Abstract
Background
There is a substantial body of evidence that prescribing for care home residents is suboptimal and requires improvement. Consequently, there is a need to identify effective interventions to optimise prescribing and resident outcomes in this context. This is an update of a previously published review (Alldred 2013).
Objectives
The objective of the review was to determine the effect of interventions to optimise overall prescribing for older people living in care homes.
Search methods
For this update, we searched the Cochrane Central Register of Controlled Trials (CENTRAL) (including the Cochrane Effective Practice and Organisation of Care (EPOC) Specialised Register), MEDLINE, EMBASE and CINAHL to May 2015. We also searched clinical trial registries for relevant studies.
Selection criteria
We included randomised controlled trials evaluating interventions aimed at optimising prescribing for older people (aged 65 years or older) living in institutionalised care facilities. Studies were included if they measured one or more of the following primary outcomes: adverse drug events; hospital admissions; mortality; or secondary outcomes, quality of life (using validated instrument); medication‐related problems; medication appropriateness (using validated instrument); medicine costs.
Data collection and analysis
Two authors independently screened titles and abstracts, assessed studies for eligibility, assessed risk of bias and extracted data. We presented a narrative summary of results.
Main results
The 12 included studies involved 10,953 residents in 355 (range 1 to 85) care homes in ten countries. Nine studies were cluster‐randomised controlled trials and three studies were patient‐randomised controlled trials. The interventions evaluated were diverse and often multifaceted. Medication review was a component of ten studies. Four studies involved multidisciplinary case‐conferencing, five studies involved an educational element for health and care professionals and one study evaluated the use of clinical decision support technology. We did not combine the results in a meta‐analysis due to heterogeneity across studies. Interventions to optimise prescribing may lead to fewer days in hospital (one study out of eight; low certainty evidence), a slower decline in health‐related quality of life (one study out of two; low certainty evidence), the identification and resolution of medication‐related problems (seven studies; low certainty evidence), and may lead to improved medication appropriateness (five studies out of five studies; low certainty evidence). We are uncertain whether the intervention improves/reduces medicine costs (five studies; very low certainty evidence) and it may make little or no difference on adverse drug events (two studies; low certainty evidence) or mortality (six studies; low certainty evidence). The risk of bias across studies was heterogeneous.
Authors' conclusions
We could not draw robust conclusions from the evidence due to variability in design, interventions, outcomes and results. The interventions implemented in the studies in this review led to the identification and resolution of medication‐related problems and improvements in medication appropriateness, however evidence of a consistent effect on resident‐related outcomes was not found. There is a need for high‐quality cluster‐randomised controlled trials testing clinical decision support systems and multidisciplinary interventions that measure well‐defined, important resident‐related outcomes.
Plain language summary
Interventions to optimise prescribing for older people in care homes
Background
Older people living in care homes (also called nursing homes, residential homes, skilled‐nursing facilities, assisted‐living facilities or aged‐care facilities) have many complex physical and mental health problems. Care home residents are prescribed many medicines compared to people who live in their own homes, with an average of eight medicines being common. International research has shown that these medicines are often not well managed, with some residents prescribed medicines inappropriately. This has the potential to lead to harmful side effects and a loss of benefit. For these reasons, it is important to make sure that care home residents are prescribed the right medicines at the right doses. This is an update of a previously published review (Alldred 2013).
Study characteristics
We found 12 studies involving 10,953 residents in 355 care homes in ten countries that evaluated interventions to optimise prescribing for care home residents. Most of the interventions had several components, often involving a review of medicines with a pharmacist and doctor. Some interventions included a teaching component and one study used Information Technology (IT).
Key results
We found no evidence of benefit of the interventions with respect to reducing adverse drug events (harmful effects caused by medicines) or death. One study led to residents having fewer days in hospital; however, the majority of studies did not show a benefit in relation to reducing hospital admissions. One study led to a slower decline in health‐related quality of life. Problems relating to medicines were found and addressed through the interventions used in the studies. Prescribing was improved based on criteria used to assess the appropriateness of prescribing in five studies.
Certainty of the evidence
We judged the overall quality of the evidence for the reported outcomes to be low for adverse drug events (harmful effects caused by medicines), hospital admissions, death, quality‐of‐life, medication‐related problems, medication appropriateness, and very low for the cost of medicines. More high‐quality studies need to be done to gather more evidence for these and other types of interventions. Further studies are needed to evaluate new technologies, including computer systems that support prescribing decisions. More work needs to be done to make sure that researchers are consistently measuring outcomes that are important to care home residents.
Summary of findings
for the main comparison.
| Interventions to optimise prescribing compared with usual GP care for care home residents | |||
|
Patient or population: older people (aged 65 years or older) living in care homes Settings: Institutionalised care facilities in Australia, Finland, Israel, Netherlands, New Zealand, Spain, Sweden, United Kingdom, and USA and Canada Intervention: Intervention to optimise prescribing (single or multicomponent intervention) Comparison: Usual care by general practitioner | |||
| Outcomes | Impact | No of Participants (studies) | Quality of the evidence (GRADE) |
| Adverse drug events | There was no evidence of an effect on adverse drug events | 1228 in 87 care homes (2 studies) | ⊕⊕⊝⊝ low |
| Hospital admissions | It is uncertain whether medication review reduces hospital admissions | 7606 in 309 care homes (8 studies) | ⊕⊕⊝⊝ low |
| Mortality | There was no evidence of an effect on mortality | 6805 in 188 care homes (6 studies) | ⊕⊕⊝⊝ low |
| Quality of life | It is uncertain whether medication review improves quality of life | 586 in 21 care homes (2 studies) | ⊕⊕⊝⊝ low |
| Medication‐related problems | Medication review may lead to the identification and resolution of medication‐related problems | 6640 in 251 care homes (7 studies) | ⊕⊕⊝⊝ low |
| Medication appropriateness | Medication review may lead to an improvement in medication appropriateness | 1566 in 152 care homes (5 studies) | ⊕⊕⊝⊝ low |
| Medicine costs | It is uncertain whether medication review decreases medication costs | 4734 in 142 care homes (5 studies) | ⊕⊝⊝⊝ very low |
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||
Quality assessment of evidence for each outcome was based on study design, risk of bias, inconsistency, indirectness and imprecision. The evidence was downgraded from high to low for adverse drug events (Crotty 2004b; Gurwitz 2008) due to a serious risk of bias and imprecision. The evidence was downgraded from high to low for hospital admissions (Furniss 2000; Roberts 2001; Crotty 2004b; Zermansky 2006; Frankenthal 2014; Garcia‐Gollarte 2014; Pitkala 2014; Connolly 2015), mortality (Furniss 2000; Roberts 2001; Zermansky 2006; Frankenthal 2014; Pitkala 2014; Connolly 2015), quality of life (Frankenthal 2014; Pitkala 2014) and medication appropriateness (Crotty 2004a; Crotty 2004b; Frankenthal 2014; Garcia‐Gollarte 2014; Pitkala 2014) due to a serious risk of bias and inconsistency. The evidence for medicines costs (Furniss 2000; Roberts 2001; Crotty 2004a; Zermansky 2006; Frankenthal 2014 was downgraded from high to very low due to a serious risk of bias, inconsistency and imprecision. The evidence for medicine‐related problems (Strikwerda 1994; Claesson 1998; Furniss 2000; Roberts 2001; Crotty 2004b; Zermansky 2006; Frankenthal 2014 was reduced from high to low due to design, risk of bias and imprecision.
Background
Globally, the proportion of older people in the population is increasing. The proportion of people aged 60 years and over was 11% in 2009 and this is projected to double by the middle of this century (United Nations 2009), with developed countries experiencing the fastest rise in number of older people. In the United Kingdom (UK), it is estimated that by 2034 nearly a quarter of the population will be aged 65 years and over. The most rapid rise has been in the 'oldest old' that is those aged 85 years and over; it is projected that by 2034 there will be a 2.5 fold increase in the number of the oldest old, representing 5% of the population (Office for National Statistics 2010). As a consequence, there will continue to be an increasing demand for long‐term care across the world.
Long‐term care may be provided in people's homes or in institutional facilities such as nursing homes or hospitals. The terminology used to describe homes that provide care for older people (defined as 65 years or older (Department of Health 2001)) differs across the world. In the UK the homes are known as 'care homes', in the United States (US) 'long‐term care facilities' and in Australia 'aged‐care facilities'. Care homes are usually classified into two main categories, those that provide 24‐hour nursing care (nursing homes in the UK, skilled‐nursing facilities in the US and aged‐care facilities providing high‐level care in Australia); and those that provide personal care (residential homes in the UK, assisted‐living in the US and aged‐care facilities providing low‐level care in Australia). Some care homes provide both types of care.
Older people living in care homes are often frail, and they are one of the most vulnerable groups in society. They have complex health needs due to multiple co‐morbidities and age‐related changes in pharmacokinetics and pharmacodynamics (Armour 2002). Polypharmacy, usually defined as greater than four or more medicines (Department of Health 2001; Rollason 2003; Patterson 2014), is common in this setting across the world with residents prescribed an increasing number of medicines over the last decade or so. In the UK, the mean number of medicines prescribed per resident was 4.9 in 1998 (Furniss 2000), 6.9 in 2003 (Zermansky 2006), and by 2007 this had risen to 8.0 (Barber 2009). Many care home residents also have cognitive impairment and this can impede their ability to communicate medicine‐related problems (Matthews 2002; Alldred 2007a).
The complexity of prescribing for this population is compounded by multiple clinicians prescribing. This may involve family physicians and community‐based consultants (for example old age psychiatrists and geriatricians) in primary care; and secondary care doctors from multiple specialities. In addition, the lack of representation of older people in clinical trials limits the evidence base and further increases the complexity (Beglinger 2008). It is, therefore, perhaps unsurprising that there is extensive evidence that prescribing is suboptimal for care home residents. Inappropriate prescribing, measured using validated, explicit and implicit definitions, has been found to be common in nursing and residential homes in several countries including the US (Beers 1992; Hanlon 1996; Sloane 2002; Gray 2003; Lau 2005; Perri 2005), Canada (Brymer 2003), the UK (Oborne 2003) and Australia (Crotty 2004a).
Perri 2005 found that over a one month duration, 47% of 1117 residents of 15 US nursing homes received at least one inappropriate medicine, with 13% of residents having at least one adverse health outcome. Inappropriate prescribing more than doubled the risk of a resident experiencing at least one adverse health outcome (odds ratio (OR) 2.34, 95% confidence interval (CI) 1.61 to 3.40). Lau 2005 reported that 50% of 3372 US nursing home residents were prescribed at least one inappropriate medicine over one year. The risks of hospitalisation and death were greater in those residents exposed to an inappropriate medicine (OR 1.27, 95% CI 1.09 to 1.47; OR 1.28, 95% CI 1.05 to 1.55, respectively). Gray 2003 found that 22% of 282 US residents of residential care facilities were prescribed at least one inappropriate medicine. There is also evidence that care home residents are under‐prescribed beneficial drugs and are poorly monitored with respect to their long‐term conditions and their medicines (Fahey 2003; Alldred 2007b; Barber 2009).
For the reasons discussed above, care home residents are particularly susceptible to adverse drug events. In two US long‐term care facilities, Gurwitz 2005 found 9.8 adverse drug events per 100 resident‐months, with 42% being judged as preventable. Drug‐related problems have been found to be responsible for 3% to 31% of hospital admissions of older people, and up to half of these are potentially avoidable (Howard 2007).
This is an update of a previously published review (Alldred 2013).
Description of the condition
As described above, suboptimal prescribing for older people living in care homes is common and may occur due to the prescribing of inappropriate medicines, the omission of beneficial medicines or the failure to appropriately monitor residents and the effects of their medicines. There are a variety of instruments that can be employed to measure the appropriateness of prescribing in older people (Spinewine 2007). However, the predictive validity of these instruments on health outcomes such as adverse drug events and hospital admissions has not been unequivocally established (Spinewine 2007).
Description of the intervention
For this review, we were interested in interventions concerned with optimising the whole medication regime for care home residents, not those concentrating solely on isolated drugs or classes such as benzodiazepines or antipsychotics nor those concentrating on one disease state. Financial and regulatory interventions tend to fall into this latter category.
There are several types of interventions that can potentially optimise prescribing in this setting, including:
professional interventions, for example educational programmes aimed at prescribers
organisational interventions, for example medication review services or specialist clinics, case conferencing, information and communication technology (ICT) interventions such as clinical decision support systems.
Medication review interventions may be aimed at specific drugs or the whole regime and can be uni‐ or multiprofessional, involving physicians, nurses and pharmacists.
How the intervention might work
Interventions designed to improve prescribing for care home residents may have an impact by discontinuing inappropriate medication; commencing beneficial medicines; and ensuring appropriate monitoring of long‐term conditions and medicines. Consequently, this may lead to a reduction in adverse drug events, improved quality of life and a reduction in medicine costs.
Why it is important to do this review
There is a substantial body of evidence that prescribing for care home residents is suboptimal and requires improvement. Furthermore, there are other Cochrane reviews being undertaken which address similar issues in different populations (Soe 2009; Christensen 2011). We evaluated the evidence for interventions to address suboptimal prescribing in this setting to identify how care can be improved for this frail and vulnerable population. We intended to achieve this by determining which interventions were effective and by identifying gaps in the evidence to inform future research.
Objectives
The objective of the review was to determine the effect of interventions to optimise overall prescribing for older people living in care homes.
Methods
Criteria for considering studies for this review
Types of studies
We included patient‐randomised controlled trials (patient‐RCT) and cluster‐randomised controlled trials (cluster‐RCT).
Types of participants
We included studies of older people (aged 65 years or older) living in institutionalised care facilities. Institutionalised care facilities include: nursing homes and residential homes (UK); skilled‐nursing facilities and assisted‐living facilities (US); and aged‐care facilities providing low‐level and high‐level care (Australia). If there was any ambiguity in the description of the institution, we clarified this with the authors of relevant papers. We considered trials for inclusion if they had a majority (80% or more) of participants aged 65 years or more, or if the mean age was greater than 65 years.
We excluded studies where the intervention focused on a single medical condition or a specific drug or class of drugs. We also excluded studies where the main focus was to reduce medication errors because such studies have a narrow focus and do not consider the whole medication regime. In addition, they do not seek to optimise prescribing, for example by adhering to evidence‐based guidelines or by reducing inappropriate prescribing, but are designed solely to reduce errors.
Types of interventions
We assessed interventions aimed at optimising prescribing for care home residents compared with usual care as defined by the study. These interventions potentially included: educational interventions aimed at prescribers; medication review services (uni‐ or multiprofessional, conducted by nurses, pharmacists or physicians); case conferencing; and ICT interventions such as clinical decision support systems. We excluded financial and regulatory interventions.
Types of outcome measures
We included a range of outcome measures including patient‐related outcomes, health service utilisation, and economic outcomes. Studies were included if they reported at least one primary outcome measure or at least one secondary outcome measure.
Primary outcomes
The primary outcome measures for the review were:
adverse drug events;
hospital admissions;
mortality.
Secondary outcomes
Secondary outcome measures were:
quality of life (using validated instrument);
medication‐related problems;
medication appropriateness (using validated instrument);
medicine costs.
Search methods for identification of studies
Paul Miller, Trials Search Co‐ordinator (TSC) for Cochrane Effective Practice and Organisation of Care (EPOC) updated the search terms used previously and conducted searches of the following electronic databases on 14 May 2015. Searches were limited by date to material published between 2012 and the search date.
Electronic searches
Cochrane Central Register of Controlled Trials (CENTRAL) 2015, Issue 4, part of The Cochrane Library. www.cochranelibrary.com, (including Cochrane Effective Practice and Organisation of Care (EPOC) Specialised Register)
MEDLINE In‐Process and Other Non‐Indexed Citations and Ovid MEDLINE 1946 to present, OvidSP
EMBASE 1996 to 2015 Week 19, OvidSP
CINAHL (Cumulative Index to Nursing and Allied Health Literature), 1981 to present, EbscoHost
Search strategies were comprised of keywords and, when available, controlled vocabulary such as MeSH (Medical Subject Headings). We applied no language restrictions. See Appendix 1 for strategies used in this update.
Searching other resources
We searched the following trial registries for relevant studies on 18 May 2015:
International Clinical Trials Registry Platform (ICTRP), World Health Organization (WHO) http://www.who.int/ictrp/en/
ClinicalTrials.gov, US National Institutes of Health (NIH) http://clinicaltrials.gov/
For search terms used in this update and number of results, see Appendix 2
We also contacted authors of relevant studies to clarify reported published information.
Data collection and analysis
Selection of studies
Two review authors (DPA and MCK) independently screened titles and abstracts to decide which studies met the inclusion criteria. Any papers not meeting the inclusion criteria were excluded at this stage. If there was uncertainty or disagreement, consensus was reached by discussion with co‐review authors. Two review authors (DPA and MCK) independently assessed the full text articles to ensure they still met the inclusion criteria. Full text articles not published in English were translated prior to being assessed for inclusion.
Data extraction and management
Two review authors (DPA and MCK) independently extracted details of articles included in the review, including the study design, the study population, the intervention, usual care, outcome measures used and length of follow‐up data, using a specially designed data extraction form based on the EPOC template (EPOC 2013). Where necessary, we contacted authors for missing information or clarification. We intended to use information from the data extraction forms to guide extraction of numerical data for meta‐analysis in Review Manager (RevMan) 5.3 (RevMan 2014).
Assessment of risk of bias in included studies
Two review authors (DPA and MCK) assessed the internal validity of each included study. We used the Cochrane Collaboration’s tool for assessing risk of bias (Higgins 2011) based on six standard criteria: adequate sequence generation; concealment of allocation; blinded or objective assessment of primary outcome(s); adequately addressed incomplete outcome data; freedom from selective reporting; freedom from other risk of bias. We used four additional criteria specified by EPOC (EPOC 2015): similar baseline outcome measurements; similar baseline characteristics; reliable primary outcome measures; and adequate protection against contamination. We made judgements as to whether studies were at low risk, high risk or unclear risk of bias and reported all included studies in the Cochrane ‘Risk of bias’ tables.
Measures of treatment effect
We initially planned to conduct a meta‐analysis, however, this was not possible due to heterogeneity (see Results). Therefore, we presented a narrative summary of the results. Wherever possible, we presented results with 95% confidence intervals.
Unit of analysis issues
We critically examined the methods of analysis of all study types. We identified cluster‐RCTs with unit of analysis errors (for example, randomisation by care home with analysis by residents without adjustments for clustering) and where appropriate, commented on unit of analysis errors in the results and discussion.
Dealing with missing data
We intended to exclude studies from a meta‐analysis if there was differential loss to follow‐up between groups, greater than 20%. However, as meta‐analysis was not appropriate, this did not apply.
Assessment of heterogeneity
See Data synthesis section.
Assessment of reporting biases
We intended to examine funnel plots corresponding to meta‐analysis of the primary outcome in order to assess the potential for small study effects such as publication bias. However, this was not possible as meta‐analysis was not undertaken.
Data synthesis
We intended to synthesise the results of the studies depending on their quality, design and heterogeneity, and we intended to pool the results of studies if at least two studies were homogeneous regarding the participants, interventions and outcomes. As stated above, this was not possible and, therefore, we presented a narrative summary. We described studies according to setting, type of intervention and study design together with an assessment of the evidence on the theoretical basis for each of the approaches described.
Certainty of the evidence
We assessed the certainty of the evidence for the main comparison using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) criteria (GRADE 2012) and presented our judgements in a 'Summary of findings' table. We downgraded the quality of the evidence when there were concerns about the design, risk of bias, inconsistency, indirectness and/or imprecision. One author (DPA) made the judgements informed by the previous version of the review (Alldred 2013) and this was agreed by a second author (MCK).
Subgroup analysis and investigation of heterogeneity
We intended to conduct subgroup analyses for professional and organisational interventions where possible. If we had found that one type of intervention was common, for example medication review, we intended to analyse this separately. If possible, we also planned to undertake subgroup meta‐analyses based on the specific nature of the intervention, for example pharmacist‐led medication review. However, subgroup analyses were not possible due to heterogeneity.
See Data synthesis section for the investigation of heterogeneity.
Sensitivity analysis
We intended to perform sensitivity analysis for pooled results based on the risk of bias. However, as we could not pool results this did not apply.
Results
Description of studies
We included 12 studies evaluating the effectiveness of interventions to optimise overall prescribing for older people living in care homes. See: Characteristics of included studies.
Results of the search
The searches identified 1469 articles for potential inclusion. Following independent screening of titles and abstracts by DPA and MCK, nine full‐text articles were assessed for eligibility and four new studies (Frankenthal 2014; Garcia‐Gollarte 2014; Pitkala 2014; Connolly 2015) met the inclusion criteria . Three studies are ongoing (Desborough ongoing; NCT02238652; Wouters ongoing) and two were excluded (Lapane 2011; Milos 2013). See PRISMA flowchart Figure 1 for details (Liberati 2009). The search yielded five related systematic reviews (Kaur 2009; Ostini 2009; Verrue 2009; LaMantia 2010; Loganathan 2011) and one narrative review (Markum 2010) and their references were reviewed along with the references from the included studies; we did not identify any further studies from these.
1.
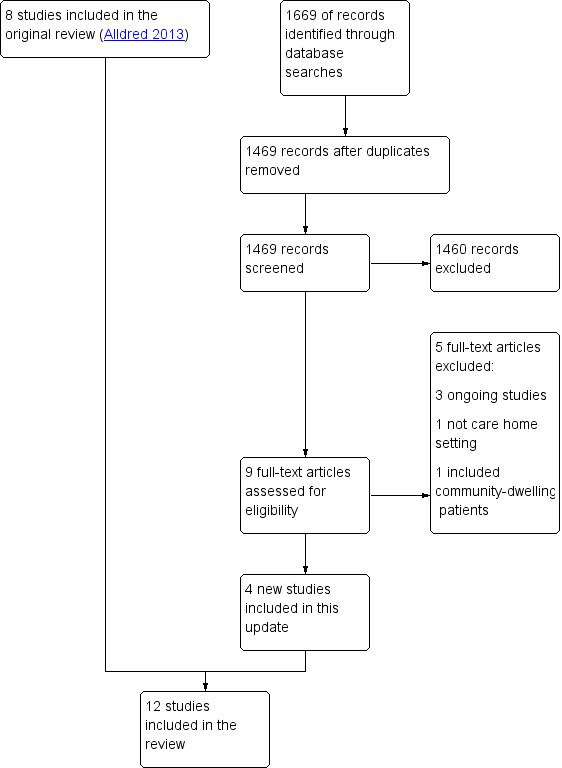
Study flow diagram.
Included studies
The 12 included studies involved 10,953 residents in 355 (range 1 to 85) care homes. Three studies were conducted in Australia (Roberts 2001; Crotty 2004a; Crotty 2004b), two in the UK (Furniss 2000; Zermansky 2006), one in Sweden (Claesson 1998), one in the Netherlands (Strikwerda 1994), one in the USA and Canada (Gurwitz 2008), one in New Zealand (Connolly 2015), one in Israel (Frankenthal 2014), one in Spain (Garcia‐Gollarte 2014) and one in Finland (Pitkala 2014).
Design
Nine studies were cluster‐RCTs (Strikwerda 1994; Claesson 1998; Furniss 2000; Roberts 2001; Crotty 2004a; Gurwitz 2008; Garcia‐Gollarte 2014; Pitkala 2014; Connolly 2015) and three studies were patient‐RCTs (Crotty 2004b; Zermansky 2006; Frankenthal 2014). Two cluster‐RCTs appeared to have made unit of analysis errors in that they did not account for the effect of clustering (Garcia‐Gollarte 2014; Pitkala 2014) and therefore, P values and 95% CIs from these two studies may be over precise.There was a wide range of study duration and follow‐up between the studies, ranging from six weeks to two years (see Table 2).
1. Summary of study characteristics.
| Study,Country, Design | Participants | Intervention | Outcome measures | Duration |
|
Claesson 1998 Sweden Cluster‐RCT |
1854 residents in 33 nursing homes | Multidisciplinary meetings with physician, pharmacist and nurse(s) | Medication‐related problems | 14 months |
|
Connolly 2015 Australia Cluster‐RCT |
1998 residents in 36 nursing homes | Multidisciplinary meetings with study geriatrician, a GP, a pharmacist and a nurse manager. Education of nurses and care‐givers | Hospital admissions Mortality |
14 months |
|
Crotty 2004a Australia Cluster‐RCT |
154 residents in 10 nursing homes | Multidisciplinary case conferencing with GP, a geriatrician, a pharmacist, residential care staff and an Alzheimer's Association representative | Medication Appropriateness Index | 3 months |
|
Crotty 2004b Australia Patient‐RCT |
110 patients discharged to 85 long‐term care facilities | Pharmacist transition co‐ordinator. Transfer of medicines information to nursing staff, family physician and community pharmacist plus medication review and case conferencing | Adverse drug events Hospital admissions Medication‐related problems Medication Appropriateness Index |
8 weeks |
|
Frankenthal 2014 Israel Patient‐RCT |
359 residents in 1 chronic care geriatric facility | Medication review by the study pharmacist | Hospital admissions Mortality Quality of life Medication appropriateness (STOPP‐START) Medication‐related problems Medicine costs |
12 months |
|
Furniss 2000 UK Cluster‐RCT |
330 residents in 14 nursing homes | Medication review by a single pharmacist | Hospital admissions Mortality Medication‐related problems Medicine costs |
8 months |
|
Garcia‐Gollarte 2014 Spain Cluster‐RCT |
716 residents in 36 nursing homes | Physician educational programme followed by on‐demand support (prescription advice) by phone | Hospital admissions (total number of days spent in hospital) Medication appropriateness (STOPP‐START) |
6 months |
|
Gurwitz 2008 USA/Canada Cluster‐RCT |
1118 residents in 29 units in 2 long‐term care facilities | Computerised provider order entry with clinical decision support | Adverse drug events | 12 months |
|
Pitkala 2014 Finland Cluster‐RCT |
227 residents in 20 assisted living facilities | Nurse training and education | Hospital admissions Mortality Health‐related Quality of Life Medication appropriateness (Beer's criteria plus others) |
12 months |
|
Roberts 2001 Australia Cluster‐RCT |
3230 residents in 52 nursing homes | Introduction of new professional role, nurse education and medication review by pharmacists | Hospital admissions Mortality Medication‐related problems Medicine costs |
24 months |
|
Strikwerda 1994 Netherlands Cluster‐RCT |
196 residents in 1 nursing home | Feedback on GP prescribing from community pharmacist | Medication‐related problems | 6 weeks |
|
Zermansky 2006 UK Patient‐RCT |
661 residents in 65 care homes | Medication review by a single pharmacist | Hospital admissions Mortality Medication‐related problems Medicine costs |
6 months |
Participants
All studies involved older people living in care homes (long‐term care facilities). Mean age ranged from 81.2 years (Furniss 2000) to 87.2 years (Gurwitz 2008) and the majority of residents were female (range 59.7% (Crotty 2004a) to 77% (Zermansky 2006)). The study by Roberts 2001 did not report mean age or gender.
Strikwerda 1994 studied 196 residents in one nursing home, Claesson 1998 studied 1854 residents in 33 nursing homes, Crotty 2004a studied 154 residents in 10 high‐level residential facilities, Crotty 2004b studied 110 residents in 85 long‐term care facilities, Furniss 2000 studied 330 residents in 14 nursing homes, Gurwitz 2008 studied 1118 residents in 29 units in two long‐term care facilities, Roberts 2001 studied 3230 residents in 52 nursing homes, Zermansky 2006 studied 661 residents in 65 nursing and residential homes for older people, Frankenthal 2014 studied 359 residents in one chronic care geriatric facility, Garcia‐Gollarte 2014 studied 716 residents in 36 nursing homes, Pitkala 2014 studied 227 residents in 20 assisted living facilities and Connolly 2015 studied 1998 residents in 36 residential aged care facilities.
Interventions
The interventions evaluated were diverse and often multifaceted. Medication review (conducted by various methods) was a component of ten studies (Strikwerda 1994; Claesson 1998; Furniss 2000; Roberts 2001; Crotty 2004a; Crotty 2004b; Zermansky 2006; Frankenthal 2014; Garcia‐Gollarte 2014; Connolly 2015). Four studies involved multidisciplinary case‐conferencing (Claesson 1998; Crotty 2004a; Crotty 2004b; Connolly 2015) and five studies involved an educational element for care home staff (Roberts 2001; Crotty 2004a; Garcia‐Gollarte 2014; Pitkala 2014; Connolly 2015). One study evaluated the use of clinical decision support technology (Gurwitz 2008). Other components of interventions included introducing a new professional role to stakeholders (Roberts 2001) and the transfer of medicines information (Crotty 2004b). Further descriptions of interventions are presented below.
Strikwerda 1994 evaluated the effect of community pharmacist feedback to GPs on their patients' prescriptions over a four‐week period.
Claesson 1998 evaluated the effectiveness of monthly multidisciplinary team meetings between the physician, pharmacist and nurse(s) over 12 months. The aim of the meetings was to discuss and improve the use of drugs. Pharmacists received a total of 65.5 hours of education and training prior to and during the intervention period.
Furniss 2000 investigated the effectiveness of pharmacist‐conducted medication review (in addition to usual care by the GP) versus usual care by the GP. The intervention was a single medication review conducted by one pharmacist with access to medical and nursing home records. No details were provided on the education and training of the pharmacist.
The intervention evaluated by Roberts 2001 had three components: (i) introducing a new professional role and relationship‐building; (ii) nurse education; (iii) medication review by pharmacists holding a postgraduate diploma in clinical pharmacy. Medication reviews were undertaken for a non‐random subsample of 500 residents (total intervention residents 905) selected by nursing staff. Most of the contact between pharmacists and GPs was indirect.
Crotty 2004a evaluated the effectiveness of an 'outreach medication advisory service'. This involved a medication review prepared by the pharmacist, followed by two multidisciplinary case conferences held six to 12 weeks apart (with the GP, geriatrician, pharmacist, care staff and an Alzheimer’s Association of South Australia representative). No details were provided on the education and training of the pharmacist.
Crotty 2004b investigated the effectiveness of a pharmacist transition co‐ordinator for residents who were being discharged from hospital to a long‐term care facility. The intervention focused on the transfer of medicines information to the nursing home staff, GP and the community pharmacist. Following this, a medication review was conducted by the community pharmacist contracted to the care home. In addition, the transition pharmacist co‐ordinated a multidisciplinary case conference 14 to 28 days after transfer involving him or herself, the GP, community pharmacist and a nurse.
Zermansky 2006 evaluated the effectiveness of a clinical medication review (in addition to usual care by the GP) undertaken by a pharmacist who held a post‐graduate clinical pharmacy qualification versus usual care by the GP. The pharmacist reviewed the medicines with the medical and care home records in conjunction with a consultation with the resident (if possible) and a nurse or carer.
The intervention investigated by Gurwitz 2008 was a clinical decision support system in facilities that had computerised provider order entry systems. The clinical decision support system was designed based on previous research on preventable adverse drug events, criteria for suboptimal prescribing in older people and drug‐drug interactions. Warning messages were displayed to prescribers in a pop‐up box in real time when medicines were entered into the computer provider order entry system. Prescribers were free to either act on alerts or ignore them.
Frankenthal 2014 investigated pharmacist‐led medication review using the Screening Tool of Older Persons’ potentially inappropriate Prescriptions (STOPP) and Screening Tool to Alert doctors to Right Treatment (START) criteria (Gallagher 2008) to identify potentially inappropriate prescriptions and potential prescription omissions. The chief physician decided whether to accept these recommendations and implemented changes.
Garcia‐Gollarte 2014 evaluated a structured educational intervention for nursing home physicians. This involved education delivered by an expert nursing home physician on drug use in older patients, medication review and adverse drug reactions. In addition, on‐demand prescribing advice was provided to physicians and educational material provided to participants.
Pitkala 2014 investigated educational information for nurses to recognise harmful medicines and adverse drug events. The nurses were then asked to identify medication‐related problems and highlight these to the physician. In addition, two‐thirds of the physicians received the training.
Connolly 2015 evaluated a multifaceted complex intervention involving: baseline facility assessment and care planning; monitoring and benchmarking of quality‐of‐care indicators; multidisciplinary team meetings including medication review (only 23% of the intervention group received medication review) by geriatrician, nurse specialist, GP, pharmacist and nurse manager; education and clinical coaching for nursing staff and caregivers.
Outcomes
Outcomes were diverse with differing definitions, methods of data collection, varying time points and different reporting methods. Studies reported measures other than those specified for this review and these are listed in the Characteristics of included studies tables.
Primary outcome measures
Adverse drug events
Only two studies specified adverse drug events as an outcome measure (Crotty 2004b; Gurwitz 2008). However, Crotty 2004b did not define adverse drug events. Adverse drug events were the primary outcome measure in the Gurwitz 2008 study and were defined as 'an injury resulting from the use of a drug'; such adverse drug events may have resulted from medication errors or from adverse drug reactions in which there was no error.
Hospital admissions
Eight studies included hospital admissions as an outcome measure (Furniss 2000; Roberts 2001; Crotty 2004b; Zermansky 2006; Frankenthal 2014; Garcia‐Gollarte 2014; Pitkala 2014; Connolly 2015). Furniss 2000 reported hospital admissions as the number of inpatient days. Roberts 2001 reported the proportion of residents hospitalised and Zermansky 2006 reported the mean number of non‐elective hospitalisations per resident. Crotty 2004b grouped together emergency department visits and hospital readmissions. Frankenthal 2014 reported hospital admissions (not defined). Garcia‐Gollarte 2014 reported the total number of days spent in hospital. Pitkala 2014 reported hospital days/per person/per year. Connolly 2015 reported ambulatory sensitive hospitalisations and all acute admissions.
Mortality
Six studies included mortality as an outcome measure (Furniss 2000; Roberts 2001; Zermansky 2006; Frankenthal 2014; Pitkala 2014; Connolly 2015). Furniss 2000 and Zermansky 2006 reported mortality as the number of deaths over eight and six months, respectively. Roberts 2001 reported the proportion of residents who had died over 12 months together with cumulative survival. Frankenthal 2014 reported the number of deaths over 12 months. Pitkala 2014 calculated hazard ratios (HR) using the Cox proportional hazard model. Connolly 2015 calculated the relative risk (RR) of death over the 14 month follow up period.
Secondary outcome measures
Quality of life
Two studies measured quality of life (Frankenthal 2014; Pitkala 2014). Pitkala 2014 used the 15 dimensional instrument of health‐related quality of life (15D) and Frankenthal 2014 used the Medical Outcomes Study 12‐item Short‐form Health survey (SF‐12).
Medication‐related problems
Medication‐related problems from the intervention arms were measured and classified in diverse ways in seven studies. Strikwerda 1994 reported the number of pharmacists' recommendations and described their type. Claesson 1998 described the type and frequency of drug‐related problems along with pharmacists' recommendations. Furniss 2000 measured the number of pharmacist's recommendations, accepted recommendations by the GP, and the number of treatment changes. Reasons were provided for the pharmacist's recommendations. Roberts 2001 measured the number of medicine changes likely to be due to medication review. Crotty 2004b identified medication‐related problems and classified them into categories. Zermansky 2006 measured the number of changes in medication per participant as the primary outcome; pharmacist's recommendations were identified, collated and classified along with GPs' acceptance of the recommendations. Frankenthal 2014 reported the number of recommendations based on the STOPP‐START criteria along with the proportion of recommendations accepted by the physician.
Medication appropriateness
Five studies assessed medication appropriateness using a validated tool (Crotty 2004a; Crotty 2004b; Frankenthal 2014; Garcia‐Gollarte 2014; Pitkala 2014). Crotty 2004a and Crotty 2004b used the Medication Appropriateness Index (MAI) (Hanlon 1992). Frankenthal 2014 and Garcia‐Gollarte 2014 used STOPP‐START (Gallagher 2008). Pitkala 2014 used a composite of Beers criteria, Anticholinergic Risk Scale, > 2 psychotropic medications, nonsteroidal anti‐inflammatory drugs (NSAIDs) and proton pump inhibitors.
Medicine costs
Five studies calculated medicine costs (Furniss 2000; Roberts 2001; Crotty 2004a; Zermansky 2006; Frankenthal 2014). Furniss 2000 calculated drug costs per resident throughout the observation and intervention phases of the study. Roberts 2001 collected yearly drug costs from prescription claims data based on the Australian Pharmaceutical Benefits Scheme. Crotty 2004a calculated monthly drug costs for all regular medicines based on the Australian Pharmaceutical Benefits Scheme. Zermansky 2006 calculated the 28‐day net ingredient cost of repeat medicines per resident. Frankenthal 2014 calculated medication costs per month.
Excluded studies
We excluded four studies (Avorn 1992; Crotty 2004c; Lapane 2011; Milos 2013) and we report reasons for their exclusion in the Characteristics of excluded studies section.
Risk of bias in included studies
Studies were heterogeneous with regard to risk of bias (see Figure 2; Figure 3). Risk of bias is summarised below for each domain.
2.
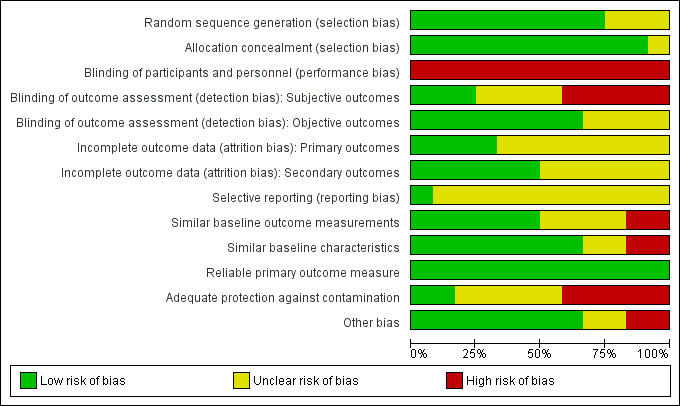
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
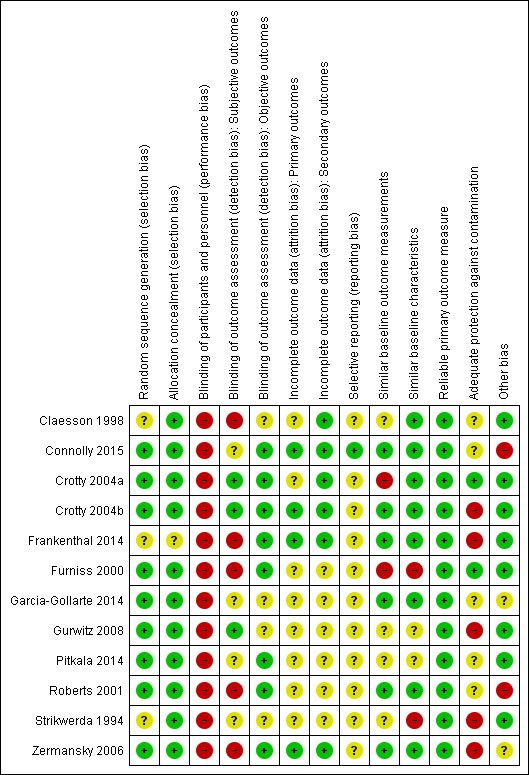
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We judged nine studies to have a low risk of bias based on random sequence generation (Furniss 2000; Roberts 2001; Crotty 2004a; Crotty 2004b; Zermansky 2006; Gurwitz 2008; Garcia‐Gollarte 2014; Pitkala 2014; Connolly 2015;). The studies by Strikwerda 1994, Claesson 1998 and Frankenthal 2014 did not report how the sequence was generated. Seven studies utilised computer‐generated random or pseudo‐random numbers (Furniss 2000; Crotty 2004a; Crotty 2004b; Zermansky 2006; Gurwitz 2008; Pitkala 2014; Connolly 2015;), Roberts 2001 drew from a hat and Garcia‐Gollarte 2014 used random number tables. Allocation was adequately concealed via centralisation in two of the patient‐RCTs (Crotty 2004b; Zermansky 2006),the study by Frankenthal 2014 did not report sufficient information on allocation concealment to permit judgement. Due to the remaining nine studies having a cluster design, we deemed them to be at low risk of bias with regard to allocation concealment (Strikwerda 1994; Claesson 1998; Furniss 2000; Roberts 2001; Crotty 2004a; Gurwitz 2008; Garcia‐Gollarte 2014; Pitkala 2014; Connolly 2015).
Blinding
Due to the nature of the interventions it was not possible to blind participants and personnel in any of the studies and, therefore, we judged performance bias to be high for each study. Three studies blinded outcome assessment for subjective outcomes (Crotty 2004a; Crotty 2004b; Gurwitz 2008) and, therefore, we judged detection bias to be low for these studies. The studies by Strikwerda 1994, Pitkala 2014 and Garcia‐Gollarte 2014 did not report if subjective outcomes were blinded and therefore, the risk was unclear, while the studies by Claesson 1998; Furniss 2000; Roberts 2001; Zermansky 2006; and Frankenthal 2014 we deemed to be high risk. We deemed detection bias to be low for objective outcomes for studies that reported them.
Incomplete outcome data
We judged five studies to be at low risk of attrition bias as they reported similar baseline characteristics with a similar number of dropouts for similar reasons (Crotty 2004a; Crotty 2004b; Zermansky 2006; Frankenthal 2014; Connolly 2015). The only outcome in the Claesson 1998 study was a description of medicine‐related problems in the intervention group and attrition bias was not relevant. We judged the risk of attrition bias to be unclear for six studies due to a lack of information (Strikwerda 1994; Furniss 2000; Roberts 2001; Gurwitz 2008; Garcia‐Gollarte 2014; Pitkala 2014).
Selective reporting
Although there was no evidence of selective reporting in the studies, that is, all outcome measures stated in the methods were reported, research protocols were not available for all but one study (Connolly 2015) and, therefore, we deemed that there was insufficient information to permit judgement for 11 out of the 12 studies. The protocol for Connolly 2015 indicated that the pre‐specified outcomes were reported in the pre‐specified way and, therefore, we judged this to be low risk of bias.
Other potential sources of bias
Similar baseline outcome measurements
We deemed six studies (Roberts 2001; Crotty 2004b; Zermansky 2006; Frankenthal 2014; Garcia‐Gollarte 2014; Connolly 2015) to be at low risk of bias as baseline outcome measurements were similar. We judged Furniss 2000 to be at high risk of bias because there were fewer deaths in the control group compared with the intervention group. We also judged Crotty 2004a to be at a high risk of bias because of baseline differences in the Medication Appropriateness Index. We deemed the study by Pitkala 2014 to be at unclear risk of bias because of baseline outcome measurement differences in health‐related quality of life and the number of harmful medicines; however, these differences were adjusted for in the analysis. We deemed the three remaining studies to be at an unclear risk of bias as outcomes were not measured at baseline (Strikwerda 1994; Claesson 1998; Gurwitz 2008).
Similar baseline characteristics
Eight studies reported similar baseline characteristics and we therefore judged them to be at low risk of bias (Claesson 1998; Roberts 2001; Crotty 2004a; Crotty 2004b; Zermansky 2006; Frankenthal 2014; Garcia‐Gollarte 2014; Connolly 2015). The study by Strikwerda 1994 reported fewer males in group A and fewer medicines in group B compared to group C and we judged this to be at high risk. We deemed the study by Furniss 2000 to be at high risk because in the control group the residents were younger and there were fewer females. We deemed Gurwitz 2008 to be at unclear risk because baseline characteristics of residents were not reported (although units were matched for general characteristics, bed size and general characteristics of residents). We also deemed the study by Pitkala 2014 to be at unclear risk because there was a higher proportion of males, a higher prevalence of 'as‐needed' medications and a higher number of co‐morbidities in the intervention group; however, these differences were adjusted for in the analysis.
Reliable primary outcome measure
We deemed all twelve studies to have reliable primary outcome measures (although not all the outcome measures were included in this review).
Adequate protection against contamination
We assessed five studies that were of a cluster design to be at an unclear risk of adequate protection against contamination because although they were randomised by care home it was unclear whether the healthcare professionals may have moved between intervention and control homes (Claesson 1998; Roberts 2001; Garcia‐Gollarte 2014; Pitkala 2014; Connolly 2015). We deemed the study by Crotty 2004a to be at low risk of contamination because in addition to the cluster design the GPs were checked to avoid contamination between intervention and control residents. We judged the study by Strikwerda 1994 to be at high risk because although residents were randomised by GP they all resided in the same nursing home. Furniss 2000 randomised care homes in different geographical areas and we therefore deemed at low risk of contamination. Gurwitz 2008 attempted to limit the crossover of prescribers between intervention and control units, however some prescribers worked simultaneously on both units and consequently we judged the trial to be at high risk of contamination. We deemed the three studies that were patient‐RCTs to be at high risk as contamination was possible (Crotty 2004b; Zermansky 2006; Frankenthal 2014).
Other bias
The medications reviews undertaken by Roberts 2001 and Connolly 2015 were completed for a non‐random subset of intervention residents; we determined this to have a high risk of bias. Garcia‐Gollarte 2014 measured medication appropriateness for a random subsample of residents, therefore the risk of bias in this study was judged to be unclear.
Effects of interventions
See: Table 1
See Table 1 for the main comparison.
Due to the heterogeneity in interventions, outcomes and risk of bias, it was deemed inappropriate to conduct a meta‐analysis. The effectiveness of the interventions are described below.
Primary outcome measures
Adverse drug events
Crotty 2004b found no evidence of an effect of a pharmacist transition co‐ordinator on adverse drug events (relative risk 1.05, 95% CI 0.66 to 1.68). Gurwitz 2008 tested a clinical decision support system and found no evidence of an effect on all adverse drug events (adjusted rate ratio 1.06, 95% CI 0.92 to 1.23) or preventable adverse drug events (adjusted rate ratio 1.02, 95% CI 0.81 to 1.30).
Hospital admissions
Furniss 2000 found fewer inpatient days per resident in the intervention group compared with the control group during the four‐month intervention phase of the study (0.55 versus 1.26); however, small numbers precluded statistical analysis. In the Roberts 2001 study, no difference was found in the mean proportion of residents hospitalised between the intervention and control groups. Crotty 2004b demonstrated a reduction in the combination of emergency room visits and hospital readmissions with a relative risk ratio of 0.38 (95% CI 0.15 to 0.99) when analysing residents who were alive at follow‐up. When residents who had died were included, there was no evidence of an effect on hospital admissions (relative risk 0.58, 95% CI 0.28 to 1.21). Zermansky 2006 showed no evidence of an effect on the mean number of hospitalisations per resident (relative risk 0.75, 95% CI 0.52 to 1.07). Frankenthal 2014 showed no evidence of an effect on the average number of hospitalisations (intervention 0.5 ± 1.0 vs 0.5 ± 0.9 control, P = 0.10). The study by Garcia‐Gollarte 2014 found a small increase in days in hospital in the control group (+ 0.38 days, P = 0.011) but no difference in the intervention group (+ 0.01 days, P = 0.822). Pitkala 2014 found that residents in the intervention group used fewer hospital days (1.4/person/year, 95% CI 1.2 to 1.6) than control residents (2.3/person/year, 95% CI 2.1 to 2.7) (adjusted RR 0.60, 95% CI 0.49 to 0.75). It is important to note that Garcia‐Gollarte 2014 and Pitkala 2014 committed unit of analysis errors and therefore, P‐values and 95% CIs may be over precise. Connolly 2015 showed no difference in ambulatory sensitive hospitalisations (RR 1.07, 95% CI 0.85 to 1.36) or total acute admissions (RR 1.02, 95% CI 0.83 to 1.26).
Mortality
Furniss 2000 found fewer deaths in the intervention group compared with the control group during the intervention phase of the study (4 versus 14, P = 0.028); however when the observation phase of the study was taken into account, the number of deaths in the control and intervention groups were 28 and 26 (P value not reported), respectively. In the Roberts 2001 study, no difference was found in the mean proportion of residents who had died between the intervention and control groups. A survival analysis found a hazard ratio of 0.85 (95% CI 0.68 to 1.06). Zermansky 2006 showed no evidence of an effect on the number of deaths (relative risk 1.06, 95% CI 0.70 to 1.64). Frankenthal 2014 reported that 15/183 (8.2%) and 17/176 (9.7%) residents died in the intervention and control groups respectively. However, this was not formally analysed as an outcome measure. Pitkala 2014 found no difference in mortality between the intervention and control groups (adjusted HR 1.04, 95% CI 0.79 to 1.36; it should be noted that the 95% CI may be over precise due to unit of analysis error). Connolly 2015 showed no evidence of an effect on mortality (RR 1.11, 95% CI 0.76 to 1.61).
Secondary outcome measures
Quality of life
Frankenthal 2014 found no difference between groups in the physical (P = 0.09) and mental (P = 0.70) components of SF‐12. Pitkala 2014 found that health‐related quality of life declined more slowly in intervention residents (‐0.038, 95% CI –0.054 to ‐0.022) than control residents (‐0.072, 95% CI ‐0.089 to ‐0.055). However, unit of analysis error was identified for this study and therefore, the confidence intervals may be over precise. Breathing, sleeping and speech were the dimensions of 15D that showed differences in favour of the intervention.
Medication‐related problems
Strikwerda 1994 reported that 122 potential medication‐related problems were identified in 61 residents. As a result, nine medicines were discontinued and four medicines had a dose reduction. The most common medication‐related problem was a potential interaction (51, 42%), followed by dose (31, 25%), indication (23, 19%) and duration of the prescription (17, 14%).
Claesson 1998 identified 819 drug‐related problems in 395 residents (2.1 per resident). The most common problem was 'choice of drug' (348, 43%), with the majority of these being inappropriate according to Swedish Medical Product Agency guidelines. Two hundred and seventy‐six (34%) problems were due to 'unclear indication' whereby the team did not know why a drug had been prescribed or the drug had not been adequately re‐evaluated. Ninety per cent (737) of the problems discussed were acted upon, with 368 (45%) resulting in stopping the medicine and 162 (20%) led to a change of medicine. This study evaluated 532 medicine changes with 404 (76%) still in place after a month, 59 (11%) discontinued and previous therapy was restored, and 69 (13%) were difficult to evaluate as partial changes had occurred.
Furniss 2000 made 261 recommendations of which 239 (92%) were accepted by the GP. This resulted in 144 actual treatment changes. Thirty residents did not require a change in therapy, and the mean number of recommendations per resident (for those who needed at least one recommendation) was 2.46 (range 0 to 7). The most common reasons for recommendations were 'indication for the medication no longer present' (85, 33%) and 'safer or more efficacious use of drug' (77, 30%).
Roberts 2001 followed up 137 of the 500 medication reviews conducted and found that 54 (39%) of the residents had changes likely to be due to the review. No further information was provided.
Crotty 2004b identified medicine‐related problems at admission to the long‐term care facility for intervention and control residents. The most common issue classified as a medicine‐related problem by the authors was that a resident had been appointed a new physician. The next most common problems identified were: discrepancy between medication discharge summary and medication (32, 57% intervention; 26, 48% control); precaution with use (18, 32% intervention; 14, 26% control); no indication for medication (18, 32% intervention; 8, 15% control).
In the study by Zermansky 2006, at least one recommendation was made in 256 (77%, 95% CI 73.1 to 81.7) residents, with a mean of 2.3 recommendations per resident. The study made 672 medication‐related recommendations, along with an additional 75 recommendations related to the residents' conditions. The most common recommendation was technical (for example generic switching, amending quantities, removing discontinued items from the repeat prescription) with 225 (30%) recommendations. Following technical reasons, the most common recommendations were to conduct a test to monitor therapy (161, 22%) and to stop a medicine (100, 13%). The GP accepted 565 (76%) of the pharmacist's recommendations and rejected 52 (7%); there was no response to the review or the resident died before the review could be actioned in the remaining cases. The GP actioned 433 (77%) of the accepted recommendations.
Frankenthal 2014 made 327 recommendations in total including 245 in 129 residents based on STOPP and 82 in 65 residents based on START. 82.4% of STOPP recommendations and 92.6% of START recommendations were accepted by the physician.
Medication appropriateness
Crotty 2004a found that, based on the Medication Appropriateness Index (MAI), medication appropriateness improved in the intervention group (MAI mean change 4.1, 95% CI 2.1 to 6.1) compared with the control group (MAI mean change 0.4, 95% CI ‐0.4 to 1.2). MAI scores were higher at baseline for intervention group residents compared with control residents (mean MAI 7.4, 95% CI 4.5 to 10.3 versus 4.1, 95% CI 2.4 to 5.7). There were no baseline differences in mean MAI scores between the control (3.7, 95% CI 2.2 to 5.2) and intervention groups (3.2, 95% CI 1.8 to 4.6) in the Crotty 2004b study. Following the intervention, there was no change in MAI in the intervention group (2.5, 95% CI 1.4 to 3.7) whereas the MAI in the control group had worsened (6.5, 95% CI 3.9 to 9.1). The effect of the intervention on MAI scores remained when controlled for baseline MAI, Charlson Comorbidity Index and the number of drugs discontinued during hospital admission.
Based on STOPP‐START criteria at six months' follow‐up, Frankenthal 2014 found a reduction in potentially inappropriate prescriptions (37.4% intervention vs 56% control, P < 0.01) and potential prescribing omissions (9.2% intervention vs 25.2% control, P < 0.01) in intervention residents at six months' follow‐up and this was sustained at 12 months.
Garcia‐Gollarte 2014 evaluated medication appropriateness using STOPP‐START criteria in a random subsample of 411 residents (200 control, 211 intervention). At follow‐up, the mean number of inappropriate drugs was lower in the intervention group than the control group (0.81 ± 1.13 vs 1.29 ± 1.56) with a decrease from baseline in the intervention group (P < 0.01) and an increase from the baseline in the control group (P < 0.01). The proportion of participants without potentially inappropriate prescriptions increased in the intervention group (33.2% at baseline vs 56.4% at follow‐up), as opposed to the control group where there was no change (37.6% at baseline vs 38.7% at follow up). Potential prescribing omissions decreased in the intervention group (0.91 ± 1.19 at baseline vs 0.13 ± 0.44 at follow up) whereas there was no change in the control group. As noted for this study previously, Garcia‐Gollarte 2014 appeared to commit a unit of analysis error and therefore, P values and confidence intervals may be over precise.
Pitkala 2014 found no change in the prevalence of harmful medication use in control residents (3.4%, 95% CI ‐3.7 to 10.6) at follow‐up, however there was a decrease in the intervention group (‐11.7, 95% CI ‐20.5 to ‐2.9). Similarly, there was a decrease in the mean number of harmful medicines in intervention residents (‐0.43, 95% CI ‐0.15 to ‐0.71) but no corresponding change in control residents (0.11, 95% CI ‐0.09 to 0.31). It should again be noted that unit of analysis error was identified in this study and therefore, confidence intervals may be over precise.
Medicine costs
The cost of medicines per resident in the observation phase of Furniss 2000 was GB Pounds (GBP) 142.53 in the control group and GBP 159.01 in the intervention group. Following the intervention phase, costs were GBP 141.24 in the control group versus GBP 131.54 in the intervention group, representing a reduction in medicine costs of GBP 27.47 per resident over a four‐month period. Accounting for the pharmacist’s time, the cost saving on medicines in the intervention group was calculated to be GBP 22 per resident. Roberts 2001 calculated a drug cost saving of Australian Dollars (AUD) 64 per resident per year in the intervention group compared to the control group. When the cost of the intervention was accounted for, the net cost saving was AUD 16 per resident per year. Crotty 2004a found no difference in mean medicine costs per month per resident between the intervention and control groups (mean change AUD 5.72 intervention vs AUD 3.37 control, P = 0.837). Zermansky 2006 reported little difference on the cost of 28 days' repeat medicines per resident (mean difference GBP ‐0.70, 95% CI GBP ‐7.28 to GBP 5.71). Frankenthal 2014 demonstrated a reduction in the average monthly medication costs in the intervention group at follow‐up compared to baseline (382.7 ± 279.3 at baseline vs 279 ± 171.9 at follow‐up, Israeli New Shekel (ILS), P < 0.01), with a difference between the intervention group and control group at follow up (279 ± 171.9 vs 402.3 ± 291.2, ILS, P < 0.01).
Certainty of the evidence
The overall quality/certainty of the evidence for the outcomes reported was judged to be low or very low, see: Table 1. The evidence was downgraded from high to low for adverse drug events (Crotty 2004b; Gurwitz 2008) due to a serious risk of bias and imprecision. The evidence was downgraded from high to low for hospital admissions (Furniss 2000; Roberts 2001; Crotty 2004b; Zermansky 2006; Frankenthal 2014; Garcia‐Gollarte 2014; Pitkala 2014; Connolly 2015), mortality (Furniss 2000; Roberts 2001; Zermansky 2006; Frankenthal 2014; Pitkala 2014; Connolly 2015), quality of life (Frankenthal 2014; Pitkala 2014) and medication appropriateness (Crotty 2004a; Crotty 2004b; Frankenthal 2014; Garcia‐Gollarte 2014; Pitkala 2014) due to a serious risk of bias and inconsistency. The evidence for medicines costs (Furniss 2000; Roberts 2001; Crotty 2004a; Zermansky 2006; Frankenthal 2014 was downgraded from high to very low due to a serious risk of bias, inconsistency and imprecision. The evidence for medicine‐related problems (Strikwerda 1994; Claesson 1998; Furniss 2000; Roberts 2001; Crotty 2004b; Zermansky 2006; Frankenthal 2014 was reduced from high to low due to design, risk of bias and imprecision.
Discussion
Summary of main results
12 studies were included in the review and three ongoing studies. The primary outcomes of the review were adverse drug events, mortality and hospital admissions.There was no evidence of an effect of the interventions on adverse drug events (Crotty 2004b; Gurwitz 2008) and mortality (Furniss 2000; Roberts 2001; Zermansky 2006; Frankenthal 2014; Pitkala 2014; Connolly 2015). There was evidence from one study that the intervention led to fewer days in hospital (Pitkala 2014); however, there was no evidence of an effect in the remaining studies (Furniss 2000; Roberts 2001; Crotty 2004b; Zermansky 2006; Frankenthal 2014; Garcia‐Gollarte 2014; Connolly 2015). One study found evidence that the intervention led to a slower decline in health‐related quality of life (Pitkala 2014) with one study showing no effect on quality of life (Frankenthal 2014). There was evidence that the interventions led to the identification and resolution of medication‐related problems (Strikwerda 1994; Claesson 1998; Furniss 2000; Roberts 2001; Crotty 2004b; Zermansky 2006; Frankenthal 2014). There was evidence from five studies that medication appropriateness was improved (Crotty 2004a; Crotty 2004b; Frankenthal 2014; Garcia‐Gollarte 2014; Pitkala 2014). However, the link between improved medication appropriateness and patient‐related outcomes is not clear. The evidence for an effect on medicine costs was mixed with three studies finding a reduction in costs (Furniss 2000; Roberts 2001; Frankenthal 2014) and two studies finding no difference (Crotty 2004a; Zermansky 2006).
Overall completeness and applicability of evidence
The review was designed to identify interventions that considered residents’ whole medication regimens to optimise prescribing. Consequently, a broad range of interventions (professional and organisational) were eligible for the review and diverse, multifaceted interventions were ultimately implemented to address the objectives of the review.
The interventions were tested in the population of interest; however, there was considerable variability in the outcomes measured with quality of life only represented in two of the included studies (Frankenthal 2014; Pitkala 2014).
Current practice varies considerably internationally. Multidisciplinary teams (involving physicians, nurses and pharmacists) play a significant role in optimising prescribing for care home residents and this was reflected in the studies; the majority of interventions involved multidisciplinary teamwork, usually with pharmacists conducting medication reviews. However, the effectiveness of this has not been demonstrated. Information and communication technology is increasingly being employed to optimise prescribing in many settings, and one study tested the impact of a clinical decision support system (Gurwitz 2008).
Quality of the evidence
We could not draw robust conclusions from the evidence due to variability in design, interventions, outcomes and results. The review included 12 studies of varying quality that included 10,953 residents living in 355 care homes in ten countries and are summarised in the 'Summary of findings' table (Table 1). The overall quality of the evidence for the outcomes reported was judged to be low or very low and therefore, there is uncertainty of the effect of interventions to optimise prescribing in this context. The interventions that were tested may reduce medication‐related problems and improve medication appropriateness; however, there may be little or no difference in adverse drug events, mortality, quality‐of‐life or hospital admissions. It is also uncertain whether the interventions decrease medication costs. The majority of the included studies were cluster‐RCTs and this was appropriate given the complex nature of interventions, the difficulty of blinding and the consequential threat of contamination. However, two of the nine cluster‐RCTs appeared to commit unit of analysis errors. The patient‐RCTs did not adequately protect against contamination and, therefore, the effects of the intervention may have potentially been diluted. Some of the studies had short follow‐up periods, which may have potentially limited the detection of effects on outcomes. None of the studies blinded participants and personnel; although this was unlikely to have been achievable due to the nature of the interventions, it may still introduce bias. The interventions tested were complex and multifaceted and none of the studies attempted to disentangle the 'black box' effect, that is to understand the effects of the contributing components. Not all the studies attempted blinding of assessment for subjective outcomes, and this could have been implemented. A major limitation of the evidence was the diversity of outcome measures and the fact that they differed in the way they were defined (if at all), collected and analysed.
Potential biases in the review process
We minimised bias when conducting this review by several methods. We conducted an extensive literature search which was guided by EPOC and we screened the included studies from published systematic reviews. We did not limit studies to those in the English language. Two review authors independently screened titles and abstracts, assessed studies for eligibility, evaluated risk of bias and extracted data.
Agreements and disagreements with other studies or reviews
We identified five previously published systematic reviews (Kaur 2009; Ostini 2009; Verrue 2009; LaMantia 2010; Loganathan 2011) and one narrative review (Markum 2010) related to the objectives of this review. We did not identify further studies from these reviews and the conclusions were similar, that is mixed results were obtained from the several intervention types tested in heterogeneous studies.
Authors' conclusions
Implications for practice.
The interventions implemented in the studies in this review led to the identification of medication‐related problems, confirming that suboptimal prescribing is prevalent in this context. The majority of medication‐related problems were resolved through the interventions employed. In addition, evidence from five studies suggested that the appropriateness of medication could be improved through multifaceted interventions involving medication review by pharmacists, transfer of information and multidisciplinary case conferencing. Despite the identification and resolution of medication‐related problems, and improvements in medication appropriateness, there is a lack of evidence on how this translates to improvements in resident‐related outcomes, namely adverse drug events, hospital admissions, mortality and quality of life. The effect of interventions on medicine costs was unclear, with three studies showing a reduction in costs and two studies showing no difference.
Implications for research.
High‐quality, adequately powered RCTs, ideally using cluster designs, need to be conducted to identify effective interventions to optimise prescribing for older care home residents. More studies are needed to investigate the effectiveness of clinical decision support systems as well as multidisciplinary interventions in this context. Further work is required to develop consensus on identifying, defining, measuring, reporting and analysing important resident‐related outcomes, including quality of life. This will enable meta‐analyses to be conducted on future RCTs.
What's new
| Date | Event | Description |
|---|---|---|
| 10 August 2015 | New citation required but conclusions have not changed | The authorship of the review has changed. This review includes 12 studies. |
| 14 May 2015 | New search has been performed | New searches performed to May 14, 2015. Four new studies identified. |
History
Protocol first published: Issue 4, 2011 Review first published: Issue 2, 2013
| Date | Event | Description |
|---|---|---|
| 22 February 2013 | Amended | Minor edits ‐ listing of 2 excluded studies |
Acknowledgements
We would like to thank Gerd Flodgren, Julia Worswick (Managing Editors EPOC Oxford, UK) and Pierre Durieux (EPOC Paris, France) for their assistance in updating the review.
We would like to acknowledge the previous authors of the review, Professor DK (Theo) Raynor, Professor Nick Barber and Ms Pat Spoor. We would also like to acknowledge the previous contributors to the review: Michelle Fiander (Trials Search Coordinator, EPOC group) in refining the original search strategy with Pat Spoor and Sally Dalton (Faculty Team Librarian, University of Leeds) for helping to run the original searches, as well as the helpful comments of peer reviewers on the protocol, Luciana Ballini, Kirby Lee, Aaron Tejani, Craig Ramsay, and the support of Lisa Bero. We would like to thank the members of the EPOC group in Canada and the UK for their help and advice. We would also like to acknowledge Mrs Julie Sowter (School of Healthcare, University of Leeds) and Noortje Arts (Institute for Linguistics, University of Utrecht) for translating the Strikwerda paper from Dutch to English and Ms Rachel Payne (School of Healthcare, University of Leeds) for secretarial support.
Appendices
Appendix 1. Electronic database search strategies
MEDLINE OvidSP 1 January 2012 ‐ 14 May 2015
| 1 | polypharmacy/ | 2628 |
| 2 | polypharm*.ti,ab. | 3944 |
| 3 | ((multi‐drug* or multidrug*) adj2 (therapy or therapies or prescribing or treatment or regime*)).ti,ab. | 3371 |
| 4 | (beer* adj1 criter*).ti,ab. | 304 |
| 5 | inappropriate prescribing/ | 1037 |
| 6 | ((appropriate or optim* or inappropriat* or suboptim* or sub‐optim* or unnecessary or incorrect* or in‐correct* or excessive or multiple or concurrent*) adj2 (medicine? or medication? or prescription* or drug*)).ti,ab. | 21359 |
| 7 | ((over adj1 prescript*) or (overprescrib* or overprescript*)).ti,ab. | 751 |
| 8 | ((under adj prescript*) or (underprescrib* or underprescript*)).ti,ab. | 276 |
| 9 | medication appropriateness index.ti,ab. | 72 |
| 10 | (quality adj (prescribing or prescription? or medication?)).ti,ab. | 85 |
| 11 | (improv* adj (prescrib* or prescription? or pharmaco*)).ti,ab. | 2066 |
| 12 | case conferencing.ti,ab. | 47 |
| 13 | medication therapy management/ | 790 |
| 14 | (medication? management or medication? therapy management or medication? strategy or medication? strategies or (medication? adj2 review*)).ti,ab. | 3596 |
| 15 | drug regimen review*.ti,ab. | 54 |
| 16 | drug utilization review/ | 3215 |
| 17 | (drug adj utili?ation adj2 (review* or evaluat*)).ti,ab. | 413 |
| 18 | drug related problem?.ti,ab. | 941 |
| 19 | ((prescribing or prescription?) adj2 pattern?).ti,ab. | 2948 |
| 20 | assessing care of vulnerable elders.ti,ab. | 56 |
| 21 | acove.ti,ab. | 46 |
| 22 | stopp.ti,ab. | 132 |
| 23 | start screening tool.ti,ab. | 18 |
| 24 | screening tool of older person's prescriptions.ti,ab. | 30 |
| 25 | screening tool to alert doctors to right treatment.ti,ab. | 29 |
| 26 | medication errors/ | 10732 |
| 27 | (pharmaceutical? or pharmacist? or prescrib*).ti,ab. | 185124 |
| 28 | pharmaceutical preparations/ | 43774 |
| 29 | pharmacists/ | 11288 |
| 30 | pharmacists' aides/ | 532 |
| 31 | prescription drugs/ | 3360 |
| 32 | drug prescriptions/ | 22654 |
| 33 | prescriptions/ | 2033 |
| 34 | pharmaceutical services/ | 4377 |
| 35 | drug toxicity/ | 22441 |
| 36 | pharmacotherap*.ti,ab. | 24486 |
| 37 | drug therapy/ | 28413 |
| 38 | drug monitoring/ | 15022 |
| 39 | or/1‐38 | 345099 |
| 40 | homes for the aged/ or "homes for the aged".tw. | 11571 |
| 41 | exp nursing homes/ or nursing home?.tw. | 40611 |
| 42 | (aged adj2 (care or nursing or healthcare or residential) adj2 (facility or facilities or home?)).ti,ab. | 708 |
| 43 | ((geriatric or elderly) adj2 (facility or facilities or care home?)).ti,ab. | 354 |
| 44 | hospitals, veterans/ | 5928 |
| 45 | or/40‐44 | 50940 |
| 46 | ((care or convalescent) adj (home? or center? or centre? or facility or facilities)).ti,ab. | 35311 |
| 47 | ((skilled or intermediate) adj (nursing facility or nursing facilities)).ti,ab. | 1609 |
| 48 | (resident* adj2 (care or facility or facilities)).ti,ab. | 6401 |
| 49 | ((nursing or group or residential) adj home?).ti,ab. | 24170 |
| 50 | long‐term care/ | 22277 |
| 51 | ((longterm or long term) adj3 (care or facility or facilities)).ti,ab. | 18133 |
| 52 | (healthcare adj2 (facility or facilities)).ti,ab. | 2669 |
| 53 | residential facilities/ | 4759 |
| 54 | assisted living facilities/ | 968 |
| 55 | assisted living.ti,ab. | 1455 |
| 56 | halfway houses/ | 1025 |
| 57 | or/46‐56 | 94250 |
| 58 | exp aged/ | 2433322 |
| 59 | geriatrics/ | 26942 |
| 60 | (gerontol* or ageing or aging or elder* or geriatric* or seniors or old age or older or late* life).ti,ab. | 583360 |
| 61 | (older adj (person* or people or adult* or patient* or inpatient* or outpatient*)).ti,ab. | 85641 |
| 62 | veterans/ | 10381 |
| 63 | veteran*.ti,ab. | 23572 |
| 64 | or/58‐63 | 2740961 |
| 65 | exp randomized controlled trial/ | 394909 |
| 66 | controlled clinical trial.pt. | 89435 |
| 67 | randomi#ed.ti,ab. | 408930 |
| 68 | placebo.ab. | 162358 |
| 69 | drug therapy.fs. | 1771119 |
| 70 | randomly.ti,ab. | 231035 |
| 71 | trial.ab. | 331228 |
| 72 | groups.ab. | 1449417 |
| 73 | or/65‐72 | 3543651 |
| 74 | exp animals/ not humans/ | 4037906 |
| 75 | 73 not 74 | 3046430 |
| 76 | 39 and 45 | 2952 |
| 77 | 39 and 57 and 64 | 2794 |
| 78 | or/76‐77 | 4142 |
| 79 | 75 and 78 | 1720 |
| 80 | limit 79 to yr="2012 ‐Current" | 393 |
Embase OvidSP 1 January 2012 ‐ 14 May 2015
| 1 | polypharmacy/ | 8098 |
| 2 | polypharm*.ti,ab. | 5772 |
| 3 | ((multi‐drug* or multidrug*) adj2 (therapy or therapies or prescribing or treatment or regime*)).ti,ab. | 3393 |
| 4 | (beer* adj1 criter*).ti,ab. | 550 |
| 5 | inappropriate prescribing/ | 1682 |
| 6 | ((appropriate or optim* or inappropriat* or suboptim* or sub‐optim* or unnecessary or incorrect* or in‐correct* or excessive or multiple or concurrent* or adverse) adj2 (medicine? or medication? or prescription* or prescrib* or drug*)).ti,ab. | 50332 |
| 7 | ((over adj1 prescript*) or (over adj1 prescrib*) or (overprescrib* or overprescript*)).ti,ab. | 1348 |
| 8 | ((under adj prescript*) or (under adj prescrib*) or (underprescrib* or underprescript*)).ti,ab. | 563 |
| 9 | medication appropriateness index/ or medication appropriateness index.ti,ab. | 113 |
| 10 | (quality adj (prescribing or prescription? or medication?)).ti,ab. | 128 |
| 11 | (improv* adj (prescrib* or prescription? or pharmaco*)).ti,ab. | 2583 |
| 12 | case conferencing.ti,ab. | 57 |
| 13 | medication therapy management/ | 4106 |
| 14 | (medication? management or medication? therapy management or drug therapy management or medication? strategy or medication? strategies or (medication? adj2 review*)).ti,ab. | 6098 |
| 15 | drug regimen review*.ti,ab. | 61 |
| 16 | (drug adj utili?ation adj2 (review* or evaluat*)).ti,ab. | 441 |
| 17 | drug utilization/ | 14141 |
| 18 | ((drug or medication) adj related problem?).ti,ab. | 1973 |
| 19 | ((prescribing or prescription?) adj2 pattern?).ti,ab. | 4150 |
| 20 | assessing care of vulnerable elders.ti,ab. | 72 |
| 21 | assessing care of vulnerable elders.mp. | 74 |
| 22 | "assessing care of vulnerable elders"/ | 2 |
| 23 | acove.ti,ab. | 89 |
| 24 | stopp.ti,ab. | 349 |
| 25 | start screening tool.ti,ab. | 46 |
| 26 | screening tool of older person's prescriptions.ti,ab. | 63 |
| 27 | screening tool to alert doctors to right treatment.ti,ab. | 56 |
| 28 | medication error/ | 11886 |
| 29 | (pharmaceutical? or pharmacist? or prescrib*).ti,ab. | 264124 |
| 30 | drug/ | 19225 |
| 31 | pharmacist/ or pharmacy technician/ | 45126 |
| 32 | prescription drug/ | 4573 |
| 33 | prescription/ | 106402 |
| 34 | pharmacy/ | 45688 |
| 35 | pharmacotherap*.ti,ab. | 31782 |
| 36 | exp drug therapy/ | 1368324 |
| 37 | drug monitoring/ | 26385 |
| 38 | drug toxicity/ | 6318 |
| 39 | "drug use"/ | 72662 |
| 40 | or/1‐39 | 1750098 |
| 41 | home for the aged/ or "home? for the aged".ti,ab. | 5316 |
| 42 | ((care or convalescent) adj (home? or center? or centre? or facility or facilities)).ti,ab. | 39224 |
| 43 | public hospital/ | 21393 |
| 44 | exp nursing homes/ | 26353 |
| 45 | ((skilled or intermediate) adj (nursing facility or nursing facilities*)).ti,ab. | 1724 |
| 46 | ((aged or geriatric or elderly) adj2 (care home? or facility or facilities or residential)).ti,ab. | 1272 |
| 47 | or/41‐46 | 86634 |
| 48 | (resident* adj2 (care or facilit*)).ti,ab. | 6772 |
| 49 | ((nursing or group or residential) adj home*).ti,ab. | 21258 |
| 50 | long term care/ | 83653 |
| 51 | ((longterm or long term) adj3 (care or facilit*)).ti,ab. | 17973 |
| 52 | residential home/ | 3955 |
| 53 | residential home*.ti,ab. | 707 |
| 54 | assisted living facility/ | 1431 |
| 55 | assisted living.ti,ab. | 1711 |
| 56 | (life care cent* or continued care cent* or extended care facilit*).ti,ab. | 283 |
| 57 | halfway house/ | 351 |
| 58 | or/48‐57 | 118205 |
| 59 | exp aged/ | 1646752 |
| 60 | geriatrics/ | 15941 |
| 61 | (aged or elder* or geriatric* or seniors or old age or older or late* life).ti,ab. | 799481 |
| 62 | (old* adj (person* or people or adult* or patient* or inpatient* or outpatient*)).ti,ab. | 126453 |
| 63 | veteran/ | 11947 |
| 64 | veteran*.ti,ab. | 22983 |
| 65 | or/59‐64 | 2164247 |
| 66 | clinical trial/ | 696973 |
| 67 | randomized controlled trial/ | 324874 |
| 68 | randomization/ | 57914 |
| 69 | single blind procedure/ | 18802 |
| 70 | double blind procedure/ | 96148 |
| 71 | crossover procedure/ | 38442 |
| 72 | randomi?ed controlled trial*.ti,ab. | 112095 |
| 73 | rct.tw. | 16460 |
| 74 | random allocation.ti,ab. | 1121 |
| 75 | randomly allocated.ti,ab. | 18340 |
| 76 | allocated randomly.ti,ab. | 1382 |
| 77 | (allocated adj2 random).ti,ab. | 292 |
| 78 | single blind*.ti,ab. | 12471 |
| 79 | double blind*.ti,ab. | 109011 |
| 80 | ((treble or triple) adj2 blind*).ti,ab. | 426 |
| 81 | prospective study/ | 267926 |
| 82 | or/66‐81 | 1145782 |
| 83 | exp animal/ not human/ | 2195026 |
| 84 | 82 not 83 | 1107995 |
| 85 | 40 and 47 | 13171 |
| 86 | 40 and 58 and 65 | 10550 |
| 87 | or/85‐86 | 20859 |
| 88 | 84 and 87 | 4153 |
| 89 | limit 88 to yr="2012 ‐Current" | 989 |
Cochrane Central Register of Controlled Trials (CENTRAL) Wiley 1 January 2012 ‐ 14 May 2015
| #1 | [mh ^polypharmacy] | 101 |
| #2 | (polypharm*):ti,ab,kw | 273 |
| #3 | (multi‐drug* or multidrug*) near/2 (therapy or therapies or prescribing or treatment or regime*):ti,ab,kw | 331 |
| #4 | (beer near/2 criter*):ti,ab,kw | 3 |
| #5 | [mh ^"inappropriate prescribing"] | 49 |
| #6 | (appropriate or optim* or inappropriat* or suboptim* or sub‐optim* or unnecessary or incorrect* or in‐correct* or excessive or multiple or concurrent*) near/2 (medicine* or medication* or prescription* or drug*):ti,ab,kw | 2588 |
| #7 | (over near/1 prescript*) or (overprescrib* or overprescript*):ti,ab,kw | 62 |
| #8 | (under near/1 prescript*) or (underprescrib* or underprescript*):ti,ab,kw | 16 |
| #9 | medication appropriateness index:ti,ab,kw | 20 |
| #10 | (quality near/1 (prescribing or prescription* or medication*)):ti,ab,kw | 59 |
| #11 | (improv* near/1 (prescrib* or prescription* or pharmaco*)):ti,ab,kw | 189 |
| #12 | case conferencing:ti,ab,kw | 12 |
| #13 | [mh ^"medication therapy management"] | 53 |
| #14 | medication* management:ti,ab,kw or "medication* therapy management":ti,ab,kw or "medication* strategy":ti,ab,kw or "medication* strategies":ti,ab,kw or (medication* near/2 review*):ti,ab,kw | 611 |
| #15 | drug regimen review*:ti,ab,kw or (drug near/1 utili?ation near/2 (review* or evaluat*)):ti,ab,kw | 153 |
| #16 | [mh ^"drug utilization review"] | 123 |
| #17 | drug related problem*:ti,ab,kw or (prescription* near/2 pattern*):ti,ab,kw or "assessing care of vulnerable elders":ti,ab,kw or (acove):ti,ab,kw or (stopp):ti,ab,kw | 194 |
| #18 | start screening tool:ti,ab,kw or "screening tool of older person's prescriptions":ti,ab,kw or "screening tool to alert doctors to right treatment":ti,ab,kw | 3 |
| #19 | [mh ^"medication errors"] | 230 |
| #20 | (pharmaceutical* or pharmacist* or prescrib*):ti,ab,kw | 15159 |
| #21 | [mh ^"pharmaceutical preparations"] | 229 |
| #22 | [mh ^pharmacists] | 452 |
| #23 | [mh ^"pharmacists' aides"] | 8 |
| #24 | [mh ^"prescription drugs"] | 92 |
| #25 | [mh ^"drug prescriptions"] | 471 |
| #26 | [mh ^prescriptions] | 91 |
| #27 | [mh ^"pharmaceutical services"] | 133 |
| #28 | [mh ^"drug toxicity"] | 780 |
| #29 | (pharmacotherap*):ti,ab,kw | 5106 |
| #30 | [mh ^"drug therapy"] | 434 |
| #31 | [mh ^"drug monitoring"] | 1129 |
| #32 | {or #1‐#31} | 25427 |
| #33 | [mh "homes for the aged"] | 498 |
| #34 | home* for the aged:ti,ab,kw or (aged near/2 (care or nursing or healthcare or residential) near/2 (facility or facilities or home*)):ti,ab,kw or (geriatric or elderly) near/2 (facility or facilities or care home*):ti,ab,kw | 990 |
| #35 | [mh "nursing homes"] | 1057 |
| #36 | [mh ^"hospitals, veterans"] | 293 |
| #37 | {or #33‐#36} | 1826 |
| #38 | (care or convalescent) next (home or homes or center* or centre* or facility or facilities):ti,ab,kw | 3372 |
| #39 | ((skilled or intermediate) near/2 (nursing facility or nursing facilities)):ti,ab,kw | 108 |
| #40 | (resident* near/2 (care or facility or facilities)):ti,ab,kw | 678 |
| #41 | (nursing or group or residential) next (home or homes):ti,ab,kw | 2301 |
| #42 | (longterm or long term) near/3 (care or facility or facilities):ti,ab,kw | 3110 |
| #43 | [mh ^"long‐term care"] | 1115 |
| #44 | [mh ^"residential facilities"] | 148 |
| #45 | (assisted living):ti,ab,kw | 315 |
| #46 | [mh ^"halfway houses"] | 18 |
| #47 | {or #38‐#46} | 8636 |
| #48 | [mh aged] | 997 |
| #49 | [mh ^geriatrics] | 202 |
| #50 | (gerontol* or ageing or aging or elder* or geriatric* or seniors or old age or older or late* life):ti,ab,kw | 44963 |
| #51 | (older next (person* or people or adult* or patient* or inpatient* or outpatient*)):ti,ab,kw | 7601 |
| #52 | [mh veterans] | 488 |
| #53 | (veteran*):ti,ab,kw | 2478 |
| #54 | {or #48‐#53} | 47508 |
| #55 | #47 and #54 | 2330 |
| #56 | #32 and (#37 or #55) Publication Year from 2012 to 2015 | 83 |
CINAHL EbscoHost 1 January 2012 ‐ 14 May 2015
| S1 | MH polypharmacy | 1,698 |
| S2 | polypharmacy | 2,183 |
| S3 | beer* n1 criter* | 150 |
| S4 | ((appropriate or optim* or inappropriat* or suboptim* or sub‐optim* or unnecessary or incorrect* or in‐correct* or excessive or multiple or concurrent*) n2 (medicine? or medication? or prescription* or drug*)) | 6,010 |
| S5 | (over n2 prescript*) or overprescrib* or overprescript* | 420 |
| S6 | "under prescript*" or underprescrib* or underprescript | 54 |
| S7 | "medication appropriateness index*" | 24 |
| S8 | quality n2 (prescri* or medication*) | 424 |
| S9 | improv* n2 (prescri* or pharmaco*) | 959 |
| S10 | "assessing care of vulnerable elders" | 37 |
| S11 | acove | 24 |
| S12 | ((multi‐drug* or multidrug*) n3 (therapy or therapies or prescribing or treatment or regime*)) | 587 |
| S13 | MH medication errors | 8,551 |
| S14 | MH inappropriate prescribing | 353 |
| S15 | pharmaceutical* or prescribing | 25,134 |
| S16 | MH pharmacists | 4,753 |
| S17 | MH "pharmacy technicians" | 205 |
| S18 | MH "drugs, prescription" | 10,395 |
| S19 | MH "prescriptions, drug” | 4,242 |
| S20 | MH "pharmacy service") or (MH "pharmaceutical care") | 2,710 |
| S21 | pharmacist* | 7,487 |
| S22 | (MH "medication management (iowa nic)") or (MH "medication managements (iowa nic) (non‐cinahl)") | 2 |
| S23 | MH drug toxicity | 3,066 |
| S24 | stopp or start screening tool | 46 |
| S25 | "screening tool of older person's prescriptions" | 7 |
| S26 | "screening tool to alert doctors to right treatment" | 8 |
| S27 | (medication* n2 (management or review* or strateg*)) | 2,550 |
| S28 | pharmacotherap* | 3,689 |
| S29 | (MH "drug therapy") | 6,126 |
| S30 | (MH "drug utilization") | 3,823 |
| S31 | "drug utili*ation" n2 (review* or evaluat*) | 216 |
| S32 | MH drug monitoring | 3,766 |
| S33 | "drug regimen review*" | 11 |
| S34 | "case conferencing" | 21 |
| S35 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 | 74,621 |
| S36 | "homes for the aged" or MH housing for the elderly | 1,945 |
| S37 | MH nursing homes+ or mw nursing home | 33,249 |
| S38 | (aged n2 ("care facilit*" or "care home*" or "nursing facilit*" or "residential facilit*")) or "aged nursing home*" or (aged n1 "healthcare facilit*") | 472 |
| S39 | "aged residential home*" or (geriatric n2 facilit*) or (geriatric* n1 "care home*") or (elderly n2 (facilit* or "care home*")) | 284 |
| S40 | (MH "hospitals, veterans") | 3,243 |
| S41 | S36 OR S37 OR S38 OR S39 OR S40 | 38,021 |
| S42 | ((care or convalescent) w1 (home* or center* or centre* or facilit*)) | 21,905 |
| S43 | ((skilled or intermediate) w1 "nursing facilit*") | 2,493 |
| S44 | (resident* n2 (care or facilit*)) | 10,260 |
| S45 | ((nursing or group or residential) n1 home*) | 36,410 |
| S46 | ((longterm or long term or long‐term) n3 (care or facilit*)) | 22,339 |
| S47 | MH residential facilities or MH long term care | 19,412 |
| S48 | "residential home*" or healthcare n2 facilit* | 1,436 |
| S49 | MH assisted living | 2,010 |
| S50 | "assisted living" | 2,521 |
| S51 | "life care cent*" or "continued care cent*" or "extended care facilit*" | 152 |
| S52 | (MH "halfway houses") | 97 |
| S53 | S42 OR S43 OR S44 OR S45 OR S46 OR S47 OR S48 OR S49 OR S50 OR S51 OR S52 | 75,485 |
| S54 | (MH "aged+") | 355,481 |
| S55 | MH geriatrics | 2,639 |
| S56 | ageing or aging or gerontol* or elder* or geriatric* or seniors or "old age" or "late* life" | 142,501 |
| S57 | old* n1 (person* or people or adult* or patient* or inpatient* or outpatient*) | 47,396 |
| S58 | MH veterans | 7,376 |
| S59 | veterans | 13,892 |
| S60 | S54 OR S55 OR S56 OR S57 OR S58 OR S59 | 408,165 |
| S61 | (MH "clinical trials") | 81,870 |
| S62 | PT clinical trial | 52,097 |
| S63 | TX clinic* n1 trial* | 121,846 |
| S64 | TX ((singl* or doubl* or tripl* or trebl*) n1 (blind* or mask*)) | 640,410 |
| S65 | TX "randomi* control* trial*" | 52,249 |
| S66 | MH random assignment | 32,044 |
| S67 | TX "random* allocat*" | 2,772 |
| S68 | MH quantitative studies | 10,797 |
| S69 | TX "allocat* random*" | 135 |
| S70 | S61 OR S62 OR S63 OR S64 OR S65 OR S66 OR S67 OR S68 OR S69 | 767,987 |
| S71 | (MH "animals+") not MH human | 28,052 |
| S72 | s70 not s71 | 764,290 |
| S73 | S35 AND S41 | 1,524 |
| S74 | S35 AND S53 AND S60 | 1,530 |
| S75 | S73 OR S74 | 2,131 |
| S76 | S72 AND S75 | 543 |
| S77 | s76 Limiters ‐ Published Date: 20120101‐20151231 | 133 |
Appendix 2. Trial registry search strategies
ClinicalTrials.gov, US National Institutes of Health (NIH) searched 18 May 2015
| ( residential homes OR nursing homes ) AND ( medicine OR medication OR prescription OR drug ) AND ( elderly OR old OR aged ) AND ( randomly OR random OR randomised OR randomized OR RCT ) | 78 |
International Clinical Trials Registry Platform (ICTRP), World Health Organization (WHO)
Number of results: 11
Each term 1 was searched with each possible combination of the other terms (2‐4). Terms were combined using AND
| Term 1 | Term 2 | Term 3 | Term 4 |
| Randomised | Nursinghomes | elderly | drugs |
| Randomized | Residentialhomes | old | medication |
| RCT | pharmacy | ||
| Randomly | polypharmacy |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Claesson 1998.
| Methods | Cluster‐RCT (randomised by nursing home) Total study duration: 14 months |
|
| Participants | 1854 residents 33 nursing homes Setting: nursing homes Age: Average 83 years Gender: Intervention 70% female; control 67% female Country: Sweden Date of study: 1994/95 |
|
| Interventions | The aim of the regular multidisciplinary meetings was to discuss and improve the use of drugs in nursing homes, and to decrease the use of drugs which, according to the advice of the workshop arranged by the Swedish Medical Products Agency, could cause confusion and impaired memory. In group discussions, the physician, pharmacist, one or more of the nursing home nurses, and in many cases, one or more of the assistant nurses and nurse aides reviewed the drug use of all residents on a monthly basis over a period of one year. The length and frequency of the meetings were adjusted by the participants to local conditions. The therapy changes that were discussed were thus based on the physician’s medical knowledge, the pharmacist’s pharmaceutical knowledge, and the nurses’ and other staff’s knowledge about the patients’ social and functional status.The selected pharmacists were educated prior to and during the intervention period. This education took the form of lectures and workshops, which took place on five occasions, twice before the intervention started and three times during the intervention period, for a total of 65.5 hours. The lectures were given by recognised experts, including clinical pharmacists, geriatricians, gerontologists, nurses and two community pharmacists with experience in nursing home consulting. Topics covered were gerontology/geriatrics (12.5 hours), drug use in the elderly (23.5 hours) and basic training in collaborative methods (18.5 hours). In addition, the pharmacists worked with patient cases in small groups, covering all the areas mentioned above (11 hours). In addition to the formal education, the pharmacists formed regional networks. The networking took place locally, whenever the pharmacist felt a need to have it. In order to make the networks constructive, the whole group was instructed by an educational specialist on one occasion. | |
| Outcomes | Medication‐related problems Not used for this review: Drug use |
|
| Notes | Supported by the National Corporation of Swedish Pharmacies and the Swedish Pharmaceutical Society | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Homes were matched in pairs then each randomised to control or intervention. [Attempted to contact author for further information but unsuccessful] |
| Allocation concealment (selection bias) | Low risk | Cluster design |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not conducted |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Blinding not conducted |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | No objective outcomes |
| Incomplete outcome data (attrition bias) Primary outcomes | Unclear risk | Not measured in this study |
| Incomplete outcome data (attrition bias) Secondary outcomes | Low risk | Medication‐related problems described for residents receiving intervention |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Similar baseline outcome measurements | Unclear risk | Medication‐related problems not measured at baseline |
| Similar baseline characteristics | Low risk | Similar baseline characteristics reported |
| Reliable primary outcome measure | Low risk | Drug use |
| Adequate protection against contamination | Unclear risk | Cluster design. [Attempted to contact author for further information but unsuccessful] |
| Other bias | Low risk | Appears to be free of other sources of bias |
Connolly 2015.
| Methods | Cluster‐RCT (randomised by care facility) Total study duration: 14 months |
|
| Participants | 36 facilities (18 intervention, 18 control). 1998 residents (1123 intervention, 875 control) Setting: Residential aged‐care (RAC) facilities Age: mean age not provided. Intervention: < 65, 6.4%; 65 to 74, 11.7%; 75 to 84, 29.5%; 85 to 94, 46.6%; 95 + 5.9%; control < 65, 7.5%; 65 to 74, 11.2%; 75 to 84, 29.1%; 85 to 94, 43.3%; 95 + 8.8% Gender: Intervention male 348 (31.0%), control male 242 (27.7%) Country: New Zealand Date of Study: 2010‐2012 |
|
| Interventions | 1. Baseline facility assessment to identify areas of need and facility care plan developed in collaboration with the gerontology nurse specialist (GNS), and RAC facility clinical leadership (anonymised example available from authors on request) 2. Monitoring and benchmarking of resident indicators linked to quality of care provided (falls, nutrition, restraint use, weight loss, urinary tract infections, residents on nine medications); benchmarking was provided on three occasions during the intervention 3. Three 1‐hour multidisciplinary team (MDT) meetings, monthly for the first three months at each facility, including medication review by study geriatrician, GNS, general practitioner (GP), pharmacist, and nurse manager. Typically, six residents were considered per meeting with priority given to new admissions, the recently hospitalised, those with recent “incidents” (e.g., fall), and those on nine or more medications 4. Gerontology education and clinical coaching for RAC nurses and caregivers, including advanced (end‐of‐life) care planning, nutrition/hydration, early detection of illness, falls prevention, end‐stage dementia care, communication with families, and practical aspects of care |
|
| Outcomes | Hospital admissions (ambulatory sensitive hospitalisations, total acute admissions) Mortality |
|
| Notes | Funded by the Health Research Council of New Zealand | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomised numbers |
| Allocation concealment (selection bias) | Low risk | Cluster design |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not conducted |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | No subjective outcomes measured |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Authors state that "'care was taken to blind investigators to facility identification wherever possible". However outcomes not likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) Primary outcomes | Low risk | Reasons for attrition reported. Described as intention‐to‐treat by authors |
| Incomplete outcome data (attrition bias) Secondary outcomes | Low risk | Reasons for attrition reported. Described as intention‐to‐treat by authors |
| Selective reporting (reporting bias) | Low risk | Pre‐specified outcomes were reported in the pre‐specified way in the protocol |
| Similar baseline outcome measurements | Low risk | Similar baseline outcome measurements (no baseline measurement of hospital admissions) |
| Similar baseline characteristics | Low risk | Similar baseline characteristics reported |
| Reliable primary outcome measure | Low risk | Hospital admissions |
| Adequate protection against contamination | Unclear risk | Cluster design. However, it was theoretically possible that some healthcare professionals may have moved between intervention and control nursing homes [author contacted] |
| Other bias | High risk | Medication reviews were undertaken for a non‐random subsample of 23% of intervention residents selected by multidisciplinary team |
Crotty 2004a.
| Methods | Cluster‐RCT (randomised by care facility) Total study duration: 3 months |
|
| Participants | 10 facilities (5 intervention, 5 control). 154 residents (50 intervention, 54 control, 50 within‐facility control) Setting: High‐level residential aged‐care facilities (nursing homes) Age: Intervention mean 85.3, control mean 83.6, within‐facility control mean 84.6 Gender: Intervention male 22 (44%), control male 23 (43%), within‐facility control male 17 (34%) Country: Australia Date of Study: 1999 [Author contacted] |
|
| Interventions | Outreach geriatric medication advisory service, case conferencing and medication review. GPs were invited to attend two multidisciplinary case conferences conducted 6 to 12 weeks apart. The resident’s GP, a geriatrician, a pharmacist, residential care staff and a representative of the Alzheimer’s Association of South Australia attended the case conferences, which were held at the facility. Residential care staff expanded on any issues in the case notes that required discussion and the Alzheimer’s Association of South Australia representative discussed non‐pharmacological management of dementia‐related behaviour. Each case conference was chaired by the GP, who used their medical records in addition to case notes from the facility. A problem list was developed by the GP in conjunction with the care staff and a medication review was conducted prior to each case conference. All facilities in the study, including those in the control group, received a half‐day workshop provided by the Alzheimer’s Association of South Australia, which examined the use of a toolkit in the management of challenging behaviours |
|
| Outcomes | Measured at baseline and three months post‐intervention: Medication appropriateness (MAI) Drug costs (based on Australian Government Pharmaceutical Benefits Scheme) Not used in this review: Nursing Home Behaviour Problem Scale (NHBPS) Number of drugs |
|
| Notes | Funded by The Quality Use of Medicines Evaluation Programme 2000‐2001, Health and Aged Care, General Practice National Innovations Funding Pool 1999‐2000, Health and Aged Care. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers used |
| Allocation concealment (selection bias) | Low risk | A researcher independent to the investigators generated the random sequence and cluster design. Staff were asked to “nominate” 20 residents from intervention sites and 10 residents from control sites. From the 20 intervention,10 were randomised to intervention and ten to within‐facility control using sequential sealed opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding conducted |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Assessed by independent pharmacist blinded to allocation [author contacted] |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | No blinding conducted, however outcomes not likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) Primary outcomes | Unclear risk | Not measured in this study |
| Incomplete outcome data (attrition bias) Secondary outcomes | Low risk | Reasons for attrition reported (all due to deaths) and no statistically significant difference found in the proportion of residents lost between groups Described as intention‐to‐treat analysis by authors |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Similar baseline outcome measurements | High risk | There were differences in the Medication Appropriateness Index between groups at baseline: Control 4.1 (95% CI 2.4‐5.7); Within‐facility control 6.0 (95% CI 3.1‐9.0); Intervention 7.4 (95% CI 4.5‐10.3) |
| Similar baseline characteristics | Low risk | Similar baseline characteristics reported |
| Reliable primary outcome measure | Low risk | Medication Appropriateness Index |
| Adequate protection against contamination | Low risk | Cluster design. Randomised by care facility. GPs were checked to avoid contamination between intervention and control residents [author contacted]. No significant differences found between the within‐facility control and the control groups, therefore no evidence of a carry‐over effect of the intervention |
| Other bias | Low risk | Appears to be free of other sources of bias |
Crotty 2004b.
| Methods | RCT (randomised by patient) Total study duration: 8 weeks |
|
| Participants | 110 patients (56 intervention, 54 control) from three hospitals discharged to 85 long‐term facilities Setting: Long‐term care facilities Age: Mean 82.7, .SD 6.4 Gender: 67 women (60.9%), 43 men (39.1%) Country: Australia Date of study: October 2002 to July 2003 |
|
| Interventions | Pharmacist transition co‐ordinator The intervention focused on transferring information on medications to care providers in the long‐term care facilities, including the nursing staff, the family physician and the accredited community pharmacist. On the patient’s discharge from the hospital to the long‐term care facility both the family physician and the community pharmacist were faxed a medication transfer summary compiled by the transition pharmacist and signed by the hospital medical officer. This communication supplemented the usual hospital discharge summary and included specific information on changes to medications that had been made in the hospital and aspects of medication management that required monitoring. After transfer of the patient to the long‐term care facility, the transition pharmacist co‐ordinated an evidence‐based medication review that was to be performed by the community pharmacist contracted to the facility within 10 to 14 days of the transfer. The transition pharmacist also co‐ordinated a case conference involving him or herself, the family physician, the community pharmacist and a registered nurse at the facility within 14 to 28 days of the transfer. At this case conference, the transition pharmacist provided information concerning medication use and appropriateness The usual hospital discharge process received by the control group included a standard hospital discharge summary |
|
| Outcomes | Measured at baseline and eight weeks post‐discharge: Adverse drug events (not defined) Hospital admissions (emergency department visits and hospital readmissions) Medication‐related problems Medication appropriateness (MAI) Not used for this review: Falls Worsening mobility Worsening behaviours Increased confusion Worsening pain |
|
| Notes | Funded by the Australian Commonwealth Department Of Health and Ageing National Demonstration Hospitals Program. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Study biostatistician provided a computer‐generated allocation sequence using block randomisation |
| Allocation concealment (selection bias) | Low risk | Randomisation was co‐ordinated by a centralised hospital pharmacy service |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding conducted |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Independent pharmacists blinded to allocation assessed Medication Appropriateness Index (MAI) |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | No blinding conducted, however outcomes not likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) Primary outcomes | Low risk | Similar attrition in both groups with similar reasons for dropouts. Described as intention‐to‐treat by authors |
| Incomplete outcome data (attrition bias) Secondary outcomes | Low risk | Similar attrition in both groups with similar reasons for dropouts. Described as intention‐to‐treat by authors |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Similar baseline outcome measurements | Low risk | Similar Medication Appropriateness Index scores at baseline. Other outcomes not measured at baseline |
| Similar baseline characteristics | Low risk | Similar baseline characteristics reported except more pre‐admission medications discontinued during hospitalisation in the control group |
| Reliable primary outcome measure | Low risk | Medication Appropriateness Index |
| Adequate protection against contamination | High risk | Randomised by patient therefore contamination possible |
| Other bias | Low risk | Appears to be free of other sources of bias |
Frankenthal 2014.
| Methods | RCT (randomised by patient) Total study duration: 1 year |
|
| Participants | 359 residents (176 control, 183 intervention) Setting: Chronic care geriatric facility Age: Mean 82.7 Gender: Intervention male 29.5%, control male 37.5% Country: Israel Date of Study: Not Stated |
|
| Interventions | The intervention consisted of a medication review by the study pharmacist for all residents at study opening and six and 12 months later. The STOPP/START criteria were applied to identify potentially inappropriate prescriptions (PIPs) and potential prescription omissions (PPOs). Interventional recommendations that the study pharmacist made for residents in the intervention group but not in the control group were discussed with the chief physician at study opening and after six months. The chief physician decided whether to accept these recommendations and implement prescribing changes | |
| Outcomes | Measured at baseline and at 12 months: Hospital admissions (not defined) Mortality Quality of life (Medical Outcomes Study 12‐item Short‐Form Health Survey [SF‐12]) Medication‐related problems (number of pharmacist recommendations, acceptance of recommendations by the physician, number of treatment changes) Medication appropriateness (STOPP‐START) Medication costs (Average monthly medication costs in Israeli Shekels) Not used for this review: Falls Functioning (Functional Indepence Measure) |
|
| Notes | Study was supported partly by a research grant from Keshet Association for the Elderly in Tel‐Aviv‐Yaffo | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not conducted |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Nurses who were unaware of group assignments assessed outcome measures. However, the study pharmacist collected data on outcome measures at follow‐up. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Nurses who were unaware of group assignments assessed outcome measures. However, the study pharmacist collected data on outcome measures at follow‐up. Outcomes not likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) Primary outcomes | Low risk | Reasons and proportions for attrition documented and similar in intervention and control. |
| Incomplete outcome data (attrition bias) Secondary outcomes | Low risk | Reasons and proportions for attrition documented and similar in intervention and control. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Similar baseline outcome measurements | Low risk | Similar baseline outcomes for falls, hospitalisations and medicine costs |
| Similar baseline characteristics | Low risk | Similar baseline characteristics reported |
| Reliable primary outcome measure | Low risk | Falls and hospitalisations |
| Adequate protection against contamination | High risk | Randomised by patient therefore contamination possible |
| Other bias | Low risk | Appears to be free from other sources of bias |
Furniss 2000.
| Methods | Cluster‐RCT (randomised by care home) Total study duration: 8 months |
|
| Participants | 330 residents (172 control, 158 intervention); 14 homes (7 matched pairs) Setting: Nursing homes Age: Control mean 78.9 SD 13.7; intervention mean 83.5 SD 9.2 Gender: Control 115 (67%) females; intervention 125 (79%) females Country: UK Date of study: Not stated |
|
| Interventions | Medication review by pharmacist Medication review by the study pharmacist in the GP’s surgery, at the nursing home or (in exceptional circumstances) over the telephone. The pharmacist collected details of current medication for each resident from the medicines administration record chart in the home, together with a brief medical history and any current problems identified by the home staff. Three weeks after the medication review, the homes were revisited, to ascertain whether there had been any immediate problems with the changes in medication and to see if the suggested changes had been implemented |
|
| Outcomes | Measured at time 0 (beginning of study), time 1 at four months (beginning of intervention) and at time 2 at eight months (end of intervention): Hospital admissions ("inpatient days") Mortality Medication‐related problems (number of pharmacist recommendations, acceptance of recommendations by the GP, number of treatment changes) Medication costs (not defined, £ sterling) Not used for this review: Mini‐Mental State Examination (MMSE) Geriatric Depression Scale (GDS) Brief Assessment Schedule Depression Cards (BASDEC) Crichton‐Royal Behaviour Rating Scale (CRBRS) Number of drugs per resident Type of drugs Reason for neuroleptic use Use of primary and secondary care resources Number of accidents Falls |
|
| Notes | Funded by the North West NHS Executive | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated pseudo random numbers used |
| Allocation concealment (selection bias) | Low risk | Homes were randomised at the start of the start of a four‐month observation phase. Cluster design |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding described |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | No blinding conducted |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | No blinding conducted, however outcomes not likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) Primary outcomes | Unclear risk | Insufficient reporting of attrition/exclusions to permit judgement |
| Incomplete outcome data (attrition bias) Secondary outcomes | Unclear risk | Insufficient reporting of attrition/exclusions to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Similar baseline outcome measurements | High risk | 14 (8.1%) deaths in control group versus 22 (13.9%) deaths in intervention group at baseline. No baseline measurements of other primary outcomes of this review |
| Similar baseline characteristics | High risk | Slightly fewer residents in the intervention group (158) versus control (172). In the control group, residents were younger (mean 78.9 SD 13.7 versus mean 83.5 SD 9.2) and there were fewer females (67% versus 79%) |
| Reliable primary outcome measure | Low risk | Crichton‐Royal Behaviour Rating Scale |
| Adequate protection against contamination | Low risk | Randomised by care home (which were in different geographical areas) |
| Other bias | Low risk | Appears to be free of other sources of bias |
Garcia‐Gollarte 2014.
| Methods | Cluster‐RCT (randomised by nursing home) Total study duration: 6 months |
|
| Participants | Control group: 17 nursing homes and 29 doctors (372 participants). Intervention Group: 19 nursing homes and 30 doctors (344 participants) Setting: Nursing homes Age: Control mean 84.5 SD 10.4 ; intervention 84.24 mean SD 14.6 Gender: Control 72.1% female; intervention 74.0% female Country: Spain Date of study: February 2010 to February 2013 |
|
| Interventions | Educational intervention delivered to 30 doctors Nursing home physician expert in drug use in older people delivered a structured educational intervention. The educational intervention included information on general aspects of prescription and drug use in geriatric patients, how to reduce the number of drugs and to perform regular reviews of medications, to avoid inappropriate drug use, to discontinue drugs that do not show benefit and to avoid under‐treatment with drugs that have shown benefit. Information also provided on adverse drug reactions in older people Educational material and references also provided to participants Educator also provided on‐demand prescription advice (via phone) for a six‐month period |
|
| Outcomes | Measured at baseline and at nine months. Hospital admissions (total number of days spent in hospital) Medication appropriateness (STOPP‐START) Not used in this review: Falls Delirium Physician and nurse visit Emergency room visits Use of antipsychotics Use of delirium drugs |
|
| Notes | Funded by the Ballesol group [author contacted] | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number tables |
| Allocation concealment (selection bias) | Low risk | Cluster design |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not conducted |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Outcomes not likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) Primary outcomes | Unclear risk | Per protocol analysis used. Dropouts were not identified by group |
| Incomplete outcome data (attrition bias) Secondary outcomes | Unclear risk | Per protocol analysis used. Dropouts were not identified by group |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Similar baseline outcome measurements | Low risk | Similar baseline outcome measurements for days in hospital and medication appropriateness |
| Similar baseline characteristics | Low risk | Similar baseline characteristics reported except worse functional status in intervention group; however, adjusting for this did not significantly change the results |
| Reliable primary outcome measure | Low risk | Objective measures of healthcare utilisation |
| Adequate protection against contamination | Unclear risk | Cluster design. However, it was theoretically possible that some physicians may have moved between intervention and control nursing homes [author contacted] |
| Other bias | Unclear risk | For prescribing appropriateness, a random sample of 311 from 1018 residents was used |
Gurwitz 2008.
| Methods | Cluster‐RCT (randomised by care unit within two long‐term care facilities) Total study duration: 12 months |
|
| Participants | 1,118 resident in 29 units in two long‐term care facilities Setting: Long‐term care facilities Age: Average 87.2 years Gender: 71.3% female Country: US and Canada Date of study: 2006‐7 [Author contacted] |
|
| Interventions | Computerised provider order entry with clinical decision support. A team of geriatricians, pharmacists, health services researchers and information system specialists designed the clinical decision support system. The team reviewed the types of preventable adverse drug events based on previous research and widely accepted published criteria for suboptimal prescribing in elderly people available at the time of this study. All serious drug–drug interactions from a standard pharmaceutical drug interaction database were also reviewed and alerts were included for a limited number of more than 600 potentially serious interactions that were reviewed. For residents on the intervention units, the alerts were displayed in a pop‐up box to prescribers in real time when a drug order was entered. The pop‐up boxes were informational; they did not require specific actions from the prescriber and did not produce or revise orders automatically. On the control units, the alerts were not displayed to the prescribers |
|
| Outcomes | Measured throughout study period (resident‐months): Adverse drug event (“an injury resulting from the use of a drug” includes medication error and adverse drug reaction) Severity of adverse drug event Preventability of adverse drug event |
|
| Notes | Supported by the Agency for Healthcare Research and Quality. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation used. Within each block, units were randomly assigned using the random function in Microsoft Excel®. [Author contacted] |
| Allocation concealment (selection bias) | Low risk | Cluster design |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not conducted |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Outcome assessors were blind to allocation |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | No objective outcomes |
| Incomplete outcome data (attrition bias) Primary outcomes | Unclear risk | Insufficient reporting of attrition/exclusions to permit judgement |
| Incomplete outcome data (attrition bias) Secondary outcomes | Unclear risk | Not measured in this study |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Similar baseline outcome measurements | Unclear risk | No baseline measurements of adverse drug effects |
| Similar baseline characteristics | Unclear risk | Baseline characteristics not reported, however, units were matched for bed size and general characteristics of residents and the unit |
| Reliable primary outcome measure | Low risk | Number of adverse drug events |
| Adequate protection against contamination | High risk | Cluster design. Efforts were made to limit crossover of prescribers between intervention and control units, however, some prescribers worked simultaneously on both intervention and control units. In an effort to assess the possibility that this may have led to changes in behaviour in the control group, the rate of responses to "unseen" alerts in the control units during the first versus the last quarter of the study was assessed at one of the study sites. The rate of response was lower in the last quarter, suggesting that prescribers did not adopt new habits due to seeing alerts on intervention units |
| Other bias | Low risk | Appears to be free of other sources of bias |
Pitkala 2014.
| Methods | Cluster‐RCT (randomised by ward) Total duration of study: 12 months |
|
| Participants | 227 residents in 20 facilities (10 control, 10 intervention) Setting: Assisted living facilities Age: Control mean 83.5 SD 6.9; intervention mean 82.9 years SD 7.5 Gender: Control 77.1% female; intervention 65.3% female Country: Finland Date of Study: Not stated |
|
| Interventions | Educational intervention: Two 4‐hour training sessions for nursing staff. Aim of session was to enable nurses to recognise harmful medications and corresponding adverse drug events. First 4‐hour session: lecture‐based, allowed participants to discuss medication‐related problems experienced by their own residents, introduced lists of harmful medications and suitable treatments. Also involved discussion about medication use for residents with real impairment and drug‐drug interactions Second 4‐hour session: case‐study‐based, demonstrate relevance and importance of topic to nurses During both training sessions nurses were encouraged to reflect on their own procedure and opportunities for improvement Those nurses that received this intervention were asked to identify potential medication‐related problem and highlight these to the consulting physician |
|
| Outcomes | Assessed at 0, 6, 12 months Hospital admissions (hospital days) Mortality Health‐related Quality of Life (15D) Medication appropriateness (composite of Beers criteria, Anticholinergic Risk Scale, > 2 psychotropic medications, NSAIDs and proton pump inhibitors) Not used in this review: Cognitive assessment (MMSE) Nutritional assessment (Mini‐nutritional assessment) |
|
| Notes | [author contacted] | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised random number generator |
| Allocation concealment (selection bias) | Low risk | Cluster design |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not conducted |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Outcomes not likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) Primary outcomes | Unclear risk | Reasons and proportions for attrition documented and similar in intervention and control. Described as intention‐to‐treat analysis by authors Overall attrition rate relatively high (27.8%) |
| Incomplete outcome data (attrition bias) Secondary outcomes | Unclear risk | Reasons and proportions for attrition documented and similar in intervention and control. Described as modified intention‐to‐treat analysis by authors Overall attrition rate relatively high (27.8%) |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Similar baseline outcome measurements | Unclear risk | Lower HRQoL in intervention group (15D score mean 0.61 [SD 0.12] vs 0.66 [0.11]) and higher mean number of harmful medications (2.9 [SD 1.8] vs 2.5 [SD 1.7]). Analyses were adjusted for these differences |
| Similar baseline characteristics | Unclear risk | More males (34.7% vs 22.9%), higher prevalence of ‘as‐needed’ medication (mean 3.6 [SD 2.3] vs 2.9 [SD2.0]), and higher number of comorbidities (Mean Charlson’s index 3.2 [2.0] vs 2.5 [1.8]) in intervention group. Analyses were adjusted for these differences |
| Reliable primary outcome measure | Low risk | Well‐defined potentially harmful medication use |
| Adequate protection against contamination | Unclear risk | Cluster design. However, it was theoretically possible that some nurses may have moved between intervention and control nursing homes, although this was deemed unlikely by the author [author contacted] |
| Other bias | Low risk | Appears to be free from other sources of bias |
Roberts 2001.
| Methods | Cluster‐RCT (randomised by care home) Total study duration: Two years |
|
| Participants | 3230 residents (905 intervention, 13 homes); 2325 control, 39 homes) Setting: Nursing homes Age: Intervention < 60 2.0%, 60‐69 6.6%, 70‐79 21.9%, 80‐89 47.4%, 90‐99 20.7%, ≥ 100 1.7% Control < 60 2.6%, 60‐69 5.4%, 70‐79 22.3%, 80‐89 46.7%, 90‐99 21.1%, ≥ 100 1.6% Gender: Not reported Country: Australia Date of Study: Not reported |
|
| Interventions | Three‐phase intervention: introducing a new professional role to stakeholders with relationship‐building; nurse education; and medication review by pharmacists The clinical pharmacy service model introduced to each nursing home was supported with activities such as focus groups facilitated by a research nurse, written and telephone communication, and face‐to‐face professional contact between nursing home staff and clinical pharmacists on issues such as drug policy and specific resident problems, together with education and medication review by pharmacists holding a postgraduate diploma in clinical pharmacy. This was a multifaceted intervention directly targeting nursing homes. Most of the contact with GPs was indirect, using the existing relationships between nursing homes and visiting GPs. A number of focus groups and personal interviews about the project were conducted with GPs. In intervention homes, problem‐based education sessions (6 ± 9 seminars totaling approximately 11 h per home) were provided to nurses. Sessions addressed basic geriatric pharmacology and some common problems in long‐term care (depression, delirium and dementia, incontinence, falls, sleep disorders, constipation and pain). Sessions were supported by wall charts, bulletins, telephone calls and clinical pharmacy visits, averaging 26 h contact per home over the study. Written, referenced drug regimen reviews were prepared by the clinical pharmacists for 500 individual residents selected by the nursing home staff. The reviews highlighted the potential for: (1) adverse drug effects, (2) ceasing one or more drugs, (3) adding drugs, (4) better use of specific drug therapy, particularly psychoactive drugs, (5) non drug interventions, and (6) adverse effect and drug response monitoring. Initial reports (61% of total) were audited by a geriatrician before dissemination. Reports were placed in each resident's nursing home records, made available to the resident's GP and discussed with nursing staff. Drugs commonly targeted in reviews and education sessions included laxatives, histamine H2‐receptor antagonists, allopurinol, quinine, antibacterials, paracetamol, nonsteroidal anti‐inflammatory drugs (NSAIDs) and psychoactive drugs |
|
| Outcomes | Measured at baseline and 12 months post‐intervention: Hospital admissions (not defined) Mortality (survival also assessed at 22 months) Medication‐related problems Medication costs (per resident per year based on prescription claims data) Not used for this review: Adverse events (from incident reports) Resident Classification Instrument (RCI) Drug use |
|
| Notes | Supported by the Commonwealth Government of Australia under the Pharmaceutical Education Program | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Homes were assigned to intervention or control by being “drawn from a hat” |
| Allocation concealment (selection bias) | Low risk | Cluster design |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding conducted |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | No blinding reported |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | No blinding reported, however outcomes not likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) Primary outcomes | Unclear risk | Insufficient reporting of attrition/exclusions to permit judgement |
| Incomplete outcome data (attrition bias) Secondary outcomes | Unclear risk | Insufficient reporting of attrition/exclusions to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Similar baseline outcome measurements | Low risk | Slight imbalance in mortality and hospitalisations at baseline; however this was accounted for in the analysis |
| Similar baseline characteristics | Low risk | Similar baseline characteristics reported |
| Reliable primary outcome measure | Low risk | Mortality and Resident Classification Instrument (RCI) |
| Adequate protection against contamination | Unclear risk | Cluster design. [Attempted to contact author for further information but no response] |
| Other bias | High risk | Medication reviews were undertaken for a non‐random subsample of 500 residents (total intervention residents 905) selected by nursing staff |
Strikwerda 1994.
| Methods | RCT (randomised by GP) Total study duration: 6 weeks |
|
| Participants | 196 residents One nursing home Age: mean 84.5 years (59‐100) Gender: 25% male Country: Netherlands Date of study: 1993 |
|
| Interventions | Feedback on GP prescribing from community pharmacist Group A received usual care, group B GPs issued with a medication list used by their patients, group C GPs received a medication list plus feedback from community pharmacist |
|
| Outcomes | Medication‐related problems Not used for this review: drug use |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Low risk | Cluster design |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding conducted |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Not measured in this study |
| Incomplete outcome data (attrition bias) Primary outcomes | Unclear risk | Not measured in this study |
| Incomplete outcome data (attrition bias) Secondary outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Similar baseline outcome measurements | Unclear risk | No baseline measurements of medication‐related problems |
| Similar baseline characteristics | High risk | Most baseline characteristics similar, however fewer males in group A and fewer medicines per resident in group B |
| Reliable primary outcome measure | Low risk | Drug use |
| Adequate protection against contamination | High risk | Randomised by GP, however control and intervention residents resided in the same nursing home |
| Other bias | Low risk | Appears to be free of other sources of bias |
Zermansky 2006.
| Methods | RCT (randomised by patient) Total study duration: 6 months |
|
| Participants | 661 (331 intervention, 330 control) care home residents, 65 care homes Setting: Nursing and residential homes for older people Age: Intervention mean 85.3 (IQR 81‐90); control mean 84.9 (IQR 80‐90) Gender: Intervention 75 (22.7%) male; control 79 (23.9%) male Country: UK Date of study: 2002 |
|
| Interventions | Medication review by a single pharmacist A clinical medication review was conducted by the study pharmacist who held a postgraduate qualification in clinical pharmacy, within 28 days of randomisation. It comprised a review of the GP clinical record and a consultation with the resident and carer. The pharmacist formulated recommendations with the resident and carer and passed them on a written proforma to the GP for acceptance and implementation. GP acceptance was signified by ticking a box on the proforma. Control patients received usual GP care |
|
| Outcomes | Measured at baseline and six months ± three weeks post‐randomisation: Hospital admissions (non‐elective) Mortality Medication‐related problems Medicine costs (cost of 28 days of repeat medicines per participant) Not used for this review: Number of changes in medicines per participant Number of medicines per participant Recorded medication reviews Falls SMMSE Barthel index Number of GP consultations |
|
| Notes | Funded by The Health Foundation, 90 Long Acre, London WC2 9RA (Registered Charity Number 286967) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomised in randomly sized blocks of 2 to 8 patients using an algorithm written in Visual Basic in Microsoft Access |
| Allocation concealment (selection bias) | Low risk | Not reported in paper. Allocation was concealed to the research pharmacist and nurse data collector by statistician [Author contacted] |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open design, no blinding attempted |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | No blinding conducted |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | No blinding conducted, however outcomes not likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) Primary outcomes | Low risk | Similar attrition in both groups with similar reasons for dropouts. Described as intention‐to‐treat by authors |
| Incomplete outcome data (attrition bias) Secondary outcomes | Low risk | Similar attrition in both groups with similar reasons for dropouts. Described as intention‐to‐treat by authors |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Similar baseline outcome measurements | Low risk | Similar baseline measurements for hospital admissions and medicine costs |
| Similar baseline characteristics | Low risk | Similar baseline characteristics reported |
| Reliable primary outcome measure | Low risk | Number of changes in medication |
| Adequate protection against contamination | High risk | Randomised by patient therefore contamination possible |
| Other bias | Unclear risk | Sample size calculation indicated that 1600 residents were required, however, only 661 residents were recruited |
IQR: Interquartile Range
MMSE: Mini‐Mental State Examination
SD: Standard Deviation
I5D: 15 Dimensional Instrument of Health‐related Quality of Life
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Avorn 1992 | Whole medication regime not considered (psychoactive medicines only) |
| Crotty 2004c | Whole medication regime not considered (psychotropic and stroke medicines only) |
| Lapane 2011 | Focus was on delirium and falls |
| Milos 2013 | Included community‐dwelling patients in addition to nursing home residents |
Characteristics of ongoing studies [ordered by study ID]
Desborough ongoing.
| Trial name or title | Multi‐professional clinical medication reviews in care homes for the elderly: study protocol for a randomised controlled trial with cost effectiveness analysis |
| Methods | Cluster RCT (randomised by care home) Total Study Duration: 12 months |
| Participants | Residents of 30 care homes for older people (average age > 65) |
| Interventions | Intervention homes will receive a multi‐professional medication review at baseline and at 6 months, with follow‐up at 12 months. Control homes will receive usual care (support they currently receive from the National Health Service), with data collection at baseline and 12 months. |
| Outcomes | Emergency hospital admissions and Accident and Emergency (A&E) visits (number of admissions in six months per patient)
Mortality
Potentially inappropriate prescribing (number of drugs which match the STOPP criteria at each data collection point) Medication costs (mean drug costs per patient ‐ net ingredient costs for 28 days) Not used for this review: Number of falls (mean per patient per month) Utilisation of primary care, secondary care and personal social services health professional time (GP, nurse and other) |
| Starting date | 2011 |
| Contact information | |
| Notes |
NCT02238652.
| Trial name or title | Improving quality of life in nursing home residents: a cluster randomized clinical trial of efficacy (KOSMOS) |
| Methods | Cluster RCT (randomised by care home) Total Study Duration: ˜ 16 months |
| Participants | Residents of 38 care homes (˜ 310 participants, average age > 65) |
| Interventions | Staff training, study guidelines and manuals |
| Outcomes | Potentially inappropriate prescribing (number of drugs which match the STOPP criteria at each collection point) Medications which should be introduced (assessed using the START criteria) Hospital admissions Mortality Quality of life in late‐stage dementia Neuropsychiatric inventory Activities of daily living |
| Starting date | 2014 |
| Contact information | |
| Notes |
Wouters ongoing.
| Trial name or title | Discontinuing inappropriate medication in nursing home residents (DIM‐NHR Study): protocol of a cluster randomised controlled trial |
| Methods | Cluster RCT (elderly care physicians and wards randomised) |
| Participants | Residents of care home (˜ 600 residents) |
| Interventions | Multidisciplinary Multistep Medication Review (3MR) will be carried out by elderly care physicians in collaboration with a pharmacist. Data will be collected at baseline and 4 months after the 3MR has taken place. |
| Outcomes | Discontinuation of inappropriate medication (according to the STOPP criteria) Starting new medication (according to the START criteria) Harm (including mortality, falls, gastrointestinal bleeding, A&E and outpatient visits, physician consultations) Quality of life (measured with Dementia Quality of Life Instrument (DQI) and EQ‐5D‐3L Cognitive function measured using the Severe Impairment Battery (SIB) and the Mini‐Mental State Examination (MMSE) Expenditure on healthcare taking into account salary costs, medication costs, laboratory examinations,additional costs |
| Starting date | 2014 |
| Contact information | |
| Notes |
EQ‐5D‐3L: EuroQol 5 Dimension Health‐related Quality of Life
Differences between protocol and review
We intended to pool results and conduct meta‐analyses if studies were homogeneous. However, as studies were heterogeneous, this was not undertaken. Following identification of unit of analysis errors, we intended to attempt to reanalyse the data and report the intra‐cluster correlation coefficient and adjust for clustering if possible. However, instead, we commented on unit of analysis errors where appropriate within the results and discussion. Similarly, subgroup analyses were not possible. We used a revised search strategy for the update (see Search methods for identification of studies). New authors for this review were Mary‐Claire Kennedy and Paul Miller. Previous authors were Professor DK (Theo) Raynor, Professor Nick Barber and Ms Pat Spoor.
Contributions of authors
David Alldred conceived and co‐ordinated the review and is the guarantor of the review. David Alldred prepared the original protocol with support and advice from Carmel Hughes, Nick Barber, David Raynor, Pat Spoor and Tim Chen. Paul Miller adapted the original search strategy (previously developed by Ms Pat Spoor with input from David Alldred) and ran the searches. All authors were involved in the retrieval of papers. David Alldred and Mary‐Claire Kennedy screened the search results, assessed retrieved papers against the eligibility criteria, assessed risk of bias and extracted data from the papers. David Alldred was responsible for entering data into RevMan and drafting the review with input from all authors.
Sources of support
Internal sources
-
School of Healthcare, University of Leeds, UK.
Funding was provided for the services of Ms Pat Spoor to develop the original search strategy and run the searches.
External sources
No sources of support supplied
Declarations of interest
David Alldred is an author on a study that was included in this review (Zermansky 2006).David Alldred ‐ none other than as indicated above. Mary‐Claire Kennedy ‐ no declarations of interest. Carmel Hughes ‐ no declarations of interest. Timothy F Chen ‐ no declarations of interest. Paul Miller ‐ no declarations of interest.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Claesson 1998 {published data only}
- Claesson CB, Schmidt IK. Drug use in Swedish nursing homes. Clinical Drug Investigation 1998;16(6):441‐52. [DOI] [PubMed] [Google Scholar]
- Schmidt IK, Claesson CB, Westerholm B, Nilsson LG. Physician and staff assessments of drug interventions and outcomes in Swedish nursing homes. Annals of Pharmacotherapy 1998;32:27‐32. [DOI] [PubMed] [Google Scholar]
Connolly 2015 {published data only}
- Connolly MJ, Boyd M, Broad JB, Kerse N, Lumley T, Whitehead N, et al. The Aged Residential Care Healthcare Utilization Study (ARCHUS): a multidisciplinary, cluster randomized controlled trial designed to reduce acute avoidable hospitalizations from long‐term care facilities. Journal of the American Medical Directors Association 2015;16(1):49‐55. [DOI] [PubMed] [Google Scholar]
- Foster SJ, Boyd M, Broad JB, Whitehead N, Kerse N, Lumley T, et al. Aged Residential Care Health Utilisation Study (ARCHUS): a randomised controlled trial to reduce acute hospitalisations from residential aged care. BMC Geriatrics 2012;12:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Crotty 2004a {published data only}
- Crotty M, Halbert J, Rowett D, Giles L, Birks R, Williams H, et al. An outreach geriatric medicine advisory service in residential aged care: a randomised controlled trial of case conferencing. Age and Ageing 2004;33:612‐7. [DOI: 10.1093/ageing/afh213] [DOI] [PubMed] [Google Scholar]
Crotty 2004b {published data only}
- Crotty M, Rowett D, Spurling L, Giles LC, Phillips PA. Does the addition of a pharmacist transition coordinator improve evidence‐based medication management and health outcomes in older adults moving from the hospital to a long‐term care facility? Results of a randomized, controlled trial. The American Journal of Geriatric Pharmacotherapy 2004;2(4):257‐64. [DOI: 10.1016/j.amjopharm.2005.01.001] [DOI] [PubMed] [Google Scholar]
Frankenthal 2014 {published data only}
- Frankenthal D, Lerman Y, Kalendaryev E, Lerman Y. Intervention with the screening tool of older persons potentially inappropriate prescriptions/screening tool to alert doctors to right treatment criteria in elderly residents of a chronic geriatric facility: a randomized clinical trial. Journal of the American Geriatrics Society 2014;62(9):1658‐65. [DOI] [PubMed] [Google Scholar]
Furniss 2000 {published data only}
- Burns A, Furniss L, Cooke J, Lloyd Craig SK, Scobie S. Pharmacist medication review in nursing homes: a cost analysis. International Journal of Geriatric Psychopharmacology 2000;2:137‐41. [Google Scholar]
- Furniss L, Burns A, Craig SKL, Scobie S, Cooke J, Faragher B. Effect of a pharmacist's medication review in nursing homes: randomised controlled trial. The British Journal of Psychiatry 2000;176:563‐7. [DOI: 10.1192/bjp.176.6.563] [DOI] [PubMed] [Google Scholar]
- Furniss L, Craig S, Scobie S, Cooke J, Burns A. Medication reviews in nursing homes: documenting and classifying the activities of a pharmacist. Pharmaceutical Journal 1998;261(7009):320‐3. [Google Scholar]
Garcia‐Gollarte 2014 {published data only}
- Garcia‐Gollarte F, Baleriola‐Julvez J, Ferrero‐Lopez I, Cuenllas‐Diaz A, Cruz‐Jentoft AJ. An educational intervention on drug use in nursing homes improves health outcomes resource utilization and reduces inappropriate drug prescription. Journal of the American Medical Directors Association 2014;15(12):885‐91. [DOI] [PubMed] [Google Scholar]
Gurwitz 2008 {published data only}
- Gurwitz JH, Field TS, Rochon P, Judge J, Harrold LR, Bell CM, et al. Effect of computerised provider order entry with clinical decision support on adverse drug events in the long‐term care setting. Journal of the American Geriatrics Society 2008;56:2225‐33. [DOI] [PubMed] [Google Scholar]
- Judge J, Field TS, DeFlorio M, Laprino J, Auger J, Rochon P, et al. Prescribers' responses to alerts during medication ordering in the long term care setting. Journal of the American Medical Informatics Association 2006;13:385‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pitkala 2014 {published data only}
- Pitkala KH, Juola AL, Kautiainen H, Soini H, Finne‐Soveri UH, Bell JS, et al. Education to reduce potentially harmful medication use among residents of assisted living facilities: a randomized controlled trial. Journal of the American Medical Directors Association 2014;15(12):892‐8. [DOI] [PubMed] [Google Scholar]
Roberts 2001 {published data only}
- Roberts M, Stokes JA, King MA, Lynne TA, Purdie DM, Glasziou PP, et al. Outcomes of a randomized controlled trial of a clinical pharmacy intervention in 52 nursing homes. British Journal of Clinical Pharmacology 2001;51:257‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Strikwerda 1994 {published data only}
- Strikwerda P, Bootsma‐De Langen AM, Berghuis F, Meyboom‐de Jong B. Drug therapy in a nursing home; favorable effect of feedback by the pharmacist on family physician's prescribing behavior [Farmacotherapie in een verzorgingshuis; gunstige invloed van feed‐back door de apotheker op het voorschrijfgedrag van de huisarts]. Nederlands Tijdschrift voor Geneeskunde 1994;138(35):1770‐4. [PubMed] [Google Scholar]
Zermansky 2006 {published data only}
- Alldred DP, Zermansky AG, Petty DR, Raynor DK, Freemantle N, Eastaugh J, et al. Clinical medication review by a pharmacist of elderly people living in care homes: pharmacist interventions. International Journal of Pharmacy Practice 2007;13:93‐9. [DOI: 10.1211/ijpp.15.2.0003] [DOI] [Google Scholar]
- Zermansky AG, Alldred DP, Petty DR, Raynor DK, Freemantle N, Eastaugh J, et al. Clinical medication review by a pharmacist of elderly people living in care homes ‐ randomised controlled trial. Age and Ageing 2006;35:586‐91. [DOI: 10.1093/ageing/afl075; ISRCTN: 45416155] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Avorn 1992 {published data only}
- Avorn J, Soumerai SB, Everitt DE, Ross‐Degnan D, Beers MH, Sherman D, Salem‐Schatz SR, Fields D. A Randomized Trial of a Program to Reduce the Use of Psychoactive Drugs in Nursing Homes. New England Journal of Medicine 1992;327(3):168‐73. [DOI] [PubMed] [Google Scholar]
Crotty 2004c {published data only}
- Crotty M, Whitehead C, Rowett D, Halbert J, Weller D, Finucane P, et al. An outreach intervention to implement evidence based practice in residential care: a randomized controlled trial. BMC Health Services Research 2004;4:6. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lapane 2011 {published data only}
- Lapane KL, Hughes CM, Daiello LA, Cameron, KA, Feinberg J. Effect of a pharmacist‐led multicomponent intervention focusing on the medication monitoring phase to prevent potential adverse drug events in nursing homes. Journal of the American Geriatrics Society 2011;59(7):1238‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Milos 2013 {published data only}
- Milos V, Rekman E, Bondesson A, Eriksson T, Jakobsson U, Westerlund T, et al. Improving the quality of pharmacotherapy in elderly primary care patients through medication reviews: a randomised controlled study. Drugs & Aging 2013;30(4):235‐46. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Desborough ongoing {published data only}
- Desborough J, Houghton J, Wood J, Wright D, Holland R, Sach T, et al. Multi‐professional clinical medication reviews in care homes for the elderly: study protocol for a randomised controlled trial with cost effectiveness analysis. Trials 2011;12:218. [ISRCTN: 90761620] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT02238652 {published data only}
- NCT02238652. Improving quality of life in nursing home residents: a cluster randomized clinical trial of efficacy. https://clinicaltrials.gov/ct2/show/NCT02238652 2014 (accessed 29 January 2016). [CTG: NCT02238652]
Wouters ongoing {published data only}
- Wouters H, Quik EH, Boersma F, Nygard P, Bosman J, Bottger WM, Mulder H, Maring JG, Wijma‐Vos L, Beerden T, Doormaal J, Postma MJ, Zuidema SU, Taxis K. Discontinuing inappropriate medication in nursing home residents (DIM‐NHR study): Protocol of a cluster randomised controlled trial. BMJ Open 2014;4(10):1. [CTG: NCT01876095; DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Alldred 2007a
- Alldred DP, Petty DR, Bowie P, Zermansky AG, Raynor DK. Antipsychotic prescribing patterns in care homes and relationship with dementia. Psychiatric Bulletin 2007;31(9):329‐32. [Google Scholar]
Alldred 2007b
- Alldred DP, Zermansky AG, Petty DR, Raynor DK, Freemantle N, Eastaugh J, et al. Clinical medication review by a pharmacist of elderly people living in care homes: Pharmacist interventions. International Journal of Pharmacy Practice 2007;15(2):93‐9. [Google Scholar]
Armour 2002
- Armour D, Cairns C (editors). Medicines in the Elderly. London: The Pharmaceutical Press, 2002. [Google Scholar]
Barber 2009
- Barber ND, Alldred DP, Raynor DK, Dickinson R, Garfield S, Jesson B, et al. Care homes' use of medicines study: prevalence, causes and potential harm of medication errors in care homes for older people. Quality and Safety in Health Care 2009;18:341‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Beers 1992
- Beers MH, Ouslander JG, Fingold SF, Morgenstern H, Reuben DB, Rogers W, et al. Inappropriate medication prescribing in skilled nursing‐home facilities. Annals of Internal Medicine 1992;117:684‐9. [DOI] [PubMed] [Google Scholar]
Beglinger 2008
- Beglinger C. Ethics related to drug therapy in the elderly. Digestive Diseases 2008;26(1):28‐31. [DOI] [PubMed] [Google Scholar]
Brymer 2003
- Brymer C, Borrie MJ, Crilly RG, Hindman D, Esbaugh J, Pavlakovic R, et al. Prevalence of and factors associated with potentially inappropriate prescribing in long‐term care facilities. Journal of the Canadian Geriatric Society 2003;6:146‐52. [Google Scholar]
Christensen 2011
- Christensen M, Lundh A. Medication review of hospitalized patients to prevent morbidity and mortality. Cochrane Database of Systematic Reviews 2011, Issue 2. [DOI: 10.1002/14651858.CD008986] [DOI] [PMC free article] [PubMed] [Google Scholar]
Department of Health 2001
- Department of Health. National Service Framework for Older People. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/198033/National_Service_Framework_for_Older_People.pdf.. London: Department of Health, 2001 (accessed 29 January 2016).
EPOC 2013
- Effective Practice, Organisation of Care (EPOC). Data collection form. EPOC Resources for review authors. Oslo: Norwegian Knowledge Centre for the Health Services. http://www.epoc.cochrane.org/epoc‐specific‐resources‐review‐authors. Oslo, 2013.
EPOC 2015
- Effective Practice, Organisation of Care (EPOC). Suggested risk of bias criteria for EPOC reviews. EPOC resources for review authors. http://www.epoc.cochrane.org/epoc‐specific‐resources‐review‐authors. Oslo: Norwegian Knowledge Centre for the Health Services, 2015. [Google Scholar]
Fahey 2003
- Fahey T, Montgomery AA, Barnes J, Protheroe J. Quality of care for elderly residents in nursing homes and elderly people living at home: controlled observational study. BMJ 2003;326:580‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gallagher 2008
- Gallagher P, Ryan C, Byrne S, Kennedy, J O'Mahony D. STOPP (Screening Tool of Older Person’s Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. International Journal of Clinical Pharmacology and Therapeutics 2008;46:72‐83. [DOI] [PubMed] [Google Scholar]
GRADE 2012
- GRADE. Grading of Recommendations Assessment, Development and Evaluation Working Group. http://www.gradeworkinggroup.org/ (accessed 29 January 2016).
Gray 2003
- Gray SL, Hedrick SC, Rhinard EE, Sales AE, Sullivan JH, Tomatore JB, et al. Potentially inappropriate medication use in community residential care facilities. The Annals of Pharmacotherapy 2003;37:988‐93. [DOI] [PubMed] [Google Scholar]
Gurwitz 2005
- Gurwitz JH, Field TS, Judge J, Rochon P, Harrold LR, Cadoret C, et al. The incidence of adverse drug events in two large academic long‐term care facilities. The American Journal of Medicine 2005;118:251‐8. [DOI] [PubMed] [Google Scholar]
Hanlon 1992
- Hanlon JT, Schmader KE, Samsa GP, Weinberger M, Uttech KM, Lewis IK, et al. A method for assessing drug therapy appropriateness. Journal of Clinical Epidemiology 1992;45(10):1045‐51. [DOI] [PubMed] [Google Scholar]
Hanlon 1996
- Hanlon JT, Weinberger M, Samsa GP, Schmader KE, Uttech KM, Lewis IK, et al. A randomised controlled trial of a clinical pharmacist intervention with elderly outpatients with polypharmacy. American Journal of Medicine 1996;100:428‐37. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.. The Cochrane Collaboration.
Howard 2007
- Howard RL, Avery AJ, Slavenburg S, Royal S, Pipe G, Lucassen P, et al. Which drugs cause preventable admission to hospital? A systematic review. British Journal of Clinical Pharmacology 2007;63:136‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kaur 2009
- Kaur S, Mitchell G, Vitetta L, Roberts MS. Interventions that can reduce inappropriate prescribing in the elderly. Drugs & Aging 2009;26(12):1013‐28. [DOI] [PubMed] [Google Scholar]
LaMantia 2010
- LaMantia MA, Schernemann LP, Viera AJ, Busby‐Whitehead J, Hanson LC. Interventions to improve transitional care between nursing homes and hospitals: a systematic review. Journal of the American Geriatrics Society 2010;58:777‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lau 2005
- Lau DT, Kasper JD, Potter DEB, Lyles A, Bennett RG. Hospitalization and death associated with potentially inappropriate medication prescriptions among elderly nursing home residents. Archives of Internal Medicine 2005;165:68‐74. [DOI] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Medicine 2009;6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Loganathan 2011
- Longanathan M, Singh S, Dean Franklin B, Bottle A, Majeed A. Interventions to optimise prescribing in care homes: systematic review. Age and Ageing 2011;40:150‐62. [DOI] [PubMed] [Google Scholar]
Markum 2010
- Markum ZA, Handler SM, Wright R, Hanlon JT. Interventions to improve suboptimal prescribing in nursing homes: a narrative review. The American Journal of Geriatric Pharmacotherapy 2010;8(3):183‐200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Matthews 2002
- Matthews FE, Dening T. Prevalence of dementia in institutional care. Lancet 2002;360:225‐6. [DOI] [PubMed] [Google Scholar]
Oborne 2003
- Oborne CA, Hooper R, Swift GC, Jackson SD. Explicit, evidence‐based criteria to assess the quality of prescribing to elderly nursing home residents. Age and Ageing 2003;32:102‐8. [DOI] [PubMed] [Google Scholar]
Office for National Statistics 2010
- Office for National Statistics. Ageing. http://www.ons.gov.uk/ons/taxonomy/index.html?nscl=Older+People 2010 (accessed 29 January 2016).
Ostini 2009
- Ostini R, Hegney D, Jackson C, Williamson M, Mackson JM, Gurman K, et al. Systematic review of interventions to improve prescribing. The Annals of Pharmacotherapy 2009;43:502‐12. [DOI] [PubMed] [Google Scholar]
Patterson 2014
- Patterson SM, Cadogan CA, Kerse N, Cardwell CR, Bradley MC, Ryan C, et al. Interventions to improve the appropriate use of polypharmacy for older people. Cochrane Database of Systematic Reviews 2014, Issue 10. [DOI: 10.1002/14651858.CD008165.pub3] [DOI] [PubMed] [Google Scholar]
Perri 2005
- Perri M, Menon AM, Deshpande AD, Shinde SB, Cooper JW, Cook CL, et al. Adverse outcomes associated with inappropriate drug use in nursing homes. The Annals of Pharmacotherapy 2005;39:405‐11. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre. Review Manager (RevMan). [Computer program]. Version 5.3. Copenhagen: The Cochrane Collaboration, 2014.
Rollason 2003
- Rollason V, Vogt N. Reduction of polypharmacy in the elderly. A systematic review of the role of the pharmacist. Drugs and Aging 2003;20(11):817‐32. [DOI] [PubMed] [Google Scholar]
Sloane 2002
- Sloane PD, Zimmerman S, Brown LC, Ives TJ, Walsh J. Inappropriate medication prescribing in residential care/assisted living facilities. Journal of the American Geriatrics Society 2002;50(6):1001‐11. [DOI] [PubMed] [Google Scholar]
Soe 2009
- Soe A, Apampa B, Fernando B, Thayyil S, Neubert A, Ghaleb MA. Interventions for reducing medication errors in children in hospital. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD006208] [DOI] [PMC free article] [PubMed] [Google Scholar]
Spinewine 2007
- Spinewine A, Schmader KE, Barber N, Hughes C, Lapane KL, Swine C, et al. Appropriate prescribing in elderly people: how well can it be measured and optimised?. Lancet 2007;370(9582):173‐84. [DOI] [PubMed] [Google Scholar]
United Nations 2009
- United Nations (Department of Economic and Social Affairs: Population Division). World Population Ageing. http://www.un.org/esa/population/publications/WPA2009/WPA2009_WorkingPaper.pdf.. New York: United Nations (Department of Economic and Social Affairs: Population Division), 2009 (accessed 29 January 2016).
Verrue 2009
- Verrue CL, Petrovic M, Mehuys E, Remon JP, Vander Stichele R. Pharmacists' interventions for optimization of medication use in nursing homes: a systematic review. Drugs & Aging 2009;26:37‐49. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Alldred 2011
- Alldred DP, Raynor DK (Theo), Hughes C, Barber N, Chen TF, Spoor P. Interventions to optimise prescribing for older people in care homes. Cochrane Database of Systematic Reviews 2011, Issue 4. [DOI: 10.1002/14651858.CD009095] [DOI] [PubMed] [Google Scholar]
Alldred 2013
- Alldred DP, Raynor DK, Hughes C, Barber N, Chen TF, Spoor P. Interventions to optimise prescribing for older people in care homes. Cochrane Database of Systematic Reviews 2013, Issue 2. [DOI: 10.1002/14651858.CD009095.pub2] [DOI] [PubMed] [Google Scholar]


