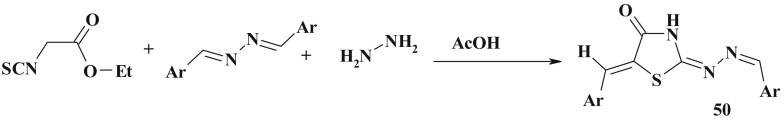Abstract
The presented review is an attempt to summarize a huge volume of data on 5-ene-4-thiazolidinones being a widely studied class of small molecules used in modern organic and medicinal chemistry. The manuscript covers approaches to the synthesis of 5-ene-4-thiazolidinone derivatives: modification of the C5 position of the basic core; synthesis of the target compounds in the one-pot or multistage reactions or transformation of other related heterocycles. The most prominent pharmacological profiles of 5-ene derivatives of different 4-thiazolidinone subtypes belonging to hit-, lead-compounds, drug-candidates and drugs as well as the most studied targets have been discussed. Currently target compounds (especially 5-en-rhodanines) are assigned as frequent hitters or pan-assay interference compounds (PAINS) within high-throughput screening campaigns. Nevertheless, the crucial impact of the presence/nature of C5 substituent (namely 5-ene) on the pharmacological effects of 5-ene-4-thiazolidinones was confirmed by the numerous listed findings from the original articles. The main directions for active 5-ene-4-thiazolidinones optimization have been shown: i) complication of the fragment in the C5 position; ii) introduction of the substituents in the N3 position (especially fragments with carboxylic group or its derivatives); iii) annealing in complex heterocyclic systems; iv) combination with other pharmacologically attractive fragments within hybrid pharmacophore approach. Moreover, the utilization of 5-ene-4-thiazolidinones in the synthesis of complex compounds with potent pharmacological application is described. The chemical transformations cover mainly the reactions which involve the exocyclic double bond in C5 position of the main core and correspond to the abovementioned direction of the 5-ene-4-thiazolidinone modification.
Keywords: 5-Ene-4-thiazolidinones, Synthesis, Biological activity
Graphical abstract
Highlights
-
•
Synthesis of 5-ene-4-thiazolidinones.
-
•
Pharmacological profiles of 5-ene-4-thiazolidinones.
-
•
5-Ene-thiazolidinones based synthesis.
1. Introduction
4-Thiazolidinones and related heterocyclic based compounds have been extensively explored as the source of antiinflammatory, antitumor, antimicrobial, antidiabetic, antibacterial agents. The findings in the medicinal chemistry and pharmacology of 4-thiazolidinones have significantly increased since [1], [2], [3] 60s being reflected in the rapid growth of the number of scientific papers [4], numerous reviews and patents covering various 4-thiazolidinone derivatives [5], [6], [7], [8], [9], [10]. The papers are dedicated to the selected 4-thiazolidinones subtypes, namely 2-(imino)amino-4-thiazolidinones [11], 4-thiazolidinones with the exocyclic C=C double bond at the C(2)-position etc [12]. This proves that 4-thiazolidinones belong to the privileged scaffolds in the modern medicinal chemistry [4], [13], [14]. Combination of several reaction centers in the structure of 4-thiazolidinone derivates makes them an effective tool for the rational diversity oriented synthesis or privileged substructure-based diversity oriented synthesis [15], [16], [17] for the new lead-compounds creation.
Major achievements in the 4-thiazolidinone field are related to the 2,4-thiazolidinedione, rhodanine (2-thioxo-4-thiazolidinone), 2-alkyl(aryl)-substituted, and 2-R-amino(imino)-substituted 4-thiazolidinone subtypes as sources of antimicrobial, antidiabetic, anti-inflammatory and anticancer lead-compounds and drug-candidates [7], [10], [11]. This is largely due to the investigation and introduction into the medical practice the antidiabetic drugs: glitazones (peroxisome proliferator-activated receptor-γ (PPAR) agonists, e.g. Rosiglitazone, Troglitazone, Pioglitazone, etc.) [18], [19] and aldose reductase inhibitor Epalrestat [20], and the simplicity in the synthesis of abovementioned compounds. Very less attention has been paid to the 4-thio- and 4-amino(imino)-derivatives (isorhodanine (4-thioxo-2-thiazolidinone), thiorhodanine (2,4-thiazolidinedithione), and especially 4-amino(imino)-2-thiazolidinone derivatives) [21], [22], [23], [24], [25].
Despite foregoing, among all 4-thiazolidinone subtypes 5 substituted thiazolidinones, namely 5-ene (5-ylidene) derivatives are of special interest in the context of chemical features and pharmacological profiles [6], [8], [26], [27].
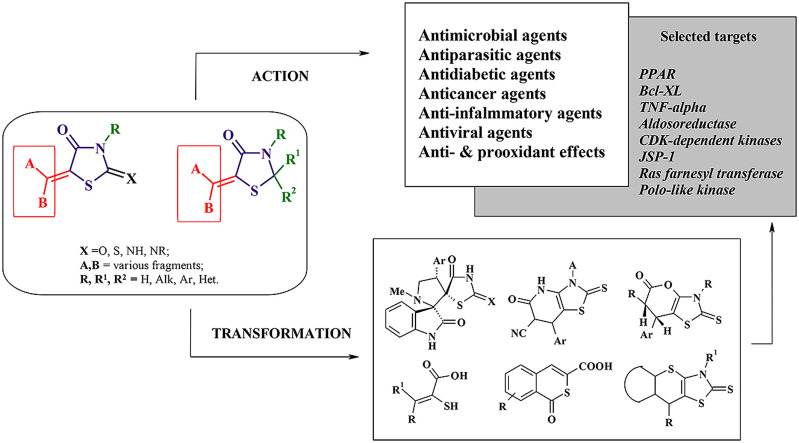
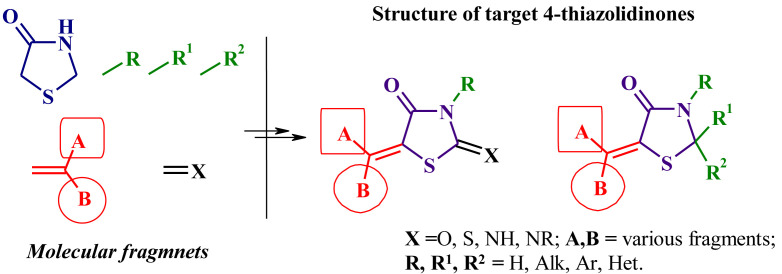
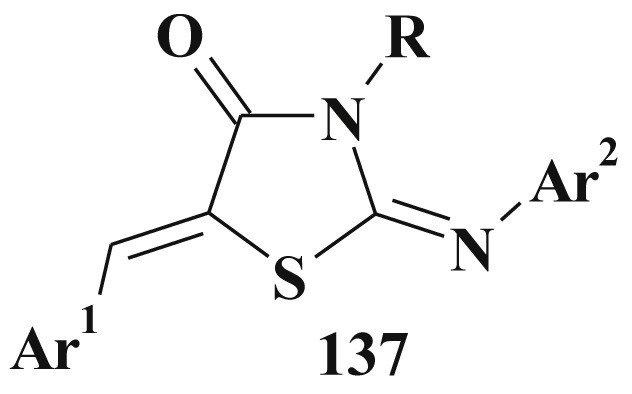
In this manuscript we tried to present the powerful pharmacological and chemical potential of 5-ene-4-thiazolidinones. Pursuing this goal we had not devided thiazolidinones according to the nature of their substituents (e.g. derivatives of 2,4-thiazolidinedione, rhodanine etc.) (Fig. 1 ).
Fig. 1.
Structure of the target 5-ene-4-thiazolidinones.
The arguments in this favour were the following: i) large number of reviews is devoted to the selected thiazolidinones subtypes; ii) generally 5-ene-derivatives of different thiazolidinone cores are characterized by the same pharmacological profiles (see below) as well as the similar synthetic protocols.
2. Synthesis of 5-ene-4-thiazolidinones
The methods used for the synthesis of 4-thiazolidinones and their derivatives depend undoubtedly on the nature of thiazolidinone subtypes and are well represented in numerous reviews and original articles (see above) [28]. Presented retro-synthetic approach (Fig. 2 ) shows wide range of synthetic routes for thiazolidine ring formation based on various condensation reactions that successfully have been employed for the 4-thiazolidinones synthesis [1], [2], [3], [6].
Fig. 2.
Retro-synthetic approach to the 4-thiazolidinone scaffold formation, adapted from Ref. [29].
These approaches are also speculatively attractive for the synthesis of 5-thiazolidinones as positional isomers of mentioned structures and they are rarely represented [30]. The most prominent and referred protocol for their synthesis is the cyclocondensation of reactants bearing the N=C=S fragment with α-halocarbonyl compounds. But, in most cases, mentioned protocol leads to the formation of 4-thiazolidinones only [31].
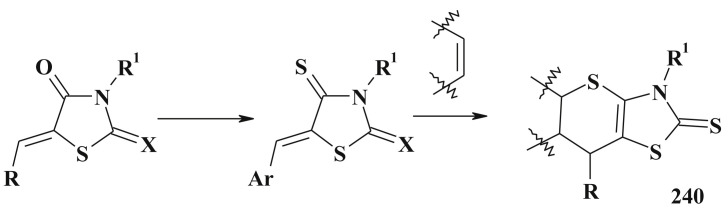
However, the protocols for synthesis of 5-ene derivatives of 4-thiazolidinones are often similar and can be divided into the next groups: i) modification of the C5 position of the basic core; ii) synthesis of the target compounds in the one-pot or multistage reactions (simultaneous formation of the core heterocycle and C5 exocyclic double bond); iii) transformation of thiazolidine derivatives or other related heterocycles in the recyclization reactions.
2.1. Modification of the C5 position of the thiazolidinone core
Knoevenagel condensation. One of the most prominent and referred protocols for 5-ene-4-thiazolidinones synthesis is the Knoevenagel condensation of thiazolidinone core and oxo-compound (Scheme 1 ). The methylene carbon atom at the С5-position possesses nucleophilic activity and can attack an electrophilic center affording the target ene-derivatives. The different aldehydes, ketones, and heterocycles (e.g. isatins, anhydrides of pyridine-3,4-dicarboxylic and phthalic acids etc.) [27], [32], [33], [34], [35], [36], [37], [38] have been used as oxo compounds. Acetic acid or its anhydride and sodium acetate; ethanol and ammonium acetate or piperidine [39]; toluene and ammonia acetate [33], [40], [41]; isopropanol and potassium tert-butylate [42], [43]; toluene and l-proline [44]; dimethylformamide and sodium acetate; ethanol and monoethanolamine as well as the solid carriers and phasetransfer catalysts etc. [45], [46] have been widely used as the medium and catalysts in this reaction. The reaction performance was also described in the medium of the aldehyde or ketone (without solvent addition) [21], polyethyleneglycol-300 [47] or based on the green chemistry approach. Moreover, performance of such condensation in aqueous solutions [48]; usage of atypical agents as well as ionic liquids [49]; the soluble polymer-supported synthesis [50] have also been investigated. Microwave assistant organic synthesis approach was also successfully employed [51], [52].
Scheme 1.
General scheme of Knoevenagel condensation of 4-thiazolidinones.
The utilization of the aromatic aldehydes leads to maximal yields of the target compounds, unlike aliphatic aldehydes and especially ketones [38], [53]. The reactivity of 4-thiazolidinones differs also and depends on the main core's substituents (2,4-thiazolidinedione, rhodanine etc.) [27], [43], [43]: rhodanines react easier than 2-amino(imino)-4-thiazolidinone, 2,4-thiazolidinone etc; 2-R-substituted-4-thiazolidinones are characterized by the lower reactivity levels. When aldehydes are utilized the preferred (and mostly only one) product had been reported to have the Z geometry based on NMR and Х-ray study regardless of the thiazolidinone subtypes [53], [54], [55]. Knoevenagel condensation between 4-thiazolidinones and ketones generally leads to the mixtures of Z- and E-isomers. The use of the dicarbonyl compounds in this reaction leads to the formation of unfused heterocycles with two thiazolidinone fragments in molecule [36], [56], [57], [58].
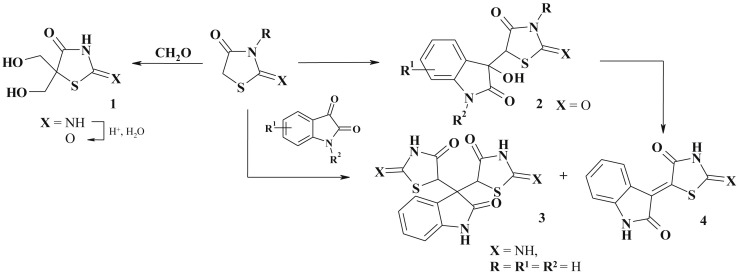
Despite the simplicity of such condensation, the formation of other products have been reported also [59]. For instance, thiopyrano[2,3-d][1,3]thiazoles were obtained from highly reactive 4-thioxo-thiazolidin(thi)one-2 and ketones (in ethanol in the presence of monoethanolamine) [21]. The condensation of 2-imino-4-thiazolidinone and formaldehyde in water (pH∼9, triethylamine) leads to the formation of 5,5-bishydroxymetyl-2-imino-4-thiazolidinone (1) [37], [60]. The uncatalyzed reaction in water with C−C bond-formation was reported to be thiazolidinedione-isatin conjugates' (2) synthetic protocol. The formation of the products was found to be thermodynamically controlled [59], [61] and further heating leads to dehydration and double bound formation at C5 position of thiozolidinone core (Scheme 2 ).
Scheme 2.
Variety of products of condensation reactions.
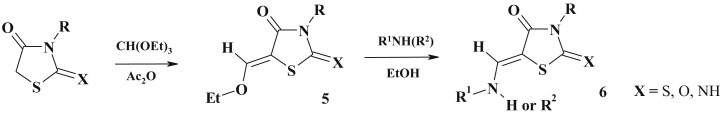
The reaction of pseudothiohydantoin (2-imino-4-thiazolidinone) and isatin (molar ratio 2:1) was carried out in absolute ethanol under refluxing affording not only target 5-ene-4-thiazolidinone (4) but also spiro-isatin conjugate (45%) 3 [57]. Based on the Knoevenagel condensation the efficient method for 5-ethoxy-4-thiazolidinones synthesis (5) using triethylorthoformate was described [32], [62], [63], [64], [65], [66], the latter (5) can be easily converted into the appropriate amines (6) (Scheme 3 ).
Scheme 3.
5-Enamine-4-thiazolidinones formation.
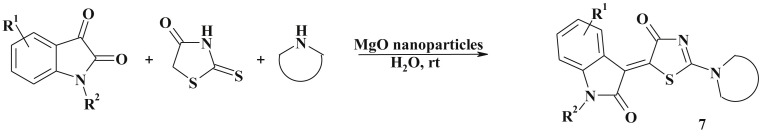
Knoevenagel condensation often may be a stage in the tandem and/or domino reactions whilst the corresponding 5-ene-thiazolidinones appear as intermediates in the synthesis of fused thiazolidinone-based heterocycles (see bellow) [67], [68], [69] or simple molecules of other thiazolidinone subtypes. For example, an efficient approach to the 5-ene-2-amino-4-thiazolidinones obtaining via sulfur/nitrogen displacement [70] was the base for the development of multicomponent reactions involving Knoevenagel condensation [71]. The one-pot reaction of isatin derivatives, rhodanine and secondary amines (magnesium oxide nanoparticles used as heterogeneous catalyst, water medium, r.t.) is an efficient green approach to the preparation of novel isatin-thiazolidinone based conjugates (7) [72] (Scheme 4 ).
Scheme 4.
One pot synthesis of 2.5-disubstituted thiazolidinones.
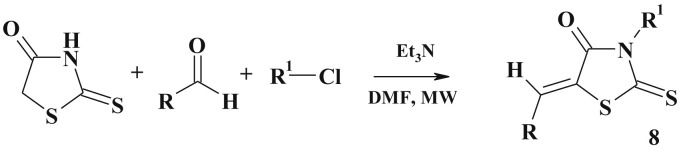
There has been developed a fast and efficient protocol for the generation of 3-substituted 5-arylidenerhodanines (8) (Scheme 5 ) in sequential one-pot, two-step process combining the Knoevenagel condensation and alkylation reaction under microwave assisted conditions [73].
Scheme 5.
One pot synthesis of 3,5-disubstituted thiazolidinones.
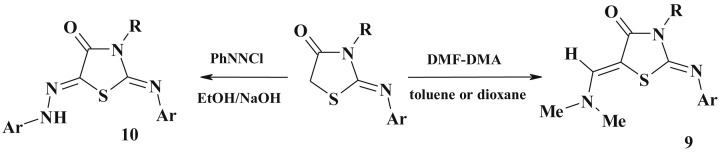
Other types of condensations. The active methylene group can be condensed with dimethylformamide-dimethylacetal (DMF-DMA) in dioxane to yield the corresponding enamines (9).
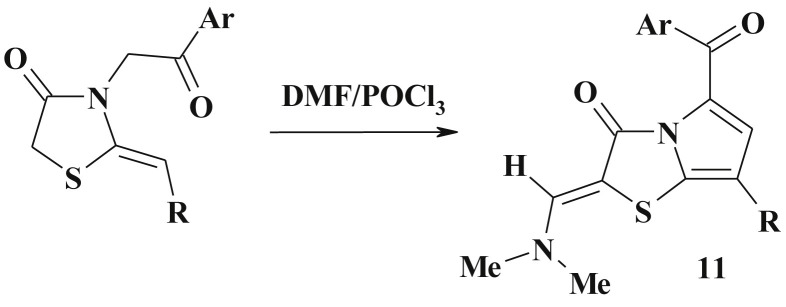
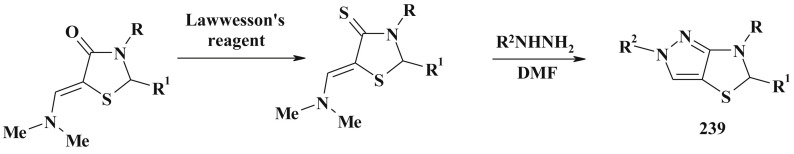
Moreover, the methylene group in 4-thiazolidinone can be coupled with the aryl-diazonium salt in EtOH/NaOH to form the corresponding arylhydrazone (10) [3], [74] (Scheme 6 ). Similar to 5-arylidene-4-thiazolidinones, in all cases only Z-isomers were formed (confirmed by X-ray data) [75]. The isosteric 2-R-substituted-4-thioxothiazolidines were also prepared in a single step on simultaneous treatment of thiazolidin-4-one with DMF-DMA and Lawesson's reagent in toluene medium [76]. Under the action of DMF/POCl3 on 3-substituted 2-ylidene-thiazolidinones the related enamine derivatives of pyrolo[2,1-b]thiazol-3-ones 11 were synthesized (Scheme 7 ) [77].
Scheme 6.
Synthesis of 4-thiazolidinone based enamines and arylhydrazones.
Scheme 7.
Synthesis of pyrolo[2,1-b]thiazol-3-ones based on 4-thiazolidinones.
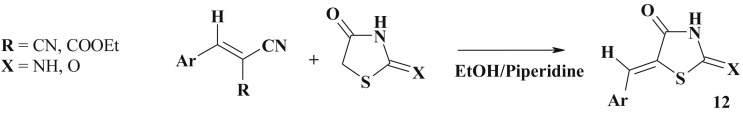
The active methylene group in the 4-thiazolidinones underwent nucleophilic addition reaction with the double bond of the various arylidene malononitriles via Michael type addition reaction (ethanol medium in the presence of piperidine) to give the same 5-ene-4-thiazolidinone derivatives (12) [75] (Scheme 8 ).
Scheme 8.
Michael type addition reaction of 4-thiazolidinones.
For instance, reaction of α-cyano-3,4,5-trimethoxycinnamonitrile and/or ethyl-α-cyano-3,4,5-trimethoxycinnamate with 2-imino-4-oxo-thiazolidine provided appropriate 5-enes’ formation (instead of the expected fused heterocycles) [78], [79]. It was proposed that the reactions proceeded via nucleophilic addition of the thiazolidinyl-C-5 to the β-carbon of the activated double bond of nitriles forming the 1:1 adduct followed by the elimination of malononitrile or ethylcyanoacetate. The same step was proposed as a mechanism of dihydrothiophene derivatives formation via the four-component reaction of aldehyde, malonitrile, 2,4-thiazolidinedione and piperidine in the presence of Bu4NOH as a basic ionic liquid in al≿ohol-aqueous medium [49] or via the triethylamine-catalyzed domino reaction [80], [81].
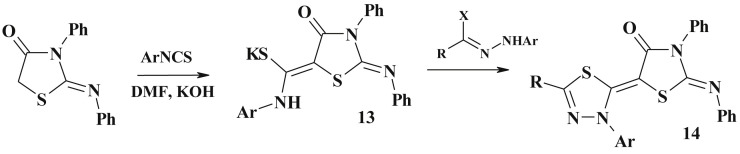
Isothiocyanate based synthesis. The reaction of 2-arylimino-3-R-4-thiazolidinones with arylisothiocyanate in DMF in the presence of KOH provided the appropriate thioamides (13) [82], [83], which may be treated as effective building blocks for the synthesis of polyfunctional compounds. The latter reacted with hydrazonoyl halides affording new 5-heterylidene derivatives 14 (Scheme 9 ).
Scheme 9.
Isothiocynate based synthesis of 5-ene-4-thiazolidinones.
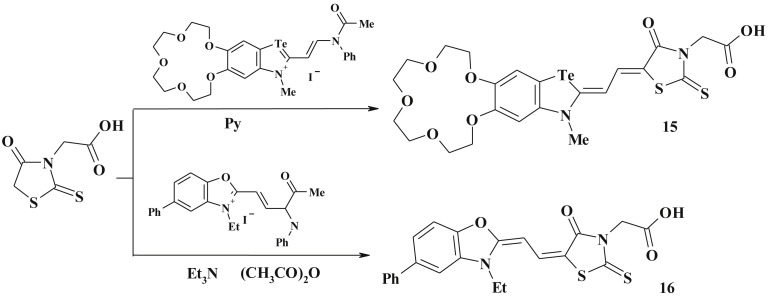
One more efficient method for 5-ene-4-thiazolidinone synthesis is the reaction of 4-thiazolidinones (e.g. rhodanine-3-acetic acid) with anilide-vinyl compounds [84] or with hemicyanines [85] with formation of dimethinemerocyanine and cyanine dyes (15, 16) (Scheme 10 ).
Scheme 10.
4-Thiazolidinone-based dimethinemerocyanine and cyanine dyes.
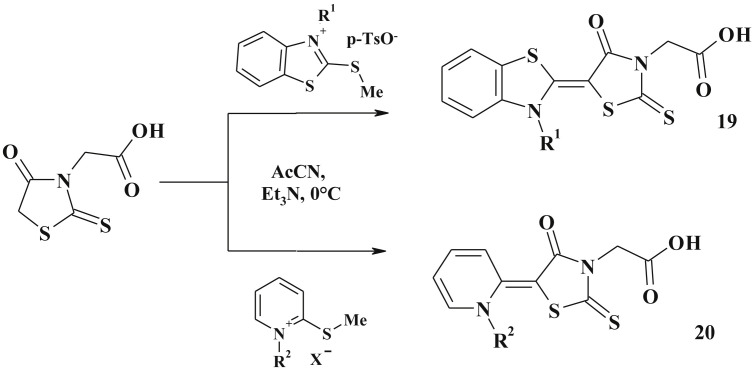
Merocyanine dyes (17–20) are other examples of polycyclic heterocycles derived from 5-ene-4-thiazolidinones. Their synthesis involves C5 active methylene group of thiazolidinone core and methylated thioxo-group (C2 position of rhodanine-3-acetic acid [86] (Scheme 11 )) or other heterocyclic S-Me salts [84], [87], [88] (Scheme 12 ).
Scheme 11.
Synthesis of 4-thiazolidinone based merocyanine dyes.
Scheme 12.
Synthesis of 5-heterylidene 4-thiazolidinones.
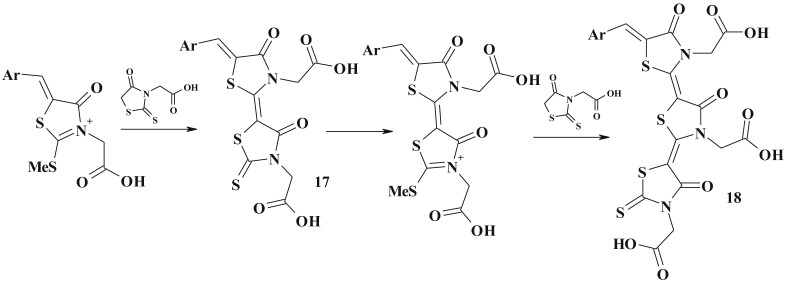
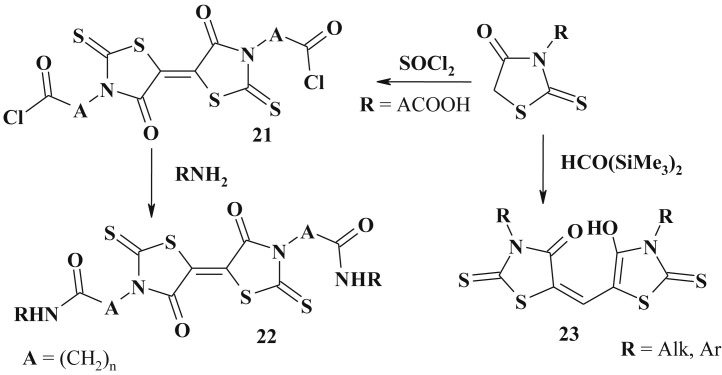
Rhodanine derivatives, such as rhodanine-3-carboxylic acids, via the action of thionyl chloride were exposed to dimerisation at the C5 position and yielded appropriate acyl chlorides (21), which can be used for further modification (22) [89] (Scheme 13 ).
Scheme 13.
Synthesis of bis-5-ene-4-thiazolidinones.
Reaction of 3-alkyl(aryl)rhodanines with bis-(trimethylsilyl)formamide also led to dimerisation and formation of the bis-(3-R-rhodaninyl-5)methinoxynes (23) [90].
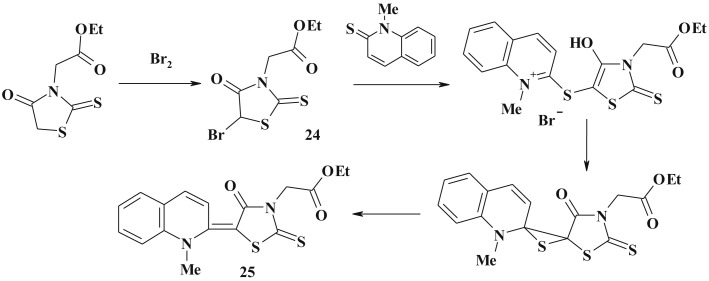
The reaction of 4-thiazolidinones with halogens led to simple 5-halogen-derivatives (24) (Scheme 14 ) which are the effective reagents for further synthetic transformation. Thus, based on ethyl ester of 5-bromo-2-thioxo-4-thiazolidone-3-acetic acid an original method for 5-ylidene derivative (25) synthesis was proposed [91].
Scheme 14.
Utilization of 5-bromo-4-thiazolidinone for synthesis of 5-heterylidene derivatives.
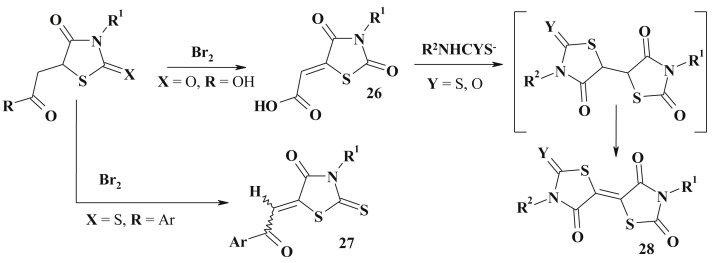
Another efficient method for the 5-ene-4-thiazolidinones (26, 27) synthesis (especially in the case of compounds containing carboxylic or carbonylic group in the C5 substituent) is a dehydrobromination reaction [92], [93], [94] (Scheme 15 ). 5-(2-Aryl-2-oxoethyl)-rhodanines reacted with bromine in acetic acid and formed a mixture of E- and Z-stereoisomers of 5-aroylmethylenerhodanines.
Scheme 15.
Synthesis and transformation of 5-carboxymethylidene-4-thiazolidinone and related derivatives.
Described esters of 5-carboxymethylidene-4-thiazolidinone (2,4-dioxothiazolidin-5-ylidene-acetic acid) (26) are attractive building blocks for the various 4-thiazolidonone subtypes synthesis [94], [95] including 5-ene derivatives. Addition of dithiocarbamates and thiocarbamates to the double bond in 5-ene-4-thiazolidinones resulted in spontaneous heterocylization to 5,5′-di-4-thiazolidinones intermediates, which were oxidized in the presence of triethylamine to 28 [96] (Scheme 15).
2.2. Simultaneous formation of heterocycle and C5 exocyclic double bond
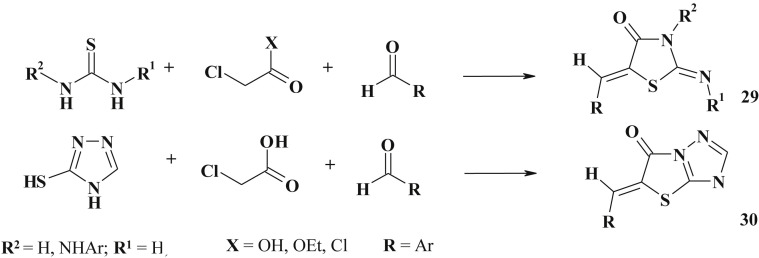
Most of the protocols presented above are rather simple and most of them explore two- or three-step procedures. Considering the current trends in organic/medicinal chemistry different one-pot multicomponent methods were proposed for 5-ene-4-thiazolidinones synthesis (29) (Scheme 16 ). The illustrative example is the one-pot method based on [2 + 3]-cyclocondensation of substituted thioureas/thiosemicarbazides with halogen-carboxylic acids followed by Knoevenagel condensation designed in our laboratory [59], [97], [98] and widely used [99] under wet chemistry conditions as well as under microwave irradiation or in the green reaction media [55], [100], [101], [102].
Scheme 16.
One-pot three component synthesis of 5-ene-4-thiazolidinones.
This approach can be successfully employed for the synthesis of fused heterocycles bearing 5-ene-4-thiazolidinones moieties e.g., 5-ylidene-[1,3]thiazolo[3,2-b]1,2,4]triazol-6-ones (30) [103] (Scheme 16).
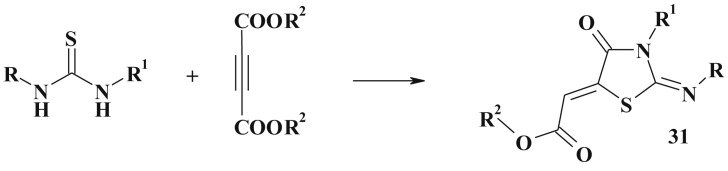
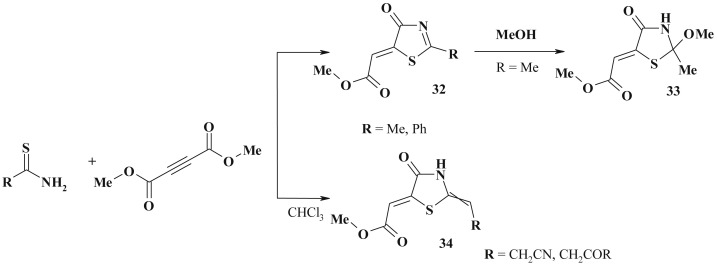
The common approach to the synthesis of 5-ylidene-4-thiazolidinones with carboxylic group (31) based on [2 + 3]-cyclocondensation reaction is the utilization of unsaturated acids and their derivatives (Scheme 17 ). This approach is also efficient in the synthesis of various C2 substituted 4-thiazolidinones [104], [105], [106] (target compounds can be N-substituted or with unsubstituted N3 position). The condensation of thioamides with dimethylacethylenedicarboxylate in benzene (or acetonitrile or acetic acid for thioacetamide) led to appropriate thiazolidinone (32, 33) [107], [108] (Scheme 18 ). But there are reports about the formation of other products, such as 2-(1-iminoethylsulfanyl)-fumarate [28], [109].
Scheme 17.
General scheme of [2 + 3]-cyclocondensation of unsaturated acids.
Scheme 18.
Condensation of thioamides with dimethylacethylenedicarboxylate.
Similar ylidene derivatives (mixture of E/Z isomers) 34 were formed in the reaction of α-carbamoyl(cyano)thioacetamides with dimethylacethylenedicarboxylate [28], [110], [111].
Thioamides of α,β-unsaturated acids in the reaction with acethylenedicarboxylic acid, propionic acid and their esters in acetone medium also yield the thiazolidinones as well as thiazanones [28], [112].
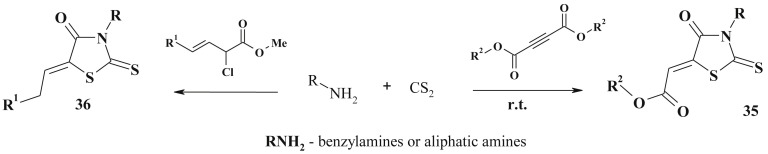
The three-component reactions of amine with dialkyl acetylenedicarboxylate and carbon disulfide yielded related rhodanine derivatives (35) [113], [114] (Scheme 19 ). Isocyanate utilization instead of carbone sulfide under the same conditions led to maleimide derivatives formation.
Scheme 19.
Carbon disulfide based synthesis of 5-ene-rhodanines.
The exploration of α-chloro-β,γ-alkenoate esters in such type of multicomponent reactions led to the formation of 5-(Z)-alkylidene-2-thioxo-1,3-thiazolidin-4-ones (36) which are uncommon compounds [115].
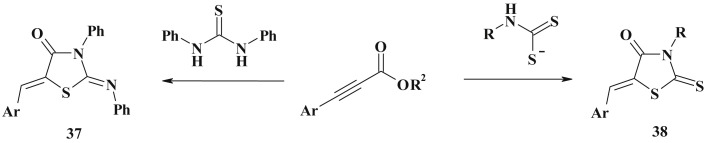
Arylpropiolates are efficient building blocks for 5-ylidenethiazolidinone constructing (Scheme 20 ). Reaction of the latter with bifunctional sulfur pronucleophiles is the phosphine-catalyzed tandem process which includes umpolung addition and intramolecular cyclization (37). Similarly, rhodanine derivatives (38) [116] were synthesized from dithiocarbamates in the phosphine catalyzed reaction (Scheme 20).
Scheme 20.
Arylpropiolates based synthesis of 5-ene-4-thiazolidinones.
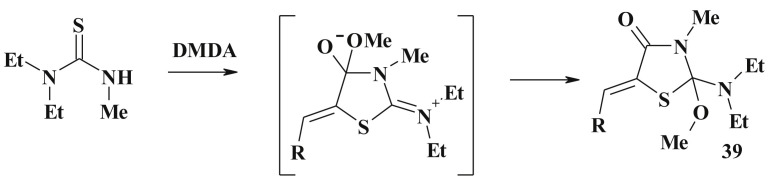
Reaction of trisubstituted thioureas with dimethylacetylenedicarboxylate (DMDA) in methanol medium can also lead to 1:1 adducts (39) [108] (Scheme 21 ).
Scheme 21.
Example of the thioureas utilization in 5-ene-4-thiazolidinone derivatives synthesis.
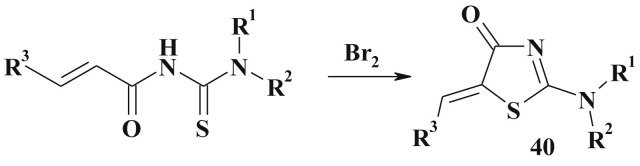
Similarly, pseudothiohydantoin (2-amino-1,3-thiazol-4(5H)-one) derivatives (40) can be easily obtained under the propenoylthioureas oxidation (e.g., by bromine action) (Scheme 22 ) [117].
Scheme 22.
Synthesis of the pseudothiohydantoin derivatives.
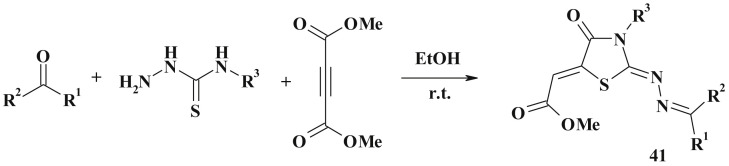
Novel 2-hydrazolyl-4-thiazolidinone-5,6-α,β-unsaturated esters (41) were synthesized in the multicomponent reaction of aldehydes, thiosemicarbazides and dimethylacetylenedicarboxylate in the ethanol medium. Interestingly, the reaction doesn't depend on the presence of electron-withdrawing or electron donating groups. It involves thiosemicarbazone formation followed by Michael addition of the sulfur atom to the triple bond and sequential cyclization (Scheme 23 ) [118].
Scheme 23.
Multicomponent reaction for 2-hydrazolyl-4-thiazolidinones synthesis.
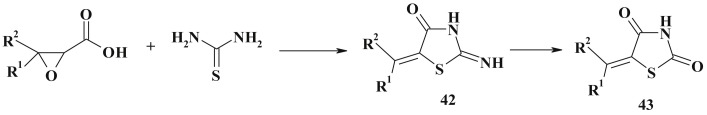
Some epoxy acids (cis- and trans-epoxysuccinic, 2,3-epoxybutyric and methyl-cis-epoxysuccinic acids) constitute another equivalent of the dielectrophilic synthon [C2]2+ in [2 + 3]-cyclocondensation reactions with thiourea when obtaining 5-substituted pseudothiohydantoines (42) and 2,4-thiazolidinediones (43) [119] (Scheme 24 ).
Scheme 24.
Synthesis of pseudothiohydantoines and 2,4-thiazolidinediones from epoxy acids.
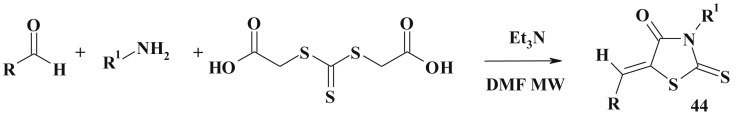
A fast and high yielding protocol for the generation of substituted 5-arylidenerhodanines (44) in sequential one-pot two-step process combining the Holmberg method and the Knoevenagel condensation under microwave assisted conditions has been developed [73] (Scheme 25 ).
Scheme 25.
Holmberg method and Knoevenagel condensation based one-pot method.
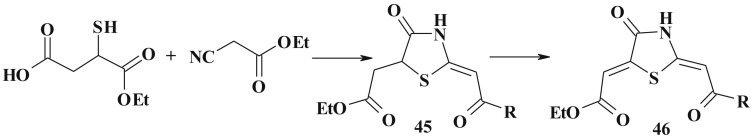
An important synthetic route to 3-substituted 4-thiazolidinones is the reaction of α-mercaptocarboxylic acid derivatives with ethylcyanoacetate, cyanoacetamides, malonodinitrile etc. [2], [3], [6], [120], [121] as well as the cyclocondensation of oxonitriles or cyanoguanidines with 2-mercaptosuccinic acid derivatives [121] (Scheme 26 ). Obtained 2-methyl-4-thiazolidinones 45 were exposed to regioselective bromination and dehydrogenation to form 5-ene-4-thiazolidinone derivatives 46 [122], [123].
Scheme 26.
Synthesis of 2,5-diene-4-thiazolidinone derivatives.
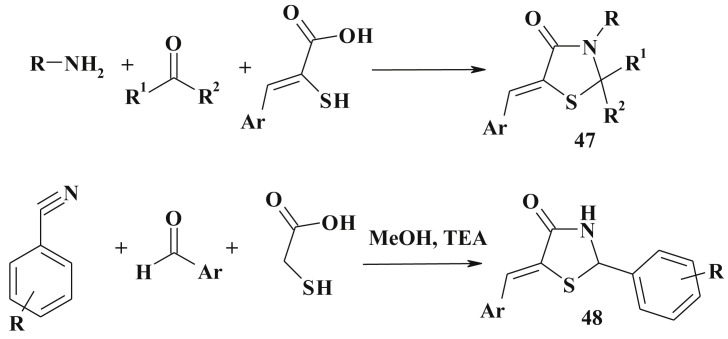
The most convenient method for 2-substituted-4-thiazolidinones synthesis is the one-pot three-component reaction of a primary amine, an oxo-compound, and a thiolic agent using various reaction conditions, such as extended heating with a dehydrating agent, using an acylation agent or microwave-assisted organic synthesis [124], [125], [126], [127], [128], [129]. Based on the retrosynthetic approach the synthesis of 5-ene-2,3-disubstituted-4-thiazolidinones (47) was proposed [43] (Scheme 27 ). 3-Substituted-2-mercaptoacrylic acids obtained from 5-arylidenerhodanine were used as thiolic agents.
Scheme 27.
Synthesis of 5-ene-2-R-4-thiazolidinones.
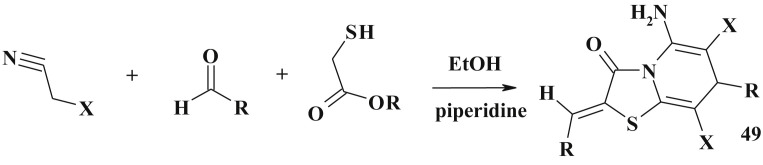
The 5-ene-4-thiazolidinones (48) were prepared in such one-pot three-component reaction where nitriles were used instead of corresponding amines [130], [131]. The similar three-component reaction was proposed for the fused heterocycles synthesis, namely thiazolo[3,2-a]pyridine derivatives (49) [132], [133] (Scheme 28 ).
Scheme 28.
Three-component reaction in the synthesis of fused heterocycles with 5-ene-4-thiazolidinone fragments.
This type of three-component domino reaction of readily available thioglycolic acid/ethylthioglycolate, aromatic aldehydes and malononitrile/ethylcyanoacetate was described in the aqueous potassium carbonate at r.t [134].
The one-pot reaction based on the condensation of ethylthiocyanoacetate, arylidenehydrazine and hydrazine hydrate in glacial acetic acid also led to the formation of 5-arylidene-2-arylidenehydrazone-4-thiazolidinone (50) [135] (Scheme 29 ).
Scheme 29.
One-pot method of 5-ene-2-hydrazone-4-thiazolidinone synthesis.
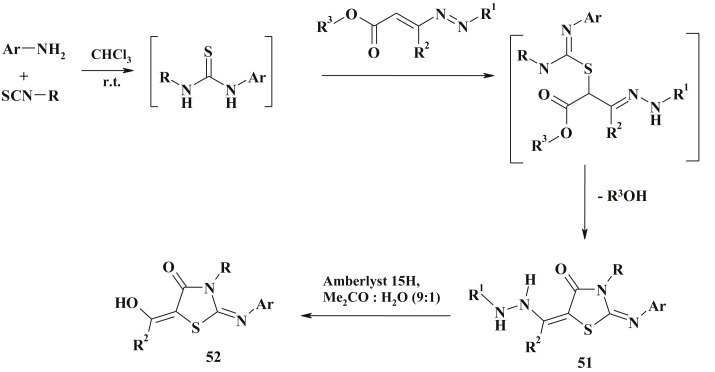
Similarly to the 5-unsubstituted 4-thiazolidinones synthesis, the sequential three-component reaction involving primary amines, isothiocyanates, and 1,2-diaza-1,3-dienes was proposed as an efficient method for the 2-iminothiazolidin-4-ones-synthesis (51) [136] (Scheme 30 ).
Scheme 30.
1,2-Diaza-1,3-dienes in 5-ene-4-thiazolidinone synthesis.
Formation of the 2-iminothiazolidin-4-one was explained by the initial regioselective S-Michael addition of the thiourea intermediate, which resulted from the coupling of amine and isothiocyanate, to the electrophilic center of 1,2-diaza-1,3-diene. The next step was the intramolecular attack of the -NH of the obtained isothiourea derivative on the ester group in C4 of the hydrazone chain with a loss of an alcohol molecule [136]. The hydrolytic cleavage of the hydrazide moiety of 51 afforded new 5-hydroxyethylidene thiazolidinones 52 [136].
2.3. Transformation of related heterocycles
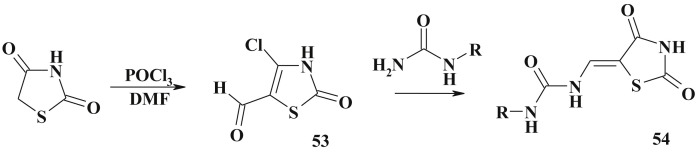
The transformation of related heterocycles aimed to form 5-ene-4-thiazolidinone is not very often explored in the 4-thiazolidinone synthesis due to the simplicity of the methods described above. However, in the reaction of monosubstituted ureas with 4-chloro-5-formylthiazolin-2-one (53) the rearrangement occurs yielding 2,4-dioxothiazolidin-5-ylidenemethyl-urea (54). The starting 4-chloro-5-formylthiazolin-2-one (53) was obtained in the reaction of 2,4-thiazolidinedione formylation [137] (Scheme 31 ).
Scheme 31.
Recyclization in the synthesis of 2,4-dioxothiazolidin-5-ylidenemethyl-urea.
3. Pharmacological profiles of 5-ene-4-thiazolidinones
Most of the pharmacologically attractive agents among 4-thiazolidinone-based compounds are exactly 5-ene-4-thiazolidinones with the exocyclic double bond. This is reflected in crucial impact of the presence/nature (namely 5-ene) of C5 substituents in thiazolidone ring on pharmacological effects [27], [34], [35], [54], [94], [138], [139], [140], [141], [142], [143]. At the same time, (ar)ylidene fragment conjugation with the carbonyl group at C4 of the thiazolidinone core makes such compounds electrophilic and potentially reactive due to possible Michael addition of the exocyclic double bond to the nucleophilic protein residues [144], [145]. Moreover, 5-ene-thiazolidinones can react with glutathione and other free thiols within a cell [145], [146], [147] (Sheme 32 ).
Scheme 32.
General scheme of thia-Michael addition of 5-ene-4-thiazolidinones.
This property characterizes 5-arylidene-4-thiazolidinones (mainly rhodanine derivatives) as frequent hitters or pan assay interference compounds (PAINS) that are thought to be useless in the modern drug discovery process because of their possible/predicted insufficient selectivity [4], [14], [148], [149], [150]. This, along with other structural features, offers high probability of polar interactions and hydrogen bonds formation. Causing a promiscuous behavior at concentrations within the “screening range” this statement should not be regarded as a general knock out criterion that excludes such screening hits from further development. It is suggested that special criteria for target affinity and selectivity must be applied to these classes of compounds so that their exceptional and potentially valuable biomolecular binding properties are consequently exploited in a useful way [14].
Interpretation of 5-arylidene-4-thiazolidinones as frequent hitters is often not confirmed in experimental studies [24], [151], [152] and a large number of lead-compounds belong to 5-ene-4-thiazolidinones. The positive perspective may also be associated with the polypharmacological approach in drug discovery, where the affinity toward various targets is regarded as an advantage [14]. Moreover, mentioned compounds as the examples of privileged scaffolds can be treated as drug-like molecules that provide baseline affinity for a whole protein family [14], [153]. Additionally, better results in drug design can be expected when responses are evoked by multipoint interventions in more than one mechanism and towards different targets [154] following the concept of multi-target drugs, also known as dual or symbiotic drugs [155]. The exploitation of 5-ene diversity allows achieving such desired combinations of molecular fragments. There have been many reports describing the presence of heterocyclic moieties, such as thiazole, pyrazole, flavones, chromone, furan etc. at C5 position of thiazolidinone core [8], [27], [34], [35], [65], [66], [138]. It is even considered that the more such fragments are in the molecule, the more effective it is when comparing with the molecules including simple aryl groups (see below). In this spirit, lack of strict specificity in ligand binding and possibility of the influence of both the compound and its metabolites on the other metaboli≿ pathways are being actively discussed. This concept is one of the arguments in favour of the search for “nonspecific” biologically active compounds. Furthermore, many compounds possess different types of activity that are apparently due to their action towards differents metabolic pathways (targets) or involving the same biological targets in metabolic pathways of various pathologies.
Thus, many authors consider that “thiazolidinones and related scaffolds should not be regarded as problematic or promiscuous binders per se” [14]. The intermolecular interaction profile of these scaffolds makes them prone to bind to a large number of targets with weak or moderate affinity. It may be that the observed moderate affinities, e.g. in screening campaigns, have been overinterpreted in the past and that these compounds have too easily been put forward as lead compounds for further development.
In this context, it is worth mentioning that exactly the same Michael acceptors are among the most effective activators of Nrf2 through the Keap1 modification that opens new perspectives in the treatment of inflammation, cancer, etc. [144], [156]. Moreover, Michael acceptors including 5-ene-thiazolidinones belong to a new class of highly specific and potent inhibitors of the mitochondrial pyruvate carrier [157].
In addition, it should be emphasized that the possibility of being Michael acceptors (calculated or predicted based on the molecular structure and used in the in silico studies) is often not confirmed experimentally under conditions close to physiological [156].
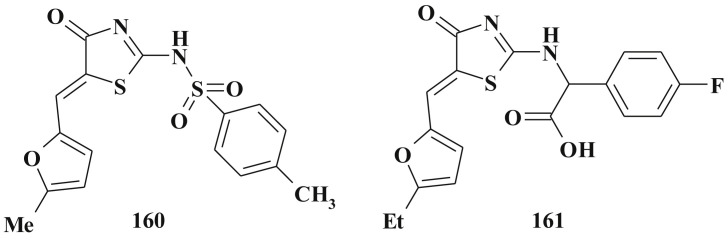
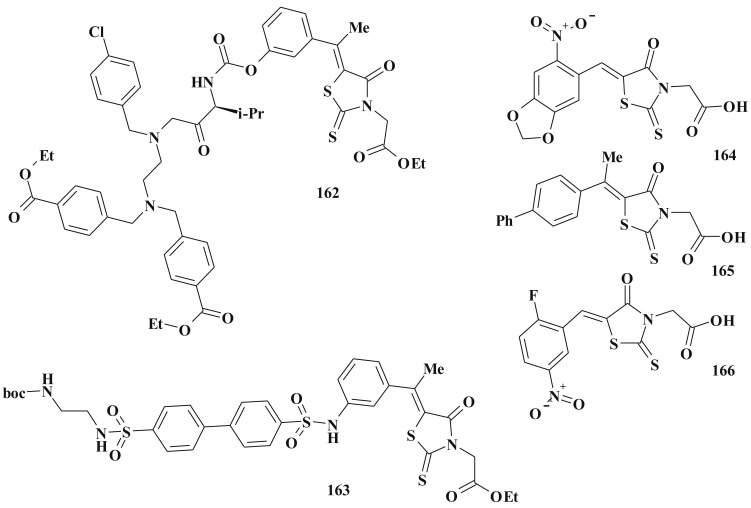
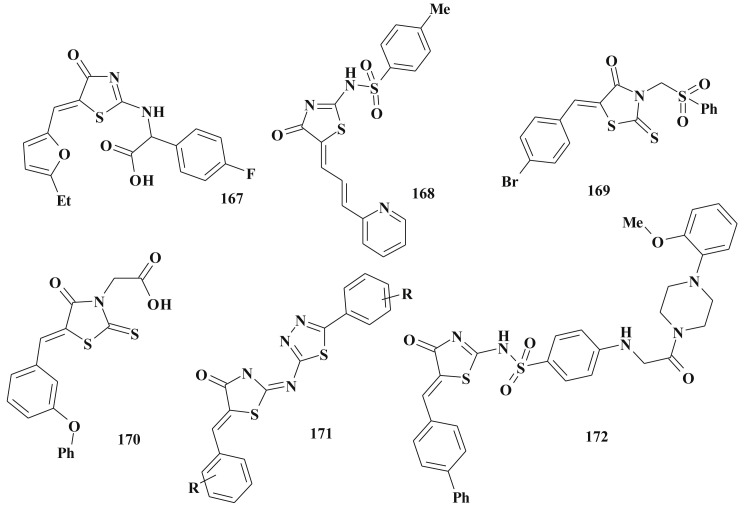
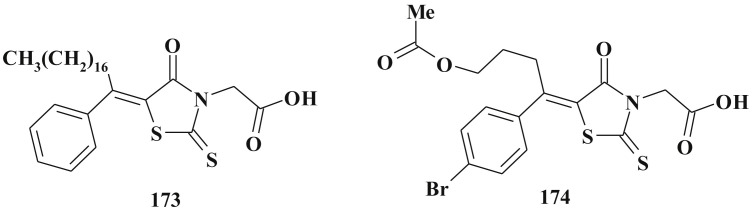
Besides, as was mentioned earlier, 5-ene-4-thiazolidinones are widely used in the design of complex heterocyclic systems within the hybride pharmacophore approach [158], [159]. The significant biological potency of compounds bearing 5-ene-4-thiazolidinone fragment in combination, for example, with heterocycles [27], [34], [65], [138], [160] or other scaffolds [161], [162] was discovered. Satisfactory toxicological parameters of such compounds may be an additional argument in favour of their development and study [163].
The next chapter represents the most prominent pharmacological profiles of 5-ene from different 4-thiazolidinone subtypes which belong to hit-, lead-compounds, drug-candidates and drugs. Two main directions of the study of biological activity of 5-ene derivatives are presented: the identification of a number of compounds possessing some types of biological activity in screening procedure (without molecular targets identification); design of high-affinity ligands to established molecular targets (for instance, a lot of thiazolidinones are proved to be ligands to the biological targets presented in protein data bank (www.rscb.org)). Moreover, several types of activity are often described for the same compounds that clearly follows from the above said.
A large number of publications are dedicated to the in silico study of 4-thiazolidinones and particularly 5-ene derivatives. In this paper we did not focus on such data as it is widely outlined in the review papers [164], [165], [166], [167], [168], [169], [170]. A wide range of established pharmacological activity of 5-ene-4-thiazolidinones does not allow to describe all its types in the given manuscript. Thereby, we focused on the most studied and described types of activity such as antimicrobial, antitumor etc. Besides, the compounds bearing fused 5-ene fragment (e.g. thiazolothiopyranes, thiazolopyridines etc.) are beyond the scope of this paper.
3.1. Antimicrobial agents
One of the earliest directions of the 5-ene-4-thiazolidinones biological assays lies in the field of antibacterial and antifungal activities search. Apparently, the structural similarity of 4-azolidinones with penicillin antibiotics was the stimulus to the study of such type of activity [1], [2], [3], [6], [167], [168]. Though, recently it was discovered that manifestation of antibacterial effect is not always related to the penicillins' mode of action. Usually, there is a tendency to move from the detection of antimicrobial activity [169], [170], [171], [172], [173], [174], [175], [176] within screening programs to identification and design of high affinity ligands to the validated molecular targets. Regardless of the nature of the basic core (2,4-thiazolidinedione, rhodanine, etc.), modification and complication of C5 ylidene fragment are the benefits for realization of such type of activity (the role of the halogen-arylidene moieties should be stressed) [141], [177].
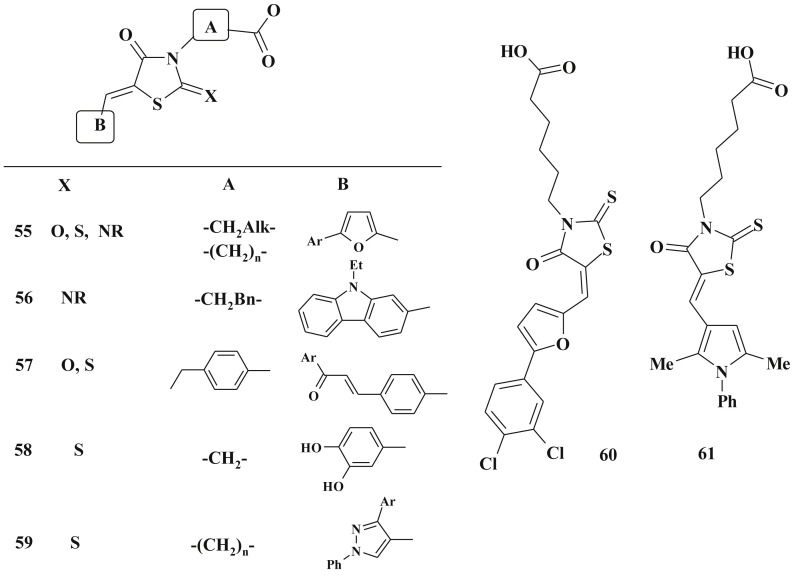
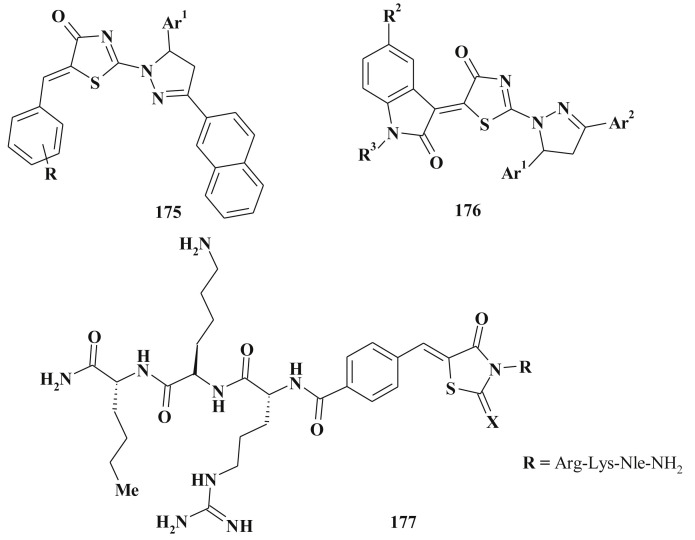
The 3-substituted 5-ylidene-4-thiazolidinones, especially 3-carboxylic acids 55–59 [171], [175], [176], [177], [178], [179], [180], [181] represent group of compounds with significant antimicrobial activity against gram-positive and gram-negative microorganisms. Their molecular mode of action is associated with the inhibition of: i) the last two stages of peptidoglycan cell wall biosynthesis (the most attractive targets for new antimicrobials design - mainly penicillin-binding proteins family, and transferases – UDP-Mur-NAc-peptidases); іі) the activity of dehydrogenases (compound 58 and related structures can cross the bacterial cell wall and lead to the identification of catechol-rhodanine core as privileged scaffold for design of the dehydrogenases inhibitors) [153]; iii) peptide deformylase [182] (for lead-compounds 60, 61 IC50 = 0.89 μM and 1.66 μM correspondly) etc (Scheme 33 ).
Scheme 33.
5-Ene-4-thiazolidinone-3-carboxylic acids with antimicrobial activity.
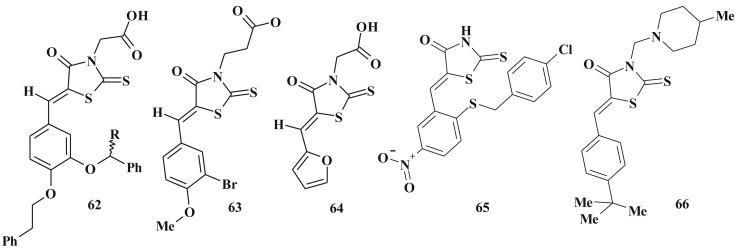
A series of 5-ene-rhodanine-3-acetic acid derivatives 62 (Scheme 34 ) were described as inhibitors of fungal protein mannosyl transferase 1 (PMT1) at micromolar levels (design of compounds was also based on the complications of C5 moiety) [183]. High-throughput screening of 5-arylidene-4-thiazolidinone-3-alkanecarboxylic acids 63, 64 proved them to be anthrax lethal factor (as one of the major virulence factors) inhibitors (IC50 ∼ 10 μМ) [45], [184].
Scheme 34.
5-Ene-rhodanines as antimicrobial agents.
Antimicrobial activity of such compounds are determined by inhibition of penicillin-binding proteins family, such as a group of transferases (including UDP-Mur-NAc-peptidases), most of their subtypes are characterized by distinct transglycosylase activity [185]. ATP-dependent amino acid ligases (MurC, MurD, MurE, and MurF, MurG) are an illustrative examples of the 5-ene-4-thiazolidinones antibacterial agents design [41], [186], [187], [188]. For instance, 65 is an efficient inhibitor of MurC (UDP-N-acetylmuramate/ l -alanine ligase) [139] and 66 – of MurG [189]. It should be emphasized that the 4-thiazolidinone and rhodanine cores are treated as novel phosphate mimics [190] (Scheme 34).
Structurally similar compounds are the inhibitors of dTDP-rhamnose synthesis. This is especially important for antimycobacterial agents search. The mycobacterial cell wall is unique because it contains an amycolylarabinogalactan layer bound to the peptidoglycan layer via a rhamnose–Glc-NAc sugar linker, where the dTDP-rhamnose can be vetreated as a precursor [191], [192].
5-Heterylidene-2,4-thiazolidinedione derivatives 67 are competitive inhibitors of recombinant bacterial arylamine-N-acetyltransferases (NATs) [193], which were designed based on the structure modification of N-(2-naphthyl)-methyl substituted 1,1-dioxo-2,3-dihydrobenzo[1,2]-thiazine-4-ylidenethiazolidine-2,4-dione (weak inhibitor of NAT) [194] (Scheme 35 ).
Scheme 35.
5-Heterylidene-4-thiazolidinedione with antimicrobial activity.
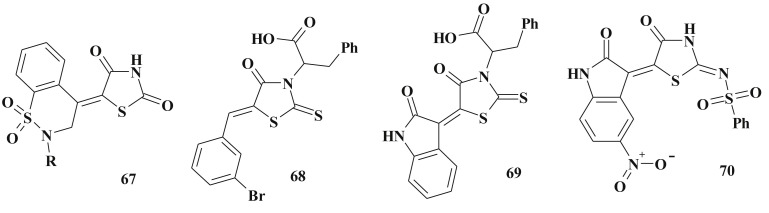
One of the pathogenetic processes of pro- and eukaryotes is the mechanism associated with galactofuranose-based conjugation which involves UDP-galactopyranose mutase (UGM). The inhibition of such process leads to inhibition of microbial growth and reduction of virulence (especially for Mycobacterium tuberculosis) and improve the antimicrobial/antimycobacterial activity of UGM inhibitors. High inhibition activity of this enzyme was also discovered for 4-thiazolidinone-3-alkanecarboxylic acids 68, 69 [180] and 2-imino derivatives 70 [145], [195] (Scheme 35).
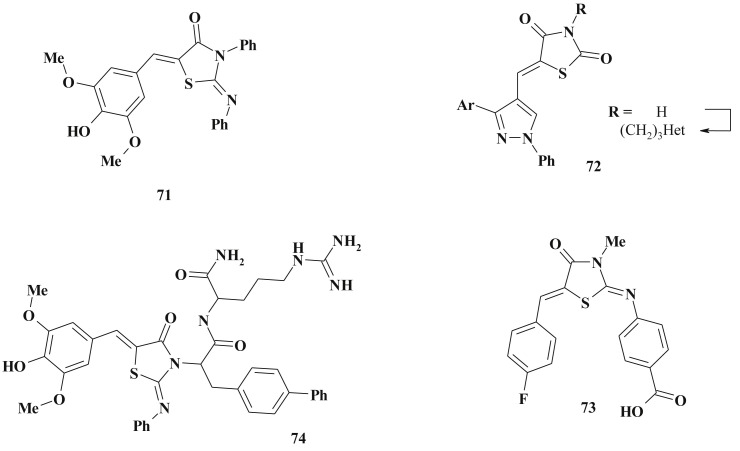
2-Amino(imino)-4-thiazolidinones have been widely investigated [141], [177], [196], [197] as antimicrobial agents, e.g. compound 71 was identified as an inhibitor of the type III secretion system of Gram-negative bacteria [196], [198]. Pseudomonas aeruginosa had shown reduction of T2S-dependent elastase secretion in the presence of 71 (Type II secretion (T2S) systems is well conserved among Gram-negative bacteria and a key virulence factor of P. aeruginosa) [199]. The structure optimization aiming the increasing of solubility led to compound 74 which possessed superior activity (10 μM) in the S. typhimurium T3S secretion assay [196]. The N-3 position was established to be the most permissive optimization direction (Scheme 36 ).
Scheme 36.
5-Ene-4-thiazolidinediones as inhibitors of the type II & III secretion systems.
The same findings are suitable for 2,4-thiazolidinedione derivatives – N3 modification provides to 5–40 folds increasing of the antimicrobial activity (72) [200], [201].
Related 2-amino-4-thiazolidinone (73) was detected as high active CysK1 inhibitor. CysK1-pyridoxal phosphate-dependent O-acetylsulfhydrylase which catalyzes the formation of l-cysteine from O-acetylserine and hydrogensulfide. This cysteine biosynthetic pathway is one of the essential pathways in microbial pathogens, providing potential targets for the development of novel antibacterial compounds [202] (Scheme 36).
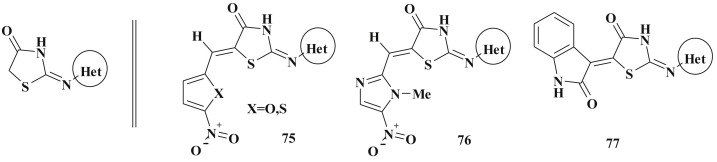
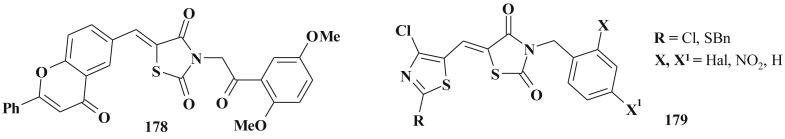
The row of 2-thiazolylimino-5-arylidene-4-thiazolidinone bearing the nitro group and small heterocyclic fragments (thiazole, benzthiazole, isatine, pyrazole, furane, thiophene) (75–77) (Scheme 37 ) in positions 2 and 5 showed sufficient antimicrobial activity levels too [203]; the nitro derivatives were characterized by high renal excretion that can be used in the design of potential diuretics [204]. The results of QSAR study revealed that the compounds with less number of atoms or less number of substituents are more likely to be active than their counterparts with higher molecular weight [205].
Scheme 37.
2,5-Disubstituted-4-thiazolidinone hybrids with antimicrobial activity.
5-Heterylidenerhodanines and simple arylidene analogs are proposed as novel class of b-lactamase inhibitors that possesed selectivity for class C b-lactamases (78 is a non-b-lactam with the IC50 ∼ 2.6 mM against the class C enzyme P99) [206].
Simple 5-alkylidene-2,4-thiazolidinediones 79 were discovered as effective inhibitors of autoinducer-2 quorum sensing (mechanism through which bacteria regulate gene expression in response to population density including regulation of the production of virulence factors etc.) Virulence targeting is representing an emerging concept in antibacterial therapy for which there are examples of compounds that inhibit virulence functions [207]. N-3-Dipeptide-thiazolidinone hybrids 80 may provide a critical step toward the validation of this strategy and the development of novel therapeutics [196] (Scheme 38 ). Compounds having bulky aromatic substituents at position 5 and a tryptophan residue at position N-3 of the rhodanine ring were the most active against InhA (trans-2-enoyl-acyl carrier protein reductase) with micromolar IC50 [208].
Scheme 38.
Structure of antimicrobial 5-ene-4-thiazolidinones.
3.2. Antiparasitic agents
The in vitro antiamoebic activity of 3-substituted 2-amino-4-thiazolidinones 81 was evaluated against HM1:IMSS strain of Entamoeba histolytica and it exhibited promising activity (IC50 - 0.11–0.172 mM being lower than that of metronidazole IC50 - 1.64 mM) and low toxicity level [209] (Scheme 39 ).
Scheme 39.
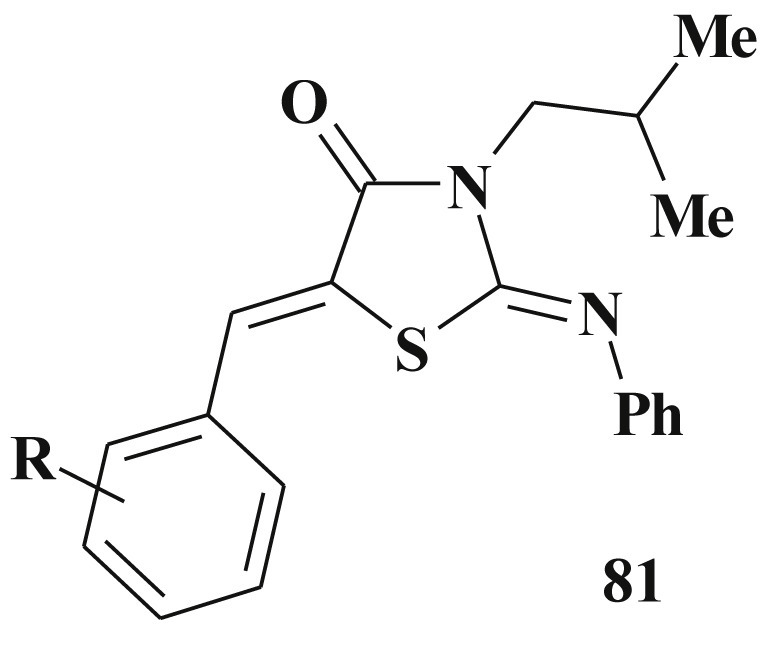
3-Substituted 2-amino-4-thiazolidinones with antiamoebic activity.
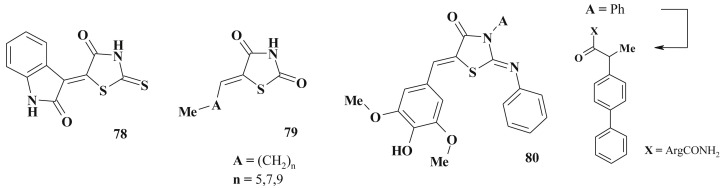
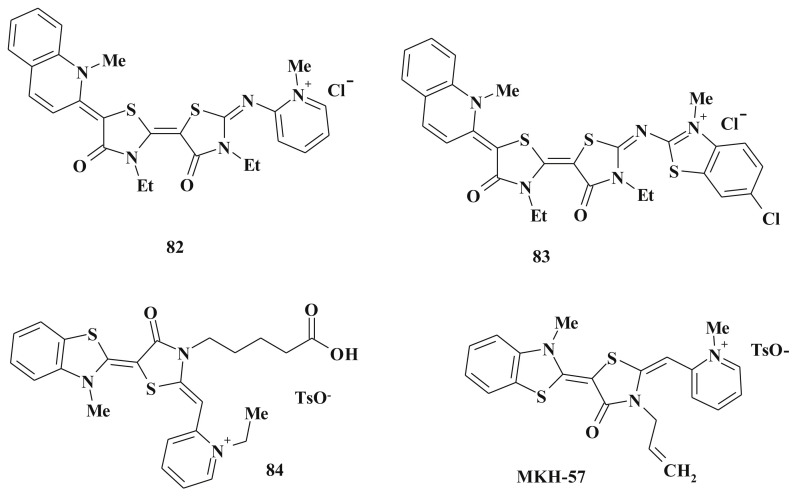
Several aza-fused rhodacyanines [210] were found as perspective agents when testing various heterocyclic rings on the antimalarial activity. Among tested compounds 82 showed excellent inhibitory activity with an IC50 of 4.4 nM (K1strain). Another compound 83 with quinoline ring and two rhodanine moieties showed 78% suppression of parasitemia (25 mg/kg/day) [211]. 4-Thiazolidinone-3-carboxylic acids belonging to rhodacyanine dyes 84 are characterized by distinct antimalarial activity [87], [212]. Significant anticancer activity of the mentioned class of compounds should be emphasized in this context (see below) (Scheme 40 ).
Scheme 40.
Rhodanine-based dyes with antimalarial activity.
An in vitro structure–activity relationship investigation showed that the rhodacyanine MKH-57 possesses high antimalarial activity (EC50 = 12 nM) and significant selective toxicity [87] as well as a series of its analogs [212].
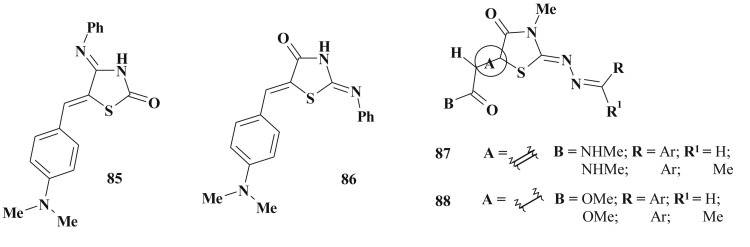
Screening of more than 13000 compounds for antimalarial activity using the agglomerative structural clustering technique allowed identifying 47 starting points for lead optimization including 4-amino(imino)thiazolidinone derivatives 85 [213]. The positional isomer of mentioned 4-amino derivatives (85) – compound 86was also described in the compound library of potential antimalarial leads [213] (Scheme 41 ).
Scheme 41.
5-Ene-4(2)-thiazolidinones as antiparasitic agents.
In the area of antiprotozoal agents search the design of antitrypanosonal agents based on thiazolidinone scaffold is of special interest [214]. Compounds 87 showed the highest antiproliferative activity in comparison with 5-saturated analog 88 when screened on Trypanosoma cruzi epimastigotes but were inactive towards cruzipain [118] (Scheme 41). While 88, obtained from a virtual screening of 500000 chemical structures (ZINC5 database) against cruzipain [215] inhibited this enzyme at micromolar concentration.
5-Benzylidenerhodanine-3-acetic acid derivatives 89 were reported to possess inhibitory activity against Trypanosoma brucei dolicholphosphate mannose synthase and glycosylphosphatidylinositol anchor synthesis as well as in vitro trypanocidal activity against the blood stream form and were non-cytotoxic against HeLa cells. Dolicholphosphatemannose synthase is a mannosyl transferase critically involved in glycoconjugate biosynthesis in T. brucei. VSG dimmers covering the parasite cell-surface are linked to the trypanosomal plasma membrane via glycosyl phosphatidylinositol anchors, which biosynthesis is essential for viability of the blood stream form of T. brucei. The 3-benzyloxy-substituted analog and the 2-hydroxyderivative 89 showed the best trypanocidal activity (ED50 ∼ 100 μM) [215] (Scheme 42 ).
Scheme 42.
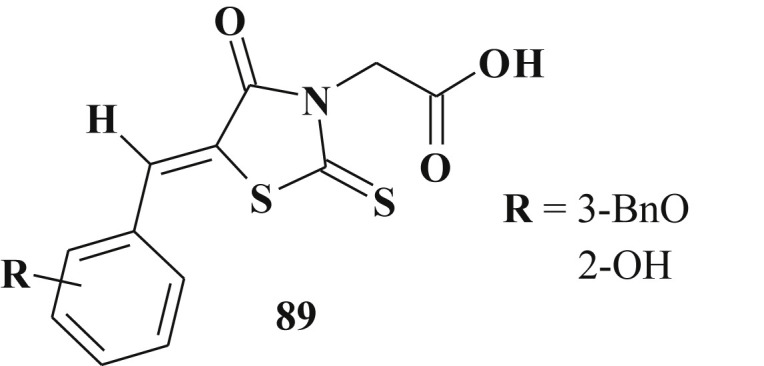
5-Benzylidenerhodanine-3-acetic acid derivatives as antitrypanosomal agents.
Simple 5-(hydroxyphenylmethylidene)thiazolidine-2,4-diones were described as novel inhibitors of Leishmania pteridine reductase 1 where the thiazolidinone ring was treated as a bioisosteric replacement for pteridine/purine ring [216].
One of the discussed modes of action of thiazolidinone based compound is the DNA-binding process. In this study 2-imino derivatives 90 were designed and the most potent molecule bound at the DNA minor groove involving Van der Waals, H-bonding and hydrophobic interactions [217] (Scheme 43 ).
Scheme 43.
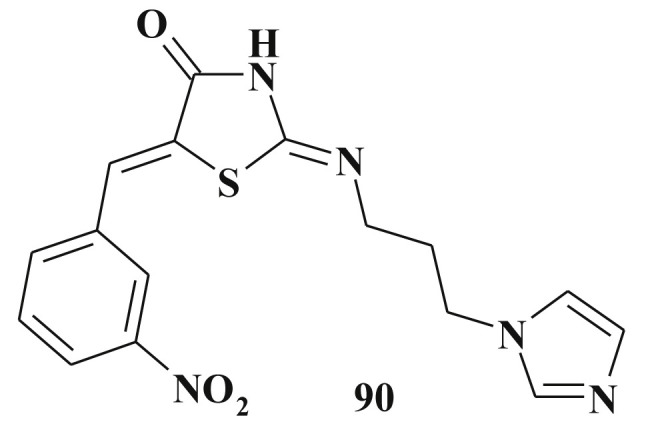
2-(3-Imidazol-1-yl-propylimino)-5-(3-nitro-benzylidene)-thiazolidin-4-one as potential DNA-binding compound.
3.3. Antidiabetic agents
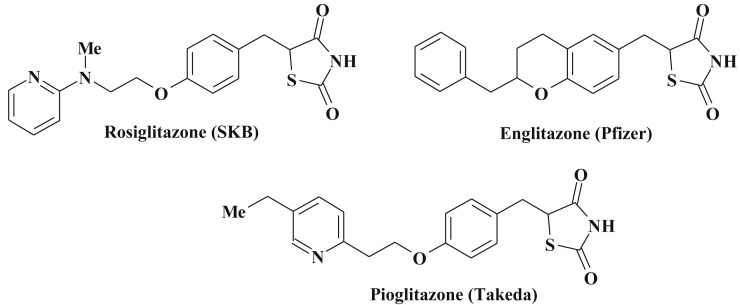
Search for new antidiabetic agents among 4-thiazolidinones is one of the most studied fields and had resulted in the introduction of new class of the antidiabetic drugs – glitazones. Their mechanism of action is associated with activation of PPARγ (peroxisome proliferation activated receptors) [19], [218], [219], [220] (Scheme 44 ).
Scheme 44.
Structures of known glitazones.
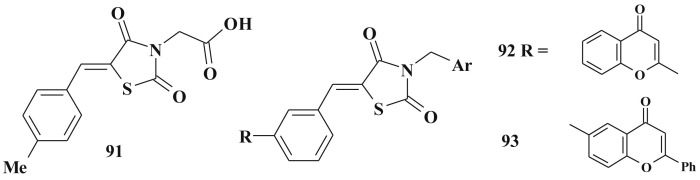
However, 5-ene derivatives including C5-unsaturated analogs of glitazones are to lesser extent activators of PPARs [221], [222]. The introduction of carboxylic acid residue in the N3 position leads to decrease of hepatotoxicity of glitazones as well as unsaturated structural analogs (5-arylidene-thiazolidine-2,4-dione-3-acetic acids 91). Compound 91 and related derivatives are reported to have low or no activity on PPAR, but have high antidiabetic activity in vivo (confirmed in sucrose-loaded model) [223].
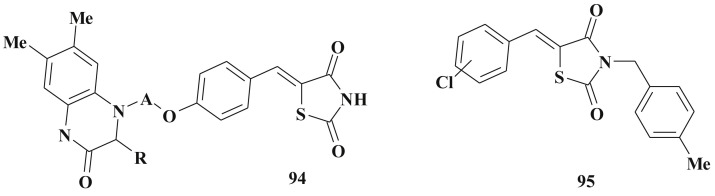
This indicates other mode of antihyperglycemic action of 5-ylidene-4-thiazolidinones which can be treated as glitasone bioisosters. 5-Arylidene-3-benzyl-2,4-thiazolidinedione derivatives (92, 93) possess antihyperglycemic activity opposed to troglitazone under the experimental conditions (evaluation of in vitro insulin releasing activity, INS-1 cells); the latter has not decreased the level of hyperglycemia [224] (Scheme 45 ). Structurally related 5-ene-2,4-thiazolidinediones 94 with bulky C5 fragments are treated as potent euglycemic and hypolipidemic agents too [225] as well as their simple analog 95 (the alloxan-induced hyperglycemia, in vivo mice model) [226] (Scheme 46 ).
Scheme 45.
5-Ene-2,4-thiazolidinediones with antihyperglycemic activity.
Scheme 46.
5-Ene-2,4-thiazolidinones based hypolipidemic agents.
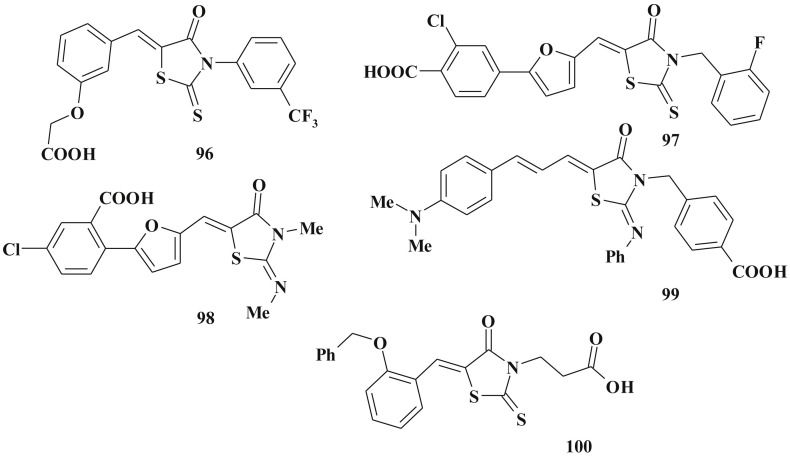
However, studies of PPAR-related actions of 5-ylidene-4-thiazolidinones are continuing. Virtual screening tools including SQUIRREL (Sophisticated QUantification of InteRaction RELationships) procedure allowed detecting a set of potential PPARs ligands [227]. 14 Compounds including 4-thiazolidinones 96–99 showed PPARα and PPARγ agonistic activity (lead-compound 99 possessed the nano-level of activity (EC50 PPARα = 0.044 μM)) (Scheme 47 ).
Scheme 47.
5-Ene-4-thiazolidinones with PPARα/γ agonistic activity.
A new series of PPARγ agonists known as (β-carboxyethyl)-rhodanine derivatives were identified based on ligand-centric and receptor-centric approaches. An in vitro assay had confirmed the nanomolar binding affinity of 100. In a cell-based transactivation assay similar PPARγ agonistic activity to that of the known PPARγ drug, pioglitazone, was shown. Based on CoMFA model and docking data it was discovered that the electrostatic interaction of the carboxyethyl group with the rhodanine core is important for its binding in the pocket of PPARγ and the rhodanine heterocycle played a different role than the thiazolidine group of rosiglitazon [228].
Currently the investigation of PPARγ antagonists is also of the great interest in the treatment of diabetes and obesity [229], [230] including study of the 5-ene-4-thiazolidinones.
The most investigated mechanism of antidiabetic mode of action of 5-ene-4-thiazolidinones is the inhibition of aldose reductase (AR). Aldose reductase is the limiting enzyme of polyol/sorbitol pathway of glucose oxidation, excessive activation of which leads to the accumulation of glucitol and the development of diabetic complications [231]. 5-Ene-rhodanine-3-alkanecarboxylic acids are high affinity inhibitors of aldose reductase [232]. The illustrative example of the mentioned compounds' row is epalrestat – (Z,E)-5-(2-methyl-3-phenyl-2-propenylidene)-2-thioxo-4-thiazolidinone-3-acetic acid. Optimization of these compounds structures is mainly associated with C5 fragment modification [53], [233], [234], [235]. Study of epalrestat analogs revealed that Z-isomers possessed higher activity level. There is a great interest in 2,4-thiazolidinedione derivatives as AR inhibitors since they can be viewed as hydantoin and rhodanine bioisosteres potentially free of the hypersensitive reactions which are linked to the presence of the hydantoin system [55], [236], [237]. In fact, to date, several thiazolidine-2,4-diones have been patented with dual activity as anti-hyperglycaemic and AR2 inhibitory agents [238], [239]. The findings suggest that the activity of these compounds might correlate with their AR2 inhibitory ability by preventing the stimulation of PKC or MAPK and the subsequent activation of NF-kB [240]. AR2 plays a pivotal role in mediating oxidative stress-induced inflammation and is implicated in the development of various inflammatory pathologies [232] (see below). This enzyme catalyzes the reduction of lipid peroxidation-derived aldehydes, thus producing metabolites which transduce inflammatory signaling by means of the activation of protein kinases such as PKC and MAPK. This in turn activates NF-kB that is responsible for the transcription of many proinflammatory genes [241], [242].
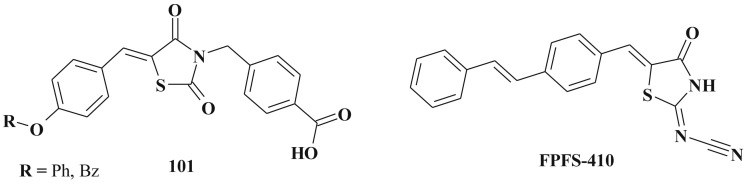
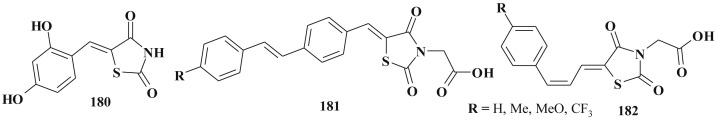
Antidiabetic activity of target compounds is often related to the inhibition of PTP 1B (protein tyrosine phosphatase 1B), which is an intracellular PTP and a key negative regulator of the insulin signaling pathway as well as to LMW-PTP (low molecular weight protein tyrosine phosphatase). This possible mode of action may be regarded as an attractive approach to the design of new therapeutic agents for the treatment of type 2 diabetes mellitus, obesity and, therefore, of the states associated with complex metabolic disorders known as metabolic syndrome. 4-(5-Arylidene-2,4-dioxothiazolidin-3-yl)methylbenzoic acids 101 were indicated as inhibitors of both PTP 1B and LMW-PTP (Scheme 48 ). One of the directions in this study is the example of utilization of phosphotyrosine-mimetics to identify effective low molecular weight nonphosphorus inhibitors of PTPs (p-methylbenzoic acid residue at N-3 position of the 5-arylidene-2,4-thiazolidinedione scaffold can act as a monoanionic pTyr-mimetic group replicating the interactions of pTyr with the catalytic site of the enzyme) [243]. In this study the authors also noted the importance of the 5-arylidene fragment. Alkylated/acylated phenolic groups and methoxy groups are desirable for the inhibitory effect.
Scheme 48.
5-Ene-4-thiazolidinones with antidiabetic activity.
5-Substituted 2-cyanimino-4-thiazolidinone (FPFS-410) – a compound related to pioglitazone (PPAR-agonist) also possesses antidiabetic activity. In vivo data revealed the ability to reduce blood glucose and triglycerides levels and reduce the obesity [244].
Screening study allowed identifying and confirming the activity of 5-arylidene derivatives as inhibitors of glycogen synthase kinase-3 (GSK-3) which, in turn, has been emerging as a key therapeutic target not only for type 2 diabetes, but also for Alzheimer'sdisease, cancer and chronic inflammation [245].
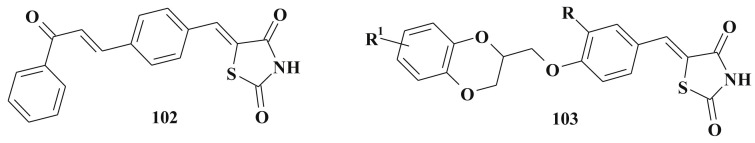
Simple 5-benzylidene-1,3-thiazolidine-2,4-dione derivatives 102 were presented as a new class of α-glucosidase inhibitors [246] (Scheme 49 ). α-Glucosidases (α-D-glucosideglucohydrolases) are membrane bound exo-acting enzymes responsible for catalyzing the final step in the digestive process of carbohydrate metabolism. α-Glucosidases are the enzymes that hydrolyze O-and S-glycosyl residues and are involved in the biosynthesis and processing of oligosaccharide chains of N-linked glycoproteins in the endoplasmic reticulum [247].
Scheme 49.
Structures of α-glucosidase and glycogen phosphorylase inhibitors.
5-Benzylidenethiazolidine-2,4-diones carrying 2,3-dihydrobenzo[1,4]dioxine fragment 103 were proved to be glycogen phosphorylase inhibitors (Scheme 49) (glycogen phosphorylase is a key enzyme in the regulation of blood sugar level and it catalyzes the formation of glucose-1-phosphate from glycogen) [248] that may be relevant to the control of blood glucose concentrations in type 2 diabetes [249].
3.4. Anticancer agents
Search for new anticancer agents is the most dynamically developing area of medicinal chemistry. A lot of papers and patents are devoted to the search for efficient anticancer agents among 4-thiazolidinones, including 5-ene derivatives (selected row of patents see) [5(ESI),250]. Research in this area could be divided into the next groups:
-
i)
in vitro screening of highly active/selective hit-compounds for further optimization, mainly with unknown mode of action (including the international programs, mainly Developmental Therapeutics Program, NCI, NIH - https://dtp.cancer.gov);
-
ii)
design of the high-affinity ligands to the “validated” anticancer biotargets;
-
iii)
creation of the hybrid molecules that combine several pharmacologically attractive scaffolds [158], [159], [251], [252];
-
iv)
search for antitumor agents among the compounds with known biological activity (anti-inflammatory, antidiabetic, anti- or prooxidant etc.).
4-Thiazolidinones are known to possess good activity against different types of cancer including relatively simple 5-ene derivatives as well as complex or hybrids/conjugates bearing non-fused 5-ene-4-thiazolidinone fragment. Mentioned compounds don't belong to any “classic” anticancer agent types [8], [94], [253], [26], [27], [254], [255], [256].
Despite the diversity of 5-ene-4-thiazolidinones, the search for antileukemic agents is one of the most promising directions. It was found the tendency of the maximum sensitivity of leukemia cell lines to various subtypes of thiazolidinones [8], [25], [26], [27], [34], [35], [66], [94], [138], [142], [143], [162], [100], [257], [258], [259], [260]. The crucial role of the C5-(ylid)ene fragment is also confirmed and complication of ene-fragment is considered as a benefit. But anti-leukemic effects of such compounds have been less documented in comparision with all other cancer types [261].
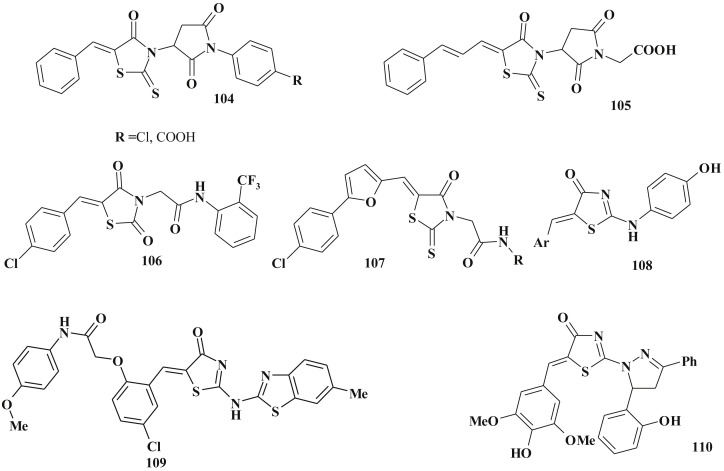
Among 4-thiazolidinone-3-carboxylic acids derivatives, amides 104–107 (Scheme 50 ) with anticancer activity were identified including samples possessing strong antileukemic activity (107). The SAR study revealed that anticancer activity has significantly decreased or disappeared after transformation into related isosteric compounds (replacement of C5 arylidene moiety by the C5-carboxymethylidene or 5-carboxymethyl fragments) or in case of compounds based on the related heterocyclic cores (e.g. 2,4-imidazolidinedione) [26], [94], [253] Moreover, novel 4-thiazolidinone-3-carboxylic acid amides 107 bearing furan moiety exhibited significant cytotoxicity and induction of apoptosis in human leukemia cells [259].
Scheme 50.
Structures of 5-ene-4-thiazolodinones with anticancer activity.
Among 5-arylidene-2-arylamino(imino)-4-thiazolidinones 108 a set of active compounds with micromolar IC50 levels has been detected (log GI50 ∼ −5.77) [143]. Based on the obtained results the compounds with bulky C5-ene fragment and benzothiazole core were designed: compound 109 (pGI50 = 4.97) selectively inhibited growth of the HOP-92 cell lines (CNS cancer, pGI50 = 6.34) [138]. Study of the anticancer activity of 4-thiazolidinones with pyrazoline moiety in the C2 position of the main core has revealed high anticancer potential of the mentioned compounds 110 (Scheme 50). SAR study confirmed the dependence of anticancer activity from the structure of C5 fragment [142]. The isomeric 4-amino thiazolidinones possessed much less activity levels [25].
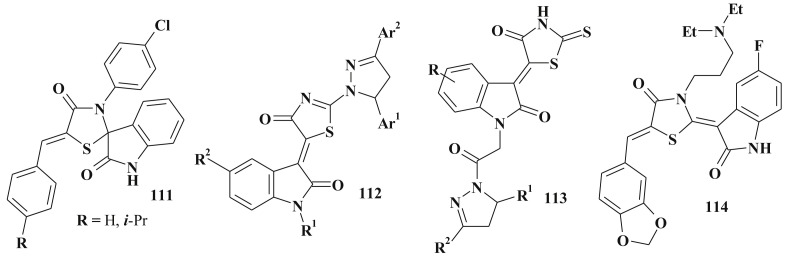
Thiazolidinone-isatin conjugates are good examples of polycyclic compounds with high anticancer potential and their design illustrates the molecular hybridization approach when two “pharmacophores” are combined into a single molecule [262]. For instance, 111 possesed micromolar activity level and also selectively inhibited the leukemia cell lines [27]. Further structure optimization led to increasing of the anticancer activity which was reflected in the design of thiazolidinone-isatin-pyrazole hybrids 112, 113 [34], [35] (Scheme 51 ). Similar isatin-pyrazoline conjugates (without thiazolidinone core) didn't show the anticancer activity.
Scheme 51.
Thiazolidinone-isatin conjugates with anticancer activity.
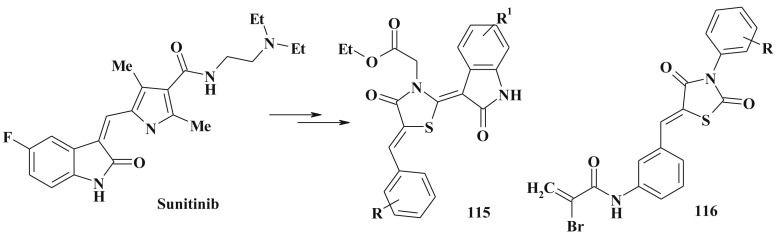
Another example of anticancer isatin-thiazolidinone hybrides are compounds 114 [263] and 115 [264] (Scheme 51, Scheme 52 ). The latter are the result of the structure optimization of sunitinib (SU11248, Sutent TM; Pfizer Inc) which is currently used in the clinics as a multi-targeting tyrosine kinase inhibitor with antiangiogenic activity [265], [266]. The same approach of molecular hybridization was used in the design of thiazolidine-2,4-diones 116 bearing α-bromoacryloylamido moiety [267], [268].
Scheme 52.
4-Thiazolidinones hybrids as anticancer agents.
Compound MKT077 is known anticancer agent [269] (Scheme 53 ) with apoptosis related mechanism of action and is an illustrative example of above mentioned compounds, though, its study has been proceeding till now. Thus, it is shown that it acts through differential interaction with the Hsp70 (Heat shock protein 70 kDa) allosteric states and reactivation of p53 function. MKT-077 therefore can be treated as an “allosteric drug” [270], [271], [272], [273].
Scheme 53.
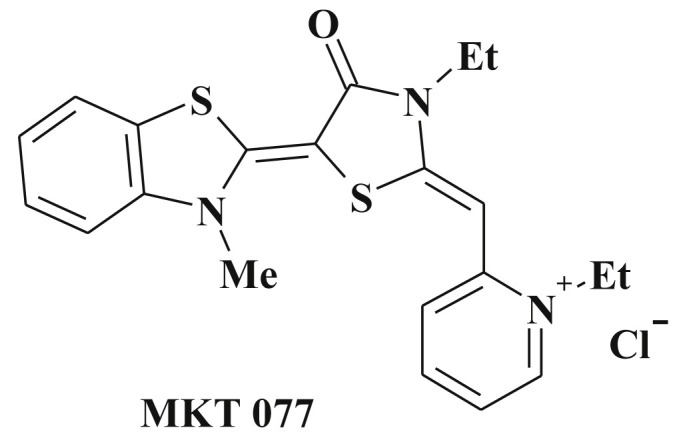
Structure of MKT 077.
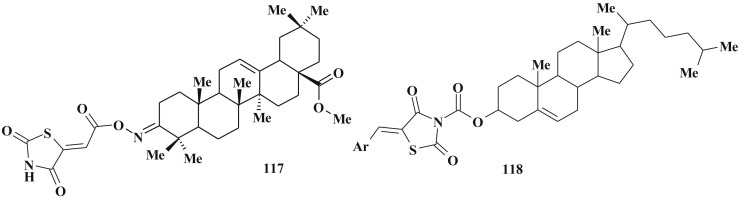
One more example of the hybrid approach in anticancer agents design is the combination of the natural compound, e.g. oleanane scaffold, with thiazolidinone core (Scheme 54 ). Among oleanane-thiazolidinone hybrids 3-(2,4-thiazolidinedione-5-ylidene)-carboxyimino]olean-12-en-28-oic acid methyl ester (117) was identified as the most active substance (pGI50 = 5.57, pTGI = 5.13 and pLC50 = 4.64 NCI NIH protocol) with low toxicity and moderate activity level in in vivo Hollow Fiber Assay [162]. Another example of this approach is 5-arylidene-2,4-thiazolidinedione bearing cholesterol fragment 118. Moreover, the level of activity and selectivity of antimitotic effects depends on the substituents in the C5 position of thiazolidinone core [161]. The maximum effect such compounds exhibited towards HeLa cancer cells line.
Scheme 54.
Combination of natural compounds and 5-ene-4-thiazolidinones fragments.
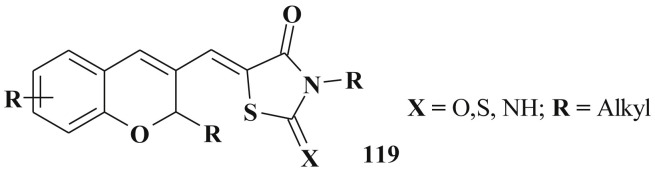
Promising active molecules based on the combination of thiazolidinone and chromene cores 119 were also found [160] (Scheme 55 ).
Scheme 55.
Combination of thiazolidinone and chromene cores.
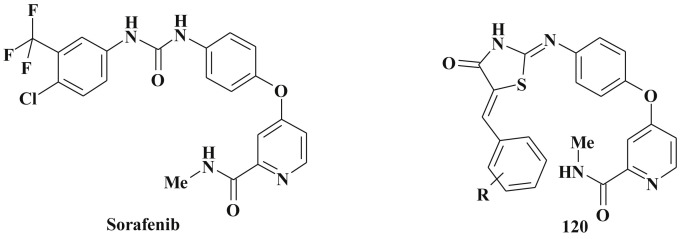
Once again it should be emphasized that for majority of mentioned compounds the tendency towards maximum sensitivity of leukemia and lung cancer cell lines is shown. This, most probably, may be a platform for the effective anti-leukemic agents design. For example, 5-isopropylidene-3-ethylrhodanine and 5-benzylidene-3-ethylrhodanine demonstrated cytotoxicity towards leukemic cell line CEM by inducing apoptosis [38], [274]. Besides this, the modification of known drugs, for example assorafenib, with 5-ene-4-thiazolidinone fragments 120 is reported [275], [276] (Scheme 56 ).
Scheme 56.
Modification of known drug with 5-ene-4-thiazolidinone fragment.
Whereas, a lot of potential biotargets for new anticancer agents design are known at present, there have been almost no attempts to systematize the 4-thiazolidinones as anticancer agents in the review papers. For example, three aspects of the PPARs-independent antitumor activities of thiazolidinones had been outlined [10]: i) inhibition of Bcl-2/Bcl-x function, ii) proteasomal degradation of target proteins, iii) transcriptional repression of AR through Sp1 degradation [277]. Though, the latter definitely does not represent the whole spectrum of experimental data and is not enough for the design of novel hit- and lead-compounds.
Taking into account the great number of papers, we tried to outline the most referred biotargets and their ligands among 5-ene-4-thiazolidinones. It should be noted that the majority of high-affinity ligands belong to 4-thiazolidinone-3-carboxylic acids, 5-benzylidenerhodanines, 2-amino(imino)derivatives and 2,3-disubstituted-4-thiazolidinones.
Based on the established role of PPARs in the cancer and inflammation progress [219], [278], [279], [280] considerable part of investigations is dedicated to the study of glitazones and related derivatives as possible anticancer agents. However, 5-ene analogs as synthetic precursors of glitazones that do not contain exocyclic double bond in the C5 position possess less expressed affinity to PPARs as their agonists.
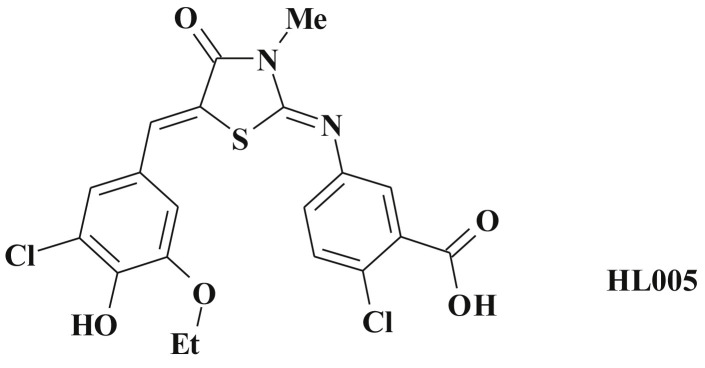
PPARγ antagonists in turn represent a new drug class that holds promise as a broadly applicable therapeutic approach for cancer treatment [229], [281]. Among them 5-ene derivatives occupy their deserved place. For instance, the novel 3-thiazolidine-modified benzoic acid derivative HL005 (Scheme 57 ) being a potent PPARγ-specific antagonist inhibits the proliferation of the MCF-7 cell line at the concentration-dependent manner, induces cell cycle arrest at the G2/M phase and interferes with cell adhesion [282].
Scheme 57.
Structure of HL005 – PPARγ-specific antagonist.
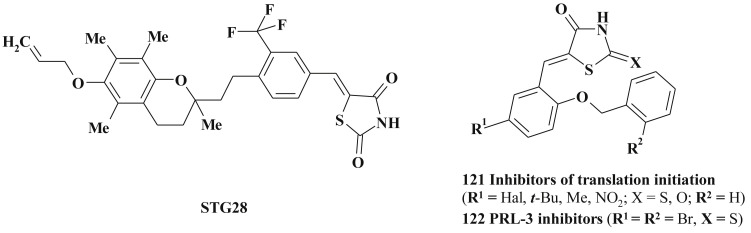
PPARs indepented anticancer effects of troglitazone are mediated mainly via the repression of cyclin D1 (MCF-7 breast cancer cells) by facilitating proteasome-facilitated proteolysis [283] and partial depletion of intracellular Ca2+ stores that leads to inhibition of translation initiation [284]. The troglitazone structure modification led to the STG28 identification – the first small-molecule agent mediating the proteasomal degradation of cyclin D1 with high specificity (exposure to STG28 did not cause any appreciable change in the expression levels of a series of other cyclins and CDK-dependent kinases) [283] (Scheme 58 ).
Scheme 58.
Structure of 5-ene-thiazolidinones with anticancer activity derived from troglitazone structure modification.
Following the structure optimization and screening data it was shown that the 5-arylidenerhodanines 121 are equipotent to troglitazone in Ca2+releasing activity, induction of eIF2a phosphorylation and more potent in inhibiting cancer cells proliferation [285]. Further modification allowed to state that a series of 5-benzylidene-2,4-thiazolidinediones and -thiones inhibited cell growth at low micromolar concentrations via the inhibition of translation initiation which involves partial depletion of intracellular Ca2+ stores and strong phosphorylation of eIF2a.
Structurally similar benzylidenerhodanines 122 showed high inhibition of protein tyrosine phosphatase PRL-3 (IC50 = 0.9 μM), which is one of the probable prognostic markers of metastatic cells [286].
Among 5-ene-rhodanines 123 was identified as a promising and selective inhibitor of enzymes of dual-specificity phosphatases group – phosphatases JSP-1 (JNK-stimulating phosphatase-1). Mentioned compounds are as well perspective agents that can be explored in the treatment of the inflammatory disorders [140] (Scheme 59 ).
Scheme 59.
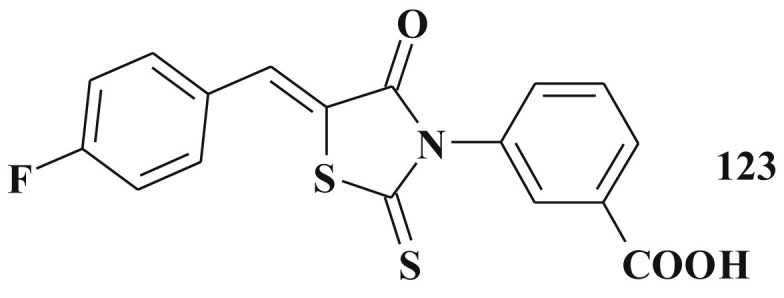
3-[5-(4-Fluorobenzylidene)-rhodanin-3-yl]-benzoic acid - JSP-1 inhibitor.
Necrostatin-7 (Nec-7) (Scheme 60 ) and related heterocycles belong to new class of “small molecules” – inhibitors of necroptosis that is regulated caspas-independent pathway of the cell death, morphological features of which are close to necrosis. This may be used in FADD-changed variant of the treatment of JurkatT cancer cells under the use of TNFα [287], [288].
Scheme 60.
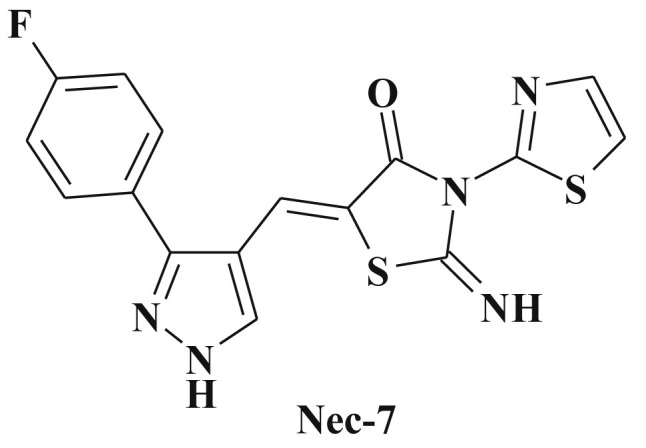
5-[3-(4-Fluorophenyl)-1H-pyrazol-4-ylmethylene]-2-imino-3-(thiazolyl-2)-4-thiazolidinone – necroptosis inhibitor.
On the other hand, derivatives of hydantoin-5-acetic acid are non-hydroxamate inhibitors of TNFα converting enzyme [289] that allows to treat them as promising anti-inflammatory agents and gives the prospects to establish anticancer potential of the given class of compounds. Moreover, the ability of hydantoincarboxylic acids, especially with the thiazole fragments, to inhibit activity of Ras farnesyl transferase (Ftase) was proved [290]. Ras proteins play an essential role in the processes of cell growth and differentiation and need post translational modification including farnesylation catalyzed by Ras farnesyl transferase. That is the reason why Ras-Ftase inhibitors are considered as potential anticancer agents [291], [292], [293]. Structural analogs of the given substances are the ligands for neuro-immunophilin FK506-binding protein (FKBP) [294].
5-Substituted rhodanine-3-carboxylic acids 124 ( Scheme 61 ) are the inhibitors of protein-protein interaction of antiapoptotic proteins of the Bcl-2 and Bax family and their binding to the appropriate receptor domains [257], [295], [296], [297], [298], [299].
Scheme 61.
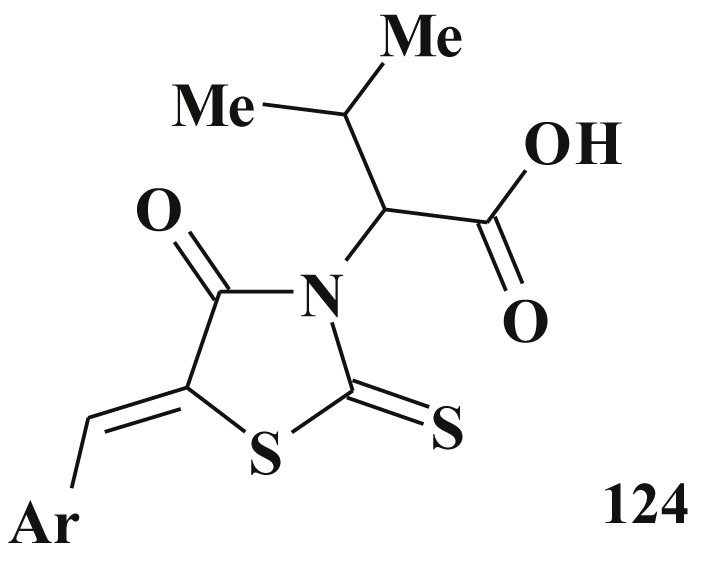
5-Arylidenerhodanine-3-carboxylic acids - Bcl-2 inhibitor.
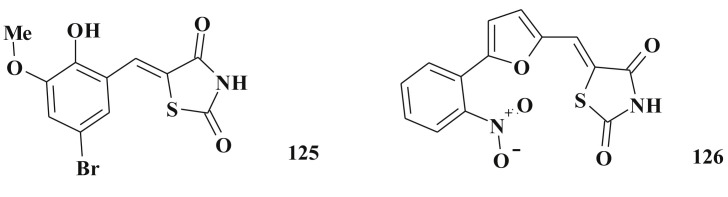
Apart from the directions shown above, 5-aryl(hetyryl)idenethiazolidine-2,4-diones 125, 126 (Scheme 62 ) were identified as potent and selective insulin-like growth factor-1 receptor (IGF-1R) inhibitors [230].
Scheme 62.
Structure of insulin-like growth factor-1 receptor inhibitors.
IGF-1R is a growth factor receptor of tyrosine kinase family, acting as a critical mediator of cell proliferation and survival. Although, being highly related to insulin receptor, it plays a different role in organism development, being responsible for normal growth and development as opposed to glucose homeostasis. Epidemiological studies indicate that the IGF-1R is overexpressed in human cancer and is primarily responsible for tumor genesis. Signaling through IGF-1R includes the activation of PI3K and Raf pathways [300]. Inhibition of both these pathways makes IGF-1R kinase a promising target for cancer therapy. Moreover, series of 3,5-disubstituted thiazolidine-2,4-dione analogs [301] and 3-(2-aminoethyl)-5-(3-phenylpropylidene)-thiazolidine-2,4-dione [302] were shown to be potential anticancer agents via the inhibition of the Raf/MEK/ERK and PI3K/Akt signaling cascades.
The inhibitors of extracellular signal-regulated kinases-1 and 2 (ERK1/2) – (e.g. (Z)-3-(2-aminoethyl)-5-(4-ethoxybenzylidene)thiazolidine-2,4-dione 127) are the examples of compounds with greater selectivity for inhibiting the proliferation of melanoma cells [303] (Scheme 63 ).
Scheme 63.
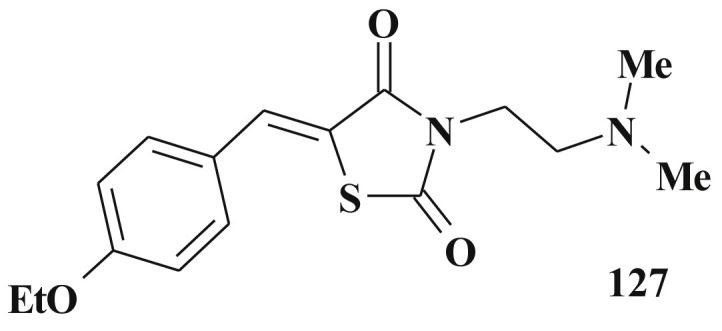
Structure of signal-regulated kinases inhibitor.
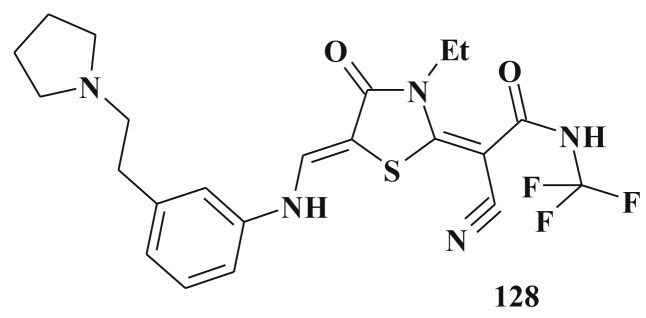
Polo-like kinase 1 (Plk1) is a key regulator of mitotic progression and cell division. In eukaryotes it acts in concert with cyclin-dependent kinase 1 – cyclin B1 and Aurora kinases to conduct a wide range of critical cell cycle events. Because Plk1 has been preclinically validated as a cancer target, small-molecule inhibitors of Plk1 have become attractive candidates for anticancer drugs development [304]. Thiazolidinone 128 (Scheme 64 ) selectively inhibits human Plk1 (IC50 19 nM) and various human and mouse tumor cell lines (IC50 0.2–1.3 μM) and cause a prometaphase-like mitotic arrest [305].
Scheme 64.
Structure of Polo-like kinase 1 inhibitor.
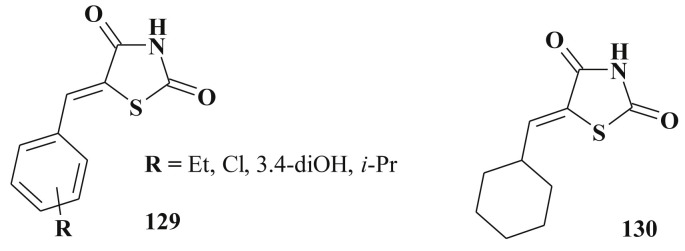
Simple 5-ene-rhodanines (129, 130) (Scheme 65 ) may also be considered as “Myc-Max compounds” that inhibit or reverse the association between c-Myc and its obligated HLH-LZ heterodimerization partner Max [306], [307], [308].
Scheme 65.
Simple 5-ene-rhodanines as “Myc-Max compounds”.
Among 5-ene derivatives of 2-iminothiazolidine, 2,4-thiazolidinedione and rhodanine the estrogen-related receptor-α (ERR-α) modulators were identified. They can be useful for the prophylaxis or treatment of ERR-α associated diseases such as breast cancer [309], [310].
Following the modification of 5-(3-trifluoromethylbenzylidene)thiazolidine-2,4-dione (high selectivity Pim-1 inhibitor) a series of substituted thiazolidine-2,4-dione derivatives were identified as highly active and selective Pim-1 and Pim-2 inhibitors (nanomolar values of IC50 for Pim-1 and ∼2.0 μM for Pim-2) [54]. Pim-1 and Pim-2 are serine/threonine protein kinases frequently overexpressed in prostate cancer and certain forms of leukemia and lymphoma [311]; Pim can phosphorylate the proapoptotic protein BAD (the Bcl-2-associated death promoter) leading to sequestration of 14-3-3 proteins and inhibits the apoptosis [312], [313].
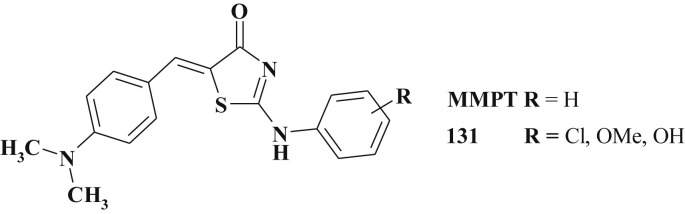
As it was already mentioned, 5-ylidene-2-amino(imino)-4-thiazolidinones 131 (Scheme 66 ) comprise one of the most investigated thiazolidinone subtype as compounds with anticancer activity [138], [142], [143], [314], [315]. Among them the derivative MMPT was identified as a hit-compound being able to effectively inhibit the growth of some lung cancer cell lines (H460 and H460/TaxR) and at the same time do not influence normal fibroblasts in a dose response manner [151], [316], [317]. Study of the structure-activity relationship in a group of 372 compounds revealed some structural peculiarities: i) the nitrogen atom of thiazolidine core has to be unsubstituted; ii) dimethylaminobenzylidene fragment is the best in the C5 position; iii) 2-phenylamino moiety may contain substituents in different positions of aromatic ring [151].
Scheme 66.
Structure of simple 5-ylidene-2-amino(imino)-4-thiazolidinones with significant anticancer activity.
5-[(4-Methylphenyl)methylene]-2-(phenylamino)-4(5H)-thiazolone (MMPT) and 5-(2,4-dihydroxybenzylidene)-2-(phenylimino)-1,3-thiazolidine (DBPT) are effective in the treatment of multidrug-resistant (MDR) cancer. Multidrug-resistance is a phenotype of cross-resistance to multiple drugs with diverse chemical structures. One of the well-documented MDR mechanisms is the overexpression of the MDR-1gene that encodes the transmembrane, ATP-dependent drug efflux transporter P-glycoprotein (P-gp) in response to chemotherapy [318], [319], [320]. P-gp prevents the intracellular accumulation of many cancer drugs by increasing thei refflux out of cancer cells as well as through hepatic, renal, or intestinal clearance pathways [319]. Attempts to coadminister P-gp modulators or inhibitors to increase cellular availability by blocking the actions of P-gp have met with limited success [321], [322], [323]. Therefore, a more promising approach lies in the design and discovery of novel compounds that are not substrates of P-gp and are effective against drug-resistant cancer while at the same time exhibit minimal toxicity to normal cellular functions. MMPT inhibited the growth of human non-small-cell lung cancer and colon cancer cells (drug-sensitive (H460) and drug-resistant (H460/TaxR cell lines) independently of P-glycoprotein and p53 status [151], [316]. Besides this, it selectively killed drug resistant cancer cells and induced apoptosis [317]. Among 5-ylidene-2-arylamino-4-thiazolidinone derivatives effective growth inhibitors of HT29 cell line (with a high COX-2 expression) were identified [324].
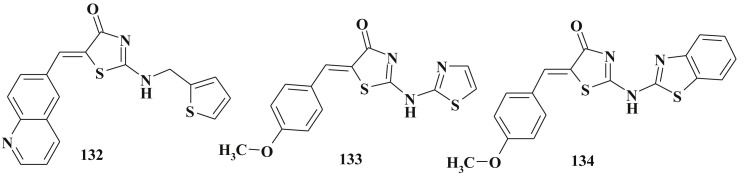
Among mentioned 4-thiazolidinone derivatives efficient CDK1 132 [325], CDK1/cyclinB inhibitors [326], [327], [328] and SHP-2 inhibitors 133 and 134 (Scheme 67 ) (SHP-2 is a non receptor protein tyrosine phosphatase that mediates cell signaling by growth factors and cytokines acting via the RAS/MAP kinase pathway) [329], [330] were discovered. Benzo[d]thiazole derivatives turned out to be more potent than their thiazole analogs.
Scheme 67.
Structures of CDK1 and SHP-2 inhibitors.
2-Substituted thiazolidinone and oxazolidinone derivatives were established as the inhibitors of phosphatases and anticancer agents [331]. Modifying the imidazolidine derivatives the new cell division cycle 7 kinase inhibitors were designed 135 and (Z)-2-(benzylamino)-5-(1H-pyrolo2,3-b]pyridin-3-ylmethylene)-1,3-thiazol-4(5H)-one] was selected as a lead compound [332] (Scheme 68 ).
Scheme 68.
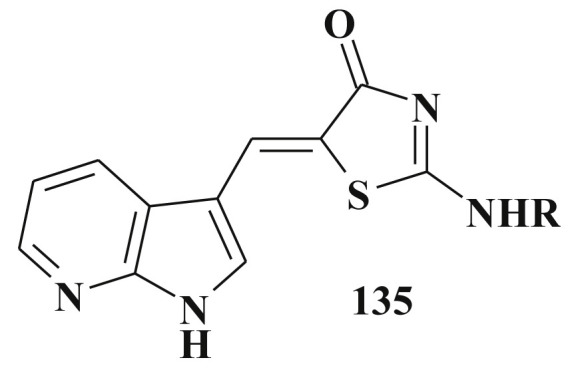
Structure of cycle 7 kinase inhibitors.
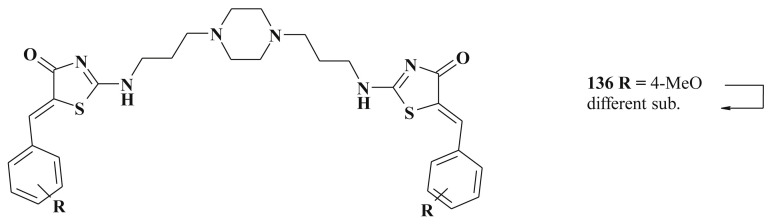
2-N,N′-Disubstituted diamines bearing 5-arylidene-4-thiazolidinone moiety 136 (Scheme 69 ) had shown nanomolar inhibition potency (IC50 40 nM) towards tyrosine phosphorylation-regulated kinases 1A [333]. This result prompted to explore the symmetric 1,2-diamino-linker grafted on N-3 position of two different 5-arylidenerhodanine platforms in order to modulate potential biological activity and led to the synthesis of unsymmetrical linked bis-5-arylidenerhodanine derivatives with anticancer effects [334].
Scheme 69.
Structure of tyrosine phosphorylation-regulated kinases 1A inhibitors.
One of the established modes of action of 4-thiazolidinones anticancer activity is their antagonistic activity towards α v β 3 receptors. The value of inhibition of the α v β 3 activity is proved by the establishing of the correlation between progression of factor's expression and cancer growth. Search for effective inhibitors was held based on the pharmacophore model and virtual screening of 600000 compounds following by further confirming of the activity in vitro. This allowed to select promising compounds including 4-azolidinones. 2-Substituted 4-thiazolidinone derivatives among selected ones appeared to be efficient integrin α v β 3 antagonists 137 [335] (Scheme 70 ).
Scheme 70.
General structure of integrin αvβ3 antagonists.
One of the aspects of anticancer effects realization of 4-azolidinone-3-alkanecarboxylic acids is the ability to influence the oxidative homeostasis of the cancer cells and the impact on the reactive oxygen species production. The modulating effect of the 5-ene-rhodanines on the proliferation and apoptosis of the cancer cells was identified as well as the influence on metabolism of NO alone with antioxidant and immunomodulating actions [336]. In this context interesting is the combining anticancer activity with anti-inflammatory effects; with antioxidant and/or other types of activity in some of the compounds [337] that is important in the development of “classic triad”: “oxidative stress – inflammation – cancer”. Additionally, the pharmacological profile of 5-arylidene-4-thiazolidinones derivatives is related to free radicals scavenging activity [106], [338], [339].
Besides this, notes about prooxidant effect of thiazolidinones with anticancer activity are frequently found in the papers. It has been shown that the derivatives of 5-ylidene-2-imino-4-thiazolidinone increased levels of reactive oxygen species (ROS) (HT29 cells), moreover, the ROS increasing was blocked by the ascorbic acid addition [340]. 5-Benzilidene-3-ethylrhodanine treatment led to increased level of ROS production and DNA strand breaks suggesting activation of apoptosis for induction of the cell death (leukemic cell line, CEM) plus by inducing a block at S phase [274]. Cell exposure to the selected compound was associated with the production of ROS, and the induction of autophagy which could lead to cell death [341].
Recent observation has been made in favour of the alternative chemotherapeutic strategy against cancer that consists in increasing the production of reactive oxygen species [340], [342]. Moreover, generation of ROS could activate the NF-2E related factor 2/Kelch like ECH-associated protein 1 (Nrf2/Keap1) pathway involved in protection of the cells against oxidative stress [343]. Activation of the Nrf2/Keap1 pathway leads to the stabilization of Nrf2 which translocates in nucleic db in ds to Maf protein. The complex, after binding to specific DNA sequence, defined as antioxidant response element localized in the promoter of Nfr2/Maf target genes, increases the transcription of genes involved in reactive species-mediated response such as HemeOxygen-1 (HO-1), NADH quinoneoxidase-1 (NQO1) or glutamylcysteyl ligase. It could also indicate the indirect impact of the given class of compounds on the pro/antioxidant balance [156]. ROS play a key role in mitochondria mediated apoptosis. Mitochondria are the prime source of ROS which are byproducts of aerobic respiration [344], [345], [346]. High levels of ROS in mitochondria can result in free radical attack of membrane phospholipids and cause mitochondrial membrane depolarization. This is an irreversible step associated with the release of mitochondrial factors triggering caspase cascades [347], [348].
On the other hand, immunoblotting analysis showed that 5-(4-hydroxy-3-dimethoxybenzylidene)-2-thioxo-4-thiazolidinone (RD-1) significantly elevated the Parkin and Miro 2 expression levels in acute MPTP treated mice and improved mitochondrial membrane potential and ATP synthesis in MPP + -treated Neuro-2a cells. Moreover, RD-1 attenuated impaired mitochondrial transport and vesicle release dysfunction has been evoked by MPP + cytotoxicity in cultured primary mesencephalic neurons. Taken together, the results indicate that improving the mitochondrial dysfunction may be a good choice to delay the neurodegenerative progression commonly associated with pakinson diseases as well as cancer [349].
Among the proposed mechanisms for the antitumor effect of target compounds apoptosis induction, cell cycle arrest and differentiation have been extensively reported [38], [66], [252], [274], [277]. Anticancer effect of such compounds can be also achieved by blocking the cell cycle progression at the G2/M phases border (not affect the G1 to S phase transition) in reversible manner and by the induction of apoptosis [252], [325]. FACS (fluorescence-activated cell sorting) analysis of 5-ylidene-rhodanine-3-carboxylic acids, which possessed strong antiproliferative activity against human leukemia cells, showed a remarkable accumulation of subploid cells, the sub G1 phase (G0/G1) followed by the decline of both G1 and G2/M phases. Morerover, the presented findings suggest that the observed growth inhibition could be due to apoptosis [259]. The related 5-ylidene thiazolidinones with heterocyclic fragments induced cell cycle arrest of leukemic cells at G2/M phase and induced cell death that resulted in increased level of SubG1 phase population, thereby affecting its proliferation and causing depolarization of mitochondrial membrane potential [260]. The similar results were found for 2-heterylamino 4-thiazolidinones that induced cell accumulation at G2/M and in sub G0/G1 phases of the cell cycle. Furthermore, dissipation of mitochondria membrane potential was observed as well as red/ox changes in the treated cells (HT29 cell lines) [340]. Treatment of cancer cells with mentioned MMPT and DBPT led to a time-dependent accumulation of cells arrested in G2/M phase [316]. 5-Ene-4-thiazolidinones caused a prometaphase-like mitotic (G2–M) arrest (as the ATP-competitive inhibitors of Plk1) [305], [350].
Despite the diversity of presented biotargets, the notes about thiazolidine derivatives being inductors of apoptosis in various cancer cells have become more frequent [351], [352], [353], [354], [355], [356], [357]. Above all, apoptosis-related mechanism is associated with the inhibition of Bcl-2/Bcl-x function. However, there has been described another feasibility of target compounds to affect diverse apoptotic signaling pathways, namely, induction of peroxisome proliferator-activated receptor-associated caspase-dependent [250], [317], [358] mitochondria-mediated apoptosis. 4-Thiazolidinones can decrease mitochondrial membrane potential which is one of the important mechanisms for the mitochondrial mediated apoptotic cell death [359] (an example of such compound is MKT 077) [269], [271].
3.5. Anti-inflammatory agents
Progress in the anti-inflammatory agents search is reached via both high affinity ligand design and pharmacological screening of the compounds with unknown molecular mode of action. Anti-inflammatory activity of 4-thiazolidinones is associated, primarily, with their ability to inhibit isoforms of cyclooxygenase (COX) and lipoxygenase (LOX). The most successful representative of anti-inflammatory agents on the base of 5-ene-4-thiazolidinones is darbufelone – (5-(3,5-ditertbutyl-4-hydroxybenzylidene)-thiazol-4-one [33], [360], [361]) (Scheme 71 ) that has attracted broad attention as dual inhibitor of cellular prostaglandin and leukotriene production via cyclooxygenase activity inhibition including inhibition of prostaglandin endoperoxide synthase-1 (PGHS-1) and PGHS-2 [362].
Scheme 71.
Darbufelone structure and directions of the structure modification.
Chemical structure of darbufelon has been considered as lead-compound for novel anti-inflammatory agents design till now. Optimization of the latter and application of the bioisostere concept allowed the elaboration of new bioactive compounds 138 (GS26) and 139 (GS28) (Scheme 71) with significant anti-inflammatory activity as well as antiedematogenic and ulcerogenic activity in vivo [363]. Further studies of related compounds allowed identifying selective inhibitors of COX-2 as promising anti-inflammatory agents with fewer side effects. Obtained data and SAR analysis resulted in identifying the most active compound 140 (Scheme 71) from the group of thiazolones and oxazolones with di-tert-butylphenol fragment. Compound 140 inhibited human recombinant COX-2 (IC50 1.7 μM) and inhibited the COX-2 activity in cell line J774A.1 (IC50 0.17 μM). This compound turned out to be inactive towards COX-1 at the concentration of 100 μM and did not inhibit the COX-1 in thrombocytes at 20 μM [360].
Among 5-ene-4-thiazolidinones CT-8 (Scheme 72 ) was found to be potent inhibitor of 15-hydroxyprostaglandin dehydrogenase (15-PGDH) [364]. 15-PGDH type I is NAD+-dependent (while Type II is NADP+ preferred) cytosolic enzyme which catalyzes oxidation of prostaglandins to 15-ketoprostaglandins. Inhibition of this enzyme is related to prostaglandin E2 action and can lead not only to anti-inflammatory effects but, for instance, to reduce hair loss [365]. Structure-activity analysis indicated that the N-methylation of CT-8 abolished the inhibitory activity. It was also established that the nature of the moiety linked to benzylidenethiazolidine-2,4-dione through another linkage plays an important role in its inhibitory activity [364]. Further modification led to the new related compound 141 identification (IC50 = 0.031 μM) [366] (Scheme 72).
Scheme 72.
Structures of 15-hydroxyprostaglandin dehydrogenase inhibitors.
Another mechanism of anti-inflammatory activity has been the inhibition of phosphodiesterase (PDE4 and PDE2). Phosphodiesterase is responsible for the hydrolysis of secondary messanger cAMP, the level of which increases under inflammation processes. Besides this, phosphodiesterase inhibitors may be effective agents in astma and obstructive lung diseases treatment. It has been established the ability of 5-arylidenerhodanines, including 3-substituted carboxylic acids [367] to inhibit above mentioned enzyme. Moreover, unlike other rhodanine derivatives, carboxylic derivatives selectively inhibit only phosphodiesterase-4 that may be an argument for their study as probable agents for Parkinson's and Alzheimer's diseases.
The antigenerative effect of 5-benzylidene-4-thiazolidinones with benzothiazole 142 and isothiazole 143 moieties (Scheme 73 ) on human chondrocytes culture was estimated when studying potent inhibitors of metalloproteinase (MMP). Given experimental model reproduces the mechanisms involved in osteoarthritis [240], [368]. The compound 143 (Ar = 4-MeO-C6H4) showed MMP-13 inhibition activity at nanomolar concentration (IC50 0,036 μМ).
Scheme 73.
Structures of 15-hydroxyprostaglandin dehydrogenase inhibitors.
Moreover, among anti-inflammatory 5-ene-4-thiazolidinones a series of furan-2-ylmethylene-2,4-thiazolidinediones have been investigated as the ATP-competitive PI3Ks inhibitors 144 (Scheme 74 ) (IC50 0.2–0.9 μM). Class I phosphoinositide 3-kinases (PI3Ks), in particular PI3Kγ, have become attractive drug targets in inflammatory and autoimmune diseases treatment [369]. An acidic NH group in the thiazolidinedione core and a hydroxyl group in the furan-2-yl-phenyl part of the molecule play crucial roles in binding to PI3K and contribute to class IBPI3K selectivity. Besides the compound 145 inhibited PI3Kγ (IC50 33 nM) (but not PI3Kα, PI3Kβ) dependent pathways inside cells and in a murine peritonitis model, it produced a similar decrease in leukocyte infiltration.
Scheme 74.
Structure of ATP-competitive PI3Ks inhibitors.
The anti-inflammatory properties of 5-arylidene-2-phenylimino-4-thiazolidinones 146 (Scheme 75 ) are related to their ability to block the production or action of the degenerative factors induced by IL-1b [370] and possessing antidegenerative activity on human chondrocyte cultures.
Scheme 75.
5-Arylidene-2-amino-4-thiazolidinones with anti-inflammatory activity.
5-Arylidine-2,4-thiazolidinedione derivatives without substituent in the N3 position [371] as well as N3-substituted analogs 147 [372] (Scheme 76 ) possessed anti-inflammatory activity and related type of activities, such as analgesic activity and in vitro antioxidant activity or even antimicrobial [167]. The structural affinity to PPARγ agonists (in silico correlation studies and PPAR-competition binding assay) indicates the possibility of PPAR mediated mode of anti-inflanmatory action [372].
Scheme 76.
5-Ene-4-thiazolidinones as active anti-inflammatory agents.
Similarly, 5-arylidene-2-imino-4-thiazolidinones 148 (Scheme 76) exhibited anti-inflammatory effect (carrageenan-induced paw edema model) causing the reduction of PGE2 level together with insignificant COX-2 inhibition when compared with celecoxib. It should be noted that 5-unsaturated thiazolidinones possess much less expressed effect or are inactive [373]. Applying procedures of virtual and highthroughput screening for 2-(thiazole-2-ylamino)-thiazol-4-ones study led to identification of compounds 149 (Scheme 76) with anti-inflammatory activity. In the in vivo cyclooxygenase and lipoxygenase inhibition assays as well as anti-inflammatory assay the compounds turned out to be promising agents for further studies in this field [374].
The row of hybrids 138 [363] (Scheme 71), 150 [375], 151 [376] (Scheme 77 ) based on indometacine, roziglitazone and darbufelone molecules was designed. The molecules possesed in vivo anti-inflammatory activity in air pouch and peritonitis models or carrageenan induced paw edema model and inhibited the cyclooxygenase-1 and 2.
Scheme 77.
5-Ene-4-thiazolidinones designed based on indometacine modification.
A number of related 3-substituted-5-arylidenethiazolidine-2,4-diones (152, 153) (Scheme 78 ) was screened for anti-inflammatory effects. Compounds exhibited significant levels of anti-inflammatory activity in the assay of induced edema in mouse paws [377].
Scheme 78.
5-Ene-4-thiazolidinones being active in induced edema paws model.
Biphenyl-4-carboxylic acid 5-(arylidene)-2-(aryl)-4-oxothiazolidin-3-yl amides 154 (Scheme 78) had also shown significant activity in carrageenan test [378].
Application of polypharmacological approach and the advances in the pathophysiological role of various biotargets had resulted in the great interest in search for new anti-inflanmatory agents. For example, it was established that aldose reductase (AR) is critically involved in inflammatory processes under both normoglycemic and diabetic conditions. This enzyme, which is overexpressed under various oxidative conditions, intervenes in multiple signaling pathways leading to inflammation and tissue degeneration. Accordingly, it was ascertained that AR inhibition prevents multiple inflammatory pathways [379], [380], [381] and, therefore, new more effective and safer AR inhibitors were designed not only as antidiabetic (see above) but also antinflammatory agents [232]. 2-(Benzylamino)-5-((thiophen-2-yl)methylene)-thiazol-4-(5H)-one showed the highest anti-inflammatory response on PBMCs (peripheral blood mononuclear cells) exerted through the NF-kB inhibition. This and related compounds also had antioxidant activity and xanthine oxidase inhibitory potency [152].
3.6. Antiviral agents
Investigation of antiviral activity of 4-thiazolidinone derivatives has been carried out mainly in two directions: search for anti HIV agents and search for agents used in hepatitis C treatment. Tested compounds are small molecule inhibitors of validated targets such as HCV NS3 and 5 proteases [39], [382], HIV RT [383] etc.
Recently, rhodanine derivatives, namely 2-aryl-5-(4-oxo-3-phenethyl-2-thioxothiazolidinylidenemethyl)-furans 155 (Scheme 79 ) were reported to exhibit anti-HIV-1 activity [384].
Scheme 79.
5-Ene-4-thiazolidinone derivatives with antiviral activity.
Among 5-arylidenerhodanine derivatives the first small molecule able to inhibit HIV replication by targeting a cellular enzyme – the RNA helicases DDX3 had been identified [385]. The precise combination of functional groups on the rhodanine scaffold was shown to be responsible for the DDX3 inhibitory activity and selectivity of the hit compound 156 (FE15) (Scheme 79).
The 2-thioxo-4-thiazolidinones and salicylic acid containing compounds were the most potent HIV-1 integrase inhibitors (HIV-1 integrase catalyzes the integration of proviral DNA in to the host genome, an essential step for viral replication) among several compounds retrieved from a database of small-molecules. Compounds 157 (Scheme 79) inhibited strand transfer activities of HIV-1 integrase with similar IC50 values (10–20 μM). The replacement of either the rhodanine or salicylic acid fragments is found to reduce HIV-1 integrase inhibitory potency [386].
Rhodanine-3-alkanecarboxylic acids, their amides and bicyclic analogs 158, 159 (Scheme 80 ) represent a new class of inhibitors of HIV-1 Integrase as antiviral agents [387]. They were identified in in silico studies when modeling HIV-1 integrase inhibitors as well as in in vitro investigations.
Scheme 80.
Structures of inhibitors of HIV-1 integrase.
Employing HCV proteins as targets, directly acting antiviral agents were identified and collectively described as specifically targeted antiviral therapy for HCV (STAT-C) [388], [389], [390].
Most approaches to small molecule inhibitors search for Hepatitis C virus treatment have mainly focused upon inhibition of essential viral targets, such as the NS3-4A protease (analogous to HIV protease), NS5B polymerase, NS3 helicase and NS5A [391], [392]. A literature survey on HCV NS5B polymerase inhibitors clearly indicates that 4-thiazolidinones could inhibit this enzyme and might be promising candidates for the development of novel antiviral agents against HCV. Screening of in-house library allowed identifying derivative 160 (Scheme 81 ) as hit-compound (IC50 2.0 μM) acting towards NS5B-polymerase of HCV. Based on the results of rational design and virtual screening (GOLD docking) novel 2-imino-4-thiazolidinone derivatives were synthesized and compound 161 (Scheme 81) with the IC50 (3.0 μM) level close to that of 160 was identified [393], [394], [395].
Scheme 81.
Structures of HCV NS5B polymerase inhibitors.
Further investigation led to N-substituted (aryl)alkylidene-rhodanines synthesis which inhibit HCV NS3 protease and also are good inhibitors of other serine proteases (chymotrypsin and plasmin). But, the selectivity of some compounds (162, 163) (Scheme 82 ) with bulkier C5 fragments bearing hydrophobic functional groups as well as simple analogs (164–166) (Scheme 82) was increased by ten fold towards HCV NS3 protease respectively [39].
Scheme 82.
Structures of HCV NS3 protease inhibitors.
The 4-thiazolidinones from the groups of 2,3-diaryl-4-thiazolidinones [382], [396]; 2-amino-5-arylidene-4-thiazolinones [397], [398]; and 5-arylidene-3-substituted rhodanines [399], [400] (167–171) (Scheme 83 ) were the initial structures for new inhibitors of HCV NS5B polymerase design, mainly 2-heteroarylimino-5-arylidene-4-thiazolidinones. In all cases the 5-ylidenethiazolidininones inhibited NS5B at lower IC50 values ranging between 19.8 and 64.9 mM. Moreover, the authors argued about the contribution of these (ar)ylidene groups in stabilizing the binding mode to NS5B active site.
Scheme 83.
5-Ene-4-thiazolidinones as inhibitors of HCV NS5B.
Further investigation led to new lead-compound identification 172 [401]. Thus the derivatives of 5-ene-rhodanine-3-acetic acids 173, 174 were active towards HCV inhibiting virus protease [402]. Additionally, it was established the ability of the derivatives of this compounds' class to inhibit human serine proteases. SAR analysis showed that substitution of arylidene moiety by the carboxy methylidene fragment led to insignificant loss of the activity (Scheme 84 ).
Scheme 84.
Structures of HCV protease inhibitors.
Based on the previous data [403] a series of 2(4)-pyrazolyl-4(2)-thiazolidinones 175, 176 (Scheme 85 ) were synthesized and their antiviral activity against Influenza viruses (Type A and B), Corona virus SARS, Tacaribe virus, Dengue virus, Rift Valley Fever virus, Respiratory Syncytial virus, Vaccinia virus and Venezuelan Equine Encephalitis virus was tested in vitro.
Scheme 85.
Structures of hit-compounds with antiviral activity.
Molecular hybridization method allowed obtaining thiazolidinone–peptide hybrids 177 (Scheme 85) that inhibited Dengue virus protease. Moreover, it was determined that thiazolidinone core (2,4-thiazolidinedione and rhodanine) and the peptides should be accomplished by relatively rigid arylidene moieties using para-substitution [404].
3.7. Anti- and prooxidant agents
Violation of the antioxidant defence system and the balance of the pro/antioxidants are described under majority of pathologies including above mentioned. Therefore, a lot of papers are dedicated to the study of the 4-thiazolidones antioxidant activity [339]. Most of the articles describe investigation of the antiradical properties of 5-ylidenethiazolidinones in the model systems and evaluation of their efficiency as free radical scavengers (e.g. scavenging activity to DPPH (1,1-diphenyl-2-picrylhydrazyde) [106]) combined with the study of other types of activity, in particular, antitumor and anti-inflammatory (see above) [405]. Such data concern all the classes of 4-thiazolidinones bearing different types of ylidene fragment. 4-Thiazolidinone derivative 178 (Scheme 86 ) possesses anti-radical activity including the ability to inhibit superoxide anion formation [406] and it is known that antioxidant activity increases when additional carboxylic group is introduced in the substituent at the position C5 of the heterocyclic core in both N3 substituted and N3 unsubstituted derivatives [407].
Scheme 86.
Structures of 4-thiazolidinones with anti-radical activity.
For the simple 5-benzylidene rhodanines antioxidant activity in various LDL oxidation models, such as TBARS assay, conjugated diene formation, REM of ox-LDL, fragmentation of apo β-100 by SDS-PAGE, radical DPPH scavenging activity, and macrophage-mediated LDL oxidation was showed [405]. It should be noted that the branched aliphatic fragments prevalence (spatially-screened phenols) largely contributes to the imitation of the known agents, e.g. BHT or probucol [336].
Antioxidant properties of a series of 2,4-dichlorothiazolylthiazolidine-2,4-dione and 4-chloro-2-benzylsulfanylthiazolyl-thiazolidine-2,4-dione derivatives 179 (Scheme 86) were reported in two different in vitro assays: superoxide anion radical formation and DPPH radical scavenging activity [408].
The presence of the benzylsulfanyl group at the second position of the thiazole ring plays a significant role in increasing the superoxide anion scavenging activity. No correlation was observed in results on superoxide radical and DPPH radical scavenger capacity.
Simple thiazolidinedione derivative (Z)-5-(2,4-dihydroxybenzylidene)-thiazolidine-2,4-dione 180 (Scheme 87 ) plays a crucial role in UV-induced melanogenesis, which is known to be related to the induction of tyrosinase enzyme. Compound inhibited nitroprusside-induced NO generation dose-dependently and suppressed tyrosinase activity and melanin synthesis (B16F10 melanoma cells) [409].
Scheme 87.
Structures of 4-thiazolidinones with dual activity.
Series of 5-arylidene-2,4-thiazolidinediones 181, 182 (Scheme 86) and their 2-phenylimino analogs inhibiting AR [236], [410], [411], [412] possess dual activity as antidiabetic and antioxidant agents for the treatment of diabetic complications [413]. The results indicate that latter possess excellent antioxidant properties inhibiting the production of TBARS in the test of compounds effect on hydroxyl radical-dependent lipoperoxidation induced in rat brain homogenate by the oxidant system Fe3+/ascorbic acid. It was observed that the presence of electron releasing substituents on the distal phenyl ring differentiates the antioxidant activity of the compounds. Detailed mechanisms of antioxidant effects of compounds of this class is still unclear and can be explained not only by the ability to inhibit superoxide anion (or other ROS) production, but also by influencing other parts of the pro/antioxidants system. Because of the changes in the classic approaches to the evaluation of pro/antioxidants [414], emerging of new approaches to the interpretation of antioxidant effects [415] and study of other (not related to free radical scavenging) mechanisms of the antioxidants [156], more attention is paid to the study of the molecular mechanisms of stimulation of antioxidant defence system [416], [417] including the possibility of these substances pro-oxidant impact with subsequent stimulation of antioxidant defence system (see above – anticancer agents).
3.8. Other types of activity
A large number of bio-targets of 5-ene-4-thiazolidinones are involved in different pathologies and there are a lot of data on the efficacy of 5-ene-thiazolidinones in different experimental models [5], [6], [7], [100], [418].
For instance, molecular docking studies indicated that the 5-ene-thiazolidinedione moiety was a likely candidate for its selectivity to monoamine oxidase-B. This hypothesis was confirmed by the identification of two lead-compounds 183 (Scheme 88 ) with IC50 13–20 μM [419].
Scheme 88.
Examples of 5-ene-4-thiazolidinones with different types of activity.
Some 5-ene-rhodanine-3-carboxylic acid derivatibes, namely 4-(5Z)-5-{5-(4-bromophenyl)-2-furyl]methylene}-4-oxo-2-thioxo-1,3-thiazolidin-3-yl-butanoic acid and 6-((5Z)-5-{5-(4-bromophenyl)-2-furylmethylene}-4-oxo-2-thioxo-1,3-thiazolidin-3-yl)hexanoic acid are efficient inhibitors of apoptosis signal-regulating kinase 1 [420] which is involved in the variety of cellular processes [421] and can be considered as a promising target for the treatment of the various cardiovascular diseases, neurodegenerative diseases, several liver and kidney diseases etc.
At the same time several other kinases, for example a cyclin-dependent kinase family are attractive targets for the design of high affinity inhibitors based on 5-alkylideenrhodanines [52]. 5-Ylidenes 184, 185 (Scheme 88) were identified in the virtual screening as representatives of novel glycogen synthase kinase-3 (GSK-3b) inhibitors and their activity was confirmed experimentally [245] (ІС50 1.56–5.56 μМ). GSK-3b has been emerging as a key therapeutic target for type-2 diabetes melitus, Alzheimer's disease, cancer and chronic inflammation. There is experimental evidence that GSK-3b inhibitors activate negative regulation of NF-kB activity, p53-dependent apoptosis, and enhance the TRAIL-induced cell death [422], [423].
3-(3-Trifluoromethyl)phenyl]-5-arylidene-2-thioxo-4-thiazolidinone was identified as a potent and selective blocker of the cystic fibrosis transmembrane conductance regulator (CFTR) Cl-channel. Moreover, it acted differently from other known blockers of the CFTR channel. Rather than blocking the channel pore, it affects the mechanism of channel gating, possibly by binding to nucleotide binding domain (NBD-1) [424], [425], [426]. CFTR, a member of the ATP-binding cassette transporter superfamily, is an epithelial chloride channel that plays a critical role in fluid absorption and secretion. Defective CFTR function causes cystic fibrosis, the most common lethal genetic disease in caucasians that produces severe lung disease, pancreatic insufficiency, neonatal intestinal obstruction and infertility where the hyperfunction of CFTR chloride channels, usually resulted from bacterial enterotoxins, constitutes the basic cause for secretory diarrhea. Thiazolidinone CFTR inhibitors may be useful in developing large-animal models of cystic fibrosis and in reducing intestinal fluid loss in cholera and other secretory diarrheas.
Benzylidenerhodanine derivatives showed good inhibitory activity against recombinant human PRL-3 (phosphatase of regenerating liver, the representant of protein tyrosine phosphatases). Compounds 186 and 187 (Scheme 88) were the most active in vitro in this series and showed the ability to reduce the invasiveness of tumor (186) [286].
Following virtual screening of potent serotonin N-acetyltransferase (member of the GCN5N-acetyltransferase superfamily catalyzing the penultimate step in the biosynthesis of melatonin) inhibitors the class of 5-ene-rhodanine-3-carboxylic acids 188 (Scheme 89 ) was identified that exhibited low micromolar competitive inhibition against acetyl-CoA and proved to be effective in blocking melatonin production in pineal cell [427].
Scheme 89.
Structures of 5-ene-4-thiazolidinones with action on different targets.
5-Arylidene-4-thiazolidinone derivatives 189 (Scheme 89) exhibited antidegenerative activity and could block multiple cartilage destruction during the osteoarthritic process by reducing NO release and restoring normal levels of glycosaminoglycans in chondrocytes treated with beta L-1, and possessed MMP-13 inhibition activity (MMPs are a large family of calcium dependent, zinc containing endopeptidases responsible for the tissue remodeling and an extracellular matrix degradation) [240]. These compounds were found to be endowed with interesting activity levels in models of acute inflammation, such as carrageenan-induced paw and pleurisy edema in rats and to be inhibitors of COX isoforms [373].
5-Benzylidene 2,4-thiazolidinedione derivatives are proposed for the treatment of Non-Alcoholic Fatty Liver Disease [428]. Compound 190 (Scheme 89) was found to upregulate the adiponectin protein expression and down regulate the secretion of leptin protein in 3T3-L1 adipocytes at a respective concentration of 10 mM.
4-{(Z)-(2Z)-2-(2-Fluorobenzylidene)-4-oxo-1,3-thiazolidin-5-ylidene]methyl}benzoic acid 191 (Scheme 89) was described as the most potent full agonist to human GPR35 [429]. A class of orphan receptors GPR35 has been described as a potentially novel drug target [430] for the design of compounds with therapeutic application in a number of diseases including inflammation, metabolic disorders, nociception and cardiovascular diseases. The olefinic thiazolidinedione 192 (Scheme 90 ) was found to be potent and selective b3-adrenergic receptor (b3-AR) (b3-Ar EC50 0.006 mM, IA = 1.03) agonists (96-fold vs b2 and 492-fold vs b1) being more active than primary hits (5-saturated analogs) [431]. b3-AR Agonists are potential drugs for the treatment of obesity, type II diabetes, frequent urination and related diseases [432]. Related thiazolidinones with a cyanamide (193), hydroxylamine (194) (Scheme 90) or 4-amino-1-benzylpiperidine substituents were generally very potent towards the b3 receptor, however, they were not very selective against both the b2 and b1 receptors. Related benzimidazolones (195) (Scheme 90) displayed a good b3 agonist selectivity profile.
Scheme 90.
Structures of 5-ene-4-thiazolodinone b3-adrenergic receptor agonists.
5-Benzylidene-2-thioxo-4-thiazolidinones 196, 197 were described as inhibitors of Aggrecanase-2 that are members of the ADAMTS (adisinte grin and metalloprotease with thrombospondin motifs) family of zinc metalloproteases. The inhibition of Aggrecanase-2, therefore, protect cartilage from damage and provide the first potential therapy to halt and/or reverse the progression of osteoarthritis and the compounds were at least 3-fold more active than 2,4-thiazolidinedione analogs [433].
In the result of virtual screening 198 (Scheme 91 ) was found as potent canabinoid receptor type 1 antagonist with promising binding affinity (IC50 125 nM) and also displayed good pharmacokinetic profile in rats as well as in human plasma [434], [435].
Scheme 91.
Structures of 5-ene-4-thiazolodinone b3-adrenergic receptor agonists and canabinoid receptor type 1 antagonist.
Novel class of sphingosine-1-phosphate (S1P1) receptor agonists based on the 2-iminothiazolidin-4-one scaffold 199 and ACT-128800 (Scheme 92 ) was found as the most active compound in in vitro and in vivo assays. Selective agonists of S1P1 receptor are of therapeutic interest for their ability to halt the exit of lymphocytes from lymph nodes. This interruption of lymphocyte migration promises a new immunomodulatory therapeutic principle for a variety of autoimmune diseases [436].
Scheme 92.
Novel class of sphingosine-1-phosphate receptor agonists.
New class of benzisothiazolylimino-5-benzylidene-4-thiazolidinones was identified as new metalloproteinase-3 inhibitors and chondroprotective agents. The mode of action is also related to the decreasing of NF-kB level [437].
Optovin ((5E)-5-[(2,5-Dimethyl-1-pyridin-3-ylpyrrol-3-yl)methylidene]-2-sulfanylidene-1,3-thiazolidin-4-one) (Scheme 93 ) was detected as a small molecule that enables repeatable photo activation of motor behaviors in wild type animals. Optovin acts as a light-sensitive ligand for anion channel involved in the detection of painful sensory stimuli. It activates human TRPA1 via structure-dependent photochemical reactions with redox-sensitive cysteine residues. Optovin treatment enables control of motor activity in the paralyzed extremities by localized illumination. These studies identify a light-based strategy for controlling endogenous TRPA1 [146], [147]. It should be mentioned that optovin is a rare example of the E-isomers of 5-heterylidenerhodanines.
Scheme 93.
Structure of optovin.
Among rhodanine-3-carboxylic (sulfonic) acids the new inhibitors of histone acetyltransferases (200–202) (Scheme 94 ) were identified. Histone acetyltransferases (HATs) are promising epigenetic drug targets involved in the pathogenesis of a wide range of diseases [427], [438].
Scheme 94.
Structures of inhibitors of histone acetyltransferases.
Summarizing all the above, 5-ene-4-thiazolidinones deservedly can be considered as privileged heterocycles with the wide spectrum of biological activity. Assigning 5-ene-thiazolidinones as PAINS due to possible Michael acceptor functionality is not so unambiguous and requires further study; therefore, most compounds can not be rejected per se. There are two main directions of the presented literature data: i) screening of the compounds activity without confirmed mode of action and ii) search for highly active and selective ligands to so-called “validated” bio-targets, which definitely don't negate each other. Search for antitumor, hypoglycemic, antimicrobial, antiviral and anti-inflammatory agents are the main fields of the 5-ene-4-thiazolidinones study. Moreover, most of the works are dedicated to the investigation of the antitumor potential of these compounds. Despite a series of established bio-targets, a detailed mode of anti-cancer effect undoubtedly depends on the structure of a particular derivative and needs further study. However, for the majority of 5-ene-thiazolidinones apoptosis-dependent as well as the ROS mediated modes of action are established.
The main directions of the active 5-ene-thiazolidinones optimization can be devided as follows: i) complications of the C5 fragment (bulky-substituents), while N3 unsubstituted position is preferred; ii) introduction of substituents in N3 position (especially fragments bearing carboxylic group or its derivatives); iii) annulation of 5-ene-thiazolidinones in complex fused heterocyclic systems; iv) combination with other pharmacologically attractive fragments within hybride pharmocophore approach.
4. 5-Ene-4-thiazolidinones based synthesis
Following the diversity of the chemical and pharmacological properties of 5-ene-4-thiazolidinones they can be treated as the building blocks for the synthesis of various derivatives and as a step of so-called privileged substructure-based diversity oriented synthesis strategy [15], [16], [17], [439], which is proved to be a fruitful tool to rapidly discover biologically active lead-compounds. Moreover, recently the following thesis was put forward: the compounds based on 5-ene-4-thiazolidinones, mainly annelated systems (e.g. thiopyrano[2,3-d]thiazoles) possess the similar to 5-ene-4-thiazolidinones pharmacological profiles and can be considered as cyclic bio-mimetics of their synthetic precursors [440], [441], [442], [443], [444]. Regardless of the type of thiazolidinone core, we tried to outline the reactions common for all 5-ene-4-thiazolidinones and in particular for those involving the C5 exocyclic double bond [445].
4.1. Addition reactions
Reduction. The C5 exocyclic double bond of 5-ene-4-thiazolidinones which formed the planar conjugated systems could be relatively easy reduced leading to the formation of corresponding unconjugated structures (Scheme 95 ). Challenging problem of such type of non-conjugated systems (that are basically most studied on the example of glitazones (see above)) is the relative simplicity of enolization at the 5-position under physiological conditions, which makes the stereochemistry difficult to maintain at this position [446].
Scheme 95.
General scheme of reduction and enolization.
Lithium and sodium borohydride and sodium hydrides [234], [303], [447], H2–Pd/C in dioxane [223], Na/Hg, THF/H2O [431], Mg in methanol or zinc in acetic acid [448] etc. have been used as reducing agents. This reaction is mainly studied as a phase of glytazone synthesis [449]. An appropriate reaction conditions allow selective reducing of the double bond leaving intact other molecular fragments, e.g. in the case of the rhodanine-3-carboxylic acids. Under reduction of the complex 5-heterylidene-4-thiazolidinones 203 (Scheme 96 ) the formation of fused heterocyclic systems 204 was described. The treatment of pyrazole–thiazolidinone derivatives with sodium hydride in N,N-dimethylformamide caused dimerisation reaction to give the corresponding spiro compounds 204 [450].
Scheme 96.
Synthesis of spiro compounds under complex 5-heterylidene-4-thiazolidinones reduction.
The exocyclic double bond of 5-arylidene-3-methyl-2-thioxo-4-thiazolidinones is a good olefin for variety addition reactions, e.g. Michael addition [451]. The reaction conditions (e.g. microwave irradiation) confirm the doubts about the realization of such reaction under physiological conditions (see above) considering 5-ene-thiazolidinones as Michael acceptors. While the Michael addition (followed the Knoevenagel condensation) [452] is a phase of domino or tandem multicomponent reactions. Reaction of 3-aroylrhodanines, aromatic aldehydes and N-dithiocarbaminates involves the consecutive Knoevenagel condensation and Michael addition (Scheme 97 ). The reaction is stereoselective and is carried out in dry conditions under microwave irradiation. Dithioester 205 under intermolecular cyclization and iodine (207), montmorillonite Li+ clay (206) or montmorillonite K-10 clay (208) action gave the appropriate heterocycles [453].
Scheme 97.
Michael addition as a phase of multicomponent reaction in the synthesis of fused heterocycles.
Utilization of the 5-carboxymethylidene-rhodanines 209 (as [C2]2+ synthones) in the known [2 + 3]-cyclocondensation reaction led to bis-thiazolidinones 210 formation. The latter can be easely reduced (adding zinc in acetic acid) to bis-thiazolidinones 211 which can readily be oxidized to starting 209 (in the presence of catalitic ammounts of triethylamine) [454], [455] (Scheme 98 ).
Scheme 98.
Synthesis of bis-thiazolidinones.
Similarly, 5-heterylidene-4-thiazolidinones 213 (Scheme 99 ) have been synthesized based on 5-methoxycarbonylmethylidene-4-thiazolidones 209 in the reaction with o-aminothiophenol and following dehydrogenation of appropriate 5-alkyl-4-thiazolidinones (212) in the presence of triethylamine in DMF [456].
Scheme 99.
Synthetic approach to C5 fragment complication.
Three-component, one-pot procedure for the synthesis of pyran-annulated thiazoles 216 involving the in situ generation of azlactone (215) and formation of pyranothiazoles is best explained by Michael addition of azlactone to 5-ene-rhodanine (214) affording the corresponding Michael adducts which underwent ring transformation to yield the final products [457] (Scheme 100 ).
Scheme 100.
Synthesis of 5-oxo-2-thioxo-2,3,6,7-tetrahydropyrano[2,3-d]thiazol-5-ones.
5-Oxo-2-thioxo-2,3,4,5,6,7-hexahydro[1,3]thiazolo[4,5-b]pyridine-6-carbonitriles 217 (Scheme 101 ) were obtained by refluxing 5-ene-rhodanines with ethyl cyanoacetate or malonodinitrile in acetic acid in the presence of ammonium acetate. First stage of the process is base-catalyzed condensation of ethyl cyanoacetate and the α,β-unsaturated ketone fragment of 5-ene-rhodanine (214). The subsequent intramolecular cyclization of Michael adduct with elimination of water leads to the formation of final product [133], [458], [459], [460].
Scheme 101.
Synthesis of 5-oxo-2-thioxo-2,3,4,5,6,7-hexahydro[1,3]thiazolo[4,5-b]pyridines.
5-Ene-rhodanines were successfully applied in cascade reactions for the construction of spiro rhodanines 218 (Scheme 102 ) with multiple consecutive chiral centers catalyzed by a simple diamine (the most efficient is 2S-N1-cyclohexyl-4,4-dimethylpentane-1,2-diamine and N-Boc-l-tryptophan), providing products with high stereoselectivities [461].
Scheme 102.
Synthesis of spiro rhodanines.
5-Ene-2-spirothiazolidine has been used as a component of Michael addition with hydroxylamine hydrochloride to yield the novel spiro (cyclohexane-isoxazolo-thiazole) derivative 219. In addition, α,β-unsaturated ketone (5-ene-2-spirothiazolidine) was used for the synthesis of corresponding oxiranyl derivatives 220 by treatment with hydrogen peroxide in the presence of sodium hydroxide. Also, starting thiazolidinone was treated with ethylcyanoacetate (medium of acetic acid in the presence of sodium acetate) under reflux to afford the corresponding pyrano-thiazole-carbonitrile derivative 221 [462] (Scheme 103 ).
Scheme 103.
Utilization of 5-ene-2-spirothiazolidine.
Similar heterocycles based on the same starting compound (5-ylidene-3-phenyl-cyclohexane(1′-2)thiazolidin-4-one) are described in the reaction with the difunctional nucleophiles (thiourea, hydrazine hydrate derivatives, malononitrile and ethylcyanoacetate) that yielded thiazolo[4,5-d]pyrimidine, pyrazolo[3,4-d]thiazole, thiazolo[4,5-b]pyridine derivatives 222 [463] (Scheme 104 ).
Scheme 104.
Reactions of 5-ene-2-spiro-4-thiazolidinones with difunctional nucleophiles yielding different fused heterocycles.
The bromination of the double bond is the efficient approach to the synthesis of compounds 223 with high antimicrobial activity [464], [465] (Scheme 105 ).
Scheme 105.
Bromination of 5- ylidene-4-thiazolidinones.
The 5-arylidene derivatives reacted with Grignard reagent via 1,4-conjugate addition to the exocyclic double bond to afford 5-alkylaryl-4-thiazolidinones 224 [466], [467], [468], [469] (Scheme 106 ). Such reaction has been rarely found in the current literature data [78].
Scheme 106.
Reaction of 5-arylidene-thiazolidin-4-one with Grignard reagent.
5-Benzylidene thiazolidinone derivatives having a conjugated carbonyl group reacted with such difunctional nucleophile as phenylhydrazine in ethanol in the presence of sodium acetate to give tetrahydro-5H-pyrazolo[3,4-d]1,3]thiazole-5-(thi)ones 225 [458], [459], [460] (Scheme 107 ).
Scheme 107.
Synthesis of pyrazolothiazole starting from 5-benzylidene thiazolidinone.
4.2. Hydrolytic cleavage
4-Thiazolidinone core is not stable in the alkali medium and this feature has been often explored in their cyclization reactions. 5-Ylidene-rhodanines are attractive starting reagents for the synthesis of 3-substituted-2-mercaptoacrylic acids 226 [43], [470]. Alkaline hydrolysis of 5-ylidenerhodanines leads to the mentioned acids formation [471] (Scheme 108 ).
Scheme 108.
Synthesis of 3-substituted-2-mercaptoacrylic acids under the alkaline hydrolysis of 5-ene-rhodanines.
The possibility of 3-substituted-2-mercaptoacrylic acids utilization as thiolic agents in the one-pot, three-component reaction for the synthesis of 5-ene-2,3-disubstituted-4-thiazolidinones was proposed based on the retro-synthetic approach [43]. The 5-arylidene-4-thiazolidinones containing substituents in the ortho position of arylidene fragment are of special interest. The efficient approach to the synthesis of isothiocoumarin derivatives 227 and related compounds 228–231 (structures of which depends on the nature of substituent in the ortho-position of aryl(heter)ylidene fragment) is based on the above mentioned 5-ene-4-thiazolidinones [472], [473], [474], [475], [476], [477] (Scheme 109 ).
Scheme 109.
Synthesis of different polycyclic compounds based on 5-arylidene-4-thiazolidinones (adapted from [477]).
In the base hydrolysis of rhodanines two-step reaction occurs in which intermediates of the hydrolysis undergo heterocyclization with the formation of the 1-oxo-1H-2-benzothiopyran-3-carboxylic acids, 3-mercaptocoumarins [478], [479], ketocinchoninic acids, 2-indole-carboxylic acids etc.
5-Arylidene-4-thiazolidinones under the action of hydrazine/thiosemicarbazone yield pyrazoline 232 and 1,2,4-triazine 233 derivatives [480] (Scheme 110 ).
Scheme 110.
Transformation of 5-arylidene-thiazolidinones under the action of hydrazine/thiosemicarbazone.
The synthesis of similar pyrazole derivatives 234 is also described starting from 2-ylidene-substituted 4-thiazolidinones [481] (Scheme 111 ).
Scheme 111.
Synthesis of pyrazoles from 2-ylidene-substituted 4-thiazolidinones.
Simple synthesis of 2-thioxo-6-azauracils was developed based on the reaction of 5-substituted 2,4-thiazolidinediones with thiosemicarbazide in alkaline medium [482]. Based on the above mentioned approach to C5-moiety optimization, the introduction of bulky heterocyclic fragments into 4-thiazolidinone molecule via the recyclization of C5 ylidene fragment containing highly reactive groups (mainly in ortho position) was proposed (Scheme 112 ).
Scheme 112.
Examples of the reactions leading to introduction of bulky heterocyclic moieties into C5 of 4-thiazolidinone core.
Though, presented protocols for 235–237 [75], [483] are not persuasive, taking into consideration the transformation (instability) of 4-thiazolidinone core in the basic medium (see below) [1], [2], [6].
4.3. Hetero-Diels-Alder reaction and related processes
The thionation is one of the effective approaches to the modification of 4-thiazolidinones (mainly in the position 4) and one of the steps of the multicomponent reactions in the synthesis of fused heterocycles. For example, thionation of 5-aroylmethylidene- or 5-aroylmethylrhodanines led to 2,3-dihydro-2-thioxothieno[2,3-d]thiazoles 238 [93] (Scheme 113 ).
Scheme 113.
Synthesis of 2,3-dihydro-2-thioxothieno[2,3-d]thiazoles.
In order to synthezise the pyrazolo[3,4-d]thiazole system the condensation of 5-ene-4-thioxothiazolidine with hydrazines were studied [484]. Thiazolidine-thione condensation with hydrazine derivatives in DMF yielded a single product in regioselective synthesis of 1,3,5-trisubstituted-5,6-dihydro-2H-pyrazolo[3,4-d]thiazoles 239 [76] (Scheme 114 ).
Scheme 114.
Utilization of 5-ene-4-thioxothiazolidine in the reaction with hydrazines.
4-Thioxothiazolidines are efficient heterodienes in hetero-Diels–Alder cycloaddition being one of the most powerful methods in the construction of fused heterocycles 240 bearing thiazolidinone fragment (Scheme 115 ).
Scheme 115.
Synthesis of fused thiopyranothiazole system.
Thiopyrano[2,3-d][1,3]thiazoles are usually synthesized via [4 + 2]-cycloaddition in hetero-Diels-Alder reaction of 5-arylidene-2,4-dithioxothiazolidines (5-arylidenethiorhodanines) or 5-arylidene-4-thioxo-2-thiazolidinones (5-arylideneisorhodanines) which contain С = С5-С4 = S group in their structure and are active heterodienes [21], [440], [441], [442], [443], [444], [482]. Thiopyrano[2,3-d][1,3]thiazoles are of a special interest as cyclic isosteric mimics of their synthetic precursors 5-arylidene-4-thiazolidinones without Michael accepting functionalities (see above) [440], [441], [442], [443], [444]. Maleic acid and its derivatives, acrylic acid and its derivatives, acrylonitrile, β-nitrostyrene, norbornene, 5-norbonene-2,3-dicarboxylic acid derivatives, cynnamic acids, propiolic acid, aroylpyruvic acid etc. have been studied in such heterodiene condensation as dienophiles [21], [440], [441], [442], [443], [444], [485], [486], [487], [488]. The cycloadditions are highly regio- and srereoselective and form products according to Frontier Orbital Theory [67], [69], [489], [490].
Interesting results were described when 5-benzylidenerhodanines with substituent in ortho-position in phenyl ring were utilized in such type of reactions. The reactions between the latter and crotonic acid, its anhydride or 4-chlorophenylamide, cynnamic acids [490], acrylic acid and its ester involved tandem hetero-Dielse-Alder and acylation processes, affording tetracyclic fused heterocycles (chromeno[4′,3′:4,5]thiopyrano[2,3-d]thiazoles) 241 (Scheme 116 ).
Scheme 116.
Synthesis of chromeno[4′,3′:4,5]thiopyrano[2,3-d]thiazoles.
Similar 2-hydroxybenzylidene rhodanine derivatives reacted with malononitrile to give fused chromeno[4′,3′:4,5]pyrano[2,3-d]thiazol-6-ones 242. Due to instability of rhodanines in basic medium at high temperatures the yields of the cyclocondensation products are low [458] (Scheme 117 ).
Scheme 117.
Synthesis of chromeno[4′,3′:4,5]pyrano[2,3-d]thiazol-6-ones.
5-Ene-4-thiazolidinones are often the intermediates in the above mentioned tandem reactions involving the hetero-Dielse-Alder reaction [67], [69], [483], [484], [490] (Scheme 118 ).
Scheme 118.
Examples of 5-ene-4-thiazolidinones as intermediates in tandem reactions.
Rare are the reports about the utilization of 2-imino-4-thiazolidinones [491] and rhodanine derivatives in the hetero-Diels-Alder reaction. For example, in the reaction of 2-imino-4-thiazolidinones and norbornene fused tiopyranothiazole derivatives are formed 244 (Scheme 119 ).
Scheme 119.
Utilization of 2-imino-4-thiazolidinones in the hetero-Diels-Alder reaction.
The reactions of arylidenerhodanines with maleic anhydride, N-phenylmaleimide, and DMAD were performed under MW irradiation and have led to pyrano[2,3-d][1,3]thiazoles formation [492].
Besides 5-arylidene derivatives, other types of 5-ene-4-thioxothiazolidines were described. For instance, 5-ethoxy-4-thioxothiazolidines are starting materials for polycyclic systems 245–247 formation [64] (Scheme 120 ). The final product formation is accompanied by the elimination of alcohol molecule and oxidation in the case of 247.
Scheme 120.
Synthesis of polycyclic molecules based on 5-ene-4-thioxothiazolidines.
Rhodanine derivatives were successfully used as the dienophiles to react with the various 2,4-dienals in the asymmetric Diels-Alder reaction leading to the construction of structurally complex compounds 248 containing the rhodanine motif [493] (Scheme 121 ). The reaction is also diastereo- and enantioselective.
Scheme 121.
Rhodanine derivatives as dienophiles in the Diels-Alder reaction.
4.4. Other cycloaddition reactions
Three-component 1,3-dipolar cycloaddition reaction of isatin, sarcosine and 5-arylidene-1,3-thiazolidine-2,4-dione or 5-arylidene-4-thioxo-1,3-thiazolidine-2-one in ethanol under ultrasound irradiation is an efficient protocol for the synthesis of dispiropyrrolidine derivatives 249 [494] (Scheme 122 ).
Scheme 122.
One-pot approach for the synthesis of spiro compounds using 5-arylidene-thiazolidinones.
The analougues two step condensation reactions affording similar complex spiro derivatives 250 are described based on 5-arylidene-thiazolo[3,2-b][1,2,4]triazol-6(5H)-ones which contain ‘fixed’ 5-arylidenethiazolidinone fragment in their structure [495] (Scheme 122).
Related dispiroindole-[3,3′-pyrrolidine-[4′,5″-1,3]thiazolidine]-2,4"(1H)-diones 251 were synthesized starting from isatin-thiazolidione conjugates in cyclocondensation reaction (Scheme 123 ). Mentioned compounds were formed as racemic mixture of two enantiomers resulting from the addition of azomethine ylide (the reaction proceeded diastereoselectively – following NMR data) [496].
Scheme 123.
Synthesis of dispiroindole-[3,3′-pyrrolidine-[4′,5″-1,3]thiazolidine]-2,4"(1H)-diones.
The 5-arylidene-4-thiazolidinones in the Michael reaction with 5-aminopyrazole in acetic acid afforded fused heterocyclic systems 252 containing anazolone moiety. The reaction involved the intramolecular cyclization of the appropriate intermediates and oxidative aromatization [497] (Scheme 124 ).
Scheme 124.
Example of 5-arylidene-4-thiazolidinones utilization in Michael reaction.
5. Conclusions
Class of 5-ene-thiazolidinones is an illustrative example of the so-called privileged heterocycles in modern medicinal chemistry, possessing wide range of pharmacological activities. The major achievements in their study have been related to antitumor, hypoclycemic, antiviral and antimicrobial activities. Despite 5-ene-4-thiazolidinone derivatives are assigned as pan-assay interference compounds (with possible low affinity to several biotargets and not sufficient selectivity), their properties of Michael acceptors in vivo should be studied more precisely and objectively. The affinity toward different targets may be considered as an advantage and basis for further modification aiming to increase the selectivity. Another benefit of the described compounds is relative simplicity of their synthesis – sufficiently important argument for the needs of medicinal chemistry. A lot of data indicates saving the biological activity of 5-ene-4-thiazolidinones in the molecules synthesized on their basis. Thereby, polyfunctional 5-ene-4-thiazolidinones are useful starting building blocks for the synthesis of complex pharmacologically attractive heterocyclic systems. Given the data presented in the article, 5-ene-thiazolidinone scaffolds are the powerful tool in medicinal chemistry and undoubtedly should not be regarded as problematic compounds per se.
References
- 1.Brown F.C. 4-Thiazolidinones. Chem. Rev. 1961;61:463–521. [Google Scholar]
- 2.Singh S., Parmar S.S., Raman K., Stenberg V.I. Chemistry and biological activity of thiazolidinones. Chem. Rev. 1981;81:175–203. [Google Scholar]
- 3.Newkome G.R., Ashutosh N. 4-Thiazolidinones. Adv. Heterocycl. Chem. 1980;25:83–112. [Google Scholar]
- 4.Tomašic T., Peterlin Mašic L. Rhodanine as a scaffold in drug discovery: a critical review of its biological activities and mechanisms of target modulation. Expert Opin. Drug Discov. 2012;7:549–560. doi: 10.1517/17460441.2012.688743. [DOI] [PubMed] [Google Scholar]
- 5.Tripathi A.C., Gupta S.J., Fatima G.N., Sonar K., Verma A., Saraf S.K. 4-Thiazolidinones: the advances continue. Eur. J. Med. Chem. 2014;72:52–77. doi: 10.1016/j.ejmech.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 6.Lesyk R.B., Zimenkovsky B.S. 4-Thiazolidones: centenarian history, current status and perspectives for modern organic and medicinal chemistry. Curr. Org. Chem. 2004;8:1547–1577. [Google Scholar]
- 7.Tomasic T., Peterlin Mašic L. Rhodanine as a privileged scaffold in drug discovery. Curr. Med. Chem. 2009;16:1596–1629. doi: 10.2174/092986709788186200. [DOI] [PubMed] [Google Scholar]
- 8.Lesyk R.B., Zimenkovsky B.S., Kaminskyy D.V., Kryshchyshyn A.P., Havrylyuk D.Ya, Atamanyuk D.V., Subtel’na I.Yu, Khyluk D.V. Thiazolidinone motif in anticancer drug discovery. experience of DH LNMU medicinal chemistry scientific group. Biopolym. Cell. 2011;27:107–117. [Google Scholar]
- 9.Verma A., Saraf S.K. 4-Thiazolidinone – a biologically active scaffold. Eur. J. Med. Chem. 2008;43:897–905. doi: 10.1016/j.ejmech.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 10.Jain V.S., Vora D.K., Ramaa C.S. Thiazolidine-2,4-diones: progress towards multifarious applications. Bioorg. Med. Chem. 2013;21:1599–1620. doi: 10.1016/j.bmc.2013.01.029. [DOI] [PubMed] [Google Scholar]
- 11.Metwally M.A., Farahat A.A., Abdel-Wahab B.F. 2-Amino-4-thiazolidinones: synthesis and reactions. J. Sulfur Chem. 2010;31:315–349. [Google Scholar]
- 12.Stojanovic M., Dzambaski Z., Bondzic B., Aleksic J., Baranac-Stojanovic M. 4-Oxothiazolidines with exocyclic C=C double bond(s): synthesis, structure, reactions and biological activity. Curr. Org. Chem. 2014;18:1108–1148. [Google Scholar]
- 13.Welsch M.E., Snyder S.A., Stockwell B.R. Privileged scaffolds for library design and drug discovery. Curr. Opin. Chem. Biol. 2010;14:1–15. doi: 10.1016/j.cbpa.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mendgen T., Steuer C., Klein C.D. Privileged scaffolds or promiscuous binders: a comparative study on rhodanines and related heterocycles in medicinal chemistry. J. Med. Chem. 2012;55:743–753. doi: 10.1021/jm201243p. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y., Wang S., Wu S., Zhu S., Dong G., Miao Z., Yao J., Zhang W., Sheng C., Wang W. Facile construction of structurally diverse thiazolidinedione-derived compounds via divergent stereoselective cascade organocatalysis and their biological exploratory studies. ACS Comb. Sci. 2013;15:298–308. doi: 10.1021/co400022r. [DOI] [PubMed] [Google Scholar]
- 16.Spandl R.J., Bender A., Spring D.R. Diversity-oriented synthesis: a spectrum of approaches and results. Org. Biomol. Chem. 2008;6:1149–1158. doi: 10.1039/b719372f. [DOI] [PubMed] [Google Scholar]
- 17.Oh S., Park S.B. A design strategy for drug-like polyheterocycles with privileged substructures for discovery of specific small-molecule modulators. Chem. Commun. 2011;47:12754–12761. doi: 10.1039/c1cc14042f. [DOI] [PubMed] [Google Scholar]
- 18.Willson T.M., Cobb J.E., Cowan D.J., Wiethe R.W., Correa I.D., Prakash S.R., Beck K.D., Moore L.B., Kliewer S.A., Lehmann J.M. The structure-activity relationship between peroxisome proliferator-activated receptor γ agonism and the antihyperglycemic activity of thiazolidinediones. J. Med. Chem. 1996;39:665–668. doi: 10.1021/jm950395a. [DOI] [PubMed] [Google Scholar]
- 19.Kaminsky D.V., Kryshchyshyn A.P., Lesyk R.B. Peroxisome proliferator activated receptors as prospective biological targets for rational design of innovative drugs. J. Org. Pharm. Chem. 2013;11:26–36. (in Ukrainian) [Google Scholar]
- 20.Ramirez M.A., Borja N.L. Epalrestat: an aldose reductase inhibitor for the treatment of diabetic neuropathy. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2008;28:646–655. doi: 10.1592/phco.28.5.646. [DOI] [PubMed] [Google Scholar]
- 21.Kaminskyy D., Vasylenko O., Atamanyuk D., Gzella A., Lesyk R. Isorhodanine and thiorhodanine motifs in the synthesis of fused thiopyrano[2,3-d][1,3]thiazoles. Synlett. 2011;10:1385–1388. [Google Scholar]
- 22.Komaritsa I.D. Studies on azolidones and their derivatives. Chem. Heterocycl. Compd. 1968;4:324–325. [Google Scholar]
- 23.Plevachuk N.E., Komaritsa I.D. A study of azolidones and their derivatives. Chem. Heterocycl. Compd. 1970;6:144–145. [Google Scholar]
- 24.Pinson J.A., Schmidt-Kittler O., Frazzetto M., Zheng Z., Jennings I.G., Kinzler K.W., Vogelstein B., Chalmers D.K., Thompson E. Synthesis and pharmacological evaluation of 4-iminothiazolidinones for inhibition of PI3 kinase. Aust. J. Chem. 2012;65:1396–1404. doi: 10.1071/CH12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaminskyy D., Subtel'na I., Zimenkovsky B., Karpenko O., Gzella A., Lesyk R. Synthesis and evaluation of anticancer activity of 5-ylidene-4-aminothiazol-2(5H)-one derivatives. Med. Chem. 2015;11:517–530. doi: 10.2174/1573406411666150211112049. [DOI] [PubMed] [Google Scholar]
- 26.Kaminskyy D.V., Lesyk R.B. Structure-anticancer activity relationships among 4-azolidinone-3-carboxylic acids derivatives. Biopolym. Cell. 2010;26:136–145. [Google Scholar]
- 27.Kaminskyy D., Khyluk D., Vasylenko O., Zaprutko L., Lesyk R. A Facile synthesis and anticancer activity evaluation of spiro-thiazolidinone-isatin conjugates. Sci. Pharm. 2011;79:763–777. doi: 10.3797/scipharm.1109-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Danilkina N.A., Mikhailov L.E., Ivin B.A. Condensations of thioamides with acetylenecarboxylic acid derivatives. Russ. J. Org. Chem. 2006;42:783–814. [Google Scholar]
- 29.Mustafa S.M., Nair V.A., Chittoor J., Krishnapillai S. Synthesis of 1,2,4-triazoles and thiazoles from thiosemicarbazide and its derivatives. Mini-Rev. Org. Chem. 2004;1:375–385. [Google Scholar]
- 30.Metwally M.A., Etman H.A., Keshk E.M., Fekry A. Thiazolidin-5-ones: synthesis and reactions. Phosphorus, Sulfur Silicon Relat. Elem. 2006;181:1039–1058. [Google Scholar]
- 31.Kaminskyy D., Gzella A.K., Lesyk R. Cyclocondensation of thioamides and haloacetic acid derivatives provides only 4-thiazolidinones. Isomeric 5-thiazolidinones were not observed. Synth. Commun. 2014;44:231–236. [Google Scholar]
- 32.Lo C., Croxall W.J. Alkoxymethylenerhodanines and their reactions with rhodanines. J. Am. Chem. Soc. 1954;76:4166–4169. [Google Scholar]
- 33.Unangst C., Connor D.T., Cetenko W.A., Sorenson R.J., Kostlan C.R., Sircar J.C., Wright C.D., Schrier D.J., Dyer R.D. Synthesis and biological evaluation of 5-[3,5-bis((1,1-dimethylethyl)-4-hydroxyphenyl)methylene]oxazoles, -thiazoles, and -imidazoles: novel dual 5-lipoxygenase and cyclooxygenase inhibitors with antiinflammatory activity. J. Med. Chem. 1994;37:322–328. doi: 10.1021/jm00028a017. [DOI] [PubMed] [Google Scholar]
- 34.Havrylyuk D., Zimenkovsky B., Vasylenko O., Gzella A., Lesyk R. Synthesis of new 4-thiazolidinone-, pyrazoline-, and isatin-based conjugates with promising antitumor activity. J. Med. Chem. 2012;55:8630–8641. doi: 10.1021/jm300789g. [DOI] [PubMed] [Google Scholar]
- 35.Havrylyuk D., Kovach N., Zimenkovsky B., Vasylenko O., Lesyk R. Synthesis and anticancer activity of isatin-based pyrazolines and thiazolidines conjugates. Arch. Pharm. 2011;344:514–522. doi: 10.1002/ardp.201100055. [DOI] [PubMed] [Google Scholar]
- 36.Pardasani R.T., Pardasani P., Jain A., Kohli S. Synthesis and semiempirical calculations of thiazolidinone and imidazolidinone derivatives of α-diones. Phosphorus, Sulfur Silicon Relat. Elem. 2004;179:1569–1575. [Google Scholar]
- 37.Ramsh S.M., Ivanenko A.G., Shpilyovy V.A., Medvedskiy N.L., Kushakova M. Synthesis of 2-aryl and 2-hetaryl derivatives of 2'-aminospiro [(1,3-dioxane)-5,5'-thiazolin]-4'-one and spiro[(1,3-dioxane)-5,5'-thiazolidine]2',4'-dione. Chem. Heterocycl. Compd. 2004;40:919–926. [Google Scholar]
- 38.Ravi S., Chiruvella K.K., Rajesh K., Prabhu V., Raghavan S.C. 5-Isopropylidene-3-ethyl rhodanine induce growth inhibition followed by apoptosis in leukemia cells. Eur. J. Med. Chem. 2010;45:2748–2752. doi: 10.1016/j.ejmech.2010.02.054. [DOI] [PubMed] [Google Scholar]
- 39.Sing W.T., Lee C.L., Yeo S.L., Lim S.P., Sim M.M. Arylalkylidene rhodanine with bulky and hydrophobic functional group as selective HCV NS3 protease inhibitor. Biorg. Med. Chem. Lett. 2001;11:91–94. doi: 10.1016/s0960-894x(00)00610-7. [DOI] [PubMed] [Google Scholar]
- 40.El-Sherif H.A.H., Mahmoud A.M., Sarhan A.A.O., Hozien Z.A., Habib O.M.A. One pot synthesis of novel thiazolo[3,2-b][1,2,4]triazoles: a useful synthetic application of the acidified acetic acid method. J. Sulfur Chem. 2006;27:65–85. [Google Scholar]
- 41.Tomasic T., Sink R., Zidar N., Fic A., Contreras-Martel C., Dessen A., Patin D., Blanot D., Muller-Premru M., Gobec S., Zega A., Kikelj D., Peterlin Masic L. Dual inhibitor of MurD and MurE ligases from Escherichia coli and Staphylococcus Aureus. ACS Med. Chem. Lett. 2012;3:626–630. doi: 10.1021/ml300047h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brown F.C., Jones R.S., Kent M. The aldol condensation with 2,3-diphenyl-4-thiazolidinone. Can. J. Chem. 1963;41:817–820. [Google Scholar]
- 43.Kaminskyy D., Khyluk D., Vasylenko O., Lesyk R. An efficient method for the transformation of 5-ylidenerhodanines into 2,3,5-trisubstituted-4-thiazolidinones. Tetrahedron Lett. 2012;53:557–559. [Google Scholar]
- 44.Riyaz S., Naidu A., Dubey K. L-Proline-catalyzed synthesis of novel 5-(1H-Indol-3-yl-methylene)-thiazolidine-2,4-dione derivatives as potential antihyperglycemic agents. Synth. Commun. 2011;41:2756–2762. [Google Scholar]
- 45.Forino M., Johnson S., Wong T.Y., Savinov A.Y., Li W., Fattorusso R., Becattini B., Orry A.J., Jung D., Abagyan R.A., Smith J.W., Alibek K., Liddington R.C., Strongin A.Y., Pellecchia M. Efficient synthetic inhibitors of anthrax lethal factor. Proc. Nat. Acad. Sci. U. S. A. 2005;102:9499–9504. doi: 10.1073/pnas.0502733102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lidstorm P., Tierney J., Wathey B., Westman J. Microwave assisted organic synthesis – a Review. Tetrahedron. 2001;57:9225–9283. [Google Scholar]
- 47.Mahalle S.R., Netankar D., Bondge S., Mane R.A. An efficient method for Knoevenagel condensation: a facile synthesis of 5-arylidenyl-2,4-thiazolidinedione. Green Chem. Lett. Rev. 2008;1:103–106. [Google Scholar]
- 48.Shelke K.F., Sapkal S.B., Kakade G.K., Sadaphal S.A., Shingate B.B., Shingare M.S. Alum catalyzed simple and efficient synthesis of 5-arylidene-2, 4-thiazolidinedione in aqueous media. Green Chem. Lett. Rev. 2010;3:17–21. [Google Scholar]
- 49.Khazaei A., Veisi H., Safaei M., Ahmadian M. Green synthesis of 5-arylidene-2,4-thiazolidinedione, 5-benzylidene rhodanine and dihydrothiophene derivatives catalyzed by hydrated ionic liquid tetrabutylammonium hydroxide in aqueous medium. J. Sulfur Chem. 2014;35:270–278. [Google Scholar]
- 50.Liu Z., Huang Y., Zhang W., Ma L., Li J., Wang X., Li J., Shen J. Soluble polymer-supported synthesis of 5-arylidene thiazolidinones and pyrimidinones using a novel traceless linker strategy. J. Comb. Chem. 2008;10:632–636. doi: 10.1021/cc800054e. [DOI] [PubMed] [Google Scholar]
- 51.Sortino M., Delgado P., Juárez S., Quiroga J., Abonía R., Insuasty B., Nogueras M., Rodero L., Garibotto F.M., Enrize R.D., Zacchino S.A. Synthesis and antifungal activity of Z-5-arylidenerhodanines. Bioorg. Med. Chem. 2007;15:484–494. doi: 10.1016/j.bmc.2006.09.038. [DOI] [PubMed] [Google Scholar]
- 52.Guiheneuf S., Paquin L., Carreaux F., Durieu E., Roisnel T., Meijer L., Bazureau J. New 5-ylidene rhodanine derivatives based on the dispacamide A model. Mol. Divers. 2014;18:375–388. doi: 10.1007/s11030-014-9509-7. [DOI] [PubMed] [Google Scholar]
- 53.Ohishi Y., Mukai T., Nagahara M., Yajima M., Kajikawa N., Miyahara K., Takano T. Preparations of 5-alkylmethylidene-3-carboxymethylrhodanine derivatives and their aldose reductase inhibitory activity. Chem. Pharm. Bull. 1990;38:1911–1919. doi: 10.1248/cpb.38.1911. [DOI] [PubMed] [Google Scholar]
- 54.Xia Z., Knaak C., Ma J., Beharry Z.M., McInnes C., Wang W., Kraft A.S., Smith C.D. Synthesis and evaluation of novel inhibitors of Pim-1 and Pim-2 protein kinases. J. Med. Chem. 2009;52:74–86. doi: 10.1021/jm800937p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bruno G., Costantino L., Curinga C., Maccari R., Monforte F., Nicolo F., Ottana R., Vigorita M.G. Synthesis and aldose reductase inhibitory activity of 5-arylidene-2,4-thiazolidinediones. Bioorg. Med. Chem. 2002;10:1077–1084. doi: 10.1016/s0968-0896(01)00366-2. [DOI] [PubMed] [Google Scholar]
- 56.Allan F.J., Allan G.G. Cyanorhodanines. J. Heterocycl. Chem. 1970;7:1091–1093. [Google Scholar]
- 57.Pardasani R.T., Pardasani P., Ojha C.K., Sherry D., Chaturvedi V., Sharma I. Syntheses of indigoid dye precursors and bioactive compounds via condensation of 1,2- and 1,4-diones with thiohydantoins. Phosphorus, Sulfur Silicon Relat. Elem. 2002;177:2435–2443. [Google Scholar]
- 58.Mushtaque M., Avecilla F., Hafeez Z.B., Jahan M., Khan M.S., Rizvi M.M.A., Khan M.S., Srivastava A., Malik A., Verma S. Synthesis, stereochemistry determination, pharmacological studies and quantum chemical analyses of bisthiazolidinone derivative. J. Mol. Struct. 2017;1127:99–113. [Google Scholar]
- 59.Gazieva G.A., Izmest'ev A.N. Oxoindolinylidene derivatives of thiazolidin-4-ones: methods of synthesis and biological activity (review) Chem. Heterocycl. Compd. 2015;50:1515–1527. [Google Scholar]
- 60.Ramsh S.M., Ivanenko A.G. Unusual hydroxymethylation of 2-amino-4-thiazolinone. Chem. Heterocycl. Compd. 2003;39:1541–1542. [Google Scholar]
- 61.Paladhi S., Bhati M., Panda D., Dash J. Thiazolidinedione-isatin conjugates via an uncatalyzed diastereoselective aldol reaction on water. J. Org. Chem. 2014;79:1473–1480. doi: 10.1021/jo402515d. [DOI] [PubMed] [Google Scholar]
- 62.Ead H.A., Abdallah S.O., Kassab N.A., Metwalli N.H., Saleh Y.E. 5-(Ethoxymethylene)-thiazolidine-2,4-dione derivatives: reactions and biological activities. Arch. Pharm. Weinh. 1987;320:1227–1232. doi: 10.1002/ardp.198700038. [DOI] [PubMed] [Google Scholar]
- 63.Yarovenko V.N., Nikitina A.S., Zavarzin I.V., Krayushkin M.M., Kovalenko L.V. Synthesis of 2-thioxo-1,3-thiazolidin-4-one derivatives. Russ. Chem. Bull. Int. Ed. 2007;56:1624–1630. [Google Scholar]
- 64.Atamanyuk D., Zimenkovsky B., Atamanyuk V., Lesyk R. 5-Ethoxymethylidene-4-thioxo-2-thiazolidinone as versatile building block for novel biorelevant small molecules with thiopyrano[2,3-d][1,3]thiazole core. Synth. Commun. 2014;44:237–244. [Google Scholar]
- 65.Havrylyuk D., Zimenkovsky B., Karpenko O., Grellier P., Lesyk R. Synthesis of pyrazoline–thiazolidinone hybrids with trypanocidal activity. Eur. J. Med. Chem. 2014;85:245–254. doi: 10.1016/j.ejmech.2014.07.103. [DOI] [PubMed] [Google Scholar]
- 66.Senkiv J., Finiuk N., Kaminskyy D., Havrylyuk D., Wojtyra M., Kril I., Stoyka R., Lesyk R. 5-Ene-4-thiazolidinones induce apoptosis in mammalian leukemia cells. Eur. J. Med. Chem. 2016;117:33–46. doi: 10.1016/j.ejmech.2016.03.089. [DOI] [PubMed] [Google Scholar]
- 67.Matiychuk V.S., Lesyk R.B., Obushak M.D., Gzella A., Atamanyuk D.V., Ostapiuk Y.V., Kryshchyshyn A. A new domino-Knoevenagel-hetero-Diels-Alder reaction. Tetrahedron Lett. 2008;49:4648–4651. [Google Scholar]
- 68.Bryhas A.O., Horak Y.I., Ostapiuk Y.V., Obushak M.D., Matiychuk V.S. A new three-step domino Knoevenagel-hetero-Diels–Alder oxidation reaction. Tetrahedron Lett. 2011;52:2324–2326. [Google Scholar]
- 69.Majumdar K.C., Taher A., Nandi R.K. Synthesis of heterocycles by domino-Knoevenagel–hetero-Diels-Alder reactions. Tetrahedron. 2012;68:5693–5718. [Google Scholar]
- 70.Bourahla K., Derdour A., Rahmouni M.F., Carreauxa, Bazureaua J. A practical access to novel 2-amino-5-arylidene-1,3-thiazol-4(5H)-ones via sulfur/nitrogen displacement under solvent-free microwave irradiation. Tetrahedron Lett. 2007;48:5785–5789. [Google Scholar]
- 71.Zvezdanovic J., Daskalova L., Yancheva D., Cvetkovic D., Markovic D., Anderluh M., Smelcerovic A. 2-Amino-5-alkylidenethiazol-4-ones as promising lipid peroxidation inhibitors. Monatsh. Chem. 2014;145:945–952. [Google Scholar]
- 72.Baharfar R., Shariati N. An efficient one-pot synthesis of novel isatin-based 2-amino thiazol-4-one conjugates using MgO nanoparticles in aqueous media. Comptes Rendus Chim. 2014;17:413–419. [Google Scholar]
- 73.Radi M., Botta L., Casaluce G., Bernardini M., Botta M. Practical one-pot two-step protocol for the microwave-assisted synthesis of highly functionalized rhodanine derivatives. J. Comb. Chem. 2009;12:200–205. doi: 10.1021/cc9001789. [DOI] [PubMed] [Google Scholar]
- 74.Chaban T.I., Ogurtsov V.V., Chaban I.G., Klenina O.V., Komarytsia J.D. Synthesis and antioxidant activity evaluation of novel 5,7-dimethyl-3H-thiazolo[4,5-b]pyridines. Phosphorus Sulfur. 2013;188:1611–1620. [Google Scholar]
- 75.Behbehani H., Ibrahim H.M. 4-Thiazolidinones in heterocyclic synthesis: synthesis of novel enaminones, azolopyrimidines and 2-arylimino-5-arylidene-4-thiazolidinones. Molecules. 2012;17:6362–6385. doi: 10.3390/molecules17066362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gautam P., Gautam D., Chaudhary R. Regioselective synthesis of new 2,5,6-trisubstituted 5,6-dihydro-2H-pyrazolo[3,4-d]thiazoles from 5-dimethylaminoethylene-thiozolidin-4-thiones. J. Sulfur Chem. 2014;35:628–640. [Google Scholar]
- 77.Tverdokhlebov A.V., Resnyanska E.V., Tolmachev A.A., Andrushko A.A. Novel approach to pyrrolo[2,1-b]thiazoles. Synthesis. 2003;17:2632–2638. [Google Scholar]
- 78.Mahmoud M.R., Madkour H.M.F., El-Bordany E.A.E.F., Soliman E.S.A. Synthesis and reactions of (Z)-2-imino-5-(3,4,5-trimethoxybenzylidene)thiazolidin-4(H)one. Eur. J. Chem. 2011;2:475–479. [Google Scholar]
- 79.de Melo Rêgo M.J.B., Galdino-Pitta M.R., Pereira D.T.M., da Silva J.C., Rabello M.M., de Lima M.C.A., Hernandes P.M.Z., Pitta I.R., Galdino S.L., da Rocha Pitta M.G. Synthesis, in vitro anticancer activity and in silico study of new disubstituted thiazolidinedione derivatives. Med. Chem. Res. 2014;23:3220–3226. [Google Scholar]
- 80.Sun J., Xia E.Y., Zhang L.L., Yan C.G. Triethylamine-catalyzed domino reactions of 1,3-thiazolidinedione: a facile access to functionalized dihydrothiophenes. Eur. J. Org. Chem. 2009;30:5247–5254. doi: 10.1021/jo900215a. [DOI] [PubMed] [Google Scholar]
- 81.Shi D.-Q., Zou Y., Hu Y., Wu H. Improved synthesis of dihydrothiophenes derivatives under ultrasound irradiation. J. Heterocycl. Chem. 2011;48:896–900. [Google Scholar]
- 82.Hassaneen H.M., Miqdad O.A., Abunada N.M., Fares A.A. Reaction of thiocarboxanilide derivatives of 2-phenylimino-3-phenyl-4-thiazolidinone and 1,3-diphenyl-2-thioxo-4-imidazolone with hydrazonoyl halides and active chloromethylene compounds. Nat. Sci. 2011;3:199–207. [Google Scholar]
- 83.Hamdy N.A., Abdel-Aziz A.H., Farag A.M., Fakhr I.M. Synthesis of some 1,3-thiazole, 1,3,4-thiadiazole, pyrazolo[5,1-c]-1,2,4-triazine, and 1,2,4-triazolo[5,1-c]-1,2,4-triazine derivatives based on the thiazolo[3,2-a]benzimidazole moiety. Monatsh Chem. 2007;138:1001–1010. [Google Scholar]
- 84.Peng Z.H., Zhou X.F., Carroll S., Geise H.J., Peng B.X., Dommisse R., Esmans E., Carleer R. Structure of rhodanine dyes, spectroscopy and performance in photographic emulsions. J. Mater. Chem. 1996;6:1325–1333. [Google Scholar]
- 85.Ke W., Luo X., Liu X., Xu H. Synthesis of cyanine dyes derived from benzotellurazo-15-crown-5. J. Heterocycl. Chem. 2000;37:1321–1324. [Google Scholar]
- 86.J.D. Mee, Method of synthesizing dyes and precursor compounds therefore, US Patent 5,679,795, (1997).
- 87.Takasu K., Inoue H., Kim H.-S., Suzuki M., Shishido T., Wataya Y., Ihara M. Rhodacyanine dyes as antimalarials 1. Preliminary evaluation of their activity and toxicity. J. Med. Chem. 2002;45:995–998. doi: 10.1021/jm0155704. [DOI] [PubMed] [Google Scholar]
- 88.Brooker L.G.S., Keyes G.H., Sprague R.H., VanDyke R.H., VanLare E., VanZandt G., White F.L. Studies in the cyanine dye series. The merocyanines. J. Am. Chem. Soc. 1951;73:5326–5332. [Google Scholar]
- 89.Horishny V.Ya, Vladzimirska O.V., Stebluk M. Synthesis and properties of bicyclic nonfused rhodanine derivatives based on amino acids. Farm. Zhurnal. 1995;2:66–70. (In Ukrainian) [Google Scholar]
- 90.Lazukina A.A., Mushkalo I.L., Nepluyev V.M., Kukhar V.D. Synthesis of 2,2-bis(arylsulphonyl)vinylamines based on bis(threemethylsilyl)-phormamide. Zhur. Org. Chim. 1983;11:2417–2420. [Google Scholar]
- 91.Knott E.B., Jeffreys R.A. Compounds containing sulphur chromophores. Part II. attempts to prepare sulphide analogues of merocyanines. J. Am. Chem. Soc. 1955;77:927–933. [Google Scholar]
- 92.Deghengni R., Daneault G. Orotic acid and its analogues: Part II. On the alkaline rearrangement of 5-carboxymethylidenehydantoin. Can. J. Chem. 1960;38:1255–1260. [Google Scholar]
- 93.Omar M.T., Nadia N., Saied K.F. A one-pot synthesis of 2,3-dihydro-2-thioxothieno[2,3-d]thiazoles. Synthesis. 2001;3:413–418. [Google Scholar]
- 94.Kaminskyy D., Zimenkovsky B., Lesyk R. Synthesis and in vitro anticancer activity of 2,4-azolidinedione-acetic acids derivatives. Eur. J. Med. Chem. 2009;44:3627–3636. doi: 10.1016/j.ejmech.2009.02.023. [DOI] [PubMed] [Google Scholar]
- 95.Zimenkovskii B.S., Kutsyk R.V., Lesyk R.B., Matyichuk V.S., Obushak N.D., Klyufinska T.I. Synthesis and antimicrobial activity of 2,4-dioxothiazolidine-5-acetic acid amides. Pharm. Chem. J. 2006;40:303–306. [Google Scholar]
- 96.Nagase H. Studies on fungicides. XXIII. Addition of dithiocarbamates and thiocarbamates to 2-thioxo-, 2-oxo- and 2-imino-5-methoxy-carbonylmethylidene-4-thiazolidones. Chem. Pharm. Bull. 1973;21:1132–1135. [Google Scholar]
- 97.Turkevich N.M., Sementsiv G.N. Derivatives of 2-benzazepine-1,3-dione. Chem. Heterocycl. Compd. 1984;20:24–26. [Google Scholar]
- 98.Turkevich N.M., Lymar O.F. Synthesis of isatin derivatives with possible antiviral or antimicrobial action. Pharm. Chem. J. 1969;3:145–146. [Google Scholar]
- 99.Mobinikhaledi A., Amiri A.K. Natural eutectic salts catalyzed one-pot synthesis of 5-arylidene-2-imino-4-thiazolidinones. Res. Chem. Intermed. 2013;39:1491–1498. [Google Scholar]
- 100.Kaminskyy D., den Hartog G.J., Wojtyra M., Lelyukh M., Gzella A., Bast A., Lesyk R. Antifibrotic and anticancer action of 5-ene amino/iminothiazolidinones. Eur. J. Med. Chem. 2016;112:180–195. doi: 10.1016/j.ejmech.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 101.Kasmi-Mir S., Djafri A., Paquin L., Hamelin J., Rahmouni M. One-pot synthesis of 5-arylidene-2-imino-4-thiazolidinones under microwave irradiation. Molecules. 2006;11:597–602. doi: 10.3390/11080597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ramsh S.M., Solov'eva S.Y., Ginak A.I. Structure of 2-imino-5-arylidene-4-thiazolidinones. Chem. Heterocycl. Compd. 1983;19:611–614. [Google Scholar]
- 103.Lesyk R., Vladzimirska O., Holota S., Zaprutko L., Gzella A. New 5-substituted thiazolo[3,2-b][1,2,4]triazol-6-ones: synthesis and anticancer evaluation. Eur. J. Med. Chem. 2007;42:641–648. doi: 10.1016/j.ejmech.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 104.Yavari I., Sabbaghan M., Porshamsian K., Bagheri M., Ali-Asgari S., Hossaini Z. Efficient synthesis of alkyl 2-(2-(arylcarbonylimino)-3-aryl-4-oxo-1,3-thiazolan-5-ylidene)-acetates. Mol. Divers. 2007;11:81–85. doi: 10.1007/s11030-007-9061-9. [DOI] [PubMed] [Google Scholar]
- 105.Hassan A.A., Aly A.A., Bedair T.I., Brown A.B., El-Emaryc T.I. A facile method for the synthesis of hydrazine-4-oxothiazolidine and imino-5-oxothiadiazine derivatives from 1,4-disubstituted thiosemicarbazides. J. Heterocycl. Chem. 2014;51:44–49. [Google Scholar]
- 106.Aly A.A., Brown A.B., Abdel-Aziz M., Abuo-Rahma G.E.D.A., Radwan M.F., Ramadan M., Gamal-Eldeen A.M. An efficient synthesis of thiazolidine-4-ones with antitumor and antioxidant activities. J. Heterocycl. Chem. 2012;49:726–731. [Google Scholar]
- 107.Mushkalo L.K., Yangol G.Ya. Condensation of carboxylic acid thioamides. Ukr. Khim. Zhurnal. 1955;21:732–737. (In Ukrainian), Chem. Abstr. 50, 16751 [1956] [Google Scholar]
- 108.Acheson R.M., Wallis J.D. Addition reactions of heterocyclic compounds. Part 74. Products from dimethyl acetylenedicarboxylate with thiourea, thioamide, and guanidine derivatives. J. Chem. Soc. Perkin Trans. 1981;1(1):415–422. [Google Scholar]
- 109.Lown J.W., Ma J.C.N. Addition reactions of acetylenic esters with substituted thioureas. Can. J. Chem. 1967;45:939–951. [Google Scholar]
- 110.Berseneva V.S., Tkachev A.V., Morzherin Y.Y., Dehaev W., Luyten I., Toppet S., Bakulev V.A. Synthesis of novel thiazolidin-4-ones by reaction of malonthioamide derivatives with dimethyl acetylenedicarboxylate. J. Chem. Soc. Perkin Trans. 1988;1(14):2133–2136. [Google Scholar]
- 111.Kosterina M.F., Morzherin Y.Y., Kramarenko O.A., Berseneva V.S., Matern A.I., Tkachev A.V., Bakulev V.A. Synthesis and complexing properties of alkyl (3-oxo-2,3-dihydrothiophen-2-ylidene)- and 4-(oxothiazolidin-5-ylidene)acetate derivatives. Rus. J. Org. Chem. 2004;40:866–869. [Google Scholar]
- 112.Coen S., Raggonen B., Vieillescazes C., Rooero J. Reactivity of conjugated enaminothioamides toward activated acetylenic compounds. Heterocycles. 1985;23:1225–1228. [Google Scholar]
- 113.Alizadeh A., Rostamnia S., Zohreh N., Hosseinpour R. A simple and effective approach to the synthesis of rhodanine derivatives via three-component reactions in water. Tetrahedron Lett. 2009;50:1533–1535. [Google Scholar]
- 114.Appalanaidu K., Dadmal T., Babu N.J., Kumbhare R.M. An improved one-pot multicomponent strategy for the preparation of thiazoline, thiazolidinone and thiazolidinol scaffolds. RSC Adv. 2015;5:88063–88069. [Google Scholar]
- 115.Jacobine A.M., Posner G.H. Three-component, one-flask synthesis of rhodanines (thiazolidinones) J. Org. Chem. 2011;76:8121–8125. doi: 10.1021/jo201561t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gabillet S., Lecerclé D., Loreau O., Carboni M., Dézard S., Gomis J.-M., Taran F. Phosphine-catalyzed construction of sulfur heterocycles. Org. Lett. 2007;9:3925–3927. doi: 10.1021/ol701563e. [DOI] [PubMed] [Google Scholar]
- 117.Dzurilla M., Kristian P., Imrich J., Stec J. Bromine oxidation of N-(3 or 4-substituted-phenyl)-N'-3-phenylpropenoylthioureas. Collect. Czech. Chem. C. 1983;11:3134–3139. [Google Scholar]
- 118.Pizzo C., Saiz C., Talevi A., Gavernet L., Palestro P., Bellera C., Blanch L.B., Benítez D., Cazzulo J.J., Chidichimo A., Wipf P., Mahler S.G. Synthesis of 2-hydrazolyl-4-thiazolidinones based on multicomponent reactions and biological evaluation against Trypanosoma Cruzi. Chem. Biol. Drug Des. 2011;77:166–172. doi: 10.1111/j.1747-0285.2010.01071.x. [DOI] [PubMed] [Google Scholar]
- 119.Choughuley A.S., Chadna M.S. Reactions of some epoxy acids with thiourea. Ind. J. Chem. 1963;1:437–440. [Google Scholar]
- 120.Isidor J.L., Mckee R.L. Synthesis of 2-methylene-4-thiazolidinones. J. Org. Chem. 1973;38:3615–3617. [Google Scholar]
- 121.Koval I.V. Heterocyclization reactions involving thiols. Russ. J. Org. Chem. 2006;42 625–251. [Google Scholar]
- 122.Markovic R., Baranac M. Regioselective synthesis of new 5-ethoxycarbonylmethyl-4-oxothiazolidyn-2-ylidene bromides and rearrangement reaction thereof. Synlett. 2000;5:607–610. [Google Scholar]
- 123.Markovic R., Baranac M., Dzambaski Z., Stojanović M., Steel J. High regioselectivity in the heterocyclization of b-oxonitriles to 4-oxothiazolidines: X-ray structure proof. Tetrahedron. 2003;59:7803–7810. [Google Scholar]
- 124.Kavitha C.V., Swamy S.N., Mantelingu K., Doreswamy S., Sridhar M.A., Prasad S.J., Rangappa K.S. Synthesis of new bioactive venlafaxine analogs: novel thiazolidin-4-ones as antimicrobials. Bioorg. Med. Chem. 2006;14 doi: 10.1016/j.bmc.2005.11.017. 229–2299. [DOI] [PubMed] [Google Scholar]
- 125.Shaker R.M. One-pot synthesis of novel 1,1′-and 1,4-bridged bis-thiazolidinone derivatives and their antimicrobial activity. Phosphorus Sulfur. 1999;149:7–14. [Google Scholar]
- 126.Smith R.L., Lee T., Gould N., Cragoe E.J. Prostaglandin isosteres. 1. (8-Aza-, 8, 10-diaza-, and 8-aza-11-thia)-9-oxoprostanoic acids and their derivatives. J. Med. Chem. 1977;20:1292–1299. doi: 10.1021/jm00220a013. [DOI] [PubMed] [Google Scholar]
- 127.Dandia A., Singh R., Khaturia S., Merienne C., Morgant G., Loupy A. Efficient microwave enhanced regioselective synthesis of a series of benzimidazolyl/triazolyl spiro [indole-thiazolidinones] as potent antifungal agents and crystal structure of spiro[3H-indole-3,2′-thiazolidine]-3′(1, 2, 4-triazol-3-yl)-2, 4′(1H)-dione. Bioorg. Med. Chem. 2006;14:2409–2417. doi: 10.1016/j.bmc.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 128.Gududuru V., Nguyen V., Dalton J.T., Miller D.D. Efficient microwave enhanced synthesis of 4-thiazolidinones. Synlett. 2004;13:2357–2358. [Google Scholar]
- 129.Srivastava T., Haq W., Katti S.B. Carbodiimide mediated synthesis of 4-thiazolidinones by one-pot three-component condensation. Tetrahedron. 2002;58:7619–7624. [Google Scholar]
- 130.Hofmann B., Barzen S., Rödl C.B., Kiehl A., Borig J., Zivkovic A., Stark H., Schneider G., Steinhilber D.A. Class of 5-benzylidene-2-phenylthiazolinones with high potency as direct 5-lipoxygenase inhibitors. J. Med. Chem. 2011;54:1943–1947. doi: 10.1021/jm101165z. [DOI] [PubMed] [Google Scholar]
- 131.Zayed E.M., Elbannany A.A.A., Ghozlan S.A.S. Studies on thiazolin-4-one: synthesis of some pyrano[2,3-b]thiazole derivatives. Pharmazie. 1985;40:194–196. [Google Scholar]
- 132.El-Maghraby A.A., El-Hag Ali G., Ahmed A.H.A., El-Gaby M.S.A. Studies on thiazolopyridines. Part 1: antimicrobial activity of some novel fluorinated thiazolo[3,2-a]pyridines and thiazolo[2′,3′:1,6]pyrido[2,3-d]pyrimidines. Phosphorus Sulfur. 2002;177:293–302. [Google Scholar]
- 133.El-Gaby M.S.A., Al-Sehemi A.G., Mohamed Y.A., Ammar Y.A. Recent trends in chemistry of thiazolopyridines. Phosphorus Sulfur. 2006;181:631–674. [Google Scholar]
- 134.Boominathan M., Nagaraj M., Maheshwaran C., Muthusubramanian S., Bhuvanesh N. One-pot green synthesis of thiazolo[3,2-a]pyridine derivatives via tandem cyclization in aqueous media. J. Hetercyclic Chem. 2014;51:244–248. [Google Scholar]
- 135.Kambe S., Hayashi T. Thiocyanoacetate. III. The synthesis of 2-hydrazono-4-thiazolidinone derivatives. Bull. Chem. Soc. Jpn. 1972;45:952–954. [Google Scholar]
- 136.Attanasi O.A., Bartoccini S., Favi G., Giorgi G., Perrulli F.R., Santeusanio S. Powerful approach to heterocyclic skeletal diversity by sequential three-component reaction of amines, isothiocyanates, and 1,2-diaza-1,3-dienes. J. Org. Chem. 2012;77:1161–1167. doi: 10.1021/jo2021949. [DOI] [PubMed] [Google Scholar]
- 137.Spitsyn N.V., Vdovichenko A.N. Rearrangement with oxide ion transfer in reactions of 4-chloro-2-oxo-2,3-dihydrothiazole-5-carbaldehyde with ureas. Cis-(Z)-trans-(E) isomerism of N-(2,4-dioxothiazolidin-5-ylidenemethyl)ureas. Russ. J. Org. Chem. 2006;42:1036–1038. [Google Scholar]
- 138.Havrylyuk D., Mosula L., Zimenkovsky B., Vasylenko O., Gzella A., Lesyk R. Synthesis and anticancer activity evaluation of 4-thiazolidinones containing benzothiazole moiety. Eur. J. Med. Chem. 2010;45:5012–5021. doi: 10.1016/j.ejmech.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 139.Sim M.M., Ng S.B., Buss A.D., Crasta S.C., Goh K.L., Lee S.K. Benzylidene rhodanines as novel inhibitors of UDP-N-acetylmuramate/L-alanine ligase. Bioorg. Med. Chem. Lett. 2002;12:697–699. doi: 10.1016/s0960-894x(01)00832-0. [DOI] [PubMed] [Google Scholar]
- 140.Cutshall N.S., O'Day C., Prezhdo M. Rhodanine derivatives as inhibitors of JSP-1. Bioorg. Med. Chem. Lett. 2005;15:3374–3379. doi: 10.1016/j.bmcl.2005.05.034. [DOI] [PubMed] [Google Scholar]
- 141.Vicini P., Geronikaki A., Incerti M., Zani F., Dearden J., Hewitt M. 2-Heteroarylimino-5-benzylidene-4-thiazolidinones analogues of 2-thiazolylimino-5-benzylidene-4-thiazolidinones with antimicrobial activity: synthesis and structure activity relationship. Bioorg. Med. Chem. 2008;16:3714–3724. doi: 10.1016/j.bmc.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 142.Havrylyuk D., Zimenkovsky B., Vasylenko O., Zaprutko L., Gzella A., Lesyk R. Synthesis of novel thiazolone-based compounds containing pyrazoline moiety and evaluation of their anticancer activity. Eur. J. Med. Chem. 2009;44:1396–1404. doi: 10.1016/j.ejmech.2008.09.032. [DOI] [PubMed] [Google Scholar]
- 143.Subtelna I., Atamanyuk D., Szymańska E., Kieć-Kononowicz K., Zimenkovsky B., Vasylenko O., Gzella A., Lesyk R. Synthesis of 5-arylidene-2-amino-4-azolones and evaluation of their anticancer activity. Bioorg. Med. Chem. 2010;18:5090–5102. doi: 10.1016/j.bmc.2010.05.073. [DOI] [PubMed] [Google Scholar]
- 144.Ahn B.Z., Sok D.E. Michael acceptors as a tool for anticancer drug design. Curr. Pharm. Des. 1996;2:247–262. [Google Scholar]
- 145.Carlson E.E., May J.F., Kiessling L.L. Chemical probes of UDP-galactopyranose mutase. Chem. Biol. 2006;13:825–837. doi: 10.1016/j.chembiol.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 146.Fajardo O., Friedrich R.W. Optopharmacology: a light switch for pain. Nat. Chem. Biol. 2013;9:219–220. doi: 10.1038/nchembio.1203. [DOI] [PubMed] [Google Scholar]
- 147.Kokel D., Cheung C.Y.J., Mills R., Coutinho-Budd J., Huang L., Setola V., Sprague J., Jin S., Jin Y.N., Huang X.-P., Bruni G., Woolf C.J., Roth B.L., Hamblin M.R., Zylka M.J., Milan D.J., Peterson R.T. Photochemical activation of TRPA1 channels in neurons and animals. Nat. Chem. Biol. 2013;9:257–263. doi: 10.1038/nchembio.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Baell J.B. Observations on screening-based research and some concerning trends in the literature. Future Med. Chem. 2010;2:1529–1546. doi: 10.4155/fmc.10.237. [DOI] [PubMed] [Google Scholar]
- 149.Baell J.B., Holloway G.A. New substructure filters for removal of pan assay interference compounds (PAINS) from screening libraries and for their exclusion in bioassays. J. Med. Chem. 2010;53:2719–2740. doi: 10.1021/jm901137j. [DOI] [PubMed] [Google Scholar]
- 150.Baell J.B., Walters M.A. Chemical con artists foil drug discovery. Nature. 2014;513:481–483. doi: 10.1038/513481a. [DOI] [PubMed] [Google Scholar]
- 151.Zhou H., Wu S., Zhai S., Liu A., Sun Y., Li R., Zhang Y., Ekins S., Swaan P.W., Fang B., Zhang B., Yan B. Design, synthesis, cytoselective toxicity, structure activity relationships, and pharmacophore of thiazolidinone derivatives targeting drug-resistant lung cancer cells. J. Med. Chem. 2008;51:1242–1251. doi: 10.1021/jm7012024. [DOI] [PubMed] [Google Scholar]
- 152.Smelcerovic Z., Veljkovic A., Kocic G., Yancheva D., Petronijevic Z., Anderluh M., Smelcerovic A. Xanthine oxidase inhibitory properties and anti-inflammatory activity of 2-amino-5-alkylidene-thiazol-4-ones. Chem. Biol. Interact. 2015;229:73–81. doi: 10.1016/j.cbi.2015.01.022. [DOI] [PubMed] [Google Scholar]
- 153.Ge X., Wakim B., Sem D.S. Chemical proteomics-based drug design: target and antitarget fishing with a catechol − rhodanine privileged scaffold for NAD(P)(H) binding proteins. J. Med. Chem. 2008;51:4571–4580. doi: 10.1021/jm8002284. [DOI] [PubMed] [Google Scholar]
- 154.Borisy A.A., Elliott P.J., Hurst N.W., Lee M.S., Lehár J., Price E.R., Serbedzija G., Zimmermann G.R., Foley M.A., Stockwell B.R., Keith C.T. Systematic discovery of multicomponent therapeutics. Proc. Natl. Acad. Sci. U. S. A. 2003;100:7977–7982. doi: 10.1073/pnas.1337088100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Morphy R., Rankovic Z. Designed multiple ligands. An emerging drug discovery paradigm. J. Med. Chem. 2005;48:6523–6543. doi: 10.1021/jm058225d. [DOI] [PubMed] [Google Scholar]
- 156.Forman H.J., Davies K.J.A., Ursini F. How do nutritional antioxidants really work: nucleophilic tone and para-hormesis versus free radical scavenging in vivo. Free Radic. Biol. Med. 2014;6:24–35. doi: 10.1016/j.freeradbiomed.2013.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hildyard J.C.W., Ämmälä C., Dukes I.D., Thomson S.A., Halestrap A.P. Identification and characterisation of a new class of highly specific and potent inhibitors of the mitochondrial pyruvate carrier. Biochim. Biophys. Acta. Bioenerg. 2005;1707:221–230. doi: 10.1016/j.bbabio.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 158.Meunier B. Hybrid molecules with a dual mode of action: dream or reality? Acc. Chem. Res. 2008;41:69–77. doi: 10.1021/ar7000843. [DOI] [PubMed] [Google Scholar]
- 159.Xu L., Vagner J., Josan J., Lynch R.M., Morse D.L., Baggett B., Han H., Mash E.A., Hruby V.J., Robert J. Enhanced targeting with heterobivalent ligands. Mol. Cancer Ther. 2009;8:2356–2365. doi: 10.1158/1535-7163.MCT-08-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Azizmohammadi M., Khoobi M., Ramazani A., Emami S., Zarrin A., Firuzi O., Miri R., Shafiee A. 2H-Chromene derivatives bearing thiazolidine-2,4-dione, rhodanine or hydantoin moieties as potential anticancer agents. Eur. J. Med. Chem. 2012;59:15–22. doi: 10.1016/j.ejmech.2012.10.044. [DOI] [PubMed] [Google Scholar]
- 161.Bogdanovi G., Koji V., Djokovi D. Synthesis and biological activity evaluation of new functionally substituted 5-arylidene-2,4-dioxotetrahydro-1,3-thiazoles. J. Serb. Chem. Soc. 2006;71:861–866. [Google Scholar]
- 162.Kaminskyy D., Bednarczyk-Cwynar B., Vasylenko O., Kazakova O., Zimenkovsky B., Zaprutko L., Lesyk R. Synthesis of new potential anticancer agents based on 4-thiazolidinone and oleanane scaffolds. Med. Chem. Res. 2012;21:3568–3580. [Google Scholar]
- 163.Tang S.Q., Lee Y.Y.I., Packiaraj D.S., Ho H.K., Chai C.L.L. Systematic evaluation of the metabolism and toxicity of thiazolidinone and imidazolidinone heterocycles. Chem. Res. Toxicol. 2015;28:2019–2033. doi: 10.1021/acs.chemrestox.5b00247. [DOI] [PubMed] [Google Scholar]
- 164.Prabhakar Y.S., Solomon V.R., Gupta M.K., Katti S.B. QSAR studies on thiazolidines: a biologically privileged scaffold. Top. Heterocycl. Chem. 2006;4:161–249. [Google Scholar]
- 165.Devinyak O., Zimenkovsky B., Lesyk R. Biologically active 4-thiazolidinones: a review of QSAR studies and QSAR modeling of antitumor activity. Curr. Top. Med. Chem. 2012;12:2763–2784. doi: 10.2174/1568026611212240006. [DOI] [PubMed] [Google Scholar]
- 166.Devinyak O., Havrylyuk D., Zimenkovsky B., Lesyk R. Computational search for possible mechanisms of 4-thiazolidinones anticancer activity. The power of visualization. Mol. Inf. 2014;33:216–229. doi: 10.1002/minf.201300086. [DOI] [PubMed] [Google Scholar]
- 167.Nastasă C., Tiperciuc B., Pârvu A., Duma M., Ionut I., Oniga O. Synthesis of new N-substituted 5-arylidene-2,4-thiazolidinediones as anti-inflammatory and antimicrobial agents. Arch. Pharm. 2013;346:481–490. doi: 10.1002/ardp.201300021. [DOI] [PubMed] [Google Scholar]
- 168.Tomašić T., Zidar N., Mueller-Premru M., Kikelj D., Mašič L.P. Synthesis and antibacterial activity of 5-ylidenethiazolidin-4-ones and 5-benzylidene-4,6-pyrimidinediones. Eur. J. Med. Chem. 2010;45:1667–1672. doi: 10.1016/j.ejmech.2009.12.030. [DOI] [PubMed] [Google Scholar]
- 169.Gouveia F.L., de-Oliveira R., de-Oliveira T.B., da-Silva I.M., do-Nascimento S.C., de-Sena K.X., de-Albuquerque J.F. Synthesis, antimicrobial and cytotoxic activities of some 5-arylidene-4-thioxo-thiazolidine-2-ones. Eur. J. Med. Chem. 2009;44:2038–2043. doi: 10.1016/j.ejmech.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 170.Zheng C.J., Song M.X., Sun L., Wu Y., Hong L., Piao H.R. Synthesis and biological evaluation of 5-aryloxypyrazole derivatives bearing a rhodanine-3-aromatic acid as potential antimicrobial agents. Bioorg. Med. Chem. Lett. 2012;22:7024–7028. doi: 10.1016/j.bmcl.2012.09.107. [DOI] [PubMed] [Google Scholar]
- 171.Xu L.L., Zheng C.J., Sun L., Miao J., Piao H.R. Synthesis of novel 1,3-diaryl pyrazole derivatives bearing rhodanine-3-fatty acid moieties as potential antibacterial agents. Eur. J. Med. Chem. 2012;48:174–178. doi: 10.1016/j.ejmech.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 172.Song M.X., Zheng C.J., Deng X.Q., Wang Q., Hou S., Liu T.T., Xing X.L., Piao H.R. Synthesis and bioactivity evaluation of rhodanine derivatives as potential anti-bacterial agents. Eur. J. Med. Chem. 2012;54:403–412. doi: 10.1016/j.ejmech.2012.05.023. [DOI] [PubMed] [Google Scholar]
- 173.Heerding D.A., Christmann L.T., Clark T.J., Holmes D.J., Rittenhouse S.F., Takata D.T., Venslavsky J.W. New benzylidenethiazolidinediones as antibacterial agents. Bioorg. Med. Chem. Lett. 2003;13:3771–3773. doi: 10.1016/j.bmcl.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 174.Bondock S., Khalifa W., Fadda A.A. Synthesis and antimicrobial evaluation of some new thiazole, thiazolidinone and thiazoline derivatives starting from 1-chloro-3,4-dihydronaphthalene-2-carboxaldehyde. Eur. J. Med. Chem. 2007;42:948–954. doi: 10.1016/j.ejmech.2006.12.025. [DOI] [PubMed] [Google Scholar]
- 175.Liu J.C., Zheng C.J., Wang M.X., Li Y.R., Ma L.X., Hou S., Piao H.R. Synthesis and evaluation of the antimicrobial activities of 3-((5-phenyl-1,3,4-oxadiazol-2-yl)methyl)-2-thioxothiazolidin-4-one derivatives. Eur. J. Med. Chem. 2014;74:405–410. doi: 10.1016/j.ejmech.2013.12.054. [DOI] [PubMed] [Google Scholar]
- 176.Jin X., Zheng C.J., Song M.X., Wu Y., Sun L.P., Li Y.J., Yu L.J., Piao H.R. Synthesis and antimicrobial evaluation of L-phenylalanine-derived C5-substituted rhodanine and chalcone derivatives containing thiobarbituric acid or 2-thioxo-4-thiazolidinone. Eur. J. Med. Chem. 2012;56:203–209. doi: 10.1016/j.ejmech.2012.08.026. [DOI] [PubMed] [Google Scholar]
- 177.Vicini P., Geronikaki A., Anastasia K., Incerti M., Zani F. Synthesis and antimicrobial activity of novel 2-thiazolylimino-5-arylidene-4-thiazolidinones. Bioorg. Med. Chem. 2006;14:3859–3864. doi: 10.1016/j.bmc.2006.01.043. [DOI] [PubMed] [Google Scholar]
- 178.Liu X.F., Zheng C.J., Sun L., Liu X.K., Piao H.R. Synthesis of new chalcone derivatives bearing 2,4-thiazolidinedione and benzoic acid moieties as potential anti-bacterial agents. Eur. J. Med. Chem. 2011;46:3469–3473. doi: 10.1016/j.ejmech.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 179.Chen Z.H., Zheng C.J., Sun L., Piao H.R. Synthesis of new chalcone derivatives containing a rhodanine-3-acetic acid moiety with potential anti-bacterial activity. Eur. J. Med. Chem. 2010;45:5739–5743. doi: 10.1016/j.ejmech.2010.09.031. [DOI] [PubMed] [Google Scholar]
- 180.Garcia-Lara J., Masalha M., Foster S.J. Staphylococcus aureus: the search for novel targets. Drug Discov. Today. 2005;10:643–651. doi: 10.1016/S1359-6446(05)03432-X. [DOI] [PubMed] [Google Scholar]
- 181.Silver L.L. Novel inhibitors of bacterial cell wall synthesis. Curr. Opin. Microbiol. 2003;6:431–438. doi: 10.1016/j.mib.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 182.Howard M.H., Cenizal T., Gutteridge S. A novel class of inhibitors of peptide deformylase discovered through high-throughput screening and virtual ligand screening. J. Med. Chem. 2004;47:6669–6672. doi: 10.1021/jm049222o. [DOI] [PubMed] [Google Scholar]
- 183.Orchard M.G., Neuss J.C., Galley C.M.S., Carr A., Porter D.W., Smith P., Scopes D.I.C., Haydon D., Vousden K., Stubberfield C.R., Young K., Page M. Rhodanine-3-acetic acid derivatives as inhibitors of fungal protein mannosyl transferase 1 (PMT1) Bioorg. Med. Chem. Lett. 2004;14:3975–3978. doi: 10.1016/j.bmcl.2004.05.050. [DOI] [PubMed] [Google Scholar]
- 184.Schepetkin I.A., Khlebnikov A.I., Kirpotina L.N., Quinn M.T. Novel small-molecule inhibitors of anthrax lethal factor identified by high-throughput screening. J. Med. Chem. 2006;49:5232–5244. doi: 10.1021/jm0605132. [DOI] [PubMed] [Google Scholar]
- 185.Zervosen A., Lu W.P., Chen Z., White R.E., Demuth T.P., Frère J.M. Interaction between penicilin-binding proteins (pbps) and two novel classes of pbp inhibitors, arylalkylidene rhodanines and arylalkylidene iminothiazolidin-4-ones. Antimicrob. Agents Chemoterap. 2004;48:961–969. doi: 10.1128/AAC.48.3.961-969.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Škedelj V., Tomašić T., Mašič L.P., Zega A. ATP-binding site of bacterial enzymes as a target for antibacterial drug design. J. Med. Chem. 2011;54:915–929. doi: 10.1021/jm101121s. [DOI] [PubMed] [Google Scholar]
- 187.Tomašić T., Kovač A., Simčič M., Blanot D., Grdadolnik S.G., Gobec S., Kikelj D., Mašič L.P. Novel 2-thioxothiazolidin-4-one inhibitors of bacterial murd ligase targeting d-glu- and diphosphate-binding sites. Eur. J. Med. Chem. 2011;46:3964–3975. doi: 10.1016/j.ejmech.2011.05.070. [DOI] [PubMed] [Google Scholar]
- 188.Zidar N., Tomasic T., Sink R., Rupnik V., Kovac A., Turk S., Patin D., Blanot D., Contreras Martel C., Dessen A., Müller Premru M., Zega A., Gobec S., Peterlin Masic L., Kikelj D. Discovery of novel 5-benzylidenerhodanine and 5-benzylidenethiazolidine-2, 4-dione inhibitors of murd ligase. J. Med. Chem. 2010;53:6584–6594. doi: 10.1021/jm100285g. [DOI] [PubMed] [Google Scholar]
- 189.Helm J.S., Hu Y., Chen L., Gross B., Walker S. Identification of active-site inhibitors of MurG using a generalizable highthroughput glycosyltransferase screen. J. Am. Chem. Soc. 2003;125:11168–11169. doi: 10.1021/ja036494s. [DOI] [PubMed] [Google Scholar]
- 190.Nottbohm A.C., Hergenrother P.J. John Wiley & Sons, Inc.; 2008. Phosphate Mimics: Cyclic Compounds, in Wiley Encyclopedia of Chemical Biology; pp. 1943–1957. [Google Scholar]
- 191.Babaoglu K., Page M.A., Jones V.C., McNeil M.R., Dong C., Naismith J.H., Lee R.E. Novel inhibitors of an emerging target in mycobacterium tuberculosis substituted thiazolidinones as inhibitors of dTDP-rhamnose synthesis. Bioorg. Med. Chem. Lett. 2003;13:3227–3230. doi: 10.1016/s0960-894x(03)00673-5. [DOI] [PubMed] [Google Scholar]
- 192.Ballell L., Field R.A., Duncan K., Young R.J. New small-molecule synthetic antimycobacterials. Antimicrob. Agents Chemother. 2005;49:2153–2163. doi: 10.1128/AAC.49.6.2153-2163.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Brooke E.W., Davies S.G., Mulvaney A.W., Okada M., Pompeo F., Sim E., Vickers R.J., Westwood I.M. Synthesis and in vitro evaluation of novel small molecule inhibitors of bacterial arylamine N-acetyltransferases (NATs) Bioorg. Med. Chem. Lett. 2003;13:2527–2530. doi: 10.1016/s0960-894x(03)00484-0. [DOI] [PubMed] [Google Scholar]
- 194.Brooke E.W., Davies S.G., Mulvaney A.W., Pompeo F., Sim E. An approach to identifying novel substrates of bacterial arylamine N-acetyltransferases. Bioorg. Med. Chem. 2003;11:1227–1234. doi: 10.1016/s0968-0896(02)00642-9. [DOI] [PubMed] [Google Scholar]
- 195.Soltero-Higgin M., Carlson E.E., Phillips J.H. Identification of inhibitors for UDP-galactopyranose mutase. J. Am. Chem. Soc. 2004;34:10532–10533. doi: 10.1021/ja048017v. [DOI] [PubMed] [Google Scholar]
- 196.Kline T., Felise H.B., Barry K.C., Jackson S.R., Nguyen H.V., Miller S.I. Substituted 2-imino-5-arylidenethiazolidin-4-one inhibitors of bacterial type III secretion. J. Med. Chem. 2008;51:7065–7074. doi: 10.1021/jm8004515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Omar K., Geronikaki A., Zoumpoulakis P., Camoutsis C., Socovic M., Ciric A., Glamoclija J. Novel 4-thiazolidinone derivatives as potential antifungal and antibacterial drugs. Bioorg. Med. Chem. 2010;18:426–432. doi: 10.1016/j.bmc.2009.10.041. [DOI] [PubMed] [Google Scholar]
- 198.Felise H.B., Nguyen H.V., Pfuetzner R.A., Barry K.C., Jackson S.R., Blanc M.-P., Bronstein P.A., Kline T., Miller S.I. An inhibitor of Gram-negative bacterial virulence protein secretion. Cell Host Microbe. 2008;4:325–336. doi: 10.1016/j.chom.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Wagner S., Sommer R., Hinsberger S., Lu C., Hartmann R.W., Empting M., Titz A. Novel Strategies for the treatment of pseudomonas aeruginosa infections. J. Med. Chem. 2016;59:5929–5969. doi: 10.1021/acs.jmedchem.5b01698. [DOI] [PubMed] [Google Scholar]
- 200.Desai N.C., Satodiya H.M., Rajpara K.M., Joshi V.V., Bhatt K., Vaghani H. Synthesis and evaluation of N-substituted thiazolidine-2,4-dione containing pyrazole as a potent antimicrobial agents. Anti-Infec. Agents. 2014;12:85–94. [Google Scholar]
- 201.Desai N.C., Satodiya H.M., Kotadiya G.M., Vaghani H.V. Synthesis and antibacterial and cytotoxic activities of new n-3 substituted thiazolidine-2,4-dione derivatives bearing the pyrazole moiety. Arch. Pharm. 2014;347:523–532. doi: 10.1002/ardp.201300466. [DOI] [PubMed] [Google Scholar]
- 202.Poyraz O., Jeankumar V.U., Saxena S., Schnell R., Haraldsson M., Yogeeswari P., Sriram D., Schneider G. Structure-guided design of novel thiazolidine inhibitors of O-acetyl serine sulfhydrylase from mycobacterium tuberculosis. J. Med. Chem. 2013;56:6457–6466. doi: 10.1021/jm400710k. [DOI] [PubMed] [Google Scholar]
- 203.Mallick S., Martin A.R., Lingard R.G. Synthesis and antimicrobial evaluation of some 5-(5-nitrofurylidene)rhodanines, 5-(5-nitrofurylidene)thiazolidine-2,4-diones, and their vinylogs. J. Med. Chem. 1971;14:528–532. doi: 10.1021/jm00288a017. [DOI] [PubMed] [Google Scholar]
- 204.Akerblom E. Nitrofurans with hight renal expression. J. Med. Chem. 1974;17:756–758. doi: 10.1021/jm00253a023. [DOI] [PubMed] [Google Scholar]
- 205.Hosseinzadeh N., Hasani M., Foroumadi A., Nadri H., Emami S., Samadi N., Ali M., Parastoo F., Farideh S., Neda S., Yasam A. 5-Nitro-heteroarylidene analogs of 2-thiazolylimino-4-thiazolidinones as a novel series of antibacterial agents. Med. Chem. Res. 2013;22:2293–2302. [Google Scholar]
- 206.Grant E.B., Guiadeen D., Baum E.Z., Foleno B.D., Jin H., Montenegro D.A., Nelson E.K., Bush A., Hlasta D.J. The synthesis and sar of rhodanines as novel class C-lactamase inhibitors. Bioorg. Med. Chem. Lett. 2000;10:2179–2182. doi: 10.1016/s0960-894x(00)00444-3. [DOI] [PubMed] [Google Scholar]
- 207.Brackman G., Quntar A.A.A.A., Enk C.D., Karalic I., Nelis H.J., Calenbergh S.V., Srebnik M., Coenye T. Synthesis and evaluation of thiazolidinedione and dioxazaborocane analogues as inhibitors of AI-2 quorum sensing in vibrio Harveyi. Bioorg. Med. Chem. 2013;21:660–667. doi: 10.1016/j.bmc.2012.11.055. [DOI] [PubMed] [Google Scholar]
- 208.Slepikas L., Chiriano G., Perozzo R., Tardy S., Kranjc-Pietrucci A., Patthey-Vuadens O., Ouertatani-Sakouhi H., Kicka S., Harrison C.F., Scrignari T., Perron K., Hilbi H., Soldati T., Cosson P., Tarasevicius E., Scapozza L. In Silico driven design and synthesis of rhodanine derivatives as novel antibacterials targeting the enoyl reductase InhA. J. Med. Chem. 2016;59:10917–10928. doi: 10.1021/acs.jmedchem.5b01620. [DOI] [PubMed] [Google Scholar]
- 209.Mushtaque M., Avecilla F., Azam A. Synthesis, characterization and structure optimization of a series of thiazolidinone derivatives as entamoeba histolytica inhibitors. Eur. J. Med. Chem. 2012;55:439–448. doi: 10.1016/j.ejmech.2012.06.052. [DOI] [PubMed] [Google Scholar]
- 210.Takasu K., Pudhom K., Kaiser M., Brun R., Ihara M. Synthesis and antimalarial efficacy of aza-fused rhodacyanines in vitro and in the P. Berghei Mouse model. J. Med. Chem. 2006;49:4795–4798. doi: 10.1021/jm0606241. [DOI] [PubMed] [Google Scholar]
- 211.Kaur K., Jain M., Reddy R.P., Jain R. Quinolines and structurally related heterocycles as antimalarials. Eur. J. Med. Chem. 2010;45:3245–3264. doi: 10.1016/j.ejmech.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 212.Pudhom K., Kasai K., Terauchi H., Inoue H., Kaiser M., Brun R., Ihara M., Takasu K. Synthesis of three classes of rhodacyanine dyes and evaluation of their in vitro and in vivo antimalarial activity. Bioorg. Med. Chem. 2006;14:8550–8563. doi: 10.1016/j.bmc.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 213.Calderón F., Barros D., Bueno J.M., Coterón J.M., Fernández E., Gamo F.J., Lavandera J.L., León M.L., Macdonald S.J.F., Mallo A., Manzano P., Porras E., Fiandor J.M., Castro J. An invitation to open innovation in malaria drug discovery: 47 quality starting points from the TCAMS. АCS Med. Chem. Lett. 2011;2:741–746. doi: 10.1021/ml200135p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Kryshchyshyn A., Kaminskyy D., Grellier P., Lesyk R. Trends in research of antitrypanosomal agents among synthetic heterocycles. Eur. J. Med. Chem. 2014;85:51–64. doi: 10.1016/j.ejmech.2014.07.092. [DOI] [PubMed] [Google Scholar]
- 215.Smith T.K., Young B.L., Denton H., Hughes D.L., Wagner G.K. First small molecular inhibitors of t. brucei dolicholphosphate mannose synthase (DPMS), a validated drug target in african sleeping sickness. Bioorg. Med. Chem. Lett. 2009;19:1749–1752. doi: 10.1016/j.bmcl.2009.01.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Leite F.H.A., da Silva Santiago P.B.G., Froes T.Q., da Silva Filho J., da Silva S.G., Ximenes R.M., de Faria A.R., Brondani D.J., de Albuquerque J.F.C., Castilho M.S. Structure-guided discovery of thiazolidine-2,4-dione derivatives as a novel class of Leishmania major pteridine reductase 1 inhibitors. Eur. J. Med. Chem. 2016;123:639–648. doi: 10.1016/j.ejmech.2016.07.060. [DOI] [PubMed] [Google Scholar]
- 217.War J.A., Srivastava S.K., Srivastava S.D. Synthesis and DNA-binding study of imidazole linked thiazolidinone derivatives. Luminescence. 2017;32:104–113. doi: 10.1002/bio.3156. [DOI] [PubMed] [Google Scholar]
- 218.Reginato M.J., Lazar M.A. Mechanisms by which thiazolidinediones enhance insulin action. Trends Endocrinol. Metab. 1999;10:9–13. doi: 10.1016/s1043-2760(98)00110-6. [DOI] [PubMed] [Google Scholar]
- 219.Moore J.T., Collins J.L., Pearce K.H. The nuclear receptor superfamily and drug discovery. Chem. Med. Chem. 2006;1:504–523. doi: 10.1002/cmdc.200600006. [DOI] [PubMed] [Google Scholar]
- 220.Desvergne B., Wahli W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr. Rev. 1999;20:649–688. doi: 10.1210/edrv.20.5.0380. [DOI] [PubMed] [Google Scholar]
- 221.Thaggikuppe Krishnamurthy P., Joghee Nanjan Chandrasekar M., Joghee Nanjana M. Newer approaches to the discovery of glitazones. Mini-Rev. Org. Chem. 2013;10:66–72. [Google Scholar]
- 222.Lohary B.B., Bhushan V., Reddy A.S., Rao P.B., Reddy N.J., Harikishore P., Haritha N., Vikramadityan R.K., Chakrabarati R., Rajagopalan R., Katneni K. Novel euglycemic and hypolipidemic agents. 4. Pyridyl- and quinolinyl-containing thiazolidinediones. J. Med. Chem. 1999;42:2569–2581. doi: 10.1021/jm980622j. [DOI] [PubMed] [Google Scholar]
- 223.Bhat B.A., Ponnala S., Sahu D.P., Tiwari P., Tripathi B.K., Srivastava A.K. Synthesis and antihyperglycemic activity profiles of novel thiazolidinedione derivatives. Bioorg. Med. Chem. 2004;12:5857–5864. doi: 10.1016/j.bmc.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 224.Tunçbilek M., Bozdağ-Dündar O., Ayhan-Kılcıgil G., Ceylan M., Waheed A., Verspohl E.J., Ertan R. Synthesis and hypoglycemic activity of some substituted flavonyl thiazolidinedione derivatives – fifth communication: flavonyl benzyl substituted 2,4-thiazolidinediones. Il Farm. 2003;58:79–83. doi: 10.1016/S0014-827X(02)01241-7. [DOI] [PubMed] [Google Scholar]
- 225.Gupta D., Ghosh N.N., Chandra R. Synthesis and pharmacological evaluation of substituted 5-(4-(2-(6,7-dimethyl-1,2,3,4-tetrahydro-2-oxo-4-quinoxalinyl)ethoxy)phenyl)methylene]thiazolidine-2,4-dione derivatives as potent euglycemic and hypolipidemic agents. Bioorg. Med. Chem. Lett. 2005;15:1019–1022. doi: 10.1016/j.bmcl.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 226.da Costa Leite L.F.C., Mourao R.H.V., de Lima M.C.A., Galdino S.L., Hernandes M.Z., Neves F.A.R., Vidal S., Barbe J., da Rocha Pitta I. Synthesis, biological evaluation and molecular modeling studies of arylidene-thiazolidinediones with potential hypoglycemic and hypolipidemic activities. Eur. J. Med. Chem. 2007;42:1263–1271. doi: 10.1016/j.ejmech.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 227.Proschak E., Zettl H., Tanrikulu Yu, Weisel M., Kriegl J.M., Rau O., Schubert-Zsilavecz M., Schneider G. From molecular shape to potent bioactive agents i: bioisosteric replacement of molecular fragments. Chem. Med. Chem. 2009;4:41–44. doi: 10.1002/cmdc.200800313. [DOI] [PubMed] [Google Scholar]
- 228.Choi J., Ko Y., Lee H.S., Park Y.S., Yang Y., Yoon S. Identification of (b-Carboxyethyl)-rhodanine derivatives exhibiting Peroxisome Proliferator-Activated Receptor γ activity. Eur. J. Med. Chem. 2010;45:193–202. doi: 10.1016/j.ejmech.2009.09.042. [DOI] [PubMed] [Google Scholar]
- 229.Ammazzalorso A., De Filippis B., Giampietro L., Amoroso R. Blocking the peroxisome proliferator-activated receptor (PPAR): an overview. Chem. Med. Chem. 2013;8:1609–1616. doi: 10.1002/cmdc.201300250. [DOI] [PubMed] [Google Scholar]
- 230.Waki H., Yamauchi T., Kadowaki T. PPAR-gamma antagonist as a potential drug for the treatment of obesity and diabetes, Nihon rinsho Japan. J. Clin. Med. 2010;68:350–355. [PubMed] [Google Scholar]
- 231.El-Kabbani O., Ruiz F., Darmanin C., Chung R.T. Aldose reductase structures: implications for mechanism and inhibition. Cell. Mol. Life Sci. 2004;61:750–762. doi: 10.1007/s00018-003-3403-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Maccari R., Vitale R.M., Ottana R., Rocchiccioli M., Marrazzo A., Cardile V., Graziano A.C.E., Amodeo P., Mura U., DelCorso A. Structure activity relationships and molecular modelling of new 5-arylidene-4-thiazolidinonederivatives as aldose reductase inhibitors and potential anti-inflammatory agents. Eur. J. Med. Chem. 2014;81:1–14. doi: 10.1016/j.ejmech.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 233.Murata M., Fujitani B., Mizuta H. Synthesis and aldose reductase inhibitory activity of a new series of 5-((2-(ω-carboxyalkoxy)aryl)methylene)-4-oxo-2-thioxothiazolidine derivatives. Eur. J. Med. Chem. 1999;34:1061–1070. [Google Scholar]
- 234.Ohishi Y., Mukai T., Nagahara M., Yajima M., Kajikawa N. Preparation of 5-alkyl-3-carboxymethylrodanines and their aldose reductase activity. Chem. Pharm. Bull. 1992;40:907–911. doi: 10.1248/cpb.40.907. [DOI] [PubMed] [Google Scholar]
- 235.Fresneau P., Cussac M., Morand J.M., Szymonski B., Tranqui D., Leclerc G. Synthesis, activity, and molecular modeling of new 2,4-Dioxo-5-(naphthylmethylene)-3-thiazolidineacetic acids and 2-thioxo analogues as potent aldose reductase inhibitors. J. Med. Chem. 1998;41:4706–4715. doi: 10.1021/jm9801399. [DOI] [PubMed] [Google Scholar]
- 236.Maccari R., Ottana R., Curinga C., Vigorita M.G., Rakowitz D., Steindl T., Langer T. Structure-activity relationships and molecular modelling of 5-arylidene-2,4-thiazolidinediones active as aldose reductase inhibitors. Bioorg. Med. Chem. 2005;13:2809–2823. doi: 10.1016/j.bmc.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 237.Bozdağ-Dündar O., Verspohl E.J., Daş-Evcimen N., Kaup R.M., Bauer K., Sarıkaya M., Evranos B., Ertan R. Synthesis and biological activity of some new flavonyl-2,4-thiazolidinediones. Bioorg. Med. Chem. 2008;16:6747–6751. doi: 10.1016/j.bmc.2008.05.059. [DOI] [PubMed] [Google Scholar]
- 238.Costantino L., Rastelli G., Cignarella G., Vianello P., Barlocco D. New aldose reductase inhibitors as potential agents for the prevention of long-term diabetic complications. Expert Opin. Ther. Pat. 1997;7:843–858. [Google Scholar]
- 239.Costantino L., Rastelli G., Gamberoni M.C., Barlocco D. Pharmacological approaches to the treatment of diabetic complications. Expet Opin. Ther. Pat. 2000;10:1245–1262. [Google Scholar]
- 240.Panico A., Maccari R., Cardile V., Crascì L., Ronsisvalle S., Ottana R. 5-Arylidene-4-thiazolidinone derivatives active as antidegenerative agents on human chondrocyte cultures. Med. Chem. 2013;9:84–90. doi: 10.2174/157340613804488378. [DOI] [PubMed] [Google Scholar]
- 241.Srivastava S.K., Yadav U.C.S., Reddy A.B.M., Saxena A., Tammali R., Shoeb M., Ansari N.H., Bhatnagar A., Petrash M.J., Srivastava S., Ramana K.V. Aldose reductase inhibitor suppresses oxidative stress-induced inflammatory disorders. Chem. Biol. Interact. 2011;191:330–338. doi: 10.1016/j.cbi.2011.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Srivastava S.K., Ramana K.V. Focus on molecules: nuclear factor-kappa B. Ex. Eye Res. 2009;88:2–3. doi: 10.1016/j.exer.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Maccari R., Paoli P., Ottanà R., Jacomelli M., Ciurleo R., Manao G., Steindl T., Langer T., Vigoritaa M.G., Camici G. 5-Arylidene-2,4-thiazolidinediones as inhibitors of protein tyrosine phosphatases. Bioorg. Med. Chem. 2007;15:5137–5149. doi: 10.1016/j.bmc.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 244.Norisada N., Masuzaki H., Fujimoto M., Inoue G., Hosoda K., Hayashi T., Watanabe M., Muraoka S., Yoneda F., Nakao K. Antidiabetic and adipogenic properties in a newly synthesized thiazolidine derivative, FPFS-410. Metabolism. 2004;12:1532–1537. doi: 10.1016/j.metabol.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 245.Kim H.J., Choo H., Cho Y.S., No K.T., Pae A.N. Novel GSK-3β inhibitors from sequential virtual screening. Bioorg. Med. Chem. 2008;16:636–643. doi: 10.1016/j.bmc.2007.10.047. [DOI] [PubMed] [Google Scholar]
- 246.Nori D.L.S., Satyanarayan K.V.V.V., Avupati V.R., Bugata B.K., Yenupuri S. Synthesis, characterization and in vitro evaluation of some new 5-benzylidene-1,3-thiazolidine-2, 4-dione analogs as new class of α-glucosidase inhibitors. Eur. J. Chem. 2014;5:144–149. [Google Scholar]
- 247.Fujisawa T., Ikegami H., Inoue K., Kawabata Y., Ogihara T. Effect of two α-glucosidase inhibitors, voglibose and acarbose, on postprandial hyperglycemia correlates with subjective abdominal symptoms. Metabolism. 2005;54:387–390. doi: 10.1016/j.metabol.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 248.Juhász L., Docsa T., Brunyászki A., Gergely P., Antus S. Synthesis and glycogen phosphorylase inhibitor activity of 2,3-dihydrobenzo[1,4]dioxin derivatives. Bioorg. Med. Chem. 2007;15:4048–4056. doi: 10.1016/j.bmc.2007.03.084. [DOI] [PubMed] [Google Scholar]
- 249.Treadway J.L., Mendys P., Hoover D.J. Glycogen phosphorylase inhibitors for treatment of type 2 Diabetes Mellitus. Expert Opin. Investig. Drugs. 2001;10:439–454. doi: 10.1517/13543784.10.3.439. [DOI] [PubMed] [Google Scholar]
- 250.Asati V., Mahapatra D.K., Bharti S.K. Thiazolidine-2,4-diones as multi-targeted scaffold in medicinal chemistry: potential anticancer agents. Eur. J. Med. Chem. 2014;87:814–833. doi: 10.1016/j.ejmech.2014.10.025. [DOI] [PubMed] [Google Scholar]
- 251.Nepali K., Sharma S., Sharma M., Bedi P.M.S., Dhar K.L. Rational approaches, design strategies, structure activity relationship and mechanistic insights for anticancer hybrids. Eur. J. Med. Chem. 2014;77:422–487. doi: 10.1016/j.ejmech.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 252.Sharma P., Reddy T.S., Thummuri D., Senwar K.R., Kumar N.P., Naidu V.G.M., Bharghava S.K., Shankaraiah N. Synthesis and biological evaluation of new benzimidazole-thiazolidinedione hybrids as potential cytotoxic and apoptosis inducing agents. Eur. J. Med. Chem. 2016;124:608–621. doi: 10.1016/j.ejmech.2016.08.029. [DOI] [PubMed] [Google Scholar]
- 253.Kaminskyy D.V., Roman O.M., Atamanyuk D.V., Lesyk R.B. 5-Ylidene-2-thioxo-4-thiazolidinone-3-succinic acids and their derivatives: synthesis, anticancer activity, QSAR-analysis. J. Org. Pharm. Chem. 2006;4:41–48. (in Ukrainian) [Google Scholar]
- 254.Patil V., Tilekar K., Mehendale-Munj S., Mohan R., Ramaa C.S. Synthesis and primary cytotoxicity evaluation of new 5-benzylidene-2,4-thiazolidinedione derivatives. Eur. J. Med. Chem. 2010;45:4539–4544. doi: 10.1016/j.ejmech.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 255.Szychowski K.A., Leja M.L., Kaminskyy D.V., Binduga U.E., Pinyazhko R., Lesyk R.B., Gmiński J. Study of novel anticancer 4-thiazolidinone derivatives. Chem. Biol. Interact. 2017;262:46–56. doi: 10.1016/j.cbi.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 256.Salamone S., Colin C., Grillier-Vuissoz I., Kuntz S., Mazerbourg S., Flament S., Martin H., Richert L., Chapleur Y., Boisbrun M. Synthesis of new troglitazone derivatives: anti-proliferative activity in breast cancer cell lines and preliminary toxicological study. Eur. J. Med. Chem. 2012;51:206–215. doi: 10.1016/j.ejmech.2012.02.044. [DOI] [PubMed] [Google Scholar]
- 257.Fu H., Hou X., Wang L., Dun Y., Yang X., Fang H. Design, synthesis and biological evaluation of 3-aryl-rhodanine benzoic acids as anti-apoptotic protein Bcl-2 inhibitors. Bioorg. Med. Chem. Lett. 2015;25:5265–5269. doi: 10.1016/j.bmcl.2015.09.051. [DOI] [PubMed] [Google Scholar]
- 258.Kavitha C.V., Chandrappa S., Narasimhamurthy K.H., Rangappa K.S. Synthesis and evaluation of 5-((5-(4-methoxyphenyl)furan-2-yl)methylene)thiazolidine-2,4-diones as a new class of cytotoxicagents for leukemia treatment. Asian J. Biochem. Pharm. Res. 2014;4:309–323. [Google Scholar]
- 259.Chandrappa S., Kavitha C.V., Shahabuddin M.S., Vinaya K., Kumar C.S.A., Ranganatha S.R., Raghavan S.C., Rangappa K.S. Synthesis of 2-(5-([5-(4-chlorophenyl)furan-2-yl]methylene)-4-oxo-2-thioxo-thiazolidin-3-yl)acetic acid derivatives and evaluation of their cytotoxicity and induction of apoptosis in human leukemia cells. Biorg. Med. Chem. 2009;17:2576–2584. doi: 10.1016/j.bmc.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 260.Kumar K.S.S., Hanumappa A., Hegde M., Narasimhamurthy K.H., Raghavan S.C., Rangappa K.S. Synthesis and antiproliferative effect of novel 4-thiazolidinone-, pyridine- and piperazine-based conjugates on human leukemic cells. Eur. J. Med. Chem. 2014;81:341–349. doi: 10.1016/j.ejmech.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 261.Suresh N., Nagesh H.N., Sekhar K.V., Kumar A., Shirazi A.N., Parang K. Synthesis of novel ciprofloxacin analogues and evaluation of their anti-proliferative effect on human cancer cell lines. Bioorg. Med. Chem. Lett. 2013;23:6292–6295. doi: 10.1016/j.bmcl.2013.09.077. [DOI] [PubMed] [Google Scholar]
- 262.Havrylyuk D., Roman O., Lesyk R. Synthetic approaches, structure activity relationship and biological applications for pharmacologically attractive pyrazole/pyrazoline–thiazolidine-based hybrids. Eur. J. Med. Chem. 2016;113:145–166. doi: 10.1016/j.ejmech.2016.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Wang S., Zhao Y., Zhang G., Lv Y., Zhang N., Gong P. Design, synthesis and biological evaluation of novel 4-thiazolidinones containing indolin-2-one moiety as potential antitumor agent. Eur. J. Med. Chem. 2011;46:3509–3518. doi: 10.1016/j.ejmech.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 264.Wang S., Zhao Y., Zhu W., Liu Y., Guo K., Gong P. Synthesis and anticancer activity of indolin-2-one derivatives bearing the 4-thiazolidinone moiety. Arch. Pharm. Chem. Life Sci. 2012;345:73–80. doi: 10.1002/ardp.201100082. [DOI] [PubMed] [Google Scholar]
- 265.Sun L., Liang C., Shirazian S., Zhou Y., Miller T., Cui J., Fukuda J.Y., Chu J.-Y., Nematalla A., Wang X.Y., Chen H., Sistla A., Luu T.C., Tang F., Wei J., Tang C. Discovery of 5-[5-fluoro-2-oxo-1,2-dihydroindol-(3Z)-ylidenemethyl]-2,4-dimethyl-1H-pyrrole-3-carboxylic acid (2-diethylaminoethyl)amide, a novel tyrosine kinase inhibitor targeting vascular endothelial and platelet-derived growth factor receptor tyrosine kinase. J. Med. Chem. 2003;46:1116–1119. doi: 10.1021/jm0204183. [DOI] [PubMed] [Google Scholar]
- 266.Sun C.L., Christensen J.G., McMahon G. Discovery and development of Sunitinib (SU 11248): multitarget tyrosine kinase inhibitor of tumor growth, survival, and angiogenesis. In: Li R., Stafford J.A., editors. Kinase Inhibitor Drugs. John Wiley Sons, Inc. Hoboken; New Jersey: 2009. pp. 1–39. [Google Scholar]
- 267.Romagnoli R., Baraldi P.G., Salvador M.K., Camacho M.E., Balzarini J., Bermejo J., Estévez F. Anticancer activity of novel hybrid molecules containing 5-benzylidene thiazolidine-2,4-dione. Eur. J. Med. Chem. 2013;63:544–557. doi: 10.1016/j.ejmech.2013.02.030. [DOI] [PubMed] [Google Scholar]
- 268.Cozzi P. The discovery of a new potential anticancer drug: a case history. Il Farm. 2003;58:213–220. doi: 10.1016/S0014-827X(03)00014-4. [DOI] [PubMed] [Google Scholar]
- 269.Kawakami M., Koya K., Ukai T., Tatsuta N., Ikegawa A., Ogawa K., Shishido T., Chen L.B. Structure-activity of novel rhodacyanine dyes as antitumor agents. J. Med. Chem. 1998;41:130–142. doi: 10.1021/jm970590k. [DOI] [PubMed] [Google Scholar]
- 270.Rousaki A., Miyata Y., Jinwal U.K., Dickey C.A., Gestwicki J.E., Zuiderweg E.R. Allosteric drugs: the interaction of anti-tumor compound mkt-077 with human hsp70 chaperones. J. Mol. Biol. 2011;411:614–632. doi: 10.1016/j.jmb.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Miyata Y., Li X., Lee H.F., Jinwal U.K., Srinivasan S.R., Seguin S., Young Z.T., Brodsky J.L., Dickey C.A., Sun D., Gestwicki J.E. Synthesis and initial evaluation of YM-08, a blood-brain barrier permeable derivative of the heat shock protein 70 (Hsp70) inhibitor mkt-077, which reduces tau levels. ACS Chem. Neurosci. 2013;4:930–939. doi: 10.1021/cn300210g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Wadhwa R., Yaguchi T., Hasan M.K., Mitsui Y., Reddel R.R., Kaul S.C. Hsp70 Family member, Mot-2/mthsp 70/GRP75, binds to the cytoplasmic sequestration domain of the p53 protein. Experim. Cell Res. 2002;274:246–253. doi: 10.1006/excr.2002.5468. [DOI] [PubMed] [Google Scholar]
- 273.Ando K., Oki E., Zhao Y., Ikawa-Yoshida A., Kitao H., Saeki H., Kimura Y., Ida S., Morita M., Kusumoto T., Maehara Y. Mortalin is a prognostic factor of gastric cancer with normal p53 function. Gastr. Cancer. 2014;17:255–262. doi: 10.1007/s10120-013-0279-1. [DOI] [PubMed] [Google Scholar]
- 274.Moorthy B.T., Ravi S., Srivastava M., Chiruvella K.K., Hemlal H., Joy O., Raghavan S.C. Novel rhodanine derivatives induce growth inhibition followed by apoptosis. Bioorg. Med. Chem. Lett. 2010;20:6297–6301. doi: 10.1016/j.bmcl.2010.08.084. [DOI] [PubMed] [Google Scholar]
- 275.Li W., Zhai X., Zhong Z., Li G., Pu Y., Gong P. Design, synthesis and evaluation of novel rhodanine containing sorafenib analogs as potential antitumor agents. Arch. Pharm. Chem. Life Sci. 2011;11:349–357. doi: 10.1002/ardp.201000326. [DOI] [PubMed] [Google Scholar]
- 276.Zhai X., Li W., Chen D., Lai R., Liu J., Gong P. Design and synthesis of 2-iminothiazolidin-4-one moiety-containing compounds as potent antiproliferative agents. Arch. Pharm. 2012;345:360–367. doi: 10.1002/ardp.201100064. [DOI] [PubMed] [Google Scholar]
- 277.Chou F.S., Wang P.S., Kulp S., Pinzone J.J. Effects of thiazolidinediones on differentiation, proliferation, and apoptosis. Mol. Cancer Res. 2007;5:523–530. doi: 10.1158/1541-7786.MCR-06-0278. [DOI] [PubMed] [Google Scholar]
- 278.Michalic L., Desverge B., Wahli W. Peroxisome-Proliferator-Activated Receptors and cancers: complexes stories. Nat. Rev. Cancer. 2004;4:61–70. doi: 10.1038/nrc1254. [DOI] [PubMed] [Google Scholar]
- 279.Gelman L., Fruchart J.-C., Auwerx J. An update on the mechanisms of action of the Peroxisome Proliferator-Activated Receptors (PPARs) and their roles in inflammation and cancer. Cell. Mol. Life Sci. 1999;55:942–943. doi: 10.1007/s000180050345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Kubota T., Koshizuka K., Williamson E.A., Asou H., Said J.W., Holden S., Miyoshi I., Koeffler P. Ligand for peroxisome proliferator-activated receptor γ (troglitazone) has potent antitumor effect against human prostate cancer both in vitro and in vivo. Cancer Res. 1998;58:3344–3352. [PubMed] [Google Scholar]
- 281.Panigrahy D., Huang S., Kieran M.W., Kaipainen A. PPARγ as a therapeutic target for tumor angiogenesis and metastasis. Cancer Biol. Ther. 2005;4:687–693. doi: 10.4161/cbt.4.7.2014. [DOI] [PubMed] [Google Scholar]
- 282.Lu W., Che P., Zhang Y., Li H., Zou S., Zhu J., Deng J., Shen X., Jiang H., Li J., Huang J. HL005 – a new selective PPARγ antagonist specifically inhibits the proliferation of MCF-7. J. Steroid. Biochem. Mol. Biol. 2011;124:112–120. doi: 10.1016/j.jsbmb.2011.01.019. [DOI] [PubMed] [Google Scholar]
- 283.Chen H., Fan Y.H., Natarajan A., Guo Y., Iyasere J., Harbinski F., Luus L., Christ W., Aktasa H., Halperina J.A. Development of small-molecule cyclin D1 – ablative agents. J. Med. Chem. 2006;49:4684–4689. doi: 10.1021/jm060057h. [DOI] [PubMed] [Google Scholar]
- 284.Palakurthi S.S., Aktas H., Grubissich L.M., Mortensen R.M., Halperin J.A. Anticancer Effects of thiazolidinediones are independent of peroxisome proliferator-activated receptor γ and mediated by inhibition of translation initiation. Cancer Res. 2001;61:6213–6218. [PubMed] [Google Scholar]
- 285.Chen H., Fan Y.H., Natarajan A., Guo Y.P., Iyasere J., Harbinski F., Luus L., Christ W., Aktasa H., Halperina J.A. Synthesis and biological evaluation of thiazolidine-2,4-dione and 2,4-thione derivatives as inhibitors of translation initiation. Bioorg. Med. Chem. Lett. 2004;14:5401–5405. doi: 10.1016/j.bmcl.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 286.Ahn J.H., Kim S.J., Park W.S., Cho S.Y., Ha J.D., Kim S.S., Kang S.K., Jeong D.G., Jung S.K., Lee S.H., Kim H.M., Park S.K., Lee K.H., Lee C.W., Ryu S.E., Choi J.K. Synthesis and biological evaluation of rhodanine derivatives as PRL-3 inhibitors. Bioorg. Med. Chem. Lett. 2006;16:2996–2999. doi: 10.1016/j.bmcl.2006.02.060. [DOI] [PubMed] [Google Scholar]
- 287.Teng X., Degterev A., Jagtap P., Xing X., Choi S., Denu R., Yuan J., Cuny G.D. Structure-activity relationship study of novel necroptosis inhibitors. Bioorg. Med. Chem. Lett. 2005;15:5039–5044. doi: 10.1016/j.bmcl.2005.07.077. [DOI] [PubMed] [Google Scholar]
- 288.Zheng W., Degterev A., Hsu E., Yuan J., Yuan C. structure-activity relationship study of a novel necroptosis inhibitor, necrostatin-7. Bioorg. Med. Chem. Lett. 2008;18:4932–4935. doi: 10.1016/j.bmcl.2008.08.058. [DOI] [PubMed] [Google Scholar]
- 289.Sheppeck J.E., Gilmore J.L., Yang A. Discovery of novel hydantoins as selective non-hydroxamate inhibitors of tumor necrosis factor-A converting enzyme (TACE) Bioorg. Med. Chem. Lett. 2007;17:1413–1417. doi: 10.1016/j.bmcl.2006.11.089. [DOI] [PubMed] [Google Scholar]
- 290.Lee J., Kim J., Koh J.S., Chung H.H., Kim K.H. Hydantoin derivatives as non-peptidic inhibitors of ras farnesyl transferase. Bioorg. Med. Chem. Lett. 2006;16:1954–1956. doi: 10.1016/j.bmcl.2005.12.074. [DOI] [PubMed] [Google Scholar]
- 291.Gibbs J.B., Oliff A. The potential of farnesyltransferase inhibitors as cancer chemotherapeutics. Annu. Rev. Pharmacol. Toxicol. 1997;37:143–166. doi: 10.1146/annurev.pharmtox.37.1.143. [DOI] [PubMed] [Google Scholar]
- 292.Haluska G.K., Dy A., Adjei A. Farnesyltransferase inhibitors as anticancer agents. Eur. J. Cancer. 2002;38:1685–1700. doi: 10.1016/s0959-8049(02)00166-1. [DOI] [PubMed] [Google Scholar]
- 293.Mazieres J., Pradines A., Favre G. Perspectives on farnesyltransferase inhibitors in cancer therapy. Cancer Lett. 2004;206:159–167. doi: 10.1016/j.canlet.2003.08.033. [DOI] [PubMed] [Google Scholar]
- 294.Zhao L., Huang W., Liu H., Wang L., Zhong W., Xiao J., Hu Y., Li S. FK506-binding protein ligands: structure-based design, synthesis, and neurotrophic/neuroprotective properties of substituted 5,5-dimethyl-2-(4-thiazolidine)carboxylates. J. Med. Chem. 2006;49:4059–4071. doi: 10.1021/jm0502384. [DOI] [PubMed] [Google Scholar]
- 295.Degterev A., Lugovskoy A., Cardone M., Mulley B., Wagner G., Mitchison T., Yuan J. Identification of small-molecule inhibitors of interaction between the BH3 domain and Bcl-xL. Nat. Cell Biol. 2001;3:173–182. doi: 10.1038/35055085. [DOI] [PubMed] [Google Scholar]
- 296.Liu W., Bulgaru A., Haigentz M., Stein C.A., Perez-Soler R., Mani S. The Bcl2-family of protein ligands as cancer drugs: the next generation of therapeutics. Curr. Med. Chem. Anti-Cancer Agents. 2003;3:217–223. doi: 10.2174/1568011033482459. [DOI] [PubMed] [Google Scholar]
- 297.Lugovskoy A.A., Degterev A.I., Fahmy A.F., Zhou P., Gross J.D., Yuan J., Wagner G. A Novel approach for characterizing protein ligand complexes: molecular basis for specificity of small-molecule Bcl-2 inhibitors. J. Am. Chem. Soc. 2002;124:1234–1240. doi: 10.1021/ja011239y. [DOI] [PubMed] [Google Scholar]
- 298.Xing C., Wang L., Tang X., Sham Y.Y. Development of selective inhibitors for anti-apoptotic Bcl-2 proteins from BHI-1. Bioorg. Med. Chem. 2007;15:2167–2176. doi: 10.1016/j.bmc.2006.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Shiau C.W., Yang C.C., Kulp S.K., Chen K.F., Chen C.S., Huang J.W., Chen C.S. Thiazolidenediones mediate apoptosis in prostate cancer cells in part through inhibition of Bcl-xL/Bcl-2 functions independently of PPARγ. Cancer Res. 2005;65:1561–1569. doi: 10.1158/0008-5472.CAN-04-1677. [DOI] [PubMed] [Google Scholar]
- 300.Liu X., Xie H., Luo C., Tong L., Wang Y., Prng T., Ding J., Jiang H., Li H. Discovery and SAR of thiazolidine-2, 4-dione analogues as insulin-like Growth Factor-1 Receptor (IGF-1R) inhibitors via hierarchical virtual screening. J. Med. Chem. 2010;53:2661–2665. doi: 10.1021/jm901798e. [DOI] [PubMed] [Google Scholar]
- 301.Liu K., Rao W., Parikh H., Li Q., Guo T.L., Grant S., Kellog G.E., Zhang S. 3,5-Disubstituted-thiazolidine-2,4-dione analogs as anticancer agents: design, synthesis and biological characterization. Eur. J. Med. Chem. 2012;47:125–137. doi: 10.1016/j.ejmech.2011.10.031. [DOI] [PubMed] [Google Scholar]
- 302.Li Q., Wu J., Zheng H., Liu K., Guo T.L., Liu Y., Eblen S.T., Grant S., Zhang S. Discovery of 3-(2-aminoethyl)-5-(3-phenyl-propylidene)-thiazolidine-2,4-dione as a dual inhibitor of the Raf/MEK/ERK and the PI3K/Akt signaling pathways. Bioorg. Med. Chem. Lett. 2010;20:4526–4530. doi: 10.1016/j.bmcl.2010.06.030. [DOI] [PubMed] [Google Scholar]
- 303.Jung K.Y., Samadani R., Chauhan J., Nevels K., Yap J.L., Zhang J., Worlikar S., Lanning M.E., Chen L., Ensey M., Shukla S., Salmo R., Heinzl G., Gordon C., Dukes T., MacKerell A.D., Shapiro P., Fletcher S. Structural modifications of (Z)-3-(2-aminoethyl)-5-(4-ethoxybenzylidene)thiazolidine-2,4-dione that improve selectivity for inhibiting the proliferation of melanoma cells containing active ERK signaling. Org. Biomol. Chem. 2013;11:3706–3732. doi: 10.1039/c3ob40199e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Strebhardt K. Multifaceted polo-like kinases: drug targets and antitargets for cancer therapy. Nat. Rev. Drug Discov. 2010;9:643–660. doi: 10.1038/nrd3184. [DOI] [PubMed] [Google Scholar]
- 305.Santamaria A., Neef R., Eberspächer U., Eis K., Husemann M., Mumberg D., Prechtl S., Schulze V., Siemeister G., Wortmann L., Barr F.A., Nigg E.A. Use of the novel Plk1 inhibitor ZK-thiazolidinone to elucidate functions of Plk1 in early and late stages of mitosis. Mol. Biol. Cell. 2007;18:4024–4036. doi: 10.1091/mbc.E07-05-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Wang H., Hammoudeh D.I., Follis A.V., Reese B.E., Lazo J.S., Metallo S.J., Prochownik E.V. Improved low molecular weight Myc-Max inhibitors. Mol. Cancer. Ther. 2007;6:2399–2408. doi: 10.1158/1535-7163.MCT-07-0005. [DOI] [PubMed] [Google Scholar]
- 307.Zhang M., Fan H.Y., Li S.C. Inhibition of c-Myc by 10058-F4 induces growth arrest and chemosensitivity in pancreatic ductal adenocarcinoma. Biomed. Pharmacother. 2015;73:123–128. doi: 10.1016/j.biopha.2015.05.019. [DOI] [PubMed] [Google Scholar]
- 308.Muvarak N., Kelley S., Robert C., Baer M.R., Perrotti D., Gambacorti-Passerini C., Civin C., Scheibner K., Rassool F.V. c-MYC Generates repair errors via increased transcription of alternative-NHEJ factors, LIG3 and PARP 1, in tyrosine kinase-activated leukemias. Mol. Cancer Res. 2015;13:699–712. doi: 10.1158/1541-7786.MCR-14-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Chisamore M.J., Wilkinson H.A., Flores O., Chen J.D. Estrogen-related receptor-α antagonist inhibits both estrogen receptor–positive and estrogen receptor-negative breast tumor grow thin mous exenografts. Mol. Cancer Ttherap. 2009;8:672–681. doi: 10.1158/1535-7163.MCT-08-1028. [DOI] [PubMed] [Google Scholar]
- 310.A. Hasuoka, K. Ono, M. Kawasaki, Prophylactic or therapeutic agent for cancer EP 2450352, 2010 U. S. Patent Application No. 13/388, 795.
- 311.Jacobs M.D., Black J., Futer O., Swenson L., Hare B., Fleming M., Saxena K. Pim-1 Ligand-bound structures reveal the mechanism of Serine/Threonine kinase inhibition by LY 294002. J. Biol. Chem. 2005;280:13728–21373. doi: 10.1074/jbc.M413155200. [DOI] [PubMed] [Google Scholar]
- 312.Aho T.L., Sandholm J., Peltola K.J., Mankonen H., Lilly M., Koskinen J. Pim-1 Kinase promotes inactivation of the pro-apoptotic bad protein byphosphorylating it on the Ser112 Gate keeper site. FEBS Lett. 2004;571:43–49. doi: 10.1016/j.febslet.2004.06.050. [DOI] [PubMed] [Google Scholar]
- 313.Macdonald A., Campbell D.G., Toth R., McLauchlan H., Hastie C.J., Arthur J.S. Pim Kinases phosphorylate multiple sites on bad and promote 14-3-3 binding and dissociation from Bcl-XL. BMC Cell Biol. 2006;7:1. doi: 10.1186/1471-2121-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Havrylyuk D., Zimenkovsky B., Lesyk R. Synthesis and anticancer activity of novel nonfused bicyclic thiazolidinone derivatives. Phosphorus Sulfur. 2009;184:638–650. [Google Scholar]
- 315.G. Attardo, S. Tripathy, M. Gagnon, Arylmethylidene heterocycles as novel an algesics, Patent WO 2009, 097695, Apl. # PCT/CA2009/000158, (2009).
- 316.Teraishi F., Wu S., Sasaki J., Zhang L., Davis J.J., Guo W., Dong F., Fang B. JNK 1-Dependent antimitotic activity of thiazolidin compounds in humannon-small-cell lung and colon cancer cells. Cell. Mol. Life Sci. 2005;62:2382–2389. doi: 10.1007/s00018-005-5365-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Teraishi F., Wu S.H., Sasaki J., Zhang L.D., Zhu H.B., Davis J.J., Fang B.L. P-Glycoprotein-independent apoptosis induction by a novel synthetic compound, MMPT 5-((4-methylphenyl)methylene)-2-(phenylamino)-4(5H)-thiazolone. J. Pharmacol. Exper. Ther. 2005;314:355–362. doi: 10.1124/jpet.105.085654. [DOI] [PubMed] [Google Scholar]
- 318.Fojo A.T., Ueda K., Slamon D.J., Poplack D.G., Gottesman M.M., Pastan I. Expression of a multidrug-resistance gene in human tumors and tissues. Proc. Natl. Acad. Sci. U. S. A. 1987;84:265–269. doi: 10.1073/pnas.84.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Gottesman M.M., Fojo T., Bates S.E. Multidrugresistance in cancer: role of ATP-dependent transporters. Nat. Rev. Cancer. 2002;2:48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 320.Kartner N., Riordan J.R., Ling V. Cell-surface-glycoprotein associated with multidrug resistance in mammalian-cell lines. Science. 1983;221:1285–1288. doi: 10.1126/science.6137059. [DOI] [PubMed] [Google Scholar]
- 321.Ferry D.R., Traunecker H., Kerr D.J. Clinical trials of P glycoprotein reversal in solid tumours. Eur. J. Cancer. 1996;32:1070–1081. doi: 10.1016/0959-8049(96)00091-3. [DOI] [PubMed] [Google Scholar]
- 322.Samuels B.L., Hollis D.R., Rosner G.L., Trump D.L., Shapiro C.L., Vogelzang N.J., Schilsky R.L. Modulation of vinblastine resistance in metastatic renal cell carcinoma with cyclosporine a or tamoxifen: a cancer and leukemia group B study. Clin. Cancer Res. 1997;3:1977–1984. [PubMed] [Google Scholar]
- 323.Smith A.J., Mayer U., Schinkel A.H., Borst A. Vailability of PSC 833, a substrate and inhibitor of P-glycoproteins, in various concentrations of serum. J. Nat. Cancer Inst. 1998;90:1161–1166. doi: 10.1093/jnci/90.15.1161. [DOI] [PubMed] [Google Scholar]
- 324.Ottanà R., Carotti S., Maccari R., Landini I., Chiricosta G., Caciagli B., Vigorita M., Mini E. In vitro antiproliferative activity against human colon cancer cell lines of representative 4-thiazolidinones. Part I. Bioorg. Med. Chem. Lett. 2005;15:3930–3933. doi: 10.1016/j.bmcl.2005.05.093. [DOI] [PubMed] [Google Scholar]
- 325.Vassilev L.T., Tovar C., Chen S., Knezevic D., Zhao X., Sun H., Heimbrook D.C., Chen L. Selective small-molecule inhibitor reveals critical mitotic functions of human CDK1. Proc. Nat. Acad. Sci. 2006;103:10660–10665. doi: 10.1073/pnas.0600447103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Chen S., Chen L., Le N.T., Zhao C., Sidduri A., Lou J., Michoud C., Portland L., Jackson N., Liu J.J., Konzelmann F., Chi F., Tovar C., Xiang Q., Chen Y., Wen Y., Vassilev L.T. Synthesis and activity of quinolinyl-methylene-thiazolinones as potent and selective Cyclin-Dependent Kinase 1 inhibitors. Bioorg. Med. Chem. Lett. 2007;17:2134–2138. doi: 10.1016/j.bmcl.2007.01.081. [DOI] [PubMed] [Google Scholar]
- 327.L. Chen, S. Chen, Thiazolinone unsubstituted quinolines U.S. Patent 7,326,786, (2008).
- 328.Lapenna S., Giordano A. Cell cycle kinases as therapeutic targets for cancer. Nat. Rev. Drug Discov. 2009;8:547–566. doi: 10.1038/nrd2907. [DOI] [PubMed] [Google Scholar]
- 329.Bruce D., Gelb L., Tartaglia M. Noonan syndrome and related disorders: dysregulated ras-mitogen activated protein kinase signal transduction. Hum. Mol. Genet. 2006;15:220–226. doi: 10.1093/hmg/ddl197. [DOI] [PubMed] [Google Scholar]
- 330.Geronikaki A., Eleftheriou P., Vicini P., Alam I., Dixit A., Saxena A.K. 2-Thiazolylimino/heteroarylimino-5-arylidene-4-thiazolidinones as new agents with SHP-2. J. Med. Chem. 2008;51:5221–5228. doi: 10.1021/jm8004306. [DOI] [PubMed] [Google Scholar]
- 331.M. Pfahl, H. Al-Shamma, A. Giachino, D. Pleynet, H. Bao, L. Spruce, C. Cow, C. Tachdjian, J. Zapf, T. Wiemann, 2-Substituted thiazolidinone and oxazolidinone derivatives for the inhibition of phosphatases and the treatment of cancer. U.S. Patent 0097566, (2004).
- 332.Ermoli A., Bargiotti A., Brasca M.G., Ciavolella A., Colombo N., Fachin G., Isacchi A., Menichincheri M., Molinari A., Montagnoli A., Pillan A., Rainoldi S., Sirtori F.R., Sola F., Thieffine S., Tibolla M., Valsasina B., Volpi D., Santocanale C., Vanotti E. Cell division cycle 7 kinase inhibitors: 1H-pyrrolo[2,3-b]pyridines, synthesis and structure - activity relationships. J. Med. Chem. 2009;52:4380–4390. doi: 10.1021/jm900248g. [DOI] [PubMed] [Google Scholar]
- 333.Coulibaly W.K., Paquin L., Benie A., Bekro Y.A., Durieux E., Rucheau S., Meijer L., Le Guevel R., Corlu A., Bazureau J.P. Synthesis of new N,N’-bis[5-arylidene-4-oxo-4,5-dihydro-thiazolidine-2-yl]piperazine derivatives under microwave irradiation and preliminary biological evaluation. Sci. Pharm. 2012;80:825–836. doi: 10.3797/scipharm.1206-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Coulibaly W.K., Paquin L., Bénie A., Békro Y.A., Le Guével R., Ravache M., Corlu A., Bazureau J.P. Prospective study directed to the synthesis of unsymmetrical linked bis-5-arylidene rhodanine derivatives via “one-pot two steps” reactions under microwave irradiation with their antitumor activity. Med. Chem. Res. 2015;24:1653–1661. [Google Scholar]
- 335.Dayam R., Aiello F., Deng J., Wu Y., Garofalo A., Chen X., Neamati N. Discovery of small molecule integrin αvβ3 antagonists as novel anticancer agents. J. Med. Chem. 2006;49:4526–4534. doi: 10.1021/jm051296s. [DOI] [PubMed] [Google Scholar]
- 336.Kesel A.J., Sonnenbichler I., Polborn K., Gürtler L., Klinkert W.E., Modolell M., Nüssler A.K., Oberthür W. A New antioxidative vitamin B6 analogue modulates pathophysiological cell proliferation and damage. Bioorg. Med. Chem. 1999;7:359–367. doi: 10.1016/s0968-0896(98)00229-6. [DOI] [PubMed] [Google Scholar]
- 337.Lesyk R., Zimenkovsky B., Lukyanchuk V. Chemistry and pharmacology of 4-thiazolidone derivates. Ann. Pol. Chem. Soc. 2003;2:293–298. [Google Scholar]
- 338.Hossain S.U., Bhattacharya S. Synthesis of O-prenylated and O-geranylated derivatives of 5-benzylidene-2,4-thiazolidinediones and evaluation of their free radical scavenging activity as well as effect on some phase ii antioxidant/detoxifying enzymes. Bioorg. Med. Chem. Lett. 2007;17:1149–1154. doi: 10.1016/j.bmcl.2006.12.040. [DOI] [PubMed] [Google Scholar]
- 339.Geronikaki A.A., Pitta E., Liaras K.S. Thiazoles and thiazolidinones as antioxidants. Curr. Med. Chem. 2013;20:4460–4480. doi: 10.2174/09298673113209990143. [DOI] [PubMed] [Google Scholar]
- 340.Revelant G., Huber-Villaume S., Dunand S., Kirsch G., Schohn H., Hesse S. Synthesis and biological evaluation of novel 2-heteroarylimino-1,3-thiazolidin-4-ones as potential anti-tumor agents. Eur. J. Med. Chem. 2015;94:102–112. doi: 10.1016/j.ejmech.2015.02.053. [DOI] [PubMed] [Google Scholar]
- 341.Trachootham D., Alexandre J., Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat. Rev. Drug Discov. 2009;8:579–591. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- 342.Hippert M.M., O'Toole S., Thorburn A. Autophagy in cancer: good, bad, or both? Cancer Res. 2006;66:9349–9351. doi: 10.1158/0008-5472.CAN-06-1597. [DOI] [PubMed] [Google Scholar]
- 343.Niture S.K., Kaspar J.W., Shen J., Jaiswal A.K. Nrf2 Signaling and cell survival. Toxicol. Appl. Pharmacol. 2010;244:37–42. doi: 10.1016/j.taap.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Rego P.C., Oliveira C.R. Mitochondrial dysfunction and reactive oxygen species in excitotoxicity and apoptosis: implications for the pathogenesis of neurodegenerative diseases. Neurochem. Res. 2003;28:1563–1574. doi: 10.1023/a:1025682611389. [DOI] [PubMed] [Google Scholar]
- 345.Chatterjee S., Kundu S., Bhattacharyya A. Mechanism of cadmium induced apoptosis in the immunocyte. Toxicol. Lett. 2008;177:83–89. doi: 10.1016/j.toxlet.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 346.Pathak N., Khandelwal S. Role of oxidative stress and apoptosis in cadmium induced thymicatrophy and splenomegaly in mice. Toxicol. Lett. 2007;169:95–108. doi: 10.1016/j.toxlet.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 347.Zhou Y.J., Zhang S., Liu C.W., Cai Y.Q. The protection of selenium on ROS mediated-apoptosis by mitochondria dysfunction in cadmium-induced LLCPK1 cells. Toxicol. Vitro. 2009;23:288–294. doi: 10.1016/j.tiv.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 348.Huppertz B., Kadyrov M., Kingdom J.C. Apoptosis and its role in the trophoblast. Am. J. Obstet. Gynecol. 2006;195:29–39. doi: 10.1016/j.ajog.2005.07.039. [DOI] [PubMed] [Google Scholar]
- 349.Ren Z., Yang N., Ji C., Zheng J., Wang T., Liu Y., Zuo P. Neuroprotective effects of 5-(4-hydroxy-3-dimethoxybenzylidene)-thiazolidinone in MPTP induced parkinsonism model in mice. Neuropharmacol. 2015;93:209–218. doi: 10.1016/j.neuropharm.2015.01.030. [DOI] [PubMed] [Google Scholar]
- 350.Schöffski P. Polo-like kinase (PLK) inhibitors in preclinical and early clinical development in oncology. Oncologist. 2009;14:559–570. doi: 10.1634/theoncologist.2009-0010. [DOI] [PubMed] [Google Scholar]
- 351.Onen-Bayram F.E., Durmaz I., Scherman D., Herscovici J., Cetin-Atalay R. A novel thiazolidine compound induces caspase-9 dependent apoptosis in cancer cells. Bioorg. Med. Chem. 2012;20:5094–5102. doi: 10.1016/j.bmc.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 352.Gududuru V., Hurh E., Dalton J.T., Miller D.D. Synthesis and antiproliferative activity of 2-aryl-4-oxo-thiazolidin-3-yl-amides for prostate cancer. Bioorg. Med. Chem. Lett. 2004;14:5289–5293. doi: 10.1016/j.bmcl.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 353.Gududuru V., Hurh E., Dalton J.T., Miller D.D. Discovery of 2-arylthiazolidine-4-carboxylic acid amides as a new class of cytotoxic agents for prostate cancer. J. Med. Chem. 2005;48:2584–2588. doi: 10.1021/jm049208b. [DOI] [PubMed] [Google Scholar]
- 354.Gududuru V., Hurh E., Sullivan J., Dalton J.T., Miller D.D. SAR studies of 2-arylthiazolidine-4-carboxylic acid amides: a novel class of cytotoxic agents for prostate cancer. Bioorg. Med. Chem. Lett. 2005;15:4010–4013. doi: 10.1016/j.bmcl.2005.06.032. [DOI] [PubMed] [Google Scholar]
- 355.Li W., Wang Z., Gududuru V., Zbytek B., Slominski A.T., Dalton J.T., Miller D.D. Structure-activity relationship studies of arylthiazolidine amides as selective cytotoxic agents for melanoma. Anticancer Res. 2007;27:883–888. [PubMed] [Google Scholar]
- 356.Li W., Lu Y., Wang Z., Dalton J.T., Miller D.D. Synthesis and antiproliferative activity of thiazolidine analogs for melanoma. Bioorg. Med. Chem. Lett. 2007;17:4113–4117. doi: 10.1016/j.bmcl.2007.05.059. [DOI] [PubMed] [Google Scholar]
- 357.Lu Y., Wang Z., Li C.M., Chen J.J., Dalton J.T., Li W., Miller D.D. Synthesis, in vitro structure–activity relationship, and in vivo studies of 2-arylthiazolidine-4-carboxylic acid amides as anticancer agents. Bioorg. Med. Chem. 2010;18:477–495. doi: 10.1016/j.bmc.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Chumak V.V., Fil М.R., Panchuk R.R., Zimenkovsky B.S., Havrylyuk D.Y., Lesyk R.B., Stoika R.S. Study of antineoplastic action of novel isomeric derivatives of 4-thiazolidinone. Ukr. Biochem. J. 2014;86:96–105. [PubMed] [Google Scholar]
- 359.Kumar K.S.S., Hanumappa A., Vetrivel M., Hegde M., Girish Y.R., Byregowda T.R., Rao S., Raghavan S.C., Rangappa K.S. Antiproliferative and tumor inhibitory studies of 2,3-disubstituted 4-thiazolidinone derivatives. Bioorg. Med. Chem. Lett. 2015;25:3616–3620. doi: 10.1016/j.bmcl.2015.06.069. [DOI] [PubMed] [Google Scholar]
- 360.Song Y., Connor D.T., Doubleday R., Sorenson R.J., Sercel A.D., Unangst C., Roth B.D., Gilbertsen R.B., Chan K., Schrier D.J., Guglietta A., Bornemeier D.A., Dyer R.D. Synthesis, structure-activity relationships, and in vivo evaluations of substituted di-tert-butylphenols as a novel class of potent, selective, and orally active cyclooxygenase-2 inhibitors. 1. Thiazolone and oxazolone series. J. Med. Chem. 1999;42:1151–1160. doi: 10.1021/jm9805081. [DOI] [PubMed] [Google Scholar]
- 361.Kulkarni S.K., Singh V.P. Positioning dual inhibitors in the treatment of pain and inflammatory disorders. Infammopharmacol. 2008;16:1–15. doi: 10.1007/s10787-006-1604-7. [DOI] [PubMed] [Google Scholar]
- 362.Johnson A.R., Marletta M.A., Dyer R.D. Slow-binding inhibition of human prostaglandin endoperoxide synthase-2 with darbufelone, an isoform-selective antiinflammatory di-tert-butyl phenol. Biochemistry. 2001;40:7736–7745. doi: 10.1021/bi002343f. [DOI] [PubMed] [Google Scholar]
- 363.Silva A.A.R., Silvagoes A.J., Lima W.T., Souzamaia M.B. Antiedematogenic activity of two thiazolidine derivatives: N-tryptophyl-5-(3,5-di-tert-butyl-4-hydroxybenzylidene)rhodanine (GS26) and N-tryptophyl-5-(3,5-di-tert-butyl-4-hydroxybenzylidene)-2,4-thiazolidinedione (GS28) Chem. Pharm. Bull. 2003;51:1351–1355. doi: 10.1248/cpb.51.1351. [DOI] [PubMed] [Google Scholar]
- 364.Cho H., Tai H.H. Thiazolidinediones as a novel class of NAD+-dependent 15-hydroxyprostaglandin dehydrogenase inhibitors. Arch. Biochem. Biophys. 2002;405:247–251. doi: 10.1016/s0003-9861(02)00352-1. [DOI] [PubMed] [Google Scholar]
- 365.Jean F.M., Laurent C., Brigitte G., Olivier G., Florence B., Rui B., Christophe D., Maria C., Roger R., Neuwels M., Bruno A.B. Expression of NAD+-dependent 15-hydroxyprostaglandin dehydrogenase and protection of prostaglandins in human hair follicle. Exp. Dermatol. 2008;17:821–828. doi: 10.1111/j.1600-0625.2008.00706.x. [DOI] [PubMed] [Google Scholar]
- 366.Wu Y., Tai H.H., Cho H. Synthesis and SAR of thiazolidinedione derivatives as 15-PGDH inhibitors. Bioorg. Med. Chem. 2010;18:1428–1433. doi: 10.1016/j.bmc.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 367.Irvine M.W., Patrick G.L., Kewney J., Hastings S.F., MacKenzie S.J. Rhodanine derivatives as novel inhibitors of PDE4. Bioorg. Med. Chem. Lett. 2008;18:2032–2037. doi: 10.1016/j.bmcl.2008.01.117. [DOI] [PubMed] [Google Scholar]
- 368.Panico A.M., Vicini P., Geronikaki A., Incerti M., Cardile V., Crascì L., Messina R., Ronsisvalle S. Heteroarylimino-4-thiazolidinones as inhibitors of cartilage degradation. Bioorg. Chem. 2011;39:48–52. doi: 10.1016/j.bioorg.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 369.Pomel V., Klicic J., Covini D., Church D.D., Shaw J., Roulin K., Burgat-Charvillon F., Valognes D., Camps M., Chabert C., Gillieron C., Francuon B., Perrin D., Leroy D., Gretener D., Nichols A., Vitte A., Carboni S., Rommel C., Schwarz M.K., Ruckle T. Furan-2-ylmethylene thiazolidinediones as novel, potent, and selective inhibitors of phosphoinositide 3-kinase. J. Med. Chem. 2006;49:3857–3871. doi: 10.1021/jm0601598. [DOI] [PubMed] [Google Scholar]
- 370.Ottana R., Maccari R., Ciurleo R., Vigorita M.G., Panico A.M., Cardile V., Garufib F., Ronsisvalle S. Synthesis and in vitro evaluation of 5-arylidene-3-hydroxyalkyl-2-phenylimino-4-thiazolidinones with antidegenerative activity on human chondrocyte cultures. Bioorg. Med. Chem. 2007;15:7618–7625. doi: 10.1016/j.bmc.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 371.Garg A., Chawla P., Panjwani D., Saraf S.A. Synthesis of some novel 5-substituted arylidene-2,4-thiazolidinediones as bioactive agents. Int. J. Pharm. Sci. Nanotechnol. 2011;4:1373–1378. [Google Scholar]
- 372.Barros C.D., Amato A.A., DeOliveira T.B., Iannini K.B.R., DaSilva A.L., DaSilva T.G., Leite E.S., Hernandes M.Z., DeLima M.C.A., Galdino S.L., Neves F.A., Pitta R.I.R. Synthesis and anti-inflammatory activity of new arylidene-thiazolidine-2,4-diones as PPARγ ligands. Bioorg. Med. Chem. 2010;18:3805–3811. doi: 10.1016/j.bmc.2010.04.045. [DOI] [PubMed] [Google Scholar]
- 373.Ottanà R., Maccari R., Barreca M.L., Bruno G., Rotondo A., Rossi A., Chiricosta G., DiPaola R., Sautebin L., Cuzzocrea S., Vigorita M.G. 5-Arylidene-2-imino-4-thiazolidinones: design and synthesis of novel anti-inflammatory agents. Bioorg. Med. Chem. 2005;13:4243–4252. doi: 10.1016/j.bmc.2005.04.058. [DOI] [PubMed] [Google Scholar]
- 374.Geronikaki A.A., Lagunin A.A., Hadjipavlou-Litina D.I., Eleftheriou P.T., Filimonov D.A., Poroikov V.V., Alam I., Saxena A.K. Computer-aided discovery of anti-inflammatory thiazolidinones with dual Cyclooxygenase/Lipoxygenase inhibition. J. Med. Chem. 2008;51:1601–1609. doi: 10.1021/jm701496h. [DOI] [PubMed] [Google Scholar]
- 375.Uchôa F.D.T., Silva T.G., Lima M.D.C.A., Galdino S.L., Pitta I.D.R., Costa T.D. Preclinical pharmacokinetic and pharmacodynamic evaluation of thiazolidinone pg15: an anti-inflammatory candidate. J. Pharm. Pharmacol. 2009;61:339–345. doi: 10.1211/jpp/61.03.0008. [DOI] [PubMed] [Google Scholar]
- 376.Cetal S.L. Synthesis and anti-inflammatory activity of new thiazolidine-2,4-dione, 4-thioxothazolidinones and 2-thioxoimazolidinones. Heterocycl. Commun. 2005;11:121–128. [Google Scholar]
- 377.Albuquerque J.F.C., Azevedo L.C., Galdino S.L., Chantegrel J., Pitta I.R., Luu-Duc C. Synthesis and structural study of 5-arylidene-thiazolidine-2,4-diones and 3-substituted-4-thio-imidazolidine-2-ones. Ann. Pharm. Fr. 1994;53:209–214. [PubMed] [Google Scholar]
- 378.Deep A., Jain S., Sharma P.C. Synthesis and anti-inflammatory activity of some novel biphenyl-4-carboxylic acid 5-(arylidene)-2-(aryl)-4-oxothiazolidin-3-yl amides. Acta Pol. Pharm. Drug Res. 2010;67:63–67. [PubMed] [Google Scholar]
- 379.Ramana K.V., Srivastava S.K. Aldosereductase: a novel therapeutic target for inflammatory pathologies. Int. J. Biochem. Cell Biol. 2010;42:17–20. doi: 10.1016/j.biocel.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Ramana K.V. Aldose reductase: new insights for an old enzyme. Biomol. Concept. 2011;2:103–114. doi: 10.1515/BMC.2011.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Ramana K.V., Srivastava S.K. Mediation of aldose reductase in lipopolysaccharide-induced inflammatory signals in mouse peritoneal macrophages. Cytokine. 2006;36:115–122. doi: 10.1016/j.cyto.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Kaushik-Basu N., Bopda-Waffo A., Talele T.T., Basu A., Chen Y., Kucukguzel S.G. 4-Thiazolidinones: a novel class of hepatitis c virus ns5b polymerase inhibitors. Front. Biosci. J. Virt. Lib. 2007;13:3857–3868. doi: 10.2741/2974. [DOI] [PubMed] [Google Scholar]
- 383.Barreca M.L., Chimirri A., DeClercq E., DeLuca L., Monforte A.M., Monforte P., Rao A., Zappala M. Anti-HIV agents: design and discovery of new potent RT inhibitors. Farmaco. 2003;58:259–263. doi: 10.1016/S0014-827X(03)00024-7. [DOI] [PubMed] [Google Scholar]
- 384.Jiang S., Tala S.R., Lu H., Abo-Dya N.E., Avan I., Gyanda K., Lu L., Katritzky A.R., Debnath A. Design, synthesis, and biological activity of novel 5-((arylfuran/1H-pyrrol-2-yl)methylene)-2-thioxo-3-(3-(trifluoromethyl)phenyl)thiazolidin-4-ones as HIV-1 fusion inhibitors targeting gp41. J. Med. Chem. 2010;54:572–579. doi: 10.1021/jm101014v. [DOI] [PubMed] [Google Scholar]
- 385.Maga G., Falchi F., Garbelli A., Belfiore A., Witvrouw M., Manetti F., Botta M. Pharmacophore modeling and molecular docking led to the discovery of inhibitors of human immunodeficiency virus-1 replication targeting the human cellular aspartic acid-glutamic acid-alanine-aspartic acid box polypeptide 3. J. Med. Chem. 2008;51:6635–6638. doi: 10.1021/jm8008844. [DOI] [PubMed] [Google Scholar]
- 386.Dayam R., Gundla R., Al-Mawsawi L.Q., Neamati N. HIV-1 integrase inhibitors: 2005-2006 update. Med. Res. Rev. 2008;28:118–154. doi: 10.1002/med.20116. [DOI] [PubMed] [Google Scholar]
- 387.Dayam R., Sanchez T., Clement O., Shoemaker R., Sei S., Neamati N. β-Diketo acid pharmacophore hypothesis. 1. Discovery of a novel class of HIV-1 integrase inhibitors. J. Med. Chem. 2005;48:111–120. doi: 10.1021/jm0496077. [DOI] [PubMed] [Google Scholar]
- 388.Lange C.M., Sarrazin C., Zeuzem S. Review article: specifically targeted anti-viral therapy for hepatitis c a new era in therapy. Aliment. Pharm. Ther. 2010;32:14–28. doi: 10.1111/j.1365-2036.2010.04317.x. [DOI] [PubMed] [Google Scholar]
- 389.Parfieniuk A., Jaroszewicz J., Flisiak R. Specifically targeted antiviral therapy for hepatitis C virus. World J. Gastroenterol. 2007;43:5673–5681. doi: 10.3748/wjg.v13.i43.5673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Thompson A.J., McHutchison J.G. Antiviral resistance and specifically targeted therapy for HCV (STAT-C) J. Viral Hepat. 2009;16:377–387. doi: 10.1111/j.1365-2893.2009.01124.x. [DOI] [PubMed] [Google Scholar]
- 391.Vermehren J., Sarrazin C. New HCV therapies on the horizon. Clin. Microbiol. Infect. 2011;17:122–134. doi: 10.1111/j.1469-0691.2010.03430.x. [DOI] [PubMed] [Google Scholar]
- 392.Küçükgüzel İ., Satılmış G., Gurukumar K.R., Basu A., Tatar E., Nichols D.B., Talele T.T., Kaushik-Basu N. 2-Heteroarylimino-5-arylidene-4-thiazolidinones as a new class of non-nucleoside inhibitors of HCV NS5B polymerase. Eur. J. Med. Chem. 2013;69:931–941. doi: 10.1016/j.ejmech.2013.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Barreca M.L., Iraci N., Manfroni G., Cecchetti V. Allosteric inhibition of the hepatitis C virus NS5B polymerase: in silico strategies for drug discovery and development. Future Med. Chem. 2011;8:1027–1055. doi: 10.4155/fmc.11.53. [DOI] [PubMed] [Google Scholar]
- 394.Yan S., Appleby T., Larson G., Wu J.Z., Hamatake R., Hong Z.Y.N. Structure-based design of a novel thiazolone scaffold as HCV NS5 B polymerase allosteric inhibitors. Bioorg. Med. Chem. Lett. 2006;16:5888–5891. doi: 10.1016/j.bmcl.2006.08.056. [DOI] [PubMed] [Google Scholar]
- 395.Yan S., Appleby T., Larson G., Wu J.Z., Hamatake R.K., Hong Z., Yao N. Thiazolone-acylsulfonamides as novel HCV NS5 B polymerase allosteric inhibitors: convergence of structure-based drug design and X-Ray crystallographic study. Bioorg. Med. Chem. Lett. 2007;17:1991–1995. doi: 10.1016/j.bmcl.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 396.Rawal R.K., Katti S.B., Kaushik-Basu N., Arora P., Pan Z. Non-nucleoside Inhibitors of the hepatitis C virus NS5B RNA-dependant RNA polymerase: 2-aryl-3-heteroaryl-1,3-thiazolidin-4-one derivatives. Bioorg. Med. Chem. Lett. 2008;18:6110–6114. doi: 10.1016/j.bmcl.2008.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Yan S., Larson G., Wu J.Z., Appleby T., Ding Y., Hamatake R., Hong Z., Yao N. Novel thiazolones as HCV NS5B polymerase allosteric inhibitors: further designs, SAR, and X-ray complex structure. Bioorg. Med. Chem. Lett. 2007;17:63–67. doi: 10.1016/j.bmcl.2006.09.095. [DOI] [PubMed] [Google Scholar]
- 398.Ding Y., Smith K.L., Varaprasad C.V., Chang E., Alexander J., Yao N. Synthesis of thiazolone-based sulfonamides as inhibitors of HCV NS5B polymerase. Bioorg. Med. Chem. Lett. 2007;17:841–845. doi: 10.1016/j.bmcl.2006.08.104. [DOI] [PubMed] [Google Scholar]
- 399.Lee G., Piper D.E., Wang Z., Anzola J., Powers J., Walker N., Li Y. Novel inhibitors of hepatitis C virus RNA-dependent RNA polymerases. J. Mol. Biol. 2006;357:1051–1057. doi: 10.1016/j.jmb.2006.01.032. [DOI] [PubMed] [Google Scholar]
- 400.Talele T.T., Arora P., Kulkarni S.S., Patel M.R., Singh S., Chudayeu M., Kaushik-Basu N. Structure-based virtual screening, synthesis and SAR of novel inhibitors of hepatitis C virus NS5B polymerase. Bioorg. Med. Chem. 2010;18:4630–4638. doi: 10.1016/j.bmc.2010.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Al-Ansary G.H., Ismail M.A., El Ella D.A.A., Eid S., Abouzid K.A. Molecular design and synthesis of HCV inhibitors based on thiazolone scaffold. Eur. J. Med. Chem. 2013;68:19–32. doi: 10.1016/j.ejmech.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 402.Sudo K., Matsumoto Y., Matsushima M., Fujiwara M., Konno K., Shimotohno K., Shigeta S., Yokota T. Novel hepatitis C virus protease inhibitors: thiazolidine derivatives. Biochem. Biophys. Res. Comm. 1997;238:643–647. doi: 10.1006/bbrc.1997.7358. [DOI] [PubMed] [Google Scholar]
- 403.Havrylyuk D., Zimenkovsky B., Vasylenko O., Lesyk R. Synthesis and anticancer and antiviral activities of new 2-pyrazoline-substituted 4-thiazolidinones. J. Heterocycl. Chem. 2013;50:55–62. [Google Scholar]
- 404.Nitsche C., Schreier V.N., Behnam M.A., Kumar A., Bartenschlager R., Klein C.D. Thiazolidinone–peptide hybrids as dengue virus protease inhibitors with antiviral activity in cell culture. J. Med. Chem. 2013;56:8389–8403. doi: 10.1021/jm400828u. [DOI] [PubMed] [Google Scholar]
- 405.Jeong T.S., Kim J.R., Kim K.S., Cho K.H., Bae K.H., Lee W.S. Inhibitory effects of multi-substituted benzylidene thiazolidine-2,4-diones on LDL oxidation. Bioorg. Med. Chem. 2004;12:4017–4023. doi: 10.1016/j.bmc.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 406.Tuncbilek M., CanEke B., Ayhan-Kilcigil G., Çoban T., Iscan M. Evaluation of the antioxidant effects of some flavonylthiazolidinediones by determining their effects on lipid peroxidation, superoxide anion formation, and 2,2-diphenyl-1-picrylhydrazyl stable free radical. Biol. Pharm. Bull. 2004;27:912–915. doi: 10.1248/bpb.27.912. [DOI] [PubMed] [Google Scholar]
- 407.Shih M.H., Ke F.Y. Syntheses and evaluation of antioxidant activity of sydnonylsubstituted thiazolidinone and thiazoline derivatives. Bioorg. Med. Chem. 2004;12:4633–4643. doi: 10.1016/j.bmc.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 408.Bozda-Dundar O., Coban T., Ceylan-Unlusoy M., Ertan R. Radical scavenging capacities of some thiazolylthiazolidine-2,4-dione derivatives. Med. Chem. Res. 2009;18:1–7. [Google Scholar]
- 409.Kim H., Choi Y.J., Moon K.M., Lee H.J., Wooc Y., Chung K.W., Jung Y., Kim S., Chun Y., Byun Y., Ha M., Moon H.R., Chung H.Y. The inhibitory effect of a synthetic compound, [Z]-5-[2,4-dihydroxybenzylidene]thiazolidine-2,4-dione [MHY498], on nitric oxide-induced melanogenesis. Bioorg. Med. Chem. Lett. 2013;23:4332–4335. doi: 10.1016/j.bmcl.2013.05.094. [DOI] [PubMed] [Google Scholar]
- 410.Maccari R., Ottana R., Ciurleo R., Vigorita M.G., Rakowitz D., Steindl T., Langer T. Evaluation of in vitro aldose redutase inhibitory activity of 5-arylidene-2,4-thiazolidinediones. Bioorg. Med. Chem. Lett. 2007;17:3886–3893. doi: 10.1016/j.bmcl.2007.04.109. [DOI] [PubMed] [Google Scholar]
- 411.Maccari R., Ottana R., Ciurleo R., Rakowitz D., Matuszczak B., Laggner C., Langer T. Synthesis, induced-fit docking investigations, and in vitro aldose reductase inhibitory activity of noncarboxylic acid containing 2,4-thiazolidinedione derivatives. Bioorg. Med. Chem. 2008;16:5840–5852. doi: 10.1016/j.bmc.2008.04.072. [DOI] [PubMed] [Google Scholar]
- 412.Maccari R., Ciurleo R., Giglio M., Cappiello M., Moschini R., Corso A.D., Mura U., Ottana R. Identification of new noncarboxylic acid containing inhibitors of aldose reductase. Bioorg. Med. Chem. 2010;18:4049–4055. doi: 10.1016/j.bmc.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 413.Ottana R., Maccari R., Giglio M., DelCorso A., Cappiello M., Mura U., Cosconati S., Marinelli L., Novellino E., Sartini S., LaMotta C., DaSettimo F. Identification of 5-arylidene-4-thiazolidinone derivatives endowed with dual activity as aldose reductase inhibitors and antioxidant agents for the treatment of diabetic complications. Eur. J. Med. Chem. 2011;46:2797–2806. doi: 10.1016/j.ejmech.2011.03.068. [DOI] [PubMed] [Google Scholar]
- 414.Halliwell B. How to characterize a biological antioxidant. Free Radic. Res. Commun. 1990;91:1–32. doi: 10.3109/10715769009148569. [DOI] [PubMed] [Google Scholar]
- 415.Bast A., Haenen G.R. Ten misconceptions about antioxidants. Trends Pharmacol. Sci. 2013;34:430–436. doi: 10.1016/j.tips.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 416.Yelisyeyeva O.P., Semen K.O., Ostrovska G.V., Kaminskyy D.V., Sirota T.V., Zarkovic N., Rybalchenko V.K., Bast A. The effect of amaranth oil on monolayers of artificial lipids and hepatocyte plasma membranes with adrenalin-induced stress. Food Chem. 2014;147:152–159. doi: 10.1016/j.foodchem.2013.09.119. [DOI] [PubMed] [Google Scholar]
- 417.Semen K.O., denHartog G.J.M., Kaminskyy D.V., Sirota T.V., Maij N.G.A.A., Yelisyeyeva O.P., Bast A. Redox modulation by amaranth oil in human lung fibroblasts. Nat. Prod. Chem. Res. 2013;2:122. [Google Scholar]
- 418.Liu Y.M., Nepali K., Liou J.P. Idiopathic pulmonary fibrosis: current status, recent progress, and emerging targets. J. Med. Chem. 2017;60:527–553. doi: 10.1021/acs.jmedchem.6b00935. [DOI] [PubMed] [Google Scholar]
- 419.Geldenhuys W.J., Darvesh A.S., Funk M.O., Van der Schyf C.J., Carroll R.T. Identification of novel monoamineoxidase B inhibitors by structure-based virtual screening. Bioorg. Med. Chem. Lett. 2010;20:5295–5298. doi: 10.1016/j.bmcl.2010.06.128. [DOI] [PubMed] [Google Scholar]
- 420.Volynets G., Bdzhola V.G., Golub A.G., Synyugin A.R., Chekanov M.A., Kukharenko O.P., Yarmoluk S.M. Rational design of apoptosis signal-regulating kinase 1 inhibitors: discovering novel structural scaffold. Eur. J. Med. Chem. 2013;61:104–115. doi: 10.1016/j.ejmech.2012.09.022. [DOI] [PubMed] [Google Scholar]
- 421.Nakamura T., Kataoka K., Fukuda M., Nako H., Tokutomi Y., Dong Y.F., Ichijo H., Ogawa H., Kim-Mitsuyama S. Critical role of apoptosis signal regulating kinase 1 in aldosterone/salt-induced cardiac inflammation and fibrosis. Hypertension. 2009;54:544–551. doi: 10.1161/HYPERTENSIONAHA.109.135392. [DOI] [PubMed] [Google Scholar]
- 422.Liao X., Zhang L., Thrasher J.B., Du J., Li B. Glycogen synthase kinase-3β Suppression eliminates tumor necrosis factor-related apoptosis-inducing ligand resistance in prostate cancer. Mol. Cancer Ther. 2003;2:1215–1222. [PubMed] [Google Scholar]
- 423.Ghosh J.C., Altieri D.C. Activation of p53-dependent apoptosis by a acute ablation of glycogen synthase kinase-3β in colorectal cancer cells. Clin. Cancer Res. 2005;11:4580–4588. doi: 10.1158/1078-0432.CCR-04-2624. [DOI] [PubMed] [Google Scholar]
- 424.Taddei A., Folli C., Zegarra-Moran O., Fanen P., Verkman A.S., Galietta L.J. Altered channel gating mechanism for CFTR inhibition by a high-affinity thiazolidinone blocker. FEBS Lett. 2004;558:52–56. doi: 10.1016/S0014-5793(04)00011-0. [DOI] [PubMed] [Google Scholar]
- 425.Kopeikin Z., Sohma Y., Li M., Hwang T.C. On the mechanism of CFTR inhibition by a thiazolidinone derivative. J. Gen. Physiol. 2010;136:6659–6671. doi: 10.1085/jgp.201010518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Ma T., Thiagarajah J.R., Yang H., Sonawane N.D., Folli C., Galietta L.J., Verkman A.S. Thiazolidinone CFTR inhibitor identified by high-throughput screening blocks cholera toxin–induced intestinal fluid secretion. J. Clin. Investig. 2002;110:1651–1658. doi: 10.1172/JCI16112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Szewczuk L.M., Saldanha S.A., Ganguly S., Bowers E.M., Javoroncov M., Karanam B., Culhane J.C., Holbert M.A., Klein D.C., Abagyan R., Cole P.A. De novo discovery of serotonin N-acetyltransferase inhibitors. J. Med. Chem. 2007;50:5330–5338. doi: 10.1021/jm0706463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Ma L., Chen J., Liang X., Xie C., Deng C., Huang L., Peng A., Wei Y., Chen L. Synthesis and evaluation of 5-benzylidenethiazolidine-2,4-dione derivatives for the treatment of non-alcoholic fatty liver disease. Arch. Pharm. 2012;345:517–524. doi: 10.1002/ardp.201100413. [DOI] [PubMed] [Google Scholar]
- 429.Neetoo-Isseljee Z., MacKenzie A.E., Southern C., Jerman J., McIver E.G., Harries N., Taylor D.L., Milligan G. High-throughput identification and characterization of novel, species-selective GPR35 agonists. J. Pharmacol. Exp. Ther. 2013;344:568–578. doi: 10.1124/jpet.112.201798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Mackenzie A.E., Lappin J.E., Taylor D.L., Nicklin S.A., Milligan G. GPR 35 as a novel therapeutic target. Front. Endocrinol. 2011;2:68. doi: 10.3389/fendo.2011.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Hu B., Malamas M., Ellingboe J., Largis E., Han S., Mulveyc R., Tillettc J. New oxadiazolidinedione derivatives as potent and selective human 3 agonists. Bioorg. Med. Chem. Lett. 2001;11:981–984. doi: 10.1016/s0960-894x(01)00147-0. [DOI] [PubMed] [Google Scholar]
- 432.Weyer C., Gautier J.F., Danforth E., Jr. Development of beta 3-adrenoceptor agonists for the treatment of obesity and diabetes - an update. Diabetes Metab. 1999;25:11–21. [PubMed] [Google Scholar]
- 433.Bursavich M.G., Gilbert A.M., Lombardi S., Georgiadis K.E., Reifenberg E., Flannery C.R., Morris E.A. Synthesis and evaluation of arylthioxothiazolidinone inhibitors of ADAMTS-5 (Aggrecanase-2) Bioorg. Med. Chem. Lett. 2007;17:1185–1188. doi: 10.1016/j.bmcl.2006.12.027. [DOI] [PubMed] [Google Scholar]
- 434.Lee G.N., Kim K.R., Ahn S.H., Bae M.A., Kang N.S. Discovery of cannabinoid-1 receptor antagonists by virtual screening. Bioorg. Med. Chem. Lett. 2010;10:5130–5132. doi: 10.1016/j.bmcl.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 435.Mishra C.B., Kumari S., Tiwari M. Thiazole: a promising heterocycle for the development of potent CNS active agents. Eur. J. Med. Chem. 2015;92:1–34. doi: 10.1016/j.ejmech.2014.12.031. [DOI] [PubMed] [Google Scholar]
- 436.Bolli M.H., Abele S., Binkert C., Bravo R., Buchmann S., Bur D., Gatfield J., Hess P., Kohl C., Mangold C., Mathys B., Menyhart K., Müller C., Nayler O., Scherz M., Schmidt G., Sippel V., Steiner B., Strasser D., Treiberand A., Mathys B. 2-Imino-thiazolidin-4-one derivatives as potent, orally active S1P1 receptor agonists. J. Med. Chem. 2010;53:4198–4211. doi: 10.1021/jm100181s. [DOI] [PubMed] [Google Scholar]
- 437.Crascì L., Vicini P., Incerti M., Cardile V., Avondo S., Panico A. 2-Benzisothiazolylimino-5-benzylidene-4-thiazolidinones as protective agents against cartilage destruction. Bioorg. Med. Chem. 2015;23:1551–1556. doi: 10.1016/j.bmc.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 438.Furdas S.D., Shekfeh S., Kannan S., Sippl W., Jung M. Rhodanine carboxylic acids as novel inhibitors of histone acetyltransferases. Med. Chem. Comm. 2012;3:305–311. [Google Scholar]
- 439.Galloway W.R., Isidro-Llobet A., Spring D.R. Diversity-oriented synthesis as a tool for the discovery of novel biologically active small molecules. Nat. Commun. 2010;1:80. doi: 10.1038/ncomms1081. [DOI] [PubMed] [Google Scholar]
- 440.Lesyk R., Zimenkovsky B., Atamanyuk D., Jensen F., Kieć-Kononowicz K., Gzella A. Anticancer thiopyrano[2,3-d][1,3]thiazol-2-ones with norbornane moiety. Synthesis, cytotoxicity, physico-chemical properties, and computational studies. Bioorg. Med. Chem. 2006;14:5230–5240. doi: 10.1016/j.bmc.2006.03.053. [DOI] [PubMed] [Google Scholar]
- 441.Atamanyuk D., Zimenkovsky B., Lesyk R. Synthesis and anticancer activity of novel thiopyrano[2,3-d]thiazole-based compounds containing norbornane moiety. J. Sulfur Chem. 2008;29:151–162. [Google Scholar]
- 442.Zelisko N., Atamanyuk D., Vasylenko O., Grellier P., Lesyk R. Synthesis and antitrypanosomal activity of new 6,6,7-trisubstituted thiopyrano[2,3-d][1,3]thiazoles. Bioorg. Med. Chem. Lett. 2012;22:7071–7074. doi: 10.1016/j.bmcl.2012.09.091. [DOI] [PubMed] [Google Scholar]
- 443.Atamanyuk D., Zimenkovsky B., Atamanyuk V., Nektegayev I., Lesyk R. Synthesis and biological activity of new thiopyrano[2,3-d]thiazoles containing a naphthoquinone moiety. Sci. Pharm. 2013;81:423–436. doi: 10.3797/scipharm.1301-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Metwally N.H. Synthesis of some new fused thiopyrano2,3-d]thiazoles and their derivatives. J. Sulf. Chem. 2007;28:275–284. [Google Scholar]
- 445.Enchev V., Chorbadjiev S., Jordanov B. Comparative study of the structure of rhodanine, isorhodanine, thiazolidine-2,4-dione, and thiorhodanine. Chem. Heterocycl. Compd. 2002;38:1110–1120. [Google Scholar]
- 446.Yu F., Hu H., Gu X., Ye J. Asymmetric michael addition of substituted rhodanines to α,β-unsaturated ketones catalyzed by bulky primary amines. Org. Lett. 2012;14:2038–2041. doi: 10.1021/ol300489q. [DOI] [PubMed] [Google Scholar]
- 447.Giles R.G., Lewis N.J., Quick J.K., Sasse M.J., Urquhart M.W., Youssef L. Regio specific reduction of 5-benzylidene-2,4-thiazolidinediones and 4-oxo-2-thiazolidinethiones using lithiumborohydride in pyridine and tetrahydrofuran. Tetrahedron. 2000;56:4531–4537. [Google Scholar]
- 448.Morzherin Y.Y., Kosterina M.F., Bezborodov A.S., Berseneva V.S., Luyten I., Dehaen W., Bakulev V.A. Selective reduction of 2,5-dimethylenethiazolidinone. Chem. Heterocycl. Com. 2000;36:113–114. [Google Scholar]
- 449.Cantello B.C.C., Gavthorue M.A., Cottom G.J., Duff P.T., Haigh D.P., Hindley R.M., Lister C.A., Smith S.A., Thurlby P.L. (ω-(Heterocyclylamino-alkoxy)benzyl)-2,4-thiazolidinediones as potent antihyperglycemic agents. J. Med. Chem. 1994;37:3977–3985. doi: 10.1021/jm00049a017. [DOI] [PubMed] [Google Scholar]
- 450.Nishida S., Maruoka H., Yoshimura Y., Goto T., Tomita R., Masumoto E., Okabe F., Yamagata K., Fujioka T. Synthesis and biological activities of some new thiazolidine derivatives containing pyrazole ring system. J. Heterocycl. Chem. 2012;49:303–309. [Google Scholar]
- 451.Villemin D., Alloum A.B. Potassium fluoride on alumina: condensation of 3-methyl-2-thiono-4-thiazolidinone with aldehydes. Synthesis of α-thioacrylic acids and phosphonothionothiazolidinones. Phosphorus Sulfur. 1993;79:33–41. [Google Scholar]
- 452.Gerencsér J., Dormán G., Darvas F. Meldrum's acid in multicomponent reactions: applications to combinatorial and diversity-oriented synthesis. QSAR Comb. Sci. 2006;25:439–448. [Google Scholar]
- 453.Yadav L.D.S., Rai V.K. Chemoselective annulation of 1,3-dithiin, -thiazine and -oxathiin rings on thiazoles using a green protocol. Tetrahedron. 2006;62:8029–8034. [Google Scholar]
- 454.Cain E.N., Warrener R.N. Preparation of 1,3-thiazines: sulphur analogues of nucleic acid pyrimidine bases. Aust. J. Chem. 1970;23:51–72. [Google Scholar]
- 455.Hiroshi N. Studies on fungicides. XXII. Reaction of dimethyl acetylenedicarboxylate with dithiocarbamates, thiolcarbamates, thiosemicarbazides and thiosemicarbazones. Chem. Pharm. Bull. 1973;21:279–286. [Google Scholar]
- 456.Hiroshi N. Studies on fungicides. XXIV. Reaction 5-methoxy-carbonylmethylidene-2-thioxo(or oxo)-4-thiazolidones with o-aminobenzenethiol and other thiols. Chem. Pharm. Bull. 1974;22:42–49. doi: 10.1248/cpb.22.42. [DOI] [PubMed] [Google Scholar]
- 457.Yadav L.D.S., Singh A. Multi-component synthesis of pyran-annulated thiazoles under solvent-free microwave irradiation. Synthesis. 2003;15:2395–2399. [Google Scholar]
- 458.Yarovenko V.N., Nikitina A.S., Zavarzin I.V., Krayushkin M.M., Kovalenko L.V. Synthesis of fused heterocyclic compounds on the basis of 2-thioxo-1,3-thiazolidin-4-ones. Rus. J. Org. Chem. 2007;43:1364–1370. [Google Scholar]
- 459.El-Shafei A.K., El-Sayed A.M., Sultan A., Abdel-Ghany H. Application of phase-transfer catalysis in reactions with some rhodanine derivatives. Gazz. Chim. Ital. 1990;120:197–203. [Google Scholar]
- 460.Naik T.R.R., Naik H.S.B., Naik H.R.P., Raghavendra M., Ramesha S. Synthesis of novel 2-seleno-1,8-naphthyridines derivatives. Phosphorus, Sulfur. 2008;183:1968–1974. [Google Scholar]
- 461.Wu W., Huang H., Yuan X., Zhu K., Ye J. Asymmetric construction of spirocyclohexanone rhodanines catalyzed by simple diamine derived from chiral tert-leucine. Chem. Commun. 2012;48:9180–9182. doi: 10.1039/c2cc34321e. [DOI] [PubMed] [Google Scholar]
- 462.Flefel E.M., Sayed H.H., Hashem A.I., Shalaby E.A., El-Sofany W., Abdel-Megeid F.M. Pharmacological evaluation of some novel synthesized compounds derived from spiro (cyclohexane-1,2′-thiazolidines) Med. Chem. Res. 2014;23:2515–2527. [Google Scholar]
- 463.Sayed H.H., Morsy E.M., Kotb E.R. Facile novel synthesis and reactions of thiazolidin-4-one derivatives for antimicrobial agents. Synt. Commun. 2010;40:2712–2722. [Google Scholar]
- 464.Dhal N., Achary T.E., Nayak A. Studies in synthesis of thiazolidinones. 2. 5-Benzal derivatives of 2-(substituted benzothiazole-2'-yl-imino)-4-thiazolidinones and their brominated products. J. Indian. Chem. Soc. 1974;10:931–933. [Google Scholar]
- 465.Rout M.K. 2-β-Nafthylamino-4-thiazolidone and its derivatives. J. Am. Chem. Soc. 1955;5:2427–2428. [Google Scholar]
- 466.Kassab N.A.L., El Nagdy M.H., Ead H.A.R. Reactions with 5-substituted 4-arylimino-2-thiazolidinones. J. Prak. Chem. 1973;315:265–273. [Google Scholar]
- 467.Mustafa A., Asker W., Shalaby A.F.A.M., Harhash A.H., Daguer R. Behaviour of the hetero-ring in substituted 2-phenylimino-4-thiazolidinones towards the action of organomagnesium compounds. Tetrahedron. 1964;20:25–31. [Google Scholar]
- 468.Kassab N.A.L., Elnagdy M.H., Messeha N.A., Ead H.A.R. Reactions with 5-substituted isorhodanines. J. Prak. Chem. 1972;314:799–805. [Google Scholar]
- 469.Mustafa A., Asker W., El-din Sobhy M.E. On the reactivity of the exocyclic double bond in 5-arylidene-3-aryl-2,4-thiazolidinediones their reaction with diazoalkanes, p-thiocresol and piperidine. J. Am. Chem. Soc. 1960;82:2597–2601. [Google Scholar]
- 470.Shvaika O.P., Artemov V.N., Baranov S.N. Reactions forming and recyclizing heterocycles. Chem. Heterocycl. Compd. 1970;6:922–927. [Google Scholar]
- 471.Dobrina V.A., Ioffe D.V. Synthesis of α-cyclopentylmandelic acid. Pharm. Chem. J. 1978;11:817–818. [Google Scholar]
- 472.Brown J.J., Newbold G.T. 1,2-Dihydro-2-thianaphthalenederivatives. Part III. Further examples of the conversion of 1,2-dihydro-1-keto-2-thianaphthalenes into indanones. J. Chem. Soc. 1952;13:4397–4403. [Google Scholar]
- 473.Dijksman D.J., Newbold G.T. 1:2-Dihydro-2-thianaphthalenederivatives. Part I. Preparation and reactions of 1:2-dihydro-1-keto-2-thianaphthalenes. J. Chem. Soc. 1951:1213–1218. [Google Scholar]
- 474.Kamal A., Robertson A., Tittensor E. Hydroxy-carbonyl compounds. Part XIV. The syntheses of some isocoumarins. J. Chem. Soc. 1950:3375–3380. [Google Scholar]
- 475.Pokhodylo N.T., Matiychuk V.S., Obushak M.D. A Convenient method for the synthesis of thiopyrano[4,3-c]quinoline, a new heterocyclic system. Chem. Heterocyc. Compd. 2009;45:121–122. [Google Scholar]
- 476.Pokhodylo N.T., Matiychuk V.S., Obushak M.D. Synthesis of isothiocoumarin derivatives. Chem. Heterocyc. Compd. 2010;46:140–145. [Google Scholar]
- 477.Kaminskyy D., Kryshchyshyn A., Nektegayev I., Vasylenko O., Grellier P., Lesyk R. Isothiocoumarin-3-carboxylic acid derivatives: synthesis, anticancer and antitrypanosomal activity evaluation. Eur. J. Med. Chem. 2014;75:57–66. doi: 10.1016/j.ejmech.2014.01.028. [DOI] [PubMed] [Google Scholar]
- 478.Eiserband G., Otteneder M., Tang W. Synthesis of N-acetyl-S-(3-coumarinyl)-cysteine and HPLC analisis of urinary coumarine metabolites. Toxicol. 2003;109:249–258. doi: 10.1016/s0300-483x(03)00204-x. [DOI] [PubMed] [Google Scholar]
- 479.Xing Q.Y., Wang W.J., He D.Y. 3-Thiolcoumarin and its derivatives. Acta Chim. Sint. 1987;45:344–353. [Google Scholar]
- 480.Artemov V.N., Baranov S.N., Kovach N.A., Shvajka O. Recyclization reactions in a series of 5-aryliden-1,3-thiazolidones under the action of hydrazines. Dokl. Acad. Nauk. SSSR. 1973;211:1369–1372. [Google Scholar]
- 481.Zapol’skii V.A., Namyslo J.C., Gjikaj M., Kaufmann D.E. Chemistry of polyhalogenated nitrobutadienes, 14: efficient synthesis of functionalized (Z)-2-allylidenethiazolidin-4-ones. Beilstein J. Org. Chem. 2014;10:1638–1644. doi: 10.3762/bjoc.10.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Slouka J., Sloukova J. Jednoduchá synthesa některých 2-thio-6-azauracilů. Acta Univ. Palack Olomoucensis. 1965;6:247–251. [Google Scholar]
- 483.Ibrahim M.A., Abdel-Hamed M.A.M., El-Gohary N.M. A new approach for the synthesis of bioactive heteroaryl thiazolidine-2,4-diones. J. Braz. Chem. Soc. 2011;22:1130–1139. [Google Scholar]
- 484.Zuhal T., Cigdem Y., Feray A., Emine B., Nuket O. Synthesis of new pyrazolothiazole derivatives from 4-thiazolidinones. Molecules. 2007;12:2151–2159. doi: 10.3390/12092151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Allah S.O.A., Hammouda H.A., Ali F.A. Heterodiene synthesis. Synthesis of fused thiopyrano[2,3-d] thiazole, benzopyrano[3′,4′:4,5]thiopyrano[2,3-d]thiazole and thiazolo[3,4-c]triazine derivative. J. Heterocycl. Chem. 1985;22:497–500. [Google Scholar]
- 486.Kryshchyshyn A., Atamanyuk D., Lesyk R. Fused thiopyrano[2,3-d]thiazole derivatives as potential anticancer agents. Sci. Pharm. 2012;80:509–529. doi: 10.3797/scipharm.1204-02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Lozynskyi A., Zimenkovsky B., Karkhut A., Polovkovych S., Gzella A.K., Lesyk R. Application of the 2(5H)furanone motif in the synthesis of new thiopyrano[2,3-d]thiazoles via the hetero-Diels–Alder reaction and related tandem processes. Tetrahedron Lett. 2016;57:3318–3321. [Google Scholar]
- 488.Zelisko N., Atamanyuk D., Ostapiuk Y., Bryhas A., Matiychuk V., Gzella A., Lesyk R. Synthesis of fused thiopyrano[2,3-d][1,3]thiazoles via hetero-Diels–Alder Reaction related tandem and domino processes. Tetrahedron. 2015;71:9501–9508. [Google Scholar]
- 489.Kowiel M., Zelisko N., Atamanyuk D., Lesyk R., Gzella A.K. 2-7-(3,5-Dibromo-2-hydroxyphenyl)-6-ethoxycarbonyl-2-oxo-5H-2,3,6,7-tetrahydrothiopyrano[2,3-d][1,3]thiazol-6-ylacetic acid ethanol monosolvate. Acta Cryst. 2012;68:2721–2722. doi: 10.1107/S1600536812035325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Zelisko N., Atamanyuk D., Vasylenko O., Bryhas A., Matiychuk V., Gzella A., Lesyk R. Crotonic, cynnamic, and propiolic acids motifs in the synthesis of thiopyrano[2,3-d][1,3]thiazoles via hetero-Diels–Alder reaction and related tandem processes. Tetrahedron. 2014;70:720–729. [Google Scholar]
- 491.Erol S., Dogan I. exo-Selective inverse-electron-demand hetero Diels–Alder reactions of norbornene with 5-benzylidine-2-arylimino-3-aryl-thiazolidine-4-thiones at room temperature. Tetrahedron. 2013;69:1337–1344. [Google Scholar]
- 492.Yarovenko V.N., Nikitina A.S., Zayakin E.S., Zavarzin I.V., Krayushkin M.M., Kovalenko L.V. 2-Thioxopyrano[2,3-d][1,3]thiazoles by Diels-Alder reaction of arylidene rhodanines under microwave irradiation. ARKIVOC. 2008;IV:103–111. [Google Scholar]
- 493.Zhu K., Huang H., Wu W., Wei Y., Ye J. Aminocatalyzed asymmetric Diels–Alder reaction of 2,4-dienals and rhodanine/hydantoin derivatives. Chem. Commun. 2013;49:2157–2159. doi: 10.1039/c3cc00023k. [DOI] [PubMed] [Google Scholar]
- 494.Liu H., Zou Y., Hu Y., Shi D.Q. An efficient one-pot synthesis of dispiropyrrolidine derivatives through 1,3-dipolar cycloaddition reactions under ultrasound irradiation. J. Heterocycl. Chem. 2011;48:877–881. [Google Scholar]
- 495.Xiaofang L., Bin L., Haochong L., Xianyong Y., Pingguia Y. Synthesis of novel spiro thiazolotriazole derivatives via 1,3-dipolar cycloaddition of azomethine ylide. J. Heterocycl. Chem. 2012;49:1050–1053. [Google Scholar]
- 496.Shvets A.A., Kurbatov S.V. New method of synthesis of β,β′-spiro pyrrolidino oxindoles. Rus. Org. Chem. 2010;46:306–308. [Google Scholar]
- 497.Komogortsev A.N., Lichitsky B.V., Dudinov A.A., Krylov K.S., Bogacheva A.M., Kobeleva O.I., Barachevskii V.A., Krayushkin M.M. Three-component condensation of iminoazolidines with aldehydes and 5-aminopyrazole. Mendeleev Commun. 2013;23:222–223. [Google Scholar]