One-third (136) of FDA-approved orphan indications between 2010 and 2018 were pediatric. Most pediatric orphan indications expanded use of existing drugs; 20 received breakthrough designation.
Abstract
BACKGROUND:
Orphan drug development is crucial for children, who are disproportionately affected by rare diseases. Data are lacking on the number, nature, and benefit of recently approved pediatric orphan indications.
METHODS:
We classified the 402 orphan indications the US Food and Drug Administration approved between 2010 and 2018 as “pediatric” if they were approved for children only or targeted pediatric diseases. We determined the number of unique diseases targeted by pediatric orphan indications and calculated the proportion that were for (1) novel drugs, (2) non-novel drugs approved to treat ≥1 common disease, and (3) non-novel drugs approved only to treat rare diseases. Among pediatric orphan indications eligible for US Food and Drug Administration breakthrough designation (granted to drugs potentially representing major therapeutic advances), we calculated the proportion receiving this designation.
RESULTS:
Of the 402 orphan indications, 136 (33.8%) were pediatric. These 136 indications targeted 87 unique diseases; 21 diseases were targeted by ≥1 indication. Of the 136 pediatric orphan indications, 60 (44.1%) were for novel drugs, 45 (33.1%) were for non-novel drugs approved to treat ≥1 common disease, and 31 (22.8%) were for non-novel drugs approved only to treat rare diseases. Among 97 indications eligible for breakthrough designation, 20 (20.6%) received this designation.
CONCLUSIONS:
Recent orphan drug development has increased the availability of treatments for pediatric rare diseases. Most pediatric orphan indications expanded use of existing drugs, and many targeted the same disease. Some indications may represent breakthroughs, but substantial unmet need for treatments remains for most pediatric rare diseases.
What’s Known on This Subject:
Orphan drug development is crucial to children, who are disproportionately affected by rare diseases. However, data are lacking on the number, nature, and benefit of recently approved pediatric orphan indications.
What This Study Adds:
Among 402 orphan indications US Food and Drug Administration approved between 2010 and 2018, 136 (33.8%) were pediatric. Of these, 44.1% were for novel drugs, and 20 received breakthrough designation. Orphan drug development has benefited children with rare diseases, mostly by expanding the use of existing drugs.
“Orphan drugs” are drugs with at least 1 US Food and Drug Administration (FDA) approved orphan indication to treat rare diseases affecting <200 000 Americans.1 These drugs are increasingly salient to patients, innovators, and payers in the United States.1,2 During the 10-year period between 2000 and 2009, 148 orphan indications were approved.3 In comparison, almost the same number (150) were approved between 2010 and 2014, and 170% more (252) were approved between 2015 and 2018.3 In 2017, orphan drug spending totaled $112 billion, or 25% of US prescription drug spending that year.1
The surge in orphan drug development has been driven by the Orphan Drug Act of 1983.4,5 The act incentivizes the development of orphan drugs by providing benefits such as tax credits and grants for clinical testing.6 Furthermore, the act allows a 7-year period of “orphan drug exclusivity” during which a manufacturer’s competitors can market a different drug for the same indication but not an alternative version of the same drug for the same indication, thus allowing the manufacturer wide latitude on pricing.6 A single drug can receive multiple orphan indications, each of which grants a new round of incentives.7 Any drug can receive an orphan indication, regardless of whether it is novel or has previous FDA-approved orphan or non-orphan indications.7,8
Understanding the number, nature, and benefit of recently approved orphan indications is particularly important for children. Half of rare diseases affect children, and it is estimated that 95% of the ∼7000 rare diseases have no treatment options.9 Despite the importance of orphan drug development to children, the only previous comprehensive analysis of pediatric orphan indications was focused on approvals between 2000 and 2009.10 In the current study, we assessed the number of pediatric orphan indications approved between 2010 and 2018, the number and types of diseases targeted by these pediatric orphan indications, the proportion of these indications that were for novel drugs, and the proportion that were for drugs receiving FDA breakthrough designation, which is an expedited review pathway granted when preliminary data suggest that a drug could substantially improve existing treatments for serious conditions.11
Methods
Data Source and Sample
We analyzed the FDA orphan drug database, which lists all orphan indications approved since 1983 (Supplemental Fig 3 describes the orphan drug approval process).3 The sample included all orphan indications for which the 7-year period of orphan drug exclusivity began between January 1, 2010, and December 31, 2018. The Institutional Review Board of the University of Michigan Medical School did not regulate this study as human subjects research because data were publicly available.
Identification of Pediatric Orphan Indications
For each orphan indication, we determined if the drug was approved for children only (limited to children aged <17 years [FDA’s definition of children]), adults only (limited to adults aged ≥17 years), or both. We considered a drug to be approved for both adults and children if both age groups were listed explicitly (eg, “adults and children with disease X”). If no age group was listed in the indication (eg, “patients with disease X”), we classified on the basis of whether the drug’s label stated that safety and efficacy had been established in adults only, children only, or both.
We also classified whether the disease targeted by the indication was a “pediatric disease,” defined as a disease that typically has its onset in childhood or predominantly affects children. Classification decisions were made independently by 2 authors (A.V., a general internist and pediatrician; K.C., a general pediatrician). A consensus-based approach was used to resolve disagreements. The 2 raters agreed on 95% of initial classification decisions.
We classified an orphan indication as a “pediatric orphan indication” if the drug was approved for children only or targeted a pediatric disease. Following a previous analysis, indications that were approved for adults only and that targeted pediatric diseases were classified as pediatric.10
Diseases Targeted by Pediatric Orphan Indications
Using a similar consensus-based approach, we assigned the diseases targeted by pediatric orphan indications into 1 of 19 disease categories (eg, cancer or cardiovascular).
Categorization of Pediatric Orphan Indications
To characterize the nature of pediatric orphan indications, we assigned them to 1 of 3 categories: (1) indications for novel drugs, (2) indications for non-novel drugs approved to treat ≥1 common disease, and (3) indications for non-novel drugs approved only to treat rare diseases.
The first category included indications for drugs without a previously approved active moiety when orphan drug exclusivity began (ie, on the date of marketing approval). These included orphan indications approved under new drug applications listed as “new molecular entities,” orphan indications on the FDA’s list of new biological license application approvals, and orphan indications for fractionated plasma products or cellular and/or gene therapy products that represented the drug’s initial approval.12–14
The second category included indications for drugs with ≥1 FDA-approved non-orphan indication to treat ≥1 common disease when orphan drug exclusivity began. We defined non-orphan indications as indications not included in the FDA orphan drug database.3
The third category included indications for drugs that only had orphan indications when orphan drug exclusivity began. We used FDA approval histories to further classify indications assigned to this category into 2 subgroups: those that targeted a different subset of a rare disease for which a drug was already approved (ie, an expansion of previous orphan indication) and those that targeted a new disease for which the drug was not already approved.13
Breakthrough Designation
We used FDA breakthrough designation as our measure of whether pediatric orphan indications may have represented major therapeutic advances. This designation, which allows for expedited review, is granted if drugs treat a serious or life-threatening condition and if preliminary clinical evidence suggests the drug may substantially improve existing treatments.11 This designation was first made available on July 9, 2012, but it is not available to products that are not reviewed by the FDA Center for Drug Evaluation and Research (CDER), such as fractionated plasma products. For eligible pediatric orphan indications approved by the CDER on or after this date, we used data from the FDA Web site to determine if the drug received breakthrough designation.11
Statistical Analysis
We used descriptive statistics to calculate the number of FDA-approved orphan indications and the number of pediatric orphan indications, both overall and by year. We determined which of the 19 disease categories were most frequently targeted by pediatric orphan indications, as well as the number of unique diseases targeted by these indications.
We calculated the proportion of pediatric orphan indications that were for novel drugs, non-novel drugs approved to treat ≥1 common disease, and non-novel drugs approved only to treat rare diseases. We determined the number of unique diseases targeted by pediatric orphan indications in each of the 3 categories. For pediatric orphan indications for non-novel drugs (ie, the second and third categories), we assessed the “vintage” of the drug by calculating the amount of time between the date on which the drug’s active moiety was first FDA approved and the date on which orphan drug exclusivity began.13,15 Finally, we calculated the proportion of eligible pediatric orphan indications that were for drugs with breakthrough designation.
Results
Number of Pediatric Orphan Indications
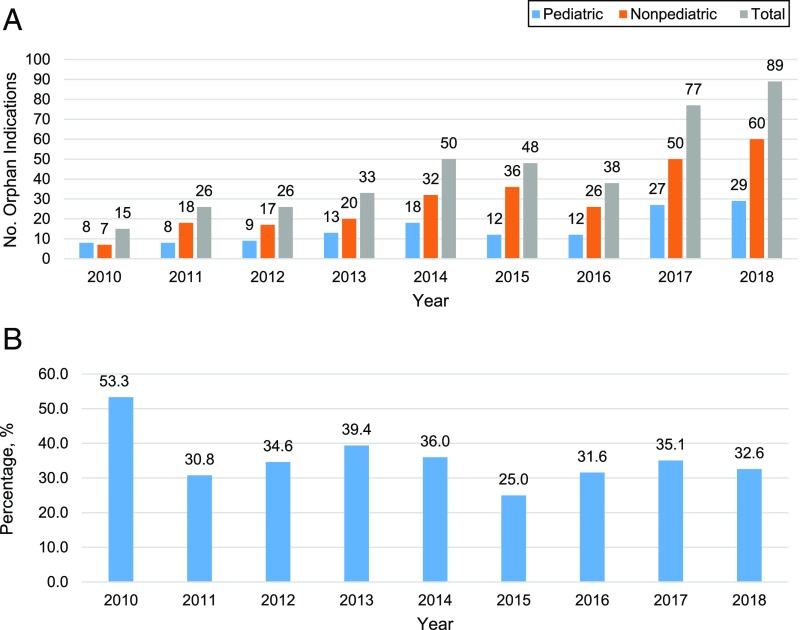
There were 402 FDA-approved orphan indications between 2010 and 2018. The number of approved orphan indications rose from 15 in 2010 to 89 in 2018. Of the 402 orphan indications, 42 (10.4%) were approved for children only, 247 (61.4%) for adults only, and 113 (28.1%) for both. Furthermore, 122 (30.3%) were classified as targeting pediatric diseases.
Of the 402 orphan indications, 136 (33.8%) were classified as pediatric (approved only for children or targeted pediatric diseases). The number of pediatric orphan indications ranged from 8 to 18 between 2010 and 2016, then increased to 27 in 2017 and 29 in 2018 (Fig 1A). The proportion of all orphan indications classified as pediatric was 53.3% in 2010 and subsequently ranged from 25.0% to 39.4% between 2011 and 2018 (Fig 1B).
FIGURE 1.
Number of pediatric orphan indications and percentage of orphan indications classified as pediatric orphan indications between 2010 and 2018. A, Number of orphan indications classified (2010–2018) as pediatric or non-pediatric and the total number of orphan indications. B, Percentage of all orphan indications classified as pediatric orphan indications per year.
Diseases Targeted by Pediatric Orphan Indications
The diseases targeted by the 136 pediatric orphan indications were most commonly categorized as genetic and/or metabolic (16.9%), hematologic (16.9%), immunologic and/or rheumatologic (12.5%), and cancer (11.8%). In contrast, of the 266 non-pediatric orphan indications, 164 (61.7%) targeted cancer (Supplemental Table 4).
The 136 pediatric orphan indications targeted 87 unique diseases (1 disease for every 1.6 indications); 21 of these diseases were targeted by ≥1 pediatric orphan indication. Among the 87 unique diseases, the most commonly targeted were cystic fibrosis (12 pediatric orphan indications), acute lymphoblastic leukemia (12), and hereditary angioedema (6) (Supplemental Table 5).
Categorization of Pediatric Orphan Indications
Of the 136 pediatric orphan indications, 60 (44.1%) were for novel drugs, 45 (33.1%) were for non-novel drugs approved to treat common diseases, and 31 (22.8%) were for non-novel drugs approved only to treat rare diseases (Fig 2). Among the 136 pediatric orphan indications, 76 (55.9%) were for non-novel drugs. For these 76 indications, the mean and median vintage was 17.9 and 11.6 years, respectively.
FIGURE 2.
Categorization of pediatric orphan indications.
The 60 pediatric orphan indications for novel drugs are listed in Table 1. These 60 indications targeted 44 unique diseases, indicating that some targeted the same disease. For example, 4 targeted hereditary angioedema (Supplemental Table 6).
TABLE 1.
Pediatric Orphan Indications for Novel Drugs, 2010–2018 (N = 60)
| Generic Name | Disease Population(s) | Generic Name | Disease Population(s) |
|---|---|---|---|
| Antihemophilic factor (recombinant), Fc fusion protein | Hemophilia A | Factor XIII concentrate (human) | Congenital factor XII deficiency |
| Anti–inhibitor coagulant complex | Hemophilia A; B | Fish oil triglycerides | Parenteral nutrition–associated cholestasis |
| Asfotase alfa | Hypophosphatasia | Icatibant | Hereditary angioedema |
| Benznidazole | Chagas disease | Inotuzumab ozogamicin | Acute lymphoblastic leukemia |
| Blinatumomab | Acute lymphoblastic leukemia | Ivacaftor | Cystic fibrosis |
| Botulism antitoxin heptavalent (A, B, C, D, E, F, G) (equine) | Botulism | Lanadelumab-flyo | Hereditary angioedema |
| Burosumab-twza | X-linked hypophosphatemia | Lomitapide | Familial hypercholesterolemia |
| C1-esterase inhibitor (recombinant) | Hereditary angioedema | Lumacaftor–ivacaftor | Cystic fibrosis |
| C1-esterase-inhibitor (human, pasteurized) | Hereditary angioedema | Macimorelin acetate | Growth hormone deficiency |
| Cannabidiol | Lennox-Gastaut syndrome | Metreleptin | Lipodystrophy |
| Carglumic acid | N-acetylglutamate synthase deficiency | Migalastat hydrochloride | Fabry disease |
| Cerliponase alfa | Tripeptidyl peptidase 1 deficiency | Miltefosine | Leishmania donovani/braziliensis/guyanensis/panamensis |
| Cholic acid | Bile acid synthesis disorders | Mipomersen | Familial hypercholesterolemia |
| Clobazam | Lennox-Gastaut syndrome | Moxidectin | Onchocerca volvulus |
| Coagulation factor IX (recombinant) | Hemophilia B | Nusinersen | Spinal muscular atrophy |
| Coagulation factor IX (recombinant), Fc fusion protein | Hemophilia B | Pegvaliase-pqpz | Phenylketonuria |
| Coagulation factor X (human) | Factor X deficiency | Ponatinib | Acute lymphoblastic leukemia |
| Coagulation factor XIII A-subunit (recombinant) | Hemophilia A | Recombinant fusion protein; coagulation factor IX with albumin | Hemophilia B |
| Deferasirox | Non–transfusion-dependent thalassemia syndromes | Recombinant human acid α-glucosidase; alglucosidase alfa | Pompe disease |
| Deferiprone | Thalassemia syndromes | Recombinant von Willebrand factor | von Willebrand disease |
| Deflazacort | Duchenne muscular dystrophy | Sebelipase alfa | Lysosomal acid lipase |
| Dinutuximab | Neuroblastoma | Stiripentol | Dravet syndrome |
| Elapegademase-lvlr | Adenosine deaminase; severe combined immunodeficiency | Taliglucerase alfa | Type 1 Gaucher disease |
| Eliglustat | Gaucher disease | Tezacaftor and ivacaftor combination therapy | Cystic fibrosis |
| Elosulfase alfa | Mucopolysaccharidosis type IVA | Tisagenlecleucel | Acute lymphoblastic leukemia |
| Emapalumab-lzsg | Hemophagocytic lymphohistiocytosis | Uridine triacetate | Orotic aciduria |
| Emicizumab-kxwh | Hemophilia A | Varicella zoster immune globulin (human) | Varicella |
| Erwinia L-asparaginase | Acute lymphoblastic leukemia | Velaglucerase alfa | Gaucher disease |
| Eteplirsen | Duchenne muscular dystrophy | Vestronidase alfa-vjbk | Mucopolysaccharidosis type VII |
| Evolocumab | Familial hypercholesterolemia | Voretigene neparvovec-rzyl | Retinal dystrophy |
The 45 pediatric orphan indications for non-novel drugs approved to treat ≥1 common disease targeted 34 unique diseases (Supplemental Table 7). Examples of these indications are listed in Table 2. For example, adalimumab (Humira) was approved for several common autoimmune conditions in adults, such as rheumatoid arthritis, at the time it received a pediatric orphan indication for juvenile idiopathic arthritis in children ages 2 to 3 years in 2014, which is 6 years after it received an orphan indication for this disease in children 4 years and older. Propranolol was initially approved in 1967 for common diseases such as hypertension before receiving a pediatric orphan indication in 2014 for proliferating infantile hemangioma (Hemangeol). Hydroxyurea was also initially approved in 1967 for multiple types of cancer and adults with sickle cell anemia before receiving a pediatric orphan indication in 2017 for children with sickle cell anemia (Siklos).
TABLE 2.
Examples of Pediatric Orphan Indications for Non-Novel Drugs
| Generic Name (Trade Name) | Pediatric Orphan Indication | Date of Initial FDA Approval for Drug’s Active Moiety | Orphan Drug Exclusivity Start Date | Years Between Initial FDA Approval and Orphan Drug Exclusivity (Vintage) | Approved Only To Treat Rare Diseases When Orphan Drug Exclusivity Began | Examples of Previous FDA-Approved Indications When Orphan Drug Exclusivity Began |
|---|---|---|---|---|---|---|
| Lumacaftor–ivacaftor (Orkambi) | Cystic fibrosis in children aged 6–11 y homozygous for the F508del CFTR mutation | July 2, 2015 | September 28, 2016 | 1.2 | Yes | Cystic fibrosis in adults and children aged ≥12 y homozygous for the F508del CFTR mutation |
| Lumacaftor–ivacaftor (Orkambi) | Cystic fibrosis in children aged 2–5 y homozygous for the F508del CFTR mutation | July 2, 2015 | August 7, 2018 | 3.1 | Yes | Cystic fibrosis in adults and children aged ≥6 y homozygous for the F508del CFTR mutation |
| Ivacaftor (Kalydeco) | Cystic fibrosis in children aged ≥2 y with specific CFTR mutations (eg, 711+3A G, E831X) | January 31, 2012 | July 31, 2017 | 5.5 | Yes | Cystic fibrosis in children aged ≥2 y with other CFTR mutations (eg, E56K, P67L, R74W) |
| Canakinumab (Ilaris) | Tumor necrosis factor receptor–associated periodic syndrome in pediatric and adult patients | June 17, 2009 | September 23, 2016 | 7.3 | Yes | Cryopyrin-associated periodic syndromes in adults and children aged ≥4 y; systemic juvenile idiopathic arthritis in adults and children aged ≥2 y |
| Ledipasvir–sofosbuvir (Harvoni) | Chronic hepatitis C in children aged 12 and older or who weigh ≥35 kg | October 10, 2014 | April 7, 2017 | 2.5 | No | Chronic hepatitis C in adults |
| Adalimumab (Humira) | Juvenile idiopathic arthritis in children aged 2–3 y | December 31, 2002 | September 30, 2014 | 11.8 | No | Adults with autoimmune diseases such as rheumatoid arthritis, ankylosing spondylitis, Crohn disease; children aged ≥4 y with juvenile idiopathic arthritis; children ≥6 y with Crohn disease |
| AbobotulinumtoxinA (Dysport)a | Lower limb spasticity in children aged ≥2 y with cerebral palsy | December 9, 1991 | July 29, 2016 | 24.7 | No | Adults with cervical dystonia; cosmesis in non-elderly adults with glabellar lines |
| Propranolol hydrochloride (Hemangeol) | Proliferating infantile hemangioma | November 13, 1967 | March 14, 2014 | 46.4 | No | Hypertension, arrhythmias migraine, myocardial infarctionb |
| Hydroxyurea (Siklos) | Children ≥2 y with sickle cell anemia | December 7, 1967 | December 21, 2017 | 50.1 | No | Adults with certain types of head and neck cancer; adults with sickle cell anemiab |
AboboulinumtoxinA is a specific formulation of botulinum toxin type A, which was first FDA approved in 1991.
Siklos and Hemangeol have no other indications other than the pediatric orphan indication listed but are not new molecular entities because their active moieties had previously been approved by the FDA for other indications (examples of which are listed in the last column).
The 31 pediatric orphan indications for non-novel drugs approved only to treat rare diseases targeted 21 diseases (Supplemental Table 8). Examples of these indications are listed in Table 2. Among the 31 indications, 25 (80.6%) targeted a different age- or biomarker-based subset of a rare disease that the drug had already been approved to treat. For example, lumacaftor–ivacaftor (Orkambi) was initially approved in 2015 as an orphan drug for patients with cystic fibrosis who were aged ≥12 years with the F508del CFTR gene mutation and subsequently gained 2 additional orphan indications for patients with cystic fibrosis with this mutation who were aged 6 to 11 years and aged 2 to 5 years. In contrast, of the 31 pediatric orphan indications for orphan-only drugs, 6 (16.1%) targeted a rare disease for which the drug had not previously been approved. One example is canakinumab (Ilaris), which received an orphan indication for tumor necrosis factor receptor–associated periodic syndrome in 2016 after previously receiving orphan indications for rare diseases such as cryopyrin-associated periodic syndrome.
Breakthrough Designation
Of the 136 pediatric orphan indications, 97 were approved by CDER after July 9, 2012, and therefore were eligible for breakthrough designation. Of these, 20 (20.6%) were for drugs that received breakthrough designation (Table 3). Among these 20 indications, 12 were for novel drugs and 8 for non-novel drugs; all of the latter drugs were approved only to treat rare diseases when orphan drug exclusivity began (Fig 2).
TABLE 3.
Pediatric Orphan Indications for Drugs Receiving FDA Breakthrough Designation
| Generic Name | Trade Name | Marketing Approval Date | Category | Disease Population(s) Targeted by Indication |
|---|---|---|---|---|
| Canakinumab | Ilaris | September 23, 2016 | Non-novel, rare disease only | Tumor necrosis factor receptor–associated periodic syndrome |
| Canakinumab | Ilaris | September 23, 2016 | Non-novel, rare disease only | Hyperimmunoglobulin D syndrome; mevalonate kinase deficiency |
| Canakinumab | Ilaris | September 23, 2016 | Non-novel, rare disease only | Familial Mediterranean fever |
| Ivacaftor | Kalydeco | February 21, 2014 | Non-novel, rare disease only | Patients with cystic fibrosis aged ≥6 y with certain CFTR mutations (eg, G1224E, G1349D) |
| Ivacaftor | Kalydeco | December 29, 2014 | Non-novel, rare disease only | Patients with cystic fibrosis aged ≥6 y with certain R117H CFTR mutation |
| Ivacaftor | Kalydeco | March 17, 2015 | Non-novel, rare disease only | Patients with cystic fibrosis aged 2–5 y with certain CFTR mutations (eg, G1224E, G1349D) |
| Lumacaftor–ivacaftor | Orkambi | September 28, 2016 | Non-novel, rare disease only | Patients with cystic fibrosis aged 6–11 y homozygous for the F508del CFTR mutation |
| Lumacaftor–ivacaftor | Orkambi | August 7, 2018 | Non-novel, rare disease only | Patients with cystic fibrosis aged 2–5 y homozygous for the F508del CFTR mutation |
| Blinatumomab | Blincyto | December 3, 2014 | Novel drug | Philadelphia chromosome–negative relapsed or refractory B-cell precursor acute lymphoblastic leukemia |
| Cerliponase alfa | Brineura | April 27, 2017 | Novel drug | Patients aged ≥3 y with tripeptidyl peptidase 1 deficiency |
| Burosumab-twza | Crysvita | April 17, 2018 | Novel drug | X-linked hypophosphatemia in patients aged ≥1 y |
| Emapalumab-lzsg | Gamifant | November 20, 2018 | Novel drug | Hemophagocytic lymphohistiocytosis |
| Emicizumab-kxwh | Hemlibra | November 16, 2017 | Novel drug | Hemophilia A |
| Sebelipase alfa | Kanuma | December 8, 2015 | Novel drug | Lysosomal acid lipase |
| Uridine triacetate | n/a | September 4, 2015 | Novel drug | Hereditary orotic aciduria |
| Inotuzumab ozogamicin | n/a | August 17, 2017 | Novel drug | Relapsed or refractory B-cell precursor acute lymphoblastic leukemia |
| Tezacaftor and ivacaftor | n/a | February 12, 2018 | Novel drug | Patients with cystic fibrosis aged ≥12 y homozygous for the F508del CFTR mutation |
| Lumacaftor–ivacaftor | Orkambi | July 2, 2015 | Novel drug | Patients with cystic fibrosis aged ≥12 y homozygous for the F508del CFTR mutation |
| Asfotase alfa | Strensiq | October 23, 2015 | Novel drug | Perinatal-, infantile-, and juvenile-onset hypophosphatasia |
| Lanadelumab-flyo | Takhzyro | August 23, 2018 | Novel drug | Patients with hereditary angioedema aged ≥12 y |
n/a, not applicable.
Discussion
In this analysis of 402 orphan indications approved between 2010 and 2018, one-third were approved for children only or targeted pediatric diseases. The 136 pediatric orphan indications targeted 87 unique diseases, and 21 of these diseases were targeted by >1 indication. The majority of pediatric orphan indications were not for novel drugs but rather represented expanded uses of existing drugs, some of which are decades old and some of which are approved to treat common diseases. Twenty pediatric orphan indications were for drugs granted FDA breakthrough designation, suggesting that the drugs potentially could substantially improve existing treatments for serious conditions.
In a previous analysis, the authors found that one-third of FDA-approved orphan indications between 2000 and 2009 were pediatric.10 Although this proportion was similar to that found in our study, the absolute number of pediatric orphan indications has risen dramatically over the past decade, coinciding with the rise in the number of orphan indications more generally. The rising number of pediatric orphan indications, coupled with the fact that many were for drugs that may have represented therapeutic breakthroughs, suggest that recent pediatric orphan drug development may have provided life-altering or life-saving benefits to many children with rare diseases.
Although the success of recent pediatric orphan drug development is cause for optimism, the fact that many pediatric orphan indications targeted the same disease suggests that innovators are not solely focused on developing treatments for the 95% of rare diseases with no therapeutic options.9 For example, 4 pediatric orphan indications for novel drugs targeted hereditary angioedema. As another example, among the 31 pediatric orphan indications for drugs approved only to treat rare diseases, 25 targeted additional age- or biomarker-based subsets of a rare disease that the drug was already approved to treat. It is desirable to invest in the discovery of multiple treatment options for a single disease, such as hereditary angioedema, and to invest in the expansion of previous indications to the maximum number of patients who could benefit. However, it is important to ensure that this investment does not divert limited resources from efforts to discover treatments for rare diseases that have none.
Every pediatric orphan indication provides a degree of benefit to patients, as approval requires formal testing of safety and efficacy in children. Every indication also entails societal costs. Although a formal cost-effectiveness analysis was beyond the scope of this analysis, in this study we identified a few examples of pediatric orphan indications for which benefits may have been modest compared to these costs. Consider hydroxyurea: in 2017, 50 years after initial approval in 1967, an orphan version of this drug (Siklos) was approved to prevent acute pain crises and reduce the need for transfusion in children with sickle cell anemia, although hydroxyurea has been used off-label for this purpose for years.16 FDA approval of previously off-label uses of prescription drugs may be clinically valuable, as approval requires the sponsor to demonstrate the drug’s efficacy, identify appropriate dosing, and determine clinical risks. However, because of the orphan approval, no new hydroxyurea-containing medications can be marketed to prevent pain crises and reduce the need for transfusion in children with sickle cell anemia until 2024, the end of the 7-year period of orphan drug exclusivity. The lack of competition during this period means that the manufacturer has considerable ability to charge high prices. Indeed, the average wholesale price for Siklos is $6.00 per 100 mg tablet, compared to $0.95 per 200 mg capsule for Droxia, a hydroxyurea formulation also used for children with sickle cell anemia.17 Because it is increasingly common for patients and their families to pay a fraction of the costs of drug therapy out of pocket, high prices for orphan drugs may create financial burden and barriers to adherence for patients and families.18–21 High prices may also strain the budgets of private insurers and state Medicaid programs, the latter of which compete for scarce annual resources with other potentially beneficial programs for children, such as public education.
Overall, our results illustrate many successes of recent pediatric orphan drug development and also suggest that additional reforms may be needed to address the remaining unmet need for treatment among children with rare diseases. To achieve this goal, the US Congress recently passed the Research to Accelerate Cures and Equity Act of 2017.22 Before 2017, the Pediatric Research Equity Act did not require drug manufacturers seeking approval for an adult indication to include children in clinical testing if the disease did not affect children or if the drug had orphan designation. The Research to Accelerate Cures and Equity Act of 2017 amended the Pediatric Research Equity Act to require pediatric testing when a drug intended to treat an adult cancer has a common molecular target in pediatric cancer and additionally eliminated the orphan exemption for cancer drugs.22 This act could increase the number of children included in cancer drug trials and therefore the number of pediatric orphan indications.
Another reform that may help address unmet need is to allow drug prices to vary by indication on the basis of factors such as benefit to patients and availability of therapeutic alternatives.23 Such pricing may incentivize investment in the discovery of treatments for pediatric rare diseases that have none. However, this policy is not without trade-offs. For example, when the benefit of a drug cannot be predicted with certainty, innovators may choose not to invest in development rather than face the prospect of lower prices if the drug is ultimately not as efficacious as hoped. Future work is needed to evaluate the effects of this and other potential reforms on pediatric orphan drug development.
Our study has several limitations. First, we used FDA breakthrough designation to measure whether drugs may have represented substantial improvements over existing treatments. However, other drugs that were substantial improvements may have lacked this designation and/or may have received other FDA designations for promising drugs (eg, priority, fast-track, and accelerated approval). Research examining the benefit of individual pediatric orphan indications is needed to quantify the number representing major advances. Second, we used a consensus-based approach to classify whether diseases targeted by orphan indications were pediatric. However, the 2 raters agreed on 95% of initial classification decisions and had expertise in both general pediatrics and general internal medicine. Third, examining the cost-effectiveness of pediatric orphan indications was beyond the scope of this analysis, as was examining the budgetary impact of pediatric orphan indications on families and payers. These topics have been examined to some degree in previous analyses but are worthy of further study.19,24
Conclusions
The number of treatments for rare pediatric diseases has increased over the past decade, and many of these potentially represented therapeutic breakthroughs. However, substantial unmet need for treatment options remains for most children with rare diseases. Policy makers should consider changes to drug development incentives to ensure that these needs are met.
Glossary
- CDER
Center for Drug Evaluation and Research
- FDA
US Food and Drug Administration
Footnotes
Ms Kimmel, Ms Conti, Dr Volerman, and Dr Chua conceptualized and designed the study, analyzed and interpreted the data, and reviewed and revised the manuscript; and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Dr Chua is supported by a career development award from the National Institute on Drug Abuse (grant 1K08DA048110-01). The other authors have not received any funding. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.The IQVIA Institute Orphan Drugs in the United States: Growth Trends in Rare Disease Treatments. Parsippany, NJ: The IQVIA Institute; 2018 [Google Scholar]
- 2.America’s Health Insurance Plans The rise of orphan drugs. 2019. Available at: https://www.ahip.org/wp-content/uploads/IB_OrphanDrugs-1004.pdf. Accessed November 21, 2019
- 3.US Food and Drug Administration. Orphan drug designations and approvals. 2017. Available at: https://www.accessdata.fda.gov/scripts/opdlisting/oopd/index.cfm. Accessed August 15, 2019
- 4.Bagley N, Berger M, Chandra A, Garthwaite C, Stern A. The Orphan Drug Act at 30 In: Lerner J, Stern S, eds. Innovation Policy and the Economy, 2018 (National Bureau of Economic Research Innovation Policy and the Economy), vol. Vol 2 Chicago, IL: University of Chicago Press; 2019 [Google Scholar]
- 5.Mikami K. Orphans in the market: the history of orphan drug policy. Soc Hist Med. 2019;32(3):609–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Srivastava G, Ashley W. Orphan Drugs: Understanding the FDA Approval Process, vol. Vol. 1 Philadelphia, PA: University of Pennsylvania Academic Entrepreneurship for Medical and Health Scientists; 2019 [Google Scholar]
- 7.Tribble SJ, Lupkin S. Drugmakers Manipulate Orphan Drug Rules to Create Prized Monopolies. San Francisco, CA: Kaiser Health News; 2017 [Google Scholar]
- 8.US Food and Drug Administration Insights into rare disease drug approval: trends and recent developments. 2017. Available at: https://www.fda.gov/files/about%20fda/published/Insights-into-Rare-Disease-Drug-Approval--Trends-and-Recent-Developments-%28October-17--2017%29.pdf. Accessed September 28, 2019
- 9.The Lancet Diabetes Endocrinology Spotlight on rare diseases. Lancet Diabetes Endocrinol. 2019;7(2):75. [DOI] [PubMed] [Google Scholar]
- 10.Thorat C, Xu K, Freeman SN, et al. What the Orphan Drug Act has done lately for children with rare diseases: a 10-year analysis. Pediatrics. 2012;129(3):516–521 [DOI] [PubMed] [Google Scholar]
- 11.US Food and Drug Administration. Breakthrough therapy approvals. 2019. Available at: https://www.fda.gov/drugs/nda-and-bla-approvals/breakthrough-therapy-approvals. Accessed September 17, 2019
- 12.US Food and Drug Administration New molecular entity (NME) drug and new biologic approvals. 2018. Available at: https://www.fda.gov/drugs/nda-and-bla-approvals/new-molecular-entity-nme-drug-and-new-biologic-approvals. Accessed September 17, 2019
- 13.US Food and Drug Administration Drugs@FDA: FDA-approved drug products. 2019. Available at: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm. Accessed September 17, 2019
- 14.US Food and Drug Administration Licensed biological products with supporting documents. 2019. Available at: https://www.fda.gov/vaccines-blood-biologics/licensed-biological-products-supporting-documents. Accessed September 17, 2019
- 15.Lichtenberg FR. The effect of pharmaceutical innovation on longevity: patient level evidence from the 1996-2002 Medical Expenditure Panel Survey and Linked Mortality Public-use Files. Forum Health Econ Policy. 2013;16(1):1–33 [DOI] [PubMed] [Google Scholar]
- 16.Reeves SL, Jary HK, Gondhi JP, Raphael JL, Lisabeth LD, Dombkowski KJ. Hydroxyurea use among children with sickle cell anemia. Pediatr Blood Cancer. 2019;66(6):e27721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.IBM Watson Health IBM Micromedex Web applications access. Available at: https://www.micromedexsolutions.com. Accessed December 6, 2019
- 18.Thomas K. This new treatment could save the lives of babies. But it costs $2.1 million. The New York Times. May 24, 2019. Available at: https://www.nytimes.com/2019/05/24/health/zolgensma-gene-therapy-drug.html. Accessed August 15, 2019.
- 19.Chua KP, Conti RM. Out-of-pocket spending on orphan drug prescriptions among commercially insured adults in 2014. J Gen Intern Med. 2019;34(3):338–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomas K, Abelson R. The $6 million drug claim. The New York Times. August 25, 2019. Available at: https://www.nytimes.com/2019/08/25/health/drug-prices-rare-diseases.html. Accessed September 17, 2019.
- 21.Kaiser Family Foundation ; Peterson Center on Healthcare. What are recent trends and characteristics of workers with high drug spending? Available at https://www.healthsystemtracker.org/chart-collection/recent-trends-characteristics-workers-high-drug-spending/#item-start. Accessed January 27, 2020
- 22.Fink JL. Will the RACE for Children Act lead to new treatments for pediatric cancer? Cancer. 2017;123(2):189. [DOI] [PubMed] [Google Scholar]
- 23.Bach PB. Indication-specific pricing for cancer drugs. JAMA. 2014;312(16):1629–1630 [DOI] [PubMed] [Google Scholar]
- 24.Institute for Clinical and Economic Review. Assessing the effectiveness and value of drugs for rare conditions: a technical brief for the ICER Orphan Drug Assessment & Pricing Summit. 2017. Available at: https://icer-review.org/wp-content/uploads/2017/02/ICER_Assessing-the-Value-of-Drugs-for-Rare-Conditions_051017.pdf. Accessed September 23, 2019