BAC is a functional antagonist of albuterol associated with delayed recovery from asthma exacerbations. Only preservative-free albuterol formulations should be used for continuous nebulization.
Abstract
Video Abstract
BACKGROUND AND OBJECTIVES:
The albuterol dropper bottle used to prepare solutions for continuous nebulization contains the preservative benzalkonium chloride (BAC). BAC, by itself, has been shown to cause bronchospasm. We hypothesized that BAC would decrease the therapeutic efficacy of albuterol in patients with acute asthma exacerbations.
METHODS:
We performed a retrospective cohort study comparing the clinical outcomes of patients <18 years of age receiving continuous nebulized albuterol with and without BAC. For the primary end point (duration of continuous albuterol nebulization), we compared the 2 groups with Kaplan-Meier estimate of survival curves, conducted a log-rank test of difference, and adjusted for baseline characteristics using multivariable Cox regression. A P value <.05 was considered significant.
RESULTS:
A total of 477 patients were included in the analysis (236 exposed to BAC and 241 controls). The duration of continuous nebulization was significantly longer in the BAC group than in the control group (median of 9 vs 6 hours; 15.7% required continuous nebulization compared to 5.8% of controls at 24 hours). The control group was 79% more likely to stop continuous nebulization at any particular point in time (hazard ratio 1.79; 95% confidence interval: 1.45 to 2.22; P < .001) and 43% more likely to stop additional respiratory support (hazard ratio 1.43; 95% confidence interval: 1.16 to 1.75; P < .001).
CONCLUSIONS:
BAC is a functional albuterol antagonist associated with a longer duration of continuous albuterol nebulization treatment and additional respiratory support, suggesting that preservative-free albuterol formulations are safer for use in continuous nebulization.
What’s Known on This Subject:
Benzalkonium chloride is a preservative contained in one formulation of albuterol used for continuous nebulization that, by itself, is known to cause clinically significant bronchospasm in patients with stable asthma.
What This Study Adds:
Continuous nebulization of albuterol containing the benzalkonium chloride preservative is associated with slower recovery from a severe acute asthma exacerbation than continuous nebulization of preservative-free albuterol solutions.
Asthma is one of the most prevalent chronic diseases in the pediatric population.1 Severe asthma exacerbations can result in respiratory distress with need for hospital admission and continuous nebulized albuterol for acute management, consistent with current guidelines.1 Benzalkonium chloride (BAC) is a chemical preservative contained in the 0.5% multidose 20-mL dropper bottle of albuterol. All other albuterol products for nebulization are single-dose sterile, preservative-free vials of concentrate or varying dilutions with saline. There is no preservative-free product available in the 20-mL vial size. Either a BAC-containing or a preservative-free albuterol product can be administered via continuous nebulization. Preparing solutions for continuous nebulization is significantly faster and easier if the larger (20-mL) vial size is used. Consequently, many children’s hospitals in the United States use the BAC-containing product to prepare solutions for continuous nebulization and may not be aware of the possible deleterious effects of this preservative. BAC, by itself, has been shown to cause bronchospasm in patients with asthma in a dose-dependent and cumulative manner.2,3
Asmus et al2 compared the airway response to nebulized EDTA versus nebulized BAC in human subjects with mild stable asthma. BAC was more likely (P < .0001) than EDTA or placebo to cause a decrease in forced viral capacity in the first second of exhalation (FEV1) of at least 20%; in some cases, the drop in FEV1 was 40% to 50%.2 Zhang et al3 demonstrated that 17 of 28 subjects with stable asthma (61%) reached the threshold for a significant drop (20% or greater) in FEV1. The effect was dose dependent and cumulative. Increased baseline airway hyperresponsiveness was associated with increased likelihood of having bronchoconstriction caused by BAC.3 The threshold dose of BAC for triggering bronchospasm in asymptomatic subjects was ∼300 μg.3 Because each 2.5 mg of albuterol from the dropper bottle contains 50 μg of BAC and the albuterol dose ranges from 10 to 20 mg/hour, patients receive 800 to 1600 μg BAC in every 4 hours of continuous nebulized albuterol administration. Because of these properties, the adverse effects of BAC in patients with severe bronchospasm are likely to be more prominent when inhaled continuously in patients with severe asthma exacerbation compared to intermittent exposure in volunteer subjects who have stable mild bronchospasm.
In October 2015, our hospital, University of Florida Health Shands Children’s Hospital, switched from the use of a 0.5-mL unit-dose preservative-free formulation of albuterol to the 20-mL dropper bottle containing the BAC preservative to prepare solutions for continuous nebulization. This was prompted by a patient safety report filed by a PICU attending physician when there was a delay in the pharmacy providing the solution for continuous albuterol nebulization. The albuterol formulation containing the BAC preservative was provided by the manufacturer in significantly larger volume containers than the preservative-free albuterol formulation, thus reducing the number of times the pharmacy technician had to withdraw solution (1 draw for the dropper bottle containing BAC versus draws from 16 to 32 vials of the preservative-free unit-dose formulation). Our aim was to determine if the change to BAC-containing albuterol products resulted in clinical consequences in children with severe acute asthma exacerbations. On the basis of the literature and the expected exposure to a large cumulative dose of BAC during continuous nebulization, we hypothesized that the use of albuterol formulations containing BAC would prolong the duration of continuous nebulization, an indirect measure of adverse effect.
Methods
We performed a retrospective cohort study comparing clinical outcomes for patients receiving continuous albuterol nebulization with and without the BAC preservative. Approval for the study was obtained from the University of Florida Institutional Review Board (IRB) with a waiver of informed consent (IRB 201701148). We identified all patients from 0 to 17 years old who had received continuous nebulized albuterol as inpatients at our institution 1.5 years before and 1.5 years after our institution’s change in the albuterol formulation. We designated all eligible patients who had received this therapy with the formulation containing the BAC preservative for management of asthma exacerbation as the “BAC” (exposed) group and all eligible patients who had received it with the preservative-free formulation as the control group. Patients intubated before admission or during the hospitalization were excluded from statistical analysis because they represent a small but distinct subgroup of patients. For this subgroup, only descriptive data are presented.
Electronic medical records of all included patients were extracted to compare baseline demographic (age, sex, race) and clinical information for both groups, as well as parameters related to study outcome measures. We evaluated the following outcome measures: (1) duration of administration of continuous albuterol, (2) total dose of albuterol administered via continuous nebulization, (3) duration of administration of supplemental respiratory support (conventional nasal cannula, high-flow nasal cannula, or non-invasive positive pressure ventilation), (4) length of inpatient stay, (5) whether intravenous terbutaline was added to treatment with continuous albuterol, (6) the number of intravenous magnesium bolus doses administered, and (7) maximum heart rate measured during administration of continuous albuterol. We used milligrams as opposed to weight-based dosing per kilogram for continuous albuterol dosing analysis to remain consistent with the clinical dosing practices at our institution and given previous studies suggesting that dosing in a weight-based manner is not of added benefit.4 In addition to the demographics, we compared the 2 groups on the above outcomes adjusting for the following baseline clinical measurements: oxygen saturation (using pulse oximetry) and initial asthma score after admission to the emergency department. The asthma score used at our institution is a clinical composite score that evaluates overall patient appearance, respiratory rate, accessory muscle use, air exchange on auscultation, wheezing, and oxygen saturation. An asthma score of ≥ 6 after 3 consecutive albuterol treatments is typically associated with the decision to start continuous nebulized albuterol per the hospital’s asthma treatment guideline. However, decisions to start or stop continuous albuterol nebulization were sometimes made on the basis of the clinical impression independent of asthma scoring. Charges for medical care were estimated for each study group by averaging the total amount of dollars billed for each patient’s hospitalization.
Baseline characteristics were compared between groups by using 2-tailed Welch’s t test for continuous variables and Pearson’s χ2 test for categorical variables. For the primary end point (duration of continuous albuterol nebulization) and other secondary time-to-event outcomes (duration of respiratory support needed and length of stay), we compared the 2 groups on the basis of Kaplan-Meier estimate of survival curves and conducted a log-rank test of difference. In addition, multivariable Cox regression was used to test between-group differences adjusting for baseline characteristics. Point estimates and 95% confidence intervals (CIs) of adjusted hazard ratios (HRs) were provided. Furthermore, multivariate regression analysis was used to compare the 2 groups on other continuous outcomes (such as total dose of albuterol administered via continuous nebulization) adjusting for the same baseline characteristics. For the binary outcome (terbutaline given: yes or no), we conducted a logistic regression adjusting for the same baseline characteristics. Results from propensity score analyses based on the inverse probability weighting method were also reported. We used R statistical software (version 3.4.4) for the analysis.5 A P value <.05 was considered as the threshold for statistical significance. Data are expressed as median (IQR) and mean (SD) unless otherwise stated.
Results
A total of 521 patients met the inclusion criteria. Twenty-nine of these patients were excluded because of having been intubated during the asthma exacerbation examined, and another 15 patients were excluded because of missing patient data regarding the primary outcome (duration of continuous albuterol nebulization). A total of 477 patients were thus included in the analysis (236 exposed to BAC and 241 controls). There were no statistically significant differences in severity of exacerbation between the 2 groups, as indicated by initial oxygen saturation and asthma scores measured (Table 1). All patients were treated with systemic corticosteroids in addition to continuous albuterol nebulization.
TABLE 1.
Comparison of Demographics and Baseline Clinical Characteristics Between Groups
| Variable | Exposed to Benzalkonium | Control | P |
|---|---|---|---|
| Oxygen saturation after initial presentation, %, mean (SD) | 94 (4) | 94 (4) | .67 |
| First asthma score documented after arrival, mean (SD) | 7 (2) | 7 (2) | .20 |
| Age, y, mean (SD) | 5.8 (4.0) | 5.9 (3.8) | .68 |
| Sex, n (%) | .26 | ||
| Male | 130 (55) | 145 (60) | |
| Female | 106 (45) | 96 (40) | |
| Race, n (%) | .36 | ||
| African American | 115 (53) | 135 (60) | |
| White | 82 (38) | 71 (32) | |
| Hispanic | 12 (6) | 14 (6) | |
| Asian American | 7 (3) | 4 (2) |
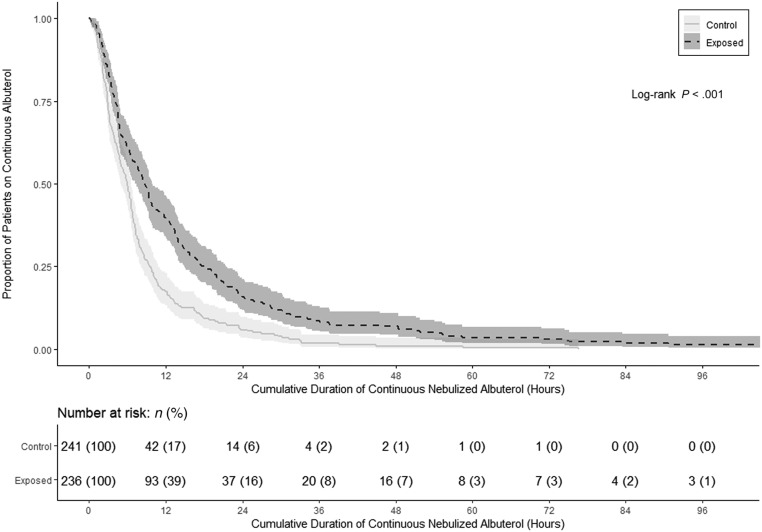
The primary end point, duration of continuous albuterol nebulization, was significantly longer in the BAC group (median [IQR]: 9 [4–18] hours) compared with the control group (6 [3–9] hours) on the basis of a log-rank test (P < .001). The Kaplan-Meier estimates indicated that at 24 hours, when continuous nebulization is often completed,6 the proportion of patients requiring continuous albuterol was 15.7% among those receiving BAC compared with only 5.8% of the control group (Fig 1). After adjusting for the potential confounding demographic and baseline clinical measurements in the analysis, multivariate Cox regression suggested that patients in the control group were 79% more likely to stop continuous nebulization than the BAC group at any particular point in time (HR of 1.79; 95% CI: 1.45 to 2.22; P < .001) (Table 2). Furthermore, propensity score analysis based on the inverse probability weighting method revealed that patients in the control group were 75% more likely to stop continuous nebulization than the BAC group at any particular point in time (HR of 1.75; 95% CI: 1.51 to 2.02; P < .001) (Supplemental Table 3). Results from the Cox regression also revealed that for the entire patient population, higher baseline asthma score and older age were associated with the longer duration of continuous albuterol nebulization (P = .03 and P = .001, respectively) (Supplemental Table 4).
FIGURE 1.
Kaplan-Meier plot comparing the proportion of patients with asthma exacerbation treated with continuous albuterol nebulization for a given cumulative duration in the benzalkonium-exposed group versus the control group. At 24 hours, the proportion continuing to require continuous nebulization was 15.7% for the BAC-exposed group compared with 5.8% for the control group (P < .001).
TABLE 2.
Summary of Clinical Outcomes by Group, Point Estimate, and 95% CIs of Group Effect From Regression Analysis
| Clinical Outcome | Exposed to Benzalkonium | Control | Adjusted HR, Mean Difference, or Odds Ratio for Control Versus Exposed (95% CI) | P |
|---|---|---|---|---|
| Duration of continuous albuterol nebulization, h, median (IQR) | 9 (4–18) | 6 (3–9) | 1.79 (1.45 to 2.22)a,c | <.001a |
| Duration of respiratory support needed, h, median (IQR) | 16 (6–33) | 8 (2–21) | 1.43 (1.16 to 1.75)a,c | <.001a |
| Length of stay, h, median (IQR) | 43 (29–65) | 44 (35–62) | 0.93 (0.76 to 1.15)c | .52 |
| Total dose of albuterol administered by continuous nebulization, mg, mean (SD) | 185 (371) | 86 (102) | −117 (−174.6 to −59.1)a,d | <.001a |
| No. magnesium bolus doses given per patient, mean (SD)b | 1 (1) | 1 (1) | −0.10 (−0.29 to 0.09)d | .32 |
| Maximum heart rate during administration of continuous albuterol, beats per min, mean (SD) | 169 (20) | 167 (19) | −0.18 (−3.03 to 2.67)d | .90 |
| Total hospital charges, $, mean (SD) | 5988.48 (6136.44) | 5941.91 (4384.21) | 3.54 (−939 to 946)d | .99 |
| IV terbutaline, No. patients (%) | .06 | |||
| Yes | 8 (3.4) | 4 (1.7) | 0.12 (0.01 to 0.75)e | |
| No | 228 (96.6) | 237 (98.3) | 0.12 (0.01 to 0.75)e |
The first 3 outcomes were analyzed by using multivariate Cox regression, the next 4 outcomes by linear regression, and the last outcome by logistic regression. IV, intravenous.
Statistically significant.
77.5% of patients in the benzalkonium-exposed group received 1 or more doses of IV magnesium, whereas 86.7% of patients in the control group received 1 or more doses of IV magnesium (no statistically significant difference based on logistic regression [P = .30]).
HR.
Mean difference.
Odds ratio.
The duration of respiratory support needed was significantly longer in the BAC group (median [IQR]: 16 [6–33] hours) compared with the control group (8 [2–21] hours). After adjusting for baseline characteristics, Cox regression analysis revealed that patients in the control group were 43% more likely to stop respiratory support than the BAC group at any particular point in time (HR of 1.43; 95% CI: 1.16 to 1.75; P < .001) (Table 2). Propensity score analysis revealed that patients in the control group were 38% more likely to stop respiratory support than those in the BAC group at any particular point in time (HR of 1.38; 95% CI: 1.20 to 1.59; P < .001) (Supplemental Table 3). Also, the total dose of albuterol administered by continuous nebulization was significantly greater in the BAC group (mean [SD]]: 185 mg [371]) than in the control group (86 mg [102]; P < .001). There were no statistically significant differences in the other clinical end points, such as length of stay and total hospital charges (Table 2).
Of the 29 patients who were excluded because of having been intubated, 18 of these patients would have been placed in the BAC group and 11 of these patients would have been placed in the control group had they not been intubated. The median time to extubation (excluding time intubated before arrival at our institution) was 118 (76, 186) hours for those who would have been in the BAC group versus 62 (35, 165) hours for those who would have been in the control group.
Discussion
The results of this retrospective analysis reveal that BAC is significantly associated with an increase in dose and duration of continuous albuterol nebulization and longer duration of respiratory support needed among children hospitalized with severe asthma exacerbations requiring continuous nebulized albuterol. An increased duration of 3 hours of continuous albuterol nebulization use and 8 hours of need for respiratory support suggests that patients in the BAC group continued to have respiratory distress, on average, for a substantially longer period of time than patients in the control group. However, the length of stay did not differ between the 2 groups despite the significant difference in median continuous albuterol duration. This is probably because of other confounding factors that affect the decision to discharge patients after the end of continuous albuterol administration. This also suggests that the deleterious effects of BAC were temporary and lasted only for the duration of the administration of continuous albuterol. Further investigation into the long-term effects of BAC-containing albuterol with future prospective studies would be helpful.
Although there is substantial evidence that BAC induces bronchospasm in clinical pharmacology studies of patients with stable asthma7 and there are a few recent case reports regarding difficulty weaning children exposed to BAC from continuous nebulized albuterol,8,9 this is the first study comparing the clinical outcomes of specifically pediatric patients with asthma exacerbation receiving continuous albuterol nebulization with an albuterol formulation containing BAC compared to a formulation that is preservative free. Previous studies have demonstrated that the effect of BAC nebulized in the airways of patients with stable asthma was dose dependent, cumulative, and more pronounced in patients with greater baseline airway reactivity.2,3 Therefore, it is not surprising that BAC exposure in children with life-threatening asthma exacerbations slowed the response to therapy as evidenced by the much longer duration of administration of continuous nebulized albuterol, a larger total dose of albuterol administered continuously, and longer duration of supplemental respiratory support required in the BAC-exposed patients. This is consistent with BAC working as a functional antagonist of the pharmacologic effects of albuterol, which may increase the risk of clinical complications or clinical deterioration in patients with severe asthma exacerbations. Also, because some patients with severe asthma may respond slowly to treatment, it may not be readily apparent to the clinician that the preservative is the cause.
In contrast to our study, a recent quality improvement project from our institution, which was determined to be exempt from the requirement for IRB review, included only 128 patients with severe asthma exacerbation who were admitted 3 months before and 3 months after the switch from preservative-free to BAC-containing continuous albuterol nebulization. The researchers in that project concluded that there was no significant difference between BAC-containing albuterol products and preservative-free albuterol products in terms of median duration of continuous albuterol nebulization administered to patients with asthma exacerbation.10 However, the larger patient population included in the current study provides sufficient statistical power to detect statistically significant differences and suggests that the former project suffered from a type II error. On the basis of the former project, our institution as well as another affiliated institution in Jacksonville continues to use the BAC-containing product to prepare continuous nebulization but use the preservative-free product for all other albuterol nebulizer treatments.
The strengths of our study include the large number of patients studied, the similarity of average severity of asthma exacerbation at initial presentation to our institution between the groups compared, and the similarity of key demographics (eg, age, sex, and race) between each group. Our study does have limitations, however. It is a retrospective study as opposed to a prospective randomized controlled trial. Asthma scores for each patient after initial emergency department evaluation could not be assessed because subsequent scores were not consistently documented after transfer from the emergency department to the pediatric hospital units in the earlier hospitalizations assessed in this study. Asthma scoring became more consistent during the later hospitalizations studied, which might be a confounding factor in our study; the likelihood that performing scores lengthened duration does not seem logical, however. Changes in practice patterns (eg, due to changes in physician and/or nursing staff over the period studied) were unable to be ruled out given the retrospective nature of this study. Also, the investigator gathering the data was not blinded to which group each patient was assigned, although only objective data were compared.
Conclusions
We found that continuous nebulization with the albuterol formulations containing BAC is associated with a longer duration of continuous nebulization and with the need for additional respiratory support. These findings suggest that using preservative-free albuterol formulations is a safer approach for continuous albuterol nebulization treatment of children hospitalized with severe asthma exacerbations. A prospective, double-blinded, placebo-controlled, randomized study to confirm our findings would be scientifically helpful but may not be ethically feasible given the findings of the current study.
Glossary
- BAC
benzalkonium chloride
- CI
confidence interval
- FEV1
forced viral capacity in the first second of exhalation
- HR
hazard ratio
- IRB
institutional review board
Footnotes
Dr Pertzborn participated in the concept and design of the study, collected data, made substantial contributions to the analysis and interpretation of the data, drafted the initial manuscript, reviewed the manuscript, and critically revised the manuscript; Drs Prabhakaran, Abu-Hasan, and Hendeles, and Ms Baker participated in the concept and design of the study, made substantial contributions to the analysis and interpretation of the data, reviewed the manuscript, and critically revised the manuscript; Dr Wu and Mr Wu made substantial contributions to the statistical analysis and interpretation of the data, reviewed the manuscript, and critically revised the statistical comments; and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: The statistical analysis was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number UL1TR001427. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The statistical analysis was also supported by the US Health Resources and Services Administration (Health Resources and Services Administration–Maternal and Child Health Bureau grant T72-MC0002 [2015–2020]). Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
COMPANION PAPER: A companion to this article can be found online at www.pediatrics.org/cgi/doi/10.1542/peds.2019-3590.
Dr Pertzborn's current affiliation is Division of Pediatric Pulmonary and Sleep Medicne, University of Arkansas for Medical Sciences, Little Rock, AR. Dr Abu-Hasan's current affiliation is Department of Pediatric Pulmonology, Sidra Medicine, Doha, Qatar
References
- 1.US Department of Health and Human Services; National Institutes of Health; National Heart, Lung, and Blood Institute. National Asthma Education and Prevention Program Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma: Summary Report 2007. Bethesda, MD: National Heart, Lung, and Blood Institute; 2007. Available at: https://www.nhlbi.nih.gov/sites/default/files/media/docs/asthsumm.pdf. Accessed November 16, 2018 [Google Scholar]
- 2.Asmus MJ, Barros MD, Liang J, Chesrown SE, Hendeles L. Pulmonary function response to EDTA, an additive in nebulized bronchodilators. J Allergy Clin Immunol. 2001;107(1):68–72 [DOI] [PubMed] [Google Scholar]
- 3.Zhang YG, Wright WJ, Tam WK, Nguyen-Dang TH, Salome CM, Woolcock AJ. Effect of inhaled preservatives on asthmatic subjects. II. Benzalkonium chloride. Am Rev Respir Dis. 1990;141(6):1405–1408 [DOI] [PubMed] [Google Scholar]
- 4.Oberklaid F, Mellis CM, Souëf PN, Geelhoed GC, Maccarrone AL. A comparison of a bodyweight dose versus a fixed dose of nebulised salbutamol in acute asthma in children. Med J Aust. 1993;158(11):751–753 [DOI] [PubMed] [Google Scholar]
- 5.R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2018. Available at: https://www.R-project.org/. Accessed September 22, 2018 [Google Scholar]
- 6.Kenyon CC, Fieldston ES, Luan X, Keren R, Zorc JJ. Safety and effectiveness of continuous aerosolized albuterol in the non-intensive care setting. Pediatrics. 2014;134(4). Available at: www.pediatrics.org/cgi/content/full/134/4/e976 [DOI] [PubMed] [Google Scholar]
- 7.Prabhakaran S, Abu-Hasan M, Hendeles L. Benzalkonium chloride: a bronchoconstricting preservative in continuous albuterol nebulizer solutions. Pharmacotherapy. 2017;37(5):607–610 [DOI] [PubMed] [Google Scholar]
- 8.Currie M, Jordan C, Loughlin C. When the nebs aren’t helping: paradoxical bronchoconstriction due to preservative in continuous albuterol nebulizer solution [abstract]. Am J Respir Crit Care Med. 2016;193:A5614 [Google Scholar]
- 9.George M, Joshi SV, Concepcion E, Lee H. Paradoxical bronchospasm from benzalkonium chloride (BAC) preservative in albuterol nebulizer solution in a patient with acute severe asthma. A case report and literature review of airway effects of BAC. Respir Med Case Rep. 2017;21:39–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Orth LE, Kelly BJ, Lagasse CA, Collins SW, Ryan MF. Safety and effectiveness of albuterol solutions with and without benzalkonium chloride when administered by continuous nebulization. Am J Health Syst Pharm. 2018;75(22):1791–1797 [DOI] [PubMed] [Google Scholar]