Abstract
Objective:
Memory impairment is an important contributor to the reduction in quality of life experienced by older adults, and genetic risk factors seem to contribute to variance in age-related cognitive decline. Brain-derived neurotrophic factor (BDNF) is an important nerve growth factor linked with development and neural plasticity. The Val66Met polymorphism in the BDNF gene has been associated with impaired episodic memory in adults, but whether this functional variant plays a role in cognitive aging remains unclear. The purpose of this study was to investigate the effects of the BDNF Val66Met polymorphism on memory performance in a sample of elderly adults.
Methods:
Eighty-seven subjects aged > 55 years were recruited using a community-based convenience sampling strategy in Porto Alegre, Brazil. The logical memory subset of the Wechsler Memory Scale-Revised was used to assess immediate verbal recall (IVR), delayed verbal recall (DVR), and memory retention rate.
Results:
BDNF Met allele carriers had lower DVR scores (p = 0.004) and a decline in memory retention (p = 0.017) when compared to Val/Val homozygotes. However, we found no significant differences in IVR between the two groups (p = 0.088).
Conclusion:
These results support the hypothesis of the BDNF Val66Met polymorphism as a risk factor associated with cognitive impairment, corroborating previous findings in young and older adults.
Keywords: Aging, brain-derived neurotrophic factor, cognition, memory, polymorphism
Introduction
Age-related cognitive decline is a natural process and has been considered an important contributor to loss of functional capacity and reduced quality of life in older adults.1 Genetic risk factors account for ∼ 50% of variance in adult cognitive ability, and still account for the majority of age-related variability in the elderly.2 The degree of cognitive decline in older adults is influenced by genetic predisposition to different cellular and molecular neurobiological factors that affect long-term cognitive ability.2,3
Brain-derived neurotrophic factor (BDNF) is a member of the nerve growth family that plays critical roles in regulating neuronal differentiation and synaptic plasticity - two complex neuronal processes implicated in learning and memory - throughout life.4 Consistent with the view that regulation of BDNF levels has effects on memory processes, previous studies of potential neurobiological factors of cognitive impairment found that memory deficits are associated with changes in peripheral BDNF levels in patients with psychiatric disorders.5,6 In addition, it has been suggested that BDNF synthesis declines throughout the life-span,7 suggesting a specific neurobiological mechanism of age-related decline in human memory.
One viable candidate gene polymorphism for understanding declarative memory is the BDNF Val66Met (rs6265) single nucleotide polymorphism (SNP), which results in a valine (Val)-to-methionine (Met) substitution at codon residue 66 in the BDNF precursor peptide sequence.8 The BDNF Val66Met polymorphism generates an alteration in BDNF trafficking to secretory granules and reduces its local secretion and distribution,8 which exerts an important effect on episodic memory functioning.9 However, recent meta-analyses investigating the relationship between the functional BDNF Val66Met polymorphism and performance on memory tasks have suggested heterogeneity in odds ratios among subjects.3,10 For example, some genetic association studies found impaired episodic memory performance in BDNF Met allele carriers compared to Val/Val homozygotes,11-13 whereas other reports found no effect of the BDNF Val66Met polymorphism on memory.14-16 Specifically in terms of verbal memory, in a sample of elderly subjects, BDNF Met allele carriers had poorer delayed verbal recall (DVR) than Val/Val homozygotes.9 On the other hand, other studies have shown no association of the Val66Met polymorphism and declarative memory in older14,16 or young adults.15,17
Considering these heterogeneous findings and the lack of evidence linking the BDNF Val66Met polymorphism with cognitive performance in older adults without psychiatry disorders, the current study was designed to investigate the effect of BDNF Val66Met polymorphism on declarative memory performance in elders from community associations without global cognitive impairment. We hypothesized that the individuals with a genetic predisposition to expression of BDNF (Met allele carriers) would show lower declarative memory performance.
Methods
Sample
The sample comprised 126 older adults recruited from Porto Alegre, state of Rio Grande do Sul, Brazil, using a community-based convenience sampling strategy. In brief, volunteers were recruited by the research team at community associations from 2013 to 2014. All subjects provided written informed consent for participation in accordance with the study protocol, as approved by the Pontifícia Universidade Católica do Rio Grande do Sul (PUCRS) Ethics Committee (protocol no. 241.863; CAAE 01764012.0.0000.5336). The inclusion criteria were as follows: age > 55 years and no diagnosis of dementia or neurological disorders (e.g., neurodegenerative disorders, stroke, epilepsy). We included volunteers with diagnoses of mild depression and who were well controlled on treatment for chronic medical conditions, including diabetes, hypertension, hypothyroidism, osteoporosis, and rheumatoid arthritis, due to the high prevalence of these clinical conditions in aging individuals.18 After enrollment, all participants were assessed for the following exclusion criteria: (A) fewer than 4 years of formal education (n=29); (B) history of traumatic brain injury (n=0); (C) Mini Mental State Examination (MMSE) below of the cutoff point of 21/22 (n=10); and (D) treatment with benzodiazepines (n=0). After application of these criteria, 87 older adults were retained for analysis. The final sample was divided by BDNF Val66Met genotype (Val/Val homozygotes and Met allele carriers).
Clinical assessment
Demographic characteristics were assessed by two well-trained psychologists through self-report during a clinical interview. Information on sociodemographic status and health history was also obtained. The MMSE19 was used as a dementia screening tool based on the cutoff point suggested by Almeida20 for Brazilian elderly individuals with some formal education.
The Mini International Neuropsychiatric Interview Plus (MINI-Plus)21,22 was used to investigate psychiatric disorders according to the DSM-IV criteria. The Geriatric Depression Scale-Short Form (GDS-15),23 a 15-item self-report assessment that measures depression symptoms in the elderly, was also applied. In addition, the Brazilian version of the Childhood Trauma Questionnaire (CTQ)24,25 was administered to investigate experiences of childhood abuse and neglect during early ages, as it has been suggested that such events could have a significant impact on neurodevelopment and, consequently, lead to long-lasting cognitive disabilities.26
Memory assessment
The logical memory (LM) subtest of the Wechsler Memory Scale-Revised (WMS-R),27 a verbal declarative memory task, was used to evaluate short-term and long-term verbal memory performance. Subjects were told two short stories and asked to freely recall their content immediately and 30 min after listening. The sum of the number of correctly recalled sentences was used to generate an immediate verbal recall (IVR) score and a DVR score. Percent memory retention (retention rate) was then calculated as DVR/IVR × 100.
BDNF genotyping
DNA was isolated from peripheral blood by the salting-out procedure.28 Prior to genotyping, DNA was assessed using a Qubit 2.0® fluorometer (Life Technologies, USA) in accordance with manufacturer instructions (dsDNA HS assay Kit; Life Technologies, USA). The BDNF Val66Met polymorphism (rs6265 SNP) was genotyped using the TaqMan® SNP assay (ID: C 11592758 10; Life Technologies, USA) in a StepOne™ real-time polymerase chain reaction (PCR) system (Applied Biosystems, USA), following manufacturer instructions. Standard PCR was carried out using TaqMan® Real-Time PCR Master Mix (Life Technologies, USA) as indicated by the manufacturer. Data acquisition was performed using the allelic discrimination analysis module of StepOne™ version 2.0 software (Applied Biosystems, USA). The BDNF Val66Met polymorphism was also tested for Hardy-Weinberg equilibrium.
Statistical analysis
Variables were tested for normality of distribution by the Kolmogorov-Smirnov test. Descriptive statistics for demographic variables, BDNF Val66Met polymorphism status, and memory measures were calculated, and results were presented as mean and standard deviation (SD), percentage, or both as appropriate. The specific statistical tests used for the demographic and clinical characteristics of the sample are given in the Results section.
Multivariate general linear models were used to test the influence of the BDNF Val66Met polymorphism on IVR, DVR, and retention memory scores. Gender, age, years of education, and MMSE, CTQ, and GDS scores were included as covariates of interest. A p-value < 0.05 was considered statistically significant. All analyses were performed using SPSS version 20 and graphs plotted in GraphPad Prism version 6.
Results
Table 1 summarizes the sociodemographic and clinical variables of the sample, stratified by BDNF Val66Met genotype. The chi-square (χ2) test was used to estimate the allelic frequency of the BDNF Val66Met polymorphism on the basis of Hardy-Weinberg equilibrium. The frequencies of the 87 subjects analyzed in our study were 29.9% Met allele carriers and 70.1% Val/Val homozygotes, which is consistent with Hardy-Weinberg equilibrium (p = 0.840) and similar to the frequencies previously reported for another elderly sample.16
Table 1. Demographic and clinical characteristics of the sample.
| Val/Val (n=61) | Met allele (n=26) | Statistics | p-value | |
|---|---|---|---|---|
| Age (years) | 68.61 (7.60) | 71.62 (8.51) | t = -1.629 | 0.107 |
| Education (years) | 8.90 (3.96) | 9.08 (4.03) | t = -0.188 | 0.851 |
| GDS score | 3.97 (3.34) | 3.65 (2.68) | t = 0.423 | 0.674 |
| MMSE | 27.98 (2.17) | 27.69 (2.16) | t = 0.573 | 0.568 |
| Gender | ||||
| Male | 13 (21.3) | 8 (30.8) | χ2 = 0.891 | 0.414 |
| Female | 48 (78.7) | 18 (69.2) | ||
| Clinical variables | ||||
| Diabetes | 9 (90.0) | 1 (10.0) | χ2 = 2.132 | 0.135 |
| Hypertension | 35 (66.0) | 18 (34.0) | χ2 = 1.076 | 0.214 |
| Hypothyroidism | 13 (76.5) | 4 (23.5) | χ2 = 0.407 | 0.768 |
| Osteoporosis | 10 (66.7) | 5 (33.3) | χ2 = 0.371 | 0.745 |
| Rheumatoid arthritis | 8 (66.7) | 4 (33.3) | χ2 = 0.268 | 0.735 |
| Depression | 20 (64.5) | 11 (35.5) | χ2 = 0.720 | 0.466 |
| Cigarette use | 18 (85.7) | 3 (14.3) | χ2 = 0.940 | 0.462 |
| Alcohol use | 12 (85.7) | 2 (14.3) | χ2 = 0.480 | 0.695 |
| CTQ | 38 (73.1) | 14 (26.9) | χ2 = 0.541 | 0.483 |
Age, years of education, and GDS and MMSE scores data presented as mean (SD). Gender and clinical variables presented as n (%).
CTQ = Childhood Trauma Questionnaire; GDS = Geriatric Depression Scale; MMSE = Mini Mental State Examination; SD = standard deviation.
Demographic and clinical profiles were compared between groups with the t test (quantitative variables) and χ2 test (categorical variables).
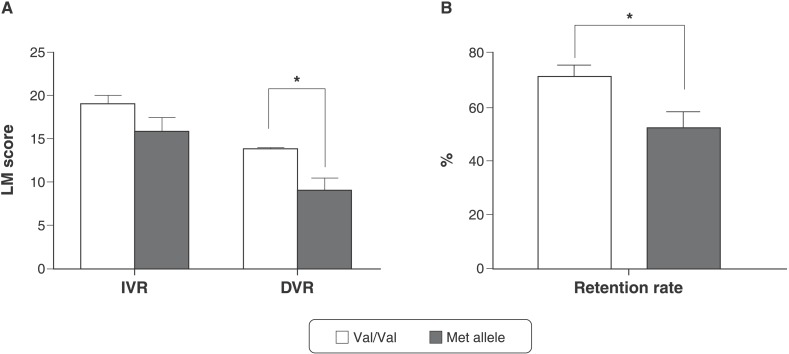
Multivariate general linear models using BDNF Val66Met genotype as predictor were fitted to the composite cognitive variables while covarying for age, gender, years of education, and MMSE, CTQ, and GDS scores. We found further evidence for the association between the BDNF Val66Met polymorphism and cognitive impairment in this sample of older adults without psychiatric disorders. Regarding LM assessment, Met allele carriers had lower DVR scores compared to participants homozygous for Val/Val (p = 0.004) (Figure 1A). In addition, Met allele carriers had a lower retention rate when compared to Val/Val homozygotes (p = 0.017) (Figure 1B). However, no significant between-group differences were found for IVR (p = 0.088) (Figure 1A).
Figure 1. Effect of BDNF Val66Met polymorphism on memory performance. Data presented as mean (standard deviation). A) IVR: Val/Val, 19.00 (7.38); Met, 15.85 (8.02); DVR: Val/Val, 13.87 (7.46); Met, 8.26 (7.25). B) Retention rate: Val/Val, 71.53 (31.20); Met: 52.06 (26.84). MANCOVA among groups adjusted for age, gender, years of education, CTQ score, GDS score, and MMSE score: A) IVR: F1,85 = 2.980, p = 0.088; DVR: F1,85 = 8.710, * p = 0.004 (Met allele carriers < Val/Val homozygotes). B) Retention rate: F1,85 = 5.934, * p = 0.017 (Met allele carriers < Val/Val homozygotes). CTQ = Childhood Trauma Questionnaire; DVR = delayed verbal recall; GDS = Geriatric Depression Scale-Short Form; IVR = immediate verbal recall; LM = logical memory; MANCOVA = multivariate analysis of covariance; MMSE = Mini Mental State Examination.
Discussion
In this study, we addressed the relationship between the BDNF Val66Met polymorphism and verbal memory processes related to IVR and DVR, as well as memory retention, through a declarative memory task in a sample of older adults. BDNF Val66Met genotype variation affected DVR and memory retention processes, but did not influence IVR performance. Our results were in line with those of some previous studies that demonstrated impairments in verbal memory in BDNF Met allele carriers when compared to Val/Val homozygotes.8,11,13,29
Taken together, these findings provide further evidence of the relationship between the BDNF Val66Met polymorphism and performance in both recall and retention processes. Since LM requires that information retention for the immediate and delayed tasks beyond that which would be possible based on models of working memory,30 it has been suggested that, to perform successfully on distinct memory phases, a wide-ranging set of processes such as encoding, storage, and retrieval would be required. Moreover, the hippocampus is considered to play major roles in DVR and retention,31 suggesting that altered BDNF function in this brain region might lead to impairment in verbal memory tasks.
Despite a growing body of evidence to support the evaluation of specific memory processes through defined memory tasks, as well as several neuroimaging studies pointing to the involvement of specific brain regions in the integrity of memory functioning, the neurobiological mechanisms underlying memory remain poorly understood. Some authors4,11 argue that learning and memory consolidation processes could be dependent of BDNF-induced activation of long-term potentiation (LTP) in the central nervous system, which would lead to a long-lasting enhancement of signal transmission between hippocampal synapses. In addition, BDNF is considered necessary for the activation of other signaling cascades involved in LTP activation, such as extracellular signal-regulated kinase, that also participate in consolidation and retrieval of encoded memories.4 Considering the evidence for the effect of the BDNF Val66Met variant in activity-dependent BDNF response in the hippocampus, it is interesting to note that BDNF Met allele carriers have been shown to have smaller hippocampal volumes,32 altered hippocampal patterns,33 and reduced hippocampal neuronal integrity8 when compared to Val/Val homozygotes. These findings are generally explained by irregular intracellular trafficking and impaired secretion of BDNF, leading to long-lasting changes in cell development and hippocampal plasticity,9,34 suggesting a possible mechanism for genetic effects on memory performance.
One previous report using data from older adults suggested that the BDNF Met allele is associated with higher memory performance,35 whereas other studies found no effect of BDNF Val66Met variant on memory in older14,16,36 or young adults.15,17 Differences in socioeconomic status as well as in age range between the samples analyzed in these studies might explain these contradictory findings. Our sample was composed of older adults with a younger mean age and from a lower-income setting when compared with the two cohorts of the elderly Scottish population published in 2006.16 Although our sample size is small, our data do not reflect a sample-specific effect, as they corroborate previous findings associating the BDNF Met allele and memory impairments in older adults.9,29
Given the complexity of neuronal processes underlying the BDNF Val66Met polymorphism and memory performance in elderly, we believe that our findings are in accordance with the modulation hypothesis proposed by a previous study.37 According to the authors, this nonlinear hypothesis assumes that the magnitude of the genetic predisposition for poorer cognition performance conditioned by the BDNF Met allele could be increased in the elderly, especially when chemical and structural brain resources are declining with the life-span. Therefore, they suggested that age-related loss of neurochemical factors could modulate the effect of the BDNF Val66Met polymorphism on memory performance. In this context, given that hippocampal-dependent functions decline during aging, we believe neuronal plasticity could be a possible mechanism for involvement of genetic risk factors in declarative memory performance.
Our results should be interpreted within the context of the limitations of our study. Although we controlled for covariates, sample size was certainly an issue, considering categorical and dimensional measures, and will have limited the reliability of our statistical analyses. In addition, the design of our study precluded assessment of individual memory impairment over time, although we could estimate the effects of the aging process. We also used a single task to assess declarative memory performance; further studies should consider using a battery of tasks to evaluate multiple types of memory. Moreover, although our sample was formally non-psychiatric, we did not exclude subjects with mild to moderate depressive symptoms, and could not exclude common clinical diseases related to the aging process (Table 1). Unfortunately, studies with healthy elderly subjects represent a challenge in this field. Finally, it is already known that the BDNF Val66Met polymorphism has effects on other cognitive domains besides memory, including distinct components of executive functions,35,38 reasoning,16 attention,39 and visual-auditory working memory.40 Therefore, we were not able to restrict the effects of this SNP to memory.
In summary, this study verified the influence of the BDNF Val66Met polymorphism in specific memory processes, as identified by modifications in DVR and retention scores. This suggests that Met allele carriers have impairments in storage and retrieval processes, supporting the modulation hypothesis of genetic effects on cognition. The present data should be relevant for future meta-analyses evaluating the relationship between BDNF Met allele carrier status and cognitive decline in older adults. Future longitudinal studies coupled with neuroimaging approaches could bring us closer to getting a clear picture of the role of BDNF variations in memory performance across the life-span, especially in older age.
Disclosure
The authors report no conflicts of interest.
Acknowledgements
This research was supported by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo è Pesquisa do Estado do Rio Grande do Sul (FAPERGS). LAA was supported by CNPq early career grants (nos. 161803/2014-8 and 150678/2015-0).
References
- 1.Kljajevic V. From cell to cognition: can changes in telomere length indicate patterns of cognitive aging? Clin Sci (Lond). 2011;121:313–4. doi: 10.1042/CS20110227. [DOI] [PubMed] [Google Scholar]
- 2.Deary IJ, Wright AF, Harris SE, Whalley LJ, Starr JM. Searching for genetic influences on normal cognitive ageing. Trends Cogn Sci. 2004;8:178–84. doi: 10.1016/j.tics.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 3.Kambeitz JP, Bhattacharyya S, Kambeitz-Ilankovic LM, Valli I, Collier DA, McGuire P. Effect of BDNF val(66)met polymorphism on declarative memory and its neural substrate: a meta-analysis. Neurosci Biobehav Rev. 2012;36:2165–77. doi: 10.1016/j.neubiorev.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Bekinschtein P, Cammarota M, Medina JH. BDNF and memory processing. Neuropharmacology. 2014;76:677–83. doi: 10.1016/j.neuropharm.2013.04.024. [DOI] [PubMed] [Google Scholar]
- 5.Grassi-Oliveira R, Stein LM, Lopes RP, Teixeira AL, Bauer ME. Low plasma brain-derived neurotrophic factor and childhood physical neglect are associated with verbal memory impairment in major depression--a preliminary report. Biol Psychiatry. 2008;64:281–5. doi: 10.1016/j.biopsych.2008.02.023. [DOI] [PubMed] [Google Scholar]
- 6.Viola TW, Tractenberg SG, Kluwe-Schiavon B, Levandowski ML, Sanvicente-Vieira B, Wearick-Silva LE, et al. Brain-derived neurotrophic factor and delayed verbal recall in crack/cocaine dependents. Eur Addict Res. 2015;21:273–8. doi: 10.1159/000430436. [DOI] [PubMed] [Google Scholar]
- 7.Erickson KI, Prakash RS, Voss MW, Chaddock L, Heo S, McLaren M, et al. Brain-derived neurotrophic factor is associated with age-related decline in hippocampal volume. J Neurosci. 2010;30:5368–75. doi: 10.1523/JNEUROSCI.6251-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–69. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- 9.Miyajima F, Ollier W, Mayes A, Jackson A, Thacker N, Rabbitt P, et al. Brain-derived neurotrophic factor polymorphism Val66Met influences cognitive abilities in the elderly. Genes Brain Behav. 2008;7:411–7. doi: 10.1111/j.1601-183X.2007.00363.x. [DOI] [PubMed] [Google Scholar]
- 10.Mandelman SD, Grigorenko EL. BDNF Val66Met and cognition: all, none, or some? A meta-analysis of the genetic association. Genes Brain Behav. 2012;11:127–36. doi: 10.1111/j.1601-183X.2011.00738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hariri AR, Goldberg TE, Mattay VS, Kolachana BS, Callicott JH, Egan MF, et al. Brain-derived neurotrophic factor val66met polymorphism affects human memory-related hippocampal activity and predicts memory performance. J Neurosci. 2003;23:6690–4. doi: 10.1523/JNEUROSCI.23-17-06690.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldberg TE, Iudicello J, Russo C, Elvevåg B, Straub R, Egan MF, et al. BDNF Val66Met polymorphism significantly affects d' in verbal recognition memory at short and long delays. Biol Psychol. 2008;77:20–4. doi: 10.1016/j.biopsycho.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 13.Ho BC, Milev P, O'Leary DS, Librant A, Andreasen NC, Wassink TH. Cognitive and magnetic resonance imaging brain morphometric correlates of brain-derived neurotrophic factor Val66Met gene polymorphism in patients with schizophrenia and healthy volunteers. Arch Gen Psychiatry. 2006;63:731–40. doi: 10.1001/archpsyc.63.7.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benjamin S, McQuoid DR, Potter GG, Payne ME, MacFall JR, Steffens DC, et al. The brain-derived neurotrophic factor Val66Met polymorphism, hippocampal volume, and cognitive function in geriatric depression. Am J Geriatr Psychiatry. 2010;18:323–31. doi: 10.1097/JGP.0b013e3181cabd2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gong P, Zheng A, Chen D, Ge W, Lv C, Zhang K, et al. Effect of BDNF Val66Met polymorphism on digital working memory and spatial localization in a healthy Chinese Han population. J Mol Neurosci. 2009;38:250–6. doi: 10.1007/s12031-009-9205-8. [DOI] [PubMed] [Google Scholar]
- 16.Harris SE, Fox H, Wright AF, Hayward C, Starr JM, Whalley LJ, et al. The brain-derived neurotrophic factor Val66Met polymorphism is associated with age-related change in reasoning skills. Mol Psychiatry. 2006;11:505–13. doi: 10.1038/sj.mp.4001799. [DOI] [PubMed] [Google Scholar]
- 17.Karnik MS, Wang L, Barch DM, Morris JC, Csernansky JG. BDNF polymorphism rs6265 and hippocampal structure and memory performance in healthy control subjects. Psychiatry Res. 2010;178:425–9. doi: 10.1016/j.psychres.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmidt MI, Duncan BB, Azevedo e Silva G, Menezes AM, Monteiro CA, Barreto SM, et al. Chronic non-communicable diseases in Brazil: burden and current challenges. Lancet. 2011;377:1949–61. doi: 10.1016/S0140-6736(11)60135-9. [DOI] [PubMed] [Google Scholar]
- 19.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 20.Almeida OP. [Mini mental state examination and the diagnosis of dementia in Brazil]. Arq Neuropsiquiatr. 1998;56:605–12. doi: 10.1590/s0004-282x1998000400014. [DOI] [PubMed] [Google Scholar]
- 21.Amorim P, Lecrubier Y, Weiller E, Hergueta T, Sheehan D. DSM-IH-R Psychotic Disorders: procedural validity of the Mini International Neuropsychiatric Interview (MINI). Concordance and causes for discordance with the CIDI. Eur Psychiatry. 1998;13:26–34. doi: 10.1016/S0924-9338(97)86748-X. [DOI] [PubMed] [Google Scholar]
- 22.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59:22–33. quiz 34-57. [PubMed] [Google Scholar]
- 23.Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;11983(17):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 24.Grassi-Oliveira R, Stein LM, Pezzi JC. [Translation and content validation of the Childhood Trauma Questionnaire into Portuguese language]. Rev Saude Publica. 2006;40:249–55. doi: 10.1590/s0034-89102006000200010. [DOI] [PubMed] [Google Scholar]
- 25.Grassi-Oliveira R, Cogo-Moreira H, Salum GA, Brietzke E, Viola TW, Manfro GG, et al. Childhood Trauma Questionnaire (CTQ) in Brazilian samples of different age groups: findings from confirmatory factor analysis. PloS one. 2014;9:e87118. doi: 10.1371/journal.pone.0087118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teicher MH, Tomoda A, Andersen SL. Neurobiological consequences of early stress and childhood maltreatment: are results from human and animal studies comparable? Ann N Y Acad Sci. 2006;1071:313–23. doi: 10.1196/annals.1364.024. [DOI] [PubMed] [Google Scholar]
- 27.Wechsler D. The Wechsler memory scale. San Antonio: The Psychological Corporation. 1987 [Google Scholar]
- 28.Lahiri DK, Nurnberger JI., Jr A rapid non-enzymatic method for the preparation of HMW DNA from blood for RFLP studies. Nucleic Acids Res. 1991;19:5444. doi: 10.1093/nar/19.19.5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kennedy KM, Reese ED, Horn MM, Sizemore AN, Unni AK, Meerbrey ME, et al. BDNF val66met polymorphism affects aging of multiple types of memory. Brain Res. 2015;1612:104–17. doi: 10.1016/j.brainres.2014.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baddeley A. The episodic buffer: a new component of working memory? Trends Cogn Sci. 2000;4:417–23. doi: 10.1016/s1364-6613(00)01538-2. [DOI] [PubMed] [Google Scholar]
- 31.Wolk DA, Dickerson BC. Alzheimer's Disease Neuroimaging Initiative. Fractionating verbal episodic memory in Alzheimer's disease. Neuroimage. 2011;54:1530–9. doi: 10.1016/j.neuroimage.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bueller JA, Aftab M, Sen S, Gomez-Hassan D, Burmeister M, Zubieta JK. BDNF Val66Met allele is associated with reduced hippocampal volume in healthy subjects. Biol Psychiatry. 2006;59:812–5. doi: 10.1016/j.biopsych.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 33.Lovden M, Schaefer S, Noack H, Kanowski M, Kaufmann J, Tempelmann C, et al. Performance-related increases in hippocampal N-acetylaspartate (NAA) induced by spatial navigation training are restricted to BDNF Val homozygotes. Cereb Cortex. 2011;21:1435–42. doi: 10.1093/cercor/bhq230. [DOI] [PubMed] [Google Scholar]
- 34.Kojima M, Takei N, Numakawa T, Ishikawa Y, Suzuki S, Matsumoto T, et al. Biological characterization and optical imaging of brain-derived neurotrophic factor-green fluorescent protein suggest an activity-dependent local release of brain-derived neurotrophic factor in neurites of cultured hippocampal neurons. J Neurosci Res. 2001;64:1–10. doi: 10.1002/jnr.1080. [DOI] [PubMed] [Google Scholar]
- 35.Gajewski PD, Hengstler JG, Golka K, Falkenstein M, Beste C. The Met-allele of the BDNF Val66Met polymorphism enhances task switching in elderly. Neurobiol Aging. 2011;32(2327):e7–19. doi: 10.1016/j.neurobiolaging.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 36.Kim A, Fagan AM, Goate AM, Benzinger TL, Morris JC, Head D, et al. Lack of an association of BDNF Val66Met polymorphism and plasma BDNF with hippocampal volume and memory. Cogn Affect Behav Neurosci. 2015;15:625–43. doi: 10.3758/s13415-015-0343-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lindenberger U, Nagel IE, Chicherio C, Li SC, Heekeren HR, Backman L. Age-related decline in brain resources modulates genetic effects on cognitive functioning. Front Neurosci. 2008;2:234–44. doi: 10.3389/neuro.01.039.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Erickson KI, Kim JS, Suever BL, Voss MW, Francis BM, Kramer AF. Genetic contributions to age-related decline in executive function: a 10-year longitudinal study of COMT and BDNF polymorphisms. Front Hum Neurosci. 2008;2:11. doi: 10.3389/neuro.09.011.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Getzmann S, Gajewski PD, Hengstler JG, Falkenstein M, Beste C. BDNF Val66Met polymorphism and goal-directed behavior in healthy elderly - evidence from auditory distraction. Neuroimage. 2013;64:290–8. doi: 10.1016/j.neuroimage.2012.08.079. [DOI] [PubMed] [Google Scholar]
- 40.Niechwiej-Szwedo E, Gonzalez D, Tapper A, Mardian E, Roy E, Duncan RE. The BDNF Val66Met polymorphism is associated with improved performance on a visual-auditory working memory task in varsity athletes. J Vis. 2015;15:676. [Google Scholar]