Highlights
-
•
Enteric coronaviruses have evolved to modulate the host innate immunity.
-
•
Viral IFN antagonists have been identified and they are mostly redundant.
-
•
For protection of intestinal epithelia from enteric viruses, type III IFN plays a major role.
Keywords: Porcine epidemic diarrhea virus, Enteric coronavirus, Interferon regulation, Antiviral innate signaling, nsp1, Immune evasion
Abstract
Porcine epidemic diarrhea virus (PEDV) and porcine deltacoronavirus (PDCoV) are emerged and reemerging viruses in pigs, and together with transmissible gastroenteritis virus (TGEV), pose significant economic concerns to the swine industry. These viruses infect epithelial cells of the small intestine and cause watery diarrhea, dehydration, and a high mortality in neonatal piglets. Type I interferons (IFN-α/β) are major antiviral cytokines forming host innate immunity, and in turn, these enteric coronaviruses have evolved to modulate the host innate immune signaling during infection. Accumulating evidence however suggests that IFN induction and signaling in the intestinal epithelial cells differ from other epithelial cells, largely due to distinct features of the gut epithelial mucosal surface and commensal microflora, and it appears that type III interferon (IFN-λ) plays a key role to maintain the antiviral state in the gut. This review describes the recent understanding on the immune evasion strategies of porcine enteric coronaviruses and the role of different types of IFNs for intestinal antiviral innate immunity.
1. Introduction
1.1. Coronavirus enteritis in pigs
Coronavirus enteritis is a highly contagious viral disease in pigs characterized by severe diarrhea, vomiting, and dehydration with a high mortality, especially in neonatal piglets less than 2 weeks of age. Transmissible gastroenteritis virus (TGEV) is a frequent cause for endemic and epidemic viral enteritis in neonates and older pigs (Kim et al., 2000, Laude et al., 1993), and porcine epidemic diarrhea virus (PEDV) has become a major concern in the swine industry in the US since its emergence in April 2013. As with TGEV, PEDV causes severe enteritis in newborn piglets but the mortality can be much higher and may reach up to 100% during epidemics (Debouck and Pensaert, 1980, Song and Park, 2012). PED was first described in the UK in the early 1970s and has since become endemic in some parts of Europe. Highly virulent PEDV has emerged in Asia and subsequently in the US (Chen et al., 2014a, Chen et al., 2014b, Marthaler et al., 2013, Mole, 2013, Stevenson et al., 2013). PEDV has since quickly spread to most states and as of April 2016, 36 states have become endemic for PEDV, raising a significant economic concern (www.aphis.usda.gov/animal-health/secd). PDCoV is a newly emerged swine enteric coronavirus causing enteritis and severe diarrhea in piglets, which was first described in a surveillance study 2012 in Hong Kong, China (Woo et al., 2012). PDCoV is an additional enteric virus emerged in the US in February 2014 (Wang et al., 2014). PDCoV has been identified in the feces and intestinal samples of pigs experiencing severe diarrhea without PEDV and TGEV (Wang et al., 2014). Retrospective studies show that PDCoV has been present in the US at least since August 2013 (McCluskey et al., 2016, Sinha et al., 2015). PDCoV has also spread to at least 20 states of the US (www.aphis.usda.gov/animal-health/secd), Korea, and China (Dong et al., 2015, Lee and Lee, 2014). PDCoV infection seems clinically milder with a lower death rate of 30–40% in neonates than typical PEDV infection (Wang et al., 2014), but co-infection with PEDV, TGEV, or porcine rotavirus is common and result in a severe form of disease (Marthaler et al., 2014, Wang et al., 2014). Clinical symptoms by PDCoV are reproducible in both gnotobiotic pigs and conventional pigs and present diarrhea accompanied by severe histopathological lesions such as villi atrophy in the absence of other pathogens (Chen et al., 2015, Jung et al., 2015c, Ma et al., 2015). Although the clinical signs and pathological lesions are indistinguishable among PEDV, TGEV, and PDCoV (Jung et al., 2014), these coronaviruses are antigenically distinct and thus the cross-protection is absent (Lin et al., 2015). Pathogenesis of these coronaviruses may include destruction of enterocytes and villous atrophy of the intestinal mucosa in the jejunum and ileum (Debouck and Pensaert, 1980, Jung et al., 2014).
Coronaviruses are enveloped viruses containing the genome of single-stranded positive-sense RNA in the family Coronaviridae. The coronavirus genome ranges of 28–30 Kb in length and is currently the largest known viral RNA genome. Coronaviruses are grouped in the Coronavirinae subfamily which is comprised of 4 genera; Alpha-, Beta-, Gamma- and Delta-coronaviruses (http://www.ictvonline.org/virustaxonoym.asp [ICTV, 2014]). Evolutionary studies suggest that Alphacoronavirus and Betacoronavirus are originated from bats while Gammacoronavirus and Deltacoronavirus are from birds (Woo et al., 2012). In humans, coronaviruses infect the respiratory tract and cause symptoms ranging from common colds to severe pneumonia and acute respiratory distress. Severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV) belong to Betacoronavirus, and cause lower respiratory tract infections of significant public concerns (Gralinski and Baric, 2015). Among swine enteric coronaviruses, TGEV and PEDV belong to Alphacoronavirus and share a similar genome organization, whereas PDCoV belongs to Deltacoronavirus (http://www.ictvonline.org/virustaxonomy.asp).
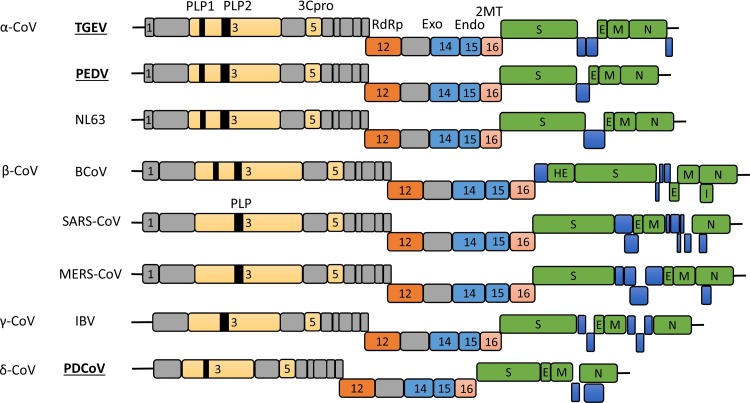
The PEDV genome is approximately 28 Kb in length with the 5′-cap and 3′-polyadenylated tail. The genome is arranged with ORF1a-ORF1b-S-ORF3-E-M-N in order with both termini flanked with untranslated regions (UTRs) (Duarte et al., 1993). ORF1a codes for a large polyprotein pp1a, while ORF1b is expressed as the pp1ab fusion protein via the ribosomal frameshifting. These polyproteins are proteolytically cleaved to 16 nonstructural proteins, nsp1 through nsp16, by the proteinase activity of nsp3 and nsp5. The TGEV genome is similar to that of PEDV and arranged as ORF1a-ORF1b-S-ORF3a-ORF3b-E-M-N-ORF7 in order (Alonso et al., 2002). For PEDV, ORF3 encodes the sole accessory protein, whereas for TGEV, three accessory proteins are encoded by ORF3a, ORF3b, and ORF7 (Brian and Baric, 2005). The PDCoV genome is the smallest of the three coronaviruses with 26 Kb in length, and includes two accessory genes of NS6 and NS7 with the gene order of ORF1a-ORF1b-S-E-M-NS6-N-NS7 (Woo et al., 2012). Notably, PDCoV lacks of the nsp1 gene and thus codes for only 15 nsps in total. Genomic similarities and dissimilarities for α-, β-, γ-, and δ-coronaviruses are illustrated in Fig. 1 .
Fig. 1.
Comparative genome organization of the representative coronaviruses from each genus in the family Coronaviridae. Swine enteric coronaviruses (TGEV, PEDV, and PDCoV) are indicated in bold underlined. Structural proteins are presented in green. Putative accessory proteins are shown in blue. Nonstructural proteins in ORF1a/b are presented in grey or other colors. Papain-like proteinases (PLP1 and PLP2), coronavirus 3C-like proteinase (3Cpro), RNA-dependent RNA polymerase (RdRp), two viral nucleases (nsp14 as exonuclease and nsp15 as endonuclease), and 2′-O-methytransferase (2MT; nsp16) are indicated by respective nsp numbers. The genome for Alaphacoronavirus and Betacoronavirus contains the nsp1 gene in different lengths, whereas the genome for Gammacoronavirus and Deltacoronavirus lacks the nsp1 gene. TGEV, transmissible gastroenteritis coronavirus; PEDV, porcine epidemic diarrhea virus; NL63, human coronavirus NL63; BCoV, bovine coronavirus; SARS-CoV, severe acute respiratory syndrome coronavirus; MERS-CoV, middle east respiratory syndrome coronavirus; IBV, avian infectious bronchitis virus; PDCoV, porcine deltacoronavirus; HE, hemagglutinin-esterase; S, spike; E, envelope; M, membrane; N, nucleocapsid; I, internal open reading frame.
1.2. Early response to viral infection
Upon virus infection, a host reacts quickly to invading virus by producing type I interferons (IFN-α/β) and elicits an antiviral state in infected cells and uninfected neighbor cells. Three different types of IFNs are known: type I (IFN-α/β), type II (IFN-γ), and type III (IFN-λ) IFNs (Table 1 ). For humans, type I IFNs contain 13 subtypes of IFN-α and a single subtype for IFN-β, IFN-ε, IFN-κ, and IFN-ω. For pigs, IFN-α is produced from as many as 17 functional genes. For type II IFN, only a single subtype of IFN-γ is reported for pigs, which is produced largely in immune cells. Type III IFNs include IFN-λ1 (interleukin 29 [IL-29]), λ2 (IL-28A), λ3 (IL-28B), and IFN-λ4 (Kotenko et al., 2003, Prokunina-Olsson et al., 2013, Sheppard et al., 2003).
Table 1.
Types of interferons, antiviral functions, and their cellular receptors.
| IFN type | Subtype | Chomosomal locus | Celluar source | Antiviral function | IFN receptor | Receptor distribution |
|---|---|---|---|---|---|---|
| Type I IFN | IFN-α (17 for pig, and 13 for human), IFN-β (1), IFN-ε (1), IFN-κ (1), IFN-ω (1) |
1 for pig, 9 for human, 4 for mouse |
Nearly all nucleated cells produce IFN-β. IFN-α subtypes are primarily produced by leukocytes. pDCs are the most potent type I IFN producers. IFN-κ and IFN-ε produced in a tissue-specific manner. |
Potent antiviral activities. | IFNAR1 and IFNAR2 | Nearly all nucleated cells, except intestinal epithelial cells (IECs). |
| Type II IFN | IFN-γ (1) | 5 for pig, 12 for human, 10 for mouse |
Made primarily by immune cells such as CD4 and CD8 T cells, NK and NKT cells, DCs, and macrophages. | More of an interleukin than an interferon. Modest antiviral activity. |
IFNGR1 and IFNGR2 | Broad tissue distribution. |
| Type III IFN | IFN-λ (4) | 14 for pig, 19 for human, 7 for mouse |
A variety of human primary cell types of hematopoietic lineage. Epithelial cells are the potent producers for type III IFNs among the nonhematopoietic cells. |
Largelyrestricted to epithelium. lung epithelium responds to both type I and III IFNs. IECs respond exclusively to type III IFNs. |
IFNLR1 and IL-10R2 |
IL-10R2 is widely distributed across cell types. IFNLR1 is mainly restricted to epithelial cells. |
Different types of IFNs signal through different receptors. IFN-α/β signal through a heterodimeric complex composed of a single chain of IFN-α receptors 1 (IFNAR1) and 2 (IFNAR2), which are believed to be expressed on the cell surface of all nucleated cells (Gibbert et al., 2013, Pestka et al., 2004). IFN-γ forms a homodimer for signaling through the IFN-γ receptor (IFNGR) complex, which is composed of dimer of two transmembrane-spanning receptors [IFN-γ receptors 1 (IFNGR1) and 2 (IFNGR2)] with broad tissue distributions. IFN-λ signals through heterodimers of interleukin-10 receptor 2 (IL10R2) and IFN-λ receptor 1 (IFNLR1). While IL-10R2 is widely distributed in different cell types, IFNLR1 is largely restricted to epithelial cells (Sommereyns et al., 2008), and thus subtypes of IFN-λ limit their antiviral functions to epithelial cells. All IFNs activate the Janus kinase (JAK)-signal transducer and activator of transcription (STAT) signaling pathway to establish an antiviral state. Since IFNGR1/2 are widely distributed, most cell types are capable of responding to IFN-γ (Valente et al., 1992). While the IFN-γ has pleiotropic effects on immune cells, a direct antiviral activity is rather limited. In contrast, both IFN-α/β and IFN-λ induce a potent antiviral state by regulating the expression of hundreds of interferon stimulating genes (ISGs). Even though IFN-α/β and IFN-λ utilize different receptors for downstream signaling, their induction and antiviral mechanism are similar, and in vivo studies for IFN-λ show a high degree of redundancy of these two IFN systems in lung epithelial cells (Ank et al., 2008, Mordstein et al., 2008, Mordstein et al., 2010), raising a question that why two seemingly redundant antiviral systems exist. IFN signaling cascades are summarized in Fig. 3.
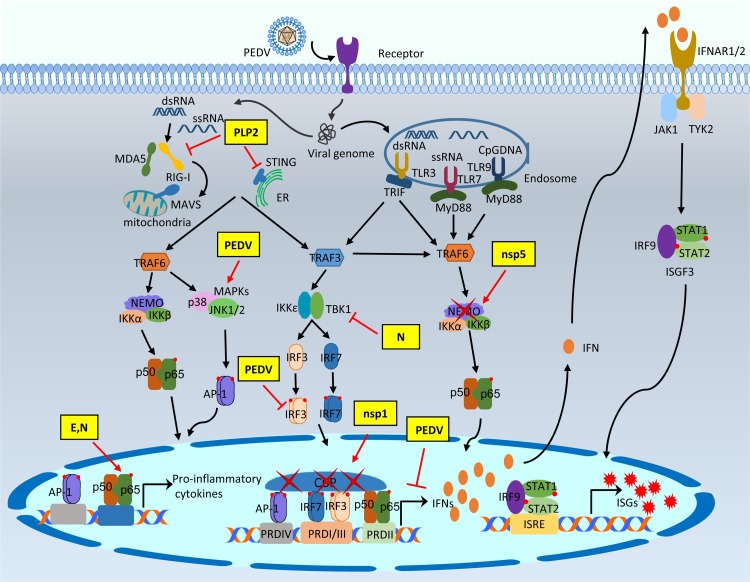
Fig. 3.
Modulation of type I IFN response by PEDV proteins. After binding to the celluar receptor APN, PEDV is internalized into target cells by direct fusion of viral-cellular membranes, and the viral genomic RNA is released into the cytosol for replication. The single-stranded viral RNA as well as dsRNA as a replicative intermediate are sensed by host innate nucleic acids sensors in the cytoplasm and the endosome. The activation of innate immune sensors further initiates the production of type I interferons or pro-inflammatory cytokines after binding of activated NF-κB, AP-1, IRF3, and IRF7 to the respective PRD regions. Type I IFNs are secreted and bind to the cell surface receptors of both virus-infected and non-infected neighbor cells to induce the nuclear localization of ISGF3 complex. Activation of JAK-STAT pathway induces the production of hundreds of interferon stimulating genes (ISGs) for establishment of the antiviral state. The PEDV IFN antagonists intervene the IFN signaling pathway at different stages. The modulation of the type I IFN response by PEDV and PEDV proteins are shown in yellow boxes.
Swine enteric viruses are mostly transmitted via the fecal-oral route and a possible aerosol transmission and some of them are highly infectious. TGEV infects and replicates primarily in small intestinal enterocytes and to a lesser extent in the respiratory tract (Kim et al., 2000). PEDV and PDCoV also infect the small intestinal enterocytes (Jung et al., 2015c, Jung and Saif, 2015), and thus intestinal epithelial surface is the first line of host defense to prevent enteric viral infections. Due to the distinctive features of the intestinal epithelial mucosal surface, induction and signaling of IFNs are unique. Coronaviruses have a large genome and have evolved to carry redundant mechanisms to counteract the host innate immune response. For SARS-CoV, at least 11 viral proteins have been identified as type I IFN antagonists (Kindler and Thiel, 2014, Shi et al., 2014a, Totura and Baric, 2012), and a recent study for PEDV shows that at least 10 viral proteins are type I IFN antagonists (Zhang et al., 2016). By contrast, acute infection of TGEV induces a high level of IFN-α in newborn pigs (La Bonnardiere and Laude, 1981). Despite the variation of genome organization, swine enteric coronaviruses seem to utilize similar but distinct mechanisms for activation and evasion of host innate immunity on the intestinal epithelial surface.
A wealth of information is available for IFNs about their mechanisms of induction, signaling cascades, and functions, but many fundamental questions still remain to be answered, especially for their roles for intestinal antiviral innate immunity. The current review discusses the activation and modulation of innate immune responses mediated by swine enteric coronaviruses. More specifically, the mechanisms of viral proteins that modulate host innate antiviral defense and the roles of different types of IFNs in the intestinal innate immunity are described.
2. Production of type I IFNs and the IFN signaling
The host innate immune system utilizes PRRs (pattern-recognition receptors) to sense and respond to PAMPs (pathogen-associated molecular patterns) of invading viruses (Kawai and Akira, 2011). This recognition of the membrane-bound PRRs triggers the activation of IFN induction pathway. Toll-like receptors (TLRs) are among the best-characterized groups of PRRs (Kawai and Akira, 2011). TLRs constitutes a family of single transmembrane proteins with ectodomains containing the leucine-rich repeats for recognition of PAMPs and a cytosolic TIR (toll/IL-1 receptor) domain as a key domain transducing signals to downstream adaptors including TRIF (TIR-containing adaptor protein inducing IFN-β) and MyD88 (myeloid differentiation primary response gene 88) (Kawai and Akira, 2010). Among TLRs, TLRs 3, 7, 8, 9, and 13 are involved in endosomal nucleic acid sensing. TLR3 is the dsRNA sensor and responds to poly(I:C), a synthetic RNA analog (Kariko et al., 2004, Okahira et al., 2005). TLR7 and TLR8 recognize ssRNA derived from RNA viruses (Diebold et al., 2004, Heil et al., 2004), whereas TLR9 senses CpG DNA motifs in the genome of DNA viruses (Vollmer et al., 2004). TLR13 has recently been identified as the sensor for bacterial 23 S ribosomal RNA (Oldenburg et al., 2012). The RLR (RIG-I-like receptor) family is responsible for the sensing of cytosolic RNA. The RLR members include RIG-I (activated retinoic acid-inducible gene I), MDA5 (melanoma differentiation gene 5), and LPG2 (laboratory of genetics and physiology 2). RIG-I and MDA5 respond to poly(I:C), with their specificities based on the length of dsRNA. RIG-I senses shorter dsRNA (∼300 bp) containing a 5′-triphosphate panhandle structure, whereas MDA5 recognizes long dsRNA ( > 4 kb) fragments (Kato et al., 2008, Schlee et al., 2009, Schmidt et al., 2009). Cytoplasmic DNA sensors have been addressed including STING (stimulator of IFN genes) and cGAS (cyclic GMP-AMP synthase) for cytosolic DNA sensing (Ishikawa and Barber, 2008, Sun et al., 2013, Wu et al., 2013). For type I IFNs production, activated RIG-I or MDA5 binds to the mitochondrial adaptor protein MAVS/IPS-1 and recruits TRAF3 (TNF receptor-associated factor 3) and TRAF6. TRAF3 activates downstream IKK (IκB kinase) related kinases such as TBK1 (TANK-binding kinase 1) and IKKε for induction of IRF3/IRF7-dependent type I IFN production (Fitzgerald et al., 2003, Sharma et al., 2003). TRAF6 leads to TAK1 activation, followed by NF-κB activation for type I IFN and other cytokine productions (Rajsbaum and Garcia-Sastre, 2013). Within the IFN-β promoter, there are four regulatory cis elements, namely, the positive regulatory domains (PRDs) I, II, III, and IV. PRD I/III, PRD II, and PRD IV are binding sites for IRFs, NF-κB, and ATF-2/c-Jun (AP-1), respectively (Honda et al., 2006). The expression of IFN-β requires the assembly of these regulatory factors on PRDs to form an enhanceosome complex, which recruits CBP for IFN production (Randall and Goodbourn, 2008). Following production and secretion, IFNs bind to the cell surface receptors to trigger the activation of JAK-STAT pathway for the IFN-signaling cascade. The three different IFN systems signal through distinct IFN receptors. Type I IFNs and type III IFNs trigger the formation of ISGF3 complex for the production of hundreds of ISGs. In contrast, type II IFN triggers the phosphorylation of STAT1 and subsequent formation of IFN-γ activation factor (GAF) for antiviral genes expression (Schneider et al., 2014).
Expression of the membrane-bound PRRs is cell type-dependent. Specific molecular features of the PRRs and the cell type that recognizes them are two main factors for the specificity in IFN subtype production (Hoffmann et al., 2015). Nearly all nucleated cells are capable of producing IFN-β through activation of IRF3 and NF-κB, but IFN-α subtypes are primarily produced by leukocytes (Cantell et al., 1981). Plasmacytoid dendritic cells (pDCs) are the most potent type I IFN producers with up to a hundred to a thousand times more IFN-α than other cell types (Siegal et al., 1999). Activated pDCs migrate to the lymph nodes and potentially influence various cell types through the secreted IFNs. How IFN responses vary during virus infection is still unclear. A recent study shows that different IFN responses depend on the activation of pDCs by interactions with infected cells or virus alone (Frenz et al., 2014). Levels of total IFN-α production in pDCs vary greatly with invading virus, time, and amount of inoculum (Thomas et al., 2014). Another study shows that pDCs produce a highly uniformed IFN-α profile regardless of the PAMP (Szubin et al., 2008). The ratios between different IFN subtypes may be optimized by evolution in a specific cell type. Similar to IFN-α/β, IFN-λ can be induced in different cell types by various viruses. IFN-λ can be expressed in a variety of cell types of the hematopoietic lineage, which also produces IFN-α/β in abundance. Among the nonhematopoietic cells, epithelial cells are potent producers for IFN-λ. The IFN-λ response is largely restricted to the epithelium such that lung epithelial cells respond to IFN-α/β and IFN-λ and intestinal epithelial cells respond exclusively to IFN-λ (Mordstein et al., 2010, Pott et al., 2011, Sommereyns et al., 2008).
3. Role of IFNs on the intestinal epithelial surface
3.1. Intestinal epithelial barrier
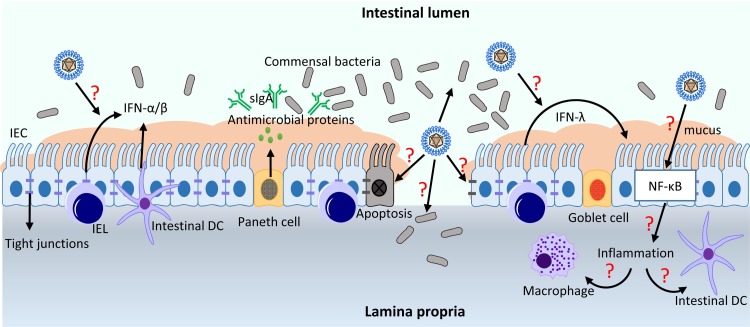
Small intestinal epithelial cells (IECs) form a physical barrier to segregate the mucosal immune system and commensal microbial communities in the lumen. The Paneth and goblet cells secrete mucus and antimicrobial proteins to exclude invading bacteria and viruses from the epithelial surface, and renewal of the epithelial cell layer is controlled by the intestinal stem cells in the crypts. Secreted IgA (sIgA) also contributes to the barrier function. The lamina propria is made of stromal cells, B cells, T cells, dendritic cells, and macrophages. Intraepithelial lymphocytes (IELs) are localized between IECs, and these cells constitute a large and highly conserved T cell compartment, which serves through cytolysis of dysregulated IECs and renewal of IECs. Microfold (M) cells are found in the gut-associated lymphoid tissues (GALT) of the Peyer’s patches and the mucosa-associated lymphoid tissue (MALT) of other parts of the gastrointestinal tract, and they are specialized for antigen sampling and initiation for mucosal immune responses (Peterson and Artis, 2014). Unlike the lung epithelium, the intestinal epithelium has to maintain the tolerance to the symbiotic gastrointestinal microflora while mounting an effective immune response during infection. The intestinal epithelial barrier is depicted in Fig. 2 .
Fig. 2.
Infection of porcine enteric coronaviruses and innate immune defense at the intestinal epithelial barrier. PEDV suppresses IFN-α/β production and PEDV-specific viral antagonists have been identified (see text). Some questions need to be explored for host-PEDV/PDCoV interactions and their pathogenic mechanisms. Question marks (red) indicate unknown mechanisms that remain to be studied as described in the text. IEC, intestinal epithelial cells; IEL, intraepithelial lymphocytes; DC, dendritic cells.
3.2. Roles of IFN-α/β and IFN-λ in the intestine
Type I IFNs (IFN-α/β) are major cytokines to restrict viral infections. Recent studies however shed a new light on the importance of IFN-λ for restricting viral infection in the intestinal epithelium. In the rotavirus model, IFN-α/β rather play a minor role for restricting viral infections, and instead IFN-λ play a major role to inhibit viral replication in the intestinal epithelium (Angel et al., 1999, Pott et al., 2011). IECs strongly respond to IFN-λ but only marginally respond to IFN-α/β in vivo. The effects of IFN-α/β and IFN-λ are compartmentalized with the major effect of IFN-α/β on the intestinal lymphocytes and IFN-λ on the epithelial cells (Pott et al., 2011). Distinct roles of IFN-α/β and IFN-λ for controlling systemic and local dissemination of rotavirus, respectively, have been suggested. IFN-α/β produced by mucosal pDCs can enhance B cell response to promote viral clearance and protection against re-infection (Deal et al., 2013). For rotavirus, significance of IFN-α/β seems to vary depending on the viral strains, host, and synergy with other types of IFNs, but IFN-λ determines localized intestinal epithelial antiviral defense. A recent finding shows that activated IELs upregulate the IFN-α/β and IFN-λ expression to mount a rapid antiviral state for protection of IECs (Swamy et al., 2015). Activation of IELs in vivo rapidly provokes ISGs expression through the IFN-α and IFN-λ receptors. Thus, activation of IELs offers an overt means to promote an innate antiviral potential of the intestinal epithelium.
Resistance of invading viruses in the gut epithelium relies mainly on IFN-λ, not IFN-α/β, which raises a question on the ubiquitous expression of receptors for IFN-α/β. A recent study shows that mouse IECs minimally express functional type I IFN receptors and thus do not respond to type I IFNs, explaining why the IFN-α/β system is unable to compensate the IFN-λ deficiency during enteric epitheliotropic viral infections (Mahlakoiv et al., 2015). This study shows that virus-infected IECs are potent producers of IFN-λ, suggesting that the gut mucosa possesses a compartmentalized IFN system in which epithelial cells respond predominantly to IFN-λ, whereas other cells of the gut rely on IFN-α/β for antiviral defense. Timely production of IFN-λ in IECs drives rapid clearance of intestinal viral infections and limits viral shedding in the feces. The intestinal epithelium responds exclusively to IFN-λ in a non-redundant fashion to control epitheliotropic viruses (Pott et al., 2011). For reovirus, IFN-λ restricts initial viral replication and reduces viral shedding in the feces, whereas IFN-α/β are critical for the prevention of systemic infection. Similarly, norovirus infection also requires IFN-λ to control persistent enteric infection, although IFN-α/β restrict the systemic spread (Nice et al., 2015).
In the gut, the protective effect of the innate immunity, in particular IFN-λ, is counteracted by intestinal commensal bacteria (Baldridge et al., 2015). PEDV infection causes the decrease of commensal bacteria normally present in the healthy gastrointestinal tract and induces an unbalanced shift of gut microbiota (Koh et al., 2015, Liu et al., 2015b), suggesting a possible modulation of IFN-λ response by PEDV. The border surface of the intestinal epithelium is the major entry site for enteric viruses and specific mechanisms are needed for the fail-safe protection of such multi-layered surfaces. Although IFN-λ production by IECs is required, it is not sufficient for protection of the epithelium from rotavirus-induced cell damages. The production and release of IL-1α by IECs following rotavirus infection enhances IL-22 production. A recent study identifies a cooperation of IFN-λ and IL-22 to synergistically curtail mucosal viral infection (Hernandez et al., 2015). The receptors for IL-22 and IFN-λ are restricted to epithelial cells and simultaneous engagement of both receptors on IECs is required for restriction of rotavirus infection, suggesting a second layer control is necessary (Hernandez et al., 2015). Downregulation of IFNLR1 and interference of the STAT1 signaling may be an important strategy for viral evasion (Sen et al., 2014).
After stimulation with poly(I:C) and infection with reovirus, both IECs and hematopoietic cells in the intestinal epithelium express IFN-λ strongly and quickly but do not express IFN-α/β genes (Mahlakoiv et al., 2015). Thus, mechanisms seem to have evolved to produce IFN-λ specifically and potently in the intestinal epithelium. Since common pathways are believed to be utilized for induction of both IFN-α/β and IFN-λ (Onoguchi et al., 2007), the IFN-α/β pathway is specifically blocked in IECs by an unknown mechanism. Another study shows that peroxisome-bound MAVS may preferentially activate IFN-λ expression (Odendall et al., 2014). Nlrp6 (nucleotide-binding oligomerization domain-like receptor 6) is an intracellular protein specific for tissues and cell-types with the highest expression in IECs (Elinav et al., 2011). Nlrp6 binds to viral RNA via the RNA helicase Dhx15 as a RNA sensor and triggers MAVS-dependent expression of IFN-α/β and IFN-λ plus ISGs, and thus plays an important role for intestinal antiviral response (Wang et al., 2015b).
Using gene-knockout mice for specific IFN receptors, IFN-λ has been identified to play an important role in the defense against SARS-CoV and other respiratory viral infections (Mordstein et al., 2010). Fecal shedding of SARS-CoV can be detected from mice lacking both receptors for IFN-α/β and IFN-λ but not from mice lacking single receptors, suggesting that IFN-λ controls viral infections in epithelial cells of both respiratory and gastrointestinal tracts. Relative contributions of the various IFN subtypes for restricting enteric coronavirus infections remain elusive. Both TGEV and PEDV can infect and replicate in PAMs in the respiratory tract in vivo (Park and Shin, 2014). PEDV has also been shown to infect macrophages infiltrated in the lamina propria and modulates immune responses after infection of monocyte-derived DCs (MoDCs) and intestinal DCs (Gao et al., 2015, Lee et al., 2000). In summary, IFN-λ seems to be critical to efficiently restrict enteric coronavirus infections in the intestinal epithelial surface, including PEDV, TGEV, and possibly PDCoV infections, and in turn, these viruses may modulate the host IFN-λ response. It is of great interest to investigate how such enteric coronaviruses regulate the IFN-λ response in pigs. A full-length infectious cDNA clone is available for PEDV, and it will be useful to study individual gene functions using replicating mutants in vivo. As such, we will be able to better understand the pathogenic mechanisms of porcine enteric coronaviruses and host responses to such infections (Beall et al., 2016, Jengarn et al., 2015).
4. Modulation of IFN response by porcine enteric coronaviruses
4.1. Aminopeptidase N (CD13) as the cellular receptor and viral tissue tropism
For coronaviruses, S protein and its interaction with a receptor on susceptible cells determine the host range and viral tropism (Bosch et al., 2003, Gallagher and Buchmeier, 2001). Unlike many other coronaviruses, cell culture adaptation of PEDV triggers mutations in the S protein sequence (Lawrence et al., 2014) and requires the supplement of an exogenous protease, typically trypsin, to activate the S protein to induce membrane fusion after binding to the cellular receptor (Hofmann and Wyler, 1988, Park et al., 2011, Wicht et al., 2014). The trypsin dependency of PEDV in vitro is consistent with the tissue tropism of trypsin-rich small intestine in pigs. Some of cell-adapted PEDV strains lose the trypsin-dependency (Nam and Lee, 2010, Wicht et al., 2014). PEDV-infected cells produce distinct cytopathic effects including cell fusion, multinucleated syncytia formation, vacuolation, and detachment due to apoptotic cell death (Kim and Lee, 2014). A recent study shows that heparan sulfate on the cell-surface may function as an attachment factor for PEDV (Huan et al., 2015). As with TGEV, PEDV also utilizes aminopeptidase N (APN; CD13) as a functional receptor. APN is a heavily glycosylated transmembrane protein and is expressed by most cells of myeloid origin including monocytes, macrophages, granulocytes, and their hematopoietic precursors (Chen et al., 1996, Mina-Osorio, 2008, Riemann et al., 1999). It is also abundantly expressed in the brush border of epithelial cells from the small intestine and renal proximal tubules and some other cell membranes. In the small intestine, it plays a role in the final digestion of peptides generated from hydrolysis of proteins by gastric and pancreatic proteases (Kruse et al., 1988). TGEV utilizes APN as receptor for entry into swine testis (ST) cells, which however cannot render efficient propagation of PEDV (Delmas et al., 1992). This differentiation may due to the relatively low level expression of APN on ST cells (Li et al., 2007). Over-expression of APN enhances PEDV infection, and an increased APN density enhances the PEDV susceptibility in cultured ST cells (Li et al., 2007, Nam and Lee, 2010, Oh et al., 2003). Porcine intestinal tissues are more sensitive for high-titer virus production for PEDV natural infection, which may due to the abundant expression of APN in porcine intestinal brush border membrane (Li et al., 2007). Collectively, the high level expression of APN at the intestinal barrier correlates with the efficient replication of both TGEV and PEDV, and infectivity of PEDV is more dependent on APN density. Additionally, PEDV also infects cultured cells of pigs, humans, monkeys, and bats, which suggests that PEDV may utilize APN of other species origin or other unidentified receptors or co-receptors for efficient entry (Liu et al., 2015a, Zhang et al., 2016). Both PEDV and TGEV infect alveolar macrophages besides small intestinal epithelial cells (Laude et al., 1984, Park and Shin, 2014). PEDV also infect infiltrated macrophages in the lamina propria in vivo (Lee et al., 2000). Even though the clinical signs of epidemic PEDV and TGEV infections are indistinguishable, it is of great interest to investigate whether the cell and tissue tropism contributes to the differential pathogenesis in vivo.
4.2. Activation of IFN-α/β by TGEV
Porcine enteric coronaviruses replicate in IECs and are transmitted via a fecal-oral route. The viral entry and release for TGEV are restricted to the apical surface of polarized epithelial cells (Rossen et al., 1994). Experimental infection of newborn piglets with TGEV induces a strong and early IFN-α production in serum and intestinal secretions (La Bonnardiere and Laude, 1981). The blocking of the viral receptor still induces IFN-α response, indicating that the entry is not needed for IFN induction in leukocytes (Riffault et al., 1997). Early and strong IFN-α production can also be induced in nonimmune cells after exposure to TGEV-infected epithelial cells (Charley and Laude, 1988). The rapid and massive release of IFN-α in serum is mediated by M and E proteins of TGEV (Charley and Laude, 1988, Laude et al., 1992, Riffault et al., 1997). A specific point mutation in the M protein results in lower IFN-α induction as compared to wild-type virus (Splichal et al., 1997), and the virus-like particles made of M and E proteins induce IFN-α production in porcine leukocytes as almost efficiently as fully infectious TGEV (Baudoux et al., 1998). However, reconstituted virosomes lose the ability to induce IFN-α production, indicating that the native envelope structure is essential (Riffault et al., 1997). Immunohistochemistry analysis of the tissues from TGEV-infected piglets show that IFN-α-producing cells (IPCs) are almost exclusively detected in the intestinal tissues and mesenteric lymph nodes (MLNs) at an early time of infection such as 6 h and disappeared by 24 h (Riffault et al., 2001). The majority of IPCs are localized between enterocytes in the epithelial layer, the lamina propria, around the Peyer’s patches, and are accumulated in the MLNs (Riffault et al., 2001). The number of IPCs in spleen or peripheral lymph nodes is limited, and thus circulating IFN-α is likely originated from the gut and MLNs triggered by virus (Riffault et al., 2001). IPCs in pigs are identified as pDCs (Summerfield et al., 2003). Interestingly, gut-associated IPCs express swine leucocyte antigen II (SLA II) and are distinct from TGEV-positive cells, suggesting that these cells may be the mucosal counterparts of the intestinal pDCs (Riffault et al., 2001). Contrary to pDCs that produce high levels of IFN-α and TNF-α after infection, porcine monocyte-derived DCs do not respond to TGEV (Guzylack-Piriou et al., 2006). The frequency of intestinal pDCs however is low comparing to other DCs, and thus the limited populations of intestinal pDCs may not be a major subset of antigen-presenting DCs. The production of IFN-α by intestinal pDCs in MLNs may flood the T cell area to influence the immune outcome (Charley et al., 2006). In addition to the production of IFN-α/β, TGEV induces phosphorylation and nuclear translocation of STAT1 in virus-infected cells and enhances the expression of ISGs (An et al., 2014). TGEV infection also promotes the p53 signaling to regulate apoptosis, and the viral nucleocapsid protein is responsible for this action and the cell cycle arrest (Ding et al., 2014a, Huang et al., 2013).
IFN-α has been shown to inhibit TGEV protein expression without reducing viral RNA in TGEV-infected cells (Jordan and Derbyshire, 1995). Infection of TGEV upregulates the expression of 2′-5′-oligoadenylate (2′-5′A) synthetase for inhibition of viral replication (Bosworth et al., 1989, Jordan and Derbyshire, 1995). Swine IFN-α has been shown to enhance the protection from TGEV and alleviate clinical signs in piglets when orally co-administered with Salmonella enterica serovar Typhimurium expressing swine IFN-α with or without cytokine IL-18 (Kim et al., 2010, Lee et al., 2011). The viral load is significantly reduced in intestinal segments of piglets when treated with an IFN inducer before challenge, but no reduction in viral yields is seen when simultaneously inoculated with TGEV and IFN-α, suggesting that IFN may be more useful prophylactically (Jordan and Derbyshire, 1994).
4.3. PEDV pathogenesis and innate signaling
PEDV is cytolytic with an acute necrosis in IECs, leading to villous atrophy in the small intestine (Jung et al., 2014). The high mortality of PEDV in piglets is associated with extensive dehydration resulting from severe villous atrophy. The severe dehydration may due to the extensive structural destruction and disorganization of tight junctions and adherens junctions during infection in nursing piglets (Jung et al., 2015b). The slower turnover of the enterocytes, which is mediated by the regeneration of crypt stem cells in nursing piglets compared to weaned piglets, is probably responsible for the age-dependent resistance to PED (Jung et al., 2015a). PEDV antigens are mainly observed in villous enterocytes of the small and large intestines. PEDV also infect macrophages infiltrated in the lamina propria in vivo (Lee et al., 2000). Experimental infection of TGEV shows that the lung is the tract sustaining viral replication and IFN synthesis (La Bonnardiere and Laude, 1983). TGEV can replicate in porcine alveolar macrophages (PAMs) in vitro and induces a marked synthesis of type I IFNs (Laude et al., 1984). Similarly, PEDV can also infect and replicate in PAMs in vivo (Park and Shin, 2014), suggesting an extra-intestinal replication of PEDV. Additionally, PEDV has been shown to induce apoptotic cell death in vitro and in vivo through the caspase-independent mitochondrial apoptosis-inducing factor (AIF) pathway (Kim and Lee, 2014). Another study claims that PEDV may not induce the death of enterocytes in the small intestine through apoptosis pathway in infected pigs (Jung and Saif, 2015).
After binding to APN, PEDV is internalized into target cells by direct membrane fusion, and the genomic RNA is released into the cytosol for replication. The sensing of viral nucleic acids by PRRs in the endosome or cytosol initiates the innate immune signaling. Like other coronaviruses, PEDV likely replicates in the double membrane vesicles to hide the viral RNA from sensing by PRRs and to attenuate the innate immune response (Angelini et al., 2013, Snijder et al., 2006). A recent study shows that TLR2, TLR3, and TLR9 may contribute to NF-κB activation in response to PEDV infection in IECs in vitro but does not cause RIG-I activation (Cao et al., 2015b). TLR2 on the plasma membrane responds to viral glycoproteins on the envelope to induce production of IFNs and inflammatory cytokines, while TLR3 and TLR9 are localized largely in the endosome to sense dsRNA and CpG DNA, respectively (Boehme and Compton, 2004). This suggests that PEDV may initiate the innate immune response through its surface glycoprotein and viral nucleic acids in the endosomes. PEDV activates the ERK (extracellular signal-regulated kinase) pathway early during infection independently from viral RNA replication (Kim and Lee, 2015). Knockdown or inhibition of ERK suppresses viral transcription, protein expression, and progeny production, which suggest that the activation of ERK pathway is beneficial and required for PEDV replication (Kim and Lee, 2015). Proteomics analysis of PEDV-infected cells shows differential expression of proteins involved in apoptosis, signal transduction, and stress responses (Zeng et al., 2015), and ERK1 is significantly downregulated during PEDV infection. Thus, the ERK pathway modulation by PEDV is of further interest to study. In one study, six different signaling pathways have been shown to be differentially regulated by PEDV, which includes the RLR, Rap1, PI3K-Akt, MAPK, JAK-STAT, and TLR signaling pathways (Sun et al., 2015), suggesting the modulation of host innate immunity by PEDV. For PDCoV, little is known about the viral modulation of host immunity. The PDCoV N protein forms non-covalently linked oligomers localized in the nucleolus (Lee and Lee, 2015). The N protein alters the expression of cellular proteins relevant to cell division, metabolism, stress response, protein biosynthesis and transport, cytoskeleton networks, and cell communication (Lee and Lee, 2015). Notably, PDCoV N protein upregulates two members of the heat shock protein 70 (HSP70) family. In two lines of swine cells (LLC-PK1 and ST), which are used to isolate and propagate PDCoV (Jung et al., 2016), apoptosis is induced in vitro, but no apoptosis is observed in vivo in PDCoV-infected intestinal enterocytes (Jung et al., 2016). This suggests that a better cell system is needed to mimic in vivo conditions for PDCoV infections, and for this purpose IECs, intestinal enteroids, or organoids may be useful (Finkbeiner et al., 2012, Jung et al., 2016).
PEDV infection in suckling pigs does not alter the natural killer (NK) cell activity compared to weaned pigs that show significantly higher NK cell activities in the blood and ileum (Annamalai et al., 2015). Interestingly, PEDV infection seems to stimulate a marked induction of serum IFN-α in suckling pigs at 1-day post-infection which then declines to the basal level by 5-day post-infection (Annamalai et al., 2015). In weaned pigs however, PEDV infection causes a slight induction of serum IFN-α at 3-day post-infection and a decrease to the basal level at 5-day post-infection. The peak production of serum IFN-α coincides with the peak of viral RNA shedding in the feces and serum of suckling pigs or serum in weaned pigs and the onset of diarrhea. PEDV infection differentially modulates inflammatory cytokines in both suckling and weaned pigs and induces a similar level of TNF-α and IFN-α (Annamalai et al., 2015). According to this study, PEDV is capable of modulating IFN-α production especially in weaned pigs. Since PEDV infection occurs in the small intestine as with TGEV, it is of interest to examine IFN-α in the intestinal secretions and how PEDV modulates type I IFN induction in the intestinal pDCs. Intestinal DCs are highly effective antigen-presenting cells distributed beneath the epithelial lining. Unlike TGEV, PEDV infects MoDCs and produces high levels of IL-12 and INF-γ without alteration of the IL-10 expression (Gao et al., 2015). Interaction between PEDV and MoDCs affects the stimulation of T cell response in vitro. PEDV may also enhance the ability of porcine intestinal DCs to sample antigens and activate T-cell proliferation in vivo (Gao et al., 2015). How the intestinal pDCs respond to PEDV infection and modulate innate immune signaling remains to be determined.
4.4. Evasion of IFN response by PEDV
IFN-α/β plays an important role in restricting viral infections, and enteric coronaviruses have evolved redundant mechanisms to counteract the host innate immunity for optimal viral adaptation and replication. Even though TGEV activates IFN-α/β production, its accessory protein 7 modulates IFN-α/β expression by interacting with PP1 and prolongs viral dissemination for increased survival (Cruz et al., 2011). Accumulating evidence shows that PEDV also has an ability to evade host IFN response. PEDV does not activate IFN-β expression even after poly(I:C) stimulation in virus-infected cells (Xing et al., 2013, Zhang et al., 2016). It appears that PEDV infection inhibits IRF3 nuclear translocation and thus blocks IRF3-mediated IFN induction. Using MARC-145 cells that are IFN-competent and PEDV-permissive, our laboratory has investigated the innate immune modulatory role of PEDV (Zhang et al., 2016). In our study, the culture supernatants from PEDV-infected cells do not suppress the growth of VSV-GFP, providing evidence for suppression of IFN-α/β by PEDV. Further studies show that PEDV has an ability to degrade the CREB-binding protein (CBP) in the nucleus and thus inhibits the enhanceosome assembly resulting in the suppression of IFN transcription (Zhang et al., 2016). IECs are major target cells of PEDV in pigs, and in these cells, PEDV blocks the activation of MAVS/IPS-1 and IRF3 nuclear translocation in the RIG-I-mediated pathway and do not induce IFN-β expression (Cao et al., 2015a). IPEC-J2 cells are epithelial cells derived from the jejunum of a colostrum-deprived neonatal pig of 12 h of age (Zhao et al., 2014; Dr. A. Blikslager, North Carolina State University). These cells however appear to be non-permissive for PEDV infection (unpublished data), and thus they are less useful than the initial anticipation for the study of PEDV innate immune evasion.
We have individually cloned all coding sequences from PEDV and expressed them to screen for IFN antagonists by luciferase reporter assays. Of 23 viral proteins, 10 proteins are found to suppress IFN induction, which includes both structural proteins and nonstructural proteins: nsp1, nsp3, nsp7, nsp14, nsp15, nsp16, E, M, N, and ORF3 (Zhang et al., 2016). These proteins antagonize the IFN-β and IRF3 activities. Among these, nsp1 is the most potent IFN antagonist, and further studies show that nsp1 does not interfere the IRF3 phosphorylation and nuclear translocation, and rather interrupts the interaction of IRF3 with CREB-binding protein (CBP) and thus inhibits the IFN enhanceosome assembly. PEDV nsp1 triggers CBP degradation in the nucleus in a proteasome-dependent manner, which seems to be the key mechanism for nsp1-mediated IFN suppression (Zhang et al., 2016). PEDV dramatically decreases the level of ubiquitin (Ub)-conjugated proteins in the cytoplasm. Papain-like proteinase 2 (PLP2) in nsp3 of PEDV contains a deubiquitinase (DUB) activity to process the cytosolic K48- and K63-linked polyubiquitin chains (Xing et al., 2013). PLP2 in nsp3 of both α-CoV and β-CoV could process both lysine-48- and lysine-63- linked polyubiquitin chains of the cytosolic proteins (Chen et al., 2014a, Chen et al., 2014b, Clementz et al., 2010). SARS-CoV PLP2 reduces the levels of ubiquitinated forms of RIG-I, STING, TRAF3, TBK1, and IRF3 in the STING-TRAF3-TBK1 complex for inhibition of the type I interferon signaling pathway (Chen et al., 2014a, Chen et al., 2014b). Similarly, PEDV PLP2 interacts with deubiquitinated RIG-I and STING to antagonize IFN response. PLP2 of SARS-CoV specifically deubiquitinates MDM2 (mouse double minute 2 homolog) and degrades p53 to suppress IFN signaling (Yuan et al., 2015). Similarly, PEDV upregulates MDM2 expression, degrades p53, and significantly inhibits IRF7 expression, suggesting that PEDV PLP2 may also target the p53 pathway for innate immune evasion and inhibit p53-dependent apoptosis (Yuan et al., 2015). Collectively, PEDV differentially modulates ubiquitination level in the cytoplasm and nucleus for modulation of innate immune responses.
PEDV infection leads to activation of the NF-κB pathway in IECs (Cao et al., 2015b). PEDV stimulates nuclear localization of exogenous p65 at 24 h post infection, and increases the nuclear localization of endogenous p65 at 12 h through 48 h post-infection. In another study, PEDV normally induces activation of NF-κB and AP-1 following poly(I:C) stimulation at 24 h post-infection (Xing et al., 2013). These studies are relatively late stage for PEDV infection. PEDV may inhibit NF-κB activation at an early stage for optimal survival and then activates it at late stage for inflammation. For porcine reproductive and respiratory syndrome virus (PRRSV), which is a porcine arterivirus of the Nidovirales order, NF-κB is activated 30 min after infection (Fu et al., 2012). Thus, how PEDV inhibits NF-κB during an early time of infection is of interest and needs to be explored. For SARS-CoV, the N protein activates NF-κB (Liao et al., 2005) whereas the M protein suppresses NF-κB, probably through a direct interaction with IKKβ (Fang et al., 2007). For PEDV, the E and N proteins cause ER stress leading to activation of the NF-κB pathway for up-regulation of IL-8 and Bcl-2 (Xu et al., 2013a, Xu et al., 2013b), and the M protein of PEDV is reported to induce a cell cycle arrest at the S phase via the cyclin A pathway (Xu et al., 2015). In other study, the N protein blocks the IFN-β production and ISGs expression, leading to suppression of the IRF3 and NF-κB activities (Ding et al., 2014b). The N protein is an RNA-binding protein and shuttles between the cytoplasm and nucleus (Shi et al., 2014b). Furthermore, N interacts with TBK1 and sequesters its association with IRF3 to block the phosphorylation and nuclear translocation of IRF3 leading to inhibition of IRF3-mediated IFN production (Ding et al., 2014b). The nsp5 protein of PEDV is a 3C-like proteinase and has been shown as an IFN antagonist. The cysteine proteinase activity of nsp5 cleaves NEMO which is an essential NF-κB modulator and disrupts the RIG-I/MDA5 signaling (Wang et al., 2015a). Modulation of innate immune response and signaling by PEDV infection is summarized in Table 2 .
Table 2.
Modulation of innate immune response and signaling by PEDV.
| Modulation of innate immune responses by PEDV | Model | References |
|---|---|---|
| Suppression of IFN-β induction upon poly(I:C) stimulation and inhibits IRF3 nuclear translocation. | Vero E6 cells | Xing et al. (2013) |
| Suppression of type I IFN induction and degrades CBP in the nucleus. | MARC-145 cells | Zhang et al. (2016) |
| Lack of poly(I:C)-mediated IFN-β induction and blocking activation of IPS-1 in RIG-I-mediated pathway. | Intestinal epithelial cells (IECs) | Cao et al. (2015a) |
| Ativation of NF-κB pathway through TLR2, TLR3, and TLR9. | IECs | Cao et al. (2015b) |
| Activation of extracellular signal-regulated kinase (ERK) pathway early during infection. | Vero cells | Kim and Lee (2015) |
| PEDV infection causes undetectable natural killer (NK) cell activity in suckling piglets compared to weaned pigs. | Pigs | Annamalai et al. (2015) |
| A marked induction of serum IFN-α in suckling pigs at 1 day post infection, but it is declined significantly to the base level at 5 days post-infection. | Pigs | Annamalai et al. (2015) |
For SARS-CoV, nsp14 is an exoribonuclease and nsp15 is an endoribonuclease, and both function through a specific digestion of dsRNA and subsequent removal of RNA-PAMPs, leading to suppression of IFN responses (Kindler and Thiel, 2014). SARS-CoV nsp16 is a 2′-O-methlytransferase that modifies the 5′ cap structure of viral RNA and functions to avoid detection by the host immune system (Totura and Baric, 2012). PEDV nsp14, nsp15, and nsp16 correspond to nsp14, nsp15, and nsp16 of SARS-CoV and are presumed to share similar functions for innate immune evasion, which needs to be verified experimentally. The M protein plays an important role in viral assembly and induction of PEDV neutralizing antibodies, and a recent study shows that M is an additional IFN antagonist (Zhang et al., 2016). Since M protein of TGEV is responsible for abundant expression of IFN-α, it is of great interest to determine how the PEDV M protein can suppress IFN response and functions differently from the TGEV M protein. ORF3 is the sole accessory protein of PEDV and its role for pathogenesis remains enigmatic. Some strains of cell-adapted PEDV display internal deletions in the ORF3 gene (Park et al., 2008), suggesting that ORF3 relates to adaptation and pathogenicity. ORF3 has also been shown to function as an ion channel protein and thus the suppression of ORF3 expression inhibits viral production (Wang et al., 2012). Additionally, ORF3 delays the S phase in dividing cell and suppresses the cell cycle progression (Ye et al., 2015). A contradictory data exists that ORF3 causes an inability to recover progeny virus from a full-length infectious clone, suggesting that ORF3 negatively modulates PEDV replication in vitro (Jengarn et al., 2015). However, the deletion of entire ORF3 by targeted RNA recombination has been possible and ORF3 appears nonessential for PEDV replication in vitro (Li et al., 2013). ORF3 inhibits type I IFN production (Zhang et al., 2016) and facilitates the vesicle formation in ORF3-expressing cells (Ye et al., 2015), implicating an important role of ORF3 for innate immune evasion. These viral antagonists may target different signaling pathways to modulate the host innate immunity synergistically and efficiently during infection. The modulation of type I IFN responses by PEDV and the IFN antagonism by PEDV proteins is shown in Fig. 3.
4.5. Coronavirus nsp1 as a multifunctional IFN antagonist
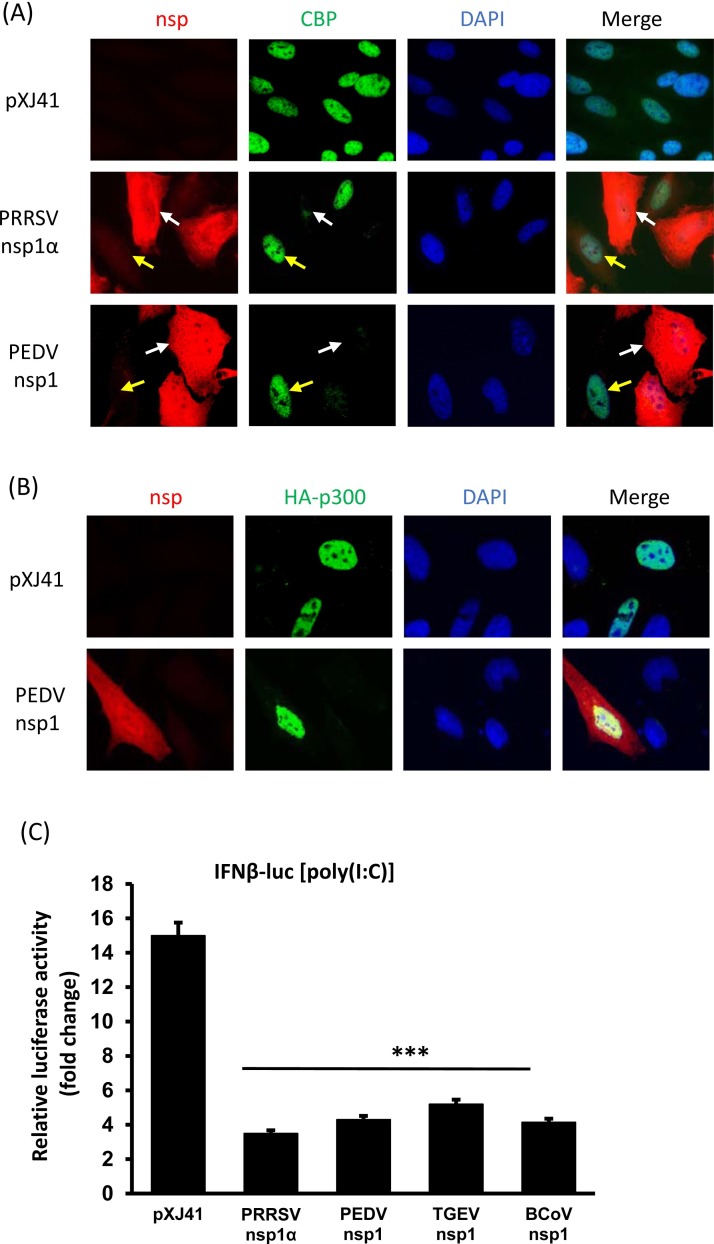
Coronavirus nsp1 is the N-terminal cleavage product of polyproteins pp1a and pp1a/b processed by the papain-like proteinase 1 (PLP1) activity of nsp3 (Ziebuhr, 2005). The nsp1 gene is one of the most divergent genes among four different genera. Only the genome of α-CoV and β-CoV codes for nsp1, whereas the genome of γ-CoV and δ-CoV lack the nsp1 gene (Woo et al., 2010, Woo et al., 2012, Ziebuhr, 2005, Ziebuhr et al., 2007). The nsp1 gene of α-CoV is much smaller than that of β-CoV and the sequence homology is also limited. The PEDV nsp1 gene encodes for 110 amino acids with the predicted molecular migration of 12 kD. The protein sequence of nsp1 does not harbor any known cellular functional motifs (Connor and Roper, 2007) and thus is a genus-specific genetic marker (Snijder et al., 2003). However, nsp1 of α-CoV and β-CoV share similar functions. Coronavirus nsp1, especially β-CoV nsp1, regulates host cell and viral gene expression (Narayanan et al., 2014). The nsp1 protein of α-CoV and β-CoV is a potent IFN antagonist, and among all coronaviruses, SARS-CoV nsp1 is best characterized. SARS-CoV nsp1 inhibits the reporter gene expression under the constitutive promoters as well as the IFN-β promoter (Kamitani et al., 2006). As with SARS-CoV nsp1, murine hepatitis coronavirus (MHV) nsp1 and HCoV-229E nsp1 affect the cellular gene expression by suppressing IFN-β, IFN-stimulated response element (ISRE), and SV40 promoter (Zust et al., 2007). MHV nsp1 facilitates viral replication in mice by suppressing IFN-α/β through an unknown mechanism. MHV nsp1 and SARS-CoV nsp1 are distributed in the cytoplasm of virus-infected cells (Brockway et al., 2004, Kamitani et al., 2006), and SARS-CoV nsp1 suppresses IFN mRNA accumulation without altering the IRF3 dimerization (Kamitani et al., 2006). SARS-CoV nsp1 blocks the NF-κB, IRF3, and IRF7 activities. SARS-CoV nsp1 also inhibits IFN-dependent signaling by interfering STAT1 phosphorylation (Wathelet et al., 2007). Bat-CoV nsp1 inhibits type I IFNs and ISGs production (Tohya et al., 2009), HCoV-229E nsp1 and HCoV-NL63 nsp1 also inhibits the IFN-β, ISRE, ISG15 activities as well as the SV40, HSV-TK and CMV promoters (Wang et al., 2010, Zust et al., 2007). PEDV nsp1 has shown to degrade the CBP in the nucleus to antagonize the type I IFN induction (Zhang et al., 2016; Fig. 4 A). The p300 protein is a transcriptional co-activator protein for CBP, which functions as histone acetyltransferase and plays a central role in coordinating and integrating multiple signal-dependent events for appropriate level of gene transcription. PEDV nsp1 does not alter the expression level for p300 (Fig. 4B), which further confirm the specificity of degradation of CBP. To examine whether the nsp1 protein of other coronaviruses also possessed an activity for type I IFN suppression, nsp1 genes were cloned from TGEV and bovine coronavirus (BCoV) and examined for an IFN suppressive activity using an IFN-β luciferase assay. Similar to PEDV nsp1, TGEV nsp1 and BCoV nsp1 also significantly suppress the IFN-β promoter activities (Fig. 4C). TGEV nsp1 has been shown to inhibit host protein synthesis without binding to 40 S ribosomal subunit (Huang et al., 2011), and BCoV infection activates host innate defense via TLR7/8-dependent pathways and inhibits type I IFNs and pro-inflammatory cytokines (Aich et al., 2007). It is of interest to investigate the detailed mechanism for TGEV-nsp1- and BCoV-nsp1-mediated IFN suppression.
Fig. 4.
Coronavirus nsp1 is a major IFN antagonist. (A) Degradation of CREB-binding protein (CBP) by PEDV nsp1. Cells were seeded on coverslips and transfected with PEDV nsp1 gene. Porcine reproductive and respiratory syndrome virus (PRRSV) nsp1α is known to degrade CBP in the nucleus and thus included as a control. Cells were stained at 24 h post-transfection for indirect immunofluorescence assay (IFA) using rabbit anti-FLAG polyclonal antibody (red) and mouse anti-CBP monoclonal antibody (green). Nuclei were stained with DAPI (blue). White arrows indicate nsp1-expressing cells. Yellow arrows indicate control cells. (B) PEDV nsp1 does not alter the expression of p300. Cells were grown to 90% confluence and HA-tagged p300 gene was co-transfected with PEDV nsp1 gene or empty vector pXJ41. Cells were stained at 24 h post-transfection for detection of nsp1 (red) using rabbit anti-FLAG polyclonal antibody and for p300 (green) using mouse anti-HA monoclonal antibody. Nuclei were stained with DAPI (blue). (C) Suppression of type I IFN induction by nsp1 of bovine coronavirus (BCoV) and transmissible gastroenteritis coronavirus (TGEV). HeLa cells were seeded in 24-well plates and co-transfected with pIFN-β-luc along with individual nsp1 gene and pRL-TK at a ratio of 1:1:0.1. PRRSV nsp1α is a known IFN suppressor and was included as a control. At 24 h post-transfection, cells were stimulated with poly(I:C) (0.5 μg/ml) for 12 h and the luciferase activities were measured. The reporter experiments were repeated three time, each in triplicate. Asterisks indicate the statistical significance. Statistical analysis was performed by Student’s t-test. *, P < 0.05, **, P < 0.01, and ***, P < 0.001.
5. Conclusion
Two emerging enteric coronaviruses, PEDV and PDCoV, have caused epidemic and endemic infections in pig populations in many countries and have become a major economic threat to the pork industry. The intestinal epithelial surface is the first line of defense to restrict the enteric viral infections, and both IFN-α/β and IFN-λ are important antiviral cytokines that viruses have evolved to counteract. For PEDV, at least 10 viral IFN-α/β antagonists have been identified in our laboratory. Since enteric coronaviruses infect intestinal epithelial surface, several questions still remain to be answered (Fig. 2). Firstly, whether PEDV and PDCoV selectively activate and/or modulate IFN-λ production and whether IFN-λ plays a protective role on the intestinal surface. Secondly, how different cell types such as IECs, pDCs, and IELs in the intestinal barrier induce different types of IFNs and function for mucosal defense. Thirdly, the intestinal epithelium constantly interacts with commensal bacteria and opportunistic viral infections, and thus IFN systems must be able to deal with these organisms without severe inflammation and tissue damages. Thus, better research models for cells and animals are needed to truly reflect viral evasions from innate immune defense. Lastly, specific cytokine mediators are produced in IECs, and how they respond to porcine enteric viral infections is of an additional interest.
Acknowledgements
This project was supported by USDA (HATCH) Multistate NC1202, and Agriculture and Food Research Initiative (AFRI) Competitive Grant no. 2013-67015-21243 of USDA National Institute of Food and Agriculture (NIFA).
References
- Aich P., Wilson H.L., Kaushik R.S., Potter A.A., Babiuk L.A., Griebel P. Comparative analysis of innate immune responses following infection of newborn calves with bovine rotavirus and bovine coronavirus. J. Gen. Virol. 2007;88(Pt 10):2749–2761. doi: 10.1099/vir.0.82861-0. [DOI] [PubMed] [Google Scholar]
- Alonso S., Izeta A., Sola I., Enjuanes L. Transcription regulatory sequences and mRNA expression levels in the coronavirus transmissible gastroenteritis virus. J. Virol. 2002;76(3):1293–1308. doi: 10.1128/JVI.76.3.1293-1308.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An K., Fang L., Luo R., Wang D., Xie L., Yang J., Chen H., Xiao S. Quantitative proteomic analysis reveals that transmissible gastroenteritis virus activates the JAK-STAT1 signaling pathway. J. Proteome Res. 2014;13(12):5376–5390. doi: 10.1021/pr500173p. [DOI] [PubMed] [Google Scholar]
- Angel J., Franco M.A., Greenberg H.B., Bass D. Lack of a role for type I and type II interferons in the resolution of rotavirus-induced diarrhea and infection in mice. J. Interferon Cytokine Res. 1999;19(6):655–659. doi: 10.1089/107999099313802. [DOI] [PubMed] [Google Scholar]
- Angelini M.M., Akhlaghpour M., Neuman B.W., Buchmeier M.J. Severe acute respiratory syndrome coronavirus nonstructural proteins 3, 4, and 6 induce double-membrane vesicles. MBio. 2013;4(4):e00524–13. doi: 10.1128/mBio.00524-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ank N., Iversen M.B., Bartholdy C., Staeheli P., Hartmann R., Jensen U.B., Dagnaes-Hansen F., Thomsen A.R., Chen Z., Haugen H., Klucher K., Paludan S.R. An important role for type III interferon (IFN-lambda/IL-28) in TLR-induced antiviral activity. J. Immunol. 2008;180(4):2474–2485. doi: 10.4049/jimmunol.180.4.2474. [DOI] [PubMed] [Google Scholar]
- Annamalai T., Saif L.J., Lu Z., Jung K. Age-dependent variation in innate immune responses to porcine epidemic diarrhea virus infection in suckling versus weaned pigs. Vet. Immunol. Immunopathol. 2015;168(3-4):193–202. doi: 10.1016/j.vetimm.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldridge M.T., Nice T.J., McCune B.T., Yokoyama C.C., Kambal A., Wheadon M., Diamond M.S., Ivanova Y., Artyomov M., Virgin H.W. Commensal microbes and interferon-lambda determine persistence of enteric murine norovirus infection. Science. 2015;347(6219):266–269. doi: 10.1126/science.1258025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudoux P., Carrat C., Besnardeau L., Charley B., Laude H. Coronavirus pseudoparticles formed with recombinant M and E proteins induce alpha interferon synthesis by leukocytes. J. Virol. 1998;72(11):8636–8643. doi: 10.1128/jvi.72.11.8636-8643.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beall A., Yount B., Lin C.M., Hou Y., Wang Q., Saif L., Baric R. Characterization of a pathogenic full-Length cDNA clone and transmission model for porcine epidemic diarrhea virus strain PC22A. MBio. 2016;7(1) doi: 10.1128/mBio.01451-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehme K.W., Compton T. Innate sensing of viruses by toll-like receptors. J. Virol. 2004;78(15):7867–7873. doi: 10.1128/JVI.78.15.7867-7873.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch B.J., van der Zee R., de Haan C.A., Rottier P.J. The coronavirus spike protein is a class I virus fusion protein: structural and functional characterization of the fusion core complex. J. Virol. 2003;77(16):8801–8811. doi: 10.1128/JVI.77.16.8801-8811.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosworth B.T., MacLachlan N.J., Johnston M.I. Induction of the 2–5A system by interferon and transmissible gastroenteritis virus. J. Interferon Res. 1989;9(6):731–739. doi: 10.1089/jir.1989.9.731. [DOI] [PubMed] [Google Scholar]
- Brian D.A., Baric R.S. Coronavirus genome structure and replication. Curr. Top. Microbiol. Immunol. 2005;287:1–30. doi: 10.1007/3-540-26765-4_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockway S.M., Lu X.T., Peters T.R., Dermody T.S., Denison M.R. Intracellular localization and protein interactions of the gene 1 protein p28 during mouse hepatitis virus replication. J. Virol. 2004;78(21):11551–11562. doi: 10.1128/JVI.78.21.11551-11562.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantell K., Hirvonen S., Kauppinen H.L., Myllyla G. Production of interferon in human leukocytes from normal donors with the use of Sendai virus. Methods Enzymol. 1981;78(Pt A):29–38. doi: 10.1016/0076-6879(81)78094-7. [DOI] [PubMed] [Google Scholar]
- Cao L., Ge X., Gao Y., Herrler G., Ren Y., Ren X., Li G. Porcine epidemic diarrhea virus inhibits dsRNA-induced interferon-beta production in porcine intestinal epithelial cells by blockade of the RIG-I-mediated pathway. Virol. J. 2015;12:127. doi: 10.1186/s12985-015-0345-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L., Ge X., Gao Y., Ren Y., Ren X., Li G. Porcine epidemic diarrhea virus infection induces NF-kappaB activation through the TLR2 TLR3, and TLR9 pathways in porcine intestinal epithelial cells. J. Gen. Virol. 2015 doi: 10.1099/vir.0.000133. [DOI] [PubMed] [Google Scholar]
- Charley B., Laude H. Induction of alpha interferon by transmissible gastroenteritis coronavirus: role of transmembrane glycoprotein E1. J. Virol. 1988;62(1):8–11. doi: 10.1128/jvi.62.1.8-11.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charley B., Riffault S., Van Reeth K. Porcine innate and adaptative immune responses to influenza and coronavirus infections. Ann. N. Y. Acad. Sci. 2006;1081:130–136. doi: 10.1196/annals.1373.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Kinzer C.A., Paul W.E. p161, a murine membrane protein expressed on mast cells and some macrophages, is mouse CD13/aminopeptidase N. J. Immunol. 1996;157(6):2593–2600. [PubMed] [Google Scholar]
- Chen Q., Li G., Stasko J., Thomas J.T., Stensland W.R., Pillatzki A.E., Gauger P.C., Schwartz K.J., Madson D., Yoon K.J., Stevenson G.W., Burrough E.R., Harmon K.M., Main R.G., Zhang J. Isolation and characterization of porcine epidemic diarrhea viruses associated with the 2013 disease outbreak among swine in the United States. J. Clin. Microbiol. 2014;52(1):234–243. doi: 10.1128/JCM.02820-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Yang X., Zheng Y., Yang Y., Xing Y., Chen Z. SARS coronavirus papain-like protease inhibits the type I interferon signaling pathway through interaction with the STING-TRAF3-TBK1 complex. Protein Cell. 2014;5(5):369–381. doi: 10.1007/s13238-014-0026-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q., Gauger P., Stafne M., Thomas J., Arruda P., Burrough E., Madson D., Brodie J., Magstadt D., Derscheid R., Welch M., Zhang J. Pathogenicity and pathogenesis of a United States porcine deltacoronavirus cell culture isolate in 5-day-old neonatal piglets. Virology. 2015;482:51–59. doi: 10.1016/j.virol.2015.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clementz M.A., Chen Z., Banach B.S., Wang Y., Sun L., Ratia K., Baez-Santos Y.M., Wang J., Takayama J., Ghosh A.K., Li K., Mesecar A.D., Baker S.C. Deubiquitinating and interferon antagonism activities of coronavirus papain-like proteases. J. Virol. 2010;84(9):4619–4629. doi: 10.1128/JVI.02406-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor R.F., Roper R.L. Unique SARS-CoV protein nsp1: bioinformatics, biochemistry and potential effects on virulence. Trends Microbiol. 2007;15(2):51–53. doi: 10.1016/j.tim.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz J.L., Sola I., Becares M., Alberca B., Plana J., Enjuanes L., Zuniga S. Coronavirus gene 7 counteracts host defenses and modulates virus virulence. PLoS Pathog. 2011;7(6):e1002090. doi: 10.1371/journal.ppat.1002090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deal E.M., Lahl K., Narvaez C.F., Butcher E.C., Greenberg H.B. Plasmacytoid dendritic cells promote rotavirus-induced human and murine B cell responses. J. Clin. Invest. 2013;123(6):2464–2474. doi: 10.1172/JCI60945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debouck P., Pensaert M. Experimental infection of pigs with a new porcine enteric coronavirus, CV 777. Am. J. Vet. Res. 1980;41(2):219–223. [PubMed] [Google Scholar]
- Delmas B., Gelfi J., L'Haridon R., Vogel L.K., Sjostrom H., Noren O., Laude H. Aminopeptidase N is a major receptor for the entero-pathogenic coronavirus TGEV. Nature. 1992;357(6377):417–420. doi: 10.1038/357417a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diebold S.S., Kaisho T., Hemmi H., Akira S., Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303(5663):1529–1531. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- Ding L., Huang Y., Du Q., Dong F., Zhao X., Zhang W., Xu X., Tong D. TGEV nucleocapsid protein induces cell cycle arrest and apoptosis through activation of p53 signaling. Biochem. Biophys. Res. Commun. 2014;445(2):497–503. doi: 10.1016/j.bbrc.2014.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Z., Fang L., Jing H., Zeng S., Wang D., Liu L., Zhang H., Luo R., Chen H., Xiao S. Porcine epidemic diarrhea virus nucleocapsid protein antagonizes beta interferon production by sequestering the interaction between IRF3 and TBK1. J. Virol. 2014;88(16):8936–8945. doi: 10.1128/JVI.00700-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong N., Fang L., Zeng S., Sun Q., Chen H., Xiao S. Porcine deltacoronavirus in mainland China. Emerg. Infect. Dis. 2015;21(12):2254–2255. doi: 10.3201/eid2112.150283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte M., Gelfi J., Lambert P., Rasschaert D., Laude H. Genome organization of porcine epidemic diarrhoea virus. Adv. Exp. Med. Biol. 1993;342:55–60. doi: 10.1007/978-1-4615-2996-5_9. [DOI] [PubMed] [Google Scholar]
- Elinav E., Strowig T., Kau A.L., Henao-Mejia J., Thaiss C.A., Booth C.J., Peaper D.R., Bertin J., Eisenbarth S.C., Gordon J.I., Flavell R.A. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell. 2011;145(5):745–757. doi: 10.1016/j.cell.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X., Gao J., Zheng H., Li B., Kong L., Zhang Y., Wang W., Zeng Y., Ye L. The membrane protein of SARS-CoV suppresses NF-kappaB activation. J. Med. Virol. 2007;79(10):1431–1439. doi: 10.1002/jmv.20953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkbeiner S.R., Zeng X.L., Utama B., Atmar R.L., Shroyer N.F., Estes M.K. Stem cell-derived human intestinal organoids as an infection model for rotaviruses. MBio. 2012;3(4):e00159–00112. doi: 10.1128/mBio.00159-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald K.A., McWhirter S.M., Faia K.L., Rowe D.C., Latz E., Golenbock D.T., Coyle A.J., Liao S.M., Maniatis T. IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat. Immunol. 2003;4(5):491–496. doi: 10.1038/ni921. [DOI] [PubMed] [Google Scholar]
- Frenz T., Graalmann L., Detje C.N., Doring M., Grabski E., Scheu S., Kalinke U. Independent of plasmacytoid dendritic cell (pDC) infection, pDC triggered by virus-infected cells mount enhanced type I IFN responses of different composition as opposed to pDC stimulated with free virus. J. Immunol. 2014;193(5):2496–2503. doi: 10.4049/jimmunol.1400215. [DOI] [PubMed] [Google Scholar]
- Fu Y., Quan R., Zhang H., Hou J., Tang J., Feng W.H. Porcine reproductive and respiratory syndrome virus induces interleukin-15 through the NF-kappaB signaling pathway. J. Virol. 2012;86(14):7625–7636. doi: 10.1128/JVI.00177-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher T.M., Buchmeier M.J. Coronavirus spike proteins in viral entry and pathogenesis. Virology. 2001;279(2):371–374. doi: 10.1006/viro.2000.0757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q., Zhao S., Qin T., Yin Y., Yang Q. Effects of porcine epidemic diarrhea virus on porcine monocyte-derived dendritic cells and intestinal dendritic cells. Vet. Microbiol. 2015;179(3-4):131–141. doi: 10.1016/j.vetmic.2015.05.016. [DOI] [PubMed] [Google Scholar]
- Gibbert K., Schlaak J.F., Yang D., Dittmer U. IFN-alpha subtypes: distinct biological activities in anti-viral therapy. Br. J. Pharmacol. 2013;168(5):1048–1058. doi: 10.1111/bph.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gralinski L.E., Baric R.S. Molecular pathology of emerging coronavirus infections. J. Pathol. 2015;235(2):185–195. doi: 10.1002/path.4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzylack-Piriou L., Piersma S., McCullough K., Summerfield A. Role of natural interferon-producing cells and T lymphocytes in porcine monocyte-derived dendritic cell maturation. Immunology. 2006;118(1):78–87. doi: 10.1111/j.1365-2567.2006.02343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil F., Hemmi H., Hochrein H., Ampenberger F., Kirschning C., Akira S., Lipford G., Wagner H., Bauer S. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303(5663):1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- Hernandez P.P., Mahlakoiv T., Yang I., Schwierzeck V., Nguyen N., Guendel F., Gronke K., Ryffel B., Holscher C., Dumoutier L., Renauld J.C., Suerbaum S., Staeheli P., Diefenbach A. Interferon-lambda and interleukin 22 act synergistically for the induction of interferon-stimulated genes and control of rotavirus infection. Nat. Immunol. 2015;16(7):698–707. doi: 10.1038/ni.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann H.H., Schneider W.M., Rice C.M. Interferons and viruses: an evolutionary arms race of molecular interactions. Trends Immunol. 2015;36(3):124–138. doi: 10.1016/j.it.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann M., Wyler R. Propagation of the virus of porcine epidemic diarrhea in cell culture. J. Clin. Microbiol. 1988;26(11):2235–2239. doi: 10.1128/jcm.26.11.2235-2239.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda K., Takaoka A., Taniguchi T. Type I inteferon gene induction by the interferon regulatory factor family of transcription factors. Immunity. 2006;25:349–360. doi: 10.1016/j.immuni.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Huan C.C., Wang Y., Ni B., Wang R., Huang L., Ren X.F., Tong G.Z., Ding C., Fan H.J., Mao X. Porcine epidemic diarrhea virus uses cell-surface heparan sulfate as an attachment factor. Arch. Virol. 2015;160(7):1621–1628. doi: 10.1007/s00705-015-2408-0. [DOI] [PubMed] [Google Scholar]
- Huang C., Lokugamage K.G., Rozovics J.M., Narayanan K., Semler B.L., Makino S. Alphacoronavirus transmissible gastroenteritis virus nsp1 protein suppresses protein translation in mammalian cells and in cell-free HeLa cell extracts but not in rabbit reticulocyte lysate. J. Virol. 2011;85(1):638–643. doi: 10.1128/JVI.01806-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Ding L., Li Z., Dai M., Zhao X., Li W., Du Q., Xu X., Tong D. Transmissible gastroenteritis virus infection induces cell apoptosis via activation of p53 signalling. J. Gen. Virol. 2013;94(Pt 8):1807–1817. doi: 10.1099/vir.0.051557-0. [DOI] [PubMed] [Google Scholar]
- Ishikawa H., Barber G.N. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature. 2008;455(7213):674–678. doi: 10.1038/nature07317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jengarn J., Wongthida P., Wanasen N., Frantz P.N., Wanitchang A., Jongkaewwattana A. Genetic manipulation of porcine epidemic diarrhoea virus recovered from a full-length infectious cDNA clone. J. Gen. Virol. 2015;96(8):2206–2218. doi: 10.1099/vir.0.000184. [DOI] [PubMed] [Google Scholar]
- Jordan L.T., Derbyshire J.B. Antiviral activity of interferon against transmissible gastroenteritis virus in cell culture and ligated intestinal segments in neonatal pigs. Vet. Microbiol. 1994;38(3):263–276. doi: 10.1016/0378-1135(94)90007-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan L.T., Derbyshire J.B. Antiviral action of interferon-alpha against porcine transmissible gastroenteritis virus. Vet. Microbiol. 1995;45(1):59–70. doi: 10.1016/0378-1135(94)00118-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung K., Saif L.J. Porcine epidemic diarrhea virus infection: etiology, epidemiology, pathogenesis and immunoprophylaxis. Vet. J. 2015;204(2):134–143. doi: 10.1016/j.tvjl.2015.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung K., Wang Q., Scheuer K.A., Lu Z., Zhang Y., Saif L.J. Pathology of US porcine epidemic diarrhea virus strain PC21A in gnotobiotic pigs. Emerg. Infect. Dis. 2014;20(4):662–665. doi: 10.3201/eid2004.131685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung K., Annamalai T., Lu Z., Saif L.J. Comparative pathogenesis of US porcine epidemic diarrhea virus (PEDV) strain PC21A in conventional 9-day-old nursing piglets vs. 26-day-old weaned pigs. Vet. Microbiol. 2015;178(1-2):31–40. doi: 10.1016/j.vetmic.2015.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung K., Eyerly B., Annamalai T., Lu Z., Saif L.J. Structural alteration of tight and adherens junctions in villous and crypt epithelium of the small and large intestine of conventional nursing piglets infected with porcine epidemic diarrhea virus. Vet. Microbiol. 2015;177(3-4):373–378. doi: 10.1016/j.vetmic.2015.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung K., Hu H., Eyerly B., Lu Z., Chepngeno J., Saif L.J. Pathogenicity of 2 porcine deltacoronavirus strains in gnotobiotic pigs. Emerg. Infect. Dis. 2015;21(4):650–654. doi: 10.3201/eid2104.141859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung K., Hu H., Saif L.J. Porcine deltacoronavirus induces apoptosis in swine testicular and LLC porcine kidney cell lines in vitro but not in infected intestinal enterocytes in vivo. Vet. Microbiol. 2016;182:57–63. doi: 10.1016/j.vetmic.2015.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamitani W., Narayanan K., Huang C., Lokugamage K., Ikegami T., Ito N., Kubo H., Makino S. Severe acute respiratory syndrome coronavirus nsp1 protein suppresses host gene expression by promoting host mRNA degradation. Proc. Natl. Acad. Sci. U. S. A. 2006;103(34):12885–12890. doi: 10.1073/pnas.0603144103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariko K., Ni H., Capodici J., Lamphier M., Weissman D. mRNA is an endogenous ligand for Toll-like receptor 3. J. Biol. Chem. 2004;279(13):12542–12550. doi: 10.1074/jbc.M310175200. [DOI] [PubMed] [Google Scholar]
- Kato H., Takeuchi O., Mikamo-Satoh E., Hirai R., Kawai T., Matsushita K., Hiiragi A., Dermody T.S., Fujita T., Akira S. Length-dependent recognition of double-stranded ribonucleic acids by retinoic acid-inducible gene-I and melanoma differentiation-associated gene 5. J. Exp. Med. 2008;205(7):1601–1610. doi: 10.1084/jem.20080091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T., Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 2010;11(5):373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- Kawai T., Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34(5):637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Kim Y., Lee C. Porcine epidemic diarrhea virus induces caspase-independent apoptosis through activation of mitochondrial apoptosis-inducing factor. Virology. 2014;460-461:180–193. doi: 10.1016/j.virol.2014.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y., Lee C. Extracellular signal-regulated kinase (ERK) activation is required for porcine epidemic diarrhea virus replication. Virology. 2015;484:181–193. doi: 10.1016/j.virol.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim L., Hayes J., Lewis P., Parwani A.V., Chang K.O., Saif L.J. Molecular characterization and pathogenesis of transmissible gastroenteritis coronavirus (TGEV) and porcine respiratory coronavirus (PRCV) field isolates co-circulating in a swine herd. Arch. Virol. 2000;145(6):1133–1147. doi: 10.1007/s007050070114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.J., Han Y.W., Rahman M.M., Kim S.B., Uyangaa E., Lee B.M., Kim J.H., Roh Y.S., Kang S.H., Kim K., Lee J.H., Kim B., Park K.I., Eo S.K. Live attenuated Salmonella enterica serovar Typhimurium expressing swine interferon-alpha has antiviral activity and alleviates clinical signs induced by infection with transmissible gastroenteritis virus in piglets. Vaccine. 2010;28(31):5031–5037. doi: 10.1016/j.vaccine.2010.05.013. [DOI] [PubMed] [Google Scholar]
- Kindler E., Thiel V. To sense or not to sense viral RNA–essentials of coronavirus innate immune evasion. Curr. Opin. Microbiol. 2014;20:69–75. doi: 10.1016/j.mib.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh H.W., Kim M.S., Lee J.S., Kim H., Park S.J. Changes in the swine gut microbiota in response to porcine epidemic diarrhea infection. Microb. Environ. 2015;30(3):284–287. doi: 10.1264/jsme2.ME15046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotenko S.V., Gallagher G., Baurin V.V., Lewis-Antes A., Shen M., Shah N.K., Langer J.A., Sheikh F., Dickensheets H., Donnelly R.P. IFN-lambdas mediate antiviral protection through a distinct class II cytokine receptor complex. Nat. Immunol. 2003;4(1):69–77. doi: 10.1038/ni875. [DOI] [PubMed] [Google Scholar]
- Kruse T.A., Bolund L., Grzeschik K.H., Ropers H.H., Olsen J., Sjostrom H., Noren O. Assignment of the human aminopeptidase N (peptidase E) gene to chromosome 15q13-qter. FEBS Lett. 1988;239(2):305–308. doi: 10.1016/0014-5793(88)80940-2. [DOI] [PubMed] [Google Scholar]
- La Bonnardiere C., Laude H. High interferon titer in newborn pig intestine during experimentally induced viral enteritis. Infect. Immun. 1981;32(1):28–31. doi: 10.1128/iai.32.1.28-31.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Bonnardiere C., Laude H. Interferon induction in rotavirus and coronavirus infections: a review of recent results. Ann. Rech. Vet. 1983;14(4):507–511. [PubMed] [Google Scholar]
- Laude H., Charley B., Gelfi J. Replication of transmissible gastroenteritis coronavirus (TGEV) in swine alveolar macrophages. J. Gen. Virol. 1984;65(Pt 2):327–332. doi: 10.1099/0022-1317-65-2-327. [DOI] [PubMed] [Google Scholar]
- Laude H., Gelfi J., Lavenant L., Charley B. Single amino acid changes in the viral glycoprotein M affect induction of alpha interferon by the coronavirus transmissible gastroenteritis virus. J. Virol. 1992;66(2):743–749. doi: 10.1128/jvi.66.2.743-749.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laude H., Van Reeth K., Pensaert M. Porcine respiratory coronavirus: molecular features and virus-host interactions. Vet. Res. 1993;24(2):125–150. [PubMed] [Google Scholar]
- Lawrence P.K., Bumgardner E., Bey R.F., Stine D., Bumgarner R.E. Genome sequences of porcine epidemic diarrhea virus: in vivo and in vitro phenotypes. Genome Announc. 2014;2(3) doi: 10.1128/genomeA.00503-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Lee C. Complete genome characterization of korean porcine deltacoronavirus strain KOR/KNU14-04/2014. Genome Announc. 2014;2(6) doi: 10.1128/genomeA.01191-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Lee C. Functional characterization and proteomic analysis of the nucleocapsid protein of porcine deltacoronavirus. Virus Res. 2015;208:136–145. doi: 10.1016/j.virusres.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.M., Lee B.J., Tae J.H., Kweon C.H., Lee Y.S., Park J.H. Detection of porcine epidemic diarrhea virus by immunohistochemistry with recombinant antibody produced in phages. J. Vet. Med. Sci. 2000;62(3):333–337. doi: 10.1292/jvms.62.333. [DOI] [PubMed] [Google Scholar]
- Lee B.M., Han Y.W., Kim S.B., Rahman M.M., Uyangaa E., Kim J.H., Roh Y.S., Kim B., Han S.B., Hong J.T., Kim K., Eo S.K. Enhanced protection against infection with transmissible gastroenteritis virus in piglets by oral co-administration of live attenuated Salmonella enterica serovar Typhimurium expressing swine interferon-alpha and interleukin-18. Comp. Immunol. Microbiol. Infect. Dis. 2011;34(4):369–380. doi: 10.1016/j.cimid.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Li B.X., Ge J.W., Li Y.J. Porcine aminopeptidase N is a functional receptor for the PEDV coronavirus. Virology. 2007;365(1):166–172. doi: 10.1016/j.virol.2007.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Li Z., Zou Y., Wicht O., van Kuppeveld F.J., Rottier P.J., Bosch B.J. Manipulation of the porcine epidemic diarrhea virus genome using targeted RNA recombination. PLoS One. 2013;8(8):e69997. doi: 10.1371/journal.pone.0069997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Q.J., Ye L.B., Timani K.A., Zeng Y.C., She Y.L., Ye L., Wu Z.H. Activation of NF-kappaB by the full-length nucleocapsid protein of the SARS coronavirus. Acta Biochim. Biophys. Sin. (Shanghai) 2005;37(9):607–612. doi: 10.1111/j.1745-7270.2005.00082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C.M., Gao X., Oka T., Vlasova A.N., Esseili M.A., Wang Q., Saif L.J. Antigenic relationships among porcine epidemic diarrhea virus and transmissible gastroenteritis virus strains. J. Virol. 2015;89(6):3332–3342. doi: 10.1128/JVI.03196-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Tang J., Ma Y., Liang X., Yang Y., Peng G., Qi Q., Jiang S., Li J., Du L., Li F. Receptor usage and cell entry of porcine epidemic diarrhea coronavirus. J. Virol. 2015;89(11):6121–6125. doi: 10.1128/JVI.00430-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Zhao L., Zhai Z., Zhao W., Ding J., Dai R., Sun T., Meng H. Porcine epidemic diarrhea virus infection induced the unbalance of gut microbiota in piglets. Curr. Microbiol. 2015;71(6):643–649. doi: 10.1007/s00284-015-0895-6. [DOI] [PubMed] [Google Scholar]
- Ma Y., Zhang Y., Liang X., Lou F., Oglesbee M., Krakowka S., Li J. Origin, evolution, and virulence of porcine deltacoronaviruses in the United States. MBio. 2015;6(2):e00064. doi: 10.1128/mBio.00064-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahlakoiv T., Hernandez P., Gronke K., Diefenbach A., Staeheli P. Leukocyte-derived IFN-alpha/beta and epithelial IFN-lambda constitute a compartmentalized mucosal defense system that restricts enteric virus infections. PLoS Pathog. 2015;11(4):e1004782. doi: 10.1371/journal.ppat.1004782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marthaler D., Jiang Y., Otterson T., Goyal S., Rossow K., Collins J. Complete genome sequence of porcine epidemic diarrhea virus strain USA/Colorado/2013 from the United States. Genome Announc. 2013;1(4) doi: 10.1128/genomeA.00555-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marthaler D., Raymond L., Jiang Y., Collins J., Rossow K., Rovira A. Rapid detection, complete genome sequencing, and phylogenetic analysis of porcine deltacoronavirus. Emerg. Infect. Dis. 2014;20(8):1347–1350. doi: 10.3201/eid2008.140526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCluskey B.J., Haley C., Rovira A., Main R., Zhang Y., Barder S. Retrospective testing and case series study of porcine delta coronavirus in U.S. swine herds. Prev. Vet. Med. 2016;123:185–191. doi: 10.1016/j.prevetmed.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mina-Osorio P. The moonlighting enzyme CD13: old and new functions to target. Trends Mol. Med. 2008;14(8):361–371. doi: 10.1016/j.molmed.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mole B. Deadly pig virus slips through US borders. Nature. 2013;499(7459):388. doi: 10.1038/499388a. [DOI] [PubMed] [Google Scholar]
- Mordstein M., Kochs G., Dumoutier L., Renauld J.C., Paludan S.R., Klucher K., Staeheli P. Interferon-lambda contributes to innate immunity of mice against influenza A virus but not against hepatotropic viruses. PLoS Pathog. 2008;4(9):e1000151. doi: 10.1371/journal.ppat.1000151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mordstein M., Neugebauer E., Ditt V., Jessen B., Rieger T., Falcone V., Sorgeloos F., Ehl S., Mayer D., Kochs G., Schwemmle M., Gunther S., Drosten C., Michiels T., Staeheli P. Lambda interferon renders epithelial cells of the respiratory and gastrointestinal tracts resistant to viral infections. J. Virol. 2010;84(11):5670–5677. doi: 10.1128/JVI.00272-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam E., Lee C. Contribution of the porcine aminopeptidase N (CD13) receptor density to porcine epidemic diarrhea virus infection. Vet. Microbiol. 2010;144(1-2):41–50. doi: 10.1016/j.vetmic.2009.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan K., Ramirez S.I., Lokugamage K.G., Makino S. Coronavirus nonstructural protein 1: Common and distinct functions in the regulation of host and viral gene expression. Virus Res. 2014 doi: 10.1016/j.virusres.2014.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nice T.J., Baldridge M.T., McCune B.T., Norman J.M., Lazear H.M., Artyomov M., Diamond M.S., Virgin H.W. Interferon-lambda cures persistent murine norovirus infection in the absence of adaptive immunity. Science. 2015;347(6219):269–273. doi: 10.1126/science.1258100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odendall C., Dixit E., Stavru F., Bierne H., Franz K.M., Durbin A.F., Boulant S., Gehrke L., Cossart P., Kagan J.C. Diverse intracellular pathogens activate type III interferon expression from peroxisomes. Nat. Immunol. 2014;15(8):717–726. doi: 10.1038/ni.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh J.S., Song D.S., Park B.K. Identification of a putative cellular receptor 150 kDa polypeptide for porcine epidemic diarrhea virus in porcine enterocytes. J. Vet. Sci. 2003;4(3):269–275. [PubMed] [Google Scholar]
- Okahira S., Nishikawa F., Nishikawa S., Akazawa T., Seya T., Matsumoto M. Interferon-beta induction through toll-like receptor 3 depends on double-stranded RNA structure. DNA Cell Biol. 2005;24(10):614–623. doi: 10.1089/dna.2005.24.614. [DOI] [PubMed] [Google Scholar]
- Oldenburg M., Kruger A., Ferstl R., Kaufmann A., Nees G., Sigmund A., Bathke B., Lauterbach H., Suter M., Dreher S., Koedel U., Akira S., Kawai T., Buer J., Wagner H., Bauer S., Hochrein H., Kirschning C.J. TLR13 recognizes bacterial 23 S rRNA devoid of erythromycin resistance-forming modification. Science. 2012;337(6098):1111–1115. doi: 10.1126/science.1220363. [DOI] [PubMed] [Google Scholar]
- Onoguchi K., Yoneyama M., Takemura A., Akira S., Taniguchi T., Namiki H., Fujita T. Viral infections activate types I and III interferon genes through a common mechanism. J. Biol. Chem. 2007;282(10):7576–7581. doi: 10.1074/jbc.M608618200. [DOI] [PubMed] [Google Scholar]
- Park J.E., Shin H.J. Porcine epidemic diarrhea virus infects and replicates in porcine alveolar macrophages. Virus Res. 2014;191:143–152. doi: 10.1016/j.virusres.2014.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.J., Moon H.J., Luo Y., Kim H.K., Kim E.M., Yang J.S., Song D.S., Kang B.K., Lee C.S., Park B.K. Cloning and further sequence analysis of the ORF3 gene of wild- and attenuated-type porcine epidemic diarrhea viruses. Virus Genes. 2008;36(1):95–104. doi: 10.1007/s11262-007-0164-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.E., Cruz D.J., Shin H.J. Receptor-bound porcine epidemic diarrhea virus spike protein cleaved by trypsin induces membrane fusion. Arch. Virol. 2011;156(10):1749–1756. doi: 10.1007/s00705-011-1044-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestka S., Krause C.D., Walter M.R. Interferons, interferon-like cytokines, and their receptors. Immunol. Rev. 2004;202:8–32. doi: 10.1111/j.0105-2896.2004.00204.x. [DOI] [PubMed] [Google Scholar]
- Peterson L.W., Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 2014;14(3):141–153. doi: 10.1038/nri3608. [DOI] [PubMed] [Google Scholar]
- Pott J., Mahlakoiv T., Mordstein M., Duerr C.U., Michiels T., Stockinger S., Staeheli P., Hornef M.W. IFN-lambda determines the intestinal epithelial antiviral host defense. Proc. Natl. Acad. Sci. U. S. A. 2011;108(19):7944–7949. doi: 10.1073/pnas.1100552108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokunina-Olsson L., Muchmore B., Tang W., Pfeiffer R.M., Park H., Dickensheets H., Hergott D., Porter-Gill P., Mumy A., Kohaar I., Chen S., Brand N., Tarway M., Liu L., Sheikh F., Astemborski J., Bonkovsky H.L., Edlin B.R., Howell C.D., Morgan T.R., Thomas D.L., Rehermann B., Donnelly R.P., O'Brien T.R. A variant upstream of IFNL3 (IL28B) creating a new interferon gene IFNL4 is associated with impaired clearance of hepatitis C virus. Nat. Genet. 2013;45(2):164–171. doi: 10.1038/ng.2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajsbaum R., Garcia-Sastre A. Viral evasion mechanisms of early antiviral responses involving regulation of ubiquitin pathways. Trends Microbiol. 2013;21(8):421–429. doi: 10.1016/j.tim.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall R.E., Goodbourn S. Interferons and viruses: an interplay between induction, signalling, antiviral responses and virus countermeasures. J. Gen. Virol. 2008;89:1–47. doi: 10.1099/vir.0.83391-0. [DOI] [PubMed] [Google Scholar]
- Riemann D., Kehlen A., Langner J. CD13–not just a marker in leukemia typing. Immunol. Today. 1999;20(2):83–88. doi: 10.1016/S0167-5699(98)01398-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riffault S., Grosclaude J., Vayssier M., Laude H., Charley B. Reconstituted coronavirus TGEV virosomes lose the virus ability to induce porcine interferon-alpha production. Vet. Res. 1997;28(2):105–114. [PubMed] [Google Scholar]
- Riffault S., Carrat C., van Reeth K., Pensaert M., Charley B. Interferon-alpha-producing cells are localized in gut-associated lymphoid tissues in transmissible gastroenteritis virus (TGEV) infected piglets. Vet. Res. 2001;32(1):71–79. doi: 10.1051/vetres:2001111. [DOI] [PubMed] [Google Scholar]
- Rossen J.W., Bekker C.P., Voorhout W.F., Strous G.J., van der Ende A., Rottier P.J. Entry and release of transmissible gastroenteritis coronavirus are restricted to apical surfaces of polarized epithelial cells. J. Virol. 1994;68(12):7966–7973. doi: 10.1128/jvi.68.12.7966-7973.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlee M., Roth A., Hornung V., Hagmann C.A., Wimmenauer V., Barchet W., Coch C., Janke M., Mihailovic A., Wardle G., Juranek S., Kato H., Kawai T., Poeck H., Fitzgerald K.A., Takeuchi O., Akira S., Tuschl T., Latz E., Ludwig J., Hartmann G. Recognition of 5' triphosphate by RIG-I helicase requires short blunt double-stranded RNA as contained in panhandle of negative-strand virus. Immunity. 2009;31(1):25–34. doi: 10.1016/j.immuni.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A., Schwerd T., Hamm W., Hellmuth J.C., Cui S., Wenzel M., Hoffmann F.S., Michallet M.C., Besch R., Hopfner K.P., Endres S., Rothenfusser S. 5'-triphosphate RNA requires base-paired structures to activate antiviral signaling via RIG-I. Proc. Natl. Acad. Sci. U. S. A. 2009;106(29):12067–12072. doi: 10.1073/pnas.0900971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider W.M., Chevillotte M.D., Rice C.M. Interferon-stimulated genes: a complex web of host defenses. Annu. Rev. Immunol. 2014;32:513–545. doi: 10.1146/annurev-immunol-032713-120231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen A., Rott L., Phan N., Mukherjee G., Greenberg H.B. Rotavirus NSP1 protein inhibits interferon-mediated STAT1 activation. J. Virol. 2014;88(1):41–53. doi: 10.1128/JVI.01501-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S., tenOever B.R., Grandvaux N., Zhou G.P., Lin R., Hiscott J. Triggering the interferon antiviral response through an IKK-related pathway. Science. 2003;300(5622):1148–1151. doi: 10.1126/science.1081315. [DOI] [PubMed] [Google Scholar]
- Sheppard P., Kindsvogel W., Xu W., Henderson K., Schlutsmeyer S., Whitmore T.E., Kuestner R., Garrigues U., Birks C., Roraback J., Ostrander C., Dong D., Shin J., Presnell S., Fox B., Haldeman B., Cooper E., Taft D., Gilbert T., Grant F.J., Tackett M., Krivan W., McKnight G., Clegg C., Foster D., Klucher K.M. IL-28, IL-29 and their class II cytokine receptor IL-28R. Nat. Immunol. 2003;4(1):63–68. doi: 10.1038/ni873. [DOI] [PubMed] [Google Scholar]
- Shi C.S., Qi H.Y., Boularan C., Huang N.N., Abu-Asab M., Shelhamer J.H., Kehrl J.H. SARS-coronavirus open reading frame-9b suppresses innate immunity by targeting mitochondria and the MAVS/TRAF3/TRAF6 signalosome. J. Immunol. 2014;193(6):3080–3089. doi: 10.4049/jimmunol.1303196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi D., Lv M., Chen J., Shi H., Zhang S., Zhang X., Feng L. Molecular characterizations of subcellular localization signals in the nucleocapsid protein of porcine epidemic diarrhea virus. Viruses. 2014;6(3):1253–1273. doi: 10.3390/v6031253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegal F.P., Kadowaki N., Shodell M., Fitzgerald-Bocarsly P.A., Shah K., Ho S., Antonenko S., Liu Y.J. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284(5421):1835–1837. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- Sinha A., Gauger P., Zhang J., Yoon K.J., Harmon K. PCR-based retrospective evaluation of diagnostic samples for emergence of porcine deltacoronavirus in US swine. Vet. Microbiol. 2015;179(3-4):296–298. doi: 10.1016/j.vetmic.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijder E.J., Bredenbeek P.J., Dobbe J.C., Thiel V., Ziebuhr J., Poon L.L., Guan Y., Rozanov M., Spaan W.J., Gorbalenya A.E. Unique and conserved features of genome and proteome of SARS-coronavirus, an early split-off from the coronavirus group 2 lineage. J. Mol. Biol. 2003;331(5):991–1004. doi: 10.1016/S0022-2836(03)00865-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijder E.J., van der Meer Y., Zevenhoven-Dobbe J., Onderwater J.J., van der Meulen J., Koerten H.K., Mommaas A.M. Ultrastructure and origin of membrane vesicles associated with the severe acute respiratory syndrome coronavirus replication complex. J. Virol. 2006;80(12):5927–5940. doi: 10.1128/JVI.02501-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommereyns C., Paul S., Staeheli P., Michiels T. IFN-lambda (IFN-lambda) is expressed in a tissue-dependent fashion and primarily acts on epithelial cells in vivo. PLoS Pathog. 2008;4(3):e1000017. doi: 10.1371/journal.ppat.1000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song D., Park B. Porcine epidemic diarrhoea virus: a comprehensive review of molecular epidemiology, diagnosis, and vaccines. Virus Genes. 2012;44(2):167–175. doi: 10.1007/s11262-012-0713-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Splichal I., Rehakova Z., Sinkora M., Sinkora J., Trebichavsky I., Laude H., Charley B. In vivo study of interferon-alpha-secreting cells in pig foetal lymphohaematopoietic organs following in utero TGEV coronavirus injection. Res. Immunol. 1997;148(4):247–256. doi: 10.1016/S0923-2494(97)80866-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson G.W., Hoang H., Schwartz K.J., Burrough E.R., Sun D., Madson D., Cooper V.L., Pillatzki A., Gauger P., Schmitt B.J., Koster L.G., Killian M.L., Yoon K.J. Emergence of Porcine epidemic diarrhea virus in the United States: clinical signs, lesions, and viral genomic sequences. J. Vet. Diagn. Invest. 2013;25(5):649–654. doi: 10.1177/1040638713501675. [DOI] [PubMed] [Google Scholar]
- Summerfield A., Guzylack-Piriou L., Schaub A., Carrasco C.P., Tache V., Charley B., McCullough K.C. Porcine peripheral blood dendritic cells and natural interferon-producing cells. Immunology. 2003;110(4):440–449. doi: 10.1111/j.1365-2567.2003.01755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L., Wu J., Du F., Chen X., Chen Z.J. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. 2013;339(6121):786–791. doi: 10.1126/science.1232458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D., Shi H., Guo D., Chen J., Shi D., Zhu Q., Zhang X., Feng L. Analysis of protein expression changes of the Vero E6 cells infected with classic PEDV strain CV777 by using quantitative proteomic technique. J. Virol. Methods. 2015;218:27–39. doi: 10.1016/j.jviromet.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swamy M., Abeler-Dorner L., Chettle J., Mahlakoiv T., Goubau D., Chakravarty P., Ramsay G., Reis e Sousa C., Staeheli P., Blacklaws B.A., Heeney J.L., Hayday A.C. Intestinal intraepithelial lymphocyte activation promotes innate antiviral resistance. Nat. Commun. 2015;6:7090. doi: 10.1038/ncomms8090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szubin R., Chang W.L., Greasby T., Beckett L., Baumgarth N. Rigid interferon-alpha subtype responses of human plasmacytoid dendritic cells. J. Interferon Cytokine Res. 2008;28(12):749–763. doi: 10.1089/jir.2008.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J.M., Pos Z., Reinboth J., Wang R.Y., Wang E., Frank G.M., Lusso P., Trinchieri G., Alter H.J., Marincola F.M., Thomas E. Differential responses of plasmacytoid dendritic cells to influenza virus and distinct viral pathogens. J. Virol. 2014;88(18):10758–10766. doi: 10.1128/JVI.01501-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohya Y., Narayanan K., Kamitani W., Huang C., Lokugamage K., Makino S. Suppression of host gene expression by nsp1 proteins of group 2 bat coronaviruses. J. Virol. 2009;83(10):5282–5288. doi: 10.1128/JVI.02485-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totura A.L., Baric R.S. SARS coronavirus pathogenesis: host innate immune responses and viral antagonism of interferon. Curr. Opin. Virol. 2012;2(3):264–275. doi: 10.1016/j.coviro.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente G., Ozmen L., Novelli F., Geuna M., Palestro G., Forni G., Garotta G. Distribution of interferon-gamma receptor in human tissues. Eur. J. Immunol. 1992;22(9):2403–2412. doi: 10.1002/eji.1830220933. [DOI] [PubMed] [Google Scholar]
- Vollmer J., Weeratna R., Payette P., Jurk M., Schetter C., Laucht M., Wader T., Tluk S., Liu M., Davis H.L., Krieg A.M. Characterization of three CpG oligodeoxynucleotide classes with distinct immunostimulatory activities. Eur. J. Immunol. 2004;34(1):251–262. doi: 10.1002/eji.200324032. [DOI] [PubMed] [Google Scholar]
- Wang Y., Shi H., Rigolet P., Wu N., Zhu L., Xi X.G., Vabret A., Wang X., Wang T. Nsp1 proteins of group I and SARS coronaviruses share structural and functional similarities. Infect. Genet. Evol. 2010;10(7):919–924. doi: 10.1016/j.meegid.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Lu W., Chen J., Xie S., Shi H., Hsu H., Yu W., Xu K., Bian C., Fischer W.B., Schwarz W., Feng L., Sun B. PEDV ORF3 encodes an ion channel protein and regulates virus production. FEBS Lett. 2012;586(4):384–391. doi: 10.1016/j.febslet.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Byrum B., Zhang Y. Detection and genetic characterization of deltacoronavirus in pigs, Ohio, USA, 2014. Emerg. Infect. Dis. 2014;20(7):1227–1230. doi: 10.3201/eid2007.140296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Fang L., Shi Y., Zhang H., Gao L., Peng G., Chen H., Li K., Xiao S. Porcine epidemic diarrhea virus 3C-Like protease regulates its interferon antagonism by cleaving NEMO. J. Virol. 2015;90(4):2090–2101. doi: 10.1128/JVI.02514-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Zhu S., Yang L., Cui S., Pan W., Jackson R., Zheng Y., Rongvaux A., Sun Q., Yang G., Gao S., Lin R., You F., Flavell R., Fikrig E. Nlrp6 regulates intestinal antiviral innate immunity. Science. 2015 doi: 10.1126/science.aab3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wathelet M.G., Orr M., Frieman M.B., Baric R.S. Severe acute respiratory syndrome coronavirus evades antiviral signaling: role of nsp1 and rational design of an attenuated strain. J. Virol. 2007;81(21):11620–11633. doi: 10.1128/JVI.00702-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicht O., Li W., Willems L., Meuleman T.J., Wubbolts R.W., van Kuppeveld F.J., Rottier P.J., Bosch B.J. Proteolytic activation of the porcine epidemic diarrhea coronavirus spike fusion protein by trypsin in cell culture. J. Virol. 2014;88(14):7952–7961. doi: 10.1128/JVI.00297-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo P.C., Huang Y., Lau S.K., Yuen K.Y. Coronavirus genomics and bioinformatics analysis. Viruses. 2010;2(8):1804–1820. doi: 10.3390/v2081803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo P.C., Lau S.K., Lam C.S., Lau C.C., Tsang A.K., Lau J.H., Bai R., Teng J.L., Tsang C.C., Wang M., Zheng B.J., Chan K.H., Yuen K.Y. Discovery of seven novel Mammalian and avian coronaviruses in the genus deltacoronavirus supports bat coronaviruses as the gene source of alphacoronavirus and betacoronavirus and avian coronaviruses as the gene source of gammacoronavirus and deltacoronavirus. J. Virol. 2012;86(7):3995–4008. doi: 10.1128/JVI.06540-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Sun L., Chen X., Du F., Shi H., Chen C., Chen Z.J. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science. 2013;339(6121):826–830. doi: 10.1126/science.1229963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y., Chen J., Tu J., Zhang B., Chen X., Shi H., Baker S.C., Feng L., Chen Z. The papain-like protease of porcine epidemic diarrhea virus negatively regulates type I interferon pathway by acting as a viral deubiquitinase. J. Gen. Virol. 2013;94(Pt 7):1554–1567. doi: 10.1099/vir.0.051169-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Zhang H., Zhang Q., Dong J., Liang Y., Huang Y., Liu H.J., Tong D. Porcine epidemic diarrhea virus E protein causes endoplasmic reticulum stress and up-regulates interleukin-8 expression. Virol. J. 2013;10:26. doi: 10.1186/1743-422X-10-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Zhang H., Zhang Q., Huang Y., Dong J., Liang Y., Liu H.J., Tong D. Porcine epidemic diarrhea virus N protein prolongs S-phase cell cycle, induces endoplasmic reticulum stress, and up-regulates interleukin-8 expression. Vet. Microbiol. 2013;164(3-4):212–221. doi: 10.1016/j.vetmic.2013.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X.G., Zhang H.L., Zhang Q., Dong J., Huang Y., Tong D.W. Porcine epidemic diarrhea virus M protein blocks cell cycle progression at S-phase and its subcellular localization in the porcine intestinal epithelial cells. Acta Virol. 2015;59(3):265–275. doi: 10.4149/av_2015_03_265. [DOI] [PubMed] [Google Scholar]
- Ye S., Li Z., Chen F., Li W., Guo X., Hu H., He Q. Porcine epidemic diarrhea virus ORF3 gene prolongs S-phase, facilitates formation of vesicles and promotes the proliferation of attenuated PEDV. Virus Genes. 2015;51(3):385–392. doi: 10.1007/s11262-015-1257-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L., Chen Z., Song S., Wang S., Tian C., Xing G., Chen X., Xiao Z.X., He F., Zhang L. p53 degradation by a coronavirus papain-like protease suppresses type I interferon signaling. J. Biol. Chem. 2015;290(5):3172–3182. doi: 10.1074/jbc.M114.619890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng S., Zhang H., Ding Z., Luo R., An K., Liu L., Bi J., Chen H., Xiao S., Fang L. Proteome analysis of porcine epidemic diarrhea virus (PEDV)-infected Vero cells. Proteomics. 2015;15(11):1819–1828. doi: 10.1002/pmic.201400458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Shi K., Yoo D. Suppression of type I interferon production by porcine epidemic diarrhea virus and degradation of CREB-binding protein by nsp1. Virology. 2016;489:252–268. doi: 10.1016/j.virol.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S., Gao J., Zhu L., Yang Q. Transmissible gastroenteritis virus and porcine epidemic diarrhoea virus infection induces dramatic changes in the tight junctions and microfilaments of polarized IPEC-J2 cells. Virus Res. 2014;192:34–45. doi: 10.1016/j.virusres.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziebuhr J., Schelle B., Karl N., Minskaia E., Bayer S., Siddell S.G., Gorbalenya A.E., Thiel V. Human coronavirus 229E papain-like proteases have overlapping specificities but distinct functions in viral replication. J. Virol. 2007;81(8):3922–3932. doi: 10.1128/JVI.02091-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziebuhr J. The coronavirus replicase. Curr. Top. Microbiol. Immunol. 2005;287:57–94. doi: 10.1007/3-540-26765-4_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zust R., Cervantes-Barragan L., Kuri T., Blakqori G., Weber F., Ludewig B., Thiel V. Coronavirus non-structural protein 1 is a major pathogenicity factor: implications for the rational design of coronavirus vaccines. PLoS Pathog. 2007;3(8):e109. doi: 10.1371/journal.ppat.0030109. [DOI] [PMC free article] [PubMed] [Google Scholar]