Abstract
Here we present hyaluronic acid (HA) hydrogels crosslinked via thiol-ene reaction initiated by visible blue light exposure in the presence of riboflavin phosphate (RFP). The gelation procedure is rapid and proceeds as effectively with exposure to blue light as it does with UV light. We successfully initiated the thiol-ene reaction by RFP with blue light, which triggered gelation that proceeds over about 5 min at 36 °C after an initial small change in modulus upon light exposure. Gel transparency was also evaluated, and the HA gel exhibited over 80% transmittance in the visible spectrum. The degradation and protein release kinetics of the photo-crosslinked HA hydrogel are also presented. The capacity of blue light to initiate thiol-ene reaction was equal to or more effective than UV light of the same energy. The cytocompatibility of hydrogels was evaluated using corneal fibroblasts, and the light-induced fabrication procedure and resultant gel materials did not affect cell viability. The results indicate that an RFP-based, BL-initiated photo-reaction to gelate HA may be an effective and promising modality for applications where in situ gelation is desired.
Keywords: Hyaluronic acid (HA), Thiol-ene reaction, Photo-click chemistry, Riboflavin phosphate (RFP), In situ-forming hydrogel
1. Introduction
Hyaluronic acid (HA) has been extensively studied as a biomaterial for therapeutic applications, and has been used in eye drops as viscosity-increasing agents that improve drug bioavailability by increasing contact time in the precorneal space [1]. However, the residence time on the ocular surface is under one hour, and 90% of HA is cleared within half an hour [2]. Crosslinking is one way to increase the residence time of macromolecules and can be accomplished through a variety of means, including the free radical crosslinking of acrylic pendant groups using UV light and a photo-initiator [3, 4]. Thiol-ene chemistry is a highly efficient reaction between a thiol and an alkene to form an alkyl sulfide that is mediated by free radicals and has been used to crosslink conjugated HA and other biomolecules [5–7]. Light-induced thiol-ene reactions (so-called “photo-click chemistry”) using UV and visible light have been developed as crosslinking methods to maintain the bioactivity of covalently linked proteins while also providing spatial and temporal control over the reaction [8–11]. Certain naturally occurring, photosensitive molecules have been explored for the purpose of crosslinking biomolecules. Eosin-Y activated by green light has also been shown to mediate thiol-ene crosslinking of HA and exhibit in vitro cytocompatibility [12]. Rose Bengal has been studied as well, as a mediator of collagen crosslinking upon exposure to green light [13]. Riboflavin phosphate (RFP), a water-soluble form of riboflavin also known as vitamin B2, has been studied extensively as a photo-initiator and was approved in 2016 by the FDA for crosslinking of collagen with UV light [14–17]. We recently reported the use of blue light to trigger the crosslinking between heterologous proteins (growth factors and collagen) via photoactivation of RFP [18].
We present here the use of blue light (BL) to trigger gelation of HA via thiol-ene reaction between methacrylated HA (MA-HA) and thiolated HA (SH-HA). The system exhibits an interesting gelation delay behavior, where the gelation is triggered but not completed during the BL exposure interval (20–40 s), and instead the gelation reaction takes place over several minutes. This provides some optionality over how the gel could be applied in various research or clinical settings, where direct light exposure over cells or tissues may not be desired. At the same time, we chose to study BL as an alternative illumination source to UV due to its safe and ubiquitous use in ophthalmology as a diagnostic aid in combination with fluorescein dyes to evaluate corneal disease and injury, to measure intraocular pressure, and to perform retinal angiography. RFP was added to a mixture of MA-HA and SH-HA solution as a photo-initiator. Due to its success as a photo-initiator for unconjugated natural collagen, we hypothesized that it could be used to accelerate the thiol-ene reaction as well, which are known to be photosensitive. The triggering capability of BL was compared to UV, and the rheological properties, transparency, degradation characteristics, protein release, and cytocompatibility of the photoreaction were evaluated.
2. Experimental
2.1. Materials
Unless otherwise noted, all chemicals and solvents were of analytical grade and used as provided by the manufacturers. Methacrylated HA and thiolated HA were purchased from Vornia Biomaterials (Tallaght, Dublin, Ireland). Riboflavin phosphate (RFP), phosphate-buffered saline (PBS), bovine serum albumin (BSA), 1,9-di-methylmethylene blue (DMMB), ethanol, glycine, sodium chloride, hydrochloric acid, Pierce BCA protein assay kit, gelatin, collagen, fibronectin, dimethyl sulfoxide (DMSO), Cholera Subunit A, and insulin were purchased from Sigma-Aldrich (St. Louis, MO, USA). Dulbecco’s phosphate-buffered saline (DPBS), antibiotic-antimycotic, Dulbecco’s modified eagle medium/nutrient mixture F-12 (DMEM/F-12) with 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), fetal bovine serum (FBS), epidermal growth factor (EGF), and LIVE/DEAD viability/ cytotoxicity kit were purchased from Thermo Fisher Scientific (Waltham, MA, USA).
2.2. Fabrication of HA gels by light-induced thiol-ene reaction
Methacrylated HA (MA-HA) and thiolated HA (SH-HA) were dissolved in PBS as 10 mg/mL, and then the solutions were mixed at a 1:1 ratio. The riboflavin dissolved in PBS and added to the mixed HA solution. Exposure times of 20 s for UV and 40 s for blue light (BL) were used through a 3W UV LED Spot Cure system (Doctor UV, Redondo Beach, CA, USA) and commercial 1.5 W dental LED curing light, respectively.
2.3. Characterization of HA gels
FTIR spectra were recorded on a Nicolet iS50 FTIR spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) at Stanford Soft & Hybrid Materials Facility (SMF, Stanford, CA, USA). MA-HA and SH-HA were measured in powder as provided without dissolving in PBS. The HA gels were gently dried at room temperature and then measured.
Rheology of the HA gel was evaluated using an ARES-G2 rheometer (TA Instruments, New Castle, DE, USA) at SMF. The UV or BL was exposed after the HA and RFP mixture solutions were mounted on the plate, and then the rheometer ran immediately. To determine gelation time, sweeps were performed at 36 °C for 1800 s at 1% strain and 1 Hz oscillatory frequency. To evaluate definitive storage and loss moduli of gels, the samples were incubated at 36 °C overnight after light exposure, and then frequency sweeps from 0.1 to 10 Hz with a fixed 1% stain were performed.
The absorbance spectra from 300 to 700 nm of RFP and resultant HA gels in 96 well plates were measured using SpectraMax M Series Multi-Mode Microplate Reader (Sunnyvale, CA, USA). The absorbance spectrum of the 96 well plate was obtained, and the absorbance spectra of samples were calibrated accordingly. The gel samples were fabricated in 96 well plate from 100 μL of precursor solution with light exposure. After absorbance measurement, 150 μL of PBS was added to each well and incubated. The absorbance spectra were measured after 24 h with removing incubation solution. The procedure was repeated and measured at 48 h.
2.4. Degradation of HA gels and BSA release
For evaluation of HA gel degradation and BSA release, BSA was added to the HA and RFP solution, and gels were fabricated in 24 well plates. After 30 min incubation at 36 °C, the gels were rinsed with PBS, and the washing solution was collected at day 0. Fresh PBS was added to each well and incubated at 36 °C. The collection and incubation were repeated at certain days until day 21.
Degraded HA was quantified using a DMMB assay. DMMB was dissolved in ethanol as 3.2 mg/mL and incubated overnight at room temperature. Hydrochloric acid was added to DI water to make pH 3.5, and then glycine and sodium chloride were added to make 40 mM, respectively. The DMMB and acidic solutions were mixed with 1:199 ratio to make DMMB assay working solution. In 96 well plate, 25 μL of collected sample solution with 10-time dilution and 200 μL of DMMB assay working solution were mixed and measured absorbance immediately.
The released BSA was quantified using a BCA protein assay. We followed the BCA protein assay kit protocol to make BCA protein assay working solution. In 96 well plate, 25 μL of collected sample solution without dilution and 200 μL of BCA protein assay working solution were mixed and incubated for 30 min at 36 °C.
The absorbance was measured using SpectraMax M Series MultiMode Microplate Reader. After the standard curves of HA and BSA solutions were measured, and the amounts of HA and BSA were accumulated and divided by the initially added amounts.
2.5. Cell culture and viability assay
Primary corneal fibroblasts were obtained from rabbit corneas. Plates were precoated with a solution of collagen and fibronectin (1:1) and BSA (1%) for 1 h at 37 °C. Rabbit eyes were washed with DPBS, in the presence of antibiotic-antimycotic. The endothelial layer was removed, and the corneas were placed side up on a sterile surface and cut into triangular wedges. The cornea was then placed upside down on the precoated plates. The tissue was allowed to dry for 20 min, and then one drop of DMEM/F12 with HEPES, FBS (15%), DMSO (0.5%), Cholera Subunit A (1 μg/mL), EGF (10ng/mL), and insulin (5 μg/mL) was added to each segment. On the next day, 1 mL of medium was added to each well. After confluence, the cells were subcultured and used at passage two.
To evaluate the cytotoxicity of HA gels, the mixture of MA-HA, SH-HA, and 0.01% RFP was prepared. After UV or BL exposure, 100 μL of precured solution was applied to each well of 24 well plates. After incubation for 30 min at 37 °C, 1 × 104 cells/cm2 were seeded and incubated overnight. Cell viability was assessed via LIVE/DEAD staining following the manufacturer’s instructions. The plates were mounted and observed using a laser scanning microscope (ZEISS LSM 880, Carl Zeiss Ag, Oberkochen, Germany).
3. Results and discussion
3.1. HA gel formation by light-induced thiol-ene reaction
In this study, we investigated the use of BL to crosslink conjugated HA through thiol-ene photochemistry. We hypothesized that a light-induced thiol-ene reaction could be used to rapidly form a crosslinked HA gel. The methacrylate and thiol groups were utilized for the light-induced thiol-ene reaction, and HA functionalized with the two chemical groups were commercially available. RFP was used as the photoinitiator for the thiol-ene reaction, and then either BL and UV light was used to irradiate the solution to form the alkyl sulfide bond (Fig. 1). Of note, RFP has two absorbance peaks at wavelengths of 370 and 445 nm (Fig. 2a) and thus absorbs visible light in the blue spectrum as well as light in the UV spectrum. A commercial dental LED curing light was used as the BL source (Fig. 2b) while a UV LED Spot Cure system was used as the UV light source.
Fig. 1.

Light-induced thiol-ene reaction of methacrylated HA (MA-HA) and thiolated HA (SH-HA) with riboflavin phosphate (RFP).
Fig. 2.

(a) The absorbance spectrum of RFP. (b) Photographic image of the LED dental blue light used in our experiments. The tip of the light source was wrapped with paper to prevent light spreading. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Here, we introduce the combination of RFP and BL to photo-initiate the thiol-ene reaction. RFP and UV have been used to strengthen the mechanical properties of the cornea through crosslinking of the collagen matrix [14, 19]. While the FDA-approved method used in the clinic applies UV light as the energy source, we use BL in this study, which we have showed previously to be effective at inducing crosslinks between collagen and growth factors [18]. UV irradiation of RFP generates singlet oxygen in an oxygenated environment, a phenomenon which has been applied to the crosslinking of the native collagen in corneal tissue [19]. We hypothesized that BL near 445 nm activates RFP in the same way that UV light does (Fig. 2a). In this study, UV (3 W/ cm2) and BL (1.5 W/cm2) were applied, and we compared the effect of the light source on the photo-click chemistry reaction and gel formation. We found that 40 s of BL exposure time initiates the thiol-ene reaction (Fig. S1). To provide the same light energy for the reaction, light exposure times were set to 20 and 40 s for UV and BL respectively.
Fourier transform infrared (FTIR) spectra were acquired to monitor the reaction between the thiol and methacrylate (Fig. 3). Generally, the S—H peak of FTIR is weak, but we could observe S—H signal at 2550 cm−1 from SH-HA. The thiol peak disappeared after the light-induced thiol-ene reaction, indicating its consumption during the reaction. The C=C signal from the methacrylate was observed at 1610 cm−1, and the peaks were slightly reduced but remained after light exposure. There was no difference in the spectra of the gels made by UV and BL exposure.
Fig. 3.

FTIR spectra of hyaluronic acid before gelation (MA-HA, SH-HA) and after gelation by photo-click chemistry with UV and BL.
The FTIR result showed that the change in the spectrum of the thiol group was greater than that of the methacrylate group before and after the thiol-ene reaction, indicating that the thiols were consumed to a greater extent than the methacrylates (Fig. 3). The degrees of substitution for MA-HA and SH-HA were 46% and 63%, so all methacrylate should be consumed if the thiol-ene reaction is to go to completion. The fact that the methacrylate peak remained after the reaction suggests that not all the methacrylates were consumed by the reaction. Thus, not all thiol and methacrylate groups were used in the reaction. This is likely due to the fact that methacrylate is more stable than thiols in ambient or aqueous environments, so unreacted methacrylate remains after the thiol-ene reaction while unreacted thiol is oxidized to form disulfide bonds.
3.2. Dynamic moduli of HA gels by light-induced thiol-ene reaction
The mixture of MA-HA and SH-HA without RFP remained as a solutions after incubation for 15min at 36 °C, while light-exposed HA mixture with RFP gradually formed gels during the same interval (Fig. 4a). After UV or BL exposure in the presence of RFP, the solution started to form gel partially, which had a slightly flowable property (yellow background region). Following incubation for 15 min at 36 °C, the gelation was completed in both the UV and BL exposure cases, and there was no significant difference macroscopically (green background region) between them.
Fig. 4.

(a) Photographic images of resultant HA gels. The ‘No RFP & Light’ group included MA-HA and SH-HA, and light was not applied. For light-induced thiol-ene reactions, 0.01% RFP was added to the mixture of MA-HA and SH-HA, and then UV and BL light was applied, respectively. (b-g) Dynamic moduli (G′: storage moduli, G″: loss moduli) plotted as a function of time (b, d, f) and frequency (c, e, g) of HA gels by light-induced thiol-ene reaction with 0.01% (b, c), 0.1% (d, e), and 0.001% RFP (f, g). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
The dynamic moduli changes induced by crosslinking of HA were measured at 36 °C as a function of time without additional light exposure (Fig. 4b). The mixture of MA-HA and SH-HA without RFP and light exposure showed gradually increased storage modulus, and it reached 40.5 Pa in 1800 s. The storage modulus was higher than the loss modulus during incubation at 36 °C, but there was no drastic increase of dynamic modulus. On the other hand, the gelation of the light-induced thiol-ene reaction with RFP as a photo-initiator exhibited delayed and steeply increased moduli during incubation. Regardless of light wavelength, the mounted samples showed higher storage moduli than loss moduli (UV G’: 18.7 Pa, UV G”: 0.8 Pa; BL G’: 5.4 Pa, BL G”: 0.6 Pa), meaning that the samples exhibit elastic behavior as a result of crosslinking. After about 300 s, the moduli of the gels were drastically increased. Upon UV exposure, the rate of increase of dynamic moduli and storage moduli at 1800 s were relatively lower than that observed with BL exposure (UV: 39.8 kPa; BL: 52.5 kPa). The storage and loss moduli of crosslinked HA gel by UV and BL were measured as a function of frequency, and both gels maintained their viscoelastic behavior at 0.1–10 Hz, which indicates completion of the gelation reaction (Fig. 4c).
We measured the dynamic modulus with different concentrations of RFP (Fig. 4d and f). The gelation of solutions including 0.1 and 0.001% RFP was completed within 1800 s similar to 0.01%, but the starting time points of the observed modulus increases were slightly different. The moduli of the HA solutions including 0.001% RFP started to increase drastically at about 300 s after light exposure, and this inflection point moved to about 900 s for 0.1% RFP. Also, immediately after light exposure, although the 0.1% (highest) concentration produced gels with low storage and loss moduli (UV G’: 0.4 Pa, UV G”: 0.3 Pa; BL G’: 2.6 Pa, BL G”: 0.1 Pa). Thus, the 0.1% RFP concentration yielded a delay in the point at which the modulus increases (Fig. 4d). This suggests that the yellow color of RFP in solution could affect the efficiency of reaction due to reduced light penetration. The 0.1% RFP was excessive for the thiol-ene reaction between MA-HA and SH-HA, so it not only initiated the reaction but also interfered with the transfer of light. Nevertheless, there were no big differences on the storage moduli after gelation compared to 0.01% RFP (Fig. 4e). Although the storage moduli of UV/BL-induced gels from 0.001% RFP showed over 10kPa, the resultant gels were not completely formed (Fig. 4g).
The gelation of MA-HA and SH-HA without RFP and light was not observed macroscopically (Fig. 4a), but there were increases of dynamic moduli from the mixture without light mediation (Fig. 4b). The increase in storage modulus is a result of two crosslinking reactions in the mixture solution. The first reaction is the formation of disulfide bonds via oxidation of thiol groups. The second reaction is Michael addition pathway between thiol and methacrylate groups, where thiolate anion addition to the electron-deficient carbon-carbon double bond occurs [20]. Our reaction proceeded at 36 °C to approximate body temperature; higher temperatures could promote the Michael addition pathway further. To determine the influence of the thiol oxidation and Michael addition, the storage modulus was measured after 1 day, and it corresponded with the storage modulus change of the mixture of MA-HA and SH-HA without RFP (Fig. S2). From the result, we confirmed that the gelation of MA-HA and SH-HA without external triggering by light and RFP has a slow reaction rate and yields gels with low moduli. Of note, BL or UV light exposure in the presence of RFP does not trigger gelation of MA-HA alone in the absence of SH-HA. This indicates that the free radicals generated by RFP do are insufficient to catalyze polymerization of the methacrylate groups. Furthermore, BL or UV light exposure does not trigger gelation of SH-HA alone either, indicating that photoactivation by RFP is not sufficiently oxidizing enough to form disulfide bonds. Thus, the light-induced activation of RFP in our system specifically targets the thiol-ene reaction.
The delayed gelation of light-induced thiol-ene reaction has unique potential in clinical situations. For example, due to the delay in complete gelation after light exposure, the precursor solution can be irradiated prior to being applied to the patient’s tissue surface, with the gel then forming in situ. Thus, the HA gel layer can be formed on the tissue surface without direct exposure of the tissue to light. The absence of light irradiation during treatment can reduce any potential harm, especially for light-sensitive tissue such as an eye. Nevertheless, blue light is safely used in medicine, such as in ophthalmic and dental applications, so the photoreaction has the potential to be used safely in situ as well.
A previous study introduced riboflavin as an initiator for thiol-norbornene reaction for synthetic hydrogel formation, but the gel formations were quick and required light until completion of the reaction [7]. The difference in gelation kinetics between this study and the present one is due to the alkene group. The photo-initiated thiol-methacrylate reaction is relatively slow compared to thiol-norbornene [21], making the light-induced thiol-methacrylate reaction potentially more convenient from a handling perspective. To evaluate the effect of light without RFP on gelation, the mixture of MA-HA and SH-HA alone was exposed to UV or BL, and the dynamic moduli were found to be the same as those of the unexposed mixtures (Fig. S3). This indicates that there is no additional crosslinking reaction without RFP despite light exposure, and this result means that RFP has an important and essential role in the light-induced thiol-ene reaction.
3.3. Transparency of HA gels by light-induced thiol-ene reaction
The transparency of the gel was measured by evaluation of transmittance after gelation. The gels were fabricated in 96 well plates at 100 μL volumes. The resultant gels were slightly yellow in color, and transmittance was determined by the concentration of RFP (Fig. 5a). HA gels with 0.001% RFP were macroscopically clear. For HA with 0.01% RFP, both UV and BL-induced gels had similar transmittance spectra, and they were over 80% in the visible wavelength range (Fig. 5b). The HA containing 0.1% RFP showed about 20% transmittance under 480 nm, but transparency increased within 2 days after gel incubation in aqueous solution (Fig. 5c). The transparency increase with time indicates that the RFP was able to diffuse out from the HA gel matrix.
Fig. 5.

(a) Photographic images of HA gels after light exposure with different concentrations of RFP. Transmittance spectra of HA gels by (b) 0.01% and (c) 0.1% RFP with time.
The transmittance spectra corresponded with the concentration of RFP, and there was a significant decrease between 0.01% and 0.1% RFP from about 80% to 20% in the visible wavelength range. The sharp decrease of transmittance in these cases supports that high concentration of RFP interferes with the thiol-ene reaction initiation by the absorbance of light, so the lowest concentration of RFP capable of initiating the reaction was deemed the most suitable condition for gelation.
3.4. Degradation of HA gels and BSA release
Bovine serum albumin (BSA) was used as a model protein, and the release profile was evaluated in this study. The BSA was encapsulated in the HA gel matrix, and the protein was released from the matrix with HA degradation. The protein release profiles were matched with HA degradation curves, so the main driving force for release was matrix degradation rather than diffusion.
We measured the degradation of HA and release of BSA from the gel matrices to evaluate both the degradation rate of the cross linked HA matrix and its capacity as a drug delivery vehicle (Fig. 6). The gels were fabricated in 24 well plates, and PBS solution was added, followed by collection and replacement of the incubation solution at certain days. For evaluation of HA degradation, the HA in the collected solution was quantified using a dimethylmethylene blue (DMMB) assay. The washing solution at day 0 contained little HA in the cases of both UV and BL photo-crosslinking (UV: 4.41 ± 1.10%, BL: 6.09 ± 0.61%), suggesting that 95% of HA molecules participated in the gel formation (Fig. 6a). Both types of HA gels were degraded to half their mass in about 2–3 days. There was no statistical difference between gels made by UV and BL, but the BL-induced gel exhibited a slightly faster degradation rate than the UV-induced gel. Most of the HA was degraded within 12 days, at which point the HA degradation rate was markedly reduced for both gels. When we evaluated the HA degradation from the gel that was fabricated with 0.001% RFP, the washing solution at day 0 contained 50.29 ± 4.46 and 55.98 ± 2.78% for UV and BL, respectively (Fig. 6b). These gels were completely degraded within 2–3 days, indicating that the low RFP concentration was not sufficient to support the thiol-ene reaction for hydrogel network crosslinking.
Fig. 6.

The degradation profiles of HA gels by (a) 0.01%, (b) 0.001% and 0.1% RFP. The BSA release profiles of HA gels by (c) 0.01%, (d) 0.001% and 0.1% RFP.
From the HA degradation results, the HA gels initiated by 0.01% and 0.1% RFP formed completely. The amount of HA that participated in the formation of gel matrix was over 90% for both concentrations of 0.01% and 0.1% RFP. On the other hand, 0.001% RFP showed similar dynamic modulus curves with 0.01% RFP (Fig. 4f), but the gels formed only partially. Almost half of HA was not included hydrogel reaction, and the HA molecules were detected in the rinsing solution (Fig. 6b). The 0.001% RFP concentration was not enough to initiate the thiol-ene reaction of MA-HA and SH-HA. Thus 0.01% was found to be the best concentration of RFP for gelation, which was the lowest concentration that could initiate the reaction.
The release profile of BSA from the HA gel was measured by quantification of protein in the collected aqueous solution. The release of BSA generally followed the HA degradation profile, with greater release as the gel was further degraded (Fig. 6c). BSA was well-incorporated into the HA gel because the washing solution at day 0 contained 7.74 ± 0.48 and 5.59 ± 0.41% for UV and BL exposure, respectively. On the other hand, at the 0.001% RFP, BSA release profiles corresponded with HA degradation. As more than 50% HA did not form a gel, more than 50% of BSA was in the washing solution at day 0 without being encapsulated in the HA gel (Fig. 6d). Most of the encapsulated BSA was released within 4 days in all cases, and HA gels with 0.1% RFP showed similar release profiles to those with 0.01% RFP (Fig. 6d).
The results of this study also showed that BL and UV light exhibit similar ability to trigger the thiol-ene reaction. Although BL and UV are interchangeable in this way, BL provides potentially a safer energy source than UV, and may be preferable when biomolecules such as proteins, or living cells are being irradiated simultaneously. Thus, the substitution of UV with BL may be advantageous for certain biomedical applications of HA gels.
3.5. Corneal fibroblast viability
The cytocompatibility of the HA precursor solution and gels were evaluated by corneal fibroblast viability. The concentration of RFP was fixed at 0.01% for the cell viability test. After light exposure, the viscous HA solutions were transferred to the well plates, and the solutions formed gels. The cells were cultured with the HA gels overnight and no cytotoxic effects due to the presence of the HA gels initiated by both UV and BL were observed (Fig. 7). The cells did not adhere on the HA gel surface, but the cells on the well plate adhered and remained viable. Moreover, there were no cytotoxic effects due to contact between the cells and the HA gel, indicated by the viability of the cells at the gel interface. The cell viability was quantitated by CCK-8 assay, and there was no significant difference in cell viability upon exposure to either the BL or UV-crosslinked HA gels (Fig. S4).
Fig. 7.
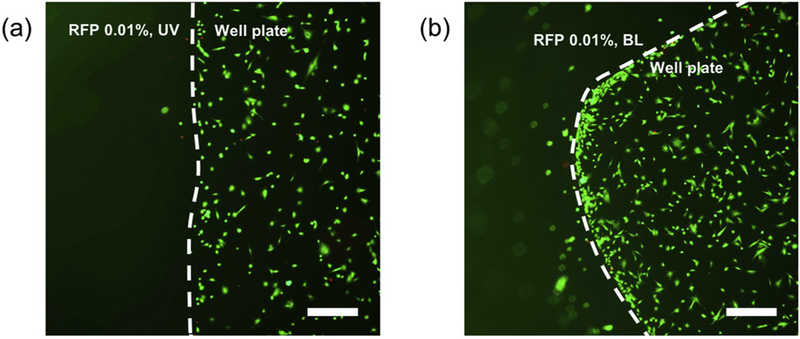
Fluorescence images of comeal fibroblasts LIVE/DEAD assay. Cells were cultured in the presence of HA gels initiated by (b) UV and (c) BL. Scale bars represent 500 μm.
HA is a widely used biomaterial in medicine, while RFP is a naturally occuring vitamin used in corneal crosslinking. Reacting HA conjugated with thiol and methacrylate groups in the presence of RFP did not appear to affect their biocompatibility.
4. Conclusion
A light-induced thiol-ene reaction was used to rapidly form an HA gel and encapsulate proteins that could be released from the matrix on the order of days. We learned that BL is sufficient to trigger the thiol-ene reaction, but that this reaction proceeds over several minutes rather than going to completion during the light exposure interval. BL was as or more effective than UV at activating the thiol-ene reaction with RFP, and a concentration of 0.01% RFP was found to be optimal for gel formation, which was the lowest concentration that could initiate the thiol-ene reaction. For several minutes immediately after light exposure, the solution takes on a viscous and flowable state, which lends the material to topical application or injection. It then exhibits a steep modulus increase. Moreover, the resultant gel is transparent, making it potentially suitable for ophthalmic applications. The fabrication procedure and resultant hydrogel had excellent cytocompatibility. The ability to crosslink HA using RFP, a known biocompatible photo-activator, with exposure to visible light makes this system promising for therapeutic applications, in particular ones where in situ gel formation is desirable.
Supplementary Material
Acknowledgement
This work was supported by a K08 award (EY028176) and a P30 core grant (EY026877) from the National Eye Institute, a Stanford SPARK Translational Research Grant, a core grant from the Research to Prevent Blindness (RPB) Foundation, and the Byers Eye Institute at Stanford. Part of this work was performed at the Stanford Nano Shared Facilities (SNSF), supported by the National Science Foundation under award ECCS-1542152.
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.reactfunctpolym.2018.06.010.
References
- [1].Eljarrat-Binstock E, Pe’er J, Domb AJ, New techniques for drug delivery to the posterior eye segment, Pharm. Res. 27 (4) (2010) 530–543. [DOI] [PubMed] [Google Scholar]
- [2].Mochizuki H, Yamada M, Hato S, Nishida T, Fluorophotometric measurement of the precorneal residence time of topically applied hyaluronic acid, Br. J. Ophthalmol. 92 (1) (2008) 108–111. [DOI] [PubMed] [Google Scholar]
- [3].Marklein RA, Burdick JA, Spatially controlled hydrogel mechanics to modulate stem cell interactions, Soft Matter 6 (1) (2010) 136–143. [Google Scholar]
- [4].Ma Y, Thiele J, Abdelmohsen L, Xu J, Huck WTS, Biocompatible macro-initiators controlling radical retention in microfluidic on-chip photo-polymerization of water-in-oil emulsions, Chem. Commun. 50 (1) (2014) 112–114. [DOI] [PubMed] [Google Scholar]
- [5].Gramlich WM, Kim IL, Burdick JA, Synthesis and orthogonal photopatterning of hyaluronic acid hydrogels with thiol-norbornene chemistry, Biomaterials 34 (38) (2013) 9803–9811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Mergy J, Fournier A, Hachet E, Auzely-Velty R, Modification of polysaccharides via thiol-ene chemistry: a versatile route to functional biomaterials, J. Polym. Sci. Part A 50 (19) (2012) 4019–4028. [Google Scholar]
- [7].Batchelor RR, Kwandou G, Spicer PT, Stenzel MH, (–)-riboflavin (vitamin B2) and flavin mononucleotide as visible light photo initiators in the thiol-ene polymerisation of PEG-based hydrogels, Polym. Chem. 8 (6) (2017) 980–984. [Google Scholar]
- [8].McCall JD, Anseth KS, Thiol-ene photopolymerizations provide a facile method to encapsulate proteins and maintain their bioactivity, Biomacromolecules 13 (8) (2012) 2410–2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hoyle CE, Bowman CN, Thiol-ene click chemistry, Angew. Chem. Int. Ed. 49 (9) (2010) 1540–1573. [DOI] [PubMed] [Google Scholar]
- [10].Liu H, Chung H, Visible-light induced thiol-Ene reaction on natural lignin, ACS Sustain. Chem. Eng. 5 (10) (2017) 9160–9168. [Google Scholar]
- [11].Ahn D, Sathe SS, Clarkson BH, Scott TF, Hexaarylbiimidazoles as visible light thiol-ene photoinitiators, Dent. Mater. 31 (9) (2015) 1075–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Shih H, Lin C-C, Visible-light-mediated thiol-ene hydrogelation using Eosin-Y as the only photoinitiator, Macromol. Rapid Commun. 34 (3) (2013) 269–273. [DOI] [PubMed] [Google Scholar]
- [13].Cherfan D, Verter EE, Melki S, Gisel TE, Doyle JFJ, Scarcelli G, Yun SH, Redmond RW, Kochevar IE, Collagen cross-linking using rose Bengal and green light to increase corneal stiffness, Invest. Ophthalmol. Vis. Sci. 54 (5) (2013) 3426–3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Spoerl E, Mrochen M, Sliney D, Trokel S, Seiler T, Safety ofUVA-riboflavin cross-linking of the cornea, Cornea 26 (4) (2007) 385–389. [DOI] [PubMed] [Google Scholar]
- [15].Huang R, Choe E, Min DB, Kinetics for singlet oxygen formation by riboflavin photosensitization and the reaction between riboflavin and singlet oxygen, J. Food Sci. 69 (9) (2004) C726–C732. [Google Scholar]
- [16].Oster G, Yang N-L, Photopolymerization of vinyl monomers, Chem. Rev. 68 (2) (1968) 125–151. [Google Scholar]
- [17].Encinas MV, Rufs AM, Bertolotti S, Previtali CM, Free radical polymerization photoinitiated by riboflavin/amines. Effect of the amine structure, Macromolecules 34 (9) (2001) 2845–2847. [Google Scholar]
- [18].Fernandes-Cunha GM, Lee HJ, Kumar A, Kreymerman A, Heilshorn S, Myung D, Immobilization of growth factors to collagen surfaces using pulsed visible light, Biomacromolecules 18 (10) (2017) 3185–3196. [DOI] [PubMed] [Google Scholar]
- [19].McCall AS, Kraft S, Edelhauser HF, Kidder GW, Lundquist RR, Bradshaw HE, Dedeic Z, Dionne MJC, Clement EM, Conrad GW, Mechanisms of corneal tissue cross-linking in response to treatment with topical riboflavin and long-wavelength ultraviolet radiation (UVA), Invest. Ophthalmol. Vis. Sci. 51 (1) (2010) 129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Yu L, Wang L-H, Hu Z-T, You Y-Z, Wu D-C, Hong C-Y, Sequential Michael addition thiol-ene and radical-mediated thiol-ene reactions in one-pot produced sequence-ordered polymers, Polym. Chem. 6 (9) (2015) 1527–1532. [Google Scholar]
- [21].Northrop BH, Coffey RN, Thiol-ene click chemistry: computational and kinetic analysis of the influence of alkene functionality, J. Am. Chem. Soc. 134 (33) (2012) 13804–13817. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


