Abstract
Liposomes are widely utilized as a carrier to improve therapeutic efficacy of agents thanks to their merits of high loading capacity, targeting delivery, reliable protection of agents, good biocompatibility, versatile structure modification and adjustable characteristics, such as size, surface charge, membrane flexibility and the agent loading mode. In particular, in recent years, through modification with immunopotentiators and targeting molecules, and in combination with innovative immunization devices, liposomes are rapidly developed as a multifunctional vaccine adjuvant-delivery system (VADS) that has a high capability in inducing desired immunoresponses, as they can target immune cells and even cellular organelles, engender lysosome escape, and promote Ag cross-presentation, thus enormously enhancing vaccination efficacy. Moreover, after decades of development, several products developed on liposome VADS have already been authorized for clinical immunization and are showing great advantages over conventional vaccines. This article describes in depth some critical issues relevant to the development of liposomes as a VADS, including principles underlying immunization, physicochemical properties of liposomes as the immunity-influencing factors, functional material modification to enhance immunostimulatory functions, the state-of-the-art liposome VADSs, as well as the marketed vaccines based on a liposome VADS. Therefore, this article provides a comprehensive reference to the development of novel liposome vaccines.
Keywords: Vaccine adjuvant-delivery system, Mucosal vaccination, Pathogen-associated molecular pattern, Antigen cross-presentation, Immunoresponse, Cellular immunity
Abbreviations: PE, Phosphatidylethanolamine; CSP, Circumsporozoite protein of the Plasmodium falciparum; VLP, Virus-like particles; DNR, Daunorubicin; Ara-C, Cytosine arabinoside; siRNA, Small interfering RNA.
Graphical abstract
Highlights
-
•
Liposomes are rapidly developed as a potent vaccine adjuvant-delivery system.
-
•
Liposomes can be modified with functional molecules and combined with innovative immunization device.
-
•
Liposomes can fulfil functions of target delivery, lysosome escape and cross-presentation.
-
•
Several liposome VADS-based products have been authorized for clinical immunization.
1. Introduction
Nowadays vaccination is considered the most economical strategy for handling various diseases, such as microbial infections, autoimmune relevant disorders, and even certain types of cancer [1]. Annually, millions of human lives are saved from lethal infections as a result of prompt immunization with vaccines, which are also playing roles in conquering other life-threatening diseases such as autoimmune disorders, allergic reactions. Notably, in the past years, some refractory cancers are also included in the scope of vaccination targets as they are subject to immunotherapy, which achieves great therapeutic effects in certain patients, thanks to advances made in immunology, biotechnology and relevant fields [2]. However, today there is still a long list of infections in urgent need of effective vaccines, which unfortunately, may not exist yet in at least a foreseeable future. The reason is that many microbial pathogens, such as HIV (human immunodeficiency virus), HCV (hepatitis C virus) and HSV (herpes simplex virus), are showing an elusive or ever-changing immunogenic feature on their appearance to continuously challenge the currently available vaccine strategies while dismantling a variety of potential arsenals. Meanwhile, some pathogens, such as Ebola virus and SARS virus (severe acute respiratory syndrome virus), may emerge abruptly in an uncertain place to make a ravage in people and then disappear before an effective strategy having been drawn up for the trial of battling them, leaving even no trace as a hint for developing an efficacious product able to tame them [3,4]. Nevertheless, the past few decades have witnessed a range of lethal infectious pathogens, such as smallpox, HPV, HBV and VZV (varicella zoster virus), that have been, or nearly, conquered by the newly developed medications including, especially, vaccines [5,6]. In particular, in the past few years immunotherapy has been established as a promising strategy in treating some refractory diseases, including certain lethal cancers, inherited disease, anaphylaxis, and disorders of autoimmunity, such as rheumatoid arthritis, multiple sclerosis and lupus, which, though are still torturing numerous patients, also wane in intensity and mortality due to the kind of vaccination treatment [7].
Vaccination as well as immunotherapy acts to battle diseases through making use of host immune system, which can be activated by the antigenic components (called antigens) of disease-causing subjects (called pathogens) to set up immunity capable of erasing the pathogens bearing the identical antigens (Ags). These processes involved in immunity establishment present the principle for developing vaccines, which however can be made with different formulations and display diverse dosage forms. Conventional vaccines are made of whole microbes that have been inactivated or attenuated and usually possess a potent power to induce the Ag-specific immunoresponses in hosts, but they are also linked to safety issues resulted mainly from reversion of administered strain to virulent mutants, as supported by the outcomes of gene sequencing in the vaccinated sufferers [8]. Notably, in the past decades, great achievements in immunology, cell biology, as well as bioengineering technology allow rapid identification and purification of novel Ags. The refined Ags are capable of being formulated with definitive ingredients to constitute subunit vaccines that can elicit the immunity to accurately target the highly matched objects while maintaining an excellent safety profile. Thus, subunit vaccines are now more and more employed to fight various diseases, including not only infections but also intractable autoimmunity and malicious cancer [9]. Compared to classical vaccines, subunit vaccines possess many distinct benefits, such as high safety, needing no dangerous microorganisms in production; very few redundant components relevant to reactogenicity; diverse therapy scope including infections, autoimmunity and cancer; feasibility of engaging several peptide epitopes in one product targeting different subtypes or life cycle stages of a pathogen [10]. However, subunit vaccines just made of highly purified Ags are often showing poor immunogenicity and therefore can hardly induce effective immunoresponses in the absence of an adjuvant, a substance that, when included in the formulation, enables a vaccine to efficiently elicit the Ag-specific immunity with a high level and long duration ensuring a reliable anti-Ag protection [11]. Moreover, subunit vaccines lack most microbial constituents including, especially, those that are in fact the adjuvanticity-bearing substances conservatively displayed on microbe surfaces while manifesting certain distinctive structural features, defined as the pathogen/damage/danger-associated molecular patterns (PAMPs/DAMPs). PAMPs or DAMPs can strongly activate the innate immunoresponses through binding to and stimulate the corresponding pattern recognition receptors (PRRs), including mainly TLRs (Toll-like receptors), CLRs (C-type lectin receptors), RLRs (retinoic-acid-inducible gene-I-like receptor), NLRs (The nucleotide-binding oligomerization domain-like receptors), and the newly identified cGAS (cGMP-AMP synthase, a cytosolic DNA sensor [12]). PRRs are, in response to detection of pathogenic microbes, evolutionarily expressed by immune cells, especially, the professional APCs (antigen presentation cells), including mainly DCs (dendritic cells) and MPs (macrophages). As such, due to lack of PAMPs/DAMPs subunit vaccines usually have a low capacity in activating the innate immune system, which is a precursor and sponsor responsible for initiating the adaptive immune system for setting up the anti-Ag immunity. Therefore, to conquer this drawback, subunit vaccines are often additionally combined with an adjuvant or incorporated in a carrier to enhance their immunostimulatory effects [13]. Notably, at present subunit vaccines are frequently constituted with various nanocarriers, such as liposomes, emulsions, cochleates, VLPs (virus-like particles), polymeric and inorganic nanoparticles (NPs), which can be further incorporated diversely with adjuvants or the adjuvanticity-bearing substances, such as different PRRas (PRR agonists), squalene, saponin as well as insoluble aluminium salts (alums), thus forming a vaccine adjuvant-delivery system (VADS) to enhance efficacy [[14], [15], [16], [17], [18]]. In fact, in as early as 1926, significant enhancement of efficacy of subunit vaccines, such as the one composed of diphtheria toxoid, was successfully achieved by Glenny and colleagues through precipitation of the Ag onto alum, which has ever since been widely adopted as an efficient strategy to improve vaccine effectiveness. As a result, alum has been employed as a classical VADS to produce various types of vaccines for clinical prophylaxis of a range of pathogens and today is still given to millions of people each year [19].
Actually, a VADS constructed with a nanocarrier proves able to fulfil multiple functions, including protecting Ags from premature degradation, maintaining ingredients with prolonged release, targeting of cargos to professional APCs, and boosting vaccines in immunostimulatory effects, thus making full use of bioactive agents to save dose. Among different categories of nanocarriers, liposomes since their discovery in 1960s [20] have fervently been exploited for constructing a DDS (drug delivery systems) or VADS owing to their many advantages, such as excellent biocompatibility, diverse loading mode, big loading capacity, easy preparation and surface modification to generate desired functions [21,22]. Also, as a VADS, liposomes are suitable for making the vaccines that are adaptive to various administration routes, such as intramuscular and subcutaneous injection, oral uptake, intranasal and other topical ways [23]. Moreover, liposomes have long been confirmed of the intrinsic adjuvanticities and, unlike other adjuvants, have showed minimal reactogenicity, therefore rarely causing hypersensitivity-associated reactions in immunized subjects [[24], [25], [26]]. In addition, it is argued that liposomes can always play well the role of an adjuvant regardless of the Ag loading mode of being entrapped within, attached onto, or even simply admixed with the vesicles, thus greatly simplifying the product manufacturing procedure owing to omitting a tedious step of removing free Ags [27,28]. Also, liposomes have been extensively explored to be combined with a variety of functional molecules, such as the ones bearing a PAMP/DAMP feature able to binding to certain PRRs, and the ones matching the receptors expressed on APCs to facilitate APC uptake of vaccines [16,17,[29], [30], [31]]. Notably, after decades of development, several type of liposomes designed as a VADS have already been successfully employed to make vaccines that have been approved for clinical immunization against different infections, including even those that are caused by the most intractable pathogens, such as malaria and shingles [5].
This review paper describes the knowledge of significance on liposomes used as a VADS, including principles on immunization and design of a liposome VADS, features and properties of liposomes that are beneficial for constructing a VADS, the state-of-the-art advances in liposome VADS, and also the approved vaccines manufactured with the most advanced liposome technology, to present a comprehensive reference on liposome vaccines.
2. Principles underlying vaccine prophylaxis of infectious diseases
The modern vaccine concept had been gradually established since the late eighteenth century when Edward Jenner took pus from the hands of milkmaids with cowpox and scratched it into the arm of some recipients, who were thus successfully protected from smallpox infection [1,32]. By doing so, Jenner preliminarily laid the primary principle for developing modern vaccines, which contain antigens (Ags) and after administration will trigger host immune system to set up a defensive network against the Ag-exhibiting pathogens. The supportive basis under establishing this defence lies in that in vertebrates and higher organisms including human beings, the complex immune system is well formed by long time survival evolution under pressure from pathogens and thus, consisting of several orders of differentiated structures and compositions, including lymphoid organs and tissues, diverse immune cells, and various chemokines and cytokines, which orchestrate to engender protective immunity [33].
In the case of dangerous signal stimulation sparked by vaccines or pathogens, the immune system will function through motivating its components to act together under influences from each other and to form throughout the body a solid defence against pathogens. As one of the main defensive components, the immune system possesses the abilities to discern, from numerous contacting subjects, the abnormal pathogens as a detrimental factor to the body through interacting with the subjects' surface structures. These structures on pathogens are usually composed of glycoproteins and may show, depending on the type of species, certain unique conserved features falling just within the range of “antigens” or “PAMPs/DAMPs”, which are evolutionarily “defined” by the host immune system as a result of the long term fights repeatedly occurred between the opposite sides of host and pathogens. Gradually, the immune system builds up the power of defence against the detrimental invaders, including various infectious microorganisms such as bacteria, viruses, fungi and parasites, and even cancerous neoplasms arising from the body itself [32,34].
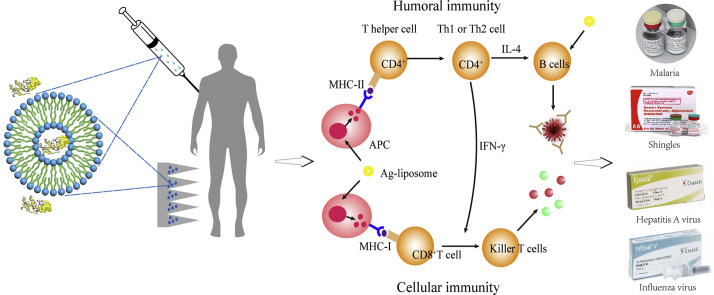
Typically, when Ags carried by a vaccine/pathogen enter a vertebrate host for the first time via the (breached) skin or mucosal barrier [35], they will be immediately recognized and internalized by tissue-resident APCs (Fig. 1 ), mainly DCs and MPs, but also Langerhans cells (a unique type of MPs inhabiting in the stratified squamous epithelium of skin and various type 2 mucosal tissues, i.e., the stratified epithelium-covered mucosa, and displaying a remarkable mixture of properties of MPs, e.g., self-maintain locally, and DCs, e.g., homeostatically migrate to draining lymph nodes and present Ags to T lymphocytes) [36]. Also, a fraction of the Ags (with the vaccine/pathogen) may traffic directly into draining lymph nodes (dLNs) wherein to be captured and endocytosed by the within APCs, including DCs, MPs and follicular B cells (FBCs). Then, the internalized Ags will be processed by APCs into smaller pieces including the epitopes (the antigenic determinants) that will be loaded onto MHC (major histocompatibility complex) molecules and then together displayed on cell surface for presentation to T lymphocytes which takes place within dLNs (Fig. 1). In the case of direct blood entrance caused by, e.g., injection vaccination, blood infusion contamination, external bleeding and insect bite, Ags are then rapidly filtered and captured by splenic APCs, also including mainly MPs, DCs and FBCs. Spleen has the anatomical organization of white pulp resembling that of the LNs, particularly with regard to the specialized compartments for B cell and T cell populations. As such, spleen and numerous LNs together present the main arenas where the innate and adaptive immunoresponses proceed to establish the anti-Ag immunity [37].
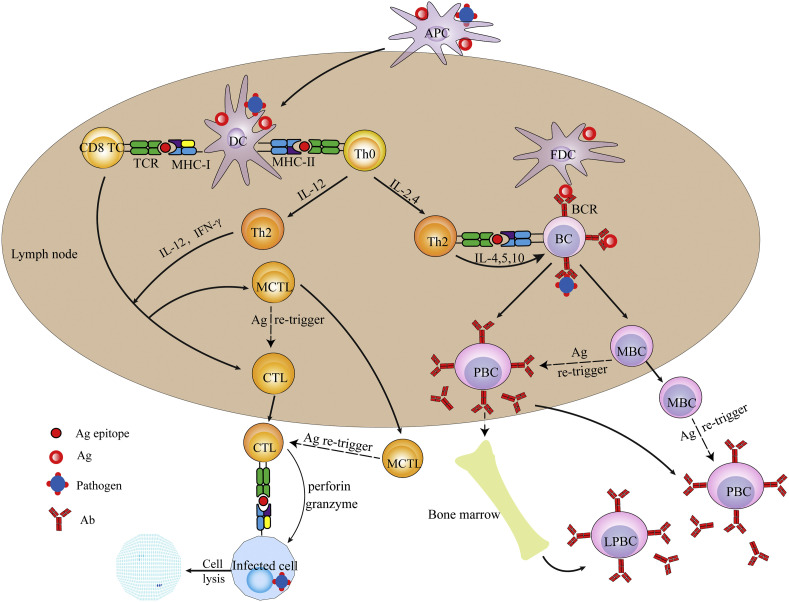
Fig. 1.
Schematic description of the process for establishing the humoral and cellular immunity triggered by a pathogen (or vaccine). Abbreviations: APC, antigen presentation cell; DC, dendritic cell; FDC, follicular dendritic cell; CTL, cytotoxic T lymphocyte; TCR, T cell receptor; BCR, B cell receptor; Th0, naïve CD4+ T cell; Th1, type 1 helper T cell; Th2, type 2 helper T cell; PBC, plasma B cell; LPBC, long-lived plasma B cell.
After Ag uptake, peripheral APCs, mainly DCs but also Langerhans cells, will be activated while they are migrating to the dLNs and processing the endocytosed cargoes into fragments including the Ag epitopes, which subsequently bind to either MHC-I, for endogenous Ags, e.g., derived from cellular wastes or viral proteins, or MHC-II molecules, for exogenous Ags, e.g., delivered by vaccines, and then displayed on APC surface in the form MHC-I/II-Ag epitope (Fig. 1). Then in the dLN/spleen, the MHC-II-Ag epitopes displayed on APCs will interact with diverse TCRs (T cell receptors) of CD4+ T cells, which thus are stimulated to differentiate mainly, depending on cytokines, into either Th1 cells (type 1 T helper cells), usually under co-stimulation by IL-12, or Th2 cells, usually under co-stimulation by IL-2 and IL-4, to secrete a collection of cytokines for further triggering two downstream responses.
One downstream contributes to the cellular responses, whereby, the APC MHC-I-Ag epitopes bind to TCRs of CD8+ T cells, which, under additional co-stimulation of, usually, IL-12 and IFN-γ secreted mainly by activated Th1 cells, will turn into via differentiation the Ag-specific CTLs (cytotoxic T lymphocytes) and memory CTLs (MCTLs) (Fig. 1) [38]. Both CTLs may migrate to circulation system and peripheral tissues and are able to recognize and bind via TCRs to the identical MHC-I-Ag epitopes presented on the encountered pathogen-hidden or diseased cells. The problematic cells targeted by CTLs are then induced to undergo a series of intracellular caspase cascade followed by apoptosis or lysis due to receiving the cytokines or chemicals such as perforin, granzyme, cytotoxin and granulysin secreted by the bound CTLs, leading to dumping of the pathogens for full eradication. MCTLs are responsible for providing the long-term protection to host, since they can survive for long time to lyse the emerging infected cells displaying MHC-I-Ag-epitopes identical to the initial triggers, thus erasing the relevant pathogens (Fig. 1).
The other downstream accounts for the T (thymus)-dependent humoral responses, whereby in vivo Ags are partially taken up by APCs which then present MHC-II-Ag-epitope and thus induce production of Th2 cells. Meanwhile, partial Ags are to traffic to dLNs wherein they may be captured by LN-resident DCs and MPs either for surface presentation to activate T cells or for transition to FDCs (follicular DCs). FDCs are able to retain Ags for long time in a non-degradative form for periodical display, allowing the Ags to bind to the highly diverse BCRs (B cell receptors). Otherwise, the Ags that have arrived in dLNs may directly enter the follicles where they will directly bind to BCRs (known as signal 1). Subsequently, the BCR-Ags are endocytosed and processed by follicular B cells (FBCs) for presentation as the MHC-II-Ag-epitopes, which, at the border of the T-cell zones and B cell follicles in LNs, are recognized and bound by TCRs of the differentiated Th2 cells (a process known as cognate T-B cell cooperation, signal 2). Meanwhile CD40 on B cells is bound for co-stimulation by CD40L (CD40 ligand) on the differentiated Th2 cells, which are thus fully activated to secrete cytokines (signal 3) such as IL-4, −5 and − 10, triggering B cells to seed in germinal centers, differentiate and proliferate into plasma cells (including short-lived and long-lived plasma cells) as well as memory B cells (MBCs) [39]. Plasma cells are producing the Ag-specific Abs which are able to neutralize the pathogenetic subjects into non-infectious organisms prior to their settlement in the host (Fig. 1) [40]. Notably, long-term production of Abs may be maintained by a combination of the short-lived plasma cells, which usually have a life of 3–5 days, and the long-lived plasma cells (PC), which acquire the long-living property through migrating to survival niches within the bone marrow (Fig. 1), wherein they receive survival factors such as APRIL (a proliferation-inducing ligand) and IL-6 and thus may exist even for the life-time of hosts depending partially on the stimulatory Ags. Alternatively, high levels of Abs may be rapidly produced as a result of prompt proliferation and differentiation of MBCs into the short-lived plasma cells upon re-encountering the identical Ags, which are usually carried by pathogens (Fig. 1) [41].
Notably, certain types of Ags bearing multiple repeating epitopes, such as some bacterial cell wall polysaccharides, glycolipids and nucleic acids, can bind, in multiple number, to and mediate BCRs first to undergo clustering and then to act as signal 2 to directly activate B cells. As such, B cells are induced, independent of T cells, to differentiate into plasma cells (the process known as T-independent B cell activation), but not memory B cells, to produce Abs of low affinity, mainly IgM or immunosuppressive sialylated IgG, which may not favour the anti-pathogen immunity [42].
Generally, the T-dependent process is able to imprint immune system with Ag features to establish the immune memory, which is marked by forming the long-lived memory T cells (including CD4+ and CD8+ MTCs) [38,43], possibly, derived from their effector counterparts [44], and long-lived memory B cells in LNs, spleen and peripheral tissues [45]. The generated immune memory is thought the most important consequence of vaccination as it enables the pathogen-experienced hosts to possess the ability of rapidly initiating responses to the encountered pathogens bearing identical Ags, thus engendering prompt immunity to remove invaders [32]. Acquiring the competence after experience by host immune system in defeating pathogens annotates the basic concept of immunity and the main principles underlying vaccination, of which a deep understanding will undoubtedly accelerate the development of novel vaccines as well as a VADS to cope with the aggressive pathogens [32]. However, at present, though it is thought that vaccine or VADS design should aim at stimulating a robust response to generate a substantial number of effector cells, from which memory cell populations might be expected to arise, the best condition that can efficiently promote formation of memory immune cells remains still to be fully defined [44].
3. Classical vaccines and adjuvants
Early vaccines were developed only for defending against the pathogenic infections and, thus, simply made of whole organisms that were inactivated or attenuated to reduce their inherent virulence and pathogenicity, while maintaining still the ability to activate the host immune system after administration to establish efficacious immunity against the matched microbes [46]. Usually, the live attenuated pathogen-based vaccines possess the capability to elicit robust and long-lasting immunity in recipients, however, they also have the potential to cause detrimental infections in recipients, as a result of occurrence of the unpredictable mutation in the engineered organisms. For example, in recent years, immunization of oral polio vaccines consisting of the live attenuated polioviruses was astonishingly found by gene sequencing to be the cause for several pandemics of polio, due to the reversion of the vaccine strains to the neurovirulent and transmissible state of wild polioviruses [47]. By comparison, the vaccines made of inactivated microorganisms are relatively safe, but they stimulate rather weak and even target-deviated immunoresponses, as a result of both the inactivation process, which may alter the sequence of authentic Ags, and the comprehensive components, which are likely to induce the irrelevant immunoresponses and even trigger unwanted inflammations due to existence of reactogenicity [48].
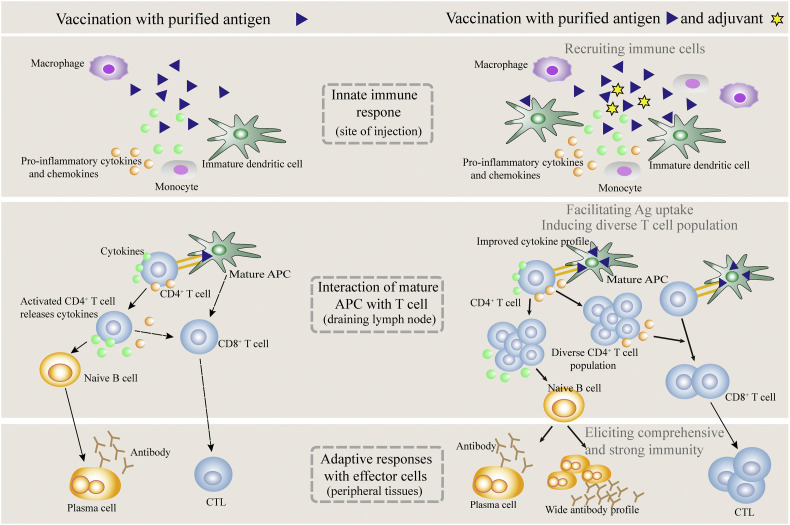
To overcome the weakness of the whole microbe-based products, at present vaccines are often formulated using purified Ags and the auxiliary ingredients, including the so-called adjuvants, which can markedly enhance the potency of vaccines by triggering the innate immunoresponses. The most frequently included adjuvant in human vaccines is alum (aluminium salt), which was first used as a vaccine adjuvant in the early 1920s by Glenny and colleagues, and is now still widely used in various vaccines against numerous infections, contributing greatly to human health [49,50]. Though alum in vaccines may cause stimulatory inflammations giving rise to certain adverse effects after administration, it has in general the acceptable safety profile and possesses the abilities to enhance the efficacy of many vaccines against the corresponding pathogens. In fact, the mechanisms underlying the adjuvant actions that are not relevant to PRR stimulation remain mostly still an elusive secrete, full unveiling of which will, undoubtedly, pave the way to discovering novel adjuvants. At present, it is thought that a vaccine adjuvant, e.g., alum, may act by a combination of various mechanisms, such as forming depot, recruiting immune cells, enhancing Ag uptake and presentation, inducing production of cytokines and chemokines, and promoting Ag and APC traffic to dLNs [51]. By and large, an adjuvant improves the immunoresponses, in some cases, as a result of: (1) recruiting immune cells such as DCs, MPs and neutrophils to vaccination site; (2) facilitating APC uptake of Ags; and (3) activating innate immune cells such as neutrophils, natural killer cells (NKCs), and especially, professional APCs (mainly DCs and MPs) (Fig. 2 ). These aspects promote not only the release of a specific myriad of cytokines and chemokines promoting generation of specialized T lymphocyte subsets such as Th1 and Th2 cells, but also the presentation of Ags in MHC-I and/or -II mode, resulting in enhanced humoral and/or cellular immunity against pathogens (Fig. 2) [52]. Also, it is argued that a classical adjuvant exerts only the short-lived effects on the adaptive immunoresponses, which may arise from the influence of a specific collection of cytokines and chemokines released by immunocytes under stimulation of the adjuvant-activated APCs with just a short life.
Fig. 2.
The immunoresponses induced by immunization with vaccines in the presence or absence of an adjuvant.
4. Subunit vaccines delivered by liposomes
The safety concern on conventional vaccines promotes scientists to develop novel subunit vaccines that are not based on a whole microorganism but its antigenic parts, such as proteins, DNA, RNA, and the synthetic carrier-vectored Ags [53]. However, lack of other components of a pathogen, which may not only play a role in protection of antigens but also act as a type of PAMP/DAMP, often makes subunit vaccines vulnerable to environmental damages and ineffective in triggering immunoresponses toward Ags [54,55]. To enhance the potency of subunit vaccines, researchers have developed various types of particulate carriers, such as liposomes, cochleates, virosomes, emulsions, lipid and polymeric NPs, some of which are constructed in just the manner of mimicking the structure of pathogenic organisms to carry, protect and deliver vaccines, thus promoting their immunostimulatory activities [14,17,29,53,56]. Also, these particulate carriers are often additionally incorporated with functional molecules, such as TLRas (toll-like receptor agonists/ligands), CLRas (C-type lectin receptor agonist/ligands), NLRas (NOD-like receptor agonists/ligands), saponin and squalene and their derivatives, and various environmental stimulus-sensitive molecules, to further expand their functions [57,58]. As such, these carriers can play roles of not only an adjuvant in boosting immunostimulatory effects, but also of a delivery vehicle for targeting immunocytes as well as for the sustained or signal-triggered release, engendering a multifunctional vaccine adjuvant-delivery system (VADS) [[14], [15], [16], [17], [18],28,30,[59], [60], [61]]. The best illumination of this concept of VADS may perhaps be presented by citing the FDA (Food and Drug Administration of USA) approved novel adjuvant entities, such as nanoemulsion/squalene-based MF59, liposome/TLR4a-based AS01, liposome/TLR4a/saponin-based AS01b, and aluminium aggregate/TLR4a-based AS04, which are used in several vaccines serving as both an adjuvant and a vehicle for delivering bioactive ingredients [5,62].
In developing vaccines, a suitable adjuvant plays a crucial role in enhancing immunostimulatory effects and may well be combined with Ags in an appropriate formulation or carrier. The thus formulated vaccines had better be feasible for large-scale manufacture and be able to deliver the components to APCs, which are ignited to launch a wave of first innate and subsequent adaptive immunoresponses with the magnitude, quality, breadth as well as persistence that are sufficient for establishing the efficacious anti-pathogen immunity. Therefore, a successful vaccine may consist of three key components: effective Ag, robust adjuvant, and efficient formulation or carrier that is able to deliver ingredients to APCs, even intracellular organelles, or at least the appropriate sites such as LNs and mucosa [63]. Whilst vaccine carriers are developed with various micro/nano-particles, different types of liposomes (such as conventional liposomes [27], the inter-bilayer cross-linked multilamellar vesicles (ICMVs) [64] and solid core liposomes [15]) with each bearing unique structures and properties, prominently represent a suitable VADS [16].
4.1. Basics of liposomes: discovery and characteristics
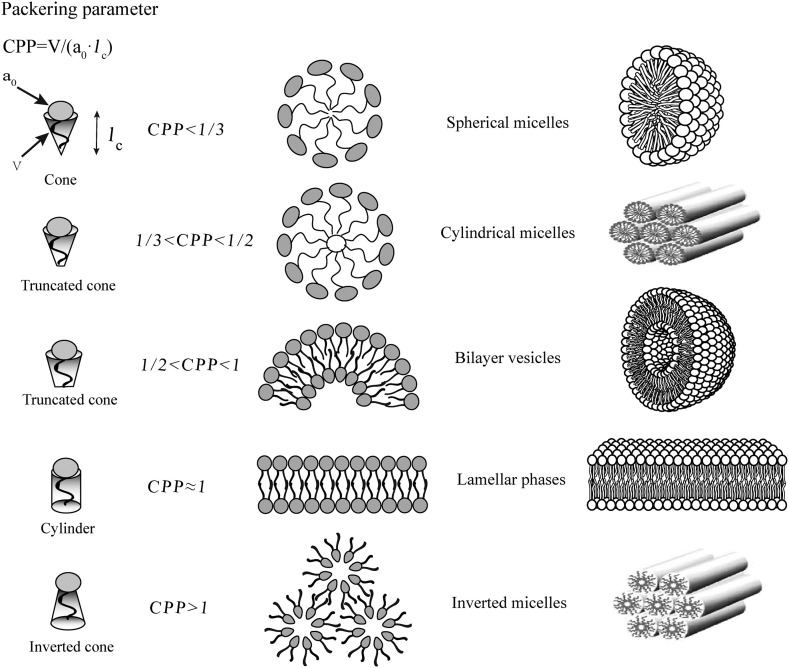
Liposomes are the phospholipid bilayer-enclosed spheres formed via self-assembly in water under a driving force of hydrophobicity. Liposomes were first discovered in the early 1960s by Bangham et al using an electron microscope, and later coined the name “liposome” by Sessa and Weissmann in 1968 [20,65]. Liposomes have such a unique architecture that is confined, as proposed by Israelachvili and Mitchell in middle 1970s, by the molecular shape of amphiphilic phospholipids which just have a critical packing parameter (CPP) in the range of from 1/2 to 1. CPP was defined as the equation of CPP = v/a o l c, where v, l c and a o are, respectively, the volume and length of the lipophilic chain, and the cross-section area of the hydrophilic core of the phospholipid expressed per molecule in the aggregates [66]. And the amphiphilic molecules with a CPP value falling out of the range will assemble into other shapes [67], as shown in Fig. 3 .
Fig. 3.
Molecular shape and critical packing parameter (CPP) of amphiphilic molecules, and the self-assembly entities formed of different amphiphiles (v, the lipophilic chain volume; ao, the cross-section area of hydrophilic head group, lc, the length of lipophilic chain).
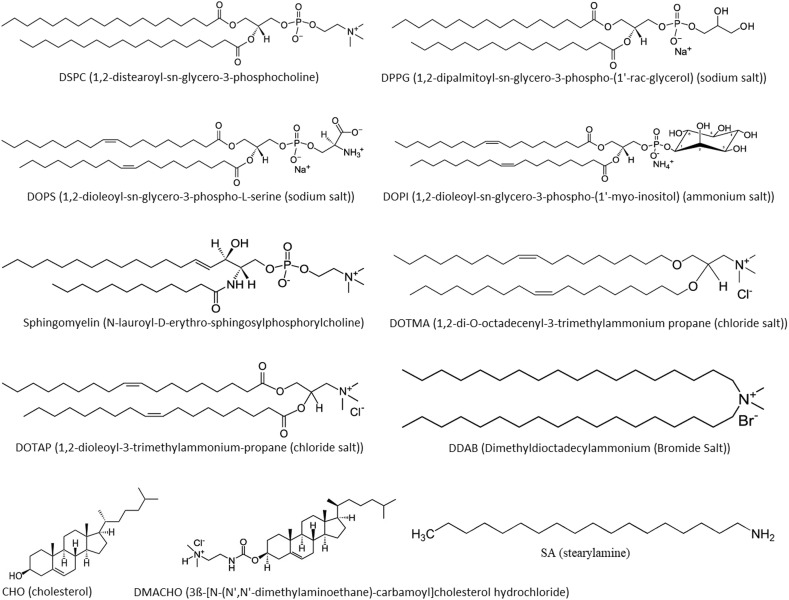
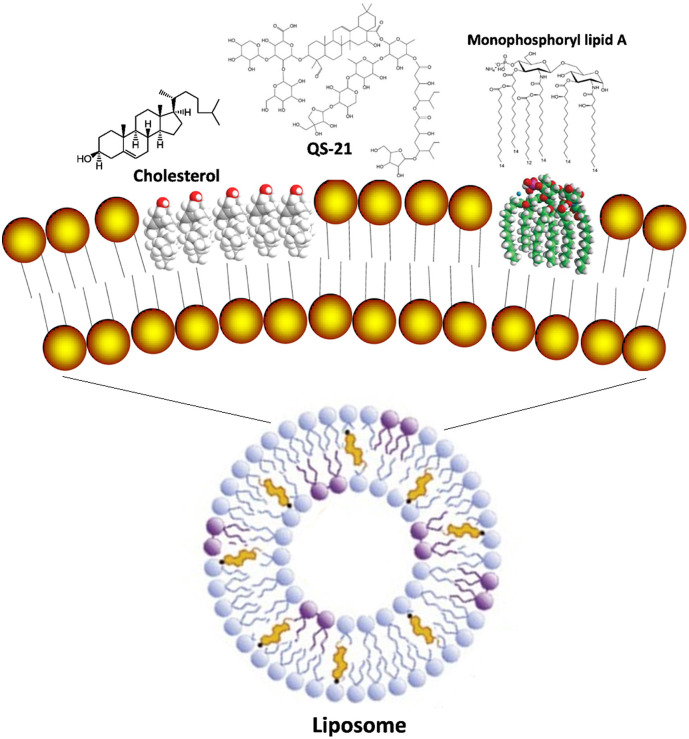
Generally, liposomes may be made of different types of amphiphilic phospholipids, such as PC (phosphatidylcholine), PS (phosphatidylserine) and SM (sphingomyelin), which may also be combined with other lipids, such as CHO (cholesterol) for membrane stabilization, and the negatively or positively charged lipids to modulate liposome structure and surface properties, thus forming a suitable carrier to be used as a DDS (drug delivery system) or a VADS. Some of the materials that are most frequently used for preparation of liposomes are shown with molecular structure in Fig. 4 .
Fig. 4.
Molecular structure of some of the lipids that are frequently used for constructing liposomes.
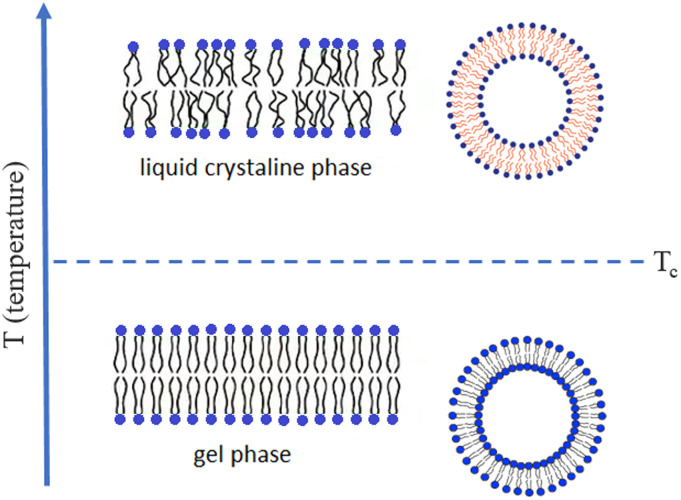
Notably, at ambient temperature, liposomes will display their bilayer membranes in a “fluid” or “rigid” fluidity state, which is determined by the phospholipid category and its hydrocarbon chain length (usually longer than 12C) as well as saturation (Fig. 5 ). These parameters together with the location of unsaturated carbon bond remarkably influence the van de Waals force, and other the interactions, among adjacent chains of phospholipids thus governing membranes to manifest the corresponding fluidity. As such, liposomes with membranes consisting of just a type of phospholipids will show a specific gel to liquid crystalline phase transition temperature (Tc), which, however, may be blurred when a mixture of lipids, such as egg PC, soy PC, or combined with CHO, is used. Thus, when liposomes are made of a phospholipid with Tc above or below the ambient temperature, their membranes will correspondingly be in the rigid gel or fluid liquid crystalline phase [26]. However, when liposomes are made of a phospholipid with Tc just equivalent to the ambient temperature, their membranes will manifest a maximal permeability and may abruptly release all loaded cargos, and this phenomenon is sometimes employed to engineer a temperature-sensitive carrier. At room temperature, whilst liposome lipids with long and saturated hydrocarbon chains have a high Tc and tend to form rigid ordered bilayer structures which may be in a gel phase, liposome lipids with short and unsaturated chains have a low Tc and will generate fluid and disorganised bilayers which may be in a liquid-crystalline phase (Fig. 5). Interestingly, incorporation of CHO may significantly influence the fluidity of membranes and, at high concentration (CHO/PC > 1/3 mol), may smear out the gel–liquid crystalline transition phenomenon in liposomes. Particularly, CHO may remarkably decrease permeability of membranes and thus, is often employed for preventing leakage of liposomes, especially, when lyophilization or elevation of temperature for remote drug loading is involved [68,69].
Fig. 5.
Phospholipid bilayer membrane fluidity of liposomes. When the ambient temperature (T) < Tc of phospholipids, liposome membrane is in the gel phase; when T > Tc, liposome membrane in the liquid crystalline phase; while T increases from below Tc to above Tc, liposome membrane changes from the gel to the liquid crystalline phase.
So far as stability is concerned, surface PEGylation (modification with PEG) is another effective way to enhance liposome quality, because it can engender on vesicles a steric stabilization effect. PEGylation not only can prevent liposomes from coagulation and fusion, but also might allow liposomes to avoid being bound by plasma proteins, or to be bound by certain specific types of plasma proteins which recently were surprisingly reported able to enormously prolong NP (nanoparticle) circulation time in vivo [70], thus generating the so-called stealth effects on liposomes [71]. In addition, liposomes can be enhanced in stability by charging with cationic or anionic lipids to generate a repulsive electrostatic force repelling each other to avoid aggregation. Also, freeze-drying of liposomes in the presence of sugar as lyoprotectant to form a dry cake fixing individual liposomes in a solid matrix provides a feasible strategy to increase product stability as high as enough to meet the clinical application needs [72].
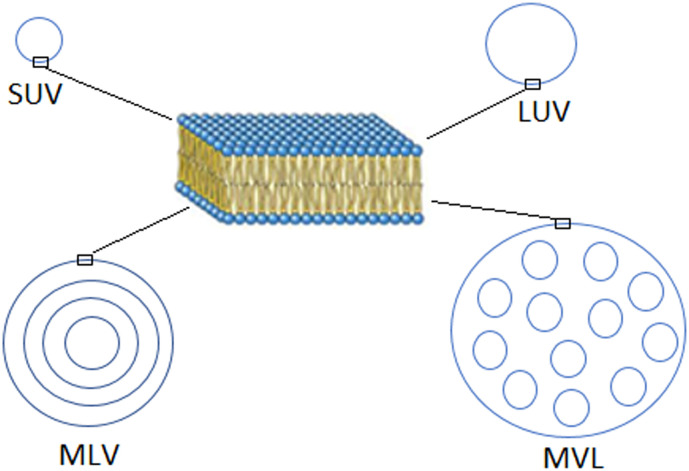
Recently, several novel types of liposomes, such as ultradeformable vesicles (Transfersome®) [73], the inter-bilayer cross-linked multilamellar vesicles (ICMVs) [64], and solid core liposomes [15], have been engineered for vaccine delivery. However, they are based on the conventional liposomes, which may be designed to fit well the function of a VADS [27], thanks to their distinctive structures classifying liposomes into three basic types. (1) Small unilamellar vesicles (SUVs) with a size <200 nm or large unilamellar vesicles (LUVs) with a size <500 nm but >200 nm, which are spheres with a single phospholipid bilayer and are frequently used as a targeting DDS or VADS. (2) Micro-sized multilamellar vesicles (MLVs), which have numerous concentric phospholipid bilayers and are usually used as a sustained release carrier. (3) Micro-sized multivesicular liposomes (MVLs), which are featured with multiple water-filled cavities separated by continuously connected lipid bilayers and are used as a reservoir for long sustained release (Fig. 6 ) [74].
Fig. 6.
Structural features of different types of liposomes.
4.2. Liposome delivery of vaccines
Since their discovery by Bangham and colleagues in 1965 [20], liposomes have always been the research focus of a variety of subjects, such as lipid membrane model, life origin, narcosis mechanism, and, especially, DDS and VADS, due to their unique properties beneficial for delivery of various bioactive agents [[21], [22], [23]].
In fact, as early as 1974, liposomes were already confirmed of intrinsic adjuvanticity by Gregoriadis et al who observed potent antibody immunoresponses to the carried Ags such as diphtheria toxoid in vaccinated mice. Meanwhile, the vaccinated mice showed at injection sites no signs of granulomas, a common side effect associated with conventional adjuvants, nor the symptoms of hypersensitivity reaction, after receiving intravenous or intra-foot pad immunization [[24], [25], [26],75]. These early investigations paved the way for exploring liposomes as a VADS and were immediately followed by numerous studies, which further revealed that the immunostimulatory activity of liposomes as a VADS is significantly influenced by their physicochemical properties and even vaccination routes [18,76,77].
Now, liposomes have firmly proven a safe and effective VADS with low reactogenicity and able to carry multiple Ags, other adjuvants and/or functional molecules, such as PRRas and even aluminium NPs, to further enhance immune-boosting effects or dictate immunoresponses toward Th1 and/or Th2 pathways for establishing desired immunity [15,28,78].
4.3. Factors influencing the efficacy of liposome VADS
As a VADS, liposomes can efficiently fulfil the vaccine delivery functions, which, however, are significantly influenced by many of liposome physicochemical attributes, including surface charge, membrane fluidity, size, and even Ag-loading mode (entrapped within or associated on surfaces of vesicles), which have been confirmed capable of altering APC uptake, process and presentation of the Ags delivered by the liposomes [27].
4.3.1. Surface property
Surface charge will always exert a significant impact on the immunostimulatory function of liposomes, because it is a key factor that not only influences the Ag load and release and liposome stability, but also affects via electrostatic forces the interactions between liposomes and biological components, immunocytes and even intracellular organelles, all of which may be the oppositely/likely charged objects [27].
Cationic liposomes are usually made (by additionally incorporating) of lipids with quaternary ammonium head groups that are positively charged in normal physiological conditions (Fig. 4). This kind of liposomes when injected intravenously into mice may generate the cell-disrupting and haemolysis effects, rendering them reduced practical applications in vessel vaccination [79]. By contrast, with local administration, the cationic liposomes may cause tissue damage at the injection site, leading to the release of endogenous materials such as nucleic acids, which may likely act as a DAMP to activate immune cells, forming the basis for the use as a VADS [80]. In addition, cationic liposomes can carry via entrapment and/or adsorption the negatively charged Ags, which after administration will not be immediately released due to electrostatic attraction, thus forming a depot effect to recruit APCs. Interestingly, APCs are usually negatively charged and therefore prone to adhesion by cationic liposomes, which thus are subjected to the accelerated cellular phagocytosis, making an enhanced contribution to APC activation via, perhaps, the intracellular FcRγ–Syk–Card9 pathway [81,82]. For example, the cationic liposomes made of DDA/TDB of 5: 1 (w/w) (DDA, dimethyl dioctadecyl-ammonium bromide; TDB, α,α'-trehalose 6,6′-dibehenate), have been successfully developed as a clinical trial VADS for delivering different Ags, such as tuberculosis (TB) antigen of Ag85B-ESAT-6 and hepatitis B virus (HBV) surface antigen (HBsAg). And the available data demonstrated that the cationic liposomes could safely induce robust cellular and humoral immunoresponses via either subcutaneous (s.c.) or intramuscular (i.m.) vaccination [83]. These outcomes strongly suggest cationic liposomes a promising VADS able to effectively deliver vaccines for eliciting the anti-pathogen immunity.
Anionic liposomes are usually made of or modified with lipids (Fig. 4) that are negatively charged in normal physiological conditions, such as PG (phosphatidylglycerol), PA and PI (phosphatidylinositol) [84]. By intuition, anionic surface charge can hardly offer benefits to delivering vaccines to APCs given the anionic nature of the latter, which due to electrostatic repulsion may repel the negatively charged particles from access to cellular uptake. However, a recent study reported that, in the presence of adjuvant GLA (glucopyranosyl lipid adjuvant, a TLR4a), the anionic liposomes made of CHO/DPPC/DPPG were more potent in enhancing Th1 responses in mice than the cationic liposomes made of CHO/DPPC/DPTAP, in the case of delivering Mycobacterium tuberculosis (Mtb) Ag of ID93. Moreover, this effect of cationic liposomes was also correlated well with the VADS in protection against Mtb challenge [85]. Notably, for mucosal vaccination, the anionic or neutral particles may be an advantageous VADS, because they will not bind to and be trapped in the negatively charged mucus gel layer, thus allowing them to easily approach the follicle-associated epithelia whereby to be endocytosed by M cells [86]. Also, in a previous report researchers confirmed in mice that both anionic and neutral NPs showed a higher affinity to the Peyer's patches than cationic particles [87], suggesting the negatively charged carriers may favour oral and, possibly, other mucosal vaccination.
When designed as a VADS, anionic liposomes are often engineered purposely by incorporation of negatively charged lipids with special functions, such as and lipid A [88] and PS (phosphatidylserine) [89], which may display special interactions with APCs. Monophosphoryl lipid A (MPLA) is an adjuvant derived by acidic hydrolysis from the highly toxic lipopolysaccharide (LPS), and proves able to safely trigger endosomal TLR4, followed by activation of the downstream TRIF-mediated cascade and the ultimate transcription factors, such as NF-κB, IRF3 and AP1, as such exhibiting immunomodulatory activities beneficial for developing a liposome VADS [90]. Moreover, the MPLA-modified liposomes are suitable for delivering a variety of vaccines, among which a few products, such as Mosqurix® against both Plasmodium falciparum and HBV, and Shringrix® against shingles virus, have been officially authorized for clinical use. Now, they are confirmed by numerous clinical cases able to efficiently trigger the Th1-biased immunoresponses in humans, resulting in strong cellular immunity against the related pathogens while causing few unacceptable side effects [62,77].
Notably, among various negatively charged phospholipids, phosphatidylserine (PS) strikingly distinguishes itself from others, since, as a cell membrane component, it is strictly kept by enzyme flippase in the inner layer of normal cell membranes. However, during apoptosis under mediation of scramblase which acts to bidirectionally transport phospholipids in membranes in an ATP-independent manner, PS will flip to the outer leaflet so as to be instantly exposed for recognition through binding to a number of specific PS receptors that are expressed on immune cells, mainly MPs and immature DCs [91]. Then, these immune cells are subsequently triggered to devour timely the apoptotic cells leaving them no chance to release noxious materials for inducing deleterious autoimmune reactions [92]. Thus, PS in vivo acts in fact as a crucial danger or “eat me” signal to immune cells, mainly APCs, providing, probably, an ideal component that may be incorporated in liposomes to facilitate APC uptake of the carried Ags and sponsoring the downstream immunoresponses. It should be noted that different PS receptors on different immune cells (not only APCs), upon PS ligation, will manifest different functions, including, particularly, the anti-inflammatory and even tolerogenic effects. These effects, on one hand, are in good accommodation to PS role in inducing rapid phagocytic clearance of dying cells in silence to avoid autoimmunity, but, on the other hand, count obviously against establishing the anti-pathogen immunity [93,94].
For instance, previous work by Hoffmann et al. showed that in mice liposomes made of PS/PC/CHO (3:3:4) specifically inhibited the immunoresponses to the post-delivered Ags in CFA (complete Freund adjuvant), as proved by decreased production of IL-2, IL-4 and IFN-γ by dLN cells, the Ag-specific CD4+ T cells and IgG in blood [95]. This inhibitory effect exerted through in vivo activating PS receptors was reversed by administration of the Abs against TGF-β (transforming growth factor-β), which showed an increased expression in PS-treated mice, implying its potential role in mediating the inhibitory function of PS. Moreover, PS-containing liposomes neither directly inhibited DC maturation in vitro in response to different stimuli, nor prevented DC migration to dLNs in vivo, hinting the complicated interactions among various immunocytes involved in the suppressive process sponsored by PS. Recently, Balu-lyer's group prepared Factor VIII-loaded liposomes with PS/PC (3:7 mol ratio), which when incubated with DCs in vitro caused increased secretion of TGF-β, and, when immunized to mice, due to PS incorporation, not only lowered the Ag-specific Ab response but also induced the hypo-responsiveness to the Ag re-challenge [96]. These outcomes suggest that PS-containing liposomes may function to exert the immune regulatory effects capable of converting an immunogen to a tolerogen, opposing the use as a VADS against pathogens.
However, recent work by Luis et al showed that addition of combinatory PS/GM3 (ganglioside of mono-sialic acid) into the Ag-loaded POPC liposomes remarkably increased the anti-Ag immune reactions in vaccinated mice, though insertion of PS alone into the carrier made a minimal contribution to the enhancement [97]. Also, Cauvi et al revealed in murine model that anionic empty PS liposomes triggered peritoneal MPs to have about 4700 genes specifically modified, resulting in changed protein expressions by peritoneal cells, including increased secretion of several chemokines (CXCL1, CXCL2, CSF-2, and CSF-3) and cytokines (TNF-a, IL-6, IL-10). And consequently, massive neutrophil infiltration into the peritoneum succeeded and proved capable of neutralizing a septic polymicrobial insult [98]. However, such neutralization of pathogens was also obtained by intraperitoneal administration of PG liposomes, suggesting that these effects might be just caused by the negative charge property of phospholipids, through possibly increasing the absolute value of the negative potential of MP plasma internal membranes. The membrane potential change may ignite the intracellular signalling cascade involved in activating the innate immunoresponses responsible for controlling infections. Obviously, these immunostimulatory effects of empty PS liposomes can hardly be directly translated into an Ag-loaded VADS, which functions well only when both the innate and adaptive immune systems are sufficiently activated.
In contrary to the above negative reports, PS-containing liposomes have also been demonstrated by different researchers able to remarkably enhance the immunostimulatory potential of their delivered Ags and thus are proposed as an effective VADS for making vaccines against pathogens. For instance, Arigita et al recently reported that vaccines delivered by the PS liposomes resulted in an improved Ag uptake by DCs and an increase of the anti-bacterial antibody response compared to those delivered by PG liposomes [99]. Cui's group also observed that, when simply admixed with model Ags, anionic liposomes prepared with DOPS or DOPA (1,2-dioleoyl-sn-glycero-3-phosphoric acid) showed comparable and even strong adjuvant activities on both humoral and cellular responses and elicited stronger Ag-specific antibody immunity than alum admixed with Ags, compared to DOPG liposomes which showed only a weak adjuvanticity [84]. However, other researchers observed that only PS-, but not PA-, containing liposomes with encapsulated Ags were noticed of the ability to enhance the Ag-specific immunoresponses. This was evidenced by the observation that, cocultured with the Ag-specific Th1 clone 42-6A cells in the presence of APCs, PS liposomes triggered the Ag-specific production of IFN-γ. And in mice only co-administration with the Ag-specific Th1 cells plus Ag-loaded PS liposomes could induce secretion of high levels of serum and splenic IFN-γ [100]. In another study, PS liposomes were demonstrated capable of promoting APC uptake of their carried Ags, as a result of liposome binding to PS-specific receptors on APCs. This conclusion was reinforced by the work from Chiantia's group, which showed that the anti-HIV Env Ab-decorated PS liposomes were bound by HIV VLPs (virus-like particles), which were facilitated, for MP internalization, in a liposome PS-dependent manner [101].
Summarily, PS-containing liposomes are reported by different groups to show complicated, even conflicting, immunostimulatory activities. This inconsistency is most likely to arise from the discrepancy in experimental formulations, derived cells and animal models. However, without a strong supportive evidence, it is hard to exclude the data from the false that were possibly resulted from unintentional errors or other issues, since some conflicting results seemed to be obtained from the experiments that were so closely designed. Nevertheless, at present it remains uncertain as to whether the PS liposomes will be further developed as a practical VADS to enhance the immunoresponses against pathogens, or as a clinical strategy to suppress the unwanted immunoresponses toward the administered therapeutic proteins, such as Factor VIII, to elevate their effectiveness.
4.3.2. Membrane fluidity
Liposome membrane fluidity, or called liposome flexibility, decreases with the saturation and length of lipids used to construct liposomes, and is also reported to have a complex impact on the activity of liposomal VADS. For example, it was reported that the Leishmania donovani antigen-encapsulated liposomes with DSPC (distearyl L-α-phosphatidylcholine Tc of 55 °C) demonstrated improved adjuvant activity and showed >90% protection to the vaccinated mice that were subjected to a lethal challenge. By contrast, the Ag-loaded liposomes with DPPC (dipalmitoyl L-α-phosphatidylcholine, Tc of 41 °C) or DMPC (dimyristoyl L-α-phosphatidylcholine, Tc of 23 °C) showed little adjuvanticity and even no protection in vaccinated mice challenged with the same type of pathogens [102].
The influence of liposome fluidity on VADS function is thought relevant to the interactions between liposome and cell membranes, and the subsequent liposome biodistribution in recipients. For instance, it was demonstrated that rigid liposomes made of DDA (dimethyl dioctadecylammonium, Tc of 47 °C) triggered more robust immune reactions than did fluid liposomes composed of unsaturated DODA (dimethyl dioleoylammonium) [103]. Further investigation revealed that the DDA rigid liposomes presented a larger amount of Ags at the injection site, thus attracting more APCs with elevated CD40 and CD86 to engender higher Th1 responses than did the fluid DODA liposomes. Also, DODA liposomes were found to readily release the Ags at the injection site, leading to formation of a population of Ag-positive but adjuvant-negative APCs which were thus less activated. However, an earlier paper reported that the haptenated liposomes made of DOPC (dioleoyl L-α-phosphatidylcholine, Tc of −17 °C) or DSPC (Tc of 55 °C) showed a low immunogenicity than liposomes made of DPPC (Tc of 41 °C) or sphingomyelin (Tc of 30–38 °C), either of which has an intermediate Tc relevant to DPPC and DOPC, suggesting the immunostimulatory activity of liposome may have little direct relevance to their membrane fluidity [104].
Inclusion of CHO within liposomes are known able to modulate bilayer membrane fluidity and therefore widely employed in formulating liposomes to elevate the stability of a DDS (drug delivery system) [23]. However, in terms of the influence of CHO on the immunostimulatory effect of a liposome VADS, the conclusion is rather elusive. Whilst in some studies inclusion of CHO in liposomes was observed to enhance immunoresponses [104], other investigations also demonstrated that liposomes containing CHO with an elevated membrane fluidity showed decreased adjuvant effects [105]. Nevertheless, inclusion of CHO seems to have a distinct impact on immunostimulatory activity of a liposomal VADS, although the available investigations reported inconsistent results [105,106]. Recent work by Jiskoot et al demonstrated that the presence of CHO significantly contributed to the adjuvant effect of the influenza HA (hemagglutinin)-loaded cationic liposomes composed of eDPPC (1,2-diacyl-sn-glycero-3-ethylphosphocholine)/CHO. In comparison to the liposomes containing no CHO, eDPPC/CHO liposomes facilitated HA uptake by DCs in vitro and enhanced anti-HA immunity in mice. However, for cationic liposomes, incorporation of CHO exerted only a moderate impact on immunoresponses triggered by the VADS [107]. Luis et al using a HIV-1 gp41 MPER (membrane proximal external region) engrafted with tetanus toxoid (TT) as Ags, and POPC (1-palmitoyl-2-oleoylphosphatidylcholine) as the matrix material, prepared the Ag-loaded liposomes as a VADS against HIV. To enhance efficacy, the liposomes were further incorporated with other lipids, including PS (phosphatidylserine), GM3, or combinatory CHO/SM (sphingomyelin) which are enriched in HIV viral membrane lipid rafts, where MPER is embedded for maintaining peptide conformation [97]. In mice, the gp41-TT-loaded POPC liposomes when combined with PS/GM3 or CHO/SM induced significantly increased anti-gp41 immune reactions which was further enhanced by the addition of MPLA. By contrast, insertion of PS or MG3 alone to the gp41-TT-loaded POPC liposomes made no contribution to enhancement of immunoresponses. These outcomes imply that CHO plays roles in a liposome VADS, as embodied not only in constructing a stable VADS but also in maintaining the specific carrier structure for displaying Ags in a distinctive/natural way, thus making an impact on immunization effects.
4.3.3. Vesicle size
The size of a carrier plays an important role in defining its specific functions in various applications, as controlling of size in a micro- or nano-scale may render the carrier unique properties entirely different from those of its macroscopic counter parts. Also, the size of liposomes has a significant impact on the immunostimulatory activity of the VADS and may even dictate the immune reactions to proceed toward the Th1- or Th2-biased pathway [27]. For example, it is reported that when administered to mice by either subcutaneous injection or oral uptake, smaller lipid vesicles (< 150 nm) enhanced the development of Th2 response, whereas larger lipid vesicles (> 200 nm) promoted IFN-γ and typical Th1 response [108,109]. In mouse model, 24 h after intra-footpad injection, whilst larger VLPs (virus-like particles with a size of 500–2000 nm) were mainly linked to DCs lingering in the injection site, smaller VLPs (20–200 nm) drained rapidly to dLNs wherein they were picked up by the LN-resident immune cells [110].
However, previous work by Perrie's group unveiled that cationic liposomes (DDA/TDB of 5:1, w/w) with different sizes ranging from 200 to 2000 nm showed similar activities in trigging in vitro APC uptake and in vivo Th1 response. These liposomes exhibited a similar slow drainage from the injection site in mice, but a size-dependent movement to popliteal dLNs, wherein large vesicles were detected at a remarkably elevated level in comparison to small vesicles [111]. The results suggest that, in contrast to neutral liposomes, cationic liposomes with a large size may tend to from a depot at injection site due to binding to cells or tissue components via electrostatic attraction, and thus be picked up more efficiently by local APCs for trafficking to LNs. The group also found cationic liposomes with a large size (2 μm) triggered high splenocyte proliferation but low IL-10 secretion, while the positively charged vesicles of 500 nm promoted production of IFN-γ from splenocytes and secretion of IL-1β at the injection site favouring Th1 responses [111]. When the cationic liposomes were PEGylated, the passive drainage was observed to cause in mouse dLNs an enhanced proportion of smaller, rather than larger, vesicles. And the PEGylated small liposomes exerted lowered depot effects and induced deceased production of IgG2b and IFN-γ but increased IL-5, in comparison to their un-PEGylated counterparts, suggesting PEGylated cationic liposomes with a small size may be in favour of Th2 response [71].
A previous investigation was made on intracellular trafficking of Ags delivered by vesicles composed of 1-monopalmitoyl glycerol/CHO/dicetyl phosphate. The results revealed that Ags carried by the vesicles with a large size (560 nm) were delivered into the early endosome-like, immature phagosomes, which are responsible for recruiting the Ag-processing apparatus, leading to efficient Ag presentation. By contrast, soluble or small vesicle (155 nm)-carried Ags rapidly localized to the late endolysosomes wherein they were degraded, leading to a reduced Ag-presentation efficiency [112]. Seeing that frequent occurrence of lysosomal degradation of Ags into useless pieces will invalidate vaccination, researchers are focusing efforts on developing liposomes as a multifunctional VADS that is capable of not only delivering Ags into APCs but also rendering the intracellular Ags lysosome escape [28]. Lysosome escape proves a feasible strategy allowing Ags to avoid lysosomal degradation and to be processed by immunoproteasomes (specialized proteasomes in immunocytes) within APCs. Cellular immunoproteasomes, owing to their distinct beta subunits of β-1, −2, −5i with special activity, have a low caspase-like proteolytic activity but a high chymotrypsin-like activity, and thus, during antigen process, can contribute to creating small fragments that are more likely to bind to MHC-I molecules for presentation, as such enhancing Th1 responses [113].
Overall, liposomes or other particulate vaccines sizing from 20 to 100 nm will preferentially traffic to dLNs, which may be further facilitated by PEGylation of the carriers [53]. Particles or molecules smaller than 20 nm may disseminate into tissues and, subsequently, enter the systemic circulation, thus offering little chance for lymphatic uptake. Larger particles (> 100 nm) are more likely to become trapped in tissues, creating depot effects in the injection site to recruit and be picked up by APCs, which will then mature and travel to dLNs for sponsoring immunoresponses. Obviously, the in vivo traffic trends and immunological activities of liposomes are simultaneously influenced by many physicochemical properties, including not only size but also membrane fluidity, surface charge and PEGylation, all of which are also mutually influenced.
4.3.4. Ag-loading mode
In a VADS based on liposomes or other NPs, whilst efficient loading of Ags into the carrier is indispensable for the object-targeted delivery, the loading mode is diverse and has an impact on the release and immunostimulatory activity of Ags. With the lipid bilayer-enclosed structure, liposomes can be loaded with Ags through several modes: adsorption on or linkage by covalent bonds onto surfaces, entrapment in interiors, intercalation between adjacent bilayers (particularly for MLVs or special lipid structures, such as cochleates), and combination of these ways. Recently, Perrie's group constructed DDA/TDB cationic liposomes as a VADS and loaded the cationic vesicles with Ags by two modes: adsorption on surface of MLVs and encapsulation within DRVs (dehydration–rehydration vesicles). The researchers showed that Ag location in liposomes did not make a difference in several aspects including vaccine biodistribution, APC recruitment and antibody response. However, when bearing 10% PEGylation, MLVs induced significantly higher IFN-γ responses than did DRV counterparts. But this effect was dramatically reversed by 25% PEGylation of the vesicles, when the DRV formulation promoted higher IFN-γ responses [71]. Previously, this group fabricated the Ag-encapsulated or -adsorbed microspheres with poly(lactide-coglycolide) (PLGA), chitosan and DDA, and showed that whilst the Ags bound to carrier surface favoured humoral responses, the entrapped Ags were more likely to trigger a cellular response [114]. By contrast, the work by Jiskoot et al demonstrated that adsorption of influenza HA on the surface of cationic liposomes composed of DC-CHO (dimethylaminoethane carbamoyl CHO)/DPPC was slightly more immunogenic than encapsulation of HA within the carrier, as proved by the induced HA inhibition titer, which was found higher in the former than in the latter [107].
Recently, Rincon-Restrepo et al using PPS-PEG (poly(propylene sulfide)-poly(ethylene glycol)), engineered two nanoparticulate systems: small solid NPs (25–40 nm) with Ags linked on surfaces via a reducible disulfide bond, and large hollow polymersomes (HPSs) (150–175 nm) with Ags encapsulated within [115]. The authors proved that small NPs preferentially augmented CTLs, as a result of instant cleavage of the disulfide linkage to release Ags in early endosomes, thus rendering Ags lysosome escape for proteasome processing and MHC-I presentation. In contrast, HPSs were better at activating CD4+ T cells, enhancing follicular CD4+ T helper (fTh) cell response to activate follicular B lymphocytes for antibody production. The reason was argued as that the Ags were protectively delivered by HPSs into lysosomes wherein to be released from the oxidative catalysis-disrupted HPSs and thus were subjected to lysosomal process and MHC-II presentation. The authors also revealed that, though HPSs travelled to dLNs at a slower speed than did NPs due to an increased interstitial resistance to larger particles, an equivalent amount of Ags was drained to dLNs after immunization with either carriers at long time point (36 h). However, at early time point (2 h), HPSs were mainly retained in lymphatic sinuses allowing Ags to be sampled by CD11b+ DCs, which are residing close to LN cortex and relevant to MHC-II presentation; by contrast, NPs smoothly reached LN paracortical area wherein to be picked up by CD11cmidMHC-IIhi migratory DCs, which processed Ags via proteasomes for MHC-I presentation [115].
In addition, the Ag loading mode and process may exert an influence on the bioactivity of Ags, since encapsulation Ags within liposomes may involve damaging steps to compromise the immunogenicity of vaccines. By contrast, the gentle adsorption process may be accomplished by just mixing of ingredients and possesses a good chance to maintain Ag activities [116]. However, Ags adsorbed on a carrier surface may be released at a relatively high rate, leaving a limited time window for APC recruitment, and this may cause inefficient and unsynchronized cellular uptake of Ags and adjuvants to form a population of APCs that are Ag-positive but adjuvant-negative and unable to properly present Ags, as mentioned above [103]. Recent studies also reported that whilst encapsulation of Ags within a carrier engendered more efficient lysosomal escape and cross-presentation of Ags in DCs than did absorption on a carrier [117], whereas the combination of Ag encapsulation and absorption of Ags with PLGA NPs induced more potent anti-Ag immunoresponses than each mode alone did, due, perhaps, to both adequate initial and persistent Ag exposure [118].
However, what is more attractive than proving which is the optimal Ag-loading mode for developing a VADS is the argument that just mixing Ags with a VADS without either encapsulation or adsorption can still boost the immunoresponses, as exemplified by numerous outcomes from both clinical and preclinical studies [119]. For examples, as mentioned above, the work by Cui's group proved that anionic liposomes also displayed robust adjuvant activity when simply admixed with Ags [84]. In fact, the approved vaccines based on a VADS, such as Fluad® based on emulsion MF59, Mosquirix® based on liposome AS01, Shingrix® based on liposome AS01b, and numerous alum-adjuvanted vaccines, are mostly developed just through simple admixing of Ags with a VADS [62].
Notably, as for aluminium-based VADSs, the mode of Ag load may represent exceptions, given that upon admixing, the Ags may be strongly adsorbed onto aluminium particles through, likely, the chemical Al—P bonds between aluminium and the phosphorus group that is often enriched in various protein Ags [15,120]. This suggests that, in designing a VADS, full exploration helps making a decision on the Ag loading mode: encapsulation or adsorption, chemical linkage or other means.
Other impressive findings on Ag load include that some VADSs or adjuvants can be administered separately from the Ag administration while achieving improved vaccination efficacy. For instances, the early work showed that administration of the mineral oil-based O/W emulsion adjuvant Marcol52 one week prior to administration of Ags still triggered effective responses to the Ag. And subcutaneous immunization with the adjuvant Quil A® alone, i.e., without co-injection of any Ags, also significantly expanded the antibody repertoire to the administered Ags [121,122]. Full activation of the innate immune system beforehand by a VADS or adjuvant may be, possibly, the prerequisite and mechanism for the success of immunization of Ags via separate administration, which is of practical significance in that it may allow the subsequent immunization, even with different vaccines, to leave out the VADS or adjuvant.
4.3.5. Ag release and liposome integrity in vivo
Unlike liposomes that are used as a DDS for treating diseases, such as solid tumour and the age-related macular degeneration (AMD), and function via delivering drugs to “actively” target the sick cells or lesions, liposomes as a VADS can take actions through being “passively” targeted by immune cells. This means that a liposome VADS can be captured by immune cells including APCs that are patrolling and searching the alien materials throughout the body while performing their defensive missions. This also means that the Ags delivered by liposomes possess a great chance to be picked up by immune cells, whether they are still carried by the VADS or not. As a result of this, the Ag release or leakage from liposomes seems to make less sense in vaccination and thus, may not receive the concern during the VADS development as enough as that of a DDS does, as reflected by many reports wherein the Ag release test was completely neglected [[123], [124], [125]]. Further, this situation may be exacerbated by the notice that the licensed vaccines based on a VADS are functioning to elicit immunity against pathogens without loading Ags to carriers via encapsulation or adsorption. And they are manufactured mostly through admixing Ags with liposomes, or other NPs, even just before immunization, as exemplified by Shingrix® vaccines which are supplied with separated packages of Ag and VADS [126].
Despite these facts, the Ag release is still one of the factors that have an important impact on the immunostimulatory activity of a liposome VADS, because rapid release will render Ags not only direct exposure to the tissue fluids which may possibly damage their bioactivities, but also wide cellular uptake by, possibly, the APCs that have not been sensitized by the VADS or adjuvant, thus leading to compromised efficacy of vaccination [103]. In addition, certain adjuvants are compounds with a low molecular weight (MW), and incorporation in liposomes or other VADSs can guarantee their co-delivery with Ags to the same population of APCs, while avoiding a systemic distribution and off-target cellular uptake. For example, resiquimod, a small water-soluble agonist of the endosome-located TLR7/8, will rapidly distribute throughout the body after injection in an aqueous solution and, when conjugated to cationic liposomes composed of DDA/TDB, has achieved co-delivery with Ags in mouse models, though this bound combination did not significantly elevate the already strong adjuvanticity of the cationic liposomes [127].
Notably, many studies on liposome VADSs have actually reported the Ag release data [15,56,59,61], however, the direct relationship between the Ag release features and the induced immunity quality has rarely been elucidated. Moreover, these investigations on Ag release were mostly conducted with in vitro experiments, which can hardly reflect the real profile that liposomes will manifest under in vivo circumstances. In vivo, liposomes will encounter numerous chemicals, proteins and enzymes, which may selectively bind to the carrier, possibly catalyse its components to degrade, and even make the carrier broken. Indeed, many types of liposomes (especially, the cationic ones) designed as a VADS are loaded with Ags via electrostatic attraction, and when injected into the body they are likely to be neutralized to lose the carried cargos. In studies relevant to the in vivo characteristics, fluorescent materials are often employed as a label for tracking the behaviour of liposomes in the body [128]. However, this hardly provides a strong evidence to verify the carrier integrity, since fluorescent materials may aggregate to emit light even after carrier collapse. It should be pointed out that even the theoretical analyses sound reasonable, the experimental data on the in vivo characteristics of carriers are yet in shortage and are needed to provide a solid support for the rational design of liposome VADSs.
4.3.6. Vaccination route
Liposomes as a versatile VADS suit various immunization routes, including mainly four ways: injection (such as intramuscular, intradermal, subcutaneous, intraperitoneal and intravenous injection), topical administration (such as cutaneous and mucosal administration); oral uptake [30], and inhalation [129]. Three reasons drive researchers to explore different immunization routes: satisfying dosage form requirement; offering convenient administration; and, most importantly, improving efficacy. Usually, vaccination is performed through administering to three sites: muscle, mucosa and skin; however, recently, intravenous injection (i.v.), by mimicking natural transmission process, such as insect bite, is also trialled for immunization of certain vaccines against some special pathogens, such as Plasmodium falciparum (Pf), which is a mosquito-borne parasite causing malady of malaria through direct entering blood of hosts [130].
The early work by Nussenzweig et al proved that defensive immunity to the pre-erythrocytic phases of Plasmodium berghei (Pb), which causes malaria in certain rodents, could be achieved through immunization by repeated intravenous injection of the X-ray irradiated Pb sporozoites (Pb-SPZ). This early research is of great significance since it primarily paved the way for the development of anti-malaria vaccines [131]. Advancing this technique, researchers recently engineered a novel malaria vaccine composed of the radiation-attenuated, metabolically active PfSPZ. When given to nonhuman primates via i.v. immunization, but not s.c. (subcutaneous), the vaccine triggered strong and durable anti-PfSPZ T cell reactions in peripheral blood, and even more potent in liver, which is the prospective site of immune protection, suggesting i.v. administration an optimal immunization route for the PfSPZ vaccine [130].
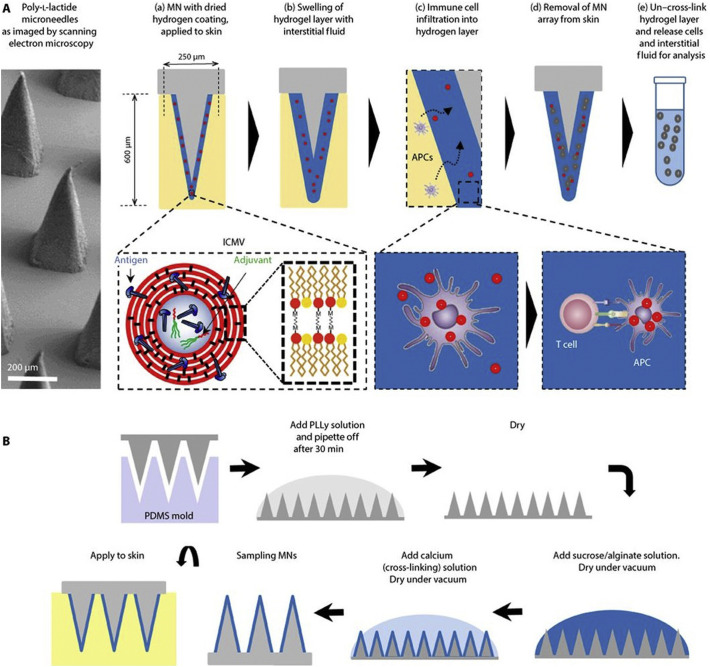
Recent work by Schmidt et al confirmed that i.p. (intraperitoneal) immunization provoked drainage of vaccine to the dLNs, wherein to activate the resident CD8α + DCs for Ag cross presentation, a prerequisite for induction of CTL responses. By comparison, s.c. (subcutaneous) or i.m. (intramuscular) immunization initiated formation of a vaccine depot at administration site, which may hamper the approach of vaccines to CD8α + DCs [132]. Also, Wang's group showed that administration of a multifunctional liposome VADS with microneedle arrays (MAs) to mouse oral cavity mucosa induced robust immunity in oral and gastrointestinal mucosa, favouring prophylaxis of pathogens transmitted via ingesting route [60]. By comparison, when a similar liposome VADS delivered by MAs were administered to mice via vaginal mucosa, it elicited strong immunity in reproductive duct and intestinal lumen, which is of peculiar significance for defending against sexually transmitted infections [28]. More recently, Fox et al. developed LecA Ag-loaded PEGylated liposomes containing two TLRas: GLA (TLR4a) and 3 M-052 (TLR7/8a), which after administered intranasally to mice elicited efficiently both intestinal mucosal and Th1 responses. Compared to s.c. administration, intranasal vaccination induced mice to produce elevated levels of fecal IgA, serum IgG2a, IFN-γ and IL-17A, and finally provided >80% protection of mice from disease challenge [133]. Therefore, immunization route, in addition to the physicochemical and immunological characteristics of a VADS, also plays a key role in eliciting the protective immunity, especially, in mucosa, whereby most pathogens preferentially invade the host.
Taken together, the physicochemical properties of liposomes have a significant influence on the adjuvant and delivery efficacy of the VADS. The crucial influencing parameters include membrane fluidity, surface charge and PEGylation, as well as physical size, each of which plays an individual role in modulating the biological behaviours of the VADS, but mutually influences each other, ultimately providing a collective effect on the immunostimulatory activity. Thus, a liposome VADS may show a depot effect, targeted biodistribution and specific intracellular trafficking, which act in combination to dictate the outcomes of the adaptive immunoresponses. As a result, liposome formulations should be optimized with full consideration on the vast array of lipids and combinations, vesicle structures, and their physicochemical characteristics. These issues may well be regarded as a multi-factorial impact and should be optimally combined in constituting an effective VADS, which is capable of not only delivering Ags but also promoting APC activation and its interaction with the downstream immunocytes to elicit the desired immunity. Though some NPs exemplified here are, in a strict sense, not liposomes, it is known that despite of the carrier type, it is the size, structure, surface properties, and Ag release feature that are the main decisive factors that define the function of a VADS. Therefore, their investigation outcomes and conclusions may provide a useful reference to developing a liposome VADS as well [27].
5. The state-of-the-art liposome VADS
5.1. Designing a liposome VADS based on immune system features
The ultimate goal of developing a VADS is to make a vaccine able to trigger the immune system to establish potent immunity that can prevent pathogen infection, erase the invaded pathogens, or cure harmful lesions [28]. As discussed above, modulation of liposomes on their physicochemical properties such as size and surface charge, can also improve their immunostimulatory effects [27]. However, in more cases, liposomes are specifically engineered in a certain way to engender special functions enabling them to efficiently activate host immune system. Vertebrate immune system according to its function includes two subtypes: the innate immune system, with NK (natural killer) cells, monocytes and, especially, APCs (MPs and DCs) as the main participants; and the adaptive immune system, with T and B cells as the main participants [134]. Whilst the adaptive immune system has evolved for around 500 million years and is confined to vertebrates, the innate immune system has been established for a much longer time and acts as the main defensive strategy among vertebrates, invertebrates, as well as in plants, under modulation by rather conserved mechanisms [135]. Innate immune system plays an important role in constructing the anti-pathogen immunity, because its activation is a precursor and prerequisite to sponsoring the Ag-specific adaptive immunoresponses. Therefore, innate immunoresponses usually occur in advance upon detection of the PAMPs/DAMPs via various PRRs, such as TLRs, CLRs, RLRs and NLRs, commonly expressed on immunocytes, especially, APCs. Notably, these PRRs are providing a collection of targets for designing a VADS to boost immunoresponses toward Ags and thus are now widely employed for engineering functional liposomes, and also other NPs, as a vaccine carrier [136].
At molecular level, liposomes are frequently engineered as a VADS bearing PRRas (PRR agonists) so that they can efficiently target and activate APCs through triggering proinflammatory transcription factors, such as NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells), CREB (cyclic AMP-responsive element-binding protein), AP1 (activator protein 1), IRF3 (IFN-regulatory factor 3) and IRF7, to secret cytokines and chemokines beneficial for promoting innate and adaptive immunoresponses [137]. Other than PRRas, bioactive molecules that have a high affinity to a distinct biomarker on APCs' surface, such as CD47 (clusters of differentiation 47), have also been explored to modify liposomes or other NPs to constitute a targeting VADS for eliciting potent immunity [138].
In practice, as a VADS, liposomes are often designed to target, through depot recruiting or bio-adhesive effects, the sentinel APCs scattered affluently in the superficial tissues such as skin and mucosa. Moreover, liposomes are also engineered to target the secondary lymphoid organ immunocytes, such as DCs, MPs, FDCs (follicular DCs) as well as FBCs (follicular B cells), which are densely clustered within the compartmentalized dLNs or spleen, through PEGylation and size reduction to generate stealth effects to tissue components [139].
Another strategy to improve the immnunostimulatory function of a liposome VADS involves rendering the carrier the ability of engendering lysosome escape, which allows the delivered Ags to avoid the endolysosome degradation. Lysosomal degradation proves a common pathway whereby APCs process the internalized NP-carried Ags into small, but often useless, fragments, and thus may cause compromised vaccination. In contrast, Ags that have successfully managed lysosome escape will enter the cytoplasm and may be handled, like endogenous Ags, by proteasomes to generate a number of epitopes that tend to adhere to MHC-I for presentation, favouring establishing cellular immunity [14,28].
5.2. Liposomes engineered with functional materials for constructing a VADS
Liposomes are known to possess the lipid bilayer membranes, which, despite bearing the intrinsic instability as a negative factor for developing a clinical product, are beneficially providing a unique structure to admit diverse modifications with functional materials in different modes, such as surface adsorption, conjugating, encapsulation or intercalation within lipid bilayers, rendering liposomes a multifunctional VADS [18]. As such, liposomes may be engineered in certain ways to possess special functions that are able to intervene some intracellular processes improving the efficacy of the delivered vaccines [28]. For example, liposomes are frequently engineered with the materials that have the intrinsic or specific adjuvanticity, such as squalene, saponin (e.g., water soluble QS-21), as well as various TLRas (e.g., MPLA and CpG ODN), to boost immunoresponses [5]. Liposomes as a VADS attract research interests thanks to their distinct immuneostimulatory functions, which may be summarized as follows. (1) They can rapidly activate innate immune system which may ultimately translate to robust humoral and cellular immunity against pathogens. (2) They can induce a broad spectrum of adaptive immunity providing protective defence against pathogens of heterovariants. For example, vaccines carried by a MPLA-incorporating liposomes could effectively protect the host from invasion by the strains of influenza or human papillomavirus (HPV) bearing antigenic components not yet included in the products [140]. (3) They can boost immunoresponses and efficacy of vaccination in certain special recipients, such as infants, feeble patients and the elderly, who have an incomplete or weakened immune system. Examples include the marketed products that are formulated with MPLA, CpG ODN and/or QS-21 and can protect these populations against HBV, influenza, or herpes zoster virus [141]. These advantages, together with feasibility of large-scale production, and long records of high safety profile, will pave the way for engineering the advanced liposome VADSs for conquering many devastating pathogens.
To develop a functional mucosal VADS, Wang's group prepared the mannosylated and MPLA-modified liposomes (MMLs) and stealth MPLA-modified liposomes (SMLs), which were fabricated together into the so-called proSMMA (proSML/MLL-constituted microneedle array) (Fig. 7 ) [28]. Vaginal administration of proSMMA to mice by mucosal patching elicited robust anti-Ag humoral as well as cellular immunity at both systemic and mucosal levels, especially, in the reproductive and intestinal tissues. Deep dissection disclosed that the novel VADS engendered the liposome-carried Ags to be phagocytosed by both mucosal and dLN-resident APCs and, thereafter, be substantially cross-presented for MHC-I exposure as a result of lysosome escape and ROS (reactive oxygen species) irritation, which were caused by the functional liposomes, leading to establishing the ultimate comprehensive and intensive immunity. These results proved that liposomes are such a versatile carrier that can be incorporated with the immune-boosting molecules, and they can be further fabricated into the administration-fitted device, like a microneedle array, to form the multifunctional VADS, which is able to elicit strong immunity at mucosa to set up the first line of defence against pathogens [27].
Fig. 7.
Schematic of proSMMA designed as a robust mucosal VADS consisting of a mixture of 50 nm stealth MPLA-liposomes and mannosylated MPLA-liposomes fabricated into a microneedle array.
Recently, Karmakar et al. made the L-rhamnose (Rha) epitope-anchored liposomes conjugated with the synthetic TLR2a Pam3Cys-linked glycopeptide Ags derived from MUC1, which is a mucin overexpressed on several types of cancers, and had been engineered to contain a GalNAcα1-Ser/Thr segment to have the immunogenic peptide epitopes exposed to immune system (Fig. 8 ) [142]. In vivo experiments demonstrated that, administered to the Rha-preimmunized C57BL/6 mice, the Rha epitope- and Ag-anchored liposomes remarkably enhanced production of the MUC1-specific CTLs as well as Abs. In particular, this kind of anti-MUC1 Abs might possess a big potential to bind to and kill tumour cells through ADCC (Ab dependent cell-mediated cytotoxicity) via FcγRs (Fcγ receptors) expressed on certain myeloid cells, such as NK cells, MPs, neutrophils and eosinophils [143]. Further, the authors showed that in vivo Rha-modified liposomes were bound by anti-Rha Abs to form the immune complexes, which facilitated APC uptake and (cross) presentation of MUC1 Ags. These results suggest the possibility of using a liposome VADS to enhance APC cross presentation of Ag epitopes on MHC-I through Ab-mediated phagocytosis via formation of anti–Rha–FcR (Fc receptor) (Fig. 8), which occurs owing to the presence of natural anti-Rha Abs. Notably, in humans the natural anti-Rha Abs exist abundantly as IgM and IgG2 (IgG3 in mice) [144], which generally bind poorly to FcγR and thus, will exert trivial inhibitory effects on the ADCC-based anticancer activities.
Fig. 8.
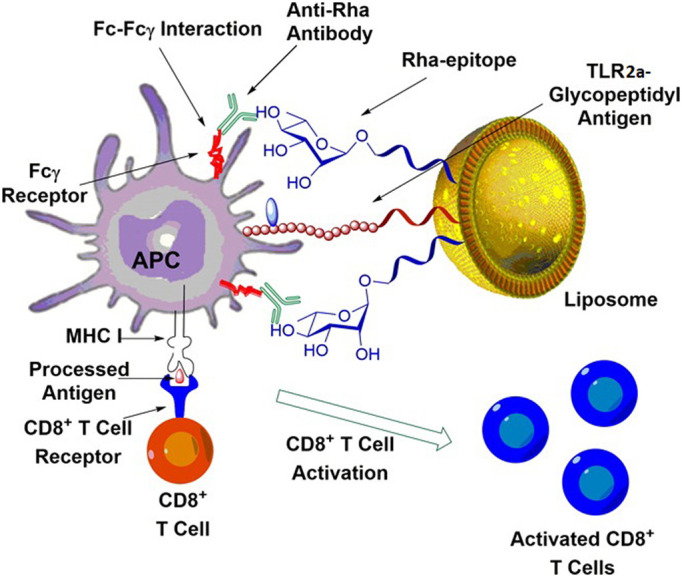
Schematic structure the VADS constructed with the Rha epitope-anchored liposomes loaded with Pam3Cys-linked MUC1 glycopeptide Ags, and the principle underlying the immunostimulatory action of the VADS. Reprinted with permission from Reference [142].
It is desirable to make a VADS that can be used to temporally monitor the immunoresponses including the activity and state of immune cell populations residing within tissues, meanwhile it can well perform immunization process. This concept of a VADS is of significance with regard to several issues involved in vaccination, such as immune reaction supervision, efficacy evaluation, regime regulation, and immunization compliance. Recently, Irvine's group created a novel VADS bearing such a concept and fabricated with poly-l-lactide-constructed microneedle array (MA), of which microneedle projections were coated with a mixture of the Ag/CpG (an adjuvant of TLR9a)-loaded liposomes (interbilayer cross-linked multilamellar vesicles, ICMVs) and the Ca2+ cross-linked alginate polymers, to form the stimulatory sampling microneedles (SSMNs) [145]. After insertion into skin, SSMN polymers swelled due to hydration into a porous matrix capable of longitudinal sampling of the interstitial fluid and the infiltrated local leukocytes, enabling parallel monitoring of immune reactions while causing trivial injury (Fig. 9 ). In mice, even after the anti-Ag T cells in systemic circulation could not be detected by conventional protocol, SSMNs still successfully sampled the tissue-resident memory T cells in the skin, while exerting no influence on the immune status of naïve or antigen-exposed animal models. Could this liposome-constructed SSMNs be successfully adapted to accomplish the vaccination regime while temporally monitoring immunocytes and collecting immunological information from the skin and mucosal tissues in a non-invasive manner, would it represent a superior functional VADS.
Fig. 9.
Schematic of description of preparation of SSMNs (stimulatory sampling microneedles) for inducing and monitoring immunoresponses. (A) Schematic of structure of a SSMN and its action mechanism. (B) Microneedle engineering process. PLLy, poly-l-lysine; PDMS, polydimethylsiloxane. Partially reprinted with permission from Reference [145].
In summary, the immune system of mammals is equipped with numerous competent components, such as various leukocytes and their subsets, cell receptors, cytokines, chemokines and compliments, which are tightly linked into a complex integrity. These components orchestrate exquisitely to fulfil the defensive functions and are also providing multiple targets for designing functional liposomes as a VADS. Modulation of each of the individual components may significantly affect the whole community of signalling pathways that are involved in immunoresponses or other biological processes. It also proves feasible to have multiple immune components targeted simultaneously by multifunctional liposomes customized as a VADS able to effectively boost the immunity against pathogens.
6. Liposome VADS-based vaccines in clinical immunization
Liposomes are a suitable API (active pharmaceutical ingredient) carrier possessing numerous advantages, including easy and large-scale preparation, as mentioned above. However, it took a rather long time to make the first drug-loaded liposomes ever come out as a clinical product. This is a bit of discouraging and far beyond the commitment of some liposome scientists, especially, in the middle of 1980s, when some researchers even doubted the feasibility of liposome DDS [146]. Fortunately, just at that time, certain liposome-based cosmetic products were successfully brought to markets by several giant fashion-focused companies. It was hopeful and never helpless for pharmaceutical scientists to see that the first batch of liposomal products were flamboyantly marketed as an anti–aging cream with an exaggerated name of ‘Capture’ introduced by Dior in 1986, followed immediately by ‘Niosome’ marketed in 1987 by Lancome, a L'Oreal company [147]. Encouraged by this, combined with great achievements in optimization of liposome formulations, such as control of vesicle size, and lyophilisation of stable dry products with disaccharides as a lyoprotectant, scientists eventually brought to European markets in 1990 the first therapeutic liposomal product, Ambisome®. It is amphotericin B-loaded SUVs with the optimized formulation of HSPC/CHO/DSPG/amphotericin B (5:2.5:2:1, mole ratio) and a size of around 40 nm, and was later authorized in USA in 1997, to treat systemic fungal infections and certain protozoan infections such as visceral leishmaniasis and primary amoebic meningoencephalitis [148,149]. Meanwhile, two breakthrough techniques, remote loading drugs into liposomes and PEGylation of liposome surfaces, were achieved and matured to clear, in the development of liposomal products, the longstanding crucial barriers, including poor encapsulation of drugs, instability of an aqueous suspension of liposomes, and very short circulation time of in vivo liposomes due to rapid removal conducted by mononuclear phagocytic system (MPS) after systemic injection. As a consequence of these achievements, the first anticancer liposomal drug Doxil®, which is the Stealth® (pegylated) liposomes composed of HSPC/CHO/DSPE-mPEG2000 (3:1:1, mass ratio), having a size of about 100 nm, and loaded by ammonium sulfate-gradient procedure with doxorubicin (total lipid/drug = 8:1, mass ratio), came ultimately into markets after final approval by FDA in 1995 for the treatment of AIDS-related Kaposi's sarcoma, and later expanded for therapy of ovarian cancer in 1999, and for multiple myeloma in 2007 [72].
Up to now (April 16, 2019) there are, world-widely, >22 liposomal products that have ever entered markets for treatment of various diseases, including infections, pains, and mostly tumours, as listed in Table 1 . Notably, 5 among these approved liposomal products are just the liposome VADS-based vaccines that have been authorized for prophylaxis of pathogenic infections in healthy populations [150]. Epaxal and Inflexal are administered intramuscularly for defending against hepatitis A virus and seasonal influenza virus, respectively [151]. Nasalflu is given uniquely by intranasal spray against also influenza virus, but was, unfortunately, withdrawn from markets shortly after its approval due to causing Bell's palsy (facial paralysis) in some recipients [152]. Other approved liposome vaccines are well introduced elsewhere [5,62], and herein, the newly authorized vaccine Shingrix® is presented with a bit of detail.
Table 1.
Marketed liposomal productsa.
| Product (manufacturer) | Drug | Indications | Year approvedb |
|---|---|---|---|
| AmBisome (Gilead) | Amphotericin B | Fungal infections and Leishmaniasis | 1990 (Europe), 1997 (USA) |
| Doxil (Johnson&Johnson) | Doxorubicin | Kaposi's sarcoma | 1995 |
| Ovarian cancer | 1999 | ||
| Breast Cancer | 2003 | ||
| Multiple myeloma + Velcade | 2007 (Europe, Canada) | ||
| DaunoXome (Galen) | Daunorubicin | Kaposi's sarcoma | 1996 (Europe), 1996 (USA) |
| Myocet (Cephalon) | Doxorubicin | Breast cancer+cyclophosphamide | 2000 (Europe) |
| Epaxal (Crucell, Switzerland) | Virosome | Hepatitis A virus | 1994 (Europe) |
| Inflexal (Crucell, Switzerland) | Virosome | Influenza virus | 1997 (Europe) |
| Nasalflu | Virosome | Influenza virus (Intranasal application) | 2000 (Europe, withdrawn in 2001) |
| Amphotec (Intermune) | Amphotericin B | Invasive aspergillosis | 1996 |
| Abelcet (Enzon) | Amphotericin B | Aspergillosis | 1995 |
| Visudyne (QLT) | Verteporphin | Wet macular degeneration | 2000 (USA), 2003 (Japan) |
| DepoDur (Pacira) | Morphine sulfate | Pain following surgery | 2004 |
| DepoCyt (Pacira) | Cytosine Arabinoside | Lymphomatous or Neoplastic meningitis | 1999 |
| Exparel (Pacira) | Bupivacaine | Post-surgical pain | 2011 |
| Lipo-Dox (TLC, Taiwan) | Doxorubicin | Kaposi's sarcoma, breast and ovarian cancer | 2002 (Taiwan) |
| Lipusu (Luye Pharma, China) | Paclitaxel | Ovarian cancer | 2003 (China) |
| Breast cancer | |||
| Non-small cell lung cancer (NSCLC) | |||
| Mepact (Takeda) | Muramyl tripeptide-PE | Nonmetastatic, resectable osteosarcoma | 2009 (Europe) |
| Marqibo (Talon) | Vincristine | Acute lymphoblastic leukemia | 2012 |
| Onivyde (Merrimack) | Irinotecan | Pancreatic cancer | 2015 |
| Vyxeos (Jazz/Celator) | Ara-C/DNR (5:1) | Acute myeloid leukemia (AML) | 2017 |
| Mosquirix (GlaxoSmithKline) | HBsAg-VLP/MPLA/CSP | Malaria | 2015 |
| Shringrix (GlaxoSmithKline) | MPLA/QS21/gE | Shingles | 2017 |
| Onpattro (Alnylam) | siRNA | Polyneuropathy (Hereditary transthyretin amyloidosis) | 2018 |
World-widely, there are also several approved generic liposome products, and they are not listed here owing to lack of novelty.
The product was licensed in USA unless an annotation indicates where it was.
Shingrix® is the state-of-the-art liposome VADS-based novel subunit vaccine developed by pharmaceutical giant GSK (GlaxoSmithKline) and was approved in October 2017 by US FDA for prophylaxis in the elderly aged 50 years and older of shingles (or herpes zoster, HZ). Shingles is caused by reactivation of the dormant DNA VZV (varicella zoster virus, a member of the herpesvirus family) that has resided in sensory dorsal root ganglia after its first infection of hosts, likely, causing relatively mild varicella (also called chickenpox) (Fig. 10 ) [62]. The primary infection triggers response to VZV leading to the release of antiviral cytokines and activation of NK cells usually able to control viral replication in the mucosa. Also, the primary infection stimulates the hosts to establish the adaptive and memory immunity forming persistence of anti-VZV IgG and VZV-specific CD4+ T cells and, particularly, CD8+ CTLs. This kind of immunity correlates best with the severity of primary infection and preventing reactivation of latent VZV residing in ganglionic cells, but, unfortunately, naturally wanes over time, allowing possible occurrence of shingles in late life [153]. Shingles may be caused by risk factors including old age, weakened cellular immunity, illness, female gender, genetic susceptibility, mechanical trauma, recent psychological stress and white race. Shingles usually manifests the prodrome of acute neuralgia for 2–100 days preceding the rash, accompanied by potentially devastating complications of secondary bacterial infection and sepsis, meningoencephalitis, visual and hearing impairment [154].
Fig. 10.
Structure of VZV virus and anatomy of sensory dorsal root ganglia, where VZV (varicella zoster virus) reside silently after primary infection. Reprinted with permission from ViralZone (Source: ViralZone: www.expasy.org/viralzone, SIB Swiss Institute of Bioinformatics).
In fact, in 2006 a live-attenuated vaccine Zostavax® for HZ was already licensed to Merck/Sanofi Pasteur for adults over 50 years of age at the price of $100–200 for a single dose regime, which can effectively reduce the incidence and complications of shingles. However, the vaccine efficacy decreases rapidly with time and the incidence of shingles may return to around baseline levels around year 8 post vaccination. Moreover, there are certain contra-indications to the live-attenuated microbe-based vaccines, e.g., in VZV naive or immunosuppressed patients, the vaccination may cause diseases [155]. This suggests that it is desirable to develop an alternative that should be a subunit vaccine with a good safety profile and high efficacy. And these issues represent the guidance and impetus to the design and development of novel vaccines against shingles, and thus comes Shingrix®.
Shingrix® is an anti-HZ subunit vaccine based on a liposome VADS and is provided with two parts: AS01b as the VADS, and the freeze-dried powder of recombinant VZV glycoprotein E (gE) as the Ag. AS01b is a VADS consisting of an aqueous suspension of DOPC/CHO/MPLA liposomes with a size of 50–100 nm [156] bound by QS21 to liposome CHO (Fig. 11 ) [157]. The Ag of gE is the most abundant glycoprotein on both VZV virions and cells infected with VZV and plays a crucial role in virus replication and cell-to-cell spread, while it can elicit a greater immune response than other glycoprotein antigens [158]. The accomplished clinical trials confirmed that the vaccine efficacy to prevent the development of shingles for up to 4 years follow up was 97% overall in subjects older than 50 years of age, and around 90% in participants older than 70 years old. More importantly, the anti-Ag response appears preserved with age since protective efficacy waned minimally over 4 years and was well maintained in adults aged over 70 years [159]. The rational design of Shingrix® can be attributed to several aspects: the strong background study results in the right choice of the proper Ag of gE; the component QS21 guarantees effective activation of innate immunoresponses, while MPLA-liposomes direct the response toward Th1 pathway via triggering TLR4 leading to robust cellular immunity, and the liposome VADS has a high stability [160].
Fig. 11.
Schematic description of AS01b which is a liposome-based VADS. It is used for boosting the efficacy of a subunit vaccine, Shingrix®, against VZV (varicella zoster virus), which contains the Ag of gE. Reprinted with a little adaption with permission from Reference [157].
The significance of success of Shingrix® to the both vaccine and liposome fields lies in that it firmly demonstrates that a subunit vaccine not only possesses a good safety profile but also has the potential to elicit the anti-pathogen immunity stronger than that by the whole microbe-based traditional vaccines, as long as it is rationally formulated as a suitable VADS based on an appropriate nanocarrier, such as, liposomes.
7. Perspectives
After decades of development, liposomes have now been successfully employed as a DDS or VADS for various clinical therapies, focusing mainly on treatment of cancer and vaccination against infectious disease, as listed in Table 1. In particular, liposomes as a VADS can be easily decorated with various immune-boosting molecules and are thus capable of remarkably improving the efficacy of a subunit vaccine [161], such as Shingrix®, even to surpass the conventional whole microbe-based vaccines, which are always concerned with reversion of virulence to cause severe complications [47].
VADSs based on other micro−/nanoparticles, such as metal (gold, silver, iron) NPs, inorganic (silica) NPs and polymeric NPs, though have also showed a few advantages over conventional formulations in fulfilling functions of delivery and adjuvant, are mostly in early development [134]. An emulsion-based VADS, MF59, approved to Novartis for delivery of influenza vaccines, is a defined formulation consisting of o/w emulsion of squalene/Span85 /Tween80/water [162], which was later reformulated by GSK as an emulsion of squalene/α-tocopherol/Tween 80/water, known as AS03 [126]. However, unlike liposomes, emulsions are in fact a very fragile carrier having a limited capacity to allow surface modification with functional materials, such as targeting molecules, to form a diverse VADS for delivering subunit vaccines. Alum is another strong VADS that has been used for nearly a century in enhancing antibody immunity [50]; unfortunately, it often fails in eliciting an effective cellular immunity while showing drawback of reactogenicity associated with unwanted inflammatory side effects. Moreover, alum is not always providing a shelter to labile ingredients but may undermine the quaternary or tertiary structure of proteins due to its potent phosphophilicity exerting strong binding to protein phosphorus groups [163]. By contrast, liposomes can be easily engineered to possess beneficial features of an effective VADS in delivery of Ags, while showing good biocompatibility and minimal side effects.
In prospect, liposomes may be further formulated into an advanced VADS that is suitable for developing the efficacious vaccines to combat many tedious pathogens, especially, some most challenging ones, such as HIV, HCV, HSV, and even the emerging super bugs [164,165]. For this, in future, liposomes may be developed to play roles of a versatile VADS that can mimic the way whereby the Ags are maintained and exposed to APCs by a pathogen during a natural infection to trigger strong immunoresponses [166]. Achieving this requires liposomes in structure to keep the Ags natural for presentation through, possibly, embedding with multiple copies of the same peptide Ags, while rendering Ags resistance to environmental violation. Other aspects that may be emphasized to improve the immunostimulatory function of liposomes may involve: acting as a depot to increase the exposure time of Ags to immune cells and enhance APC uptake of vaccines; targeting immunocytes or lymph nodes to save Ags; fitting mucosal vaccination to set up both systemic and mucosal immunity; rendering Ags lysosome escape to facilitate cross-presentation of Ags via MHC-I presentation to establish cellular immunity for erasing cell-hidden pathogens or tumour cells [53]. Notably, some functions such as engendering lysosome escape, enhancing Ag cross presentation, and controlling immunological expression in a specific pathway, can hardly be obtained unless the related intracellular biological features are fully understood and able to be explored to test the activity of a VADS that is tailored with the corresponding functions, such as response to environmental stimulus, sensing of physiological factor, and targeting intracellular structures [167].
Also, numerous questions still remain open with regard to the development of a liposome VADS for producing clinical products. A few opening questions may be listed here to call on attention and considerations from researchers who are committed to making liposome vaccines. Also, a list of common issues still exists there as the bottleneck in translation of the laboratory work on liposome VADS into the clinical application, while often being ignored by researchers.
First, safety should be put forward in the front of the rank, given novel liposomes incorporating synthetic materials may possibly generate the potential cytotoxicity in vivo. Moreover, most of novel liposome VADSs do not have a (long) record of a safety profile in even animal models, let alone human use, leaving an open question on toxicity to be fully addressed before their acceptance as an alternative method for delivering novel human vaccines [167].
Second, stability is another outstanding concern, considering liposomes as a colloidal system, though have been conquered of aggregation instability by PEGylation and lyophilization, are often customized with functional materials, especially the environment-sensitive ones, which render the carrier unendurable to a long-term storage.
Third, scale-up production of the liposomes that are subjected to several levels of modification is of practical difficulty, because the complexed procedure with too many steps may make inconsistency in batch-to-batch production. Meanwhile, modification with the expensive materials may enhance the production cost to a completely unacceptable level, representing another problem.
Fourth, translation of the efficacy of novel liposome VADS from animal studies to human trials confronts also many obstacles. At present, various types of the liposome VADSs have been elaborately designed and presented with promising outcomes of immune-boosting activities in, unfortunately, just murine and other small animal models, which may be yet the inappropriate one for a specific vaccine [26]. In most cases, it is yet impractical to directly interpret the results from such studies as documented only in rodents to a clinical reference, whereas solid conclusions drawn from investigations using different animal models, such as pigs, rhesus monkeys, and even chimpanzees, are often lacking in studying a novel liposome VADS.
Fifth, the prevalence of low reproducibility of the currently published outcomes, especially, in drug development field [168], resulted from the inappropriate designs with little practical rationality combined with the positive result-oriented publishing bias and the misconception of “research for publication” [169], sets up also a huge barrier to translation of bench work to bed therapy. Notably, the unreproducible results not only cause a substantial waste of valuable resources but also badly discourage industrial partners to devote to translation of the findings by researchers from the academia circle [170]. Although the reproducibility of the published results on liposome VADSs remains unclear, the reality can hardly be optimistic based on the disappointed outcomes from such investigations as performed in drug development and other fields. This concern is also indirectly reflected by the professional comments on certain publications complaining the difficulty in interpreting the reported results and also by the disturbance arising from the observations in contradiction to the printed outcomes [171,172]. Obviously, these are a kind of “side effects” arising from the development of vaccines, even when they are based on a liposome VADS claimed of high safety profiles.
8. Conclusions
Liposomes are now still extensively employed as an efficient carrier for delivering vaccines owing to their beneficial properties, such as high safety, big loading capacity, and easy preparation and decoration to engender multiple functions. In particular, when incorporated with functional molecules, such as ligands to TLRs and adjuvanticity-bearing chemicals, liposomes can actually act as a versatile VADS to greatly enhance the immunity induced by the carried vaccines. As researchers continuously commit their efforts to developing optimal formulations combined with the emergence of novel materials, and the inspiration of deep understanding of the processes involved in the immunological reactions triggered by vaccination, numerous problems and barriers to developing functional liposome VADSs will be handled. As a result, many novel vaccines that are based on a liposome VADS will eventually be produced for clinical immunization to conquer a variety of the life-threatening diseases.
Acknowledgments
Acknowledgements
The authors are grateful to the anonymous reviewers for their precious time and valuable suggestions on improving the quality of the manuscript. The authors thank the family of T Wang for supplying him plenty of time dedicated to writing and editing the manuscript.
Conflict of interest
All the authors declared no conflict of interests.
Funding
This work was financially supported by National Natural Science Foundation of China (Grant number 81703449), Natural Science Foundation of Anhui Province (Grant number 1708085QH196), the Department of Education of Anhui Province (Grant numbers KJ2016SD28 and gxfxZD2016045), and also by the Department of Human Resource & Social Security of Anhui Province (Grant number 2018H173).
References
- 1.De Gregorio E., Rappuoli R. From empiricism to rational design: a personal perspective of the evolution of vaccine development. Nat. Rev. Immunol. 2014;14(7):505–514. doi: 10.1038/nri3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Plotkin S.A. Vaccines: the fourth century. Clin. Vaccine Immunol. 2009;16(12):1709–1719. doi: 10.1128/CVI.00290-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gao Y., McKay P.F., Mann J.F.S. Advances in HIV-1 vaccine development. Viruses. 2018;10(4) doi: 10.3390/v10040167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koff W.C., Burton D.R., Johnson P.R., Walker B.D., King C.R., Nabel G.J., Ahmed R., Bhan M.K., Plotkin S.A. Accelerating next-generation vaccine development for global disease prevention. Science. 2013;340(6136) doi: 10.1126/science.1232910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Del Giudice G., Rappuoli R., Didierlaurent A.M. Correlates of adjuvanticity: A review on adjuvants in licensed vaccines. Semin. Immunol. 2018;39:14–21. doi: 10.1016/j.smim.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Gupta S.B., Coller B.A., Feinberg M. Unprecedented pace and partnerships: the story of and lessons learned from one Ebola vaccine program. Expert Rev. Vaccines. 2018;17(10):913–923. doi: 10.1080/14760584.2018.1527692. [DOI] [PubMed] [Google Scholar]
- 7.Germain R.N. Vaccines and the future of human immunology. Immunity. 2010;33(4):441–450. doi: 10.1016/j.immuni.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 8.Sarnquist C., Holubar M., Garcia-Garcia L., Ferreyra-Reyes L., Delgado-Sanchez G., Cruz-Hervert L.P., Montero-Campos R., Altamirano J., Purington N., Boyle S., Modlin J., Ferreira-Guerrero E., Canizales-Quintero S., Diaz Ortega J.L., Desai M., Maldonado Y.A. Protocol paper: Oral poliovirus vaccine transmissibility in communities after cessation of routine Oral poliovirus vaccine immunization. Clin. Infect. Dis. 2018;67(suppl_1):S115–S120. doi: 10.1093/cid/ciy606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gregory A.E., Titball R., Williamson D. Vaccine delivery using nanoparticles. Front Cell Infect. Mi. 2013;3 doi: 10.3389/fcimb.2013.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Skwarczynski M., Toth I. Recent advances in peptide-based subunit nanovaccines. Nanomedicine (London) 2014;9(17):2657–2669. doi: 10.2217/nnm.14.187. [DOI] [PubMed] [Google Scholar]
- 11.Perrie Y., Mohammed A.R., Kirby D.J., McNeil S.E., Bramwell V.W. Vaccine adjuvant systems: enhancing the efficacy of sub-unit protein antigens. Int. J. Pharm. 2008;364(2):272–280. doi: 10.1016/j.ijpharm.2008.04.036. [DOI] [PubMed] [Google Scholar]
- 12.Sun L.J., Wu J.X., Du F.H., Chen X., Chen Z.J.J. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. 2013;339(6121):786–791. doi: 10.1126/science.1232458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coffman R.L., Sher A., Seder R.A. Vaccine adjuvants: putting innate immunity to work. Immunity. 2010;33(4):492–503. doi: 10.1016/j.immuni.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang X., Wang N., Li N., Zhen Y., Wang T. Multifunctional particle-constituted microneedle arrays as cutaneous or mucosal vaccine adjuvant-delivery systems. Hum Vaccin Immunother. 2016;12(8):2075–2089. doi: 10.1080/21645515.2016.1158368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang T., Zhen Y.Y., Ma X.Y., Wei B., Wang N. Phospholipid bilayer-coated aluminum nanoparticles as an effective vaccine adjuvant-delivery system. ACS Appl. Mater. Interfaces. 2015;7(12):6391–6396. doi: 10.1021/acsami.5b00348. [DOI] [PubMed] [Google Scholar]
- 16.Wang T., Wang N. Preparation of the multifunctional liposome-containing microneedle arrays as an Oral cavity mucosal vaccine adjuvant-delivery system. Methods Mol. Biol. 2016;1404:651–667. doi: 10.1007/978-1-4939-3389-1_42. [DOI] [PubMed] [Google Scholar]
- 17.Wang T., Wang N. Biocompatible mater constructed microneedle arrays as a novel vaccine adjuvant-delivery system for cutaneous and mucosal vaccination. Curr. Pharm. Design. 2015;21(36):5245–5255. doi: 10.2174/1381612821666150923100147. [DOI] [PubMed] [Google Scholar]
- 18.Wang N., Wang T. Preparation of multifunctional liposomes as a stable vaccine delivery-adjuvant system by procedure of emulsification-Lyophilization. Methods Mol. Biol. 2016;1404:635–649. doi: 10.1007/978-1-4939-3389-1_41. [DOI] [PubMed] [Google Scholar]
- 19.HogenEsch H., O'Hagan D.T., Fox C.B. Optimizing the utilization of aluminum adjuvants in vaccines: you might just get what you want. NPJ vaccines. 2018;3 doi: 10.1038/s41541-018-0089-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bangham A.D., Standish M.M., Watkins J.C. Diffusion of univalent ions across the lamellae of swollen phospholipids. J. Mol. Biol. 1965;13(1):238–252. doi: 10.1016/s0022-2836(65)80093-6. [DOI] [PubMed] [Google Scholar]
- 21.Weissig V. Liposomes came first: the early history of Liposomology. Methods Mol. Biol. 2017;1522:1–15. doi: 10.1007/978-1-4939-6591-5_1. [DOI] [PubMed] [Google Scholar]
- 22.Gregoriadis G. Liposome research in drug delivery: the early days. J. Drug Target. 2008;16(7–8):520–524. doi: 10.1080/10611860802228350. [DOI] [PubMed] [Google Scholar]
- 23.Allen T.M., Cullis P.R. Liposomal drug delivery systems: from concept to clinical applications. Adv. Drug Deliv. Rev. 2013;65(1):36–48. doi: 10.1016/j.addr.2012.09.037. [DOI] [PubMed] [Google Scholar]
- 24.Allison A.G., Gregoriadis G. Liposomes as immunological adjuvants. Nature. 1974;252(5480):252. doi: 10.1038/252252a0. [DOI] [PubMed] [Google Scholar]
- 25.Gregoriadis G., Allison A.C. Entrapment of proteins in liposomes prevents allergic reactions in pre-immunised mice. FEBS Lett. 1974;45(1):71–74. doi: 10.1016/0014-5793(74)80813-6. [DOI] [PubMed] [Google Scholar]
- 26.Gregoriadis G., McCormack B., Obrenovic M., Perrie Y., Saffie R. Liposomes as immunological adjuvants and vaccine carriers. In: O'Hagan D.T., editor. Vaccine Adjuvants: Preparation Methods and Research Protocols. Springer; New York: 2000. pp. 137–150. [Google Scholar]
- 27.Perrie Y., Crofts F., Devitt A., Griffiths H.R., Kastner E., Nadella V. Designing liposomal adjuvants for the next generation of vaccines. Adv. Drug Deliv. Rev. 2016;99:85–96. doi: 10.1016/j.addr.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 28.Wang N., Zhen Y., Jin Y., Wang X., Li N., Jiang S., Wang T. Combining different types of multifunctional liposomes loaded with ammonium bicarbonate to fabricate microneedle arrays as a vaginal mucosal vaccine adjuvant-dual delivery system (VADDS) J. Control. Release. 2017;246:12–29. doi: 10.1016/j.jconrel.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 29.Wang N., Wang T., Zhang M.L., Chen R.N., Niu R.W., Deng Y.H. Mannose derivative and lipid A dually decorated cationic liposomes as an effective cold chain free oral mucosal vaccine adjuvant-delivery system. Eur. J. Pharm. Biopharm. 2014;88(1):194–206. doi: 10.1016/j.ejpb.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 30.Wang N., Wang T., Zhang M., Chen R., Deng Y. Using procedure of emulsification-lyophilization to form lipid A-incorporating cochleates as an effective oral mucosal vaccine adjuvant-delivery system (VADS) Int. J. Pharm. 2014;468(1–2):39–49. doi: 10.1016/j.ijpharm.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 31.Wang T., Zhen Y.Y., Ma X.Y., Wei B.A., Li S.Q., Wang N.N. Mannosylated and lipid A-incorporating cationic liposomes constituting microneedle arrays as an effective oral mucosal HBV vaccine applicable in the controlled temperature chain. Colloid Surf. B. 2015;126:520–530. doi: 10.1016/j.colsurfb.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 32.Pulendran B., Ahmed R. Immunological mechanisms of vaccination. Nat. Immunol. 2011;12(6):509–517. doi: 10.1038/ni.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andre F.E. Vaccinology: past achievements, present roadblocks and future promises. Vaccine. 2003;21(7–8):593–595. doi: 10.1016/s0264-410x(02)00702-8. [DOI] [PubMed] [Google Scholar]
- 34.Saalmuller A. New understanding of immunological mechanisms. Vet. Microbiol. 2006;117(1):32–38. doi: 10.1016/j.vetmic.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 35.Kuka M., Iannacone M. Viral subversion of B cell responses within secondary lymphoid organs. Nat. Rev. Immunol. 2018;18(4):255–265. doi: 10.1038/nri.2017.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kashem S.W., Haniffa M., Kaplan D.H. Antigen-presenting cells in the skin. Annu. Rev. Immunol. 2017;35:469–499. doi: 10.1146/annurev-immunol-051116-052215. [DOI] [PubMed] [Google Scholar]
- 37.Heesters B.A., van der Poel C.E., Das A., Carroll M.C. Antigen presentation to B cells. Trends Immunol. 2016;37(12):844–854. doi: 10.1016/j.it.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 38.Martin M.D., Badovinac V.P. Defining memory CD8 T cell. Front. Immunol. 2018;9:2692. doi: 10.3389/fimmu.2018.02692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yuseff M.I., Lennon-Dumenil A.M. B cells use conserved polarity cues to regulate their antigen processing and presentation functions. Front. Immunol. 2015;6 doi: 10.3389/fimmu.2015.00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grimm S.K., Ackerman M.E. Vaccine design: emerging concepts and renewed optimism. Curr. Opin. Biotechnol. 2013;24(6):1078–1088. doi: 10.1016/j.copbio.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nutt S.L., Hodgkin P.D., Tarlinton D.M., Corcoran L.M. The generation of antibody-secreting plasma cells. Nat. Rev. Immunol. 2015;15(3):160–171. doi: 10.1038/nri3795. [DOI] [PubMed] [Google Scholar]
- 42.Hess C., Winkler A., Lorenz A.K., Holecska V., Blanchard V., Eiglmeier S., Schoen A.L., Bitterling J., Stoehr A.D., Petzold D., Schommartz T., Mertes M.M., Schoen C.T., Tiburzy B., Herrmann A., Kohl J., Manz R.A., Madaio M.P., Berger M., Wardemann H., Ehlers M. T cell-independent B cell activation induces immunosuppressive sialylated IgG antibodies. J. Clin. Invest. 2013;123(9):3788–3796. doi: 10.1172/JCI65938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schreiner D., King C.G. CD4+memory T cells at home in the tissue: mechanisms for health and disease. Front. Immunol. 2018;9 doi: 10.3389/fimmu.2018.02394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Omilusik K.D., Goldrath A.W. The origins of memory T cells. Nature. 2017;552(7685):337–339. doi: 10.1038/d41586-017-08280-8. [DOI] [PubMed] [Google Scholar]
- 45.Seifert M., Kuppers R. Human memory B cells. Leukemia. 2016;30(12):2283–2292. doi: 10.1038/leu.2016.226. [DOI] [PubMed] [Google Scholar]
- 46.Plotkin S.A., Plotkin S.L. The development of vaccines: how the past led to the future. Nat. Rev. Microbiol. 2011;9(12):889–893. doi: 10.1038/nrmicro2668. [DOI] [PubMed] [Google Scholar]
- 47.Bandyopadhyay A.S., Garon J., Seib K., Orenstein W.A. Polio vaccination: past, present and future. Future Microbiol. 2015;10(5):791–808. doi: 10.2217/fmb.15.19. [DOI] [PubMed] [Google Scholar]
- 48.Vetter V., Denizer G., Friedland L.R., Krishnan J., Shapiro M. Understanding modern-day vaccines: what you need to know. Ann. Med. 2018;50(2):110–120. doi: 10.1080/07853890.2017.1407035. [DOI] [PubMed] [Google Scholar]
- 49.Hem S.L., HogenEsch H. Relationship between physical and chemical properties of aluminum-containing adjuvants and immunopotentiation. Expert Rev. Vaccines. 2007;6(5):685–698. doi: 10.1586/14760584.6.5.685. [DOI] [PubMed] [Google Scholar]
- 50.Glenny A., Pope C., Waddington H., Wallace U. The antigenic value of toxoid precipitated by potassium alum. J. Pathol. 1926;29:31–40. [Google Scholar]
- 51.Awate S., Babiuk L.A., Mutwiri G. Mechanisms of action of adjuvants. Front. Immunol. 2013;4 doi: 10.3389/fimmu.2013.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Di Pasquale A., Preiss S., Tavares Da Silva F., Garcon N. Vaccine adjuvants: from 1920 to 2015 and beyond. Vaccines (Basel) 2015;3(2):320–343. doi: 10.3390/vaccines3020320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moyer T.J., Zmolek A.C., Irvine D.J. Beyond antigens and adjuvants: formulating future vaccines. J. Clin. Invest. 2016;126(3):799–808. doi: 10.1172/JCI81083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sahdev P., Ochyl L.J., Moon J.J. Biomaterials for nanoparticle vaccine delivery systems. Pharm. Res. 2014;31(10):2563–2582. doi: 10.1007/s11095-014-1419-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leroux-Roels G. Unmet needs in modern vaccinology: adjuvants to improve the immune response. Vaccine. 2010;28(Suppl. 3):C25–C36. doi: 10.1016/j.vaccine.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 56.Li N., Wang N., Wang X., Zhen Y., Wang T. Microneedle arrays delivery of the conventional vaccines based on nonvirulent viruses. Drug Deliv. 2016;23(9):3234–3247. doi: 10.3109/10717544.2016.1165311. [DOI] [PubMed] [Google Scholar]
- 57.Pati R., Shevtsov M., Sonawane A. Nanoparticle vaccines against infectious diseases. Front. Immunol. 2018;9:2224. doi: 10.3389/fimmu.2018.02224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kammona O., Bourganis V., Karamanidou T., Kiparissides C. Recent developments in nanocarrier-aided mucosal vaccination. Nanomedicine (London) 2017;12(9):1057–1074. doi: 10.2217/nnm-2017-0015. [DOI] [PubMed] [Google Scholar]
- 59.Zhen Y.Y., Wang N., Gao Z.B., Ma X.Y., Wei B.A., Deng Y.H., Wang T. Multifunctional liposomes constituting microneedles induced robust systemic and mucosal immunoresponses against the loaded antigens via oral mucosal vaccination. Vaccine. 2015;33(35):4330–4340. doi: 10.1016/j.vaccine.2015.03.081. [DOI] [PubMed] [Google Scholar]
- 60.Wang T., Zhen Y., Ma X., Wei B., Li S., Wang N. Mannosylated and lipid A-incorporating cationic liposomes constituting microneedle arrays as an effective oral mucosal HBV vaccine applicable in the controlled temperature chain. Colloids Surf. B: Biointerfaces. 2015;126:520–530. doi: 10.1016/j.colsurfb.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 61.Wang N., Wang T., Zhang M., Chen R., Niu R., Deng Y. Mannose derivative and lipid A dually decorated cationic liposomes as an effective cold chain free oral mucosal vaccine adjuvant-delivery system. Eur. J. Pharm. Biopharm. 2014;88(1):194–206. doi: 10.1016/j.ejpb.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 62.Weinberger B. Adjuvant strategies to improve vaccination of the elderly population. Curr. Opin. Pharmacol. 2018;41:34–41. doi: 10.1016/j.coph.2018.03.014. [DOI] [PubMed] [Google Scholar]
- 63.Vasilakos J.P., Tomai M.A. The use of toll-like receptor 7/8 agonists as vaccine adjuvants. Expert Rev. Vaccines. 2013;12(7):809–819. doi: 10.1586/14760584.2013.811208. [DOI] [PubMed] [Google Scholar]
- 64.Moon J.J., Suh H., Bershteyn A., Stephan M.T., Liu H., Huang B., Sohail M., Luo S., Ho Um S., Khant H., Goodwin J.T., Ramos J., Chiu W., Irvine D.J. Interbilayer-crosslinked multilamellar vesicles as synthetic vaccines for potent humoral and cellular immune responses. Nat. Mater. 2011;10(3):243–251. doi: 10.1038/nmat2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sessa G., Weissmann G. Phospholipid spherules (liposomes) as a model for biological membranes. J. Lipid Res. 1968;9(3):310–318. [PubMed] [Google Scholar]
- 66.Israelachvili J.N., Mitchell D.J. A model for the packing of lipids in bilayer membranes. Biochim. Biophys. Acta. 1975;389(1):13–19. doi: 10.1016/0005-2736(75)90381-8. [DOI] [PubMed] [Google Scholar]
- 67.Dutt S., Siril P.F., Remita S. Swollen liquid crystals (SLCs): a versatile template for the synthesis of nano structured materials. RSC Adv. 2017;7(10):5733–5750. [Google Scholar]
- 68.Raffy S., Teissie J. Control of lipid membrane stability by cholesterol content. Biophys. J. 1999;76(4):2072–2080. doi: 10.1016/S0006-3495(99)77363-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sulkowski W.W., Pentak D., Nowak K., Sulkowska A. The influence of temperature, cholesterol content and pH on liposome stability. J. Mol. Struct. 2005;744:737–747. [Google Scholar]
- 70.Schottler S., Becker G., Winzen S., Steinbach T., Mohr K., Landfester K., Mailander V., Wurm F.R. Protein adsorption is required for stealth effect of poly(ethylene glycol)- and poly(phosphoester)-coated nanocarriers. Nat. Nanotechnol. 2016;11(4):372–377. doi: 10.1038/nnano.2015.330. [DOI] [PubMed] [Google Scholar]
- 71.Kaur R., Bramwell V.W., Kirby D.J., Perrie Y. Pegylation of DDA:TDB liposomal adjuvants reduces the vaccine depot effect and alters the Th1/Th2 immune responses. J. Control. Release. 2012;158(1):72–77. doi: 10.1016/j.jconrel.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 72.Barenholz Y. Doxil(R)—the first FDA-approved nano-drug: lessons learned. J. Control. Release. 2012;160(2):117–134. doi: 10.1016/j.jconrel.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 73.Chopra A., Cevc G. Non-invasive, epicutaneous immunisation with toxoid in deformable vesicles protects mice against tetanus, chiefly owing to a Th2 response. Eur. J. Pharm. Sci. 2014;56:55–64. doi: 10.1016/j.ejps.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 74.Pattni B.S., Chupin V.V., Torchilin V.P. New developments in liposomal drug delivery. Chem. Rev. 2015;115(19):10938–10966. doi: 10.1021/acs.chemrev.5b00046. [DOI] [PubMed] [Google Scholar]
- 75.Gregoriadis G., Wills E.J., Swain C.P., Tavill A.S. Drug-carrier potential of liposomes in cancer chemotherapy. Lancet. 1974;1(7870):1313–1316. doi: 10.1016/s0140-6736(74)90682-5. [DOI] [PubMed] [Google Scholar]
- 76.Mohanan D., Slutter B., Henriksen-Lacey M., Jiskoot W., Bouwstra J.A., Perrie Y., Kundig T.M., Gander B., Johansen P. Administration routes affect the quality of immune responses: A cross-sectional evaluation of particulate antigen-delivery systems. J. Control. Release. 2010;147(3):342–349. doi: 10.1016/j.jconrel.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 77.Alving C.R., Rao M., Steers N.J., Matyas G.R., Mayorov A.V. Liposomes containing lipid A: an effective, safe, generic adjuvant system for synthetic vaccines. Expert Rev. Vaccines. 2012;11(6):733–744. doi: 10.1586/erv.12.35. [DOI] [PubMed] [Google Scholar]
- 78.Hassane F.S., Phalipon A., Tanguy M., Guerreiro C., Belot F., Frisch B., Mulard L.A., Schuber F. Rational design and immunogenicity of liposome-based diepitope constructs: application to synthetic oligosaccharides mimicking the Shigella flexneri 2a O-antigen. Vaccine. 2009;27(39):5419–5426. doi: 10.1016/j.vaccine.2009.06.031. [DOI] [PubMed] [Google Scholar]
- 79.Gall D. The adjuvant activity of aliphatic nitrogenous bases. Immunology. 1966;11(4):369–386. [PMC free article] [PubMed] [Google Scholar]
- 80.Shi Y., Rock K.L. Cell death releases endogenous adjuvants that selectively enhance immune surveillance of particulate antigens. Eur. J. Immunol. 2002;32(1):155–162. doi: 10.1002/1521-4141(200201)32:1<155::AID-IMMU155>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 81.Werninghaus K., Babiak A., Gross O., Holscher C., Dietrich H., Agger E.M., Mages J., Mocsai A., Schoenen H., Finger K., Nimmerjahn F., Brown G.D., Kirschning C., Heit A., Andersen P., Wagner H., Ruland J., Lang R. Adjuvanticity of a synthetic cord factor analogue for subunit mycobacterium tuberculosis vaccination requires FcRgamma-Syk-Card9-dependent innate immune activation. J. Exp. Med. 2009;206(1):89–97. doi: 10.1084/jem.20081445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Henriksen-Lacey M., Christensen D., Bramwell V.W., Lindenstrom T., Agger E.M., Andersen P., Perrie Y. Liposomal cationic charge and antigen adsorption are important properties for the efficient deposition of antigen at the injection site and ability of the vaccine to induce a CMI response. J. Control. Release. 2010;145(2):102–108. doi: 10.1016/j.jconrel.2010.03.027. [DOI] [PubMed] [Google Scholar]
- 83.Vangala A., Bramwell V.W., McNeil S., Christensen D., Agger E.M., Perrie Y. Comparison of vesicle based antigen delivery systems for delivery of hepatitis B surface antigen. J. Control. Release. 2007;119(1):102–110. doi: 10.1016/j.jconrel.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 84.Yanasarn N., Sloat B.R., Cui Z. Negatively charged liposomes show potent adjuvant activity when simply admixed with protein antigens. Mol. Pharm. 2011;8(4):1174–1185. doi: 10.1021/mp200016d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Orr M.T., Fox C.B., Baldwin S.L., Sivananthan S.J., Lucas E., Lin S., Phan T., Moon J.J., Vedvick T.S., Reed S.G., Coler R.N. Adjuvant formulation structure and composition are critical for the development of an effective vaccine against tuberculosis. J. Control. Release. 2013;172(1):190–200. doi: 10.1016/j.jconrel.2013.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Norris D.A., Puri N., Sinko P.J. The effect of physical barriers and properties on the oral absorption of particulates. Adv. Drug Deliv. Rev. 1998;34(2–3):135–154. doi: 10.1016/s0169-409x(98)00037-4. [DOI] [PubMed] [Google Scholar]
- 87.Shakweh M., Besnard M., Nicolas V., Fattal E. Poly (lactide-co-glycolide) particles of different physicochemical properties and their uptake by Peyer's patches in mice. Eur. J. Pharm. Biopharm. 2005;61(1–2):1–13. doi: 10.1016/j.ejpb.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 88.Beck Z., Torres O.B., Matyas G.R., Lanar D.E., Alving C.R. Immune response to antigen adsorbed to aluminum hydroxide particles: effects of co-adsorption of ALF or ALFQ adjuvant to the aluminum-antigen complex. J. Control. Release. 2018;275:12–19. doi: 10.1016/j.jconrel.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nagata S., Hanayama R., Kawane K. Autoimmunity and the clearance of dead cells. Cell. 2010;140(5):619–630. doi: 10.1016/j.cell.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 90.Casella C.R., Mitchell T.C. Putting endotoxin to work for us: monophosphoryl lipid A as a safe and effective vaccine adjuvant. Cell. Mol. Life Sci. 2008;65(20):3231–3240. doi: 10.1007/s00018-008-8228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Moller-Tank S., Maury W. Phosphatidylserine receptors: enhancers of enveloped virus entry and infection. Virology. 2014;468-470:565–580. doi: 10.1016/j.virol.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Verhoven B., Schlegel R.A., Williamson P. Mechanisms of phosphatidylserine exposure, a phagocyte recognition signal, on apoptotic T lymphocytes. J. Exp. Med. 1995;182(5):1597–1601. doi: 10.1084/jem.182.5.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Freeman G.J., Casasnovas J.M., Umetsu D.T., DeKruyff R.H. TIM genes: a family of cell surface phosphatidylserine receptors that regulate innate and adaptive immunity. Immunol. Rev. 2010;235:172–189. doi: 10.1111/j.0105-2896.2010.00903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bagalkot V., Deiuliis J.A., Rajagopalan S., Maiseyeu A. "Eat me" imaging and therapy. Adv. Drug Deliv. Rev. 2016;99:2–11. doi: 10.1016/j.addr.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hoffmann P.R., Kench J.A., Vondracek A., Kruk E., Daleke D.L., Jordan M., Marrack P., Henson P.M., Fadok V.A. Interaction between phosphatidylserine and the phosphatidylserine receptor inhibits immune responses in vivo. J. Immunol. 2005;174(3):1393–1404. doi: 10.4049/jimmunol.174.3.1393. [DOI] [PubMed] [Google Scholar]
- 96.Schneider J.L., Balu-Iyer S.V. Phosphatidylserine converts immunogenic recombinant human acid alpha-glucosidase to a Tolerogenic form in a mouse model of Pompe disease. J. Pharm. Sci. 2016;105(10):3097–3104. doi: 10.1016/j.xphs.2016.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Molinos-Albert L.M., Bilbao E., Agullo L., Marfil S., Garcia E., Rodriguez de la Concepcion M.L., Izquierdo-Useros N., Vilaplana C., Nieto-Garai J.A., Contreras F.X., Floor M., Cardona P.J., Martinez-Picado J., Clotet B., Villa-Freixa J., Lorizate M., Carrillo J., Blanco J. Proteoliposomal formulations of an HIV-1 gp41-based miniprotein elicit a lipid-dependent immunodominant response overlapping the 2F5 binding motif. Sci. Rep. 2017;7 doi: 10.1038/srep40800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cauvi D.M., Hawisher D., Dores-Silva P.R., Lizardo R.E., De Maio A. Macrophage reprogramming by negatively charged membrane phospholipids controls infection. Faseb J. 2018;33(2):2995–3009. doi: 10.1096/fj.201801579R. fj201801579R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Arigita C., Bevaart L., Everse L.A., Koning G.A., Hennink W.E., Crommelin D.J., van de Winkel J.G., van Vugt M.J., Kersten G.F., Jiskoot W. Liposomal meningococcal B vaccination: role of dendritic cell targeting in the development of a protective immune response. Infect. Immun. 2003;71(9):5210–5218. doi: 10.1128/IAI.71.9.5210-5218.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yotsumoto S., Kakiuchi T., Aramaki Y. Enhancement of IFN-gamma production for Th1-cell therapy using negatively charged liposomes containing phosphatidylserine. Vaccine. 2007;25(29):5256–5262. doi: 10.1016/j.vaccine.2007.05.037. [DOI] [PubMed] [Google Scholar]
- 101.Petazzi R.A., Gramatica A., Herrmann A., Chiantia S. Time-controlled phagocytosis of asymmetric liposomes: application to phosphatidylserine immunoliposomes binding HIV-1 virus-like particles. Nanomedicine. 2015;11(8):1985–1992. doi: 10.1016/j.nano.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 102.Mazumdar T., Anam K., Ali N. Influence of phospholipid composition on the adjuvanticity and protective efficacy of liposome-encapsulated Leishmania donovani antigens. J. Parasitol. 2005;91(2):269–274. doi: 10.1645/GE-356R1. [DOI] [PubMed] [Google Scholar]
- 103.Christensen D., Henriksen-Lacey M., Kamath A.T., Lindenstrom T., Korsholm K.S., Christensen J.P., Rochat A.F., Lambert P.H., Andersen P., Siegrist C.A., Perrie Y., Agger E.M. A cationic vaccine adjuvant based on a saturated quaternary ammonium lipid have different in vivo distribution kinetics and display a distinct CD4 T cell-inducing capacity compared to its unsaturated analog. J. Control. Release. 2012;160(3):468–476. doi: 10.1016/j.jconrel.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 104.van Houte A.J., Snippe H., Schmitz M.G., Willers J.M. Characterization of immunogenic properties of haptenated liposomal model membranes in mice. V. Effect of membrane composition on humoral and cellular immunogenicity. Immunology. 1981;44(3):561–568. [PMC free article] [PubMed] [Google Scholar]
- 105.Nakano Y., Mori M., Yamamura H., Naito S., Kato H., Taneichi M., Tanaka Y., Komuro K., Uchida T. Cholesterol inclusion in liposomes affects induction of antigen-specific IgG and IgE antibody production in mice by a surface-linked liposomal antigen. Bioconjug. Chem. 2002;13(4):744–749. doi: 10.1021/bc0155667. [DOI] [PubMed] [Google Scholar]
- 106.Bakouche O., Gerlier D. Enhancement of immunogenicity of tumour virus antigen by liposomes: the effect of lipid composition. Immunology. 1986;58(3):507–513. [PMC free article] [PubMed] [Google Scholar]
- 107.Barnier-Quer C., Elsharkawy A., Romeijn S., Kros A., Jiskoot W. Adjuvant effect of cationic liposomes for subunit influenza vaccine: influence of antigen loading method, cholesterol and immune modulators. Pharmaceutics. 2013;5(3):392–410. doi: 10.3390/pharmaceutics5030392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Brewer J.M., Tetley L., Richmond J., Liew F.Y., Alexander J. Lipid vesicle size determines the Th1 or Th2 response to entrapped antigen. J. Immunol. 1998;161(8):4000–4007. [PubMed] [Google Scholar]
- 109.Mann J.F., Shakir E., Carter K.C., Mullen A.B., Alexander J., Ferro V.A. Lipid vesicle size of an oral influenza vaccine delivery vehicle influences the Th1/Th2 bias in the immune response and protection against infection. Vaccine. 2009;27(27):3643–3649. doi: 10.1016/j.vaccine.2009.03.040. [DOI] [PubMed] [Google Scholar]
- 110.Manolova V., Flace A., Bauer M., Schwarz K., Saudan P., Bachmann M.F. Nanoparticles target distinct dendritic cell populations according to their size. Eur. J. Immunol. 2008;38(5):1404–1413. doi: 10.1002/eji.200737984. [DOI] [PubMed] [Google Scholar]
- 111.Henriksen-Lacey M., Devitt A., Perrie Y. The vesicle size of DDA:TDB liposomal adjuvants plays a role in the cell-mediated immune response but has no significant effect on antibody production. J. Control. Release. 2011;154(2):131–137. doi: 10.1016/j.jconrel.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 112.Brewer J.M., Pollock K.G., Tetley L., Russell D.G. Vesicle size influences the trafficking, processing, and presentation of antigens in lipid vesicles. J. Immunol. 2004;173(10):6143–6150. doi: 10.4049/jimmunol.173.10.6143. [DOI] [PubMed] [Google Scholar]
- 113.Kimura H., Caturegli P., Takahashi M., Suzuki K. New insights into the function of the immunoproteasome in immune and nonimmune cells. J Immunol Res. 2015:1–8. doi: 10.1155/2015/541984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kirby D.J., Kaur R., Agger E.M., Andersen P., Bramwell V.W., Perrie Y. Developing solid particulate vaccine adjuvants: surface bound antigen favours a humoural response, whereas entrapped antigen shows a tendency for cell mediated immunity. Curr. Drug Deliv. 2013;10(3):268–278. doi: 10.2174/1567201811310030003. [DOI] [PubMed] [Google Scholar]
- 115.Rincon-Restrepo M., Mayer A., Hauert S., Bonner D.K., Phelps E.A., Hubbell J.A., Swartz M.A., Hirosue S. Vaccine nanocarriers: coupling intracellular pathways and cellular biodistribution to control CD4 vs CD8 T cell responses. Biomaterials. 2017;132:48–58. doi: 10.1016/j.biomaterials.2017.03.047. [DOI] [PubMed] [Google Scholar]
- 116.Yadav S.C., Kumari A., Yadav R. Development of peptide and protein nanotherapeutics by nanoencapsulation and nanobioconjugation. Peptides. 2011;32(1):173–187. doi: 10.1016/j.peptides.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 117.Liu L., Ma P., Wang H., Zhang C., Sun H., Wang C., Song C., Leng X., Kong D., Ma G. Immune responses to vaccines delivered by encapsulation into and/or adsorption onto cationic lipid-PLGA hybrid nanoparticles. J. Control. Release. 2016;225:230–239. doi: 10.1016/j.jconrel.2016.01.050. [DOI] [PubMed] [Google Scholar]
- 118.Zhang W., Wang L., Liu Y., Chen X., Liu Q., Jia J., Yang T., Qiu S., Ma G. Immune responses to vaccines involving a combined antigen-nanoparticle mixture and nanoparticle-encapsulated antigen formulation. Biomaterials. 2014;35(23):6086–6097. doi: 10.1016/j.biomaterials.2014.04.022. [DOI] [PubMed] [Google Scholar]
- 119.Bielinska A.U., Janczak K.W., Landers J.J., Makidon P., Sower L.E., Peterson J.W., Baker J.R., Jr. Mucosal immunization with a novel nanoemulsion-based recombinant anthrax protective antigen vaccine protects against bacillus anthracis spore challenge. Infect. Immun. 2007;75(8):4020–4029. doi: 10.1128/IAI.00070-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhao Q., Sitrin R. Surface phosphophilicity of aluminum-containing adjuvants probed by their efficiency for catalyzing the P--O bond cleavage with chromogenic and fluorogenic substrates. Anal. Biochem. 2001;295(1):76–81. doi: 10.1006/abio.2001.5175. [DOI] [PubMed] [Google Scholar]
- 121.van der Heijden P.J., Bokhout B.A., Bianchi A.T., Scholten J.W., Stok W. Separate application of adjuvant and antigen: the effect of a water-in-oil emulsion on the splenic plaque-forming cell response to sheep red blood cells in mice. Immunobiology. 1986;171(1–2):143–154. doi: 10.1016/s0171-2985(86)80023-7. [DOI] [PubMed] [Google Scholar]
- 122.Orbegozo-Medina R.A., Martinez-Sernandez V., Gonzalez-Warleta M., Castro-Hermida J.A., Mezo M., Ubeira F.M. Vaccination of sheep with Quil-A(R) adjuvant expands the antibody repertoire to the Fasciola MF6p/FhHDM-1 antigen and administered together impair the growth and antigen release of flukes. Vaccine. 2018;36(15):1949–1957. doi: 10.1016/j.vaccine.2018.02.115. [DOI] [PubMed] [Google Scholar]
- 123.Fereig R.M., Abdelbaky H.H., Kuroda Y., Nishikawa Y. Critical role of TLR2 in triggering protective immunity with cyclophilin entrapped in oligomannose-coated liposomes against Neospora caninum infection in mice. Vaccine. 2019;37(7):937–944. doi: 10.1016/j.vaccine.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 124.Jacoberger-Foissac C., Saliba H., Seguin C., Brion A., Kakhi Z., Frisch B., Fournel S., Heurtault B. Optimization of peptide-based cancer vaccine compositions, by sequential screening, using versatile liposomal platform. Int. J. Pharm. 2019;562:342–350. doi: 10.1016/j.ijpharm.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 125.Wasan E.K., Syeda J., Strom S., Cawthray J., Hancock R.E., Wasan K.M., Gerdts V. A lipidic delivery system of a triple vaccine adjuvant enhances mucosal immunity following nasal administration in mice. Vaccine. 2019;37(11):1503–1515. doi: 10.1016/j.vaccine.2019.01.058. [DOI] [PubMed] [Google Scholar]
- 126.Wilkins A.L., Kazmin D., Napolitani G., Clutterbuck E.A., Pulendran B., Siegrist C.A., Pollard A.J. AS03- and MF59-Adjuvanted influenza vaccines in children. Front. Immunol. 2017;8:1760. doi: 10.3389/fimmu.2017.01760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wilkinson A., Lattmann E., Roces C.B., Pedersen G.K., Christensen D., Perrie Y. Lipid conjugation of TLR7 agonist Resiquimod ensures co-delivery with the liposomal cationic adjuvant formulation 01 (CAF01) but does not enhance immunopotentiation compared to non-conjugated Resiquimod+CAF01. J. Control. Release. 2018;291:1–10. doi: 10.1016/j.jconrel.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 128.Le Moignic A., Malard V., Benvegnu T., Lemiegre L., Berchel M., Jaffres P.A., Baillou C., Delost M., Macedo R., Rochefort J., Lescaille G., Pichon C., Lemoine F.M., Midoux P., Mateo V. Preclinical evaluation of mRNA trimannosylated lipopolyplexes as therapeutic cancer vaccines targeting dendritic cells. J. Control. Release. 2018;278:110–121. doi: 10.1016/j.jconrel.2018.03.035. [DOI] [PubMed] [Google Scholar]
- 129.Trentini M.M., de Oliveira F.M., Gaeti M.P., Batista A.C., Lima E.M., Kipnis A., Junqueira-Kipnis A.P. Microstructured liposome subunit vaccines reduce lung inflammation and bacterial load after mycobacterium tuberculosis infection. Vaccine. 2014;32(34):4324–4332. doi: 10.1016/j.vaccine.2014.06.037. [DOI] [PubMed] [Google Scholar]
- 130.Seder R.A., Chang L.J., Enama M.E., Zephir K.L., Sarwar U.N., Gordon I.J., Holman L.A., James E.R., Billingsley P.F., Gunasekera A., Richman A., Chakravarty S., Manoj A., Velmurugan S., Li M., Ruben A.J., Li T., Eappen A.G., Stafford R.E., Plummer S.H., Hendel C.S., Novik L., Costner P.J., Mendoza F.H., Saunders J.G., Nason M.C., Richardson J.H., Murphy J., Davidson S.A., Richie T.L., Sedegah M., Sutamihardja A., Fahle G.A., Lyke K.E., Laurens M.B., Roederer M., Tewari K., Epstein J.E., Sim B.K., Ledgerwood J.E., Graham B.S., Hoffman S.L., V.R.C.S. Team Protection against malaria by intravenous immunization with a nonreplicating sporozoite vaccine. Science. 2013;341(6152):1359–1365. doi: 10.1126/science.1241800. [DOI] [PubMed] [Google Scholar]
- 131.Nussenzweig R.S., Vanderberg J., Most H., Orton C. Protective immunity produced by the injection of x-irradiated sporozoites of plasmodium berghei. Nature. 1967;216(5111):160–162. doi: 10.1038/216160a0. [DOI] [PubMed] [Google Scholar]
- 132.Schmidt S.T., Khadke S., Korsholm K.S., Perrie Y., Rades T., Andersen P., Foged C., Christensen D. The administration route is decisive for the ability of the vaccine adjuvant CAF09 to induce antigen-specific CD8(+) T-cell responses: the immunological consequences of the biodistribution profile. J. Control. Release. 2016;239:107–117. doi: 10.1016/j.jconrel.2016.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Abhyankar M.M., Orr M.T., Lin S., Suraju M.O., Simpson A., Blust M., Pham T., Guderian J.A., Tomai M.A., Elvecrog J., Pedersen K., Petri W.A., Jr., Fox C.B. Adjuvant composition and delivery route shape immune response quality and protective efficacy of a recombinant vaccine for Entamoeba histolytica. NPJ vaccines. 2018;3:22. doi: 10.1038/s41541-018-0060-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Du J., Zhang Y.S., Hobson D., Hydbring P. Nanoparticles for immune system targeting. Drug Discov. Today. 2017;16:343–353. doi: 10.1016/j.drudis.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 135.Iwasaki A., Medzhitov R. Control of adaptive immunity by the innate immune system. Nat. Immunol. 2015;16(4):343–353. doi: 10.1038/ni.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kumar H., Kawai T., Akira S. Pathogen recognition by the innate immune system. Int. Rev. Immunol. 2011;30(1):16–34. doi: 10.3109/08830185.2010.529976. [DOI] [PubMed] [Google Scholar]
- 137.Akira S. Innate immunity and adjuvants. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2011;366(1579):2748–2755. doi: 10.1098/rstb.2011.0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Silva J.M., Videira M., Gaspar R., Preat V., Florindo H.F. Immune system targeting by biodegradable nanoparticles for cancer vaccines. J. Control. Release. 2013;168(2):179–199. doi: 10.1016/j.jconrel.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 139.Oberoi H.S., Yorgensen Y.M., Morasse A., Evans J.T., Burkhart D.J. PEG modified liposomes containing CRX-601 adjuvant in combination with methylglycol chitosan enhance the murine sublingual immune response to influenza vaccination. J. Control. Release. 2016;223:64–74. doi: 10.1016/j.jconrel.2015.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bonam S.R., Partidos C.D., Halmuthur S.K.M., Muller S. An overview of novel adjuvants designed for improving vaccine efficacy. Trends Pharmacol. Sci. 2017;38(9):771–793. doi: 10.1016/j.tips.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 141.Chen W.H., Kozlovsky B.F., Effros R.B., Grubeck-Loebenstein B., Edelman R., Sztein M.B. Vaccination in the elderly: an immunological perspective. Trends Immunol. 2009;30(7):351–359. doi: 10.1016/j.it.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Karmakar P., Lee K., Sarkar S., Wall K.A., Sucheck S.J. Synthesis of a liposomal MUC1 Glycopeptide-based immunotherapeutic and evaluation of the effect of l-Rhamnose targeting on cellular immune responses. Bioconjug. Chem. 2016;27(1):110–120. doi: 10.1021/acs.bioconjchem.5b00528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wang W., Erbe A.K., Hank J.A., Morris Z.S., Sondel P.M. NK Cell-mediated antibody-dependent cellular cytotoxicity in cancer immunotherapy. Front. Immunol. 2015;6:368. doi: 10.3389/fimmu.2015.00368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Jakobsche C.E., Parker C.G., Tao R.N., Kolesnikova M.D., Douglass E.F., Spiegel D.A. Exploring binding and effector functions of natural human antibodies using synthetic Immunomodulators. ACS Chem. Biol. 2013;8(11):2404–2411. doi: 10.1021/cb4004942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mandal A., Boopathy A.V., Lam L.K.W., Moynihan K.D., Welch M.E., Bennett N.R., Turvey M.E., Thai N., Van J.H., Love J.C., Hammond P.T., Irvine D.J. Cell and fluid sampling microneedle patches for monitoring skin-resident immunity. Sci. Transl. Med. 2018;10(467) doi: 10.1126/scitranslmed.aar2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lasic D.D. On the history of liposomes. In: Lasic D.D., Barenholz Y., editors. Handbook of Nonmedical Applications of Liposomes, Volume I: Theory and Basic Sciences. 1st edition. CRC-Press; Florida, USA: 1996. pp. 1–11. [Google Scholar]
- 147.Lasic D.D. Novel applications of liposomes. Trends Biotechnol. 1998;16(7):307–321. doi: 10.1016/s0167-7799(98)01220-7. [DOI] [PubMed] [Google Scholar]
- 148.Gulati M., Bajad S., Singh S., Ferdous A.J., Singh M. Development of liposomal amphotericin B formulation. J. Microencapsul. 1998;15(2):137–151. doi: 10.3109/02652049809006844. [DOI] [PubMed] [Google Scholar]
- 149.Pearson R.D., Jeronimo S.M., Lareau S.M. US Food and Drug Administration approval of liposomal amphotericin B for the treatment of visceral Leishmaniasis: A model for orphan drug development. Curr. Infect. Dis. Rep. 1999;1(5):415–416. doi: 10.1007/s11908-999-0052-0. [DOI] [PubMed] [Google Scholar]
- 150.Bulbake U., Doppalapudi S., Kommineni N., Khan W. Liposomal formulations in clinical use: an updated review. Pharmaceutics. 2017;9(2) doi: 10.3390/pharmaceutics9020012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Moser C., Muller M., Kaeser M.D., Weydemann U., Amacker M. Influenza virosomes as vaccine adjuvant and carrier system. Expert Rev. Vaccines. 2013;12(7):779–791. doi: 10.1586/14760584.2013.811195. [DOI] [PubMed] [Google Scholar]
- 152.Glueck R. Review of intranasal influenza vaccine. Adv. Drug Deliv. Rev. 2001;51(1–3):203–211. doi: 10.1016/s0169-409x(01)00174-0. [DOI] [PubMed] [Google Scholar]
- 153.Kawai K., Gebremeskel B.G., Acosta C.J. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open. 2014;4(6) doi: 10.1136/bmjopen-2014-004833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gershon A.A., Gershon M.D., Breuer J., Levin M.J., Oaklander A.L., Griffiths P.D. Advances in the understanding of the pathogenesis and epidemiology of herpes zoster. J. Clin. Virol. 2010;48(Suppl. 1):S2–S7. doi: 10.1016/S1386-6532(10)70002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gagliardi A.M., Andriolo B.N., Torloni M.R., Soares B.G. Vaccines for preventing herpes zoster in older adults. Cochrane Database Syst. Rev. 2016;3 doi: 10.1002/14651858.CD008858.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Alving C.R., Beck Z., Matyas G.R., Rao M. Liposomal adjuvants for human vaccines. Expert Opin. Drug Deliv. 2016;13(6):807–816. doi: 10.1517/17425247.2016.1151871. [DOI] [PubMed] [Google Scholar]
- 157.Beck Z., Matyas G.R., Alving C.R. Detection of liposomal cholesterol and monophosphoryl lipid A by QS-21 saponin and Limulus polyphemus amebocyte lysate. Biochim. Biophys. Acta. 2015;1848(3):775–780. doi: 10.1016/j.bbamem.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 158.Syed Y.Y. Recombinant zoster vaccine (Shingrix((R))): a review in herpes zoster. Drugs Aging. 2018;35:1031–1040. doi: 10.1007/s40266-018-0603-x. [DOI] [PubMed] [Google Scholar]
- 159.Cunningham A.L., Lal H., Kovac M., Chlibek R., Hwang S.J., Diez-Domingo J., Godeaux O., Levin M.J., McElhaney J.E., Puig-Barbera J., Vanden Abeele C., Vesikari T., Watanabe D., Zahaf T., Ahonen A., Athan E., Barba-Gomez J.F., Campora L., de Looze F., Downey H.J., Ghesquiere W., Gorfinkel I., Korhonen T., Leung E., McNeil S.A., Oostvogels L., Rombo L., Smetana J., Weckx L., Yeo W., Heineman T.C., Z.O.E.S. Group Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N. Engl. J. Med. 2016;375(11):1019–1032. doi: 10.1056/NEJMoa1603800. [DOI] [PubMed] [Google Scholar]
- 160.Dendouga N., Fochesato M., Lockman L., Mossman S., Giannini S.L. Cell-mediated immune responses to a varicella-zoster virus glycoprotein E vaccine using both a TLR agonist and QS21 in mice. Vaccine. 2012;30(20):3126–3135. doi: 10.1016/j.vaccine.2012.01.088. [DOI] [PubMed] [Google Scholar]
- 161.James S.F., Chahine E.B., Sucher A.J., Hanna C. Shingrix: the new Adjuvanted recombinant herpes zoster vaccine. Ann. Pharmacother. 2018;52(7):673–680. doi: 10.1177/1060028018758431. [DOI] [PubMed] [Google Scholar]
- 162.O'Hagan D.T., Ott G.S., Van Nest G., Rappuoli R., Del Giudice G. The history of MF59 (R) adjuvant: a phoenix that arose from the ashes. Expert Rev Vaccines. 2013;12(1):13–30. doi: 10.1586/erv.12.140. [DOI] [PubMed] [Google Scholar]
- 163.Tomljenovic L., A. Shaw C. Aluminum vaccine adjuvants: are they safe? Curr. Med. Chem. 2011;18(17):2630–2637. doi: 10.2174/092986711795933740. [DOI] [PubMed] [Google Scholar]
- 164.Tokatlian T., Kulp D.W., Mutafyan A.A., Jones C.A., Menis S., Georgeson E., Kubitz M., Zhang M.H., Melo M.B., Silva M., Yun D.S., Schief W.R., Irvine D.J. Enhancing humoral responses against HIV envelope trimers via nanoparticle delivery with stabilized synthetic liposomes. Sci. Rep. 2018;8(1) doi: 10.1038/s41598-018-34853-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Vincent L.R., Jerse A.E. Biological feasibility and importance of a gonorrhea vaccine for global public health. Vaccine. 2018 doi: 10.1016/j.vaccine.2018.02.081. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hanson M.C., Abraham W., Crespo M.P., Chen S.H., Liu H., Szeto G.L., Kim M., Reinherz E.L., Irvine D.J. Liposomal vaccines incorporating molecular adjuvants and intrastructural T-cell help promote the immunogenicity of HIV membrane-proximal external region peptides. Vaccine. 2015;33(7):861–868. doi: 10.1016/j.vaccine.2014.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Liu H., Irvine D.J. Guiding principles in the design of molecular bioconjugates for vaccine applications. Bioconjug. Chem. 2015;26(5):791–801. doi: 10.1021/acs.bioconjchem.5b00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Begley C.G., Ellis L.M. Drug development: raise standards for preclinical cancer research. Nature. 2012;483(7391):531–533. doi: 10.1038/483531a. [DOI] [PubMed] [Google Scholar]
- 169.Mogil J.S., Macleod M.R. No publication without confirmation. Nature. 2017;542(7642):409–411. doi: 10.1038/542409a. [DOI] [PubMed] [Google Scholar]
- 170.Baker M. Biotech giant publishes failures to confirm high-profile science. Nature. 2016;530(7589):141. doi: 10.1038/nature.2016.19269. [DOI] [PubMed] [Google Scholar]
- 171.Tiefenboeck P., Kim J.A., Trunk F., Leroux J.C. Comment on "A liposomal system capable of generating CO2 bubbles to induce transient cavitation, lysosomal rupturing and cell necrosis". Angew. Chem. Int. Ed. 2017;56(39):11686–11689. doi: 10.1002/anie.201703740. [DOI] [PubMed] [Google Scholar]
- 172.Leroux J.C. Editorial: drug delivery: too much complexity, not enough reproducibility? Angew. Chem. Int. Ed. Eng. 2017;56(48):15170–15171. doi: 10.1002/anie.201709002. [DOI] [PubMed] [Google Scholar]