AJ. 2002. CREB required for the stability of new and reactivated fear memories. Nat Neurosci 5: 348–355. 10.1038/nn819 [DOI] [PubMed] [Google Scholar]
- Kim SK, Nabekura J, Koizumi S. 2017. Astrocyte-mediated synapse remodeling in the pathological brain. Glia 65: 1719–1727. 10.1002/glia.23169 [DOI] [PubMed] [Google Scholar]
- Konradi C, Leveque JC, Hyman SE. 1996. Amphetamine and dopamine-induced immediate early gene expression in striatal neurons depends on postsynaptic NMDA receptors and calcium. J Neurosci 16: 4231–4239. 10.1523/jneurosci.16-13-04231.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppel I, Jaanson K, Klasche A, Tuvikene J, Tiirik T, Pärn A, Timmusk T. 2018. Dopamine cross-reacts with adrenoreceptors in cortical astrocytes to induce BDNF expression, CREB signaling and morphological transformation. Glia 66: 206–216. 10.1002/glia.23238 [DOI] [PubMed] [Google Scholar]
- Kumar V, Jong YJ, O'Malley KL. 2008. Activated nuclear metabotropic glutamate receptor mGlu5 couples to nuclear Gq/11 proteins to generate inositol 1,4,5-trisphosphate-mediated nuclear Ca2+ release. J Biol Chem 283: 14072–14083. 10.1074/jbc.M708551200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuner R. 2010. Central mechanisms of pathological pain. Nat Med 16: 1258–1266. 10.1038/nm.2231 [DOI] [PubMed] [Google Scholar]
- Lacagnina MJ, Rivera PD, Bilbo SD. 2017. Glial and neuroimmune mechanisms as critical modulators of drug use and abuse. Neuropsychopharmacology 42: 156–177. 10.1038/npp.2016.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacar B, Linker SB, Jaeger BN, Krishnaswami SR, Barron JJ, Kelder MJE, Parylak SL, Paquola ACM, Venepally P, Novotny M, et al. 2016. Nuclear RNA-seq of single neurons reveals molecular signatures of activation. Nat Commun 7: 11022 10.1038/ncomms11022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamsa K, Lau P. 2019. Long-term plasticity of hippocampal interneurons during in vivo memory processes. Curr Opin Neurobiol 54: 20–27. 10.1016/j.conb.2018.08.006 [DOI] [PubMed] [Google Scholar]
- Lau D, Bading H. 2009. Synaptic activity-mediated suppression of p53 and induction of nuclear calcium-regulated neuroprotective genes promote survival through inhibition of mitochondrial permeability transition. J Neurosci 29: 4420–4429. 10.1523/jneurosci.0802-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau D, Bengtson CP, Buchthal B, Bading H. 2015. BDNF reduces toxic extrasynaptic NMDA receptor signaling via synaptic NMDA receptors and nuclear-calcium-induced transcription of inhba/activin A. Cell Rep 12: 1353–1366. 10.1016/j.celrep.2015.07.038 [DOI] [PubMed] [Google Scholar]
- Lee B, Butcher GQ, Hoyt KR, Impey S, Obrietan K. 2005. Activity-dependent neuroprotection and cAMP response element-binding protein (CREB): Kinase coupling, stimulus intensity, and temporal regulation of CREB phosphorylation at serine 133. J Neurosci 25: 1137–1148. 10.1523/jneurosci.4288-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leveille F, Papadia S, Fricker M, Bell KF, Soriano FX, Martel MA, Puddifoot C, Habel M, Wyllie DJ, Ikonomidou C, et al. 2010. Suppression of the intrinsic apoptosis pathway by synaptic activity. J Neurosci 30: 2623–2635. 10.1523/jneurosci.5115-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limback-Stokin K, Korzus E, Nagaoka-Yasuda R, Mayford M. 2004. Nuclear calcium/calmodulin regulates memory consolidation. J Neurosci 24: 10858–10867. 10.1523/jneurosci.1022-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Bloodgood BL, Hauser JL, Lapan AD, Koon AC, Kim TK, Hu LS, Malik AN, Greenberg ME. 2008. Activity-dependent regulation of inhibitory synapse development by Npas4. Nature 455: 1198–1204. 10.1038/nature07319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J, Cooper K, Sehgal M, Silva AJ. 2018. Memory formation depends on both synapse-specific modifications of synaptic strength and cell-specific increases in excitability. Nat Neurosci 21: 309–314. 10.1038/s41593-018-0076-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litke C, Bading H, Mauceri D. 2018. Histone deacetylase 4 shapes neuronal morphology via a mechanism involving regulation of expression of vascular endothelial growth factor D. J Biol Chem 293: 8196–8207. 10.1074/jbc.RA117.001613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Chan SL, de Souza-Pinto NC, Slevin JR, Wersto RP, Zhan M, Mustafa K, de Cabo R, Mattson MP. 2006. Mitochondrial UCP4 mediates an adaptive shift in energy metabolism and increases the resistance of neurons to metabolic and oxidative stress. Neuromolecular Med 8: 389–414. 10.1385/NMM:8:3:389 [DOI] [PubMed] [Google Scholar]
- Long I, Suppian R, Ismail Z. 2011. Increases in mRNA and DREAM protein expression in the rat spinal cord after formalin induced pain. Neurochem Res 36: 533–539. 10.1007/s11064-010-0375-0 [DOI] [PubMed] [Google Scholar]
- López-Hurtado A, Burgos DF, Gonzalez P, Dopazo XM, Gonzalez V, Rabano A, Mellstrom B, Naranjo JR. 2018. Inhibition of DREAM-ATF6 interaction delays onset of cognition deficit in a mouse model of Huntington's disease. Mol Brain 11: 13 10.1186/s13041-018-0359-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Cohen S, Li B, Tsien RW. 2012. Exploring the dominant role of Cav1 channels in signalling to the nucleus. Biosci Rep 33: 97–101. 10.1042/bsr20120099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Groth RD, Cohen SM, Emery JF, Li B, Hoedt E, Zhang G, Neubert TA, Tsien RW. 2014. γCaMKII shuttles Ca2+/CaM to the nucleus to trigger CREB phosphorylation and gene expression. Cell 159: 281–294. 10.1016/j.cell.2014.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madabhushi R, Gao F, Pfenning AR, Pan L, Yamakawa S, Seo J, Rueda R, Phan TX, Yamakawa H, Pao PC, et al. 2015. Activity-induced DNA breaks govern the expression of neuronal early-response genes. Cell 161: 1592–1605. 10.1016/j.cell.2015.05.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magistretti PJ, Allaman I. 2018. Lactate in the brain: From metabolic end-product to signalling molecule. Nat Rev Neurosci 19: 235–249. 10.1038/nrn.2018.19 [DOI] [PubMed] [Google Scholar]
- Maher F, Simpson IA. 1994. Modulation of expression of glucose transporters GLUT3 and GLUT1 by potassium and N-methyl-d-aspartate in cultured cerebellar granule neurons. Mol Cell Neurosci 5: 369–375. 10.1006/mcne.1994.1044 [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, Verhoog MB, Goriounova NA. 2019. Synaptic plasticity in human cortical circuits: Cellular mechanisms of learning and memory in the human brain? Curr Opin Neurobiol 54: 186–193. 10.1016/j.conb.2018.06.013 [DOI] [PubMed] [Google Scholar]
- Marcello E, Di Luca M, Gardoni F. 2018. Synapse-to-nucleus communication: From developmental disorders to Alzheimer's disease. Curr Opin Neurobiol 48: 160–166. 10.1016/j.conb.2017.12.017 [DOI] [PubMed] [Google Scholar]
- Marchenko SM, Thomas RC. 2006. Nuclear Ca2+ signalling in cerebellar Purkinje neurons. Cerebellum 5: 36–42. 10.1080/14734220600554438 [DOI] [PubMed] [Google Scholar]
- Marchenko SM, Yarotskyy VV, Kovalenko TN, Kostyuk PG, Thomas RC. 2005. Spontaneously active and InsP3-activated ion channels in cell nuclei from rat cerebellar Purkinje and granule neurones. J Physiol 565: 897–910. 10.1113/jphysiol.2004.081299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margineanu MB, Mahmood H, Fiumelli H, Magistretti PJ. 2018. L-lactate regulates the expression of synaptic plasticity and neuroprotection genes in cortical neurons: A transcriptome analysis. Front Mol Neurosci 11: 375 10.3389/fnmol.2018.00375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie H, Morishita W, Yu X, Calakos N, Malenka RC. 2005. Generation of silent synapses by acute in vivo expression of CaMKIV and CREB. Neuron 45: 741–752. 10.1016/j.neuron.2005.01.039 [DOI] [PubMed] [Google Scholar]
- Martins TV, Evans MJ, Wysham DB, Morris RJ. 2016. Nuclear pores enable sustained perinuclear calcium oscillations. BMC Syst Biol 10: 55 10.1186/s12918-016-0289-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauceri D, Freitag HE, Oliveira AM, Bengtson CP, Bading H. 2011. Nuclear calcium-VEGFD signaling controls maintenance of dendrite arborization necessary for memory formation. Neuron 71: 117–130. 10.1016/j.neuron.2011.04.022 [DOI] [PubMed] [Google Scholar]
- Mauceri D, Hagenston AM, Schramm K, Weiss U, Bading H. 2015. Nuclear calcium buffering capacity shapes neuronal architecture. J Biol Chem 290: 23039–23049. 10.1074/jbc.M115.654962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGann JC, Mandel G. 2018. Neuronal activity induces glutathione metabolism gene expression in astrocytes. Glia 66: 2024–2039. 10.1002/glia.23455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNulty SE, Barrett RM, Vogel-Ciernia A, Malvaez M, Hernandez N, Davatolhagh MF, Matheos DP, Schiffman A, Wood MA. 2012. Differential roles for Nr4a1 and Nr4a2 in object location vs. object recognition long-term memory. Learn Mem 19: 588–592. 10.1101/lm.026385.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Means AR. 2000. Regulatory cascades involving calmodulin-dependent protein kinases. Mol Endocrinol 14: 4–13. 10.1210/mend.14.1.0414 [DOI] [PubMed] [Google Scholar]
- Mederos S, González-Arias C, Perea G. 2018. Astrocyte-neuron networks: A multilane highway of signaling for homeostatic brain function. Front Synaptic Neurosci 10: 45 10.3389/fnsyn.2018.00045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellström B, Sahún I, Ruiz-Nuño A, Murtra P, Gomez-Villafuertes R, Savignac M, Oliveros JC, Gonzalez P, Kastanauskaite A, Knafo S, et al. 2014. DREAM controls the on/off switch of specific activity-dependent transcription pathways. Mol Cell Biol 34: 877–887. 10.1128/MCB.00360-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellström B, Kastanauskaite A, Knafo S, Gonzalez P, Dopazo XM, Ruiz-Nuño A, Jefferys JG, Zhuo M, Bliss TV, Naranjo JR, et al. 2016. Specific cytoarchitectureal changes in hippocampal subareas in daDREAM mice. Mol Brain 9: 22 10.1186/s13041-016-0204-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan AJ. 2016. Ca2+ dialogue between acidic vesicles and ER. Biochem Soc Trans 44: 546–553. 10.1042/BST20150290 [DOI] [PubMed] [Google Scholar]
- Morgan JI, Curran T. 1986. Role of ion flux in the control of c-fos expression. Nature 322: 552–555. 10.1038/322552a0 [DOI] [PubMed] [Google Scholar]
- Morgan JI, Curran T. 1988. Calcium as a modulator of the immediate-early gene cascade in neurons. Cell Calcium 9: 303–311. 10.1016/0143-4160(88)90011-5 [DOI] [PubMed] [Google Scholar]
- Morisot N, Ron D. 2017. Alcohol-dependent molecular adaptations of the NMDA receptor system. Genes Brain Behav 16: 139–148. 10.1111/gbb.12363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Okuno S, Sato F, Fujisawa H. 1995. An immunohistochemical study of Ca2+/calmodulin-dependent protein kinase IV in the rat central nervous system: Light and electron microscopic observations. Neuroscience 68: 181–194. 10.1016/0306-4522(95)00092-W [DOI] [PubMed] [Google Scholar]
- Nestler EJ. 2001. Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci 2: 119–128. 10.1038/35053570 [DOI] [PubMed] [Google Scholar]
- Newington JT, Rappon T, Albers S, Wong DY, Rylett RJ, Cumming RC. 2012. Overexpression of pyruvate dehydrogenase kinase 1 and lactate dehydrogenase A in nerve cells confers resistance to amyloid β and other toxins by decreasing mitochondrial respiration and reactive oxygen species production. J Biol Chem 287: 37245–37258. 10.1074/jbc.M112.366195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivares-Florez S, Czolbe M, Riediger F, Seidlmayer L, Williams T, Nordbeck P, Strasen J, Glocker C, Jansch M, Eder-Negrin P, et al. 2018. Nuclear calcineurin is a sensor for detecting Ca2+ release from the nuclear envelope via IP3R. J Mol Med (Berl) 96: 1239–1249. 10.1007/s00109-018-1701-2 [DOI] [PubMed] [Google Scholar]
- Oliveira AM, Hemstedt TJ, Bading H. 2012. Rescue of aging-associated decline in Dnmt3a2 expression restores cognitive abilities. Nat Neurosci 15: 1111–1113. 10.1038/nn.3151 [DOI] [PubMed] [Google Scholar]
- Oliveira AM, Hemstedt TJ, Freitag HE, Bading H. 2016. Dnmt3a2: A hub for enhancing cognitive functions. Mol Psychiatry 21: 1130–1136. 10.1038/mp.2015.175 [DOI] [PubMed] [Google Scholar]
- Oliveira AM, Litke C, Paldy E, Hagenston AM, Lu J, Kuner R, Bading H, Mauceri D. 2019. Epigenetic control of hypersensitivity in chronic inflammatory pain by the de novo DNA methyltransferase Dnmt3a2. Mol Pain 15: 1744806919827469 10.1177/1744806919827469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Malley KL, Jong YJ, Gonchar Y, Burkhalter A, Romano C. 2003. Activation of metabotropic glutamate receptor mGlu5 on nuclear membranes mediates intranuclear Ca2+ changes in heterologous cell types and neurons. J Biol Chem 278: 28210–28219. 10.1074/jbc.M300792200 [DOI] [PubMed] [Google Scholar]
- Osawa M, Tong KI, Lilliehook C, Wasco W, Buxbaum JD, Cheng HY, Penninger JM, Ikura M, Ames JB. 2001. Calcium-regulated DNA binding and oligomerization of the neuronal calcium-sensing protein, calsenilin/DREAM/KChIP3. J Biol Chem 276: 41005–41013. 10.1074/jbc.M105842200 [DOI] [PubMed] [Google Scholar]
- Pacelli C, Giguere N, Bourque MJ, Levesque M, Slack RS, Trudeau LE. 2015. Elevated mitochondrial bioenergetics and axonal arborization size are key contributors to the vulnerability of dopamine neurons. Curr Biol 25: 2349–2360. 10.1016/j.cub.2015.07.050 [DOI] [PubMed] [Google Scholar]
- Padamsey Z, McGuinness L, Bardo SJ, Reinhart M, Tong R, Hedegaard A, Hart ML, Emptage NJ. 2017. Activity-dependent exocytosis of lysosomes regulates the structural plasticity of dendritic spines. Neuron 93: 132–146. 10.1016/j.neuron.2016.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadia S, Stevenson P, Hardingham NR, Bading H, Hardingham GE. 2005. Nuclear Ca2+ and the cAMP response element-binding protein family mediate a late phase of activity-dependent neuroprotection. J Neurosci 25: 4279–4287. 10.1523/jneurosci.5019-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadia S, Soriano FX, Léveillé F, Martel MA, Dakin KA, Hansen HH, Kaindl A, Sifringer M, Fowler J, Stefovska V, et al. 2008. Synaptic NMDA receptor activity boosts intrinsic antioxidant defenses. Nat Neurosci 11: 476–487. 10.1038/nn2071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo L, Valor LM, Eraso-Pichot A, Barco A, Golbano A, Hardingham GE, Masgrau R, Galea E. 2017. CREB regulates distinct adaptive transcriptional programs in astrocytes and neurons. Sci Rep 7: 6390 10.1038/s41598-017-06231-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer T, Schuster S, Bonhoeffer S. 2001. Cooperation and competition in the evolution of ATP-producing pathways. Science 292: 504–507. 10.1126/science.1058079 [DOI] [PubMed] [Google Scholar]
- Pittenger C, Huang YY, Paletzki RF, Bourtchouladze R, Scanlin H, Vronskaya S, Kandel ER. 2002. Reversible inhibition of CREB/ATF transcription factors in region CA1 of the dorsal hippocampus disrupts hippocampus-dependent spatial memory. Neuron 34: 447–462. 10.1016/S0896-6273(02)00684-0 [DOI] [PubMed] [Google Scholar]
- Ploski JE, Monsey MS, Nguyen T, DiLeone RJ, Schafe GE. 2011. The neuronal PAS domain protein 4 (Npas4) is required for new and reactivated fear memories. PLoS ONE 6: e23760 10.1371/journal.pone.0023760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JM, Sah P. 2002. Nuclear calcium signaling evoked by cholinergic stimulation in hippocampal CA1 pyramidal neurons. J Neurosci 22: 3454–3462. 10.1523/jneurosci.22-09-03454.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presumey J, Bialas AR, Carroll MC. 2017. Complement system in neural synapse elimination in development and disease. Adv Immunol 135: 53–79. 10.1016/bs.ai.2017.06.004 [DOI] [PubMed] [Google Scholar]
- Price TJ, Inyang KE. 2015. Commonalities between pain and memory mechanisms and their meaning for understanding chronic pain. Prog Mol Biol Transl Sci 131: 409–434. 10.1016/bs.pmbts.2014.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruunsild P, Bengtson CP, Bading H. 2017. Networks of cultured iPSC-derived neurons reveal the human synaptic activity-regulated adaptive gene program. Cell Rep 18: 122–135. 10.1016/j.celrep.2016.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Tan YW, Hagenston AM, Martel MA, Kneisel N, Skehel PA, Wyllie DJ, Bading H, Hardingham GE. 2013. Mitochondrial calcium uniporter Mcu controls excitotoxicity and is transcriptionally repressed by neuroprotective nuclear calcium signals. Nat Commun 4: 2034 10.1038/ncomms3034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, McQueen J, Bilican B, Dando O, Magnani D, Punovuori K, Selvaraj BT, Livesey M, Haghi G, Heron S, et al. 2016. Evidence for evolutionary divergence of activity-dependent gene expression in developing neurons. eLife 5: e20337 10.7554/elife.20337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahn EJ, Guzman-Karlsson MC, David Sweatt J. 2013. Cellular, molecular, and epigenetic mechanisms in non-associative conditioning: implications for pain and memory. Neurobiol Learn Mem 105: 133–150. 10.1016/j.nlm.2013.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajakumar A, Thamotharan S, Raychaudhuri N, Menon RK, Devaskar SU. 2004. Trans-activators regulating neuronal glucose transporter isoform-3 gene expression in mammalian neurons. J Biol Chem 279: 26768–26779. 10.1074/jbc.M402735200 [DOI] [PubMed] [Google Scholar]
- Ramamoorthi K, Fropf R, Belfort GM, Fitzmaurice HL, McKinney RM, Neve RL, Otto T, Lin Y. 2011. Npas4 regulates a transcriptional program in CA3 required for contextual memory formation. Science 334: 1669–1675. 10.1126/science.1208049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsden DB, Ho PW, Ho JW, Liu HF, So DH, Tse HM, Chan KH, Ho SL. 2012. Human neuronal uncoupling proteins 4 and 5 (UCP4 and UCP5): Structural properties, regulation, and physiological role in protection against oxidative stress and mitochondrial dysfunction. Brain Behav 2: 468–478. 10.1002/brb3.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangaraju V, Calloway N, Ryan TA. 2014. Activity-driven local ATP synthesis is required for synaptic function. Cell 156: 825–835. 10.1016/j.cell.2013.12.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas M, Villar D, González P, Dopazo XM, Mellstrom B, Naranjo JR. 2011. Building the DREAM interactome. Sci China Life Sci 54: 786–792. 10.1007/s11427-011-4196-4 [DOI] [PubMed] [Google Scholar]
- Rivera-Arconada I, Benedet T, Roza C, Torres B, Barrio J, Krzyzanowska A, Avendaño C, Mellström B, Lopez-Garcia JA, Naranjo JR. 2010. DREAM regulates BDNF-dependent spinal sensitization. Mol Pain 6: 95 10.1186/1744-8069-6-95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison AJ, Nestler EJ. 2011. Transcriptional and epigenetic mechanisms of addiction. Nat Rev Neurosci 12: 623–637. 10.1038/nrn3111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues MA, Gomes DA, Nathanson MH, Leite MF. 2009. Nuclear calcium signaling: A cell within a cell. Braz J Med Biol Res 42: 17–20. 10.1590/S0100-879X2008005000050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruchti E, Roach PJ, DePaoli-Roach AA, Magistretti PJ, Allaman I. 2016. Protein targeting to glycogen is a master regulator of glycogen synthesis in astrocytes. IBRO Rep 1: 46–53. 10.1016/j.ibror.2016.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez I, Duran J, Sinadinos C, Beltran A, Yanes O, Tevy MF, Martinez-Pons C, Milan M, Guinovart JJ. 2014. Neurons have an active glycogen metabolism that contributes to tolerance to hypoxia. J Cereb Blood Flow Metab 34: 945–955. 10.1038/jcbfm.2014.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandkühler J. 2009. Models and mechanisms of hyperalgesia and allodynia. Physiol Rev 89: 707–758. 10.1152/physrev.00025.2008 [DOI] [PubMed] [Google Scholar]
- Sando R III, Gounko N, Pieraut S, Liao L, Yates J III, Maximov A. 2012. HDAC4 governs a transcriptional program essential for synaptic plasticity and memory. Cell 151: 821–834. 10.1016/j.cell.2012.09.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saneyoshi T, Fortin DA, Soderling TR. 2010. Regulation of spine and synapse formation by activity-dependent intracellular signaling pathways. Curr Opin Neurobiol 20: 108–115. 10.1016/j.conb.2009.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santello M, Toni N, Volterra A. 2019. Astrocyte function from information processing to cognition and cognitive impairment. Nat Neurosci 22: 154–166. 10.1038/s41593-018-0325-8 [DOI] [PubMed] [Google Scholar]
- Sanz E, Yang L, Su T, Morris DR, McKnight GS, Amieux PS. 2009. Cell-type-specific isolation of ribosome-associated mRNA from complex tissues. Proc Natl Acad Sci 106: 13939–13944. 10.1073/pnas.0907143106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathyamurthy A, Johnson KR, Matson KJE, Dobrott CI, Li L, Ryba AR, Bergman TB, Kelly MC, Kelley MW, Levine AJ. 2018. Massively parallel single nucleus transcriptional profiling defines spinal cord neurons and their activity during behavior. Cell Rep 22: 2216–2225. 10.1016/j.celrep.2018.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlumm F, Mauceri D, Freitag HE, Bading H. 2013. Nuclear calcium signaling regulates nuclear export of a subset of class IIa histone deacetylases following synaptic activity. J Biol Chem 288: 8074–8084. 10.1074/jbc.M112.432773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segarra-Mondejar M, Casellas-Diaz S, Ramiro-Pareta M, Muller-Sanchez C, Martorell-Riera A, Hermelo I, Reina M, Aragones J, Martinez-Estrada OM, Soriano FX. 2018. Synaptic activity-induced glycolysis facilitates membrane lipid provision and neurite outgrowth. EMBO J 37: e97368 10.15252/embj.201797368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servili E, Trus M, Maayan D, Atlas D. 2018. β-Subunit of the voltage-gated Ca2+ channel Cav1.2 drives signaling to the nucleus via H-Ras. Proc Natl Acad Sci 115: E8624–E8633. 10.1073/pnas.1805380115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalizi A, Gaudilliere B, Yuan Z, Stegmuller J, Shirogane T, Ge Q, Tan Y, Schulman B, Harper JW, Bonni A. 2006. A calcium-regulated MEF2 sumoylation switch controls postsynaptic differentiation. Science 311: 1012–1017. 10.1126/science.1122513 [DOI] [PubMed] [Google Scholar]
- Shang S, Zhu F, Liu B, Chai Z, Wu Q, Hu M, Wang Y, Huang R, Zhang X, Wu X, et al. 2016. Intracellular TRPA1 mediates Ca2+ release from lysosomes in dorsal root ganglion neurons. J Cell Biol 215: 369–381. 10.1083/jcb.201603081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Y, McCarthy KD. 1994. Plasticity of astrocytes. Glia 11: 147–155. 10.1002/glia.440110209 [DOI] [PubMed] [Google Scholar]
- Simonetti M, Hagenston AM, Vardeh D, Freitag HE, Mauceri D, Lu J, Satagopam VP, Schneider R, Costigan M, Bading H, et al. 2013. Nuclear calcium signaling in spinal neurons drives a genomic program required for persistent inflammatory pain. Neuron 77: 43–57. 10.1016/j.neuron.2012.10.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan SA, Barres BA. 2018. Assembling a cellular user manual for the brain. J Neurosci 38: 3149–3153. 10.1523/jneurosci.2479-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderling TR. 1999. The Ca2+-calmodulin-dependent protein kinase cascade. Trends Biochem Sci 24: 232–236. 10.1016/S0968-0004(99)01383-3 [DOI] [PubMed] [Google Scholar]
- Soriano FX, Papadia S, Bell KF, Hardingham GE. 2009. Role of histone acetylation in the activity-dependent regulation of sulfiredoxin and sestrin 2. Epigenetics 4: 152–158. 10.4161/epi.4.3.8753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel I, Mardinly AR, Gabel HW, Bazinet JE, Couch CH, Tzeng CP, Harmin DA, Greenberg ME. 2014. Npas4 regulates excitatory-inhibitory balance within neural circuits through cell-type-specific gene programs. Cell 157: 1216–1229. 10.1016/j.cell.2014.03.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suberbielle E, Sanchez PE, Kravitz AV, Wang X, Ho K, Eilertson K, Devidze N, Kreitzer AC, Mucke L. 2013. Physiologic brain activity causes DNA double-strand breaks in neurons, with exacerbation by amyloid-β. Nat Neurosci 16: 613–621. 10.1038/nn.3356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Lin Y. 2016. Npas4: Linking neuronal activity to memory. Trends Neurosci 39: 264–275. 10.1016/j.tins.2016.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Ren X, Qi W, Yuan D, Simpkins JW. 2016. Geissoschizine methyl ether protects oxidative stress-mediated cytotoxicity in neurons through the “Neuronal Warburg Effect.” J Ethnopharmacol 187: 249–258. 10.1016/j.jep.2016.04.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadi M, Allaman I, Lengacher S, Grenningloh G, Magistretti PJ. 2015. Learning-induced gene expression in the hippocampus reveals a role of neuron -astrocyte metabolic coupling in long term memory. PLoS ONE 10: e0141568 10.1371/journal.pone.0141568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto-Kimura S, Suzuki K, Horigane SI, Kamijo S, Inoue M, Sakamoto M, Fujii H, Bito H. 2017. Calmodulin kinases: Essential regulators in health and disease. J Neurochem 141: 808–818. 10.1111/jnc.14020 [DOI] [PubMed] [Google Scholar]
- Taniguchi M, Carreira MB, Cooper YA, Bobadilla AC, Heinsbroek JA, Koike N, Larson EB, Balmuth EA, Hughes BW, Penrod RD, et al. 2017. HDAC5 and its target gene, Npas4, function in the nucleus accumbens to regulate cocaine-conditioned behaviors. Neuron 96: 130–144.e6. 10.1016/j.neuron.2017.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodosis DT, Poulain DA, Oliet SH. 2008. Activity-dependent structural and functional plasticity of astrocyte-neuron interactions. Physiol Rev 88: 983–1008. 10.1152/physrev.00036.2007 [DOI] [PubMed] [Google Scholar]
- Tiwari VK, Burger L, Nikoletopoulou V, Deogracias R, Thakurela S, Wirbelauer C, Kaut J, Terranova R, Hoerner L, Mielke C, et al. 2012. Target genes of topoisomerase IIβ regulate neuronal survival and are defined by their chromatin state. Proc Natl Acad Sci 109: E934–E943. 10.1073/pnas.1119798109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tochiki KK, Cunningham J, Hunt SP, Géranton SM. 2012. The expression of spinal methyl-CpG-binding protein 2, DNA methyltransferases and histone deacetylases is modulated in persistent pain states. Mol Pain 8: 14 10.1186/1744-8069-8-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida S, Shumyatsky GP. 2018. Synaptically localized transcriptional regulators in memory formation. Neuroscience 370: 4–13. 10.1016/j.neuroscience.2017.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waitt AE, Reed L, Ransom BR, Brown AM. 2017. Emerging roles for glycogen in the CNS. Front Mol Neurosci 10: 73 10.3389/fnmol.2017.00073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DM, Nestler EJ. 2018. Neuroepigenetics and addiction. Handb Clin Neurol 148: 747–765. 10.1016/B978-0-444-64076-5.00048-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Wang Y. 2012. Negative modulation of NMDA receptor channel function by DREAM/calsenilin/KChIP3 provides neuroprotection? Front Mol Neurosci 5: 39 10.3389/fnmol.2012.00039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Marks CR, Perfitt TL, Nakagawa T, Lee A, Jacobson DA, Colbran RJ. 2017. A novel mechanism for Ca2+/calmodulin-dependent protein kinase II targeting to L-type Ca2+ channels that initiates long-range signaling to the nucleus. J Biol Chem 292: 17324–17336. 10.1074/jbc.M117.788331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S, Hong M, Lasser-Ross N, Ross WN. 2006. Modulation of calcium wave propagation in the dendrites and to the soma of rat hippocampal pyramidal neurons. J Physiol 575: 455–468. 10.1113/jphysiol.2006.114231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayman GA, Impey S, Marks D, Saneyoshi T, Grant WF, Derkach V, Soderling TR. 2006. Activity-dependent dendritic arborization mediated by CaM-kinase I activation and enhanced CREB-dependent transcription of Wnt-2. Neuron 50: 897–909. 10.1016/j.neuron.2006.05.008 [DOI] [PubMed] [Google Scholar]
- Weislogel JM, Bengtson CP, Muller MK, Hortzsch JN, Bujard M, Schuster CM, Bading H. 2013. Requirement for nuclear calcium signaling in Drosophila long-term memory. Sci Signal 6: ra33 10.1126/scisignal.2003598 [DOI] [PubMed] [Google Scholar]
- Weng FJ, Garcia RI, Lutzu S, Alvina K, Zhang Y, Dushko M, Ku T, Zemoura K, Rich D, Garcia-Dominguez D, et al. 2018. Npas4 is a critical regulator of learning-induced plasticity at mossy fiber-CA3 synapses during contextual memory formation. Neuron 97: 1137–1152.e5. 10.1016/j.neuron.2018.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegert JS, Bading H. 2011. Activity-dependent calcium signaling and ERK-MAP kinases in neurons: A link to structural plasticity of the nucleus and gene transcription regulation. Cell Calcium 49: 296–305. 10.1016/j.ceca.2010.11.009 [DOI] [PubMed] [Google Scholar]
- Wood MA, Kaplan MP, Park A, Blanchard EJ, Oliveira AM, Lombardi TL, Abel T. 2005. Transgenic mice expressing a truncated form of CREB-binding protein (CBP) exhibit deficits in hippocampal synaptic plasticity and memory storage. Learn Mem 12: 111–119. 10.1101/lm.86605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YE, Pan L, Zuo Y, Li X, Hong W. 2017. Detecting activated cell populations using single-cell RNA-Seq. Neuron 96: 313–329.e6. 10.1016/j.neuron.2017.09.026 [DOI] [PubMed] [Google Scholar]
- Yellen G. 2018. Fueling thought: Management of glycolysis and oxidative phosphorylation in neuronal metabolism. J Cell Biol 217: 2235–2246. 10.1083/jcb.201803152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin JC, Wallach JS, Del Vecchio M, Wilder EL, Zhou H, Quinn WG, Tully T. 1994. Induction of a dominant negative CREB transgene specifically blocks long-term memory in Drosophila. Cell 79: 49–58. 10.1016/0092-8674(94)90399-9 [DOI] [PubMed] [Google Scholar]
- Yu Y, Oberlaender K, Bengtson CP, Bading H. 2017. One nuclear calcium transient induced by a single burst of action potentials represents the minimum signal strength in activity-dependent transcription in hippocampal neurons. Cell Calcium 65: 14–21. 10.1016/j.ceca.2017.03.003 [DOI] [PubMed] [Google Scholar]
- Zalcman G, Federman N, Romano A. 2018. CaMKII isoforms in learning and memory: Localization and function. Front Mol Neurosci 11: 445 10.3389/fnmol.2018.00445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisel A, Hochgerner H, Lonnerberg P, Johnsson A, Memic F, van der Zwan J, Haring M, Braun E, Borm LE, La Manno G, et al. 2018. Molecular architecture of the mouse nervous system. Cell 174: 999–1014.e22. 10.1016/j.cell.2018.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SJ, Steijaert MN, Lau D, Schütz G, Delucinge-Vivier C, Descombes P, Bading H. 2007. Decoding NMDA receptor signaling: Identification of genomic programs specifying neuronal survival and death. Neuron 53: 549–562. 10.1016/j.neuron.2007.01.025 [DOI] [PubMed] [Google Scholar]
- Zhang SJ, Zou M, Lu L, Lau D, Ditzel DA, Delucinge-Vivier C, Aso Y, Descombes P, Bading H. 2009. Nuclear calcium signaling controls expression of a large gene pool: Identification of a gene program for acquired neuroprotection induced by synaptic activity. PLoS Genet 5: e1000604 10.1371/journal.pgen.1000604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SJ, Buchthal B, Lau D, Hayer S, Dick O, Schwaninger M, Veltkamp R, Zou M, Weiss U, Bading H. 2011a. A signaling cascade of nuclear calcium-CREB-ATF3 activated by synaptic NMDA receptors defines a gene repression module that protects against extrasynaptic NMDA receptor-induced neuronal cell death and ischemic brain damage. J Neurosci 31: 4978–4990. 10.1523/jneurosci.2672-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Cai YQ, Zou F, Bie B, Pan ZZ. 2011b. Epigenetic suppression of GAD65 expression mediates persistent pain. Nat Med 17: 1448–1455. 10.1038/nm.2442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Reichel JM, Han C, Zuniga-Hertz JP, Cai D. 2017. Astrocytic process plasticity and IKKβ/NF-κB in central control of blood glucose, blood pressure, and body weight. Cell Metab 25: 1091–1102.e4. 10.1016/j.cmet.2017.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]