Highlights
-
•
An attenuated QX-like IBV strain was developed through multiple passages.
-
•
We tested the safety and efficacy of the attenuated YN strain.
-
•
The attenuated strain has a clear decrease in pathogenicity for chickens.
-
•
The vaccine based on strain IB YN was efficacious against challenge.
Keywords: Infectious bronchitis virus, Attenuated virus, Pathogenicity, Safety, Efficacy
Abstract
Infectious bronchitis (IB) is a highly contagious respiratory and urogenital disease of chickens caused by infectious bronchitis virus (IBV). This disease is of considerable economic importance and is primarily controlled through biosecurity and immunization with live attenuated and inactivated IB vaccines of various serotypes. In the present study, we tested the safety and efficacy of an attenuated predominant Chinese QX-like IBV strain. The results revealed that the attenuated strain has a clear decrease in pathogenicity for specific-pathogen-free (SPF) chickens compared with the parent strain. Strain YN-inoculated birds had clinical signs of varying severity with 30% mortality, while the attenuated group appeared healthy, with less tissue damage. The attenuated strain also had relatively low tissue replication rates and higher antibody levels. The superior protective efficacy of the attenuated strain was observed when vaccinated birds were challenged with a homologous or heterologous field IBV strain, indicating the potential of the attenuated YN (aYN) as a vaccine. Producing a vaccine targeting the abundant serotype in China is essential to reducing the economic impact of IB on the poultry industry.
1. Introduction
Infectious bronchitis virus (IBV) belongs to the order Nidovirales, family Coronaviridae, and genus Gammacoronavirus. Infectious bronchitis (IB) is an important worldwide viral disease of poultry. This poses a major economic threat to the poultry industry because of poor weight gain and lost feed efficiency in broilers and reduced numbers and quality of eggs in layers. In addition, some virulent strains cause high mortality in young chickens due to renal disease.
While chicks are the most susceptible, IBV affects chickens of all ages. Despite a predilection towards the respiratory tract, IBV also infects a wide range of organs, including the kidneys, gastrointestinal tract, and oviduct, causing respiratory disease, interstitial nephritis, and decreased egg production (Cavanagh and Naqi, 2003). Clinical signs include coughing, sneezing, tracheal rales, and watery eyes (Cavanagh, 2007, Raj and Jones, 1997). Lesions in infected birds include degeneration of renal epithelium, renal interstitial lymphoplasmacytic inflammation, and degeneration and necrosis of the ciliated respiratory epithelium.
The main method of protecting chickens from IB is inoculation with both attenuated live and inactivated vaccines. Although inactivated vaccines are cheaper and easier to administer, the poultry industry prefers to use live vaccines rather than inactivated ones, as they are more effective (Huang and Wang, 2006). Despite widespread vaccination in China and other countries, IB outbreaks still occur sporadically because of little or no cross protection between different IBV serotypes (Cook et al., 2001, Liu et al., 2009). Specifically, a new IBV variant has been circulating in China since 1998 (Wang et al., 1998). This virus has been identified as the QX strain and has been primarily associated with various renal pathologies (Terregino et al., 2008 Zsofia et al., 2009). Phylogenetic analysis showed that the IBV isolates that clustered with QX were mainly Chinese isolates. These results further indicated that IBV isolates that are prevalent in China were significantly evolutionarily distant from Mass-type strains. However, Mass-type vaccine strains (e.g., H52, H120, Ma5, and W93) are mainly IBV vaccine strains typically administered in China today, which may not be able to provide efficient protection against field strains. Other vaccine strategies including combination of Mass type vaccine with 4/91, Conn type etc., were attempted before and could get a broader protection than the Mass type vaccine alone (Cook et al., 2001, Cook et al., 1999). However, the 4/91 and Conn type vaccine have not been licensed as commercial vaccines in China nowadays. Thus, it will be necessary to rapidly develop new vaccines against the QX-like field strains.
It has been shown that the spike glycoprotein (S) of coronavirus is a determinant of tissue tropism and virulence and a tiny change in the S gene may lead to vaccine failure (Cavanagh and Cook, 1997, Cavanagh et al., 1986). Thus, until a universal vaccine can be developed, the ongoing determination of epidemic serotypes and production of new vaccines are key factors in controlling infectious bronchitis (Jackwood et al., 2005).
Early on, it was recognized that live-attenuated IB vaccines could be developed by reducing virus virulence by multiple serial passages in 10–11-day-old embryonated eggs, and this method is still widely applied today (Cook et al., 2012, Gelb and Cloud, 1983). Commercial IB vaccines are developed by multiple passage of a field isolate in specific-pathogen-free (SPF) embryonated eggs until the desired blend of non-pathogenicity and immunogenicity has been achieved (Bijlenga et al., 2011, Jackwood et al., 2003).
We have previously isolated and characterized a virulent IBV strain from 30-day-old vaccinated broiler chickens in the Yunnan Province of China. This isolate is genetically similar to most of the prevalent strains of IBV found in China, albeit with increased pathogenicity than previously characterized strains (Feng et al., 2012). To determine its utility as a vaccine, this YN strain was attenuated by 118 serial passages in 10-day-old SPF embryonated chicken eggs. We tested the safety and efficacy of the attenuated strain in this study.
2. Materials and methods
2.1. Virus and attenuation
The IBV QX-like strain YN (GenBank accession no.: JF893452) originated from a H120 vaccinated broiler flock with respiratory and renal disease, with a death rate of 30% (Feng et al., 2012). The virus was purified and passaged by inoculating 10-day-old SPF embryonated chicken eggs via the allantoic sac route. Inoculated eggs were incubated for 40 h at 37 °C and the allantoic fluid harvested for subsequent passages. Eggs that died within 24 h of inoculation were discarded. At every 10th passage, eggs were examined using reverse transcription polymerase chain reaction (RT-PCR) for the presence of virus. The resulting attenuated YN strain was shorted as aYN and was titrated by inoculating 10-fold serial dilutions with phosphate-buffered saline (PBS) of the virus stocks into the allantoic sac of 10-day-old SPF embryonated eggs. The embryo 50% infectious doses (EID50) were determined according to the method of (Reed and Muench, 1938).
The homologous (YN strain) and heterologous (SD strain) challenged IBV strains were used for determine the protective efficacy of aYN. The heterologous challenged IBV SD strain was isolated from Mass-type vaccinated chickens of Shandong province in 2013. Based on the phylogenetic analysis, it has the highest homology with LX4 (GenBank accession number: AY189157), and has a 89.7% similarity with the YN strain. The EID50 for strain YN and SD was 106.83 EID50/0.2 mL and 105.63 EID50/0.2 mL, respectively.
2.2. Animal and ethics statement
One day or 5-week-old SPF chickens were used to determine the safety and efficiency of aYN. All chickens were kept in isolators at China Agricultural University throughout the experiment and the animal rearing facilities were approved by the Beijing Administration Committee of Laboratory Animals under the leadership of the Beijing Association for Science and Technology (approval ID SYXK [Jing] 2013-0013). All of the animals used in this study were cared for in accordance with established guidelines, and the experimental protocols were performed with the approval of the Animal Welfare and Ethical Censor Committee at China Agricultural University.
2.3. Safety in SPF chickens
A total of sixty 5-week-old SPF white leghorn chickens were assigned to 3 groups of 20 chickens each. The chickens were maintained in isolators with positive pressure in air-conditioned rooms. Of the three groups, two were inoculated with 200 μL YN or YN attenuated containing 105.0 EID50 by intranasal and intraocular routes. The third group was maintained as a negative control. Birds were housed separately in isolators for chickens with consistent conditions, and food and water were provided ad libitum.
2.3.1. Clinical observations and sampling
To determine the pathology produced by the two IBV strains, all birds were observed daily for 21 days post-inoculation (dpi) for clinical signs. Clinical signs indicative of infection with IBV strains consisted of hunched posture, depression, reluctance to move, emaciation, diarrhea and soiled vent were given daily clinical scores: 0 for normal, 1 for mild depression, 2 for severe depressed, 3 for paralysis/prostration, and 4 for death. Two chicks from each group were sacrificed at 3, 5, 7, 10 and 15 dpi. Gross changes were noted and samples of trachea, kidney, lung, proventriculus, duodenum, and bursa of Fabricius were collected for virus detection via real-time quantitative reverse transcription polymerase chain reaction (RT-qPCR) and in 10% neutral formalin for histopathological examination. Necropsies were carried out, and external and internal abnormalities were recorded. Lesions in the trachea, kidney, lung, proventriculus, duodenum, and bursa of Fabricius were scored as 0 for no lesions, 1 for mild, 2 for moderate and 3 for severe lesions. Mean lesion scores (MLSs) were calculated for each group. Blood samples from surviving chicks in each group were collected at the end of the experiment for antibody detection at 21 dpi.
2.3.2. Histopathology and immunohistochemistry
Tissues collected above were routinely processed, embedded in paraffin wax, and cut into 5 μm sections. Sections were stained with hematoxylin and eosin and examined for lesions using light microscopy. The slides were examined by light microscopy and the lesions were scored according to the severity of the observed lesions. Absence of injury was classified as -, while mild, moderate and severe were classified as +, ++, and +++ respectively.
Duplicate tissue sections were prepared for immunohistochemistry (IHC) to detect viral antigen via the following protocol. Briefly, 5 μm tissue sections were subjected to antigen retrieval and then incubated in 10% normal goat serum in PBS for 30 min to block nonspecific binding. Slides were further incubated with chicken anti-IBV hyperimmune serum at 1:500 dilutions in PBS for 2 h followed by incubation with a horseradish peroxidase-conjugated rabbit chicken IgG for 1 h. The reaction was visualized by the addition of 3,3-diaminobenzidine (DAB, Sigma, St.Louis, Mo, USA) for 15 min. Finally, sections were counterstained with hematoxylin, air dried, and examined by light microscopy.
2.3.3. Transmission electron microscopy (TEM)
The trachea was removed under strict aseptic conditions. The tracheal rings were cut into semicircles and quarter-circles. They were immersed in 2% paraformaldehyde and 2.5% glutaraldehyde in 0.1 M phosphate buffer, pH 5.5 and fixed for 2 h at 4 °C. After primary fixation, samples were then washed with PBS, post-fixed in 1% osmium tetroxide, washed, and dehydrated in an increasing series of ethanol solutions. Dehydrated pellets were embedded in an epoxy resin and sections were cut at 70 nm. Finally, sections were placed on copper sieves and stained with uranyl acetate and lead citrate. Sections were imaged using a JEM-1230 TEM microscope (JEOL, Tokyo, Japan).
2.3.4. Scanning electron microscopy (SEM)
Tracheal samples were fixed as described above. After dehydration in an increasingly concentrated series of alcohol, samples were critical point-dried (Polaron E3000) with carbon dioxide and mounted on aluminum stubs. The mounted samples were gold-coated (Polaron E5100) and imaged using a JEOL JSM-5310LV (JEOL, Tokyo, Japan).
2.3.5. IBV detection by real time RT-qPCR
Total RNA was extracted from trachea, kidney, lung, proventriculus, duodenum, and bursa of Fabricius using Trizol (Invitrogen, Carlsbad, CA, USA). cDNA was obtained via reverse transcription as previously described [20]. cDNA samples were analyzed using SYBR Green I real-time RT-qPCR to determine viral loads. Primers were designed using Primer Premier 5.0 based on the conserved region of N gene of the YN strain (GenBank accession no.: JF893452). The 20-μL PCR mixture was composed of 10 μLof Power SYBR Green PCR Master Mix (Applied Biosystems, Foster, CA, USA), 0.5 μl of each primer, 1 μl of template, and 8 μl of double-distilled water. Real-time PCR was performed on a 7500 System SDS Software (Applied Biosystems) using the following thermal cycles: a 10 min hot start at 95 °C, followed by 40 cycles of denaturation at 95 °C for 15 s, annealing at 55 °C for 35 s and extension at 72 °C for 40 s.
All qPCR reactions were carried out in triplicate and repeated at least twice. A linear regression was determined plotting the logarithmic values of the number of copies of plasmid DNA containing the insert against the cycle threshold (Ct) values to convert Ct values into copy numbers. The relative N gene expression was analyzed using GraphPad Prism version 6.0 (GraphPad Software Inc., San Diego, CA, USA).
2.3.6. Serology
A commercial enzyme-linked immunosorbent assay (ELISA) kit (IDEXX Laboratories, Westbrook, ME, USA) was used to measure IBV antibody levels according to the manufacturer's instructions. The viral titer was calculated using the formula provided by IDEXX.
2.4. Protective efficacy of aYN
Ninety 1-day-old SPF chickens were divided into five groups of 18 birds as follows. Group aYN-YN and aYN-SD were vaccinated intranasally with 105.0 EID50 aYN and challenged intranasally with homologous field strain YN or heterologous field strain SD at a dose of 105EID50/bird at 21 days post vaccination, respectively. Group control-YN and control-SD were left unvaccinated and challenged with YN or SD at the same time. Group control-NC was maintained as a negative control. All birds were housed separately in isolators with consistent conditions, and food and water were provided ad libitum.
2.4.1. Clinical observations and sampling
Following challenge, all birds were observed daily for clinical signs attributable to IB infection for two weeks. Two birds from each group were killed humanely at 3 and 5 day postchallenge (dpc) for gross lesions observation and the evaluation of trachea ciliary activity.
2.4.2. Detection of virus shedding in oral swabs
Ten oral swabs from each group were randomly collected at 5dpc for the detection of virus shedding via RT-PCR. For PCR, 10 μl Taq SuperMix (TransGen, Beijing, China) and 10 pmol of each primer were added to 100 ng cDNA as template in a total of 20 μl reaction volume. PCR was performed at 95°C for 5 min, followed by 30 cycles of denaturation (95 °C, 45 s), annealing (53 °C, 45 s), and polymerization (72°C, 1 min), and the postpolymerization step was performed at 72 °C for 10 min. A pair of primers (forward: 5′-TTTTGGTGATGACAAGATGAA-3′; reverse: 5′-CGCATTGTTCCTCTCCTC-3′), which amplify and detect a 403-bp fragment of the M gene of IBV was used in the procedure. Amplified sequences were analyzed by 1.0% agarose gel electrophoresis.
2.4.3. Inhibition of ciliary activity
For evaluation of tracheal ciliostasis, three sections of the upper, middle and lower part of the upper, middle and lower part of the trachea respectively, nine rings per bird, were analyzed. The rings were placed in a Petri dish containing Eagle culture medium with 10% fetal bovine serum. They were then determined by inverted light microscope at a magnification of 400×, observing the degree of integrity and preservation of the ciliary movement of the tracheal epithelial cells. A score of 0 was given if the cilia in the complete tracheal section showed movement; a score of 1 was given if the cilia of 75–100% of the tracheal section showed movement; a score of 2 if the cilia of 50–75% of the trachea showed movement; a score of 3 if the cilia of 25– 50% of the trachea section showed movement; and a score of 4 if the cilia of less than 25% of the trachea section showed movement or no movement at all. For each group, the average ciliostasis score was calculated.
2.5. Statistical analysis
Data were analyzed using unpaired t-test in GraphPad Prism version 6.0 for Windows to obtain a statistical analysis of the differences between aYN and YN inoculated groups. In the case of the serology test and ciliary activity inhibition test, collected data were analysed using two-way ANOVA to see whether there was any significant difference between the different groups. Multiple comparison between the groups was performed using Tukey's multiple comparisons test; The significance was considered as follows: significant at P ≤ 0.05 (*); highly significant at P ≤ 0.01 (**); and very highly significant at P ≤ 0.001 (***).
3. Results
3.1. Safety in SPF chickens
3.1.1. Clinical signs
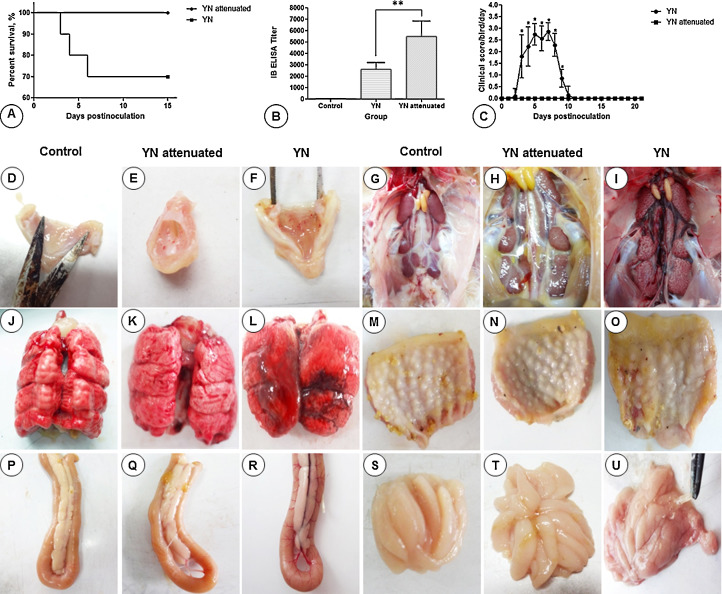
Chicks inoculated with the YN strain showed clinical signs as early as 2 dpi persisting until 7 dpi. Diseased chicks showed signs of coughing, sneezing, tracheal and bronchiolar rales, listlessness, huddling, ruffled feathers, increased water intake, and slight watery diarrhea. Three birds in the YN inoculated group died during the experiment, and the mortality reached 30% (Fig. 1 A). No obvious clinical signs or deaths attributable to IBV were observed in either the control group or the YN attenuated group (Fig. 1C).
Fig. 1.
Survival percentage (A), mean antibody titers at 21 dpi (B), clinical scores (C) and representative gross lesions (D–U) in chickens experimentally infected with the YN and YN attenuated strains. Each column represents the mean titers± standard deviation (SD) (n = 10). Bars indicate mean ± SD. The significance was considered as follows: significant at P ≤ 0.05 (*), highly significant at P ≤ 0.01 (**), and very highly significant at P ≤ 0.001 (***).
3.1.2. Gross lesions
At necropsy, lesions were detected in the respiratory, urinary, and digestive systems in chickens inoculated with YN, including punctate hemorrhages and catarrhal exudates in the throat and trachea (Fig. 1F). The kidneys were swollen with urate deposits frequently observed in the tubules and ureters (Fig. 1I). The lungs of several chickens had congestion and edema (Fig. 1L). Digestive tract lesions consisted of thickening of the wall of the proventriculus and duodenum (Figs. 1O and R), in some cases associated with mucosal congestion. The bursas had heavy exudates of mucus and hemorrhage (Fig. 1U). Chickens inoculated with the attenuated strain had only occasional petechiae in the throat (Fig. 1E) and a slight amount of mucus adhered to the bursa (Fig. 1T). No gross lesions were observed in any birds in the control group (Figs. 1D, G, J, M, P, and S). Number of birds with gross lesions and mean lesion scores at necropsy were showed in Table 1 .
Table 1.
Number of birds with gross lesions and mean lesion scores at necropsy.
| Lesions | Groupa | Days of sampling (post-inoculation) |
|||||
|---|---|---|---|---|---|---|---|
| 3 | 5 | 7 | 10 | 15 | Cumulative total | ||
| Trachea |
YN | 1b (1.5)c |
2 (2.0) |
2 (2.0) |
2 (2.0) |
2 (2.0) |
9 (1.9) |
| aYN | 2 (2.0) |
0 (0) |
1 (0.5) |
0 (0) |
2 (1.0) |
5 (0.7)** |
|
| Proventriculus |
YN | 1 (0.5) |
1 (0.5) |
2 (1.0) |
2 (2.0) |
0 (0) |
6 (0.8) |
| aYN | 0 (0) |
0 (0) |
0 (0) |
0 (0) |
0 (0) |
0 (0)** |
|
| Duodenum |
YN | 2 (1.5) |
1 (1.5) |
2 (1.0) |
1 (1.0) |
1 (1.0) |
7 (1.2) |
| aYN | 0 (0) |
0 (0) |
0 (0) |
0 (0) |
0 (0) |
0 (0)** |
|
| Bursa of Fabricius |
YN | 0 (0) |
1 (0.5) |
2 (2.0) |
2 (3.0) |
2 (2.0) |
7 (1.5) |
| aYN | 0 (0) |
0 (0) |
1 (0.5) |
0 (0) |
1 (0.5) |
2 (0.2)** |
|
| Lung |
YN | 2 (2.0) |
2 (2.0) |
1 (1.5) |
2 (2.0) |
2 (1.5) |
9 (1.8) |
| aYN | 0 (0) |
0 (0) |
0 (0) |
0 (0) |
0 (0) |
0 (0)*** |
|
| Kidney |
YN | 2 (2.0) |
1 (1.5) |
2 (3.0) |
2 (3.0) |
2 (2.0) |
9 (2.3) |
| aYN | 0 (0) |
0 (0) |
0 (0) |
0 (0) |
0 (0) |
0 (0)*** |
|
The significance was considered as follows: significant at P≤0.05 (*), highly significant at P ≤ 0.01 (**), and very highly significant at P ≤ 0.001 (***).
YN group infected with YN strain; aYN group infected with attenuated YN strain. There were no lesions in birds in the control group.
No. of birds with lesions out of two birds.
Mean lesion scores out of two birds.
3.1.3. Histopathology and immunohistochemistry
The microscopic lesions were consistent with the gross lesions described above. Moderate to severe lesions were predominantly found between 3 and 10 dpi and were more common in the chickens infected with the wild-type than the attenuated strain (Fig. 2 ). Inflammatory cell infiltrates and varying degrees of epithelial desquamation of ciliated cells were detected in the trachea (Fig. 2B and C), with hemorrhage and mucosa injury in some chickens.
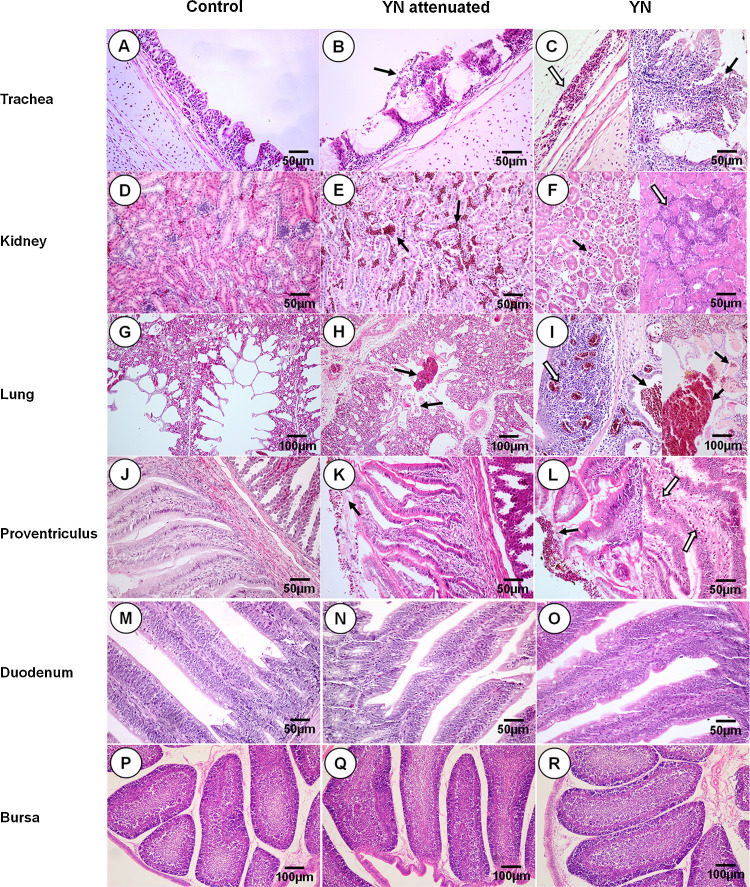
Fig. 2.
Histopathologic changes detected 7 dpi. B and C: black arrows indicate extensive drop out, degeneration, and necrosis of the ciliated epithelial cells; the open arrow indicates subserosal hemorrhage. E: black arrows indicate tubulointerstitial co;1;ngestion. F: black arrows indicate tubulointerstitial expansion and the open arrow indicates tubulointerstitial lymphocytic infiltrates. H and I: black arrows indicate hemorrhages; the open arrow indicates bronchial mucosal infiltration and congestion. K and L: black arrows indicate erythrocytes, inflammation, and exudates adhered to the mucous membrane surfaces; the open arrow indicates inflammatory infiltration of the lamina propria. A–F, J–O: scale bar = 50 μm; G–I, P–R: scale bar = 100 μm.
Foci of necrosis and intense multifocal nephritis, interstitial congestion, with lymphoplasmacytic infiltration and tubular dilation were scattered throughout the kidneys of YN-inoculated chickens (Fig. 2E and F). Proteinaceous material was detected in the tubules of a number of YN-inoculated chickens. Congestion, hemorrhages, erythrocytes and lymphocytes were frequently detected in the bronchial and air capillary lumina of the lungs. A number of YN-inoculated chickens exhibited bronchiectasis (Fig. 2H and I).
In the proventriculus, lesions included loss of mucosal epithelium with inflammatory cell infiltration (Fig. 2K and L). No histologic evidence of damage was detected in the duodenum and bursa (Fig. 2N, O, Q, and R). No IBV-related lesions were observed in the control birds (Fig. 2A, D, G, J, M, and P). Histological scores in different organs of SPF chicks inoculated with YN or aYN were showed in Table 2 .
Table 2.
Histology in different organs of SPF chicks inoculated with YN or aYN.
| Organs | Group | Days of sampling (post-inoculation) |
||||
|---|---|---|---|---|---|---|
| 3 | 5 | 7 | 10 | 15 | ||
| Trachea | YN | ++a | +++ | +++ | ++ | + |
| aYN | + | – | + | – | + | |
| Proventriculus | YN | + | + | ++ | + | – |
| aYN | – | – | + | – | – | |
| Duodenum | YN | – | – | – | – | – |
| aYN | – | – | – | – | – | |
| Bursa of Fabricius | YN | – | – | – | – | – |
| aYN | – | – | – | – | – | |
| Lung | YN | ++ | +++ | ++ | ++ | + |
| aYN | – | + | + | + | – | |
| Kidney | YN | +++ | ++ | +++ | ++ | + |
| aYN | – | + | + | – | – | |
Mean of the severity index from two chicks: -, no change; +, mild; + +, moderate; +++, severe.
The presence of IBV antigen was widely detected in all IBV target organs. The cytoplasm of epithelial cells of the mucosa and lamina propria of the trachea, air capillaries, renal tubules, proventriculus, duodenum, and bursa of Fabricius all had immunoreactivity. More intense immunoreactivity was noted within the YN-inoculated group than in the attenuated group (Fig. 3 ).
Fig. 3.
Immunohistochemical detection of IBV antigens at 7 dpi. Black arrows indicate viral antigen immunoreactivity. Scale bar = 50 μm.
3.1.4. TEM
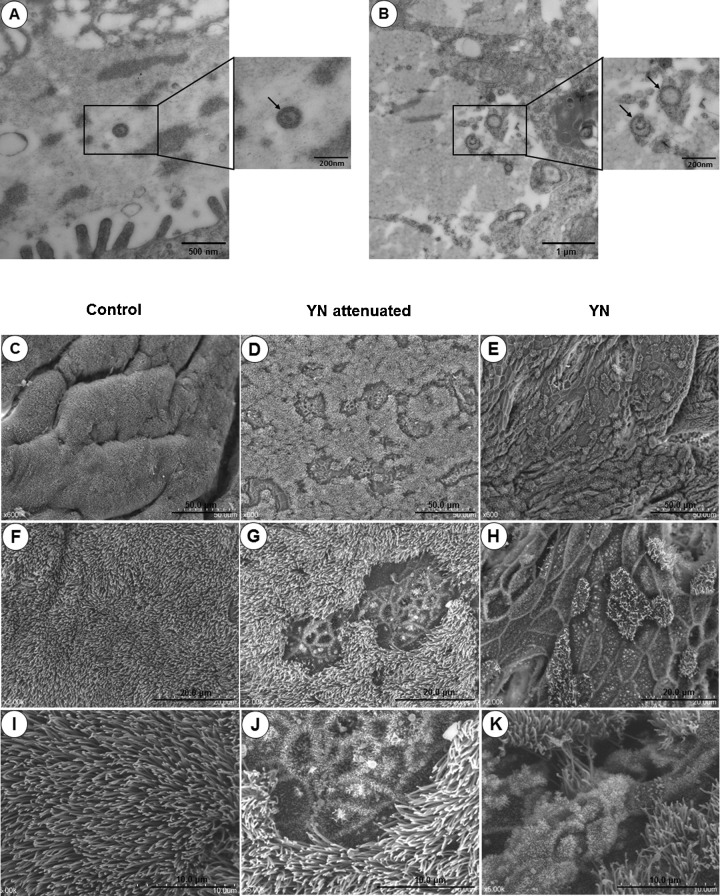
To further examine IBV replication in the respiratory tract, we observed the virus particles using transmission electron microscopy. As shown in Fig. 4 A and B, typical coronavirus particles were noted in the lumina of tracheas inoculated with each of the virus strains.
Fig. 4.
Transmission (A–B) and scanning (C–K) electron micrographs of tracheal epithelium at 7 dpi. A, B: virus particles in the tracheal lumen. C, F, I: control epithelium (×600, ×2.0K, and ×20.0K, respectively). D, G, J: respiratory epithelium from YN attenuated infected chickens (×600, ×2.0K, and ×5.0K, respectively). E, H, K: respiratory epithelium from YN infected chickens (×600, ×2.0K, and ×5.0K, respectively).
3.1.5. SEM
SEM examination of YN-infected tracheas revealed severe lesions in the respiratory mucosa caused by replication of the inoculated virus (Fig. 4E, H, and K). This was evidenced by severe erosion of the respiratory epithelium with associated inflammation. In the YN attenuated group, relatively mild tracheal damage was observed (Fig. 4D, G, and J). No lesions were observed in the tracheas of control birds (Fig. 4C, F, and I).
3.1.6. Viral genome copy number in tissues
No viral DNA was detected in tissues from groups before inoculation and in mock-infected chickens. Table 3 shows results of viral DNA testing from both infected groups. The proportion of positive samples in the group infected with the YN strain was significantly higher than that of the attenuated group.
Table 3.
Tissue tropisms in 35-day-old SPF chickens.
| Group | Organ |
|||||
|---|---|---|---|---|---|---|
| Proventriculus | Lung | Tracheal | Duodenum | Kidney | Bursa | |
| YN attenuated | 4/10 | 2/10 | 6/10 | 2/10 | 5/10 | 7/10 |
| YN | 6/10 | 4/10 | 8/10 | 6/14 | 6/10 | 8/10 |
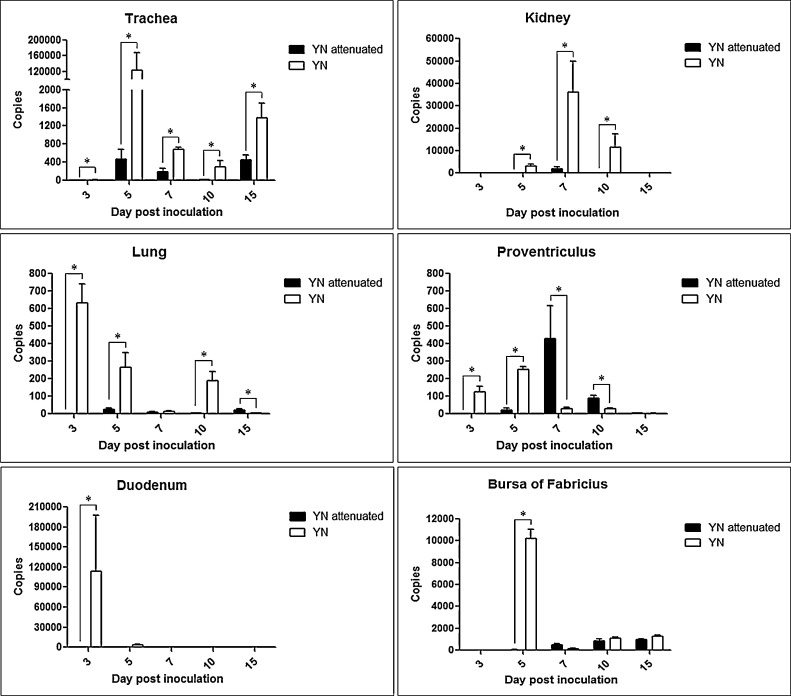
The time-dependent viral load levels in different organs of infected groups are shown in Fig. 5 . The viral DNA levels in the spleen, lung, and duodenum in the YN group peaked at 3 dpi and subsequently gradually decreased. No virus was detected in the YN attenuated samples from similar time points. The maximum amount of viral DNA was detected at 5 dpi in the tracheas of both groups and the proventriculus and bursa from the YN group. Viral loads in the kidneys of both groups increased starting at 5 dpi and peaking at 7 and 10 dpi for the YN and attenuated strains, respectively. The proventriculus and lung samples from the YN attenuated group peaked at 7 dpi, and the duodenum and bursa of fabricius samples from the YN attenuated group had peak viral numbers at 10 dpi. All samples except those from the proventriculus contained more viral copies in the YN group. In addition, the number of copies of viral DNA in the trachea and kidneys were significantly greater than other organs at the same time.
Fig. 5.
Viral loads in samples after YN or YN attenuated strain infection. The significance was considered as follows: significant at P ≤ 0.05 (*), highly significant at P ≤ 0.01 (**), and very highly significant at P ≤ 0.001 (***).
3.1.7. Serology
Antibody responses were measured using a commercial ELISA kit (IDEXX Laboratories). Antibodies against IBV were not detected in any groups before inoculation (0 dpi) and were never detected in the mock-infected group. Chickens in both infected groups seroconverted by 21 dpi and the mean titers induced by the YN and YN attenuated were 2587 and 5504, respectively. The differences in the mean titers between birds inoculated with the YN and YN attenuated strains were significantly different (P < 0.05) (Fig. 1B).
3.2. Protective efficacy of aYN
3.2.1. Clinical observations
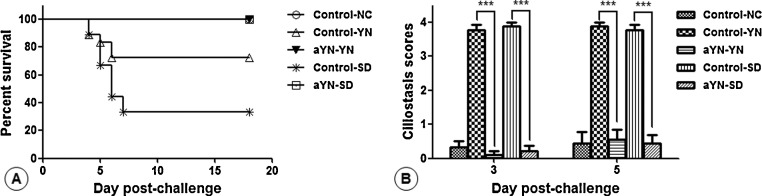
Birds in unvaccinated groups which challenged with the YN or SD strain showed clinical signs and death as early as 4dpc. Diseased chicks showed signs of listlessness, huddling, ruffled feathers, increased water intake, and slight watery diarrhea. During the observation period, the mortality in group control-YN and control-SD were 28% and 67%, respectively (Fig. 6 A). No obvious clinical signs or deaths attributable to IBV were observed in aYN vaccinated groups and group control-NC.
Fig. 6.
Survival percentage (A) and trachea ciliostasis scores (B) in chickens experimentally challenged with IBVYN and SD strains. Bars indicate mean ± SD. The significance was considered as follows: significant at P ≤ 0.05 (*), highly significant at P ≤ 0.01 (**), and very highly significant at P ≤ 0.001 (***).
At necropsy, all euthanized chickens showed the slight hemorrhage with serous catarrhal exudate in the trachea and typical kidney lesions characterized by obvious swelling and urate deposition in the tubules and ureters in group control-YN and control-SD. No gross lesions were observed in any birds in aYN vaccinated groups and group control-NC.
3.2.2. Inhibition of the ciliary activity
Inhibition of the ciliary activity was measured at 3 and 5 dpc in the trachea. The group control-YN and control-SD showed a maximum average ciliostasis score of 4, while the average ciliostasis score in group aYN-YN and aYN-SD were below 1 (Fig. 6B). The difference of ciliostasis was extremely significant between aYN vaccinated groups and unvaccinated groups (P < 0.0001).
3.2.3. Detection of virus shedding in oral swabs
Detected by RT-PCR method, virus shedding rate was 100% in group control-YN and control-SD at 5dpc, while the rates were 0 and 30% in group aYN-YN and aYN-SD, respectively. No virus was detected in the unchallenged group (Control-NC).
4. Discussion
The best protection against challenge is achieved by a vaccine containing homologous strains (Gelb et al., 1990). As previously demonstrated in some reports, cross-protection is poor between different serotypes and genotypes of IBV strains. Consequently, currently available vaccines cannot provide sufficient protection for heterologous challenge (Cavanagh and Cook, 1997, Gelb et al., 2005, Liu et al., 2006). The S1 gene of IBV has serotype-specific and neutralization-specific epitopes. Serotype evolution and the genetic diversity of IBV are monitored by analysis of the S1 gene. The majority of QX-like IBV isolates present in the field in China had poor similarity in the S1 part of the spike protein with vaccine strains, indicating the antigenic differences and large evolutionary distances between vaccine strains and IBV field strains in China (Liu et al., 2006, Zhao et al., 2014). Despite the widespread use of live attenuated IBV vaccine (mass serotype), such as strains H120, H52, and Ma5, vaccinated chicken flocks usually fail to achieve complete protection against field virulent IBV challenge. The poultry industry has, in recent years, detected an increasing incidence of outbreaks related to QX-like IBV strains of different serotypes in many countries (Abro et al., 2012, Terregino et al., 2008, Valastro et al., 2010, Xu et al., 2007). Optimal vaccines against circulating IBV strains in China require attenuated vaccines designed from local strains in China. A previous IBV isolate (the YN strain) was passaged 118 times through SPF chicken embryonated eggs. As a result of this process, the virus becomes more adapted to the embryo, reflected by more efficient replication and higher pathogenicity for the embryo (data not shown). This attenuated YN strain was inoculated via the oral or oculonasal route into SPF chickens to compare the tissue tropism and pathogenicity to its parent strain.
After infection, birds in the attenuated group showed no common clinical signs as those observed in birds inoculated with the YN strain. However, gross lesions, although much milder and in fewer organs than those in chicks infected with the YN strain, were still detectible at necropsy. Histopathology indicated lower pathogenicity of the attenuated strain, which showed moderate inflammatory infiltration and epithelial degeneration. Lesions caused by the YN strain were similar to those described in the previous report, which characterized it as a virulent QX-like IBV strain (Feng et al., 2012).
In general, the systemic distribution of YN antigen was demonstrated by IHC staining and was most abundant in the respiratory and urinary systems. The epithelia of the trachea and alimentary tract of the YN-infected group were strongly immunoreactive to IBV antigens compared with the attenuated group. The presence of virus particles in both the infected groups post-inoculation indicates that a significant viral infection was delivered to the experimental SPF chickens and suggests that ciliated respiratory tract cells play a significant role in viral replication. These lesions appeared to damage the host cell, leading to the loss of cellular functions and decreasing immunity, increasing the opportunities for secondary or multiple infections (Davies et al., 2009). However, the increased antigen production and cilia loss in the YN strain supports it being more pathogenic than the attenuated strain.
In terms of the real-time RT-qPCR examination, both strains were detected in respiratory and non-respiratory tissues, including the kidney, trachea, lungs, proventriculus, duodenum, and bursa of Fabricius, indicating viral replication in these organs; however, this was relatively limited in the birds infected with the YN attenuated strain. We also observed that the viral RNA levels in the tracheas of birds in both groups declined after the peak, then reached another small peak. Loss of ciliated columnar epithelium, and, presumably, the associated viral replication in those cells, is a common lesion with IBV infection (Callison et al., 2006, Geerligs et al., 2011). The small peak may be related to the recovery of the epithelial cells in the upper-respiratory tract after extensive damage at the early infection period.
Among the tissues examined by histopathology, the damage was most severe in the trachea and kidney, the primary target organs of IBV (Uenaka et al., 1998). The viral loads were highest in these two organs. Therefore, the higher the viral load, the more severe the histologic damage, and the stronger the positive signal.
The YN attenuated strain was able to induce a higher humoral antibody response following inoculation. This suggests that this strain may be useful in vaccination programs under field conditions to reduce the economic losses caused by QX-like IBV infections on commercial layer and broiler farms.
Information regarding vaccine efficacy against circulating infectious bronchitis virus strains of China will provide valuable knowledge for the poultry industry when considering vaccine types. Therefore we invested the efficiency of the YN attenuated strain to determine if it could provide protection against the homologous and heterologous virulent strains. The results indicated that the strain aYN protected SPF birds against morbidity and mortality from challenge with the highly virulent strains YN and SD.
Assessment of immunity to challenge with IBV is most commonly done by removal of trachea at 4 or 5 days after challenge followed by either quantification of ciliostasis (Cavanagh et al., 1997, Cook et al., 1976) or detection of viral shedding. There was a clear decrease in ciliostasis scores in the YN attenuated vaccinated groups compared with the unvaccinated groups after challenge with homologous and heterologous virulent strains, indicating the YN attenuated strain could protect the respiratory tract efficiently. In addition, virus shedding in the infected chickens is a big challenge to the control of infectious bronchitis virus. It would encourage viral spread among chickens and support virus persistence. Thus the reduction of virus excretion should be taken into consideration when choosing the vaccine type and program. In our experiment, a significant decrease was observed in the vaccinated groups after challenge. We also noticed that the virus shedding inhibition in YN challenged group was more significant than the SD challenged group, which indicating that the vaccine strain could provide a better protection against the challenge with homologous strains.
The genetic mechanism responsible for loss of pathogenicity is still not well understood. The S1 gene is responsible for induction of protective immunity, and small differences in S1 contribute to poor cross protection(Cavanagh and Cook, 1997). It has been shown that a number of amino acid residues in S1 contribute to IBV attenuation (Cavanagh et al., 2005, Liu et al., 2007). Besides the S1 subunit, the nucleocapsid protein can also induce protective immune responses in chickens(Boots et al., 1992). Previous studies have shown that amino acid substitutions within the replicase gene may result in attenuation following serial passage in embryonated eggs (Armesto et al., 2009). Further genetic investigation will be necessary to determine the key mutations responsible for the attenuation in this sample. Moreover, the stability of the vaccine and its tendency to revert should be investigated in the future study before administration in the field to ensure the security and reliability of this vaccine.
The attenuated YN strain showed lower replicate ability and decreased pathogenicity than its parent strain, with an efficacious protection against homologous and heterologous field strains, indicating the potential to become an alternative vaccine candidate for controlling IB infections in China.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgement
This study was supported by Beijing Agriculture Innovation Consortium of Poultry Research System.
References
- Abro S.H., Renstrom L.H., Ullman K., Belak S., Baule C. Characterization and analysis of the full-length genome of a strain of the European QX-like genotype of infectious bronchitis virus. Arch. Virol. 2012;157:1211–1215. doi: 10.1007/s00705-012-1284-0. [DOI] [PubMed] [Google Scholar]
- Armesto M., Cavanagh D., Britton P. The replicase gene of avian coronavirus infectious bronchitis virus is a determinant of pathogenicity. PloS ONE. 2009;4:e7384. doi: 10.1371/journal.pone.0007384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijlenga G., Cook J.K.A., Gelb J., Jr, Wit J.J., d Development and use of the H strain of avian infectious bronchitis virus from the Netherlands as a vaccine: a review. Avian Pathol. 2011;33:550–557. doi: 10.1080/03079450400013154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boots A.M.H., Benaissa Trouw B.J., Hesselink W., Rijke E., Schrier C., Hensen E.J. Induction of anti-viral immune responses by immunization with recombinant-DNA encoded avian coronavirus nucleocapsid protein. Vaccine. 1992;10:119–124. doi: 10.1016/0264-410X(92)90028-I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callison S.A., Hilt D.A., Boynton T.O., Sample B.F., Swayne D.E., Jackwood M.W. Development and evaluation of a real-time Taqman RT-PCR assay for the detection of infectious bronchitis virus from infected chickens. J. Virol. Methods. 2006;138:60–65. doi: 10.1016/j.jviromet.2006.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh D. Coronavirus avian infectious bronchitis virus. Vet. Res. 2007;38:281–297. doi: 10.1051/vetres:2006055. [DOI] [PubMed] [Google Scholar]
- Cavanagh D., Cook J. Relationship between sequence variation in the S1 spike protein of infectious bronchitis virus and the extent of cross‐protection in vivo. Avian Pathol. 1997;26:63–74. doi: 10.1080/03079459708419194. [DOI] [PubMed] [Google Scholar]
- Cavanagh D., Davis P.J., Darbyshire J.H., Peters R.W. Coronavirus IBV: virus retaining spike glycopolypeptide S2 but not S1 is unable to induce virus-neutralizing or haemagglutination-inhibiting antibody, or induce chicken tracheal protection. J. Gen. Virol. 1986;67:1435–1442. doi: 10.1099/0022-1317-67-7-1435. [DOI] [PubMed] [Google Scholar]
- Cavanagh D., Elus M., Cook J. Relationship between sequence variation in the S1 spike protein of infectious bronchitis virus and the extent of cross‐protection in vivo. Avian Pathol. 1997;26:63–74. doi: 10.1080/03079459708419194. [DOI] [PubMed] [Google Scholar]
- Cavanagh D., Naqi S. Infectious bronchitis. Dis. Poult. 2003;11:101–119. [Google Scholar]
- Cavanagh D., Picault J.P., Gough R.E., Hess† M., Mawditt K., Britton P. Variation in the spike protein of the 793/B type of infectious bronchitis virus, in the field and during alternate passage in chickens and embryonated eggs. Avian Pathol. 2005;34:20–25. doi: 10.1080/03079450400025414. [DOI] [PubMed] [Google Scholar]
- Cook J.K., Darbyshire J., Peters R. The use of chicken tracheal organ cultures for the isolation and assay of avian infectious bronchitis virus. Arch. Virol. 1976;50:109–118. doi: 10.1007/BF01318005. [DOI] [PubMed] [Google Scholar]
- Cook J.K.A., Orbell S.J., Woods M.A., Huggins M.B. Breadth of protection of the respiratory tract provided by different live-attenuated infectious bronchitis vaccines against challenge with infectious bronchitis viruses of heterologous serotypes. Avian Pathol. 1999;28:477–485. doi: 10.1080/03079459994506. [DOI] [PubMed] [Google Scholar]
- Cook J., Chesher J., Baxendale W., Greenwood N., Huggins M., Orbell S. Protection of chickens against renal damage caused by a nephropathogenic infectious bronchitis virus. Avian Pathol. 2001;30:423–426. doi: 10.1080/03079450120066421. [DOI] [PubMed] [Google Scholar]
- Cook J., Jackwood M., Jones R. The long view: 40 years of infectious bronchitis research. Avian Pathol. 2012;41:239–250. doi: 10.1080/03079457.2012.680432. [DOI] [PubMed] [Google Scholar]
- Davies G., Genini S., Bishop S., Giuffra E. An assessment of opportunities to dissect host genetic variation in resistance to infectious diseases in livestock. Animal. 2009;3:415–436. doi: 10.1017/S1751731108003522. [DOI] [PubMed] [Google Scholar]
- Feng J.L., Hu Y.X., Ma Z.J., Yu Q., Zhao J.X., Zhang G.Z. Virulent avian infectious bronchitis virus, People's Republic of China. Emerg. Infect. Dis. 2012;18:1994–2001. doi: 10.3201/eid1812.120552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerligs H.J., Boelm G.J., Meinders C.A.M., Stuurman B.G.E., Symons J., Karaca K. Efficacy and safety of an attenuated live QX-like infectious bronchitis virus strain as a vaccine for chickens. Avian Pathol. 2011;40:93–102. doi: 10.1080/03079457.2010.542742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelb J., Jr, Cloud S. Effect of serial embryo passage of an Arkansas-type avian infectious bronchitis virus isolate on clinical response, virus recovery, and immunity. Avian Dis. 1983:679–687. [PubMed] [Google Scholar]
- Gelb J., Jr, Wolff J., Moran C. Variant serotypes of infectious bronchitis virus isolated from commercial layer and broiler chickens. Avian Dis. 1990;35:82–87. [PubMed] [Google Scholar]
- Gelb J., Jr, Weisman Y., Ladman B., Meir R. S1 gene characteristics and efficacy of vaccination against infectious bronchitis virus field isolates from the United States and Israel (1996 to 2000) Avian Pathol. 2005;34:194–203. doi: 10.1080/03079450500096539. [DOI] [PubMed] [Google Scholar]
- Huang Y.P., Wang C.H. Development of attenuated vaccines from Taiwanese infectious bronchitis virus strains. Vaccine. 2006;24:785–791. doi: 10.1016/j.vaccine.2005.08.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackwood M.W., Hilt D.A., Brown T.P. Attenuation, safety, and efficacy of an infectious bronchitis virus GA98 serotype vaccine. Avian Dis. 2003;47:627–632. doi: 10.1637/6094. [DOI] [PubMed] [Google Scholar]
- Jackwood M.W., Hilt D.A., Lee C.W., Kwon H.M., Callison S.A., Moore K.M. Data from 11 years of molecular typing infectious bronchitis virus field isolates. Avian Dis. 2005;49:614–618. doi: 10.1637/7389-052905R.1. [DOI] [PubMed] [Google Scholar]
- Liu S.W., Chen J.F., Han Z.X., Zhang Q.X., Shao Y.H., Tong G.Z. Infectious bronchitis virus: S1 gene characteristics of vaccines used in China and efficacy of vaccination against heterologous strains from China. Avian Pathol. 2006;35:394–399. doi: 10.1080/03079450600920984. [DOI] [PubMed] [Google Scholar]
- Liu S.W., Han Z.X., Chen J.F., Liu X., Shao Y.H., Rong J.G. S1 gene sequence heterogeneity of a pathogenic infectious bronchitis virus strain and its embryo-passaged, attenuated derivatives. Avian Pathol. 2007;36:231–234. doi: 10.1080/03079450701338730. [DOI] [PubMed] [Google Scholar]
- Liu X.L., Su J.L., Zhao J.X., Zhang G.Z. Complete genome sequence analysis of a predominant infectious bronchitis virus (IBV) strain in China. Virus Genes. 2009;38:56–65. doi: 10.1007/s11262-008-0282-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj G.D., Jones R. Infectious bronchitis virus: immunopathogenesis of infection in the chicken. Avian Pathol. 1997;26:677–706. doi: 10.1080/03079459708419246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed L.J., Muench H. A simple method of estimating fifty per cent endpoints. Am. J. Epidemiol. 1938;27:493–497. [Google Scholar]
- Terregino C., Toffan A., Beato M.S., De Nardi R., Meini A., Ortali G. Pathogenicity of a QX strain of infectious bronchitis virus in specific pathogen free and commercial broiler chickens, and evaluation of protection induced by a vaccination programme based on the Ma5 and 4/91 serotypes. Avian Pathol. 2008;37:487–493. doi: 10.1080/03079450802356938. [DOI] [PubMed] [Google Scholar]
- Uenaka T., Kishimoto I., Sato S., Animas S., Ito T., Cook J.K.A. Intracloacal infection with avian infectious bronchitis virus. Avian Pathol. 1998;27:309–312. doi: 10.1080/03079459808419342. [DOI] [PubMed] [Google Scholar]
- Valastro V., Monne I., Fasolato M., Cecchettin K., Terregino C., Cattoli G. QX-type infectious bronchitis virus in commercial flocks in the UK. Vet. Rec. 2010;167:865. doi: 10.1136/vr.c6001. [DOI] [PubMed] [Google Scholar]
- Wang Y.D., Wang Y.L., Zhang Z.C., Fan G.C., Jiang Y.H., Liu X.E. Isolation and identification of glandular stomach type IBV (QX IBV) in chickens. Chin. J. Anim. Quarantine. 1998;15:1–3. [Google Scholar]
- Xu C.P., Zhao J.X., Hu X.D., Zhang G.Z. Isolation and identification of four infectious bronchitis virus strains in China and analyses of their S1 glycoprotein gene. Vet. Microbiol. 2007;122:61–71. doi: 10.1016/j.vetmic.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Liu X.Y., Cheng J.L., Wu Y.P., Zhang G.Z. Molecular characterization of an infectious bronchitis virus strain isolated from northern China in 2012. Arch. Virol. 2014;159:3457–3461. doi: 10.1007/s00705-014-2213-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zsofia B., Tamas M., Tibor S., Eva S., Veronika K., Zsolt A.-T. Comparison of the pathogenicity of QX-like, M41 and 793/B infectious bronchitis strains from different pathological conditions. Avian Pathol. 2009;38:449–456. doi: 10.1080/03079450903349196. [DOI] [PubMed] [Google Scholar]