Abstract
Lassa virus (LASV) causes Lassa hemorrhagic fever in humans and poses a significant threat to public health in West Africa. Current therapeutic treatments for Lassa fever are limited, making the development of novel countermeasures an urgent priority. In this study, we identified losmapimod, a p38 mitogen-activated protein kinase (MAPK) inhibitor, from 102 screened compounds as an inhibitor of LASV infection. Losmapimod exerted its inhibitory effect against LASV after p38 MAPK down-regulation, and, interestingly, had no effect on other arenaviruses capable of causing viral hemorrhagic fever. Mechanistic studies showed that losmapimod inhibited LASV entry by affecting the stable signal peptide (SSP)-GP2 subunit interface of the LASV glycoprotein, thereby blocking pH-dependent viral fusion. As an aryl heteroaryl bis-carboxyamide derivative, losmapimod represents a novel chemical scaffold with anti-LASV activity, and it provides a new lead structure for the future development of LASV fusion inhibitors.
Keywords: Losmapimod, Lassa virus, Entry inhibitor, SSP-GP2, Drug repurposing
Abbreviations: BSL-4, biosafety level 4; CAD, cation amphiphilic drug; CC50, half maximal cytotoxic concentration; CHPV, Chapare virus; COPD, chronic obstructive pulmonary diseases; CPE, cytopathic effect; α-DG, α-dystroglycan; EC50, half maximal effective concentration; GEQ, genome equivalent; GPC, glycoprotein complex; GTOV, Guanarito virus; JUNV, Junin virus; LAMP1, lysosome-associated membrane protein 1; LASV, Lassa virus; LCMV, Lymphocytic choriomeningitis virus; LUJV, Lujo virus; MACV, Machupo virus; SlP, site 1 protease; SABV, Sabia virus; SSP, stable signal peptide; hTfR1, Human transferrin receptor 1; SI, selectivity index; TMD, transmembrane domain; V-ATPase, vacuolar-type H+-ATPase; VHF, viral hemorrhagic fever; WWAV, Whitewater Arroyo virus
Highlights
-
•
Losmapimod was identified as a Lassa virus entry inhibitor following the screening of 102 clinical compounds.
-
•
Losmapimod inhibited Lassa virus infection by affecting the SSP-GP2 interface of the Lassa virus glycoprotein.
-
•
Losmapimod preferentially inhibits the entry of Lassa virus among arenaviruses known to cause human disease.
1. Introduction
Lassa virus (LASV) is the causative agent of Lassa hemorrhagic fever, and it is responsible for 100,000 to 300,000 human infections and around 5000 deaths in West Africa per year (Houlihan and Behrens, 2017). Indeed, the 2019 outbreak of Lassa fever in Nigeria remains ongoing, and has already resulted in 472 confirmed cases with a case fatality rate of 23.3% as of March 2019 (Nigeria Centre for Disease Control, 2019). Currently there are no FDA-approved LASV-specific antivirals or vaccines. Ribavirin is commonly used for Lassa fever in the early course of illness, but it is not suggested for post-exposure prophylaxis (Houlihan and Behrens, 2017). In pre-clinical studies, treatment with 300 mg/kg/day favipiravir (T-705), an RNA polymerase inhibitor, resulted in 100% survival in mice (Oestereich et al., 2016) and guinea pig (Safronetz et al., 2015) infected with LASV. Moreover, when used synergistically with ribavirin, favipiravir significantly improved disease outcome in LASV-infected mice (Carrillo-Bustamante et al., 2017). Human monoclonal antibodies that target LASV glycoproteins also represent a possible future treatment strategy for Lassa fever (Warner et al., 2018).
The Mammarenavirus genus (family Arenaviridae) is serologically and geographically classified into Old World and New World complexes (Oldstone, 2002). LASV, along with the prototype arenavirus lymphocytic choriomeningitis virus (LCMV) and Lujo virus (LUJV), belong to the Old World mammarenaviruses (Radoshitzky et al., 2015). The New World complex also contains several viruses that cause viral hemorrhagic fever (VHF) in humans, including Machupo virus (MACV), Guanarito virus (GTOV), Junin virus (JUNV), Sabia virus (SABV), and Chapare virus (CHPV) (Sarute and Ross, 2017). LASV is phylogenetically closer to LCMV than the New World mammarenaviruses (Oldstone, 2002).
LASV is an enveloped RNA virus that contains two ambisense RNA genome segments, encoding the viral polymerase (L), matrix protein (Z), nucleoprotein (NP), and the glycoprotein complex (GPC) (Knipe and Howley, 2013). LASV entry is mediated solely by the GPC, which is first synthesized as a single polypeptide and then cleaved by signal peptidase and subtilisin/hexin-isoenzyme-1/site-1 protease (SKI-1/S1P) into three segments: the stable signal peptide (SSP), the receptor-binding subunit GP1, and the membrane fusion subunit GP2 (Bederka et al., 2014). The SSP, GP1, and GP2 are noncovalently bound to each other and together form trimers that are packed onto the virion surface (Bederka et al., 2014). The SSP consists of 58 amino acids with two transmembrane domains and one ectodomain (Bederka et al., 2014). The GP1 subunit is associated with receptor recognition, and it interacts with cellular receptors alpha-dystroglycan (α-DG) (Acciani et al., 2017) and lysosome-associated membrane protein 1 (LAMP1) (Jae et al., 2014). The GP2 subunit is a class I viral fusion protein and upon exposure to acidic environment undergoes a conformational change that triggers membrane fusion (Igonet et al., 2011). SSP is essential in GPC maturation (Burri et al., 2013) and promotes GPC-mediated pH-dependent membrane fusion by interacting with the GP2 subunit (Messina et al., 2012).
LASV is one of the category A agents that requires biosafety level 4 (BSL-4) containment (Centers for Disease Control and Prevention, 2010), which poses significant obstacles for antiviral discovery. Using recombinant or pseudotyped virus systems is therefore an accepted alternative for antiviral screening (Larson et al., 2008; Tani et al., 2014; Chen et al., 2018). In fact, a number of arenavirus entry inhibitors, such as the broad-spectrum arenavirus entry inhibitor ST-193 (Larson et al., 2008) and Lujo virus entry inhibitor desipramine (Tani et al., 2014), were discovered by pseudovirus screening.
The spread of Lassa fever in Africa and the lack of efficacious therapeutics impose a dire need for antiviral development. Drug repurposing has become popular in the field with the aim to fast-track the development process (Ashburn and Thor, 2004). This strategy has been widely used for identifying Ebola, Zika, and severe acute respiratory syndrome (SARS) virus inhibitors, with several promising hit/lead-antivirals reported (Cheng et al., 2016; Dyall et al., 2014; Johansen et al., 2015; Kouznetsova et al., 2014; Madrid et al., 2013), and lacidipine was identified from an approved drug library as a compound with anti-LASV activity (Wang et al., 2018). In this study, we examined a clinical compound library in a search for LASV inhibitors and identified losmapimod, a p38 mitogen-activated protein kinase (MAPK) inhibitor originally intended for cardiovascular diseases and chronic obstructive pulmonary diseases (COPD), as an inhibitor of LASV entry.
2. Materials and methods
2.1. Cells and plasmids
HEK293T, A549, HeLa, HepG2, Huh7, U-87 MG, BHK-21, and Vero cells were obtained from the China Infrastructure of Cell Line Resource (Beijing, China). Vero E6 cells were obtained from the American Type Culture Collection (ATCC). The cells were cultured in Dulbecco's modified Eagle's medium (DMEM; Invitrogen, USA) supplemented with 10% FBS, 100 IU/mL penicillin, and 100 μg/mL streptomycin. U-87 MG cells were cultured in minimal essential medium (MEM; Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS; Gibco, USA), 100 IU/mL penicillin, and 100 μg/mL streptomycin. Cells were maintained in a humidified atmosphere containing 5% CO2 at 37 °C.
The codon-optimized GPC genes of LASV (strain Josiah, GenBank Accession No. NP_694870.1; strain LP, GenBank Accession No. AAF86701.1; strain 803213, GenBank Accession No. AAF86703.1 and strain GA391, GenBank Accession No. CAA36645.1), LCMV (strain Armstrong 53b, GenBank Accession No. AAX49341.1), LUJV (GenBank Accession No. ACR56359.1), MACV (strain Carvallo, GenBank Accession No. NP_899212.1), GTOV (strain S-56764, GenBank Accession No. AAT72103.1), JUNV (strain XJ13, GenBank Accession No. ACO52428.1), SABV (strain SPH114202, GenBank Accession No. YP_089665.1), WWAV (strain 9310141, GenBank Accession No. AAN09950.1), and CHPV (strain 810419, GenBank Accession No. YP_001816782.1) were synthesized and inserted into eukaryotic expression vectors as described previously (Tang et al., 2018; Zhang et al., 2018). The env-deficient HIV core plasmid (pNL4-3.Luc.R_E_) was obtained from the National Institutes of Health AIDS Research and Reference Reagent Program (Germantown, MD, USA).
2.2. Compounds
The clinical compound library was obtained from TargetMol (Boston, MA, USA), with all compounds guaranteed to be more than 95% pure. Losmapimod (purity > 98%) and desipramine hydrochloride (purity > 98%) were obtained from TargetMol. ST-193 (purity > 98%) was obtained from MedChemExpress (NJ, USA). F3406-2010 (purity > 98%) was obtained from Life Chemicals (Woodbridge, CT, USA). Bafilomycin A1 (purity > 98%) was obtained from LC Laboratories (Boston, MA, USA). Compound purity was provided by the suppliers. The compounds were dissolved in dimethyl sulfoxide (DMSO, Sigma, St. Louis, MO, USA) and stored at −20 °C until use. DMSO was used as a vehicle control in all experiments at a final concentration of 0.1%.
2.3. Cell viability assay
To evaluate the effect of compounds on cell viability, a CellTiter-Glo® Luminescent Cell Viability Assay (Promega, Madison, WI, USA) was performed as previously described (Tang et al., 2018). Briefly, HEK293T cells were treated with 10 μM final concentration of compounds for cell viability or serial dilutions of compounds for half maximal cytotoxic concentration (CC50) assessment in a 96-well plate format with a starting cell density of 8 × 103 cells/well. Forty-eight hours later, the CellTiter-Glo® Assay was performed and relative luminescence units (RLUs) were recorded. Cells treated with DMSO (0.1% v/v) served as the solvent control and their mean RLU value was set as 100% cell viability. Doxorubicin was used as the positive control. Cells with medium served as the negative control. Percentage cell viability was calculated as follows: RLUs of compound/RLUs of DMSO control × 100%. The assay was performed in triplicate wells for each compound concentration, and the mean and standard deviation (SD) were calculated by GraphPad Prism software.
Cell viability was also evaluated on Vero E6 cells using losmapimod concentrations that ranged from 0.39 μM to 50 μM. Briefly, Vero E6 cells were seeded in 96-well plates on the day before the assay. Cells were then treated with the compound at the indicated concentrations and incubated for 72 h, after which cell viability was evaluated using the WST-1 Assay kit (Roche, Cat. No.11644807001). Cells with medium were used as the negative control, and 1% DMSO (v/v) served as the solvent control. Puromycin was used as the positive control as described previously (Tang et al., 2018). Percentage cell viability was calculated as follows: RUs of compound/RUs of DMSO control × 100%. The assay was performed in triplicate wells for each compound concentration, and the mean and standard deviation (SD) were calculated by GraphPad Prism software.
2.4. Pseudo-arenavirus preparation, infection and compound activity assay in vitro
The HIV pseudotyped viruses bearing the glycoprotein (GP) of each of the nine arenaviruses were generated and used for virus infection assay and compound activity assay as previously reported (Tang et al., 2018; Zhang et al., 2018). Briefly, the plasmids encoding arenavirus-GP (2 μg) and the HIV vector (pNL4.3.Luc-R-E-, 8 μg) were co-transfected into HEK293T cells and 48 h later, the arenavirus-GP/HIV-luc were collected, quantified by an HIV-1 p24 ELISA kit (Sino Biological Inc., Beijing, China) and stored at −80 °C until use. For the virus infection assay, cells were infected with the pseudoviruses and 48 h later virus infectivity was measured by a Luciferase Assay System (Promega, Madison, WI, USA). For the compound activity assay, cells were treated with compounds 15 min prior to arenavirus-GP/HIV-luc infection. Cells treated with DMSO (0.1% v/v) served as the solvent control and their RLU value was set as 100% infectivity. All assays were performed in duplicate wells for each virus infection or compound concentration, and the mean and standard deviation (SD) were calculated by GraphPad Prism software.
2.5. VSVΔG-LASV-GP-GFP infection assay
Vero E6 cells were pretreated with losmapimod (0.07–50 μM) or DMSO for 1 h at 37 °C and infected at a multiplicity of infection (MOI) of 0.1 with wild type vesicular stomatitis virus expressing the enhanced green fluorescent protein (EGFP) (VSV-EGFP) or recombinant VSV lacking its native glycoprotein (VSVΔG) and expressing, instead, LASV-GP (strain Josiah) along with EGFP (VSVΔG-LASV-GP-EGFP) in the presence of drug or DMSO for 1 h at 37 °C. Following the 1-h incubation, the inoculum was removed and replaced with fresh medium (DMEM containing 2% FBS). Cells were then incubated for 72 h in the presence of drug or DMSO, after which they were monitored with an Evos FL microscope, and the GFP signal was quantified on a Biotek Synergy HTX plate reader. This assay was performed in duplicate wells and compound dose-response curves were generated by GraphPad Prism software.
2.6. Authentic LASV infection assay
All work with infectious live virus was performed in the containment level 4 facility at the National Microbiology Laboratory (NML) of the Public Health Agency of Canada (PHAC) in the Canadian Science Centre for Human and Animal Health (CSCHAH), Winnipeg, Canada. All procedures were conducted in accordance with protocols appropriate for this level of biosafety. Briefly, Vero E6 cells were grown in 48-well plates to 95–100% confluency and were pretreated with serial dilutions of losmapimod (0.02–50 μM) for 1 h at 37 °C. Following pretreatment, cells were infected with LASV (strain Josiah) at an MOI of 0.01 in the presence of drug for 1 h at 37 °C before the inoculum was removed and replaced with fresh medium containing drug and 2% FBS. At 72 h post infection, cell culture supernatants were harvested and viral titration was determined by RT-qPCR as described previously (Tang et al., 2018), or by calculating the 50% tissue culture infectious dose (TCID50) using the Reed and Muench method (Reed and Muench, 1938). This assay was performed in triplicate wells and compound dose-response curves were generated by GraphPad Prism software.
2.7. p38 MAPK knock down by RNAi
HEK293T cells grown in 6-well plates to 70% confluency were co-transfected with p38-α and p38-β siRNAs (p38-α/MAPK14 siRNA: 5′-GGUCUCUGGAGGAAUUCAATT-3′ and p38-β/MAPK11 siRNA: 5′-GCCAUAGACCUCCUUGGAATT-3′ were synthesized by GenePharma, Suzhou, China), or mock transfected with only the transfection reagent, JetPRIME (Polyplus, Illkirch, France). Twenty-four hours post-transfection, cells were passaged to 48-well plates. Sixty hours post transfection, cells were infected by LASV-GP (strain Josiah)/HIV-luc in duplicate wells, and luciferase activity was tested 48 h post infection. At 60 h post-transfection, the infected cells were tested for p38 expression by Western blot (rabbit anti-p38 MAPK polyclonal antibody, Cat. 9212 from Cell Signaling Technology, Danvers, MA, USA; mouse anti-β-actin monoclonal antibody from OriGene Technologies, Inc., Rockville, MD, USA). The infectivity of LASV pseudovirus on p38 knock-down HEK293T cells was normalized to mock which is set as 100% infectivity.
2.8. Time-of-addition assay
U-87 MG cells were incubated with LASV-GP (strain Josiah)/HIV-luc at 4 °C for 2 h to permit attachment. The supernatant containing unattached virions was removed, and fresh medium containing 10% FBS was added to the wells. Losmapimod (30 μM), bafilomycin A1 (3 nM) and solvent control DMSO (0.1% v/v) were added to the cells at pre-attachment, during attachment, post-attachment, or during attachment and post-attachment of LASV-GP/HIV-luc. The cells were incubated in a 5% CO2 incubator at 37 °C for 2 days, after which the cells were lysed and luciferase activity was measured with a Luciferase Assay System. The assay was performed in duplicate wells for each compound concentration, and the mean and standard deviation were calculated by GraphPad Prism software.
2.9. Cell-cell fusion assay
HEK293T cells were co-transfected with a LASV-GP (strain Josiah) expression plasmid and pEGFP or with an LCMV-GP expression plasmid and pEGFP using jetPRIME transfection reagent according to the manufacturer's protocol (Polyplus, Illkirch, France). The transfected cells were seeded into 48-well plates 24 h post-transfection. Twenty-four hours later, the transfected cells were treated with PBS (pH 4.8) for 20 min. Losmapimod, ST-193, or bafilomycin A1 were added to the cells in triplicate wells 4 h before low pH treatment, during the 20-min low pH treatment, or after low-pH treatment (4 h). Cells were washed with PBS (pH 7.5) before and after low pH treatment, and cells were then replenished with fresh medium containing 10% FBS. Four hours later, syncytium formation was observed under a fluorescence microscope.
A T7 RNA polymerase-based luciferase reporter cell-cell fusion assay was also performed. Briefly, HEK293T cells were co-transfected with the T7 RNA polymerase expression plasmid pCAGGS-T7 and LASV-GP (strain Josiah) expression plasmid. U-87 MG cells were transfected with the firefly luciferase expression plasmid pT7EMCV-luc under the control of the T7 promoter. Twenty-four hours post-transfection, cells were passaged and co-cultured in 24-well plates (HEK293T:U-87 MG = 4:1). The co-cultured cells were treated with compounds prior to 20-min low pH treatment (PBS, pH 4.8). Cells were washed with PBS (pH 7.5) before and after low pH treatment, and 4 h later, cells were lysed and luciferase activity was determined. Cells treated with DMSO were used as 100% fusion. The assay was performed in triplicate wells for each compound concentration, and the mean and standard deviation were calculated by GraphPad Prism software.
2.10. LASV-GPmut construction, mutant pseudovirus production and compound activity assay
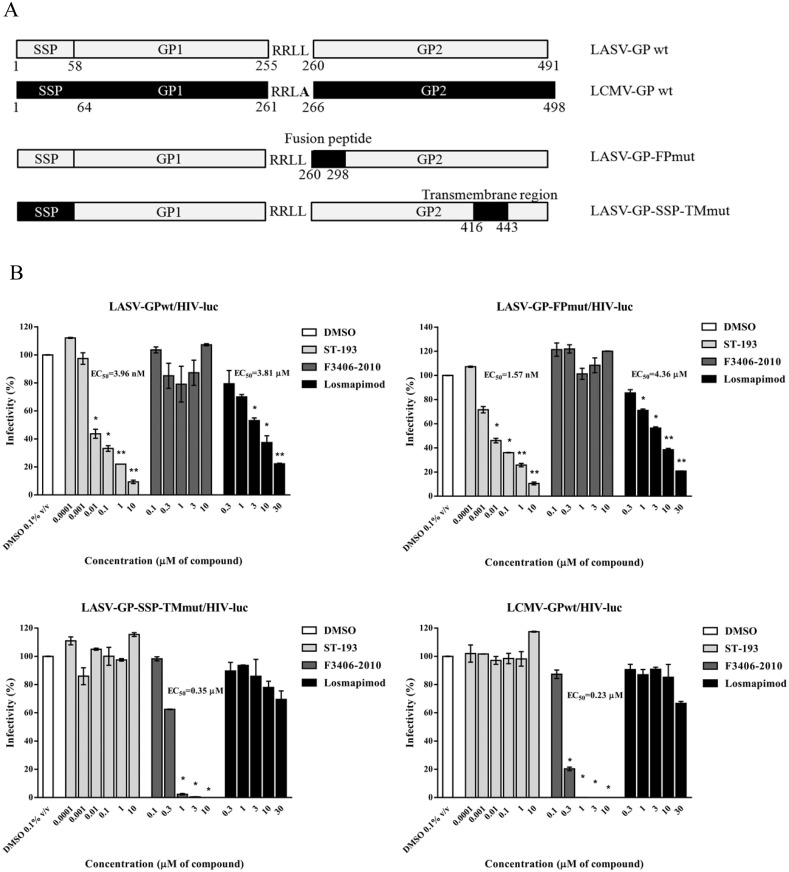
The LASV-GP (strain Josiah, GenBank accession no. NP_694870.1) fragment replacements by LCMV-GP (strain Arm53b, GenBank accession no. AAX49341.1) were created by overlapping PCR. The fusion peptide (FP, 5′ 826 nt to 5′ 879 nt, S276 to A293) of the LCMV-GP open reading frame were amplified by PCR and then fused to replace the corresponding LASV-GP region (FP, 5′ 808 nt to 5′ 861 nt, E270 to E287) to get LASV-GP-FPmut. The stable signal peptide (SSP, 5′ 19 nt to 5′ 174 nt, M7 to G58) and transmembrane domain (TM, 5′ 1264 nt to 5′ 1347 nt, R422 to V449) of the LCMV-GP open reading frame were amplified by PCR and then were both fused to replace the corresponding LASV-GP region (SSP, 5′ 19 nt to 5′ 174 nt, F7 to T58; TM, 5′ 1246 nt to 5′ 1329 nt, Q416 to I443) to get LASV-SSP-TMmut.
The constructed LASV-GP-FPmut and LASV-SSP-TMmut were then used to produce HIV based pseudoviruses, and these mutant viruses were used for compound activity assays as described in section 2.4.
2.11. Statistical analysis
Mean values, standard deviation (SD), and the half maximal effective concentration (EC50) values were calculated by GraphPad Prism software. Data were analyzed for statistical significance using a Student's t-test. The asterisks represent significant differences: *P < 0.05, and **P < 0.01.
3. Results
3.1. Losmapimod is an inhibitor of LASV entry
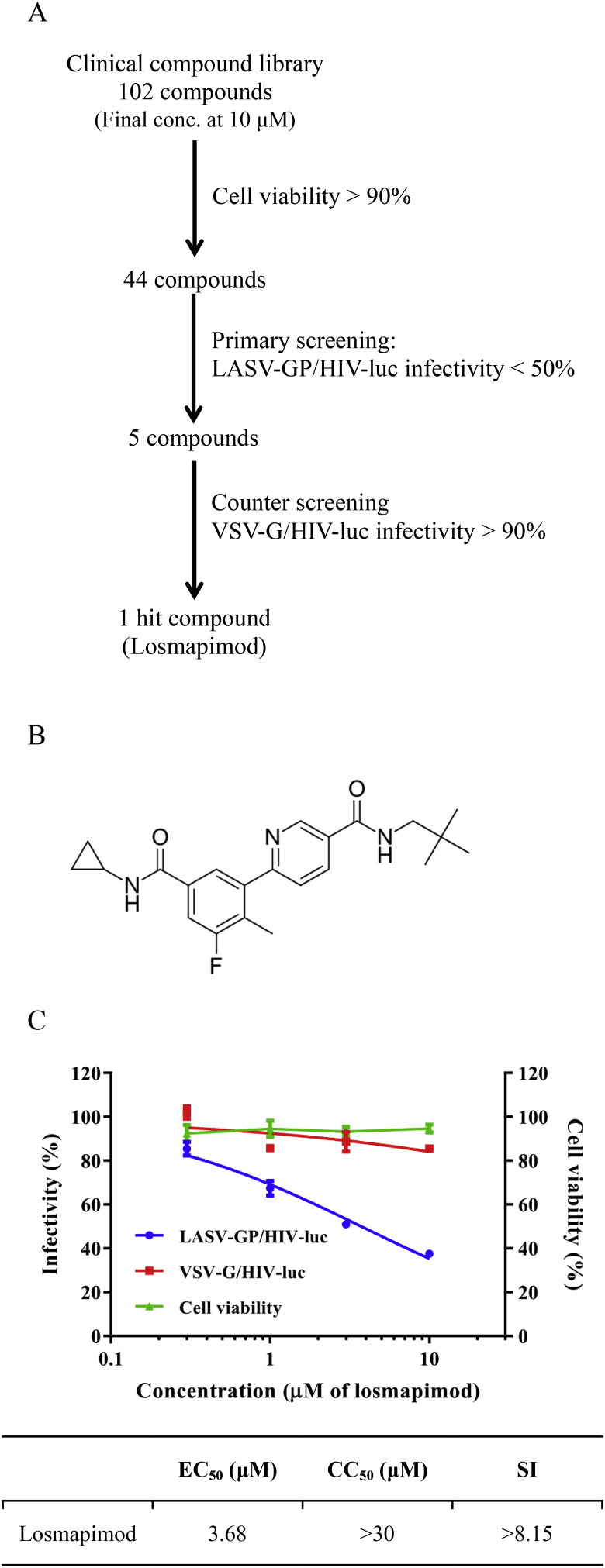
A library of 102 compounds that are in various stages of clinical trials was screened for anti-LASV (strain Josiah) activity (Fig. 1 A; Table S1). All compounds were first tested at a final concentration of 10 μM for cytotoxicity on HEK293T using the CellTiter-Glo assay, and 58 compounds causing over 10% reduction of cell viability were excluded from the remainder of the study. The rest of the compounds were then tested for their inhibitory effect on LASV using the HIV pseudotyped virus, LASV-GP/HIV-luc. HEK293T cells were treated with compound 15 min prior to LASV-GP/HIV-luc infection and compound inhibitory effect was calculated 48 h post infection in relation to the solvent control DMSO (0.1% v/v). After the initial screening, five compounds showed more than 50% inhibitory activity against LASV-GP/HIV-luc (Table S1). A vesicular stomatitis virus (VSV) glycoprotein pseudotyped HIV (VSV-G/HIV-luc) was used to exclude compounds that target the HIV-luc core. In the end, losmapimod (Fig. 1B) was the only compound with a specific inhibitory effect against LASV-GP/HIV-luc, exhibiting an EC50 of 3.68 μM (Fig. 1C). Losmapimod was also tested by using a recombinant VSV pseudotyped model and a similar result was obtained (Fig. S1).
Fig. 1.
Identification of losmapimod as a LASV entry inhibitor by clinical compound library screening. (A) Screening flowchart. (B) The chemical structure of losmapimod. (C) The effects of losmapimod on LASV-GP/HIV-luc and VSV-G/HIV-luc pseudovirus infection, as well as cell viability. The EC50, CC50 and SI values for losmapimod against LASV-GP/HIV-luc are provided. Library screening and losmapimod infectivity and cell viability assays were conducted using HEK293T cells.
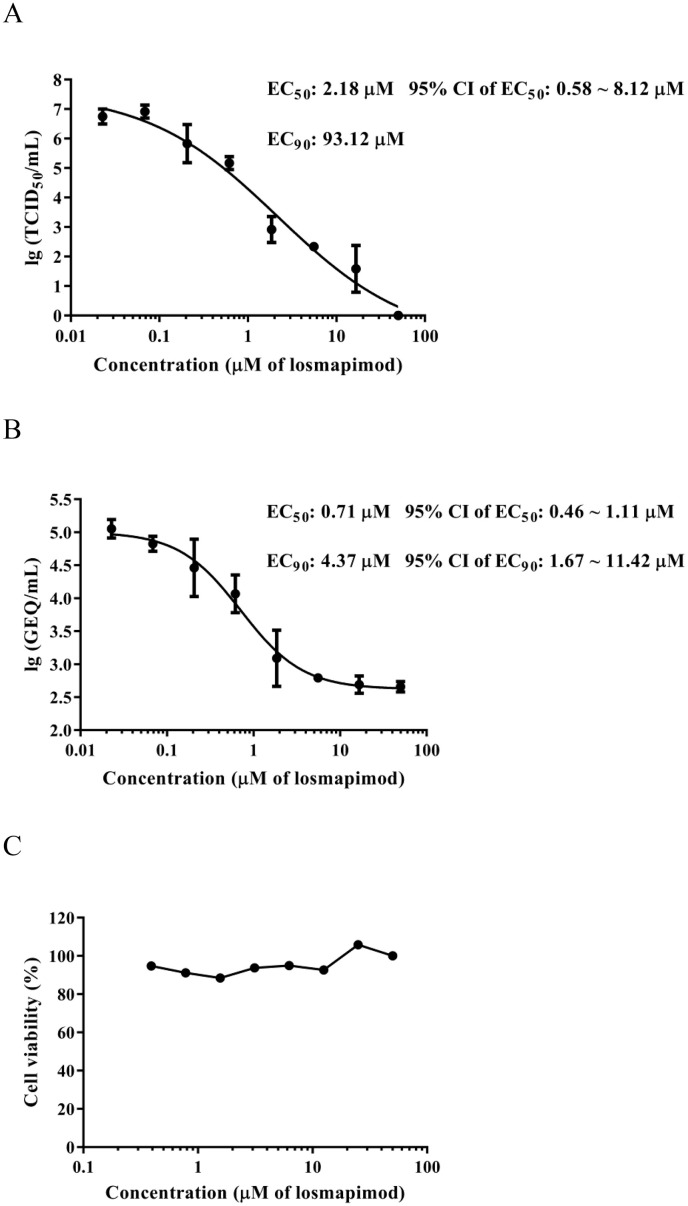
The use of an authentic virus infection assay is essential for the confirmation of compound activity. In this study, authentic LASV (strain Josiah) infection assays were conducted, and losmapimod exhibited activity against LASV infection on Vero E6 cells, as assessed by both TCID50 and RT-qPCR, with EC50 values of 2.18 μM and 0.71 μM, respectively (Fig. 2 ). These data are comparable to what was observed using LASV-GP/HIV-luc, and they demonstrate that losmapimod effectively inhibits authentic LASV.
Fig. 2.
Validation of the inhibitory activity of losmapimod against authentic LASV. Vero E6 cells were pretreated with serial dilutions of losmapimod (0.02–50 μM) and then infected at an MOI of 0.01 LASV (strain Josiah) in the presence of losmapimod. At 72 h post infection, cell culture supernatants were harvested and viral titration was determined by (A) TCID50 or (B) RT-qPCR. LASV titration was represented by genome equivalent (GEQ) per millilitre in the RT-qPCR method. EC50, 95% confidence intervals (CI) and EC90 values were calculated by GraphPad Prism software. (C) The effect of losmapimod on the cell viability of Vero E6 cells was determined by WST-1 assay at 72 h incubation endpoint.
To test whether the activity of losmapimod is cell type specific, losmapimod was then tested on cell lines originating from different tissues (such as lung, liver, and kidney, etc.) and animal species (human, hamster and monkey). The results showed that losmapimod is effective on all these cell lines tested (Fig. S2), indicating that the anti-LASV effect of this drug is not cell-type specific. Together, these data suggest that losmapimod is capable of inhibiting LASV infection by directly blocking viral entry.
3.2. Losmapimod preferentially inhibits LASV among arenaviruses known to cause human disease
LASV strain Josiah is a member of lineage IV of LASV (Bowen et al., 2000). To test the efficacy of losmapimod on other strains of LASV belonging to different lineages, we generated three additional HIV pseudotyped viruses each expressing a LASV-GP representative of a distinct lineage: strain LP (lineage I), strain 803213 (lineage II), and strain GA391 (lineage III). Losmapimod showed an inhibitory effect against all three viruses similar to what was observed against LASV strain Josiah, with EC50 values ranging from 0.9 to 2.7 μM (Table 1 and Fig. S3A). These data suggest that losmapimod is capable of inhibiting multiple different strains of LASV.
Table 1.
Losmapimod inhibits the entry of Lassa virus but not other arenaviruses.
| HIV based pseudotyped arenaviruses | Losmapimod |
ST-193 |
||||
|---|---|---|---|---|---|---|
| Infectivity (%) ± SD at 30 μM | EC50 (μM) | EC50 (μM) | 95% CI (μM) | |||
| OWAc | LASV | strain LP lineage I | 13.2 ± 0.7 | 2.7 | 0.024 | 0.015–0.038 |
| strain 803213 lineage II | 11.0 ± 0.2 | 1.0 | 0.0038 | 0.0020–0.0073 | ||
| strain GA391 lineage III | 9.7 ± 0.5 | 0.9 | 0.0047 | 0.0021–0.011 | ||
| strain Josiah lineage IV | 8.5 ± 0.6 | 1.7 | 0.0062 | 0.0043–0.0088 | ||
| LCMV | 93.7 ± 3.0 | / | NDa | / | ||
| LUJV | 99.2 ± 1.6 | / | NDb | / | ||
| NWAc | MACV | 70.7 ± 1.1 | / | 0.00091 | 0.00074–0.0011 | |
| WWAV | 67.0 ± 1.1 | / | 0.0036 | 0.0033–0.0039 | ||
| GTOV | 81.5 ± 1.2 | / | 0.00018 | 0.00015–0.00023 | ||
| JUNV | 55.8 ± 1.5 | / | 0.00040 | 0.00027–0.00059 | ||
| SABV | 85.5 ± 0.8 | / | 0.013 | 0.011–0.016 | ||
| CHPV | 78.1 ± 6.6 | / | 0.023 | 0.012–0.043 | ||
F3406-2010 was used as a positive compound for LCMV-GP/HIV-luc inhibition, with an EC50 of 0.19 μM.
Desipramine hydrochloride was used as a positive compound for LUJV-GP/HIV-luc infection, with an EC50 of 6.5 μM.
OWA: Old World Arenavirus; NWA: New World Arenavirus.
Since LASV is an Old World arenavirus that can cause viral hemorrhagic fever in humans, we next asked whether losmapimod also had the ability to inhibit other arenaviruses that can cause human disease. Eight arenaviruses, including two Old World arenaviruses (LCMV and Lujo virus) and six VHF-causing New World arenaviruses, were tested for their sensitivity to losmapimod using HIV-based pseudotyped arenaviruses. Arenavirus-GP/HIV-luc (including LCMV-GP/HIV-luc, LUJV-GP/HIV-luc, MACV-GP/HIV-luc, GTOV-GP/HIV-luc, JUNV-GP/HIV-luc, SABV-GP/HIV-luc, WWAV-GP/HIV-luc and CHPV-GP/HIV-luc) were used to infect A549 cells in the presence of losmapimod. As shown in Table 1, at 30 μM, the highest concentration tested, losmapimod preferentially inhibited LASV among these arenaviruses. The dose-response curves of losmapimod against eight other arenaviruses are shown in Fig. S3B.
3.3. Down-regulation of p38 MAPKs does not affect the anti-LASV activity of losmapimod
Losmapimod was initially developed as a p38 MAPK inhibitor intended for treatment of cardiovascular disease and COPD. P38 MAPKs are a class of mitogen-activated protein kinases that are responsive to stress stimuli, such as cytokines, ultraviolet irradiation, and heat shock, and are involved in cell differentiation, apoptosis and autophagy (Segales et al., 2016). Four p38 MAPKs have been identified, including p38-α (MAPK14), -β (MAPK11), -γ (MAPK12/ERK6), and -δ (MAPK13/SAPK4). Losmapimod is a selective inhibitor of p38-α (MAPK14) and -β (MAPK11) with EC50 values in the sub-micromolar range (Norman, 2015).
To determine whether the anti-LASV effect of losmapimod is mediated through p38 MAPK repression, we tested the infectivity of LASV-GP/HIV-luc on p38 knock-down HEK293T cells. As shown in Fig. S4, knock-down of p38 MAPK expression by co-transfection of p38-α and p38-β siRNAs did not hinder LASV-GP/HIV-luc infection, and the activity of losmapimod was unaffected. Moreover, three other p38 MAPK inhibitors were assessed for their anti-LASV activity, and none displayed any inhibitory activity against LASV-GP/HIV-luc infection (Table S2). Thus, our results indicate that the anti-LASV activity of losmapimod is not achieved through the inhibition of p38 MAPKs.
3.4. Losmapimod inhibits LASV-GP mediated viral fusion
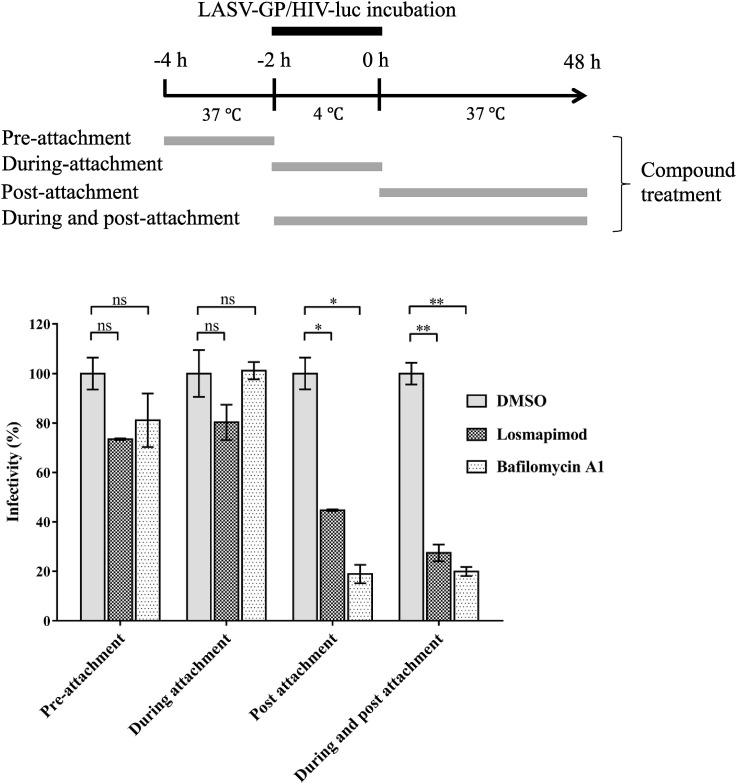
LASV entry initiates with recognition and binding of the cellular receptor by GP1, followed by endocytosis and low pH-triggered membrane fusion. To identify the step at which losmapimod exerts its effect, a time-of-addition assay was performed (Tai et al., 2015). Losmapimod was incubated with cells at pre-attachment, during attachment, post-attachment, or during & post-attachment of LASV-GP/HIV-luc (Fig. 3 ), and virus infectivity was determined 48 h post infection. Similar to the broadly active fusion inhibitor bafilomycin A1, a vacuolar-type H+-ATPase (V-ATPase) inhibitor that blocks endosomal acidification, losmapimod only inhibited LASV infection at the post-attachment stage (Fig. 3), indicating that it takes effect at the viral fusion step.
Fig. 3.
Losmapimod blocked LASV entry at the stage of post-attachment. Losmapimod, bafilomycin A1 and solvent control DMSO were added to the U-87MG cells as indicated. The infectivity of virus was determined 48 h post infection by measurement of luciferase activity. The experiments were repeated twice, and similar results were obtained. Statistical significance between two groups was calculated by Student's t-test using GraphPad Prism software, with statistical significance set at *P < 0.05 and **P < 0.01. ns: not significant (P ≥ 0.05).
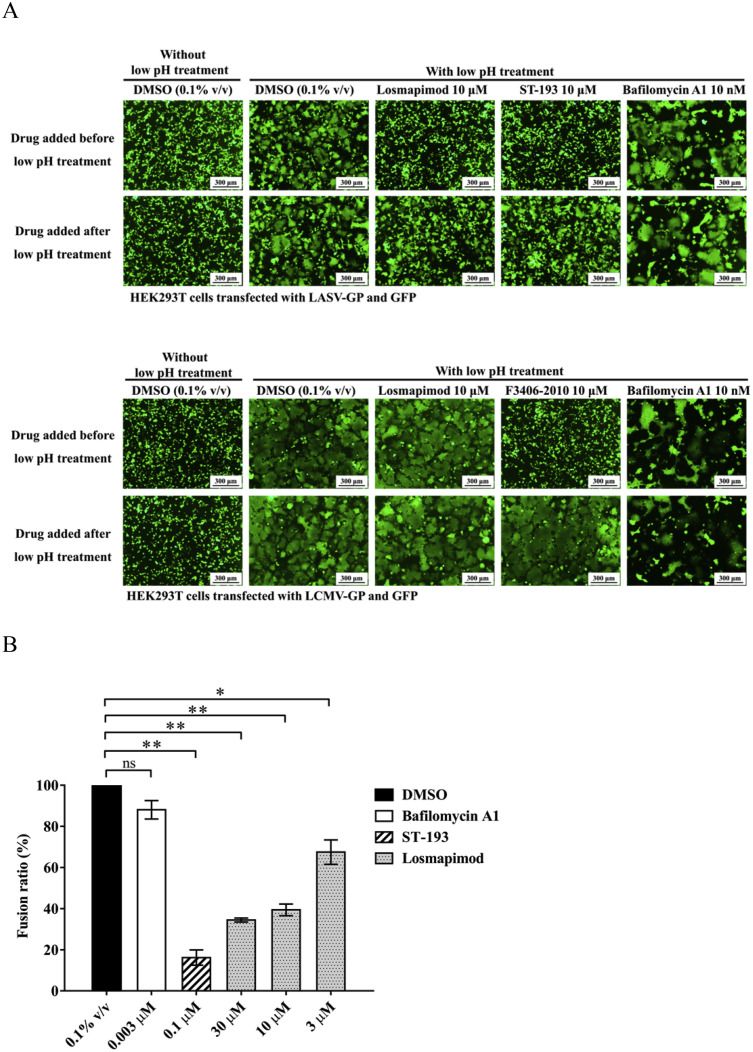
After GP1-receptor recognition and binding, LASV undergoes macropinocytosis followed by a GP2 conformational change triggered by the high proton concentration in the endosome (Oppliger et al., 2016). The conformational change leads to the exposure of the hydrophobic fusion peptide, resulting in fusion between the viral and endo-lysosomal membranes (White and Whittaker, 2016). To examine whether losmapimod could directly inhibit LASV-GP mediated fusion, a cell-cell fusion assay was performed. HEK293T cells expressing LASV-GP or LCMV-GP and the green fluorescent protein (GFP) were subjected to a low-pH treatment and syncytium formation was observed by fluorescence microscopy. ST-193 is known as a viral fusion inhibitor that targets LASV-GP2 (Larson et al., 2008), while F3406-2010 is a LCMV-specific fusion inhibitor (Ngo et al., 2015), and these two compounds were used as controls for the fusion assays. As mentioned before, the vacuolar-type H+-ATPase (V-ATPase) inhibitor, bafilomycin A1 was able to block LASV entry by preventing acidification of the endo-lysosome pathway (Fig. 3), but was unable to block cell-to-cell membrane fusion triggered directly by a high extracellular proton concentration, and thus was used as a negative control in the fusion assays (Fig. 4 ). As shown in Fig. 4A, losmapimod inhibited fusion that was mediated by LASV-GP but not LCMV-GP, which is consistent with the compound specificity displayed in Table 1. The same result was observed on a T7 RNA polymerase-based luciferase reporter cell-cell fusion assay (Fig. 4B). In this assay, losmapimod dose-dependently inhibited the reporter gene expression driven by T7 polymerase, which functioned in syncytium formed by LASV-GP mediated cell-cell fusion.
Fig. 4.
Losmapimod specifically inhibited low pH-triggered LASV-GP mediated cell-cell fusion. (A) Losmapimod inhibited low pH-triggered LASV-GP (strain Josiah), but not LCMV-GP mediated cell-cell fusion. HEK293T cells co-expressing LASV-GP or LCMV-GP and the enhanced green fluorescent protein (EGFP) were incubated with DMSO as solvent control, losmapimod, ST-193, and LCMV entry inhibitor F3406-2010 as indicated. The transfected cells were treated with PBS (pH 4.8) for 20 min. Syncytium formation was observed under a fluorescence microscope 4 h after low pH treatment. (B) Losmapimod inhibited LASV-GP mediated cell-cell fusion in a dose-dependent manner. HEK293T cells expressing T7 RNA polymerase and LASV-GP (strain Josiah) were fused with U-87 MG cells expressing firefly luciferase under the control of the T7 promoter following low pH trigger. Cells were treated with losmapimod (30 μM, 10 μM, 3 μM), bafilomycin A1 (3 nM) or ST-193 (100 nM) prior to low pH trigger. Four hours following low pH treatment, cells were lysed and luciferase activity was determined. Cells treated with DMSO (0.1% v/v) were used for 100% fusion. The experiments were repeated once, and similar results were obtained. Statistical significance between two groups was calculated by Student's t-test using GraphPad Prism software, with statistical significance set at *P < 0.05 and **P < 0.01. ns: not significant (P ≥ 0.05).
Our time-of-addition assay and cell-cell fusion assay results together showed that losmapimod acted similarly to ST-193, which suggests that losmapimod blocks the viral fusion process by affecting LASV-GP2 conformation.
3.5. Losmapimod targets the SSP-GP2 interface of LASV glycoprotein
The arenavirus GP complex is formed by noncovalent interactions between SSP, GP1, and GP2 subunits, of which the pH-sensing SSP-GP2 is critical to the viral fusion process. To determine if losmapimod acts on the LASV SSP-GP2 interaction, we created LASV-GP constructs with specific regions replaced by corresponding regions from LCMV-GP, which closely resembles LASV-GP but is insensitive to losmapimod. As shown in Fig. 5 A, the LASV-GP-FP mutant was constructed by replacing the LASV-GP fusion peptide (E270 to E287, LASV-GP numbering) with the LCMV fusion peptide (S276 to A293, LCMV-GP numbering); the LASV-GP-SSP-TM mutant was constructed by a replacement of both LASV-GP-SSP (F7 to T58, LASV-GP numbering) and LASV-GP-TM (Q416 to I443, LASV-GP numbering) with LCMV-GP-SSP (M7 to G58, LCMV-GP numbering) and LCMV-GP-TM (R422 to V449, LCMV-GP numbering). The LASV/LCMV-GP mutant constructs were then used to produce HIV-based pseudotyped viruses, which were used to test losmapimod activity. We used ST-193 and F3406-2010 as the reference compounds, which act on the SSP-TM region of LASV-GP and LCMV-GP respectively. As shown in Fig. 5B, consistent with previously published results (Larson et al., 2008; Ngo et al., 2015), as a LASV inhibitor, ST-193 retained its activity against LASV-GPwt/HIV-luc and LASV-GP-FPmut/HIV-luc, while it was ineffective at inhibiting LCMV-GPwt and LASV-GP-SSP-TMmut/HIV-luc. Conversely, the LCMV specific inhibitor F3406-2010 was ineffective at inhibiting LASV-GPwt/HIV-luc and LASV-GP-FPmut/HIV-luc, although it remained active against LCMV-GPwt/HIV-luc and LASV-GP-SSP-TMmut/HIV-luc. As shown in Fig. 5B, similar to ST-193, the activity of losmapimod against LASV-GP-FPmut/HIV-luc was comparable to that of LASV-GPwt/HIV-luc, indicating that the fusion peptide of LASV-GP2 is not the region targeted by losmapimod. Consequentially, losmapimod was ineffective against LASV-GP-SSP-TMmut/HIV-luc infection, suggesting that the SSP-TM region of LASV-GP is where losmapimod exerts its function.
Fig. 5.
Losmapimod inhibited LASV-GP entry by affecting SSP-GP2 interface. (A) A diagram of fragment replacement mutation based on protein sequence alignment between LASV-GP (strain Josiah) and LCMV-GP (strain Arm53b). The LASV-GP fragments replaced by the corresponding LCMV-GP fragments are indicated in black. (B) Losmapimod targets the SSP-TM region of LASV-GP. HEK293T cells were treated with losmapimod 15 min prior to virus infection and 48 h post infection, cells were lysed and luciferase activity was measured. DMSO solvent control was set as 100% infectivity, and statistical significance between each compound treatment and DMSO was calculated by Student's t-test, with statistical significance set at *P < 0.05 and **P < 0.01. The EC50 values were calculated by GraphPad Prism software.
4. Discussion
The lack of approved and effective therapeutics for Lassa hemorrhagic fever has created a dire need for the discovery of new antivirals. Drug repurposing has become an attractive approach in antiviral discovery and development in recent years (Ashburn and Thor, 2004). Losmapimod was developed as an oral p38 MAPK inhibitor for the treatment of COPD or cardiovascular diseases under phase II/III clinical investigations. Our p38 knock-down assay showed that the inhibitory effect of losmapimod against LASV entry is not dependent upon the down regulation of its original therapeutic target, p38 MAPK. We then considered whether losmapimod inhibited LASV entry by interacting with cellular receptors. Arenaviruses employ different cellular receptors for entry. The New World arenaviruses use human transferrin receptor (hTfR1) (Sarute and Ross, 2017), while Lujo virus, an Old World arenavirus, uses NRP2 and CD63 for entry (Raaben et al., 2017). Conversely, LCMV, which is a prototype arenavirus belonging to the Old World serotype, uses α-DG as the receptor for entry, the same as LASV (Cao et al., 1998). Among the nine arenaviruses tested in our study, losmapimod only displayed activity against LASV. Since losmapimod inhibited the entry of LASV but not LCMV, despite the fact that the two viruses share the same cellular receptor, we postulate that losmapimod is not targeting the cell surface receptor α-DG but rather LASV GPC itself.
The fact that losmapimod could directly inhibit LASV-GP mediated fusion in an artificial extracellular low pH condition suggests that the inhibitory effect of losmapimod on LASV fusion is not achieved by affecting the proton influx. Instead, these data imply a direct interaction with the LASV glycoprotein. The unique SSP-GP2 interface of the arenavirus glycoprotein is crucial for viral fusion and is therefore an attractive target for antivirals, as many previously discovered arenavirus entry inhibitors, such as ST-193, F3406-2010, lassamycin-1 and lacidipine, all target this sensitive domain (Shankar et al., 2016; Thomas et al., 2011; Wang et al., 2018; York et al., 2008). Our fragment replacing mutational study of LASV-GP and LCMV-GP demonstrated that losmapimod also targets the LASV-SSP-GP2 interface. Interestingly, losmapimod showed relatively similar inhibitory activity among four strains of LASV, while ST-193 was less efficacious against LASV strain LP (Table 1 and Fig. S3A). Sequence analyses revealed that GP from LASV strain LP possessed an isoleucine at position 435 (within the TM region), whereas the GPs from the three other LASV strains possessed a valine at this position (Fig. S6). Not only does this suggest that the amino acid at position 435 plays a critical role in resistance to ST-193, which is consistent with a previous report (Larson et al., 2008), but it also implies that the mode of function of losmapimod is different from that of ST-193 and that the sequence variation within the SSP region does not affect losmapimod activity. Previous studies have demonstrated that mutations within the SSP or TM regions confer resistance to drugs such as lacidipine (Wang et al., 2018) and ST-193 (Larson et al., 2008), and it will be interesting to determine whether similar mutations arise in response to treatment with losmapimod.
A major benefit of drug repurposing, compared with the traditional de novo discovery process, is the high success rate due to available safety data from clinical studies (Ashburn and Thor, 2004). As a repurposed drug, the dose regimens for new applications are often required to be comparable or lower than the original therapeutic dose. In this study, the efficacy of losmapimod against LASV in vitro (EC50: 0.71–3.68 μM, Fig. 1, Fig. 2) was not as potent as that against its original target p38 MAPK (EC50: 25 nM, Table S2), making it difficult to repurpose losmapimod as a treatment of Lassa hemorrhagic fever. However, as an aryl heteroaryl bis-carboxyamide derivative, a structurally distinctive LASV fusion inhibitor, losmapimod is also a cation amphiphilic drug (CAD) with a logP of 4.012 (Funk and Krise, 2012), which potentially enables it to accumulate in the endo-lysosomal compartments, where it displays its anti-LASV fusion effect. Indeed, these unique structural characteristics make losmapimod a valuable lead for the future development of anti-LASV agents.
Conflicts of interest
The authors declare no conflicts of interests.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Nos. 81473256 and 81273561), the CAMS Innovation Fund for Medical Sciences (No. 2016-I2M-1-014), the Science and Technology Program of Beijing (No. Z151100000115008), the Beijing Key Laboratory of New Drug Mechanisms and Pharmacological Evaluation Study (No. BZ0150), and the Drug Innovation Major Project (No. 2015ZX09102-023 and No. 2018ZX09711001-003-002). This work was partially supported by the Public Health Agency of Canada and CIHR grant IER-143487 to Xiangguo Qiu.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.antiviral.2019.03.014.
Contributor Information
Xiangguo Qiu, Email: xiangguo.qiu@canada.ca.
Ying Guo, Email: yingguo6@imm.ac.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Acciani M., Alston J.T., Zhao G., Reynolds H., Ali A.M., Xu B., Brindley M.A. Mutational analysis of Lassa virus glycoprotein highlights regions required for alpha-dystroglycan utilization. J. Virol. 2017;91 doi: 10.1128/JVI.00574-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburn T.T., Thor K.B. Drug repositioning: identifying and developing new uses for existing drugs. Nat. Rev. Drug Discov. 2004;3:673–683. doi: 10.1038/nrd1468. [DOI] [PubMed] [Google Scholar]
- Bederka L.H., Bonhomme C.J., Ling E.L., Buchmeier M.J. Arenavirus stable signal peptide is the keystone subunit for glycoprotein complex organization. mBio. 2014;5 doi: 10.1128/mBio.02063-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen M.D., Rollin P.E., Ksiazek T.G., Hustad H.L., Bausch D.G., Demby A.H., Bajani M.D., Peters C.J., Nichol S.T. Genetic diversity among Lassa virus strains. J. Virol. 2000;74:6992–7004. doi: 10.1128/jvi.74.15.6992-7004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burri D.J., Pasquato A., da Palma J.R., Igonet S., Oldstone M.B., Kunz S. The role of proteolytic processing and the stable signal peptide in expression of the Old World arenavirus envelope glycoprotein ectodomain. Virology. 2013;436:127–133. doi: 10.1016/j.virol.2012.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W., Henry M.D., Borrow P., Yamada H., Elder J.H., Ravkov E.V., Nichol S.T., Compans R.W., Campbell K.P., Oldstone M.B. Identification of alpha-dystroglycan as a receptor for lymphocytic choriomeningitis virus and Lassa fever virus. Science. 1998;282:2079–2081. doi: 10.1126/science.282.5396.2079. [DOI] [PubMed] [Google Scholar]
- Carrillo-Bustamante P., Nguyen T.H.T., Oestereich L., Gunther S., Guedj J., Graw F. Determining Ribavirin's mechanism of action against Lassa virus infection. Sci. Rep. 2017;7:11693. doi: 10.1038/s41598-017-10198-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q., Tang K., Zhang X., Chen P., Guo Y. Establishment of pseudovirus infection mouse models for in vivo pharmacodynamics evaluation of filovirus entry inhibitors. Acta Pharm. Sin. B. 2018;8:200–208. doi: 10.1016/j.apsb.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng F., Murray J.L., Rubin D.H. Drug repurposing: new treatments for Zika virus infection? Trends Mol. Med. 2016;22:919–921. doi: 10.1016/j.molmed.2016.09.006. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . fifth ed. 2010. Biosafety in Microbiological and Biomedical Laboratories; pp. 246–265. [Google Scholar]
- Dyall J., Coleman C.M., Hart B.J., Venkataraman T., Holbrook M.R., Kindrachuk J., Johnson R.F., Olinger G.G., Jr., Jahrling P.B., Laidlaw M., Johansen L.M., Lear-Rooney C.M., Glass P.J., Hensley L.E., Frieman M.B. Repurposing of clinically developed drugs for treatment of Middle East respiratory syndrome coronavirus infection. Antimicrob. Agents Chemother. 2014;58:4885–4893. doi: 10.1128/AAC.03036-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk R.S., Krise J.P. Cationic amphiphilic drugs cause a marked expansion of apparent lysosomal volume: implications for an intracellular distribution-based drug interaction. Mol. Pharm. 2012;9:1384–1395. doi: 10.1021/mp200641e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houlihan C., Behrens R. Lassa fever. BMJ. 2017;358:j2986. doi: 10.1136/bmj.j2986. [DOI] [PubMed] [Google Scholar]
- Igonet S., Vaney M.C., Vonrhein C., Bricogne G., Stura E.A., Hengartner H., Eschli B., Rey F.A. X-ray structure of the arenavirus glycoprotein GP2 in its postfusion hairpin conformation. Proc. Natl. Acad. Sci. U. S. A. 2011;108:19967–19972. doi: 10.1073/pnas.1108910108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jae L.T., Raaben M., Herbert A.S., Kuehne A.I., Wirchnianski A.S., Soh T.K., Stubbs S.H., Janssen H., Damme M., Saftig P., Whelan S.P., Dye J.M., Brummelkamp T.R. Virus entry. Lassa virus entry requires a trigger-induced receptor switch. Science. 2014;344:1506–1510. doi: 10.1126/science.1252480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen L.M., DeWald L.E., Shoemaker C.J., Hoffstrom B.G., Lear-Rooney C.M., Stossel A., Nelson E., Delos S.E., Simmons J.A., Grenier J.M., Pierce L.T., Pajouhesh H., Lehar J., Hensley L.E., Glass P.J., White J.M., Olinger G.G. A screen of approved drugs and molecular probes identifies therapeutics with anti-Ebola virus activity. Sci. Transl. Med. 2015;7 doi: 10.1126/scitranslmed.aaa5597. 290ra289. [DOI] [PubMed] [Google Scholar]
- Knipe D.M., Howley P. sixth ed. 2013. Fields Virology. 1283 - 1281 1303. [Google Scholar]
- Kouznetsova J., Sun W., Martinez-Romero C., Tawa G., Shinn P., Chen C.Z., Schimmer A., Sanderson P., McKew J.C., Zheng W., Garcia-Sastre A. Identification of 53 compounds that block Ebola virus-like particle entry via a repurposing screen of approved drugs. Emerg. Microb. Infect. 2014;3:e84. doi: 10.1038/emi.2014.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson R.A., Dai D., Hosack V.T., Tan Y., Bolken T.C., Hruby D.E., Amberg S.M. Identification of a broad-spectrum arenavirus entry inhibitor. J. Virol. 2008;82:10768–10775. doi: 10.1128/JVI.00941-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madrid P.B., Chopra S., Manger I.D., Gilfillan L., Keepers T.R., Shurtleff A.C., Green C.E., Iyer L.V., Dilks H.H., Davey R.A., Kolokoltsov A.A., Carrion R., Jr., Patterson J.L., Bavari S., Panchal R.G., Warren T.K., Wells J.B., Moos W.H., Burke R.L., Tanga M.J. A systematic screen of FDA-approved drugs for inhibitors of biological threat agents. PLoS One. 2013;8 doi: 10.1371/journal.pone.0060579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messina E.L., York J., Nunberg J.H. Dissection of the role of the stable signal peptide of the arenavirus envelope glycoprotein in membrane fusion. J. Virol. 2012;86:6138–6145. doi: 10.1128/JVI.07241-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo N., Henthorn K.S., Cisneros M.I., Cubitt B., Iwasaki M., de la Torre J.C., Lama J. Identification and mechanism of action of a novel small-molecule inhibitor of arenavirus multiplication. J. Virol. 2015;89:10924–10933. doi: 10.1128/JVI.01587-15. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Nigeria Centre for Disease Control . 2019. An Update of Lassa Fever Outbreak in Nigeria.http://www.ncdc.gov.ng/diseases/sitreps/ [Google Scholar]
- Norman P. Investigational p38 inhibitors for the treatment of chronic obstructive pulmonary disease. Expert Opin. Investig. Drugs. 2015;24:383–392. doi: 10.1517/13543784.2015.1006358. [DOI] [PubMed] [Google Scholar]
- Oestereich L., Rieger T., Ludtke A., Ruibal P., Wurr S., Pallasch E., Bockholt S., Krasemann S., Munoz-Fontela C., Gunther S. Efficacy of favipiravir alone and in combination with ribavirin in a lethal, immunocompetent mouse model of Lassa fever. J. Infect. Dis. 2016;213:934–938. doi: 10.1093/infdis/jiv522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldstone M.B. Arenaviruses. I. The epidemiology molecular and cell biology of arenaviruses. Introduction. Curr. Top. Microbiol. Immunol. 2002;262:V–XII. [PubMed] [Google Scholar]
- Oppliger J., Torriani G., Herrador A., Kunz S. Lassa virus cell entry via dystroglycan involves an unusual pathway of macropinocytosis. J. Virol. 2016;90:6412–6429. doi: 10.1128/JVI.00257-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radoshitzky S.R., Bao Y., Buchmeier M.J., Charrel R.N., Clawson A.N., Clegg C.S., DeRisi J.L., Emonet S., Gonzalez J.P., Kuhn J.H., Lukashevich I.S., Peters C.J., Romanowski V., Salvato M.S., Stenglein M.D., de la Torre J.C. Past, present, and future of arenavirus taxonomy. Arch. Virol. 2015;160:1851–1874. doi: 10.1007/s00705-015-2418-y. [DOI] [PubMed] [Google Scholar]
- Raaben M., Jae L.T., Herbert A.S., Kuehne A.I., Stubbs S.H., Chou Y.Y., Blomen V.A., Kirchhausen T., Dye J.M., Brummelkamp T.R., Whelan S.P. NRP2 and CD63 are host factors for Lujo virus cell entry. Cell Host Microbe. 2017;22 doi: 10.1016/j.chom.2017.10.002. 688-696 e685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed L.J., Muench H. A simple method of estimating fifty per cent endpoints12. Am. J. Epidemiol. 1938;27:493–497. [Google Scholar]
- Safronetz D., Rosenke K., Westover J.B., Martellaro C., Okumura A., Furuta Y., Geisbert J., Saturday G., Komeno T., Geisbert T.W., Feldmann H., Gowen B.B. The broad-spectrum antiviral favipiravir protects Guinea pigs from lethal Lassa virus infection post-disease onset. Sci. Rep. 2015;5:14775. doi: 10.1038/srep14775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarute N., Ross S.R. New World arenavirus biology. Ann. Rev. Virol. 2017;4:141–158. doi: 10.1146/annurev-virology-101416-042001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segales J., Perdiguero E., Munoz-Canoves P. Regulation of muscle stem cell functions: a focus on the p38 MAPK signaling pathway. Front. Cell Dev. Biol. 2016;4:91. doi: 10.3389/fcell.2016.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar S., Whitby L.R., Casquilho-Gray H.E., York J., Boger D.L., Nunberg J.H. Small-molecule fusion inhibitors bind the pH-sensing SSP-GP2 subunit interface of the Lassa virus envelope glycoprotein. J. Virol. 2016;90:6799–6807. doi: 10.1128/JVI.00597-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai C.J., Li C.L., Tai C.J., Wang C.K., Lin L.T. Early viral entry assays for the identification and evaluation of antiviral compounds. J. Vis. Exp. 2015 doi: 10.3791/53124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang K., He S., Zhang X., Guo J., Chen Q., Yan F., Banadyga L., Zhu W., Qiu X., Guo Y. Tangeretin, an extract from Citrus peels, blocks cellular entry of arenaviruses that cause viral hemorrhagic fever. Antivir. Res. 2018;160:87–93. doi: 10.1016/j.antiviral.2018.10.011. [DOI] [PubMed] [Google Scholar]
- Tani H., Iha K., Shimojima M., Fukushi S., Taniguchi S., Yoshikawa T., Kawaoka Y., Nakasone N., Ninomiya H., Saijo M., Morikawa S. Analysis of Lujo virus cell entry using pseudotype vesicular stomatitis virus. J. Virol. 2014;88:7317–7330. doi: 10.1128/JVI.00512-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C.J., Casquilho-Gray H.E., York J., DeCamp D.L., Dai D., Petrilli E.B., Boger D.L., Slayden R.A., Amberg S.M., Sprang S.R., Nunberg J.H. A specific interaction of small molecule entry inhibitors with the envelope glycoprotein complex of the Junin hemorrhagic fever arenavirus. J. Biol. Chem. 2011;286:6192–6200. doi: 10.1074/jbc.M110.196428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Liu Y., Zhang G., Wang S., Guo J., Cao J., Jia X., Zhang L., Xiao G., Wang W. Screening and identification of Lassa virus entry inhibitors from an FDA-approved drug library. J. Virol. 2018;92 doi: 10.1128/JVI.00954-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner B.M., Safronetz D., Stein D.R. Current research for a vaccine against Lassa hemorrhagic fever virus. Drug Des. Dev. Ther. 2018;12:2519–2527. doi: 10.2147/DDDT.S147276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J.M., Whittaker G.R. Fusion of enveloped viruses in endosomes. Traffic. 2016;17:593–614. doi: 10.1111/tra.12389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- York J., Dai D., Amberg S.M., Nunberg J.H. pH-induced activation of arenavirus membrane fusion is antagonized by small-molecule inhibitors. J. Virol. 2008;82:10932–10939. doi: 10.1128/JVI.01140-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Tang K., Guo J., Chen Q., Guo Y. Establishment of a cell-based evaluation system for arenavirus entry inhibitor. Acta Pharm. Sin. 2018;53(5):735–742. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.