Abstract
Objective:
Bipolar disorder (BD) has been associated with increased rates of age-related diseases, such as type II diabetes, metabolic syndrome, osteoporosis, and cardiovascular disorders. Several biological findings have been associated with age-related disorders, including increased oxidative stress, inflammation, and telomere shortening. The objective of this study was to compare telomere length among participants with BD at early and late stages and age- and gender-matched healthy controls.
Methods:
Twenty-six euthymic subjects with BD and 34 healthy controls were recruited. Genomic DNA was extracted from peripheral blood and mean telomere length was measured using real-time quantitative polymerase chain reaction.
Results:
Telomere length was significantly shorter in both the early and late subgroups of BD subjects when compared to the respective controls (p = 0.002 and p = 0.005, respectively). The sample size prevented additional subgroup analyses, including potential effects of medication, smoking status, and lifestyle.
Conclusion:
This study is concordant with previous evidence of telomere shortening in BD, in both early and late stages of the disorder, and supports the notion of accelerated aging in BD.
Keywords: Bipolar disorder; telomeres; telomere shortening; senescence; genetics; oxidative stress; inflammation; mania, depression; aging
Introduction
Bipolar disorder (BD) is a chronic, severe, and disabling disorder.1,2 It is also associated with increased risk for multiple general medical conditions3 and higher mortality rates.4 Patients with BD experience higher rates of illnesses associated with aging, such as type II diabetes, metabolic syndrome, cancer, immune dysregulation, dementia, osteoporosis, and cardiovascular and cerebrovascular illness.5 Several explanations for these age-related comorbidities in BD have been proposed, such as increased oxidative stress, a chronic inflammatory process, and stress-related dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis, culminating in the allostatic load (AL).2,6-9 Furthermore, unhealthy lifestyles or environmental factors (e.g., smoking, unhealthy diet, physical inactivity, psychological or physical stress) - which are frequent among bipolar patients - and adverse events of pharmacotherapy may contribute to the additional medical morbidity associated with psychiatric disorders.5
Telomeres are highly specialized DNA-protein structures located at the end of linear chromosomes in eukaryotic organisms. They preserve genetic information by mitigating non-homologous recombination, end-to-end fusion, and nucleolytic degradation.10 Telomere shortening is a natural physiologic process that occurs with each round of somatic cell division11 and is progressive with aging.12 However, under conditions of chronic stress, telomere shortening can be prematurely induced or accelerated.13 In this regard, an unhealthy lifestyle or environmental factors can have a negative effect on telomere maintenance, inducing a premature aging phenotype that can lead to cell death or eventual organ failure.
Studies investigating a connection between telomere shortening and psychiatric disorders have shown inconsistent findings. For instance, Simon et al.14 showed that patients with severe psychiatric disorders (major depression, BD, and anxiety) had shorter telomeres than healthy controls. Likewise, patients with bipolar depression presented shorter telomeres.15 These findings have not been replicated in other studies that showed no changes in telomere length (measured as the T/S ratio) in subjects with BD or schizophrenia.16 Hoen et al.17 found that anxiety disorders were strongly associated with telomere shortening after 2 years of follow-up, while no prospective correlation was observed in patients with major depression. More recently, Garcia-Rizo et al.18 reported significantly decreased telomere length in newly diagnosed, antidepressant-naïve patients with depression compared to controls. Recent evidence from a BD cohort further demonstrated that patients on long-term lithium therapy had longer telomeres (about 10%) than those not responding well to lithium or healthy controls.19 A recent study reported immunosenescent cells and shortened telomeres in female patients with BD.20
A 2015 multicenter study and meta-analysis addressing whether BD can be considered an accelerated aging (AA) disease included seven cohorts with 1,115 patients.21 Although the authors did not find shorter telomeres in individuals with BD, they detected high heterogeneity among the methodologies employed, which could explain the unexpected similarities. To date, no studies have addressed telomere shortening in early and late stages of BD patients. Given that the issue of telomere length among BD patients is still under debate, the present study aimed to investigate the association of BD with mean telomere length in a sample of euthymic bipolar patients in the early and late stages of the disorder and thus provide additional knowledge on the impacts of early-stage BD on health and cellular aging.
Methods
Subjects
Outpatients were recruited from the Bipolar Disorder Program at Hospital de Clínicas de Porto Alegre, Universidade Federal do Rio Grande do Sul, Porto Alegre, southern Brazil. Inclusion criteria were: 1) age > 18 years; 2) meeting DSM-IV criteria for BD type I; and 3) meeting remission criteria, defined as a score < 7 on both the 17-item Hamilton Depression Rating Scale (17-HAM-D)22 and the Young Mania Rating Scale (YMRS)23 for at least 1 month before assessment. All patients received pharmacotherapy in accordance with predetermined protocols. Patients were classified as being in the early or late stage of BD following the Kapczinski et al. classification.24
Healthy volunteers (n=34) were divided into two age- and sex-matched groups to control for age differences in the early- and late-stage experimental groups (early and late groups, age [range] = 32 [25-57] and 36 [28-64] years, respectively). All volunteers were recruited at HCPA. They had no current or previous history and no first-degree family history of major psychiatric disorders, including dementia or mental retardation, as assessed by the non-patient version of the Structured Clinical Interview for DSM-IV (SCID).
This study was approved by the HCPA Ethics Committee. Participants were informed of the goals and procedures of the study and were only included after signing an informed consent form. The investigation was carried out in accordance with the latest version of the Declaration of Helsinki.
Assessments
The SCID for DSM-IV Axis I and Axis II Disorders (SCID-I and SCID-II) assessments were administered to confirm diagnosis. Sociodemographic and clinical data were collected by administering a structured interview and examining patients’ clinical records. Raters experienced in the assessment of depressive and manic symptoms administered the 17-HAM-D and YMRS questionnaires, as well as the Functioning Assessment Short Test (FAST) to assess functioning.25
Measurement of relative T/S
Laboratory staff were blinded to all clinical information. Whole peripheral venous blood was used for genomic DNA (gDNA) extraction with a commercial kit (Illustra blood genomicPrep Mini Spin Kit, GE Healthcare, Little Chalfont, England) following manufacturer instructions. Nucleic acid quantification and purity were checked spectrophotometrically (BioPhotometer plus; Eppendorf, Hamburg, Germany) and samples were stored at -20 °C for subsequent analysis. gDNA (25 ng/reaction) was used as template for quantification of relative mean T/S ratio by real-time quantitative polymerase chain reaction (qPCR), with minor modifications from a previously reported protocol.26 In summary, for each sample, two separate qPCR runs were performed in triplicate in separate 96-well plates in the same position. One reaction amplified the telomere (T) repeat sequence, while the other amplified a single copy gene, 36B4 (S), which served as a quantitative control. For each participant, relative telomere length was expressed as the T/S ratio. Previously published primer sequences26 were (5′→3′): tel 1, GGTTTTTGAGGGTGAGGGTGAGGGTGAGGGTGAGGGT; tel 2, TCCCGACTATCCCTATCCCTATCCCTATCCCTATCCCTA; 36B4u, CAGCAAGTGGGAAGGTGTAATCC; and 36B4d, CCCATTCTATCATCAACGGGTACAA. T and S master mix reactions were identical in a final volume of 20 µL with 0.1 x SYBR® Green (Molecular Probes, CA, USA), 2 mM MgCl2, and 0.1 mM each dNTP, 1% DMSO, and 0.5 U of Platinum® Taq DNA Polymerase (Invitrogen, Carlsbad, USA). Final primer concentrations for telomere amplification were 270 and 1,125 nM for telomere primers, respectively, and 300 and 500 nM for 36B4u and 36B4d primers. PCR reactions were performed in a StepOnePlus™ Real-Time PCR System (Applied Biosystems, Carlsbad, USA) and analyzed in StepOne™ Software v.2.3. (Applied Biosystems). The thermal cycling profile for amplification consisted of an initial incubation step for 2 min at 94 °C to activate hot-start Platinum Taq DNA polymerase, followed by 22 cycles of denaturing at 94 °C for 15 s, and annealing and extension for 2 min at 54 °C for telomere amplification. For 36B4 amplification, the profile consisted of 30 cycles of denaturing at 94°C for 15 s followed by annealing and extension for 2 min at 60°C. The specificity of amplification was confirmed at the end of each run using melting curve analysis. We additionally confirmed PCR products by agarose gel electrophoresis. In each run, a reference sample was included as a calibrator to normalize the participants’ T/S ratio and calculate the final T/S ratio. Finally, to check for PCR amplification efficiency, standard curves for telomere and 36B4 amplification were generated from the reference sample over a fivefold range by serial dilution from 100 to 0.16 ng of gDNA. Inter-plate variability was 2.7%.
Statistical analysis
All statistical analysis was performed in PASW Statistics version 18. Demographic and clinical characteristics were analyzed using the chi-square test and Student t-test. Because telomere length was not always normally distributed, as tested by the Kolmogorov-Smirnov test, we compared T/S between groups nonparametrically, using the Mann-Whitney U test. A p-value < 0.005 was considered significant, and 95% confidence intervals (95%CIs) were used.
Results
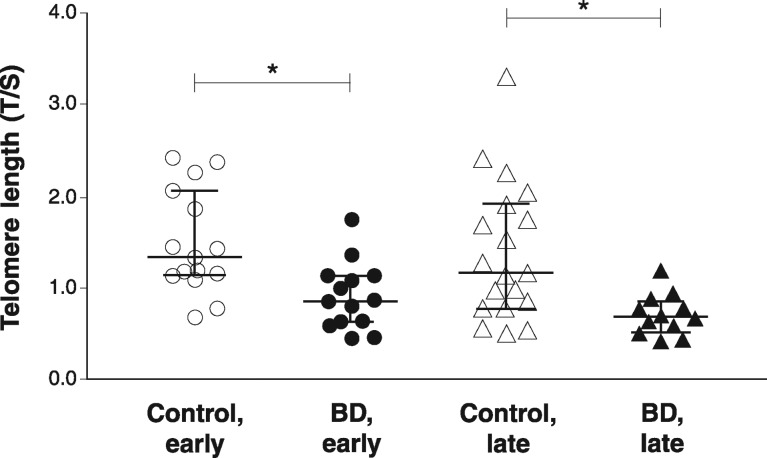
The BD (n=26) and control (n=34) groups were similar regarding age and gender distribution. There were statistically significant differences in functioning in the late-stage group (p = 0.001), which was expected in view of BD progression. Other clinical and demographic characteristics of the sample are shown in Table 1. The small sample size prevented us from analyzing the effects of pharmacotherapy on telomere length. All patients were on one or more drugs for BD management, except for two receiving lithium monotherapy and one taking only olanzapine. Of the 23 other patients, 13 were on lithium, 14 were taking some antipsychotic, and four were using some antidepressant. As demonstrated in Figure 1, significantly shorter telomere length was found in early- and late-stage patients as compared with the respective control groups (p = 0.002 and p = 0.005, respectively; Mann-Whitney U test).
Table 1. Characteristics of healthy controls and patients with bipolar disorder.
| Early-stage BD | Late-stage BD | |||||
|---|---|---|---|---|---|---|
| BD (n=14) | Controls (n=15) | p-value | BD (n=12) | Controls (n=19) | p-value | |
| Gender (male/female) | 3/11 | 6/9 | 0.427* | 4/8 | 7/12 | 1.000* |
| Age (years), mean (SD) | 40.71 (11.77) | 38.13 (11.44) | 0.555† | 53.83 (11.44) | 50.26 (9.48) | 0.354† |
| BD duration (years) | 8.00 (5-11.7) | - | - | 22.50 (11.2-34.7) | - | - |
| Number of mood episodes | 4.0 (2.2-5.7) | - | - | 17.0 (10-40) | - | - |
| Education (years/study) | 11.0 (8.25-12.0) | 12.0 (9.0-14.0) | 0.366† | 8.0 (6.0-9.0) | 8.0 (6.0-10.0) | 0.673† |
| FAST | 12.00 (6.0-32.0) | 6.00 (4.0-12.0) | 0.055‡ | 46.00 (35.7-54.5) | 7.50 (4.2-22.5) | < 0.001 ‡ |
| HAM-D | 1.0 (0.0-2.25) | - | - | 5.0 (3.0-2.0) | - | - |
| YMRS | 0.0 (0.0-3.0) | - | - | 1.0 (1.0-3.0) | - | - |
| BMI, mean (SD) | 26.94 (5.99) | 26.75 (3.46) | 0.920† | 31.56 (7.32) | 26.73 (4.71) | - |
| Telomere length | 0.85 (0.6-1.1) | 1.32 (1.1-2.0) | 0.002 ‡ | 0.68 (0.5-0.8) | 1.15 (0.7-1.9) | 0.058† |
Data expressed as median (interquartile range), unless otherwise specified.
BD = bipolar disorder; BMI = body mass index; FAST = Functioning Assessment Short Test; HAM-D = Hamilton Depression Rating Scale; YMRS =Young Mania Rating Scale.
Chi-square,
Student’s t-test,
Mann-Whitney U.
Figure 1. Telomere length (median and interquartile range [95% confidence interval]) in patients with BD and healthy subjects. BD = bipolar disorder; T/S ratio = telomere repeat (T) copy number to single (S) copy number (T/S). * p > 0.005, Mann-Whitney U test.
Discussion
Our results are in agreement with most previous studies that analyzed telomere length in BD, insofar as patients with BD presented shorter telomeres when compared to healthy subjects. In 2006, Simon et al.14 were the first to report shortened telomeres in subjects with mood disorders vs. healthy subjects. Three other studies subsequently reported similar findings, including a higher load of short telomeres in BD patients when compared to controls, which was associated with number of depressive episodes (many vs. few), and a mean telomere length approximately 552 base pairs shorter in BD patients.27 Shorter telomeres in mononuclear cells from female euthymic bipolar patients were associated with cellular senescence.20 Shorter telomere length in a sample of moderately depressed BD patients seemed not to be influenced by medication, although a decreased mean telomere length was observed in drug-free patients when compared to controls.15 In contrast, Mansour et al.16 reported no difference in telomere length between BD patients and a healthy control group.
Multiple mechanisms might be implicated in telomere shortening in BD, including oxidative stress28 leading to secondary DNA damage,29 inflammation,30 and glucocorticoid load.7 Mechanistically, oxidative stress can lead to the formation of 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodG) at the GGG triplet in telomere sequences,31 which is noted to be increased in patients with BD.32 Chronic systemic inflammation has also been shown to accelerate aging via reactive oxygen species-mediated exacerbation of telomere dysfunction.33 Of note, exacerbated oxidative stress might be a consequence of chronic activation of the autonomic and neuroendocrine stress responses,13 which is thought to occur in BD.9
Telomere shortening has also been associated with reduced activity and levels of telomerase, which is the enzyme responsible for catalyzing addition of the necessary telomeric DNA repeats onto the 3′ ends of the telomere after each cell division.13 Accordingly, chronically stressed individuals have shown lower telomerase activity,13 and ex vivo exposure of lymphocytes to cortisol has been shown to reduce telomerase activity.34 HPA axis impairment has been found in patients with BD,7,35 as demonstrated by increased cortisol levels36 and cortisol non-suppression in the dexamethasone suppression test.37 Increased stress exposure and cortisol levels might thus accelerate telomere shortening. In this same vein, telomerase may be a promising therapeutic target among BD patients.
Interestingly, shorter telomeres can trigger cell senescence38 and apoptosis,39 which is consistent with the reported increase in early apoptosis in patients with BD.40 In addition, cell senescence is associated with increased cellular secretion of proinflammatory cytokines41,42 contributing to the senescence-associated secretory phenotype (SASP), which may also link telomere shortening with age-associated medical conditions, most of which are highly prevalent in patients with BD.5
Our results add to the evidence base because we classified BD participants into early and late stages of illness, with control groups matched for age and gender. This staging system allowed us to suggest that telomere shortening occurs early in the course of BD. This contributes to the theory that shortened telomeres in psychiatric illness may be a risk factor, both preceding and leading to all the changes in oxidative stress and inflammatory response reported in BD.43 However, the precise timing of this phenomenon - whether, e.g., it is evident at the first (or the first few) episodes, or even at the prodromal stage - is unclear. Longitudinal studies are necessary to explore this question and clarify whether shortened telomeres are a result of chronic exposure to inflammation and oxidative stress or a factor leading to these systemic maladaptations. These findings may contribute to the theory of AA in BD.5
This finding is concordant with several aspects already known to be related to BD: increased prevalence of comorbid medical illness, structural brain alterations, cognitive and functioning impairments, oxidative stress and immunological imbalance, neurotrophic deficiencies that form the basis of neuroprogression,44 and, ultimately, decreased telomere length.5 A relation between AA and AL has been proposed,5,9 whereby saturation of the body’s ability to maintain homeostasis would lead to a series of maladaptive systemic alterations (AL) resulting in an increased occurrence of comorbidities, appearing as an AA process and, possibly, resulting in decreased life expectancy due to natural causes in patients with BD.45
Limitations of this study include the small sample size, which precluded analysis of differences in effects of medication, smoking status,46 and lifestyle factors47 between specific subgroups of patients. Ethnic background is a possible confounder47; however, we did not have information about this variable. A potential gender difference, with longer telomeres in females, has been an issue of intense debate. A recent systematic review and meta-analysis of 36,230 participants may have clarified this problem, concluding that gender differences are detectable with Southern blot methods, but not when real-time PCR or flow-FISH are used.48 Accordingly, in our sample there was no difference in T/S between males and females. Additionally, because of the cross-sectional nature of the study, causal associations could not be inferred. Finally, the lack of data on inflammation and oxidative stress might hinder a mechanistic exploration of our results.
In conclusion, our data support previous studies showing telomere shortening in a sample of euthymic bipolar patients, suggesting this is a critical mechanism present since BD onset49 and which may play a role in the AA seen in these patients. Further studies using longitudinal designs and the possibility of including non-BD relatives will allow us to explore additional heritable traits.
Disclosure
MB has received grant/research support from Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, the Meat and Livestock Board, Organon, Novartis, Mayne Pharma, Servier, and Woolworths; has been a speaker for AstraZeneca, Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, Janssen-Cilag, Lundbeck, Merck, Pfizer, Sanofi-Synthélabo, Servier, Solvay, and Wyeth; and served as a consultant to AstraZeneca, BioAdvantex, Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, Janssen-Cilag, Lundbeck, Merck, and Servier. CSG has been a paid consultant/speaker for Actelion, Janssen-Cilag, and Lundbeck. ARR has served as speaker for Eli Lilly. The other authors report no conflicts of interest.
Acknowledgements
This study received financial support from the Ministério da Ciência, Tecnologia e Inovação (MCT)/Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq)-Universal (grants 479305/2009-9, 473515/2013-0, and 443526/2014-1), CNPq Produtividade em Pesquisa (304443/2014-0), and Ciências sem Fronteiras (CSF) (40.00032/2012-0). FMB-T and BSP received fellowships from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). MMP and GRF received a fellowship from CNPq. BSP received a scholarship from CAPES/CSF and CNPq/GD. MB is supported by the National Health and Medical Research Council (NHMRC) Senior Principal Research Fellowship (1059660). ARR is grateful for the support provided by L’Oréal Brasil, Academia Brasileira de Ciências, and the UNESCO National Commission For Women in Science.
References
- 1.Rosa AR, Magalhães PV, Czepielewski L, Sulzbach MV, Goi PD, Vieta E, et al. Clinical staging in bipolar disorder: focus on cognition and functioning. J Clin Psychiatry. 2014;75:e450–6. doi: 10.4088/JCP.13m08625. [DOI] [PubMed] [Google Scholar]
- 2.Vieta E, Popovic D, Rosa AR, Solé B, Grande I, Frey BN, et al. The clinical implications of cognitive impairment and allostatic load in bipolar disorder. Eur Psychiatry. 2013;28:21–9. doi: 10.1016/j.eurpsy.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Kupfer DJ. The increasing medical burden in bipolar disorder. JAMA. 2005;293:2528–30. doi: 10.1001/jama.293.20.2528. [DOI] [PubMed] [Google Scholar]
- 4.Crump C, Sundquist K, Winkleby MA, Sundquist J. Comorbidities and mortality in bipolar disorder: a Swedish national cohort study. JAMA Psychiatry. 2013;70:931–9. doi: 10.1001/jamapsychiatry.2013.1394. [DOI] [PubMed] [Google Scholar]
- 5.Rizzo LB, Costa LG, Mansur RB, Swardfager W, Belangero SI, Grassi-Oliveira R, et al. The theory of bipolar disorder as an illness of accelerated aging: implications for clinical care and research. Neurosci Biobehav Rev. 2014;42:157–69. doi: 10.1016/j.neubiorev.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Frey BN, Andreazza AC, Kunz M, Gomes FA, Quevedo J, Salvador M, et al. Increased oxidative stress and DNA damage in bipolar disorder: a twin-case report. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:283–5. doi: 10.1016/j.pnpbp.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 7.Fries GR, Vasconcelos-Moreno MP, Gubert C, dos Santos BT, Sartori J, Eisele B, et al. Hypothalamic-pituitary-adrenal axis dysfunction and illness progression in bipolar disorder. Int J Neuropsychopharmacol. 2014 Oct 31;18(1) doi: 10.1093/ijnp/pyu043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grande I, Magalhães PV, Kunz M, Vieta E, Kapczinski F. Mediators of allostasis and systemic toxicity in bipolar disorder. Physiol Behav. 2012;106:46–50. doi: 10.1016/j.physbeh.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 9.Kapczinski F, Vieta E, Andreazza AC, Frey BN, Gomes FA, Tramontina J, et al. Allostatic load in bipolar disorder: implications for pathophysiology and treatment. Neurosci Biobehav Rev. 2008;32:675–92. doi: 10.1016/j.neubiorev.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 10.de Lange T. Protection of mammalian telomeres. Oncogene. 2002;21:532–40. doi: 10.1038/sj.onc.1205080. [DOI] [PubMed] [Google Scholar]
- 11.Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–60. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 12.Cawthon RM, Smith KR, O’Brien E, Sivatchenko A, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003;361:393–5. doi: 10.1016/S0140-6736(03)12384-7. [DOI] [PubMed] [Google Scholar]
- 13.Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, et al. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A. 2004;101:17312–5. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simon NM, Smoller JW, McNamara KL, Maser RS, Zalta AK, Pollack MH, et al. Telomere shortening and mood disorders: preliminary support for a chronic stress model of accelerated aging. Biol Psychiatry. 2006;60:432–5. doi: 10.1016/j.biopsych.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Lima IM, Barros A, Rosa DV, Albuquerque M, Malloy-Diniz L, Neves FS, et al. Analysis of telomere attrition in bipolar disorder. J Affect Disord. 2015;172:43–7. doi: 10.1016/j.jad.2014.09.043. [DOI] [PubMed] [Google Scholar]
- 16.Mansour H, Chowdari K, Fathi W, Elassy M, Ibrahim I, Wood J, et al. Does telomere length mediate associations between inbreeding and increased risk for bipolar I disorder and schizophrenia? Psychiatry Res. 2011;188:129–32. doi: 10.1016/j.psychres.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 17.Hoen PW, Rosmalen JGM, Schoevers RA, Huzen J, van der Harst P, de Jonge P. Association between anxiety but not depressive disorders and leukocyte telomere length after 2 years of follow-up in a population-based sample. Psychol Med. 2013;43:689–97. doi: 10.1017/S0033291712001766. [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Rizo C, Fernandez-Egea E, Miller BJ, Oliveira C, Justicia A, Griffith JK, et al. Abnormal glucose tolerance, white blood cell count, and telomere length in newly diagnosed, antidepressant-naïve patients with depression. Brain Behav Immun. 2013;28:49–53. doi: 10.1016/j.bbi.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martinsson L, Wei Y, Xu D, Melas PA, Mathé AA, Schalling M, et al. Long-term lithium treatment in bipolar disorder is associated with longer leukocyte telomeres. Transl Psychiatry. 2013;3:e261. doi: 10.1038/tp.2013.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rizzo LB, Do Prado CH, Grassi-Oliveira R, Wieck A, Correa BL, Teixeira AL, et al. Immunosenescence is associated with human cytomegalovirus and shortened telomeres in type I bipolar disorder. Bipolar Disord. 2013;15:832–8. doi: 10.1111/bdi.12121. [DOI] [PubMed] [Google Scholar]
- 21.Colpo GD, Leffa DD, Köhler CA, Kapczinski F, Quevedo J, Carvalho AF. Is bipolar disorder associated with accelerating aging? A meta-analysis of telomere length studies. J Affect Disord. 2015;186:241–8. doi: 10.1016/j.jad.2015.06.034. [DOI] [PubMed] [Google Scholar]
- 22.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–35. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 24.Kapczinski F, Magalhães PV, Balanzá-Martinez V, Dias VV, Frangou S, Gama CS, et al. Staging systems in bipolar disorder: an International Society for Bipolar Disorders Task Force Report. Acta Psychiatr Scand. 2014;130:354–63. doi: 10.1111/acps.12305. [DOI] [PubMed] [Google Scholar]
- 25.Rosa AR, Sánchez-Moreno J, Martínez-Aran A, Salamero M, Torrent C, Reinares M, et al. Validity and reliability of the Functioning Assessment Short Test (FAST) in bipolar disorder. Clin Pract Epidemiol Ment Health. 2007;3:5. doi: 10.1186/1745-0179-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30:e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elvsåshagen T, Vera E, Bøen E, Bratlie J, Andreassen OA, Josefsen D, et al. The load of short telomeres is increased and associated with lifetime number of depressive episodes in bipolar II disorder. J Affect Disord. 2011;135:43–50. doi: 10.1016/j.jad.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 28.Brown NC, Andreazza AC, Young LT. An updated meta-analysis of oxidative stress markers in bipolar disorder. Psychiatry Res. 2014;218:61–8. doi: 10.1016/j.psychres.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 29.Andreazza AC, Frey BN, Erdtmann B, Salvador M, Rombaldi F, Santin A, et al. DNA damage in bipolar disorder. Psychiatry Res. 2007;153:27–32. doi: 10.1016/j.psychres.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 30.Modabbernia A, Taslimi S, Brietzke E, Ashrafi M. Cytokine alterations in bipolar disorder: a meta-analysis of 30 studies. Biol Psychiatry. 2013;74:15–25. doi: 10.1016/j.biopsych.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 31.Kawanishi S, Oikawa S. Mechanism of telomere shortening by oxidative stress. Ann N Y Acad Sci. 2004;1019:278–84. doi: 10.1196/annals.1297.047. [DOI] [PubMed] [Google Scholar]
- 32.Munkholm K, Peijs L, Kessing LV, Vinberg M. Reduced mRNA expression of PTGDS in peripheral blood mononuclear cells of rapid-cycling bipolar disorder patients compared with healthy control subjects. Int J Neuropsychopharmacol. 2014 Dec 7;18(5) doi: 10.1093/ijnp/pyu101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jurk D, Wilson C, Passos JF, Oakley F, Correia-Melo C, Greaves L, et al. Chronic inflammation induces telomere dysfunction and accelerates ageing in mice. Nat Commun. 2014;2:4172. doi: 10.1038/ncomms5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choi J, Fauce SR, Effros RB. Reduced telomerase activity in human T lymphocytes exposed to cortisol. Brain Behav Immun. 2008;22:600–5. doi: 10.1016/j.bbi.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Daban C, Vieta E, Mackin P, Young AH. Hypothalamic-pituitary-adrenal axis and bipolar disorder. Psychiatr Clin North Am. 2005;28:469–80. doi: 10.1016/j.psc.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 36.Cervantes P, Gelber S, Kin FN, Nair VN, Schwartz G. Circadian secretion of cortisol in bipolar disorder. J Psychiatry Neurosci. 2001;26:411–6. [PMC free article] [PubMed] [Google Scholar]
- 37.Watson S, Gallagher P, Ritchie JC, Ferrier IN, Young AH. Hypothalamic-pituitary-adrenal axis function in patients with bipolar disorder. Br J Psychiatry. 2004;184:496–502. doi: 10.1192/bjp.184.6.496. [DOI] [PubMed] [Google Scholar]
- 38.Herbig U, Jobling WA, Chen BP, Chen DJ, Sedivy JM. Telomere shortening triggers senescence of human cells through a pathway involving ATM, p53, and p21(CIP1), but not p16(INK4a). Mol Cell. 2004;14:501–13. doi: 10.1016/s1097-2765(04)00256-4. [DOI] [PubMed] [Google Scholar]
- 39.Mondello C, Scovassi AI. Telomeres, telomerase, and apoptosis. Biochem Cell Biol. 2004;82:498–507. doi: 10.1139/o04-048. [DOI] [PubMed] [Google Scholar]
- 40.Fries GR, Vasconcelos-Moreno MP, Gubert C, Santos BT, da Rosa AL, Eisele B, et al. Early apoptosis in peripheral blood mononuclear cells from patients with bipolar disorder. J Affect Disord. 2014;152-154:474–7. doi: 10.1016/j.jad.2013.07.027. [DOI] [PubMed] [Google Scholar]
- 41.Effros RB, Dagarag M, Spaulding C, Man J. The role of CD8+ T-cell replicative senescence in human aging. Immunol Rev. 2005;205:147–57. doi: 10.1111/j.0105-2896.2005.00259.x. [DOI] [PubMed] [Google Scholar]
- 42.Puterman E, Lin J, Blackburn E, O’Donovan A, Adler N, Epel E. The power of exercise: buffering the effect of chronic stress on telomere length. PloS One. 2010;5:e10837. doi: 10.1371/journal.pone.0010837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lindqvist D, Epel ES, Mellon SH, Penninx BW, Révész D, Verhoeven JE, et al. Psychiatric disorders and leukocyte telomere length: underlying mechanisms linking mental illness with cellular aging. Neurosci Biobehav Rev. 2015;55:333–64. doi: 10.1016/j.neubiorev.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Berk M, Kapczinski F, Andreazza AC, Dean OM, Giorlando F, Maes M, et al. Pathways underlying neuroprogression in bipolar disorder: focus on inflammation, oxidative stress and neurotrophic factors. Neurosci Biobehav Rev. 2011;35:804–17. doi: 10.1016/j.neubiorev.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 45.Kessing LV, Vradi E, McIntyre RS, Andersen PK. Causes of decreased life expectancy over the life span in bipolar disorder. J Affect Disord. 2015;180:142–7. doi: 10.1016/j.jad.2015.03.027. [DOI] [PubMed] [Google Scholar]
- 46.Valdes AM, Andrew T, Gardner JP, Kimura M, Oelsner E, Cherkas LF, et al. Obesity, cigarette smoking, and telomere length in women. Lancet. 2005;366:662–4. doi: 10.1016/S0140-6736(05)66630-5. [DOI] [PubMed] [Google Scholar]
- 47.Adler N, Pantell MS, O’Donovan A, Blackburn E, Cawthon R, Koster A, et al. Educational attainment and late life telomere length in the Health, Aging and Body Composition Study. Brain Behav Immun. 2013;27:15–21. doi: 10.1016/j.bbi.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gardner M, Bann D, Wiley L, Cooper R, Hardy R, Nitsch D, et al. Gender and telomere length: systematic review and meta-analysis. Exp Gerontol. 2014;51:15–27. doi: 10.1016/j.exger.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Berk M, Berk L, Dodd S, Cotton S, Macneil C, Daglas R, et al. Stage managing bipolar disorder. Bipolar Disord. 2014;16:471–7. doi: 10.1111/bdi.12099. [DOI] [PubMed] [Google Scholar]