Abstract
It has been well documented that BST2 restricts the release of enveloped viruses by cross-linking newly produced virions to the cell membrane. However, it is less clear whether and how BST2 inhibits the release of enveloped viruses which bud via the secretory pathway. Here, we demonstrated that BST2 restricts the release of Japanese encephalitis virus (JEV) whose budding occurs at the ER-Golgi intermediate compartment, and in turn, JEV infection downregulates BST2 expression. We further found that the JEV envelope protein E, but not other viral components, significantly downregulates BST2 with the viral protein M playing an auxiliary role in the process. Envelope protein E-mediated BST2 downregulation appears to undergo lysosomal degradation pathway. Additional study revealed that the transmembrane domain and the coiled-coil domain (CC) of BST2 are the target domains of viral protein E and that the N- and C-terminal membrane anchors and the CC domain of BST2 are essential for blocking JEV release. Our results together indicate that the release of enveloped viruses whose budding take place in an intracellular compartment can be restricted by BST2.
Keywords: JEV, BST2, Restriction, Envelope protein E, Antagonism
Highlights
-
•
BST2 restricts the release of JEV virus particles.
-
•
JEV protein E downregulates the expression of BST2 through the lysosomal pathway.
-
•
The TM and CC domains of BST2 are important targets of JEV protein E.
1. Introduction
Bone marrow stromal cell antigen 2 (BST2) is an unusual type II glycoprotein composed of a short N-terminal cytoplasmic domain, a transmembrane domain, an extracellular loop and a C-terminal Glycosylphosphatidylinositol (GPI) anchor (Hinz et al., 2010, Kupzig et al., 2003, Perez-Caballero et al., 2009). A number of viruses have been reported to be restricted by BST2, including retroviruses (alpha-, beta-, delta-, lenti-, and spuma-)(Jouvenet et al., 2009), arenaviruses (Lassa virus and Machupo virus)(Radoshitzky et al., 2010; Sakuma et al., 2009), herpesviruses (Kaposi's sarcoma-associated herpesvirus and human simplex virus) (Blondeau et al., 2013, Liu et al., 2015, Mansouri et al., 2009, Zenner et al., 2013), filoviruses (Ebola virus and Marburg virus)(Jouvenet et al., 2009; Sakuma et al., 2009), rhabdoviruses (vesicular stomatitis virus)(Weidner et al., 2010), paramyxoviruses (Nipah virus), orthomyxoviruses (influenza A virus)(Watanabe et al., 2011; Yondola et al., 2011), and flaviviruses (Dengue virus)(Pan et al., 2012). To date, several viral antagonists have been shown to counteract the restriction of BST2, including the Vpu (Neil et al., 2008), Env (Gupta et al., 2009) and Nef (Jia et al., 2009, Sakuma et al., 2009) proteins from primate lentiviruses, the K5 protein from KHSV (Mansouri et al., 2009, Pardieu et al., 2010), the glycoprotein (GP) from Ebola virus (Sakuma et al., 2009), the virion host shutoff protein (vhs) and glycoprotein M (gM) from HSV-1 (Blondeau et al., 2013, Zenner et al., 2013), and multiple viral glycoproteins (gB, gD, gH, gL) from HSV-2 (Liu et al., 2015). The fate of BST2 counteracted by viruses remains inconclusive. For instance, while some studies reported that Vpu mediates BST2 degradation through proteasomal pathway (Douglas et al., 2009, Mangeat et al., 2009), others suggested that Vpu induces the retention of BST2 within the endolysosomal system with concomitant partial lysosomal degradation (Iwabu et al., 2009). KSHV K5 promotes BST2 lysosomal degradation through the ubiquitination of a single lysine residue in the cytoplasmic domain of the protein, which targets BST2 for transport to lysosomes via the ESCRT pathway (Mansouri et al., 2009, Pardieu et al., 2010), whereas HIV-2 Env exclusively sequesters BST2 within the TGN with no concomitant degradation (Hauser et al., 2010, Le Tortorec and Neil, 2009).
As a broad-spectrum restriction factor against enveloped viruses, the antiviral activity of BST2 is primarily thought to act on the cell surface, where most viruses acquire their envelopes by directly budding from the plasma membrane. Of interest, recent studies reported that human coronavirus 229E and hepatitis C virus, whose assembly take place in the ER and release from cells via secretory pathway, are inhibited at intracellular membranes by BST2 (Pan et al., 2013, Wang et al., 2014). BST2 localizes in the plasma membrane, TGN, and some early endosomal compartments, such as the recycling endosome compartment (Kupzig et al., 2003). Nevertheless, it remains to be determined whether the restriction of BST2 on viruses releasing via secretory pathway is viral species specific.
Japanese encephalitis virus (JEV) is a member of the Flaviviridae family. The infection of JEV can cause nervous system disease with irreversible neurological damage in humans and animals (Vaughn and Hoke, 1992). The genome of JEV has one open reading frame (ORF) encoding a single polyprotein, which is cleaved into 3 structural proteins - capsid protein (C), precursor membrane protein (prM) and envelope protein (E), and 7 non-structural proteins - NS1, NS2A, NS2B, NS3, NS4A, NS4B and NS5. The genomic RNA of JEV is organized within multiple copies of the C protein, which forms a nucleocapsid surrounded by a host-derived lipid bilayer containing two viral surface proteins, prM/M and E (Heinz and Allison, 2003, Kim et al., 2008). JEV assembly and release are similar to those of human coronavirus 229E and hepatitis C virus (Pan et al., 2013, Wang et al., 2014). JEV progeny virion uses the intrinsic secretory pathways to bud from the membranes of the endoplasmic reticulum (ER) and Golgi apparatus (Rice, 1996). Whether BST2 restricts the release of JEV has yet to be determined.
In the current study, we investigated whether BST2 plays a role in the release of JEV progeny virions and the potential mechanisms by which JEV counteracts BST2. Our data together indicated that BST2 is capable of restricting the release of JEV progeny virions and that the JEV protein E functions as an antagonist to counteract the restriction of BST2 by interacting with the TM and CC domains of BST2, leading to its degradation in the lysosomal pathway. Our findings together support that BST2 is capable of inhibiting the release of enveloped viruses at both the plasma membrane and intracellular membranes.
2. Results
2.1. BST2 reduces the release of JEV progeny virions
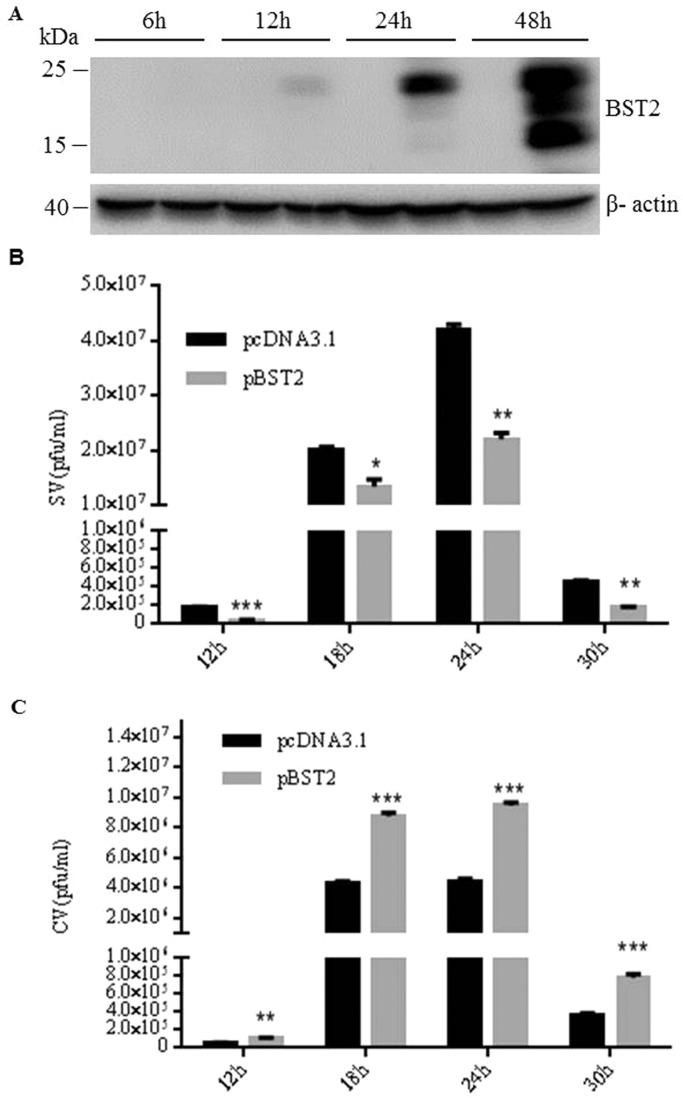
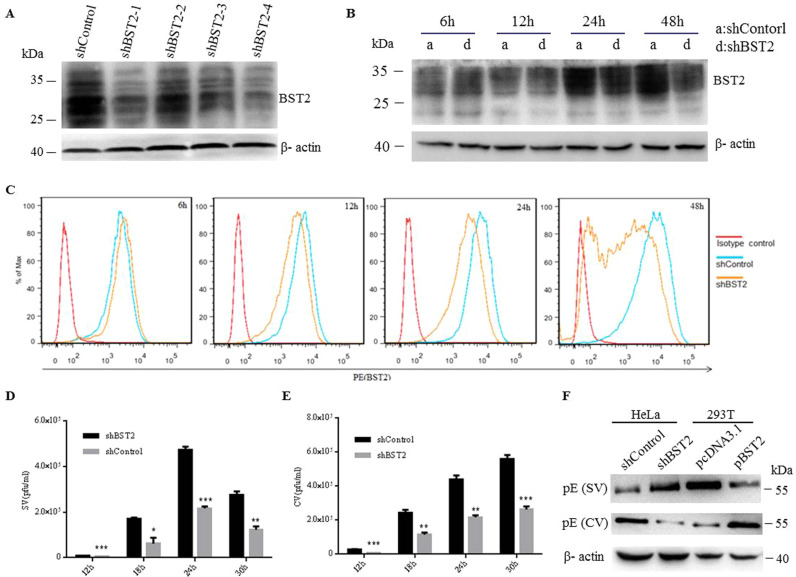
To determine whether BST2 is capable of inhibiting the release of JEV progeny virions, we conducted the virus release assay using two different cell lines, 293T cells which do not express detectable level of BST2 and HeLa cells which constitutively express BST2. Firstly, 293T cells were transfected with BST2 expression plasmid (pBST2) or pcDNA3.1 and then collected at different time points post transfection. The expression of BST2 was confirmed by Western blot. As shown in Fig. 1A, BST2 displayed several isoforms, likely due to heterogeneity of glycosylation during post-translational modification (Andrew et al., 2009). There was no detectable BST2 at 6 h post transfection. 293T cells transfected with pBST2 or pcDNA3.1 were infected with JEV at an MOI of 10 at 6 h post transfection. At different time points post infection, plaque assays were conducted to assess the amounts of infectious cell-free virions (SV) and cell-associated progeny virions (CV) containing both extracellular viruses attached to the cell and intracellular viruses. As shown in Fig. 1B, there was about 2-fold reduction of infectious cell-free virions in the presence of BST2. In contrast, the titer of CV in BST2-expressing cells was higher than that from pcDNA3.1 transfected cells (Fig. 1C), suggesting that exogenous expression of BST2 restricts the release of JEV progeny virions. The antiviral property of endogenous BST2 was also examined in HeLa cells. Retroviral vectors expressing BST2 shRNA or control shRNA were used. Western blot showed that, at 48 h post transfection, all of the four BST2 shRNAs reduced the expression of BST2 with different efficiency and the most effective shRNA was the #4BST2 shRNA ( Fig. 2A). Subsequently, the #4BST2 shRNA was used in the following experiments. Flow cytometry and western blot analysis indicated that BST2 shRNA reduced cell-surface and total expression levels of BST2 at 12 h post-transfection (Fig. 2B and C). HeLa cells transfected with BST2 shRNA or control shRNA were infected with JEV at an MOI of 25 at 6 h post shRNA transfection. The amounts of SV and CV were analyzed at different time points post infection. As shown in Fig. 2D, down-regulation of BST2 led to a 2.5-fold increase of SV production, whereas the CV of the control cells was more than that in the BST2-depleted HeLa cells (Fig. 2E). The level of protein E in SV or CV were determined by western blot assay at 24 h post infection. As shown in Fig. 2F, there was much more protein E in SV in the absence of BST2. In contrast, the level of protein E in CV of BST2-expressing cells was higher than that from BST2 shRNA or pcDNA3.1 transfected cells. These data together demonstrated that BST2 can function as a restriction factor to inhibit the release of JEV progeny virions.
Fig. 1.
Overexpression of BST2 decreases the release of JEV progeny virions. (A) 293T cells were transfected with pBST2 or pcDNA3.1, harvested at different time points, lysed and the total expression of BST2 was detected by western blot. (B and C) 293T cells were transfected with pBST2 or pcDNA3.1. At 6 h post transfection, cells were infected with JEV at an MOI of 10. The titers of supernatant virus (SV) or cell-associated virus (CV) were determined by plaque assay at the indicated time points post infection. Data shown are mean ± SD of three independent experiments with each condition performed in triplicate. Compared to pcDNA3.1, *P < 0.05; **P < 0.01; ***P < 0.001.
Fig. 2.
Downregulation of endogenous BST2 enhances the release of JEV progeny virions. (A) HeLa cells were transfected with BST2-specific shRNA or control shRNA. At 48 h post infection, cells were harvested and lysed for western blot analysis. (B and C) HeLa cells transfected with BST2 shRNA or non-targeting shRNA were collected at different time points and analyzed by western blot (B) and flow cytometry (C). (D and E) HeLa cells were infected with JEV at an MOI of 25 following 6 h transfection with BST2-specific shRNA or control shRNA, and the titers of supernatant virus (SV) or cell-associated virus (CV) were determined by plaque assay at the indicated time points. (F) HeLa cells were infected with JEV at an MOI of 25 following 6 h transfection with BST2 shRNA or control shRNA. 293T cells were transfected with pBST2 or pcDNA3.1. The levels of protein E in SV or CV were determined by western blot assay at 24 h post infection. Data shown are mean ± SD of three independent experiments with each condition performed in triplicate. Compared to control shRNA, *P < 0.05; **P < 0.01; ***P < 0.001.
2.2. JEV infection downregulates the expression of BST2
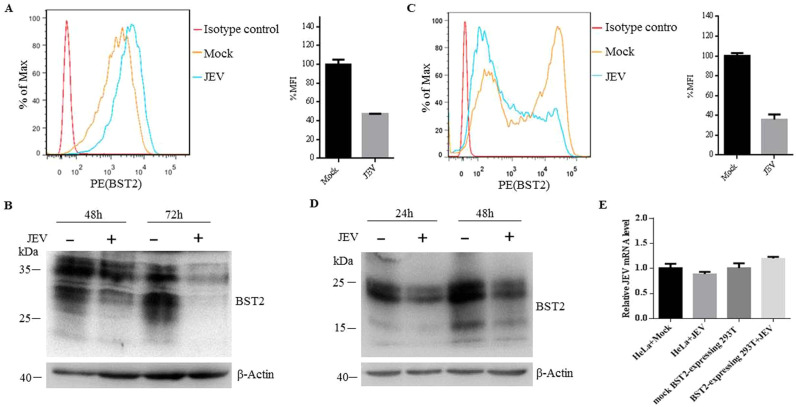
Different viruses have evolved a variety of countermeasures against BST2, the majority of which cause the removal of BST2 from the cell surface followed by either sequestration in intracellular compartments or degradation in the lysosomes or proteasome. For instance, HIV-1 and Sendai virus can downregulate the expression level of BST2 on the cell surface (Bampi et al., 2013, Douglas et al., 2009). To investigate whether JEV has a similar capability, we first examined whether JEV infection can reduce the endogenous expression of BST2. HeLa cells were infected with JEV at an MOI of 25. At different time points post infection, cells were collected and analyzed. The total expression level of BST2 was decreased at 36 h post infection (Fig. S1A), while the cellular localization of BST2 did not changed (Fig. S1B). As shown in Fig. 3A, the mean fluorescence intensity (MFI) of cell-surface BST2 in infected cells was lower than that in the mock-infected cells, indicating that the surface expression level of BST2 was significantly downregulated following JEV infection. Western blot analysis showed that the total expression level of BST2 was also decreased in the parallel samples (Fig. 3B). We subsequently investigated whether JEV infection reduced the exogenous expression of BST2. After 6 h post transfection with pBST2, 293T cells were infected with JEV at an MOI of 10. Consistent with the results shown in Fig. 3A and B, JEV infection significantly decreased cell surface and total expression of BST2 in 293T cells (Fig. 3C and D). To examine whether BST2 downregulation was due to the depletion of BST2 mRNA as suggested by others (Zenner et al., 2013), BST2 mRNA was measured by real-time PCR. As shown in Fig. 3E, the BST2 mRNA level in JEV-infected cells had no significant difference compared with that in uninfected cells. These data suggested that the expression level of BST2 was reduced by JEV infection.
Fig. 3.
JEV infection downregulates the expression of BST2. (A) HeLa cells were mock-infected or infected with JEV at an MOI of 25. At 48 h post infection, cells were fixed and probed with PE-conjugated anti-BST2 antibody. Cells were then analyzed by flow cytometry. The bar graph is mean ± SD of MFI from three independent experiments with one representative histogram being shown. (B) At 48 or 72 h post infection, HeLa cells infected or uninfected with JEV were collected and analyzed by western blot. (C) 293T cells were transfected with plasmid expressing BST2 or empty plasmid. At 6 h post transfection, cells were infected with JEV, collected at 24 h post infection and stained with anti-BST2 antibody for flow cytometry analysis. The bar graph is mean ± SD of MFI from three independent experiments with one representative histogram being shown. (D) At 24 or 48 h post infection, 293T cells infected or uninfected with JEV were collected and analyzed by western blot. One representative experiment out of three is shown. (E) HeLa cells were transfected with control shRNA or BST2 specific shRNA, infected with JEV at an MOI of 25. 293T cells were transfected with empty plasmid or plasmid expressing BST2, infected with JEV at an MOI of 10. The total RNA of the samples was extracted, and the BST2 mRNA was determined by RT-PCR. The mRNA level of GAPDH was scored in parallel and used as an internal control. Data shown are mean ± SD of three independent experiments.
2.3. JEV protein E downregulates the expression of BST2 through the lysosomal pathway
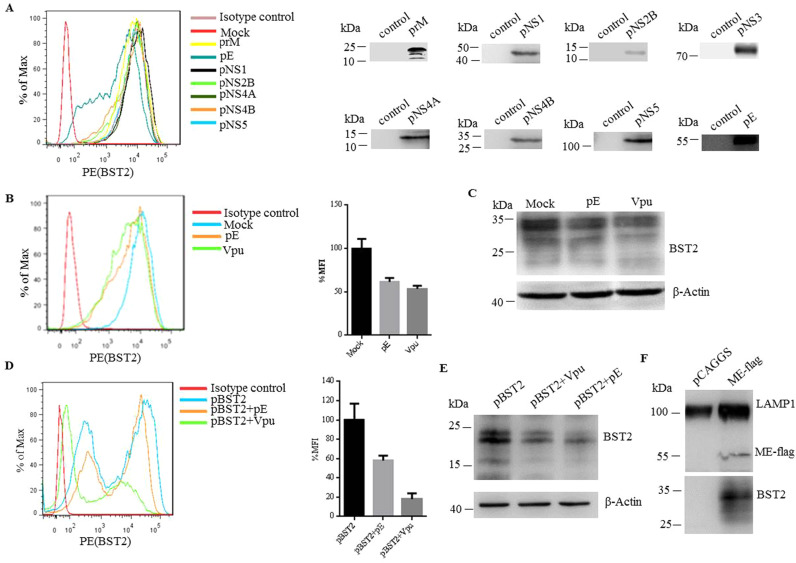
Many viruses counteract the function of BST2 to promote the surface removal of BST2. For instance, HIV-1 encodes Vpu which interacts with BST2 and leads to its degradation (Douglas et al., 2009, Mansouri et al., 2009). In an attempt to clarify the mechanism of BST2 degradation caused by JEV, expressing vectors encoding different JEV structural and non-structural proteins were transfected into HeLa cells, respectively. At 48 h post transfection, the surface expression of BST2 was examined by flow cytometry. We observed that BST2 was downregulated on the cell surface following transfection of plasmid expressing envelope protein E ( Fig. 4A). We compared the downregulation effects of protein E in both HeLa cells and BST2-transfected 293T cells, while HIV-1 Vpu was used as a positive control. As shown in Fig. 4B and C, both cell surface and total expression levels of BST2 were markedly decreased in protein E-expressing HeLa cells at 48 h post transfection. Similarly, the expression of protein E profoundly reduced the surface and total expression of BST2 in BST2-transfected 293T cells (Fig. 4D and E). To examine whether envelope protein E mediated-downregulation of BST2 undergoes lysosomal degradation, we used a lysosome enrichment kit (Thermo) to isolate and enrich intact lysosomes from cells transfected with pCAGGS or plasmids expressing ME-flag. The prepared cell extracts were ultracentrifuged by density gradient centrifugation. The corresponding bands were collected and the harvested lysosome pellets were detected by western blot. As shown in Fig. 4F, the lysosome marker LAMP1, BST2 and protein E all existed in the separated samples from ME-flag-transfected cells. In contrast, only LAMP1 existed in the samples from pCAGGS transfected cells. To examine whether protein E mediated-downregulation of BST2 undergoes proteasomal degradation pathway, HeLa cells were transfected with pCAGGS or prME plasmid followed by cultivation in the presence of the proteasome protease inhibitor (MG132). At 24 h post transfection, cells were processed for immunofluorescence staining for subcellular localization of BST2 and a proteasome marker 20S proteasome, showing that BST2 colocalized with the 20S proteasome in the presence or absence of protein E (Fig. S2). These data together indicated that protein E mediated-downregulation of BST2 undergoes lysosomal degradation.
Fig. 4.
JEV envelope protein E antagonizes BST2. (A) HeLa cells were transfected with plasmid expressing the structural or non-structural proteins of JEV. The surface expression of BST2 was analyzed by flow cytometry (left), while the total expression of JEV proteins was analyzed by western blot (right). (B) HeLa cells were transfected with plasmid expressing HIV-1 Vpu, JEV envelope protein E and control plasmid pcDNA3.1(+), and the cell surface expression of BST2 was analyzed by flow cytometry. The bar graph is mean ± SD of MFI from three independent experiments with one representative histogram being shown. (C) The total expression level of BST2 in the parallel samples of (B) was analyzed by western blot. (D) The cell surface expression level of BST2 on 293T cells transfected with plasmid expressing BST2 alone or cotransfected with plasmids expressing BST2 and JEV protein E or HIV-1 Vpu. The bar graph is mean ± SD of MFI from three independent experiments with one representative histogram being shown. (E) The total expression level of BST2 in the parallel samples of (D) was analyzed by western blot. (F) Examination of protein E-mediated BST2 degradation. HeLa cells were transfected with pcDNA3.1 or plasmid expressing gE-flag. The prepared cell extracts were ultracentrifuged by density gradient centrifugation and the lysosome band was located in the top 2 mL of the gradient. The corresponding bands were collected and the finally harvested lysosome pellets were detected by western blot. One representative experiment out of three is shown.
2.4. JEV protein M is involved in BST2 downregulation mediated by protein E
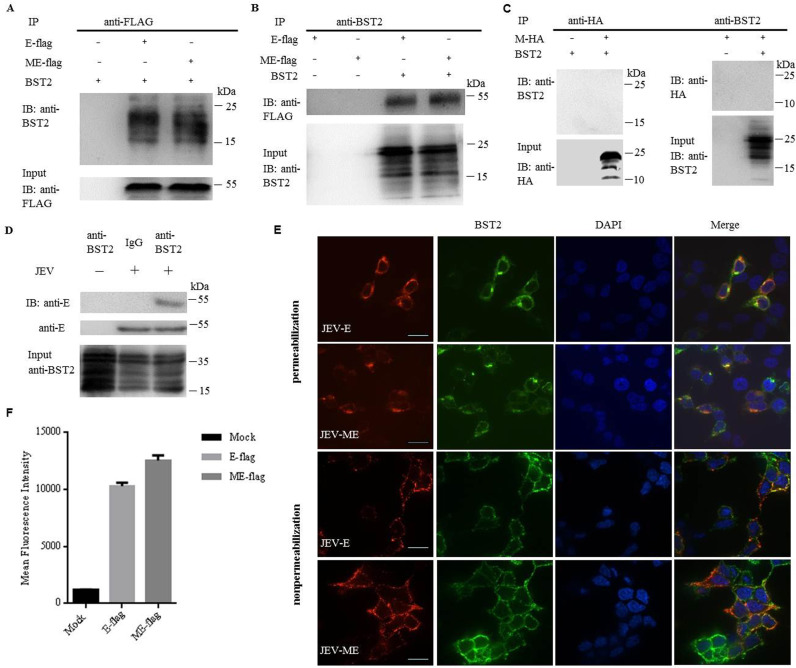
It has been reported that the prM plays an important role in the intracellular localization of JEV protein E and that the cell surface expression of protein E could hardly be detected in the absence of prM (Du et al., 2015). Although prM alone did not downregulate BST2, whether it plays a role in facilitating the function of protein E on BST2 downregulation was unclear. Therefore, co-IP experiments were carried out to assess the role of prM during the process of BST2 downregulation. 293T cells were cotransfected with plasmids encoding E-flag (the protein E with a flag tag) or prME-flag (the fusion protein of E and prM with a flag tag) and BST2. Precleared cell lysates were incubated with an anti-flag antibody, and the resulting complexes were analyzed by western blotting using the antibody against BST2. As shown in Fig. 5A, BST2 was specifically and effectively precipitated in the presence of protein E. The co-IP experiments were also performed by pulling down with the anti-BST2 antibody, followed by western blot with the anti-flag antibody. The flag antibody was able to specifically precipitate the immune complex that contained the BST2 and E-flag or ME-flag (Fig. 5B). To preclude a possible interaction between prM and BST2, 293T cells were contransfected with prM-HA and BST2. The lysates were immunoprecipitated with the anti-HA antibody and the anti-BST2 antibody, respectively, showing that prM did not interact with BST2 (Fig. 5C). To further confirm the interaction of protein E with BST2 in JEV-infected cells, we used JEV to infect HeLa cells at an MOI of 25. At 48 h post infection, cells were collected and assayed by co-IP. The interaction between BST2 and protein E was observed in JEV-infected cells (Fig. 5D). Immunofluorescence assay was also carried out in parallel. 293T cells were cotransfected with plasmids encoding E-flag (the protein E with a flag tag) or prME-flag (the fusion protein of E and prM with a flag tag) and BST2. At 24 h post transfection, cells were analyzed for colocalization by confocal microscopy. As shown in Fig. 5E, the JEV protein E co-localized with BST2 on cell surfaces of both non-permeabilized and permeabilized cells. In the presence of prM, more protein E was detected on the cell surface than that in the absence of prM (Fig. 5F). Taken together, these results confirmed a specific interaction between protein E and BST2 and the auxiliary role of prM in this process.
Fig. 5.
JEV protein E physically interacts with BST2. 293T cells were cotransfected with pBST2 and plasmid expressing E-flag, ME-flag or M-HA. At 48 h post transfection, cell lysates were analyzed by co-IP. Co-IP was pulled down using the anti-BST2, anti-flag or anti-HA antibody. Proteins were immunoprecipitated with the anti-flag (A) or anti-BST2 antibody (B) as indicated. (C) Proteins were immunoprecipitated with the anti-HA or anti-BST2 antibody as indicated. (D) HeLa cells were infected with JEV at a MOI of 25. At 48 h post infection, cell lysates were analyzed by co-IP. Co-IP was pulled down using the anti-BST2 antibody. One representative experiment out of three is shown. (E) Colocalization of BST2 with JEV E-flag or ME-flag. 293T cells cotransfected with pBST2 and plasmid expressing E-flag or ME-flag were costained with anti-flag (red) and anti-BST2 (green) antibodies. Nuclei were counterstained with DAPI (blue). Representative confocal images from three independent experiments are shown. Scale bars in all panels represent 10 µm. (F) HeLa cells were transfected with plasmid expressing protein E. The surface expression of BST2 was analyzed by flow cytometry.
2.5. The TM and CC domains of BST2 are important targets of JEV protein E
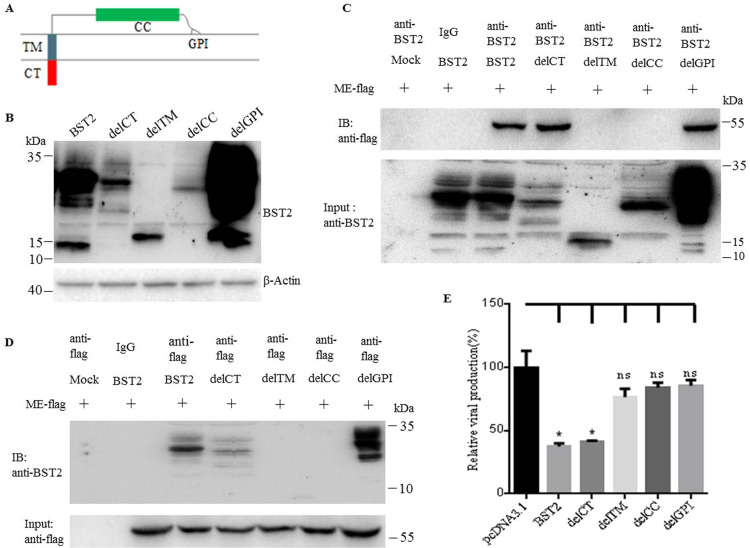
To investigate which domain of BST2 is the target of JEV protein E, the interaction between different BST2 mutants and protein E was examined by coimmunoprecipitation assay. A panel of BST2 mutants (delCT, delTM, delCC and delGPI) were constructed as depicted in Fig. 6A and the expression was confirmed by western blot (Fig. 6B). The precleared cell lysates from transfected cells were incubated with the anti-BST2 antibody or an isotype control antibody. The precipitates were subsequently analyzed by western blot using the anti-flag antibody. As shown in Fig. 6C, the BST2 mutants delCT and delGPI but not delTM and delCC were specifically and effectively precipitated in the presence of protein E. Furthermore, co-IP assays were performed by pulling down with the antibody against flag followed by western blot with the antibody against the BST2, showing that the delCT and delGPI but not delTM and delCC were specifically coimmunoprecipitated (Fig. 6D). These results suggested that the TM and CC domains of BST2 are required for its physical interaction with protein E.
Fig. 6.
Mapping of BST2 domains essential for its anti-JEV activity. (A) Schematic representation of the BST2 mutants. (B) 293T cells were cotransfected with pME-flag and plasmid expressing wild type BST2 or BST2 mutants. At 48 h post transfection, cells were collected and lysed. The expression of BST2 mutants in 293T cells was confirmed by Western blot. (C) and (D) The interaction between protein E and BST2 mutants in the parallel samples of (B) was analyzed by Co-IP. (E) 293T cells were transfected with pcDNA3.1 or wild type BST2 or BST2 mutants. At 6 h post transfection, cells were infected with JEV at an MOI of 10 and the relative viral production of JEV was assessed by viral release assay. One representative experiment out of three is shown.
To further determine which domain of BST2 was essential for its antiviral activity. 293T cells were tranfected with pcDNA3.1, plasmid expressing wild type BST2 or its mutants (delCT, delTM, delCC and delGPI), respectively. At 6 h post transfection, cells were infected with JEV at an MOI of 10, and the supernatants were collected at 24 h post infection followed by the determination of virus titers. As shown in Fig. 6E, like wild type BST2, mutant delCT inhibited the release of JEV progeny virions, while mutants delTM, delGPI and delCC did not show such antiviral activity. These data together demonstrated that the N- and C-terminal membrane anchors and the coiled-coiled domain of BST2 are required for its inhibition of JEV release.
3. Discussion
It has been reported that human coronavirus 229E and hepatitis C virus whose assembly take place in the ER and release from cells occurs via secretory pathway are inhibited by BST2 at intracellular membranes, although the underlying mechanism is less clear (Pan et al., 2013, Wang et al., 2014). In the current study, we found that BST2 restricts the release of JEV, which core particles bud through the endoplasmic reticulum membrane and are transported to the secretory pathway via the trans-Golgi network, suggesting that virions linked to vesicle membranes via BST2 are retained on cells surfaces following the exocytotic fusion of virion-containing vesicles with plasma membranes. Our findings indicate that BST2 is a broad-spectrum restriction factor capable of inhibiting virus budding at both the plasma membrane and the intracellular membranes. JEV infection downregulated both cell surface and total expression of BST2, implying that the reduction of BST2 mediated by JEV infection was likely due to protein degradation.
Although BST2 can restrict the release of enveloped viruses, many viruses have evolved specific antagonists to counteract such antiviral activity. Examples include HIV-1 Vpu, HIV-2 Env, simian immunodeficiency virus Nef and Env, Ebola and Sendai virus GP, and KSHV K5. We found that JEV infection significantly downregulated BST2 expression. After examining the structural and non-structural proteins of JEV, we identified envelope proteins E as the only viral component that downregulated the cell surface and total expression levels of BST2. Co-IP assays demonstrated a specific interaction between the viral protein E and BST2, revealing that protein E functions as a viral antagonist to counteract the restriction by the host. JEV infection or viral protein E resulted in BST2 degradation in both HeLa cells which constitutively express BST2 and 293T cells exogenously expressing BST2, indicating that JEV counteracts BST2 in a cell type and expression mode independent fashion. There are several proposed mechanisms for viral proteins to antagonize BST2, including lysosomal degradation, proteasomal degradation, and/or sequestration/retargeting of BST2 to the trans-Golgi network. Using proteasomal protease inhibitors, immunofluorescence imaging revealed that BST2 colocalized with the proteasome marker 20S proteasome in the presence or absence of protein E. Western blot assay showed that protein E and BST2 existed in the isolated lysosomes of protein E-transfected cells. These together indicate that protein E promotes the degradation of BST2 via lysosomal pathway.
JEV consists of a nucleocapsid surrounded by a lipid bilayer containing the envelope glycoprotein and the membrane protein (M). The M protein is derived from a glycosylated precursor membrane protein (prM) following a cleavage by a furin-like protease when immature virions are released via the secretory pathway (Mukhopadhyay et al., 2005). It has been demonstrated that prM is required for the proper folding, membrane association and assembly of the flavivirus envelope protein E (Konishi and Mason, 1993, Mukhopadhyay et al., 2005). We found that higher expression level of protein E was detected in the presence of prM than that in absence of prM, indicating that prM likely plays an auxiliary role in protein E-mediated BST2 downregulation, likely by promoting more protein E to interact with BST2.
The N-terminal transmembrane domain (TM) and the C-terminal GPI anchor domain (GPI) at either end of the coiled-coil domain (CC) of BST2 were showed to be important for BST2 mediated restriction of HIV-1 and Xenotropic murine leukemia virus-related virus (XMRV) release (Hu et al., 2012, Perez-Caballero et al., 2009). In our study, following construction of a panel of BST2 mutants, co-IP and virus release assays indicated that the TM and CC domains of BST2 are also essential for the interaction with protein E and for inhibiting JEV release. Of note, although the GPI anchor does not directly interact with protein E, it appears to be essential for the antiviral activity of BST2.
In conclusion, our data demonstrated that BST2 is capable of restricting the release of JEV and in turn JEV infection downregulates the expression of BST2. JEV envelope protein E functions as an antagonist to counteract the antiviral activity of BST2 while prM plays an auxiliary role in this process. The TM and CC domains of BST2 are important for the interaction between protein E and BST2, while the N- and C-terminal membrane anchors and the coiled-coiled domain of BST2 are required for its inhibition of JEV infection.
4. Materials and methods
4.1. Cells and virus
HeLa, human embryonic kidney 293T (HEK-293T) and baby hamster kidney cell lines (BHK-21) were maintained in Dulbecco's Modified Eagle's medium (DMEM, HyClone) supplemented with 10% fetal bovina serum (FBS, GIBCO), 100U/mL penicillin/streptomycin and 2 mM glutamine. All cell lines were grown at 37 °C in the presence of 5% CO2.
The JEV strain SA14-14 was propagated in BHK-21 cells utilizing DMEM medium containing 2% FBS. Virus titer was determined by a plaque-forming assay on BHK-21 cells as previously described (Anand et al., 2010).
4.2. Antibodies, plasmids and shRNAs
Rabbit anti-human BST2 antibody was kindly provided by the National Institutes of Health (NIH). BST2 expressing plasmid (pBST2) was from Origene. Mouse monoclonal antibody against FLAG was purchased from Sigma (F1804). Mouse monoclonal antibody against HA (sc-7392) and mouse monoclonal antibody against β-actin (sc-81178) were purchased from Santa Cruz Biotechnology. Rabbit polyclonal antibody against LAMP1 (ab24170) and mouse monoclonal antibody against JEV glycoprotein E (ab41671) were purchased from Abcam.
The BST2 mutants delCT, delTM, delCC and delGPI were generated according to the methods described previously (Perez-Caballero et al., 2009). The ORFs of envelope protein E, prM and both envelope protein E and prM were cloned into pCAGGS (Tani et al., 2010) with a flag or a HA tag at the C terminal, termed E-flag, prM-HA and ME-flag, respectively. BST2 shRNA (TG314427) and control shRNA (TG314427) were purchased from Origene.
4.3. JEV release assay
HeLa monolayers were transfected with BST2 or control shRNA using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. At 6 h post transfection, cells were infected with JEV at an MOI of 25. 293T monolayers were transfected with plasmid expressing BST2 or control plasmid followed by infection with JEV at an MOI of 10. Two hours later, the medium was removed and replaced with acid-citrate buffer (pH=3) to inactivate extracellular viruses, followed by three washes with PBS and the addition of fresh medium. The supernatants were collected at different time points post infection, centrifuged to remove cellular debris and virus titers were determined by plaque assay on BHK-21 cells. For cell-associated virus titers, cells were lysed by 3 freeze-thaw cycles, cleared by centrifugation and titered as described above.
4.4. Flow cytometry
For BST2 cell surface staining, cells were resuspended in flow cytometry buffer (1×PBS–3% fetal bovine serum), and incubated with a phycoerythrin (PE)-conjugated antibody against BST2 (12–3179; eBioscience) or isotype-matched PE conjugated IgG1 (eBioscience) for 1 h on ice. Cells were washed three times with flow cytometry buffer and fixed with 1% paraformaldehyde. At least 20,000 events were collected for each sample using FACS Calibur flow cytometer (BD). The mean fluorescence intensity (MFI) was determined by software Flowjo.
4.5. Western blotting
Cells were lysed in RIPA buffer (0.1% SDS, 1% Triton X-100, 1% sodium deoxycholate, 150 mM NaCl, 10 mM Tris[pH7.5], 1 mM EDTA), and the samples were separated by 12% SDS-PAGE, followed by transferring onto PVDF membranes. The membranes were probed with anti-BST2 (1:2000), anti-flag (1:2000), anti-HA (1:2000), anti-protein E (1:1000), anti-LAMP1 (1:1000) or anti-β-actin (1:1000) antibody for 1 h at room temperature, and subsequently washed three times with 0.1% Tween 20/PBS, followed by an incubation for 1 h with HRP conjugated goat anti–rabbit secondary antibody (1:10,000; BA1054, Boster) or HRP conjugated goat anti–mouse secondary antibody (1:10,000; BA1050, Boster). Following three washes with 0.1% Tween 20/PBS, the bands were visualized by exposure to FluorChem HD2 Imaging System (Alpha Innotech) after the addition of chemiluminescent substrate (SuperSignal® West Dura Extended Duration Substrate; 34075; Thermo Scientific Pierce).
4.6. RP-CTR
HeLa cells were infected with JEV at an MOI of 25 or mock infected. At 48 h post infection, cells were collected and the total RNA was extracted using Trizol (Invitrogen). RNase-free DNase I (Fermentas) was used to eliminate the contamination of genomic DNA. cDNA was then synthesized using moloney murine leukemia virus transcriptase (Promega). The newly synthesized cDNA was used as the template for the amplification of a highly specific nucleotide region of BST2 gene. Primers 5′-CAAACTCCTGCAACCTGACC-3′ and 5′-CATTCTCAAGCTCCTTGATGC-3′ were used for BST2 amplification. GAPDH was used as an internal control amplified with primers 5′-GGGAAGCTCACTGGCATGG-3′ and 5′-TTACTCCTTGGAGGCCATGT-3′ (Moltedo et al., 2011). Relative quantitative PCR was performed using a SYBR Green Real-Time PCR Master Mix (Toyobo) Dye and an ABI step one real-time PCR system (Applied Biosystems) as previously described (Chen et al., 2013). The final reaction conditions were as follow: 95 °C for 1 min, followed by 40 cycles of 95 °C for 15 s, 60 °C for 15 s, and 72 °C for 45 s. The difference in gene expression was calculated on the basis of 2-ΔΔCT values.
4.7. Immunofluorescence
To detect the cellular localization of BST2 and JEV protein E, cells on 35 mm glass bottom culture dishes were washed three times with PBS, followed by fixation with 4% (w/v) cold paraformaldehyde for 10 min at room temperature. Cells were permeabilized with PBST (PBS-0.2% (v/v) Triton X-100) for 10 min at room temperature and then blocked with PBS-2% (w/v) BSA for 1 h at room temperature. Cells were incubated for 1 h at 37 °C with the anti-flag antibody at a dilution of 1:200 and anti-BST2 antibody at a dilution of 1:200, followed by an incubation for 1 h at room temperature with a Alexa Fluor 647-labeled Goat Anti-Mouse IgG(H+L) (A0473, Beyotime) at a dilution of 1:200 and a Alexa Fluor 488-labeled Goat Anti-Rabbit IgG(H+L) (A0423, Beyotime) at a dilution of 1:200 in PBS-2% (w/v) BSA. To assess the localization of JEV protein E on cell surface, cells on 35 mm glass bottom culture dishes were washed three times with PBS, followed by incubation with PBS-2% (w/v) BSA for 1 h at room temperature. Cells were then incubated for 1 h at 4 °C with the anti-flag antibody at a dilution of 1:200 and the anti-BST2 antibody at a dilution of 1:200, followed by incubation with the Alexa Fluor 647-labeled Goat Anti-Mouse IgG(H+L)(A0473, Beyotime) at a dilution of 1:200 and the Alexa Fluor 488-labeled Goat Anti-Rabbit IgG(H+L)(A0423, Beyotime) at a dilution of 1:200 in PBS-2% (w/v) BSA. Cells were washed three times with PBS after each incubation. Nuclei were dyed with DAPI (AR1177, Boster). Stained cells were analyzed using confocal microscopy (PerkinElmer UltraViewVoX) using a 60 × oil objective with 1.5-fold optical zoom.
4.8. Coimmunoprecipitation
Cells were lysed in RIPA buffer [50 mM Tris·HCl, 150 mM NaCl, 0.1% SDS, 0.5% sodium deoxycholate, 1% Nonidet P-40, protease inhibitors (Roche), pH 7.4] and sonicated. Lysates were incubated with the anti-flag or the anti-BST2 antibody for 1 h at 4 °C. Thereafter, sepharose Protein-G beads (Invitrogen) were added and samples were incubated for an additional 4 h at 4 °C with rotation. Samples were washed 4 times with RIPA buffer and resuspended in 2× SDS/PAGE sample buffer. Proteins were analyzed by immunoblotting with the anti-flag, anti-BST2 or anti-HA antibody.
4.9. Statistical analysis
All experiments were repeated for at least three times, and the data are presented as mean ± SD unless otherwise specified. The difference of mean value was analyzed by a paired Student's t-test. All statistical analysis was performed by GraphPad Prism. P < 0.05 was considered statistically significant.
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2016YFC1200400), the National Natural Science Foundation of China Grants 81572009 and 31570165, the State Key Laboratory of Virology (klv-2016-02) and the Chinese Academy of Sciences (CXJJ-16M120). We thank the Core Facility and Technical Support at Wuhan Institute of Virology for technique supports of Confocal Microscopy (Dr. Ding Gao) and Flow Cytometry (Ms. Juan Min).
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.virol.2017.07.008.
Appendix A. Supplementary material
Supplementary material
References
- Anand N., Kumar S., Gowal D. Standardization of plaque assay of Japanese encephalitis virus (Nakayama NIH strain) on BHK-21 (Cl-13) cell line. Am. J. Biomed. Sci. 2010:43–50. doi: 10.4149/av_2015_03_234. [DOI] [PubMed] [Google Scholar]
- Andrew A.J., Miyagi E., Kao S., Strebel K. The formation of cysteine-linked dimers of BST-2/tetherin is important for inhibition of HIV-1 virus release but not for sensitivity to Vpu. Retrovirology. 2009;6:80. doi: 10.1186/1742-4690-6-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bampi C., Rasga L., Roux L. Antagonism to human BST-2/tetherin by Sendai virus glycoproteins. J. General. Virol. 2013;94:1211–1219. doi: 10.1099/vir.0.051771-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blondeau C., Pelchen-Matthews A., Mlcochova P., Marsh M., Milne R.S., Towers G.J. Tetherin restricts herpes simplex virus 1 and is antagonized by glycoprotein M. J. Virol. 2013;87:13124–13133. doi: 10.1128/JVI.02250-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Pei R., Chen X. Different responses of two highly permissive cell lines upon HCV infection. Virol. Sin. 2013;28:202–208. doi: 10.1007/s12250-013-3342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas J.L., Viswanathan K., McCarroll M.N., Gustin J.K., Fruh K., Moses A.V. Vpu directs the degradation of the human immunodeficiency virus restriction factor BST-2/Tetherin via a {beta}TrCP-dependent mechanism. J. Virol. 2009;83:7931–7947. doi: 10.1128/JVI.00242-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du R., Yin F., Wang M., Hu Z., Wang H., Deng F. Glycoprotein E of the Japanese encephalitis virus forms virus-like particles and induces syncytia when expressed by a baculovirus. J. General. Virol. 2015;96:1006–1014. doi: 10.1099/vir.0.000052. [DOI] [PubMed] [Google Scholar]
- Gupta R.K., Mlcochova P., Pelchen-Matthews A., Petit S.J., Mattiuzzo G., Pillay D., Takeuchi Y., Marsh M., Towers G.J. Simian immunodeficiency virus envelope glycoprotein counteracts tetherin/BST-2/CD317 by intracellular sequestration. Proc. Natl. Acad. Sci. USA. 2009;106:20889–20894. doi: 10.1073/pnas.0907075106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser H., Lopez L.A., Yang S.J., Oldenburg J.E., Exline C.M., Guatelli J.C., Cannon P.M. HIV-1 Vpu and HIV-2 Env counteract BST-2/tetherin by sequestration in a perinuclear compartment. Retrovirology. 2010;7:51. doi: 10.1186/1742-4690-7-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz F.X., Allison S.L. Flavivirus structure and membrane fusion. Adv. Virus Res. 2003;59:63–97. doi: 10.1016/s0065-3527(03)59003-0. [DOI] [PubMed] [Google Scholar]
- Hinz A., Miguet N., Natrajan G., Usami Y., Yamanaka H., Renesto P., Hartlieb B., McCarthy A.A., Simorre J.P., Gottlinger H., Weissenhorn W. Structural basis of HIV-1 tethering to membranes by the BST-2/tetherin ectodomain. Cell Host Microbe. 2010;7:314–323. doi: 10.1016/j.chom.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S., Pang X., Li J., Cen S., Jin Q., Guo F. The role of the structural domains of human BST-2 in inhibiting the release of xenotropic murine leukemia virus-related virus. Biochem. Biophys. Res. Commun. 2012;428:17–23. doi: 10.1016/j.bbrc.2012.10.001. [DOI] [PubMed] [Google Scholar]
- Iwabu Y., Fujita H., Kinomoto M., Kaneko K., Ishizaka Y., Tanaka Y., Sata T., Tokunaga K. HIV-1 accessory protein Vpu internalizes cell-surface BST-2/tetherin through transmembrane interactions leading to lysosomes. J. Biol. Chem. 2009;284:35060–35072. doi: 10.1074/jbc.M109.058305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia B., Serra-Moreno R., Neidermyer W., Rahmberg A., Mackey J., Fofana I.B., Johnson W.E., Westmoreland S., Evans D.T. Species-specific activity of SIV Nef and HIV-1 Vpu in overcoming restriction by tetherin/BST2. PLoS Pathog. 2009;5:e1000429. doi: 10.1371/journal.ppat.1000429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouvenet N., Neil S.J., Zhadina M., Zang T., Kratovac Z., Lee Y., McNatt M., Hatziioannou T., Bieniasz P.D. Broad-spectrum inhibition of retroviral and filoviral particle release by tetherin. J. Virol. 2009;83:1837–1844. doi: 10.1128/JVI.02211-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.M., Yun S.I., Song B.H., Hahn Y.S., Lee C.H., Oh H.W., Lee Y.M. A single N-linked glycosylation site in the Japanese encephalitis virus prM protein is critical for cell type-specific prM protein biogenesis, virus particle release, and pathogenicity in mice. J. Virol. 2008;82:7846–7862. doi: 10.1128/JVI.00789-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi E., Mason P.W. Proper maturation of the Japanese encephalitis virus envelope glycoprotein requires cosynthesis with the premembrane protein. J. Virol. 1993;67:1672–1675. doi: 10.1128/jvi.67.3.1672-1675.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupzig S., Korolchuk V., Rollason R., Sugden A., Wilde A., Banting G. Bst-2/HM1.24 is a raft-associated apical membrane protein with an unusual topology. Traffic. 2003;4:694–709. doi: 10.1034/j.1600-0854.2003.00129.x. [DOI] [PubMed] [Google Scholar]
- Le Tortorec A., Neil S.J. Antagonism to and intracellular sequestration of human tetherin by the human immunodeficiency virus type 2 envelope glycoprotein. J. Virol. 2009;83:11966–11978. doi: 10.1128/JVI.01515-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Luo S., He S., Zhang M., Wang P., Li C., Huang W., Hu B., Griffin G.E., Shattock R.J., Hu Q. Tetherin restricts HSV-2 release and is counteracted by multiple viral glycoproteins. Virology. 2015;475:96–109. doi: 10.1016/j.virol.2014.11.005. [DOI] [PubMed] [Google Scholar]
- Mangeat B., Gers-Huber G., Lehmann M., Zufferey M., Luban J., Piguet V. HIV-1 Vpu neutralizes the antiviral factor Tetherin/BST-2 by binding it and directing its beta-TrCP2-dependent degradation. PLoS Pathog. 2009;5:e1000574. doi: 10.1371/journal.ppat.1000574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansouri M., Viswanathan K., Douglas J.L., Hines J., Gustin J., Moses A.V., Fruh K. Molecular mechanism of BST2/tetherin downregulation by K5/MIR2 of Kaposi's sarcoma-associated herpesvirus. J. Virol. 2009;83:9672–9681. doi: 10.1128/JVI.00597-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moltedo B., Li W., Yount J.S., Moran T.M. Unique type I interferon responses determine the functional fate of migratory lung dendritic cells during influenza virus infection. PLoS Pathog. 2011;7:e1002345. doi: 10.1371/journal.ppat.1002345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay S., Kuhn R.J., Rossmann M.G. A structural perspective of the flavivirus life cycle. Nat. Rev. Microbiol. 2005;3:13–22. doi: 10.1038/nrmicro1067. [DOI] [PubMed] [Google Scholar]
- Neil S.J., Zang T., Bieniasz P.D. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature. 2008;451:425–430. doi: 10.1038/nature06553. [DOI] [PubMed] [Google Scholar]
- Pan X.B., Han J.C., Cong X., Wei L. BST2/tetherin inhibits dengue virus release from human hepatoma cells. PloS One. 2012;7:e51033. doi: 10.1371/journal.pone.0051033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X.B., Qu X.W., Jiang D., Zhao X.L., Han J.C., Wei L. BST2/Tetherin inhibits hepatitis C virus production in human hepatoma cells. Antivir. Res. 2013;98:54–60. doi: 10.1016/j.antiviral.2013.01.009. [DOI] [PubMed] [Google Scholar]
- Pardieu C., Vigan R., Wilson S.J., Calvi A., Zang T., Bieniasz P., Kellam P., Towers G.J., Neil S.J. The RING-CH ligase K5 antagonizes restriction of KSHV and HIV-1 particle release by mediating ubiquitin-dependent endosomal degradation of tetherin. PLoS Pathog. 2010;6:e1000843. doi: 10.1371/journal.ppat.1000843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Caballero D., Zang T., Ebrahimi A., McNatt M.W., Gregory D.A., Johnson M.C., Bieniasz P.D. Tetherin inhibits HIV-1 release by directly tethering virions to cells. Cell. 2009;139:499–511. doi: 10.1016/j.cell.2009.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radoshitzky S.R., Dong L., Chi X., Clester J.C., Retterer C., Spurgers K., Kuhn J.H., Sandwick S., Ruthel G., Kota K., Boltz D., Warren T., Kranzusch P.J., Whelan S.P., Bavari S. Infectious Lassa virus, but not filoviruses, is restricted by BST-2/tetherin. J. Virol. 2010;84:10569–10580. doi: 10.1128/JVI.00103-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice C.M. Flaviviridae: the viruses and their replication. Fields Virol. 1996;3:931–959. [Google Scholar]
- Sakuma T., Noda T., Urata S., Kawaoka Y., Yasuda J. Inhibition of Lassa and Marburg virus production by tetherin. J. Virol. 2009;83:2382–2385. doi: 10.1128/JVI.01607-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tani H., Shiokawa M., Kaname Y., Kambara H., Mori Y., Abe T., Moriishi K., Matsuura Y. Involvement of ceramide in the propagation of Japanese encephalitis virus. J. Virol. 2010;84:2798–2807. doi: 10.1128/JVI.02499-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn D.W., Hoke C.H., Jr. The epidemiology of Japanese encephalitis: prospects for prevention. Epidemiol. Rev. 1992;14:197–221. doi: 10.1093/oxfordjournals.epirev.a036087. [DOI] [PubMed] [Google Scholar]
- Wang S.M., Huang K.J., Wang C.T. BST2/CD317 counteracts human coronavirus 229E productive infection by tethering virions at the cell surface. Virology. 2014;449:287–296. doi: 10.1016/j.virol.2013.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe R., Leser G.P., Lamb R.A. Influenza virus is not restricted by tetherin whereas influenza VLP production is restricted by tetherin. Virology. 2011;417:50–56. doi: 10.1016/j.virol.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidner J.M., Jiang D., Pan X.B., Chang J., Block T.M., Guo J.T. Interferon-induced cell membrane proteins, IFITM3 and tetherin, inhibit vesicular stomatitis virus infection via distinct mechanisms. J. Virol. 2010;84:12646–12657. doi: 10.1128/JVI.01328-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yondola M.A., Fernandes F., Belicha-Villanueva A., Uccelini M., Gao Q., Carter C., Palese P. Budding capability of the influenza virus neuraminidase can be modulated by tetherin. J. Virol. 2011;85:2480–2491. doi: 10.1128/JVI.02188-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenner H.L., Mauricio R., Banting G., Crump C.M. Herpes simplex virus 1 counteracts tetherin restriction via its virion host shutoff activity. J. Virol. 2013;87:13115–13123. doi: 10.1128/JVI.02167-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material