Abstract
Background and Purpose
Orthostatic intolerance and falls differ between sexes and change with age. However, it remains unclear what role cerebral autoregulation may play in this response. This study was designed to determine whether or not cerebral autoregulation, assessed using transcranial Doppler ultrasound (TCD), is more effective in elderly females than in males.
Methods
We used TCD to evaluate cerebral autoregulation in 544 (236 male) subjects over the age of 70 recruited as part of the MOBILIZE Boston study (MBS). The MBS is a prospective cohort study of a unique set of risk factors for falls in seniors in the Boston area. We assessed CO2 reactivity and transfer function gain, phase and coherence during five minutes of quiet sitting and autoregulatory index (ARI) during sit-to-stand tests.
Results
Male subjects had significantly lower CO2 reactivity (Males: 1.10±0.03, Females: 1.32±0.43 cm.s−1/%CO2, p<0.001), ARI indices (Males: 4.41±2.44, Female: 5.32±2.47, p<0.001) and higher transfer function gain (Males: 1.34±0.49, Females: 1.19±0.43, p=0.002) and lower phase (Males: 42.7±23.6, Females: 49.4±24.9, p=0.002) in the autoregulatory band, implying less effective cerebral autoregulation. However, reduced autoregulation in males did not fall below the normal range, indicating autoregulation was intact, but less effective.
Conclusion
Female subjects were better able to maintain cerebral flow velocities during postural changes and demonstrated better cerebral autoregulation. The mechanisms of sex based differences in autoregulation remain unclear, but may partially explain the higher rates of orthostatic hypotension related hospitalizations in elderly men.
Keywords: cerebral blood flow, blood pressure, cerebral autoregulation, sex, sit-to-stand
Introduction
Cerebral autoregulation (CA) is an intrinsic mechanism that maintains cerebral blood flow (CBF) relatively constant over a wide range of blood pressures.1 If autoregulatory responses are slow, the likelihood that transient decreases in blood pressure during postural changes would result in transient cerebral hypoperfusion increases, potentially causing symptoms including dizziness, light-headedness, and syncope.
Orthostatic intolerance is three to four times higher in young women than young men.2 Similarly, post-spaceflight orthostatic intolerance also occurs more frequently in women.3 However, in older populations, orthostatic hypotension-related hospitalization rates are higher in men than women.4 One explanation for these differences could be differences in CA between men and women.
Previous studies have shown higher cerebral flow velocity,5 cerebral vasomotor reactivity,6 and cerebrovascular reactivity7 in females. Gender related differences in autoregulation have also been observed in adolescent8 and young adult females.9 However, previous data is somewhat conflicting and involves relatively small numbers of subjects (<25). In addition, no work has compared sex differences in autoregulation in older populations. To address this issue, we analysed the autoregulatory responses of subjects taking part in the MOBILIZE Boston study (Maintenance of Balance, Independent Living, Intellect, and Zest in the Elderly of Boston). The Mobilize Boston study (MBS) is a study of 800 older adults in the Boston area, designed to improve understanding of how older adults can maintain their health and independence for longer.
We hypothesized that CA would be more effective in female subjects when compared to males.
Methods
Subjects
The study sample was taken from the MBS population. In total, 816 subjects (292 male) over the age of 70 were enrolled. Transcranial Doppler (TCD) testing was completed in 63% of the sample, and partially completed in another 11% of participants. TCD data could not be obtained in some subjects because of the absence of a suitable temporal window to insonate the middle cerebral artery. Subjects with a history of stroke (N=78, 34 male) were excluded from the study. The MBS was approved by the Institutional Review Board of Hebrew SeniorLife and all subjects provided written informed consent.
Instrumentation
Each subject was instrumented with a three lead ECG and finger cuff continuous blood pressure monitor (Finometer, FMS Medical Systems), held at heart level with a sling. Cerebral blood flow velocity (CBFV) was measured continuously in the middle cerebral artery (MCA) using transcranial Doppler ultrasonography (MultiDop, DWL). Continuous end-tidal CO2 levels were measured by a gas analyzer through a sampling tube attached to a nasal cannula. All physiological signals were digitized at 500Hz (Windaq) and stored for offline analysis.
CO2 reactivity protocol
The cerebrovascular response to CO2 was assessed by asking subjects to breathe normally for two minutes, inspire a gas mixture of 8% CO2, 21% O2, and balance nitrogen for two minutes and then mildly hyperventilate to an end-tidal CO2 of approximately 25mmHg for two minutes.
Sit-to-stand protocol
For posture change, a sit-to-stand maneuver was performed. Subjects sat with their legs elevated at 90° on a stool for five minutes followed by moving from sit-to-stand for one minute. Initiation of stand was considered to be the point at which both feet touched the floor. This was performed twice. Subjects were not if data quality was considered too poor for analysis.
Data Processing and Analysis
Postprocessing was done using custom-written Matlab scripts. To evaluate beat-to-beat dynamics of mean arterial pressure (MAP) and CBFV, we calculated the differences between the sitting value (averaged over 50 seconds) and the nadir of blood pressure (average of five values surrounding the nadir), in both absolute and percentage change. In addition we estimated the cerebrovascular resistance (CVR) (ΔMAP/CBFV) over the same period. End-tidal CO2 levels were calculated while sitting and 10–30 seconds after standing. The average of both sit-to-stand responses for each subject was calculated to produce one response per subject.
To quantify autoregulation we calculated the dynamic autoregulatory index (ARI)10 based on the transient changes in CBFV and MAP when standing. In addition we calculated transfer function gain, phase and coherence on the five minutes seated periods. Subjects with 3 or more ectopic beats per minute were excluded from transfer function analysis.11
To calculate CO2 reactivity, we plotted the CBFV of each beat during the cerebrovascular reactivity test and the corresponding end-tidal CO2 value. The slope of this relationship was used as an index of CO2 reactivity (cm.s−1/mmHg).
Statistical Analysis
The effects of group (male vs. female) on CBFV, heart rate, MAP, end-tidal CO2, CVR, ARI and transfer function indices were assessed by independent samples t-tests. Data are presented as mean±standard deviation and levels of P<0.05 are considered statistically significant.
Results
Subjects
In total, 544 subjects (236 male) subjects were included in this study. Body mass index (BMI), incidence of hypertension, diabetes, and hyperlipidemia were compared between genders (Table 1). Females had higher cholesterol levels (p<0.001), and lower rates of diabetes (p=0.006). CO2 reactivity data was obtained in 383 (164 male) subjects, transfer function analysis in 407 subjects (163 male), sit-to-stand in 440 subjects (193 male). Of these 440 subjects, 353 subjects (165 males) had data considered suitable for ARI analysis. Remaining subjects were excluded due to inadequate signal quality.
Table I.
Summary of subject characteristics
| Male | Female | P value | |
|---|---|---|---|
| Age (years) | 78.3±5.3 (N=236) | 77.9±5.6 (N=308) | 0.328 |
| BMI | 27.0±4.3 (N=236) | 26.9±5.4 (N=308) | 0.761 |
| Systolic BP (mmHg) | 127.5±15.2 (N=235) | 130.2±18.5 (N=304) | 0.059 |
| Diastolic BP (mmHg) | 69.8±8.7 (N=235) | 70.0±8.7 (N=304) | 0.791 |
| Diabetes, with (%) | 18.3 (N=230) | 8.1 (N=297) | 0.006 |
| Hyperlipidemia, with (%) | 40.3 (N=236) | 53.6 (N=308) | 0.002 |
| Hypertension, with (%) | 17.9 (N=235) | 25.3 (N=304) | 0.974 |
BMI=Body Mass Index, BP=blood pressure. N=number of subjects for whom data is available.
A greater proportion of male subjects had diabetes (p=0.006). We therefore analysed autoregulatory data for all subjects and subjects without diabetes, because previous studies have shown that CA is impaired in diabetic subjects.12 A higher proportion of females had hyperlipidemia, but a multiple analysis of variance (MANOVA) analysis found no interaction between gender and hyperlipidemia. Therefore we do not believe that this difference in hyperlipidemia rates affected the study outcome.
Sit-to-stand protocol
Figure 1 shows a comparison of male versus female sit-to-stand responses (Table 2) Males had a slightly larger drop in MAP (p=0.006), and a significantly larger drop in MCA flow velocity (p<0.001). Females had significantly higher ARI values than males (Figure 2), demonstrating that CBFV returned to baseline faster even considering the difference in blood pressure changes (Males: 4.41±2.44, females: 5.32±2.46, p=0.001). Changes in heart rate and end-tidal CO2 on standing were not significantly different between male and female subjects.
Figure 1.

Normalised middle cerebral artery cerebral blood flow velocity (MCA CBFV), mean arterial pressure (MAP), heart rate and end-tidal CO2 levels during sit-to-stand (at 60 seconds). bpm = beats per minute.
Table II.
Baseline Hemodynamics and response to sit-to-stand Procedure
| Male (N=193) | Female (N=247) | P value | |
|---|---|---|---|
| MAP (mmHg) | 67.9±13.7 | 70.4±13.4 | 0.048 |
| HR (bpm) | 63.1±9.6 | 68.2±10.5 | <0.001 |
| CBFV (cms−1) | 39.2±10.0 | 43.1±9.8 | <0.001 |
| CVR (mmHg/cms−1) | 1.85±0.60 | 1.73±0.60 | 0.041 |
| End-tidal CO2 (mmHg) | 35.1±4.5 | 35.9±3.8 | 0.044 |
| ΔMAP (mmHg) | −18.2±8.7 | −16.0±8.3 | 0.006 |
| ΔCBFV (%) | −22.2±13.2 | −14.5±10.9 | <0.001 |
| Ratio of % change | 0.746±0.1.48 | 0.702±0.970 | 0.71 |
| CBFV to % change MAP | |||
| ΔCVR (mmHg/cms−1) | −0.073±0.444 | −0.145±0.254 | 0.044 |
| Δheart rate (bpm) | 12.60±5.80 | 13.24±6.41 | 0.281 |
| Time to blood pressure nadir (s) | 11.44±4.42 | 10.92±3.57 | 0.179 |
| Time to CBFV nadir (s) | 8.90±4.74 | 9.02±5.14 | 0.801 |
| Δend-tidal CO2 (mmHg) | −1.14±2.18 | −1.17±1.50 | 0.885 |
MAP=mean arterial pressure, HR=heart rate, CBFV=cerebral blood flow velocity, CVR=cerebrovascular resistance. bpm=beats per minute. P value=male versus female comparison.
Figure 2.
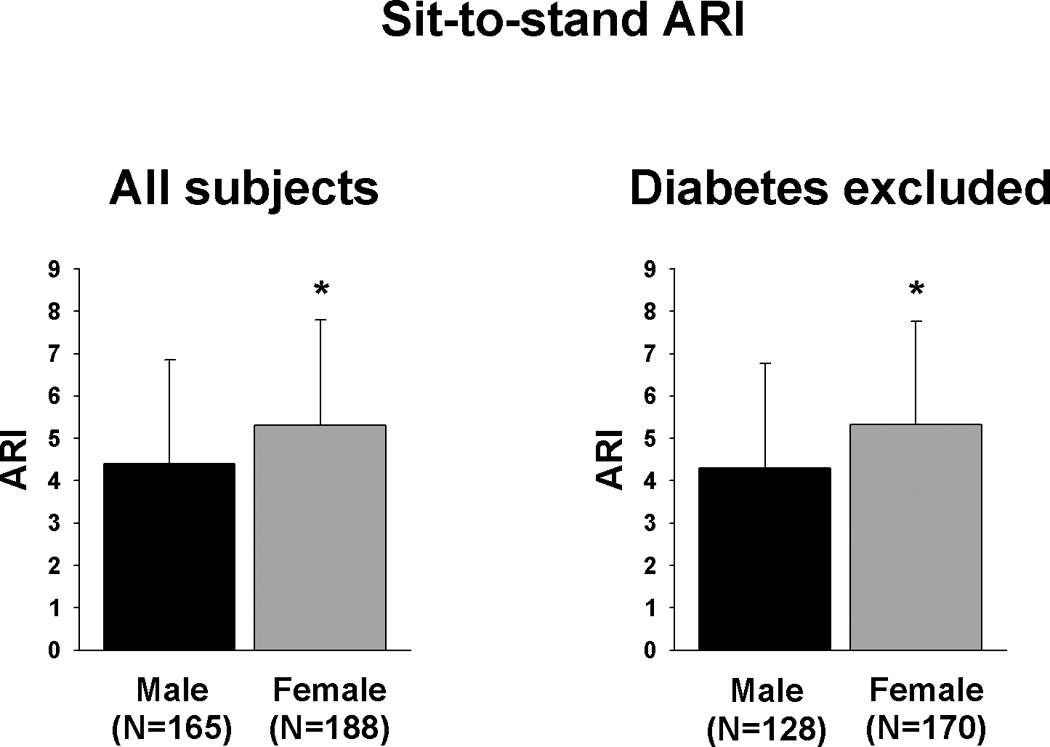
ARI during sit-to-stand.
Linear regression analysis of baseline sitting CBFV by age showed that females had a significant decrease of −4.60cm.s−1 per decade. In contrast, male subjects showed a non-significant change of −1.32cm.s−1 per decade. This significantly greater decrease in females (p<0.001) resulted in much higher CBFV values in the younger females reaching similar values to those of the males by the last decade.
Transfer Function Response
Transfer functions were calculated with the subject in the seated position in three frequency bands: very low frequency (0.03–0.07Hz); low frequency (0.07–0.15Hz), and cardiac frequency surrounding the heart rate (~1Hz). Previous research has suggested that gains in the very low frequency range represent autoregulatory processes.13
Males had significantly higher gain (Males: 1.34±0.49, Females: 1.19±0.43, p=0.002) and lower phase (Males: 42.7±23.6, Females: 49.4±24.9, p=0.007) in the very low frequency band. In contrast, there were no significant differences in the low frequency band (Figure 3). To determine the pressure-flow relations within the beat, we examined transfer functions at the cardiac frequency. Male subjects had higher cardiac frequency gains (Males: 2.36±0.64, Females: 2.04±0.55, p<0.001).
Figure 3.

Male versus female transfer function in the very low (0.03–0.07Hz); low (0.07–0.15Hz), and cardiac (~1Hz) frequency bands.
CO2 reactivity protocol
Females had significantly higher CO2 reactivity slopes (All subjects:-Males: 1.10±0.03cm.s−1/mmHg, Females: 1.32±0.43cm.s−1/mmHg, p<0.001. Diabetes excluded:- Males: 1.08±0.32cm.s−1/mmHg, Females: 1.31±0.42cm.s−1/mmHg). To control for greater baseline CBFV in the females, we also examined the cerebrovascular reactivity calculated from normalized CBFV data and females also showed significantly greater values (All subjects: Males: 2.88±0.65%/mmHg, Females: 3.15±0.77%/mmHg p<0.001. Diabetes excluded:- Males: 2.89±0.64cm.s−1/mmHg, Females: 3.21±0.71cm.s−1/mmHg).
Discussion
The main findings of this study are that, in an older population, females were better able to regulate CBF, demonstrating higher ARI values during a sit-to-stand maneuver, and better transfer function CA indices. Females also had higher baseline MCA flow velocities and greater cerebrovascular reactivity to CO2. Higher rates of diabetes in men did not affect the outcome of this study. Indices of CA were within the normal healthy range for both genders, indicating that CA is not impaired in healthy aging.
A recent epidemiological study showed a higher rate of orthostatic hypotension related hospitalizations in elderly males.4 This could be the result of either greater hypotension in males, resulting in greater cerebral hypoperfusion, and/or less effective autoregulation, resulting in an inability to compensate for orthostatic blood pressure drops. In fact, our results found that males tended to have larger blood pressure drops during sit-to-stand, as well as lower ARI values, reduced phase shift and higher gains than females, indicating less effective autoregulation than females. This raises the possibility that reduced autoregulation capacity may be related to this increased incidence of orthostatic hypotension related hospitalizations in elderly men.
Previous work in young adult females found that MCA transfer function coherence in the low frequency band was lower in females, when tested in the upright position, suggesting better autoregulation.9 Other studies in children have produced conflicting results. No sex differences in either the basilar or MCA were found in young children (4–8 years),14 but in older children (10–16 years), the same group found that females are better able at maintain basilar artery flow when upright while males were better at maintaining MCA.8 However in both these studies, measures of autoregulation where based on steady state values rather than dynamic changes as we have reported.
The reasons for improved autoregulation in our female cohort remain unclear. One possibility is that the MCA territory in females was more vasoconstricted. Previous work has demonstrated that autoregulation is improved when the cerebrovascular bed is constricted, such as during hypocapnia15 and is correlated to CVR.11 However, females in our study had lower CVR values than males, indicating their cerebrovascular beds were more dilated and thus autoregulation should have been worse.
Another possibility is that sex hormone levels could have affected the autoregulatory response. This appears to be unlikely since our females were post-menopausal and those on hormone replacement therapy did not significantly differ in response. Previous work has also found no difference in the autoregulatory response to orthostatic stress in premenopausal women when tested at various times throughout the menstrual cycle.16 Thus, it appears unlikely that estrogen levels had an effect on the autoregulatory response.
The relationship between gender and CO2 cerebrovascular reactivity remains unclear. The Rotterdam study found no difference in cerebrovascular reactivity between males and females greater than 65 years, but lower reactivity in young females.17 Similarly, postmenopausal women <65 years of age were found to have lower breath hold indexes, indicating reduced cerebrovascular reactivity, to a group of age matched men.18 In contrast, we found greater cerebrovascular reactivity in females >65 years of age, similar to previous findings in younger women <65 years,7, 19 while Vriens et al found no significant difference between genders.20 It remains unclear why these differences exist. One possibility is that methodology issues may underlie the differences. Our technique used 8% CO2 with 21% O2, causing increased arterial CO2 levels without changing arterial oxygen levels. Other studies have used 5% CO2 with 95% O2, resulting in hypercapnia but also causing hyperoxia, or acetazolamide possibly affecting the result. It remains to be determined if methodological issues may explain the contradictory findings.
Regardless, the results of this study, as well as previous work in stroke patients has found that cerebrovascular reactivity was not correlated to autoregulatory measures.21 Thus differences in cerebrovascular reactivity likely do not explain differences in cerebral autoregulation between genders.
A potential explanation for the higher MCA flow velocities in females is the possible effect of sex hormone levels. HRT in postmenopausal women has also been reported to increase CBF,22 as has controlled ovarian stimulation.23 However, higher CBFV has also been shown in prepubital girls,14 and in the older population in this study. Since both these groups are known to have lower levels of estrogen, it appears unlikely that sex hormone differences can explain the differences in MCA flow velocities.
The difference in baseline CBFV between genders could also be explained by differences in MCA vessel diameter. Smaller vessel diameter would lead to increased flow velocity in female subjects, assuming no difference in CBF. Muller et al24 reported that MCA vessel diameter was 9.3% larger in male subjects. In contrast, Orlandini et al25 reported no significant sex differences in cerebral vessels. Since neither MCA vessel diameter nor absolute MCA flow were measured in this study, we cannot exclude the possibility that differences in global CBF were present. In addition, if anatomical differences explained the higher flow in females, we would expect that this difference would be unaffected by age. We found that females demonstrated a significant decrease in CBFV with age, consistent with data previously reported by Bakker et al,17 suggesting that anatomical differences cannot explain the differing CBFV.
Aging is associated with decreased baroreflex function related to vascular stiffening.26 Since, like the baroreflex, CA is a myogenic response to stretch of the vessel, it would seem logical that CA would be impaired with age. In fact, it has been suggested that impairment in CA associated with aging, may contribute to increased rates of syncope in the elderly.27 However, the results of several studies have shown that dynamic CA is maintained in healthy aging.11, 28–29 This may be related to the fact that some data has demonstrated that cerebral vessels may not show the same stiffening effects as peripheral vessels.11 This is the largest cross-sectional study to date on CA in the elderly, and ARI values for both males and females were similar to those of younger healthy controls in previous studies.11, 28–29 This is in contrast to the longitudinal study of Brodie et al30 which showed a decline in ARI of 1.1 over ten years in ten healthy subjects. Thus, it is possible that our subjects may have had higher ARI values when younger. Regardless, even if there was a decline, indices of autoregulation in this population still remained within the expected range for healthy subjects, further supporting the finding that autoregulation is not impaired in healthy aging.
A limitation of this study is the potential for selection bias, as not all subjects recruited for the MOBILIZE Boston study completed TCD analysis. To address this, we compared age, BMI, incidences of diabetes, hypertension, and hyperlipidemia for both males and females in the TCD (N=544) and no-TCD (N=221) groups. There were no clinically significant differences between groups. Thus selection bias seems unlikely, but cannot be dismissed completely.
Conclusion
In summary, female subjects were better able to maintain CBFV during postural changes and demonstrated better CA. Females also had higher MCA flow velocities, and CO2 cerebrovascular reactivity than males. Finally, females appeared to have greater cerebral vessel compliance. It remains unclear why older females demonstrate improved CA. Future work is needed to examine gender differences in various cerebral arterial beds to better understand these underlying autoregulatory differences.
Acknowledgements
The authors acknowledge the MOBILIZE Boston research team and study participants for the contribution of their time, effort, and dedication.
Funding
This work was supported by the HRC/ Harvard Research Nursing Home Program Project, funded by NIH grant number AG004390.
Brian Deegan was funded by an Irish Research Council for Science Engineering and Technology postgraduate scholarship, and Faculty Scholarship.
Dr. Sorond was supported by NIH/National Institute on Aging (K23-AG030967 [PI] and P01-AG004390 [Co-I]). Dr. Lipsitz was supported by NIH/ National Institute on Aging (P01 AG004390 [PI]).
Dr. Serrador was the recipient of an E.T.S. Walton Visitor Award from Science Foundation Ireland.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
There were no conflicts of interest in this study.
References
- 1.Paulson OB, Strandgaard S, Edvinsson L. Cerebral autoregulation. Cerebrovasc. Brain Metab. Rev. 1990;2:161–192. [PubMed] [Google Scholar]
- 2.Schondorf R, Benoit J, Stein R. Cerebral autoregulation in orthostatic intolerance. Ann. N. Y. Acad. Sci. 2001;940:514–526. doi: 10.1111/j.1749-6632.2001.tb03702.x. [DOI] [PubMed] [Google Scholar]
- 3.Fritsch-Yelle JM, Whitson PA, Bondar RL, Brown TE. Subnormal norepinephrine release relates to presyncope in astronauts after spaceflight. J. Appl. Physiol. 1996;81:2134–2141. doi: 10.1152/jappl.1996.81.5.2134. [DOI] [PubMed] [Google Scholar]
- 4.Shibao C, Grijalva CG, Raj SR, Biaggioni I, Griffin MR. Orthostatic hypotension-related hospitalizations in the united states. Am. J. Med. 2007;120:975–980. doi: 10.1016/j.amjmed.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 5.Ackerstaff RG, Keunen RW, van Pelt W, Montauban van Swijndregt AD, Stijnen T. Influence of biological factors on changes in mean cerebral blood flow velocity in normal ageing: A transcranial doppler study. Neurol. Res. 1990;12:187–191. doi: 10.1080/01616412.1990.11739941. [DOI] [PubMed] [Google Scholar]
- 6.Karnik R, Valentin A, Winkler WB, Khaffaf N, Donath P, Slany J. Sex-related differences in acetazolamide-induced cerebral vasomotor reactivity. Stroke. 1996;27:56–58. doi: 10.1161/01.str.27.1.56. [DOI] [PubMed] [Google Scholar]
- 7.Kastrup A, Thomas C, Hartmann C, Schabet M. Sex dependency of cerebrovascular co2 reactivity in normal subjects. Stroke. 1997;28:2353–2356. doi: 10.1161/01.str.28.12.2353. [DOI] [PubMed] [Google Scholar]
- 8.Vavilala MS, Kincaid MS, Muangman SL, Suz P, Rozet I, Lam AM. Gender differences in cerebral blood flow velocity and autoregulation between the anterior and posterior circulations in healthy children. Pediatr. Res. 2005;58:574–578. doi: 10.1203/01.PDR.0000179405.30737.0F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang X, Krishnamurthy S, Evans J, Bhakta D, Justice L, Bruce E, et al. Transfer function analysis of gender-related differences in cerebral autoregulation. Biomed. Sci. Instrum. 2005;41:48–53. [PubMed] [Google Scholar]
- 10.Tiecks FP, Lam AM, Aaslid R, Newell DW. Comparison of static and dynamic cerebral autoregulation measurements. Stroke. 1995;26:1014–1019. doi: 10.1161/01.str.26.6.1014. [DOI] [PubMed] [Google Scholar]
- 11.Serrador JM, Sorond FA, Vyas M, Gagnon M, Iloputaife ID, Lipsitz LA. Cerebral pressure-flow relations in hypertensive elderly humans: Transfer gain in different frequency domains. J. Appl. Physiol. 2005;98:151–159. doi: 10.1152/japplphysiol.00471.2004. [DOI] [PubMed] [Google Scholar]
- 12.Cencetti S, Lagi A, Cipriani M, Fattorini L, Bandinelli G, Bernardi L. Autonomic control of the cerebral circulation during normal and impaired peripheral circulatory control. Heart. 1999;82:365–372. doi: 10.1136/hrt.82.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang R, Zuckerman JH, Giller CA, Levine BD. Transfer function analysis of dynamic cerebral autoregulation in humans. Am. J. Physiol. 1998;274:H233–H241. doi: 10.1152/ajpheart.1998.274.1.h233. [DOI] [PubMed] [Google Scholar]
- 14.Tontisirin N, Muangman SL, Suz P, Pihoker C, Fisk D, Moore A, et al. Early childhood gender differences in anterior and posterior cerebral blood flow velocity and autoregulation. Pediatrics. 2007;119:e610–e615. doi: 10.1542/peds.2006-2110. [DOI] [PubMed] [Google Scholar]
- 15.Aaslid R, Lindegaard KF, Sorteberg W, Nornes H. Cerebral autoregulation dynamics in humans. Stroke. 1989;20:45–52. doi: 10.1161/01.str.20.1.45. [DOI] [PubMed] [Google Scholar]
- 16.Claydon VE, Younis NR, Hainsworth R. Phase of the menstrual cycle does not affect orthostatic tolerance in healthy women. Clin. Auton. Res. 2006;16:98–104. doi: 10.1007/s10286-006-0330-y. [DOI] [PubMed] [Google Scholar]
- 17.Bakker SL, de Leeuw FE, den Heijer T, Koudstaal PJ, Hofman A, Breteler MM. Cerebral haemodynamics in the elderly: The rotterdam study. Neuroepidemiology. 2004;23:178–184. doi: 10.1159/000078503. [DOI] [PubMed] [Google Scholar]
- 18.Matteis M, Troisi E, Monaldo BC, Caltagirone C, Silvestrini M. Age and sex differences in cerebral hemodynamics: A transcranial doppler study. Stroke. 1998;29:963–967. doi: 10.1161/01.str.29.5.963. [DOI] [PubMed] [Google Scholar]
- 19.Karnik R, Valentin A, Winkler WB, Khaffaf N, Donath P, Slany J. Sex-related differences in acetazolamide-induced cerebral vasomotor reactivity. Stroke. 1996;27:56–58. doi: 10.1161/01.str.27.1.56. [DOI] [PubMed] [Google Scholar]
- 20.Vriens EM, Kraaier V, Musbach M, Wieneke GH, van Huffelen AC. Transcranial pulsed doppler measurements of blood velocity in the middle cerebral artery: Reference values at rest and during hyperventilation in healthy volunteers in relation to age and sex. Ultrasound Med. Biol. 1989;15:1–8. doi: 10.1016/0301-5629(89)90125-7. [DOI] [PubMed] [Google Scholar]
- 21.Gommer ED, Staals J, van Oostenbrugge RJ, Lodder J, Mess WH, Reulen JP. Dynamic cerebral autoregulation and cerebrovascular reactivity: A comparative study in lacunar infarct patients. Physiol. Meas. 2008;29:1293–1303. doi: 10.1088/0967-3334/29/11/005. [DOI] [PubMed] [Google Scholar]
- 22.Penotti M, Nencioni T, Gabrielli L, Farina M, Castiglioni E, Polvani F. Blood flow variations in internal carotid and middle cerebral arteries induced by postmenopausal hormone replacement therapy. Am. J. Obstet. Gynecol. 1993;169:1226–1232. doi: 10.1016/0002-9378(93)90287-s. [DOI] [PubMed] [Google Scholar]
- 23.Nevo O, Soustiel JF, Thaler I. Cerebral blood flow is increased during controlled ovarian stimulation. Am. J. Physiol. Heart Circ. Physiol. 2007;293:H3265–H3269. doi: 10.1152/ajpheart.00633.2007. [DOI] [PubMed] [Google Scholar]
- 24.Muller HR, Brunholzl C, Radu EW, Buser M. Sex and side differences of cerebral arterial caliber. Neuroradiology. 1991;33:212–216. doi: 10.1007/BF00588220. [DOI] [PubMed] [Google Scholar]
- 25.Orlandini GE, Ruggiero C, Orlandini SZ, Gulisano M. Blood vessel size of circulus arteriosus cerebri (circle of willis): A statistical research on 100 human subjects. Acta anatomica. 1985;123:72–76. doi: 10.1159/000146042. [DOI] [PubMed] [Google Scholar]
- 26.Hunt BE, Farquhar WB, Taylor JA. Does reduced vascular stiffening fully explain preserved cardiovagal baroreflex function in older, physically active men? Circulation. 2001;103:2424. doi: 10.1161/01.cir.103.20.2424. [DOI] [PubMed] [Google Scholar]
- 27.Wollner L, McCarthy ST, Soper ND, Macy DJ. Failure of cerebral autoregulation as a cause of brain dysfunction in the elderly. Br. Med. J. 1979;1:1117. doi: 10.1136/bmj.1.6171.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carey BJ, Eames PJ, Blake MJ, Panerai RB, Potter JF. Dynamic cerebral autoregulation is unaffected by aging. Stroke. 2000;31:2895–2900. doi: 10.1161/01.str.31.12.2895. [DOI] [PubMed] [Google Scholar]
- 29.van Beek A, Claassen J, Rikkert M, Jansen R. Cerebral autoregulation: An overview of current concepts and methodology with special focus on the elderly. J. Cereb. Blood Flow Metab. 2008;28:1071–1085. doi: 10.1038/jcbfm.2008.13. [DOI] [PubMed] [Google Scholar]
- 30.Brodie FG, Panerai RB, Foster S, Evans DH, Robinson TG. Long-term changes in dynamic cerebral autoregulation: A 10 years follow up study. Clin. Physiol. Funct. Imaging. 2009;29:366–371. doi: 10.1111/j.1475-097X.2009.00880.x. [DOI] [PubMed] [Google Scholar]


