Abstract
Background
Foot ulcers in people with diabetes are a prevalent and serious global health issue. Wound dressings are regarded as important components of ulcer treatment, with clinicians and patients having many different types to choose from including hydrocolloid dressings. There is a range of different hydrocolloids available including fibrous‐hydrocolloid and hydrocolloid (matrix) dressings. A clear and current overview of current evidence is required to facilitate decision‐making regarding dressing use.
Objectives
To compare the effects of hydrocolloid wound dressings with no dressing or alternative dressings on the healing of foot ulcers in people with diabetes.
Search methods
For this first update, in April 2013, we searched the following databases the Cochrane Wounds Group Specialised Register; The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library); Ovid MEDLINE; Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations); Ovid EMBASE; and EBSCO CINAHL. There were no restrictions based on language or date of publication.
Selection criteria
Published or unpublished randomised controlled trials (RCTs) that have compared the effects on ulcer healing of hydrocolloid with alternative wound treatments in the treatment of foot ulcers in people with diabetes.
Data collection and analysis
Two review authors independently performed study selection, risk of bias assessment and data extraction.
Main results
We included five studies (535 participants) in the review: these compared hydrocolloids with basic wound contact dressings, foam dressings, alginate dressings and a topical treatment. Meta‐analysis of two studies indicated no statistically significant difference in ulcer healing between fibrous‐hydrocolloids and basic wound contact dressings: risk ratio 1.01 (95% CI 0.74 to 1.38). One of these studies found that a basic wound contact dressing was more cost‐effective than a fibrous‐hydrocolloid dressing. One study compared a hydrocolloid‐matrix dressing with a foam dressing and found no statistically significant difference in the number of ulcers healed. There was no statistically significant difference in healing between an antimicrobial (silver) fibrous‐hydrocolloid dressing and standard alginate dressing; an antimicrobial dressing (iodine‐impregnated) and a standard fibrous hydrocolloid dressing or a standard fibrous hydrocolloid dressing and a topical cream containing plant extracts.
Authors' conclusions
Currently there is no research evidence to suggest that any type of hydrocolloid wound dressing is more effective in healing diabetic foot ulcers than other types of dressing or a topical cream containing plant extracts. Decision makers may wish to consider aspects such as dressing cost and the wound management properties offered by each dressing type e.g. exudate management.
Keywords: Humans; Bandages, Hydrocolloid; Wound Healing; Amputation, Surgical; Diabetic Foot; Diabetic Foot/therapy; Randomized Controlled Trials as Topic
Plain language summary
Hydrocolloid dressings to promote foot ulcer healing in people with diabetes when compared with other dressing types
Diabetes, a condition which leads to high blood glucose concentrations, is a common condition with around 2.8 million people affected in the UK (approximately 4.3% of the population). Dressings are commonly used to treat foot ulcers in people with diabetes. There are many types of dressings that can be used, which also vary considerably in cost.This review (four studies involving a total of 511 participants) identified no research evidence to suggest that any type of hydrocolloid wound dressing is more effective in healing diabetic foot ulcers than other types of dressing.
Summary of findings
Summary of findings for the main comparison. Fibrous‐hydrocolloid (hydrofibre) dressing compared to basic wound contact dressing for healing diabetic foot ulcers.
| Fibrous‐hydrocolloid (hydrofibre) dressing compared to basic wound contact dressing for healing diabetic foot ulcers | ||||||
| Patient or population: patients with healing diabetic foot ulcers Settings: Any Intervention: Fibrous‐hydrocolloid (hydrofibre) dressing Comparison: basic wound contact dressing | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Basic wound contact dressing | Fibrous‐hydrocolloid (hydrofibre) dressing | |||||
| Number of ulcers healed | Low1 | RR 1.01 (0.74 to 1.38) | 229 (2 studies) | ⊕⊕⊕⊝ moderate2 | ||
| 340 per 1000 | 343 per 1000 (252 to 469) | |||||
| Moderate1 | ||||||
| 530 per 1000 | 535 per 1000 (392 to 731) | |||||
| High1 | ||||||
| 650 per 1000 | 657 per 1000 (481 to 897) | |||||
| HRQoL | See comment | See comment | Not estimable | 0 (0) | See comment | One study measured HRQoL at 24 weeks follow‐up. Data from several domains are presented in the report, with no statistically significant difference observed. |
| Adverse events | See comment | See comment | Not estimable | 0 (0) | See comment | AEs for two studies ‐ very similar numbers in each arms. Data not analysed here as not independent ‐ that is one person could have multiple events or due to limited data. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Baseline risk of healing obtained from external source in which data from 27,630 patients with a diabetic neuropathic foot ulcer was used to develop a simple prognostic model to predict likelihood of ulcer healing (Margolis DJ, Allen‐Taylor L, Hoffstad O, Berlin JA. Diabetic neuropathic foot ulcers: predicting which ones will not heal. Am J Med. 2003;115:627‐31). It is important to note that given an outcome of ulcer healing, low risk refers to a low risk of healing and thus reflects the most severe patient populations. Conversely high risk refers to a high risk of healing. 2 108 participants achieved the endpoint of healing in the two studies, this is an underpowered comparison. The confidence interval around the estimate of relative risk is consistent with a 26% relative reduction in healing with hydrocolloid and a 38% relative increase in healing with hydrocolloid.
Background
Description of the condition
Diabetes, a condition which leads to high blood glucose concentrations is common and affects around 2.8 million people in the UK (approximately 4.3% of the population) (Diabetes UK 2011). This number is set to increase over the next 25 years as the incidence of diabetes increases rapidly (WHO 2005). Global projections suggest that the worldwide prevalence of diabetes is expected to rise to 4.4% by 2030, meaning that approximately 366 million people will be affected (Wild 2004).
Success in treating people with diabetes has improved their life expectancy. However, the increased prevalence of diabetes coupled with the extended time people live with the disease has led to a rise in the number of diabetes‐related complications, such as neuropathy and peripheral arterial disease (PAD). It is estimated that lower extremity disease (defined as lower‐extremity PAD, lower‐extremity peripheral neuropathy or history of foot ulcer or lower‐extremity amputations) is twice as common in people with diabetes compared with people without (Gregg 2004). Both neuropathy and PAD are risk factors for diabetic foot ulceration (Pecoraro 1990; Reiber 1999), which is a problem reported to affect 15% or more of the diabetic population at some time in their lives (Reiber 1996; Singh 2005). Around 1% to 4% of people with diabetes have foot ulcers at any given time (Abbott 2002; Kumar 1994). An ulcer forms as a result of damage to the epidermis and subsequent loss of underlying tissue. Specifically, the International Consensus on the Diabetic Foot defines a foot ulcer as a wound extending through the full thickness of the skin below the level of the ankle (Apelqvist 2000a). This is irrespective of duration and the ulcer can extend to muscle, tendon and bone. The Wagner wound classification system is well established and widely used for grading diabetic foot ulcers. The system assesses ulcer depth and the presence of osteomyelitis or gangrene in the following grades: grade 0 (pre‐ or post‐ulcerative lesion), grade 1 (partial/full thickness ulcer), grade 2 (probing to tendon or capsule), grade 3 (deep with osteitis), grade 4 (partial foot gangrene) and grade 5 (whole foot gangrene) (Wagner 1981). However, newer grading systems, such as the PEDIS system (Schaper 2004) and the University of Texas Wound Classification System (Oyibo 2001) have been developed.
PAD and neuropathy can occur separately (ischaemic foot and neuropathic foot) or in combination (in the neuroischaemic foot). The over‐arching term 'diabetic neuropathy' refers to a number of neuropathic syndromes. Chronic distal sensorimotor symmetrical neuropathy (abbreviated to distal symmetrical neuropathy) is the most common, affecting around 28% of people with diabetes. It can lead to ulceration through the following route(s) (Tesfaye 1996).
Sympathetic autonomic neuropathy leads to decreased sweating causing anhidrotic (dry) skin, which is prone to cracks and fissures causing a break in the dermal barrier (Tesfaye 1996).
Motor neuropathy causes wasting of the small, intrinsic muscles of the foot by de‐enervation. As the muscles waste they cause retraction of the toes and lead to a subsequent deformity. The abnormal foot shape can promote ulcer development due to an increase in plantar pressures (Murray 1996).
Sensory neuropathy results in impaired sensation, making the patient unaware of potentially dangerous foreign bodies and injuries.
People with diabetes‐related foot ulceration are treated in a variety of settings, for example community clinics, surgeries and their own homes, by a variety of practitioners; this can make data collection challenging. A UK study estimated that 2% of community‐based diabetic patients develop new foot ulcers each year (Abbott 2002). In terms of healing, a meta‐analysis of trials in which people with neuropathic ulcers received good wound care reported that 24% of ulcers attained complete healing by 12 weeks and 31% by 20 weeks (Margolis 1999). However, the risk of ulcer recurrence post‐healing is high. Pound 2005 reported that 62% of ulcer patients (n = 231) became ulcer‐free at some stage over a 31‐month observation period. However, of the ulcer‐free group 40% went on to develop a new or recurrent ulcer after a median of 126 days. The ulcer recurrence rate over five years can be as high as 70% (Dorresteijn 2010; Van Gils 1999).
Diabetic foot ulcers can seriously impact on an individual's quality of life and as many as 85% of foot‐related amputations are preceded by ulceration (Apelqvist 2000b; Pecoraro 1990). Patients with diabetes have a 10 to 20‐fold higher risk of losing a lower limb or part of a lower limb due to non‐traumatic amputation than those without diabetes (Morris 1998; Wrobel 2001).
Diabetic foot ulcers represent a major use of health resources, incurring costs not only for dressings applied, but also staff costs (for podiatry, nurses, doctors), tests and investigations, antibiotics and specialist footwear. Currie 1998 estimated the cost of healing a foot ulcer in a patient with diabetes at around GBP 1451. Hospital admissions add further to the costs. Ten years ago the cost of diabetic foot ulceration to the UK National Health Service was believed to be about GBP 12.9 million per year (Spencer 2000) and this figure is likely to have increased significantly. The economic impact is also high in terms of the personal costs to patients and carers, for example costs associated with lost work time and productivity while the patient is non‐weight bearing or hospitalised.
Description of the intervention
Broadly, the treatment of diabetic foot ulcers includes pressure relief (or off‐loading) by resting the foot or wearing special footwear or shoe inserts (or both); the removal of dead cellular material from the surface of the wound (debridement or desloughing)(Edwards 2010); infection control (Storm‐Versloot 2007); and the use of wound dressings (Bergin 2006; Dumville 2011a; Dumville 2011b; Dumville 2012). Other general strategies in the treatment of diabetic foot ulcers include: patient education (Dorresteijn 2010; Dorresteijn 2001); optimisation of blood glucose control; correction (where possible) of arterial insufficiency; and surgical interventions (debridement, drainage of pus, revascularisation, amputation).
Dressings are widely used in wound care, both to protect the wound and to promote healing. Classification of a dressing normally depends on the key material used. Several attributes of an ideal wound dressing have been described (BNF 2010), including:
the ability of the dressing to absorb and contain exudate without leakage or strike‐through;
lack of particulate contaminants left in the wound by the dressing;
thermal insulation;
permeability to water and bacteria;
avoidance of wound trauma on dressing removal;
frequency with which the dressing needs to be changed;
provision of pain relief; and
comfort.
There is a vast choice of dressings available to treat chronic wounds such as diabetic foot ulcers. For ease of comparison this review has categorised dressings according to the British National Formulary 2010 (BNF 2010) which is freely available via the Internet, although there are alternative classifications. We will use 'generic' names where possible, also providing UK trade names and manufacturers where these are available to allow cross‐referencing with the BNF. However, it is important to note that the way dressings are categorised as well as dressing names, manufacturers and distributors may vary from country to country, so these are provided as a guide only. Below is a description of all categories of dressings and includes the category of dressing (hydrocolloid) which is the focus of this review:
Basic wound contact dressings
Low‐adherence dressings and wound contact materials: usually cotton pads which are placed directly in contact with the wound. They can be either non‐medicated (e.g. paraffin gauze dressing) or medicated (e.g. containing povidone iodine or chlorhexidine). Examples are paraffin gauze dressing, BP 1993 and Xeroform (Covidien) dressing ‐ a non‐adherent petrolatum blend with 3% bismuth tribromophenate on fine mesh gauze.
Absorbent dressings: applied directly to the wound or used as secondary absorbent layers in the management of heavily exuding wounds. Examples include Primapore (Smith & Nephew), Mepore (Mölnlycke) and absorbent cotton gauze (BP 1988).
Advanced wound dressings
Hydrocolloid dressings: are occlusive dressings usually composed of a hydrocolloid matrix bonded onto a vapour‐permeable film or foam backing. When in contact with the wound surface this matrix forms a gel to provide a moist environment. Examples are: Granuflex (ConvaTec) and Duoderm (Smith and Nephew). Fibrous hydrocolloids have been developed which resemble alginates and are not occlusive but which are more absorbant than standard hydrocolloid dressings: Aquacel (ConvaTec).
Hydrogel dressings: consist of a cross‐linked insoluable polymers (i.e. starch or carboxymethylcellulose) and up to 96% water. These dressings are designed to absorb wound exudate or rehydrate a wound depending on the wound moisture levels. They are supplied in either flat sheets, an amorphous hydrogel or as beads. Examples are: ActiformCool (Activa) and Aquaflo (Covidien)
Films ‐ permeable film and membrane dressings: permeable to water vapour and oxygen but not to water or microorganisms. Examples are Tegaderm (3M) and Opsite (Smith & Nephew).
Soft polymer dressings: dressings composed of a soft silicone polymer held in a non‐adherent layer. They are moderately absorbent. Examples are: Mepitel (Mölnlycke) and Urgotul (Urgo).
Foam dressings: normally contain hydrophilic polyurethane foam and are designed to absorb wound exudate and maintain moist wound surface. There are various versions and some foam dressings that include additional absorbent materials, such as viscose and acrylate fibres or particles of superabsorbent polyacrylate, or which are silicone‐coated for non‐traumatic removal. Examples are: Allevyn (Smith & Nephew), Biatain (Coloplast) and Tegaderm (3M).
Alginate dressings: highly absorbent and come in the form of calcium alginate or calcium sodium alginate and can be combined with collagen. The alginate forms a gel when in contact with the wound surface which can be lifted off with dressing removal or rinsed away with sterile saline. Bonding to a secondary viscose pad increases absorbency. Examples are: Curasorb (Covidien), SeaSorb (Coloplast) and Sorbsan (Unomedical).
Capillary‐action dressings: consist of an absorbent core of hydrophilic fibres held between two low‐adherent contact layers. Examples are: Advadraw (Advancis) and Vacutx (Protex).
Odour‐absorbent dressings: dressings that contain charcoal and are used to absorb wound odour. Often these types of wound dressings are used in conjunction with a secondary dressing to improve absorbency. Example: CarboFLEX (ConvaTec).
Antimicrobial dressings
Honey‐impregnated dressings: contain medical‐grade honey which is proposed to have antimicrobial and anti‐inflammatory properties and can be used for acute or chronic wounds. Examples are: Medihoney (Medihoney) and Activon Tulle (Advancis).
Iodine‐impregnated dressings: release free iodine when exposed to wound exudate, which is thought to act as a wound antiseptic. Examples are Iodoflex (Smith & Nephew) and Iodozyme (Insense).
Silver‐impregnated dressings: used to treat infected wounds as silver ions are thought to have antimicrobial properties. Silver versions of most dressing types are available (e.g. silver foam, silver hydrocolloid etc). Examples are: Acticoat (Smith & Nephew) and Urgosorb Silver (Urgo).
Other antimicrobial dressings: these dressings are composed of a gauze or low‐adherent dressing impregnated with an ointment thought to have antimicrobial properties. Examples are: chlorhexidine gauze dressing (Smith & Nephew).
Specialist dressings
Protease‐modulating matrix dressings: alter the activity of proteolytic enzymes in chronic wounds. Examples are: Promogran (Systagenix) and Sorbion (H & R).
The diversity of dressings available to clinicians (including variation within each type listed above) makes evidence‐based decision‐making difficult when deciding the best treatment regimen for the patient. In a UK survey undertaken to determine treatments used for debriding diabetic foot ulcers, a diversity of treatments was reported (Smith 2003). It is possible that a similar scenario is true for dressing choice. A survey of Diabetes Specialist Nurses found that low/non‐adherent dressings, hydrocolloids and alginate dressings were the most popular for all wound types, despite a paucity of evidence for either of these dressing types (Fiskin 1996). However, several new dressing types have been made available and heavily promoted in recent years. Some dressings now have an 'active' ingredient such as silver that are promoted as dressing treatment options to reduce infection and thus possibly also promote healing in this way. With increasingly sophisticated technology being applied to wound care, practitioners need to know how effective these often expensive dressings are compared with more traditional dressings.
How the intervention might work
Animal experiments conducted over 40 years ago suggest that acute wounds heal more quickly when their surface is kept moist, rather than left to dry and scab (Winter 1963). A moist environment is thought to provide optimal conditions for the cells involved in the healing process as well as allowing autolytic debridement, which is thought to be an important part of the healing pathway (Cardinal 2009). The desire to maintain a moist wound environment is a key driver for the use of wound dressings. Different wound dressings vary in their level of absorbency so that a very wet wound can be treated with an absorbent dressing (such as a foam dressing) to draw excess moisture away from the wound to avoid skin damage, whilst a drier wound can be treated with a more occlusive dressing to maintain a moist environment. Hydrocolloid dressings are composed of a layer of sodium carboxymethylcellulose (or similar material which forms a gel when wet) bounded onto a vapour‐permeable film or foam pad. When in contact with the wound dressings form a gel whilst maintaining a moist wound environment. Fibrous‐hydrocolloids are a sub‐set of dressings that are designed for use in wounds with heavy exudate in lieu of alternate dressing types such as alginates.
Why it is important to do this review
Diabetic foot ulcers are a common consequence of diabetes internationally. Treatment with dressings forms a key part of the treatment pathway when caring for people with diabetic foot ulcers and there are many types of dressings that can be used, which also vary considerably in cost. Guidelines for the treatment of diabetic ulcer (e.g. Steed 2006) maintain that clinical judgement should be used to select a moist wound dressing.
However, previous reviews of the evidence for wound dressings as treatments for diabetic foot ulcers have not found evidence to support a specific dressing choice. Ten trials were eligible for inclusion in a UK Health Technology Assessment review of wound dressings published in 2000 (O'Meara 2000). The review included nine trials that investigated a dressing or topical treatment for healing diabetic foot ulcers. The review did not find any evidence to suggest that one dressing type was more or less effective in terms of treating diabetic foot ulcers. The methodological quality of trials was poor and all were small. Only one comparison was repeated in more than one trial. A further systematic review, conducted some years ago reported similar findings (Mason 1999). A more recent systematic review on the effectiveness of interventions to enhance the healing of chronic ulcers of the foot (Hinchliffe 2008) (search date December 2006) included only eight trials (randomised and non‐randomised) did not identify any evidence that one dressing type was superior to another in terms of promoting ulcer healing. A Cochrane review of silver‐based wound dressings and topical agents for treating diabetic foot ulcers (Bergin 2006; search date 2010) did not find any studies that met its inclusion criteria. Finally, a review of antimicrobial treatments for diabetic foot ulcers (Nelson 2006) included dressings and found that existing evidence was too weak to recommend any antimicrobial product.
This review is part of a suite of Cochrane Reviews investigating the use of dressings in the treatment of foot ulcers in people with diabetes. Each review will focus on a particular dressing type which in this review is the hydrocolloid dressing. These reviews will be summarised in an overview of reviews (Becker 2011) which will draw together all existing Cochrane review evidence regarding the use of dressings to treat foot ulcers in people with diabetes. Whilst other existing review evidence may also be included in this overview, following Cochrane guidance, this will only occur in the absence of a relevant Cochrane intervention review (Becker 2011).
Objectives
To compare the effects of all types of hydrocolloid wound dressings with no dressing or alternative dressings on the healing of foot ulcers in people with diabetes.
Methods
Criteria for considering studies for this review
Types of studies
Published or unpublished randomised controlled trials (RCTs) that evaluate the effects of any type of hydrocolloid wound dressing in the treatment of diabetic foot ulcers, irrespective of publication status or language.
Types of participants
Trials recruiting people with Type I or Type II diabetes, with an open foot ulcer. Since study‐specific classifications of ulcer diagnosis were likely to be too restrictive, we accepted study authors' definitions of what was classed a diabetic foot ulcer. There was no restriction in relation to the aetiology of the ulcer; trials recruiting people with ulcers of neuropathic, ischaemic or neuroischaemic causes were all eligible for inclusion.
We included trials involving participants of any age. We excluded trials which included patients with a number of different wound aetiologies in addition to diabetic foot ulcers (e.g. pressure ulcers, mixed arterial/venous arterial) unless the results for the subgroup of patients with a diabetic foot ulcer were reported separately or available from authors on contact.
Types of interventions
The primary intervention was all types of hydrocolloid wound dressings (BNF 2010). We included any RCT in which the presence or absence of a hydrocolloid dressing was the only systematic difference between treatment groups. We anticipated that likely comparisons would include hydrocolloid dressings compared with other dressing types and/or other interventions (which could be non‐dressing treatments, i.e. topical applications). We did not consider differences in timings of applications to be an issue thus where dressings or creams were applied at different frequencies e.g. once a day in one trial arm and twice a day in the other arm ‐ studies were still included.
Types of outcome measures
Primary outcomes
Time to ulcer healing.
Number of ulcers completely healed within a specific time period (we assumed that the period of time in which healing occurred was the duration of the trial unless otherwise stated).
Secondary outcomes
Health‐related quality of life (measured using a standardised generic questionnaire such as EQ‐5D, SF‐36, SF‐12 or SF‐6 or disease‐specific questionnaire). We did not include ad‐hoc measures of quality of life which are likely not to be validated and will not be common to multiple trials.
Number and level of amputations.
Adverse events, including infection and pain (measured using survey/questionnaire/data capture process or visual analogue scale).
Cost (including measurements of resource use such as number of dressing changes and nurse time).
Ulcer recurrence.
Change in ulcer area expressed as absolute changes (e.g. surface area changes in cm2 since baseline) or relative changes (e.g. percentage change in area relative to baseline).
Search methods for identification of studies
For the search methods used in the original version of this review see Appendix 1
Electronic searches
For this first update we searched the following databases in April 2013:
The Cochrane Wounds Group Specialised Register (searched 11 April 2013);
The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2013, Issue 3);
Ovid MEDLINE (1950 to March Week 4 2013);
Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations, April 10, 2013);
Ovid EMBASE (1980 to 2013 April 05);
EBSCO CINAHL (1982 to 4 April 2013).
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) using the following exploded MeSH headings and keywords:
#1 MeSH descriptor Occlusive Dressings explode all trees #2 MeSH descriptor Biological Dressings explode all trees #3 MeSH descriptor Alginates explode all trees #4 MeSH descriptor Hydrogels explode all trees #5 MeSH descriptor Silver explode all trees #6 MeSH descriptor Honey explode all trees #7 (dressing* or alginate* or hydrogel* or "foam" or "bead" or "film" or "films" or tulle or gauze or non‐adherent or "non adherent" or silver or honey or matrix):ti,ab,kw #8 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7) #9 MeSH descriptor Foot Ulcer explode all trees #10 MeSH descriptor Diabetic Foot explode all trees #11 diabet* NEAR/3 ulcer*:ti,ab,kw #12 diabet* NEAR/3 (foot or feet):ti,ab,kw #13 diabet* NEAR/3 wound*:ti,ab,kw #14 (#9 OR #10 OR #11 OR #12 OR #13) #15 (#8 AND #14)
The search strategies used in Ovid MEDLINE, Ovid EMBASE and EBSCO CINAHL can be found in Appendix 2, Appendix 3 and Appendix 4 respectively. We combined the Ovid MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision) (Lefebvre 2011). We also combined the EMBASE and CINAHL searches with the trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN 2011). There were no restrictions on the basis of date or language of publication.
Searching other resources
In the original version of this review we attempted to contact researchers to obtain any unpublished data when needed. We also searched the reference lists of the included studies and previous systematic reviews. We contacted appropriate manufacturers (Smith & Nephew, Convatec Ltd, Mölnlycke Health Care, 3M Healthcare, Coloplast Ltd) for details of any unpublished studies.
Data collection and analysis
Selection of studies
Two review authors independently assessed the titles and abstracts of retrieved studies for relevance. After this initial assessment, we obtained all studies felt to be potentially relevant, in full. Two review authors then independently checked the full papers for eligibility, with disagreements resolved by discussion and, where required, the input of a third review author. We recorded all reasons for exclusion.
Data extraction and management
We extracted and summarised details of the eligible studies using a data extraction sheet. Two review authors extracted data independently and resolved disagreements by discussion. Where data were missing from reports we attempted to contact the study authors to obtain the missing information. We included studies published in duplicate once but maximally extracted data. We extracted the following data:
country of origin;
type of ulcer;
unit of investigation (per patient) ‐ single ulcer or foot or patient or multiple ulcers on the same patient;
care setting;
number of participants randomised to each trial arm;
eligibility criteria and key baseline participant data;
details of the dressing/treatment regimen received by each group;
details of any co‐interventions;
primary and secondary outcome(s) (with definitions);
outcome data for primary and secondary outcomes (by group);
duration of follow up;
number of withdrawals (by group);
adverse events, including amputation; and
source of funding.
Assessment of risk of bias in included studies
Two review authors independently assessed each included study using the Cochrane Collaboration tool for assessing risk of bias (Higgins 2011). This tool addresses six specific domains, namely sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and other issues (e.g. extreme baseline imbalance, issues with unit of investigation) (see Appendix 5 for details of the criteria on which the judgement was based). We assessed blinding and completeness of outcome data for each outcome separately. We completed a 'Risk of bias' table for each eligible study. We resolved disagreements about risk of bias assessment by discussion. Where a lack of reported information resulted in an unclear decision, where possible we contacted authors for clarification.
We have presented our assessment of risk of bias findings using a 'Risk of bias' summary figure, which presents all of the judgements in a cross‐tabulation of study by entry. This display of internal validity indicates the weight the reader may give the results of each study. We also aimed to present this assessment in the narrative review.
We classified trials as being at high risk of bias if they are rated 'high' for any of three key criteria (randomisation sequence, allocation concealment and blinded outcome assessment).
Measures of treatment effect
Where possible, we present the outcome results for each trial with 95% confidence intervals (CI). We report estimates for dichotomous outcomes (e.g. ulcers healed during time period) as risk ratio (RR). We used the RR rather than odds ratio (OR), since ORs (when interpreted as RR) can give an inflated impression of the effect size when event rates are high, as is the case for many trials reporting healing of chronic wounds (Deeks 2002). We planned to report outcomes relating to continuous data (e.g. percentage change in ulcer area) as mean difference (MD) and overall effect size (with 95% CI calculated). Where a study reported time to healing data (the probability of healing over a consecutive time period) we planned to report and plot these data (where possible) using hazard ratio estimates. If studies reporting time‐to‐event data (e.g. time to healing) did not report a hazard ratio or reported these data incorrectly as a continuous variable then, where feasible, we planned to estimate this using other reported outcomes such as the numbers of events through the application of available statistical methods (Tierney 2007).
Unit of analysis issues
We recorded whether trials measured outcomes in relation to an ulcer, a foot, a participant or whether multiple ulcers on the same participant were studied. We also recorded where multiple ulcers on a participant had been (incorrectly) treated as independent in a study, rather than within‐patient analysis methods being applied. We have recorded this as part of the risk of bias assessment. Unless otherwise stated, where the number of wounds appeared to equal the number of participants we treated the ulcer as the unit of analysis in this review.
Dealing with missing data
Missing data are common in trial reports. Excluding participants post‐randomisation from the analysis or ignoring those participants lost to follow up can, in effect, compromise the process of randomisation and thus potentially introduce bias into the trial. In individual studies, where "proportion of ulcers healed" data were presented, we assumed that where randomised participants were not included in an analysis, their wound did not heal (that is, they will be considered in the denominator but not the numerator). Where a trial did not specify participant group numbers prior to dropout, we planned to present only complete case data. We planned to present data for time to healing, area change and for all secondary outcomes as a complete case analysis.
Assessment of heterogeneity
We considered both clinical and statistical heterogeneity. Wherever appropriate, we pooled data using meta‐analysis (conducted using RevMan 5.1 (RevMan 2011)), that is where studies appeared similar in terms of level of participants, intervention type and duration and outcome type. We assessed statistical heterogeneity using the Chi² test (a significance level of P < 0.1 was considered to indicate heterogeneity) and the I² statistic (Higgins 2003). The I² statistic examines the percentage of total variation across studies due to heterogeneity rather than to chance. Values of I² over 50% indicate a high level of heterogeneity. In the absence of clinical heterogeneity and in the presence of statistical heterogeneity (I² over 50%), we used a random‐effects model. However, we did not pool studies where heterogeneity was substantial (I² over 75%). Where there was no clinical or statistical heterogeneity we envisaged using a fixed‐effect model.
Data synthesis
We combined studies using a narrative overview with meta‐analyses of outcome data where appropriate (in RevMan 5.1). The decision to include studies in a meta‐analysis depended on the availability of treatment effect data and assessment of heterogeneity. For time‐to‐event data, we planned to plot log rank observed minus expected events estimates using a fixed‐effect model (a random‐effects model for time to event data is not available for this analysis in RevMan 5.1). Where relevant and possible we planned to conduct sensitivity analysis to investigate the potential impact of studies at high risk of bias on pooled results.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies.
Results of the search
The systematic search yielded 346 abstracts which we screened for potential inclusion in the review. Of these, we obtained 103 reports (for 84 studies) for a more detailed assessment and four studies were eligible for inclusion in the review. No eligible studies were obtained from the five commercial companies that were contacted. The update search conducted in April 2013 yielded 116 citations of which two studies were obtained for further information (Turns 2012) (excluded) and Kuo 2012 (included).
One study is awaiting translation from Turkish (Ogce 2007). We are not aware of any relevant ongoing studies (checked ISRCTN register 25 April 2013).
Included studies
Five studies (535 participants) were included in this review (Clever 1995; Jeffcoate 2009; Jude 2007;Kuo 2012; Piaggesi 2001). The dressings evaluated are detailed in Table 2 (three trials evaluated a fibrous hydrocolloid dressing, one a hydrocolloid‐matrix dressing and one a silver fibrous‐hydrocolloid dressing). Two studies were single‐centred (Kuo 2012; Piaggesi 2001), two were multi‐centred (Jeffcoate 2009; Jude 2007) and the remaining study did not detail the number of centres (Clever 1995). One study was undertaken in the UK (Jeffcoate 2009); one in Germany (Clever 1995); one in Italy (Piaggesi 2001); one in Taiwan (Kuo 2012) and one study was multi‐national, taking place in Italy, France, Germany and Sweden (Jude 2007).
1. Summary of studies.
| First author | Group A | Group B | Group C | Duration of follow up | % healed data |
| Clever 1995 | Hydrocolloid (polyurethane matrix) dressing (Cutinova Hydro, S&N Hlth) | Foam dressing (Allevyn, S&N Hlth) | 16 weeks | yes | |
| Jeffcoate 2009 | Fibrous‐hydrocolloid (hydrofibre) dressing (Aquacel, ConvaTec) | Iodine‐impregnated dressing (Inadine, Johnson & Johnson) | Non‐adherent dressing (Johnson & Johnson) | 24 weeks | yes |
| Jude 2007 | Fibrous‐hydrocolloid (hydrofibre) dressing with 1.2% ionic silver (Aquacel Ag, ConvaTec) | Calcium‐alginate dressing (Algosteril, S&N Hlth) | 8 weeks | yes | |
| Kuo 2012 | Fibrous‐hydrocolloid (hydrofibre) dressing (Aquacel, ConvaTec) | Cream contained extracts from two botanical raw materials, P. amboinicus and C. asiatica.(Active ingredient 1.25%) | 2 weeks | No | |
| Piaggesi 2001 | Fibrous‐hydrocolloid (Hydrofibre) dressing (Aquacel, ConvaTec) | Saline‐moistened gauze | Not reported (maximum follow up was 350 days) | yes |
All studies were undertaken in adults with diabetes, three studies specified that they included people with both Type 1 and Type 2 diabetes (Jeffcoate 2009;Kuo 2012Piaggesi 2001). One study specified that it included only people with Wagner grade 1 or 2 ulcers (Jude 2007) and all four studies specified that they only included participants with ulcers that were neuropathic or neuroischaemic in origin and/or specified that participants had to have an ankle brachial pressure index (ABPI) above a certain value (Clever 1995; Jeffcoate 2009; Jude 2007; Piaggesi 2001). Three studies excluded participants that had infected, sloughy or deep ulcers (Clever 1995; Jeffcoate 2009; Piaggesi 2001). In general it seems that studies aimed to include participants with relatively non‐complex diabetic foot ulcers although Kuo 2012 only included ulcers that were classified as Wagner stage 3. The duration of trial follow up ranged from two weeks (Kuo 2012) to approximately 350 days (Piaggesi 2001); full details are presented in Table 2. Of the five included studies, four were two‐arm and one was three‐armed (Jeffcoate 2009). For this three‐armed trial, as each study group received a different intervention all relevant comparisons were included. Four studies reported the number of ulcers healed: only Kuo 2012 did not: the study had only two weeks follow up and after this time all ulcers were either skin grafted or closed surgically. Mean time to healing was reported in three studies (Clever 1995; Jeffcoate 2009; Piaggesi 2001), however, the use of mean vales can result in biased estimates since to calculate mean time to healing either all participants must have healed and/or assumptions need to be made about the shape of the survival curve. The more appropriate summary measure, median time to healing, was reported for one study only (Clever 1995). The reporting of secondary outcomes was limited. Adverse event reporting appeared systematic in three studies: Jeffcoate 2009; Jude 2007 and Kuo 2012. Only one study conducted a robust economic evaluation (Jeffcoate 2009).
Excluded studies
We excluded 80 studies from the review. The main reasons for exclusion were: the participants in the study were not randomised (n = 10), no single, identifiable dressing type was evaluated (n = 11); another intervention, not a dressing, differed between study groups (n = 27); the dressing(s) evaluated were not hydrocolloid (n = 26). Another reason was recorded for six studies.
Risk of bias in included studies
We classified studies rated 'High Risk' for any of three key domains: randomisation sequence, allocation concealment and blinded outcome assessment, as being at high risk of bias. (Characteristics of included studies; Figure 1; Figure 2). One study (Jeffcoate 2009) was regarded as being at low risk of bias for the three key domains. The remaining three studies were rated unclear for one or more key domains and hence we could not confidently judge them to be at high or low risk of bias.
1.
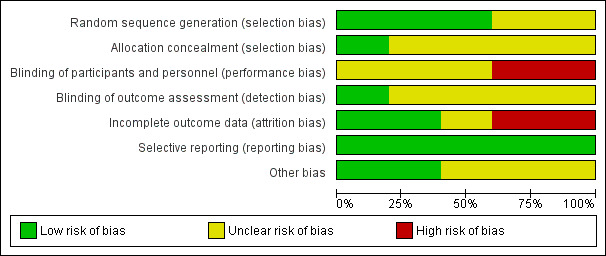
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.
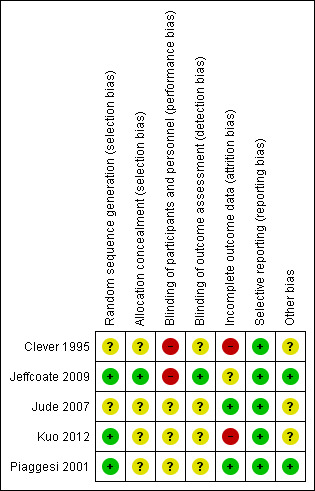
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Adequacy of randomisation process
All five studies were described as "randomised" with three reporting the method used to generate randomisation sequence and hence judged to be at low risk of bias for this domain (Jeffcoate 2009; Kuo 2012; Piaggesi 2001). Piaggesi 2001 reported the use of computer‐generated randomisation; Jeffcoate 2009 used a randomisation sequence created using SPSS (SPSS Inc., Version 14). Whilst Jude 2007 reported use of sealed envelopes the method of randomisation sequence generation remained unclear. The randomisation method was not reported in the remaining study (Clever 1995).
Allocation concealment
Jeffcoate 2009 utilised central office allocation and was the only included study to describe allocation concealment adequately. Jude 2007 reported the use of sealed envelopes for allocation, however, it was unclear if these envelopes were sequentially numbered and opaque. Likewise, the remaining studies did not clearly report the allocation concealment procedure such that we could assess the degree of concealment.
Blinding
Assessment of wound healing can be subjective and thus has the potential to be influenced if the outcome assessor is aware of the treatment allocation. In this review we focused on whether included studies had conducted blinded outcome assessment. One study (Jeffcoate 2009) reported adequate blinding of the outcome assessors and hence was judged to be at low risk of bias for this domain; the remaining four studies were judged to be at unclear risk of bias. Piaggesi 2001 reported blinding of outcome assessment for some trial outcomes, however, it was unclear if this included ulcer healing data and in Jude 2007; Kuo 2012 and Clever 1995 the blinding of outcome assessment was not explicitly mentioned.
Incomplete outcome data
Three studies were judged to have high loss to follow up (Clever 1995; Jeffcoate 2009; Kuo 2012). Clever 1995 reported six of 40 participants (15%) were lost to follow up; Jeffcoate 2009 reported that 88 of 317 (28%) participants were lost to follow up with significant differences between groups. And in Kuo 2012 3/24 randomised participants (12.5%) were not included in the analysis. In terms of conducting intention‐to‐treat (ITT) analysis, Clever 1995 stated that withdrawals were excluded from the final analyses and was hence deemed to be at high risk of bias for this domain. Jeffcoate 2009 conducted ITT analysis dealing with missing data using the last value carried forward method, which was judged to be at unclear risk of bias. This method is not a robust way of imputing missing data and has the potential to introduce bias (Moher 2010). Jude 2007 and Piaggesi 2001 were deemed to have conducted ITT analysis (thus at low risk of bias for this domain).
Selective reporting
All studies reported outcomes adequately and were deemed to be at low risk of bias. However, it is important to note that judgement for this domain may be of limited value given it was made at face value based on the reporting of outcomes in the results that were described in the methods. Study reports were not compared to study protocols, which were not actively sought out.
Other potential sources of bias
Two studies were funded by non‐commercial organisations (Jeffcoate 2009; Piaggesi 2001) and two studies were funded by commercial organisations (Clever 1995; Jude 2007). Kuo 2012 did not report funding information.
Effects of interventions
See: Table 1
Dressing compared with dressing
Advanced wound dressing compared with basic wound contact dressing
Comparison 1: fibrous‐hydrocolloid dressing compared with basic wound contact dressing (two trials; 229 participants)
Two studies (Jeffcoate 2009; Piaggesi 2001) compared a fibrous‐hydrocolloid dressing with a basic wound contact dressing. Both studies compared the same brand of fibrous‐hydrocolloid dressing (Table 2) with either a dry, non‐adherent dressing (Jeffcoate 2009) or saline‐moistened gauze (Piaggesi 2001). Jeffcoate 2009 was a three‐armed study in which two groups were relevant to this comparison; in total 229 participants were included in this comparison, however only 20 of these participants were recruited in Piaggesi 2001.
Primary outcome: ulcer healing
Jeffcoate 2009 had a follow‐up time of 24 weeks. There was no statistically significant difference in the number of ulcers healed in the fibrous‐hydrocolloid‐dressed group (46/103; 45%) compared with the basic wound contact dressed‐group (41/106; 39%): risk ratio (RR) 1.15, 95% confidence interval (CI) 0.84 to 1.59 (Analysis 1.1). The mean time to healing was reported as 125.8 days (standard deviation (SD) 55.9) for the fibrous hydrocolloid‐dressed group and 130.7 days (SD 52.4) for the basic wound contact‐dressed group. The mean time to healing was obtained by fixing the maximum duration of trial involvement at 168 days. This trial reported a large number of losses to follow up (88 participants, 28% of total). ITT analysis was carried out by the trialists using the last value carried forward method to deal with missing data resulting from withdrawal of participants. It is important to note that this method of dealing with missing data is not robust and has the potential to bias treatment effects especially where loss of data is unequal between trial arms (Moher 2010).
1.1. Analysis.
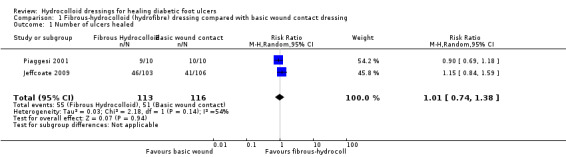
Comparison 1 Fibrous‐hydrocolloid (hydrofibre) dressing compared with basic wound contact dressing, Outcome 1 Number of ulcers healed.
Piaggesi 2001 did not report the study follow‐up time; the maximum period reported (graphically) in the study report was approximately 350 days. There was no statistically significant difference in the number of ulcers healed in the fibrous‐hydrocolloid‐dressed group (9/10; 90%) compared with the basic wound contact dressed‐group (10/10; 100%): RR 0.90, 95% CI 0.69 to 1.18 (Analysis 1.1). Mean time to healing data were presented: 127 days (SD 46) in the fibrous‐hydrocolloid‐dressed group and 234 days (SD 61) in the basic wound contact‐dressed group. The study authors analysed these data (log rank test) reporting a statistically different difference in time to healing (p <0.001). Whilst it is usually incorrect to treat healing data as continuous since in most studies not all patients will heal and thus will not have a time to healing value from which to calculate the mean; in this small study 19 of the 20 participants did heal and one underwent an amputation (not clear if amputation date was used in calculation of mean healing values). However, such small participant numbers limit the conclusions that can be drawn from these data; although we note there was no apparent baseline imbalance for duration and size of ulcer.
We pooled ulcer healed data from Jeffcoate 2009 and Piaggesi 2001 using a random‐effects model (Chi²: P = 0.14; I² = 54%) (Analysis 1.1). There was no statistically significant difference in the number of ulcers healed in the fibrous‐hydrocolloid‐dressed groups compared with the basic wound contact‐dressed groups: RR 1.01 (95% CI 0.74 to 1.38). Thus, on average, there was no difference in treatment effect between fibrous‐hydrocolloid and basic wound contact dressings although confidence intervals were wide. In terms of the source of heterogeneity, the two studies had different ankle brachial pressure index (ABPI) thresholds for study inclusion (> 0.7 in Jeffcoate 2009 and > 0.9 in Piaggesi 2001). Additionally the Piaggesi 2001 study was small with only 20 participants, compared with over 300 in Jeffcoate 2009 . Whilst this should not make a difference in terms of heterogeneity per se, the small number of participants could lead to differences between the study populations even though they had similar inclusion criteria. Comparing the baseline variables suggests that, on average, the patients in Piaggesi 2001 were slightly younger than in Jeffcoate 2009). Finally it is important to note that these trials had different follow‐up times, with one (Piaggesi 2001) being twice as long as the other. This, as well as the participants having higher ABPI values, may explain the higher rates of healing in the Piaggesi 2001 study. Jeffcoate 2009 does not present information about ulcer duration and/or size at baseline so it is not clear if these characteristics differed between studies.
Secondary outcomes
Jeffcoate 2009: There were four amputations reported in the fibrous‐hydrocolloid‐dressed group compared with two amputations in the basic wound contact‐dressed group. We did not analyse these data as it was not clear from the report if there was one amputation per person or if one person had undergone two amputations. The cost of generating a healed ulcer was estimated to be GBP 362 in the basic wound contact group, with the cost of an additional ulcer healed increasing to GBP 836 for the fibrous‐hydrocolloid group. This increase in cost was likely due to the incremental mean cost difference in per patient dressing management (higher costs associated the with fibrous‐hydrocolloid dressing) and the limited incremental difference in healing between the study groups. In terms of adverse events, both groups had similar numbers of serious (28 in the fibrous‐hydrocolloid‐dressed group compared with 35 in the basic wound contact‐dressed group) and non‐serious (227 in the fibrous‐hydrocolloid‐dressed group compared with 244 in the basic wound contact‐dressed group) events. There was no difference in quality of life (disease‐specific and generic) nor in recurrence rates.
Piaggesi 2001: There were five amputations in the fibrous‐hydrocolloid‐dressed group compared with three amputations in the basic wound contract‐dressed group. Adverse events recording was minimal with two specific adverse events being reported for the fibrous‐hydrocolloid group compared with five for the basic wound contact‐dressed group. The average number of days between dressings changes was similar for both groups (2.1 compared with 2.4).
Summary: fibrous‐hydrocolloid dressing compared with basic wound contact dressing
There was no statistically significant difference in the number of diabetic foot ulcers healed when treated with a fibrous‐hydrocolloid dressing compared with basic wound contact dressings in these studies with good length of follow up. There was a statistically significant difference in mean time to healing reported in Piaggesi 2001 however the small size of this study and potential issues with analysis mean that limited conclusions can be drawn. In terms of secondary outcome data, Jeffcoate 2009 suggests that the basic wound contact dressing was a more cost‐effective treatment compared with a fibrous‐hydrocolloid dressing. There did not appear to be any difference in the number of adverse events, the quality of life or ulcer recurrence between the groups.
Comparisons between alternative advanced dressings
Comparison 2: hydrocolloid (matrix) dressing compared with foam dressing (one trial; 40 participants)
Clever 1995 recruited 40 participants and compared a hydrocolloid‐matrix dressing with a foam dressing (Table 2).
Primary outcome: ulcer healing
Clever 1995 had a maximum follow‐up of 16 weeks. There was no statistically significant difference in the number of ulcers healed in the hydrocolloid‐matrix‐dressed group (16/20; 80%): compared with the foam‐dressed group (14/20; 70%): RR 1.14, 95% CI 0.80 to 1.64 (Analysis 2.1). The median time to healing was similar in both groups: 15.5 (range 4 to 76) days in the hydrocolloid‐matrix dressed group compared with 16.5 days (range 4 to 52) in the foam‐dressed group.
2.1. Analysis.
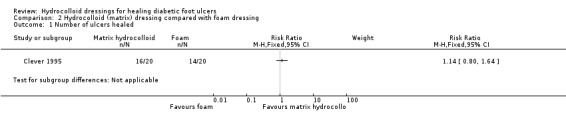
Comparison 2 Hydrocolloid (matrix) dressing compared with foam dressing, Outcome 1 Number of ulcers healed.
Secondary outcomes
Clever 1995: There was limited reporting of adverse events, with one event reported in the hydrocolloid‐matrix‐dressed group and five events in the foam‐dressed group. The mean number of dressing changes between clinical visits was similar for both groups: 2.23 changes in the hydrocolloid‐matrix‐dressed group compared with 2.37 changes in the foam‐dressed group.
Summary: hydrocolloid (matrix) dressing compared with foam dressing
Limited data from one small study found no difference in healing between ulcers treated with hydrocolloid matrix and foam dressings.
Antimicrobial dressing compared with non antimicrobial dressing
Jude 2007 recruited 134 participants and compared a silver fibrous‐hydrocolloid dressing with an alginate dressing (Table 2). Jeffcoate 2009 was a three‐armed study with 317 participants, with two arms (number of participants 211) that compared an iodine‐impregnated dressing with a fibrous‐hydrocolloid dressing.
Comparison 3: silver‐hydrocolloid dressing compared with an alginate dressing (one trial; 134 participants)
Primary outcome: ulcer healing
Jude 2007 had a follow‐up period of eight weeks. There was no statistically significant difference in the number of ulcers healed in the silver fibrous‐hydrocolloid‐dressed group (21/67; 31%) compared with the alginate‐dressed group (15/67; 22%): RR 1.40, 95% CI 0.79 to 2.47 (Analysis 3.1). The mean time to healing was reported as 52.6 days (SD 1.8) in the silver fibrous‐hydrocolloid‐dressed group compared with 57.7 days (SD 1.7) in the alginate‐dressed group.
3.1. Analysis.
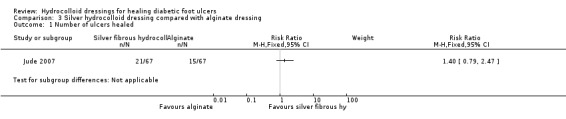
Comparison 3 Silver hydrocolloid dressing compared with alginate dressing, Outcome 1 Number of ulcers healed.
Secondary outcomes
Jude 2007: 25 participants experienced one or more events in the silver fibrous‐hydrocolloid‐dressed group (including one death) compared with 26 participants in the alginate‐dressed group (including one death). The mean number of dressing changes during study were similar for both group (21.9 for the silver fibrous‐hydrocolloid‐dressed group and 20.8 for the alginate‐dressed group). There were more infections in the fibrous hydrocolloid group (14 versus 8).
Comparison 4: iodine‐impregnated dressing compared with fibrous‐hydrocolloid dressing (one trial; 211 participants)
Primary outcome: ulcer healing
Jeffcoate 2009 was a three‐armed study, in which two groups were relevant to this comparison and had a follow‐up time of 24 weeks. There was no statistically significant difference in the number of ulcers healed in the iodine‐impregnated dressing group (48/108; 44%) compared with the fibrous‐hydrocolloid group (46/103; 45%): RR 1.00, 95% CI 0.74 to 1.34 (Analysis 4.1). The mean time to healing was reported as 127.8 days (SD 54.2) for the iodine‐dressed group and 125.8 days (SD 55.9) for the fibrous‐hydrocolloid dressed‐group. The mean time to healing was obtained by fixing the maximum duration of trial involvement at 168 days.
4.1. Analysis.
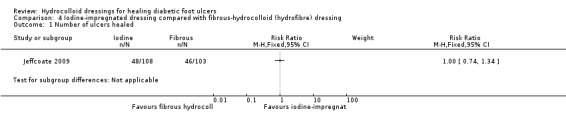
Comparison 4 Iodine‐impregnated dressing compared with fibrous‐hydrocolloid (hydrofibre) dressing, Outcome 1 Number of ulcers healed.
Secondary outcomes
There was one amputation in the iodine‐dressed group compared with four amputations in the fibrous‐hydrocolloid‐dressed group. We did not analyse these data as it was not clear if it was the same people who had undergone amputation (thus introducing clustering). The cost of healing an additional ulcer healed was GBP 848 for the iodine‐dressed group. In terms of adverse events, both groups had similar numbers of serious (37 in the iodine‐dressed group compared with 28 in the fibrous‐hydrocolloid‐dressed group) and non‐serious (239 in the iodine group compared with 227 in the fibrous‐hydrocolloid dressed group) events. There was no difference in quality of life (disease‐specific and generic) nor in recurrence rates. There was a possible difference in recurrence rates, more in iodine group (seven compared with three) but these numbers of events were small.
Given the different dressing type we did not pool these data in an antimicrobial compared with non‐antimicrobial meta‐analysis.
Summary: antimicrobial fibrous‐hydrocolloid dressing compared with non‐antimicrobial dressing
There was no statistically significant difference in the number of ulcers healed when treated with an antimicrobial (silver) fibrous‐hydrocolloid dressing compared with a standard alginate dressings. Nor was there any statistically significant difference in ulcer healing between an antimicrobial (iodine impregnated)‐dressed group when compared with a standard fibrous hydrocolloid‐dressed group. In terms of secondary outcome data, Jeffcoate 2009 conducted a detailed cost‐effectiveness analysis and concluded that the costs of using fibrous‐hydrocolloid and an iodine‐impregnated dressing were similar. There did not appear to be any difference in the number of adverse events, the quality of life or ulcer recurrence between the groups, although number of recurrence events were small. This trial was of adequate statistical power and good methodological quality.
Dressing compared with topical treatment
Advanced wound dressing compared with plant‐based topical treatment
Comparison 4: fibrous‐hydrocolloid dressing compared with Plectranthus amboinicus and Centella asiatica Cream (one trial; 24 participants)
Primary outcome: ulcer healing
Kuo 2012 had a maximum follow‐up of 2 weeks with all ulcers being treated surgically after this point (grafting or surgical closure for healing by primary intention). Number of ulcers healed was not reported however the median percent change in wound size (assume from baseline to 14 days) was reported. The median % change was reported as ‐22.64% in the hydrocolloid group and ‐27.18% in the topical treatment group. This difference was stated as not statistically significant in the trial report (p=0.673). Given the limited data reported we have not analysed further (Analysis 5.1)
5.1. Analysis.
Comparison 5 Trial data, Outcome 1 Trial data.
| Trial data | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Groups | Primary outcome: ulcer healing | Secondary: health‐related quality of life | Number and level of amputations | Adverse events, including pain | Cost | Ulcer recurrence | Heading 8 |
| Clever 1995 | Group A (n = 20): Hydrocolloid (polyurethane matrix) dressing Group B (n = 20): Foam dressing |
Number of ulcers healed:
Group A: 16 Group B: 14 Mean time to healing in days (SD) Group A: 25.19 (23.52) Group B: 20.43 (14.74) Median time to healing in days Group A: 15.5 (range 4 to 76) Group B: 16.5 (range 4 to 52) Wound size at 4 weeks mm2 (SD): Group A: 32.37 (54.12) Group B: 33.46 (75.22) |
n/r | n/r | (Reasons not reported separately for 2 groups):
Group A: 1 Group B: 5 |
Mean number of dressing changes between clinical visits (SD):
Group A: 2.23 (2.19) Group B: 2.37 (2.18) |
n/r | |
| Jeffcoate 2009 | Group A (n = 103): Fibrous‐hydrocolloid (hydrofibre) dressing Group B (n = 108): Iodine‐impregnated dressing Group C (n = 106): non‐adherent dressing, viscose filament gauze |
Number of ulcers healed at 24 weeks:
Group A: 46 Group B: 48 Group C: 41 Mean time to healing in days (SD) (fixed at max of 168 days) Group A: 125.8 (55.9) Group B: 127.8 (54.2) Group C 130.7 (52.4) |
Mean Cardiff Wound Impact Schedule score at 24 weeks (SD) Group A: Physical functioning: 71.4 (19.5). Social functioning: 70.3 (25.4). Well being: 53.1 (19.9) Group B: Physical functioning: 67.1 (23.6). Social functioning: 69.7 (24.1). Well being: 51.0 (22.3) Group C: Physical functioning: 68.9 (19.1). Social functioning: 69.8 (23.5). Well being: 50.2 (21.1) Other Study also reports mean and SD for each of the eight domains of the SF‐36. No significant different between the groups for any domain. |
Minor amputations (Below ankle): Group A: 3 Group B: 1 Group C: 1 Major amputations (Above knee) Group A: 1 Group B: 0 Group C: 1 |
Non‐serious adverse events
Group A: 227 Group B: 239 Group C: 244 Serious adverse events Group A: 28 Group B: 37 Group C: 35 |
Cost in GBP per patient for dressing management:
Mean (95% CI) Group A: 191.33 (148.41 to 234.25) Group B: 183.60 (128.92 to 238.21) Group C: 141.18 (108.18 to 174.17) Cost in GBP of professional time in managing foot ulcers: Mean (95% CI) Group A: 459.87 (354.78 to 564.97) Group B: 556.90 (422.32 to 691.48) Group C: 448.86 (348.68 to 549.03 Cost in GBP of generating a healed ulcer using non‐adherent dressing (Group C): 362. Cost in GBP of generating an additional healed ulcer: Group A: 836 Group B: 848 |
At same site Group A: 3 Group B: 7 Group C: 3 | |
| Jude 2007 | Group A (n = 67): Fibrous‐hydrocolloid (hydrofibre) dressing with 1.2% ionic silver Group B (n = 67): Calcium‐alginate dressing | Number of ulcers healed in 8 weeks Group A: 21 Group B: 15 Velocity of healing cm2/week (SD): Group A: 0.29 (0.33) (n = 61) Group B: 0.26 (0.90) (n = 61) Velocity of healing as % per week (SD): Group A: 11.6 (17.7) (n = 61) Group B: 10.0 (15.5) (n = 61) Mean time in days to healing (SD): Group A: 52.6 (1.8) Group B: 57.7 (1.7) Reduction in ulcer area in 8 weeks: Group A: 58.1 (53.1). Group B: 60.5 (42.7). Reduction in ulcer depth (cm) at 8 weeks (SD): Group A: 0.25 (0.49) Group B: 0.13 (0.37) | n/r | n/r | Group A: 25 participants experienced one or more events. Death = 1; Infection = 14. 8 participants discontinued treatment due to AE. Group B: 26 participants experienced adverse event. Death = 1; Infection = 8. 13 participants discontinued treatment due to AE. | Mean number of dressing changes during study: Group A: 21.9 Group B: 20.8 | n/r | |
| Kuo 2012 | Group A (n = 12): fibrous‐hydrocolloid (hydrofibre) dressing Group B (n = 12): cream contained extracts from two botanical raw materials, P. amboinicus and C. asiatica. |
Percent change in wound size (from baseline ‐ assumed to 14 days). Median and (IQR) Group A: ‐22.64 (‐36.90 to ‐3.20) Group B: ‐ 27.18(‐38.86 to 36.10) Reported in paper as not statistically significant from Mann‐Whitney U test. P = 0.673 |
n/r | n/r |
Number of people with one or more adverse events Group A: 5/12 (41.7%) Group B: 5/12 (41.7%) |
|||
| Piaggesi 2001 | Group A (n = 10): Fibrous‐hydrocolloid (hydrofibre) dressing Group B (n = 10): Saline‐moistened gauze dressing |
Number of ulcers healed (during period of study): Group A: 9 Group B: 10 Healing time in days (mean) (SD) Group A: 127 (46 days) Group B: 234 (61 days) Median % reduction in lesion volume at 8 weeks follow up (interquartile range): Group A: 50 (26) Group B: 35 (15) Median % of granulation tissue at 8 weeks follow up (interquartile range) Group A: 60 (40) Group B: 32.5 (10) | n/r | Group A: Amputation of a lesser toe = 3; Amputation of 2 lesser toes = 1; Metatarsal resection = 1 Group B: Amputation of a lesser toe = 2; Metatarsal resection = 1 | Group A: Maceration of peri‐lesional skin = 1; Infective complications = 1 Group B: Maceration of peri‐lesional skin = 2; Infective complications = 3 | Average number of days between dressings changes (SD) Group A: 2.1 (0.6) Group B: 2.4 (0.3) | n/r | |
Kuo 2012: It was reported that 5/12 (41.7%) participants in each group had one or more adverse events. No further analysis was undertaken (Analysis 5.1).
Summary of Findings Table
We have included a Summary of Findings table (Table 1) in this review for the comparisons informed by more than one trial (fibrous‐hydrocolloid dressing compared with basic wound contact dressing): this aims to give a concise overview and synthesis of the volume and quality of the evidence for this comparison. The Summary of Findings table confirm our conclusion that the quality of evidence is of moderate quality and on balance there is no strong evidence of a benefit of using hydrocolloid dressings for healing foot ulcers in people with diabetes.
Discussion
Summary of main results
This review has identified, appraised and presented all available RCT evidence (Clever 1995; Jeffcoate 2009; Jude 2007; Kuo 2012; Piaggesi 2001) regarding the clinical and cost‐effectiveness of all types of hydrocolloid wound dressings in the treatment of diabetic foot ulcers.
When data from two studies comparing fibrous‐hydrocolloid and basic wound contact dressings were pooled, there was no statistically significant difference in ulcer healing between the treatments. We also found no evidence of any difference in ulcer healing between a hydrocolloid‐matrix dressing and a foam dressing. Similarly, there was no evidence of any difference in the number of diabetic foot ulcers healed when treated with an antimicrobial (silver) fibrous‐hydrocolloid dressing compared with a standard alginate dressing; nor between an antimicrobial dressing (iodine‐impregnated) and a standard fibrous‐hydrocolloid dressing. One robust study with an adequate follow‐up period (24 weeks) found that a basic wound contact dressing was more cost‐effective in healing diabetic foot ulcers than a fibrous hydrocolloid (hydrofibre) dressing (Jeffcoate 2009). Four of the included studies (Clever 1995; Piaggesi 2001; Jude 2007; Kuo 2012) were small and therefore statistically underpowered to detect important treatment differences should they exist and one study did not follow wounds up to healing (Kuo 2012). However, the pooling of data from Piaggesi 2001 with the much larger Jeffcoate 2009 study increased the power of this comparison. We note that most included studies were evaluating treatments on people who appeared to have relatively non‐complex foot ulcers. This means the body of literature presented may be of limited use to health professionals in the treatment of people with harder to heal foot ulcers as it is difficult to generalise from the included studies to people with more co‐morbidities or complications; this is a limitation of the RCTs that have been undertaken in this field thus far.
Quality of the evidence
One included study in this review was of deemed to be at low risk of bias (Jeffcoate 2009); the remaining studies were at unclear risk of bias due to poor reporting since studies did not follow good practice conduct and reporting guidelines, e.g. CONSORT (Schulz 2010). Key areas of good practice are the robust generation of a randomisation sequence, for example, computer‐generated, robust allocation concealment, the use of a telephone randomisation service and blinded outcome assessment where possible. All this information should be clearly stated in the study report as all trial authors should anticipate the inclusion of their trials in systematic reviews. In terms of analysis, where possible, data from all participants should be included, that is an intention‐to‐treat analysis is conducted. Steps should be taken during trial conduct to prevent missing data as far as is possible. Where missing data are an issue, imputation methods should be considered and clearly reported when implemented. Finally, where possible robust economic data should be collected.
Potential biases in the review process
The review considered as much evidence as it was possible to obtain, including studies that were not published in the English language. We contacted relevant pharmaceutical companies but did not receive any RCT data from them. There is the potential for publication bias, however, this is likely to be a limited issue in this review given the large number of negative findings that have been published. It is also important to note that two studies are awaiting assessment and may be included in future reviews.
Agreements and disagreements with other studies or reviews
The existing evidence‐base to help clinicians in their decision‐making processes suggests that there is no evidence to suggest that hydrocolloid dressings are better than alternative dressings for diabetic foot ulcers. This agrees with the most recent systematic review in this area (Hinchliffe 2008), which did not find any evidence that any one dressing type was more effective than others in healing diabetic foot ulcers. However, we note that Hinchliffe 2008 included only one trial of hydrocolloid dressings, compared with the four studies included in this review .
Authors' conclusions
Implications for practice.
Based on a comprehensive review of current evidence, fibrous‐hydrocolloid dressings (with or without antimicrobial components) and hydrocolloid‐matrix dressings do not appear to increase the healing rates of diabetic foot ulcers compared with alternative dressings. Practitioners may therefore elect to consider other characteristics such as costs and symptom management properties when choosing between alternatives. We note that most included studies were evaluating treatments on people who appeared to have relatively non‐complex foot ulcers. This means the body of literature presented may be of limited use to health professionals in the treatment of people with harder to heal foot ulcers as it is difficult to generalise from the included studies to people with more co‐morbidities or complications; this is a limitation of the RCTs that have been undertaken in this field thus far.
Implications for research.
Current evidence suggests that there is no difference in ulcer healing between hydrocolloid dressings and alternatives; it is important to note that included studies have evaluated only fibrous‐hydrocolloid and matrix hydrocolloid dressings. It is unclear if this is due to limited used of occlusive hydrocolloid dressings on diabetic foot ulcers due to the perceived (but untested) risk of increased infection risk from anaerobic micro‐organisms with these treatments. The importance of including robust cost‐effectiveness analyses is highlighted by Jeffcoate 2009, who did not find that treatment with advanced wound management dressings reduced the number of clinic visits. In terms of dressing choice, any investment in future research must maximise its value to decision‐makers. Given the large number of dressing options, the design of future trials should be driven by the questions of high priority to patients and other decision makers. It is also important for research to ensure that the outcomes that are collected in research studies are those that matter to patients, carers and health professionals. It may be that dressings should be viewed as management tools and that other treatments that address patient lifestyle issues deserve attention. Where trials are conducted, good practice guidelines must be followed in their design, implementation and reporting. Further reviews are being conducted to synthesise evidence regarding the effect of other dressings on the treatment of diabetic foot ulcers. It would then be useful to conduct further evidence synthesis (overviews of reviews, mixed treatment comparisons or both) to aid decision‐making about the choice of dressings for diabetic foot ulcers across all dressing options.
What's new
| Date | Event | Description |
|---|---|---|
| 11 April 2013 | New citation required but conclusions have not changed | One new study included (Kuo 2012), no change to conclusions. |
| 11 April 2013 | New search has been performed | First update, new search, summary of findings table added. |
Acknowledgements
The authors would like to thank the following people who reviewed the protocol and review for clarity, readability and rigour: Wounds Group editors (Julie Bruce, Andrea Nelson and Gill Worthy) and peer referees (David Armstrong, Duncan Chambers and Janet Yarrow). In addition copy editor Jenny Bellorini; Nikki Stubbs for clinical advice; Xun Li Xun for translation of Chinese language papers. We would like to thank Sally E.M. Bell‐Syer and Ruth Foxlee for all their expertise and support during the review process.
Appendices
Appendix 1. Search methods for the original version of the review ‐ January 2011
Electronic searches
We searched the following databases:
The Cochrane Wounds Group Specialised Register (searched 4 January 2012);
The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2011, Issue 4);
Ovid MEDLINE (1950 to December Week 3 2011);
Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations, January 03, 2012);
Ovid EMBASE (1980 to 2011 Week 52);
EBSCO CINAHL (1982 to 30 December 2011).
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) using the following exploded MeSH headings and keywords:
#1 MeSH descriptor Occlusive Dressings explode all trees #2 MeSH descriptor Biological Dressings explode all trees #3 MeSH descriptor Alginates explode all trees #4 MeSH descriptor Hydrogels explode all trees #5 MeSH descriptor Silver explode all trees #6 MeSH descriptor Honey explode all trees #7 (dressing* or alginate* or hydrogel* or "foam" or "bead" or "film" or "films" or tulle or gauze or non‐adherent or "non adherent" or silver or honey or matrix):ti,ab,kw #8 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7) #9 MeSH descriptor Foot Ulcer explode all trees #10 MeSH descriptor Diabetic Foot explode all trees #11 diabet* NEAR/3 ulcer*:ti,ab,kw #12 diabet* NEAR/3 (foot or feet):ti,ab,kw #13 diabet* NEAR/3 wound*:ti,ab,kw #14 (#9 OR #10 OR #11 OR #12 OR #13) #15 (#8 AND #14)
The search strategies used in Ovid MEDLINE, Ovid EMBASE and EBSCO CINAHL can be found in Appendix 1, Appendix 2 and Appendix 3 respectively. We combined the Ovid MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision) (Lefebvre 2011). We also combined the EMBASE and CINAHL searches with the trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN 2011). There were no restrictions on the basis of date or language of publication.
We searched for on‐going studies on the ISRCTN register (http://www.controlled‐trials.com/isrctn/) (last searched 22nd May 2011).
Searching other resources
We attempted to contact researchers to obtain any unpublished data when needed. We also searched the reference lists of the included studies and previous systematic reviews. We contacted appropriate manufacturers (Smith & Nephew, Convatec Ltd, Mölnlycke Health Care, 3M Healthcare, Coloplast Ltd) for details of any unpublished studies.
Appendix 2. Ovid MEDLINE search strategy
1 exp Occlusive Dressings/ 2 exp Biological Dressings/ 3 exp Alginates/ 4 exp Hydrogels/ 5 exp Silver/ 6 exp Honey/ 7 (dressing* or hydrocolloid* or alginate* or hydrogel* or foam or bead or film*1 or tulle or gauze or non‐adherent or non adherent or silver or honey or matrix).tw. 8 or/1‐7 9 exp Foot Ulcer/ 10 exp Diabetic Foot/ 11 (diabet* adj3 ulcer*).tw. 12 (diabet* adj3 (foot or feet)).tw. 13 (diabet* adj3 wound*).tw. 14 or/9‐13 15 8 and 14
Appendix 3. Ovid EMBASE search strategy
1 exp wound dressing/ 2 exp alginic acid/ 3 exp hydrogel/ 4 exp SILVER/ 5 exp HONEY/ 6 (dressing* or hydrocolloid* or alginate* or hydrogel* or foam or bead or film*1 or tulle or gauze or non‐adherent or non adherent or silver or honey or matrix).tw. 7 or/1‐6 8 exp foot ulcer/ 9 exp diabetic foot/ 10 (diabet* adj3 ulcer*).tw. 11 (diabet* adj3 (foot or feet)).tw. 12 (diabet* adj3 wound*).tw. 13 or/8‐12 14 7 and 13
Appendix 4. EBSCO CINAHL search strategy
S11 S4 and S10 S10 S5 or S6 or S7 or S8 or S9 S9 TI diabet* N3 wound* or AB diabet* N3 wound* S8 TI (diabet* N3 foot OR diabet* N3 feet) or AB (diabet* N3 foot OR diabet* N3 feet) S7 TI diabet* N3 ulcer* or AB diabet* N3 ulcer* S6 (MH "Foot Ulcer+") S5 (MH "Diabetic Foot") S4 S1 or S2 or S3 S3 TI (dressing* or alginate* or hydrogel* or foam or bead or film or films or tulle or gauze or non‐adherent or non adherent or honey or silver or matrix) or AB (dressing* or alginate* or hydrogel* or foam or bead or film or films or tulle or gauze or non‐adherent or non adherent or honey or silver or matrix) S2 (MH "Honey") S1 (MH "Bandages and Dressings+")
Appendix 5. Risk of bias criteria
1. Was the allocation sequence randomly generated?
Low risk of bias
The investigators describe a random component in the sequence generation process such as: referring to a random number table; using a computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots.
High risk of bias
The investigators describe a non‐random component in the sequence generation process. Usually, the description would involve some systematic, non‐random approach, for example: sequence generated by odd or even date of birth; sequence generated by some rule based on date (or day) of admission; sequence generated by some rule based on hospital or clinic record number.
Unclear
Insufficient information about the sequence generation process to permit judgement of low or high risk of bias.
2. Was the treatment allocation adequately concealed?
Low risk of bias
Participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based and pharmacy‐controlled randomisation); sequentially‐numbered drug containers of identical appearance; sequentially‐numbered, opaque, sealed envelopes.
High risk of bias
Participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on: using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or nonopaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure.
Unclear
Insufficient information to permit judgement of low or high risk of bias. This is usually the case if the method of concealment is not described or not described in sufficient detail to allow a definite judgement, for example if the use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially numbered, opaque and sealed.
3. Blinding ‐ was knowledge of the allocated interventions adequately prevented during the study?
Low risk of bias
Any one of the following.
No blinding, but the review authors judge that the outcome and the outcome measurement are not likely to be influenced by lack of blinding.
Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken.
Either participants or some key study personnel were not blinded, but outcome assessment was blinded and the non‐blinding of others unlikely to introduce bias.
High risk of bias
Any one of the following.
No blinding or incomplete blinding, and the outcome or outcome measurement is likely to be influenced by lack of blinding.
Blinding of key study participants and personnel attempted, but likely that the blinding could have been broken.
Either participants or some key study personnel were not blinded, and the non‐blinding of others likely to introduce bias.
Unclear
Any one of the following.
Insufficient information to permit judgement of low or high risk of bias.
The study did not address this outcome.
4. Were incomplete outcome data adequately addressed?
Low risk of bias
Any one of the following.
No missing outcome data.
Reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias).
Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups.
For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate.
For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size.
Missing data have been imputed using appropriate methods.
High risk of bias
Any one of the following.
Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups.
For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate.
For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size.
‘As‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation.
Potentially inappropriate application of simple imputation.
Unclear
Any one of the following.
Insufficient reporting of attrition/exclusions to permit judgement of low or high risk of bias (e.g. number randomised not stated, no reasons for missing data provided).
The study did not address this outcome.
5. Are reports of the study free of suggestion of selective outcome reporting?
Low risk of bias
Any of the following.
The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way.
The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon).
High risk of bias
Any one of the following.
Not all of the study’s pre‐specified primary outcomes have been reported.
One or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified.
One or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect).
One or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis.
The study report fails to include results for a key outcome that would be expected to have been reported for such a study.
Unclear
Insufficient information to permit judgement of low or high risk of bias. It is likely that the majority of studies will fall into this category.
6. Other sources of potential bias
Low risk of bias
The study appears to be free of other sources of bias.
High risk of bias
There is at least one important risk of bias. For example, the study:
had a potential source of bias related to the specific study design used; or
had extreme baseline imbalance; or
has been claimed to have been fraudulent; or
had some other problem.
Unclear
There may be a risk of bias, but there is either:
insufficient information to assess whether an important risk of bias exists; or
insufficient rationale or evidence that an identified problem will introduce bias.
Data and analyses
Comparison 1. Fibrous‐hydrocolloid (hydrofibre) dressing compared with basic wound contact dressing.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of ulcers healed | 2 | 229 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.74, 1.38] |
Comparison 2. Hydrocolloid (matrix) dressing compared with foam dressing.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of ulcers healed | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
Comparison 3. Silver hydrocolloid dressing compared with alginate dressing.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of ulcers healed | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
Comparison 4. Iodine‐impregnated dressing compared with fibrous‐hydrocolloid (hydrofibre) dressing.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of ulcers healed | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
Comparison 5. Trial data.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Trial data | Other data | No numeric data |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Clever 1995.
| Methods | RCT (not clear if single‐centre or multi‐centred) comparing a foam dressing (Allevyn, Smith & Nephew) with a hydrocolloid (polyurethane matrix) dressing (Cutinova Hydro, S&N Hlth, previously Beiersdorf) undertaken in Germany Duration of follow up: until healing occurred or for a maximum of 16 weeks | |
| Participants | 40 participants Inclusion criteria: patients aged 18 to 80 years with a pure neuropathic superficial ulcer 1 to 5 cm in diameter and with no clinical and radiological signs of osteomyelitis or tendon involvement Exclusion criteria: patients with an ankle‐brachial pressure index < 0.8 (measured using doppler ultrasound) and with clinical or radiological signs of osteomyelitis or tendon involvement. Ulcers requiring topical treatment were also excluded, as were patients with know allergies to any product being used. | |
| Interventions | Group A (n = 20): hydrocolloid (polyurethane matrix) dressing (Cutinova Hydro, Smith & Nephew) Group B (n = 20): foam dressing (Allevyn, Smith & Nephew) In both groups, dressing changes were performed as often as required but at least once a week Co‐intervention: pressure relief comprising a half‐shoe or so‐called 'heal sandal', therapeutic footwear with cushioned insoles, and crutches as required to meet individual needs, infection control with systemic antibiotics if required, wound cleansing with Ringer's solution and debridement with removal of callus if needed |
|
| Outcomes | Primary outcome: ulcer healing (number of ulcers healed; mean time to healing; median time to healing; wound size) Secondary outcomes: adverse events (number); costs (mean number of dressing changes between clinical visits) Health‐related quality of life; amputations and ulcer recurrence not reported |
|
| Notes | Trial data: Analysis 5.1 Funding source: Beiersdorf AG, Hamburg |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote:"Conducted an open, randomised, controlled study" Comment: method of generation of random schedule not reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: the process of randomising participants, including who did this is not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Conducted an open, randomised, controlled study" Comment: the trial was stated as being open‐labelled. No other details in the text |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote:"Conducted an open, randomised, controlled study" Comment: this was labelled an open trial not clear if blinded evaluation was conducted. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: in total 6 participants were withdrawn, or 15% of the total study population. The study report also states that withdrawals were excluded from the analyses. |
| Selective reporting (reporting bias) | Low risk | Comment: based on paper only, protocol not obtained |
| Other bias | Unclear risk | Comment: funded by commercial organisation |
Jeffcoate 2009.
| Methods | Three‐armed RCT comparing an iodine‐impregnated dressing (Inadine, Johnson and Johnson) and fibrous‐hydrocolloid (hydrofibre) dressing (Aquacel, ConvaTec) with a non‐adherent dressing, viscose filament gauze (Johnson & Johnson) undertaken in the UK Duration of follow up: ulcers once healed were followed bi‐weekly for 4 weeks to ensure they remained healed. Ulcers that recurred within the 4 weeks were regarded as unhealed and continued in the study. All participants with healed ulcers were re‐assessed by the clinicians in charge of their care 12 weeks after healing to assess for recurrence. Patients with persistent ulcers were assessed by clinicians in charge by 24 weeks and withdrawn from the intervention phase at that time. They did attend for a final assessment 36 weeks after recruitment. | |
| Participants | 317 participants Inclusion criteria: patients with Type 1 or 2 diabetes, aged 18 years or more having a foot ulcer present for at least 6 weeks. Ulcer cross‐sectional area of between 25 and 2500 mm2. Able and willing to give informed consent. Reasonably accessible by car to the hospital base and under routine review by the multidisciplinary clinic. If there was more than one ulcer on the foot, the largest ulcer that conformed to the inclusion criteria was selected as the index ulcer. Exclusion criteria: patients with a known allergy to any of the trial preparations (including iodine). Any ulcer on either foot extending to tendon, periosteum or bone. Patients with infection of the bone, soft tissue infection requiring treatment with systemic antibiotics. An ulcer on a limb being considered for revascularisation. Ulcers chosen for management with a non‐removable cast without a dressing window. Gangrene on the affected foot. Eschar which was not removable by clinical debridement. Patients with evidence of a sinus or deep track. Patients in whom the hallux had been amputated on the affected side (preventing the measurement of toe pressure). Those with an ankle:brachial pressure index of less than 0.7 or toe systolic pressure less than 30 mmHg. Ulceration judged to be caused primarily by disease other than diabetes. Patients with any other serious disease likely to compromise the outcome of the trial. Patients with critical renal disease (creatinine greater than 300 mmol/l) and those receiving immunosuppressants, systemic corticosteroid therapy (other than by inhalation) or any other preparation which could, in the opinion of the supervising clinician, have interfered with wound healing. Patients living at such a distance (generally further than 10 miles) from the clinic as would have made frequent assessment visits inappropriately expensive and/or impractical. Patients who withheld consent. | |
| Interventions | Group A (n = 103): fibrous‐hydrocolloid (hydrofibre) dressing (Aquacel, ConvaTec) Group B (n = 108): iodine‐impregnated dressing (Inadine, Systagenix) Group C (n = 106): non‐adherent dressing, viscose filament gauze (Johnson & Johnson) For all groups, patients and carers were shown the dressing to be used and asked if they wished to change their own dressings (either entirely or just on some occasions), but with fortnightly monitoring by a trial nurse. Those who wished to do so received further training to ensure the dressings were applied correctly. Those who chose not to be responsible for this aspect of their care had their dressings changed by the district nurse or practice nurse, according to usual procedure, or by the trial nurse. Dressings were changed daily, on alternate days or 3 times a week according to need and/or availability of professional staff. Co‐intervention: ulcer management was in line with current guidelines for good practice, including appropriate and regular use of debridement and with a removable fibreglass or polyester boot being recommended for off‐loading. Participants were advised to have a bath or shower as often as they wished provided the ulcer could be redressed afterwards, and provided the ulcerated foot was not immersed in water for more than 5 minutes. |
|
| Outcomes | Primary outcome: ulcer healing (number of ulcers healed at 24 weeks; mean time to healing in days) Secondary outcomes: health‐related quality of life (Mean Cardiff Wound Impact Schedule score); amputations (minor and major); adverse events (serious and non‐serious); cost (cost per patient); ulcer recurrence | |
| Notes | Trial data: Analysis 5.1 In total, 88 were withdrawn from this study. The study methods note that an ITT analysis for % healed was conducted using last entry carried forward, with participants only considered healed if this was confirmed after 4 weeks. Thus, the analysis assumed that those withdrawn did not heal (they are in denominator but not the numerator) Funding source: non‐commercial organisation (United Kingdom National Health Service Health Technology Assessment Programme) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote:"Randomisation lists were created using SPSS (SPSS Inc., Version 14), using blinded dressing codes " Comment: method of generation of random schedule reported |
| Allocation concealment (selection bias) | Low risk | Quote:"using blinded dressing codes. The lists were held at Cardiff University and each recruiting centre telephoned a designated number during working hours" Comment: central allocation using telephone |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote:"The nurse was not blinded to the randomisation and dressed the wound at the end of the visit" Comment: no mention about blinding of participants. Healthcare providers were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote:"Dressings were removed prior to the examination by investigators who were not involved in the conduct of the trial and who were blind to the randomisation group." Comment: outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote:"Intention to treat analysis was carried out using the last value carried forward method, with strict adherence to the protocol such that only those who attended for a healing verification visit and reported as still healed at 28 days have been coded as ‘healed’ for the outcome classification." Comment: ITT analysis was done but imputing missing data due to withdrawal of trial participants due to adverse events and protocol violations |
| Selective reporting (reporting bias) | Low risk | Comment: based on full report, protocol not obtained |
| Other bias | Low risk | Comment: funded by non‐commercial organisation (UK Health Technology Assessment Programme) |
Jude 2007.
| Methods | Multi‐centred, 2‐armed trial, RCT comparing a fibrous‐hydrocolloid (hydrofibre) dressing with 1.2% ionic silver (Aquacel Ag, ConvaTec) with a calcium‐alginate dressing (Algosteril, Smith & Nephew) undertaken in the UK, France, Germany, Sweden Duration of follow up: 8 weeks | |
| Participants | 134 participants Inclusion criteria: patients with type 1 or type 2 diabetes mellitus (HbA1c ≤ 12%), serum creatinine ≤ 200 mol/l diabetic foot ulcers classed as Wagner grade 1 or 2 and of neuropathic or neuro‐ischaemic aetiology. All wounds > 1 cm2 in area. Exclusion criteria: patients with known allergies to dressings under study; if there was a known or suspected malignancy near ulcer. Also, if patient had been on systemic antibiotics > 7 days prior to enrolment or with inadequate arterial perfusion as defined by ankle‐to‐brachial index < 0.8, great toe systolic blood pressure < 40 mmHg or forefoot TcPO2 < 30 mmHg (subject supine) or < 40 mmHg (subject sitting) | |
| Interventions | Group A (n = 67): fibrous‐hydrocolloid (hydrofibre) dressing with 1.2% ionic silver (Aquacel® Ag, ConvaTec). Left in place and changed on leakage or at evaluation or every 7 days as indicated. Group B (n = 67): calcium‐alginate dressing (Algosteril, Smith & Nephew). Manufacturers instructions were followed and the dressing was moistened before use on dry wounds and changed on leakage or at evaluation or every 7 days as indicated (except if the wound was infected when dressed changed daily). In both groups, ulcers were cleansed using sterile saline, each dressing was covered with a sterile, non‐adherent foam dressing Co‐intervention: accommodative footwear for non‐plantar ulcers and off‐loading for planter ulcers was delivered as required. | |
| Outcomes | Primary outcome: ulcer healing (number of ulcers healed; velocity of healing; mean time in days to healing; reduction in ulcer area; reduction in ulcer depth) Secondary outcomes: adverse events (number); costs (mean number of dressing changes) Health‐related quality of life; amputations and ulcer recurrence not reported |
|
| Notes | Trial data: Analysis 5.1 22 participants had clinically infected ulcers at baseline, 9 in Group A and 13 in Group B . On enrolment antibiotics were prescribed to 13 in Group A and 8 in Group B. Funding source: ConvaTec (Bristol Myers Squibb) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote:"Individuals were randomly assigned to receive either ** or ** dressings according to instructions in a sealed envelope and stratified according to whether or not systemic antibiotics were being administered for treatment of the study ulcer" Comment: method of generation of random schedule not clear from this description |
| Allocation concealment (selection bias) | Unclear risk | Comment: not clear if envelopes were sequentially numbered, opaque and sealed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: no mention of blinding in study report |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no mention of blinding in study report |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 21 participants recorded as discontinuing treatment, however, it does not seem like these were study withdrawals. All randomised included in the analysis. |
| Selective reporting (reporting bias) | Low risk | Comment: based on study report, protocol not obtained |
| Other bias | Unclear risk | Comment: funded by commercial organisation |
Kuo 2012.
| Methods | 2‐arm trial, RCT comparing a fibrous‐hydrocolloid (hydrofibre) dressing with a topical cream containing P. amboinicus (Lour.) Spreng. (Lamiaceae) and C. asiatica (L.) Urban (Umbelliferae) undertaken in Taiwan. Duration of follow up: 2 weeks. After two weeks, the wounds in both groups were all reconstructed by split‐thickness skin graft or primary closure. |
|
| Participants | 24 participants Inclusion criteria: Inclusion criteria were patients with type 1 or type 2 diabetes, aged 20 years or older, and having Wagner grade 3 foot ulcers postsurgical debridement. Wagner grade 3 was defined as “deep ulcer involving osteitis, abscess, or osteomyelitis”. Exclusion criteria: Patients with poor nutritional status (albumin <3 g/dL), poor diabetic control (HbA1c >10%), anaemia (hemoglobin <10 g/dL), and leukocyte counts <1,000/cu mm; presence of connective tissue disease; known or suspected malignancy local to the study ulcer; renal failure insufficiency (serum creatinine >1.5mg/dL) or abnormal liver function (AST, ALT >2.5 × upper limit of normal range); requiring treatment with immunosuppressive agents, corticosteroids, chemotherapy or radiotherapy; female patients with positive pregnancy test or breastfeeding or unwilling to use appropriate contraceptive methods during study; patients with known sensitivity to essential oils or lanolin cream. | |
| Interventions | Group A (n = 12): fibrous‐hydrocolloid (hydrofibre) dressing (Aquacel ConvaTec, Valencia, CA, USA). Hydrocolloid fiber dressing group and left in place for up to 7 days or changed earlier as clinically indicated.
Group B (n = 12): cream contained extracts from two botanical raw materials, P. amboinicus and C. asiatica. The plants of
P. amboinicus were collected on 2007 according to good agricultural and good collection practices. C. asiatica. extract
was sourced commercially with certificate of analysis of the extract and herbal material. the most active fractions from P. amboinicus and from C. asiatica, were combined in a 1 : 4 ratio to form the drug substance. The final cream, contained
1.25% of drug substance in a cream base, 15 g per tube. The cream base contained cetostearyl alcohol, ireine, liquid
petrolatum, methyl paraben propyl paraben, Span 60, Tween 60, white petrolatum, water, and pigments. The cream was applied topically twice daily in an amount to fully cover the ulcer area in a thin and even layer (not exceed 2 millimetres in thickness). In both groups, After applying cream or fibrous‐hydrocolloid dressing, the wound was covered with a transparent, adhesive, waterproof dressing (Opsite, Smith & Nephew, Taipei, Taiwan). After two weeks, the wounds in both groups were all reconstructed by split‐thickness skin graft or primary closure. Co‐intervention: sharp surgical debridement (including resection of necrotic soft tissue and bone, sinus tracts, fistulae, undermined borders, callus) to form viable wound margins was performed before randomization and repeated as needed during the dosing period. Systemic antimicrobial agents were allowed for treatment of infections. Nonweight bearing or offloading was required for all subjects. Prohibited treatments during the study period included immunosuppressive agents, corticosteroids, chemotherapy and radiotherapy. |
|
| Outcomes | Primary outcome: Percent change in wound size Secondary outcomes: Adverse event (no. of participants with at least one adverse event). |
|
| Notes | Trial data: Analysis 5.1 Funding source: No details | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: Treatment allocation was performed before site initiation. Permuted‐block treatment allocation was used to assign participants to each group. A list of sequential numbers was generated using a permuted‐block randomization procedure with a block size of 4 in SAS 9.1, with each number randomly
assigned to one group. Comment: Adequate |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Patients meeting the inclusion and exclusion criteria were randomly assigned in a 1:1 ratio to the WH‐1 cream group or the hydrocolloid fiber dressing group according to a predefined randomization schedule" Comment: No mention of how the randomisation sequence was implemented |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: No mention of blinding in report |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: No mention of blinding in report |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 24 participants were randomised and 21 were included in the analysis: the 3 exclusions represent 12.5% of the total sample size. Classed as high risk of bias. |
| Selective reporting (reporting bias) | Low risk | No evidence. Healed wounds were not planned in this study. |
| Other bias | Unclear risk | Possibility some baseline imbalance perhaps due to the small sample size. |
Piaggesi 2001.
| Methods | Single‐centred, 2‐armed RCT comparing a fibrous‐hydrocolloid (hydrofibre) dressing (Aquacel, ConvaTec) with saline moistened gauze undertaken in Italy Duration of follow up: patients were followed until complete re‐epithelialisation occurred. Maximum calculated by review authors as approximately 350 days. | |
| Participants | 20 participants Inclusion criteria: diabetic patients (type 1 or type 2) for over 5 years, between age 18 to 75 years, foot ulcer more than 3 weeks, > 1 cm wide and 1 cm deep, good peripheral blood supply (palpable peripheral pulses or ABPI > 0.9). Ulcers were due to diabetic neuropathy, or surgical drainage of a previous infection or both. Exclusion criteria: active infection documented by clinical local (redness, swelling, tenderness, purulent discharge, odour) or systemic (fever, malaise, leukocytosis) and confirmed with culture exams. Serum creatinine > 2 mg/dl, recent episodes of ketoacidosis, malignancies and any systemic therapy or chronic pathology which could obstruct the healing process. | |
| Interventions | Group A (n = 10): fibrous‐hydrocolloid (hydrofibre) dressing (Aquacel, ConvaTec); dressing changed every second or third day, depending on the extent of wound exudate Group B (n = 10): saline‐moistened gauze; dressing renewed twice a day with saline to prevent drying out Both trial dressings were covered by several layers of gauze Co‐interventions: participants received special postoperative shoes to relieve the pressure from the ulcerated foot. Participants were trained to walk with crutches until there was satisfactory healing. | |
| Outcomes | Primary outcome: ulcer healing (number of ulcers healed; healing time in days; median % reduction in lesion volume; median % of granulation tissue) Secondary outcomes: amputation; adverse events; cost/resource use (average number of days between dressings changes) Health‐related quality of life and ulcer recurrence not reported | |
| Notes | Trial data: Analysis 5.1 Study authors have also reported the results for healing time excluding the patients suffering from infection (NOT extracted) Funding source: grant from Italian health board (Ministero della Sanita: Ricerca Finalizzata 1999 ‐ Convenzione no. RF 99.52). Dressing material and devices were supplied by the hospital during the study as part of the routine therapy: manufacturers were not involved in any part of the experiment. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote:"Patients were randomly sorted into two different groups using a computer ‐generated list". Comment: method of generation of random schedule reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: the process of randomising participants, including who did this is not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: no mention of blinding in study report |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote:"After 8 weeks patients were blindly evaluated by one of the authors (M.R) for rate of RVL and rate of GT". Comment: it is not clear if healing assessment was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no indication of incomplete outcome data in paper |
| Selective reporting (reporting bias) | Low risk | Comment: based on study report, protocol not obtained |
| Other bias | Low risk | Comment: funded by non‐commercial organisation |
ABPI: ankle brachial pressure index ITT: intention‐to‐treat RCT: randomised controlled trial
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Agas 2006 | Study did not randomise participants |
| Ahroni 1993 | The dressing groups evaluated in this study were not hydrocolloid dressings |
| Altman 1993 | No single, identifiable dressing type evaluated. |
| Alvarez 2003 | The dressing groups evaluated in this study were not hydrocolloid dressings |
| Apelqvist 1990 | Relevant outcome data are not reported: study outcome was limited to change in size of necrotic material on the wound. Study authors were unable to provide the original healing outcome data. |
| Apelqvist 1996 | No single, identifiable dressing type evaluated. |
| Apelqvist 2004 | No single, identifiable dressing type evaluated. |
| Armstrong 2004 | No single, identifiable dressing type evaluated. |
| Baker 1993 | The dressing groups evaluated in this study were not hydrocolloid dressings |
| Belcaro 2010 | The dressing groups evaluated in this study were not hydrocolloid dressings |
| Blackman 1994 | The dressing groups evaluated in this study were not hydrocolloid dressings |
| Bogaert 2004 | Study did not randomise participants |
| Bradshaw 1989 | Trial stopped after recruiting six participants. No data presented. Authors not contacted for healing data. |
| Caravaggi 2003 | Other intervention, not dressings, differs between trial arms |
| Chang 2000 | Study did not include diabetic foot ulcers |
| Chauhan 2003 | Other intervention, not dressings, differ between trial arms |
| Chirwa 2010 | Study did not randomise participants |
| Cuevas 2007 | No single, identifiable dressing type evaluated. |
| D'Hemecourt 1998 | The dressing groups evaluated in this study were not hydrocolloid dressings |
| Dash 2009 | Other intervention, not dressings, differ between trial arms |
| Diehm 2005 | Study did not randomise participants |
| Donaghue 1998 | The dressing groups evaluated in this study were not hydrocolloid dressings |
| Driver 2006 | Other intervention, not dressings, differs between trial arms |
| Edmonds 2009 | Other intervention, not dressings, differs between trial arms |
| Eginton 2003 | No single, identifiable dressing type evaluated. |
| Etoz 2003 | Study did not randomise participants |
| Farac 1999 | Author contacted: study not suitable for inclusion due to data quality issues |
| Foo 2004 | The dressing groups evaluated in this study were not hydrocolloid dressings |
| Foster 1994 | The dressing groups evaluated in this study were not hydrocolloid dressings |
| Foster 1999 | Other intervention, not dressings, differs between trial arms |
| Gao 2007 | Other intervention, not dressings, differs between trial arms |
| Gentzkow 1996 | Other intervention, not dressings, differs between trial arms |
| Gottrup 2011 | The dressing groups evaluated in this study were not hydrocolloid dressings |
| Hanft 2002 | Other intervention, not dressings, differs between trial arms |
| Jeffery 2008 | Study did not randomise participants |
| Jensen 1998 | The dressing groups evaluated in this study were not hydrocolloid dressings |
| Kordestani 2008 | The dressing groups evaluated in this study were not hydrocolloid dressings |
| Lalau 2002 | The dressing groups evaluated in this study were not hydrocolloid dressings |
| Landsman 2010 | Other intervention, not dressings, differs between trial arms |
| Lazaro‐Martinez 2007 | No single, identifiable dressing type evaluated. |
| Lipkin 2003 | Other intervention, not dressings, differs between trial arms |
| Markevich 2000 | The dressing groups evaluated in this study were not hydrocolloid dressings |
| Marston 2001 | Other intervention, not dressings, differs between trial arms |
| Mazzone 1993 | The dressing groups evaluated in this study were not hydrocolloid dressings |
| McCallon 2000 | Study did not randomise participants |
| Mody 2008 | Study did not include diabetic foot ulcers |
| Moretti 2009 | Other intervention, not dressings, differs between trial arms |
| Mueller 1989 | Other intervention, not dressings, differs between trial arms |
| Mulder 1994 | The dressing groups evaluated in this study were not hydrocolloid dressings |
| Munter 2006 | The dressing groups evaluated in this study were not hydrocolloid dressings |
| Novinscak 2010 | No single, identifiable dressing type evaluated. |
| Palao i Domenech 2008 | The dressing groups evaluated in this study were not hydrocolloid dressings |
| Parish 2009 | Other intervention, not dressings, differs between trial arms |
| Pham 1999 | Other intervention, not dressings, differs between trial arms |
| Piaggesi 1997 | Study did not randomise participants |
| Reyzelman 2009 | No single, identifiable dressing type evaluated. |
| Roberts 2001 | The dressing groups evaluated in this study were not hydrocolloid dressings |
| Robson 2005 | Other intervention, not dressings, differs between trial arms |
| Robson 2009 | Study did not include diabetic foot ulcers |
| Sabolinski 2000 | Other intervention, not dressings, differs between trial arms |
| Sabolinski 2001 | Other intervention, not dressings, differs between trial arms |
| Shaw 2010 | Other intervention, not dressings, differs between trial arms |
| Shukrimi 2008 | Other intervention, not dressings, differs between trial arms |
| Sibbald 2011 | The dressing groups evaluated in this study were not hydrocolloid dressings |
| Solway 2011 | Study did not randomise participants |
| Steed 1992 | Other intervention, not dressings, differs between trial arms |
| Steed 1995 | Other intervention, not dressings, differs between trial arms |
| Steed 1996 | Other intervention, not dressings, differs between trial arms |
| Subrahmanyam 1993 | The dressing groups evaluated in this study were not hydrocolloid dressings |
| Trial 2010 | The dressing groups evaluated in this study were not hydrocolloid dressings |
| Turns 2012 | Study did not randomise participants |
| Urbaneie‐Rovan 1999 | No single, identifiable dressing type evaluated. |
| Vandeputte 1997 | The dressing groups evaluated in this study were not hydrocolloid dressings |
| Varma 2006 | No single, identifiable dressing type evaluated. |
| Veves 2001 | Other intervention, not dressings, differs between trial arms |
| Veves 2002 | The dressing groups evaluated in this study were not hydrocolloid dressings |
| Whalley 2001 | The dressing groups evaluated in this study were not hydrocolloid dressings |
| Woo 2010 | The dressing groups evaluated in this study were not hydrocolloid dressings |
| Yao 2007 | Other intervention, not dressings, differs between trial arms |
| Zimny 2003 | Other intervention, not dressings, differs between trial arms |
Characteristics of studies awaiting assessment [ordered by study ID]
Ogce 2007.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Translation required |
Contributions of authors
Jo Dumville developed the review and co‐ordinated development, completed the first draft of the review, made an intellectual contribution, approved the final version prior to submission and is the guarantor of the review and the update. Sohan Deshpande completed the first draft of the review, made an intellectual contribution and approved the final version of the review prior to submission. Susan O’Meara edited the review, made an intellectual contribution and approved the final version of the review and the update prior to submission. Katharine Speak made an intellectual contribution to the review, advised on the review and approved the final version prior to submission.
Contributions of editorial base:
Nicky Cullum: edited the protocol and review; advised on methodology, interpretation and content. Approved the final review prior to submission. Joan Webster, Editor: approved the review update prior to submission. Sally Bell‐Syer: co‐ordinated the editorial process. Advised on methodology, interpretation and content. Edited the review. Ruth Foxlee: designed the search strategy and edited the search methods section.
Sources of support
Internal sources
Department of Health Sciences, University of York, UK.
External sources
NIHR Programme Grants for Applied Research, UK.
NIHR/Department of Health (England), Cochrane Wounds Group, UK.
Declarations of interest
Susan O'Meara and Jo Dumville receive funding from the National Institute for Health Research (NIHR) under its Programme Grants for Applied Research programme. This study presents independent research funded by the National Institute for Health Research (NIHR) under its Programme Grants for Applied Research programme (RP‐PG‐0407‐10428). The views expressed in this presentation are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Sohan Deshpande and Katharine Speak: none declared.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Clever 1995 {published data only}
- Clever HU, Dreyer M. Comparing two wound dressings for the treatment of neuropathic diabetic foot ulcers. Proceedings of the 5th European Conference on Advances in Wound Management; 1995, 21‐24 November; Harrogate, UK. 1995:201‐3.
Jeffcoate 2009 {published data only}
- Jeffcoate WJ, Price PE, Phillips CJ, Game FL, Mudge E, Davies S, et al. Randomised controlled trial of the use of three dressing preparations in the management of chronic ulceration of the foot in diabetes. Health Technology Assessment 2009;13(54):1‐110. [DOI] [PubMed] [Google Scholar]
Jude 2007 {published data only}
- Jude EB, Apelqvist, Spraul M, Martini J. Prospective randomized controlled study of Hydrofiber dressing containing ionic silver or calcium alginate dressings in non‐ischaemic diabetic foot ulcers. Diabetic Medicine 2007;24(3):280‐8. [DOI] [PubMed] [Google Scholar]
Kuo 2012 {published data only}
- Kuo Y‐S, Chien H‐F, Lu W. Plectranthus amboinicus and Centella asiatica Creamforthe Treatment of Diabetic Foot Ulcers. Evidence‐Based Complementary and Alternative Medicine 2012;418679:doi: 10.1155/2012/418679. [DOI] [PMC free article] [PubMed] [Google Scholar]
Piaggesi 2001 {published data only}
- Piaggesi A, Baccetti F, Rizzo L, Romanelli, Navalesi R, Benzi L. Sodium carboxyl‐methyl‐cellulose dressings in the management of deep ulcerations of diabetic foot. Diabetic Medicine 2001;18(4):320‐4. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Agas 2006 {published data only}
- Agas CM, Bui TD, Driver VR, Gordon IL. Effect of window casts on healing rates of diabetic foot ulcers. Journal of Wound Care 2006;15(2):80‐3. [DOI] [PubMed] [Google Scholar]
Ahroni 1993 {published data only}
- Ahroni JH, Boyko EJ, Pecoraro RE. Diabetic foot ulcer healing: extrinsic vs intrinsic factors. Wounds 1993;5(5):245‐55. [Google Scholar]
Altman 1993 {published data only}
- Altman MI, Mulder GD. Multi‐centre evaluation of Nu‐Gel wound dressing in full‐thickness chronic wounds of the lower extremities. 3rd European Conference on Advances in Wound Management; 1993, 19‐22 October; Harrogate, UK. 1993:164‐5.
Alvarez 2003 {published data only}
- Alvarez OM, Roger RS, Booker JG, Patel M. Effect of non contact normothermic wound therapy on the healing of neuropathic (diabetic) foot ulcers: an interim analysis of 20 patients. Journal of Foot and Ankle Surgery 2003;42(1):30‐5. [DOI] [PubMed] [Google Scholar]
Apelqvist 1990 {published data only}
- Apelqvist J, Larsson J, Stenstrom A. Topical treatment of necrotic foot ulcers in diabetic patients: a comparative trial of DuoDerm and MeZinc. British Journal of Dermatology 1990;123:787‐92. [DOI] [PubMed] [Google Scholar]
Apelqvist 1996 {published data only}
- Apelqvist J, Ragnarson‐Tennvall G. Cavity foot ulcers in diabetic patients: a comparative study of cadexomer iodine ointment and standard treatment. An economic analysis alongside a clinical trial. Acta Dermato‐Venereologica 1996;76(3):321‐5. [DOI] [PubMed] [Google Scholar]
Apelqvist 2004 {published data only}
- Apelqvist J, Piaggesi A. Enamel matrix protein in diabetic foot ulcers, a controlled multicentre study. 2nd World Union of Wound Healing Societies Meeting; 2004 ,8‐13 July; Paris. 2004:8‐13.
Armstrong 2004 {published data only}
- Armstrong DG, Lavery LA, Frykberg RG, Andros G, Attinger CE, Boulton AJM. VAC therapy appears to heal complex DFU. 2nd World Union of Wound Healing Societies Meeting; 2004 ,8‐13 July; Paris. 2004:22.
Baker 1993 {unpublished data only}
- Baker NR, Creevy J. A randomised comparative pilot study to evaluate Allevyn hydrocellular dressings and Sorbsan calcium‐alginate dressings in the treatment of diabetic foot ulcers. Unpublished 1993.
Belcaro 2010 {published data only}
- Belcaro G, Cesarone MR, Errichi BM, Ricci A, Dugall M, Pellegrini L, et al. Venous and diabetic ulcerations: management with topical multivalent silver oxide ointment. Panminerva Medica 2010;52(2 (Suppl 1)):37‐42. [PubMed] [Google Scholar]
Blackman 1994 {published data only}
- Blackman JD, Senseng D, Quinn L, Mazzone T. Clinical evaluation of a semipermeable polymeric membrane dressing for the treatment of chronic diabetic foot ulcers. Diabetic Care 1994;17(4):322‐5. [DOI] [PubMed] [Google Scholar]
Bogaert 2004 {published data only}
- Bogaert T, Meert S, Derre B, Goethals E. Topical autologous platelet gel enhances healing of chronic diabetic ulcer: Preliminary report. 2nd World Union of Wound Healing Societies Meeting; 2004 ,8‐13 July; Paris. 2004:114.
Bradshaw 1989 {published data only}
- Bradshaw T, Gem J, Boulton A. The use of Kaltostat in the treatment of ulceration in the diabetic foot. Chiropodist 1989;44(9):204‐7. [Google Scholar]
Caravaggi 2003 {published data only}
- Caravaggi C, Giglio R, Pritelli C, Sommaria M, Dalla Noce S, Faglia E, et al. HYAFF 11‐based autologous dermal and epidermal grafts in the treatment of noninfected diabetic plantar and dorsal foot ulcers: a prospective, multicenter, controlled, randomized clinical trial. Diabetes Care 2006;10:2853‐9. [DOI] [PubMed] [Google Scholar]
Chang 2000 {published data only}
- Chang DW, Sanchez LA, Veith FJ, Wain RA, Okhi T, Suggs WD. Can a tissue‐engineered skin graft improve healing of lower extremity foot wounds after revascularization?. Annals of Vascular Surgery 2000;14(1):44‐9. [DOI] [PubMed] [Google Scholar]
Chauhan 2003 {published data only}
- Chauhan VS, Rasheed MA, Pandley SS, Shukla VK. Nonhealing wounds ‐ a therapeutic dilemma. International Journal of Lower Extremity Wounds 2003;2(1):40‐5. [DOI] [PubMed] [Google Scholar]
Chirwa 2010 {published and unpublished data}
- Chirwa Z, Bhengu T, Litiane K. The cost effectiveness of using calcium alginate silver matrix in the treatment of wounds. European Wound Management Association Journal 2010;10(2):257, Abstract P299. [Google Scholar]
Cuevas 2007 {published data only}
- Cuevas FR, Velázquez Méndez AA, Andrade IC. Zinc hyaluronate effects on ulcers in diabetic patients [Efecto delhialuronato de zinc sobre las úlceras en pacientes con diabetes]. Gerokomos 2007;18(2):91‐105. [Google Scholar]
D'Hemecourt 1998 {published data only}
- D'Hemecourt PA, Smiell JM, Karim MR. Sodium carboxymethyl cellulose aqueous‐based gel vs becaplermin gel in patients with nonhealing lower extremity diabetic ulcers. Wounds 1998;10(3):69‐75. [Google Scholar]
Dash 2009 {published data only}
- Dash NR, Dash SN, Routray P, Mohapatra S, Mohapatra PC. Targeting nonhealing ulcers of lower extremity in human through autologous bone marrow‐derived mesenchymal stem cells. Rejuvenation Research 2009;12(5):359‐66. [DOI] [PubMed] [Google Scholar]
Diehm 2005 {published data only}
- Diehm C, Lawall H. Evaluation of Tielle hydro polymer dressings in the management of chronic exuding wounds in primary care. International Wound Journal 2005;2(1):26‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Donaghue 1998 {published data only}
- Donaghue VM, Chrzan JS, Rosenblum BI, Giurini JM, Habershaw GM, Veves A. Evaluation of a collagen‐alginate wound dressing in the management of diabetic foot ulcers. Advances in Wound Care 1998;11(3):114‐9. [PubMed] [Google Scholar]
Driver 2006 {published data only}
- Driver VR, Hanft J, Fylling CP, Beriou JM, and Autologel Diabetic Foot Ulcer Study Group. A prospective randomized controlled trial of autologous platelet‐rich plasma gel for the treatment of diabetic foot ulcers. Ostomy/Wound Management 2006;52(6):68‐70, 72, 74. [PubMed] [Google Scholar]
Edmonds 2009 {published data only}
- Edmonds M. Apligraf in the treatment of neuropathic diabetic foot ulcers. International Journal of Lower Extremity Wounds 2009;8(1):11‐8. [DOI] [PubMed] [Google Scholar]
Eginton 2003 {published data only}
- Eginton MT, Brown KR, Seabrook GR, Towne JB, Cambria RA. A prospective randomized evaluation of negative‐pressure wound dressings for diabetic foot wounds. Annals of Vascular Surgery 2003;17(6):645‐9. [DOI] [PubMed] [Google Scholar]
Etoz 2003 {published data only}
- Etoz A, Ozgenel Y, Ozcan M. The use of negative pressure wound therapy on diabetic foot ulcers: a preliminary controlled trial. Wounds: A Compendium of Clinical Research and Practice 2004;16(8):264‐9. [Google Scholar]
Farac 1999 {published data only}
- Farac KP, Grief R, Sessler DI. Radiant heat bandage speeds healing of diabetic‐neuropathic foot ulcers. 31st Annual Wound, Ostomy and Continence Conference; 1999 June; Minneapolis, MN. 1999:488.
Foo 2004 {published data only}
- Foo LSS, Chua BSY, Chia GT, Tan SB, Howe TS. Vacuum assisted closure vs moist gauze dressing in post‐operative diabetic foot wounds: early results from a randomised controlled trial. 2nd World Union of Wound Healing Societies Meeting; 2004 ,8‐13 July; Paris. 2004:8‐9.
Foster 1994 {published data only}
- Foster AVM. Comparing dressings for diabetic foot ulcers ‐ letter. Journal of Wound Care 1995;4(1):10. [Google Scholar]
- Foster AVM, Greenhill MT, Edmonds ME. A randomised comparative study to compare Allevyn hydrocellular dressings and Kaltostat calcium‐sodium alginate dressings in the treatment of diabetic foot ulcers. 5th Annual Symposium on Advanced Wound Care; 1992, 23‐25 April; New Orleans, Lousiana. 1992:146.
- Foster AVM, Greenhill MT, Edmonds ME. A randomised comparative study to compatre Allevyn hydrocellular dressings and Kaltostat calcium‐sodium alginate dressings in the treatment of diabetic foot ulcers. 2nd European Conference on Advances in Wound Management; 1992, 20‐23 October; Harrogate, UK. 1993:77.
- Foster AVM, Greenhill MT, Edmonds ME. Comparing two dressings in the treatment of diabetic foot ulcers. Journal of Wound Care 1994;3(5):224‐8. [DOI] [PubMed] [Google Scholar]
Foster 1999 {published data only}
- Foster A, Bates M, Doxford M, Edmonds ME. Treatment of indolent neuropathic ulceration of the diabetic foot with Hyaff. The Diabetic Foot 1999;2(2):72. [Google Scholar]
Gao 2007 {published data only}
- Gao L, Xu GX, Zhou HB. Efficacy evaluation of diabetic foot ulceration treated with iodophors dressings therapy. Journal of Clinical Nursing 2007;6(4):21‐2. [Google Scholar]
Gentzkow 1996 {published data only}
- Gentzkow GD, Iwasaki SD, Hershon KS, Mengel M, Prendergast JJ, Ricotta JJ, et al. Use of Dermagraft, a cultured human dermis, to treat diabetic foot ulcers. Diabetes Care 1996;19(4):350‐4. [DOI] [PubMed] [Google Scholar]
Gottrup 2011 {published data only}
- Gottrup F, Gibson M, Karlsmark T, Bishoff‐Mikkelsen M, Nisbet L, Cullen B. Collagen/ORC/silver treatment of diabetic foot ulcers; a randomised controlled trial. Wound Repair and Regeneration 2011;19(2):A24. [DOI] [PubMed] [Google Scholar]
- Gottrup F, Karlsmark T, Cullen B, Gibson M, Nisbet L. Comparative clinical study to determine the effects of collagen/orc+silver therapy on wound healing of diabetic foot ulcers. EWMA Journal 2010;10(2):83 Abstract 126. [Google Scholar]
Hanft 2002 {published data only}
- Hanft JR, Surprenant MS. Healing of chronic foot ulcers in diabetic patients treated with a human fibroblast‐derived dermis. Journal of Foot and Ankle Surgery 2002;41(5):291‐1. [DOI] [PubMed] [Google Scholar]
Jeffery 2008 {published data only}
- Jeffery S. A honey‐based dressing for diabetic foot ulcers: a controlled study. Diabetic Foot Journal 2008;11(2):87‐91. [Google Scholar]
Jensen 1998 {published data only}
- Jensen JL, Seeley J, Gillin B. A controlled, randomized comparison of two moist wound healing protocols: Carrasyn hydrogel wound dressing and wet‐to‐moist saline gauze. Advances in Wound Care 1998;11(7 (Suppl)):1‐4. [PubMed] [Google Scholar]
Kordestani 2008 {published data only}
- Kordestani S, Shahrezaee M, Tahmasebi MN, Hajimahmodi H, Ghasemali DH, Abyaneh MS. A randomised controlled trial on the effectiveness of an advanced wound dressing used in Iran. Journal of Wound Care 2008;17(7):323‐7. [DOI] [PubMed] [Google Scholar]
Lalau 2002 {published data only}
- Lalau JD, Bresson R, Charpentier P, Coliche V, Erlher S, Ha Van G, et al. Efficacy and tolerance of calcium alginate versus Vaseline gauze dressings in the treatment of diabetic foot lesions. Diabetes and Metabolism 2002;28(3):223‐9. [PubMed] [Google Scholar]
- Lalau JD, Samardzic M. Algoderm dressing versus Vaseline gauze for the treatment of diabetic foot lesions. First World Wound Healing Congress; 2000, 10‐13 September; Melbourne, Australia. 2000:96.
Landsman 2010 {published data only}
- Landsman A, Agnew P, Parish L, Joseph R, Galiano RD. Diabetic foot ulcers treated with becaplermin and TheraGauze, a moisture‐controlling smart dressing: a randomized, multicenter, prospective analysis. Journal of the American Podiatric Medical Association 2010;100(3):155‐60. [DOI] [PubMed] [Google Scholar]
Lazaro‐Martinez 2007 {published data only}
- Lázaro‐Martíneza JL, García‐Moralesa E, Aragón‐Sánchezc FJ. Randomized comparative trial of a collagen/oxidized regenerated cellulose dressing in the treatment of neuropathic diabetic foot ulcers. EWMA Journal 2008;8(2 (Suppl)):Poster 371. [Google Scholar]
- Lázaro‐Martíneza JL, García‐Moralesa E, Beneit‐Montesinosa JV, Martínez‐de‐Jesúsb FR, Aragón‐Sánchezc FJ. Randomized comparative trial of a collagen/oxidized regenerated cellulose dressing in the treatment of neuropathic diabetic foot ulcers [Estudio aleatorizado y comparativo de unapósito de colágeno y celulosa oxidadaregenerada en el tratamiento de úlcerasneuropáticas de pie diabético]. Cirugia Espanola 2007;82(1):27‐31. [DOI] [PubMed] [Google Scholar]
Lipkin 2003 {published data only}
- Lipkin S, Chaikof E, Isseroff Z, Silverstein P. Effectiveness of bilayered cellular matrix in healing of neuropathic diabetic foot ulcers: results of a multicenter pilot trial. Wounds: A Compendium of Clinical Research and Practice 2003;15(7):230‐6. [Google Scholar]
Markevich 2000 {published data only}
- Markevich YO, McLeod‐Roberts J, Mousley M, Melloy E. Maggot therapy for diabetic neuropathic foot wounds. Diabetologia. Northampton, UK, 2000; Vol. 43, issue Suppl 1:A15.
Marston 2001 {published data only}
- Marston W, Foushee K, Farber M. Prospective randomized study of a cryopreserved, human fibroblast‐derived dermis in the treatment of chronic plantar foot ulcers associated with diabetes mellitus. 14th Annual Symposium on Advances in Wound Care and Medical Research Forum on Wound Repair; 2001 30 April ‐ 3 May; Las Vegas, Nevada. 2001.
Mazzone 1993 {published data only}
- Mazzone T, Blackman JD. Evaluation of a new loaded foam membrane on the healing rate of diabetic foot ulcers. 1st Joint Meeting of the Wound Healing Society and the European Tissue Repair Society; 1993, August; Amsterdam, the Netherlands 1993:88.
McCallon 2000 {published data only}
- McCallon SK, Knight CA, Valiulus JP, Cunningham MW, McCulloch, Farinas LP. Vacuum‐assisted closure versus saline moistened gauze in the healing of postoperative diabetic foot wounds. Ostomy/Wound Management 2000;46(8):28‐34. [PubMed] [Google Scholar]
Mody 2008 {published data only}
- Mody GN, Nirmal IA, Duraisamy S, Perakath B. A blinded, prospective, randomized controlled trial of topical negative pressure wound closure in India. Ostomy/Wound Management 2008;54(12):36‐46. [PubMed] [Google Scholar]
Moretti 2009 {published data only}
- Moretti B, Notarnicola A, Maggio G, Moretti L, Pascone M, Tafuri S, et al. The management of neuropathic ulcers of the foot in diabetes by shock wave therapy. BMC Musculoskeletal Disorders 2009; Vol. 10, issue 54. [DOI: 10.1186/1471-2474-10-54] [DOI] [PMC free article] [PubMed]
Mueller 1989 {published data only}
- Mueller MJ, Diamond JE, Sinacore DR, Delitto A, Blair VP, Drury DA, et al. Total contact casting in treatment of diabetic plantar ulcers. Diabetes Care 1989;12(6):184‐8. [DOI] [PubMed] [Google Scholar]
Mulder 1994 {published data only}
- Mulder GD, Jensen JL, Seeley JE, Peak Andrews K. A controlled randomized study of an amorphous hydrogel to expedite closure of diabetic ulcers. 4th European Tissue Repair Society Meeting; 1994, 25‐28 August; Oxford, England. 1994:130.
Munter 2006 {published data only}
- Munter KC, Beele H, Russell L, Basse E, Grochenig E, Crespi A, et al. The CONTOP study: improved healing of delayed healing ulcers with sustained silver‐releasing foam dressing versus other silver dressings. 16th Conference of the European Wound Management Association; 2006, 18‐20 May; Prague, Czech Republic. 2006:210, Abstract No.P068.
- Munter KC, Beele H, Russell L, Basse PB, Groechenig E, Crespi A, et al. The CONTOP study: a large‐scale,comparative, randomized study in patients treated with a sustained silver‐releasing foam dressing. SAWC; 30 April‐3 May 2006; San Antonio,Texas. 2006:Poster 11.
- Munter KC, Beele H, Russell L, Crespi A, Grocenig E, Basse P, et al. Effect of a sustained silver‐releasing dressing on ulcers with delayed healing: the CONTOP study. Journal of Wound Care 2006;15(5):199‐205. [DOI] [PubMed] [Google Scholar]
- Russell L, Nebbioso G, Munter KC, Beele H, Basse PB, Dienst H. The Contop study: a hydro‐activated silver containing foam dressing versus standard care. 2nd World Union of Wound Healing Societies Meeting; 2004 ,8‐13 July; Paris. 2004:89.
- Scalise A, Forma O, Happe M, Hahn TW. The Contop study: real life experiences from an international study comparing a silver containing hydro‐activated foam dressing with standard wound care. 13th Conference of the European Wound Management Association; 2003, 22‐24 May; Pisa, Italy. 2003:212.
Novinscak 2010 {published data only}
- Novinscak T, Zvorc M, Trojko S, Jozinovic E, Filipovic M, Grudic R. Comparison of cost‐benefit of the three methods of diabetic ulcer treatment: dry, moist and negative pressure. Acta Medica Croatica 2010;64(Suppl 1):113‐5. [Google Scholar]
Palao i Domenech 2008 {published data only}
- Palao i Domenech R, Romanelli M, Tsiftsis DD, Slonkova V, Jortikka A, Johannesen N, et al. Effect of an ibuprofen‐releasing foam dressing on wound pain: a real‐life RCT. Journal of Wound Care 2008;17(8):342, 344‐8. [DOI] [PubMed] [Google Scholar]
Parish 2009 {published data only}
- Parish L, Routh H, Parish J. Diabetic foot ulcers: a randomized multicenter study comparing a moisture‐controlling dressing with a topical growth factor. Journal of the American Academy of Dermatology 2009;60(Suppl 3):AB202. [Google Scholar]
Pham 1999 {published data only}
- Pham HT, Rosenblum BI, Lyons TE, Giurini JM, Chrzan JS, Habershaw GM, et al. Evaluation of a human skin equivalent for the treatment of diabetic foot ulcers in a prospective, randomized, clinical trial. Wounds: A Compendium of Clinical Research and Practice 1999;11(4):76‐86. [Google Scholar]
Piaggesi 1997 {published data only}
- Piaggesi A. A thin hydrocolloid occlusive dressing in diabetic foot ulcerations. Journal of Wound Care 1997;6(3):10. [Google Scholar]
Reyzelman 2009 {published data only}
- Reyzelman A, Crews RT, Moore JC, Moore L, Mukker JS, Offutt S, et al. Clinical effectiveness of an acellular dermal regenerative tissue matrix compared to standard wound management in healing diabetic foot ulcers: a prospective, randomised, multicentre study. International Wound Journal 2009;6(3):196‐208. [DOI] [PMC free article] [PubMed] [Google Scholar]
Roberts 2001 {published data only}
- Roberts GH, Hammad LH, Haggan G, Baker N, Sandeman D, Mani R, et al. Hydrocellular against non‐adherent dressings to treat diabetic foot ulcers ‐ a randomised controlled study. 11th European Tissue Repair Society Annual Conference; 2001 5‐8 September; Cardiff, Wales. 2001:50.
Robson 2005 {published data only}
- Robson MC, Payne WG, Garner WL, Biundo J, Giacalone VF, Cooper DM, et al. Integrating the results of phase IV(post marketing) clinical trial with four previous trials reinforces the position that Regranex (Becaplermin) gel 0.01% is an effective adjunct to the treatment of diabetic foot ulcers. Journal of Applied Research 2005;5(1):35‐45. [Google Scholar]
Robson 2009 {published data only}
- Robson V, Dodd S, Thomas S. Standardized antibacterial honey (Medihoney) with standard therapy in wound care: randomized clinical trial. Journal of Advanced Nursing 2009 ;65 (3):565‐75. [DOI] [PubMed] [Google Scholar]
Sabolinski 2000 {published data only}
- Sabolinski M, Giovino K, Graftskin Diabetic Foot Ulcer Study Group. Risk factors associated with the healing of diabetic foot ulcers. Wound Healing Society Educational Symposium; 2000, 4‐6 June; Toronto, Canada. 2000:14.
- Sabolinski M, Toole T, Giovino K, Apligraft Diabetic Foot Ulcer Study Group. Apligraf (Graftskin) bilayered living skin construct in the treatment of diabetic foot ulcers. First World Wound Healing Congress; 2000, 10‐13 September; Melbourne, Australia. 2000:68‐9.
- Sabolinski ML, Veves A. Graftskin (Apligraf) in neuropathic diabetic foot ulcers. Wounds: A Compendium of Clinical Research and Practice 2000;12(5 (Suppl A)):33A‐6A. [Google Scholar]
- Sabolinski ML, Veves A. Graftskin bilayered living skin construct in the treatment of diabetic foot ulcers. 13th Annual Symposium on Advanced Wound Care and 10th Annual Medical Research Forum on Wound Repair; 2000, 1‐4 April; Dallas, Texas. 2000:C72‐C73.
- Veves A, Falango V, Armstrong DG, Sabolinski ML. Graftskin (Apligraf) a human skin equivalent, promotes wound healing in diabetic foot ulcers in a prospective, randomized, multicenter clinical trial. Tenth Annual Meeting of the European Tissue Repair Society; 24–27 May 2000; Brussels, Belgium. 2000.
Sabolinski 2001 {published data only}
- Sabolinski M, Falanga V. The healing of diabetic plantar foot ulcers of greater than two‐month duration with Graftskin in a randomized prospective study. Eleventh Annual Meeting and Educational Symposuim Wound Healing Society; 2001, 16‐18 May; Albuquerque, New Mexico. 2001:148.
Shaw 2010 {published data only}
- Shaw J, Hughes CM, Lagan KM, Stevenson MR, Irwin CR, Bell PM. The effect of topical phenytoin on healing in diabetic foot ulcers: a randomised controlled trial. Diabetologia 2010;53(Suppl 1):S463. [DOI] [PubMed] [Google Scholar]
Shukrimi 2008 {published data only}
- Shukrimi A, Sulaiman AR, Halim AY, Azril A. A comparative study between honey and povidone iodine as dressing solution for Wagner type II diabetic foot ulcers. Medical Journal of Malaysia 2008;63(1):44‐6. [PubMed] [Google Scholar]
Sibbald 2011 {published data only}
- Sibbald RG, Coutts P, Woo KY. Reduction of bacterial burden and pain in chronic wounds using a new polyhexamethylene biguanide antimicrobial foam dressing‐clinical trial results. Advances in Skin and Wound Care 2011;24(2):78‐84. [DOI] [PubMed] [Google Scholar]
Solway 2011 {published data only}
- Solway DR, Clark WA, Levinson DJ. A parallel open‐label trial to evaluate microbial cellulose wound dressing in the treatment of diabetic foot ulcers. International Wound Journal 2011;8(1):69‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Steed 1992 {published data only}
- Holloway GA, Steed DL, DeMarco MJ, Matsumoto T, Moosa HH, Webster MW, et al. A randomized controlled multicenter dose response trial of activated platelet supernatant topical CT‐102 in chronic nonhealing diabetic wounds. Wounds: A Compendium of Clinical Research and Practice 1993;5(4):198‐206. [Google Scholar]
- Steed DL. Growth factors in managing diabetic foot ulcers. 5th Annual Symposium on Advanced Wound Care; 1992, 23‐25 April; New Orleans, Lousiana. 1992.
- Steed DL, Goslen JB, Holloway GA, Malone JM, Bunt TJ, Webster MW. Randomized prospective double‐blind trial in healing chronic diabetic foot ulcers. Diabetic Care 1992;15(11):1598‐604. [DOI] [PubMed] [Google Scholar]
Steed 1995 {published data only}
- Steed DL, Ricotta JJ, Prendergast JJ, Kaplan RJ, Webstar MW, McGill JB. Promotion and acceleration of diabetic ulcer healing by arginine‐glycine‐aspartic acid (RGD) peptide matrix. RGD Study Group. Diabetes Care 1995;18(1):39‐46. [DOI] [PubMed] [Google Scholar]
Steed 1996 {published data only}
- Steed DL, Edington HD, Webster MW. Recurrence rate of diabetic neurotrophic foot ulcers healed using topical application of growth factors released from platelets. Wound Repair and Regeneration 1996;4(2):230‐3. [DOI] [PubMed] [Google Scholar]
Subrahmanyam 1993 {published data only}
- Subrahmanyam M. Honey as a surgical dressing for burns and ulcers. Indian Journal of Surgery 1993;55(9):468‐73. [Google Scholar]
Trial 2010 {published data only}
- Teot L, Trial C, Lavigne JP. Results of RCT on the antimicrobial effectiveness of a new silver alginate wound dressing. European Wound Management Association Journal 2008;8(Suppl 2):54 Abstract no. 69. [Google Scholar]
- Trial C, Darbas H, Lavigne J. Assessment of the antimicrobial effectiveness of a new silver alginate wound dressing: a RCT. Journal of Wound Care 2010;19(1):20‐6. [DOI] [PubMed] [Google Scholar]
Turns 2012 {published data only}
- Turns M. Evaluation of NOSF in neuropathic diabetic. Wounds UK 2012;8(1):100‐6. [Google Scholar]
Urbaneie‐Rovan 1999 {published data only}
- Urbaneie‐Rovan V. Efficacy and cost of different dressings. Practical Diabetes International 1999;16(2):S3. [Google Scholar]
Vandeputte 1997 {published data only}
- Vandeputte J, Gryson L. Clinical trial on the control of diabetic foot infection by an immunomodulating hydrogel containing 65% glycerine. Proceedings of the 6th European Conference on Advances in Wound Management; 1‐4 October, 1996; Amsterdam. 1996:50‐3.
- Vandeputte J, Gryson L. Diabetic foot infection controlled by immuno‐modulating hydrogel containing 65% glycerine. Personal Communication: Presentation of a clinical trial. 1996.
Varma 2006 {published data only}
- Varma AK, Bal A, Kumar H, Kesav R, Nair S. Efficacy of polyurethane foam dressing in debrided diabetic lower limb wounds. Wounds: A Compendium of Clinical Research and Practice 2006;18(10):300‐6. [Google Scholar]
Veves 2001 {published data only}
- Veves A, Falanga V, Armstrong DG, Sabolinski ML. Graftskin, a human skin equivalent, is effective in the management of neuropathic diabetic foot ulcers. Diabetes Care 2001;24(2):290‐5. [DOI] [PubMed] [Google Scholar]
Veves 2002 {published data only}
- Sheehan P, Jones P, Caselli A, Giurini JM, Veves A. Percent change in wound area of diabetic foot ulcers over a 4‐week period is a robust predictor of complete healing in a 12‐week prospective trial. Diabetes Care 2003;26(6):1879‐82. [DOI] [PubMed] [Google Scholar]
- Veves A. Promogran clinical trial. The Diabetic Foot 2001;4(4):S4‐S5. [Google Scholar]
- Veves A. Wound care device expert meeting. Summary report. Promogran ‐ clinical trial data. The Diabetic Foot 2001;4(3):S8‐S10. [Google Scholar]
- Veves A, Sheehan P, Pham HT. A randomized controlled trial of Promogran (a collagen / oxidised regenerated cellulose dressing) vs standard treatment in the management of diabetic foot ulcers. Archives of Surgery 2002;137:822‐7. [DOI] [PubMed] [Google Scholar]
Whalley 2001 {published data only}
- Capillas R, Whalley A, Boulton AMJ, Dargis V, Harding K, Acker K. Performance characteristics and safety of hydrogels using a non‐adhesive foam dressing as secondary dressing in the treatment of diabetic foot ulcers. 12th Conference of the European Wound Management Association;2002, 23‐25 May; Granada, Spain. 2002:261.
- Whalley A, Boulton AJM, Dargis V, Harding K, Acker K, Capillas R. Characteristics of the efficacy and safety of a hydrogen using a hydropolymer as the secondary dressing in the treatment of ulcers of the diabetic foot. 12th Conference of the European Wound Management Association;2002, 23‐25 May; Granada, Spain. 2002:322.
- Whalley A, Boulton AJM, Dargis V, Harding K, Acker K, Capillas R. Performance characteristics and safety of Purilon gel versus Intrasite using Biatain non‐adhesive dressing as secondary dressing in the treatment of diabetic foot ulcers. 11th European Tissue Repair Society Annual Conference; 2001 5‐8 September; Cardiff, Wales. 2001:49.
Woo 2010 {published data only}
- Woo K, Sibbald G, Coutts P. Reduction of infection and pain in chronic wounds using a new antimicrobial foam dressing. EWMA Journal 2010;10(2):59 Abstract 78. [DOI] [PubMed] [Google Scholar]
Yao 2007 {published data only}
- Yao XZ, Chen W. Effectiveness of feet soaking with Chinese herbs combined with changing dressings in the treatment of diabetic foot ulceration. Journal of Nursing Science 2007;22(5):42‐3. [Google Scholar]
Zimny 2003 {published data only}
- Zimny S, Schatz H, Pfohl U. The effects of applied felted foam on wound healing and healing times in the therapy of neuropathic diabetic foot ulcers. Diabetic Medicine 2003;20(8):622‐5. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Ogce 2007 {published data only}
- Ogce F, Dramali A. The treatment of diabetic foot ulcers. Sendrom 2007;19(12):79‐81. [Google Scholar]
Additional references
Abbott 2002
- Abbott CA, Carrington AL, Ashe H, Bath S, Every LC, Griffiths J, et al. The North West Diabetes Foot Care Study: incidence of and risk factors for, new diabetic foot ulceration in a community‐based patient cohort. Diabetic Medicine 2002;19:377‐84. [DOI] [PubMed] [Google Scholar]
Apelqvist 2000a
- Apelqvist J, Bakker K, Houtum WH, Nabuurs‐Franssen MH, Schaper NC. International consensus and practical guidelines on the management and the prevention of the diabetic foot: International Working Group on the Diabetic Foot. Diabetes Metabolism Research and Review 2000;16(Suppl 1):S84‐92. [DOI] [PubMed] [Google Scholar]
Apelqvist 2000b
- Apelqvist J, Larsson J. What is the most effective way to reduce incidence of amputation in the diabetic foot?. Diabetes Metabolism Research and Reviews 2000;16(Suppl 1):S75‐83. [DOI] [PubMed] [Google Scholar]
Becker 2011
- Becker LA, Oxman AD. Chapter 22: Overviews of reviews. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Bergin 2006
- Bergin SM, Wraight P. Silver based wound dressings and topical agents for treating diabetic foot ulcers. Cochrane Database of Systematic Reviews 2006, Issue 1. [DOI: 10.1002/14651858.CD005082.pub2] [DOI] [PubMed] [Google Scholar]
BNF 2010
- British Medical Association and Royal Pharmaceutical Society of Great Britain. British National Formulary Appendix 8: Wound management products and elastic hosiery. http://www.bnf.org.uk/bnf/bnf/current Sept 2010;60.
Cardinal 2009
- Cardinal M, Eisenbud DE, Armstrong DG, Zelen C, Driver V, Attinger C, et al. Serial surgical debridement: a retrospective study on clinical outcomes in chronic lower extremity wounds. Wound Repair and Regeneration 2009;17(3):306‐11. [DOI] [PubMed] [Google Scholar]
Currie 1998
- Currie CJ, Morgan CL, Peters JR. The epidemiology and cost of inpatient care for peripheral vascular disease, infection, neuropathy, and ulceration in diabetes. Diabetes Care 1998;21(1):42‐8. [DOI] [PubMed] [Google Scholar]
Deeks 2002
- Deeks JJ. Issues in the selection of a summary statistic for meta‐analysis of clinical trials with binary outcomes. Statistics in Medicine 2002;21:1575‐600. [DOI] [PubMed] [Google Scholar]
Diabetes UK 2011
- Reports and statistics. http://www.diabetes.org.uk/Professionals/Publications‐reports‐and‐resources/Reports‐statistics‐and‐case‐studies/Reports/. www.diabetes.org.uk, (accessed July 2011).
Dorresteijn 2001
- Dorresteijn JA N, Kriegsman DM W, Assendelft WJJ, Valk GD. Patient education for preventing diabetic foot ulceration. Cochrane Database of Systematic Reviews 2001, Issue 4. [DOI: 10.1002/14651858.CD001488.pub2] [DOI] [PubMed] [Google Scholar]
Dorresteijn 2010
- Dorresteijn JAN, Kriegsman DMW, Valk GD. Complex interventions for preventing diabetic foot ulceration. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD007610.pub2] [DOI] [PubMed] [Google Scholar]
Dumville 2011a
- Dumville JC, Deshpande S, O'Meara S, Speak K. Foam dressings for healing diabetic foot ulcers. Cochrane Database of Systematic Reviews 2011, Issue 9. [DOI: 10.1002/14651858.CD009111.pub2.] [DOI] [PubMed] [Google Scholar]
Dumville 2011b
- Dumville JC, O'Meara S, Deshpande S, Speak K. Hydrogel dressings for healing diabetic foot ulcers. Cochrane Database of Systematic Reviews 2011, Issue 5. [DOI: 10.1002/14651858.CD009101.pub2] [DOI] [PubMed] [Google Scholar]
Dumville 2012
- Dumville JC, O'Meara S, Deshpande S, Speak K. Alginate dressings for healing diabetic foot ulcers. Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI: 10.1002/14651858.CD009110] [DOI] [PubMed] [Google Scholar]
Edwards 2010
- Edwards J, Stapley S. Debridement of diabetic foot ulcers. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD003556.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fiskin 1996
- Fiskin R, Digby M. Which dressing for diabetic foot ulcers?. Practical Diabetes International 1996;13(4):107‐9. [Google Scholar]
Gregg 2004
- Gregg EW, Sorlie P, Paulose‐Ram R, Gu Q, Eberhardt MS, Wolz M, et al. Prevalence of lower‐extremity disease in the U.S. adult population >40 years of age with and without diabetes. Diabetes Care 2004;27(7):1591‐7. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group (Editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hinchliffe 2008
- Hinchliffe R, Valk GD, Apelqvist J, Armstrong DG, Bakker K, Game FL, et al. A systematic review of the effectiveness of interventions to enhance the healing of chronic ulcers of the foot in diabetes. Diabetes Metabolism Research Review 2008;24(Suppl 1):S119‐44. [DOI] [PubMed] [Google Scholar]
Kumar 1994
- Kumar S, Ashe HA, Parnell LN, Fernando DJ, Tsigos C, Young RJ, et al. The prevalence of foot ulceration and its correlates in type 2 diabetic patients: a population‐based study. Diabetic Medicine 1994;11:480‐4. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J, on behalf of the Cochrane Information Retrieval Methods Group. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Margolis 1999
- Margolis D, Kantor J, Berlin J. Healing of diabetic neuropathic foot ulcers receiving standard treatment. A meta‐analysis. Diabetes Care 1999;22:692‐5. [DOI] [PubMed] [Google Scholar]
Mason 1999
- Mason J, O'Keeffe C, Hutchinson A, McIntosh A, Young R, Booth A. A systematic review of foot ulcer in patients with type 2 diabetes mellitus. II: treatment. Diabetes Medicine 1999;16(11):889‐909. [DOI] [PubMed] [Google Scholar]
Moher 2010
- Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, Elbourne D, Egger M, Altman DG, for the CONSORT Group. CONSORT 2010 Explanation and Elaboration: updated guidelines for reporting parallel group randomised trial. BMJ 2010;340:c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
Morris 1998
- Morris AD, McAlpine R, Steinke D, Boyle DI, Ebrahim AR, Vasudev N, et al. Diabetes and lower limb amputations in the community. A retrospective cohort study. DARTS/MEMO Collaboration. Diabetes Audit and Research in Tayside Scotland/Medicines Monitoring Unit. Diabetes Care 1998;21:738‐43. [DOI] [PubMed] [Google Scholar]
Murray 1996
- Murray HJ, Young MJ, Hollis S, Boulton AJ. The association between callus formation, high pressures and neuropathy in diabetic foot ulceration. Diabetic Medicine 1996;13:979‐82. [DOI] [PubMed] [Google Scholar]
Nelson 2006
- Nelson EA, O'Meara S, Golder S, Dalton J, Craig D, Iglesias C, DASIDU Steering Group. Systematic review of antimicrobial treatments for diabetic foot ulcers. Diabetes Medicine 2006;23(4):348‐59. [DOI] [PubMed] [Google Scholar]
O'Meara 2000
- Meara S, Cullum N, Majid M, Sheldon T. Systematic reviews of wound care management: (3) antimicrobial agents for chronic wounds; (4) diabetic foot ulceration. Health Technology Assessment 2000;4(21):1‐237. [PubMed] [Google Scholar]
Oyibo 2001
- Oyibo SO, Jude EB, Tarawneh I, Nguyen HC, Harkless LB, Boulton AJ. A comparison of two diabetic foot ulcer classification systems: the Wagner and the University of Texas wound classification systems. Diabetes Care 2001;24(1):84‐8. [DOI] [PubMed] [Google Scholar]
Pecoraro 1990
- Pecoraro RE, Reiber GE, Burgess EM. Pathways to diabetic limb amputation: basis for prevention. Diabetes Care 1990;13:513‐21. [DOI] [PubMed] [Google Scholar]
Pound 2005
- Pound N, Chipchase S, Treece K, Game F, Jeffcoate W. Ulcer‐free survival following management of foot ulcers in diabetes. Diabetic Medicine 2005;22(10):1306‐9. [DOI] [PubMed] [Google Scholar]
Reiber 1996
- Reiber G. The epidemiology of diabetic foot problems. Diabetic Medicine 1996;13S:S6‐11. [PubMed] [Google Scholar]
Reiber 1999
- Reiber GE, Vileikyte L, Boyko EJ, Aguila M, Smith DG, Lavery LA, et al. Causal pathways for incident lower extremity ulcers in patients with diabetes from two settings. Diabetes Care 1999;22:157‐62. [DOI] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre. Review Manager (RevMan) Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration 2011.
Schaper 2004
- Schaper NC. Diabetic foot ulcer classification system for research purposes: a progress report on criteria for including patients in research studies. Diabetes Metabolism and Research Review 2004;20(S1):S90‐5. [DOI] [PubMed] [Google Scholar]
Schulz 2010
- Schulz KF, Altman DG, Moher D, CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. PLoS Medicine 2010;7(3):e1000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
SIGN 2011
- Scottish Intercollegiate Guidelines Network (SIGN). Search filters. http://www.sign.ac.uk/methodology/filters.html#random.
Singh 2005
- Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA 2005;293:217‐28. [DOI] [PubMed] [Google Scholar]
Smith 2003
- Smith J. A national survey of podiatry practice in the treatment of diabetic foot ulcers. Unpublished.
Spencer 2000
- Spencer S. Pressure relieving interventions for preventing and treating diabetic foot ulcers. Cochrane Database of Systematic Reviews 2000, Issue 3. [DOI: 10.1002/14651858.CD002302] [DOI] [PubMed] [Google Scholar]
Steed 2006
- Steed DL, Attinger C, Colaizzi T, Crossland M, Franz M, Harkless L, et al. Guidelines for the treatment of diabetic ulcers. Wound Repair and Regeneration 2006;14(6):680‐92. [DOI] [PubMed] [Google Scholar]
Storm‐Versloot 2007
- Storm‐Versloot MN, Vos CG, Ubbink DT, Vermeulen H. Topical silver for preventing wound infection. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD006478] [DOI] [PubMed] [Google Scholar]
Tesfaye 1996
- Tesfaye S, Stephens L, Stephenson J, Fuller J, Platter ME, Ionescu‐Tirgoviste C, et al. The prevalence of diabetic neuropathy and its relationship to glycaemic control and potential risk factors: The EURODIAB IDDM complications study. Diabetalogia 1996;39:1377‐84. [DOI] [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials 2007;7(8):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Gils 1999
- Gils C, Wheeler LA, Mellsrom M, Brinton EA, Mason S, Wheeler CG. Amputation prevention by vascular surgery and podiatry collaboration in high risk diabetic and non‐diabetic patients ‐ the operation desert foot experience. Diabetes Care 1999;22(5):678‐83. [DOI] [PubMed] [Google Scholar]
Wagner 1981
- Wagner FW. The dysvascular foot: a system of diagnosis and treatment. Foot and Ankle 1981;2:64‐122. [DOI] [PubMed] [Google Scholar]
WHO 2005
- Diabetes estimates and projections. http//www.who.int/diabetes/facts/world‐figures/en/index4.html (accessed 23 February 2011).
Wild 2004
- Wild S. Global prevalence of diabetes estimates for the year 2000 and projections for 2030. Diabetes Care 2004;27(5):1047‐53. [DOI] [PubMed] [Google Scholar]
Winter 1963
- Winter GD, Scales JT. Effect of air drying and dressings on the surface of a wound. Nature 1963;197:91. [DOI] [PubMed] [Google Scholar]
Wrobel 2001
- Wrobel JS, Mayfield JA, Reiber GE. Geographic variation of lower limb extremity major amputation in individuals with and without diabetes in the Medicare population. Diabetes Care 2001;24:860‐4. [DOI] [PubMed] [Google Scholar]


