Abstract
Background
Foot ulcers in people with diabetes mellitus are a common and serious global health issue. Dressings form a key part of ulcer treatment, with clinicians and patients having many different types to choose from including alginate dressings. A clear and current overview of current evidence is required to facilitate decision‐making regarding dressing use.
Objectives
To compare the effects of alginate wound dressings with no wound dressing or alternative dressings on the healing of foot ulcers in people with diabetes mellitus.
Search methods
For this first update, in April 2013, we searched the following databases the Cochrane Wounds Group Specialised Register; The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library); Ovid MEDLINE; Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations); Ovid EMBASE; and EBSCO CINAHL. There were no restrictions based on language or date of publication.
Selection criteria
Published or unpublished randomised controlled trials (RCTs) that have compared the effects on ulcer healing of alginate dressings with alternative wound dressings or no dressing in the treatment of foot ulcers in people with diabetes.
Data collection and analysis
Two review authors independently performed study selection, risk of bias assessment and data extraction.
Main results
We included six studies (375 participants) in this review; these compared alginate dressings with basic wound contact dressings, foam dressings and a silver‐containing, fibrous‐hydrocolloid dressing. Meta analysis of two studies found no statistically significant difference between alginate dressings and basic wound contact dressings: risk ratio (RR) 1.09 (95% CI 0.66 to 1.80). Pooled data from two studies comparing alginate dressings with foam dressings found no statistically significant difference in ulcer healing (RR 0.67, 95% CI 0.41 to 1.08). There was no statistically significant difference in the number of diabetic foot ulcers healed when an anti‐microbial (silver) hydrocolloid dressing was compared with a standard alginate dressing (RR 1.40, 95% CI 0.79 to 2.47). All studies had short follow‐up times (six to 12 weeks), and small sample sizes.
Authors' conclusions
Currently there is no research evidence to suggest that alginate wound dressings are more effective in healing foot ulcers in people with diabetes than other types of dressing however many trials in this field are very small. Decision makers may wish to consider aspects such as dressing cost and the wound management properties offered by each dressing type e.g. exudate management.
Plain language summary
Alginate dressings for healing foot ulcers in people with diabetes mellitus
Diabetes mellitus, a condition which leads to high blood glucose concentrations, is a common condition with around 2.8 million people affected in the UK (approximately 4.3% of the population). Wound dressings are widely used to treat foot ulcers in people with diabetes. There are many types of dressings that can be used, which also vary considerably in cost. This review (six studies involving a total of 375 participants) identified no research evidence to suggest that alginate wound dressings are more effective in healing diabetic foot ulcers than other types of dressing. More, better quality research is needed.
Summary of findings
Summary of findings for the main comparison. Alginate dressing compared to basic wound contact dressing for healing diabetic foot ulcers.
| Alginate dressing compared to basic wound contact dressing for healing diabetic foot ulcers | ||||||
| Patient or population: patients with Settings: Any Intervention: Alginate dressing Comparison: basic wound contact dressing | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Basic wound contact dressing | Alginate dressing | |||||
| Number of ulcers healed | Low1 | RR 1.09 (0.66 to 1.8) | 114 (2 studies) | ⊕⊕⊝⊝ low2,3 | ||
| 340 per 1000 | 371 per 1000 (224 to 612) | |||||
| Moderate1 | ||||||
| 530 per 1000 | 578 per 1000 (350 to 954) | |||||
| High1 | ||||||
| 650 per 1000 | 708 per 1000 (429 to 1000) | |||||
| HRQoL ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | Foot ulcers in people with diabetes adversely affect quality of life however no study measured (or reported) this. |
| Adverse events | See comment | See comment | Not estimable | 0 (0) | See comment | Reporting of adverse events appeared to be ad hoc and the number of events were extremely small. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Baseline risk of healing obtained from external source in which data from 27,630 patients with a diabetic neuropathic foot ulcer was used to develop a simple prognostic model to predict likelihood of ulcer healing (Margolis DJ, Allen‐Taylor L, Hoffstad O, Berlin JA. Diabetic neuropathic foot ulcers: predicting which ones will not heal. Am J Med. 2003;115:627‐31). It is important to note that given an outcome of ulcer healing, low risk refers to a low risk of healing and thus reflects the most severe patient populations. Conversely high risk refers to a high risk of healing. 2 Both studies at unclear or high risk of bias 3 Studies were small, total number of participants 114 with only 40% achieving healing; this is an underpowered comparison.
Summary of findings 2. Alginate dressings compared to foam dressings for healing diabetic foot ulcers.
| Alginate dressings compared to foam dressings for healing diabetic foot ulcers | ||||||
| Patient or population: patients with healing diabetic foot ulcers Settings: Any Intervention: Alginate dressings Comparison: foam dressings | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Foam dressings | Alginate dressings | |||||
| Number of ulcers healed | Low1 | RR 0.67 (0.41 to 1.08) | 50 (2 studies) | ⊕⊕⊝⊝ low2,3 | ||
| 340 per 1000 | 228 per 1000 (139 to 367) | |||||
| Moderate1 | ||||||
| 530 per 1000 | 355 per 1000 (217 to 572) | |||||
| High1 | ||||||
| 650 per 1000 | 435 per 1000 (266 to 702) | |||||
| HRQoL ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | Foot ulcers in people with diabetes adversely affect quality of life however no study measured (or reported) this. |
| Adverse events | See comment | See comment | Not estimable | 0 (0) | See comment | Reporting of adverse events appeared to be ad hoc and the number of events were extremely small and only reported in one trial. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Baseline risk of healing obtained from external source in which data from 27,630 patients with a diabetic neuropathic foot ulcer was used to develop a simple prognostic model to predict likelihood of ulcer healing (Margolis DJ, Allen‐Taylor L, Hoffstad O, Berlin JA. Diabetic neuropathic foot ulcers: predicting which ones will not heal. Am J Med. 2003;115:627‐31). It is important to note that given an outcome of ulcer healing, low risk refers to a low risk of healing and thus reflects the most severe patient populations. Conversely high risk refers to a high risk of healing. 2 Both studies overall at unclear risk of bias, however one study was at low risk of bias for one domain (random sequence generation). 3 Only 50 participants were included in this comparison.
Background
Description of the condition
Diabetes mellitus, a condition which leads to high blood glucose concentrations is common and affects around 2.8 million people in the UK (approximately 4.3% of the population) (Diabetes UK 2011). This number is set to increase over the next 25 years as the incidence of diabetes increases rapidly (WHO 2005). Global projections suggest that the worldwide prevalence of diabetes is expected to rise to 4.4% by 2030, meaning that approximately 366 million people will be affected (Wild 2004).
Success in treating people with diabetes has improved their life expectancy. However, the increased prevalence of diabetes coupled with the extended time people live with the disease has led to a rise in the number of diabetes‐related complications, such as neuropathy and peripheral arterial disease (PAD). It is estimated that lower extremity disease (defined as lower‐extremity PAD, lower‐extremity peripheral neuropathy or history of foot ulcer or lower‐extremity amputations) is twice as common in people with diabetes compared with people without (Gregg 2004). Both neuropathy and PAD are risk factors for diabetic foot ulceration (Pecoraro 1990; Reiber 1999), which is a problem reported to affect 15% or more of the diabetic population at some time in their lives (Reiber 1996; Singh 2005). Around 1% to 4% of people with diabetes have foot ulcers at any given time (Abbott 2002; Kumar 1994). An ulcer forms as a result of damage to the epidermis and subsequent loss of underlying tissue. Specifically, the International Consensus on the Diabetic Foot defines a foot ulcer as a wound extending through the full thickness of the skin below the level of the ankle (Apelqvist 2000a). This is irrespective of duration and the ulcer can extend to muscle, tendon and bone. The Wagner wound classification system is well established and widely used for grading diabetic foot ulcers. The system assesses ulcer depth and the presence of osteomyelitis or gangrene in the following grades: grade 0 (pre‐ or post‐ulcerative lesion), grade 1 (partial/full thickness ulcer), grade 2 (probing to tendon or capsule), grade 3 (deep with osteitis), grade 4 (partial foot gangrene) and grade 5 (whole foot gangrene) (Wagner 1981). However, newer grading systems, such as the PEDIS system (Schaper 2004) and the University of Texas Wound Classification System (Oyibo 2001) have been developed.
PAD and neuropathy can occur separately (ischaemic foot and neuropathic foot) or in combination (in the neuroischaemic foot). The over‐arching term 'diabetic neuropathy' refers to a number of neuropathic syndromes. Chronic distal sensorimotor symmetrical neuropathy (abbreviated to distal symmetrical neuropathy) is the most common, affecting around 28% of people with diabetes. It can lead to ulceration through the following route(s) (Tesfaye 1996).
Sympathetic autonomic neuropathy leads to decreased sweating causing anhidrotic (dry) skin, which is prone to cracks and fissures causing a break in the dermal barrier (Tesfaye 1996).
Motor neuropathy causes wasting of the small, intrinsic muscles of the foot by de‐enervation. As the muscles waste they cause retraction of the toes and lead to a subsequent deformity. The abnormal foot shape can promote ulcer development due to an increase in plantar pressures (Murray 1996).
Sensory neuropathy results in impaired sensation, making the patient unaware of potentially dangerous foreign bodies and injuries.
People with diabetes‐related foot ulceration are treated in a variety of settings, for example community clinics, surgeries and their own homes, by a variety of practitioners; this can make data collection challenging. A UK study estimated that 2% of community‐based diabetic patients develop new foot ulcers each year (Abbott 2002). In terms of healing, a meta‐analysis of trials in which people with neuropathic ulcers received good wound care reported that 24% of ulcers attained complete healing by 12 weeks and 31% by 20 weeks (Margolis 1999). However, the risk of ulcer recurrence post‐healing is high. Pound 2005 reported that 62% of ulcer patients (n = 231) became ulcer‐free at some stage over a 31‐month observation period. However, of the ulcer‐free group 40% went on to develop a new or recurrent ulcer after a median of 126 days. The ulcer recurrence rate over five years can be as high as 70% (Dorresteijn 2010; Van Gils 1999).
Diabetic foot ulcers can seriously impact on an individual's quality of life and as many as 85% of foot‐related amputations are preceded by ulceration (Apelqvist 2000b; Pecoraro 1990). Patients with diabetes have a 10 to 20‐fold higher risk of losing a lower limb or part of a lower limb due to non‐traumatic amputation than those without diabetes (Morris 1998; Wrobel 2001).
Diabetic foot ulcers represent a major use of health resources, incurring costs not only for dressings applied, but also staff costs (for podiatry, nurses, doctors), tests and investigations, antibiotics and specialist footwear. Currie 1998 estimated the cost of healing a foot ulcer in a patient with diabetes at around GBP 1451. Hospital admissions add further to the costs. Ten years ago the cost of diabetic foot ulceration to the UK National Health Service was believed to be about GBP 12.9 million per year (Spencer 2000) and this figure is likely to have increased significantly. The economic impact is also high in terms of the personal costs to patients and carers, for example costs associated with lost work time and productivity while the patient is non‐weight bearing or hospitalised.
Description of the intervention
Broadly, the treatment of diabetic foot ulcers includes pressure relief (or off‐loading) by resting the foot, or wearing special footwear or shoe inserts (or both); the removal of dead cellular material from the surface of the wound (debridement or desloughing)(Edwards 2010); infection control (Storm‐Versloot 2007); and the use of wound dressings (Bergin 2006; Dumville 2011a; Dumville 2011b). Other general strategies in the treatment of diabetic foot ulcers include: patient education (Dorresteijn 2010; Dorrestein 2001); optimisation of blood glucose control; correction (where possible) of arterial insufficiency; and surgical interventions (debridement, drainage of pus, revascularisation, amputation).
Dressings are widely used in wound care, both to protect the wound and to promote healing. Classification of a dressing normally depends on the key material used. Several attributes of an ideal wound dressing have been described (BNF 2010), including:
the ability of the dressing to absorb and contain exudate without leakage or strike‐through;
lack of particulate contaminants left in the wound by the dressing;
thermal insulation;
permeability to water and impermeability to bacteria;
avoidance of wound trauma on dressing removal;
low frequency of required dressing changes required;
provision of pain relief; and
comfort.
There is a vast choice of dressings available to treat chronic wounds such as diabetic foot ulcers. For ease of comparison this review has categorised dressings according to the British National Formulary 2010 (BNF 2010), which is freely available via the Internet. We will use generic names where possible, also providing UK trade names and manufacturers where these are available to allow cross reference with the BNF. However, it is important to note, however, that as a result of the way dressings are categorised, dressing names, manufacturers and distributors of dressings may vary from country to country, so these are provided as a guide only. Below is a description of all categories of dressings and includes the category of dressing (alginates) which is the focus of this review:
Basic wound contact dressings
Low‐adherence dressings and wound contact materials: usually cotton pads which are placed directly in contact with the wound. They can be either non‐medicated (e.g. paraffin gauze dressing) or medicated (e.g. containing povidone iodine or chlorhexidine). Examples are paraffin gauze dressing, BP 1993 and Xeroform (Covidien) dressing ‐ a non‐adherent petrolatum blend with 3% bismuth tribromophenate on fine mesh gauze.
Absorbent dressings: applied directly to the wound or used as secondary absorbent layers in the management of heavily exuding wounds. Examples include Primapore (Smith & Nephew), Mepore (Mölnlycke) and absorbent cotton gauze (BP 1988).
Advanced wound dressings
Alginate dressings: highly absorbent and come in the form of calcium alginate or calcium sodium alginate and can be combined with collagen. The alginate forms a gel when in contact with the wound surface which can be lifted off with dressing removal or rinsed away with sterile saline. Bonding to a secondary viscose pad increases absorbency. Examples are: Curasorb (Covidien), SeaSorb (Coloplast) and Sorbsan (Unomedical).
Foam dressings: normally contain hydrophilic polyurethane foam and are designed to absorb wound exudate and maintain a moist wound surface. There are various versions and some foam dressings include additional absorbent materials, such as viscose and acrylate fibres or particles of superabsorbent polyacrylate, or which are silicone‐coated for non‐traumatic removal. Examples are: Allevyn (Smith & Nephew), Biatain (Coloplast) and Tegaderm (3M).
Hydrogel dressings: consist of cross‐linked insoluable polymers (i.e. starch or carboxymethylcellulose) and up to 96% water. These dressings are designed to absorb wound exudate or rehydrate a wound depending on the wound moisture levels. They are supplied in either flat sheets, an amorphous hydrogel or as beads. Examples are: ActiformCool (Activa) and Aquaflo (Covidien).
Films ‐ permeable film and membrane dressings: permeable to water vapour and oxygen but not to water or microorganisms. Examples are Tegaderm (3M) and Opsite (Smith & Nephew).
Soft polymer dressings: dressings composed of a soft silicone polymer held in a non‐adherent layer. They are moderately absorbent. Examples are: Mepitel (Mölnlycke) and Urgotul (Urgo).
Hydrocolloid dressings: are occlusive dressings usually composed of a hydrocolloid matrix bonded onto a vapour‐permeable film or foam backing. When in contact with the wound surface this matrix forms a gel to provide a moist environment. Examples are: Granuflex (ConvaTec) and NU DERM (Systagenix). Fibrous alternatives have been developed which resemble alginates and are not occlusive but which are more absorbant than standard hydrocolloid dressings: Aquacel (ConvaTec).
Capillary‐action dressings: consist of an absorbent core of hydrophilic fibres held between two low‐adherent contact layers. Examples are: Advadraw (Advancis) and Vacutx (Protex).
Odour‐absorbent dressings: dressings that contain charcoal and are used to absorb wound odour. Often these types of wound dressings are used in conjunction with a secondary dressing to improve absorbency. Example: CarboFLEX (ConvaTec).
Anti‐microbial dressings
Honey‐impregnated dressings: contain medical‐grade honey which is proposed to have antimicrobial and anti‐inflammatory properties and can be used for acute or chronic wounds. Examples are: Medihoney (Medihoney) and Activon Tulle (Advancis).
Iodine‐impregnated dressings: release free iodine when exposed to wound exudate, which is thought to act as a wound antiseptic. Examples are Iodoflex (Smith & Nephew) and Iodozyme (Insense).
Silver‐impregnated dressings: used to treat infected wounds as silver ions are thought to have antimicrobial properties. Silver versions of most dressing types are available (e.g. silver foam, silver hydrocolloid etc). Examples are: Acticoat (Smith & Nephew) and Urgosorb Silver (Urgo).
Other antimicrobial dressings: these dressings are composed of a gauze or low‐adherent dressing impregnated with an ointment thought to have antimicrobial properties. Examples are: chlorhexidine gauze dressing (Smith & Nephew) and Cutimed Sorbact (BSN Medical).
Specialist dressings
Protease‐modulating matrix dressings: alter the activity of proteolytic enzymes in chronic wounds. Examples are: Promogran (Systagenix) and Sorbion (H & R).
The diversity of dressings available to clinicians (including variation within each type, listed above) makes evidence‐based decision‐making difficult when deciding the best treatment regimen for the patient. In a UK survey undertaken to determine treatments used for debriding diabetic foot ulcers, a diversity of treatments was reported (Smith 2003). It is possible that a similar scenario is true for dressing choice. A survey of Diabetes Specialist Nurses found that low/non‐adherent dressings, hydrocolloids and alginate dressings were the most popular for all wound types, despite a paucity of evidence for any of these dressing types (Fiskin 1996). However, several new dressing types have been made available and heavily promoted in recent years. Some dressings now have an 'active' ingredient such as silver that are promoted as dressing treatment options to reduce infection and thus possibly also promote healing in this way. With increasingly sophisticated technology being applied to wound care, practitioners need to know how effective these often expensive dressings are compared with more traditional dressings.
How the intervention might work
Animal experiments conducted over 40 years ago suggested that an acute wound heals more quickly when its surface is kept moist, rather than left to dry and scab (Winter 1963). A moist environment is thought to provide optimal conditions for the cells involved in the healing process, as well as allowing autolytic debridement (removal/digestion of dead matter via the body), which is thought to be an important part of the healing pathway (Cardinal 2009). The desire to maintain a moist wound environment is a key driver for the use of wound dressings. Different wound dressings vary in their level of absorbency, so that a very wet wound can be treated with an absorbent dressing (such as an alginate dressing) to draw excess moisture away from the wound to avoid skin maceration, whilst a drier wound can be treated with a more occlusive dressing to maintain a moist environment. Alginate dressings contain sodium, or sodium and calcium, salts of alginic acid. These alginate salts are highly hydrophilic and can absorb large volumes of wound exudate.
Why it is important to do this review
Diabetic foot ulcers are a common consequence of diabetes internationally. Treatment with dressings forms a key part of the treatment pathway when caring for people with diabetic foot ulcers and there are many types of dressings that can be used, which also vary considerably in cost. Guidelines for the treatment of diabetic ulcer (e.g. Steed 2006) maintain that clinical judgement should be used to select a moist wound dressing.
However, previous reviews of the evidence for wound dressings as treatments for diabetic foot ulcers have not found evidence to support a specific dressing choice. Ten trials were eligible for inclusion in a UK Health Technology Assessment review of wound dressings published in 2000 (O'Meara 2000). The review included nine trials that investigated a dressing or topical treatment for healing diabetic foot ulcers. The review did not find any evidence to suggest that one dressing type was more or less effective in terms of treating diabetic foot ulcers. The methodological quality of trials was poor and all were small. Only one comparison was repeated in more than one trial. A further systematic review, conducted some years ago reported similar findings (Mason 1999). A more recent systematic review on the effectiveness of interventions to enhance the healing of chronic ulcers of the foot (Hinchliffe 2008) (search date December 2006) included only eight trials (randomised and non‐randomised) did not identify any evidence that one dressing type was superior to another in terms of promoting ulcer healing. A Cochrane Review of silver‐based wound dressings and topical agents for treating diabetic foot ulcers (Bergin 2006; search date 2010) did not find any studies that met its inclusion criteria. Finally, a review of antimicrobial treatments for diabetic foot ulcers (Nelson 2006) included dressings and found that existing evidence was too weak to recommend any antimicrobial product.
This review is part of a suite of Cochrane reviews investigating the use of dressings in the treatment of foot ulcers in people with diabetes mellitus. Each review will focus on a particular dressing type which in this review is the alginate dressing. Other reviews have considered hydrocolloid, hydrogel and foam dressings (Dumville 2011a; Dumville 2011b). These reviews will be summarised in an overview of reviews (Becker 2011), which will draw together all existing Cochrane review evidence regarding the use of dressings to treat foot ulcers in people with diabetes. While other existing review evidence may also be included in this overview, following Cochrane guidance, this will only occur in the absence of a relevant Cochrane Intervention review (Higgins 2011).
Objectives
To compare the effects of alginate wound dressings with no dressing or alternative dressings on the healing of foot ulcers in people with diabetes mellitus.
Methods
Criteria for considering studies for this review
Types of studies
Published or unpublished randomised controlled trials (RCTs) that evaluated the effects of any type of alginate wound dressing in the treatment of diabetic foot ulcers, irrespective of publication status or language.
Types of participants
Trials recruiting people with Type 1 or Type 2 diabetes, with an open foot ulcer. Since we anticipated that study‐specific classifications of ulcer diagnosis were likely to be too restrictive, we accepted the study authors' definitions of what they classed as diabetic foot ulcers. There was no restriction in relation to the aetiology of the ulcer, so trials recruiting people with ulcers of neuropathic, ischaemic or neuroischaemic causes were all eligible for inclusion.
We included trials involving participants of any age. We excluded trials that included patients with a number of different wound aetiologies in addition to diabetic foot ulcers (e.g. pressure ulcers, venous leg ulcers, mixed arterial/venous leg ulcers, arterial leg ulcers), unless the results for the subgroup of patients with a diabetic foot ulcer were reported separately or were available from authors on request.
Types of interventions
The primary intervention was the alginate wound dressing (BNF 2010). We included any RCT in which the presence or absence of a alginate dressing was the only systematic difference between treatment groups. We anticipated that likely comparisons would include alginate dressings compared with other dressing types or other interventions (which could be non‐dressing treatments i.e. topical applications), or both.
Types of outcome measures
Primary outcomes
Time to ulcer healing; and,
number of ulcers completely healed within a specific time period (we assumed that the period of time in which healing occurred was the duration of the trial unless otherwise stated)
Secondary outcomes
Health‐related quality of life (measured using a standardised generic questionnaire such as EQ‐5D, SF‐36, SF‐12 or SF‐6 or disease‐specific questionnaire). We did not include ad hoc measures of quality of life which are likely not to be validated and will not be common to multiple trials;
number and level of amputations;
adverse events, including amputations, infection and pain (measured using survey/questionnaire/data capture process or visual analogue scale);
cost (including measurements of resource use such as number of dressing changes and nurse time);
ulcer recurrence;
change in ulcer area expressed as absolute changes (e.g. surface area changes in cm2 since baseline) or relative changes (e.g. percentage change in area relative to baseline).
Search methods for identification of studies
For the search methods used in the original version of this review see Appendix 1
Electronic searches
For this first update we searched the following databases in April 2013:
The Cochrane Wounds Group Specialised Register (searched 11 April 2013);
The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2013, Issue 3);
Ovid MEDLINE (1950 to March Week 4 2013);
Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations, April 10, 2013);
Ovid EMBASE (1980 to 2013 April 05);
EBSCO CINAHL (1982 to 4 April 2013).
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) using the following exploded MeSH headings and keywords:
#1 MeSH descriptor Occlusive Dressings explode all trees #2 MeSH descriptor Biological Dressings explode all trees #3 MeSH descriptor Alginates explode all trees #4 MeSH descriptor Hydrogels explode all trees #5 MeSH descriptor Silver explode all trees #6 MeSH descriptor Honey explode all trees #7 (dressing* or alginate* or hydrogel* or "foam" or "bead" or "film" or "films" or tulle or gauze or non‐adherent or "non adherent" or silver or honey or matrix):ti,ab,kw #8 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7) #9 MeSH descriptor Foot Ulcer explode all trees #10 MeSH descriptor Diabetic Foot explode all trees #11 diabet* NEAR/3 ulcer*:ti,ab,kw #12 diabet* NEAR/3 (foot or feet):ti,ab,kw #13 diabet* NEAR/3 wound*:ti,ab,kw #14 (#9 OR #10 OR #11 OR #12 OR #13) #15 (#8 AND #14)
The search strategies used in Ovid MEDLINE, Ovid EMBASE and EBSCO CINAHL can be found in Appendix 2, Appendix 3 and Appendix 4 respectively. We combined the Ovid MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision) (Lefebvre 2011). We also combined the EMBASE and CINAHL searches with the trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN 2011). There were no restrictions on the basis of date or language of publication.
Searching other resources
In the original version of this review we attempted to contact researchers to obtain any unpublished data when needed. We also searched the reference lists of the included studies and previous systematic reviews. We contacted appropriate manufacturers (Smith & Nephew, Convatec Ltd, Mölnlycke Health Care, 3M Healthcare, Coloplast Ltd) for details of any unpublished studies.
Data collection and analysis
Selection of studies
Two review authors independently assessed the titles and abstracts of retrieved studies for relevance. After this initial assessment, we obtained all studies felt to be potentially relevant in full. Two review authors independently checked the full papers for eligibility, with disagreements resolved by discussion and, where required, the input of a third review author. We recorded all reasons for exclusion.
Data extraction and management
We extracted and summarised details of the eligible studies using a data extraction sheet. Two review authors extracted data independently and resolved disagreements by discussion. Where data were missing from reports we attempted to contact the study authors to obtain the missing information. We included studies published in duplicate once, but extracted the maximal amount of data. We extracted the following data :
country of origin;
type of ulcer;
unit of investigation (per patient) ‐ single ulcer or foot or patient or multiple ulcers on the same patient;
care setting;
number of participants randomised to each trial arm;
eligibility criteria and key baseline participant data;
details of the dressing/treatment regimen received by each group;
details of any co‐interventions;
primary and secondary outcome(s) (with definitions);
outcome data for primary and secondary outcomes (by group);
duration of follow‐up;
number of withdrawals (by group);
adverse events, including amputation; and,
source of funding for trial.
Assessment of risk of bias in included studies
Two review authors independently assessed each included study using the Cochrane Collaboration tool for assessing risk of bias (Higgins 2011). This tool addresses six specific domains, namely sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and other issues (e.g. extreme baseline imbalance, issues with unit of investigation) (see Appendix 5 for details of the criteria on which the judgement was based). We assessed blinding and completeness of outcome data for each outcome separately. We completed a 'Risk of bias' table for each eligible study. We resolved disagreements about risk of bias assessment by discussion. Where possible, where a lack of reported information resulted in an unclear decision, authors were contacted for clarification.
We have presented our assessment of risk of bias findings using a 'Risk of bias' summary figure, which presents all of the judgements in a cross‐tabulation of study by entry. This display of internal validity indicates the weight readers may give the results of each study. We also aimed to present this assessment in the narrative review.
We classified trials as being at high risk of bias if they were rated 'high' for any one of three key criteria, i.e. randomisation sequence, allocation concealment and blinded outcome assessment.
Measures of treatment effect
Where possible, we have presented the outcome results for each trial with 95% confidence intervals (CI). We report estimates for dichotomous outcomes (e.g. ulcers healed during a particular time period) as risk ratios (RR). We used the RR rather than odds ratio (OR), since ORs (when interpreted as RR) can give an inflated impression of the effect size when event rates are high, as is the case for many trials reporting healing of chronic wounds (Deeks 2002). We planned to report outcomes relating to continuous data (e.g. percentage change in ulcer area) as mean differences (MD) and overall effect size (with 95% CI calculated). Where a study reported time to healing data (the probability of healing over a consecutive time period) we planned to report and plot these data (where possible) using hazard ratio estimates. If studies reporting time to event data (e.g. time to healing) did not report a hazard ratio, or reported these data incorrectly as a continuous variable, then, where feasible, we planned to estimate this using other reported outcomes, such as the numbers of events, through the application of available statistical methods (Tierney 2007).
Unit of analysis issues
We recorded whether trials measured outcomes in relation to an ulcer, a foot, a participant or whether multiple ulcers on the same participant were studied. We also recorded occasions where multiple ulcers on a participant had been (incorrectly) treated as independent within a study, rather than having within‐patient analysis methods applied. We recorded this as part of the risk of bias assessment. Unless otherwise stated, where the number of wounds appeared to equal the number of participants, we treated the ulcer as the unit of analysis in this review.
Dealing with missing data
Missing data are common in trial reports. Excluding participants post‐randomisation from the analysis or ignoring those participants lost to follow‐up can, in effect, compromise the process of randomisation, and thus potentially introduce bias into the trial. In individual studies, where proportion of ulcers healed data were presented, we assumed that where randomised participants were not included in an analysis, their wound did not heal (that is, they would be considered in the denominator but not the numerator). Where a trial did not specify participant group numbers prior to dropout, we planned to present only complete case data. We planned to present data for time to healing, area change and for all secondary outcomes as a complete case analysis.
Assessment of heterogeneity
We considered both clinical and statistical heterogeneity. Wherever appropriate, we pooled data using meta‐analysis (conducted using RevMan 5.1 (RevMan 2011)), that is where studies appeared similar in terms of level of participants, intervention type and duration, and outcome type. We assessed statistical heterogeneity using the chi² test (a significance level of P < 0.1 was considered to indicate heterogeneity) and the I² estimate (Higgins 2003). The I² estimate examines the percentage of total variation across studies due to heterogeneity rather than to chance. Values of I² over 50% indicate a high level of heterogeneity. In the absence of clinical heterogeneity and in the presence of statistical heterogeneity (I² over 50%), we used a random‐effects model, however, we did not pool studies where heterogeneity was very high (I² over 75%). Where there was no clinical or statistical heterogeneity we envisaged using a fixed‐effect model.
Data synthesis
We combined studies using a narrative overview with meta‐analyses of outcome data where appropriate (in RevMan 5). The decision to include studies in a meta‐analysis depended on the availability of treatment effect data and assessment of heterogeneity. For time to event data, we planned to plot log rank observed minus expected events estimates using a fixed‐effect model (a random‐effects model is not available for this analysis in RevMan 5). Where relevant, and possible, we planned to conduct sensitivity analysis to investigate the potential impact of studies at high risk of bias on pooled results.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies
Results of the search
The search yielded 346 abstracts which were screened for potential inclusion in the review. Of these, 103 reports (for 85 studies) were obtained for a more detailed assessment and six studies were found to be eligible for inclusion in the review. No information about further eligible studies were obtained from the five commercial companies we contacted. We are not aware of any relevant on‐going studies (ISRCTN register checked 24th April 2013). The update search conducted in April 2013 yielded 116 citations of which one study was obtained for further information (Turns 2012): this was excluded.
Included studies
Six studies (375 participants) were included in this review (Ahroni 1993; Baker 1993; Donaghue 1998; Foster 1994; Jude 2007; Lalau 2002): a summary is presented in Table 3. Two studies were single‐centred (Ahroni 1993; Baker 1993), two were multi‐centred (Jude 2007; Lalau 2002), and the remaining studies did not detail the number of centres (Donaghue 1998; Foster 1994). Two studies were undertaken in the USA (Ahroni 1993; Donaghue 1998); two in the UK (Baker 1993; Foster 1994); and one in France (Lalau 2002); one study was multi‐national, taking place in UK, France, Germany and Sweden (Jude 2007).
1. Summary of studies.
| First Author | Group A | Group B | Duration of follow‐up | % healed data |
| Ahroni 1993 | Calcium‐alginate dressing (Sorbsan, Aspen Medical) | Dry, fine mesh gauze (Owens Non‐Adherent dressing) | 4 weeks | Yes |
| Baker 1993 | Calcium‐alginate dressing (Sorbsan, Aspen Medical) | Foam dressing (Allevyn, Smith and Nephew ) | 12 weeks | Yes |
| Donaghue 1998 | Collagen‐alginate dressing (Fibracol, Johnson & Johnson Medical) | Saline moistened gauze | 8 weeks | Yes |
| Foster 1994 | Calcium‐alginate dressing (Kaltostat, ConvaTec) | Foam dressing (Allevyn, Smith and Nephew ) | 8 weeks | Yes |
| Jude 2007 | Calcium‐alginate dressing (Alosteril, Smith and Nephew) | Fibrous‐hydrocolloid (hydrofibre) dressing with 1.2% ionic silver (Aquacel Ag, ConvaTec) | 8 weeks | Yes |
| Lalau 2002 | Calcium‐alginate dressing (Algosteril, Smith and Nephew) | Vaseline gauze (Vaselitulle, Solvay Pharma) | 4 weeks | No |
All studies were undertaken in adults with diabetes. One study included people with both Type 1 and Type 2 diabetes (Lalau 2002). One study (Jude 2007) specified inclusion of only people with a Wagner grade 1 or 2 ulcer and an ankle brachial pressure index (ABPI) above 0.8. Five studies excluded participants that had infected, sloughy or deep ulcers (Ahroni 1993; Baker 1993; Donaghue 1998; Foster 1994; Lalau 2002). In general it seems that studies aimed to include participants with relatively non‐complex diabetic foot ulcers. The duration of trial follow‐up ranged from four weeks (Ahroni 1993) to 12 weeks (Baker 1993), full details are presented in Table 3. All included studies were two‐armed, and one study employed unequal (2:1) randomisation with the treatment group being the larger group (Donaghue 1998). The most widely reported healing outcome was the number of ulcers healed (five studies); only Lalau 2002 did not provide these data. Mean time to healing was reported in two studies (Donaghue 1998; Jude 2007), while the more appropriate summary measure ‐ median time to healing ‐ was reported in two studies (Baker 1993; Foster 1994). The reporting of secondary outcomes was limited. Adverse event reporting did not appear to be systematic in most studies (with the exception of Jude 2007).
Excluded studies
Eighty studies were excluded from the review. The main reasons for exclusion were:
participants in the study were not randomised (10 trials);
no single, identifiable dressing group was evaluated (11 trials);
other intervention, not dressings, differ between trial arms (26 trials);
the dressing(s) evaluated were not alginate dressing (26 trials).
Various other reasons were recorded for the seven remaining studies (Characteristics of excluded studies).
Risk of bias in included studies
We classified studies rated 'High Risk' for any of three key domains: randomisation sequence, allocation concealment and blinded outcome assessment as being at high risk of bias (Characteristics of included studies; Figure 1; Figure 2). One included study was deemed to be at high risk of bias (Ahroni 1993). We rated the remaining five studies as being at unclear risk of bias due to poor reporting.
1.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Adequacy of randomisation process
All six studies were described as "randomised" however, only two reported the method used to generate the randomisation sequence; Baker 1993 reported the use of computer‐generated randomisation, and Jude 2007 reported use of sealed envelopes. Both these trials were judged to be at low risk of bias for this domain. The randomisation method was not reported in the remaining four studies.
Allocation concealment
Jude 2007 reported the use of sealed envelopes for allocation, however, it was unclear whether these envelopes were sequentially numbered and opaque. The five remaining studies did not report the allocation procedure such that we could assess the degree of concealment.
Blinding
Assessment of wound healing can be subjective, and thus has the potential to be influenced if the outcome assessor is aware of the treatment allocation. In this review we focused on whether the studies had conducted a blinded outcome assessment. The study classed as being at high risk of bias reported that blinding was not possible because of the distinct appearance of the dressings without specifying the use of blinded outcome assessment (Ahroni 1993). One study reported adequate blinding of the outcome assessors (Lalau 2002), and hence was judged to be at low risk of bias for this domain. Blinding was not discussed In the remaining studies.
Incomplete outcome data
One study was judged to have high loss to follow‐up (Lalau 2002), reporting that 13 out of 77 participants (17%) did not complete the full six weeks of the study. It seems that the authors conducted the main analysis at four weeks to 'gain' more data although this was not clearly reported. Ahroni 1993; Donaghue 1998 and Jude 2007 were deemed to have conducted an intention‐to‐treat analysis (thus at low risk of bias for this domain). The remaining studies did not report enough information to enable us to make a judgement about intention‐to‐treat analysis and so were classed as unclear (Baker 1993; Foster 1994).
Selective reporting
There was no evidence of selective reporting, and studies were deemed to be at unclear or low risk of bias. It is important to note, however, that judgements for this domain may be of limited value given that they were made by taking the reporting of outcomes in the results that were described in the methods at face value. Study reports were not compared to study protocols, which we did not actively seek out.
Other potential sources of bias
One study did not report its funding source (Baker 1993). The remaining five studies were funded by commercial organisations. Three studies reported some baseline imbalances for different baseline characteristics (Ahroni 1993; Foster 1994; Lalau 2002).
Effects of interventions
Dressing compared with dressing
Advanced wound dressings compared with basic wound contact dressing
Comparison 1: Alginate dressings compared with basic wound contact dressings (three trials; 191 participants)
Three studies (Ahroni 1993; Donaghue 1998; Lalau 2002), involving a total of 191 participants, compared alginate dressings with basic wound contact dressings. Three different brands of alginate dressing were evaluated (Table 3), namely two calcium‐alginate dressings (Ahroni 1993; Lalau 2002), and a collagen‐alginate dressing (Donaghue 1998). The three studies included different basic wound contact dressings: Ahroni 1993 used dry gauze, Donaghue 1998 saline gauze, and Lalau 2002 paraffin gauze.
Primary outcome: ulcer healing
Ahroni 1993 had a follow‐up time of four weeks. There was no statistically significant difference in the number of ulcers healed between the alginate‐dressed group (5/20; 25%) and the basic wound contact‐dressed group (7/19; 37%): RR 0.68, 95% CI 0.26 to 1.77 (Analysis 1.1). This study had a notable difference in baseline ulcer duration with the alginate‐dressed group, on average, having older ulcers (mean ulcer duration: 132.9 days in the alginate‐dressed group and 74.9 days in the basic wound contact‐dressed group). This study was recorded as being at high risk of bias due to non‐blinded outcome assessment. Donaghue 1998 had a maximum follow‐up time of eight weeks. There was no statistically significant difference in the number of ulcers healed in the alginate‐dressed group (24/50; 48%) compared with the basic wound contact‐dressed group (9/25; 36%): RR 1.33, 95% CI 0.73 to 2.42 (Analysis 1.1). The mean time to healing was reported as 6.2 weeks (standard deviation (SD) 0.4) for the alginate‐dressed group and 5.8 weeks (SD 0.4) for the basic wound contact‐dressed group. However, it is important to note that median rather than mean time to healing is normally the more appropriate time‐to‐healing measure since it allows the inclusion of data from participants who do not heal during the course of the study (and thus cannot provide a healing time). It is not clear how these data were dealt with in the calculation of the mean value presented. This study was recorded as being at unclear risk of bias. Lalau 2002 had a follow‐up time of six weeks, although it was unclear whether only the four‐week outcome data were analysed. The study did not report number of ulcers healed, but reported surrogate outcomes. The number of ulcers with granulated tissue over 75% of wound area and 40% decrease in wound surface area was 42.8% in the alginate‐dressed group and 28.5% in the basic wound contact‐dressed group (raw data not provided). The mean change in ulcer area at the four‐week follow‐up was similar between the two groups: a 35.7% reduction in area in the alginate‐dressed group and a 34.9% reduction in the basic wound contact‐dressed group. We pooled ulcer healed data from Ahroni 1993 and Donaghue 1998 (heterogeneity Chi² 1.37, P value 0.24; I² value 27%) in a fixed‐effect meta‐analysis (Analysis 1.1). There was no statistically significant difference in the number of ulcers healed in the alginate‐dressed groups compared with the basic wound contact‐dressed groups: RR 1.09 (95% CI 0.66 to 1.80).
1.1. Analysis.
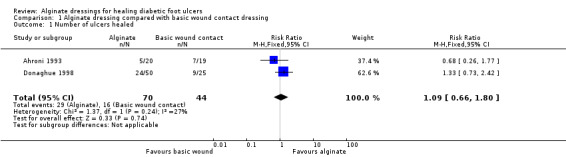
Comparison 1 Alginate dressing compared with basic wound contact dressing, Outcome 1 Number of ulcers healed.
Secondary outcomes
Ahroni 1993 reported two amputations in each trial group (four amputations in total), but these occurred after the four‐week follow‐up. Six additional adverse events were noted in the study report for the alginate‐dressed group (four antibiotic treatments, one death and one septicaemia), and four in the basic wound contact‐dressed group (three required antibiotic treatment and one death). Donaghue 1998 reported that six trial participants had adverse events, but it was not clear to which groups these participants belonged, and the adverse events were not described. Summary: Alginate dressings compared with basic wound contact dressings
There was no statistically significant difference in the number of diabetic foot ulcers healed when treated with an alginate dressing compared with basic wound contact dressings in studies with four to eight weeks of follow‐up. One reported study was classed as being at high risk of bias. Limited secondary outcome data were presented, so we could not draw any conclusions about the advantages or disadvantages of these treatments in terms of cost, quality of life, adverse events or ulcer recurrence.
Advanced dressing compared with advanced dressing
Comparison 2: Alginate dressing compared with foam dressing (two trials; 50 participants)
Two studies (Baker 1993; Foster 1994), with a total of 50 participants compared alginate dressings with foam dressings. Both studies compared the same foam dressing, but two different brands of calcium‐alginate dressings (Table 3).
Primary outcome: ulcer healing
Baker 1993 had a maximum follow‐up period of 12 weeks. Significantly more ulcers in the foam‐dressed group healed compared with the alginate‐dressed group (foam: 9/10; 90%; alginate: 4/10; 40%). This difference was statistically significant: RR 0.44, 95% CI 0.20 to 0.98 in favour of the foam dressing (Analysis 2.1). The study report also noted that a Cox's proportional hazards model adjusted for initial ulcer size and duration of ulcer at baseline (as well as treatment effect) returned a hazard ratio of 4.04 in favour of foam dressing (95% CI 1.18 to 13.84). We did not have the raw data to replicate this analysis, however, the median time to healing was reported as 28 days in the foam‐dressed group and had not been reached by 84 days in the alginate‐dressed group (i.e. less than half of participants in this group had healed by the end of the follow‐up period, so the median could not be calculated). This study was classed as being at unclear risk of bias due to the limited information available in the study report.
2.1. Analysis.

Comparison 2 Alginate dressings compared with foam dressings, Outcome 1 Number of ulcers healed.
Foster 1994 had a maximum follow‐up period of eight weeks. There was no statistically significant difference in the number of ulcers healed between the alginate‐dressed group (8/15; 53%) and the foam‐dressed group (9/15; 60%): RR 0.89, 95% CI 0.47 to 1.67 (Analysis 2.1). The median time to healing was estimated by the review authors from a graph presented in the study report as being 42 days for the alginate‐dressed group and 40 days for the foam‐dressed group. This study was also classed as being at unclear risk of bias due to the limited information available in the study report. Differences in baseline characteristics between groups were noted, specifically that participants in the alginate‐dressed group had a mean age of 70 years, while those in the foam‐dressed group had a mean age of 61 years.
We pooled the data on number of ulcers healed from Baker 1993 and Foster 1994 using a fixed effect model (heterogeneity Chi² 1.83: P value 0.18; I² value 45%). There was no statistically significant difference in the risk of healing between the alginate‐ and foam‐dressed groups: RR 0.67, 95% CI 0.41 to 1.08.
Secondary outcomes
The Baker 1993 trial did not report secondary outcomes. There was limited reporting of adverse events in the Foster 1994 trial, with no events reported in the foam‐dressed group and four in the alginate‐dressed group.
Summary: Foam dressings compared with alginate dressings
Limited data from two small studies at unclear risk of bias found no statistically significant difference in ulcer healing between alginate and foam dressings. It is important to note the short follow‐up times and small sample sizes for these trials.
Antimicrobial dressing compared with non anti‐microbial dressing
Jude 2007 was a two‐armed study with 134 participants that compared a silver fibrous‐hydrocolloid dressing with a calcium‐alginate dressing (Table 3).
Comparison 3: Silver fibrous‐hydrocolloid dressing compared with an alginate dressing (one trial; 134 participants)
Primary outcome: ulcer healing
The Jude 2007 trial had a follow‐up period of eight weeks. There was no statistically significant difference in the number of ulcers healed in the silver fibrous‐hydrocolloid‐dressed group (21/67; 31%) compared with the alginate‐dressed group (15/67; 22%): RR 1.40, 95% CI 0.79 to 2.47 (Analysis 3.1). The mean time to healing was reported as 52.6 days (SD 1.8) in the silver fibrous‐hydrocolloid‐dressed group compared with 57.7 days (SD 1.7) in the alginate‐dressed group.
3.1. Analysis.
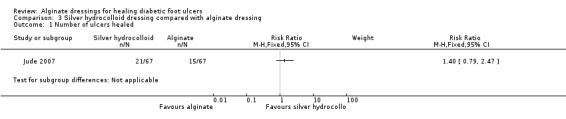
Comparison 3 Silver hydrocolloid dressing compared with alginate dressing, Outcome 1 Number of ulcers healed.
Secondary outcomes
In the Jude 2007 trial 25 participants experienced one or more adverse events in the silver fibrous‐hydrocolloid‐dressed group (including one death) compared with 26 participants in the alginate‐dressed group (including one death). The mean number of dressing changes during the study were similar for both group (21.9 for the silver fibrous‐hydrocolloid‐dressed group and 20.8 for the alginate‐dressed group). There were more infections (type unclear) in the fibrous hydrocolloid group (14 versus eight).
Summary: Silver fibrous‐hydrocolloid dressing compared with an alginate dressing
There was no statistically significant difference in the number of ulcers healed between antimicrobial (silver) hydrocolloid dressings and standard alginate dressings.
Summary of Findings Table
We have included Summary of Findings tables (Table 1; Table 2) in this review, which give a concise overview and synthesis of the volume and quality of the evidence. The Summary of Findings tables (one each for alginate dressing compared with basic wound contact dressing and alginate dressing compared with foam dressing) confirm our conclusion that the volume and quality of the evidence are low and on balance there is no strong evidence of a benefit of using alginate dressings for healing foot ulcers in people with diabetes.
Discussion
Summary of main results
This review has identified, appraised and presented all available RCT evidence (six studies) regarding the clinical effectiveness of alginate wound dressings in the treatment of diabetic foot ulcers.
We found no evidence that alginate dressings promote the healing of diabetic foot ulcers compared with basic wound contact dressings. When data from two studies (eight and 12 weeks follow‐up) were pooled, there was no statistically significant difference in ulcer healing between alginate and foam dressings. Finally, there was no evidence of any difference in the number of diabetic foot ulcers healed when treated with an anti‐microbial (silver) hydrocolloid dressing compared with a standard alginate dressing (eight‐week follow‐up). We note that most included studies evaluated treatments on participants with non‐complex foot ulcers. This means that the body of literature presented may be of limited use to health professionals in the treatment of patients with harder to heal foot ulcers, as it is difficult to generalise from the studies included to patients with more co‐morbidities or complications; this is a limitation of the RCTs that have been undertaken in this field thus far. Included trials were small and therefore statistically underpowered to detect important treatment differences should they exist.
Quality of the evidence
All studies included study in this review were of high or unclear risk of bias. In general studies did not follow good practice conduct and reporting guidelines e.g. CONSORT (Schulz 2010). Key areas of good practice are the robust generation of a randomisation sequence, for example, computer generated, robust allocation concealment, for example the use of a telephone randomisation service and blinded outcome assessment where possible. All this information should be clearly stated in the study report as all trial authors should anticipate the inclusion of their trials in systematic reviews. In terms of analysis, where possible, data from all participants should be included, that is an ITT analysis should be conducted. Steps should be taken during trial conduct to prevent missing data as far as is possible. Where missing data are an issue, imputation methods should be considered and clearly reported when implemented. Finally, where possible robust economic data should be collected.
Potential biases in the review process
The review considered as much evidence as it was possible to obtain, including studies that were not published in English‐language journals. We contacted relevant pharmaceutical companies but did not receive any RCT data from them. There is a potential for publication bias, however, this is likely to be a limited issue in this review given the large number of negative findings that have been published. It is also important to note that two studies are awaiting assessment and may be included in future reviews.
Agreements and disagreements with other studies or reviews
Current evidence does not indicate a difference between alginate dressings and other types of dressing in terms of ability to heal diabetic foot ulcers. This finding is consistent with that of the most recent systematic review in the area (Hinchliffe 2008), which did not find evidence that any dressing type was more effective than others in healing diabetic foot ulcers.
Authors' conclusions
Implications for practice.
A comprehensive review of current evidence did not find evidence that alginate dressings promote the healing of diabetic foot ulcers compared with alternative dressings. Practitioners may therefore elect to consider other characteristics such as costs and symptom management properties when choosing between alternatives.
Implications for research.
Current evidence suggests that there is no difference in ulcer healing between alginate dressings and the other dressing types that have been evaluated. In terms of dressing choice, any investment in future research must maximise its value to decision‐makers. Given the large number of dressing options, the design of future trials should be driven by the questions of high priority to patients and other decision makers. It is also important for research to ensure that the outcomes that are collected in research studies are those that matter to patients, carers and health professionals. It may be that dressings should be viewed as management tools and that other treatments that address patient lifestyle issues deserve attention. Where trials are conducted, good practice guidelines must be followed in their design, implementation and reporting. Further reviews are being conducted to synthesise evidence regarding the effect of other dressings on the treatment of diabetic foot ulcers. It would then be useful to conduct further evidence synthesis (overviews of reviews, mixed treatment comparisons or both) to aid decision‐making about the choice of dressings for diabetic foot ulcers across all dressing options.
What's new
| Date | Event | Description |
|---|---|---|
| 26 March 2015 | Amended | Contact details updated. |
History
Protocol first published: Issue 5, 2011 Review first published: Issue 2, 2012
| Date | Event | Description |
|---|---|---|
| 25 June 2013 | New citation required but conclusions have not changed | First update, no change to conclusions. |
| 25 June 2013 | New search has been performed | New search, no new studies identified. |
Acknowledgements
The authors would like to thank the following people who reviewed the protocol and review for clarity, readability and rigour: Wounds Group editors (Julie Bruce, Andrea Nelson and Gill Worthy) and peer referees (David Armstrong, Duncan Chambers and Janet Yarrow), and also copy editor Jenny Bellorini.
Appendices
Appendix 1. Search methods for the original version of the review ‐ January 2011
Electronic searches
We searched the following databases:
The Cochrane Wounds Group Specialised Register (searched 4 January 2012);
The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2011, Issue 4);
Ovid MEDLINE (1950 to December Week 3 2011);
Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations, January 03, 2012);
Ovid EMBASE (1980 to 2011 Week 52);
EBSCO CINAHL (1982 to 30 December 2011).
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) using the following exploded MeSH headings and keywords:
#1 MeSH descriptor Occlusive Dressings explode all trees #2 MeSH descriptor Biological Dressings explode all trees #3 MeSH descriptor Alginates explode all trees #4 MeSH descriptor Hydrogels explode all trees #5 MeSH descriptor Silver explode all trees #6 MeSH descriptor Honey explode all trees #7 (dressing* or alginate* or hydrogel* or "foam" or "bead" or "film" or "films" or tulle or gauze or non‐adherent or "non adherent" or silver or honey or matrix):ti,ab,kw #8 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7) #9 MeSH descriptor Foot Ulcer explode all trees #10 MeSH descriptor Diabetic Foot explode all trees #11 diabet* NEAR/3 ulcer*:ti,ab,kw #12 diabet* NEAR/3 (foot or feet):ti,ab,kw #13 diabet* NEAR/3 wound*:ti,ab,kw #14 (#9 OR #10 OR #11 OR #12 OR #13) #15 (#8 AND #14)
The search strategies used in Ovid MEDLINE, Ovid EMBASE and EBSCO CINAHL can be found in Appendix 1, Appendix 2 and Appendix 3 respectively. We combined the Ovid MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision). We also combined the EMBASE and CINAHL searches with the trial filters developed by the Scottish Intercollegiate Guidelines Network. There were no restrictions on the basis of date or language of publication.
We searched for on‐going studies on the ISRCTN register (http://www.controlled‐trials.com/isrctn/) (last searched 22nd May 2011).
Searching other resources
We attempted to contact researchers to obtain unpublished data when needed. We also searched the reference lists of the included studies and of previous systematic reviews. We contacted appropriate manufacturers (Smith & Nephew, Convatec Ltd, Mölnlycke Health Care, 3M Healthcare, Coloplast Ltd) for details of any unpublished studies.
Appendix 2. Ovid MEDLINE search strategy
1 exp Occlusive Dressings/ 2 exp Biological Dressings/ 3 exp Alginates/ 4 exp Hydrogels/ 5 exp Silver/ 6 exp Honey/ 7 (dressing* or hydrocolloid* or alginate* or hydrogel* or foam or bead or film*1 or tulle or gauze or non‐adherent or non adherent or silver or honey or matrix).tw. 8 or/1‐7 9 exp Foot Ulcer/ 10 exp Diabetic Foot/ 11 (diabet* adj3 ulcer*).tw. 12 (diabet* adj3 (foot or feet)).tw. 13 (diabet* adj3 wound*).tw. 14 or/9‐13 15 8 and 14
Appendix 3. Ovid EMBASE search strategy
1 exp wound dressing/ 2 exp alginic acid/ 3 exp hydrogel/ 4 exp SILVER/ 5 exp HONEY/ 6 (dressing* or hydrocolloid* or alginate* or hydrogel* or foam or bead or film*1 or tulle or gauze or non‐adherent or non adherent or silver or honey or matrix).tw. 7 or/1‐6 8 exp foot ulcer/ 9 exp diabetic foot/ 10 (diabet* adj3 ulcer*).tw. 11 (diabet* adj3 (foot or feet)).tw. 12 (diabet* adj3 wound*).tw. 13 or/8‐12 14 7 and 13
Appendix 4. EBSCO CINAHL search strategy
S11 S4 and S10 S10 S5 or S6 or S7 or S8 or S9 S9 TI diabet* N3 wound* or AB diabet* N3 wound* S8 TI (diabet* N3 foot OR diabet* N3 feet) or AB (diabet* N3 foot OR diabet* N3 feet) S7 TI diabet* N3 ulcer* or AB diabet* N3 ulcer* S6 (MH "Foot Ulcer+") S5 (MH "Diabetic Foot") S4 S1 or S2 or S3 S3 TI (dressing* or alginate* or hydrogel* or foam or bead or film or films or tulle or gauze or non‐adherent or non adherent or honey or silver or matrix) or AB (dressing* or alginate* or hydrogel* or foam or bead or film or films or tulle or gauze or non‐adherent or non adherent or honey or silver or matrix) S2 (MH "Honey") S1 (MH "Bandages and Dressings+")
Appendix 5. Risk of bias criteria
1. Was the allocation sequence randomly generated?
Low risk of bias
The investigators describe a random component in the sequence generation process such as: referring to a random number table; using a computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots.
High risk of bias
The investigators describe a non‐random component in the sequence generation process. Usually, the description would involve some systematic, non‐random approach, for example: sequence generated by odd or even date of birth; sequence generated by some rule based on date (or day) of admission; sequence generated by some rule based on hospital or clinic record number.
Unclear
Insufficient information about the sequence generation process to permit judgement of low or high risk of bias.
2. Was the treatment allocation adequately concealed?
Low risk of bias
Participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based and pharmacy‐controlled randomisation); sequentially‐numbered drug containers of identical appearance; sequentially‐numbered, opaque, sealed envelopes.
High risk of bias
Participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on: using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure.
Unclear
Insufficient information to permit judgement of low or high risk of bias. This is usually the case if the method of concealment is not described or not described in sufficient detail to allow a definite judgement, for example if the use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially numbered, opaque and sealed.
3. Blinding ‐ was knowledge of the allocated interventions adequately prevented during the study?
Low risk of bias
Any one of the following.
No blinding, but the review authors judge that the outcome and the outcome measurement are not likely to be influenced by lack of blinding.
Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken.
Either participants or some key study personnel were not blinded, but outcome assessment was blinded and the non‐blinding of others unlikely to introduce bias.
High risk of bias
Any one of the following.
No blinding or incomplete blinding, and the outcome or outcome measurement is likely to be influenced by lack of blinding.
Blinding of key study participants and personnel attempted, but likely that the blinding could have been broken.
Either participants or some key study personnel were not blinded, and the non‐blinding of others likely to introduce bias.
Unclear
Any one of the following.
Insufficient information to permit judgement of low or high risk of bias.
The study did not address this outcome.
4. Were incomplete outcome data adequately addressed?
Low risk of bias
Any one of the following.
No missing outcome data.
Reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias).
Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups.
For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate.
For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size.
Missing data have been imputed using appropriate methods.
High risk of bias
Any one of the following.
Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups.
For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate.
For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size.
‘As‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation.
Potentially inappropriate application of simple imputation.
Unclear
Any one of the following.
Insufficient reporting of attrition/exclusions to permit judgement of low or high risk of bias (e.g. number randomised not stated, no reasons for missing data provided).
The study did not address this outcome.
5. Are reports of the study free of suggestion of selective outcome reporting?
Low risk of bias
Any of the following.
The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way.
The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon)
High risk of bias
Any one of the following.
Not all of the study’s pre‐specified primary outcomes have been reported.
One or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified.
One or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect).
One or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis.
The study report fails to include results for a key outcome that would be expected to have been reported for such a study.
Unclear
Insufficient information to permit judgement of low or high risk of bias. It is likely that the majority of studies will fall into this category.
6. Other sources of potential bias
Low risk of bias
The study appears to be free of other sources of bias.
High risk of bias
There is at least one important risk of bias. For example, the study:
had a potential source of bias related to the specific study design used; or
had extreme baseline imbalance; or
has been claimed to have been fraudulent; or
had some other problem.
Unclear
There may be a risk of bias, but there is either:
insufficient information to assess whether an important risk of bias exists; or
insufficient rationale or evidence that an identified problem will introduce bias.
Data and analyses
Comparison 1. Alginate dressing compared with basic wound contact dressing.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of ulcers healed | 2 | 114 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.66, 1.80] |
Comparison 2. Alginate dressings compared with foam dressings.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of ulcers healed | 2 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.41, 1.08] |
Comparison 3. Silver hydrocolloid dressing compared with alginate dressing.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of ulcers healed | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
Comparison 4. Trial data.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Trial data | Other data | No numeric data |
4.1. Analysis.
Comparison 4 Trial data, Outcome 1 Trial data.
| Trial data | |||||||
|---|---|---|---|---|---|---|---|
| Study | Groups | Primary outcome: ulcer healing (SD = standard deviation) | Secondary: health‐related quality of life | Number and level of amputations | Adverse events, including pain | Cost (SD = standard deviation) | Ulcer recurrence |
| Ahroni 1993 | Group A (n = 20): two layers of calcium‐alginate dressing; Group B (n = 19): single layer of dry, fine mesh gauze. | Number of ulcers healed at 4 weeks: Group A: 5 Group B: 7 Number of ulcers healed post 4‐week follow‐up: Group A: 12 Group B: 14 Healing rate mm2/day (SD)(duration of measurements unclear): Group A: (data from 5 participants missing): area, ‐2.19 (4.0); linear: 0.094 (0.147); Group B: (data from 3 participants missing): area, ‐2.04 (2.61); linear: 0.084 (0.100). Relative odds of non‐healing (Group A vs Group B, adjusted for ulcer duration): 1.2 (CI not presented). | not reported | (All post 4‐week follow‐up period): Group A: 2 Group B: 2. | Within 4‐week follow‐up Group A: hospitalised due to foot infection for parenteral antibiotics = 1, hospitalised for septicaemia = 1, treated with oral antibiotics = 3. Group B: hospitalised due to foot infection for parenteral antibiotics = 1, treated with oral antibiotics = 2. Post 4‐week follow‐up Group A: 1 death; Group B: 1 death. | not reported | not reported |
| Baker 1993 | Group A (n = 10): calcium alginate dressing; Group B (n = 10): foam dressing. |
Ulcer healing:
Number of ulcers healed at 12 weeks
Group A: 4 Group B: 9 Median healing time (days) Group A: median time to healing not reached by 84 days Group B: 28 Cox's proportional hazards model adjusted for initial ulcer size and duration of ulcer at baseline and treatment effect gave a hazard ratio of 4.04 in favour of foam dressing (95% CI 1.18 to 13.84). |
not reported | not reported | . not reported. | not reported | not reported |
| Donaghue 1998 | Group A (n = 50): collagen‐alginate dressing (Fibracol, Johnson & Johnson Medical); Group B (n = 25): saline‐moistened gauze. | Number of ulcers healed: Group A: 24 Group B: 9 Mean time to healing in weeks (SD): Group A: 6.2 (0.4) Group B: 5.8 (0.4) Reduction in wound area 75% or greater reduction in wound area: Group A: 39 Group B: 15 Mean time to 75% healing in weeks (SD): Group A: 3.46 (0.4) Group B: 3.72 (0.5) | not reported | not reported | Number of adverse events: 6 patients in total from both groups were not listed and reported for both groups separately. | not reported | not reported |
| Foster 1994 | Group A (n = 15): Calcium‐alginate dressing; Group B (n = 15): Foam dressing. |
Number of ulcers healed:
Group A: 8 Group B: 9 Ulcers improved (not defined): Group A: 3 Group B: 6 Median time to healing (days) (K‐M plot presented but no median time to healing or HR from a Cox's Proportional Hazards analysis presented. The median time to healing was estimated by the reviewer author from the graph). Group A: 42 Group B: 40 |
not reported | not reported | Group A: severe pain = 1; dressings plugged a plantar lesion, preventing free drainage of exudate = 3 (1 developed cellulitis); Group B: 0 . |
not reported | not reported |
| Jude 2007 | Group A (n = 67): calcium‐alginate dressing; Group B (n = 67): fibrous‐hydrocolloid (hydrofibre) dressing with 1.2% ionic silver. |
Number of ulcers healed in 8 weeks:
Group A:15 Group B: 21 Velocity of healing cm2/week (SD): Group A: 0.26 (0.90) (n = 61) Group B: 0.29 (0.33) (n = 61). Velocity of healing as % per week (SD): Group A: 10.0 (15.5) (n = 61) Group B: 11.6 (17.7) (n = 61) Mean time in days to healing (SD): Group A: 57.7 (1.7) Group B: 52.6 (1.8) Reduction in ulcer area in 8 weeks: Group A: 60.5 (42.7) Group B: 58.1 (53.1) Reduction in ulcer depth (cm) at 8 weeks (SD): Group A: 0.13 (0.37) Group B: 0.25 (0.49) |
not reported | not reported |
Group A: 26 participants experienced adverse event: death = 1; infection = 8. 13 participants discontinued treatment due to adverse events. Group B: 25 participants experienced one or more events: death = 1; infection = 14. 8 participants discontinued treatment due to AE. |
Mean number of dressing changes during study:
Group A: 20.8 Group B: 21.9 |
not reported |
| Lalau 2002 | Group A (n = 39): calcium‐alginate dressing; Group B (n = 38): Vaseline gauze. | % of ulcers with granulated tissue over 75% of wound area and 40% decrease in wound surface area (not clear whether this was at 4 or 6 weeks): Group A: 42.8 Group B: 28.5 Mean change in wound area at 4 weeks (SD) : Group A: ‐35.7 (30.7) Group B: ‐34.9 (41.1) | not reported | not reported | Pain on dressing change removal (self‐reported using a 10 cm visual analogue scale not reported whether 0 equalled no pain and 10 highest pain, or not) (SD): Group A: 0.3 (0.1) Group B: 1.8 (0.6) Other events: Group A: 4 participants experienced adverse events: osteitis = 1, osteoarthritis = 1, cardiac arrhyhtmia = 1, fatal myocardial infraction = 1). Group B: 6 participants experienced adverse events: osteitis = 1, wound infection = 2, aggravation of arteriopathy = 1, occurrence of renal failure = 1, fatal pulmonary embolism = 1. | Total number of dressing changes (SD): Group A: 20 (1) Group B: 23 (1) | not reported |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ahroni 1993.
| Methods | Single‐centred, 2‐armed RCT comparing a calcium‐alginate dressing (Sorbsan, Aspen Medical, previously Dow B Hickman Inc.) with dry gauze (Owens Non‐Adherent dressing) undertaken in the USA. Duration of follow‐up: stated as 4 weeks, although some data collected beyond this point, but duration of this post‐4 week period not specified. | |
| Participants | 39 participants. Inclusion criteria: patients with diabetic foot ulcers that penetrated epidermis but did not significantly involve joint spaces, tendons or bone. Exclusion criteria: patients previously enrolled in the study; those with evidence of systemic toxicity (high fever, hypotension or metabolic decompensation); patients who required inpatient management of ulcers at time of initial evaluation (severe infection, ischaemia, extensive cellulitis, lymphangitis, deep necrosis, gangrene, crepitus or gas in tissue, or osteomyelitis or presumed deep infection); those unable or unwilling to comply with either daily wound care regimen or to come to weekly clinic visits. | |
| Interventions | Group A (n = 20): 2 layers of calcium‐alginate dressing (Sorbsan, Aspen Medical) changed daily. Group B (n = 19): single layer of dry, fine mesh gauze (Owens Non‐Adherent dressing) changed twice a day. Co‐intervention: all wounds were cleansed with a half‐strength hydrogen peroxide solution and rinsed with normal saline. | |
| Outcomes | Primary outcome: ulcer healing (number of ulcers healed at 4 weeks; number of ulcers healed at post 4 week follow‐up; healing rate mm2/day; relative odds of non‐healing).
Secondary outcomes: amputations; adverse events. Health‐related quality of life; costs and ulcer recurrence not reported. |
|
| Notes | Trial data: Analysis 4.1 Funding source: Dow B Hickam Inc and Veterans Affair Rehabilitation: research and development. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote:"Subjects were randomly assigned to four weeks of daily dressing change with..." Comment: method of generating the random schedule not reported. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no mention of allocation concealment in study report. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote:"Because of differences in the appearance of the two dressings a blinded study was not feasible" Comment: blinding of participants and personnel not done. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote:"Because of differences in the appearance of the two dressings a blinded study was not feasible" Comment: blinding of outcome assessors not done. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no indication of incomplete outcome data/attrition in paper. |
| Selective reporting (reporting bias) | Low risk | Comment: based on paper only, protocol not obtained. |
| Other bias | Unclear risk | Comment: some differences in baseline characteristics between groups. Mean duration of ulcer 132.9 days in Group A and 74.9 days in Group B. Adjusted for in one analysis (logistic regression). Study was funded by commercial organisation. |
Baker 1993.
| Methods | Single‐centred, 2‐armed RCT comparing a calcium alginate dressing (Sorbsan, Aspen Medical) with a foam dressing (Allevyn, Smith and Nephew) undertaken in the UK. Duration of follow‐up: until the wound healed, or for a maximum period of 12 weeks. | |
| Participants | 20 participants. Inclusion criteria: patients > 18 years old with clean diabetic foot ulcers, neuropathic in origin, located on weight‐bearing areas of foot; able to give informed consent; willingly compliant to requirements of study protocol; geographically able to comply with the study's demands. Exclusion criteria: patients with necrotic, sloughy ulcers or peripheral vascular disease; presence of infection in ulcerative foot; history of poor compliance; unable to attend regularly, or unable to follow simple instructions; those who could not comprehend the nature of the trial. | |
| Interventions | Group A (n = 10): calcium alginate dressing (Sorbsan, Aspen Medical). A secondary low adherent absorbant dressing was used. Group B (n = 10): foam dressing (Allevyn, Smith & Nephew). No secondary dressing was applied . In both groups, dressings were cut to the required size, but they did not overlap the wound margins by more than 3 cm or by less than 0.5 cm. The dressings were secured by standard podiatric methods; e.g. padding and or strapping. Frequency of dressing change depended on the quantity of exudate produced by the ulcers. If, upon removal, either dressing should appear to be stuck, they were to be irrigated, as necessary, with sterile saline. Co‐intervention: ulcers were cleansed with warm sterile saline only, and debridement was undertaken where required. |
|
| Outcomes | Primary outcome: ulcer healing (number of ulcers healed; median healing time). Secondary outcomes: not reported. | |
| Notes | Trial data: Analysis 4.1 Funding source: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Each subject will be randomly allocated to one of the two treatment groups either to the Allevyn or the Sorbsan group. This will be determined by the randomisation code which is computer generated." Comment: method of generation of random schedule reported. |
| Allocation concealment (selection bias) | Unclear risk | Comment: the process of randomising participants, including who did this was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: no mention of blinding in study report. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no mention of blinding in study report. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: one withdrawal (1/20 = 5%). Viewed as limited attrition. |
| Selective reporting (reporting bias) | Low risk | Comment: based on paper only, protocol not obtained. |
| Other bias | Unclear risk | Comment: funding source not reported. |
Donaghue 1998.
| Methods | 2‐armed RCT (not clear if single‐ or multi‐centred) comparing a collagen‐alginate dressing (Fibracol, Johnson & Johnson Medical) with saline‐moistened gauze undertaken in the USA. Duration of follow‐up: until the target ulcer healed, or for a maximum of 8 weeks. | |
| Participants | 75 participants. Inclusion criteria: diabetic patients ≥ 21 years old with foot ulceration of at least 1 cm2 in size after initial debridement; adequate nutritional intake (indicated by a serum albumin of > 2.5 g/dl) and adequate blood flow to the lower extremities (indicated by palpable pulse and/or normal noninvasive tests). Exclusion criteria: patients with severe renal or liver impairment (indicated by creatinine levels and liver function tests 2 or more times higher than normal); presence of any serious medical disorders that could interfere with wound healing; evidence of osteomyelitis (diagnosed by the existence of a deep ulcer probing to bone, or by radiographic findings); clinical signs of infection; history of drug or alcohol abuse. | |
| Interventions | Group A (n = 50): collagen‐alginate dressing (Fibracol, Johnson & Johnson Medical). Group B (n = 25): saline‐moistened gauze. In both group, patients or caregivers also were given explicit instructions for dressing changes and were told to change the wound dressing as often as required. Co‐intervention: weight‐bearing limitation in all participants was achieved by employing the standard procedure of applying a self‐adhesive felted foam dressing to the foot with a window at the site of the ulcer and the use of healing sandals. | |
| Outcomes | Primary outcome: ulcer healing (number of ulcers healed; mean time to healing; % ulcers with 75% or greater reduction in wound area; mean time to 75% healing in weeks).
Secondary outcomes: adverse events. Health‐related quality of life; amputations; costs and ulcer recurrence not reported. |
|
| Notes | Trial data: Analysis 4.1 Adverse events not reported for each arm. Also important to note that whilst an ITT analysis was undertaken, including the 14 people that were withdrawn, the treatment of this missing data was not discussed. We assumed that withdrawals were treated as not having healed. Funding Source: Johnson & Johnson Medical Arlington, TX. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were assigned randomly in a 2:1 ratio open‐label design" Comment: method of generation of random schedule not reported. |
| Allocation concealment (selection bias) | Unclear risk | Comment: the process of randomising participants, including who did this, was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Patients were assigned randomly in a 2:1 ratio open‐label design" Comment: This was labelled an open trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Patients were assigned randomly in a 2:1 ratio open‐label design" Comment: This was labelled an open trial not clear if blinded evaluation was conducted. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Quote: "sixty‐one of the original 75 (81%) enrolled patients completed the study." Quote: "All 75 patients enrolled were included in this intention to treat analysis" Comment: it was unclear what assumptions were made about the missing data. |
| Selective reporting (reporting bias) | Unclear risk | Comment: based on paper only, protocol not obtained. |
| Other bias | Unclear risk | Comment: funded by commercial organisation. |
Foster 1994.
| Methods | 2‐armed RCT (not clear whether single‐ or multi‐centred) comparing a calcium‐alginate dressing (Kaltostat, ConvaTec) with a foam dressing (Allevyn, Smith & Nephew) undertaken in the UK. Duration of follow‐up: until ulcer healed, or for a maximum of 8 weeks. | |
| Participants | 30 participants. Inclusion criteria: patients > 18 years old with clean diabetic foot ulcers, willing and able to comply with study protocol. Exclusion criteria: sloughy, necrotic or infected ulcers. | |
| Interventions | Group A (n = 15): calcium‐alginate dressing (Kaltostat, ConvaTec). The dressing was moistened with saline. A perforated film absorbent dressing was used as a secondary dressing, this was secured with hypo‐allergic tape or a conforming bandage, depending on the state of the skin. Group B (n = 15): foam dressing (Allevyn, Smith & Nephew). Where surrounding skin was in good condition, the dressing was secured with hypo‐allergic tape. If the skin was atrophic or fragile, then no tape was applied, but a conforming bandage was used to secure the dressing. To apply the dressing to patients' lesser toes, a strip of polyurethane foam dressing was doubled over and fastened at the sides to form a sleeve that fitted over the toe. Co‐interventions: none reported. |
|
| Outcomes | Primary outcome: ulcer healing (number of ulcers healed; ulcers improved; median time to healing).
Secondary outcomes: adverse events. Health‐related quality of life, amputations, costs and ulcer recurrence not reported. |
|
| Notes | Trial data: Analysis 4.1 Dressing performance parameters such as patient comfort and ease of removal were assessed using 3‐point graded categorical scores. For each parameter the mean category score for each patient over the repeated dressing assessment was calculated. These data were not extracted, as this approach has not been validated and does not facilitate comparison between studies. Funding source: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote:"Thirty out patients entered the study and 15 were randomised to each dressing" Comment: method of generation of random schedule not reported. |
| Allocation concealment (selection bias) | Unclear risk | Comment: the process of randomising participants, including who did this was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: no mention of blinding in study report. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no mention of blinding in study report. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: 4 participants were withdrawn from the alginate group (4/30 = 13%). There were no withdrawals from the foam group. It is not clear how data from the withdrawn participants were used. |
| Selective reporting (reporting bias) | Low risk | Comment: based on paper only, protocol not obtained. |
| Other bias | Unclear risk | Comment: some differences in baseline characteristics between groups, e.g. mean age 61 years in Group A and 70 years in group Group B. Small sample size meant trial was at high risk of chance imbalance. Funding source: not reported |
Jude 2007.
| Methods | Multi‐centred, 2‐armed RCT comparing a calcium‐alginate dressing (Algosteril, Smith & Nephew) with a fibrous‐hydrocolloid (hydrofibre) dressing with 1.2% ionic silver (Aquacel Ag, ConvaTec) undertaken in the UK, France, Germany, and Sweden. Duration of follow‐up: 8 weeks. | |
| Participants | 134 participants. Inclusion criteria: patients with Type 1 or Type 2 diabetes mellitus (HbA1c ≤ 12%); serum creatinine ≤ 200 mol/l; neuropathic or neuro‐Ischaemic diabetic foot ulcers classed as Wagner grade 1 or 2; all wounds > 1 cm2 in area. Exclusion criteria: patients with known allergies to dressings being investigated; known or suspected malignancy near ulcer; taking systemic antibiotics > 7 days prior to enrolment; inadequate arterial perfusion defined by ankle‐to‐brachial index < 0.8, or great toe systolic blood pressure < 40 mmHg or forefoot TcPO2 < 30 mmHg (subject supine) or < 40 mmHg (participant sitting). | |
| Interventions | Group A (n = 67): calcium‐alginate dressing (Algosteril, Smith & Nephew). Manufacturers' instructions were followed, and dressing was moistened before use on dry wounds, and changed on leakage or at evaluation or every 7 days as indicated (except for infected wounds on which the dressing was changed daily). Group B (n = 67): fibrous‐hydrocolloid (hydrofibre) dressing with 1.2% ionic silver (AQUACEL® Ag, ConvaTec). Left in place and changed on leakage or at evaluation or every 7 days as indicated. In both groups, ulcers were cleansed using sterile saline, each dressing was covered with a sterile, non‐adherent foam dressing. Co‐intervention: accommodative footwear for non‐plantar ulcers and off‐loading for planter ulcers delivered as required. |
|
| Outcomes | Priamry outcome: ulcer healing (number of ulcers healed; velocity of healing; mean time in days to healing; reduction in ulcer area; reduction in ulcer depth).
Secondary outcomes: adverse events, costs (mean number of dressing changes during study). Health‐related quality of life, amputations, and ulcer recurrence not reported. |
|
| Notes | Trial data: Analysis 4.1 22 participants had clinically infected ulcers at baseline, 13 in Group A and 9 in Group B. On enrolment antibiotics were prescribed to 8 in Group A and 13 in Group B. Funding source: ConvaTec (Bristol Myers Squibb). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote:" Individuals were randomly assigned to receive either...(dressing details)... according to instructions in a sealed envelope and stratified according to whether or not systemic antibiotics were being administered for treatment of the study ulcer" Comment: method of generation of random schedule reported. |
| Allocation concealment (selection bias) | Unclear risk | Comment: not clear if envelopes were sequentially numbered, opaque and sealed. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk |
Comment: no mention of blinding in study report. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no mention of blinding in study report. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 21 participants recorded as discontinuing treatment, however, it does not seem that these were study withdrawals. All randomised participants included in the analysis. |
| Selective reporting (reporting bias) | Low risk | Comment: based on study report, protocol not obtained. |
| Other bias | Unclear risk | Comment: funded by commercial organisation. |
Lalau 2002.
| Methods | A multi‐centred, 2‐armed RCT comparing a calcium alginate dressing (Algosteril, Smith & Nephew) with Vaseline gauze (Vaselitulle, Solvay Pharma), undertaken in France. Duration of follow‐up: 6 weeks. The study report stated, however, that, due to premature cessation of treatment in 13 out of 77 patients, it was decided to shorten the period of the efficacy analysis to 4 weeks. The authors said that "this reduction was not accompanied by a revision of the criterion of efficacy and all patients remained included in the 6‐week tolerance analysis study" (it was not clear to what the mention of "tolerance analysis" refers). | |
| Participants | 77 participants. Inclusion criteria: patients < 75 years old, with Type 1 or 2 diabetes and a foot lesion in phase of cleansing (granulation tissue surface of less than 50% of wound area) with surface area between 1 cm2 and 50 cm2. Exclusion criteria: HBA1c level of more than 10%; presence of clinical infection with redness, swelling, warmth and peri‐wound erythema; osteomyelitis on plain radiography or probing of bone; a tunnelled wound; any severe hypovascularisation. | |
| Interventions | Group A (n = 39): calcium‐alginate dressing (Algosteril, Smith & Nephew). Group B (n = 38): Vaseline gauze (Vaselitulle, Solvay Pharma). In both groups dressings were applied directly to the wound for up to 6 weeks. Dressing were changed every day until debridement, and then every 2 to 3 days. No other treatment was permitted except the use of saline solution. In both groups sterile gauze was applied as secondary dressing. Co‐interventions: conservative management was carried out using appropriate pressure‐relieving methods. | |
| Outcomes | Primary outcome: ulcer healing (% of ulcers with granulated tissue over 75% of wound area and 40% decrease in wound surface area; mean change in wound area). Secondary outcomes: adverse events (pain on dressing change and other events); costs (total number of dressing changes). Health‐related quality of life, amputations and ulcer recurrence not reported. | |
| Notes | Trial data: Analysis 4.1 Funding source: laboratories Brothier (Parris‐Nanterre, France). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote:"Patients were randomly assigned to receive treatment with either calcium alginate or Vaseline gauze". Comment: method of generation of random schedule not reported. |
| Allocation concealment (selection bias) | Unclear risk | Comment: the process of randomising participants, including who did this was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote:"This was an opened controlled trial for 6 weeks with blinded evaluation." Comment: the trial was stated as being open‐label. No other details provided in the text. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote:"An independent investigator, blind to allocated treatment, was assigned to analyse wound area surfaces." Comment: outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: In total 13 patients did not complete the full 6 weeks of the study. It was unclear how these were analysed — it seems that the author conducted the main analysis at four weeks to 'gain' more data. |
| Selective reporting (reporting bias) | Low risk | Comment: based on study report, protocol not obtained. |
| Other bias | Unclear risk | Comment: there was some baseline imbalance in wound duration with 4.9 ± 7.8 months in alginate group vs 9.1 ± 13.1 months in Vaseline gauze group. Funded by a commercial company. |
Abbreviations
± = plus or minus > = greater than ≥ = greater than or equal to < = less than ≤ = less than or equal to HBA1c = glycate haemoglobin ITT = intention‐to‐treat analysis n = number in sample/group RCT = randomised controlled trial TcPO2 = transcutaneous oxygen pressure
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Agas 2006 | Study did not randomise participants. |
| Altman 1993 | No single, identifiable dressing type evaluated |
| Alvarez 2003 | The dressing groups evaluated in this study were not alginate dressings. |
| Apelqvist 1990 | Relevant outcome data are not reported: study outcome was limited to change in size of necrotic material on the wound. Study authors were unable to provide the original healing outcome data. |
| Apelqvist 1996 | No single, identifiable dressing type evaluated. |
| Apelqvist 2004 | No single, identifiable dressing type evaluated. |
| Armstrong 2004 | No single, identifiable dressing type evaluated. |
| Belcaro 2010 | The dressing groups evaluated in this study were not alginate dressings. |
| Blackman 1994 | The dressing groups evaluated in this study were not alginate dressings. |
| Bogaert 2004 | Study did not randomise participants. |
| Bradshaw 1989 | Trial stopped after recruiting six participants. No data presented. Authors not contacted for healing data. |
| Caravaggi 2003 | Other intervention, not dressings, differed between trial arms. |
| Chang 2000 | Study did not include diabetic foot ulcers. |
| Chauhan 2003 | Other intervention, not dressings, differ between trial arms |
| Chirwa 2010 | Study did not randomise participants. |
| Clever 1996 | The dressing groups evaluated in this study were not alginate dressings. |
| Cuevas 2007 | No single, identifiable dressing type evaluated. |
| D'Hemecourt 1998 | The dressing groups evaluated in this study were not alginate dressings. |
| Dash 2009 | Other intervention, not dressings, differ between trial arms |
| Diehm 2005 | Study did not randomise participants. |
| Driver 2006 | Other intervention, not dressings, differs between trial arms. |
| Edmonds 2009 | Other intervention, not dressings, differed between trial arms. |
| Eginton 2003 | No single, identifiable dressing type evaluated. |
| Etoz 2003 | Study did not randomise participants. |
| Farac 1999 | Author contacted: study not suitable for inclusion due to data quality issues. |
| Foo 2004 | The dressing groups evaluated in this study were not alginate dressings. |
| Foster 1999 | Other intervention, not dressings, differed between trial arms. |
| Gao 2007 | Other intervention, not dressings, differed between trial arms. |
| Gentzkow 1996 | Other intervention, not dressings, differed between trial arms. |
| Gottrup 2011 | The dressing groups evaluated in this study were not alginate dressings. |
| Hanft 2002 | Other intervention, not dressings, differed between trial arms. |
| Jeffcoate 2009 | The dressing groups evaluated in this study were not alginate dressings. |
| Jeffery 2008 | Study did not randomise participants. |
| Jensen 1998 | The dressing groups evaluated in this study were not alginate dressings. |
| Kordestani 2008 | The dressing groups evaluated in this study were not alginate dressings. |
| Landsman 2010 | Other intervention, not dressings, differed between trial arms. |
| Lazaro‐Martinez 2007 | No single, identifiable dressing type evaluated. |
| Lipkin 2003 | Other intervention, not dressings, differed between trial arms. |
| Markevich 2000 | The dressing groups evaluated in this study were not alginate dressings. |
| Marston 2001 | Other intervention, not dressings, differed between trial arms. |
| Mazzone 1993 | The dressing groups evaluated in this study were not alginate dressings. |
| McCallon 2000 | Study did not randomise participants. |
| Mody 2008 | Study did not include diabetic foot ulcers. |
| Moretti 2009 | Other intervention, not dressings, differed between trial arms. |
| Mueller 1989 | Other intervention, not dressings, differed between trial arms. |
| Mulder 1994 | The dressing groups evaluated in this study were not alginate dressings. |
| Munter 2006 | The dressing groups evaluated in this study were not alginate dressings. |
| Novinscak 2010 | No single, identifiable dressing type evaluated. |
| Ogce 2007 | The dressing groups evaluated in this study were not alginate dressings. |
| Palao i Domenech 2008 | The dressing groups evaluated in this study were not alginate dressings. |
| Parish 2009 | Other intervention, not dressings, differed between trial arms. |
| Pham 1999 | Other intervention, not dressings, differed between trial arms. |
| Piaggesi 1997 | Study did not randomise participants. |
| Piaggesi 2001 | The dressing groups evaluated in this study were not alginate dressings. |
| Reyzelman 2009 | No single, identifiable dressing type evaluated. |
| Roberts 2001 | The dressing groups evaluated in this study were not alginate dressings. |
| Robson 2005 | Other intervention, not dressings, differed between trial arms. |
| Robson 2009 | Study did not include diabetic foot ulcers. |
| Sabolinski 2000 | Other intervention, not dressings, differed between trial arms. |
| Sabolinski 2001 | Other intervention, not dressings, differed between trial arms. |
| Shaw 2010 | Other intervention, not dressings, differed between trial arms. |
| Shukrimi 2008 | Other intervention, not dressings, differed between trial arms. |
| Sibbald 2011 | The dressing groups evaluated in this study were not alginate dressings. |
| Solway 2011 | Study did not randomise participants. |
| Steed 1992 | Other intervention, not dressings, differed between trial arms. |
| Steed 1995 | Other intervention, not dressings, differed between trial arms. |
| Steed 1996 | Other intervention, not dressings, differed between trial arms. |
| Subrahmanyam 1993 | The dressing groups evaluated in this study were not alginate dressings |
| Teot 2008 | The dressing groups evaluated in this study were not alginate dressings. |
| Trial 2010 | The dressing groups evaluated in this study were not alginate dressings. |
| Turns 2012 | Study did not randomise participants |
| Urbaneie 1999 | No single, identifiable dressing type evaluated. |
| Vandeputte 1997 | The dressing groups evaluated in this study were not alginate dressings. |
| Varma 2006 | No single, identifiable dressing type evaluated. |
| Veves 2001 | Other intervention, not dressings, differed between trial arms. |
| Veves 2002 | The dressing groups evaluated in this study were not alginate dressings. |
| Whalley 2001 | The dressings groups evaluated in this study were not alginate dressings. |
| Woo 2010 | The dressing groups evaluated in this study were not alginate dressings. |
| Yao 2007 | Other intervention, not dressings, differed between trial arms. |
| Zimny 2003 | Other intervention, not dressings, differed between trial arms. |
Abbreviations
DFU = diabetic foot ulcer (patients)
Contributions of authors
Jo Dumville developed the review and co‐ordinated its development, completed the first draft of the review, made an intellectual contribution, approved the final version prior to submission and is the guarantor of the review. Sohan Deshpande completed the first draft of the review, made an intellectual contribution and approved the final version of the review prior to submission. Susan O’Meara edited the review, made an intellectual contribution and approved the final version of the review prior to submission. Katharine Speak made an intellectual contribution to the review, advised on the review and approved the final version prior to submission.
Contributions of editorial base
Nicky Cullum: edited the review; advised on methodology, interpretation and content. Approved the final review prior to submission. Sally Bell‐Syer: co‐ordinated the editorial process. Advised on methodology, interpretation and content. Edited and copy edited the review. Ruth Foxlee: designed the search strategy, edited the search methods section and ran the searches.
Sources of support
Internal sources
Department of Health Sciences, University of York, UK.
External sources
NIHR Programme Grants for Applied Research, UK.
NIHR/Department of Health (England), Cochrane Wounds Group, UK.
Declarations of interest
Susan O'Meara and Jo Dumville receive funding from the National Institute for Health Research (NIHR) under its Programme Grants for Applied Research funding programme. This review presents independent research funded by the National Institute for Health Research (NIHR) under its Programme Grants for Applied Research funding programme (RP‐PG‐0407‐10428). The views expressed in this presentation are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Sohan Deshpande and Katherine Speak have no interests to declare.
Edited (no change to conclusions)
References
References to studies included in this review
Ahroni 1993 {published data only}
- Ahroni JH, Boyko EJ, Pecoraro RE. Diabetic foot ulcer healing: extrinsic vs intrinsic factors. Wounds 1993;5(5):245‐55. [Google Scholar]
Baker 1993 {unpublished data only}
- Baker NR, Creevy J. A randomised comparative pilot study to evaluate Allevyn hydrocellular dressings and Sorbsan calcium‐alginate dressings in the treatment of diabetic foot ulcers. Unpublished 1993.
Donaghue 1998 {published data only}
- Donaghue VM, Chrzan JS, Rosenblum BI, Giurini JM, Habershaw GM, Veves A. Evaluation of a collagen‐alginate wound dressing in the management of diabetic foot ulcers. Advances in Wound Care 1998;11(3):114‐9. [PubMed] [Google Scholar]
Foster 1994 {published data only}
- Foster AVM, Greenhill MT, Edmonds ME. Comparing two dressings in the treatment of diabetic foot ulcers. Journal of Wound Care 1994;3(5):224‐8. [DOI] [PubMed] [Google Scholar]
Jude 2007 {published data only}
- Jude EB, Apelqvist J, Spraul M, Martini J. Prospective randomized controlled study of Hydrofiber dressing containing ionic silver or calcium alginate dressings in non‐ischaemic diabetic foot ulcers. Diabetic Medicine 2007;24(3):280‐8. [DOI] [PubMed] [Google Scholar]
Lalau 2002 {published data only}
- Lalau JD, Bresson R, Charpentier P, Coliche V, Erlher S, Ha Van G, et al. Efficacy and tolerance of calcium alginate versus Vaseline gauze dressings in the treatment of diabetic foot lesions. Diabetes and Metabolism 2002;28(3):223‐9. [PubMed] [Google Scholar]
References to studies excluded from this review
Agas 2006 {published data only}
- Agas CM, Bui TD, Driver VR, Gordon IL. Effect of window casts on healing rates of diabetic foot ulcers. Journal of Wound Care 2006;15(2):80‐3. [DOI] [PubMed] [Google Scholar]
Altman 1993 {published data only}
- Altman MI, Mulder GD. Multi‐centre evaluation of Nu‐Gel wound dressing in full‐thickness chronic wounds of the lower extremities. 3rd European Conference on Advances in Wound Management; 1993, 19‐22 October; Harrogate, UK. 1993:164‐5.
Alvarez 2003 {published data only}
- Alvarez OM, Roger RS, Booker JG, Patel M. Effect of non contact normothermic wound therapy on the healing of neuropathic (diabetic) foot ulcers: An interim analysis of 20 patients. Journal of Foot and Ankle Surgery 2003;42(1):30‐5. [DOI] [PubMed] [Google Scholar]
Apelqvist 1990 {published data only}
- Apelqvist J, Larsson J, Stenstrom A. Topical treatment of necrotic foot ulcers in diabetic patients:a comparative trial of DuoDerm and MeZinc. British Journal of Dermatology 1990;123:787‐92. [DOI] [PubMed] [Google Scholar]
Apelqvist 1996 {published data only}
- Apelqvist J, Ragnarson‐Tennvall G. Cavity foot ulcers in diabetic patients: a comparative study of cadexomer iodine ointment and standard treatment. An economic analysis alongside a clinical trial. Acta Dermato‐Venereologica 1996;76(3):321‐5. [DOI] [PubMed] [Google Scholar]
Apelqvist 2004 {published data only}
- Apelqvist J, Piaggesi A. Enamel Matrix Protein in Diabetic Foot Ulcers, A Controlled Multicentre Study. 2nd World Union of Wound Healing Societies Meeting; 8‐13 July 2004. 2004:8‐13.
Armstrong 2004 {published data only}
- Armstrong DG, Lavery LA, Frykberg RG, Andros G, Attinger CE, Boulton AJM. VAC Therapy appears to heal complex DFU. 2nd World Union of Wound Healing Societies Meeting; 8‐13 July 2004. 2004:22.
Belcaro 2010 {published data only}
- Belcaro G, Cesarone MR, Errichi BM, Ricci A, Dugall M, Pellegrini L, et al. Venous and diabetic ulcerations: management with topical multivalent silver oxide ointment. Panminerva Medica 2010;52(2 (Suppl 1)):37‐42. [PubMed] [Google Scholar]
Blackman 1994 {published data only}
- Blackman JD, Senseng D, Quinn L, Mazzone T. Clinical evaluation of a semipermeable polymeric membrane dressing for the treatment of chronic diabetic foot ulcers. Diabetic Care 1994;17(4):322‐5. [DOI] [PubMed] [Google Scholar]
Bogaert 2004 {published data only}
- Bogaert T, Meert S, Derre B, Goethals E. Topical autologous platelet gel enhances healing of chronic diabetic ulcer: Preliminary report. 2nd World Union of Wound Healing Societies Meeting; 8‐13 July 2004. 2004:114.
Bradshaw 1989 {published data only}
- Bradshaw T, Gem J, Boulton A. The use of Kaltostat in the treatment of ulceration in the diabetic foot. Chiropodist 1989;44(9):204‐7. [Google Scholar]
Caravaggi 2003 {published data only}
- Caravaggi C, Giglio R, Pritelli C, Sommaria M, Dalla Noce S, Faglia E, et al. HYAFF 11‐based autologous dermal and epidermal grafts in the treatment of noninfected diabetic plantar and dorsal foot ulcers: a prospective, multicenter, controlled,randomized clinical trial. Diabetes Care 2006;10:2853‐9. [DOI] [PubMed] [Google Scholar]
Chang 2000 {published data only}
- Chang DW, Sanchez LA, Veith FJ, Wain RA, Okhi T, Suggs WD. Can a tissue‐engineered skin graft improve healing of lower extremity foot wounds after revascularization?. Annals of Vascular Surgery 2000;14(1):44‐9. [DOI] [PubMed] [Google Scholar]
Chauhan 2003 {published data only}
- Chauhan VS, Rasheed MA, Pandley SS, Shukla VK. Nonhealing wounds — a therapeutic dilemma. International Journal of Lower Extremity Wounds 2003;2(1):40‐5. [DOI] [PubMed] [Google Scholar]
Chirwa 2010 {published and unpublished data}
- Chirwa Z, Bhengu T, Litiane K. The cost effectiveness of using calcium alginate silver matrix in the treatment of wounds. EWMA Journal 2010;10(2):257, Abstract P299. [Google Scholar]
Clever 1996 {published data only}
- Clever HU, Dreyer M. Comparing two wound dressings for the treatment of neuropathic diabetic foot ulcers. Proceedings of the 5th European Conference on Advances in Wound Management; 21‐24 November, 1995; Harrogate, UK. 1996:201‐3.
Cuevas 2007 {published data only}
- Cuevas FR, Velázquez Méndez AA, Andrade IC. Zinc hyaluronate effects on ulcers in diabetic patients [Efecto delhialuronato de zinc sobre las úlceras en pacientes con diabetes]. Gerokomos 2007;18(2):91‐105. [Google Scholar]
D'Hemecourt 1998 {published data only}
- D'Hemecourt PA, Smiell JM, Karim MR. Sodium carboxymethyl cellulose aqueous‐based gel vs becaplermin gel in patients with nonhealing lower extremity diabetic ulcers. Wounds 1998;10(3):69‐75. [Google Scholar]
Dash 2009 {published data only}
- Dash NR, Dash SN, Routray P, Mohapatra S, Mohapatra PC. Targeting nonhealing ulcers of lower extremity in human through autologous bone marrow‐derived mesenchymal stem cells. Rejuvenation Research 2009;12(5):359‐66. [DOI] [PubMed] [Google Scholar]
Diehm 2005 {published data only}
- Diehm C, Lawall H. Evaluation of Tielle hydro polymer dressings in the management of chronic exuding wounds in primary care. International Wound Journal 2005;2(1):26‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Driver 2006 {published data only}
- Driver VR, Hanft J, Fylling CP, Beriou JM, and Autologel Diabetic Foot Ulcer Study Group. A prospective, randomized,controlled trial of autologous platelet‐rich plasma gel for the treatment of diabetic foot ulcers. Ostomy/Wound Management 2006;52(6):68‐70, 72, 74. [PubMed] [Google Scholar]
Edmonds 2009 {published data only}
- Edmonds M. Apligraf in the treatment of neuropathic diabetic foot ulcers. International Journal of Lower Extremity Wounds 2009;8(1):11‐8. [DOI] [PubMed] [Google Scholar]
Eginton 2003 {published data only}
- Eginton MT, Brown KR, Seabrook GR, Towne JB, Cambria RA. A prospective randomized evaluation of negative‐pressure wound dressings for diabetic foot wounds. Annals of Vascular Surgery 2003;17(6):645‐9. [DOI] [PubMed] [Google Scholar]
Etoz 2003 {published data only}
- Etoz A, Ozgenel Y, Ozcan M. The use of negative pressure wound therapy on diabetic foot ulcers: a preliminary controlled trial. Wounds: A Compendium of Clinical Research and Practice 2004;16(8):264‐9. [Google Scholar]
Farac 1999 {published data only}
- Farac KP, Grief R, Sessler DI. Radiant heat bandage speeds healing of diabetic‐neuropathic foot ulcers. 31st Annual Wound, Ostomy and Continence Conference; 1999 June; Minneapolis, MN. 1999:488.
Foo 2004 {published data only}
- Foo LSS, Chua BSY, Chia GT, Tan SB, Howe TS. Vacuum assisted closure vs moist gauze dressing in post‐operative diabetic foot wounds: Early results from a randomised controlled trial. 2nd World Union of Wound Healing Societies Meeting; 2004 ,8‐13 July; Paris. 2004:8‐9.
Foster 1999 {published data only}
- Foster A, Bates M, Doxford M, Edmonds ME. Treatment of indolent neuropathic ulceration of the diabetic foot with Hyaff. The Diabetic Foot 1999;2(2):72. [Google Scholar]
Gao 2007 {published data only}
- Gao L, Xu GX, Zhou HB. Efficacy evaluation of diabetic foot ulceration treated with iodophors dressings therapy. Journal of Clinical Nursing 2007;6(4):21‐2. [Google Scholar]
Gentzkow 1996 {published data only}
- Gentzkow GD, Iwasaki SD, Hershon KS, Mengel M, Prendergast JJ, Ricotta JJ, et al. Use of Dermagraft, a cultured human dermis, to treat diabetic foot ulcers. Diabetes Care 1996;19(4):350‐4. [DOI] [PubMed] [Google Scholar]
Gottrup 2011 {published data only}
- Gottrup F, Gibson M, Karlsmark T, Bishoff‐Mikkelsen M, Nisbet L, Cullen B. Collagen/ORC/silver treatment of diabetic foot ulcers; A randomised controlled trial. Wound Repair and Regeneration 2011;19(2):A24. [DOI] [PubMed] [Google Scholar]
- Gottrup F, Karlsmark T, Cullen B, Gibson M, Nisbet L. Comparative clinical study to determine the effects of collagen/orc+silver therapy on wound healing of diabetic foot ulcers. EWMA Journal 26‐28 May 2010;10(2):83 Abstract 126. [Google Scholar]
Hanft 2002 {published data only}
- Hanft JR, Surprenant MS. Healing of chronic foot ulcers in diabetic patients treated with a human fibroblast‐derived dermis. Journal of Foot and Ankle Surgery 2002;41(5):291. [DOI] [PubMed] [Google Scholar]
Jeffcoate 2009 {published data only}
- Jeffcoate WJ, Price PE, Phillips CJ, Game FL, Mudge E, Davies S, et al. Randomised controlled trial of the use of three dressing preparations in the management of chronic ulceration of the foot in diabetes. Health Technology Assessment 2009;13(54):1‐110. [DOI] [PubMed] [Google Scholar]
Jeffery 2008 {published data only}
- Jeffery S. A honey‐based dressing for diabetic foot ulcers: A controlled study. The Diabetic Foot Journal 2008;11(2):87‐91. [Google Scholar]
Jensen 1998 {published data only}
- Jensen JL, Seeley J, Brian Gillin. A controlled, randomized comparison of two moist wound healing protocols: Carrasyn hydrogel wound dressing and wet‐to‐moist saline gauze. Advances in Wound Care 1998;11(7 (Suppl)):1‐4. [PubMed] [Google Scholar]
Kordestani 2008 {published data only}
- Kordestani S, Shahrezaee M, Tahmasebi MN, Hajimahmodi H, Ghasemali DH, Abyaneh MS. A randomised controlled trial on the effectiveness of an advanced wound dressing used in Iran. Journal of Wound Care 2008;17(7):323‐7. [DOI] [PubMed] [Google Scholar]
Landsman 2010 {published data only}
- Landsman A, Agnew P, Parish L, Joseph R, Galiano RD. Diabetic foot ulcers treated with becaplermin and TheraGauze, a moisture‐controlling smart dressing: a randomized, multicenter, prospective analysis. Journal of the American Podiatric Medical Association 2010;100(3):155‐60. [DOI] [PubMed] [Google Scholar]
Lazaro‐Martinez 2007 {published data only}
- Lázaro‐Martínez JL, García‐Moralesa E, Aragón‐Sánchezc FJ. Randomized comparative trial of a collagen/oxidized regenerated cellulose dressing in the treatment of neuropathic diabetic foot ulcers. EWMA Journal. 2008; Vol. 8, issue 2 (Supp):Abstract no. P371.
- Lázaro‐Martínez JL, García‐Moralesa E, Beneit‐Montesinosa JV, Martínez‐de‐Jesúsb FR, Aragón‐Sánchezc FJ. Randomized comparative trial of a collagen/oxidized regenerated cellulose dressing in the treatment of neuropathic diabetic foot ulcers [Estudio aleatorizado y comparativo de unapósito de colágeno y celulosa oxidadaregenerada en el tratamiento de úlcerasneuropáticas de pie diabético]. Cirugia Espanola 2007;82(1):27‐31. [DOI] [PubMed] [Google Scholar]
Lipkin 2003 {published data only}
- Lipkin S, Chaikof E, Isseroff Z, Silverstein P. Effectiveness of bilayered cellular matrix in healing of neuropathic diabetic foot ulcers: results of a multicenter pilot trial. Wounds: A Compendium of Clinical Research and Practice 2003;15(7):230‐6. [Google Scholar]
Markevich 2000 {published data only}
- Markevich YO, McLeod‐Roberts J, Mousley M, Melloy E. Maggot therapy for diabetic neuropathic foot wounds. Diabetologia. 2000; Vol. 43, issue Supp 11:A15.
Marston 2001 {published data only}
- Marston W, Foushee K, Farber M. Prospective randomized study of a cryopreserved, human fibroblast‐derived dermis in the treatment of chronic plantar foot ulcers associated with diabetes mellitus. 14th Annual Symposium on Advances in Wound Care and Medical Research Forum on Wound Repair; 2001 30 April ‐ 3 May; Las Vegas, Nevada. 2001.
Mazzone 1993 {published data only}
- Mazzone T, Blackman JD. Evaluation of a new loaded foam membrane on the healing rate of diabetic foot ulcers. 1st Joint Meeting of the Wound Healing Society and the European Tissue Repair Society; 1993, August; Amsterdam, the Netherlands. 1993.
McCallon 2000 {published data only}
- McCallon SK, Knight CA, Valiulus JP, Cunningham MW, McCulloch JM, Farinas LP. Vacuum‐assisted closure versus saline‐moistened gauze in the healing of postoperative diabetic foot wounds. Ostomy/Wound Management 2000;46(8):28‐34. [PubMed] [Google Scholar]
Mody 2008 {published data only}
- Mody GN, Nirmal IA, Duraisamy S, Perakath B. A blinded, prospective, randomized controlled trial of topical negative pressure wound closure in India. Ostomy/Wound Management 2008;54(12):36‐46. [PubMed] [Google Scholar]
Moretti 2009 {published data only}
- Moretti B, Notarnicola A, Maggio G, Moretti L, Pascone M, Tafuri S, et al. The management of neuropathic ulcers of the foot in diabetes by shock wave therapy. BMC Musculoskeletal Disorders 2009; Vol. 10, issue 54. [DOI: 10.1186/1471-2474-10-54] [DOI] [PMC free article] [PubMed]
Mueller 1989 {published data only}
- Mueller MJ, Diamond JE, Sinacore DR, Delitto A, Blair VP, Drury DA, et al. Total contact casting in treatment of diabetic plantar ulcers. Diabetes Care 1989;12:184‐8. [DOI] [PubMed] [Google Scholar]
Mulder 1994 {published data only}
- Mulder GD, Jensen JL, Seeley JE, Peak Andrews K. A controlled randomized study of an amorphous hydrogel to expedite closure of diabetic ulcers. 4th European Tissue Repair Society Meeting; 1994, 25‐28 August; Oxford, England. 1994:130.
Munter 2006 {published data only}
- Munter KC, Beele H, Russell L, Basse E, Grochenig E, Crespi A, et al. The CONTOP study: improved healing of delayed healing ulcers with sustained silver‐releasing foam dressing versus other silver dressings. 16th Conference of the European Wound Management Association; 2006, 18‐20 May; Prague, Czech Republic. 2006:Abstract No.P068.
- Munter KC, Beele H, Russell L, Basse PB, Groechenig E, Crespi A, et al. The CONTOP study: a large‐scale,comparative, randomized study in patients treated with a sustained silver‐releasing foam dressing. SAWC; 30 April‐3 May 2006; San Antonio,Texas. 2006.
- Munter KC, Beele H, Russell L, Crespi A, Grocenig E, Basse P, et al. Effect of a sustained silver‐releasing dressing on ulcers with delayed healing:The CONTOP study. Journal of Wound Care 2006;15(5):199‐205. [DOI] [PubMed] [Google Scholar]
- Russell L, Nebbioso G, Munter KC, Beele H, Basse PB. Dienst H. The Contop Study: A hydro‐activated silver containing foam dressing versus standard care. 2nd World Union of Wound Healing Societies Meeting; 8–13 July 2004; Paris. 2004.
- Scalise A, Forma O, Happe M, Hahn TW. The Contop study: real life experiences from an international study comparing a silver containing hydro‐activated foam dressing with standard wound care. 13th Conference of the European Wound Management Association. Pisa, Italy, 22‐23 May 2003.
Novinscak 2010 {published data only}
- Novinscak T, Zvorc M, Trojko S, Jozinovic E, Filipovic M, Grudic R. Comparison of cost‐benefit of the three methods of diabetic ulcer treatment: Dry, moist and negative pressure. Acta Medica Croatica 2010;64(Suppl. 1):113‐5. [Google Scholar]
Ogce 2007 {published data only}
- Ogce F, Dramali A. The treatment of diabetic foot ulcers. Sendrom 2007;19(12):79‐81. [Google Scholar]
Palao i Domenech 2008 {published data only}
- Palao i Domenech R, Romanelli M, Tsiftsis DD, Slonkova V, Jortikka A, Johannesen N, et al. Effect of an ibuprofen‐releasing foam dressing on wound pain: a real‐life RCT. Journal of Wound Care 2008;17(8):342, 344‐8. [DOI] [PubMed] [Google Scholar]
Parish 2009 {published data only}
- Parish L, Routh H, Parish J. Diabetic foot ulcers: A randomized multicenter study comparing a moisture‐controlling dressing with a topical growth factor. Journal of the American Academy of Dermatology 2009;60(Suppl 3):AB202. [Google Scholar]
Pham 1999 {published data only}
- Pham HT, Rosenblum BI, Lyons TE, Giurini JM, Chrzan JS, Habershaw GM, et al. Evaluation of a human skin equivalent for the treatment of diabetic foot ulcers in a prospective, randomized, clinical trial. Wounds: A Compendium of Clinical Research and Practice 1999;11(4):76‐86. [Google Scholar]
Piaggesi 1997 {published data only}
- Piaggesi A. A thin hydrocolloid occlusive dressing in diabetic foot ulcerations. Journal of Wound Care 1997;6(3):10. [Google Scholar]
Piaggesi 2001 {published data only}
- Piaggesi A, Baccetti F, Rizzo L, Romanelli M, Navalesi R, Benzi L. Sodium carboxyl‐methyl‐cellulose dressings in the management of deep ulcerations of diabetic foot. Diabetic Medicine 2001;18:320‐4. [DOI] [PubMed] [Google Scholar]
Reyzelman 2009 {published data only}
- Reyzelman A, Crews RT, Moore JC, Moore L, Mukker JS, Offutt S, et al. Clinical effectiveness of an acellular dermal regenerative tissue matrix compared to standard wound management in healing diabetic foot ulcers: a prospective, randomised, multicentre study. International Wound Journal 2009;6(3):196‐208. [DOI] [PMC free article] [PubMed] [Google Scholar]
Roberts 2001 {published data only}
- Roberts GH, Hammad LH, Haggan G, Baker N, Sandeman D, Mani R, et al. Hydrocellular against non‐adherent dressings to treat diabetic foot ulcers‐ A randomised controlled study. 11th ETRS Annual Conference. 2001.
Robson 2005 {published data only}
- Robson MC, Payne WG, Garner WL, Biundo J, Giacalone VF, Cooper DM, et al. Integrating the results of phase IV (post marketing) clinical trial with four previous trials reinforces the position that Regranex (Becaplermin) Gel 0.01% is an effective adjunct to the treatment of diabetic foot ulcers. Journal of Applied Research 2005;5(1):35‐45. [Google Scholar]
Robson 2009 {published data only}
- Robson V, Dodd S, Thomas S. Standardized antibacterial honey (Medihoney) with standard therapy in wound care: randomized clinical trial. Journal of Advanced Nursing 2009 ;65 (3 ):565‐75. [DOI] [PubMed] [Google Scholar]
Sabolinski 2000 {published data only}
- Sabolinski M, Giovino K, and Graftskin Diabetic Foot Ulcer Study Group. Risk factors associated with the healing of diabetic foot ulcers. Wound Healing Society Educational Symposium. Toronto, Canada, 2000, 4‐6 June.
- Sabolinski M, Toole T, Giovino K, and Apligraft Diabetic Foot Ulcer Study Group. Apligraf (Graftskin) bilayered living skin construct in the treatment of diabetic foot ulcers. First World Wound Healing Congress. Melbourne, Australia, 2000, 10‐13 September.
- Sabolinski ML, Veves A. Graftskin (Apligraf) in neuropathic diabetic foot ulcers. Wounds: A Compendium of Clinical Research and Practice 2000;12(5):Suppl A 33A‐6A. [Google Scholar]
- Sabolinski ML, Veves A. Graftskin bilayered living skin construct in the treatment of diabetic foot ulcers. 13th Annual Symposium on Advanced Wound Care and 10th Annual Medical Research Forum on Wound Repair. Dallas,USA, 1‐4 April 2000.
- Veves A, Falango V, Armstrong DG, Sabolinski ML. Graftskin (Apligraf) a human skin equivalent, promotes wound healing in diabetic foot ulcers in a prospective, randomized, multicenter clinical trial. Tenth Annual Meeting of the European Tissue Repair Society. Brussels, Belgium, 24–27 May 2000.
Sabolinski 2001 {published data only}
- Sabolinski M, Falanga V. The healing of diabetic plantar foot ulcers of greater than two‐month duration with Graftskin in a randomized prospective study. Eleventh Annual Meeting and Educational Symposuim Wound Healing Society. Albuquerque, New Mexico, 2001, 16‐18 May.
Shaw 2010 {published data only}
- Shaw J, Hughes CM, Lagan KM, Stevenson MR, Irwin CR, Bell PM. The effect of topical phenytoin on healing in diabetic foot ulcers: a randomised controlled trial. Diabetologia 2010;53(Suppl 1):S463. [DOI] [PubMed] [Google Scholar]
Shukrimi 2008 {published data only}
- Shukrimi A, Sulaiman AR, Halim AY, Azril A. A comparative study between honey and povidone iodine as dressing solution for Wagner type II diabetic foot ulcers. Medical Journal of Malaysia 2008;63(1):44‐6. [PubMed] [Google Scholar]
Sibbald 2011 {published data only}
- Sibbald RG, Coutts P, Woo KY. Reduction of bacterial burden and pain in chronic wounds using a new polyhexamethylene biguanide antimicrobial foam dressing‐clinical trial results. Advances in Skin and Wound Care 2011 ;24 (2 ):78‐84. [DOI] [PubMed] [Google Scholar]
Solway 2011 {published data only}
- Solway DR, Clark WA, Levinson DJ. A parallel open‐label trial to evaluate microbial cellulose wound dressing in the treatment of diabetic foot ulcers. International Wound Journal 2011;8(1):69‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Steed 1992 {published data only}
- Holloway GA, Steed DL, DeMarco MJ, Matsumoto T, Moosa HH, Webster MW, et al. A randomized controlled multicenter dose response trial of activated platelet supernatant topical CT‐102 in chronic nonhealing diabetic wounds. Wounds: A Compendium of Clinical Research and Practice 1993;5(4):198‐206. [Google Scholar]
- Steed DL. Growth factors in managing diabetic foot ulcers. 5th Annual Symposium on Advanced Wound Care. New Orleans, Lousiana, 23‐25 April, 1992.
- Steed DL, Goslen JB, Holloway GA, Malone JM, Bunt TJ, Webster MW. Randomized prospective double‐blind trial in healing chronic diabetic foot ulcers. Diabetic Care 1992;15(11):1598‐1604. [DOI] [PubMed] [Google Scholar]
Steed 1995 {published data only}
- Steed DL, Ricotta JJ, Prendergast JJ, Kaplan RJ, Webstar MW, McGill JB. Promotion and acceleration of diabetic ulcer healing by arginine‐glycine‐aspartic acid (RGD) peptide matrix. RGD Study Group. Diabetes care 1995;18(1):39‐46. [DOI] [PubMed] [Google Scholar]
Steed 1996 {published data only}
- Steed DL, Edington HD, Webster MW. Recurrence rate of diabetic neurotrophic foot ulcers healed using topical application of growth factors released from platelets. Wound Repair and Regeneration 1996;4(2):230‐3. [DOI] [PubMed] [Google Scholar]
Subrahmanyam 1993 {published data only}
- Subrahmanyam M. Honey as a surgical dressing for burns and ulcers. Indian Journal of Surgery 1993;55(9):468‐73. [Google Scholar]
Teot 2008 {published data only}
- Teot L, Trial C, Lavigne JP. Results of RCT on the antimicrobial effectiveness of a new silver alginate wound dressing. EWMA Journal 2008;8(Supp 2):54 Abstract no. 69. [DOI] [PubMed] [Google Scholar]
Trial 2010 {published data only}
- Trial C, Darbas H, Lavigne J. Assessment of the antimicrobial effectiveness of a new silver alginate wound dressing: a RCT. Journal of Wound Care 2010;19(1):20‐6. [DOI] [PubMed] [Google Scholar]
Turns 2012 {published data only}
- Turns M. Evaluation of NOSF in neuropathic diabetic. Wounds UK 2012;8(1):100‐106. [Google Scholar]
Urbaneie 1999 {published data only}
- Urbaneie‐Rovan V. Efficacy and cost of different dressings. Practical Diabetes International 1999;16(2):S3. [Google Scholar]
Vandeputte 1997 {published data only}
- Vandeputte J, Gryson L. Clinical trial on the control of diabetic foot infection by an immunomodulating hydrogel containing 65% glycerine. Proceedings of the 6th European Conference on Advances in Wound Management. Harrogate, UK, 1995, 21–24 November.
- Vandeputte J, Gryson L. Diabetic foot infection controlled by immuno‐modulating hydrogel containing 65% glycerine. Presentation of a clinical trial. Personal Communication 1996.
Varma 2006 {published data only}
- Varma AK, Bal A, Kumar H, Kesav R, Nair S. Efficacy of polyurethane foam dressing in debrided diabetic lower limb wounds. Wounds: A Compendium of Clinical Research and Practice 2006;18(10):300‐6. [Google Scholar]
Veves 2001 {published data only}
- Veves A, Falanga V, Armstrong DG, Sabolinski ML. Graftskin, a human skin equivalent, is effective in the management of neuropathic diabetic foot ulcers. Diabetes Care 2001;24(2):290‐5. [DOI] [PubMed] [Google Scholar]
Veves 2002 {published data only}
- Sheehan P, Jones P, Caselli A, Giurini JM, Veves A. Percent change in wound area of diabetic foot ulcers over a 4‐week period is a robust predictor of complete healing in a 12‐week prospective trial. Diabetes Care 2003;26:1879‐82. [DOI] [PubMed] [Google Scholar]
- Veves A. Promogran clinical trial. The Diabetic Foot 2001;4(4):S4‐S5. [Google Scholar]
- Veves A. Wound care device expert meeting. Summary report. Promogran ‐ Clinical trial data. The Diabetic Foot 2001;4(3):S8‐S10. [Google Scholar]
- Veves A, Sheehan P, Pham HT. A randomized controlled trial of Promogran (a collagen/oxidised regenerated cellulose dressing) vs standard treatment in the management of diabetic foot ulcers. Archives of Surgery 2002;137:822‐7. [DOI] [PubMed] [Google Scholar]
Whalley 2001 {published data only}
- Capillas R, Whalley A, Boulton AMJ, Dargis V, Harding K, Acker K. Performance characteristics and safety of hydrogels using a non‐adhesive foam dressing as secondary dressing in the treatment of diabetic foot ulcers. 12th Conference of the European Wound Management Association;2002, 23‐25 May; Granada, Spain. 2002:261.
- Whalley A, Boulton AJM, Dargis V, Harding K, Acker K, Capillas R. Characteristics of the efficacy and safety of a hydrogen using a hydropolymer as the secondary dressing in the treatment of ulcers of the diabetic foot. 12th Conference of the European Wound Management Association;2002, 23‐25 May; Granada, Spain. 2002:322.
- Whalley A, Boulton AJM, Dargis V, Harding K, Acker K, Capillas R. Performance characteristics and safety of Purilon gel versus Intrasite using Biatain non‐adhesive dressing as secondary dressing in the treatment of diabetic foot ulcers. 11th European Tissue Repair Society Annual Conference; 2001 5‐8 September; Cardiff, Wales. 2001:49.
Woo 2010 {published data only}
- Woo K, Sibbald G, Coutts P. Reduction of infection and pain in chronic wounds using a new antimicrobial foam dressing. EWMA Journal 2010;10(2):59 Abstract 78. [DOI] [PubMed] [Google Scholar]
Yao 2007 {published data only}
- Yao XZ, Chen W. Effectiveness of feet soaking with Chinese herbs combined with changing dressings in the treatment of diabetic foot ulceration. Journal of Nursing Science 2007;22(5):42‐3. [Google Scholar]
Zimny 2003 {published data only}
- Zimny S, Schatz H, Pfohl U. The effects of applied felted foam on wound healing and healing times in the therapy of neuropathic diabetic foot ulcers. Diabetic Medicine 2003;20(8):622‐5. [DOI] [PubMed] [Google Scholar]
Additional references
Abbott 2002
- Abbott CA, Carrington AL, Ashe H, Bath S, Every LC, Griffiths J, et al. The North West Diabetes Foot Care Study: incidence of and risk factors for, new diabetic foot ulceration in a community‐based patient cohort. Diabetic Medicine 2002;19:377‐84. [DOI] [PubMed] [Google Scholar]
Apelqvist 2000a
- Apelqvist J, Bakker K, Houtum WH, Nabuurs‐Franssen MH, Schaper NC. International consensus and practical guidelines on the management and the prevention of the diabetic foot. International Working Group on the Diabetic Foot. Diabetes Metabolism Research and Reviews 2000;16(Suppl 1):S84‐92. [DOI] [PubMed] [Google Scholar]
Apelqvist 2000b
- Apelqvist J, Larsson J. What is the most effective way to reduce incidence of amputation in the diabetic foot?. Diabetes Metabolism Research and Reviews 2000;16(Suppl 1):S75‐83. [DOI] [PubMed] [Google Scholar]
Becker 2011
- Becker LA, Oxman AD. Chapter 22: Overviews of reviews. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Bergin 2006
- Bergin SM, Wraight P. Silver based wound dressings and topical agents for treating diabetic foot ulcers. Cochrane Database of Systematic Reviews 2006, Issue 1. [DOI: 10.1002/14651858.CD005082.pub2] [DOI] [PubMed] [Google Scholar]
BNF 2010
- British Medical Association and Royal Pharmaceutical Society of Great Britain. British National Formulary Appendix 8: Wound management products and elastic hosiery. http://www.bnf.org.uk/bnf/bnf/current Sept 2010;60.
Cardinal 2009
- Cardinal M, Eisenbud DE, Armstrong DG, Zelen C, Driver V, Attinger C, et al. Serial surgical debridement: a retrospective study on clinical outcomes in chronic lower extremity wounds. Wound Repair and Regeneration 2009;17(3):306‐11. [DOI] [PubMed] [Google Scholar]
Currie 1998
- Currie CJ, Morgan CL, Peters JR. The epidemiology and cost of inpatient care for peripheral vascular disease, infection, neuropathy, and ulceration in diabetes. Diabetes Care 1998;21(1):42‐8. [DOI] [PubMed] [Google Scholar]
Deeks 2002
- Deeks JJ. Issues in the selection of a summary statistic for meta‐analysis of clinical trials with binary outcomes. Statistics in Medicine 2002;21(1):575‐600. [DOI] [PubMed] [Google Scholar]
Diabetes UK 2011
- Reports and statistics. http://www.diabetes.org.uk/Professionals/Publications‐reports‐and‐resources/Reports‐statistics‐and‐case‐studies/Reports/. www.diabetes.org.uk, (accessed July 2011).
Dorresteijn 2010
- Dorresteijn JAN, Kriegsman DMW, Valk GD. Complex interventions for preventing diabetic foot ulceration. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD007610.pub2] [DOI] [PubMed] [Google Scholar]
Dorrestein 2001
- Dorresteijn JA N, Kriegsman DM W, Assendelft WJJ, Valk GD. Patient education for preventing diabetic foot ulceration. Cochrane Database of Systematic Reviews 2001, Issue 4. [DOI: 10.1002/14651858.CD001488.pub2] [DOI] [PubMed] [Google Scholar]
Dumville 2011a
- Dumville JC, Deshpande S, O'Meara S, Speak K. Foam dressings for healing diabetic foot ulcers. Cochrane Database of Systematic Reviews 2011, Issue 9. [DOI: 10.1002/14651858.CD009111.pub2.] [DOI] [PubMed] [Google Scholar]
Dumville 2011b
- Dumville JC, O'Meara S, Deshpande S, Speak K. Hydrogel dressings for healing diabetic foot ulcers. Cochrane Database of Systematic Reviews 2011, Issue 5. [DOI: 10.1002/14651858.CD009101.pub2.] [DOI] [PubMed] [Google Scholar]
Edwards 2010
- Edwards J, Stapley S. Debridement of diabetic foot ulcers. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD003556.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fiskin 1996
- Fiskin R, Digby M. Which dressing for diabetic foot ulcers?. Practical Diabetes International 1996;13(4):107‐9. [Google Scholar]
Gregg 2004
- Gregg EW, Sorlie P, Paulose‐Ram R, Gu Q, Eberhardt MS, Wolz M, et al. Prevalence of lower‐extremity disease in the U.S. adult population >40 years of age with and without diabetes. Diabetes Care 2004;27(7):1591‐7. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group (Editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hinchliffe 2008
- Hinchliffe R, Valk GD, Apelqvist J, Armstrong DG, Bakker K, Game FL, et al. A systematic review of the effectiveness of interventions to enhance the healing of chronic ulcers of the foot in diabetes. Diabetes Metabolism Research Review 2008;24(Suppl 1):S119‐44. [DOI] [PubMed] [Google Scholar]
Kumar 1994
- Kumar S, Ashe HA, Parnell LN, Fernando DJ, Tsigos C, Young RJ, et al. The prevalence of foot ulceration and its correlates in type 2 diabetic patients: a population‐based study. Diabetic Medicine 1994;11:480‐4. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J, on behalf of the Cochrane Information Retrieval Methods Group. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011.
Margolis 1999
- Margolis D, Kantor J, Berlin J. Healing of diabetic neuropathic foot ulcers receiving standard treatment. A meta‐analysis. Diabetes Care 1999;22:692‐5. [DOI] [PubMed] [Google Scholar]
Mason 1999
- Mason J, O'Keeffe C, Hutchinson A, McIntosh A, Young R, Booth A. A systematic review of foot ulcer in patients with type 2 diabetes mellitus. II: treatment. Diabetes Medicine 1999;16(11):889‐909. [DOI] [PubMed] [Google Scholar]
Morris 1998
- Morris AD, McAlpine R, Steinke D, Boyle DI, Ebrahim AR, Vasudev N, et al. Diabetes and lower limb amputations in the community. A retrospective cohort study. DARTS/MEMO Collaboration. Diabetes Audit and Research in Tayside Scotland/Medicines Monitoring Unit. Diabetes Care 1998;21:738‐43. [DOI] [PubMed] [Google Scholar]
Murray 1996
- Murray HJ, Young MJ, Hollis S, Boulton AJ. The association between callus formation, high pressures and neuropathy in diabetic foot ulceration. Diabetic Medicine 1996;13:979‐82. [DOI] [PubMed] [Google Scholar]
Nelson 2006
- Nelson EA, O'Meara S, Golder S, Dalton J, Craig D, Iglesias C, DASIDU Steering Group. Systematic review of antimicrobial treatments for diabetic foot ulcers. Diabetes Medicine 2006;23(4):348‐59. [DOI] [PubMed] [Google Scholar]
O'Meara 2000
- Meara S, Cullum N, Majid M, Sheldon T. Systematic reviews of wound care management: (3) antimicrobial agents for chronic wounds; (4) diabetic foot ulceration. Health Technology Assessment 2000;4(21):1‐237. [PubMed] [Google Scholar]
Oyibo 2001
- Oyibo SO, Jude EB, Tarawneh I, Nguyen HC, Harkless LB, Boulton AJ. A comparison of two diabetic foot ulcer classification systems: the Wagner and the University of Texas wound classification systems. Diabetes Care 2001;24(1):84‐8. [DOI] [PubMed] [Google Scholar]
Pecoraro 1990
- Pecoraro RE, Reiber GE, Burgess EM. Pathways to diabetic limb amputation: basis for prevention. Diabetes Care 1990;13:513‐21. [DOI] [PubMed] [Google Scholar]
Pound 2005
- Pound N, Chipchase S, Treece K, Game F, Jeffcoate W. Ulcer‐free survival following management of foot ulcers in diabetes. Diabetic Medicine 2005;22(10):1306‐9. [DOI] [PubMed] [Google Scholar]
Reiber 1996
- Reiber G. The epidemiology of diabetic foot problems. Diabetic Medicine 1996;13S:S6‐11. [PubMed] [Google Scholar]
Reiber 1999
- Reiber GE, Vileikyte L, Boyko EJ, Aguila M, Smith DG, Lavery LA, et al. Causal pathways for incident lower extremity ulcers in patients with diabetes from two settings. Diabetes Care 1999;22:157‐62. [DOI] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre. Review Manager (RevMan) Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, issue 2011.
Schaper 2004
- Schaper NC. Diabetic foot ulcer classification system for research purposes: a progress report on criteria for including patients in research studies. Diabetes Metabolism and Research Review 2004;20(S1):S90‐5. [DOI] [PubMed] [Google Scholar]
Schulz 2010
- Schulz KF, Altman DG, Moher D, CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. PLoS Medicine 2010;7(3):e1000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
SIGN 2011
- Scottish Intercollegiate Guidelines Network (SIGN). Search filters. http://www.sign.ac.uk/methodology/filters.html#random.
Singh 2005
- Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA 2005;293:217‐28. [DOI] [PubMed] [Google Scholar]
Smith 2003
- Smith J. A national survey of podiatry practice in the treatment of diabetic foot ulcers. Unpublished.
Spencer 2000
- Spencer S. Pressure relieving interventions for preventing and treating diabetic foot ulcers. Cochrane Database of Systematic Reviews 2000, Issue 3. [DOI: 10.1002/14651858.CD002302] [DOI] [PubMed] [Google Scholar]
Steed 2006
- Steed DL, Attinger C, Colaizzi T, Crossland M, Franz M, Harkless L, et al. Guidelines for the treatment of diabetic ulcers. Wound Repair and Regeneration 2006;14(6):680‐92. [DOI] [PubMed] [Google Scholar]
Storm‐Versloot 2007
- Storm‐Versloot MN, Vos CG, Ubbink DT, Vermeulen H. Topical silver for preventing wound infection. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD006478] [DOI] [PubMed] [Google Scholar]
Tesfaye 1996
- Tesfaye S, Stephens L, Stephenson J, Fuller J, Platter ME, Ionescu‐Tirgoviste C, et al. The prevalence of diabetic neuropathy and its relationship to glycaemic control and potential risk factors: The EURODIAB IDDM complications study. Diabetalogia 1996;39:1377‐84. [DOI] [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials 2007;7(8):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Gils 1999
- Gils C, Wheeler LA, Mellsrom M, Brinton EA, Mason S, Wheeler CG. Amputation prevention by vascular surgery and podiatry collaboration in high risk diabetic and non‐diabetic patients ‐ the operation desert foot experience. Diabetes Care 1999;22(5):678‐83. [DOI] [PubMed] [Google Scholar]
Wagner 1981
- Wagner FW. The dysvascular foot: a system of diagnosis and treatment. Foot and Ankle 1981;2:64‐122. [DOI] [PubMed] [Google Scholar]
WHO 2005
- World Health Organisation. Diabetes estimates and projections. http//www.who.int/diabetes/facts/world‐figures/en/index4.html (Accessed 23rd February 2011).
Wild 2004
- Wild S. Global prevalence of diabetes estimates for the year 2000 and projections for 2030. Diabetes Care 2004;27(5):1047‐53. [DOI] [PubMed] [Google Scholar]
Winter 1963
- Winter GD, Scales JT. Effect of air drying and dressings on the surface of a wound. Nature 1963;197:91. [DOI] [PubMed] [Google Scholar]
Wrobel 2001
- Wrobel JS, Mayfield JA, Reiber GE. Geographic variation of lower limb extremity major amputation in individuals with and without diabetes in the Medicare population. Diabetes Care 2001;24:860‐4. [DOI] [PubMed] [Google Scholar]


