Version Changes
Revised. Amendments from Version 1
This version is a minor revision and improvement of the already accepted manuscript, based on the comments from the two reviewers. The main change is the inclusion of accuracy measures for germline variants based on the Genome In a Bottle HG001 gold standard dataset, presented in the text and in the new Table 4. In addition, we have also added information about which tools are used for each type of variant calling in the revised Table 1. Other edits to the text are minor clarifications of i) the selection of the included software, ii) the usage of the “-profile” parameter, iii) the yet limited benchmarking of exome sequencing data, iv) the availability of a small test dataset, v) the user responsibility to adjust the downstream filtering of variants, vi) how Docker, Singularity and Conda environments are provided, and vii) the workflow error handling.
Abstract
Whole-genome sequencing (WGS) is a fundamental technology for research to advance precision medicine, but the limited availability of portable and user-friendly workflows for WGS analyses poses a major challenge for many research groups and hampers scientific progress. Here we present Sarek, an open-source workflow to detect germline variants and somatic mutations based on sequencing data from WGS, whole-exome sequencing (WES), or gene panels. Sarek features (i) easy installation, (ii) robust portability across different computer environments, (iii) comprehensive documentation, (iv) transparent and easy-to-read code, and (v) extensive quality metrics reporting. Sarek is implemented in the Nextflow workflow language and supports both Docker and Singularity containers as well as Conda environments, making it ideal for easy deployment on any POSIX-compatible computers and cloud compute environments. Sarek follows the GATK best-practice recommendations for read alignment and pre-processing, and includes a wide range of software for the identification and annotation of germline and somatic single-nucleotide variants, insertion and deletion variants, structural variants, tumour sample purity, and variations in ploidy and copy number. Sarek offers easy, efficient, and reproducible WGS analyses, and can readily be used both as a production workflow at sequencing facilities and as a powerful stand-alone tool for individual research groups. The Sarek source code, documentation and installation instructions are freely available at https://github.com/nf-core/sarek and at https://nf-co.re/sarek/.
Keywords: Analysis workflow, Whole Genome Sequencing, Germline variants, Somatic variants, Cancer
Introduction
Whole-genome sequencing (WGS) and whole-exome sequencing (WES) technologies opens up new avenues for research and for clinical applications, with many large initiatives launched worldwide. While much effort has been invested in novel sequencing analysis software, the importance of providing and maintaining workflows to combine software in an efficient and reproducible manner has been underestimated and too few resources are typically dedicated to address this issue. This is of particular importance for somatic variant analysis and especially for analysis of complex cancer genomes, where a combination of tools is still required for optimal sensitivity and specificity and to detect various types of gene mutations and other abnormalities ( Alioto et al., 2015). Some encouraging solutions have been presented in recent years, including SeqMule ( Guo et al., 2015), SpeedSeq ( Chiang et al., 2015), Bcbio-nextgen, and DNAp ( Causey et al., 2018). While all of the above represent commendable and important efforts, we have not found any workflow solution that in our opinion fulfils all of the following important user aspects: (i) easy installation, (ii) robust portability across different compute environments, (iii) comprehensive documentation, (iv) transparent and easy-to-read code, and (v) extensive quality metrics reporting. Here we present Sarek, an easy-to-install community-maintained workflow, offering a complete and scalable solution for germline and somatic variant detection, annotation and quality control. Sarek supports several reference genomes and can handle data from WGS, WES and gene panels, and is intended to be used both as a production workflow at core facilities and as a stand-alone tool for individual research groups. By using Docker or Singularity containers, Sarek installs easily on all POSIX compatible systems such as Linux and Mac OS X and is designed to work on compute environments dedicated to handle sensitive personal data without direct internet access—a situation expected to become increasingly common with growing data security awareness.
Methods
Operation: workflow overview and software
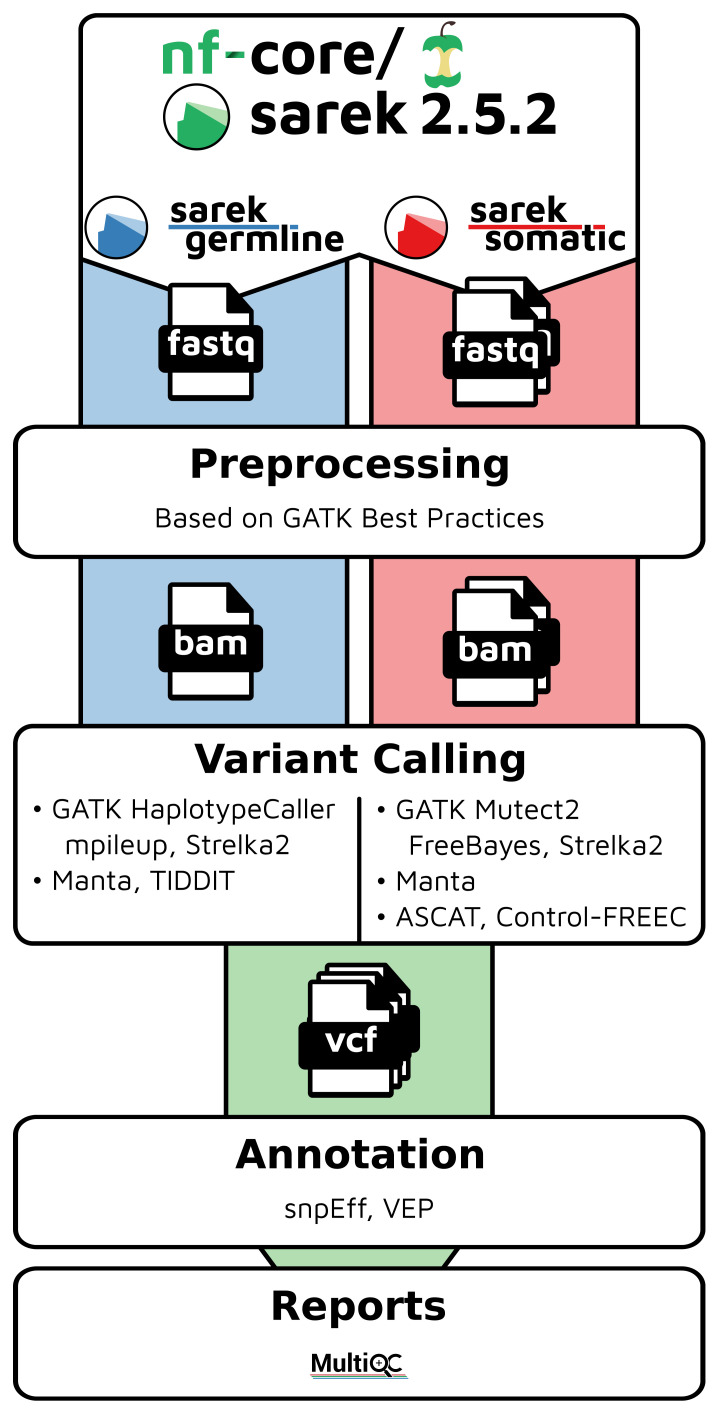
Sarek offers a portable workflow for germline and somatic variant detection, annotation and quality control based on WGS, WES or gene panel data, using a range of state-of-the-art software and data resources in the field ( Table 1, Figure 1). In the pre-processing step, sequence reads are aligned to the reference genome with BWA-MEM ( Li, 2013), followed by deduplication and recalibration with GATK ( McKenna et al., 2010). For germline samples, single-nucleotide variants and small insertion/deletions are detected with HaplotypeCaller ( McKenna et al., 2010) and Strelka2 ( Kim et al., 2018), and structural variations are detected with Manta ( Chen et al., 2016) and TIDDIT ( Eisfeldt et al., 2017). For somatic samples, somatic single-base mutations (SSM) and small somatic insertion/deletion mutations (SIM) are detected by GATK4 Mutect2 ( Cibulskis et al., 2013) and Strelka2 ( Kim et al., 2018). Somatic structural variants (including copy-number variation), as well as ploidy and sample purity are detected by Manta ( Chen et al., 2016), ASCAT ( Van Loo et al., 2010), and Control-FREEC ( Boeva et al., 2012). All variants are annotated for potential functional effects with snpEff ( Cingolani et al., 2012) and VEP ( McLaren et al., 2016). Importantly, Sarek also generates a wide range of quality control metrics using FastQC, QualiMap ( Okonechnikov et al., 2016), BCFtools ( Li, 2011), Samtools ( Li et al., 2009), and VCFtools ( Danecek et al., 2011), visualized as an aggregated quality control review across samples with MultiQC ( Ewels et al., 2016). All software currently included in Sarek are selected based on the criteria that they should be of high quality, well-maintained, and with robust installation and running performances. Additional alternative or complementing software will be added to Sarek in later updates, based on the input and engagement of the user community.
Table 1. Software required and implemented in Sarek.
A list of all the software required and currently implemented in Sarek. All analysis and quality metrics software are installed automatically when Sarek is launched. P, Preprocessing; G, Germline; S, Somatic; snv, Single-nucleotide variants and small indels; sv, Structural variants; pp, Ploidy and sample purity; a, Annotation.
Figure 1. Schematic overview of the Sarek workflow for analysis of germline and somatic variants.
A schematic overview including some of the main analysis software implemented in the Sarek workflow. A more comprehensive list of the currently implemented software is given in Table 1.
Portability and reproducibility
Sarek is implemented in Nextflow ( Di Tommaso et al., 2017), a workflow language designed specifically for bioinformatics applications. Nextflow has a transparent design, making the Sarek code easy to read, adjust and extend. Sarek has well-functioning error reporting to diagnose e.g. software or hardware errors during a run, and incomplete runs are easily restarted from any stage in the workflow process. Compared to the Bpipe workflow language (used in for example DNAp), Nextflow offers superior support for different execution environments, like Slurm, Sun Grid Engine, LSF and Kubernetes, and includes native support for cloud compute environments including Google Cloud and AWS. Support for AWS batch gives the possibility to easily distribute thousands of batch jobs on Amazon Web Services. Sarek is part of a rapidly growing community effort of well documented and community-tested Nextflow pipelines, and adheres to the nf-core portability and documentation guidelines ( Ewels et al., 2019). To facilitate easy installation and to ensure reproducibility, all Sarek required tools are installed in Conda, and then pushed to DockerHub ( https://hub.docker.com/), making Sarek and all its dependencies directly accessible from a Conda environment, or as Docker or Singularity ( Kurtzer et al., 2017) containers. While Docker is a widely appreciated container solution, it is not always allowed at high-performance computing centers because of the involved security risks, making Singularity the preferred choice at these sites ( Kurtzer et al., 2017). This is of particular importance for computer environments designed for handling of sensitive personal data, where a high level of data security has to be maintained across multiple projects and users.
Implementation: equipment and resource usage
Sarek can be installed and executed on any POSIX-compatible computer system. To run a full WGS analysis, including both germline and somatic variants from a tumour/normal dataset with 90x/90x read coverage, we recommend a minimum of 16 cores on a node with 128 GB RAM, and at least 4 TB available free storage (in addition to the initial FASTQ files) in the input/output working directory. Of this, about 1.4 TB will be allocated for BAM files, annotated VCF files and CNV files, but excluding GVCF files ( Table 2). At the end of the run, 2.3 TB temporary data can be removed, unless the user plans to perform re-runs from intermediate processing states. Many processes are distributed across cores by dividing the genome into smaller chunks, each being handled as a separate core job, with all the results being merged and sorted in a final step. Some of the used software are parallelized by design, while for others Sarek uses a scatter-gather approach to efficiently distribute the processing load across CPU cores and reduce the wall clock runtime.
Table 2. Sarek resource usage.
Resource usage during a Sarek run on a WGS 90X/90X coverage medulloblastoma dataset on a 48-threaded computer node, starting from compressed FASTQ files. The storage resources refer to result files only. The total storage including all temporary data was 3.7 TB.
| Input
data |
Mapping, merging,
deduplication |
Quality score
recalibration |
Variant calling,
annotation |
Total | |
|---|---|---|---|---|---|
| Storage | 458 GB | 530 GB | 386 GB | 4 GB | 1378 GB |
| Process time | 1081 CPU h | 95 CPU h | 614 CPU h | 1790 CPU h | |
| Wall clock time | 35h 26m | 3h 26m | 13h 29m | 48h 21m | |
| Peak memory | 119 GB | 18 GB | 128 GB |
GB, gigabyte; CPU, central processing unit; h, hours; m, minutes.
Installation and testing
Sarek is run from a computer system with a local installation of Nextflow and support for either Conda environments, Docker or Singularity containers. Nextflow can automatically fetch the Sarek source code from GitHub. All software dependencies are encapsulated in Docker or Singularity containers which are downloaded from Docker Hub, or built in a new Conda environment using Bioconda ( Grüning et al., 2018). As such, cumbersome software installations by the user are completely avoided. Configuration files allow tailoring to specific user needs. Sarek comes with a small test dataset and a suite of tests to verify the installation. This is also used for Continuous Integration testing with GitHub Actions.
Results
To test performance in terms of resource usage and biological results, Sarek was run on a medulloblastoma WGS tumour/normal dataset from a sample with high tumour cell content (∼98%), and with a curated “Gold Set” of verified somatic mutations from a previous benchmark study ( Alioto et al., 2015). In line with the above benchmark study, Sarek (version 2.5.2) was executed with WGS germline and somatic variant calling using a 90X/90X tumour/normal dataset (accession number EGAD00001001859, read sets EGAR00001387019-24 and EGAR00001387025-32). Runs were performed on a single 48-thread node with a local direct attached storage (DAS): A Dell PowerEdge R740 server, with two Intel Xeon Gold 6126 with a total of 24 cores (48 threads) CPUs, 756 GB memory, and 100 TB SCv3020 Compellent Storage. The complete Sarek run including preprocessing followed by both germline and somatic variant calling and annotation took 48 hours and 21 minutes, and required about three times more storage than the original input data ( Table 2). Notably, the complete Sarek run was executed by a single command, with fully automated installation, execution, and efficient job distributions of the more than 15 different software tools to complete the analysis and provide quality control metrics, without any manual intervention needed during the two-day run. To ensure that the Sarek output was biologically sound, we calculated precision, recall and F1 statistics for the Sarek output based on the “Gold Set” of somatic single-base mutations (SSM) and somatic insertion/deletion mutations (SIM) as previously defined ( Alioto et al., 2015). Using the intersection of the output from the two somatic variant callers (GATK4 Mutect2 and Strelka2), Sarek provided accuracy measures for SSMs (F1 score = 0.80) and SIMs (F1 score = 0.58) in the top range of the 18 somatic variant calling procedures included in the original benchmarking study on this data set ( Table 3), indicating that the workflow operates as intended. The sample purity was estimated to be 100%, as compared to 98% previously reported for this sample. For somatic structural variants and ploidy, no relevant benchmark data was available, and therefore no quantitative assessment beyond previously published results for the implemented software could be performed, but the integrity of the runs were checked by comparing the results of Manta, ASCAT, and Control-FREEC run within Sarek and as stand-alone. To benchmark Sarek on germline single-nucleotide variants and small insertions/deletions, we used 46X WGS data for the well-studied individual NA12878:HG001 ( ftp://ftp-trace.ncbi.nlm.nih.gov/giab/ftp/data/NA12878/NIST_NA12878_HG001_HiSeq_300x/, read set folders 131219_D00360_005_BH814YADXX [accession number SRR2052337 - SRR2052339, SRR2052342, SRR2052345, SRR20523428], and 131219_D00360_006_AH81VLADXX [accession number SRX1049774 -SRX1049779]) and a “Gold Set” of variants from the Genome in a Bottle project ( Zook et al., 2019), showing overall high accuracy ( Table 4).
Table 3. Sarek WGS somatic variant benchmarking.
Summary of accuracy measures for the two somatic variant callers used in Sarek to detect somatic single-base mutations (SSMs) and somatic insertion/deletion mutations (SIMs), as well as their union and intersection.
| Somatic caller | Recall | Precision | F1-score |
|---|---|---|---|
| SSM (Gold Set: n=1263) | |||
| GATK4 Mutect2 | 0.80 | 0.45 | 0.58 |
| Strelka2 | 0.77 | 0.29 | 0.42 |
| Union (GATK4 Mutect2, Strelka2) | 0.82 | 0.23 | 0.36 |
| Intersection (GATK4 Mutect2, Strelka2) | 0.74 | 0.88 | 0.80 |
| Benchmark median * | 0.68 | 0.78 | 0.71 |
| SIM (Gold Set: n=347) | |||
| GATK4 Mutect2 | 0.48 | 0.38 | 0.42 |
| Strelka2 | 0.74 | 0.31 | 0.44 |
| Union (GATK4 Mutect2, Strelka2) | 0.77 | 0.25 | 0.38 |
| Intersection (GATK4 Mutect2, Strelka2) | 0.46 | 0.77 | 0.58 |
| Benchmark median * | 0.34 | 0.71 | 0.48 |
* The median accuracy measures across 18 somatic variant calling procedures as previously reported ( Alioto et al., 2015)
Table 4. Sarek WGS germline variant benchmarking.
Summary of accuracy measures for the two variant callers used in Sarek to detect germline single-nucleotide variants (SNVs) and germline insertion/deletion variants (INDELs), as well as their union and intersection.
| Germline caller | Recall | Precision | F1-score |
|---|---|---|---|
| SNV (Gold Set: n=3088156) | |||
| GATK4 HaplotypeCaller | 0.93 | 1.00 | 0.96 |
| Strelka2 | 0.98 | 1.00 | 0.99 |
| Union (GATK4 HaplotypeCaller, Strelka2) | 0.99 | 0.94 | 0.96 |
| Intersection (GATK4 HaplotypeCaller, Strelka2) | 0.93 | 1.00 | 0.96 |
| INDEL (Gold Set: n=530423) | |||
| GATK4 HaplotypeCaller | 0.91 | 0.99 | 0.95 |
| Strelka2 | 0.92 | 0.99 | 0.95 |
| Union (GATK4 HaplotypeCaller, Strelka2) | 0.93 | 0.98 | 0.96 |
| Intersection (GATK4 HaplotypeCaller, Strelka2) | 0.90 | 1.00 | 0.94 |
Use case
Sarek has been extensively tested and applied on various WGS datasets, including thousands of samples for germline variant analyses, and hundreds of paired tumour/normal samples for somatic mutation analyses. In addition, Sarek has also been adapted to run on WES data and gene panels, and has been reported to work well in pilot user projects, although no systematic testing has yet been performed on such data. Below we present a standard use case with a tumour/normal WGS dataset as input, running both germline and somatic variant analyses.
Input data
For a somatic variant analysis, the user should provide the sequencing FASTQ files from both tumour and normal control tissue from the same individual, described in a tab-delimited TSV file (here: samples.tsv). Each line of the TSV file contains information about a sequence data file, including: The identifier of the individual, the gender (XX or XY), the status of the sample (0 for Normal or 1 for Tumour), the identifier of the sample, the sequencing lane (if samples are multiplexed across multiple lanes), and the paths to the FASTQ file of the first and second read in the read-pair. Relapse samples from the same individual are also supported.
Running sarek on WGS data with singularity containers
Running Sarek with Singularity container on a computer system supporting Java 8 requires only installation of Nextflow and Singularity. A full analysis run starting from FASTQ files including mapping, recalibration, variant calling and annotation, as well as generating a full QC report can be invoked by a single Nextflow command:
> nextflow run nf-core/sarek -r 2.5.2 -profile singularity --input samples.tsv --tools Mutect2,Strelka,Manta,TIDDIT,ASCAT,ControlFREEC,snpEff,VEP
Nextflow will recognize the workflow name and will download the specified version (2.5.2) of the pipeline from GitHub, including the corresponding container, as well as fetching the required reference files from AWS-iGenomes. The default reference genome is human GRCh38, but Sarek also supports GRCh37 and nearly 30 other genomes directly accessible from iGenomes. Alternatively, users can manually supply Sarek with other reference genomes. Non-default parameters and links to local reference files are handled in accordance with nf-core guidelines. User configuration profiles can be stored locally or centrally at https://github.com/nf-core/configs.
Output
A full Sarek run will produce a large number of output files, but the main results consist of (i) a set of annotated variants in VCF files from the various included tools for both germline and somatic variants, (ii) tumour sample purity and ploidy results for somatic samples, and (iii) a broad set of QC metrics. A detailed description of all output files is given at the Sarek documentation pages. While Sarek will report variants from all callers included in the run, it is up to the user to decide how to combine and filter the results from different callers, since the optimal post-processing will depend on the particular samples and research questions at hand.
Discussion
Human WGS is transforming medical research, and provides a foundation to develop novel clinical applications and improve health care. An important aspect to harvesting the potential of WGS is however to empower the research community with adequate bioinformatics tools, and reproducible bioinformatics workflows are important drivers of scientific progress by making complex processing of large datasets feasible for a wide range of researchers. While we are highly appreciative of existing workflows for cancer and non-cancer variant detection, we argue that there is no one-size-fits-all solution and more initiatives are needed to serve the large and diverse research user community, especially for WGS data. Sarek builds on a philosophy of reasonably narrow, independent workflows, written in the domain-specific language Nextflow. In our experience, this is an effective strategy to simplify workflow maintenance at sequencing core facilities, and to allow easy deployment and modifications by individual research groups. Sarek efficiently utilizes cloud and high-performance compute clusters and installs easily across compute environments. Sarek provides annotated VCF files, CNV reports and quality metrics for germline and cancer samples from raw FASTQ sequencing data in about 48 hours for 90X/90X WGS data (as demonstrated here), in a few hours for WES data, and within minutes for gene panels (in-house data, not presented here). It should be noted that while Sarek can substantially reduce the labor and management time of running and maintaining a large collection of software, and help users to perform quality-controlled reporting in an organized manner, careful parameter tuning, downstream variant filtering, and qualitative assessments by the user remains important. Ongoing efforts aim to develop add-on ranking and visualization modules and to efficiently extract clinically and biologically relevant findings, to help advance basic and translational research.
Conclusion
Sarek is a portable and reproducible workflow to detect germline and somatic variants from WGS, WES and gene panel data. It includes extensive analysis and quality control metrics, while still being limited to a relatively narrow scope to achieve optimal usability, functionality and transparency. Sarek is flexible with a low threshold for user modifications, and is thus well adapted to the current requirements in the research community. Thanks to its design, it installs easily and reproducibly on all POSIX compatible computer systems, including secure compute environments for sensitive personal data with indirect Internet access.
Data availability
Source data
European Genome-phenome Archive: A comprehensive assessment of somatic mutation detection in cancer using whole genome sequencing. https://www.ebi.ac.uk/ega/datasets/EGAD00001001859. Read sets EGAR00001387019-24 and EGAR00001387025-32 were analysed. These data are held under restricted access. Readers wishing to apply for access to the data must first apply through the ICGC Data Access Compliance Office ( https://icgc.org/daco) and complete the data access form. Access will be granted to those whose projects conform to the goals and policies of ICGC. Help with completing the data access form is available at https://icgc.org/daco/help-guide-section.
Sequence Read Archive: NIST Genome in a Bottle, ~300X sequencing of HG001 (NA12878). ftp://ftp-trace.ncbi.nlm.nih.gov/giab/ftp/data/NA12878/NIST_NA12878_HG001_HiSeq_300x/, read set folders 131219_D00360_005_BH814YADXX [SRA accession number SRR2052337 - SRR2052339, SRR2052342, SRR2052345, SRR20523428], and 131219_D00360_006_AH81VLADXX [SRA accession number SRX1049774 -SRX1049779]). These data are publicly available for direct download.
The workflow itself comes with a prebuilt profile with a complete configuration for automated testing, including links to a small test dataset.
Software availability
Sarek is available at: https://nf-co.re/sarek.
Source code available at: https://github.com/nf-core/sarek.
Archived source code at time of publication: https://doi.org/10.5281/zenodo.3579102 ( Garcia et al., 2019).
License: MIT License.
Acknowledgements
We are grateful for the valuable input from the Oslo University Hospital bioinformatics core facility (Oslo University Hospital), the T Martinsson lab (Gothenburg University), the A–C Syvänen lab (Uppsala University), and Alex Peltzer (Quantitative Biology Center, University of Tübingen). The National Genomics Infrastructure (NGI) and Uppsala Multidisciplinary Centre for Advanced Computational Science (UPPMAX) provided computational resources. Help with graphical design was provided by Dr. Jonas Söderberg (Uppsala university).
Funding Statement
This study was supported by the Swedish Research Council (NGI: 2017-00630, NBIS: 2017-00656), the Swedish Childhood Cancer Fund (The Swedish Childhood Tumor Biobank (BTB): BB2017-0001; BB2018-0001; BB2019-0001), and the Knut and Alice Wallenberg Foundation (KAW 2014.0278).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 2; peer review: 2 approved]
References
- Alioto TS, Buchhalter I, Derdak S, et al. : A comprehensive assessment of somatic mutation detection in cancer using whole-genome sequencing. Nat Commun. 2015;6:10001. 10.1038/ncomms10001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeva V, Popova T, Bleakley K, et al. : Control-FREEC: a tool for assessing copy number and allelic content using next-generation sequencing data. Bioinformatics. 2012;28(3):423–5. 10.1093/bioinformatics/btr670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Causey JL, Ashby C, Walker K, et al. : DNAp: A Pipeline for DNA-seq Data Analysis. Sci Rep. 2018;8(1):6793. 10.1038/s41598-018-25022-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Schulz-Trieglaff O, Shaw R, et al. : Manta: rapid detection of structural variants and indels for germline and cancer sequencing applications. Bioinformatics. 2016;32(8):1220–1222. 10.1093/bioinformatics/btv710 [DOI] [PubMed] [Google Scholar]
- Chiang C, Layer RM, Faust GG, et al. : SpeedSeq: ultra-fast personal genome analysis and interpretation. Nat Methods. 2015;12(10):966–968. 10.1038/nmeth.3505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cibulskis K, Lawrence MS, Carter SL, et al. : Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nat Biotechnol. 2013;31(3):213–219. 10.1038/nbt.2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cingolani P, Platts A, Wang le L, et al. : A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w 1118; iso-2; iso-3. Fly (Austin). 2012;6(2):80–92. 10.4161/fly.19695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danecek P, Auton A, Abecasis G, et al. : The variant call format and VCFtools. Bioinformatics. 2011;27(15):2156–2158. 10.1093/bioinformatics/btr330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Tommaso P, Chatzou M, Floden EW, et al. : Nextflow enables reproducible computational workflows. Nat Biotechnol. 2017;35(4):316–319. 10.1038/nbt.3820 [DOI] [PubMed] [Google Scholar]
- Eisfeldt J, Vezzi F, Olason P, et al. : TIDDIT, an efficient and comprehensive structural variant caller for massive parallel sequencing data [version 2; peer review: 2 approved]. F1000Res. 2017;6:664. 10.12688/f1000research.11168.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewels P, Magnusson M, Lundin S, et al. : MultiQC: Summarize analysis results for multiple tools and samples in a single report. Bioinformatics. 2016;32(19):3047–3048. 10.1093/bioinformatics/btw354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewels PA, Peltzer A, Fillinger S, et al. : nf-core: Community curated bioinformatics pipelines. bioRxiv. 2019;610741 10.1101/610741 [DOI] [PubMed] [Google Scholar]
- Garcia M, Peltzer A, Alneberg J: nf-core/sarek: Sarek 2.5.2 - Jåkkåtjkaskajekna (Version 2.5.2). Zenodo. 2019. 10.5281/zenodo.3579102 [DOI] [Google Scholar]
- Grüning B, Dale R, Sjödin A, et al. : Bioconda: sustainable and comprehensive software distribution for the life sciences. Nat Methods. 2018;15(7):475–476. 10.1038/s41592-018-0046-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Ding X, Shen Y, et al. : SeqMule: automated pipeline for analysis of human exome/genome sequencing data. Sci Rep. 2015;5:14283. 10.1038/srep14283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Scheffler K, Halpern AL, et al. : Strelka2: fast and accurate calling of germline and somatic variants. Nat Methods. 2018;15(8):591–594. 10.1038/s41592-018-0051-x [DOI] [PubMed] [Google Scholar]
- Kurtzer GM, Sochat V, Bauer MW: Singularity: Scientific containers for mobility of compute. PLoS One. 2017;12(5):e0177459. 10.1371/journal.pone.0177459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H: A statistical framework for SNP calling, mutation discovery, association mapping and population genetical parameter estimation from sequencing data. Bioinformatics. 2011;27(21):2987–2993. 10.1093/bioinformatics/btr509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H: Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv 1303.3997v2. 2013. Reference Source [Google Scholar]
- Li H, Handsaker B, Wysoker A, et al. : The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25(16):2078–2079. 10.1093/bioinformatics/btp352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna A, Hanna M, Banks E, et al. : The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20(9):1297–1303. 10.1101/gr.107524.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren W, Gil L, Hunt SE, et al. : The Ensembl Variant Effect Predictor. Genome Biol. 2016;17(1):122. 10.1186/s13059-016-0974-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okonechnikov K, Conesa A, García-Alcalde F: Qualimap 2: advanced multi-sample quality control for high-throughput sequencing data. Bioinformatics. 2016;32(2):292–294. 10.1093/bioinformatics/btv566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Loo P, Nordgard SH, Lingjærde OC, et al. : Allele-specific copy number analysis of tumors. Proc Natl Acad Sci U S A. 2010;107(39):16910–16915. 10.1073/pnas.1009843107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zook JM, McDaniel J, Olson ND, et al. : An open resource for accurately benchmarking small variant and reference calls. Nat Biotechnol. 2019;37(5):561–566. 10.1038/s41587-019-0074-6 [DOI] [PMC free article] [PubMed] [Google Scholar]