Abstract
Importance
Transcranial Doppler (TCD) screening identifies children with sickle cell anemia with the highest risk of stroke. An accurate claims-based methodology for identifying children with sickle cell anemia was recently developed and validated, which establishes the necessary groundwork to enable large population-based assessments of health services use among children with sickle cell anemia using administrative claims.
Objective
To assess the feasibility of using administrative claims to identify receipt of TCD screening among children with sickle cell anemia, describe the receipt of TCD screening among children with sickle cell anemia, and characterize opportunities for intervention.
Design
Cross-sectional study using Medicaid claims from 2005 to 2010.
Setting
Medicaid claims were obtained from the following states: Florida, Illinois, Louisiana, Michigan, South Carolina and Texas.
Participants
Children 2–16 years with sickle cell anemia were identified through the presence of ≥3 Medicaid claims with a diagnosis of HbSS within a calendar year (2005–2010). A total of 4,775 children contributed 10,787 person-years throughout the study period.
Exposure(s)
A subset of children enrolled for ≥2 consecutive years was identified to examine potential predictors of TCD screening, which included age, gender, previous receipt of TCD screening, state of residence, and health services utilization (well child visits, outpatient, ED, inpatient).
Main Outcome and Measure(s)
Receipt of TCD screening and sickle cell disease (SCD)-related healthcare utilization was assessed by year and state. Using logistic regression with generalized estimating equations, associated predictors were included in a multivariable model to estimate odds of TCD screening.
Results
TCD screening rates increased over the six-year study period from 22% to 44% (p<0.0001); rates varied substantially across states (p=0.0037). A subset of 2,388 children with sickle cell anemia (50%) was enrolled for ≥2 consecutive years. Each year of increasing child’s age was associated with 3% lower odds of TCD screening (p=0.002). Previous receipt of TCD screening (OR=2.44, p<0.0001) or well child visits (OR=1.10, p=0.007) were associated with higher odds of receiving a TCD screening.
Conclusions and Relevance
Despite national recommendations, TCD screening rates remain low. Successful strategies to improve TCD screening rates may capitalize on the numerous healthcare interactions among children with sickle cell anemia.
Introduction
Sickle cell disease (SCD) is a hereditary chronic condition primarily affecting minority children in the US and associated with substantial morbidity and mortality.1–4 SCD exists in multiple subtypes; the subtype conferring the highest risk of morbidity and mortality is sickle cell anemia (hemoglobin [Hb] SS). Sickle cell anemia is associated with an elevated risk of stroke; without intervention, reports indicate that 10% of children with sickle cell anemia will have a stroke prior to the age of 20.5,6 Elevated cerebral blood velocities are associated with an increased risk of stroke, which can be assessed using Transcranial Doppler (TCD) screening.7 The Stroke Prevention Trial in Sickle cell anemia (STOP) demonstrated that among children with high blood velocities as detected by TCD screening, receipt of chronic blood transfusions reduced the risk of stroke by 92% compared to the standard care.8
Recently, the National Heart, Lung and Blood Institute (NHLBI) released an Expert Panel Report detailing evidence-based guidelines for the treatment of SCD.9,10 These guidelines strongly recommend that children with sickle cell anemia receive annual TCD screenings from 2–16 years of age. Although this recommendation is consistent with those published for the prior decade,11 prior studies indicate that TCD screening rates remain low.10–14 Most reports of TCD screening rates have been limited to studies from comprehensive sickle cell centers (CSCC) where the identification of the target population is enabled either through chart review or registries within a CSCC, or limited to a state Medicaid program.12–15
Achieving a population-based perspective on TCD screening has been hampered by the inherent challenges of identifying children with sickle cell anemia among large populations where medical chart review would be cost prohibitive. In population-based studies of other chronic conditions, administrative claims data have been successfully employed to conduct asthma surveillance16,17 and a wide array of studies characterizing health services use.18–20 A claims-based methodology for identifying children with sickle cell anemia with a high degree of sensitivity and specificity was recently developed and validated.21 The accuracy of this claims-based method establishes the necessary groundwork to enable large population-based assessments of health services use among children with sickle cell anemia using administrative claims. With that in mind, our objective was to assess receipt of TCD screening among a multi-state population of children with sickle cell anemia and to characterize opportunities for intervention.
Methods
We performed a cross-sectional study of TCD screening rates among children with sickle cell anemia using a multi-state set of Medicaid administrative claims data.
Data source and Study Population
Our target population was drawn from the Medicaid programs for six states with average to high prevalence of SCD: Florida, Illinois, Louisiana, Michigan, South Carolina and Texas.22 Administrative data from Medicaid Analytic eXtract (MAX) for the years 2005 to 2010 were acquired from the Centers for Medicare and Medicaid Services (CMS), including enrollment history and all claims for inpatient, outpatient, emergency department (ED), laboratory, and outpatient pharmacy health services. At the time of this study, these were the most recent MAX data available. Using a validated methodology, our study population consisted of children with three or more claims for HbSS within a year.21,23–25 In a similar manner, we acquired all administrative claims for all services provided to these children.
Children 2–16 years old were eligible to be in the study population, consistent with recommendations for annual TCD screening.10 Eligible children were required to be enrolled in Medicaid for at least one calendar year (January 1 – December 31) from 2005 to 2010. Children could contribute multiple years of enrollment during the study period. As chronic blood transfusions are likely to be indicative of prior stroke or treatment for children with high blood velocities as detected by previous TCD,7,8 we excluded children with receipt of more than 6 chronic blood transfusions in a year using procedure codes (Table 1).
Table 1.
Claims-Based Definitions of Variables using Medicaid Analytic eXtract data; 2005–2010
| Variable | Dataset | Definition |
|---|---|---|
| Inpatient Visit | Inpatient File | Any Event within file |
| Outpatient Visit | Other Services File | Event labeled Physician, Other Practitioner, Outpatient hospital, Clinic (Other categories such as dental, laboratory, home health excluded) |
| Transcranial Doppler | Other Services File | HCPCS* Codes: 93886, 93888, 93890, 93892, 93893 |
| Blood Transfusion | Other Services File | HCPCS Codes: 09883, 36455, 86999, S3906, S9538, 09882, 36430 |
| Well-Child Visit | Other Services File | HCPCS Codes: 99381–5; 99391–5; 99432, 83655 |
| Emergency Department Visit | Other Services File | ICD-9 Codes: V202, V700, V703, V705–9 |
| Sickle Cell Disease-related Healthcare | Inpatient and Other Services File | Place of Service: Emergency Department |
| Sickle Cell Anemia | Inpatient and Other Services File | ICD-9 Codes: 282.61, 282.61 |
HCPCS: Healthcare Common Procedure Coding System, a set of procedure codes based on the Current Procedural Terminology (CPT) from the American Medical Association
TCD Screening and Health Services Utilization
Receipt of TCD was classified dichotomously (yes/no) within each calendar year using procedure codes. The accuracy of this approach has previously been validated; as compared to the gold standard of medical charts, Medicaid administrative claims for receipt of TCD screening have a sensitivity of 94% (95% CI: 83%−99%) and a specificity of 100% (95% CI: 91%−100%) among children enrolled in Michigan Medicaid.26
Potential Predictors of TCD Screening
To assess potential predictors of TCD screening, we identified a subgroup of the study population specified above. This subset consisted of children enrolled for at least 2 consecutive years in Medicaid; children could contribute multiple intervals. Successive 2-year intervals could include a common year (e.g., 2007–08, 2008–09). The requirement of 2 years of enrollment was necessary to ensure that the potential predictors occurred prior to the assessment of receipt of TCD screening. Our primary outcome, receipt of TCD screening, was assessed during the second year of enrollment of each 2-year interval; predictors were measured in the first year of enrollment with the exception of age, which was classified as of January 1 of the second enrollment year. We evaluated potential associations between receipt of TCD screening and the following predictors: child’s age, sex, prior use of health services (SCD-related inpatient, SCD-related outpatient, ED, well child visit), previous receipt of TCD screening, year, and state of residence. Table 1 describes the procedure and diagnosis codes used to classify these health services.23–25
Statistical Analysis
Frequencies and percentages (or means and standard deviations) were determined for demographic characteristics, overall and by state. Among the study population of children with sickle cell anemia enrolled in Medicaid for at least one year, the proportion of children receiving annual TCD screening was calculated by state for each year in the study period (2005 to 2010). The presence of trends in TCD screening rates were also assessed over time and by state using linear regression and ANOVA. Potential predictors of TCD screening were assessed only among the subset of the study population enrolled for at least 2 years. Means and standard deviations of the number of annual health services visits were assessed; health services were measured in the first year of enrollment. Logistic regression was used to estimate the bivariate associations between each potential predictor and receipt of TCD screening. Since multiple periods of enrollment were allowed for each child, generalized estimating equations (GEE) models with robust standard errors were used to account for the correlation among children. Counts of healthcare services and age were modeled as continuous variables; predictors showing an association (p<0.20) with receipt of TCD screening were included in a final multivariable model. Odds ratios with 95% confidence intervals were used to assess the final associations. For all models, regression diagnostics were performed to assess normality of error variances.
This study was approved by the Institutional Review Board at the University of Michigan (HUM00048799).
Results
A total of 4,887 children with sickle cell anemia between the ages of 2–16 were identified during the study period, contributing a total of 11,542 person-years of enrollment. Overall, 112 children (2%) representing 755 (7%) person-years were excluded due to receipt of 6 or more blood transfusions during a calendar year. The final study population consisted of a total of 4,775 children (98%) contributing 10,787 person-years (93%). States varied in the total number of person-years contributed to the study population as follows: Florida: 3,186 (30%), Texas: 1,948 (18%), Louisiana: 1,800 (17%), Illinois: 1,722 (16%), Michigan: 1,281 (12%), and South Carolina: 850 (8%). In 2005, the average age of children in the study population was 8.0 years (SD=3.8) and 47% were female (Table 2).
Table 2.
Demographics by State, Children with Sickle Cell Anemia Enrolled in Medicaid in 2005
| FL | IL | LA | MI | SC | TX | Overall | ||
|---|---|---|---|---|---|---|---|---|
| n=509 | n=249 | n = 321 | n = 238 | n = 200 | n = 258 | n = 1,775 | ||
| (%) | (%) | (%) | (%) | (%) | (%) | (%) | ||
| Gender | Female | 49 | 47 | 47 | 45 | 49 | 46 | 47 |
| Male | 51 | 53 | 53 | 55 | 51 | 54 | 53 | |
| Age (years) | 2 to 6 | 46 | 33 | 40 | 32 | 36 | 40 | 39 |
| 7 to 11 | 34 | 43 | 40 | 40 | 33 | 38 | 38 | |
| 12 to 16 | 20 | 24 | 20 | 28 | 31 | 23 | 23 |
Overall, among children enrolled in Medicaid for at least one year, TCD screening rates increased from 22% (2005) to 44% (2010) during the study period (Figure 1). Screening rates varied by year and by state, with Texas having the lowest screening rate at any time point (7% in 2005). Overall, these rates increased over time (p<0.0001) and varied by state (p=0.0037).
Figure 1.
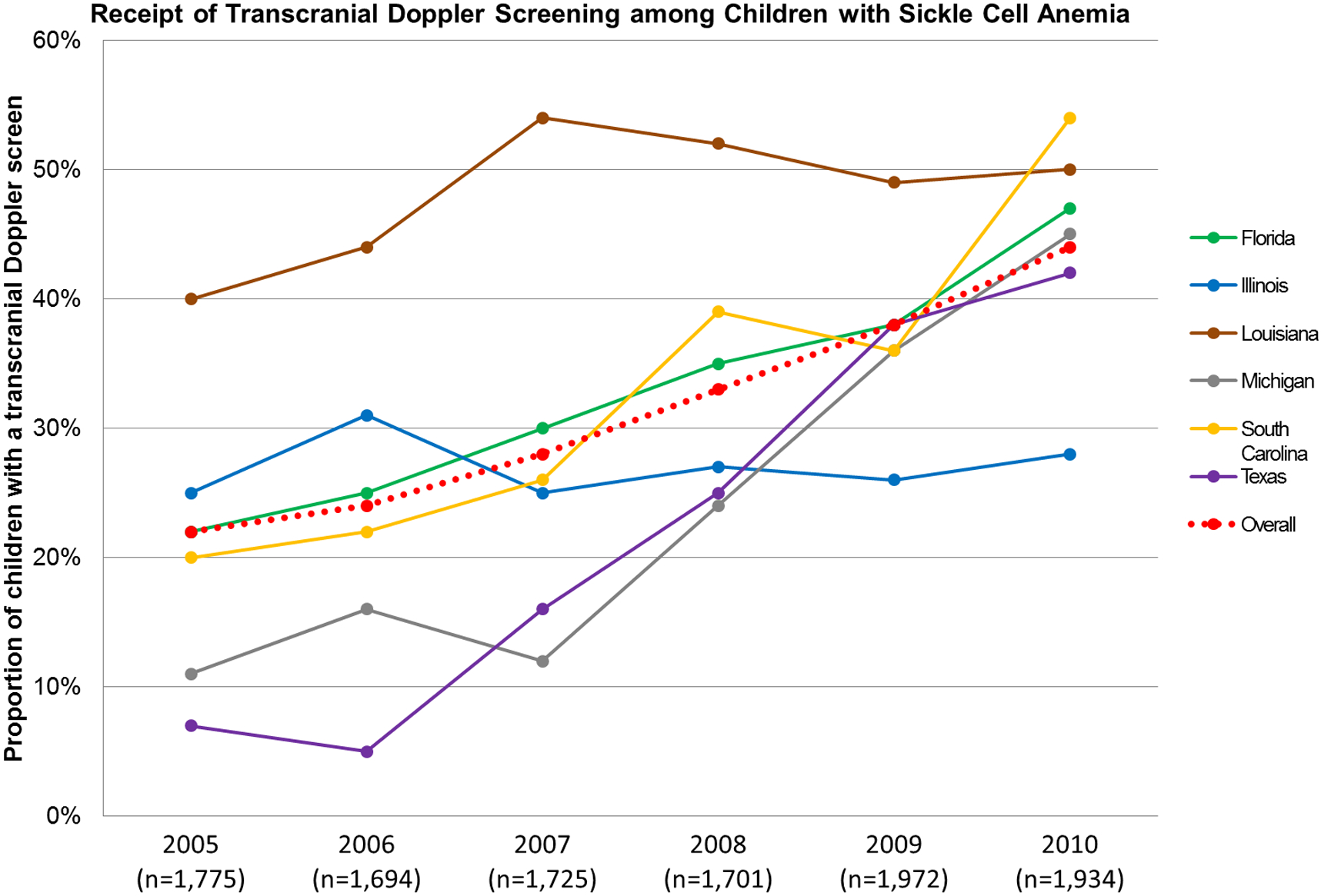
Proportion of Children and Adolescents With Sickle Cell Anemia in Medicaid Receiving at Least 1 Transcranial Doppler (TCD) Screening by State and Year
Predictors of TCD Screening
The evaluation of predictors of TCD screening was performed among the subset of the study population enrolled for at least 2 consecutive years, which consisted of 2,388 children (50%). On average, eligible children had 2.1 sickle cell disease-related inpatient hospitalizations, 20.0 sickle cell disease-related outpatient visits, 3.7 emergency deparment visits, and 0.7 well child visits within the first year of their enrollment (Table 3). Bivariate analysis indicated that age, count of well child visits, outpatient, ED and inpatient stays, previous receipt of TCD screening, state of residence, and year were independently associated with receipt of TCD screening. The multivariable model predicting receipt of TCD screening confirmed this association for age, number of well child and inpatient visits, receipt of TCD screening in the first year of the enrollment period, state, and year (Table 4). Each year of increasing age (OR=0.97, p=0.002) was associated with decreased odds of receiving a TCD screening. Previous receipt of TCD screening (OR=2.44, p<0.0001) and increasing number of well child visits (OR=1.10, p=0.007) were associated with higher odds of receiving a TCD screening. Compared to children with sickle cell anemia in Louisiana, the odds of receiving a TCD were lower in each remaining state in the study population. The odds of receiving a TCD screening in 2009 and 2010 were higher compared to receiving a TCD screening in 2005 (Table 4).
Table 3.
Annual Healthcare Utilization among Children with Sickle Cell Anemia Enrolled for at Least 2 Consecutive Years from 2005–2010 (n = 2,338 children)*
| Mean Count of Visits (Standard Deviation) | |
|---|---|
| SCD-related Inpatient | 2.1 (2.2) |
| SCD-related Outpatient | 20.0 (16.6) |
| Emergency Department | 3.7 (3.6) |
| Well Child | 0.7 (0.9) |
Healthcare Measured in 1st year of Enrollment; Children eligible to contribute multiple 2-year intervals
Table 4.
Multivariable Model Predicting Receipt of TCD Screening (n = 2,338)
| OR | Lower 95% CI | Upper 95% CI | ||
|---|---|---|---|---|
| Age | 0.97 | 0.95 | 0.98 | |
| Inpatient | 0.96 | 0.93 | 1.00 | |
| Outpatient | 1.00 | 0.99 | 1.00 | |
| ED | 1.01 | 0.99 | 1.03 | |
| Well-child | 1.10 | 1.03 | 1.18 | |
| Previous TCD | 2.44 | 2.11 | 2.81 | |
| State | FL | 0.63 | 0.52 | 0.76 |
| IL | 0.42 | 0.34 | 0.53 | |
| LA | 1.00 | Reference | Reference | |
| MI | 0.44 | 0.35 | 0.56 | |
| SC | 0.76 | 0.57 | 1.05 | |
| TX | 0.42 | 0.33 | 0.52 | |
| Year | 2005 | 1.00 | Reference | Reference |
| 2006 | 0.84 | 0.72 | 0.98 | |
| 2007 | 1.01 | 0.86 | 1.19 | |
| 2008 | 1.01 | 0.85 | 1.19 | |
| 2009 | 1.32 | 1.09 | 1.60 | |
| 2010 | 1.30 | 1.10 | 1.53 |
Discussion
TCD screening is the only method to identify children with sickle cell anemia at the highest risk of stroke, and its importance has recently been underscored by NHLBI guidelines that strongly recommend children with sickle cell anemia receive annual TCD screening. Consistent assessment of TCD screening as well as identification of strategies to improve these rates are essential to improve the health of these high-risk children.10 Our findings indicate that although TCD screening rates have increased over time, even the highest rates we report are suboptimal. Substantial opportunity for improvement exists; potentially successful strategies may capitalize on the numerous healthcare interactions of these children within a year, particularly among children that have not previously received a TCD screen. However, numerous challenges also exist to increasing TCD screening rates; addressing these barriers on multiple levels may be the most successful strategy to increase these rates.
Despite increasing over time, TCD screening rates remained suboptimal across each state in our sample, and this was especially evident among older children. Screening rates differed by state at baseline, but the majority of states had increased to similar screening rates in 2010, with the exception of Illinois which performed below. Differences in screening rates across states could potentially be attributable to variation in insurance policies and sickle cell clinic-specific factors by state. Few factors affected the receipt of TCD screening, as a similar proportion of children received a TCD irrespective of the presence of an SCD-related inpatient, SCD-related outpatient, or ED visit. A decreased likelihood of receipt of TCD screening with increasing age is consistent with other studies;12–14 however, we reported that increasing outpatient visits are not associated with receipt of TCD screening. This differs from a previous study at a CSSC which indicated that increased outpatient visits were associated with increased TCD screening, although our study also includes care provided at sites other than a CSSCs and may differ for that reason.27 The increased likelihood of receipt of screening with the presence of a well-child visit may indicate that children receiving well-child visits are more likely to utilize other preventive care services. Our findings also indicate that the likelihood of receiving a TCD screening increase among those who have previously received a TCD screen, suggesting that a subgroup of children are consistently receiving TCD screening. As a consequence, interventions focused on initiating TCD screening among children who are previously unscreened may be an effective mechanism to increase the likelihood of receiving subsequent TCD screens.
As substantial gaps exist between the NHLBI recommendations of annual TCD screening and the proportion of children receiving TCD screening, emphasis should be placed on mechanisms to improve TCD screening and reduce the incidence of pediatric stroke.10,12 We found that although TCD screening rates are low, overall healthcare utilization is substantially higher than previously reported.28 As a consequence, that patient’s contact with the healthcare system is not a barrier to the receipt of TCD screening among this population. This suggests that interventions to effectively encourage TCD screenings during existing healthcare encounters may be more successful than other strategies such as reminder/recall letters, which are aimed prompting a future healthcare encounter. A recent study which assessed the effectiveness of reminders for TCD showed that these letters did not increase baseline rates of TCD screening, which is consistent with patients having adequate contact with healthcare providers, but experiencing missed opportunities.29–32 One quality improvement strategy that has demonstrated improvements in rates of TCD screening is the integration of TCD screening into a comprehensive sickle cell visit as opposed to receipt of TCD in an off-site, separately scheduled appointment reported increased TCD rates.33 In addition, given the apparently high incidence of missed opportunities for TCD screening during healthcare encounters, interventions that employ provider prompts and facilitate TCD scheduling using health information technology and electronic health record (EHR)-based may be effective strategies. Such mechanisms can assist in the identification of TCD-eligible children and facilitate efficient scheduling.34,35
Successful strategies to increase TCD screening rates will also likely need to target barriers that may be experienced by both providers and patient caregivers. For example, a survey of pediatric hematologists, neurologists, and primary care physicians treating children with sickle cell disease indicated that these physicians may lack of self-efficacy, outcome expectancy, and knowledge in respect to specific TCD screening guidelines. In addition, a need to increase caregiver knowledge about risk of stroke among children with SCD has been previously identified.36,37 Additional barriers facing these families may include health literacy, motivation, and competing priorities. It will be imperative to include mixed methods research, such as patient surveys and focus groups of key stakeholders, to gain clarity on the most effective use of resources to improve TCD screening rates in this high-risk population. Strategies that have proven successful in increasing the receipt of preventive care in other pediatric chronic conditions should additionally be explored. For example, school-based interventions have been successful in improving asthma self-management skills, and web-based interventions have been successful in encouraging behavior change among adolescents with diabetes.38,39
Limitations to this study exist. This study relies on the completeness and accuracy of administrative data to determine receipt of TCD screening. However, a prior study demonstrated that administrative claims are highly sensitive and specific in identifying receipt of TCD screening as compared to the gold standard of medical record review.26 Although our claims-based method is believed to be accurate, we were unable to ascertain the reasons behind the lack of receipt of TCD screening. For example, we were not able to determine if a provider chose to initiate transfusions for stroke prevention irrespective of receipt of TCD screening, or if a parent refused the TCD screening recommendation. In addition, we were unable to account for specific practices at sickle cell centers which may influence TCD screening, such as physician prescribing behaviors and availability of facilities in which to perform a TCD screen. Our study population consists of children that that have a history of utilizing sickle cell anemia-related healthcare. There may be a subgroup of children with sickle cell anemia that do not utilize healthcare reported as being related to this condition, although we expect this group to be small.21 We also assume the subgroup of children without sickle cell anemia-related healthcare would also be less likely to receive TCD screening; therefore, our rates may be overestimating the true rate of TCD screening among children with SCD.
Conclusion
Despite national recommendations, annual TCD screening rates remain low, particularly among adolescents. Successful strategies to increase screening among children with sickle cell anemia may be aimed at more effective prompts to identify TCD-eligible patients during the numerous healthcare encounters typically experienced by this population. However, the effectiveness of specific mechanisms by which TCD missed opportunities can be remediated requires additional study.
Acknowledgments
Funding/Support: This study was funded by the Agency for Healthcare Research and Quality (AHRQ) and the Centers for Medicare & Medicaid Services (CMS) under the CHIPRA Pediatric Quality Measures Program Centers of Excellence grant number U18 HS020516.
Role of the Sponsor: The funding agencies had no role in design and conduct of the study; collection, management, analysis, or interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflict of Interest Disclosure: None.
References
- 1.Fullerton HJ, Chetkovich DM, Wu YW, Smith WS, Johnston SC. Deaths from stroke in US children, 1979 to 1998. Neurology. July 9 2002;59(1):34–39. [DOI] [PubMed] [Google Scholar]
- 2.Goldenberg NA, Bernard TJ, Fullerton HJ, Gordon A, deVeber G. Antithrombotic treatments, outcomes, and prognostic factors in acute childhood-onset arterial ischaemic stroke: a multicentre, observational, cohort study. Lancet Neurol. December 2009;8(12):1120–1127. [DOI] [PubMed] [Google Scholar]
- 3.Cnossen MH, Aarsen FK, Akker SL, et al. Paediatric arterial ischaemic stroke: functional outcome and risk factors. Dev Med Child Neurol. April;52(4):394–399. [DOI] [PubMed] [Google Scholar]
- 4.Lanthier S, Kirkham FJ, Mitchell LG, et al. Increased anticardiolipin antibody IgG titers do not predict recurrent stroke or TIA in children. Neurology. January 27 2004;62(2):194–200. [DOI] [PubMed] [Google Scholar]
- 5.Verduzco LA, Nathan DG. Sickle cell disease and stroke. Blood. December 10 2009;114(25):5117–5125. [DOI] [PubMed] [Google Scholar]
- 6.Ohene-Frempong K, Weiner SJ, Sleeper LA, et al. Cerebrovascular accidents in sickle cell disease: rates and risk factors. Blood. January 1 1998;91(1):288–294. [PubMed] [Google Scholar]
- 7.Adams RJ, McKie VC, Carl EM, et al. Long-term stroke risk in children with sickle cell disease screened with transcranial Doppler. Ann Neurol. November 1997;42(5):699–704. [DOI] [PubMed] [Google Scholar]
- 8.Adams RJ, McKie VC, Hsu L, et al. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. The New England journal of medicine. July 2 1998;339(1):5–11. [DOI] [PubMed] [Google Scholar]
- 9.Yawn BP, Buchanan GR, Afenyi-Annan AN, et al. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA. September 10 2014;312(10):1033–1048. [DOI] [PubMed] [Google Scholar]
- 10.National Heart Lung and Blood Institute. Evidence Based Management of Sickle Cell Disease. 2014; http://www.nhlbi.nih.gov/health-pro/guidelines/sickle-cell-disease-guidelines/sickle-cell-disease-report.pdf. Accessed 11/11, 2014.
- 11.National Heart Lung and Blood Institute. The Management of Sickle Cell Disease. 2002; http://www.nhlbi.nih.gov/health/prof/blood/sickle/sc_mngt.pdf. Accessed 11/19, 2014.
- 12.Armstrong-Wells J, Grimes B, Sidney S, et al. Utilization of TCD screening for primary stroke prevention in children with sickle cell disease. Neurology. April 14 2009;72(15):1316–1321. [DOI] [PubMed] [Google Scholar]
- 13.Raphael JL, Shetty PB, Liu H, Mahoney DH, Mueller BU. A critical assessment of transcranial doppler screening rates in a large pediatric sickle cell center: opportunities to improve healthcare quality. Pediatr Blood Cancer. November 2008;51(5):647–651. [DOI] [PubMed] [Google Scholar]
- 14.Enninful-Eghan H, Moore RH, Ichord R, Smith-Whitley K, Kwiatkowski JL. Transcranial Doppler ultrasonography and prophylactic transfusion program is effective in preventing overt stroke in children with sickle cell disease. J Pediatr. September 2010;157(3):479–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reeves SL, Braun TM, Dombkowski KJ, Fullerton HJ, Boulton ML, Lisabeth LD. The Role of Neighborhoods in the Receipt of Transcranial Doppler Screening Among Children With Sickle Cell Disease. J Pediatr Hematol Oncol. May 2015;37(4):269–273. [DOI] [PubMed] [Google Scholar]
- 16.Gershon AS, Wang C, Guan J, Vasilevska-Ristovska J, Cicutto L, To T. Identifying patients with physician-diagnosed asthma in health administrative databases. Can Respir J. Nov-Dec 2009;16(6):183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dombkowski KJ, Wasilevich EA, Lyon-Callo SK. Pediatric asthma surveillance using Medicaid claims. Public Health Rep. Sep-Oct 2005;120(5):515–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krumholz HM, Wang Y, Mattera JA, et al. An administrative claims model suitable for profiling hospital performance based on 30-day mortality rates among patients with an acute myocardial infarction. Circulation. April 4 2006;113(13):1683–1692. [DOI] [PubMed] [Google Scholar]
- 19.Schneeweiss S, Avorn J. A review of uses of health care utilization databases for epidemiologic research on therapeutics. J Clin Epidemiol. 2005;58(4):323–337. [DOI] [PubMed] [Google Scholar]
- 20.Riley GF. Administrative and claims records as sources of health care cost data. Med Care. 2009;47(7_Supplement_1):S51–S55. [DOI] [PubMed] [Google Scholar]
- 21.Reeves S, Garcia E, Kleyn M, et al. Identifying sickle cell disease cases using administrative claims. Acad Pediatr. Sep-Oct 2014;14(5 Suppl):S61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Newborn Screening and Genetics Resource Center in Cooperation with the Maternal and Child Health Bureau Genetic Services Branch and the Association of Public Health Laboratories. National newborn screening 2006 incidence report. http://genes-r-us.uthscsa.edu/sites/genes-r-us/files/resources/genetics/2006datareport.Accessed 1/28, 2013.
- 23.Yusuf HR, Atrash HK, Grosse SD, Parker CS, Grant AM. Emergency department visits made by patients with sickle cell disease: a descriptive study, 1999–2007. Am J Prev Med. April;38(4 Suppl):S536–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ovbiagele B, Adams RJ. Trends in comorbid sickle cell disease among stroke patients. J Neurol Sci. February 15 2012;313(1–2):86–91. [DOI] [PubMed] [Google Scholar]
- 25.Leschke J, Panepinto JA, Nimmer M, Hoffmann RG, Yan K, Brousseau DC. Outpatient follow-up and rehospitalizations for sickle cell disease patients. Pediatr Blood Cancer. March 2012;58(3):406–409. [DOI] [PubMed] [Google Scholar]
- 26.Dombkowski KJ, Reeves SL, Madden BW, Shevrin CA, McCormick J, Freed GL for the Quality Measurement, Evaluation, Testing, Review, and Implementation Consortium. Transcranial Doppler ultrasonography screening for children with sickle cell disease National Quality Measures Clearinghouse (NQMC). Rockville (MD): Agency for Healthcare Research and Quality (AHRQ) Published November 3, 2014 http://www.qualitymeasures.ahrq.gov/content.aspx?id=47887. [Google Scholar]
- 27.Eckrich MJ, Wang WC, Yang E, et al. Adherence to transcranial Doppler screening guidelines among children with sickle cell disease. Pediatric blood & cancer. 2013;60(2):270–274. [DOI] [PubMed] [Google Scholar]
- 28.Raphael JL, Dietrich CL, Whitmire D, Mahoney DH, Mueller BU, Giardino AP. Healthcare utilization and expenditures for low income children with sickle cell disease. Pediatr Blood Cancer. February 2009;52(2):263–267. [DOI] [PubMed] [Google Scholar]
- 29.Dombkowski KJ, Davis MM, Cohn LM, Clark SJ. Effect of missed opportunities on influenza vaccination rates among children with asthma. Arch Pediatr Adolesc Med. September 2006;160(9):966–971. [DOI] [PubMed] [Google Scholar]
- 30.Thomas CM, Loewen A, Coffin C, Campbell NR. Improving rates of pneumococcal vaccination on discharge from a tertiary center medical teaching unit: a prospective intervention. BMC Public Health. October 14 2005;5:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verani JR, Irigoyen M, Chen S, Chimkin F. Influenza vaccine coverage and missed opportunities among inner-city children aged 6 to 23 months: 2000–2005. Pediatrics. March 2007;119(3):e580–586. [DOI] [PubMed] [Google Scholar]
- 32.Walton S, Elliman D, Bedford H. Missed opportunities to vaccinate children admitted to a paediatric tertiary hospital. Arch Dis Child. July 2007;92(7):620–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCarville MB, Goodin GS, Fortner G, et al. Evaluation of a comprehensive transcranial doppler screening program for children with sickle cell anemia. Pediatr Blood Cancer. April 2008;50(4):818–821. [DOI] [PubMed] [Google Scholar]
- 34.Boyle RG, Solberg LI, Fiore MC. Electronic medical records to increase the clinical treatment of tobacco dependence: a systematic review. Am J Prev Med. December 2010;39(6 Suppl 1):S77–82. [DOI] [PubMed] [Google Scholar]
- 35.Hsu L, Bowlus CL, Stewart SL, et al. Electronic messages increase hepatitis B screening in at-risk Asian American patients: a randomized, controlled trial. Dig Dis Sci. March 2013;58(3):807–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reeves SL, Fullerton HJ, Dombkowski KJ, Boulton ML, Braun TM, Lisabeth LD. Physician Attitude, Awareness, and Knowledge Regarding Guidelines for Transcranial Doppler Screening in Sickle Cell Disease. Clin Pediatr (Phila). October 15 2014. [DOI] [PubMed] [Google Scholar]
- 37.Bollinger LM, Nire KG, Rhodes MM, Chisolm DJ, O’Brien SH. Caregivers’ perspectives on barriers to transcranial Doppler screening in children with sickle-cell disease. Pediatr Blood Cancer. January;56(1):99–102. [DOI] [PubMed] [Google Scholar]
- 38.Mosnaim GS, Pappalardo AA, Resnick SE, et al. Behavioral Interventions to Improve Asthma Outcomes for Adolescents: A Systematic Review. J Allergy Clin Immunol Pract. November 7 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patrick K, Norman GJ, Davila EP, et al. Outcomes of a 12-month technology-based intervention to promote weight loss in adolescents at risk for type 2 diabetes. J Diabetes Sci Technol. May 2013;7(3):759–770. [DOI] [PMC free article] [PubMed] [Google Scholar]


