Abstract
Background
Ankle fractures, which usually occur after a twisting incident, are a diverse collection of injuries with different levels of complexity and severity. They have an incidence of 1 in 1000 a year in children. Treatment generally involves splints and casts for minor fractures and surgical fixation with screws, plates and pins followed by immobilisation for more serious fractures.
Objectives
To assess the effects (benefits and harms) of different interventions for treating ankle fractures in children.
Search methods
We searched the Cochrane Bone, Joint and Muscle Trauma Group Specialised Register (22 September 2015), the Cochrane Central Register of Controlled Trials (CENTRAL) (2015, Issue 8), MEDLINE (1946 to September Week 2 2015), MEDLINE In‐Process & Other Non‐Indexed Citations (21 September 2015), EMBASE (1980 to 2015 Week 38), CINAHL (1937 to 22 September 2015), trial registers (17 February 2015), conference proceedings and reference lists of articles.
Selection criteria
We included randomised and quasi‐randomised controlled trials evaluating interventions for treating ankle fractures in children.
Data collection and analysis
Two review authors independently screened titles, abstracts and full articles for inclusion, assessed risk of bias and collected data. We undertook no meta‐analysis.
Main results
We included three randomised controlled trials reporting results for 189 children, all of whom had a clinical diagnosis of a "low risk" ankle fracture. These were predominantly classified as undisplaced Salter‐Harris type I fractures of the distal fibula. All three trials compared non‐surgical management options. The three trials were at high risk of bias, primarily relating to the impracticality of blinding participants and treating clinicians to the allocated interventions.
Two trials compared the Aircast Air‐Stirrup ankle brace versus a rigid cast, which was a removable fibreglass posterior splint in one trial (trial A) and a below‐knee fibreglass walking cast in the other trial (trial B). In trial A, both devices were removed at around two weeks. In trial B, removal of the brace was optional after five days, while the walking cast was removed after three weeks. There was low‐quality evidence of clinically important differences in function scores at four weeks in favour of the brace groups of both trials. Function was measured using the Activities Scale for Kids‐performance (ASKp; score range 0 to 100, higher scores mean better function) in trial A and using a modified version of the ASKp score (range 0 to 100%, higher percentages mean better function) in trial B. The results for trial A (40 participants) were median 91.9 in the brace group versus 84.2 in the splint group. The results for trial B (104 participants) were 91.3% versus 85.3%; mean difference (MD) 6.00% favouring brace (95% confidence interval (CI) 1.38% to 10.62%). Trial B indicated that 5% amounted to a clinically relevant difference in the modified ASKp score. Neither trial reported on unacceptable anatomy or related outcomes or long‐term follow‐up. There was very low‐quality evidence relating to adverse events, none of which were serious. Trial A found twice as many children with pressure‐related complications in the brace group (10 of 20 versus 5 of 20). In contrast, trial B found four times as many children in the cast group had adverse outcomes assessed in terms of an unscheduled visit to a healthcare provider (4 of 54 versus 16 of 50). Both trials linked some of the adverse events in the brace group with the failure to wear a protective sock. There was very low‐quality evidence indicating an earlier return to pre‐injury activity in the brace groups in both trials. Trial B provided low‐quality evidence that children much prefer five days or more wearing an ankle brace than three weeks immobilised in a walking ankle cast. There was moderate‐quality evidence of a lack of difference between the two groups in pain at four weeks.
The third trial compared the Tubigrip bandage plus crutches and advice versus a plaster of Paris walking cast for two weeks and reported results at four weeks' follow‐up for 45 children with an inversion injury of the ankle. The trial found very low‐quality evidence of little difference in pain and function between the two groups, measured using a non‐validated pain and function score at four weeks. The trial did not report on adverse effects. There was very low‐quality evidence of an earlier return to normal activities, averaging six days, in children treated with Tubigrip (mean 14.17 days for Tubigrip versus 20.19 days for cast; MD ‐6.02 days, 95% CI ‐8.92 to ‐3.12 days).
Recent evidence from magnetic resonance imaging studies of the main category of injury evaluated in these three trials suggests that most of the injuries in these trials were sprains or bone bruises rather than fractures of the distal fibular growth plate.
Authors' conclusions
There is low‐quality evidence of a quicker recovery of self reported function at four weeks in children with clinically diagnosed low‐risk ankle fractures who are treated with an ankle brace compared with those treated with a rigid cast, especially a non‐removable walking cast. There is otherwise a lack of evidence from randomised controlled trials to inform clinical practice for children with ankle fractures. Research to identify and address priority questions on the treatment of these common fractures is needed.
Plain language summary
Treatments for broken ankles in children
Background
A broken ankle, also called an ankle fracture, involves a break in one or more of the three bones that make up the ankle. It often results from a twisted ankle. Ankle fracture is a common injury in children. Some fractures are minor, and the bones remain in place. Other fractures are more serious, such as when the broken bones are displaced from each other or even come through the skin. These fractures can affect the way the bones grow. Serious disruption of the growth plates may result in leg deformity.
Minor fractures are often treated by placing the injured leg in a removable fibreglass splint or a plaster cast. These devices may also be used for some displaced fractures after the displaced fracture parts have been put back into place. However, displaced fractures often require surgery. An operation enables the surgeon to put the broken bone pieces back into their correct places. Screws, plates and pins are typically used to hold the bones in place. The leg is usually placed in a plaster cast while the bones heal.
Results of the search
We searched medical databases up to September 2015 and included three randomised studies reporting results for 189 children. All the children were considered by the treating clinicians to have minor ankle fractures that were at low risk of growth‐plate complications.
Key results
Two studies compared the use of a removable prefabricated ankle brace with a rigid cast. One study used a removable fibreglass splint for two weeks, and the other used a below‐the‐knee plaster walking cast for three weeks. Both studies provided some evidence of a quicker recovery of self reported function at four weeks in children who were treated with an ankle brace compared with those treated with a rigid cast. One study reported more complications, such as pressure marks and blisters, in the brace group. Most of these were attributed to a protective sock not being worn with the brace. The other study reported more unscheduled visits to healthcare providers for problems in the rigid‐cast group. In this study, children much preferred the brace, which could be removed after five days, than the cast, which remained on for three weeks. Neither study reported results in the long term.
The third study compared the Tubigrip bandage plus crutches and advice versus a plaster of Paris walking cast for two weeks. This study found some weak evidence of an earlier return to former activities of around six days (14 compared with 20 days) in children in the Tubigrip group. The study did not report on complications or long‐term outcome.
Quality of the evidence
All three studies had weaknesses that could have affected the reliability of their results. We considered the evidence to be generally of low or very low quality, which means we are unsure of these results.
Conclusions
Using an ankle brace rather than a rigid cast, in particular a non‐removable walking cast, may result in quicker recovery in children with minor ankle fractures. Further studies are required to identify the best treatment for broken ankles in children.
Summary of findings
Summary of findings for the main comparison. Ankle brace compared with rigid cast for 'low risk' ankle fractures in children.
| Ankle brace compared with rigid cast for 'low risk' ankle fractures in children | ||||||
|
Patient or population: children with acute 'low risk' (undisplaced) ankle fractures Settings: acute‐care setting in children's hospitals Intervention: ankle brace (this was Aircast Air‐Strirrup in both trials)1 Comparison: rigid cast (this was fibreglass in both trials; one was a posterior splint, the other a below‐knee walking cast)1 | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Cast | Brace | |||||
| Modified Activities Scale for Kids‐performance (ASKp) score (0 to 100%: best outcome) at 4 weeks | The mean ASKp score was 85.3% in the cast group of 1 trial | The mean ASKp score in the brace group was 6.00% higher (1.38% to 10.62% higher) | ‐ | 104 (1) | ⊕⊕⊝⊝ low2 | The MD was greater than the 5% value used by the trial as a measure of a clinically relevant change for the purposes of their sample size calculation. The second trial also reported higher ASKp scores (score (0 to 100: best outcome) in the brace group (median 91.9 versus 84.2). However, the difference between the 2 groups was reported not to be statistically significant (reported P = 0.13) |
| Unacceptable anatomy: leg‐length discrepancy, limp, abnormal gait | See comment | See comment | ‐ | ‐ | ‐ | Not reported. Although these outcomes are unlikely for 'low risk' ankle fractures, the follow‐up of both trials was too short to check for this outcome |
| Number of children experiencing adverse outcomes at 4 weeks | Fibreglass posterior splint | Brace |
RR 2.0 (0.83 to 4.81) |
40 (1) | ⊕⊝⊝⊝ very low4 | Adverse effects5 listed are likely to be linked with the interventions used.1 Both trials linked some of the adverse events in the brace group with not wearing a protective sock. 1 trial stated that no serious adverse events were reported at 3 months in 94 children |
| 250 per 10003 | 500 per 1000 (208 to 1000) | |||||
| Fibreglass walking cast | Brace | RR 0.23 (0.08 to 0.65) | 104 (1) | |||
| 320 per 10003 | 74 per 1000 (26 to 208) | |||||
| Time to resume pre‐injury level of activity (days) | median 20.0 days | median 12.5 days | ‐ | 40 (1) |
⊕⊝⊝⊝ very low6 | |
| Return to pre‐injury levels of activity at 4 weeks | 586 per 10003 | 797 per 1000 (598 to 1000) | RR 1.36 (1.02 to 1.80) | 94 (1) | ⊕⊝⊝⊝ very low7 | |
| Patient satisfaction: would have preferred the other device | 540 per 10003 | 29 per 1000 (16 to 173) |
RR 0.10 (0.03 to 0.32) |
103 (1) | ⊕⊕⊝⊝ low8 | This trial compared Aircast Air‐Stirrup for 5 days versus walking cast for 3 weeks. The second trial comparing Aircast Air‐Stirrup versus backslab for 2 weeks reported that similar numbers would be happy to have the same intervention again |
| Faces Pain Scale score at 4 weeks' post ‐injury (0 to 10: greatest pain) | The mean pain score was 0.33 in the cast group | The mean pain score in the brace group was 0.01 lower (0.33 lower to 0.31 higher) | ‐ | 104 (1) | ⊕⊕⊕⊝ moderate9 | Duration of analgesic use (paracetamol or ibuprofen) in the first 14 days was similar in both groups (median 2.0 versus 1.6 days) in the second trial1 (40 participants) |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; MD: Mean difference; RR: Risk Ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1One trial compared Aircast Air‐Stirrup ankle brace versus a removable moulded fibreglass posterior splint (backslab); both were removed at 12 to 16 days. The second trial compared Aircast Air‐Stirrup ankle brace with optional removal after five days versus a below‐knee fibreglass walking cast removed after three weeks.
2The evidence was downgraded one level for risk of bias (mainly performance and detection bias) and one level for indirectness (the timing of the outcome was too short considering that the cast was retained for three weeks).
3The control group estimate was based on that of the trial providing data for this outcome.
4The evidence was downgraded one level for risk of bias (mainly performance and detection bias), one level for inconsistency (however, the data from the two trials were not pooled), and one level for indirectness (issues relating to reporting and definition of adverse events).
5Listed adverse events were pressure marks, blisters and heel pain in the first trial, and unscheduled visits to a healthcare provider for reasons such as poor cast fit, itchy leg and "strength and range‐of‐motion issues" in the second trial.
6The evidence was downgraded one level for risk of bias (mainly performance and detection bias), one level for imprecision (small trial size), and one level for indirectness (study population included children with sprains only).
7The evidence was downgraded two levels for risk of bias (mainly performance and detection bias, but also attrition bias), and one level for imprecision (single trial).
8The evidence was downgraded one level for risk of bias (mainly performance and detection bias) and one level for inconsistency (the data from the second trial, where the timing of the interventions was equivalent and the cast could be removed for washing, showed similar satisfaction).
9The evidence was downgraded one level for risk of bias (mainly performance and detection bias).
Background
Description of the condition
The ankle is a hinge joint between the two lower‐leg bones (the tibia and fibula) and the talus bone, or ankle bone. It allows the foot to flex (pull up) and extend (push down) (Blackburn 2012). Paediatric ankle fractures occur with an incidence of 1 in 1000 children per year (Bible 2009). An epidemiological study of fractures in children in southeast Scotland showed that ankle fractures made up 3.5% of all children's fractures (Rennie 2007). They are the third most common cause of epiphyseal plate, or growth plate injury (Peterson 1994) (the epiphyseal plate or physis, herein referred to as the growth plate, is the area of a long bone at which bone lengthening occurs (Salter 1963)). The average age of children who sustain ankle fractures is 10.9 years, and 58% are male. Ankle fractures occur most commonly as a result of twisting injuries, often during football (Rennie 2007).
X‐rays are typically used to diagnose ankle fractures (Simanovsky 2005; Taggart 2012). Magnetic resonance imaging (MRI) may be used to detect occult or hidden fractures, or to characterise soft‐tissue injuries such as those to the ligaments that stabilise the distal tibia and fibula (Hermans 2012). A computed tomography scan may be useful in those whose fracture patterns are intra‐articular (across a joint surface) (Cutler 2004).
Ankle fractures represent a varied group of injuries, which differ in their severity and complexity. Fractures of the ankle are classified under a variety of systems. The most commonly used system for fractures in children and adolescents is the Salter‐Harris system, which is based on the relation of the fracture with the growth plate and carries with it prognostic significance. In this system, a fracture that follows the plane of the growth plate is classified as Salter‐Harris type I. Type II fractures occur through the growth plate and then enter the shaft of the bone. A type III fracture involves the growth plate and then exits the bone end, whereas a type IV fracture traverses the growth plate, involving both the shaft and the end of the bone. A fracture with compression of the growth plate is classified as Salter‐Harris type V (Salter 1963). Characteristics of ankle fractures in any age group that are understood to influence outcome are the displacement (misalignment) of the fracture fragments, damage to or significant incongruity of the joint surface, and damage to the growth plate. For example, displaced fragments of bone do not heal together in an anatomical way, and the resultant deformity may cause abnormal biomechanical loading of the joint, with a risk of secondary osteoarthrosis. The same concerns apply to joint surface incongruity. Damage to the growth plate in children may interrupt growth at that site and cause it to fuse early (premature physeal closure) (Barmada 2003;Leary 2009). This may result in the affected leg being shorter than the other and angular deformity (or bend) if only one part of the growth plate is affected and the other part continues to grow (partial physeal arrest) (Blackburn 2012). This is important in subsequent biomechanical loading and may give the child a limp, joint instability and secondary osteoarthrosis. This accounts for the focus on the growth plate in the Salter‐Harris classification system for children and adolescents.
The less commonly used Lauge‐Hansen classification system links foot position, such as whether it is pronated or supinated, and which direction the applied force is impacting the ankle (external rotation) to a described injury with suggested therapeutic strategies (Aiyenuro 2013). The most common type of injury to an ankle is while it is in supination and the force is external‐rotation (SER), which results in a typical series of injuries: firstly to the anterior inferior tibiofibular ligament (SER1), then a short spiral fracture of the fibula (SER2), then a tear to the posterior inferior tibiofibular ligament (SER3), and a medial injury to either the deltoid ligament or the medial malleolus (SER4) (Aiyenuro 2013). Thus this system provides information on both mechanism and severity of injury.
Of particular importance in adolescents is injury to growth plates whilst they are fusing. The ankle growth plate typically begins to fuse at 15 years of age in girls, and at 17 years in boys. In both cases, complete fusion usually occurs within 18 months of onset (Blackburn 2012). A fracture through the growth plate occurring during this period of fusion is called a transitional fracture. Examples of transitional fractures are triplane and Tillaux fractures. A triplane fracture is a fracture that extends into the shaft of the bone in one plane as well as through the transitional growth plate into the joint in an orthogonal plane (Jones 2003). Tillaux fractures are Salter‐Harris type III fractures occurring during fusion, where the fracture involves avulsion of a fragment of epiphyseal bone due to force exerted on it by the anterior tibiofibular ligament (Blackburn 2012).
Classifying such a diverse group of injuries as ankle fractures is important as it provides an indication of the complexity of the fracture, allows for accurate communication about the fracture, and informs appropriate treatments for it. It also informs prognosis.
Description of the intervention
If a fracture is stable and undisplaced, treatment generally involves the immobilisation of the affected leg in an above‐ or below‐knee cast with regular radiographic follow‐up in the community to ensure the fracture remains stable and undisplaced. A cast can be constructed of diverse materials, such as fibreglass or plaster of Paris. Strapping, prefabricated braces and prefabricated boots may also be used. The cast may be partial (back slab) or full (encircling the limb) and can be made either to extend above the knee or remain just below it, thereby allowing the knee to bend. The period of time a cast is worn, generally several weeks, may be influenced by the clinical situation (fracture mechanism, configuration and associated injury), the age of the child and social circumstances. Some children are required to avoid applying any weight through the leg, especially in the early stages of healing, but may be allowed partial and then full weight‐bearing status later.
Surgery is generally reserved for displaced fractures, especially where the joint surface is disrupted. This typically involves open surgery to expose the fractured bone and, under direct vision, re‐position the bone fragments; these are then fixed in place using devices such as screws, pins and plates. After surgery, a cast may be applied to support the position of fragments whilst the fracture heals. Where there is significant damage to the soft tissue, external fixation, whereby the fracture is immobilised using pins and screws that go through the skin and connect with a frame outside the body, may be used. This may be a preliminary procedure to hold the bones in place while the child's local soft tissue or general condition improves, or it may be a definitive operation that holds the bones in place to allow healing. In the former situation, it is likely that secondary surgery will follow when the child's condition is more stable.
How the intervention might work
The choice of treatment for ankle fractures will be influenced by the type of fracture (fracture pattern) and any damage to surrounding tissue, such as swelling, bruising and damage to nerves or vessels. Splinting immobilises the bony fragments and allows them to heal together. Examples of non‐surgical immobilisation include strapping, prefabricated braces, prefabricated boots, and casts constructed of plaster of Paris or other, synthetic materials. These differ with regard to levels of rigidity and immobilisation, convenience and availability, conformity to underlying anatomy and cost. Prefabricated boots and braces will not conform exactly to the individual and may not be available at all centres. Plaster of Paris is widely available and provides a bespoke cast, but can be heavy and weakens if it gets wet or with prolonged loading (weight‐bearing). Synthetic cast materials are generally lighter than plaster of Paris but are more elastic and generally do not conform as well to underlying anatomy as plaster of Paris. Synthetic materials may not be readily available and may be more expensive. Some splintage can be removed or easily adjusted in the community, whereas other splintage, notably full casts in any material, require return to a clinic.
The risks of casts include discomfort, pressure sores, neurovascular compromise and, most seriously, compartment syndrome (a medical emergency whereby the limb's survival is at risk). In some instances, the cast may loosen, break or weaken over time, and a replacement may be needed. Partial casts (known as 'back slabs') are weaker than full casts. Their main advantage is ease of removal; they do not, however, reduce the risk of compartment syndrome. With a non‐surgical approach, there is a greater risk of requiring later surgery if the bone fragments become misaligned during the initial stages of healing.
A non‐surgical approach avoids the potential complications associated with surgery, which include damage to local nerves and vessels, stripping of periosteal tissue (with adverse impact on healing), infection and scarring. Surgical treatment allows for more accurate reduction of the fracture fragments, and the resultant stability may promote better healing. For ankle fractures in children, surgery is usually indicated if the fracture is unstable, if there is a step in the joint surface that is greater than 2 millimetres, or if there is significant disruption to the growth plate. Even after surgery, it is likely that a cast will be used to immobilise the fixation.
Surgeons have a wide array of tools available to them for surgical intervention, including: lag screws, screws with washers, pin fixation, nailing, tension band wiring and plating (Cottalorda 2008). External fixation including fine‐wire frames is considered useful where there is significant soft‐tissue damage, as it provides relatively rigid fixation and the potential for lengthening with minimal disruption to the soft tissue. However, external fixation devices are cumbersome, with pin infection considered inevitable, and further operations are required to adjust and remove the frame. With any surgical intervention using non‐biodegradable implants, there is a risk that some or all of the metalwork will need to be removed in time, although this is seldom routine.
The duration of immobilisation depends on a number of factors, including the child's age, the fracture configuration (displacement and stability), radiographic (X‐ray) evidence of healing, and the clinical condition of the child (pain reduction and return to functional weight‐bearing). The initial period of immobilisation roughly coincides with the period of time required for bony callus to form around the fracture fragments in both children and adults. Thereafter, most individuals are allowed to partially or fully bear weight. There is also variation in whether the knee is also immobilised in a cast. This decision is likely to be based on the weight‐bearing status and inherent stability of the fracture, as immobilising the knee can improve the stability of the fracture and may deter the individual from bearing weight prematurely.
Within these general guidelines, the type of cast, extent of cast (involving the knee or not), duration of immobilisation, duration of non‐weight‐bearing status, and decision regarding the need for surgery can vary widely between centres and between clinicians.
Why it is important to do this review
Suboptimal management of ankle fractures, especially where they involve the growth plate or joint surface, can have significant long‐term complications that include leg‐length discrepancy, deformity, pain, abnormal gait and secondary osteoarthrosis (Barmada 2003; Leary 2009). Several questions regarding the management of paediatric ankle fractures remain to be answered:
What is the most effective and appropriate non‐surgical immobilisation for a given type of fracture (e.g. strapping, prefabricated braces, prefabricated boots, plaster of Paris or other, synthetic cast, backslab or full cast)?
What is the optimal duration of immobilisation for a given intervention and fracture type?
Should the leg be immobilised in an above‐ or below‐knee cast?
How long, if at all, should a leg remain non‐weight‐bearing?
What are the indications for surgical versus non‐surgical treatment?
If surgery is indicated, what is the safest and most effective surgical intervention for a given fracture pattern?
The purpose of this review was to collate and appraise high‐level evidence from randomised controlled trials in order to explore the variations and reduce uncertainty in, and to assess the appropriateness of, current clinical practice in treating different types of paediatric ankle fractures (including Salter‐Harris type I fibula fractures, Salter‐Harris type II and III medial malleolar fractures, transitional fractures and open ankle fractures). In doing so, we hope to inform patient, parent and clinician decisions in the treatment of ankle fractures in children and also to highlight where there is insufficient evidence to endorse or inform changes to practice.
Objectives
To compare the effects (benefits and harms) of different interventions for treating ankle fractures in children.
We made comparisons in the following main categories:
different conservative (non‐surgical) interventions;
surgical versus conservative treatment;
different surgical interventions;
different types of postsurgical immobilisation.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised and quasi‐randomised (method of allocating participants to a treatment that is not strictly random, for example by hospital number) controlled clinical trials evaluating interventions for treating ankle fractures in children.
Types of participants
Children and adolescents presenting with acute (as defined by study authors) ankle fracture. Ideally, radiological confirmation should be reported in the study. We included trials of adults or skeletally mature adolescents, provided they were clearly less than 10% of the total participants, or separate data were available. We excluded studies where participants with congenital musculoskeletal conditions that affect healing or rate of fracture were more than 10% of the total participants, unless we could obtain separate data from the study authors.
As treatment decisions and outcomes are likely to vary according to the type of ankle fracture (Salter‐Harris type I fibular fractures, Salter‐Harris type II and III medial malleolar fractures, transitional fractures and open ankle fractures), this review made clear, where possible and necessary, to which category of fracture the evidence applies. For trials including ankle injuries for which there was a clinical suspicion of a fracture but 'normal X‐rays' (radiographs) or no radiological confirmation, we included all randomised participants but also presented separate data for those children for whom the fracture diagnosis was confirmed or strengthened, such as via another imaging modality.
Types of interventions
We planned to include all interventions used for treating ankle fractures in children. In our protocol, we set out the following main comparisons.
Different methods of conservative (non‐surgical) management. Our prespecified main comparisons were: below‐knee casts versus above‐knee casts; newer casting methods (e.g. fibreglass casts) versus plaster of Paris casts; prefabricated boots and braces versus rigid casts (such as plaster of Paris casts); partial weight‐bearing versus full non‐weight‐bearing; and shorter periods of immobilisation versus longer periods of immobilisation.
Surgical versus conservative treatment.
Different surgical interventions. Our prespecified main comparisons were: plates and screws versus percutaneous pins; and plates and screws versus external fixation.
Different types of postsurgical immobilisation. Our prespecified main comparisons were: shorter periods of immobilisation versus longer periods of immobilisation; and partial weight‐bearing versus full non‐weight‐bearing.
Types of outcome measures
Primary outcomes
Functional outcome measures, such as the Foot Function Index (FFI) (Budiman‐Mak 1991), Musculoskeletal Function Assessment Questionnaire (Swiontkowski 1999), Activity Scale for Kids (ASK) (Young 2000), Pediatric Outcomes Data Collection Instrument (PODCI) (Daltroy 1998), The Oxford Ankle Foot Questionnaire for Children (OxAFQ‐C) (Morris 2008)
Unacceptable anatomy (angular or rotational deformity, shortening), leg‐length discrepancy, limp, abnormal gait
Adverse effects: infection (joint, osteomyelitis), nerve and soft‐tissue injury (including pressure sores), need for new/further surgical intervention (other than routine implant removal)
Secondary outcomes
Time to return to normal activities (or interim stages of recovery)
Patient and parent satisfaction
Pain (visual analogue scale)
Resource use (e.g. duration of hospitalisation) and other costs
Timing of outcome measurement
We collected data for short‐term (less than three months), medium‐term (between three months and one year) and long‐term (ideally at least one year) follow‐up times.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Bone, Joint and Muscle Trauma Group Specialised Register (22 September 2015), the Cochrane Central Register of Controlled Trials (CENTRAL) (2015, Issue 8), MEDLINE (1946 to September Week 2 2015), MEDLINE In‐Process & Other Non‐Indexed Citations (21 September 2015), EMBASE (1980 to 2015 Week 38), and CINAHL (1937 to 22 September 2015). We also searched Current Controlled Trials (no longer available) and the WHO International Clinical Trials Registry Platform (WHO ICTRP) for ongoing and recently completed trials (17 February 2015). We did not apply any language restrictions.
In MEDLINE (Ovid Online), a subject‐specific strategy was combined with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials (sensitivity‐maximising version) (Lefebvre 2011). Search strategies for CENTRAL, MEDLINE, EMBASE, CINAHL, Current Controlled Trials and the WHO ICTRP can be found in Appendix 1.
Searching other resources
We searched reference lists of articles deemed eligible and contacted expert researchers in the field. We searched websites of conferences and meetings that might report relevant trials, specifically the American Association of Orthopaedic Surgeons Annual Meetings (2012 to 2015), the British Orthopaedic Foot and Ankle Society Annual Scientific Meetings (2009 to 2014), British Orthopaedic Association Annual Congresses (2013 to 2015), Canadian Orthopaedic Association Annual Meetings (2003 to 2015), European Federation of National Associations of Orthopaedics and Traumatology Annual Congresses (1999, 2005, 2007 to 2015), European Foot and Ankle Society Advanced Symposiums (2013 to 2014), and New Zealand Orthopaedic Association Annual Scientific Meeting (2015).
Data collection and analysis
We described in our protocol our intended methodology for data collection and analysis (Yeung 2013), which we based on the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Selection of studies
Three review authors (DEY, CAM, XJ) independently screened titles and abstracts obtained from the electronic searches for potentially eligible studies. We obtained full‐text articles of the potentially eligible studies. The same review authors (DEY, CAM, XJ) independently selected studies according to the inclusion criteria of the review. Disagreements were resolved by discussion and consultation with another review author (SLB).
Data extraction and management
Three review authors (DEY, CAM, XJ) independently extracted data from each trial using a data extraction form and entered data into Review Manager 5.3 (RevMan 2014). We recorded qualitative details and data regarding the study groups, interventions and outcomes. We contacted trial authors for further details. Any differences in the data extraction between the review authors were resolved by reviewing trial reports and discussion among the review authors (DEY, CAM, XJ).
Assessment of risk of bias in included studies
Two review authors (DEY, CAM) independently assessed risk of bias using The Cochrane Collaboration's 'Risk of bias' tool (Higgins 2011). We contacted study authors to help clarify the 'Risk of bias' categories. Any discrepancies were resolved through discussion amongst all the review authors. We assessed the risk of bias as low risk, unclear risk or high risk for the following domains:
sequence generation;
allocation concealment;
blinding of participants and personnel;
blinding of outcome assessment;
incomplete outcome data;
selective outcome reporting;
other bias.
We considered risk of bias associated with patient‐rated outcomes separately from clinician‐rated outcomes for the two blinding and incomplete outcome data domains. We recorded other sources of bias that could potentially affect the outcomes and thus increase the risk of bias.
Measures of treatment effect
We calculated risk ratios and 95% confidence intervals (CIs) for dichotomous outcomes (for example growth plate change) and mean differences and 95% CIs for continuous outcomes reported on the same scale (for example pain scores). We planned to calculate standardised mean differences and 95% CIs when pooling outcomes measured in different ways or using different scales (for example different reporting methods for articular damage or different functional outcome scales).
Unit of analysis issues
As anticipated, the unit of randomisation in the trials was the individual child. Children may occasionally have bilateral injuries, and the results may be presented for fractures or limbs. If such a unit‐of‐analysis issue had arisen and appropriate corrections had not been made, we would have considered presenting the data for such trials only where the disparity between the units of analysis and randomisation was small. Where the data were pooled, we planned to perform a sensitivity analysis to examine the effect of excluding incorrectly reported trials from the analysis.
We were aware of other potential unit‐of‐analysis issues, including multiple observations of the same outcome (such as when a child experienced multiple complications or received multiple modes of treatment) or multiple time points. For the latter, we used data from clinically relevant time points and analysed these separately.
Dealing with missing data
We sought missing data from trial authors. However, we did not consider sensitivity analysis to assess the effect of the data that remained missing to be required. Should we have performed sensitivity analyses, we would have stated the assumptions underlying the methods used (Higgins 2011). Where possible, we reported intention‐to‐treat analyses and were alert to the possibility of unreported loss to follow‐up. We did not impute missing standard deviations nor were there data (standard errors, exact P values or 95% confidence intervals) available to calculate these.
Assessment of heterogeneity
We combined trial results only where the interventions, participant groups and outcome measures were sufficiently similar, as judged by clinical criteria and consideration of the statistical heterogeneity. We assessed statistical heterogeneity by visual inspection of forest plots and consideration of the Chi² test (statistically significant at P value < 0.10) and the I² statistic. Our interpretation of the I² statistic result followed definitions suggested in Higgins 2011: 0% to 40% was not considered to be important; 30% to 60% represented moderate heterogeneity; 50% to 90% represented substantial heterogeneity; and 75% to 100% represented considerable heterogeneity.
Assessment of reporting biases
If more than 10 studies had reported data in a forest plot, we would have attempted to assess publication bias by generating funnel plots (trial effect versus standard error). We planned to assess funnel plot asymmetry using Egger's test (Egger 1997).
Data synthesis
When considered appropriate, we planned to pool results of comparable groups of trials using both fixed‐effect and random‐effects models. We were to choose the model to report in the review based on careful consideration of the extent of heterogeneity and whether it could be explained, in addition to other factors, such as the number and size of the included studies. We were to use 95% confidence intervals throughout. We considered not pooling data where there was considerable heterogeneity (I² greater than 75%) that could not be explained by the diversity of methodological or clinical features in the trials. When meta‐analyses were not possible or appropriate, we reported the data from the relevant trials individually and presented the data in forest plots.
Subgroup analysis and investigation of heterogeneity
The data available from the included trials were insufficient to carry out out preplanned subgroup analyses:
children and adolescents (from birth to 13 years versus 14 to 18 years);
types of fractures (transitional versus not);
open versus closed fractures;
multiple versus isolated injuries.
We planned to investigate whether the results of subgroups were significantly different by inspecting the overlap of 95% confidence intervals and performing the test for subgroup differences available in Review Manager (RevMan 2014).
Sensitivity analysis
In future updates where data allow, we plan sensitivity analysis to explore aspects of trial and review methodology, including the inclusion of trials at high or unclear risk of bias from lack of allocation concealment or assessor blinding or both; the selection of statistical model (fixed‐effect versus random‐effects) for meta‐analysis; the inclusion of trials only reported in conference abstracts; and the effects of missing data.
We conducted two sensitivity analyses to show the findings of:
the majority subgroup of fractures (Salter‐Harris type I) in Barnett 2012; and
a subgroup of ultrasound‐confirmed ankle fractures in Gleeson 1996.
Assessing the quality of the evidence
We used the GRADE approach to assess the quality of evidence (very low, low, moderate, high) for each of the key outcomes listed in the Types of outcome measures (GRADEpro; Higgins 2011). We used five GRADE considerations (limitations in the design and implementation of the studies, indirectness of evidence, unexplained heterogeneity/inconsistency of results, imprecision of results and the probability of publication bias) to assess the quality of evidence for each outcome.
'Summary of findings' tables
We prepared a 'Summary of findings' table for the comparison of ankle brace versus rigid cast for children with "low risk" ankle fractures. We selected for presentation the first six outcomes listed in Types of outcome measures, but also presented return to pre‐injury levels of activity at four weeks as well as time to resume pre‐injury level of activity (days).
Results
Description of studies
Results of the search
We identified and screened a total of 680 records from the following databases: Cochrane Bone, Joint and Muscle Trauma Group Specialised Register (15 records); Cochrane Central Register of Controlled Trials (CENTRAL) (116), MEDLINE (128), EMBASE (167), CINAHL (202), the WHO ICTRP (14) and Current Controlled Trials (38). We identified no relevant studies from searching conference proceedings or the reference lists of the included studies.
We obtained a total of 40 full reports. We included three studies (Barnett 2012, Boutis 2007 (published in two articles), Gleeson 1996), and excluded the remaining 36 (Excluded studies). We did not identify any ongoing studies, and no studies await classification.
A flow diagram summarising the study selection process is shown in Figure 1.
1.
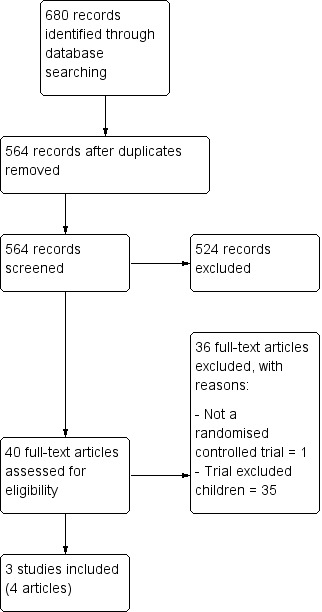
Study flow diagram.
Included studies
We have presented full details of the three included studies in Characteristics of included studies.
Settings
Barnett 2012 was conducted in Australia, Boutis 2007 in Canada, and Gleeson 1996 in the United Kingdom. The three single‐centre trials were carried out in the acute‐care setting of children's hospitals.
Participants
The three trials included a total of 207 children, reporting results for 189 children, who had a clinical diagnosis of "low risk" ankle fracture. The mean age of the 45 children included in Barnett 2012 was 9.2 years (range defined by inclusion criteria 5 to 15 years); 25 children (56%) were female. The mean age of the 104 children followed up in Boutis 2007 was 10.3 years (range 5 to 17 years); there were no data on the gender distribution. The mean age of the 45 children followed up in Gleeson 1996 was 9.2 years (range 3 to 14 years); 26 children (58%) were female.
All three trials recruited children presenting within 72 hours of their injury.
Barnett 2012 included low‐risk ankle fractures, which they defined as an avulsion fracture of the distal fibula, an undisplaced Salter‐Harris type I or II fracture, or an avulsion fracture of the lateral talus. Barnett 2012 excluded sprains defined as where there was no tenderness over the growth plate but tenderness over the distal edge of the fibula or over the deltoid ligament. The majority of fractures (33 of 45: 73%) were Salter‐Harris type I fractures, which were defined as isolated tenderness over the fibula growth plate and normal X‐rays (radiographs). No other imaging modality was used in Barnett 2012.
Boutis 2007 included low‐risk ankle fractures, which they defined as undisplaced distal fibular Salter‐Harris type I and II fractures, avulsion fractures of the distal fibula or fibular epiphysis. The majority of fractures (75 of 104: 72%) were Salter‐Harris type I fractures, which are not evident on normal radiographs. Boutis 2007 provided predefined criteria for "a presumptive diagnosis of this fracture" (see Characteristics of included studies). No other imaging modality was used in Boutis 2007.
Gleeson 1996 included children who presented with painful ankle injuries with swelling and tenderness over the lateral malleolus, with a normal ankle radiograph with no evidence of growth plate fusion, and who were unable to, or could only partially, weight bear. The study used an assessment tool to estimate the likelihood of the child having experienced an undisplaced distal fibular growth plate injury, or Salter‐Harris I fracture. The tool, which was also used to assess outcome, included a visual analogue pain score based on faces, and scores for swelling, tenderness and weight‐bearing. Gleeson 1996 also performed an ultrasound examination of the ankle within 72 hours in 34 of the 45 children included in the follow‐up analyses. They reported that 19 children had a subperiosteal haematoma, which was considered definite evidence of a growth‐plate injury (Salter‐Harris type I fracture). The remaining 15 children had soft‐tissue swelling alone as detected by ultrasound.
Comparisons
All three trials compared non‐surgical interventions.
Barnett 2012 and Boutis 2007 compared Aircast Air‐Stirrup ankle brace versus a rigid cast. However, there were important differences between the two trials in the duration of use of the interventions and choice of rigid casting. In Barnett 2012, both the brace and the cast (comprising a moulded fibreglass posterior splint (backslab), held in place using crepe bandage) were removed after 12 to 16 days. Both the brace and backslab could be removed for bathing. In Boutis 2007, the brace was removed after five days as tolerated, and the below‐knee fibreglass walking cast was removed after three weeks.
Gleeson 1996 compared the Tubigrip bandage with crutches versus plaster of Paris walking cast for two weeks. Those children using Tubigrip were given instructions on elevating their injured limb and applying ice for two weeks.
Outcomes
Only short‐term data (up to three months) were available for all three trials.
Barnett 2012 reviewed children two and four weeks after injury. The primary outcome was mean functional activity, as measured by the change in Activities Scale for Kids‐performance (ASKp) score in the interim period. Boutis 2007 reviewed children using a blinded research physiotherapist who visited children's homes after four weeks and measured their physical function using a modified ASKp, which included the original ASKp questionnaire with eight additional questions specific for ankle activity. Both trials reported on adverse effects. In Boutis 2007, a follow‐up telephone call was made at three months to assess subsequent complications.
Gleeson 1996 reviewed the children two and four weeks post‐injury and reassessed their pain, swelling, growth plate tenderness and degree of weight‐bearing using an unvalidated assessment scale described by the study authors. Gleeson 1996 also reported the number of days before the children stated resuming normal activities, but the study did not report on adverse effects.
Excluded studies
We excluded a total of 36 studies (Ahl 1989; Avci 1998; Bauer 1985; Dijkema 1993; Egol 2000; Eventov 1978; Gorodetskyi 2010; Handolin 2005a; Handolin 2005b; Hedström 1994; Hoelsbrekken 2013; Høiness 2004; Honigmann 2007; Joukainen 2007; Kaukonen 2005; Kimmel 2012; Konrad 2005; Lehtonen 2003; Lin 2008; Mayich 2013; Moore 2006; Moseley 2005; Noh 2012; Pakarinen 2011; Phillips 1985; Rowley 1986; Sanders 2012; Søndenaa 1986; Sun 2014; Thordarson 2001a; Thordarson 2001b; Tsukada 2013; Van Laarhoven 1996; Vioreanu 2007; White 2008; Wikerøy 2010), because the participants in these studies were adults (see Characteristics of excluded studies for details). In addition, one study was not a randomised controlled trial (Eventov 1978).
Risk of bias in included studies
See the 'Risk of bias' tables in Characteristics of included studies and Figure 2; Figure 3.
2.
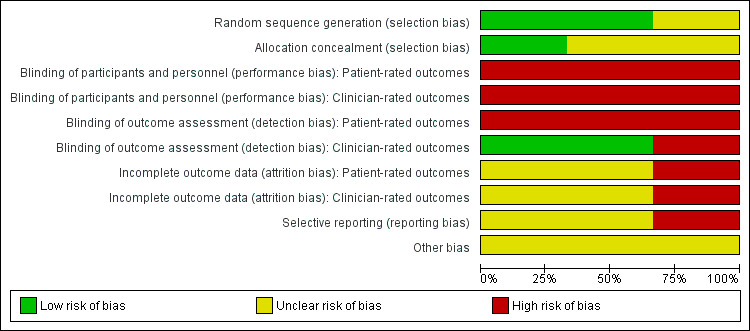
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We deemed random sequence generation to be adequate in two trials (Barnett 2012; Boutis 2007), which we rated as at low risk of sequence generation bias. Although Barnett 2012 stated that the trial investigators were blinded to the random block sizes to ensure allocation concealment, they did not describe measures to safeguard allocation concealment, and so we rated this study as at unclear risk of bias relating to allocation concealment. Boutis 2007, which maintained allocation concealment by using an online randomisation service with immediate email notification of treatment group assignment, was rated as at low risk of bias relating to allocation concealment. Gleeson 1996 provided no information about their method of randomisation and so was rated at unclear risk of bias related to sequence generation and allocation concealment.
Blinding
Since blinding of children was not feasible, patient‐rated outcomes were judged to be at high risk for both performance and detection biases in all three studies. Blinding of personnel applying the interventions was also not done, and thus clinician‐rated outcomes were judged to be at high risk for performance bias for all three trials. Outcome assessment of clinician‐rated outcomes was blind in both Barnett 2012 and Boutis 2007, which we rated as at low risk of bias for this item. In both trials, children were asked to take measures to ensure assessor blinding. In Barnett 2012, children were asked to remove their ankle brace or splint before the arrival of the assessor. In Boutis 2007, children were given an opaque stocking to cover the intervention. Gleeson 1996, which did not report any assessor blinding, was rated as at high risk of detection bias for clinician‐rated outcomes.
Incomplete outcome data
We judged both Barnett 2012 and Boutis 2007 to be at unclear risk of bias for both patient‐ and clinician‐rated outcomes, as the exclusions and loss to follow‐up were comparable in their two intervention groups. We rated Gleeson 1996 as at high risk of bias because they did not report the treatment assignment of the six children (12%) lost to follow‐up and because their account of the ultrasound results was incomplete.
Selective reporting
None of the three trials had prospective trial registration or protocols available. We rated the risk of selective reporting bias as unclear for both Barnett 2012 and Boutis 2007, reflecting some under‐reporting of outcomes described in their methods sections. We rated Gleeson 1996 as at high risk of selective reporting bias due to lack of definition of the outcome measures collected in the methods section, the high likelihood of post‐hoc analyses relating to the ultrasound findings, and lack of report on adverse effects.
Other potential sources of bias
We rated all three trials as at unclear risk of other bias, for different reasons. Barnett 2012 did not randomise sufficient numbers of children to fulfil their a priori power analysis; in Boutis 2007, the Air‐Stirrup ankle braces used in the study were provided free of charge by Aircast; and no sample‐size analysis was provided in Gleeson 1996.
Effects of interventions
See: Table 1
Aircast Air‐Stirrup ankle brace versus rigid cast
Two studies compared the Aircast Air‐Stirrup ankle brace versus rigid cast in low‐risk ankle fractures (Barnett 2012; Boutis 2007). The rigid cast was a fibreglass posterior splint worn for two weeks in Barnett 2012, and a below‐knee fibreglass walking cast worn for three weeks in Boutis 2007.
Primary outcomes
Barnett 2012 measured function using the ASKp score (range 0 to 100, higher scores mean better function) and Boutis 2007 used a modified version of the ASKp score (range 0% to 100%, higher percentages mean better function). However, only medians and interquartile range data were available for 40 children in Barnett 2012 (see Analysis 1.1). The median ASKp scores were higher in the brace group at baseline (pre‐injury value) and two and four weeks' follow‐up (median 91.9 in the brace group versus 84.2 in the splint group), but none of the differences between the two groups were reported as being statistically significant; see Analysis 1.1. There was also little between‐group difference at four weeks in the ASKp scores of children with clinically diagnosed Salter‐Harris type I fractures in Barnett 2012. Boutis 2007 found significantly higher modified ASKp scores in the ankle brace group compared with the walking cast group at four weeks (91.3% versus 85.3%; mean difference (MD) 6.00%, 95% confidence interval (CI) 1.38% to 10.62%, 104 participants; see Analysis 1.2). Barnett 2012 estimated that a difference in the ASKp score of 7 was the difference between normal and mildly disabled, whereas Boutis 2007 considered that a 5% difference in he modified ASKp score represented this distinction. Thus the best estimates and spreads of both trials were likely to include clinically important differences in ASKp or modified ASKp scores favouring the ankle brace at four weeks for at least part of the population.
1.1. Analysis.
Comparison 1 Ankle brace versus rigid cast for the treatment of 'low risk' ankle fractures, Outcome 1 Activities Scale for Kids‐performance (ASKp): 0 to 100: best outcome.
| Activities Scale for Kids‐performance (ASKp): 0 to 100: best outcome | ||||
|---|---|---|---|---|
| Study | Time |
Ankle Brace ASKp scores: median (IQR) (n) |
Rigid cast ASKp scores: median (IQR) (n) |
P value |
| Barnett 2012 | pre‐injury | 97.1 (93.9 to 98.7); n = 20 | 94.5 (91.7 to 99.3); n = 20 | 0.26 |
| Barnett 2012 | 2 weeks | 60.6 (46.8 to 72.8); n = 20 | 56.0 (44.3 to 92.6); n = 20 | 0.26 |
| Barnett 2012 | 4 weeks | 91.9 (75.7 to 98.0); n = 20 | 84.2 (70.6 to 92.6); n = 20 | 0.13 |
| Barnett 2012 | 4 weeks (SH type I fractures) | 93.8 (85.7 to 100.0); n = 15 | 90.2 (80.3 to 92.6); n = 18 | 0.26 |
1.2. Analysis.
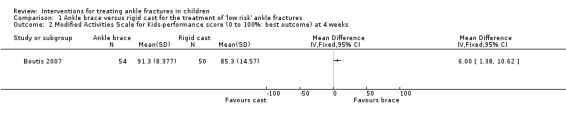
Comparison 1 Ankle brace versus rigid cast for the treatment of 'low risk' ankle fractures, Outcome 2 Modified Activities Scale for Kids‐performance score (0 to 100%: best outcome) at 4 weeks.
Neither trial reported on unacceptable anatomy and related outcomes.
Since there was substantial statistical heterogeneity (I² = 90%) when the results of the two trials were pooled, we presented their results separately in Analysis 1.3. More children in the brace group experienced an adverse outcome (pressure marks, blisters, heel pain) in Barnett 2012: 10/20 versus 5/20; risk ratio (RR) 2.0, 95% CI 0.83 to 4.81. Barnett 2012 observed that the pressure‐related complications were related to not wearing a protective sock with the device in 6 of 10 children in the brace group. Two children in each group of Barnett 2012 required additional follow‐up because of their reluctance to weight bear. Additionally, another child in each group, each of whom was listed as being lost to follow‐up, was indicated as being under orthopaedic review. Based on unscheduled visits to a healthcare provider for reasons such as poor cast fit, itchy leg, and "strength and range‐of‐motion issues", Boutis 2007 reported fewer children in the brace group experienced an adverse event: 4/54 versus 16/50; RR 0.23, 95% CI 0.08 to 0.65. Boutis 2007 also reported that one child in the brace group developed a leg rash, probably because he or she had not worn socks. A telephone follow‐up at three months found no reports of subsequent complications in the 94 children who responded.
1.3. Analysis.
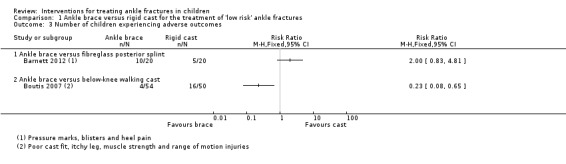
Comparison 1 Ankle brace versus rigid cast for the treatment of 'low risk' ankle fractures, Outcome 3 Number of children experiencing adverse outcomes.
Secondary outcomes
Both studies reported on the return to pre‐injury level of activity. Barnett 2012 found children in the brace group returned to pre‐injury level of activity earlier than those in the cast group (median 12.5 days versus 20.0 days; 40 children, see Analysis 1.4). Boutis 2007 found more children in the brace group had returned to their pre‐injury level of activity at four weeks (42/52 versus 25/42; RR 1.36, 95% CI 1.02 to 1.80; see Analysis 1.5). However, similar numbers of children in Boutis 2007 were able to fully weight bear without pain (39/52 versus 39/50; RR 0.96, 95% CI 0.78 to 1.19).
1.4. Analysis.
Comparison 1 Ankle brace versus rigid cast for the treatment of 'low risk' ankle fractures, Outcome 4 Time to resume pre‐injury level of activity (days).
| Time to resume pre‐injury level of activity (days) | ||
|---|---|---|
| Study |
Ankle brace Time (days): median (IQR), (n) |
Rigid cast Time (days): median (IQR), (n) |
| Barnett 2012 | 12.5 (8.0 to 17.5) days (n = 20) | 20.0 (15.5 to 23.0) days (n = 20) |
1.5. Analysis.
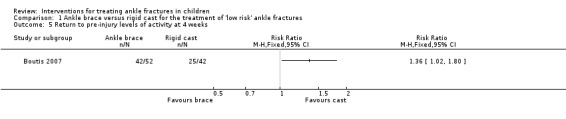
Comparison 1 Ankle brace versus rigid cast for the treatment of 'low risk' ankle fractures, Outcome 5 Return to pre‐injury levels of activity at 4 weeks.
Barnett 2012 reported that most children (or their parents) stated that they would use their allocated intervention again if necessary: 92% of brace group versus 90% of cast group (denominators were not reported). In Boutis 2007, fewer children in the ankle brace group said they would have preferred the other intervention compared with those in the walking‐cast group at four weeks (3/53 versus 27/50; RR 0.10, 95% CI 0.03 to 0.32; see Analysis 1.7). Boutis 2007 found greater patient satisfaction in the ankle brace group, with the majority (37 (87%)) being happy or very happy with their device in the brace group compared with under half of participants (22 (44%)) indicating these levels of satisfaction in the cast group (see Analysis 1.8). Parents in Barnett 2012 reported greater ease in looking after their child's device in the brace group than in the cast group. Boutis 2007 reported, without providing data, that there were no differences in parental preferences between the two groups.
1.7. Analysis.
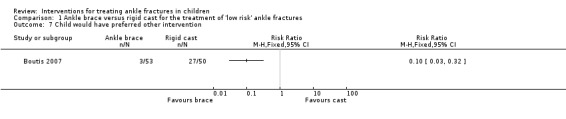
Comparison 1 Ankle brace versus rigid cast for the treatment of 'low risk' ankle fractures, Outcome 7 Child would have preferred other intervention.
1.8. Analysis.
Comparison 1 Ankle brace versus rigid cast for the treatment of 'low risk' ankle fractures, Outcome 8 Patient satisfaction with device: 'not at all happy', 'a little happy', 'happy', or 'very happy'.
| Patient satisfaction with device: 'not at all happy', 'a little happy', 'happy', or 'very happy' | |||
|---|---|---|---|
| Study | Patient Satisfaction | Ankle brace (% of total = 54) | Rigid cast (% of total = 50) |
| Boutis 2007 | Not at all happy | 4 (7.5) | 15 (30.0) |
| Boutis 2007 | A little happy | 2 (3.8) | 13 (26.0) |
| Boutis 2007 | Happy | 19 (35.8) | 13 (26.0) |
| Boutis 2007 | Very happy | 28 (52.8) | 9 (18.0) |
Duration of analgesic use (paracetamol or ibuprofen) in the first 14 days was similar in the two groups in Barnett 2012; see Analysis 1.9. Boutis 2007 found no difference between the groups in terms of pain, as measured by the Bieri Faces Pain Scale (score 0 to 10; higher scores mean worse pain): mean 0.32 in the brace group versus 0.33 in the cast group; MD ‐0.01, 95% CI ‐0.33 to 0.31; 104 participants; see Analysis 1.10).
1.9. Analysis.
Comparison 1 Ankle brace versus rigid cast for the treatment of 'low risk' ankle fractures, Outcome 9 Duration of analgesia use (paracetamol or ibuprofen), (days).
| Duration of analgesia use (paracetamol or ibuprofen), (days) | ||
|---|---|---|
| Study |
Ankle brace Duration (days): median (IQR) |
Rigid cast Duration (days): median (IQR) |
| Barnett 2012 | 2 (0 to 6) days | 1.5 (1 to 4) days |
1.10. Analysis.
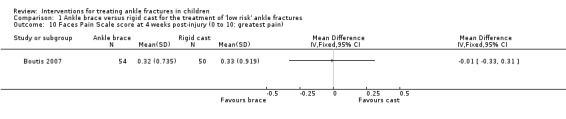
Comparison 1 Ankle brace versus rigid cast for the treatment of 'low risk' ankle fractures, Outcome 10 Faces Pain Scale score at 4 weeks post‐injury (0 to 10: greatest pain).
Boutis 2007 reported that the mean total cost in Canadian dollars during the study period 2003 to 2005 was lower in the brace group (mean 278.3 CAD versus 322.4 CAD; MD ‐44.10, 95% CI ‐142.26 to 52.06 CAD; 104 participants; see Analysis 1.11). Boutis 2007 calculated that the healthcare costs for the ankle brace were significantly lower than for the fibreglass walking cast (90.88 CAD versus 156.60 CAD), but also noted that while total costs and healthcare costs were lower for the ankle brace participants, the parental work loss costs were higher in the ankle brace group (149.60 CAD versus 121.10 CAD). Considering the cost results in the context of more favourable clinical results for the brace group, and referring to a cost‐effectiveness acceptability curve based on direct healthcare costs, Boutis 2007 concluded that the brace was cost‐effective compared with the cast.
1.11. Analysis.
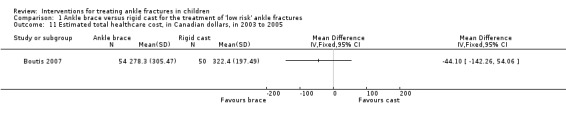
Comparison 1 Ankle brace versus rigid cast for the treatment of 'low risk' ankle fractures, Outcome 11 Estimated total healthcare cost, in Canadian dollars, in 2003 to 2005.
Checks of data for range of ankle motion at four weeks obtained from the lead author of Boutis 2007 endorsed the claimed lack of differences between the two treatment arms in the trial report (data not presented).
Tubigrip versus plaster of Paris walking cast
Gleeson 1996 compared Tubigrip (Seton Healthcare PLC) together with crutches and advice versus plaster of Paris walking cast in 51 children with symptoms and radiographic investigations suggestive of undisplaced distal fibular growth plate injury after an inversion injury of the ankle. Of the 45 children followed up for four weeks, 19 had ultrasound‐diagnosed subperiosteal haematoma consistent with a growth‐plate injury (Salter‐Harris I fractures).
Primary outcomes
Based on a non‐validated composite score (range 3 to 17; worst outcome) that included a 6‐point visual analogue pain score based on faces, and scores for swelling (1 to 4), tenderness (1 to 4) and weight‐bearing (1 to 4), Gleeson 1996 reported there were no statistically significant between‐group differences in the scores at both two weeks (mean scores 5.0 (Tubigrip) versus 6.3 (cast)) and four weeks (mean scores 3.4 versus 3.8).
Gleeson 1996 did not report on longer‐term outcome, unacceptable anatomy or on adverse effects.
Secondary outcomes
At the follow‐up assessment, children were questioned as to when they had been able to resume normal activities. Gleeson 1996 found a significant difference in favour of the Tubigrip group for all 45 children followed up (mean 14.17 days for Tubigrip versus 20.19 days for cast; MD ‐6.02 days favouring Tubigrip, 95% CI ‐8.92 to ‐3.12 days; see Analysis 2.1). A similar result was found for the 19 children with ultrasound‐confirmed fractures (mean 14.22 days versus 21.6 days; MD ‐7.38 days, 95% CI ‐11.59 to ‐3.17 days; see Analysis 2.1).
2.1. Analysis.
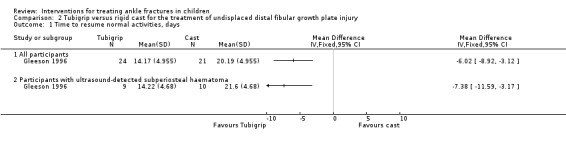
Comparison 2 Tubigrip versus rigid cast for the treatment of undisplaced distal fibular growth plate injury, Outcome 1 Time to resume normal activities, days.
Gleeson 1996 did not report on patient parent satisfaction, pain or resource use.
Subgroup analyses
A lack of data prevented us from performing subgroup analyses as initially planned.
Discussion
Summary of main results
Our search resulted in the inclusion of three randomised controlled trials (RCTs), reporting results for 189 children with low‐risk ankle fractures. Two trials compared the use of the Aircast Air‐Stirrup ankle brace against rigid casts, either a posterior splint, in Barnett 2012, or a walking cast, in Boutis 2007. One trial compared the Tubigrip bandage versus a plaster of Paris cast (Gleeson 1996). We have summarised the results of these two comparisons below.
Aircast Air‐Stirrup ankle brace versus rigid cast
We have summarised the results of this comparison in Table 1. We did not pool data from the two trials, mainly because the results from Barnett 2012 were presented as non‐parametric data (that is medians and interquartile ranges). There was low‐quality evidence of higher, and thus better, ASKp (used in Barnett 2012) or modified ASKp (used in Boutis 2007) scores in the brace groups of both trials at four weeks. The best‐estimate values for between‐group differences in both trials exceeded the minimally important difference estimates based on a clinically relevant change provided by the individual trials. Neither trial reported on unacceptable anatomy or related outcomes, although this decision is in keeping with these being "low risk" ankle fractures. There was very low‐quality evidence relating to adverse events due to brace and cast use, none of which were serious. The trial comparing similar durations of use of brace and posterior splint, both of which were removable for washing, found twice as many children with pressure‐related complications in the brace group. In contrast, the trial comparing brace with optional disuse after five days versus a walking cast for three weeks, found four times as many children in the cast group had adverse outcomes assessed in terms of an unscheduled visit to a healthcare provider. Both trials linked some of the adverse events in the brace group with the failure to wear a protective sock. There was very low‐quality evidence indicating an earlier return to pre‐injury activity in the brace groups in both trials. As with adverse outcomes, there were contrasting findings between the two trials in patient dissatisfaction with their allocated intervention. The trial comparing similar durations of use of brace and a removable posterior splint found similar high percentages of children who would select the same intervention again. However, the trial comparing brace with optional disuse after five days versus a walking cast for three weeks found far fewer children in the brace group would have preferred the other intervention. There was moderate‐quality evidence showing no difference between the two groups in pain at four weeks. Not included in Table 1 are the cost data results, which showed lower direct (healthcare) costs for the brace group.
Tubigrip versus plaster of Paris walking cast
One trial comparing the Tubigrip bandage plus crutches and advice versus a plaster of Paris walking cast for two weeks reported results at four weeks' follow‐up for 45 children with an inversion injury of the ankle, of whom 19 had an ultrasound finding suggestive of undisplaced distal fibular growth plate injury (Gleeson 1996). The trial failed to report on adverse events or longer‐term outcome. The trial provided very low‐quality evidence of little difference in pain and function between the two groups, measured using a composite and non‐validated pain and function score at four weeks. There was very low‐quality evidence of an earlier return to normal activities, averaging six days, in children treated with Tubigrip (mean 14.17 days for Tubigrip versus 20.19 days for cast; MD ‐6.02 days, 95% CI ‐8.92 to ‐3.12 days). A similar finding applied to the subgroup of 19 children with ultrasound‐diagnosed fractures.
Overall completeness and applicability of evidence
At the outset, this review sought to address four general areas in the treatment of ankle fractures in children: comparisons of different methods of non‐surgical management; surgical versus non‐surgical management; different surgical interventions; and different types of postsurgical immobilisation. However, our search found only three small trials, all of which focused on non‐surgical management of "low risk" ankle fractures (Barnett 2012; Boutis 2007; Gleeson 1996). We identified no ongoing trials, and no trials await assessment.
The three trials presented results for a total of 189 children. We undertook no pooling of outcome data, usually due to the reporting of medians rather than means by Barnett 2012, and the main follow‐up was four weeks. Although Boutis 2007 reported telephone follow‐up at three months, this was incompletely reported, and overall there was a lack of longer‐term follow‐up to confirm the expectation that the low‐risk ankle fractures were at low risk of complications relating to growth plate damage.
The three trials included injuries at the lower end of the fracture spectrum, the clinical diagnosis of the majority of fractures being undisplaced Salter‐Harris type I fractures of the distal fibula with normal radiographs. Barnett 2012 suggested in their discussion that it was likely from the results of a magnetic resonance imaging (MRI) study that the majority of the clinically diagnosed Salter‐Harris type I fractures were sprains. These 'fractures' formed 83% (33 of 40) of the fracture population. The majority of fractures in Boutis 2007 (72%) were clinically diagnosed Salter‐Harris type I fractures, however this trial used more extensive criteria for diagnosing these "rule out Salter‐Harris I" that may have reduced the number of sprain‐only injuries. Furthermore, two children with sprains only were excluded after randomisation. In Gleeson 1996, ultrasound examination at three days of 35 children revealed that only 19 had signs that were indicative of a growth‐plate injury. If, in agreement with Barnett 2012, we suppose that sprains would fare better treated by braces, then it is noteworthy that ASKp results for the Salter‐Harris type I 'fractures' showed less difference between the two intervention groups than for the whole population in Barnett 2012 (see Analysis 1.1). There was a similar lack of difference between the results of the whole trial population and the 19 children with ultrasound‐diagnosed fractures in Gleeson 1996. However, the available data are insufficient for these to be more than observations and for any statistical analysis. Further discussion on the diagnosis and characteristics of ankle inversion injuries is provided in the context of more recent evidence in Agreements and disagreements with other studies or reviews.
Quality of the evidence
All three trials were at high risk of bias relating to the impracticality of blinding children and treating clinicians to the allocated interventions. Two trials reported blinding for clinician‐rated outcome. Appropriate random sequence generation was described in two trials (Barnett 2012; Boutis 2007), and secure allocation concealment in one trial (Boutis 2007). The risks of attrition and selective reporting biases were unclear in two trials (Barnett 2012; Boutis 2007), but high in Gleeson 1996.
We have summarised the results of the GRADE assessment of the quality of evidence for the two comparisons below.
Aircast Air‐Stirrup ankle brace versus rigid cast
We have summarised the quality of evidence for each outcome in Table 1. We downgraded the evidence for all outcomes usually one level for study limitations, reflecting the high risk of performance and detection biases. We also downgraded the quality of the evidence for various outcomes for imprecision, reflecting that the data were always from the single trials, inconsistency and indirectness. We downgraded for inconsistency, even though we did not pool data. The clear heterogeneity in the finding for adverse events and patient satisfaction between the two trials is very likely to reflect differences in their two comparisons, including with regard to duration of use of the devices. We downgraded for indirectness because of inadequate definition or timing of outcome measurement or because of the high proportion of children without ankle fractures in the study population.
Tubigrip versus plaster of Paris walking cast
The quality of evidence assessments for both reported outcomes was very low. We downgraded the evidence two levels for study limitations (lack of blinding, incomplete outcome data, selective outcome reporting), reflecting a serious risk of bias, and one level for imprecision (single small trial). The quality of the evidence was impaired by indirectness, reflecting the non‐validated outcome measures and mixed population of ankle injuries, less than half of which were diagnosed using ultrasound as "low risk" ankle fractures.
Potential biases in the review process
While our search was comprehensive, it is likely that we have failed to identify some randomised trials, particularly those reported only in abstracts or in non‐English language publications.
The decision to consider under the same general comparison (brace versus cast), two trials that tested markedly different applications of the brace, mainly in terms of duration of use and cast (removable backslab for two weeks versus walking cast for three weeks) can be questioned in terms of interpretation. Differences in the results of Barnett 2012 and Boutis 2007 in terms of patient satisfaction with the device and adverse outcomes are likely to be related to the differences between the two comparisons. While this resulted in a downgrading of the quality of the evidence due to inconsistency, it is unlikely to be a source of bias.
A potential, but unavoidable, bias resides in our inclusion of trials that recruited children with clinically suspected "low risk" ankle fractures that had normal radiological findings. The recruitment strategies and inclusion criteria of all three included trials indicated awareness of a potential misdiagnosis, with Gleeson 1996 finding that under half of those children followed up had ultrasound confirmation of a growth‐plate injury. Where possible, we performed sensitivity analyses to explore the results of different injury populations.
Agreements and disagreements with other studies or reviews
All three included trials involved children with "low risk" clinically diagnosed fractures of the distal fibula, the majority of which were defined as Salter‐Harris type I fractures (injuries with tenderness and swelling over the distal fibula that do not show fractures on plain X‐ray films). Given the findings of two recently reported MRI studies that aimed to determine the frequency of Salter‐Harris type I fractures of the distal fibula, it is questionable how many of the injuries included in these trials were actually fractures (Boutis 2016;Hofsli 2016). Of the 135 children, aged between 5 and 12 years old, with clinically diagnosed Salter‐Harris type I fractures of the distal fibula in Boutis 2016, just four had MRI‐confirmed Salter‐Harris type I fractures, only two of which had injury along the whole length of the growth plate. None of the 31 children, aged 5 to 15 years, in Hofsli 2016 had MRI‐proven Salter‐Harris type I fractures of the distal fibular.
The majority (108 children) of injuries in Boutis 2016 were diagnosed as ligament injuries, 38 of which were associated with radiologically undetected avulsion fractures of the fibula. Bone bruises (contusions) were evident in 107 cases, of which this was the only diagnosis in 27 cases. Hofsli 2016 reported that 26% (8 children) had a ligamentous injury, bone contusion or both, and 74% had subcutaneous oedema around the lateral malleolus. These new developments in the diagnosis of low‐risk ankle injuries in children demonstrate the importance of using newer technologies to investigate and test long‐held maxims, in this case that non‐displaced ankle injuries with localised tenderness and swelling over the distal fibular physis automatically represent a Salter‐Harris type I injury to that physis. However, this does not undermine the importance of the findings of the trials performed in this area, which still represent the clinical diagnosis of a "low risk" ankle injury, even if not a fracture.
Authors' conclusions
Implications for practice.
There was low‐quality evidence of a quicker recovery of self reported function at four weeks in children with clinically diagnosed low‐risk ankle fractures who were treated with an ankle brace compared with those treated with a rigid cast, especially a non‐removable walking cast. There was very low‐quality evidence of an earlier return to former activities in children with clinically diagnosed low‐risk ankle fractures treated with the Tubigrip bandage plus crutches and advice compared with those treated with a plaster of Paris walking cast for two weeks. There was otherwise no evidence from RCTs to inform clinical practice for children with ankle fractures. Recent MRI research shows that many of the presumed Salter‐Harris type I fractures of the distal fibula are in fact ligamentous injury, subcutaneous oedema and bony contusions. However, the existing evidence from RCTs and clinical experience showing that the patients heal without deformity suggests that accurate diagnosis of this group of low‐risk ankle injuries may be academic.
Implications for research.
Further well‐designed and well‐reported, large‐scale RCTs are required to examine the longer‐term clinical effectiveness and cost‐effectiveness of the various non‐surgical immobilisation devices, the optimal duration of immobilisation for each type of intervention and fracture type, the comparison between above‐ and below‐knee immobilisation, the indications for surgical versus non‐surgical management, and what are the safest and most effective surgical interventions for particular fracture patterns.
The selection of priority areas for research should consider current evidence, current practice and variations in practice, and should involve consultation with patients and their families regarding their preferences and values. Multicentre trials, with long‐term follow‐up, that reflect professional consensus on treatment uncertainties should enable sufficient recruitment and implementation of treatments. Identifying priority topics for research requires input from others, but we suggest the following priority topics for research, as they may impact on economic, treatment or clinical outcome.
The validation and assessment of accuracy of triage tools to help determine which radiographic imaging modality to use in paediatric patients. Although this in itself is not an 'intervention', it is a part of the diagnosis of an ankle injury and it is important that accurate diagnosis is balanced against radiation exposure and financial expenditure. Such studies should be powered for both preschool, school‐aged and adolescent children.
Internal fixation versus external splintage. This comparison should be performed with a focus on adolescent children.
Surgical versus non‐surgical treatment in children with small intra‐articular cartilage fracture steps. This comparison should be performed for children from preschool through to adolescence.
Acknowledgements
The review authors extend their sincere thanks to Helen Handoll, Michael Rieder and Lisa Winer for valuable feedback and comments about drafts of the review. We are also grateful to Lindsey Elstub, Laura MacDonald and especially Joanne Elliott of the Cochrane Bone, Joint and Muscle Trauma Group for their guidance and support throughout this process. We acknowledge Lisa Schell with gratitude for her translation of two articles from German to English.
We are grateful to Helen Handoll, Colin Bruce, Laura MacDonald, Gillian Gummer and Elizabeth Royle for their feedback on the protocol. We also thank the UK Cochrane Centre for their systematic review courses, particularly Sheena Derry and Anne‐Marie Bagnall.
This project was supported by the National Institute for Health Research via Cochrane Infrastructure funding to the Cochrane Bone, Joint and Muscle Trauma Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Search strategies
CENTRAL (Wiley Online Library)
#1 [mh Ankle] or [mh "Ankle Joint"] or [mh "Ankle Injuries"] (1227) #2 (ankle or malleol* or unimalleo*or bimalleo* or trimalleo* or talus or talar or triplan* or tillaux or transitional or (distal near/2 (tibia* or fibula*))):ti,ab,kw (Word variations have been searched) (5610) #3 #1 or #2 (5610) #4 [mh "Fractures, Bone"] or [mh "Fracture Fixation"] or [mh "Fracture Healing"] (4425) #5 fracture*:ti,ab,kw (Word variations have been searched) (11288) #6 #4 or #5 (11305) #7 #3 and #6 (512) #8 ((physis or physeal or growth plate or salter harris) near/3 (injur* or fracture*) near/3 (ankle or tibia*)):ti,ab,kw (Word variations have been searched) (1 #9 #7 or #8 (512) #10 [mh Pediatrics] (564) #11 [mh Child] or [mh Infant] or ([mh Adolescent] not [mh Adult]) (89826) #12 neonate* or newborn or baby or babies or infant* or child* or teenage* or teen* or adolescen* or schoolchild* or school age or preschool* or toddler* or boy* or girl* or minors or pubert* or pubescen* or prepubescent* or p?ediatric* or youth*:ti,ab,kw (Word variations have been searched) (180200) #13 #10 or #11 or #12 (180283) #14 #9 and #13 (116)
MEDLINE (Ovid Online)
1 Ankle/ or Ankle Joint/ or Ankle Injuries/ (23564) 2 (ankle or malleol* or unimalleo*or bimalleo* or trimalleo* or talus or talar or triplan* or tillaux or transitional or (distal adj2 (tibia* or fibula*))).tw. (72074) 3 1 or 2 (78034) 4 exp Fractures, Bone/ or exp Fracture Fixation/ or Fracture Healing/ (155625) 5 fracture*.tw. (187375) 6 4 or 5 (232902) 7 3 and 6 (9347) 8 ((physis or physeal or growth plate or salter harris) adj3 (injur* or fracture*) adj3 (ankle or tibia*)).tw. (82) 9 7 or 8 (9384) 10 exp Pediatrics/ (46861) 11 exp Child/ or exp Infant/ or (Adolescent/ not Adult/) (2320762) 12 (neonate* or newborn or baby or babies or infant* or child* or teenage* or teen* or adolescen* or schoolchild* or school age or preschool* or toddler* or boy* or girl* or minors or pubert* or pubescen* or prepubescent* or p?ediatric* or youth*).tw. (1761745) 13 10 or 11 or 12 (2822715) 14 9 and 13 (1620) 15 Randomized controlled trial.pt. (411031) 16 Controlled clinical trial.pt. (91634) 17 randomized.ab. (334739) 18 placebo.ab. (168590) 19 Drug therapy.fs. (1834137) 20 randomly.ab. (241010) 21 trial.ab. (349377) 22 groups.ab. (1502432) 23 or/15‐22 (3660330) 24 exp Animals/ not Humans/ (4113127) 25 23 not 24 (3150132) 26 14 and 25 (128)
EMBASE (Ovid Online)
1 Ankle Fracture/ or Distal Tibia Fracture/ (3312) 2 (ankle or malleol* or unimalleo*or bimalleo* or trimalleo* or talus or talar or triplan* or tillaux or transitional or (distal adj2 (tibia* or fibula*))).tw. (88452) 3 exp Fractures, Bone/ or exp Fracture Fixation/ or Fracture Healing/ (233125) 4 fracture*.tw. (216905) 5 3 or 4 (293260) 6 2 and 5 (11163) 7 1 or 6 (11939) 8 ((physis or physeal or growth plate or salter harris) adj3 (injur* or fracture*) adj3 (ankle or tibia*)).tw. (94) 9 7 or 8 (11976) 10 Pediatrics/ (59815) 11 exp Child/ or exp Newborn/ or (exp Adolescent/ not Adult/) (2351728) 12 (neonate* or newborn or baby or babies or infant* or child* or teenage* or teen* or adolescen* or schoolchild* or school age or preschool* or toddler* or boy* or girl* or minors or pubert* or pubescen* or prepubescent* or p?ediatric* or youth*).tw. (2064951) 13 10 or 11 or 12 (2995369) 14 9 and 13 (1767) 15 Randomized controlled trial/ (383450) 16 Clinical trial/ (850503) 17 Controlled clinical trial/ (392609) 18 Randomization/ (67939) 19 Single blind procedure/ (20967) 20 Double blind procedure/ (123428) 21 Crossover procedure/ (44434) 22 Placebo/ (262958) 23 Prospective study/ (306809) 24 ((clinical or controlled or comparative or placebo or prospective* or randomi#ed) adj3 (trial or study)).tw. (855441) 25 (random* adj7 (allocat* or allot* or assign* or basis* or divid* or order*)).tw. (212383) 26 ((singl* or doubl* or trebl* or tripl*) adj7 (blind* or mask*)).tw. (176132) 27 (cross?over* or (cross adj1 over*)).tw. (76058) 28 ((allocat* or allot* or assign* or divid*) adj3 (condition* or experiment* or intervention* or treatment* or therap* or control* or group*)).tw. (281875) 29 RCT.tw. (18225) 30 or/15‐29 (2095333) 31 Case Study/ or Abstract Report/ or Letter/ (970997) 32 30 not 31 (2054643) 33 14 and 32 (167)
CINAHL (Ebsco)
S1 (MH "Ankle Fractures") (775) S2 TX (ankle or malleol* or unimalleo*or bimalleo* or trimalleo* or talus or talar or triplan* or tillaux or transitional or (distal n2 (tibia* or fibula*))) (28,141) S3 (MH "Fractures+") or (MH "Fracture Fixation") or (MH "Fracture Healing") (38,085) S4 TX fracture* (49,469) S5 S3 OR S4 (49,659) S6 S2 AND S5 (3,545) S7 S1 OR S6 (3,545) S8 PT Clinical Trial (78,685) S9 (MH "Clinical Trials+") (192,364) S10 TI clinical trial* OR AB clinical trial* (47,305) S11 TI ( (single blind* or double blind*) ) OR AB ( (single blind* or double blind*) ) (22,162) S12 TI random* OR AB random* (155,402) S13 S8 OR S9 OR S10 OR S11 OR S12 (286,765) S14 S7 AND S13 (202)
Current Controlled Trials
Basic search
ankle* AND fracture* = 38
WHO ICTRP
Basic search
ankle* AND fracture* AND child* = 6
Advanced search
Title: ankle* AND fracture* in Title (check box ‘Search for clinical trials in children’, Recruitment Status: All) = 8
Data and analyses
Comparison 1. Ankle brace versus rigid cast for the treatment of 'low risk' ankle fractures.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Activities Scale for Kids‐performance (ASKp): 0 to 100: best outcome | Other data | No numeric data | ||
| 2 Modified Activities Scale for Kids‐performance score (0 to 100%: best outcome) at 4 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Number of children experiencing adverse outcomes | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Ankle brace versus fibreglass posterior splint | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Ankle brace versus below‐knee walking cast | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Time to resume pre‐injury level of activity (days) | Other data | No numeric data | ||
| 5 Return to pre‐injury levels of activity at 4 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Able to weight bear with no pain at 4 weeks post‐injury | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7 Child would have preferred other intervention | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8 Patient satisfaction with device: 'not at all happy', 'a little happy', 'happy', or 'very happy' | Other data | No numeric data | ||
| 9 Duration of analgesia use (paracetamol or ibuprofen), (days) | Other data | No numeric data | ||
| 10 Faces Pain Scale score at 4 weeks post‐injury (0 to 10: greatest pain) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11 Estimated total healthcare cost, in Canadian dollars, in 2003 to 2005 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
1.6. Analysis.
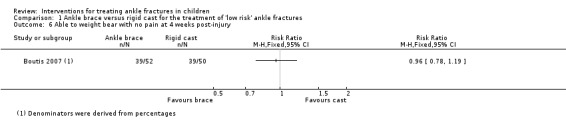
Comparison 1 Ankle brace versus rigid cast for the treatment of 'low risk' ankle fractures, Outcome 6 Able to weight bear with no pain at 4 weeks post‐injury.
Comparison 2. Tubigrip versus rigid cast for the treatment of undisplaced distal fibular growth plate injury.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time to resume normal activities, days | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 All participants | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Participants with ultrasound‐detected subperiosteal haematoma | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Barnett 2012.
| Methods | Randomised controlled study (parallel) | |
| Participants | 45 children Setting and recruitment period: Royal Children's Hospital (Melbourne, Australia); August 2007 to March 2009 Inclusion criteria: aged 5 to 15 years, inclusive, who attended with a "low‐risk ankle fracture" (avulsion fracture of distal fibula, undisplaced Salter‐Harris I fracture, and type 2 fracture of fibula or avulsion fracture of the lateral talus) Exclusion criteria:
Gender: 25 females; 20 males Age: mean 10.7 years Fracture type: "A low‐risk ankle fracture was defined as (1) an avulsion fracture of the distal fibula, (2) an undisplaced SHI (defined as isolated tenderness over fibula growth plate and normal x‐radiography) and type II fracture of the fibula, or (3) an avulsion fracture of the lateral talus." SHI stands for Salter‐Harris type I. The majority of fractures (33 of 45) were Salter‐Harris type I fractures |
|
| Interventions |
Children were allowed to mobilise using their devices and weight bear as tolerated. Both brace and splint could be removed for bathing. Both brace and splint were removed after 12 to 16 days. Allocation: 22 (brace); 23 (backslab) Analysed: 20 (brace); 20 (backslab) |
|
| Outcomes | Follow‐up schedule: At 12 to 16 days post‐injury, the children attended clinic, removed their device, had their ankle radiographed, and were seen by the blinded research physiotherapist, who gave them a second diary. The device may have been removed at this point if clinically appropriate. A second follow‐up appointment was conducted at 4 weeks, at which point the child was assessed, a second ASKp was recorded, and the second diary collected (no second radiograph). Children were given a diary to record the degree of pain and analgesia used and ease of care of the device. Primary outcome: Change in mean functional activity as measured by the ASKp. Secondary outcomes: Physiotherapy assessment at 2 and 4 weeks, degree of pain during the first 2 weeks, amount of analgesia used in the first 2 weeks, ease of caring for the device |
|
| Notes | Although separate ASKp data were provided for age subgroups (5 to 10 years and 11 to 15 years), the numbers in each group were not reported, but would in any case have been too small for meaningful subgroup analysis. Requests for raw ASKp data were sent but required ethics committee approval for the author to send these to us (personal communication from Peter Barnett on 29 September 2014) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation stratified by age group (5 to 10 and 11 to 15 years). Random block sizes of 2, 4 and 6 to generate a randomisation schedule. |
| Allocation concealment (selection bias) | Unclear risk | Study report states that investigators were blinded to the block sizes to ensure allocation concealment, but it was not explained how this was ensured |
| Blinding of participants and personnel (performance bias) Patient‐rated outcomes | High risk | Blinding children to the allocated intervention was not feasible |
| Blinding of participants and personnel (performance bias) Clinician‐rated outcomes | High risk | Personnel applying the intervention were not blinded |
| Blinding of outcome assessment (detection bias) Patient‐rated outcomes | High risk | Patient‐reported outcomes would be at high risk of bias due to the lack of blinding |
| Blinding of outcome assessment (detection bias) Clinician‐rated outcomes | Low risk | Children removed ankle brace or splint before assessment; this was ensured by the research assistants. Assessors providing physician‐reported outcomes were blinded |
| Incomplete outcome data (attrition bias) Patient‐rated outcomes | Unclear risk | Participant flow provided: the numbers not included in the analyses (2 versus 3) were comparable in the 2 groups. However, no data were provided regarding the rates of diary completion by children |
| Incomplete outcome data (attrition bias) Clinician‐rated outcomes | Unclear risk | Participant flow provided: the numbers not included in the analyses (2 versus 3) were comparable in the 2 groups |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available. Data for daily pain scores, which was a key (secondary) outcome, were not fully presented. Duration of analgesia use rather than amount of analgesia used in the first 2 weeks not provided (key, secondary outcome) |
| Other bias | Unclear risk | Not enough study participants to satisfy power calculation criteria |
Boutis 2007.
| Methods | Randomised controlled trial (parallel) | |
| Participants | 111 children Setting and recruitment period: emergency department of the Hospital for Sick Children (Toronto, Canada) between July 2003 and September 2005 Inclusion criteria: aged 5 to 18 years with acute, symptomatic low‐risk ankle fractures within 72 hours of the injury (undisplaced Salter‐Harris I and II fractures, avulsion fractures of distal fibula or fibular epiphysis) Exclusion criteria:
Gender ratio (F:M): not stated Age: mean 10.3 years Fracture type: Isolated low‐risk fractures of the ankle including undisplaced distal fibular types I and II Salter‐Harris fractures and avulsion fractures of the distal fibula or fibular epiphysis. "Because undisplaced Salter‐Harris type I fractures are not evident on radiographs and the accepted standard for diagnosis of this fracture is based on clinical findings, a presumptive diagnosis of this fracture was made using the following predefined criteria: age equal to or less than 12 years, inability to bear weight, an examination consistent with maximal tenderness and swelling over the distal fibular growth plate, and a radiograph demonstrating the absence of bony fracture with evidence of soft tissue swelling over the open distal fibular growth plate." The majority of fractures (75 of 104) were "rule out" Salter‐Harris type I fractures |
|
| Interventions |
Children were given the allocated device upon randomisation and instructed to use a sock and shoe in conjunction with the device. All children were given crutches and instructed not to weight bear for 5 days, followed by weight‐bearing as tolerated. They were given a diary to record expense, amount of analgesia, weekly pain scores and weekly return to baseline activities. Patients were telephoned weekly to address concerns and encourage completion of the diaries. Allocation: 57 (brace); 54 (cast) Analysed: 54 (brace); 50 (cast) |
|
| Outcomes | Follow‐up schedule: After 4 weeks, a blinded research physiotherapist attended the children's homes to complete the 4‐week assessments and collect the diaries. A 3‐month telephone call was made to assess subsequent complications. Primary outcomes: Modified ASKp scores at 4 weeks Secondary outcomes: Range‐of‐motion measurements, pain with walking using a Bieri Faces Pain Scale (revised), return to baseline activities, and patient preference for one immobilization device over the other |
|
| Notes | The study authors were successfully contacted regarding missing range‐of‐motion outcome data, which confirmed their claim of no significant differences between treatment groups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Online randomisation program using block randomisation with random block sizes of 6 and 8, with immediate email notification of treatment group to the research co‐ordinator |
| Allocation concealment (selection bias) | Low risk | Immediate email notification of treatment group to the research co‐ordinator was used to conceal the allocation |
| Blinding of participants and personnel (performance bias) Patient‐rated outcomes | High risk | Blinding of children and personnel to the allocated intervention was not feasible |
| Blinding of participants and personnel (performance bias) Clinician‐rated outcomes | High risk | Blinding of personnel to the allocated intervention was not feasible |
| Blinding of outcome assessment (detection bias) Patient‐rated outcomes | High risk | Patient‐reported outcomes were subject to bias because children were not blinded to the therapy received |
| Blinding of outcome assessment (detection bias) Clinician‐rated outcomes | Low risk | Children were provided with an opaque stocking to cover device before assessor's visit. Outcome‐assessing physiotherapist was blinded to the allocated therapy at the 4‐week assessment |
| Incomplete outcome data (attrition bias) Patient‐rated outcomes | Unclear risk | Participant flow provided: the numbers not included in the analyses (3 versus 4) were comparable in the 2 groups |
| Incomplete outcome data (attrition bias) Clinician‐rated outcomes | Unclear risk | Participant flow provided: the numbers not included in the analyses (3 versus 4) were comparable in the 2 groups. Analyses were by intention to treat, but 7 children were excluded from the final analysis: 5 children who were initially randomised were later found to have been misdiagnosed (foot fracture; proximal fibular fracture; tibia fracture; 2 sprains), 1 was lost to follow‐up, and 1 dropped out. Intention‐to‐treat analysis: 4 children in the cast group had their casts removed at 2 weeks because of a premature visit; 2 of these were placed into a brace. All 4 retained in the cast group for the analyses. |
| Selective reporting (reporting bias) | Unclear risk | Trial registration was retrospective. The description of adverse events was incomplete. Full data regarding goniometer‐measured range of movement (secondary outcome) were not presented in the report, however tabulated data received from the lead trialists confirmed the stated no significant differences between the 2 groups |
| Other bias | Unclear risk | The Air‐Stirrup ankle braces were provided free of charge by Aircast for the purposes of this study |
Gleeson 1996.
| Methods | Randomised controlled trial (parallel) | |
| Participants | 51 children Setting and recruitment period: Booth Hall Children's Hospital between February and May 1994 Inclusion criteria: Children with swelling after an inversion injury to the ankle and tenderness over lateral malleolus, normal ankle radiograph with no evidence of growth‐plate fusion, and who were unable to, or who could only partially, weight bear Exclusion criteria:
Gender (of 45): 26 females, 19 males Age (of 45): mean 9.2 years, range 3 to 14 years Fracture type: An ultrasound of the ankle was performed in 40 children within 72 hours of presentation to determine injury to the growth plate. Various anomalities were reported: soft‐tissue swelling, subperiosteal haematoma, swelling of peroneus longus, venous congestion, joint effusion, metaphyseal irregularity, which suggested undisplaced Salter‐Harris I growth‐plate injuries. Of the 34 children with ultrasound results at follow‐up, 19 had "definite evidence of growth‐plate injury" (subperiosteal haematoma) |
|
| Interventions |
Allocation: 51 in all Analysed: 24 (Tubigrip); 21 (cast) |
|
| Outcomes | Follow‐up schedule: After 2 weeks and 4 weeks Primary outcome: Time to return to normal activities Secondary outcome: Assessment score (3 to 17; higher scores = worse outcome) documenting pain (visual analogue score 0 to 5), swelling (1 to 4), tenderness over lateral malleolus (1 to 4) and degree of weight‐bearing (1 to 4) |
|
| Notes | The inclusion criteria corresponded to an assessment score of ≥ 10 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Authors stated "After initial assessment were randomly allocated ..." but gave no further details regarding method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Authors stated "After initial assessment were randomly allocated ..." but gave no further details regarding method of randomisation or measures to ensure allocation concealment |
| Blinding of participants and personnel (performance bias) Patient‐rated outcomes | High risk | Blinding of children and personnel to the allocated intervention was not feasible |
| Blinding of participants and personnel (performance bias) Clinician‐rated outcomes | High risk | Blinding of children and personnel to the allocated intervention was not feasible |
| Blinding of outcome assessment (detection bias) Patient‐rated outcomes | High risk | No blinding of children was noted in the study, thus patient‐reported outcomes are at high risk of bias |
| Blinding of outcome assessment (detection bias) Clinician‐rated outcomes | High risk | No blinding of the assessors was noted in the study, thus personnel‐reported outcomes are at high risk of bias |
| Incomplete outcome data (attrition bias) Patient‐rated outcomes | High risk | Group allocation not provided for 6 children lost to follow‐up (12% of 51). Of the 51 children initially recruited, 6 were lost to follow‐up. Of the remaining 45 who completed the study, 34 had ultrasound scans. The incomplete data may be a source of bias |
| Incomplete outcome data (attrition bias) Clinician‐rated outcomes | High risk | Group allocation not provided for 6 children lost to follow‐up (12% of 51). Of the 51 children initially recruited, 6 were lost to follow‐up. Of the remaining 45 who completed the study, 34 had ultrasound scans. The incomplete data may be a source of bias |
| Selective reporting (reporting bias) | High risk | No protocol available. It seems likely that the analyses relating to ultrasound findings were post‐hoc. Incomplete description of outcome measurement in methods. No report of adverse effects |
| Other bias | Unclear risk | No sample size calculation provided |
ASKp: Activities Scale for Kids‐performance
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ahl 1989 | Trial excluded children |
| Avci 1998 | Trial excluded children |
| Bauer 1985 | Trial excluded children |
| Dijkema 1993 | Trial excluded children |
| Egol 2000 | Trial excluded children |
| Eventov 1978 | Not a randomised trial; study excluded children |
| Gorodetskyi 2010 | Trial excluded children |
| Handolin 2005a | Trial excluded children |
| Handolin 2005b | Trial excluded children |
| Hedström 1994 | Trial excluded children |
| Hoelsbrekken 2013 | Trial excluded children |
| Honigmann 2007 | Trial excluded children |
| Høiness 2004 | Trial excluded children |
| Joukainen 2007 | Trial excluded children |
| Kaukonen 2005 | Trial excluded children |
| Kimmel 2012 | Trial excluded children |
| Konrad 2005 | Trial excluded children |
| Lehtonen 2003 | Trial excluded children |
| Lin 2008 | Trial excluded children |
| Mayich 2013 | Trial excluded children |
| Moore 2006 | Trial excluded children |
| Moseley 2005 | Trial excluded children |
| Noh 2012 | Trial excluded children |
| Pakarinen 2011 | Trial excluded children |
| Phillips 1985 | Trial excluded children |
| Rowley 1986 | Trial excluded children |
| Sanders 2012 | Trial excluded children |
| Sun 2014 | Trial excluded children |
| Søndenaa 1986 | Trial excluded children |
| Thordarson 2001a | Trial excluded children |
| Thordarson 2001b | Trial excluded children |
| Tsukada 2013 | Trial excluded children |
| Van Laarhoven 1996 | Trial excluded children |
| Vioreanu 2007 | Trial excluded children |
| White 2008 | Trial excluded children |
| Wikerøy 2010 | Trial excluded children |
Differences between protocol and review
Types of participants
We clarified that we would include all participants of trials including those with ankle injuries for which there was a clinical suspicion of a fracture but with normal X‐rays (radiographs) or no radiological confirmation. In such trials, we sought separate data for those children for whom the diagnosis was confirmed or strengthened using MRI or another imaging modality.
Contributions of authors
Denise Yeung and Xueli Jia initiated the review. Denise Yeung researched the background. Denise Yeung and Simon Barker drafted the protocol. Xueli Jia and Clare Miller provided feedback on drafts of the protocol. Denise Yeung, Clare Miller and Xueli Jia screened the potential studies. Denise Yeung and Clare Miller extracted data and independently assessed risk of bias in the included studies, which were agreed with Xueli Jia. Denise Yeung entered the study details into Review Manager, produced the figures and tables, and wrote the remainder of the review. All four authors read the review and provided feedback. Denise Yeung is the guarantor.
Declarations of interest
Denise E Yeung: none known Xueli Jia: none known Clare A Miller: none known Simon L Barker: none known
New
References
References to studies included in this review
Barnett 2012 {published data only}
- Barnett P. Data requiring ethics department clearance [personal communication]. Email to: D Yeung 29 September 2014.
- Barnett PL, Lee MH, Oh L, Cull G, Babl F. Functional outcome after air‐stirrup ankle brace or fiberglass backslab for pediatric low‐risk ankle fractures: a randomized observer‐blinded controlled trial. Pediatric Emergency Care 2012;28(8):745‐9. [DOI] [PubMed] [Google Scholar]
Boutis 2007 {published data only (unpublished sought but not used)}
- Boutis K. Clarification on participant flow [personal communication]. Email to: D Yeung 17 September 2014.
- Boutis K. Range of motion data [personal communication]. Email to: D Yeung 24 February 2016.
- Boutis K, Willan AR, Babyn P, Narayanan UG, Alman B, Schuh S. A randomized, controlled trial of a removable brace versus casting in children with low‐risk ankle fractures. Pediatrics 2007;119(6):e1256‐63. [DOI] [PubMed] [Google Scholar]
- NCT00132964. Brace versus casting in pediatric low risk ankle fractures. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=NCT00132964 (accessed 16 January 2015).
Gleeson 1996 {published data only}
- Gleeson AP, Stuart J, Wilson B, Phillips B. Ultrasound assessment and conservative management of inversion injuries of the ankle in children: plaster of Paris versus Tubigrip. Journal of Bone and Joint Surgery (British Volume) 1996;78‐B(3):484‐7. [PubMed] [Google Scholar]
References to studies excluded from this review
Ahl 1989 {published data only}
- Ahl T, Dalén N, Selvik G. Ankle fractures. A clinical and roentgenographic stereophotogrammetric study. Clinical Orthopaedics and Related Research 1989;245:246‐55. [PubMed] [Google Scholar]
Avci 1998 {published data only}
- Avci S, Sayli U. Comparison of the results of short‐term rigid and semi‐rigid cast immobilization for the treatment of grade 3 inversion injuries of the ankle. Injury 1998;29(8):581‐4. [DOI] [PubMed] [Google Scholar]
Bauer 1985 {published data only}
- Bauer M, Bergström B, Hemborg A, Sandegård J. Malleolar fractures: nonoperative versus operative treatment. A controlled study. Clinical Orthopeadics and Related Research 1985;199:17‐27. [PubMed] [Google Scholar]
Dijkema 1993 {published data only}
- Dijkema AR, Elst M, Breederveld RS, Verspui G, Patka P, Haarman HJ. Surgical treatment of fracture‐dislocations of the ankle joint with biodegradable implants: a prospective randomized study. Journal of Trauma and Acute Care Surgery 1993;34(1):82‐4. [DOI] [PubMed] [Google Scholar]
Egol 2000 {published data only}
- Egol KA, Dolan R, Koval KJ. Functional outcome of surgery for fractures of the ankle. A prospective, randomised comparison of management in a cast or a functional brace. Journal of Bone and Joint Surgery (British Volume) 2000;82(2):246‐9. [PubMed] [Google Scholar]
Eventov 1978 {published data only}
- Eventov I, Salama R, Goodwin DRA, Weissman SL. An evaluation of surgical and conservative treatment of fractures of the ankle in 200 patients. Journal of Trauma and Acute Care Surgery 1978;18(4):271‐74. [DOI] [PubMed] [Google Scholar]
Gorodetskyi 2010 {published data only}
- Gorodetskyi IG, Gorodnichenko AI, Tursin PS, Reshetnyak VK, Uskov ON. Use of noninvasive interactive neurostimulation to improve short‐term recovery in patients with surgically repaired bimalleolar ankle fractures: a prospective, randomized clinical trial. Journal of Foot and Ankle Surgery 2010;49(5):432‐7. [DOI] [PubMed] [Google Scholar]
Handolin 2005a {published data only}
- Handolin L, Kiljunen V, Arnala I, Kiuru MJ, Pajarinen J, Partio EK, et al. No long‐term effects of ultrasound therapy on bioabsorbable screw‐fixed lateral malleolar fracture. Scandinavian Journal of Surgery 2005;94(3):239‐42. [DOI] [PubMed] [Google Scholar]
Handolin 2005b {published data only}
- Handolin L, Kiljunen V, Arnala I, Kiuru MJ, Pajarinen J, Partio EK, et al. Effect of ultrasound therapy on bone healing of lateral malleolar fractures of the ankle joint fixed with bioabsorbable screws. Journal of Orthopaedic Science 2005;10(4):391‐5. [DOI] [PubMed] [Google Scholar]
Hedström 1994 {published data only}
- Hedström M, Ahl T, Dalén N. Early postoperative ankle exercise. A study of postoperative lateral malleolar fractures. Clinical Orthopaedics and Related Research 1994;300:193‐6. [PubMed] [Google Scholar]
Hoelsbrekken 2013 {published data only}
- Hoelsbrekken SE, Kaul‐Jensen K, Mørch T, Vika H, Clementsen T, Paulsrud Ø, et al. Nonoperative treatment of the medial malleolus in bimalleolar and trimalleolar ankle fractures: a randomized controlled trial. Journal of Orthopaedic Trauma 2013;27(11):633‐7. [DOI] [PubMed] [Google Scholar]
Honigmann 2007 {published data only}
- Honigmann P, Goldhahn S, Rosenkranz J, Audigé L, Geissmann D, Babst R. Aftertreatment of malleolar fractures following ORIF ‐ functional compared to protected functional in a vacuum‐stabilized orthesis: a randomized controlled trial. Archives of Orthopaedic and Trauma Surgery 2007;127(3):195‐203. [DOI] [PubMed] [Google Scholar]
Høiness 2004 {published data only}
- Høiness P, Strømsøe K. Tricortical versus quadricortical syndesmosis fixation in ankle fractures: a prospective, randomized study comparing two methods of syndesmosis fixation. Journal of Orthopaedic Trauma 2004;18(6):331‐7. [DOI] [PubMed] [Google Scholar]
Joukainen 2007 {published data only}
- Joukainen A, Partio EK, Waris P, Joukainen J, Kröger H, Törmälä P, et al. Bioabsorbable screw fixation for the treatment of ankle fractures. Journal of Orthopaedic Science 2007;12(1):28‐34. [DOI] [PubMed] [Google Scholar]
Kaukonen 2005 {published data only}
- Kaukonen JP, Lamberg T, Korkala O, Pajarinen J. Fixation of syndesmotic ruptures in 38 patients with a malleolar fracture: a randomized study comparing a metallic and a bioabsorbable screw. Journal of Orthopaedic Trauma 2005;19(6):392‐5. [DOI] [PubMed] [Google Scholar]
Kimmel 2012 {published data only}
- Kimmel LA, Edwards ER, Liew SM, Oldmeadow LB, Webb MJ, Holland AE. Rest easy? Is bed rest really necessary after surgical repair of an ankle fracture?. Injury 2012;43(6):766‐71. [DOI] [PubMed] [Google Scholar]
Konrad 2005 {published data only}
- Konrad G, Markmiller M, Lenich A, Mayr E, Rüter A. Tourniquets may increase postoperative swelling and pain after internal fixation of ankle fractures. Clinical Orthopaedics and Related Research 2005;433:189‐94. [DOI] [PubMed] [Google Scholar]
Lehtonen 2003 {published data only}
- Lehtonen H, Järvinen TL, Honkonen S, Nyman M, Vihtonen K, Järvinen M. Use of a cast compared with a functional ankle brace after operative treatment of an ankle fracture. A prospective, randomized study. Journal of Bone and Joint Surgery (American Volume) 2003;85(2):205‐11. [DOI] [PubMed] [Google Scholar]
Lin 2008 {published data only}
- Lin CW, Moseley AM, Haas M, Refshauge KM, Herbert RD. Manual therapy in addition to physiotherapy does not improve clinical or economic outcomes after ankle fracture. Journal of Rehabilitation Medicine 2008;40(6):433‐9. [DOI] [PubMed] [Google Scholar]
Mayich 2013 {published data only}
- Mayich DJ, Tieszer C, Lawendy A, McCormick W, Sanders D. Role of patient information handouts following operative treatment of ankle fractures: a prospective randomized study. Foot and Ankle International 2013;31(1):2‐7. [DOI] [PubMed] [Google Scholar]
Moore 2006 {published data only}
- Moore JA Jr, Shank JR, Morgan SJ, Smith WR. Syndesmosis fixation: a comparison of three and four cortices of screw fixation without hardware removal. Foot and Ankle International 2006;27(8):567‐72. [DOI] [PubMed] [Google Scholar]
Moseley 2005 {published data only}
- Moseley AM, Herbert RD, Nightingale EJ, Taylor DA, Evans TM, Robertson GJ, et al. Passive stretching does not enhance outcomes in patients with plantarflexion contracture after cast immobilization for ankle fracture: a randomized controlled trial. Archives of Physical Medicine and Rehabilitation 2005;86(6):1118‐26. [DOI] [PubMed] [Google Scholar]
Noh 2012 {published data only}
- Noh JH, Roh YH, Yang BG, Kim SW, Lee JS, Oh MK. Outcomes of operative treatment of unstable ankle fractures: a comparison of metallic and biodegradable implants. Journal of Bone and Joint Surgery (American Volume) 2012;94(22):e166. [DOI] [PubMed] [Google Scholar]
Pakarinen 2011 {published data only}
- Pakarinen HJ, Flinkkilä TE, Ohtonen PP, Hyvönen PH, Lakovaara MT, Leppilahti JI, et al. Syndesmotic fixation in supination‐external rotation ankle fractures: a prospective randomized study. Foot and Ankle International 2011;32(12):1103‐9. [DOI] [PubMed] [Google Scholar]
Phillips 1985 {published data only}
- Phillips WA, Schwartz HS, Keller CS, Woodward HR, Rudd WS, Spiegel PG, et al. A prospective, randomized study of the management of severe ankle fractures. Journal of Bone and Joint Surgery (American Volume) 1985;67(1):67‐78. [PubMed] [Google Scholar]
Rowley 1986 {published data only}
- Rowley DI, Norris SH, Duckworth T. A prospective trial comparing operative and manipulative treatment of ankle fractures. Journal of Bone and Joint Surgery (British Volume) 1986;68(4):610‐3. [DOI] [PubMed] [Google Scholar]
Sanders 2012 {published data only}
- Sanders DW, Tieszer C, Corbett B, Canadian Orthopaedic Trauma Society. Operative versus nonoperative treatment of unstable lateral malleolar fractures: a randomized multicenter trial. Journal of Orthopaedic Trauma 2012;26(3):129‐34. [DOI] [PubMed] [Google Scholar]
Sun 2014 {published data only}
- Sun H, Luo CF, Zhong B, Shi HP, Zhang CQ, Zeng BF. A prospective, randomised trial comparing the use of absorbable and metallic screws in the fixation of distal tibiofibular syndesmosis injuries: A mid‐term follow‐up. Bone and Joint Journal 2014;96:548‐54. [DOI] [PubMed] [Google Scholar]
Søndenaa 1986 {published data only}
- Søndenaa K, Høigaard U, Smith D, Alho A. Immobilization of operated ankle fractures. Acta Orthopaedica Scandinavica 1986;57(1):59‐61. [DOI] [PubMed] [Google Scholar]
Thordarson 2001a {published data only}
- Thordarson DB, Bains R, Shepherd LE. The role of ankle arthroscopy on the surgical management of ankle fractures. Foot and Ankle International 2001;22(2):123‐5. [DOI] [PubMed] [Google Scholar]
Thordarson 2001b {published data only}
- Thordarson DB, Samuelson M, Shepherd LE, Merkle PF, Lee J. Bioabsorbable versus stainless steel screw fixation of the syndesmosis in pronation‐lateral rotation ankle fractures: a prospective randomized trial. Foot and Ankle International 2001;22(4):335‐8. [DOI] [PubMed] [Google Scholar]
Tsukada 2013 {published data only}
- Tsukada S, Otsuji M, Shiozaki A, Yamamoto A, Komatsu S, Yoshimura H, et al. Locking versus non‐locking neutralization plates for treatment of lateral malleolar fractures: a randomized controlled trial. International Orthopaedics 2013;37:2451‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Laarhoven 1996 {published data only}
- Laarhoven CJ, Meeuwis JD, Werken C. Postoperative treatment of internally fixed ankle fractures: a prospective randomised study. Journal of Bone and Joint Surgery (British Volume) 1996;78(3):395‐9. [PubMed] [Google Scholar]
Vioreanu 2007 {published data only}
- Vioreanu M, Dudeney S, Hurson B, Kelly E, O'Rourke K, Quinlan W. Early mobilization in a removable cast compared with immobilization in a cast after operative treatment of ankle fractures: a prospective randomized study. Foot and Ankle International 2007;28(1):13‐9. [DOI] [PubMed] [Google Scholar]
White 2008 {published data only}
- White BJ, Walsh M, Egol KA, Tejwani NC. Intra‐articular block compared with conscious sedation for closed reduction of ankle fracture‐dislocations. A prospective randomized trial. Journal of Bone and Joint Surgery (American Volume) 2008;90(4):731‐4. [DOI] [PubMed] [Google Scholar]
Wikerøy 2010 {published data only}
- Wikerøy AK, Høiness PR, Andreassen GS, Hellund JC, Madsen JE. No difference in functional and radiographic results 8.4 years after quadricortical compared with tricortical syndesmosis fixation in ankle fractures. Journal of Orthopaedic Trauma 2010;24(1):17‐23. [DOI] [PubMed] [Google Scholar]
Additional references
Aiyenuro 2013
- Aiyenuro O, Goldberg AJ. Fractures of the foot and ankle. Surgery 2013;31(9):474‐81. [Google Scholar]
Barmada 2003
- Barmada A, Gaynor T, Mubarak SJ. Premature physeal closure following distal tibia physeal fractures: A new radiographic predictor. Journal of Pediatric Orthopaedics 2003;23(6):733‐9. [DOI] [PubMed] [Google Scholar]
Bible 2009
- Bible J, Smith B. Ankle fractures in children and adolescents. Techniques in Orthopaedics 2009;24(3):211‐9. [Google Scholar]
Blackburn 2012
- Blackburn EW, Aronsson DD, Rubright TJH, Lisle JW. Current concepts review: Ankle fractures in children. Journal of Bone and Joint Surgery (American Volume) 2012;94(13):1234‐44. [DOI] [PubMed] [Google Scholar]
Boutis 2016
- Boutis K, Plint A, Stimec J, Miller E, Babyn P, Schuh S, et al. Radiograph‐negative lateral ankle injuries in children: occult growth plate fracture or sprain?. JAMA Pediatrics 2016;170(1):e154114. [DOI] [PubMed] [Google Scholar]
Budiman‐Mak 1991
- Budiman‐Mak E, Conrad KJ, Roach KE. The foot function index: a measure of foot pain and disability. Journal of Clinical Epidemiology 1991;44(6):561‐70. [DOI] [PubMed] [Google Scholar]
Cottalorda 2008
- Cottalorda J, Beranger V, Louahem D, Camilleri JP, Launay F, Dimeglio A, et al. Salter‐Harris type III and IV medial malleolar fractures: growth arrest: is it a fate?: a retrospective study of 48 cases with open reduction. Journal of Pediatric Orthopaedics 2008;28(6):652‐5. [DOI] [PubMed] [Google Scholar]
Cutler 2004
- Cutler A, Molloy A, Dhukuram V, Bass A. Do CT scans aid assessment of distal tibial physeal fractures?. Journal of Bone and Joint Surgery (British Volume) 2004;86(2):239‐43. [DOI] [PubMed] [Google Scholar]
Daltroy 1998
- Daltroy LH, Liang MH, Fossel AH, Goldberg MJ. The POSNA pediatric musculoskeletal functional health questionnaire: report on reliability, validity, and sensitivity to change. Pediatric Outcomes Instrument Development Group. Pediatric Orthopaedic Society of North America. Journal of Pediatric Orthopaedics 1998;18(5):561‐71. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple graphical test. BMJ 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro [Computer program]
- Jan Brozek, Andrew Oxman, Holger Schünemann. GRADEpro. Version 3.2 for Windows. Jan Brozek, Andrew Oxman, Holger Schünemann, 2008.
Hermans 2012
- Hermans JJ, Wentink N, Beumer A, Hop WCJ, Heijboer MP, Moonen AFCM, et al. Correlation between radiological assessment of acute ankle fractures and syndesmotic injury on MRI. Skeletal Radiology 2012;41(7):787‐801. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hofsli 2016
- Hofsli M, Torfing T, Al‐Aubaidi Z. The proportion of distal fibula Salter–Harris type I epiphyseal fracture in the paediatric population with acute ankle injury: a prospective MRI study. Journal of Pediatric Orthopaedics B 2016;25(2):126‐32. [DOI] [PubMed] [Google Scholar]
Jones 2003
- Jones S, Phillips N, Ali F, Fernandes JA, Flowers MJ, Smith TW. Triplane fractures of the distal tibia requiring open reduction and internal fixation. Pre‐operative planning using computed tomography. Injury 2003;34(4):293‐8. [DOI] [PubMed] [Google Scholar]
Leary 2009
- Leary JT, Handling M, Talerico M, Yong L, Bowe JA. Physeal fractures of the distal tibia: predictive factors of premature physeal closure and growth arrest. Journal of Pediatric Orthopaedics 2009;29(4):356‐61. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Morris 2008
- Morris C, Doll HA, Wainwright A, Theologis T, Fitzpatrick R. The Oxford ankle foot questionnaire for children: scaling, reliability and validity. Journal of Bone and Joint Surgery (British Volume) 2008;90(11):1451‐6. [DOI] [PubMed] [Google Scholar]
Peterson 1994
- Peterson HA, Madhok R, Benson JT, Ilstrup DM, Melton LJ III. Physeal fractures: part 1: epidemiology in Olmsted County, Minnesota, 1978‐1988. Journal of Pediatric Orthopaedics 1994;14(4):423‐30. [DOI] [PubMed] [Google Scholar]
Rennie 2007
- Rennie L, Court‐Brown CM, Mok JY, Beattie TF. The epidemiology of fractures in children. Injury 2007;38(8):913‐22. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Salter 1963
- Salter RB, Harris WR. Injuries involving the epiphyseal plate. Journal of Bone and Joint Surgery (American Volume) 1963;45(3):587‐622. [Google Scholar]
Simanovsky 2005
- Simanovsky N, Hiller N, Leibner E, Simanovsky N. Sonographic detection of radiographically occult fractures in paediatric ankle injuries. Pediatric Radiology 2005;35(11):1062‐5. [DOI] [PubMed] [Google Scholar]
Swiontkowski 1999
- Swiontkowski MF, Engelberg R, Martin DP, Agel J. Short musculoskeletal function assessment questionnaire: Validity, reliability and responsiveness. Journal of Bone and Joint Surgery (American Volume) 1999;81(9):1245‐60. [DOI] [PubMed] [Google Scholar]
Taggart 2012
- Taggart I, Voskoboynik N, Shah S, Liebmann O. ED point‐of‐care ultrasound in the diagnosis of ankle fractures in children. American Journal of Emergency Medicine 2012;30(7):1328.e1‐1328.e3. [DOI] [PubMed] [Google Scholar]
Young 2000
- Young NL, Williams JI, Yoshida KK, Wright JG. Measurement properties of the activities scale for kids. Journal of Clinical Epidemiology 2000;53(2):125‐37. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Yeung 2013
- Yeung DE, Miller CA, Jia X, Barker SL. Interventions for treating ankle fractures in children. Cochrane Database of Systematic Reviews 2013, Issue 11. [DOI: 10.1002/14651858.CD010836] [DOI] [PMC free article] [PubMed] [Google Scholar]


