Abstract
Objective:
To assess the effectiveness of a cognitive-behavioral therapy-based intervention (Superwellness Program) on weight gain compared with a treatment-as-usual (TAU) approach in patients treated with antipsychotics, and to evaluate the relationship between body mass index (BMI) variation and clinical variables.
Method:
Eighty-five patients treated with antipsychotics were allocated across two groups, experimental (n=59) and control (n=26). The Superwellness Program (experimental group) consisted of 32 twice-weekly 1-hour sessions, conducted by a psychologist and a nutritionist/nurse, concurrently with moderate food intake and moderate physical activity plans. Sociodemographic, clinical, and biological variables were collected at baseline, at the end of intervention (16 weeks), and after 6 months.
Results:
BMI change from baseline differed significantly between the experimental and control groups, with a larger decrease in the experimental group (F = 5.5, p = 0.021). Duration of illness moderated the effect of treatment on BMI (p = 0.026). No significant (p = 0.499) effect of intervention during the follow-up period was found. Interestingly, the intervention indirectly induced a significant (p = 0.024) reduction in metabolic risk by reducing BMI.
Conclusion:
A cognitive-behavioral therapy-based intervention could be useful in reducing weight in a clinical population taking antipsychotics, with consequent benefit to physical and mental health.
Keywords: Antipsychotic, cognitive-behavioral therapy, obesity, weight gain
Introduction
Overweight and obesity are common concerns in individuals with severe mental disorders, and represent a common adverse effect of many psychiatric drugs. In particular, antipsychotic drugs increase the risk of overweight, obesity,1 diabetes mellitus,2 and cardiovascular diseases,3 leading to a significant reduction in quality of life4 and greater morbidity and mortality.5 The prevalence of obesity among people with psychotic disorder is 40-60%, higher than the 35% prevalence in the general U.S. adult population.6 Furthermore, weight gain is one of the major reasons for cessation of antipsychotic treatment.7 Indeed, medication adherence is associated with perceived weight status.8 Moreover, being overweight or obese has an impact on important aspects of health-related quality of life9 and increases the social stigma associated with mental disorder.10 For these reasons, management of weight gain should become an intervention target. There is growing interest in developing strategies to control or mitigate weight gain with pharmacologic and nonpharmacologic interventions.
Several pharmacologic interventions to attenuate antipsychotic-induced weight gain have been proposed, but with only moderate success. Out of 15 agents examined in a recent meta-analysis,11 only five showed significant benefit versus placebo. The other two established pharmacologic approaches, metformin and topiramate, reduce antipsychotic-related weight gain compared to placebo but can cause additional side effects.12,13
Conversely, nonpharmacologic interventions do not have such side effects and seem to be promising. A meta-analysis of 17 randomized clinical trials (RCTs)14 including 810 participants reported that nonpharmacologic interventions were significantly more effective than the respective control conditions. In particular, the pooled weight change of the experimental groups compared to the controls was around 3 kg in these studies, and the magnitude of the weight loss was comparable to that achieved with metformin and topiramate,11 without their adverse effects. The recent STRIDE RCT showed that patients taking antipsychotic could lose significant amounts of weight with a comprehensive weight-loss and lifestyle-change program,15 and that losing weight also improved perceived health and health-related self-efficacy.16 Thus, expanding research on this topic is an important step in improving health interventions for people with serious mental illnesses.
The principal aim of this study was to assess the effectiveness of a cognitive-behavioral therapy (CBT)-based intervention (Superwellness Program) on weight gain compared with a treatment-as-usual (TAU) approach, within a psychiatric and psychosocial treatment regimen representative of the usual setting and modality of care in Italian psychiatric rehabilitative centers.
Materials and methods
This study was approved by the local ethics commission (Comitato Etico delle Istituzioni Ospedaliere Cattoliche, CEIOC) and all patients provided written informed consent.
A prospective, multicenter design was used, with a 6-month follow-up period. Participants were recruited into five psychiatric centers: four inpatient units and one outpatient service.
The inclusion criteria were: diagnosis of schizophrenia, schizophreniform disorder, or schizoaffective disorder, in treatment with antipsychotic drugs; age 18-65 years; body mass index (BMI) > 25; and ability to give informant consent. The exclusion criteria were anorexia nervosa, bulimia nervosa, alcohol or substance dependence, and mental retardation.
Patients were enrolled consecutively into the experimental group (to a maximum of eight patients per group) and compared with controls.
Interventions
The Superwellness Program follows a model previously developed and published in the context of an informative campaign on safe nutritional habits supported by the Coop-Lombardia food retail group in 2005. To ensure intervention homogeneity among centers, each session was manualized.
The program consisted of: 32 twice-weekly, 1-hour sessions conducted by a psychologist and a nutritionist/nurse; 2) a moderate food intake plan, without any specific food prohibitions, based on Mediterranean diet (MedDiet)17 recommendations; and 3) a moderate physical activity plan to promote self-care by participants.
The CBT-oriented intervention included four different aspects: 1) psychoeducation on nutrition: information on different type of foods, the food pyramid, daily nutritional needs, as well as recommendations for a successful moderate calorie-restricted diet; 2) cognitive aspects: to help patients to recognize dysfunctional beliefs and cognitive bias on food habits (such as dichotomous thoughts, overgeneralization) and to replace them with more functional beliefs; 3) emotional aspects: to increase self-observation of eating behaviors (aided by a dietary journal) and emotional states and sensations associated with satiety and hunger, in order to increase participants’ ability to discriminate these conditions; and 4) behavioral aspects: to help participants develop functional alternative behavioral skills to reduce out-of-meal food intake and to promote other behaviors instead of eating. Activities for each session were scheduled in detail. Each session included a psychoeducational part and a review of dietary journals. Particular attention was given to the specific problems or difficulties of each participant and to promote alternative skills and solutions using cognitive and behavioral strategies. In the TAU group, the intervention consisted of an individual session in which participants received information about foods and nutritional needs, and were encouraged to maintain a safe lifestyle.
Both groups were led by a psychologist and a nutritionist (or, where a nutritionist was not available, a specially trained nurse). Intensive training on the Superwellness program was provided by the reference psychologist before the start of the intervention; in particular, two sessions were devoted exclusively to familiarizing the research protocol with all involved staff, followed by a single session, for psychologists, to homogenize style and techniques of conduction.
Assessments
Assessments were performed at baseline, after 16 weeks (i.e., post-intervention for the Superwellness group), and after 6 months. Demographic and clinical features were collected at baseline using a standardized data collection form. Diagnoses were made according to the DSM-IV criteria by the psychiatrists in charge.
Outcome measures
Weight and body mass index
Height was measured once at baseline. Body weight was measured on a digital scale at baseline, after 16 weeks, and after 6 months. BMI was computed, by definition, as the weight in kilograms divided by the height squared in meters. The change in weight and in BMI was computed as the difference between values measured at the end of the intervention and values measured at baseline.
Clinical assessment
Clinical assessment was conducted at baseline, after 16 weeks, and after 6 months. The following scales were administered: the Positive and Negative Syndrome Scale (PANSS)18 to assess positive and negative symptomatology; the Global Assessment of Functioning (GAF) Scale to assess psychological, social, and occupational functioning19; the WHO Quality of Life-BREF (WHOQOL-BREF)20,21 to measure quality of life; and the Short Form-36 Health Survey (SF-36) to assess patient health.22,23
Other parameters
Patients underwent blood tests consistent with their clinical assessment in order to monitor the following cholesterol (total) and triglyceride levels. Waist circumference was also measured. These parameters were then stratified into risk levels to define a comprehensive cardiovascular risk index.
Statistical analyses
Descriptive statistics (mean and standard deviation for continuous variables, absolute and relative frequency for categorical variables) were used to summarize the demographic and clinical features of the study sample. Parametric t-tests were used to assess absence of differences in demographic and clinical variables (age, weight, duration of illness, and metabolic parameters) between two groups before the intervention. Similarly, chi-square tests were used to ascertain absence of associations between the two groups and the categorical sociodemographic and clinical variables (sex, marital status, diagnosis, type of antipsychotic drug) before intervention.
A repeated-measures analysis of variance (ANOVA) model was employed to assess progression of the main (BMI) and other outcome variables (WHOQOL-BREF, PANSS, GAF, SF-36 Physical and Mental indices) over time (pre- and post-intervention as within-time factor) and between groups. Moreover, the associations and correlations of sociodemographic and clinical characteristics with the difference in the significant outcomes from baseline, highlighted in previous analyses, were evaluated by ANOVA models and Pearson’s correlation coefficients. To define the metabolic risk index used as a compound variable to assess all three metabolic parameters (cholesterol level, triglyceride level, and waist circumference), we first categorized each of the three metabolic parameters into low, medium and high (assigning a score of zero, one, or two, respectively) according to the Executive Summary of the Third Report of the National Cholesterol Education Program24 and to Han et al.25 Successively, the metabolic risk index (ranging from 0 = no risk to 6 = highest risk) was obtained as the sum of scores for the three parameters.
Lastly, a structural equation model (SEM) was used to analyze interrelations between intervention groups and other sociodemographic variables found to be associated or correlated with changes in BMI. The main advantage of using SEM is the flexibility to model complex relationships between one or more independent, or exogenous, variables (intervention, GAF scale, illness duration, sex) and one or more dependent, or endogenous, variables (change in BMI, change in metabolic risk index) simultaneously.26
To test whether the hypothesized model is a plausible explanatory model for the empirical data, its goodness of fit was checked by several measures27: chi-square test, relative chi-square test (< 2.5 indicates good fit), comparative fit index (CFI; ∼1 indicates good fit), root mean square error of approximation (RMSEA; < 0.05 indicates good fit), and the Tucker-Lewis coefficient (TLI; > 0.9 indicates good fit).
Missing data (less than 15%) for variables included in SEM were handled by stochastic regression imputation.28 All statistical analysis were carried out in SPSS version 21.0. SEM was implemented through the AMOS version 21.0. Statistical significance was set at p < 0.05.
Results
Participant characteristics
A total of 85 subjects were enrolled in the study: 59 were allocated to the experimental intervention (Superwellness group) and 26 to the control group. The two groups were homogeneous in terms of sociodemographic and clinical characteristics at baseline (Table 1). Most of the participants had a diagnosis of schizophrenia, with a long history of disease (17.3 years, standard deviation [SD] = 10.6 years), and were unmarried. All were receiving pharmacological treatment and most (93%) received at least one atypical (second-generation) antipsychotic, while 7% of patients received only a first-generation antipsychotic. Patients receiving second-generation antipsychotics were divided into three groups, in accordance with the 2004 Consensus Development Conference on Antipsychotic Drugs and Obesity and Diabetes,29 namely: clozapine/olanzapine (high propensity for weight gain); quetiapine/risperidone (medium propensity for weight gain); and aripiprazole (low propensity for weight gain). As the chi-square statistic was not significant across groups (χ2 = 1.654, p = 0.437; Fisher’s exact test), they can be considered similar on this variable.
Table 1. Sociodemographic and clinical characteristics of the Superwellness and TAU groups at baseline.
| Superwellness (n=59) | TAU (n=26) | ||
|---|---|---|---|
| Variable | n (%) | n (%) | χ2 p-value |
| Sex (female) | 32 (54.2) | 11 (42.3) | 0.353 |
| Marital status | |||
| Married | 5 (8.5) | 3 (11.5) | 0.347 |
| Single | 41 (69.5) | 21 (80.8) | |
| Divorced | 11 (18.6) | 1 (3.8) | |
| Diagnosis | |||
| Schizophrenia/schizophrenic disorder | 46 (78.0) | 21 (80.7) | 0.465 |
| Schizoaffective disorder | 12 (20.3) | 5 (19.2) | |
| BMI | |||
| Overweight (BMI ≤ 30) | 22 (37.3) | 5 (19.2) | 0.093 |
| Mild obesity (30 < BMI < 35) | 21 (35.6) | 8 (30.8) | |
| Moderate/severe obesity (BMI ≥ 35) | 16 (27.1) | 13 (50.0) | |
| Antipsychotic drugs | |||
| First-generation only | 4 (6.8) | 2 (7.7) | 0.626 |
| Second-generation | 55 (93.2) | 24 (92.3) | |
| Second-generation agents* | |||
| Clozapine/olanzapine | 18 (32.7) | 9 (37.5) | 0.553 |
| Risperidone/quetiapine | 26 (47.3) | 13 (54.2) | |
| Aripiprazole | 11 (20.0) | 2 (8.3) | |
| Variable | Mean (SD) | Mean (SD) | t-test p-value |
| Age (years) | 43.1 (9.0) | 41.8 (10.1) | 0.545 |
| Age at illness onset (years) | 23.6 (6.2) | 25.8 (7.8) | 0.227 |
| Duration of illness (years) | 17.0 (10.7) | 17.9 (11.1) | 0.687 |
| Weight (kg) | 91.4 (16.2) | 99.0 (17.5) | 0.075 |
| Metabolic parameters | |||
| Cholesterol, total (mg/dL)† | 214.1 (41.3) | 208.2 (42.3) | 0.581 |
| Triglycerides‡ | 162.8 (103.5) | 164.1 (71.5) | 0.888 |
| Waist circumference§ | 111.2 (13.5) | 118.8 (11.8) | 0.127 |
BMI = body mass index; SD = standard deviation; TAU = treatment as usual.
In accordance with American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, North American Association for the Study of Obesity, 2004.29
Cholesterol level: low < 200; 200 ≤ medium < 240; high ≥ 240.
Triglyceride level: low < 200; 200 ≤ medium < 400; high ≥ 400.
Waist circumference cutoff (for males): small < 95; 95 ≤ medium < 110; large ≥ 110.
Regarding the main outcome, the mean BMI at baseline was 32.3 kg/m2 (SD = 4.7) in the Superwellness group and 34.7 kg/m2 (SD = 5.3) in the TAU group, both corresponding to the same level of “mild obesity.”
Considering the metabolic parameters, both groups at baseline had medium cholesterol levels, low triglyceride levels, and very large waist circumference measurements.
Longitudinal evaluation of outcomes
The mean change in BMI after 16 weeks was -1.9% for the intervention group (decreasing from 32.6 to 32) and +0.6% for the control group (increasing from 35 to 35.2). The repeated-measures ANOVA model for BMI revealed significant effects for both principal effects (time and group) and the interaction effect (time x group) (Table 2), indicating actual effectiveness of the intervention on BMI change. Similarly, a significant interaction effect of treatment on GAF changes was observed (p = 0.050). No significant effect of intervention on the other outcomes (PANSS total score, WHOQOL-BREF subscales, and SF-36 Physical-Mental indices) were found.
Table 2. Results of repeated-measures ANOVA for BMI and clinical outcomes.
| Superwellness group Mean (SD) | TAU group Mean (SD) | Time | Group | Interaction | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T0 | T1 | T0 | T1 | F | p-value | F | p-value | F | p-value | |
| BMI | 32.6 (4.6) | 32.0 (4.6) | 35.0 (5.3) | 35.2 (5.5) | 5.5 | 0.022* | 5.8 | 0.018* | 5.5 | 0.021* |
| PANSS, total score | 79.3 (28.9) | 79.1 (25.3) | 88.5 (25.6) | 89.9 (27.7) | 2.5 | 0.116 | 0.03 | 0.859 | 0.2 | 0.676 |
| GAF | 48.9 (11.2) | 50.6 (12.1) | 52.4 (10.5) | 50.8 (9.4) | 0.5 | 0.486 | 0.3 | 0.570 | 3.8 | 0.050* |
| WHOQOL, Physical Health | 65.1 (16.9) | 62.3 (22.1) | 57.9 (17.8) | 57.1 (22.9) | 2.4 | 0.122 | 0.8 | 0.362 | 0.1 | 0.707 |
| WHOQOL, Psychological | 56.9 (18.5) | 52.9 (20.8) | 48.7 (21.2) | 47.4 (22.1) | 2.7 | 0.102 | 2.0 | 0.166 | 0.3 | 0.591 |
| WHOQOL, Social Relationship | 54.4 (21.1) | 54.9 (22.7) | 57.3 (18.8) | 52.3 (24.7) | 0.0002 | 0.968 | 0.2 | 0.689 | 0.8 | 0.381 |
| WHOQOL, Environment | 63.4 (16.4) | 56.9 (19.9) | 59.6 (16.6) | 55.5 (23.6) | 0.5 | 0.486 | 5.9 | 0.017* | 0.2 | 0.646 |
| SF-36, Physical Index | 60.8 (17.1) | 58.2 (23.1) | 57.0 (17.1) | 59.1 (22.3) | 0.1 | 0.712 | 0.193 | 0.662 | 0.723 | 0.397 |
| SF-36, Mental Index | 53.4 (16.3) | 51.9 (22.5) | 51.6 (18.9) | 50.5 (22.1) | 0.2 | 0.676 | 0.318 | 0.575 | 0.004 | 0.952 |
BMI = body mass index; GAF = Global Assessment of Functioning; SD = standard deviation; SF-36 = Short Form-36; WHOQOL = World Health Organization Quality of Life.
p < 0.05.
Considering the two outcomes significantly affected by the intervention (Table 3), we found that the difference from baseline in BMI was associated with the variables sex (males experienced greater reductions in BMI than females, p = 0.037), duration of illness (shorter illness correlated positively with reduction in BMI, r = 0.25, p = 0.026), and metabolic risk index (reduction in risk correlated positively with reduction in BMI, r = 0.23, p = 0.033). Conversely, no associations were found between GAF and other sociodemographic and clinical characteristics.
Table 3. Associations between changes in outcomes (BMI and GAF) from baseline and sociodemographic and clinical characteristics.
| BMI (difference from baseline) | GAF (difference from baseline) | |||
|---|---|---|---|---|
| Variable | Mean (SD) | ANOVA p-value | Mean (SD) | ANOVA p-value |
| Sex | ||||
| Female | -0.1 (1.6) | 0.037* | 0.4 (7.2) | 0.859 |
| Male | -0.8 (1.3) | 0.3 (5.5) | ||
| Marital status | ||||
| Married | -1.0 (0.5) | 0.143 | -2.4 (14.7) | 0.448 |
| Single | -0.5 (0.2) | 0.9 (5.2) | ||
| Divorced | 0.3 (0.5) | 0.5 (4.5) | ||
| Diagnosis | ||||
| Schizophrenia/schizophrenic disorder | -0.4 (1.4) | 0.611 | 0.8 (5.7) | 0.486 |
| Schizoaffective disorder | -0.3 (1.7) | -1.4 (10.2) | ||
| Variable | Correlation | p-value | Correlation | p-value |
| Age | -0.15 | 0.194 | 0.17 | 0.106 |
| Age at illness onset | -0.02 | 0.922 | -0.09 | 0.356 |
| Duration of illness | 0.25 | 0.026* | 0.20 | 0.184 |
| Metabolic risk index (difference from baseline) | 0.23 | 0.033* | -0.15 | 0.282 |
ANOVA = analysis of variance; BMI = body mass index; GAF = Global Assessment of Functioning; SD = standard deviation.
p < 0.05.
Regarding follow-up data, the mean BMI after 6 months was 31.7 (SD = 5.1) in the intervention group and 35.6 (SD = 5.6) in the control group. No significant changes in clinical outcomes, PANSS total score, GAF, WHOQOL-BREF subcales, or SF-36 Physical-Mental indices were found.
Structural equation model
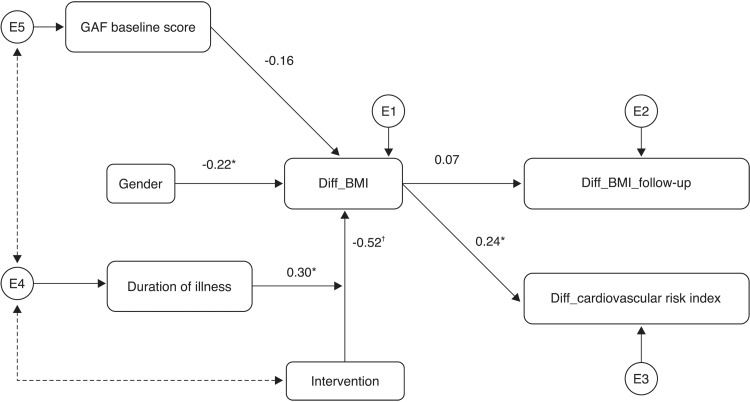
All variables significantly associated with changes in the main outcome (BMI) were included in the SEM. In addition, we also included the GAF scale as an exogenous variable. Although changes in GAF were not related to sociodemographic and clinical variables, we decided to include it in the model to investigate if and how the level of patients’ functioning at baseline affected changes in the main outcome. Besides, from a methodological point of view, the inclusion of that exogenous variable improved the goodness of fit of the SEM. Finally, regarding the variables sex and duration of illness, we tested their association with BMI change both as exogenous variables and as moderators30 of the relation with the intervention. The final model (i.e., that with the best fit) is displayed in Figure 1.
Figure 1. Structural equation model for longitudinal analysis of body mass index (BMI) changes: standardized estimates. Black dotted line depicts the covariance parameters between Global Assessment of Functioning (GAF), duration of illness, and intervention. E1-E5 indicate the measurement errors. The categorical variables gender and intervention refer to the male and Superwellness groups respectively. The number of different parameters to be estimated was 22, including covariance parameters for improving the model fit. *p < 0.05; † p < 0.001.
The number of estimated parameters was 22, including covariance parameters between exogenous variables for improving the model fit. Model fit indices showed good fit to the data (χ2 = 17.26, degrees of freedom [df] = 13, p = 0.188, relative χ2 = 1.33, CFI = 0.95, TLI = 0.92, RSMEA = 0.048 [90%CI 0.000-0.067]).
The intervention was the variable most predictive of BMI change: patients in the Superwellness group experienced a reduction of about 0.3 kg/m2 (corresponding to a 0.52 standardized coefficient) with respect to patients in the TAU group (p<0.001). Among the other clinical and demographic variables, we found that males experienced greater reductions in BMI than females (p=0.022) in both groups, and that duration of illness moderated the relationship between BMI change and group: the longer the duration of illness, the less the effect of intervention (p=0.026) in reducing BMI.
Regarding functioning, there was no evidence of association (p = 0.124) between the level of functioning at baseline and change in BMI from baseline.
Interestingly, the change in BMI after intervention correlated positively with change in metabolic risk index: a BMI decrease induced a significant (p = 0.024) reduction of metabolic risk.
Finally, no significant relation (p = 0.499) was observed between BMI change from baseline (diff_BMI in Figure 1) and BMI change during follow-up period (diff_BMI_follow-up in Figure 1), indicating no significant indirect effect of intervention during follow-up.
Discussion
The present study showed that a CBT-based group intervention was useful in reducing weight in an unselected clinical population taking antipsychotic drugs. We used a relatively short, well replicable, and cost-effective intervention that is able to modify the natural course of weight gain in patients treated with antipsychotic drugs.
Our results showed a significant difference between treated patients and the control group. In particular, in the experimental group, BMI decreased 0.6 kg/m2, while BMI increased in the TAU group. These results were similar to those reported in previous studies.1,5,31,32 Although the percentage of reduction could be considered small, it is noteworthy for a population expected to continue to gain weight each month,33 as happened in the control group.
Obesity and its consequences entail serious health risks, and antipsychotics, especially second-generation ones, have major weight gain-inducing potential. This adverse effect has recently become a major concern in the treatment of psychosis, because weight gain not only influences treatment adherence but is also associated with substantial medical morbidity and mortality.34 Results of studies on nonpharmacologic interventions designed to induce weight loss are promising, and these interventions do not have the adverse effects of pharmacologic treatments.
A key issue regards the maintenance of the effect. Indeed, an extended recent meta-analysis based on 25 RCT on nonpharmacologic interventions on weight management in people with psychotic disorder35 showed that they were effective both for weight loss and for weight-gain prevention, and had significant long-term effects (2 to 6 months post-intervention), but results were heterogeneous. In the present study, the improvement in BMI was recorded only during the intervention; no effect of intervention was observed during the 6-month follow-up period. This findings could be due to the short intervention period, but a similar BMI trajectory was found in a study by Kalarchian et al.,36 in which participants’ BMI decreased significantly during weekly behavioral treatment and remained stable through 12-month post-treatment follow-up. Obesity is now a public health problem in large parts of the industrialized world, and weight-control interventions such as diet and exercise have only provided short-term health improvements in the general population, without significant mental health problems.37 A fortiori, people with mental illness will have even more difficulties in following a proper diet while taking medications which increase their appetite and weight. In fact, patients who gain weight under antipsychotic treatment report regularly unsuccessful dietary trials.38 This aspect should be taken into account in the implementation of interventions, and some booster sessions could be planned to improve maintenance of results, as proposed for another treatment strategy.39
Physical health is a crucial dimension of quality of life in these patients, and access to physical health comparable (in terms of quality) to that enjoyed by the rest of the population is a basic target.40 In particular, obesity in schizophrenic patients treated with antipsychotic medications could lead to development of cardiovascular disease risk factors 10-15 years before the general population41 and to 23% higher cardiovascular risk compared to that of obese persons without schizophrenia.42 For these reasons, the promotion of physical health in these patients represents a key issue.40 To this end, in our sample, we collected data on other metabolic parameters in addition to weight and BMI, as suggested by Bruins et al.,35 who underlined the need to test the effect of nonpharmacologic interventions on such parameters. The Superwellness Program reduced cardiovascular risk index, associated with a reduction in cholesterol, triglycerides, and waist circumference. These differences were consistent with literature results both concerning lifestyle changes in the general population43 and nonpharmacologic interventions in a psychiatric population.14
However, changes in physical health did not correspond to a significant improvement in WHOQOL and SF-36 scores, which did not differ statistically within the two groups. Quality of life is a complex construct, including different aspects. As noted by Chen et al.,44 we could hypothesize that such a brief and specific intervention would not have a direct influence on quality of life considered as a whole. In line with our findings, a meta-analysis45 on weight loss in the general population showed only little improvement in health-related quality of life, and suggested that the most consistent effects are found only when using obesity-specific measures of quality of life. We can speculate that improvement in quality of life is harder to achieve in psychiatric patients with a long history of illness. Moreover, it is possible that minor changes occurred in our sample, but were not sufficiently large to be detected by the instruments used in this study. Furthermore, the lack of improvement in these variables could also be attributable to the brief duration of the intervention, with some changes occurring later; further investigation is needed to address this.
Another interesting result is that the reduction in BMI in the treated group was also associated with gender, with better responses observed in men, as in previous studies of the general population. Recently, Leblanc et al.46 reported that a 12-week nutritional intervention program based on the MedDiet led to more pronounced benefits in men than in women, contributing also to greater improvements in metabolic profile. Possible explanations are based on insulin homeostasis, with improvements found in men but not in women47 or in decreases in adiponectin concentration in men, which affects cardiovascular risk.48
Finally, the duration of illness, and, consequently, the duration of pharmacological treatment, also influenced the decrease in BMI; the longer the duration of illness, the smaller the effect of the intervention in reducing BMI. Data regarding duration of illness and its effect on nonpharmacologic interventions are few and contrasting. Kalarchian et al.36 found a trend for patients who had been taking antipsychotics for many years to exhibit a more favorable BMI trajectory during a nonpharmacologic intervention, but the BMI at baseline in their sample was higher than in our Superwellness group; thus, we can hypothesize that a different pattern of change occurred. Another study49 did not find a significant role of duration of antipsychotic treatment. Further research is needed.
Results from the SEM model confirmed that a CBT-based intervention could modify BMI in patients taking antipsychotics and, consequently, influence their cardiovascular risk. Duration of illness and gender should be taken into account in planning such interventions, as these factors can influence outcome. The advantage of using SEM is the possibility of modeling complex relationships between one or more independent variables and one or more dependent variables simultaneously. In particular, reduction of cardiovascular risk was found to be associated with change in BMI at the end of treatment. In turn, change in BMI depended on the intervention, and was moderated by the duration of illness. Finally, gender, but not general level of functioning, influenced the BMI reduction.
In general, it should be recommended that such interventions be integrated into treatment at the very earliest stages of pharmacologic therapy, so as to better prevent weight gain and reduce cardiovascular risk. Indirect advantages of these interventions may include prevention of medical comorbidity, as well as reduction of obesity risk and related social stigma. Furthermore, the Superwellness Program in particular is a cost-effective intervention, as weight gain is one of the major reasons for nonadherence, with consequences such as relapse and rehospitalization, which, in turn, increase the cost of psychosis treatment.50
This study has both strengths and limitations. The major strength is that the Superwellness Program has been proven effective in a routine clinical setting with an unselected population. Regardless of whether the inclusion of an unselected population could be considered a limitation and a possible confounder, it should be noted that our sample was representative of the clinical population admitted to rehabilitation units in our country. The use of restricted criteria would likely limit recruitment to small numbers of participants who might not have been representative of the chronically ill populations managed in most clinical settings. Furthermore, such interventions could easily be integrated into psychiatric rehabilitation programs and, being manualized, are amenable to implementation in different clinical settings. Some caveats should be taken into account when interpreting our data. The principal limitation of this study was its nonrandomized design. Although we did not find any clinically or statistically significant differences at baseline for the outcomes of interest, the lack of randomization makes our study less reliable and generalizable than a randomized trial. Furthermore, randomization should guarantee an ideal balance between groups for the variables of interest. Indeed, it should be noted that our control group had a slightly higher prevalence of BMIs indicative of more severe obesity.
Another possible bias that could affect generalization of our results is the small sample size. Nevertheless, a meta-analysis on this topic14 that included larger RCTs reported similar results. Finally, the brief duration of the intervention and the lack of long-term follow-up prevented us from drawing conclusions on the long-term effects of the treatment. In light of the modest weight loss reported by this and other similar studies, future interventions on weight gain should be integrated with pharmacologic treatment at early stages in order to prevent or reduce this adverse effect of antipsychotic therapy.
In conclusion, beyond the limited results of this study, it seems clear that weight-management interventions for psychiatric patients should be incorporated into clinical practice. Future research efforts could focus on the development of longer interventions, specifically preventing young patients from becoming obese at treatment onset, and on direct comparisons between different types of intervention. Long-term effects could also influence medication adherence, subsequent relapse rate, and social isolation, and further data on these topics are needed.
Disclosure
The study received partial financial support from Eli Lilly. The authors report no conflicts of interest.
References
- 1.Attux C, Martini LC, Elkis H, Tamai S, Freirias A, Camargo Md, et al. A 6-month randomized controlled trial to test the efficacy of a lifestyle intervention for weight gain management in schizophrenia. BMC Psychiatry. 2013;13:60. doi: 10.1186/1471-244X-13-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Henderson DC, Cagliero E, Gray C, Nasrallah RA, Hayden DL, Schoenfeld DA, et al. Clozapine, diabetes mellitus, weight gain, and lipid abnormalities: a five-year naturalistic study. Am J Psychiatry. 2000;157:975–81. doi: 10.1176/appi.ajp.157.6.975. [DOI] [PubMed] [Google Scholar]
- 3.Casey DE, Hansen TE. Excessive mortality and morbidity associated with schizophrenia. In: Meyer JM, Nasrallah HA, editors. Medical illness and schizophrenia. 2nd ed. Washington: American Psychiatric Publishing; 2009. pp. 17–36. [Google Scholar]
- 4.Wirshing DA. Schizophrenia and obesity: impact of antipsychotic medications. J Clin Psychiatry. 2004;65:13–26. [PubMed] [Google Scholar]
- 5.Brown S, Kim M, Mitchell C, Inskip H. Twenty-five year mortality of a community cohort with schizophrenia. Br J Psychiatry. 2010;196:116–21. doi: 10.1192/bjp.bp.109.067512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014;311:806–14. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Usher K, Park T, Foster K. The experience of weight gain as a result of taking second-generation antipsychotic medications: the mental health consumer perspective. J Psychiatr Ment Health Nurs. 2013;20:801–6. doi: 10.1111/jpm.12019. [DOI] [PubMed] [Google Scholar]
- 8.Wong MM, Chen EY, Lui SS, Tso S. Medication adherence and subjective weight perception in patients with first-episode psychotic disorder. Clin Schizophr Relat Psychoses. 2011;5:135–41. doi: 10.3371/CSRP.5.3.3. [DOI] [PubMed] [Google Scholar]
- 9.Kolotkin RL, Crosby RD, Williams GR, Hartley GG, Nicol S. The relationship between health-related quality of life and weight loss. Obes Res. 2001;9:564–71. doi: 10.1038/oby.2001.73. [DOI] [PubMed] [Google Scholar]
- 10.Russell JM, Mackell JA. Bodyweight gain associated with atypical antipsychotics: epidemiology and therapeutic implications. CNS Drugs. 2001;15:537–51. doi: 10.2165/00023210-200115070-00004. [DOI] [PubMed] [Google Scholar]
- 11.Maayan L, Correll CU. Management of antipsychotic-related weight gain. Expert Rev Neurother. 2010;10:1175–200. doi: 10.1586/ern.10.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chakrabarti R. Pharmacotherapy of obesity: emerging drugs and targets. Expert Opin Ther Targets. 2009;13:195–207. doi: 10.1517/14728220802637063. [DOI] [PubMed] [Google Scholar]
- 13.Narula PK, Rehan HS, Unni KE, Gupta N. Topiramate for prevention of olanzapine associated weight gain and metabolic dysfunction in schizophrenia: a double-blind, placebo-controlled trial. Schizophr Res. 2010;118:218–23. doi: 10.1016/j.schres.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Caemmerer J, Correll CU, Maayan L. Acute and maintenance effects of non-pharmacologic interventions for antipsychotic associated weight gain and metabolic abnormalities: a meta-analytic comparison of randomized controlled trials. Schizophr Res. 2012;140:159–68. doi: 10.1016/j.schres.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Green CA, Yarborough BJ, Leo MC, Yarborough MT, Stumbo SP, Janoff SL, et al. The STRIDE weight loss and lifestyle intervention for individuals taking antipsychotic medications: a randomized trial. Am J Psychiatry. 2015;172:71–81. doi: 10.1176/appi.ajp.2014.14020173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yarborough BJ, Leo MC, Yarborough MT, Stumbo S, Janoff SL, Perrin NA, et al. Improvement in body image, perceived health, and health-related self-efficacy among people with serious mental illness: the STRIDE study. Psychiatr Serv. 2016;67:296–301. doi: 10.1176/appi.ps.201400535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Willett WC, Sacks F, Trichopoulou A, Drescher G, Ferro-Luzzi A, Helsing E, et al. Mediterranean diet pyramid: a cultural model for healthy eating. Am J Clin Nutr. 1995;61:1402S–6S. doi: 10.1093/ajcn/61.6.1402S. [DOI] [PubMed] [Google Scholar]
- 18.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–76. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 19.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR). Arlington: American Psychiatric Publishing; 2000. [Google Scholar]
- 20.Development of the World Health Organization WHOQOL-BREF quality of life assessment The WHOQOL Group. Psychol Med. 1998;28:551–8. doi: 10.1017/s0033291798006667. [DOI] [PubMed] [Google Scholar]
- 21.De Girolamo G, Rucci P, Scocco P, Becchi A, Coppa F, D'Addario A, et al. [Quality of life assessment: validation of the Italian version of the WHOQOL-Brief]. Epidemiol Psichiatr Soc. 2000;9:45–55. doi: 10.1017/s1121189x00007740. [DOI] [PubMed] [Google Scholar]
- 22.Ware JE, Snow KK, Kosinski M, Gandek B. SF-36 health survey manual and interpretation guide. Boston: New England Medical Center; 1993. [Google Scholar]
- 23.Apolone G, Mosconi P. The Italian SF-36 Health Survey: translation, validation and norming. J Clin Epidemiol. 1998;51:1025–36. doi: 10.1016/s0895-4356(98)00094-8. [DOI] [PubMed] [Google Scholar]
- 24.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 25.Han TS, van Leer EM, Seidell JC, Lean ME. Waist circumference action levels in the identification of cardiovascular risk factors: prevalence study in a random sample. BMJ. 1995;311:1401–5. doi: 10.1136/bmj.311.7017.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bollen KA. New York: John Wiley & Sons; 2014. Structural equations with latent variables. [Google Scholar]
- 27.Hancock GR, Mueller RO. Structural equation modeling: a second course. 2nd ed. Charlotte: Information Age Publishing; 2013. [Google Scholar]
- 28.Enders CK. Applied missing data analysis. New York: The Guilford Press; 2010. [Google Scholar]
- 29.American Diabetes Association; American Psychiatric Association; American Association of Clinical Endocrinologists; North American Association for the Study of Obesity Consensus development conference on antipsychotic drugs and obesity and diabetes. J Clin Psychiatry. 2004;65:267–72. doi: 10.4088/jcp.v65n0219. [DOI] [PubMed] [Google Scholar]
- 30.Fairchild AJ, MacKinnon DP. A general model for testing mediation and moderation effects. Prev Sci. 2009;10:87–99. doi: 10.1007/s11121-008-0109-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khazaal Y, Fresard E, Rabia S, Chatton A, Rothen S, Pomini V, et al. Cognitive behavioural therapy for weight gain associated with antipsychotic drugs. Schizophr Res. 2007;91:169–77. doi: 10.1016/j.schres.2006.12.025. [DOI] [PubMed] [Google Scholar]
- 32.Skrinar GS, Huxley NA, Hutchinson DS, Menninger E, Glew P. The role of a fitness intervention on people with serious psychiatric disabilities. Psychiatr Rehabil J. 2005;29:122–7. doi: 10.2975/29.2005.122.127. [DOI] [PubMed] [Google Scholar]
- 33.Weber M, Wyne K. A cognitive/behavioral group intervention for weight loss in patients treated with atypical antipsychotics. Schizophr Res. 2006;83:95–101. doi: 10.1016/j.schres.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 34.Manu P, Dima L, Shulman M, Vancampfort D, De Hert M, Correll CU. Weight gain and obesity in schizophrenia: epidemiology, pathobiology, and management. Acta Psychiatr Scand. 2015;132:97–108. doi: 10.1111/acps.12445. [DOI] [PubMed] [Google Scholar]
- 35.Bruins J, Jörg F, Bruggeman R, Slooff C, Corpeleijn E, Pijnenborg M. The effects of lifestyle interventions on (long-term) weight management, cardiometabolic risk and depressive symptoms in people with psychotic disorders: a meta-analysis. PLoS One. 2014;9:e112276. doi: 10.1371/journal.pone.0112276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kalarchian MA, Marcus MD, Levine MD, Haas GL, Greeno CG, Weissfeld LA, et al. Behavioral treatment of obesity in patients taking antipsychotic medications. J Clin Psychiatry. 2005;66:1058–63. doi: 10.4088/jcp.v66n0815. [DOI] [PubMed] [Google Scholar]
- 37.Mann T, Tomiyama AJ, Westling E, Lew AM, Samuels B, Chatman J. Medicare's search for effective obesity treatments: diets are not the answer. Am Psychol. 2007;62:220–33. doi: 10.1037/0003-066X.62.3.220. [DOI] [PubMed] [Google Scholar]
- 38.Theisen FM, Beyenburg S, Gebhardt S, Kluge M, Blum WF, Remschmidt H, et al. A prospective study of body weight and serum leptin levels in patients treated with topiramate. Clin Neuropharmacol. 2008;31:226–30. doi: 10.1097/WNF.0b013e318157c5ce. [DOI] [PubMed] [Google Scholar]
- 39.Gearing RE, Schwalbe CS, Lee R, Hoagwood KE. The effectiveness of booster sessions in CBT treatment for child and adolescent mood and anxiety disorders. Depress Anxiety. 2013;30:800–8. doi: 10.1002/da.22118. [DOI] [PubMed] [Google Scholar]
- 40.Maj M. Physical health care in persons with severe mental illness: a public health and ethical priority. World Psychiatry. 2009;8:1–2. doi: 10.1002/j.2051-5545.2009.tb00196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bobes J, Garcia-Portilla MP, Bascaran MT, Saiz PA, Bousoño M. Quality of life in schizophrenic patients. Dialogues Clin Neurosci. 2007;9:215–26. doi: 10.31887/DCNS.2007.9.2/jbobes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ratliff JC, Palmese LB, Tonizzo KM, Chwastiak L, Tek C. Contingency management for the treatment of antipsychotic-induced weight gain: a randomized controlled pilot study. Obes Facts. 2012;5:919–27. doi: 10.1159/000345975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Desroches S, Lapointe A, Ratté S, Gravel K, Légaré F, Turcotte S. Interventions to enhance adherence to dietary advice for preventing and managing chronic diseases in adults. Cochrane Database Syst Rev. 2013;2:CD008722. doi: 10.1002/14651858.CD008722.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen CK, Chen YC, Huang YS. Effects of a 10-week weight control program on obese patients with schizophrenia or schizoaffective disorder: a 12-month follow up. Psychiatry Clin Neurosci. 2009;63:17–22. doi: 10.1111/j.1440-1819.2008.01886.x. [DOI] [PubMed] [Google Scholar]
- 45.Maciejewski ML, Patrick DL, Williamson DF. A structured review of randomized controlled trials of weight loss showed little improvement in health-related quality of life. J Clin Epidemiol. 2005;58:568–78. doi: 10.1016/j.jclinepi.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 46.Leblanc V, Hudon AM, Royer MM, Corneau L, Dodin S, Bégin C, et al. Differences between men and women in dietary intakes and metabolic profile in response to a 12-week nutritional intervention promoting the Mediterranean diet. J Nutr Sci. 2015;4:e13. doi: 10.1017/jns.2015.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bédard A, Riverin M, Dodin S, Corneau L, Lemieux S. Sex differences in the impact of the Mediterranean diet on cardiovascular risk profile. Br J Nutr. 2012;108:1428–34. doi: 10.1017/S0007114511006969. [DOI] [PubMed] [Google Scholar]
- 48.Bédard A, Tchernof A, Lamarche B, Corneau L, Dodin S, Lemieux S. Effects of the traditional Mediterranean diet on adiponectin and leptin concentrations in men and premenopausal women: do sex differences exist? Eur J Clin Nutr. 2014;68:561–6. doi: 10.1038/ejcn.2014.27. [DOI] [PubMed] [Google Scholar]
- 49.Brown S, Chan K. A randomized controlled trial of a brief health promotion intervention in a population with serious mental illness. J Ment Health. 2006;15:543–9. [Google Scholar]
- 50.Haddad PM, Brain C, Scott J. Nonadherence with antipsychotic medication in schizophrenia: challenges and management strategies. Patient Relat Outcome Meas. 2014;5:43–62. doi: 10.2147/PROM.S42735. [DOI] [PMC free article] [PubMed] [Google Scholar]