Abstract
Objective:
Major depressive disorder (MDD) is a prevalent psychiatric condition characterized by multiple symptoms that cause great distress. Uncovering the brain areas involved in MDD is essential for improving therapeutic strategies and predicting response to interventions. This systematic review discusses recent findings regarding cortical alterations in depressed patients during emotional or cognitive tasks, as measured by electroencephalography (EEG).
Methods:
A search of the MEDLINE/PubMed and Cochrane databases was carried out using the keywords EEG and depression, confined to article title.
Results:
The studies identified reveal the frontal cortex as an important brain structure involved in the complex neural processes associated with MDD. Findings point to disorganization of right-hemisphere activity and deficient cognitive processing in MDD. Depressed individuals tend to ruminate on negative information and respond with a pattern of relatively higher right frontal activity to emotional stimuli associated with withdrawal and isolation.
Conclusion:
Patients with MDD may have altered dynamic patterns of activity in several neuroanatomical structures, especially in prefrontal and limbic areas involved in affective regulation. Identification of these alterations might help predict the response of patients to different interventions more effectively and thus maximize the effects both of pharmacotherapeutic and of psychotherapeutic strategies.
Keywords: Mood disorders, unipolar, emotion, neuroanatomy, memory, cognitive neuroscience
Introduction
Major depressive disorder (MDD) is a highly prevalent psychiatric condition characterized by multiple symptoms that lead to significant distress and impair the individual’s cognitive, emotional, social, and occupational performance.1 It is usually expressed by both psychological complaints, such as sadness, anxiety, and irritability, and by somatic symptoms, such as pain and malaise. Other symptoms involve tiredness, lack of energy, difficulty concentrating, social isolation, and unpleasant thoughts.1 A dysfunctionality of multiple biological factors is thought to play a key role in the etiology and development of this severe psychiatric condition.
Unraveling the neurobiological aspects of depression is very important, since knowledge of the relationship between, for instance, the cortical systems and cognitive functions may help explain the dysfunctions that occur in MDD. This, in turn, may contribute to the delineation of more effective techniques for diagnosis, treatment, and relapse prevention.
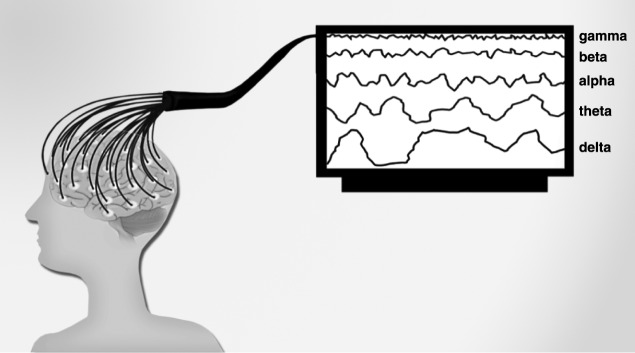
Technical limitations currently preclude direct in vivo analysis of cellular and molecular candidates underlying depressive symptoms in human subjects. However, other neurobiological aspects of depression, such as cortical activation patterns, can be investigated in the living human brain through non-invasive techniques such as electroencephalography (EEG) (Figure 1).
Figure 1. EEG is a non-invasive method invented by neuropsychiatrist Hans Berger (1873-1941) that records brain electrical activity. The first EEG studies with humans began in 1924.2 In clinical practice, EEG is useful for functional mapping of cortical areas, both in the healthy brain and in psychiatric contexts, given that it correlates psychological dysfunctions with abnormal patterns of brain functioning. The EEG method comprises a large frequency spectrum with waves of different lengths and amplitudes. From a psychophysiological standpoint, the most important parameter is the clinically relevant frequency range. EEG-identified frequencies can be broken down into the following bands or ranges: alpha, beta, gamma, theta, and delta.2 Alpha (7-14 Hz) is the frequency range which occurs during wakefulness, emerging when the eyes are closed, and in a state of relaxation. The alpha wave is attenuated by eye opening or mental activity.2 Beta (15-30 Hz) is the frequency range linked to motor behavior and is attenuated during active movements. Low-amplitude beta waves are often associated with active, busy, or anxious thinking and active concentration.3 Gamma rhythms (30-100 Hz) emerge when different populations of neurons are connected into a network to perform a certain cognitive or motor function.2 Theta waves (4-7 Hz) are normally seen in young children, but may emerge in older children and adults during drowsiness or arousal. This range is also associated with reports of relaxed, meditative, and creative states.2,4 Finally, the delta frequency range comprises the highest amplitude and the slowest waves (up to 4 Hz), and normally emerges in adults during slow-wave sleep.2 EEG = electroencephalography.
The cerebral cortex plays a key role in the complex cognitive functions of the human brain, such as memory, attention, interpretation, and perception.5 As the diagnostic criteria for MDD include cognitive deficits in the domains of memory, attention and executive function,1 a growing body of empirical research seeks to examine the nature of these deficits and investigate the brain structures implicated in the disorder.6-8
Studies using EEG observed that, at the cortical level, MDD might be related to the action of a large-scale cortical system comprising a number of functionally connected cortical regions,9 and that individuals with this disorder tend to have altered brain networks.10 The current scientific literature has validated EEG alpha as a readout for describing specific cognitive states and emotional processes, and has suggested the influence of cortical asymmetry on depression and affect.11 An extensive body of research has examined the associations between resting EEG asymmetry and predisposition for psychopathology. However, many EEG-based studies emphasize that individual responses to cognitive or emotional demands are mediated both by asymmetry and by different levels of cortical activity.11 Nevertheless, Coan et al.12 emphasized that baseline EEG assessments cannot provide specific information about the individual’s capability to react to affective challenges. However, such information can be obtained by assessing the individual’s cortical response to specific stressors during cognitive or emotional tasks, for example. From this point of view, EEG asymmetry may better elucidate behavioral tendencies when investigated in response to a relevant stimulus.11
In this paper, we reviewed studies investigating cortical alterations during emotional or cognitive tasks, as measured by EEG, in patients with MDD. Such studies are essential not only to expand knowledge on how the brain works in health and disease, but also to support the development of strategies that can restore patterns of activation in depressed patients.
Methods
We aimed to review and discuss recent findings on the cortical alterations found in depressed patients during emotional or cognitive tasks, as measured by EEG. Specifically, we focused on studies in which EEG measures were obtained in depressed patients aged 17 to 65 years, during wakefulness, and without the following comorbid conditions: psychotic symptoms, human immunodeficiency virus, or internet addiction. Specific populations, such as children and menopausal women, were also excluded, as were studies investigating the effects of specific interventions, such as transcranial magnetic stimulation. However, due to the high prevalence of comorbid depressive and anxiety disorders, studies with participants diagnosed with both conditions were included.
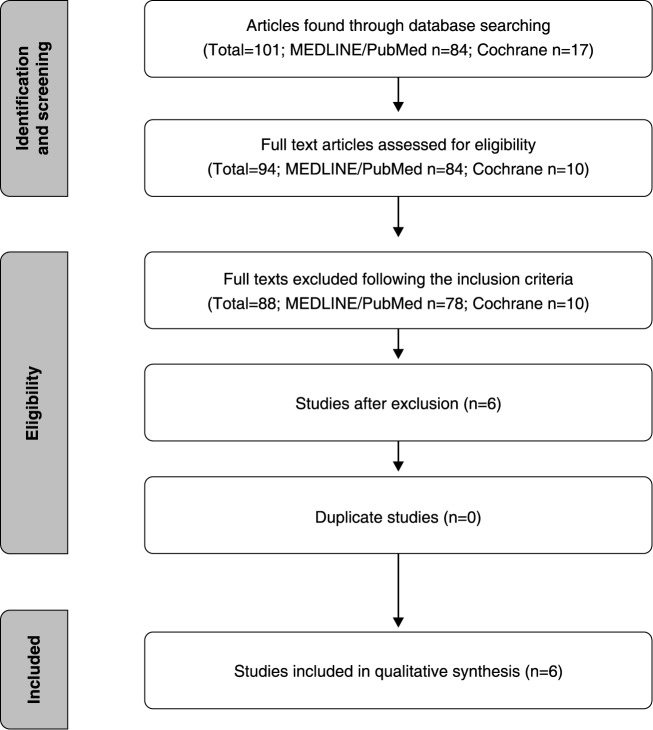
A literature search was carried out in the MEDLINE (via PubMed) and Cochrane databases to identify studies about EEG alterations during emotional or cognitive tasks in patients with depression. The keywords used were EEG and depression, confined to the article title. The search results are displayed in Figure 2. Only original papers published in English from 2005 to 2015, and meeting the eligibility criteria described above, were included. Of the 84 articles found in the PubMed database, six were included in accordance with the aforementioned criteria. None of the 17 articles found in the Cochrane Library met our inclusion criteria.
Figure 2. Flowchart of the article search process employed in this review.
Results
Specific brain networks mediate different emotions and behaviors, and changes in patterns of interaction are associated with differential cerebral activation.13 Although most of the EEG studies identified in this systematic review had small sample sizes, their results provide useful information about patterns of brain functioning during cognitive or emotional tasks in patients with depression. The main findings of our review are summarized in Table 1 and discussed in greater detail in the following sections.
Table 1. Cortical alterations in MDD during cognitive or emotional tasks, as measured by EEG.
| Reference | Subjects | M/F | Findings | Cognitive/emotional task |
|---|---|---|---|---|
| Li14 | 16 with MDD 14 HC |
6 M/10 F 4 M/10 F |
In the MDD group, abnormally increased EEG gamma coherence was found in both gamma bands, especially in the high gamma band, during emotional processing.Brain networks appeared regular during processing in MDD patients and HC. Conversely, compared with those of HCs, the networks of MDD patients tend to show a shift toward randomization.Negative bias was found in HC from the results of coherence and topological structures. Negative bias was not detected in MDD patients. | Spatial search task for facial expressions (emotional face processing) |
| Stewart15 | 143 with MDD 163 HC |
95 M/211 F | MDD individuals showed relatively less left frontal activity than never-depressed individuals when performing approach- and withdrawal-related facial expressions. | DFA task |
| Beeney16 | 13 with MDD 23 with BPD 21 HC |
All F (57) | MDD group showed greater right asymmetry, reflecting withdrawal tendencies in response to rejection. The HC group moved toward balance in terms of cortical alpha. | Cyberball task |
| Deldin & Chiu11 | 15 with MDD 18 HC |
12 F/3 M13 F/5 M | Responders showed decreased alpha power even at pre-task baseline, with no additional decrease during either unguided or guided thinking. Depressed responders (who reported greater scores on a range of scales assessing depressogenic symptoms) exhibited a baseline frontal asymmetry of greater right than left activity compared with depressed non-responders. Greater right frontal activity was found in individuals with greater depression severity. | Cognitive restructuring task based on CT techniques for depression |
| Siegle17 | 14 with MDD 15 with schizophrenia 24 HC |
6 F/8 M 7 F/8 M 12 F/12 M |
MDD individuals displayed sustained and increased gamma-band EEG throughout the task, particularly in the seconds following negative words. | Lexical emotion identification task |
| Wei18 | 16 with MDD 16 HC |
11 F/5 M 10 F/6 M |
Both groups had different WE during a recognition task with emotional faces. | Recognition task with three facial expressions: happy (positive), sad (negative), and neutral |
BPD = borderline personality disorder; CT = cognitive restructuring; DFA = directed facial action; EEG = electroencephalogram; F = female; HC = healthy controls; M = male; MDD = major depressive disorder; WE = wavelet entropy.
The altered brain network in depressive states
Li et al.14 investigated the differences in brain functional networks between patients with depression and healthy controls while they were processing emotional stimuli. In this study, EEG activities were recorded from 16 patients with depression and 14 healthy controls when they performed a spatial search task for facial expressions. Global EEG coherence, which is an efficient method to calculate the linear-dependent interaction of EEG signals between two channels or brain regions, was significantly higher in patients with depression than in healthy controls in both gamma bands, especially in the high gamma band. Results suggested that, although the brain networks of patients with depression and healthy controls had regular networks during emotional processing, the brain networks of the depressed group tended toward randomization. In the depressed group, activated edges were presented in the frontal regions but, according to other studies, this finding suggests that the frontal cortex is not alone in the complex neuronal processes associated with the depressive state; the superior occipital cortex also seems to be associated with cognitive processes involved in depression.14
To support their findings, Li et al.14 discuss the location of the amygdala and hippocampus within two specific regions: the parietal region and the right occipitotemporal cortex. The amygdala is one of the most relevant brain regions mediating stress-related emotions, but it is also associated with cognitive processes, such as attention, associative learning, and working memory.19 The hippocampus is also thought to be related to emotional regulation, and some studies have shown that hippocampal volume is significantly decreased in patients with depression as compared with healthy controls, and that the number of hippocampal neurons in these patients declines.20 In addition, the right hemisphere seems to be able to produce a larger network of associations than the left hemisphere,21 and the physiological overactivation of the right hemisphere in patients with MDD compared to healthy controls likely reflects an unsuccessful effort to overcome its functional insufficiency in depression.22 These findings point to a shift toward randomization in the brain networks of patients with depression and provide evidence that MDD could be a disorder of disrupted neuronal network organization and deficient cognitive processing.14
Gamma-band (35-45 Hz) EEG is associated with semantic functions such as feature binding and elaborative processing.17 Siegle et al.17 examined whether differences in baseline and sustained gamma-band EEG occurred following emotional stimuli in healthy adults, adults with depression, and adults with schizophrenia. In this study, 24 healthy controls, 14 patients with MDD, and 15 patients with schizophrenia completed a lexical emotion identification task while under EEG assessment. The results suggested that gamma-band EEG may serve as a useful index of sustained semantic information processing when measured over several seconds. In addition, the correlation of sustained gamma-band activity seconds after a stimulus with self-reported rumination reveals sustained elaborative processing. Depressed individuals in the study displayed sustained and increased gamma-band EEG throughout the task, particularly in the seconds following negative words.17
The finding of increased sustained gamma-band responses to negative stimuli, apparently only in depressed individuals,17 supports the argument that depressed individuals may elaborate or ruminate specifically on negative information. This finding is consistent with other studies that have revealed sustained and increased physiological reactivity to negative information23 and sustained amygdala activity24 during valence identification tasks. The frontal distribution of increased sustained gamma in depressed participants was consistent with sustained working memory processes that occur in semantic elaboration. According to the authors, these results suggest that increased and sustained elaborative processing of emotional information in depressed patients can be considered a hallmark of this disorder.17
Considering that depression is characterized as a mental disease with typical deficits in emotional processing, Wei et al.18 investigated how emotions can influence cognition. For that purpose, the authors used wavelet entropy (WE) as a tool to analyze event-related EEG during a cognitive task. According to the authors, WE can measure the frequency stabilization of the different EEG oscillations and distinguishes disordered signals (higher entropy) from ordered ones. Sixteen subjects with MDD and 16 healthy controls performed a recognition task with three types of facial expressions – happy (positive), sad (negative), and neutral – to assess the influence of emotions on working memory. The results suggested that the patients with depression had significantly higher entropies in overall brain regions than normal controls. The emotion effect was found in the right anterior and right center of the brain by WE analysis, and the authors concluded that patients with depression had much higher emotion-induced responses than normal individuals about 300 ms after the stimulus onset. Moreover, patients mobilized more neurons to perform a cognitive task than did healthy participants. The higher entropy observed in the EEG of depressive patients was found in the prefrontal region, especially in the right hemisphere, during processing of negative stimuli.18
Many studies have suggested that the prefrontal cortex is an important component of a circuit critical to emotional regulation. In line with this suggestion, Wei et al. mentioned studies that confirmed that depressive patients exhibit abnormal brain activity in the prefrontal cortex12 and that the right prefrontal cortex is associated with withdrawal and depressive states.25 In short, the findings of Wei et al. provide important additional evidence for right-hemisphere disorganization in MDD.18
Cortical activation of the approach and withdrawal systems
Stewart et al.15 examined the relationship between asymmetries in frontal brain activity and depression in the resting state and during an emotional challenge task involving approach and withdrawal-related facial expressions, to test the capability model of individual differences in frontal EEG asymmetry. This model predicted that individual differences in EEG asymmetry (associated with depressive status) would be stronger during emotional challenge than during a baseline resting session. In pursuit of this goal, EEG data were recorded during resting baseline and a facial emotion task, wherein 143 subjects with MDD and 163 healthy controls performed approach (angry and happy) and withdrawal (afraid and sad) facial expressions. Results supported the capability model and showed that depressed individuals had relatively less left frontal activity than never-depressed individuals during approach- and withdrawal-related facial expressions.15
To support these results, the authors discussed other studies that reported differences in left frontal activity between depressive patients and healthy controls. In this context, individual differences in frontal brain asymmetry may influence an individual’s vulnerability to developing depression, considering that these differences can be a diathesis that biases affective style or a tendency to engage in aspects of these motivational systems.23 According to Davidson,26 these individual differences are associated with a dispositional affective style, which refers to a predisposition that individuals may have to respond emotionally to contexts according to an approach system (which is associated to a relatively higher left than right frontal activity) or a withdrawal system (which is associated to a relatively higher right than left frontal activity).26,27 According to this model, depressed individuals tend to respond with a pattern of relatively higher right than left frontal activity, indicating withdrawal emotions, reduced approach motivation, and decreased sensitivity to reward.28,29
Beeney et al.16 conducted a study comparing the response of patients with MDD and patients with borderline personality disorder (BPD) to an emotional task. Briefly, 13 subjects with MDD, 23 subjects with BPD, and 21 healthy controls were engaged in a social rejection challenge. In line with the findings of Stewart et al.,15 these results also suggested that individuals with MDD were more likely to respond to social stress with withdrawal and isolation. In contrast, patients with BPD tended to become angry in response to rejection, a state supported by approach motivation. Although no differences were found at baseline, results demonstrated that, following rejection, individuals with MDD exhibited greater right cortical activation, consistent with withdrawal motivation.16
According to Coan & Allen,30 the study of motivated behavior and its association with frontal EEG alpha asymmetry supports greater left frontal asymmetry as a marker of approach motivation and greater right frontal asymmetry as a marker of withdrawal motivation. In addition, a number of studies about these motivational tendencies and their relationship with psychopathology have found associations between greater withdrawal motivation and greater right frontal asymmetry in individuals with MDD.30 As reported by Beeney et al.,16 these results could help provide better therapeutic strategies in order to reduce this style of reactivity – such as clinical strategies that may involve promoting approach behaviors following rejection in patients with MDD. This recommendation is in agreement with the literature, which states that behavioral activation is a useful treatment for depression31 and that facilitating approach behavior provides the possibility of expressing feelings and developing new interpersonal skills that are not possible with withdrawal and isolation.16
Cortical activity and cognitive restructuring
According to Deldin & Chiu,11 MDD seems to be the result of an intricate interaction between psychosocial and biological variables, so it is reasonable to suggest that therapeutic response is associated with cortical physiology. To confirm this relationship, the authors conducted a study that combined EEG techniques and cognitive restructuring to examine whether baseline patterns and levels of cortical activity may be useful indices of mood response to a cognitive therapy analog. Cognitive restructuring is a technique that includes identification of maladaptive thinking associated with distressing moods combined with questioning and challenging the validity of those beliefs in order to modify the maladaptive thoughts.32
Thirty subjects (15 with MDD and 15 healthy controls) participated in four EEG recording blocks: two blocks were resting baselines and the other two blocks occurred during the cognitive restructuring task. After the experiment, the response of participants on a specific scale developed by the authors (the Happiness Scale, which refers to a visual analog scale of happiness ranging from “the happiest I have ever been” to “not happy at all”) was recorded and used to divide participants into two groups: Responders (individuals who exhibited positive values of happiness response after the cognitive task) or Non-responders (individuals who reported no change or negative values of happiness after the cognitive task).
The results suggested that subjects who reported greater post- than pre-intervention happiness (i.e., Responders) exhibited greater overall cortical activity than non-responders, even at pre-task baseline, indicating the presence and predictive utility of overall greater cortical activity for identifying those who reported greater improvement after a cognitive intervention. Since cognitive therapy strategies can be considered tasks that involve high cognitive demand, an individual’s levels and patterns of cortical activity are likely to moderate the therapeutic response, as the authors showed.11 These findings raise relevant questions about cortical alterations that may occur after long-term therapy. Studies about this relationship aiming to delineate more effective therapeutic practices may therefore be warranted.11
In addition, and in agreement with other studies,15,16,18 Deldin & Chiu11 found that depressed responders – those who reported greater post- than pre-intervention happiness – were the participants who exhibited the most severe depression symptoms before the cognitive task, and that these same participants exhibited a baseline frontal asymmetry of greater right than left activity compared with depressed non-responders. This result is consistent with evidence that an asymmetry toward greater right relative to left activity in frontal areas is associated with greater negative affect.26 However, according to the authors’ discussion, the observed mood reactivity in the sample seems inconsistent with the literature, which suggests that individuals with such patterns of frontal asymmetry are predisposed to respond to negative stimuli with greater negative affect.26,33
These results suggest not only that patients with depression do not necessarily respond to negative stimuli with negative emotions, but also that the cognitive task can be processed not as a negative stimulus, as long as it provides cognitive restructuring that could improve positive affect. To investigate this assumption, other studies focusing on how depressive patients respond to positive stimuli, and which brain areas are involved in this processing, should be conducted. Such studies would enable evaluation of differential processing of positive emotions between depressed patients and healthy controls, and allow assessment of whether patterns of brain functioning differ after a positive psychotherapy, for example, during baseline, and during emotional or cognitive tasks.
Discussion
Depression is a mood disorder that causes great distress and impairs cognitive, social, and occupational performance. Due to its high prevalence, it is also one of the most relevant public health problems worldwide. Uncovering the neurobiological aspects of depression is essential not only to expand knowledge on how the brain works in health and disease, but also to support the development of strategies that can restore patterns of activation in depressed patients.
However, technical limitations mean that such biological alterations cannot yet be studied directly and in vivo in human subjects. Nevertheless, other neurobiological aspects of depression, such as cortical activation patterns, can be investigated in the living human brain through non-invasive techniques, such as EEG. Considering that maladaptive emotional and cognitive processing is a common psychological feature underlying depressive symptoms,34-36 this systematic review aimed to discuss recent findings on the cortical alterations found in depressed patients during emotional or cognitive tasks, as measured by EEG.
Overall, the reviewed studies suggest that the frontal cortex might be one of the most important brain structures involved in the complex neural processes associated with depressive states. Research findings point to right-hemisphere disorganization and deficient cognitive processing as features of in MDD.14,17,18 In addition, results suggest that depressed individuals tend to ruminate specifically on negative information and respond with a pattern of relatively higher right frontal activity to emotional stimuli associated with withdrawal and isolation motivation.11,15-17
The studies included in this review have some limitations that should be addressed in future research. Only one study was conducted on a large sample.15 Sample sizes of future investigations need to be substantially larger. In addition, most studies (except Stewart et al.15) failed to control for either comorbidities, medication, or both. Criteria to include participants in the depression group must also be defined more clearly in the studies, with additional information about the severity of depression in the patient group and clear definition of whether patients are experiencing a first episode, relapse, or recurrence.
Although further studies are needed to evaluate the effectiveness of different interventions for MDD (for a review and meta-analysis on the effectiveness of psychotherapy for depression, see Linde et al.37), available data allow us to hypothesize that some psychological interventions have potential efficacy for the treatment of MDD. Among these, we can highlight the well-documented cognitive-behavioral therapy (CBT)38,39 and positive psychotherapy.40,41
CBT is a widely researched form of psychotherapy. The proposition of CBT is that symptoms and dysfunctional behaviors are cognitively mediated. One of the most important techniques in CBT is cognitive restructuring, which includes the identification of maladaptive thinking that accompanies and precedes the experience of distressing moods and questioning/challenging the validity of those beliefs in order to modify the maladaptive thoughts.32
As previously described herein, the findings by Deldin et al.11 suggested that subjects who reported greater post-intervention results after a cognitive restructuring task revealed greater overall cortical activity than non-responders, raising relevant questions about cortical alterations that may occur after long-term therapy. Another recent study, conducted by Zaunmüller et al.,42 investigated the impact of a 90-minute psychotherapeutic microintervention on affect and electrocortical correlates of cognitive restructuring. Although both the intervention group and the control group reported increases in positive and decreases in negative affect from pre- to post-intervention, the results indicated that even such a time-limited cognitive intervention could have specific and reliably detectable effects on cortical measures. Dysphoric participants who received the restructuring microintervention seemed to reflect an intervention-specific strengthening and intensified utilization of prefrontal regulatory brain mechanisms that could serve to attenuate emotional arousal.42
Based on these data, and in agreement with Zaunmüller et al.,42 future studies investigating the repetitive practice of cognitive restructuring by using multiple training sessions and additional homework between them could help identify aspects of the brain that would be altered by longer psychotherapeutic interventions. Unraveling the mechanisms of change that mediate the complex processes of psychotherapeutic interventions is essential to enhancing the overall efficacy of treatment and to developing more tailored interventions, focused on the specific needs and neural profiles of MDD patients.
The science of positive psychology refers to the study of positive emotion, positive character, and positive institutions.43 Positive interventions intend to supplement traditional psychology by focusing on the investigation of well-being and of what makes life most worth living.44 According to Seligman et al.,32 the practice of psychology should include an understanding of the interaction of suffering and happiness and validated interventions that both relieve suffering and increase happiness.
Regarding depressive disorder, positive psychotherapy rests on the hypothesis that it can be treated effectively not only by reducing negative symptoms but also by building positive emotions, character strengths, and meaning. The direct building of positive resources may successfully reduce negative symptoms and buffer against their future reoccurrence.45 If patients with depression exhibit increased activation of brain areas responsible for negative emotions and withdrawal (especially the right prefrontal cortex), it would be relevant to assess whether positive psychotherapies (as well as other positive-based interventions, such as cognitive-behavioral solution-focused coaching [CB-SFC]) are able to change this pattern of brain functioning.
Still in the context of positive emotions, some studies suggest that positive mood might influence specific cognitive processes. According to Fredrickson & Kurtz,46 positive emotions minimize the detrimental effects of negative emotion on physiology, attention, and creativity, and can be linked to broadened scopes of attention, cognition, and action, as well as to enhanced physical, cognitive, and social resources. Results of a study with healthy individuals suggested that positive affects related to determination and satisfaction were associated with greater left frontal activity relative to baseline EEG recordings, and that these positive affects were also related to persistence on insolvable tasks presented to participants.47 Based on these findings, novel studies in the same line of that by Beeney et al.,16 for example, which investigated the reaction of MDD patients following social rejection, could be especially relevant to investigate possible improvements in social resources after positive therapy/CB-SFC. Other tasks, such as the Alternative Uses Task (AUT),48 could be interesting tools to evaluate whether creativity – an important cognitive function – improves after interventions that elicit positive emotions. The AUT is a creativity task where the individual gives as many uses as possible to an everyday object (such as a brick or pencil) within a certain period of time. Previous studies have shown that, compared to neutral or negative mood, positive mood could have beneficial effects on cognitive flexibility and, in turn, on learning performance.49
As previously suggested, another intervention aiming to enhance cognitive resources and positive emotions could be CB-SFC, an integrated coaching approach that works to help individuals perceive and cope with challenging stimuli by both identifying and putting into practice key personal strengths and restructuring unhelpful thinking.50 However, no studies have been conducted to establish neural correlates of coaching efficacy.51 Studies correlating EEG and CB-SFC could provide a better understanding of how the coaching process can change perceptions and behavior by changing the brain, as well as contribute to the development and optimization of coaching techniques that could modulate certain identified brain areas.51 Ascertaining which brain areas could be systematically activated by psychological interventions such as CB-SFC would be an important step toward enhanced, evidence-based psychological practice in the field of mental health.
In summary, the aim of the present review was to discuss some key recent findings regarding cortical alterations in patients with MDD, as measured by EEG performed during emotional or cognitive tasks. MDD is a highly prevalent and incapacitating illness characterized by a number of neurobiological substrates and downregulation of neural plasticity parameters, involving structural, functional, and molecular alterations in several areas of the brain. In some animal studies, depressive phenotypes have been associated with a decreased ability of the hippocampus to generate newly functional neurons in postnatal life, among other factors. In humans, patients with MDD exhibit alterations in dynamic patterns of activity in neuroanatomical structures, especially in the prefrontal and limbic areas of the brain that are involved in affective regulation. These structures have significant implications for the pathogenesis of MDD. In this sense, it would be reasonable to believe that interventions capable of inducing neural plasticity could be effective for ameliorating the symptoms of MDD. Increasing our understanding of the neurobiological underpinnings of depression might help predict patient response to different interventions, thus maximizing the effects of pharmacotherapeutic and psychotherapeutic strategies alike.
Disclosure
The authors report no conflicts of interest.
Acknowledgements
The authors would like to thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) for the financial support provided.
References
- 1.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). Arlington: American Psychiatric Publishing; 2013. [Google Scholar]
- 2.Niedermeyer E, da Silva FL. Electroencephalography: basic principles, clinical applications, and related fields. Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 3.Pfurtscheller G, Lopes da Silva FH. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol. 1999;110:1842–57. doi: 10.1016/s1388-2457(99)00141-8. [DOI] [PubMed] [Google Scholar]
- 4.Cahn BR, Polich J. Meditation states and traits: EEG, ERP, and neuroimaging studies. Psychol Bull. 2006;132:180–211. doi: 10.1037/0033-2909.132.2.180. [DOI] [PubMed] [Google Scholar]
- 5.Kandel ER, Schwartz JH, Jessell TM. Principles of neural science. New York: McGraw-Hill; 2000. [Google Scholar]
- 6.Kircanski K, Joormann J, Gotlib IH. Cognitive aspects of depression. Wiley Interdiscip Rev Cogn Sci. 2012;3:301–13. doi: 10.1002/wcs.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamilton JP, Furman DJ, Gotlib IH. The neural foundations of major depression: classical approaches and new frontiers. In: Lopez-Munoz FF, Alamo C, editors. Neurobiology of depression. Florida: CRC; 2012. pp. 57–74. [Google Scholar]
- 8.Ottowitz WE, Dougherty DD, Savage CR. The neural network basis for abnormalities of attention and executive function in major depressive disorder: implications for application of the medical disease model to psychiatric disorders. Harv Rev Psychiatry. 2002;10:86–99. doi: 10.1080/10673220216210. [DOI] [PubMed] [Google Scholar]
- 9.Wang L, Hermens DF, Hickie IB, Lagopoulos J. A systematic review of resting-state functional-MRI studies in major depression. J Affect Disord. 2012;142:6–12. doi: 10.1016/j.jad.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 10.Berman MG, Peltier S, Nee DE, Kross E, Deldin PJ, Jonides J. Depression, rumination and the default network. Soc Cogn Affect Neurosci. 2011;6:548–55. doi: 10.1093/scan/nsq080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deldin PJ, Chiu P. Cognitive restructuring and EEG in major depression. Biol Psychol. 2005;70:141–51. doi: 10.1016/j.biopsycho.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 12.Coan JA, Allen JJ, McKnight PE. A capability model of individual differences in frontal EEG asymmetry. Biol Psychol. 2006;72:198–207. doi: 10.1016/j.biopsycho.2005.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galvao-de Almeida A, Araujo GM, Filho, Berberian Ade A, Trezsniak C, Nery-Fernandes F, Araujo CA, Neto, et al. The impacts of cognitive-behavioral therapy on the treatment of phobic disorders measured by functional neuroimaging techniques: a systematic review. Rev Bras Psiquiatr. 2013;35:279–83. doi: 10.1590/1516-4446-2012-0922. [DOI] [PubMed] [Google Scholar]
- 14.Li Y, Cao D, Wei L, Tang Y, Wang J. Abnormal functional connectivity of EEG gamma band in patients with depression during emotional face processing. Clin Neurophysiol. 2015;126:2078–89. doi: 10.1016/j.clinph.2014.12.026. [DOI] [PubMed] [Google Scholar]
- 15.Stewart JL, Coan JA, Towers DN, Allen JJ. Resting and task-elicited prefrontal EEG alpha asymmetry in depression: support for the capability model. Psychophysiology. 2014;51:446–55. doi: 10.1111/psyp.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beeney JE, Levy KN, Gatzke-Kopp LM, Hallquist MN. EEG asymmetry in borderline personality disorder and depression following rejection. Personal Disord. 2014;5:178–85. doi: 10.1037/per0000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siegle GJ, Condray R, Thase ME, Keshavan M, Steinhauer SR. Sustained gamma-band EEG following negative words in depression and schizophrenia. Int J Psychophysiol. 2010;75:107–18. doi: 10.1016/j.ijpsycho.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei L, Li Y, Ye J, Yang X, Wang J. Emotion-induced higher wavelet entropy in the EEG with depression during a cognitive task. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:5018–21. doi: 10.1109/IEMBS.2009.5334603. [DOI] [PubMed] [Google Scholar]
- 19.Salzman CD, Fusi S. Emotion, cognition, and mental state representation in amygdala and prefrontal cortex. Annu Rev Neurosci. 2010;33:173–202. doi: 10.1146/annurev.neuro.051508.135256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee AL, Ogle WO, Sapolsky RM. Stress and depression: possible links to neuron death in the hippocampus. Bipolar Disord. 2002;4:117–28. doi: 10.1034/j.1399-5618.2002.01144.x. [DOI] [PubMed] [Google Scholar]
- 21.Beeman M, Friedman RB, Grafman J, Perez E, Diamond S, Lindsay MB. Summation priming and coarse semantic coding in the right hemisphere. J Cogn Neurosci. 1994;6:26–45. doi: 10.1162/jocn.1994.6.1.26. [DOI] [PubMed] [Google Scholar]
- 22.Rotenberg VS. The peculiarity of the right-hemisphere function in depression: solving the paradoxes. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:1–13. doi: 10.1016/S0278-5846(03)00163-5. [DOI] [PubMed] [Google Scholar]
- 23.Siegle GJ, Steinhauer SR, Carter CS, Ramel W, Thase ME. Do the seconds turn into hours? Relationships between sustained pupil dilation in response to emotional information and self-reported rumination. Cognitive Ther Res. 2003;27:365–82. [Google Scholar]
- 24.Siegle GJ, Hasselmo ME. Using connectionist models to guide assessment of psychological disorder. Psychol Assess. 2002;14:263–78. [PubMed] [Google Scholar]
- 25.Davidson RJ, Putnam KM, Larson CL. Dysfunction in the neural circuitry of emotion regulation--a possible prelude to violence. Science. 2000;289:591–4. doi: 10.1126/science.289.5479.591. [DOI] [PubMed] [Google Scholar]
- 26.Davidson RJ. Affective style and affective disorders: perspectives from affective neuroscience. Cogn Emot. 1998;12:307–30. [Google Scholar]
- 27.Davidson RJ. Emotion and affective style: hemispheric substrates. Psychol Sci. 1992;3:39–43. [Google Scholar]
- 28.Davidson RJ, Pizzagalli D, Nitschke JB, Putnam K. Depression: perspectives from affective neuroscience. Annu Rev Psychol. 2002;53:545–74. doi: 10.1146/annurev.psych.53.100901.135148. [DOI] [PubMed] [Google Scholar]
- 29.Diego MA, Field T, Hernandez-Reif M. BIS/BAS scores are correlated wi th frontal eeg asymmetry in intrusive and withdrawn depressed mothers. Inf Mental Health J. 2001;22:665–75. [Google Scholar]
- 30.Coan JA, Allen JJ. Frontal EEG asymmetry as a moderator and mediator of emotion. Biol Psychol. 2004;67:7–49. doi: 10.1016/j.biopsycho.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 31.Lejuez CW, Hopko DR, Hopko SD. A brief behavioral activation treatment for depression. Treatment manual. Behav Modif. 2001;25:255–86. doi: 10.1177/0145445501252005. [DOI] [PubMed] [Google Scholar]
- 32.Seligman ME, Steen TA, Park N, Peterson C. Positive psychology progress: empirical validation of interventions. Am Psychol. 2005;60:410–21. doi: 10.1037/0003-066X.60.5.410. [DOI] [PubMed] [Google Scholar]
- 33.Davidson RJ. Cerebral asymmetry, emotion and affective style. In: Davidson RJ, Hugdahl K, editors. Brain asymmetry. Cambridge: MIT Press; 1995. pp. 361–87. [Google Scholar]
- 34.Shi H, Wang X, Yi J, Zhu X, Zhang X, Yang J, et al. Default mode network alterations during implicit emotional faces processing in first-episode, treatment-naive major depression patients. Front Psychol. 2015;6:1198. doi: 10.3389/fpsyg.2015.01198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roca M, Vives M, Lopez-Navarro E, Garcia-Campayo J, Gili M. Cognitive impairments and depression: a critical review. Actas Esp Psiquiatr. 2015;43:187–93. [PubMed] [Google Scholar]
- 36.Joormann J. Cognitive aspects of depression. In: Gotlib LH, Hammen CL., editors. Handbook of depression. 2nd ed. New York: Guilford Press; pp. 298–321. [Google Scholar]
- 37.Linde K, Rucker G, Sigterman K, Jamil S, Meissner K, Schneider A, et al. Comparative effectiveness of psychological treatments for depressive disorders in primary care: network meta-analysis. BMC Fam Pract. 2015;16:103. doi: 10.1186/s12875-015-0314-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fernie BA, Kollmann J, Brown RG. Cognitive behavioural interventions for depression in chronic neurological conditions: a systematic review. J Psychosom Res. 2015;78:411–9. doi: 10.1016/j.jpsychores.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 39.Foroushani PS, Schneider J, Assareh N. Meta-review of the effectiveness of computerised CBT in treating depression. BMC Psychiatry. 2011;11:131. doi: 10.1186/1471-244X-11-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lau MA. New developments in psychosocial interventions for adults with unipolar depression. Curr Opin Psychiatry. 2008;21:30–6. doi: 10.1097/YCO.0b013e3282f1ae53. [DOI] [PubMed] [Google Scholar]
- 41.Schrank B, Brownell T, Tylee A, Slade M. Positive psychology: an approach to supporting recovery in mental illness. East Asian Arch Psychiatry. 2014;24:95–103. [PubMed] [Google Scholar]
- 42.Zaunmuller L, Lutz W, Strauman TJ. Affective impact and electrocortical correlates of a psychotherapeutic microintervention: an ERP study of cognitive restructuring. Psychother Res. 2014;24:550–64. doi: 10.1080/10503307.2013.847986. [DOI] [PubMed] [Google Scholar]
- 43.Seligman ME, Csikszentmihalyi M. Positive psychology. An introduction. Am Psychol. 2000;55:5–14. doi: 10.1037//0003-066x.55.1.5. [DOI] [PubMed] [Google Scholar]
- 44.Peterson C, Park N. Positive psychology as the evenhanded positive psychologist views it. Psychol Inq. 2003;14:143–7. [Google Scholar]
- 45.Seligman ME, Rashid T, Parks AC. Positive psychotherapy. Am Psychol. 2006;61:774–88. doi: 10.1037/0003-066X.61.8.774. [DOI] [PubMed] [Google Scholar]
- 46.Fredrickson BL, Kurtz LE. Cultivating positive emotions to enhance human flourishing. In: Donaldson SI, Csikszentmihalyi M, Nakamura J, editors. Applied positive psychology. New York: Psychology Press; 2011. pp. 33–45. [Google Scholar]
- 47.Price TF, Hortensius R, Harmon-Jones E. Neural and behavioral associations of manipulated determination facial expressions. Biol Psychol. 2013;94:221–7. doi: 10.1016/j.biopsycho.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 48.Guilford JP. The nature of human intelligence. New York: McGraw-Hill; 1967. [Google Scholar]
- 49.Nadler RT, Rabi R, Minda JP. Better mood and better performance. Learning rule-described categories is enhanced by positive mood. Psychol Sci. 2010;21:1770–6. doi: 10.1177/0956797610387441. [DOI] [PubMed] [Google Scholar]
- 50.Palmer S, Grant A, O’Connell B. Solution-focused coaching: lost and found. Coach Work. 2007;2:22–9. [Google Scholar]
- 51.Dias GP, Plamer S, O'Riordan S, Freitas SB, Habib LR, Bevilaqua MCN, et al. Perspectives and challenges for the study of brain responses to coaching: enhancing the dialogue between the fields of neuroscience and coaching psychology. Coach Psychol. 2015;11:11–9. [Google Scholar]